User login
NCCN unveils 'Evidence Blocks' to facilitate treatment discussions
SAN FRANCISCO – The National Comprehensive Cancer Network (NCCN) has introduced an easy-to-use visual tool called Evidence Blocks to help physicians and patients compare various treatment options and individualize the selection among them.
“This information can serve as a starting point for shared decision making between the patient and health care team based on individual patients’ value systems,” chief executive officer Dr. Robert W. Carlson said at the NCCN Annual Congress: Hematologic Malignancies, where the tool was unveiled in a session and related press conference.
The first two NCCN guidelines to incorporate the Evidence Blocks – those for multiple myeloma and chronic myelogenous leukemia – were released at the same time. The organization hopes to incorporate them into all of its guidelines by early 2017, he said.
Development of the Evidence Blocks
The NCCN developed the Evidence Blocks to address requests from various stakeholders, according to Dr. Carlson. Guideline users wanted to know more about the rationale behind recommended therapies, asked for inclusion of information on costs, and sought an aid that would allow patients to make decisions based on their individual values.
“The patient perception of value is what should be most important to us,” he commented. “But even among patients, the concept of value differs greatly from patient to patient,” based on factors such as age, comorbidities, treatment goals, and health insurance coverage.
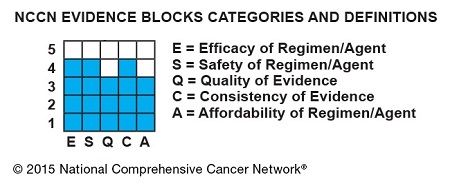
Each Evidence Block graphically displays five measures of information on a recommended therapy: efficacy, safety, quality of the evidence supporting the recommendation, consistency of the evidence supporting the recommendation, and affordability. Each column in the block represents one measure.
Blue shading indicates panelists’ average numeric score for the therapy on that measure rounded to the nearest integer, ranging from 1 (least favorable) to 5 (most favorable). Therefore, the more shading a therapy has, the more favorable its score.
The Evidence Blocks are added to the guidelines and aligned vertically on pages. “This display of the information graphically allows for very efficient scanning of multiple options for therapy. Comparisons across several regimens can be done very quickly and intuitively,” Dr. Carlson noted.
“We believe that the presentation of this type of information allows the health care provider and patient to make their own judgments of the value of specific interventions,” he added.
The affordability measure has generated the most discussion among panelists and stakeholders, according to Dr. Carlson. For this measure, panelists estimated the total cost of care for a therapy, including the costs of drugs, administration, required supportive care, toxicity monitoring, and care associated with management of toxicity. Scores range from very expensive to very inexpensive.
“We don’t use a dollar amount. Rather, it’s sort of what’s the total cost to society, if you will, of the medical intervention part of this,” he explained. “It’s important to understand that these estimates are not necessarily what a patient would pay because many patients have insurance programs that cover all of this cost.… However, we felt it was important to give patients as well as providers an estimate of what the overall magnitude of expense is because there are patients who have huge deductibles, there’s the doughnut hole within Medicare, and there are patients who have no insurance.”
The Evidence Blocks may help address a “conspiracy of silence” between physicians and patients when it comes to discussing treatment costs, whereby neither party wants to bring up this thorny issue, according to Dr. Carlson. “The Evidence Blocks demystify the discussion of cost because the affordability issue is there in front of you. So it gives people permission to talk about cost and affordability.”
It should be relatively easy to teach patients to use the Evidence Blocks. “I think you’ll find your patients will actually be interested in this and that they will not have as much difficulty interpreting this as you think they will, because the patient advocacy groups and the patient advocates that we have spoken with about this, they get this almost instantly,” he said.
Oncologist perspective
There is a critical need for tools such as the Evidence Blocks in making treatment decisions today, according to Dr. George Somlo, professor in the department of medical oncology and therapeutics research at the City of Hope Comprehensive Cancer Center in Duarte, Calif., and also a member of the NCCN multiple myeloma and breast cancer guideline panels.
Treatment options for multiple myeloma, as for many cancers, have exploded in the past few decades, he noted. “How do you go from making sense of having two drugs with a very poor outcome predicted to having literally dozens of agents approved and used in combination, and in essence being at the verge of curing patients with multiple myeloma?”
Dr. Somlo agreed that inclusion of costs in the Evidence Blocks would likely be beneficial as a conversation starter, recalling, “I’ve had patients who did not fill their prescription for a potentially curative medication because they were worried about the $2,500 or $3,500 copay.”
Patient-physician discussion will be important when it comes to using information from the new tool, he said. For example, in the NCCN guideline for multiple myeloma, some of the first-line regimens have identical Evidence Blocks; thus, consideration of factors such as comorbidities will become important.
“This kind of evidence-based scoring system can guide that kind of discussion with the patient and can tailor the individual therapeutic regimens,” he concluded.
Patient perspective
Breast cancer survivor Marta Nichols, who is vice president of investor relations at GoDaddy and a member of the California Breast Cancer Research Council based in San Francisco, welcomed the Evidence Blocks as a tool that will allow patients to make more informed decisions according to what matters most to them.
Only 33 years old at diagnosis, she and her husband had just begun to think about starting a family. “So my primary concern coming into my physician’s office was my fertility and what impact the treatment would have on my fertility. Certainly most physicians are concerned with efficacy – they want to see you survive. My concern was not just surviving, but also thriving and being able to give birth to children down the line,” she explained.
Patients today are overwhelmed not only by their cancer diagnosis, but also by the many treatment options and the new emphasis on shared decision making, Ms. Nichols noted. And that’s where the Evidence Blocks can make a difference.
“When I was diagnosed, it would have been hugely helpful for me to have information laid out in this very clear and systematic way… It would have given us the ability to make a much more informed decision,” she commented.
Multiple myeloma survivor Donald B. Orosco, who is president and chief financial officer of Orosco & Associates and owner of Monterey (Calif.) Speed and Sport, agreed, noting that his priorities when given the diagnosis more than two decades ago at age 47 differed somewhat.
“I adopted the feeling early on that I probably wasn’t going to see a cure for the disease in my lifetime, but I could accept that,” he elaborated. “I just said ‘Really, I’m interested in quality-of-life issues. I’d like to see my kids go into high school or possibly college.’ So I adopted [an approach of] trying to find something for me that would keep me alive and give me a relatively comfortable quality of life, that would allow me to continue to race cars or do whatever I had to do.”
Dr. Carlson, Dr. Somlo, Ms. Nichols, and Mr. Orosco disclosed no relevant conflicts of interest.
SAN FRANCISCO – The National Comprehensive Cancer Network (NCCN) has introduced an easy-to-use visual tool called Evidence Blocks to help physicians and patients compare various treatment options and individualize the selection among them.
“This information can serve as a starting point for shared decision making between the patient and health care team based on individual patients’ value systems,” chief executive officer Dr. Robert W. Carlson said at the NCCN Annual Congress: Hematologic Malignancies, where the tool was unveiled in a session and related press conference.
The first two NCCN guidelines to incorporate the Evidence Blocks – those for multiple myeloma and chronic myelogenous leukemia – were released at the same time. The organization hopes to incorporate them into all of its guidelines by early 2017, he said.
Development of the Evidence Blocks
The NCCN developed the Evidence Blocks to address requests from various stakeholders, according to Dr. Carlson. Guideline users wanted to know more about the rationale behind recommended therapies, asked for inclusion of information on costs, and sought an aid that would allow patients to make decisions based on their individual values.
“The patient perception of value is what should be most important to us,” he commented. “But even among patients, the concept of value differs greatly from patient to patient,” based on factors such as age, comorbidities, treatment goals, and health insurance coverage.
Each Evidence Block graphically displays five measures of information on a recommended therapy: efficacy, safety, quality of the evidence supporting the recommendation, consistency of the evidence supporting the recommendation, and affordability. Each column in the block represents one measure.
Blue shading indicates panelists’ average numeric score for the therapy on that measure rounded to the nearest integer, ranging from 1 (least favorable) to 5 (most favorable). Therefore, the more shading a therapy has, the more favorable its score.
The Evidence Blocks are added to the guidelines and aligned vertically on pages. “This display of the information graphically allows for very efficient scanning of multiple options for therapy. Comparisons across several regimens can be done very quickly and intuitively,” Dr. Carlson noted.
“We believe that the presentation of this type of information allows the health care provider and patient to make their own judgments of the value of specific interventions,” he added.
The affordability measure has generated the most discussion among panelists and stakeholders, according to Dr. Carlson. For this measure, panelists estimated the total cost of care for a therapy, including the costs of drugs, administration, required supportive care, toxicity monitoring, and care associated with management of toxicity. Scores range from very expensive to very inexpensive.
“We don’t use a dollar amount. Rather, it’s sort of what’s the total cost to society, if you will, of the medical intervention part of this,” he explained. “It’s important to understand that these estimates are not necessarily what a patient would pay because many patients have insurance programs that cover all of this cost.… However, we felt it was important to give patients as well as providers an estimate of what the overall magnitude of expense is because there are patients who have huge deductibles, there’s the doughnut hole within Medicare, and there are patients who have no insurance.”
The Evidence Blocks may help address a “conspiracy of silence” between physicians and patients when it comes to discussing treatment costs, whereby neither party wants to bring up this thorny issue, according to Dr. Carlson. “The Evidence Blocks demystify the discussion of cost because the affordability issue is there in front of you. So it gives people permission to talk about cost and affordability.”
It should be relatively easy to teach patients to use the Evidence Blocks. “I think you’ll find your patients will actually be interested in this and that they will not have as much difficulty interpreting this as you think they will, because the patient advocacy groups and the patient advocates that we have spoken with about this, they get this almost instantly,” he said.
Oncologist perspective
There is a critical need for tools such as the Evidence Blocks in making treatment decisions today, according to Dr. George Somlo, professor in the department of medical oncology and therapeutics research at the City of Hope Comprehensive Cancer Center in Duarte, Calif., and also a member of the NCCN multiple myeloma and breast cancer guideline panels.
Treatment options for multiple myeloma, as for many cancers, have exploded in the past few decades, he noted. “How do you go from making sense of having two drugs with a very poor outcome predicted to having literally dozens of agents approved and used in combination, and in essence being at the verge of curing patients with multiple myeloma?”
Dr. Somlo agreed that inclusion of costs in the Evidence Blocks would likely be beneficial as a conversation starter, recalling, “I’ve had patients who did not fill their prescription for a potentially curative medication because they were worried about the $2,500 or $3,500 copay.”
Patient-physician discussion will be important when it comes to using information from the new tool, he said. For example, in the NCCN guideline for multiple myeloma, some of the first-line regimens have identical Evidence Blocks; thus, consideration of factors such as comorbidities will become important.
“This kind of evidence-based scoring system can guide that kind of discussion with the patient and can tailor the individual therapeutic regimens,” he concluded.
Patient perspective
Breast cancer survivor Marta Nichols, who is vice president of investor relations at GoDaddy and a member of the California Breast Cancer Research Council based in San Francisco, welcomed the Evidence Blocks as a tool that will allow patients to make more informed decisions according to what matters most to them.
Only 33 years old at diagnosis, she and her husband had just begun to think about starting a family. “So my primary concern coming into my physician’s office was my fertility and what impact the treatment would have on my fertility. Certainly most physicians are concerned with efficacy – they want to see you survive. My concern was not just surviving, but also thriving and being able to give birth to children down the line,” she explained.
Patients today are overwhelmed not only by their cancer diagnosis, but also by the many treatment options and the new emphasis on shared decision making, Ms. Nichols noted. And that’s where the Evidence Blocks can make a difference.
“When I was diagnosed, it would have been hugely helpful for me to have information laid out in this very clear and systematic way… It would have given us the ability to make a much more informed decision,” she commented.
Multiple myeloma survivor Donald B. Orosco, who is president and chief financial officer of Orosco & Associates and owner of Monterey (Calif.) Speed and Sport, agreed, noting that his priorities when given the diagnosis more than two decades ago at age 47 differed somewhat.
“I adopted the feeling early on that I probably wasn’t going to see a cure for the disease in my lifetime, but I could accept that,” he elaborated. “I just said ‘Really, I’m interested in quality-of-life issues. I’d like to see my kids go into high school or possibly college.’ So I adopted [an approach of] trying to find something for me that would keep me alive and give me a relatively comfortable quality of life, that would allow me to continue to race cars or do whatever I had to do.”
Dr. Carlson, Dr. Somlo, Ms. Nichols, and Mr. Orosco disclosed no relevant conflicts of interest.
SAN FRANCISCO – The National Comprehensive Cancer Network (NCCN) has introduced an easy-to-use visual tool called Evidence Blocks to help physicians and patients compare various treatment options and individualize the selection among them.
“This information can serve as a starting point for shared decision making between the patient and health care team based on individual patients’ value systems,” chief executive officer Dr. Robert W. Carlson said at the NCCN Annual Congress: Hematologic Malignancies, where the tool was unveiled in a session and related press conference.
The first two NCCN guidelines to incorporate the Evidence Blocks – those for multiple myeloma and chronic myelogenous leukemia – were released at the same time. The organization hopes to incorporate them into all of its guidelines by early 2017, he said.
Development of the Evidence Blocks
The NCCN developed the Evidence Blocks to address requests from various stakeholders, according to Dr. Carlson. Guideline users wanted to know more about the rationale behind recommended therapies, asked for inclusion of information on costs, and sought an aid that would allow patients to make decisions based on their individual values.
“The patient perception of value is what should be most important to us,” he commented. “But even among patients, the concept of value differs greatly from patient to patient,” based on factors such as age, comorbidities, treatment goals, and health insurance coverage.
Each Evidence Block graphically displays five measures of information on a recommended therapy: efficacy, safety, quality of the evidence supporting the recommendation, consistency of the evidence supporting the recommendation, and affordability. Each column in the block represents one measure.
Blue shading indicates panelists’ average numeric score for the therapy on that measure rounded to the nearest integer, ranging from 1 (least favorable) to 5 (most favorable). Therefore, the more shading a therapy has, the more favorable its score.
The Evidence Blocks are added to the guidelines and aligned vertically on pages. “This display of the information graphically allows for very efficient scanning of multiple options for therapy. Comparisons across several regimens can be done very quickly and intuitively,” Dr. Carlson noted.
“We believe that the presentation of this type of information allows the health care provider and patient to make their own judgments of the value of specific interventions,” he added.
The affordability measure has generated the most discussion among panelists and stakeholders, according to Dr. Carlson. For this measure, panelists estimated the total cost of care for a therapy, including the costs of drugs, administration, required supportive care, toxicity monitoring, and care associated with management of toxicity. Scores range from very expensive to very inexpensive.
“We don’t use a dollar amount. Rather, it’s sort of what’s the total cost to society, if you will, of the medical intervention part of this,” he explained. “It’s important to understand that these estimates are not necessarily what a patient would pay because many patients have insurance programs that cover all of this cost.… However, we felt it was important to give patients as well as providers an estimate of what the overall magnitude of expense is because there are patients who have huge deductibles, there’s the doughnut hole within Medicare, and there are patients who have no insurance.”
The Evidence Blocks may help address a “conspiracy of silence” between physicians and patients when it comes to discussing treatment costs, whereby neither party wants to bring up this thorny issue, according to Dr. Carlson. “The Evidence Blocks demystify the discussion of cost because the affordability issue is there in front of you. So it gives people permission to talk about cost and affordability.”
It should be relatively easy to teach patients to use the Evidence Blocks. “I think you’ll find your patients will actually be interested in this and that they will not have as much difficulty interpreting this as you think they will, because the patient advocacy groups and the patient advocates that we have spoken with about this, they get this almost instantly,” he said.
Oncologist perspective
There is a critical need for tools such as the Evidence Blocks in making treatment decisions today, according to Dr. George Somlo, professor in the department of medical oncology and therapeutics research at the City of Hope Comprehensive Cancer Center in Duarte, Calif., and also a member of the NCCN multiple myeloma and breast cancer guideline panels.
Treatment options for multiple myeloma, as for many cancers, have exploded in the past few decades, he noted. “How do you go from making sense of having two drugs with a very poor outcome predicted to having literally dozens of agents approved and used in combination, and in essence being at the verge of curing patients with multiple myeloma?”
Dr. Somlo agreed that inclusion of costs in the Evidence Blocks would likely be beneficial as a conversation starter, recalling, “I’ve had patients who did not fill their prescription for a potentially curative medication because they were worried about the $2,500 or $3,500 copay.”
Patient-physician discussion will be important when it comes to using information from the new tool, he said. For example, in the NCCN guideline for multiple myeloma, some of the first-line regimens have identical Evidence Blocks; thus, consideration of factors such as comorbidities will become important.
“This kind of evidence-based scoring system can guide that kind of discussion with the patient and can tailor the individual therapeutic regimens,” he concluded.
Patient perspective
Breast cancer survivor Marta Nichols, who is vice president of investor relations at GoDaddy and a member of the California Breast Cancer Research Council based in San Francisco, welcomed the Evidence Blocks as a tool that will allow patients to make more informed decisions according to what matters most to them.
Only 33 years old at diagnosis, she and her husband had just begun to think about starting a family. “So my primary concern coming into my physician’s office was my fertility and what impact the treatment would have on my fertility. Certainly most physicians are concerned with efficacy – they want to see you survive. My concern was not just surviving, but also thriving and being able to give birth to children down the line,” she explained.
Patients today are overwhelmed not only by their cancer diagnosis, but also by the many treatment options and the new emphasis on shared decision making, Ms. Nichols noted. And that’s where the Evidence Blocks can make a difference.
“When I was diagnosed, it would have been hugely helpful for me to have information laid out in this very clear and systematic way… It would have given us the ability to make a much more informed decision,” she commented.
Multiple myeloma survivor Donald B. Orosco, who is president and chief financial officer of Orosco & Associates and owner of Monterey (Calif.) Speed and Sport, agreed, noting that his priorities when given the diagnosis more than two decades ago at age 47 differed somewhat.
“I adopted the feeling early on that I probably wasn’t going to see a cure for the disease in my lifetime, but I could accept that,” he elaborated. “I just said ‘Really, I’m interested in quality-of-life issues. I’d like to see my kids go into high school or possibly college.’ So I adopted [an approach of] trying to find something for me that would keep me alive and give me a relatively comfortable quality of life, that would allow me to continue to race cars or do whatever I had to do.”
Dr. Carlson, Dr. Somlo, Ms. Nichols, and Mr. Orosco disclosed no relevant conflicts of interest.
AT NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
NCCN creates tool to aid treatment decisions

