User login
What is the significance of the head-to-body delivery interval in shoulder dystocia?
- Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
Shoulder dystocia is a well-described obstetric complication that occurs in approximately 1% of deliveries.1 It has been associated with adverse maternal outcomes as well as adverse perinatal outcomes, including fracture, nerve palsy, and hypoxic ischemic encephalopathy.
Although multiple risk factors for shoulder dystocia have been described, experts have not yet been able to combine them into an accurate, discriminating, clinically useful shoulder dystocia prediction model; therefore, shoulder dystocia remains an unpredictable event.2 We also lack a strategy to prevent shoulder dystocia. Because we cannot predict or prevent it, a provider’s response to shoulder dystocia, once it occurs, is seminal, in terms of management.
Details of the study
As Lerner and colleagues concisely state, when shoulder dystocia occurs, there is a need for caution in the application of force during maneuvers and a “countervailing need to achieve delivery.” It is in a provider’s interest, then, to have knowledge of whether there is a time at which that countervailing need to achieve delivery takes on greater relative significance.
In an effort to address this issue, the authors examined the relationship between the duration of shoulder dystocia and neonatal depression (defined as the need for cardiopulmonary resuscitation or intubation; a pH level below 7.0; an Apgar score below 6 at 5 minutes; or death).
In their study, 127 births involving uncomplicated shoulder dystocia (i.e., no evidence of neonatal trauma or depression) from a single institution were compared with 55 births involving complicated shoulder dystocia (i.e., the occurrence of brachial plexus palsy with or without neonatal depression).
Lerner and colleagues found a correlation between the duration of shoulder dystocia and the extent of neonatal complications. For example, the median interval from head-to-body delivery for uncomplicated births was 1.0 minute; for births complicated by brachial plexus palsy alone, it was 2.0 minutes; and for births complicated by brachial plexus palsy and neonatal depression, the interval was 5.3 minutes (P <.001). There was no single cutoff, however, that was completely discriminating with regard to whether neonatal depression would occur.
Strengths and weaknesses of the trial
As the authors note, one limitation of their study is a lack of precision in the recorded duration of shoulder dystocia cases, given that “it appears that clinicians often rounded” the stated times.
Other types of bias that may have affected the findings include:
- Selection bias. In an observational study such as this, it is typically ideal to draw the cases and controls from the same underlying population in an effort to limit the occurrence of other potentially confounding factors, both known and unknown. In this study, however, the uncomplicated cases came from one institution over 10 years, whereas the complicated cases came from a medicolegal database one author had accumulated over 15 years. Because these clearly are very different populations, the reported association between head-to-body delivery interval and brachial plexus palsy or neonatal depression may be related to characteristics other than, or in addition to, duration of the dystocia. For example, there may have been complicated cases that did not result in legal action. If the duration of the dystocia is in any way related to the chance that medicolegal action occurs, the relationship between duration and the presence of complication will be affected.
- Ascertainment bias. Because this study lacked a standard approach to the recording of duration, ascertainment bias may have affected the results. It is possible, for example, that the knowledge that a complication did or did not occur could have affected whether the duration was recorded or how much time was documented.
Complications of shoulder dystocia are rare
Ultimately, the primary question posed in this article is difficult to answer. Although shoulder dystocia occurs in approximately 1% of births, major adverse perinatal outcomes occur in only a fraction of these cases. That fact means that an event such as permanent brachial plexus palsy or neonatal depression, let alone actual hypoxic ischemic encephalopathy, occurs only in the context of thousands of births.
The data published to date,3,4 including this study, should offer some reassurance to obstetric care providers. Long-term adverse outcomes are uncommon in shoulder dystocia. Even intermediate outcomes such as neonatal depression, when they do occur, appear to be uncommon when the shoulder dystocia is of relatively short duration.
When shoulder dystocia does occur, however, providers should maintain situational awareness, being cognizant of the time that elapses, so that the continuation of appropriate and coordinated maneuvers can be ensured.
WILLIAM A. GROBMAN, MD, MBA
We want to hear from you! Tell us what you think.
1. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetric nightmare. Clin Obstet Gynecol. 2002;45(2):345-362.
2. Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194(3):888-894.
3. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839-842.
4. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474-479.
- Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
Shoulder dystocia is a well-described obstetric complication that occurs in approximately 1% of deliveries.1 It has been associated with adverse maternal outcomes as well as adverse perinatal outcomes, including fracture, nerve palsy, and hypoxic ischemic encephalopathy.
Although multiple risk factors for shoulder dystocia have been described, experts have not yet been able to combine them into an accurate, discriminating, clinically useful shoulder dystocia prediction model; therefore, shoulder dystocia remains an unpredictable event.2 We also lack a strategy to prevent shoulder dystocia. Because we cannot predict or prevent it, a provider’s response to shoulder dystocia, once it occurs, is seminal, in terms of management.
Details of the study
As Lerner and colleagues concisely state, when shoulder dystocia occurs, there is a need for caution in the application of force during maneuvers and a “countervailing need to achieve delivery.” It is in a provider’s interest, then, to have knowledge of whether there is a time at which that countervailing need to achieve delivery takes on greater relative significance.
In an effort to address this issue, the authors examined the relationship between the duration of shoulder dystocia and neonatal depression (defined as the need for cardiopulmonary resuscitation or intubation; a pH level below 7.0; an Apgar score below 6 at 5 minutes; or death).
In their study, 127 births involving uncomplicated shoulder dystocia (i.e., no evidence of neonatal trauma or depression) from a single institution were compared with 55 births involving complicated shoulder dystocia (i.e., the occurrence of brachial plexus palsy with or without neonatal depression).
Lerner and colleagues found a correlation between the duration of shoulder dystocia and the extent of neonatal complications. For example, the median interval from head-to-body delivery for uncomplicated births was 1.0 minute; for births complicated by brachial plexus palsy alone, it was 2.0 minutes; and for births complicated by brachial plexus palsy and neonatal depression, the interval was 5.3 minutes (P <.001). There was no single cutoff, however, that was completely discriminating with regard to whether neonatal depression would occur.
Strengths and weaknesses of the trial
As the authors note, one limitation of their study is a lack of precision in the recorded duration of shoulder dystocia cases, given that “it appears that clinicians often rounded” the stated times.
Other types of bias that may have affected the findings include:
- Selection bias. In an observational study such as this, it is typically ideal to draw the cases and controls from the same underlying population in an effort to limit the occurrence of other potentially confounding factors, both known and unknown. In this study, however, the uncomplicated cases came from one institution over 10 years, whereas the complicated cases came from a medicolegal database one author had accumulated over 15 years. Because these clearly are very different populations, the reported association between head-to-body delivery interval and brachial plexus palsy or neonatal depression may be related to characteristics other than, or in addition to, duration of the dystocia. For example, there may have been complicated cases that did not result in legal action. If the duration of the dystocia is in any way related to the chance that medicolegal action occurs, the relationship between duration and the presence of complication will be affected.
- Ascertainment bias. Because this study lacked a standard approach to the recording of duration, ascertainment bias may have affected the results. It is possible, for example, that the knowledge that a complication did or did not occur could have affected whether the duration was recorded or how much time was documented.
Complications of shoulder dystocia are rare
Ultimately, the primary question posed in this article is difficult to answer. Although shoulder dystocia occurs in approximately 1% of births, major adverse perinatal outcomes occur in only a fraction of these cases. That fact means that an event such as permanent brachial plexus palsy or neonatal depression, let alone actual hypoxic ischemic encephalopathy, occurs only in the context of thousands of births.
The data published to date,3,4 including this study, should offer some reassurance to obstetric care providers. Long-term adverse outcomes are uncommon in shoulder dystocia. Even intermediate outcomes such as neonatal depression, when they do occur, appear to be uncommon when the shoulder dystocia is of relatively short duration.
When shoulder dystocia does occur, however, providers should maintain situational awareness, being cognizant of the time that elapses, so that the continuation of appropriate and coordinated maneuvers can be ensured.
WILLIAM A. GROBMAN, MD, MBA
We want to hear from you! Tell us what you think.
- Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
Shoulder dystocia is a well-described obstetric complication that occurs in approximately 1% of deliveries.1 It has been associated with adverse maternal outcomes as well as adverse perinatal outcomes, including fracture, nerve palsy, and hypoxic ischemic encephalopathy.
Although multiple risk factors for shoulder dystocia have been described, experts have not yet been able to combine them into an accurate, discriminating, clinically useful shoulder dystocia prediction model; therefore, shoulder dystocia remains an unpredictable event.2 We also lack a strategy to prevent shoulder dystocia. Because we cannot predict or prevent it, a provider’s response to shoulder dystocia, once it occurs, is seminal, in terms of management.
Details of the study
As Lerner and colleagues concisely state, when shoulder dystocia occurs, there is a need for caution in the application of force during maneuvers and a “countervailing need to achieve delivery.” It is in a provider’s interest, then, to have knowledge of whether there is a time at which that countervailing need to achieve delivery takes on greater relative significance.
In an effort to address this issue, the authors examined the relationship between the duration of shoulder dystocia and neonatal depression (defined as the need for cardiopulmonary resuscitation or intubation; a pH level below 7.0; an Apgar score below 6 at 5 minutes; or death).
In their study, 127 births involving uncomplicated shoulder dystocia (i.e., no evidence of neonatal trauma or depression) from a single institution were compared with 55 births involving complicated shoulder dystocia (i.e., the occurrence of brachial plexus palsy with or without neonatal depression).
Lerner and colleagues found a correlation between the duration of shoulder dystocia and the extent of neonatal complications. For example, the median interval from head-to-body delivery for uncomplicated births was 1.0 minute; for births complicated by brachial plexus palsy alone, it was 2.0 minutes; and for births complicated by brachial plexus palsy and neonatal depression, the interval was 5.3 minutes (P <.001). There was no single cutoff, however, that was completely discriminating with regard to whether neonatal depression would occur.
Strengths and weaknesses of the trial
As the authors note, one limitation of their study is a lack of precision in the recorded duration of shoulder dystocia cases, given that “it appears that clinicians often rounded” the stated times.
Other types of bias that may have affected the findings include:
- Selection bias. In an observational study such as this, it is typically ideal to draw the cases and controls from the same underlying population in an effort to limit the occurrence of other potentially confounding factors, both known and unknown. In this study, however, the uncomplicated cases came from one institution over 10 years, whereas the complicated cases came from a medicolegal database one author had accumulated over 15 years. Because these clearly are very different populations, the reported association between head-to-body delivery interval and brachial plexus palsy or neonatal depression may be related to characteristics other than, or in addition to, duration of the dystocia. For example, there may have been complicated cases that did not result in legal action. If the duration of the dystocia is in any way related to the chance that medicolegal action occurs, the relationship between duration and the presence of complication will be affected.
- Ascertainment bias. Because this study lacked a standard approach to the recording of duration, ascertainment bias may have affected the results. It is possible, for example, that the knowledge that a complication did or did not occur could have affected whether the duration was recorded or how much time was documented.
Complications of shoulder dystocia are rare
Ultimately, the primary question posed in this article is difficult to answer. Although shoulder dystocia occurs in approximately 1% of births, major adverse perinatal outcomes occur in only a fraction of these cases. That fact means that an event such as permanent brachial plexus palsy or neonatal depression, let alone actual hypoxic ischemic encephalopathy, occurs only in the context of thousands of births.
The data published to date,3,4 including this study, should offer some reassurance to obstetric care providers. Long-term adverse outcomes are uncommon in shoulder dystocia. Even intermediate outcomes such as neonatal depression, when they do occur, appear to be uncommon when the shoulder dystocia is of relatively short duration.
When shoulder dystocia does occur, however, providers should maintain situational awareness, being cognizant of the time that elapses, so that the continuation of appropriate and coordinated maneuvers can be ensured.
WILLIAM A. GROBMAN, MD, MBA
We want to hear from you! Tell us what you think.
1. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetric nightmare. Clin Obstet Gynecol. 2002;45(2):345-362.
2. Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194(3):888-894.
3. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839-842.
4. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474-479.
1. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetric nightmare. Clin Obstet Gynecol. 2002;45(2):345-362.
2. Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194(3):888-894.
3. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839-842.
4. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474-479.
How to repair bladder injury at the time of cesarean delivery
You are performing the fourth cesarean delivery on your patient, a 30-year-old G4P3. The abdominal and uterine incisions go well, and delivery is uneventful. As you prepare to close the uterus, you notice that there is a hole in the bladder 5 cm long. You realize that the injury likely occurred before you made the uterine incision, at the time you mobilized the bladder from the lower uterine segment.
Now, how will you repair the injury to the bladder?
Injury to the bladder or ureter occurs in about 0.3% of cesarean deliveries and about 1% of gynecologic surgical procedures. Bladder injury is more common than injury to the ureter.1,2
Intraoperative recognition of the injury usually permits prompt and successful repair. Delayed recognition of the injury—in the postoperative period—is associated with serious complications, however.
Preoperative planning—that ounce of prevention
Risk factors for cesarean-related bladder injury include:
- repeat delivery
- emergency delivery
- delivery after a protracted second stage of labor.
Bladder injury has been reported to occur in about 0.2% of primary cesarean deliveries and in 0.6% of repeat cesarean deliveries.3
During repeat cesarean delivery, two surgical challenges are often encountered: The bladder 1) is densely adherent to the underlying lower uterine segment and 2) might have healed in a position much higher on the uterus, thereby blocking access to the lower uterine segment.
During emergency cesarean delivery for placental abruption or fetal bradycardia, bladder injury might occur because visualization and dissection of each surgical plane is less than optimal.
In cesarean delivery after a protracted second stage, the vagina—rather than the lower uterine segment—might be incised because of difficulty identifying the interface between uterus and vagina, resulting in an increased risk of bladder injury.
Tips. You can reduce the risk of injury to the bladder as follows:
- Enter the peritoneal cavity at the most superior segment of the abdominal incision; use sharp, rather than blunt, dissection to separate a densely adherent bladder from the uterus.
- When your patient is carrying a large fetus and experiencing a prolonged second stage of labor—and requires cesarean delivery—consider performing a low vertical uterine incision to reduce the risk of extensions into the broad ligament.
Repairing injury to the dome
The bladder comprises the dome superiorly and the base inferiorly. The base of the bladder contains the trigone, which includes the ureters that enter posteriorly and the urethra that exits at the most inferior portion of the bladder (see the FIGURE).
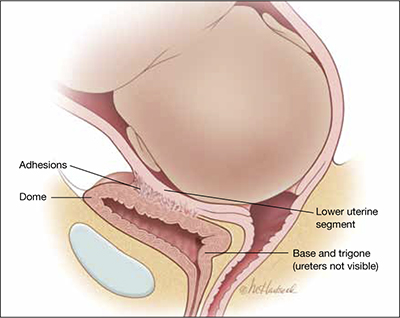
The bladder at delivery: Position and parts
During cesarean delivery, most injuries to the bladder occur in the dome, far from the trigone.During cesarean delivery, most injuries to the bladder occur in the dome, far from the trigone. You can demonstrate this by trying to feel the tip of the Foley catheter bulb through a bladder injury that occurs during cesarean delivery: The trigone is, typically, more than 6 cm—and often more than 10 cm—distant from injury in the dome.
ObGyns are highly qualified to repair an injury in the dome. The surgical steps often used to repair cystotomy in the dome of the bladder include:
- Identify the extent of injury; ensure that it is limited to the dome (that is, does not involve the trigone or is not a large one that extends deep into the posterior bladder).
- Repair the cystotomy in two layers, using absorbable suture. Never use non-absorbable suture because it might act as a nidus for bladder calculi to form. Close the first layer with simple running 3-0 absorbable suture. Close the second layer using running imbricating 2-0 or 3-0 absorbable suture.
- To ensure integrity of the repair and to detect the presence of any other bladder injuries, backfill the bladder with a sterile milk or indigo carmine dye through the Foley catheter.
- The bladder should be drained by Foley catheter for approximately 7 days.
Repairing injury to the trigone or ureters
In most cases, an urologist or urogynecologist should be called in to repair an injury to the trigone or ureter. A possible exception: When it is clear that a stitch has kinked one of the ureters, and removing that stitch resolves the problem.
If you detect a large injury to the bladder—one that involves the posterior wall—consider that the trigone might be injured. Repair of the bladder should then be delayed until a ureteral dye test can be performed.
