User login
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
Global project reveals cancer’s genomic playbook
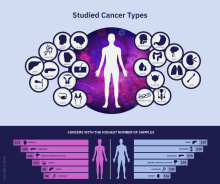
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE
Lenvatinib/pembrolizumab has good activity in advanced RCC, other solid tumors
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
New nomogram better predicts bladder cancer risk
A new and simple nomogram for predicting the risk of bladder cancer in patients with microscopic hematuria could optimize the diagnostic work up process, according to a recent study.
The tool may help improve patient understanding about their risk of bladder cancer, as well as alleviate unnecessary diagnostic evaluations for some patients.
“The goal of this study was to identify objective clinical factors associated with a bladder cancer diagnosis and to use these factors to create a nomogram that accurately predicts risk of bladder cancer,” wrote Richard S. Matulewicz, MD, MS, of Northwestern University, Chicago, and colleagues in Urologic Oncology.
Researchers identified 4,178 patients with a new diagnosis of microscopic hematuria from 2007 to 2015. Data was collected from an enterprise data repository of the Northwestern Medicine healthcare system. Study participants who underwent a full microhematuria evaluation were randomized to either a training or validation subgroup. In the training cohort, logistic regression analysis was used to detect factors linked to the diagnosis of bladder cancer. In the model, receiver operating curves were built to predict a diagnosis of bladder cancer among participants. In addition, calibration plots were computed for both subgroups to evaluate the discriminative ability of the model. After analysis, the researchers found significant differences in urinalysis results and demographics among patients with and without a diagnosis of bladder cancer. Patients with bladder cancer had a higher amount of microhematuria (RBC/hpf) on urinalysis (P less than .0001), were more likely previous or current smokers (P = .001), were more often male (68.2% vs. 49.7%; P = .0002), and were older (69.1 vs. 58.2 years; P less than .0001).
With respect to the predictive ability of the model, the area under the curve (AUC) in the training and validation set was 0.79 (95% confidence interval, 0.75-0.83) and 0.74 (95% CI, 0.67-0.80), respectively.
In addition, calibration plots demonstrated that the tool was able to predict the risk of bladder cancer diagnosis for patients with a probability of 0.3 or below.
“These results indicate that the model works best for a range of probabilities of (0-0.30), which is the vast majority of patients clinically and in our data,” the researchers explained.
The team acknowledged that characterizing risk beyond these levels should be done with caution given poor calibration beyond this threshold.
“External validation [of the model] and continued evolution of risk stratification models are needed,” they concluded.
The study was funded by the National Institutes of Health and the American Association of Medical Colleges. The authors reported having no conflicts of interest.
SOURCE: Matulewicz RS et al. Urol Oncol. 2020 Jan 14. doi: 10.1016/j.urolonc.2019.12.010.
A new and simple nomogram for predicting the risk of bladder cancer in patients with microscopic hematuria could optimize the diagnostic work up process, according to a recent study.
The tool may help improve patient understanding about their risk of bladder cancer, as well as alleviate unnecessary diagnostic evaluations for some patients.
“The goal of this study was to identify objective clinical factors associated with a bladder cancer diagnosis and to use these factors to create a nomogram that accurately predicts risk of bladder cancer,” wrote Richard S. Matulewicz, MD, MS, of Northwestern University, Chicago, and colleagues in Urologic Oncology.
Researchers identified 4,178 patients with a new diagnosis of microscopic hematuria from 2007 to 2015. Data was collected from an enterprise data repository of the Northwestern Medicine healthcare system. Study participants who underwent a full microhematuria evaluation were randomized to either a training or validation subgroup. In the training cohort, logistic regression analysis was used to detect factors linked to the diagnosis of bladder cancer. In the model, receiver operating curves were built to predict a diagnosis of bladder cancer among participants. In addition, calibration plots were computed for both subgroups to evaluate the discriminative ability of the model. After analysis, the researchers found significant differences in urinalysis results and demographics among patients with and without a diagnosis of bladder cancer. Patients with bladder cancer had a higher amount of microhematuria (RBC/hpf) on urinalysis (P less than .0001), were more likely previous or current smokers (P = .001), were more often male (68.2% vs. 49.7%; P = .0002), and were older (69.1 vs. 58.2 years; P less than .0001).
With respect to the predictive ability of the model, the area under the curve (AUC) in the training and validation set was 0.79 (95% confidence interval, 0.75-0.83) and 0.74 (95% CI, 0.67-0.80), respectively.
In addition, calibration plots demonstrated that the tool was able to predict the risk of bladder cancer diagnosis for patients with a probability of 0.3 or below.
“These results indicate that the model works best for a range of probabilities of (0-0.30), which is the vast majority of patients clinically and in our data,” the researchers explained.
The team acknowledged that characterizing risk beyond these levels should be done with caution given poor calibration beyond this threshold.
“External validation [of the model] and continued evolution of risk stratification models are needed,” they concluded.
The study was funded by the National Institutes of Health and the American Association of Medical Colleges. The authors reported having no conflicts of interest.
SOURCE: Matulewicz RS et al. Urol Oncol. 2020 Jan 14. doi: 10.1016/j.urolonc.2019.12.010.
A new and simple nomogram for predicting the risk of bladder cancer in patients with microscopic hematuria could optimize the diagnostic work up process, according to a recent study.
The tool may help improve patient understanding about their risk of bladder cancer, as well as alleviate unnecessary diagnostic evaluations for some patients.
“The goal of this study was to identify objective clinical factors associated with a bladder cancer diagnosis and to use these factors to create a nomogram that accurately predicts risk of bladder cancer,” wrote Richard S. Matulewicz, MD, MS, of Northwestern University, Chicago, and colleagues in Urologic Oncology.
