User login
Hemodialysis Access and Age-Related Outcomes
NATIONAL HARBOR, MD. – The National Kidney Foundation recommends the preferential creation of radiocephalic fistulas over that of brachiocephalic fistulas for hemodialysis access, but in patients older than 68 years, radiocephalic fistulas are associated with more postoperative complications, according to a retrospective analysis of 287 patients.
Dr. Steven Abramowitz and his colleagues at the Mount Sinai Medical Center, New York, studied patients older than 68 years of age who had preoperative vein mapping and regular follow-up after creation of arteriovenous fistulas. Within this group of 287 patients, 164 underwent radiocephalic fistula (RCF) creation and 123 underwent brachiocephalic fistula (BCF) creation. Dr. Abramowitz presented their findings at the Vascular Annual Meeting.
The researchers analyzed medical records to determine the number of central venous catheter days, the number of fistula-related procedures recorded, and the number of access-related hospitalizations for each patient. Bivariate analysis using linear modeling and one-way analysis of variance was used to assess cohort differences.
Among those patients who had a BCF, the average number of central venous catheter days was 53.3 days per patient, the average number of fistula-related procedures recorded was 0.6 per patient, and the number of hemodialysis-related hospitalizations was 0.3 per patient.
In comparison, among those patients who had an RCF, the average number of central venous catheter days was 83.4 days per patient, the average number of fistula-related procedures recorded was 1.8 per patient, and the average number of hemodialysis-related hospitalizations was 0.8 per patient.
These results indicated a significant difference in postoperative course between those patients who underwent BCF vs. RCF creation.
Patients older than 68 years of age who undergo RCF creation "may have a greater likelihood of increased central venous catheter days, a greater number of hospitalizations related to hemodialysis access, and a greater number of postoperative procedures than those who undergo BCF creation," said Dr. Abramowitz.
He reported that he had no disclosures.
NATIONAL HARBOR, MD. – The National Kidney Foundation recommends the preferential creation of radiocephalic fistulas over that of brachiocephalic fistulas for hemodialysis access, but in patients older than 68 years, radiocephalic fistulas are associated with more postoperative complications, according to a retrospective analysis of 287 patients.
Dr. Steven Abramowitz and his colleagues at the Mount Sinai Medical Center, New York, studied patients older than 68 years of age who had preoperative vein mapping and regular follow-up after creation of arteriovenous fistulas. Within this group of 287 patients, 164 underwent radiocephalic fistula (RCF) creation and 123 underwent brachiocephalic fistula (BCF) creation. Dr. Abramowitz presented their findings at the Vascular Annual Meeting.
The researchers analyzed medical records to determine the number of central venous catheter days, the number of fistula-related procedures recorded, and the number of access-related hospitalizations for each patient. Bivariate analysis using linear modeling and one-way analysis of variance was used to assess cohort differences.
Among those patients who had a BCF, the average number of central venous catheter days was 53.3 days per patient, the average number of fistula-related procedures recorded was 0.6 per patient, and the number of hemodialysis-related hospitalizations was 0.3 per patient.
In comparison, among those patients who had an RCF, the average number of central venous catheter days was 83.4 days per patient, the average number of fistula-related procedures recorded was 1.8 per patient, and the average number of hemodialysis-related hospitalizations was 0.8 per patient.
These results indicated a significant difference in postoperative course between those patients who underwent BCF vs. RCF creation.
Patients older than 68 years of age who undergo RCF creation "may have a greater likelihood of increased central venous catheter days, a greater number of hospitalizations related to hemodialysis access, and a greater number of postoperative procedures than those who undergo BCF creation," said Dr. Abramowitz.
He reported that he had no disclosures.
NATIONAL HARBOR, MD. – The National Kidney Foundation recommends the preferential creation of radiocephalic fistulas over that of brachiocephalic fistulas for hemodialysis access, but in patients older than 68 years, radiocephalic fistulas are associated with more postoperative complications, according to a retrospective analysis of 287 patients.
Dr. Steven Abramowitz and his colleagues at the Mount Sinai Medical Center, New York, studied patients older than 68 years of age who had preoperative vein mapping and regular follow-up after creation of arteriovenous fistulas. Within this group of 287 patients, 164 underwent radiocephalic fistula (RCF) creation and 123 underwent brachiocephalic fistula (BCF) creation. Dr. Abramowitz presented their findings at the Vascular Annual Meeting.
The researchers analyzed medical records to determine the number of central venous catheter days, the number of fistula-related procedures recorded, and the number of access-related hospitalizations for each patient. Bivariate analysis using linear modeling and one-way analysis of variance was used to assess cohort differences.
Among those patients who had a BCF, the average number of central venous catheter days was 53.3 days per patient, the average number of fistula-related procedures recorded was 0.6 per patient, and the number of hemodialysis-related hospitalizations was 0.3 per patient.
In comparison, among those patients who had an RCF, the average number of central venous catheter days was 83.4 days per patient, the average number of fistula-related procedures recorded was 1.8 per patient, and the average number of hemodialysis-related hospitalizations was 0.8 per patient.
These results indicated a significant difference in postoperative course between those patients who underwent BCF vs. RCF creation.
Patients older than 68 years of age who undergo RCF creation "may have a greater likelihood of increased central venous catheter days, a greater number of hospitalizations related to hemodialysis access, and a greater number of postoperative procedures than those who undergo BCF creation," said Dr. Abramowitz.
He reported that he had no disclosures.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Patients older than 68 years of age with radiocephalic fistulas had significantly increased central venous catheter days, more hospitalizations related to hemodialysis access, and more postoperative procedures than did those who underwent brachiocephalic fistula creation.
Data Source: Researchers retrospectively analyzed 287 patients older than 68 years of age, 164 of whom underwent radiocephalic fistula creation and 123 of whom underwent brachiocephalic fistula creation.
Disclosures: Dr. Abramowitz reported that he had no disclosures.
Secondhand Smoke Increases Bladder Problems in Children
ATLANTA – Children who are exposed to secondhand tobacco smoke have an increased risk of urinary urgency, frequency, and incontinence, prospective data from a small study have shown.
Among children with these bladder symptoms, 28% were exposed to tobacco smoke on a daily basis – 13% higher than the overall child exposure rate in New Jersey, Dr. Kelly Johnson said at the annual meeting of the American Urological Association.
In addition to irritating a child’s bladder, childhood exposure to tobacco smoke is directly linked to the development of bladder cancer as an adult, she said in a press briefing.
Dr. Johnson, chief urology resident at the Robert Wood Johnson University Hospital, New Brunswick, N.J., presented prospective data on 45 children, aged 4-17 years, who presented with irritative bladder symptoms – frequency, urgency, and incontinence.
She used the Harvard Children’s Health and the Children’s Neurotoxicant Exposure studies to classify tobacco smoke exposure. The patients’ symptom severity was scored with the Dysfunctional Voiding Scoring System and classified as very mild, mild, Drmoderate, or severe.
About half of the group (21) had very mild or mild symptoms, while the remainder had symptoms scored as moderate or severe.
None of the children with mild scores were exposed to secondhand smoke on a daily basis, and none had mothers who smoked. However, 23% of those with moderate to severe scores had mothers who smoked, and 50% were exposed to smoke in a car on a regular basis.
"On our measures of environmental tobacco smoke exposure, children with greater exposure had significantly higher symptom severity scores than children who weren’t exposed," Dr. Johnson said. "This relationship was particularly striking for the younger children aged 4-10 years old."
Physicians who see children with bladder dysfunction should ask parents about smoke exposure, she advised – not only because of its effect on the current problems, but because of its proven dangers as the child grows up.
"Tobacco smoke contains chemicals that are known bladder irritants in both children and adults, and European studies have shown a strong relationship between adult bladder cancer and childhood tobacco smoke exposure.
The discussion of a child’s urinary symptoms provides a very good opportunity to speak to parents about smoking cessation, she added.
"It’s a teachable moment," that can have a long-lasting positive impact on both the child and the parent. "Unlike other risky behaviors, which affect only the person who engages in them, smoking poses substantial health risks to those not involved in the process," she said.
Dr. Johnson said she had had no relevant financial disclosures.
ATLANTA – Children who are exposed to secondhand tobacco smoke have an increased risk of urinary urgency, frequency, and incontinence, prospective data from a small study have shown.
Among children with these bladder symptoms, 28% were exposed to tobacco smoke on a daily basis – 13% higher than the overall child exposure rate in New Jersey, Dr. Kelly Johnson said at the annual meeting of the American Urological Association.
In addition to irritating a child’s bladder, childhood exposure to tobacco smoke is directly linked to the development of bladder cancer as an adult, she said in a press briefing.
Dr. Johnson, chief urology resident at the Robert Wood Johnson University Hospital, New Brunswick, N.J., presented prospective data on 45 children, aged 4-17 years, who presented with irritative bladder symptoms – frequency, urgency, and incontinence.
She used the Harvard Children’s Health and the Children’s Neurotoxicant Exposure studies to classify tobacco smoke exposure. The patients’ symptom severity was scored with the Dysfunctional Voiding Scoring System and classified as very mild, mild, Drmoderate, or severe.
About half of the group (21) had very mild or mild symptoms, while the remainder had symptoms scored as moderate or severe.
None of the children with mild scores were exposed to secondhand smoke on a daily basis, and none had mothers who smoked. However, 23% of those with moderate to severe scores had mothers who smoked, and 50% were exposed to smoke in a car on a regular basis.
"On our measures of environmental tobacco smoke exposure, children with greater exposure had significantly higher symptom severity scores than children who weren’t exposed," Dr. Johnson said. "This relationship was particularly striking for the younger children aged 4-10 years old."
Physicians who see children with bladder dysfunction should ask parents about smoke exposure, she advised – not only because of its effect on the current problems, but because of its proven dangers as the child grows up.
"Tobacco smoke contains chemicals that are known bladder irritants in both children and adults, and European studies have shown a strong relationship between adult bladder cancer and childhood tobacco smoke exposure.
The discussion of a child’s urinary symptoms provides a very good opportunity to speak to parents about smoking cessation, she added.
"It’s a teachable moment," that can have a long-lasting positive impact on both the child and the parent. "Unlike other risky behaviors, which affect only the person who engages in them, smoking poses substantial health risks to those not involved in the process," she said.
Dr. Johnson said she had had no relevant financial disclosures.
ATLANTA – Children who are exposed to secondhand tobacco smoke have an increased risk of urinary urgency, frequency, and incontinence, prospective data from a small study have shown.
Among children with these bladder symptoms, 28% were exposed to tobacco smoke on a daily basis – 13% higher than the overall child exposure rate in New Jersey, Dr. Kelly Johnson said at the annual meeting of the American Urological Association.
In addition to irritating a child’s bladder, childhood exposure to tobacco smoke is directly linked to the development of bladder cancer as an adult, she said in a press briefing.
Dr. Johnson, chief urology resident at the Robert Wood Johnson University Hospital, New Brunswick, N.J., presented prospective data on 45 children, aged 4-17 years, who presented with irritative bladder symptoms – frequency, urgency, and incontinence.
She used the Harvard Children’s Health and the Children’s Neurotoxicant Exposure studies to classify tobacco smoke exposure. The patients’ symptom severity was scored with the Dysfunctional Voiding Scoring System and classified as very mild, mild, Drmoderate, or severe.
About half of the group (21) had very mild or mild symptoms, while the remainder had symptoms scored as moderate or severe.
None of the children with mild scores were exposed to secondhand smoke on a daily basis, and none had mothers who smoked. However, 23% of those with moderate to severe scores had mothers who smoked, and 50% were exposed to smoke in a car on a regular basis.
"On our measures of environmental tobacco smoke exposure, children with greater exposure had significantly higher symptom severity scores than children who weren’t exposed," Dr. Johnson said. "This relationship was particularly striking for the younger children aged 4-10 years old."
Physicians who see children with bladder dysfunction should ask parents about smoke exposure, she advised – not only because of its effect on the current problems, but because of its proven dangers as the child grows up.
"Tobacco smoke contains chemicals that are known bladder irritants in both children and adults, and European studies have shown a strong relationship between adult bladder cancer and childhood tobacco smoke exposure.
The discussion of a child’s urinary symptoms provides a very good opportunity to speak to parents about smoking cessation, she added.
"It’s a teachable moment," that can have a long-lasting positive impact on both the child and the parent. "Unlike other risky behaviors, which affect only the person who engages in them, smoking poses substantial health risks to those not involved in the process," she said.
Dr. Johnson said she had had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN UROLOGICAL ASSOCIATION
Major Finding: Half of children with moderate to severe bladder symptoms are exposed to secondhand tobacco smoke on a regular basis.
Data Source: The data were from a prospective cohort study of 45 children.
Disclosures: Dr. Johnson said she had no relevant financial disclosures.
Renal Failure Risk Is Key in Albumin for Spontaneous Bacterial Peritonitis
SAN DIEGO – Cirrhotics with spontaneous bacterial peritonitis probably need albumin only if their total bilirubin is above 4 mg/dL and/or their blood urea nitrogen (BUN) is above 30 mg/dL, according to transplant hepatologist Dr. James Burton.
Supplementation expands plasma volume to attenuate the splanchnic and systemic vasodilation associated with spontaneous bacterial peritonitis (SBP), increasing blood flow to the kidneys and preserving renal function, said Dr. Burton, medical director of liver transplantation at the University of Colorado Hospital, Aurora.
But it seems to be needed only when SBP patients are at high risk for renal failure, according to two studies.
The first randomized 63 cirrhotic SBP patients to cefotaxime alone and 63 to cefotaxime plus albumin, 1.5 g/kg on day 1 and 1.0 g/kg on day 3. The regimen wiped out the infection in most patients. Renal impairment and mortality were significantly lower in the albumin group, but only patients with total baseline bilirubins above 4 mg/dL and/or BUNs greater than 30 mg/dL benefited from albumin, Dr. Burton said (N. Engl. J. Med. 1999;341:403-9).
A follow-up 28-subject study limited albumin supplementation, again 1.5 g/kg on day 1 and 1.0 g/kg on day 3, to SBP patients with similar parameters or creatinines above 1 mg/dL. It was the right choice; none of the 15 patients with lower baseline values developed renal impairment, Dr. Burton said (Gut 2007;56:597-9).
He noted that it’s possible for patients to have SBP without obvious symptoms, so "if you’re sick enough to have cirrhosis and be in the hospital, you need a needle in your abdomen to rule out" the condition.
In general, cirrhotics have low levels of albumin, which is responsible for about 80% of plasma’s oncotic pressure. Also, what they have "may not work as well" to transport fatty acids, hormones, and enzymes, and drugs.
Outpatient supplementation for cirrhotics not only seems to reduce the need for paracentesis, but also makes patients feel better. "I am a believer because my patients tell me it works. Some of my patients refer to it as ‘nectar of the gods.’ This is more than just the oncotic things. I also think they are transporting hormones, carrying drugs, and other stuff better. So I am a big believer," Dr. Burton said.
He opts for 25% albumin instead of 5%, to reduce fluid load and also because "there is a lot of sodium in 5% albumin. Obviously, these patients need to have their sodium restricted," he said.
When paracentesis is needed, "studies suggest you can probably [remove] 4-6 liters without giving albumin." Even so, "we often give albumin during paracentesis," he said.
Dr. Burton said he has no relevant financial disclosures.
SAN DIEGO – Cirrhotics with spontaneous bacterial peritonitis probably need albumin only if their total bilirubin is above 4 mg/dL and/or their blood urea nitrogen (BUN) is above 30 mg/dL, according to transplant hepatologist Dr. James Burton.
Supplementation expands plasma volume to attenuate the splanchnic and systemic vasodilation associated with spontaneous bacterial peritonitis (SBP), increasing blood flow to the kidneys and preserving renal function, said Dr. Burton, medical director of liver transplantation at the University of Colorado Hospital, Aurora.
But it seems to be needed only when SBP patients are at high risk for renal failure, according to two studies.
The first randomized 63 cirrhotic SBP patients to cefotaxime alone and 63 to cefotaxime plus albumin, 1.5 g/kg on day 1 and 1.0 g/kg on day 3. The regimen wiped out the infection in most patients. Renal impairment and mortality were significantly lower in the albumin group, but only patients with total baseline bilirubins above 4 mg/dL and/or BUNs greater than 30 mg/dL benefited from albumin, Dr. Burton said (N. Engl. J. Med. 1999;341:403-9).
A follow-up 28-subject study limited albumin supplementation, again 1.5 g/kg on day 1 and 1.0 g/kg on day 3, to SBP patients with similar parameters or creatinines above 1 mg/dL. It was the right choice; none of the 15 patients with lower baseline values developed renal impairment, Dr. Burton said (Gut 2007;56:597-9).
He noted that it’s possible for patients to have SBP without obvious symptoms, so "if you’re sick enough to have cirrhosis and be in the hospital, you need a needle in your abdomen to rule out" the condition.
In general, cirrhotics have low levels of albumin, which is responsible for about 80% of plasma’s oncotic pressure. Also, what they have "may not work as well" to transport fatty acids, hormones, and enzymes, and drugs.
