User login
FDA approves rifamycin for treatment of traveler’s diarrhea
The Food and Drug Administration has approved rifamycin (Aemcolo) for the treatment of traveler’s diarrhea caused by noninvasive strains of Escherichia coli.
FDA approval was based on results of three clinical trials. The efficacy of rifamycin was shown in a trial of 264 adults with traveler’s diarrhea in Guatemala and Mexico. Compared with placebo, rifamycin significantly reduced symptoms of the condition. The safety of rifamycin was illustrated in a pair of studies including 619 adults with traveler’s diarrhea who took rifamycin orally for 3-4 days. The most common adverse events were headache and constipation.
Traveler’s diarrhea is the most common travel-related illness, affecting 10%-40% of travelers. It can be caused by a multitude of pathogens, but bacteria from food or water is the most common source. High-risk areas include much of Asia, the Middle East, Mexico, Central and South America, and Africa.
Rifamycin was not effective in patients with diarrhea complicated by fever and/or bloody stool or in diarrhea caused by a pathogen other than E. coli.
“Travelers’ diarrhea affects millions of people each year, and having treatment options for this condition can help reduce symptoms of the condition,” Edward Cox, MD, MPH, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has approved rifamycin (Aemcolo) for the treatment of traveler’s diarrhea caused by noninvasive strains of Escherichia coli.
FDA approval was based on results of three clinical trials. The efficacy of rifamycin was shown in a trial of 264 adults with traveler’s diarrhea in Guatemala and Mexico. Compared with placebo, rifamycin significantly reduced symptoms of the condition. The safety of rifamycin was illustrated in a pair of studies including 619 adults with traveler’s diarrhea who took rifamycin orally for 3-4 days. The most common adverse events were headache and constipation.
Traveler’s diarrhea is the most common travel-related illness, affecting 10%-40% of travelers. It can be caused by a multitude of pathogens, but bacteria from food or water is the most common source. High-risk areas include much of Asia, the Middle East, Mexico, Central and South America, and Africa.
Rifamycin was not effective in patients with diarrhea complicated by fever and/or bloody stool or in diarrhea caused by a pathogen other than E. coli.
“Travelers’ diarrhea affects millions of people each year, and having treatment options for this condition can help reduce symptoms of the condition,” Edward Cox, MD, MPH, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has approved rifamycin (Aemcolo) for the treatment of traveler’s diarrhea caused by noninvasive strains of Escherichia coli.
FDA approval was based on results of three clinical trials. The efficacy of rifamycin was shown in a trial of 264 adults with traveler’s diarrhea in Guatemala and Mexico. Compared with placebo, rifamycin significantly reduced symptoms of the condition. The safety of rifamycin was illustrated in a pair of studies including 619 adults with traveler’s diarrhea who took rifamycin orally for 3-4 days. The most common adverse events were headache and constipation.
Traveler’s diarrhea is the most common travel-related illness, affecting 10%-40% of travelers. It can be caused by a multitude of pathogens, but bacteria from food or water is the most common source. High-risk areas include much of Asia, the Middle East, Mexico, Central and South America, and Africa.
Rifamycin was not effective in patients with diarrhea complicated by fever and/or bloody stool or in diarrhea caused by a pathogen other than E. coli.
“Travelers’ diarrhea affects millions of people each year, and having treatment options for this condition can help reduce symptoms of the condition,” Edward Cox, MD, MPH, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
FDA approves adalimumab biosimilar Hyrimoz
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
AGA is taking the lead in educating health care providers and patients about biosimilars and how they can be used for IBD patient care. Learn more at www.gastro.org/biosimilars.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
AGA is taking the lead in educating health care providers and patients about biosimilars and how they can be used for IBD patient care. Learn more at www.gastro.org/biosimilars.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
AGA is taking the lead in educating health care providers and patients about biosimilars and how they can be used for IBD patient care. Learn more at www.gastro.org/biosimilars.
Budesonide topped placebo for treating lymphocytic colitis
Among patients with lymphocytic colitis, 8 weeks of oral budesonide therapy was associated with significantly higher rates of clinical and histologic remission versus placebo in a multicenter, double-blind clinical trial.
Fully 79% of patients achieved clinical remission with budesonide, compared with only 42% of patients in the placebo arm (P = .01), reported Stephan Miehlke, MD, of the Center for Digestive Diseases in Hamburg, Germany, and his associates. A third group of patients received oral mesalazine therapy, which induced clinical remission in 68% of cases (P = .09). Budesonide also induced histologic remission significantly more often (68%) than did mesalazine (26%; P = .02) or placebo (21%; P = .008).
“The study population was not large, but the trial was adequately powered,” the researchers wrote. The report was published online in Gastroenterology. “These results confirm the efficacy of budesonide for the induction of remission in active lymphocytic colitis and are consistent with expert recommendations for its use as first-line therapy.”
Lymphocytic colitis is a subtype of microscopic colitis that is characterized by an increase in intraepithelial lymphocytes. This condition has substantial negative effects on quality of life – the most common symptom is chronic diarrhea, and some patients also experience fecal incontinence and abdominal pain. Expert guidelines recommend first-line treatment with budesonide and second-line treatment with mesalazine, but evidence supporting either recommendation is sparse and low-quality, the investigators wrote.
For the study, they compared 8 weeks of treatment with pH-modified release oral budesonide granules (9 mg once daily), oral mesalazine granules (3 g once daily) or placebo in 57 patients (19 per arm) with histologically confirmed, newly diagnosed or relapsed lymphocytic colitis. All patients had at least a 12-week history of watery, nonbloody diarrhea, no other documented diarrheal conditions, and no recent history of antidiarrheal therapy. Nearly three-quarters were female and the mean age was 59 years. The primary endpoint was clinical remission, defined as no more than 21 stools in the 7 days before week 8, including no more than 6 watery stools.
After 8 weeks of double-blinded treatment, all clinically remitted patients stopped treatment and were followed for another 16 weeks. Those who were not in remission or who relapsed were offered 4 weeks of open-label budesonide therapy, which led to clinical remission in 88% of cases, the researchers said. “This study confirms that budesonide is effective for the induction of remission in active lymphocytic colitis,” they concluded. “Strikingly, a substantial improvement in symptoms, including a profound reduction in the number of watery stools, was seen within a median of 3 days after starting budesonide therapy.”
Serious adverse events were uncommon in all three groups, and each arm had a similar rate of adverse events considered secondary to treatment. In the budesonide group, these included one case each of weight gain, transient ischemic attack, and affective disturbance with sleep disorder. In the mesalazine group, three patients developed acute pancreatitis, increased hepatic enzymes, or dizziness. Eleven percent of budesonide recipients and 16% of mesalazine recipients stopped treatment because of adverse events. “No patient in any group had a clinically significant shift in cortisol level between baseline and week 8 that was considered related to the study drug,” the investigators said. “Other changes in laboratory parameters were not considered clinically relevant in any treatment group.”
The study was funded by Dr. Falk Pharma GmbH, Freiburg, Germany. Dr. Miehlke and two coauthors received speaker fees from Dr. Falk Pharma. Dr. Miehlke and one coauthor received consultancy fees from Tillots. One coauthor received speaker fees, has been a member of the advisory board, and has received grants from Tillots.
SOURCE: Miehlke S et al. Gastroenterology. 2018 Sep 6. doi: 10.1053/j.gastro.2018.08.042.
Among patients with lymphocytic colitis, 8 weeks of oral budesonide therapy was associated with significantly higher rates of clinical and histologic remission versus placebo in a multicenter, double-blind clinical trial.
Fully 79% of patients achieved clinical remission with budesonide, compared with only 42% of patients in the placebo arm (P = .01), reported Stephan Miehlke, MD, of the Center for Digestive Diseases in Hamburg, Germany, and his associates. A third group of patients received oral mesalazine therapy, which induced clinical remission in 68% of cases (P = .09). Budesonide also induced histologic remission significantly more often (68%) than did mesalazine (26%; P = .02) or placebo (21%; P = .008).
“The study population was not large, but the trial was adequately powered,” the researchers wrote. The report was published online in Gastroenterology. “These results confirm the efficacy of budesonide for the induction of remission in active lymphocytic colitis and are consistent with expert recommendations for its use as first-line therapy.”
Lymphocytic colitis is a subtype of microscopic colitis that is characterized by an increase in intraepithelial lymphocytes. This condition has substantial negative effects on quality of life – the most common symptom is chronic diarrhea, and some patients also experience fecal incontinence and abdominal pain. Expert guidelines recommend first-line treatment with budesonide and second-line treatment with mesalazine, but evidence supporting either recommendation is sparse and low-quality, the investigators wrote.
For the study, they compared 8 weeks of treatment with pH-modified release oral budesonide granules (9 mg once daily), oral mesalazine granules (3 g once daily) or placebo in 57 patients (19 per arm) with histologically confirmed, newly diagnosed or relapsed lymphocytic colitis. All patients had at least a 12-week history of watery, nonbloody diarrhea, no other documented diarrheal conditions, and no recent history of antidiarrheal therapy. Nearly three-quarters were female and the mean age was 59 years. The primary endpoint was clinical remission, defined as no more than 21 stools in the 7 days before week 8, including no more than 6 watery stools.
After 8 weeks of double-blinded treatment, all clinically remitted patients stopped treatment and were followed for another 16 weeks. Those who were not in remission or who relapsed were offered 4 weeks of open-label budesonide therapy, which led to clinical remission in 88% of cases, the researchers said. “This study confirms that budesonide is effective for the induction of remission in active lymphocytic colitis,” they concluded. “Strikingly, a substantial improvement in symptoms, including a profound reduction in the number of watery stools, was seen within a median of 3 days after starting budesonide therapy.”
Serious adverse events were uncommon in all three groups, and each arm had a similar rate of adverse events considered secondary to treatment. In the budesonide group, these included one case each of weight gain, transient ischemic attack, and affective disturbance with sleep disorder. In the mesalazine group, three patients developed acute pancreatitis, increased hepatic enzymes, or dizziness. Eleven percent of budesonide recipients and 16% of mesalazine recipients stopped treatment because of adverse events. “No patient in any group had a clinically significant shift in cortisol level between baseline and week 8 that was considered related to the study drug,” the investigators said. “Other changes in laboratory parameters were not considered clinically relevant in any treatment group.”
The study was funded by Dr. Falk Pharma GmbH, Freiburg, Germany. Dr. Miehlke and two coauthors received speaker fees from Dr. Falk Pharma. Dr. Miehlke and one coauthor received consultancy fees from Tillots. One coauthor received speaker fees, has been a member of the advisory board, and has received grants from Tillots.
SOURCE: Miehlke S et al. Gastroenterology. 2018 Sep 6. doi: 10.1053/j.gastro.2018.08.042.
Among patients with lymphocytic colitis, 8 weeks of oral budesonide therapy was associated with significantly higher rates of clinical and histologic remission versus placebo in a multicenter, double-blind clinical trial.
Fully 79% of patients achieved clinical remission with budesonide, compared with only 42% of patients in the placebo arm (P = .01), reported Stephan Miehlke, MD, of the Center for Digestive Diseases in Hamburg, Germany, and his associates. A third group of patients received oral mesalazine therapy, which induced clinical remission in 68% of cases (P = .09). Budesonide also induced histologic remission significantly more often (68%) than did mesalazine (26%; P = .02) or placebo (21%; P = .008).
“The study population was not large, but the trial was adequately powered,” the researchers wrote. The report was published online in Gastroenterology. “These results confirm the efficacy of budesonide for the induction of remission in active lymphocytic colitis and are consistent with expert recommendations for its use as first-line therapy.”
Lymphocytic colitis is a subtype of microscopic colitis that is characterized by an increase in intraepithelial lymphocytes. This condition has substantial negative effects on quality of life – the most common symptom is chronic diarrhea, and some patients also experience fecal incontinence and abdominal pain. Expert guidelines recommend first-line treatment with budesonide and second-line treatment with mesalazine, but evidence supporting either recommendation is sparse and low-quality, the investigators wrote.
For the study, they compared 8 weeks of treatment with pH-modified release oral budesonide granules (9 mg once daily), oral mesalazine granules (3 g once daily) or placebo in 57 patients (19 per arm) with histologically confirmed, newly diagnosed or relapsed lymphocytic colitis. All patients had at least a 12-week history of watery, nonbloody diarrhea, no other documented diarrheal conditions, and no recent history of antidiarrheal therapy. Nearly three-quarters were female and the mean age was 59 years. The primary endpoint was clinical remission, defined as no more than 21 stools in the 7 days before week 8, including no more than 6 watery stools.
After 8 weeks of double-blinded treatment, all clinically remitted patients stopped treatment and were followed for another 16 weeks. Those who were not in remission or who relapsed were offered 4 weeks of open-label budesonide therapy, which led to clinical remission in 88% of cases, the researchers said. “This study confirms that budesonide is effective for the induction of remission in active lymphocytic colitis,” they concluded. “Strikingly, a substantial improvement in symptoms, including a profound reduction in the number of watery stools, was seen within a median of 3 days after starting budesonide therapy.”
