User login
Difficult fetal extraction at cesarean delivery: What should you do?
Do you have a clinical pearl for delivering a deflexed, deeply impacted fetal head at cesarean delivery?
CASE At 2 Am, the director of nursing pages you, asking you to attend to a 26-year-old gravida 1 para 0 who has just been brought by ambulance to labor and delivery after attempting home birth.
The midwife caring for the patient reports that she has been fully dilated for 7 hours and has been pushing for 4 hours. You confirm that the patient is fully dilated, with the presenting part at +1 station; significant caput succedaneum; and molding of the fetal head.
Estimated fetal weight is 3,600 g. The fetal heart-rate tracing is Category II.
You recommend a cesarean delivery. The exhausted patient agrees, reluctantly.
But when you attempt to deliver the fetal head, you realize that it is deflexed and stuck deep in the pelvis. It’s going to be very difficult to deliver the head without trauma to the lower uterine segment, upper vagina and, possibly, the bladder.
What should you do now?
Experienced clinicians often recognize, instinctively, a looming catastrophe before it happens because we have seen a similar situation earlier in our career.1 After we identify the potential for trouble, we attempt to avert disaster by taking preventive action.
One very good occasion to use that “clinical sixth sense”
Consider the situation in which labor has been complicated by a long second stage, with a large fetus—a disaster waiting to happen. In such a case, cesarean delivery, if it is necessary, can be difficult to perform because the fetal head is stuck deep in the pelvis. Attempting to deliver a deeply impacted fetal head, using standard delivery maneuvers, may cause extensive trauma to the lower uterine segment, vagina, and bladder, and fetal injury. In turn, ureteral injury or postpartum hemorrhage may occur during your repair of damage to the lower uterine segment, vagina, or bladder.
In the scenario described a moment ago, the fact that the patient was in the second stage of labor for 7 hours, at home, without anesthesia, and with failure to progress to vaginal delivery increases the likelihood that the fetal head is impacted deep in the pelvis. Before you perform cesarean delivery, you might find it helpful to perform a vaginal examination to answer two questions:
- On vaginal examination, between contractions, can the fetal head be gently moved out of the pelvis? Or is it deeply impacted?
- Is there sufficient space between the fetal head and symphysis pubis to permit delivery with standard cesarean maneuvers?
If the head is impacted deep in the pelvis, I encourage you to consider alternative approaches to cesarean delivery, including reverse breech extraction (FIGURE 1) or an assist from a vaginal hand (FIGURE 2) to facilitate delivery.
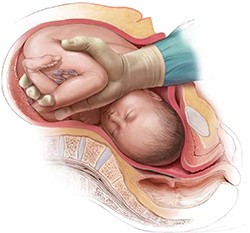
FIGURE 1 Reverse breech extraction—the “pull technique”
Once the uterus has been opened, reach immediately into the upper segment for a fetal leg. Apply gentle traction on the leg until the other leg appears. With two legs held together, deliver (pull) the body of the fetus out of the uterus.
The “pull technique”: Reverse breech extraction
One randomized trial and one retrospective study have evaluated the use of reverse breech extraction (the pull technique) in comparison to pushing up with a vaginal hand from below (the push technique) for managing a difficult cesarean delivery after obstructed labor.
Results of a clinical trial. 108 Nigerian women who had obstructed labor were randomly assigned to a pull technique (reverse breech extraction) or a push technique (assist from a vaginal hand).2
The push technique in this study was reportedly performed with a “finger” in the vagina pushing up on the fetal head while the surgeon attempted to deliver the head in a standard fashion.
The pull technique was performed by opening the uterus, immediately reaching into the upper uterus for a fetal leg, and applying gentle traction on the leg until the second leg appeared. Then, with two legs held together, the body of the fetus was delivered (pulled) out of the uterus. The delivery was then completed using a technique similar to that used for a breech delivery. Standard breech delivery maneuvers were used to assist with the delivery of the fetal shoulders and fetal head.
Comparing the push technique with the pull technique, the push technique was associated with longer operative time (89 minutes compared with 56 minutes [p <.001]); greater blood loss (1,257 mL and 899 mL [p <.001]); and more extensions involving the uterus (30% and 11% [p <.05]) and vagina (17% and 4% [p <.05]) that required surgical repair. The rate of fetal injury was similar using either technique: 6% (push) and 7% (pull).
Results of a retrospective review. A study of 48 difficult cesarean deliveries reported that the push technique resulted in a higher rate of extensions of the uterine incision (50%) than the pull technique (15%).3
A third retrospective study is relevant. Investigators compared reverse breech extraction and standard direct delivery of the impacted fetal head, without assistance from a vaginal hand. In 182 laboring women in whom the fetal head was deeply impacted, reverse breech extraction was associated with a lower rate of extension of the uterine incision (2%) than the conventional approach of direct delivery of the impacted fetal head (23%).4
My recommendation. When you intend to use a reverse breech delivery technique to deliver a deeply impacted fetal head, I recommend a low vertical uterine incision so that you are able to extend the incision superiorly in case there is difficulty delivering the breech. Many clinicians report, however, that it is relatively easy to perform a reverse breech extraction through a transverse uterine incision.5 If you have made a transverse hysterotomy incision and it becomes difficult to deliver the breech, consider making a J or T incision in the uterus to provide additional room to deliver the breech.
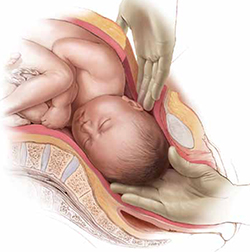
FIGURE 2 Assist from a vaginal hand: The “push technique”
When pushing the head up from the vagina, try to flex the fetal head. If possible, use three or four fingers—or a cupped hand or the palm of the hand—to apply force spread widely across the presenting part.
The “push technique”: An assist from a vaginal hand
Using a hand in the vagina to push the head up toward the uterine incision can be performed by an assistant or the primary surgeon.6 If the need for assistance with a hand from below is recognized before the cesarean is undertaken, the mother’s legs can be placed in a supine frog-leg or modified lithotomy position.7
The assistant pushing the head up from the vagina should try to flex the fetal head. If possible, three or four fingers—or a cupped hand or the palm of the hand—should be used to apply force spread widely across the presenting part.
Caution: Using only one or two fingers for this technique, with the pushing focused on one small area of the head, may increase the risk of fetal skull fracture.
Using the obstetrical spoon
Some clinicians who routinely use a Coyne spoon to deliver the fetal head at the time of cesarean delivery prefer to use the spoon to deliver the deeply impacted fetal head. Using two fingers (not the entire hand), the spoon is gently placed through the uterine incision to a position below the fetal head. The spoon is then used to help release and elevate the head from the pelvis, and the fetus is delivered in the usual manner with the spoon.
Caution: After an excessively prolonged labor, it may be difficult to place the spoon below the fetal head without damaging the lower uterine segment.
Other techniques to consider
When a transverse uterine incision is performed after prolonged labor, a fetal shoulder often appears in the hysterotomy as soon as the incision is made. This so-called shoulder sign is another indication that the fetal head is deeply impacted. Clinicians have reported that it can be helpful to have an assistant gently push the shoulder cephalad, while the surgeon attempts the direct extraction of the fetal head in the classical manner.
A more formal method of using the shoulder that presents in the hysterotomy incision to facilitate delivery has been reported8:
- The shoulder presenting in the hysterotomy is delivered
- The opposite shoulder is delivered
- The fetal body is delivered
- The fetal head is delivered last.
There are risks and consequences to extending the second stage
Trends in OB practice have resulted in more instances of labor in which the second stage extends past 3 hours. Prolonged labor markedly increases the likelihood that an obstetrician will encounter cases in which the fetal head is deflexed and deeply impacted in the pelvis, making extraction of the fetal head very difficult. Prolonged labor also increases the likelihood that the lower uterine segment and upper vagina will be edematous and very thin, increasing the likelihood of trauma to these, and adjacent, organs.
One approach to reduce the risk of difficult fetal extraction is to limit the second stage of labor to 3 hours or less in most situations. If you are asked to perform a cesarean delivery on a patient whose provider has allowed the second stage to extend well beyond 3 hours, be prepared to perform a reverse breech extraction!
in his memorable 2011 Editorials
- Consider denosumab for postmenopausal osteoporosis (January)
- What can “meaningful use” of an EHR mean for your bottom line? (February)
- Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other? (March)
- Stop staring at that Category-II fetal heart-rate tracing … (April)
- Big step forward and downward: An OC with 10 μg of estrogen (May)
- OB and neonatal medicine practices are evolving—in ways that might surprise you (June)
- Have you made best use of the Bakri balloon in PPH? (July)
- Not all contraceptives are suitable immediately postpartum (September)
- Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander (October)
- Insomnia is a troubling and under-treated problem (November)
- How to repair bladder injury at the time of cesarean delivery (December)
1. Stuebe AM. Level IV evidence—adverse anecdote and clinical practice. N Engl J Med. 2011;365(1):8-9.
2. Fasubaa OB, Ezechi OC, Orji O, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375-378.
3. Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol. 2005;121(1):24-26.
4. Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scan. 2009;88(10):1163-1166.
5. Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynecol Obstet. 1997;56(2):183-184.
6. Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the mid cavity. Arch Gynecol. 1983;234(1):59-60.
7. Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707-710.
8. Khosla AH, Dahiya K, Sangwan K. Cesarean section in a wedged head. Indian J Med Sci. 2003;57:187-191.
Do you have a clinical pearl for delivering a deflexed, deeply impacted fetal head at cesarean delivery?
CASE At 2 Am, the director of nursing pages you, asking you to attend to a 26-year-old gravida 1 para 0 who has just been brought by ambulance to labor and delivery after attempting home birth.
The midwife caring for the patient reports that she has been fully dilated for 7 hours and has been pushing for 4 hours. You confirm that the patient is fully dilated, with the presenting part at +1 station; significant caput succedaneum; and molding of the fetal head.
Estimated fetal weight is 3,600 g. The fetal heart-rate tracing is Category II.
You recommend a cesarean delivery. The exhausted patient agrees, reluctantly.
But when you attempt to deliver the fetal head, you realize that it is deflexed and stuck deep in the pelvis. It’s going to be very difficult to deliver the head without trauma to the lower uterine segment, upper vagina and, possibly, the bladder.
What should you do now?
Experienced clinicians often recognize, instinctively, a looming catastrophe before it happens because we have seen a similar situation earlier in our career.1 After we identify the potential for trouble, we attempt to avert disaster by taking preventive action.
One very good occasion to use that “clinical sixth sense”
Consider the situation in which labor has been complicated by a long second stage, with a large fetus—a disaster waiting to happen. In such a case, cesarean delivery, if it is necessary, can be difficult to perform because the fetal head is stuck deep in the pelvis. Attempting to deliver a deeply impacted fetal head, using standard delivery maneuvers, may cause extensive trauma to the lower uterine segment, vagina, and bladder, and fetal injury. In turn, ureteral injury or postpartum hemorrhage may occur during your repair of damage to the lower uterine segment, vagina, or bladder.
In the scenario described a moment ago, the fact that the patient was in the second stage of labor for 7 hours, at home, without anesthesia, and with failure to progress to vaginal delivery increases the likelihood that the fetal head is impacted deep in the pelvis. Before you perform cesarean delivery, you might find it helpful to perform a vaginal examination to answer two questions:
- On vaginal examination, between contractions, can the fetal head be gently moved out of the pelvis? Or is it deeply impacted?
- Is there sufficient space between the fetal head and symphysis pubis to permit delivery with standard cesarean maneuvers?
If the head is impacted deep in the pelvis, I encourage you to consider alternative approaches to cesarean delivery, including reverse breech extraction (FIGURE 1) or an assist from a vaginal hand (FIGURE 2) to facilitate delivery.

FIGURE 1 Reverse breech extraction—the “pull technique”
Once the uterus has been opened, reach immediately into the upper segment for a fetal leg. Apply gentle traction on the leg until the other leg appears. With two legs held together, deliver (pull) the body of the fetus out of the uterus.
The “pull technique”: Reverse breech extraction
One randomized trial and one retrospective study have evaluated the use of reverse breech extraction (the pull technique) in comparison to pushing up with a vaginal hand from below (the push technique) for managing a difficult cesarean delivery after obstructed labor.
Results of a clinical trial. 108 Nigerian women who had obstructed labor were randomly assigned to a pull technique (reverse breech extraction) or a push technique (assist from a vaginal hand).2
The push technique in this study was reportedly performed with a “finger” in the vagina pushing up on the fetal head while the surgeon attempted to deliver the head in a standard fashion.
The pull technique was performed by opening the uterus, immediately reaching into the upper uterus for a fetal leg, and applying gentle traction on the leg until the second leg appeared. Then, with two legs held together, the body of the fetus was delivered (pulled) out of the uterus. The delivery was then completed using a technique similar to that used for a breech delivery. Standard breech delivery maneuvers were used to assist with the delivery of the fetal shoulders and fetal head.
Comparing the push technique with the pull technique, the push technique was associated with longer operative time (89 minutes compared with 56 minutes [p <.001]); greater blood loss (1,257 mL and 899 mL [p <.001]); and more extensions involving the uterus (30% and 11% [p <.05]) and vagina (17% and 4% [p <.05]) that required surgical repair. The rate of fetal injury was similar using either technique: 6% (push) and 7% (pull).
Results of a retrospective review. A study of 48 difficult cesarean deliveries reported that the push technique resulted in a higher rate of extensions of the uterine incision (50%) than the pull technique (15%).3
A third retrospective study is relevant. Investigators compared reverse breech extraction and standard direct delivery of the impacted fetal head, without assistance from a vaginal hand. In 182 laboring women in whom the fetal head was deeply impacted, reverse breech extraction was associated with a lower rate of extension of the uterine incision (2%) than the conventional approach of direct delivery of the impacted fetal head (23%).4
My recommendation. When you intend to use a reverse breech delivery technique to deliver a deeply impacted fetal head, I recommend a low vertical uterine incision so that you are able to extend the incision superiorly in case there is difficulty delivering the breech. Many clinicians report, however, that it is relatively easy to perform a reverse breech extraction through a transverse uterine incision.5 If you have made a transverse hysterotomy incision and it becomes difficult to deliver the breech, consider making a J or T incision in the uterus to provide additional room to deliver the breech.

FIGURE 2 Assist from a vaginal hand: The “push technique”
When pushing the head up from the vagina, try to flex the fetal head. If possible, use three or four fingers—or a cupped hand or the palm of the hand—to apply force spread widely across the presenting part.
The “push technique”: An assist from a vaginal hand
Using a hand in the vagina to push the head up toward the uterine incision can be performed by an assistant or the primary surgeon.6 If the need for assistance with a hand from below is recognized before the cesarean is undertaken, the mother’s legs can be placed in a supine frog-leg or modified lithotomy position.7
The assistant pushing the head up from the vagina should try to flex the fetal head. If possible, three or four fingers—or a cupped hand or the palm of the hand—should be used to apply force spread widely across the presenting part.
Caution: Using only one or two fingers for this technique, with the pushing focused on one small area of the head, may increase the risk of fetal skull fracture.
Using the obstetrical spoon
Some clinicians who routinely use a Coyne spoon to deliver the fetal head at the time of cesarean delivery prefer to use the spoon to deliver the deeply impacted fetal head. Using two fingers (not the entire hand), the spoon is gently placed through the uterine incision to a position below the fetal head. The spoon is then used to help release and elevate the head from the pelvis, and the fetus is delivered in the usual manner with the spoon.
Caution: After an excessively prolonged labor, it may be difficult to place the spoon below the fetal head without damaging the lower uterine segment.
Other techniques to consider
When a transverse uterine incision is performed after prolonged labor, a fetal shoulder often appears in the hysterotomy as soon as the incision is made. This so-called shoulder sign is another indication that the fetal head is deeply impacted. Clinicians have reported that it can be helpful to have an assistant gently push the shoulder cephalad, while the surgeon attempts the direct extraction of the fetal head in the classical manner.
A more formal method of using the shoulder that presents in the hysterotomy incision to facilitate delivery has been reported8:
- The shoulder presenting in the hysterotomy is delivered
- The opposite shoulder is delivered
- The fetal body is delivered
- The fetal head is delivered last.
There are risks and consequences to extending the second stage
Trends in OB practice have resulted in more instances of labor in which the second stage extends past 3 hours. Prolonged labor markedly increases the likelihood that an obstetrician will encounter cases in which the fetal head is deflexed and deeply impacted in the pelvis, making extraction of the fetal head very difficult. Prolonged labor also increases the likelihood that the lower uterine segment and upper vagina will be edematous and very thin, increasing the likelihood of trauma to these, and adjacent, organs.
One approach to reduce the risk of difficult fetal extraction is to limit the second stage of labor to 3 hours or less in most situations. If you are asked to perform a cesarean delivery on a patient whose provider has allowed the second stage to extend well beyond 3 hours, be prepared to perform a reverse breech extraction!
in his memorable 2011 Editorials
- Consider denosumab for postmenopausal osteoporosis (January)
- What can “meaningful use” of an EHR mean for your bottom line? (February)
- Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other? (March)
- Stop staring at that Category-II fetal heart-rate tracing … (April)
- Big step forward and downward: An OC with 10 μg of estrogen (May)
- OB and neonatal medicine practices are evolving—in ways that might surprise you (June)
- Have you made best use of the Bakri balloon in PPH? (July)
- Not all contraceptives are suitable immediately postpartum (September)
- Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander (October)
- Insomnia is a troubling and under-treated problem (November)
- How to repair bladder injury at the time of cesarean delivery (December)
Do you have a clinical pearl for delivering a deflexed, deeply impacted fetal head at cesarean delivery?