Ureteral dye test. Inject 5 mL of indigo carmine dye intravenously, and directly observe the flow of dye-stained urine from both ureteral orifices. If you see that dye is flowing from both ureteral orifices and you do not see dye in the retroperitoneal and intra-abdominal spaces, ureteral integrity is likely and you can proceed to repair the injury to the dome.
An alternative test. Urologists often place ureteral catheters under direct visualization, using the injury in the dome to obtain access to the ureteral orifices.4 If a ureteral catheter cannot be advanced to the renal pelvis, a kink or occlusion of the ureter is a possibility. The ureteral catheters not only aid in diagnosing injury but help maintain ureteral patency and prevent ureteral obstruction caused by postoperative swelling.
Ureteral injuries that occur during cesarean delivery often go undetected intraoperatively5; most occur when a transverse uterine incision extends into the broad ligament or vagina. If you suspect ureteral injury and have determined that the bladder is intact, you can take either of two approaches to assessing ureteral patency: 1) perform cystoscopy and insert ureteral catheters in a retrograde manner or 2) incise the dome and insert ureteral catheters under direct visualization.6
After a complex bladder injury has been repaired, intraperitoneal drains may be placed to monitor for extravasation of urine into the peritoneal cavity and to actively drain fluid that might accumulate there.
Postop care The bladder should be drained by Foley catheter for approximately 7 days after the repair. Most experts recommend that a voiding cystogram be performed before the Foley catheter is discontinued; some, however, note that complete healing after repair of a bladder injury at cesarean delivery is to be expected, making a cystogram unnecessary.
Late detection. Postoperative events that might alert you to undetected ureteral injury include pain at the costovertebral angle, oliguria, hematuria, watery vaginal discharge, persistent fever, and abdominal distention.
“I need help!” Sorry—it may not be on the way.
According to the American College of Surgeons, the median age of urologists is older than 50 years7; in many communities, increasing numbers of senior urologists are reluctant to take night and weekend call for emergencies that occur on their hospital’s OB service. Consequently, you and your colleagues might find yourselves compelled to care for a greater number of the urinary tract injuries that occur during cesarean delivery without the immediately available aid of an urologist. But, as I noted, ObGyns are well-trained to take primary responsibility for the repair of most of these injuries.
in his memorable 2011 Editorials
- Consider denosumab for postmenopausal osteoporosis (January)
- hat can “meaningful use” of an EHR mean for your bottom line? (February)
- Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other? (March)
- Stop staring at that Category-II fetal heart-rate tracing … (April)
- Big step forward and downward: An OC with 10 μg of estrogen (May)
- OB and neonatal medicine practices are evolving—in ways that might surprise you (June)
- Have you made best use of the Bakri balloon in PPH? (July)
- Not all contraceptives are suitable immediately postpartum (September)
- Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander (October)
- Insomnia is a troubling and under-treated problem (November)
- How to repair bladder injury at the time of cesarean delivery (December)
1. Gilmour DT, Das S, Flowerdew G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol. 2006;107(6):1366-1372.
2. Phipps MG, Watabe B, Clemons JL, Weitzen S, Myers DL. Risk factors for bladder injury during cesarean delivery. Obstet Gynecol. 2005;105(1):156-160.
3. Eisenkop SM, Richman R, Platt LD, Paul RH. Urinary tract injury during cesarean section. Obstet Gynecol. 1982;60(5):591-596.
4. Yossepowitch O, Baniel J, Livne PM. Urological injuries during cesarean section: intraoperative diagnosis and management. J Urol. 2004;172(1):196-199.
5. Rajasekar D, Hall M. Urinary tract injuries during obstetric intervention. Br J Obstet Gyneaecol. 1997;104(6):731-734.
6. Mendez LE. Iatrogenic injuries in gynecologic cancer surgery. Surg Clin North Am. 2001;81(4):897-923.
7. Walker E, Poley S, Ricketts T. The Aging Surgeon Population. Chapel Hill, NC: American College of Surgeons Health Policy Research Institute; May 2010. http://operationpatientaccess.facs.org/userfiles/file/ACS%20HPRI%20 %20The%20Aging%20Surgeon%20Population%20-%20May%202010.pdf. Accessed November 17, 2011.
You are performing the fourth cesarean delivery on your patient, a 30-year-old G4P3. The abdominal and uterine incisions go well, and delivery is uneventful. As you prepare to close the uterus, you notice that there is a hole in the bladder 5 cm long. You realize that the injury likely occurred before you made the uterine incision, at the time you mobilized the bladder from the lower uterine segment.
Now, how will you repair the injury to the bladder?
Injury to the bladder or ureter occurs in about 0.3% of cesarean deliveries and about 1% of gynecologic surgical procedures. Bladder injury is more common than injury to the ureter.1,2
Intraoperative recognition of the injury usually permits prompt and successful repair. Delayed recognition of the injury—in the postoperative period—is associated with serious complications, however.
Preoperative planning—that ounce of prevention
Risk factors for cesarean-related bladder injury include:
- repeat delivery
- emergency delivery
- delivery after a protracted second stage of labor.
Bladder injury has been reported to occur in about 0.2% of primary cesarean deliveries and in 0.6% of repeat cesarean deliveries.3
During repeat cesarean delivery, two surgical challenges are often encountered: The bladder 1) is densely adherent to the underlying lower uterine segment and 2) might have healed in a position much higher on the uterus, thereby blocking access to the lower uterine segment.
During emergency cesarean delivery for placental abruption or fetal bradycardia, bladder injury might occur because visualization and dissection of each surgical plane is less than optimal.
In cesarean delivery after a protracted second stage, the vagina—rather than the lower uterine segment—might be incised because of difficulty identifying the interface between uterus and vagina, resulting in an increased risk of bladder injury.
Tips. You can reduce the risk of injury to the bladder as follows:
- Enter the peritoneal cavity at the most superior segment of the abdominal incision; use sharp, rather than blunt, dissection to separate a densely adherent bladder from the uterus.
- When your patient is carrying a large fetus and experiencing a prolonged second stage of labor—and requires cesarean delivery—consider performing a low vertical uterine incision to reduce the risk of extensions into the broad ligament.
Repairing injury to the dome
The bladder comprises the dome superiorly and the base inferiorly. The base of the bladder contains the trigone, which includes the ureters that enter posteriorly and the urethra that exits at the most inferior portion of the bladder (see the FIGURE).

The bladder at delivery: Position and parts
During cesarean delivery, most injuries to the bladder occur in the dome, far from the trigone.During cesarean delivery, most injuries to the bladder occur in the dome, far from the trigone. You can demonstrate this by trying to feel the tip of the Foley catheter bulb through a bladder injury that occurs during cesarean delivery: The trigone is, typically, more than 6 cm—and often more than 10 cm—distant from injury in the dome.
ObGyns are highly qualified to repair an injury in the dome. The surgical steps often used to repair cystotomy in the dome of the bladder include:
- Identify the extent of injury; ensure that it is limited to the dome (that is, does not involve the trigone or is not a large one that extends deep into the posterior bladder).
- Repair the cystotomy in two layers, using absorbable suture. Never use non-absorbable suture because it might act as a nidus for bladder calculi to form. Close the first layer with simple running 3-0 absorbable suture. Close the second layer using running imbricating 2-0 or 3-0 absorbable suture.
- To ensure integrity of the repair and to detect the presence of any other bladder injuries, backfill the bladder with a sterile milk or indigo carmine dye through the Foley catheter.
- The bladder should be drained by Foley catheter for approximately 7 days.
Repairing injury to the trigone or ureters
In most cases, an urologist or urogynecologist should be called in to repair an injury to the trigone or ureter. A possible exception: When it is clear that a stitch has kinked one of the ureters, and removing that stitch resolves the problem.
If you detect a large injury to the bladder—one that involves the posterior wall—consider that the trigone might be injured. Repair of the bladder should then be delayed until a ureteral dye test can be performed.
Ureteral dye test. Inject 5 mL of indigo carmine dye intravenously, and directly observe the flow of dye-stained urine from both ureteral orifices. If you see that dye is flowing from both ureteral orifices and you do not see dye in the retroperitoneal and intra-abdominal spaces, ureteral integrity is likely and you can proceed to repair the injury to the dome.
An alternative test. Urologists often place ureteral catheters under direct visualization, using the injury in the dome to obtain access to the ureteral orifices.4 If a ureteral catheter cannot be advanced to the renal pelvis, a kink or occlusion of the ureter is a possibility. The ureteral catheters not only aid in diagnosing injury but help maintain ureteral patency and prevent ureteral obstruction caused by postoperative swelling.
Ureteral injuries that occur during cesarean delivery often go undetected intraoperatively5; most occur when a transverse uterine incision extends into the broad ligament or vagina. If you suspect ureteral injury and have determined that the bladder is intact, you can take either of two approaches to assessing ureteral patency: 1) perform cystoscopy and insert ureteral catheters in a retrograde manner or 2) incise the dome and insert ureteral catheters under direct visualization.6
After a complex bladder injury has been repaired, intraperitoneal drains may be placed to monitor for extravasation of urine into the peritoneal cavity and to actively drain fluid that might accumulate there.
Postop care The bladder should be drained by Foley catheter for approximately 7 days after the repair. Most experts recommend that a voiding cystogram be performed before the Foley catheter is discontinued; some, however, note that complete healing after repair of a bladder injury at cesarean delivery is to be expected, making a cystogram unnecessary.
Late detection. Postoperative events that might alert you to undetected ureteral injury include pain at the costovertebral angle, oliguria, hematuria, watery vaginal discharge, persistent fever, and abdominal distention.
“I need help!” Sorry—it may not be on the way.
According to the American College of Surgeons, the median age of urologists is older than 50 years7; in many communities, increasing numbers of senior urologists are reluctant to take night and weekend call for emergencies that occur on their hospital’s OB service. Consequently, you and your colleagues might find yourselves compelled to care for a greater number of the urinary tract injuries that occur during cesarean delivery without the immediately available aid of an urologist. But, as I noted, ObGyns are well-trained to take primary responsibility for the repair of most of these injuries.
in his memorable 2011 Editorials
- Consider denosumab for postmenopausal osteoporosis (January)
- hat can “meaningful use” of an EHR mean for your bottom line? (February)
- Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other? (March)
- Stop staring at that Category-II fetal heart-rate tracing … (April)
- Big step forward and downward: An OC with 10 μg of estrogen (May)
- OB and neonatal medicine practices are evolving—in ways that might surprise you (June)
- Have you made best use of the Bakri balloon in PPH? (July)
- Not all contraceptives are suitable immediately postpartum (September)
- Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander (October)
- Insomnia is a troubling and under-treated problem (November)
- How to repair bladder injury at the time of cesarean delivery (December)
You are performing the fourth cesarean delivery on your patient, a 30-year-old G4P3. The abdominal and uterine incisions go well, and delivery is uneventful. As you prepare to close the uterus, you notice that there is a hole in the bladder 5 cm long. You realize that the injury likely occurred before you made the uterine incision, at the time you mobilized the bladder from the lower uterine segment.
Now, how will you repair the injury to the bladder?
Injury to the bladder or ureter occurs in about 0.3% of cesarean deliveries and about 1% of gynecologic surgical procedures. Bladder injury is more common than injury to the ureter.1,2
Intraoperative recognition of the injury usually permits prompt and successful repair. Delayed recognition of the injury—in the postoperative period—is associated with serious complications, however.
Preoperative planning—that ounce of prevention
Risk factors for cesarean-related bladder injury include:
- repeat delivery
- emergency delivery
- delivery after a protracted second stage of labor.
Bladder injury has been reported to occur in about 0.2% of primary cesarean deliveries and in 0.6% of repeat cesarean deliveries.3
During repeat cesarean delivery, two surgical challenges are often encountered: The bladder 1) is densely adherent to the underlying lower uterine segment and 2) might have healed in a position much higher on the uterus, thereby blocking access to the lower uterine segment.
During emergency cesarean delivery for placental abruption or fetal bradycardia, bladder injury might occur because visualization and dissection of each surgical plane is less than optimal.
In cesarean delivery after a protracted second stage, the vagina—rather than the lower uterine segment—might be incised because of difficulty identifying the interface between uterus and vagina, resulting in an increased risk of bladder injury.
Tips. You can reduce the risk of injury to the bladder as follows:
- Enter the peritoneal cavity at the most superior segment of the abdominal incision; use sharp, rather than blunt, dissection to separate a densely adherent bladder from the uterus.
- When your patient is carrying a large fetus and experiencing a prolonged second stage of labor—and requires cesarean delivery—consider performing a low vertical uterine incision to reduce the risk of extensions into the broad ligament.
Repairing injury to the dome
The bladder comprises the dome superiorly and the base inferiorly. The base of the bladder contains the trigone, which includes the ureters that enter posteriorly and the urethra that exits at the most inferior portion of the bladder (see the FIGURE).

The bladder at delivery: Position and parts
During cesarean delivery, most injuries to the bladder occur in the dome, far from the trigone.During cesarean delivery, most injuries to the bladder occur in the dome, far from the trigone. You can demonstrate this by trying to feel the tip of the Foley catheter bulb through a bladder injury that occurs during cesarean delivery: The trigone is, typically, more than 6 cm—and often more than 10 cm—distant from injury in the dome.
ObGyns are highly qualified to repair an injury in the dome. The surgical steps often used to repair cystotomy in the dome of the bladder include:
- Identify the extent of injury; ensure that it is limited to the dome (that is, does not involve the trigone or is not a large one that extends deep into the posterior bladder).
- Repair the cystotomy in two layers, using absorbable suture. Never use non-absorbable suture because it might act as a nidus for bladder calculi to form. Close the first layer with simple running 3-0 absorbable suture. Close the second layer using running imbricating 2-0 or 3-0 absorbable suture.
- To ensure integrity of the repair and to detect the presence of any other bladder injuries, backfill the bladder with a sterile milk or indigo carmine dye through the Foley catheter.
- The bladder should be drained by Foley catheter for approximately 7 days.
Repairing injury to the trigone or ureters
In most cases, an urologist or urogynecologist should be called in to repair an injury to the trigone or ureter. A possible exception: When it is clear that a stitch has kinked one of the ureters, and removing that stitch resolves the problem.
If you detect a large injury to the bladder—one that involves the posterior wall—consider that the trigone might be injured. Repair of the bladder should then be delayed until a ureteral dye test can be performed.
Ureteral dye test. Inject 5 mL of indigo carmine dye intravenously, and directly observe the flow of dye-stained urine from both ureteral orifices. If you see that dye is flowing from both ureteral orifices and you do not see dye in the retroperitoneal and intra-abdominal spaces, ureteral integrity is likely and you can proceed to repair the injury to the dome.
An alternative test. Urologists often place ureteral catheters under direct visualization, using the injury in the dome to obtain access to the ureteral orifices.4 If a ureteral catheter cannot be advanced to the renal pelvis, a kink or occlusion of the ureter is a possibility. The ureteral catheters not only aid in diagnosing injury but help maintain ureteral patency and prevent ureteral obstruction caused by postoperative swelling.
Ureteral injuries that occur during cesarean delivery often go undetected intraoperatively5; most occur when a transverse uterine incision extends into the broad ligament or vagina. If you suspect ureteral injury and have determined that the bladder is intact, you can take either of two approaches to assessing ureteral patency: 1) perform cystoscopy and insert ureteral catheters in a retrograde manner or 2) incise the dome and insert ureteral catheters under direct visualization.6
After a complex bladder injury has been repaired, intraperitoneal drains may be placed to monitor for extravasation of urine into the peritoneal cavity and to actively drain fluid that might accumulate there.
Postop care The bladder should be drained by Foley catheter for approximately 7 days after the repair. Most experts recommend that a voiding cystogram be performed before the Foley catheter is discontinued; some, however, note that complete healing after repair of a bladder injury at cesarean delivery is to be expected, making a cystogram unnecessary.
Late detection. Postoperative events that might alert you to undetected ureteral injury include pain at the costovertebral angle, oliguria, hematuria, watery vaginal discharge, persistent fever, and abdominal distention.
“I need help!” Sorry—it may not be on the way.
