User login
In mantle cell lymphoma, triple therapy proves too toxic
Combined idelalisib, lenalidomide, and rituximab proved excessively toxic for the treatment of relapsed and refractory mantle cell and follicular lymphoma in two phase I trials conducted by the Alliance for Clinical Trials in Oncology.
The unexpected outcome, which led to early study termination, underscores the need for caution as new treatment combinations are proposed, Sonali M. Smith, MD, of the University of Chicago and her colleagues said in The Lancet Haematology.
In four of the first eight patients enrolled in the mantle cell lymphoma (A051201) and follicular lymphoma (A051202) phase I trials between July 9, 2013, and Sept. 30, 2014, unexpected dose-limiting toxicities occurred, including grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash.
The adverse events occurred between 9 and 20 days after treatment initiation and coincided with rituximab infusions, the researchers said. No treatment-related deaths occurred (Lancet Haematol. 2017 Apr;4:e176-82).
Overall, 8 of 11 patients were removed from treatment because of an adverse event, and 3 of those required intensive care unit level of care.
Although rituximab was removed in both trials, two of the remaining three patients in the studies, including three with mantle cell lymphoma and eight with follicular lymphoma, experienced grade 3 rashes, and one had grade 3 AST elevations. In those with mantle cell lymphoma, the most common grade 3-4 adverse events were ALT elevations and rash. In those with follicular lymphoma, the most common grade 3-4 adverse events were neutropenia and rash.
“Given the inability to deliver treatment due to toxicity, both studies were permanently closed,” the researchers wrote, noting that the primary endpoint of safety and tolerability was not met.
The trials had the overall goal of developing targeted regimens to replace cytotoxic therapy.
“Both ... trials were designed to capitalize on the clinical synergy of lenalidomide and rituximab observed in previous trials by adding the highly specific PI3K delta inhibitor, idelalisib, for patients with relapsed mantle cell lymphoma and follicular lymphoma,” they said.
Previously available data implied that lenalidomide plus rituximab would be a safe backbone for therapy, and there was clinical rationale for adding idelalisib to that combination, they explained.
“Overall, our brief experience underscores the limited knowledge regarding drug interactions and off-target effects and serves as a cautionary note in developing biological agents in combination and against ad-hoc combinations outside of carefully monitored clinical trials,” they said.
The researchers noted that the nature of the toxicities observed in these trials supports an immune-activated state characterized by excessive inflammation.
“A more detailed assessment of effect on cytokines, T-cell subsets, natural killer cells, and clinical features predictive of toxicity and response should be included in any further testing of these classes of agents, and they should never be combined outside of a carefully designed and diligently monitored clinical trial setting,” they concluded.
The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
The findings by Dr. Smith and her colleagues add to several other reported studies that involved unexpected toxicities with various combinations of targeted agents in lymphoid malignancies.
Combinations of B-cell receptor signaling inhibitors can lead to immune dysregulation, which can be acute and severe when combined with immunomodulatory agents.
While the study of rational targeted combinations continues to hold immense potential in both untreated and relapsed/refractory disease, the combination must be thoroughly studied in the context of carefully and conservatively designed clinical trials.
Given the unpredictable nature of adverse events, the use of novel combinations outside of a clinical trial should be strongly discouraged.
Patrick M. Reagan, MD , and Paul M. Barr, MD , are with the James P. Wilmot Cancer Institute, University of Rochester, New York. Dr. Reagan reported having no disclosures. Dr. Barr has consulted for Gilead, Pharmacyclics, AbbVie, and Celgene. They made their remarks in an editorial that accompanied the article.
The findings by Dr. Smith and her colleagues add to several other reported studies that involved unexpected toxicities with various combinations of targeted agents in lymphoid malignancies.
Combinations of B-cell receptor signaling inhibitors can lead to immune dysregulation, which can be acute and severe when combined with immunomodulatory agents.
While the study of rational targeted combinations continues to hold immense potential in both untreated and relapsed/refractory disease, the combination must be thoroughly studied in the context of carefully and conservatively designed clinical trials.
Given the unpredictable nature of adverse events, the use of novel combinations outside of a clinical trial should be strongly discouraged.
Patrick M. Reagan, MD , and Paul M. Barr, MD , are with the James P. Wilmot Cancer Institute, University of Rochester, New York. Dr. Reagan reported having no disclosures. Dr. Barr has consulted for Gilead, Pharmacyclics, AbbVie, and Celgene. They made their remarks in an editorial that accompanied the article.
The findings by Dr. Smith and her colleagues add to several other reported studies that involved unexpected toxicities with various combinations of targeted agents in lymphoid malignancies.
Combinations of B-cell receptor signaling inhibitors can lead to immune dysregulation, which can be acute and severe when combined with immunomodulatory agents.
While the study of rational targeted combinations continues to hold immense potential in both untreated and relapsed/refractory disease, the combination must be thoroughly studied in the context of carefully and conservatively designed clinical trials.
Given the unpredictable nature of adverse events, the use of novel combinations outside of a clinical trial should be strongly discouraged.
Patrick M. Reagan, MD , and Paul M. Barr, MD , are with the James P. Wilmot Cancer Institute, University of Rochester, New York. Dr. Reagan reported having no disclosures. Dr. Barr has consulted for Gilead, Pharmacyclics, AbbVie, and Celgene. They made their remarks in an editorial that accompanied the article.
Combined idelalisib, lenalidomide, and rituximab proved excessively toxic for the treatment of relapsed and refractory mantle cell and follicular lymphoma in two phase I trials conducted by the Alliance for Clinical Trials in Oncology.
The unexpected outcome, which led to early study termination, underscores the need for caution as new treatment combinations are proposed, Sonali M. Smith, MD, of the University of Chicago and her colleagues said in The Lancet Haematology.
In four of the first eight patients enrolled in the mantle cell lymphoma (A051201) and follicular lymphoma (A051202) phase I trials between July 9, 2013, and Sept. 30, 2014, unexpected dose-limiting toxicities occurred, including grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash.
The adverse events occurred between 9 and 20 days after treatment initiation and coincided with rituximab infusions, the researchers said. No treatment-related deaths occurred (Lancet Haematol. 2017 Apr;4:e176-82).
Overall, 8 of 11 patients were removed from treatment because of an adverse event, and 3 of those required intensive care unit level of care.