CASE At 2 Am, the director of nursing pages you, asking you to attend to a 26-year-old gravida 1 para 0 who has just been brought by ambulance to labor and delivery after attempting home birth.
The midwife caring for the patient reports that she has been fully dilated for 7 hours and has been pushing for 4 hours. You confirm that the patient is fully dilated, with the presenting part at +1 station; significant caput succedaneum; and molding of the fetal head.
Estimated fetal weight is 3,600 g. The fetal heart-rate tracing is Category II.
You recommend a cesarean delivery. The exhausted patient agrees, reluctantly.
But when you attempt to deliver the fetal head, you realize that it is deflexed and stuck deep in the pelvis. It’s going to be very difficult to deliver the head without trauma to the lower uterine segment, upper vagina and, possibly, the bladder.
What should you do now?
Experienced clinicians often recognize, instinctively, a looming catastrophe before it happens because we have seen a similar situation earlier in our career.1 After we identify the potential for trouble, we attempt to avert disaster by taking preventive action.
One very good occasion to use that “clinical sixth sense”
Consider the situation in which labor has been complicated by a long second stage, with a large fetus—a disaster waiting to happen. In such a case, cesarean delivery, if it is necessary, can be difficult to perform because the fetal head is stuck deep in the pelvis. Attempting to deliver a deeply impacted fetal head, using standard delivery maneuvers, may cause extensive trauma to the lower uterine segment, vagina, and bladder, and fetal injury. In turn, ureteral injury or postpartum hemorrhage may occur during your repair of damage to the lower uterine segment, vagina, or bladder.
In the scenario described a moment ago, the fact that the patient was in the second stage of labor for 7 hours, at home, without anesthesia, and with failure to progress to vaginal delivery increases the likelihood that the fetal head is impacted deep in the pelvis. Before you perform cesarean delivery, you might find it helpful to perform a vaginal examination to answer two questions:
- On vaginal examination, between contractions, can the fetal head be gently moved out of the pelvis? Or is it deeply impacted?
- Is there sufficient space between the fetal head and symphysis pubis to permit delivery with standard cesarean maneuvers?
If the head is impacted deep in the pelvis, I encourage you to consider alternative approaches to cesarean delivery, including reverse breech extraction (FIGURE 1) or an assist from a vaginal hand (FIGURE 2) to facilitate delivery.

FIGURE 1 Reverse breech extraction—the “pull technique”
Once the uterus has been opened, reach immediately into the upper segment for a fetal leg. Apply gentle traction on the leg until the other leg appears. With two legs held together, deliver (pull) the body of the fetus out of the uterus.
The “pull technique”: Reverse breech extraction
One randomized trial and one retrospective study have evaluated the use of reverse breech extraction (the pull technique) in comparison to pushing up with a vaginal hand from below (the push technique) for managing a difficult cesarean delivery after obstructed labor.
Results of a clinical trial. 108 Nigerian women who had obstructed labor were randomly assigned to a pull technique (reverse breech extraction) or a push technique (assist from a vaginal hand).2
The push technique in this study was reportedly performed with a “finger” in the vagina pushing up on the fetal head while the surgeon attempted to deliver the head in a standard fashion.
The pull technique was performed by opening the uterus, immediately reaching into the upper uterus for a fetal leg, and applying gentle traction on the leg until the second leg appeared. Then, with two legs held together, the body of the fetus was delivered (pulled) out of the uterus. The delivery was then completed using a technique similar to that used for a breech delivery. Standard breech delivery maneuvers were used to assist with the delivery of the fetal shoulders and fetal head.
Comparing the push technique with the pull technique, the push technique was associated with longer operative time (89 minutes compared with 56 minutes [p <.001]); greater blood loss (1,257 mL and 899 mL [p <.001]); and more extensions involving the uterus (30% and 11% [p <.05]) and vagina (17% and 4% [p <.05]) that required surgical repair. The rate of fetal injury was similar using either technique: 6% (push) and 7% (pull).
Results of a retrospective review. A study of 48 difficult cesarean deliveries reported that the push technique resulted in a higher rate of extensions of the uterine incision (50%) than the pull technique (15%).3
A third retrospective study is relevant. Investigators compared reverse breech extraction and standard direct delivery of the impacted fetal head, without assistance from a vaginal hand. In 182 laboring women in whom the fetal head was deeply impacted, reverse breech extraction was associated with a lower rate of extension of the uterine incision (2%) than the conventional approach of direct delivery of the impacted fetal head (23%).4
My recommendation. When you intend to use a reverse breech delivery technique to deliver a deeply impacted fetal head, I recommend a low vertical uterine incision so that you are able to extend the incision superiorly in case there is difficulty delivering the breech. Many clinicians report, however, that it is relatively easy to perform a reverse breech extraction through a transverse uterine incision.5 If you have made a transverse hysterotomy incision and it becomes difficult to deliver the breech, consider making a J or T incision in the uterus to provide additional room to deliver the breech.

FIGURE 2 Assist from a vaginal hand: The “push technique”
When pushing the head up from the vagina, try to flex the fetal head. If possible, use three or four fingers—or a cupped hand or the palm of the hand—to apply force spread widely across the presenting part.
The “push technique”: An assist from a vaginal hand
Using a hand in the vagina to push the head up toward the uterine incision can be performed by an assistant or the primary surgeon.6 If the need for assistance with a hand from below is recognized before the cesarean is undertaken, the mother’s legs can be placed in a supine frog-leg or modified lithotomy position.7
The assistant pushing the head up from the vagina should try to flex the fetal head. If possible, three or four fingers—or a cupped hand or the palm of the hand—should be used to apply force spread widely across the presenting part.
Caution: Using only one or two fingers for this technique, with the pushing focused on one small area of the head, may increase the risk of fetal skull fracture.
Using the obstetrical spoon
Some clinicians who routinely use a Coyne spoon to deliver the fetal head at the time of cesarean delivery prefer to use the spoon to deliver the deeply impacted fetal head. Using two fingers (not the entire hand), the spoon is gently placed through the uterine incision to a position below the fetal head. The spoon is then used to help release and elevate the head from the pelvis, and the fetus is delivered in the usual manner with the spoon.
Caution: After an excessively prolonged labor, it may be difficult to place the spoon below the fetal head without damaging the lower uterine segment.
Other techniques to consider
When a transverse uterine incision is performed after prolonged labor, a fetal shoulder often appears in the hysterotomy as soon as the incision is made. This so-called shoulder sign is another indication that the fetal head is deeply impacted. Clinicians have reported that it can be helpful to have an assistant gently push the shoulder cephalad, while the surgeon attempts the direct extraction of the fetal head in the classical manner.
A more formal method of using the shoulder that presents in the hysterotomy incision to facilitate delivery has been reported8:
- The shoulder presenting in the hysterotomy is delivered
- The opposite shoulder is delivered
- The fetal body is delivered
- The fetal head is delivered last.
There are risks and consequences to extending the second stage
Trends in OB practice have resulted in more instances of labor in which the second stage extends past 3 hours. Prolonged labor markedly increases the likelihood that an obstetrician will encounter cases in which the fetal head is deflexed and deeply impacted in the pelvis, making extraction of the fetal head very difficult. Prolonged labor also increases the likelihood that the lower uterine segment and upper vagina will be edematous and very thin, increasing the likelihood of trauma to these, and adjacent, organs.
One approach to reduce the risk of difficult fetal extraction is to limit the second stage of labor to 3 hours or less in most situations. If you are asked to perform a cesarean delivery on a patient whose provider has allowed the second stage to extend well beyond 3 hours, be prepared to perform a reverse breech extraction!
in his memorable 2011 Editorials
- Consider denosumab for postmenopausal osteoporosis (January)
- What can “meaningful use” of an EHR mean for your bottom line? (February)
- Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other? (March)
- Stop staring at that Category-II fetal heart-rate tracing … (April)
- Big step forward and downward: An OC with 10 μg of estrogen (May)
- OB and neonatal medicine practices are evolving—in ways that might surprise you (June)
- Have you made best use of the Bakri balloon in PPH? (July)
- Not all contraceptives are suitable immediately postpartum (September)
- Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander (October)
- Insomnia is a troubling and under-treated problem (November)
- How to repair bladder injury at the time of cesarean delivery (December)
1. Stuebe AM. Level IV evidence—adverse anecdote and clinical practice. N Engl J Med. 2011;365(1):8-9.
2. Fasubaa OB, Ezechi OC, Orji O, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375-378.
3. Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol. 2005;121(1):24-26.
4. Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scan. 2009;88(10):1163-1166.
5. Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynecol Obstet. 1997;56(2):183-184.
6. Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the mid cavity. Arch Gynecol. 1983;234(1):59-60.
7. Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707-710.
8. Khosla AH, Dahiya K, Sangwan K. Cesarean section in a wedged head. Indian J Med Sci. 2003;57:187-191.
1. Stuebe AM. Level IV evidence—adverse anecdote and clinical practice. N Engl J Med. 2011;365(1):8-9.
2. Fasubaa OB, Ezechi OC, Orji O, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375-378.
3. Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol. 2005;121(1):24-26.
4. Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scan. 2009;88(10):1163-1166.
5. Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynecol Obstet. 1997;56(2):183-184.
6. Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the mid cavity. Arch Gynecol. 1983;234(1):59-60.
7. Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707-710.
8. Khosla AH, Dahiya K, Sangwan K. Cesarean section in a wedged head. Indian J Med Sci. 2003;57:187-191.
Overweight and Obese Women Deliver Fewer IVF Live Births
ORLANDO – Obesity significantly lowers a woman's chance of delivering a live birth after in vitro fertilization, according to a retrospective study of more than 4,500 women.
Up to a 68% lower chance for a live birth was the major finding when researchers compared overweight and obese women to those with a normal body mass index (BMI). Women with a BMI greater than 25 kg/m
The live birth rate declined as BMI increased, Dr. Stephanie Jones said. Compared with women with a normal BMI (18.50-24.99), the adjusted odds ratio (OR) for a live birth was 0.96 among overweight women (25-29.99); 0.63 for obesity class I (30-34.99); 0.39 for obesity class II (35-39.99); and 0.32 for those in obesity class III (BMI of 40 kg/m
The clinical message is to counsel patients that even “a modest amount of weight loss might improve IVF success rates,” Dr. Jones said at the meeting.
Dr. Jones and her associates examined outcomes after the first, fresh, autologous procedure for 4,609 women treated at Boston IVF in Brookline, Mass. from 2006 to 2010. Patients were aged 20-45 years.
A secondary outcome, the likelihood of implantation, was significantly different by BMI, compared with those with a normal BMI. Chances dropped for underweight women (BMI less than 18 kg/m
The likelihood of clinical pregnancy dropped only slightly for underweight women (adjusted OR, 0.98). However, it decreased significantly for overweight women (0.90) and for women in obesity class I (0.70), class II (0.41), and class III (0.43).
Interestingly, the miscarriage rate did not differ significantly according to maternal BMI, said Dr. Jones, a third-year resident in the department of obstetrics and gynecology, Beth Israel Deaconess Medical Center, Boston.
The normal-weight reference group included 2,605 patients with a BMI of 18.5-24.99 kg/m
In addition to its large sample size, the single institution design of the study is an advantage, Dr. Jones said. Previous researchers reported an association between increasing obesity and lower IVF success, but most of these studies were small, unadjusted, and focused on pregnancy rates.
“The live birth rate is the outcome most significant to our patients,” she said.
A systematic literature review found a decreased chance of IVF pregnancy (OR, 0.71) for overweight or obese women compared with normal weight women (Hum. Reprod. Update 2007;13:433-44). “But they only compared women in two groups – those with a BMI of 25 or less versus 25 plus,” Dr. Jones said.
In another study reported at the 2009 ASRM meeting, researchers found a lower clinical pregnancy rate and lower birth weights as maternal BMI increased (Hum. Reprod. 2011;26:245-52). This report was multicenter “and they did not necessarily control for differences in provider factors,” she said.
Dr. Jones and her associates also controlled for multiple potential confounders, including maternal age, paternal age, baseline follicle stimulating hormone levels, duration of stimulation, mean daily gonadotropin dose, peak estradiol, number of oocytes retrieved, use of intracytoplasmic sperm injection, embryo quality and number, transfer day, and number of embryos transferred.
ORLANDO – Obesity significantly lowers a woman's chance of delivering a live birth after in vitro fertilization, according to a retrospective study of more than 4,500 women.
Up to a 68% lower chance for a live birth was the major finding when researchers compared overweight and obese women to those with a normal body mass index (BMI). Women with a BMI greater than 25 kg/m
The live birth rate declined as BMI increased, Dr. Stephanie Jones said. Compared with women with a normal BMI (18.50-24.99), the adjusted odds ratio (OR) for a live birth was 0.96 among overweight women (25-29.99); 0.63 for obesity class I (30-34.99); 0.39 for obesity class II (35-39.99); and 0.32 for those in obesity class III (BMI of 40 kg/m
The clinical message is to counsel patients that even “a modest amount of weight loss might improve IVF success rates,” Dr. Jones said at the meeting.
Dr. Jones and her associates examined outcomes after the first, fresh, autologous procedure for 4,609 women treated at Boston IVF in Brookline, Mass. from 2006 to 2010. Patients were aged 20-45 years.
A secondary outcome, the likelihood of implantation, was significantly different by BMI, compared with those with a normal BMI. Chances dropped for underweight women (BMI less than 18 kg/m
The likelihood of clinical pregnancy dropped only slightly for underweight women (adjusted OR, 0.98). However, it decreased significantly for overweight women (0.90) and for women in obesity class I (0.70), class II (0.41), and class III (0.43).
Interestingly, the miscarriage rate did not differ significantly according to maternal BMI, said Dr. Jones, a third-year resident in the department of obstetrics and gynecology, Beth Israel Deaconess Medical Center, Boston.
The normal-weight reference group included 2,605 patients with a BMI of 18.5-24.99 kg/m
In addition to its large sample size, the single institution design of the study is an advantage, Dr. Jones said. Previous researchers reported an association between increasing obesity and lower IVF success, but most of these studies were small, unadjusted, and focused on pregnancy rates.
“The live birth rate is the outcome most significant to our patients,” she said.
A systematic literature review found a decreased chance of IVF pregnancy (OR, 0.71) for overweight or obese women compared with normal weight women (Hum. Reprod. Update 2007;13:433-44). “But they only compared women in two groups – those with a BMI of 25 or less versus 25 plus,” Dr. Jones said.
In another study reported at the 2009 ASRM meeting, researchers found a lower clinical pregnancy rate and lower birth weights as maternal BMI increased (Hum. Reprod. 2011;26:245-52). This report was multicenter “and they did not necessarily control for differences in provider factors,” she said.
Dr. Jones and her associates also controlled for multiple potential confounders, including maternal age, paternal age, baseline follicle stimulating hormone levels, duration of stimulation, mean daily gonadotropin dose, peak estradiol, number of oocytes retrieved, use of intracytoplasmic sperm injection, embryo quality and number, transfer day, and number of embryos transferred.
ORLANDO – Obesity significantly lowers a woman's chance of delivering a live birth after in vitro fertilization, according to a retrospective study of more than 4,500 women.
Up to a 68% lower chance for a live birth was the major finding when researchers compared overweight and obese women to those with a normal body mass index (BMI). Women with a BMI greater than 25 kg/m
The live birth rate declined as BMI increased, Dr. Stephanie Jones said. Compared with women with a normal BMI (18.50-24.99), the adjusted odds ratio (OR) for a live birth was 0.96 among overweight women (25-29.99); 0.63 for obesity class I (30-34.99); 0.39 for obesity class II (35-39.99); and 0.32 for those in obesity class III (BMI of 40 kg/m
The clinical message is to counsel patients that even “a modest amount of weight loss might improve IVF success rates,” Dr. Jones said at the meeting.
Dr. Jones and her associates examined outcomes after the first, fresh, autologous procedure for 4,609 women treated at Boston IVF in Brookline, Mass. from 2006 to 2010. Patients were aged 20-45 years.
A secondary outcome, the likelihood of implantation, was significantly different by BMI, compared with those with a normal BMI. Chances dropped for underweight women (BMI less than 18 kg/m
The likelihood of clinical pregnancy dropped only slightly for underweight women (adjusted OR, 0.98). However, it decreased significantly for overweight women (0.90) and for women in obesity class I (0.70), class II (0.41), and class III (0.43).
Interestingly, the miscarriage rate did not differ significantly according to maternal BMI, said Dr. Jones, a third-year resident in the department of obstetrics and gynecology, Beth Israel Deaconess Medical Center, Boston.
The normal-weight reference group included 2,605 patients with a BMI of 18.5-24.99 kg/m
In addition to its large sample size, the single institution design of the study is an advantage, Dr. Jones said. Previous researchers reported an association between increasing obesity and lower IVF success, but most of these studies were small, unadjusted, and focused on pregnancy rates.
“The live birth rate is the outcome most significant to our patients,” she said.
A systematic literature review found a decreased chance of IVF pregnancy (OR, 0.71) for overweight or obese women compared with normal weight women (Hum. Reprod. Update 2007;13:433-44). “But they only compared women in two groups – those with a BMI of 25 or less versus 25 plus,” Dr. Jones said.
In another study reported at the 2009 ASRM meeting, researchers found a lower clinical pregnancy rate and lower birth weights as maternal BMI increased (Hum. Reprod. 2011;26:245-52). This report was multicenter “and they did not necessarily control for differences in provider factors,” she said.