Researchers identified 4,178 patients with a new diagnosis of microscopic hematuria from 2007 to 2015. Data was collected from an enterprise data repository of the Northwestern Medicine healthcare system. Study participants who underwent a full microhematuria evaluation were randomized to either a training or validation subgroup. In the training cohort, logistic regression analysis was used to detect factors linked to the diagnosis of bladder cancer. In the model, receiver operating curves were built to predict a diagnosis of bladder cancer among participants. In addition, calibration plots were computed for both subgroups to evaluate the discriminative ability of the model. After analysis, the researchers found significant differences in urinalysis results and demographics among patients with and without a diagnosis of bladder cancer. Patients with bladder cancer had a higher amount of microhematuria (RBC/hpf) on urinalysis (P less than .0001), were more likely previous or current smokers (P = .001), were more often male (68.2% vs. 49.7%; P = .0002), and were older (69.1 vs. 58.2 years; P less than .0001).
With respect to the predictive ability of the model, the area under the curve (AUC) in the training and validation set was 0.79 (95% confidence interval, 0.75-0.83) and 0.74 (95% CI, 0.67-0.80), respectively.
In addition, calibration plots demonstrated that the tool was able to predict the risk of bladder cancer diagnosis for patients with a probability of 0.3 or below.
“These results indicate that the model works best for a range of probabilities of (0-0.30), which is the vast majority of patients clinically and in our data,” the researchers explained.
The team acknowledged that characterizing risk beyond these levels should be done with caution given poor calibration beyond this threshold.
“External validation [of the model] and continued evolution of risk stratification models are needed,” they concluded.
The study was funded by the National Institutes of Health and the American Association of Medical Colleges. The authors reported having no conflicts of interest.
SOURCE: Matulewicz RS et al. Urol Oncol. 2020 Jan 14. doi: 10.1016/j.urolonc.2019.12.010.
FROM UROLOGIC ONCOLOGY
Patient-reported outcomes highlight long-term impacts of prostatectomy
For men with prostate cancer, prostatectomy is more likely to cause long-term, clinically meaningful incontinence than are other treatment modalities, based on patient-reported outcomes from more than 2,000 men.
Patients with high-risk disease who underwent prostatectomy also reported worse sexual function at every time point over 5 years, reported Karen E. Hoffman, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues. Other functional differences between treatments faded over time, they said.
“These estimates of the long-term bowel, bladder, and sexual function after localized prostate cancer treatment may clarify expectations and enable men to make informed choices about care,” the investigators wrote. Their report is in JAMA.
The prospective, population-based cohort study involved 1,386 men with favorable-risk prostate cancer and 619 men with unfavorable-risk disease. For men with favorable-risk disease, treatments included low-dose brachytherapy, external beam radiation therapy (EBRT), nerve-sparing prostatectomy, and active surveillance. Men with unfavorable-risk disease were treated with either prostatectomy or EBRT plus androgen deprivation therapy (ADT).
At multiple time points for 5 years, patients completed the 26-item Expanded Prostate Index Composite questionnaire (EPIC; range, 0-100). Clinically significant functional differences between treatment modalities were defined for bowel and hormonal function (4-6), urinary irritative symptoms (5-7), urinary incontinence (6-9), and sexual function (10-12). All 2,005 men completed at least one questionnaire, with about 3 out of 4 men (77%) still responding after 5 years.
Across all patients, prostatectomy was associated with a relatively higher risk of long-term, clinically meaningful urinary incontinence.
For men with favorable-risk disease, the adjusted mean difference for incontinence between nerve-sparing prostatectomy and active surveillance was –10.9 (P less than .001). Patients in this group who elected prostatectomy over active surveillance had a higher rate of urinary leakage (10% vs. 7%; P = .04); however, there was no significant difference in rates of moderate or big problems with urinary function, which suggests that incontinence issues were typically mild.
For men with unfavorable risk disease, prostatectomy carried a higher risk of more severe incontinence. The adjusted mean difference between prostatectomy and EBRT plus ADT was –23.2 (P less than .001), and patients treated with prostatectomy were more likely to report moderate or big problems with urinary function (17% vs. 13%; P = .005). In addition to these urinary issues, men with unfavorable-risk disease who underwent prostatectomy were more likely to report worse sexual function at 5 years, compared with those who were treated with EBRT plus ADT (adjusted mean difference, –12.5).
While other clinically meaningful differences were found between treatments for other functional categories, these differences lost statistical significance by the 5-year time point.
The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
SOURCE: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
For men with prostate cancer, prostatectomy is more likely to cause long-term, clinically meaningful incontinence than are other treatment modalities, based on patient-reported outcomes from more than 2,000 men.
Patients with high-risk disease who underwent prostatectomy also reported worse sexual function at every time point over 5 years, reported Karen E. Hoffman, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues. Other functional differences between treatments faded over time, they said.
“These estimates of the long-term bowel, bladder, and sexual function after localized prostate cancer treatment may clarify expectations and enable men to make informed choices about care,” the investigators wrote. Their report is in JAMA.
The prospective, population-based cohort study involved 1,386 men with favorable-risk prostate cancer and 619 men with unfavorable-risk disease. For men with favorable-risk disease, treatments included low-dose brachytherapy, external beam radiation therapy (EBRT), nerve-sparing prostatectomy, and active surveillance. Men with unfavorable-risk disease were treated with either prostatectomy or EBRT plus androgen deprivation therapy (ADT).
