User login
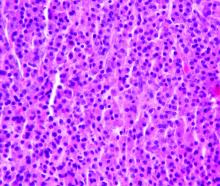
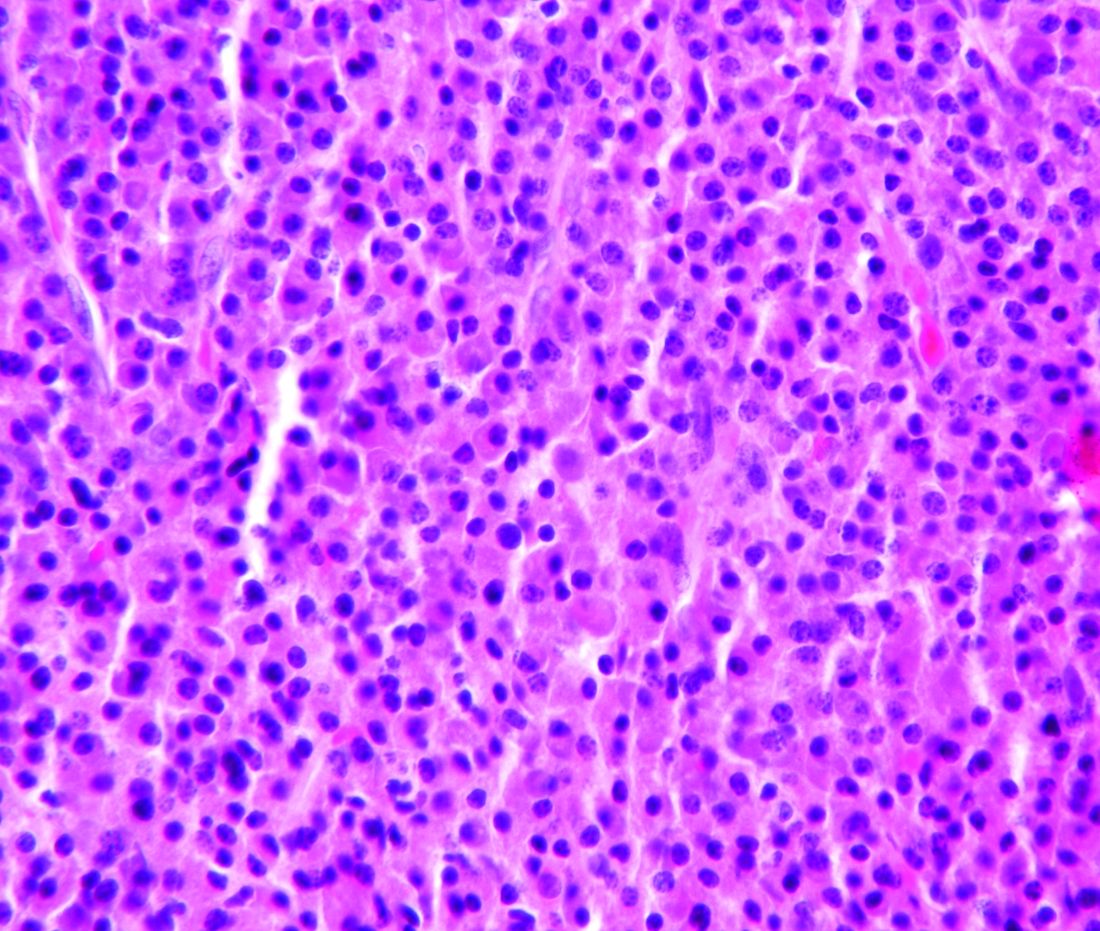
Study halted; ‘hyperprogression’ seen with nivolumab for R/R PTCL
ORLANDO – There is an urgent need for new therapies to treat relapsed or refractory peripheral T-cell lymphoma, but results of a phase 2 study suggest that monotherapy with the immune checkpoint inhibitor nivolumab (Opdivo) is not the hoped-for salvage treatment.
An interim analysis of data on 12 patients with peripheral T-cell lymphoma (PTCL) treated with nivolumab monotherapy showed an overall response rate of 33%, consisting of 2 complete responses and 2 partial responses. But the responses were short lived, and one patient had hyperprogressive disease – dramatic progression within one cycle of treatment – while two more had progression within two cycles, leading to a trial halt, reported N. Nora Bennani, MD, from the Mayo Clinic in Rochester, Minn.
“These findings likely reflect the distinct biology of PTCL and should be considered when designing future studies using checkpoint inhibitors in these diseases,” she said at the annual meeting of the American Society of Hematology.
The rationale for using an immune checkpoint inhibitor directed against the programmed death–1 protein and its ligands (PD and PD-L1/2) is that malignant cells in PTCL induce a profoundly immunosuppressive tumor microenvironment. Checkpoint inhibitors have shown strong activity against relapsed Hodgkin lymphoma, and the Mayo Clinic researchers speculated that an anti-PD-1 agent could have a similar effect in PTCL.
They had originally planned to enroll 29 patients into a phase 2 trial with nivolumab delivered 240 mg every 2 weeks for eight cycles, followed by a dose of 480 mg given every 4 weeks until disease progression or intolerable toxicities.
Patients were eligible if they had biopsy-confirmed relapsed or refractory PTCL, measurable disease on cross-sectional imaging of at least 1.5 cm, and prior systemic chemoimmunotherapy and/or autologous stem cell transplantation.
The interim analysis included 12 patients who received at least one dose of nivolumab. Of the 12 patients, 6 had angioimmunoblastic T-cell lymphoma (AITL), 3 had PTCL not otherwise specified, and 1 each had ALK-negative anaplastic large cell lymphoma (ALK-ALCL), enteropathy-associated T-cell lymphoma (EATL), or hepatosplenic gamma/delta T-cell lymphoma.
All patients had Ann Arbor stage III/IV disease, and 11 had extranodal involvement.
As noted, there were 4 responses among the 12 patients, consisting of 1 complete response in the patient with ALK-ALCL and 1 in a patient with AITL, and 2 partial responses – 1 in a patient with PTCL-NOS, and 1 in the patient with EATL.
The median progression-free survival for all 12 patients was short at 2.7 months, and the median overall survival was estimated at 6.7 months.
“It was staggering to see this: The duration of response was significantly short, less than 2 months,” Dr. Bennani said.
Nonhematologic toxicities were seen in 5 of the 12 patients (42%), and hematologic adverse events occurred in 3 (25%). All patients are now off treatment, 10 because of disease progression, 1 because of acute pancreatitis, and the aforementioned patient with hyperprogressive disease.
The patient with hyperprogressive disease had significant progression in tonsillar and cervical lymphadenopathy within 7-10 days of nivolumab infusion, with biopsy-proven AITL in the involved nodes.
“I believe that, in this patient population, combination therapies will be key. I think checkpoint blockers alone are not going to be sufficient to see meaningful outcomes in these patients,” Dr. Bennani said in an interview.
“An overall response rate of 33% is significant, because most other agents that were FDA approved in this patient population have response rates around 30%,” she said, adding that it’s possible that the patients with rapid progression had disease too advanced to be effectively treated with a checkpoint inhibitor.
“Ideally however, if we want to move forward, it will need to be with combinations of checkpoint inhibitors with HDAC [histone deacetylase] inhibitors, hypomethylating agents, or even PI3 kinase inhibitors,” she said.
The study was supported by Bristol-Myers Squibb. Dr. Bennani reported research funding and advisory board activities for Bristol-Myers Squibb and others.
SOURCE: Bennani NN et al. ASH 2019, Abstract 467.
ORLANDO – There is an urgent need for new therapies to treat relapsed or refractory peripheral T-cell lymphoma, but results of a phase 2 study suggest that monotherapy with the immune checkpoint inhibitor nivolumab (Opdivo) is not the hoped-for salvage treatment.
An interim analysis of data on 12 patients with peripheral T-cell lymphoma (PTCL) treated with nivolumab monotherapy showed an overall response rate of 33%, consisting of 2 complete responses and 2 partial responses. But the responses were short lived, and one patient had hyperprogressive disease – dramatic progression within one cycle of treatment – while two more had progression within two cycles, leading to a trial halt, reported N. Nora Bennani, MD, from the Mayo Clinic in Rochester, Minn.