Dr. Jones and her associates also controlled for multiple potential confounders, including maternal age, paternal age, baseline follicle stimulating hormone levels, duration of stimulation, mean daily gonadotropin dose, peak estradiol, number of oocytes retrieved, use of intracytoplasmic sperm injection, embryo quality and number, transfer day, and number of embryos transferred.
From the Annual Meeting of the American Society for Reproductive Medicine
FDA: Appropriate SSRI Use OK in Pregnancy
Pregnant women taking selective serotonin reuptake inhibitors for depression may continue to do so, despite a 2006 warning that the drugs may predispose infants to persistent pulmonary hypertension, the Food and Drug Administration has announced.
That earlier warning was based on a single study indicating that infants exposed to the drug in utero after the 20th week of pregnancy were six times more likely to develop persistent pulmonary hypertension (PPHN) than nonexposed infants (N. Engl. J. Med. 2006;354:579-87).
“Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of SSRIs during pregnancy can cause persistent pulmonary hypertension,” the FDA said in a press statement.
The agency will update the drugs' warning labels to reflect data from new studies, which have produced conflicting results about the risk SSRIs may pose to an unborn child. Those studies include a large retrospective database study in 2009 that found no association between SSRI use and PPHN (Pharmacoepidemiol. Drug Saf. 2009;18:246-52), and a 2011 case-control study of 11,923 births that showed PPHN was associated with cesarean delivery but not with SSRI use in the second half of pregnancy (Am. J. Perinatol. 2011;28:19-24).
FDA officials concluded that the evidence is not sufficient to withhold SSRI treatment from pregnant women or take them off the antidepressants. “At present, FDA … recommends that health care providers treat depression during pregnancy as clinically appropriate,” according to the agency's statement.
Dr. Gideon Koren, professor of pediatrics, pharmacology, pharmacy, medicine, and medical genetics at the University of Toronto, commented in an interview, “I support FDA's hesitation in confirming causation of SSRIs in causing PPHN. The available studies are split in their ability to show an association between SSRIs taken in late pregnancy.
“Critically, several studies have shown that depression itself is also associated with increased risk of PPHN. Hence it is quite possible that depression and not its treatment cause this rare risk ('confounding by indication').” Dr. Koren also heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk Program.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are “substantial risks associated with undertreatment or no treatment of depression during pregnancy.” Risks of untreated maternal depression can include low birth weight, preterm delivery, lower Apgar scores, poor prenatal care, failure to recognize or report impending labor, and increased risks of fetal abuse, neonaticide or maternal suicide, the FDA warned.
Both the American Psychiatric Association and the American College of Obstetricians and Gynecologists recommend monitoring pregnant women for depression and treating them appropriately.
Physicians should continue to report any possible adverse effects to the FDA's MedWatch program, www.fda.gov/MedWatch/report.htm
Reporting forms can also be requested by calling 800-332-1088.
Dr. Koren said he had no relevant financial disclosures.
View on the News
Cautious Treatment Makes Sense
Even at the time of the first publication regarding the link between SSRIs and PPHN in 2006, the conclusion of the authors was that if the link is causal, the absolute risk for PPHN following late pregnancy exposure to SSRIs is very low. Thus, the recommendation that clinicians treat pregnant women appropriately for their symptoms was consistent with the initial findings, and continues to be so.
Since the initial publication, three others have appeared in full manuscript form and one in abstract form (now in press). The two published U.S. studies were either underpowered, or had limitations in classifying the outcomes, whereas the two Scandinavian studies confirmed the initial findings in large cohort or linked database studies. Importantly, the European studies that confirmed the association also came to the conclusion that SSRIs pose a small increased risk for a very rare outcome of pregnancy. Thus, the recommendation to treat only if needed, but not to avoid necessary treatment because of concern for PPHN continues to make sense.
CHRISTINA CHAMBERS, Ph.D., M.P.H., is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she currently receives grant funding for two studies unrelated to SSRIs from GlaxoSmithKline and GlaxoSmithKline Bio.
Pregnant women taking selective serotonin reuptake inhibitors for depression may continue to do so, despite a 2006 warning that the drugs may predispose infants to persistent pulmonary hypertension, the Food and Drug Administration has announced.
That earlier warning was based on a single study indicating that infants exposed to the drug in utero after the 20th week of pregnancy were six times more likely to develop persistent pulmonary hypertension (PPHN) than nonexposed infants (N. Engl. J. Med. 2006;354:579-87).
“Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of SSRIs during pregnancy can cause persistent pulmonary hypertension,” the FDA said in a press statement.
The agency will update the drugs' warning labels to reflect data from new studies, which have produced conflicting results about the risk SSRIs may pose to an unborn child. Those studies include a large retrospective database study in 2009 that found no association between SSRI use and PPHN (Pharmacoepidemiol. Drug Saf. 2009;18:246-52), and a 2011 case-control study of 11,923 births that showed PPHN was associated with cesarean delivery but not with SSRI use in the second half of pregnancy (Am. J. Perinatol. 2011;28:19-24).
FDA officials concluded that the evidence is not sufficient to withhold SSRI treatment from pregnant women or take them off the antidepressants. “At present, FDA … recommends that health care providers treat depression during pregnancy as clinically appropriate,” according to the agency's statement.
Dr. Gideon Koren, professor of pediatrics, pharmacology, pharmacy, medicine, and medical genetics at the University of Toronto, commented in an interview, “I support FDA's hesitation in confirming causation of SSRIs in causing PPHN. The available studies are split in their ability to show an association between SSRIs taken in late pregnancy.
“Critically, several studies have shown that depression itself is also associated with increased risk of PPHN. Hence it is quite possible that depression and not its treatment cause this rare risk ('confounding by indication').” Dr. Koren also heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk Program.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are “substantial risks associated with undertreatment or no treatment of depression during pregnancy.” Risks of untreated maternal depression can include low birth weight, preterm delivery, lower Apgar scores, poor prenatal care, failure to recognize or report impending labor, and increased risks of fetal abuse, neonaticide or maternal suicide, the FDA warned.
Both the American Psychiatric Association and the American College of Obstetricians and Gynecologists recommend monitoring pregnant women for depression and treating them appropriately.
Physicians should continue to report any possible adverse effects to the FDA's MedWatch program, www.fda.gov/MedWatch/report.htm
Reporting forms can also be requested by calling 800-332-1088.
Dr. Koren said he had no relevant financial disclosures.
View on the News
Cautious Treatment Makes Sense
Even at the time of the first publication regarding the link between SSRIs and PPHN in 2006, the conclusion of the authors was that if the link is causal, the absolute risk for PPHN following late pregnancy exposure to SSRIs is very low. Thus, the recommendation that clinicians treat pregnant women appropriately for their symptoms was consistent with the initial findings, and continues to be so.
Since the initial publication, three others have appeared in full manuscript form and one in abstract form (now in press). The two published U.S. studies were either underpowered, or had limitations in classifying the outcomes, whereas the two Scandinavian studies confirmed the initial findings in large cohort or linked database studies. Importantly, the European studies that confirmed the association also came to the conclusion that SSRIs pose a small increased risk for a very rare outcome of pregnancy. Thus, the recommendation to treat only if needed, but not to avoid necessary treatment because of concern for PPHN continues to make sense.
CHRISTINA CHAMBERS, Ph.D., M.P.H., is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she currently receives grant funding for two studies unrelated to SSRIs from GlaxoSmithKline and GlaxoSmithKline Bio.
Pregnant women taking selective serotonin reuptake inhibitors for depression may continue to do so, despite a 2006 warning that the drugs may predispose infants to persistent pulmonary hypertension, the Food and Drug Administration has announced.
That earlier warning was based on a single study indicating that infants exposed to the drug in utero after the 20th week of pregnancy were six times more likely to develop persistent pulmonary hypertension (PPHN) than nonexposed infants (N. Engl. J. Med. 2006;354:579-87).
“Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of SSRIs during pregnancy can cause persistent pulmonary hypertension,” the FDA said in a press statement.
The agency will update the drugs' warning labels to reflect data from new studies, which have produced conflicting results about the risk SSRIs may pose to an unborn child. Those studies include a large retrospective database study in 2009 that found no association between SSRI use and PPHN (Pharmacoepidemiol. Drug Saf. 2009;18:246-52), and a 2011 case-control study of 11,923 births that showed PPHN was associated with cesarean delivery but not with SSRI use in the second half of pregnancy (Am. J. Perinatol. 2011;28:19-24).
FDA officials concluded that the evidence is not sufficient to withhold SSRI treatment from pregnant women or take them off the antidepressants. “At present, FDA … recommends that health care providers treat depression during pregnancy as clinically appropriate,” according to the agency's statement.
Dr. Gideon Koren, professor of pediatrics, pharmacology, pharmacy, medicine, and medical genetics at the University of Toronto, commented in an interview, “I support FDA's hesitation in confirming causation of SSRIs in causing PPHN. The available studies are split in their ability to show an association between SSRIs taken in late pregnancy.
“Critically, several studies have shown that depression itself is also associated with increased risk of PPHN. Hence it is quite possible that depression and not its treatment cause this rare risk ('confounding by indication').” Dr. Koren also heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk Program.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are “substantial risks associated with undertreatment or no treatment of depression during pregnancy.” Risks of untreated maternal depression can include low birth weight, preterm delivery, lower Apgar scores, poor prenatal care, failure to recognize or report impending labor, and increased risks of fetal abuse, neonaticide or maternal suicide, the FDA warned.
Both the American Psychiatric Association and the American College of Obstetricians and Gynecologists recommend monitoring pregnant women for depression and treating them appropriately.
Physicians should continue to report any possible adverse effects to the FDA's MedWatch program, www.fda.gov/MedWatch/report.htm
Reporting forms can also be requested by calling 800-332-1088.
Dr. Koren said he had no relevant financial disclosures.
View on the News
Cautious Treatment Makes Sense
Even at the time of the first publication regarding the link between SSRIs and PPHN in 2006, the conclusion of the authors was that if the link is causal, the absolute risk for PPHN following late pregnancy exposure to SSRIs is very low. Thus, the recommendation that clinicians treat pregnant women appropriately for their symptoms was consistent with the initial findings, and continues to be so.
Since the initial publication, three others have appeared in full manuscript form and one in abstract form (now in press). The two published U.S. studies were either underpowered, or had limitations in classifying the outcomes, whereas the two Scandinavian studies confirmed the initial findings in large cohort or linked database studies. Importantly, the European studies that confirmed the association also came to the conclusion that SSRIs pose a small increased risk for a very rare outcome of pregnancy. Thus, the recommendation to treat only if needed, but not to avoid necessary treatment because of concern for PPHN continues to make sense.
CHRISTINA CHAMBERS, Ph.D., M.P.H., is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she currently receives grant funding for two studies unrelated to SSRIs from GlaxoSmithKline and GlaxoSmithKline Bio.
No Chemo if hCG Falls After Molar Pregnancy
Women with raised but falling human chorionic gonadotropin concentrations 6 months after a molar pregnancy do not need chemotherapy, as almost all of them will spontaneously remit, the results of a large retrospective cohort study have shown.
The findings, published online in the Lancet (Lancet 2011 [doi:10.1016/S0140-6736(11)61265-8]), challenge current international clinical guidelines (Int. J. Gynecol. Obstet. 2002;77:285-7) that consider chemotherapy to be indicated when hCG concentrations are high for 6 months or more following evacuation of a hydatidiform mole.
The new findings argue that an elevated hCG level at 6 months is not an indicator of malignancy when values are falling, and that instead of initiating chemotherapy, women with raised but falling hCG can undergo surveillance of their hCG levels, with testing until they return to normal.
Chemotherapy is needed only if hCG levels are still rising at 6 months, have plateaued, or are greater than 345 IU/L, or if there is radiologic evidence of neoplasia.
If the surveillance approach were to become standard, more women could avoid exposure to toxic chemotherapy drugs and could safely conceive sooner after evacuation of a molar pregnancy, said the authors of the study led by Dr. Roshan Agarwal of Imperial College London.
Current U.K. guidelines advise women not to become pregnant until 12 months following chemotherapy, whereas they would have to wait only 6 months after a spontaneous return to normal hCG.
For their research, Dr. Agarwal and colleagues retrospectively identified 13,960 women registered at London's Charing Cross Hospital between January 1993 and May 2008 who had undergone evacuation of a complete or partial hydatidiform mole. Of these, 974 (7%) required chemotherapy within 6 months, and hCG normalized spontaneously in 12,910 (92%) within 6 months.
The remaining 76 women still had high hCG concentrations (more than 5 IU/L) 6 months after evacuation of the molar pregnancy. Sixty-six patients underwent surveillance, in which blood and urine samples were collected and evaluated every 2 weeks until normal hCG was achieved, followed by monthly urine samples for 6 months. Ten patients underwent chemotherapy.
In the surveillance group, 98% of patients (n = 65) saw hCG values return to normal without chemotherapy (in all but 6 of them within a year), and the remaining patient, who had chronic renal failure, remained healthy despite having elevated hCG, Dr. Agarwal and colleagues reported.
Among the 10 patients who received chemotherapy, 6 had complete responses, and 4 had partial or no responses but remained well, even though hCG concentrations in 2 patients continued to be elevated. There was no significant difference in time to normalization between the groups and no deaths had occurred in either group after a median 2 years' follow-up.
The investigators acknowledged that a weakness of their study is retrospective design, the use of a single study site, and the small number of patients with raised hCG at 6 months.
However, they said, the findings “directly challenge the present clinical dogma” to show that the surveillance model is clinically acceptable. The results “will change international practice and spare women unnecessary exposure to chemotherapy,” they wrote in their analysis.
In a case study linked to Dr. Agarwal and colleagues' article, Rosemary A. Fisher, also of Imperial College London, described a woman who had a miscarriage and three consecutive molar pregnancies, yet was able to achieve a normal pregnancy with use of a donor egg.
“To the best of our knowledge, this report establishes for the first time that oocyte donation can enable women with familial recurrent hydatidiform moles due to NLRP7 mutations to achieve a normal pregnancy,” Ms. Fisher and colleagues wrote, adding that this finding offered “hope to other women with this condition.”
The study also shows “that the major role of NLRP7 in pregnancy is in the developing oocyte,” they said.
I think this study is
interesting. The number of patients who were treated with chemotherapy
was very small (10 of 76 patients). Therefore, comparison of the
patients who received chemotherapy versus surveillance is difficult due
to the size of the study, the retrospective nature of the study, as well
as the fact that the patients who received chemotherapy had higher
median hCG levels than those under surveillance (157 vs. 13). HCG levels
also were not well correlated with remission (r = 0.233; p = 0.049).
Patients
with low hCG levels at 6 months can probably be offered surveillance as
an alternative to chemotherapy in select circumstances. However, due to
the aforementioned concerns, before these findings are applied
wholesale in clinical practice, additional studies including a
prospective study are necessary.
E. ALBERT REECE, M.D., Ph.D, M.B.A., is
vice president for medical affairs at the University of Maryland,
Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished
Professor and dean of the school of medicine. He said he had no relevant
financial disclosures.
Treatment Still a Bit of a Conundrum
The data from Dr.
Agarwal and colleagues' study are reassuring. However, 13% of the cohort
received chemotherapy, and how these individuals would have progressed
if no treatment were given is unknown. Hence, the key question remains:
When and to whom should chemotherapy be given? The investigators propose
a cutoff hCG concentration of 345 IU/L at 6 months, which was the
median hCG value in patients who responded to chemotherapy in their
cohort.
Meanwhile, investigators in an earlier study (Gynecol.
Oncol. 2010;116:3-9) proposed that chemotherapy should be started only
when total hCG begins to rise and is greater than 3,000 IU/L, because
chemotherapy would probably be ineffective below this value. Direct
comparison between the two cohorts is inappropriate because the group in
the earlier study was heterogeneous (patients had been given various
previous treatments), whereas Dr. Agarwal and colleagues' group was
unique in its homogeneity (all patients had persistently raised but
falling hCG levels after a molar pregnancy). Centers treating this
condition and using a particular protocol should report their findings
so recommendations can be updated for improved treatment of these
patients.
ANNIE N.Y. Cheung, M.D., and KAREN K.L. CHAN, M.D., are
both with the University of Hong Kong, Queen Mary Hospital, Hong Kong.
They said they had no relevant financial disclosures. They wrote an
editorial accompanying the Agarwal article (Lancet 2011
[doi:10.1016/S0140-6736(11)61518-3]).
I think this study is
interesting. The number of patients who were treated with chemotherapy
was very small (10 of 76 patients). Therefore, comparison of the
patients who received chemotherapy versus surveillance is difficult due
to the size of the study, the retrospective nature of the study, as well
as the fact that the patients who received chemotherapy had higher
median hCG levels than those under surveillance (157 vs. 13). HCG levels
also were not well correlated with remission (r = 0.233; p = 0.049).
Patients
with low hCG levels at 6 months can probably be offered surveillance as
an alternative to chemotherapy in select circumstances. However, due to
the aforementioned concerns, before these findings are applied
wholesale in clinical practice, additional studies including a
prospective study are necessary.
E. ALBERT REECE, M.D., Ph.D, M.B.A., is
vice president for medical affairs at the University of Maryland,
Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished
Professor and dean of the school of medicine. He said he had no relevant
financial disclosures.
Treatment Still a Bit of a Conundrum
The data from Dr.
