User login
Providers endorse medical marijuana for kids with cancer
A survey of nearly 300 US medical providers revealed that many were open to helping children with cancer access medical marijuana (MM).
However, most of the providers surveyed did not know state-specific regulations pertaining to MM.
Providers who were legally eligible to certify (ETC) for MM were less open to endorsing its use.
The lack of standards on formulations, dosing, and potency of MM was identified as the greatest barrier to recommending MM for children with cancer.
Kelly Michelson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois, and her colleagues reported these findings in Pediatrics.
The researchers used a 32-item survey to assess MM practices, knowledge, attitudes, and barriers for pediatric oncology providers in Illinois, Massachusetts, and Washington.
The survey was sent to providers at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Seattle Children’s Cancer and Blood Disorders Center, and Lurie Children’s Center for Cancer and Blood Disorders.
There were 288 respondents, and 33% were legally ETC for MM. Eighty-six percent of ETC providers were physicians, and 14% were nurse practitioners or physician assistants.
Of the non-ETC providers, 89% were nurses, 8% were nurse practitioners or physician assistants, 2% were psychosocial providers, and 2% were “other” providers.
Thirty percent of all providers said they had received at least 1 request for MM in the previous month. And 14% of these providers facilitated patient access to MM.
Ninety-two percent of providers said they were willing to help pediatric cancer patients access MM. Fifty-seven percent of providers approved of patients smoking MM, 89% approved of oral formulations, 67% approved of using MM as cancer-directed therapy, and 92% approved of using MM to manage symptoms.
Fifty-nine percent of providers knew that MM is against federal laws, and 86% knew that their state had legalized MM, but only 5% knew state-specific regulations.
ETC providers were less likely to report willingness to help patients access MM. These providers were also less likely to approve of MM use by smoking, oral formulations, as cancer-directed therapy, or to manage symptoms.
“It is not surprising that providers who are eligible to certify for medical marijuana were more cautious about recommending it, given that their licensure could be jeopardized due to federal prohibition,” Dr Michelson said.
“Institutional policies also may have influenced their attitudes. Lurie Children’s, for example, prohibits pediatric providers from facilitating medical marijuana access in accordance with the federal law, even though it is legal in Illinois.”
Most providers considered MM more permissible for use in children with advanced cancer or near the end of life than in earlier stages of cancer treatment. This is consistent with the current American Academy of Pediatrics position that sanctions MM use for “children with life-limiting or seriously debilitating conditions.”
Only 2% of providers reported that MM was never appropriate for a child with cancer.
Most providers (63%) were not concerned about substance abuse in children who receive MM or about being prosecuted for helping patients access MM (80%).
The greatest concern (listed by 46% of providers) was the absence of standards around prescribing MM to children with cancer.
“In addition to unclear dosage guidelines, the lack of high quality scientific data that medical marijuana benefits outweigh possible harm is a huge concern for providers accustomed to evidence-based practice,” Dr Michelson said. “We need rigorously designed clinical trials on the use of medical marijuana in children with cancer.” ![]()
A survey of nearly 300 US medical providers revealed that many were open to helping children with cancer access medical marijuana (MM).
However, most of the providers surveyed did not know state-specific regulations pertaining to MM.
Providers who were legally eligible to certify (ETC) for MM were less open to endorsing its use.
The lack of standards on formulations, dosing, and potency of MM was identified as the greatest barrier to recommending MM for children with cancer.
Kelly Michelson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois, and her colleagues reported these findings in Pediatrics.
The researchers used a 32-item survey to assess MM practices, knowledge, attitudes, and barriers for pediatric oncology providers in Illinois, Massachusetts, and Washington.
The survey was sent to providers at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Seattle Children’s Cancer and Blood Disorders Center, and Lurie Children’s Center for Cancer and Blood Disorders.
There were 288 respondents, and 33% were legally ETC for MM. Eighty-six percent of ETC providers were physicians, and 14% were nurse practitioners or physician assistants.
Of the non-ETC providers, 89% were nurses, 8% were nurse practitioners or physician assistants, 2% were psychosocial providers, and 2% were “other” providers.
Thirty percent of all providers said they had received at least 1 request for MM in the previous month. And 14% of these providers facilitated patient access to MM.
Ninety-two percent of providers said they were willing to help pediatric cancer patients access MM. Fifty-seven percent of providers approved of patients smoking MM, 89% approved of oral formulations, 67% approved of using MM as cancer-directed therapy, and 92% approved of using MM to manage symptoms.
Fifty-nine percent of providers knew that MM is against federal laws, and 86% knew that their state had legalized MM, but only 5% knew state-specific regulations.
ETC providers were less likely to report willingness to help patients access MM. These providers were also less likely to approve of MM use by smoking, oral formulations, as cancer-directed therapy, or to manage symptoms.
“It is not surprising that providers who are eligible to certify for medical marijuana were more cautious about recommending it, given that their licensure could be jeopardized due to federal prohibition,” Dr Michelson said.
“Institutional policies also may have influenced their attitudes. Lurie Children’s, for example, prohibits pediatric providers from facilitating medical marijuana access in accordance with the federal law, even though it is legal in Illinois.”
Most providers considered MM more permissible for use in children with advanced cancer or near the end of life than in earlier stages of cancer treatment. This is consistent with the current American Academy of Pediatrics position that sanctions MM use for “children with life-limiting or seriously debilitating conditions.”
Only 2% of providers reported that MM was never appropriate for a child with cancer.
Most providers (63%) were not concerned about substance abuse in children who receive MM or about being prosecuted for helping patients access MM (80%).
The greatest concern (listed by 46% of providers) was the absence of standards around prescribing MM to children with cancer.
“In addition to unclear dosage guidelines, the lack of high quality scientific data that medical marijuana benefits outweigh possible harm is a huge concern for providers accustomed to evidence-based practice,” Dr Michelson said. “We need rigorously designed clinical trials on the use of medical marijuana in children with cancer.” ![]()
A survey of nearly 300 US medical providers revealed that many were open to helping children with cancer access medical marijuana (MM).
However, most of the providers surveyed did not know state-specific regulations pertaining to MM.
Providers who were legally eligible to certify (ETC) for MM were less open to endorsing its use.
The lack of standards on formulations, dosing, and potency of MM was identified as the greatest barrier to recommending MM for children with cancer.
Kelly Michelson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois, and her colleagues reported these findings in Pediatrics.
The researchers used a 32-item survey to assess MM practices, knowledge, attitudes, and barriers for pediatric oncology providers in Illinois, Massachusetts, and Washington.
The survey was sent to providers at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Seattle Children’s Cancer and Blood Disorders Center, and Lurie Children’s Center for Cancer and Blood Disorders.
There were 288 respondents, and 33% were legally ETC for MM. Eighty-six percent of ETC providers were physicians, and 14% were nurse practitioners or physician assistants.
Of the non-ETC providers, 89% were nurses, 8% were nurse practitioners or physician assistants, 2% were psychosocial providers, and 2% were “other” providers.
Thirty percent of all providers said they had received at least 1 request for MM in the previous month. And 14% of these providers facilitated patient access to MM.
Ninety-two percent of providers said they were willing to help pediatric cancer patients access MM. Fifty-seven percent of providers approved of patients smoking MM, 89% approved of oral formulations, 67% approved of using MM as cancer-directed therapy, and 92% approved of using MM to manage symptoms.
Fifty-nine percent of providers knew that MM is against federal laws, and 86% knew that their state had legalized MM, but only 5% knew state-specific regulations.
