User login
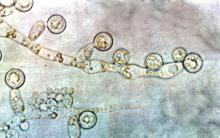
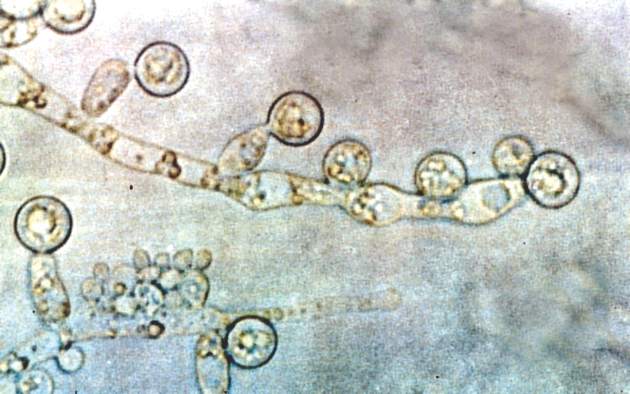
Blood Aspergillus RNA a promising biomarker for invasive aspergillosis
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
FROM MEDICAL MYCOLOGY
Key clinical point: Elevated Aspergillus RNA blood levels during the first 4-6 weeks of antifungal treatment predicts poor response at week 12.
Major finding: Eleven of 14 patients who did not respond to antifungals at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, versus 11 of 27 (41%) who did respond (P = .046).
Data source: Small observational study of patients with proven or probable invasive aspergillosis.
Disclosures: This work was funded by Merck. Four investigators are current or former employees.
PCR better at diagnosing fungal chronic rhinosinusitis than cultures
ITS1/ITS2 polymerase chain reaction sequencing was significantly better at identifying fungal chronic rhinosinusitis (CRS) than were cultures, according to Dr. Pauline Comacle and her associates.
A total of 70 samples from 61 patients with CRS were tested via the use of polymerase chain reaction (PCR) testing and cultures. Fungal CRS was identified in 42 patients, of whom 37 had a fungus ball, 3 had allergic fungal CRS, and 2 had unspecified fungal CRS. ITS1/ITS2 PCR sequencing successfully identified fungal CRS in all patients, while cultures were positive for fungal CRS in 20 of the 42 patients.
Aspergillus fumigatus was the most common fungus in the study, occurring in 69% of patients. Cladosporium cladosporoides occurred in 9.5% of patients, and A. nidulans, A. flavus, and Scedosporium species each occurred in 7% of patients.
“This study nicely shows that molecular methods are powerful tools for the diagnosis of chronic rhinosinusitis and help in characterizing the accurate epidemiology of fungal CRS,” the investigators concluded.
Find the full study in Medical Mycology (doi: 10.1093/mmy/myw041).
ITS1/ITS2 polymerase chain reaction sequencing was significantly better at identifying fungal chronic rhinosinusitis (CRS) than were cultures, according to Dr. Pauline Comacle and her associates.
A total of 70 samples from 61 patients with CRS were tested via the use of polymerase chain reaction (PCR) testing and cultures. Fungal CRS was identified in 42 patients, of whom 37 had a fungus ball, 3 had allergic fungal CRS, and 2 had unspecified fungal CRS. ITS1/ITS2 PCR sequencing successfully identified fungal CRS in all patients, while cultures were positive for fungal CRS in 20 of the 42 patients.
Aspergillus fumigatus was the most common fungus in the study, occurring in 69% of patients. Cladosporium cladosporoides occurred in 9.5% of patients, and A. nidulans, A. flavus, and Scedosporium species each occurred in 7% of patients.
“This study nicely shows that molecular methods are powerful tools for the diagnosis of chronic rhinosinusitis and help in characterizing the accurate epidemiology of fungal CRS,” the investigators concluded.
Find the full study in Medical Mycology (doi: 10.1093/mmy/myw041).