Serious adverse events were uncommon in all three groups, and each arm had a similar rate of adverse events considered secondary to treatment. In the budesonide group, these included one case each of weight gain, transient ischemic attack, and affective disturbance with sleep disorder. In the mesalazine group, three patients developed acute pancreatitis, increased hepatic enzymes, or dizziness. Eleven percent of budesonide recipients and 16% of mesalazine recipients stopped treatment because of adverse events. “No patient in any group had a clinically significant shift in cortisol level between baseline and week 8 that was considered related to the study drug,” the investigators said. “Other changes in laboratory parameters were not considered clinically relevant in any treatment group.”
The study was funded by Dr. Falk Pharma GmbH, Freiburg, Germany. Dr. Miehlke and two coauthors received speaker fees from Dr. Falk Pharma. Dr. Miehlke and one coauthor received consultancy fees from Tillots. One coauthor received speaker fees, has been a member of the advisory board, and has received grants from Tillots.
SOURCE: Miehlke S et al. Gastroenterology. 2018 Sep 6. doi: 10.1053/j.gastro.2018.08.042.
FROM GASTROENTEROLOGY
Key clinical point: Budesonide significantly outperformed placebo for inducing clinical remission of lymphocytic colitis.
Major finding: Rates of clinical remission were 79% with budesonide, 42% with placebo (P = .01), and 68% with mesalazine (P = .09 vs. placebo).
Study details: Multicenter double-blind trial of 57 patients with chronic lymphocytic colitis.
Disclosures: The study was funded by Dr. Falk Pharma GmbH, Freiburg, Germany. Dr. Miehlke and two coauthors received speaker fees from Dr. Falk Pharma. Dr. Miehlke and one coauthor received consultancy fees from Tillots. One coauthor received speaker fees, has been a member of the advisory board, and has received grants from Tillots.
Source: Miehlke S et al. Gastroenterology. 2018 Sep 6.
Crohn’s disease tied to anal canal high-risk HPV infection
Crohn’s disease was significantly associated with anal canal high-risk human papillomavirus (HPV) infection in a prospective, single-center study of patients undergoing colonoscopy for various indications.
High-risk HPV and HPV strain 16 were detected in 30% of patients with Crohn’s disease and 18% of patients without Crohn’s disease (P = .005), said Lucine Vuitton, MD, of University Hospital of Besançon (France) and her associates. “Increasing our knowledge of HPV infection of anal tissues could help physicians identify populations at risk and promote prophylaxis with vaccination and adequate screening,” the investigators wrote in the November issue of Clinical Gastroenterology and Hepatology.
Most anal cancers are squamous cell carcinomas, for which infection with high-risk HPV (especially high-risk HPV16) is a driving risk factor. Case studies and literature reviews have linked Crohn’s disease to increased rates of anal canal cancers, but population-based data were lacking, the researchers wrote. Therefore, they prospectively analyzed anal tissue samples from 467 consecutive patients undergoing colonoscopy at a tertiary care center in France. Median age was 54 years (interquartile range, 18-86 years), and 52% of patients were women. No patient had detectable macroscopic neoplastic lesions at the anal margin at baseline.
The researchers used the QIAamp DNA Blood minikit (Qiagen) for DNA extraction and the INNO-LiPA HPV Genotyping Extra kit (Fujirebio Diagnostics) for HPV DNA detection and genotyping. These methods identified HPV DNA in anal tissue samples from 34% of the patients and high-risk HPV DNA in 18% of patients. The most prevalent genotype was HPV16 (detected in 7% of samples), followed by HPV51, HPV52, and HPV39.
A total of 112 patients were receiving at least one immunosuppressive treatment for inflammatory bowel disease or another condition. Seventy patients had Crohn’s disease, and 29 patients had ulcerative colitis. The prevalence of anal canal high-risk HPV and HPV16 infection in patients with ulcerative colitis was similar to that seen in those without inflammatory bowel disease. However, patients with Crohn’s disease were more likely to have anal canal high-risk HPV infection (30%) and HPV16 infection (14%), compared with patients without Crohn’s disease (18% and 7%, respectively). Additionally, among 22 patients with Crohn’s disease and perianal involvement, 11 had HPV DNA in the anal canal versus 30% of other patients with inflammatory bowel disease.
Women were more likely to have anal canal high-risk HPV (23%) infection than were men (13%; P = .004). In a multivariable analysis of self-reported data and medical data, significant risk factors for high-risk HPV infection included female sex, a history of sexually transmitted infections, having more than 10 sexual partners over the life course, having at least one sexual partner during the past year, current smoking, and immunosuppressive therapy. The multivariable analysis also linked Crohn’s disease with anal canal high-risk HPV16 infection (odds ratio, 3.8), but the association did not reach statistical significance (95% confidence interval, 0.9-16.9).
Most patients with Crohn’s disease were on immunosuppressive therapy, “which markedly affected statistical power,” the researchers commented. Nonetheless, their findings support HPV vaccination for patients with Crohn’s disease, as well as efforts to target high-risk patients who could benefit from anal cancer screening, they said.
The work was funded by the APICHU research grant from Besançon (France) University Hospital and by the Région de Franche-Comté. Dr. Vuitton disclosed ties to AbbVie, Ferring, MSD, Hospira, Janssen, and Takeda. Three coinvestigators disclosed relationships with AbbVie, MSD, Hospira, Mayoli, and Roche.
SOURCE: Vuitton L et al. Clin Gastroenterol Hepatol. 2018 Nov. doi: 10.1016/j.cgh.2018.03.008.
Crohn’s disease was significantly associated with anal canal high-risk human papillomavirus (HPV) infection in a prospective, single-center study of patients undergoing colonoscopy for various indications.
High-risk HPV and HPV strain 16 were detected in 30% of patients with Crohn’s disease and 18% of patients without Crohn’s disease (P = .005), said Lucine Vuitton, MD, of University Hospital of Besançon (France) and her associates. “Increasing our knowledge of HPV infection of anal tissues could help physicians identify populations at risk and promote prophylaxis with vaccination and adequate screening,” the investigators wrote in the November issue of Clinical Gastroenterology and Hepatology.
Most anal cancers are squamous cell carcinomas, for which infection with high-risk HPV (especially high-risk HPV16) is a driving risk factor. Case studies and literature reviews have linked Crohn’s disease to increased rates of anal canal cancers, but population-based data were lacking, the researchers wrote. Therefore, they prospectively analyzed anal tissue samples from 467 consecutive patients undergoing colonoscopy at a tertiary care center in France. Median age was 54 years (interquartile range, 18-86 years), and 52% of patients were women. No patient had detectable macroscopic neoplastic lesions at the anal margin at baseline.
The researchers used the QIAamp DNA Blood minikit (Qiagen) for DNA extraction and the INNO-LiPA HPV Genotyping Extra kit (Fujirebio Diagnostics) for HPV DNA detection and genotyping. These methods identified HPV DNA in anal tissue samples from 34% of the patients and high-risk HPV DNA in 18% of patients. The most prevalent genotype was HPV16 (detected in 7% of samples), followed by HPV51, HPV52, and HPV39.
A total of 112 patients were receiving at least one immunosuppressive treatment for inflammatory bowel disease or another condition. Seventy patients had Crohn’s disease, and 29 patients had ulcerative colitis. The prevalence of anal canal high-risk HPV and HPV16 infection in patients with ulcerative colitis was similar to that seen in those without inflammatory bowel disease. However, patients with Crohn’s disease were more likely to have anal canal high-risk HPV infection (30%) and HPV16 infection (14%), compared with patients without Crohn’s disease (18% and 7%, respectively). Additionally, among 22 patients with Crohn’s disease and perianal involvement, 11 had HPV DNA in the anal canal versus 30% of other patients with inflammatory bowel disease.
Women were more likely to have anal canal high-risk HPV (23%) infection than were men (13%; P = .004). In a multivariable analysis of self-reported data and medical data, significant risk factors for high-risk HPV infection included female sex, a history of sexually transmitted infections, having more than 10 sexual partners over the life course, having at least one sexual partner during the past year, current smoking, and immunosuppressive therapy. The multivariable analysis also linked Crohn’s disease with anal canal high-risk HPV16 infection (odds ratio, 3.8), but the association did not reach statistical significance (95% confidence interval, 0.9-16.9).
Most patients with Crohn’s disease were on immunosuppressive therapy, “which markedly affected statistical power,” the researchers commented. Nonetheless, their findings support HPV vaccination for patients with Crohn’s disease, as well as efforts to target high-risk patients who could benefit from anal cancer screening, they said.
The work was funded by the APICHU research grant from Besançon (France) University Hospital and by the Région de Franche-Comté. Dr. Vuitton disclosed ties to AbbVie, Ferring, MSD, Hospira, Janssen, and Takeda. Three coinvestigators disclosed relationships with AbbVie, MSD, Hospira, Mayoli, and Roche.
SOURCE: Vuitton L et al. Clin Gastroenterol Hepatol. 2018 Nov. doi: 10.1016/j.cgh.2018.03.008.
Crohn’s disease was significantly associated with anal canal high-risk human papillomavirus (HPV) infection in a prospective, single-center study of patients undergoing colonoscopy for various indications.
High-risk HPV and HPV strain 16 were detected in 30% of patients with Crohn’s disease and 18% of patients without Crohn’s disease (P = .005), said Lucine Vuitton, MD, of University Hospital of Besançon (France) and her associates. “Increasing our knowledge of HPV infection of anal tissues could help physicians identify populations at risk and promote prophylaxis with vaccination and adequate screening,” the investigators wrote in the November issue of Clinical Gastroenterology and Hepatology.
Most anal cancers are squamous cell carcinomas, for which infection with high-risk HPV (especially high-risk HPV16) is a driving risk factor. Case studies and literature reviews have linked Crohn’s disease to increased rates of anal canal cancers, but population-based data were lacking, the researchers wrote. Therefore, they prospectively analyzed anal tissue samples from 467 consecutive patients undergoing colonoscopy at a tertiary care center in France. Median age was 54 years (interquartile range, 18-86 years), and 52% of patients were women. No patient had detectable macroscopic neoplastic lesions at the anal margin at baseline.
The researchers used the QIAamp DNA Blood minikit (Qiagen) for DNA extraction and the INNO-LiPA HPV Genotyping Extra kit (Fujirebio Diagnostics) for HPV DNA detection and genotyping. These methods identified HPV DNA in anal tissue samples from 34% of the patients and high-risk HPV DNA in 18% of patients. The most prevalent genotype was HPV16 (detected in 7% of samples), followed by HPV51, HPV52, and HPV39.
A total of 112 patients were receiving at least one immunosuppressive treatment for inflammatory bowel disease or another condition. Seventy patients had Crohn’s disease, and 29 patients had ulcerative colitis. The prevalence of anal canal high-risk HPV and HPV16 infection in patients with ulcerative colitis was similar to that seen in those without inflammatory bowel disease. However, patients with Crohn’s disease were more likely to have anal canal high-risk HPV infection (30%) and HPV16 infection (14%), compared with patients without Crohn’s disease (18% and 7%, respectively). Additionally, among 22 patients with Crohn’s disease and perianal involvement, 11 had HPV DNA in the anal canal versus 30% of other patients with inflammatory bowel disease.
Women were more likely to have anal canal high-risk HPV (23%) infection than were men (13%; P = .004). In a multivariable analysis of self-reported data and medical data, significant risk factors for high-risk HPV infection included female sex, a history of sexually transmitted infections, having more than 10 sexual partners over the life course, having at least one sexual partner during the past year, current smoking, and immunosuppressive therapy. The multivariable analysis also linked Crohn’s disease with anal canal high-risk HPV16 infection (odds ratio, 3.8), but the association did not reach statistical significance (95% confidence interval, 0.9-16.9).
Most patients with Crohn’s disease were on immunosuppressive therapy, “which markedly affected statistical power,” the researchers commented. Nonetheless, their findings support HPV vaccination for patients with Crohn’s disease, as well as efforts to target high-risk patients who could benefit from anal cancer screening, they said.
The work was funded by the APICHU research grant from Besançon (France) University Hospital and by the Région de Franche-Comté. Dr. Vuitton disclosed ties to AbbVie, Ferring, MSD, Hospira, Janssen, and Takeda. Three coinvestigators disclosed relationships with AbbVie, MSD, Hospira, Mayoli, and Roche.
SOURCE: Vuitton L et al. Clin Gastroenterol Hepatol. 2018 Nov. doi: 10.1016/j.cgh.2018.03.008.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Crohn’s disease was associated with high-risk human papillomavirus infection.
Major finding: High-risk HPV and HPV16 were detected in 30% of patients with Crohn’s disease versus 18% of those without Crohn’s disease (P = .005).
Study details: Analyses of anal tissue samples from 467 consecutive patients, including 70 with Crohn’s disease.
