User login
Inhibitor exhibits ‘modest’ activity in lymphoma

A small-molecule inhibitor has shown modest anticancer activity in a phase 1 trial of patients with relapsed/refractory lymphoma or multiple myeloma (MM), according to an investigator involved in the study.
A majority of patients who recieved the drug, pevonedistat, achieved stable disease, and a few patients with lymphoma experienced partial responses.
Investigators said pevonedistat could be given safely, although 100% of patients experienced adverse events (AEs), and a majority experienced grade 3 or higher AEs.
“The most important findings from our study are that pevonedistat hits its target in cancer cells in patients, can be given safely, and has modest activity in heavily pretreated patients with relapsed/refractory lymphoma, suggesting that we are on the right path,” said Jatin J. Shah, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Shah and his colleagues reported these findings in Clinical Cancer Research. The trial was sponsored by Millennium Pharmaceuticals, Inc., the company developing pevonedistat.
Pevonedistat is a first-in-class, investigational, small-molecule inhibitor of the NEDD8-activating enzyme.
“This enzyme is part of the ubiquitin-proteasome system, which is the target of a number of FDA-approved anticancer therapeutics, including bortezomib . . . ,” Dr Shah explained. “Pevonedistat also alters the ability of cancer cells to repair damaged DNA.”
Patient and treatment characteristics
Dr Shah and his colleagues enrolled 44 patients on this trial. Seventeen patients had relapsed/refractory MM, and 27 had relapsed/refractory lymphoma.
The types of lymphoma were diffuse large B-cell lymphoma (n=10), follicular lymphoma (n=5), Hodgkin lymphoma (n=5), small lymphocytic lymphoma/chronic lymphocytic leukemia (n=2), mantle cell lymphoma (n=1), lymphoplasmacytic lymphoma (n=1), peripheral T-cell lymphoma (n=1), splenic marginal zone B-cell lymphoma (n=1), and “other” (n=1).
Twenty-seven patients received escalating doses of pevonedistat on schedule A, which was days 1, 2, 8, and 9 of a 21-day cycle, and 17 patients received escalating doses of the drug on schedule B, which was days 1, 4, 8, and 11 of a 21-day cycle.
Safety
The maximum tolerated doses were 110 mg/m2 and 196 mg/m2 on schedule A and B, respectively. The dose-limiting toxicities were febrile neutropenia, transaminase elevations, and muscle cramps on schedule A, and thrombocytopenia on schedule B.
On schedule A, 100% of patients experienced AEs, and 59% had AEs of grade 3 or higher. The grade 3 or higher AEs were anemia (19%), thrombocytopenia (4%), neutropenia (7%), fatigue (7%), ALT increase (4%), AST increase (7%), diarrhea (4%), pain (4%), dyspnea (4%), hypercalcemia (7%), hypophosphatemia (11%), hyperkalemia (4%), muscle spasms (4%), abdominal discomfort (4%), and pneumonia (4%).
On schedule B, 100% of patients experienced AEs, and 71% had grade 3 or higher AEs. The grade 3 or higher AEs were anemia (6%), thrombocytopenia (6%), neutropenia (12%), fatigue (6%), ALT increase (6%), pyrexia (6%), pain (6%), dyspnea (6%), hypophosphatemia (6%), muscle spasms (6%), upper respiratory tract infection (6%), dehydration (6%), hyperbilirubinemia (6%), and pneumonia (12%).
Efficacy
Three patients had a partial response to treatment, including 1 patient with relapsed nodular sclerosis Hodgkin lymphoma, 1 with relapsed diffuse large B-cell lymphoma, and 1 with relapsed peripheral T-cell lymphoma.
Another 30 patients, 17 with lymphoma and 13 with MM, had stable disease.
“Although pevonedistat had modest activity as a single-agent treatment, we expect greater activity when it is given in combination with standard therapy,” Dr Shah said.
“The pharmacodynamics data showed that pevonedistat hit its target in cancer cells in patients at low doses. This is important because it may mean that we do not need to escalate the dose in future trials to increase anticancer activity. This has the potential to increase the risk-benefit ratio of pevonedistat.”
Dr Shah said a limitation of this study is that it included a small number of patients, all of whom were very heavily pretreated, which may limit assessment of how active pevonedistat could be. ![]()

A small-molecule inhibitor has shown modest anticancer activity in a phase 1 trial of patients with relapsed/refractory lymphoma or multiple myeloma (MM), according to an investigator involved in the study.
A majority of patients who recieved the drug, pevonedistat, achieved stable disease, and a few patients with lymphoma experienced partial responses.
Investigators said pevonedistat could be given safely, although 100% of patients experienced adverse events (AEs), and a majority experienced grade 3 or higher AEs.
“The most important findings from our study are that pevonedistat hits its target in cancer cells in patients, can be given safely, and has modest activity in heavily pretreated patients with relapsed/refractory lymphoma, suggesting that we are on the right path,” said Jatin J. Shah, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Shah and his colleagues reported these findings in Clinical Cancer Research. The trial was sponsored by Millennium Pharmaceuticals, Inc., the company developing pevonedistat.
Pevonedistat is a first-in-class, investigational, small-molecule inhibitor of the NEDD8-activating enzyme.
“This enzyme is part of the ubiquitin-proteasome system, which is the target of a number of FDA-approved anticancer therapeutics, including bortezomib . . . ,” Dr Shah explained. “Pevonedistat also alters the ability of cancer cells to repair damaged DNA.”
Patient and treatment characteristics
Dr Shah and his colleagues enrolled 44 patients on this trial. Seventeen patients had relapsed/refractory MM, and 27 had relapsed/refractory lymphoma.
The types of lymphoma were diffuse large B-cell lymphoma (n=10), follicular lymphoma (n=5), Hodgkin lymphoma (n=5), small lymphocytic lymphoma/chronic lymphocytic leukemia (n=2), mantle cell lymphoma (n=1), lymphoplasmacytic lymphoma (n=1), peripheral T-cell lymphoma (n=1), splenic marginal zone B-cell lymphoma (n=1), and “other” (n=1).
Twenty-seven patients received escalating doses of pevonedistat on schedule A, which was days 1, 2, 8, and 9 of a 21-day cycle, and 17 patients received escalating doses of the drug on schedule B, which was days 1, 4, 8, and 11 of a 21-day cycle.
Safety
The maximum tolerated doses were 110 mg/m2 and 196 mg/m2 on schedule A and B, respectively. The dose-limiting toxicities were febrile neutropenia, transaminase elevations, and muscle cramps on schedule A, and thrombocytopenia on schedule B.
On schedule A, 100% of patients experienced AEs, and 59% had AEs of grade 3 or higher. The grade 3 or higher AEs were anemia (19%), thrombocytopenia (4%), neutropenia (7%), fatigue (7%), ALT increase (4%), AST increase (7%), diarrhea (4%), pain (4%), dyspnea (4%), hypercalcemia (7%), hypophosphatemia (11%), hyperkalemia (4%), muscle spasms (4%), abdominal discomfort (4%), and pneumonia (4%).
On schedule B, 100% of patients experienced AEs, and 71% had grade 3 or higher AEs. The grade 3 or higher AEs were anemia (6%), thrombocytopenia (6%), neutropenia (12%), fatigue (6%), ALT increase (6%), pyrexia (6%), pain (6%), dyspnea (6%), hypophosphatemia (6%), muscle spasms (6%), upper respiratory tract infection (6%), dehydration (6%), hyperbilirubinemia (6%), and pneumonia (12%).
Efficacy
Three patients had a partial response to treatment, including 1 patient with relapsed nodular sclerosis Hodgkin lymphoma, 1 with relapsed diffuse large B-cell lymphoma, and 1 with relapsed peripheral T-cell lymphoma.
Another 30 patients, 17 with lymphoma and 13 with MM, had stable disease.
“Although pevonedistat had modest activity as a single-agent treatment, we expect greater activity when it is given in combination with standard therapy,” Dr Shah said.
“The pharmacodynamics data showed that pevonedistat hit its target in cancer cells in patients at low doses. This is important because it may mean that we do not need to escalate the dose in future trials to increase anticancer activity. This has the potential to increase the risk-benefit ratio of pevonedistat.”
Dr Shah said a limitation of this study is that it included a small number of patients, all of whom were very heavily pretreated, which may limit assessment of how active pevonedistat could be. ![]()

