User login
Women, older patients at greater risk of more aggressive PBC
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: Women, older individuals, and patients with other autoimmune diseases are more likely to have worse primary biliary cholangitis (PBC).
Major finding: More than one-third of patients with PBC have high levels of alkaline phosphatase.
Study details: An analysis of medical records for 15,875 patients with PBC.
Disclosures: Two authors declared research funding or consulting fees from the pharmaceutical industry; one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
Source: Younossi ZM et al. J Clin Gastroenterol. 2018 August 24. doi: 10.1097/MCG.0000000000001120.
Outpatient costs soar for Medicare patients with chronic hepatitis B
The average cost of outpatient care for Medicare recipients with chronic hepatitis B (CH-B) rose by 400% from 2005 to 2014, according to investigators.
Inpatient costs also increased, although less dramatically, reported lead author Min Kim, MD, of the Inova Fairfax Hospital Center for Liver Diseases in Falls Church, Virginia, and her colleagues. The causes of these spending hikes may range from policy changes and expanded screening to an aging immigrant population.
“According to the National Health and Nutrition Examination Survey, from 1988 to 2012 most people with CH-B in the United States were foreign born and accounted for up to 70% of all CH-B infections,” the authors wrote in the Journal of Clinical Gastroenterology. “The Centers for Disease Control [and Prevention] estimates that Asians, who comprise 5% of the U.S. population, account for 50% of all chronic CH-B infections.” Despite these statistics, the clinical and economic impacts of an aging immigrant population are unknown. The investigators therefore assessed patient characteristics associated with increased 1-year mortality and the impact of demographic changes on Medicare costs.
The retrospective study began with a random sample of Medicare beneficiaries from 2005 to 2014. From this group, 18,603 patients with CH-B were identified by ICD-9 codes V02.61, 070.2, 070.3, 070.42, and 070.52. Patients with ICD-9-CM codes of 197.7, 155.1, or 155.2 were excluded, as were records containing insufficient information about year, region, or race. Patients were analyzed collectively and as inpatients (n = 6,550) or outpatients (n = 13,648).
Cost of care (per patient, per year) and 1-year mortality were evaluated. Patient characteristics included age, sex, race/ethnicity, geographic region, type of Medicare eligibility, length of stay, Charlson comorbidity index, presence of decompensated cirrhosis, and/or hepatocellular carcinoma (HCC).
Most dramatically, outpatient charges rose more than 400% during the study period, from $9,257 in 2005 to $47,864 in 2014 (P less than .001). Inpatient charges increased by almost 50%, from $66,610 to $94,221 (P less than .001). (All values converted to 2016 dollars.)
Although the increase in outpatient costs appears seismic, the authors noted that costs held steady from 2005 to 2010 before spiking dramatically, reaching a peak of $58,450 in 2013 before settling down to $47,864 the following year. This spike may be caused by changes in screening measures and policies. In 2009, the American Association for the Study of Liver Diseases expanded screening guidelines to include previously ineligible patients with CH-B, and in 2010, the Centers for Medicare & Medicaid Services expanded ICD-9 and ICD-10 codes for CH-B from 9 to 25.
“It seems plausible that the increase in CH-B prevalence, and its associated costs, might actually be a reflection of [these factors],” the authors noted. Still, “additional studies are needed to clarify this observation.”
Turning to patient characteristics, the authors reported that 1-year mortality was independently associated most strongly with decompensated cirrhosis (odds ratio, 3.02) and hepatocellular carcinoma (OR, 2.64). In comparison with white patients, Asians were less likely to die (OR, 0.47).
“It is possible that this can be explained through differences in transmission mode and disease progression of CH-B between these two demographics,” the authors wrote. “A majority of Asian Medicare recipients with CH-B likely acquired it perinatally and did not develop significant liver disease. In contrast, whites with CH-B generally acquired it in adulthood, increasing the chance of developing liver disease.”
Over the 10-year study period, Medicare beneficiaries with CH-B became more frequently Asian and less frequently male. While the number of outpatient visits and average Charlson comorbidity index increased, decreases were reported for length of stay, rates of 1-year mortality, hospitalization, and HCC – the latter of which is most closely associated with higher costs of care.
The investigators suggested that the decreased incidence of HCC was caused by “better screening programs for HCC and/or more widespread use of antiviral treatment for CH-B.”
“Although advances in antiviral treatment have effectively reduced hospitalization and disease progression,” the authors wrote, “vulnerable groups – especially immigrants and individuals living in poverty – present an important challenge for better identification of infected individuals and their linkage to care. In this context, it is vital to target these cohorts to reduce further mortality and resource utilization, as well as optimize long-term public health and financial benefits.”
Study funding was provided by Seattle Genetics. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
SOURCE: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
The average cost of outpatient care for Medicare recipients with chronic hepatitis B (CH-B) rose by 400% from 2005 to 2014, according to investigators.
Inpatient costs also increased, although less dramatically, reported lead author Min Kim, MD, of the Inova Fairfax Hospital Center for Liver Diseases in Falls Church, Virginia, and her colleagues. The causes of these spending hikes may range from policy changes and expanded screening to an aging immigrant population.
“According to the National Health and Nutrition Examination Survey, from 1988 to 2012 most people with CH-B in the United States were foreign born and accounted for up to 70% of all CH-B infections,” the authors wrote in the Journal of Clinical Gastroenterology. “The Centers for Disease Control [and Prevention] estimates that Asians, who comprise 5% of the U.S. population, account for 50% of all chronic CH-B infections.” Despite these statistics, the clinical and economic impacts of an aging immigrant population are unknown. The investigators therefore assessed patient characteristics associated with increased 1-year mortality and the impact of demographic changes on Medicare costs.
The retrospective study began with a random sample of Medicare beneficiaries from 2005 to 2014. From this group, 18,603 patients with CH-B were identified by ICD-9 codes V02.61, 070.2, 070.3, 070.42, and 070.52. Patients with ICD-9-CM codes of 197.7, 155.1, or 155.2 were excluded, as were records containing insufficient information about year, region, or race. Patients were analyzed collectively and as inpatients (n = 6,550) or outpatients (n = 13,648).
Cost of care (per patient, per year) and 1-year mortality were evaluated. Patient characteristics included age, sex, race/ethnicity, geographic region, type of Medicare eligibility, length of stay, Charlson comorbidity index, presence of decompensated cirrhosis, and/or hepatocellular carcinoma (HCC).
Most dramatically, outpatient charges rose more than 400% during the study period, from $9,257 in 2005 to $47,864 in 2014 (P less than .001). Inpatient charges increased by almost 50%, from $66,610 to $94,221 (P less than .001). (All values converted to 2016 dollars.)
Although the increase in outpatient costs appears seismic, the authors noted that costs held steady from 2005 to 2010 before spiking dramatically, reaching a peak of $58,450 in 2013 before settling down to $47,864 the following year. This spike may be caused by changes in screening measures and policies. In 2009, the American Association for the Study of Liver Diseases expanded screening guidelines to include previously ineligible patients with CH-B, and in 2010, the Centers for Medicare & Medicaid Services expanded ICD-9 and ICD-10 codes for CH-B from 9 to 25.
“It seems plausible that the increase in CH-B prevalence, and its associated costs, might actually be a reflection of [these factors],” the authors noted. Still, “additional studies are needed to clarify this observation.”
Turning to patient characteristics, the authors reported that 1-year mortality was independently associated most strongly with decompensated cirrhosis (odds ratio, 3.02) and hepatocellular carcinoma (OR, 2.64). In comparison with white patients, Asians were less likely to die (OR, 0.47).
“It is possible that this can be explained through differences in transmission mode and disease progression of CH-B between these two demographics,” the authors wrote. “A majority of Asian Medicare recipients with CH-B likely acquired it perinatally and did not develop significant liver disease. In contrast, whites with CH-B generally acquired it in adulthood, increasing the chance of developing liver disease.”