“These findings likely reflect the distinct biology of PTCL and should be considered when designing future studies using checkpoint inhibitors in these diseases,” she said at the annual meeting of the American Society of Hematology.
The rationale for using an immune checkpoint inhibitor directed against the programmed death–1 protein and its ligands (PD and PD-L1/2) is that malignant cells in PTCL induce a profoundly immunosuppressive tumor microenvironment. Checkpoint inhibitors have shown strong activity against relapsed Hodgkin lymphoma, and the Mayo Clinic researchers speculated that an anti-PD-1 agent could have a similar effect in PTCL.
They had originally planned to enroll 29 patients into a phase 2 trial with nivolumab delivered 240 mg every 2 weeks for eight cycles, followed by a dose of 480 mg given every 4 weeks until disease progression or intolerable toxicities.
Patients were eligible if they had biopsy-confirmed relapsed or refractory PTCL, measurable disease on cross-sectional imaging of at least 1.5 cm, and prior systemic chemoimmunotherapy and/or autologous stem cell transplantation.
The interim analysis included 12 patients who received at least one dose of nivolumab. Of the 12 patients, 6 had angioimmunoblastic T-cell lymphoma (AITL), 3 had PTCL not otherwise specified, and 1 each had ALK-negative anaplastic large cell lymphoma (ALK-ALCL), enteropathy-associated T-cell lymphoma (EATL), or hepatosplenic gamma/delta T-cell lymphoma.
All patients had Ann Arbor stage III/IV disease, and 11 had extranodal involvement.
As noted, there were 4 responses among the 12 patients, consisting of 1 complete response in the patient with ALK-ALCL and 1 in a patient with AITL, and 2 partial responses – 1 in a patient with PTCL-NOS, and 1 in the patient with EATL.
The median progression-free survival for all 12 patients was short at 2.7 months, and the median overall survival was estimated at 6.7 months.
“It was staggering to see this: The duration of response was significantly short, less than 2 months,” Dr. Bennani said.
Nonhematologic toxicities were seen in 5 of the 12 patients (42%), and hematologic adverse events occurred in 3 (25%). All patients are now off treatment, 10 because of disease progression, 1 because of acute pancreatitis, and the aforementioned patient with hyperprogressive disease.
The patient with hyperprogressive disease had significant progression in tonsillar and cervical lymphadenopathy within 7-10 days of nivolumab infusion, with biopsy-proven AITL in the involved nodes.
“I believe that, in this patient population, combination therapies will be key. I think checkpoint blockers alone are not going to be sufficient to see meaningful outcomes in these patients,” Dr. Bennani said in an interview.
“An overall response rate of 33% is significant, because most other agents that were FDA approved in this patient population have response rates around 30%,” she said, adding that it’s possible that the patients with rapid progression had disease too advanced to be effectively treated with a checkpoint inhibitor.
“Ideally however, if we want to move forward, it will need to be with combinations of checkpoint inhibitors with HDAC [histone deacetylase] inhibitors, hypomethylating agents, or even PI3 kinase inhibitors,” she said.
The study was supported by Bristol-Myers Squibb. Dr. Bennani reported research funding and advisory board activities for Bristol-Myers Squibb and others.
SOURCE: Bennani NN et al. ASH 2019, Abstract 467.
ORLANDO – There is an urgent need for new therapies to treat relapsed or refractory peripheral T-cell lymphoma, but results of a phase 2 study suggest that monotherapy with the immune checkpoint inhibitor nivolumab (Opdivo) is not the hoped-for salvage treatment.
An interim analysis of data on 12 patients with peripheral T-cell lymphoma (PTCL) treated with nivolumab monotherapy showed an overall response rate of 33%, consisting of 2 complete responses and 2 partial responses. But the responses were short lived, and one patient had hyperprogressive disease – dramatic progression within one cycle of treatment – while two more had progression within two cycles, leading to a trial halt, reported N. Nora Bennani, MD, from the Mayo Clinic in Rochester, Minn.
“These findings likely reflect the distinct biology of PTCL and should be considered when designing future studies using checkpoint inhibitors in these diseases,” she said at the annual meeting of the American Society of Hematology.
The rationale for using an immune checkpoint inhibitor directed against the programmed death–1 protein and its ligands (PD and PD-L1/2) is that malignant cells in PTCL induce a profoundly immunosuppressive tumor microenvironment. Checkpoint inhibitors have shown strong activity against relapsed Hodgkin lymphoma, and the Mayo Clinic researchers speculated that an anti-PD-1 agent could have a similar effect in PTCL.
They had originally planned to enroll 29 patients into a phase 2 trial with nivolumab delivered 240 mg every 2 weeks for eight cycles, followed by a dose of 480 mg given every 4 weeks until disease progression or intolerable toxicities.
Patients were eligible if they had biopsy-confirmed relapsed or refractory PTCL, measurable disease on cross-sectional imaging of at least 1.5 cm, and prior systemic chemoimmunotherapy and/or autologous stem cell transplantation.
The interim analysis included 12 patients who received at least one dose of nivolumab. Of the 12 patients, 6 had angioimmunoblastic T-cell lymphoma (AITL), 3 had PTCL not otherwise specified, and 1 each had ALK-negative anaplastic large cell lymphoma (ALK-ALCL), enteropathy-associated T-cell lymphoma (EATL), or hepatosplenic gamma/delta T-cell lymphoma.
All patients had Ann Arbor stage III/IV disease, and 11 had extranodal involvement.
As noted, there were 4 responses among the 12 patients, consisting of 1 complete response in the patient with ALK-ALCL and 1 in a patient with AITL, and 2 partial responses – 1 in a patient with PTCL-NOS, and 1 in the patient with EATL.
The median progression-free survival for all 12 patients was short at 2.7 months, and the median overall survival was estimated at 6.7 months.
“It was staggering to see this: The duration of response was significantly short, less than 2 months,” Dr. Bennani said.
Nonhematologic toxicities were seen in 5 of the 12 patients (42%), and hematologic adverse events occurred in 3 (25%). All patients are now off treatment, 10 because of disease progression, 1 because of acute pancreatitis, and the aforementioned patient with hyperprogressive disease.
The patient with hyperprogressive disease had significant progression in tonsillar and cervical lymphadenopathy within 7-10 days of nivolumab infusion, with biopsy-proven AITL in the involved nodes.
“I believe that, in this patient population, combination therapies will be key. I think checkpoint blockers alone are not going to be sufficient to see meaningful outcomes in these patients,” Dr. Bennani said in an interview.
“An overall response rate of 33% is significant, because most other agents that were FDA approved in this patient population have response rates around 30%,” she said, adding that it’s possible that the patients with rapid progression had disease too advanced to be effectively treated with a checkpoint inhibitor.
“Ideally however, if we want to move forward, it will need to be with combinations of checkpoint inhibitors with HDAC [histone deacetylase] inhibitors, hypomethylating agents, or even PI3 kinase inhibitors,” she said.
The study was supported by Bristol-Myers Squibb. Dr. Bennani reported research funding and advisory board activities for Bristol-Myers Squibb and others.
SOURCE: Bennani NN et al. ASH 2019, Abstract 467.
REPORTING FROM ASH 2019
Care coordination, equity can eliminate disparities for nonwhite patients with DLBCL
ORLANDO – Patients with diffuse large B-cell lymphoma (DLBCL) who are members of an ethnic or racial minority do not have worse outcomes than whites when they receive appropriate treatment and institutional support, a study on disparities in cancer care shows.
Although previous studies have shown that minorities with DLBCL have worse outcomes than do whites, results of a study comparing outcomes from 155 patients of white heritage with those of 41 patients from black, Hispanic, or other minority backgrounds found no significant differences in either progression-free survival (PFS) or overall survival in 2 years over follow-up, reported Nilanjan Ghosh, MD, PhD, from the Levine Cancer Institute, Atrium Health, in Charlotte, N.C.