A small-molecule inhibitor has shown modest anticancer activity in a phase 1 trial of patients with relapsed/refractory lymphoma or multiple myeloma (MM), according to an investigator involved in the study.
A majority of patients who recieved the drug, pevonedistat, achieved stable disease, and a few patients with lymphoma experienced partial responses.
Investigators said pevonedistat could be given safely, although 100% of patients experienced adverse events (AEs), and a majority experienced grade 3 or higher AEs.
“The most important findings from our study are that pevonedistat hits its target in cancer cells in patients, can be given safely, and has modest activity in heavily pretreated patients with relapsed/refractory lymphoma, suggesting that we are on the right path,” said Jatin J. Shah, MD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Shah and his colleagues reported these findings in Clinical Cancer Research. The trial was sponsored by Millennium Pharmaceuticals, Inc., the company developing pevonedistat.
Pevonedistat is a first-in-class, investigational, small-molecule inhibitor of the NEDD8-activating enzyme.
“This enzyme is part of the ubiquitin-proteasome system, which is the target of a number of FDA-approved anticancer therapeutics, including bortezomib . . . ,” Dr Shah explained. “Pevonedistat also alters the ability of cancer cells to repair damaged DNA.”
Patient and treatment characteristics
Dr Shah and his colleagues enrolled 44 patients on this trial. Seventeen patients had relapsed/refractory MM, and 27 had relapsed/refractory lymphoma.
The types of lymphoma were diffuse large B-cell lymphoma (n=10), follicular lymphoma (n=5), Hodgkin lymphoma (n=5), small lymphocytic lymphoma/chronic lymphocytic leukemia (n=2), mantle cell lymphoma (n=1), lymphoplasmacytic lymphoma (n=1), peripheral T-cell lymphoma (n=1), splenic marginal zone B-cell lymphoma (n=1), and “other” (n=1).
Twenty-seven patients received escalating doses of pevonedistat on schedule A, which was days 1, 2, 8, and 9 of a 21-day cycle, and 17 patients received escalating doses of the drug on schedule B, which was days 1, 4, 8, and 11 of a 21-day cycle.
Safety
The maximum tolerated doses were 110 mg/m2 and 196 mg/m2 on schedule A and B, respectively. The dose-limiting toxicities were febrile neutropenia, transaminase elevations, and muscle cramps on schedule A, and thrombocytopenia on schedule B.
On schedule A, 100% of patients experienced AEs, and 59% had AEs of grade 3 or higher. The grade 3 or higher AEs were anemia (19%), thrombocytopenia (4%), neutropenia (7%), fatigue (7%), ALT increase (4%), AST increase (7%), diarrhea (4%), pain (4%), dyspnea (4%), hypercalcemia (7%), hypophosphatemia (11%), hyperkalemia (4%), muscle spasms (4%), abdominal discomfort (4%), and pneumonia (4%).
On schedule B, 100% of patients experienced AEs, and 71% had grade 3 or higher AEs. The grade 3 or higher AEs were anemia (6%), thrombocytopenia (6%), neutropenia (12%), fatigue (6%), ALT increase (6%), pyrexia (6%), pain (6%), dyspnea (6%), hypophosphatemia (6%), muscle spasms (6%), upper respiratory tract infection (6%), dehydration (6%), hyperbilirubinemia (6%), and pneumonia (12%).
Efficacy
Three patients had a partial response to treatment, including 1 patient with relapsed nodular sclerosis Hodgkin lymphoma, 1 with relapsed diffuse large B-cell lymphoma, and 1 with relapsed peripheral T-cell lymphoma.
Another 30 patients, 17 with lymphoma and 13 with MM, had stable disease.
“Although pevonedistat had modest activity as a single-agent treatment, we expect greater activity when it is given in combination with standard therapy,” Dr Shah said.
“The pharmacodynamics data showed that pevonedistat hit its target in cancer cells in patients at low doses. This is important because it may mean that we do not need to escalate the dose in future trials to increase anticancer activity. This has the potential to increase the risk-benefit ratio of pevonedistat.”
Dr Shah said a limitation of this study is that it included a small number of patients, all of whom were very heavily pretreated, which may limit assessment of how active pevonedistat could be. ![]()
Multiple myeloma advances in diagnosis, staging, therapy extend survival
SAN FRANCISCO – With multiple therapies and refinements in diagnostic and staging criteria, risk stratification, and transplantation, “we have made dramatic improvements in survival” for malignant myeloma, Dr. Damian J. Green told attendees of the NCCN Annual Congress: Hematologic Malignancies. In fact, he said, these advances have propelled the field toward a once unthinkable question: Can myeloma be cured?
Diagnostic criteria
The criteria used to diagnose active myeloma recently changed, noted Dr. Green of the University of Washington, the Multiple Myeloma and Stem Cell Transplant Program at the Seattle Cancer Care Alliance, and the Fred Hutchinson Cancer Research Center, all in Seattle.
Long-used CRAB criteria (calcium elevation, renal failure, anemia, and bone lesions) have been updated to incorporate three additional biomarkers – a bone marrow plasma cell percentage of 60% or greater, a serum free light chain ratio of 100 or greater, and a skeletal MRI or CT showing more than one focal lesion – conferring a very high risk of progression (Lancet Oncol. 2014;15[12]:e538-48).
“Many of us are using these new, independently validated factors, I would say, in select cases, not all the time. But they are now becoming part of the accepted dogma for determining in whom you might initiate therapy,” he said.
This change is likely to affect the epidemiology of smoldering myeloma, he noted. “We are taking the people at highest risk of progression and shifting them now, potentially, into the active group. What that means is whoever remains in the smoldering group, their prognosis is actually going to be better in the future.”
Staging criteria
The criteria used to stage myeloma have also changed, just in the past few months. The International Staging System (ISS) is about 15 years old. “The problem is it predated the era of novel therapy, and it predated our understanding of high-risk cytogenetics. That has been a long-term criticism,” Dr. Green said.
The new revised system, termed R-ISS, incorporates cytogenetics – designating 17p deletion, translocation 4;14, and translocation 14;16 as high-risk cytogenetics – as well as lactate dehydrogenase (J Clin Oncol. 2015;33:2863-9).
“I think there’s going to be uniform acceptance of this change. It’s a big deal in terms of how we manage these folks and in terms of what tests need to be ordered,” he said. “But it’s going to change things because lots of our interpretation of prior data is based on the old [system].”
Primary therapy
Numerous regimens are effective as primary therapy in myeloma, with expert consensus favoring three drugs over two for fit patients. Triple combinations achieve a greater depth of response, and deeper responses – whether assessed with multiparameter high-sensitivity flow cytometry (Blood. 2015;125:1932-5) or deep sequencing (Blood. 2014;123:3073-9) – correlate with better outcomes.
“Now I don’t know if that is just telling us about the basic biology of the disease – you respond better, therefore you have a better outcome – or if three drugs are definitively better than two up front,” Dr. Green said. “But until we know that, I think the consensus from the myeloma community is, three drugs in patients who can tolerate that.”
Forthcoming data to be presented at the ASH meeting will likely shed more light on the comparative efficacy of various primary regimens, he said.
The therapeutic options also are likely to increase soon, as two or three new drugs are likely to be approved for multiple myeloma in the next 6 months, he added.
Risk-adapted management
Another area of rapid change has been therapy that is adapted to a patient’s risk of progression, Dr. Green said. “Because we have all these new agents, that keeps changing. Is it high risk or isn’t it high risk based on cytogenetics? Maybe it was yesterday and it’s not today because some new agent is improving outcomes for a specific subset of patients.”
There is some disagreement on where, exactly, certain cytogenetics fall. But 17p deletion is generally viewed as high risk, and a recent study suggested that the survival benefit of bortezomib (Velcade) induction followed by maintenance after stem cell transplant in newly diagnosed myeloma was especially pronounced among patients with this cytogenetic abnormality (J Clin Oncol. 2012;30:2946-55).
“Although there’s not a randomized trial powered to prove this directly, we are beginning to understand and see that difference clinically. Patients who have 17p disease should see proteasome inhibitor therapy up front and I believe as part of their maintenance, unless they can’t tolerate it or are resistant to it,” Dr. Green recommended.
Stem cell transplant
“The data continue to support the use of an autologous stem cell transplant up front in the management of patients with myeloma after induction,” he contended.
Studies establishing the efficacy of transplant were done before the era of novel therapies. “Some people said all these novel therapies make transplant less important, but that really hasn’t been borne out. That debate is sort of falling away because we now have some new studies that have come out demonstrating a continued benefit in survival for patients who are able to and undergo an autologous stem cell transplant as part of their care,” he said.
“It is a standard of care and if you want proof of it, you can just look at the number of transplants we are doing of multiple myeloma in the United States every year,” he said. “It continues to increase and continues to be the No. 1 indication for transplant.”
Maintenance therapy post transplant
The best approach to maintenance therapy after transplant remains controversial, according to Dr. Green. Lenalidomide (Revlimid) is the standard of care in the United States based on three large trials, all of which showed a progression-free survival benefit of the drug, and one of which showed an overall survival benefit.
“That’s been the rationale for keeping patients on it,” he said, while noting that trials have differed with respect to patient populations and duration on the drug. However, patients with high-risk features may be good candidates for alternate agents.
Options for relapsed disease
Clonal evolution has become an area of interest as it pertains to treatment decisions in the relapsed myeloma setting. “Myeloma is a wily foe, it evolves over time: We find a good treatment against it and it evolves and there is progression,” Dr. Green said. For example, patients may be found to have a 17p deletion when they previously didn’t have one, which could tilt the treatment decision to bortezomib.
Hematologists should consider putting their patients with relapse on clinical trials testing salvage regimens, he said. “Only 4% of patients in the United States are enrolled in a clinical trial, and 40% of trials are closed due to low accrual. If you can get a patient on a trial, please do.”
A regimen that was successful previously in a given patient can be used again. And the roughly one dozen other options for relapsed disease now include the newcomers carfilzomib (Kyprolis), pomalidomide (Pomalyst), and panobinostat (Farydak).
The old drug melphalan (Alkeran) should not be overlooked either. “Melphalan should still be considered a part of salvage regimens for patients. If they have already undergone transplant or are not transplant candidates, at some point, they should receive melphalan, in my opinion,” he said.
Investigational agents
Various investigational agents are being evaluated in trials in myeloma. They include, for example, daratumumab, an anti-CD38 antibody that achieved a 36% response rate in patients with relapsed or relapsed, refractory disease (N Engl J Med. 2015;24;373[13]:1207-19), and elotuzumab, an anti-SLAM F7 antibody that when combined with lenalidomide and dexamethasone improved progression-free survival in patients with relapsed or refractory disease, both overall and among those with high-risk features (N Engl J Med. 2015;373[7]:621-31).
Chimeric antigen receptor (CAR) T cells also have been tested in myeloma (N Engl J Med. 2015;373[11]:1040-7). “I don’t think that this is going to be the home-run approach, but I do think it’s an interesting proof of principle,” Dr. Green said.
Taken together, data suggest that today, cure is within reach for at least a subset of patients with myeloma. For example, more than a third of those undergoing stem cell transplantation who have a complete response are still alive at 12 years, with some having long-term survival (Blood 2011;118:529-34).
“I’m betting that those are the patients who, if we were able to look back in time, we would have seen they had no evidence of minimal residual disease by looking with more of those technologies we now have available for depth of response,” proposed Dr. Green, who disclosed that he had no relevant conflicts of interest.
SAN FRANCISCO – With multiple therapies and refinements in diagnostic and staging criteria, risk stratification, and transplantation, “we have made dramatic improvements in survival” for malignant myeloma, Dr. Damian J. Green told attendees of the NCCN Annual Congress: Hematologic Malignancies. In fact, he said, these advances have propelled the field toward a once unthinkable question: Can myeloma be cured?
Diagnostic criteria
The criteria used to diagnose active myeloma recently changed, noted Dr. Green of the University of Washington, the Multiple Myeloma and Stem Cell Transplant Program at the Seattle Cancer Care Alliance, and the Fred Hutchinson Cancer Research Center, all in Seattle.
Long-used CRAB criteria (calcium elevation, renal failure, anemia, and bone lesions) have been updated to incorporate three additional biomarkers – a bone marrow plasma cell percentage of 60% or greater, a serum free light chain ratio of 100 or greater, and a skeletal MRI or CT showing more than one focal lesion – conferring a very high risk of progression (Lancet Oncol. 2014;15[12]:e538-48).
“Many of us are using these new, independently validated factors, I would say, in select cases, not all the time. But they are now becoming part of the accepted dogma for determining in whom you might initiate therapy,” he said.
This change is likely to affect the epidemiology of smoldering myeloma, he noted. “We are taking the people at highest risk of progression and shifting them now, potentially, into the active group. What that means is whoever remains in the smoldering group, their prognosis is actually going to be better in the future.”
Staging criteria
The criteria used to stage myeloma have also changed, just in the past few months. The International Staging System (ISS) is about 15 years old. “The problem is it predated the era of novel therapy, and it predated our understanding of high-risk cytogenetics. That has been a long-term criticism,” Dr. Green said.
The new revised system, termed R-ISS, incorporates cytogenetics – designating 17p deletion, translocation 4;14, and translocation 14;16 as high-risk cytogenetics – as well as lactate dehydrogenase (J Clin Oncol. 2015;33:2863-9).
“I think there’s going to be uniform acceptance of this change. It’s a big deal in terms of how we manage these folks and in terms of what tests need to be ordered,” he said. “But it’s going to change things because lots of our interpretation of prior data is based on the old [system].”
Primary therapy
Numerous regimens are effective as primary therapy in myeloma, with expert consensus favoring three drugs over two for fit patients. Triple combinations achieve a greater depth of response, and deeper responses – whether assessed with multiparameter high-sensitivity flow cytometry (Blood. 2015;125:1932-5) or deep sequencing (Blood. 2014;123:3073-9) – correlate with better outcomes.
“Now I don’t know if that is just telling us about the basic biology of the disease – you respond better, therefore you have a better outcome – or if three drugs are definitively better than two up front,” Dr. Green said. “But until we know that, I think the consensus from the myeloma community is, three drugs in patients who can tolerate that.”
Forthcoming data to be presented at the ASH meeting will likely shed more light on the comparative efficacy of various primary regimens, he said.
The therapeutic options also are likely to increase soon, as two or three new drugs are likely to be approved for multiple myeloma in the next 6 months, he added.
Risk-adapted management
Another area of rapid change has been therapy that is adapted to a patient’s risk of progression, Dr. Green said. “Because we have all these new agents, that keeps changing. Is it high risk or isn’t it high risk based on cytogenetics? Maybe it was yesterday and it’s not today because some new agent is improving outcomes for a specific subset of patients.”
There is some disagreement on where, exactly, certain cytogenetics fall. But 17p deletion is generally viewed as high risk, and a recent study suggested that the survival benefit of bortezomib (Velcade) induction followed by maintenance after stem cell transplant in newly diagnosed myeloma was especially pronounced among patients with this cytogenetic abnormality (J Clin Oncol. 2012;30:2946-55).
“Although there’s not a randomized trial powered to prove this directly, we are beginning to understand and see that difference clinically. Patients who have 17p disease should see proteasome inhibitor therapy up front and I believe as part of their maintenance, unless they can’t tolerate it or are resistant to it,” Dr. Green recommended.
Stem cell transplant
“The data continue to support the use of an autologous stem cell transplant up front in the management of patients with myeloma after induction,” he contended.
Studies establishing the efficacy of transplant were done before the era of novel therapies. “Some people said all these novel therapies make transplant less important, but that really hasn’t been borne out. That debate is sort of falling away because we now have some new studies that have come out demonstrating a continued benefit in survival for patients who are able to and undergo an autologous stem cell transplant as part of their care,” he said.
“It is a standard of care and if you want proof of it, you can just look at the number of transplants we are doing of multiple myeloma in the United States every year,” he said. “It continues to increase and continues to be the No. 1 indication for transplant.”
Maintenance therapy post transplant
The best approach to maintenance therapy after transplant remains controversial, according to Dr. Green. Lenalidomide (Revlimid) is the standard of care in the United States based on three large trials, all of which showed a progression-free survival benefit of the drug, and one of which showed an overall survival benefit.
“That’s been the rationale for keeping patients on it,” he said, while noting that trials have differed with respect to patient populations and duration on the drug. However, patients with high-risk features may be good candidates for alternate agents.
Options for relapsed disease
Clonal evolution has become an area of interest as it pertains to treatment decisions in the relapsed myeloma setting. “Myeloma is a wily foe, it evolves over time: We find a good treatment against it and it evolves and there is progression,” Dr. Green said. For example, patients may be found to have a 17p deletion when they previously didn’t have one, which could tilt the treatment decision to bortezomib.
Hematologists should consider putting their patients with relapse on clinical trials testing salvage regimens, he said. “Only 4% of patients in the United States are enrolled in a clinical trial, and 40% of trials are closed due to low accrual. If you can get a patient on a trial, please do.”
A regimen that was successful previously in a given patient can be used again. And the roughly one dozen other options for relapsed disease now include the newcomers carfilzomib (Kyprolis), pomalidomide (Pomalyst), and panobinostat (Farydak).
The old drug melphalan (Alkeran) should not be overlooked either. “Melphalan should still be considered a part of salvage regimens for patients. If they have already undergone transplant or are not transplant candidates, at some point, they should receive melphalan, in my opinion,” he said.
Investigational agents
Various investigational agents are being evaluated in trials in myeloma. They include, for example, daratumumab, an anti-CD38 antibody that achieved a 36% response rate in patients with relapsed or relapsed, refractory disease (N Engl J Med. 2015;24;373[13]:1207-19), and elotuzumab, an anti-SLAM F7 antibody that when combined with lenalidomide and dexamethasone improved progression-free survival in patients with relapsed or refractory disease, both overall and among those with high-risk features (N Engl J Med. 2015;373[7]:621-31).
Chimeric antigen receptor (CAR) T cells also have been tested in myeloma (N Engl J Med. 2015;373[11]:1040-7). “I don’t think that this is going to be the home-run approach, but I do think it’s an interesting proof of principle,” Dr. Green said.
Taken together, data suggest that today, cure is within reach for at least a subset of patients with myeloma. For example, more than a third of those undergoing stem cell transplantation who have a complete response are still alive at 12 years, with some having long-term survival (Blood 2011;118:529-34).
“I’m betting that those are the patients who, if we were able to look back in time, we would have seen they had no evidence of minimal residual disease by looking with more of those technologies we now have available for depth of response,” proposed Dr. Green, who disclosed that he had no relevant conflicts of interest.
SAN FRANCISCO – With multiple therapies and refinements in diagnostic and staging criteria, risk stratification, and transplantation, “we have made dramatic improvements in survival” for malignant myeloma, Dr. Damian J. Green told attendees of the NCCN Annual Congress: Hematologic Malignancies. In fact, he said, these advances have propelled the field toward a once unthinkable question: Can myeloma be cured?
Diagnostic criteria
The criteria used to diagnose active myeloma recently changed, noted Dr. Green of the University of Washington, the Multiple Myeloma and Stem Cell Transplant Program at the Seattle Cancer Care Alliance, and the Fred Hutchinson Cancer Research Center, all in Seattle.
Long-used CRAB criteria (calcium elevation, renal failure, anemia, and bone lesions) have been updated to incorporate three additional biomarkers – a bone marrow plasma cell percentage of 60% or greater, a serum free light chain ratio of 100 or greater, and a skeletal MRI or CT showing more than one focal lesion – conferring a very high risk of progression (Lancet Oncol. 2014;15[12]:e538-48).
“Many of us are using these new, independently validated factors, I would say, in select cases, not all the time. But they are now becoming part of the accepted dogma for determining in whom you might initiate therapy,” he said.
This change is likely to affect the epidemiology of smoldering myeloma, he noted. “We are taking the people at highest risk of progression and shifting them now, potentially, into the active group. What that means is whoever remains in the smoldering group, their prognosis is actually going to be better in the future.”
Staging criteria
The criteria used to stage myeloma have also changed, just in the past few months. The International Staging System (ISS) is about 15 years old. “The problem is it predated the era of novel therapy, and it predated our understanding of high-risk cytogenetics. That has been a long-term criticism,” Dr. Green said.
The new revised system, termed R-ISS, incorporates cytogenetics – designating 17p deletion, translocation 4;14, and translocation 14;16 as high-risk cytogenetics – as well as lactate dehydrogenase (J Clin Oncol. 2015;33:2863-9).
“I think there’s going to be uniform acceptance of this change. It’s a big deal in terms of how we manage these folks and in terms of what tests need to be ordered,” he said. “But it’s going to change things because lots of our interpretation of prior data is based on the old [system].”
Primary therapy
Numerous regimens are effective as primary therapy in myeloma, with expert consensus favoring three drugs over two for fit patients. Triple combinations achieve a greater depth of response, and deeper responses – whether assessed with multiparameter high-sensitivity flow cytometry (Blood. 2015;125:1932-5) or deep sequencing (Blood. 2014;123:3073-9) – correlate with better outcomes.
“Now I don’t know if that is just telling us about the basic biology of the disease – you respond better, therefore you have a better outcome – or if three drugs are definitively better than two up front,” Dr. Green said. “But until we know that, I think the consensus from the myeloma community is, three drugs in patients who can tolerate that.”
Forthcoming data to be presented at the ASH meeting will likely shed more light on the comparative efficacy of various primary regimens, he said.
The therapeutic options also are likely to increase soon, as two or three new drugs are likely to be approved for multiple myeloma in the next 6 months, he added.
Risk-adapted management
Another area of rapid change has been therapy that is adapted to a patient’s risk of progression, Dr. Green said. “Because we have all these new agents, that keeps changing. Is it high risk or isn’t it high risk based on cytogenetics? Maybe it was yesterday and it’s not today because some new agent is improving outcomes for a specific subset of patients.”
There is some disagreement on where, exactly, certain cytogenetics fall. But 17p deletion is generally viewed as high risk, and a recent study suggested that the survival benefit of bortezomib (Velcade) induction followed by maintenance after stem cell transplant in newly diagnosed myeloma was especially pronounced among patients with this cytogenetic abnormality (J Clin Oncol. 2012;30:2946-55).
“Although there’s not a randomized trial powered to prove this directly, we are beginning to understand and see that difference clinically. Patients who have 17p disease should see proteasome inhibitor therapy up front and I believe as part of their maintenance, unless they can’t tolerate it or are resistant to it,” Dr. Green recommended.
Stem cell transplant
“The data continue to support the use of an autologous stem cell transplant up front in the management of patients with myeloma after induction,” he contended.
Studies establishing the efficacy of transplant were done before the era of novel therapies. “Some people said all these novel therapies make transplant less important, but that really hasn’t been borne out. That debate is sort of falling away because we now have some new studies that have come out demonstrating a continued benefit in survival for patients who are able to and undergo an autologous stem cell transplant as part of their care,” he said.
“It is a standard of care and if you want proof of it, you can just look at the number of transplants we are doing of multiple myeloma in the United States every year,” he said. “It continues to increase and continues to be the No. 1 indication for transplant.”
Maintenance therapy post transplant
The best approach to maintenance therapy after transplant remains controversial, according to Dr. Green. Lenalidomide (Revlimid) is the standard of care in the United States based on three large trials, all of which showed a progression-free survival benefit of the drug, and one of which showed an overall survival benefit.
“That’s been the rationale for keeping patients on it,” he said, while noting that trials have differed with respect to patient populations and duration on the drug. However, patients with high-risk features may be good candidates for alternate agents.
Options for relapsed disease
Clonal evolution has become an area of interest as it pertains to treatment decisions in the relapsed myeloma setting. “Myeloma is a wily foe, it evolves over time: We find a good treatment against it and it evolves and there is progression,” Dr. Green said. For example, patients may be found to have a 17p deletion when they previously didn’t have one, which could tilt the treatment decision to bortezomib.
Hematologists should consider putting their patients with relapse on clinical trials testing salvage regimens, he said. “Only 4% of patients in the United States are enrolled in a clinical trial, and 40% of trials are closed due to low accrual. If you can get a patient on a trial, please do.”
A regimen that was successful previously in a given patient can be used again. And the roughly one dozen other options for relapsed disease now include the newcomers carfilzomib (Kyprolis), pomalidomide (Pomalyst), and panobinostat (Farydak).
The old drug melphalan (Alkeran) should not be overlooked either. “Melphalan should still be considered a part of salvage regimens for patients. If they have already undergone transplant or are not transplant candidates, at some point, they should receive melphalan, in my opinion,” he said.
Investigational agents
Various investigational agents are being evaluated in trials in myeloma. They include, for example, daratumumab, an anti-CD38 antibody that achieved a 36% response rate in patients with relapsed or relapsed, refractory disease (N Engl J Med. 2015;24;373[13]:1207-19), and elotuzumab, an anti-SLAM F7 antibody that when combined with lenalidomide and dexamethasone improved progression-free survival in patients with relapsed or refractory disease, both overall and among those with high-risk features (N Engl J Med. 2015;373[7]:621-31).
Chimeric antigen receptor (CAR) T cells also have been tested in myeloma (N Engl J Med. 2015;373[11]:1040-7). “I don’t think that this is going to be the home-run approach, but I do think it’s an interesting proof of principle,” Dr. Green said.
Taken together, data suggest that today, cure is within reach for at least a subset of patients with myeloma. For example, more than a third of those undergoing stem cell transplantation who have a complete response are still alive at 12 years, with some having long-term survival (Blood 2011;118:529-34).
“I’m betting that those are the patients who, if we were able to look back in time, we would have seen they had no evidence of minimal residual disease by looking with more of those technologies we now have available for depth of response,” proposed Dr. Green, who disclosed that he had no relevant conflicts of interest.
EXPERT ANALYSIS FROM NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
Drug protects fertility in chemo-treated mice