According to the American College of Surgeons, the median age of urologists is older than 50 years7; in many communities, increasing numbers of senior urologists are reluctant to take night and weekend call for emergencies that occur on their hospital’s OB service. Consequently, you and your colleagues might find yourselves compelled to care for a greater number of the urinary tract injuries that occur during cesarean delivery without the immediately available aid of an urologist. But, as I noted, ObGyns are well-trained to take primary responsibility for the repair of most of these injuries.
in his memorable 2011 Editorials
- Consider denosumab for postmenopausal osteoporosis (January)
- hat can “meaningful use” of an EHR mean for your bottom line? (February)
- Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other? (March)
- Stop staring at that Category-II fetal heart-rate tracing … (April)
- Big step forward and downward: An OC with 10 μg of estrogen (May)
- OB and neonatal medicine practices are evolving—in ways that might surprise you (June)
- Have you made best use of the Bakri balloon in PPH? (July)
- Not all contraceptives are suitable immediately postpartum (September)
- Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander (October)
- Insomnia is a troubling and under-treated problem (November)
- How to repair bladder injury at the time of cesarean delivery (December)
1. Gilmour DT, Das S, Flowerdew G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol. 2006;107(6):1366-1372.
2. Phipps MG, Watabe B, Clemons JL, Weitzen S, Myers DL. Risk factors for bladder injury during cesarean delivery. Obstet Gynecol. 2005;105(1):156-160.
3. Eisenkop SM, Richman R, Platt LD, Paul RH. Urinary tract injury during cesarean section. Obstet Gynecol. 1982;60(5):591-596.
4. Yossepowitch O, Baniel J, Livne PM. Urological injuries during cesarean section: intraoperative diagnosis and management. J Urol. 2004;172(1):196-199.
5. Rajasekar D, Hall M. Urinary tract injuries during obstetric intervention. Br J Obstet Gyneaecol. 1997;104(6):731-734.
6. Mendez LE. Iatrogenic injuries in gynecologic cancer surgery. Surg Clin North Am. 2001;81(4):897-923.
7. Walker E, Poley S, Ricketts T. The Aging Surgeon Population. Chapel Hill, NC: American College of Surgeons Health Policy Research Institute; May 2010. http://operationpatientaccess.facs.org/userfiles/file/ACS%20HPRI%20 %20The%20Aging%20Surgeon%20Population%20-%20May%202010.pdf. Accessed November 17, 2011.
1. Gilmour DT, Das S, Flowerdew G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol. 2006;107(6):1366-1372.
2. Phipps MG, Watabe B, Clemons JL, Weitzen S, Myers DL. Risk factors for bladder injury during cesarean delivery. Obstet Gynecol. 2005;105(1):156-160.
3. Eisenkop SM, Richman R, Platt LD, Paul RH. Urinary tract injury during cesarean section. Obstet Gynecol. 1982;60(5):591-596.
4. Yossepowitch O, Baniel J, Livne PM. Urological injuries during cesarean section: intraoperative diagnosis and management. J Urol. 2004;172(1):196-199.
5. Rajasekar D, Hall M. Urinary tract injuries during obstetric intervention. Br J Obstet Gyneaecol. 1997;104(6):731-734.
6. Mendez LE. Iatrogenic injuries in gynecologic cancer surgery. Surg Clin North Am. 2001;81(4):897-923.
7. Walker E, Poley S, Ricketts T. The Aging Surgeon Population. Chapel Hill, NC: American College of Surgeons Health Policy Research Institute; May 2010. http://operationpatientaccess.facs.org/userfiles/file/ACS%20HPRI%20 %20The%20Aging%20Surgeon%20Population%20-%20May%202010.pdf. Accessed November 17, 2011.
Purity of Compounded 17P Is Questioned
The Food and Drug Administration is reviewing the results of tests conducted by the manufacturer of Makena, the approved version of a compounded product for preventing preterm births, which the company said has identified some problems with other compounded versions.
Makena, a compounded formulation of 17-alpha hydroxyprogesterone caproate (17P), was approved by the FDA in February 2011, providing for the first time an approved version of product that had previously been compounded in local pharmacies and used to reduce the risk of certain preterm births in patients who had already experienced a prior preterm birth.
The Nov. 8 FDA statement says that Makena manufacturer K-V Pharmaceutical provided information about tests that identified “variability” in the purity and potency of bulk hydroxyprogesterone caproate and compounded products that use it as the active ingredient. The FDA “has not validated or otherwise confirmed” these results, but “has carefully reviewed the data and will conduct an onsite review of the laboratory analyses.” In addition, the FDA has begun “its own sampling and analysis of compounded hydroxyprogesterone caproate products and the bulk [active pharmaceutical ingredients] used to make them,” a process that is ongoing, according to the statement.
“In the meantime, we remind physicians and patients that before approving the Makena new drug application, FDA reviewed manufacturing information, such as the source of the [active pharmaceutical ingredients] used by its manufacturer, proposed manufacturing processes, and the firm's adherence to current good manufacturing practice,” the statement said, adding: “Therefore, as with other approved drugs, greater assurance of safety and effectiveness is generally provided by the approved product than by a compounded product.”
Soon after Makena was approved, the initial enthusiasm over the availability of a more reliable product was tempered once it became clear that Makena would cost much more than the previously available products, eliciting concerns that this would affect access to the product among poor women, who are considered at greatest risk of preterm labor.
Despite the availability of an approved product, the FDA released a statement in March that said, “in order to support access to this important drug,” the agency had no plans to take enforcement actions against pharmacies when there was a valid prescription for hydroxyprogesterone caproate for an individual patient, unless there were problems with the quality or safety of the product, or they were not being compounded in accordance with the appropriate standards for compounding sterile products. The FDA issued that statement after learning that the company had sent letters to pharmacists saying that the agency would no longer exercise such discretion, according to the current statement.
The Food and Drug Administration is reviewing the results of tests conducted by the manufacturer of Makena, the approved version of a compounded product for preventing preterm births, which the company said has identified some problems with other compounded versions.
Makena, a compounded formulation of 17-alpha hydroxyprogesterone caproate (17P), was approved by the FDA in February 2011, providing for the first time an approved version of product that had previously been compounded in local pharmacies and used to reduce the risk of certain preterm births in patients who had already experienced a prior preterm birth.
The Nov. 8 FDA statement says that Makena manufacturer K-V Pharmaceutical provided information about tests that identified “variability” in the purity and potency of bulk hydroxyprogesterone caproate and compounded products that use it as the active ingredient. The FDA “has not validated or otherwise confirmed” these results, but “has carefully reviewed the data and will conduct an onsite review of the laboratory analyses.” In addition, the FDA has begun “its own sampling and analysis of compounded hydroxyprogesterone caproate products and the bulk [active pharmaceutical ingredients] used to make them,” a process that is ongoing, according to the statement.
“In the meantime, we remind physicians and patients that before approving the Makena new drug application, FDA reviewed manufacturing information, such as the source of the [active pharmaceutical ingredients] used by its manufacturer, proposed manufacturing processes, and the firm's adherence to current good manufacturing practice,” the statement said, adding: “Therefore, as with other approved drugs, greater assurance of safety and effectiveness is generally provided by the approved product than by a compounded product.”
Soon after Makena was approved, the initial enthusiasm over the availability of a more reliable product was tempered once it became clear that Makena would cost much more than the previously available products, eliciting concerns that this would affect access to the product among poor women, who are considered at greatest risk of preterm labor.
Despite the availability of an approved product, the FDA released a statement in March that said, “in order to support access to this important drug,” the agency had no plans to take enforcement actions against pharmacies when there was a valid prescription for hydroxyprogesterone caproate for an individual patient, unless there were problems with the quality or safety of the product, or they were not being compounded in accordance with the appropriate standards for compounding sterile products. The FDA issued that statement after learning that the company had sent letters to pharmacists saying that the agency would no longer exercise such discretion, according to the current statement.
The Food and Drug Administration is reviewing the results of tests conducted by the manufacturer of Makena, the approved version of a compounded product for preventing preterm births, which the company said has identified some problems with other compounded versions.
Makena, a compounded formulation of 17-alpha hydroxyprogesterone caproate (17P), was approved by the FDA in February 2011, providing for the first time an approved version of product that had previously been compounded in local pharmacies and used to reduce the risk of certain preterm births in patients who had already experienced a prior preterm birth.
The Nov. 8 FDA statement says that Makena manufacturer K-V Pharmaceutical provided information about tests that identified “variability” in the purity and potency of bulk hydroxyprogesterone caproate and compounded products that use it as the active ingredient. The FDA “has not validated or otherwise confirmed” these results, but “has carefully reviewed the data and will conduct an onsite review of the laboratory analyses.” In addition, the FDA has begun “its own sampling and analysis of compounded hydroxyprogesterone caproate products and the bulk [active pharmaceutical ingredients] used to make them,” a process that is ongoing, according to the statement.
“In the meantime, we remind physicians and patients that before approving the Makena new drug application, FDA reviewed manufacturing information, such as the source of the [active pharmaceutical ingredients] used by its manufacturer, proposed manufacturing processes, and the firm's adherence to current good manufacturing practice,” the statement said, adding: “Therefore, as with other approved drugs, greater assurance of safety and effectiveness is generally provided by the approved product than by a compounded product.”
Soon after Makena was approved, the initial enthusiasm over the availability of a more reliable product was tempered once it became clear that Makena would cost much more than the previously available products, eliciting concerns that this would affect access to the product among poor women, who are considered at greatest risk of preterm labor.
Despite the availability of an approved product, the FDA released a statement in March that said, “in order to support access to this important drug,” the agency had no plans to take enforcement actions against pharmacies when there was a valid prescription for hydroxyprogesterone caproate for an individual patient, unless there were problems with the quality or safety of the product, or they were not being compounded in accordance with the appropriate standards for compounding sterile products. The FDA issued that statement after learning that the company had sent letters to pharmacists saying that the agency would no longer exercise such discretion, according to the current statement.
From the Food and Drug Administration
Foley Catheter Bests Prostaglandin E2 Gel
Use of a Foley catheter for labor induction in women with an unfavorable cervix at term resulted in a similar cesarean section rate to use of prostaglandin E2 gel for labor induction, but with fewer maternal and neonatal side effects, according to findings from an open-label randomized controlled trial involving more than 800 women.
Cesarean section, most often done for failure to progress during the first stage of labor, was performed in 23% of 411 women induced using a Foley catheter, and in 20% of 408 women induced using vaginal prostaglandin E2 gel (relative risk, 1.13), Dr. Marta Jozwiak of Groene Hart Hospital, Gouda, the Netherlands, and her colleagues from the PROBAAT Study Group reported.
The frequency of vaginal instrumental deliveries was similar in the two groups as well (11% and 13% in the Foley catheter and prostaglandin E2 groups, respectively), the investigators said (Lancet 2011 [doi:10.1016/S0140-6736(11)61484-0]).
Two serious maternal adverse events occurred, both in the prostaglandin group. These included one uterine perforation after insertion of an intrauterine pressure catheter and one uterine rupture during oxytocin augmentation, they said, noting that both neonates were born in good clinical condition, but were admitted to the neonatal ward with suspected infection.
Four minor maternal adverse events occurred, including three allergic reactions (in one woman in the Foley catheter group and in two women in the prostaglandin E2 group), and blood loss on a second insertion of the catheter in one woman. Also, the rate of suspected intrapartum infection was significantly lower in the Foley catheter group (1% vs. 3%).
As for neonatal adverse events, the rate of admissions to the neonatal ward was significantly higher in the prostaglandin E2 group (12% vs. 20%), Dr. Jozwiak and her associates said.
Patients in the PROBAAT trial included women beyond 37 weeks' gestation with a singleton pregnancy in cephalic presentation, with intact membranes and an unfavorable cervix as defined by a Bishop score less than 6. The women were enrolled at 12 centers throughout the Netherlands between Feb. 10, 2009, and May 17, 2010, and all had an indication for labor induction, and had no prior cesarean sections.
The findings in regard to cesarean section rates when labor induction is performed using a Foley catheter vs. prostaglandin E2 were confirmed by a meta-analysis that included data from this study. The meta-analysis also demonstrated that Foley catheter induction was associated with reduced rates of hyperstimulation (odds ratio, 0.44) and postpartum hemorrhage (OR, 0.60), the investigators reported, noting that although the use of a Foley catheter for labor induction did not increase the vaginal delivery rate when compared with use of prostaglandin E2 as they had hypothesized, the findings nonetheless support the use of Foley catheters.
“After induction with a Foley catheter, the overall number of operative deliveries for suspected fetal distress was lower, fewer mothers were treated with intrapartum antibiotics, and significantly fewer neonates were admitted to the neonatal ward,” they said, adding: “We therefore think that a Foley catheter should be considered for induction of labor in women with an unfavorable cervix at term.”
Also, in light of the low cost and easy storage of Foley catheters, their use could be suitable for developing countries and low-resource settings, Dr. Jozwiak and her associates noted.
Future studies should compare the use of Foley catheters with other prostaglandin preparations such as prostaglandin E1 (misoprostol) which is becoming increasingly popular worldwide, and should evaluate the use of Foley catheters in women with a prior cesarean section, they suggested.
Dr. Jane E.
Norman and Dr. Sarah Stock note that the findings that intracervical
placement of a Foley catheter induces cervical ripening without inducing
uterine contractions and is as successful as prostaglandin for inducing
labor, could have important implications for women with a prior
cesarean section.
In these women, induction with prostaglandins is
associated with uterine rupture, they noted, explaining that
prostaglandins affect both cervical ripening and contractions
simultaneously, whereas the ideal strategy for induction is likely
administration of a cervical ripening agent before stimulation of
contractions. This, they argue, would decrease the need for fetal
monitoring during ripening and reduce the risk of uterine rupture.
“Although
women with a previous cesarean section were excluded from Jozwiak and
colleagues' study, a Foley catheter could be the ideal induction agent
in this situation and should be assessed further in randomized trials,”
they said, adding that if such trials are done, avoidance of maternal
and neonatal mortality and morbidity should be included as primary
outcome measures, as they are “arguably as important as speed and
avoidance of cesarean section.”
DR. NORMAN and DR. STOCK are
with the MRC Centre for Reproductive Health and the Queen's Medical
Research Center at the University of Edinburgh. Their comments were
taken from an editorial accompanying the article by Dr. Jozwiak and
colleagues (Lancet 2011 [doi:10.1016/S0140-6736(11)61581-X]). Dr. Norman
reported receiving fees for acting as a consultant for Preglem and is
an unpaid member of an advisory board for Hologic. Dr. Stock had no
relevant financial disclosures.
Dr. Jane E.
Norman and Dr. Sarah Stock note that the findings that intracervical
placement of a Foley catheter induces cervical ripening without inducing
uterine contractions and is as successful as prostaglandin for inducing
labor, could have important implications for women with a prior
cesarean section.
In these women, induction with prostaglandins is
associated with uterine rupture, they noted, explaining that
prostaglandins affect both cervical ripening and contractions
simultaneously, whereas the ideal strategy for induction is likely
administration of a cervical ripening agent before stimulation of
contractions. This, they argue, would decrease the need for fetal
monitoring during ripening and reduce the risk of uterine rupture.
“Although
women with a previous cesarean section were excluded from Jozwiak and
colleagues' study, a Foley catheter could be the ideal induction agent
in this situation and should be assessed further in randomized trials,”
they said, adding that if such trials are done, avoidance of maternal
and neonatal mortality and morbidity should be included as primary
outcome measures, as they are “arguably as important as speed and
avoidance of cesarean section.”
DR. NORMAN and DR. STOCK are
with the MRC Centre for Reproductive Health and the Queen's Medical
Research Center at the University of Edinburgh. Their comments were
taken from an editorial accompanying the article by Dr. Jozwiak and
colleagues (Lancet 2011 [doi:10.1016/S0140-6736(11)61581-X]). Dr. Norman
reported receiving fees for acting as a consultant for Preglem and is
an unpaid member of an advisory board for Hologic. Dr. Stock had no
relevant financial disclosures.
Dr. Jane E.
Norman and Dr. Sarah Stock note that the findings that intracervical
placement of a Foley catheter induces cervical ripening without inducing
uterine contractions and is as successful as prostaglandin for inducing
labor, could have important implications for women with a prior
cesarean section.
In these women, induction with prostaglandins is
associated with uterine rupture, they noted, explaining that
prostaglandins affect both cervical ripening and contractions
simultaneously, whereas the ideal strategy for induction is likely
administration of a cervical ripening agent before stimulation of
contractions. This, they argue, would decrease the need for fetal
monitoring during ripening and reduce the risk of uterine rupture.