Agarwal and colleagues' study are reassuring. However, 13% of the cohort
received chemotherapy, and how these individuals would have progressed
if no treatment were given is unknown. Hence, the key question remains:
When and to whom should chemotherapy be given? The investigators propose
a cutoff hCG concentration of 345 IU/L at 6 months, which was the
median hCG value in patients who responded to chemotherapy in their
cohort.
Meanwhile, investigators in an earlier study (Gynecol.
Oncol. 2010;116:3-9) proposed that chemotherapy should be started only
when total hCG begins to rise and is greater than 3,000 IU/L, because
chemotherapy would probably be ineffective below this value. Direct
comparison between the two cohorts is inappropriate because the group in
the earlier study was heterogeneous (patients had been given various
previous treatments), whereas Dr. Agarwal and colleagues' group was
unique in its homogeneity (all patients had persistently raised but
falling hCG levels after a molar pregnancy). Centers treating this
condition and using a particular protocol should report their findings
so recommendations can be updated for improved treatment of these
patients.
ANNIE N.Y. Cheung, M.D., and KAREN K.L. CHAN, M.D., are
both with the University of Hong Kong, Queen Mary Hospital, Hong Kong.
They said they had no relevant financial disclosures. They wrote an
editorial accompanying the Agarwal article (Lancet 2011
[doi:10.1016/S0140-6736(11)61518-3]).
I think this study is
interesting. The number of patients who were treated with chemotherapy
was very small (10 of 76 patients). Therefore, comparison of the
patients who received chemotherapy versus surveillance is difficult due
to the size of the study, the retrospective nature of the study, as well
as the fact that the patients who received chemotherapy had higher
median hCG levels than those under surveillance (157 vs. 13). HCG levels
also were not well correlated with remission (r = 0.233; p = 0.049).
Patients
with low hCG levels at 6 months can probably be offered surveillance as
an alternative to chemotherapy in select circumstances. However, due to
the aforementioned concerns, before these findings are applied
wholesale in clinical practice, additional studies including a
prospective study are necessary.
E. ALBERT REECE, M.D., Ph.D, M.B.A., is
vice president for medical affairs at the University of Maryland,
Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished
Professor and dean of the school of medicine. He said he had no relevant
financial disclosures.
Treatment Still a Bit of a Conundrum
The data from Dr.
Agarwal and colleagues' study are reassuring. However, 13% of the cohort
received chemotherapy, and how these individuals would have progressed
if no treatment were given is unknown. Hence, the key question remains:
When and to whom should chemotherapy be given? The investigators propose
a cutoff hCG concentration of 345 IU/L at 6 months, which was the
median hCG value in patients who responded to chemotherapy in their
cohort.
Meanwhile, investigators in an earlier study (Gynecol.
Oncol. 2010;116:3-9) proposed that chemotherapy should be started only
when total hCG begins to rise and is greater than 3,000 IU/L, because
chemotherapy would probably be ineffective below this value. Direct
comparison between the two cohorts is inappropriate because the group in
the earlier study was heterogeneous (patients had been given various
previous treatments), whereas Dr. Agarwal and colleagues' group was
unique in its homogeneity (all patients had persistently raised but
falling hCG levels after a molar pregnancy). Centers treating this
condition and using a particular protocol should report their findings
so recommendations can be updated for improved treatment of these
patients.
ANNIE N.Y. Cheung, M.D., and KAREN K.L. CHAN, M.D., are
both with the University of Hong Kong, Queen Mary Hospital, Hong Kong.
They said they had no relevant financial disclosures. They wrote an
editorial accompanying the Agarwal article (Lancet 2011
[doi:10.1016/S0140-6736(11)61518-3]).
Women with raised but falling human chorionic gonadotropin concentrations 6 months after a molar pregnancy do not need chemotherapy, as almost all of them will spontaneously remit, the results of a large retrospective cohort study have shown.
The findings, published online in the Lancet (Lancet 2011 [doi:10.1016/S0140-6736(11)61265-8]), challenge current international clinical guidelines (Int. J. Gynecol. Obstet. 2002;77:285-7) that consider chemotherapy to be indicated when hCG concentrations are high for 6 months or more following evacuation of a hydatidiform mole.
The new findings argue that an elevated hCG level at 6 months is not an indicator of malignancy when values are falling, and that instead of initiating chemotherapy, women with raised but falling hCG can undergo surveillance of their hCG levels, with testing until they return to normal.
Chemotherapy is needed only if hCG levels are still rising at 6 months, have plateaued, or are greater than 345 IU/L, or if there is radiologic evidence of neoplasia.
If the surveillance approach were to become standard, more women could avoid exposure to toxic chemotherapy drugs and could safely conceive sooner after evacuation of a molar pregnancy, said the authors of the study led by Dr. Roshan Agarwal of Imperial College London.
Current U.K. guidelines advise women not to become pregnant until 12 months following chemotherapy, whereas they would have to wait only 6 months after a spontaneous return to normal hCG.
For their research, Dr. Agarwal and colleagues retrospectively identified 13,960 women registered at London's Charing Cross Hospital between January 1993 and May 2008 who had undergone evacuation of a complete or partial hydatidiform mole. Of these, 974 (7%) required chemotherapy within 6 months, and hCG normalized spontaneously in 12,910 (92%) within 6 months.
The remaining 76 women still had high hCG concentrations (more than 5 IU/L) 6 months after evacuation of the molar pregnancy. Sixty-six patients underwent surveillance, in which blood and urine samples were collected and evaluated every 2 weeks until normal hCG was achieved, followed by monthly urine samples for 6 months. Ten patients underwent chemotherapy.
In the surveillance group, 98% of patients (n = 65) saw hCG values return to normal without chemotherapy (in all but 6 of them within a year), and the remaining patient, who had chronic renal failure, remained healthy despite having elevated hCG, Dr. Agarwal and colleagues reported.
Among the 10 patients who received chemotherapy, 6 had complete responses, and 4 had partial or no responses but remained well, even though hCG concentrations in 2 patients continued to be elevated. There was no significant difference in time to normalization between the groups and no deaths had occurred in either group after a median 2 years' follow-up.
The investigators acknowledged that a weakness of their study is retrospective design, the use of a single study site, and the small number of patients with raised hCG at 6 months.
However, they said, the findings “directly challenge the present clinical dogma” to show that the surveillance model is clinically acceptable. The results “will change international practice and spare women unnecessary exposure to chemotherapy,” they wrote in their analysis.
In a case study linked to Dr. Agarwal and colleagues' article, Rosemary A. Fisher, also of Imperial College London, described a woman who had a miscarriage and three consecutive molar pregnancies, yet was able to achieve a normal pregnancy with use of a donor egg.
“To the best of our knowledge, this report establishes for the first time that oocyte donation can enable women with familial recurrent hydatidiform moles due to NLRP7 mutations to achieve a normal pregnancy,” Ms. Fisher and colleagues wrote, adding that this finding offered “hope to other women with this condition.”
The study also shows “that the major role of NLRP7 in pregnancy is in the developing oocyte,” they said.
Women with raised but falling human chorionic gonadotropin concentrations 6 months after a molar pregnancy do not need chemotherapy, as almost all of them will spontaneously remit, the results of a large retrospective cohort study have shown.
The findings, published online in the Lancet (Lancet 2011 [doi:10.1016/S0140-6736(11)61265-8]), challenge current international clinical guidelines (Int. J. Gynecol. Obstet. 2002;77:285-7) that consider chemotherapy to be indicated when hCG concentrations are high for 6 months or more following evacuation of a hydatidiform mole.
The new findings argue that an elevated hCG level at 6 months is not an indicator of malignancy when values are falling, and that instead of initiating chemotherapy, women with raised but falling hCG can undergo surveillance of their hCG levels, with testing until they return to normal.
Chemotherapy is needed only if hCG levels are still rising at 6 months, have plateaued, or are greater than 345 IU/L, or if there is radiologic evidence of neoplasia.
If the surveillance approach were to become standard, more women could avoid exposure to toxic chemotherapy drugs and could safely conceive sooner after evacuation of a molar pregnancy, said the authors of the study led by Dr. Roshan Agarwal of Imperial College London.
Current U.K. guidelines advise women not to become pregnant until 12 months following chemotherapy, whereas they would have to wait only 6 months after a spontaneous return to normal hCG.
For their research, Dr. Agarwal and colleagues retrospectively identified 13,960 women registered at London's Charing Cross Hospital between January 1993 and May 2008 who had undergone evacuation of a complete or partial hydatidiform mole. Of these, 974 (7%) required chemotherapy within 6 months, and hCG normalized spontaneously in 12,910 (92%) within 6 months.
The remaining 76 women still had high hCG concentrations (more than 5 IU/L) 6 months after evacuation of the molar pregnancy. Sixty-six patients underwent surveillance, in which blood and urine samples were collected and evaluated every 2 weeks until normal hCG was achieved, followed by monthly urine samples for 6 months. Ten patients underwent chemotherapy.
In the surveillance group, 98% of patients (n = 65) saw hCG values return to normal without chemotherapy (in all but 6 of them within a year), and the remaining patient, who had chronic renal failure, remained healthy despite having elevated hCG, Dr. Agarwal and colleagues reported.
Among the 10 patients who received chemotherapy, 6 had complete responses, and 4 had partial or no responses but remained well, even though hCG concentrations in 2 patients continued to be elevated. There was no significant difference in time to normalization between the groups and no deaths had occurred in either group after a median 2 years' follow-up.
The investigators acknowledged that a weakness of their study is retrospective design, the use of a single study site, and the small number of patients with raised hCG at 6 months.
However, they said, the findings “directly challenge the present clinical dogma” to show that the surveillance model is clinically acceptable. The results “will change international practice and spare women unnecessary exposure to chemotherapy,” they wrote in their analysis.
In a case study linked to Dr. Agarwal and colleagues' article, Rosemary A. Fisher, also of Imperial College London, described a woman who had a miscarriage and three consecutive molar pregnancies, yet was able to achieve a normal pregnancy with use of a donor egg.
“To the best of our knowledge, this report establishes for the first time that oocyte donation can enable women with familial recurrent hydatidiform moles due to NLRP7 mutations to achieve a normal pregnancy,” Ms. Fisher and colleagues wrote, adding that this finding offered “hope to other women with this condition.”
The study also shows “that the major role of NLRP7 in pregnancy is in the developing oocyte,” they said.
FROM THE LANCET
National Texting Program for New Moms Continues Growth
A nationwide texting program for new moms continues to grow in its second year, and an initial evaluation of the enrollees' feedback is showing promising results.
The public-private partnership called text4baby sends free educational text messages to expecting and new moms. The program now has over 260,000 enrollees, up from more than 150,000 in April.
With the advancement of technology and the widespread access to mobile phones, national agencies are trying to use tools such as texting to promote healthy behaviors.
The U.S. Department of Health and Human Services created a Text4Health Task Force in 2010, trying to “capitalize on the rapid proliferation of mobile phone technology and platforms, such as text messaging,” and reach underserved groups, according to one of the agency's recent announcements.
Maternal and child health, domestic violence and sexual abuse prevention, tobacco control, emergency preparedness, and diabetes and asthma education are among the agency's texting projects.
Although it is too soon to tell whether such texting initiatives will improve health outcomes, positive feedback from women and physicians who use text4baby has turned some skeptics into believers.
“The overwhelming response was that the program brought information into their hands,” said Dr. Yvette LaCoursiere, an assistant clinical professor in the reproductive medicine department at University of California, San Diego. She was involved in the multiagency partnership that conducted a small-scale evaluation of text4baby enrollees in San Diego.
Dr. LaCoursiere calls herself “a bit of a devil's advocate,” and before conducting the survey she had some concerns. For one, she wondered whether the program was well received and whether it would create more work for physicians.
But she found out otherwise, she said.
With the goal of reaching women, especially those who are uninsured or underinsured, text4baby sends three free text messages daily to enrollees, many of which are relevant to their due date (nationally, around 46% of women signed up during their first trimester). It sends out phone numbers of relevant resources, and it alerts women of an outbreak or recall.
In the telephone survey of 122 text4baby users (roughly 10% of San Diego County's text4baby enrollees), 63% of the respondents said that the service helped them remember appointments or immunizations for themselves or their child, 75% said the messages informed them of “medical warning signs that they did not know,” and 71% said the messages promoted a conversation with their physician.
More than half of underinsured respondents (53%) said they called a phone number that was sent in a text4baby message.
“The messages support the messages ob.gyns provide to their patients,” said Dr. LaCoursiere. “I tell my patients it's the text version of a [maternity book].”
Dr. LaCoursiere said that some of her physician colleagues who have signed up for the service have also “picked up some tips” from the messages.
To become more attractive to users and gather their insights, the messaging program is now trying to become interactive.
One of its first interactive projects was a flu module, which asked enrollees whether or not they were planning to get a flu shot this season.
Of the 31% of over 100,000 active text4baby users currently in the “pregnancy” or “new baby' protocol who responded, 40% said they had already gotten the shot, 29% said they were planning to, and 31% said they were not. More than half of those who said they were planning to get the flu shot requested a reminder provided by the module.
Such interactivity can help engage the users and also reinforce key health concepts, according to Dr. Carolyn B. Bridges, associate director of adult immunization at the Centers for Disease Control and Prevention, who spoke about the module at a recent briefing.
Text4baby, which is a program of the National Healthy Mothers, Healthy Babies Coalition, is planning to reach 1 million women by the end of 2012. The program was developed as a free tool to reach mothers across the nation and help reduce the risk of negative birth outcomes, according to the organization. With more than 28,000 infant deaths each year, the United States has one of the highest infant mortality rates among the industrialized nations.
While there are various texting projects underway, maternal and child health might have one of the more eager audiences.
“Pregnant women are hungry for knowledge,” said Dr. LaCoursiere. “They want to learn how to be a good mom. So you have a population who's very interested in learning.”
Text4baby is planning to release radio and television Public Service Announcements and increase its presence on social media in 2012, according to the program's organizers.
Several national evaluations are underway, and the results could be available within the next 2 years.
'The [text4baby] messages support the messages ob.gyns. provide to their patients.'
Source DR. LaCOURSIERE
A sample of text4baby messages is shown on a cellphone screen.
Source Naseem S. Miller/Elsevier Global Medical News
A nationwide texting program for new moms continues to grow in its second year, and an initial evaluation of the enrollees' feedback is showing promising results.
The public-private partnership called text4baby sends free educational text messages to expecting and new moms. The program now has over 260,000 enrollees, up from more than 150,000 in April.
With the advancement of technology and the widespread access to mobile phones, national agencies are trying to use tools such as texting to promote healthy behaviors.
The U.S. Department of Health and Human Services created a Text4Health Task Force in 2010, trying to “capitalize on the rapid proliferation of mobile phone technology and platforms, such as text messaging,” and reach underserved groups, according to one of the agency's recent announcements.
Maternal and child health, domestic violence and sexual abuse prevention, tobacco control, emergency preparedness, and diabetes and asthma education are among the agency's texting projects.
Although it is too soon to tell whether such texting initiatives will improve health outcomes, positive feedback from women and physicians who use text4baby has turned some skeptics into believers.
“The overwhelming response was that the program brought information into their hands,” said Dr. Yvette LaCoursiere, an assistant clinical professor in the reproductive medicine department at University of California, San Diego. She was involved in the multiagency partnership that conducted a small-scale evaluation of text4baby enrollees in San Diego.
Dr. LaCoursiere calls herself “a bit of a devil's advocate,” and before conducting the survey she had some concerns. For one, she wondered whether the program was well received and whether it would create more work for physicians.
But she found out otherwise, she said.
With the goal of reaching women, especially those who are uninsured or underinsured, text4baby sends three free text messages daily to enrollees, many of which are relevant to their due date (nationally, around 46% of women signed up during their first trimester). It sends out phone numbers of relevant resources, and it alerts women of an outbreak or recall.
In the telephone survey of 122 text4baby users (roughly 10% of San Diego County's text4baby enrollees), 63% of the respondents said that the service helped them remember appointments or immunizations for themselves or their child, 75% said the messages informed them of “medical warning signs that they did not know,” and 71% said the messages promoted a conversation with their physician.
More than half of underinsured respondents (53%) said they called a phone number that was sent in a text4baby message.
“The messages support the messages ob.gyns provide to their patients,” said Dr. LaCoursiere. “I tell my patients it's the text version of a [maternity book].”
Dr. LaCoursiere said that some of her physician colleagues who have signed up for the service have also “picked up some tips” from the messages.
To become more attractive to users and gather their insights, the messaging program is now trying to become interactive.
One of its first interactive projects was a flu module, which asked enrollees whether or not they were planning to get a flu shot this season.
Of the 31% of over 100,000 active text4baby users currently in the “pregnancy” or “new baby' protocol who responded, 40% said they had already gotten the shot, 29% said they were planning to, and 31% said they were not. More than half of those who said they were planning to get the flu shot requested a reminder provided by the module.
Such interactivity can help engage the users and also reinforce key health concepts, according to Dr. Carolyn B. Bridges, associate director of adult immunization at the Centers for Disease Control and Prevention, who spoke about the module at a recent briefing.
Text4baby, which is a program of the National Healthy Mothers, Healthy Babies Coalition, is planning to reach 1 million women by the end of 2012. The program was developed as a free tool to reach mothers across the nation and help reduce the risk of negative birth outcomes, according to the organization. With more than 28,000 infant deaths each year, the United States has one of the highest infant mortality rates among the industrialized nations.
While there are various texting projects underway, maternal and child health might have one of the more eager audiences.
“Pregnant women are hungry for knowledge,” said Dr. LaCoursiere. “They want to learn how to be a good mom. So you have a population who's very interested in learning.”