ITS1/ITS2 polymerase chain reaction sequencing was significantly better at identifying fungal chronic rhinosinusitis (CRS) than were cultures, according to Dr. Pauline Comacle and her associates.
A total of 70 samples from 61 patients with CRS were tested via the use of polymerase chain reaction (PCR) testing and cultures. Fungal CRS was identified in 42 patients, of whom 37 had a fungus ball, 3 had allergic fungal CRS, and 2 had unspecified fungal CRS. ITS1/ITS2 PCR sequencing successfully identified fungal CRS in all patients, while cultures were positive for fungal CRS in 20 of the 42 patients.
Aspergillus fumigatus was the most common fungus in the study, occurring in 69% of patients. Cladosporium cladosporoides occurred in 9.5% of patients, and A. nidulans, A. flavus, and Scedosporium species each occurred in 7% of patients.
“This study nicely shows that molecular methods are powerful tools for the diagnosis of chronic rhinosinusitis and help in characterizing the accurate epidemiology of fungal CRS,” the investigators concluded.
Find the full study in Medical Mycology (doi: 10.1093/mmy/myw041).
FROM MEDICAL MYCOLOGY
Déjà vu: An FDA warning about oral ketoconazole ... again
The Food and Drug Administration issued a health warning on May 16th, 2016, regarding the use of oral ketoconazole for the treatment of skin and nail dermatophyte and candidal infections – wait what? Why is this even an active discussion? Let’s take a step back: In July 2013, the FDA strengthened its warnings and withdrew FDA indications for ketoconazole, specifically stating that its use for Candida and dermatophyte infections is no longer indicated and that it should only be considered in fungal infections, such as blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis, when other antifungals are not available.
The reality is that for, most clinicians, it has been well accepted for years that this drug has significant toxicities, probably the most among the azole class, especially in terms of liver issues and drug interactions being one of the most potent inhibitors of the hepatic CYP (cytochrome P450) system.
Endocrinologists have been preaching that the drug can impair adrenal function, causing insufficiency. So this warning begs the question … who is still actively prescribing this medication? Sadly, this advisory was in response to data showing that this drug was still being prescribed for skin and nail fungal infections in 2015, as well as one documented death associated with its use for this indication. Seriously? Terbinafine (250 mg daily for 6 weeks for fingernails, 12 weeks for toenails) and fluconazole (300 mg weekly for 6 months for fingernails and 9 months for toenails) are both safe and cheap means to treat onychomycosis. If you can get it covered, we even have effective topical treatments for onychomycosis, not to mention we have ALWAYS had topical options for superficial cutaneous mycoses. This is a no brainer. Just say NO to oral ketoconazole.
Dr. Adam Friedman is the residency program director and director of translational research in the department of dermatology, George Washington University, Washington. He is on the editorial advisory board of Dermatology News. He has no related disclosures.
The Food and Drug Administration issued a health warning on May 16th, 2016, regarding the use of oral ketoconazole for the treatment of skin and nail dermatophyte and candidal infections – wait what? Why is this even an active discussion? Let’s take a step back: In July 2013, the FDA strengthened its warnings and withdrew FDA indications for ketoconazole, specifically stating that its use for Candida and dermatophyte infections is no longer indicated and that it should only be considered in fungal infections, such as blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis, when other antifungals are not available.
The reality is that for, most clinicians, it has been well accepted for years that this drug has significant toxicities, probably the most among the azole class, especially in terms of liver issues and drug interactions being one of the most potent inhibitors of the hepatic CYP (cytochrome P450) system.
Endocrinologists have been preaching that the drug can impair adrenal function, causing insufficiency. So this warning begs the question … who is still actively prescribing this medication? Sadly, this advisory was in response to data showing that this drug was still being prescribed for skin and nail fungal infections in 2015, as well as one documented death associated with its use for this indication. Seriously? Terbinafine (250 mg daily for 6 weeks for fingernails, 12 weeks for toenails) and fluconazole (300 mg weekly for 6 months for fingernails and 9 months for toenails) are both safe and cheap means to treat onychomycosis. If you can get it covered, we even have effective topical treatments for onychomycosis, not to mention we have ALWAYS had topical options for superficial cutaneous mycoses. This is a no brainer. Just say NO to oral ketoconazole.
