User login
MELD 3.0 Reduces Sex-Based Liver Transplant Disparities
SAN DIEGO — , according to new research.
In particular, women are now more likely to be added to the waitlist for a liver transplant, more likely to receive a transplant, and less likely to fall off the waitlist because of death.
“MELD 3.0 improved access to transplantation for women, and now waitlist mortality and transplant rates for women more closely approximate the rates for men,” said lead author Allison Kwong, MD, assistant professor of medicine and transplant hepatologist at Stanford Medicine in California.
“Overall transplant outcomes have also improved year over year,” said Kwong, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Changes in MELD and Transplant Numbers
MELD, which estimates liver failure severity and short-term survival in patients with chronic liver disease, has been used since 2002 to determine organ allocation priority for patients in the United States awaiting liver transplantation. Originally, the score incorporated three variables: creatinine, bilirubin, and the international normalized ratio (INR). MELDNa1, or MELD 2.0, was adopted in 2016 to add sodium.
“Under this system, however, there have been sex-based disparities” with women receiving lower priority scores despite similar disease severity, said Kwong.
“This has been attributed to several factors, such as the creatinine term in the MELD score underestimating renal dysfunction in women, height and body size differences, and differences in disease etiology, and how we’ve assigned exception points historically,” she reported.
Men have had a lower pretransplant mortality rate and higher deceased donor transplant rates, she added.
MELD 3.0 was developed to address these gender differences and other determinants of waitlist outcomes. The updated equation added 1.33 points for women, as well as adding other variables, such as albumin, interactions between bilirubin and sodium, and interactions between albumin and creatinine, to increase prediction accuracy.
To observe the effects of the new system, Kwong and colleagues analyzed OPTN data for patients aged 12 years or older, focusing on the records of more than 20,300 newly registered liver transplant candidates, and about 18,700 transplant recipients, during the 12 months before and 12 months after MELD 3.0 was implemented.
After the switch, 43.7% of newly registered liver transplant candidates were women, compared with 40.4% before the switch. At registration, the median age was 55, both before and after the change in policy, and the median MELD score changed from 23 to 22 after implementation.
In addition, 42.1% of transplants occurred among women after MELD 3.0 implementation, as compared with 37.3% before. Overall, deceased donor transplant rates were similar for men and women after MELD 3.0 implementation.
The 90-day waitlist dropout rate — patients who died or became too sick to receive a transplant — decreased from 13.5% to 9.1% among women, which may be partially attributable to MELD 3.0, said Kwong.
However, waitlist dropout rates also decreased among men, from 9.8% to 7.4%, probably because of improvements in technology, such as machine perfusion, which have increased the number of available livers, she added.
Disparities Continue to Exist
Some disparities still exist. Although the total median MELD score at transplant decreased from 29 to 27, women still had a higher median score of 29 at transplant, compared with a median score of 27 among men.
“This indicates that there may still be differences in transplant access between the sexes,” Kwong said. “There are still body size differences that can affect the probability of transplant, and this would not be addressed by MELD 3.0.”
Additional transplant disparities exist related to other patient characteristics, such as age, race, and ethnicity.
Future versions of MELD could potentially consider these factors, said session moderator Aleksander Krag, MD, PhD, MBA, professor of clinical medicine at the University of Southern Denmark, Odense, and secretary general of the European Association for the Study of the Liver, 2023-2025.
“There are infinite versions of MELD that can be made,” Kwong said. “It’s still early to see how MELD 3.0 will serve the system, but so far, so good.”
In a comment, Tamar Taddei, MD, professor of medicine in digestive diseases at Yale School of Medicine, New Haven, Connecticut, who comoderated the session, noted the importance of using a MELD score that considers sex-based differences.
This study brings MELD 3.0 to its fruition by reducing the disparities experienced by women who were underserved by the previous scoring systems, she said.
It was lovely to see that MELD 3.0 reduced the disparities with transplants, and also that the waitlist dropout was reduced — for both men and women,” Taddei said. “This change is a no-brainer.”
Kwong, Krag, and Taddei reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — , according to new research.
In particular, women are now more likely to be added to the waitlist for a liver transplant, more likely to receive a transplant, and less likely to fall off the waitlist because of death.
“MELD 3.0 improved access to transplantation for women, and now waitlist mortality and transplant rates for women more closely approximate the rates for men,” said lead author Allison Kwong, MD, assistant professor of medicine and transplant hepatologist at Stanford Medicine in California.
“Overall transplant outcomes have also improved year over year,” said Kwong, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Changes in MELD and Transplant Numbers
MELD, which estimates liver failure severity and short-term survival in patients with chronic liver disease, has been used since 2002 to determine organ allocation priority for patients in the United States awaiting liver transplantation. Originally, the score incorporated three variables: creatinine, bilirubin, and the international normalized ratio (INR). MELDNa1, or MELD 2.0, was adopted in 2016 to add sodium.
“Under this system, however, there have been sex-based disparities” with women receiving lower priority scores despite similar disease severity, said Kwong.
“This has been attributed to several factors, such as the creatinine term in the MELD score underestimating renal dysfunction in women, height and body size differences, and differences in disease etiology, and how we’ve assigned exception points historically,” she reported.
Men have had a lower pretransplant mortality rate and higher deceased donor transplant rates, she added.
MELD 3.0 was developed to address these gender differences and other determinants of waitlist outcomes. The updated equation added 1.33 points for women, as well as adding other variables, such as albumin, interactions between bilirubin and sodium, and interactions between albumin and creatinine, to increase prediction accuracy.
To observe the effects of the new system, Kwong and colleagues analyzed OPTN data for patients aged 12 years or older, focusing on the records of more than 20,300 newly registered liver transplant candidates, and about 18,700 transplant recipients, during the 12 months before and 12 months after MELD 3.0 was implemented.
After the switch, 43.7% of newly registered liver transplant candidates were women, compared with 40.4% before the switch. At registration, the median age was 55, both before and after the change in policy, and the median MELD score changed from 23 to 22 after implementation.
In addition, 42.1% of transplants occurred among women after MELD 3.0 implementation, as compared with 37.3% before. Overall, deceased donor transplant rates were similar for men and women after MELD 3.0 implementation.
The 90-day waitlist dropout rate — patients who died or became too sick to receive a transplant — decreased from 13.5% to 9.1% among women, which may be partially attributable to MELD 3.0, said Kwong.
However, waitlist dropout rates also decreased among men, from 9.8% to 7.4%, probably because of improvements in technology, such as machine perfusion, which have increased the number of available livers, she added.
Disparities Continue to Exist
Some disparities still exist. Although the total median MELD score at transplant decreased from 29 to 27, women still had a higher median score of 29 at transplant, compared with a median score of 27 among men.
“This indicates that there may still be differences in transplant access between the sexes,” Kwong said. “There are still body size differences that can affect the probability of transplant, and this would not be addressed by MELD 3.0.”
Additional transplant disparities exist related to other patient characteristics, such as age, race, and ethnicity.
Future versions of MELD could potentially consider these factors, said session moderator Aleksander Krag, MD, PhD, MBA, professor of clinical medicine at the University of Southern Denmark, Odense, and secretary general of the European Association for the Study of the Liver, 2023-2025.
“There are infinite versions of MELD that can be made,” Kwong said. “It’s still early to see how MELD 3.0 will serve the system, but so far, so good.”
In a comment, Tamar Taddei, MD, professor of medicine in digestive diseases at Yale School of Medicine, New Haven, Connecticut, who comoderated the session, noted the importance of using a MELD score that considers sex-based differences.
This study brings MELD 3.0 to its fruition by reducing the disparities experienced by women who were underserved by the previous scoring systems, she said.
It was lovely to see that MELD 3.0 reduced the disparities with transplants, and also that the waitlist dropout was reduced — for both men and women,” Taddei said. “This change is a no-brainer.”
Kwong, Krag, and Taddei reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — , according to new research.
In particular, women are now more likely to be added to the waitlist for a liver transplant, more likely to receive a transplant, and less likely to fall off the waitlist because of death.
“MELD 3.0 improved access to transplantation for women, and now waitlist mortality and transplant rates for women more closely approximate the rates for men,” said lead author Allison Kwong, MD, assistant professor of medicine and transplant hepatologist at Stanford Medicine in California.
“Overall transplant outcomes have also improved year over year,” said Kwong, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Changes in MELD and Transplant Numbers
MELD, which estimates liver failure severity and short-term survival in patients with chronic liver disease, has been used since 2002 to determine organ allocation priority for patients in the United States awaiting liver transplantation. Originally, the score incorporated three variables: creatinine, bilirubin, and the international normalized ratio (INR). MELDNa1, or MELD 2.0, was adopted in 2016 to add sodium.
“Under this system, however, there have been sex-based disparities” with women receiving lower priority scores despite similar disease severity, said Kwong.
“This has been attributed to several factors, such as the creatinine term in the MELD score underestimating renal dysfunction in women, height and body size differences, and differences in disease etiology, and how we’ve assigned exception points historically,” she reported.
Men have had a lower pretransplant mortality rate and higher deceased donor transplant rates, she added.
MELD 3.0 was developed to address these gender differences and other determinants of waitlist outcomes. The updated equation added 1.33 points for women, as well as adding other variables, such as albumin, interactions between bilirubin and sodium, and interactions between albumin and creatinine, to increase prediction accuracy.
To observe the effects of the new system, Kwong and colleagues analyzed OPTN data for patients aged 12 years or older, focusing on the records of more than 20,300 newly registered liver transplant candidates, and about 18,700 transplant recipients, during the 12 months before and 12 months after MELD 3.0 was implemented.
After the switch, 43.7% of newly registered liver transplant candidates were women, compared with 40.4% before the switch. At registration, the median age was 55, both before and after the change in policy, and the median MELD score changed from 23 to 22 after implementation.
In addition, 42.1% of transplants occurred among women after MELD 3.0 implementation, as compared with 37.3% before. Overall, deceased donor transplant rates were similar for men and women after MELD 3.0 implementation.
The 90-day waitlist dropout rate — patients who died or became too sick to receive a transplant — decreased from 13.5% to 9.1% among women, which may be partially attributable to MELD 3.0, said Kwong.
However, waitlist dropout rates also decreased among men, from 9.8% to 7.4%, probably because of improvements in technology, such as machine perfusion, which have increased the number of available livers, she added.