“Although
women with a previous cesarean section were excluded from Jozwiak and
colleagues' study, a Foley catheter could be the ideal induction agent
in this situation and should be assessed further in randomized trials,”
they said, adding that if such trials are done, avoidance of maternal
and neonatal mortality and morbidity should be included as primary
outcome measures, as they are “arguably as important as speed and
avoidance of cesarean section.”
DR. NORMAN and DR. STOCK are
with the MRC Centre for Reproductive Health and the Queen's Medical
Research Center at the University of Edinburgh. Their comments were
taken from an editorial accompanying the article by Dr. Jozwiak and
colleagues (Lancet 2011 [doi:10.1016/S0140-6736(11)61581-X]). Dr. Norman
reported receiving fees for acting as a consultant for Preglem and is
an unpaid member of an advisory board for Hologic. Dr. Stock had no
relevant financial disclosures.
Use of a Foley catheter for labor induction in women with an unfavorable cervix at term resulted in a similar cesarean section rate to use of prostaglandin E2 gel for labor induction, but with fewer maternal and neonatal side effects, according to findings from an open-label randomized controlled trial involving more than 800 women.
Cesarean section, most often done for failure to progress during the first stage of labor, was performed in 23% of 411 women induced using a Foley catheter, and in 20% of 408 women induced using vaginal prostaglandin E2 gel (relative risk, 1.13), Dr. Marta Jozwiak of Groene Hart Hospital, Gouda, the Netherlands, and her colleagues from the PROBAAT Study Group reported.
The frequency of vaginal instrumental deliveries was similar in the two groups as well (11% and 13% in the Foley catheter and prostaglandin E2 groups, respectively), the investigators said (Lancet 2011 [doi:10.1016/S0140-6736(11)61484-0]).
Two serious maternal adverse events occurred, both in the prostaglandin group. These included one uterine perforation after insertion of an intrauterine pressure catheter and one uterine rupture during oxytocin augmentation, they said, noting that both neonates were born in good clinical condition, but were admitted to the neonatal ward with suspected infection.
Four minor maternal adverse events occurred, including three allergic reactions (in one woman in the Foley catheter group and in two women in the prostaglandin E2 group), and blood loss on a second insertion of the catheter in one woman. Also, the rate of suspected intrapartum infection was significantly lower in the Foley catheter group (1% vs. 3%).
As for neonatal adverse events, the rate of admissions to the neonatal ward was significantly higher in the prostaglandin E2 group (12% vs. 20%), Dr. Jozwiak and her associates said.
Patients in the PROBAAT trial included women beyond 37 weeks' gestation with a singleton pregnancy in cephalic presentation, with intact membranes and an unfavorable cervix as defined by a Bishop score less than 6. The women were enrolled at 12 centers throughout the Netherlands between Feb. 10, 2009, and May 17, 2010, and all had an indication for labor induction, and had no prior cesarean sections.
The findings in regard to cesarean section rates when labor induction is performed using a Foley catheter vs. prostaglandin E2 were confirmed by a meta-analysis that included data from this study. The meta-analysis also demonstrated that Foley catheter induction was associated with reduced rates of hyperstimulation (odds ratio, 0.44) and postpartum hemorrhage (OR, 0.60), the investigators reported, noting that although the use of a Foley catheter for labor induction did not increase the vaginal delivery rate when compared with use of prostaglandin E2 as they had hypothesized, the findings nonetheless support the use of Foley catheters.
“After induction with a Foley catheter, the overall number of operative deliveries for suspected fetal distress was lower, fewer mothers were treated with intrapartum antibiotics, and significantly fewer neonates were admitted to the neonatal ward,” they said, adding: “We therefore think that a Foley catheter should be considered for induction of labor in women with an unfavorable cervix at term.”
Also, in light of the low cost and easy storage of Foley catheters, their use could be suitable for developing countries and low-resource settings, Dr. Jozwiak and her associates noted.
Future studies should compare the use of Foley catheters with other prostaglandin preparations such as prostaglandin E1 (misoprostol) which is becoming increasingly popular worldwide, and should evaluate the use of Foley catheters in women with a prior cesarean section, they suggested.
Use of a Foley catheter for labor induction in women with an unfavorable cervix at term resulted in a similar cesarean section rate to use of prostaglandin E2 gel for labor induction, but with fewer maternal and neonatal side effects, according to findings from an open-label randomized controlled trial involving more than 800 women.
Cesarean section, most often done for failure to progress during the first stage of labor, was performed in 23% of 411 women induced using a Foley catheter, and in 20% of 408 women induced using vaginal prostaglandin E2 gel (relative risk, 1.13), Dr. Marta Jozwiak of Groene Hart Hospital, Gouda, the Netherlands, and her colleagues from the PROBAAT Study Group reported.
The frequency of vaginal instrumental deliveries was similar in the two groups as well (11% and 13% in the Foley catheter and prostaglandin E2 groups, respectively), the investigators said (Lancet 2011 [doi:10.1016/S0140-6736(11)61484-0]).
Two serious maternal adverse events occurred, both in the prostaglandin group. These included one uterine perforation after insertion of an intrauterine pressure catheter and one uterine rupture during oxytocin augmentation, they said, noting that both neonates were born in good clinical condition, but were admitted to the neonatal ward with suspected infection.
Four minor maternal adverse events occurred, including three allergic reactions (in one woman in the Foley catheter group and in two women in the prostaglandin E2 group), and blood loss on a second insertion of the catheter in one woman. Also, the rate of suspected intrapartum infection was significantly lower in the Foley catheter group (1% vs. 3%).
As for neonatal adverse events, the rate of admissions to the neonatal ward was significantly higher in the prostaglandin E2 group (12% vs. 20%), Dr. Jozwiak and her associates said.
Patients in the PROBAAT trial included women beyond 37 weeks' gestation with a singleton pregnancy in cephalic presentation, with intact membranes and an unfavorable cervix as defined by a Bishop score less than 6. The women were enrolled at 12 centers throughout the Netherlands between Feb. 10, 2009, and May 17, 2010, and all had an indication for labor induction, and had no prior cesarean sections.
The findings in regard to cesarean section rates when labor induction is performed using a Foley catheter vs. prostaglandin E2 were confirmed by a meta-analysis that included data from this study. The meta-analysis also demonstrated that Foley catheter induction was associated with reduced rates of hyperstimulation (odds ratio, 0.44) and postpartum hemorrhage (OR, 0.60), the investigators reported, noting that although the use of a Foley catheter for labor induction did not increase the vaginal delivery rate when compared with use of prostaglandin E2 as they had hypothesized, the findings nonetheless support the use of Foley catheters.
“After induction with a Foley catheter, the overall number of operative deliveries for suspected fetal distress was lower, fewer mothers were treated with intrapartum antibiotics, and significantly fewer neonates were admitted to the neonatal ward,” they said, adding: “We therefore think that a Foley catheter should be considered for induction of labor in women with an unfavorable cervix at term.”
Also, in light of the low cost and easy storage of Foley catheters, their use could be suitable for developing countries and low-resource settings, Dr. Jozwiak and her associates noted.
Future studies should compare the use of Foley catheters with other prostaglandin preparations such as prostaglandin E1 (misoprostol) which is becoming increasingly popular worldwide, and should evaluate the use of Foley catheters in women with a prior cesarean section, they suggested.
From the Lancet
Fibroids Foretell Worse Maternal and Fetal Outcomes
ORLANDO – Uterine fibroids are bad for pregnancy and neonatal outcomes, and a new study shows just how bad.
Women diagnosed with fibroids on their first obstetric ultrasound examination, for example, were significantly more likely to experience preterm labor or preterm premature rupture of the membranes (pPROM). Also, significantly more deliver before 37 weeks' gestation or via cesarean section, compared with a group of women without these noncancerous growths of the uterus.
Dr. Radwan Asaad and his colleagues compared 152 women with fibroids to another 165 matched controls in a retrospective cohort analysis conducted at Wayne State University, Detroit. They also found fibroids weren't good news for the baby either.
“Uterine fibroids complicate the pregnancy course as evidenced by a considerable impact on the obstetrical and neonatal outcomes,” Dr. Asaad said at the meeting.
In terms of the significantly different maternal numbers, women with fibroids were more likely to experience preterm labor (16.4% vs. 2.4% of controls), and pPROM (15.8% vs. 3.6%), and to deliver preterm (33.3% vs. 10.1%).
Fetal malpresentation also was significantly more likely in the fibroid group (22% vs. 6% in controls). Cesarean delivery occurred in 54.3% of the fibroid group vs. 28.0% of the control group, another significant difference.
Gestational age at delivery was significantly less when the mother had fibroids (mean 35.3 weeks) vs. without (38.6 weeks).
Children born to women in the fibroid group had a mean birth weight of 2,634 g, compared with 3,181 g for those born to control group women. Apgar scores at 1 minute were a mean 6.7 vs. 7.8 in the control group and at 5 minutes were a mean 7.9 vs. 8.8.
Pregnancy loss was higher in the fibroid group during the first trimester (7.9% vs. 3.6% in controls) and during the second trimester (5.9% vs. 1.2%), but these differences were not statistically significant to the P less than .001 level. A trend toward more arrested dilation in the fibroid group likewise did not reach significance.
“Uterine myomas are the most common pelvic tumor in reproductive-age women,” said Dr. Asaad, a laparoscopic and minimally invasive surgeon in the department of obstetrics and gynecology at Hutzel Women's Hospital and Wayne State University/Detroit Medical Center. Prevalence in published studies varies from 2% to 11%, depending on the trimester in which they are measured and the size threshold chosen by researchers.
A meeting attendee asked for information on the size and anatomic location of the fibroids. Dr. Asaad replied that he was only able to categorize women dichotomously as yes/no for presence of fibroids in this retrospective study.
Dr. Asaad and his associates reviewed all department ultrasounds from 1998 to 2006 at their tertiary care center. Women with complete records in the fibroid group were matched to controls for age, gravidity, parity, and year of delivery.
He said he had no relevant financial disclosures.
ORLANDO – Uterine fibroids are bad for pregnancy and neonatal outcomes, and a new study shows just how bad.
Women diagnosed with fibroids on their first obstetric ultrasound examination, for example, were significantly more likely to experience preterm labor or preterm premature rupture of the membranes (pPROM). Also, significantly more deliver before 37 weeks' gestation or via cesarean section, compared with a group of women without these noncancerous growths of the uterus.
Dr. Radwan Asaad and his colleagues compared 152 women with fibroids to another 165 matched controls in a retrospective cohort analysis conducted at Wayne State University, Detroit. They also found fibroids weren't good news for the baby either.
“Uterine fibroids complicate the pregnancy course as evidenced by a considerable impact on the obstetrical and neonatal outcomes,” Dr. Asaad said at the meeting.
In terms of the significantly different maternal numbers, women with fibroids were more likely to experience preterm labor (16.4% vs. 2.4% of controls), and pPROM (15.8% vs. 3.6%), and to deliver preterm (33.3% vs. 10.1%).
Fetal malpresentation also was significantly more likely in the fibroid group (22% vs. 6% in controls). Cesarean delivery occurred in 54.3% of the fibroid group vs. 28.0% of the control group, another significant difference.
Gestational age at delivery was significantly less when the mother had fibroids (mean 35.3 weeks) vs. without (38.6 weeks).
Children born to women in the fibroid group had a mean birth weight of 2,634 g, compared with 3,181 g for those born to control group women. Apgar scores at 1 minute were a mean 6.7 vs. 7.8 in the control group and at 5 minutes were a mean 7.9 vs. 8.8.
Pregnancy loss was higher in the fibroid group during the first trimester (7.9% vs. 3.6% in controls) and during the second trimester (5.9% vs. 1.2%), but these differences were not statistically significant to the P less than .001 level. A trend toward more arrested dilation in the fibroid group likewise did not reach significance.
“Uterine myomas are the most common pelvic tumor in reproductive-age women,” said Dr. Asaad, a laparoscopic and minimally invasive surgeon in the department of obstetrics and gynecology at Hutzel Women's Hospital and Wayne State University/Detroit Medical Center. Prevalence in published studies varies from 2% to 11%, depending on the trimester in which they are measured and the size threshold chosen by researchers.
A meeting attendee asked for information on the size and anatomic location of the fibroids. Dr. Asaad replied that he was only able to categorize women dichotomously as yes/no for presence of fibroids in this retrospective study.
Dr. Asaad and his associates reviewed all department ultrasounds from 1998 to 2006 at their tertiary care center. Women with complete records in the fibroid group were matched to controls for age, gravidity, parity, and year of delivery.
He said he had no relevant financial disclosures.
ORLANDO – Uterine fibroids are bad for pregnancy and neonatal outcomes, and a new study shows just how bad.
Women diagnosed with fibroids on their first obstetric ultrasound examination, for example, were significantly more likely to experience preterm labor or preterm premature rupture of the membranes (pPROM). Also, significantly more deliver before 37 weeks' gestation or via cesarean section, compared with a group of women without these noncancerous growths of the uterus.
Dr. Radwan Asaad and his colleagues compared 152 women with fibroids to another 165 matched controls in a retrospective cohort analysis conducted at Wayne State University, Detroit. They also found fibroids weren't good news for the baby either.
“Uterine fibroids complicate the pregnancy course as evidenced by a considerable impact on the obstetrical and neonatal outcomes,” Dr. Asaad said at the meeting.
In terms of the significantly different maternal numbers, women with fibroids were more likely to experience preterm labor (16.4% vs. 2.4% of controls), and pPROM (15.8% vs. 3.6%), and to deliver preterm (33.3% vs. 10.1%).
Fetal malpresentation also was significantly more likely in the fibroid group (22% vs. 6% in controls). Cesarean delivery occurred in 54.3% of the fibroid group vs. 28.0% of the control group, another significant difference.
Gestational age at delivery was significantly less when the mother had fibroids (mean 35.3 weeks) vs. without (38.6 weeks).
Children born to women in the fibroid group had a mean birth weight of 2,634 g, compared with 3,181 g for those born to control group women. Apgar scores at 1 minute were a mean 6.7 vs. 7.8 in the control group and at 5 minutes were a mean 7.9 vs. 8.8.
Pregnancy loss was higher in the fibroid group during the first trimester (7.9% vs. 3.6% in controls) and during the second trimester (5.9% vs. 1.2%), but these differences were not statistically significant to the P less than .001 level. A trend toward more arrested dilation in the fibroid group likewise did not reach significance.
“Uterine myomas are the most common pelvic tumor in reproductive-age women,” said Dr. Asaad, a laparoscopic and minimally invasive surgeon in the department of obstetrics and gynecology at Hutzel Women's Hospital and Wayne State University/Detroit Medical Center. Prevalence in published studies varies from 2% to 11%, depending on the trimester in which they are measured and the size threshold chosen by researchers.
A meeting attendee asked for information on the size and anatomic location of the fibroids. Dr. Asaad replied that he was only able to categorize women dichotomously as yes/no for presence of fibroids in this retrospective study.
Dr. Asaad and his associates reviewed all department ultrasounds from 1998 to 2006 at their tertiary care center. Women with complete records in the fibroid group were matched to controls for age, gravidity, parity, and year of delivery.
He said he had no relevant financial disclosures.
From the Annual Meeting of the American Society for Reproductive Medicine
First-Trimester Cervical Length Predicts IVF Preterm Deliveries
ORLANDO – Short cervical length during the first trimester predicts preterm delivery of an in vitro fertilization pregnancy, according to two retrospective studies involving a total of 167 women.
Although they used slightly different parameters, researchers who assessed 113 women at Montreal Fertility Center and others who studied 54 women at Detroit Medical Center reached the same conclusion: A cervical length shorter than approximately 4 cm is associated with greater risk of delivery before 37 weeks' gestation.
These studies are part of a move to identify women at risk for preterm delivery earlier, when clinicians would have more time to intervene. Some previous researchers report associations between shorter midterm cervical length and preterm delivery (Ultrasound Obstet. Gynecol. 2008;32:640-5), while others point to a need for additional evidence (Cochrane Database Syst. Rev. 2009;CD007235 [doi:10.1002/14651858.CD007235.pub2]).
“Little is known about the predictive value of a first-trimester cervical length measurement,” Dr. Olivia Vincent-Boulay of the Montreal Fertility Centre said at the meeting.
