User login
EC approves mogamulizumab for MF, SS
The European Commission (EC) has granted marketing authorization for mogamulizumab (Poteligeo), a humanized monoclonal antibody directed against CCR4.
This means mogamulizumab is approved for use in adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
This approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
Kyowa Kirin plans to launch mogamulizumab in various European markets starting next year.
The EC’s decision to approve mogamulizumab was supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to an independent review committee, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Commission (EC) has granted marketing authorization for mogamulizumab (Poteligeo), a humanized monoclonal antibody directed against CCR4.
This means mogamulizumab is approved for use in adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
This approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
Kyowa Kirin plans to launch mogamulizumab in various European markets starting next year.
The EC’s decision to approve mogamulizumab was supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to an independent review committee, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Commission (EC) has granted marketing authorization for mogamulizumab (Poteligeo), a humanized monoclonal antibody directed against CCR4.
This means mogamulizumab is approved for use in adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
This approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
Kyowa Kirin plans to launch mogamulizumab in various European markets starting next year.
The EC’s decision to approve mogamulizumab was supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to an independent review committee, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
Pegfilgrastim biosimilar approved by EC
The European Commission (EC) has granted marketing authorization for Sandoz’s pegfilgrastim product Ziextenzo®, a biosimilar of Amgen’s Neulasta.
Ziextenzo is approved for the same use as the reference medicine—to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval was based on research suggesting Ziextenzo is comparable to Neulasta in terms of safety, efficacy, pharmacokinetics, and pharmacodynamics.1,2,3,4
1. Blackwell K. et al. Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol 28, 2272-2277 (2017).
2. Nakov R. et al. Proposed biosimilar pegfilgrastim LA-EP2006 shows similarity in pharmacokinetics and pharmacodynamics to reference pegfilgrastim in healthy subjects. 2017 San Antonio Breast Cancer Symposium, abstract P3-14-10.
3. Blackwell K. et al. A Comparison of Proposed Biosimilar LA-EP2006 and Reference Pegfilgrastim for the Prevention of Neutropenia in Patients With Early-Stage Breast Cancer Receiving Myelosuppressive Adjuvant or Neoadjuvant Chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a Phase III, Randomized, Double-Blind Trial. Oncologist 21, 789-794 (2016).
4. Harbeck N. et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 12, 1359-1367 (2016).
The European Commission (EC) has granted marketing authorization for Sandoz’s pegfilgrastim product Ziextenzo®, a biosimilar of Amgen’s Neulasta.
Ziextenzo is approved for the same use as the reference medicine—to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval was based on research suggesting Ziextenzo is comparable to Neulasta in terms of safety, efficacy, pharmacokinetics, and pharmacodynamics.1,2,3,4
1. Blackwell K. et al. Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol 28, 2272-2277 (2017).
2. Nakov R. et al. Proposed biosimilar pegfilgrastim LA-EP2006 shows similarity in pharmacokinetics and pharmacodynamics to reference pegfilgrastim in healthy subjects. 2017 San Antonio Breast Cancer Symposium, abstract P3-14-10.
3. Blackwell K. et al. A Comparison of Proposed Biosimilar LA-EP2006 and Reference Pegfilgrastim for the Prevention of Neutropenia in Patients With Early-Stage Breast Cancer Receiving Myelosuppressive Adjuvant or Neoadjuvant Chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a Phase III, Randomized, Double-Blind Trial. Oncologist 21, 789-794 (2016).
4. Harbeck N. et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 12, 1359-1367 (2016).
The European Commission (EC) has granted marketing authorization for Sandoz’s pegfilgrastim product Ziextenzo®, a biosimilar of Amgen’s Neulasta.
Ziextenzo is approved for the same use as the reference medicine—to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval was based on research suggesting Ziextenzo is comparable to Neulasta in terms of safety, efficacy, pharmacokinetics, and pharmacodynamics.1,2,3,4
1. Blackwell K. et al. Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol 28, 2272-2277 (2017).
2. Nakov R. et al. Proposed biosimilar pegfilgrastim LA-EP2006 shows similarity in pharmacokinetics and pharmacodynamics to reference pegfilgrastim in healthy subjects. 2017 San Antonio Breast Cancer Symposium, abstract P3-14-10.
3. Blackwell K. et al. A Comparison of Proposed Biosimilar LA-EP2006 and Reference Pegfilgrastim for the Prevention of Neutropenia in Patients With Early-Stage Breast Cancer Receiving Myelosuppressive Adjuvant or Neoadjuvant Chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a Phase III, Randomized, Double-Blind Trial. Oncologist 21, 789-794 (2016).
4. Harbeck N. et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 12, 1359-1367 (2016).
Patterns of malignancies in patients with HIV-AIDS: a single institution observational study
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
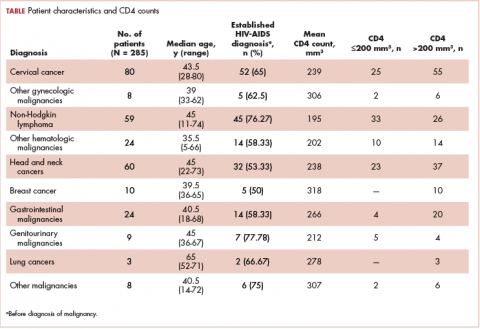
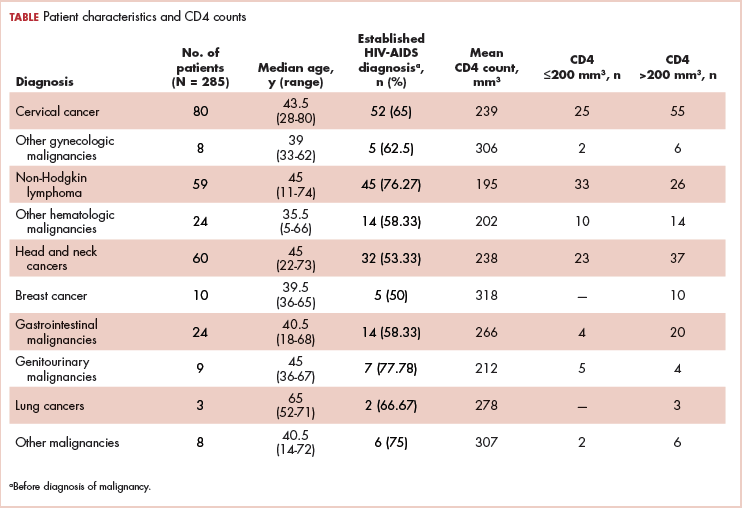
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
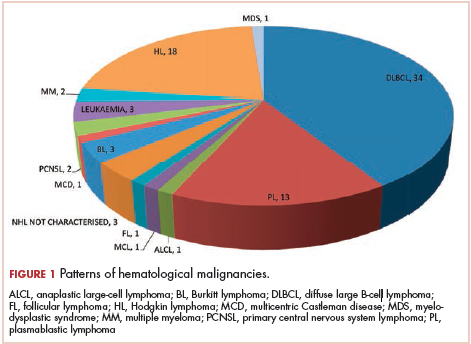
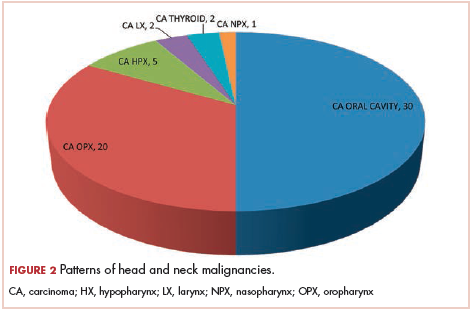
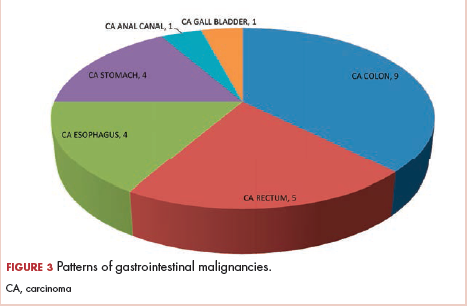
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
EC approves pegfilgrastim biosimilar
The European Commission (EC) has approved Mundipharma’s pegfilgrastim product Pelmeg, a biosimilar of Amgen’s Neulasta.
Pelmeg is approved for use in reducing the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy for malignancies, with the exceptions of chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of Pelmeg was supported by research showing pharmacokinetic comparability between Pelmeg and Neulasta at a dose of 6 mg, pharmacodynamic comparability at doses of 6 mg and 3 mg, and no clinically meaningful differences in the safety and immunogenicity profiles of Pelmeg and Neulasta.1,2,3
1. Roth K. et al. Demonstration of pharmacokinetic and pharmacodynamic comparability in healthy volunteers for B12019, a proposed pegfilgrastim biosimilar. ECCO 2017, abstract 241.
2. Roth K. et al. Comparability of pharmacodynamics and immunogenicity of B12019, a proposed pegfilgrastim biosimilar to Neulasta®. ASH 2017, abstract 1002.
3. Roth K. et al. Pharmacokinetic and pharmacodynamic comparability of B12019, a proposed pegfilgrastim biosimilar. ESMO 2017, poster 1573.