Over the 10-year study period, Medicare beneficiaries with CH-B became more frequently Asian and less frequently male. While the number of outpatient visits and average Charlson comorbidity index increased, decreases were reported for length of stay, rates of 1-year mortality, hospitalization, and HCC – the latter of which is most closely associated with higher costs of care.
The investigators suggested that the decreased incidence of HCC was caused by “better screening programs for HCC and/or more widespread use of antiviral treatment for CH-B.”
“Although advances in antiviral treatment have effectively reduced hospitalization and disease progression,” the authors wrote, “vulnerable groups – especially immigrants and individuals living in poverty – present an important challenge for better identification of infected individuals and their linkage to care. In this context, it is vital to target these cohorts to reduce further mortality and resource utilization, as well as optimize long-term public health and financial benefits.”
Study funding was provided by Seattle Genetics. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
SOURCE: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
The average cost of outpatient care for Medicare recipients with chronic hepatitis B (CH-B) rose by 400% from 2005 to 2014, according to investigators.
Inpatient costs also increased, although less dramatically, reported lead author Min Kim, MD, of the Inova Fairfax Hospital Center for Liver Diseases in Falls Church, Virginia, and her colleagues. The causes of these spending hikes may range from policy changes and expanded screening to an aging immigrant population.
“According to the National Health and Nutrition Examination Survey, from 1988 to 2012 most people with CH-B in the United States were foreign born and accounted for up to 70% of all CH-B infections,” the authors wrote in the Journal of Clinical Gastroenterology. “The Centers for Disease Control [and Prevention] estimates that Asians, who comprise 5% of the U.S. population, account for 50% of all chronic CH-B infections.” Despite these statistics, the clinical and economic impacts of an aging immigrant population are unknown. The investigators therefore assessed patient characteristics associated with increased 1-year mortality and the impact of demographic changes on Medicare costs.
The retrospective study began with a random sample of Medicare beneficiaries from 2005 to 2014. From this group, 18,603 patients with CH-B were identified by ICD-9 codes V02.61, 070.2, 070.3, 070.42, and 070.52. Patients with ICD-9-CM codes of 197.7, 155.1, or 155.2 were excluded, as were records containing insufficient information about year, region, or race. Patients were analyzed collectively and as inpatients (n = 6,550) or outpatients (n = 13,648).
Cost of care (per patient, per year) and 1-year mortality were evaluated. Patient characteristics included age, sex, race/ethnicity, geographic region, type of Medicare eligibility, length of stay, Charlson comorbidity index, presence of decompensated cirrhosis, and/or hepatocellular carcinoma (HCC).
Most dramatically, outpatient charges rose more than 400% during the study period, from $9,257 in 2005 to $47,864 in 2014 (P less than .001). Inpatient charges increased by almost 50%, from $66,610 to $94,221 (P less than .001). (All values converted to 2016 dollars.)
Although the increase in outpatient costs appears seismic, the authors noted that costs held steady from 2005 to 2010 before spiking dramatically, reaching a peak of $58,450 in 2013 before settling down to $47,864 the following year. This spike may be caused by changes in screening measures and policies. In 2009, the American Association for the Study of Liver Diseases expanded screening guidelines to include previously ineligible patients with CH-B, and in 2010, the Centers for Medicare & Medicaid Services expanded ICD-9 and ICD-10 codes for CH-B from 9 to 25.
“It seems plausible that the increase in CH-B prevalence, and its associated costs, might actually be a reflection of [these factors],” the authors noted. Still, “additional studies are needed to clarify this observation.”
Turning to patient characteristics, the authors reported that 1-year mortality was independently associated most strongly with decompensated cirrhosis (odds ratio, 3.02) and hepatocellular carcinoma (OR, 2.64). In comparison with white patients, Asians were less likely to die (OR, 0.47).
“It is possible that this can be explained through differences in transmission mode and disease progression of CH-B between these two demographics,” the authors wrote. “A majority of Asian Medicare recipients with CH-B likely acquired it perinatally and did not develop significant liver disease. In contrast, whites with CH-B generally acquired it in adulthood, increasing the chance of developing liver disease.”
Over the 10-year study period, Medicare beneficiaries with CH-B became more frequently Asian and less frequently male. While the number of outpatient visits and average Charlson comorbidity index increased, decreases were reported for length of stay, rates of 1-year mortality, hospitalization, and HCC – the latter of which is most closely associated with higher costs of care.
The investigators suggested that the decreased incidence of HCC was caused by “better screening programs for HCC and/or more widespread use of antiviral treatment for CH-B.”
“Although advances in antiviral treatment have effectively reduced hospitalization and disease progression,” the authors wrote, “vulnerable groups – especially immigrants and individuals living in poverty – present an important challenge for better identification of infected individuals and their linkage to care. In this context, it is vital to target these cohorts to reduce further mortality and resource utilization, as well as optimize long-term public health and financial benefits.”
Study funding was provided by Seattle Genetics. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
SOURCE: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: Outpatient care for patients with chronic hepatitis B is becoming more expensive; the trend may be tied to an aging immigrant population.
Major finding: The average Medicare charge for outpatient care per patient increased from $9,257 in 2005 to $47,864 in 2014 (P less than .001).
Study details: A retrospective study involving 18,603 Medicare recipients with chronic hepatitis B who filed claims between 2005 and 2014.
Disclosures: Study funding was provided by the Beatty Center for Integrated Research. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
Source: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
A postgraduate tour through the biliary tree, pancreas, and liver
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
Even modest alcohol use may worsen NAFLD
Patients with nonalcoholic fatty liver disease who consumed modest quantities of alcohol had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis, compared with nondrinkers, according to the results of a longitudinal cohort study published in the Clinical Gastroenterology and Hepatology.
Modest drinkers also had significantly less improvement in their AST levels, compared with nondrinkers, said Veeral Ajmera, MD, of the University of California, San Diego, and his associates. “Importantly, our results suggest that cessation of alcohol use may mitigate these changes,” they wrote. Clinicians should consider the spectrum of nonalcoholic fatty liver disease (NAFLD), and especially nonalcoholic steatohepatitis (NASH), when making recommendations about alcohol use. “More advanced NAFLD severity may warrant counseling against [even] modest alcohol use.”
More than one in three adults in the United States has NAFLD and about two-thirds drink alcohol, almost always in moderation, the researchers noted. Modest alcohol use has been linked to decreased cardiovascular risk, which is particularly relevant because patients with NAFLD tend to have risk factors for cardiovascular disease. Results from at least two cross-sectional studies also suggest modest drinkers with NAFLD have less severe histology, including less NASH and fibrosis. However, modest drinkers tend to be more physically active, with lower body mass indices, higher physical activity levels, and less obesity, which are potential confounders. To better understand the effects of modest alcohol consumption on NAFLD, the researchers studied adults with NAFLD who participated in studies conducted by the multicenter NASH Clinical Research Network.
The 285 participants were typically aged in their late 40s, female, white, and obese, with an average body mass index of 34.7 kg/m2. In all, 168 participants (59%) reported consuming up to two drinks per day, while 41% abstained from alcohol use. During an average of 47 months between biopsies (standard deviation, 26 months), nondrinkers averaged a 0.49 reduction in steatosis grade, significantly more than that of modest drinkers (reduction, 0.30; P = .04). Nondrinkers also had a greater decrease in mean AST level (7 U/L), compared with drinkers (2 U/L; P = .04).
A total of 64% of patients were classified as having definite NASH, 19% had NAFLD without NASH, and 17% had borderline NASH. At baseline, 23% of patients did not have fibrosis, 32% had stage 1 fibrosis, 21% had stage 2, 21% had stage 3, and 3% had stage 4. Modest drinkers were more likely to be white and were less likely to be diagnosed with definitive NASH at baseline. After controlling for these potential confounders, modest drinkers had significantly lower odds of NASH resolution, compared with nondrinkers (adjusted odds ratio, 0.32; 95% confidence interval, 0.11-0.92; P = .04).