Dr. Adam Friedman is the residency program director and director of translational research in the department of dermatology, George Washington University, Washington. He is on the editorial advisory board of Dermatology News. He has no related disclosures.
The Food and Drug Administration issued a health warning on May 16th, 2016, regarding the use of oral ketoconazole for the treatment of skin and nail dermatophyte and candidal infections – wait what? Why is this even an active discussion? Let’s take a step back: In July 2013, the FDA strengthened its warnings and withdrew FDA indications for ketoconazole, specifically stating that its use for Candida and dermatophyte infections is no longer indicated and that it should only be considered in fungal infections, such as blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis, when other antifungals are not available.
The reality is that for, most clinicians, it has been well accepted for years that this drug has significant toxicities, probably the most among the azole class, especially in terms of liver issues and drug interactions being one of the most potent inhibitors of the hepatic CYP (cytochrome P450) system.
Endocrinologists have been preaching that the drug can impair adrenal function, causing insufficiency. So this warning begs the question … who is still actively prescribing this medication? Sadly, this advisory was in response to data showing that this drug was still being prescribed for skin and nail fungal infections in 2015, as well as one documented death associated with its use for this indication. Seriously? Terbinafine (250 mg daily for 6 weeks for fingernails, 12 weeks for toenails) and fluconazole (300 mg weekly for 6 months for fingernails and 9 months for toenails) are both safe and cheap means to treat onychomycosis. If you can get it covered, we even have effective topical treatments for onychomycosis, not to mention we have ALWAYS had topical options for superficial cutaneous mycoses. This is a no brainer. Just say NO to oral ketoconazole.
Dr. Adam Friedman is the residency program director and director of translational research in the department of dermatology, George Washington University, Washington. He is on the editorial advisory board of Dermatology News. He has no related disclosures.
FDA: No oral ketoconazole for skin, nail fungus
The Food and Drug Administration is warning health care professionals not to prescribe oral ketoconazole for patients with fungal infections of the skin and nails, because of "the risks of serious liver damage, adrenal gland problems, and harmful interactions with other medicines that outweigh its benefit in treating these conditions."
The advisory, issued on May 19, points out that oral ketoconazole (Nizoral) is no longer approved for treating nail or skin fungal infections. Topical forms of ketoconazole have not been associated with liver damage, adrenal problems, or drug interactions, the advisory adds.
"Health care professionals should use ketoconazole tablets only to treat serious fungal infections when no other antifungal therapies are available," according to the FDA. "Skin and nail fungal infections in otherwise healthy persons are not life-threatening, and so the risks associated with oral ketoconazole outweigh the benefits. Other treatment options are available over-the-counter and by prescription, but are also associated with risks that should be weighed against their benefits."
The advisory updates one issued in July 2013 when the drug's label was changed to reflect these safety concerns, including dropping the nail and skin infections from the approved indications. Since then, the FDA has received one report of a patient who died of liver failure associated with oral ketoconazole used to treat nail fungus. Furthermore, a survey of office-based physicians found that in the 18 months ending in June 2015, "skin and nail fungal infections were the only diagnoses cited for the use of oral ketoconazole."
Serious adverse events associated with oral ketoconazole should be reported to the FDA's MedWatch program online or call 800-332-1088.
The Food and Drug Administration is warning health care professionals not to prescribe oral ketoconazole for patients with fungal infections of the skin and nails, because of "the risks of serious liver damage, adrenal gland problems, and harmful interactions with other medicines that outweigh its benefit in treating these conditions."
The advisory, issued on May 19, points out that oral ketoconazole (Nizoral) is no longer approved for treating nail or skin fungal infections. Topical forms of ketoconazole have not been associated with liver damage, adrenal problems, or drug interactions, the advisory adds.