Disparities Continue to Exist
Some disparities still exist. Although the total median MELD score at transplant decreased from 29 to 27, women still had a higher median score of 29 at transplant, compared with a median score of 27 among men.
“This indicates that there may still be differences in transplant access between the sexes,” Kwong said. “There are still body size differences that can affect the probability of transplant, and this would not be addressed by MELD 3.0.”
Additional transplant disparities exist related to other patient characteristics, such as age, race, and ethnicity.
Future versions of MELD could potentially consider these factors, said session moderator Aleksander Krag, MD, PhD, MBA, professor of clinical medicine at the University of Southern Denmark, Odense, and secretary general of the European Association for the Study of the Liver, 2023-2025.
“There are infinite versions of MELD that can be made,” Kwong said. “It’s still early to see how MELD 3.0 will serve the system, but so far, so good.”
In a comment, Tamar Taddei, MD, professor of medicine in digestive diseases at Yale School of Medicine, New Haven, Connecticut, who comoderated the session, noted the importance of using a MELD score that considers sex-based differences.
This study brings MELD 3.0 to its fruition by reducing the disparities experienced by women who were underserved by the previous scoring systems, she said.
It was lovely to see that MELD 3.0 reduced the disparities with transplants, and also that the waitlist dropout was reduced — for both men and women,” Taddei said. “This change is a no-brainer.”
Kwong, Krag, and Taddei reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AASLD 24
‘Watershed Moment’: Semaglutide Shown to Be Effective in MASH
SAN DIEGO — according to interim results from a phase 3 trial.
At 72 weeks, a 2.4-mg once-weekly subcutaneous dose of semaglutide demonstrated superiority, compared with placebo, for the two primary endpoints: Resolution of steatohepatitis with no worsening of fibrosis and improvement in liver fibrosis with no worsening of steatohepatitis.
“It’s been a long journey. I’ve been working with GLP-1s for 16 years, and it’s great to be able to report the first GLP-1 receptor agonist to demonstrate efficacy in a phase 3 trial for MASH,” said lead author Philip Newsome, MD, PhD, director of the Roger Williams Institute of Liver Studies at King’s College London in England.
“There were also improvements in a slew of other noninvasive markers,” said Newsome, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Although already seen in a broader context, “it’s nice to see a demonstration of the cardiometabolic benefits in the context of MASH and a reassuring safety profile,” he added.
Interim ESSENCE Trial Analysis
ESSENCE (NCT04822181) is an ongoing multicenter, phase 3 randomized, double-blind, placebo-controlled outcome trial studying semaglutide for the potential treatment of MASH.
The trial includes 1200 participants with biopsy-defined MASH and fibrosis, stages F2 and F3, who were randomized 2:1 to a once-weekly subcutaneous injection of 2.4 mg of semaglutide or placebo for 240 weeks. After initiation, the semaglutide dosage was increased every 4 weeks up to 16 weeks when the full dose (2.4 mg) was reached.
In a planned interim analysis, the trial investigators evaluated the primary endpoints at week 72 for the first 800 participants, with biopsies taken at weeks 1 and 72.
A total of 534 people were randomized to the semaglutide group, including 169 with F2 fibrosis and 365 with F3 fibrosis. Among the 266 participants randomized to placebo, 81 had F2 fibrosis and 185 had F3 fibrosis.
At baseline, the patient characteristics were similar between the groups (mean age, 56 years; body mass index, 34.6). A majority of participants also were White (67.5%), women (57.1%), had type 2 diabetes (55.9%), F3 fibrosis (68.8%), and enhanced liver fibrosis (ELF) scores around 10 (55.5%).
For the first primary endpoint, 62.9% of those in the semaglutide group and 34.1% of those in the placebo group reached resolution of steatohepatitis with no worsening of fibrosis. This represented an estimated difference in responder proportions (EDP) of 28.9%.
In addition, 37% of those in the semaglutide group and 22.5% of those in the placebo group met the second primary endpoint of improvement in liver fibrosis with no worsening of steatohepatitis (EDP, 14.4%).
Among the secondary endpoints, combined resolution of steatohepatitis with a one-stage improvement in liver fibrosis occurred in 32.8% of the semaglutide group and 16.2% of the placebo group (EDP, 16.6%).
In additional analyses, Newsome and colleagues found 20%-40% improvements in liver enzymes and noninvasive fibrosis markers, such as ELF and vibration-controlled transient elastography liver stiffness.
Weight loss was also significant, with a 10.5% reduction in the semaglutide group compared with a 2% reduction in the placebo group.
Cardiometabolic risk factors improved as well, with changes in blood pressure measurements, hemoglobin A1c scores, and cholesterol values.
Although not considered statistically significant, patients in the semaglutide group also reported greater reductions in body pain.
In a safety analysis of 1195 participants at 96 weeks, adverse events, severe adverse events, and discontinuations were similar in both groups. Not surprisingly, gastrointestinal side effects were more commonly reported in the semaglutide group, Newsome said.
Highly Anticipated Results
After Newsome’s presentation, attendees applauded.
Rohit Loomba, MD, a gastroenterologist at the University of California, San Diego, who was not involved with the study, called the results the “highlight of the meeting.”
This sentiment was echoed by Naga Chalasani, MD, AGAF, a gastroenterologist at Indiana University Medical Center, Indianapolis, who called the results a “watershed moment in the MASH field” with “terrific data.”
Based on questions after the presentation, Newsome indicated that future ESSENCE reports would look at certain aspects of the results, such as the 10% weight loss among those in the semaglutide group, as well as the mechanisms of histological and fibrosis improvement.
“We know from other GLP-1 trials that more weight loss occurs in those who don’t have type 2 diabetes, and we’re still running those analyses,” he said. “Weight loss is clearly a major contributor to MASH improvement, but there seem to be some weight-independent effects here, which are likely linked to insulin sensitivity or inflammation. We look forward to presenting those analyses in due course.”
In a comment, Kimberly Ann Brown, MD, AGAF, chief of gastroenterology and hepatology at Henry Ford Health System in Detroit, Michigan, AASLD Foundation chair, and comoderator of the late-breaking abstract session, spoke about the highly anticipated presentation.
“This study was really the pinnacle of this meeting. We’ve all been waiting for this data, in large part because many of our patients are already using these medications,” Brown said. “Seeing the benefit for the liver, as well as lipids and other cardiovascular measures, is so important. Having this confirmatory study will hopefully lead to the availability of the medication for this indication among our patients.”
Newsome reported numerous disclosures, including consultant relationships with pharmaceutical companies, such as Novo Nordisk, Boehringer Ingelheim, and Madrigal Pharmaceuticals. Loomba has research grant relationships with numerous companies, including Hanmi, Gilead, Galmed Pharmaceuticals, Galectin Therapeutics, Eli Lilly, Bristol-Myers Squibb, and Boehringer Ingelheim. Chalasani has consultant relationships with Ipsen, Pfizer, Merck, Altimmune, GSK, Madrigal Pharmaceuticals, and Zydus. Brown reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to interim results from a phase 3 trial.
At 72 weeks, a 2.4-mg once-weekly subcutaneous dose of semaglutide demonstrated superiority, compared with placebo, for the two primary endpoints: Resolution of steatohepatitis with no worsening of fibrosis and improvement in liver fibrosis with no worsening of steatohepatitis.
“It’s been a long journey. I’ve been working with GLP-1s for 16 years, and it’s great to be able to report the first GLP-1 receptor agonist to demonstrate efficacy in a phase 3 trial for MASH,” said lead author Philip Newsome, MD, PhD, director of the Roger Williams Institute of Liver Studies at King’s College London in England.
“There were also improvements in a slew of other noninvasive markers,” said Newsome, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Although already seen in a broader context, “it’s nice to see a demonstration of the cardiometabolic benefits in the context of MASH and a reassuring safety profile,” he added.
Interim ESSENCE Trial Analysis
ESSENCE (NCT04822181) is an ongoing multicenter, phase 3 randomized, double-blind, placebo-controlled outcome trial studying semaglutide for the potential treatment of MASH.
The trial includes 1200 participants with biopsy-defined MASH and fibrosis, stages F2 and F3, who were randomized 2:1 to a once-weekly subcutaneous injection of 2.4 mg of semaglutide or placebo for 240 weeks. After initiation, the semaglutide dosage was increased every 4 weeks up to 16 weeks when the full dose (2.4 mg) was reached.
In a planned interim analysis, the trial investigators evaluated the primary endpoints at week 72 for the first 800 participants, with biopsies taken at weeks 1 and 72.
A total of 534 people were randomized to the semaglutide group, including 169 with F2 fibrosis and 365 with F3 fibrosis. Among the 266 participants randomized to placebo, 81 had F2 fibrosis and 185 had F3 fibrosis.
At baseline, the patient characteristics were similar between the groups (mean age, 56 years; body mass index, 34.6). A majority of participants also were White (67.5%), women (57.1%), had type 2 diabetes (55.9%), F3 fibrosis (68.8%), and enhanced liver fibrosis (ELF) scores around 10 (55.5%).
For the first primary endpoint, 62.9% of those in the semaglutide group and 34.1% of those in the placebo group reached resolution of steatohepatitis with no worsening of fibrosis. This represented an estimated difference in responder proportions (EDP) of 28.9%.
In addition, 37% of those in the semaglutide group and 22.5% of those in the placebo group met the second primary endpoint of improvement in liver fibrosis with no worsening of steatohepatitis (EDP, 14.4%).
Among the secondary endpoints, combined resolution of steatohepatitis with a one-stage improvement in liver fibrosis occurred in 32.8% of the semaglutide group and 16.2% of the placebo group (EDP, 16.6%).
In additional analyses, Newsome and colleagues found 20%-40% improvements in liver enzymes and noninvasive fibrosis markers, such as ELF and vibration-controlled transient elastography liver stiffness.
Weight loss was also significant, with a 10.5% reduction in the semaglutide group compared with a 2% reduction in the placebo group.
Cardiometabolic risk factors improved as well, with changes in blood pressure measurements, hemoglobin A1c scores, and cholesterol values.
Although not considered statistically significant, patients in the semaglutide group also reported greater reductions in body pain.
In a safety analysis of 1195 participants at 96 weeks, adverse events, severe adverse events, and discontinuations were similar in both groups. Not surprisingly, gastrointestinal side effects were more commonly reported in the semaglutide group, Newsome said.
Highly Anticipated Results
After Newsome’s presentation, attendees applauded.