Outpatient supplementation for cirrhotics not only seems to reduce the need for paracentesis, but also makes patients feel better. "I am a believer because my patients tell me it works. Some of my patients refer to it as ‘nectar of the gods.’ This is more than just the oncotic things. I also think they are transporting hormones, carrying drugs, and other stuff better. So I am a big believer," Dr. Burton said.
He opts for 25% albumin instead of 5%, to reduce fluid load and also because "there is a lot of sodium in 5% albumin. Obviously, these patients need to have their sodium restricted," he said.
When paracentesis is needed, "studies suggest you can probably [remove] 4-6 liters without giving albumin." Even so, "we often give albumin during paracentesis," he said.
Dr. Burton said he has no relevant financial disclosures.
SAN DIEGO – Cirrhotics with spontaneous bacterial peritonitis probably need albumin only if their total bilirubin is above 4 mg/dL and/or their blood urea nitrogen (BUN) is above 30 mg/dL, according to transplant hepatologist Dr. James Burton.
Supplementation expands plasma volume to attenuate the splanchnic and systemic vasodilation associated with spontaneous bacterial peritonitis (SBP), increasing blood flow to the kidneys and preserving renal function, said Dr. Burton, medical director of liver transplantation at the University of Colorado Hospital, Aurora.
But it seems to be needed only when SBP patients are at high risk for renal failure, according to two studies.
The first randomized 63 cirrhotic SBP patients to cefotaxime alone and 63 to cefotaxime plus albumin, 1.5 g/kg on day 1 and 1.0 g/kg on day 3. The regimen wiped out the infection in most patients. Renal impairment and mortality were significantly lower in the albumin group, but only patients with total baseline bilirubins above 4 mg/dL and/or BUNs greater than 30 mg/dL benefited from albumin, Dr. Burton said (N. Engl. J. Med. 1999;341:403-9).
A follow-up 28-subject study limited albumin supplementation, again 1.5 g/kg on day 1 and 1.0 g/kg on day 3, to SBP patients with similar parameters or creatinines above 1 mg/dL. It was the right choice; none of the 15 patients with lower baseline values developed renal impairment, Dr. Burton said (Gut 2007;56:597-9).
He noted that it’s possible for patients to have SBP without obvious symptoms, so "if you’re sick enough to have cirrhosis and be in the hospital, you need a needle in your abdomen to rule out" the condition.
In general, cirrhotics have low levels of albumin, which is responsible for about 80% of plasma’s oncotic pressure. Also, what they have "may not work as well" to transport fatty acids, hormones, and enzymes, and drugs.
Outpatient supplementation for cirrhotics not only seems to reduce the need for paracentesis, but also makes patients feel better. "I am a believer because my patients tell me it works. Some of my patients refer to it as ‘nectar of the gods.’ This is more than just the oncotic things. I also think they are transporting hormones, carrying drugs, and other stuff better. So I am a big believer," Dr. Burton said.
He opts for 25% albumin instead of 5%, to reduce fluid load and also because "there is a lot of sodium in 5% albumin. Obviously, these patients need to have their sodium restricted," he said.
When paracentesis is needed, "studies suggest you can probably [remove] 4-6 liters without giving albumin." Even so, "we often give albumin during paracentesis," he said.
Dr. Burton said he has no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE SOCIETY OF HOSPITAL MEDICINE
The Treatment of Gout
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Ruoff has disclosed that he is on the speakers’ bureau for and has received research grants from Takeda Pharmaceuticals.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from URL Pharma, Inc.
DB is a 50-year-old obese male visiting the clinic for symptoms suggestive of allergic rhinitis. The nurse has informed the family physician that DB was limping from the waiting room to the examination room. DB reports that he has been experiencing pain in his left big toe and ankle over the past few days. The last time this happened, the pain resolved within 7 to 10 days.
DB reports that he has experienced 4 or 5 similar episodes over the past 3 years. The first attacks affected his left big toe, but he now also experiences some pain in his left ankle. The pain is moderate, peaks in 1 to 2 days, and resolves within 7 to 10 days. Acetaminophen provided little pain relief so DB now takes ibuprofen 400 mg 3 times daily, as it “helps take the edge off.” Other medications include aspirin 81 mg per day and an oral antihistamine as needed for hay fever. DB reports that he eats seafood 2 to 3 times per week and red meat 1 to 2 times per week; he drinks 2 six-packs of beer per week.
Physical examination: weight, 186 lb (body mass index [BMI], 27 kg/m2); blood pressure, 126/76 mm Hg; and temperature, 98.8°F. His left big toe and ankle are red, slightly swollen, and warm with a small subcutaneous nodule noted on the first metatarsophalangeal joint. There is no sign of skin or joint infection.
The impression from his history and physical exam is that DB is suffering from an acute attack of gout, but the family physician also considers other diagnoses.
Background
Gout is a heterogeneous disorder that peaks in incidence in the fifth decade. Gout is caused by hyperuricemia, generally as a result of reduced excretion of uric acid by the kidneys; hyperuricemia may also result from overproduction of uric acid. Data from the National Health and Nutrition Examination Survey (NHANES) 2007-2008 indicate that the prevalence of gout continues to rise in the United States, likely related to the increasing frequency of adiposity and hypertension. Overall, about 75% of the 8.3 million people with gout are men.1
Risk Factors
Clinically defined hyperuricemia—a serum urate (sUA) level greater than 6.8 mg/dL, the concentration at which urate exceeds its solubility in most biological fluids—is the major risk factor for gout. However, not all persons with hyperuricemia have gout. Data from NHANES 2007-2008, in which the definition of hyperuricemia was an sUA level greater than 7.0 mg/dL for men and greater than 5.7 mg/dL for women, showed the mean sUA level to be 6.1 mg/dL in men and 4.9 mg/dL in women, corresponding to hyperuricemia prevalences of 21.2% and 21.6%, respectively.1
There are several other risk factors for gout, including hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease (CVD), and metabolic syndrome.2 For a man with hypertension, the relative risk (RR) of gout is 2.3 compared with a normotensive man.3 Furthermore, it is well established that the use of diuretics increases the risk of gout (RR, 1.8).3 Several other medications increase sUA level as well: aspirin (including low-dose), cyclosporine, pyrazinamide, ethambutol, and niacin.2
Lifestyle and diet also pose a risk for gout. The risk of gout increases with BMI such that, compared with a man with a BMI of 21 to 22.9 kg/m2, the RR of gout is doubled for a man with a BMI of 25 to 29.9 kg/m2; for a man with a BMI of 35 kg/m2 or more, the RR is tripled.3 Sugar-sweetened soft drinks (but not diet soft drinks) and fructose-rich fruits and fruit juices also increase the risk of gout, as do a high alcohol intake, particularly beer, and a diet rich in meat (especially organ meat, turkey, or wild game) or seafood.4 A moderate intake of purine-rich vegetables (eg, peas, beans, lentils, spinach, mushrooms, oatmeal, and cauliflower) or protein is not associated with an increased risk of gout, while a high consumption of dairy products is associated with a decreased risk.5,6
Untreated or poorly treated gout usually leads to further acute attacks and progressive joint and tissue damage. In addition, gout and hyperuricemia serve as risk factors for other diseases. Adults with gout are 3 times as likely to develop metabolic syndrome as adults without gout.7 An elevated sUA level is also an independent risk factor for the development of hypertension (RR, 1.1), as well as myocardial infarction (MI; RR, 1.9), and stroke (RR, 1.6).8,9 An increasing sUA level also increases the risk of renal failure.10,11 In a study of 49,413 men followed for a mean of 5.4 years, the age-adjusted RR of renal failure was 1.5 in men with an sUA level of 6.5 to 8.4 mg/dL and 8.5 in men with an sUA level of 8.5 to 13.0 mg/dL compared with men with an sUA level of 5.0 to 6.4 mg/dL.11
Clinical Presentation
The deposition of monosodium urate (MSU) crystals in joints and tissues is very common and typically causes no signs or symptoms in the majority of persons. Even in men with an sUA level of 9 mg/dL or greater, the cumulative incidence of gouty arthritis has been found to be 22% over 5 years.12 However, as crystal deposition progresses, acute, painful attacks occur more frequently, with the development of chronic tophaceous gout after several years.13
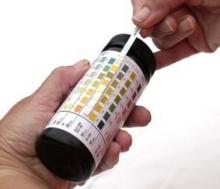
Laboratory results for DB:
- Serum uric acid, 7.9 mg/dL
- White blood cell count, 15,800/mm3
- Serum creatinine, 1.2 mg/dL (estimated creatinine clearance, 90 mL/min)
- Erythrocyte sedimentation rate, 23 mm/h
- Low-density lipoprotein cholesterol (nonfasting), 127 mg/dL
Laboratory confirmation of hyperuricemia together with the pain, swelling, and tenderness of DB’s toe and ankle, other findings from his medical history and physical exam (eg, the use of aspirin daily), and exclusion of alternative diagnoses, such as septic arthritis, enable the family physician to arrive at a presumptive diagnosis of gouty arthritis. Aspiration of MSU crystals from DB’s toe or ankle is the gold standard and would allow for a definitive diagnosis. Although the sUA level was found to be high, it should be noted that a normal sUA level is often found during an acute attack; should this occur, the sUA level should be checked again 1 to 2 weeks after the acute attack has resolved.
Goals of Treatment
The cornerstone of gout management is daily, long-term treatment with urate-lowering therapy (ULT) combined with as-needed treatment for an acute attack. In addition, since initiation of ULT mobilizes MSU crystals, which often leads to a short-term increase in acute attacks, prophylaxis with an appropriate anti-inflammatory therapy is recommended at the time ULT is initiated.14
The therapeutic goals of gout treatment are 2-pronged: treatment of an acute gout attack and management of chronic gout. For an acute attack, the goals are to exclude a diagnosis of septic arthritis; reduce inflammation and terminate the attack; and seek, assess, and control associated diseases, such as diabetes mellitus, hypertension, hyperlipidemia, and CVD. If this latter goal is not possible during the acute attack, plans should be made to assess associated diseases once the acute attack has resolved.14 Lowering the sUA level is not a goal of therapy for an acute attack, but it is the primary goal of ULT for chronic gout. Lowering the sUA level to less than 6.0 mg/dL, which is well below the saturation point of urate in most biological fluids, is intended to prevent further acute attacks, tophus formation, and tissue damage.14
Treatment of an Acute Attack
The mainstay of treatment for an acute attack is anti-inflammatory therapy to reduce pain and inflammation.14 Therapy should be initiated at the onset of the attack and continued until the attack is terminated, which is typically 1 to 2 weeks. Anti-inflammatory therapy traditionally has in-cluded colchicine, a nonsteroidal anti-inflammatory drug (NSAID), or a corticosteroid.14
Nonsteroidal Anti-inflammatory Drugs
The NSAIDs are all thought to provide similar efficacy when used in maximum doses.15,16 Since gastrointestinal toxicity is a concern with NSAIDs, coadministration of a proton pump inhibitor, H2 antagonist, or misoprostol is advised for patients with an increased risk of peptic ulcers, bleeds, or perforations.17 The risk of MI, stroke, cardiovascular death, and atrial fibrillation/flutter with NSAID therapy should be considered, especially because gout often coexists with cardiovascular disorders.15,18,19 Furthermore, NSAIDs are contraindicated in patients with heart failure or renal insufficiency.20,21
Corticosteroids. A systematic review of clinical trials involving systemic corticosteroids that found a few prospective trials of low to moderate quality concluded that there was inconclusive evidence for the efficacy and effectiveness of corticosteroids in the treatment of acute gout.22 No serious adverse events (AEs) were reported. A more recent prospective trial found comparable pain reduction and incidence of AEs with naproxen 500 mg twice daily and prednisolone 35 mg once daily for 5 days in patients with monoarticular gout.23 Furthermore, clinical experience indicates that intra-articular aspiration and injection of a long-acting corticosteroid is an effective and safe treatment for an acute attack.14,15 Corticosteroids may be useful in patients who have an inadequate response to, are intolerant of, or have a contraindication to NSAIDs and colchicine.14,15
Colchicine. Much of the recent clinical investigation regarding pharmacologic treatment of an acute gout attack has involved colchicine. To overcome the limitations of the standard dose-to-toxicity regimen of colchicine, a low-dose regimen of colchicine (1.2 mg followed by 0.6 mg 1 hour later) was investigated and subsequently approved by the US Food and Drug Administration (FDA).24
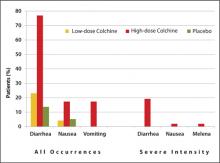
Approval was based on a randomized, double-blind comparison with high-dose colchicine (1.2 mg followed by 0.6 mg every hour for 6 hours) and placebo in 184 patients with an acute gout attack.25 The primary endpoint, a 50% or greater reduction in pain at 24 hours without the use of rescue medication, was reached in 28 of 74 patients (38%) in the low-dose group, 17 of 52 patients (33%) in the high-dose group, and 9 of 58 patients (16%) in the placebo group (P = .005 and P = .034, respectively, versus placebo). An AE occurred in 36.5% and 76.9% of study participants in the low-dose and high-dose colchicine groups, respectively, and in 27.1% of participants in the placebo group. Gastrointestinal AEs (eg, diarrhea, nausea, and vomiting) were less common in the low-dose colchicine group ( FIGURE ). All AEs in the low-dose group were mild to moderate in intensity, while 10 of 52 patients (19.2%) in the high-dose group had an AE of severe intensity. Concomitant use of numerous drugs can increase the concentration of colchicine. Examples include atorvastatin, fluvastatin, pravastatin, simvastatin, fibrates, gemfibrozil, digoxin, clarithromycin, erythromycin, fluconazole, itraconazole, ketoconazole, protease inhibitors, diltiazem, verapamil, and cyclosporine, as well as grapefruit juice.26
FIGURE
Frequency of selected adverse events occurring over 24 hours with low-dose vs high-dose colchicine25
Treatment plan:
TABLE
Care plan for a patient with gout27
| Acute flare | Chronic gout | |
|---|---|---|
| Goals |
|
|
| Educational points |
|
|
| sUA, serum uric acid; ULT, urate-lowering therapy. Source: Reproduced with permission. Becker MA, et al. J Fam Pract. 2010;59(6):S1-S8. Quadrant HealthCom Inc. Copyright 2010. | ||
Urate-Lowering Therapy
Urate lowering therapy is indicated for most, but not all, patients with gout. ULT is generally not recommended for those who have suffered a single attack of gout and have no complications, since 40% of these patients will not experience another attack within a year. However, should a second attack occur within a year of the first attack, ULT is recommended. Some patients who have experienced a single attack may elect to initiate ULT after being educated about the risks of the disease and the risks and benefits of ULT.14 Patients who have had an attack of gout and also have a comorbidity (eg, visible gouty tophi, renal insufficiency, uric acid stones, or use of a diuretic for hypertension) should begin ULT, since the risk of further attacks is higher in these patients, and kidney or joint damage is more likely.17
Initiation of ULT should not occur until 1 to 2 weeks after an acute attack has resolved, since beginning ULT during an acute attack is thought to prolong the attack.17 Because gout is a chronic, largely self-managed disease, patient education is a cornerstone of successful long-term treatment. Implementation of a care plan for both an acute flare and chronic gout is recommended ( TABLE ).27
Anti-inflammatory prophylaxis should begin at the same time that ULT is initiated, since an acute attack is likely due to a transient rise in the sUA level resulting from mobilization of MSU crystals. Colchicine, which is the only drug approved by the FDA for prophylaxis of an acute gout attack, can be used daily in a low-dose regimen (0.6 mg once or twice daily) for up to 6 months.17,26 Alternatively, an NSAID can be used.17
One recent investigation pooled the results of 3 phase III clinical trials of ULT in 4101 patients with gout.28 Patients received prophylaxis for 8 weeks or 6 months with low-dose colchicine 0.6 mg once daily or the combination of naproxen 250 mg twice daily with lansoprazole 15 mg once daily. The incidence of acute gout attacks increased sharply (up to 40%) at the end of 8 weeks of prophylaxis with either colchicine or naproxen and then declined steadily, whereas the rates of acute attacks were consistently low (3% to 5%) at the end of 6 months of prophylaxis with either colchicine or naproxen/lansoprazole. With the 8-week prophylaxis regimen, diarrhea was more common in the colchicine group (n = 993) than in the naproxen group (n = 829) (8.4% vs 2.7%, respectively; P < .001). With the 6-month prophylaxis regimen, liver function abnormalities (7.7% vs 4.3%; P = .023) and headache (2.8% vs 0.9%; P = .037) were more common with colchicine (n = 1807) than naproxen, while gastrointestinal/abdominal pains (3.2% vs 1.2%; P = .012) and dental/oral soft tissue infections (2.3% vs 0.6%; P = .006) were more common with naproxen (n = 346) than colchicine.