Dr. Vincent-Boulay and her associates reviewed transvaginal ultrasound findings for 113 women who conceived via in vitro fertilization (IVF) at their center. Cervical length measurements were taken between 6 and 12 weeks' gestation. A total 60% delivered full term, at 37 weeks or longer. Another 23% delivered at 34-36 weeks; 10% at 30-33 weeks; and 7% of women delivered before 30 weeks' gestation. Thus, 40% delivered prior to 37 weeks' gestation.
As their average first-trimester cervical length decreased, so did mean gestational age. For example, women with a cervix of 5 cm or longer delivered at a mean of 37 weeks. This decreased to 35 weeks for those with a cervical length from 4.0 cm to 4.9 cm and to 32 weeks for mothers with a cervix shorter than 4.0 cm.
“Our sample size was pretty small, which is why we chose 5-cm and below 4-cm groups,” Dr. Vincent-Boulay said. “It would be great to do further studies with a larger sample to do a more stratified analysis.”
There were some interesting differences between the 86 singleton and 27 twin pregnancies. Cervical length below 5 cm during the first trimester significantly correlated with preterm delivery in twin pregnancies. In the case of singletons, only a measurement below 4 cm was a significant predictor. The researchers assessed serial ultrasound measurements, and found, for example, that cervical length at 10 weeks predicted a preterm delivery of twins but not singletons.
Of the 113 IVF pregnancies, 86 or (76%) were singletons and 27 (24%) were twins. A greater number of singletons were delivered at full term (58 children), compared with twins (10 sets) in the study.
Put into clinical terms, Dr. Vincent-Boulay said: “Less than 4 cm at any time during pregnancy, whether it is single or twins, may be a cause for alarm. A cervical length less than 5 cm at 10 weeks for twins is also significant risk for preterm delivery.”
A second study presented at the ASRM meeting revealed a very similar cervical length cutoff during the first trimester, 38.5 mm (or 3.85 cm) or less. Dr. Zain Al-Safi and his colleagues reviewed the records for 54 women who conceived via IVF at Wayne State University/Detroit Medical Center.
Specifically, they found 17 or 32% of pregnant women with a short cervical length measured at the first ultrasound (between 5 and 9 weeks' gestation) delivered before 37 weeks' gestation. Fifteen women who delivered preterm had an ultrasound measurement of 38.5 mm or less, giving this cutoff a sensitivity of 47% and a specificity of 81%.
This study is important because “premature delivery is a major obstetric complication and a substantial contributor to neonatal morbidity,” said Dr. Al-Safi, an ob.gyn. at Wayne State University.
A total 21 women (39%) had multiple gestations. A meeting attendee commented that this was a high percentage and asked about IVF embryo transfer protocol.
“We did not include that in our results. We looked at twins, but did not go back and look at how many embryos were transferred,” Dr. Al-Safi replied.
A follow-up question was asked about the findings without multiples. “We took out multiples and frozen embryos and found similar results,” Dr. Al-Safi said.
A lack of follow-up ultrasound assessment in the second trimester, a small sample size, and the retrospective design are potential limitations, Dr. Al-Safi said. The findings should be considered experimental until replicated in larger, prospective studies, he added.
“If we know cervical length in the future correlates to preterm delivery … we could prevent it,” Dr. Al-Safi said.
Future studies also should evaluate the optimal management for patients with a short cervix, Dr. Vincent-Boulay said.
ORLANDO – Short cervical length during the first trimester predicts preterm delivery of an in vitro fertilization pregnancy, according to two retrospective studies involving a total of 167 women.
Although they used slightly different parameters, researchers who assessed 113 women at Montreal Fertility Center and others who studied 54 women at Detroit Medical Center reached the same conclusion: A cervical length shorter than approximately 4 cm is associated with greater risk of delivery before 37 weeks' gestation.
These studies are part of a move to identify women at risk for preterm delivery earlier, when clinicians would have more time to intervene. Some previous researchers report associations between shorter midterm cervical length and preterm delivery (Ultrasound Obstet. Gynecol. 2008;32:640-5), while others point to a need for additional evidence (Cochrane Database Syst. Rev. 2009;CD007235 [doi:10.1002/14651858.CD007235.pub2]).
“Little is known about the predictive value of a first-trimester cervical length measurement,” Dr. Olivia Vincent-Boulay of the Montreal Fertility Centre said at the meeting.
Dr. Vincent-Boulay and her associates reviewed transvaginal ultrasound findings for 113 women who conceived via in vitro fertilization (IVF) at their center. Cervical length measurements were taken between 6 and 12 weeks' gestation. A total 60% delivered full term, at 37 weeks or longer. Another 23% delivered at 34-36 weeks; 10% at 30-33 weeks; and 7% of women delivered before 30 weeks' gestation. Thus, 40% delivered prior to 37 weeks' gestation.
As their average first-trimester cervical length decreased, so did mean gestational age. For example, women with a cervix of 5 cm or longer delivered at a mean of 37 weeks. This decreased to 35 weeks for those with a cervical length from 4.0 cm to 4.9 cm and to 32 weeks for mothers with a cervix shorter than 4.0 cm.
“Our sample size was pretty small, which is why we chose 5-cm and below 4-cm groups,” Dr. Vincent-Boulay said. “It would be great to do further studies with a larger sample to do a more stratified analysis.”
There were some interesting differences between the 86 singleton and 27 twin pregnancies. Cervical length below 5 cm during the first trimester significantly correlated with preterm delivery in twin pregnancies. In the case of singletons, only a measurement below 4 cm was a significant predictor. The researchers assessed serial ultrasound measurements, and found, for example, that cervical length at 10 weeks predicted a preterm delivery of twins but not singletons.
Of the 113 IVF pregnancies, 86 or (76%) were singletons and 27 (24%) were twins. A greater number of singletons were delivered at full term (58 children), compared with twins (10 sets) in the study.
Put into clinical terms, Dr. Vincent-Boulay said: “Less than 4 cm at any time during pregnancy, whether it is single or twins, may be a cause for alarm. A cervical length less than 5 cm at 10 weeks for twins is also significant risk for preterm delivery.”
A second study presented at the ASRM meeting revealed a very similar cervical length cutoff during the first trimester, 38.5 mm (or 3.85 cm) or less. Dr. Zain Al-Safi and his colleagues reviewed the records for 54 women who conceived via IVF at Wayne State University/Detroit Medical Center.
Specifically, they found 17 or 32% of pregnant women with a short cervical length measured at the first ultrasound (between 5 and 9 weeks' gestation) delivered before 37 weeks' gestation. Fifteen women who delivered preterm had an ultrasound measurement of 38.5 mm or less, giving this cutoff a sensitivity of 47% and a specificity of 81%.
This study is important because “premature delivery is a major obstetric complication and a substantial contributor to neonatal morbidity,” said Dr. Al-Safi, an ob.gyn. at Wayne State University.
A total 21 women (39%) had multiple gestations. A meeting attendee commented that this was a high percentage and asked about IVF embryo transfer protocol.
“We did not include that in our results. We looked at twins, but did not go back and look at how many embryos were transferred,” Dr. Al-Safi replied.
A follow-up question was asked about the findings without multiples. “We took out multiples and frozen embryos and found similar results,” Dr. Al-Safi said.
A lack of follow-up ultrasound assessment in the second trimester, a small sample size, and the retrospective design are potential limitations, Dr. Al-Safi said. The findings should be considered experimental until replicated in larger, prospective studies, he added.
“If we know cervical length in the future correlates to preterm delivery … we could prevent it,” Dr. Al-Safi said.
Future studies also should evaluate the optimal management for patients with a short cervix, Dr. Vincent-Boulay said.
ORLANDO – Short cervical length during the first trimester predicts preterm delivery of an in vitro fertilization pregnancy, according to two retrospective studies involving a total of 167 women.
Although they used slightly different parameters, researchers who assessed 113 women at Montreal Fertility Center and others who studied 54 women at Detroit Medical Center reached the same conclusion: A cervical length shorter than approximately 4 cm is associated with greater risk of delivery before 37 weeks' gestation.
These studies are part of a move to identify women at risk for preterm delivery earlier, when clinicians would have more time to intervene. Some previous researchers report associations between shorter midterm cervical length and preterm delivery (Ultrasound Obstet. Gynecol. 2008;32:640-5), while others point to a need for additional evidence (Cochrane Database Syst. Rev. 2009;CD007235 [doi:10.1002/14651858.CD007235.pub2]).
“Little is known about the predictive value of a first-trimester cervical length measurement,” Dr. Olivia Vincent-Boulay of the Montreal Fertility Centre said at the meeting.
Dr. Vincent-Boulay and her associates reviewed transvaginal ultrasound findings for 113 women who conceived via in vitro fertilization (IVF) at their center. Cervical length measurements were taken between 6 and 12 weeks' gestation. A total 60% delivered full term, at 37 weeks or longer. Another 23% delivered at 34-36 weeks; 10% at 30-33 weeks; and 7% of women delivered before 30 weeks' gestation. Thus, 40% delivered prior to 37 weeks' gestation.
As their average first-trimester cervical length decreased, so did mean gestational age. For example, women with a cervix of 5 cm or longer delivered at a mean of 37 weeks. This decreased to 35 weeks for those with a cervical length from 4.0 cm to 4.9 cm and to 32 weeks for mothers with a cervix shorter than 4.0 cm.
“Our sample size was pretty small, which is why we chose 5-cm and below 4-cm groups,” Dr. Vincent-Boulay said. “It would be great to do further studies with a larger sample to do a more stratified analysis.”
There were some interesting differences between the 86 singleton and 27 twin pregnancies. Cervical length below 5 cm during the first trimester significantly correlated with preterm delivery in twin pregnancies. In the case of singletons, only a measurement below 4 cm was a significant predictor. The researchers assessed serial ultrasound measurements, and found, for example, that cervical length at 10 weeks predicted a preterm delivery of twins but not singletons.
Of the 113 IVF pregnancies, 86 or (76%) were singletons and 27 (24%) were twins. A greater number of singletons were delivered at full term (58 children), compared with twins (10 sets) in the study.
Put into clinical terms, Dr. Vincent-Boulay said: “Less than 4 cm at any time during pregnancy, whether it is single or twins, may be a cause for alarm. A cervical length less than 5 cm at 10 weeks for twins is also significant risk for preterm delivery.”
A second study presented at the ASRM meeting revealed a very similar cervical length cutoff during the first trimester, 38.5 mm (or 3.85 cm) or less. Dr. Zain Al-Safi and his colleagues reviewed the records for 54 women who conceived via IVF at Wayne State University/Detroit Medical Center.
Specifically, they found 17 or 32% of pregnant women with a short cervical length measured at the first ultrasound (between 5 and 9 weeks' gestation) delivered before 37 weeks' gestation. Fifteen women who delivered preterm had an ultrasound measurement of 38.5 mm or less, giving this cutoff a sensitivity of 47% and a specificity of 81%.
This study is important because “premature delivery is a major obstetric complication and a substantial contributor to neonatal morbidity,” said Dr. Al-Safi, an ob.gyn. at Wayne State University.
A total 21 women (39%) had multiple gestations. A meeting attendee commented that this was a high percentage and asked about IVF embryo transfer protocol.
“We did not include that in our results. We looked at twins, but did not go back and look at how many embryos were transferred,” Dr. Al-Safi replied.
A follow-up question was asked about the findings without multiples. “We took out multiples and frozen embryos and found similar results,” Dr. Al-Safi said.
A lack of follow-up ultrasound assessment in the second trimester, a small sample size, and the retrospective design are potential limitations, Dr. Al-Safi said. The findings should be considered experimental until replicated in larger, prospective studies, he added.
“If we know cervical length in the future correlates to preterm delivery … we could prevent it,” Dr. Al-Safi said.
Future studies also should evaluate the optimal management for patients with a short cervix, Dr. Vincent-Boulay said.
From the Annual Meeting of the American Society for Reproductive Medicine
10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia
Because eclampsia occurs but rarely during pregnancy and the postpartum period, most health-care providers have little to no personal experience with management of this life-threatening obstetric emergency. Knowledge about maternal resuscitation during and after an eclamptic seizure is critical for improving maternal and perinatal outcomes.
In this round-up, I present 10 practical recommendations for prompt diagnosis and management of women who have eclampsia. Immediate implementation of these recommendations can lead to improved maternal and perinatal outcomes (both acute and long-term).
1. Practice. Practice again.
Implement regular monthly simulation training sessions
Fisher N, Bernstein PS, Satin A, et al. Resident training for eclampsia and magnesium toxicity management: simulation or traditional lecture? Am J Obstet Gynecol. 2010;203(4):379.e1–5.
Eclampsia is unpredictable and can develop rapidly at home, in labor and delivery, on the antepartum/postpartum ward, and in the emergency room. Therefore, it is prudent that all health-care providers who treat pregnant or postpartum women on a daily basis be trained and knowledgeable about early detection and management of eclampsia. This goal can be achieved by developing drills for rehearsal and by testing the response and skills of all providers.
2. Preventive: Magnesium sulfate
Do not attempt to arrest the seizure. Use MgSO4 to prevent recurrent convulsions.
Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulfate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;(12):CD000127.
Most eclamptic seizures are self-limiting. Therefore, there is no need to administer bolus drugs such as diazepam or midazolam. These drugs are usually used in the emergency room, but they inhibit maternal laryngeal reflexes and may lead to aspiration. They also suppress the central nervous system respiratory centers and can cause apnea, requiring intubation.
When used in the management of eclampsia, magnesium sulfate is associated with a lower rate of recurrent seizures and maternal death than is diazepam.
3. FHR changes? Be patient.
Do not rush the patient to emergent cesarean section because of an abnormal FHR tracing
Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
During an eclamptic convulsion, there is usually prolonged fetal heart rate (FHR) deceleration or even bradycardia—with or without an increase in both frequency and uterine tone. After the convulsion, as a result of maternal hypoxia and hypercarbia, the FHR tracing can show tachycardia, reduced beat-to-beat variability, and transient recurrent decelerations. When this happens, concern about fetal status can distract the obstetric provider from resuscitation of the mother. However, these FHR changes usually return to normal after maternal resuscitation. If the FHR changes persist for longer than 15 minutes, consider abruptio placentae and move to delivery.
4. Target: Lower BP
Reduce maternal blood pressure to a safe level to prevent stroke, but without compromising uteroplacental perfusion
Zwart JJ, Richters A, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Eclampsia in the Netherlands. Obstet Gynecol. 2008;112(4):820–827.
In this nationwide review of complications from eclampsia in the Netherlands, the authors found that failure to treat persistent severe hypertension was associated with hypertensive encephalopathy, cerebral infarction, bleeding, or congestive heart failure. They also found that 35.2% of women had systolic or diastolic blood pressure at or above 170/110 mm Hg at admission, but fewer than half were given antihypertensive drugs at that time. Among the cases deemed to have received substandard care, one third involved inadequate treatment of hypertension.
5. Know your antihypertensives
Learn which agents are best to control severe hypertension in eclampsia
Sibai BM. Hypertensive Emergencies. In: Foley MR, Strong TH, Garite TJ, eds. Obstetric Intensive Care Manual. 3rd ed. New York, NY: The McGraw-Hill Companies; 2010.
It is critical to familiarize oneself with the mechanism of action, dose, and potential side effects of agents used to control hypertension. For example, neither hydralazine nor nifedipine should be used in patients who have severe headache and persistent tachycardia (pulse, >100 bpm). Labetalol should be avoided in women who have persistent bradycardia (pulse, <60 bpm), asthma, or congestive heart failure.
For women who have persistent headache and tachycardia, I suggest intravenous (IV) labetalol, starting at a dose of 20 mg, 40 mg, or 80 mg every 10 minutes as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg. The maximum dose of labetalol should not exceed 300 mg in 1 hour.
For patients who have bradycardia and severe asthma, I suggest oral, rapid-acting nifedipine, starting at 10 mg to 20 mg, to be repeated in 20 to 30 minutes as needed, up to a maximum of 50 mg to 60 mg in 1 hour. Oral nifedipine can be used with magnesium sulfate. An alternative is an IV bolus injection of hydralazine, starting at a dose of 5 mg to 10 mg, to be repeated every 15 minutes, up to a maximum dose of 25 mg.
6. Avoid general anesthesia
Use neuraxial anesthesia for labor and delivery in eclampsia
Turner JA. Severe preeclampsia: anesthetic implications of the disease and its management. Am J Ther. 2009;16(4):284–248.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Cesarean delivery: a population-based study. Br J Anaesth. 2010;105(6):818–826.