He attributes the results to his center’s robust nurse navigation program, equal access among all patients – regardless of ability to pay – to standard treatments, and to the availability of clinical trial participation and stem cell transplantation.
“I think a key message is that if you are able to offer the same treatment and clinical trials to people irrespective of their race or socioeconomic status and can provide support, you can get equal outcomes as long as the biology is the same in both groups,” he said at a briefing prior to presentation of data in an oral abstract session at the annual meeting of the American Society of Hematology.
Dr. Ghosh pointed to four separate studies that showed that minority populations with DLBCL have worse outcomes than did whites, and noted that both uninsured and Medicaid-insured patients have also been shown to have poorer results, suggesting a role of socioeconomic factors in determining who gets optimum care and who does not.
The investigators compared PFS and OS among white and nonwhite patients with DLBCL treated in their institution, which has a safety-net cancer center. They also looked at the frequencies of clinical trial participation and stem cell transplantation between the groups.
The study included all patients with de novo DLBCL who presented to their center during January 2016–January 2019. They used patient-reported descriptors of race/ethnicity to create one of two cohorts: either self-identified whites (155 patients) or nonwhites (41), a group that included black patients, Hispanic patients, Asian Americans, and Native Americans.
The authors collected data on demographics, disease characteristics (including revised International Prognostic Index and double-hit status), insurance data, treatment, trial enrollment, progression, and death.
They found that nonwhites were significantly younger at diagnosis (median 56 vs. 64 years; P = .007), with an even distribution between the sexes in each group.
Two-thirds of both white and nonwhite patients had government insurance (Medicare or Medicaid). Of the remaining patients, 33% of white had private insurance, compared with 27% of nonwhites. No whites were uninsured, but 3 of the 41 nonwhites (7%) had no insurance.
Of the 155 white patients, 121 (86%) received nurse navigation services, as did 33 of 41 (81%) of nonwhites. The services include lodging assistance for homeless patients, transportation services for patients without cars, and care coordination among primary care physicians, oncologists, and other specialists. The services are part of the center’s standard practice, with excess costs, if any, folded into the budget, Dr. Ghosh said.
Looking at disease characteristics and treatment, the investigators found that risk profiles were similar between the groups. A higher percentage of whites had double-hit lymphoma (11% vs. 7%), but this difference was not statistically significant.
The investigators also found that in their program race was not a barrier to optimum therapy, with 96% of whites and 98% of nonwhites receiving frontline therapy with an anthracycline and rituximab-based regimen, and 4% and 2%, respectively received a non–anthracycline based regimen.
In each group, 39% of patients had disease that either relapsed or was refractory to frontline therapy.
In all, 11% of whites and 12% of nonwhites enrolled in clinical trials, 11% and 19%, respectively, underwent stem cell transplantation.
For patients with relapsed/refractory disease, the 2-year PFS rates were 60% for whites, and 63% for nonwhites, and the 2-year OS rates were 74% and 81%, respectively.
Dr. Ghosh and colleagues concluded that “our safety net cancer center, with extensive nurse navigator support and access to standard treatments, stem cell transplants, and cutting-edge clinical trials may abrogate the inferior outcomes in minority populations that have been previously reported.”
The study was internally funded. Dr. Ghosh reported consulting fees, research funding, speakers bureau activity, and/or honoraria from multiple companies.
SOURCE: Hu B et al. ASH 2019. Abstract 425.
ORLANDO – Patients with diffuse large B-cell lymphoma (DLBCL) who are members of an ethnic or racial minority do not have worse outcomes than whites when they receive appropriate treatment and institutional support, a study on disparities in cancer care shows.
Although previous studies have shown that minorities with DLBCL have worse outcomes than do whites, results of a study comparing outcomes from 155 patients of white heritage with those of 41 patients from black, Hispanic, or other minority backgrounds found no significant differences in either progression-free survival (PFS) or overall survival in 2 years over follow-up, reported Nilanjan Ghosh, MD, PhD, from the Levine Cancer Institute, Atrium Health, in Charlotte, N.C.
He attributes the results to his center’s robust nurse navigation program, equal access among all patients – regardless of ability to pay – to standard treatments, and to the availability of clinical trial participation and stem cell transplantation.
“I think a key message is that if you are able to offer the same treatment and clinical trials to people irrespective of their race or socioeconomic status and can provide support, you can get equal outcomes as long as the biology is the same in both groups,” he said at a briefing prior to presentation of data in an oral abstract session at the annual meeting of the American Society of Hematology.
Dr. Ghosh pointed to four separate studies that showed that minority populations with DLBCL have worse outcomes than did whites, and noted that both uninsured and Medicaid-insured patients have also been shown to have poorer results, suggesting a role of socioeconomic factors in determining who gets optimum care and who does not.
The investigators compared PFS and OS among white and nonwhite patients with DLBCL treated in their institution, which has a safety-net cancer center. They also looked at the frequencies of clinical trial participation and stem cell transplantation between the groups.
The study included all patients with de novo DLBCL who presented to their center during January 2016–January 2019. They used patient-reported descriptors of race/ethnicity to create one of two cohorts: either self-identified whites (155 patients) or nonwhites (41), a group that included black patients, Hispanic patients, Asian Americans, and Native Americans.
The authors collected data on demographics, disease characteristics (including revised International Prognostic Index and double-hit status), insurance data, treatment, trial enrollment, progression, and death.
They found that nonwhites were significantly younger at diagnosis (median 56 vs. 64 years; P = .007), with an even distribution between the sexes in each group.
Two-thirds of both white and nonwhite patients had government insurance (Medicare or Medicaid). Of the remaining patients, 33% of white had private insurance, compared with 27% of nonwhites. No whites were uninsured, but 3 of the 41 nonwhites (7%) had no insurance.
Of the 155 white patients, 121 (86%) received nurse navigation services, as did 33 of 41 (81%) of nonwhites. The services include lodging assistance for homeless patients, transportation services for patients without cars, and care coordination among primary care physicians, oncologists, and other specialists. The services are part of the center’s standard practice, with excess costs, if any, folded into the budget, Dr. Ghosh said.
Looking at disease characteristics and treatment, the investigators found that risk profiles were similar between the groups. A higher percentage of whites had double-hit lymphoma (11% vs. 7%), but this difference was not statistically significant.
The investigators also found that in their program race was not a barrier to optimum therapy, with 96% of whites and 98% of nonwhites receiving frontline therapy with an anthracycline and rituximab-based regimen, and 4% and 2%, respectively received a non–anthracycline based regimen.
In each group, 39% of patients had disease that either relapsed or was refractory to frontline therapy.
In all, 11% of whites and 12% of nonwhites enrolled in clinical trials, 11% and 19%, respectively, underwent stem cell transplantation.
For patients with relapsed/refractory disease, the 2-year PFS rates were 60% for whites, and 63% for nonwhites, and the 2-year OS rates were 74% and 81%, respectively.
Dr. Ghosh and colleagues concluded that “our safety net cancer center, with extensive nurse navigator support and access to standard treatments, stem cell transplants, and cutting-edge clinical trials may abrogate the inferior outcomes in minority populations that have been previously reported.”
The study was internally funded. Dr. Ghosh reported consulting fees, research funding, speakers bureau activity, and/or honoraria from multiple companies.
SOURCE: Hu B et al. ASH 2019. Abstract 425.
ORLANDO – Patients with diffuse large B-cell lymphoma (DLBCL) who are members of an ethnic or racial minority do not have worse outcomes than whites when they receive appropriate treatment and institutional support, a study on disparities in cancer care shows.
Although previous studies have shown that minorities with DLBCL have worse outcomes than do whites, results of a study comparing outcomes from 155 patients of white heritage with those of 41 patients from black, Hispanic, or other minority backgrounds found no significant differences in either progression-free survival (PFS) or overall survival in 2 years over follow-up, reported Nilanjan Ghosh, MD, PhD, from the Levine Cancer Institute, Atrium Health, in Charlotte, N.C.