Uricostatic Agents
Uricostatic therapy with a xanthine oxidase inhibitor (ie, allopurinol or febuxostat) is the most commonly used ULT. Allopurinol is effective in lowering the sUA level and has been shown to lower the rates of all-cause mortality and cardiovascular events, and, in patients with chronic kidney disease, slow the progression of renal disease.29,30 One key point that must be kept in mind is that the efficacy of allopurinol to lower the sUA level is dose-dependent, although limited safety data are available for doses >300 mg per day.14,31,32 One recent prospective clinical trial showed that 26% of patients achieved an sUA level of 5 mg/dL or less following 2 months of treatment with allopurinol 300 mg per day compared with 78% of those who subsequently doubled the dose to 300 mg twice daily.31 Two patients discontinued treatment with allopurinol because of an AE. Finally, the dose of allopurinol must be adjusted based on renal function to minimize the risk of AEs, particularly skin rashes.33
Febuxostat is also effective in lowering the sUA level. In patients with an sUA level of 8.0 mg/dL or higher and a creatinine clearance of 50 mL/min or higher at baseline, an sUA level of less than 6.0 mg/dL was achieved in 53% of patients treated with febuxostat 80 mg (n = 256) versus 21% of patients treated with allopurinol 300 mg once daily (n = 253) after 1 year (P < .001).34 The most frequent treatment-related AE was liver function abnormality, which occurred in 4% of patients in each group. Results of a 6-month trial showed that achievement of an sUA level of less than 6.0 mg/dL was achieved in 45% and 67% of patients treated with febuxostat 40 mg or 80 mg daily, respectively, and 42% of those treated with allopurinol 300 mg (200 mg in moderate renal impairment) daily.35 Febuxostat also has been shown to slow the progression of, or even stabilize, renal function.36
Treatment plan (continued):
- For an acute gout attack: Continue colchicine as needed
- ULT: Initiate allopurinol 100 mg once daily; increase to 200 mg once daily in 1 week, and 300 mg once daily in another week
- -Alternatively, initiate febuxostat 40 mg once daily; increase to 80 mg once daily if an sUA level of less than 6.0 mg/dL is not achieved within 2 weeks
- For prophylaxis of an acute attack when initiating ULT: Initiate colchicine 0.6 mg once daily; may increase to 0.6 mg twice daily if needed
- -Alternatively, initiate naproxen 250 mg twice daily with a proton pump inhibitor
- Measure sUA in 1 month; if the sUA level is greater than 6.0 mg/dL, increase allopurinol to 200 mg twice daily
- -Measure sUA in 1 month; if the sUA level is still greater than 6.0 mg/dL, increase allopurinol to 300 mg twice daily
- Implement the care plan ( TABLE )27
- -Inquire about and address issues to promote adherence and self-management
- -Discuss the most common AEs with allopurinol and colchicine and the actions the patient should take if an AE occurs
- Once the sUA level is 6.0 mg/dL or less, monitor sUA annually (including serum creatinine)14
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(suppl 5):S9-S12.
3. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742-748.
4. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309-312.
5. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
6. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277-1281.
7. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109-115.
8. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031-1036.
9. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503-1507.
10. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642-650.
11. Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10(6):403-409.
12. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421-426.
13. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(Suppl 5):S5-S8.
14. Hamburger M, Baraf HS, Adamson TC III, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123 (6 suppl 1):3-36.
15. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312-1324.
16. Schumacher HR Jr, Boice JA, Daikh DI, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324(7352):1488-1492.
17. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46(8):1372-1374.
18. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.-
19. Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.-
20. NSAIDS and chronic kidney disease. US Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/news/docs/nsaid_video.htm. Published 2012. Accessed April 22, 2012.
21. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
22. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
23. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854-1860.
24. Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
25. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
26. Colcrys [package insert]. Philadelphia, PA: AR Scientific, Inc.; 2011.
27. Becker MA, Ruoff GE. What do I need to know about gout? J Fam Pract. 2010;59(6 suppl):S1-S8.
28. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386-2397.
29. Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009;48(7):804-806.
30. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388-1393.
31. Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892-897.
32. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421.
33. Zyloprim [package insert]. San Diego, CA: Prometheus Laboratories Inc.; 2003.
34. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450-2461.
35. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:doi:10.1186/ar2978.
36. Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7-13.
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Ruoff has disclosed that he is on the speakers’ bureau for and has received research grants from Takeda Pharmaceuticals.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from URL Pharma, Inc.
DB is a 50-year-old obese male visiting the clinic for symptoms suggestive of allergic rhinitis. The nurse has informed the family physician that DB was limping from the waiting room to the examination room. DB reports that he has been experiencing pain in his left big toe and ankle over the past few days. The last time this happened, the pain resolved within 7 to 10 days.
DB reports that he has experienced 4 or 5 similar episodes over the past 3 years. The first attacks affected his left big toe, but he now also experiences some pain in his left ankle. The pain is moderate, peaks in 1 to 2 days, and resolves within 7 to 10 days. Acetaminophen provided little pain relief so DB now takes ibuprofen 400 mg 3 times daily, as it “helps take the edge off.” Other medications include aspirin 81 mg per day and an oral antihistamine as needed for hay fever. DB reports that he eats seafood 2 to 3 times per week and red meat 1 to 2 times per week; he drinks 2 six-packs of beer per week.
Physical examination: weight, 186 lb (body mass index [BMI], 27 kg/m2); blood pressure, 126/76 mm Hg; and temperature, 98.8°F. His left big toe and ankle are red, slightly swollen, and warm with a small subcutaneous nodule noted on the first metatarsophalangeal joint. There is no sign of skin or joint infection.
The impression from his history and physical exam is that DB is suffering from an acute attack of gout, but the family physician also considers other diagnoses.
Background
Gout is a heterogeneous disorder that peaks in incidence in the fifth decade. Gout is caused by hyperuricemia, generally as a result of reduced excretion of uric acid by the kidneys; hyperuricemia may also result from overproduction of uric acid. Data from the National Health and Nutrition Examination Survey (NHANES) 2007-2008 indicate that the prevalence of gout continues to rise in the United States, likely related to the increasing frequency of adiposity and hypertension. Overall, about 75% of the 8.3 million people with gout are men.1
Risk Factors
Clinically defined hyperuricemia—a serum urate (sUA) level greater than 6.8 mg/dL, the concentration at which urate exceeds its solubility in most biological fluids—is the major risk factor for gout. However, not all persons with hyperuricemia have gout. Data from NHANES 2007-2008, in which the definition of hyperuricemia was an sUA level greater than 7.0 mg/dL for men and greater than 5.7 mg/dL for women, showed the mean sUA level to be 6.1 mg/dL in men and 4.9 mg/dL in women, corresponding to hyperuricemia prevalences of 21.2% and 21.6%, respectively.1
There are several other risk factors for gout, including hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease (CVD), and metabolic syndrome.2 For a man with hypertension, the relative risk (RR) of gout is 2.3 compared with a normotensive man.3 Furthermore, it is well established that the use of diuretics increases the risk of gout (RR, 1.8).3 Several other medications increase sUA level as well: aspirin (including low-dose), cyclosporine, pyrazinamide, ethambutol, and niacin.2
Lifestyle and diet also pose a risk for gout. The risk of gout increases with BMI such that, compared with a man with a BMI of 21 to 22.9 kg/m2, the RR of gout is doubled for a man with a BMI of 25 to 29.9 kg/m2; for a man with a BMI of 35 kg/m2 or more, the RR is tripled.3 Sugar-sweetened soft drinks (but not diet soft drinks) and fructose-rich fruits and fruit juices also increase the risk of gout, as do a high alcohol intake, particularly beer, and a diet rich in meat (especially organ meat, turkey, or wild game) or seafood.4 A moderate intake of purine-rich vegetables (eg, peas, beans, lentils, spinach, mushrooms, oatmeal, and cauliflower) or protein is not associated with an increased risk of gout, while a high consumption of dairy products is associated with a decreased risk.5,6
Untreated or poorly treated gout usually leads to further acute attacks and progressive joint and tissue damage. In addition, gout and hyperuricemia serve as risk factors for other diseases. Adults with gout are 3 times as likely to develop metabolic syndrome as adults without gout.7 An elevated sUA level is also an independent risk factor for the development of hypertension (RR, 1.1), as well as myocardial infarction (MI; RR, 1.9), and stroke (RR, 1.6).8,9 An increasing sUA level also increases the risk of renal failure.10,11 In a study of 49,413 men followed for a mean of 5.4 years, the age-adjusted RR of renal failure was 1.5 in men with an sUA level of 6.5 to 8.4 mg/dL and 8.5 in men with an sUA level of 8.5 to 13.0 mg/dL compared with men with an sUA level of 5.0 to 6.4 mg/dL.11
Clinical Presentation
The deposition of monosodium urate (MSU) crystals in joints and tissues is very common and typically causes no signs or symptoms in the majority of persons. Even in men with an sUA level of 9 mg/dL or greater, the cumulative incidence of gouty arthritis has been found to be 22% over 5 years.12 However, as crystal deposition progresses, acute, painful attacks occur more frequently, with the development of chronic tophaceous gout after several years.13
Laboratory results for DB:
- Serum uric acid, 7.9 mg/dL
- White blood cell count, 15,800/mm3
- Serum creatinine, 1.2 mg/dL (estimated creatinine clearance, 90 mL/min)
- Erythrocyte sedimentation rate, 23 mm/h
- Low-density lipoprotein cholesterol (nonfasting), 127 mg/dL
Laboratory confirmation of hyperuricemia together with the pain, swelling, and tenderness of DB’s toe and ankle, other findings from his medical history and physical exam (eg, the use of aspirin daily), and exclusion of alternative diagnoses, such as septic arthritis, enable the family physician to arrive at a presumptive diagnosis of gouty arthritis. Aspiration of MSU crystals from DB’s toe or ankle is the gold standard and would allow for a definitive diagnosis. Although the sUA level was found to be high, it should be noted that a normal sUA level is often found during an acute attack; should this occur, the sUA level should be checked again 1 to 2 weeks after the acute attack has resolved.
Goals of Treatment
The cornerstone of gout management is daily, long-term treatment with urate-lowering therapy (ULT) combined with as-needed treatment for an acute attack. In addition, since initiation of ULT mobilizes MSU crystals, which often leads to a short-term increase in acute attacks, prophylaxis with an appropriate anti-inflammatory therapy is recommended at the time ULT is initiated.14
The therapeutic goals of gout treatment are 2-pronged: treatment of an acute gout attack and management of chronic gout. For an acute attack, the goals are to exclude a diagnosis of septic arthritis; reduce inflammation and terminate the attack; and seek, assess, and control associated diseases, such as diabetes mellitus, hypertension, hyperlipidemia, and CVD. If this latter goal is not possible during the acute attack, plans should be made to assess associated diseases once the acute attack has resolved.14 Lowering the sUA level is not a goal of therapy for an acute attack, but it is the primary goal of ULT for chronic gout. Lowering the sUA level to less than 6.0 mg/dL, which is well below the saturation point of urate in most biological fluids, is intended to prevent further acute attacks, tophus formation, and tissue damage.14
Treatment of an Acute Attack
The mainstay of treatment for an acute attack is anti-inflammatory therapy to reduce pain and inflammation.14 Therapy should be initiated at the onset of the attack and continued until the attack is terminated, which is typically 1 to 2 weeks. Anti-inflammatory therapy traditionally has in-cluded colchicine, a nonsteroidal anti-inflammatory drug (NSAID), or a corticosteroid.14
Nonsteroidal Anti-inflammatory Drugs
The NSAIDs are all thought to provide similar efficacy when used in maximum doses.15,16 Since gastrointestinal toxicity is a concern with NSAIDs, coadministration of a proton pump inhibitor, H2 antagonist, or misoprostol is advised for patients with an increased risk of peptic ulcers, bleeds, or perforations.17 The risk of MI, stroke, cardiovascular death, and atrial fibrillation/flutter with NSAID therapy should be considered, especially because gout often coexists with cardiovascular disorders.15,18,19 Furthermore, NSAIDs are contraindicated in patients with heart failure or renal insufficiency.20,21
Corticosteroids. A systematic review of clinical trials involving systemic corticosteroids that found a few prospective trials of low to moderate quality concluded that there was inconclusive evidence for the efficacy and effectiveness of corticosteroids in the treatment of acute gout.22 No serious adverse events (AEs) were reported. A more recent prospective trial found comparable pain reduction and incidence of AEs with naproxen 500 mg twice daily and prednisolone 35 mg once daily for 5 days in patients with monoarticular gout.23 Furthermore, clinical experience indicates that intra-articular aspiration and injection of a long-acting corticosteroid is an effective and safe treatment for an acute attack.14,15 Corticosteroids may be useful in patients who have an inadequate response to, are intolerant of, or have a contraindication to NSAIDs and colchicine.14,15
Colchicine. Much of the recent clinical investigation regarding pharmacologic treatment of an acute gout attack has involved colchicine. To overcome the limitations of the standard dose-to-toxicity regimen of colchicine, a low-dose regimen of colchicine (1.2 mg followed by 0.6 mg 1 hour later) was investigated and subsequently approved by the US Food and Drug Administration (FDA).24
Approval was based on a randomized, double-blind comparison with high-dose colchicine (1.2 mg followed by 0.6 mg every hour for 6 hours) and placebo in 184 patients with an acute gout attack.25 The primary endpoint, a 50% or greater reduction in pain at 24 hours without the use of rescue medication, was reached in 28 of 74 patients (38%) in the low-dose group, 17 of 52 patients (33%) in the high-dose group, and 9 of 58 patients (16%) in the placebo group (P = .005 and P = .034, respectively, versus placebo). An AE occurred in 36.5% and 76.9% of study participants in the low-dose and high-dose colchicine groups, respectively, and in 27.1% of participants in the placebo group. Gastrointestinal AEs (eg, diarrhea, nausea, and vomiting) were less common in the low-dose colchicine group ( FIGURE ). All AEs in the low-dose group were mild to moderate in intensity, while 10 of 52 patients (19.2%) in the high-dose group had an AE of severe intensity. Concomitant use of numerous drugs can increase the concentration of colchicine. Examples include atorvastatin, fluvastatin, pravastatin, simvastatin, fibrates, gemfibrozil, digoxin, clarithromycin, erythromycin, fluconazole, itraconazole, ketoconazole, protease inhibitors, diltiazem, verapamil, and cyclosporine, as well as grapefruit juice.26
FIGURE
Frequency of selected adverse events occurring over 24 hours with low-dose vs high-dose colchicine25
Treatment plan:
TABLE
Care plan for a patient with gout27
| Acute flare | Chronic gout | |
|---|---|---|
| Goals |
|
|
| Educational points |
|
|
| sUA, serum uric acid; ULT, urate-lowering therapy. Source: Reproduced with permission. Becker MA, et al. J Fam Pract. 2010;59(6):S1-S8. Quadrant HealthCom Inc. Copyright 2010. | ||
Urate-Lowering Therapy
Urate lowering therapy is indicated for most, but not all, patients with gout. ULT is generally not recommended for those who have suffered a single attack of gout and have no complications, since 40% of these patients will not experience another attack within a year. However, should a second attack occur within a year of the first attack, ULT is recommended. Some patients who have experienced a single attack may elect to initiate ULT after being educated about the risks of the disease and the risks and benefits of ULT.14 Patients who have had an attack of gout and also have a comorbidity (eg, visible gouty tophi, renal insufficiency, uric acid stones, or use of a diuretic for hypertension) should begin ULT, since the risk of further attacks is higher in these patients, and kidney or joint damage is more likely.17
Initiation of ULT should not occur until 1 to 2 weeks after an acute attack has resolved, since beginning ULT during an acute attack is thought to prolong the attack.17 Because gout is a chronic, largely self-managed disease, patient education is a cornerstone of successful long-term treatment. Implementation of a care plan for both an acute flare and chronic gout is recommended ( TABLE ).27
Anti-inflammatory prophylaxis should begin at the same time that ULT is initiated, since an acute attack is likely due to a transient rise in the sUA level resulting from mobilization of MSU crystals. Colchicine, which is the only drug approved by the FDA for prophylaxis of an acute gout attack, can be used daily in a low-dose regimen (0.6 mg once or twice daily) for up to 6 months.17,26 Alternatively, an NSAID can be used.17
One recent investigation pooled the results of 3 phase III clinical trials of ULT in 4101 patients with gout.28 Patients received prophylaxis for 8 weeks or 6 months with low-dose colchicine 0.6 mg once daily or the combination of naproxen 250 mg twice daily with lansoprazole 15 mg once daily. The incidence of acute gout attacks increased sharply (up to 40%) at the end of 8 weeks of prophylaxis with either colchicine or naproxen and then declined steadily, whereas the rates of acute attacks were consistently low (3% to 5%) at the end of 6 months of prophylaxis with either colchicine or naproxen/lansoprazole. With the 8-week prophylaxis regimen, diarrhea was more common in the colchicine group (n = 993) than in the naproxen group (n = 829) (8.4% vs 2.7%, respectively; P < .001). With the 6-month prophylaxis regimen, liver function abnormalities (7.7% vs 4.3%; P = .023) and headache (2.8% vs 0.9%; P = .037) were more common with colchicine (n = 1807) than naproxen, while gastrointestinal/abdominal pains (3.2% vs 1.2%; P = .012) and dental/oral soft tissue infections (2.3% vs 0.6%; P = .006) were more common with naproxen (n = 346) than colchicine.