Photo courtesy of UW Health
The heart medication dexrazoxane can protect fertility and improve survival in female mice receiving the chemotherapeutic agent doxorubicin, according to research published in PLOS ONE.
Dexrazoxane prevented ovarian damage, increased the number of healthy offspring mice had, and prolonged their survival.
If dexrazoxane has the same effects in humans, it could save young lives while also overcoming limitations to fertility treatments currently used during cancer treatment, according to study author Sana Salih, MD, of the University of Wisconsin School of Medicine and Public Health in Madison.
“Fertility preservation following chemotherapy for children and women diagnosed with cancer is a formidable challenge,” Dr Salih said. “For pre-pubescent girls, the only option to prevent chemo-induced ovarian failure is to preserve ovarian tissue by freezing.”
Unfortunately, that is an experimental procedure that requires surgery to harvest and again to re-implant the tissue after cancer treatment. The transplantation carries a small risk of cancer recurrence and provides only a short-term solution.
“The transplanted ovarian tissue can only function for 3 to 7 years,” Dr Salih said.
So she was pleased to discover that pre-administration of dexrazoxane diminished ovarian damage and preserved ovarian function and fertility in mice whose ovaries were exposed to doxorubicin.
“What really surprised us is that a very small dose of dexrazoxane was enough to give full ovarian protection,” Dr Salih said.
Mice treated with dexrazoxane also gave birth to healthier litters, with more pups and higher birth weights than mice that received doxorubicin alone.
In addition, the mice that received dexrazoxane and doxorubicin lived much longer than mice that only received doxorubicin. And mice given only dexrazoxane lived longer than control mice receiving no interventions.
“The [US Food and Drug Administration] currently limits the use of dexrazoxane to adults to protect their hearts from the toxic side effects of chemotherapy,” Dr Salih said. “But these patients are receiving very high doses of dexrazoxane that may actually be contributing to increased toxicity, leading to decreased survival in some patients.”
Dr Salih and her colleagues found they could achieve high mouse survival rates, ovarian protection, and birthing successes using a dose of dexrazoxane 10 times lower than what is used for adult human cardiac protection. Post-mortem studies on the mice showed protection of ovarian, heart, and other cells and tissues.
“This is exciting,” Dr Salih said. “We are now submitting a grant to look at low-dose dexrazoxane protection in nonhuman primates as a stepping stone to clinical translation in pediatric cancer patients.”
Dr Salih has begun studies needed to show that safe doses of dexrazoxane can protect developing primate ovaries. Nonhuman primate ovarian development, cycle time, and gestation are very similar to that of humans.
“My goal is to present data so that physicians can come up with dosage recommendations and safety profiles for early clinical trials in humans,” Dr Salih said. “Up to 6% of young girls with childhood cancers and 50% of women with breast cancer who endure chemotherapy face ovarian failure. We need to give more cancer survivors real hope that they can conceive a healthy child.” ![]()

Photo courtesy of UW Health
The heart medication dexrazoxane can protect fertility and improve survival in female mice receiving the chemotherapeutic agent doxorubicin, according to research published in PLOS ONE.
Dexrazoxane prevented ovarian damage, increased the number of healthy offspring mice had, and prolonged their survival.
If dexrazoxane has the same effects in humans, it could save young lives while also overcoming limitations to fertility treatments currently used during cancer treatment, according to study author Sana Salih, MD, of the University of Wisconsin School of Medicine and Public Health in Madison.
“Fertility preservation following chemotherapy for children and women diagnosed with cancer is a formidable challenge,” Dr Salih said. “For pre-pubescent girls, the only option to prevent chemo-induced ovarian failure is to preserve ovarian tissue by freezing.”
Unfortunately, that is an experimental procedure that requires surgery to harvest and again to re-implant the tissue after cancer treatment. The transplantation carries a small risk of cancer recurrence and provides only a short-term solution.
“The transplanted ovarian tissue can only function for 3 to 7 years,” Dr Salih said.
So she was pleased to discover that pre-administration of dexrazoxane diminished ovarian damage and preserved ovarian function and fertility in mice whose ovaries were exposed to doxorubicin.
“What really surprised us is that a very small dose of dexrazoxane was enough to give full ovarian protection,” Dr Salih said.
Mice treated with dexrazoxane also gave birth to healthier litters, with more pups and higher birth weights than mice that received doxorubicin alone.
In addition, the mice that received dexrazoxane and doxorubicin lived much longer than mice that only received doxorubicin. And mice given only dexrazoxane lived longer than control mice receiving no interventions.
“The [US Food and Drug Administration] currently limits the use of dexrazoxane to adults to protect their hearts from the toxic side effects of chemotherapy,” Dr Salih said. “But these patients are receiving very high doses of dexrazoxane that may actually be contributing to increased toxicity, leading to decreased survival in some patients.”
Dr Salih and her colleagues found they could achieve high mouse survival rates, ovarian protection, and birthing successes using a dose of dexrazoxane 10 times lower than what is used for adult human cardiac protection. Post-mortem studies on the mice showed protection of ovarian, heart, and other cells and tissues.
“This is exciting,” Dr Salih said. “We are now submitting a grant to look at low-dose dexrazoxane protection in nonhuman primates as a stepping stone to clinical translation in pediatric cancer patients.”
Dr Salih has begun studies needed to show that safe doses of dexrazoxane can protect developing primate ovaries. Nonhuman primate ovarian development, cycle time, and gestation are very similar to that of humans.
“My goal is to present data so that physicians can come up with dosage recommendations and safety profiles for early clinical trials in humans,” Dr Salih said. “Up to 6% of young girls with childhood cancers and 50% of women with breast cancer who endure chemotherapy face ovarian failure. We need to give more cancer survivors real hope that they can conceive a healthy child.” ![]()