Disclosures: The work was funded by the APICHU research grant from Besançon (France) University Hospital and by the Région de Franche-Comté. Dr. Vuitton disclosed ties to AbbVie, Ferring, MSD, Hospira, Janssen, and Takeda. Three coinvestigators disclosed relationships with AbbVie, MSD, Hospira, Mayoli, and Roche.
Source: Vuitton L et al. Clin Gastroenterol Hepatol. 2018 Nov. doi: 10.1016/j.cgh.2018.03.008.
H. pylori antibiotic resistance reaches ‘alarming levels’
Over the past decade, Helicobacter pylori strains have reached “alarming levels” of antimicrobial resistance worldwide, investigators reported in the November issue of Gastroenterology.
In a large meta-analysis spanning 2007-2017, H. pylori isolates showed a 15% or higher pooled prevalence of primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin in almost all World Health Organization (WHO) regions. “Local surveillance networks are required to select appropriate eradication regimens for each region,” concluded Alessia Savoldi, MD, of the University of Tübingen (Germany) and her associates.
Typically, the threshold of antimicrobial resistance for choosing empiric regimens is 15%, Dr. Savoldi and her associates noted. Their systematic review and meta-analysis included 178 studies comprising 66,142 isolates from 65 countries. They defined H. pylori infection as a positive histology, serology, stool antigen, urea breath test, or rapid urease test. They excluded studies of fewer than 50 isolates, studies that only reported resistance as a percentage with no denominator, studies that failed to specify time frames or clustered data over more than 3 years, and data reported in guidelines, conference presentations, or letters without formal publication.
The prevalence of primary clarithromycin resistance exceeded 15% in the WHO European Region (18%; 95% confidence interval, 16%-20%), the Eastern Mediterranean Region (33%), and the Western Pacific Region (34%) and reached 10% in the Americas and the South East Asia region. Furthermore, primary resistance to metronidazole exceeded 15% in all WHO regions, ranging from 56% in the Eastern Mediterranean Region to 23% in the Americas. Resistance to levofloxacin was at least 15% in all WHO regions except the European region (11%).
In most regions, H. pylori also accrued substantially more antimicrobial resistance over time, the investigators said. Clarithromycin resistance rose from 13% during 2006 through 2008 to 21% during 2012 through 2016 (P less than .001). Levofloxacin resistance in the Western Pacific region increased from 12% to 31% during the same two time periods (P less than .001). Several other WHO regions showed less significant trends toward increasing resistance. Multidrug resistance also rose. Resistance to both clarithromycin and metronidazole increased markedly in all WHO areas with available data, reaching 14% in the Eastern Mediterranean and Western Pacific regions and 23% in the European region.
Secondary analyses linked resistance with dramatic increases in the odds of treatment failure. For example, clarithromycin resistance conferred a sevenfold increase in the odds of treatment failure for regimens containing clarithromycin (odds ratio, 7.0; 95% CI, 5.2 to 9.3; P less than .001). Corresponding ORs were 8.2 for levofloxacin resistance, 2.5 for metronidazole resistance, and 9.4 for dual clarithromycin-metronidazole resistance.
The investigators acknowledged several limitations. Of publications in this meta-analysis, 85% represented single-center studies with limited sample sizes, they wrote. Studies often excluded demographic and endoscopic details. Furthermore, only three studies provided prevalence data for the WHO Africa Region and these only provided overall estimates without stratifying by resistance type.
The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
SOURCE: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007.
The first-line treatment of individuals with Helicobacter pylori infection using clarithromycin-based triple therapies or, if penicillin allergic, bismuth-based quadruple therapies is generally effective. However, reports of declining therapeutic efficacy have led to published guidelines to recommend confirmation of H. pylori eradication after completing a course of antibiotics. It is believed that increasing antibiotic use in agriculture and medicine around the globe have contributed to the increasing H. pylori antibiotic resistance and declining efficacy of standard H. pylori regimens.
Indeed, most H. pylori guidelines recommend antibiotic sensitivity testing after failing two courses of treatment; however, performing such testing successfully may require sending fresh gastric biopsy samples to an in-house H. pylori culture lab within 1 hour, which is generally not available to most clinicians. Clearly, the gap in knowledge of local antibiotic resistance could be addressed by having a readily accessible culture facility and the testing should be reimbursed by health insurance.
Single-center experiences with antibiotic sensitivity–guided salvage therapy in the United States, however, registered a lower efficacy rate of approximately 50%, which indicates that other host factors (such as gastric acidity pH less than 5.5 or body mass index greater than 30 kg/m2) may affect the minimum inhibitory concentration (MIC) of the antibiotics against H. pylori.
In order to better study the effects of these host factors relative to the effect of antibiotic resistance on therapeutic efficacy, it is critical that we practice precision medicine by determining the antibiotic sensitivity of the H. pylori strain prior to initiating the antibiotic treatment. It may be possible to achieve more than 90% therapeutic efficacy given known antibiotic sensitivities of the bacteria and optimized host factors to lower the MIC. In addition, with the increasing awareness of the importance of gut microbiota in health and disease, clinicians should strive to narrow the antibiotic coverage that will be possible if antibiotic sensitivity is known (for example, use high-dose amoxicillin and proton-pump inhibitor dual therapy).
John Y. Kao, MD, AGAF, is the current chair of the AGA Institute Council Esophageal, Gastric and Duodenal Disorders Section, a physician investigator in the University of Michigan Center for Gastrointestinal Research, and an associate professor in the department of medicine in the division of gastroenterology & hepatology and an associate program director of the GI Fellowship Program at Michigan Medicine at the University of Michigan, Ann Arbor. He has no conflicts.
The first-line treatment of individuals with Helicobacter pylori infection using clarithromycin-based triple therapies or, if penicillin allergic, bismuth-based quadruple therapies is generally effective. However, reports of declining therapeutic efficacy have led to published guidelines to recommend confirmation of H. pylori eradication after completing a course of antibiotics. It is believed that increasing antibiotic use in agriculture and medicine around the globe have contributed to the increasing H. pylori antibiotic resistance and declining efficacy of standard H. pylori regimens.
Indeed, most H. pylori guidelines recommend antibiotic sensitivity testing after failing two courses of treatment; however, performing such testing successfully may require sending fresh gastric biopsy samples to an in-house H. pylori culture lab within 1 hour, which is generally not available to most clinicians. Clearly, the gap in knowledge of local antibiotic resistance could be addressed by having a readily accessible culture facility and the testing should be reimbursed by health insurance.
Single-center experiences with antibiotic sensitivity–guided salvage therapy in the United States, however, registered a lower efficacy rate of approximately 50%, which indicates that other host factors (such as gastric acidity pH less than 5.5 or body mass index greater than 30 kg/m2) may affect the minimum inhibitory concentration (MIC) of the antibiotics against H. pylori.
In order to better study the effects of these host factors relative to the effect of antibiotic resistance on therapeutic efficacy, it is critical that we practice precision medicine by determining the antibiotic sensitivity of the H. pylori strain prior to initiating the antibiotic treatment. It may be possible to achieve more than 90% therapeutic efficacy given known antibiotic sensitivities of the bacteria and optimized host factors to lower the MIC. In addition, with the increasing awareness of the importance of gut microbiota in health and disease, clinicians should strive to narrow the antibiotic coverage that will be possible if antibiotic sensitivity is known (for example, use high-dose amoxicillin and proton-pump inhibitor dual therapy).
John Y. Kao, MD, AGAF, is the current chair of the AGA Institute Council Esophageal, Gastric and Duodenal Disorders Section, a physician investigator in the University of Michigan Center for Gastrointestinal Research, and an associate professor in the department of medicine in the division of gastroenterology & hepatology and an associate program director of the GI Fellowship Program at Michigan Medicine at the University of Michigan, Ann Arbor. He has no conflicts.
The first-line treatment of individuals with Helicobacter pylori infection using clarithromycin-based triple therapies or, if penicillin allergic, bismuth-based quadruple therapies is generally effective. However, reports of declining therapeutic efficacy have led to published guidelines to recommend confirmation of H. pylori eradication after completing a course of antibiotics. It is believed that increasing antibiotic use in agriculture and medicine around the globe have contributed to the increasing H. pylori antibiotic resistance and declining efficacy of standard H. pylori regimens.
Indeed, most H. pylori guidelines recommend antibiotic sensitivity testing after failing two courses of treatment; however, performing such testing successfully may require sending fresh gastric biopsy samples to an in-house H. pylori culture lab within 1 hour, which is generally not available to most clinicians. Clearly, the gap in knowledge of local antibiotic resistance could be addressed by having a readily accessible culture facility and the testing should be reimbursed by health insurance.
Single-center experiences with antibiotic sensitivity–guided salvage therapy in the United States, however, registered a lower efficacy rate of approximately 50%, which indicates that other host factors (such as gastric acidity pH less than 5.5 or body mass index greater than 30 kg/m2) may affect the minimum inhibitory concentration (MIC) of the antibiotics against H. pylori.
In order to better study the effects of these host factors relative to the effect of antibiotic resistance on therapeutic efficacy, it is critical that we practice precision medicine by determining the antibiotic sensitivity of the H. pylori strain prior to initiating the antibiotic treatment. It may be possible to achieve more than 90% therapeutic efficacy given known antibiotic sensitivities of the bacteria and optimized host factors to lower the MIC. In addition, with the increasing awareness of the importance of gut microbiota in health and disease, clinicians should strive to narrow the antibiotic coverage that will be possible if antibiotic sensitivity is known (for example, use high-dose amoxicillin and proton-pump inhibitor dual therapy).
John Y. Kao, MD, AGAF, is the current chair of the AGA Institute Council Esophageal, Gastric and Duodenal Disorders Section, a physician investigator in the University of Michigan Center for Gastrointestinal Research, and an associate professor in the department of medicine in the division of gastroenterology & hepatology and an associate program director of the GI Fellowship Program at Michigan Medicine at the University of Michigan, Ann Arbor. He has no conflicts.
Over the past decade, Helicobacter pylori strains have reached “alarming levels” of antimicrobial resistance worldwide, investigators reported in the November issue of Gastroenterology.
In a large meta-analysis spanning 2007-2017, H. pylori isolates showed a 15% or higher pooled prevalence of primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin in almost all World Health Organization (WHO) regions. “Local surveillance networks are required to select appropriate eradication regimens for each region,” concluded Alessia Savoldi, MD, of the University of Tübingen (Germany) and her associates.
Typically, the threshold of antimicrobial resistance for choosing empiric regimens is 15%, Dr. Savoldi and her associates noted. Their systematic review and meta-analysis included 178 studies comprising 66,142 isolates from 65 countries. They defined H. pylori infection as a positive histology, serology, stool antigen, urea breath test, or rapid urease test. They excluded studies of fewer than 50 isolates, studies that only reported resistance as a percentage with no denominator, studies that failed to specify time frames or clustered data over more than 3 years, and data reported in guidelines, conference presentations, or letters without formal publication.
The prevalence of primary clarithromycin resistance exceeded 15% in the WHO European Region (18%; 95% confidence interval, 16%-20%), the Eastern Mediterranean Region (33%), and the Western Pacific Region (34%) and reached 10% in the Americas and the South East Asia region. Furthermore, primary resistance to metronidazole exceeded 15% in all WHO regions, ranging from 56% in the Eastern Mediterranean Region to 23% in the Americas. Resistance to levofloxacin was at least 15% in all WHO regions except the European region (11%).
In most regions, H. pylori also accrued substantially more antimicrobial resistance over time, the investigators said. Clarithromycin resistance rose from 13% during 2006 through 2008 to 21% during 2012 through 2016 (P less than .001). Levofloxacin resistance in the Western Pacific region increased from 12% to 31% during the same two time periods (P less than .001). Several other WHO regions showed less significant trends toward increasing resistance. Multidrug resistance also rose. Resistance to both clarithromycin and metronidazole increased markedly in all WHO areas with available data, reaching 14% in the Eastern Mediterranean and Western Pacific regions and 23% in the European region.
Secondary analyses linked resistance with dramatic increases in the odds of treatment failure. For example, clarithromycin resistance conferred a sevenfold increase in the odds of treatment failure for regimens containing clarithromycin (odds ratio, 7.0; 95% CI, 5.2 to 9.3; P less than .001). Corresponding ORs were 8.2 for levofloxacin resistance, 2.5 for metronidazole resistance, and 9.4 for dual clarithromycin-metronidazole resistance.
The investigators acknowledged several limitations. Of publications in this meta-analysis, 85% represented single-center studies with limited sample sizes, they wrote. Studies often excluded demographic and endoscopic details. Furthermore, only three studies provided prevalence data for the WHO Africa Region and these only provided overall estimates without stratifying by resistance type.
The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
SOURCE: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007.
Over the past decade, Helicobacter pylori strains have reached “alarming levels” of antimicrobial resistance worldwide, investigators reported in the November issue of Gastroenterology.