He attributes the results to his center’s robust nurse navigation program, equal access among all patients – regardless of ability to pay – to standard treatments, and to the availability of clinical trial participation and stem cell transplantation.
“I think a key message is that if you are able to offer the same treatment and clinical trials to people irrespective of their race or socioeconomic status and can provide support, you can get equal outcomes as long as the biology is the same in both groups,” he said at a briefing prior to presentation of data in an oral abstract session at the annual meeting of the American Society of Hematology.
Dr. Ghosh pointed to four separate studies that showed that minority populations with DLBCL have worse outcomes than did whites, and noted that both uninsured and Medicaid-insured patients have also been shown to have poorer results, suggesting a role of socioeconomic factors in determining who gets optimum care and who does not.
The investigators compared PFS and OS among white and nonwhite patients with DLBCL treated in their institution, which has a safety-net cancer center. They also looked at the frequencies of clinical trial participation and stem cell transplantation between the groups.
The study included all patients with de novo DLBCL who presented to their center during January 2016–January 2019. They used patient-reported descriptors of race/ethnicity to create one of two cohorts: either self-identified whites (155 patients) or nonwhites (41), a group that included black patients, Hispanic patients, Asian Americans, and Native Americans.
The authors collected data on demographics, disease characteristics (including revised International Prognostic Index and double-hit status), insurance data, treatment, trial enrollment, progression, and death.
They found that nonwhites were significantly younger at diagnosis (median 56 vs. 64 years; P = .007), with an even distribution between the sexes in each group.
Two-thirds of both white and nonwhite patients had government insurance (Medicare or Medicaid). Of the remaining patients, 33% of white had private insurance, compared with 27% of nonwhites. No whites were uninsured, but 3 of the 41 nonwhites (7%) had no insurance.
Of the 155 white patients, 121 (86%) received nurse navigation services, as did 33 of 41 (81%) of nonwhites. The services include lodging assistance for homeless patients, transportation services for patients without cars, and care coordination among primary care physicians, oncologists, and other specialists. The services are part of the center’s standard practice, with excess costs, if any, folded into the budget, Dr. Ghosh said.
Looking at disease characteristics and treatment, the investigators found that risk profiles were similar between the groups. A higher percentage of whites had double-hit lymphoma (11% vs. 7%), but this difference was not statistically significant.
The investigators also found that in their program race was not a barrier to optimum therapy, with 96% of whites and 98% of nonwhites receiving frontline therapy with an anthracycline and rituximab-based regimen, and 4% and 2%, respectively received a non–anthracycline based regimen.
In each group, 39% of patients had disease that either relapsed or was refractory to frontline therapy.
In all, 11% of whites and 12% of nonwhites enrolled in clinical trials, 11% and 19%, respectively, underwent stem cell transplantation.
For patients with relapsed/refractory disease, the 2-year PFS rates were 60% for whites, and 63% for nonwhites, and the 2-year OS rates were 74% and 81%, respectively.
Dr. Ghosh and colleagues concluded that “our safety net cancer center, with extensive nurse navigator support and access to standard treatments, stem cell transplants, and cutting-edge clinical trials may abrogate the inferior outcomes in minority populations that have been previously reported.”
The study was internally funded. Dr. Ghosh reported consulting fees, research funding, speakers bureau activity, and/or honoraria from multiple companies.
SOURCE: Hu B et al. ASH 2019. Abstract 425.
REPORTING FROM ASH 2019
Myeloma patients over age 70 can benefit from auto-HC transplant
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
REPORTING FROM ASH 2019
Bispecific CAR T-cells yield high response rate in relapsed/refractory myeloma
ORLANDO – A dual-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated a high overall response rate, a long response duration, and manageable safety in patients with relapsed or refractory multiple myeloma, according to an investigator in a phase 1 study.
The overall response rate exceeded 90%, and about three-quarters of patients remained progression-free at 9 months after treatment with the CAR T-cell therapy, which targets both B-cell maturation antigen (BCMA) and CD38, the study investigator reported.
Grade 3 or greater cytokine release syndrome (CRS) occurred in about one-quarter of the patients, and no neurotoxicity was observed, according to investigator Yu Hu, MD, of Tongji Medical College in Hubei, China.
“,” Dr. Hu said in a press conference.
Short-term relapse has been a “major challenge” with current CAR T-cell therapies currently under investigation for myeloma, most of which target BCMA, according to Dr. Hu.
He said the bispecific CAR T-cell therapy under investigation, known as BM38, was designed to target antigen loss and increase persistence of effector cells. According to the investigator, this was the first study to focus on an anti-BCMA and CD38 dual-targeted CAR T-cell therapy for patients with relapsed or refractory multiple myeloma.
Gary J. Schiller, MD, of UCLA Health, who moderated the press conference, said that while dual-targeting is a potentially “attractive” approach in these hard-to-treat patients, further follow-up is needed to see duration of response and to see if antigen escape re-emerges.
“Cellular therapy is costly, in terms of toxicity as well as financial costs, so you would like to see what the durability of responses is before engaging in that as a late-stage therapy, not to mention moving it up front,” Dr. Schiller said in an interview.
The median progression-free survival (PFS) duration had not been reached at the time of this report, though the 9-month PFS rate was 78.87%, according to the data presented by Dr. Hu.
In the phase 1 study, 22 patients received BM38 CAR T-cell infusions following a fludarabine and cyclophosphamide preconditioning regimen. The median patient age was 59 years, and 50% were male. Nearly three-quarters (72%) had a cytogenetic abnormality, and the median number of prior therapies approached four (range, two to nine prior therapies).
Twenty of the patients (90.9%) had a response: 12 who achieved stringent complete remission, 2 with very good partial response, 5 with partial responses, and 1 with a minimal response.
Of 9 patients with extramedullary disease, 8 achieved partial or complete elimination of tumors, Dr. Hu said in his presentation.
Cytokine release syndrome occurred in 20 patients (90.91%), 5 of whom experienced severe cases (22.73%), according to the reported data. There was no observed neurotoxicity, according to the report, while almost all had hematologic toxicities. Three experienced hepatotoxicity and one had nephrotoxicity, according to Dr. Hu.
The phase 1 study was supported by the National Natural Science Foundation of China, the Major Technological Innovation Special Project Fund of Hubei Province of China, and Cellyan Therapeutics. The senior author of the study was affiliated with Cellyan Therapeutics. Dr. Hu and coauthors reported that they had no relevant conflicts of interest to declare.
SOURCE: Li C et al. ASH 2019. Abstract 930.
ORLANDO – A dual-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated a high overall response rate, a long response duration, and manageable safety in patients with relapsed or refractory multiple myeloma, according to an investigator in a phase 1 study.
The overall response rate exceeded 90%, and about three-quarters of patients remained progression-free at 9 months after treatment with the CAR T-cell therapy, which targets both B-cell maturation antigen (BCMA) and CD38, the study investigator reported.
Grade 3 or greater cytokine release syndrome (CRS) occurred in about one-quarter of the patients, and no neurotoxicity was observed, according to investigator Yu Hu, MD, of Tongji Medical College in Hubei, China.
“,” Dr. Hu said in a press conference.
Short-term relapse has been a “major challenge” with current CAR T-cell therapies currently under investigation for myeloma, most of which target BCMA, according to Dr. Hu.
He said the bispecific CAR T-cell therapy under investigation, known as BM38, was designed to target antigen loss and increase persistence of effector cells. According to the investigator, this was the first study to focus on an anti-BCMA and CD38 dual-targeted CAR T-cell therapy for patients with relapsed or refractory multiple myeloma.
Gary J. Schiller, MD, of UCLA Health, who moderated the press conference, said that while dual-targeting is a potentially “attractive” approach in these hard-to-treat patients, further follow-up is needed to see duration of response and to see if antigen escape re-emerges.
“Cellular therapy is costly, in terms of toxicity as well as financial costs, so you would like to see what the durability of responses is before engaging in that as a late-stage therapy, not to mention moving it up front,” Dr. Schiller said in an interview.