Photo courtesy of UW Health
The heart medication dexrazoxane can protect fertility and improve survival in female mice receiving the chemotherapeutic agent doxorubicin, according to research published in PLOS ONE.
Dexrazoxane prevented ovarian damage, increased the number of healthy offspring mice had, and prolonged their survival.
If dexrazoxane has the same effects in humans, it could save young lives while also overcoming limitations to fertility treatments currently used during cancer treatment, according to study author Sana Salih, MD, of the University of Wisconsin School of Medicine and Public Health in Madison.
“Fertility preservation following chemotherapy for children and women diagnosed with cancer is a formidable challenge,” Dr Salih said. “For pre-pubescent girls, the only option to prevent chemo-induced ovarian failure is to preserve ovarian tissue by freezing.”
Unfortunately, that is an experimental procedure that requires surgery to harvest and again to re-implant the tissue after cancer treatment. The transplantation carries a small risk of cancer recurrence and provides only a short-term solution.
“The transplanted ovarian tissue can only function for 3 to 7 years,” Dr Salih said.
So she was pleased to discover that pre-administration of dexrazoxane diminished ovarian damage and preserved ovarian function and fertility in mice whose ovaries were exposed to doxorubicin.
“What really surprised us is that a very small dose of dexrazoxane was enough to give full ovarian protection,” Dr Salih said.
Mice treated with dexrazoxane also gave birth to healthier litters, with more pups and higher birth weights than mice that received doxorubicin alone.
In addition, the mice that received dexrazoxane and doxorubicin lived much longer than mice that only received doxorubicin. And mice given only dexrazoxane lived longer than control mice receiving no interventions.
“The [US Food and Drug Administration] currently limits the use of dexrazoxane to adults to protect their hearts from the toxic side effects of chemotherapy,” Dr Salih said. “But these patients are receiving very high doses of dexrazoxane that may actually be contributing to increased toxicity, leading to decreased survival in some patients.”
Dr Salih and her colleagues found they could achieve high mouse survival rates, ovarian protection, and birthing successes using a dose of dexrazoxane 10 times lower than what is used for adult human cardiac protection. Post-mortem studies on the mice showed protection of ovarian, heart, and other cells and tissues.
“This is exciting,” Dr Salih said. “We are now submitting a grant to look at low-dose dexrazoxane protection in nonhuman primates as a stepping stone to clinical translation in pediatric cancer patients.”
Dr Salih has begun studies needed to show that safe doses of dexrazoxane can protect developing primate ovaries. Nonhuman primate ovarian development, cycle time, and gestation are very similar to that of humans.
“My goal is to present data so that physicians can come up with dosage recommendations and safety profiles for early clinical trials in humans,” Dr Salih said. “Up to 6% of young girls with childhood cancers and 50% of women with breast cancer who endure chemotherapy face ovarian failure. We need to give more cancer survivors real hope that they can conceive a healthy child.” ![]()
Breast cancer drug may also work in MCL, myeloma
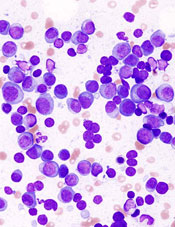
showing multiple myeloma
NEW YORK—Targeting the cell cycle with cyclin-dependent kinase (CDK) inhibitors may be an effective strategy to treat lymphoma and myeloma, according to a presentation at Lymphoma & Myeloma 2015.
Palbociclib, an inhibitor of CDK4 and CDK6, received accelerated approval from the US Food and Drug Administration to treat advanced breast cancer.
Now, it is showing promise in mantle cell lymphoma (MCL) and multiple myeloma (MM) as well.
CDK family members are important regulators of cell-cycle progression. Dysregulation of CDK4 and CDK6 is one of the most common genomic aberrations in human cancer, including myeloma, lymphoma, leukemia, breast cancer, metastatic lung adenocarcinoma, and glioblastoma.
MCL, which accounts for 6% of non-Hodgkin lymphomas, has an overall poor prognosis, with most patients eventually becoming resistant to drugs. MCL expresses cyclin D1 as a consequence of the t(11;14) translocation and overexpresses CDK4.
“So this is a perfect disease for the development of targeting CDK4,” said Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, New York, who presented this information at the meeting.
CDK4 and CDK6 signaling occur at the beginning of the cell cycle and bring the cell from the resting state into early G2.
“If we could control that,” Dr Chen-Kiang explained, “we reasoned that we could control the DNA replication and cell division” and increase tumor-specific cell death.
Palbociclib (PD0332991; Ibrance), an orally bioavailable, selective CDK4/CDK6 inhibitor, induces early G1 arrest. It is reversible and low in toxicity, according to Dr Chen-Kiang.
Currently, it’s being tested in phase 1 trials in MCL with ibrutinib and in MM with lenalidomide-dexamethasone. Phase 1 trials have also been completed at Cornell with palbociclib as a single agent in MCL, with bortezomib in MCL, and with bortezomib-dexamethasone in MM.
Palbociclib in MCL
Investigators conducted a phase 1, single-agent study of palbociclib to determine whether it could be tolerated in humans.
“CDK4/CDK6 is expressed in every cell,” Dr Chen-Kiang noted, “and you are targeting 2 proteins that are needed for every cell.”
Seventeen patients received 125 mg of palbociclib per day for 21 of 28 days.
“And surprisingly,” Dr Chen-Kiang said, “it’s very well tolerated.”
The most common adverse events were neutropenia, fatigue, and diarrhea.
“And even more surprising,” she added “is that we actually had a complete response (CR) [and] 2 partial responses, in addition to 5 stable diseases.”
The investigators hypothesized that blocking the cell cycle in G1 creates an imbalance in gene expression.
They then conducted a trial of palbociclib plus bortezomib in 17 patients with recurrent MCL. Patients received palbociclib on days 1–12 and low-dose bortezomib on days 8, 11, 15, and 18.
The palbociclib dose ranged from 75 mg to 125 mg, and the bortezomib dose ranged from 1.0 mg/m2 to 1.3 mg/m2.
Eleven patients experienced a reduction in tumor volume, the majority at the 125-mg dose.
Using whole-exome and whole-transcriptome sequencing of serial biopsies, investigators determined that CDK4/CDK6 inhibition induces early G1 arrest in MCL cells in both responders and non-responders initially.
This may occur because the cell cycle is perfectly controlled, and there’s no mutation in CDK4 in any of the patients.
Investigators attempted to identify genes that could differentiate sensitivity from resistance to CDK4 targeting. They found that a very small number of genes display opposite regulation in prolonged early G1 arrest in responding versus non-responding patients.
These genes are involved in metabolism and redox homeostasis and include a gene called PIK3IP1, an inhibitor of PI3 kinase.
In earlier analyses, the investigators had discovered a relapse-specific C481S mutation at the ibrutinib binding site of Bruton’s tyrosine kinase (BTK) in MCL cells. The mutation occurred at progression following a durable response to ibrutinib.
The mutation is absent, however, in patients with transient ibrutinib responses or in primary resistance.
The team observed that early cell-cycle arrest by CDK4 inhibition reprograms MCL cells for killing by ibrutinib. This occurs through inhibition of BTK and AKT (protein kinase B).
So the investigators undertook to study palbociclib in combination with ibrutinib, “with extraordinary results,” Dr Chen-Kiang said.
The trial is ongoing and, at the moment, has a 60% CR rate with durable responses.
“We’re very happy with this,” Dr Chen-Kiang said.
Palbociclib in MM
In addition to the phase 1/2 study of palbociclib with bortezomib and dexamethasone, investigators are also pursuing palbociclib in combination with lenalidomide and dexamethasone, which Dr Chen-Kiang briefly elaborated upon.
Lenalidomide rarely produces a complete remission on its own, she explained, so the team decided to study palbociclib in combination with immunomodulatory drugs in MM (NCT02030483).
The strategy is to prime the cell cycle with palbociclib and then use lenalidomide and dexamethasone to increase efficacy.
Twenty patients have received this combination thus far, and investigators found that palbociclib enhances the activity of lenalidomide in the killing of primary bone marrow myeloma cells.
Lenalidomide reduces the MEIS2/CRBN ratio in these cells. The drug changes the ratio, reduces the blocker, and allows the CRBN to work.
“The whole principle here is to control the gene coupling to the cell cycle and induce imbalance in gene expression,” Dr Chen-Kiang said. “And this weakens the tumor cells.”
Two-thirds of the samples respond to palboclib, she said, and those patients go on to be treated with lenalidomide or pomalidomide.
One third will not respond, but their in vivo clinical response and the ex vivo responders’ purified cells mimic one another.
“Now, this is very exciting to us,” she said.
The combination study with lenalidomide and dexamethasone is currently underway.
Palbociclib is being developed by Pfizer. ![]()

showing multiple myeloma
NEW YORK—Targeting the cell cycle with cyclin-dependent kinase (CDK) inhibitors may be an effective strategy to treat lymphoma and myeloma, according to a presentation at Lymphoma & Myeloma 2015.
Palbociclib, an inhibitor of CDK4 and CDK6, received accelerated approval from the US Food and Drug Administration to treat advanced breast cancer.
Now, it is showing promise in mantle cell lymphoma (MCL) and multiple myeloma (MM) as well.
CDK family members are important regulators of cell-cycle progression. Dysregulation of CDK4 and CDK6 is one of the most common genomic aberrations in human cancer, including myeloma, lymphoma, leukemia, breast cancer, metastatic lung adenocarcinoma, and glioblastoma.
MCL, which accounts for 6% of non-Hodgkin lymphomas, has an overall poor prognosis, with most patients eventually becoming resistant to drugs. MCL expresses cyclin D1 as a consequence of the t(11;14) translocation and overexpresses CDK4.
“So this is a perfect disease for the development of targeting CDK4,” said Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, New York, who presented this information at the meeting.
CDK4 and CDK6 signaling occur at the beginning of the cell cycle and bring the cell from the resting state into early G2.
“If we could control that,” Dr Chen-Kiang explained, “we reasoned that we could control the DNA replication and cell division” and increase tumor-specific cell death.
Palbociclib (PD0332991; Ibrance), an orally bioavailable, selective CDK4/CDK6 inhibitor, induces early G1 arrest. It is reversible and low in toxicity, according to Dr Chen-Kiang.
Currently, it’s being tested in phase 1 trials in MCL with ibrutinib and in MM with lenalidomide-dexamethasone. Phase 1 trials have also been completed at Cornell with palbociclib as a single agent in MCL, with bortezomib in MCL, and with bortezomib-dexamethasone in MM.
Palbociclib in MCL
Investigators conducted a phase 1, single-agent study of palbociclib to determine whether it could be tolerated in humans.
“CDK4/CDK6 is expressed in every cell,” Dr Chen-Kiang noted, “and you are targeting 2 proteins that are needed for every cell.”
Seventeen patients received 125 mg of palbociclib per day for 21 of 28 days.
“And surprisingly,” Dr Chen-Kiang said, “it’s very well tolerated.”
The most common adverse events were neutropenia, fatigue, and diarrhea.
“And even more surprising,” she added “is that we actually had a complete response (CR) [and] 2 partial responses, in addition to 5 stable diseases.”
The investigators hypothesized that blocking the cell cycle in G1 creates an imbalance in gene expression.
They then conducted a trial of palbociclib plus bortezomib in 17 patients with recurrent MCL. Patients received palbociclib on days 1–12 and low-dose bortezomib on days 8, 11, 15, and 18.
The palbociclib dose ranged from 75 mg to 125 mg, and the bortezomib dose ranged from 1.0 mg/m2 to 1.3 mg/m2.
Eleven patients experienced a reduction in tumor volume, the majority at the 125-mg dose.
Using whole-exome and whole-transcriptome sequencing of serial biopsies, investigators determined that CDK4/CDK6 inhibition induces early G1 arrest in MCL cells in both responders and non-responders initially.
This may occur because the cell cycle is perfectly controlled, and there’s no mutation in CDK4 in any of the patients.
Investigators attempted to identify genes that could differentiate sensitivity from resistance to CDK4 targeting. They found that a very small number of genes display opposite regulation in prolonged early G1 arrest in responding versus non-responding patients.
These genes are involved in metabolism and redox homeostasis and include a gene called PIK3IP1, an inhibitor of PI3 kinase.
In earlier analyses, the investigators had discovered a relapse-specific C481S mutation at the ibrutinib binding site of Bruton’s tyrosine kinase (BTK) in MCL cells. The mutation occurred at progression following a durable response to ibrutinib.
The mutation is absent, however, in patients with transient ibrutinib responses or in primary resistance.
The team observed that early cell-cycle arrest by CDK4 inhibition reprograms MCL cells for killing by ibrutinib. This occurs through inhibition of BTK and AKT (protein kinase B).
So the investigators undertook to study palbociclib in combination with ibrutinib, “with extraordinary results,” Dr Chen-Kiang said.
The trial is ongoing and, at the moment, has a 60% CR rate with durable responses.
“We’re very happy with this,” Dr Chen-Kiang said.
Palbociclib in MM
In addition to the phase 1/2 study of palbociclib with bortezomib and dexamethasone, investigators are also pursuing palbociclib in combination with lenalidomide and dexamethasone, which Dr Chen-Kiang briefly elaborated upon.
Lenalidomide rarely produces a complete remission on its own, she explained, so the team decided to study palbociclib in combination with immunomodulatory drugs in MM (NCT02030483).
The strategy is to prime the cell cycle with palbociclib and then use lenalidomide and dexamethasone to increase efficacy.
Twenty patients have received this combination thus far, and investigators found that palbociclib enhances the activity of lenalidomide in the killing of primary bone marrow myeloma cells.
Lenalidomide reduces the MEIS2/CRBN ratio in these cells. The drug changes the ratio, reduces the blocker, and allows the CRBN to work.
“The whole principle here is to control the gene coupling to the cell cycle and induce imbalance in gene expression,” Dr Chen-Kiang said. “And this weakens the tumor cells.”
Two-thirds of the samples respond to palboclib, she said, and those patients go on to be treated with lenalidomide or pomalidomide.
One third will not respond, but their in vivo clinical response and the ex vivo responders’ purified cells mimic one another.
“Now, this is very exciting to us,” she said.
The combination study with lenalidomide and dexamethasone is currently underway.
Palbociclib is being developed by Pfizer. ![]()