In a large meta-analysis spanning 2007-2017, H. pylori isolates showed a 15% or higher pooled prevalence of primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin in almost all World Health Organization (WHO) regions. “Local surveillance networks are required to select appropriate eradication regimens for each region,” concluded Alessia Savoldi, MD, of the University of Tübingen (Germany) and her associates.
Typically, the threshold of antimicrobial resistance for choosing empiric regimens is 15%, Dr. Savoldi and her associates noted. Their systematic review and meta-analysis included 178 studies comprising 66,142 isolates from 65 countries. They defined H. pylori infection as a positive histology, serology, stool antigen, urea breath test, or rapid urease test. They excluded studies of fewer than 50 isolates, studies that only reported resistance as a percentage with no denominator, studies that failed to specify time frames or clustered data over more than 3 years, and data reported in guidelines, conference presentations, or letters without formal publication.
The prevalence of primary clarithromycin resistance exceeded 15% in the WHO European Region (18%; 95% confidence interval, 16%-20%), the Eastern Mediterranean Region (33%), and the Western Pacific Region (34%) and reached 10% in the Americas and the South East Asia region. Furthermore, primary resistance to metronidazole exceeded 15% in all WHO regions, ranging from 56% in the Eastern Mediterranean Region to 23% in the Americas. Resistance to levofloxacin was at least 15% in all WHO regions except the European region (11%).
In most regions, H. pylori also accrued substantially more antimicrobial resistance over time, the investigators said. Clarithromycin resistance rose from 13% during 2006 through 2008 to 21% during 2012 through 2016 (P less than .001). Levofloxacin resistance in the Western Pacific region increased from 12% to 31% during the same two time periods (P less than .001). Several other WHO regions showed less significant trends toward increasing resistance. Multidrug resistance also rose. Resistance to both clarithromycin and metronidazole increased markedly in all WHO areas with available data, reaching 14% in the Eastern Mediterranean and Western Pacific regions and 23% in the European region.
Secondary analyses linked resistance with dramatic increases in the odds of treatment failure. For example, clarithromycin resistance conferred a sevenfold increase in the odds of treatment failure for regimens containing clarithromycin (odds ratio, 7.0; 95% CI, 5.2 to 9.3; P less than .001). Corresponding ORs were 8.2 for levofloxacin resistance, 2.5 for metronidazole resistance, and 9.4 for dual clarithromycin-metronidazole resistance.
The investigators acknowledged several limitations. Of publications in this meta-analysis, 85% represented single-center studies with limited sample sizes, they wrote. Studies often excluded demographic and endoscopic details. Furthermore, only three studies provided prevalence data for the WHO Africa Region and these only provided overall estimates without stratifying by resistance type.
The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
SOURCE: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007.
FROM GASTROENTEROLOGY
Key clinical point: Helicobacter pylori now shows significant levels of antibiotic resistance worldwide, complicating choices of empiric therapy.
Major finding: Primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin was 15% or more in all WHO regions except for primary clarithromycin resistance in the Americas (10%) and South East Asia (10%) and primary levofloxacin resistance in Europe (11%).
Study details: Meta-analysis of 178 studies comprising 66,142 isolates from 65 countries.
Disclosures: The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
Source: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007
Thiopurines linked to zoster in IBD patients
For patients with inflammatory bowel disease (IBD), thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid (5-ASA) monotherapy, according to the results of two large retrospective cohort studies.
In the multivariable analysis, thiopurine monotherapy was linked to about a 47% increase in the risk of herpes zoster, compared with 5-ASA monotherapy (adjusted hazard ratio, 1.47; 95% confidence interval, 1.31-1.65; P less than .001). Combination therapy with thiopurines and tumor necrosis factor antagonists conferred about a 65% increase in zoster risk (aHR, 1.65; 95% CI, 1.22-2.23; P = .001). However, tumor necrosis factor–antagonist monotherapy did not appear to significantly increase the risk of zoster when compared with 5-ASA monotherapy, reported Nabeel Khan, MD, of the University of Pennsylvania in Philadelphia, and his associates.
“Compared to [patients without] IBD, ulcerative colitis (UC) and Crohn’s disease (CD) each were associated with significantly increased risk of herpes zoster infection,” the researchers wrote online in Clinical Gastroenterology and Hepatology. “With the approval of a new and potentially safer vaccine for herpes zoster, the effects of immunization of patients with IBD should be investigated.”
Past studies have linked IBD with a 1.2- to 1.8-fold increase in the risk of zoster, but these studies date to the prebiologic era or excluded patients who were in their midsixties or older, the researchers wrote. “Additionally, these prior studies have not assessed the validity of the codes used to identify herpes zoster and also did not account for the impact of vaccination,” they added. “They also did not take into consideration the severity of the disease or degree of steroid exposure.”
Therefore, the researchers conducted two retrospective cohort studies of patients in the United States Department of Veterans Affairs between 2000 and 2016. The first cohort study compared the incidence of herpes zoster among patients with IBD who received 5-ASA alone with matched patients without IBD. The second cohort study measured the incidence of herpes zoster in patients with IBD who received various medications and combination regimen. “The VA has a predominantly older population, which makes it an ideal cohort to study herpes zoster incidence in a high-risk population,” the investigators noted. “Unlike insurance databases, the VA database can be validated internally and vaccination records are documented.”
After adjusting for age, race, sex, geographic region, disease flare, corticosteroid use, and baseline comorbidities, the estimated hazard of developing herpes zoster was 1.81 (95% confidence interval, 1.56-2.11) among patients with ulcerative colitis and 1.56 (95% CI, 1.28-1.91) among patients with Crohn’s disease, as compared with patients without IBD. Regardless of their age or the medications they were receiving, patients with IBD had a higher incidence of zoster than the oldest group of patients without IBD (older than 60 years), regardless of age or medication. “The highest risk of herpes zoster was observed in patients with IBD who were less than 60 years of age and on combination therapy,” the investigators wrote. “Patients with IBD younger than 50 years who were on combination therapy had higher risk of herpes zoster, compared with patients with IBD older than 60 years of age who were not on immunosuppressive therapy.” Based on the findings, they recommended studying the efficacy of widespread use of the new herpes zoster vaccine in patients with IBD.
Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
Patients with inflammatory bowel disease are thought to have altered immune regulation, which may increase the risk of systemic complications including infections like herpes zoster. Many of the prior studies assessing the risk of herpes zoster in IBD patients were done before the advent of biologics and excluded older patients, thereby limiting their utility. This study by Khan et al. aimed to better estimate the incidence and risk factors for development of herpes zoster and to determine the effect of immunosuppressant use on this risk. In two large, retrospective cohort studies they found that, compared with patients without IBD, patients with IBD had a significantly increased risk of developing herpes zoster. Furthermore, this risk was higher in those with recent or cumulative steroid use and in those treated with thiopurines (as monotherapy or in combination with anti-TNF agents). Interestingly, exposure to TNF antagonists alone was not associated with an increased risk of herpes zoster infection.
Richa Shukla, MD, assistant professor, section of gastroenterology and hepatology, Baylor College of Medicine, Houston.
Patients with inflammatory bowel disease are thought to have altered immune regulation, which may increase the risk of systemic complications including infections like herpes zoster. Many of the prior studies assessing the risk of herpes zoster in IBD patients were done before the advent of biologics and excluded older patients, thereby limiting their utility. This study by Khan et al. aimed to better estimate the incidence and risk factors for development of herpes zoster and to determine the effect of immunosuppressant use on this risk. In two large, retrospective cohort studies they found that, compared with patients without IBD, patients with IBD had a significantly increased risk of developing herpes zoster. Furthermore, this risk was higher in those with recent or cumulative steroid use and in those treated with thiopurines (as monotherapy or in combination with anti-TNF agents). Interestingly, exposure to TNF antagonists alone was not associated with an increased risk of herpes zoster infection.
Richa Shukla, MD, assistant professor, section of gastroenterology and hepatology, Baylor College of Medicine, Houston.
Patients with inflammatory bowel disease are thought to have altered immune regulation, which may increase the risk of systemic complications including infections like herpes zoster. Many of the prior studies assessing the risk of herpes zoster in IBD patients were done before the advent of biologics and excluded older patients, thereby limiting their utility. This study by Khan et al. aimed to better estimate the incidence and risk factors for development of herpes zoster and to determine the effect of immunosuppressant use on this risk. In two large, retrospective cohort studies they found that, compared with patients without IBD, patients with IBD had a significantly increased risk of developing herpes zoster. Furthermore, this risk was higher in those with recent or cumulative steroid use and in those treated with thiopurines (as monotherapy or in combination with anti-TNF agents). Interestingly, exposure to TNF antagonists alone was not associated with an increased risk of herpes zoster infection.
Richa Shukla, MD, assistant professor, section of gastroenterology and hepatology, Baylor College of Medicine, Houston.
For patients with inflammatory bowel disease (IBD), thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid (5-ASA) monotherapy, according to the results of two large retrospective cohort studies.
In the multivariable analysis, thiopurine monotherapy was linked to about a 47% increase in the risk of herpes zoster, compared with 5-ASA monotherapy (adjusted hazard ratio, 1.47; 95% confidence interval, 1.31-1.65; P less than .001). Combination therapy with thiopurines and tumor necrosis factor antagonists conferred about a 65% increase in zoster risk (aHR, 1.65; 95% CI, 1.22-2.23; P = .001). However, tumor necrosis factor–antagonist monotherapy did not appear to significantly increase the risk of zoster when compared with 5-ASA monotherapy, reported Nabeel Khan, MD, of the University of Pennsylvania in Philadelphia, and his associates.
“Compared to [patients without] IBD, ulcerative colitis (UC) and Crohn’s disease (CD) each were associated with significantly increased risk of herpes zoster infection,” the researchers wrote online in Clinical Gastroenterology and Hepatology. “With the approval of a new and potentially safer vaccine for herpes zoster, the effects of immunization of patients with IBD should be investigated.”
Past studies have linked IBD with a 1.2- to 1.8-fold increase in the risk of zoster, but these studies date to the prebiologic era or excluded patients who were in their midsixties or older, the researchers wrote. “Additionally, these prior studies have not assessed the validity of the codes used to identify herpes zoster and also did not account for the impact of vaccination,” they added. “They also did not take into consideration the severity of the disease or degree of steroid exposure.”
Therefore, the researchers conducted two retrospective cohort studies of patients in the United States Department of Veterans Affairs between 2000 and 2016. The first cohort study compared the incidence of herpes zoster among patients with IBD who received 5-ASA alone with matched patients without IBD. The second cohort study measured the incidence of herpes zoster in patients with IBD who received various medications and combination regimen. “The VA has a predominantly older population, which makes it an ideal cohort to study herpes zoster incidence in a high-risk population,” the investigators noted. “Unlike insurance databases, the VA database can be validated internally and vaccination records are documented.”
After adjusting for age, race, sex, geographic region, disease flare, corticosteroid use, and baseline comorbidities, the estimated hazard of developing herpes zoster was 1.81 (95% confidence interval, 1.56-2.11) among patients with ulcerative colitis and 1.56 (95% CI, 1.28-1.91) among patients with Crohn’s disease, as compared with patients without IBD. Regardless of their age or the medications they were receiving, patients with IBD had a higher incidence of zoster than the oldest group of patients without IBD (older than 60 years), regardless of age or medication. “The highest risk of herpes zoster was observed in patients with IBD who were less than 60 years of age and on combination therapy,” the investigators wrote. “Patients with IBD younger than 50 years who were on combination therapy had higher risk of herpes zoster, compared with patients with IBD older than 60 years of age who were not on immunosuppressive therapy.” Based on the findings, they recommended studying the efficacy of widespread use of the new herpes zoster vaccine in patients with IBD.
Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
For patients with inflammatory bowel disease (IBD), thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid (5-ASA) monotherapy, according to the results of two large retrospective cohort studies.
In the multivariable analysis, thiopurine monotherapy was linked to about a 47% increase in the risk of herpes zoster, compared with 5-ASA monotherapy (adjusted hazard ratio, 1.47; 95% confidence interval, 1.31-1.65; P less than .001). Combination therapy with thiopurines and tumor necrosis factor antagonists conferred about a 65% increase in zoster risk (aHR, 1.65; 95% CI, 1.22-2.23; P = .001). However, tumor necrosis factor–antagonist monotherapy did not appear to significantly increase the risk of zoster when compared with 5-ASA monotherapy, reported Nabeel Khan, MD, of the University of Pennsylvania in Philadelphia, and his associates.
“Compared to [patients without] IBD, ulcerative colitis (UC) and Crohn’s disease (CD) each were associated with significantly increased risk of herpes zoster infection,” the researchers wrote online in Clinical Gastroenterology and Hepatology. “With the approval of a new and potentially safer vaccine for herpes zoster, the effects of immunization of patients with IBD should be investigated.”
Past studies have linked IBD with a 1.2- to 1.8-fold increase in the risk of zoster, but these studies date to the prebiologic era or excluded patients who were in their midsixties or older, the researchers wrote. “Additionally, these prior studies have not assessed the validity of the codes used to identify herpes zoster and also did not account for the impact of vaccination,” they added. “They also did not take into consideration the severity of the disease or degree of steroid exposure.”