patient and her father
Photo by Rhoda Baer
The National Comprehensive Cancer Network (NCCN) has developed a new tool to accompany its clinical practice guidelines.
The tool—known as NCCN Evidence Blocks™—is designed to provide additional information about guideline recommendations and help inform treatment decisions.
NCCN has already added Evidence Blocks to its guidelines for chronic myelogenous leukemia and multiple myeloma.
The organization hopes to have Evidence Blocks for all of its guidelines by early 2017.
The Evidence Blocks provide a visual representation of 5 key value measures pertaining to guideline recommendations:
- Efficacy of treatment regimens
- Safety of regimens
- Quality and quantity of evidence supporting regimens
- Consistency of evidence supporting regimens
- Affordability of regimens. (This represents an estimate of overall total cost of a therapy, including but not limited to acquisition, administration, inpatient vs outpatient care, supportive care, infusions, toxicity monitoring, antiemetics and growth factors, and hospitalization.)
The Evidence Blocks are graphics of actual blocks that consist of 25 small squares. So each block has 5 rows and 5 columns.
Each of the 5 value measures—efficacy, safety, etc.—has a dedicated column within an Evidence Block, and each row of the Evidence Block represents a rating on a scale of 1 to 5. A score of 1 is unfavorable and a score of 5 is most favorable.
The rating of each value measure is shown by filling in the squares of the dedicated column—such as efficacy—up to the row that represents its assigned score—such as 4.
For example:
NCCN hopes this visual rating system will help patients and their physicians identify the optimal treatment based on clinical and economic considerations that are of the most value to the patient.
“Some patients will want an emerging therapy even with limited data,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“Others will be most concerned about the expected side effects of the treatment indicated in the safety column. Still others may be very sensitive to cost. By considering the attributes of the range of possible therapies, the healthcare provider and the patient can discuss the benefits and drawbacks of each option and come to a decision most acceptable to the individual.”
By the end of 2015, NCCN expects to publish NCCN Evidence Blocks for systemic therapies (not surgery or radiation therapy) in the NCCN guidelines for breast, colon, non-small cell lung, and rectal cancers.
NCCN Evidence Blocks for systemic therapies are expected to be contained within the complete library of NCCN guidelines by the end of 2016.
In the near term, NCCN will continue to publish 2 sets of guidelines: those including NCCN Evidence Blocks and those without. The Evidence Blocks are not currently published in the NCCN Guidelines for Patients® and are intended for use in the US only.
For more information about NCCN Evidence Blocks, visit NCCN.org/EvidenceBlocks. ![]()