The European Commission (EC) has approved Mundipharma’s pegfilgrastim product Pelmeg, a biosimilar of Amgen’s Neulasta.
Pelmeg is approved for use in reducing the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy for malignancies, with the exceptions of chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of Pelmeg was supported by research showing pharmacokinetic comparability between Pelmeg and Neulasta at a dose of 6 mg, pharmacodynamic comparability at doses of 6 mg and 3 mg, and no clinically meaningful differences in the safety and immunogenicity profiles of Pelmeg and Neulasta.1,2,3
1. Roth K. et al. Demonstration of pharmacokinetic and pharmacodynamic comparability in healthy volunteers for B12019, a proposed pegfilgrastim biosimilar. ECCO 2017, abstract 241.
2. Roth K. et al. Comparability of pharmacodynamics and immunogenicity of B12019, a proposed pegfilgrastim biosimilar to Neulasta®. ASH 2017, abstract 1002.
3. Roth K. et al. Pharmacokinetic and pharmacodynamic comparability of B12019, a proposed pegfilgrastim biosimilar. ESMO 2017, poster 1573.
The European Commission (EC) has approved Mundipharma’s pegfilgrastim product Pelmeg, a biosimilar of Amgen’s Neulasta.
Pelmeg is approved for use in reducing the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy for malignancies, with the exceptions of chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of Pelmeg was supported by research showing pharmacokinetic comparability between Pelmeg and Neulasta at a dose of 6 mg, pharmacodynamic comparability at doses of 6 mg and 3 mg, and no clinically meaningful differences in the safety and immunogenicity profiles of Pelmeg and Neulasta.1,2,3
1. Roth K. et al. Demonstration of pharmacokinetic and pharmacodynamic comparability in healthy volunteers for B12019, a proposed pegfilgrastim biosimilar. ECCO 2017, abstract 241.
2. Roth K. et al. Comparability of pharmacodynamics and immunogenicity of B12019, a proposed pegfilgrastim biosimilar to Neulasta®. ASH 2017, abstract 1002.
3. Roth K. et al. Pharmacokinetic and pharmacodynamic comparability of B12019, a proposed pegfilgrastim biosimilar. ESMO 2017, poster 1573.
Malignant olecranon bursitis in the setting of multiple myeloma relapse
Multiple myeloma is the most common plasma cell neoplasm, with an estimated 24,000 cases occurring annually.1 Symptomatic multiple myeloma most commonly presents with one or more of the cardinal CRAB phenomena of hypercalcemia, renal dysfunction, anemia, or lytic bone lesions.2 Less commonly, patients may present with plasmacytomas (focal lesions of malignant plasma cells), which may involve bony or soft tissues.1
Plasma cell neoplasms occasionally involve the joints, including the elbows, typically as plasmacytomas. The elbow is an unusual but reported location of plasmacytomas.3,4 A case of multiple myeloma and amyloid light-chain (AL) amyloidosis has been reported, with manifestations including pseudomyopathy, bone marrow plasmacytosis, and bilateral trochanteric bursitis.5Bursitis is defined as inflammation of the synovial-fluid–containing sacs that lubricate joints. The olecranon bursa is commonly affected. Etiologies include infection, inflammatory disease, trauma, and malignancy. Furthermore, there is an association between bursitis and immunosuppression.6,7 The most common modes of therapy used to treat bursitis are nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgical management.
Trochanteric bursitis has been attributed to multiple myeloma in one previous case report, but we are not aware of any previous cases of olecranon bursitis caused by multiple myeloma. Here, we present the case of a 46-year-old man with heavily pretreated multiple myeloma and amyloidosis who developed left olecranon bursitis contemporaneously with disease relapse; flow cytometric analysis of the bursal fluid demonstrated an abnormal plasma cell population, establishing the etiology.
Case presentation and summary
A 46-year-old man with a longstanding history of multiple myeloma developed swelling of the left elbow that was initially painless in September 2016. He had been diagnosed with IgA kappa multiple myeloma and AL deposition in 2011. Over the course of his disease, he was treated with the following sequence of therapies: cyclophosphamide, bortezomib, and dexamethasone, followed by melphalan-conditioned autologous peripheral blood stem cell transplant; lenalidomide and dexamethasone; carfilzomib and dexamethasone; pomalidomide, bortezomib, and dexamethasone; and bortezomib, lenalidomide, dexamethasone, doxorubicin, cyclophosphamide, and etoposide, followed by second melphalan-conditioned autologous peripheral blood stem cell transplant. In addition to treatment with numerous novel and chemotherapeutic agents, his disease course was notable for amyloid deposition in the liver, bone marrow, and kidneys, which resulted in dialysis dependence.
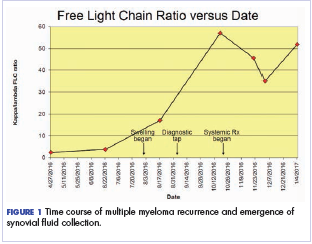
After the second autologous transplant, he achieved a very good partial response and experienced about 9 months of remission, after which laboratory evaluation indicated recurrence of IgA kappa monoclonal protein and free kappa light-chains, which increased slowly over several months without focal symptoms, cytopenias, or decline in organ function (Figure 1).
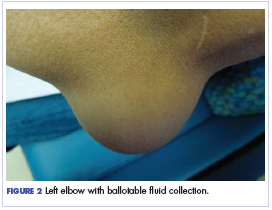
Twelve months after his second transplant, he presented in September 2016 with 4 weeks of left elbow swelling, with the appearance suggesting a fluid collection over the left olecranon process (Figure 2). The fluid collection was not painful unless bumped or pushed. The maximum pain level was 1-2 on a scale of 0-10. His daughter drained the fluid collection on 2 occasions, but it reaccumulated over 2 to 3 days. He reported no fevers, chills, or sweats. He did not have any redness at the site. He did not report any systemic symptoms.
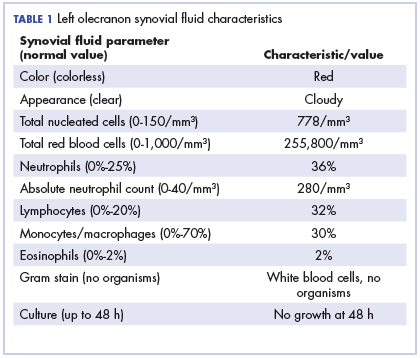
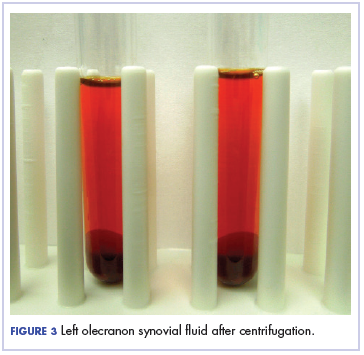
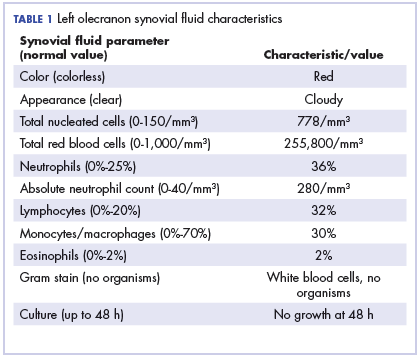
Physical examination of the left elbow demonstrated a ballotable fluid collection associated with the olecranon, with no associated warmth, tenderness, or erythema. Bursal fluid was sampled, yielding orange-colored serous fluid with bland characteristics (Figure 3). Microbiologic studies were negative (Table 1). We did not suspect a malignant cause initially.
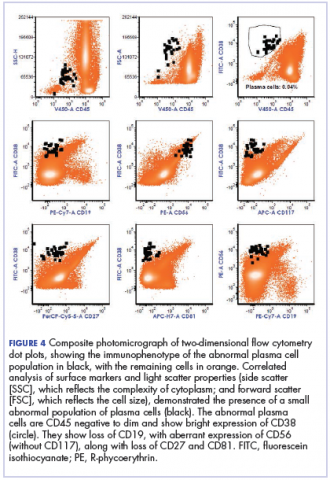
The fluid collection persisted despite treatment with nonsteroidal anti-inflammatory drugs and serial drainage procedures approximately twice per week. It became more erythematous and uncomfortable. We repeated diagnostic sampling at 13 months post-transplant. Cytospin revealed scant plasma cells. A multiparametric 8-color flow cytometric analysis was performed on the bursal fluid. It demonstrated the presence of a small abnormal population of plasma cells (0.04%). The abnormal plasma cells showed expression of CD138 and bright CD38 with aberrant expression of CD56, dim CD45, and loss of CD19, CD81 and CD27. They did not express CD117 or CD20 (Figure 4).
Because of the patient’s discomfort and his history of multidrug-refractory multiple myeloma, we obtained computed tomography imaging of the axial and appendicular skeleton, which demonstrated diffuse small lytic lesions, none larger than 3 mm, including the left elbow joint. The patient began systemic treatment with ixazomib, pomalidomide, and dexamethasone and then received radiation therapy of 20 Gy in 4 fractions to the left olecranon area. The bursal fluid collection remained stable in size but required periodic, though less frequent, drainage procedures. Unfortunately, the patient only tolerated 2 cycles of systemic therapy before experiencing hypercalcemia, exacerbation of hepatic amyloidosis, and a decline in performance status. He died 17 months after the transplant.