Although rituximab was removed in both trials, two of the remaining three patients in the studies, including three with mantle cell lymphoma and eight with follicular lymphoma, experienced grade 3 rashes, and one had grade 3 AST elevations. In those with mantle cell lymphoma, the most common grade 3-4 adverse events were ALT elevations and rash. In those with follicular lymphoma, the most common grade 3-4 adverse events were neutropenia and rash.
“Given the inability to deliver treatment due to toxicity, both studies were permanently closed,” the researchers wrote, noting that the primary endpoint of safety and tolerability was not met.
The trials had the overall goal of developing targeted regimens to replace cytotoxic therapy.
“Both ... trials were designed to capitalize on the clinical synergy of lenalidomide and rituximab observed in previous trials by adding the highly specific PI3K delta inhibitor, idelalisib, for patients with relapsed mantle cell lymphoma and follicular lymphoma,” they said.
Previously available data implied that lenalidomide plus rituximab would be a safe backbone for therapy, and there was clinical rationale for adding idelalisib to that combination, they explained.
“Overall, our brief experience underscores the limited knowledge regarding drug interactions and off-target effects and serves as a cautionary note in developing biological agents in combination and against ad-hoc combinations outside of carefully monitored clinical trials,” they said.
The researchers noted that the nature of the toxicities observed in these trials supports an immune-activated state characterized by excessive inflammation.
“A more detailed assessment of effect on cytokines, T-cell subsets, natural killer cells, and clinical features predictive of toxicity and response should be included in any further testing of these classes of agents, and they should never be combined outside of a carefully designed and diligently monitored clinical trial setting,” they concluded.
The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
Combined idelalisib, lenalidomide, and rituximab proved excessively toxic for the treatment of relapsed and refractory mantle cell and follicular lymphoma in two phase I trials conducted by the Alliance for Clinical Trials in Oncology.
The unexpected outcome, which led to early study termination, underscores the need for caution as new treatment combinations are proposed, Sonali M. Smith, MD, of the University of Chicago and her colleagues said in The Lancet Haematology.
In four of the first eight patients enrolled in the mantle cell lymphoma (A051201) and follicular lymphoma (A051202) phase I trials between July 9, 2013, and Sept. 30, 2014, unexpected dose-limiting toxicities occurred, including grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash.
The adverse events occurred between 9 and 20 days after treatment initiation and coincided with rituximab infusions, the researchers said. No treatment-related deaths occurred (Lancet Haematol. 2017 Apr;4:e176-82).
Overall, 8 of 11 patients were removed from treatment because of an adverse event, and 3 of those required intensive care unit level of care.
Although rituximab was removed in both trials, two of the remaining three patients in the studies, including three with mantle cell lymphoma and eight with follicular lymphoma, experienced grade 3 rashes, and one had grade 3 AST elevations. In those with mantle cell lymphoma, the most common grade 3-4 adverse events were ALT elevations and rash. In those with follicular lymphoma, the most common grade 3-4 adverse events were neutropenia and rash.
“Given the inability to deliver treatment due to toxicity, both studies were permanently closed,” the researchers wrote, noting that the primary endpoint of safety and tolerability was not met.
The trials had the overall goal of developing targeted regimens to replace cytotoxic therapy.
“Both ... trials were designed to capitalize on the clinical synergy of lenalidomide and rituximab observed in previous trials by adding the highly specific PI3K delta inhibitor, idelalisib, for patients with relapsed mantle cell lymphoma and follicular lymphoma,” they said.
Previously available data implied that lenalidomide plus rituximab would be a safe backbone for therapy, and there was clinical rationale for adding idelalisib to that combination, they explained.
“Overall, our brief experience underscores the limited knowledge regarding drug interactions and off-target effects and serves as a cautionary note in developing biological agents in combination and against ad-hoc combinations outside of carefully monitored clinical trials,” they said.
The researchers noted that the nature of the toxicities observed in these trials supports an immune-activated state characterized by excessive inflammation.
“A more detailed assessment of effect on cytokines, T-cell subsets, natural killer cells, and clinical features predictive of toxicity and response should be included in any further testing of these classes of agents, and they should never be combined outside of a carefully designed and diligently monitored clinical trial setting,” they concluded.
The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
Key clinical point:
Major finding: Of 11 patients, 8 were removed from treatment because of an adverse event, and 3 of those required intensive care unit–level care.
Data source: Two phase I trials involving 11 patients.
Disclosures: The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
Newly diagnosed mantle cell lymphoma is ‘one of the hardest consultations’
NEW YORK – Relatively young patients with newly diagnosed, average-risk mantle cell lymphoma who go into remission on induction therapy face a difficult choice on their next management step: undergo immediate autologous stem cell transplantation or defer the stem cell transplant and continue on maintenance therapy.
The choice is especially difficult because both are currently considered reasonable options and each choice has certain attractions and downsides, experts highlighted in discussing this fork-in-the-road decision patients face.
Immediate autologous stem cell transplantation (ASCT) has a good chance to allow the patient to remain treatment free and in remission for as long as about 10 years, but it involves intensive upfront treatment for 6-9 months, during which the patient will likely not be able to work or carry on many usual activities. Deferring the transplant with maintenance therapy puts off this life-disrupting initial period of intensive therapy for what may be several years, but relapse on maintenance therapy is inevitable and once it happens the patient may not have as successful an outcome from an ASCT. It also means several years of ongoing drug therapy with a maintenance regimen.
“I tell my fellows that patients with newly diagnosed mantle cell lymphoma [are] one of the hardest consultations because, unlike most other lymphomas, there is no established standard therapy but a range of options,” Timothy S. Fenske, MD, said at the conference held by Imedex. “I go through the pros and cons with patients, and it comes down to the patient’s perceived quality of life and their lifestyle.”
“It’s a very difficult decision [for patients] because we don’t have the data we’d like to have,” observed Peter Martin, MD, director of the clinical research program in lymphoma at Weill Cornell Medicine, New York.
“There is a lot of upfront toxicity with transplantation, with 6-9 months out of work in my experience. Patients often tell me that they can’t afford to do that; they’ll lose their employment insurance and won’t be able to pay for replacement insurance. But then they will hopefully go 6-10 years without more treatment, which is a real benefit. With less intensive upfront treatment they have a chance for similar overall survival, but they’ll need more ongoing treatment. It’s pretty complicated and challenging” for patients to make a decision, he said. “It depends a lot on where patients are in their lives and what they are willing to accept,” Dr. Martin said.