ETC providers were less likely to report willingness to help patients access MM. These providers were also less likely to approve of MM use by smoking, oral formulations, as cancer-directed therapy, or to manage symptoms.
“It is not surprising that providers who are eligible to certify for medical marijuana were more cautious about recommending it, given that their licensure could be jeopardized due to federal prohibition,” Dr Michelson said.
“Institutional policies also may have influenced their attitudes. Lurie Children’s, for example, prohibits pediatric providers from facilitating medical marijuana access in accordance with the federal law, even though it is legal in Illinois.”
Most providers considered MM more permissible for use in children with advanced cancer or near the end of life than in earlier stages of cancer treatment. This is consistent with the current American Academy of Pediatrics position that sanctions MM use for “children with life-limiting or seriously debilitating conditions.”
Only 2% of providers reported that MM was never appropriate for a child with cancer.
Most providers (63%) were not concerned about substance abuse in children who receive MM or about being prosecuted for helping patients access MM (80%).
The greatest concern (listed by 46% of providers) was the absence of standards around prescribing MM to children with cancer.
“In addition to unclear dosage guidelines, the lack of high quality scientific data that medical marijuana benefits outweigh possible harm is a huge concern for providers accustomed to evidence-based practice,” Dr Michelson said. “We need rigorously designed clinical trials on the use of medical marijuana in children with cancer.” ![]()
VIDEO: Joint FDA-ASH session highlights new AML drugs
ATLANTA – The past year brought a flurry of new drug approvals for the treatment of acute myeloid leukemia (AML), including CPX-351, midostaurin, gemtuzumab ozogamicin, and enasidenib.
During a special interest session at the annual meeting of the American Society of Hematology, Food and Drug Administration representatives discussed the available data and approval process for these drugs, and clinicians discussed their use in the real-world setting.
In this video interview, – a liposome-encapsulated combination of daunorubicin and cytarabine approved in August for patients with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes, and midostaurin (Rydapt), which was approved in April for the treatment of newly diagnosed AML patients who are FLT3 mutation-positive. She also discussed future directions for these agents.
“So what clinicians are faced with is, all of a sudden, a number of new agents, and no particularly vetted or data-based algorithm by which to assign patients from one to the other,” said Dr. Michaelis, of the Medical College of Wisconsin, Milwaukee, adding that none of the drugs have been compared against one another.
In her own practice, when it comes to CPX-351, she said she first discusses the pros and cons with patients.
“This drug is used for older individuals ... with very adverse risk disease, and so the first question is do you fit the trial entry criteria, do you want to go through induction, do you understand what that’s going to mean, and am I going to take you to transplant after we go through this.”
As for midostaurin, she said she tries to use it on anyone who fits the trial criteria and is FLT-3 positive.
“The trick with that is that we don’t know the FLT-3 status at the time we have to start induction, so it’s hard to determine the exact right doses of your induction regimen knowing that you’re not going to get the test back until day 6, 7, 8, and you’re supposed to start delivering the drug on day 8, so we still have a ways as a care community to catch up with being able to give these drugs in a manner that was the same as what was delivered in the trials that led to approval.”
She also discussed the potential for combining treatments.
“I think there’s really room for studies on combinations of inhibitors plus the CPX, the safety of using a variety of induction regimens alongside midostaurin, and safety of combining things, like with midostaurin for example, with some of our antifungals ... and to make sure that that’s safe. So yeah, we’ve got a lot more to do,” she said.
Dr. Michaelis serves on an advisory board for Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – The past year brought a flurry of new drug approvals for the treatment of acute myeloid leukemia (AML), including CPX-351, midostaurin, gemtuzumab ozogamicin, and enasidenib.
During a special interest session at the annual meeting of the American Society of Hematology, Food and Drug Administration representatives discussed the available data and approval process for these drugs, and clinicians discussed their use in the real-world setting.
In this video interview, – a liposome-encapsulated combination of daunorubicin and cytarabine approved in August for patients with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes, and midostaurin (Rydapt), which was approved in April for the treatment of newly diagnosed AML patients who are FLT3 mutation-positive. She also discussed future directions for these agents.
“So what clinicians are faced with is, all of a sudden, a number of new agents, and no particularly vetted or data-based algorithm by which to assign patients from one to the other,” said Dr. Michaelis, of the Medical College of Wisconsin, Milwaukee, adding that none of the drugs have been compared against one another.
In her own practice, when it comes to CPX-351, she said she first discusses the pros and cons with patients.
“This drug is used for older individuals ... with very adverse risk disease, and so the first question is do you fit the trial entry criteria, do you want to go through induction, do you understand what that’s going to mean, and am I going to take you to transplant after we go through this.”
As for midostaurin, she said she tries to use it on anyone who fits the trial criteria and is FLT-3 positive.
“The trick with that is that we don’t know the FLT-3 status at the time we have to start induction, so it’s hard to determine the exact right doses of your induction regimen knowing that you’re not going to get the test back until day 6, 7, 8, and you’re supposed to start delivering the drug on day 8, so we still have a ways as a care community to catch up with being able to give these drugs in a manner that was the same as what was delivered in the trials that led to approval.”
She also discussed the potential for combining treatments.
“I think there’s really room for studies on combinations of inhibitors plus the CPX, the safety of using a variety of induction regimens alongside midostaurin, and safety of combining things, like with midostaurin for example, with some of our antifungals ... and to make sure that that’s safe. So yeah, we’ve got a lot more to do,” she said.
Dr. Michaelis serves on an advisory board for Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – The past year brought a flurry of new drug approvals for the treatment of acute myeloid leukemia (AML), including CPX-351, midostaurin, gemtuzumab ozogamicin, and enasidenib.
During a special interest session at the annual meeting of the American Society of Hematology, Food and Drug Administration representatives discussed the available data and approval process for these drugs, and clinicians discussed their use in the real-world setting.
In this video interview, – a liposome-encapsulated combination of daunorubicin and cytarabine approved in August for patients with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes, and midostaurin (Rydapt), which was approved in April for the treatment of newly diagnosed AML patients who are FLT3 mutation-positive. She also discussed future directions for these agents.
“So what clinicians are faced with is, all of a sudden, a number of new agents, and no particularly vetted or data-based algorithm by which to assign patients from one to the other,” said Dr. Michaelis, of the Medical College of Wisconsin, Milwaukee, adding that none of the drugs have been compared against one another.
In her own practice, when it comes to CPX-351, she said she first discusses the pros and cons with patients.
“This drug is used for older individuals ... with very adverse risk disease, and so the first question is do you fit the trial entry criteria, do you want to go through induction, do you understand what that’s going to mean, and am I going to take you to transplant after we go through this.”
As for midostaurin, she said she tries to use it on anyone who fits the trial criteria and is FLT-3 positive.
“The trick with that is that we don’t know the FLT-3 status at the time we have to start induction, so it’s hard to determine the exact right doses of your induction regimen knowing that you’re not going to get the test back until day 6, 7, 8, and you’re supposed to start delivering the drug on day 8, so we still have a ways as a care community to catch up with being able to give these drugs in a manner that was the same as what was delivered in the trials that led to approval.”
She also discussed the potential for combining treatments.
“I think there’s really room for studies on combinations of inhibitors plus the CPX, the safety of using a variety of induction regimens alongside midostaurin, and safety of combining things, like with midostaurin for example, with some of our antifungals ... and to make sure that that’s safe. So yeah, we’ve got a lot more to do,” she said.
Dr. Michaelis serves on an advisory board for Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM ASH 2017
EC approves new formulation of pegaspargase
The European Commission (EC) has granted marketing authorization for a lyophilized formulation of pegaspargase (ONCASPAR).