patient and her father
Photo by Rhoda Baer
The National Comprehensive Cancer Network (NCCN) has developed a new tool to accompany its clinical practice guidelines.
The tool—known as NCCN Evidence Blocks™—is designed to provide additional information about guideline recommendations and help inform treatment decisions.
NCCN has already added Evidence Blocks to its guidelines for chronic myelogenous leukemia and multiple myeloma.
The organization hopes to have Evidence Blocks for all of its guidelines by early 2017.
The Evidence Blocks provide a visual representation of 5 key value measures pertaining to guideline recommendations:
- Efficacy of treatment regimens
- Safety of regimens
- Quality and quantity of evidence supporting regimens
- Consistency of evidence supporting regimens
- Affordability of regimens. (This represents an estimate of overall total cost of a therapy, including but not limited to acquisition, administration, inpatient vs outpatient care, supportive care, infusions, toxicity monitoring, antiemetics and growth factors, and hospitalization.)
The Evidence Blocks are graphics of actual blocks that consist of 25 small squares. So each block has 5 rows and 5 columns.
Each of the 5 value measures—efficacy, safety, etc.—has a dedicated column within an Evidence Block, and each row of the Evidence Block represents a rating on a scale of 1 to 5. A score of 1 is unfavorable and a score of 5 is most favorable.
The rating of each value measure is shown by filling in the squares of the dedicated column—such as efficacy—up to the row that represents its assigned score—such as 4.
For example:

NCCN hopes this visual rating system will help patients and their physicians identify the optimal treatment based on clinical and economic considerations that are of the most value to the patient.
“Some patients will want an emerging therapy even with limited data,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“Others will be most concerned about the expected side effects of the treatment indicated in the safety column. Still others may be very sensitive to cost. By considering the attributes of the range of possible therapies, the healthcare provider and the patient can discuss the benefits and drawbacks of each option and come to a decision most acceptable to the individual.”
By the end of 2015, NCCN expects to publish NCCN Evidence Blocks for systemic therapies (not surgery or radiation therapy) in the NCCN guidelines for breast, colon, non-small cell lung, and rectal cancers.
NCCN Evidence Blocks for systemic therapies are expected to be contained within the complete library of NCCN guidelines by the end of 2016.
In the near term, NCCN will continue to publish 2 sets of guidelines: those including NCCN Evidence Blocks and those without. The Evidence Blocks are not currently published in the NCCN Guidelines for Patients® and are intended for use in the US only.
For more information about NCCN Evidence Blocks, visit NCCN.org/EvidenceBlocks. ![]()

patient and her father
Photo by Rhoda Baer
The National Comprehensive Cancer Network (NCCN) has developed a new tool to accompany its clinical practice guidelines.
The tool—known as NCCN Evidence Blocks™—is designed to provide additional information about guideline recommendations and help inform treatment decisions.
NCCN has already added Evidence Blocks to its guidelines for chronic myelogenous leukemia and multiple myeloma.
The organization hopes to have Evidence Blocks for all of its guidelines by early 2017.
The Evidence Blocks provide a visual representation of 5 key value measures pertaining to guideline recommendations:
- Efficacy of treatment regimens
- Safety of regimens
- Quality and quantity of evidence supporting regimens
- Consistency of evidence supporting regimens
- Affordability of regimens. (This represents an estimate of overall total cost of a therapy, including but not limited to acquisition, administration, inpatient vs outpatient care, supportive care, infusions, toxicity monitoring, antiemetics and growth factors, and hospitalization.)
The Evidence Blocks are graphics of actual blocks that consist of 25 small squares. So each block has 5 rows and 5 columns.
Each of the 5 value measures—efficacy, safety, etc.—has a dedicated column within an Evidence Block, and each row of the Evidence Block represents a rating on a scale of 1 to 5. A score of 1 is unfavorable and a score of 5 is most favorable.
The rating of each value measure is shown by filling in the squares of the dedicated column—such as efficacy—up to the row that represents its assigned score—such as 4.
For example:

NCCN hopes this visual rating system will help patients and their physicians identify the optimal treatment based on clinical and economic considerations that are of the most value to the patient.
“Some patients will want an emerging therapy even with limited data,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“Others will be most concerned about the expected side effects of the treatment indicated in the safety column. Still others may be very sensitive to cost. By considering the attributes of the range of possible therapies, the healthcare provider and the patient can discuss the benefits and drawbacks of each option and come to a decision most acceptable to the individual.”
By the end of 2015, NCCN expects to publish NCCN Evidence Blocks for systemic therapies (not surgery or radiation therapy) in the NCCN guidelines for breast, colon, non-small cell lung, and rectal cancers.
NCCN Evidence Blocks for systemic therapies are expected to be contained within the complete library of NCCN guidelines by the end of 2016.
In the near term, NCCN will continue to publish 2 sets of guidelines: those including NCCN Evidence Blocks and those without. The Evidence Blocks are not currently published in the NCCN Guidelines for Patients® and are intended for use in the US only.
For more information about NCCN Evidence Blocks, visit NCCN.org/EvidenceBlocks. ![]()
Cancer survivors have poor diets, study suggests

New research suggests that cancer survivors in the US may need dietary interventions to improve their health.
The study showed that, overall, cancer survivors did not adhere to federal dietary guidelines as well as a matched control population.
Cancer survivors tended to consume more empty calories and less fiber than controls.
Fang Fang Zhang, MD, PhD, of Tufts University in Boston, Massachusetts, and his colleagues reported these findings in Cancer.
The team evaluated the diets of 1533 adult cancer survivors who participated in the National Health and Nutrition Examination Survey from 1999 to 2010. The investigators also assessed the diets of 3075 individuals who had no history of cancer and were matched to the cancer survivors by age, sex, and race/ethnicity.
The goal was to determine how subjects’ diets aligned with advice from the 2010 Dietary Guidelines for Americans, which was jointly issued by the Department of Agriculture and the Department of Health and Human Services.
Cancer survivors had poor adherence to the guidelines, with a total Healthy Eating Index score of 47.2 out of 100, compared with a score of 48.3 in the adults without a history of cancer (P=0.03).
Cancer survivors had a significantly lower mean score for empty calories compared to the noncancer group—13.6 and 14.4, respectively (P=0.001). This suggested the cancer group had a higher consumption of calories from solid fats, alcohol, and added sugars.
Cancer survivors also had significantly lower dietary intake of fiber than the noncancer group—15.0 and 15.9 g per day, respectively (P=0.02).
Compared to recommended values, cancer survivors had low dietary intakes of vitamin D (31% of the recommended intake), vitamin E (47%), potassium (55%), and calcium (73%) but high intakes of saturated fat (112%) and sodium (133%).
The investigators noted that diet quality in cancer survivors increased linearly with age. The older the age, the better the diet quality.
Survivors with lower education (high school or less) had significantly worse diet quality than those with higher education. And survivors who were current smokers had significantly worse diet quality than non-smokers or former smokers.
For the 4 major cancer types in the US (breast, prostate, lung, and colorectal), breast cancer survivors had the best diet quality, and lung cancer survivors had the worst diet quality.
The investigators said that knowing how well cancer survivors adhere to federal dietary guidelines can help inform evidence-based priorities for improving their nutritional intake.
“Dietary changes that include more fiber, fruit, and vegetables in the diet and less fat, sodium, and added sugar would be important for cancer survivors,” Dr Zhang said.
“Oncology care providers can play critical roles in reinforcing the importance of a healthful diet and can refer patients to registered dietitians who are experts in oncology care or to other reputable sources in order to improve survivors’ overall health.” ![]()