Therefore, the researchers conducted two retrospective cohort studies of patients in the United States Department of Veterans Affairs between 2000 and 2016. The first cohort study compared the incidence of herpes zoster among patients with IBD who received 5-ASA alone with matched patients without IBD. The second cohort study measured the incidence of herpes zoster in patients with IBD who received various medications and combination regimen. “The VA has a predominantly older population, which makes it an ideal cohort to study herpes zoster incidence in a high-risk population,” the investigators noted. “Unlike insurance databases, the VA database can be validated internally and vaccination records are documented.”
After adjusting for age, race, sex, geographic region, disease flare, corticosteroid use, and baseline comorbidities, the estimated hazard of developing herpes zoster was 1.81 (95% confidence interval, 1.56-2.11) among patients with ulcerative colitis and 1.56 (95% CI, 1.28-1.91) among patients with Crohn’s disease, as compared with patients without IBD. Regardless of their age or the medications they were receiving, patients with IBD had a higher incidence of zoster than the oldest group of patients without IBD (older than 60 years), regardless of age or medication. “The highest risk of herpes zoster was observed in patients with IBD who were less than 60 years of age and on combination therapy,” the investigators wrote. “Patients with IBD younger than 50 years who were on combination therapy had higher risk of herpes zoster, compared with patients with IBD older than 60 years of age who were not on immunosuppressive therapy.” Based on the findings, they recommended studying the efficacy of widespread use of the new herpes zoster vaccine in patients with IBD.
Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: For patients with inflammatory bowel disease, thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid monotherapy.
Major finding: The adjusted hazard ratio was 1.47 (95% confidence interval, 1.31-1.65; P less than .001).
Study details: Two large retrospective cohort studies of veterans with and without inflammatory bowel disease.
Disclosures: Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
Source: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
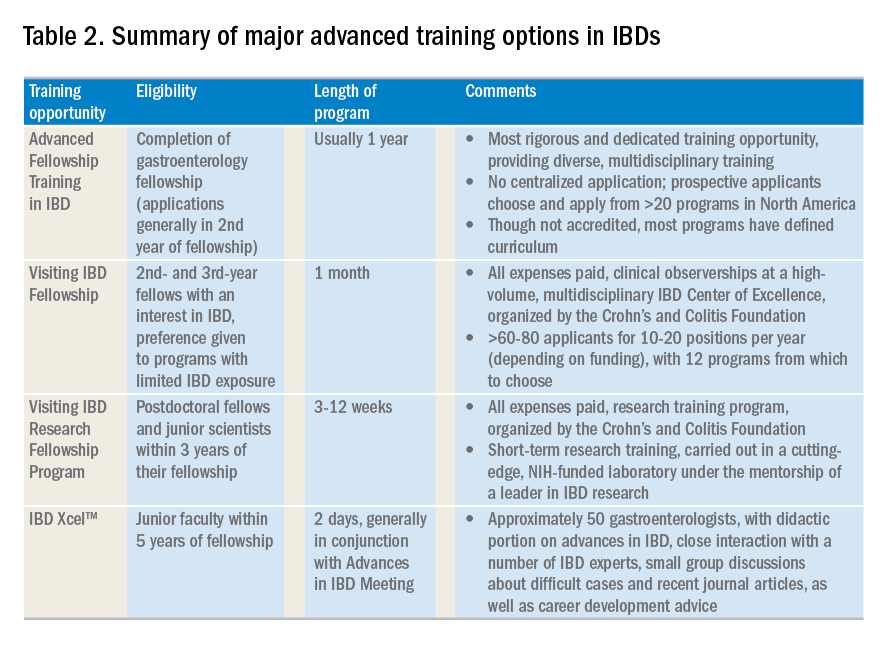
Advanced training options in inflammatory bowel disease
The global incidence and prevalence of inflammatory bowel disease (IBD) is rising, and it is estimated that by 2025, approximately 2.2 million Americans will be living with this disease. At the same time, there have been several paradigm-changing scientific and medical advances in the understanding and management of IBD. As the diagnostic, therapeutic, and monitoring armamentarium in the management of IBD increases, so is the complexity of the decision making. Advanced concepts and training are often not covered adequately during a general gastroenterology fellowship. In a survey of 160 trainees, more than one-third of fellows did not feel “confident” or “mostly comfortable” with their level of IBD training. Yet, efficient dissemination, effective translation and integration of these advances into clinical practice is paramount to improving quality of care. To facilitate multiple goals as listed in Table 1, advanced training in the field of IBD is increasingly important. In this article, I review different training options available for young gastroenterologists.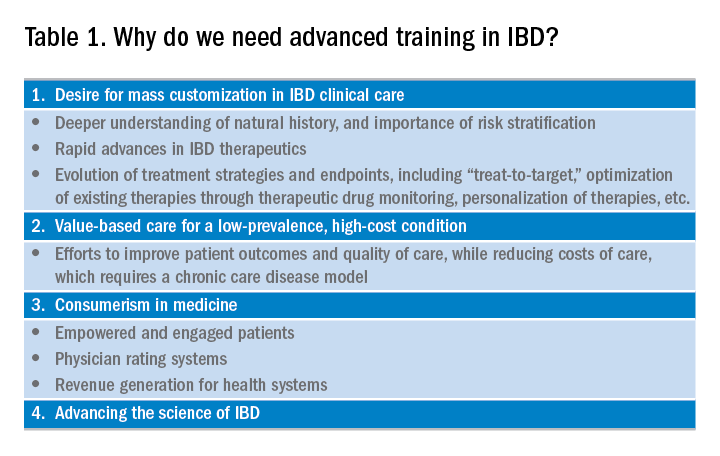
Readers are also directed to an excellent article by David Rubin, MD, published in Gastroenterology in 2015.
Advanced fellowship training in IBD
The most rigorous training in IBD is offered through dedicated advanced fellowships. Currently, there are more than 20 such fellowships in North America, most of them offered at large academic centers with nationally and internationally renowned faculty. These training positions are generally 1 year long, offered after completion of gastroenterology fellowship. The Accreditation Council of the Graduate Medical Education (ACGME) does not accredit these advanced training programs, and there is not a separate American Board of Internal Medicine (ABIM) certification for IBD. Funding of such programs comes from different sources including endowments, private foundations, institutional funds, pharmaceutical company grants, and even limited faculty appointments of the trainees. Though there is currently no official regulatory oversight and requirements, most programs have well-defined curricula covering diverse aspects of IBD care. This core curriculum has been nicely summarized in a recent article by Uma Mahadevan, MD, in Gastroenterology.
Clinical training in these programs is offered through a mix of outpatient IBD clinics (generally three to five clinics/week, with one or more senior IBD-focused faculty member), supervising general gastroenterology fellows for inpatient IBD care, dedicated IBD-focused endoscopy sessions (generally one or two sessions/week) including chromoendoscopy and stricture dilation, as well as formal and informal mentorship by one or more senior faculty members, time and mentorship for scholarly activities, and appropriate evaluation and feedback systems. In addition, most programs offer multidisciplinary training through dedicated clinics with colorectal surgeons (such as pouch clinics, etc.), opportunities for observing and interacting with radiologists, pathologists, psychologists, and dietitians.
There is no centralized application process and prospective applicants should reach out to their program directors and mentors regarding guidance, as well as program directors of specific training programs to learn more about these programs, generally in the second half of their gastroenterology training. The Crohn’s and Colitis Foundation maintains a list of fellowship training programs and appropriate contacts. In choosing a specific program, prospective fellows should consider the rigor and diversity of training, balance between service and scholarship, mentorship opportunities as well as the experience and outcomes of previous fellows in the program. Besides formal interviews at prospective program, fellows should utilize the networking opportunities afforded through the American Gastroenterological Association (both with senior faculty as well as through the Trainee and Early-Career Committee), the Crohn’s and Colitis Foundation as well as other organizations in learning more about programs.
Visiting IBD Fellow Program: Clinical observership, through the Crohn’s and Colitis Foundation
The Visiting IBD Fellow Program – with the support of the Crohn’s and Colitis Foundation – which launched in 2006, arose from the need for immersive training in IBD, especially for fellows for whom IBD exposure may be limited. In this 1-month “observership,” interested 2nd and 3rd year fellows get the opportunity to observe faculty at a high-volume, multidisciplinary IBD Center of Excellence. Besides providing additional knowledge and expertise in the field, this also allows fellows the chance to understand how IBD Centers are set up, so they may seek to replicate similar models as local or regional IBD experts. Currently, 12 centers participate in this program. There is no cost to the fellows who are selected to participate, and all travel expenses and lodging are covered. The program significantly improved the fellows’ knowledge, skills, and attitudes toward IBD and has steadily gained in popularity, with more than 60-80 applicants for 10-20 positions per year (depending on funding). In addition to the clinical exposure, this experience also facilitates networking with faculty and other fellows at participating institutions. Full details of this program can be accessed from the Crohn’s and Colitis Foundation website.
A similar, expenses-paid, abbreviated 3-day program of IBD preceptorship has been launched for advanced practice providers (qualified advanced-practice nurses, nurse practitioners, and physician assistants). This program provides preceptee exposure to medical, surgical, outpatient, and inpatient experiences with patients at a leading academic IBD center.
Visiting IBD Research Fellowship Program, through the Crohn’s and Colitis Foundation
The Crohn’s and Colitis Foundation recently launched a new, short-term, mentored research initiative designed to promote career advancement for talented junior investigators dedicated to IBD research, and to enable knowledge-sharing among leaders in the IBD field. The Foundation encourages outstanding young scientists (postdoctoral studies in the first 3 years of their fellowship), who would like to expand their expertise in IBD research to participate in this short-term research training, carried out in a cutting-edge, NIH-funded laboratory under the mentorship of a leader in IBD research. This all-expense covered 3-12 week rotation provides mentorship and technical training in a state-of-the-art research lab relevant to IBD, with an emphasis on preclinical research most closely relevant to human disease. Details of the program can be found on the Crohn’s and Colitis Foundation website.
IBD Xcel
In 2013, Cornerstones Health, a nonprofit medical education organization, launched a 2-day program dedicated to advances in the field of IBD for junior gastroenterologists within 5 years of completion of their fellowship training. The program includes a didactic component as well as close interaction with a number of IBD experts, small-group discussions about difficult cases, and recent journal articles, as well as career-development advice. The education component is free of cost to selected participants, though travel and housing expenses are not covered.
Besides these dedicated advanced training opportunities, there are major conferences that cover IBD extensively and exclusively. These include the annual Crohn’s and Colitis Congress® conducted jointly by the Crohn’s and Colitis Foundation and the American Gastroenterological Association, the annual Advances in Inflammatory Bowel Diseases through Imedex, the annual European Crohn’s and Colitis Congress, the American College of Gastroenterology’s IBD School, as well as several regional courses conducted throughout the country. In terms of networking opportunities for gastroenterology fellows interested in IBD and junior faculty, REACH-IBD (Rising Educators, Academicians and Clinicians Helping Inflammatory Bowel Disease), founded under the auspices of the Crohn’s and Colitis Foundation in 2013, provides a unique resource. This group is open to all clinical fellows, postdoctoral scientists, and junior faculty (pediatric and adult; medical and surgical specialties, as well as PhDs) less than 7 years out of training with a rank not higher than assistant professor. The mission is to facilitate networking and career development for clinical fellows, postdoctoral scientists, and junior faculty with an interest in IBD; increase active participation of our members in the clinical, educational, scientific, and research programs within the Crohn’s and Colitis Foundation; and foster collaborative research among our members within the Foundation. The group organizes specific breakout events at the Digestive Disease Week® and the annual Crohn’s and Colitis Congress, covering diverse topics such as setting up an IBD practice, funding opportunities, paper and grant writing, career advancement guidance. More information on this can be found on the Crohn’s and Colitis Foundation website.
To summarize, there are numerous opportunities of varying lengths to receive training in inflammatory bowel diseases. This exciting field is expanding at a rapid pace, and instead of limiting management to dedicated IBD Centers of Excellence, there is clear need for effective dissemination of new management approaches and incorporation of quality measures will likely raise the bar for all patients and physicians who care for them.
AGA offers IBD education
Check out AGA’s on-demand IBD education available in AGA University.
Dr. Singh is assistant professor of medicine, division of gastroenterology, University of California, San Diego. He is supported by the American College of Gastroenterology and Crohn’s and Colitis Foundation, has received research grants from Pfizer and AbbVie, and consulting fees from AbbVie, Takeda, and AMAG Pharmaceuticals.