Text4baby is planning to release radio and television Public Service Announcements and increase its presence on social media in 2012, according to the program's organizers.
Several national evaluations are underway, and the results could be available within the next 2 years.
'The [text4baby] messages support the messages ob.gyns. provide to their patients.'
Source DR. LaCOURSIERE
A sample of text4baby messages is shown on a cellphone screen.
Source Naseem S. Miller/Elsevier Global Medical News
A nationwide texting program for new moms continues to grow in its second year, and an initial evaluation of the enrollees' feedback is showing promising results.
The public-private partnership called text4baby sends free educational text messages to expecting and new moms. The program now has over 260,000 enrollees, up from more than 150,000 in April.
With the advancement of technology and the widespread access to mobile phones, national agencies are trying to use tools such as texting to promote healthy behaviors.
The U.S. Department of Health and Human Services created a Text4Health Task Force in 2010, trying to “capitalize on the rapid proliferation of mobile phone technology and platforms, such as text messaging,” and reach underserved groups, according to one of the agency's recent announcements.
Maternal and child health, domestic violence and sexual abuse prevention, tobacco control, emergency preparedness, and diabetes and asthma education are among the agency's texting projects.
Although it is too soon to tell whether such texting initiatives will improve health outcomes, positive feedback from women and physicians who use text4baby has turned some skeptics into believers.
“The overwhelming response was that the program brought information into their hands,” said Dr. Yvette LaCoursiere, an assistant clinical professor in the reproductive medicine department at University of California, San Diego. She was involved in the multiagency partnership that conducted a small-scale evaluation of text4baby enrollees in San Diego.
Dr. LaCoursiere calls herself “a bit of a devil's advocate,” and before conducting the survey she had some concerns. For one, she wondered whether the program was well received and whether it would create more work for physicians.
But she found out otherwise, she said.
With the goal of reaching women, especially those who are uninsured or underinsured, text4baby sends three free text messages daily to enrollees, many of which are relevant to their due date (nationally, around 46% of women signed up during their first trimester). It sends out phone numbers of relevant resources, and it alerts women of an outbreak or recall.
In the telephone survey of 122 text4baby users (roughly 10% of San Diego County's text4baby enrollees), 63% of the respondents said that the service helped them remember appointments or immunizations for themselves or their child, 75% said the messages informed them of “medical warning signs that they did not know,” and 71% said the messages promoted a conversation with their physician.
More than half of underinsured respondents (53%) said they called a phone number that was sent in a text4baby message.
“The messages support the messages ob.gyns provide to their patients,” said Dr. LaCoursiere. “I tell my patients it's the text version of a [maternity book].”
Dr. LaCoursiere said that some of her physician colleagues who have signed up for the service have also “picked up some tips” from the messages.
To become more attractive to users and gather their insights, the messaging program is now trying to become interactive.
One of its first interactive projects was a flu module, which asked enrollees whether or not they were planning to get a flu shot this season.
Of the 31% of over 100,000 active text4baby users currently in the “pregnancy” or “new baby' protocol who responded, 40% said they had already gotten the shot, 29% said they were planning to, and 31% said they were not. More than half of those who said they were planning to get the flu shot requested a reminder provided by the module.
Such interactivity can help engage the users and also reinforce key health concepts, according to Dr. Carolyn B. Bridges, associate director of adult immunization at the Centers for Disease Control and Prevention, who spoke about the module at a recent briefing.
Text4baby, which is a program of the National Healthy Mothers, Healthy Babies Coalition, is planning to reach 1 million women by the end of 2012. The program was developed as a free tool to reach mothers across the nation and help reduce the risk of negative birth outcomes, according to the organization. With more than 28,000 infant deaths each year, the United States has one of the highest infant mortality rates among the industrialized nations.
While there are various texting projects underway, maternal and child health might have one of the more eager audiences.
“Pregnant women are hungry for knowledge,” said Dr. LaCoursiere. “They want to learn how to be a good mom. So you have a population who's very interested in learning.”
Text4baby is planning to release radio and television Public Service Announcements and increase its presence on social media in 2012, according to the program's organizers.
Several national evaluations are underway, and the results could be available within the next 2 years.
'The [text4baby] messages support the messages ob.gyns. provide to their patients.'
Source DR. LaCOURSIERE
A sample of text4baby messages is shown on a cellphone screen.
Source Naseem S. Miller/Elsevier Global Medical News
Meta-Analysis: Pregnancy-Associated Breast Cancer Fares Poorly
SAN ANTONIO – Pregnancy-associated breast cancer carries a relatively poor prognosis regardless of whether the malignancy is diagnosed during pregnancy or post partum, a meta-analysis has shown.
The meta-analysis included 29 matched case-control studies totaling 3,529 women with pregnancy-associated breast cancer – defined as breast cancer diagnosed during pregnancy or within 1 year afterward – and 36,914 controls whose breast cancer was unrelated to pregnancy.
This is believed to be the largest-ever meta-analysis addressing the prognosis of pregnancy-associated breast cancer, a relatively rare disease, Dr. Hatem A. Azim Jr. reported at the annual San Antonio Breast Cancer Symposium. In addition to the published reports, Dr. Azim and coauthors contacted the various study investigators to obtain unpublished data.
Women with pregnancy-associated breast cancer had a significantly greater risk of death than did controls, Dr. Azim and colleagues found: In a multivariate analysis, pregnancy increased the overall mortality rate by 46%. This held true even after adjustment for differences in tumor size, nodal status, and systemic therapy, according to Dr. Azim of the Jules Bordet Institute at the Free University of Brussels.
The secondary outcome measure in the meta-analysis was disease-free survival. Patients with pregnancy-associated breast cancer had a 59% increased risk of relapse compared with women with nonpregnancy-related breast cancer.
Roughly 6% of breast cancers are diagnosed in women before the age of 40, and of those only about 10% are diagnosed during pregnancy or within a year after. The relative rarity of this condition precludes conducting definitive large prospective clinical trials addressing patient prognosis.
Despite the rarity of pregnancy-associated breast cancer, however, it’s an important condition for a couple of reasons: The incidence of pregnancy-associated breast cancer is increasing because of the popular trend for delay of childbirth until later in life, and the condition poses a thorny therapeutic challenge because of the poor prognosis.
Dr. Azim reported no relevant financial disclosures.
SAN ANTONIO – Pregnancy-associated breast cancer carries a relatively poor prognosis regardless of whether the malignancy is diagnosed during pregnancy or post partum, a meta-analysis has shown.
The meta-analysis included 29 matched case-control studies totaling 3,529 women with pregnancy-associated breast cancer – defined as breast cancer diagnosed during pregnancy or within 1 year afterward – and 36,914 controls whose breast cancer was unrelated to pregnancy.
This is believed to be the largest-ever meta-analysis addressing the prognosis of pregnancy-associated breast cancer, a relatively rare disease, Dr. Hatem A. Azim Jr. reported at the annual San Antonio Breast Cancer Symposium. In addition to the published reports, Dr. Azim and coauthors contacted the various study investigators to obtain unpublished data.
Women with pregnancy-associated breast cancer had a significantly greater risk of death than did controls, Dr. Azim and colleagues found: In a multivariate analysis, pregnancy increased the overall mortality rate by 46%. This held true even after adjustment for differences in tumor size, nodal status, and systemic therapy, according to Dr. Azim of the Jules Bordet Institute at the Free University of Brussels.
The secondary outcome measure in the meta-analysis was disease-free survival. Patients with pregnancy-associated breast cancer had a 59% increased risk of relapse compared with women with nonpregnancy-related breast cancer.
Roughly 6% of breast cancers are diagnosed in women before the age of 40, and of those only about 10% are diagnosed during pregnancy or within a year after. The relative rarity of this condition precludes conducting definitive large prospective clinical trials addressing patient prognosis.
Despite the rarity of pregnancy-associated breast cancer, however, it’s an important condition for a couple of reasons: The incidence of pregnancy-associated breast cancer is increasing because of the popular trend for delay of childbirth until later in life, and the condition poses a thorny therapeutic challenge because of the poor prognosis.
Dr. Azim reported no relevant financial disclosures.
SAN ANTONIO – Pregnancy-associated breast cancer carries a relatively poor prognosis regardless of whether the malignancy is diagnosed during pregnancy or post partum, a meta-analysis has shown.
The meta-analysis included 29 matched case-control studies totaling 3,529 women with pregnancy-associated breast cancer – defined as breast cancer diagnosed during pregnancy or within 1 year afterward – and 36,914 controls whose breast cancer was unrelated to pregnancy.
This is believed to be the largest-ever meta-analysis addressing the prognosis of pregnancy-associated breast cancer, a relatively rare disease, Dr. Hatem A. Azim Jr. reported at the annual San Antonio Breast Cancer Symposium. In addition to the published reports, Dr. Azim and coauthors contacted the various study investigators to obtain unpublished data.
Women with pregnancy-associated breast cancer had a significantly greater risk of death than did controls, Dr. Azim and colleagues found: In a multivariate analysis, pregnancy increased the overall mortality rate by 46%. This held true even after adjustment for differences in tumor size, nodal status, and systemic therapy, according to Dr. Azim of the Jules Bordet Institute at the Free University of Brussels.
The secondary outcome measure in the meta-analysis was disease-free survival. Patients with pregnancy-associated breast cancer had a 59% increased risk of relapse compared with women with nonpregnancy-related breast cancer.
Roughly 6% of breast cancers are diagnosed in women before the age of 40, and of those only about 10% are diagnosed during pregnancy or within a year after. The relative rarity of this condition precludes conducting definitive large prospective clinical trials addressing patient prognosis.
Despite the rarity of pregnancy-associated breast cancer, however, it’s an important condition for a couple of reasons: The incidence of pregnancy-associated breast cancer is increasing because of the popular trend for delay of childbirth until later in life, and the condition poses a thorny therapeutic challenge because of the poor prognosis.
Dr. Azim reported no relevant financial disclosures.
FROM THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Pregnancy-associated breast cancer is associated with a 46% increased risk of death and a 59% greater relapse risk than breast cancer unrelated to pregnancy.
Data Source: A meta-analysis of 29 matched case-control studies totaling 3,529 women whose breast cancer was diagnosed during pregnancy or within 1 year afterward and 36,914 controls whose breast cancer was unrelated to pregnancy.
Disclosures: Dr. Azim reported no relevant financial disclosures.
FDA: Appropriate SSRI Use OK in Pregnancy
Pregnant women taking selective serotonin reuptake inhibitors for depression may continue to do so, despite a 2006 warning that the drugs may predispose infants to persistent pulmonary hypertension, the Food and Drug Administration announced Dec. 14.
That earlier warning was based on a single study indicating that infants exposed to the drug in utero after the 20th week of pregnancy were six times more likely to develop persistent pulmonary hypertension (PPHN) than nonexposed infants (N. Engl. J. Med. 2006;354:579-87).
"Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of SSRIs during pregnancy can cause persistent pulmonary hypertension," the FDA said in a press statement.
The agency will update the drugs’ warning labels to reflect data from new studies, which have produced conflicting results about the risk SSRIs may pose to an unborn child.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are "substantial risks associated with undertreatment or no treatment of depression during pregnancy."
Those studies include a large retrospective database study in 2009 that found no association between SSRI use and PPHN (Pharmacoepidemiol. Drug Saf. 2009;18:246-52), and a 2011 case-control study of 11,923 births that showed PPHN was associated with cesarean delivery but not with SSRI use in the second half of pregnancy (Am. J. Perinatol. 2011;28:19-24).
FDA officials concluded that the evidence is not sufficient to withhold SSRI treatment from pregnant women or take them off the antidepressants. "At present, FDA ... recommends that health care providers treat depression during pregnancy as clinically appropriate," according to the agency’s statement.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are "substantial risks associated with undertreatment or no treatment of depression during pregnancy."
Risks of untreated maternal depression can include low birth weight, preterm delivery, lower Apgar scores, poor prenatal care, failure to recognize or report impending labor, and increased risks of fetal abuse, neonaticide, or maternal suicide, the FDA warned.
Both the American Psychiatric Association and the American College of Obstetricians and Gynecologists recommend monitoring pregnant women for depression and treating them appropriately.
Physicians should continue to report any possible adverse effects to the FDA’s MedWatch program. Reporting forms can also be requested by calling 800-332-1088. The form can be submitted online or by fax to 800-FDA-0178.
Pregnant women taking selective serotonin reuptake inhibitors for depression may continue to do so, despite a 2006 warning that the drugs may predispose infants to persistent pulmonary hypertension, the Food and Drug Administration announced Dec. 14.
That earlier warning was based on a single study indicating that infants exposed to the drug in utero after the 20th week of pregnancy were six times more likely to develop persistent pulmonary hypertension (PPHN) than nonexposed infants (N. Engl. J. Med. 2006;354:579-87).
"Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of SSRIs during pregnancy can cause persistent pulmonary hypertension," the FDA said in a press statement.
The agency will update the drugs’ warning labels to reflect data from new studies, which have produced conflicting results about the risk SSRIs may pose to an unborn child.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are "substantial risks associated with undertreatment or no treatment of depression during pregnancy."
Those studies include a large retrospective database study in 2009 that found no association between SSRI use and PPHN (Pharmacoepidemiol. Drug Saf. 2009;18:246-52), and a 2011 case-control study of 11,923 births that showed PPHN was associated with cesarean delivery but not with SSRI use in the second half of pregnancy (Am. J. Perinatol. 2011;28:19-24).
FDA officials concluded that the evidence is not sufficient to withhold SSRI treatment from pregnant women or take them off the antidepressants. "At present, FDA ... recommends that health care providers treat depression during pregnancy as clinically appropriate," according to the agency’s statement.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are "substantial risks associated with undertreatment or no treatment of depression during pregnancy."
Risks of untreated maternal depression can include low birth weight, preterm delivery, lower Apgar scores, poor prenatal care, failure to recognize or report impending labor, and increased risks of fetal abuse, neonaticide, or maternal suicide, the FDA warned.
Both the American Psychiatric Association and the American College of Obstetricians and Gynecologists recommend monitoring pregnant women for depression and treating them appropriately.
Physicians should continue to report any possible adverse effects to the FDA’s MedWatch program. Reporting forms can also be requested by calling 800-332-1088. The form can be submitted online or by fax to 800-FDA-0178.
Pregnant women taking selective serotonin reuptake inhibitors for depression may continue to do so, despite a 2006 warning that the drugs may predispose infants to persistent pulmonary hypertension, the Food and Drug Administration announced Dec. 14.
That earlier warning was based on a single study indicating that infants exposed to the drug in utero after the 20th week of pregnancy were six times more likely to develop persistent pulmonary hypertension (PPHN) than nonexposed infants (N. Engl. J. Med. 2006;354:579-87).
"Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of SSRIs during pregnancy can cause persistent pulmonary hypertension," the FDA said in a press statement.
The agency will update the drugs’ warning labels to reflect data from new studies, which have produced conflicting results about the risk SSRIs may pose to an unborn child.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are "substantial risks associated with undertreatment or no treatment of depression during pregnancy."
Those studies include a large retrospective database study in 2009 that found no association between SSRI use and PPHN (Pharmacoepidemiol. Drug Saf. 2009;18:246-52), and a 2011 case-control study of 11,923 births that showed PPHN was associated with cesarean delivery but not with SSRI use in the second half of pregnancy (Am. J. Perinatol. 2011;28:19-24).
FDA officials concluded that the evidence is not sufficient to withhold SSRI treatment from pregnant women or take them off the antidepressants. "At present, FDA ... recommends that health care providers treat depression during pregnancy as clinically appropriate," according to the agency’s statement.
Physicians and their patients should carefully weigh the risks and benefits of any antidepressant use in pregnancy, the FDA added, given that there are "substantial risks associated with undertreatment or no treatment of depression during pregnancy."
Risks of untreated maternal depression can include low birth weight, preterm delivery, lower Apgar scores, poor prenatal care, failure to recognize or report impending labor, and increased risks of fetal abuse, neonaticide, or maternal suicide, the FDA warned.
Both the American Psychiatric Association and the American College of Obstetricians and Gynecologists recommend monitoring pregnant women for depression and treating them appropriately.
Physicians should continue to report any possible adverse effects to the FDA’s MedWatch program. Reporting forms can also be requested by calling 800-332-1088. The form can be submitted online or by fax to 800-FDA-0178.
Oxytocin System Functioning Mediates Effects of Maternal Depression
Children exposed to maternal depression throughout their first year of life are more likely than nonexposed children to develop mental disorders by age 6 years, particularly if their oxytocin system functioning is disordered, according to findings from a longitudinal study of 155 mother-child pairs.
Of the children in the study who were exposed to depression throughout their first year, 60% exhibited mental disorders by age 6 years, compared with only 15% of those born to mothers with no depression or other mental disorders, Ruth Feldman, Ph.D., reported at the annual meeting of the American College of Neuropsychopharmacology.
Anxiety disorders and conduct disorders were the most common conditions exhibited by the exposed children, although depression is likely to show more prominently at the next assessment when the children are aged 9 or 10 years, noted Dr. Feldman of Bar-llan University in Ramat Gan, Israel.
The exposed children also demonstrated lower social engagement with their mothers, lower playfulness and creativity, and diminished social involvement, compared with non-exposed children, and also were less verbal and expressed less empathy to the pain, suffering, and embarrassment of strangers, she said at a press briefing held in conjunction with the meeting.
Like their mothers, who had an increased likelihood of having disordered oxytocin functioning and who produced less peripheral oxytocin in their saliva, the children were found to have disordered functioning of the oxytocin system and lower salivary oxytocin levels.