New research suggests that cancer survivors in the US may need dietary interventions to improve their health.
The study showed that, overall, cancer survivors did not adhere to federal dietary guidelines as well as a matched control population.
Cancer survivors tended to consume more empty calories and less fiber than controls.
Fang Fang Zhang, MD, PhD, of Tufts University in Boston, Massachusetts, and his colleagues reported these findings in Cancer.
The team evaluated the diets of 1533 adult cancer survivors who participated in the National Health and Nutrition Examination Survey from 1999 to 2010. The investigators also assessed the diets of 3075 individuals who had no history of cancer and were matched to the cancer survivors by age, sex, and race/ethnicity.
The goal was to determine how subjects’ diets aligned with advice from the 2010 Dietary Guidelines for Americans, which was jointly issued by the Department of Agriculture and the Department of Health and Human Services.
Cancer survivors had poor adherence to the guidelines, with a total Healthy Eating Index score of 47.2 out of 100, compared with a score of 48.3 in the adults without a history of cancer (P=0.03).
Cancer survivors had a significantly lower mean score for empty calories compared to the noncancer group—13.6 and 14.4, respectively (P=0.001). This suggested the cancer group had a higher consumption of calories from solid fats, alcohol, and added sugars.
Cancer survivors also had significantly lower dietary intake of fiber than the noncancer group—15.0 and 15.9 g per day, respectively (P=0.02).
Compared to recommended values, cancer survivors had low dietary intakes of vitamin D (31% of the recommended intake), vitamin E (47%), potassium (55%), and calcium (73%) but high intakes of saturated fat (112%) and sodium (133%).
The investigators noted that diet quality in cancer survivors increased linearly with age. The older the age, the better the diet quality.
Survivors with lower education (high school or less) had significantly worse diet quality than those with higher education. And survivors who were current smokers had significantly worse diet quality than non-smokers or former smokers.
For the 4 major cancer types in the US (breast, prostate, lung, and colorectal), breast cancer survivors had the best diet quality, and lung cancer survivors had the worst diet quality.
The investigators said that knowing how well cancer survivors adhere to federal dietary guidelines can help inform evidence-based priorities for improving their nutritional intake.
“Dietary changes that include more fiber, fruit, and vegetables in the diet and less fat, sodium, and added sugar would be important for cancer survivors,” Dr Zhang said.
“Oncology care providers can play critical roles in reinforcing the importance of a healthful diet and can refer patients to registered dietitians who are experts in oncology care or to other reputable sources in order to improve survivors’ overall health.” ![]()

New research suggests that cancer survivors in the US may need dietary interventions to improve their health.
The study showed that, overall, cancer survivors did not adhere to federal dietary guidelines as well as a matched control population.
Cancer survivors tended to consume more empty calories and less fiber than controls.
Fang Fang Zhang, MD, PhD, of Tufts University in Boston, Massachusetts, and his colleagues reported these findings in Cancer.
The team evaluated the diets of 1533 adult cancer survivors who participated in the National Health and Nutrition Examination Survey from 1999 to 2010. The investigators also assessed the diets of 3075 individuals who had no history of cancer and were matched to the cancer survivors by age, sex, and race/ethnicity.
The goal was to determine how subjects’ diets aligned with advice from the 2010 Dietary Guidelines for Americans, which was jointly issued by the Department of Agriculture and the Department of Health and Human Services.
Cancer survivors had poor adherence to the guidelines, with a total Healthy Eating Index score of 47.2 out of 100, compared with a score of 48.3 in the adults without a history of cancer (P=0.03).
Cancer survivors had a significantly lower mean score for empty calories compared to the noncancer group—13.6 and 14.4, respectively (P=0.001). This suggested the cancer group had a higher consumption of calories from solid fats, alcohol, and added sugars.
Cancer survivors also had significantly lower dietary intake of fiber than the noncancer group—15.0 and 15.9 g per day, respectively (P=0.02).
Compared to recommended values, cancer survivors had low dietary intakes of vitamin D (31% of the recommended intake), vitamin E (47%), potassium (55%), and calcium (73%) but high intakes of saturated fat (112%) and sodium (133%).
The investigators noted that diet quality in cancer survivors increased linearly with age. The older the age, the better the diet quality.
Survivors with lower education (high school or less) had significantly worse diet quality than those with higher education. And survivors who were current smokers had significantly worse diet quality than non-smokers or former smokers.
For the 4 major cancer types in the US (breast, prostate, lung, and colorectal), breast cancer survivors had the best diet quality, and lung cancer survivors had the worst diet quality.
The investigators said that knowing how well cancer survivors adhere to federal dietary guidelines can help inform evidence-based priorities for improving their nutritional intake.
“Dietary changes that include more fiber, fruit, and vegetables in the diet and less fat, sodium, and added sugar would be important for cancer survivors,” Dr Zhang said.
“Oncology care providers can play critical roles in reinforcing the importance of a healthful diet and can refer patients to registered dietitians who are experts in oncology care or to other reputable sources in order to improve survivors’ overall health.” ![]()
Regimen may lengthen survival in AL amyloidosis