The global incidence and prevalence of inflammatory bowel disease (IBD) is rising, and it is estimated that by 2025, approximately 2.2 million Americans will be living with this disease. At the same time, there have been several paradigm-changing scientific and medical advances in the understanding and management of IBD. As the diagnostic, therapeutic, and monitoring armamentarium in the management of IBD increases, so is the complexity of the decision making. Advanced concepts and training are often not covered adequately during a general gastroenterology fellowship. In a survey of 160 trainees, more than one-third of fellows did not feel “confident” or “mostly comfortable” with their level of IBD training. Yet, efficient dissemination, effective translation and integration of these advances into clinical practice is paramount to improving quality of care. To facilitate multiple goals as listed in Table 1, advanced training in the field of IBD is increasingly important. In this article, I review different training options available for young gastroenterologists.
Readers are also directed to an excellent article by David Rubin, MD, published in Gastroenterology in 2015.
Advanced fellowship training in IBD
The most rigorous training in IBD is offered through dedicated advanced fellowships. Currently, there are more than 20 such fellowships in North America, most of them offered at large academic centers with nationally and internationally renowned faculty. These training positions are generally 1 year long, offered after completion of gastroenterology fellowship. The Accreditation Council of the Graduate Medical Education (ACGME) does not accredit these advanced training programs, and there is not a separate American Board of Internal Medicine (ABIM) certification for IBD. Funding of such programs comes from different sources including endowments, private foundations, institutional funds, pharmaceutical company grants, and even limited faculty appointments of the trainees. Though there is currently no official regulatory oversight and requirements, most programs have well-defined curricula covering diverse aspects of IBD care. This core curriculum has been nicely summarized in a recent article by Uma Mahadevan, MD, in Gastroenterology.
Clinical training in these programs is offered through a mix of outpatient IBD clinics (generally three to five clinics/week, with one or more senior IBD-focused faculty member), supervising general gastroenterology fellows for inpatient IBD care, dedicated IBD-focused endoscopy sessions (generally one or two sessions/week) including chromoendoscopy and stricture dilation, as well as formal and informal mentorship by one or more senior faculty members, time and mentorship for scholarly activities, and appropriate evaluation and feedback systems. In addition, most programs offer multidisciplinary training through dedicated clinics with colorectal surgeons (such as pouch clinics, etc.), opportunities for observing and interacting with radiologists, pathologists, psychologists, and dietitians.
There is no centralized application process and prospective applicants should reach out to their program directors and mentors regarding guidance, as well as program directors of specific training programs to learn more about these programs, generally in the second half of their gastroenterology training. The Crohn’s and Colitis Foundation maintains a list of fellowship training programs and appropriate contacts. In choosing a specific program, prospective fellows should consider the rigor and diversity of training, balance between service and scholarship, mentorship opportunities as well as the experience and outcomes of previous fellows in the program. Besides formal interviews at prospective program, fellows should utilize the networking opportunities afforded through the American Gastroenterological Association (both with senior faculty as well as through the Trainee and Early-Career Committee), the Crohn’s and Colitis Foundation as well as other organizations in learning more about programs.

Visiting IBD Fellow Program: Clinical observership, through the Crohn’s and Colitis Foundation
The Visiting IBD Fellow Program – with the support of the Crohn’s and Colitis Foundation – which launched in 2006, arose from the need for immersive training in IBD, especially for fellows for whom IBD exposure may be limited. In this 1-month “observership,” interested 2nd and 3rd year fellows get the opportunity to observe faculty at a high-volume, multidisciplinary IBD Center of Excellence. Besides providing additional knowledge and expertise in the field, this also allows fellows the chance to understand how IBD Centers are set up, so they may seek to replicate similar models as local or regional IBD experts. Currently, 12 centers participate in this program. There is no cost to the fellows who are selected to participate, and all travel expenses and lodging are covered. The program significantly improved the fellows’ knowledge, skills, and attitudes toward IBD and has steadily gained in popularity, with more than 60-80 applicants for 10-20 positions per year (depending on funding). In addition to the clinical exposure, this experience also facilitates networking with faculty and other fellows at participating institutions. Full details of this program can be accessed from the Crohn’s and Colitis Foundation website.
A similar, expenses-paid, abbreviated 3-day program of IBD preceptorship has been launched for advanced practice providers (qualified advanced-practice nurses, nurse practitioners, and physician assistants). This program provides preceptee exposure to medical, surgical, outpatient, and inpatient experiences with patients at a leading academic IBD center.
Visiting IBD Research Fellowship Program, through the Crohn’s and Colitis Foundation
The Crohn’s and Colitis Foundation recently launched a new, short-term, mentored research initiative designed to promote career advancement for talented junior investigators dedicated to IBD research, and to enable knowledge-sharing among leaders in the IBD field. The Foundation encourages outstanding young scientists (postdoctoral studies in the first 3 years of their fellowship), who would like to expand their expertise in IBD research to participate in this short-term research training, carried out in a cutting-edge, NIH-funded laboratory under the mentorship of a leader in IBD research. This all-expense covered 3-12 week rotation provides mentorship and technical training in a state-of-the-art research lab relevant to IBD, with an emphasis on preclinical research most closely relevant to human disease. Details of the program can be found on the Crohn’s and Colitis Foundation website.
IBD Xcel
In 2013, Cornerstones Health, a nonprofit medical education organization, launched a 2-day program dedicated to advances in the field of IBD for junior gastroenterologists within 5 years of completion of their fellowship training. The program includes a didactic component as well as close interaction with a number of IBD experts, small-group discussions about difficult cases, and recent journal articles, as well as career-development advice. The education component is free of cost to selected participants, though travel and housing expenses are not covered.
Besides these dedicated advanced training opportunities, there are major conferences that cover IBD extensively and exclusively. These include the annual Crohn’s and Colitis Congress® conducted jointly by the Crohn’s and Colitis Foundation and the American Gastroenterological Association, the annual Advances in Inflammatory Bowel Diseases through Imedex, the annual European Crohn’s and Colitis Congress, the American College of Gastroenterology’s IBD School, as well as several regional courses conducted throughout the country. In terms of networking opportunities for gastroenterology fellows interested in IBD and junior faculty, REACH-IBD (Rising Educators, Academicians and Clinicians Helping Inflammatory Bowel Disease), founded under the auspices of the Crohn’s and Colitis Foundation in 2013, provides a unique resource. This group is open to all clinical fellows, postdoctoral scientists, and junior faculty (pediatric and adult; medical and surgical specialties, as well as PhDs) less than 7 years out of training with a rank not higher than assistant professor. The mission is to facilitate networking and career development for clinical fellows, postdoctoral scientists, and junior faculty with an interest in IBD; increase active participation of our members in the clinical, educational, scientific, and research programs within the Crohn’s and Colitis Foundation; and foster collaborative research among our members within the Foundation. The group organizes specific breakout events at the Digestive Disease Week® and the annual Crohn’s and Colitis Congress, covering diverse topics such as setting up an IBD practice, funding opportunities, paper and grant writing, career advancement guidance. More information on this can be found on the Crohn’s and Colitis Foundation website.
To summarize, there are numerous opportunities of varying lengths to receive training in inflammatory bowel diseases. This exciting field is expanding at a rapid pace, and instead of limiting management to dedicated IBD Centers of Excellence, there is clear need for effective dissemination of new management approaches and incorporation of quality measures will likely raise the bar for all patients and physicians who care for them.
AGA offers IBD education
Check out AGA’s on-demand IBD education available in AGA University.
Dr. Singh is assistant professor of medicine, division of gastroenterology, University of California, San Diego. He is supported by the American College of Gastroenterology and Crohn’s and Colitis Foundation, has received research grants from Pfizer and AbbVie, and consulting fees from AbbVie, Takeda, and AMAG Pharmaceuticals.
The global incidence and prevalence of inflammatory bowel disease (IBD) is rising, and it is estimated that by 2025, approximately 2.2 million Americans will be living with this disease. At the same time, there have been several paradigm-changing scientific and medical advances in the understanding and management of IBD. As the diagnostic, therapeutic, and monitoring armamentarium in the management of IBD increases, so is the complexity of the decision making. Advanced concepts and training are often not covered adequately during a general gastroenterology fellowship. In a survey of 160 trainees, more than one-third of fellows did not feel “confident” or “mostly comfortable” with their level of IBD training. Yet, efficient dissemination, effective translation and integration of these advances into clinical practice is paramount to improving quality of care. To facilitate multiple goals as listed in Table 1, advanced training in the field of IBD is increasingly important. In this article, I review different training options available for young gastroenterologists.
Readers are also directed to an excellent article by David Rubin, MD, published in Gastroenterology in 2015.
Advanced fellowship training in IBD
The most rigorous training in IBD is offered through dedicated advanced fellowships. Currently, there are more than 20 such fellowships in North America, most of them offered at large academic centers with nationally and internationally renowned faculty. These training positions are generally 1 year long, offered after completion of gastroenterology fellowship. The Accreditation Council of the Graduate Medical Education (ACGME) does not accredit these advanced training programs, and there is not a separate American Board of Internal Medicine (ABIM) certification for IBD. Funding of such programs comes from different sources including endowments, private foundations, institutional funds, pharmaceutical company grants, and even limited faculty appointments of the trainees. Though there is currently no official regulatory oversight and requirements, most programs have well-defined curricula covering diverse aspects of IBD care. This core curriculum has been nicely summarized in a recent article by Uma Mahadevan, MD, in Gastroenterology.
Clinical training in these programs is offered through a mix of outpatient IBD clinics (generally three to five clinics/week, with one or more senior IBD-focused faculty member), supervising general gastroenterology fellows for inpatient IBD care, dedicated IBD-focused endoscopy sessions (generally one or two sessions/week) including chromoendoscopy and stricture dilation, as well as formal and informal mentorship by one or more senior faculty members, time and mentorship for scholarly activities, and appropriate evaluation and feedback systems. In addition, most programs offer multidisciplinary training through dedicated clinics with colorectal surgeons (such as pouch clinics, etc.), opportunities for observing and interacting with radiologists, pathologists, psychologists, and dietitians.
There is no centralized application process and prospective applicants should reach out to their program directors and mentors regarding guidance, as well as program directors of specific training programs to learn more about these programs, generally in the second half of their gastroenterology training. The Crohn’s and Colitis Foundation maintains a list of fellowship training programs and appropriate contacts. In choosing a specific program, prospective fellows should consider the rigor and diversity of training, balance between service and scholarship, mentorship opportunities as well as the experience and outcomes of previous fellows in the program. Besides formal interviews at prospective program, fellows should utilize the networking opportunities afforded through the American Gastroenterological Association (both with senior faculty as well as through the Trainee and Early-Career Committee), the Crohn’s and Colitis Foundation as well as other organizations in learning more about programs.

Visiting IBD Fellow Program: Clinical observership, through the Crohn’s and Colitis Foundation
The Visiting IBD Fellow Program – with the support of the Crohn’s and Colitis Foundation – which launched in 2006, arose from the need for immersive training in IBD, especially for fellows for whom IBD exposure may be limited. In this 1-month “observership,” interested 2nd and 3rd year fellows get the opportunity to observe faculty at a high-volume, multidisciplinary IBD Center of Excellence. Besides providing additional knowledge and expertise in the field, this also allows fellows the chance to understand how IBD Centers are set up, so they may seek to replicate similar models as local or regional IBD experts. Currently, 12 centers participate in this program. There is no cost to the fellows who are selected to participate, and all travel expenses and lodging are covered. The program significantly improved the fellows’ knowledge, skills, and attitudes toward IBD and has steadily gained in popularity, with more than 60-80 applicants for 10-20 positions per year (depending on funding). In addition to the clinical exposure, this experience also facilitates networking with faculty and other fellows at participating institutions. Full details of this program can be accessed from the Crohn’s and Colitis Foundation website.
A similar, expenses-paid, abbreviated 3-day program of IBD preceptorship has been launched for advanced practice providers (qualified advanced-practice nurses, nurse practitioners, and physician assistants). This program provides preceptee exposure to medical, surgical, outpatient, and inpatient experiences with patients at a leading academic IBD center.
Visiting IBD Research Fellowship Program, through the Crohn’s and Colitis Foundation
The Crohn’s and Colitis Foundation recently launched a new, short-term, mentored research initiative designed to promote career advancement for talented junior investigators dedicated to IBD research, and to enable knowledge-sharing among leaders in the IBD field. The Foundation encourages outstanding young scientists (postdoctoral studies in the first 3 years of their fellowship), who would like to expand their expertise in IBD research to participate in this short-term research training, carried out in a cutting-edge, NIH-funded laboratory under the mentorship of a leader in IBD research. This all-expense covered 3-12 week rotation provides mentorship and technical training in a state-of-the-art research lab relevant to IBD, with an emphasis on preclinical research most closely relevant to human disease. Details of the program can be found on the Crohn’s and Colitis Foundation website.
IBD Xcel
In 2013, Cornerstones Health, a nonprofit medical education organization, launched a 2-day program dedicated to advances in the field of IBD for junior gastroenterologists within 5 years of completion of their fellowship training. The program includes a didactic component as well as close interaction with a number of IBD experts, small-group discussions about difficult cases, and recent journal articles, as well as career-development advice. The education component is free of cost to selected participants, though travel and housing expenses are not covered.