The median progression-free survival (PFS) duration had not been reached at the time of this report, though the 9-month PFS rate was 78.87%, according to the data presented by Dr. Hu.
In the phase 1 study, 22 patients received BM38 CAR T-cell infusions following a fludarabine and cyclophosphamide preconditioning regimen. The median patient age was 59 years, and 50% were male. Nearly three-quarters (72%) had a cytogenetic abnormality, and the median number of prior therapies approached four (range, two to nine prior therapies).
Twenty of the patients (90.9%) had a response: 12 who achieved stringent complete remission, 2 with very good partial response, 5 with partial responses, and 1 with a minimal response.
Of 9 patients with extramedullary disease, 8 achieved partial or complete elimination of tumors, Dr. Hu said in his presentation.
Cytokine release syndrome occurred in 20 patients (90.91%), 5 of whom experienced severe cases (22.73%), according to the reported data. There was no observed neurotoxicity, according to the report, while almost all had hematologic toxicities. Three experienced hepatotoxicity and one had nephrotoxicity, according to Dr. Hu.
The phase 1 study was supported by the National Natural Science Foundation of China, the Major Technological Innovation Special Project Fund of Hubei Province of China, and Cellyan Therapeutics. The senior author of the study was affiliated with Cellyan Therapeutics. Dr. Hu and coauthors reported that they had no relevant conflicts of interest to declare.
SOURCE: Li C et al. ASH 2019. Abstract 930.
ORLANDO – A dual-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated a high overall response rate, a long response duration, and manageable safety in patients with relapsed or refractory multiple myeloma, according to an investigator in a phase 1 study.
The overall response rate exceeded 90%, and about three-quarters of patients remained progression-free at 9 months after treatment with the CAR T-cell therapy, which targets both B-cell maturation antigen (BCMA) and CD38, the study investigator reported.
Grade 3 or greater cytokine release syndrome (CRS) occurred in about one-quarter of the patients, and no neurotoxicity was observed, according to investigator Yu Hu, MD, of Tongji Medical College in Hubei, China.
“,” Dr. Hu said in a press conference.
Short-term relapse has been a “major challenge” with current CAR T-cell therapies currently under investigation for myeloma, most of which target BCMA, according to Dr. Hu.
He said the bispecific CAR T-cell therapy under investigation, known as BM38, was designed to target antigen loss and increase persistence of effector cells. According to the investigator, this was the first study to focus on an anti-BCMA and CD38 dual-targeted CAR T-cell therapy for patients with relapsed or refractory multiple myeloma.
Gary J. Schiller, MD, of UCLA Health, who moderated the press conference, said that while dual-targeting is a potentially “attractive” approach in these hard-to-treat patients, further follow-up is needed to see duration of response and to see if antigen escape re-emerges.
“Cellular therapy is costly, in terms of toxicity as well as financial costs, so you would like to see what the durability of responses is before engaging in that as a late-stage therapy, not to mention moving it up front,” Dr. Schiller said in an interview.
The median progression-free survival (PFS) duration had not been reached at the time of this report, though the 9-month PFS rate was 78.87%, according to the data presented by Dr. Hu.
In the phase 1 study, 22 patients received BM38 CAR T-cell infusions following a fludarabine and cyclophosphamide preconditioning regimen. The median patient age was 59 years, and 50% were male. Nearly three-quarters (72%) had a cytogenetic abnormality, and the median number of prior therapies approached four (range, two to nine prior therapies).
Twenty of the patients (90.9%) had a response: 12 who achieved stringent complete remission, 2 with very good partial response, 5 with partial responses, and 1 with a minimal response.
Of 9 patients with extramedullary disease, 8 achieved partial or complete elimination of tumors, Dr. Hu said in his presentation.
Cytokine release syndrome occurred in 20 patients (90.91%), 5 of whom experienced severe cases (22.73%), according to the reported data. There was no observed neurotoxicity, according to the report, while almost all had hematologic toxicities. Three experienced hepatotoxicity and one had nephrotoxicity, according to Dr. Hu.
The phase 1 study was supported by the National Natural Science Foundation of China, the Major Technological Innovation Special Project Fund of Hubei Province of China, and Cellyan Therapeutics. The senior author of the study was affiliated with Cellyan Therapeutics. Dr. Hu and coauthors reported that they had no relevant conflicts of interest to declare.
SOURCE: Li C et al. ASH 2019. Abstract 930.
REPORTING FROM ASH 2019
High complete response rate seen with novel CAR-T for myeloma
ORLANDO – A novel chimeric antigen receptor T (CAR T) cell construct is associated with deep clinical responses in patients with multiple myeloma for whom prior lines of therapy – some numbering in the double digits – have failed.
Among 29 patients with multiple myeloma enrolled in a phase 1b/2 trial of JNJ-4528, the overall response rate (ORR) at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression at a median of 6 months, reported Deepu Madduri, MD, of Icahn School of Medicine at Mount Sinai, New York.
“These are very heavily pretreated patients, and so getting early and deep responses is quite amazing,” she said at a briefing prior to presentation of the data at the annual meeting of the American Society of Hematology.
JNJ-4528 is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). As previously reported, an identical CAR T cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma. JNJ-4528 was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration on Dec. 6, 2019, and a priority medicines (PRIME) designation by the European Medicines Agency in April 2019.
BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells. Several research groups are currently investigating CAR T cells or monoclonal antibodies targeted to BCMA. The product closest to receiving FDA approval is likely BB2121.
At ASH 2019, Dr. Madduri presented results from the phase 1b portion of the CARTITUDE-1 trial. The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least 3 prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug (IMiD), and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
A total of 29 patients, median age 60, were evaluable for the safety and efficacy endpoints. One-fourth of the patients had a high-risk cytogenetic profile. The patients had received a median of 5 prior lines of therapy, with one patient receiving 18 prior lines. Of the 29 patients, 25 (86%) had previously undergone autologous transplantation.
As noted before, the ORR after a median follow-up of 6 months was 100%, with 69% completer responses, 17% very good partial responses, and 14% partial responses. The median time to complete response was 1 month (range 1 to 9 months). All but two patients remained free of disease progression at the median 6-month follow-up.
Nearly all patients (27) developed cytokine release syndrome (CRS), and one patient with prolonged grade 4 CRS died from related complications 99 days after infusion.
The median time to onset of CRS was 7 days with more than 90% of cases occurring between days 5 and 9.
Neurotoxicities, specifically immune effector cell–associated neurotoxicity syndrome (ICANS), were infrequent in CRS, and when they did occur were generally low grade, with only 1 grade 3 ICANS event.
Asked in an interview whether the impressive response rates seen with JNJ-4528 might persist over time, Dr. Madduri acknowledged that follow-up is still relatively short.
“This product is unique in that has a CD8 central memory phenotype preferentially, and we’re hoping that this would play a central role in the durability of response because they’re memory cells, but I think at this time we don’t know,” she said.
The CARTITUDE-1 trial is funded by Janssen Research & Development. Dr. Madduri disclosed serving as a consultant to Janssen and to Takeda, Foundation Medicine, AbbVie, and Celgene.
SOURCE: Madduri D et al. ASH 2019. Abstract 577.
ORLANDO – A novel chimeric antigen receptor T (CAR T) cell construct is associated with deep clinical responses in patients with multiple myeloma for whom prior lines of therapy – some numbering in the double digits – have failed.
Among 29 patients with multiple myeloma enrolled in a phase 1b/2 trial of JNJ-4528, the overall response rate (ORR) at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression at a median of 6 months, reported Deepu Madduri, MD, of Icahn School of Medicine at Mount Sinai, New York.
“These are very heavily pretreated patients, and so getting early and deep responses is quite amazing,” she said at a briefing prior to presentation of the data at the annual meeting of the American Society of Hematology.
JNJ-4528 is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). As previously reported, an identical CAR T cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma. JNJ-4528 was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration on Dec. 6, 2019, and a priority medicines (PRIME) designation by the European Medicines Agency in April 2019.
BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells. Several research groups are currently investigating CAR T cells or monoclonal antibodies targeted to BCMA. The product closest to receiving FDA approval is likely BB2121.
At ASH 2019, Dr. Madduri presented results from the phase 1b portion of the CARTITUDE-1 trial. The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least 3 prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug (IMiD), and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
A total of 29 patients, median age 60, were evaluable for the safety and efficacy endpoints. One-fourth of the patients had a high-risk cytogenetic profile. The patients had received a median of 5 prior lines of therapy, with one patient receiving 18 prior lines. Of the 29 patients, 25 (86%) had previously undergone autologous transplantation.
As noted before, the ORR after a median follow-up of 6 months was 100%, with 69% completer responses, 17% very good partial responses, and 14% partial responses. The median time to complete response was 1 month (range 1 to 9 months). All but two patients remained free of disease progression at the median 6-month follow-up.
Nearly all patients (27) developed cytokine release syndrome (CRS), and one patient with prolonged grade 4 CRS died from related complications 99 days after infusion.
The median time to onset of CRS was 7 days with more than 90% of cases occurring between days 5 and 9.
Neurotoxicities, specifically immune effector cell–associated neurotoxicity syndrome (ICANS), were infrequent in CRS, and when they did occur were generally low grade, with only 1 grade 3 ICANS event.
Asked in an interview whether the impressive response rates seen with JNJ-4528 might persist over time, Dr. Madduri acknowledged that follow-up is still relatively short.
“This product is unique in that has a CD8 central memory phenotype preferentially, and we’re hoping that this would play a central role in the durability of response because they’re memory cells, but I think at this time we don’t know,” she said.
The CARTITUDE-1 trial is funded by Janssen Research & Development. Dr. Madduri disclosed serving as a consultant to Janssen and to Takeda, Foundation Medicine, AbbVie, and Celgene.
SOURCE: Madduri D et al. ASH 2019. Abstract 577.
ORLANDO – A novel chimeric antigen receptor T (CAR T) cell construct is associated with deep clinical responses in patients with multiple myeloma for whom prior lines of therapy – some numbering in the double digits – have failed.
Among 29 patients with multiple myeloma enrolled in a phase 1b/2 trial of JNJ-4528, the overall response rate (ORR) at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression at a median of 6 months, reported Deepu Madduri, MD, of Icahn School of Medicine at Mount Sinai, New York.
“These are very heavily pretreated patients, and so getting early and deep responses is quite amazing,” she said at a briefing prior to presentation of the data at the annual meeting of the American Society of Hematology.
JNJ-4528 is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). As previously reported, an identical CAR T cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma. JNJ-4528 was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration on Dec. 6, 2019, and a priority medicines (PRIME) designation by the European Medicines Agency in April 2019.
BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells. Several research groups are currently investigating CAR T cells or monoclonal antibodies targeted to BCMA. The product closest to receiving FDA approval is likely BB2121.
At ASH 2019, Dr. Madduri presented results from the phase 1b portion of the CARTITUDE-1 trial. The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least 3 prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug (IMiD), and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
A total of 29 patients, median age 60, were evaluable for the safety and efficacy endpoints. One-fourth of the patients had a high-risk cytogenetic profile. The patients had received a median of 5 prior lines of therapy, with one patient receiving 18 prior lines. Of the 29 patients, 25 (86%) had previously undergone autologous transplantation.
As noted before, the ORR after a median follow-up of 6 months was 100%, with 69% completer responses, 17% very good partial responses, and 14% partial responses. The median time to complete response was 1 month (range 1 to 9 months). All but two patients remained free of disease progression at the median 6-month follow-up.
Nearly all patients (27) developed cytokine release syndrome (CRS), and one patient with prolonged grade 4 CRS died from related complications 99 days after infusion.
The median time to onset of CRS was 7 days with more than 90% of cases occurring between days 5 and 9.
Neurotoxicities, specifically immune effector cell–associated neurotoxicity syndrome (ICANS), were infrequent in CRS, and when they did occur were generally low grade, with only 1 grade 3 ICANS event.
Asked in an interview whether the impressive response rates seen with JNJ-4528 might persist over time, Dr. Madduri acknowledged that follow-up is still relatively short.
“This product is unique in that has a CD8 central memory phenotype preferentially, and we’re hoping that this would play a central role in the durability of response because they’re memory cells, but I think at this time we don’t know,” she said.
The CARTITUDE-1 trial is funded by Janssen Research & Development. Dr. Madduri disclosed serving as a consultant to Janssen and to Takeda, Foundation Medicine, AbbVie, and Celgene.
SOURCE: Madduri D et al. ASH 2019. Abstract 577.
REPORTING FROM ASH 2019
The clinical impact of new approvals in sickle cell, MCL
In this edition of “How I Will Treat My Next Patient,” I highlight two recent drug approvals by the Food and Drug Administration – crizanlizumab for sickle cell patients with painful crises and zanubrutinib for mantle cell lymphoma (MCL) patients in relapse.
Crizanlizumab
P-selectin is an adhesion molecule expressed on activated vascular endothelial cells and platelets. It is a key molecule in the initiation of leukocyte rolling on vessel walls and promotes firm attachment and extravasation to underlying tissues during inflammation. Up-regulation of P-selectin on endothelial cells and platelets contributes to the cell-cell interactions involved in the pathogenesis of sickle cell pain crises.
The SUSTAIN study was a multisite, placebo-controlled, randomized phase 2 trial of two different dosage levels of intravenous crizanlizumab (2.5 mg/kg or 5 mg/kg for 52 weeks), a humanized anti–P-selectin antibody, examining its effect on pain crises in patients with sickle cell disease. The primary endpoint was the annual rate of sickle cell pain crises, with a variety of clinically relevant secondary endpoints. The target population had 2-10 pain crises in the 12 months before enrollment. Patients on a stable dose of hydroxyurea for at least the most recent 3 months were allowed to enter, but if patients were not receiving hydroxyurea, it could not be initiated during the trial. Patients who were undergoing chronic red-cell transfusion therapy were excluded.
Among 198 enrolled patients, 35% did not complete the 52 weeks of treatment. Discontinuations were equally balanced among patients assigned to the high-dose, low-dose, and placebo cohorts. Adverse events associated with crizanlizumab included back pain, nausea, pyrexia, and arthralgia. Serious adverse events occurred in 55 patients, with 5 deaths, all of which were unrelated to treatment. Crizanlizumab did not augment hemolysis or bacterial infections.
In the efficacy analysis, patients receiving high-dose crizanlizumab had a median annual rate of 1.63 health care visits for sickle cell pain crises, compared with 2.98 visits for placebo patients (P = .01). In comparison with placebo, high-dose crizanlizumab also delayed the first pain crisis after starting treatment (4.1 months vs. 1.4 months), delayed the median time to a second pain crisis, and decreased the median number of pain crises annually.
More than twice as many high-dose crizanlizumab patients had no pain crisis episodes, compared with placebo patients. In general, differences were more striking in patients who were not taking hydroxyurea and who had non–hemoglobin SS disease. Differences in the primary endpoint between low-dose crizanlizumab and placebo were numerically, but not statistically, different.
How these results influence practice
It has been over 20 years since a new agent (hydroxyurea) was approved for sickle cell patients and, despite its use, sickle cell pain crises remain a frequent problem. Pain crises are associated with worse quality of life and increased risk of death. A promising advance is badly needed, especially in an era in which sensitivity to providers’ role in the opioid addiction crisis is highly scrutinized and may contribute to future undertreatment of pain episodes. This is especially true for patients from areas with high levels of opioid misuse.
The SUSTAIN trial was international, multi-institutional, placebo-controlled, and inclusive. These attributes enhance the likelihood that crizanlizumab will enhance patient care in routine practice. As an intravenous agent, monitoring adherence and toxicity are less challenging than with hydroxyurea. Despite these factors, however, there are some concerns. Crizanlizumab was not free of toxicity, quality of life via the Brief Pain Inventory used in the trial was not improved, and changes in the pain-severity and pain-interference domains were small. Treatment in SUSTAIN ensued for 52 weeks, so the emergence of late neutralizing antibodies and late toxicities with longer-term therapy will require careful postmarketing assessment.
These concerns notwithstanding, anyone who has cared for sickle cell patients would be excited about the potential benefits crizanlizumab could bring to patient care.
Zanubrutinib
The FDA has approved zanubrutinib for the treatment of MCL in adult patients who have received at least one prior therapy. The approval is based on the results of two studies in which overall response rate was the primary endpoint.
BGB-3111-206 (NCT03206970) was a phase 2, open-label, multicenter, single-arm trial of 86 patients with MCL who received at least one prior therapy. Zanubrutinib was given orally at 160 mg twice daily until disease progression or unacceptable toxicity. BGB-3111-AU-003 (NCT 02343120) was a phase 1/2, open-label, dose-escalation trial of B-cell malignancies, including 32 previously treated MCL patients treated with zanubrutinib at 160 mg twice daily or 320 mg once daily.
In the phase 2 trial, 18fluorodeoxyglucose (FDG)–PET scans were required and the ORR was 84% (95% confidence interval, 74%-91%), with a complete response rate of 59% (95% CI, 48%-70%) and a median response duration of 19.5 months (95% CI, 16.6% to not estimable). In the phase 1/2 dose-escalation trial, FDG-PET scans were not required and the ORR was 84% (95% CI, 67%-95%), with a complete response rate of 22% (95% CI, 9%-40%) and a median response duration of 18.5 months (95% CI, 12.6% to not estimable). In both trials, median follow-up on study was about 18 months.
The most common adverse reactions were cytopenias, upper respiratory tract infection, rash, bruising, diarrhea, and cough. The most common serious adverse reactions were pneumonia in 11% and hemorrhage in 5% of patients. Of 118 MCL patients, 8 stopped therapy because of an adverse event, most frequently pneumonia (3.4%).
How these results influence practice
Unfortunately, the therapy of recurrent MCL is noncurative, because of the rapid development of treatment resistance. There are multiple single-and multiagent chemotherapy regimens that may be tried, many incorporating immunotherapy options such as anti-CD20- or Bruton tyrosine kinase (BTK)–targeted agents. Given the limited efficacy of these agents, temporary nature of remissions, and paucity of data comparing these various treatment options, participation in clinical trials is encouraged whenever possible.
Outside of a clinical trial, zanubrutinib joins ibrutinib and acalabrutinib as approved single-agent BTK inhibitors for adult MCL patients in relapse. The impressive ORR and response duration reported for zanubrutinib are similar to the results achieved with the other agents, but the toxicity pattern may be slightly different.
As in the treatment of hormonally sensitive breast cancer, clinicians and patients benefit when they have multiple similar, equally efficacious oral agents with slightly different toxicity patterns so that quality of life can be improved and treatment duration maximized before treatment resistance develops and a more toxic and/or inconvenient therapy needs to be employed.
Whether zanubrutinib has benefits beyond those for MCL patients in relapse will depend on the results of confirmatory trials and patient-reported outcome data.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two recent drug approvals by the Food and Drug Administration – crizanlizumab for sickle cell patients with painful crises and zanubrutinib for mantle cell lymphoma (MCL) patients in relapse.
Crizanlizumab
P-selectin is an adhesion molecule expressed on activated vascular endothelial cells and platelets. It is a key molecule in the initiation of leukocyte rolling on vessel walls and promotes firm attachment and extravasation to underlying tissues during inflammation. Up-regulation of P-selectin on endothelial cells and platelets contributes to the cell-cell interactions involved in the pathogenesis of sickle cell pain crises.
The SUSTAIN study was a multisite, placebo-controlled, randomized phase 2 trial of two different dosage levels of intravenous crizanlizumab (2.5 mg/kg or 5 mg/kg for 52 weeks), a humanized anti–P-selectin antibody, examining its effect on pain crises in patients with sickle cell disease. The primary endpoint was the annual rate of sickle cell pain crises, with a variety of clinically relevant secondary endpoints. The target population had 2-10 pain crises in the 12 months before enrollment. Patients on a stable dose of hydroxyurea for at least the most recent 3 months were allowed to enter, but if patients were not receiving hydroxyurea, it could not be initiated during the trial. Patients who were undergoing chronic red-cell transfusion therapy were excluded.
Among 198 enrolled patients, 35% did not complete the 52 weeks of treatment. Discontinuations were equally balanced among patients assigned to the high-dose, low-dose, and placebo cohorts. Adverse events associated with crizanlizumab included back pain, nausea, pyrexia, and arthralgia. Serious adverse events occurred in 55 patients, with 5 deaths, all of which were unrelated to treatment. Crizanlizumab did not augment hemolysis or bacterial infections.
In the efficacy analysis, patients receiving high-dose crizanlizumab had a median annual rate of 1.63 health care visits for sickle cell pain crises, compared with 2.98 visits for placebo patients (P = .01). In comparison with placebo, high-dose crizanlizumab also delayed the first pain crisis after starting treatment (4.1 months vs. 1.4 months), delayed the median time to a second pain crisis, and decreased the median number of pain crises annually.
More than twice as many high-dose crizanlizumab patients had no pain crisis episodes, compared with placebo patients. In general, differences were more striking in patients who were not taking hydroxyurea and who had non–hemoglobin SS disease. Differences in the primary endpoint between low-dose crizanlizumab and placebo were numerically, but not statistically, different.
How these results influence practice
It has been over 20 years since a new agent (hydroxyurea) was approved for sickle cell patients and, despite its use, sickle cell pain crises remain a frequent problem. Pain crises are associated with worse quality of life and increased risk of death. A promising advance is badly needed, especially in an era in which sensitivity to providers’ role in the opioid addiction crisis is highly scrutinized and may contribute to future undertreatment of pain episodes. This is especially true for patients from areas with high levels of opioid misuse.
The SUSTAIN trial was international, multi-institutional, placebo-controlled, and inclusive. These attributes enhance the likelihood that crizanlizumab will enhance patient care in routine practice. As an intravenous agent, monitoring adherence and toxicity are less challenging than with hydroxyurea. Despite these factors, however, there are some concerns. Crizanlizumab was not free of toxicity, quality of life via the Brief Pain Inventory used in the trial was not improved, and changes in the pain-severity and pain-interference domains were small. Treatment in SUSTAIN ensued for 52 weeks, so the emergence of late neutralizing antibodies and late toxicities with longer-term therapy will require careful postmarketing assessment.
These concerns notwithstanding, anyone who has cared for sickle cell patients would be excited about the potential benefits crizanlizumab could bring to patient care.
Zanubrutinib
The FDA has approved zanubrutinib for the treatment of MCL in adult patients who have received at least one prior therapy. The approval is based on the results of two studies in which overall response rate was the primary endpoint.
BGB-3111-206 (NCT03206970) was a phase 2, open-label, multicenter, single-arm trial of 86 patients with MCL who received at least one prior therapy. Zanubrutinib was given orally at 160 mg twice daily until disease progression or unacceptable toxicity. BGB-3111-AU-003 (NCT 02343120) was a phase 1/2, open-label, dose-escalation trial of B-cell malignancies, including 32 previously treated MCL patients treated with zanubrutinib at 160 mg twice daily or 320 mg once daily.
In the phase 2 trial, 18fluorodeoxyglucose (FDG)–PET scans were required and the ORR was 84% (95% confidence interval, 74%-91%), with a complete response rate of 59% (95% CI, 48%-70%) and a median response duration of 19.5 months (95% CI, 16.6% to not estimable). In the phase 1/2 dose-escalation trial, FDG-PET scans were not required and the ORR was 84% (95% CI, 67%-95%), with a complete response rate of 22% (95% CI, 9%-40%) and a median response duration of 18.5 months (95% CI, 12.6% to not estimable). In both trials, median follow-up on study was about 18 months.
The most common adverse reactions were cytopenias, upper respiratory tract infection, rash, bruising, diarrhea, and cough. The most common serious adverse reactions were pneumonia in 11% and hemorrhage in 5% of patients. Of 118 MCL patients, 8 stopped therapy because of an adverse event, most frequently pneumonia (3.4%).