Epidural, spinal, or combined anesthesia is safe in the absence of coagulopathy or severe thrombocytopenia. General anesthesia increases the risk of aspiration, failed intubation due to pharyngolaryngeal edema, and stroke secondary to the increase in systemic and intracerebral pressures during intubation and extubation.
Eclampsia is not an indication for cesarean delivery
Repke JT, Sibai BM. Preeclampsia and eclampsia. OBG Manage. 2009;21(4):44–55.
Once the mother has been resuscitated and stabilized, the provider should choose a mode of delivery that is based on fetal condition, gestational age, presence or absence of labor, and the cervical Bishop score. Vaginal delivery can be achieved in most patients who have a gestational age of 34 weeks or greater.
8. Late presentation happens
Be aware that eclampsia can develop for the first time as long as 28 days postpartum
Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e31–37.
Atypical eclampsia is any eclampsia that develops beyond 48 hours postpartum. A history of diagnosed predelivery preeclampsia is not necessary for development of late postpartum eclampsia. In general, more than 50% of patients who develop late postpartum eclampsia have no evidence of preeclampsia prior to delivery.
Be aware that the clinical and neuro-imaging features of eclampsia overlap with those of reversible cerebral vasoconstriction syndrome (angiopathy)
Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care. 2009;11(2):199–209.
Women who have reversible cerebral vasoconstriction syndrome have clinical findings (acute onset of recurrent headaches, visual changes, seizures, and hypertension) and cerebral magnetic resonance imaging (MRI) findings (posterior reversible encephalopathy syndrome) that are similar to those of women who have late postpartum eclampsia (FIGURE). However, in women who have postpartum cerebral angiopathy, cerebral angiography will show the presence of bead-like vasoconstriction—which is usually absent in eclampsia.
Posterior reversible encephalopathy syndrome
Green arrows point to vasogenic edema in the occipital lobes and, partially, the parietal lobes. The edema is gone on repeat magnetic resonance imaging (see Recommendation #9).
10. Act today, see a better outcome tomorrow
Avoid long-term maternal neurologic injury by managing eclampsia properly
Zeeman GG. Neurologic complications of preeclampsia. Semin Perinatol. 2009;33(3):166–172.
Residual neurologic damage is rare in the majority of women who have eclampsia. However, long-term cerebral white-matter injury (cytotoxic edema, infarction) on MRI imaging and impaired memory and cognitive function may develop in some women who have multiple seizures and who have inadequately controlled persistent severe hypertension.
We want to hear from you! Tell us what you think.
Because eclampsia occurs but rarely during pregnancy and the postpartum period, most health-care providers have little to no personal experience with management of this life-threatening obstetric emergency. Knowledge about maternal resuscitation during and after an eclamptic seizure is critical for improving maternal and perinatal outcomes.
In this round-up, I present 10 practical recommendations for prompt diagnosis and management of women who have eclampsia. Immediate implementation of these recommendations can lead to improved maternal and perinatal outcomes (both acute and long-term).
1. Practice. Practice again.
Implement regular monthly simulation training sessions
Fisher N, Bernstein PS, Satin A, et al. Resident training for eclampsia and magnesium toxicity management: simulation or traditional lecture? Am J Obstet Gynecol. 2010;203(4):379.e1–5.
Eclampsia is unpredictable and can develop rapidly at home, in labor and delivery, on the antepartum/postpartum ward, and in the emergency room. Therefore, it is prudent that all health-care providers who treat pregnant or postpartum women on a daily basis be trained and knowledgeable about early detection and management of eclampsia. This goal can be achieved by developing drills for rehearsal and by testing the response and skills of all providers.
2. Preventive: Magnesium sulfate
Do not attempt to arrest the seizure. Use MgSO4 to prevent recurrent convulsions.
Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulfate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;(12):CD000127.
Most eclamptic seizures are self-limiting. Therefore, there is no need to administer bolus drugs such as diazepam or midazolam. These drugs are usually used in the emergency room, but they inhibit maternal laryngeal reflexes and may lead to aspiration. They also suppress the central nervous system respiratory centers and can cause apnea, requiring intubation.
When used in the management of eclampsia, magnesium sulfate is associated with a lower rate of recurrent seizures and maternal death than is diazepam.
3. FHR changes? Be patient.
Do not rush the patient to emergent cesarean section because of an abnormal FHR tracing
Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
During an eclamptic convulsion, there is usually prolonged fetal heart rate (FHR) deceleration or even bradycardia—with or without an increase in both frequency and uterine tone. After the convulsion, as a result of maternal hypoxia and hypercarbia, the FHR tracing can show tachycardia, reduced beat-to-beat variability, and transient recurrent decelerations. When this happens, concern about fetal status can distract the obstetric provider from resuscitation of the mother. However, these FHR changes usually return to normal after maternal resuscitation. If the FHR changes persist for longer than 15 minutes, consider abruptio placentae and move to delivery.
4. Target: Lower BP
Reduce maternal blood pressure to a safe level to prevent stroke, but without compromising uteroplacental perfusion
Zwart JJ, Richters A, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Eclampsia in the Netherlands. Obstet Gynecol. 2008;112(4):820–827.
In this nationwide review of complications from eclampsia in the Netherlands, the authors found that failure to treat persistent severe hypertension was associated with hypertensive encephalopathy, cerebral infarction, bleeding, or congestive heart failure. They also found that 35.2% of women had systolic or diastolic blood pressure at or above 170/110 mm Hg at admission, but fewer than half were given antihypertensive drugs at that time. Among the cases deemed to have received substandard care, one third involved inadequate treatment of hypertension.
5. Know your antihypertensives
Learn which agents are best to control severe hypertension in eclampsia
Sibai BM. Hypertensive Emergencies. In: Foley MR, Strong TH, Garite TJ, eds. Obstetric Intensive Care Manual. 3rd ed. New York, NY: The McGraw-Hill Companies; 2010.
It is critical to familiarize oneself with the mechanism of action, dose, and potential side effects of agents used to control hypertension. For example, neither hydralazine nor nifedipine should be used in patients who have severe headache and persistent tachycardia (pulse, >100 bpm). Labetalol should be avoided in women who have persistent bradycardia (pulse, <60 bpm), asthma, or congestive heart failure.
For women who have persistent headache and tachycardia, I suggest intravenous (IV) labetalol, starting at a dose of 20 mg, 40 mg, or 80 mg every 10 minutes as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg. The maximum dose of labetalol should not exceed 300 mg in 1 hour.
For patients who have bradycardia and severe asthma, I suggest oral, rapid-acting nifedipine, starting at 10 mg to 20 mg, to be repeated in 20 to 30 minutes as needed, up to a maximum of 50 mg to 60 mg in 1 hour. Oral nifedipine can be used with magnesium sulfate. An alternative is an IV bolus injection of hydralazine, starting at a dose of 5 mg to 10 mg, to be repeated every 15 minutes, up to a maximum dose of 25 mg.
6. Avoid general anesthesia
Use neuraxial anesthesia for labor and delivery in eclampsia
Turner JA. Severe preeclampsia: anesthetic implications of the disease and its management. Am J Ther. 2009;16(4):284–248.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Cesarean delivery: a population-based study. Br J Anaesth. 2010;105(6):818–826.
Epidural, spinal, or combined anesthesia is safe in the absence of coagulopathy or severe thrombocytopenia. General anesthesia increases the risk of aspiration, failed intubation due to pharyngolaryngeal edema, and stroke secondary to the increase in systemic and intracerebral pressures during intubation and extubation.
Eclampsia is not an indication for cesarean delivery
Repke JT, Sibai BM. Preeclampsia and eclampsia. OBG Manage. 2009;21(4):44–55.
Once the mother has been resuscitated and stabilized, the provider should choose a mode of delivery that is based on fetal condition, gestational age, presence or absence of labor, and the cervical Bishop score. Vaginal delivery can be achieved in most patients who have a gestational age of 34 weeks or greater.
8. Late presentation happens
Be aware that eclampsia can develop for the first time as long as 28 days postpartum
Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e31–37.
Atypical eclampsia is any eclampsia that develops beyond 48 hours postpartum. A history of diagnosed predelivery preeclampsia is not necessary for development of late postpartum eclampsia. In general, more than 50% of patients who develop late postpartum eclampsia have no evidence of preeclampsia prior to delivery.
Be aware that the clinical and neuro-imaging features of eclampsia overlap with those of reversible cerebral vasoconstriction syndrome (angiopathy)
Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care. 2009;11(2):199–209.
Women who have reversible cerebral vasoconstriction syndrome have clinical findings (acute onset of recurrent headaches, visual changes, seizures, and hypertension) and cerebral magnetic resonance imaging (MRI) findings (posterior reversible encephalopathy syndrome) that are similar to those of women who have late postpartum eclampsia (FIGURE). However, in women who have postpartum cerebral angiopathy, cerebral angiography will show the presence of bead-like vasoconstriction—which is usually absent in eclampsia.
Posterior reversible encephalopathy syndrome
Green arrows point to vasogenic edema in the occipital lobes and, partially, the parietal lobes. The edema is gone on repeat magnetic resonance imaging (see Recommendation #9).
10. Act today, see a better outcome tomorrow
Avoid long-term maternal neurologic injury by managing eclampsia properly
Zeeman GG. Neurologic complications of preeclampsia. Semin Perinatol. 2009;33(3):166–172.
Residual neurologic damage is rare in the majority of women who have eclampsia. However, long-term cerebral white-matter injury (cytotoxic edema, infarction) on MRI imaging and impaired memory and cognitive function may develop in some women who have multiple seizures and who have inadequately controlled persistent severe hypertension.
We want to hear from you! Tell us what you think.
Because eclampsia occurs but rarely during pregnancy and the postpartum period, most health-care providers have little to no personal experience with management of this life-threatening obstetric emergency. Knowledge about maternal resuscitation during and after an eclamptic seizure is critical for improving maternal and perinatal outcomes.
In this round-up, I present 10 practical recommendations for prompt diagnosis and management of women who have eclampsia. Immediate implementation of these recommendations can lead to improved maternal and perinatal outcomes (both acute and long-term).
1. Practice. Practice again.
Implement regular monthly simulation training sessions
Fisher N, Bernstein PS, Satin A, et al. Resident training for eclampsia and magnesium toxicity management: simulation or traditional lecture? Am J Obstet Gynecol. 2010;203(4):379.e1–5.
Eclampsia is unpredictable and can develop rapidly at home, in labor and delivery, on the antepartum/postpartum ward, and in the emergency room. Therefore, it is prudent that all health-care providers who treat pregnant or postpartum women on a daily basis be trained and knowledgeable about early detection and management of eclampsia. This goal can be achieved by developing drills for rehearsal and by testing the response and skills of all providers.
2. Preventive: Magnesium sulfate
Do not attempt to arrest the seizure. Use MgSO4 to prevent recurrent convulsions.
Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulfate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;(12):CD000127.
Most eclamptic seizures are self-limiting. Therefore, there is no need to administer bolus drugs such as diazepam or midazolam. These drugs are usually used in the emergency room, but they inhibit maternal laryngeal reflexes and may lead to aspiration. They also suppress the central nervous system respiratory centers and can cause apnea, requiring intubation.
When used in the management of eclampsia, magnesium sulfate is associated with a lower rate of recurrent seizures and maternal death than is diazepam.
3. FHR changes? Be patient.
Do not rush the patient to emergent cesarean section because of an abnormal FHR tracing
Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
During an eclamptic convulsion, there is usually prolonged fetal heart rate (FHR) deceleration or even bradycardia—with or without an increase in both frequency and uterine tone. After the convulsion, as a result of maternal hypoxia and hypercarbia, the FHR tracing can show tachycardia, reduced beat-to-beat variability, and transient recurrent decelerations. When this happens, concern about fetal status can distract the obstetric provider from resuscitation of the mother. However, these FHR changes usually return to normal after maternal resuscitation. If the FHR changes persist for longer than 15 minutes, consider abruptio placentae and move to delivery.
4. Target: Lower BP
Reduce maternal blood pressure to a safe level to prevent stroke, but without compromising uteroplacental perfusion
Zwart JJ, Richters A, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Eclampsia in the Netherlands. Obstet Gynecol. 2008;112(4):820–827.
In this nationwide review of complications from eclampsia in the Netherlands, the authors found that failure to treat persistent severe hypertension was associated with hypertensive encephalopathy, cerebral infarction, bleeding, or congestive heart failure. They also found that 35.2% of women had systolic or diastolic blood pressure at or above 170/110 mm Hg at admission, but fewer than half were given antihypertensive drugs at that time. Among the cases deemed to have received substandard care, one third involved inadequate treatment of hypertension.
5. Know your antihypertensives
Learn which agents are best to control severe hypertension in eclampsia
Sibai BM. Hypertensive Emergencies. In: Foley MR, Strong TH, Garite TJ, eds. Obstetric Intensive Care Manual. 3rd ed. New York, NY: The McGraw-Hill Companies; 2010.
It is critical to familiarize oneself with the mechanism of action, dose, and potential side effects of agents used to control hypertension. For example, neither hydralazine nor nifedipine should be used in patients who have severe headache and persistent tachycardia (pulse, >100 bpm). Labetalol should be avoided in women who have persistent bradycardia (pulse, <60 bpm), asthma, or congestive heart failure.
For women who have persistent headache and tachycardia, I suggest intravenous (IV) labetalol, starting at a dose of 20 mg, 40 mg, or 80 mg every 10 minutes as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg. The maximum dose of labetalol should not exceed 300 mg in 1 hour.
For patients who have bradycardia and severe asthma, I suggest oral, rapid-acting nifedipine, starting at 10 mg to 20 mg, to be repeated in 20 to 30 minutes as needed, up to a maximum of 50 mg to 60 mg in 1 hour. Oral nifedipine can be used with magnesium sulfate. An alternative is an IV bolus injection of hydralazine, starting at a dose of 5 mg to 10 mg, to be repeated every 15 minutes, up to a maximum dose of 25 mg.
6. Avoid general anesthesia
Use neuraxial anesthesia for labor and delivery in eclampsia
Turner JA. Severe preeclampsia: anesthetic implications of the disease and its management. Am J Ther. 2009;16(4):284–248.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Cesarean delivery: a population-based study. Br J Anaesth. 2010;105(6):818–826.
Epidural, spinal, or combined anesthesia is safe in the absence of coagulopathy or severe thrombocytopenia. General anesthesia increases the risk of aspiration, failed intubation due to pharyngolaryngeal edema, and stroke secondary to the increase in systemic and intracerebral pressures during intubation and extubation.
Eclampsia is not an indication for cesarean delivery
Repke JT, Sibai BM. Preeclampsia and eclampsia. OBG Manage. 2009;21(4):44–55.
Once the mother has been resuscitated and stabilized, the provider should choose a mode of delivery that is based on fetal condition, gestational age, presence or absence of labor, and the cervical Bishop score. Vaginal delivery can be achieved in most patients who have a gestational age of 34 weeks or greater.
8. Late presentation happens
Be aware that eclampsia can develop for the first time as long as 28 days postpartum
Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e31–37.
Atypical eclampsia is any eclampsia that develops beyond 48 hours postpartum. A history of diagnosed predelivery preeclampsia is not necessary for development of late postpartum eclampsia. In general, more than 50% of patients who develop late postpartum eclampsia have no evidence of preeclampsia prior to delivery.
Be aware that the clinical and neuro-imaging features of eclampsia overlap with those of reversible cerebral vasoconstriction syndrome (angiopathy)
Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care. 2009;11(2):199–209.
Women who have reversible cerebral vasoconstriction syndrome have clinical findings (acute onset of recurrent headaches, visual changes, seizures, and hypertension) and cerebral magnetic resonance imaging (MRI) findings (posterior reversible encephalopathy syndrome) that are similar to those of women who have late postpartum eclampsia (FIGURE). However, in women who have postpartum cerebral angiopathy, cerebral angiography will show the presence of bead-like vasoconstriction—which is usually absent in eclampsia.
Posterior reversible encephalopathy syndrome
Green arrows point to vasogenic edema in the occipital lobes and, partially, the parietal lobes. The edema is gone on repeat magnetic resonance imaging (see Recommendation #9).
10. Act today, see a better outcome tomorrow
Avoid long-term maternal neurologic injury by managing eclampsia properly
Zeeman GG. Neurologic complications of preeclampsia. Semin Perinatol. 2009;33(3):166–172.