The product is intended for use as a component of antineoplastic combination therapy in acute lymphoblastic leukemia patients of all ages.
The EC’s approval authorizes Shire to market lyophilized pegaspargase in the 28 member states of the European Union as well as Iceland, Liechtenstein, and Norway.
Lyophilized pegaspargase works the same way as the liquid formulation. By depleting serum L-asparagine levels and thereby interfering with protein synthesis, pegaspargase deprives lymphoblasts of L-asparagine, resulting in cell death.
The lyophilized formulation offers the same dosing regimen as liquid pegaspargase but also provides a shelf life of up to 24 months—3 times longer than that of the liquid formulation.
Shire expects lyophilized pegaspargase to be available in European markets beginning in the first half of 2018.
“With this lyophilized formulation, we aim to make pegylated asparaginase, part of the pediatric standard therapy in acute lymphoblastic leukemia, available to patients in countries where liquid ONCASPAR is not currently offered,” said Howard B. Mayer, MD, senior vice-president and ad-interim head of global research and development at Shire.
“Additionally, with extended shelf life up to 24 months, treatment centers will have flexibility in inventory management to help ensure continuous treatment supply for patients.” ![]()
The European Commission (EC) has granted marketing authorization for a lyophilized formulation of pegaspargase (ONCASPAR).
The product is intended for use as a component of antineoplastic combination therapy in acute lymphoblastic leukemia patients of all ages.
The EC’s approval authorizes Shire to market lyophilized pegaspargase in the 28 member states of the European Union as well as Iceland, Liechtenstein, and Norway.
Lyophilized pegaspargase works the same way as the liquid formulation. By depleting serum L-asparagine levels and thereby interfering with protein synthesis, pegaspargase deprives lymphoblasts of L-asparagine, resulting in cell death.
The lyophilized formulation offers the same dosing regimen as liquid pegaspargase but also provides a shelf life of up to 24 months—3 times longer than that of the liquid formulation.
Shire expects lyophilized pegaspargase to be available in European markets beginning in the first half of 2018.
“With this lyophilized formulation, we aim to make pegylated asparaginase, part of the pediatric standard therapy in acute lymphoblastic leukemia, available to patients in countries where liquid ONCASPAR is not currently offered,” said Howard B. Mayer, MD, senior vice-president and ad-interim head of global research and development at Shire.
“Additionally, with extended shelf life up to 24 months, treatment centers will have flexibility in inventory management to help ensure continuous treatment supply for patients.” ![]()
The European Commission (EC) has granted marketing authorization for a lyophilized formulation of pegaspargase (ONCASPAR).
The product is intended for use as a component of antineoplastic combination therapy in acute lymphoblastic leukemia patients of all ages.
The EC’s approval authorizes Shire to market lyophilized pegaspargase in the 28 member states of the European Union as well as Iceland, Liechtenstein, and Norway.
Lyophilized pegaspargase works the same way as the liquid formulation. By depleting serum L-asparagine levels and thereby interfering with protein synthesis, pegaspargase deprives lymphoblasts of L-asparagine, resulting in cell death.
The lyophilized formulation offers the same dosing regimen as liquid pegaspargase but also provides a shelf life of up to 24 months—3 times longer than that of the liquid formulation.
Shire expects lyophilized pegaspargase to be available in European markets beginning in the first half of 2018.
“With this lyophilized formulation, we aim to make pegylated asparaginase, part of the pediatric standard therapy in acute lymphoblastic leukemia, available to patients in countries where liquid ONCASPAR is not currently offered,” said Howard B. Mayer, MD, senior vice-president and ad-interim head of global research and development at Shire.
“Additionally, with extended shelf life up to 24 months, treatment centers will have flexibility in inventory management to help ensure continuous treatment supply for patients.” ![]()
VIDEO: Venetoclax/rituximab prolongs PFS in relapsed/refractory CLL
ATLANTA – Relapsed or refractory chronic lymphocytic leukemia (CLL) often has a suboptimal response to conventional chemotherapy, because of adverse biological features that can accumulate in cells.
The combination of bendamustine (Treanda) and rituximab has been associated with about 60% overall responses rates, median progression-free survival of approximately 15 months, and overall survival of nearly 3 years in patients with CLL, and there is now evidence that substituting venetoclax (Venclexta) for bendamustine could improve outcomes even further.
In a video interview at the annual meeting of the American Society of Hematology, comparing bendamustine plus rituximab with venetoclax plus rituximab in patients with relapsed/refractory CLL.
Venetoclax/rituximab was superior to bendamustine/rituximab for prolonging progression-free survival, with effects consistent across subgroups, regardless of mutation status, and for having a clinically meaningful improvement in overall survival.
The MURANO trial was funded by AbbVie. Dr. Seymour reported honoraria, research funding, and advisory committee and speakers bureau participation for AbbVie and other companies.
ATLANTA – Relapsed or refractory chronic lymphocytic leukemia (CLL) often has a suboptimal response to conventional chemotherapy, because of adverse biological features that can accumulate in cells.
The combination of bendamustine (Treanda) and rituximab has been associated with about 60% overall responses rates, median progression-free survival of approximately 15 months, and overall survival of nearly 3 years in patients with CLL, and there is now evidence that substituting venetoclax (Venclexta) for bendamustine could improve outcomes even further.
In a video interview at the annual meeting of the American Society of Hematology, comparing bendamustine plus rituximab with venetoclax plus rituximab in patients with relapsed/refractory CLL.
Venetoclax/rituximab was superior to bendamustine/rituximab for prolonging progression-free survival, with effects consistent across subgroups, regardless of mutation status, and for having a clinically meaningful improvement in overall survival.
The MURANO trial was funded by AbbVie. Dr. Seymour reported honoraria, research funding, and advisory committee and speakers bureau participation for AbbVie and other companies.
ATLANTA – Relapsed or refractory chronic lymphocytic leukemia (CLL) often has a suboptimal response to conventional chemotherapy, because of adverse biological features that can accumulate in cells.
The combination of bendamustine (Treanda) and rituximab has been associated with about 60% overall responses rates, median progression-free survival of approximately 15 months, and overall survival of nearly 3 years in patients with CLL, and there is now evidence that substituting venetoclax (Venclexta) for bendamustine could improve outcomes even further.
In a video interview at the annual meeting of the American Society of Hematology, comparing bendamustine plus rituximab with venetoclax plus rituximab in patients with relapsed/refractory CLL.
Venetoclax/rituximab was superior to bendamustine/rituximab for prolonging progression-free survival, with effects consistent across subgroups, regardless of mutation status, and for having a clinically meaningful improvement in overall survival.
The MURANO trial was funded by AbbVie. Dr. Seymour reported honoraria, research funding, and advisory committee and speakers bureau participation for AbbVie and other companies.
REPORTING FROM ASH 2017
Azacitidine maintenance improves PFS in older AML patients
ATLANTA – In older patients with acute myeloid leukemia (AML) in complete remission after intensive chemotherapy, the addition of maintenance therapy with azacitidine significantly improved disease-free survival (DFS), according to results of a randomized, placebo-controlled phase 3 study.
Compared with observation, DFS was significantly improved in the maintenance azacitidine arm, according to results from the 116-patient HOVON97 trial presented at the annual meeting of the American Society of Hematology.
Overall survival was not significantly different between arms, possibly because of an excess of allogeneic transplant in the observation arm, according to Geert Huls, MD, PhD, of the department of hematology, University Medical Center Groningen, the Netherlands.
“When censored for allogeneic transplant, maintenance with azacitidine improves overall survival,” Dr. Huls said during an oral presentation on the findings.