In general, Dr. Martin took a more skeptical view of ASCT than Dr. Fenske. “There is no evidence that ASCT cures patients or prolongs their survival. It improves progression-free survival, but not necessarily overall survival,” Dr. Martin noted.
In fact, a report in December 2016 at the American Society of Hematology annual meeting (Blood. 2016 Dec 5;abstract 1095) suggested that “biology is the primary driver of outcomes in mantle cell lymphoma, not treatment,” said Dr. Martin. The results from a limited number of patients enrolled in the Nordic mantle cell lymphoma trials provided good but preliminary evidence that “if you have good biology it doesn’t matter what the treatment is, you will do well,” he explained.
In fact, a report in December 2016 at the American Society of Hematology annual meeting (Blood. 2016 Dec 5;abstract 1095) suggested that “biology is the primary driver of outcomes in mantle cell lymphoma, not treatment,” said Dr. Martin. The results from a limited number of patients enrolled in the Nordic mantle cell lymphoma trials provided good but preliminary evidence that “less intense therapy works just as well” as more intense therapy, as long as the patient has a favorable genetic profile, he explained.
In contrast, Dr. Fenske put a much more positive spin on more intensive treatment upfront with ASCT.
“There is not much debate that you get longer progression-free survival with the more intensive approach. The question is, does progression-free survival matter in mantle cell lymphoma? I argue that it does because relapse in patients with mantle cell lymphoma is no picnic. What you can expect in patients with relapsed or refractory mantle cell lymphoma is a progression-free survival of about 1-2 years, and an overall survival of about 2-3 years,” said Dr. Fenske, head of the section of bone marrow transplant and hematologic malignancies at the Medical College of Wisconsin in Milwaukee.
As an example of the poor prognosis of relapsed or refractory mantle cell lymphoma patients Dr. Fenske cited a review he coauthored of 97 patients treated with ibrutinib (Imbruvica. Their median duration of response was 17 months and median progression-free survival was 15 months. Once ibrutinib treatment failure occurred their median overall survival was less than 3 months. (Hematol Oncol. 2017 Jan 8.doi:10.1002/hon.2380).
“It’s easy to get carried away” when patients temporarily respond to a drug like ibrutinib or other new agents with a degree of efficacy for lymphomas, Dr. Fenske said, but these transient responses “don’t solve the problem. The patient is headed for trouble,” usually within a couple of years.
“I would argue that, especially for younger patients, the goal is to try to achieve the longest first remission, and that means an ASCT.” Dr. Fenske admitted that this strategy won’t work for very-high-risk patients, but for these patients no good treatment options currently exist.
He also stressed that research is just beginning to explore using measurement of negative minimal residual disease to identify patients with the best outcomes following initial induction treatment. It is possible that patients with undetectable minimal residual disease can avoid immediate ASCT and instead receive maintenance therapy, a hypothesis slated for testing in a randomized trial, he said.
Dr. Martin has been a consultant to Celgene, Gilead, Janssen, Novartis, Pharmacyclics, and Verastem. Dr. Fenske has been a consultant to Abbvie, Celgene, Pharmacyclics, Sanofi, and Seattle Genetics.
[email protected]
On Twitter @mitchelzoler
This article was updated May 30, 2017 .
NEW YORK – Relatively young patients with newly diagnosed, average-risk mantle cell lymphoma who go into remission on induction therapy face a difficult choice on their next management step: undergo immediate autologous stem cell transplantation or defer the stem cell transplant and continue on maintenance therapy.
The choice is especially difficult because both are currently considered reasonable options and each choice has certain attractions and downsides, experts highlighted in discussing this fork-in-the-road decision patients face.
Immediate autologous stem cell transplantation (ASCT) has a good chance to allow the patient to remain treatment free and in remission for as long as about 10 years, but it involves intensive upfront treatment for 6-9 months, during which the patient will likely not be able to work or carry on many usual activities. Deferring the transplant with maintenance therapy puts off this life-disrupting initial period of intensive therapy for what may be several years, but relapse on maintenance therapy is inevitable and once it happens the patient may not have as successful an outcome from an ASCT. It also means several years of ongoing drug therapy with a maintenance regimen.
“I tell my fellows that patients with newly diagnosed mantle cell lymphoma [are] one of the hardest consultations because, unlike most other lymphomas, there is no established standard therapy but a range of options,” Timothy S. Fenske, MD, said at the conference held by Imedex. “I go through the pros and cons with patients, and it comes down to the patient’s perceived quality of life and their lifestyle.”
“It’s a very difficult decision [for patients] because we don’t have the data we’d like to have,” observed Peter Martin, MD, director of the clinical research program in lymphoma at Weill Cornell Medicine, New York.
“There is a lot of upfront toxicity with transplantation, with 6-9 months out of work in my experience. Patients often tell me that they can’t afford to do that; they’ll lose their employment insurance and won’t be able to pay for replacement insurance. But then they will hopefully go 6-10 years without more treatment, which is a real benefit. With less intensive upfront treatment they have a chance for similar overall survival, but they’ll need more ongoing treatment. It’s pretty complicated and challenging” for patients to make a decision, he said. “It depends a lot on where patients are in their lives and what they are willing to accept,” Dr. Martin said.
In general, Dr. Martin took a more skeptical view of ASCT than Dr. Fenske. “There is no evidence that ASCT cures patients or prolongs their survival. It improves progression-free survival, but not necessarily overall survival,” Dr. Martin noted.
In fact, a report in December 2016 at the American Society of Hematology annual meeting (Blood. 2016 Dec 5;abstract 1095) suggested that “biology is the primary driver of outcomes in mantle cell lymphoma, not treatment,” said Dr. Martin. The results from a limited number of patients enrolled in the Nordic mantle cell lymphoma trials provided good but preliminary evidence that “if you have good biology it doesn’t matter what the treatment is, you will do well,” he explained.
In fact, a report in December 2016 at the American Society of Hematology annual meeting (Blood. 2016 Dec 5;abstract 1095) suggested that “biology is the primary driver of outcomes in mantle cell lymphoma, not treatment,” said Dr. Martin. The results from a limited number of patients enrolled in the Nordic mantle cell lymphoma trials provided good but preliminary evidence that “less intense therapy works just as well” as more intense therapy, as long as the patient has a favorable genetic profile, he explained.
In contrast, Dr. Fenske put a much more positive spin on more intensive treatment upfront with ASCT.