How these results influence practice
Unfortunately, the therapy of recurrent MCL is noncurative, because of the rapid development of treatment resistance. There are multiple single-and multiagent chemotherapy regimens that may be tried, many incorporating immunotherapy options such as anti-CD20- or Bruton tyrosine kinase (BTK)–targeted agents. Given the limited efficacy of these agents, temporary nature of remissions, and paucity of data comparing these various treatment options, participation in clinical trials is encouraged whenever possible.
Outside of a clinical trial, zanubrutinib joins ibrutinib and acalabrutinib as approved single-agent BTK inhibitors for adult MCL patients in relapse. The impressive ORR and response duration reported for zanubrutinib are similar to the results achieved with the other agents, but the toxicity pattern may be slightly different.
As in the treatment of hormonally sensitive breast cancer, clinicians and patients benefit when they have multiple similar, equally efficacious oral agents with slightly different toxicity patterns so that quality of life can be improved and treatment duration maximized before treatment resistance develops and a more toxic and/or inconvenient therapy needs to be employed.
Whether zanubrutinib has benefits beyond those for MCL patients in relapse will depend on the results of confirmatory trials and patient-reported outcome data.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two recent drug approvals by the Food and Drug Administration – crizanlizumab for sickle cell patients with painful crises and zanubrutinib for mantle cell lymphoma (MCL) patients in relapse.
Crizanlizumab
P-selectin is an adhesion molecule expressed on activated vascular endothelial cells and platelets. It is a key molecule in the initiation of leukocyte rolling on vessel walls and promotes firm attachment and extravasation to underlying tissues during inflammation. Up-regulation of P-selectin on endothelial cells and platelets contributes to the cell-cell interactions involved in the pathogenesis of sickle cell pain crises.
The SUSTAIN study was a multisite, placebo-controlled, randomized phase 2 trial of two different dosage levels of intravenous crizanlizumab (2.5 mg/kg or 5 mg/kg for 52 weeks), a humanized anti–P-selectin antibody, examining its effect on pain crises in patients with sickle cell disease. The primary endpoint was the annual rate of sickle cell pain crises, with a variety of clinically relevant secondary endpoints. The target population had 2-10 pain crises in the 12 months before enrollment. Patients on a stable dose of hydroxyurea for at least the most recent 3 months were allowed to enter, but if patients were not receiving hydroxyurea, it could not be initiated during the trial. Patients who were undergoing chronic red-cell transfusion therapy were excluded.
Among 198 enrolled patients, 35% did not complete the 52 weeks of treatment. Discontinuations were equally balanced among patients assigned to the high-dose, low-dose, and placebo cohorts. Adverse events associated with crizanlizumab included back pain, nausea, pyrexia, and arthralgia. Serious adverse events occurred in 55 patients, with 5 deaths, all of which were unrelated to treatment. Crizanlizumab did not augment hemolysis or bacterial infections.
In the efficacy analysis, patients receiving high-dose crizanlizumab had a median annual rate of 1.63 health care visits for sickle cell pain crises, compared with 2.98 visits for placebo patients (P = .01). In comparison with placebo, high-dose crizanlizumab also delayed the first pain crisis after starting treatment (4.1 months vs. 1.4 months), delayed the median time to a second pain crisis, and decreased the median number of pain crises annually.
More than twice as many high-dose crizanlizumab patients had no pain crisis episodes, compared with placebo patients. In general, differences were more striking in patients who were not taking hydroxyurea and who had non–hemoglobin SS disease. Differences in the primary endpoint between low-dose crizanlizumab and placebo were numerically, but not statistically, different.
How these results influence practice
It has been over 20 years since a new agent (hydroxyurea) was approved for sickle cell patients and, despite its use, sickle cell pain crises remain a frequent problem. Pain crises are associated with worse quality of life and increased risk of death. A promising advance is badly needed, especially in an era in which sensitivity to providers’ role in the opioid addiction crisis is highly scrutinized and may contribute to future undertreatment of pain episodes. This is especially true for patients from areas with high levels of opioid misuse.
The SUSTAIN trial was international, multi-institutional, placebo-controlled, and inclusive. These attributes enhance the likelihood that crizanlizumab will enhance patient care in routine practice. As an intravenous agent, monitoring adherence and toxicity are less challenging than with hydroxyurea. Despite these factors, however, there are some concerns. Crizanlizumab was not free of toxicity, quality of life via the Brief Pain Inventory used in the trial was not improved, and changes in the pain-severity and pain-interference domains were small. Treatment in SUSTAIN ensued for 52 weeks, so the emergence of late neutralizing antibodies and late toxicities with longer-term therapy will require careful postmarketing assessment.
These concerns notwithstanding, anyone who has cared for sickle cell patients would be excited about the potential benefits crizanlizumab could bring to patient care.
Zanubrutinib
The FDA has approved zanubrutinib for the treatment of MCL in adult patients who have received at least one prior therapy. The approval is based on the results of two studies in which overall response rate was the primary endpoint.
BGB-3111-206 (NCT03206970) was a phase 2, open-label, multicenter, single-arm trial of 86 patients with MCL who received at least one prior therapy. Zanubrutinib was given orally at 160 mg twice daily until disease progression or unacceptable toxicity. BGB-3111-AU-003 (NCT 02343120) was a phase 1/2, open-label, dose-escalation trial of B-cell malignancies, including 32 previously treated MCL patients treated with zanubrutinib at 160 mg twice daily or 320 mg once daily.
In the phase 2 trial, 18fluorodeoxyglucose (FDG)–PET scans were required and the ORR was 84% (95% confidence interval, 74%-91%), with a complete response rate of 59% (95% CI, 48%-70%) and a median response duration of 19.5 months (95% CI, 16.6% to not estimable). In the phase 1/2 dose-escalation trial, FDG-PET scans were not required and the ORR was 84% (95% CI, 67%-95%), with a complete response rate of 22% (95% CI, 9%-40%) and a median response duration of 18.5 months (95% CI, 12.6% to not estimable). In both trials, median follow-up on study was about 18 months.
The most common adverse reactions were cytopenias, upper respiratory tract infection, rash, bruising, diarrhea, and cough. The most common serious adverse reactions were pneumonia in 11% and hemorrhage in 5% of patients. Of 118 MCL patients, 8 stopped therapy because of an adverse event, most frequently pneumonia (3.4%).
How these results influence practice
Unfortunately, the therapy of recurrent MCL is noncurative, because of the rapid development of treatment resistance. There are multiple single-and multiagent chemotherapy regimens that may be tried, many incorporating immunotherapy options such as anti-CD20- or Bruton tyrosine kinase (BTK)–targeted agents. Given the limited efficacy of these agents, temporary nature of remissions, and paucity of data comparing these various treatment options, participation in clinical trials is encouraged whenever possible.
Outside of a clinical trial, zanubrutinib joins ibrutinib and acalabrutinib as approved single-agent BTK inhibitors for adult MCL patients in relapse. The impressive ORR and response duration reported for zanubrutinib are similar to the results achieved with the other agents, but the toxicity pattern may be slightly different.
As in the treatment of hormonally sensitive breast cancer, clinicians and patients benefit when they have multiple similar, equally efficacious oral agents with slightly different toxicity patterns so that quality of life can be improved and treatment duration maximized before treatment resistance develops and a more toxic and/or inconvenient therapy needs to be employed.
Whether zanubrutinib has benefits beyond those for MCL patients in relapse will depend on the results of confirmatory trials and patient-reported outcome data.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
SOX11 shows value as diagnostic marker in MCL
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
FROM PLOS ONE
Acalabrutinib may outperform other targeted therapies in MCL
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
FROM CLINICAL THERAPEUTICS
ASH preview: Key themes include tackling CAR T obstacles, sickle cell advances, VTE
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Early lenalidomide may delay progression of smoldering myeloma
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
FROM JOURNAL OF CLINICAL ONCOLOGY