showing multiple myeloma
NEW YORK—Targeting the cell cycle with cyclin-dependent kinase (CDK) inhibitors may be an effective strategy to treat lymphoma and myeloma, according to a presentation at Lymphoma & Myeloma 2015.
Palbociclib, an inhibitor of CDK4 and CDK6, received accelerated approval from the US Food and Drug Administration to treat advanced breast cancer.
Now, it is showing promise in mantle cell lymphoma (MCL) and multiple myeloma (MM) as well.
CDK family members are important regulators of cell-cycle progression. Dysregulation of CDK4 and CDK6 is one of the most common genomic aberrations in human cancer, including myeloma, lymphoma, leukemia, breast cancer, metastatic lung adenocarcinoma, and glioblastoma.
MCL, which accounts for 6% of non-Hodgkin lymphomas, has an overall poor prognosis, with most patients eventually becoming resistant to drugs. MCL expresses cyclin D1 as a consequence of the t(11;14) translocation and overexpresses CDK4.
“So this is a perfect disease for the development of targeting CDK4,” said Selina Chen-Kiang, PhD, of Weill Cornell Medical College in New York, New York, who presented this information at the meeting.
CDK4 and CDK6 signaling occur at the beginning of the cell cycle and bring the cell from the resting state into early G2.
“If we could control that,” Dr Chen-Kiang explained, “we reasoned that we could control the DNA replication and cell division” and increase tumor-specific cell death.
Palbociclib (PD0332991; Ibrance), an orally bioavailable, selective CDK4/CDK6 inhibitor, induces early G1 arrest. It is reversible and low in toxicity, according to Dr Chen-Kiang.
Currently, it’s being tested in phase 1 trials in MCL with ibrutinib and in MM with lenalidomide-dexamethasone. Phase 1 trials have also been completed at Cornell with palbociclib as a single agent in MCL, with bortezomib in MCL, and with bortezomib-dexamethasone in MM.
Palbociclib in MCL
Investigators conducted a phase 1, single-agent study of palbociclib to determine whether it could be tolerated in humans.
“CDK4/CDK6 is expressed in every cell,” Dr Chen-Kiang noted, “and you are targeting 2 proteins that are needed for every cell.”
Seventeen patients received 125 mg of palbociclib per day for 21 of 28 days.
“And surprisingly,” Dr Chen-Kiang said, “it’s very well tolerated.”
The most common adverse events were neutropenia, fatigue, and diarrhea.
“And even more surprising,” she added “is that we actually had a complete response (CR) [and] 2 partial responses, in addition to 5 stable diseases.”
The investigators hypothesized that blocking the cell cycle in G1 creates an imbalance in gene expression.
They then conducted a trial of palbociclib plus bortezomib in 17 patients with recurrent MCL. Patients received palbociclib on days 1–12 and low-dose bortezomib on days 8, 11, 15, and 18.
The palbociclib dose ranged from 75 mg to 125 mg, and the bortezomib dose ranged from 1.0 mg/m2 to 1.3 mg/m2.
Eleven patients experienced a reduction in tumor volume, the majority at the 125-mg dose.
Using whole-exome and whole-transcriptome sequencing of serial biopsies, investigators determined that CDK4/CDK6 inhibition induces early G1 arrest in MCL cells in both responders and non-responders initially.
This may occur because the cell cycle is perfectly controlled, and there’s no mutation in CDK4 in any of the patients.
Investigators attempted to identify genes that could differentiate sensitivity from resistance to CDK4 targeting. They found that a very small number of genes display opposite regulation in prolonged early G1 arrest in responding versus non-responding patients.
These genes are involved in metabolism and redox homeostasis and include a gene called PIK3IP1, an inhibitor of PI3 kinase.
In earlier analyses, the investigators had discovered a relapse-specific C481S mutation at the ibrutinib binding site of Bruton’s tyrosine kinase (BTK) in MCL cells. The mutation occurred at progression following a durable response to ibrutinib.
The mutation is absent, however, in patients with transient ibrutinib responses or in primary resistance.
The team observed that early cell-cycle arrest by CDK4 inhibition reprograms MCL cells for killing by ibrutinib. This occurs through inhibition of BTK and AKT (protein kinase B).
So the investigators undertook to study palbociclib in combination with ibrutinib, “with extraordinary results,” Dr Chen-Kiang said.
The trial is ongoing and, at the moment, has a 60% CR rate with durable responses.
“We’re very happy with this,” Dr Chen-Kiang said.
Palbociclib in MM
In addition to the phase 1/2 study of palbociclib with bortezomib and dexamethasone, investigators are also pursuing palbociclib in combination with lenalidomide and dexamethasone, which Dr Chen-Kiang briefly elaborated upon.
Lenalidomide rarely produces a complete remission on its own, she explained, so the team decided to study palbociclib in combination with immunomodulatory drugs in MM (NCT02030483).
The strategy is to prime the cell cycle with palbociclib and then use lenalidomide and dexamethasone to increase efficacy.
Twenty patients have received this combination thus far, and investigators found that palbociclib enhances the activity of lenalidomide in the killing of primary bone marrow myeloma cells.
Lenalidomide reduces the MEIS2/CRBN ratio in these cells. The drug changes the ratio, reduces the blocker, and allows the CRBN to work.
“The whole principle here is to control the gene coupling to the cell cycle and induce imbalance in gene expression,” Dr Chen-Kiang said. “And this weakens the tumor cells.”
Two-thirds of the samples respond to palboclib, she said, and those patients go on to be treated with lenalidomide or pomalidomide.
One third will not respond, but their in vivo clinical response and the ex vivo responders’ purified cells mimic one another.
“Now, this is very exciting to us,” she said.
The combination study with lenalidomide and dexamethasone is currently underway.
Palbociclib is being developed by Pfizer. ![]()
MM-related ESRD on the decline
Photo by Anna Frodesiak
New research suggests the risk of end-stage renal disease (ESRD) caused by multiple myeloma (MM) is declining, and survival is lengthening for patients who do develop ESRD due to MM.
Researchers said these findings are encouraging, but efforts are still needed to develop effective MM treatments with fewer side effects.
They noted that MM treatment has changed substantially in the last decade.
But it hasn’t been clear whether the burden of ESRD due to MM has changed or whether survival has improved for patients with ESRD due to MM.
To gain some insight, Robert Foley, MD, of the University of Minnesota in Minneapolis, and his colleagues examined data from the US Renal Data System database spanning the period from 2001 to 2010.
They reported their findings in the Journal of the American Society of Nephrology.
The team found that, of the 1,069,343 patients with ESRD who were on renal replacement therapy (RRT), 12,703 had developed ESRD due to MM.
However, the incidence of ESRD from MM decreased from 2001 to 2010. Compared to 2001-2002 (1.00), the standardized incidence ratios of ESRD due to MM were 0.96 for 2003-2004 (P<0.05), 0.99 for 2005-2006 (P>0.05), 0.89 for 2007-2008 (P<0.001), and 0.82 for 2009-2010 (P<0.001).
The demography-adjusted incidence ratio for ESRD due to MM decreased (P<0.05) between 2001-2002 and 2009-2010 in the overall population and in most of the subgroups examined.
The exceptions were patients younger than 40, Hispanic patients, and those belonging to the “other” race category. (The race categories were “white,” “black,” and “other,” while the ethnicity categories were “Hispanic” and “non-Hispanic.”)
The data also showed improvements in survival over time among patients with ESRD due to MM.
Compared to 2001-2002, the adjusted hazard ratios (AHRs) for death were 1.02 for 2003-2004 (P=0.5), 0.93 for 2005-2006 (P=0.02), 0.86 for 2007-2008 (P<0.001), and 0.74 for 2009-2010 (P<0.001). (This analysis was adjusted for age, sex, race, ethnicity, ischemic heart disease, diabetes, mode of RRT, estimated glomerular filtration rate, body mass index, serum albumin, and hemoglobin.)
AHRs for death were highest in the first year after RRT initiation (AHR=2.6; P<0.001) and decreased in year 3 (AHR=1.59; P<0.001).
The AHR for death in patients with ESRD due to MM compared to those with ESRD due to all other causes was 2.05 (P<0.001).
“Myeloma is the commonest malignancy leading to kidney failure,” Dr Foley said. “It’s encouraging that we found that kidney failure due to multiple myeloma declined considerably over the last decade.” ![]()

Photo by Anna Frodesiak
New research suggests the risk of end-stage renal disease (ESRD) caused by multiple myeloma (MM) is declining, and survival is lengthening for patients who do develop ESRD due to MM.
Researchers said these findings are encouraging, but efforts are still needed to develop effective MM treatments with fewer side effects.
They noted that MM treatment has changed substantially in the last decade.
But it hasn’t been clear whether the burden of ESRD due to MM has changed or whether survival has improved for patients with ESRD due to MM.
To gain some insight, Robert Foley, MD, of the University of Minnesota in Minneapolis, and his colleagues examined data from the US Renal Data System database spanning the period from 2001 to 2010.
They reported their findings in the Journal of the American Society of Nephrology.
The team found that, of the 1,069,343 patients with ESRD who were on renal replacement therapy (RRT), 12,703 had developed ESRD due to MM.
However, the incidence of ESRD from MM decreased from 2001 to 2010. Compared to 2001-2002 (1.00), the standardized incidence ratios of ESRD due to MM were 0.96 for 2003-2004 (P<0.05), 0.99 for 2005-2006 (P>0.05), 0.89 for 2007-2008 (P<0.001), and 0.82 for 2009-2010 (P<0.001).
The demography-adjusted incidence ratio for ESRD due to MM decreased (P<0.05) between 2001-2002 and 2009-2010 in the overall population and in most of the subgroups examined.
The exceptions were patients younger than 40, Hispanic patients, and those belonging to the “other” race category. (The race categories were “white,” “black,” and “other,” while the ethnicity categories were “Hispanic” and “non-Hispanic.”)
The data also showed improvements in survival over time among patients with ESRD due to MM.
Compared to 2001-2002, the adjusted hazard ratios (AHRs) for death were 1.02 for 2003-2004 (P=0.5), 0.93 for 2005-2006 (P=0.02), 0.86 for 2007-2008 (P<0.001), and 0.74 for 2009-2010 (P<0.001). (This analysis was adjusted for age, sex, race, ethnicity, ischemic heart disease, diabetes, mode of RRT, estimated glomerular filtration rate, body mass index, serum albumin, and hemoglobin.)
AHRs for death were highest in the first year after RRT initiation (AHR=2.6; P<0.001) and decreased in year 3 (AHR=1.59; P<0.001).
The AHR for death in patients with ESRD due to MM compared to those with ESRD due to all other causes was 2.05 (P<0.001).
“Myeloma is the commonest malignancy leading to kidney failure,” Dr Foley said. “It’s encouraging that we found that kidney failure due to multiple myeloma declined considerably over the last decade.” ![]()

Photo by Anna Frodesiak
New research suggests the risk of end-stage renal disease (ESRD) caused by multiple myeloma (MM) is declining, and survival is lengthening for patients who do develop ESRD due to MM.
Researchers said these findings are encouraging, but efforts are still needed to develop effective MM treatments with fewer side effects.
They noted that MM treatment has changed substantially in the last decade.
But it hasn’t been clear whether the burden of ESRD due to MM has changed or whether survival has improved for patients with ESRD due to MM.
To gain some insight, Robert Foley, MD, of the University of Minnesota in Minneapolis, and his colleagues examined data from the US Renal Data System database spanning the period from 2001 to 2010.
They reported their findings in the Journal of the American Society of Nephrology.
The team found that, of the 1,069,343 patients with ESRD who were on renal replacement therapy (RRT), 12,703 had developed ESRD due to MM.
However, the incidence of ESRD from MM decreased from 2001 to 2010. Compared to 2001-2002 (1.00), the standardized incidence ratios of ESRD due to MM were 0.96 for 2003-2004 (P<0.05), 0.99 for 2005-2006 (P>0.05), 0.89 for 2007-2008 (P<0.001), and 0.82 for 2009-2010 (P<0.001).
The demography-adjusted incidence ratio for ESRD due to MM decreased (P<0.05) between 2001-2002 and 2009-2010 in the overall population and in most of the subgroups examined.
The exceptions were patients younger than 40, Hispanic patients, and those belonging to the “other” race category. (The race categories were “white,” “black,” and “other,” while the ethnicity categories were “Hispanic” and “non-Hispanic.”)
The data also showed improvements in survival over time among patients with ESRD due to MM.
Compared to 2001-2002, the adjusted hazard ratios (AHRs) for death were 1.02 for 2003-2004 (P=0.5), 0.93 for 2005-2006 (P=0.02), 0.86 for 2007-2008 (P<0.001), and 0.74 for 2009-2010 (P<0.001). (This analysis was adjusted for age, sex, race, ethnicity, ischemic heart disease, diabetes, mode of RRT, estimated glomerular filtration rate, body mass index, serum albumin, and hemoglobin.)
AHRs for death were highest in the first year after RRT initiation (AHR=2.6; P<0.001) and decreased in year 3 (AHR=1.59; P<0.001).
The AHR for death in patients with ESRD due to MM compared to those with ESRD due to all other causes was 2.05 (P<0.001).
“Myeloma is the commonest malignancy leading to kidney failure,” Dr Foley said. “It’s encouraging that we found that kidney failure due to multiple myeloma declined considerably over the last decade.” ![]()
Cancer care in Latin America