“[The] presence of NASH has consistently been shown to predict increased risk for fibrosis progression, and therefore, our finding of less NASH resolution among consistent modest drinkers is clinically relevant,” the investigators wrote. “Although we were unable to assess the association between modest alcohol consumption and cardiovascular risk, we did not see any significant changes in measured metabolic risk factors with known associations with cardiovascular disease including low-density lipoprotein and high-density lipoprotein cholesterol and insulin resistance.”
Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
SOURCE: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.
Patients with nonalcoholic fatty liver disease who consumed modest quantities of alcohol had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis, compared with nondrinkers, according to the results of a longitudinal cohort study published in the Clinical Gastroenterology and Hepatology.
Modest drinkers also had significantly less improvement in their AST levels, compared with nondrinkers, said Veeral Ajmera, MD, of the University of California, San Diego, and his associates. “Importantly, our results suggest that cessation of alcohol use may mitigate these changes,” they wrote. Clinicians should consider the spectrum of nonalcoholic fatty liver disease (NAFLD), and especially nonalcoholic steatohepatitis (NASH), when making recommendations about alcohol use. “More advanced NAFLD severity may warrant counseling against [even] modest alcohol use.”
More than one in three adults in the United States has NAFLD and about two-thirds drink alcohol, almost always in moderation, the researchers noted. Modest alcohol use has been linked to decreased cardiovascular risk, which is particularly relevant because patients with NAFLD tend to have risk factors for cardiovascular disease. Results from at least two cross-sectional studies also suggest modest drinkers with NAFLD have less severe histology, including less NASH and fibrosis. However, modest drinkers tend to be more physically active, with lower body mass indices, higher physical activity levels, and less obesity, which are potential confounders. To better understand the effects of modest alcohol consumption on NAFLD, the researchers studied adults with NAFLD who participated in studies conducted by the multicenter NASH Clinical Research Network.
The 285 participants were typically aged in their late 40s, female, white, and obese, with an average body mass index of 34.7 kg/m2. In all, 168 participants (59%) reported consuming up to two drinks per day, while 41% abstained from alcohol use. During an average of 47 months between biopsies (standard deviation, 26 months), nondrinkers averaged a 0.49 reduction in steatosis grade, significantly more than that of modest drinkers (reduction, 0.30; P = .04). Nondrinkers also had a greater decrease in mean AST level (7 U/L), compared with drinkers (2 U/L; P = .04).
A total of 64% of patients were classified as having definite NASH, 19% had NAFLD without NASH, and 17% had borderline NASH. At baseline, 23% of patients did not have fibrosis, 32% had stage 1 fibrosis, 21% had stage 2, 21% had stage 3, and 3% had stage 4. Modest drinkers were more likely to be white and were less likely to be diagnosed with definitive NASH at baseline. After controlling for these potential confounders, modest drinkers had significantly lower odds of NASH resolution, compared with nondrinkers (adjusted odds ratio, 0.32; 95% confidence interval, 0.11-0.92; P = .04).
“[The] presence of NASH has consistently been shown to predict increased risk for fibrosis progression, and therefore, our finding of less NASH resolution among consistent modest drinkers is clinically relevant,” the investigators wrote. “Although we were unable to assess the association between modest alcohol consumption and cardiovascular risk, we did not see any significant changes in measured metabolic risk factors with known associations with cardiovascular disease including low-density lipoprotein and high-density lipoprotein cholesterol and insulin resistance.”
Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
SOURCE: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.
Patients with nonalcoholic fatty liver disease who consumed modest quantities of alcohol had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis, compared with nondrinkers, according to the results of a longitudinal cohort study published in the Clinical Gastroenterology and Hepatology.
Modest drinkers also had significantly less improvement in their AST levels, compared with nondrinkers, said Veeral Ajmera, MD, of the University of California, San Diego, and his associates. “Importantly, our results suggest that cessation of alcohol use may mitigate these changes,” they wrote. Clinicians should consider the spectrum of nonalcoholic fatty liver disease (NAFLD), and especially nonalcoholic steatohepatitis (NASH), when making recommendations about alcohol use. “More advanced NAFLD severity may warrant counseling against [even] modest alcohol use.”
More than one in three adults in the United States has NAFLD and about two-thirds drink alcohol, almost always in moderation, the researchers noted. Modest alcohol use has been linked to decreased cardiovascular risk, which is particularly relevant because patients with NAFLD tend to have risk factors for cardiovascular disease. Results from at least two cross-sectional studies also suggest modest drinkers with NAFLD have less severe histology, including less NASH and fibrosis. However, modest drinkers tend to be more physically active, with lower body mass indices, higher physical activity levels, and less obesity, which are potential confounders. To better understand the effects of modest alcohol consumption on NAFLD, the researchers studied adults with NAFLD who participated in studies conducted by the multicenter NASH Clinical Research Network.
The 285 participants were typically aged in their late 40s, female, white, and obese, with an average body mass index of 34.7 kg/m2. In all, 168 participants (59%) reported consuming up to two drinks per day, while 41% abstained from alcohol use. During an average of 47 months between biopsies (standard deviation, 26 months), nondrinkers averaged a 0.49 reduction in steatosis grade, significantly more than that of modest drinkers (reduction, 0.30; P = .04). Nondrinkers also had a greater decrease in mean AST level (7 U/L), compared with drinkers (2 U/L; P = .04).
A total of 64% of patients were classified as having definite NASH, 19% had NAFLD without NASH, and 17% had borderline NASH. At baseline, 23% of patients did not have fibrosis, 32% had stage 1 fibrosis, 21% had stage 2, 21% had stage 3, and 3% had stage 4. Modest drinkers were more likely to be white and were less likely to be diagnosed with definitive NASH at baseline. After controlling for these potential confounders, modest drinkers had significantly lower odds of NASH resolution, compared with nondrinkers (adjusted odds ratio, 0.32; 95% confidence interval, 0.11-0.92; P = .04).
“[The] presence of NASH has consistently been shown to predict increased risk for fibrosis progression, and therefore, our finding of less NASH resolution among consistent modest drinkers is clinically relevant,” the investigators wrote. “Although we were unable to assess the association between modest alcohol consumption and cardiovascular risk, we did not see any significant changes in measured metabolic risk factors with known associations with cardiovascular disease including low-density lipoprotein and high-density lipoprotein cholesterol and insulin resistance.”
Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
SOURCE: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Consider counseling patients with more advanced nonalcoholic fatty liver disease to avoid alcohol.
Major finding: Compared with nondrinkers, patients who reported modest alcohol use had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis (P = .04 for both comparisons).
Study details: A longitudinal cohort study of 285 adults with nonalcoholic fatty liver disease with paired biopsy specimens obtained an average of 4 years apart.
Disclosures: Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
Source: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.
Avatrombopag cut procedure-related transfusions in patients with thrombocytopenia, chronic liver disease
Once-daily treatment with the oral second-generation thrombopoietin agonist avatrombopag (Doptelet) significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures, according to the results of two international, randomized, double-blind, phase III, placebo-controlled trials reported in the September issue of Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia, versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group, versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
These results led the Food and Drug Administration to approve avatrombopag in May 2018 under its priority review process. The novel therapy “may be a safe and effective alternative to platelet transfusions” that could simplify the clinical management of patients with chronic liver disease and thrombocytopenia, Norah Terrault, MD, MPH, and her associates wrote in Gastroenterology.
The ADAPT-1 study included 231 patients, while ADAPT-2 included 204 patients. In each trial, patients were randomized on a 2:1 basis to receive oral avatrombopag or placebo once daily for 5 consecutive days. Patients in the intervention arms received 60 mg avatrombopag if their baseline platelet count was less than 40 x 109 per liter, and 40 mg if their baseline platelet count was 40-50 x 109 per liter. Procedures were scheduled for 10-13 days after treatment initiation.