Uricostatic Agents
Uricostatic therapy with a xanthine oxidase inhibitor (ie, allopurinol or febuxostat) is the most commonly used ULT. Allopurinol is effective in lowering the sUA level and has been shown to lower the rates of all-cause mortality and cardiovascular events, and, in patients with chronic kidney disease, slow the progression of renal disease.29,30 One key point that must be kept in mind is that the efficacy of allopurinol to lower the sUA level is dose-dependent, although limited safety data are available for doses >300 mg per day.14,31,32 One recent prospective clinical trial showed that 26% of patients achieved an sUA level of 5 mg/dL or less following 2 months of treatment with allopurinol 300 mg per day compared with 78% of those who subsequently doubled the dose to 300 mg twice daily.31 Two patients discontinued treatment with allopurinol because of an AE. Finally, the dose of allopurinol must be adjusted based on renal function to minimize the risk of AEs, particularly skin rashes.33
Febuxostat is also effective in lowering the sUA level. In patients with an sUA level of 8.0 mg/dL or higher and a creatinine clearance of 50 mL/min or higher at baseline, an sUA level of less than 6.0 mg/dL was achieved in 53% of patients treated with febuxostat 80 mg (n = 256) versus 21% of patients treated with allopurinol 300 mg once daily (n = 253) after 1 year (P < .001).34 The most frequent treatment-related AE was liver function abnormality, which occurred in 4% of patients in each group. Results of a 6-month trial showed that achievement of an sUA level of less than 6.0 mg/dL was achieved in 45% and 67% of patients treated with febuxostat 40 mg or 80 mg daily, respectively, and 42% of those treated with allopurinol 300 mg (200 mg in moderate renal impairment) daily.35 Febuxostat also has been shown to slow the progression of, or even stabilize, renal function.36
Treatment plan (continued):
- For an acute gout attack: Continue colchicine as needed
- ULT: Initiate allopurinol 100 mg once daily; increase to 200 mg once daily in 1 week, and 300 mg once daily in another week
- -Alternatively, initiate febuxostat 40 mg once daily; increase to 80 mg once daily if an sUA level of less than 6.0 mg/dL is not achieved within 2 weeks
- For prophylaxis of an acute attack when initiating ULT: Initiate colchicine 0.6 mg once daily; may increase to 0.6 mg twice daily if needed
- -Alternatively, initiate naproxen 250 mg twice daily with a proton pump inhibitor
- Measure sUA in 1 month; if the sUA level is greater than 6.0 mg/dL, increase allopurinol to 200 mg twice daily
- -Measure sUA in 1 month; if the sUA level is still greater than 6.0 mg/dL, increase allopurinol to 300 mg twice daily
- Implement the care plan ( TABLE )27
- -Inquire about and address issues to promote adherence and self-management
- -Discuss the most common AEs with allopurinol and colchicine and the actions the patient should take if an AE occurs
- Once the sUA level is 6.0 mg/dL or less, monitor sUA annually (including serum creatinine)14
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Ruoff has disclosed that he is on the speakers’ bureau for and has received research grants from Takeda Pharmaceuticals.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from URL Pharma, Inc.
DB is a 50-year-old obese male visiting the clinic for symptoms suggestive of allergic rhinitis. The nurse has informed the family physician that DB was limping from the waiting room to the examination room. DB reports that he has been experiencing pain in his left big toe and ankle over the past few days. The last time this happened, the pain resolved within 7 to 10 days.
DB reports that he has experienced 4 or 5 similar episodes over the past 3 years. The first attacks affected his left big toe, but he now also experiences some pain in his left ankle. The pain is moderate, peaks in 1 to 2 days, and resolves within 7 to 10 days. Acetaminophen provided little pain relief so DB now takes ibuprofen 400 mg 3 times daily, as it “helps take the edge off.” Other medications include aspirin 81 mg per day and an oral antihistamine as needed for hay fever. DB reports that he eats seafood 2 to 3 times per week and red meat 1 to 2 times per week; he drinks 2 six-packs of beer per week.
Physical examination: weight, 186 lb (body mass index [BMI], 27 kg/m2); blood pressure, 126/76 mm Hg; and temperature, 98.8°F. His left big toe and ankle are red, slightly swollen, and warm with a small subcutaneous nodule noted on the first metatarsophalangeal joint. There is no sign of skin or joint infection.
The impression from his history and physical exam is that DB is suffering from an acute attack of gout, but the family physician also considers other diagnoses.
Background
Gout is a heterogeneous disorder that peaks in incidence in the fifth decade. Gout is caused by hyperuricemia, generally as a result of reduced excretion of uric acid by the kidneys; hyperuricemia may also result from overproduction of uric acid. Data from the National Health and Nutrition Examination Survey (NHANES) 2007-2008 indicate that the prevalence of gout continues to rise in the United States, likely related to the increasing frequency of adiposity and hypertension. Overall, about 75% of the 8.3 million people with gout are men.1
Risk Factors
Clinically defined hyperuricemia—a serum urate (sUA) level greater than 6.8 mg/dL, the concentration at which urate exceeds its solubility in most biological fluids—is the major risk factor for gout. However, not all persons with hyperuricemia have gout. Data from NHANES 2007-2008, in which the definition of hyperuricemia was an sUA level greater than 7.0 mg/dL for men and greater than 5.7 mg/dL for women, showed the mean sUA level to be 6.1 mg/dL in men and 4.9 mg/dL in women, corresponding to hyperuricemia prevalences of 21.2% and 21.6%, respectively.1
There are several other risk factors for gout, including hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease (CVD), and metabolic syndrome.2 For a man with hypertension, the relative risk (RR) of gout is 2.3 compared with a normotensive man.3 Furthermore, it is well established that the use of diuretics increases the risk of gout (RR, 1.8).3 Several other medications increase sUA level as well: aspirin (including low-dose), cyclosporine, pyrazinamide, ethambutol, and niacin.2
Lifestyle and diet also pose a risk for gout. The risk of gout increases with BMI such that, compared with a man with a BMI of 21 to 22.9 kg/m2, the RR of gout is doubled for a man with a BMI of 25 to 29.9 kg/m2; for a man with a BMI of 35 kg/m2 or more, the RR is tripled.3 Sugar-sweetened soft drinks (but not diet soft drinks) and fructose-rich fruits and fruit juices also increase the risk of gout, as do a high alcohol intake, particularly beer, and a diet rich in meat (especially organ meat, turkey, or wild game) or seafood.4 A moderate intake of purine-rich vegetables (eg, peas, beans, lentils, spinach, mushrooms, oatmeal, and cauliflower) or protein is not associated with an increased risk of gout, while a high consumption of dairy products is associated with a decreased risk.5,6
Untreated or poorly treated gout usually leads to further acute attacks and progressive joint and tissue damage. In addition, gout and hyperuricemia serve as risk factors for other diseases. Adults with gout are 3 times as likely to develop metabolic syndrome as adults without gout.7 An elevated sUA level is also an independent risk factor for the development of hypertension (RR, 1.1), as well as myocardial infarction (MI; RR, 1.9), and stroke (RR, 1.6).8,9 An increasing sUA level also increases the risk of renal failure.10,11 In a study of 49,413 men followed for a mean of 5.4 years, the age-adjusted RR of renal failure was 1.5 in men with an sUA level of 6.5 to 8.4 mg/dL and 8.5 in men with an sUA level of 8.5 to 13.0 mg/dL compared with men with an sUA level of 5.0 to 6.4 mg/dL.11
Clinical Presentation
The deposition of monosodium urate (MSU) crystals in joints and tissues is very common and typically causes no signs or symptoms in the majority of persons. Even in men with an sUA level of 9 mg/dL or greater, the cumulative incidence of gouty arthritis has been found to be 22% over 5 years.12 However, as crystal deposition progresses, acute, painful attacks occur more frequently, with the development of chronic tophaceous gout after several years.13
Laboratory results for DB:
- Serum uric acid, 7.9 mg/dL
- White blood cell count, 15,800/mm3
- Serum creatinine, 1.2 mg/dL (estimated creatinine clearance, 90 mL/min)
- Erythrocyte sedimentation rate, 23 mm/h
- Low-density lipoprotein cholesterol (nonfasting), 127 mg/dL
Laboratory confirmation of hyperuricemia together with the pain, swelling, and tenderness of DB’s toe and ankle, other findings from his medical history and physical exam (eg, the use of aspirin daily), and exclusion of alternative diagnoses, such as septic arthritis, enable the family physician to arrive at a presumptive diagnosis of gouty arthritis. Aspiration of MSU crystals from DB’s toe or ankle is the gold standard and would allow for a definitive diagnosis. Although the sUA level was found to be high, it should be noted that a normal sUA level is often found during an acute attack; should this occur, the sUA level should be checked again 1 to 2 weeks after the acute attack has resolved.
Goals of Treatment
The cornerstone of gout management is daily, long-term treatment with urate-lowering therapy (ULT) combined with as-needed treatment for an acute attack. In addition, since initiation of ULT mobilizes MSU crystals, which often leads to a short-term increase in acute attacks, prophylaxis with an appropriate anti-inflammatory therapy is recommended at the time ULT is initiated.14
The therapeutic goals of gout treatment are 2-pronged: treatment of an acute gout attack and management of chronic gout. For an acute attack, the goals are to exclude a diagnosis of septic arthritis; reduce inflammation and terminate the attack; and seek, assess, and control associated diseases, such as diabetes mellitus, hypertension, hyperlipidemia, and CVD. If this latter goal is not possible during the acute attack, plans should be made to assess associated diseases once the acute attack has resolved.14 Lowering the sUA level is not a goal of therapy for an acute attack, but it is the primary goal of ULT for chronic gout. Lowering the sUA level to less than 6.0 mg/dL, which is well below the saturation point of urate in most biological fluids, is intended to prevent further acute attacks, tophus formation, and tissue damage.14
Treatment of an Acute Attack
The mainstay of treatment for an acute attack is anti-inflammatory therapy to reduce pain and inflammation.14 Therapy should be initiated at the onset of the attack and continued until the attack is terminated, which is typically 1 to 2 weeks. Anti-inflammatory therapy traditionally has in-cluded colchicine, a nonsteroidal anti-inflammatory drug (NSAID), or a corticosteroid.14
Nonsteroidal Anti-inflammatory Drugs
The NSAIDs are all thought to provide similar efficacy when used in maximum doses.15,16 Since gastrointestinal toxicity is a concern with NSAIDs, coadministration of a proton pump inhibitor, H2 antagonist, or misoprostol is advised for patients with an increased risk of peptic ulcers, bleeds, or perforations.17 The risk of MI, stroke, cardiovascular death, and atrial fibrillation/flutter with NSAID therapy should be considered, especially because gout often coexists with cardiovascular disorders.15,18,19 Furthermore, NSAIDs are contraindicated in patients with heart failure or renal insufficiency.20,21
Corticosteroids. A systematic review of clinical trials involving systemic corticosteroids that found a few prospective trials of low to moderate quality concluded that there was inconclusive evidence for the efficacy and effectiveness of corticosteroids in the treatment of acute gout.22 No serious adverse events (AEs) were reported. A more recent prospective trial found comparable pain reduction and incidence of AEs with naproxen 500 mg twice daily and prednisolone 35 mg once daily for 5 days in patients with monoarticular gout.23 Furthermore, clinical experience indicates that intra-articular aspiration and injection of a long-acting corticosteroid is an effective and safe treatment for an acute attack.14,15 Corticosteroids may be useful in patients who have an inadequate response to, are intolerant of, or have a contraindication to NSAIDs and colchicine.14,15
Colchicine. Much of the recent clinical investigation regarding pharmacologic treatment of an acute gout attack has involved colchicine. To overcome the limitations of the standard dose-to-toxicity regimen of colchicine, a low-dose regimen of colchicine (1.2 mg followed by 0.6 mg 1 hour later) was investigated and subsequently approved by the US Food and Drug Administration (FDA).24
Approval was based on a randomized, double-blind comparison with high-dose colchicine (1.2 mg followed by 0.6 mg every hour for 6 hours) and placebo in 184 patients with an acute gout attack.25 The primary endpoint, a 50% or greater reduction in pain at 24 hours without the use of rescue medication, was reached in 28 of 74 patients (38%) in the low-dose group, 17 of 52 patients (33%) in the high-dose group, and 9 of 58 patients (16%) in the placebo group (P = .005 and P = .034, respectively, versus placebo). An AE occurred in 36.5% and 76.9% of study participants in the low-dose and high-dose colchicine groups, respectively, and in 27.1% of participants in the placebo group. Gastrointestinal AEs (eg, diarrhea, nausea, and vomiting) were less common in the low-dose colchicine group ( FIGURE ). All AEs in the low-dose group were mild to moderate in intensity, while 10 of 52 patients (19.2%) in the high-dose group had an AE of severe intensity. Concomitant use of numerous drugs can increase the concentration of colchicine. Examples include atorvastatin, fluvastatin, pravastatin, simvastatin, fibrates, gemfibrozil, digoxin, clarithromycin, erythromycin, fluconazole, itraconazole, ketoconazole, protease inhibitors, diltiazem, verapamil, and cyclosporine, as well as grapefruit juice.26
FIGURE
Frequency of selected adverse events occurring over 24 hours with low-dose vs high-dose colchicine25
Treatment plan:
TABLE
Care plan for a patient with gout27
| Acute flare | Chronic gout | |
|---|---|---|
| Goals |
|
|
| Educational points |
|
|
| sUA, serum uric acid; ULT, urate-lowering therapy. Source: Reproduced with permission. Becker MA, et al. J Fam Pract. 2010;59(6):S1-S8. Quadrant HealthCom Inc. Copyright 2010. | ||
Urate-Lowering Therapy
Urate lowering therapy is indicated for most, but not all, patients with gout. ULT is generally not recommended for those who have suffered a single attack of gout and have no complications, since 40% of these patients will not experience another attack within a year. However, should a second attack occur within a year of the first attack, ULT is recommended. Some patients who have experienced a single attack may elect to initiate ULT after being educated about the risks of the disease and the risks and benefits of ULT.14 Patients who have had an attack of gout and also have a comorbidity (eg, visible gouty tophi, renal insufficiency, uric acid stones, or use of a diuretic for hypertension) should begin ULT, since the risk of further attacks is higher in these patients, and kidney or joint damage is more likely.17
Initiation of ULT should not occur until 1 to 2 weeks after an acute attack has resolved, since beginning ULT during an acute attack is thought to prolong the attack.17 Because gout is a chronic, largely self-managed disease, patient education is a cornerstone of successful long-term treatment. Implementation of a care plan for both an acute flare and chronic gout is recommended ( TABLE ).27
Anti-inflammatory prophylaxis should begin at the same time that ULT is initiated, since an acute attack is likely due to a transient rise in the sUA level resulting from mobilization of MSU crystals. Colchicine, which is the only drug approved by the FDA for prophylaxis of an acute gout attack, can be used daily in a low-dose regimen (0.6 mg once or twice daily) for up to 6 months.17,26 Alternatively, an NSAID can be used.17
One recent investigation pooled the results of 3 phase III clinical trials of ULT in 4101 patients with gout.28 Patients received prophylaxis for 8 weeks or 6 months with low-dose colchicine 0.6 mg once daily or the combination of naproxen 250 mg twice daily with lansoprazole 15 mg once daily. The incidence of acute gout attacks increased sharply (up to 40%) at the end of 8 weeks of prophylaxis with either colchicine or naproxen and then declined steadily, whereas the rates of acute attacks were consistently low (3% to 5%) at the end of 6 months of prophylaxis with either colchicine or naproxen/lansoprazole. With the 8-week prophylaxis regimen, diarrhea was more common in the colchicine group (n = 993) than in the naproxen group (n = 829) (8.4% vs 2.7%, respectively; P < .001). With the 6-month prophylaxis regimen, liver function abnormalities (7.7% vs 4.3%; P = .023) and headache (2.8% vs 0.9%; P = .037) were more common with colchicine (n = 1807) than naproxen, while gastrointestinal/abdominal pains (3.2% vs 1.2%; P = .012) and dental/oral soft tissue infections (2.3% vs 0.6%; P = .006) were more common with naproxen (n = 346) than colchicine.