patient and her father
Photo by Rhoda Baer
Despite progress made in cancer care in Latin America in the last 2 years, substantial barriers remain to ensure optimal clinical management, according to a report commissioned by The Lancet Oncology.
The report, an update from a report published in 2013, details a number of improvements in cancer care in Latin America, either specifically related to cancer or to general healthcare initiatives that will also benefit cancer patients.
However, the updated report also suggests that major changes are needed in many areas to increase the standard of cancer care in Latin America.
Progress made
According to the report, progress has been made in the following areas.
The proportion of people in Latin America affiliated with any kind of health insurance program grew from 46% to 60% between 2008 and 2013.
For 2014, the World Health Organization (WHO) reported an 8% increase in the number of countries (60% of the whole region) with a National Cancer Plan. The following countries have newly adopted plans: Suriname, Ecuador, Dominican Republic, Trinidad and Tobago, Puerto Rico, Peru, El Salvador, and Colombia.
In addition, Latin America—most notably, Brazil and Argentina—has begun to address the shortage of cancer specialists.
Brazil has shown an increase of 77% in oncologists since 2011 (from 1457 to 2577). Concurrently, the number of hematologists has also increased by 40% (from 1420 in 2011 to 1985 in 2015), and that of radiotherapists by 12% (from 444 in 2011 to 497 in 2015). These rises are in the context of an 11% increase in cancer cases in Brazil (from 518,000 new cases in 2012 to 576,000 in 2014).
Many countries across Latin America have signed on to the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020, endorsed by the WHO, which aims to achieve a 25% reduction in premature mortality from non-communicable diseases (including cancer) by 2025.
The Colombian Ministry of Health and Social Protection has expanded its social insurance program to cover all types of cancer.
Since January 2014, the administration of chemotherapy and radiation treatments is free of charge in Uruguay.
The Atlas of Palliative Care was published in Latin America, which revealed a growth of more than 400% in the number of palliative services since 2006.
Room for improvement
The report indicates that the following issues are still problems in Latin America.
Compared with high-income countries, Latin America in 2015 remains behind in terms of public expenditure on health and cancer care.
Argentina and Mexico spend around 6% of their gross national product on healthcare, compared to 9% for the UK, 11% for Germany, and 17% for the US, which reflects a gap between Latin American and other countries not only proportionately but also in terms of absolute dollars. Only Brazil, at 9%, is close to the proportion spent in high-income countries.
In Latin America, only Brazil, Cuba, Costa Rica, and Uruguay are considered to have integration of social security and public insurance, and only Brazil, Cuba, and Costa Rica can be judged to have universal healthcare.
Many countries still have no specific training in palliative care (including Bolivia, El Salvador, Honduras, and Nicaragua).
Additionally, data from 2002 showed that Latin America accounted for less than 1% of the world’s opioid drug consumption for pain relief. Consumption of strong opioids still lags behind developed countries today, with no Latin American country exceeding 15 mg/capita per year.
Under-implementation of new technologies has not improved substantially since the previous Lancet Oncology Commission in 2013. There are a few exceptions, however, such as PET scanning technology improvements in Uruguay.
Pharmaceutical trials for expensive new anticancer therapies are largely unhelpful to most patients in Latin America. Patients participating in trials of expensive new anticancer therapies sometimes cannot complete treatment once their trial ends, and the trials often do not lead to approval in these regions.
There are often geographical disparities where most cancer specialists are located in major hospitals in big cities, requiring patients from rural and remote areas to travel far distances to these hospitals for cancer care.
In addition, waiting times in these centers can be unacceptably long, with reports from Mexico and Brazil describing median waiting times of 7 months or more for patients with breast cancer from symptomatic presentation to initial treatment.
Better cancer registries are desperately needed in all Latin American countries to more accurately quantify the cancer burden in the region and the resources required to combat it, according to the report. ![]()

patient and her father
Photo by Rhoda Baer
Despite progress made in cancer care in Latin America in the last 2 years, substantial barriers remain to ensure optimal clinical management, according to a report commissioned by The Lancet Oncology.
The report, an update from a report published in 2013, details a number of improvements in cancer care in Latin America, either specifically related to cancer or to general healthcare initiatives that will also benefit cancer patients.
However, the updated report also suggests that major changes are needed in many areas to increase the standard of cancer care in Latin America.
Progress made
According to the report, progress has been made in the following areas.
The proportion of people in Latin America affiliated with any kind of health insurance program grew from 46% to 60% between 2008 and 2013.
For 2014, the World Health Organization (WHO) reported an 8% increase in the number of countries (60% of the whole region) with a National Cancer Plan. The following countries have newly adopted plans: Suriname, Ecuador, Dominican Republic, Trinidad and Tobago, Puerto Rico, Peru, El Salvador, and Colombia.
In addition, Latin America—most notably, Brazil and Argentina—has begun to address the shortage of cancer specialists.
Brazil has shown an increase of 77% in oncologists since 2011 (from 1457 to 2577). Concurrently, the number of hematologists has also increased by 40% (from 1420 in 2011 to 1985 in 2015), and that of radiotherapists by 12% (from 444 in 2011 to 497 in 2015). These rises are in the context of an 11% increase in cancer cases in Brazil (from 518,000 new cases in 2012 to 576,000 in 2014).
Many countries across Latin America have signed on to the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020, endorsed by the WHO, which aims to achieve a 25% reduction in premature mortality from non-communicable diseases (including cancer) by 2025.
The Colombian Ministry of Health and Social Protection has expanded its social insurance program to cover all types of cancer.
Since January 2014, the administration of chemotherapy and radiation treatments is free of charge in Uruguay.
The Atlas of Palliative Care was published in Latin America, which revealed a growth of more than 400% in the number of palliative services since 2006.
Room for improvement
The report indicates that the following issues are still problems in Latin America.
Compared with high-income countries, Latin America in 2015 remains behind in terms of public expenditure on health and cancer care.
Argentina and Mexico spend around 6% of their gross national product on healthcare, compared to 9% for the UK, 11% for Germany, and 17% for the US, which reflects a gap between Latin American and other countries not only proportionately but also in terms of absolute dollars. Only Brazil, at 9%, is close to the proportion spent in high-income countries.
In Latin America, only Brazil, Cuba, Costa Rica, and Uruguay are considered to have integration of social security and public insurance, and only Brazil, Cuba, and Costa Rica can be judged to have universal healthcare.
Many countries still have no specific training in palliative care (including Bolivia, El Salvador, Honduras, and Nicaragua).
Additionally, data from 2002 showed that Latin America accounted for less than 1% of the world’s opioid drug consumption for pain relief. Consumption of strong opioids still lags behind developed countries today, with no Latin American country exceeding 15 mg/capita per year.
Under-implementation of new technologies has not improved substantially since the previous Lancet Oncology Commission in 2013. There are a few exceptions, however, such as PET scanning technology improvements in Uruguay.
Pharmaceutical trials for expensive new anticancer therapies are largely unhelpful to most patients in Latin America. Patients participating in trials of expensive new anticancer therapies sometimes cannot complete treatment once their trial ends, and the trials often do not lead to approval in these regions.
There are often geographical disparities where most cancer specialists are located in major hospitals in big cities, requiring patients from rural and remote areas to travel far distances to these hospitals for cancer care.
In addition, waiting times in these centers can be unacceptably long, with reports from Mexico and Brazil describing median waiting times of 7 months or more for patients with breast cancer from symptomatic presentation to initial treatment.
Better cancer registries are desperately needed in all Latin American countries to more accurately quantify the cancer burden in the region and the resources required to combat it, according to the report. ![]()

patient and her father
Photo by Rhoda Baer
Despite progress made in cancer care in Latin America in the last 2 years, substantial barriers remain to ensure optimal clinical management, according to a report commissioned by The Lancet Oncology.
The report, an update from a report published in 2013, details a number of improvements in cancer care in Latin America, either specifically related to cancer or to general healthcare initiatives that will also benefit cancer patients.
However, the updated report also suggests that major changes are needed in many areas to increase the standard of cancer care in Latin America.
Progress made
According to the report, progress has been made in the following areas.
The proportion of people in Latin America affiliated with any kind of health insurance program grew from 46% to 60% between 2008 and 2013.
For 2014, the World Health Organization (WHO) reported an 8% increase in the number of countries (60% of the whole region) with a National Cancer Plan. The following countries have newly adopted plans: Suriname, Ecuador, Dominican Republic, Trinidad and Tobago, Puerto Rico, Peru, El Salvador, and Colombia.
In addition, Latin America—most notably, Brazil and Argentina—has begun to address the shortage of cancer specialists.
Brazil has shown an increase of 77% in oncologists since 2011 (from 1457 to 2577). Concurrently, the number of hematologists has also increased by 40% (from 1420 in 2011 to 1985 in 2015), and that of radiotherapists by 12% (from 444 in 2011 to 497 in 2015). These rises are in the context of an 11% increase in cancer cases in Brazil (from 518,000 new cases in 2012 to 576,000 in 2014).
Many countries across Latin America have signed on to the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020, endorsed by the WHO, which aims to achieve a 25% reduction in premature mortality from non-communicable diseases (including cancer) by 2025.
The Colombian Ministry of Health and Social Protection has expanded its social insurance program to cover all types of cancer.
Since January 2014, the administration of chemotherapy and radiation treatments is free of charge in Uruguay.
The Atlas of Palliative Care was published in Latin America, which revealed a growth of more than 400% in the number of palliative services since 2006.
Room for improvement
The report indicates that the following issues are still problems in Latin America.
Compared with high-income countries, Latin America in 2015 remains behind in terms of public expenditure on health and cancer care.
Argentina and Mexico spend around 6% of their gross national product on healthcare, compared to 9% for the UK, 11% for Germany, and 17% for the US, which reflects a gap between Latin American and other countries not only proportionately but also in terms of absolute dollars. Only Brazil, at 9%, is close to the proportion spent in high-income countries.
In Latin America, only Brazil, Cuba, Costa Rica, and Uruguay are considered to have integration of social security and public insurance, and only Brazil, Cuba, and Costa Rica can be judged to have universal healthcare.
Many countries still have no specific training in palliative care (including Bolivia, El Salvador, Honduras, and Nicaragua).
Additionally, data from 2002 showed that Latin America accounted for less than 1% of the world’s opioid drug consumption for pain relief. Consumption of strong opioids still lags behind developed countries today, with no Latin American country exceeding 15 mg/capita per year.
Under-implementation of new technologies has not improved substantially since the previous Lancet Oncology Commission in 2013. There are a few exceptions, however, such as PET scanning technology improvements in Uruguay.
Pharmaceutical trials for expensive new anticancer therapies are largely unhelpful to most patients in Latin America. Patients participating in trials of expensive new anticancer therapies sometimes cannot complete treatment once their trial ends, and the trials often do not lead to approval in these regions.
There are often geographical disparities where most cancer specialists are located in major hospitals in big cities, requiring patients from rural and remote areas to travel far distances to these hospitals for cancer care.
In addition, waiting times in these centers can be unacceptably long, with reports from Mexico and Brazil describing median waiting times of 7 months or more for patients with breast cancer from symptomatic presentation to initial treatment.
Better cancer registries are desperately needed in all Latin American countries to more accurately quantify the cancer burden in the region and the resources required to combat it, according to the report. ![]()
Ibrutinib may prove useful in MM, research shows

showing multiple myeloma
NEW YORK—Results of an open-label, phase 2, dose-escalation study of ibrutinib combined with low-dose dexamethasone suggest the Bruton’s tyrosine kinase (BTK) inhibitor may be useful in treating relapsed or relapsed and refractory patients with multiple myeloma (MM).
In the highest dose cohort, 23% of patients experienced a clinical benefit, which was defined as a minimal response or better by International Myeloma Working Group criteria.
“Ibrutinib is a remarkable agent and, through BTK inhibition, has been a game-changer in CLL [chronic lymphocytic leukemia],” said Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Richardson reported results with ibrutinib in MM at Lymphoma & Myeloma 2015.
The rationale for the use of ibrutinib in myeloma is the robust BTK expression in the plasma cells of MM patients as well as its regulation of myeloma “stemness” in the bone marrow.
Ibrutinib plus low-dose dexamethasone has previously demonstrated activity in myeloma at the highest dose tested in a phase 1 trial.
So investigators continued to evaluate ibrutinib in 4 dose cohorts, with and without dexamathesone, and enrolled 92 relapsed/refractory MM patients. Dr Richardson described the results of cohort 4, which met criteria for expansion as of March 2015.
The investigators enrolled 43 patients in cohort 4 to receive 840 mg daily of ibrutinib plus 40 mg of dexamethasone weekly. This dose of ibrutinib is double the dose approved by the US Food and Drug Administration (FDA) for treatment of CLL and Waldenström’s macroglobulinemia.
The patient population was “very typical” for a relapsed/refractory patient population, Dr Richardson noted. The median age was 65 years (range, 43–81), almost two-thirds were male, and they had a median of 4 (range, 2–10) prior therapies.
More than half of patients had an ECOG performance status of 0 or 1, and their median time since diagnosis was 6.5 years.
Nineteen percent had t(11;14), and other chromosomal abnormalities included del 13q14, t(4:14), and del 17p. Twenty-three patients were ISS stage I, 16 were ISS stage II, and 4 were ISS stage III.
Seventy-seven percent of patients had received an autologous stem cell transplant, 95% had prior alkylator treatment, 91% prior lenalidomide, 58% prior thalidomide, and 91% prior bortezomib treatment.
All patients were steroid-refractory. Thirty-five percent were refractory to alkylator treatment, 61% were refractory to lenalidomide, and 49% were refractory to bortezomib.
“And even a number of them had been exposed to pomalidomide and also to carfilzomib,” Dr Richardson said, “recognizing that these are the new and exciting drugs on the block, but, at the same time, a significant number of our patients already received those therapies.”
Five percent of patients achieved a partial response to ibrutinib and low-dose dexamethasone, and 18% achieved a minimal response, for a clinical benefit rate of 23%. Thirty percent had stable disease after 4 or more cycles.
“What was particularly striking,” Dr Richardson said, “was at the top dose, the 840-mg dose, the progression-free survival.”
The median time to disease progression was 5.4 months (range, 0.0–16.4), which the investigators thought was compelling in this large study.
Dr Richardson noted that there were no significant differences in safety observed across all dose cohorts, although 55% of patients experienced a grade 3 or greater treatment-emergent adverse event (AE), while 29% experienced at least 1 serious AE. And 10% of patients experienced peripheral neuropathy (PN), 8 of whom had a prior history of PN.
Treatment-emergent hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included anemia (23%) and thrombocytopenia (21%). Grade 3/4 anemia and thrombocytopenia occurred in 9% of patients in each category.
Treatment-emergent non-hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included diarrhea (63%), fatigue (49%), cough (26%), nausea (23%), and muscle spasms (21%). Two percent of patients had grade 3/4 diarrhea. There were no other grade 3/4 adverse events in the cohort.
Eighty-six percent of patients in cohort 4 discontinued treatment, 60% due to progressive disease, 12% due to an AE, and 2% at the discretion of the investigator. Twelve percent withdrew, were noncompliant, or required concomitant medication that was not permitted by the protocol.
Overall, Dr Richardson said, the safety profile was manageable, similar across the dosing cohorts, and consistent with those seen in CLL and Waldenström’s macroglobulinemia. The 840-mg dose did not increase toxicity and demonstrated activity in this heavily pretreated population.
“The real signal that struck us,” Dr Richardson emphasized, “was the progression-free survival at 5.4 months . . . for a 92-patient, multicenter experience, this is obviously, I think, an encouraging start.”
Investigators continue to explore ibrutinib in 2 ongoing combination studies, one with carfilzomib and dexamethasone (PCYC-1119) and another with pomalidomide and dexamethasone (PCYC-1138). Another trial with lenalidomide and dexamethasone is planned.
Ibrutinib now has 4 FDA-approved indications: patients with CLL who have received at least 1 prior therapy, CLL patients with del 17p, patients with mantle cell lymphoma, and patients with Waldenström’s macroglobulinemia.
Ibrutinib is distributed and marketed as Imbruvica by Pharmacyclics and also marketed by Janssen Biotech, Inc. ![]()