Besides these dedicated advanced training opportunities, there are major conferences that cover IBD extensively and exclusively. These include the annual Crohn’s and Colitis Congress® conducted jointly by the Crohn’s and Colitis Foundation and the American Gastroenterological Association, the annual Advances in Inflammatory Bowel Diseases through Imedex, the annual European Crohn’s and Colitis Congress, the American College of Gastroenterology’s IBD School, as well as several regional courses conducted throughout the country. In terms of networking opportunities for gastroenterology fellows interested in IBD and junior faculty, REACH-IBD (Rising Educators, Academicians and Clinicians Helping Inflammatory Bowel Disease), founded under the auspices of the Crohn’s and Colitis Foundation in 2013, provides a unique resource. This group is open to all clinical fellows, postdoctoral scientists, and junior faculty (pediatric and adult; medical and surgical specialties, as well as PhDs) less than 7 years out of training with a rank not higher than assistant professor. The mission is to facilitate networking and career development for clinical fellows, postdoctoral scientists, and junior faculty with an interest in IBD; increase active participation of our members in the clinical, educational, scientific, and research programs within the Crohn’s and Colitis Foundation; and foster collaborative research among our members within the Foundation. The group organizes specific breakout events at the Digestive Disease Week® and the annual Crohn’s and Colitis Congress, covering diverse topics such as setting up an IBD practice, funding opportunities, paper and grant writing, career advancement guidance. More information on this can be found on the Crohn’s and Colitis Foundation website.
To summarize, there are numerous opportunities of varying lengths to receive training in inflammatory bowel diseases. This exciting field is expanding at a rapid pace, and instead of limiting management to dedicated IBD Centers of Excellence, there is clear need for effective dissemination of new management approaches and incorporation of quality measures will likely raise the bar for all patients and physicians who care for them.
AGA offers IBD education
Check out AGA’s on-demand IBD education available in AGA University.
Dr. Singh is assistant professor of medicine, division of gastroenterology, University of California, San Diego. He is supported by the American College of Gastroenterology and Crohn’s and Colitis Foundation, has received research grants from Pfizer and AbbVie, and consulting fees from AbbVie, Takeda, and AMAG Pharmaceuticals.
Etrasimod improves clinical, endoscopic outcomes in patients with UC
PHILADELPHIA – Use of etrasimod was associated with improved clinical and endoscopic results, and was generally safe and well tolerated compared with placebo in patients with moderate to severe ulcerative colitis, according to a recent award-winning presentation at the annual meeting of the American College of Gastroenterology.
“Patients with moderate to severe ulcerative colitis receiving etrasimod 2 mg per day achieved statistically significant and clinically meaningful differences in all of the primary and secondary endpoints, and most exploratory endpoints were also significantly proved,” William J. Sandborn, MD, AGAF, FACG, professor of clinical medicine at the University of California, San Diego, stated in his presentation at the meeting. “A dose-response relationship was observed in virtually all of the measures of treatment efficacy.”
The abstract received the ACG Auxiliary Award (Member), which is given to ACG members each year with outstanding abstract submissions.
Dr. Sandborn and his colleagues enrolled 156 patients with ulcerative colitis (UC) into the OASIS study, a randomized, double-blind, parallel-group, phase 2 study of etrasimod, an oral sphingosine 1-phosphate (S1P) receptor modulator, compared with placebo. Patients were aged 18-80 years, with moderate to severe UC as defined by a three-component Mayo Clinic Score (MCS) comprising rectal bleeding, frequency of stool, and endoscopy.
Those patients who achieved an MCS score between 4 and 9 points with an endoscopic subscore of at least 2 and rectal bleeding (RB) subscore of at least 1 were included. Patients were divided into once-daily etrasimod 1 mg (52 patients), once-daily etrasimod 2 mg (50 patients) and placebo (54 patients) groups and treated over a 12-week period.
At 12 weeks, the least-squares mean difference for change in baseline in three-component MCS was 1.94 in the 1-mg etrasimod group and 2.49 in the 2-mg etrasimod group compared with placebo (1.50). Endoscopic improvement was greater in the 1-mg etrasimod (22.5%) and 2-mg (41.8%) groups compared with placebo (17.8%); endoscopic remission rates also improved in the 1-mg etrasimod (13.7%) and 2-mg (15.3%) groups compared with placebo (5.3%). Lymphocyte count circulation significantly decreased in the 1-mg etrasimod (37.2%) and 2-mg (57.3%) groups compared with the placebo group. With regard to rectal bleeding, the rectal bleeding subscore also decreased in the 1-mg etrasimod and 2-mg groups compared with placebo at 12 weeks from baseline.
The researchers noted no significant differences in adverse events among groups, with the placebo group showing a higher rate of major adverse events (11.1%) compared with the 1-mg etrasimod (5.8%) and 2-mg etrasimod (0%) groups.
“The OASIS trial results for etrasimod would support proceeding to a phase 3 program for this drug in patients with moderate to severe ulcerative colitis,” Dr. Sandborn concluded.
Dr. Sandborn reports consultancies, speaker bureau memberships, and research support from AbbVie, Biogen, Celgene, Ferring, Genentech, Gilead Sciences, Immune Pharmaceuticals, Janssen, Lilly, MedImmune, Novartis, Pfizer, Regeneron, Ritter Pharmaceuticals, Salix, Theradiag, UCB Pharma, and Vascular Biogenics, among others.
SOURCE: Sandborn WJ et al. ACG 2018. Presentation 11.
PHILADELPHIA – Use of etrasimod was associated with improved clinical and endoscopic results, and was generally safe and well tolerated compared with placebo in patients with moderate to severe ulcerative colitis, according to a recent award-winning presentation at the annual meeting of the American College of Gastroenterology.
“Patients with moderate to severe ulcerative colitis receiving etrasimod 2 mg per day achieved statistically significant and clinically meaningful differences in all of the primary and secondary endpoints, and most exploratory endpoints were also significantly proved,” William J. Sandborn, MD, AGAF, FACG, professor of clinical medicine at the University of California, San Diego, stated in his presentation at the meeting. “A dose-response relationship was observed in virtually all of the measures of treatment efficacy.”
The abstract received the ACG Auxiliary Award (Member), which is given to ACG members each year with outstanding abstract submissions.
Dr. Sandborn and his colleagues enrolled 156 patients with ulcerative colitis (UC) into the OASIS study, a randomized, double-blind, parallel-group, phase 2 study of etrasimod, an oral sphingosine 1-phosphate (S1P) receptor modulator, compared with placebo. Patients were aged 18-80 years, with moderate to severe UC as defined by a three-component Mayo Clinic Score (MCS) comprising rectal bleeding, frequency of stool, and endoscopy.
Those patients who achieved an MCS score between 4 and 9 points with an endoscopic subscore of at least 2 and rectal bleeding (RB) subscore of at least 1 were included. Patients were divided into once-daily etrasimod 1 mg (52 patients), once-daily etrasimod 2 mg (50 patients) and placebo (54 patients) groups and treated over a 12-week period.
At 12 weeks, the least-squares mean difference for change in baseline in three-component MCS was 1.94 in the 1-mg etrasimod group and 2.49 in the 2-mg etrasimod group compared with placebo (1.50). Endoscopic improvement was greater in the 1-mg etrasimod (22.5%) and 2-mg (41.8%) groups compared with placebo (17.8%); endoscopic remission rates also improved in the 1-mg etrasimod (13.7%) and 2-mg (15.3%) groups compared with placebo (5.3%). Lymphocyte count circulation significantly decreased in the 1-mg etrasimod (37.2%) and 2-mg (57.3%) groups compared with the placebo group. With regard to rectal bleeding, the rectal bleeding subscore also decreased in the 1-mg etrasimod and 2-mg groups compared with placebo at 12 weeks from baseline.
The researchers noted no significant differences in adverse events among groups, with the placebo group showing a higher rate of major adverse events (11.1%) compared with the 1-mg etrasimod (5.8%) and 2-mg etrasimod (0%) groups.
“The OASIS trial results for etrasimod would support proceeding to a phase 3 program for this drug in patients with moderate to severe ulcerative colitis,” Dr. Sandborn concluded.
Dr. Sandborn reports consultancies, speaker bureau memberships, and research support from AbbVie, Biogen, Celgene, Ferring, Genentech, Gilead Sciences, Immune Pharmaceuticals, Janssen, Lilly, MedImmune, Novartis, Pfizer, Regeneron, Ritter Pharmaceuticals, Salix, Theradiag, UCB Pharma, and Vascular Biogenics, among others.
SOURCE: Sandborn WJ et al. ACG 2018. Presentation 11.
PHILADELPHIA – Use of etrasimod was associated with improved clinical and endoscopic results, and was generally safe and well tolerated compared with placebo in patients with moderate to severe ulcerative colitis, according to a recent award-winning presentation at the annual meeting of the American College of Gastroenterology.
“Patients with moderate to severe ulcerative colitis receiving etrasimod 2 mg per day achieved statistically significant and clinically meaningful differences in all of the primary and secondary endpoints, and most exploratory endpoints were also significantly proved,” William J. Sandborn, MD, AGAF, FACG, professor of clinical medicine at the University of California, San Diego, stated in his presentation at the meeting. “A dose-response relationship was observed in virtually all of the measures of treatment efficacy.”
The abstract received the ACG Auxiliary Award (Member), which is given to ACG members each year with outstanding abstract submissions.
Dr. Sandborn and his colleagues enrolled 156 patients with ulcerative colitis (UC) into the OASIS study, a randomized, double-blind, parallel-group, phase 2 study of etrasimod, an oral sphingosine 1-phosphate (S1P) receptor modulator, compared with placebo. Patients were aged 18-80 years, with moderate to severe UC as defined by a three-component Mayo Clinic Score (MCS) comprising rectal bleeding, frequency of stool, and endoscopy.
Those patients who achieved an MCS score between 4 and 9 points with an endoscopic subscore of at least 2 and rectal bleeding (RB) subscore of at least 1 were included. Patients were divided into once-daily etrasimod 1 mg (52 patients), once-daily etrasimod 2 mg (50 patients) and placebo (54 patients) groups and treated over a 12-week period.
At 12 weeks, the least-squares mean difference for change in baseline in three-component MCS was 1.94 in the 1-mg etrasimod group and 2.49 in the 2-mg etrasimod group compared with placebo (1.50). Endoscopic improvement was greater in the 1-mg etrasimod (22.5%) and 2-mg (41.8%) groups compared with placebo (17.8%); endoscopic remission rates also improved in the 1-mg etrasimod (13.7%) and 2-mg (15.3%) groups compared with placebo (5.3%). Lymphocyte count circulation significantly decreased in the 1-mg etrasimod (37.2%) and 2-mg (57.3%) groups compared with the placebo group. With regard to rectal bleeding, the rectal bleeding subscore also decreased in the 1-mg etrasimod and 2-mg groups compared with placebo at 12 weeks from baseline.
The researchers noted no significant differences in adverse events among groups, with the placebo group showing a higher rate of major adverse events (11.1%) compared with the 1-mg etrasimod (5.8%) and 2-mg etrasimod (0%) groups.
“The OASIS trial results for etrasimod would support proceeding to a phase 3 program for this drug in patients with moderate to severe ulcerative colitis,” Dr. Sandborn concluded.
Dr. Sandborn reports consultancies, speaker bureau memberships, and research support from AbbVie, Biogen, Celgene, Ferring, Genentech, Gilead Sciences, Immune Pharmaceuticals, Janssen, Lilly, MedImmune, Novartis, Pfizer, Regeneron, Ritter Pharmaceuticals, Salix, Theradiag, UCB Pharma, and Vascular Biogenics, among others.
SOURCE: Sandborn WJ et al. ACG 2018. Presentation 11.
REPORTING FROM ACG 2018
Key clinical point: Compared with placebo, etrasimod was associated with endoscopic and clinical improvement in patients with ulcerative colitis.
Major finding: Patients taking etrasimod 2 mg (41.8%) and etrasimod 1 mg (22.5%) saw greater endoscopic improvement compared with placebo (17.8%).
Study details: A randomized, double-blind, parallel-group, phase 2 study of 156 patients with ulcerative colitis receiving etrasimod or placebo.
Disclosures: Dr. Sandborn reports consultancies, speaker bureau memberships, and research support from AbbVie, Biogen, Celgene, Ferring, Genentech, Gilead Sciences, Immune Pharmaceuticals, Janssen, Lilly, MedImmune, Novartis, Pfizer, Regeneron, Ritter Pharmaceuticals, Salix, Theradiag, UCB Pharma, and Vascular Biogenics, among others.
Source: Sandborn WJ et al. ACG 2018. Presentation 11.
Low-FODMAP diet improves fecal incontinence in patients with loose stool
PHILADELPHIA – A low fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet was associated with improvement in fecal incontinence in 64.6% of patients who received dietary training, according to recent research presented at the American College of Gastroenterology annual meeting.
“In this case series, the low-FODMAP diet improved fecal incontinence in two-thirds of patients with fecal incontinence and loose stools,” Stacy Menees, MD, MS, assistant professor at the University of Michigan in Ann Arbor, said in her presentation.