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()
Baseline factors predict early response to multiple myeloma therapy
CHICAGO – Age and the results of select baseline lab measures reflected early response to initial therapy in a review of 1,304 newly diagnosed patients with symptomatic multiple myeloma.
Characteristics associated with increased odds of achieving very good partial response or better (VGPR+) within four cycles of treatment in the study participants, who were seen between 2001 and 2013, were absolute free light chain difference of more than 175 mg/dL (odds ratio, 2.38), age less than 75 years at diagnosis (OR, 2.18), hemoglobin concentration less than 10/12.5 (OR, 1.68), and IgA vs. IgG serum heavy chain secretion (OR, 1.66), Dr. Moritz Binder of the Mayo Clinic, Rochester, Minn., reported in a poster at the American Society of Hematology Meeting on Hematologic Malignancies.
In patients receiving proteasome inhibitors, better response was associated with creatinine concentrations greater than 1.5 mg/dL (OR, 3.83), calcium concentration less than 9.0 mg/dL (OR, 3.37), and greater absolute free light chain difference greater than 175 mg/dL (OR, 2.50), Dr. Binder noted.
High-risk cytogenetic features, conversely, were not associated with treatment response, he said.
The findings are important because the initial response to therapy in newly diagnosed disease can have an effect on long-term outcomes; achieving at least a VGPR to initial treatment can improve progression-free and overall survival. Further, novel agents and risk-adapted treatment strategies now in use have improved response rates and overall survival, Dr. Binder said, adding that it has been unclear, however, whether baseline laboratory parameters could predict the likelihood of early, deep response to initial therapy.
“The ability to predict the likelihood of response to the current therapies can have implications for the treatment approaches in NDMM,” he wrote.
In this study, 288 patients achieved VGPR+ after 4 months, and those patients had a decreased risk of subsequent mortality (hazard ratio, 0.69). This remained true after adjusting for sex, age, International Staging System stage, bone marrow plasma cell involvement, lactate dehydrogenase concentration, initial treatment regimen group, and hematopoietic stem cell transplantation during the disease course (HR, 0.68), he said.
The three most common regimens with immunomodulators that were used in the cohort were lenalidomide-dexamethasone, thalidomide-dexamethasone, and cyclophosphamide-lenalidomide-dexamethasone, and the three most common regimens with proteasome inhibitors were bortezomib-cyclophosphamide-dexamethasone, bortezomib-lenalidomide-dexamethasone, and bortezomib-dexamethasone.
Dr. Binder reported receiving research funding from ASH. Coauthors reported receiving research funding from Celgene, Janssen, Millennium, and Pfizer, and/or serving in an advisory role for Pfizer.
CHICAGO – Age and the results of select baseline lab measures reflected early response to initial therapy in a review of 1,304 newly diagnosed patients with symptomatic multiple myeloma.
Characteristics associated with increased odds of achieving very good partial response or better (VGPR+) within four cycles of treatment in the study participants, who were seen between 2001 and 2013, were absolute free light chain difference of more than 175 mg/dL (odds ratio, 2.38), age less than 75 years at diagnosis (OR, 2.18), hemoglobin concentration less than 10/12.5 (OR, 1.68), and IgA vs. IgG serum heavy chain secretion (OR, 1.66), Dr. Moritz Binder of the Mayo Clinic, Rochester, Minn., reported in a poster at the American Society of Hematology Meeting on Hematologic Malignancies.
In patients receiving proteasome inhibitors, better response was associated with creatinine concentrations greater than 1.5 mg/dL (OR, 3.83), calcium concentration less than 9.0 mg/dL (OR, 3.37), and greater absolute free light chain difference greater than 175 mg/dL (OR, 2.50), Dr. Binder noted.
High-risk cytogenetic features, conversely, were not associated with treatment response, he said.
The findings are important because the initial response to therapy in newly diagnosed disease can have an effect on long-term outcomes; achieving at least a VGPR to initial treatment can improve progression-free and overall survival. Further, novel agents and risk-adapted treatment strategies now in use have improved response rates and overall survival, Dr. Binder said, adding that it has been unclear, however, whether baseline laboratory parameters could predict the likelihood of early, deep response to initial therapy.
“The ability to predict the likelihood of response to the current therapies can have implications for the treatment approaches in NDMM,” he wrote.
In this study, 288 patients achieved VGPR+ after 4 months, and those patients had a decreased risk of subsequent mortality (hazard ratio, 0.69). This remained true after adjusting for sex, age, International Staging System stage, bone marrow plasma cell involvement, lactate dehydrogenase concentration, initial treatment regimen group, and hematopoietic stem cell transplantation during the disease course (HR, 0.68), he said.
The three most common regimens with immunomodulators that were used in the cohort were lenalidomide-dexamethasone, thalidomide-dexamethasone, and cyclophosphamide-lenalidomide-dexamethasone, and the three most common regimens with proteasome inhibitors were bortezomib-cyclophosphamide-dexamethasone, bortezomib-lenalidomide-dexamethasone, and bortezomib-dexamethasone.
Dr. Binder reported receiving research funding from ASH. Coauthors reported receiving research funding from Celgene, Janssen, Millennium, and Pfizer, and/or serving in an advisory role for Pfizer.
CHICAGO – Age and the results of select baseline lab measures reflected early response to initial therapy in a review of 1,304 newly diagnosed patients with symptomatic multiple myeloma.
Characteristics associated with increased odds of achieving very good partial response or better (VGPR+) within four cycles of treatment in the study participants, who were seen between 2001 and 2013, were absolute free light chain difference of more than 175 mg/dL (odds ratio, 2.38), age less than 75 years at diagnosis (OR, 2.18), hemoglobin concentration less than 10/12.5 (OR, 1.68), and IgA vs. IgG serum heavy chain secretion (OR, 1.66), Dr. Moritz Binder of the Mayo Clinic, Rochester, Minn., reported in a poster at the American Society of Hematology Meeting on Hematologic Malignancies.
In patients receiving proteasome inhibitors, better response was associated with creatinine concentrations greater than 1.5 mg/dL (OR, 3.83), calcium concentration less than 9.0 mg/dL (OR, 3.37), and greater absolute free light chain difference greater than 175 mg/dL (OR, 2.50), Dr. Binder noted.
High-risk cytogenetic features, conversely, were not associated with treatment response, he said.
The findings are important because the initial response to therapy in newly diagnosed disease can have an effect on long-term outcomes; achieving at least a VGPR to initial treatment can improve progression-free and overall survival. Further, novel agents and risk-adapted treatment strategies now in use have improved response rates and overall survival, Dr. Binder said, adding that it has been unclear, however, whether baseline laboratory parameters could predict the likelihood of early, deep response to initial therapy.
“The ability to predict the likelihood of response to the current therapies can have implications for the treatment approaches in NDMM,” he wrote.
In this study, 288 patients achieved VGPR+ after 4 months, and those patients had a decreased risk of subsequent mortality (hazard ratio, 0.69). This remained true after adjusting for sex, age, International Staging System stage, bone marrow plasma cell involvement, lactate dehydrogenase concentration, initial treatment regimen group, and hematopoietic stem cell transplantation during the disease course (HR, 0.68), he said.
The three most common regimens with immunomodulators that were used in the cohort were lenalidomide-dexamethasone, thalidomide-dexamethasone, and cyclophosphamide-lenalidomide-dexamethasone, and the three most common regimens with proteasome inhibitors were bortezomib-cyclophosphamide-dexamethasone, bortezomib-lenalidomide-dexamethasone, and bortezomib-dexamethasone.
Dr. Binder reported receiving research funding from ASH. Coauthors reported receiving research funding from Celgene, Janssen, Millennium, and Pfizer, and/or serving in an advisory role for Pfizer.
AT MHM 2015
Key clinical point: A number of factors predict early response to initial therapy in patients with symptomatic multiple myeloma, according to findings from a review of 1,304 cases.
Major finding: Predictors of early response were greater absolute free light chain difference (OR, 2.38), younger age (OR, 2.18), hemoglobin concentration (OR, 1.68), and IgA vs. IgG heavy chain secretion (OR, 1.66).
Data source: A retrospective cohort study involving 1,304 patients.
Disclosures: Dr. Binder reported receiving research funding from ASH. Coauthors reported receiving research funding from Celgene, Janssen, Millennium, and Pfizer, and/or serving in an advisory role for Pfizer.
New guidelines address multiple myeloma–related complications
New guidelines on managing the complications of multiple myeloma and its treatment recommend whole-body, low-dose computed tomography over conventional radiography because of its superior sensitivity for detecting osteolytic lesions. The guidelines, drafted by the European Myeloma Network, also provide an imaging algorithm to address various clinical scenarios.
This is just one of the recommendations from the interdisciplinary panel that reviewed published randomized trials, guidelines, meta-analyses, systematic reviews, observational studies, and case reports on the topic. They graded their recommendations according to the strength of the evidence and, when evidence was insufficient, expert consensus.
“Multiple myeloma … is characterized by bone destruction, anemia, renal and immunological impairment. These complications may lead to severe impairment of the quality of life of myeloma patients and may deteriorate their life expectancy,” note the authors, who were led by Dr. Evangelos Terpos of the department of clinical therapeutics, National and Kapodistrian University of Athens (Haematologica. 2015;100:1254-66).
The panel endorsed addition of zoledronic acid (Zometa) or pamidronate (Aredia) to specific antimyeloma therapy for patients with adequate renal function who have bone disease at diagnosis. Although evidence is weaker, they note that symptomatic patients who do not have lytic lesions on conventional radiography can be treated with zoledronic acid; however, its use in patients with no bone involvement on computed tomography or magnetic resonance imaging has uncertain benefit. Additionally, they do not recommend use of bisphosphonates in asymptomatic patients.
The panel recommends that zoledronic acid be given continuously, although they add that benefit of continuous use is not clear in patients who achieve at least a very good partial response.
Treatment can be initiated with erythropoiesis-stimulating agents such as epoetin alfa (various brand names) and darbepoetin (Aranesp) in patients who have persistent symptomatic anemia (defined as a hemoglobin level of less than 10 g/dL) without any other apparent cause. However, these agents should be stopped after 6 to 8 weeks if hemoglobin response has not been adequate.
The panel notes that bortezomib(Velcade)-based regimens are the standard of care for patients with multiple myeloma who have renal impairment. Lenalidomide is an option, albeit with weaker evidence, in cases of mild to moderate renal impairment.
In patients who experience treatment-induced peripheral neuropathy, therapy should be modified by either altering the schedule or route of administration (as appropriate) or reducing the dose, according to the guidelines.
Patients with multiple myeloma (and their contacts) should be vaccinated against influenza, the panel recommends; vaccination against Streptococcus pneumonia and Haemophilus influenzae is “appropriate,” although efficacy is not guaranteed as patients are immunologically compromised.
The panel endorsed acyclovir (Zovirax) or valacyclovir (Valtrex) for herpes zoster virus prophylaxis in patients receiving proteasome inhibitors or undergoing autologous or allogeneic transplantation, mainly directed to patients who are seropositive.
Finally, clinicians should assess risk of venous thromboembolism in patients who are due to start immunomodulatory drug therapy and should use appropriate risk-based antiplatelet or anticoagulation therapy throughout treatment, according to the guidelines.
Dr. Terpos reported having no relevant disclosures.
New guidelines on managing the complications of multiple myeloma and its treatment recommend whole-body, low-dose computed tomography over conventional radiography because of its superior sensitivity for detecting osteolytic lesions. The guidelines, drafted by the European Myeloma Network, also provide an imaging algorithm to address various clinical scenarios.
This is just one of the recommendations from the interdisciplinary panel that reviewed published randomized trials, guidelines, meta-analyses, systematic reviews, observational studies, and case reports on the topic. They graded their recommendations according to the strength of the evidence and, when evidence was insufficient, expert consensus.
“Multiple myeloma … is characterized by bone destruction, anemia, renal and immunological impairment. These complications may lead to severe impairment of the quality of life of myeloma patients and may deteriorate their life expectancy,” note the authors, who were led by Dr. Evangelos Terpos of the department of clinical therapeutics, National and Kapodistrian University of Athens (Haematologica. 2015;100:1254-66).
The panel endorsed addition of zoledronic acid (Zometa) or pamidronate (Aredia) to specific antimyeloma therapy for patients with adequate renal function who have bone disease at diagnosis. Although evidence is weaker, they note that symptomatic patients who do not have lytic lesions on conventional radiography can be treated with zoledronic acid; however, its use in patients with no bone involvement on computed tomography or magnetic resonance imaging has uncertain benefit. Additionally, they do not recommend use of bisphosphonates in asymptomatic patients.
The panel recommends that zoledronic acid be given continuously, although they add that benefit of continuous use is not clear in patients who achieve at least a very good partial response.
Treatment can be initiated with erythropoiesis-stimulating agents such as epoetin alfa (various brand names) and darbepoetin (Aranesp) in patients who have persistent symptomatic anemia (defined as a hemoglobin level of less than 10 g/dL) without any other apparent cause. However, these agents should be stopped after 6 to 8 weeks if hemoglobin response has not been adequate.
The panel notes that bortezomib(Velcade)-based regimens are the standard of care for patients with multiple myeloma who have renal impairment. Lenalidomide is an option, albeit with weaker evidence, in cases of mild to moderate renal impairment.
In patients who experience treatment-induced peripheral neuropathy, therapy should be modified by either altering the schedule or route of administration (as appropriate) or reducing the dose, according to the guidelines.
Patients with multiple myeloma (and their contacts) should be vaccinated against influenza, the panel recommends; vaccination against Streptococcus pneumonia and Haemophilus influenzae is “appropriate,” although efficacy is not guaranteed as patients are immunologically compromised.
The panel endorsed acyclovir (Zovirax) or valacyclovir (Valtrex) for herpes zoster virus prophylaxis in patients receiving proteasome inhibitors or undergoing autologous or allogeneic transplantation, mainly directed to patients who are seropositive.
Finally, clinicians should assess risk of venous thromboembolism in patients who are due to start immunomodulatory drug therapy and should use appropriate risk-based antiplatelet or anticoagulation therapy throughout treatment, according to the guidelines.
Dr. Terpos reported having no relevant disclosures.
New guidelines on managing the complications of multiple myeloma and its treatment recommend whole-body, low-dose computed tomography over conventional radiography because of its superior sensitivity for detecting osteolytic lesions. The guidelines, drafted by the European Myeloma Network, also provide an imaging algorithm to address various clinical scenarios.
This is just one of the recommendations from the interdisciplinary panel that reviewed published randomized trials, guidelines, meta-analyses, systematic reviews, observational studies, and case reports on the topic. They graded their recommendations according to the strength of the evidence and, when evidence was insufficient, expert consensus.
“Multiple myeloma … is characterized by bone destruction, anemia, renal and immunological impairment. These complications may lead to severe impairment of the quality of life of myeloma patients and may deteriorate their life expectancy,” note the authors, who were led by Dr. Evangelos Terpos of the department of clinical therapeutics, National and Kapodistrian University of Athens (Haematologica. 2015;100:1254-66).
The panel endorsed addition of zoledronic acid (Zometa) or pamidronate (Aredia) to specific antimyeloma therapy for patients with adequate renal function who have bone disease at diagnosis. Although evidence is weaker, they note that symptomatic patients who do not have lytic lesions on conventional radiography can be treated with zoledronic acid; however, its use in patients with no bone involvement on computed tomography or magnetic resonance imaging has uncertain benefit. Additionally, they do not recommend use of bisphosphonates in asymptomatic patients.
The panel recommends that zoledronic acid be given continuously, although they add that benefit of continuous use is not clear in patients who achieve at least a very good partial response.
Treatment can be initiated with erythropoiesis-stimulating agents such as epoetin alfa (various brand names) and darbepoetin (Aranesp) in patients who have persistent symptomatic anemia (defined as a hemoglobin level of less than 10 g/dL) without any other apparent cause. However, these agents should be stopped after 6 to 8 weeks if hemoglobin response has not been adequate.
The panel notes that bortezomib(Velcade)-based regimens are the standard of care for patients with multiple myeloma who have renal impairment. Lenalidomide is an option, albeit with weaker evidence, in cases of mild to moderate renal impairment.
In patients who experience treatment-induced peripheral neuropathy, therapy should be modified by either altering the schedule or route of administration (as appropriate) or reducing the dose, according to the guidelines.
Patients with multiple myeloma (and their contacts) should be vaccinated against influenza, the panel recommends; vaccination against Streptococcus pneumonia and Haemophilus influenzae is “appropriate,” although efficacy is not guaranteed as patients are immunologically compromised.
The panel endorsed acyclovir (Zovirax) or valacyclovir (Valtrex) for herpes zoster virus prophylaxis in patients receiving proteasome inhibitors or undergoing autologous or allogeneic transplantation, mainly directed to patients who are seropositive.
Finally, clinicians should assess risk of venous thromboembolism in patients who are due to start immunomodulatory drug therapy and should use appropriate risk-based antiplatelet or anticoagulation therapy throughout treatment, according to the guidelines.
Dr. Terpos reported having no relevant disclosures.
FROM HAEMATOLOGICA
Aberrant miRNA levels may reflect progression to multiple myeloma
Levels of specific microRNAs (miRNAs) detected in the peripheral blood of patients with monoclonal gammopathy of undetermined significance (MGUS), and smoldering multiple myeloma may prove to predict progression to multiple myeloma, according to Weixin Wang of the Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, Md., and colleagues (J Mol Diagn. 2015;17:669-78).
The research team analyzed bone marrow aspirates from 20 patients with multiple myeloma and 8 healthy controls and found 11 miRNAs with significantly lower expression. Serum was then analyzed in 17 patients with MGUS, 17 with smoldering multiple myeloma, 13 with multiple myeloma, and 12 healthy controls. Four of 11 miRNAs (let-7i, miR-15a, miR-16, and miR-106b) were significantly decreased in MGUS, suggesting that aberrant expression of these miRNAs may be associated with early neoplastic events. Eight of 11 miRNAs (let-7a, let-7b, let-7i, miR-15a, miR-15b, miR-16, miR-106b, and miR-20a) were decreased in smoldering disease. The other three miRNAs (miR-21, miR-223, and miR-361) were significantly decreased in multiple myeloma but not in MGUS/SMM, suggesting that down-regulation of this group of miRNAs may be related to later events in disease progression, including malignant transformation from precursor disease to myeloma, the researchers wrote.
Small, noncoding miRNA molecules function in posttranscriptional gene regulation through RNA silencing, and many of the gene targets of the 11 miRNAs encode proteins that regulate cell proliferation.
Previous studies have shown that the let-7 family of miRNAs are expressed at low levels in human cancer and stem cells, and these miRNAs were implicated in the current study as well. Let-7 miRNAs silence many genes involved in oncogenesis, cell cycle, proliferation, and apoptosis, including MYC. The researchers found that let-7a and let-7b expression was normal in MGUS and decreased in smoldering disease and multiple myeloma, which may be related to increased MYC expression in disease progression.
Dr. Wang and coauthors reported having no disclosures.
Levels of specific microRNAs (miRNAs) detected in the peripheral blood of patients with monoclonal gammopathy of undetermined significance (MGUS), and smoldering multiple myeloma may prove to predict progression to multiple myeloma, according to Weixin Wang of the Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, Md., and colleagues (J Mol Diagn. 2015;17:669-78).
The research team analyzed bone marrow aspirates from 20 patients with multiple myeloma and 8 healthy controls and found 11 miRNAs with significantly lower expression. Serum was then analyzed in 17 patients with MGUS, 17 with smoldering multiple myeloma, 13 with multiple myeloma, and 12 healthy controls. Four of 11 miRNAs (let-7i, miR-15a, miR-16, and miR-106b) were significantly decreased in MGUS, suggesting that aberrant expression of these miRNAs may be associated with early neoplastic events. Eight of 11 miRNAs (let-7a, let-7b, let-7i, miR-15a, miR-15b, miR-16, miR-106b, and miR-20a) were decreased in smoldering disease. The other three miRNAs (miR-21, miR-223, and miR-361) were significantly decreased in multiple myeloma but not in MGUS/SMM, suggesting that down-regulation of this group of miRNAs may be related to later events in disease progression, including malignant transformation from precursor disease to myeloma, the researchers wrote.
Small, noncoding miRNA molecules function in posttranscriptional gene regulation through RNA silencing, and many of the gene targets of the 11 miRNAs encode proteins that regulate cell proliferation.
Previous studies have shown that the let-7 family of miRNAs are expressed at low levels in human cancer and stem cells, and these miRNAs were implicated in the current study as well. Let-7 miRNAs silence many genes involved in oncogenesis, cell cycle, proliferation, and apoptosis, including MYC. The researchers found that let-7a and let-7b expression was normal in MGUS and decreased in smoldering disease and multiple myeloma, which may be related to increased MYC expression in disease progression.
Dr. Wang and coauthors reported having no disclosures.
Levels of specific microRNAs (miRNAs) detected in the peripheral blood of patients with monoclonal gammopathy of undetermined significance (MGUS), and smoldering multiple myeloma may prove to predict progression to multiple myeloma, according to Weixin Wang of the Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, Md., and colleagues (J Mol Diagn. 2015;17:669-78).
The research team analyzed bone marrow aspirates from 20 patients with multiple myeloma and 8 healthy controls and found 11 miRNAs with significantly lower expression. Serum was then analyzed in 17 patients with MGUS, 17 with smoldering multiple myeloma, 13 with multiple myeloma, and 12 healthy controls. Four of 11 miRNAs (let-7i, miR-15a, miR-16, and miR-106b) were significantly decreased in MGUS, suggesting that aberrant expression of these miRNAs may be associated with early neoplastic events. Eight of 11 miRNAs (let-7a, let-7b, let-7i, miR-15a, miR-15b, miR-16, miR-106b, and miR-20a) were decreased in smoldering disease. The other three miRNAs (miR-21, miR-223, and miR-361) were significantly decreased in multiple myeloma but not in MGUS/SMM, suggesting that down-regulation of this group of miRNAs may be related to later events in disease progression, including malignant transformation from precursor disease to myeloma, the researchers wrote.
Small, noncoding miRNA molecules function in posttranscriptional gene regulation through RNA silencing, and many of the gene targets of the 11 miRNAs encode proteins that regulate cell proliferation.
Previous studies have shown that the let-7 family of miRNAs are expressed at low levels in human cancer and stem cells, and these miRNAs were implicated in the current study as well. Let-7 miRNAs silence many genes involved in oncogenesis, cell cycle, proliferation, and apoptosis, including MYC. The researchers found that let-7a and let-7b expression was normal in MGUS and decreased in smoldering disease and multiple myeloma, which may be related to increased MYC expression in disease progression.
Dr. Wang and coauthors reported having no disclosures.
FROM THE JOURNAL OF MOLECULAR DIAGNOSTICS
Key clinical point: Low levels of specific miRNAs in peripheral blood may reflect disease progression from MGUS and smoldering disease to multiple myeloma.
Major finding: Of 11 miRNAs found to be significantly decreased in the serum of patients with multiple myeloma compared to healthy controls, 8 were decreased in smoldering multiple myeloma and 4 were decreased in MGUS.
Data source: Serum for miRNA analysis came from 13 patients with multiple myeloma, 17 with smoldering disease, 17 with MGUS, and 12 healthy controls.
Disclosures: Dr. Wang and coauthors reported having no disclosures.
CHMP grants accelerated assessment for MM drug