At multiple time points for 5 years, patients completed the 26-item Expanded Prostate Index Composite questionnaire (EPIC; range, 0-100). Clinically significant functional differences between treatment modalities were defined for bowel and hormonal function (4-6), urinary irritative symptoms (5-7), urinary incontinence (6-9), and sexual function (10-12). All 2,005 men completed at least one questionnaire, with about 3 out of 4 men (77%) still responding after 5 years.
Across all patients, prostatectomy was associated with a relatively higher risk of long-term, clinically meaningful urinary incontinence.
For men with favorable-risk disease, the adjusted mean difference for incontinence between nerve-sparing prostatectomy and active surveillance was –10.9 (P less than .001). Patients in this group who elected prostatectomy over active surveillance had a higher rate of urinary leakage (10% vs. 7%; P = .04); however, there was no significant difference in rates of moderate or big problems with urinary function, which suggests that incontinence issues were typically mild.
For men with unfavorable risk disease, prostatectomy carried a higher risk of more severe incontinence. The adjusted mean difference between prostatectomy and EBRT plus ADT was –23.2 (P less than .001), and patients treated with prostatectomy were more likely to report moderate or big problems with urinary function (17% vs. 13%; P = .005). In addition to these urinary issues, men with unfavorable-risk disease who underwent prostatectomy were more likely to report worse sexual function at 5 years, compared with those who were treated with EBRT plus ADT (adjusted mean difference, –12.5).
While other clinically meaningful differences were found between treatments for other functional categories, these differences lost statistical significance by the 5-year time point.
The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
SOURCE: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
For men with prostate cancer, prostatectomy is more likely to cause long-term, clinically meaningful incontinence than are other treatment modalities, based on patient-reported outcomes from more than 2,000 men.
Patients with high-risk disease who underwent prostatectomy also reported worse sexual function at every time point over 5 years, reported Karen E. Hoffman, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues. Other functional differences between treatments faded over time, they said.
“These estimates of the long-term bowel, bladder, and sexual function after localized prostate cancer treatment may clarify expectations and enable men to make informed choices about care,” the investigators wrote. Their report is in JAMA.
The prospective, population-based cohort study involved 1,386 men with favorable-risk prostate cancer and 619 men with unfavorable-risk disease. For men with favorable-risk disease, treatments included low-dose brachytherapy, external beam radiation therapy (EBRT), nerve-sparing prostatectomy, and active surveillance. Men with unfavorable-risk disease were treated with either prostatectomy or EBRT plus androgen deprivation therapy (ADT).
At multiple time points for 5 years, patients completed the 26-item Expanded Prostate Index Composite questionnaire (EPIC; range, 0-100). Clinically significant functional differences between treatment modalities were defined for bowel and hormonal function (4-6), urinary irritative symptoms (5-7), urinary incontinence (6-9), and sexual function (10-12). All 2,005 men completed at least one questionnaire, with about 3 out of 4 men (77%) still responding after 5 years.
Across all patients, prostatectomy was associated with a relatively higher risk of long-term, clinically meaningful urinary incontinence.
For men with favorable-risk disease, the adjusted mean difference for incontinence between nerve-sparing prostatectomy and active surveillance was –10.9 (P less than .001). Patients in this group who elected prostatectomy over active surveillance had a higher rate of urinary leakage (10% vs. 7%; P = .04); however, there was no significant difference in rates of moderate or big problems with urinary function, which suggests that incontinence issues were typically mild.
For men with unfavorable risk disease, prostatectomy carried a higher risk of more severe incontinence. The adjusted mean difference between prostatectomy and EBRT plus ADT was –23.2 (P less than .001), and patients treated with prostatectomy were more likely to report moderate or big problems with urinary function (17% vs. 13%; P = .005). In addition to these urinary issues, men with unfavorable-risk disease who underwent prostatectomy were more likely to report worse sexual function at 5 years, compared with those who were treated with EBRT plus ADT (adjusted mean difference, –12.5).
While other clinically meaningful differences were found between treatments for other functional categories, these differences lost statistical significance by the 5-year time point.
The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
SOURCE: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
FROM JAMA
Key clinical point: For men with prostate cancer, prostatectomy is significantly more likely to cause long-term incontinence than are other treatment modalities.
Major finding: For each time point through 5 years, men with unfavorable-risk prostate cancer who underwent prostatectomy had significantly worse incontinence scores than did those who underwent external beam radiation therapy (EBRT) and androgen deprivation therapy (ADT) (adjusted mean difference, –23.2).
Study details: A prospective, population-based cohort study involving 2,005 men prostate cancer.
Disclosures: The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
Source: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
HRQoL deteriorates during chemoradiotherapy for bladder cancer
Chemoradiotherapy impairs health-related quality of life (HRQoL) after the initiation of treatment in patients with muscle-invasive bladder cancer, according to an exploratory analysis of trial data.
However, HRQoL scores improved to pretreatment levels within 6 months, and improvements were maintained at 5-year follow up.
“[We] planned a prospective assessment of patient-reported outcomes within BC2001, using the Functional Assessment of Cancer Therapy–Bladder (FACT-BL) questionnaire,” wrote Robert A. Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research, England, and colleagues. Their report is in European Urology.
The exploratory analyses of HRQoL data included 452 patients from the phase 3 BC2001 trial. The objective of the analysis was to evaluate the impact of chemoradiotherapy on HRQoL in trial participants.
The BC2001 trial randomized patients to either radiotherapy (standard or reduced high-dose volume radiotherapy) and/or chemotherapy (radiotherapy or chemoradiotherapy) comparison groups. The primary outcome was the change in bladder cancer subscale (BLCS) scores from baseline to 12 months.
At trial baseline, study subjects were enrolled into the optional substudy, which involved completion of the FACT-BL questionnaire. Follow-up assessment was conducted at various time points after the start of radiotherapy.