showing multiple myeloma
NEW YORK—Results of an open-label, phase 2, dose-escalation study of ibrutinib combined with low-dose dexamethasone suggest the Bruton’s tyrosine kinase (BTK) inhibitor may be useful in treating relapsed or relapsed and refractory patients with multiple myeloma (MM).
In the highest dose cohort, 23% of patients experienced a clinical benefit, which was defined as a minimal response or better by International Myeloma Working Group criteria.
“Ibrutinib is a remarkable agent and, through BTK inhibition, has been a game-changer in CLL [chronic lymphocytic leukemia],” said Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Richardson reported results with ibrutinib in MM at Lymphoma & Myeloma 2015.
The rationale for the use of ibrutinib in myeloma is the robust BTK expression in the plasma cells of MM patients as well as its regulation of myeloma “stemness” in the bone marrow.
Ibrutinib plus low-dose dexamethasone has previously demonstrated activity in myeloma at the highest dose tested in a phase 1 trial.
So investigators continued to evaluate ibrutinib in 4 dose cohorts, with and without dexamathesone, and enrolled 92 relapsed/refractory MM patients. Dr Richardson described the results of cohort 4, which met criteria for expansion as of March 2015.
The investigators enrolled 43 patients in cohort 4 to receive 840 mg daily of ibrutinib plus 40 mg of dexamethasone weekly. This dose of ibrutinib is double the dose approved by the US Food and Drug Administration (FDA) for treatment of CLL and Waldenström’s macroglobulinemia.
The patient population was “very typical” for a relapsed/refractory patient population, Dr Richardson noted. The median age was 65 years (range, 43–81), almost two-thirds were male, and they had a median of 4 (range, 2–10) prior therapies.
More than half of patients had an ECOG performance status of 0 or 1, and their median time since diagnosis was 6.5 years.
Nineteen percent had t(11;14), and other chromosomal abnormalities included del 13q14, t(4:14), and del 17p. Twenty-three patients were ISS stage I, 16 were ISS stage II, and 4 were ISS stage III.
Seventy-seven percent of patients had received an autologous stem cell transplant, 95% had prior alkylator treatment, 91% prior lenalidomide, 58% prior thalidomide, and 91% prior bortezomib treatment.
All patients were steroid-refractory. Thirty-five percent were refractory to alkylator treatment, 61% were refractory to lenalidomide, and 49% were refractory to bortezomib.
“And even a number of them had been exposed to pomalidomide and also to carfilzomib,” Dr Richardson said, “recognizing that these are the new and exciting drugs on the block, but, at the same time, a significant number of our patients already received those therapies.”
Five percent of patients achieved a partial response to ibrutinib and low-dose dexamethasone, and 18% achieved a minimal response, for a clinical benefit rate of 23%. Thirty percent had stable disease after 4 or more cycles.
“What was particularly striking,” Dr Richardson said, “was at the top dose, the 840-mg dose, the progression-free survival.”
The median time to disease progression was 5.4 months (range, 0.0–16.4), which the investigators thought was compelling in this large study.
Dr Richardson noted that there were no significant differences in safety observed across all dose cohorts, although 55% of patients experienced a grade 3 or greater treatment-emergent adverse event (AE), while 29% experienced at least 1 serious AE. And 10% of patients experienced peripheral neuropathy (PN), 8 of whom had a prior history of PN.
Treatment-emergent hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included anemia (23%) and thrombocytopenia (21%). Grade 3/4 anemia and thrombocytopenia occurred in 9% of patients in each category.
Treatment-emergent non-hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included diarrhea (63%), fatigue (49%), cough (26%), nausea (23%), and muscle spasms (21%). Two percent of patients had grade 3/4 diarrhea. There were no other grade 3/4 adverse events in the cohort.
Eighty-six percent of patients in cohort 4 discontinued treatment, 60% due to progressive disease, 12% due to an AE, and 2% at the discretion of the investigator. Twelve percent withdrew, were noncompliant, or required concomitant medication that was not permitted by the protocol.
Overall, Dr Richardson said, the safety profile was manageable, similar across the dosing cohorts, and consistent with those seen in CLL and Waldenström’s macroglobulinemia. The 840-mg dose did not increase toxicity and demonstrated activity in this heavily pretreated population.
“The real signal that struck us,” Dr Richardson emphasized, “was the progression-free survival at 5.4 months . . . for a 92-patient, multicenter experience, this is obviously, I think, an encouraging start.”
Investigators continue to explore ibrutinib in 2 ongoing combination studies, one with carfilzomib and dexamethasone (PCYC-1119) and another with pomalidomide and dexamethasone (PCYC-1138). Another trial with lenalidomide and dexamethasone is planned.
Ibrutinib now has 4 FDA-approved indications: patients with CLL who have received at least 1 prior therapy, CLL patients with del 17p, patients with mantle cell lymphoma, and patients with Waldenström’s macroglobulinemia.
Ibrutinib is distributed and marketed as Imbruvica by Pharmacyclics and also marketed by Janssen Biotech, Inc. ![]()