Uricostatic Agents
Uricostatic therapy with a xanthine oxidase inhibitor (ie, allopurinol or febuxostat) is the most commonly used ULT. Allopurinol is effective in lowering the sUA level and has been shown to lower the rates of all-cause mortality and cardiovascular events, and, in patients with chronic kidney disease, slow the progression of renal disease.29,30 One key point that must be kept in mind is that the efficacy of allopurinol to lower the sUA level is dose-dependent, although limited safety data are available for doses >300 mg per day.14,31,32 One recent prospective clinical trial showed that 26% of patients achieved an sUA level of 5 mg/dL or less following 2 months of treatment with allopurinol 300 mg per day compared with 78% of those who subsequently doubled the dose to 300 mg twice daily.31 Two patients discontinued treatment with allopurinol because of an AE. Finally, the dose of allopurinol must be adjusted based on renal function to minimize the risk of AEs, particularly skin rashes.33
Febuxostat is also effective in lowering the sUA level. In patients with an sUA level of 8.0 mg/dL or higher and a creatinine clearance of 50 mL/min or higher at baseline, an sUA level of less than 6.0 mg/dL was achieved in 53% of patients treated with febuxostat 80 mg (n = 256) versus 21% of patients treated with allopurinol 300 mg once daily (n = 253) after 1 year (P < .001).34 The most frequent treatment-related AE was liver function abnormality, which occurred in 4% of patients in each group. Results of a 6-month trial showed that achievement of an sUA level of less than 6.0 mg/dL was achieved in 45% and 67% of patients treated with febuxostat 40 mg or 80 mg daily, respectively, and 42% of those treated with allopurinol 300 mg (200 mg in moderate renal impairment) daily.35 Febuxostat also has been shown to slow the progression of, or even stabilize, renal function.36
Treatment plan (continued):
- For an acute gout attack: Continue colchicine as needed
- ULT: Initiate allopurinol 100 mg once daily; increase to 200 mg once daily in 1 week, and 300 mg once daily in another week
- -Alternatively, initiate febuxostat 40 mg once daily; increase to 80 mg once daily if an sUA level of less than 6.0 mg/dL is not achieved within 2 weeks
- For prophylaxis of an acute attack when initiating ULT: Initiate colchicine 0.6 mg once daily; may increase to 0.6 mg twice daily if needed
- -Alternatively, initiate naproxen 250 mg twice daily with a proton pump inhibitor
- Measure sUA in 1 month; if the sUA level is greater than 6.0 mg/dL, increase allopurinol to 200 mg twice daily
- -Measure sUA in 1 month; if the sUA level is still greater than 6.0 mg/dL, increase allopurinol to 300 mg twice daily
- Implement the care plan ( TABLE )27
- -Inquire about and address issues to promote adherence and self-management
- -Discuss the most common AEs with allopurinol and colchicine and the actions the patient should take if an AE occurs
- Once the sUA level is 6.0 mg/dL or less, monitor sUA annually (including serum creatinine)14
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(suppl 5):S9-S12.
3. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742-748.
4. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309-312.
5. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
6. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277-1281.
7. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109-115.
8. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031-1036.
9. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503-1507.
10. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642-650.
11. Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10(6):403-409.
12. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421-426.
13. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(Suppl 5):S5-S8.
14. Hamburger M, Baraf HS, Adamson TC III, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123 (6 suppl 1):3-36.
15. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312-1324.
16. Schumacher HR Jr, Boice JA, Daikh DI, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324(7352):1488-1492.
17. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46(8):1372-1374.
18. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.-
19. Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.-
20. NSAIDS and chronic kidney disease. US Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/news/docs/nsaid_video.htm. Published 2012. Accessed April 22, 2012.
21. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
22. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
23. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854-1860.
24. Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
25. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
26. Colcrys [package insert]. Philadelphia, PA: AR Scientific, Inc.; 2011.
27. Becker MA, Ruoff GE. What do I need to know about gout? J Fam Pract. 2010;59(6 suppl):S1-S8.
28. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386-2397.
29. Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009;48(7):804-806.
30. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388-1393.
31. Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892-897.
32. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421.
33. Zyloprim [package insert]. San Diego, CA: Prometheus Laboratories Inc.; 2003.
34. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450-2461.
35. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:doi:10.1186/ar2978.
36. Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7-13.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(suppl 5):S9-S12.
3. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742-748.
4. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309-312.
5. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
6. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277-1281.
7. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109-115.
8. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031-1036.
9. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503-1507.
10. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642-650.
11. Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10(6):403-409.
12. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421-426.
13. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(Suppl 5):S5-S8.
14. Hamburger M, Baraf HS, Adamson TC III, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123 (6 suppl 1):3-36.
15. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312-1324.
16. Schumacher HR Jr, Boice JA, Daikh DI, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324(7352):1488-1492.
17. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46(8):1372-1374.
18. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.-
19. Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.-
20. NSAIDS and chronic kidney disease. US Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/news/docs/nsaid_video.htm. Published 2012. Accessed April 22, 2012.
21. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
22. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
23. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854-1860.
24. Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
25. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
26. Colcrys [package insert]. Philadelphia, PA: AR Scientific, Inc.; 2011.
27. Becker MA, Ruoff GE. What do I need to know about gout? J Fam Pract. 2010;59(6 suppl):S1-S8.
28. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386-2397.
29. Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009;48(7):804-806.
30. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388-1393.
31. Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892-897.
32. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421.
33. Zyloprim [package insert]. San Diego, CA: Prometheus Laboratories Inc.; 2003.
34. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450-2461.
35. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:doi:10.1186/ar2978.
36. Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7-13.
Dipstick Proteinuria Predicts Acute Kidney Injury in Septic Patients
NATIONAL HARBOR, MD. – De novo dipstick proteinuria accurately predicted acute kidney injury among 328 critically ill septic patients, a retrospective chart study has shown.
With sepsis, inflammation results in increased capillary permeability to plasma proteins, manifesting in an increased excretion of albumin into the urine. Because the production of creatinine from the muscle is reduced in septic patients, relying on changes in serum creatinine could delay the diagnosis of this acute kidney injury (AKI), according to Dr. Javier Neyra.
"It is highly important to identify biomarkers that are sensitive, specific, and provide timely and early diagnosis of acute kidney injury before substantial damage has already been done. ... De novo dipstick proteinuria represents a simple, inexpensive biomarker in sepsis with predictive power for AKI," said Dr. Neyra of the Henry Ford Hospital, Detroit.
Charts from a total of 2,252 patients admitted to the intensive care unit for severe sepsis between January 2004 and July 2011 were analyzed retrospectively. Patients with a baseline serum creatinine level greater than 1.5 mg/dL, the presence of dipstick proteinuria within 3 months of the admission date, or common causes of false-positive dipstick tests such as urinary tract infection or gross hematuria were excluded. Of the remaining 470 patients, 328 had undergone dipstick testing on admission. Of those, 46% (152) had dipstick proteinuria.
Serum creatinine increased by at least 0.3 mg/dL in 210 (64%) patients within the first 72 hours of admission, signifying the first stage of acute kidney injury. In this group, new-onset dipstick proteinuria was found in 114 (54%) patients, for a positive predictive value of 75%. Dipstick proteinuria was found in 91 (55%) of 166 patients who met the Acute Kidney Injury Network criteria for AKI, for a positive predictive value of 60%.
After adjustment for age, sex, race, comorbidities, hemodynamic status, and other variables, de novo dipstick proteinuria at the time of admission independently predicted AKI with an odds ratio of 2.3 (95% confidence interval, 1.4-3.8), Dr. Neyra reported in a poster at a meeting sponsored by the National Kidney Foundation.
In an interview, Dr. Neyra explained that such information identifies septic patients who would benefit from more careful monitoring of kidney function and hemodynamic stability. In addition, in those patients it would be important to avoid nephrotoxic agents such as aminoglycoside antibiotics and nonsteroidal anti-inflammatory agents, as well as exposure to contrast material unless it was absolutely necessary.
"The dipstick is a test that you already have in your hospital that you can utilize. It’s simple, inexpensive, and it’s already there," he said.
Dr. Neyra stated that he had no relevant financial disclosures.
NATIONAL HARBOR, MD. – De novo dipstick proteinuria accurately predicted acute kidney injury among 328 critically ill septic patients, a retrospective chart study has shown.
With sepsis, inflammation results in increased capillary permeability to plasma proteins, manifesting in an increased excretion of albumin into the urine. Because the production of creatinine from the muscle is reduced in septic patients, relying on changes in serum creatinine could delay the diagnosis of this acute kidney injury (AKI), according to Dr. Javier Neyra.
"It is highly important to identify biomarkers that are sensitive, specific, and provide timely and early diagnosis of acute kidney injury before substantial damage has already been done. ... De novo dipstick proteinuria represents a simple, inexpensive biomarker in sepsis with predictive power for AKI," said Dr. Neyra of the Henry Ford Hospital, Detroit.
Charts from a total of 2,252 patients admitted to the intensive care unit for severe sepsis between January 2004 and July 2011 were analyzed retrospectively. Patients with a baseline serum creatinine level greater than 1.5 mg/dL, the presence of dipstick proteinuria within 3 months of the admission date, or common causes of false-positive dipstick tests such as urinary tract infection or gross hematuria were excluded. Of the remaining 470 patients, 328 had undergone dipstick testing on admission. Of those, 46% (152) had dipstick proteinuria.
Serum creatinine increased by at least 0.3 mg/dL in 210 (64%) patients within the first 72 hours of admission, signifying the first stage of acute kidney injury. In this group, new-onset dipstick proteinuria was found in 114 (54%) patients, for a positive predictive value of 75%. Dipstick proteinuria was found in 91 (55%) of 166 patients who met the Acute Kidney Injury Network criteria for AKI, for a positive predictive value of 60%.
After adjustment for age, sex, race, comorbidities, hemodynamic status, and other variables, de novo dipstick proteinuria at the time of admission independently predicted AKI with an odds ratio of 2.3 (95% confidence interval, 1.4-3.8), Dr. Neyra reported in a poster at a meeting sponsored by the National Kidney Foundation.
In an interview, Dr. Neyra explained that such information identifies septic patients who would benefit from more careful monitoring of kidney function and hemodynamic stability. In addition, in those patients it would be important to avoid nephrotoxic agents such as aminoglycoside antibiotics and nonsteroidal anti-inflammatory agents, as well as exposure to contrast material unless it was absolutely necessary.
"The dipstick is a test that you already have in your hospital that you can utilize. It’s simple, inexpensive, and it’s already there," he said.
Dr. Neyra stated that he had no relevant financial disclosures.
NATIONAL HARBOR, MD. – De novo dipstick proteinuria accurately predicted acute kidney injury among 328 critically ill septic patients, a retrospective chart study has shown.
With sepsis, inflammation results in increased capillary permeability to plasma proteins, manifesting in an increased excretion of albumin into the urine. Because the production of creatinine from the muscle is reduced in septic patients, relying on changes in serum creatinine could delay the diagnosis of this acute kidney injury (AKI), according to Dr. Javier Neyra.
"It is highly important to identify biomarkers that are sensitive, specific, and provide timely and early diagnosis of acute kidney injury before substantial damage has already been done. ... De novo dipstick proteinuria represents a simple, inexpensive biomarker in sepsis with predictive power for AKI," said Dr. Neyra of the Henry Ford Hospital, Detroit.
Charts from a total of 2,252 patients admitted to the intensive care unit for severe sepsis between January 2004 and July 2011 were analyzed retrospectively. Patients with a baseline serum creatinine level greater than 1.5 mg/dL, the presence of dipstick proteinuria within 3 months of the admission date, or common causes of false-positive dipstick tests such as urinary tract infection or gross hematuria were excluded. Of the remaining 470 patients, 328 had undergone dipstick testing on admission. Of those, 46% (152) had dipstick proteinuria.
Serum creatinine increased by at least 0.3 mg/dL in 210 (64%) patients within the first 72 hours of admission, signifying the first stage of acute kidney injury. In this group, new-onset dipstick proteinuria was found in 114 (54%) patients, for a positive predictive value of 75%. Dipstick proteinuria was found in 91 (55%) of 166 patients who met the Acute Kidney Injury Network criteria for AKI, for a positive predictive value of 60%.
After adjustment for age, sex, race, comorbidities, hemodynamic status, and other variables, de novo dipstick proteinuria at the time of admission independently predicted AKI with an odds ratio of 2.3 (95% confidence interval, 1.4-3.8), Dr. Neyra reported in a poster at a meeting sponsored by the National Kidney Foundation.
In an interview, Dr. Neyra explained that such information identifies septic patients who would benefit from more careful monitoring of kidney function and hemodynamic stability. In addition, in those patients it would be important to avoid nephrotoxic agents such as aminoglycoside antibiotics and nonsteroidal anti-inflammatory agents, as well as exposure to contrast material unless it was absolutely necessary.
"The dipstick is a test that you already have in your hospital that you can utilize. It’s simple, inexpensive, and it’s already there," he said.
Dr. Neyra stated that he had no relevant financial disclosures.
FROM A MEETING SPONSORED BY THE NATIONAL KIDNEY FOUNDATION
Major Finding: Dipstick proteinuria was found in 55% of patients who met the AKIN criteria for acute kidney injury, for a positive predictive value of 60%.
Data Source: The findings come from a retrospective chart study of 328 ICU patients with sepsis who had dipstick testing done on admission.
Disclosures: Dr. Neyra reported having no relevant financial disclosures.
PSA Level Could Determine Screening Frequency in 40s
ATLANTA – Men in their 40s with a low prostate specific antigen can probably safely delay additional testing for 10-15 years.
Young men with a higher baseline level, however, are twice as likely to develop prostate cancer over the same time period and should probably have their PSA tested at regular intervals, Dr. Christopher Weight said at the annual meeting of the American Urologic Association.
His prospective study of 268 men in their 40s showed that none of the men with a baseline PSA of 1.0 ng/mL or less developed high-risk disease by 10 years and only 3% developed it by 15 years. The findings could provide an effective way to risk-stratify young populations, reducing unnecessary testing and the consequences that sometimes follow it, he said.
"We have to admit that we overdiagnose and overtreat men," Dr. Weight said at a press briefing. "But there is danger in completely throwing out the PSA test. Testing men early can help us identify those who can safely delay additional testing and those who will benefit from more frequent tests."
Dr. Weight, a urology oncology fellow at the Mayo Clinic, Rochester, Minn., turned to the Olmsted County cohort for the study data. Since 1990, most of the residents in the county have received their medical care through the Mayo Clinic and its affiliate centers. A linked health records database provides information for long-term population-based studies.
His analysis included 268 men, all of whom had a baseline PSA drawn sometime during their 40s (median age 45 years). The men also had a transrectal ultrasound and digital rectal exam. They have been followed now for up to 20 years, with a median time of 16 years.
Among the cohort, 192 had a baseline PSA of 1.0 ng/mL or lower and 76 had a level of more than 1.0 ng/mL. There were no significant between-group differences in either family history or the results of the rectal exam.
Over the full follow-up period, men with the lower PSA level had a significantly lower risk of exceeding the age-specific cut points for PSA than did men with the higher levels (10% vs. 50%).
By the end of the follow-up period, there were six incident cases of prostate cancer in the low-PSA group, all of which were low-risk disease. This translated to an incidence rate of 1.6 per 1,000 patient/ years, with a mean of 15 years until diagnosis.
Twice as many men in the high-PSA group developed prostate cancer (12). Of these cases, 10 were low-risk disease and 2 high-risk. This translated into a rate of 8/1,000 patient-years, with a mean of 10 years to diagnosis.
The baseline measurement was fairly predictive of 15-year outcomes, Dr. Weight said. A cutoff of 1.0 ng/mL at the initial test had a sensitivity of 67% and a specificity of 74% for predicting the occurrence of prostate cancer. Changing the cutoff to 0.7 ng/mL on the initial test resulted in a sensitivity of 83% and a specificity of 46%
The results show that this single, early PSA level may be helpful in counseling patients about follow-up, said Dr. Scott Eggener, who moderated the briefing.
"The goals of any test are to identify the cohort of people most likely to benefit, and those people in whom screening can be limited to minimize the potential harms of the test," said Dr. Eggener, director of urology outcomes and translational research at the University of Chicago Medical Center. "If a young man has a very low PSA, we can feel comfortable in recommending that he have another test in several years, somewhat like what’s done with a screening colonoscopy. If the level is higher, this patient probably needs to be followed more frequently."
Neither Dr. Weight nor Dr. Eggener had any financial disclosures.
ATLANTA – Men in their 40s with a low prostate specific antigen can probably safely delay additional testing for 10-15 years.
Young men with a higher baseline level, however, are twice as likely to develop prostate cancer over the same time period and should probably have their PSA tested at regular intervals, Dr. Christopher Weight said at the annual meeting of the American Urologic Association.
His prospective study of 268 men in their 40s showed that none of the men with a baseline PSA of 1.0 ng/mL or less developed high-risk disease by 10 years and only 3% developed it by 15 years. The findings could provide an effective way to risk-stratify young populations, reducing unnecessary testing and the consequences that sometimes follow it, he said.
"We have to admit that we overdiagnose and overtreat men," Dr. Weight said at a press briefing. "But there is danger in completely throwing out the PSA test. Testing men early can help us identify those who can safely delay additional testing and those who will benefit from more frequent tests."
Dr. Weight, a urology oncology fellow at the Mayo Clinic, Rochester, Minn., turned to the Olmsted County cohort for the study data. Since 1990, most of the residents in the county have received their medical care through the Mayo Clinic and its affiliate centers. A linked health records database provides information for long-term population-based studies.
His analysis included 268 men, all of whom had a baseline PSA drawn sometime during their 40s (median age 45 years). The men also had a transrectal ultrasound and digital rectal exam. They have been followed now for up to 20 years, with a median time of 16 years.
Among the cohort, 192 had a baseline PSA of 1.0 ng/mL or lower and 76 had a level of more than 1.0 ng/mL. There were no significant between-group differences in either family history or the results of the rectal exam.
Over the full follow-up period, men with the lower PSA level had a significantly lower risk of exceeding the age-specific cut points for PSA than did men with the higher levels (10% vs. 50%).
By the end of the follow-up period, there were six incident cases of prostate cancer in the low-PSA group, all of which were low-risk disease. This translated to an incidence rate of 1.6 per 1,000 patient/ years, with a mean of 15 years until diagnosis.
Twice as many men in the high-PSA group developed prostate cancer (12). Of these cases, 10 were low-risk disease and 2 high-risk. This translated into a rate of 8/1,000 patient-years, with a mean of 10 years to diagnosis.