The depressed mothers, as well as their children, had a threefold greater prevalence of a risky variant (a variant with two "G" alleles) of the oxytocin receptor gene.
Of note, the 40% of exposed children who did not develop a mental disorder by age 6 years demonstrated more normal functioning of the oxytocin system, and they had better social engagements and higher levels of empathy, Dr. Feldman said.
Furthermore, these children were born to women who had less disruption of the oxytocin system and a less risky variant (the "A" allele variant) of the oxytocin receptor gene, as well as typical levels of oxytocin in their saliva.
These women, despite their depression, had better emotional skills and an improved capacity for providing adequate care, Dr. Feldman said.
It appears that a properly functioning oxytocin system offers protection against the effects of chronic maternal depression in some children, she added.
Study participants were recruited from a larger sample of nearly 2,000 mothers who participated in a mental health survey when they delivered, and again at 6 and 9 months after delivery. The oxytocin measures and in-home observations between parents and children were conducted in those who participated in this portion of the study. In addition to the 20% of participants who had depression throughout the first postpartum year, 4% were diagnosed with subclinical depression and 4% with subclinical anxiety, and 62% had no signs of mental disorders or symptoms during the first year.
The findings are of interest because the oxytocin system is an open system with cross-generation effects, Dr. Feldman said, explaining that if the system is known to be disrupted – in cases of postpartum depression, for example – oxytocin-related interventions could be provided. Mothers could be instructed to increase maternal touch and gaze, or intranasal oxytocin could be administered, for example.
Such interventions could provide a protective barrier against some of the psychopathologies associated with maternal depression. Indeed, intranasal oxytocin administration to both infants and fathers (whose oxytocin levels also were shown in this study to be lower in the setting of maternal depression), was shown to improve vagal tone, duration of social engagement behavior, and to markedly increase salivary oxytocin, Dr. Feldman concluded.
She reported no disclosures.
conduct disorder in children
Children exposed to maternal depression throughout their first year of life are more likely than nonexposed children to develop mental disorders by age 6 years, particularly if their oxytocin system functioning is disordered, according to findings from a longitudinal study of 155 mother-child pairs.
Of the children in the study who were exposed to depression throughout their first year, 60% exhibited mental disorders by age 6 years, compared with only 15% of those born to mothers with no depression or other mental disorders, Ruth Feldman, Ph.D., reported at the annual meeting of the American College of Neuropsychopharmacology.
Anxiety disorders and conduct disorders were the most common conditions exhibited by the exposed children, although depression is likely to show more prominently at the next assessment when the children are aged 9 or 10 years, noted Dr. Feldman of Bar-llan University in Ramat Gan, Israel.
The exposed children also demonstrated lower social engagement with their mothers, lower playfulness and creativity, and diminished social involvement, compared with non-exposed children, and also were less verbal and expressed less empathy to the pain, suffering, and embarrassment of strangers, she said at a press briefing held in conjunction with the meeting.
Like their mothers, who had an increased likelihood of having disordered oxytocin functioning and who produced less peripheral oxytocin in their saliva, the children were found to have disordered functioning of the oxytocin system and lower salivary oxytocin levels.
The depressed mothers, as well as their children, had a threefold greater prevalence of a risky variant (a variant with two "G" alleles) of the oxytocin receptor gene.
Of note, the 40% of exposed children who did not develop a mental disorder by age 6 years demonstrated more normal functioning of the oxytocin system, and they had better social engagements and higher levels of empathy, Dr. Feldman said.
Furthermore, these children were born to women who had less disruption of the oxytocin system and a less risky variant (the "A" allele variant) of the oxytocin receptor gene, as well as typical levels of oxytocin in their saliva.
These women, despite their depression, had better emotional skills and an improved capacity for providing adequate care, Dr. Feldman said.
It appears that a properly functioning oxytocin system offers protection against the effects of chronic maternal depression in some children, she added.
Study participants were recruited from a larger sample of nearly 2,000 mothers who participated in a mental health survey when they delivered, and again at 6 and 9 months after delivery. The oxytocin measures and in-home observations between parents and children were conducted in those who participated in this portion of the study. In addition to the 20% of participants who had depression throughout the first postpartum year, 4% were diagnosed with subclinical depression and 4% with subclinical anxiety, and 62% had no signs of mental disorders or symptoms during the first year.
The findings are of interest because the oxytocin system is an open system with cross-generation effects, Dr. Feldman said, explaining that if the system is known to be disrupted – in cases of postpartum depression, for example – oxytocin-related interventions could be provided. Mothers could be instructed to increase maternal touch and gaze, or intranasal oxytocin could be administered, for example.
Such interventions could provide a protective barrier against some of the psychopathologies associated with maternal depression. Indeed, intranasal oxytocin administration to both infants and fathers (whose oxytocin levels also were shown in this study to be lower in the setting of maternal depression), was shown to improve vagal tone, duration of social engagement behavior, and to markedly increase salivary oxytocin, Dr. Feldman concluded.
She reported no disclosures.
Children exposed to maternal depression throughout their first year of life are more likely than nonexposed children to develop mental disorders by age 6 years, particularly if their oxytocin system functioning is disordered, according to findings from a longitudinal study of 155 mother-child pairs.
Of the children in the study who were exposed to depression throughout their first year, 60% exhibited mental disorders by age 6 years, compared with only 15% of those born to mothers with no depression or other mental disorders, Ruth Feldman, Ph.D., reported at the annual meeting of the American College of Neuropsychopharmacology.
Anxiety disorders and conduct disorders were the most common conditions exhibited by the exposed children, although depression is likely to show more prominently at the next assessment when the children are aged 9 or 10 years, noted Dr. Feldman of Bar-llan University in Ramat Gan, Israel.
The exposed children also demonstrated lower social engagement with their mothers, lower playfulness and creativity, and diminished social involvement, compared with non-exposed children, and also were less verbal and expressed less empathy to the pain, suffering, and embarrassment of strangers, she said at a press briefing held in conjunction with the meeting.
Like their mothers, who had an increased likelihood of having disordered oxytocin functioning and who produced less peripheral oxytocin in their saliva, the children were found to have disordered functioning of the oxytocin system and lower salivary oxytocin levels.
The depressed mothers, as well as their children, had a threefold greater prevalence of a risky variant (a variant with two "G" alleles) of the oxytocin receptor gene.
Of note, the 40% of exposed children who did not develop a mental disorder by age 6 years demonstrated more normal functioning of the oxytocin system, and they had better social engagements and higher levels of empathy, Dr. Feldman said.
Furthermore, these children were born to women who had less disruption of the oxytocin system and a less risky variant (the "A" allele variant) of the oxytocin receptor gene, as well as typical levels of oxytocin in their saliva.
These women, despite their depression, had better emotional skills and an improved capacity for providing adequate care, Dr. Feldman said.
It appears that a properly functioning oxytocin system offers protection against the effects of chronic maternal depression in some children, she added.
Study participants were recruited from a larger sample of nearly 2,000 mothers who participated in a mental health survey when they delivered, and again at 6 and 9 months after delivery. The oxytocin measures and in-home observations between parents and children were conducted in those who participated in this portion of the study. In addition to the 20% of participants who had depression throughout the first postpartum year, 4% were diagnosed with subclinical depression and 4% with subclinical anxiety, and 62% had no signs of mental disorders or symptoms during the first year.
The findings are of interest because the oxytocin system is an open system with cross-generation effects, Dr. Feldman said, explaining that if the system is known to be disrupted – in cases of postpartum depression, for example – oxytocin-related interventions could be provided. Mothers could be instructed to increase maternal touch and gaze, or intranasal oxytocin could be administered, for example.
Such interventions could provide a protective barrier against some of the psychopathologies associated with maternal depression. Indeed, intranasal oxytocin administration to both infants and fathers (whose oxytocin levels also were shown in this study to be lower in the setting of maternal depression), was shown to improve vagal tone, duration of social engagement behavior, and to markedly increase salivary oxytocin, Dr. Feldman concluded.
She reported no disclosures.
conduct disorder in children
conduct disorder in children
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF NEUROPSYCHO-PHARMACOLOGY
Major Finding: Of the children in the study who were exposed to depression throughout their first year of life, 60% exhibited mental disorders by age 6 years, compared with only 15% of those born to mothers with no depression or other mental disorders. Of note, the 40% of exposed children who did not develop a mental disorder by age 6 years demonstrated more normal functioning of the oxytocin system, as did their mothers (despite their depression).
Data Source: A prospective longitudinal study of 155 mother-child pairs.
Disclosures: Dr. Feldman reported no disclosures.
The birth rate among US teens fell to a record low in 2010
The birth rate among US teens 15 to 19 years old was 34.3 births for every 1,000 teenagers in 2010—a 9% decline from 2009 and the lowest rate ever recorded in nearly seven decades of collecting data, according to a report released November 17, 2011, by the Centers for Disease Control and Prevention (CDC).
The report, Births: Preliminary Data for 2010, from the CDC’s National Center for Health Statistics, is based on an analysis of nearly 100% of birth records collected in the 50 states, the District of Columbia, and US territories.
The birth rate for teenagers 15 to 19 years old has declined for the past 3 years and 17 of the past 19 years. Birth rates for younger and older teenagers and for all race and ethnic groups reached historic lows in 2010.
Other findings:
- The rate of cesarean delivery declined for the first time since 1996. In 2010, it was 32.8%, down from 32.9% in 2009
- Total births declined 3%, from 4,130,665 in 2009 to 4,000,279 in 2010
- The overall fertility rate fell 3%, from 66.2 births for every 1,000 females 15 to 44 years old in 2009 to 64.1 in 2010. This is the third straight decline for overall fertility in the United States.
- The total number of births to unmarried mothers declined for the second year in a row, to 1,633,785, down from 1,693,685 in 2009. Similarly, the birth rate for unmarried mothers declined to 47.7 for every 1,000 women in 2010, compared with 49.9 in 2009. The percentage of births to unmarried mothers also declined slightly in 2010, to 40.8%, compared with 41% in 2009.
- The birth rate for women in their early 20s fell 6% in 2010. The rates also fell for women in their late 20s and 30s. However, the birth rate for women in their early 40s increased to 10.2 per 1,000 women in 2010, compared with 10.0 in 2009, making it the highest birth rate for this age group since 1967
- The preterm birth rate declined for the fourth straight year in 2010, to just under 12% of all births (11.99%)—a 6% drop from 2006
- The rate of low birth-weight infants remained essentially unchanged between 2009 and 2010 at less than 8.2%, but is down slightly from the record high of 8.3 in 2006.
The full report is available at http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_02.pdf.
We want to hear from you! Tell us what you think.
The birth rate among US teens 15 to 19 years old was 34.3 births for every 1,000 teenagers in 2010—a 9% decline from 2009 and the lowest rate ever recorded in nearly seven decades of collecting data, according to a report released November 17, 2011, by the Centers for Disease Control and Prevention (CDC).
The report, Births: Preliminary Data for 2010, from the CDC’s National Center for Health Statistics, is based on an analysis of nearly 100% of birth records collected in the 50 states, the District of Columbia, and US territories.
The birth rate for teenagers 15 to 19 years old has declined for the past 3 years and 17 of the past 19 years. Birth rates for younger and older teenagers and for all race and ethnic groups reached historic lows in 2010.
Other findings:
- The rate of cesarean delivery declined for the first time since 1996. In 2010, it was 32.8%, down from 32.9% in 2009
- Total births declined 3%, from 4,130,665 in 2009 to 4,000,279 in 2010
- The overall fertility rate fell 3%, from 66.2 births for every 1,000 females 15 to 44 years old in 2009 to 64.1 in 2010. This is the third straight decline for overall fertility in the United States.
- The total number of births to unmarried mothers declined for the second year in a row, to 1,633,785, down from 1,693,685 in 2009. Similarly, the birth rate for unmarried mothers declined to 47.7 for every 1,000 women in 2010, compared with 49.9 in 2009. The percentage of births to unmarried mothers also declined slightly in 2010, to 40.8%, compared with 41% in 2009.
- The birth rate for women in their early 20s fell 6% in 2010. The rates also fell for women in their late 20s and 30s. However, the birth rate for women in their early 40s increased to 10.2 per 1,000 women in 2010, compared with 10.0 in 2009, making it the highest birth rate for this age group since 1967
- The preterm birth rate declined for the fourth straight year in 2010, to just under 12% of all births (11.99%)—a 6% drop from 2006
- The rate of low birth-weight infants remained essentially unchanged between 2009 and 2010 at less than 8.2%, but is down slightly from the record high of 8.3 in 2006.
The full report is available at http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_02.pdf.
We want to hear from you! Tell us what you think.
The birth rate among US teens 15 to 19 years old was 34.3 births for every 1,000 teenagers in 2010—a 9% decline from 2009 and the lowest rate ever recorded in nearly seven decades of collecting data, according to a report released November 17, 2011, by the Centers for Disease Control and Prevention (CDC).
The report, Births: Preliminary Data for 2010, from the CDC’s National Center for Health Statistics, is based on an analysis of nearly 100% of birth records collected in the 50 states, the District of Columbia, and US territories.
The birth rate for teenagers 15 to 19 years old has declined for the past 3 years and 17 of the past 19 years. Birth rates for younger and older teenagers and for all race and ethnic groups reached historic lows in 2010.
Other findings:
- The rate of cesarean delivery declined for the first time since 1996. In 2010, it was 32.8%, down from 32.9% in 2009
- Total births declined 3%, from 4,130,665 in 2009 to 4,000,279 in 2010
- The overall fertility rate fell 3%, from 66.2 births for every 1,000 females 15 to 44 years old in 2009 to 64.1 in 2010. This is the third straight decline for overall fertility in the United States.
- The total number of births to unmarried mothers declined for the second year in a row, to 1,633,785, down from 1,693,685 in 2009. Similarly, the birth rate for unmarried mothers declined to 47.7 for every 1,000 women in 2010, compared with 49.9 in 2009. The percentage of births to unmarried mothers also declined slightly in 2010, to 40.8%, compared with 41% in 2009.
- The birth rate for women in their early 20s fell 6% in 2010. The rates also fell for women in their late 20s and 30s. However, the birth rate for women in their early 40s increased to 10.2 per 1,000 women in 2010, compared with 10.0 in 2009, making it the highest birth rate for this age group since 1967
- The preterm birth rate declined for the fourth straight year in 2010, to just under 12% of all births (11.99%)—a 6% drop from 2006
- The rate of low birth-weight infants remained essentially unchanged between 2009 and 2010 at less than 8.2%, but is down slightly from the record high of 8.3 in 2006.
The full report is available at http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_02.pdf.
We want to hear from you! Tell us what you think.
A reasoned plan to manage a persistent Category-II FHR tracing
- Stop staring at that Category-II fetal heart-rate tracing…
Robert L. Barbieri, MD (Editorial, April 2011) - Guidelines on fetal monitoring aim to codify normal, abnormal FHR
Robert L. Barbieri, MD (Editorial, October 2008)
CASE An uncertain interlude during labor
An obstetrician checks on her laboring patient, only to discover that the fetal heart-rate (FHR) tracing has moved from Category I, a normal classification, into Category II—a gray zone. The OB decides to be proactive, not simply to wait for the tracing to return to normal. She has the patient move from a supine to a lateral position, provides oxygen, and administers a bolus of 500 to 1,000 mL of lactated Ringer’s solution over 20 minutes.
The tracing remains in Category II.
What should the OB do next?
When a fetal heart-rate tracing remains in Category II despite well-considered conservative corrective measures, a reasoned, rather than passive, approach is recommended.In 2008, the National Institute of Child Health and Human Development proposed a three-tier classification system for electronic FHR tracings (TABLE 1).1 Tracings in Category I are considered normal and can be managed routinely.1-3 Category-III tracings are considered abnormal and require additional attention; if corrective measures do not result in improvement, rapid delivery usually is warranted.1-3 Category II includes all FHR tracings that do not fit into either of the other categories. Because Category II encompasses such a wide range of FHR tracings, there are many options for management.
TABLE 1
3-tier fetal heart-rate classification system
| Category | Description |
|---|---|
| I | Fetal heart-rate (FHR) tracings include all of the following:
|
| II | Includes all FHR tracings not included in Category I or Category III |
| III | FHR tracings include:
|
| Source: Adapted from Macones GA, et al.1 | |
If the case described above sounds familiar, it may be that you read Editor in Chief Dr. Robert L. Barbieri’s editorial on Category-II FHR tracings in the April 2011 issue of OBG Management.4 That essay described a number of common conservative corrective measures applicable for Category-II tracings, including the three interventions the OB performed.