Photo by Linda Bartlett
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has agreed to provide accelerated assessment for daratumumab.
The drug is under review as monotherapy for patients with relapsed and refractory multiple myeloma (MM).
The CHMP grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days.
About daratumumab
Daratumumab is an investigational monoclonal antibody that works by binding to CD38 on the surface of MM cells. In doing so, daratumumab triggers the patient’s own immune system to attack MM cells, resulting in cell death through multiple mechanisms of action.
In July 2013, daratumumab was granted orphan drug status by the European Medicines Agency for the treatment of plasma cell myeloma.
The drug has been accepted for priority review in the US as monotherapy for MM patients who are refractory to both a proteasome inhibitor and an immunomodulatory agent or who have received 3 or more prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
In August 2012, Janssen Biotech, Inc. and Genmab entered an agreement that granted Janssen an exclusive worldwide license to develop, manufacture, and commercialize daratumumab.
Daratumumab trials
The marketing authorization application for daratumumab includes data from the phase 2 MMY2002 (SIRIUS) study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested that daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%.
Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 of the patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events. ![]()

Photo by Linda Bartlett
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has agreed to provide accelerated assessment for daratumumab.
The drug is under review as monotherapy for patients with relapsed and refractory multiple myeloma (MM).
The CHMP grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days.
About daratumumab
Daratumumab is an investigational monoclonal antibody that works by binding to CD38 on the surface of MM cells. In doing so, daratumumab triggers the patient’s own immune system to attack MM cells, resulting in cell death through multiple mechanisms of action.
In July 2013, daratumumab was granted orphan drug status by the European Medicines Agency for the treatment of plasma cell myeloma.
The drug has been accepted for priority review in the US as monotherapy for MM patients who are refractory to both a proteasome inhibitor and an immunomodulatory agent or who have received 3 or more prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
In August 2012, Janssen Biotech, Inc. and Genmab entered an agreement that granted Janssen an exclusive worldwide license to develop, manufacture, and commercialize daratumumab.
Daratumumab trials
The marketing authorization application for daratumumab includes data from the phase 2 MMY2002 (SIRIUS) study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested that daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%.
Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 of the patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events. ![]()