“There is not much debate that you get longer progression-free survival with the more intensive approach. The question is, does progression-free survival matter in mantle cell lymphoma? I argue that it does because relapse in patients with mantle cell lymphoma is no picnic. What you can expect in patients with relapsed or refractory mantle cell lymphoma is a progression-free survival of about 1-2 years, and an overall survival of about 2-3 years,” said Dr. Fenske, head of the section of bone marrow transplant and hematologic malignancies at the Medical College of Wisconsin in Milwaukee.
As an example of the poor prognosis of relapsed or refractory mantle cell lymphoma patients Dr. Fenske cited a review he coauthored of 97 patients treated with ibrutinib (Imbruvica. Their median duration of response was 17 months and median progression-free survival was 15 months. Once ibrutinib treatment failure occurred their median overall survival was less than 3 months. (Hematol Oncol. 2017 Jan 8.doi:10.1002/hon.2380).
“It’s easy to get carried away” when patients temporarily respond to a drug like ibrutinib or other new agents with a degree of efficacy for lymphomas, Dr. Fenske said, but these transient responses “don’t solve the problem. The patient is headed for trouble,” usually within a couple of years.
“I would argue that, especially for younger patients, the goal is to try to achieve the longest first remission, and that means an ASCT.” Dr. Fenske admitted that this strategy won’t work for very-high-risk patients, but for these patients no good treatment options currently exist.
He also stressed that research is just beginning to explore using measurement of negative minimal residual disease to identify patients with the best outcomes following initial induction treatment. It is possible that patients with undetectable minimal residual disease can avoid immediate ASCT and instead receive maintenance therapy, a hypothesis slated for testing in a randomized trial, he said.
Dr. Martin has been a consultant to Celgene, Gilead, Janssen, Novartis, Pharmacyclics, and Verastem. Dr. Fenske has been a consultant to Abbvie, Celgene, Pharmacyclics, Sanofi, and Seattle Genetics.
[email protected]
On Twitter @mitchelzoler
This article was updated May 30, 2017 .
NEW YORK – Relatively young patients with newly diagnosed, average-risk mantle cell lymphoma who go into remission on induction therapy face a difficult choice on their next management step: undergo immediate autologous stem cell transplantation or defer the stem cell transplant and continue on maintenance therapy.
The choice is especially difficult because both are currently considered reasonable options and each choice has certain attractions and downsides, experts highlighted in discussing this fork-in-the-road decision patients face.
Immediate autologous stem cell transplantation (ASCT) has a good chance to allow the patient to remain treatment free and in remission for as long as about 10 years, but it involves intensive upfront treatment for 6-9 months, during which the patient will likely not be able to work or carry on many usual activities. Deferring the transplant with maintenance therapy puts off this life-disrupting initial period of intensive therapy for what may be several years, but relapse on maintenance therapy is inevitable and once it happens the patient may not have as successful an outcome from an ASCT. It also means several years of ongoing drug therapy with a maintenance regimen.
“I tell my fellows that patients with newly diagnosed mantle cell lymphoma [are] one of the hardest consultations because, unlike most other lymphomas, there is no established standard therapy but a range of options,” Timothy S. Fenske, MD, said at the conference held by Imedex. “I go through the pros and cons with patients, and it comes down to the patient’s perceived quality of life and their lifestyle.”
“It’s a very difficult decision [for patients] because we don’t have the data we’d like to have,” observed Peter Martin, MD, director of the clinical research program in lymphoma at Weill Cornell Medicine, New York.
“There is a lot of upfront toxicity with transplantation, with 6-9 months out of work in my experience. Patients often tell me that they can’t afford to do that; they’ll lose their employment insurance and won’t be able to pay for replacement insurance. But then they will hopefully go 6-10 years without more treatment, which is a real benefit. With less intensive upfront treatment they have a chance for similar overall survival, but they’ll need more ongoing treatment. It’s pretty complicated and challenging” for patients to make a decision, he said. “It depends a lot on where patients are in their lives and what they are willing to accept,” Dr. Martin said.
In general, Dr. Martin took a more skeptical view of ASCT than Dr. Fenske. “There is no evidence that ASCT cures patients or prolongs their survival. It improves progression-free survival, but not necessarily overall survival,” Dr. Martin noted.
In fact, a report in December 2016 at the American Society of Hematology annual meeting (Blood. 2016 Dec 5;abstract 1095) suggested that “biology is the primary driver of outcomes in mantle cell lymphoma, not treatment,” said Dr. Martin. The results from a limited number of patients enrolled in the Nordic mantle cell lymphoma trials provided good but preliminary evidence that “if you have good biology it doesn’t matter what the treatment is, you will do well,” he explained.
In fact, a report in December 2016 at the American Society of Hematology annual meeting (Blood. 2016 Dec 5;abstract 1095) suggested that “biology is the primary driver of outcomes in mantle cell lymphoma, not treatment,” said Dr. Martin. The results from a limited number of patients enrolled in the Nordic mantle cell lymphoma trials provided good but preliminary evidence that “less intense therapy works just as well” as more intense therapy, as long as the patient has a favorable genetic profile, he explained.
In contrast, Dr. Fenske put a much more positive spin on more intensive treatment upfront with ASCT.
“There is not much debate that you get longer progression-free survival with the more intensive approach. The question is, does progression-free survival matter in mantle cell lymphoma? I argue that it does because relapse in patients with mantle cell lymphoma is no picnic. What you can expect in patients with relapsed or refractory mantle cell lymphoma is a progression-free survival of about 1-2 years, and an overall survival of about 2-3 years,” said Dr. Fenske, head of the section of bone marrow transplant and hematologic malignancies at the Medical College of Wisconsin in Milwaukee.
As an example of the poor prognosis of relapsed or refractory mantle cell lymphoma patients Dr. Fenske cited a review he coauthored of 97 patients treated with ibrutinib (Imbruvica. Their median duration of response was 17 months and median progression-free survival was 15 months. Once ibrutinib treatment failure occurred their median overall survival was less than 3 months. (Hematol Oncol. 2017 Jan 8.doi:10.1002/hon.2380).
“It’s easy to get carried away” when patients temporarily respond to a drug like ibrutinib or other new agents with a degree of efficacy for lymphomas, Dr. Fenske said, but these transient responses “don’t solve the problem. The patient is headed for trouble,” usually within a couple of years.
“I would argue that, especially for younger patients, the goal is to try to achieve the longest first remission, and that means an ASCT.” Dr. Fenske admitted that this strategy won’t work for very-high-risk patients, but for these patients no good treatment options currently exist.