Dr. Menees and her colleagues performed a retrospective chart review of 65 patients in the Michigan Bowel Control clinic with fecal incontinence (FI) “without alarming features” who were recommended a low FODMAP diet and received formal dietary teaching between August 2012 and December 2017. Patients received the teaching from a Michigan Medicine gastrointestinal dietitian. The dietitians performed a semi-quantitative analysis of patient response, including complete response, Dr. Menees said.
If we are going to help people with fecal incontinence, the key is to concentrate on the area of their stool consistency,” she explained. “We know that foods high in fermentable oligo-, di-, and monosaccharides and polyols, otherwise known as FODMAPS, can cause diarrhea and urgency.”
These FODMAP foods can include fruits with fructose exceeding glucose, fructan-containing vegetables, wheat-based products, sorbitol- and lactose-containing foods, and raffinose-containing foods, Dr. Menees said.
“We know from observations in data and research from the Monash University and our own group that patients with irritable bowel syndrome who are on a [low-]FODMAP diet [get relief from] their symptoms,” she added.
Most of the patients were female, and most were white. The mean age was 61.9 years, and mean body mass index was 26.7 kg/m2. Among the patients, 27.7% had irritable bowel syndrome (IBS), 9.2% had inflammatory bowel disease (IBD), 16.9% had diabetes mellitus, and 20% had a prior cholecystectomy.
With regard to reports of loose stool and FI, 46.1% of patients said they had loose stool daily with a mean of 2.8 loose bowel movements daily. More than half of the patients (60.5%) reported FI daily; 37.8% said they experienced weekly FI, and 4.6% reported monthly FI. The number of patients reporting FI was higher than 100% because some patients reported in the chart that they had FI daily or weekly, Dr. Menees said.
FI improvement was reported by 64.6% of patients, with 88.1% of responders having a greater than 50% reduction in FI and 35.7% of patients experiencing complete resolution of FI. There were no serious adverse events in the study, Dr. Menees noted.
“The three patients with postinfection IBS and fecal incontinence had no response to a low-FODMAP diet,” she said. “Our dietitians noted that onion and garlic were the triggers that were most often identified.”
Limitations include the retrospective chart review design and small number of patients, which may overestimate or underestimate the study results, and the fact that the study was done at a tertiary care center, Dr. Menees said.
“We are nearing the completion of a confirmatory, prospective, pilot randomized clinical trial to confirm the efficacy of a low-FODMAP diet in patients who suffer from fecal incontinence and loose stools,” she concluded.
Dr. Menees reports being a consultant for Synergy.
Use AGA’s patient education materials to help your patients better understand the low-FODMAP diet, which can be found at http://ow.ly/Xfqj30maODu
SOURCE: Menees SB et al. ACG 2018. Presentation 9.
PHILADELPHIA – A low fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet was associated with improvement in fecal incontinence in 64.6% of patients who received dietary training, according to recent research presented at the American College of Gastroenterology annual meeting.
“In this case series, the low-FODMAP diet improved fecal incontinence in two-thirds of patients with fecal incontinence and loose stools,” Stacy Menees, MD, MS, assistant professor at the University of Michigan in Ann Arbor, said in her presentation.
Dr. Menees and her colleagues performed a retrospective chart review of 65 patients in the Michigan Bowel Control clinic with fecal incontinence (FI) “without alarming features” who were recommended a low FODMAP diet and received formal dietary teaching between August 2012 and December 2017. Patients received the teaching from a Michigan Medicine gastrointestinal dietitian. The dietitians performed a semi-quantitative analysis of patient response, including complete response, Dr. Menees said.
If we are going to help people with fecal incontinence, the key is to concentrate on the area of their stool consistency,” she explained. “We know that foods high in fermentable oligo-, di-, and monosaccharides and polyols, otherwise known as FODMAPS, can cause diarrhea and urgency.”
These FODMAP foods can include fruits with fructose exceeding glucose, fructan-containing vegetables, wheat-based products, sorbitol- and lactose-containing foods, and raffinose-containing foods, Dr. Menees said.
“We know from observations in data and research from the Monash University and our own group that patients with irritable bowel syndrome who are on a [low-]FODMAP diet [get relief from] their symptoms,” she added.
Most of the patients were female, and most were white. The mean age was 61.9 years, and mean body mass index was 26.7 kg/m2. Among the patients, 27.7% had irritable bowel syndrome (IBS), 9.2% had inflammatory bowel disease (IBD), 16.9% had diabetes mellitus, and 20% had a prior cholecystectomy.
With regard to reports of loose stool and FI, 46.1% of patients said they had loose stool daily with a mean of 2.8 loose bowel movements daily. More than half of the patients (60.5%) reported FI daily; 37.8% said they experienced weekly FI, and 4.6% reported monthly FI. The number of patients reporting FI was higher than 100% because some patients reported in the chart that they had FI daily or weekly, Dr. Menees said.
FI improvement was reported by 64.6% of patients, with 88.1% of responders having a greater than 50% reduction in FI and 35.7% of patients experiencing complete resolution of FI. There were no serious adverse events in the study, Dr. Menees noted.
“The three patients with postinfection IBS and fecal incontinence had no response to a low-FODMAP diet,” she said. “Our dietitians noted that onion and garlic were the triggers that were most often identified.”
Limitations include the retrospective chart review design and small number of patients, which may overestimate or underestimate the study results, and the fact that the study was done at a tertiary care center, Dr. Menees said.
“We are nearing the completion of a confirmatory, prospective, pilot randomized clinical trial to confirm the efficacy of a low-FODMAP diet in patients who suffer from fecal incontinence and loose stools,” she concluded.
Dr. Menees reports being a consultant for Synergy.
Use AGA’s patient education materials to help your patients better understand the low-FODMAP diet, which can be found at http://ow.ly/Xfqj30maODu
SOURCE: Menees SB et al. ACG 2018. Presentation 9.
PHILADELPHIA – A low fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet was associated with improvement in fecal incontinence in 64.6% of patients who received dietary training, according to recent research presented at the American College of Gastroenterology annual meeting.
“In this case series, the low-FODMAP diet improved fecal incontinence in two-thirds of patients with fecal incontinence and loose stools,” Stacy Menees, MD, MS, assistant professor at the University of Michigan in Ann Arbor, said in her presentation.
Dr. Menees and her colleagues performed a retrospective chart review of 65 patients in the Michigan Bowel Control clinic with fecal incontinence (FI) “without alarming features” who were recommended a low FODMAP diet and received formal dietary teaching between August 2012 and December 2017. Patients received the teaching from a Michigan Medicine gastrointestinal dietitian. The dietitians performed a semi-quantitative analysis of patient response, including complete response, Dr. Menees said.
If we are going to help people with fecal incontinence, the key is to concentrate on the area of their stool consistency,” she explained. “We know that foods high in fermentable oligo-, di-, and monosaccharides and polyols, otherwise known as FODMAPS, can cause diarrhea and urgency.”
These FODMAP foods can include fruits with fructose exceeding glucose, fructan-containing vegetables, wheat-based products, sorbitol- and lactose-containing foods, and raffinose-containing foods, Dr. Menees said.
“We know from observations in data and research from the Monash University and our own group that patients with irritable bowel syndrome who are on a [low-]FODMAP diet [get relief from] their symptoms,” she added.
Most of the patients were female, and most were white. The mean age was 61.9 years, and mean body mass index was 26.7 kg/m2. Among the patients, 27.7% had irritable bowel syndrome (IBS), 9.2% had inflammatory bowel disease (IBD), 16.9% had diabetes mellitus, and 20% had a prior cholecystectomy.
With regard to reports of loose stool and FI, 46.1% of patients said they had loose stool daily with a mean of 2.8 loose bowel movements daily. More than half of the patients (60.5%) reported FI daily; 37.8% said they experienced weekly FI, and 4.6% reported monthly FI. The number of patients reporting FI was higher than 100% because some patients reported in the chart that they had FI daily or weekly, Dr. Menees said.
FI improvement was reported by 64.6% of patients, with 88.1% of responders having a greater than 50% reduction in FI and 35.7% of patients experiencing complete resolution of FI. There were no serious adverse events in the study, Dr. Menees noted.
“The three patients with postinfection IBS and fecal incontinence had no response to a low-FODMAP diet,” she said. “Our dietitians noted that onion and garlic were the triggers that were most often identified.”
Limitations include the retrospective chart review design and small number of patients, which may overestimate or underestimate the study results, and the fact that the study was done at a tertiary care center, Dr. Menees said.
“We are nearing the completion of a confirmatory, prospective, pilot randomized clinical trial to confirm the efficacy of a low-FODMAP diet in patients who suffer from fecal incontinence and loose stools,” she concluded.
Dr. Menees reports being a consultant for Synergy.
Use AGA’s patient education materials to help your patients better understand the low-FODMAP diet, which can be found at http://ow.ly/Xfqj30maODu
SOURCE: Menees SB et al. ACG 2018. Presentation 9.
REPORTING FROM ACG 2018
Key clinical point: Patients who underwent formal dietary teaching for a fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet showed associated improvements in fecal incontinence.
Major finding: Among patients with fecal incontinence, 64.6% experienced improvement; of these, 88.1% of responders had a greater than 50% reduction in fecal incontinence and 35.7% experienced complete resolution of fecal incontinence.
Study details: A retrospective chart review of 65 patients with fecal incontinence who received formal dietary teaching at the Michigan Bowel Control clinic.
Disclosures: Dr. Menees reports being a consultant for Synergy.
Source: Menees SB et al. ACG 2018. Presentation 9.
How to vaccinate patients on biologics
SAN FRANCISCO – The new herpes zoster subunit vaccine (Shingrix) is on the short list of essential vaccines for immunocompromised adults, including those on biologics.
Ongoing research is demonstrating efficacy and safety in renal transplants patients, as well as those with hematologic cancer and stem cell transplants, according to Lorry Rubin, MD, director of pediatric infectious diseases at Cohen Children’s Medical Center, Queens, and professor of pediatrics at Hofstra University, Hempstead, N.Y.
Immunocompromised people, including those on biologics, should be immunized against a variety of diseases just like everyone else, but it’s tricky. There’s considerable variability in how biologics affect the immune system and subsequent vaccine potency. Timing is important, and although live vaccines are generally a no-go, there’s one class of biologics with which they’re safe, he said.
In a wide-ranging interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Dr. Rubin shared his advice on immunizing the immunocompromised, including the other vaccines on the short list. He also tackled the common concern that vaccinations might trigger rejection in transplant patients.
He’s well qualified to address the issues: Dr. Rubin was lead author on the 2013 Infectious Diseases Society of America guidelines on vaccinating immunocompromised patients.
SAN FRANCISCO – The new herpes zoster subunit vaccine (Shingrix) is on the short list of essential vaccines for immunocompromised adults, including those on biologics.
Ongoing research is demonstrating efficacy and safety in renal transplants patients, as well as those with hematologic cancer and stem cell transplants, according to Lorry Rubin, MD, director of pediatric infectious diseases at Cohen Children’s Medical Center, Queens, and professor of pediatrics at Hofstra University, Hempstead, N.Y.
Immunocompromised people, including those on biologics, should be immunized against a variety of diseases just like everyone else, but it’s tricky. There’s considerable variability in how biologics affect the immune system and subsequent vaccine potency. Timing is important, and although live vaccines are generally a no-go, there’s one class of biologics with which they’re safe, he said.
In a wide-ranging interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Dr. Rubin shared his advice on immunizing the immunocompromised, including the other vaccines on the short list. He also tackled the common concern that vaccinations might trigger rejection in transplant patients.
He’s well qualified to address the issues: Dr. Rubin was lead author on the 2013 Infectious Diseases Society of America guidelines on vaccinating immunocompromised patients.
SAN FRANCISCO – The new herpes zoster subunit vaccine (Shingrix) is on the short list of essential vaccines for immunocompromised adults, including those on biologics.
Ongoing research is demonstrating efficacy and safety in renal transplants patients, as well as those with hematologic cancer and stem cell transplants, according to Lorry Rubin, MD, director of pediatric infectious diseases at Cohen Children’s Medical Center, Queens, and professor of pediatrics at Hofstra University, Hempstead, N.Y.
Immunocompromised people, including those on biologics, should be immunized against a variety of diseases just like everyone else, but it’s tricky. There’s considerable variability in how biologics affect the immune system and subsequent vaccine potency. Timing is important, and although live vaccines are generally a no-go, there’s one class of biologics with which they’re safe, he said.
In a wide-ranging interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Dr. Rubin shared his advice on immunizing the immunocompromised, including the other vaccines on the short list. He also tackled the common concern that vaccinations might trigger rejection in transplant patients.
He’s well qualified to address the issues: Dr. Rubin was lead author on the 2013 Infectious Diseases Society of America guidelines on vaccinating immunocompromised patients.
REPORTING FROM IDWEEK 2018