Rohit Loomba, MD, a gastroenterologist at the University of California, San Diego, who was not involved with the study, called the results the “highlight of the meeting.”
This sentiment was echoed by Naga Chalasani, MD, AGAF, a gastroenterologist at Indiana University Medical Center, Indianapolis, who called the results a “watershed moment in the MASH field” with “terrific data.”
Based on questions after the presentation, Newsome indicated that future ESSENCE reports would look at certain aspects of the results, such as the 10% weight loss among those in the semaglutide group, as well as the mechanisms of histological and fibrosis improvement.
“We know from other GLP-1 trials that more weight loss occurs in those who don’t have type 2 diabetes, and we’re still running those analyses,” he said. “Weight loss is clearly a major contributor to MASH improvement, but there seem to be some weight-independent effects here, which are likely linked to insulin sensitivity or inflammation. We look forward to presenting those analyses in due course.”
In a comment, Kimberly Ann Brown, MD, AGAF, chief of gastroenterology and hepatology at Henry Ford Health System in Detroit, Michigan, AASLD Foundation chair, and comoderator of the late-breaking abstract session, spoke about the highly anticipated presentation.
“This study was really the pinnacle of this meeting. We’ve all been waiting for this data, in large part because many of our patients are already using these medications,” Brown said. “Seeing the benefit for the liver, as well as lipids and other cardiovascular measures, is so important. Having this confirmatory study will hopefully lead to the availability of the medication for this indication among our patients.”
Newsome reported numerous disclosures, including consultant relationships with pharmaceutical companies, such as Novo Nordisk, Boehringer Ingelheim, and Madrigal Pharmaceuticals. Loomba has research grant relationships with numerous companies, including Hanmi, Gilead, Galmed Pharmaceuticals, Galectin Therapeutics, Eli Lilly, Bristol-Myers Squibb, and Boehringer Ingelheim. Chalasani has consultant relationships with Ipsen, Pfizer, Merck, Altimmune, GSK, Madrigal Pharmaceuticals, and Zydus. Brown reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to interim results from a phase 3 trial.
At 72 weeks, a 2.4-mg once-weekly subcutaneous dose of semaglutide demonstrated superiority, compared with placebo, for the two primary endpoints: Resolution of steatohepatitis with no worsening of fibrosis and improvement in liver fibrosis with no worsening of steatohepatitis.
“It’s been a long journey. I’ve been working with GLP-1s for 16 years, and it’s great to be able to report the first GLP-1 receptor agonist to demonstrate efficacy in a phase 3 trial for MASH,” said lead author Philip Newsome, MD, PhD, director of the Roger Williams Institute of Liver Studies at King’s College London in England.
“There were also improvements in a slew of other noninvasive markers,” said Newsome, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Although already seen in a broader context, “it’s nice to see a demonstration of the cardiometabolic benefits in the context of MASH and a reassuring safety profile,” he added.
Interim ESSENCE Trial Analysis
ESSENCE (NCT04822181) is an ongoing multicenter, phase 3 randomized, double-blind, placebo-controlled outcome trial studying semaglutide for the potential treatment of MASH.
The trial includes 1200 participants with biopsy-defined MASH and fibrosis, stages F2 and F3, who were randomized 2:1 to a once-weekly subcutaneous injection of 2.4 mg of semaglutide or placebo for 240 weeks. After initiation, the semaglutide dosage was increased every 4 weeks up to 16 weeks when the full dose (2.4 mg) was reached.
In a planned interim analysis, the trial investigators evaluated the primary endpoints at week 72 for the first 800 participants, with biopsies taken at weeks 1 and 72.
A total of 534 people were randomized to the semaglutide group, including 169 with F2 fibrosis and 365 with F3 fibrosis. Among the 266 participants randomized to placebo, 81 had F2 fibrosis and 185 had F3 fibrosis.
At baseline, the patient characteristics were similar between the groups (mean age, 56 years; body mass index, 34.6). A majority of participants also were White (67.5%), women (57.1%), had type 2 diabetes (55.9%), F3 fibrosis (68.8%), and enhanced liver fibrosis (ELF) scores around 10 (55.5%).
For the first primary endpoint, 62.9% of those in the semaglutide group and 34.1% of those in the placebo group reached resolution of steatohepatitis with no worsening of fibrosis. This represented an estimated difference in responder proportions (EDP) of 28.9%.
In addition, 37% of those in the semaglutide group and 22.5% of those in the placebo group met the second primary endpoint of improvement in liver fibrosis with no worsening of steatohepatitis (EDP, 14.4%).
Among the secondary endpoints, combined resolution of steatohepatitis with a one-stage improvement in liver fibrosis occurred in 32.8% of the semaglutide group and 16.2% of the placebo group (EDP, 16.6%).
In additional analyses, Newsome and colleagues found 20%-40% improvements in liver enzymes and noninvasive fibrosis markers, such as ELF and vibration-controlled transient elastography liver stiffness.
Weight loss was also significant, with a 10.5% reduction in the semaglutide group compared with a 2% reduction in the placebo group.
Cardiometabolic risk factors improved as well, with changes in blood pressure measurements, hemoglobin A1c scores, and cholesterol values.
Although not considered statistically significant, patients in the semaglutide group also reported greater reductions in body pain.
In a safety analysis of 1195 participants at 96 weeks, adverse events, severe adverse events, and discontinuations were similar in both groups. Not surprisingly, gastrointestinal side effects were more commonly reported in the semaglutide group, Newsome said.
Highly Anticipated Results
After Newsome’s presentation, attendees applauded.
Rohit Loomba, MD, a gastroenterologist at the University of California, San Diego, who was not involved with the study, called the results the “highlight of the meeting.”
This sentiment was echoed by Naga Chalasani, MD, AGAF, a gastroenterologist at Indiana University Medical Center, Indianapolis, who called the results a “watershed moment in the MASH field” with “terrific data.”
Based on questions after the presentation, Newsome indicated that future ESSENCE reports would look at certain aspects of the results, such as the 10% weight loss among those in the semaglutide group, as well as the mechanisms of histological and fibrosis improvement.
“We know from other GLP-1 trials that more weight loss occurs in those who don’t have type 2 diabetes, and we’re still running those analyses,” he said. “Weight loss is clearly a major contributor to MASH improvement, but there seem to be some weight-independent effects here, which are likely linked to insulin sensitivity or inflammation. We look forward to presenting those analyses in due course.”
In a comment, Kimberly Ann Brown, MD, AGAF, chief of gastroenterology and hepatology at Henry Ford Health System in Detroit, Michigan, AASLD Foundation chair, and comoderator of the late-breaking abstract session, spoke about the highly anticipated presentation.
“This study was really the pinnacle of this meeting. We’ve all been waiting for this data, in large part because many of our patients are already using these medications,” Brown said. “Seeing the benefit for the liver, as well as lipids and other cardiovascular measures, is so important. Having this confirmatory study will hopefully lead to the availability of the medication for this indication among our patients.”
Newsome reported numerous disclosures, including consultant relationships with pharmaceutical companies, such as Novo Nordisk, Boehringer Ingelheim, and Madrigal Pharmaceuticals. Loomba has research grant relationships with numerous companies, including Hanmi, Gilead, Galmed Pharmaceuticals, Galectin Therapeutics, Eli Lilly, Bristol-Myers Squibb, and Boehringer Ingelheim. Chalasani has consultant relationships with Ipsen, Pfizer, Merck, Altimmune, GSK, Madrigal Pharmaceuticals, and Zydus. Brown reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AASLD 24
How to Discuss Lifestyle Modifications in MASLD
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).
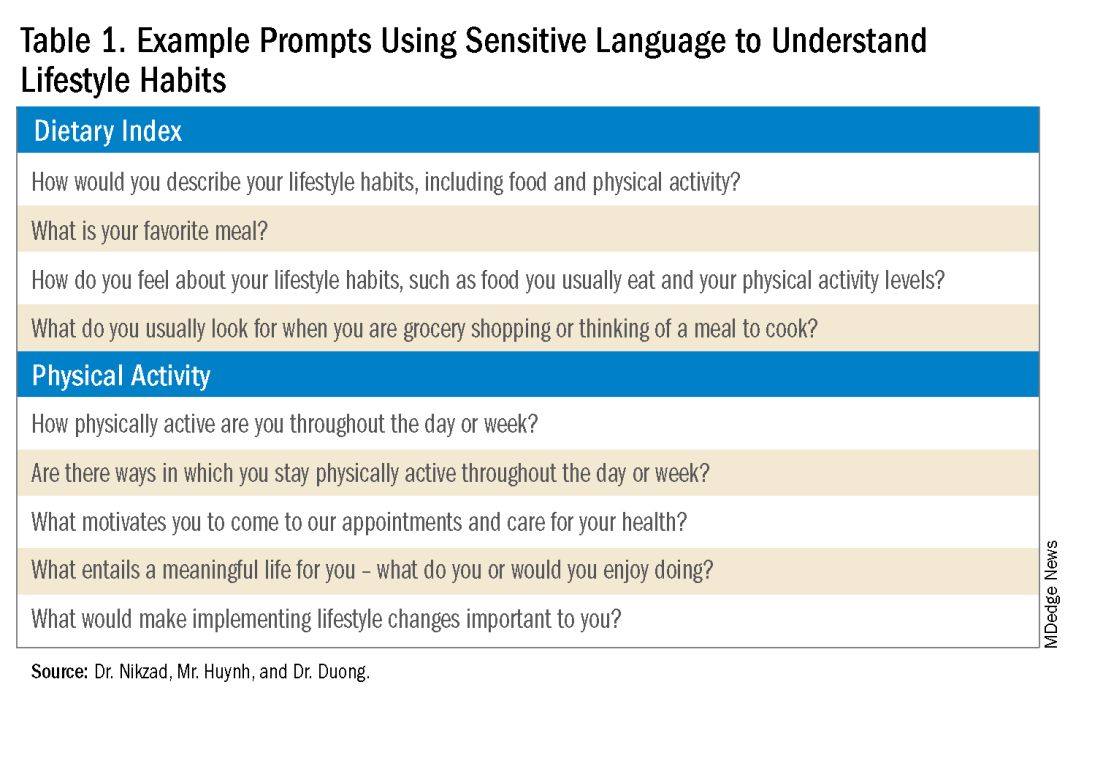
Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.