The randomized maintenance therapy trial was designed to include 126 patients aged 60 years or older who had a confirmed diagnosis of AML and refractory anemia with excess of blasts (RAEB, RAEB-t) and who were in complete remission or in complete remission with incomplete blood count recovery after two cycles of therapy.
Investigators randomly assigned 116 patients to maintenance versus observation. Researchers intended to assign a total of 126 patients, but the trial was stopped early because of slow accrual, Dr. Huls said.
Maintenance treatment with azacitidine was given until relapse for no more than 12 cycles, according to the study protocol. Disease-free survival, the primary endpoint, was measured from the date of randomization to relapse or death from any cause.
Azacitidine maintenance therapy significantly improved DFS (P = .03), Dr. Huls said. After researchers adjusted for poor risk cytogenetic abnormalities at diagnosis and platelet count at study entry, the DFS difference remained significant (hazard ratio, 0.61; 95% confidence interval, 0.4-0.92; P = .019).
Overall survival, a secondary endpoint of the trial, was not significantly different between arms, even after adjustment for cytogenetic abnormalities and platelet counts, Dr. Huls said.
However, investigators found an excess of allogeneic transplant in the observation arm (11 patients, vs. 3 in the azacitidine arm). After they censored those 14 patients, they saw a difference in overall survival favoring azacitidine maintenance that approached significance (P = .07).
Dr. Huls speculated that the excess of transplant may have been related to “the psychology of the doctors.” In the maintenance arm, the physician’s thought process may have been that “ ‘this patient has now had two lines of treatment and has a relapse, and we are done,’ and in the [observation] arm he says, ‘well, the patient has had one arm of treatment, let’s go for another,’ ” Dr. Huls said.
Tolerability data showed that 14 adverse events were reported in the azacitidine maintenance arm, versus 4 for observation. One serious adverse event of grade 3 was reported in the azacitidine arm. The proportion of patients without platelet transfusions during the study was 86% for azacitidine and 93% for observation, and the proportion of patients without red blood cell transfusions was similarly 86% and 92% for the azacitidine and observation arms, respectively.
Dr. Huls reported financial relationships with Janssen and Celgene.
SOURCE: Huls G et al. ASH 2017 Abstract 463.
ATLANTA – In older patients with acute myeloid leukemia (AML) in complete remission after intensive chemotherapy, the addition of maintenance therapy with azacitidine significantly improved disease-free survival (DFS), according to results of a randomized, placebo-controlled phase 3 study.
Compared with observation, DFS was significantly improved in the maintenance azacitidine arm, according to results from the 116-patient HOVON97 trial presented at the annual meeting of the American Society of Hematology.
Overall survival was not significantly different between arms, possibly because of an excess of allogeneic transplant in the observation arm, according to Geert Huls, MD, PhD, of the department of hematology, University Medical Center Groningen, the Netherlands.
“When censored for allogeneic transplant, maintenance with azacitidine improves overall survival,” Dr. Huls said during an oral presentation on the findings.
The randomized maintenance therapy trial was designed to include 126 patients aged 60 years or older who had a confirmed diagnosis of AML and refractory anemia with excess of blasts (RAEB, RAEB-t) and who were in complete remission or in complete remission with incomplete blood count recovery after two cycles of therapy.
Investigators randomly assigned 116 patients to maintenance versus observation. Researchers intended to assign a total of 126 patients, but the trial was stopped early because of slow accrual, Dr. Huls said.
Maintenance treatment with azacitidine was given until relapse for no more than 12 cycles, according to the study protocol. Disease-free survival, the primary endpoint, was measured from the date of randomization to relapse or death from any cause.
Azacitidine maintenance therapy significantly improved DFS (P = .03), Dr. Huls said. After researchers adjusted for poor risk cytogenetic abnormalities at diagnosis and platelet count at study entry, the DFS difference remained significant (hazard ratio, 0.61; 95% confidence interval, 0.4-0.92; P = .019).
Overall survival, a secondary endpoint of the trial, was not significantly different between arms, even after adjustment for cytogenetic abnormalities and platelet counts, Dr. Huls said.
However, investigators found an excess of allogeneic transplant in the observation arm (11 patients, vs. 3 in the azacitidine arm). After they censored those 14 patients, they saw a difference in overall survival favoring azacitidine maintenance that approached significance (P = .07).
Dr. Huls speculated that the excess of transplant may have been related to “the psychology of the doctors.” In the maintenance arm, the physician’s thought process may have been that “ ‘this patient has now had two lines of treatment and has a relapse, and we are done,’ and in the [observation] arm he says, ‘well, the patient has had one arm of treatment, let’s go for another,’ ” Dr. Huls said.
Tolerability data showed that 14 adverse events were reported in the azacitidine maintenance arm, versus 4 for observation. One serious adverse event of grade 3 was reported in the azacitidine arm. The proportion of patients without platelet transfusions during the study was 86% for azacitidine and 93% for observation, and the proportion of patients without red blood cell transfusions was similarly 86% and 92% for the azacitidine and observation arms, respectively.
Dr. Huls reported financial relationships with Janssen and Celgene.
SOURCE: Huls G et al. ASH 2017 Abstract 463.
ATLANTA – In older patients with acute myeloid leukemia (AML) in complete remission after intensive chemotherapy, the addition of maintenance therapy with azacitidine significantly improved disease-free survival (DFS), according to results of a randomized, placebo-controlled phase 3 study.
Compared with observation, DFS was significantly improved in the maintenance azacitidine arm, according to results from the 116-patient HOVON97 trial presented at the annual meeting of the American Society of Hematology.
Overall survival was not significantly different between arms, possibly because of an excess of allogeneic transplant in the observation arm, according to Geert Huls, MD, PhD, of the department of hematology, University Medical Center Groningen, the Netherlands.
“When censored for allogeneic transplant, maintenance with azacitidine improves overall survival,” Dr. Huls said during an oral presentation on the findings.
The randomized maintenance therapy trial was designed to include 126 patients aged 60 years or older who had a confirmed diagnosis of AML and refractory anemia with excess of blasts (RAEB, RAEB-t) and who were in complete remission or in complete remission with incomplete blood count recovery after two cycles of therapy.
Investigators randomly assigned 116 patients to maintenance versus observation. Researchers intended to assign a total of 126 patients, but the trial was stopped early because of slow accrual, Dr. Huls said.
Maintenance treatment with azacitidine was given until relapse for no more than 12 cycles, according to the study protocol. Disease-free survival, the primary endpoint, was measured from the date of randomization to relapse or death from any cause.
Azacitidine maintenance therapy significantly improved DFS (P = .03), Dr. Huls said. After researchers adjusted for poor risk cytogenetic abnormalities at diagnosis and platelet count at study entry, the DFS difference remained significant (hazard ratio, 0.61; 95% confidence interval, 0.4-0.92; P = .019).
Overall survival, a secondary endpoint of the trial, was not significantly different between arms, even after adjustment for cytogenetic abnormalities and platelet counts, Dr. Huls said.
However, investigators found an excess of allogeneic transplant in the observation arm (11 patients, vs. 3 in the azacitidine arm). After they censored those 14 patients, they saw a difference in overall survival favoring azacitidine maintenance that approached significance (P = .07).
Dr. Huls speculated that the excess of transplant may have been related to “the psychology of the doctors.” In the maintenance arm, the physician’s thought process may have been that “ ‘this patient has now had two lines of treatment and has a relapse, and we are done,’ and in the [observation] arm he says, ‘well, the patient has had one arm of treatment, let’s go for another,’ ” Dr. Huls said.