He also stressed that research is just beginning to explore using measurement of negative minimal residual disease to identify patients with the best outcomes following initial induction treatment. It is possible that patients with undetectable minimal residual disease can avoid immediate ASCT and instead receive maintenance therapy, a hypothesis slated for testing in a randomized trial, he said.
Dr. Martin has been a consultant to Celgene, Gilead, Janssen, Novartis, Pharmacyclics, and Verastem. Dr. Fenske has been a consultant to Abbvie, Celgene, Pharmacyclics, Sanofi, and Seattle Genetics.
[email protected]
On Twitter @mitchelzoler
This article was updated May 30, 2017 .
EXPERT ANALYSIS FROM A MEETING ON HEMATOLOGIC MALIGNANCIES
MAGNIFY in relapsed/refractory mantle cell lymphoma
MAGNIFY is a phase IIIB randomized trial actively recruiting patients with relapsed/refractory mantle cell lymphoma, based on studies posted at ClinicalTrials.gov.
MAGNIFY (NCT01996865) is a study of lenalidomide (CC-5013) plus rituximab maintenance therapy, followed by lenalidomide single-agent maintenance therapy, versus rituximab. Sponsored by Celgene, the maker of lenalidomide (Revlimid), the trial’s primary outcome measure is progression-free survival at up to 8 years.
The MAGNIFY trial includes patients with grades 1-3b or transformed follicular lymphoma, marginal zone lymphoma, or mantle cell lymphoma who had received at least one prior therapy and had stage I-IV measurable disease. About 500 patients are planned to enroll in 12 cycles of R2 induction, and slightly more than 300 patients are projected to be randomized after induction to the two maintenance arms. Induction includes oral lenalidomide 20 mg/day, days 1-21 per 28-day cycle; plus intravenous rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 3, 5, 7, 9, and 11 (28-day cycles).
Patients will then be randomized to maintenance lenalidomide 10 mg/day, given on days 1-21 per 28-day cycle for cycles 13-30; plus rituximab 375 mg/m2, given on day 1 of cycles 13, 15, 17, 19, 21, 23, 25, 27, and 29 (R2, Arm A), or rituximab alone (same schedule, Arm B). Patients receiving R2 maintenance after 18 cycles may continue maintenance lenalidomide monotherapy at 10 mg/day, days 1-21 per 28-day cycle, at the discretion of the patient and/or investigator, until disease progression as tolerated.
The primary endpoint is progression-free survival. Secondary endpoints include safety, overall survival, response rates, duration of response, and quality of life. Patients will be followed for at least 5 years after the last patient-initiated induction therapy. Enrollment in MAGNIFY began in March 2014; as of January 2016, 133 patients are enrolled, according to the study page at ClinicalTrials.gov.
MAGNIFY is a phase IIIB randomized trial actively recruiting patients with relapsed/refractory mantle cell lymphoma, based on studies posted at ClinicalTrials.gov.
MAGNIFY (NCT01996865) is a study of lenalidomide (CC-5013) plus rituximab maintenance therapy, followed by lenalidomide single-agent maintenance therapy, versus rituximab. Sponsored by Celgene, the maker of lenalidomide (Revlimid), the trial’s primary outcome measure is progression-free survival at up to 8 years.
The MAGNIFY trial includes patients with grades 1-3b or transformed follicular lymphoma, marginal zone lymphoma, or mantle cell lymphoma who had received at least one prior therapy and had stage I-IV measurable disease. About 500 patients are planned to enroll in 12 cycles of R2 induction, and slightly more than 300 patients are projected to be randomized after induction to the two maintenance arms. Induction includes oral lenalidomide 20 mg/day, days 1-21 per 28-day cycle; plus intravenous rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 3, 5, 7, 9, and 11 (28-day cycles).
Patients will then be randomized to maintenance lenalidomide 10 mg/day, given on days 1-21 per 28-day cycle for cycles 13-30; plus rituximab 375 mg/m2, given on day 1 of cycles 13, 15, 17, 19, 21, 23, 25, 27, and 29 (R2, Arm A), or rituximab alone (same schedule, Arm B). Patients receiving R2 maintenance after 18 cycles may continue maintenance lenalidomide monotherapy at 10 mg/day, days 1-21 per 28-day cycle, at the discretion of the patient and/or investigator, until disease progression as tolerated.
The primary endpoint is progression-free survival. Secondary endpoints include safety, overall survival, response rates, duration of response, and quality of life. Patients will be followed for at least 5 years after the last patient-initiated induction therapy. Enrollment in MAGNIFY began in March 2014; as of January 2016, 133 patients are enrolled, according to the study page at ClinicalTrials.gov.
MAGNIFY is a phase IIIB randomized trial actively recruiting patients with relapsed/refractory mantle cell lymphoma, based on studies posted at ClinicalTrials.gov.
MAGNIFY (NCT01996865) is a study of lenalidomide (CC-5013) plus rituximab maintenance therapy, followed by lenalidomide single-agent maintenance therapy, versus rituximab. Sponsored by Celgene, the maker of lenalidomide (Revlimid), the trial’s primary outcome measure is progression-free survival at up to 8 years.
The MAGNIFY trial includes patients with grades 1-3b or transformed follicular lymphoma, marginal zone lymphoma, or mantle cell lymphoma who had received at least one prior therapy and had stage I-IV measurable disease. About 500 patients are planned to enroll in 12 cycles of R2 induction, and slightly more than 300 patients are projected to be randomized after induction to the two maintenance arms. Induction includes oral lenalidomide 20 mg/day, days 1-21 per 28-day cycle; plus intravenous rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 3, 5, 7, 9, and 11 (28-day cycles).
Patients will then be randomized to maintenance lenalidomide 10 mg/day, given on days 1-21 per 28-day cycle for cycles 13-30; plus rituximab 375 mg/m2, given on day 1 of cycles 13, 15, 17, 19, 21, 23, 25, 27, and 29 (R2, Arm A), or rituximab alone (same schedule, Arm B). Patients receiving R2 maintenance after 18 cycles may continue maintenance lenalidomide monotherapy at 10 mg/day, days 1-21 per 28-day cycle, at the discretion of the patient and/or investigator, until disease progression as tolerated.
The primary endpoint is progression-free survival. Secondary endpoints include safety, overall survival, response rates, duration of response, and quality of life. Patients will be followed for at least 5 years after the last patient-initiated induction therapy. Enrollment in MAGNIFY began in March 2014; as of January 2016, 133 patients are enrolled, according to the study page at ClinicalTrials.gov.