Residual neurologic damage is rare in the majority of women who have eclampsia. However, long-term cerebral white-matter injury (cytotoxic edema, infarction) on MRI imaging and impaired memory and cognitive function may develop in some women who have multiple seizures and who have inadequately controlled persistent severe hypertension.
We want to hear from you! Tell us what you think.
New data: Foley catheter is as effective as prostaglandin gel for induction of labor—with fewer side effects
The Foley catheter is one of the oldest mechanical methods used for inducing labor. New data show that it produces a vaginal delivery rate similar to that of prostaglandin E2 gel, the current treatment of choice in the United States and United Kingdom—but carries fewer side effects.1
The newly published PROBAAT trial suggests that a mechanical approach to induction of labor could reduce complications; it also challenges the belief that mechanical methods increase the risk of infection for mothers and newborns.1
In the open-label trial of 824 women in 12 hospitals in the Netherlands, women who had a singleton gestation in cephalic presentation, with intact membranes and an unfavorable cervix, were randomized to induction of labor with a 30-mL Foley catheter (n=412) or vaginal prostaglandin E2 gel (n=412). The rate of cesarean delivery was similar between groups (23% for the Foley catheter vs 20% for prostaglandin gel; risk ratio, 1.13; 95% confidence interval [CI], 0.87–1.47). Two serious maternal adverse events were recorded in the prostaglandin group—one uterine perforation after insertion of a uterine pressure catheter and one uterine rupture during augmentation with oxytocin—versus none in the Foley-catheter group, though this difference was not statistically significant.1
In addition, the women randomized to the Foley catheter had a lower rate of operative delivery for suspected fetal distress, fewer mothers required intrapartum antibiotics, and fewer newborns were admitted to the NICU. However, the time from the start of induction of labor to birth was longer with the Foley catheter (median of 29 versus 18 hours; relative risk, 1.66; 95% CI, 1.34-1.61; P < .0001).
A meta-analysis that included these new data was then conducted by the researchers; it confirmed that induction of labor with the Foley catheter produces a vaginal delivery rate similar to that of prostaglandin E2 gel and significantly reduces the rates of uterine hyperstimulation and postpartum hemorrhage. This finding is in line with another recent meta-analysis.2
In a commentary accompanying the study, Jane Norman and Sarah Stock of the University of Edinburgh in Edinburgh, Scotland, conclude: “These data should prompt a revision of the recommendation that ‘mechanical procedures (balloon catheters and laminaria tents) should not be used routinely for induction of [labor].’”3
The authors of the study itself conclude: “We think that a Foley catheter should at least be considered for induction of labor in women with an unfavorable cervix at term.”
We want to hear from you! Tell us what you think.
1. Jozwiak M, Rengerink KO, Benthem M, et al. for the PROBAAT Study Group. Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT trial): an open-label, randomised controlled trial [published online ahead of print October 25, 2011]. Lancet. doi: 10.1016/S0140-6736(11)61484-0.
2. Vaknin Z, Kurzweil Y, Sherman D, et al. Foley catheter balloon vs locally applied prostaglandins for cervical ripening and labor induction: a systematic review and meta-analysis. Am J Obstet Gynecol. 2010;203(5):418-429.
3. Norman JE, Stock S. Intracervical Foley catheter for induction of labour [published online ahead of print October 25, 2011]. Lancet. doi: 10.1016/S0140-6736(11)61581-X.
The Foley catheter is one of the oldest mechanical methods used for inducing labor. New data show that it produces a vaginal delivery rate similar to that of prostaglandin E2 gel, the current treatment of choice in the United States and United Kingdom—but carries fewer side effects.1
The newly published PROBAAT trial suggests that a mechanical approach to induction of labor could reduce complications; it also challenges the belief that mechanical methods increase the risk of infection for mothers and newborns.1
In the open-label trial of 824 women in 12 hospitals in the Netherlands, women who had a singleton gestation in cephalic presentation, with intact membranes and an unfavorable cervix, were randomized to induction of labor with a 30-mL Foley catheter (n=412) or vaginal prostaglandin E2 gel (n=412). The rate of cesarean delivery was similar between groups (23% for the Foley catheter vs 20% for prostaglandin gel; risk ratio, 1.13; 95% confidence interval [CI], 0.87–1.47). Two serious maternal adverse events were recorded in the prostaglandin group—one uterine perforation after insertion of a uterine pressure catheter and one uterine rupture during augmentation with oxytocin—versus none in the Foley-catheter group, though this difference was not statistically significant.1
In addition, the women randomized to the Foley catheter had a lower rate of operative delivery for suspected fetal distress, fewer mothers required intrapartum antibiotics, and fewer newborns were admitted to the NICU. However, the time from the start of induction of labor to birth was longer with the Foley catheter (median of 29 versus 18 hours; relative risk, 1.66; 95% CI, 1.34-1.61; P < .0001).
A meta-analysis that included these new data was then conducted by the researchers; it confirmed that induction of labor with the Foley catheter produces a vaginal delivery rate similar to that of prostaglandin E2 gel and significantly reduces the rates of uterine hyperstimulation and postpartum hemorrhage. This finding is in line with another recent meta-analysis.2
In a commentary accompanying the study, Jane Norman and Sarah Stock of the University of Edinburgh in Edinburgh, Scotland, conclude: “These data should prompt a revision of the recommendation that ‘mechanical procedures (balloon catheters and laminaria tents) should not be used routinely for induction of [labor].’”3
The authors of the study itself conclude: “We think that a Foley catheter should at least be considered for induction of labor in women with an unfavorable cervix at term.”
We want to hear from you! Tell us what you think.
The Foley catheter is one of the oldest mechanical methods used for inducing labor. New data show that it produces a vaginal delivery rate similar to that of prostaglandin E2 gel, the current treatment of choice in the United States and United Kingdom—but carries fewer side effects.1
The newly published PROBAAT trial suggests that a mechanical approach to induction of labor could reduce complications; it also challenges the belief that mechanical methods increase the risk of infection for mothers and newborns.1
In the open-label trial of 824 women in 12 hospitals in the Netherlands, women who had a singleton gestation in cephalic presentation, with intact membranes and an unfavorable cervix, were randomized to induction of labor with a 30-mL Foley catheter (n=412) or vaginal prostaglandin E2 gel (n=412). The rate of cesarean delivery was similar between groups (23% for the Foley catheter vs 20% for prostaglandin gel; risk ratio, 1.13; 95% confidence interval [CI], 0.87–1.47). Two serious maternal adverse events were recorded in the prostaglandin group—one uterine perforation after insertion of a uterine pressure catheter and one uterine rupture during augmentation with oxytocin—versus none in the Foley-catheter group, though this difference was not statistically significant.1
In addition, the women randomized to the Foley catheter had a lower rate of operative delivery for suspected fetal distress, fewer mothers required intrapartum antibiotics, and fewer newborns were admitted to the NICU. However, the time from the start of induction of labor to birth was longer with the Foley catheter (median of 29 versus 18 hours; relative risk, 1.66; 95% CI, 1.34-1.61; P < .0001).
A meta-analysis that included these new data was then conducted by the researchers; it confirmed that induction of labor with the Foley catheter produces a vaginal delivery rate similar to that of prostaglandin E2 gel and significantly reduces the rates of uterine hyperstimulation and postpartum hemorrhage. This finding is in line with another recent meta-analysis.2
In a commentary accompanying the study, Jane Norman and Sarah Stock of the University of Edinburgh in Edinburgh, Scotland, conclude: “These data should prompt a revision of the recommendation that ‘mechanical procedures (balloon catheters and laminaria tents) should not be used routinely for induction of [labor].’”3
The authors of the study itself conclude: “We think that a Foley catheter should at least be considered for induction of labor in women with an unfavorable cervix at term.”
We want to hear from you! Tell us what you think.
1. Jozwiak M, Rengerink KO, Benthem M, et al. for the PROBAAT Study Group. Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT trial): an open-label, randomised controlled trial [published online ahead of print October 25, 2011]. Lancet. doi: 10.1016/S0140-6736(11)61484-0.
2. Vaknin Z, Kurzweil Y, Sherman D, et al. Foley catheter balloon vs locally applied prostaglandins for cervical ripening and labor induction: a systematic review and meta-analysis. Am J Obstet Gynecol. 2010;203(5):418-429.
3. Norman JE, Stock S. Intracervical Foley catheter for induction of labour [published online ahead of print October 25, 2011]. Lancet. doi: 10.1016/S0140-6736(11)61581-X.
1. Jozwiak M, Rengerink KO, Benthem M, et al. for the PROBAAT Study Group. Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT trial): an open-label, randomised controlled trial [published online ahead of print October 25, 2011]. Lancet. doi: 10.1016/S0140-6736(11)61484-0.
2. Vaknin Z, Kurzweil Y, Sherman D, et al. Foley catheter balloon vs locally applied prostaglandins for cervical ripening and labor induction: a systematic review and meta-analysis. Am J Obstet Gynecol. 2010;203(5):418-429.
3. Norman JE, Stock S. Intracervical Foley catheter for induction of labour [published online ahead of print October 25, 2011]. Lancet. doi: 10.1016/S0140-6736(11)61581-X.
What is the optimal interval between administration of antenatal corticosteroids and preterm delivery?
In the early 1970s, as a direct by-product of basic science research on preterm birth using a sheep model, Liggins and Howie discovered that the administration of corticosteroids to women facing impending preterm birth could lower the risk not only of respiratory morbidity but also of intraventricular hemorrhage, necrotizing colitis, and death for their newborns. This discovery was confirmed in other clinical trials, and the novel strategy of a secondary therapy to reduce the sequelae of preterm birth became part of standard perinatal care. Despite this advance, numerous questions remain, including the proper dosing interval and number of doses of corticosteroids necessary to prevent the sequelae of preterm birth.
Authors explored births before 34 weeks’ gestation
To elucidate the proper dosing interval, Wilms and colleagues conducted a retrospective cohort study among women who received antenatal corticosteroids and delivered before 34 weeks’ gestation. Of the 254 infants who were delivered prematurely, those delivered within 7 days of the administration of corticosteroids had a reduced risk of requiring intervention in the NICU. The authors concluded that the efficacy of antenatal corticosteroids diminishes when the treatment-to-delivery interval exceeds 7 days.
Limitations of the study
The investigators admonish readers to carefully consider the timing of the first dose of corticosteroid therapy. Their conclusions are limited by 1) the retrospective nature of their research and 2) the fact that clinicians who cared for newborns in this study were aware of the timing of maternal corticosteroid administration.
Nevertheless, clinicians can draw pertinent points for day-to-day practice:
- The window of benefit seems to be time-limited, with a break-point and diminution at 7 days after administration of corticosteroids
- A second dose of antenatal corticosteroids may be of benefit in selected situations.
Repetitive dosing beyond two rounds 1 week apart appears to have no benefit, according to clinical trials of weekly versus one-time dosing. Moreover, repetitive dosing causes small but significant decrements in birth weight and head circumference. Whether these changes are associated with long-term harm remains unknown.
Other authors have drawn similar conclusions—but we need prospective randomized, controlled trials to clarify the issue of whether to repeat corticosteroid administration and, if so, how many doses are optimal and how often they should be given.
In women who are given antenatal corticosteroids for impending preterm birth, pay attention to the interval between administration and delivery. In this study, an interval of 7 days or less was associated with a reduced need for intervention in the NICU.
John M. Thorp, Jr, MD
We want to hear from you! Tell us what you think.
In the early 1970s, as a direct by-product of basic science research on preterm birth using a sheep model, Liggins and Howie discovered that the administration of corticosteroids to women facing impending preterm birth could lower the risk not only of respiratory morbidity but also of intraventricular hemorrhage, necrotizing colitis, and death for their newborns. This discovery was confirmed in other clinical trials, and the novel strategy of a secondary therapy to reduce the sequelae of preterm birth became part of standard perinatal care. Despite this advance, numerous questions remain, including the proper dosing interval and number of doses of corticosteroids necessary to prevent the sequelae of preterm birth.
Authors explored births before 34 weeks’ gestation
To elucidate the proper dosing interval, Wilms and colleagues conducted a retrospective cohort study among women who received antenatal corticosteroids and delivered before 34 weeks’ gestation. Of the 254 infants who were delivered prematurely, those delivered within 7 days of the administration of corticosteroids had a reduced risk of requiring intervention in the NICU. The authors concluded that the efficacy of antenatal corticosteroids diminishes when the treatment-to-delivery interval exceeds 7 days.
Limitations of the study
The investigators admonish readers to carefully consider the timing of the first dose of corticosteroid therapy. Their conclusions are limited by 1) the retrospective nature of their research and 2) the fact that clinicians who cared for newborns in this study were aware of the timing of maternal corticosteroid administration.
Nevertheless, clinicians can draw pertinent points for day-to-day practice:
- The window of benefit seems to be time-limited, with a break-point and diminution at 7 days after administration of corticosteroids
- A second dose of antenatal corticosteroids may be of benefit in selected situations.
Repetitive dosing beyond two rounds 1 week apart appears to have no benefit, according to clinical trials of weekly versus one-time dosing. Moreover, repetitive dosing causes small but significant decrements in birth weight and head circumference. Whether these changes are associated with long-term harm remains unknown.
Other authors have drawn similar conclusions—but we need prospective randomized, controlled trials to clarify the issue of whether to repeat corticosteroid administration and, if so, how many doses are optimal and how often they should be given.
In women who are given antenatal corticosteroids for impending preterm birth, pay attention to the interval between administration and delivery. In this study, an interval of 7 days or less was associated with a reduced need for intervention in the NICU.
John M. Thorp, Jr, MD
We want to hear from you! Tell us what you think.
In the early 1970s, as a direct by-product of basic science research on preterm birth using a sheep model, Liggins and Howie discovered that the administration of corticosteroids to women facing impending preterm birth could lower the risk not only of respiratory morbidity but also of intraventricular hemorrhage, necrotizing colitis, and death for their newborns. This discovery was confirmed in other clinical trials, and the novel strategy of a secondary therapy to reduce the sequelae of preterm birth became part of standard perinatal care. Despite this advance, numerous questions remain, including the proper dosing interval and number of doses of corticosteroids necessary to prevent the sequelae of preterm birth.
Authors explored births before 34 weeks’ gestation
To elucidate the proper dosing interval, Wilms and colleagues conducted a retrospective cohort study among women who received antenatal corticosteroids and delivered before 34 weeks’ gestation. Of the 254 infants who were delivered prematurely, those delivered within 7 days of the administration of corticosteroids had a reduced risk of requiring intervention in the NICU. The authors concluded that the efficacy of antenatal corticosteroids diminishes when the treatment-to-delivery interval exceeds 7 days.
Limitations of the study
The investigators admonish readers to carefully consider the timing of the first dose of corticosteroid therapy. Their conclusions are limited by 1) the retrospective nature of their research and 2) the fact that clinicians who cared for newborns in this study were aware of the timing of maternal corticosteroid administration.
Nevertheless, clinicians can draw pertinent points for day-to-day practice:
- The window of benefit seems to be time-limited, with a break-point and diminution at 7 days after administration of corticosteroids
- A second dose of antenatal corticosteroids may be of benefit in selected situations.
Repetitive dosing beyond two rounds 1 week apart appears to have no benefit, according to clinical trials of weekly versus one-time dosing. Moreover, repetitive dosing causes small but significant decrements in birth weight and head circumference. Whether these changes are associated with long-term harm remains unknown.
Other authors have drawn similar conclusions—but we need prospective randomized, controlled trials to clarify the issue of whether to repeat corticosteroid administration and, if so, how many doses are optimal and how often they should be given.
In women who are given antenatal corticosteroids for impending preterm birth, pay attention to the interval between administration and delivery. In this study, an interval of 7 days or less was associated with a reduced need for intervention in the NICU.
John M. Thorp, Jr, MD
We want to hear from you! Tell us what you think.
Noninvasive test identifies more than 98% of Down syndrome cases
The first noninvasive maternal blood test for Down syndrome is available to physicians on request in 20 major metropolitan regions in the United States, with more widespread availability planned in the coming months.
According to manufacturer Sequenom, Inc., the MaterniT21 assay is indicated for use in pregnant women at “high risk” of carrying a fetus with Down syndrome and can accurately test maternal blood as early as 10 weeks of gestation.