Discussion
Our patient experienced left olecranon bursitis simultaneously with relapse of multiple myeloma and AL amyloidosis. Evaluation for infectious causes was negative, and the bursal fluid did not have strongly inflammatory characteristics. Furthermore, a small plasma cell population was isolated from the fluid. Imaging did not reveal an underlying dominant lytic lesion. Although we do not have direct pathologic confirmation, the clinical scenario and flow cytometry findings support our interpretation that the patient’s bursitis was caused by or at least related to underlying multiple myeloma. While reactive plasma cells are also CD38 positive and CD138 positive, they maintain the expression of CD19 and CD45 without aberrant expression of CD56 or CD117 and do not show loss of expression of CD81 or CD27. In this situation, we suspect that either a plasmacytoma involving the soft tissue of the bursa or amyloid infiltration of the synovium may have occurred. Anti-myeloma therapies and radiation therapy did not result in control of the bursitis, though it should be noted that the patient’s highly refractory disease progressed despite treatment with a combination of later-generation immunomodulatory imide and proteasome inhibitor therapies.
Cases of malignant bursitis have been reported several times in the literature, though nearly all of the instances involved connective tissue or metastatic tumors. Tumor histologies include osteochondroma,8,9 malignant fibrous histiocytoma,10 synovial sarcoma,11 and metastatic breast cancer.12
Hematologic malignancies are more rare causes of bursitis; our literature search identified a report of 2 cases of non-Hodgkin lymphoma mimicking rheumatoid arthritis. The joints were the knee and elbow. Synovial fluid from one case was clear and yellow, with leukocytosis with a neutrophilic predominance (similar to our case). In both cases, pathology confirmed lymphomatous infiltration of the synovium.13 Notably, we identified a case of a previously healthy 35-year-old woman with bilateral trochanteric bursitis. Biopsy of tissue from the right trochanteric bursa demonstrated positive birefringence, diagnostic of AL amyloidosis. The patient also had a biclonal paraprotein accompanied by calvarial lytic lesions. She was treated with a corticosteroid pulse and bisphosphonates, followed by autologous hematopoietic stem cell transplant. 5 Our case shares features with the above case, including the relatively young age of the patient and the presence of AL amyloidosis.
Our patient wished to avoid a surgical biopsy procedure, and therefore we utilized flow cytometry of the bursal fluid to establish that the etiology of fluid collection was consistent with his concurrent relapse of multiple myeloma. We believe that we are reporting the second case of multiple myeloma-associated bursitis and the first case associated with multiple myeloma relapse; to our knowledge, it is the first to be diagnosed with the aid of flow cytometry.
Because of our patient’s reliance on hemodialysis beginning one year prior to his presentation with olecranon bursitis, we entertain “dialysis elbow” within the differential diagnosis. Dialysis elbow is a relatively uncommon complication of dialysis, in which patients develop olecranon bursitis on the same side as the hemodialysis access after a prolonged (months to years) duration of hemodialysis. Serositis and mechanical forces are the hypothesized etiologies14; infectious and rheumatologic causes were excluded from the reported cases. Nevertheless, we favor a malignant cause based upon the flow cytometry findings indicating involvement by immunophenotypically abnormal plasma cells.
Our patient was treated initially with serial drainage and nonsteroidals, which had little impact. After diagnosis of a plasma cell population in the fluid, we offered local treatment with radiation and systemic treatment of multiple myeloma, which offered better but suboptimal control. Possible treatments for olecranon bursitis include surgery, corticosteroid injections, anti-inflammatories, and serial drainage. Nonsurgical management may be more effective than surgical management, and corticosteroid injection carries significant risks. On the other hand, serial drainage does not confer additional infection risk in cases with aseptic etiology.15 We combined conservative measures as well as treatment of the underlying disease, but we believe that our patient did not derive significant benefit because of the refractory nature of his disease; he also expressed a preference to avoid surgical intervention.
Conclusion
Bursitis is a rare but thought-provoking potential manifestation of multiple myeloma and AL amyloidosis; we believe that our patient’s bursitis was related to plasma cell neoplasia based upon co-occurrence with disease relapse. His bursitis turned out to be an early indicator of impending systemic relapse. In this particular case, in which the patient wished to avoid surgical intervention, flow cytometry was of great value, and we believe that our case is the first report of malignant bursitis being diagnosed by flow cytometry. Our patient’s case shares similarities with other biopsy-confirmed cases of malignant bursitis, but we were able to avoid the need for surgical biopsy or bursal stripping.
The authors thank Jennifer Wilham MT (ASCP), Pat Byrd MT (ASCP), and Darlene Mann MT (ASCP) for their technical support.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459.
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
3. Gozzetti A, Coviello G, Fabbri A, et al. Unusual localizations of plasmacytoma. Leuk Res. 2011;35(7):e104-e105.
4. Kivioja AH, Karaharju EO, Elomaa I, Böhling TO. Surgical treatment of myeloma of bone. Eur J Cancer. 1992;28(11):1865-1869.
5. Santos MS, Soares B, Mendes O, Carvalho CM, Casimiro RF. Multiple myeloma-amyloidosis presenting as pseudomyopathy. Rev Bras Reumatol. 2011;51(6):651-654. 6. Blackwell JR, Hay BA, Bolt AM, May SM. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6(3):182-190.
7. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25(1):158-167.
8. De Groote J, Geerts B, Mermuys K, Verstraete K. Osteochondroma of the proximal humerus with frictional bursitis and secondary synovial osteochondromatosis. JBR-BTR. 2015;98(1):45-47. 9. Kumar R, Anjana, Kundan M. Retrocalcaneal bursitis due to rare calcaneal osteochrondroma in adult male: excision and outcome. J Orthop Case Rep. 2016;6(2):16-19.
10. Yoon PW, Jang WY, Yoo JJ, Yoon KS, Kim HJ. Malignant fibrous histiocytoma at the site of an alumina-on-alumina-bearing total hip arthroplasty mimicking infected trochanteric bursitis. J Arthroplasty. 2012;27(2):324.e9-324.e12.
11. Hutchison CW, Kling DH. Malignant synovioma. Am J Cancer. 1940;40(1):8-84.
12. Hutchings C, Hull R. Metastatic bone disease presenting as trochanteric bursitis. J R Soc Med. 1997;90(12):685-686.
13. Dorfman HD, Siegel HL, Perry MC, Oxenhandler R. Non-Hodgkin’s lymphoma of the synovium simulating rheumatoid arthritis. Arthritis Rheum. 1987;30(2):155-161.
14. Chao CT, Wu MS. Dialysis elbow. QJM. 2012;105(5):485-486.
15. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536.
Multiple myeloma is the most common plasma cell neoplasm, with an estimated 24,000 cases occurring annually.1 Symptomatic multiple myeloma most commonly presents with one or more of the cardinal CRAB phenomena of hypercalcemia, renal dysfunction, anemia, or lytic bone lesions.2 Less commonly, patients may present with plasmacytomas (focal lesions of malignant plasma cells), which may involve bony or soft tissues.1
Plasma cell neoplasms occasionally involve the joints, including the elbows, typically as plasmacytomas. The elbow is an unusual but reported location of plasmacytomas.3,4 A case of multiple myeloma and amyloid light-chain (AL) amyloidosis has been reported, with manifestations including pseudomyopathy, bone marrow plasmacytosis, and bilateral trochanteric bursitis.5Bursitis is defined as inflammation of the synovial-fluid–containing sacs that lubricate joints. The olecranon bursa is commonly affected. Etiologies include infection, inflammatory disease, trauma, and malignancy. Furthermore, there is an association between bursitis and immunosuppression.6,7 The most common modes of therapy used to treat bursitis are nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgical management.
Trochanteric bursitis has been attributed to multiple myeloma in one previous case report, but we are not aware of any previous cases of olecranon bursitis caused by multiple myeloma. Here, we present the case of a 46-year-old man with heavily pretreated multiple myeloma and amyloidosis who developed left olecranon bursitis contemporaneously with disease relapse; flow cytometric analysis of the bursal fluid demonstrated an abnormal plasma cell population, establishing the etiology.
Case presentation and summary
A 46-year-old man with a longstanding history of multiple myeloma developed swelling of the left elbow that was initially painless in September 2016. He had been diagnosed with IgA kappa multiple myeloma and AL deposition in 2011. Over the course of his disease, he was treated with the following sequence of therapies: cyclophosphamide, bortezomib, and dexamethasone, followed by melphalan-conditioned autologous peripheral blood stem cell transplant; lenalidomide and dexamethasone; carfilzomib and dexamethasone; pomalidomide, bortezomib, and dexamethasone; and bortezomib, lenalidomide, dexamethasone, doxorubicin, cyclophosphamide, and etoposide, followed by second melphalan-conditioned autologous peripheral blood stem cell transplant. In addition to treatment with numerous novel and chemotherapeutic agents, his disease course was notable for amyloid deposition in the liver, bone marrow, and kidneys, which resulted in dialysis dependence.