The baseline measurement was fairly predictive of 15-year outcomes, Dr. Weight said. A cutoff of 1.0 ng/mL at the initial test had a sensitivity of 67% and a specificity of 74% for predicting the occurrence of prostate cancer. Changing the cutoff to 0.7 ng/mL on the initial test resulted in a sensitivity of 83% and a specificity of 46%
The results show that this single, early PSA level may be helpful in counseling patients about follow-up, said Dr. Scott Eggener, who moderated the briefing.
"The goals of any test are to identify the cohort of people most likely to benefit, and those people in whom screening can be limited to minimize the potential harms of the test," said Dr. Eggener, director of urology outcomes and translational research at the University of Chicago Medical Center. "If a young man has a very low PSA, we can feel comfortable in recommending that he have another test in several years, somewhat like what’s done with a screening colonoscopy. If the level is higher, this patient probably needs to be followed more frequently."
Neither Dr. Weight nor Dr. Eggener had any financial disclosures.
ATLANTA – Men in their 40s with a low prostate specific antigen can probably safely delay additional testing for 10-15 years.
Young men with a higher baseline level, however, are twice as likely to develop prostate cancer over the same time period and should probably have their PSA tested at regular intervals, Dr. Christopher Weight said at the annual meeting of the American Urologic Association.
His prospective study of 268 men in their 40s showed that none of the men with a baseline PSA of 1.0 ng/mL or less developed high-risk disease by 10 years and only 3% developed it by 15 years. The findings could provide an effective way to risk-stratify young populations, reducing unnecessary testing and the consequences that sometimes follow it, he said.
"We have to admit that we overdiagnose and overtreat men," Dr. Weight said at a press briefing. "But there is danger in completely throwing out the PSA test. Testing men early can help us identify those who can safely delay additional testing and those who will benefit from more frequent tests."
Dr. Weight, a urology oncology fellow at the Mayo Clinic, Rochester, Minn., turned to the Olmsted County cohort for the study data. Since 1990, most of the residents in the county have received their medical care through the Mayo Clinic and its affiliate centers. A linked health records database provides information for long-term population-based studies.
His analysis included 268 men, all of whom had a baseline PSA drawn sometime during their 40s (median age 45 years). The men also had a transrectal ultrasound and digital rectal exam. They have been followed now for up to 20 years, with a median time of 16 years.
Among the cohort, 192 had a baseline PSA of 1.0 ng/mL or lower and 76 had a level of more than 1.0 ng/mL. There were no significant between-group differences in either family history or the results of the rectal exam.
Over the full follow-up period, men with the lower PSA level had a significantly lower risk of exceeding the age-specific cut points for PSA than did men with the higher levels (10% vs. 50%).
By the end of the follow-up period, there were six incident cases of prostate cancer in the low-PSA group, all of which were low-risk disease. This translated to an incidence rate of 1.6 per 1,000 patient/ years, with a mean of 15 years until diagnosis.
Twice as many men in the high-PSA group developed prostate cancer (12). Of these cases, 10 were low-risk disease and 2 high-risk. This translated into a rate of 8/1,000 patient-years, with a mean of 10 years to diagnosis.
The baseline measurement was fairly predictive of 15-year outcomes, Dr. Weight said. A cutoff of 1.0 ng/mL at the initial test had a sensitivity of 67% and a specificity of 74% for predicting the occurrence of prostate cancer. Changing the cutoff to 0.7 ng/mL on the initial test resulted in a sensitivity of 83% and a specificity of 46%
The results show that this single, early PSA level may be helpful in counseling patients about follow-up, said Dr. Scott Eggener, who moderated the briefing.
"The goals of any test are to identify the cohort of people most likely to benefit, and those people in whom screening can be limited to minimize the potential harms of the test," said Dr. Eggener, director of urology outcomes and translational research at the University of Chicago Medical Center. "If a young man has a very low PSA, we can feel comfortable in recommending that he have another test in several years, somewhat like what’s done with a screening colonoscopy. If the level is higher, this patient probably needs to be followed more frequently."
Neither Dr. Weight nor Dr. Eggener had any financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN UROLOGICAL ASSOCIATION
U.S. Task Force Confirms Stance Against Universal PSA Screening
"Do not use prostate-specific antigen (PSA)–based screening for prostate cancer."
With these words, the U.S. Preventive Services Task Force (USPSTF) finalized its stance against using this blood test to screen men universally in the United States for prostate cancer.
This firm position is unlikely to end the controversy that ensued after the task force released its draft recommendations in October 2011 ("U.S. Task Force: No PSA Testing for Healthy Men.") Editorials opposing and supporting universal prostate specific antigen (PSA) screening accompany publication early online of the final recommendations in the May 22 issue of Annals of Internal Medicine.
The 16-member task force gave population-based PSA screening a grade D recommendation, after a public comment period yielded no new, overwhelming evidence countering the draft recommendations against universal testing. This means the members believe there is moderate or high certainty that the harms of the intervention are equal to or outweigh the benefits.
"Science shows us the benefit is small and the harms significant," Dr. Virginia A. Moyer, the task force chair, said in an interview.
Fever, blood in the urine, transient urinary difficulties, and moderate-to-severe pain associated with biopsy are potential harms associated with screening cited in the recommendations. Risk for perioperative death, cardiovascular events, urinary incontinence, erectile dysfunction, and bowel dysfunction are some of the harms associated with treatment of prostate cancer.
The recommendation applies to "men in the general U.S. population, regardless of age." The new position replaces the 2008 recommendations, which cited insufficient evidence to support improved health outcomes associated with prostate cancer screening for men younger than 75 years and more conclusive evidence pointing to more harm than benefit for men aged 75 years or older.
The new literature-based recommendations leave room for physicians and patients particularly concerned about risk of prostate cancer to consider individual PSA testing. The USPSTF learned during the public comment period that it needed to emphasize that the recommendations do not preclude such discussions, said Dr. Moyer, professor of pediatrics at Baylor College of Medicine, Houston.
"One of the main things we clarified was ... that this is not a recommendation not to talk to patients about it or not to address the patient’s concerns," she said.
Opponents Step Forward
Coming out against the new recommendations is an ad hoc group of nine oncologists, primary care physicians, and preventive medicine specialists. In an accompanying editorial they argue against curtailment of PSA screening, criticize a lack of specialty physician involvement on the task force, and question the quality of evidence in two large trials heavily weighted in the task force’s decision making: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (N. Engl. J. Med. 2009;360:1310-9) and the European Randomized Study for Prostate Cancer (ERSCP) (N. Engl. J. Med. 2012;366:981-90).
"The most important flaws of the PLCO are the greater than 50% ‘contamination’ rate by nonprotocol PSA measurements in the control group, prescreening of 40% of study participants before enrollment in the trial, and the fact that two thirds of patients with abnormal screening tests did not have prompt biopsy," the ad hoc group wrote.
"The study has a known flaw – that a fair number in the control group got screened – which would tend to make the intervention and control groups look more alike," Dr. Moyer acknowledged.
The task force, however, offset this potential bias with inclusion of the European study, she said. The different methodologies used in the two studies provided balance.
The task force does not include urologists or cancer specialists, the ad hoc group also pointed out.
Dr. Moyer said that these specialty physicians were involved in the evidence report initially developed for review by the task force. The members of the task force have "precisely the expertise needed" to advise these doctors on management of asymptomatic men in the primary care setting, she added.
"We are experts in primary care and prevention, and we advise primary care physicians," Dr. Moyer said.
Dr. Otis Brawley Backs Task Force
Backing the task force’s recommendations is Dr. Otis Brawley, chief medical officer of the American Cancer Society. "What many people, doctors as well as lay people, have not truly appreciated is that there are significant harms associated with prostate cancer screening," he said in an interview. "Those harms are seen consistently in every clinical trial that has been done to assess screening."
Dr. Brawley supports individual PSA screening as long as it follows a thorough and balanced informed consent process. "I and the American Cancer Society hold out that there are some people at high risk and some people who are so concerned about prostate cancer that, if they understand the considerable harms and understand that the possibility of benefit is a possibility and not proven, and they want to be screened ... they should be allowed to get screened."
"Part of the reason the task force came down so hard is because this [informed consent] has not been happening," said Dr. Brawley, professor of hematology and oncology at the Emory Clinic in Atlanta.
Another reason, he said, is that "we have all these mass screening events going on. Not only is there no informed decision-making done and not only is there no information regarding harms, but men are being told screening can only benefit them."
Dr. Brawley outlined these and other considerations in a second editorial that accompanies the recommendations.
Going forward, Dr. Brawley recommends that physicians provide patients with a brief, written handout on prostate cancer that summarizes the task force recommendations, including potential benefits and harms of PSA screening. Patients could review the information prior to seeing their physician and then opt in or opt out of this screening, he said.
The American Cancer Society provides a patient handout on "Testing for Prostate Cancer" online. The USPSTF also is posting guidance for physicians on counseling patients in light of its new recommendations.
Dr. Moyer acknowledged that the issues surrounding PSA screening are emotionally charged. "Change is stunningly hard," she said. Our understanding of cancer dates back to the 1950s or earlier. Our thinking has been that you get one cancer cell in your body and then it’s a march to imminent death. We now recognize that cancer is not a monolithic thing."
Regarding prostate cancer, she added, "For the vast majority of men, it will not affect them in their lifetime."
"Unfortunately, at the moment, the PSA is the only test we have," Dr. Moyer said. "The test itself is not very good. The PSA test is too sensitive – it picks up almost anything that happens to the prostate." She added. "The dream is that a better test is developed."
Dr. Moyer and Dr. Brawley had no relevant financial disclosures.
"Do not use prostate-specific antigen (PSA)–based screening for prostate cancer."
With these words, the U.S. Preventive Services Task Force (USPSTF) finalized its stance against using this blood test to screen men universally in the United States for prostate cancer.
This firm position is unlikely to end the controversy that ensued after the task force released its draft recommendations in October 2011 ("U.S. Task Force: No PSA Testing for Healthy Men.") Editorials opposing and supporting universal prostate specific antigen (PSA) screening accompany publication early online of the final recommendations in the May 22 issue of Annals of Internal Medicine.
The 16-member task force gave population-based PSA screening a grade D recommendation, after a public comment period yielded no new, overwhelming evidence countering the draft recommendations against universal testing. This means the members believe there is moderate or high certainty that the harms of the intervention are equal to or outweigh the benefits.
"Science shows us the benefit is small and the harms significant," Dr. Virginia A. Moyer, the task force chair, said in an interview.
Fever, blood in the urine, transient urinary difficulties, and moderate-to-severe pain associated with biopsy are potential harms associated with screening cited in the recommendations. Risk for perioperative death, cardiovascular events, urinary incontinence, erectile dysfunction, and bowel dysfunction are some of the harms associated with treatment of prostate cancer.
The recommendation applies to "men in the general U.S. population, regardless of age." The new position replaces the 2008 recommendations, which cited insufficient evidence to support improved health outcomes associated with prostate cancer screening for men younger than 75 years and more conclusive evidence pointing to more harm than benefit for men aged 75 years or older.
The new literature-based recommendations leave room for physicians and patients particularly concerned about risk of prostate cancer to consider individual PSA testing. The USPSTF learned during the public comment period that it needed to emphasize that the recommendations do not preclude such discussions, said Dr. Moyer, professor of pediatrics at Baylor College of Medicine, Houston.
"One of the main things we clarified was ... that this is not a recommendation not to talk to patients about it or not to address the patient’s concerns," she said.
Opponents Step Forward
Coming out against the new recommendations is an ad hoc group of nine oncologists, primary care physicians, and preventive medicine specialists. In an accompanying editorial they argue against curtailment of PSA screening, criticize a lack of specialty physician involvement on the task force, and question the quality of evidence in two large trials heavily weighted in the task force’s decision making: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (N. Engl. J. Med. 2009;360:1310-9) and the European Randomized Study for Prostate Cancer (ERSCP) (N. Engl. J. Med. 2012;366:981-90).
"The most important flaws of the PLCO are the greater than 50% ‘contamination’ rate by nonprotocol PSA measurements in the control group, prescreening of 40% of study participants before enrollment in the trial, and the fact that two thirds of patients with abnormal screening tests did not have prompt biopsy," the ad hoc group wrote.
"The study has a known flaw – that a fair number in the control group got screened – which would tend to make the intervention and control groups look more alike," Dr. Moyer acknowledged.
The task force, however, offset this potential bias with inclusion of the European study, she said. The different methodologies used in the two studies provided balance.
The task force does not include urologists or cancer specialists, the ad hoc group also pointed out.
Dr. Moyer said that these specialty physicians were involved in the evidence report initially developed for review by the task force. The members of the task force have "precisely the expertise needed" to advise these doctors on management of asymptomatic men in the primary care setting, she added.
"We are experts in primary care and prevention, and we advise primary care physicians," Dr. Moyer said.
Dr. Otis Brawley Backs Task Force
Backing the task force’s recommendations is Dr. Otis Brawley, chief medical officer of the American Cancer Society. "What many people, doctors as well as lay people, have not truly appreciated is that there are significant harms associated with prostate cancer screening," he said in an interview. "Those harms are seen consistently in every clinical trial that has been done to assess screening."
Dr. Brawley supports individual PSA screening as long as it follows a thorough and balanced informed consent process. "I and the American Cancer Society hold out that there are some people at high risk and some people who are so concerned about prostate cancer that, if they understand the considerable harms and understand that the possibility of benefit is a possibility and not proven, and they want to be screened ... they should be allowed to get screened."
"Part of the reason the task force came down so hard is because this [informed consent] has not been happening," said Dr. Brawley, professor of hematology and oncology at the Emory Clinic in Atlanta.
Another reason, he said, is that "we have all these mass screening events going on. Not only is there no informed decision-making done and not only is there no information regarding harms, but men are being told screening can only benefit them."
Dr. Brawley outlined these and other considerations in a second editorial that accompanies the recommendations.
Going forward, Dr. Brawley recommends that physicians provide patients with a brief, written handout on prostate cancer that summarizes the task force recommendations, including potential benefits and harms of PSA screening. Patients could review the information prior to seeing their physician and then opt in or opt out of this screening, he said.
The American Cancer Society provides a patient handout on "Testing for Prostate Cancer" online. The USPSTF also is posting guidance for physicians on counseling patients in light of its new recommendations.
Dr. Moyer acknowledged that the issues surrounding PSA screening are emotionally charged. "Change is stunningly hard," she said. Our understanding of cancer dates back to the 1950s or earlier. Our thinking has been that you get one cancer cell in your body and then it’s a march to imminent death. We now recognize that cancer is not a monolithic thing."
Regarding prostate cancer, she added, "For the vast majority of men, it will not affect them in their lifetime."
"Unfortunately, at the moment, the PSA is the only test we have," Dr. Moyer said. "The test itself is not very good. The PSA test is too sensitive – it picks up almost anything that happens to the prostate." She added. "The dream is that a better test is developed."
Dr. Moyer and Dr. Brawley had no relevant financial disclosures.
"Do not use prostate-specific antigen (PSA)–based screening for prostate cancer."
With these words, the U.S. Preventive Services Task Force (USPSTF) finalized its stance against using this blood test to screen men universally in the United States for prostate cancer.
This firm position is unlikely to end the controversy that ensued after the task force released its draft recommendations in October 2011 ("U.S. Task Force: No PSA Testing for Healthy Men.") Editorials opposing and supporting universal prostate specific antigen (PSA) screening accompany publication early online of the final recommendations in the May 22 issue of Annals of Internal Medicine.
The 16-member task force gave population-based PSA screening a grade D recommendation, after a public comment period yielded no new, overwhelming evidence countering the draft recommendations against universal testing. This means the members believe there is moderate or high certainty that the harms of the intervention are equal to or outweigh the benefits.
"Science shows us the benefit is small and the harms significant," Dr. Virginia A. Moyer, the task force chair, said in an interview.
Fever, blood in the urine, transient urinary difficulties, and moderate-to-severe pain associated with biopsy are potential harms associated with screening cited in the recommendations. Risk for perioperative death, cardiovascular events, urinary incontinence, erectile dysfunction, and bowel dysfunction are some of the harms associated with treatment of prostate cancer.
The recommendation applies to "men in the general U.S. population, regardless of age." The new position replaces the 2008 recommendations, which cited insufficient evidence to support improved health outcomes associated with prostate cancer screening for men younger than 75 years and more conclusive evidence pointing to more harm than benefit for men aged 75 years or older.
The new literature-based recommendations leave room for physicians and patients particularly concerned about risk of prostate cancer to consider individual PSA testing. The USPSTF learned during the public comment period that it needed to emphasize that the recommendations do not preclude such discussions, said Dr. Moyer, professor of pediatrics at Baylor College of Medicine, Houston.
"One of the main things we clarified was ... that this is not a recommendation not to talk to patients about it or not to address the patient’s concerns," she said.
Opponents Step Forward
Coming out against the new recommendations is an ad hoc group of nine oncologists, primary care physicians, and preventive medicine specialists. In an accompanying editorial they argue against curtailment of PSA screening, criticize a lack of specialty physician involvement on the task force, and question the quality of evidence in two large trials heavily weighted in the task force’s decision making: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (N. Engl. J. Med. 2009;360:1310-9) and the European Randomized Study for Prostate Cancer (ERSCP) (N. Engl. J. Med. 2012;366:981-90).
"The most important flaws of the PLCO are the greater than 50% ‘contamination’ rate by nonprotocol PSA measurements in the control group, prescreening of 40% of study participants before enrollment in the trial, and the fact that two thirds of patients with abnormal screening tests did not have prompt biopsy," the ad hoc group wrote.
"The study has a known flaw – that a fair number in the control group got screened – which would tend to make the intervention and control groups look more alike," Dr. Moyer acknowledged.
The task force, however, offset this potential bias with inclusion of the European study, she said. The different methodologies used in the two studies provided balance.
The task force does not include urologists or cancer specialists, the ad hoc group also pointed out.
Dr. Moyer said that these specialty physicians were involved in the evidence report initially developed for review by the task force. The members of the task force have "precisely the expertise needed" to advise these doctors on management of asymptomatic men in the primary care setting, she added.
"We are experts in primary care and prevention, and we advise primary care physicians," Dr. Moyer said.
Dr. Otis Brawley Backs Task Force
Backing the task force’s recommendations is Dr. Otis Brawley, chief medical officer of the American Cancer Society. "What many people, doctors as well as lay people, have not truly appreciated is that there are significant harms associated with prostate cancer screening," he said in an interview. "Those harms are seen consistently in every clinical trial that has been done to assess screening."
Dr. Brawley supports individual PSA screening as long as it follows a thorough and balanced informed consent process. "I and the American Cancer Society hold out that there are some people at high risk and some people who are so concerned about prostate cancer that, if they understand the considerable harms and understand that the possibility of benefit is a possibility and not proven, and they want to be screened ... they should be allowed to get screened."
"Part of the reason the task force came down so hard is because this [informed consent] has not been happening," said Dr. Brawley, professor of hematology and oncology at the Emory Clinic in Atlanta.
Another reason, he said, is that "we have all these mass screening events going on. Not only is there no informed decision-making done and not only is there no information regarding harms, but men are being told screening can only benefit them."
Dr. Brawley outlined these and other considerations in a second editorial that accompanies the recommendations.
Going forward, Dr. Brawley recommends that physicians provide patients with a brief, written handout on prostate cancer that summarizes the task force recommendations, including potential benefits and harms of PSA screening. Patients could review the information prior to seeing their physician and then opt in or opt out of this screening, he said.
The American Cancer Society provides a patient handout on "Testing for Prostate Cancer" online. The USPSTF also is posting guidance for physicians on counseling patients in light of its new recommendations.
Dr. Moyer acknowledged that the issues surrounding PSA screening are emotionally charged. "Change is stunningly hard," she said. Our understanding of cancer dates back to the 1950s or earlier. Our thinking has been that you get one cancer cell in your body and then it’s a march to imminent death. We now recognize that cancer is not a monolithic thing."
Regarding prostate cancer, she added, "For the vast majority of men, it will not affect them in their lifetime."
"Unfortunately, at the moment, the PSA is the only test we have," Dr. Moyer said. "The test itself is not very good. The PSA test is too sensitive – it picks up almost anything that happens to the prostate." She added. "The dream is that a better test is developed."
Dr. Moyer and Dr. Brawley had no relevant financial disclosures.
FROM ANNALS OF INTERNAL MEDICINE
NICE Reverses Course on Abiraterone
After initially turning it down – and being pressured to reevaluate by the U.K. Department of Health – the clinical effectiveness agency for England and Wales has decided to recommend abiraterone, in combination with glucocorticoids, as a second-line treatment for metastatic prostate cancer.
The National Institute for Health and Clinical Excellence announced on May 16 that its new draft guidance recommending abiraterone (Zytiga, Janssen) for castration-resistant prostate cancer came in response to both a new manufacturer pricing agreement and additional information on how many patients were likely to be eligible for treatment.
In February, NICE had deemed abiraterone, which has been shown to prolong survival by a median 4.6 months, not cost-effective at an estimated £63,200 per quality-adjusted life year.
The following month, the U.K. Department of Health asked NICE to reevaluate its decision with regard to its estimates of the number of men eligible to be treated with abiraterone. NICE evaluates differently end-of-life treatments for patients with a life expectancy of less than 2 years, depending on the size of the population affected.
A revised pricing scheme in addition to revised estimates of the population eligible for abiraterone treatment lowered NICE’s estimates to £46,800 per QALY, just under its threshold for an end-of-life treatment in a small population.
Abiraterone works by blocking androgen synthesis in the adrenal glands, prostate tissue, and prostate tumors. It is indicated for men whose disease has progressed following docetaxel-containing chemotherapy regimens, and who have been deemed "castration resistant" because their tumors do not respond to androgen-deprivation treatments that may or may not include surgical castration.
The list price of abiraterone is £2,930 for a 30-day supply of 120 tablets; NICE did not disclose the new discounted price. It is taken as a single dose of 1,000 mg daily, in four tablets. In a manufacturer-sponsored randomized controlled trial (n = 1,195), subjects receiving abiraterone plus prednisone or prednisolone saw a median overall survival gain of 14.8 months compared with 10.9 months for those taking placebo plus either prednisone or prednisolone after 1 year follow-up (HR 0.65; 95% confidence interval, 0.54-0.77; P less than .001).
The trial (N. Engl. J. Med. 2011;364:1995-2005) was stopped due to significant evidence of benefit, but follow-up continued, and an updated analysis after 20.2 months showed that median survival continued to be significantly longer in the abiraterone group than the prednisolone group (15.8 months compared with 11.2 months; HR 0.74; 95% CI 0.64 to 0.86).
In its earlier draft guidance NICE had estimated the number of men eligible for second-line treatment with abiraterone to be at least 3,500 in 2011. The revised estimate suggests that only 2,500 would have been eligible – a small population, by NICE’s calculations, and therefore meeting its cost-effectiveness criteria for an end-of-life treatment.
After initially turning it down – and being pressured to reevaluate by the U.K. Department of Health – the clinical effectiveness agency for England and Wales has decided to recommend abiraterone, in combination with glucocorticoids, as a second-line treatment for metastatic prostate cancer.
The National Institute for Health and Clinical Excellence announced on May 16 that its new draft guidance recommending abiraterone (Zytiga, Janssen) for castration-resistant prostate cancer came in response to both a new manufacturer pricing agreement and additional information on how many patients were likely to be eligible for treatment.
In February, NICE had deemed abiraterone, which has been shown to prolong survival by a median 4.6 months, not cost-effective at an estimated £63,200 per quality-adjusted life year.
The following month, the U.K. Department of Health asked NICE to reevaluate its decision with regard to its estimates of the number of men eligible to be treated with abiraterone. NICE evaluates differently end-of-life treatments for patients with a life expectancy of less than 2 years, depending on the size of the population affected.
A revised pricing scheme in addition to revised estimates of the population eligible for abiraterone treatment lowered NICE’s estimates to £46,800 per QALY, just under its threshold for an end-of-life treatment in a small population.
Abiraterone works by blocking androgen synthesis in the adrenal glands, prostate tissue, and prostate tumors. It is indicated for men whose disease has progressed following docetaxel-containing chemotherapy regimens, and who have been deemed "castration resistant" because their tumors do not respond to androgen-deprivation treatments that may or may not include surgical castration.
The list price of abiraterone is £2,930 for a 30-day supply of 120 tablets; NICE did not disclose the new discounted price. It is taken as a single dose of 1,000 mg daily, in four tablets. In a manufacturer-sponsored randomized controlled trial (n = 1,195), subjects receiving abiraterone plus prednisone or prednisolone saw a median overall survival gain of 14.8 months compared with 10.9 months for those taking placebo plus either prednisone or prednisolone after 1 year follow-up (HR 0.65; 95% confidence interval, 0.54-0.77; P less than .001).
The trial (N. Engl. J. Med. 2011;364:1995-2005) was stopped due to significant evidence of benefit, but follow-up continued, and an updated analysis after 20.2 months showed that median survival continued to be significantly longer in the abiraterone group than the prednisolone group (15.8 months compared with 11.2 months; HR 0.74; 95% CI 0.64 to 0.86).
In its earlier draft guidance NICE had estimated the number of men eligible for second-line treatment with abiraterone to be at least 3,500 in 2011. The revised estimate suggests that only 2,500 would have been eligible – a small population, by NICE’s calculations, and therefore meeting its cost-effectiveness criteria for an end-of-life treatment.
After initially turning it down – and being pressured to reevaluate by the U.K. Department of Health – the clinical effectiveness agency for England and Wales has decided to recommend abiraterone, in combination with glucocorticoids, as a second-line treatment for metastatic prostate cancer.
The National Institute for Health and Clinical Excellence announced on May 16 that its new draft guidance recommending abiraterone (Zytiga, Janssen) for castration-resistant prostate cancer came in response to both a new manufacturer pricing agreement and additional information on how many patients were likely to be eligible for treatment.
In February, NICE had deemed abiraterone, which has been shown to prolong survival by a median 4.6 months, not cost-effective at an estimated £63,200 per quality-adjusted life year.
The following month, the U.K. Department of Health asked NICE to reevaluate its decision with regard to its estimates of the number of men eligible to be treated with abiraterone. NICE evaluates differently end-of-life treatments for patients with a life expectancy of less than 2 years, depending on the size of the population affected.
A revised pricing scheme in addition to revised estimates of the population eligible for abiraterone treatment lowered NICE’s estimates to £46,800 per QALY, just under its threshold for an end-of-life treatment in a small population.
Abiraterone works by blocking androgen synthesis in the adrenal glands, prostate tissue, and prostate tumors. It is indicated for men whose disease has progressed following docetaxel-containing chemotherapy regimens, and who have been deemed "castration resistant" because their tumors do not respond to androgen-deprivation treatments that may or may not include surgical castration.
The list price of abiraterone is £2,930 for a 30-day supply of 120 tablets; NICE did not disclose the new discounted price. It is taken as a single dose of 1,000 mg daily, in four tablets. In a manufacturer-sponsored randomized controlled trial (n = 1,195), subjects receiving abiraterone plus prednisone or prednisolone saw a median overall survival gain of 14.8 months compared with 10.9 months for those taking placebo plus either prednisone or prednisolone after 1 year follow-up (HR 0.65; 95% confidence interval, 0.54-0.77; P less than .001).
The trial (N. Engl. J. Med. 2011;364:1995-2005) was stopped due to significant evidence of benefit, but follow-up continued, and an updated analysis after 20.2 months showed that median survival continued to be significantly longer in the abiraterone group than the prednisolone group (15.8 months compared with 11.2 months; HR 0.74; 95% CI 0.64 to 0.86).
In its earlier draft guidance NICE had estimated the number of men eligible for second-line treatment with abiraterone to be at least 3,500 in 2011. The revised estimate suggests that only 2,500 would have been eligible – a small population, by NICE’s calculations, and therefore meeting its cost-effectiveness criteria for an end-of-life treatment.
ACR Gives Special Consideration to Pregnancy in Nephritis Guidelines
DESTIN, FLA. – Management of lupus nephritis during pregnancy gets close attention in ACR’s new nephritis guidelines.
No treatment is necessary in pregnant women with prior lupus nephritis who have no current evidence of systemic or renal disease activity, while those with mild systemic activity may be treated with hydroxychloroquine, according to the guidelines, which are published in the June issue of Arthritis Care & Research.
"There are good data suggesting hydroxychloroquine controls lupus in women who are pregnant, resulting in fewer flares" Dr. Bevra H. Hahn said at the Congress of Clinical Rheumatology. Dr. Hahn, professor of medicine and chief of the division of rheumatology at the University of California, Los Angeles, led the ACR core working group that helped with development of the guidelines.
In patients with clinically active nephritis or with substantial extrarenal disease activity, glucocorticoids may be prescribed at doses necessary to control disease activity (Arthritis Care Res. 2012;64:797-08).
"I start at 0.5 mg/kg per day," Dr. Hahn said of glucocorticoids under these circumstances. She noted that only steroids that are metabolized by placental enzymes should be used so that the drug does not reach the fetus.
She and her coauthors cautioned, however, that high-dose glucocorticoid therapy is associated with a high risk of maternal complications – including hypertension and diabetes mellitus – in patients with systemic lupus erythematosus (SLE). They also stress that mycophenolate mofetil, cyclophosphamide, and methotrexate should be avoided in pregnancy, because they are established human teratogens.
Azathioprine, though listed as pregnancy category D indicating teratogenic risk, has been shown in cross-sectional studies to be associated with very low risk of fetal abnormalities and can be added if necessary, according to the task force panel charged with developing the guidelines.
The azathioprine dose, however, should not exceed 2 mg/kg per day in pregnant women, Dr. Hahn said.
The task force has recommended that pregnant patients with a persistently active nephritis and documented or suspected class III or IV disease with crescents may be candidates for delivery after 28 weeks if the fetus is viable. The recommendations with respect to pregnancy were based on level C evidence, indicating they were based on consensus, expert opinion, and case series.
For women with SLE and nephritis who are not pregnant, but who have concerns about fertility preservation, the task force panel recommended that mycophenolate mofetil was preferable to cyclophosphamide for induction therapy, because cyclophosphamide has been shown to cause permanent infertility in both women and men.
For example, one study showed that 6 months of high-dose intravenous cyclophosphamide with a cumulative dose of 4.4 g-10 g was associated with sustained amenorrhea in about 10% of young women, and the risk increased with age.
However, the physician should be certain the patient is not pregnant before prescribing mycophenolate mofetil or mycophenolic acid, and treatment should be stopped for at least 6 weeks before pregnancy is attempted.
The guidelines were sponsored by the American College of Rheumatology via a competitive grant mechanism. Dr. Hahn has received consultant fees, speaking fees, and/or honoraria from UCB and Abbott and has served on the data and safety monitoring board for Anthera. The complete list of disclosures for the guideline authors is available with the full text of the article.
Azathioprine, teratogenic risk,
DESTIN, FLA. – Management of lupus nephritis during pregnancy gets close attention in ACR’s new nephritis guidelines.
No treatment is necessary in pregnant women with prior lupus nephritis who have no current evidence of systemic or renal disease activity, while those with mild systemic activity may be treated with hydroxychloroquine, according to the guidelines, which are published in the June issue of Arthritis Care & Research.
"There are good data suggesting hydroxychloroquine controls lupus in women who are pregnant, resulting in fewer flares" Dr. Bevra H. Hahn said at the Congress of Clinical Rheumatology. Dr. Hahn, professor of medicine and chief of the division of rheumatology at the University of California, Los Angeles, led the ACR core working group that helped with development of the guidelines.
In patients with clinically active nephritis or with substantial extrarenal disease activity, glucocorticoids may be prescribed at doses necessary to control disease activity (Arthritis Care Res. 2012;64:797-08).
"I start at 0.5 mg/kg per day," Dr. Hahn said of glucocorticoids under these circumstances. She noted that only steroids that are metabolized by placental enzymes should be used so that the drug does not reach the fetus.
She and her coauthors cautioned, however, that high-dose glucocorticoid therapy is associated with a high risk of maternal complications – including hypertension and diabetes mellitus – in patients with systemic lupus erythematosus (SLE). They also stress that mycophenolate mofetil, cyclophosphamide, and methotrexate should be avoided in pregnancy, because they are established human teratogens.
Azathioprine, though listed as pregnancy category D indicating teratogenic risk, has been shown in cross-sectional studies to be associated with very low risk of fetal abnormalities and can be added if necessary, according to the task force panel charged with developing the guidelines.
The azathioprine dose, however, should not exceed 2 mg/kg per day in pregnant women, Dr. Hahn said.
The task force has recommended that pregnant patients with a persistently active nephritis and documented or suspected class III or IV disease with crescents may be candidates for delivery after 28 weeks if the fetus is viable. The recommendations with respect to pregnancy were based on level C evidence, indicating they were based on consensus, expert opinion, and case series.
For women with SLE and nephritis who are not pregnant, but who have concerns about fertility preservation, the task force panel recommended that mycophenolate mofetil was preferable to cyclophosphamide for induction therapy, because cyclophosphamide has been shown to cause permanent infertility in both women and men.
For example, one study showed that 6 months of high-dose intravenous cyclophosphamide with a cumulative dose of 4.4 g-10 g was associated with sustained amenorrhea in about 10% of young women, and the risk increased with age.
However, the physician should be certain the patient is not pregnant before prescribing mycophenolate mofetil or mycophenolic acid, and treatment should be stopped for at least 6 weeks before pregnancy is attempted.
The guidelines were sponsored by the American College of Rheumatology via a competitive grant mechanism. Dr. Hahn has received consultant fees, speaking fees, and/or honoraria from UCB and Abbott and has served on the data and safety monitoring board for Anthera. The complete list of disclosures for the guideline authors is available with the full text of the article.
DESTIN, FLA. – Management of lupus nephritis during pregnancy gets close attention in ACR’s new nephritis guidelines.
No treatment is necessary in pregnant women with prior lupus nephritis who have no current evidence of systemic or renal disease activity, while those with mild systemic activity may be treated with hydroxychloroquine, according to the guidelines, which are published in the June issue of Arthritis Care & Research.
"There are good data suggesting hydroxychloroquine controls lupus in women who are pregnant, resulting in fewer flares" Dr. Bevra H. Hahn said at the Congress of Clinical Rheumatology. Dr. Hahn, professor of medicine and chief of the division of rheumatology at the University of California, Los Angeles, led the ACR core working group that helped with development of the guidelines.
In patients with clinically active nephritis or with substantial extrarenal disease activity, glucocorticoids may be prescribed at doses necessary to control disease activity (Arthritis Care Res. 2012;64:797-08).
"I start at 0.5 mg/kg per day," Dr. Hahn said of glucocorticoids under these circumstances. She noted that only steroids that are metabolized by placental enzymes should be used so that the drug does not reach the fetus.
She and her coauthors cautioned, however, that high-dose glucocorticoid therapy is associated with a high risk of maternal complications – including hypertension and diabetes mellitus – in patients with systemic lupus erythematosus (SLE). They also stress that mycophenolate mofetil, cyclophosphamide, and methotrexate should be avoided in pregnancy, because they are established human teratogens.
Azathioprine, though listed as pregnancy category D indicating teratogenic risk, has been shown in cross-sectional studies to be associated with very low risk of fetal abnormalities and can be added if necessary, according to the task force panel charged with developing the guidelines.
The azathioprine dose, however, should not exceed 2 mg/kg per day in pregnant women, Dr. Hahn said.
The task force has recommended that pregnant patients with a persistently active nephritis and documented or suspected class III or IV disease with crescents may be candidates for delivery after 28 weeks if the fetus is viable. The recommendations with respect to pregnancy were based on level C evidence, indicating they were based on consensus, expert opinion, and case series.
For women with SLE and nephritis who are not pregnant, but who have concerns about fertility preservation, the task force panel recommended that mycophenolate mofetil was preferable to cyclophosphamide for induction therapy, because cyclophosphamide has been shown to cause permanent infertility in both women and men.
For example, one study showed that 6 months of high-dose intravenous cyclophosphamide with a cumulative dose of 4.4 g-10 g was associated with sustained amenorrhea in about 10% of young women, and the risk increased with age.
However, the physician should be certain the patient is not pregnant before prescribing mycophenolate mofetil or mycophenolic acid, and treatment should be stopped for at least 6 weeks before pregnancy is attempted.
The guidelines were sponsored by the American College of Rheumatology via a competitive grant mechanism. Dr. Hahn has received consultant fees, speaking fees, and/or honoraria from UCB and Abbott and has served on the data and safety monitoring board for Anthera. The complete list of disclosures for the guideline authors is available with the full text of the article.
Azathioprine, teratogenic risk,
Azathioprine, teratogenic risk,
FROM THE CONGRESS OF CLINICAL RHEUMATOLOGY
ACR Releases Updated Lupus Nephritis Guidelines
DESTIN, Fla. – Renal biopsy, unless strongly contraindicated, should be performed in every patient with clinical evidence of active lupus nephritis that has not been previously treated so that glomerular disease can be classified by current International Society of Nephrology/Renal Pathology Society classification, according to updated lupus nephritis treatment guidelines from the American College of Rheumatology.
Biopsy also will allow evaluation of disease for activity and chronicity and for tubular and vascular changes, as well as for the identification of additional or alternative causes of renal disease, according to the guidelines, which are published in the June issue of Arthritis Care and Research (Arthritis Care Res. 2012;54:797-808).
In fact, the recommended therapeutic strategies in the updated guidelines require knowledge of the classification of nephritis based on renal biopsy, according to Dr. Bevra H. Hahn, speaking at a meeting that happened to coincide with the online release of the guidelines on May 4.
For example, histologic class I and class II disease generally do not require immunosuppressive treatment; class III and class IV disease – and class V disease when combined with class III and IV disease – require aggressive therapy with glucocorticoids and immunosuppressive agents; and patients with class V disease alone (pure membranous lupus nephritis) with nephritic range proteinuria should be started on prednisone at 0.5mg/kg per day plus mycophenolate mofetil at 2-3 g total daily. Class VI disease generally requires preparation for renal replacement therapy.
The guidelines update those published in 1999, which represented a more general approach to systemic lupus erythematosus (SLE). These new guidelines more directly address nephritis, including case identification, treatment, and monitoring, and they include data on newer therapeutic modalities, including mycophenolate mofetil, mycophenolic acid, and rituximab, which were not available at the time the previous guidelines were developed, said Dr. Hahn, who led the core working group that helped develop the new guidelines.
They also address special situations such as pregnancy.
The core working group, along with a core executive group and a task force panel of experts used the validated modified RAND/University of California at Los Angeles Appropriateness Method, which involves a systematic literature view and expert opinion (based on voting by the task force panel) to develop the new guidelines.
The biopsy recommendation and the related therapeutic recommendations are based on level C evidence, indicating they were derived by consensus, expert opinion, and case series. Indications for renal biopsy, according to the task force panel include increasing serum creatinine without compelling alternative causes, confirmed proteinuria of 1 g or more/24 hours, and combinations of proteinuria of 0.5 g or more/24 hours plus hematuria (defined as 5 or more red blood cells per high power field) and proteinuria of 0.5 g or more/24 hours plus cellular casts – as long at these findings are confirmed in at least two tests conducted within a short time period and in the absence of alternative causes.
The task force panel also addressed adjunctive treatments, specifically recommending that:
• All patients with SLE be treated with a background of hydroxychloroquine unless contraindicated. This level C recommendation is based on recent cross-sectional and prospective controlled trial data indicating it is of benefit for reducing flare rates, is associated with significantly lower damage accrual (including renal damage), and may be associated with reduced risk of clotting events.
• Careful attention be paid to control of hypertension, with a target of no more than 130/80 mm Hg. This recommendation is based on level A evidence for nondiabetic chronic renal disease, indicating it is derived from multiple randomized controlled trials or a meta-analysis.
• Women of child-bearing potential who have active or prior lupus nephritis be counseled about the pregnancy risks conferred by the disease and its treatments. This recommendation is based on level C evidence.
Other task force panel recommendations specifically address induction of improvement in patients with International Society of Nephrology class III/IV lupus glomerulonephritis, induction of improvement in those with class IV or IV/V disease with cellular crescents, maintaining improvement in those who respond to induction therapy, and changing therapies in those who do not. Additional recommendations address the identification of vascular disease in patients with SLE and renal abnormalities, treatment of lupus nephritis patients who are pregnant, and monitoring the activity of lupus nephritis, Dr. Hahn said.
The new recommendations are "heavily based on induction with mycophenolate mofetil or cyclophosphamide, and on maintenance with mycophenolate mofetil or azathioprine," she noted.
"Nephritis remains one of the most devastating complications of lupus," she and her coauthors wrote, noting that the incidence increased during the 1980s and 1990s, with no decline seen as of 2004, despite the availability of new therapeutic regimens.
Standardized incidence rates for end-stage renal disease in the United States have risen in a number of populations, including younger patients, African Americans, and those living in the South.
"We hope that institution of these recommendations might lead to reductions in these trends. Furthermore, they may allow us to evaluate whether those who receive the recommended therapies are less likely to develop end-stage renal disease," they continued, concluding that while much progress has been made since lupus nephritis was associated with a near-terminal prognosis, these recommendations represent an effort to further improve outcomes and decrease morbidity and mortality in SLE.
The guidelines were sponsored by the American College of Rheumatology via a competitive grant mechanism. Dr. Hahn has received consultant fees, speaking fees, and/or honoraria from UCB and Abbott and has served on the data and safety monitoring board for Anthera. The complete list of disclosures for the guideline authors is available with the full text of the article.
DESTIN, Fla. – Renal biopsy, unless strongly contraindicated, should be performed in every patient with clinical evidence of active lupus nephritis that has not been previously treated so that glomerular disease can be classified by current International Society of Nephrology/Renal Pathology Society classification, according to updated lupus nephritis treatment guidelines from the American College of Rheumatology.
Biopsy also will allow evaluation of disease for activity and chronicity and for tubular and vascular changes, as well as for the identification of additional or alternative causes of renal disease, according to the guidelines, which are published in the June issue of Arthritis Care and Research (Arthritis Care Res. 2012;54:797-808).
In fact, the recommended therapeutic strategies in the updated guidelines require knowledge of the classification of nephritis based on renal biopsy, according to Dr. Bevra H. Hahn, speaking at a meeting that happened to coincide with the online release of the guidelines on May 4.
For example, histologic class I and class II disease generally do not require immunosuppressive treatment; class III and class IV disease – and class V disease when combined with class III and IV disease – require aggressive therapy with glucocorticoids and immunosuppressive agents; and patients with class V disease alone (pure membranous lupus nephritis) with nephritic range proteinuria should be started on prednisone at 0.5mg/kg per day plus mycophenolate mofetil at 2-3 g total daily. Class VI disease generally requires preparation for renal replacement therapy.
The guidelines update those published in 1999, which represented a more general approach to systemic lupus erythematosus (SLE). These new guidelines more directly address nephritis, including case identification, treatment, and monitoring, and they include data on newer therapeutic modalities, including mycophenolate mofetil, mycophenolic acid, and rituximab, which were not available at the time the previous guidelines were developed, said Dr. Hahn, who led the core working group that helped develop the new guidelines.
They also address special situations such as pregnancy.
The core working group, along with a core executive group and a task force panel of experts used the validated modified RAND/University of California at Los Angeles Appropriateness Method, which involves a systematic literature view and expert opinion (based on voting by the task force panel) to develop the new guidelines.
The biopsy recommendation and the related therapeutic recommendations are based on level C evidence, indicating they were derived by consensus, expert opinion, and case series. Indications for renal biopsy, according to the task force panel include increasing serum creatinine without compelling alternative causes, confirmed proteinuria of 1 g or more/24 hours, and combinations of proteinuria of 0.5 g or more/24 hours plus hematuria (defined as 5 or more red blood cells per high power field) and proteinuria of 0.5 g or more/24 hours plus cellular casts – as long at these findings are confirmed in at least two tests conducted within a short time period and in the absence of alternative causes.
The task force panel also addressed adjunctive treatments, specifically recommending that:
• All patients with SLE be treated with a background of hydroxychloroquine unless contraindicated. This level C recommendation is based on recent cross-sectional and prospective controlled trial data indicating it is of benefit for reducing flare rates, is associated with significantly lower damage accrual (including renal damage), and may be associated with reduced risk of clotting events.
• Careful attention be paid to control of hypertension, with a target of no more than 130/80 mm Hg. This recommendation is based on level A evidence for nondiabetic chronic renal disease, indicating it is derived from multiple randomized controlled trials or a meta-analysis.
• Women of child-bearing potential who have active or prior lupus nephritis be counseled about the pregnancy risks conferred by the disease and its treatments. This recommendation is based on level C evidence.
Other task force panel recommendations specifically address induction of improvement in patients with International Society of Nephrology class III/IV lupus glomerulonephritis, induction of improvement in those with class IV or IV/V disease with cellular crescents, maintaining improvement in those who respond to induction therapy, and changing therapies in those who do not. Additional recommendations address the identification of vascular disease in patients with SLE and renal abnormalities, treatment of lupus nephritis patients who are pregnant, and monitoring the activity of lupus nephritis, Dr. Hahn said.
The new recommendations are "heavily based on induction with mycophenolate mofetil or cyclophosphamide, and on maintenance with mycophenolate mofetil or azathioprine," she noted.
"Nephritis remains one of the most devastating complications of lupus," she and her coauthors wrote, noting that the incidence increased during the 1980s and 1990s, with no decline seen as of 2004, despite the availability of new therapeutic regimens.
Standardized incidence rates for end-stage renal disease in the United States have risen in a number of populations, including younger patients, African Americans, and those living in the South.
"We hope that institution of these recommendations might lead to reductions in these trends. Furthermore, they may allow us to evaluate whether those who receive the recommended therapies are less likely to develop end-stage renal disease," they continued, concluding that while much progress has been made since lupus nephritis was associated with a near-terminal prognosis, these recommendations represent an effort to further improve outcomes and decrease morbidity and mortality in SLE.
The guidelines were sponsored by the American College of Rheumatology via a competitive grant mechanism. Dr. Hahn has received consultant fees, speaking fees, and/or honoraria from UCB and Abbott and has served on the data and safety monitoring board for Anthera. The complete list of disclosures for the guideline authors is available with the full text of the article.
DESTIN, Fla. – Renal biopsy, unless strongly contraindicated, should be performed in every patient with clinical evidence of active lupus nephritis that has not been previously treated so that glomerular disease can be classified by current International Society of Nephrology/Renal Pathology Society classification, according to updated lupus nephritis treatment guidelines from the American College of Rheumatology.
Biopsy also will allow evaluation of disease for activity and chronicity and for tubular and vascular changes, as well as for the identification of additional or alternative causes of renal disease, according to the guidelines, which are published in the June issue of Arthritis Care and Research (Arthritis Care Res. 2012;54:797-808).
In fact, the recommended therapeutic strategies in the updated guidelines require knowledge of the classification of nephritis based on renal biopsy, according to Dr. Bevra H. Hahn, speaking at a meeting that happened to coincide with the online release of the guidelines on May 4.
For example, histologic class I and class II disease generally do not require immunosuppressive treatment; class III and class IV disease – and class V disease when combined with class III and IV disease – require aggressive therapy with glucocorticoids and immunosuppressive agents; and patients with class V disease alone (pure membranous lupus nephritis) with nephritic range proteinuria should be started on prednisone at 0.5mg/kg per day plus mycophenolate mofetil at 2-3 g total daily. Class VI disease generally requires preparation for renal replacement therapy.
The guidelines update those published in 1999, which represented a more general approach to systemic lupus erythematosus (SLE). These new guidelines more directly address nephritis, including case identification, treatment, and monitoring, and they include data on newer therapeutic modalities, including mycophenolate mofetil, mycophenolic acid, and rituximab, which were not available at the time the previous guidelines were developed, said Dr. Hahn, who led the core working group that helped develop the new guidelines.
They also address special situations such as pregnancy.
The core working group, along with a core executive group and a task force panel of experts used the validated modified RAND/University of California at Los Angeles Appropriateness Method, which involves a systematic literature view and expert opinion (based on voting by the task force panel) to develop the new guidelines.
The biopsy recommendation and the related therapeutic recommendations are based on level C evidence, indicating they were derived by consensus, expert opinion, and case series. Indications for renal biopsy, according to the task force panel include increasing serum creatinine without compelling alternative causes, confirmed proteinuria of 1 g or more/24 hours, and combinations of proteinuria of 0.5 g or more/24 hours plus hematuria (defined as 5 or more red blood cells per high power field) and proteinuria of 0.5 g or more/24 hours plus cellular casts – as long at these findings are confirmed in at least two tests conducted within a short time period and in the absence of alternative causes.
The task force panel also addressed adjunctive treatments, specifically recommending that:
• All patients with SLE be treated with a background of hydroxychloroquine unless contraindicated. This level C recommendation is based on recent cross-sectional and prospective controlled trial data indicating it is of benefit for reducing flare rates, is associated with significantly lower damage accrual (including renal damage), and may be associated with reduced risk of clotting events.
• Careful attention be paid to control of hypertension, with a target of no more than 130/80 mm Hg. This recommendation is based on level A evidence for nondiabetic chronic renal disease, indicating it is derived from multiple randomized controlled trials or a meta-analysis.
• Women of child-bearing potential who have active or prior lupus nephritis be counseled about the pregnancy risks conferred by the disease and its treatments. This recommendation is based on level C evidence.
Other task force panel recommendations specifically address induction of improvement in patients with International Society of Nephrology class III/IV lupus glomerulonephritis, induction of improvement in those with class IV or IV/V disease with cellular crescents, maintaining improvement in those who respond to induction therapy, and changing therapies in those who do not. Additional recommendations address the identification of vascular disease in patients with SLE and renal abnormalities, treatment of lupus nephritis patients who are pregnant, and monitoring the activity of lupus nephritis, Dr. Hahn said.
The new recommendations are "heavily based on induction with mycophenolate mofetil or cyclophosphamide, and on maintenance with mycophenolate mofetil or azathioprine," she noted.
"Nephritis remains one of the most devastating complications of lupus," she and her coauthors wrote, noting that the incidence increased during the 1980s and 1990s, with no decline seen as of 2004, despite the availability of new therapeutic regimens.
Standardized incidence rates for end-stage renal disease in the United States have risen in a number of populations, including younger patients, African Americans, and those living in the South.
"We hope that institution of these recommendations might lead to reductions in these trends. Furthermore, they may allow us to evaluate whether those who receive the recommended therapies are less likely to develop end-stage renal disease," they continued, concluding that while much progress has been made since lupus nephritis was associated with a near-terminal prognosis, these recommendations represent an effort to further improve outcomes and decrease morbidity and mortality in SLE.
The guidelines were sponsored by the American College of Rheumatology via a competitive grant mechanism. Dr. Hahn has received consultant fees, speaking fees, and/or honoraria from UCB and Abbott and has served on the data and safety monitoring board for Anthera. The complete list of disclosures for the guideline authors is available with the full text of the article.
FROM THE CONGRESS OF CLINICAL RHEUMATOLOGY