Photo by Linda Bartlett
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has agreed to provide accelerated assessment for daratumumab.
The drug is under review as monotherapy for patients with relapsed and refractory multiple myeloma (MM).
The CHMP grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days.
About daratumumab
Daratumumab is an investigational monoclonal antibody that works by binding to CD38 on the surface of MM cells. In doing so, daratumumab triggers the patient’s own immune system to attack MM cells, resulting in cell death through multiple mechanisms of action.
In July 2013, daratumumab was granted orphan drug status by the European Medicines Agency for the treatment of plasma cell myeloma.
The drug has been accepted for priority review in the US as monotherapy for MM patients who are refractory to both a proteasome inhibitor and an immunomodulatory agent or who have received 3 or more prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
In August 2012, Janssen Biotech, Inc. and Genmab entered an agreement that granted Janssen an exclusive worldwide license to develop, manufacture, and commercialize daratumumab.
Daratumumab trials
The marketing authorization application for daratumumab includes data from the phase 2 MMY2002 (SIRIUS) study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested that daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%.
Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 of the patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events. ![]()
Signature may predict progression to MM

Photo by Graham Colm
New research has revealed a microRNA (miRNA) signature in the bone marrow of patients with multiple myeloma (MM) that is also detectable in peripheral blood.
Investigators believe this signature may mark the onset of MM and predict progression to MM in patients with monoclonal gammopathy of undetermined significance (MGUS) or smoldering myeloma (SMM).
This research has been published in The Journal of Molecular Diagnostics.
“Currently, there is no single factor that can predict patients with MGUS or SMM who are likely to progress to myeloma,” said Katherine R. Calvo, MD, PhD, of the National Institutes of Health in Bethesda, Maryland.
“A biomarker of disease progression in the peripheral blood could assist in the early identification of patients evolving to multiple myeloma.”
With this in mind, Dr Calvo and her colleagues studied miRNAs as possible biomarkers of MM. Previous research has shown increased levels of specific miRNAs in the blood and plasma of MM patients.
In this study, the investigators analyzed bone marrow, plasma, and serum samples from healthy controls and patients with MM, MGUS, or SMM.
The team analyzed fluid from the bone marrow of 20 patients with MM and identified 111 miRNAs that showed a 2-fold or greater difference from levels observed in 8 control samples. Sixty-nine of the miRNAs were downregulated, and 42 were upregulated.
Further analysis revealed a unique miRNA signature indicative of MM. The bone marrow signature included 8 members of the let-7 family of miRNAs, each of which showed significant decreases ranging from 6-fold to 17-fold (P<0.03) in patients with MM.
Other experiments revealed the miRNA profiles characteristic of MM in peripheral blood, serum, and plasma samples.
Using quantitative real-time PCR, the investigators identified 18 miRNAs that were significantly decreased in bone marrow MM samples. Of these, 11 (60%) miRNAs were also significantly decreased in serum samples, and 6 of the 11 were also found to be lower in plasma samples (including 3 members of the let-7 miRNA family).
The investigators further explored whether the miRNA pattern of MM in precursor diseases changes as the disease progresses. They analyzed serum samples in 17 patients with MGUS, 17 with SMM, 13 with MM, and 12 healthy controls.
Only 4 of the 11 miRNAs (36%) that were reduced in the MM serum samples were lower in the MGUS samples.
“This suggests that aberrant expression of these [4] miRNAs may be associated with early events in plasma cell neoplasia,” Dr Calvo said.
Eight of the 11 (73%) miRNAs were decreased in SMM plasma samples. However, 3 (27%) were significantly reduced only in the MM samples, suggesting that downregulation of this group of miRNAs may be related to later events during evolution from precursor disease to MM.
“Our findings suggest that the antiproliferative and proapoptotic miRNAs, such as the let-7 family members, are downregulated in multiple myeloma’s microenvironment,” Dr Calvo said.
“These findings suggest that measuring expression of miRNAs associated with myeloma progression in the peripheral blood may hold promise for predicting disease progression in MGUS and SMM.” ![]()

Photo by Graham Colm
New research has revealed a microRNA (miRNA) signature in the bone marrow of patients with multiple myeloma (MM) that is also detectable in peripheral blood.
Investigators believe this signature may mark the onset of MM and predict progression to MM in patients with monoclonal gammopathy of undetermined significance (MGUS) or smoldering myeloma (SMM).
This research has been published in The Journal of Molecular Diagnostics.
“Currently, there is no single factor that can predict patients with MGUS or SMM who are likely to progress to myeloma,” said Katherine R. Calvo, MD, PhD, of the National Institutes of Health in Bethesda, Maryland.
“A biomarker of disease progression in the peripheral blood could assist in the early identification of patients evolving to multiple myeloma.”
With this in mind, Dr Calvo and her colleagues studied miRNAs as possible biomarkers of MM. Previous research has shown increased levels of specific miRNAs in the blood and plasma of MM patients.
In this study, the investigators analyzed bone marrow, plasma, and serum samples from healthy controls and patients with MM, MGUS, or SMM.
The team analyzed fluid from the bone marrow of 20 patients with MM and identified 111 miRNAs that showed a 2-fold or greater difference from levels observed in 8 control samples. Sixty-nine of the miRNAs were downregulated, and 42 were upregulated.
Further analysis revealed a unique miRNA signature indicative of MM. The bone marrow signature included 8 members of the let-7 family of miRNAs, each of which showed significant decreases ranging from 6-fold to 17-fold (P<0.03) in patients with MM.
Other experiments revealed the miRNA profiles characteristic of MM in peripheral blood, serum, and plasma samples.
Using quantitative real-time PCR, the investigators identified 18 miRNAs that were significantly decreased in bone marrow MM samples. Of these, 11 (60%) miRNAs were also significantly decreased in serum samples, and 6 of the 11 were also found to be lower in plasma samples (including 3 members of the let-7 miRNA family).
The investigators further explored whether the miRNA pattern of MM in precursor diseases changes as the disease progresses. They analyzed serum samples in 17 patients with MGUS, 17 with SMM, 13 with MM, and 12 healthy controls.
Only 4 of the 11 miRNAs (36%) that were reduced in the MM serum samples were lower in the MGUS samples.
“This suggests that aberrant expression of these [4] miRNAs may be associated with early events in plasma cell neoplasia,” Dr Calvo said.
Eight of the 11 (73%) miRNAs were decreased in SMM plasma samples. However, 3 (27%) were significantly reduced only in the MM samples, suggesting that downregulation of this group of miRNAs may be related to later events during evolution from precursor disease to MM.
“Our findings suggest that the antiproliferative and proapoptotic miRNAs, such as the let-7 family members, are downregulated in multiple myeloma’s microenvironment,” Dr Calvo said.
“These findings suggest that measuring expression of miRNAs associated with myeloma progression in the peripheral blood may hold promise for predicting disease progression in MGUS and SMM.” ![]()

Photo by Graham Colm
New research has revealed a microRNA (miRNA) signature in the bone marrow of patients with multiple myeloma (MM) that is also detectable in peripheral blood.
Investigators believe this signature may mark the onset of MM and predict progression to MM in patients with monoclonal gammopathy of undetermined significance (MGUS) or smoldering myeloma (SMM).
This research has been published in The Journal of Molecular Diagnostics.
“Currently, there is no single factor that can predict patients with MGUS or SMM who are likely to progress to myeloma,” said Katherine R. Calvo, MD, PhD, of the National Institutes of Health in Bethesda, Maryland.
“A biomarker of disease progression in the peripheral blood could assist in the early identification of patients evolving to multiple myeloma.”
With this in mind, Dr Calvo and her colleagues studied miRNAs as possible biomarkers of MM. Previous research has shown increased levels of specific miRNAs in the blood and plasma of MM patients.
In this study, the investigators analyzed bone marrow, plasma, and serum samples from healthy controls and patients with MM, MGUS, or SMM.
The team analyzed fluid from the bone marrow of 20 patients with MM and identified 111 miRNAs that showed a 2-fold or greater difference from levels observed in 8 control samples. Sixty-nine of the miRNAs were downregulated, and 42 were upregulated.
Further analysis revealed a unique miRNA signature indicative of MM. The bone marrow signature included 8 members of the let-7 family of miRNAs, each of which showed significant decreases ranging from 6-fold to 17-fold (P<0.03) in patients with MM.
Other experiments revealed the miRNA profiles characteristic of MM in peripheral blood, serum, and plasma samples.
Using quantitative real-time PCR, the investigators identified 18 miRNAs that were significantly decreased in bone marrow MM samples. Of these, 11 (60%) miRNAs were also significantly decreased in serum samples, and 6 of the 11 were also found to be lower in plasma samples (including 3 members of the let-7 miRNA family).
The investigators further explored whether the miRNA pattern of MM in precursor diseases changes as the disease progresses. They analyzed serum samples in 17 patients with MGUS, 17 with SMM, 13 with MM, and 12 healthy controls.
Only 4 of the 11 miRNAs (36%) that were reduced in the MM serum samples were lower in the MGUS samples.
“This suggests that aberrant expression of these [4] miRNAs may be associated with early events in plasma cell neoplasia,” Dr Calvo said.
Eight of the 11 (73%) miRNAs were decreased in SMM plasma samples. However, 3 (27%) were significantly reduced only in the MM samples, suggesting that downregulation of this group of miRNAs may be related to later events during evolution from precursor disease to MM.
“Our findings suggest that the antiproliferative and proapoptotic miRNAs, such as the let-7 family members, are downregulated in multiple myeloma’s microenvironment,” Dr Calvo said.
“These findings suggest that measuring expression of miRNAs associated with myeloma progression in the peripheral blood may hold promise for predicting disease progression in MGUS and SMM.” ![]()
Group calls for more investment in radiotherapy