After analysis, the researchers found that HRQoL scores deteriorated at end of treatment (BLCS: –5.06; 99% confidence interval, –6.12 to –4.00; overall FACT-BL: –8.22; 99% CI, –10.76 to –5.68), but improved to baseline levels at 6 months, and were maintained thereafter.
“Two-thirds of patients report stable or improved HRQoL on long-term follow-up,” they reported. “There [was] no evidence of impairment in HRQoL resulting from the addition of chemotherapy,” the researchers said.
In addition, the team found that pretrial administration of neoadjuvant chemotherapy had no significant impact on HRQoL outcomes.
One key limitation of the analysis was incomplete follow-up with HRQoL questionnaires. Over time, response rates declined, with a 60% expected response rate at 5 years.
“Addition of concomitant chemotherapy or use of neoadjuvant chemotherapy has no significant impact on HRQoL, further supporting the routine use of 5-FU and mitomycin C in this setting,” Dr. Huddart and coauthors concluded.
Cancer Research UK funded the study. The authors reported having no conflicts of interest.
SOURCE: Huddart RA et al. Eur Urol. 2019 Dec 13. doi: 10.1016/j.eururo.2019.11.001.
Chemoradiotherapy impairs health-related quality of life (HRQoL) after the initiation of treatment in patients with muscle-invasive bladder cancer, according to an exploratory analysis of trial data.
However, HRQoL scores improved to pretreatment levels within 6 months, and improvements were maintained at 5-year follow up.
“[We] planned a prospective assessment of patient-reported outcomes within BC2001, using the Functional Assessment of Cancer Therapy–Bladder (FACT-BL) questionnaire,” wrote Robert A. Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research, England, and colleagues. Their report is in European Urology.
The exploratory analyses of HRQoL data included 452 patients from the phase 3 BC2001 trial. The objective of the analysis was to evaluate the impact of chemoradiotherapy on HRQoL in trial participants.
The BC2001 trial randomized patients to either radiotherapy (standard or reduced high-dose volume radiotherapy) and/or chemotherapy (radiotherapy or chemoradiotherapy) comparison groups. The primary outcome was the change in bladder cancer subscale (BLCS) scores from baseline to 12 months.
At trial baseline, study subjects were enrolled into the optional substudy, which involved completion of the FACT-BL questionnaire. Follow-up assessment was conducted at various time points after the start of radiotherapy.
After analysis, the researchers found that HRQoL scores deteriorated at end of treatment (BLCS: –5.06; 99% confidence interval, –6.12 to –4.00; overall FACT-BL: –8.22; 99% CI, –10.76 to –5.68), but improved to baseline levels at 6 months, and were maintained thereafter.
“Two-thirds of patients report stable or improved HRQoL on long-term follow-up,” they reported. “There [was] no evidence of impairment in HRQoL resulting from the addition of chemotherapy,” the researchers said.
In addition, the team found that pretrial administration of neoadjuvant chemotherapy had no significant impact on HRQoL outcomes.
One key limitation of the analysis was incomplete follow-up with HRQoL questionnaires. Over time, response rates declined, with a 60% expected response rate at 5 years.
“Addition of concomitant chemotherapy or use of neoadjuvant chemotherapy has no significant impact on HRQoL, further supporting the routine use of 5-FU and mitomycin C in this setting,” Dr. Huddart and coauthors concluded.
Cancer Research UK funded the study. The authors reported having no conflicts of interest.
SOURCE: Huddart RA et al. Eur Urol. 2019 Dec 13. doi: 10.1016/j.eururo.2019.11.001.
Chemoradiotherapy impairs health-related quality of life (HRQoL) after the initiation of treatment in patients with muscle-invasive bladder cancer, according to an exploratory analysis of trial data.
However, HRQoL scores improved to pretreatment levels within 6 months, and improvements were maintained at 5-year follow up.
“[We] planned a prospective assessment of patient-reported outcomes within BC2001, using the Functional Assessment of Cancer Therapy–Bladder (FACT-BL) questionnaire,” wrote Robert A. Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research, England, and colleagues. Their report is in European Urology.
The exploratory analyses of HRQoL data included 452 patients from the phase 3 BC2001 trial. The objective of the analysis was to evaluate the impact of chemoradiotherapy on HRQoL in trial participants.
The BC2001 trial randomized patients to either radiotherapy (standard or reduced high-dose volume radiotherapy) and/or chemotherapy (radiotherapy or chemoradiotherapy) comparison groups. The primary outcome was the change in bladder cancer subscale (BLCS) scores from baseline to 12 months.
At trial baseline, study subjects were enrolled into the optional substudy, which involved completion of the FACT-BL questionnaire. Follow-up assessment was conducted at various time points after the start of radiotherapy.
After analysis, the researchers found that HRQoL scores deteriorated at end of treatment (BLCS: –5.06; 99% confidence interval, –6.12 to –4.00; overall FACT-BL: –8.22; 99% CI, –10.76 to –5.68), but improved to baseline levels at 6 months, and were maintained thereafter.
“Two-thirds of patients report stable or improved HRQoL on long-term follow-up,” they reported. “There [was] no evidence of impairment in HRQoL resulting from the addition of chemotherapy,” the researchers said.
In addition, the team found that pretrial administration of neoadjuvant chemotherapy had no significant impact on HRQoL outcomes.
One key limitation of the analysis was incomplete follow-up with HRQoL questionnaires. Over time, response rates declined, with a 60% expected response rate at 5 years.
“Addition of concomitant chemotherapy or use of neoadjuvant chemotherapy has no significant impact on HRQoL, further supporting the routine use of 5-FU and mitomycin C in this setting,” Dr. Huddart and coauthors concluded.
Cancer Research UK funded the study. The authors reported having no conflicts of interest.
SOURCE: Huddart RA et al. Eur Urol. 2019 Dec 13. doi: 10.1016/j.eururo.2019.11.001.
FROM EUROPEAN UROLOGY
FDA approves pembrolizumab for BCG-unresponsive NMIBC with CIS
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guérin (BCG)–unresponsive, high-risk, non–muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors, who are ineligible for or have elected not to undergo cystectomy.
The approval was based on response in a single-arm trial of 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors, the FDA said in a statement.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41% (95% confidence interval, 31-51) and median response duration was 16.2 months.
The most common adverse reactions in patients who received pembrolizumab in the trial were fatigue, diarrhea, rash, pruritus, musculoskeletal pain, hematuria, cough, arthralgia, nausea, constipation, urinary tract infection, peripheral edema, hypothyroidism, and nasopharyngitis.
The recommended dose is 200 mg every 3 weeks, the FDA said.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guérin (BCG)–unresponsive, high-risk, non–muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors, who are ineligible for or have elected not to undergo cystectomy.
The approval was based on response in a single-arm trial of 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors, the FDA said in a statement.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41% (95% confidence interval, 31-51) and median response duration was 16.2 months.
The most common adverse reactions in patients who received pembrolizumab in the trial were fatigue, diarrhea, rash, pruritus, musculoskeletal pain, hematuria, cough, arthralgia, nausea, constipation, urinary tract infection, peripheral edema, hypothyroidism, and nasopharyngitis.
The recommended dose is 200 mg every 3 weeks, the FDA said.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guérin (BCG)–unresponsive, high-risk, non–muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors, who are ineligible for or have elected not to undergo cystectomy.
The approval was based on response in a single-arm trial of 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors, the FDA said in a statement.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41% (95% confidence interval, 31-51) and median response duration was 16.2 months.
The most common adverse reactions in patients who received pembrolizumab in the trial were fatigue, diarrhea, rash, pruritus, musculoskeletal pain, hematuria, cough, arthralgia, nausea, constipation, urinary tract infection, peripheral edema, hypothyroidism, and nasopharyngitis.
The recommended dose is 200 mg every 3 weeks, the FDA said.
Study simplifies bladder cancer molecular subtypes
Muscle-invasive bladder cancer (MIBC) can be divided into six molecular classes that may eventually guide clinical decision making, according to an international consensus group.
The six classes are based on a variety of disease factors, including immune and stromal cell infiltration, oncogenic mechanisms, histologic features, and clinical characteristics, reported lead author Aurélie Kamoun, PhD, of Ligue Nationale Contre le Cancer in Paris, and colleagues.
Although several molecular classification systems for MIBC have been previously published, subtypes have varied widely, the investigators explained in European Urology.
“This diversity has impeded transferring subtypes into clinical practice and highlights that establishing a single consensus set of molecular subtypes would facilitate achieving such a transfer,” the investigators wrote.
In order to reach a consensus, the investigators drew data from the six previously published classification systems, including 1,750 transcriptomic profiles from 16 published datasets and 2 additional patient populations.
Using a network-based approach, the investigators identified consensus classes that reconciled differences between the previously published systems. This process revealed a core set of 1,084 consensus samples, from which the investigators built a single-sample transcriptomic classifier. This free tool is now available online at https://github.com/cit-bioinfo/consensusMIBC.
The six consensus molecular classes (with prevalence among samples) are basal/squamous (35%), luminal papillary (24%), luminal unstable (15%), luminal nonspecified (8%), stroma rich (15%), and neuroendocrine like (3%).
The investigators described a number of disease characteristics that correlate with each molecular class, from the genomic to the clinical level. For example, basal/squamous tumors were associated with TP53 mutations and immune infiltration involving natural killer cells and cytotoxic lymphocytes.
Similar conclusions were described at the clinical level.
Consensus class predicted overall survival, with neuroendocrine-like tumors having the worst prognosis relative to luminal papillary tumors (hazard ratio, 2.34; P less than .03).
The investigators also highlighted some possible treatment implications related to subtype. For example, patients with basal/squamous or luminal nonspecified tumors derived benefit from neoadjuvant chemotherapy. Similarly, patients with luminal nonspecified, luminal unstable, or neuroendocrine-like tumors were more likely to respond when given atezolizumab.
“We expect that this consensus classification will help the development of MIBC precision medicine by providing a robust framework to connect clinical findings to molecular contexts and to identify clinically relevant biomarkers for patient management,” Dr. Kamoun and coauthors concluded.
The investigators reported relationships with Bayer, Astellas, Genentech, and others.
SOURCE: Kamoun A et al. Eur Urol. 2019 Sep 26. doi: 10.1016/j.eururo.2019.09.006.
Muscle-invasive bladder cancer (MIBC) can be divided into six molecular classes that may eventually guide clinical decision making, according to an international consensus group.
The six classes are based on a variety of disease factors, including immune and stromal cell infiltration, oncogenic mechanisms, histologic features, and clinical characteristics, reported lead author Aurélie Kamoun, PhD, of Ligue Nationale Contre le Cancer in Paris, and colleagues.
Although several molecular classification systems for MIBC have been previously published, subtypes have varied widely, the investigators explained in European Urology.
“This diversity has impeded transferring subtypes into clinical practice and highlights that establishing a single consensus set of molecular subtypes would facilitate achieving such a transfer,” the investigators wrote.
In order to reach a consensus, the investigators drew data from the six previously published classification systems, including 1,750 transcriptomic profiles from 16 published datasets and 2 additional patient populations.
Using a network-based approach, the investigators identified consensus classes that reconciled differences between the previously published systems. This process revealed a core set of 1,084 consensus samples, from which the investigators built a single-sample transcriptomic classifier. This free tool is now available online at https://github.com/cit-bioinfo/consensusMIBC.
The six consensus molecular classes (with prevalence among samples) are basal/squamous (35%), luminal papillary (24%), luminal unstable (15%), luminal nonspecified (8%), stroma rich (15%), and neuroendocrine like (3%).
The investigators described a number of disease characteristics that correlate with each molecular class, from the genomic to the clinical level. For example, basal/squamous tumors were associated with TP53 mutations and immune infiltration involving natural killer cells and cytotoxic lymphocytes.
Similar conclusions were described at the clinical level.
Consensus class predicted overall survival, with neuroendocrine-like tumors having the worst prognosis relative to luminal papillary tumors (hazard ratio, 2.34; P less than .03).
The investigators also highlighted some possible treatment implications related to subtype. For example, patients with basal/squamous or luminal nonspecified tumors derived benefit from neoadjuvant chemotherapy. Similarly, patients with luminal nonspecified, luminal unstable, or neuroendocrine-like tumors were more likely to respond when given atezolizumab.
“We expect that this consensus classification will help the development of MIBC precision medicine by providing a robust framework to connect clinical findings to molecular contexts and to identify clinically relevant biomarkers for patient management,” Dr. Kamoun and coauthors concluded.
The investigators reported relationships with Bayer, Astellas, Genentech, and others.
SOURCE: Kamoun A et al. Eur Urol. 2019 Sep 26. doi: 10.1016/j.eururo.2019.09.006.
Muscle-invasive bladder cancer (MIBC) can be divided into six molecular classes that may eventually guide clinical decision making, according to an international consensus group.
The six classes are based on a variety of disease factors, including immune and stromal cell infiltration, oncogenic mechanisms, histologic features, and clinical characteristics, reported lead author Aurélie Kamoun, PhD, of Ligue Nationale Contre le Cancer in Paris, and colleagues.
Although several molecular classification systems for MIBC have been previously published, subtypes have varied widely, the investigators explained in European Urology.
“This diversity has impeded transferring subtypes into clinical practice and highlights that establishing a single consensus set of molecular subtypes would facilitate achieving such a transfer,” the investigators wrote.
In order to reach a consensus, the investigators drew data from the six previously published classification systems, including 1,750 transcriptomic profiles from 16 published datasets and 2 additional patient populations.
Using a network-based approach, the investigators identified consensus classes that reconciled differences between the previously published systems. This process revealed a core set of 1,084 consensus samples, from which the investigators built a single-sample transcriptomic classifier. This free tool is now available online at https://github.com/cit-bioinfo/consensusMIBC.
The six consensus molecular classes (with prevalence among samples) are basal/squamous (35%), luminal papillary (24%), luminal unstable (15%), luminal nonspecified (8%), stroma rich (15%), and neuroendocrine like (3%).
The investigators described a number of disease characteristics that correlate with each molecular class, from the genomic to the clinical level. For example, basal/squamous tumors were associated with TP53 mutations and immune infiltration involving natural killer cells and cytotoxic lymphocytes.
Similar conclusions were described at the clinical level.
Consensus class predicted overall survival, with neuroendocrine-like tumors having the worst prognosis relative to luminal papillary tumors (hazard ratio, 2.34; P less than .03).
The investigators also highlighted some possible treatment implications related to subtype. For example, patients with basal/squamous or luminal nonspecified tumors derived benefit from neoadjuvant chemotherapy. Similarly, patients with luminal nonspecified, luminal unstable, or neuroendocrine-like tumors were more likely to respond when given atezolizumab.
“We expect that this consensus classification will help the development of MIBC precision medicine by providing a robust framework to connect clinical findings to molecular contexts and to identify clinically relevant biomarkers for patient management,” Dr. Kamoun and coauthors concluded.
The investigators reported relationships with Bayer, Astellas, Genentech, and others.
SOURCE: Kamoun A et al. Eur Urol. 2019 Sep 26. doi: 10.1016/j.eururo.2019.09.006.
FROM EUROPEAN UROLOGY
Urothelial cancer: Less gained from immunotherapy in patients with poor performance scores
Patients with advanced urothelial cancer and a poor performance status may have little to gain from immune checkpoint inhibitor therapy, suggests a multicenter real-world retrospective cohort study.
“The perceived favorable toxicity profile of immune checkpoint inhibitors has led to the selection of these agents for patients otherwise unfit for systemic chemotherapy,” noted the investigators, led by Ali Raza Khaki, MD, a fellow in the division of oncology, department of medicine, University of Washington, Seattle. “However, there is a paucity of data supporting the use of immune checkpoint inhibitors in patients with a poor performance status, who were not very well represented in the clinical trials that led to their approval, with no trial enrolling patients with an ECOG [Eastern Cooperative Oncology Group] performance status greater than or equal to 3 and only 3 trials including patients with an ECOG performance status of 2.”
Dr. Khaki and coinvestigators analyzed data from 499 patients with advanced urothelial cancer treated with immune checkpoint inhibitors at 18 institutions during 2013-2019. Slightly more than one-quarter had an ECOG performance status of 2 or higher.
Study results, reported in Cancer, showed that the overall response rate to immune checkpoint inhibitor therapy was similar regardless of performance status, across patients being treated in different lines of therapy.
However, among patients being treated in the first line, overall survival was better for those with a performance status of 0 to 1 than for those with a performance status of 2 or higher (median, 15.2 vs. 7.2 months; hazard ratio for death, 0.62; P = .01). There was no significant difference in this outcome among patients being treated in subsequent lines (median, 9.8 vs. 8.2 months; hazard ratio, 0.78; P = .27).
Among the 288 patients who died, 10% started immune checkpoint inhibitors in the last 30 days of life and 33% started them in the last 90 days of life. Patients initiating this therapy in the last 30 days of life were almost three time more likely to die in a hospital (odds ratio, 2.89; P = .04).
Notably, among the 11 patients with an ECOG performance status of 3, none had a response to immune checkpoint inhibitors and only two achieved stable disease. Moreover, two patients in this subgroup died within a week of receiving these agents.
“Despite comparable overall response rates, immune checkpoint inhibitors may not overcome the negative prognostic role of a poor performance status, particularly in the first-line setting,” Dr. Khaki and coinvestigators wrote. “Our study underscores the importance of developing prospectively validated predictive biomarkers to aid in identifying those patients most and least likely to benefit from immune checkpoint inhibitors.
“Overall, our data suggest that the decision of immune checkpoint inhibitor initiation near the end of life, akin to the practice for chemotherapy, should be considered carefully, and it should be accompanied by a detailed discussion of the data, rationale, and risks and benefits to minimize unnecessary potential adverse events and the cost and intensity of end-of-life care,” they recommended.
Dr. Khaki did not disclose any conflicts of interest. The study was supported by the National Cancer Institute, the National Center for Advancing Translational Sciences of the National Institutes of Health, the Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, the Wellcome Trust Strategic Fund, and Merck.
SOURCE: Khaki AR et al. Cancer. 2019 Dec 12. doi: 10.1002/cncr.32645.
Patients with advanced urothelial cancer and a poor performance status may have little to gain from immune checkpoint inhibitor therapy, suggests a multicenter real-world retrospective cohort study.
“The perceived favorable toxicity profile of immune checkpoint inhibitors has led to the selection of these agents for patients otherwise unfit for systemic chemotherapy,” noted the investigators, led by Ali Raza Khaki, MD, a fellow in the division of oncology, department of medicine, University of Washington, Seattle. “However, there is a paucity of data supporting the use of immune checkpoint inhibitors in patients with a poor performance status, who were not very well represented in the clinical trials that led to their approval, with no trial enrolling patients with an ECOG [Eastern Cooperative Oncology Group] performance status greater than or equal to 3 and only 3 trials including patients with an ECOG performance status of 2.”
Dr. Khaki and coinvestigators analyzed data from 499 patients with advanced urothelial cancer treated with immune checkpoint inhibitors at 18 institutions during 2013-2019. Slightly more than one-quarter had an ECOG performance status of 2 or higher.
Study results, reported in Cancer, showed that the overall response rate to immune checkpoint inhibitor therapy was similar regardless of performance status, across patients being treated in different lines of therapy.
However, among patients being treated in the first line, overall survival was better for those with a performance status of 0 to 1 than for those with a performance status of 2 or higher (median, 15.2 vs. 7.2 months; hazard ratio for death, 0.62; P = .01). There was no significant difference in this outcome among patients being treated in subsequent lines (median, 9.8 vs. 8.2 months; hazard ratio, 0.78; P = .27).
Among the 288 patients who died, 10% started immune checkpoint inhibitors in the last 30 days of life and 33% started them in the last 90 days of life. Patients initiating this therapy in the last 30 days of life were almost three time more likely to die in a hospital (odds ratio, 2.89; P = .04).
Notably, among the 11 patients with an ECOG performance status of 3, none had a response to immune checkpoint inhibitors and only two achieved stable disease. Moreover, two patients in this subgroup died within a week of receiving these agents.
“Despite comparable overall response rates, immune checkpoint inhibitors may not overcome the negative prognostic role of a poor performance status, particularly in the first-line setting,” Dr. Khaki and coinvestigators wrote. “Our study underscores the importance of developing prospectively validated predictive biomarkers to aid in identifying those patients most and least likely to benefit from immune checkpoint inhibitors.
“Overall, our data suggest that the decision of immune checkpoint inhibitor initiation near the end of life, akin to the practice for chemotherapy, should be considered carefully, and it should be accompanied by a detailed discussion of the data, rationale, and risks and benefits to minimize unnecessary potential adverse events and the cost and intensity of end-of-life care,” they recommended.
Dr. Khaki did not disclose any conflicts of interest. The study was supported by the National Cancer Institute, the National Center for Advancing Translational Sciences of the National Institutes of Health, the Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, the Wellcome Trust Strategic Fund, and Merck.
SOURCE: Khaki AR et al. Cancer. 2019 Dec 12. doi: 10.1002/cncr.32645.
Patients with advanced urothelial cancer and a poor performance status may have little to gain from immune checkpoint inhibitor therapy, suggests a multicenter real-world retrospective cohort study.
“The perceived favorable toxicity profile of immune checkpoint inhibitors has led to the selection of these agents for patients otherwise unfit for systemic chemotherapy,” noted the investigators, led by Ali Raza Khaki, MD, a fellow in the division of oncology, department of medicine, University of Washington, Seattle. “However, there is a paucity of data supporting the use of immune checkpoint inhibitors in patients with a poor performance status, who were not very well represented in the clinical trials that led to their approval, with no trial enrolling patients with an ECOG [Eastern Cooperative Oncology Group] performance status greater than or equal to 3 and only 3 trials including patients with an ECOG performance status of 2.”
Dr. Khaki and coinvestigators analyzed data from 499 patients with advanced urothelial cancer treated with immune checkpoint inhibitors at 18 institutions during 2013-2019. Slightly more than one-quarter had an ECOG performance status of 2 or higher.
Study results, reported in Cancer, showed that the overall response rate to immune checkpoint inhibitor therapy was similar regardless of performance status, across patients being treated in different lines of therapy.
However, among patients being treated in the first line, overall survival was better for those with a performance status of 0 to 1 than for those with a performance status of 2 or higher (median, 15.2 vs. 7.2 months; hazard ratio for death, 0.62; P = .01). There was no significant difference in this outcome among patients being treated in subsequent lines (median, 9.8 vs. 8.2 months; hazard ratio, 0.78; P = .27).
Among the 288 patients who died, 10% started immune checkpoint inhibitors in the last 30 days of life and 33% started them in the last 90 days of life. Patients initiating this therapy in the last 30 days of life were almost three time more likely to die in a hospital (odds ratio, 2.89; P = .04).
Notably, among the 11 patients with an ECOG performance status of 3, none had a response to immune checkpoint inhibitors and only two achieved stable disease. Moreover, two patients in this subgroup died within a week of receiving these agents.
“Despite comparable overall response rates, immune checkpoint inhibitors may not overcome the negative prognostic role of a poor performance status, particularly in the first-line setting,” Dr. Khaki and coinvestigators wrote. “Our study underscores the importance of developing prospectively validated predictive biomarkers to aid in identifying those patients most and least likely to benefit from immune checkpoint inhibitors.
“Overall, our data suggest that the decision of immune checkpoint inhibitor initiation near the end of life, akin to the practice for chemotherapy, should be considered carefully, and it should be accompanied by a detailed discussion of the data, rationale, and risks and benefits to minimize unnecessary potential adverse events and the cost and intensity of end-of-life care,” they recommended.
Dr. Khaki did not disclose any conflicts of interest. The study was supported by the National Cancer Institute, the National Center for Advancing Translational Sciences of the National Institutes of Health, the Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, the Wellcome Trust Strategic Fund, and Merck.
SOURCE: Khaki AR et al. Cancer. 2019 Dec 12. doi: 10.1002/cncr.32645.
FROM CANCER
FDA approves antibody-drug conjugate for advanced urothelial cancer
The Food and Drug Administration has granted accelerated approval to enfortumab vedotin-ejfv (Padcev) for the treatment of adult patients with locally advanced or metastatic urothelial cancer that has previously been treated with a programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.
The conjugate was approved based on overall response rate in a trial of 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy, the FDA said in a press statement.
The overall response rate was 44%, with 12% having a complete response and 32% having a partial response. The median duration of response was 7.6 months.
The most common side effects for patients were fatigue, peripheral neuropathy, decreased appetite, rash, alopecia, nausea, altered taste, diarrhea, dry eye, pruritis, and dry skin. Patients may experience hyperglycemia, and blood sugar levels should be monitored closely in patients receiving enfortumab vedotin-ejfv, the FDA said.
Patients may experience eye disorders, and health care professionals may consider prophylactic artificial tears for dry eyes and referral to an ophthalmologist for any new symptoms related to the eye, the agency said. The FDA also advises telling patients of reproductive age to use effective contraception during treatment, and for a period of time thereafter. Women who are pregnant or breastfeeding should not take the antibody-drug conjugate because it may cause harm to a developing fetus or newborn baby or cause delivery complications.
The Food and Drug Administration has granted accelerated approval to enfortumab vedotin-ejfv (Padcev) for the treatment of adult patients with locally advanced or metastatic urothelial cancer that has previously been treated with a programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.
The conjugate was approved based on overall response rate in a trial of 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy, the FDA said in a press statement.
The overall response rate was 44%, with 12% having a complete response and 32% having a partial response. The median duration of response was 7.6 months.
The most common side effects for patients were fatigue, peripheral neuropathy, decreased appetite, rash, alopecia, nausea, altered taste, diarrhea, dry eye, pruritis, and dry skin. Patients may experience hyperglycemia, and blood sugar levels should be monitored closely in patients receiving enfortumab vedotin-ejfv, the FDA said.
Patients may experience eye disorders, and health care professionals may consider prophylactic artificial tears for dry eyes and referral to an ophthalmologist for any new symptoms related to the eye, the agency said. The FDA also advises telling patients of reproductive age to use effective contraception during treatment, and for a period of time thereafter. Women who are pregnant or breastfeeding should not take the antibody-drug conjugate because it may cause harm to a developing fetus or newborn baby or cause delivery complications.
The Food and Drug Administration has granted accelerated approval to enfortumab vedotin-ejfv (Padcev) for the treatment of adult patients with locally advanced or metastatic urothelial cancer that has previously been treated with a programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.
The conjugate was approved based on overall response rate in a trial of 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy, the FDA said in a press statement.
The overall response rate was 44%, with 12% having a complete response and 32% having a partial response. The median duration of response was 7.6 months.
The most common side effects for patients were fatigue, peripheral neuropathy, decreased appetite, rash, alopecia, nausea, altered taste, diarrhea, dry eye, pruritis, and dry skin. Patients may experience hyperglycemia, and blood sugar levels should be monitored closely in patients receiving enfortumab vedotin-ejfv, the FDA said.
Patients may experience eye disorders, and health care professionals may consider prophylactic artificial tears for dry eyes and referral to an ophthalmologist for any new symptoms related to the eye, the agency said. The FDA also advises telling patients of reproductive age to use effective contraception during treatment, and for a period of time thereafter. Women who are pregnant or breastfeeding should not take the antibody-drug conjugate because it may cause harm to a developing fetus or newborn baby or cause delivery complications.