Other measures:
- reduce or stop infusion of oxytocin
- discontinue cervical ripening agents
- consider administering a tocolytic, such as terbutaline, if tachysystole is present or if uterine contractions are prolonged or coupled
- consider the option of amnioinfusion if variable decelerations are present.4,5
Systematic review of the oxygen pathway, from the environment to the fetus (maternal lungs, heart, vasculature, uterus, placenta, and umbilical cord), can facilitate recollection of all of these measures. In addition, a simplified “A-B-C-D” approach to the management of a Category-II FHR tracing is helpful (TABLES 2 and 3):
- Assess the oxygen pathway
- Begin conservative corrective measures
- Clear obstacles to rapid delivery
- Determine decision-to-delivery time.6,7
TABLE 2
Conservative corrective measures to improve fetal oxygenation
| “A” Assess oxygen pathway | “B” Begin corrective measures if indicated | |
|---|---|---|
| Lungs | Airway and breathing | Supplemental oxygen (10 L) using a tight-fitting, non-rebreather face mask for at least 15 minutes |
| Heart | Heart rate and rhythm | Position changes IV fluid bolus (500–1,000 cc of isotonic fluid over 20 min) Correct hypotension |
| Vasculature | Blood pressure Volume status | |
| Uterus | Contraction strength Contraction frequency Baseline uterine tone Exclude uterine rupture | Stop or reduce uterine stimulants (oxytocin, prostaglandin) Consider uterine relaxant (terbutaline) |
| Placenta | Placental separation Bleeding vasa previa | |
| Cord | Vaginal exam Exclude cord prolapse | Consider amnioinfusion |
| Courtesy of David A. Miller, MD | ||
As the obstetrician in the opening scenario knows all too well, conservative corrective measures do not always transform FHR tracings from Category II to Category I. In fact, it is extremely common for a Category-II tracing to remain in Category II despite every conservative corrective measure in the book. This article presents a practical, systematic, standardized approach to the management of a persistent Category-II FHR tracing.
TABLE 3
Steps involved in preparing for delivery
| “C” Clear obstacles to rapid delivery | “D” Determine decision-to-delivery time | |
|---|---|---|
| Facility | Operating room availability Equipment | Facility response time |
| Staff | Notify: Obstetrician Surgical assistant Anesthesiologist Neonatologist Pediatrician Nursing staff | Consider staff: Availability Training Experience |
| Mother | Informed consent Anesthesia options Laboratory tests Blood products Intravenous access Urinary catheter Abdominal prep Transfer to OR | Surgical considerations (prior abdominal or uterine surgery) Medical considerations (obesity, hypertension, diabetes, SLE) Obstetric considerations (parity, pelvimetry, placental location) |
| Fetus | Confirm: Estimated fetal weight Gestational age Presentation Position | Consider factors such as: Estimated fetal weight Gestational age Presentation Position |
| Labor | Confirm adequate monitoring of uterine contractions | Consider factors such as: Arrest disorder Protracted labor Remote from delivery Poor expulsive efforts |
| Courtesy of David A. Miller, MD | ||
CASE Continued
When the OB’s preliminary interventions fail to nudge the FHR tracing back to Category I, she stops oxytocin and administers terbutaline. She even tries amnioinfusion. Still, the FHR tracing remains in Category II.
“What now?,” she wonders.
If conservative measures do not correct the FHR tracing to the satisfaction of the clinician, it is prudent to plan ahead for the possible need for rapid delivery. In a standardized “A-B-C-D” approach to FHR management, the next step is “C”: Clear obstacles to rapid delivery. This step does not constitute a commitment to a particular time or method of delivery. It simply serves as a reminder of common sources of unnecessary delay so that they can be addressed in a standardized, timely manner (FIGURE).

Decision model for management of intrapartum fetal heart rate (FHR)Standardization has long been recognized as an essential element of patient safety, and a growing body of contemporary evidence confirms that standardization can reduce adverse outcomes and malpractice claims.8-10 In FHR monitoring, standardization can help ensure that common obstacles to rapid delivery are not overlooked and that decisions are made in a timely fashion. TABLE 3 identifies common obstacles to rapid delivery, groups them in five major categories, and organizes them in non-random order. From largest to smallest, these categories include the facility, staff, mother, fetus, and labor.
Because many of these examples are viewed by clinicians as “common sense,” they do not always receive the serious, systematic attention they deserve. Instead, they are often left to the vagaries of random recall and are frequently overlooked, jeopardizing patient safety and inviting criticism. An easy way to minimize the error inherent in random recall is to use a simple checklist and to post it in a conspicuous location on the labor-and-delivery unit.
Next step: “D” – Determine the decision-to-delivery time
After appropriate conservative measures have been implemented and obstacles to rapid delivery have been cleared away, it is sensible to take a moment to estimate the time needed to accomplish delivery in the event of a sudden emergency. This step should be addressed by the clinician who is ultimately responsible for performing operative delivery, should it become necessary. The time between decision and delivery can be estimated systematically by considering individual characteristics of the facility, staff, mother, fetus, and labor. TABLE 3 summarizes examples of factors that can have an impact on this estimate.
Clinical judgment is required
Management steps A, B, C, and D are relatively uncontroversial, readily amenable to standardization, and represent the overwhelming majority of decisions that must be made during labor. These steps do not replace clinical judgment. On the contrary, they encourage the systematic, timely application of clinical judgment.
However, if the FHR tracing has not returned to Category I by the time A, B, C, and D are completed, the clinician must make a decision about whether to continue to wait for spontaneous vaginal delivery or to expedite delivery by other means. This decision balances the estimated time until vaginal delivery against the estimated time until the onset of metabolic acidemia and potential injury.
The estimate of the time until vaginal delivery is guided by the usual obstetric considerations, including the three “P’s”:
- Power – uterine contractions
- Passenger – the fetus
- Passage – the pelvis.
The estimate of the time until the onset of metabolic acidemia and potential injury is guided by limited data suggesting that metabolic acidemia usually does not appear suddenly, but can evolve gradually over a period of approximately 60 minutes.15 This general statement applies only to FHR tracings that are normal initially and subsequently develop minimal to absent variability with recurrent decelerations and no acute events.15 It does not constitute a “safe harbor.”
The inherent imprecision of these estimates can make the decision difficult. One of the most common preventable errors at this stage of FHR management is to postpone a difficult but clinically necessary decision in the hope that the situation will resolve on its own. Despite the difficulty, the standard of care mandates that a decision must be made using the best information available.
If a decision is made to expedite delivery, the rationale should be documented, and the plan should be implemented as rapidly and safely as feasible. If a decision is made to continue to wait, the rationale and plan should be documented, and the decision should be revisited after a reasonable period of time, usually in the range of 5 to 15 minutes in the second stage of labor.
“Deciding to wait” is distinctly different from “waiting to decide.” The former reflects the timely application of clinical judgment; the latter suggests procrastination.
CASE Resolved
The OB evaluates the patient again. The FHR tracing remains in Category II. The baseline rate is 150 bpm, variability is moderate, accelerations are present, and there are variable decelerations with every other contraction. The cervix remains dilated to 6 cm despite more than 2 hours of adequate contractions. Secondary arrest of dilatation is diagnosed, and cesarean delivery is recommended. Shortly thereafter, a vigorous baby is born. As the presence of moderate variability and accelerations predicted, the 5-minute Apgar score is normal. Assessment of the umbilical artery blood gas confirms the absence of metabolic acidemia, and the newborn course is uneventful.
The paradox of FHR monitoring
The greatest strength of intrapartum FHR monitoring is the ability of moderate variability or accelerations, or both, to predict normal neurologic outcome with an extremely high degree of reliability.1,11,12 One of the greatest weaknesses of FHR monitoring is the inability of an “abnormal” tracing to predict abnormal neurologic outcome with any clinically relevant degree of accuracy. The false-positive rate of FHR monitoring for predicting cerebral palsy has been reported to exceed 99%, yielding a positive predictive value of less than 1%.1,13 This imprecision is explained in part by the relative rarity of intrapartum hypoxic neurologic injury, and in part by the mitigating interventions that are frequently prompted by FHR “abnormalities.”14 However, these explanations do not alter the fact that the positive predictive value of intrapartum FHR monitoring, as it is used in actual clinical practice, is essentially zero.
Reasonable management decisions simply cannot be based on the results of a test that is virtually always wrong. On the other hand, the negative predictive value of intrapartum FHR monitoring is nearly 100%. A test that is virtually always right is the ideal foundation for rational decision-making.
Standardization of intrapartum FHR monitoring promotes safety by reducing unnecessary complexity and minimizing the error inherent in random recall. However, the technology can achieve its potential only if it is used appropriately. Trying to use intrapartum FHR monitoring to diagnose neurologic injury is a recipe for failure. In contrast, relying on the presence of moderate variability or accelerations, or both, to confirm adequate fetal oxygenation allows the clinician to formulate and articulate a rational, evidence-based plan of management that reflects consensus in the literature.
We want to hear from you! Tell us what you think.
1. Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661-666.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin number 106: Intrapartum fetal heart rate monitoring: nomenclature interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192-202.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 116: Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116(5):1232-1240.
4. Barbieri RL. Stop staring at that Category-II fetal heart-rate tracing…and do something instead to improve fetal status! OBG Manage. 2011;23(4):6-9.
5. Simpson KR, James DC. Efficacy of intrauterine resuscitation techniques in improving fetal oxygen status during labor. Obstet Gynecol. 2005;105(6):1362-1368.
6. Miller DA. Intrapartum fetal heart rate definitions and interpretation: evolving consensus. Clin Obstet Gynecol. 2011;54(1):16-21.
7. Miller DA. Intrapartum fetal heart monitoring: a standardized approach to management. Clin Obstet Gynecol. 2011;54(1):22-27.
8. To Err is Human: Building a Safer Health System. Kohn LT Corrigan JM, Donaldson MS, eds. Committee on Quality of Health Care in America. Institute of Medicine. Washington, DC: National Academy of Sciences;1999:14.
9. Pettker CM, Thung SF, Norwitz ER, et al. Impact of a comprehensive patient safety strategy on obstetric adverse events. Am J Obstet Gynecol. 2009;200(5):492.e1-8.
10. Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279-1283.
11. MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: International consensus statement. BMJ. 1999;319(7216):1054-1059.
12. American College of Obstetricians and Gynecologists. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: American College of Obstetricians and Gynecologists; 2003.
13. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613-618.
14. Freeman RK, Nageotte MP. Comments on American College of Obstetricians and Gynecologists Practice Bulletin No. 106. Am J Obstet Gynecol. 2010;202(5):411-412.
15. Parer JT, King T, Flanders S, Fox M, Kilpatrick SJ. Fetal acidemia and electronic fetal heart rate patterns: is there evidence of an association? J Matern Fetal Neonatal Med. 2006;19(5):289-294.
- Stop staring at that Category-II fetal heart-rate tracing…
Robert L. Barbieri, MD (Editorial, April 2011) - Guidelines on fetal monitoring aim to codify normal, abnormal FHR
Robert L. Barbieri, MD (Editorial, October 2008)
CASE An uncertain interlude during labor
An obstetrician checks on her laboring patient, only to discover that the fetal heart-rate (FHR) tracing has moved from Category I, a normal classification, into Category II—a gray zone. The OB decides to be proactive, not simply to wait for the tracing to return to normal. She has the patient move from a supine to a lateral position, provides oxygen, and administers a bolus of 500 to 1,000 mL of lactated Ringer’s solution over 20 minutes.
The tracing remains in Category II.
What should the OB do next?

When a fetal heart-rate tracing remains in Category II despite well-considered conservative corrective measures, a reasoned, rather than passive, approach is recommended.In 2008, the National Institute of Child Health and Human Development proposed a three-tier classification system for electronic FHR tracings (TABLE 1).1 Tracings in Category I are considered normal and can be managed routinely.1-3 Category-III tracings are considered abnormal and require additional attention; if corrective measures do not result in improvement, rapid delivery usually is warranted.1-3 Category II includes all FHR tracings that do not fit into either of the other categories. Because Category II encompasses such a wide range of FHR tracings, there are many options for management.
TABLE 1
3-tier fetal heart-rate classification system
| Category | Description |
|---|---|
| I | Fetal heart-rate (FHR) tracings include all of the following:
|
| II | Includes all FHR tracings not included in Category I or Category III |
| III | FHR tracings include:
|
| Source: Adapted from Macones GA, et al.1 | |
If the case described above sounds familiar, it may be that you read Editor in Chief Dr. Robert L. Barbieri’s editorial on Category-II FHR tracings in the April 2011 issue of OBG Management.4 That essay described a number of common conservative corrective measures applicable for Category-II tracings, including the three interventions the OB performed.
Other measures:
- reduce or stop infusion of oxytocin
- discontinue cervical ripening agents
- consider administering a tocolytic, such as terbutaline, if tachysystole is present or if uterine contractions are prolonged or coupled
- consider the option of amnioinfusion if variable decelerations are present.4,5
Systematic review of the oxygen pathway, from the environment to the fetus (maternal lungs, heart, vasculature, uterus, placenta, and umbilical cord), can facilitate recollection of all of these measures. In addition, a simplified “A-B-C-D” approach to the management of a Category-II FHR tracing is helpful (TABLES 2 and 3):
- Assess the oxygen pathway
- Begin conservative corrective measures
- Clear obstacles to rapid delivery
- Determine decision-to-delivery time.6,7
TABLE 2
Conservative corrective measures to improve fetal oxygenation
| “A” Assess oxygen pathway | “B” Begin corrective measures if indicated | |
|---|---|---|
| Lungs | Airway and breathing | Supplemental oxygen (10 L) using a tight-fitting, non-rebreather face mask for at least 15 minutes |
| Heart | Heart rate and rhythm | Position changes IV fluid bolus (500–1,000 cc of isotonic fluid over 20 min) Correct hypotension |
| Vasculature | Blood pressure Volume status | |
| Uterus | Contraction strength Contraction frequency Baseline uterine tone Exclude uterine rupture | Stop or reduce uterine stimulants (oxytocin, prostaglandin) Consider uterine relaxant (terbutaline) |
| Placenta | Placental separation Bleeding vasa previa | |
| Cord | Vaginal exam Exclude cord prolapse | Consider amnioinfusion |
| Courtesy of David A. Miller, MD | ||
As the obstetrician in the opening scenario knows all too well, conservative corrective measures do not always transform FHR tracings from Category II to Category I. In fact, it is extremely common for a Category-II tracing to remain in Category II despite every conservative corrective measure in the book. This article presents a practical, systematic, standardized approach to the management of a persistent Category-II FHR tracing.
TABLE 3
Steps involved in preparing for delivery
| “C” Clear obstacles to rapid delivery | “D” Determine decision-to-delivery time | |
|---|---|---|
| Facility | Operating room availability Equipment | Facility response time |
| Staff | Notify: Obstetrician Surgical assistant Anesthesiologist Neonatologist Pediatrician Nursing staff | Consider staff: Availability Training Experience |
| Mother | Informed consent Anesthesia options Laboratory tests Blood products Intravenous access Urinary catheter Abdominal prep Transfer to OR | Surgical considerations (prior abdominal or uterine surgery) Medical considerations (obesity, hypertension, diabetes, SLE) Obstetric considerations (parity, pelvimetry, placental location) |
| Fetus | Confirm: Estimated fetal weight Gestational age Presentation Position | Consider factors such as: Estimated fetal weight Gestational age Presentation Position |
| Labor | Confirm adequate monitoring of uterine contractions | Consider factors such as: Arrest disorder Protracted labor Remote from delivery Poor expulsive efforts |
| Courtesy of David A. Miller, MD | ||
CASE Continued
When the OB’s preliminary interventions fail to nudge the FHR tracing back to Category I, she stops oxytocin and administers terbutaline. She even tries amnioinfusion. Still, the FHR tracing remains in Category II.
“What now?,” she wonders.
If conservative measures do not correct the FHR tracing to the satisfaction of the clinician, it is prudent to plan ahead for the possible need for rapid delivery. In a standardized “A-B-C-D” approach to FHR management, the next step is “C”: Clear obstacles to rapid delivery. This step does not constitute a commitment to a particular time or method of delivery. It simply serves as a reminder of common sources of unnecessary delay so that they can be addressed in a standardized, timely manner (FIGURE).

Decision model for management of intrapartum fetal heart rate (FHR)Standardization has long been recognized as an essential element of patient safety, and a growing body of contemporary evidence confirms that standardization can reduce adverse outcomes and malpractice claims.8-10 In FHR monitoring, standardization can help ensure that common obstacles to rapid delivery are not overlooked and that decisions are made in a timely fashion. TABLE 3 identifies common obstacles to rapid delivery, groups them in five major categories, and organizes them in non-random order. From largest to smallest, these categories include the facility, staff, mother, fetus, and labor.
Because many of these examples are viewed by clinicians as “common sense,” they do not always receive the serious, systematic attention they deserve. Instead, they are often left to the vagaries of random recall and are frequently overlooked, jeopardizing patient safety and inviting criticism. An easy way to minimize the error inherent in random recall is to use a simple checklist and to post it in a conspicuous location on the labor-and-delivery unit.
Next step: “D” – Determine the decision-to-delivery time
After appropriate conservative measures have been implemented and obstacles to rapid delivery have been cleared away, it is sensible to take a moment to estimate the time needed to accomplish delivery in the event of a sudden emergency. This step should be addressed by the clinician who is ultimately responsible for performing operative delivery, should it become necessary. The time between decision and delivery can be estimated systematically by considering individual characteristics of the facility, staff, mother, fetus, and labor. TABLE 3 summarizes examples of factors that can have an impact on this estimate.
Clinical judgment is required
Management steps A, B, C, and D are relatively uncontroversial, readily amenable to standardization, and represent the overwhelming majority of decisions that must be made during labor. These steps do not replace clinical judgment. On the contrary, they encourage the systematic, timely application of clinical judgment.
However, if the FHR tracing has not returned to Category I by the time A, B, C, and D are completed, the clinician must make a decision about whether to continue to wait for spontaneous vaginal delivery or to expedite delivery by other means. This decision balances the estimated time until vaginal delivery against the estimated time until the onset of metabolic acidemia and potential injury.
The estimate of the time until vaginal delivery is guided by the usual obstetric considerations, including the three “P’s”:
- Power – uterine contractions
- Passenger – the fetus
- Passage – the pelvis.