Unavoidable, random DNA replication errors are the most common cancer drivers
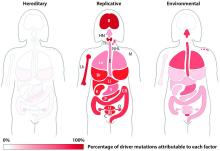
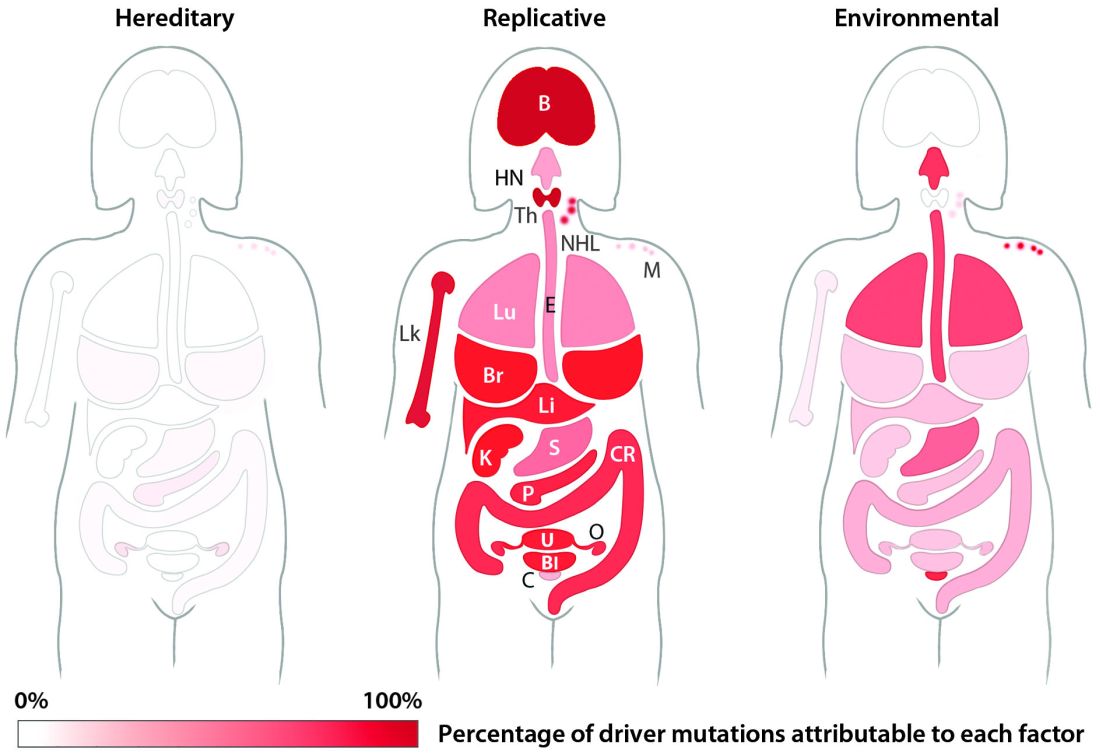
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Key clinical point:
Major finding: Two-thirds (66%) of cancer drivers are replication errors, 29% are environmentally induced, and 5% are hereditary.
Data source: The researchers examined cancer mutation drivers in two cohorts that spanned 69 countries.
Disclosures: Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
Phase III trial: VZV protects auto-HCT patients
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Overall incidence of herpes zoster was 32.8 cases/1,000 patient-years vs. 91.8/1,000 patient-years in patients in the vaccine and placebo groups, respectively.
Data source: A randomized, placebo-controlled phase III trial involving 1,230 patients.
Disclosures: Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
For mantle cell lymphoma, VR-CAP beat R-CHOP
For patients with newly diagnosed mantle cell lymphoma, duration and quality of response were superior with a regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), based on a post hoc analysis of the randomized, phase III LYM-3002 trial.
The difference was especially evident among patients who had a low- or medium-risk mantle cell lymphoma international prognostic index, Gregor Verhoef, MD, of University Hospital Leuven (Belgium) and his associates wrote in Haematologica.
In LYM-3002, 487 patients with newly diagnosed stage II-IV mantle cell lymphoma received six to eight 21-day cycles of intravenous VR-CAP or R-CHOP. Although overall response rates were similar for both groups, VR-CAP was associated with better duration of response and progression-free survival (PFS) and extended time to next treatment. To further explore these differences, the post hoc analysis stratified outcomes by response categories and analyzed depth of response based on computed tomography (CT) scans. Patients had a median age of about 65 years, and most were white males with stage-IV disease at diagnosis and an Eastern Cooperative Oncology Group performance status of 1 (Haematologica. 2017 Feb 9. doi: 10.3324/haematol.2016.152496).The superiority of VR-CAP held up across response categories. Complete responders to VR-CAP had more than twice the median PFS as did complete responders to R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004). Among partial responders, median PFS was 17.1 vs. 11.7 months, respectively (HR, 0.62; 95% CI, 0.43-0.89; P = .01). Respective median duration of overall response was 42.1 months for VR-CAP vs. 18.5 months among complete responders (HR, 0.42; P less than .001), and 20.2 vs. 9.6 months among partial responders (HR, 0.57; P = .006).
Median time to next treatment also favored VR-CAP over R-CHOP among both complete responders (not evaluable vs. 26.6 months; HR, 0.42; P less than .001) and partial responders (35.3 vs. 24.3 months; HR, 0.57; P = .006), the researchers said. Further, CT scans showed that proportionally more patients in each response category became lesion-negative on VR-CAP than on R-CHOP. Among complete responders, rates of lesion negativity were 72% and 59%, respectively. Among partial responders, rates were 48% and 28%.
The effects of VR-CAP were most evident among patients with a low or medium-risk mantle cell lymphoma international prognostic index. Perhaps high-risk status signifies more rapidly proliferative disease, which negates the deeper responses with VR-CAP, compared with R-CHOP, they added.
The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
For patients with newly diagnosed mantle cell lymphoma, duration and quality of response were superior with a regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), based on a post hoc analysis of the randomized, phase III LYM-3002 trial.
The difference was especially evident among patients who had a low- or medium-risk mantle cell lymphoma international prognostic index, Gregor Verhoef, MD, of University Hospital Leuven (Belgium) and his associates wrote in Haematologica.