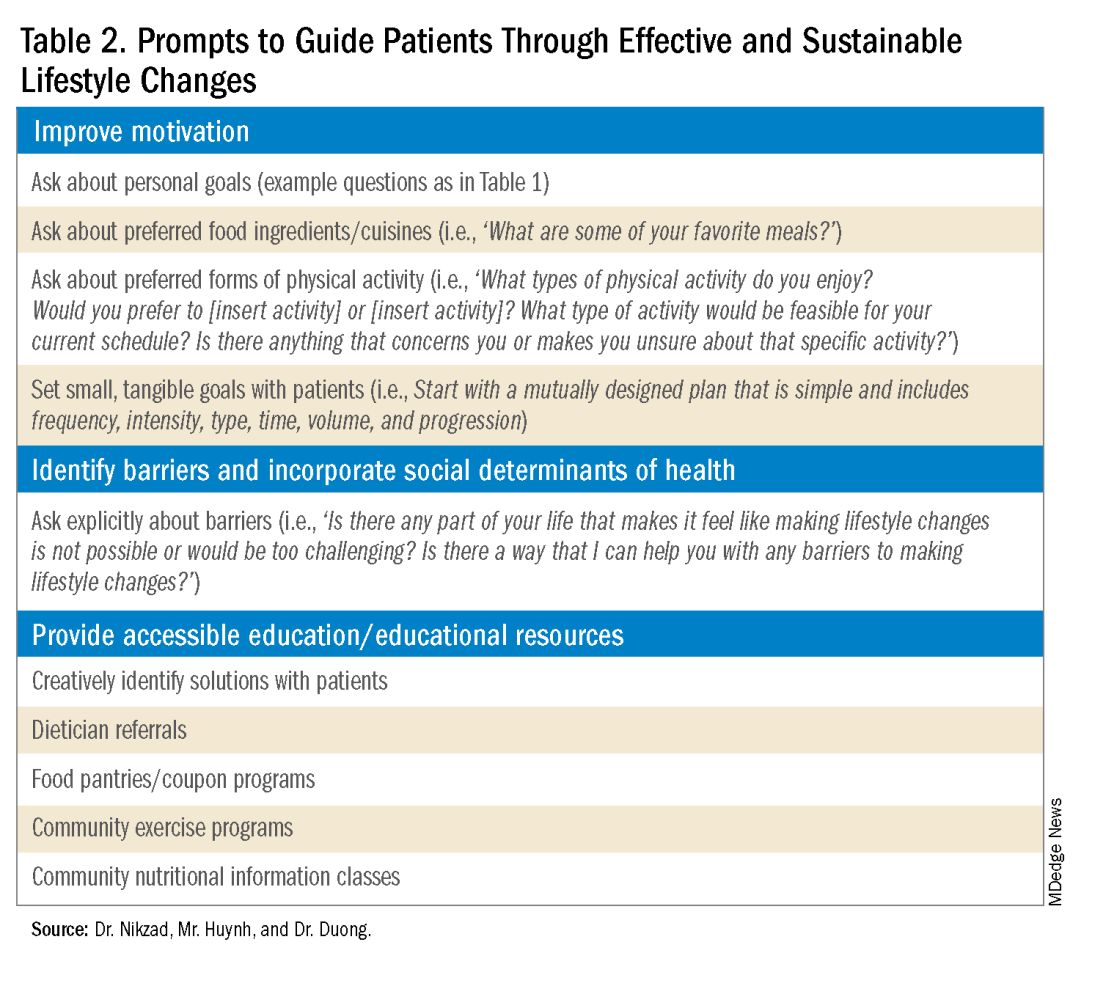
Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Journal Highlights: Sept.-Oct. 2024
Upper GI
Levinthal DJ et al. AGA Clinical Practice Update on Diagnosis and Management of Cyclic Vomiting Syndrome: Commentary. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.05.031.
Geeratragool T et al. Comparison of Vonoprazan Versus Intravenous Proton Pump Inhibitor for Prevention of High-Risk Peptic Ulcers Rebleeding After Successful Endoscopic Hemostasis: A Multicenter Randomized Noninferiority Trial. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.03.036.
Goodoory VC et al. Effect of Brain-Gut Behavioral Treatments on Abdominal Pain in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gastroenterology. 2024 Oct. doi: 10.1053/j.gastro.2024.05.010.
Kurlander JE et al; Gastrointestinal Bleeding Working Group. Prescribing of Proton Pump Inhibitors for Prevention of Upper Gastrointestinal Bleeding in US Outpatient Visits. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.01.047.
Oliva S et al. Crafting a Therapeutic Pyramid for Eosinophilic Esophagitis in the Age of Biologics. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.020.
Lower GI
Redd WD et al. Follow-Up Colonoscopy for Detection of Missed Colorectal Cancer After Diverticulitis. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.03.036.
Peyrin-Biroulet L et al. Upadacitinib Achieves Clinical and Endoscopic Outcomes in Crohn’s Disease Regardless of Prior Biologic Exposure. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.02.026.
Chang PW et al. ChatGPT4 Outperforms Endoscopists for Determination of Postcolonoscopy Rescreening and Surveillance Recommendations. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.022.
Liver
Wang L et al. Association of GLP-1 Receptor Agonists and Hepatocellular Carcinoma Incidence and Hepatic Decompensation in Patients With Type 2 Diabetes. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.04.029.
Bajaj JS et al. Serum Ammonia Levels Do Not Correlate With Overt Hepatic Encephalopathy Severity in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.02.015.
Endoscopy
Steinbrück I, et al. Cold Versus Hot Snare Endoscopic Resection of Large Nonpedunculated Colorectal Polyps: Randomized Controlled German CHRONICLE Trial. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.05.013.
Misc.
Kothari S et al. AGA Clinical Practice Update on Pregnancy-Related Gastrointestinal and Liver Disease: Expert Review. Gastroenterology. 2024 Oct. doi: 10.1053/j.gastro.2024.06.014.
Chavannes M et al. AGA Clinical Practice Update on the Role of Intestinal Ultrasound in Inflammatory Bowel Disease: Commentary. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.039.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Upper GI
Levinthal DJ et al. AGA Clinical Practice Update on Diagnosis and Management of Cyclic Vomiting Syndrome: Commentary. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.05.031.
Geeratragool T et al. Comparison of Vonoprazan Versus Intravenous Proton Pump Inhibitor for Prevention of High-Risk Peptic Ulcers Rebleeding After Successful Endoscopic Hemostasis: A Multicenter Randomized Noninferiority Trial. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.03.036.
Goodoory VC et al. Effect of Brain-Gut Behavioral Treatments on Abdominal Pain in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gastroenterology. 2024 Oct. doi: 10.1053/j.gastro.2024.05.010.
Kurlander JE et al; Gastrointestinal Bleeding Working Group. Prescribing of Proton Pump Inhibitors for Prevention of Upper Gastrointestinal Bleeding in US Outpatient Visits. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.01.047.
Oliva S et al. Crafting a Therapeutic Pyramid for Eosinophilic Esophagitis in the Age of Biologics. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.020.
Lower GI
Redd WD et al. Follow-Up Colonoscopy for Detection of Missed Colorectal Cancer After Diverticulitis. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.03.036.
Peyrin-Biroulet L et al. Upadacitinib Achieves Clinical and Endoscopic Outcomes in Crohn’s Disease Regardless of Prior Biologic Exposure. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.02.026.
Chang PW et al. ChatGPT4 Outperforms Endoscopists for Determination of Postcolonoscopy Rescreening and Surveillance Recommendations. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.022.
Liver
Wang L et al. Association of GLP-1 Receptor Agonists and Hepatocellular Carcinoma Incidence and Hepatic Decompensation in Patients With Type 2 Diabetes. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.04.029.
Bajaj JS et al. Serum Ammonia Levels Do Not Correlate With Overt Hepatic Encephalopathy Severity in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.02.015.
Endoscopy
Steinbrück I, et al. Cold Versus Hot Snare Endoscopic Resection of Large Nonpedunculated Colorectal Polyps: Randomized Controlled German CHRONICLE Trial. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.05.013.
Misc.
Kothari S et al. AGA Clinical Practice Update on Pregnancy-Related Gastrointestinal and Liver Disease: Expert Review. Gastroenterology. 2024 Oct. doi: 10.1053/j.gastro.2024.06.014.
Chavannes M et al. AGA Clinical Practice Update on the Role of Intestinal Ultrasound in Inflammatory Bowel Disease: Commentary. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.039.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Upper GI
Levinthal DJ et al. AGA Clinical Practice Update on Diagnosis and Management of Cyclic Vomiting Syndrome: Commentary. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.05.031.
Geeratragool T et al. Comparison of Vonoprazan Versus Intravenous Proton Pump Inhibitor for Prevention of High-Risk Peptic Ulcers Rebleeding After Successful Endoscopic Hemostasis: A Multicenter Randomized Noninferiority Trial. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.03.036.
Goodoory VC et al. Effect of Brain-Gut Behavioral Treatments on Abdominal Pain in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gastroenterology. 2024 Oct. doi: 10.1053/j.gastro.2024.05.010.
Kurlander JE et al; Gastrointestinal Bleeding Working Group. Prescribing of Proton Pump Inhibitors for Prevention of Upper Gastrointestinal Bleeding in US Outpatient Visits. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.01.047.
Oliva S et al. Crafting a Therapeutic Pyramid for Eosinophilic Esophagitis in the Age of Biologics. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.020.
Lower GI
Redd WD et al. Follow-Up Colonoscopy for Detection of Missed Colorectal Cancer After Diverticulitis. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.03.036.
Peyrin-Biroulet L et al. Upadacitinib Achieves Clinical and Endoscopic Outcomes in Crohn’s Disease Regardless of Prior Biologic Exposure. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.02.026.
Chang PW et al. ChatGPT4 Outperforms Endoscopists for Determination of Postcolonoscopy Rescreening and Surveillance Recommendations. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.022.
Liver
Wang L et al. Association of GLP-1 Receptor Agonists and Hepatocellular Carcinoma Incidence and Hepatic Decompensation in Patients With Type 2 Diabetes. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.04.029.
Bajaj JS et al. Serum Ammonia Levels Do Not Correlate With Overt Hepatic Encephalopathy Severity in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.02.015.
Endoscopy
Steinbrück I, et al. Cold Versus Hot Snare Endoscopic Resection of Large Nonpedunculated Colorectal Polyps: Randomized Controlled German CHRONICLE Trial. Gastroenterology. 2024 Sep. doi: 10.1053/j.gastro.2024.05.013.