The test is based on the presence of cell-free fetal nucleic acids in maternal plasma. The health provider draws a small sample of whole blood from a pregnant woman and ships the sample to the Sequenom Center for Molecular Medicine in San Diego, California, where massively parallel sequencing (MPS) is used to measure a possible overabundance of chromosome 21, or Trisomy 21 (Down syndrome).
Down syndrome affects approximately 6,000 infants or 1 in every 691 pregnancies each year in the United States.1
Currently, identification of Trisomy 21 requires assessment of several maternal serum screening markers and sonographic measurement of nuchal translucency. If these tests identify a high risk of Trisomy 21, chorionic villus sampling (CVS) or amniocentesis follows. First-trimester screening identifies as many as 90% of all cases of Down syndrome, with a false-positive rate of 2%.1 Because CVS and amniocentesis are invasive, however, they carry a small risk of pregnancy loss.
All MaterniT21 tests must be interpreted at one of the manufacturer’s laboratories. Approximate cost: $1,900—roughly equivalent to the cost of amniocentesis. Sequenom expects the out-of-pocket cost for patients with insurance to be no more than $235.
The test is not approved by the US Food and Drug Administration, which does not regulate tests when only one laboratory is involved.
Similar tests for Down syndrome are reportedly being developed by other laboratories.
Data on the new test
MaterniT21 was evaluated in a validation study of 4,664 women who had a high risk of Down syndrome.2 Fetal karyotyping was compared with the MaterniT21 test in 212 cases of Down syndrome and 1,484 matched euploid cases. The Down syndrome detection rate for the MaterniT21 test was 98.6% (209 of 212 cases); the false-positive rate was 0.20% (three of 1,471 cases); and testing failed in 13 pregnancies (0.8%), all of which were euploid. Researchers concluded that the new test “can substantially reduce the need for invasive diagnostic procedures and attendant procedure-related fetal losses.”2
“The results of this large clinical validation study are extremely promising,” said Allan T. Bombard, MD, laboratory director of Sequenom Center for Molecular Medicine and one of the authors of the study. “We believe perinatal specialists and obstetricians will appreciate the introduction of a test that is noninvasive and highly specific.”
The manufacturer is planning outreach to clinicians through its sales force, medical science liaisons, managed care team, and genetic counselors.
Is the test ready for widespread use?
“I certainly think this test needs to be confirmed by other studies before it becomes ready for prime time,” says Jeffrey A. Kuller, MD, a maternal-fetal medicine specialist and clinical geneticist at Duke University Medical Center in Durham, NC. Dr. Kuller notes that the MaterniT21 test detects only Trisomy 21, whereas conventional first-trimester screening assesses Trisomy 18 and, usually, Trisomy 13 as well. Dr. Kuller is professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke.
“Another big question mark is what does one do with an abnormal result? We have a pretty standardized algorithm for what we do if a patient has an abnormal first-trimester screen,” he points out—”they’re offered invasive testing.” But it’s unclear whether this test is intended to be diagnostic if it detects Trisomy 21.
“Can we essentially tell a patient, ‘You’re carrying a fetus with Down syndrome?’ Or do we need to do invasive testing like we do right now?”
Another unresolved issue is whether the test can be used reliably in a “low-risk” population. The creators of the MaterniT21 assay tested the product only in a “high-risk” population, Dr. Kuller notes. “I don’t think we know whether this would be a reasonable test for all patients.”
These three issues remain unresolved, says Dr. Kuller:
- confirmation of the test’s efficacy in additional studies
- clarification of whether it is diagnostic of Down syndrome
- evaluation of the test in a population at low risk of Trisomy 21.
Panel offers recommendations for genetic counseling
The International Society for Prenatal Diagnosis (ISPD) issued a “rapid response statement” on the new test on October 24: “At this time, individual women who might be considering the MPS test need to receive detailed genetic counseling that explains the benefits and limitations of the test. Testing should only be provided after an informed consent.”3
The ISPD recommends that the following information be conveyed to the patient:
- The test identifies only Down syndrome, or only “about half of the fetal aneuploidy that would be identified through amniocentesis or CVS”.
- The test is not perfect; that is, it does not identify all cases of fetal Down syndrome.
- Because false-positive results are possible, women who test positive still need the result confirmed by amniocentesis or CVS.
- If a woman tests positive for Down syndrome on the MPS test, the waiting period for the results of confirmatory testing may be “highly stressful”.
- The MPS test may not be informative in all cases.
- Women who have an elevated risk of having a child with a “prenatally diagnosable disorder with Mendelian pattern of inheritance, microdeletion syndrome, or some other conditions” still need to undergo amniocentesis or CVS.3
We want to hear from you! Tell us what you think.
1. Centers for Disease Control and Prevention Facts about Down Syndrome.http://www.cdc.gov/ncbddd/birthdefects/DownSyndrome.html. Updated June 8 2011. Accessed October 24, 2011.
2. Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study [published online ahead of print October 14, 2011. Genet Med. 2011. doi: 10.1097/GIM.0b013e3182368a0e.
3. International Society for Prenatal Diagnosis. ISPD Rapid Response Statement: Prenatal Detection of Down Syndrome using Massively Parallel Sequencing (MPS): a rapid response statement from a committee on behalf of the Board of the International Society for Prenatal Diagnosis 24 October 2011. Charlottesville, Va: ISPD; 2011.
The first noninvasive maternal blood test for Down syndrome is available to physicians on request in 20 major metropolitan regions in the United States, with more widespread availability planned in the coming months.
According to manufacturer Sequenom, Inc., the MaterniT21 assay is indicated for use in pregnant women at “high risk” of carrying a fetus with Down syndrome and can accurately test maternal blood as early as 10 weeks of gestation.
The test is based on the presence of cell-free fetal nucleic acids in maternal plasma. The health provider draws a small sample of whole blood from a pregnant woman and ships the sample to the Sequenom Center for Molecular Medicine in San Diego, California, where massively parallel sequencing (MPS) is used to measure a possible overabundance of chromosome 21, or Trisomy 21 (Down syndrome).
Down syndrome affects approximately 6,000 infants or 1 in every 691 pregnancies each year in the United States.1
Currently, identification of Trisomy 21 requires assessment of several maternal serum screening markers and sonographic measurement of nuchal translucency. If these tests identify a high risk of Trisomy 21, chorionic villus sampling (CVS) or amniocentesis follows. First-trimester screening identifies as many as 90% of all cases of Down syndrome, with a false-positive rate of 2%.1 Because CVS and amniocentesis are invasive, however, they carry a small risk of pregnancy loss.
All MaterniT21 tests must be interpreted at one of the manufacturer’s laboratories. Approximate cost: $1,900—roughly equivalent to the cost of amniocentesis. Sequenom expects the out-of-pocket cost for patients with insurance to be no more than $235.
The test is not approved by the US Food and Drug Administration, which does not regulate tests when only one laboratory is involved.
Similar tests for Down syndrome are reportedly being developed by other laboratories.
Data on the new test
MaterniT21 was evaluated in a validation study of 4,664 women who had a high risk of Down syndrome.2 Fetal karyotyping was compared with the MaterniT21 test in 212 cases of Down syndrome and 1,484 matched euploid cases. The Down syndrome detection rate for the MaterniT21 test was 98.6% (209 of 212 cases); the false-positive rate was 0.20% (three of 1,471 cases); and testing failed in 13 pregnancies (0.8%), all of which were euploid. Researchers concluded that the new test “can substantially reduce the need for invasive diagnostic procedures and attendant procedure-related fetal losses.”2
“The results of this large clinical validation study are extremely promising,” said Allan T. Bombard, MD, laboratory director of Sequenom Center for Molecular Medicine and one of the authors of the study. “We believe perinatal specialists and obstetricians will appreciate the introduction of a test that is noninvasive and highly specific.”
The manufacturer is planning outreach to clinicians through its sales force, medical science liaisons, managed care team, and genetic counselors.
Is the test ready for widespread use?
“I certainly think this test needs to be confirmed by other studies before it becomes ready for prime time,” says Jeffrey A. Kuller, MD, a maternal-fetal medicine specialist and clinical geneticist at Duke University Medical Center in Durham, NC. Dr. Kuller notes that the MaterniT21 test detects only Trisomy 21, whereas conventional first-trimester screening assesses Trisomy 18 and, usually, Trisomy 13 as well. Dr. Kuller is professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke.
“Another big question mark is what does one do with an abnormal result? We have a pretty standardized algorithm for what we do if a patient has an abnormal first-trimester screen,” he points out—”they’re offered invasive testing.” But it’s unclear whether this test is intended to be diagnostic if it detects Trisomy 21.
“Can we essentially tell a patient, ‘You’re carrying a fetus with Down syndrome?’ Or do we need to do invasive testing like we do right now?”
Another unresolved issue is whether the test can be used reliably in a “low-risk” population. The creators of the MaterniT21 assay tested the product only in a “high-risk” population, Dr. Kuller notes. “I don’t think we know whether this would be a reasonable test for all patients.”
These three issues remain unresolved, says Dr. Kuller:
- confirmation of the test’s efficacy in additional studies
- clarification of whether it is diagnostic of Down syndrome
- evaluation of the test in a population at low risk of Trisomy 21.
Panel offers recommendations for genetic counseling
The International Society for Prenatal Diagnosis (ISPD) issued a “rapid response statement” on the new test on October 24: “At this time, individual women who might be considering the MPS test need to receive detailed genetic counseling that explains the benefits and limitations of the test. Testing should only be provided after an informed consent.”3
The ISPD recommends that the following information be conveyed to the patient:
- The test identifies only Down syndrome, or only “about half of the fetal aneuploidy that would be identified through amniocentesis or CVS”.
- The test is not perfect; that is, it does not identify all cases of fetal Down syndrome.
- Because false-positive results are possible, women who test positive still need the result confirmed by amniocentesis or CVS.
- If a woman tests positive for Down syndrome on the MPS test, the waiting period for the results of confirmatory testing may be “highly stressful”.
- The MPS test may not be informative in all cases.
- Women who have an elevated risk of having a child with a “prenatally diagnosable disorder with Mendelian pattern of inheritance, microdeletion syndrome, or some other conditions” still need to undergo amniocentesis or CVS.3
We want to hear from you! Tell us what you think.
The first noninvasive maternal blood test for Down syndrome is available to physicians on request in 20 major metropolitan regions in the United States, with more widespread availability planned in the coming months.
According to manufacturer Sequenom, Inc., the MaterniT21 assay is indicated for use in pregnant women at “high risk” of carrying a fetus with Down syndrome and can accurately test maternal blood as early as 10 weeks of gestation.
The test is based on the presence of cell-free fetal nucleic acids in maternal plasma. The health provider draws a small sample of whole blood from a pregnant woman and ships the sample to the Sequenom Center for Molecular Medicine in San Diego, California, where massively parallel sequencing (MPS) is used to measure a possible overabundance of chromosome 21, or Trisomy 21 (Down syndrome).
Down syndrome affects approximately 6,000 infants or 1 in every 691 pregnancies each year in the United States.1
Currently, identification of Trisomy 21 requires assessment of several maternal serum screening markers and sonographic measurement of nuchal translucency. If these tests identify a high risk of Trisomy 21, chorionic villus sampling (CVS) or amniocentesis follows. First-trimester screening identifies as many as 90% of all cases of Down syndrome, with a false-positive rate of 2%.1 Because CVS and amniocentesis are invasive, however, they carry a small risk of pregnancy loss.
All MaterniT21 tests must be interpreted at one of the manufacturer’s laboratories. Approximate cost: $1,900—roughly equivalent to the cost of amniocentesis. Sequenom expects the out-of-pocket cost for patients with insurance to be no more than $235.
The test is not approved by the US Food and Drug Administration, which does not regulate tests when only one laboratory is involved.
Similar tests for Down syndrome are reportedly being developed by other laboratories.
Data on the new test
MaterniT21 was evaluated in a validation study of 4,664 women who had a high risk of Down syndrome.2 Fetal karyotyping was compared with the MaterniT21 test in 212 cases of Down syndrome and 1,484 matched euploid cases. The Down syndrome detection rate for the MaterniT21 test was 98.6% (209 of 212 cases); the false-positive rate was 0.20% (three of 1,471 cases); and testing failed in 13 pregnancies (0.8%), all of which were euploid. Researchers concluded that the new test “can substantially reduce the need for invasive diagnostic procedures and attendant procedure-related fetal losses.”2
“The results of this large clinical validation study are extremely promising,” said Allan T. Bombard, MD, laboratory director of Sequenom Center for Molecular Medicine and one of the authors of the study. “We believe perinatal specialists and obstetricians will appreciate the introduction of a test that is noninvasive and highly specific.”
The manufacturer is planning outreach to clinicians through its sales force, medical science liaisons, managed care team, and genetic counselors.
Is the test ready for widespread use?
“I certainly think this test needs to be confirmed by other studies before it becomes ready for prime time,” says Jeffrey A. Kuller, MD, a maternal-fetal medicine specialist and clinical geneticist at Duke University Medical Center in Durham, NC. Dr. Kuller notes that the MaterniT21 test detects only Trisomy 21, whereas conventional first-trimester screening assesses Trisomy 18 and, usually, Trisomy 13 as well. Dr. Kuller is professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke.
“Another big question mark is what does one do with an abnormal result? We have a pretty standardized algorithm for what we do if a patient has an abnormal first-trimester screen,” he points out—”they’re offered invasive testing.” But it’s unclear whether this test is intended to be diagnostic if it detects Trisomy 21.
“Can we essentially tell a patient, ‘You’re carrying a fetus with Down syndrome?’ Or do we need to do invasive testing like we do right now?”
Another unresolved issue is whether the test can be used reliably in a “low-risk” population. The creators of the MaterniT21 assay tested the product only in a “high-risk” population, Dr. Kuller notes. “I don’t think we know whether this would be a reasonable test for all patients.”
These three issues remain unresolved, says Dr. Kuller:
- confirmation of the test’s efficacy in additional studies
- clarification of whether it is diagnostic of Down syndrome
- evaluation of the test in a population at low risk of Trisomy 21.
Panel offers recommendations for genetic counseling
The International Society for Prenatal Diagnosis (ISPD) issued a “rapid response statement” on the new test on October 24: “At this time, individual women who might be considering the MPS test need to receive detailed genetic counseling that explains the benefits and limitations of the test. Testing should only be provided after an informed consent.”3
The ISPD recommends that the following information be conveyed to the patient:
- The test identifies only Down syndrome, or only “about half of the fetal aneuploidy that would be identified through amniocentesis or CVS”.
- The test is not perfect; that is, it does not identify all cases of fetal Down syndrome.
- Because false-positive results are possible, women who test positive still need the result confirmed by amniocentesis or CVS.
- If a woman tests positive for Down syndrome on the MPS test, the waiting period for the results of confirmatory testing may be “highly stressful”.
- The MPS test may not be informative in all cases.
- Women who have an elevated risk of having a child with a “prenatally diagnosable disorder with Mendelian pattern of inheritance, microdeletion syndrome, or some other conditions” still need to undergo amniocentesis or CVS.3
We want to hear from you! Tell us what you think.
1. Centers for Disease Control and Prevention Facts about Down Syndrome.http://www.cdc.gov/ncbddd/birthdefects/DownSyndrome.html. Updated June 8 2011. Accessed October 24, 2011.
2. Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study [published online ahead of print October 14, 2011. Genet Med. 2011. doi: 10.1097/GIM.0b013e3182368a0e.
3. International Society for Prenatal Diagnosis. ISPD Rapid Response Statement: Prenatal Detection of Down Syndrome using Massively Parallel Sequencing (MPS): a rapid response statement from a committee on behalf of the Board of the International Society for Prenatal Diagnosis 24 October 2011. Charlottesville, Va: ISPD; 2011.
1. Centers for Disease Control and Prevention Facts about Down Syndrome.http://www.cdc.gov/ncbddd/birthdefects/DownSyndrome.html. Updated June 8 2011. Accessed October 24, 2011.
2. Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study [published online ahead of print October 14, 2011. Genet Med. 2011. doi: 10.1097/GIM.0b013e3182368a0e.
3. International Society for Prenatal Diagnosis. ISPD Rapid Response Statement: Prenatal Detection of Down Syndrome using Massively Parallel Sequencing (MPS): a rapid response statement from a committee on behalf of the Board of the International Society for Prenatal Diagnosis 24 October 2011. Charlottesville, Va: ISPD; 2011.