The estimate of the time until the onset of metabolic acidemia and potential injury is guided by limited data suggesting that metabolic acidemia usually does not appear suddenly, but can evolve gradually over a period of approximately 60 minutes.15 This general statement applies only to FHR tracings that are normal initially and subsequently develop minimal to absent variability with recurrent decelerations and no acute events.15 It does not constitute a “safe harbor.”
The inherent imprecision of these estimates can make the decision difficult. One of the most common preventable errors at this stage of FHR management is to postpone a difficult but clinically necessary decision in the hope that the situation will resolve on its own. Despite the difficulty, the standard of care mandates that a decision must be made using the best information available.
If a decision is made to expedite delivery, the rationale should be documented, and the plan should be implemented as rapidly and safely as feasible. If a decision is made to continue to wait, the rationale and plan should be documented, and the decision should be revisited after a reasonable period of time, usually in the range of 5 to 15 minutes in the second stage of labor.
“Deciding to wait” is distinctly different from “waiting to decide.” The former reflects the timely application of clinical judgment; the latter suggests procrastination.
CASE Resolved
The OB evaluates the patient again. The FHR tracing remains in Category II. The baseline rate is 150 bpm, variability is moderate, accelerations are present, and there are variable decelerations with every other contraction. The cervix remains dilated to 6 cm despite more than 2 hours of adequate contractions. Secondary arrest of dilatation is diagnosed, and cesarean delivery is recommended. Shortly thereafter, a vigorous baby is born. As the presence of moderate variability and accelerations predicted, the 5-minute Apgar score is normal. Assessment of the umbilical artery blood gas confirms the absence of metabolic acidemia, and the newborn course is uneventful.
The paradox of FHR monitoring
The greatest strength of intrapartum FHR monitoring is the ability of moderate variability or accelerations, or both, to predict normal neurologic outcome with an extremely high degree of reliability.1,11,12 One of the greatest weaknesses of FHR monitoring is the inability of an “abnormal” tracing to predict abnormal neurologic outcome with any clinically relevant degree of accuracy. The false-positive rate of FHR monitoring for predicting cerebral palsy has been reported to exceed 99%, yielding a positive predictive value of less than 1%.1,13 This imprecision is explained in part by the relative rarity of intrapartum hypoxic neurologic injury, and in part by the mitigating interventions that are frequently prompted by FHR “abnormalities.”14 However, these explanations do not alter the fact that the positive predictive value of intrapartum FHR monitoring, as it is used in actual clinical practice, is essentially zero.
Reasonable management decisions simply cannot be based on the results of a test that is virtually always wrong. On the other hand, the negative predictive value of intrapartum FHR monitoring is nearly 100%. A test that is virtually always right is the ideal foundation for rational decision-making.
Standardization of intrapartum FHR monitoring promotes safety by reducing unnecessary complexity and minimizing the error inherent in random recall. However, the technology can achieve its potential only if it is used appropriately. Trying to use intrapartum FHR monitoring to diagnose neurologic injury is a recipe for failure. In contrast, relying on the presence of moderate variability or accelerations, or both, to confirm adequate fetal oxygenation allows the clinician to formulate and articulate a rational, evidence-based plan of management that reflects consensus in the literature.
We want to hear from you! Tell us what you think.
- Stop staring at that Category-II fetal heart-rate tracing…
Robert L. Barbieri, MD (Editorial, April 2011) - Guidelines on fetal monitoring aim to codify normal, abnormal FHR
Robert L. Barbieri, MD (Editorial, October 2008)
CASE An uncertain interlude during labor
An obstetrician checks on her laboring patient, only to discover that the fetal heart-rate (FHR) tracing has moved from Category I, a normal classification, into Category II—a gray zone. The OB decides to be proactive, not simply to wait for the tracing to return to normal. She has the patient move from a supine to a lateral position, provides oxygen, and administers a bolus of 500 to 1,000 mL of lactated Ringer’s solution over 20 minutes.
The tracing remains in Category II.
What should the OB do next?

When a fetal heart-rate tracing remains in Category II despite well-considered conservative corrective measures, a reasoned, rather than passive, approach is recommended.In 2008, the National Institute of Child Health and Human Development proposed a three-tier classification system for electronic FHR tracings (TABLE 1).1 Tracings in Category I are considered normal and can be managed routinely.1-3 Category-III tracings are considered abnormal and require additional attention; if corrective measures do not result in improvement, rapid delivery usually is warranted.1-3 Category II includes all FHR tracings that do not fit into either of the other categories. Because Category II encompasses such a wide range of FHR tracings, there are many options for management.
TABLE 1
3-tier fetal heart-rate classification system
| Category | Description |
|---|---|
| I | Fetal heart-rate (FHR) tracings include all of the following:
|
| II | Includes all FHR tracings not included in Category I or Category III |
| III | FHR tracings include:
|
| Source: Adapted from Macones GA, et al.1 | |
If the case described above sounds familiar, it may be that you read Editor in Chief Dr. Robert L. Barbieri’s editorial on Category-II FHR tracings in the April 2011 issue of OBG Management.4 That essay described a number of common conservative corrective measures applicable for Category-II tracings, including the three interventions the OB performed.
Other measures:
- reduce or stop infusion of oxytocin
- discontinue cervical ripening agents
- consider administering a tocolytic, such as terbutaline, if tachysystole is present or if uterine contractions are prolonged or coupled
- consider the option of amnioinfusion if variable decelerations are present.4,5
Systematic review of the oxygen pathway, from the environment to the fetus (maternal lungs, heart, vasculature, uterus, placenta, and umbilical cord), can facilitate recollection of all of these measures. In addition, a simplified “A-B-C-D” approach to the management of a Category-II FHR tracing is helpful (TABLES 2 and 3):
- Assess the oxygen pathway
- Begin conservative corrective measures
- Clear obstacles to rapid delivery
- Determine decision-to-delivery time.6,7
TABLE 2
Conservative corrective measures to improve fetal oxygenation
| “A” Assess oxygen pathway | “B” Begin corrective measures if indicated | |
|---|---|---|
| Lungs | Airway and breathing | Supplemental oxygen (10 L) using a tight-fitting, non-rebreather face mask for at least 15 minutes |
| Heart | Heart rate and rhythm | Position changes IV fluid bolus (500–1,000 cc of isotonic fluid over 20 min) Correct hypotension |
| Vasculature | Blood pressure Volume status | |
| Uterus | Contraction strength Contraction frequency Baseline uterine tone Exclude uterine rupture | Stop or reduce uterine stimulants (oxytocin, prostaglandin) Consider uterine relaxant (terbutaline) |
| Placenta | Placental separation Bleeding vasa previa | |
| Cord | Vaginal exam Exclude cord prolapse | Consider amnioinfusion |
| Courtesy of David A. Miller, MD | ||
As the obstetrician in the opening scenario knows all too well, conservative corrective measures do not always transform FHR tracings from Category II to Category I. In fact, it is extremely common for a Category-II tracing to remain in Category II despite every conservative corrective measure in the book. This article presents a practical, systematic, standardized approach to the management of a persistent Category-II FHR tracing.
TABLE 3
Steps involved in preparing for delivery
| “C” Clear obstacles to rapid delivery | “D” Determine decision-to-delivery time | |
|---|---|---|
| Facility | Operating room availability Equipment | Facility response time |
| Staff | Notify: Obstetrician Surgical assistant Anesthesiologist Neonatologist Pediatrician Nursing staff | Consider staff: Availability Training Experience |
| Mother | Informed consent Anesthesia options Laboratory tests Blood products Intravenous access Urinary catheter Abdominal prep Transfer to OR | Surgical considerations (prior abdominal or uterine surgery) Medical considerations (obesity, hypertension, diabetes, SLE) Obstetric considerations (parity, pelvimetry, placental location) |
| Fetus | Confirm: Estimated fetal weight Gestational age Presentation Position | Consider factors such as: Estimated fetal weight Gestational age Presentation Position |
| Labor | Confirm adequate monitoring of uterine contractions | Consider factors such as: Arrest disorder Protracted labor Remote from delivery Poor expulsive efforts |
| Courtesy of David A. Miller, MD | ||
CASE Continued
When the OB’s preliminary interventions fail to nudge the FHR tracing back to Category I, she stops oxytocin and administers terbutaline. She even tries amnioinfusion. Still, the FHR tracing remains in Category II.
“What now?,” she wonders.
If conservative measures do not correct the FHR tracing to the satisfaction of the clinician, it is prudent to plan ahead for the possible need for rapid delivery. In a standardized “A-B-C-D” approach to FHR management, the next step is “C”: Clear obstacles to rapid delivery. This step does not constitute a commitment to a particular time or method of delivery. It simply serves as a reminder of common sources of unnecessary delay so that they can be addressed in a standardized, timely manner (FIGURE).

Decision model for management of intrapartum fetal heart rate (FHR)Standardization has long been recognized as an essential element of patient safety, and a growing body of contemporary evidence confirms that standardization can reduce adverse outcomes and malpractice claims.8-10 In FHR monitoring, standardization can help ensure that common obstacles to rapid delivery are not overlooked and that decisions are made in a timely fashion. TABLE 3 identifies common obstacles to rapid delivery, groups them in five major categories, and organizes them in non-random order. From largest to smallest, these categories include the facility, staff, mother, fetus, and labor.
Because many of these examples are viewed by clinicians as “common sense,” they do not always receive the serious, systematic attention they deserve. Instead, they are often left to the vagaries of random recall and are frequently overlooked, jeopardizing patient safety and inviting criticism. An easy way to minimize the error inherent in random recall is to use a simple checklist and to post it in a conspicuous location on the labor-and-delivery unit.
Next step: “D” – Determine the decision-to-delivery time
After appropriate conservative measures have been implemented and obstacles to rapid delivery have been cleared away, it is sensible to take a moment to estimate the time needed to accomplish delivery in the event of a sudden emergency. This step should be addressed by the clinician who is ultimately responsible for performing operative delivery, should it become necessary. The time between decision and delivery can be estimated systematically by considering individual characteristics of the facility, staff, mother, fetus, and labor. TABLE 3 summarizes examples of factors that can have an impact on this estimate.
Clinical judgment is required
Management steps A, B, C, and D are relatively uncontroversial, readily amenable to standardization, and represent the overwhelming majority of decisions that must be made during labor. These steps do not replace clinical judgment. On the contrary, they encourage the systematic, timely application of clinical judgment.
However, if the FHR tracing has not returned to Category I by the time A, B, C, and D are completed, the clinician must make a decision about whether to continue to wait for spontaneous vaginal delivery or to expedite delivery by other means. This decision balances the estimated time until vaginal delivery against the estimated time until the onset of metabolic acidemia and potential injury.
The estimate of the time until vaginal delivery is guided by the usual obstetric considerations, including the three “P’s”:
- Power – uterine contractions
- Passenger – the fetus
- Passage – the pelvis.
The estimate of the time until the onset of metabolic acidemia and potential injury is guided by limited data suggesting that metabolic acidemia usually does not appear suddenly, but can evolve gradually over a period of approximately 60 minutes.15 This general statement applies only to FHR tracings that are normal initially and subsequently develop minimal to absent variability with recurrent decelerations and no acute events.15 It does not constitute a “safe harbor.”
The inherent imprecision of these estimates can make the decision difficult. One of the most common preventable errors at this stage of FHR management is to postpone a difficult but clinically necessary decision in the hope that the situation will resolve on its own. Despite the difficulty, the standard of care mandates that a decision must be made using the best information available.
If a decision is made to expedite delivery, the rationale should be documented, and the plan should be implemented as rapidly and safely as feasible. If a decision is made to continue to wait, the rationale and plan should be documented, and the decision should be revisited after a reasonable period of time, usually in the range of 5 to 15 minutes in the second stage of labor.
“Deciding to wait” is distinctly different from “waiting to decide.” The former reflects the timely application of clinical judgment; the latter suggests procrastination.
CASE Resolved
The OB evaluates the patient again. The FHR tracing remains in Category II. The baseline rate is 150 bpm, variability is moderate, accelerations are present, and there are variable decelerations with every other contraction. The cervix remains dilated to 6 cm despite more than 2 hours of adequate contractions. Secondary arrest of dilatation is diagnosed, and cesarean delivery is recommended. Shortly thereafter, a vigorous baby is born. As the presence of moderate variability and accelerations predicted, the 5-minute Apgar score is normal. Assessment of the umbilical artery blood gas confirms the absence of metabolic acidemia, and the newborn course is uneventful.
The paradox of FHR monitoring
The greatest strength of intrapartum FHR monitoring is the ability of moderate variability or accelerations, or both, to predict normal neurologic outcome with an extremely high degree of reliability.1,11,12 One of the greatest weaknesses of FHR monitoring is the inability of an “abnormal” tracing to predict abnormal neurologic outcome with any clinically relevant degree of accuracy. The false-positive rate of FHR monitoring for predicting cerebral palsy has been reported to exceed 99%, yielding a positive predictive value of less than 1%.1,13 This imprecision is explained in part by the relative rarity of intrapartum hypoxic neurologic injury, and in part by the mitigating interventions that are frequently prompted by FHR “abnormalities.”14 However, these explanations do not alter the fact that the positive predictive value of intrapartum FHR monitoring, as it is used in actual clinical practice, is essentially zero.
Reasonable management decisions simply cannot be based on the results of a test that is virtually always wrong. On the other hand, the negative predictive value of intrapartum FHR monitoring is nearly 100%. A test that is virtually always right is the ideal foundation for rational decision-making.
Standardization of intrapartum FHR monitoring promotes safety by reducing unnecessary complexity and minimizing the error inherent in random recall. However, the technology can achieve its potential only if it is used appropriately. Trying to use intrapartum FHR monitoring to diagnose neurologic injury is a recipe for failure. In contrast, relying on the presence of moderate variability or accelerations, or both, to confirm adequate fetal oxygenation allows the clinician to formulate and articulate a rational, evidence-based plan of management that reflects consensus in the literature.
We want to hear from you! Tell us what you think.
1. Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661-666.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin number 106: Intrapartum fetal heart rate monitoring: nomenclature interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192-202.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 116: Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116(5):1232-1240.
4. Barbieri RL. Stop staring at that Category-II fetal heart-rate tracing…and do something instead to improve fetal status! OBG Manage. 2011;23(4):6-9.
5. Simpson KR, James DC. Efficacy of intrauterine resuscitation techniques in improving fetal oxygen status during labor. Obstet Gynecol. 2005;105(6):1362-1368.
6. Miller DA. Intrapartum fetal heart rate definitions and interpretation: evolving consensus. Clin Obstet Gynecol. 2011;54(1):16-21.
7. Miller DA. Intrapartum fetal heart monitoring: a standardized approach to management. Clin Obstet Gynecol. 2011;54(1):22-27.
8. To Err is Human: Building a Safer Health System. Kohn LT Corrigan JM, Donaldson MS, eds. Committee on Quality of Health Care in America. Institute of Medicine. Washington, DC: National Academy of Sciences;1999:14.
9. Pettker CM, Thung SF, Norwitz ER, et al. Impact of a comprehensive patient safety strategy on obstetric adverse events. Am J Obstet Gynecol. 2009;200(5):492.e1-8.
10. Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279-1283.
11. MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: International consensus statement. BMJ. 1999;319(7216):1054-1059.
12. American College of Obstetricians and Gynecologists. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: American College of Obstetricians and Gynecologists; 2003.
13. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613-618.
14. Freeman RK, Nageotte MP. Comments on American College of Obstetricians and Gynecologists Practice Bulletin No. 106. Am J Obstet Gynecol. 2010;202(5):411-412.
15. Parer JT, King T, Flanders S, Fox M, Kilpatrick SJ. Fetal acidemia and electronic fetal heart rate patterns: is there evidence of an association? J Matern Fetal Neonatal Med. 2006;19(5):289-294.
1. Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661-666.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin number 106: Intrapartum fetal heart rate monitoring: nomenclature interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192-202.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 116: Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116(5):1232-1240.
4. Barbieri RL. Stop staring at that Category-II fetal heart-rate tracing…and do something instead to improve fetal status! OBG Manage. 2011;23(4):6-9.
5. Simpson KR, James DC. Efficacy of intrauterine resuscitation techniques in improving fetal oxygen status during labor. Obstet Gynecol. 2005;105(6):1362-1368.
6. Miller DA. Intrapartum fetal heart rate definitions and interpretation: evolving consensus. Clin Obstet Gynecol. 2011;54(1):16-21.
7. Miller DA. Intrapartum fetal heart monitoring: a standardized approach to management. Clin Obstet Gynecol. 2011;54(1):22-27.
8. To Err is Human: Building a Safer Health System. Kohn LT Corrigan JM, Donaldson MS, eds. Committee on Quality of Health Care in America. Institute of Medicine. Washington, DC: National Academy of Sciences;1999:14.
9. Pettker CM, Thung SF, Norwitz ER, et al. Impact of a comprehensive patient safety strategy on obstetric adverse events. Am J Obstet Gynecol. 2009;200(5):492.e1-8.
10. Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279-1283.
11. MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: International consensus statement. BMJ. 1999;319(7216):1054-1059.
12. American College of Obstetricians and Gynecologists. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: American College of Obstetricians and Gynecologists; 2003.
13. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613-618.
14. Freeman RK, Nageotte MP. Comments on American College of Obstetricians and Gynecologists Practice Bulletin No. 106. Am J Obstet Gynecol. 2010;202(5):411-412.
15. Parer JT, King T, Flanders S, Fox M, Kilpatrick SJ. Fetal acidemia and electronic fetal heart rate patterns: is there evidence of an association? J Matern Fetal Neonatal Med. 2006;19(5):289-294.