"Health care professionals should use ketoconazole tablets only to treat serious fungal infections when no other antifungal therapies are available," according to the FDA. "Skin and nail fungal infections in otherwise healthy persons are not life-threatening, and so the risks associated with oral ketoconazole outweigh the benefits. Other treatment options are available over-the-counter and by prescription, but are also associated with risks that should be weighed against their benefits."
The advisory updates one issued in July 2013 when the drug's label was changed to reflect these safety concerns, including dropping the nail and skin infections from the approved indications. Since then, the FDA has received one report of a patient who died of liver failure associated with oral ketoconazole used to treat nail fungus. Furthermore, a survey of office-based physicians found that in the 18 months ending in June 2015, "skin and nail fungal infections were the only diagnoses cited for the use of oral ketoconazole."
Serious adverse events associated with oral ketoconazole should be reported to the FDA's MedWatch program online or call 800-332-1088.
The Food and Drug Administration is warning health care professionals not to prescribe oral ketoconazole for patients with fungal infections of the skin and nails, because of "the risks of serious liver damage, adrenal gland problems, and harmful interactions with other medicines that outweigh its benefit in treating these conditions."
The advisory, issued on May 19, points out that oral ketoconazole (Nizoral) is no longer approved for treating nail or skin fungal infections. Topical forms of ketoconazole have not been associated with liver damage, adrenal problems, or drug interactions, the advisory adds.
"Health care professionals should use ketoconazole tablets only to treat serious fungal infections when no other antifungal therapies are available," according to the FDA. "Skin and nail fungal infections in otherwise healthy persons are not life-threatening, and so the risks associated with oral ketoconazole outweigh the benefits. Other treatment options are available over-the-counter and by prescription, but are also associated with risks that should be weighed against their benefits."
The advisory updates one issued in July 2013 when the drug's label was changed to reflect these safety concerns, including dropping the nail and skin infections from the approved indications. Since then, the FDA has received one report of a patient who died of liver failure associated with oral ketoconazole used to treat nail fungus. Furthermore, a survey of office-based physicians found that in the 18 months ending in June 2015, "skin and nail fungal infections were the only diagnoses cited for the use of oral ketoconazole."
Serious adverse events associated with oral ketoconazole should be reported to the FDA's MedWatch program online or call 800-332-1088.
Fungi may exacerbate asthma, chronic sinusitis
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
AT AAAAI
Key clinical point: Consider antifungal therapy if asthma or chronic sinusitis patients don’t respond well to conventional treatment.
Major finding: With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center.
Data source: A single-center case review.
Disclosures: The investigators had no relevant financial disclosures, and there was no outside funding for the work.
Serious infections are increasing among psoriasis inpatients
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis is an independent risk factor for serious infections, and serious infections are increasing among inpatients with psoriasis.
Major finding: Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients in the United States with psoriasis between 2002 and 2012 (all P-values less than .05). In the United Kingdom during the same time period, patients with severe psoriasis had a 63% greater risk of serious infection than patients without psoriasis.
Data source: Analyses of data from the Nationwide Inpatient Sample for 2002 through 2012, and from The Health Improvement Network for 2003 through 2012.
Disclosures: The Nationwide Inpatient Sample analysis was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. The analysis of The Health Improvement Network was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
Candida linked to sex-specific schizophrenia symptoms
Exposure to Candida albicans significantly increased the odds of a schizophrenia diagnosis in men, according to case-control data from two cohorts of 947 adults.
“Fungal pathogens have not been extensively evaluated in studies of psychiatric disorders,” wrote Dr. Emily G. Severance of Johns Hopkins University in Baltimore, and her colleagues, in njp (Nature Partner Journals) Schizophrenia.