“Platelet counts increased by [treatment] day 4, peaked at days 10-13, and then returned to baseline levels by day 35,” the researchers reported. Among ADAPT-1 patients with low baseline counts, 69% of avatrombopag recipients reached a prespecified target of at least 50 x 109 platelets per liter on their procedure day, versus 4% of placebo recipients (P less than .0001). Corresponding proportions in ADAPT-2 were 67% and 7%, respectively (P less than .0001). Among patients with higher baseline counts, 88% and 20% achieved the target, respectively, in ADAPT-1 (P less than .0001), as did 93% versus 39%, respectively, in ADAPT-2 (P less than .0001).
Avatrombopag and placebo produced similar rates of treatment-emergent adverse events. These most often consisted of abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache. Only three avatrombopag patients developed platelet counts above 200 x 109 per liter, and they all remained asymptomatic, the investigators said.
Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, Bristol-Myers Squibb, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
SOURCE: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
Thrombocytopenia in cirrhosis is frequent and multifactorial and includes sequestration in the spleen, reduced liver-derived thrombopoietin, bone marrow toxicity, and autoimmunity towards platelets. Severe thrombocytopenia (less than 50/nL) is rare in cirrhotic patients, but when it occurs may prevent required procedures from being performed or require platelet transfusions, which are associated with significant risks.
Previous attempts to increase platelets in cirrhotic patients with thrombopoietin agonists were halted because of increased frequency of portal vein thrombosis and hepatic decompensation.
Now avatrombopag has been specifically licensed with a 5-day regimen to increase platelets prior to elective interventions in severely thrombocytopenic (less than 50/nL) patients with chronic liver disease with a seemingly better safety profile than earlier treatments and good efficacy. The patient groups studied in the licensing trial had slightly milder but not significantly different liver disease, compared with those in the eltrombopag studies. The key difference was a pretreatment requirement of a portal vein flow of more than 10 cm/sec prior to enrollment, which likely reduced the risk of portal vein thrombosis. It is important that providers ready to use avatrombopag are aware of this.
Importantly, no data are currently available for patients with a Model for End-Stage Liver Disease score greater than 24, and very limited data are available for patients with Child B and Child C cirrhosis.
Given this limitation, careful judgment will be needed; a pretreatment portal vein flow may be advisable, though not a label requirement.
An observational study, NCT03554759, in patients with chronic liver disease and thrombocytopenia is ongoing and will further confirm the likely safety of avatrombopag.
Hans L. Tillmann, MD, is a clinical associate professor, East Carolina University, Greenville, and staff physician, Greenville (N.C.) VA Health Care Center. He has no relevant conflicts of interest.
Thrombocytopenia in cirrhosis is frequent and multifactorial and includes sequestration in the spleen, reduced liver-derived thrombopoietin, bone marrow toxicity, and autoimmunity towards platelets. Severe thrombocytopenia (less than 50/nL) is rare in cirrhotic patients, but when it occurs may prevent required procedures from being performed or require platelet transfusions, which are associated with significant risks.
Previous attempts to increase platelets in cirrhotic patients with thrombopoietin agonists were halted because of increased frequency of portal vein thrombosis and hepatic decompensation.
Now avatrombopag has been specifically licensed with a 5-day regimen to increase platelets prior to elective interventions in severely thrombocytopenic (less than 50/nL) patients with chronic liver disease with a seemingly better safety profile than earlier treatments and good efficacy. The patient groups studied in the licensing trial had slightly milder but not significantly different liver disease, compared with those in the eltrombopag studies. The key difference was a pretreatment requirement of a portal vein flow of more than 10 cm/sec prior to enrollment, which likely reduced the risk of portal vein thrombosis. It is important that providers ready to use avatrombopag are aware of this.
Importantly, no data are currently available for patients with a Model for End-Stage Liver Disease score greater than 24, and very limited data are available for patients with Child B and Child C cirrhosis.
Given this limitation, careful judgment will be needed; a pretreatment portal vein flow may be advisable, though not a label requirement.
An observational study, NCT03554759, in patients with chronic liver disease and thrombocytopenia is ongoing and will further confirm the likely safety of avatrombopag.
Hans L. Tillmann, MD, is a clinical associate professor, East Carolina University, Greenville, and staff physician, Greenville (N.C.) VA Health Care Center. He has no relevant conflicts of interest.
Thrombocytopenia in cirrhosis is frequent and multifactorial and includes sequestration in the spleen, reduced liver-derived thrombopoietin, bone marrow toxicity, and autoimmunity towards platelets. Severe thrombocytopenia (less than 50/nL) is rare in cirrhotic patients, but when it occurs may prevent required procedures from being performed or require platelet transfusions, which are associated with significant risks.
Previous attempts to increase platelets in cirrhotic patients with thrombopoietin agonists were halted because of increased frequency of portal vein thrombosis and hepatic decompensation.
Now avatrombopag has been specifically licensed with a 5-day regimen to increase platelets prior to elective interventions in severely thrombocytopenic (less than 50/nL) patients with chronic liver disease with a seemingly better safety profile than earlier treatments and good efficacy. The patient groups studied in the licensing trial had slightly milder but not significantly different liver disease, compared with those in the eltrombopag studies. The key difference was a pretreatment requirement of a portal vein flow of more than 10 cm/sec prior to enrollment, which likely reduced the risk of portal vein thrombosis. It is important that providers ready to use avatrombopag are aware of this.
Importantly, no data are currently available for patients with a Model for End-Stage Liver Disease score greater than 24, and very limited data are available for patients with Child B and Child C cirrhosis.
Given this limitation, careful judgment will be needed; a pretreatment portal vein flow may be advisable, though not a label requirement.
An observational study, NCT03554759, in patients with chronic liver disease and thrombocytopenia is ongoing and will further confirm the likely safety of avatrombopag.
Hans L. Tillmann, MD, is a clinical associate professor, East Carolina University, Greenville, and staff physician, Greenville (N.C.) VA Health Care Center. He has no relevant conflicts of interest.
Once-daily treatment with the oral second-generation thrombopoietin agonist avatrombopag (Doptelet) significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures, according to the results of two international, randomized, double-blind, phase III, placebo-controlled trials reported in the September issue of Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia, versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group, versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
These results led the Food and Drug Administration to approve avatrombopag in May 2018 under its priority review process. The novel therapy “may be a safe and effective alternative to platelet transfusions” that could simplify the clinical management of patients with chronic liver disease and thrombocytopenia, Norah Terrault, MD, MPH, and her associates wrote in Gastroenterology.
The ADAPT-1 study included 231 patients, while ADAPT-2 included 204 patients. In each trial, patients were randomized on a 2:1 basis to receive oral avatrombopag or placebo once daily for 5 consecutive days. Patients in the intervention arms received 60 mg avatrombopag if their baseline platelet count was less than 40 x 109 per liter, and 40 mg if their baseline platelet count was 40-50 x 109 per liter. Procedures were scheduled for 10-13 days after treatment initiation.
“Platelet counts increased by [treatment] day 4, peaked at days 10-13, and then returned to baseline levels by day 35,” the researchers reported. Among ADAPT-1 patients with low baseline counts, 69% of avatrombopag recipients reached a prespecified target of at least 50 x 109 platelets per liter on their procedure day, versus 4% of placebo recipients (P less than .0001). Corresponding proportions in ADAPT-2 were 67% and 7%, respectively (P less than .0001). Among patients with higher baseline counts, 88% and 20% achieved the target, respectively, in ADAPT-1 (P less than .0001), as did 93% versus 39%, respectively, in ADAPT-2 (P less than .0001).
Avatrombopag and placebo produced similar rates of treatment-emergent adverse events. These most often consisted of abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache. Only three avatrombopag patients developed platelet counts above 200 x 109 per liter, and they all remained asymptomatic, the investigators said.
Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, Bristol-Myers Squibb, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
SOURCE: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
Once-daily treatment with the oral second-generation thrombopoietin agonist avatrombopag (Doptelet) significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures, according to the results of two international, randomized, double-blind, phase III, placebo-controlled trials reported in the September issue of Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia, versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group, versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
These results led the Food and Drug Administration to approve avatrombopag in May 2018 under its priority review process. The novel therapy “may be a safe and effective alternative to platelet transfusions” that could simplify the clinical management of patients with chronic liver disease and thrombocytopenia, Norah Terrault, MD, MPH, and her associates wrote in Gastroenterology.
The ADAPT-1 study included 231 patients, while ADAPT-2 included 204 patients. In each trial, patients were randomized on a 2:1 basis to receive oral avatrombopag or placebo once daily for 5 consecutive days. Patients in the intervention arms received 60 mg avatrombopag if their baseline platelet count was less than 40 x 109 per liter, and 40 mg if their baseline platelet count was 40-50 x 109 per liter. Procedures were scheduled for 10-13 days after treatment initiation.
“Platelet counts increased by [treatment] day 4, peaked at days 10-13, and then returned to baseline levels by day 35,” the researchers reported. Among ADAPT-1 patients with low baseline counts, 69% of avatrombopag recipients reached a prespecified target of at least 50 x 109 platelets per liter on their procedure day, versus 4% of placebo recipients (P less than .0001). Corresponding proportions in ADAPT-2 were 67% and 7%, respectively (P less than .0001). Among patients with higher baseline counts, 88% and 20% achieved the target, respectively, in ADAPT-1 (P less than .0001), as did 93% versus 39%, respectively, in ADAPT-2 (P less than .0001).
Avatrombopag and placebo produced similar rates of treatment-emergent adverse events. These most often consisted of abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache. Only three avatrombopag patients developed platelet counts above 200 x 109 per liter, and they all remained asymptomatic, the investigators said.
Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, Bristol-Myers Squibb, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
SOURCE: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
FROM GASTROENTEROLOGY
Key clinical point: Once-daily treatment with oral avatrombopag significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures.
Major finding: In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
Study details: ADAPT-1 and ADAPT-2, international, randomized, double-blind, placebo-controlled, phase III trials.
Disclosures: Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, BMS, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
Source: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
Study examines the world of alcohol use
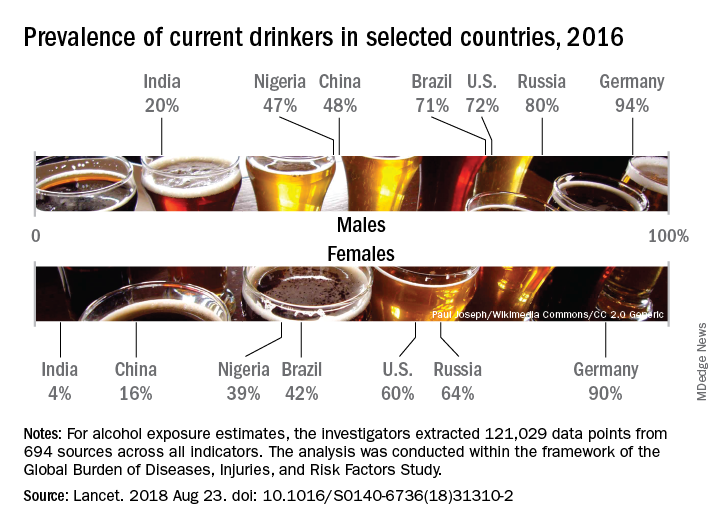
Considerable variations were seen in alcohol consumption. In 2016, males overall consumed more than twice as many standard drinks per day as females: 1.70 versus 0.73. Alcohol consumption in those aged 15-95 years was highest in the top quintile of countries according to sociodemographic development for both males (2.9 drinks per day) and females (1.9) and lowest in the bottom quintile of countries for males (1.4) and the second-lowest quintile for females (0.3), Max G. Griswold, MA, of the University of Washington, Seattle, and his associates said in the Lancet.
Denmark had the highest prevalence of current drinkers of any country for both males (97%) and females (95%) in 2016; Pakistan was lowest for males (0.8%) and Bangladesh was lowest for females (0.3%). The United States had a prevalence of 72% for males and 60% for females, along with consumption rates of 3.2 drinks per day for males and 1.9 for females. Alcohol-related diseases caused 6.7% of male deaths and 2.3% of female deaths in the United States, both close to the global numbers of 6.8% for males and 2.2% for females, the investigators said.
The analysis, conducted within the framework of the Global Burden of Diseases, Injuries, and Risk Factors Study, showed that even a single alcoholic drink a day increases the risk of developing 1 of the 23 alcohol-related health problems by 0.5% a year for people aged 15-95 years, which translates into a rate of 918 per 100,000 population, compared with 914 per 100,000 for nondrinkers. Consuming two drinks a day raises the risk to 7%, which would be an incidence of 977 per 100,000, and those who have five drinks a day increase their risk by 37%, which works out to 1,252 people per 100,000 who would develop an alcohol-related disease, Mr. Griswold and his associates said.
In an editorial comment, Robyn Burton, PhD, of King’s College London and Nick Sheron, MD, of the University of Southampton (England), wrote that “the conclusions of the study are clear and unambiguous: Alcohol is a colossal global health issue and small reductions in health-related harms at low levels of alcohol intake are outweighed by the increased risk of other health-related harms, including cancer. … These diseases of unhealthy behaviors, facilitated by unhealthy environments and fueled by commercial interests putting shareholder value ahead of the tragic human consequences, are the dominant health issue of the 21st century. The solutions are straightforward: Increasing taxation creates income for hard-pressed health ministries, and reducing the exposure of children to alcohol marketing has no downsides.”
The study was funded by the Bill and Melinda Gates Foundation. Mr. Griswold did not have any conflicts to disclose, but six of his several hundred coauthors did make such disclosures.
SOURCE: Griswold MG et al. Lancet. 2018 Aug 23. doi: 10.1016/S0140-6736(18)31310-2.

Considerable variations were seen in alcohol consumption. In 2016, males overall consumed more than twice as many standard drinks per day as females: 1.70 versus 0.73. Alcohol consumption in those aged 15-95 years was highest in the top quintile of countries according to sociodemographic development for both males (2.9 drinks per day) and females (1.9) and lowest in the bottom quintile of countries for males (1.4) and the second-lowest quintile for females (0.3), Max G. Griswold, MA, of the University of Washington, Seattle, and his associates said in the Lancet.
Denmark had the highest prevalence of current drinkers of any country for both males (97%) and females (95%) in 2016; Pakistan was lowest for males (0.8%) and Bangladesh was lowest for females (0.3%). The United States had a prevalence of 72% for males and 60% for females, along with consumption rates of 3.2 drinks per day for males and 1.9 for females. Alcohol-related diseases caused 6.7% of male deaths and 2.3% of female deaths in the United States, both close to the global numbers of 6.8% for males and 2.2% for females, the investigators said.
The analysis, conducted within the framework of the Global Burden of Diseases, Injuries, and Risk Factors Study, showed that even a single alcoholic drink a day increases the risk of developing 1 of the 23 alcohol-related health problems by 0.5% a year for people aged 15-95 years, which translates into a rate of 918 per 100,000 population, compared with 914 per 100,000 for nondrinkers. Consuming two drinks a day raises the risk to 7%, which would be an incidence of 977 per 100,000, and those who have five drinks a day increase their risk by 37%, which works out to 1,252 people per 100,000 who would develop an alcohol-related disease, Mr. Griswold and his associates said.