In LYM-3002, 487 patients with newly diagnosed stage II-IV mantle cell lymphoma received six to eight 21-day cycles of intravenous VR-CAP or R-CHOP. Although overall response rates were similar for both groups, VR-CAP was associated with better duration of response and progression-free survival (PFS) and extended time to next treatment. To further explore these differences, the post hoc analysis stratified outcomes by response categories and analyzed depth of response based on computed tomography (CT) scans. Patients had a median age of about 65 years, and most were white males with stage-IV disease at diagnosis and an Eastern Cooperative Oncology Group performance status of 1 (Haematologica. 2017 Feb 9. doi: 10.3324/haematol.2016.152496).The superiority of VR-CAP held up across response categories. Complete responders to VR-CAP had more than twice the median PFS as did complete responders to R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004). Among partial responders, median PFS was 17.1 vs. 11.7 months, respectively (HR, 0.62; 95% CI, 0.43-0.89; P = .01). Respective median duration of overall response was 42.1 months for VR-CAP vs. 18.5 months among complete responders (HR, 0.42; P less than .001), and 20.2 vs. 9.6 months among partial responders (HR, 0.57; P = .006).
Median time to next treatment also favored VR-CAP over R-CHOP among both complete responders (not evaluable vs. 26.6 months; HR, 0.42; P less than .001) and partial responders (35.3 vs. 24.3 months; HR, 0.57; P = .006), the researchers said. Further, CT scans showed that proportionally more patients in each response category became lesion-negative on VR-CAP than on R-CHOP. Among complete responders, rates of lesion negativity were 72% and 59%, respectively. Among partial responders, rates were 48% and 28%.
The effects of VR-CAP were most evident among patients with a low or medium-risk mantle cell lymphoma international prognostic index. Perhaps high-risk status signifies more rapidly proliferative disease, which negates the deeper responses with VR-CAP, compared with R-CHOP, they added.
The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
For patients with newly diagnosed mantle cell lymphoma, duration and quality of response were superior with a regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), based on a post hoc analysis of the randomized, phase III LYM-3002 trial.
The difference was especially evident among patients who had a low- or medium-risk mantle cell lymphoma international prognostic index, Gregor Verhoef, MD, of University Hospital Leuven (Belgium) and his associates wrote in Haematologica.
In LYM-3002, 487 patients with newly diagnosed stage II-IV mantle cell lymphoma received six to eight 21-day cycles of intravenous VR-CAP or R-CHOP. Although overall response rates were similar for both groups, VR-CAP was associated with better duration of response and progression-free survival (PFS) and extended time to next treatment. To further explore these differences, the post hoc analysis stratified outcomes by response categories and analyzed depth of response based on computed tomography (CT) scans. Patients had a median age of about 65 years, and most were white males with stage-IV disease at diagnosis and an Eastern Cooperative Oncology Group performance status of 1 (Haematologica. 2017 Feb 9. doi: 10.3324/haematol.2016.152496).The superiority of VR-CAP held up across response categories. Complete responders to VR-CAP had more than twice the median PFS as did complete responders to R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004). Among partial responders, median PFS was 17.1 vs. 11.7 months, respectively (HR, 0.62; 95% CI, 0.43-0.89; P = .01). Respective median duration of overall response was 42.1 months for VR-CAP vs. 18.5 months among complete responders (HR, 0.42; P less than .001), and 20.2 vs. 9.6 months among partial responders (HR, 0.57; P = .006).
Median time to next treatment also favored VR-CAP over R-CHOP among both complete responders (not evaluable vs. 26.6 months; HR, 0.42; P less than .001) and partial responders (35.3 vs. 24.3 months; HR, 0.57; P = .006), the researchers said. Further, CT scans showed that proportionally more patients in each response category became lesion-negative on VR-CAP than on R-CHOP. Among complete responders, rates of lesion negativity were 72% and 59%, respectively. Among partial responders, rates were 48% and 28%.
The effects of VR-CAP were most evident among patients with a low or medium-risk mantle cell lymphoma international prognostic index. Perhaps high-risk status signifies more rapidly proliferative disease, which negates the deeper responses with VR-CAP, compared with R-CHOP, they added.
The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
FROM HAEMATOLOGICA
Key clinical point: A regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) led to superior duration and quality of response when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with newly diagnosed mantle cell lymphoma.
Major finding: Among complete responders, median progression-free survival on VR-CAP was nearly twice that of R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004).
Data source: A post hoc analysis of a phase III trial comparing VR-CAP with R-CHOP in 487 patients with newly diagnosed, measurable stage II-IV mantle cell lymphoma (LYM-3002).
Disclosures: The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
Ibrutinib, palbociclib yield durable complete responses in pretreated mantle cell lymphoma
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
AT ASH 2016
Key clinical point: Combination therapy with ibrutinib and palbociclib was generally well tolerated and induced complete responses in patients with pretreated mantle cell lymphoma.
Major finding: A total of 44% of patients had complete responses, and 67% remained alive and progression-free after a median of 11 months of follow-up. Severe rashes occurred at the highest dose studied (420 mg ibrutinib/100 mg palbociclib).
Data source: A phase I trial of 20 patients with previously treated mantle cell lymphoma.
Disclosures: The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
Bortezomib-based regimen led to durable remissions in mantle cell lymphoma
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
Key clinical point: First-line therapy with bortezomib plus modified hyper-CVAD followed by rituximab maintenance induced durable remissions in mantle cell lymphoma.
Major finding: Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years.
Data source: A multicenter phase II trial of 30 adults with mantle cell lymphoma who were treatment-naïve or had received only one cycle of CHOP or CHOP-like chemotherapy.
Disclosures: Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
Rituximab after ASCT boosted survival in mantle cell lymphoma
SAN DIEGO – Maintenance therapy every other month with rituximab significantly prolonged event-free and overall survival after autologous stem cell transplantation among younger patients with mantle cell lymphoma, based on results from a multicenter, randomized, phase 3 trial.
After a median follow-up period of 50 months, 79% of the rituximab maintenance arm remained alive and free of progression, relapse, and severe infection, compared with 61% of the no-maintenance arm (P = .001), said Steven Le Gouill, MD, PhD, at the 2016 meeting of the American Society of Hematology.
This is the first study linking rituximab maintenance after ASCT to improved survival in younger patients with mantle cell lymphoma, said Dr. Le Gouill of Nantes University Hospital in Nantes, France.
The trial “demonstrates for the first time that rituximab maintenance after ASCT prolongs event-free survival, progression-free survival, and overall survival” in younger patients with treatment-naïve mantle cell lymphoma, Dr. Le Gouill said. The findings confirm rituximab maintenance as “a new standard of care” in younger patients with mantle cell lymphoma, he concluded.