After the second autologous transplant, he achieved a very good partial response and experienced about 9 months of remission, after which laboratory evaluation indicated recurrence of IgA kappa monoclonal protein and free kappa light-chains, which increased slowly over several months without focal symptoms, cytopenias, or decline in organ function (Figure 1).
Twelve months after his second transplant, he presented in September 2016 with 4 weeks of left elbow swelling, with the appearance suggesting a fluid collection over the left olecranon process (Figure 2). The fluid collection was not painful unless bumped or pushed. The maximum pain level was 1-2 on a scale of 0-10. His daughter drained the fluid collection on 2 occasions, but it reaccumulated over 2 to 3 days. He reported no fevers, chills, or sweats. He did not have any redness at the site. He did not report any systemic symptoms.
Physical examination of the left elbow demonstrated a ballotable fluid collection associated with the olecranon, with no associated warmth, tenderness, or erythema. Bursal fluid was sampled, yielding orange-colored serous fluid with bland characteristics (Figure 3). Microbiologic studies were negative (Table 1). We did not suspect a malignant cause initially.
The fluid collection persisted despite treatment with nonsteroidal anti-inflammatory drugs and serial drainage procedures approximately twice per week. It became more erythematous and uncomfortable. We repeated diagnostic sampling at 13 months post-transplant. Cytospin revealed scant plasma cells. A multiparametric 8-color flow cytometric analysis was performed on the bursal fluid. It demonstrated the presence of a small abnormal population of plasma cells (0.04%). The abnormal plasma cells showed expression of CD138 and bright CD38 with aberrant expression of CD56, dim CD45, and loss of CD19, CD81 and CD27. They did not express CD117 or CD20 (Figure 4).
Because of the patient’s discomfort and his history of multidrug-refractory multiple myeloma, we obtained computed tomography imaging of the axial and appendicular skeleton, which demonstrated diffuse small lytic lesions, none larger than 3 mm, including the left elbow joint. The patient began systemic treatment with ixazomib, pomalidomide, and dexamethasone and then received radiation therapy of 20 Gy in 4 fractions to the left olecranon area. The bursal fluid collection remained stable in size but required periodic, though less frequent, drainage procedures. Unfortunately, the patient only tolerated 2 cycles of systemic therapy before experiencing hypercalcemia, exacerbation of hepatic amyloidosis, and a decline in performance status. He died 17 months after the transplant.
Discussion
Our patient experienced left olecranon bursitis simultaneously with relapse of multiple myeloma and AL amyloidosis. Evaluation for infectious causes was negative, and the bursal fluid did not have strongly inflammatory characteristics. Furthermore, a small plasma cell population was isolated from the fluid. Imaging did not reveal an underlying dominant lytic lesion. Although we do not have direct pathologic confirmation, the clinical scenario and flow cytometry findings support our interpretation that the patient’s bursitis was caused by or at least related to underlying multiple myeloma. While reactive plasma cells are also CD38 positive and CD138 positive, they maintain the expression of CD19 and CD45 without aberrant expression of CD56 or CD117 and do not show loss of expression of CD81 or CD27. In this situation, we suspect that either a plasmacytoma involving the soft tissue of the bursa or amyloid infiltration of the synovium may have occurred. Anti-myeloma therapies and radiation therapy did not result in control of the bursitis, though it should be noted that the patient’s highly refractory disease progressed despite treatment with a combination of later-generation immunomodulatory imide and proteasome inhibitor therapies.
Cases of malignant bursitis have been reported several times in the literature, though nearly all of the instances involved connective tissue or metastatic tumors. Tumor histologies include osteochondroma,8,9 malignant fibrous histiocytoma,10 synovial sarcoma,11 and metastatic breast cancer.12
Hematologic malignancies are more rare causes of bursitis; our literature search identified a report of 2 cases of non-Hodgkin lymphoma mimicking rheumatoid arthritis. The joints were the knee and elbow. Synovial fluid from one case was clear and yellow, with leukocytosis with a neutrophilic predominance (similar to our case). In both cases, pathology confirmed lymphomatous infiltration of the synovium.13 Notably, we identified a case of a previously healthy 35-year-old woman with bilateral trochanteric bursitis. Biopsy of tissue from the right trochanteric bursa demonstrated positive birefringence, diagnostic of AL amyloidosis. The patient also had a biclonal paraprotein accompanied by calvarial lytic lesions. She was treated with a corticosteroid pulse and bisphosphonates, followed by autologous hematopoietic stem cell transplant. 5 Our case shares features with the above case, including the relatively young age of the patient and the presence of AL amyloidosis.
Our patient wished to avoid a surgical biopsy procedure, and therefore we utilized flow cytometry of the bursal fluid to establish that the etiology of fluid collection was consistent with his concurrent relapse of multiple myeloma. We believe that we are reporting the second case of multiple myeloma-associated bursitis and the first case associated with multiple myeloma relapse; to our knowledge, it is the first to be diagnosed with the aid of flow cytometry.
Because of our patient’s reliance on hemodialysis beginning one year prior to his presentation with olecranon bursitis, we entertain “dialysis elbow” within the differential diagnosis. Dialysis elbow is a relatively uncommon complication of dialysis, in which patients develop olecranon bursitis on the same side as the hemodialysis access after a prolonged (months to years) duration of hemodialysis. Serositis and mechanical forces are the hypothesized etiologies14; infectious and rheumatologic causes were excluded from the reported cases. Nevertheless, we favor a malignant cause based upon the flow cytometry findings indicating involvement by immunophenotypically abnormal plasma cells.
Our patient was treated initially with serial drainage and nonsteroidals, which had little impact. After diagnosis of a plasma cell population in the fluid, we offered local treatment with radiation and systemic treatment of multiple myeloma, which offered better but suboptimal control. Possible treatments for olecranon bursitis include surgery, corticosteroid injections, anti-inflammatories, and serial drainage. Nonsurgical management may be more effective than surgical management, and corticosteroid injection carries significant risks. On the other hand, serial drainage does not confer additional infection risk in cases with aseptic etiology.15 We combined conservative measures as well as treatment of the underlying disease, but we believe that our patient did not derive significant benefit because of the refractory nature of his disease; he also expressed a preference to avoid surgical intervention.
Conclusion
Bursitis is a rare but thought-provoking potential manifestation of multiple myeloma and AL amyloidosis; we believe that our patient’s bursitis was related to plasma cell neoplasia based upon co-occurrence with disease relapse. His bursitis turned out to be an early indicator of impending systemic relapse. In this particular case, in which the patient wished to avoid surgical intervention, flow cytometry was of great value, and we believe that our case is the first report of malignant bursitis being diagnosed by flow cytometry. Our patient’s case shares similarities with other biopsy-confirmed cases of malignant bursitis, but we were able to avoid the need for surgical biopsy or bursal stripping.
The authors thank Jennifer Wilham MT (ASCP), Pat Byrd MT (ASCP), and Darlene Mann MT (ASCP) for their technical support.
Multiple myeloma is the most common plasma cell neoplasm, with an estimated 24,000 cases occurring annually.1 Symptomatic multiple myeloma most commonly presents with one or more of the cardinal CRAB phenomena of hypercalcemia, renal dysfunction, anemia, or lytic bone lesions.2 Less commonly, patients may present with plasmacytomas (focal lesions of malignant plasma cells), which may involve bony or soft tissues.1
Plasma cell neoplasms occasionally involve the joints, including the elbows, typically as plasmacytomas. The elbow is an unusual but reported location of plasmacytomas.3,4 A case of multiple myeloma and amyloid light-chain (AL) amyloidosis has been reported, with manifestations including pseudomyopathy, bone marrow plasmacytosis, and bilateral trochanteric bursitis.5Bursitis is defined as inflammation of the synovial-fluid–containing sacs that lubricate joints. The olecranon bursa is commonly affected. Etiologies include infection, inflammatory disease, trauma, and malignancy. Furthermore, there is an association between bursitis and immunosuppression.6,7 The most common modes of therapy used to treat bursitis are nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgical management.
Trochanteric bursitis has been attributed to multiple myeloma in one previous case report, but we are not aware of any previous cases of olecranon bursitis caused by multiple myeloma. Here, we present the case of a 46-year-old man with heavily pretreated multiple myeloma and amyloidosis who developed left olecranon bursitis contemporaneously with disease relapse; flow cytometric analysis of the bursal fluid demonstrated an abnormal plasma cell population, establishing the etiology.
Case presentation and summary
A 46-year-old man with a longstanding history of multiple myeloma developed swelling of the left elbow that was initially painless in September 2016. He had been diagnosed with IgA kappa multiple myeloma and AL deposition in 2011. Over the course of his disease, he was treated with the following sequence of therapies: cyclophosphamide, bortezomib, and dexamethasone, followed by melphalan-conditioned autologous peripheral blood stem cell transplant; lenalidomide and dexamethasone; carfilzomib and dexamethasone; pomalidomide, bortezomib, and dexamethasone; and bortezomib, lenalidomide, dexamethasone, doxorubicin, cyclophosphamide, and etoposide, followed by second melphalan-conditioned autologous peripheral blood stem cell transplant. In addition to treatment with numerous novel and chemotherapeutic agents, his disease course was notable for amyloid deposition in the liver, bone marrow, and kidneys, which resulted in dialysis dependence.
After the second autologous transplant, he achieved a very good partial response and experienced about 9 months of remission, after which laboratory evaluation indicated recurrence of IgA kappa monoclonal protein and free kappa light-chains, which increased slowly over several months without focal symptoms, cytopenias, or decline in organ function (Figure 1).
Twelve months after his second transplant, he presented in September 2016 with 4 weeks of left elbow swelling, with the appearance suggesting a fluid collection over the left olecranon process (Figure 2). The fluid collection was not painful unless bumped or pushed. The maximum pain level was 1-2 on a scale of 0-10. His daughter drained the fluid collection on 2 occasions, but it reaccumulated over 2 to 3 days. He reported no fevers, chills, or sweats. He did not have any redness at the site. He did not report any systemic symptoms.
Physical examination of the left elbow demonstrated a ballotable fluid collection associated with the olecranon, with no associated warmth, tenderness, or erythema. Bursal fluid was sampled, yielding orange-colored serous fluid with bland characteristics (Figure 3). Microbiologic studies were negative (Table 1). We did not suspect a malignant cause initially.
The fluid collection persisted despite treatment with nonsteroidal anti-inflammatory drugs and serial drainage procedures approximately twice per week. It became more erythematous and uncomfortable. We repeated diagnostic sampling at 13 months post-transplant. Cytospin revealed scant plasma cells. A multiparametric 8-color flow cytometric analysis was performed on the bursal fluid. It demonstrated the presence of a small abnormal population of plasma cells (0.04%). The abnormal plasma cells showed expression of CD138 and bright CD38 with aberrant expression of CD56, dim CD45, and loss of CD19, CD81 and CD27. They did not express CD117 or CD20 (Figure 4).
Because of the patient’s discomfort and his history of multidrug-refractory multiple myeloma, we obtained computed tomography imaging of the axial and appendicular skeleton, which demonstrated diffuse small lytic lesions, none larger than 3 mm, including the left elbow joint. The patient began systemic treatment with ixazomib, pomalidomide, and dexamethasone and then received radiation therapy of 20 Gy in 4 fractions to the left olecranon area. The bursal fluid collection remained stable in size but required periodic, though less frequent, drainage procedures. Unfortunately, the patient only tolerated 2 cycles of systemic therapy before experiencing hypercalcemia, exacerbation of hepatic amyloidosis, and a decline in performance status. He died 17 months after the transplant.
Discussion
Our patient experienced left olecranon bursitis simultaneously with relapse of multiple myeloma and AL amyloidosis. Evaluation for infectious causes was negative, and the bursal fluid did not have strongly inflammatory characteristics. Furthermore, a small plasma cell population was isolated from the fluid. Imaging did not reveal an underlying dominant lytic lesion. Although we do not have direct pathologic confirmation, the clinical scenario and flow cytometry findings support our interpretation that the patient’s bursitis was caused by or at least related to underlying multiple myeloma. While reactive plasma cells are also CD38 positive and CD138 positive, they maintain the expression of CD19 and CD45 without aberrant expression of CD56 or CD117 and do not show loss of expression of CD81 or CD27. In this situation, we suspect that either a plasmacytoma involving the soft tissue of the bursa or amyloid infiltration of the synovium may have occurred. Anti-myeloma therapies and radiation therapy did not result in control of the bursitis, though it should be noted that the patient’s highly refractory disease progressed despite treatment with a combination of later-generation immunomodulatory imide and proteasome inhibitor therapies.
Cases of malignant bursitis have been reported several times in the literature, though nearly all of the instances involved connective tissue or metastatic tumors. Tumor histologies include osteochondroma,8,9 malignant fibrous histiocytoma,10 synovial sarcoma,11 and metastatic breast cancer.12
Hematologic malignancies are more rare causes of bursitis; our literature search identified a report of 2 cases of non-Hodgkin lymphoma mimicking rheumatoid arthritis. The joints were the knee and elbow. Synovial fluid from one case was clear and yellow, with leukocytosis with a neutrophilic predominance (similar to our case). In both cases, pathology confirmed lymphomatous infiltration of the synovium.13 Notably, we identified a case of a previously healthy 35-year-old woman with bilateral trochanteric bursitis. Biopsy of tissue from the right trochanteric bursa demonstrated positive birefringence, diagnostic of AL amyloidosis. The patient also had a biclonal paraprotein accompanied by calvarial lytic lesions. She was treated with a corticosteroid pulse and bisphosphonates, followed by autologous hematopoietic stem cell transplant. 5 Our case shares features with the above case, including the relatively young age of the patient and the presence of AL amyloidosis.
Our patient wished to avoid a surgical biopsy procedure, and therefore we utilized flow cytometry of the bursal fluid to establish that the etiology of fluid collection was consistent with his concurrent relapse of multiple myeloma. We believe that we are reporting the second case of multiple myeloma-associated bursitis and the first case associated with multiple myeloma relapse; to our knowledge, it is the first to be diagnosed with the aid of flow cytometry.
Because of our patient’s reliance on hemodialysis beginning one year prior to his presentation with olecranon bursitis, we entertain “dialysis elbow” within the differential diagnosis. Dialysis elbow is a relatively uncommon complication of dialysis, in which patients develop olecranon bursitis on the same side as the hemodialysis access after a prolonged (months to years) duration of hemodialysis. Serositis and mechanical forces are the hypothesized etiologies14; infectious and rheumatologic causes were excluded from the reported cases. Nevertheless, we favor a malignant cause based upon the flow cytometry findings indicating involvement by immunophenotypically abnormal plasma cells.
Our patient was treated initially with serial drainage and nonsteroidals, which had little impact. After diagnosis of a plasma cell population in the fluid, we offered local treatment with radiation and systemic treatment of multiple myeloma, which offered better but suboptimal control. Possible treatments for olecranon bursitis include surgery, corticosteroid injections, anti-inflammatories, and serial drainage. Nonsurgical management may be more effective than surgical management, and corticosteroid injection carries significant risks. On the other hand, serial drainage does not confer additional infection risk in cases with aseptic etiology.15 We combined conservative measures as well as treatment of the underlying disease, but we believe that our patient did not derive significant benefit because of the refractory nature of his disease; he also expressed a preference to avoid surgical intervention.
Conclusion
Bursitis is a rare but thought-provoking potential manifestation of multiple myeloma and AL amyloidosis; we believe that our patient’s bursitis was related to plasma cell neoplasia based upon co-occurrence with disease relapse. His bursitis turned out to be an early indicator of impending systemic relapse. In this particular case, in which the patient wished to avoid surgical intervention, flow cytometry was of great value, and we believe that our case is the first report of malignant bursitis being diagnosed by flow cytometry. Our patient’s case shares similarities with other biopsy-confirmed cases of malignant bursitis, but we were able to avoid the need for surgical biopsy or bursal stripping.
The authors thank Jennifer Wilham MT (ASCP), Pat Byrd MT (ASCP), and Darlene Mann MT (ASCP) for their technical support.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459.
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
3. Gozzetti A, Coviello G, Fabbri A, et al. Unusual localizations of plasmacytoma. Leuk Res. 2011;35(7):e104-e105.
4. Kivioja AH, Karaharju EO, Elomaa I, Böhling TO. Surgical treatment of myeloma of bone. Eur J Cancer. 1992;28(11):1865-1869.
5. Santos MS, Soares B, Mendes O, Carvalho CM, Casimiro RF. Multiple myeloma-amyloidosis presenting as pseudomyopathy. Rev Bras Reumatol. 2011;51(6):651-654. 6. Blackwell JR, Hay BA, Bolt AM, May SM. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6(3):182-190.
7. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25(1):158-167.
8. De Groote J, Geerts B, Mermuys K, Verstraete K. Osteochondroma of the proximal humerus with frictional bursitis and secondary synovial osteochondromatosis. JBR-BTR. 2015;98(1):45-47. 9. Kumar R, Anjana, Kundan M. Retrocalcaneal bursitis due to rare calcaneal osteochrondroma in adult male: excision and outcome. J Orthop Case Rep. 2016;6(2):16-19.
10. Yoon PW, Jang WY, Yoo JJ, Yoon KS, Kim HJ. Malignant fibrous histiocytoma at the site of an alumina-on-alumina-bearing total hip arthroplasty mimicking infected trochanteric bursitis. J Arthroplasty. 2012;27(2):324.e9-324.e12.
11. Hutchison CW, Kling DH. Malignant synovioma. Am J Cancer. 1940;40(1):8-84.
12. Hutchings C, Hull R. Metastatic bone disease presenting as trochanteric bursitis. J R Soc Med. 1997;90(12):685-686.
13. Dorfman HD, Siegel HL, Perry MC, Oxenhandler R. Non-Hodgkin’s lymphoma of the synovium simulating rheumatoid arthritis. Arthritis Rheum. 1987;30(2):155-161.
14. Chao CT, Wu MS. Dialysis elbow. QJM. 2012;105(5):485-486.
15. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459.
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
3. Gozzetti A, Coviello G, Fabbri A, et al. Unusual localizations of plasmacytoma. Leuk Res. 2011;35(7):e104-e105.
4. Kivioja AH, Karaharju EO, Elomaa I, Böhling TO. Surgical treatment of myeloma of bone. Eur J Cancer. 1992;28(11):1865-1869.
5. Santos MS, Soares B, Mendes O, Carvalho CM, Casimiro RF. Multiple myeloma-amyloidosis presenting as pseudomyopathy. Rev Bras Reumatol. 2011;51(6):651-654. 6. Blackwell JR, Hay BA, Bolt AM, May SM. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6(3):182-190.
7. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25(1):158-167.
8. De Groote J, Geerts B, Mermuys K, Verstraete K. Osteochondroma of the proximal humerus with frictional bursitis and secondary synovial osteochondromatosis. JBR-BTR. 2015;98(1):45-47. 9. Kumar R, Anjana, Kundan M. Retrocalcaneal bursitis due to rare calcaneal osteochrondroma in adult male: excision and outcome. J Orthop Case Rep. 2016;6(2):16-19.
10. Yoon PW, Jang WY, Yoo JJ, Yoon KS, Kim HJ. Malignant fibrous histiocytoma at the site of an alumina-on-alumina-bearing total hip arthroplasty mimicking infected trochanteric bursitis. J Arthroplasty. 2012;27(2):324.e9-324.e12.
11. Hutchison CW, Kling DH. Malignant synovioma. Am J Cancer. 1940;40(1):8-84.
12. Hutchings C, Hull R. Metastatic bone disease presenting as trochanteric bursitis. J R Soc Med. 1997;90(12):685-686.
13. Dorfman HD, Siegel HL, Perry MC, Oxenhandler R. Non-Hodgkin’s lymphoma of the synovium simulating rheumatoid arthritis. Arthritis Rheum. 1987;30(2):155-161.
14. Chao CT, Wu MS. Dialysis elbow. QJM. 2012;105(5):485-486.
15. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536.
ASH expands late-breaking abstract session
An additional presentation has been added to the late-breaking abstract session of the 2018 ASH Annual Meeting.
The session was expanded from six abstracts to seven this year because of a record number of “exciting” submissions, according to ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland.
“We received 98 late-breaking abstracts, which is a record,” Dr. Brodsky said.
“They were so exciting this year that we added a seventh, and, quite frankly, we could have added several more, but we just didn’t have time in the meeting.”
Dr. Brodsky discussed this year’s late-breaking abstracts during a recent press briefing.
Abstract LBA-1 reports results of rivaroxaban thromboprophylaxis in cancer patients with an increased risk of venous thromboembolism (VTE). Compared to placebo, rivaroxaban significantly reduced VTE and VTE-related death during treatment but not over the entire study period.
Abstract LBA-2 describes a phase 3, randomized trial comparing daratumumab plus lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who are ineligible for transplant. The addition of daratumumab reduced the risk of disease progression or death by 45%.
Abstract LBA-3 details results with HemoTypeSC, a test used to detect sickle cell trait and sickle cell disease. The test correctly identified all phenotypes in the 1,000 children studied.
Abstract LBA-4 describes a randomized, phase 3 study comparing ibrutinib plus rituximab to fludarabine, cyclophosphamide, and rituximab in younger patients with untreated chronic lymphocytic leukemia (CLL). Investigators found that ibrutinib plus rituximab provided significantly better progression-free and overall survival than the three-drug combination.
Abstract LBA-5 details a strategy for direct oral anticoagulant use in patients with atrial fibrillation undergoing surgery. Investigators say the strategy is likely to be “practice-changing” and incorporated into guidelines.
Abstract LBA-6 covers a trial of emapalumab in patients with primary hemophagocytic lymphohistiocytosis. Emapalumab, which was recently approved by the U.S. Food and Drug Administration, produced responses in most of the 34 patients studied and had a favorable safety profile, according to investigators.
Abstract LBA-7 reports the discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax in patients with progressive CLL. Investigators say this mutation could be a biomarker of CLL relapse.
An additional presentation has been added to the late-breaking abstract session of the 2018 ASH Annual Meeting.
The session was expanded from six abstracts to seven this year because of a record number of “exciting” submissions, according to ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland.
“We received 98 late-breaking abstracts, which is a record,” Dr. Brodsky said.
“They were so exciting this year that we added a seventh, and, quite frankly, we could have added several more, but we just didn’t have time in the meeting.”
Dr. Brodsky discussed this year’s late-breaking abstracts during a recent press briefing.
Abstract LBA-1 reports results of rivaroxaban thromboprophylaxis in cancer patients with an increased risk of venous thromboembolism (VTE). Compared to placebo, rivaroxaban significantly reduced VTE and VTE-related death during treatment but not over the entire study period.
Abstract LBA-2 describes a phase 3, randomized trial comparing daratumumab plus lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who are ineligible for transplant. The addition of daratumumab reduced the risk of disease progression or death by 45%.
Abstract LBA-3 details results with HemoTypeSC, a test used to detect sickle cell trait and sickle cell disease. The test correctly identified all phenotypes in the 1,000 children studied.
Abstract LBA-4 describes a randomized, phase 3 study comparing ibrutinib plus rituximab to fludarabine, cyclophosphamide, and rituximab in younger patients with untreated chronic lymphocytic leukemia (CLL). Investigators found that ibrutinib plus rituximab provided significantly better progression-free and overall survival than the three-drug combination.
Abstract LBA-5 details a strategy for direct oral anticoagulant use in patients with atrial fibrillation undergoing surgery. Investigators say the strategy is likely to be “practice-changing” and incorporated into guidelines.
Abstract LBA-6 covers a trial of emapalumab in patients with primary hemophagocytic lymphohistiocytosis. Emapalumab, which was recently approved by the U.S. Food and Drug Administration, produced responses in most of the 34 patients studied and had a favorable safety profile, according to investigators.
Abstract LBA-7 reports the discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax in patients with progressive CLL. Investigators say this mutation could be a biomarker of CLL relapse.
An additional presentation has been added to the late-breaking abstract session of the 2018 ASH Annual Meeting.
The session was expanded from six abstracts to seven this year because of a record number of “exciting” submissions, according to ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland.
“We received 98 late-breaking abstracts, which is a record,” Dr. Brodsky said.
“They were so exciting this year that we added a seventh, and, quite frankly, we could have added several more, but we just didn’t have time in the meeting.”
Dr. Brodsky discussed this year’s late-breaking abstracts during a recent press briefing.
Abstract LBA-1 reports results of rivaroxaban thromboprophylaxis in cancer patients with an increased risk of venous thromboembolism (VTE). Compared to placebo, rivaroxaban significantly reduced VTE and VTE-related death during treatment but not over the entire study period.
Abstract LBA-2 describes a phase 3, randomized trial comparing daratumumab plus lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who are ineligible for transplant. The addition of daratumumab reduced the risk of disease progression or death by 45%.
Abstract LBA-3 details results with HemoTypeSC, a test used to detect sickle cell trait and sickle cell disease. The test correctly identified all phenotypes in the 1,000 children studied.
Abstract LBA-4 describes a randomized, phase 3 study comparing ibrutinib plus rituximab to fludarabine, cyclophosphamide, and rituximab in younger patients with untreated chronic lymphocytic leukemia (CLL). Investigators found that ibrutinib plus rituximab provided significantly better progression-free and overall survival than the three-drug combination.
Abstract LBA-5 details a strategy for direct oral anticoagulant use in patients with atrial fibrillation undergoing surgery. Investigators say the strategy is likely to be “practice-changing” and incorporated into guidelines.
Abstract LBA-6 covers a trial of emapalumab in patients with primary hemophagocytic lymphohistiocytosis. Emapalumab, which was recently approved by the U.S. Food and Drug Administration, produced responses in most of the 34 patients studied and had a favorable safety profile, according to investigators.
Abstract LBA-7 reports the discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax in patients with progressive CLL. Investigators say this mutation could be a biomarker of CLL relapse.
Immunotherapy may hold the key to defeating virally associated cancers
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
CAR T-cell studies to be presented at ASH
Several studies set to be presented at the 2018 ASH Annual Meeting provide new insights regarding chimeric antigen receptor (CAR) T-cell therapies.
One study suggests ibrutinib may enhance CAR T-cell therapy in patients with chronic lymphocytic leukemia (CLL), and another suggests checkpoint inhibitors can augment CAR T-cell therapy in certain patients with B-cell acute lymphoblastic leukemia (ALL).
Two additional studies indicate that responses to tisagenlecleucel are durable in both ALL and diffuse large B-cell lymphoma (DLBCL).
A fifth study suggests hematopoietic stem cell transplant (HSCT) may reduce the risk of relapse after CAR T-cell therapy.
ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland, discussed these studies during a media briefing ahead of the ASH Annual Meeting.
Ibrutinib
In the ibrutinib study (abstract 299), patients received the BTK inhibitor starting 2 weeks prior to leukapheresis and continued until 3 months after treatment with JCAR014.
Data suggest this strategy may improve responses and decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory CLL.
Responses occurred in 88% of patients who received ibrutinib and 56% of those who did not.
Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients (26%) in the no-ibrutinib cohort and 0 of 17 patients in the ibrutinib cohort.
These findings are “early and preliminary but very exciting” Dr. Brodsky said.
Checkpoint inhibitors
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy.
The 14 patients studied had early CAR T-cell loss, partial response, or no response to CAR T-cell therapy. Thirteen patients had B-cell ALL, and one had B lymphoblastic lymphoma.
CD19-directed CAR T-cell therapy consisted of tisagenlecleucel in four patients and CTL119 in 10. Thirteen patients received pembrolizumab, and one received nivolumab.
Three of six patients who had early B-cell recovery re-established B-cell aplasia with the addition of a checkpoint inhibitor. In two patients, B-cell aplasia persists with ongoing pembrolizumab.
Four patients who did not respond to or relapsed after their initial CAR T-cell therapy had a partial (n=2) or complete response (n=2) with the addition of pembrolizumab.
There were additional partial responses in the remaining four patients. However, one of these patients (with CD19-dim/negative disease) progressed.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said.
“[This is a] very small [study with] preliminary data but very exciting that it is safe to give checkpoint inhibitors with CAR T cells, and it may be efficacious at getting the immune response back.”
Tisagenlecleucel follow-up
One of the two tisagenlecleucel updates (abstract 895) consists of data from the ELIANA trial, which includes pediatric and young adult patients with relapsed/refractory ALL.
The overall response rate was 82% (65/79). Of the 65 responders, 29 were still in response at follow-up.
The probability of relapse-free survival was 66% at 12 months and 18 months.
“These are some very fast-growing tumors, and these are refractory, resistant patients, so, as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
The other tisagenlecleucel update (abstract 1684) is from the JULIET trial, which includes adults with relapsed or refractory DLBCL (n=99).
The overall response rate was 54%. The probability of relapse-free survival was 66% at 6 months and 64% at both 12 months and 18 months.
HSCT consolidation
Dr. Brodsky also discussed long-term follow-up from a phase 1/2 trial of SCRI-CAR19v1, a CD19-specific CAR T-cell product, in patients with relapsed/refractory ALL (abstract 967).
Of the 50 evaluable patients, 17 had no history of HSCT prior to CAR T-cell therapy.
Three of the 17 patients did not proceed to HSCT after CAR T-cell therapy, and two of these patients relapsed. Of the 14 patients who did undergo HSCT after CAR T-cell therapy, two relapsed.
There were 33 patients with a prior history of HSCT, and 10 of them had another HSCT after CAR T-cell therapy. Five of them are still alive and in remission.
Of the 23 patients who did not undergo another HSCT, eight are still in remission.
“This study is very small, and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
Several studies set to be presented at the 2018 ASH Annual Meeting provide new insights regarding chimeric antigen receptor (CAR) T-cell therapies.
One study suggests ibrutinib may enhance CAR T-cell therapy in patients with chronic lymphocytic leukemia (CLL), and another suggests checkpoint inhibitors can augment CAR T-cell therapy in certain patients with B-cell acute lymphoblastic leukemia (ALL).
Two additional studies indicate that responses to tisagenlecleucel are durable in both ALL and diffuse large B-cell lymphoma (DLBCL).
A fifth study suggests hematopoietic stem cell transplant (HSCT) may reduce the risk of relapse after CAR T-cell therapy.
ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland, discussed these studies during a media briefing ahead of the ASH Annual Meeting.
Ibrutinib
In the ibrutinib study (abstract 299), patients received the BTK inhibitor starting 2 weeks prior to leukapheresis and continued until 3 months after treatment with JCAR014.
Data suggest this strategy may improve responses and decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory CLL.
Responses occurred in 88% of patients who received ibrutinib and 56% of those who did not.
Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients (26%) in the no-ibrutinib cohort and 0 of 17 patients in the ibrutinib cohort.
These findings are “early and preliminary but very exciting” Dr. Brodsky said.
Checkpoint inhibitors
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy.
The 14 patients studied had early CAR T-cell loss, partial response, or no response to CAR T-cell therapy. Thirteen patients had B-cell ALL, and one had B lymphoblastic lymphoma.
CD19-directed CAR T-cell therapy consisted of tisagenlecleucel in four patients and CTL119 in 10. Thirteen patients received pembrolizumab, and one received nivolumab.
Three of six patients who had early B-cell recovery re-established B-cell aplasia with the addition of a checkpoint inhibitor. In two patients, B-cell aplasia persists with ongoing pembrolizumab.
Four patients who did not respond to or relapsed after their initial CAR T-cell therapy had a partial (n=2) or complete response (n=2) with the addition of pembrolizumab.
There were additional partial responses in the remaining four patients. However, one of these patients (with CD19-dim/negative disease) progressed.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said.
“[This is a] very small [study with] preliminary data but very exciting that it is safe to give checkpoint inhibitors with CAR T cells, and it may be efficacious at getting the immune response back.”
Tisagenlecleucel follow-up
One of the two tisagenlecleucel updates (abstract 895) consists of data from the ELIANA trial, which includes pediatric and young adult patients with relapsed/refractory ALL.
The overall response rate was 82% (65/79). Of the 65 responders, 29 were still in response at follow-up.
The probability of relapse-free survival was 66% at 12 months and 18 months.
“These are some very fast-growing tumors, and these are refractory, resistant patients, so, as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
The other tisagenlecleucel update (abstract 1684) is from the JULIET trial, which includes adults with relapsed or refractory DLBCL (n=99).
The overall response rate was 54%. The probability of relapse-free survival was 66% at 6 months and 64% at both 12 months and 18 months.
HSCT consolidation
Dr. Brodsky also discussed long-term follow-up from a phase 1/2 trial of SCRI-CAR19v1, a CD19-specific CAR T-cell product, in patients with relapsed/refractory ALL (abstract 967).
Of the 50 evaluable patients, 17 had no history of HSCT prior to CAR T-cell therapy.
Three of the 17 patients did not proceed to HSCT after CAR T-cell therapy, and two of these patients relapsed. Of the 14 patients who did undergo HSCT after CAR T-cell therapy, two relapsed.
There were 33 patients with a prior history of HSCT, and 10 of them had another HSCT after CAR T-cell therapy. Five of them are still alive and in remission.
Of the 23 patients who did not undergo another HSCT, eight are still in remission.
“This study is very small, and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
Several studies set to be presented at the 2018 ASH Annual Meeting provide new insights regarding chimeric antigen receptor (CAR) T-cell therapies.
One study suggests ibrutinib may enhance CAR T-cell therapy in patients with chronic lymphocytic leukemia (CLL), and another suggests checkpoint inhibitors can augment CAR T-cell therapy in certain patients with B-cell acute lymphoblastic leukemia (ALL).
Two additional studies indicate that responses to tisagenlecleucel are durable in both ALL and diffuse large B-cell lymphoma (DLBCL).
A fifth study suggests hematopoietic stem cell transplant (HSCT) may reduce the risk of relapse after CAR T-cell therapy.
ASH Secretary Robert A. Brodsky, MD, of Johns Hopkins University in Baltimore, Maryland, discussed these studies during a media briefing ahead of the ASH Annual Meeting.
Ibrutinib
In the ibrutinib study (abstract 299), patients received the BTK inhibitor starting 2 weeks prior to leukapheresis and continued until 3 months after treatment with JCAR014.
Data suggest this strategy may improve responses and decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory CLL.
Responses occurred in 88% of patients who received ibrutinib and 56% of those who did not.
Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients (26%) in the no-ibrutinib cohort and 0 of 17 patients in the ibrutinib cohort.
These findings are “early and preliminary but very exciting” Dr. Brodsky said.
Checkpoint inhibitors
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy.
The 14 patients studied had early CAR T-cell loss, partial response, or no response to CAR T-cell therapy. Thirteen patients had B-cell ALL, and one had B lymphoblastic lymphoma.
CD19-directed CAR T-cell therapy consisted of tisagenlecleucel in four patients and CTL119 in 10. Thirteen patients received pembrolizumab, and one received nivolumab.
Three of six patients who had early B-cell recovery re-established B-cell aplasia with the addition of a checkpoint inhibitor. In two patients, B-cell aplasia persists with ongoing pembrolizumab.
Four patients who did not respond to or relapsed after their initial CAR T-cell therapy had a partial (n=2) or complete response (n=2) with the addition of pembrolizumab.
There were additional partial responses in the remaining four patients. However, one of these patients (with CD19-dim/negative disease) progressed.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said.
“[This is a] very small [study with] preliminary data but very exciting that it is safe to give checkpoint inhibitors with CAR T cells, and it may be efficacious at getting the immune response back.”
Tisagenlecleucel follow-up
One of the two tisagenlecleucel updates (abstract 895) consists of data from the ELIANA trial, which includes pediatric and young adult patients with relapsed/refractory ALL.
The overall response rate was 82% (65/79). Of the 65 responders, 29 were still in response at follow-up.
The probability of relapse-free survival was 66% at 12 months and 18 months.
“These are some very fast-growing tumors, and these are refractory, resistant patients, so, as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
The other tisagenlecleucel update (abstract 1684) is from the JULIET trial, which includes adults with relapsed or refractory DLBCL (n=99).
The overall response rate was 54%. The probability of relapse-free survival was 66% at 6 months and 64% at both 12 months and 18 months.
HSCT consolidation
Dr. Brodsky also discussed long-term follow-up from a phase 1/2 trial of SCRI-CAR19v1, a CD19-specific CAR T-cell product, in patients with relapsed/refractory ALL (abstract 967).
Of the 50 evaluable patients, 17 had no history of HSCT prior to CAR T-cell therapy.
Three of the 17 patients did not proceed to HSCT after CAR T-cell therapy, and two of these patients relapsed. Of the 14 patients who did undergo HSCT after CAR T-cell therapy, two relapsed.
There were 33 patients with a prior history of HSCT, and 10 of them had another HSCT after CAR T-cell therapy. Five of them are still alive and in remission.
Of the 23 patients who did not undergo another HSCT, eight are still in remission.
“This study is very small, and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
Biosimilar deemed equivalent to rituximab in FL
Phase 3 results suggest the biosimilar product CT-P10 is equivalent to rituximab in patients with low-tumor-burden follicular lymphoma (FL).
Overall response rates were similar—both exceeding 80%—in patients who received CT-P10 and those who received rituximab.
In addition, adverse event (AE) profiles were comparable between the treatment arms.
Larry W. Kwak, MD, PhD, of City of Hope in Duarte, California, and his colleagues reported these results in The Lancet Haematology.
CT-P10 was approved by the European Commission in 2017 and was recommended for approval by the U.S. Food and Drug Administration’s Oncologic Drugs Advisory Committee last month.
The phase 3 trial of CT-P10 included 258 patients with stage II-IV low-tumor-burden FL. They were randomized to receive CT-P10 (n=130) or rituximab (n=128).
Patients received intravenous CT-P10 or rituximab weekly for 4 weeks as induction therapy. Patients experiencing disease control went on to a maintenance phase with their assigned treatment, given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
Efficacy
The overall response rate at 7 months was 83% in patients randomized to CT-P10 and 81% in those randomized to rituximab.
The complete response rates were 28% and 34%, respectively. The unconfirmed complete response rates were 5% and 2%, respectively. And the partial response rates were 51% and 46%, respectively.
The two treatments were deemed therapeutically equivalent, as the two-sided 90% confidence intervals for the difference in proportion of responders between CT-P10 and rituximab were within the prespecified equivalence margin of 17%.
Safety
Treatment-emergent AEs occurred in 71% of patients in the CT-P10 arm and 67% of those in the rituximab arm.
The most common treatment-emergent AEs (in the CT-P10 and rituximab arms, respectively) were:
- Infusion-related reactions (31% and 29%)
- Infections (27% and 21%)
- Worsening neutropenia (22% for both)
- Upper respiratory tract infection (12% and 11%)
- Worsening anemia (10% and 14%)
- Worsening thrombocytopenia (8% and 7%)
- Fatigue (7% and 9%)
- Diarrhea (5% for both)
- Nausea (5% for both)
- Urinary tract infection (4% and 5%)
- Headache (3% and 5%).
Serious AEs were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
Two serious AEs—myocardial infarction and constipation—in the CT-P10 arm were considered related to treatment. None of the serious AEs in the rituximab arm were considered treatment-related.
Two patients in the CT-P10 arm discontinued treatment due to AEs—one due to myocardial infarction and one due to dermatitis. There were no AE-related discontinuations in the rituximab arm.
There were two deaths in the CT-P10 arm as of the cutoff date (January 4, 2018). One was due to myocardial infarction, and one was due to respiratory failure. The myocardial infarction was considered possibly related to treatment.
This trial was sponsored by Celltrion, the company developing CT-P10. Three study authors are employees of the company.
Dr. Kwak and several other authors not employed by Celltrion reported disclosures related to the company. Authors also reported relationships with Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
Phase 3 results suggest the biosimilar product CT-P10 is equivalent to rituximab in patients with low-tumor-burden follicular lymphoma (FL).
Overall response rates were similar—both exceeding 80%—in patients who received CT-P10 and those who received rituximab.
In addition, adverse event (AE) profiles were comparable between the treatment arms.
Larry W. Kwak, MD, PhD, of City of Hope in Duarte, California, and his colleagues reported these results in The Lancet Haematology.
CT-P10 was approved by the European Commission in 2017 and was recommended for approval by the U.S. Food and Drug Administration’s Oncologic Drugs Advisory Committee last month.
The phase 3 trial of CT-P10 included 258 patients with stage II-IV low-tumor-burden FL. They were randomized to receive CT-P10 (n=130) or rituximab (n=128).
Patients received intravenous CT-P10 or rituximab weekly for 4 weeks as induction therapy. Patients experiencing disease control went on to a maintenance phase with their assigned treatment, given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
Efficacy
The overall response rate at 7 months was 83% in patients randomized to CT-P10 and 81% in those randomized to rituximab.
The complete response rates were 28% and 34%, respectively. The unconfirmed complete response rates were 5% and 2%, respectively. And the partial response rates were 51% and 46%, respectively.
The two treatments were deemed therapeutically equivalent, as the two-sided 90% confidence intervals for the difference in proportion of responders between CT-P10 and rituximab were within the prespecified equivalence margin of 17%.
Safety
Treatment-emergent AEs occurred in 71% of patients in the CT-P10 arm and 67% of those in the rituximab arm.
The most common treatment-emergent AEs (in the CT-P10 and rituximab arms, respectively) were:
- Infusion-related reactions (31% and 29%)
- Infections (27% and 21%)
- Worsening neutropenia (22% for both)
- Upper respiratory tract infection (12% and 11%)
- Worsening anemia (10% and 14%)
- Worsening thrombocytopenia (8% and 7%)
- Fatigue (7% and 9%)
- Diarrhea (5% for both)
- Nausea (5% for both)
- Urinary tract infection (4% and 5%)
- Headache (3% and 5%).
Serious AEs were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
Two serious AEs—myocardial infarction and constipation—in the CT-P10 arm were considered related to treatment. None of the serious AEs in the rituximab arm were considered treatment-related.
Two patients in the CT-P10 arm discontinued treatment due to AEs—one due to myocardial infarction and one due to dermatitis. There were no AE-related discontinuations in the rituximab arm.
There were two deaths in the CT-P10 arm as of the cutoff date (January 4, 2018). One was due to myocardial infarction, and one was due to respiratory failure. The myocardial infarction was considered possibly related to treatment.
This trial was sponsored by Celltrion, the company developing CT-P10. Three study authors are employees of the company.
Dr. Kwak and several other authors not employed by Celltrion reported disclosures related to the company. Authors also reported relationships with Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
Phase 3 results suggest the biosimilar product CT-P10 is equivalent to rituximab in patients with low-tumor-burden follicular lymphoma (FL).
Overall response rates were similar—both exceeding 80%—in patients who received CT-P10 and those who received rituximab.
In addition, adverse event (AE) profiles were comparable between the treatment arms.
Larry W. Kwak, MD, PhD, of City of Hope in Duarte, California, and his colleagues reported these results in The Lancet Haematology.
CT-P10 was approved by the European Commission in 2017 and was recommended for approval by the U.S. Food and Drug Administration’s Oncologic Drugs Advisory Committee last month.
The phase 3 trial of CT-P10 included 258 patients with stage II-IV low-tumor-burden FL. They were randomized to receive CT-P10 (n=130) or rituximab (n=128).
Patients received intravenous CT-P10 or rituximab weekly for 4 weeks as induction therapy. Patients experiencing disease control went on to a maintenance phase with their assigned treatment, given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
Efficacy
The overall response rate at 7 months was 83% in patients randomized to CT-P10 and 81% in those randomized to rituximab.
The complete response rates were 28% and 34%, respectively. The unconfirmed complete response rates were 5% and 2%, respectively. And the partial response rates were 51% and 46%, respectively.
The two treatments were deemed therapeutically equivalent, as the two-sided 90% confidence intervals for the difference in proportion of responders between CT-P10 and rituximab were within the prespecified equivalence margin of 17%.
Safety
Treatment-emergent AEs occurred in 71% of patients in the CT-P10 arm and 67% of those in the rituximab arm.
The most common treatment-emergent AEs (in the CT-P10 and rituximab arms, respectively) were:
- Infusion-related reactions (31% and 29%)
- Infections (27% and 21%)
- Worsening neutropenia (22% for both)
- Upper respiratory tract infection (12% and 11%)
- Worsening anemia (10% and 14%)
- Worsening thrombocytopenia (8% and 7%)
- Fatigue (7% and 9%)
- Diarrhea (5% for both)
- Nausea (5% for both)
- Urinary tract infection (4% and 5%)
- Headache (3% and 5%).
Serious AEs were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
Two serious AEs—myocardial infarction and constipation—in the CT-P10 arm were considered related to treatment. None of the serious AEs in the rituximab arm were considered treatment-related.
Two patients in the CT-P10 arm discontinued treatment due to AEs—one due to myocardial infarction and one due to dermatitis. There were no AE-related discontinuations in the rituximab arm.
There were two deaths in the CT-P10 arm as of the cutoff date (January 4, 2018). One was due to myocardial infarction, and one was due to respiratory failure. The myocardial infarction was considered possibly related to treatment.
This trial was sponsored by Celltrion, the company developing CT-P10. Three study authors are employees of the company.
Dr. Kwak and several other authors not employed by Celltrion reported disclosures related to the company. Authors also reported relationships with Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
Elderly NHL patients have higher NRM after HSCT
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.