Tolerability data showed that 14 adverse events were reported in the azacitidine maintenance arm, versus 4 for observation. One serious adverse event of grade 3 was reported in the azacitidine arm. The proportion of patients without platelet transfusions during the study was 86% for azacitidine and 93% for observation, and the proportion of patients without red blood cell transfusions was similarly 86% and 92% for the azacitidine and observation arms, respectively.
Dr. Huls reported financial relationships with Janssen and Celgene.
SOURCE: Huls G et al. ASH 2017 Abstract 463.
AT ASH 2017
Key clinical point:
Major finding: Disease-free survival was significantly improved (HR, 0.61; 95% CI, 0.4-0.92; P = .019)
Data source: A randomized, multicenter phase 3 trial including 116 older patients (60 years or older) with AML and refractory anemia with excess of blasts (RAEB, RAEB-t).
Disclosures: Dr. Huls reported financial relationships with Janssen and Celgene.
Source: Huls G et al. ASH 2017 Abstract 463.
Flu vaccine did not protect children with acute leukemia
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
FROM THE JOURNAL OF PEDIATRICS
CLARITY: Ibrutinib/venetoclax combo results look promising for relapsed/refractory CLL
ATLANTA – Combination therapy with ibrutinib and venetoclax is well tolerated and shows promise for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL), according to initial results from the CLARITY feasibility trial.
Of 38 patients who received at least 6 months of treatment with combination ibrutinib (Imbruvica)/venetoclax (Venclexta) and reached month 8 – and therefore had computed tomography, clinical data, and peripheral blood and marrow assessments available – 15 (37%) achieved peripheral blood minimal residual disease (MRD) negativity, and 12 (32%) achieved bone marrow MRD negativity, Peter Hillmen, MBChB, PhD, reported during a press briefing at the annual meeting of the American Society of Hematology.
The rates of MRD negativity in the blood and marrow, and of normal trephine biopsy, were similar in subsets of patients who relapsed within 36 months of prior treatment with fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab (BR), and with prior idelalisib exposure, he noted.
“In terms of [International Workshop on Chronic Lymphocytic Leukemia] response criteria, which is a secondary endpoint, 47% of patients achieved a [complete remission or complete remission with incomplete hematologic recovery] and every patient has had an overall response, which for this group of patients is impressive,” he said.
Again, the findings were similar in those who were refractory to prior FCR/BR or to previous idelalisib, he noted.
Both ibrutinib and venetoclax are approved as single agents for the treatment of CLL. Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has had a major effect on patient outcomes, showing overall survival advantages in numerous trials, Dr. Hillmen said.
“However, ibrutinib does not eradicate disease, and patients remain on treatment indefinitely or until progression,” he said.
Venetoclax is a highly selective B cell lymphoma–2 inhibitor approved for refractory CLL in patients with 17p deletion. It has a rapid effect, which can lead to tumor lysis syndrome, but also leads to eradication of MRD in some patients, which can lead to prolonged survival, he said.
The CLARITY trial was designed to investigate the safety and efficacy of the two in combination in relapsed/refractory CLL patients.
The primary endpoint of the study is MRD eradication in the marrow after 12 months of treatment. The current analysis looks at a key secondary endpoint of the study – MRD eradication in the marrow after 6 months of treatment.
The study enrolled 54 patients, including 37 men and 17 women with a median age of 64 years; 20% have 17p deletion, and the population was heavily pretreated, with 81% having prior FCR or BR (44% with relapse within 3 years of treatment), and 20% with previous idelalisib exposure. Patients were excluded if they had prior exposure to ibrutinib or venetoclax.
Treatment involves ibrutinib monotherapy at a dose of 420 mg/day for 2 months to debulk the disease, after which venetoclax is added at a dose escalating from 20 mg to 400 mg/day over 2 months to reduce the risk of tumor lysis syndrome.
Bone marrow biopsies are performed at 6, 12, and 24 months. Treatment is discontinued at 12 months in those who achieve MRD negativity at 6 months, and is discontinued at 24 months in those who achieve MRD negativity at 12 months.
The combination treatment was well tolerated in the first 38 patients. Bruising (mainly grade 1) occurred in 33 patients, and neutropenia (including 16 grade 3 cases and 6 grade 4 cases) occurred in 25, and some GI toxicity occurred, but was largely grade 1 or 2, Dr. Hillmen said.
“There really was otherwise very acceptable toxicity,” he added, noting that a single case of tumor lysis syndrome occurred, but was managed successfully by delaying venetoclax.
“That patient re-escalated back onto treatment and is doing well,” he said.
No patients stopped treatment, and only seven had treatment interruption, and then only for a few days, he noted.
The findings are encouraging, and suggest a potent synergy between ibrutinib and venetoclax, said Dr. Hillmen.
“We’re seeing, even at this very early stage, over 30% of patients achieving MRD negative remission, which was our target at the 12-month bone marrow stage with this combination,” he said.
In light of these results, the ongoing phase 3 FLAIR trial, which is actively recruiting, has been modified to include combination ibrutinib and venetoclax in front-line CLL, he said.
Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham (England).
[email protected]
SOURCE: Hillmen P et al., ASH abstract 428.
ATLANTA – Combination therapy with ibrutinib and venetoclax is well tolerated and shows promise for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL), according to initial results from the CLARITY feasibility trial.
Of 38 patients who received at least 6 months of treatment with combination ibrutinib (Imbruvica)/venetoclax (Venclexta) and reached month 8 – and therefore had computed tomography, clinical data, and peripheral blood and marrow assessments available – 15 (37%) achieved peripheral blood minimal residual disease (MRD) negativity, and 12 (32%) achieved bone marrow MRD negativity, Peter Hillmen, MBChB, PhD, reported during a press briefing at the annual meeting of the American Society of Hematology.
The rates of MRD negativity in the blood and marrow, and of normal trephine biopsy, were similar in subsets of patients who relapsed within 36 months of prior treatment with fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab (BR), and with prior idelalisib exposure, he noted.
“In terms of [International Workshop on Chronic Lymphocytic Leukemia] response criteria, which is a secondary endpoint, 47% of patients achieved a [complete remission or complete remission with incomplete hematologic recovery] and every patient has had an overall response, which for this group of patients is impressive,” he said.
Again, the findings were similar in those who were refractory to prior FCR/BR or to previous idelalisib, he noted.
Both ibrutinib and venetoclax are approved as single agents for the treatment of CLL. Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has had a major effect on patient outcomes, showing overall survival advantages in numerous trials, Dr. Hillmen said.
“However, ibrutinib does not eradicate disease, and patients remain on treatment indefinitely or until progression,” he said.
Venetoclax is a highly selective B cell lymphoma–2 inhibitor approved for refractory CLL in patients with 17p deletion. It has a rapid effect, which can lead to tumor lysis syndrome, but also leads to eradication of MRD in some patients, which can lead to prolonged survival, he said.
The CLARITY trial was designed to investigate the safety and efficacy of the two in combination in relapsed/refractory CLL patients.
The primary endpoint of the study is MRD eradication in the marrow after 12 months of treatment. The current analysis looks at a key secondary endpoint of the study – MRD eradication in the marrow after 6 months of treatment.
The study enrolled 54 patients, including 37 men and 17 women with a median age of 64 years; 20% have 17p deletion, and the population was heavily pretreated, with 81% having prior FCR or BR (44% with relapse within 3 years of treatment), and 20% with previous idelalisib exposure. Patients were excluded if they had prior exposure to ibrutinib or venetoclax.