Prior research http://www.nejm.org/doi/full/10.1056/NEJMoa1200920#t=abstract supports maintenance therapy with rituximab rather than interferon alfa for older patients whose mantle cell lymphoma responded to induction with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), noted Dr. Le Gouill. To examine outcomes in younger patients with treatment-naïve mantle cell lymphoma, he and his associates treated 299 individuals aged 65 years and younger (median age, 57 years) with standard induction consisting of 4 courses of rituximab, dexamethasone, high-dose cytarabine, and salt platinum (R-DHAP) every 21 days, followed by conditioning with rituximab plus BiCNU, etoposide, cytarabine, and melphalan (R-BEAM) and ASCT. Patients without at least a partial response to R-DHAP received 4 additional courses of R-CHOP-14 before ASCT. Patients then were randomized either to no maintenance or to infusions of 375 mg R per m2 every 2 months for 3 years.
A total of 53% of patients were mantle cell lymphoma international prognostic index (MIPI) low risk, 27% were intermediate and 19% were high-risk. Rituximab maintenance was associated with a 60% lower risk of progression (HR, 0.4; 95% CI, 0.23 to 0.68; P = .0007) and a 50% lower risk of death (HR, 0.5; 95% CI, 0.25 to 0.98; P = .04).
The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
SAN DIEGO – Maintenance therapy every other month with rituximab significantly prolonged event-free and overall survival after autologous stem cell transplantation among younger patients with mantle cell lymphoma, based on results from a multicenter, randomized, phase 3 trial.
After a median follow-up period of 50 months, 79% of the rituximab maintenance arm remained alive and free of progression, relapse, and severe infection, compared with 61% of the no-maintenance arm (P = .001), said Steven Le Gouill, MD, PhD, at the 2016 meeting of the American Society of Hematology.
This is the first study linking rituximab maintenance after ASCT to improved survival in younger patients with mantle cell lymphoma, said Dr. Le Gouill of Nantes University Hospital in Nantes, France.
The trial “demonstrates for the first time that rituximab maintenance after ASCT prolongs event-free survival, progression-free survival, and overall survival” in younger patients with treatment-naïve mantle cell lymphoma, Dr. Le Gouill said. The findings confirm rituximab maintenance as “a new standard of care” in younger patients with mantle cell lymphoma, he concluded.
Prior research http://www.nejm.org/doi/full/10.1056/NEJMoa1200920#t=abstract supports maintenance therapy with rituximab rather than interferon alfa for older patients whose mantle cell lymphoma responded to induction with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), noted Dr. Le Gouill. To examine outcomes in younger patients with treatment-naïve mantle cell lymphoma, he and his associates treated 299 individuals aged 65 years and younger (median age, 57 years) with standard induction consisting of 4 courses of rituximab, dexamethasone, high-dose cytarabine, and salt platinum (R-DHAP) every 21 days, followed by conditioning with rituximab plus BiCNU, etoposide, cytarabine, and melphalan (R-BEAM) and ASCT. Patients without at least a partial response to R-DHAP received 4 additional courses of R-CHOP-14 before ASCT. Patients then were randomized either to no maintenance or to infusions of 375 mg R per m2 every 2 months for 3 years.
A total of 53% of patients were mantle cell lymphoma international prognostic index (MIPI) low risk, 27% were intermediate and 19% were high-risk. Rituximab maintenance was associated with a 60% lower risk of progression (HR, 0.4; 95% CI, 0.23 to 0.68; P = .0007) and a 50% lower risk of death (HR, 0.5; 95% CI, 0.25 to 0.98; P = .04).
The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
SAN DIEGO – Maintenance therapy every other month with rituximab significantly prolonged event-free and overall survival after autologous stem cell transplantation among younger patients with mantle cell lymphoma, based on results from a multicenter, randomized, phase 3 trial.
After a median follow-up period of 50 months, 79% of the rituximab maintenance arm remained alive and free of progression, relapse, and severe infection, compared with 61% of the no-maintenance arm (P = .001), said Steven Le Gouill, MD, PhD, at the 2016 meeting of the American Society of Hematology.
This is the first study linking rituximab maintenance after ASCT to improved survival in younger patients with mantle cell lymphoma, said Dr. Le Gouill of Nantes University Hospital in Nantes, France.
The trial “demonstrates for the first time that rituximab maintenance after ASCT prolongs event-free survival, progression-free survival, and overall survival” in younger patients with treatment-naïve mantle cell lymphoma, Dr. Le Gouill said. The findings confirm rituximab maintenance as “a new standard of care” in younger patients with mantle cell lymphoma, he concluded.
Prior research http://www.nejm.org/doi/full/10.1056/NEJMoa1200920#t=abstract supports maintenance therapy with rituximab rather than interferon alfa for older patients whose mantle cell lymphoma responded to induction with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), noted Dr. Le Gouill. To examine outcomes in younger patients with treatment-naïve mantle cell lymphoma, he and his associates treated 299 individuals aged 65 years and younger (median age, 57 years) with standard induction consisting of 4 courses of rituximab, dexamethasone, high-dose cytarabine, and salt platinum (R-DHAP) every 21 days, followed by conditioning with rituximab plus BiCNU, etoposide, cytarabine, and melphalan (R-BEAM) and ASCT. Patients without at least a partial response to R-DHAP received 4 additional courses of R-CHOP-14 before ASCT. Patients then were randomized either to no maintenance or to infusions of 375 mg R per m2 every 2 months for 3 years.
A total of 53% of patients were mantle cell lymphoma international prognostic index (MIPI) low risk, 27% were intermediate and 19% were high-risk. Rituximab maintenance was associated with a 60% lower risk of progression (HR, 0.4; 95% CI, 0.23 to 0.68; P = .0007) and a 50% lower risk of death (HR, 0.5; 95% CI, 0.25 to 0.98; P = .04).
The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
AT ASH 2016
Key clinical point: Maintenance therapy with rituximab after autologous stem cell transplantation was associated with significantly increased survival among younger patients with mantle cell lymphoma.
Major finding: After a median follow-up time of 50 months, 79% of patients who received rituximab maintenance remained alive and free of progression, relapse, and severe infection, compared with 61% of those who received no maintenance therapy (P = .001).
Data source: A multicenter randomized phase 3 trial of 299 adults up to 65 years old with mantle cell lymphoma.
Disclosures: The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
VIDEO: First multicenter trial of CAR T cells shows response in DLBCL
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales