User login
Would you prescribe antenatal steroids to a pregnant patient at high risk for delivering at 22 weeks’ gestation?
For many decades, the limit of newborn viability was at approximately 24 weeks’ gestation. Recent advances in pregnancy and neonatal care suggest that the new limit of viability is 22 (22 weeks and 0 days to 22 weeks and 6 days) or 23 (23 weeks and 0 days to 23 weeks and 6 days) weeks of gestation. In addition, data from observational cohort studies indicate that for infants born at 22 and 23 weeks’ gestation, survival is dependent on a course of antenatal steroids administered prior to birth plus intensive respiratory and cardiovascular support at delivery and in the neonatal intensive care unit (NICU).
Antenatal steroids: Critical for survival at 22 and 23 weeks of gestation
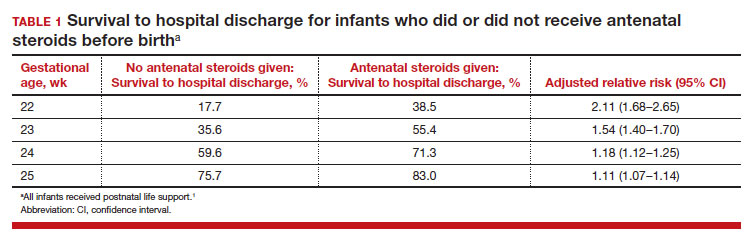
Most studies of birth outcomes at 22 and 23 weeks’ gestation rely on observational cohorts where unmeasured differences among the maternal-fetal dyads that received or did not receive a specific treatment confounds the interpretation of the data. However, data from multiple large observational cohorts suggest that between 22 and 24 weeks of gestation, completion of a course of antenatal steroids will optimize infant outcomes. Particularly noteworthy was the observation that the incremental survival benefit of antenatal steroids was greatest at 22 and 23 weeks’ gestation (TABLE 1).1 Similar results have been reported by Rossi and colleagues (TABLE 2).2
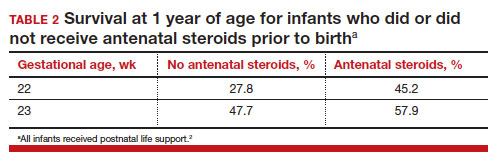
The importance of a completed course of antenatal steroids before birth was confirmed in another cohort study of 431 infants born in 2016 to 2019 at 22 weeks and 0 days’ to 23 weeks and 6 days’ gestation.3 Survival to discharge occurred in 53.9% of infants who received a full course of antenatal steroids before birth and 35.5% among those who did not receive antenatal steroids..3 Survival to discharge without major neonatal morbidities was 26.9% in those who received a full course of antenatal steroids and 10% among those who did not. In this cohort, major neonatal morbidities included severe intracranial hemorrhage, cystic periventricular leukomalacia, severe bronchopulmonary dysplasia, surgical necrotizing enterocolitis, or severe retinopathy of prematurity requiring treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends against antenatal steroids prior to 22 weeks and 0 days gestation.4 However, some neonatologists might recommend that antenatal steroids be given starting at 21 weeks and 5 days of gestation if birth is anticipated in the 22nd week of gestation and the patient prefers aggressive treatment of the newborn.
Active respiratory and cardiovascular support improves newborn outcomes
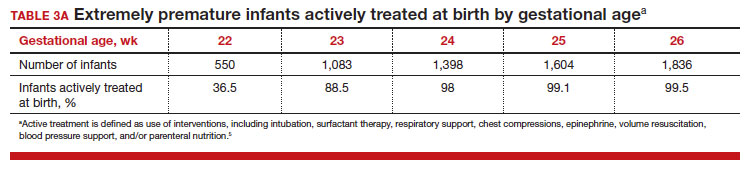
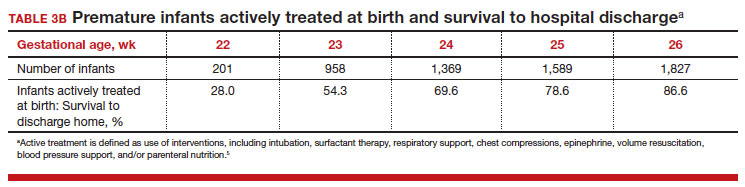
To maximize survival, infants born at 22 and 23 weeks’ gestation always require intensive active treatment at birth and in the following days in the NICU. Active treatment may include respiratory support, surfactant treatment, pressors, closure of a patent ductus arteriosus, transfusion of red blood cells, and parenteral nutrition. In one observational cohort study, active treatment at birth was not routinely provided at 22 and 23 weeks’ gestation but was routinely provided at later gestational ages (TABLE 3A).5 Not surprisingly, active treatment, especially at early gestational ages, is associated with improved survival to discharge. For example, at 22 weeks’ gestation, survival to discharge in infants who received or did not receive intensive active treatment was 28% and 0%, respectively.5 However, specific clinical characteristics of the pregnant patient and newborn may have influenced which infants were actively treated, confounding interpretation of the observation. In this cohort of extremely premature newborns, survival to hospital discharge increased substantially between 22 weeks and 26 weeks of gestational age (TABLE 3B).5
Many of the surviving infants needed chronic support treatment. Among surviving infants born at 22 weeks and 26 weeks, chronic support treatments were being used by 22.6% and 10.6% of infants, respectively, 2 years after birth.5 For surviving infants born at 22 weeks, the specific chronic support treatments included gastrostomy or feeding tube (19.4%), oxygen (9.7%), pulse oximeter (9.7%), and/or tracheostomy (3.2%). For surviving infants born at 26 weeks’ gestation, the specific chronic support treatments included gastrotomy or feeding tube (8.5%), pulse oximeter (4.4%), oxygen (3.2%), tracheostomy (2.3%), an apnea monitor (1.5%), and/or ventilator or continuous positive airway pressure (1.1%).5
Continue to: Evolving improvement in infant outcomes...
Evolving improvement in infant outcomes
In 1963, Jacqueline Bouvier Kennedy went into preterm labor at 34 weeks of gestation and delivered her son Patrick at a community facility. Due to severe respiratory distress syndrome, Patrick was transferred to the Boston Children’s Hospital, and he died shortly thereafter.6 Sixty years later, due to advances in obstetric and neonatal care, death from respiratory distress syndrome at 34 weeks of gestation is uncommon in the United States.
Infant outcomes following birth at 22 and 23 weeks’ gestation continue to improve. An observational cohort study from Sweden reported that at 22 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 10% and 30%, respectively.7 Similarly, at 23 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 52% and 61%, respectively.7 However, most of the surviving infants in this cohort had one or more major neonatal morbidities, including intraventricular hemorrhage grade 3 or 4; periventricular leukomalacia; necrotizing enterocolitis; retinopathy of prematurity grade 3, 4, or 5; or severe bronchopulmonary dysplasia.7
In a cohort of infants born in Japan at 22 to 24 weeks of gestation, there was a notable decrease in major neurodisability at 3 years of age for births occurring in 2 epochs, 2003 to 2007 and 2008 to 2012.8 When comparing outcomes in 2003 to 2007 versus 2008 to 2012, the change in rate of various major complications included the following: cerebral palsy (15.9% vs 9.5%), visual impairment (13.6% vs 4.4%), blindness (4.8% vs 1.3%), and hearing impairment (2.6% vs 1.0%). In contrast, the rate of cognitive impairment, defined as less than 70% of standard test performance for chronological age, was similar in the 2 time periods (36.5% and 37.9%, respectively).8 Based on data reported between 2000 and 2020, a systematic review and meta-analysis by Backes and colleagues concluded that there has been substantial improvement in the survival of infants born at 22 weeks of gestation.9
The small baby unit
A feature of modern medicine is the relentless evolution of new clinical subspecialties and sub-subspecialties. NICUs evolved from newborn nurseries to serve the needs of the most severely ill newborns, with care provided by a cadre of highly trained subspecialized neonatologists and neonatal nurses. A new era is dawning, with some NICUs developing a sub-subspecialized small baby unit to care for infants born between 22 and 26 weeks of gestation. These units often are staffed by clinicians with a specific interest in optimizing the care of extremely preterm infants, providing continuity of care over a long hospitalization.10 The benefits of a small baby unit may include:
- relentless standardization and adherence to the best intensive care practices
- daily use of checklists
- strict adherence to central line care
- timely extubation and transition to continuous positive airway pressure
- adherence to breastfeeding guidelines
- limiting the number of clinicians responsible for the patient
- promotion of kangaroo care
- avoidance of noxious stimuli.10,11
Continue to: Ethical and clinical issues...
Ethical and clinical issues
Providing clinical care to infants born at the edge of viability is challenging and raises many ethical and clinical concerns.12,13 For an infant born at the edge of viability, clinicians and parents do not want to initiate a care process that improves survival but results in an extremely poor quality of life. At the same time, clinicians and parents do not want to withhold care that could help an extremely premature newborn survive and thrive. Consequently, the counseling process is complex and requires coordination between the obstetrical and neonatology disciplines, involving physicians and nurses from both. A primary consideration in deciding to institute active treatment at birth is the preference of the pregnant patient and the patient’s trusted family members. A thorough discussion of these issues is beyond the scope of this editorial. ACOG provides detailed advice about the approach to counseling patients who face the possibility of a periviable birth.14
To help standardize the counseling process, institutions may find it helpful to recommend that clinicians consistently use a calculator to provide newborn outcome data to patients. The National Institute of Child Health and Human Development’s Extremely Preterm Birth Outcomes calculator uses the following inputs:
- gestational age
- estimated birth weight
- sex
- singleton/multiple gestation
- antenatal steroid treatment.
It also provides the following outputs as percentages:
- survival with active treatment at birth
- survival without active treatment at birth
- profound neurodevelopmental impairment
- moderate to severe neurodevelopmental impairment
- blindness
- deafness
- moderate to severe cerebral palsy
- cognitive developmental delay.15
A full assessment of all known clinical factors should influence the interpretation of the output from the clinical calculator. An alternativeis to use data from the Vermont Oxford Network. NICUs with sufficient clinical volume may prefer to use their own outcome data in the counseling process.
Institutions and clinical teams may improve the consistency of the counseling process by identifying criteria for 3 main treatment options:
- clinical situations where active treatment at birth is not generally offered (eg, <22 weeks’ gestation)
- clinical situations where active treatment at birth is almost always routinely provided (eg, >25 weeks’ gestation)
- clinical situations where patient preferences are especially important in guiding the use of active treatment.
Most institutions do not routinely offer active treatment of the newborn at a gestational age of less than 22 weeks and 0 days. Instead, comfort care often is provided for these newborns. Most institutions routinely provide active treatment at birth beginning at 24 or 25 weeks’ gestation unless unique risk factors or comorbidities warrant not providing active treatment (TABLE 3A). Some professional societies recommend setting a threshold for recommending active treatment at birth. For example, the British Association of Perinatal Medicine recommends that if there is 50% or higher probability of survival without severe disability, active treatment at birth should be considered because it is in the best interest of the newborn.16 In the hours and days following birth, the clinical course of the newborn greatly influences the treatment plan and care goals. After the initial resuscitation, if the clinical condition of an extremely preterm infant worsens and the prognosis is grim, a pivot to palliative care may be considered.
Final thoughts
Periviability is the earliest stage of fetal development where there is a reasonable chance, but not a high likelihood, of survival outside the womb. For decades, the threshold for periviability was approximately 24 weeks of gestation. With current obstetrical and neonatal practice, the new threshold for periviability is 22 to 23 weeks of gestation, but death prior to hospital discharge occurs in approximately half of these newborns. For the survivors, lifelong neurodevelopmental complications and pulmonary disease are common. Obstetricians play a key role in counseling patients who are at risk of giving birth before 24 weeks of gestation. Given the challenges faced by an infant born at 22 and 23 weeks’ gestation, pregnant patients and trusted family members should approach the decision to actively resuscitate the newborn with caution. However, if the clinical team, patient, and trusted family members agree to pursue active treatment, completion of a course of antenatal steroids and appropriate respiratory and cardiovascular support at birth are key to improving long-term outcomes. ●
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks gestation. JAMA Network Open. 2018;E183235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks [published online November 28, 2021]. Am J Perinatol. doi:10.1055/s-004-1740062
- Chawla S, Wyckoff MH, Rysavy MA, et al. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Network Open. 2022;5:E2233331.
- Use of antenatal corticosteroids at 22 weeks of gestation. ACOG website. Published September 2021. Accessed April 10, 2023. https://www.acog .org/clinical/clinical-guidance/practice -advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation
- Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248-263.
- The tragic death of Patrick, JFK and Jackie’s newborn son, in 1963. Irish Central website. Published November 6, 2022. Accessed April 10, 2023. https://www.irishcentral.com/roots/history /tragic-death-patrick-kennedy-jfk-jackie
- Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;32:1188-1199.
- Kono Y, Yonemoto N, Nakanishi H, et al. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatrics Open. 2018;2:E000211.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Morris M, Cleary JP, Soliman A. Small baby unit improves quality and outcomes in extremely low birth weight infants. Pediatrics. 2015;136:E1007-E1015.
- Fathi O, Nelin LD, Shephard EG, et al. Development of a small baby unit to improve outcomes for the extremely premature infant. J Perinatology. 2002;42:157-164.
- Lantos JD. Ethical issues in treatment of babies born at 22 weeks of gestation. Arch Dis Child. 2021;106:1155-1157.
- Shinwell ES. Ethics of birth at the limit of viability: the risky business of prediction. Neonatology. 2015;107:317-320.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No 6. periviable birth. Obstet Gynecol. 2017;E187-E199.
- Extremely preterm birth outcomes tool. NICHD website. Updated March 2, 2020. Accessed April 10, 2023. https://www.nichd.nih.gov/research /supported/EPBO/use#
- Mactier H, Bates SE, Johnston T, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. 2020;105:F232-F239.

For many decades, the limit of newborn viability was at approximately 24 weeks’ gestation. Recent advances in pregnancy and neonatal care suggest that the new limit of viability is 22 (22 weeks and 0 days to 22 weeks and 6 days) or 23 (23 weeks and 0 days to 23 weeks and 6 days) weeks of gestation. In addition, data from observational cohort studies indicate that for infants born at 22 and 23 weeks’ gestation, survival is dependent on a course of antenatal steroids administered prior to birth plus intensive respiratory and cardiovascular support at delivery and in the neonatal intensive care unit (NICU).
Antenatal steroids: Critical for survival at 22 and 23 weeks of gestation
Most studies of birth outcomes at 22 and 23 weeks’ gestation rely on observational cohorts where unmeasured differences among the maternal-fetal dyads that received or did not receive a specific treatment confounds the interpretation of the data. However, data from multiple large observational cohorts suggest that between 22 and 24 weeks of gestation, completion of a course of antenatal steroids will optimize infant outcomes. Particularly noteworthy was the observation that the incremental survival benefit of antenatal steroids was greatest at 22 and 23 weeks’ gestation (TABLE 1).1 Similar results have been reported by Rossi and colleagues (TABLE 2).2


The importance of a completed course of antenatal steroids before birth was confirmed in another cohort study of 431 infants born in 2016 to 2019 at 22 weeks and 0 days’ to 23 weeks and 6 days’ gestation.3 Survival to discharge occurred in 53.9% of infants who received a full course of antenatal steroids before birth and 35.5% among those who did not receive antenatal steroids..3 Survival to discharge without major neonatal morbidities was 26.9% in those who received a full course of antenatal steroids and 10% among those who did not. In this cohort, major neonatal morbidities included severe intracranial hemorrhage, cystic periventricular leukomalacia, severe bronchopulmonary dysplasia, surgical necrotizing enterocolitis, or severe retinopathy of prematurity requiring treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends against antenatal steroids prior to 22 weeks and 0 days gestation.4 However, some neonatologists might recommend that antenatal steroids be given starting at 21 weeks and 5 days of gestation if birth is anticipated in the 22nd week of gestation and the patient prefers aggressive treatment of the newborn.
Active respiratory and cardiovascular support improves newborn outcomes
To maximize survival, infants born at 22 and 23 weeks’ gestation always require intensive active treatment at birth and in the following days in the NICU. Active treatment may include respiratory support, surfactant treatment, pressors, closure of a patent ductus arteriosus, transfusion of red blood cells, and parenteral nutrition. In one observational cohort study, active treatment at birth was not routinely provided at 22 and 23 weeks’ gestation but was routinely provided at later gestational ages (TABLE 3A).5 Not surprisingly, active treatment, especially at early gestational ages, is associated with improved survival to discharge. For example, at 22 weeks’ gestation, survival to discharge in infants who received or did not receive intensive active treatment was 28% and 0%, respectively.5 However, specific clinical characteristics of the pregnant patient and newborn may have influenced which infants were actively treated, confounding interpretation of the observation. In this cohort of extremely premature newborns, survival to hospital discharge increased substantially between 22 weeks and 26 weeks of gestational age (TABLE 3B).5


Many of the surviving infants needed chronic support treatment. Among surviving infants born at 22 weeks and 26 weeks, chronic support treatments were being used by 22.6% and 10.6% of infants, respectively, 2 years after birth.5 For surviving infants born at 22 weeks, the specific chronic support treatments included gastrostomy or feeding tube (19.4%), oxygen (9.7%), pulse oximeter (9.7%), and/or tracheostomy (3.2%). For surviving infants born at 26 weeks’ gestation, the specific chronic support treatments included gastrotomy or feeding tube (8.5%), pulse oximeter (4.4%), oxygen (3.2%), tracheostomy (2.3%), an apnea monitor (1.5%), and/or ventilator or continuous positive airway pressure (1.1%).5
Continue to: Evolving improvement in infant outcomes...
Evolving improvement in infant outcomes
In 1963, Jacqueline Bouvier Kennedy went into preterm labor at 34 weeks of gestation and delivered her son Patrick at a community facility. Due to severe respiratory distress syndrome, Patrick was transferred to the Boston Children’s Hospital, and he died shortly thereafter.6 Sixty years later, due to advances in obstetric and neonatal care, death from respiratory distress syndrome at 34 weeks of gestation is uncommon in the United States.
Infant outcomes following birth at 22 and 23 weeks’ gestation continue to improve. An observational cohort study from Sweden reported that at 22 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 10% and 30%, respectively.7 Similarly, at 23 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 52% and 61%, respectively.7 However, most of the surviving infants in this cohort had one or more major neonatal morbidities, including intraventricular hemorrhage grade 3 or 4; periventricular leukomalacia; necrotizing enterocolitis; retinopathy of prematurity grade 3, 4, or 5; or severe bronchopulmonary dysplasia.7
In a cohort of infants born in Japan at 22 to 24 weeks of gestation, there was a notable decrease in major neurodisability at 3 years of age for births occurring in 2 epochs, 2003 to 2007 and 2008 to 2012.8 When comparing outcomes in 2003 to 2007 versus 2008 to 2012, the change in rate of various major complications included the following: cerebral palsy (15.9% vs 9.5%), visual impairment (13.6% vs 4.4%), blindness (4.8% vs 1.3%), and hearing impairment (2.6% vs 1.0%). In contrast, the rate of cognitive impairment, defined as less than 70% of standard test performance for chronological age, was similar in the 2 time periods (36.5% and 37.9%, respectively).8 Based on data reported between 2000 and 2020, a systematic review and meta-analysis by Backes and colleagues concluded that there has been substantial improvement in the survival of infants born at 22 weeks of gestation.9
The small baby unit
A feature of modern medicine is the relentless evolution of new clinical subspecialties and sub-subspecialties. NICUs evolved from newborn nurseries to serve the needs of the most severely ill newborns, with care provided by a cadre of highly trained subspecialized neonatologists and neonatal nurses. A new era is dawning, with some NICUs developing a sub-subspecialized small baby unit to care for infants born between 22 and 26 weeks of gestation. These units often are staffed by clinicians with a specific interest in optimizing the care of extremely preterm infants, providing continuity of care over a long hospitalization.10 The benefits of a small baby unit may include:
- relentless standardization and adherence to the best intensive care practices
- daily use of checklists
- strict adherence to central line care
- timely extubation and transition to continuous positive airway pressure
- adherence to breastfeeding guidelines
- limiting the number of clinicians responsible for the patient
- promotion of kangaroo care
- avoidance of noxious stimuli.10,11
Continue to: Ethical and clinical issues...
Ethical and clinical issues
Providing clinical care to infants born at the edge of viability is challenging and raises many ethical and clinical concerns.12,13 For an infant born at the edge of viability, clinicians and parents do not want to initiate a care process that improves survival but results in an extremely poor quality of life. At the same time, clinicians and parents do not want to withhold care that could help an extremely premature newborn survive and thrive. Consequently, the counseling process is complex and requires coordination between the obstetrical and neonatology disciplines, involving physicians and nurses from both. A primary consideration in deciding to institute active treatment at birth is the preference of the pregnant patient and the patient’s trusted family members. A thorough discussion of these issues is beyond the scope of this editorial. ACOG provides detailed advice about the approach to counseling patients who face the possibility of a periviable birth.14
To help standardize the counseling process, institutions may find it helpful to recommend that clinicians consistently use a calculator to provide newborn outcome data to patients. The National Institute of Child Health and Human Development’s Extremely Preterm Birth Outcomes calculator uses the following inputs:
- gestational age
- estimated birth weight
- sex
- singleton/multiple gestation
- antenatal steroid treatment.
It also provides the following outputs as percentages:
- survival with active treatment at birth
- survival without active treatment at birth
- profound neurodevelopmental impairment
- moderate to severe neurodevelopmental impairment
- blindness
- deafness
- moderate to severe cerebral palsy
- cognitive developmental delay.15
A full assessment of all known clinical factors should influence the interpretation of the output from the clinical calculator. An alternativeis to use data from the Vermont Oxford Network. NICUs with sufficient clinical volume may prefer to use their own outcome data in the counseling process.
Institutions and clinical teams may improve the consistency of the counseling process by identifying criteria for 3 main treatment options:
- clinical situations where active treatment at birth is not generally offered (eg, <22 weeks’ gestation)
- clinical situations where active treatment at birth is almost always routinely provided (eg, >25 weeks’ gestation)
- clinical situations where patient preferences are especially important in guiding the use of active treatment.
Most institutions do not routinely offer active treatment of the newborn at a gestational age of less than 22 weeks and 0 days. Instead, comfort care often is provided for these newborns. Most institutions routinely provide active treatment at birth beginning at 24 or 25 weeks’ gestation unless unique risk factors or comorbidities warrant not providing active treatment (TABLE 3A). Some professional societies recommend setting a threshold for recommending active treatment at birth. For example, the British Association of Perinatal Medicine recommends that if there is 50% or higher probability of survival without severe disability, active treatment at birth should be considered because it is in the best interest of the newborn.16 In the hours and days following birth, the clinical course of the newborn greatly influences the treatment plan and care goals. After the initial resuscitation, if the clinical condition of an extremely preterm infant worsens and the prognosis is grim, a pivot to palliative care may be considered.
Final thoughts
Periviability is the earliest stage of fetal development where there is a reasonable chance, but not a high likelihood, of survival outside the womb. For decades, the threshold for periviability was approximately 24 weeks of gestation. With current obstetrical and neonatal practice, the new threshold for periviability is 22 to 23 weeks of gestation, but death prior to hospital discharge occurs in approximately half of these newborns. For the survivors, lifelong neurodevelopmental complications and pulmonary disease are common. Obstetricians play a key role in counseling patients who are at risk of giving birth before 24 weeks of gestation. Given the challenges faced by an infant born at 22 and 23 weeks’ gestation, pregnant patients and trusted family members should approach the decision to actively resuscitate the newborn with caution. However, if the clinical team, patient, and trusted family members agree to pursue active treatment, completion of a course of antenatal steroids and appropriate respiratory and cardiovascular support at birth are key to improving long-term outcomes. ●

For many decades, the limit of newborn viability was at approximately 24 weeks’ gestation. Recent advances in pregnancy and neonatal care suggest that the new limit of viability is 22 (22 weeks and 0 days to 22 weeks and 6 days) or 23 (23 weeks and 0 days to 23 weeks and 6 days) weeks of gestation. In addition, data from observational cohort studies indicate that for infants born at 22 and 23 weeks’ gestation, survival is dependent on a course of antenatal steroids administered prior to birth plus intensive respiratory and cardiovascular support at delivery and in the neonatal intensive care unit (NICU).
Antenatal steroids: Critical for survival at 22 and 23 weeks of gestation
Most studies of birth outcomes at 22 and 23 weeks’ gestation rely on observational cohorts where unmeasured differences among the maternal-fetal dyads that received or did not receive a specific treatment confounds the interpretation of the data. However, data from multiple large observational cohorts suggest that between 22 and 24 weeks of gestation, completion of a course of antenatal steroids will optimize infant outcomes. Particularly noteworthy was the observation that the incremental survival benefit of antenatal steroids was greatest at 22 and 23 weeks’ gestation (TABLE 1).1 Similar results have been reported by Rossi and colleagues (TABLE 2).2


The importance of a completed course of antenatal steroids before birth was confirmed in another cohort study of 431 infants born in 2016 to 2019 at 22 weeks and 0 days’ to 23 weeks and 6 days’ gestation.3 Survival to discharge occurred in 53.9% of infants who received a full course of antenatal steroids before birth and 35.5% among those who did not receive antenatal steroids..3 Survival to discharge without major neonatal morbidities was 26.9% in those who received a full course of antenatal steroids and 10% among those who did not. In this cohort, major neonatal morbidities included severe intracranial hemorrhage, cystic periventricular leukomalacia, severe bronchopulmonary dysplasia, surgical necrotizing enterocolitis, or severe retinopathy of prematurity requiring treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends against antenatal steroids prior to 22 weeks and 0 days gestation.4 However, some neonatologists might recommend that antenatal steroids be given starting at 21 weeks and 5 days of gestation if birth is anticipated in the 22nd week of gestation and the patient prefers aggressive treatment of the newborn.
Active respiratory and cardiovascular support improves newborn outcomes
To maximize survival, infants born at 22 and 23 weeks’ gestation always require intensive active treatment at birth and in the following days in the NICU. Active treatment may include respiratory support, surfactant treatment, pressors, closure of a patent ductus arteriosus, transfusion of red blood cells, and parenteral nutrition. In one observational cohort study, active treatment at birth was not routinely provided at 22 and 23 weeks’ gestation but was routinely provided at later gestational ages (TABLE 3A).5 Not surprisingly, active treatment, especially at early gestational ages, is associated with improved survival to discharge. For example, at 22 weeks’ gestation, survival to discharge in infants who received or did not receive intensive active treatment was 28% and 0%, respectively.5 However, specific clinical characteristics of the pregnant patient and newborn may have influenced which infants were actively treated, confounding interpretation of the observation. In this cohort of extremely premature newborns, survival to hospital discharge increased substantially between 22 weeks and 26 weeks of gestational age (TABLE 3B).5


Many of the surviving infants needed chronic support treatment. Among surviving infants born at 22 weeks and 26 weeks, chronic support treatments were being used by 22.6% and 10.6% of infants, respectively, 2 years after birth.5 For surviving infants born at 22 weeks, the specific chronic support treatments included gastrostomy or feeding tube (19.4%), oxygen (9.7%), pulse oximeter (9.7%), and/or tracheostomy (3.2%). For surviving infants born at 26 weeks’ gestation, the specific chronic support treatments included gastrotomy or feeding tube (8.5%), pulse oximeter (4.4%), oxygen (3.2%), tracheostomy (2.3%), an apnea monitor (1.5%), and/or ventilator or continuous positive airway pressure (1.1%).5
Continue to: Evolving improvement in infant outcomes...
Evolving improvement in infant outcomes
In 1963, Jacqueline Bouvier Kennedy went into preterm labor at 34 weeks of gestation and delivered her son Patrick at a community facility. Due to severe respiratory distress syndrome, Patrick was transferred to the Boston Children’s Hospital, and he died shortly thereafter.6 Sixty years later, due to advances in obstetric and neonatal care, death from respiratory distress syndrome at 34 weeks of gestation is uncommon in the United States.
Infant outcomes following birth at 22 and 23 weeks’ gestation continue to improve. An observational cohort study from Sweden reported that at 22 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 10% and 30%, respectively.7 Similarly, at 23 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 52% and 61%, respectively.7 However, most of the surviving infants in this cohort had one or more major neonatal morbidities, including intraventricular hemorrhage grade 3 or 4; periventricular leukomalacia; necrotizing enterocolitis; retinopathy of prematurity grade 3, 4, or 5; or severe bronchopulmonary dysplasia.7
In a cohort of infants born in Japan at 22 to 24 weeks of gestation, there was a notable decrease in major neurodisability at 3 years of age for births occurring in 2 epochs, 2003 to 2007 and 2008 to 2012.8 When comparing outcomes in 2003 to 2007 versus 2008 to 2012, the change in rate of various major complications included the following: cerebral palsy (15.9% vs 9.5%), visual impairment (13.6% vs 4.4%), blindness (4.8% vs 1.3%), and hearing impairment (2.6% vs 1.0%). In contrast, the rate of cognitive impairment, defined as less than 70% of standard test performance for chronological age, was similar in the 2 time periods (36.5% and 37.9%, respectively).8 Based on data reported between 2000 and 2020, a systematic review and meta-analysis by Backes and colleagues concluded that there has been substantial improvement in the survival of infants born at 22 weeks of gestation.9
The small baby unit
A feature of modern medicine is the relentless evolution of new clinical subspecialties and sub-subspecialties. NICUs evolved from newborn nurseries to serve the needs of the most severely ill newborns, with care provided by a cadre of highly trained subspecialized neonatologists and neonatal nurses. A new era is dawning, with some NICUs developing a sub-subspecialized small baby unit to care for infants born between 22 and 26 weeks of gestation. These units often are staffed by clinicians with a specific interest in optimizing the care of extremely preterm infants, providing continuity of care over a long hospitalization.10 The benefits of a small baby unit may include:
- relentless standardization and adherence to the best intensive care practices
- daily use of checklists
- strict adherence to central line care
- timely extubation and transition to continuous positive airway pressure
- adherence to breastfeeding guidelines
- limiting the number of clinicians responsible for the patient
- promotion of kangaroo care
- avoidance of noxious stimuli.10,11
Continue to: Ethical and clinical issues...
Ethical and clinical issues
Providing clinical care to infants born at the edge of viability is challenging and raises many ethical and clinical concerns.12,13 For an infant born at the edge of viability, clinicians and parents do not want to initiate a care process that improves survival but results in an extremely poor quality of life. At the same time, clinicians and parents do not want to withhold care that could help an extremely premature newborn survive and thrive. Consequently, the counseling process is complex and requires coordination between the obstetrical and neonatology disciplines, involving physicians and nurses from both. A primary consideration in deciding to institute active treatment at birth is the preference of the pregnant patient and the patient’s trusted family members. A thorough discussion of these issues is beyond the scope of this editorial. ACOG provides detailed advice about the approach to counseling patients who face the possibility of a periviable birth.14
To help standardize the counseling process, institutions may find it helpful to recommend that clinicians consistently use a calculator to provide newborn outcome data to patients. The National Institute of Child Health and Human Development’s Extremely Preterm Birth Outcomes calculator uses the following inputs:
- gestational age
- estimated birth weight
- sex
- singleton/multiple gestation
- antenatal steroid treatment.
It also provides the following outputs as percentages:
- survival with active treatment at birth
- survival without active treatment at birth
- profound neurodevelopmental impairment
- moderate to severe neurodevelopmental impairment
- blindness
- deafness
- moderate to severe cerebral palsy
- cognitive developmental delay.15
A full assessment of all known clinical factors should influence the interpretation of the output from the clinical calculator. An alternativeis to use data from the Vermont Oxford Network. NICUs with sufficient clinical volume may prefer to use their own outcome data in the counseling process.
Institutions and clinical teams may improve the consistency of the counseling process by identifying criteria for 3 main treatment options:
- clinical situations where active treatment at birth is not generally offered (eg, <22 weeks’ gestation)
- clinical situations where active treatment at birth is almost always routinely provided (eg, >25 weeks’ gestation)
- clinical situations where patient preferences are especially important in guiding the use of active treatment.
Most institutions do not routinely offer active treatment of the newborn at a gestational age of less than 22 weeks and 0 days. Instead, comfort care often is provided for these newborns. Most institutions routinely provide active treatment at birth beginning at 24 or 25 weeks’ gestation unless unique risk factors or comorbidities warrant not providing active treatment (TABLE 3A). Some professional societies recommend setting a threshold for recommending active treatment at birth. For example, the British Association of Perinatal Medicine recommends that if there is 50% or higher probability of survival without severe disability, active treatment at birth should be considered because it is in the best interest of the newborn.16 In the hours and days following birth, the clinical course of the newborn greatly influences the treatment plan and care goals. After the initial resuscitation, if the clinical condition of an extremely preterm infant worsens and the prognosis is grim, a pivot to palliative care may be considered.
Final thoughts
Periviability is the earliest stage of fetal development where there is a reasonable chance, but not a high likelihood, of survival outside the womb. For decades, the threshold for periviability was approximately 24 weeks of gestation. With current obstetrical and neonatal practice, the new threshold for periviability is 22 to 23 weeks of gestation, but death prior to hospital discharge occurs in approximately half of these newborns. For the survivors, lifelong neurodevelopmental complications and pulmonary disease are common. Obstetricians play a key role in counseling patients who are at risk of giving birth before 24 weeks of gestation. Given the challenges faced by an infant born at 22 and 23 weeks’ gestation, pregnant patients and trusted family members should approach the decision to actively resuscitate the newborn with caution. However, if the clinical team, patient, and trusted family members agree to pursue active treatment, completion of a course of antenatal steroids and appropriate respiratory and cardiovascular support at birth are key to improving long-term outcomes. ●
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks gestation. JAMA Network Open. 2018;E183235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks [published online November 28, 2021]. Am J Perinatol. doi:10.1055/s-004-1740062
- Chawla S, Wyckoff MH, Rysavy MA, et al. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Network Open. 2022;5:E2233331.
- Use of antenatal corticosteroids at 22 weeks of gestation. ACOG website. Published September 2021. Accessed April 10, 2023. https://www.acog .org/clinical/clinical-guidance/practice -advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation
- Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248-263.
- The tragic death of Patrick, JFK and Jackie’s newborn son, in 1963. Irish Central website. Published November 6, 2022. Accessed April 10, 2023. https://www.irishcentral.com/roots/history /tragic-death-patrick-kennedy-jfk-jackie
- Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;32:1188-1199.
- Kono Y, Yonemoto N, Nakanishi H, et al. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatrics Open. 2018;2:E000211.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Morris M, Cleary JP, Soliman A. Small baby unit improves quality and outcomes in extremely low birth weight infants. Pediatrics. 2015;136:E1007-E1015.
- Fathi O, Nelin LD, Shephard EG, et al. Development of a small baby unit to improve outcomes for the extremely premature infant. J Perinatology. 2002;42:157-164.
- Lantos JD. Ethical issues in treatment of babies born at 22 weeks of gestation. Arch Dis Child. 2021;106:1155-1157.
- Shinwell ES. Ethics of birth at the limit of viability: the risky business of prediction. Neonatology. 2015;107:317-320.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No 6. periviable birth. Obstet Gynecol. 2017;E187-E199.
- Extremely preterm birth outcomes tool. NICHD website. Updated March 2, 2020. Accessed April 10, 2023. https://www.nichd.nih.gov/research /supported/EPBO/use#
- Mactier H, Bates SE, Johnston T, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. 2020;105:F232-F239.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks gestation. JAMA Network Open. 2018;E183235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks [published online November 28, 2021]. Am J Perinatol. doi:10.1055/s-004-1740062
- Chawla S, Wyckoff MH, Rysavy MA, et al. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Network Open. 2022;5:E2233331.
- Use of antenatal corticosteroids at 22 weeks of gestation. ACOG website. Published September 2021. Accessed April 10, 2023. https://www.acog .org/clinical/clinical-guidance/practice -advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation
- Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248-263.
- The tragic death of Patrick, JFK and Jackie’s newborn son, in 1963. Irish Central website. Published November 6, 2022. Accessed April 10, 2023. https://www.irishcentral.com/roots/history /tragic-death-patrick-kennedy-jfk-jackie
- Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;32:1188-1199.
- Kono Y, Yonemoto N, Nakanishi H, et al. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatrics Open. 2018;2:E000211.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Morris M, Cleary JP, Soliman A. Small baby unit improves quality and outcomes in extremely low birth weight infants. Pediatrics. 2015;136:E1007-E1015.
- Fathi O, Nelin LD, Shephard EG, et al. Development of a small baby unit to improve outcomes for the extremely premature infant. J Perinatology. 2002;42:157-164.
- Lantos JD. Ethical issues in treatment of babies born at 22 weeks of gestation. Arch Dis Child. 2021;106:1155-1157.
- Shinwell ES. Ethics of birth at the limit of viability: the risky business of prediction. Neonatology. 2015;107:317-320.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No 6. periviable birth. Obstet Gynecol. 2017;E187-E199.
- Extremely preterm birth outcomes tool. NICHD website. Updated March 2, 2020. Accessed April 10, 2023. https://www.nichd.nih.gov/research /supported/EPBO/use#
- Mactier H, Bates SE, Johnston T, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. 2020;105:F232-F239.
News & Perspectives from Ob.Gyn. News
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
Gestational HTN, preeclampsia worsen long-term risk for ischemic, nonischemic heart failure
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
sFlt-1:PlGF ratio normal at 24 to 28 weeks: Discontinue aspirin for preterm preeclampsia prevention?

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
Early gestational diabetes treatment may improve neonatal outcomes
Screening and treatment for gestational diabetes are currently recommended at 24-28 weeks’ gestation, with earlier testing recommended for women at increased risk, but the potential benefits of earlier intervention remain debatable, wrote David Simmons, MD, of Western Sydney University, Campbelltown, Australia, and colleagues.
“Until now, there has been complete equipoise over whether to treat hyperglycemia below that of overt diabetes early in pregnancy,” Dr. Simmons said in an interview. The conflicting questions: “Would early treatment reduce the excess deposition of fat on the baby with all of its sequelae; but would early treatment reduce fuel supply to some babies at a critical time and lead to SGA [small for gestational age]?” Dr. Simmons noted.
In a study published in the New England Journal of Medicine, Dr. Simmons and colleagues randomized 406 women aged 18 years and older with singleton pregnancies to immediate treatment for gestational diabetes. Another 396 women were randomized to a control group for deferred treatment or no treatment, based on results of an oral glucose tolerance test at 24-28 weeks’ gestation. All participants had at least one risk factor for hyperglycemia, and met the World Health Organization criteria for gestational diabetes. Women with preexisting diabetes or contraindicating comorbid medical conditions were excluded.
The study had three primary outcomes. The first was a composite of neonatal outcomes including birth before 37 weeks’ gestation, birth weight of 4,500 g or higher, birth trauma, neonatal respiratory distress, phototherapy, stillbirth or neonatal death, or shoulder dystocia.
The final sample included 748 women for adverse neonatal outcomes, 750 for pregnancy-related hypertension, and 492 for neonatal lean body mass. The mean age of the participants was 32 years; approximately one-third were white European and another third were South Asian. Overall baseline demographics were similar between the groups, and the initial oral glucose tolerance tests were performed at a mean of 15.6 weeks’ gestation.
Overall, 24.9% of women in the early treatment group experienced an adverse neonatal event vs. 30.5% of controls, for an adjusted risk difference of –5.6% and adjusted relative risk of 0.82.
Notably, in an exploratory subgroup analysis, respiratory distress occurred in 9.8% of infants born to women in the immediate treatment group vs. 17.0% of infants in the control group. “Neonatal respiratory distress was the main driver of the between-group difference observed for the first primary outcome,” the researchers wrote. A prespecified subgroup analysis suggested that the impact of an earlier intervention on adverse neonatal outcomes might be greater among women with a higher glycemic value and those whose oral glucose tolerance tests occurred at less than 14 weeks’ gestation, they noted. Stillbirths or neonatal deaths were similar and infrequent in both groups.
Pregnancy-related hypertension occurred in 10.6% of the immediate-treatment group and 9.9% of the controls group (adjusted risk difference, 0.7%). For the third outcome, the mean neonatal lean body mass was 2.86 g in the immediate-treatment group and 2.91 g for the controls (adjusted mean difference, −0.04 g).
No differences in serious adverse events related to either screening or treatment were noted between the groups.
Impact on neonatal outcomes merits further study
Dr. Simmons said that he was surprised by the study findings. “We thought if there was an effect, it would be small, but it isn’t,” he told this publication.
“If you combine the severe adverse outcomes, the perineal trauma and the reduction in days in NICU/special care unit, this is a significant impact on morbidity and likely on cost,” and researchers are currently examining data for cost-effectiveness, he said.
“We did not expect the likely large impact on reducing respiratory distress and perineal trauma,” he noted. “These findings have not been previously reported, perhaps because they were not looked for.” By contrast, “we thought here might be reductions in lower gestational age and cesarean delivery, but there was not,” he added.
The findings were limited by several factors including the nonstandardized approach to gestational diabetes treatment and the use of third-trimester treatment targets that had not been tested in earlier trimesters, the researchers noted. Other limitations included the focus on women already at high risk for hyperglycemia; therefore, the results might not generalize to women not at risk, they wrote.
The current study represents a beginning of answers, with data suggesting that early treatment for gestational diabetes reduces severe adverse pregnancy outcomes, days in NICU/special care unit, and perineal trauma, likely from the first trimester, said Dr. Simmons. However, the findings must be interpreted with caution, as criteria that are too low “might lead to more small babies,” he said. “We look forward to working with others to translate these findings into practice,” he added.
Much more research is needed to answer the many questions prompted by the current study, including who did and did not have complications, Dr. Simmons told this publication. Other studies are needed to collect data on cost-effectiveness, as well as consumer views, especially “different perspectives from different parts of the globe,” he said. Although there is not enough evidence yet to draw conclusions about the role of continuous glucose monitoring (CGM) in managing gestational diabetes, many studies are underway; “we look forward to the results,” of these studies, Dr. Simmons added.
Findings support early screening
Gestational diabetes is one of the most common medical complications of pregnancy, and accounts for more than 80% of diabetes-related diagnoses in pregnancy, said Emily Fay, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, in an interview.
“Previous studies have found that women with gestational diabetes are at higher risk in their pregnancy, including higher chance of developing preeclampsia, higher chance of cesarean delivery, and higher risks for their baby, including risk of shoulder dystocia, birth trauma, and jaundice, and higher birth weights,” she said. “Fortunately, studies have also shown that treatment of gestational diabetes helps lower these risks,” she noted. Currently, patients undergo routine screening for gestational diabetes between 24 and 28 weeks of pregnancy, but some who have risk factors for gestational diabetes may have screening in the early part of pregnancy, said Dr. Fay.
The current findings were not surprising overall, said Dr. Fay, who was not involved in the study. “The study authors looked at a variety of outcomes including neonatal adverse outcomes, neonatal body weight, and pregnancy-related hypertension,” she said.
The researchers found that patients treated early had a lower rate of adverse neonatal outcomes, which was to be expected, Dr. Fay said. “They did not find a difference in neonatal body weight; this also was not surprising, as the women who were not in the early treatment group still received treatment at the time of diagnosis later in pregnancy, which likely helped normalize the weights,” she explained.
“My takeaway from this study is that we should continue to screen patients with risk factors for gestational diabetes early in pregnancy and treat them at the time of diagnosis,” Dr. Fay told this publication. However, barriers that may exist to early treatment involve access to care, including being able to see a provider early in pregnancy, she said. “The treatment for gestational diabetes includes dietary education with diet changes and checking blood sugars frequently. Access to nutrition education can be limited and access to healthy foods can be expensive and difficult to obtain,” she noted. “Checking blood sugars throughout the day can also be difficult for those who are busy or working and who may not have the ability to take time to do this,” she said. However, “these barriers may be overcome by health care reform that improves patient access to and coverage of pregnancy care, improved access and affordability of healthy foods, and employer flexibility to allow the time and space to check blood sugars if needed,” she added.
Looking ahead, the use of continuous glucose monitors in pregnancy is an expanding area of research, said Dr. Fay. “Patients can quickly view their blood sugar without the use of finger sticks, which may help overcome some of the barriers patients may have with using finger sticks,” she noted. “Continuous glucose monitors have been used for those with type 1 and type 2 diabetes with success, and we need to better understand if these can also be helpful in gestational diabetes,” she said. Dr. Fay and colleagues at the University of Washington are currently conducting an ongoing study to explore the use of CGM in gestational diabetes.
The study was supported by the National Health and Medical Research Council, the Region Örebro Research Committee, the Medical Scientific Fund of the Mayor of Vienna, the South Western Sydney Local Health District Academic Unit, and a Western Sydney University Ainsworth Trust Grant. The researchers had no financial conflicts to disclose. Dr. Fay had no relevant financial conflicts to disclose.
Screening and treatment for gestational diabetes are currently recommended at 24-28 weeks’ gestation, with earlier testing recommended for women at increased risk, but the potential benefits of earlier intervention remain debatable, wrote David Simmons, MD, of Western Sydney University, Campbelltown, Australia, and colleagues.
“Until now, there has been complete equipoise over whether to treat hyperglycemia below that of overt diabetes early in pregnancy,” Dr. Simmons said in an interview. The conflicting questions: “Would early treatment reduce the excess deposition of fat on the baby with all of its sequelae; but would early treatment reduce fuel supply to some babies at a critical time and lead to SGA [small for gestational age]?” Dr. Simmons noted.
In a study published in the New England Journal of Medicine, Dr. Simmons and colleagues randomized 406 women aged 18 years and older with singleton pregnancies to immediate treatment for gestational diabetes. Another 396 women were randomized to a control group for deferred treatment or no treatment, based on results of an oral glucose tolerance test at 24-28 weeks’ gestation. All participants had at least one risk factor for hyperglycemia, and met the World Health Organization criteria for gestational diabetes. Women with preexisting diabetes or contraindicating comorbid medical conditions were excluded.
The study had three primary outcomes. The first was a composite of neonatal outcomes including birth before 37 weeks’ gestation, birth weight of 4,500 g or higher, birth trauma, neonatal respiratory distress, phototherapy, stillbirth or neonatal death, or shoulder dystocia.
The final sample included 748 women for adverse neonatal outcomes, 750 for pregnancy-related hypertension, and 492 for neonatal lean body mass. The mean age of the participants was 32 years; approximately one-third were white European and another third were South Asian. Overall baseline demographics were similar between the groups, and the initial oral glucose tolerance tests were performed at a mean of 15.6 weeks’ gestation.
Overall, 24.9% of women in the early treatment group experienced an adverse neonatal event vs. 30.5% of controls, for an adjusted risk difference of –5.6% and adjusted relative risk of 0.82.
Notably, in an exploratory subgroup analysis, respiratory distress occurred in 9.8% of infants born to women in the immediate treatment group vs. 17.0% of infants in the control group. “Neonatal respiratory distress was the main driver of the between-group difference observed for the first primary outcome,” the researchers wrote. A prespecified subgroup analysis suggested that the impact of an earlier intervention on adverse neonatal outcomes might be greater among women with a higher glycemic value and those whose oral glucose tolerance tests occurred at less than 14 weeks’ gestation, they noted. Stillbirths or neonatal deaths were similar and infrequent in both groups.
Pregnancy-related hypertension occurred in 10.6% of the immediate-treatment group and 9.9% of the controls group (adjusted risk difference, 0.7%). For the third outcome, the mean neonatal lean body mass was 2.86 g in the immediate-treatment group and 2.91 g for the controls (adjusted mean difference, −0.04 g).
No differences in serious adverse events related to either screening or treatment were noted between the groups.
Impact on neonatal outcomes merits further study
Dr. Simmons said that he was surprised by the study findings. “We thought if there was an effect, it would be small, but it isn’t,” he told this publication.
“If you combine the severe adverse outcomes, the perineal trauma and the reduction in days in NICU/special care unit, this is a significant impact on morbidity and likely on cost,” and researchers are currently examining data for cost-effectiveness, he said.
“We did not expect the likely large impact on reducing respiratory distress and perineal trauma,” he noted. “These findings have not been previously reported, perhaps because they were not looked for.” By contrast, “we thought here might be reductions in lower gestational age and cesarean delivery, but there was not,” he added.
The findings were limited by several factors including the nonstandardized approach to gestational diabetes treatment and the use of third-trimester treatment targets that had not been tested in earlier trimesters, the researchers noted. Other limitations included the focus on women already at high risk for hyperglycemia; therefore, the results might not generalize to women not at risk, they wrote.
The current study represents a beginning of answers, with data suggesting that early treatment for gestational diabetes reduces severe adverse pregnancy outcomes, days in NICU/special care unit, and perineal trauma, likely from the first trimester, said Dr. Simmons. However, the findings must be interpreted with caution, as criteria that are too low “might lead to more small babies,” he said. “We look forward to working with others to translate these findings into practice,” he added.
Much more research is needed to answer the many questions prompted by the current study, including who did and did not have complications, Dr. Simmons told this publication. Other studies are needed to collect data on cost-effectiveness, as well as consumer views, especially “different perspectives from different parts of the globe,” he said. Although there is not enough evidence yet to draw conclusions about the role of continuous glucose monitoring (CGM) in managing gestational diabetes, many studies are underway; “we look forward to the results,” of these studies, Dr. Simmons added.
Findings support early screening
Gestational diabetes is one of the most common medical complications of pregnancy, and accounts for more than 80% of diabetes-related diagnoses in pregnancy, said Emily Fay, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, in an interview.
“Previous studies have found that women with gestational diabetes are at higher risk in their pregnancy, including higher chance of developing preeclampsia, higher chance of cesarean delivery, and higher risks for their baby, including risk of shoulder dystocia, birth trauma, and jaundice, and higher birth weights,” she said. “Fortunately, studies have also shown that treatment of gestational diabetes helps lower these risks,” she noted. Currently, patients undergo routine screening for gestational diabetes between 24 and 28 weeks of pregnancy, but some who have risk factors for gestational diabetes may have screening in the early part of pregnancy, said Dr. Fay.
The current findings were not surprising overall, said Dr. Fay, who was not involved in the study. “The study authors looked at a variety of outcomes including neonatal adverse outcomes, neonatal body weight, and pregnancy-related hypertension,” she said.
The researchers found that patients treated early had a lower rate of adverse neonatal outcomes, which was to be expected, Dr. Fay said. “They did not find a difference in neonatal body weight; this also was not surprising, as the women who were not in the early treatment group still received treatment at the time of diagnosis later in pregnancy, which likely helped normalize the weights,” she explained.
“My takeaway from this study is that we should continue to screen patients with risk factors for gestational diabetes early in pregnancy and treat them at the time of diagnosis,” Dr. Fay told this publication. However, barriers that may exist to early treatment involve access to care, including being able to see a provider early in pregnancy, she said. “The treatment for gestational diabetes includes dietary education with diet changes and checking blood sugars frequently. Access to nutrition education can be limited and access to healthy foods can be expensive and difficult to obtain,” she noted. “Checking blood sugars throughout the day can also be difficult for those who are busy or working and who may not have the ability to take time to do this,” she said. However, “these barriers may be overcome by health care reform that improves patient access to and coverage of pregnancy care, improved access and affordability of healthy foods, and employer flexibility to allow the time and space to check blood sugars if needed,” she added.
Looking ahead, the use of continuous glucose monitors in pregnancy is an expanding area of research, said Dr. Fay. “Patients can quickly view their blood sugar without the use of finger sticks, which may help overcome some of the barriers patients may have with using finger sticks,” she noted. “Continuous glucose monitors have been used for those with type 1 and type 2 diabetes with success, and we need to better understand if these can also be helpful in gestational diabetes,” she said. Dr. Fay and colleagues at the University of Washington are currently conducting an ongoing study to explore the use of CGM in gestational diabetes.
The study was supported by the National Health and Medical Research Council, the Region Örebro Research Committee, the Medical Scientific Fund of the Mayor of Vienna, the South Western Sydney Local Health District Academic Unit, and a Western Sydney University Ainsworth Trust Grant. The researchers had no financial conflicts to disclose. Dr. Fay had no relevant financial conflicts to disclose.
Screening and treatment for gestational diabetes are currently recommended at 24-28 weeks’ gestation, with earlier testing recommended for women at increased risk, but the potential benefits of earlier intervention remain debatable, wrote David Simmons, MD, of Western Sydney University, Campbelltown, Australia, and colleagues.
“Until now, there has been complete equipoise over whether to treat hyperglycemia below that of overt diabetes early in pregnancy,” Dr. Simmons said in an interview. The conflicting questions: “Would early treatment reduce the excess deposition of fat on the baby with all of its sequelae; but would early treatment reduce fuel supply to some babies at a critical time and lead to SGA [small for gestational age]?” Dr. Simmons noted.
In a study published in the New England Journal of Medicine, Dr. Simmons and colleagues randomized 406 women aged 18 years and older with singleton pregnancies to immediate treatment for gestational diabetes. Another 396 women were randomized to a control group for deferred treatment or no treatment, based on results of an oral glucose tolerance test at 24-28 weeks’ gestation. All participants had at least one risk factor for hyperglycemia, and met the World Health Organization criteria for gestational diabetes. Women with preexisting diabetes or contraindicating comorbid medical conditions were excluded.
The study had three primary outcomes. The first was a composite of neonatal outcomes including birth before 37 weeks’ gestation, birth weight of 4,500 g or higher, birth trauma, neonatal respiratory distress, phototherapy, stillbirth or neonatal death, or shoulder dystocia.
The final sample included 748 women for adverse neonatal outcomes, 750 for pregnancy-related hypertension, and 492 for neonatal lean body mass. The mean age of the participants was 32 years; approximately one-third were white European and another third were South Asian. Overall baseline demographics were similar between the groups, and the initial oral glucose tolerance tests were performed at a mean of 15.6 weeks’ gestation.
Overall, 24.9% of women in the early treatment group experienced an adverse neonatal event vs. 30.5% of controls, for an adjusted risk difference of –5.6% and adjusted relative risk of 0.82.
Notably, in an exploratory subgroup analysis, respiratory distress occurred in 9.8% of infants born to women in the immediate treatment group vs. 17.0% of infants in the control group. “Neonatal respiratory distress was the main driver of the between-group difference observed for the first primary outcome,” the researchers wrote. A prespecified subgroup analysis suggested that the impact of an earlier intervention on adverse neonatal outcomes might be greater among women with a higher glycemic value and those whose oral glucose tolerance tests occurred at less than 14 weeks’ gestation, they noted. Stillbirths or neonatal deaths were similar and infrequent in both groups.
Pregnancy-related hypertension occurred in 10.6% of the immediate-treatment group and 9.9% of the controls group (adjusted risk difference, 0.7%). For the third outcome, the mean neonatal lean body mass was 2.86 g in the immediate-treatment group and 2.91 g for the controls (adjusted mean difference, −0.04 g).
No differences in serious adverse events related to either screening or treatment were noted between the groups.
Impact on neonatal outcomes merits further study
Dr. Simmons said that he was surprised by the study findings. “We thought if there was an effect, it would be small, but it isn’t,” he told this publication.
“If you combine the severe adverse outcomes, the perineal trauma and the reduction in days in NICU/special care unit, this is a significant impact on morbidity and likely on cost,” and researchers are currently examining data for cost-effectiveness, he said.
“We did not expect the likely large impact on reducing respiratory distress and perineal trauma,” he noted. “These findings have not been previously reported, perhaps because they were not looked for.” By contrast, “we thought here might be reductions in lower gestational age and cesarean delivery, but there was not,” he added.
The findings were limited by several factors including the nonstandardized approach to gestational diabetes treatment and the use of third-trimester treatment targets that had not been tested in earlier trimesters, the researchers noted. Other limitations included the focus on women already at high risk for hyperglycemia; therefore, the results might not generalize to women not at risk, they wrote.
The current study represents a beginning of answers, with data suggesting that early treatment for gestational diabetes reduces severe adverse pregnancy outcomes, days in NICU/special care unit, and perineal trauma, likely from the first trimester, said Dr. Simmons. However, the findings must be interpreted with caution, as criteria that are too low “might lead to more small babies,” he said. “We look forward to working with others to translate these findings into practice,” he added.
Much more research is needed to answer the many questions prompted by the current study, including who did and did not have complications, Dr. Simmons told this publication. Other studies are needed to collect data on cost-effectiveness, as well as consumer views, especially “different perspectives from different parts of the globe,” he said. Although there is not enough evidence yet to draw conclusions about the role of continuous glucose monitoring (CGM) in managing gestational diabetes, many studies are underway; “we look forward to the results,” of these studies, Dr. Simmons added.
Findings support early screening
Gestational diabetes is one of the most common medical complications of pregnancy, and accounts for more than 80% of diabetes-related diagnoses in pregnancy, said Emily Fay, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, in an interview.
“Previous studies have found that women with gestational diabetes are at higher risk in their pregnancy, including higher chance of developing preeclampsia, higher chance of cesarean delivery, and higher risks for their baby, including risk of shoulder dystocia, birth trauma, and jaundice, and higher birth weights,” she said. “Fortunately, studies have also shown that treatment of gestational diabetes helps lower these risks,” she noted. Currently, patients undergo routine screening for gestational diabetes between 24 and 28 weeks of pregnancy, but some who have risk factors for gestational diabetes may have screening in the early part of pregnancy, said Dr. Fay.
The current findings were not surprising overall, said Dr. Fay, who was not involved in the study. “The study authors looked at a variety of outcomes including neonatal adverse outcomes, neonatal body weight, and pregnancy-related hypertension,” she said.
The researchers found that patients treated early had a lower rate of adverse neonatal outcomes, which was to be expected, Dr. Fay said. “They did not find a difference in neonatal body weight; this also was not surprising, as the women who were not in the early treatment group still received treatment at the time of diagnosis later in pregnancy, which likely helped normalize the weights,” she explained.
“My takeaway from this study is that we should continue to screen patients with risk factors for gestational diabetes early in pregnancy and treat them at the time of diagnosis,” Dr. Fay told this publication. However, barriers that may exist to early treatment involve access to care, including being able to see a provider early in pregnancy, she said. “The treatment for gestational diabetes includes dietary education with diet changes and checking blood sugars frequently. Access to nutrition education can be limited and access to healthy foods can be expensive and difficult to obtain,” she noted. “Checking blood sugars throughout the day can also be difficult for those who are busy or working and who may not have the ability to take time to do this,” she said. However, “these barriers may be overcome by health care reform that improves patient access to and coverage of pregnancy care, improved access and affordability of healthy foods, and employer flexibility to allow the time and space to check blood sugars if needed,” she added.
Looking ahead, the use of continuous glucose monitors in pregnancy is an expanding area of research, said Dr. Fay. “Patients can quickly view their blood sugar without the use of finger sticks, which may help overcome some of the barriers patients may have with using finger sticks,” she noted. “Continuous glucose monitors have been used for those with type 1 and type 2 diabetes with success, and we need to better understand if these can also be helpful in gestational diabetes,” she said. Dr. Fay and colleagues at the University of Washington are currently conducting an ongoing study to explore the use of CGM in gestational diabetes.
The study was supported by the National Health and Medical Research Council, the Region Örebro Research Committee, the Medical Scientific Fund of the Mayor of Vienna, the South Western Sydney Local Health District Academic Unit, and a Western Sydney University Ainsworth Trust Grant. The researchers had no financial conflicts to disclose. Dr. Fay had no relevant financial conflicts to disclose.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Transcranial magnetic stimulation during pregnancy: An alternative to antidepressant treatment?
A growing number of women ask about nonpharmacologic approaches for either the treatment of acute perinatal depression or for relapse prevention during pregnancy.
The last several decades have brought an increasing level of comfort with respect to antidepressant use during pregnancy, which derives from several factors.
First, it’s been well described that there’s an increased risk of relapse and morbidity associated with discontinuation of antidepressants proximate to pregnancy, particularly in women with histories of recurrent disease (JAMA Psychiatry. 2023;80[5]:441-50 and JAMA. 2006;295[5]:499-507).
Second, there’s an obvious increased confidence about using antidepressants during pregnancy given the robust reproductive safety data about antidepressants with respect to both teratogenesis and risk for organ malformation. Other studies also fail to demonstrate a relationship between fetal exposure to antidepressants and risk for subsequent development of attention-deficit/hyperactivity disorder (ADHD) and autism. These latter studies have been reviewed extensively in systematic reviews of meta-analyses addressing this question.
However, there are women who, as they approach the question of antidepressant use during pregnancy, would prefer a nonpharmacologic approach to managing depression in the setting of either a planned pregnancy, or sometimes in the setting of acute onset of depressive symptoms during pregnancy. Other women are more comfortable with the data in hand regarding the reproductive safety of antidepressants and continue antidepressants that have afforded emotional well-being, particularly if the road to well-being or euthymia has been a long one.
Still, we at Massachusetts General Hospital (MGH) Center for Women’s Mental Health along with multidisciplinary colleagues with whom we engage during our weekly Virtual Rounds community have observed a growing number of women asking about nonpharmacologic approaches for either the treatment of acute perinatal depression or for relapse prevention during pregnancy. They ask about these options for personal reasons, regardless of what we may know (and what we may not know) about existing pharmacologic interventions. In these scenarios, it is important to keep in mind that it is not about what we as clinicians necessarily know about these medicines per se that drives treatment, but rather about the private calculus that women and their partners apply about risk and benefit of pharmacologic treatment during pregnancy.
Nonpharmacologic treatment options
Mindfulness-based cognitive therapy (MBCT), cognitive behavioral therapy (CBT), and behavioral activation are therapies all of which have an evidence base with respect to their effectiveness for either the acute treatment of both depression (and perinatal depression specifically) or for mitigating risk for depressive relapse (MBCT). Several investigations are underway evaluating digital apps that utilize MBCT and CBT in these patient populations as well.
New treatments for which we have none or exceedingly sparse data to support use during pregnancy are neurosteroids. We are asked all the time about the use of neurosteroids such as brexanolone or zuranolone during pregnancy. Given the data on effectiveness of these agents for treatment of postpartum depression, the question about use during pregnancy is intuitive. But at this point in time, absent data, their use during pregnancy cannot be recommended.
With respect to newer nonpharmacologic approaches that have been looked at for treatment of major depressive disorder, the Food and Drug Administration has approved transcranial magnetic stimulation (TMS), a noninvasive form of neuromodulating therapy that use magnetic pulses to stimulate specific regions of the brain that have been implicated in psychiatric illness.
While there are no safety concerns that have been noted about use of TMS, the data regarding its use during pregnancy are still relatively limited, but it has been used to treat certain neurologic conditions during pregnancy. We now have a small randomized controlled study using TMS during pregnancy and multiple small case series suggesting a signal of efficacy in women with perinatal major depressive disorder. Side effects of TMS use during pregnancy have included hypotension, which has sometimes required repositioning of subjects, particularly later in pregnancy. Unlike electroconvulsive therapy, (ECT), often used when clinicians have exhausted other treatment options, TMS has no risk of seizure associated with its use.
TMS is now entering into the clinical arena in a more robust way. In certain settings, insurance companies are reimbursing for TMS treatment more often than was the case previously, making it a more viable option for a larger number of patients. There are also several exciting newer protocols, including theta burst stimulation, a new form of TMS treatment with less of a time commitment, and which may be more cost effective. However, data on this modality of treatment remain limited.
Where TMS fits in treating depression during pregnancy
The real question we are getting asked in clinic, both in person and during virtual rounds with multidisciplinary colleagues from across the world, is where TMS might fit into the algorithm for treating of depression during pregnancy. Where is it appropriate to be thinking about TMS in pregnancy, and where should it perhaps be deferred at this moment (and where is it not appropriate)?
It is probably of limited value (and possibly of potential harm) to switch to TMS in patients who have severe recurrent major depression and who are on maintenance antidepressant, and who believe that a switch to TMS will be effective for relapse prevention; there are simply no data currently suggesting that TMS can be used as a relapse prevention tool, unlike certain other nonpharmacologic interventions.
What about managing relapse of major depressive disorder during pregnancy in a patient who had responded to an antidepressant? We have seen patients with histories of severe recurrent disease who are managed well on antidepressants during pregnancy who then have breakthrough symptoms and inquire about using TMS as an augmentation strategy. Although we don’t have clear data supporting the use of TMS as an adjunct in that setting, in those patients, one could argue that a trial of TMS may be appropriate – as opposed to introducing multiple medicines to recapture euthymia during pregnancy where the benefit is unclear and where more exposure is implied by having to do potentially multiple trials.
Other patients with new onset of depression during pregnancy who, for personal reasons, will not take an antidepressant or pursue other nonpharmacologic interventions will frequently ask about TMS. and the increased availability of TMS in the community in various centers – as opposed to previously where it was more restricted to large academic medical centers.
I think it is a time of excitement in reproductive psychiatry where we have a growing number of tools to treat perinatal depression – from medications to digital tools. These tools – either alone or in combination with medicines that we’ve been using for years – are able to afford women a greater number of choices with respect to the treatment of perinatal depression than was available even 5 years ago. That takes us closer to an ability to use interventions that truly combine patient wishes and “precision perinatal psychiatry,” where we can match effective therapies with the individual clinical presentations and wishes with which patients come to us.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
A growing number of women ask about nonpharmacologic approaches for either the treatment of acute perinatal depression or for relapse prevention during pregnancy.
The last several decades have brought an increasing level of comfort with respect to antidepressant use during pregnancy, which derives from several factors.
First, it’s been well described that there’s an increased risk of relapse and morbidity associated with discontinuation of antidepressants proximate to pregnancy, particularly in women with histories of recurrent disease (JAMA Psychiatry. 2023;80[5]:441-50 and JAMA. 2006;295[5]:499-507).
Second, there’s an obvious increased confidence about using antidepressants during pregnancy given the robust reproductive safety data about antidepressants with respect to both teratogenesis and risk for organ malformation. Other studies also fail to demonstrate a relationship between fetal exposure to antidepressants and risk for subsequent development of attention-deficit/hyperactivity disorder (ADHD) and autism. These latter studies have been reviewed extensively in systematic reviews of meta-analyses addressing this question.
However, there are women who, as they approach the question of antidepressant use during pregnancy, would prefer a nonpharmacologic approach to managing depression in the setting of either a planned pregnancy, or sometimes in the setting of acute onset of depressive symptoms during pregnancy. Other women are more comfortable with the data in hand regarding the reproductive safety of antidepressants and continue antidepressants that have afforded emotional well-being, particularly if the road to well-being or euthymia has been a long one.
Still, we at Massachusetts General Hospital (MGH) Center for Women’s Mental Health along with multidisciplinary colleagues with whom we engage during our weekly Virtual Rounds community have observed a growing number of women asking about nonpharmacologic approaches for either the treatment of acute perinatal depression or for relapse prevention during pregnancy. They ask about these options for personal reasons, regardless of what we may know (and what we may not know) about existing pharmacologic interventions. In these scenarios, it is important to keep in mind that it is not about what we as clinicians necessarily know about these medicines per se that drives treatment, but rather about the private calculus that women and their partners apply about risk and benefit of pharmacologic treatment during pregnancy.
Nonpharmacologic treatment options
Mindfulness-based cognitive therapy (MBCT), cognitive behavioral therapy (CBT), and behavioral activation are therapies all of which have an evidence base with respect to their effectiveness for either the acute treatment of both depression (and perinatal depression specifically) or for mitigating risk for depressive relapse (MBCT). Several investigations are underway evaluating digital apps that utilize MBCT and CBT in these patient populations as well.
New treatments for which we have none or exceedingly sparse data to support use during pregnancy are neurosteroids. We are asked all the time about the use of neurosteroids such as brexanolone or zuranolone during pregnancy. Given the data on effectiveness of these agents for treatment of postpartum depression, the question about use during pregnancy is intuitive. But at this point in time, absent data, their use during pregnancy cannot be recommended.
With respect to newer nonpharmacologic approaches that have been looked at for treatment of major depressive disorder, the Food and Drug Administration has approved transcranial magnetic stimulation (TMS), a noninvasive form of neuromodulating therapy that use magnetic pulses to stimulate specific regions of the brain that have been implicated in psychiatric illness.
While there are no safety concerns that have been noted about use of TMS, the data regarding its use during pregnancy are still relatively limited, but it has been used to treat certain neurologic conditions during pregnancy. We now have a small randomized controlled study using TMS during pregnancy and multiple small case series suggesting a signal of efficacy in women with perinatal major depressive disorder. Side effects of TMS use during pregnancy have included hypotension, which has sometimes required repositioning of subjects, particularly later in pregnancy. Unlike electroconvulsive therapy, (ECT), often used when clinicians have exhausted other treatment options, TMS has no risk of seizure associated with its use.
TMS is now entering into the clinical arena in a more robust way. In certain settings, insurance companies are reimbursing for TMS treatment more often than was the case previously, making it a more viable option for a larger number of patients. There are also several exciting newer protocols, including theta burst stimulation, a new form of TMS treatment with less of a time commitment, and which may be more cost effective. However, data on this modality of treatment remain limited.
Where TMS fits in treating depression during pregnancy
The real question we are getting asked in clinic, both in person and during virtual rounds with multidisciplinary colleagues from across the world, is where TMS might fit into the algorithm for treating of depression during pregnancy. Where is it appropriate to be thinking about TMS in pregnancy, and where should it perhaps be deferred at this moment (and where is it not appropriate)?
It is probably of limited value (and possibly of potential harm) to switch to TMS in patients who have severe recurrent major depression and who are on maintenance antidepressant, and who believe that a switch to TMS will be effective for relapse prevention; there are simply no data currently suggesting that TMS can be used as a relapse prevention tool, unlike certain other nonpharmacologic interventions.
What about managing relapse of major depressive disorder during pregnancy in a patient who had responded to an antidepressant? We have seen patients with histories of severe recurrent disease who are managed well on antidepressants during pregnancy who then have breakthrough symptoms and inquire about using TMS as an augmentation strategy. Although we don’t have clear data supporting the use of TMS as an adjunct in that setting, in those patients, one could argue that a trial of TMS may be appropriate – as opposed to introducing multiple medicines to recapture euthymia during pregnancy where the benefit is unclear and where more exposure is implied by having to do potentially multiple trials.
Other patients with new onset of depression during pregnancy who, for personal reasons, will not take an antidepressant or pursue other nonpharmacologic interventions will frequently ask about TMS. and the increased availability of TMS in the community in various centers – as opposed to previously where it was more restricted to large academic medical centers.
I think it is a time of excitement in reproductive psychiatry where we have a growing number of tools to treat perinatal depression – from medications to digital tools. These tools – either alone or in combination with medicines that we’ve been using for years – are able to afford women a greater number of choices with respect to the treatment of perinatal depression than was available even 5 years ago. That takes us closer to an ability to use interventions that truly combine patient wishes and “precision perinatal psychiatry,” where we can match effective therapies with the individual clinical presentations and wishes with which patients come to us.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
A growing number of women ask about nonpharmacologic approaches for either the treatment of acute perinatal depression or for relapse prevention during pregnancy.
The last several decades have brought an increasing level of comfort with respect to antidepressant use during pregnancy, which derives from several factors.
First, it’s been well described that there’s an increased risk of relapse and morbidity associated with discontinuation of antidepressants proximate to pregnancy, particularly in women with histories of recurrent disease (JAMA Psychiatry. 2023;80[5]:441-50 and JAMA. 2006;295[5]:499-507).
Second, there’s an obvious increased confidence about using antidepressants during pregnancy given the robust reproductive safety data about antidepressants with respect to both teratogenesis and risk for organ malformation. Other studies also fail to demonstrate a relationship between fetal exposure to antidepressants and risk for subsequent development of attention-deficit/hyperactivity disorder (ADHD) and autism. These latter studies have been reviewed extensively in systematic reviews of meta-analyses addressing this question.
However, there are women who, as they approach the question of antidepressant use during pregnancy, would prefer a nonpharmacologic approach to managing depression in the setting of either a planned pregnancy, or sometimes in the setting of acute onset of depressive symptoms during pregnancy. Other women are more comfortable with the data in hand regarding the reproductive safety of antidepressants and continue antidepressants that have afforded emotional well-being, particularly if the road to well-being or euthymia has been a long one.
Still, we at Massachusetts General Hospital (MGH) Center for Women’s Mental Health along with multidisciplinary colleagues with whom we engage during our weekly Virtual Rounds community have observed a growing number of women asking about nonpharmacologic approaches for either the treatment of acute perinatal depression or for relapse prevention during pregnancy. They ask about these options for personal reasons, regardless of what we may know (and what we may not know) about existing pharmacologic interventions. In these scenarios, it is important to keep in mind that it is not about what we as clinicians necessarily know about these medicines per se that drives treatment, but rather about the private calculus that women and their partners apply about risk and benefit of pharmacologic treatment during pregnancy.
Nonpharmacologic treatment options
Mindfulness-based cognitive therapy (MBCT), cognitive behavioral therapy (CBT), and behavioral activation are therapies all of which have an evidence base with respect to their effectiveness for either the acute treatment of both depression (and perinatal depression specifically) or for mitigating risk for depressive relapse (MBCT). Several investigations are underway evaluating digital apps that utilize MBCT and CBT in these patient populations as well.
New treatments for which we have none or exceedingly sparse data to support use during pregnancy are neurosteroids. We are asked all the time about the use of neurosteroids such as brexanolone or zuranolone during pregnancy. Given the data on effectiveness of these agents for treatment of postpartum depression, the question about use during pregnancy is intuitive. But at this point in time, absent data, their use during pregnancy cannot be recommended.
With respect to newer nonpharmacologic approaches that have been looked at for treatment of major depressive disorder, the Food and Drug Administration has approved transcranial magnetic stimulation (TMS), a noninvasive form of neuromodulating therapy that use magnetic pulses to stimulate specific regions of the brain that have been implicated in psychiatric illness.
While there are no safety concerns that have been noted about use of TMS, the data regarding its use during pregnancy are still relatively limited, but it has been used to treat certain neurologic conditions during pregnancy. We now have a small randomized controlled study using TMS during pregnancy and multiple small case series suggesting a signal of efficacy in women with perinatal major depressive disorder. Side effects of TMS use during pregnancy have included hypotension, which has sometimes required repositioning of subjects, particularly later in pregnancy. Unlike electroconvulsive therapy, (ECT), often used when clinicians have exhausted other treatment options, TMS has no risk of seizure associated with its use.
TMS is now entering into the clinical arena in a more robust way. In certain settings, insurance companies are reimbursing for TMS treatment more often than was the case previously, making it a more viable option for a larger number of patients. There are also several exciting newer protocols, including theta burst stimulation, a new form of TMS treatment with less of a time commitment, and which may be more cost effective. However, data on this modality of treatment remain limited.
Where TMS fits in treating depression during pregnancy
The real question we are getting asked in clinic, both in person and during virtual rounds with multidisciplinary colleagues from across the world, is where TMS might fit into the algorithm for treating of depression during pregnancy. Where is it appropriate to be thinking about TMS in pregnancy, and where should it perhaps be deferred at this moment (and where is it not appropriate)?
It is probably of limited value (and possibly of potential harm) to switch to TMS in patients who have severe recurrent major depression and who are on maintenance antidepressant, and who believe that a switch to TMS will be effective for relapse prevention; there are simply no data currently suggesting that TMS can be used as a relapse prevention tool, unlike certain other nonpharmacologic interventions.
What about managing relapse of major depressive disorder during pregnancy in a patient who had responded to an antidepressant? We have seen patients with histories of severe recurrent disease who are managed well on antidepressants during pregnancy who then have breakthrough symptoms and inquire about using TMS as an augmentation strategy. Although we don’t have clear data supporting the use of TMS as an adjunct in that setting, in those patients, one could argue that a trial of TMS may be appropriate – as opposed to introducing multiple medicines to recapture euthymia during pregnancy where the benefit is unclear and where more exposure is implied by having to do potentially multiple trials.
Other patients with new onset of depression during pregnancy who, for personal reasons, will not take an antidepressant or pursue other nonpharmacologic interventions will frequently ask about TMS. and the increased availability of TMS in the community in various centers – as opposed to previously where it was more restricted to large academic medical centers.
I think it is a time of excitement in reproductive psychiatry where we have a growing number of tools to treat perinatal depression – from medications to digital tools. These tools – either alone or in combination with medicines that we’ve been using for years – are able to afford women a greater number of choices with respect to the treatment of perinatal depression than was available even 5 years ago. That takes us closer to an ability to use interventions that truly combine patient wishes and “precision perinatal psychiatry,” where we can match effective therapies with the individual clinical presentations and wishes with which patients come to us.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The nation’s health secretary has this obstetrician on call
She’s seen progress, albeit slow, over three decades, yet the number of maternal deaths each year continues to rise.
Luckily, she’s got the ear of President Joe Biden’s health secretary.
Dr. Reyes, 64, is married to Health and Human Services Secretary Xavier Becerra, who is championing the administration’s initiative to require all states to provide Medicaid coverage to mothers for a year after giving birth. In March, the Centers for Disease Control and Prevention released data showing a 40% increase in U.S. maternal deaths from 2020 to 2021. The mortality rate among Black women was 2.6 times that of white women, no matter their economic status.
Over the years, Mr. Becerra has spoken highly of his wife’s expertise, but she downplays her influence, saying her husband of nearly 35 years “had it in him to begin with” to improve health care for women and to demand fewer pregnancy-related deaths. She, too, describes the nation’s high maternal mortality rate as unacceptable and preventable.
Dr. Reyes, a Latina who grew up as one of eight children in California’s agricultural heartland, now practices perinatology at the University of California, Davis. She is a member of a California Department of Public Health panel that reviews cases of maternal deaths and recommends improvements. And she chairs the board of the California Health Care Foundation, a nonprofit that works to increase health care access. (California Healthline is an editorially independent service of the California Health Care Foundation.)
Her work has been a blend of medicine and advocacy, and she worries recent federal court rulings will erode hard-fought victories regarding the safety of pregnant women and their babies. She discussed the nation’s maternal health crisis and health care disparities in an interview, which has been edited for length and clarity.
Question: When did you first realize there are disparities in the health care system?
Answer: When I was in high school in the Fresno Unified School District, we were under a consent decree to desegregate. And I was, at the time, student body president at Roosevelt High School. I was asked to be on this unified school district desegregation task force, where the district had to come up with a plan.
It was a time when I really had incredible exposure to how policies are made at a larger level, societal level, that really determine where people live, where they can seek health care, where they go to school. That experience had a tremendous impact on my life in terms of what I wanted to do in a career and how to give back.
Q: The U.S. has one of the best health care systems in the world, yet the maternal mortality rate is high compared with other developed countries. Why do think that is?
A. What we know by the CDC and maternal mortality review committees is that about 60% of maternal deaths are considered preventable. And that’s really been a lot of what I’ve tried to focus on: What can we do to reduce the severity of disease? Or what can we do within the role that we play in maternal health that can reduce that?
We know that there are societal issues absolutely that increase women’s risks and there are public health issues. But there’s a role that hospitals play in helping reduce that risk. Ten years ago, I was on the maternal mortality review committee for the state of California when we started reviewing cases of women who died within hospital systems to see, “Is there a role that we can play in a hospital system to reduce that risk?”
We recognized that sometimes there were conditions that were not recognized early enough so that there was a delay in the care. Sometimes there was a misdiagnosis. Or in some hospital systems, especially rural systems where there aren’t as many resources, sometimes there was the lack of specialists available. So, we’ve identified these risks and said, “We can do something about them.”
Q: You served on a federal panel 20 years ago that published a groundbreaking report identifying racism in health care. It seems as if we could be much further along.
A. The purpose of that committee was to really answer the question: Do patients receive a different level of care based on race? Looking back, we knew there was something there, but we really didn’t know. And it took months for the committee to come to that agreement, that there was a difference. I mean, that was honestly monumental, because we just didn’t have that level of consensus before. And so just to say “That treatment is unequal and it’s unacceptable” was really profound.
We thought that the 700-page report was going to be a time period where there was going to be tremendous movement, and I think I’ve learned over 20 years that change doesn’t happen quickly, especially when providers and health systems don’t see that they play a role. It’s like … “OK, so maybe it exists, but not for me.”
We all saw George Floyd and how he was treated. And during COVID we saw a tremendous difference in who was dying, right? Underrepresented minorities – certainly much higher. It was that culmination that made us realize the elephant in the room. We can’t ignore that this does exist, that there is a difference in how people are treated, even in our health care system.
Q: When addressing racism in health care, you talk about diversifying not just the health care workforce, but also the boardrooms of hospitals and health systems. Why is that important?
A. At the board level, change is hard. But we all play a role because leadership really helps determine much of what’s carried out. So, to have a leadership that is understanding and representative of the communities they serve, I think it has been demonstrated that we do make a difference.
Q: As a health care provider, do you have a wish list of policies you’d like the government to take up?
A. There was tremendous effort around offering preventive health services as a part of what was covered under the Affordable Care Act. And individuals exhaled, finally thinking this is a tremendous win, especially for women in pregnancy. Because we fought for preventive health services to help them have access so they can prepare for their pregnancy. So, for women, this was huge. But now with the Texas federal court ruling that the U.S. Preventive Services Task Force didn’t have any authority, it is a tremendous step backward.
We have culturally, linguistically appropriate standards in place, but it’s a matter in terms of how they’re carried out by state and by individual hospital systems. My wish list is that we really do listen to our patients, speak to them in a language of their choice, and provide them written materials in the language of their choice. We don’t fully do that.
Q: You mentioned one Texas ruling on the ACA. What’s your take on the ruling by another Texas judge suspending the abortion pill? And the U.S. Supreme Court’s overturning of Roe v. Wade?A. As a maternal-fetal medicine specialist who tries to help women plan for pregnancies, those rulings are a tremendous setback.
Q: And what about women of color? Will they find access to abortion services more difficult?
A. Oh, absolutely. When we speak of underrepresented minorities or those with less resources, they have less resources to then seek the appropriate care. Some women may have the opportunity to go to a different state or seek care elsewhere if their state doesn’t provide it. Many women just don’t have those resources to devote to them and don’t have a choice. So, we will see that disparity widen.
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
She’s seen progress, albeit slow, over three decades, yet the number of maternal deaths each year continues to rise.
Luckily, she’s got the ear of President Joe Biden’s health secretary.
Dr. Reyes, 64, is married to Health and Human Services Secretary Xavier Becerra, who is championing the administration’s initiative to require all states to provide Medicaid coverage to mothers for a year after giving birth. In March, the Centers for Disease Control and Prevention released data showing a 40% increase in U.S. maternal deaths from 2020 to 2021. The mortality rate among Black women was 2.6 times that of white women, no matter their economic status.
Over the years, Mr. Becerra has spoken highly of his wife’s expertise, but she downplays her influence, saying her husband of nearly 35 years “had it in him to begin with” to improve health care for women and to demand fewer pregnancy-related deaths. She, too, describes the nation’s high maternal mortality rate as unacceptable and preventable.
Dr. Reyes, a Latina who grew up as one of eight children in California’s agricultural heartland, now practices perinatology at the University of California, Davis. She is a member of a California Department of Public Health panel that reviews cases of maternal deaths and recommends improvements. And she chairs the board of the California Health Care Foundation, a nonprofit that works to increase health care access. (California Healthline is an editorially independent service of the California Health Care Foundation.)
Her work has been a blend of medicine and advocacy, and she worries recent federal court rulings will erode hard-fought victories regarding the safety of pregnant women and their babies. She discussed the nation’s maternal health crisis and health care disparities in an interview, which has been edited for length and clarity.
Question: When did you first realize there are disparities in the health care system?
Answer: When I was in high school in the Fresno Unified School District, we were under a consent decree to desegregate. And I was, at the time, student body president at Roosevelt High School. I was asked to be on this unified school district desegregation task force, where the district had to come up with a plan.
It was a time when I really had incredible exposure to how policies are made at a larger level, societal level, that really determine where people live, where they can seek health care, where they go to school. That experience had a tremendous impact on my life in terms of what I wanted to do in a career and how to give back.
Q: The U.S. has one of the best health care systems in the world, yet the maternal mortality rate is high compared with other developed countries. Why do think that is?
A. What we know by the CDC and maternal mortality review committees is that about 60% of maternal deaths are considered preventable. And that’s really been a lot of what I’ve tried to focus on: What can we do to reduce the severity of disease? Or what can we do within the role that we play in maternal health that can reduce that?
We know that there are societal issues absolutely that increase women’s risks and there are public health issues. But there’s a role that hospitals play in helping reduce that risk. Ten years ago, I was on the maternal mortality review committee for the state of California when we started reviewing cases of women who died within hospital systems to see, “Is there a role that we can play in a hospital system to reduce that risk?”
We recognized that sometimes there were conditions that were not recognized early enough so that there was a delay in the care. Sometimes there was a misdiagnosis. Or in some hospital systems, especially rural systems where there aren’t as many resources, sometimes there was the lack of specialists available. So, we’ve identified these risks and said, “We can do something about them.”
Q: You served on a federal panel 20 years ago that published a groundbreaking report identifying racism in health care. It seems as if we could be much further along.
A. The purpose of that committee was to really answer the question: Do patients receive a different level of care based on race? Looking back, we knew there was something there, but we really didn’t know. And it took months for the committee to come to that agreement, that there was a difference. I mean, that was honestly monumental, because we just didn’t have that level of consensus before. And so just to say “That treatment is unequal and it’s unacceptable” was really profound.
We thought that the 700-page report was going to be a time period where there was going to be tremendous movement, and I think I’ve learned over 20 years that change doesn’t happen quickly, especially when providers and health systems don’t see that they play a role. It’s like … “OK, so maybe it exists, but not for me.”
We all saw George Floyd and how he was treated. And during COVID we saw a tremendous difference in who was dying, right? Underrepresented minorities – certainly much higher. It was that culmination that made us realize the elephant in the room. We can’t ignore that this does exist, that there is a difference in how people are treated, even in our health care system.
Q: When addressing racism in health care, you talk about diversifying not just the health care workforce, but also the boardrooms of hospitals and health systems. Why is that important?
A. At the board level, change is hard. But we all play a role because leadership really helps determine much of what’s carried out. So, to have a leadership that is understanding and representative of the communities they serve, I think it has been demonstrated that we do make a difference.
Q: As a health care provider, do you have a wish list of policies you’d like the government to take up?
A. There was tremendous effort around offering preventive health services as a part of what was covered under the Affordable Care Act. And individuals exhaled, finally thinking this is a tremendous win, especially for women in pregnancy. Because we fought for preventive health services to help them have access so they can prepare for their pregnancy. So, for women, this was huge. But now with the Texas federal court ruling that the U.S. Preventive Services Task Force didn’t have any authority, it is a tremendous step backward.
We have culturally, linguistically appropriate standards in place, but it’s a matter in terms of how they’re carried out by state and by individual hospital systems. My wish list is that we really do listen to our patients, speak to them in a language of their choice, and provide them written materials in the language of their choice. We don’t fully do that.
Q: You mentioned one Texas ruling on the ACA. What’s your take on the ruling by another Texas judge suspending the abortion pill? And the U.S. Supreme Court’s overturning of Roe v. Wade?A. As a maternal-fetal medicine specialist who tries to help women plan for pregnancies, those rulings are a tremendous setback.
Q: And what about women of color? Will they find access to abortion services more difficult?
A. Oh, absolutely. When we speak of underrepresented minorities or those with less resources, they have less resources to then seek the appropriate care. Some women may have the opportunity to go to a different state or seek care elsewhere if their state doesn’t provide it. Many women just don’t have those resources to devote to them and don’t have a choice. So, we will see that disparity widen.
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
She’s seen progress, albeit slow, over three decades, yet the number of maternal deaths each year continues to rise.
Luckily, she’s got the ear of President Joe Biden’s health secretary.
Dr. Reyes, 64, is married to Health and Human Services Secretary Xavier Becerra, who is championing the administration’s initiative to require all states to provide Medicaid coverage to mothers for a year after giving birth. In March, the Centers for Disease Control and Prevention released data showing a 40% increase in U.S. maternal deaths from 2020 to 2021. The mortality rate among Black women was 2.6 times that of white women, no matter their economic status.
Over the years, Mr. Becerra has spoken highly of his wife’s expertise, but she downplays her influence, saying her husband of nearly 35 years “had it in him to begin with” to improve health care for women and to demand fewer pregnancy-related deaths. She, too, describes the nation’s high maternal mortality rate as unacceptable and preventable.
Dr. Reyes, a Latina who grew up as one of eight children in California’s agricultural heartland, now practices perinatology at the University of California, Davis. She is a member of a California Department of Public Health panel that reviews cases of maternal deaths and recommends improvements. And she chairs the board of the California Health Care Foundation, a nonprofit that works to increase health care access. (California Healthline is an editorially independent service of the California Health Care Foundation.)
Her work has been a blend of medicine and advocacy, and she worries recent federal court rulings will erode hard-fought victories regarding the safety of pregnant women and their babies. She discussed the nation’s maternal health crisis and health care disparities in an interview, which has been edited for length and clarity.
Question: When did you first realize there are disparities in the health care system?
Answer: When I was in high school in the Fresno Unified School District, we were under a consent decree to desegregate. And I was, at the time, student body president at Roosevelt High School. I was asked to be on this unified school district desegregation task force, where the district had to come up with a plan.
It was a time when I really had incredible exposure to how policies are made at a larger level, societal level, that really determine where people live, where they can seek health care, where they go to school. That experience had a tremendous impact on my life in terms of what I wanted to do in a career and how to give back.
Q: The U.S. has one of the best health care systems in the world, yet the maternal mortality rate is high compared with other developed countries. Why do think that is?
A. What we know by the CDC and maternal mortality review committees is that about 60% of maternal deaths are considered preventable. And that’s really been a lot of what I’ve tried to focus on: What can we do to reduce the severity of disease? Or what can we do within the role that we play in maternal health that can reduce that?
We know that there are societal issues absolutely that increase women’s risks and there are public health issues. But there’s a role that hospitals play in helping reduce that risk. Ten years ago, I was on the maternal mortality review committee for the state of California when we started reviewing cases of women who died within hospital systems to see, “Is there a role that we can play in a hospital system to reduce that risk?”
We recognized that sometimes there were conditions that were not recognized early enough so that there was a delay in the care. Sometimes there was a misdiagnosis. Or in some hospital systems, especially rural systems where there aren’t as many resources, sometimes there was the lack of specialists available. So, we’ve identified these risks and said, “We can do something about them.”
Q: You served on a federal panel 20 years ago that published a groundbreaking report identifying racism in health care. It seems as if we could be much further along.
A. The purpose of that committee was to really answer the question: Do patients receive a different level of care based on race? Looking back, we knew there was something there, but we really didn’t know. And it took months for the committee to come to that agreement, that there was a difference. I mean, that was honestly monumental, because we just didn’t have that level of consensus before. And so just to say “That treatment is unequal and it’s unacceptable” was really profound.
We thought that the 700-page report was going to be a time period where there was going to be tremendous movement, and I think I’ve learned over 20 years that change doesn’t happen quickly, especially when providers and health systems don’t see that they play a role. It’s like … “OK, so maybe it exists, but not for me.”
We all saw George Floyd and how he was treated. And during COVID we saw a tremendous difference in who was dying, right? Underrepresented minorities – certainly much higher. It was that culmination that made us realize the elephant in the room. We can’t ignore that this does exist, that there is a difference in how people are treated, even in our health care system.
Q: When addressing racism in health care, you talk about diversifying not just the health care workforce, but also the boardrooms of hospitals and health systems. Why is that important?
A. At the board level, change is hard. But we all play a role because leadership really helps determine much of what’s carried out. So, to have a leadership that is understanding and representative of the communities they serve, I think it has been demonstrated that we do make a difference.
Q: As a health care provider, do you have a wish list of policies you’d like the government to take up?
A. There was tremendous effort around offering preventive health services as a part of what was covered under the Affordable Care Act. And individuals exhaled, finally thinking this is a tremendous win, especially for women in pregnancy. Because we fought for preventive health services to help them have access so they can prepare for their pregnancy. So, for women, this was huge. But now with the Texas federal court ruling that the U.S. Preventive Services Task Force didn’t have any authority, it is a tremendous step backward.
We have culturally, linguistically appropriate standards in place, but it’s a matter in terms of how they’re carried out by state and by individual hospital systems. My wish list is that we really do listen to our patients, speak to them in a language of their choice, and provide them written materials in the language of their choice. We don’t fully do that.
Q: You mentioned one Texas ruling on the ACA. What’s your take on the ruling by another Texas judge suspending the abortion pill? And the U.S. Supreme Court’s overturning of Roe v. Wade?A. As a maternal-fetal medicine specialist who tries to help women plan for pregnancies, those rulings are a tremendous setback.
Q: And what about women of color? Will they find access to abortion services more difficult?
A. Oh, absolutely. When we speak of underrepresented minorities or those with less resources, they have less resources to then seek the appropriate care. Some women may have the opportunity to go to a different state or seek care elsewhere if their state doesn’t provide it. Many women just don’t have those resources to devote to them and don’t have a choice. So, we will see that disparity widen.
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
Experts outline comprehensive preeclampsia prevention strategy
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Understanding clinic-reported IVF success rates
The field of assisted reproductive technologies (ART) continues to evolve from its first successful birth in 1978 in England, and then in 1981 in the United States. Over the last 6 years, the total number of cycles in the U.S. has increased by 44% to nearly 370,000.
SART membership consists of more than 350 clinics throughout the United States, representing 80% of ART clinics. Over 95% of ART cycles in 2021 in the United States were performed in SART-member clinics.
SART is an invaluable resource for both patients and physicians. Their website includes a “Predict My Success” calculator that allows patients and physicians to enter individualized data to calculate the chance of having a baby over one or more complete cycles of IVF. To help us understand the pregnancy outcome data from ART – cycles per clinic along with national results – I posed the questions below to Amy Sparks, PhD, HCLD, director of the IVF and Andrology Laboratories and the Center for Advanced Reproductive Care at University of Iowa Hospitals and Clinics, Iowa City. Dr. Sparks is past president of SART and former chairperson of the SART Registry committee when the current Clinic Summary Report format was initially released.
Question: The Fertility Clinic Success Rate and Certification Act (FCSRCA) of 1992 mandated that all ART clinics report success rate data to the federal government, through the Centers for Disease Control and Prevention, in a standardized manner. As ART is the only field in medicine to be required to annually report their patient outcomes, that is, all initiated cycles and live births, why do you believe this law was enacted and is limited to reproductive medicine?
Answer: The FCSRCA of 1992 was enacted in response to the lack of open and reliable pregnancy success rate information for patients seeking infertility care using assisted reproductive technologies. Success rates of 25%-50% were being advertised by independent clinics when, nationally, fewer than 15% of ART procedures led to live births. The Federal Trade Commission said such claims were deceptive and filed charges against five clinics, saying they misrepresented their success in helping women become pregnant. The government won one case by court order and the other four cases were settled out of court.
This field of medicine was in the spotlight as the majority of patients lacked insurance coverage for their ART cycles, and there was a strong desire to protect consumers paying out of pocket for relatively low success. Recognizing that the FTC’s mission is to ensure truth in advertising and not regulate medical care, Congress passed the FCSRCA, mandating that all centers providing ART services report all initiated cycles and their outcomes. The CDC was appointed as the agency responsible for collecting cycle data and reporting outcomes. Centers not reporting their cycles are listed as nonreporting centers.
This act also established standards for accreditation of embryology laboratories including personnel and traditional clinical laboratory management requirements. These standards serve as the foundation for embryology laboratory accrediting agencies.
Q: Why have live-birth rates on SART appeared to be focused on “per IVF cycle” as opposed to the CDC reporting of live births “per embryo transfer?”
A: An ART cycle “start” is defined as the initiation of ovarian stimulation with medication that may or may not include administration of exogenous gonadotropins, followed by oocyte retrieval and embryo transfer. Not every patient beginning a cycle will undergo an oocyte retrieval and not all patients who undergo oocyte retrieval have an embryo transfer. The live-birth rates (LBR) for each of these steps of progression in the ART process are available in the SART and CDC reports.
In 2016, SART recognized that practices were foregoing fresh embryo transfer after oocyte retrieval, opting to cryopreserve all embryos to either accommodate genetic testing of the embryos prior to transfer or to avoid embryo transfer to an unfavorable uterine environment. In response to changes in practice and in an effort to deemphasize live birth per transfer, thereby alleviating a potential motivator or pressure for practitioners to transfer multiple embryos, SART moved to a report that displays the cumulative live-birth rate per cycle start for oocyte retrieval. The cumulative live-birth rate per cycle start for oocyte retrieval is the chance of live birth from transfers of embryos derived from the oocyte retrieval and performed within 1 year of the oocyte retrieval.
This change in reporting further reduced the pressure to transfer multiple embryos and encouraged elective, single-embryo transfer. The outcome per transfer is no longer the report’s primary focus.
Q: The latest pregnancy outcomes statistics are from the year 2020 and are finalized by the CDC. Why does the SART website have this same year labeled “preliminary” outcomes?
A: Shortly after the 2016 SART report change, the CDC made similar changes to their report. The difference is that SART provides a “preliminary” report of outcomes within the year of the cycle start for oocyte retrieval. The cumulative outcome is not “finalized” until the following year as transfers may be performed as late as 12 months after the oocyte retrieval.
SART has opted to report both the “preliminary” or interim outcome and the “final” outcome a year later. The CDC has opted to limit their report to “final” outcomes. I’m happy to report that SART recently released the final report for 2021 cycles.
Q: Have national success rates in the United States continued to rise or have they plateaued?
A: It appears that success rates have plateaued; however, we find ourselves at another point where practice patterns and patients’ approach to using ART for family building have changed.
Recognizing the impact of maternal aging on reproductive potential, patients are opting to undergo multiple ART cycles to cryopreserve embryos for family building before they attempt to get pregnant. This family-building path reduces the value of measuring the LBR per cycle start as we may not know the outcome for many years. SART leaders are deliberating intently as to how to best represent this growing patient population in outcome reporting.
Q: Can you comment on the reduction of multiple gestations with the increasing use of single-embryo transfer?
A: The reduction in emphasis on live births per transfer, emphasis on singleton live-birth rates in both the SART and CDC reports, and American Society for Reproductive Medicine practice committee guidelines strongly supporting single embryo transfer have significantly reduced the rate of multiple gestations.
A decade ago, only a third of the transfers were single-embryo transfers and over 25% of live births resulted in a multiple birth. Today, the majority of embryo transfers are elective, single-embryo transfers, and the multiple birth rate has been reduced by nearly 80%. In 2020, 93% of live births from IVF were singletons.
Q: SART offers an online IVF calculator so both patients and physicians can plug in data for an approximate cumulative success rate for up to three IVF cycles. The calculator pools data from all U.S.-reporting IVF centers. Can you explain what an “IVF cycle” is and what patient information is required? Why do success rates increase over time?
A: Each “IVF cycle” is a cycle start for an oocyte retrieval and all transfers of embryos from that cycle within a year of the oocyte retrieval. If the first cycle and subsequent transfers do not lead to a live birth, patients still have a chance to achieve a live birth with a second or third cycle. The success rate increases over time as it reflects the chance of success for a population of patients, with some achieving a live birth after the first cycle and additional patients who achieve success following their third cycle.
Q: The SART IVF calculator can be used with no prior IVF cycles or following an unsuccessful cycle. Are there data to support an estimation of outcome following two or even more unsuccessful cycles?
A: The variables in the SART IVF calculator are based upon the cycle-specific data from patients seeking care at SART member clinics. The current predictor was built with data from cycles performed in 2015-2016. SART is adjusting the predictor and developing a calculator that will be routinely updated, accordingly.
Q: Only approximately 40% of states have some form of infertility coverage law in place; however the number of IVF cycles in the United States continues to increase on an annual basis. What do you think are the driving factors behind this?
A: Advocacy efforts to improve patients’ access to infertility care have included giving patients tools to encourage their employers to include infertility care in their health care benefits package. More recently, the “Great Resignation” has led to the “Great Recruitment” and employers are recognizing that the addition of infertility care to health care benefits is a powerful recruitment tool.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
The field of assisted reproductive technologies (ART) continues to evolve from its first successful birth in 1978 in England, and then in 1981 in the United States. Over the last 6 years, the total number of cycles in the U.S. has increased by 44% to nearly 370,000.
SART membership consists of more than 350 clinics throughout the United States, representing 80% of ART clinics. Over 95% of ART cycles in 2021 in the United States were performed in SART-member clinics.
SART is an invaluable resource for both patients and physicians. Their website includes a “Predict My Success” calculator that allows patients and physicians to enter individualized data to calculate the chance of having a baby over one or more complete cycles of IVF. To help us understand the pregnancy outcome data from ART – cycles per clinic along with national results – I posed the questions below to Amy Sparks, PhD, HCLD, director of the IVF and Andrology Laboratories and the Center for Advanced Reproductive Care at University of Iowa Hospitals and Clinics, Iowa City. Dr. Sparks is past president of SART and former chairperson of the SART Registry committee when the current Clinic Summary Report format was initially released.
Question: The Fertility Clinic Success Rate and Certification Act (FCSRCA) of 1992 mandated that all ART clinics report success rate data to the federal government, through the Centers for Disease Control and Prevention, in a standardized manner. As ART is the only field in medicine to be required to annually report their patient outcomes, that is, all initiated cycles and live births, why do you believe this law was enacted and is limited to reproductive medicine?
Answer: The FCSRCA of 1992 was enacted in response to the lack of open and reliable pregnancy success rate information for patients seeking infertility care using assisted reproductive technologies. Success rates of 25%-50% were being advertised by independent clinics when, nationally, fewer than 15% of ART procedures led to live births. The Federal Trade Commission said such claims were deceptive and filed charges against five clinics, saying they misrepresented their success in helping women become pregnant. The government won one case by court order and the other four cases were settled out of court.
This field of medicine was in the spotlight as the majority of patients lacked insurance coverage for their ART cycles, and there was a strong desire to protect consumers paying out of pocket for relatively low success. Recognizing that the FTC’s mission is to ensure truth in advertising and not regulate medical care, Congress passed the FCSRCA, mandating that all centers providing ART services report all initiated cycles and their outcomes. The CDC was appointed as the agency responsible for collecting cycle data and reporting outcomes. Centers not reporting their cycles are listed as nonreporting centers.
This act also established standards for accreditation of embryology laboratories including personnel and traditional clinical laboratory management requirements. These standards serve as the foundation for embryology laboratory accrediting agencies.
Q: Why have live-birth rates on SART appeared to be focused on “per IVF cycle” as opposed to the CDC reporting of live births “per embryo transfer?”
A: An ART cycle “start” is defined as the initiation of ovarian stimulation with medication that may or may not include administration of exogenous gonadotropins, followed by oocyte retrieval and embryo transfer. Not every patient beginning a cycle will undergo an oocyte retrieval and not all patients who undergo oocyte retrieval have an embryo transfer. The live-birth rates (LBR) for each of these steps of progression in the ART process are available in the SART and CDC reports.
In 2016, SART recognized that practices were foregoing fresh embryo transfer after oocyte retrieval, opting to cryopreserve all embryos to either accommodate genetic testing of the embryos prior to transfer or to avoid embryo transfer to an unfavorable uterine environment. In response to changes in practice and in an effort to deemphasize live birth per transfer, thereby alleviating a potential motivator or pressure for practitioners to transfer multiple embryos, SART moved to a report that displays the cumulative live-birth rate per cycle start for oocyte retrieval. The cumulative live-birth rate per cycle start for oocyte retrieval is the chance of live birth from transfers of embryos derived from the oocyte retrieval and performed within 1 year of the oocyte retrieval.
This change in reporting further reduced the pressure to transfer multiple embryos and encouraged elective, single-embryo transfer. The outcome per transfer is no longer the report’s primary focus.
Q: The latest pregnancy outcomes statistics are from the year 2020 and are finalized by the CDC. Why does the SART website have this same year labeled “preliminary” outcomes?
A: Shortly after the 2016 SART report change, the CDC made similar changes to their report. The difference is that SART provides a “preliminary” report of outcomes within the year of the cycle start for oocyte retrieval. The cumulative outcome is not “finalized” until the following year as transfers may be performed as late as 12 months after the oocyte retrieval.
SART has opted to report both the “preliminary” or interim outcome and the “final” outcome a year later. The CDC has opted to limit their report to “final” outcomes. I’m happy to report that SART recently released the final report for 2021 cycles.
Q: Have national success rates in the United States continued to rise or have they plateaued?
A: It appears that success rates have plateaued; however, we find ourselves at another point where practice patterns and patients’ approach to using ART for family building have changed.
Recognizing the impact of maternal aging on reproductive potential, patients are opting to undergo multiple ART cycles to cryopreserve embryos for family building before they attempt to get pregnant. This family-building path reduces the value of measuring the LBR per cycle start as we may not know the outcome for many years. SART leaders are deliberating intently as to how to best represent this growing patient population in outcome reporting.
Q: Can you comment on the reduction of multiple gestations with the increasing use of single-embryo transfer?
A: The reduction in emphasis on live births per transfer, emphasis on singleton live-birth rates in both the SART and CDC reports, and American Society for Reproductive Medicine practice committee guidelines strongly supporting single embryo transfer have significantly reduced the rate of multiple gestations.
A decade ago, only a third of the transfers were single-embryo transfers and over 25% of live births resulted in a multiple birth. Today, the majority of embryo transfers are elective, single-embryo transfers, and the multiple birth rate has been reduced by nearly 80%. In 2020, 93% of live births from IVF were singletons.
Q: SART offers an online IVF calculator so both patients and physicians can plug in data for an approximate cumulative success rate for up to three IVF cycles. The calculator pools data from all U.S.-reporting IVF centers. Can you explain what an “IVF cycle” is and what patient information is required? Why do success rates increase over time?
A: Each “IVF cycle” is a cycle start for an oocyte retrieval and all transfers of embryos from that cycle within a year of the oocyte retrieval. If the first cycle and subsequent transfers do not lead to a live birth, patients still have a chance to achieve a live birth with a second or third cycle. The success rate increases over time as it reflects the chance of success for a population of patients, with some achieving a live birth after the first cycle and additional patients who achieve success following their third cycle.
Q: The SART IVF calculator can be used with no prior IVF cycles or following an unsuccessful cycle. Are there data to support an estimation of outcome following two or even more unsuccessful cycles?
A: The variables in the SART IVF calculator are based upon the cycle-specific data from patients seeking care at SART member clinics. The current predictor was built with data from cycles performed in 2015-2016. SART is adjusting the predictor and developing a calculator that will be routinely updated, accordingly.
Q: Only approximately 40% of states have some form of infertility coverage law in place; however the number of IVF cycles in the United States continues to increase on an annual basis. What do you think are the driving factors behind this?
A: Advocacy efforts to improve patients’ access to infertility care have included giving patients tools to encourage their employers to include infertility care in their health care benefits package. More recently, the “Great Resignation” has led to the “Great Recruitment” and employers are recognizing that the addition of infertility care to health care benefits is a powerful recruitment tool.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
The field of assisted reproductive technologies (ART) continues to evolve from its first successful birth in 1978 in England, and then in 1981 in the United States. Over the last 6 years, the total number of cycles in the U.S. has increased by 44% to nearly 370,000.
SART membership consists of more than 350 clinics throughout the United States, representing 80% of ART clinics. Over 95% of ART cycles in 2021 in the United States were performed in SART-member clinics.
SART is an invaluable resource for both patients and physicians. Their website includes a “Predict My Success” calculator that allows patients and physicians to enter individualized data to calculate the chance of having a baby over one or more complete cycles of IVF. To help us understand the pregnancy outcome data from ART – cycles per clinic along with national results – I posed the questions below to Amy Sparks, PhD, HCLD, director of the IVF and Andrology Laboratories and the Center for Advanced Reproductive Care at University of Iowa Hospitals and Clinics, Iowa City. Dr. Sparks is past president of SART and former chairperson of the SART Registry committee when the current Clinic Summary Report format was initially released.
Question: The Fertility Clinic Success Rate and Certification Act (FCSRCA) of 1992 mandated that all ART clinics report success rate data to the federal government, through the Centers for Disease Control and Prevention, in a standardized manner. As ART is the only field in medicine to be required to annually report their patient outcomes, that is, all initiated cycles and live births, why do you believe this law was enacted and is limited to reproductive medicine?
Answer: The FCSRCA of 1992 was enacted in response to the lack of open and reliable pregnancy success rate information for patients seeking infertility care using assisted reproductive technologies. Success rates of 25%-50% were being advertised by independent clinics when, nationally, fewer than 15% of ART procedures led to live births. The Federal Trade Commission said such claims were deceptive and filed charges against five clinics, saying they misrepresented their success in helping women become pregnant. The government won one case by court order and the other four cases were settled out of court.
This field of medicine was in the spotlight as the majority of patients lacked insurance coverage for their ART cycles, and there was a strong desire to protect consumers paying out of pocket for relatively low success. Recognizing that the FTC’s mission is to ensure truth in advertising and not regulate medical care, Congress passed the FCSRCA, mandating that all centers providing ART services report all initiated cycles and their outcomes. The CDC was appointed as the agency responsible for collecting cycle data and reporting outcomes. Centers not reporting their cycles are listed as nonreporting centers.
This act also established standards for accreditation of embryology laboratories including personnel and traditional clinical laboratory management requirements. These standards serve as the foundation for embryology laboratory accrediting agencies.
Q: Why have live-birth rates on SART appeared to be focused on “per IVF cycle” as opposed to the CDC reporting of live births “per embryo transfer?”
A: An ART cycle “start” is defined as the initiation of ovarian stimulation with medication that may or may not include administration of exogenous gonadotropins, followed by oocyte retrieval and embryo transfer. Not every patient beginning a cycle will undergo an oocyte retrieval and not all patients who undergo oocyte retrieval have an embryo transfer. The live-birth rates (LBR) for each of these steps of progression in the ART process are available in the SART and CDC reports.
In 2016, SART recognized that practices were foregoing fresh embryo transfer after oocyte retrieval, opting to cryopreserve all embryos to either accommodate genetic testing of the embryos prior to transfer or to avoid embryo transfer to an unfavorable uterine environment. In response to changes in practice and in an effort to deemphasize live birth per transfer, thereby alleviating a potential motivator or pressure for practitioners to transfer multiple embryos, SART moved to a report that displays the cumulative live-birth rate per cycle start for oocyte retrieval. The cumulative live-birth rate per cycle start for oocyte retrieval is the chance of live birth from transfers of embryos derived from the oocyte retrieval and performed within 1 year of the oocyte retrieval.
This change in reporting further reduced the pressure to transfer multiple embryos and encouraged elective, single-embryo transfer. The outcome per transfer is no longer the report’s primary focus.
Q: The latest pregnancy outcomes statistics are from the year 2020 and are finalized by the CDC. Why does the SART website have this same year labeled “preliminary” outcomes?
A: Shortly after the 2016 SART report change, the CDC made similar changes to their report. The difference is that SART provides a “preliminary” report of outcomes within the year of the cycle start for oocyte retrieval. The cumulative outcome is not “finalized” until the following year as transfers may be performed as late as 12 months after the oocyte retrieval.
SART has opted to report both the “preliminary” or interim outcome and the “final” outcome a year later. The CDC has opted to limit their report to “final” outcomes. I’m happy to report that SART recently released the final report for 2021 cycles.
Q: Have national success rates in the United States continued to rise or have they plateaued?
A: It appears that success rates have plateaued; however, we find ourselves at another point where practice patterns and patients’ approach to using ART for family building have changed.
Recognizing the impact of maternal aging on reproductive potential, patients are opting to undergo multiple ART cycles to cryopreserve embryos for family building before they attempt to get pregnant. This family-building path reduces the value of measuring the LBR per cycle start as we may not know the outcome for many years. SART leaders are deliberating intently as to how to best represent this growing patient population in outcome reporting.
Q: Can you comment on the reduction of multiple gestations with the increasing use of single-embryo transfer?
A: The reduction in emphasis on live births per transfer, emphasis on singleton live-birth rates in both the SART and CDC reports, and American Society for Reproductive Medicine practice committee guidelines strongly supporting single embryo transfer have significantly reduced the rate of multiple gestations.
A decade ago, only a third of the transfers were single-embryo transfers and over 25% of live births resulted in a multiple birth. Today, the majority of embryo transfers are elective, single-embryo transfers, and the multiple birth rate has been reduced by nearly 80%. In 2020, 93% of live births from IVF were singletons.
Q: SART offers an online IVF calculator so both patients and physicians can plug in data for an approximate cumulative success rate for up to three IVF cycles. The calculator pools data from all U.S.-reporting IVF centers. Can you explain what an “IVF cycle” is and what patient information is required? Why do success rates increase over time?
A: Each “IVF cycle” is a cycle start for an oocyte retrieval and all transfers of embryos from that cycle within a year of the oocyte retrieval. If the first cycle and subsequent transfers do not lead to a live birth, patients still have a chance to achieve a live birth with a second or third cycle. The success rate increases over time as it reflects the chance of success for a population of patients, with some achieving a live birth after the first cycle and additional patients who achieve success following their third cycle.
Q: The SART IVF calculator can be used with no prior IVF cycles or following an unsuccessful cycle. Are there data to support an estimation of outcome following two or even more unsuccessful cycles?
A: The variables in the SART IVF calculator are based upon the cycle-specific data from patients seeking care at SART member clinics. The current predictor was built with data from cycles performed in 2015-2016. SART is adjusting the predictor and developing a calculator that will be routinely updated, accordingly.
Q: Only approximately 40% of states have some form of infertility coverage law in place; however the number of IVF cycles in the United States continues to increase on an annual basis. What do you think are the driving factors behind this?
A: Advocacy efforts to improve patients’ access to infertility care have included giving patients tools to encourage their employers to include infertility care in their health care benefits package. More recently, the “Great Resignation” has led to the “Great Recruitment” and employers are recognizing that the addition of infertility care to health care benefits is a powerful recruitment tool.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Remote weight monitoring minimizes office visits for newborns
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT PAS 2023