showing multiple myeloma
NEW YORK—Results of an open-label, phase 2, dose-escalation study of ibrutinib combined with low-dose dexamethasone suggest the Bruton’s tyrosine kinase (BTK) inhibitor may be useful in treating relapsed or relapsed and refractory patients with multiple myeloma (MM).
In the highest dose cohort, 23% of patients experienced a clinical benefit, which was defined as a minimal response or better by International Myeloma Working Group criteria.
“Ibrutinib is a remarkable agent and, through BTK inhibition, has been a game-changer in CLL [chronic lymphocytic leukemia],” said Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Richardson reported results with ibrutinib in MM at Lymphoma & Myeloma 2015.
The rationale for the use of ibrutinib in myeloma is the robust BTK expression in the plasma cells of MM patients as well as its regulation of myeloma “stemness” in the bone marrow.
Ibrutinib plus low-dose dexamethasone has previously demonstrated activity in myeloma at the highest dose tested in a phase 1 trial.
So investigators continued to evaluate ibrutinib in 4 dose cohorts, with and without dexamathesone, and enrolled 92 relapsed/refractory MM patients. Dr Richardson described the results of cohort 4, which met criteria for expansion as of March 2015.
The investigators enrolled 43 patients in cohort 4 to receive 840 mg daily of ibrutinib plus 40 mg of dexamethasone weekly. This dose of ibrutinib is double the dose approved by the US Food and Drug Administration (FDA) for treatment of CLL and Waldenström’s macroglobulinemia.
The patient population was “very typical” for a relapsed/refractory patient population, Dr Richardson noted. The median age was 65 years (range, 43–81), almost two-thirds were male, and they had a median of 4 (range, 2–10) prior therapies.
More than half of patients had an ECOG performance status of 0 or 1, and their median time since diagnosis was 6.5 years.
Nineteen percent had t(11;14), and other chromosomal abnormalities included del 13q14, t(4:14), and del 17p. Twenty-three patients were ISS stage I, 16 were ISS stage II, and 4 were ISS stage III.
Seventy-seven percent of patients had received an autologous stem cell transplant, 95% had prior alkylator treatment, 91% prior lenalidomide, 58% prior thalidomide, and 91% prior bortezomib treatment.
All patients were steroid-refractory. Thirty-five percent were refractory to alkylator treatment, 61% were refractory to lenalidomide, and 49% were refractory to bortezomib.
“And even a number of them had been exposed to pomalidomide and also to carfilzomib,” Dr Richardson said, “recognizing that these are the new and exciting drugs on the block, but, at the same time, a significant number of our patients already received those therapies.”
Five percent of patients achieved a partial response to ibrutinib and low-dose dexamethasone, and 18% achieved a minimal response, for a clinical benefit rate of 23%. Thirty percent had stable disease after 4 or more cycles.
“What was particularly striking,” Dr Richardson said, “was at the top dose, the 840-mg dose, the progression-free survival.”
The median time to disease progression was 5.4 months (range, 0.0–16.4), which the investigators thought was compelling in this large study.
Dr Richardson noted that there were no significant differences in safety observed across all dose cohorts, although 55% of patients experienced a grade 3 or greater treatment-emergent adverse event (AE), while 29% experienced at least 1 serious AE. And 10% of patients experienced peripheral neuropathy (PN), 8 of whom had a prior history of PN.
Treatment-emergent hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included anemia (23%) and thrombocytopenia (21%). Grade 3/4 anemia and thrombocytopenia occurred in 9% of patients in each category.
Treatment-emergent non-hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included diarrhea (63%), fatigue (49%), cough (26%), nausea (23%), and muscle spasms (21%). Two percent of patients had grade 3/4 diarrhea. There were no other grade 3/4 adverse events in the cohort.
Eighty-six percent of patients in cohort 4 discontinued treatment, 60% due to progressive disease, 12% due to an AE, and 2% at the discretion of the investigator. Twelve percent withdrew, were noncompliant, or required concomitant medication that was not permitted by the protocol.
Overall, Dr Richardson said, the safety profile was manageable, similar across the dosing cohorts, and consistent with those seen in CLL and Waldenström’s macroglobulinemia. The 840-mg dose did not increase toxicity and demonstrated activity in this heavily pretreated population.
“The real signal that struck us,” Dr Richardson emphasized, “was the progression-free survival at 5.4 months . . . for a 92-patient, multicenter experience, this is obviously, I think, an encouraging start.”
Investigators continue to explore ibrutinib in 2 ongoing combination studies, one with carfilzomib and dexamethasone (PCYC-1119) and another with pomalidomide and dexamethasone (PCYC-1138). Another trial with lenalidomide and dexamethasone is planned.
Ibrutinib now has 4 FDA-approved indications: patients with CLL who have received at least 1 prior therapy, CLL patients with del 17p, patients with mantle cell lymphoma, and patients with Waldenström’s macroglobulinemia.
Ibrutinib is distributed and marketed as Imbruvica by Pharmacyclics and also marketed by Janssen Biotech, Inc. ![]()
Rapid, cheap test prognostic in multiple myeloma
A rapid, low-cost assay measuring DNA and immunoglobulin in multiple myeloma cells is both a powerful predictor of prognosis and a tool for identifying new, previously unidentified therapeutic targets, its developers say.
The test, labeled DNA/CIG, uses two-color flow cytometry to evaluate DNA in the nucleus and immunoglobulin in the cytoplasm of bone marrow taken from patients with multiple myeloma (MM) at baseline, before the start of the aggressive “Total Therapy 3b” (TT3b) protocol developed at the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences in Little Rock.
The light chain cytoplasmic immunoglobulin (CIg) index generated by the test, combined with an associated 12-gene panel, serves as an independent prognostic factor in patients with myeloma. The CIg index is a marker for immunoglobulin production in myeloma cells, with lower index scores suggesting ineffective immunologic responses.
“We show that the presence of low CIg as detected by DNA/CIG is a major adverse prognostic factor in TT3b, even when other GEP [gene expression profiling]–derived prognostic factors were accounted for,” Dr. Xenon Papanikolaou of the University of Arkansas and colleagues wrote in the journal Leukemia (2015;29:1713-20).
The investigators performed DNA/CIG as part of the routine work-up of patients with newly diagnosed MM who were scheduled to undergo the current iteration of the Total Therapy protocol, consisting of two induction chemotherapy cycles of VTD-PACE (bortezomib, thalidomide, dexamethasone and 4-day continuous infusions of cisplatin, doxorubicin, cyclophosphamide and etoposide), two planned hematopoietic stem cell transplants, and 3 years of planned maintenance with bortezomib, lenalidomide and dexamethasone.
The authors looked at the association of the CIg index, gene-expression profiling, and standard prognostic measures with both overall and progression-free survival (OS and PFS).
They found that both the percentage of light chain–restricted cells and the CIg as determined by DNA/CIG were associated with poor clinical outcomes. Additionally, a CIg lower than 2.8, albumin lower than 3.5 g/dL, and age 65 and older were significantly associated with worse PFS and OS, independent of gene-expression profiling data.
Any CIg lower than 2.8 was associated with a twofold risk for worse OS (hazard ratio, 2.08; P = .012) and a nearly doubling of risk for worse PFS (HR, 1.84; P = .001).
In multivariate models, CIg remained an independent predictor for prognosis when GEP-defined high-risk and low albumin were added in. CIg was also linked to other prognostic factors, including beta2-microglobulin greater than 5.5 mg/L, percentage of LCR cells exceeding 50%, C-reactive protein equal to or greater than 8 mg/L, and GEP-derived high centrosome index.
Low CIg was also associated with an experimental group of 12 gene probes believed to be involved in cell cycle regulation, cell differentiation, and cellular drug transport. The investigators used the gene signatures to develop a risk score, called GEP12, that offered important prognostic information for patients in TT3b and in earlier clinical studies.
The finding that a low CIg index was linked to a high centrosome index as defined by GEP points to a possible role for CIg index as a treatment selection tool.
“Interestingly, a centrosome inhibitor has shown promising activity in preclinical models of MM, thus potentially providing a selective drug for patients with low CIg myeloma,” the authors write.
The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
A rapid, low-cost assay measuring DNA and immunoglobulin in multiple myeloma cells is both a powerful predictor of prognosis and a tool for identifying new, previously unidentified therapeutic targets, its developers say.
The test, labeled DNA/CIG, uses two-color flow cytometry to evaluate DNA in the nucleus and immunoglobulin in the cytoplasm of bone marrow taken from patients with multiple myeloma (MM) at baseline, before the start of the aggressive “Total Therapy 3b” (TT3b) protocol developed at the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences in Little Rock.
The light chain cytoplasmic immunoglobulin (CIg) index generated by the test, combined with an associated 12-gene panel, serves as an independent prognostic factor in patients with myeloma. The CIg index is a marker for immunoglobulin production in myeloma cells, with lower index scores suggesting ineffective immunologic responses.
“We show that the presence of low CIg as detected by DNA/CIG is a major adverse prognostic factor in TT3b, even when other GEP [gene expression profiling]–derived prognostic factors were accounted for,” Dr. Xenon Papanikolaou of the University of Arkansas and colleagues wrote in the journal Leukemia (2015;29:1713-20).
The investigators performed DNA/CIG as part of the routine work-up of patients with newly diagnosed MM who were scheduled to undergo the current iteration of the Total Therapy protocol, consisting of two induction chemotherapy cycles of VTD-PACE (bortezomib, thalidomide, dexamethasone and 4-day continuous infusions of cisplatin, doxorubicin, cyclophosphamide and etoposide), two planned hematopoietic stem cell transplants, and 3 years of planned maintenance with bortezomib, lenalidomide and dexamethasone.
The authors looked at the association of the CIg index, gene-expression profiling, and standard prognostic measures with both overall and progression-free survival (OS and PFS).
They found that both the percentage of light chain–restricted cells and the CIg as determined by DNA/CIG were associated with poor clinical outcomes. Additionally, a CIg lower than 2.8, albumin lower than 3.5 g/dL, and age 65 and older were significantly associated with worse PFS and OS, independent of gene-expression profiling data.
Any CIg lower than 2.8 was associated with a twofold risk for worse OS (hazard ratio, 2.08; P = .012) and a nearly doubling of risk for worse PFS (HR, 1.84; P = .001).
In multivariate models, CIg remained an independent predictor for prognosis when GEP-defined high-risk and low albumin were added in. CIg was also linked to other prognostic factors, including beta2-microglobulin greater than 5.5 mg/L, percentage of LCR cells exceeding 50%, C-reactive protein equal to or greater than 8 mg/L, and GEP-derived high centrosome index.
Low CIg was also associated with an experimental group of 12 gene probes believed to be involved in cell cycle regulation, cell differentiation, and cellular drug transport. The investigators used the gene signatures to develop a risk score, called GEP12, that offered important prognostic information for patients in TT3b and in earlier clinical studies.
The finding that a low CIg index was linked to a high centrosome index as defined by GEP points to a possible role for CIg index as a treatment selection tool.
“Interestingly, a centrosome inhibitor has shown promising activity in preclinical models of MM, thus potentially providing a selective drug for patients with low CIg myeloma,” the authors write.
The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
A rapid, low-cost assay measuring DNA and immunoglobulin in multiple myeloma cells is both a powerful predictor of prognosis and a tool for identifying new, previously unidentified therapeutic targets, its developers say.
The test, labeled DNA/CIG, uses two-color flow cytometry to evaluate DNA in the nucleus and immunoglobulin in the cytoplasm of bone marrow taken from patients with multiple myeloma (MM) at baseline, before the start of the aggressive “Total Therapy 3b” (TT3b) protocol developed at the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences in Little Rock.
The light chain cytoplasmic immunoglobulin (CIg) index generated by the test, combined with an associated 12-gene panel, serves as an independent prognostic factor in patients with myeloma. The CIg index is a marker for immunoglobulin production in myeloma cells, with lower index scores suggesting ineffective immunologic responses.
“We show that the presence of low CIg as detected by DNA/CIG is a major adverse prognostic factor in TT3b, even when other GEP [gene expression profiling]–derived prognostic factors were accounted for,” Dr. Xenon Papanikolaou of the University of Arkansas and colleagues wrote in the journal Leukemia (2015;29:1713-20).
The investigators performed DNA/CIG as part of the routine work-up of patients with newly diagnosed MM who were scheduled to undergo the current iteration of the Total Therapy protocol, consisting of two induction chemotherapy cycles of VTD-PACE (bortezomib, thalidomide, dexamethasone and 4-day continuous infusions of cisplatin, doxorubicin, cyclophosphamide and etoposide), two planned hematopoietic stem cell transplants, and 3 years of planned maintenance with bortezomib, lenalidomide and dexamethasone.
The authors looked at the association of the CIg index, gene-expression profiling, and standard prognostic measures with both overall and progression-free survival (OS and PFS).
They found that both the percentage of light chain–restricted cells and the CIg as determined by DNA/CIG were associated with poor clinical outcomes. Additionally, a CIg lower than 2.8, albumin lower than 3.5 g/dL, and age 65 and older were significantly associated with worse PFS and OS, independent of gene-expression profiling data.
Any CIg lower than 2.8 was associated with a twofold risk for worse OS (hazard ratio, 2.08; P = .012) and a nearly doubling of risk for worse PFS (HR, 1.84; P = .001).
In multivariate models, CIg remained an independent predictor for prognosis when GEP-defined high-risk and low albumin were added in. CIg was also linked to other prognostic factors, including beta2-microglobulin greater than 5.5 mg/L, percentage of LCR cells exceeding 50%, C-reactive protein equal to or greater than 8 mg/L, and GEP-derived high centrosome index.
Low CIg was also associated with an experimental group of 12 gene probes believed to be involved in cell cycle regulation, cell differentiation, and cellular drug transport. The investigators used the gene signatures to develop a risk score, called GEP12, that offered important prognostic information for patients in TT3b and in earlier clinical studies.
The finding that a low CIg index was linked to a high centrosome index as defined by GEP points to a possible role for CIg index as a treatment selection tool.
“Interestingly, a centrosome inhibitor has shown promising activity in preclinical models of MM, thus potentially providing a selective drug for patients with low CIg myeloma,” the authors write.
The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
FROM LEUKEMIA
Key clinical point: Cytoplasmic immunoglobulin index (CIg) is prognostic of clinical outcomes in patients with multiple myeloma.
Major finding: A CIg lower than 2.8 was prognostic for poor overall and progression-free survival, with hazard ratios of 2.08 and 1.84, respectively.
Data source: Prospective study in 177 patients with newly diagnosed multiple myeloma.
Disclosures: The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
How a cancer diagnosis affects income

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with cancer, they experience significant decreases in the probability of working, in the number of hours they work, and correspondingly, in their incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with cancer, they experience significant decreases in the probability of working, in the number of hours they work, and correspondingly, in their incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with cancer, they experience significant decreases in the probability of working, in the number of hours they work, and correspondingly, in their incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.”
New melphalan formulation denied approval

Photo by Chad McNeeley
The US Food and Drug Administration (FDA) has said that, at present, it cannot approve a propylene glycol-free melphalan formulation (Evomela) for use in patients with multiple myeloma (MM).
Spectrum Pharmaceuticals is seeking approval for Evomela as a high-dose conditioning treatment for MM patients undergoing hematopoietic stem cell transplant (HSCT) and for palliative treatment in MM patients for whom oral therapy is not appropriate.
The FDA issued a Complete Response Letter stating that the new drug application (NDA) for Evomela cannot be approved in its present form.
However, the FDA did not identify any clinical deficiency in the NDA package.
“We will work swiftly with the FDA to address the Complete Response Letter,” said Rajesh C. Shrotriya, MD, chairman and chief executive officer of Spectrum Pharmaceuticals. “We remain committed to bringing Evomela to the market for patients and plan to work closely with the FDA.”
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time to 5 hours. This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
Captisol is a patent-protected, chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013. Spectrum assumed responsibility for completing the pivotal phase 2 clinical trial and was responsible for filing the NDA. Spectrum filed the NDA in December 2014, and the FDA accepted the application the following March.
The FDA has granted Evomela orphan drug designation for use as a high-dose conditioning regimen for MM patients undergoing HSCT.
Phase 2 study
Researchers have evaluated Evomela in a phase 2, multicenter trial. Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation last month.
The latest publication includes data on 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT. The patients received Evomela at 200 mg/m2 given as 2 doses on Day -3 and Day -2 prior to HSCT (Day 0).
Efficacy was assessed by clinical response at Day +100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
All 5 patients who had previously relapsed from a prior HSCT responded to Evomela.
All patients in the study achieved myeloablation with a median of 5 days post-HSCT. All patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified.

Photo by Chad McNeeley
The US Food and Drug Administration (FDA) has said that, at present, it cannot approve a propylene glycol-free melphalan formulation (Evomela) for use in patients with multiple myeloma (MM).
Spectrum Pharmaceuticals is seeking approval for Evomela as a high-dose conditioning treatment for MM patients undergoing hematopoietic stem cell transplant (HSCT) and for palliative treatment in MM patients for whom oral therapy is not appropriate.
The FDA issued a Complete Response Letter stating that the new drug application (NDA) for Evomela cannot be approved in its present form.
However, the FDA did not identify any clinical deficiency in the NDA package.
“We will work swiftly with the FDA to address the Complete Response Letter,” said Rajesh C. Shrotriya, MD, chairman and chief executive officer of Spectrum Pharmaceuticals. “We remain committed to bringing Evomela to the market for patients and plan to work closely with the FDA.”
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time to 5 hours. This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
Captisol is a patent-protected, chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013. Spectrum assumed responsibility for completing the pivotal phase 2 clinical trial and was responsible for filing the NDA. Spectrum filed the NDA in December 2014, and the FDA accepted the application the following March.
The FDA has granted Evomela orphan drug designation for use as a high-dose conditioning regimen for MM patients undergoing HSCT.
Phase 2 study
Researchers have evaluated Evomela in a phase 2, multicenter trial. Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation last month.
The latest publication includes data on 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT. The patients received Evomela at 200 mg/m2 given as 2 doses on Day -3 and Day -2 prior to HSCT (Day 0).
Efficacy was assessed by clinical response at Day +100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
All 5 patients who had previously relapsed from a prior HSCT responded to Evomela.
All patients in the study achieved myeloablation with a median of 5 days post-HSCT. All patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified.

Photo by Chad McNeeley
The US Food and Drug Administration (FDA) has said that, at present, it cannot approve a propylene glycol-free melphalan formulation (Evomela) for use in patients with multiple myeloma (MM).
Spectrum Pharmaceuticals is seeking approval for Evomela as a high-dose conditioning treatment for MM patients undergoing hematopoietic stem cell transplant (HSCT) and for palliative treatment in MM patients for whom oral therapy is not appropriate.
The FDA issued a Complete Response Letter stating that the new drug application (NDA) for Evomela cannot be approved in its present form.
However, the FDA did not identify any clinical deficiency in the NDA package.
“We will work swiftly with the FDA to address the Complete Response Letter,” said Rajesh C. Shrotriya, MD, chairman and chief executive officer of Spectrum Pharmaceuticals. “We remain committed to bringing Evomela to the market for patients and plan to work closely with the FDA.”
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time to 5 hours. This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
Captisol is a patent-protected, chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013. Spectrum assumed responsibility for completing the pivotal phase 2 clinical trial and was responsible for filing the NDA. Spectrum filed the NDA in December 2014, and the FDA accepted the application the following March.
The FDA has granted Evomela orphan drug designation for use as a high-dose conditioning regimen for MM patients undergoing HSCT.
Phase 2 study
Researchers have evaluated Evomela in a phase 2, multicenter trial. Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation last month.
The latest publication includes data on 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT. The patients received Evomela at 200 mg/m2 given as 2 doses on Day -3 and Day -2 prior to HSCT (Day 0).
Efficacy was assessed by clinical response at Day +100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
All 5 patients who had previously relapsed from a prior HSCT responded to Evomela.
All patients in the study achieved myeloablation with a median of 5 days post-HSCT. All patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified.