woman for radiotherapy
Photo by Rhoda Baer
VIENNA—Millions of people throughout the world are dying from potentially treatable cancers because of a chronic underinvestment in radiotherapy resources, according to a new report.
The report suggests that expanding access to radiotherapy services will require a sizeable investment upfront, but that investment could bring economic benefits of up to $365 billion in developing countries over the next 20 years.
The report was published in The Lancet Oncology and presented at the 2015 European Cancer Congress.
The report estimates that 204 million fractions of radiotherapy will be needed to treat the 12 million cancer patients worldwide who could benefit from treatment in 2035.
But the cost per fraction is highly cost-effective and low compared to the price of many new cancer drugs, according to the report’s authors.
They estimate that full access to radiotherapy could be achieved for all patients in need in low-and middle income countries (LMIC) by 2035 for $97 billion, with potential health benefits of 27 million life-years saved and economic benefits ranging from $278 billion to $365 billion over the next 20 years.
“There is a widespread misconception that the costs of providing radiotherapy put it beyond the reach of all but the richest countries, [but] nothing could be further from the truth,” said Rifat Atun, MBBS, of Harvard University in Boston, Massachusetts.
“Our work . . . clearly shows that not only can this essential service be deployed safely and high quality treatment delivered in low- and middle-income countries, but that scale-up of radiotherapy capacity is a feasible and highly cost-effective investment.”
The report provides details on access to radiotherapy services across the world, on a country-by-country basis. The authors calculated the costs and benefits of meeting the worldwide shortfall in resources and bridging the gap in access to effective treatment.
Estimates suggest that, at present, about 40% to 60% of cancer patients worldwide have access to radiotherapy. Even in high-income countries like Canada, Australia, and the UK, numbers of radiotherapy facilities, equipment, and trained staff are inadequate.
Access is worst in low-income countries, where as many as 9 out of 10 people cannot access radiotherapy. The problem of access is especially acute in Africa. In most African countries, radiotherapy is virtually non-existent. Forty countries have no radiotherapy facilities at all.
“The time has come to agree and implement immediate actions to tackle the global shortfall in radiotherapy services and the crisis of access to this highly effective treatment,” Dr Atun said.
With that in mind, he and his colleagues called for the following 6 targets to be met.
By 2020:
- 80% of countries to have comprehensive cancer plans that include radiotherapy.
- Each LMIC to create 1 new center for treatment and training.
- 80% of LMICs to include radiotherapy services in their universal health coverage plans.
By 2025:
- A 25% increase in radiotherapy treatment capacity.
- LMICs to train 7500 radiation oncologists, 20,000 radiotherapy radiographers, and 6000 medical physicists.
- $46 billion of upfront investment to be raised to establish radiotherapy infrastructure and training in LMICs (with help from international banks and the private sector).
“The evidence outlined in the [report] reinforces the case for investing in radiotherapy as an essential component of cancer control,” said Mary Gospodarowicz, MD, co-chair of the UICC Global Task Force on Radiotherapy for Cancer Control.
“The building of radiotherapy capacity will require large initial investment. However, the treatment is more cost-effective than chemotherapy and surgery for treating cancer, and the health and economic benefits will be realized in just 10 to 15 years.” ![]()

woman for radiotherapy
Photo by Rhoda Baer
VIENNA—Millions of people throughout the world are dying from potentially treatable cancers because of a chronic underinvestment in radiotherapy resources, according to a new report.
The report suggests that expanding access to radiotherapy services will require a sizeable investment upfront, but that investment could bring economic benefits of up to $365 billion in developing countries over the next 20 years.
The report was published in The Lancet Oncology and presented at the 2015 European Cancer Congress.
The report estimates that 204 million fractions of radiotherapy will be needed to treat the 12 million cancer patients worldwide who could benefit from treatment in 2035.
But the cost per fraction is highly cost-effective and low compared to the price of many new cancer drugs, according to the report’s authors.
They estimate that full access to radiotherapy could be achieved for all patients in need in low-and middle income countries (LMIC) by 2035 for $97 billion, with potential health benefits of 27 million life-years saved and economic benefits ranging from $278 billion to $365 billion over the next 20 years.
“There is a widespread misconception that the costs of providing radiotherapy put it beyond the reach of all but the richest countries, [but] nothing could be further from the truth,” said Rifat Atun, MBBS, of Harvard University in Boston, Massachusetts.
“Our work . . . clearly shows that not only can this essential service be deployed safely and high quality treatment delivered in low- and middle-income countries, but that scale-up of radiotherapy capacity is a feasible and highly cost-effective investment.”
The report provides details on access to radiotherapy services across the world, on a country-by-country basis. The authors calculated the costs and benefits of meeting the worldwide shortfall in resources and bridging the gap in access to effective treatment.
Estimates suggest that, at present, about 40% to 60% of cancer patients worldwide have access to radiotherapy. Even in high-income countries like Canada, Australia, and the UK, numbers of radiotherapy facilities, equipment, and trained staff are inadequate.
Access is worst in low-income countries, where as many as 9 out of 10 people cannot access radiotherapy. The problem of access is especially acute in Africa. In most African countries, radiotherapy is virtually non-existent. Forty countries have no radiotherapy facilities at all.
“The time has come to agree and implement immediate actions to tackle the global shortfall in radiotherapy services and the crisis of access to this highly effective treatment,” Dr Atun said.
With that in mind, he and his colleagues called for the following 6 targets to be met.
By 2020:
- 80% of countries to have comprehensive cancer plans that include radiotherapy.
- Each LMIC to create 1 new center for treatment and training.
- 80% of LMICs to include radiotherapy services in their universal health coverage plans.
By 2025:
- A 25% increase in radiotherapy treatment capacity.
- LMICs to train 7500 radiation oncologists, 20,000 radiotherapy radiographers, and 6000 medical physicists.
- $46 billion of upfront investment to be raised to establish radiotherapy infrastructure and training in LMICs (with help from international banks and the private sector).
“The evidence outlined in the [report] reinforces the case for investing in radiotherapy as an essential component of cancer control,” said Mary Gospodarowicz, MD, co-chair of the UICC Global Task Force on Radiotherapy for Cancer Control.
“The building of radiotherapy capacity will require large initial investment. However, the treatment is more cost-effective than chemotherapy and surgery for treating cancer, and the health and economic benefits will be realized in just 10 to 15 years.” ![]()

woman for radiotherapy
Photo by Rhoda Baer
VIENNA—Millions of people throughout the world are dying from potentially treatable cancers because of a chronic underinvestment in radiotherapy resources, according to a new report.
The report suggests that expanding access to radiotherapy services will require a sizeable investment upfront, but that investment could bring economic benefits of up to $365 billion in developing countries over the next 20 years.
The report was published in The Lancet Oncology and presented at the 2015 European Cancer Congress.
The report estimates that 204 million fractions of radiotherapy will be needed to treat the 12 million cancer patients worldwide who could benefit from treatment in 2035.
But the cost per fraction is highly cost-effective and low compared to the price of many new cancer drugs, according to the report’s authors.
They estimate that full access to radiotherapy could be achieved for all patients in need in low-and middle income countries (LMIC) by 2035 for $97 billion, with potential health benefits of 27 million life-years saved and economic benefits ranging from $278 billion to $365 billion over the next 20 years.
“There is a widespread misconception that the costs of providing radiotherapy put it beyond the reach of all but the richest countries, [but] nothing could be further from the truth,” said Rifat Atun, MBBS, of Harvard University in Boston, Massachusetts.
“Our work . . . clearly shows that not only can this essential service be deployed safely and high quality treatment delivered in low- and middle-income countries, but that scale-up of radiotherapy capacity is a feasible and highly cost-effective investment.”
The report provides details on access to radiotherapy services across the world, on a country-by-country basis. The authors calculated the costs and benefits of meeting the worldwide shortfall in resources and bridging the gap in access to effective treatment.
Estimates suggest that, at present, about 40% to 60% of cancer patients worldwide have access to radiotherapy. Even in high-income countries like Canada, Australia, and the UK, numbers of radiotherapy facilities, equipment, and trained staff are inadequate.
Access is worst in low-income countries, where as many as 9 out of 10 people cannot access radiotherapy. The problem of access is especially acute in Africa. In most African countries, radiotherapy is virtually non-existent. Forty countries have no radiotherapy facilities at all.
“The time has come to agree and implement immediate actions to tackle the global shortfall in radiotherapy services and the crisis of access to this highly effective treatment,” Dr Atun said.
With that in mind, he and his colleagues called for the following 6 targets to be met.
By 2020:
- 80% of countries to have comprehensive cancer plans that include radiotherapy.
- Each LMIC to create 1 new center for treatment and training.
- 80% of LMICs to include radiotherapy services in their universal health coverage plans.
By 2025:
- A 25% increase in radiotherapy treatment capacity.
- LMICs to train 7500 radiation oncologists, 20,000 radiotherapy radiographers, and 6000 medical physicists.
- $46 billion of upfront investment to be raised to establish radiotherapy infrastructure and training in LMICs (with help from international banks and the private sector).
“The evidence outlined in the [report] reinforces the case for investing in radiotherapy as an essential component of cancer control,” said Mary Gospodarowicz, MD, co-chair of the UICC Global Task Force on Radiotherapy for Cancer Control.
“The building of radiotherapy capacity will require large initial investment. However, the treatment is more cost-effective than chemotherapy and surgery for treating cancer, and the health and economic benefits will be realized in just 10 to 15 years.” ![]()