Misc.
Kothari S et al. AGA Clinical Practice Update on Pregnancy-Related Gastrointestinal and Liver Disease: Expert Review. Gastroenterology. 2024 Oct. doi: 10.1053/j.gastro.2024.06.014.
Chavannes M et al. AGA Clinical Practice Update on the Role of Intestinal Ultrasound in Inflammatory Bowel Disease: Commentary. Clin Gastroenterol Hepatol. 2024 Sep. doi: 10.1016/j.cgh.2024.04.039.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Alcohol-Associated Liver Disease and Alcohol Use Disorder on the Rise in Older Adults
SAN DIEGO — according to the results of a new study.
Even as mortality rates decline globally, AUD deaths rose in the United States, increasing 1.63% per year between 2010 and 2019. Deaths from cirrhosis increased by 0.56% each year, and deaths from primary liver cancer associated with alcohol increased by 3.09% per year.
Several factors, such as an aging US population and increasing alcohol consumption, play a major role in the uptick in mortality, said lead author Pojsakorn Danpanichkul, MD, an internal medicine resident at Texas Tech University Health Sciences Center, Lubbock, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“Healthcare providers should increase screening for alcohol use among older adults and consider the added risks of alcohol consumption. Public health strategies should target alcohol prevention and treatment programs tailored to older adults,” he said.
“Older adults are more vulnerable to the harmful effects of alcohol due to natural declines in liver function and metabolism, leading to a higher risk of liver disease and complications,” he explained. However, “little research has focused on this issue.”
Trends in US Not Seen Globally
Danpanichkul and colleagues analyzed data from the Global Burden of Disease Study for 2010-2019, calculating the annual percent change for the burden of AUD, ALD, and liver cancer from alcohol in patients age 70 and older. The research team then compared data in the United States to global estimates for these same diseases.
In 2019, there were 556,340 cases of AUD, 112,560 cases of ALD, and 3720 cases of liver cancer from alcohol in older adults in the United States. In addition, there were 1750 deaths attributed to AUD, 4860 deaths from ALD, and 3010 deaths caused by primary liver cancer from alcohol.
The age-standardized prevalence rates (ASPRs) per 100,000 people were 1547 cases of AUD, 313 cases of ALD, and 10 cases of primary liver cancer caused by alcohol.
The age-standardized death rates (ASDRs) per 100,000 people were 4.88 for AUD, 13.52 for ALD, and 8.38 for primary liver cancer.
During the time period studied, upward trends occurred in the United States, with annual ASPRs increasing by 2.52% for AUD, 1.78% for ALD, and 3.31% for primary liver cancer due to alcohol. Globally, the trends were lower, with annual increases of 0.2% for AUD, 0.38% for ALD, and 0.67% for primary liver cancer from alcohol.
During the same time, ASDRs also increased in all three categories in the United States, while global trends showed a 0.91% decline in AUD deaths and 0.6% decline in ALD deaths. Liver cancer deaths, however, increased by 0.3% worldwide.
Targeted strategies are essential to reduce this growing health burden, especially in an aging population, Danpanichkul said. “These interventions should focus on early detection, intervention, and management for individuals at risk or already affected by ALD and AUD.”
Future studies should investigate alcohol consumption and mortality trends in other age groups, including by sex, location (such as state or territory), and race and ethnicity, he said. Data for more recent years would be compelling as well.
Increased Alcohol Use During and After Pandemic
Numerous studies have indicated that alcohol use increased in 2020 during the COVID-19 pandemic and has remained elevated since then.
In a study published in the Annals of Internal Medicine, for instance, alcohol use per 100 people increased 2.69% in 2020 and 2.96% in 2022, as compared with 2018. Increases occurred across all subgroups, including age, sex, race, ethnicity, and US region.
“During the COVID-19 pandemic, many people stayed at home, watched the television, and increased their alcohol intake” — in the United States and also in Japan — said Hisanori Muto, MD, senior assistant professor of gastroenterology at Fujita Health University in Nagoya, Japan, who wasn’t involved with this study.
“Although the global numbers may appear lower, we’re also seeing an increase in AUD and ALD in Japan, similar to the United States,” he said. “It’s very important to watch these trends and address these diseases.”
Danpanichkul and Muto reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to the results of a new study.
Even as mortality rates decline globally, AUD deaths rose in the United States, increasing 1.63% per year between 2010 and 2019. Deaths from cirrhosis increased by 0.56% each year, and deaths from primary liver cancer associated with alcohol increased by 3.09% per year.
Several factors, such as an aging US population and increasing alcohol consumption, play a major role in the uptick in mortality, said lead author Pojsakorn Danpanichkul, MD, an internal medicine resident at Texas Tech University Health Sciences Center, Lubbock, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“Healthcare providers should increase screening for alcohol use among older adults and consider the added risks of alcohol consumption. Public health strategies should target alcohol prevention and treatment programs tailored to older adults,” he said.
“Older adults are more vulnerable to the harmful effects of alcohol due to natural declines in liver function and metabolism, leading to a higher risk of liver disease and complications,” he explained. However, “little research has focused on this issue.”
Trends in US Not Seen Globally
Danpanichkul and colleagues analyzed data from the Global Burden of Disease Study for 2010-2019, calculating the annual percent change for the burden of AUD, ALD, and liver cancer from alcohol in patients age 70 and older. The research team then compared data in the United States to global estimates for these same diseases.
In 2019, there were 556,340 cases of AUD, 112,560 cases of ALD, and 3720 cases of liver cancer from alcohol in older adults in the United States. In addition, there were 1750 deaths attributed to AUD, 4860 deaths from ALD, and 3010 deaths caused by primary liver cancer from alcohol.
The age-standardized prevalence rates (ASPRs) per 100,000 people were 1547 cases of AUD, 313 cases of ALD, and 10 cases of primary liver cancer caused by alcohol.
The age-standardized death rates (ASDRs) per 100,000 people were 4.88 for AUD, 13.52 for ALD, and 8.38 for primary liver cancer.
During the time period studied, upward trends occurred in the United States, with annual ASPRs increasing by 2.52% for AUD, 1.78% for ALD, and 3.31% for primary liver cancer due to alcohol. Globally, the trends were lower, with annual increases of 0.2% for AUD, 0.38% for ALD, and 0.67% for primary liver cancer from alcohol.
During the same time, ASDRs also increased in all three categories in the United States, while global trends showed a 0.91% decline in AUD deaths and 0.6% decline in ALD deaths. Liver cancer deaths, however, increased by 0.3% worldwide.
Targeted strategies are essential to reduce this growing health burden, especially in an aging population, Danpanichkul said. “These interventions should focus on early detection, intervention, and management for individuals at risk or already affected by ALD and AUD.”
Future studies should investigate alcohol consumption and mortality trends in other age groups, including by sex, location (such as state or territory), and race and ethnicity, he said. Data for more recent years would be compelling as well.
Increased Alcohol Use During and After Pandemic
Numerous studies have indicated that alcohol use increased in 2020 during the COVID-19 pandemic and has remained elevated since then.
In a study published in the Annals of Internal Medicine, for instance, alcohol use per 100 people increased 2.69% in 2020 and 2.96% in 2022, as compared with 2018. Increases occurred across all subgroups, including age, sex, race, ethnicity, and US region.
“During the COVID-19 pandemic, many people stayed at home, watched the television, and increased their alcohol intake” — in the United States and also in Japan — said Hisanori Muto, MD, senior assistant professor of gastroenterology at Fujita Health University in Nagoya, Japan, who wasn’t involved with this study.
“Although the global numbers may appear lower, we’re also seeing an increase in AUD and ALD in Japan, similar to the United States,” he said. “It’s very important to watch these trends and address these diseases.”
Danpanichkul and Muto reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to the results of a new study.
Even as mortality rates decline globally, AUD deaths rose in the United States, increasing 1.63% per year between 2010 and 2019. Deaths from cirrhosis increased by 0.56% each year, and deaths from primary liver cancer associated with alcohol increased by 3.09% per year.
Several factors, such as an aging US population and increasing alcohol consumption, play a major role in the uptick in mortality, said lead author Pojsakorn Danpanichkul, MD, an internal medicine resident at Texas Tech University Health Sciences Center, Lubbock, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“Healthcare providers should increase screening for alcohol use among older adults and consider the added risks of alcohol consumption. Public health strategies should target alcohol prevention and treatment programs tailored to older adults,” he said.
“Older adults are more vulnerable to the harmful effects of alcohol due to natural declines in liver function and metabolism, leading to a higher risk of liver disease and complications,” he explained. However, “little research has focused on this issue.”
Trends in US Not Seen Globally
Danpanichkul and colleagues analyzed data from the Global Burden of Disease Study for 2010-2019, calculating the annual percent change for the burden of AUD, ALD, and liver cancer from alcohol in patients age 70 and older. The research team then compared data in the United States to global estimates for these same diseases.
In 2019, there were 556,340 cases of AUD, 112,560 cases of ALD, and 3720 cases of liver cancer from alcohol in older adults in the United States. In addition, there were 1750 deaths attributed to AUD, 4860 deaths from ALD, and 3010 deaths caused by primary liver cancer from alcohol.
The age-standardized prevalence rates (ASPRs) per 100,000 people were 1547 cases of AUD, 313 cases of ALD, and 10 cases of primary liver cancer caused by alcohol.
The age-standardized death rates (ASDRs) per 100,000 people were 4.88 for AUD, 13.52 for ALD, and 8.38 for primary liver cancer.
During the time period studied, upward trends occurred in the United States, with annual ASPRs increasing by 2.52% for AUD, 1.78% for ALD, and 3.31% for primary liver cancer due to alcohol. Globally, the trends were lower, with annual increases of 0.2% for AUD, 0.38% for ALD, and 0.67% for primary liver cancer from alcohol.
During the same time, ASDRs also increased in all three categories in the United States, while global trends showed a 0.91% decline in AUD deaths and 0.6% decline in ALD deaths. Liver cancer deaths, however, increased by 0.3% worldwide.
Targeted strategies are essential to reduce this growing health burden, especially in an aging population, Danpanichkul said. “These interventions should focus on early detection, intervention, and management for individuals at risk or already affected by ALD and AUD.”
Future studies should investigate alcohol consumption and mortality trends in other age groups, including by sex, location (such as state or territory), and race and ethnicity, he said. Data for more recent years would be compelling as well.
Increased Alcohol Use During and After Pandemic
Numerous studies have indicated that alcohol use increased in 2020 during the COVID-19 pandemic and has remained elevated since then.
In a study published in the Annals of Internal Medicine, for instance, alcohol use per 100 people increased 2.69% in 2020 and 2.96% in 2022, as compared with 2018. Increases occurred across all subgroups, including age, sex, race, ethnicity, and US region.
“During the COVID-19 pandemic, many people stayed at home, watched the television, and increased their alcohol intake” — in the United States and also in Japan — said Hisanori Muto, MD, senior assistant professor of gastroenterology at Fujita Health University in Nagoya, Japan, who wasn’t involved with this study.
“Although the global numbers may appear lower, we’re also seeing an increase in AUD and ALD in Japan, similar to the United States,” he said. “It’s very important to watch these trends and address these diseases.”
Danpanichkul and Muto reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AASLD 2024
Alcohol Use Disorder Therapy Remains Underutilized in Alcohol-Associated Liver Disease
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Short-Course Vasoconstrictors After EVL: Time for a New Standard of Care?
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
MASH: Experts Offer Noninvasive Cutoffs for Prescribing Resmetirom
This guidance document allows clinicians to use a variety of NITs to start and monitor resmetirom therapy, precluding the need for a biopsy, lead author Mazen Noureddin, MD, of Houston Research Institute, Houston Methodist Hospital in Texas, and colleagues reported.
“The recent conditional approval by the [Food and Drug Administration] of resmetirom ... presents a much-anticipated therapeutic option for patients with noncirrhotic advanced MASH,” the investigators wrote in Clinical Gastroenterology and Hepatology.
However, the approval also “presents important challenges,” they noted, “including how to noninvasively identify patients with fibrosis stages 2-3, and how to exclude patients with more advanced disease who should not be treated until further data emerge on the use of resmetirom in this population.”
To help identify which patients should get this new intervention, Noureddin and colleagues considered benchmarks from published literature, and conducted a post hoc analysis of phase 3 MASTERO-NASH trial data. Trial enrollment required at least three cardiometabolic risk factors and a vibration-controlled transient elastography (VCTE) prescreening within the past 3 months. The population included 888 patients with F2 or F3 disease.
Recommendations were split into three categories: treat with resmetirom, consider treating with resmetirom, and do not treat with resmetirom.
The recommendation to treat calls for a VCTE of 10-15 kPa, a magnetic resonance elastography (MRE) of 3.3-4.2 kPa, or an Enhanced Liver Fibrosis (ELF) score of 9.2-10.4, with the caveat that an ELF score below 9.8 requires a second NIT for confirmation. Alternatively, a positive composite score such as FibroScan–aspartate aminotransferase (FAST), MRI–AST (MAST), or MRE + Fibrosis-4 (MEFIB) may serve as grounds for treatment. For any of the previous, platelets must concurrently be at least 140 with no evidence of portal hypertension.
The recommendation to consider treatment depends upon a VCTE of 15.1-19.9 kPa, an MRE of 4.3-4.9 kPa, an ELF score of 10.5-11.3, or positive FAST, MAST, or MEFIB. Again, these require a concurrent platelet count of 140 and no portal hypertension.
Finally, patients should not be treated with resmetirom if they have a VCTE of 20 kPa or greater, an MRE of 5 kPa or greater, and an ELF score greater than 11.3.
Noureddin and colleagues also offered guidance on monitoring strategies, including follow-up at 3, 6, and 12 months.
At 3 months, the focus should be safety, including screening for drug-related liver injury and other adverse events that warrant cessation.
At 6 months, alanine aminotransferase (ALT) levels, VCTE, or MRI–proton density fat fraction (PDFF) tests can indicate early response, but treatment should generally continue regardless of results.
At 12 months, efficacy can be fully evaluated. ALT normalization, or improvement of more than 17 IU/L or more than 20%, along with a 30% or greater drop in VCTE, or at least 30% drop in liver fat on MRI-PDFF, serve as grounds for continuation.
Noureddin and colleagues noted that ALT improvement should be paired with corresponding improvements in imaging, such as a 30% reduction in MRI-PDFF. Even if ALT levels do not improve, a 30% or greater reduction in MRI-PDFF can still indicate a positive response; however, VCTE alone may not be sufficient to fully assess treatment response.
“Emerging data, particularly regarding the noninvasive assessment of treatment response, are likely to further modify patient selection, safety signals, and efficacy algorithms,” they concluded.This study was supported by the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung, and Blood Institute, the John C. Martin Foundation, and the National Institute on Alcohol Abuse and Alcoholism. The investigators disclosed additional relationships with Novo Nordisk, Pfizer, Shire, and others.
The approval of resmetirom as the first registered treatment for metabolic dysfunction–associated steatohepatitis (MASH) marks a historic moment. This expert panel recommendation document offers valuable guidance on patient selection for resmetirom treatment, monitoring responses, and managing potential side effects and drug-drug interactions. It also highlights the complexities of applying noninvasive tests for treatment initiation. Clinicians must identify MASH patients with significant or advanced fibrosis while avoiding those with cirrhosis and hepatic decompensation. Management will be simplified if the MAESTRO-OUTCOMES trial confirms that resmetirom is safe and effective for patients with compensated MASH cirrhosis.
The most reliable response biomarkers in the MAESTRO-NASH trial include reductions in MRI–proton density fat fraction (MRI-PDFF) and serum alanine aminotransferase, despite MRI-PDFF being limited by cost and availability. Worsening liver stiffness measurement via vibration-controlled transient elastography is suggested as a stopping rule, although this is not supported by resmetirom trial data. Short-term increases in liver stiffness may yield false positives, so it is advisable to repeat or use alternative noninvasive tests before discontinuing treatment.
Vincent Wai-Sun Wong, MD, is Mok Hing Yiu Professor of Medicine at the Chinese University of Hong Kong, China. He reported his role as a consultant or advisory board member for AbbVie, AstraZeneca, Boehringer Ingelheim, Echosens, Eli Lilly, Gilead Sciences, Intercept, Inventiva, Merck, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions, and Visirna; and a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk, and Unilab. He has received a research grant from Gilead Sciences, and is the cofounder of Illuminatio Medical Technology.
The approval of resmetirom as the first registered treatment for metabolic dysfunction–associated steatohepatitis (MASH) marks a historic moment. This expert panel recommendation document offers valuable guidance on patient selection for resmetirom treatment, monitoring responses, and managing potential side effects and drug-drug interactions. It also highlights the complexities of applying noninvasive tests for treatment initiation. Clinicians must identify MASH patients with significant or advanced fibrosis while avoiding those with cirrhosis and hepatic decompensation. Management will be simplified if the MAESTRO-OUTCOMES trial confirms that resmetirom is safe and effective for patients with compensated MASH cirrhosis.
The most reliable response biomarkers in the MAESTRO-NASH trial include reductions in MRI–proton density fat fraction (MRI-PDFF) and serum alanine aminotransferase, despite MRI-PDFF being limited by cost and availability. Worsening liver stiffness measurement via vibration-controlled transient elastography is suggested as a stopping rule, although this is not supported by resmetirom trial data. Short-term increases in liver stiffness may yield false positives, so it is advisable to repeat or use alternative noninvasive tests before discontinuing treatment.
Vincent Wai-Sun Wong, MD, is Mok Hing Yiu Professor of Medicine at the Chinese University of Hong Kong, China. He reported his role as a consultant or advisory board member for AbbVie, AstraZeneca, Boehringer Ingelheim, Echosens, Eli Lilly, Gilead Sciences, Intercept, Inventiva, Merck, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions, and Visirna; and a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk, and Unilab. He has received a research grant from Gilead Sciences, and is the cofounder of Illuminatio Medical Technology.
The approval of resmetirom as the first registered treatment for metabolic dysfunction–associated steatohepatitis (MASH) marks a historic moment. This expert panel recommendation document offers valuable guidance on patient selection for resmetirom treatment, monitoring responses, and managing potential side effects and drug-drug interactions. It also highlights the complexities of applying noninvasive tests for treatment initiation. Clinicians must identify MASH patients with significant or advanced fibrosis while avoiding those with cirrhosis and hepatic decompensation. Management will be simplified if the MAESTRO-OUTCOMES trial confirms that resmetirom is safe and effective for patients with compensated MASH cirrhosis.
The most reliable response biomarkers in the MAESTRO-NASH trial include reductions in MRI–proton density fat fraction (MRI-PDFF) and serum alanine aminotransferase, despite MRI-PDFF being limited by cost and availability. Worsening liver stiffness measurement via vibration-controlled transient elastography is suggested as a stopping rule, although this is not supported by resmetirom trial data. Short-term increases in liver stiffness may yield false positives, so it is advisable to repeat or use alternative noninvasive tests before discontinuing treatment.
Vincent Wai-Sun Wong, MD, is Mok Hing Yiu Professor of Medicine at the Chinese University of Hong Kong, China. He reported his role as a consultant or advisory board member for AbbVie, AstraZeneca, Boehringer Ingelheim, Echosens, Eli Lilly, Gilead Sciences, Intercept, Inventiva, Merck, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions, and Visirna; and a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk, and Unilab. He has received a research grant from Gilead Sciences, and is the cofounder of Illuminatio Medical Technology.
This guidance document allows clinicians to use a variety of NITs to start and monitor resmetirom therapy, precluding the need for a biopsy, lead author Mazen Noureddin, MD, of Houston Research Institute, Houston Methodist Hospital in Texas, and colleagues reported.
“The recent conditional approval by the [Food and Drug Administration] of resmetirom ... presents a much-anticipated therapeutic option for patients with noncirrhotic advanced MASH,” the investigators wrote in Clinical Gastroenterology and Hepatology.
However, the approval also “presents important challenges,” they noted, “including how to noninvasively identify patients with fibrosis stages 2-3, and how to exclude patients with more advanced disease who should not be treated until further data emerge on the use of resmetirom in this population.”
To help identify which patients should get this new intervention, Noureddin and colleagues considered benchmarks from published literature, and conducted a post hoc analysis of phase 3 MASTERO-NASH trial data. Trial enrollment required at least three cardiometabolic risk factors and a vibration-controlled transient elastography (VCTE) prescreening within the past 3 months. The population included 888 patients with F2 or F3 disease.
Recommendations were split into three categories: treat with resmetirom, consider treating with resmetirom, and do not treat with resmetirom.
The recommendation to treat calls for a VCTE of 10-15 kPa, a magnetic resonance elastography (MRE) of 3.3-4.2 kPa, or an Enhanced Liver Fibrosis (ELF) score of 9.2-10.4, with the caveat that an ELF score below 9.8 requires a second NIT for confirmation. Alternatively, a positive composite score such as FibroScan–aspartate aminotransferase (FAST), MRI–AST (MAST), or MRE + Fibrosis-4 (MEFIB) may serve as grounds for treatment. For any of the previous, platelets must concurrently be at least 140 with no evidence of portal hypertension.
The recommendation to consider treatment depends upon a VCTE of 15.1-19.9 kPa, an MRE of 4.3-4.9 kPa, an ELF score of 10.5-11.3, or positive FAST, MAST, or MEFIB. Again, these require a concurrent platelet count of 140 and no portal hypertension.
Finally, patients should not be treated with resmetirom if they have a VCTE of 20 kPa or greater, an MRE of 5 kPa or greater, and an ELF score greater than 11.3.
Noureddin and colleagues also offered guidance on monitoring strategies, including follow-up at 3, 6, and 12 months.
At 3 months, the focus should be safety, including screening for drug-related liver injury and other adverse events that warrant cessation.
At 6 months, alanine aminotransferase (ALT) levels, VCTE, or MRI–proton density fat fraction (PDFF) tests can indicate early response, but treatment should generally continue regardless of results.
At 12 months, efficacy can be fully evaluated. ALT normalization, or improvement of more than 17 IU/L or more than 20%, along with a 30% or greater drop in VCTE, or at least 30% drop in liver fat on MRI-PDFF, serve as grounds for continuation.
Noureddin and colleagues noted that ALT improvement should be paired with corresponding improvements in imaging, such as a 30% reduction in MRI-PDFF. Even if ALT levels do not improve, a 30% or greater reduction in MRI-PDFF can still indicate a positive response; however, VCTE alone may not be sufficient to fully assess treatment response.
“Emerging data, particularly regarding the noninvasive assessment of treatment response, are likely to further modify patient selection, safety signals, and efficacy algorithms,” they concluded.This study was supported by the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung, and Blood Institute, the John C. Martin Foundation, and the National Institute on Alcohol Abuse and Alcoholism. The investigators disclosed additional relationships with Novo Nordisk, Pfizer, Shire, and others.
This guidance document allows clinicians to use a variety of NITs to start and monitor resmetirom therapy, precluding the need for a biopsy, lead author Mazen Noureddin, MD, of Houston Research Institute, Houston Methodist Hospital in Texas, and colleagues reported.
“The recent conditional approval by the [Food and Drug Administration] of resmetirom ... presents a much-anticipated therapeutic option for patients with noncirrhotic advanced MASH,” the investigators wrote in Clinical Gastroenterology and Hepatology.
However, the approval also “presents important challenges,” they noted, “including how to noninvasively identify patients with fibrosis stages 2-3, and how to exclude patients with more advanced disease who should not be treated until further data emerge on the use of resmetirom in this population.”
To help identify which patients should get this new intervention, Noureddin and colleagues considered benchmarks from published literature, and conducted a post hoc analysis of phase 3 MASTERO-NASH trial data. Trial enrollment required at least three cardiometabolic risk factors and a vibration-controlled transient elastography (VCTE) prescreening within the past 3 months. The population included 888 patients with F2 or F3 disease.
Recommendations were split into three categories: treat with resmetirom, consider treating with resmetirom, and do not treat with resmetirom.
The recommendation to treat calls for a VCTE of 10-15 kPa, a magnetic resonance elastography (MRE) of 3.3-4.2 kPa, or an Enhanced Liver Fibrosis (ELF) score of 9.2-10.4, with the caveat that an ELF score below 9.8 requires a second NIT for confirmation. Alternatively, a positive composite score such as FibroScan–aspartate aminotransferase (FAST), MRI–AST (MAST), or MRE + Fibrosis-4 (MEFIB) may serve as grounds for treatment. For any of the previous, platelets must concurrently be at least 140 with no evidence of portal hypertension.
The recommendation to consider treatment depends upon a VCTE of 15.1-19.9 kPa, an MRE of 4.3-4.9 kPa, an ELF score of 10.5-11.3, or positive FAST, MAST, or MEFIB. Again, these require a concurrent platelet count of 140 and no portal hypertension.
Finally, patients should not be treated with resmetirom if they have a VCTE of 20 kPa or greater, an MRE of 5 kPa or greater, and an ELF score greater than 11.3.
Noureddin and colleagues also offered guidance on monitoring strategies, including follow-up at 3, 6, and 12 months.
At 3 months, the focus should be safety, including screening for drug-related liver injury and other adverse events that warrant cessation.
At 6 months, alanine aminotransferase (ALT) levels, VCTE, or MRI–proton density fat fraction (PDFF) tests can indicate early response, but treatment should generally continue regardless of results.
At 12 months, efficacy can be fully evaluated. ALT normalization, or improvement of more than 17 IU/L or more than 20%, along with a 30% or greater drop in VCTE, or at least 30% drop in liver fat on MRI-PDFF, serve as grounds for continuation.
Noureddin and colleagues noted that ALT improvement should be paired with corresponding improvements in imaging, such as a 30% reduction in MRI-PDFF. Even if ALT levels do not improve, a 30% or greater reduction in MRI-PDFF can still indicate a positive response; however, VCTE alone may not be sufficient to fully assess treatment response.
“Emerging data, particularly regarding the noninvasive assessment of treatment response, are likely to further modify patient selection, safety signals, and efficacy algorithms,” they concluded.This study was supported by the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung, and Blood Institute, the John C. Martin Foundation, and the National Institute on Alcohol Abuse and Alcoholism. The investigators disclosed additional relationships with Novo Nordisk, Pfizer, Shire, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Can We Repurpose Obesity Drugs to Reverse Liver Disease?
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
MASLD Healthcare Costs Climbing Fast in Canada
according to a new study.
The expected surge reflects the growing prevalence of MASLD and its associated conditions, emphasizing the necessity for a comprehensive approach to address this escalating public health issue, reported lead author K. Ally Memedovich, BHSc, of the University of Calgary in Alberta, Canada, and colleagues.
“The costs associated with the management of MASLD in Canada remain unknown but have been estimated as being very high,” the investigators wrote in Gastro Hep Advances. “Specifically, in one study from the United States, the healthcare costs and utilization of those with MASLD was nearly double that of patients without MASLD but with similar health status. This difference was largely due to increases in imaging, hospitalization, liver fibrosis assessment, laboratory tests, and outpatient visits.”
Although projections are available to estimate the future prevalence of MASLD in Canada, no models are available to predict the growing national economic burden, prompting the present study.
Memedovich and colleagues analyzed healthcare usage data from 6,358 patients diagnosed with MASLD disease in Calgary from 2018 to 2020. Using provincial administrative data, they calculated both liver-specific and total healthcare costs associated with different stages of liver fibrosis, ranging from F0/F1 (minimal fibrosis) to F4 (advanced fibrosis or cirrhosis).
The patients’ liver fibrosis stages were determined using liver stiffness measurements obtained through shear wave elastography. Average annual cost per patient was then calculated for each fibrosis stage by analyzing hospitalizations, ambulatory care, and physician claims data.
The annual average liver-specific cost per patient increased with severity of liver fibrosis; costs for patients with fibrosis stages F0/F1, F2, F3, and F4 were C$7.02, C$35.30, C$60.46, and C$72.55, respectively. By 2050, liver-specific healthcare costs are projected to increase by C$51 million, reaching C$136 million Canada-wide.
Total healthcare costs were markedly higher; annual costs for patients with fibrosis stages F0/F1, F2, F3, and F4 were C$397.90, C$781.53, C$2,881.84, and C$1,598.82, respectively. As a result, total healthcare costs are expected to rise by nearly C$2 billion, contributing to a Canadian healthcare burden of C$5.81 billion annually by 2050.
The study revealed that over 90% of the healthcare costs for MASLD patients were attributed not to liver disease itself but to the management of associated comorbidities such as diabetes, hypertension, mental illness, and obesity. For instance, diabetes was the most common reason for physician visits among MASLD patients, accounting for 65.2% of cases. One study limitation was exclusion of decompensated cirrhosis, liver cancer, or a liver transplant recipient because of low prevalence in this cohort, potentially contributing to low liver specific healthcare costs.
Memedovich and colleagues noted that chronic diseases account for approximately C$68 billion annually in direct healthcare costs in Canada, representing around 58% of total healthcare expenditures. Estimates suggest that 1% annual reduction in chronic disease prevalence could save C$107 billion over the course of 20 years.
“Therefore, an approach that focuses on preventing and managing chronic diseases overall is needed to reduce the burden of MASLD on the healthcare system,” they wrote. This study was funded by LiveRx via an Alberta Innovates grant. The investigators disclosed relationships with Gilead, Abbott, GSK, and others.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is the most common chronic liver disease, and its clinical burden is expected to mirror the rising rates of obesity and diabetes over the next couple decades. The cost analysis by Memdovich and colleagues provides a timely report on the healthcare burden of MASLD in Canada. Their results are, nevertheless, generalizable to other healthcare systems.
The authors found that nearly 98% of total healthcare costs of patients with MASLD were not specifically related to liver treatment, but rather linked to the management of patients’ cardiometabolic comorbidities. Projection estimates based on this cohort suggests a steep rise in the total healthcare costs over the coming decades reflecting increasing rates of comorbidities, with largest changes expected in the advanced fibrosis patient group. These findings highlight the need for early recognition of MASLD followed by a collaborative effort in management of MASLD in conjunction with its associated cardiometabolic comorbidities.
As rates for obesity, diabetes, and MASLD continue to rise, there is an urgency to create a global strategy for MASLD management that focuses on both prevention and treatment. Public health strategies are needed to increase awareness and focus on the treatment and prevention cardiometabolic risk factors that appear to be the main drivers of healthcare costs among patients with MASLD. A concerted effort is needed from providers, both primary care and specialists, for early recognition and treatment of MASLD. Such a public health response combined with recent advent in pharmacotherapy for weight loss and metabolic dysfunction–associated steatohepatitis may alter the projected costs and hopefully decrease the disease burden associated advanced MASLD.
Akshay Shetty, MD, is assistant professor of medicine and surgery at the David Geffen School of Medicine, University of California, San Francisco. He has no conflicts of interest to declare. Sammy Saab, MD, MPH, AGAF, is professor of medicine and surgery at the David Geffen School of Medicine at UCLA. He is on the speakers bureau for AbbVie, Gilead, Eisai, Intercept, Ipsen, Salix, Mallinckrodt, and Takeda, and has been a consultant for Gilead, Ipsen, Mallinckrodt, Madrigal, and Orphalan.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is the most common chronic liver disease, and its clinical burden is expected to mirror the rising rates of obesity and diabetes over the next couple decades. The cost analysis by Memdovich and colleagues provides a timely report on the healthcare burden of MASLD in Canada. Their results are, nevertheless, generalizable to other healthcare systems.
The authors found that nearly 98% of total healthcare costs of patients with MASLD were not specifically related to liver treatment, but rather linked to the management of patients’ cardiometabolic comorbidities. Projection estimates based on this cohort suggests a steep rise in the total healthcare costs over the coming decades reflecting increasing rates of comorbidities, with largest changes expected in the advanced fibrosis patient group. These findings highlight the need for early recognition of MASLD followed by a collaborative effort in management of MASLD in conjunction with its associated cardiometabolic comorbidities.
As rates for obesity, diabetes, and MASLD continue to rise, there is an urgency to create a global strategy for MASLD management that focuses on both prevention and treatment. Public health strategies are needed to increase awareness and focus on the treatment and prevention cardiometabolic risk factors that appear to be the main drivers of healthcare costs among patients with MASLD. A concerted effort is needed from providers, both primary care and specialists, for early recognition and treatment of MASLD. Such a public health response combined with recent advent in pharmacotherapy for weight loss and metabolic dysfunction–associated steatohepatitis may alter the projected costs and hopefully decrease the disease burden associated advanced MASLD.
Akshay Shetty, MD, is assistant professor of medicine and surgery at the David Geffen School of Medicine, University of California, San Francisco. He has no conflicts of interest to declare. Sammy Saab, MD, MPH, AGAF, is professor of medicine and surgery at the David Geffen School of Medicine at UCLA. He is on the speakers bureau for AbbVie, Gilead, Eisai, Intercept, Ipsen, Salix, Mallinckrodt, and Takeda, and has been a consultant for Gilead, Ipsen, Mallinckrodt, Madrigal, and Orphalan.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is the most common chronic liver disease, and its clinical burden is expected to mirror the rising rates of obesity and diabetes over the next couple decades. The cost analysis by Memdovich and colleagues provides a timely report on the healthcare burden of MASLD in Canada. Their results are, nevertheless, generalizable to other healthcare systems.
The authors found that nearly 98% of total healthcare costs of patients with MASLD were not specifically related to liver treatment, but rather linked to the management of patients’ cardiometabolic comorbidities. Projection estimates based on this cohort suggests a steep rise in the total healthcare costs over the coming decades reflecting increasing rates of comorbidities, with largest changes expected in the advanced fibrosis patient group. These findings highlight the need for early recognition of MASLD followed by a collaborative effort in management of MASLD in conjunction with its associated cardiometabolic comorbidities.
As rates for obesity, diabetes, and MASLD continue to rise, there is an urgency to create a global strategy for MASLD management that focuses on both prevention and treatment. Public health strategies are needed to increase awareness and focus on the treatment and prevention cardiometabolic risk factors that appear to be the main drivers of healthcare costs among patients with MASLD. A concerted effort is needed from providers, both primary care and specialists, for early recognition and treatment of MASLD. Such a public health response combined with recent advent in pharmacotherapy for weight loss and metabolic dysfunction–associated steatohepatitis may alter the projected costs and hopefully decrease the disease burden associated advanced MASLD.
Akshay Shetty, MD, is assistant professor of medicine and surgery at the David Geffen School of Medicine, University of California, San Francisco. He has no conflicts of interest to declare. Sammy Saab, MD, MPH, AGAF, is professor of medicine and surgery at the David Geffen School of Medicine at UCLA. He is on the speakers bureau for AbbVie, Gilead, Eisai, Intercept, Ipsen, Salix, Mallinckrodt, and Takeda, and has been a consultant for Gilead, Ipsen, Mallinckrodt, Madrigal, and Orphalan.
according to a new study.
The expected surge reflects the growing prevalence of MASLD and its associated conditions, emphasizing the necessity for a comprehensive approach to address this escalating public health issue, reported lead author K. Ally Memedovich, BHSc, of the University of Calgary in Alberta, Canada, and colleagues.
“The costs associated with the management of MASLD in Canada remain unknown but have been estimated as being very high,” the investigators wrote in Gastro Hep Advances. “Specifically, in one study from the United States, the healthcare costs and utilization of those with MASLD was nearly double that of patients without MASLD but with similar health status. This difference was largely due to increases in imaging, hospitalization, liver fibrosis assessment, laboratory tests, and outpatient visits.”
Although projections are available to estimate the future prevalence of MASLD in Canada, no models are available to predict the growing national economic burden, prompting the present study.
Memedovich and colleagues analyzed healthcare usage data from 6,358 patients diagnosed with MASLD disease in Calgary from 2018 to 2020. Using provincial administrative data, they calculated both liver-specific and total healthcare costs associated with different stages of liver fibrosis, ranging from F0/F1 (minimal fibrosis) to F4 (advanced fibrosis or cirrhosis).
The patients’ liver fibrosis stages were determined using liver stiffness measurements obtained through shear wave elastography. Average annual cost per patient was then calculated for each fibrosis stage by analyzing hospitalizations, ambulatory care, and physician claims data.
The annual average liver-specific cost per patient increased with severity of liver fibrosis; costs for patients with fibrosis stages F0/F1, F2, F3, and F4 were C$7.02, C$35.30, C$60.46, and C$72.55, respectively. By 2050, liver-specific healthcare costs are projected to increase by C$51 million, reaching C$136 million Canada-wide.
Total healthcare costs were markedly higher; annual costs for patients with fibrosis stages F0/F1, F2, F3, and F4 were C$397.90, C$781.53, C$2,881.84, and C$1,598.82, respectively. As a result, total healthcare costs are expected to rise by nearly C$2 billion, contributing to a Canadian healthcare burden of C$5.81 billion annually by 2050.
The study revealed that over 90% of the healthcare costs for MASLD patients were attributed not to liver disease itself but to the management of associated comorbidities such as diabetes, hypertension, mental illness, and obesity. For instance, diabetes was the most common reason for physician visits among MASLD patients, accounting for 65.2% of cases. One study limitation was exclusion of decompensated cirrhosis, liver cancer, or a liver transplant recipient because of low prevalence in this cohort, potentially contributing to low liver specific healthcare costs.
Memedovich and colleagues noted that chronic diseases account for approximately C$68 billion annually in direct healthcare costs in Canada, representing around 58% of total healthcare expenditures. Estimates suggest that 1% annual reduction in chronic disease prevalence could save C$107 billion over the course of 20 years.
“Therefore, an approach that focuses on preventing and managing chronic diseases overall is needed to reduce the burden of MASLD on the healthcare system,” they wrote. This study was funded by LiveRx via an Alberta Innovates grant. The investigators disclosed relationships with Gilead, Abbott, GSK, and others.
according to a new study.
The expected surge reflects the growing prevalence of MASLD and its associated conditions, emphasizing the necessity for a comprehensive approach to address this escalating public health issue, reported lead author K. Ally Memedovich, BHSc, of the University of Calgary in Alberta, Canada, and colleagues.
“The costs associated with the management of MASLD in Canada remain unknown but have been estimated as being very high,” the investigators wrote in Gastro Hep Advances. “Specifically, in one study from the United States, the healthcare costs and utilization of those with MASLD was nearly double that of patients without MASLD but with similar health status. This difference was largely due to increases in imaging, hospitalization, liver fibrosis assessment, laboratory tests, and outpatient visits.”
Although projections are available to estimate the future prevalence of MASLD in Canada, no models are available to predict the growing national economic burden, prompting the present study.
Memedovich and colleagues analyzed healthcare usage data from 6,358 patients diagnosed with MASLD disease in Calgary from 2018 to 2020. Using provincial administrative data, they calculated both liver-specific and total healthcare costs associated with different stages of liver fibrosis, ranging from F0/F1 (minimal fibrosis) to F4 (advanced fibrosis or cirrhosis).
The patients’ liver fibrosis stages were determined using liver stiffness measurements obtained through shear wave elastography. Average annual cost per patient was then calculated for each fibrosis stage by analyzing hospitalizations, ambulatory care, and physician claims data.
The annual average liver-specific cost per patient increased with severity of liver fibrosis; costs for patients with fibrosis stages F0/F1, F2, F3, and F4 were C$7.02, C$35.30, C$60.46, and C$72.55, respectively. By 2050, liver-specific healthcare costs are projected to increase by C$51 million, reaching C$136 million Canada-wide.
Total healthcare costs were markedly higher; annual costs for patients with fibrosis stages F0/F1, F2, F3, and F4 were C$397.90, C$781.53, C$2,881.84, and C$1,598.82, respectively. As a result, total healthcare costs are expected to rise by nearly C$2 billion, contributing to a Canadian healthcare burden of C$5.81 billion annually by 2050.
The study revealed that over 90% of the healthcare costs for MASLD patients were attributed not to liver disease itself but to the management of associated comorbidities such as diabetes, hypertension, mental illness, and obesity. For instance, diabetes was the most common reason for physician visits among MASLD patients, accounting for 65.2% of cases. One study limitation was exclusion of decompensated cirrhosis, liver cancer, or a liver transplant recipient because of low prevalence in this cohort, potentially contributing to low liver specific healthcare costs.
Memedovich and colleagues noted that chronic diseases account for approximately C$68 billion annually in direct healthcare costs in Canada, representing around 58% of total healthcare expenditures. Estimates suggest that 1% annual reduction in chronic disease prevalence could save C$107 billion over the course of 20 years.
“Therefore, an approach that focuses on preventing and managing chronic diseases overall is needed to reduce the burden of MASLD on the healthcare system,” they wrote. This study was funded by LiveRx via an Alberta Innovates grant. The investigators disclosed relationships with Gilead, Abbott, GSK, and others.
FROM GASTRO HEP ADVANCES