In an editorial comment, Robyn Burton, PhD, of King’s College London and Nick Sheron, MD, of the University of Southampton (England), wrote that “the conclusions of the study are clear and unambiguous: Alcohol is a colossal global health issue and small reductions in health-related harms at low levels of alcohol intake are outweighed by the increased risk of other health-related harms, including cancer. … These diseases of unhealthy behaviors, facilitated by unhealthy environments and fueled by commercial interests putting shareholder value ahead of the tragic human consequences, are the dominant health issue of the 21st century. The solutions are straightforward: Increasing taxation creates income for hard-pressed health ministries, and reducing the exposure of children to alcohol marketing has no downsides.”
The study was funded by the Bill and Melinda Gates Foundation. Mr. Griswold did not have any conflicts to disclose, but six of his several hundred coauthors did make such disclosures.
SOURCE: Griswold MG et al. Lancet. 2018 Aug 23. doi: 10.1016/S0140-6736(18)31310-2.

Considerable variations were seen in alcohol consumption. In 2016, males overall consumed more than twice as many standard drinks per day as females: 1.70 versus 0.73. Alcohol consumption in those aged 15-95 years was highest in the top quintile of countries according to sociodemographic development for both males (2.9 drinks per day) and females (1.9) and lowest in the bottom quintile of countries for males (1.4) and the second-lowest quintile for females (0.3), Max G. Griswold, MA, of the University of Washington, Seattle, and his associates said in the Lancet.
Denmark had the highest prevalence of current drinkers of any country for both males (97%) and females (95%) in 2016; Pakistan was lowest for males (0.8%) and Bangladesh was lowest for females (0.3%). The United States had a prevalence of 72% for males and 60% for females, along with consumption rates of 3.2 drinks per day for males and 1.9 for females. Alcohol-related diseases caused 6.7% of male deaths and 2.3% of female deaths in the United States, both close to the global numbers of 6.8% for males and 2.2% for females, the investigators said.
The analysis, conducted within the framework of the Global Burden of Diseases, Injuries, and Risk Factors Study, showed that even a single alcoholic drink a day increases the risk of developing 1 of the 23 alcohol-related health problems by 0.5% a year for people aged 15-95 years, which translates into a rate of 918 per 100,000 population, compared with 914 per 100,000 for nondrinkers. Consuming two drinks a day raises the risk to 7%, which would be an incidence of 977 per 100,000, and those who have five drinks a day increase their risk by 37%, which works out to 1,252 people per 100,000 who would develop an alcohol-related disease, Mr. Griswold and his associates said.
In an editorial comment, Robyn Burton, PhD, of King’s College London and Nick Sheron, MD, of the University of Southampton (England), wrote that “the conclusions of the study are clear and unambiguous: Alcohol is a colossal global health issue and small reductions in health-related harms at low levels of alcohol intake are outweighed by the increased risk of other health-related harms, including cancer. … These diseases of unhealthy behaviors, facilitated by unhealthy environments and fueled by commercial interests putting shareholder value ahead of the tragic human consequences, are the dominant health issue of the 21st century. The solutions are straightforward: Increasing taxation creates income for hard-pressed health ministries, and reducing the exposure of children to alcohol marketing has no downsides.”
The study was funded by the Bill and Melinda Gates Foundation. Mr. Griswold did not have any conflicts to disclose, but six of his several hundred coauthors did make such disclosures.
SOURCE: Griswold MG et al. Lancet. 2018 Aug 23. doi: 10.1016/S0140-6736(18)31310-2.
FROM THE LANCET
Barriers loom for HCV care in young people who inject drugs
Young adults who inject drugs and are infected with hepatitis C virus “face unique barriers to HCV testing, counseling, and treatment,” according to Margie R. Skeer, ScD, of Tufts University, Boston, and her fellow researchers.
Dr. Skeer and her colleagues found five themes in 24 in-depth interviews with people aged 22-30 years who inject drugs and have HCV infection. At the time of the interviews, none of the patients had received the newer HCV treatment regimens (Drug Alcohol Depend. 2018 Sep 1;190:246-54).
These themes captured the knowledge of and experience of HCV along the continuum of care:
1. Deservingness of HCV treatment and stigma.
2. Dissatisfaction with provider interactions.
3. Perceived lack of referral to treatment and care continuity.
4. Disincentives around HCV treatment for PWID.
5. Perceived need for treatment.
The interviewees were largely uninformed about HCV prior to diagnosis and reported learning more about the virus after their diagnosis. They also tended to affirm the belief that they did not deserve treatment. They felt stigmatized by insurance companies and clinicians, thereby reducing their engagement in the care continuum. And, at the time, insurance companies enforced “sobriety” restrictions dictating the length of time patients had to be off drugs before qualifying for HCV treatment. In the words of one interviewee: “[Caregivers] have a big stigma when it comes to addicts. ... Their whole demeanor changes. They rush you, they slam things, they are very impatient with you, and it is very saddening to see.”
Interviewees reported no or incomplete referrals or being given pamphlets and flyers. They reported little follow-up as to whether they sought additional care, and experienced a lack of confidence from medical professionals that they could be counted on to adhere to an HCV treatment regimen.
Interviewees stated that injection drug use and HCV are inevitably linked, and that IV drug users will eventually contract HCV infections.
“Hep C’s no big deal, Hep C’s like the common cold for the junkie. ... It might take 5 years away from your, you know, life but, you know, we’re not even gonna live that long anyways, so who cares about it anyway,” remarked a 28-year old woman, who was not currently injecting drugs.
The study authors said there is an increased need to provide patient-oriented care for young injection drug users and described the potential benefits of some insurance companies reducing their sobriety and disease severity restrictions.
“Reducing stigma among healthcare professionals, which cuts across the different levels of the HCV care continuum, improving referral patterns and continuity of care, better informing people about their HCV status through patient-oriented testing and disclosure experiences, and reducing perceptions of personal responsibility for disease are crucial next steps to increasing treatment as prevention,” Dr. Skeer and her colleagues concluded.
The authors reported that they had no conflicts of interest.
AGA patient materials can help your patients better understand and manage living with hepatitis C. Learn more at patient.gastro.org.
Young adults who inject drugs and are infected with hepatitis C virus “face unique barriers to HCV testing, counseling, and treatment,” according to Margie R. Skeer, ScD, of Tufts University, Boston, and her fellow researchers.
Dr. Skeer and her colleagues found five themes in 24 in-depth interviews with people aged 22-30 years who inject drugs and have HCV infection. At the time of the interviews, none of the patients had received the newer HCV treatment regimens (Drug Alcohol Depend. 2018 Sep 1;190:246-54).
These themes captured the knowledge of and experience of HCV along the continuum of care:
1. Deservingness of HCV treatment and stigma.
2. Dissatisfaction with provider interactions.
3. Perceived lack of referral to treatment and care continuity.
4. Disincentives around HCV treatment for PWID.
5. Perceived need for treatment.
The interviewees were largely uninformed about HCV prior to diagnosis and reported learning more about the virus after their diagnosis. They also tended to affirm the belief that they did not deserve treatment. They felt stigmatized by insurance companies and clinicians, thereby reducing their engagement in the care continuum. And, at the time, insurance companies enforced “sobriety” restrictions dictating the length of time patients had to be off drugs before qualifying for HCV treatment. In the words of one interviewee: “[Caregivers] have a big stigma when it comes to addicts. ... Their whole demeanor changes. They rush you, they slam things, they are very impatient with you, and it is very saddening to see.”
Interviewees reported no or incomplete referrals or being given pamphlets and flyers. They reported little follow-up as to whether they sought additional care, and experienced a lack of confidence from medical professionals that they could be counted on to adhere to an HCV treatment regimen.
Interviewees stated that injection drug use and HCV are inevitably linked, and that IV drug users will eventually contract HCV infections.
“Hep C’s no big deal, Hep C’s like the common cold for the junkie. ... It might take 5 years away from your, you know, life but, you know, we’re not even gonna live that long anyways, so who cares about it anyway,” remarked a 28-year old woman, who was not currently injecting drugs.