The researchers compared C. albicans IgG levels of individuals with schizophrenia and bipolar disorder with controls and found no diagnostic differences. However, stratification by sex showed a significantly increased risk of schizophrenia in men with elevated C. albicans IgG levels, with a ninefold increase in risk at the highest of three levels of seropositivity (odds ratio, 9.53). Elevated C. albicans levels in males with bipolar disorder were attributed to a history of homelessness.
C. albicans antibodies in women were not significantly different between groups. However, C. albicans was significantly associated with cognitive impairment in women with schizophrenia vs. control women (OR, 1.12), but no such difference was noted in men.
High levels of C. albicans antibodies were associated with comorbid gastrointestinal, genitourinary, and neoplastic conditions among individuals with psychiatric disorders overall.
“It may be premature to list this pathogen as a risk factor for disease causation, but its status as a comorbidity requires clinical attention,” the researchers wrote. “In the long term, more research is required to understand the mechanisms that trigger pathogenicity of fungal commensals and how this might impact brain function in psychiatric disorders.”
The findings were published in npj Schizophrenia. Read the full study here (npj Schizophrenia 2016 May 4. doi: 10.1038/npjschz.2016.18).
Exposure to Candida albicans significantly increased the odds of a schizophrenia diagnosis in men, according to case-control data from two cohorts of 947 adults.
“Fungal pathogens have not been extensively evaluated in studies of psychiatric disorders,” wrote Dr. Emily G. Severance of Johns Hopkins University in Baltimore, and her colleagues, in njp (Nature Partner Journals) Schizophrenia.
The researchers compared C. albicans IgG levels of individuals with schizophrenia and bipolar disorder with controls and found no diagnostic differences. However, stratification by sex showed a significantly increased risk of schizophrenia in men with elevated C. albicans IgG levels, with a ninefold increase in risk at the highest of three levels of seropositivity (odds ratio, 9.53). Elevated C. albicans levels in males with bipolar disorder were attributed to a history of homelessness.
C. albicans antibodies in women were not significantly different between groups. However, C. albicans was significantly associated with cognitive impairment in women with schizophrenia vs. control women (OR, 1.12), but no such difference was noted in men.
High levels of C. albicans antibodies were associated with comorbid gastrointestinal, genitourinary, and neoplastic conditions among individuals with psychiatric disorders overall.
“It may be premature to list this pathogen as a risk factor for disease causation, but its status as a comorbidity requires clinical attention,” the researchers wrote. “In the long term, more research is required to understand the mechanisms that trigger pathogenicity of fungal commensals and how this might impact brain function in psychiatric disorders.”
The findings were published in npj Schizophrenia. Read the full study here (npj Schizophrenia 2016 May 4. doi: 10.1038/npjschz.2016.18).
Exposure to Candida albicans significantly increased the odds of a schizophrenia diagnosis in men, according to case-control data from two cohorts of 947 adults.
“Fungal pathogens have not been extensively evaluated in studies of psychiatric disorders,” wrote Dr. Emily G. Severance of Johns Hopkins University in Baltimore, and her colleagues, in njp (Nature Partner Journals) Schizophrenia.
The researchers compared C. albicans IgG levels of individuals with schizophrenia and bipolar disorder with controls and found no diagnostic differences. However, stratification by sex showed a significantly increased risk of schizophrenia in men with elevated C. albicans IgG levels, with a ninefold increase in risk at the highest of three levels of seropositivity (odds ratio, 9.53). Elevated C. albicans levels in males with bipolar disorder were attributed to a history of homelessness.
C. albicans antibodies in women were not significantly different between groups. However, C. albicans was significantly associated with cognitive impairment in women with schizophrenia vs. control women (OR, 1.12), but no such difference was noted in men.
High levels of C. albicans antibodies were associated with comorbid gastrointestinal, genitourinary, and neoplastic conditions among individuals with psychiatric disorders overall.
“It may be premature to list this pathogen as a risk factor for disease causation, but its status as a comorbidity requires clinical attention,” the researchers wrote. “In the long term, more research is required to understand the mechanisms that trigger pathogenicity of fungal commensals and how this might impact brain function in psychiatric disorders.”
