User login
Prepare for ‘the coming tsunami’ of NAFLD
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
REPORTING FROM ACP INTERNAL MEDICINE
Weight gain linked to progression of fibrosis in NAFLD patients
according to recent research published in Clinical Gastroenterology and Hepatology.
Researchers evaluated 40,700 Korean adults (minimum age, 18 years) with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up. Patients were categorized and placed into weight quintiles based on whether they lost weight (quintile 1, 2.3-kg or greater weight loss; quintile 2, 2.2-kg to 0.6-kg weight loss), gained weight (quintile 4, 0.7- to 2.1-kg weight gain; quintile 5, at least 2.2-kg or greater weight gain) or whether their weight remained stable (quintile 3, 0.5-kg weight loss to 0.6-kg weight gain). Researchers followed patients from baseline to fibrosis progression or last visit, calculated as person-years, and used the aspartate aminotransferase to platelet ratio index (APRI) to measure outcomes. They defined body mass index based on criteria specific to Asian populations, with underweight categorized as less than 18.5 kg/m2, normal weight as 18.5-23 kg/m2, overweight as 23-25 kg/m2, and obese as at least 25 kg/m2.
“Our findings from mostly asymptomatic, relatively young individuals with ultrasonographically detected steatosis, possibly reflecting low-risk NAFLD patients, are less likely to be affected by survivor bias and biases related to comorbidities, compared with previous findings from cohorts of high-risk groups that underwent liver biopsy,” Seungho Ryu, MD, PhD, from Kangbuk Samsung Hospital in Seoul, South Korea, and colleagues wrote in the study.
There were 5,454 participants who progressed from a low APRI to an intermediate or high APRI within 275,451.5 person-years, researchers said. Compared with the stable-weight group, hazard ratios for APRI progression in the first weight-change quintile were 0.68 (95% confidence interval, 0.62-0.74) and 0.86 in the second weight-change quintile (95% CI, 0.78-0.94). In the weight-gain groups, an increase in weight was associated with APRI progression in the fourth quintile (HR, 1.17; 95% CI, 1.07-1.28) and fifth quintile (HR, 1.71; 95% CI, 1.58-1.85) groups.
After multivariable adjustment, there was an increase in APRI progression among patients with BMIs between 23 and 24.9 kg/m2 (HR, 1.13; 95% CI, 1.02-1.26), between 25 and 29.9 kg/m2 (HR, 1.41; 95% CI, 1.28-1.55), and greater than or equal to 30 kg/m2 (HR, 2.09; 95% CI, 1.86-2.36) compared with patients with a BMI between 18.5 and 22.9 kg/m2,.
Limitations of the study included the use of ultrasonography in place of liver biopsy for diagnosing NAFLD and the use of APRI to predict fibrosis in individuals with NAFLD, researchers said.
“APRI has demonstrated a reasonable utility as a noninvasive method for the prediction of histologically confirmed advanced fibrosis,” Dr. Ryu and colleagues wrote. “Nonetheless, we acknowledge that there is no currently available longitudinal data to support the use of worsening noninvasive fibrosis markers as an indicator of histological progression of fibrosis stage over time.”
Other limitations included the study’s retrospective design, lack of availability of medication use and dietary intake, and lack of generalization based on a young, healthy population of mostly Korean employees who were employed by companies or local government. However, researchers said clinicians should encourage their patients with NAFLD to maintain a healthy weight to avoid progression of fibrosis.
The authors reported no relevant financial disclosures.
SOURCE: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
according to recent research published in Clinical Gastroenterology and Hepatology.
Researchers evaluated 40,700 Korean adults (minimum age, 18 years) with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up. Patients were categorized and placed into weight quintiles based on whether they lost weight (quintile 1, 2.3-kg or greater weight loss; quintile 2, 2.2-kg to 0.6-kg weight loss), gained weight (quintile 4, 0.7- to 2.1-kg weight gain; quintile 5, at least 2.2-kg or greater weight gain) or whether their weight remained stable (quintile 3, 0.5-kg weight loss to 0.6-kg weight gain). Researchers followed patients from baseline to fibrosis progression or last visit, calculated as person-years, and used the aspartate aminotransferase to platelet ratio index (APRI) to measure outcomes. They defined body mass index based on criteria specific to Asian populations, with underweight categorized as less than 18.5 kg/m2, normal weight as 18.5-23 kg/m2, overweight as 23-25 kg/m2, and obese as at least 25 kg/m2.
“Our findings from mostly asymptomatic, relatively young individuals with ultrasonographically detected steatosis, possibly reflecting low-risk NAFLD patients, are less likely to be affected by survivor bias and biases related to comorbidities, compared with previous findings from cohorts of high-risk groups that underwent liver biopsy,” Seungho Ryu, MD, PhD, from Kangbuk Samsung Hospital in Seoul, South Korea, and colleagues wrote in the study.
There were 5,454 participants who progressed from a low APRI to an intermediate or high APRI within 275,451.5 person-years, researchers said. Compared with the stable-weight group, hazard ratios for APRI progression in the first weight-change quintile were 0.68 (95% confidence interval, 0.62-0.74) and 0.86 in the second weight-change quintile (95% CI, 0.78-0.94). In the weight-gain groups, an increase in weight was associated with APRI progression in the fourth quintile (HR, 1.17; 95% CI, 1.07-1.28) and fifth quintile (HR, 1.71; 95% CI, 1.58-1.85) groups.
After multivariable adjustment, there was an increase in APRI progression among patients with BMIs between 23 and 24.9 kg/m2 (HR, 1.13; 95% CI, 1.02-1.26), between 25 and 29.9 kg/m2 (HR, 1.41; 95% CI, 1.28-1.55), and greater than or equal to 30 kg/m2 (HR, 2.09; 95% CI, 1.86-2.36) compared with patients with a BMI between 18.5 and 22.9 kg/m2,.
Limitations of the study included the use of ultrasonography in place of liver biopsy for diagnosing NAFLD and the use of APRI to predict fibrosis in individuals with NAFLD, researchers said.
“APRI has demonstrated a reasonable utility as a noninvasive method for the prediction of histologically confirmed advanced fibrosis,” Dr. Ryu and colleagues wrote. “Nonetheless, we acknowledge that there is no currently available longitudinal data to support the use of worsening noninvasive fibrosis markers as an indicator of histological progression of fibrosis stage over time.”
Other limitations included the study’s retrospective design, lack of availability of medication use and dietary intake, and lack of generalization based on a young, healthy population of mostly Korean employees who were employed by companies or local government. However, researchers said clinicians should encourage their patients with NAFLD to maintain a healthy weight to avoid progression of fibrosis.
The authors reported no relevant financial disclosures.
SOURCE: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
according to recent research published in Clinical Gastroenterology and Hepatology.
Researchers evaluated 40,700 Korean adults (minimum age, 18 years) with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up. Patients were categorized and placed into weight quintiles based on whether they lost weight (quintile 1, 2.3-kg or greater weight loss; quintile 2, 2.2-kg to 0.6-kg weight loss), gained weight (quintile 4, 0.7- to 2.1-kg weight gain; quintile 5, at least 2.2-kg or greater weight gain) or whether their weight remained stable (quintile 3, 0.5-kg weight loss to 0.6-kg weight gain). Researchers followed patients from baseline to fibrosis progression or last visit, calculated as person-years, and used the aspartate aminotransferase to platelet ratio index (APRI) to measure outcomes. They defined body mass index based on criteria specific to Asian populations, with underweight categorized as less than 18.5 kg/m2, normal weight as 18.5-23 kg/m2, overweight as 23-25 kg/m2, and obese as at least 25 kg/m2.
“Our findings from mostly asymptomatic, relatively young individuals with ultrasonographically detected steatosis, possibly reflecting low-risk NAFLD patients, are less likely to be affected by survivor bias and biases related to comorbidities, compared with previous findings from cohorts of high-risk groups that underwent liver biopsy,” Seungho Ryu, MD, PhD, from Kangbuk Samsung Hospital in Seoul, South Korea, and colleagues wrote in the study.
There were 5,454 participants who progressed from a low APRI to an intermediate or high APRI within 275,451.5 person-years, researchers said. Compared with the stable-weight group, hazard ratios for APRI progression in the first weight-change quintile were 0.68 (95% confidence interval, 0.62-0.74) and 0.86 in the second weight-change quintile (95% CI, 0.78-0.94). In the weight-gain groups, an increase in weight was associated with APRI progression in the fourth quintile (HR, 1.17; 95% CI, 1.07-1.28) and fifth quintile (HR, 1.71; 95% CI, 1.58-1.85) groups.
After multivariable adjustment, there was an increase in APRI progression among patients with BMIs between 23 and 24.9 kg/m2 (HR, 1.13; 95% CI, 1.02-1.26), between 25 and 29.9 kg/m2 (HR, 1.41; 95% CI, 1.28-1.55), and greater than or equal to 30 kg/m2 (HR, 2.09; 95% CI, 1.86-2.36) compared with patients with a BMI between 18.5 and 22.9 kg/m2,.
Limitations of the study included the use of ultrasonography in place of liver biopsy for diagnosing NAFLD and the use of APRI to predict fibrosis in individuals with NAFLD, researchers said.
“APRI has demonstrated a reasonable utility as a noninvasive method for the prediction of histologically confirmed advanced fibrosis,” Dr. Ryu and colleagues wrote. “Nonetheless, we acknowledge that there is no currently available longitudinal data to support the use of worsening noninvasive fibrosis markers as an indicator of histological progression of fibrosis stage over time.”
Other limitations included the study’s retrospective design, lack of availability of medication use and dietary intake, and lack of generalization based on a young, healthy population of mostly Korean employees who were employed by companies or local government. However, researchers said clinicians should encourage their patients with NAFLD to maintain a healthy weight to avoid progression of fibrosis.
The authors reported no relevant financial disclosures.
SOURCE: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Obesity and weight gain were linked to progression of fibrosis in adults with NAFLD.
Major finding: Degree of weight change was associated with risk of fibrosis progression; patients who gained weight in quintile 4 and quintile 5 had hazard ratios of 1.17 and 1.71, respectively, when compared with the quintile of patients whose weight remained stable.
Data source: A retrospective study of 40,700 Korean adults with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up.
Disclosures: The authors reported no relevant financial disclosures.
Source: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
Liver enzymes: No trivial elevations, even if asymptomatic
Elevated levels of circulating enzymes that are frequently of hepatic origin (aminotransferases and alkaline phosphatase) and bilirubin in the absence of symptoms are common in clinical practice. A dogmatic but true statement holds that there are no trivial elevations in these substances. All persistent elevations of liver enzymes need a methodical evaluation and an appropriate working diagnosis.1
Here, we outline a framework for the workup and treatment of common causes of liver enzyme elevations.
PATTERN OF ELEVATION: CHOLESTATIC OR HEPATOCELLULAR
Based on the pattern of elevation, causes of elevated liver enzymes can be sorted into disorders of cholestasis and disorders of hepatocellular injury (Table 1).1
Cholestatic disorders tend to cause elevations in alkaline phosphatase, bilirubin, and gamma-glutamyl transferase (GGT).
Hepatocellular injury raises levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
HOW SHOULD ABNORMAL RESULTS BE EVALUATED?
When approaching liver enzyme elevations, the clinician should develop a working differential diagnosis based on the medical and social history and physical examination.
Think about alcohol, drugs, and fat
The most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
Alcohol intake should be ascertained. “Significant” consumption is defined as more than 21 drinks per week in men or more than 14 drinks per week in women, over a period of at least 2 years.2
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
Alcoholic liver disease can be difficult to diagnose, as many people are initially reluctant to fully disclose how much they drink, but it should be suspected when the ratio of AST to ALT is 2 or greater.
In a classic study, a ratio greater than 2 was found in 70% of patients with alcoholic hepatitis and cirrhosis, compared with 26% of patients with postnecrotic cirrhosis, 8% with chronic hepatitis, 4% with viral hepatitis, and none with obstructive jaundice.3 Importantly, the disorder is often correctable if the patient is able to remain abstinent from alcohol over time.