Treatment involves ibrutinib monotherapy at a dose of 420 mg/day for 2 months to debulk the disease, after which venetoclax is added at a dose escalating from 20 mg to 400 mg/day over 2 months to reduce the risk of tumor lysis syndrome.
Bone marrow biopsies are performed at 6, 12, and 24 months. Treatment is discontinued at 12 months in those who achieve MRD negativity at 6 months, and is discontinued at 24 months in those who achieve MRD negativity at 12 months.
The combination treatment was well tolerated in the first 38 patients. Bruising (mainly grade 1) occurred in 33 patients, and neutropenia (including 16 grade 3 cases and 6 grade 4 cases) occurred in 25, and some GI toxicity occurred, but was largely grade 1 or 2, Dr. Hillmen said.
“There really was otherwise very acceptable toxicity,” he added, noting that a single case of tumor lysis syndrome occurred, but was managed successfully by delaying venetoclax.
“That patient re-escalated back onto treatment and is doing well,” he said.
No patients stopped treatment, and only seven had treatment interruption, and then only for a few days, he noted.
The findings are encouraging, and suggest a potent synergy between ibrutinib and venetoclax, said Dr. Hillmen.
“We’re seeing, even at this very early stage, over 30% of patients achieving MRD negative remission, which was our target at the 12-month bone marrow stage with this combination,” he said.
In light of these results, the ongoing phase 3 FLAIR trial, which is actively recruiting, has been modified to include combination ibrutinib and venetoclax in front-line CLL, he said.
Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham (England).
[email protected]
SOURCE: Hillmen P et al., ASH abstract 428.
ATLANTA – Combination therapy with ibrutinib and venetoclax is well tolerated and shows promise for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL), according to initial results from the CLARITY feasibility trial.
Of 38 patients who received at least 6 months of treatment with combination ibrutinib (Imbruvica)/venetoclax (Venclexta) and reached month 8 – and therefore had computed tomography, clinical data, and peripheral blood and marrow assessments available – 15 (37%) achieved peripheral blood minimal residual disease (MRD) negativity, and 12 (32%) achieved bone marrow MRD negativity, Peter Hillmen, MBChB, PhD, reported during a press briefing at the annual meeting of the American Society of Hematology.
The rates of MRD negativity in the blood and marrow, and of normal trephine biopsy, were similar in subsets of patients who relapsed within 36 months of prior treatment with fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab (BR), and with prior idelalisib exposure, he noted.
“In terms of [International Workshop on Chronic Lymphocytic Leukemia] response criteria, which is a secondary endpoint, 47% of patients achieved a [complete remission or complete remission with incomplete hematologic recovery] and every patient has had an overall response, which for this group of patients is impressive,” he said.
Again, the findings were similar in those who were refractory to prior FCR/BR or to previous idelalisib, he noted.
Both ibrutinib and venetoclax are approved as single agents for the treatment of CLL. Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has had a major effect on patient outcomes, showing overall survival advantages in numerous trials, Dr. Hillmen said.
“However, ibrutinib does not eradicate disease, and patients remain on treatment indefinitely or until progression,” he said.
Venetoclax is a highly selective B cell lymphoma–2 inhibitor approved for refractory CLL in patients with 17p deletion. It has a rapid effect, which can lead to tumor lysis syndrome, but also leads to eradication of MRD in some patients, which can lead to prolonged survival, he said.
The CLARITY trial was designed to investigate the safety and efficacy of the two in combination in relapsed/refractory CLL patients.
The primary endpoint of the study is MRD eradication in the marrow after 12 months of treatment. The current analysis looks at a key secondary endpoint of the study – MRD eradication in the marrow after 6 months of treatment.
The study enrolled 54 patients, including 37 men and 17 women with a median age of 64 years; 20% have 17p deletion, and the population was heavily pretreated, with 81% having prior FCR or BR (44% with relapse within 3 years of treatment), and 20% with previous idelalisib exposure. Patients were excluded if they had prior exposure to ibrutinib or venetoclax.
Treatment involves ibrutinib monotherapy at a dose of 420 mg/day for 2 months to debulk the disease, after which venetoclax is added at a dose escalating from 20 mg to 400 mg/day over 2 months to reduce the risk of tumor lysis syndrome.
Bone marrow biopsies are performed at 6, 12, and 24 months. Treatment is discontinued at 12 months in those who achieve MRD negativity at 6 months, and is discontinued at 24 months in those who achieve MRD negativity at 12 months.
The combination treatment was well tolerated in the first 38 patients. Bruising (mainly grade 1) occurred in 33 patients, and neutropenia (including 16 grade 3 cases and 6 grade 4 cases) occurred in 25, and some GI toxicity occurred, but was largely grade 1 or 2, Dr. Hillmen said.
“There really was otherwise very acceptable toxicity,” he added, noting that a single case of tumor lysis syndrome occurred, but was managed successfully by delaying venetoclax.
“That patient re-escalated back onto treatment and is doing well,” he said.
No patients stopped treatment, and only seven had treatment interruption, and then only for a few days, he noted.
The findings are encouraging, and suggest a potent synergy between ibrutinib and venetoclax, said Dr. Hillmen.
“We’re seeing, even at this very early stage, over 30% of patients achieving MRD negative remission, which was our target at the 12-month bone marrow stage with this combination,” he said.
In light of these results, the ongoing phase 3 FLAIR trial, which is actively recruiting, has been modified to include combination ibrutinib and venetoclax in front-line CLL, he said.
Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham (England).
[email protected]
SOURCE: Hillmen P et al., ASH abstract 428.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: 37% and 32% of patients achieved peripheral blood and marrow MRD negativity, respectively.
Study details: Initial results from 38 patients in the CLARITY feasibility trial.
Disclosures: Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham.
Source: Hillmen P et al. ASH Abstract 428.
Avapritinib yields high response rate in patients with systemic mastocytosis
ATLANTA – An oral investigational drug with specific activity against a mutation frequently found in advanced systemic mastocytosis (ASM) produced clinical responses in the majority treated patients, according to preliminary data presented at the annual meeting of the American Society of Hematology.
that included a 56% rate of complete or partial response, according to lead study author Daniel J. DeAngelo, MD, PhD, director of clinical and translational research at Dana-Farber Cancer Institute, Boston.
Currently, midostaurin, a multikinase inhibitor, is the only Food and Drug Administration–approved drug for the treatment of systemic mastocytosis. That approval, announced in April 2017, was based in part on a 17% rate of complete or partial response, Dr. DeAngelo noted at a press briefing.
The primary goal of the phase 1 trial was to evaluate the safety profile and establish a maximum-tolerated dose for once-daily oral avapritinib administration. Treatment-emergent side effects were primarily grade 1-2, according to Dr. DeAngelo. Most hematologic toxicities were mild to moderate, and the most common grade 3 nonhematologic toxicities were periorbital edema and fatigue.
This part of the phase 1 trial enrolled 18 patients with ASM, systemic mastocytosis with associated hematologic neoplasm (SM-AHN), and mantle cell lymphoma (MCL). Efficacy of avapritinib was assessed on International Working Group criteria for response rate in myelodysplasia.
The overall response rate was 72% (13 of 18 patients saw complete response, partial response, or clinical improvement), and a 56% rate of complete and partial response (10 of 18 patients), Dr. DeAngelo said.
Avapritinib was active in all ASM subtypes evaluated, including in patients who had previously been treated with midostaurin or chemotherapy, according to the investigators.
The data on avapritinib suggests the drug “has a potent and clinically important activity in systemic mastocytosis,” he said. “It has been a wonderful success in terms of getting the majority of patients into complete and partial remissions, and so as this evolves, having better targeted agents, I think, can improve the outcome for these patients.”
More patients are being enrolled as the phase 1 study continues into the dose-expansion phase at 300 mg once daily, and 30 of 32 patients remain on treatment with median duration of 9 months, Dr. DeAngelo said.
A phase 2 study in advanced systemic mastocytosis is planned for 2018, as well as phase 1 and phase 2 studies that will include patients with indolent or smoldering disease, he added.
Avapritinib is manufactured by Blueprint Medicines, which also supported the study. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
SOURCE: DeAngelo D et al. ASH 2017 Abstract 2.
ATLANTA – An oral investigational drug with specific activity against a mutation frequently found in advanced systemic mastocytosis (ASM) produced clinical responses in the majority treated patients, according to preliminary data presented at the annual meeting of the American Society of Hematology.
that included a 56% rate of complete or partial response, according to lead study author Daniel J. DeAngelo, MD, PhD, director of clinical and translational research at Dana-Farber Cancer Institute, Boston.
Currently, midostaurin, a multikinase inhibitor, is the only Food and Drug Administration–approved drug for the treatment of systemic mastocytosis. That approval, announced in April 2017, was based in part on a 17% rate of complete or partial response, Dr. DeAngelo noted at a press briefing.
The primary goal of the phase 1 trial was to evaluate the safety profile and establish a maximum-tolerated dose for once-daily oral avapritinib administration. Treatment-emergent side effects were primarily grade 1-2, according to Dr. DeAngelo. Most hematologic toxicities were mild to moderate, and the most common grade 3 nonhematologic toxicities were periorbital edema and fatigue.
This part of the phase 1 trial enrolled 18 patients with ASM, systemic mastocytosis with associated hematologic neoplasm (SM-AHN), and mantle cell lymphoma (MCL). Efficacy of avapritinib was assessed on International Working Group criteria for response rate in myelodysplasia.
The overall response rate was 72% (13 of 18 patients saw complete response, partial response, or clinical improvement), and a 56% rate of complete and partial response (10 of 18 patients), Dr. DeAngelo said.
Avapritinib was active in all ASM subtypes evaluated, including in patients who had previously been treated with midostaurin or chemotherapy, according to the investigators.
The data on avapritinib suggests the drug “has a potent and clinically important activity in systemic mastocytosis,” he said. “It has been a wonderful success in terms of getting the majority of patients into complete and partial remissions, and so as this evolves, having better targeted agents, I think, can improve the outcome for these patients.”
More patients are being enrolled as the phase 1 study continues into the dose-expansion phase at 300 mg once daily, and 30 of 32 patients remain on treatment with median duration of 9 months, Dr. DeAngelo said.
A phase 2 study in advanced systemic mastocytosis is planned for 2018, as well as phase 1 and phase 2 studies that will include patients with indolent or smoldering disease, he added.
Avapritinib is manufactured by Blueprint Medicines, which also supported the study. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
SOURCE: DeAngelo D et al. ASH 2017 Abstract 2.
ATLANTA – An oral investigational drug with specific activity against a mutation frequently found in advanced systemic mastocytosis (ASM) produced clinical responses in the majority treated patients, according to preliminary data presented at the annual meeting of the American Society of Hematology.
that included a 56% rate of complete or partial response, according to lead study author Daniel J. DeAngelo, MD, PhD, director of clinical and translational research at Dana-Farber Cancer Institute, Boston.
Currently, midostaurin, a multikinase inhibitor, is the only Food and Drug Administration–approved drug for the treatment of systemic mastocytosis. That approval, announced in April 2017, was based in part on a 17% rate of complete or partial response, Dr. DeAngelo noted at a press briefing.
The primary goal of the phase 1 trial was to evaluate the safety profile and establish a maximum-tolerated dose for once-daily oral avapritinib administration. Treatment-emergent side effects were primarily grade 1-2, according to Dr. DeAngelo. Most hematologic toxicities were mild to moderate, and the most common grade 3 nonhematologic toxicities were periorbital edema and fatigue.
This part of the phase 1 trial enrolled 18 patients with ASM, systemic mastocytosis with associated hematologic neoplasm (SM-AHN), and mantle cell lymphoma (MCL). Efficacy of avapritinib was assessed on International Working Group criteria for response rate in myelodysplasia.
The overall response rate was 72% (13 of 18 patients saw complete response, partial response, or clinical improvement), and a 56% rate of complete and partial response (10 of 18 patients), Dr. DeAngelo said.
Avapritinib was active in all ASM subtypes evaluated, including in patients who had previously been treated with midostaurin or chemotherapy, according to the investigators.
The data on avapritinib suggests the drug “has a potent and clinically important activity in systemic mastocytosis,” he said. “It has been a wonderful success in terms of getting the majority of patients into complete and partial remissions, and so as this evolves, having better targeted agents, I think, can improve the outcome for these patients.”
More patients are being enrolled as the phase 1 study continues into the dose-expansion phase at 300 mg once daily, and 30 of 32 patients remain on treatment with median duration of 9 months, Dr. DeAngelo said.
A phase 2 study in advanced systemic mastocytosis is planned for 2018, as well as phase 1 and phase 2 studies that will include patients with indolent or smoldering disease, he added.
Avapritinib is manufactured by Blueprint Medicines, which also supported the study. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
SOURCE: DeAngelo D et al. ASH 2017 Abstract 2.
REPORTING FROM ASH 2017
Key clinical point: Avapritinib produced complete or partial responses in the majority of patients with advanced systemic mastocytosis.
Major finding: The overall response rate was 72%, including a 56% rate of complete or partial response.
Data source: Phase 1 dose-escalation study of 18 patients with advanced systemic mastocytosis.
Disclosures: The study was supported by Blueprint Medicines. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
Source: DeAngelo D et al. ASH 2017 Abstract 2
Hallmark tumor metabolism becomes a validated therapeutic target
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
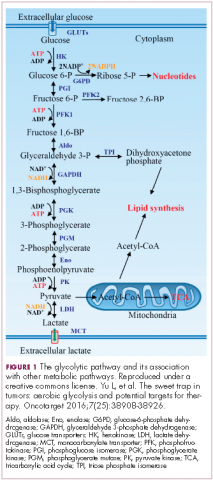
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
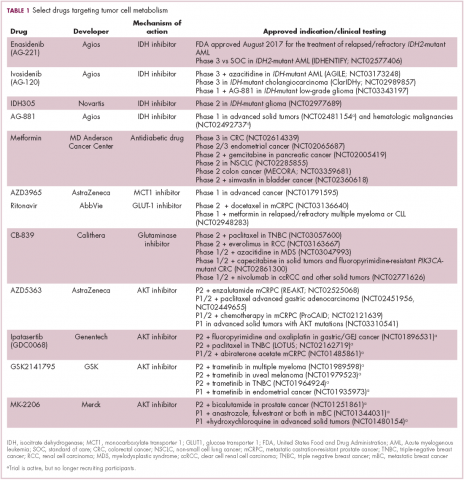
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
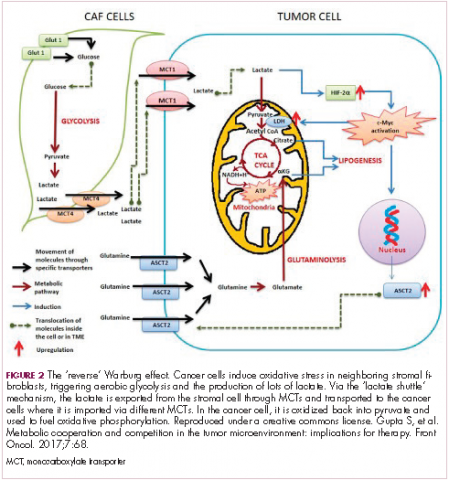
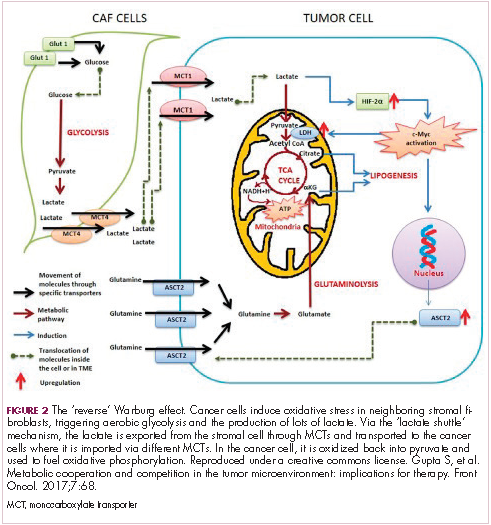
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
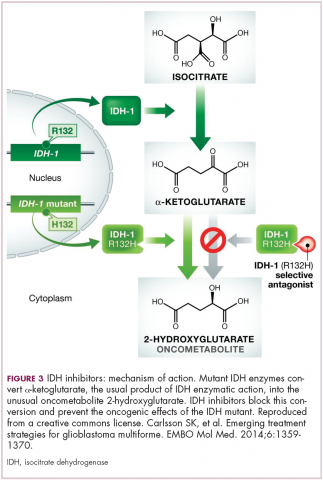
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
Company launches digital PCR test for monitoring CML
Bio-Rad Laboratories, Inc., has launched the QXDx BCR-ABL %IS Kit, a digital polymerase chain reaction (PCR) test that can monitor molecular response to therapy in patients with chronic myeloid leukemia (CML).
The kit has a CE-IVD mark and is available for in vitro diagnostic use in Europe, Hong Kong, and New Zealand.
The QXDx BCR-ABL %IS Kit uses Bio-Rad’s Droplet Digital PCR (ddPCR) technology to provide an absolute measure of BCR-ABL transcripts.
The kit measures BCR-ABL1 and ABL1 chromosomal transcripts in total RNA from whole blood of t(9;22)-positive CML patients expressing BCR-ABL1 fusion transcripts type e13a2 and/or e14a2.
The QXDx BCR-ABL %IS Kit measures the e13a2 and/or e14a2 transcripts of BCR-ABL1, normalized to the ABL1 endogenous control. The kit does not differentiate between e13a2 and e14a2 transcripts and does not monitor other rare fusion transcripts resulting from t(9;22).
The kit’s results are reported as percent reduction from a baseline of 100% on the International Scale (%IS) and on a log molecular reduction (MR) scale.
The kit is able to detect deep molecular response values of MR 4.7 (%IS 0.002) or MR 5.0 (%IS 0.001) in 2- or 4-well formats. This exceeds the typical limitations of reverse transcription quantitative PCR-based tests that are reliable down to MR 4.5 (%IS 0.0032).
Bio-Rad says the QXDx BCR-ABL %IS Kit delivers absolute quantitation, which eliminates the need for standard curves and minimizes variation between samples. The kit also provides scalable throughput, allowing for testing of 8 to 48 samples per run.
The QXDx BCR-ABL %IS Kit can be used with Bio-Rad’s QX200 AutoDG ddPCR Dx System or with the QX200 ddPCR Dx System. The QXDx BCR-ABL %IS Kit uses QuantaSoft Software v1.7 for data acquisition and output. ![]()
Bio-Rad Laboratories, Inc., has launched the QXDx BCR-ABL %IS Kit, a digital polymerase chain reaction (PCR) test that can monitor molecular response to therapy in patients with chronic myeloid leukemia (CML).
The kit has a CE-IVD mark and is available for in vitro diagnostic use in Europe, Hong Kong, and New Zealand.
The QXDx BCR-ABL %IS Kit uses Bio-Rad’s Droplet Digital PCR (ddPCR) technology to provide an absolute measure of BCR-ABL transcripts.
The kit measures BCR-ABL1 and ABL1 chromosomal transcripts in total RNA from whole blood of t(9;22)-positive CML patients expressing BCR-ABL1 fusion transcripts type e13a2 and/or e14a2.
The QXDx BCR-ABL %IS Kit measures the e13a2 and/or e14a2 transcripts of BCR-ABL1, normalized to the ABL1 endogenous control. The kit does not differentiate between e13a2 and e14a2 transcripts and does not monitor other rare fusion transcripts resulting from t(9;22).
The kit’s results are reported as percent reduction from a baseline of 100% on the International Scale (%IS) and on a log molecular reduction (MR) scale.
The kit is able to detect deep molecular response values of MR 4.7 (%IS 0.002) or MR 5.0 (%IS 0.001) in 2- or 4-well formats. This exceeds the typical limitations of reverse transcription quantitative PCR-based tests that are reliable down to MR 4.5 (%IS 0.0032).
Bio-Rad says the QXDx BCR-ABL %IS Kit delivers absolute quantitation, which eliminates the need for standard curves and minimizes variation between samples. The kit also provides scalable throughput, allowing for testing of 8 to 48 samples per run.
The QXDx BCR-ABL %IS Kit can be used with Bio-Rad’s QX200 AutoDG ddPCR Dx System or with the QX200 ddPCR Dx System. The QXDx BCR-ABL %IS Kit uses QuantaSoft Software v1.7 for data acquisition and output. ![]()
Bio-Rad Laboratories, Inc., has launched the QXDx BCR-ABL %IS Kit, a digital polymerase chain reaction (PCR) test that can monitor molecular response to therapy in patients with chronic myeloid leukemia (CML).
The kit has a CE-IVD mark and is available for in vitro diagnostic use in Europe, Hong Kong, and New Zealand.
The QXDx BCR-ABL %IS Kit uses Bio-Rad’s Droplet Digital PCR (ddPCR) technology to provide an absolute measure of BCR-ABL transcripts.
The kit measures BCR-ABL1 and ABL1 chromosomal transcripts in total RNA from whole blood of t(9;22)-positive CML patients expressing BCR-ABL1 fusion transcripts type e13a2 and/or e14a2.
The QXDx BCR-ABL %IS Kit measures the e13a2 and/or e14a2 transcripts of BCR-ABL1, normalized to the ABL1 endogenous control. The kit does not differentiate between e13a2 and e14a2 transcripts and does not monitor other rare fusion transcripts resulting from t(9;22).
The kit’s results are reported as percent reduction from a baseline of 100% on the International Scale (%IS) and on a log molecular reduction (MR) scale.
The kit is able to detect deep molecular response values of MR 4.7 (%IS 0.002) or MR 5.0 (%IS 0.001) in 2- or 4-well formats. This exceeds the typical limitations of reverse transcription quantitative PCR-based tests that are reliable down to MR 4.5 (%IS 0.0032).
Bio-Rad says the QXDx BCR-ABL %IS Kit delivers absolute quantitation, which eliminates the need for standard curves and minimizes variation between samples. The kit also provides scalable throughput, allowing for testing of 8 to 48 samples per run.
The QXDx BCR-ABL %IS Kit can be used with Bio-Rad’s QX200 AutoDG ddPCR Dx System or with the QX200 ddPCR Dx System. The QXDx BCR-ABL %IS Kit uses QuantaSoft Software v1.7 for data acquisition and output. ![]()