The study authors said there is an increased need to provide patient-oriented care for young injection drug users and described the potential benefits of some insurance companies reducing their sobriety and disease severity restrictions.
“Reducing stigma among healthcare professionals, which cuts across the different levels of the HCV care continuum, improving referral patterns and continuity of care, better informing people about their HCV status through patient-oriented testing and disclosure experiences, and reducing perceptions of personal responsibility for disease are crucial next steps to increasing treatment as prevention,” Dr. Skeer and her colleagues concluded.
The authors reported that they had no conflicts of interest.
AGA patient materials can help your patients better understand and manage living with hepatitis C. Learn more at patient.gastro.org.
Young adults who inject drugs and are infected with hepatitis C virus “face unique barriers to HCV testing, counseling, and treatment,” according to Margie R. Skeer, ScD, of Tufts University, Boston, and her fellow researchers.
Dr. Skeer and her colleagues found five themes in 24 in-depth interviews with people aged 22-30 years who inject drugs and have HCV infection. At the time of the interviews, none of the patients had received the newer HCV treatment regimens (Drug Alcohol Depend. 2018 Sep 1;190:246-54).
These themes captured the knowledge of and experience of HCV along the continuum of care:
1. Deservingness of HCV treatment and stigma.
2. Dissatisfaction with provider interactions.
3. Perceived lack of referral to treatment and care continuity.
4. Disincentives around HCV treatment for PWID.
5. Perceived need for treatment.
The interviewees were largely uninformed about HCV prior to diagnosis and reported learning more about the virus after their diagnosis. They also tended to affirm the belief that they did not deserve treatment. They felt stigmatized by insurance companies and clinicians, thereby reducing their engagement in the care continuum. And, at the time, insurance companies enforced “sobriety” restrictions dictating the length of time patients had to be off drugs before qualifying for HCV treatment. In the words of one interviewee: “[Caregivers] have a big stigma when it comes to addicts. ... Their whole demeanor changes. They rush you, they slam things, they are very impatient with you, and it is very saddening to see.”
Interviewees reported no or incomplete referrals or being given pamphlets and flyers. They reported little follow-up as to whether they sought additional care, and experienced a lack of confidence from medical professionals that they could be counted on to adhere to an HCV treatment regimen.
Interviewees stated that injection drug use and HCV are inevitably linked, and that IV drug users will eventually contract HCV infections.
“Hep C’s no big deal, Hep C’s like the common cold for the junkie. ... It might take 5 years away from your, you know, life but, you know, we’re not even gonna live that long anyways, so who cares about it anyway,” remarked a 28-year old woman, who was not currently injecting drugs.
The study authors said there is an increased need to provide patient-oriented care for young injection drug users and described the potential benefits of some insurance companies reducing their sobriety and disease severity restrictions.
“Reducing stigma among healthcare professionals, which cuts across the different levels of the HCV care continuum, improving referral patterns and continuity of care, better informing people about their HCV status through patient-oriented testing and disclosure experiences, and reducing perceptions of personal responsibility for disease are crucial next steps to increasing treatment as prevention,” Dr. Skeer and her colleagues concluded.
The authors reported that they had no conflicts of interest.
AGA patient materials can help your patients better understand and manage living with hepatitis C. Learn more at patient.gastro.org.
FROM DRUG AND ALCOHOL DEPENDENCE
Prealbumin level predicts outcomes for HCC resection
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
FROM HPB
Key clinical point: Preoperative prealbumin levels independently predicted survival after curative liver resection for HCC, while preoperative albumin levels did not.
Major finding: The hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
Study details: Retrospective analysis that included 1,483 patients with HCC newly diagnosed at one of six institutions in China during 2001-2014.
Disclosures: The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. The study authors had no conflicts of interest to disclose.
Source: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Model finds spontaneous HCV clearance higher than previous estimates
Up to 40% of hepatitis C virus (HCV)–infected individuals clear their infection spontaneously, based on the results of a new mathematical model of HCV transmission and clearance, according to a report published online in the International Journal of Infectious Diseases.
Houssein H. Ayoub, PhD, of Cornell University, New York, and his colleagues conducted a study on HCV clearance. Previous estimates using empirical data indicated that the HCV clearance rate was about 25% after and acute infection duration of 16.5 weeks, according to Dr. Ayoub and his colleagues.
They developed a model to describe HCV transmission and a virus clearance rate (fclearance), defined as the proportion of HCV-infected persons who spontaneously clear their infection after the acute stage. The rest of the infected population (1–fclearance) become chronically infected and positive for both HCV antibodies and HCV RNA.This was estimated by fitting the model to probability-based and nationally representative, population-based data from Egypt (2008 and 2015), and the U.S. National Health and Nutrition Examination Surveys (NHANES A and NHANES B) data. Their model showed that fclearance was related to the HCV viremic rate approximately as fclearance = 1.16 x (1–HCV viremic rate). The HCV viremic rate was defined as the proportion of individuals who were positive for HCV antibodies and HCV RNA out of all who were positive for HCV antibodies positive, regardless of RNA status, as measured in a cross-sectional survey.
Antibody prevalence in Egypt was estimated at 14.7% in 2008 and 10.0% in 2015, while the viremic rate was assessed as 67.1% and 70.2%, respectively. For the United States, the pooled antibody prevalence from the NHANES A data between 1999 and 2012 was an estimated 1.4% and the pooled viremic rate was estimated at around 74%. The NHANES B data used as the denominator for HCV viremic rate both individuals confirmed as HCV Ab positive and those with an undetermined HCV antibody status. (NHANES laboratory procedures can provide this added information because of their subsequent testing of undetermined HCV antibody results for HCV RNA positivity.) This change to the formula yielded a viremic rate of 64.6%.
They found that fclearance was an estimated at 39.9% and 33.5% for Egypt in 2008 and 2015, respectively, and 29.6% and 49.9% for NHANES A and NHANES B, respectively.
“Empirical measures from longitudinal cohort studies may have underestimated the ability of the host immune system to clear HCV infection. This finding may have also implications for our understanding of the biological determinants of HCV spontaneous clearance. It may hint that a strategy for HCV vaccine development could be a vaccine that does not necessarily prevent infection, but modulates immune response towards conditions that increase the capacity of the host immune system to clear HCV infection spontaneously,” the researchers concluded.
The study was funded by the Qatar National Research Fund and Cornell University. The authors reported no conflicts of interest.
SOURCE: Ayoub HH et al. Int J Infect Dis. 2018 Jul 18. doi: 10.1016/j.ijid.2018.07.013.
Up to 40% of hepatitis C virus (HCV)–infected individuals clear their infection spontaneously, based on the results of a new mathematical model of HCV transmission and clearance, according to a report published online in the International Journal of Infectious Diseases.
Houssein H. Ayoub, PhD, of Cornell University, New York, and his colleagues conducted a study on HCV clearance. Previous estimates using empirical data indicated that the HCV clearance rate was about 25% after and acute infection duration of 16.5 weeks, according to Dr. Ayoub and his colleagues.
They developed a model to describe HCV transmission and a virus clearance rate (fclearance), defined as the proportion of HCV-infected persons who spontaneously clear their infection after the acute stage. The rest of the infected population (1–fclearance) become chronically infected and positive for both HCV antibodies and HCV RNA.This was estimated by fitting the model to probability-based and nationally representative, population-based data from Egypt (2008 and 2015), and the U.S. National Health and Nutrition Examination Surveys (NHANES A and NHANES B) data. Their model showed that fclearance was related to the HCV viremic rate approximately as fclearance = 1.16 x (1–HCV viremic rate). The HCV viremic rate was defined as the proportion of individuals who were positive for HCV antibodies and HCV RNA out of all who were positive for HCV antibodies positive, regardless of RNA status, as measured in a cross-sectional survey.
Antibody prevalence in Egypt was estimated at 14.7% in 2008 and 10.0% in 2015, while the viremic rate was assessed as 67.1% and 70.2%, respectively. For the United States, the pooled antibody prevalence from the NHANES A data between 1999 and 2012 was an estimated 1.4% and the pooled viremic rate was estimated at around 74%. The NHANES B data used as the denominator for HCV viremic rate both individuals confirmed as HCV Ab positive and those with an undetermined HCV antibody status. (NHANES laboratory procedures can provide this added information because of their subsequent testing of undetermined HCV antibody results for HCV RNA positivity.) This change to the formula yielded a viremic rate of 64.6%.
They found that fclearance was an estimated at 39.9% and 33.5% for Egypt in 2008 and 2015, respectively, and 29.6% and 49.9% for NHANES A and NHANES B, respectively.
“Empirical measures from longitudinal cohort studies may have underestimated the ability of the host immune system to clear HCV infection. This finding may have also implications for our understanding of the biological determinants of HCV spontaneous clearance. It may hint that a strategy for HCV vaccine development could be a vaccine that does not necessarily prevent infection, but modulates immune response towards conditions that increase the capacity of the host immune system to clear HCV infection spontaneously,” the researchers concluded.
The study was funded by the Qatar National Research Fund and Cornell University. The authors reported no conflicts of interest.
SOURCE: Ayoub HH et al. Int J Infect Dis. 2018 Jul 18. doi: 10.1016/j.ijid.2018.07.013.
Up to 40% of hepatitis C virus (HCV)–infected individuals clear their infection spontaneously, based on the results of a new mathematical model of HCV transmission and clearance, according to a report published online in the International Journal of Infectious Diseases.
Houssein H. Ayoub, PhD, of Cornell University, New York, and his colleagues conducted a study on HCV clearance. Previous estimates using empirical data indicated that the HCV clearance rate was about 25% after and acute infection duration of 16.5 weeks, according to Dr. Ayoub and his colleagues.
They developed a model to describe HCV transmission and a virus clearance rate (fclearance), defined as the proportion of HCV-infected persons who spontaneously clear their infection after the acute stage. The rest of the infected population (1–fclearance) become chronically infected and positive for both HCV antibodies and HCV RNA.This was estimated by fitting the model to probability-based and nationally representative, population-based data from Egypt (2008 and 2015), and the U.S. National Health and Nutrition Examination Surveys (NHANES A and NHANES B) data. Their model showed that fclearance was related to the HCV viremic rate approximately as fclearance = 1.16 x (1–HCV viremic rate). The HCV viremic rate was defined as the proportion of individuals who were positive for HCV antibodies and HCV RNA out of all who were positive for HCV antibodies positive, regardless of RNA status, as measured in a cross-sectional survey.
Antibody prevalence in Egypt was estimated at 14.7% in 2008 and 10.0% in 2015, while the viremic rate was assessed as 67.1% and 70.2%, respectively. For the United States, the pooled antibody prevalence from the NHANES A data between 1999 and 2012 was an estimated 1.4% and the pooled viremic rate was estimated at around 74%. The NHANES B data used as the denominator for HCV viremic rate both individuals confirmed as HCV Ab positive and those with an undetermined HCV antibody status. (NHANES laboratory procedures can provide this added information because of their subsequent testing of undetermined HCV antibody results for HCV RNA positivity.) This change to the formula yielded a viremic rate of 64.6%.
They found that fclearance was an estimated at 39.9% and 33.5% for Egypt in 2008 and 2015, respectively, and 29.6% and 49.9% for NHANES A and NHANES B, respectively.
“Empirical measures from longitudinal cohort studies may have underestimated the ability of the host immune system to clear HCV infection. This finding may have also implications for our understanding of the biological determinants of HCV spontaneous clearance. It may hint that a strategy for HCV vaccine development could be a vaccine that does not necessarily prevent infection, but modulates immune response towards conditions that increase the capacity of the host immune system to clear HCV infection spontaneously,” the researchers concluded.
The study was funded by the Qatar National Research Fund and Cornell University. The authors reported no conflicts of interest.
SOURCE: Ayoub HH et al. Int J Infect Dis. 2018 Jul 18. doi: 10.1016/j.ijid.2018.07.013.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Key clinical point: The hepatitis C virus clearance rate was 39.9% and 33.5% for Egypt in 2008 and 2015, respectively, and 29.6% and 49.9% for two U.S. populations.
Major finding: A new model of HCV-infected persons indicates that up to 40% clear their infection spontaneously, higher than earlier estimates.
Study details: A mathematical model was developed to describe HCV transmission and clearance based on nationally representative population data for Egypt and the United States.
Disclosures: The study was funded by the Qatar National Research Fund and Cornell University. The authors reported no conflicts of interest.
Source: Ayoub HH et al. Int J Infect Dis. 2018 Jul 18. doi: 10.1016/j.ijid.2018.07.013.
FDA approves lenvatinib for HCC
Approval was based on a noninferiority trial of 954 patients with previously untreated, metastatic or unresectable HCC, comparing treatment with lenvatinib to sorafenib, according to an FDA statement.
Lenvatinib was found noninferior but not statistically superior to sorafenib for overall survival (hazard ratio, 0.92; 95% confidence interval, 0.79-1.06). Median overall survival was 13.6 months for patients in the lenvatinib arm, compared with 12.3 months for patients in the sorafenib arm.
The most common adverse reactions with lenvatinib were hypertension, fatigue, diarrhea, decreased appetite, arthralgia/myalgia, decreased weight, abdominal pain, palmar-plantar erythrodysesthesia syndrome, proteinuria, dysphonia, hemorrhagic events, hypothyroidism, and nausea.
The recommended lenvatinib dosages are 12 mg orally once daily in patients weighing 60 kg or greater actual body weight or 8 mg orally once daily in patients weighing less than 60 kg actual body weight, the FDA said.
Lenvatinib is marketed as Lenvima by Eisai.
Approval was based on a noninferiority trial of 954 patients with previously untreated, metastatic or unresectable HCC, comparing treatment with lenvatinib to sorafenib, according to an FDA statement.
Lenvatinib was found noninferior but not statistically superior to sorafenib for overall survival (hazard ratio, 0.92; 95% confidence interval, 0.79-1.06). Median overall survival was 13.6 months for patients in the lenvatinib arm, compared with 12.3 months for patients in the sorafenib arm.
The most common adverse reactions with lenvatinib were hypertension, fatigue, diarrhea, decreased appetite, arthralgia/myalgia, decreased weight, abdominal pain, palmar-plantar erythrodysesthesia syndrome, proteinuria, dysphonia, hemorrhagic events, hypothyroidism, and nausea.
The recommended lenvatinib dosages are 12 mg orally once daily in patients weighing 60 kg or greater actual body weight or 8 mg orally once daily in patients weighing less than 60 kg actual body weight, the FDA said.
Lenvatinib is marketed as Lenvima by Eisai.
Approval was based on a noninferiority trial of 954 patients with previously untreated, metastatic or unresectable HCC, comparing treatment with lenvatinib to sorafenib, according to an FDA statement.
Lenvatinib was found noninferior but not statistically superior to sorafenib for overall survival (hazard ratio, 0.92; 95% confidence interval, 0.79-1.06). Median overall survival was 13.6 months for patients in the lenvatinib arm, compared with 12.3 months for patients in the sorafenib arm.
The most common adverse reactions with lenvatinib were hypertension, fatigue, diarrhea, decreased appetite, arthralgia/myalgia, decreased weight, abdominal pain, palmar-plantar erythrodysesthesia syndrome, proteinuria, dysphonia, hemorrhagic events, hypothyroidism, and nausea.
The recommended lenvatinib dosages are 12 mg orally once daily in patients weighing 60 kg or greater actual body weight or 8 mg orally once daily in patients weighing less than 60 kg actual body weight, the FDA said.
Lenvatinib is marketed as Lenvima by Eisai.