A detailed medication history is important and should focus especially on recently added medications, dosage changes, medication overuse, and use of nonprescription drugs and herbal supplements. Common medications that affect liver enzyme levels include statins, which cause hepatic dysfunction primarily during the first 3 months of therapy, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, antibiotics, anabolic steroids, and acetaminophen (Table 2).1 Use of illicit drugs and herbal remedies should be discussed, as they may cause toxin-mediated hepatitis.
Although inflammation from drug toxicity will resolve if the offending agent is discontinued, complete recovery may take weeks to months.4
A pertinent social history includes exposure to environmental hepatotoxins such as amatoxin (contained in some wild mushrooms) and occupational hazards (eg, vinyl chloride). Risk factors for viral hepatitis should be evaluated, including intravenous drug use, blood transfusions, unprotected sexual contact, organ transplant, perinatal transmission, and a history of work in healthcare facilities or travel to regions in which hepatitis A or E is endemic.
The medical and family history should include details of associated conditions, such as:
- Right heart failure (a cause of congestive hepatopathy)
- Metabolic syndrome (associated with fatty liver disease)
- Inflammatory bowel disease and primary sclerosing cholangitis
- Early-onset emphysema and alpha-1 antitrypsin deficiency.
The physical examination should be thorough, with emphasis on the abdomen, and search for stigmata of advanced liver disease such as hepatomegaly, splenomegaly, ascites, edema, spider angiomata, jaundice, and asterixis. Any patient with evidence of chronic liver disease should be referred to a subspecialist for further evaluation.
Further diagnostic workup
Abnormal liver enzyme findings or physical examination findings should direct the subsequent diagnostic workup with laboratory testing and imaging.5
For cholestasis. If laboratory data are consistent with cholestasis or abnormal bile flow, it should be further characterized as extrahepatic or intrahepatic. Common causes of extrahepatic cholestasis include biliary tree obstruction due to stones or malignancy, often visualized as intraductal biliary dilation on ultrasonography of the right upper quadrant. Common causes of intrahepatic cholestasis include viral and alcoholic hepatitis, nonalcoholic steatohepatitis, certain drugs and toxins such as alkylated steroids and herbal medications, infiltrative diseases such as amyloid, sarcoid, lymphoma, and tuberculosis, and primary biliary cholangitis.
Abnormal findings on ultrasonography should be further pursued with advanced imaging, ie, computed tomography or magnetic resonance cholangiopancreatography (MRCP). The confirmation of a lesion on imaging is often followed by endoscopic retrograde cholangiopancreatography (ERCP) in an attempt to obtain biopsy samples, remove obstructions, and place therapeutic stents. In instances when endoscopic attempts fail to relieve the obstruction, surgical referral may be appropriate.
For nonhepatobiliary problems. Depending on clinical presentation, it may also be important to consider nonhepatobiliary causes of elevated liver enzymes.
Alkaline phosphatase is found in many other tissue types, including bone, kidney, and the placenta, and can be elevated during pregnancy, adolescence, and even after fatty meals due to intestinal release.6 After screening for the aforementioned physiologic conditions, isolated elevated alkaline phosphatase should be further evaluated by obtaining GGT or 5-nucleotidase levels, which are more specifically of hepatic origin. If these levels are within normal limits, further evaluation for conditions of bone growth and cellular turnover such as Paget disease, hyperparathyroidism, and malignancy should be considered. Specifically, Stauffer syndrome should be considered when there is a paraneoplastic rise in the alkaline phosphatase level in the setting of renal cell carcinoma without liver metastases.
AST and ALT levels may also be elevated in clinical situations and syndromes unrelated to liver disease. Rhabdomyolysis, for instance, may be associated with elevations of AST in more than 90% of cases, and ALT in more than 75%.7 Markers of muscle injury including serum creatine kinase should be obtained in the setting of heat stroke, muscle weakness, strenuous activity, or seizures, as related elevations in AST and ALT may not always be clinically indicative of liver injury.
Given the many conditions that may cause elevated liver enzymes, evaluation and treatment should focus on identifying and removing offending agents and targeting the underlying process with appropriate medical therapy.
FATTY LIVER
With rates of obesity and type 2 diabetes on the rise in the general population, identifying and treating nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) require increased awareness and close coordination between primary care providers and subspecialists.
According to current estimates, up to one-third of the US population (100 million people) may have NAFLD, and 1% to 3% of the population (4–6 million people) likely have NASH, defined as steatosis with inflammation. Development of NASH places patients at a significantly higher risk of fibrosis, hepatocellular injury, and cancer.8
NAFLD is more common in men than in women. It is present in around 80% to 90% of obese adults, two-thirds of adults with type 2 diabetes, and many people with hyperlipidemia. It is also becoming more common in children, with 40% to 70% of obese children likely having some element of NAFLD.
Diagnosis of fatty liver
Although liver enzymes are more likely to be abnormal in individuals with NAFLD, many individuals with underlying NAFLD may have normal laboratory evaluations. ALT may be elevated in only up to 20% of cases and does not likely correlate with the level of underlying liver damage, although increasing GGT may serve as a marker of fibrosis over time.9–11 In contrast to alcohol injury, however, the AST-ALT ratio is usually less than 1.0.
Noninvasive tools for diagnosing NAFLD include the NAFLD fibrosis score, which incorporates age, hyperglycemia, body mass index, platelet count, albumin level, and AST-ALT ratio. This and related scoring algorithms may be useful in differentiating patients with minimal fibrosis from those with advanced fibrosis.12,13
Ultrasonography is a first-line diagnostic test for steatosis, although it may demonstrate fatty infiltration only around 60% of the time. Computed tomography and magnetic resonance imaging are more sensitive, but costlier. Transient elastography (FibroScan; Echosens, Paris, France) has become more popular and has been shown to correlate with findings on liver biopsy in diagnosing or excluding advanced liver fibrosis.14,15
The gold standard for diagnosing NAFLD and NASH is identifying fat-laden hepatocytes or portal inflammation on biopsy; however, biopsy is generally reserved for cases in which the diagnosis remains uncertain.
Behavioral treatment
The primary treatment for NAFLD consists of behavioral modification including weight loss, exercise, and adherence to a low-fat diet, in addition to tight glycemic control and treatment of any underlying lipid abnormalities. Studies have shown that a reduction of 7% to 10% of body weight is associated with a decrease in the inflammation of NAFLD, though no strict guidelines have been established.16
Given the prevalence of NAFLD and the need for longitudinal treatment, primary care physicians will play a significant role in long-term monitoring and management of patients with fatty liver disease.
OTHER DISORDERS OF LIVER FUNCTION
Hereditary hemochromatosis
Hereditary hemochromatosis is the most common inherited liver disorder in adults of European descent,17 and can be effectively treated if discovered early. But its clinical diagnosis can be challenging, as many patients have no symptoms at presentation despite abnormal liver enzyme levels. Early symptoms may include severe fatigue, arthralgias, and, in men, impotence, before the appearance of the classic triad of “bronze diabetes” with cirrhosis, diabetes, and darkening of the skin.18
If hemochromatosis is suspected, laboratory tests should include a calculation of percent transferrin saturation, with saturation greater than 45% warranting serum ferritin measurement to evaluate for iron overload (ferritin > 200–300 ng/mL in men, > 150–200 ng/mL in women).19 If iron overload is confirmed, referral to a gastroenterologist is recommended.
Genetic evaluation is often pursued, but patients may ultimately require liver biopsy regardless of the findings, as some patients homozygous for the HFE mutation C282Y may not have clinical hemochromatosis, whereas others with hereditary hemochromatosis may not have the HFE mutation.
Therapeutic phlebotomy is the treatment of choice, and most patients tolerate it well.
Chronic hepatitis B virus and hepatitis C virus infections
Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are common in the United States, with HBV affecting more than 1 million people and HCV affecting an estimated 3.5 million.
Chronic HCV infection. Direct-acting antiviral drugs have revolutionized HCV treatment and have led to a sustained viral response and presumed cure at 12 weeks in more than 95% of cases across all HCV genotypes.20 Given the recent development of effective and well-tolerated treatments, primary care physicians have assumed a pivotal role in screening for HCV.
The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America21 recommend screening for HCV in people who have risk factors for it, ie:
- HCV exposure
- HIV infection
- Behavioral or environmental risks for contracting the virus such as intravenous drug use or incarceration
- Birth between 1945 and 1965 (one-time testing).
If HCV antibody screening is positive, HCV RNA should be obtained to quantify the viral load and confirm active infection, and genotype testing should be performed to guide treatment. Among the 6 most common HCV genotypes, genotype 1 is the most common in North America, accounting for over 70% of cases in the United States.
Although recommendations and therapies are constantly evolving, the selection of a treatment regimen and the duration of therapy are determined by viral genotype, history of prior treatment, stage of liver fibrosis, potential drug interactions, and frequently, medication cost and insurance coverage.
HBV infection. The treatment for acute HBV infection is generally supportive, though viral suppression with tenofovir or entecavir may be required for those who develop coagulopathy, bilirubinemia, or liver failure. Treatment of chronic HBV infection may not be required and is generally considered for those with elevated ALT, high viral load, or evidence of liver fibrosis on noninvasive measurements such as transient elastography.
Autoimmune hepatitis
Autoimmune causes of liver enzyme elevations should also be considered during initial screening. Positive antinuclear antibody and positive antismooth muscle antibody tests are common in cases of autoimmune hepatitis.22 Autoimmune hepatitis affects women more often than men, with a ratio of 4:1. The peaks of incidence occur during adolescence and between ages 30 and 45.23
Primary biliary cholangitis
Additionally, an elevated alkaline phosphatase level should raise concern for underlying primary biliary cholangitis (formerly called primary biliary cirrhosis), an autoimmune disorder that affects the small and medium intrahepatic bile ducts. Diagnosis of primary biliary cholangitis can be assisted by a positive test for antimitochondrial antibody, present in almost 90% of patients.24
Primary sclerosing cholangitis
Elevated alkaline phosphatase is also the hallmark of primary sclerosing cholangitis, which is associated with inflammatory bowel disease.25 Primary sclerosing cholangitis is characterized by inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which are visualized on MRCP and confirmed by biopsy if needed.
REFERRAL
Subspecialty referral should be considered if the cause remains ambiguous or unknown, if there is concern for a rare hepatic disorder such as an autoimmune condition, Wilson disease, or alpha-1 antitrypsin deficiency, or if there is evidence of advanced or chronic liver disease.
Primary care physicians are at the forefront of detecting and diagnosing liver disease, and close coordination with subspecialists will remain crucial in delivering patient care.
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010; 77(3):195–204. doi:10.3949/ccjm.77a.09064
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142(7):1592–1609. doi:10.1053/j.gastro.2012.04.001
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24(11):835–838. pmid:520102
- Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med 2002; 137(1):49-51. pmid:12093245
- Pratt DS, Kaplan MM. Evaluation of abnormal liver enzyme results in asymptomatic patients. N Engl J Med 2000; 342(17):1266–1271. doi:10.1056/NEJM200004273421707
- Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014; 29(3):269–278. doi:10.1007/s12291-013-0408-y
- Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol 2010; 6(3):294–300. doi:10.1007/s13181-010-0075-9
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010; 28(1):155–161. doi:10.1159/000282080
- Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis 2011; 12(1):10–16. doi:10.1111/j.1751-2980.2010.00471.x
- Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008; 48(3):792–798. doi:10.1002/hep.22429
- Tahan V, Canbakan B, Balci H, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterolgoy 2008; 55(85):1433-1438. pmid:18795706
- McPherson S, Stewart S, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015; 35(5):1566–1573. doi:10.1111/liv.12584
- Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Intern Med 2016; 7(4):242–252. pmid:27999641
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51(1):121–129. doi:10.1002/hep.23276
- Adams PH, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005; 352(17):1769-1778. doi:10.1056/NEJMoa041534
- Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program 2006; 2006(1):36–41. doi:10.1182/asheducation-2006.1.36
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54(1):328–343. doi:10.1002/hep.24330
- Weiler N, Zeuzem S, Welker MW. Concise review: interferon-free treatment of hepatitis C virus-associated cirrhosis and liver graft infection. World J Gastroenterol 2016; 22(41):9044–9056. doi:10.3748/wjg.v22.i41.9044
- American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed July 16, 2018.
- Manns MP, Czaja AJ, Gorham JD, et al; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51(6):2193–2213. doi:10.1002/hep.23584
- Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016; 75:6–19. doi:10.1016/j.jaut.2016.07.005
- Mousa HS, Carbone M, Malinverno F, Ronca V, Gershwin ME, Invernizzi P. Novel therapeutics for primary biliary cholangitis: Toward a disease-stage-based approach. Autoimmun Rev 2016; 15(9):870–876. doi:10.1016/j.autrev.2016.07.003
- de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015; 21(6):1956–1971. doi:10.3748/wjg.v21.i6.1956
Elevated levels of circulating enzymes that are frequently of hepatic origin (aminotransferases and alkaline phosphatase) and bilirubin in the absence of symptoms are common in clinical practice. A dogmatic but true statement holds that there are no trivial elevations in these substances. All persistent elevations of liver enzymes need a methodical evaluation and an appropriate working diagnosis.1
Here, we outline a framework for the workup and treatment of common causes of liver enzyme elevations.
PATTERN OF ELEVATION: CHOLESTATIC OR HEPATOCELLULAR
Based on the pattern of elevation, causes of elevated liver enzymes can be sorted into disorders of cholestasis and disorders of hepatocellular injury (Table 1).1
Cholestatic disorders tend to cause elevations in alkaline phosphatase, bilirubin, and gamma-glutamyl transferase (GGT).
Hepatocellular injury raises levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
HOW SHOULD ABNORMAL RESULTS BE EVALUATED?
When approaching liver enzyme elevations, the clinician should develop a working differential diagnosis based on the medical and social history and physical examination.
Think about alcohol, drugs, and fat
The most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
Alcohol intake should be ascertained. “Significant” consumption is defined as more than 21 drinks per week in men or more than 14 drinks per week in women, over a period of at least 2 years.2
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
Alcoholic liver disease can be difficult to diagnose, as many people are initially reluctant to fully disclose how much they drink, but it should be suspected when the ratio of AST to ALT is 2 or greater.
In a classic study, a ratio greater than 2 was found in 70% of patients with alcoholic hepatitis and cirrhosis, compared with 26% of patients with postnecrotic cirrhosis, 8% with chronic hepatitis, 4% with viral hepatitis, and none with obstructive jaundice.3 Importantly, the disorder is often correctable if the patient is able to remain abstinent from alcohol over time.
A detailed medication history is important and should focus especially on recently added medications, dosage changes, medication overuse, and use of nonprescription drugs and herbal supplements. Common medications that affect liver enzyme levels include statins, which cause hepatic dysfunction primarily during the first 3 months of therapy, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, antibiotics, anabolic steroids, and acetaminophen (Table 2).1 Use of illicit drugs and herbal remedies should be discussed, as they may cause toxin-mediated hepatitis.
Although inflammation from drug toxicity will resolve if the offending agent is discontinued, complete recovery may take weeks to months.4
A pertinent social history includes exposure to environmental hepatotoxins such as amatoxin (contained in some wild mushrooms) and occupational hazards (eg, vinyl chloride). Risk factors for viral hepatitis should be evaluated, including intravenous drug use, blood transfusions, unprotected sexual contact, organ transplant, perinatal transmission, and a history of work in healthcare facilities or travel to regions in which hepatitis A or E is endemic.
The medical and family history should include details of associated conditions, such as:
- Right heart failure (a cause of congestive hepatopathy)
- Metabolic syndrome (associated with fatty liver disease)
- Inflammatory bowel disease and primary sclerosing cholangitis
- Early-onset emphysema and alpha-1 antitrypsin deficiency.
The physical examination should be thorough, with emphasis on the abdomen, and search for stigmata of advanced liver disease such as hepatomegaly, splenomegaly, ascites, edema, spider angiomata, jaundice, and asterixis. Any patient with evidence of chronic liver disease should be referred to a subspecialist for further evaluation.
Further diagnostic workup
Abnormal liver enzyme findings or physical examination findings should direct the subsequent diagnostic workup with laboratory testing and imaging.5
For cholestasis. If laboratory data are consistent with cholestasis or abnormal bile flow, it should be further characterized as extrahepatic or intrahepatic. Common causes of extrahepatic cholestasis include biliary tree obstruction due to stones or malignancy, often visualized as intraductal biliary dilation on ultrasonography of the right upper quadrant. Common causes of intrahepatic cholestasis include viral and alcoholic hepatitis, nonalcoholic steatohepatitis, certain drugs and toxins such as alkylated steroids and herbal medications, infiltrative diseases such as amyloid, sarcoid, lymphoma, and tuberculosis, and primary biliary cholangitis.
Abnormal findings on ultrasonography should be further pursued with advanced imaging, ie, computed tomography or magnetic resonance cholangiopancreatography (MRCP). The confirmation of a lesion on imaging is often followed by endoscopic retrograde cholangiopancreatography (ERCP) in an attempt to obtain biopsy samples, remove obstructions, and place therapeutic stents. In instances when endoscopic attempts fail to relieve the obstruction, surgical referral may be appropriate.
For nonhepatobiliary problems. Depending on clinical presentation, it may also be important to consider nonhepatobiliary causes of elevated liver enzymes.
Alkaline phosphatase is found in many other tissue types, including bone, kidney, and the placenta, and can be elevated during pregnancy, adolescence, and even after fatty meals due to intestinal release.6 After screening for the aforementioned physiologic conditions, isolated elevated alkaline phosphatase should be further evaluated by obtaining GGT or 5-nucleotidase levels, which are more specifically of hepatic origin. If these levels are within normal limits, further evaluation for conditions of bone growth and cellular turnover such as Paget disease, hyperparathyroidism, and malignancy should be considered. Specifically, Stauffer syndrome should be considered when there is a paraneoplastic rise in the alkaline phosphatase level in the setting of renal cell carcinoma without liver metastases.
AST and ALT levels may also be elevated in clinical situations and syndromes unrelated to liver disease. Rhabdomyolysis, for instance, may be associated with elevations of AST in more than 90% of cases, and ALT in more than 75%.7 Markers of muscle injury including serum creatine kinase should be obtained in the setting of heat stroke, muscle weakness, strenuous activity, or seizures, as related elevations in AST and ALT may not always be clinically indicative of liver injury.
Given the many conditions that may cause elevated liver enzymes, evaluation and treatment should focus on identifying and removing offending agents and targeting the underlying process with appropriate medical therapy.
FATTY LIVER
With rates of obesity and type 2 diabetes on the rise in the general population, identifying and treating nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) require increased awareness and close coordination between primary care providers and subspecialists.
According to current estimates, up to one-third of the US population (100 million people) may have NAFLD, and 1% to 3% of the population (4–6 million people) likely have NASH, defined as steatosis with inflammation. Development of NASH places patients at a significantly higher risk of fibrosis, hepatocellular injury, and cancer.8
NAFLD is more common in men than in women. It is present in around 80% to 90% of obese adults, two-thirds of adults with type 2 diabetes, and many people with hyperlipidemia. It is also becoming more common in children, with 40% to 70% of obese children likely having some element of NAFLD.
Diagnosis of fatty liver
Although liver enzymes are more likely to be abnormal in individuals with NAFLD, many individuals with underlying NAFLD may have normal laboratory evaluations. ALT may be elevated in only up to 20% of cases and does not likely correlate with the level of underlying liver damage, although increasing GGT may serve as a marker of fibrosis over time.9–11 In contrast to alcohol injury, however, the AST-ALT ratio is usually less than 1.0.
Noninvasive tools for diagnosing NAFLD include the NAFLD fibrosis score, which incorporates age, hyperglycemia, body mass index, platelet count, albumin level, and AST-ALT ratio. This and related scoring algorithms may be useful in differentiating patients with minimal fibrosis from those with advanced fibrosis.12,13
Ultrasonography is a first-line diagnostic test for steatosis, although it may demonstrate fatty infiltration only around 60% of the time. Computed tomography and magnetic resonance imaging are more sensitive, but costlier. Transient elastography (FibroScan; Echosens, Paris, France) has become more popular and has been shown to correlate with findings on liver biopsy in diagnosing or excluding advanced liver fibrosis.14,15
The gold standard for diagnosing NAFLD and NASH is identifying fat-laden hepatocytes or portal inflammation on biopsy; however, biopsy is generally reserved for cases in which the diagnosis remains uncertain.
Behavioral treatment
The primary treatment for NAFLD consists of behavioral modification including weight loss, exercise, and adherence to a low-fat diet, in addition to tight glycemic control and treatment of any underlying lipid abnormalities. Studies have shown that a reduction of 7% to 10% of body weight is associated with a decrease in the inflammation of NAFLD, though no strict guidelines have been established.16
Given the prevalence of NAFLD and the need for longitudinal treatment, primary care physicians will play a significant role in long-term monitoring and management of patients with fatty liver disease.
OTHER DISORDERS OF LIVER FUNCTION
Hereditary hemochromatosis
Hereditary hemochromatosis is the most common inherited liver disorder in adults of European descent,17 and can be effectively treated if discovered early. But its clinical diagnosis can be challenging, as many patients have no symptoms at presentation despite abnormal liver enzyme levels. Early symptoms may include severe fatigue, arthralgias, and, in men, impotence, before the appearance of the classic triad of “bronze diabetes” with cirrhosis, diabetes, and darkening of the skin.18
If hemochromatosis is suspected, laboratory tests should include a calculation of percent transferrin saturation, with saturation greater than 45% warranting serum ferritin measurement to evaluate for iron overload (ferritin > 200–300 ng/mL in men, > 150–200 ng/mL in women).19 If iron overload is confirmed, referral to a gastroenterologist is recommended.
Genetic evaluation is often pursued, but patients may ultimately require liver biopsy regardless of the findings, as some patients homozygous for the HFE mutation C282Y may not have clinical hemochromatosis, whereas others with hereditary hemochromatosis may not have the HFE mutation.
Therapeutic phlebotomy is the treatment of choice, and most patients tolerate it well.
Chronic hepatitis B virus and hepatitis C virus infections
Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are common in the United States, with HBV affecting more than 1 million people and HCV affecting an estimated 3.5 million.
Chronic HCV infection. Direct-acting antiviral drugs have revolutionized HCV treatment and have led to a sustained viral response and presumed cure at 12 weeks in more than 95% of cases across all HCV genotypes.20 Given the recent development of effective and well-tolerated treatments, primary care physicians have assumed a pivotal role in screening for HCV.
The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America21 recommend screening for HCV in people who have risk factors for it, ie:
- HCV exposure
- HIV infection
- Behavioral or environmental risks for contracting the virus such as intravenous drug use or incarceration
- Birth between 1945 and 1965 (one-time testing).
If HCV antibody screening is positive, HCV RNA should be obtained to quantify the viral load and confirm active infection, and genotype testing should be performed to guide treatment. Among the 6 most common HCV genotypes, genotype 1 is the most common in North America, accounting for over 70% of cases in the United States.
Although recommendations and therapies are constantly evolving, the selection of a treatment regimen and the duration of therapy are determined by viral genotype, history of prior treatment, stage of liver fibrosis, potential drug interactions, and frequently, medication cost and insurance coverage.
HBV infection. The treatment for acute HBV infection is generally supportive, though viral suppression with tenofovir or entecavir may be required for those who develop coagulopathy, bilirubinemia, or liver failure. Treatment of chronic HBV infection may not be required and is generally considered for those with elevated ALT, high viral load, or evidence of liver fibrosis on noninvasive measurements such as transient elastography.
Autoimmune hepatitis
Autoimmune causes of liver enzyme elevations should also be considered during initial screening. Positive antinuclear antibody and positive antismooth muscle antibody tests are common in cases of autoimmune hepatitis.22 Autoimmune hepatitis affects women more often than men, with a ratio of 4:1. The peaks of incidence occur during adolescence and between ages 30 and 45.23
Primary biliary cholangitis
Additionally, an elevated alkaline phosphatase level should raise concern for underlying primary biliary cholangitis (formerly called primary biliary cirrhosis), an autoimmune disorder that affects the small and medium intrahepatic bile ducts. Diagnosis of primary biliary cholangitis can be assisted by a positive test for antimitochondrial antibody, present in almost 90% of patients.24
Primary sclerosing cholangitis
Elevated alkaline phosphatase is also the hallmark of primary sclerosing cholangitis, which is associated with inflammatory bowel disease.25 Primary sclerosing cholangitis is characterized by inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which are visualized on MRCP and confirmed by biopsy if needed.
REFERRAL
Subspecialty referral should be considered if the cause remains ambiguous or unknown, if there is concern for a rare hepatic disorder such as an autoimmune condition, Wilson disease, or alpha-1 antitrypsin deficiency, or if there is evidence of advanced or chronic liver disease.
Primary care physicians are at the forefront of detecting and diagnosing liver disease, and close coordination with subspecialists will remain crucial in delivering patient care.
Elevated levels of circulating enzymes that are frequently of hepatic origin (aminotransferases and alkaline phosphatase) and bilirubin in the absence of symptoms are common in clinical practice. A dogmatic but true statement holds that there are no trivial elevations in these substances. All persistent elevations of liver enzymes need a methodical evaluation and an appropriate working diagnosis.1
Here, we outline a framework for the workup and treatment of common causes of liver enzyme elevations.
PATTERN OF ELEVATION: CHOLESTATIC OR HEPATOCELLULAR
Based on the pattern of elevation, causes of elevated liver enzymes can be sorted into disorders of cholestasis and disorders of hepatocellular injury (Table 1).1
Cholestatic disorders tend to cause elevations in alkaline phosphatase, bilirubin, and gamma-glutamyl transferase (GGT).
Hepatocellular injury raises levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
HOW SHOULD ABNORMAL RESULTS BE EVALUATED?
When approaching liver enzyme elevations, the clinician should develop a working differential diagnosis based on the medical and social history and physical examination.
Think about alcohol, drugs, and fat
The most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
Alcohol intake should be ascertained. “Significant” consumption is defined as more than 21 drinks per week in men or more than 14 drinks per week in women, over a period of at least 2 years.2
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
Alcoholic liver disease can be difficult to diagnose, as many people are initially reluctant to fully disclose how much they drink, but it should be suspected when the ratio of AST to ALT is 2 or greater.
In a classic study, a ratio greater than 2 was found in 70% of patients with alcoholic hepatitis and cirrhosis, compared with 26% of patients with postnecrotic cirrhosis, 8% with chronic hepatitis, 4% with viral hepatitis, and none with obstructive jaundice.3 Importantly, the disorder is often correctable if the patient is able to remain abstinent from alcohol over time.
A detailed medication history is important and should focus especially on recently added medications, dosage changes, medication overuse, and use of nonprescription drugs and herbal supplements. Common medications that affect liver enzyme levels include statins, which cause hepatic dysfunction primarily during the first 3 months of therapy, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, antibiotics, anabolic steroids, and acetaminophen (Table 2).1 Use of illicit drugs and herbal remedies should be discussed, as they may cause toxin-mediated hepatitis.
Although inflammation from drug toxicity will resolve if the offending agent is discontinued, complete recovery may take weeks to months.4
A pertinent social history includes exposure to environmental hepatotoxins such as amatoxin (contained in some wild mushrooms) and occupational hazards (eg, vinyl chloride). Risk factors for viral hepatitis should be evaluated, including intravenous drug use, blood transfusions, unprotected sexual contact, organ transplant, perinatal transmission, and a history of work in healthcare facilities or travel to regions in which hepatitis A or E is endemic.
The medical and family history should include details of associated conditions, such as:
- Right heart failure (a cause of congestive hepatopathy)
- Metabolic syndrome (associated with fatty liver disease)
- Inflammatory bowel disease and primary sclerosing cholangitis
- Early-onset emphysema and alpha-1 antitrypsin deficiency.
The physical examination should be thorough, with emphasis on the abdomen, and search for stigmata of advanced liver disease such as hepatomegaly, splenomegaly, ascites, edema, spider angiomata, jaundice, and asterixis. Any patient with evidence of chronic liver disease should be referred to a subspecialist for further evaluation.
Further diagnostic workup
Abnormal liver enzyme findings or physical examination findings should direct the subsequent diagnostic workup with laboratory testing and imaging.5
For cholestasis. If laboratory data are consistent with cholestasis or abnormal bile flow, it should be further characterized as extrahepatic or intrahepatic. Common causes of extrahepatic cholestasis include biliary tree obstruction due to stones or malignancy, often visualized as intraductal biliary dilation on ultrasonography of the right upper quadrant. Common causes of intrahepatic cholestasis include viral and alcoholic hepatitis, nonalcoholic steatohepatitis, certain drugs and toxins such as alkylated steroids and herbal medications, infiltrative diseases such as amyloid, sarcoid, lymphoma, and tuberculosis, and primary biliary cholangitis.
Abnormal findings on ultrasonography should be further pursued with advanced imaging, ie, computed tomography or magnetic resonance cholangiopancreatography (MRCP). The confirmation of a lesion on imaging is often followed by endoscopic retrograde cholangiopancreatography (ERCP) in an attempt to obtain biopsy samples, remove obstructions, and place therapeutic stents. In instances when endoscopic attempts fail to relieve the obstruction, surgical referral may be appropriate.
For nonhepatobiliary problems. Depending on clinical presentation, it may also be important to consider nonhepatobiliary causes of elevated liver enzymes.
Alkaline phosphatase is found in many other tissue types, including bone, kidney, and the placenta, and can be elevated during pregnancy, adolescence, and even after fatty meals due to intestinal release.6 After screening for the aforementioned physiologic conditions, isolated elevated alkaline phosphatase should be further evaluated by obtaining GGT or 5-nucleotidase levels, which are more specifically of hepatic origin. If these levels are within normal limits, further evaluation for conditions of bone growth and cellular turnover such as Paget disease, hyperparathyroidism, and malignancy should be considered. Specifically, Stauffer syndrome should be considered when there is a paraneoplastic rise in the alkaline phosphatase level in the setting of renal cell carcinoma without liver metastases.
AST and ALT levels may also be elevated in clinical situations and syndromes unrelated to liver disease. Rhabdomyolysis, for instance, may be associated with elevations of AST in more than 90% of cases, and ALT in more than 75%.7 Markers of muscle injury including serum creatine kinase should be obtained in the setting of heat stroke, muscle weakness, strenuous activity, or seizures, as related elevations in AST and ALT may not always be clinically indicative of liver injury.
Given the many conditions that may cause elevated liver enzymes, evaluation and treatment should focus on identifying and removing offending agents and targeting the underlying process with appropriate medical therapy.
FATTY LIVER
With rates of obesity and type 2 diabetes on the rise in the general population, identifying and treating nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) require increased awareness and close coordination between primary care providers and subspecialists.
According to current estimates, up to one-third of the US population (100 million people) may have NAFLD, and 1% to 3% of the population (4–6 million people) likely have NASH, defined as steatosis with inflammation. Development of NASH places patients at a significantly higher risk of fibrosis, hepatocellular injury, and cancer.8
NAFLD is more common in men than in women. It is present in around 80% to 90% of obese adults, two-thirds of adults with type 2 diabetes, and many people with hyperlipidemia. It is also becoming more common in children, with 40% to 70% of obese children likely having some element of NAFLD.
Diagnosis of fatty liver
Although liver enzymes are more likely to be abnormal in individuals with NAFLD, many individuals with underlying NAFLD may have normal laboratory evaluations. ALT may be elevated in only up to 20% of cases and does not likely correlate with the level of underlying liver damage, although increasing GGT may serve as a marker of fibrosis over time.9–11 In contrast to alcohol injury, however, the AST-ALT ratio is usually less than 1.0.
Noninvasive tools for diagnosing NAFLD include the NAFLD fibrosis score, which incorporates age, hyperglycemia, body mass index, platelet count, albumin level, and AST-ALT ratio. This and related scoring algorithms may be useful in differentiating patients with minimal fibrosis from those with advanced fibrosis.12,13
Ultrasonography is a first-line diagnostic test for steatosis, although it may demonstrate fatty infiltration only around 60% of the time. Computed tomography and magnetic resonance imaging are more sensitive, but costlier. Transient elastography (FibroScan; Echosens, Paris, France) has become more popular and has been shown to correlate with findings on liver biopsy in diagnosing or excluding advanced liver fibrosis.14,15
The gold standard for diagnosing NAFLD and NASH is identifying fat-laden hepatocytes or portal inflammation on biopsy; however, biopsy is generally reserved for cases in which the diagnosis remains uncertain.
Behavioral treatment
The primary treatment for NAFLD consists of behavioral modification including weight loss, exercise, and adherence to a low-fat diet, in addition to tight glycemic control and treatment of any underlying lipid abnormalities. Studies have shown that a reduction of 7% to 10% of body weight is associated with a decrease in the inflammation of NAFLD, though no strict guidelines have been established.16
Given the prevalence of NAFLD and the need for longitudinal treatment, primary care physicians will play a significant role in long-term monitoring and management of patients with fatty liver disease.
OTHER DISORDERS OF LIVER FUNCTION
Hereditary hemochromatosis
Hereditary hemochromatosis is the most common inherited liver disorder in adults of European descent,17 and can be effectively treated if discovered early. But its clinical diagnosis can be challenging, as many patients have no symptoms at presentation despite abnormal liver enzyme levels. Early symptoms may include severe fatigue, arthralgias, and, in men, impotence, before the appearance of the classic triad of “bronze diabetes” with cirrhosis, diabetes, and darkening of the skin.18
If hemochromatosis is suspected, laboratory tests should include a calculation of percent transferrin saturation, with saturation greater than 45% warranting serum ferritin measurement to evaluate for iron overload (ferritin > 200–300 ng/mL in men, > 150–200 ng/mL in women).19 If iron overload is confirmed, referral to a gastroenterologist is recommended.
Genetic evaluation is often pursued, but patients may ultimately require liver biopsy regardless of the findings, as some patients homozygous for the HFE mutation C282Y may not have clinical hemochromatosis, whereas others with hereditary hemochromatosis may not have the HFE mutation.
Therapeutic phlebotomy is the treatment of choice, and most patients tolerate it well.
Chronic hepatitis B virus and hepatitis C virus infections
Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are common in the United States, with HBV affecting more than 1 million people and HCV affecting an estimated 3.5 million.
Chronic HCV infection. Direct-acting antiviral drugs have revolutionized HCV treatment and have led to a sustained viral response and presumed cure at 12 weeks in more than 95% of cases across all HCV genotypes.20 Given the recent development of effective and well-tolerated treatments, primary care physicians have assumed a pivotal role in screening for HCV.
The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America21 recommend screening for HCV in people who have risk factors for it, ie:
- HCV exposure
- HIV infection
- Behavioral or environmental risks for contracting the virus such as intravenous drug use or incarceration
- Birth between 1945 and 1965 (one-time testing).
If HCV antibody screening is positive, HCV RNA should be obtained to quantify the viral load and confirm active infection, and genotype testing should be performed to guide treatment. Among the 6 most common HCV genotypes, genotype 1 is the most common in North America, accounting for over 70% of cases in the United States.
Although recommendations and therapies are constantly evolving, the selection of a treatment regimen and the duration of therapy are determined by viral genotype, history of prior treatment, stage of liver fibrosis, potential drug interactions, and frequently, medication cost and insurance coverage.
HBV infection. The treatment for acute HBV infection is generally supportive, though viral suppression with tenofovir or entecavir may be required for those who develop coagulopathy, bilirubinemia, or liver failure. Treatment of chronic HBV infection may not be required and is generally considered for those with elevated ALT, high viral load, or evidence of liver fibrosis on noninvasive measurements such as transient elastography.
Autoimmune hepatitis
Autoimmune causes of liver enzyme elevations should also be considered during initial screening. Positive antinuclear antibody and positive antismooth muscle antibody tests are common in cases of autoimmune hepatitis.22 Autoimmune hepatitis affects women more often than men, with a ratio of 4:1. The peaks of incidence occur during adolescence and between ages 30 and 45.23
Primary biliary cholangitis
Additionally, an elevated alkaline phosphatase level should raise concern for underlying primary biliary cholangitis (formerly called primary biliary cirrhosis), an autoimmune disorder that affects the small and medium intrahepatic bile ducts. Diagnosis of primary biliary cholangitis can be assisted by a positive test for antimitochondrial antibody, present in almost 90% of patients.24
Primary sclerosing cholangitis
Elevated alkaline phosphatase is also the hallmark of primary sclerosing cholangitis, which is associated with inflammatory bowel disease.25 Primary sclerosing cholangitis is characterized by inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which are visualized on MRCP and confirmed by biopsy if needed.
REFERRAL
Subspecialty referral should be considered if the cause remains ambiguous or unknown, if there is concern for a rare hepatic disorder such as an autoimmune condition, Wilson disease, or alpha-1 antitrypsin deficiency, or if there is evidence of advanced or chronic liver disease.
Primary care physicians are at the forefront of detecting and diagnosing liver disease, and close coordination with subspecialists will remain crucial in delivering patient care.
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010; 77(3):195–204. doi:10.3949/ccjm.77a.09064
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142(7):1592–1609. doi:10.1053/j.gastro.2012.04.001
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24(11):835–838. pmid:520102
- Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med 2002; 137(1):49-51. pmid:12093245
- Pratt DS, Kaplan MM. Evaluation of abnormal liver enzyme results in asymptomatic patients. N Engl J Med 2000; 342(17):1266–1271. doi:10.1056/NEJM200004273421707
- Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014; 29(3):269–278. doi:10.1007/s12291-013-0408-y
- Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol 2010; 6(3):294–300. doi:10.1007/s13181-010-0075-9
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010; 28(1):155–161. doi:10.1159/000282080
- Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis 2011; 12(1):10–16. doi:10.1111/j.1751-2980.2010.00471.x
- Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008; 48(3):792–798. doi:10.1002/hep.22429
- Tahan V, Canbakan B, Balci H, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterolgoy 2008; 55(85):1433-1438. pmid:18795706
- McPherson S, Stewart S, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015; 35(5):1566–1573. doi:10.1111/liv.12584
- Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Intern Med 2016; 7(4):242–252. pmid:27999641
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51(1):121–129. doi:10.1002/hep.23276
- Adams PH, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005; 352(17):1769-1778. doi:10.1056/NEJMoa041534
- Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program 2006; 2006(1):36–41. doi:10.1182/asheducation-2006.1.36
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54(1):328–343. doi:10.1002/hep.24330
- Weiler N, Zeuzem S, Welker MW. Concise review: interferon-free treatment of hepatitis C virus-associated cirrhosis and liver graft infection. World J Gastroenterol 2016; 22(41):9044–9056. doi:10.3748/wjg.v22.i41.9044
- American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed July 16, 2018.
- Manns MP, Czaja AJ, Gorham JD, et al; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51(6):2193–2213. doi:10.1002/hep.23584
- Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016; 75:6–19. doi:10.1016/j.jaut.2016.07.005
- Mousa HS, Carbone M, Malinverno F, Ronca V, Gershwin ME, Invernizzi P. Novel therapeutics for primary biliary cholangitis: Toward a disease-stage-based approach. Autoimmun Rev 2016; 15(9):870–876. doi:10.1016/j.autrev.2016.07.003
- de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015; 21(6):1956–1971. doi:10.3748/wjg.v21.i6.1956
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010; 77(3):195–204. doi:10.3949/ccjm.77a.09064
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142(7):1592–1609. doi:10.1053/j.gastro.2012.04.001
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24(11):835–838. pmid:520102
- Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med 2002; 137(1):49-51. pmid:12093245
- Pratt DS, Kaplan MM. Evaluation of abnormal liver enzyme results in asymptomatic patients. N Engl J Med 2000; 342(17):1266–1271. doi:10.1056/NEJM200004273421707
- Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014; 29(3):269–278. doi:10.1007/s12291-013-0408-y
- Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol 2010; 6(3):294–300. doi:10.1007/s13181-010-0075-9
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010; 28(1):155–161. doi:10.1159/000282080
- Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis 2011; 12(1):10–16. doi:10.1111/j.1751-2980.2010.00471.x
- Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008; 48(3):792–798. doi:10.1002/hep.22429
- Tahan V, Canbakan B, Balci H, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterolgoy 2008; 55(85):1433-1438. pmid:18795706
- McPherson S, Stewart S, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015; 35(5):1566–1573. doi:10.1111/liv.12584
- Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Intern Med 2016; 7(4):242–252. pmid:27999641
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51(1):121–129. doi:10.1002/hep.23276
- Adams PH, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005; 352(17):1769-1778. doi:10.1056/NEJMoa041534
- Brissot P, de Bels F. Current approaches to the management of hemochromatosis. Hematology Am Soc Hematol Educ Program 2006; 2006(1):36–41. doi:10.1182/asheducation-2006.1.36
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54(1):328–343. doi:10.1002/hep.24330
- Weiler N, Zeuzem S, Welker MW. Concise review: interferon-free treatment of hepatitis C virus-associated cirrhosis and liver graft infection. World J Gastroenterol 2016; 22(41):9044–9056. doi:10.3748/wjg.v22.i41.9044
- American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed July 16, 2018.
- Manns MP, Czaja AJ, Gorham JD, et al; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51(6):2193–2213. doi:10.1002/hep.23584
- Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016; 75:6–19. doi:10.1016/j.jaut.2016.07.005
- Mousa HS, Carbone M, Malinverno F, Ronca V, Gershwin ME, Invernizzi P. Novel therapeutics for primary biliary cholangitis: Toward a disease-stage-based approach. Autoimmun Rev 2016; 15(9):870–876. doi:10.1016/j.autrev.2016.07.003
- de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015; 21(6):1956–1971. doi:10.3748/wjg.v21.i6.1956
KEY POINTS
- Disorders of hepatocellular injury tend to elevate levels of aminotransferases, whereas cholestatic disorders cause elevations of alkaline phosphatase and bilirubin.
- The three most common causes of liver enzyme elevation are alcohol toxicity, medication overdose, and fatty liver disease.
- Other disorders of liver dysfunction include hereditary hemochromatosis, viral hepatitis, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, and alpha-1 antitrypsin disease.
- Nonhepatic causes of elevated “liver enzymes” also need to be considered. For instance, rhabdomyolysis causes elevations in aminotransferase levels.
The bias of word choice and the interpretation of laboratory tests
In the current sociopolitical environment in the United States, the slogan “words matter” has become a battle cry for several groups and causes, emphasizing that our choice of words can influence the way we assess a specific person or situation. We are not immune to the subliminal bias of words, even as we evaluate such seemingly objective components of clinical management as laboratory test results.
Several years ago, I was supervising teaching rounds on a general medicine service. It was the first rounds of the month, and the patients were relatively new to the residents and totally unknown to me. One patient was an elderly man with weight loss, fatigue, weakness, and a history of excessive alcohol ingestion. His family had corroborated the last detail, but he had stopped drinking a long time before his admission. He had normal creatinine, minimal anemia, and markedly elevated and unexplained “liver function tests.” Liver biopsy was planned.
As we entered his room, we saw a gaunt man struggle to rise from the bedside chair to get back into bed. He rocked several times and then pushed himself up from the chair using his arms. Then, after a few short steps, he plopped back into bed and greeted us. His breakfast tray was untouched at the bedside. I introduced myself, we chatted for a short while as I examined him in front of our team, and we left.
In the hallway I asked, “Who would like to get an additional blood test before we do a liver biopsy?” Without waiting for a response I asked a second question, “What exactly are liver function tests?”
Words do matter, and they influence the way we analyze clinical scenarios. It could be argued that a complete and careful history would have established that our patient’s fatigue and weakness were due to proximal muscle weakness and not general asthenia, and that detailed questioning would have revealed that his weight loss was mainly from difficulty in swallowing without a sense of choking and coughing. But faced with an elderly man, a likely explanation for liver disease, and markedly elevated aspartate and alanine aminotransferase (AST and ALT) levels, there was premature closure of the diagnosis, and the decision was made to obtain a liver biopsy—which our hepatology consultants surely would not have done. I believe that a major contributor to the premature diagnosis was the choice of the words “liver function tests” and the default assumption that elevated serum levels of these enzymes always reflect liver disease.
Aminotransferases are fairly ubiquitous, likely present in various concentrations in all cells in our body. AST exists in mitochondrial and cytosolic forms, and ALT in the cytosol. The concentration of ALT is higher in the liver than in other organs, and its enzymatic activity is suppressed by hepatic exposure to alcohol. Both enzymes are present in muscle, and although AST is more abundant in cells other than hepatocytes, the longer serum half-life of ALT may result in roughly equal serum levels in the setting of chronic muscle injury such as myositis (the true diagnosis in our weak patient).
While a meticulous history and examination would indeed have led to the diagnosis of muscle disease in this man, they alone could not have determined whether he had coexistent liver and muscle disease. And this is a real challenge when acute muscle toxicity and liver toxicity are equally possible (eg, statin or immune checkpoint autoimmune tissue damage, or after significant trauma).
There are many nuances in the interpretation of even the most common laboratory tests. In this issue of the Journal, Agganis et al discuss liver enzymes (a term slightly more acceptable to me than liver function tests). In future issues, we will address the interpretation of other laboratory tests.
In the current sociopolitical environment in the United States, the slogan “words matter” has become a battle cry for several groups and causes, emphasizing that our choice of words can influence the way we assess a specific person or situation. We are not immune to the subliminal bias of words, even as we evaluate such seemingly objective components of clinical management as laboratory test results.
Several years ago, I was supervising teaching rounds on a general medicine service. It was the first rounds of the month, and the patients were relatively new to the residents and totally unknown to me. One patient was an elderly man with weight loss, fatigue, weakness, and a history of excessive alcohol ingestion. His family had corroborated the last detail, but he had stopped drinking a long time before his admission. He had normal creatinine, minimal anemia, and markedly elevated and unexplained “liver function tests.” Liver biopsy was planned.
As we entered his room, we saw a gaunt man struggle to rise from the bedside chair to get back into bed. He rocked several times and then pushed himself up from the chair using his arms. Then, after a few short steps, he plopped back into bed and greeted us. His breakfast tray was untouched at the bedside. I introduced myself, we chatted for a short while as I examined him in front of our team, and we left.
In the hallway I asked, “Who would like to get an additional blood test before we do a liver biopsy?” Without waiting for a response I asked a second question, “What exactly are liver function tests?”
Words do matter, and they influence the way we analyze clinical scenarios. It could be argued that a complete and careful history would have established that our patient’s fatigue and weakness were due to proximal muscle weakness and not general asthenia, and that detailed questioning would have revealed that his weight loss was mainly from difficulty in swallowing without a sense of choking and coughing. But faced with an elderly man, a likely explanation for liver disease, and markedly elevated aspartate and alanine aminotransferase (AST and ALT) levels, there was premature closure of the diagnosis, and the decision was made to obtain a liver biopsy—which our hepatology consultants surely would not have done. I believe that a major contributor to the premature diagnosis was the choice of the words “liver function tests” and the default assumption that elevated serum levels of these enzymes always reflect liver disease.
Aminotransferases are fairly ubiquitous, likely present in various concentrations in all cells in our body. AST exists in mitochondrial and cytosolic forms, and ALT in the cytosol. The concentration of ALT is higher in the liver than in other organs, and its enzymatic activity is suppressed by hepatic exposure to alcohol. Both enzymes are present in muscle, and although AST is more abundant in cells other than hepatocytes, the longer serum half-life of ALT may result in roughly equal serum levels in the setting of chronic muscle injury such as myositis (the true diagnosis in our weak patient).
While a meticulous history and examination would indeed have led to the diagnosis of muscle disease in this man, they alone could not have determined whether he had coexistent liver and muscle disease. And this is a real challenge when acute muscle toxicity and liver toxicity are equally possible (eg, statin or immune checkpoint autoimmune tissue damage, or after significant trauma).
There are many nuances in the interpretation of even the most common laboratory tests. In this issue of the Journal, Agganis et al discuss liver enzymes (a term slightly more acceptable to me than liver function tests). In future issues, we will address the interpretation of other laboratory tests.
In the current sociopolitical environment in the United States, the slogan “words matter” has become a battle cry for several groups and causes, emphasizing that our choice of words can influence the way we assess a specific person or situation. We are not immune to the subliminal bias of words, even as we evaluate such seemingly objective components of clinical management as laboratory test results.
Several years ago, I was supervising teaching rounds on a general medicine service. It was the first rounds of the month, and the patients were relatively new to the residents and totally unknown to me. One patient was an elderly man with weight loss, fatigue, weakness, and a history of excessive alcohol ingestion. His family had corroborated the last detail, but he had stopped drinking a long time before his admission. He had normal creatinine, minimal anemia, and markedly elevated and unexplained “liver function tests.” Liver biopsy was planned.
As we entered his room, we saw a gaunt man struggle to rise from the bedside chair to get back into bed. He rocked several times and then pushed himself up from the chair using his arms. Then, after a few short steps, he plopped back into bed and greeted us. His breakfast tray was untouched at the bedside. I introduced myself, we chatted for a short while as I examined him in front of our team, and we left.
In the hallway I asked, “Who would like to get an additional blood test before we do a liver biopsy?” Without waiting for a response I asked a second question, “What exactly are liver function tests?”
Words do matter, and they influence the way we analyze clinical scenarios. It could be argued that a complete and careful history would have established that our patient’s fatigue and weakness were due to proximal muscle weakness and not general asthenia, and that detailed questioning would have revealed that his weight loss was mainly from difficulty in swallowing without a sense of choking and coughing. But faced with an elderly man, a likely explanation for liver disease, and markedly elevated aspartate and alanine aminotransferase (AST and ALT) levels, there was premature closure of the diagnosis, and the decision was made to obtain a liver biopsy—which our hepatology consultants surely would not have done. I believe that a major contributor to the premature diagnosis was the choice of the words “liver function tests” and the default assumption that elevated serum levels of these enzymes always reflect liver disease.
Aminotransferases are fairly ubiquitous, likely present in various concentrations in all cells in our body. AST exists in mitochondrial and cytosolic forms, and ALT in the cytosol. The concentration of ALT is higher in the liver than in other organs, and its enzymatic activity is suppressed by hepatic exposure to alcohol. Both enzymes are present in muscle, and although AST is more abundant in cells other than hepatocytes, the longer serum half-life of ALT may result in roughly equal serum levels in the setting of chronic muscle injury such as myositis (the true diagnosis in our weak patient).
While a meticulous history and examination would indeed have led to the diagnosis of muscle disease in this man, they alone could not have determined whether he had coexistent liver and muscle disease. And this is a real challenge when acute muscle toxicity and liver toxicity are equally possible (eg, statin or immune checkpoint autoimmune tissue damage, or after significant trauma).
There are many nuances in the interpretation of even the most common laboratory tests. In this issue of the Journal, Agganis et al discuss liver enzymes (a term slightly more acceptable to me than liver function tests). In future issues, we will address the interpretation of other laboratory tests.
Palmoplantar exanthema and liver dysfunction
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
Who are the 'high-need, high-cost' patients?
Among patients hospitalized with gastrointestinal and liver diseases, a clearly identifiable subset uses significantly more health care resources, which incurs significantly greater costs, according to the results of a national database analysis published in the August issue of Clinical Gastroenterology and Hepatology.
Compared with otherwise similar inpatients, these “high-need, high-cost” individuals are significantly more likely to be enrolled in Medicare or Medicaid, to have lower income, to initially be admitted to a large, rural hospital, to have multiple comorbidities, to be obese, or to be hospitalized for infection, said Nghia Nguyen, MD, and his associates. “[A] small fraction of high-need, high-cost patients contribute disproportionately to hospitalization costs,” they wrote. “Population health management directed toward these patients would facilitate high-value care.”
Gastrointestinal and liver diseases incur more than $100 billion in health care expenses annually in the United States, of which more than 60% is related to inpatient care, the researchers noted. However, few studies have comprehensively evaluated the annual burden and costs of hospitalization in patients with chronic gastrointestinal and liver diseases. Therefore, using the Nationwide Readmissions Database, the investigators studied patients with inflammatory bowel disease (IBD), chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases who were hospitalized at least once during the first 6 months of 2013. All patients were diagnosed with IBD, chronic liver diseases, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases and followed for at least 6 months. The researchers stratified hospital days and costs and characterized the subset of patients who fell into the highest decile of days spent in the hospital per month.
The most common reason for hospitalization was chronic liver disease (nearly 377,000 patients), followed by functional gastrointestinal disorders (more than 351,000 patients), gastrointestinal hemorrhage (nearly 191,000 patients), pancreatic diseases (more than 98,000 patients), and IBD (more than 47,000 patients). Patients spent a median of 6-7 days in the hospital per year, with an interquartile range of 3-14 days. Compared with patients in the lowest decile for annual hospital stay (median, 0.13-0.14 days per month), patients in the highest decile spent a median of 3.7-5.1 days in the hospital per month. In this high-cost, high-need subset of patients, the costs of each hospitalization ranged from $7,438 per month to $11,425 per month, and they were typically hospitalized once every 2 months.
“Gastrointestinal diseases, infections, and cardiopulmonary causes were leading reasons for hospitalization of these patients,” the researchers wrote. “At a patient level, modifiable risk factors may include tackling the obesity epidemic and mental health issues and minimizing risk of iatrogenic or health care–associated infections, whereas at a health system level, interventions may include better access to care and connectivity between rural and specialty hospitals.”
Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
SOURCE: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
Understanding the reasons underlying variations in health care utilization is central to any plan to reduce costs at the population level. To this end, Nguyen et al. provide crucial data for the patients for whom we care as gastroenterologists. Studying a longitudinal database of hospitalizations in 2013, the authors provide comprehensive demographic data for the top decile of inpatient health care utilizers (defined by hospital-days/month) with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, and pancreatic diseases. Although constrained by the limits of administrative data and the lack of outpatient/pharmaceutical data linkage, these findings are strengthened by their consistency across conditions. Indeed, despite the heterogeneous disorders surveyed, a remarkably consistent high-need/high-cost "phenotype" emerges: publicly insured, low-income, rural, obese but malnourished, and beset by infections and the complications of diabetes.
What are the next steps?
When a minority of the patients are responsible for a substantial portion of the costs (i.e., the 80/20 rule), one strategy for cost containment is "hot-spotting." Hot-spotting is a two-step process: Identify high-need, high-cost patients, and then deploy interventions tailored to their needs. Nguyen and colleague's work is a landmark for the first step. However, before these findings may be translated into policy or intervention, we need granular data to explain these associations and suggest clear action items. Solutions will likely be multifactorial including early, intensified care for obesity and diabetes (before end-stage complications arise), novel care delivery methods for gastroenterology specialty care in rural hospitals, and intensified outpatient resources for high-need patients in order to coordinate alternatives to hospitalization.
Elliot B. Tapper, MD, is assistant professor, division of gastroenterology and hepatology, University of Michigan, Ann Arbor. He reports consulting for Novartis and receiving unrestricted research grants from Valeant and Gilead, all unrelated to this work.
Understanding the reasons underlying variations in health care utilization is central to any plan to reduce costs at the population level. To this end, Nguyen et al. provide crucial data for the patients for whom we care as gastroenterologists. Studying a longitudinal database of hospitalizations in 2013, the authors provide comprehensive demographic data for the top decile of inpatient health care utilizers (defined by hospital-days/month) with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, and pancreatic diseases. Although constrained by the limits of administrative data and the lack of outpatient/pharmaceutical data linkage, these findings are strengthened by their consistency across conditions. Indeed, despite the heterogeneous disorders surveyed, a remarkably consistent high-need/high-cost "phenotype" emerges: publicly insured, low-income, rural, obese but malnourished, and beset by infections and the complications of diabetes.
What are the next steps?
When a minority of the patients are responsible for a substantial portion of the costs (i.e., the 80/20 rule), one strategy for cost containment is "hot-spotting." Hot-spotting is a two-step process: Identify high-need, high-cost patients, and then deploy interventions tailored to their needs. Nguyen and colleague's work is a landmark for the first step. However, before these findings may be translated into policy or intervention, we need granular data to explain these associations and suggest clear action items. Solutions will likely be multifactorial including early, intensified care for obesity and diabetes (before end-stage complications arise), novel care delivery methods for gastroenterology specialty care in rural hospitals, and intensified outpatient resources for high-need patients in order to coordinate alternatives to hospitalization.
Elliot B. Tapper, MD, is assistant professor, division of gastroenterology and hepatology, University of Michigan, Ann Arbor. He reports consulting for Novartis and receiving unrestricted research grants from Valeant and Gilead, all unrelated to this work.
Understanding the reasons underlying variations in health care utilization is central to any plan to reduce costs at the population level. To this end, Nguyen et al. provide crucial data for the patients for whom we care as gastroenterologists. Studying a longitudinal database of hospitalizations in 2013, the authors provide comprehensive demographic data for the top decile of inpatient health care utilizers (defined by hospital-days/month) with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, and pancreatic diseases. Although constrained by the limits of administrative data and the lack of outpatient/pharmaceutical data linkage, these findings are strengthened by their consistency across conditions. Indeed, despite the heterogeneous disorders surveyed, a remarkably consistent high-need/high-cost "phenotype" emerges: publicly insured, low-income, rural, obese but malnourished, and beset by infections and the complications of diabetes.
What are the next steps?
When a minority of the patients are responsible for a substantial portion of the costs (i.e., the 80/20 rule), one strategy for cost containment is "hot-spotting." Hot-spotting is a two-step process: Identify high-need, high-cost patients, and then deploy interventions tailored to their needs. Nguyen and colleague's work is a landmark for the first step. However, before these findings may be translated into policy or intervention, we need granular data to explain these associations and suggest clear action items. Solutions will likely be multifactorial including early, intensified care for obesity and diabetes (before end-stage complications arise), novel care delivery methods for gastroenterology specialty care in rural hospitals, and intensified outpatient resources for high-need patients in order to coordinate alternatives to hospitalization.
Elliot B. Tapper, MD, is assistant professor, division of gastroenterology and hepatology, University of Michigan, Ann Arbor. He reports consulting for Novartis and receiving unrestricted research grants from Valeant and Gilead, all unrelated to this work.
Among patients hospitalized with gastrointestinal and liver diseases, a clearly identifiable subset uses significantly more health care resources, which incurs significantly greater costs, according to the results of a national database analysis published in the August issue of Clinical Gastroenterology and Hepatology.
Compared with otherwise similar inpatients, these “high-need, high-cost” individuals are significantly more likely to be enrolled in Medicare or Medicaid, to have lower income, to initially be admitted to a large, rural hospital, to have multiple comorbidities, to be obese, or to be hospitalized for infection, said Nghia Nguyen, MD, and his associates. “[A] small fraction of high-need, high-cost patients contribute disproportionately to hospitalization costs,” they wrote. “Population health management directed toward these patients would facilitate high-value care.”
Gastrointestinal and liver diseases incur more than $100 billion in health care expenses annually in the United States, of which more than 60% is related to inpatient care, the researchers noted. However, few studies have comprehensively evaluated the annual burden and costs of hospitalization in patients with chronic gastrointestinal and liver diseases. Therefore, using the Nationwide Readmissions Database, the investigators studied patients with inflammatory bowel disease (IBD), chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases who were hospitalized at least once during the first 6 months of 2013. All patients were diagnosed with IBD, chronic liver diseases, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases and followed for at least 6 months. The researchers stratified hospital days and costs and characterized the subset of patients who fell into the highest decile of days spent in the hospital per month.
The most common reason for hospitalization was chronic liver disease (nearly 377,000 patients), followed by functional gastrointestinal disorders (more than 351,000 patients), gastrointestinal hemorrhage (nearly 191,000 patients), pancreatic diseases (more than 98,000 patients), and IBD (more than 47,000 patients). Patients spent a median of 6-7 days in the hospital per year, with an interquartile range of 3-14 days. Compared with patients in the lowest decile for annual hospital stay (median, 0.13-0.14 days per month), patients in the highest decile spent a median of 3.7-5.1 days in the hospital per month. In this high-cost, high-need subset of patients, the costs of each hospitalization ranged from $7,438 per month to $11,425 per month, and they were typically hospitalized once every 2 months.
“Gastrointestinal diseases, infections, and cardiopulmonary causes were leading reasons for hospitalization of these patients,” the researchers wrote. “At a patient level, modifiable risk factors may include tackling the obesity epidemic and mental health issues and minimizing risk of iatrogenic or health care–associated infections, whereas at a health system level, interventions may include better access to care and connectivity between rural and specialty hospitals.”
Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
SOURCE: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
Among patients hospitalized with gastrointestinal and liver diseases, a clearly identifiable subset uses significantly more health care resources, which incurs significantly greater costs, according to the results of a national database analysis published in the August issue of Clinical Gastroenterology and Hepatology.
Compared with otherwise similar inpatients, these “high-need, high-cost” individuals are significantly more likely to be enrolled in Medicare or Medicaid, to have lower income, to initially be admitted to a large, rural hospital, to have multiple comorbidities, to be obese, or to be hospitalized for infection, said Nghia Nguyen, MD, and his associates. “[A] small fraction of high-need, high-cost patients contribute disproportionately to hospitalization costs,” they wrote. “Population health management directed toward these patients would facilitate high-value care.”
Gastrointestinal and liver diseases incur more than $100 billion in health care expenses annually in the United States, of which more than 60% is related to inpatient care, the researchers noted. However, few studies have comprehensively evaluated the annual burden and costs of hospitalization in patients with chronic gastrointestinal and liver diseases. Therefore, using the Nationwide Readmissions Database, the investigators studied patients with inflammatory bowel disease (IBD), chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases who were hospitalized at least once during the first 6 months of 2013. All patients were diagnosed with IBD, chronic liver diseases, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases and followed for at least 6 months. The researchers stratified hospital days and costs and characterized the subset of patients who fell into the highest decile of days spent in the hospital per month.
The most common reason for hospitalization was chronic liver disease (nearly 377,000 patients), followed by functional gastrointestinal disorders (more than 351,000 patients), gastrointestinal hemorrhage (nearly 191,000 patients), pancreatic diseases (more than 98,000 patients), and IBD (more than 47,000 patients). Patients spent a median of 6-7 days in the hospital per year, with an interquartile range of 3-14 days. Compared with patients in the lowest decile for annual hospital stay (median, 0.13-0.14 days per month), patients in the highest decile spent a median of 3.7-5.1 days in the hospital per month. In this high-cost, high-need subset of patients, the costs of each hospitalization ranged from $7,438 per month to $11,425 per month, and they were typically hospitalized once every 2 months.
“Gastrointestinal diseases, infections, and cardiopulmonary causes were leading reasons for hospitalization of these patients,” the researchers wrote. “At a patient level, modifiable risk factors may include tackling the obesity epidemic and mental health issues and minimizing risk of iatrogenic or health care–associated infections, whereas at a health system level, interventions may include better access to care and connectivity between rural and specialty hospitals.”
Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
SOURCE: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: For patients with gastrointestinal or liver disease, significant predictors of high need and cost during hospitalization included Medicare or Medicaid insurance, lower income, first hospitalization in a large rural hospital, high comorbidity burden, obesity, and hospitalization for infection.
Major finding: Patients in the highest decile spent a median of 3.7-4.1 days in the hospital per month for all causes. Gastrointestinal disease, infections, and cardiopulmonary morbidity were the most common reasons for hospitalization.
Study details: Analysis of patients with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases hospitalized at least once during 2013.
Disclosures: Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
Source: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
Liver cancer death rates down for Asians and Pacific Islanders
Liver cancer death rates are dropping for Asians/Pacific Islanders, but that is the exception to a larger trend, according to the National Center for Health Statistics.
The age-adjusted death rate for liver cancer is down 22% among Asian or Pacific Islander adults aged 25 years and older since the turn of the century, falling from 17.5 per 100,000 population in 2000 – when it was the highest of the four racial/ethnic groups included in the report – to 13.6 per 100,000 in 2016, by which time it was just middle of the pack, the NCHS reported.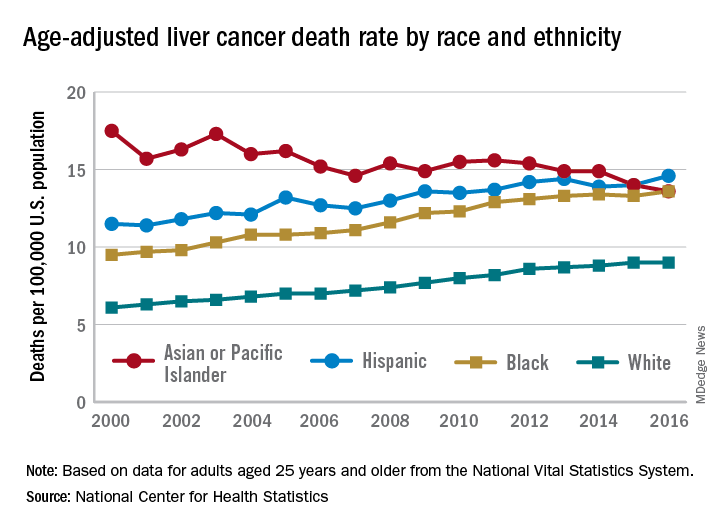
That shift resulted as much from increases for the other groups as from the decreased rate for Asians/Pacific Islanders. White adults were dying of liver cancer at a 48% higher rate in 2016 (9.0 per 100,000) than they were in 2000 (6.1), blacks saw their death rate go from 9.5 to 13.6 – a 43% increase – and the rate for Hispanics rose by 27% from 2000 (11.5) to 2016 (14.6), said Jiaquan Xu, MD, of the NCHS mortality statistics branch.
The adjusted death rate from liver cancer for all adults went from 7.2 per 100,000 in 2000 to 10.3 in 2016 for an increase of 43%. Over that period, the rate for men was always at least twice as high as it was for women: It rose from 10.5 per 100,000 for men and 4.9 for women in 2000 to 15.0 for men and 6.3 for women in 2016, Dr. Xu said based on data from the mortality files of the National Vital Statistics System.
Geographically, the District of Columbia had the highest rate at 16.8 per 100,000 in 2016, followed by Louisiana (13.8), Hawaii (12.7), and Mississippi and New Mexico (12.4 each). Vermont’s rate of 6.0 was the lowest in the country, with Maine second at 7.4, Montana third at 7.7, and Utah and Nebraska tied for fourth at 7.8, according to Dr. Xu.
Liver cancer death rates are dropping for Asians/Pacific Islanders, but that is the exception to a larger trend, according to the National Center for Health Statistics.
The age-adjusted death rate for liver cancer is down 22% among Asian or Pacific Islander adults aged 25 years and older since the turn of the century, falling from 17.5 per 100,000 population in 2000 – when it was the highest of the four racial/ethnic groups included in the report – to 13.6 per 100,000 in 2016, by which time it was just middle of the pack, the NCHS reported.
That shift resulted as much from increases for the other groups as from the decreased rate for Asians/Pacific Islanders. White adults were dying of liver cancer at a 48% higher rate in 2016 (9.0 per 100,000) than they were in 2000 (6.1), blacks saw their death rate go from 9.5 to 13.6 – a 43% increase – and the rate for Hispanics rose by 27% from 2000 (11.5) to 2016 (14.6), said Jiaquan Xu, MD, of the NCHS mortality statistics branch.
The adjusted death rate from liver cancer for all adults went from 7.2 per 100,000 in 2000 to 10.3 in 2016 for an increase of 43%. Over that period, the rate for men was always at least twice as high as it was for women: It rose from 10.5 per 100,000 for men and 4.9 for women in 2000 to 15.0 for men and 6.3 for women in 2016, Dr. Xu said based on data from the mortality files of the National Vital Statistics System.
Geographically, the District of Columbia had the highest rate at 16.8 per 100,000 in 2016, followed by Louisiana (13.8), Hawaii (12.7), and Mississippi and New Mexico (12.4 each). Vermont’s rate of 6.0 was the lowest in the country, with Maine second at 7.4, Montana third at 7.7, and Utah and Nebraska tied for fourth at 7.8, according to Dr. Xu.
Liver cancer death rates are dropping for Asians/Pacific Islanders, but that is the exception to a larger trend, according to the National Center for Health Statistics.
The age-adjusted death rate for liver cancer is down 22% among Asian or Pacific Islander adults aged 25 years and older since the turn of the century, falling from 17.5 per 100,000 population in 2000 – when it was the highest of the four racial/ethnic groups included in the report – to 13.6 per 100,000 in 2016, by which time it was just middle of the pack, the NCHS reported.
That shift resulted as much from increases for the other groups as from the decreased rate for Asians/Pacific Islanders. White adults were dying of liver cancer at a 48% higher rate in 2016 (9.0 per 100,000) than they were in 2000 (6.1), blacks saw their death rate go from 9.5 to 13.6 – a 43% increase – and the rate for Hispanics rose by 27% from 2000 (11.5) to 2016 (14.6), said Jiaquan Xu, MD, of the NCHS mortality statistics branch.
The adjusted death rate from liver cancer for all adults went from 7.2 per 100,000 in 2000 to 10.3 in 2016 for an increase of 43%. Over that period, the rate for men was always at least twice as high as it was for women: It rose from 10.5 per 100,000 for men and 4.9 for women in 2000 to 15.0 for men and 6.3 for women in 2016, Dr. Xu said based on data from the mortality files of the National Vital Statistics System.
Geographically, the District of Columbia had the highest rate at 16.8 per 100,000 in 2016, followed by Louisiana (13.8), Hawaii (12.7), and Mississippi and New Mexico (12.4 each). Vermont’s rate of 6.0 was the lowest in the country, with Maine second at 7.4, Montana third at 7.7, and Utah and Nebraska tied for fourth at 7.8, according to Dr. Xu.
Deaths from liver disease surged in U.S. since 1999
Cirrhosis mortality showed a sharp rise beginning in 2009, with a 3.6% annual increase driven entirely by a surge in alcoholic cirrhosis among young people aged 25-34 years, Elliot B. Tapper, MD, and Neehar D. Parikh, MD, reported in the BMJ. The uptick in hepatocellular carcinoma, however, was gradual and consistent, with a 2% annual increase felt mostly in older people, wrote Dr. Tapper and Dr. Parikh, both at the University of Michigan, Ann Arbor.
“The increasing mortality due to cirrhosis and hepatocellular carcinoma speaks to the expanding socioeconomic impact of liver disease,” the colleagues wrote. “Adverse trends in liver-related mortality are particularly unfortunate given that in most cases the liver disease is preventable. Understanding the factors associated with mortality due to these conditions will inform how best to allocate resources.”
The study extracted its data from the Vital Statistic Cooperative and the Centers for Disease Control and Prevention. The investigators not only examined raw mortality numbers, but analyzed them for demographic and geographic trends, in analyses that controlled for age.
Cirrhosis
During the study period, 460,760 patients died from cirrhosis (20,661 in 1999 and 34,174 in 2016, an increase of 65.4%).
Men were twice as likely to die from cirrhosis. Young people aged 25-34 years had the highest rate of increase (3.7% over the entire period and 10.5% from 2009 to 2016). This was directly driven by parallel increases in both alcohol use disorder and alcohol-related liver diseases, which increased by about 16% and 10%, respectively, in this group.
Native Americans had the highest mortality rate (25.8 per 100,000) followed by whites (12.7 per 100,000). “Notably, by 2016, cirrhosis accounted for 6.3% [up from 4.3% in 2009] and 7% [up from 5.8% in 2009] of deaths for Native Americans aged 25-34 and 35 or more, respectively,” and 2.3% of all deaths among adults aged 25-34 years, the authors wrote.
The increases were largely felt in the southern and western states (about 13 per 100,000 in each region). The greatest increases occurred in Kentucky (6.8%), New Mexico (6%), Arkansas (5.7%), Indiana (5%), and Alabama (5%). There was a statistically significant 1.2% decrease in deaths from cirrhosis in Maryland.
Hepatocellular carcinoma
Hepatocellular carcinoma accounted for 136,442 deaths during the study period (5,112 in 1999 and 11,073 in 2016 – an increase of 116.6%). This represented an average annual increase of 2%.
Men were four times more likely to die from hepatocellular carcinoma. The increase manifested mostly in older people, decreasing in those younger than 55 years. Mortality was highest among Asians and Pacific Islanders (6 per 100,000), followed by blacks (4.94 per 100,000).
The increases were largely felt in western states, with an overall increase of 4.2 per 100,000.
“Many of the same states with worsening cirrhosis-related mortality also experienced worsening mortality from hepatocellular carcinoma, including Oregon and Iowa,” the authors wrote. But mortality from the disease also increased significantly in Arizona (5.1%), Kansas (4.3%), Kentucky (4%), and Washington (3.9%).
“Potential explanations supported by these data include increasing early detection of hepatocellular carcinoma, application of curative or locoregional therapies, and, because hepatitis B is the principal cause of hepatocellular carcinoma worldwide and among Asian Americans, effectiveness of vaccination programs and the efficacy of antiviral therapy for hepatitis B in preventing the development of hepatocellular carcinoma.”
However, they noted, “it is unclear how these trends are, or will be, affected by direct-acting antivirals for hepatitis C virus ... eradication of hepatitis C virus will prevent the development of cirrhosis and its complications, potentially changing these trends in the next 5-10 years. However, therapy for hepatitis C viral infection cannot modify the statistically significant trends observed related to alcohol or the expected increase in the burden of nonalcoholic fatty liver disease.”
Neither author had any financial disclosure relevant to the work.
SOURCE: Tapper EB et al. BMJ 2018;362:k2817.
Cirrhosis mortality showed a sharp rise beginning in 2009, with a 3.6% annual increase driven entirely by a surge in alcoholic cirrhosis among young people aged 25-34 years, Elliot B. Tapper, MD, and Neehar D. Parikh, MD, reported in the BMJ. The uptick in hepatocellular carcinoma, however, was gradual and consistent, with a 2% annual increase felt mostly in older people, wrote Dr. Tapper and Dr. Parikh, both at the University of Michigan, Ann Arbor.
“The increasing mortality due to cirrhosis and hepatocellular carcinoma speaks to the expanding socioeconomic impact of liver disease,” the colleagues wrote. “Adverse trends in liver-related mortality are particularly unfortunate given that in most cases the liver disease is preventable. Understanding the factors associated with mortality due to these conditions will inform how best to allocate resources.”
The study extracted its data from the Vital Statistic Cooperative and the Centers for Disease Control and Prevention. The investigators not only examined raw mortality numbers, but analyzed them for demographic and geographic trends, in analyses that controlled for age.
Cirrhosis
During the study period, 460,760 patients died from cirrhosis (20,661 in 1999 and 34,174 in 2016, an increase of 65.4%).
Men were twice as likely to die from cirrhosis. Young people aged 25-34 years had the highest rate of increase (3.7% over the entire period and 10.5% from 2009 to 2016). This was directly driven by parallel increases in both alcohol use disorder and alcohol-related liver diseases, which increased by about 16% and 10%, respectively, in this group.
Native Americans had the highest mortality rate (25.8 per 100,000) followed by whites (12.7 per 100,000). “Notably, by 2016, cirrhosis accounted for 6.3% [up from 4.3% in 2009] and 7% [up from 5.8% in 2009] of deaths for Native Americans aged 25-34 and 35 or more, respectively,” and 2.3% of all deaths among adults aged 25-34 years, the authors wrote.
The increases were largely felt in the southern and western states (about 13 per 100,000 in each region). The greatest increases occurred in Kentucky (6.8%), New Mexico (6%), Arkansas (5.7%), Indiana (5%), and Alabama (5%). There was a statistically significant 1.2% decrease in deaths from cirrhosis in Maryland.
Hepatocellular carcinoma
Hepatocellular carcinoma accounted for 136,442 deaths during the study period (5,112 in 1999 and 11,073 in 2016 – an increase of 116.6%). This represented an average annual increase of 2%.
Men were four times more likely to die from hepatocellular carcinoma. The increase manifested mostly in older people, decreasing in those younger than 55 years. Mortality was highest among Asians and Pacific Islanders (6 per 100,000), followed by blacks (4.94 per 100,000).
The increases were largely felt in western states, with an overall increase of 4.2 per 100,000.
“Many of the same states with worsening cirrhosis-related mortality also experienced worsening mortality from hepatocellular carcinoma, including Oregon and Iowa,” the authors wrote. But mortality from the disease also increased significantly in Arizona (5.1%), Kansas (4.3%), Kentucky (4%), and Washington (3.9%).
“Potential explanations supported by these data include increasing early detection of hepatocellular carcinoma, application of curative or locoregional therapies, and, because hepatitis B is the principal cause of hepatocellular carcinoma worldwide and among Asian Americans, effectiveness of vaccination programs and the efficacy of antiviral therapy for hepatitis B in preventing the development of hepatocellular carcinoma.”
However, they noted, “it is unclear how these trends are, or will be, affected by direct-acting antivirals for hepatitis C virus ... eradication of hepatitis C virus will prevent the development of cirrhosis and its complications, potentially changing these trends in the next 5-10 years. However, therapy for hepatitis C viral infection cannot modify the statistically significant trends observed related to alcohol or the expected increase in the burden of nonalcoholic fatty liver disease.”
Neither author had any financial disclosure relevant to the work.
SOURCE: Tapper EB et al. BMJ 2018;362:k2817.
Cirrhosis mortality showed a sharp rise beginning in 2009, with a 3.6% annual increase driven entirely by a surge in alcoholic cirrhosis among young people aged 25-34 years, Elliot B. Tapper, MD, and Neehar D. Parikh, MD, reported in the BMJ. The uptick in hepatocellular carcinoma, however, was gradual and consistent, with a 2% annual increase felt mostly in older people, wrote Dr. Tapper and Dr. Parikh, both at the University of Michigan, Ann Arbor.
“The increasing mortality due to cirrhosis and hepatocellular carcinoma speaks to the expanding socioeconomic impact of liver disease,” the colleagues wrote. “Adverse trends in liver-related mortality are particularly unfortunate given that in most cases the liver disease is preventable. Understanding the factors associated with mortality due to these conditions will inform how best to allocate resources.”
The study extracted its data from the Vital Statistic Cooperative and the Centers for Disease Control and Prevention. The investigators not only examined raw mortality numbers, but analyzed them for demographic and geographic trends, in analyses that controlled for age.
Cirrhosis
During the study period, 460,760 patients died from cirrhosis (20,661 in 1999 and 34,174 in 2016, an increase of 65.4%).
Men were twice as likely to die from cirrhosis. Young people aged 25-34 years had the highest rate of increase (3.7% over the entire period and 10.5% from 2009 to 2016). This was directly driven by parallel increases in both alcohol use disorder and alcohol-related liver diseases, which increased by about 16% and 10%, respectively, in this group.
Native Americans had the highest mortality rate (25.8 per 100,000) followed by whites (12.7 per 100,000). “Notably, by 2016, cirrhosis accounted for 6.3% [up from 4.3% in 2009] and 7% [up from 5.8% in 2009] of deaths for Native Americans aged 25-34 and 35 or more, respectively,” and 2.3% of all deaths among adults aged 25-34 years, the authors wrote.
The increases were largely felt in the southern and western states (about 13 per 100,000 in each region). The greatest increases occurred in Kentucky (6.8%), New Mexico (6%), Arkansas (5.7%), Indiana (5%), and Alabama (5%). There was a statistically significant 1.2% decrease in deaths from cirrhosis in Maryland.
Hepatocellular carcinoma
Hepatocellular carcinoma accounted for 136,442 deaths during the study period (5,112 in 1999 and 11,073 in 2016 – an increase of 116.6%). This represented an average annual increase of 2%.
Men were four times more likely to die from hepatocellular carcinoma. The increase manifested mostly in older people, decreasing in those younger than 55 years. Mortality was highest among Asians and Pacific Islanders (6 per 100,000), followed by blacks (4.94 per 100,000).
The increases were largely felt in western states, with an overall increase of 4.2 per 100,000.
“Many of the same states with worsening cirrhosis-related mortality also experienced worsening mortality from hepatocellular carcinoma, including Oregon and Iowa,” the authors wrote. But mortality from the disease also increased significantly in Arizona (5.1%), Kansas (4.3%), Kentucky (4%), and Washington (3.9%).
“Potential explanations supported by these data include increasing early detection of hepatocellular carcinoma, application of curative or locoregional therapies, and, because hepatitis B is the principal cause of hepatocellular carcinoma worldwide and among Asian Americans, effectiveness of vaccination programs and the efficacy of antiviral therapy for hepatitis B in preventing the development of hepatocellular carcinoma.”
However, they noted, “it is unclear how these trends are, or will be, affected by direct-acting antivirals for hepatitis C virus ... eradication of hepatitis C virus will prevent the development of cirrhosis and its complications, potentially changing these trends in the next 5-10 years. However, therapy for hepatitis C viral infection cannot modify the statistically significant trends observed related to alcohol or the expected increase in the burden of nonalcoholic fatty liver disease.”
Neither author had any financial disclosure relevant to the work.
SOURCE: Tapper EB et al. BMJ 2018;362:k2817.
FROM BMJ
Key clinical point: Deaths from cirrhosis and hepatocellular carcinoma have surged in the United States since 1999.
Major finding: Liver cirrhosis mortality increased by 65% and hepatocellular carcinoma mortality by about 117%.
Study details: The study extracted data from the National Vital Statistics database and the CDC.
Disclosures: Neither author had relevant financial disclosures.
Source: Tapper EB et al. BMJ 2018;362:k2817.
NAFLD less common, more severe in black children
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
REPORTING FROM ADA 2018
Key clinical point: Obese black children are less likely than others to develop non-alcoholic fatty liver disease, but more likely to suffer its consequences if they do.
Major finding: Black children with NAFLD had a higher prevalence of prediabetes and type 2 diabetes (66.6%), compared with white (24.4%) and Hispanic children (31.1%).
Study details: Review of 503 obese adolescents
Disclosures: The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
Source: Trico D et al. ADA 2018, Abstract 313-OR.
AGA Clinical Practice Update: Statins are safe, effective, and important for most patients with liver disease and dyslipidemia
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
EXPERT ANALYSIS FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY