User login
High healthcare costs follow CCSs into adulthood
New research suggests survivors of childhood cancer can incur high out-of-pocket medical costs into adulthood and may forgo healthcare to lessen this financial burden.
The study showed that childhood cancer survivors (CCSs) were more likely than non-CCSs to have out-of-pocket medical costs that were at least 10% of their annual income.
And these high costs were associated with delaying care or skipping it altogether.
These findings were published in the Journal of Clinical Oncology.
“Survivors who reported spending a higher percentage of their income on out-of-pocket medical costs were not only more likely to report financial burden, they also were at risk for undertaking behaviors potentially detrimental to their health in order to save money,” said study author Ryan Nipp, MD, of Massachusetts General Hospital Cancer Center in Boston.
“While studies have identified associations between financial burden and patients’ treatment outcomes, quality of life, and even survival among adults with cancer, as far as we know, this is the first to report these associations in survivors of childhood cancer.”
For this research, Dr Nipp and his colleagues surveyed participants in the Childhood Cancer Survivor Study. This included adults who had been treated for childhood cancers between 1970 and 1986 along with a control group of siblings not affected by cancer.
In 2011 and 2012, participants were asked to provide information about their health insurance, the out-of-pocket healthcare costs they paid during the previous year, and sociodemographic information such as annual income and employment status.
The researchers also asked participants whether medical costs posed a financial burden and, if so, what measures they had taken to deal with that burden.
Study population
The researchers received complete responses from 580 CCSs and 173 of their siblings without a history of cancer. The most common cancer diagnosis was leukemia (33%), followed by Hodgkin lymphoma (14%), while non-Hodgkin lymphoma was less common (7%).
CCSs were a mean of 30.2 years from diagnosis. Use of chemotherapy (77%), radiation (66%), and surgery (81%) were common. Few patients had cancer recurrence (13%) or second cancers (5%).
There was no significant difference between CCSs and their siblings with regard to age at the time of the survey (P=0.071), household income (P=0.053), education (P=0.345), health insurance status (P=0.317), or having at least 1 hospitalization in the past year (P=0.270).
However, CCSs were significantly more likely than siblings to have chronic health conditions (P<0.001). Forty percent of CCSs had severe or life-threatening chronic conditions, compared to 17% of siblings.
Seventy-six percent of CCSs and 80% of siblings were employed. Twenty-nine percent of CCSs and 39% of siblings had household incomes exceeding $100,000. Twelve percent of CCSs and 5% of siblings had household incomes below $20,000.
Ninety-one percent of CCSs and 93% of siblings were insured. Most subjects in both groups (81% and 87%, respectively) had employer-sponsored insurance.
Results
CCSs were significantly more likely than their siblings to have out-of-pocket medical costs that were at least 10% of their annual income—10% and 3%, respectively (P<0.001).
Among CCSs, those with higher out-of-pocket costs (≥10% vs <10% of income) were more likely to have household incomes below $50,000 (odds ratio [OR]=5.5) and to report being hospitalized in the past year (OR=2.3).
CCSs with a higher percentage of their income spent on out-of-pocket costs were also more likely to:
- Have problems paying their medical bills (OR=8.8)
- Report inability to pay for basic costs of living such as food, heat, or rent (OR=6.1)
- Defer healthcare for a medical problem (OR=3.1)
- Skip a test, treatment, or follow-up (OR=2.1)
- Consider filing for bankruptcy (OR=6.4).
“A more comprehensive understanding of the relationship between high out-of-pocket medical costs and the adverse effects of increased financial burden on cancer survivors could be instrumental in helping us identify those at risk for higher costs to help us address their financial challenges and improve health outcomes,” Dr Nipp said.
“It could also help inform policy changes to help meet the unique needs of cancer survivors and improve our understanding of how both higher costs and resulting financial burden influence patients’ approach to their medical care and decision-making.” ![]()
New research suggests survivors of childhood cancer can incur high out-of-pocket medical costs into adulthood and may forgo healthcare to lessen this financial burden.
The study showed that childhood cancer survivors (CCSs) were more likely than non-CCSs to have out-of-pocket medical costs that were at least 10% of their annual income.
And these high costs were associated with delaying care or skipping it altogether.
These findings were published in the Journal of Clinical Oncology.
“Survivors who reported spending a higher percentage of their income on out-of-pocket medical costs were not only more likely to report financial burden, they also were at risk for undertaking behaviors potentially detrimental to their health in order to save money,” said study author Ryan Nipp, MD, of Massachusetts General Hospital Cancer Center in Boston.
“While studies have identified associations between financial burden and patients’ treatment outcomes, quality of life, and even survival among adults with cancer, as far as we know, this is the first to report these associations in survivors of childhood cancer.”
For this research, Dr Nipp and his colleagues surveyed participants in the Childhood Cancer Survivor Study. This included adults who had been treated for childhood cancers between 1970 and 1986 along with a control group of siblings not affected by cancer.
In 2011 and 2012, participants were asked to provide information about their health insurance, the out-of-pocket healthcare costs they paid during the previous year, and sociodemographic information such as annual income and employment status.
The researchers also asked participants whether medical costs posed a financial burden and, if so, what measures they had taken to deal with that burden.
Study population
The researchers received complete responses from 580 CCSs and 173 of their siblings without a history of cancer. The most common cancer diagnosis was leukemia (33%), followed by Hodgkin lymphoma (14%), while non-Hodgkin lymphoma was less common (7%).
CCSs were a mean of 30.2 years from diagnosis. Use of chemotherapy (77%), radiation (66%), and surgery (81%) were common. Few patients had cancer recurrence (13%) or second cancers (5%).
There was no significant difference between CCSs and their siblings with regard to age at the time of the survey (P=0.071), household income (P=0.053), education (P=0.345), health insurance status (P=0.317), or having at least 1 hospitalization in the past year (P=0.270).
However, CCSs were significantly more likely than siblings to have chronic health conditions (P<0.001). Forty percent of CCSs had severe or life-threatening chronic conditions, compared to 17% of siblings.
Seventy-six percent of CCSs and 80% of siblings were employed. Twenty-nine percent of CCSs and 39% of siblings had household incomes exceeding $100,000. Twelve percent of CCSs and 5% of siblings had household incomes below $20,000.
Ninety-one percent of CCSs and 93% of siblings were insured. Most subjects in both groups (81% and 87%, respectively) had employer-sponsored insurance.
Results
CCSs were significantly more likely than their siblings to have out-of-pocket medical costs that were at least 10% of their annual income—10% and 3%, respectively (P<0.001).
Among CCSs, those with higher out-of-pocket costs (≥10% vs <10% of income) were more likely to have household incomes below $50,000 (odds ratio [OR]=5.5) and to report being hospitalized in the past year (OR=2.3).
CCSs with a higher percentage of their income spent on out-of-pocket costs were also more likely to:
- Have problems paying their medical bills (OR=8.8)
- Report inability to pay for basic costs of living such as food, heat, or rent (OR=6.1)
- Defer healthcare for a medical problem (OR=3.1)
- Skip a test, treatment, or follow-up (OR=2.1)
- Consider filing for bankruptcy (OR=6.4).
“A more comprehensive understanding of the relationship between high out-of-pocket medical costs and the adverse effects of increased financial burden on cancer survivors could be instrumental in helping us identify those at risk for higher costs to help us address their financial challenges and improve health outcomes,” Dr Nipp said.
“It could also help inform policy changes to help meet the unique needs of cancer survivors and improve our understanding of how both higher costs and resulting financial burden influence patients’ approach to their medical care and decision-making.” ![]()
New research suggests survivors of childhood cancer can incur high out-of-pocket medical costs into adulthood and may forgo healthcare to lessen this financial burden.
The study showed that childhood cancer survivors (CCSs) were more likely than non-CCSs to have out-of-pocket medical costs that were at least 10% of their annual income.
And these high costs were associated with delaying care or skipping it altogether.
These findings were published in the Journal of Clinical Oncology.
“Survivors who reported spending a higher percentage of their income on out-of-pocket medical costs were not only more likely to report financial burden, they also were at risk for undertaking behaviors potentially detrimental to their health in order to save money,” said study author Ryan Nipp, MD, of Massachusetts General Hospital Cancer Center in Boston.
“While studies have identified associations between financial burden and patients’ treatment outcomes, quality of life, and even survival among adults with cancer, as far as we know, this is the first to report these associations in survivors of childhood cancer.”
For this research, Dr Nipp and his colleagues surveyed participants in the Childhood Cancer Survivor Study. This included adults who had been treated for childhood cancers between 1970 and 1986 along with a control group of siblings not affected by cancer.
In 2011 and 2012, participants were asked to provide information about their health insurance, the out-of-pocket healthcare costs they paid during the previous year, and sociodemographic information such as annual income and employment status.
The researchers also asked participants whether medical costs posed a financial burden and, if so, what measures they had taken to deal with that burden.
Study population
The researchers received complete responses from 580 CCSs and 173 of their siblings without a history of cancer. The most common cancer diagnosis was leukemia (33%), followed by Hodgkin lymphoma (14%), while non-Hodgkin lymphoma was less common (7%).
CCSs were a mean of 30.2 years from diagnosis. Use of chemotherapy (77%), radiation (66%), and surgery (81%) were common. Few patients had cancer recurrence (13%) or second cancers (5%).
There was no significant difference between CCSs and their siblings with regard to age at the time of the survey (P=0.071), household income (P=0.053), education (P=0.345), health insurance status (P=0.317), or having at least 1 hospitalization in the past year (P=0.270).
However, CCSs were significantly more likely than siblings to have chronic health conditions (P<0.001). Forty percent of CCSs had severe or life-threatening chronic conditions, compared to 17% of siblings.
Seventy-six percent of CCSs and 80% of siblings were employed. Twenty-nine percent of CCSs and 39% of siblings had household incomes exceeding $100,000. Twelve percent of CCSs and 5% of siblings had household incomes below $20,000.
Ninety-one percent of CCSs and 93% of siblings were insured. Most subjects in both groups (81% and 87%, respectively) had employer-sponsored insurance.
Results
CCSs were significantly more likely than their siblings to have out-of-pocket medical costs that were at least 10% of their annual income—10% and 3%, respectively (P<0.001).
Among CCSs, those with higher out-of-pocket costs (≥10% vs <10% of income) were more likely to have household incomes below $50,000 (odds ratio [OR]=5.5) and to report being hospitalized in the past year (OR=2.3).
CCSs with a higher percentage of their income spent on out-of-pocket costs were also more likely to:
- Have problems paying their medical bills (OR=8.8)
- Report inability to pay for basic costs of living such as food, heat, or rent (OR=6.1)
- Defer healthcare for a medical problem (OR=3.1)
- Skip a test, treatment, or follow-up (OR=2.1)
- Consider filing for bankruptcy (OR=6.4).
“A more comprehensive understanding of the relationship between high out-of-pocket medical costs and the adverse effects of increased financial burden on cancer survivors could be instrumental in helping us identify those at risk for higher costs to help us address their financial challenges and improve health outcomes,” Dr Nipp said.
“It could also help inform policy changes to help meet the unique needs of cancer survivors and improve our understanding of how both higher costs and resulting financial burden influence patients’ approach to their medical care and decision-making.” ![]()
Health Canada approves new use for brentuximab vedotin
Health Canada has issued a non-conditional marketing authorization for brentuximab vedotin (Adcetris).
This means the drug is now approved for use as consolidation after autologous stem cell transplant (ASCT) in patients with Hodgkin lymphoma (HL) who have an increased risk of relapse or progression.
Brentuximab vedotin previously received approval with conditions in Canada to treat HL patients who relapse after ASCT or HL patients who are not ASCT candidates and relapse after at least 2 multi-agent chemotherapy regimens.
Brentuximab vedotin also has conditional approval in Canada to treat patients with systemic anaplastic large-cell lymphoma who relapse after at least 1 multi-agent chemotherapy regimen.
Brentuximab vedotin is an antibody-drug conjugate consisting of an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E.
Brentuximab vedotin has marketing authorization in 67 countries for the treatment of relapsed or refractory HL and systemic anaplastic large-cell lymphoma.
Seattle Genetics and Takeda are jointly developing brentuximab vedotin. Seattle Genetics has US and Canadian commercialization rights, and Takeda has rights to commercialize the drug in the rest of the world.
AETHERA trial
Health Canada’s decision to extend the marketing authorization of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015.
The study enrolled 329 HL patients at risk of relapse or progression—165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()
Health Canada has issued a non-conditional marketing authorization for brentuximab vedotin (Adcetris).
This means the drug is now approved for use as consolidation after autologous stem cell transplant (ASCT) in patients with Hodgkin lymphoma (HL) who have an increased risk of relapse or progression.
Brentuximab vedotin previously received approval with conditions in Canada to treat HL patients who relapse after ASCT or HL patients who are not ASCT candidates and relapse after at least 2 multi-agent chemotherapy regimens.
Brentuximab vedotin also has conditional approval in Canada to treat patients with systemic anaplastic large-cell lymphoma who relapse after at least 1 multi-agent chemotherapy regimen.
Brentuximab vedotin is an antibody-drug conjugate consisting of an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E.
Brentuximab vedotin has marketing authorization in 67 countries for the treatment of relapsed or refractory HL and systemic anaplastic large-cell lymphoma.
Seattle Genetics and Takeda are jointly developing brentuximab vedotin. Seattle Genetics has US and Canadian commercialization rights, and Takeda has rights to commercialize the drug in the rest of the world.
AETHERA trial
Health Canada’s decision to extend the marketing authorization of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015.
The study enrolled 329 HL patients at risk of relapse or progression—165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()
Health Canada has issued a non-conditional marketing authorization for brentuximab vedotin (Adcetris).
This means the drug is now approved for use as consolidation after autologous stem cell transplant (ASCT) in patients with Hodgkin lymphoma (HL) who have an increased risk of relapse or progression.
Brentuximab vedotin previously received approval with conditions in Canada to treat HL patients who relapse after ASCT or HL patients who are not ASCT candidates and relapse after at least 2 multi-agent chemotherapy regimens.
Brentuximab vedotin also has conditional approval in Canada to treat patients with systemic anaplastic large-cell lymphoma who relapse after at least 1 multi-agent chemotherapy regimen.
Brentuximab vedotin is an antibody-drug conjugate consisting of an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E.
Brentuximab vedotin has marketing authorization in 67 countries for the treatment of relapsed or refractory HL and systemic anaplastic large-cell lymphoma.
Seattle Genetics and Takeda are jointly developing brentuximab vedotin. Seattle Genetics has US and Canadian commercialization rights, and Takeda has rights to commercialize the drug in the rest of the world.
AETHERA trial
Health Canada’s decision to extend the marketing authorization of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015.
The study enrolled 329 HL patients at risk of relapse or progression—165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()
Avelumab induces response in Hodgkin lymphoma after failed allo-SCT
LUGANO, SWITZERLAND – The immune checkpoint inhibitor avelumab showed efficacy against classical Hodgkin lymphoma among patients with disease progression following allogeneic stem cell transplants (allo-SCT), based on results of a phase 1 trial.
Two of eight patients with disease progression following an allogeneic transplant (allo-SCT) had complete responses (CR) to the programmed death ligand-1 (PD-L1) inhibitor avelumab (Bavencio), three had partial responses (PRs), and two had stable disease, reported Robert Chen, MD, of City of Hope Medical Center in Duarte, California.
“The overall response rate observed in the postallo population of 62.5% suggests that the PD-L1 blockade inhibitor may potentiate a graft-vs.-lymphoma response,” he said at the 14th International Conference on Malignant Lymphoma.
Amplification of the chromosome 9p24.1 locus is frequent in classical Hodgkin lymphoma, and the amplicon contains the genes encoding for PD-L1 and PD-L2, resulting in the over expression of both ligands, Dr. Chen said.
Both nivolumab (Opdivo) and pembrolizumab (Keytruda) are indicated for the treatment of relapsed/refractory classical Hodgkin lymphoma. Both agents block the interactions between PD-1 and both PD-L1 and PD-L2.
“However, it has not been established whether blockade of the PD-1/PD-L1 interaction is necessary and/or sufficient for the therapeutic effect observed in classical Hodgkin lymphoma,” he said.
Avelumab is an anti–PD-L1, immunoglobulin G1 monoclonal antibody that inhibits PD-1/PD-L1 interactions but leaves PD-1/PD-L2 interactions intact. This agent, which recently received FDA approval for the treatment of Merkel cell carcinoma and locally advanced or metastatic urothelial carcinoma, targets tumor cells rather than the T cells targeted by nivolumab and pembrolizumab.
In the phase 1b JAVELIN Hodgkin study, patients with histologically confirmed relapsed/refractory classical Hodgkin lymphoma who were ineligible for transplant or for whom allogeneic or autologous stem cell transplants had failed were enrolled and were assigned to one of five cohorts to receive avelumab in doses ranging from 70 mg intravenously to 10 mg/kg IV every 2 weeks (or every 3 weeks for the 500 mg dose cohort).
A total of 31 patients were randomized in the dose-finding phase of the study. The median patient age was 38 years, 24 patients were younger than 65, and 7 were 65 or older. Only 1 of the 31 patients had received a single prior line of therapy. Of those, 3 had received two prior therapies, 7 had been treated with three prior lines, and 20 had four or more prior lines of therapy. All patients had received brentuximab vedotin (Adcetris).
The median follow-up was 43.3 weeks. In all, nine patients were continuing on avelumab at the time of the data analysis. Because of disease progression, 10 patients discontinued therapy. Additionally, four discontinued because of adverse events, two chose to withdraw, one was removed from the study by the treating physicians, one did not receive treatment, and four others discontinued because of unspecified reasons.
The median treatment duration was 16.9 weeks. The mean number of cycles was 8.6.
The objective response rate was 42%, including five CRs and eight PRs. Three of the CRs were in patients treated at the 70 mg every 2 week dose, and two were in patients treated at the 500 mg every 3 week level.
In all, 23 patients experienced some degree of tumor shrinkage, and 13 had shrinkage greater than 50%.
In an analysis of best overall response among patients whose disease progressed following SCT, the investigators found that two of eight patients (25%) who had disease progression following allo-SCT had a complete response. Three of these patients had a PR, two had stable disease, and one was not eligible for response evaluation.
In contrast, there was only one objective response, a PR, among five patients who had relapses following autologous SCT.
Grade 3 or 4 treatment-related adverse events occurred in 37% of patients. There were no treatment-related deaths. The incidence of treatment-related adverse events was similar across the five dosing cohorts.
“Based on the observed efficacy and safety profiles and unmet need, this study has recently been amended to focus the expansion of patients who progressed post allo-SCT,” Dr. Chen said.
The study was sponsored by Pfizer in collaboration with Merck KGaA, Germany. Dr. Chen has consulted and served in a speakers’ bureau for Seattle Genetics, Millennium, and Genentech. He has also received research funding from Pharmacyclics, Seattle Genetics, Millennium, and Merck.
LUGANO, SWITZERLAND – The immune checkpoint inhibitor avelumab showed efficacy against classical Hodgkin lymphoma among patients with disease progression following allogeneic stem cell transplants (allo-SCT), based on results of a phase 1 trial.
Two of eight patients with disease progression following an allogeneic transplant (allo-SCT) had complete responses (CR) to the programmed death ligand-1 (PD-L1) inhibitor avelumab (Bavencio), three had partial responses (PRs), and two had stable disease, reported Robert Chen, MD, of City of Hope Medical Center in Duarte, California.
“The overall response rate observed in the postallo population of 62.5% suggests that the PD-L1 blockade inhibitor may potentiate a graft-vs.-lymphoma response,” he said at the 14th International Conference on Malignant Lymphoma.
Amplification of the chromosome 9p24.1 locus is frequent in classical Hodgkin lymphoma, and the amplicon contains the genes encoding for PD-L1 and PD-L2, resulting in the over expression of both ligands, Dr. Chen said.
Both nivolumab (Opdivo) and pembrolizumab (Keytruda) are indicated for the treatment of relapsed/refractory classical Hodgkin lymphoma. Both agents block the interactions between PD-1 and both PD-L1 and PD-L2.
“However, it has not been established whether blockade of the PD-1/PD-L1 interaction is necessary and/or sufficient for the therapeutic effect observed in classical Hodgkin lymphoma,” he said.
Avelumab is an anti–PD-L1, immunoglobulin G1 monoclonal antibody that inhibits PD-1/PD-L1 interactions but leaves PD-1/PD-L2 interactions intact. This agent, which recently received FDA approval for the treatment of Merkel cell carcinoma and locally advanced or metastatic urothelial carcinoma, targets tumor cells rather than the T cells targeted by nivolumab and pembrolizumab.
In the phase 1b JAVELIN Hodgkin study, patients with histologically confirmed relapsed/refractory classical Hodgkin lymphoma who were ineligible for transplant or for whom allogeneic or autologous stem cell transplants had failed were enrolled and were assigned to one of five cohorts to receive avelumab in doses ranging from 70 mg intravenously to 10 mg/kg IV every 2 weeks (or every 3 weeks for the 500 mg dose cohort).
A total of 31 patients were randomized in the dose-finding phase of the study. The median patient age was 38 years, 24 patients were younger than 65, and 7 were 65 or older. Only 1 of the 31 patients had received a single prior line of therapy. Of those, 3 had received two prior therapies, 7 had been treated with three prior lines, and 20 had four or more prior lines of therapy. All patients had received brentuximab vedotin (Adcetris).
The median follow-up was 43.3 weeks. In all, nine patients were continuing on avelumab at the time of the data analysis. Because of disease progression, 10 patients discontinued therapy. Additionally, four discontinued because of adverse events, two chose to withdraw, one was removed from the study by the treating physicians, one did not receive treatment, and four others discontinued because of unspecified reasons.
The median treatment duration was 16.9 weeks. The mean number of cycles was 8.6.
The objective response rate was 42%, including five CRs and eight PRs. Three of the CRs were in patients treated at the 70 mg every 2 week dose, and two were in patients treated at the 500 mg every 3 week level.
In all, 23 patients experienced some degree of tumor shrinkage, and 13 had shrinkage greater than 50%.
In an analysis of best overall response among patients whose disease progressed following SCT, the investigators found that two of eight patients (25%) who had disease progression following allo-SCT had a complete response. Three of these patients had a PR, two had stable disease, and one was not eligible for response evaluation.
In contrast, there was only one objective response, a PR, among five patients who had relapses following autologous SCT.
Grade 3 or 4 treatment-related adverse events occurred in 37% of patients. There were no treatment-related deaths. The incidence of treatment-related adverse events was similar across the five dosing cohorts.
“Based on the observed efficacy and safety profiles and unmet need, this study has recently been amended to focus the expansion of patients who progressed post allo-SCT,” Dr. Chen said.
The study was sponsored by Pfizer in collaboration with Merck KGaA, Germany. Dr. Chen has consulted and served in a speakers’ bureau for Seattle Genetics, Millennium, and Genentech. He has also received research funding from Pharmacyclics, Seattle Genetics, Millennium, and Merck.
LUGANO, SWITZERLAND – The immune checkpoint inhibitor avelumab showed efficacy against classical Hodgkin lymphoma among patients with disease progression following allogeneic stem cell transplants (allo-SCT), based on results of a phase 1 trial.
Two of eight patients with disease progression following an allogeneic transplant (allo-SCT) had complete responses (CR) to the programmed death ligand-1 (PD-L1) inhibitor avelumab (Bavencio), three had partial responses (PRs), and two had stable disease, reported Robert Chen, MD, of City of Hope Medical Center in Duarte, California.
“The overall response rate observed in the postallo population of 62.5% suggests that the PD-L1 blockade inhibitor may potentiate a graft-vs.-lymphoma response,” he said at the 14th International Conference on Malignant Lymphoma.
Amplification of the chromosome 9p24.1 locus is frequent in classical Hodgkin lymphoma, and the amplicon contains the genes encoding for PD-L1 and PD-L2, resulting in the over expression of both ligands, Dr. Chen said.
Both nivolumab (Opdivo) and pembrolizumab (Keytruda) are indicated for the treatment of relapsed/refractory classical Hodgkin lymphoma. Both agents block the interactions between PD-1 and both PD-L1 and PD-L2.
“However, it has not been established whether blockade of the PD-1/PD-L1 interaction is necessary and/or sufficient for the therapeutic effect observed in classical Hodgkin lymphoma,” he said.
Avelumab is an anti–PD-L1, immunoglobulin G1 monoclonal antibody that inhibits PD-1/PD-L1 interactions but leaves PD-1/PD-L2 interactions intact. This agent, which recently received FDA approval for the treatment of Merkel cell carcinoma and locally advanced or metastatic urothelial carcinoma, targets tumor cells rather than the T cells targeted by nivolumab and pembrolizumab.
In the phase 1b JAVELIN Hodgkin study, patients with histologically confirmed relapsed/refractory classical Hodgkin lymphoma who were ineligible for transplant or for whom allogeneic or autologous stem cell transplants had failed were enrolled and were assigned to one of five cohorts to receive avelumab in doses ranging from 70 mg intravenously to 10 mg/kg IV every 2 weeks (or every 3 weeks for the 500 mg dose cohort).
A total of 31 patients were randomized in the dose-finding phase of the study. The median patient age was 38 years, 24 patients were younger than 65, and 7 were 65 or older. Only 1 of the 31 patients had received a single prior line of therapy. Of those, 3 had received two prior therapies, 7 had been treated with three prior lines, and 20 had four or more prior lines of therapy. All patients had received brentuximab vedotin (Adcetris).
The median follow-up was 43.3 weeks. In all, nine patients were continuing on avelumab at the time of the data analysis. Because of disease progression, 10 patients discontinued therapy. Additionally, four discontinued because of adverse events, two chose to withdraw, one was removed from the study by the treating physicians, one did not receive treatment, and four others discontinued because of unspecified reasons.
The median treatment duration was 16.9 weeks. The mean number of cycles was 8.6.
The objective response rate was 42%, including five CRs and eight PRs. Three of the CRs were in patients treated at the 70 mg every 2 week dose, and two were in patients treated at the 500 mg every 3 week level.
In all, 23 patients experienced some degree of tumor shrinkage, and 13 had shrinkage greater than 50%.
In an analysis of best overall response among patients whose disease progressed following SCT, the investigators found that two of eight patients (25%) who had disease progression following allo-SCT had a complete response. Three of these patients had a PR, two had stable disease, and one was not eligible for response evaluation.
In contrast, there was only one objective response, a PR, among five patients who had relapses following autologous SCT.
Grade 3 or 4 treatment-related adverse events occurred in 37% of patients. There were no treatment-related deaths. The incidence of treatment-related adverse events was similar across the five dosing cohorts.
“Based on the observed efficacy and safety profiles and unmet need, this study has recently been amended to focus the expansion of patients who progressed post allo-SCT,” Dr. Chen said.
The study was sponsored by Pfizer in collaboration with Merck KGaA, Germany. Dr. Chen has consulted and served in a speakers’ bureau for Seattle Genetics, Millennium, and Genentech. He has also received research funding from Pharmacyclics, Seattle Genetics, Millennium, and Merck.
AT 14-ICML
Key clinical point: Avelumab showed efficacy in patients with classical Hodgkin lymphoma that relapsed following allogeneic stem cell transplant.
Major finding: The objective response rate among all patients in the study was 41.9%.
Data source: A phase 1 dose-finding and expansion study in 31 patients with relapsed/refractory classical Hodgkin lymphoma who were ineligible for SCT or experienced relapses following SCT.
Disclosures: The study was sponsored by Pfizer in collaboration with Merck KGaA, Germany. Dr. Chen has consulted and served in a speakers’ bureau for Seattle Genetics, Millennium, and Genentech. He has also received research funding from Pharmacyclics, Seattle Genetics, Millennium, and Merck.
Nivolumab for long-term treatment of cHL after auto-HSCT
LUGANO, SWITZERLAND—Nivolumab can provide long-term treatment for a broad range of adults who have relapsed or refractory classical Hodgkin lymphoma (cHL) after autologous hematopoietic stem cell transplant (auto-HSCT), according to a presentation at the 14th International Conference on Malignant Lymphoma (ICML).
In the phase 2 CheckMate-205 study, cHL patients achieved durable responses regardless of the depth of response, previous exposure to brentuximab vedotin (BV), and refractoriness to prior therapies.
Researchers observed sustained progression-free survival (PFS) in patients with stable disease (SD) or better, and the safety profile of nivolumab was considered acceptable.
“Nivolumab offers a favorable treatment outcome for patients who have relapsed disease after autologous stem cell transplant,” said Michelle Fanale, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Fanale presented results from CheckMate-205 at 14-ICML. The study was sponsored by Bristol-Myers Squibb Company.
CheckMate-205 enrolled 243 adults with relapsed or refractory cHL who had undergone auto-HSCT. Patients were divided into 3 cohorts:
- Cohort A included patients who were naïve to BV (n=63)
- Cohort B included patients who received BV only after auto-HSCT (n=80)
- Cohort C included patients who received BV before and/or after auto-HSCT (n=100).
All patients received nivolumab at 3 mg/kg once every 2 weeks until disease progression or unacceptable toxicity.
In cohort C, patients who were in complete response (CR) for 1 year were to discontinue nivolumab, but they could resume treatment with the drug if they relapsed within 2 years.
Patient characteristics
The median age was 33 (range, 18-65) in cohort A, 37 (range, 18-72) in cohort B, and 32 (range, 19-69) in cohort C.
ECOG performance status was 0 for 62% of patients in cohort A, 53% in cohort B, and 50% in cohort C. The remaining patients had a performance status of 1.
The percentage of patients with stage IV disease was 38% in cohort A, 68% in cohort B, and 61% in cohort C.
The median number of prior therapies was 2 (range, 2-8) in cohort A, 4 (range, 3-15) in cohort B, and 4 (range, 2-9) in cohort C. Fifty-nine percent, 74%, and 69% of patients, respectively, had received prior radiotherapy.
The median time from diagnosis to the first dose of nivolumab was 3.1 years (range, 1.0-30.6) in cohort A, 6.2 years (range, 1.3-25.1) in cohort B, and 3.5 years (range, 1.0-24.9) in cohort C.
The median time from auto-HSCT to the first dose of nivolumab was 1.0 years (range, 0.3-18.2) in cohort A, 3.4 years (range, 0.2-19.0) in cohort B, and 1.7 years (range, 0.2-17.0) in cohort C.
Safety
The most common drug-related adverse events (AEs) were fatigue (23% any grade, 1% grade 3/4), diarrhea (15% any grade, 1% grade 3/4), infusion-related reactions (14% any grade, <1% grade 3/4), rash (12% any grade, 1% grade 3/4), nausea (10% grade 1/2), and pruritus (10% grade 1/2).
The most common drug-related serious AEs were infusion-related reactions (2% any grade, <1% grade 3/4) and pneumonitis (1% grade 1/2).
Drug-related AEs leading to treatment discontinuation were pneumonitis (2% grade 1/2) and autoimmune hepatitis (1% grade 3/4).
There were no deaths due to drug-related AEs.
Response
The objective response rate was 69% overall, 65% in cohort A, 68% in cohort B, and 73% in cohort C.
CR was the best response for 16% of all patients, 29% of cohort A, 13% of cohort B, and 12% of cohort C.
Partial response (PR) was the best response for 53% of all patients, 37% of patients in cohort A, 55% in cohort B, and 61% in cohort C.
SD was the best response for 19% of all patients, 24% of patients in cohort A, 21% in cohort B, and 15% in cohort C.
In post-hoc analyses, responses were similar irrespective of BV treatment sequence.
The median duration of response was 17 months overall, 20 months for cohort A, 16 months for cohort B, and 15 months for cohort C.
The median duration of response in patients with a CR was 20 months overall and for cohorts A and B, but it was 15 months for cohort C.
The median duration of response in patients with a PR was 13 months overall, 17 months for cohort A, 11 months for cohort B, and 13 months for cohort C.
Survival
The median PFS for all patients was 15 months (range, 11-19). The median PFS was 22 months (range, 19-not reached) for patients who achieved a CR, 15 months (range, 11-19) for those who achieved a PR, and 11 months (range, 6-18) for those who had SD.
The median PFS was 18 months (range, 11-22) for patients in cohort A, 15 months (range, 11-20) for cohort B, and 12 months (range, 11-18) for cohort C.
The median overall survival (OS) has not been reached in any of the cohorts. The 12-months OS is 92% overall, 93% in cohort A, 95% in cohort B, and 90% in cohort C.
Patient status after extended follow-up
Forty percent of all patients were still on treatment after extended follow-up, as were 48% of patients in cohort A, 40% in cohort B, and 35% in cohort C.
The most common reason for stopping treatment was disease progression—25% of cohort A, 28% of cohort B, and 24% of cohort C.
Patients also stopped treatment due to nivolumab-related toxicity—5% in cohort A, 11% in cohort B, and 7% in cohort C. Three percent, 1%, and 1%, respectively, stopped due to AEs unrelated to nivolumab.
Three percent of patients in cohort C stopped because they had attained the maximum clinical benefit, and 8% in cohort C completed treatment. This includes 7 patients who discontinued treatment because they were in CR for 1 year.
None of the patients in cohort A or B discontinued because they attained the maximum clinical benefit or because they completed treatment.
Eight percent of patients in cohort A, 10% in cohort B, and 17% in cohort C discontinued so they could proceed to HSCT.
Outcomes after allo-HSCT
Forty-four patients received allogeneic (allo-) HSCT after nivolumab. The median post-HSCT follow-up was 5.5 months (range, 0-19), and the median time from last dose of nivolumab to allo-HSCT was 1.6 months (range, 0.5-13.5).
At 100 days, the rate of grade 2-4 acute graft-vs-host disease (GVHD) was 27%. The rate of grade 3-4 acute GVHD was 17%, and the rate of chronic GVHD was 10%. At 6 months, the rates were 30%, 20%, and 15%, respectively.
The incidence of transplant-related mortality was 13% at 100 days and at 6 months.
“While there are risks, potentially, for acute GVHD and transplant-related mortality, these aren’t necessarily significantly different from what we’ve seen from other historical publications,” Dr Fanale said.
She cited data showing that the 100-day incidence of acute GVHD in cHL patients who underwent allo-HSCT ranges from 26% to 60%, and the incidence of transplant-related mortality in these patients ranges from 6% to 28%. ![]()
LUGANO, SWITZERLAND—Nivolumab can provide long-term treatment for a broad range of adults who have relapsed or refractory classical Hodgkin lymphoma (cHL) after autologous hematopoietic stem cell transplant (auto-HSCT), according to a presentation at the 14th International Conference on Malignant Lymphoma (ICML).
In the phase 2 CheckMate-205 study, cHL patients achieved durable responses regardless of the depth of response, previous exposure to brentuximab vedotin (BV), and refractoriness to prior therapies.
Researchers observed sustained progression-free survival (PFS) in patients with stable disease (SD) or better, and the safety profile of nivolumab was considered acceptable.
“Nivolumab offers a favorable treatment outcome for patients who have relapsed disease after autologous stem cell transplant,” said Michelle Fanale, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Fanale presented results from CheckMate-205 at 14-ICML. The study was sponsored by Bristol-Myers Squibb Company.
CheckMate-205 enrolled 243 adults with relapsed or refractory cHL who had undergone auto-HSCT. Patients were divided into 3 cohorts:
- Cohort A included patients who were naïve to BV (n=63)
- Cohort B included patients who received BV only after auto-HSCT (n=80)
- Cohort C included patients who received BV before and/or after auto-HSCT (n=100).
All patients received nivolumab at 3 mg/kg once every 2 weeks until disease progression or unacceptable toxicity.
In cohort C, patients who were in complete response (CR) for 1 year were to discontinue nivolumab, but they could resume treatment with the drug if they relapsed within 2 years.
Patient characteristics
The median age was 33 (range, 18-65) in cohort A, 37 (range, 18-72) in cohort B, and 32 (range, 19-69) in cohort C.
ECOG performance status was 0 for 62% of patients in cohort A, 53% in cohort B, and 50% in cohort C. The remaining patients had a performance status of 1.
The percentage of patients with stage IV disease was 38% in cohort A, 68% in cohort B, and 61% in cohort C.
The median number of prior therapies was 2 (range, 2-8) in cohort A, 4 (range, 3-15) in cohort B, and 4 (range, 2-9) in cohort C. Fifty-nine percent, 74%, and 69% of patients, respectively, had received prior radiotherapy.
The median time from diagnosis to the first dose of nivolumab was 3.1 years (range, 1.0-30.6) in cohort A, 6.2 years (range, 1.3-25.1) in cohort B, and 3.5 years (range, 1.0-24.9) in cohort C.
The median time from auto-HSCT to the first dose of nivolumab was 1.0 years (range, 0.3-18.2) in cohort A, 3.4 years (range, 0.2-19.0) in cohort B, and 1.7 years (range, 0.2-17.0) in cohort C.
Safety
The most common drug-related adverse events (AEs) were fatigue (23% any grade, 1% grade 3/4), diarrhea (15% any grade, 1% grade 3/4), infusion-related reactions (14% any grade, <1% grade 3/4), rash (12% any grade, 1% grade 3/4), nausea (10% grade 1/2), and pruritus (10% grade 1/2).
The most common drug-related serious AEs were infusion-related reactions (2% any grade, <1% grade 3/4) and pneumonitis (1% grade 1/2).
Drug-related AEs leading to treatment discontinuation were pneumonitis (2% grade 1/2) and autoimmune hepatitis (1% grade 3/4).
There were no deaths due to drug-related AEs.
Response
The objective response rate was 69% overall, 65% in cohort A, 68% in cohort B, and 73% in cohort C.
CR was the best response for 16% of all patients, 29% of cohort A, 13% of cohort B, and 12% of cohort C.
Partial response (PR) was the best response for 53% of all patients, 37% of patients in cohort A, 55% in cohort B, and 61% in cohort C.
SD was the best response for 19% of all patients, 24% of patients in cohort A, 21% in cohort B, and 15% in cohort C.
In post-hoc analyses, responses were similar irrespective of BV treatment sequence.
The median duration of response was 17 months overall, 20 months for cohort A, 16 months for cohort B, and 15 months for cohort C.
The median duration of response in patients with a CR was 20 months overall and for cohorts A and B, but it was 15 months for cohort C.
The median duration of response in patients with a PR was 13 months overall, 17 months for cohort A, 11 months for cohort B, and 13 months for cohort C.
Survival
The median PFS for all patients was 15 months (range, 11-19). The median PFS was 22 months (range, 19-not reached) for patients who achieved a CR, 15 months (range, 11-19) for those who achieved a PR, and 11 months (range, 6-18) for those who had SD.
The median PFS was 18 months (range, 11-22) for patients in cohort A, 15 months (range, 11-20) for cohort B, and 12 months (range, 11-18) for cohort C.
The median overall survival (OS) has not been reached in any of the cohorts. The 12-months OS is 92% overall, 93% in cohort A, 95% in cohort B, and 90% in cohort C.
Patient status after extended follow-up
Forty percent of all patients were still on treatment after extended follow-up, as were 48% of patients in cohort A, 40% in cohort B, and 35% in cohort C.
The most common reason for stopping treatment was disease progression—25% of cohort A, 28% of cohort B, and 24% of cohort C.
Patients also stopped treatment due to nivolumab-related toxicity—5% in cohort A, 11% in cohort B, and 7% in cohort C. Three percent, 1%, and 1%, respectively, stopped due to AEs unrelated to nivolumab.
Three percent of patients in cohort C stopped because they had attained the maximum clinical benefit, and 8% in cohort C completed treatment. This includes 7 patients who discontinued treatment because they were in CR for 1 year.
None of the patients in cohort A or B discontinued because they attained the maximum clinical benefit or because they completed treatment.
Eight percent of patients in cohort A, 10% in cohort B, and 17% in cohort C discontinued so they could proceed to HSCT.
Outcomes after allo-HSCT
Forty-four patients received allogeneic (allo-) HSCT after nivolumab. The median post-HSCT follow-up was 5.5 months (range, 0-19), and the median time from last dose of nivolumab to allo-HSCT was 1.6 months (range, 0.5-13.5).
At 100 days, the rate of grade 2-4 acute graft-vs-host disease (GVHD) was 27%. The rate of grade 3-4 acute GVHD was 17%, and the rate of chronic GVHD was 10%. At 6 months, the rates were 30%, 20%, and 15%, respectively.
The incidence of transplant-related mortality was 13% at 100 days and at 6 months.
“While there are risks, potentially, for acute GVHD and transplant-related mortality, these aren’t necessarily significantly different from what we’ve seen from other historical publications,” Dr Fanale said.
She cited data showing that the 100-day incidence of acute GVHD in cHL patients who underwent allo-HSCT ranges from 26% to 60%, and the incidence of transplant-related mortality in these patients ranges from 6% to 28%. ![]()
LUGANO, SWITZERLAND—Nivolumab can provide long-term treatment for a broad range of adults who have relapsed or refractory classical Hodgkin lymphoma (cHL) after autologous hematopoietic stem cell transplant (auto-HSCT), according to a presentation at the 14th International Conference on Malignant Lymphoma (ICML).
In the phase 2 CheckMate-205 study, cHL patients achieved durable responses regardless of the depth of response, previous exposure to brentuximab vedotin (BV), and refractoriness to prior therapies.
Researchers observed sustained progression-free survival (PFS) in patients with stable disease (SD) or better, and the safety profile of nivolumab was considered acceptable.
“Nivolumab offers a favorable treatment outcome for patients who have relapsed disease after autologous stem cell transplant,” said Michelle Fanale, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Fanale presented results from CheckMate-205 at 14-ICML. The study was sponsored by Bristol-Myers Squibb Company.
CheckMate-205 enrolled 243 adults with relapsed or refractory cHL who had undergone auto-HSCT. Patients were divided into 3 cohorts:
- Cohort A included patients who were naïve to BV (n=63)
- Cohort B included patients who received BV only after auto-HSCT (n=80)
- Cohort C included patients who received BV before and/or after auto-HSCT (n=100).
All patients received nivolumab at 3 mg/kg once every 2 weeks until disease progression or unacceptable toxicity.
In cohort C, patients who were in complete response (CR) for 1 year were to discontinue nivolumab, but they could resume treatment with the drug if they relapsed within 2 years.
Patient characteristics
The median age was 33 (range, 18-65) in cohort A, 37 (range, 18-72) in cohort B, and 32 (range, 19-69) in cohort C.
ECOG performance status was 0 for 62% of patients in cohort A, 53% in cohort B, and 50% in cohort C. The remaining patients had a performance status of 1.
The percentage of patients with stage IV disease was 38% in cohort A, 68% in cohort B, and 61% in cohort C.
The median number of prior therapies was 2 (range, 2-8) in cohort A, 4 (range, 3-15) in cohort B, and 4 (range, 2-9) in cohort C. Fifty-nine percent, 74%, and 69% of patients, respectively, had received prior radiotherapy.
The median time from diagnosis to the first dose of nivolumab was 3.1 years (range, 1.0-30.6) in cohort A, 6.2 years (range, 1.3-25.1) in cohort B, and 3.5 years (range, 1.0-24.9) in cohort C.
The median time from auto-HSCT to the first dose of nivolumab was 1.0 years (range, 0.3-18.2) in cohort A, 3.4 years (range, 0.2-19.0) in cohort B, and 1.7 years (range, 0.2-17.0) in cohort C.
Safety
The most common drug-related adverse events (AEs) were fatigue (23% any grade, 1% grade 3/4), diarrhea (15% any grade, 1% grade 3/4), infusion-related reactions (14% any grade, <1% grade 3/4), rash (12% any grade, 1% grade 3/4), nausea (10% grade 1/2), and pruritus (10% grade 1/2).
The most common drug-related serious AEs were infusion-related reactions (2% any grade, <1% grade 3/4) and pneumonitis (1% grade 1/2).
Drug-related AEs leading to treatment discontinuation were pneumonitis (2% grade 1/2) and autoimmune hepatitis (1% grade 3/4).
There were no deaths due to drug-related AEs.
Response
The objective response rate was 69% overall, 65% in cohort A, 68% in cohort B, and 73% in cohort C.
CR was the best response for 16% of all patients, 29% of cohort A, 13% of cohort B, and 12% of cohort C.
Partial response (PR) was the best response for 53% of all patients, 37% of patients in cohort A, 55% in cohort B, and 61% in cohort C.
SD was the best response for 19% of all patients, 24% of patients in cohort A, 21% in cohort B, and 15% in cohort C.
In post-hoc analyses, responses were similar irrespective of BV treatment sequence.
The median duration of response was 17 months overall, 20 months for cohort A, 16 months for cohort B, and 15 months for cohort C.
The median duration of response in patients with a CR was 20 months overall and for cohorts A and B, but it was 15 months for cohort C.
The median duration of response in patients with a PR was 13 months overall, 17 months for cohort A, 11 months for cohort B, and 13 months for cohort C.
Survival
The median PFS for all patients was 15 months (range, 11-19). The median PFS was 22 months (range, 19-not reached) for patients who achieved a CR, 15 months (range, 11-19) for those who achieved a PR, and 11 months (range, 6-18) for those who had SD.
The median PFS was 18 months (range, 11-22) for patients in cohort A, 15 months (range, 11-20) for cohort B, and 12 months (range, 11-18) for cohort C.
The median overall survival (OS) has not been reached in any of the cohorts. The 12-months OS is 92% overall, 93% in cohort A, 95% in cohort B, and 90% in cohort C.
Patient status after extended follow-up
Forty percent of all patients were still on treatment after extended follow-up, as were 48% of patients in cohort A, 40% in cohort B, and 35% in cohort C.
The most common reason for stopping treatment was disease progression—25% of cohort A, 28% of cohort B, and 24% of cohort C.
Patients also stopped treatment due to nivolumab-related toxicity—5% in cohort A, 11% in cohort B, and 7% in cohort C. Three percent, 1%, and 1%, respectively, stopped due to AEs unrelated to nivolumab.
Three percent of patients in cohort C stopped because they had attained the maximum clinical benefit, and 8% in cohort C completed treatment. This includes 7 patients who discontinued treatment because they were in CR for 1 year.
None of the patients in cohort A or B discontinued because they attained the maximum clinical benefit or because they completed treatment.
Eight percent of patients in cohort A, 10% in cohort B, and 17% in cohort C discontinued so they could proceed to HSCT.
Outcomes after allo-HSCT
Forty-four patients received allogeneic (allo-) HSCT after nivolumab. The median post-HSCT follow-up was 5.5 months (range, 0-19), and the median time from last dose of nivolumab to allo-HSCT was 1.6 months (range, 0.5-13.5).
At 100 days, the rate of grade 2-4 acute graft-vs-host disease (GVHD) was 27%. The rate of grade 3-4 acute GVHD was 17%, and the rate of chronic GVHD was 10%. At 6 months, the rates were 30%, 20%, and 15%, respectively.
The incidence of transplant-related mortality was 13% at 100 days and at 6 months.
“While there are risks, potentially, for acute GVHD and transplant-related mortality, these aren’t necessarily significantly different from what we’ve seen from other historical publications,” Dr Fanale said.
She cited data showing that the 100-day incidence of acute GVHD in cHL patients who underwent allo-HSCT ranges from 26% to 60%, and the incidence of transplant-related mortality in these patients ranges from 6% to 28%. ![]()
Single-dose NEPA found non-inferior to aprepitant/granisetron
WASHINGTON, DC—In a head-to-head study comparing a single-dose oral antiemetic to a 3-day oral regimen, the single dose has shown itself to be non-inferior to the multi-day regimen in preventing chemotherapy-induced nausea and vomiting (CINV).
The investigators evaluated netupitant/palonosetron (NEPA) against aprepitant/granisetron (APR/GRAN) in patients on highly emetogenic chemotherapy.
They found the data suggest “that NEPA, in a single dose, had equivalent efficacy to a 3-day oral aprepitant/granisetron regimen,” according to the lead investigator and abstract presenter.
Li Zhang, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China, presented the data at the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) Congress (abstract PS049, pp S55 – S56).
NEPA is a combination of the selective NK1RA netupitant (300 mg) and the clinically and pharmacologically active 5-HT3RA, palonosetron (0.5 mg) for the prevention of CINV.
Oral palonosetron prevents nausea and vomiting during the acute phase of treatment.
Netupitant prevents nausea and vomiting during both the acute and delayed phase after cancer chemotherapy.
It is formulated into a single oral capsule.
Study design
The study was a phase 3 randomized, double-blind, double-dummy study conducted in 828 chemotherapy-naïve Asian patients receiving cisplatin-based highly emetogenic chemotherapy (HEC) agents.
Patients received a single oral dose of NEPA on day 1 or a 3-day oral APR/GRAN regimen (days 1-3).
All patients received oral dexamethasone on days 1-4.
The primary efficacy endpoint was complete response (CR), defined as no emesis or rescue medication needed during the overall (0-120 hour) phase.
The investigators defined non-inferiority to be the lower limit of the two-sided 95% confidence interval greater than the non-inferiority margin set at ̶ 10%.
Secondary endpoints included no emesis, no rescue medication, and no significant nausea (NSN), defined as <25 mm on 100 mm visual analog scale (VAS).
Results
The baseline demographics were comparable between the NEPA (n=413) and APR/GRAN (n=416) arms: 71% of the patients were male, a mean age of 55 years, and a little more than half were ECOG performance status 1.
The most common cancer types were lung and head and neck cancer.
Patients had received a median cisplatin dose of 73 and 72 mg/m2 in the NEPA and APR/GRAN arms, respectively.
Within the first 24 hours (acute phase), NEPA was non-inferior to APR/GRAN. NEPA had a CR rate of 84.5% and APR/GRAN had a CR rate of 87.0%. The risk difference between the 2 agents was -2.5% (range, -7.2%, 2.3%).
In the delayed phase (25-120 hours), NEPA had a CR rate of 77.9% and APR/GRAN, 74.3%. The risk difference was 3.7% (range, -2.1%, 9.5%).
Overall, for both phases, the CR rate was 73.8% for NEPA and 72.4% for APR/GRAN. The risk difference was 1.5% (range, -4.5%, 7.5%).
Dr Zhang pointed out that although the overall CR rates were similar, the daily rates of patients experiencing CINV remained in the range of 13% - 15% for patients in the APR/GRAN arm.
However, daily rates of CINV for patients receiving NEPA declined from 16% to 8% over the 5 days. The investigators believe this suggests a benefit for delayed CINV.
Regarding secondary endpoints, significantly more patients receiving NEPA did not require rescue medication in the delayed phase and overall than patients in the APR/GRAN arm.
Treatment-emergent adverse events (TEAEs) were comparable between the arms—58.1% in the NEPA arm and 57.5% in the APR/GRAN arm, as were serious TEAS, at 4.5% and 4.6% for NEPA and APR/GRAN, respectively. And the no emesis and no significant nausea rates favored NEPA.
The most common treatment-emergent adverse events occurring in 2% or more of the patients in both arms were constipation and hiccups.
Two serious treatment-related adverse events occurred in each arm, 1 leading to discontinuation in the NEPA arm.
The investigators concluded that NEPA, as a convenient capsule administered once per cycle, is at least as effective as the 3-day regimen of APR/GRAN in patients receiving HEC.
NEPA (Akynzeo®) is approved by the US Food and Drug Administration and marketed globally by Helsinn, Lugano, Switzerland, the sponsor of the trial.
For the full US prescribing information, see the package insert.
WASHINGTON, DC—In a head-to-head study comparing a single-dose oral antiemetic to a 3-day oral regimen, the single dose has shown itself to be non-inferior to the multi-day regimen in preventing chemotherapy-induced nausea and vomiting (CINV).
The investigators evaluated netupitant/palonosetron (NEPA) against aprepitant/granisetron (APR/GRAN) in patients on highly emetogenic chemotherapy.
They found the data suggest “that NEPA, in a single dose, had equivalent efficacy to a 3-day oral aprepitant/granisetron regimen,” according to the lead investigator and abstract presenter.
Li Zhang, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China, presented the data at the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) Congress (abstract PS049, pp S55 – S56).
NEPA is a combination of the selective NK1RA netupitant (300 mg) and the clinically and pharmacologically active 5-HT3RA, palonosetron (0.5 mg) for the prevention of CINV.
Oral palonosetron prevents nausea and vomiting during the acute phase of treatment.
Netupitant prevents nausea and vomiting during both the acute and delayed phase after cancer chemotherapy.
It is formulated into a single oral capsule.
Study design
The study was a phase 3 randomized, double-blind, double-dummy study conducted in 828 chemotherapy-naïve Asian patients receiving cisplatin-based highly emetogenic chemotherapy (HEC) agents.
Patients received a single oral dose of NEPA on day 1 or a 3-day oral APR/GRAN regimen (days 1-3).
All patients received oral dexamethasone on days 1-4.
The primary efficacy endpoint was complete response (CR), defined as no emesis or rescue medication needed during the overall (0-120 hour) phase.
The investigators defined non-inferiority to be the lower limit of the two-sided 95% confidence interval greater than the non-inferiority margin set at ̶ 10%.
Secondary endpoints included no emesis, no rescue medication, and no significant nausea (NSN), defined as <25 mm on 100 mm visual analog scale (VAS).
Results
The baseline demographics were comparable between the NEPA (n=413) and APR/GRAN (n=416) arms: 71% of the patients were male, a mean age of 55 years, and a little more than half were ECOG performance status 1.
The most common cancer types were lung and head and neck cancer.
Patients had received a median cisplatin dose of 73 and 72 mg/m2 in the NEPA and APR/GRAN arms, respectively.
Within the first 24 hours (acute phase), NEPA was non-inferior to APR/GRAN. NEPA had a CR rate of 84.5% and APR/GRAN had a CR rate of 87.0%. The risk difference between the 2 agents was -2.5% (range, -7.2%, 2.3%).
In the delayed phase (25-120 hours), NEPA had a CR rate of 77.9% and APR/GRAN, 74.3%. The risk difference was 3.7% (range, -2.1%, 9.5%).
Overall, for both phases, the CR rate was 73.8% for NEPA and 72.4% for APR/GRAN. The risk difference was 1.5% (range, -4.5%, 7.5%).
Dr Zhang pointed out that although the overall CR rates were similar, the daily rates of patients experiencing CINV remained in the range of 13% - 15% for patients in the APR/GRAN arm.
However, daily rates of CINV for patients receiving NEPA declined from 16% to 8% over the 5 days. The investigators believe this suggests a benefit for delayed CINV.
Regarding secondary endpoints, significantly more patients receiving NEPA did not require rescue medication in the delayed phase and overall than patients in the APR/GRAN arm.
Treatment-emergent adverse events (TEAEs) were comparable between the arms—58.1% in the NEPA arm and 57.5% in the APR/GRAN arm, as were serious TEAS, at 4.5% and 4.6% for NEPA and APR/GRAN, respectively. And the no emesis and no significant nausea rates favored NEPA.
The most common treatment-emergent adverse events occurring in 2% or more of the patients in both arms were constipation and hiccups.
Two serious treatment-related adverse events occurred in each arm, 1 leading to discontinuation in the NEPA arm.
The investigators concluded that NEPA, as a convenient capsule administered once per cycle, is at least as effective as the 3-day regimen of APR/GRAN in patients receiving HEC.
NEPA (Akynzeo®) is approved by the US Food and Drug Administration and marketed globally by Helsinn, Lugano, Switzerland, the sponsor of the trial.
For the full US prescribing information, see the package insert.
WASHINGTON, DC—In a head-to-head study comparing a single-dose oral antiemetic to a 3-day oral regimen, the single dose has shown itself to be non-inferior to the multi-day regimen in preventing chemotherapy-induced nausea and vomiting (CINV).
The investigators evaluated netupitant/palonosetron (NEPA) against aprepitant/granisetron (APR/GRAN) in patients on highly emetogenic chemotherapy.
They found the data suggest “that NEPA, in a single dose, had equivalent efficacy to a 3-day oral aprepitant/granisetron regimen,” according to the lead investigator and abstract presenter.
Li Zhang, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China, presented the data at the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) Congress (abstract PS049, pp S55 – S56).
NEPA is a combination of the selective NK1RA netupitant (300 mg) and the clinically and pharmacologically active 5-HT3RA, palonosetron (0.5 mg) for the prevention of CINV.
Oral palonosetron prevents nausea and vomiting during the acute phase of treatment.
Netupitant prevents nausea and vomiting during both the acute and delayed phase after cancer chemotherapy.
It is formulated into a single oral capsule.
Study design
The study was a phase 3 randomized, double-blind, double-dummy study conducted in 828 chemotherapy-naïve Asian patients receiving cisplatin-based highly emetogenic chemotherapy (HEC) agents.
Patients received a single oral dose of NEPA on day 1 or a 3-day oral APR/GRAN regimen (days 1-3).
All patients received oral dexamethasone on days 1-4.
The primary efficacy endpoint was complete response (CR), defined as no emesis or rescue medication needed during the overall (0-120 hour) phase.
The investigators defined non-inferiority to be the lower limit of the two-sided 95% confidence interval greater than the non-inferiority margin set at ̶ 10%.
Secondary endpoints included no emesis, no rescue medication, and no significant nausea (NSN), defined as <25 mm on 100 mm visual analog scale (VAS).
Results
The baseline demographics were comparable between the NEPA (n=413) and APR/GRAN (n=416) arms: 71% of the patients were male, a mean age of 55 years, and a little more than half were ECOG performance status 1.
The most common cancer types were lung and head and neck cancer.
Patients had received a median cisplatin dose of 73 and 72 mg/m2 in the NEPA and APR/GRAN arms, respectively.
Within the first 24 hours (acute phase), NEPA was non-inferior to APR/GRAN. NEPA had a CR rate of 84.5% and APR/GRAN had a CR rate of 87.0%. The risk difference between the 2 agents was -2.5% (range, -7.2%, 2.3%).
In the delayed phase (25-120 hours), NEPA had a CR rate of 77.9% and APR/GRAN, 74.3%. The risk difference was 3.7% (range, -2.1%, 9.5%).
Overall, for both phases, the CR rate was 73.8% for NEPA and 72.4% for APR/GRAN. The risk difference was 1.5% (range, -4.5%, 7.5%).
Dr Zhang pointed out that although the overall CR rates were similar, the daily rates of patients experiencing CINV remained in the range of 13% - 15% for patients in the APR/GRAN arm.
However, daily rates of CINV for patients receiving NEPA declined from 16% to 8% over the 5 days. The investigators believe this suggests a benefit for delayed CINV.
Regarding secondary endpoints, significantly more patients receiving NEPA did not require rescue medication in the delayed phase and overall than patients in the APR/GRAN arm.
Treatment-emergent adverse events (TEAEs) were comparable between the arms—58.1% in the NEPA arm and 57.5% in the APR/GRAN arm, as were serious TEAS, at 4.5% and 4.6% for NEPA and APR/GRAN, respectively. And the no emesis and no significant nausea rates favored NEPA.
The most common treatment-emergent adverse events occurring in 2% or more of the patients in both arms were constipation and hiccups.
Two serious treatment-related adverse events occurred in each arm, 1 leading to discontinuation in the NEPA arm.
The investigators concluded that NEPA, as a convenient capsule administered once per cycle, is at least as effective as the 3-day regimen of APR/GRAN in patients receiving HEC.
NEPA (Akynzeo®) is approved by the US Food and Drug Administration and marketed globally by Helsinn, Lugano, Switzerland, the sponsor of the trial.
For the full US prescribing information, see the package insert.
Less is more in PET-negative, advanced HL
MADRID—Patients with advanced Hodgkin lymphoma (HL) who are PET-negative after initial treatment with 2 cycles of eBEACOPP* should only receive 2 additional cycles of the therapy, new research suggests.
In the HD18 trial, PET-2-negative patients who received 4 cycles of eBEACOPP had non-inferior progression-free survival (PFS) and significantly better overall survival (OS) than PET-2-negative patients who received 6 or 8 cycles of the treatment.
Patients who received 4 cycles also had less severe toxicity and fewer second neoplasms than patients who received more cycles of eBEACOPP.
“When balancing efficacy and safety, results compare favorably with any other published treatment strategy so far,” said Peter Borchmann, MD, of University Hospital of Cologne in Germany.
“That’s why we recommend treatment with PET-2-guided eBEACOPP for patients with newly diagnosed, advanced-stage Hodgkin lymphoma.”
Dr Borchmann presented results from HD18 at the 22nd Congress of the European Hematology Association (EHA) as abstract S150.
Patients and treatment
Dr Borchmann and his colleagues set out to determine if they could decrease the number of eBEACOPP cycles in patients with negative PET-2 without compromising treatment efficacy.
From May 2008 to July 2014, the researchers recruited 2101 patients with newly diagnosed, advanced-stage HL.
Patients who were PET-negative after 2 cycles of eBEACOPP (n=1005) were initially randomized to receive 6 additional cycles of eBEACOPP or 2 additional cycles—a total of 8 cycles or 4 cycles, respectively. The protocol was later amended (in June 2011), and the total number of cycles in the standard therapy arm was reduced to 6.
There were 504 patients in the standard therapy arm—288 who received 8 cycles of eBEACOPP and 216 who received 6 cycles. There were 501 patients who received 4 cycles of eBEACOPP.
Sixteen patients in the standard therapy arm and 17 in the 4-cycle arm also received radiotherapy.
The median age was 32 (range, 18-60) in the standard therapy arm and 33 (range, 18-60) in the 4-cycle arm. Sixty-three percent and 62% of patients, respectively, were male.
Eight percent of patients in both arms had Ann Arbor stage IIB disease. Fifty-seven percent in the standard therapy arm and 55% in the 4-cycle arm had stage IIIA/B. And 35% and 36%, respectively, had stage IVA/B disease.
Thirty-five percent of patients in both arms had an IPS stage of 0-1. Fifty-one percent in the standard therapy arm and 52% in the 4-cycle arm had an IPS stage of 2-3. And 14% in both arms had an IPS stage of 4-7.
The median duration of therapy was 173 days (range, 41-266) for patients randomized to receive 8 cycles of eBEACOPP, 129 days (range, 35-178) in patients randomized to receive 6 cycles, and 85 days (range, 42-133) in patients randomized to receive 4 cycles.
One patient in the 6-cycle group received more than 6 cycles, and 6 patients in the 4-cycle arm received more than 4 cycles.
PFS and OS
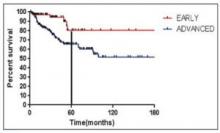
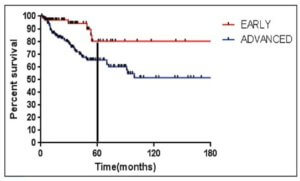
The median follow-up was 55 months. The estimated 3-year PFS was 92.3% in the standard therapy arm and 94.8% in the 4-cycle arm. The estimated 5-year PFS was 91.2% and 91.8%, respectively. The hazard ratio was 0.88.
“[Based on these data,] we can conclude that 4 cycles are as effective as 6 or 8 cycles,” Dr Borchmann said.
The estimated 3-year OS was 95.9% in the standard therapy arm and 98.7% in the 4-cycle arm. The estimated 5-year OS was 95.4% and 97.6%, respectively. The hazard ratio was 0.36 (P=0.006).
Toxicity and second neoplasms
Grade 3/4 organ toxicity occurred in 22% of patients in the 8-cycle group, 13% in the 6-cycle group, and 8% in the 4-cycle group. Grade 4 anemia, thrombocytopenia, or infection occurred in 59%, 53%, and 38%, respectively.
Treatment-related morbidity occurred in 66% of patients in the 8-cycle group, 61% in the 6-cycle group, and 41% in the 4-cycle group.
Eighteen patients in the standard therapy arm had second neoplasms—8 with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), 5 with non-Hodgkin lymphoma (NHL), and 5 with solid tumors.
Thirteen patients in the 4-cycle arm had second neoplasms—2 with AML/MDS, 8 with NHL, and 3 with solid tumors.
Causes of death
The cause of death was HL for 0.6% of patients (n=3) in the standard therapy arm and for 0.8% of patients (n=4) in the 4-cycle arm.
The cause of death was second malignancy for 2.2% of patients (n=11) in the standard therapy arm. Five patients died of AML, 3 of NHL, and 3 of solid tumor malignancies.
One patient (0.2%) in the 4-cycle arm died as a result of a second malignancy, which was AML.
Toxicity related to study treatment was the cause of death for 1.2% (n=6) of patients in the standard therapy arm. Five of the patients died of infection, and the sixth died of ischemic stroke.
None of the patients in the 4-cycle arm died of toxicity related to study treatment.
Other causes of death included toxicity of salvage treatment (0.4%, n=2 in both arms), other disease (0.2%, n=1 in both arms), accident (1 patient [0.2%] in the 4-cycle arm), and unknown cause (2 patients [0.4%] in the standard arm).
“[With 4 cycles of therapy,] we had a significant and very relevant reduction of severe, acute hematological and non-hematological toxicities, and this was accompanied by a relevant reduction of mortality for other reasons than HL,” Dr Borchmann said. “And we’ve almost eliminated HL as a cause of death in this trial.” ![]()
*dose-escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone
MADRID—Patients with advanced Hodgkin lymphoma (HL) who are PET-negative after initial treatment with 2 cycles of eBEACOPP* should only receive 2 additional cycles of the therapy, new research suggests.
In the HD18 trial, PET-2-negative patients who received 4 cycles of eBEACOPP had non-inferior progression-free survival (PFS) and significantly better overall survival (OS) than PET-2-negative patients who received 6 or 8 cycles of the treatment.
Patients who received 4 cycles also had less severe toxicity and fewer second neoplasms than patients who received more cycles of eBEACOPP.
“When balancing efficacy and safety, results compare favorably with any other published treatment strategy so far,” said Peter Borchmann, MD, of University Hospital of Cologne in Germany.
“That’s why we recommend treatment with PET-2-guided eBEACOPP for patients with newly diagnosed, advanced-stage Hodgkin lymphoma.”
Dr Borchmann presented results from HD18 at the 22nd Congress of the European Hematology Association (EHA) as abstract S150.
Patients and treatment
Dr Borchmann and his colleagues set out to determine if they could decrease the number of eBEACOPP cycles in patients with negative PET-2 without compromising treatment efficacy.
From May 2008 to July 2014, the researchers recruited 2101 patients with newly diagnosed, advanced-stage HL.
Patients who were PET-negative after 2 cycles of eBEACOPP (n=1005) were initially randomized to receive 6 additional cycles of eBEACOPP or 2 additional cycles—a total of 8 cycles or 4 cycles, respectively. The protocol was later amended (in June 2011), and the total number of cycles in the standard therapy arm was reduced to 6.
There were 504 patients in the standard therapy arm—288 who received 8 cycles of eBEACOPP and 216 who received 6 cycles. There were 501 patients who received 4 cycles of eBEACOPP.
Sixteen patients in the standard therapy arm and 17 in the 4-cycle arm also received radiotherapy.
The median age was 32 (range, 18-60) in the standard therapy arm and 33 (range, 18-60) in the 4-cycle arm. Sixty-three percent and 62% of patients, respectively, were male.
Eight percent of patients in both arms had Ann Arbor stage IIB disease. Fifty-seven percent in the standard therapy arm and 55% in the 4-cycle arm had stage IIIA/B. And 35% and 36%, respectively, had stage IVA/B disease.
Thirty-five percent of patients in both arms had an IPS stage of 0-1. Fifty-one percent in the standard therapy arm and 52% in the 4-cycle arm had an IPS stage of 2-3. And 14% in both arms had an IPS stage of 4-7.
The median duration of therapy was 173 days (range, 41-266) for patients randomized to receive 8 cycles of eBEACOPP, 129 days (range, 35-178) in patients randomized to receive 6 cycles, and 85 days (range, 42-133) in patients randomized to receive 4 cycles.
One patient in the 6-cycle group received more than 6 cycles, and 6 patients in the 4-cycle arm received more than 4 cycles.
PFS and OS
The median follow-up was 55 months. The estimated 3-year PFS was 92.3% in the standard therapy arm and 94.8% in the 4-cycle arm. The estimated 5-year PFS was 91.2% and 91.8%, respectively. The hazard ratio was 0.88.
“[Based on these data,] we can conclude that 4 cycles are as effective as 6 or 8 cycles,” Dr Borchmann said.
The estimated 3-year OS was 95.9% in the standard therapy arm and 98.7% in the 4-cycle arm. The estimated 5-year OS was 95.4% and 97.6%, respectively. The hazard ratio was 0.36 (P=0.006).
Toxicity and second neoplasms
Grade 3/4 organ toxicity occurred in 22% of patients in the 8-cycle group, 13% in the 6-cycle group, and 8% in the 4-cycle group. Grade 4 anemia, thrombocytopenia, or infection occurred in 59%, 53%, and 38%, respectively.
Treatment-related morbidity occurred in 66% of patients in the 8-cycle group, 61% in the 6-cycle group, and 41% in the 4-cycle group.
Eighteen patients in the standard therapy arm had second neoplasms—8 with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), 5 with non-Hodgkin lymphoma (NHL), and 5 with solid tumors.
Thirteen patients in the 4-cycle arm had second neoplasms—2 with AML/MDS, 8 with NHL, and 3 with solid tumors.
Causes of death
The cause of death was HL for 0.6% of patients (n=3) in the standard therapy arm and for 0.8% of patients (n=4) in the 4-cycle arm.
The cause of death was second malignancy for 2.2% of patients (n=11) in the standard therapy arm. Five patients died of AML, 3 of NHL, and 3 of solid tumor malignancies.
One patient (0.2%) in the 4-cycle arm died as a result of a second malignancy, which was AML.
Toxicity related to study treatment was the cause of death for 1.2% (n=6) of patients in the standard therapy arm. Five of the patients died of infection, and the sixth died of ischemic stroke.
None of the patients in the 4-cycle arm died of toxicity related to study treatment.
Other causes of death included toxicity of salvage treatment (0.4%, n=2 in both arms), other disease (0.2%, n=1 in both arms), accident (1 patient [0.2%] in the 4-cycle arm), and unknown cause (2 patients [0.4%] in the standard arm).
“[With 4 cycles of therapy,] we had a significant and very relevant reduction of severe, acute hematological and non-hematological toxicities, and this was accompanied by a relevant reduction of mortality for other reasons than HL,” Dr Borchmann said. “And we’ve almost eliminated HL as a cause of death in this trial.” ![]()
*dose-escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone
MADRID—Patients with advanced Hodgkin lymphoma (HL) who are PET-negative after initial treatment with 2 cycles of eBEACOPP* should only receive 2 additional cycles of the therapy, new research suggests.
In the HD18 trial, PET-2-negative patients who received 4 cycles of eBEACOPP had non-inferior progression-free survival (PFS) and significantly better overall survival (OS) than PET-2-negative patients who received 6 or 8 cycles of the treatment.
Patients who received 4 cycles also had less severe toxicity and fewer second neoplasms than patients who received more cycles of eBEACOPP.
“When balancing efficacy and safety, results compare favorably with any other published treatment strategy so far,” said Peter Borchmann, MD, of University Hospital of Cologne in Germany.
“That’s why we recommend treatment with PET-2-guided eBEACOPP for patients with newly diagnosed, advanced-stage Hodgkin lymphoma.”
Dr Borchmann presented results from HD18 at the 22nd Congress of the European Hematology Association (EHA) as abstract S150.
Patients and treatment
Dr Borchmann and his colleagues set out to determine if they could decrease the number of eBEACOPP cycles in patients with negative PET-2 without compromising treatment efficacy.
From May 2008 to July 2014, the researchers recruited 2101 patients with newly diagnosed, advanced-stage HL.
Patients who were PET-negative after 2 cycles of eBEACOPP (n=1005) were initially randomized to receive 6 additional cycles of eBEACOPP or 2 additional cycles—a total of 8 cycles or 4 cycles, respectively. The protocol was later amended (in June 2011), and the total number of cycles in the standard therapy arm was reduced to 6.
There were 504 patients in the standard therapy arm—288 who received 8 cycles of eBEACOPP and 216 who received 6 cycles. There were 501 patients who received 4 cycles of eBEACOPP.
Sixteen patients in the standard therapy arm and 17 in the 4-cycle arm also received radiotherapy.
The median age was 32 (range, 18-60) in the standard therapy arm and 33 (range, 18-60) in the 4-cycle arm. Sixty-three percent and 62% of patients, respectively, were male.
Eight percent of patients in both arms had Ann Arbor stage IIB disease. Fifty-seven percent in the standard therapy arm and 55% in the 4-cycle arm had stage IIIA/B. And 35% and 36%, respectively, had stage IVA/B disease.
Thirty-five percent of patients in both arms had an IPS stage of 0-1. Fifty-one percent in the standard therapy arm and 52% in the 4-cycle arm had an IPS stage of 2-3. And 14% in both arms had an IPS stage of 4-7.
The median duration of therapy was 173 days (range, 41-266) for patients randomized to receive 8 cycles of eBEACOPP, 129 days (range, 35-178) in patients randomized to receive 6 cycles, and 85 days (range, 42-133) in patients randomized to receive 4 cycles.
One patient in the 6-cycle group received more than 6 cycles, and 6 patients in the 4-cycle arm received more than 4 cycles.
PFS and OS
The median follow-up was 55 months. The estimated 3-year PFS was 92.3% in the standard therapy arm and 94.8% in the 4-cycle arm. The estimated 5-year PFS was 91.2% and 91.8%, respectively. The hazard ratio was 0.88.
“[Based on these data,] we can conclude that 4 cycles are as effective as 6 or 8 cycles,” Dr Borchmann said.
The estimated 3-year OS was 95.9% in the standard therapy arm and 98.7% in the 4-cycle arm. The estimated 5-year OS was 95.4% and 97.6%, respectively. The hazard ratio was 0.36 (P=0.006).
Toxicity and second neoplasms
Grade 3/4 organ toxicity occurred in 22% of patients in the 8-cycle group, 13% in the 6-cycle group, and 8% in the 4-cycle group. Grade 4 anemia, thrombocytopenia, or infection occurred in 59%, 53%, and 38%, respectively.
Treatment-related morbidity occurred in 66% of patients in the 8-cycle group, 61% in the 6-cycle group, and 41% in the 4-cycle group.
Eighteen patients in the standard therapy arm had second neoplasms—8 with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), 5 with non-Hodgkin lymphoma (NHL), and 5 with solid tumors.
Thirteen patients in the 4-cycle arm had second neoplasms—2 with AML/MDS, 8 with NHL, and 3 with solid tumors.
Causes of death
The cause of death was HL for 0.6% of patients (n=3) in the standard therapy arm and for 0.8% of patients (n=4) in the 4-cycle arm.
The cause of death was second malignancy for 2.2% of patients (n=11) in the standard therapy arm. Five patients died of AML, 3 of NHL, and 3 of solid tumor malignancies.
One patient (0.2%) in the 4-cycle arm died as a result of a second malignancy, which was AML.
Toxicity related to study treatment was the cause of death for 1.2% (n=6) of patients in the standard therapy arm. Five of the patients died of infection, and the sixth died of ischemic stroke.
None of the patients in the 4-cycle arm died of toxicity related to study treatment.
Other causes of death included toxicity of salvage treatment (0.4%, n=2 in both arms), other disease (0.2%, n=1 in both arms), accident (1 patient [0.2%] in the 4-cycle arm), and unknown cause (2 patients [0.4%] in the standard arm).
“[With 4 cycles of therapy,] we had a significant and very relevant reduction of severe, acute hematological and non-hematological toxicities, and this was accompanied by a relevant reduction of mortality for other reasons than HL,” Dr Borchmann said. “And we’ve almost eliminated HL as a cause of death in this trial.” ![]()
*dose-escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone
Brentuximab meets phase 3 primary endpoint in frontline advanced HL
Brentuximab vedotin (Adcetris®) in combination with a 3-drug chemotherapy regimen has met its primary endpoint of statistically significant improvement in modified progression-free survival (mPFS) compared with standard therapy in frontline treatment of advanced stage Hodgkin lymphoma (HL).
The ECHELON-1 trial tested brentuximab vedotin plus Adriamycin, vinblastine, and dacarbazine (AVD) against Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) in 1334 patients with previously untreated advanced HL.
Patients treated with brentuximab showed an 82% lower risk of disease progression compared with 77% in the ABVD arm.
Brentuximab vedotin is currently not approved as a frontline therapy for HL.
“Notably, this is the first clinical trial in frontline advanced Hodgkin lymphoma to show superior efficacy of a regimen that eliminates bleomycin,” said Clay Siegall, PhD, president and CEO of Seattle Genetics.
Dirk Huebner, MD, executive medical director of oncology at Takeda Pharmaceutical Company, said the results of the trial “have the potential to change the treatment approach of frontline advanced Hodgkin lymphoma.”
Seattle Genetics and Takeda are jointly developing brentuximab vedotin. Seattle Genetics has US and Canadian commercialization rights and Takeda has rights to commercialize it in the rest of the world.
Brentuximab vedotin is an antibody-drug conjugate (ADC) made up of an anti-CD30 monoclonal antibody attached by a linker to monomethyl auristatin E (MMAE). The linker system is stable in the bloodstream but releases MMAE when internalized into CD30-expressing tumor cells.
ECHELON-1
ECHELON-1 (NCT01712490) is a randomized, 2-arm, multicenter phase 3 trial comparing brentuximab vedotin plus AVD to ABVD as frontline therapy in treatment-naïve advanced HL.
The trial enrolled 1334 patients with histologically confirmed advanced HL.
The primary endpoint is mPFS by independent review facility.
The investigators, regulatory bodies, and trial sponsors defined mPFS as the time to progression, death, or receipt of additional anticancer therapy for patients who were not in complete response (CR) after completion of frontline therapy.
They chose mPFS instead of PFS because they say it provides a clearer picture of the efficacy of primary anticancer therapy by eliminating the confounding effects of additional anticancer therapy.
Secondary endpoints include overall survival (OS), CR, and safety.
The results demonstrated that combination treatment with brentuximab resulted in a statistically significant improvement in mPFS versus the control arm (hazard ratio=0.770; P=0.035).
Interim analysis of OS, the key secondary endpoint, also trended in favor of the brentuximab plus AVD arm.
The safety profile of the brentuximab combination was consistent with that of the single-agent components of the regimen.
Patients in the brentuximab arm experienced an increased incidence of febrile neutropenia and peripheral neuropathy compared to the ABVD arm.
Febrile neutropenia was reduced with the use of prophylactic growth factors.
Peripheral neuropathy was managed through dose modifications.
Patients treated with ABVD had an increased rate and severity of pulmonary toxicity.
The companies plan to submit an abstract for presentation at the American Society of Hematology annual meeting in December.
Brentuximab is currently approved by the US Food and Drug Administration (FDA) for the treatment of patients with classical HL who have received a prior stem cell transplant or 2 prior chemotherapy treatments.
Brentuximab is also approved to treat patient with anaplastic large cell lymphoma who have failed one prior treatment.
For more on brentuximab vedotin, see the full prescribing informtion. ![]()
Brentuximab vedotin (Adcetris®) in combination with a 3-drug chemotherapy regimen has met its primary endpoint of statistically significant improvement in modified progression-free survival (mPFS) compared with standard therapy in frontline treatment of advanced stage Hodgkin lymphoma (HL).
The ECHELON-1 trial tested brentuximab vedotin plus Adriamycin, vinblastine, and dacarbazine (AVD) against Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) in 1334 patients with previously untreated advanced HL.
Patients treated with brentuximab showed an 82% lower risk of disease progression compared with 77% in the ABVD arm.
Brentuximab vedotin is currently not approved as a frontline therapy for HL.
“Notably, this is the first clinical trial in frontline advanced Hodgkin lymphoma to show superior efficacy of a regimen that eliminates bleomycin,” said Clay Siegall, PhD, president and CEO of Seattle Genetics.
Dirk Huebner, MD, executive medical director of oncology at Takeda Pharmaceutical Company, said the results of the trial “have the potential to change the treatment approach of frontline advanced Hodgkin lymphoma.”
Seattle Genetics and Takeda are jointly developing brentuximab vedotin. Seattle Genetics has US and Canadian commercialization rights and Takeda has rights to commercialize it in the rest of the world.
Brentuximab vedotin is an antibody-drug conjugate (ADC) made up of an anti-CD30 monoclonal antibody attached by a linker to monomethyl auristatin E (MMAE). The linker system is stable in the bloodstream but releases MMAE when internalized into CD30-expressing tumor cells.
ECHELON-1
ECHELON-1 (NCT01712490) is a randomized, 2-arm, multicenter phase 3 trial comparing brentuximab vedotin plus AVD to ABVD as frontline therapy in treatment-naïve advanced HL.
The trial enrolled 1334 patients with histologically confirmed advanced HL.
The primary endpoint is mPFS by independent review facility.
The investigators, regulatory bodies, and trial sponsors defined mPFS as the time to progression, death, or receipt of additional anticancer therapy for patients who were not in complete response (CR) after completion of frontline therapy.
They chose mPFS instead of PFS because they say it provides a clearer picture of the efficacy of primary anticancer therapy by eliminating the confounding effects of additional anticancer therapy.
Secondary endpoints include overall survival (OS), CR, and safety.
The results demonstrated that combination treatment with brentuximab resulted in a statistically significant improvement in mPFS versus the control arm (hazard ratio=0.770; P=0.035).
Interim analysis of OS, the key secondary endpoint, also trended in favor of the brentuximab plus AVD arm.
The safety profile of the brentuximab combination was consistent with that of the single-agent components of the regimen.
Patients in the brentuximab arm experienced an increased incidence of febrile neutropenia and peripheral neuropathy compared to the ABVD arm.
Febrile neutropenia was reduced with the use of prophylactic growth factors.
Peripheral neuropathy was managed through dose modifications.
Patients treated with ABVD had an increased rate and severity of pulmonary toxicity.
The companies plan to submit an abstract for presentation at the American Society of Hematology annual meeting in December.
Brentuximab is currently approved by the US Food and Drug Administration (FDA) for the treatment of patients with classical HL who have received a prior stem cell transplant or 2 prior chemotherapy treatments.
Brentuximab is also approved to treat patient with anaplastic large cell lymphoma who have failed one prior treatment.
For more on brentuximab vedotin, see the full prescribing informtion. ![]()
Brentuximab vedotin (Adcetris®) in combination with a 3-drug chemotherapy regimen has met its primary endpoint of statistically significant improvement in modified progression-free survival (mPFS) compared with standard therapy in frontline treatment of advanced stage Hodgkin lymphoma (HL).
The ECHELON-1 trial tested brentuximab vedotin plus Adriamycin, vinblastine, and dacarbazine (AVD) against Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) in 1334 patients with previously untreated advanced HL.
Patients treated with brentuximab showed an 82% lower risk of disease progression compared with 77% in the ABVD arm.
Brentuximab vedotin is currently not approved as a frontline therapy for HL.
“Notably, this is the first clinical trial in frontline advanced Hodgkin lymphoma to show superior efficacy of a regimen that eliminates bleomycin,” said Clay Siegall, PhD, president and CEO of Seattle Genetics.
Dirk Huebner, MD, executive medical director of oncology at Takeda Pharmaceutical Company, said the results of the trial “have the potential to change the treatment approach of frontline advanced Hodgkin lymphoma.”
Seattle Genetics and Takeda are jointly developing brentuximab vedotin. Seattle Genetics has US and Canadian commercialization rights and Takeda has rights to commercialize it in the rest of the world.
Brentuximab vedotin is an antibody-drug conjugate (ADC) made up of an anti-CD30 monoclonal antibody attached by a linker to monomethyl auristatin E (MMAE). The linker system is stable in the bloodstream but releases MMAE when internalized into CD30-expressing tumor cells.
ECHELON-1
ECHELON-1 (NCT01712490) is a randomized, 2-arm, multicenter phase 3 trial comparing brentuximab vedotin plus AVD to ABVD as frontline therapy in treatment-naïve advanced HL.
The trial enrolled 1334 patients with histologically confirmed advanced HL.
The primary endpoint is mPFS by independent review facility.
The investigators, regulatory bodies, and trial sponsors defined mPFS as the time to progression, death, or receipt of additional anticancer therapy for patients who were not in complete response (CR) after completion of frontline therapy.
They chose mPFS instead of PFS because they say it provides a clearer picture of the efficacy of primary anticancer therapy by eliminating the confounding effects of additional anticancer therapy.
Secondary endpoints include overall survival (OS), CR, and safety.
The results demonstrated that combination treatment with brentuximab resulted in a statistically significant improvement in mPFS versus the control arm (hazard ratio=0.770; P=0.035).
Interim analysis of OS, the key secondary endpoint, also trended in favor of the brentuximab plus AVD arm.
The safety profile of the brentuximab combination was consistent with that of the single-agent components of the regimen.
Patients in the brentuximab arm experienced an increased incidence of febrile neutropenia and peripheral neuropathy compared to the ABVD arm.
Febrile neutropenia was reduced with the use of prophylactic growth factors.
Peripheral neuropathy was managed through dose modifications.
Patients treated with ABVD had an increased rate and severity of pulmonary toxicity.
The companies plan to submit an abstract for presentation at the American Society of Hematology annual meeting in December.
Brentuximab is currently approved by the US Food and Drug Administration (FDA) for the treatment of patients with classical HL who have received a prior stem cell transplant or 2 prior chemotherapy treatments.
Brentuximab is also approved to treat patient with anaplastic large cell lymphoma who have failed one prior treatment.
For more on brentuximab vedotin, see the full prescribing informtion. ![]()
CARs race for supremacy against aggressive non-Hodgkin lymphoma
MADRID – Two chimeric antigen receptor T cell (CAR-T) constructs are showing promising activity against treatment-refractory, aggressive forms of non-Hodgkin lymphoma in multicenter clinical trials.
In the ZUMA-1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR-T product, was associated with an 82% objective response rate (ORR), including 54% complete responses, in patients with refractory diffuse large B cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL), reported Yi Lin, MD, PhD, from the Mayo Clinic in Rochester Minnesota.
In an interim analysis from the JULIET study, a different anti-CD19 CAR-T construct labeled CTL019 was associated with a 59% ORR, consisting of 43% complete responses and 16% partial responses (PR) in patients with relapsed or refractory DLBCL, reported Gilles Salles, MD, PhD, from the University of Lyon, France.
The analysis “confirms the high response rates and durable responses observed in the previous single-center trial,” Dr. Salles said.
Although the CAR-T cell constructs in the study have different costimulatory molecules, each is created in a centralized facility, which allows for consistent manufacturing of cells sufficient for harvesting, transfecting, expanding, and reinfusing into heavily pretreated patients.
The construct used in ZUMA-1, also called KTE-C19 (Kite Pharma), has CD28 and CD3-zeta signaling domains. CTL019 (Novarits, U. Pennsylvania, and Oxford Biomedica) has CD3-zeta and 4-1BB costimulatory domains.
ZUMA-1
Dr. Lin reported phase II results from ZUMA-1, investigating axi-cel at a target dose of 2 x 106 cells per kilogram in 72 patients with refractory DLBCL (cohort 1), and 20 patients with refractory PMBCL or TFL (cohort 2).
The median patient age was 58 years. Patients had stage III or IV disease, 47% had International Prognostic Index (IPI) scores of 3-4, 77% had disease that was refractory to second-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
The axi-cel construct was successfully manufactured in 99% of patients, with an average turnaround time from apheresis to the clinical site of 17 days.
As noted before, the trial met its primary endpoint with an 82% ORR, consisting of 54% complete responses and 28% partial responses.
The median duration of response was 8.2 months, and for patients with complete responses the median duration has not been reached.
Median overall survival has also not been reached.
The treatment was generally safe, with only 13% of patients experiencing grade 3 or greater cytokine release syndrome (CRS), and 28% having grade 3 or greater neurologic events. The events were generally reversible, and the rates of each declined over time. The use of tociluzumab or steroids to control adverse events did not have a negative effect on responses, Dr. Lin said.
JULIET
In the ongoing JULIET study, patients with relapsed/refractory DLBCL after at least two prior lines of therapy and who are not candidates for stem cell transplants are enrolled.
In a safety analysis including 85 patients, the CRS was seen in 57% of all patients, including grade 3 in 17% and grade 4 in 9%.
Other common adverse events occurring within 8 weeks of CTL019 infusion were infections in 26% of patients, cytopenias lasting longer than 28 days in 26%, neurologic events in 21%, febrile neutropenia in 14%, and tumor lysis syndrome in 1%.
There were no cases of cerebral edema, and no deaths attributable to the CAR-T cell construct, Dr. Salles said.
Peter Borchmann, MD, from the University of Cologne, Germany, who attended the briefing but was not involved with either study, commented that investigators in ZUMA-1 need to monitor patients carefully, because previous clinical trials using other CAR-T cells with CD28 costimuatory domains have been associated with several cases of fatal cerebral edema.
“I think you can use CD28 in lymphoma, and it’s highly active as we have seen, but my personal impression is that you have to be aware that this might happen,” he said in an interview.
The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
MADRID – Two chimeric antigen receptor T cell (CAR-T) constructs are showing promising activity against treatment-refractory, aggressive forms of non-Hodgkin lymphoma in multicenter clinical trials.
In the ZUMA-1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR-T product, was associated with an 82% objective response rate (ORR), including 54% complete responses, in patients with refractory diffuse large B cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL), reported Yi Lin, MD, PhD, from the Mayo Clinic in Rochester Minnesota.
In an interim analysis from the JULIET study, a different anti-CD19 CAR-T construct labeled CTL019 was associated with a 59% ORR, consisting of 43% complete responses and 16% partial responses (PR) in patients with relapsed or refractory DLBCL, reported Gilles Salles, MD, PhD, from the University of Lyon, France.
The analysis “confirms the high response rates and durable responses observed in the previous single-center trial,” Dr. Salles said.
Although the CAR-T cell constructs in the study have different costimulatory molecules, each is created in a centralized facility, which allows for consistent manufacturing of cells sufficient for harvesting, transfecting, expanding, and reinfusing into heavily pretreated patients.
The construct used in ZUMA-1, also called KTE-C19 (Kite Pharma), has CD28 and CD3-zeta signaling domains. CTL019 (Novarits, U. Pennsylvania, and Oxford Biomedica) has CD3-zeta and 4-1BB costimulatory domains.
ZUMA-1
Dr. Lin reported phase II results from ZUMA-1, investigating axi-cel at a target dose of 2 x 106 cells per kilogram in 72 patients with refractory DLBCL (cohort 1), and 20 patients with refractory PMBCL or TFL (cohort 2).
The median patient age was 58 years. Patients had stage III or IV disease, 47% had International Prognostic Index (IPI) scores of 3-4, 77% had disease that was refractory to second-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
The axi-cel construct was successfully manufactured in 99% of patients, with an average turnaround time from apheresis to the clinical site of 17 days.
As noted before, the trial met its primary endpoint with an 82% ORR, consisting of 54% complete responses and 28% partial responses.
The median duration of response was 8.2 months, and for patients with complete responses the median duration has not been reached.
Median overall survival has also not been reached.
The treatment was generally safe, with only 13% of patients experiencing grade 3 or greater cytokine release syndrome (CRS), and 28% having grade 3 or greater neurologic events. The events were generally reversible, and the rates of each declined over time. The use of tociluzumab or steroids to control adverse events did not have a negative effect on responses, Dr. Lin said.
JULIET
In the ongoing JULIET study, patients with relapsed/refractory DLBCL after at least two prior lines of therapy and who are not candidates for stem cell transplants are enrolled.
In a safety analysis including 85 patients, the CRS was seen in 57% of all patients, including grade 3 in 17% and grade 4 in 9%.
Other common adverse events occurring within 8 weeks of CTL019 infusion were infections in 26% of patients, cytopenias lasting longer than 28 days in 26%, neurologic events in 21%, febrile neutropenia in 14%, and tumor lysis syndrome in 1%.
There were no cases of cerebral edema, and no deaths attributable to the CAR-T cell construct, Dr. Salles said.
Peter Borchmann, MD, from the University of Cologne, Germany, who attended the briefing but was not involved with either study, commented that investigators in ZUMA-1 need to monitor patients carefully, because previous clinical trials using other CAR-T cells with CD28 costimuatory domains have been associated with several cases of fatal cerebral edema.
“I think you can use CD28 in lymphoma, and it’s highly active as we have seen, but my personal impression is that you have to be aware that this might happen,” he said in an interview.
The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
MADRID – Two chimeric antigen receptor T cell (CAR-T) constructs are showing promising activity against treatment-refractory, aggressive forms of non-Hodgkin lymphoma in multicenter clinical trials.
In the ZUMA-1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR-T product, was associated with an 82% objective response rate (ORR), including 54% complete responses, in patients with refractory diffuse large B cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL), reported Yi Lin, MD, PhD, from the Mayo Clinic in Rochester Minnesota.
In an interim analysis from the JULIET study, a different anti-CD19 CAR-T construct labeled CTL019 was associated with a 59% ORR, consisting of 43% complete responses and 16% partial responses (PR) in patients with relapsed or refractory DLBCL, reported Gilles Salles, MD, PhD, from the University of Lyon, France.
The analysis “confirms the high response rates and durable responses observed in the previous single-center trial,” Dr. Salles said.
Although the CAR-T cell constructs in the study have different costimulatory molecules, each is created in a centralized facility, which allows for consistent manufacturing of cells sufficient for harvesting, transfecting, expanding, and reinfusing into heavily pretreated patients.
The construct used in ZUMA-1, also called KTE-C19 (Kite Pharma), has CD28 and CD3-zeta signaling domains. CTL019 (Novarits, U. Pennsylvania, and Oxford Biomedica) has CD3-zeta and 4-1BB costimulatory domains.
ZUMA-1
Dr. Lin reported phase II results from ZUMA-1, investigating axi-cel at a target dose of 2 x 106 cells per kilogram in 72 patients with refractory DLBCL (cohort 1), and 20 patients with refractory PMBCL or TFL (cohort 2).
The median patient age was 58 years. Patients had stage III or IV disease, 47% had International Prognostic Index (IPI) scores of 3-4, 77% had disease that was refractory to second-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
The axi-cel construct was successfully manufactured in 99% of patients, with an average turnaround time from apheresis to the clinical site of 17 days.
As noted before, the trial met its primary endpoint with an 82% ORR, consisting of 54% complete responses and 28% partial responses.
The median duration of response was 8.2 months, and for patients with complete responses the median duration has not been reached.
Median overall survival has also not been reached.
The treatment was generally safe, with only 13% of patients experiencing grade 3 or greater cytokine release syndrome (CRS), and 28% having grade 3 or greater neurologic events. The events were generally reversible, and the rates of each declined over time. The use of tociluzumab or steroids to control adverse events did not have a negative effect on responses, Dr. Lin said.
JULIET
In the ongoing JULIET study, patients with relapsed/refractory DLBCL after at least two prior lines of therapy and who are not candidates for stem cell transplants are enrolled.
In a safety analysis including 85 patients, the CRS was seen in 57% of all patients, including grade 3 in 17% and grade 4 in 9%.
Other common adverse events occurring within 8 weeks of CTL019 infusion were infections in 26% of patients, cytopenias lasting longer than 28 days in 26%, neurologic events in 21%, febrile neutropenia in 14%, and tumor lysis syndrome in 1%.
There were no cases of cerebral edema, and no deaths attributable to the CAR-T cell construct, Dr. Salles said.
Peter Borchmann, MD, from the University of Cologne, Germany, who attended the briefing but was not involved with either study, commented that investigators in ZUMA-1 need to monitor patients carefully, because previous clinical trials using other CAR-T cells with CD28 costimuatory domains have been associated with several cases of fatal cerebral edema.
“I think you can use CD28 in lymphoma, and it’s highly active as we have seen, but my personal impression is that you have to be aware that this might happen,” he said in an interview.
The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
AT EHA 2017
Key clinical point: CAR-T cell therapies are showing good activity against relapsed/refractory non-Hodgkin lymphomas.
Major finding: In ZUMA-1, the objective response rate was 82%. In JULIET, it was 59%
Data source: Two multicenter trials of CAR-T cells in patients with relapsed/refractory DLBCL, PMBCL, and TFL.
Disclosures: The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
Overall survival better in advanced Hodgkin lymphoma with shorter eBEACOPP
MADRID – Patients with advanced Hodgkin lymphoma who have a metabolic response after the first two cycles of extended-dose(e)BEACOPP can be spared from undergoing more than two additional cycles of the highly intensive and toxic regimen, investigators from the German Hodgkin Study Group (GHSG) contend.
Among 1,005 patients with Hodgkin lymphoma who had negative PET scans after the second cycle of eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, prednisone, procarbazine), progression-free survival (PFS) was virtually identical whether patients were randomized to undergo a total of six-to-eight cycles or only four cycles, reported Peter Borchmann, MD, from the University of Cologne, Germany.
“For patients with negative PET-2 after initial treatment with eBEACOPP. Therapy with only two additional cycles of eBEACOPP is very effective, obviously, very safe, very short – it just takes 12 weeks – and it’s affordable,” he said.
“When balancing efficacy and safety, results compare favorably with any other published treatment strategy so far. That’s why we recommend this treatment, PET-guided extended BEACOPP in patients with newly diagnosed, advanced-stage Hodgkin lymphoma,” he added.
Although most patients in the United States with newly diagnosed Hodgkin lymphoma receive ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), BEACOPP is sometimes used for high-risk patients. BEACOPP is associated with considerable toxicities, however, including increased risk of secondary malignancies.
To see whether select patients could be cured with fewer cycles of therapy, the GHSG investigators designed the GHSG HD18 study in which patient with metabolic responses determined by fluorodeoxyglucose-PET after two eBEACOPP cycles were randomized to either two or six-to-eight additional cycles.
A total of 2,101 patients from the ages of 18-60 years with newly diagnosed advanced-stage Hodgkin lymphoma were enrolled from centers in Germany, Switzerland, Austria, the Czech Republic, and the Netherlands.
After the second cycle of therapy, patients underwent fluorodeoxyglucose-PET scans, and those with negative results were then randomized.
The trial was designed and powered for noninferiority of the shortened regimen, with a maximum allowable difference of 6%.
The trial met its primary endpoint and then some. After a median observation time of 53 months, the 5-year PFS rate for patients who received only four cycles was 91.2%, compared with 91.8% for patients who underwent six-to-eight cycles. As shown on a Kaplan-Meier curve, the two lines were superimposable and virtually impossible to tell apart.
Interestingly, 5-year overall survival was significantly better with the shorter, less toxic regimen, at 97.6% vs. 95.4%, respectively, translating into a hazard ratio favoring the shorter regimen of 0.36 (P = .006).
In addition, the four-cycle regimen was associated with fewer severe infections (8% vs 15%), and lower degrees of organ toxicity (8% vs. 18%). In addition, the rate of secondary acute myeloid leukemia or the myelodysplastic syndrome was 0.4% among the 501 patients treated with only four cycles, compared with 1.6% for the 504 patients who received six to eight cycles.
There were no treatment-related deaths among patients who underwent four cycles, compared with six deaths among patients treated with additional cycles.
In an interview, Dr. Borchmann said that the findings are likely to change the standard of care for those centers that use the BEACOPP regimen. He acknowledged that the regimen is highly toxic and requires intensive patient surveillance and management, which may be more practical in Europe where patients generally live closer to major cancer centers than in the more spacious United States.
The study was supported by German Cancer Aid, the Swiss State Secratariate for Education, Research and Innovation, and by Roche Pharma AG. Dr, Borchmann reported having no relevant disclosures.
MADRID – Patients with advanced Hodgkin lymphoma who have a metabolic response after the first two cycles of extended-dose(e)BEACOPP can be spared from undergoing more than two additional cycles of the highly intensive and toxic regimen, investigators from the German Hodgkin Study Group (GHSG) contend.
Among 1,005 patients with Hodgkin lymphoma who had negative PET scans after the second cycle of eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, prednisone, procarbazine), progression-free survival (PFS) was virtually identical whether patients were randomized to undergo a total of six-to-eight cycles or only four cycles, reported Peter Borchmann, MD, from the University of Cologne, Germany.
“For patients with negative PET-2 after initial treatment with eBEACOPP. Therapy with only two additional cycles of eBEACOPP is very effective, obviously, very safe, very short – it just takes 12 weeks – and it’s affordable,” he said.
“When balancing efficacy and safety, results compare favorably with any other published treatment strategy so far. That’s why we recommend this treatment, PET-guided extended BEACOPP in patients with newly diagnosed, advanced-stage Hodgkin lymphoma,” he added.
Although most patients in the United States with newly diagnosed Hodgkin lymphoma receive ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), BEACOPP is sometimes used for high-risk patients. BEACOPP is associated with considerable toxicities, however, including increased risk of secondary malignancies.
To see whether select patients could be cured with fewer cycles of therapy, the GHSG investigators designed the GHSG HD18 study in which patient with metabolic responses determined by fluorodeoxyglucose-PET after two eBEACOPP cycles were randomized to either two or six-to-eight additional cycles.
A total of 2,101 patients from the ages of 18-60 years with newly diagnosed advanced-stage Hodgkin lymphoma were enrolled from centers in Germany, Switzerland, Austria, the Czech Republic, and the Netherlands.
After the second cycle of therapy, patients underwent fluorodeoxyglucose-PET scans, and those with negative results were then randomized.
The trial was designed and powered for noninferiority of the shortened regimen, with a maximum allowable difference of 6%.
The trial met its primary endpoint and then some. After a median observation time of 53 months, the 5-year PFS rate for patients who received only four cycles was 91.2%, compared with 91.8% for patients who underwent six-to-eight cycles. As shown on a Kaplan-Meier curve, the two lines were superimposable and virtually impossible to tell apart.
Interestingly, 5-year overall survival was significantly better with the shorter, less toxic regimen, at 97.6% vs. 95.4%, respectively, translating into a hazard ratio favoring the shorter regimen of 0.36 (P = .006).
In addition, the four-cycle regimen was associated with fewer severe infections (8% vs 15%), and lower degrees of organ toxicity (8% vs. 18%). In addition, the rate of secondary acute myeloid leukemia or the myelodysplastic syndrome was 0.4% among the 501 patients treated with only four cycles, compared with 1.6% for the 504 patients who received six to eight cycles.
There were no treatment-related deaths among patients who underwent four cycles, compared with six deaths among patients treated with additional cycles.
In an interview, Dr. Borchmann said that the findings are likely to change the standard of care for those centers that use the BEACOPP regimen. He acknowledged that the regimen is highly toxic and requires intensive patient surveillance and management, which may be more practical in Europe where patients generally live closer to major cancer centers than in the more spacious United States.
The study was supported by German Cancer Aid, the Swiss State Secratariate for Education, Research and Innovation, and by Roche Pharma AG. Dr, Borchmann reported having no relevant disclosures.
MADRID – Patients with advanced Hodgkin lymphoma who have a metabolic response after the first two cycles of extended-dose(e)BEACOPP can be spared from undergoing more than two additional cycles of the highly intensive and toxic regimen, investigators from the German Hodgkin Study Group (GHSG) contend.
Among 1,005 patients with Hodgkin lymphoma who had negative PET scans after the second cycle of eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, prednisone, procarbazine), progression-free survival (PFS) was virtually identical whether patients were randomized to undergo a total of six-to-eight cycles or only four cycles, reported Peter Borchmann, MD, from the University of Cologne, Germany.
“For patients with negative PET-2 after initial treatment with eBEACOPP. Therapy with only two additional cycles of eBEACOPP is very effective, obviously, very safe, very short – it just takes 12 weeks – and it’s affordable,” he said.
“When balancing efficacy and safety, results compare favorably with any other published treatment strategy so far. That’s why we recommend this treatment, PET-guided extended BEACOPP in patients with newly diagnosed, advanced-stage Hodgkin lymphoma,” he added.
Although most patients in the United States with newly diagnosed Hodgkin lymphoma receive ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), BEACOPP is sometimes used for high-risk patients. BEACOPP is associated with considerable toxicities, however, including increased risk of secondary malignancies.
To see whether select patients could be cured with fewer cycles of therapy, the GHSG investigators designed the GHSG HD18 study in which patient with metabolic responses determined by fluorodeoxyglucose-PET after two eBEACOPP cycles were randomized to either two or six-to-eight additional cycles.
A total of 2,101 patients from the ages of 18-60 years with newly diagnosed advanced-stage Hodgkin lymphoma were enrolled from centers in Germany, Switzerland, Austria, the Czech Republic, and the Netherlands.
After the second cycle of therapy, patients underwent fluorodeoxyglucose-PET scans, and those with negative results were then randomized.
The trial was designed and powered for noninferiority of the shortened regimen, with a maximum allowable difference of 6%.
The trial met its primary endpoint and then some. After a median observation time of 53 months, the 5-year PFS rate for patients who received only four cycles was 91.2%, compared with 91.8% for patients who underwent six-to-eight cycles. As shown on a Kaplan-Meier curve, the two lines were superimposable and virtually impossible to tell apart.
Interestingly, 5-year overall survival was significantly better with the shorter, less toxic regimen, at 97.6% vs. 95.4%, respectively, translating into a hazard ratio favoring the shorter regimen of 0.36 (P = .006).
In addition, the four-cycle regimen was associated with fewer severe infections (8% vs 15%), and lower degrees of organ toxicity (8% vs. 18%). In addition, the rate of secondary acute myeloid leukemia or the myelodysplastic syndrome was 0.4% among the 501 patients treated with only four cycles, compared with 1.6% for the 504 patients who received six to eight cycles.
There were no treatment-related deaths among patients who underwent four cycles, compared with six deaths among patients treated with additional cycles.
In an interview, Dr. Borchmann said that the findings are likely to change the standard of care for those centers that use the BEACOPP regimen. He acknowledged that the regimen is highly toxic and requires intensive patient surveillance and management, which may be more practical in Europe where patients generally live closer to major cancer centers than in the more spacious United States.
The study was supported by German Cancer Aid, the Swiss State Secratariate for Education, Research and Innovation, and by Roche Pharma AG. Dr, Borchmann reported having no relevant disclosures.
AT EHA 2017
Key clinical point: For patients with newly diagnosed advanced Hodgkin lymphoma with PET-confirmed metabolic responses, four cycles of BEACOPP were at least as good as six to eight cycles for progression-free and overall survival.
Major finding: 5-year overall survival with four cycles of extended-dose BEACOPP was 97.6%, compared with. 95.4% for six to eight cycles (P = .006).
Data source: Randomized trial in 1,005 patients with newly diagnosed Hodgkin lymphoma from five European nations.
Disclosures: The study was supported by German Cancer Aid, the Swiss State Secretariate for Education, Research and Innovation, and by Roche Pharma AG. Dr. Borchmann reported having no relevant disclosures.
New frontline treatments needed for Hodgkin lymphoma
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.