The findings were published in npj Schizophrenia. Read the full study here (npj Schizophrenia 2016 May 4. doi: 10.1038/npjschz.2016.18).
FROM NPJ SCHIZOPHRENIA
FDA evaluating the use of oral fluconazole in pregnancy
The Food and Drug Administration is reviewing the results of a Danish study that concludes there is a possible increased risk of miscarriage with the use of oral fluconazole (Diflucan) in pregnancy, according to a safety alert issued April 26.
The current drug label for oral fluconazole states that data from studies in women does not suggest an increased risk of problems during pregnancy or abnormalities in developing babies when women used a single 150-mg dose to treat vaginal yeast infections. However, reports of abnormalities at birth have resulted from high doses (400-800 mg/day) taken during pregnancy for much longer than a single dose. The Danish study had most pregnant women use one or two doses of 150 mg.
Oral fluconazole is used to treat yeast infections of the vaginal area, mouth, and esophagus. It can also be used to treat a fungal infection of the brain and spinal cord.
The FDA is also evaluating additional data and will make recommendations when the review is complete.
“Until FDA’s review is complete and more is understood about this study and other available data, FDA advises cautious prescribing of oral fluconazole in pregnancy,” the safety alert states.
The FDA also noted that the Centers for Disease Control and Prevention recommends using topical antifungal products only when treating pregnant women with vulvovaginal yeast infections.
Read more about the investigation on the FDA website.
The Food and Drug Administration is reviewing the results of a Danish study that concludes there is a possible increased risk of miscarriage with the use of oral fluconazole (Diflucan) in pregnancy, according to a safety alert issued April 26.
The current drug label for oral fluconazole states that data from studies in women does not suggest an increased risk of problems during pregnancy or abnormalities in developing babies when women used a single 150-mg dose to treat vaginal yeast infections. However, reports of abnormalities at birth have resulted from high doses (400-800 mg/day) taken during pregnancy for much longer than a single dose. The Danish study had most pregnant women use one or two doses of 150 mg.
Oral fluconazole is used to treat yeast infections of the vaginal area, mouth, and esophagus. It can also be used to treat a fungal infection of the brain and spinal cord.
The FDA is also evaluating additional data and will make recommendations when the review is complete.
“Until FDA’s review is complete and more is understood about this study and other available data, FDA advises cautious prescribing of oral fluconazole in pregnancy,” the safety alert states.
The FDA also noted that the Centers for Disease Control and Prevention recommends using topical antifungal products only when treating pregnant women with vulvovaginal yeast infections.
Read more about the investigation on the FDA website.
The Food and Drug Administration is reviewing the results of a Danish study that concludes there is a possible increased risk of miscarriage with the use of oral fluconazole (Diflucan) in pregnancy, according to a safety alert issued April 26.
The current drug label for oral fluconazole states that data from studies in women does not suggest an increased risk of problems during pregnancy or abnormalities in developing babies when women used a single 150-mg dose to treat vaginal yeast infections. However, reports of abnormalities at birth have resulted from high doses (400-800 mg/day) taken during pregnancy for much longer than a single dose. The Danish study had most pregnant women use one or two doses of 150 mg.
Oral fluconazole is used to treat yeast infections of the vaginal area, mouth, and esophagus. It can also be used to treat a fungal infection of the brain and spinal cord.
The FDA is also evaluating additional data and will make recommendations when the review is complete.
“Until FDA’s review is complete and more is understood about this study and other available data, FDA advises cautious prescribing of oral fluconazole in pregnancy,” the safety alert states.
The FDA also noted that the Centers for Disease Control and Prevention recommends using topical antifungal products only when treating pregnant women with vulvovaginal yeast infections.
Read more about the investigation on the FDA website.
Number of U.S. tuberculosis cases increased in 2015
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
FROM THE MMWR
FDA approves first generic form of oxiconazole nitrate cream
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.