User login
Have you made best use of the Bakri balloon in PPH?
- Read a related case in July’s Medical Verdicts
- “10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage”
Baha M. Sibai, MD (June 2011) - “Postpartum hemorrhage: 11 critical questions, answered by an expert”
Q&A with Haywood L. Brown, MD (January 2011) - “What you can do to optimize blood conservation in ObGyn practice”
Eric J. Bieber, MD; Linda Scott, RN; Corinna Muller, DO; Nancy Nuss, RN; and Edie L. Derian, MD (February 2010) - “Planning reduces the risk of maternal death. This tool helps.”
Robert L. Barbieri, MD (Editorial; August 2009) - “Consider retroperitoneal packing for postpartum hemorrhage”
Maj. William R. Fulton, DO (July 2008)
Obstetricians know that postpartum hemorrhage (PPH) must be treated decisively and swiftly.1 To review:
Active management of the third stage after vaginal delivery, with a uterotonic such as oxytocin, helps to reduce the frequency of PPH2; in a recent randomized trial involving vaginal delivery, the rate of PPH was 10% in women who received postpartum oxytocin and 17% in those who did not (P<.001).3
When hemorrhage occurs despite oxytocin, having been given postpartum, the standard treatment algorithm (see the TABLE) calls for:
- uterine massage
- additional uterotonics
- identification and repair of vaginal and cervical lacerations
- removal of any retained products of conception.
Sequential interventions for managing postpartum hemorrhage
| This sequence of steps applies 1) after vaginal delivery (including the left hand-side interventions) and 2) after cesarean delivery, with the abdominal incision still open (including the right hand-side interventions). | |
| Hemorrhage after vaginal birth | Hemorrhage after cesarean delivery |
| Administer oxytocin | |
| Perform uterine massage | |
| Administer additional uterotonics (methergine, misoprostol, carboprost [Hemabate]) | |
| Bring 2 units of packed RBCs and 2 units of fresh frozen plasma (FFP) to point of care Transfuse based on the clinical condition Consider transfusing RBCs and FFP at a 1:1 ratio until clotting parameters are evaluated Obtain Stat clotting studies Start an additional intravenous line | |
| Move the patient to the operating room |
|
| Repair any tears |
|
| Place uterine compression suture(s), such as a B-Lynch suture |
| Place an intrauterine balloon | Consider bilateral ligation of the internal iliac artery |
| If indicated, call for additional specialists: second anesthesiologist, gyn surgeon, interventional radiologist, blood bank director | |
| Selective embolization by interventional radiology | Hysterectomy |
| Exploratory laparotomy—follow steps (along the right-hand side of this table) for treating hemorrhage after cesarean delivery | Pelvic packing |
| Adapted from: California Maternal Quality Care Collaborative (www.CMQCC.org). | |
Placing an intrauterine balloon, such as the Bakri Postpartum Balloon (Cook Medical) or the BT-Cath (Utah Medical Products), is strongly recommended if these steps do not control bleeding.
In this Editorial, I review tips and tricks for using the Bakri balloon, building on my earlier OBG Management Editorial (February 2009), in which I outlined a basic approach to intrauterine balloon tamponade.4
TIP: Place the Bakri balloon early in the PPH treatment algorithm
For postpartum hemorrhage after vaginal delivery, typical initial steps include, as noted, fundal massage, administration of uterotonics, and curettage to remove retained products of conception. If these steps are ineffective, don’t wait: Immediately consider placing a Bakri balloon. Time is precious, and wasting time with less effective interventions results in excessive blood loss and consumption of clotting factors and increases the risk of a coagulopathy.
I’ve found that clinicians often spend too much time trying to determine whether postpartum bleeding originates in the uterus or from a cervical or vaginal laceration. But heavy bleeding can obscure anatomic structures, making it difficult to identify the site of bleeding with precision. Fruitlessly perseverating to differentiate uterine, cervical, and upper vaginal sources of bleeding can waste valuable time and lead to unnecessary blood loss.
By placing a Bakri balloon and inflating it early in the PPH treatment algorithm, you will significantly reduce uterine bleeding. You will also be able to assess the cervix and upper vagina more effectively for lacerations.
My recommendation. Place the Bakri balloon as soon as it is apparent that bleeding is so heavy that you are going to have difficulty assessing the cervix and upper vagina for lacerations. Note that this stands in contrast to the usual recommendation that you assess the cervix and vagina for lacerations before you place the Bakri balloon.
TIP: Have 3 clinicians place the balloon
Teamwork is a key to success here: The Bakri balloon is most quickly and elegantly inserted and inflated when three clinicians team up, as follows:
Clinician#1 scans the uterus, assessing for retained products of conception and providing real-time imaging as the balloon is placed and inflated. This team member provides feedback to the others about correct placement and filling of the balloon.
Clinician#2 inserts the balloon into the uterus by placing her hand into the vagina and guiding the balloon into the proper intrauterine position. (Most often, clinician#2 is the delivering clinician.) This technique is performed in a manner similar to how one places an intrauterine pressure catheter.5
Clinician #2 stabilizes the balloon in position by maintaining her hand in the vagina. She ensures that the balloon does not “pop” out of the lower uterus as the balloon is filled (FIGURE).
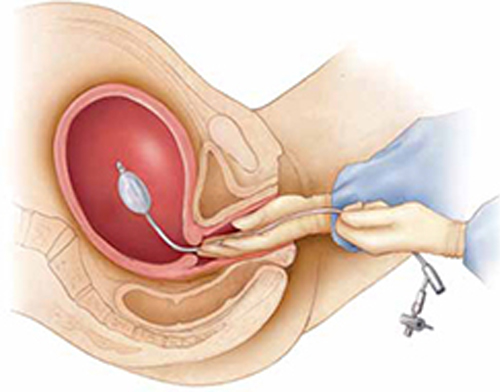
Manual insertion of a Bakri balloon
Insert the balloon into the uterus by 1) placing your hand into the vagina and guiding the balloon into the proper intrauterine position (similar to the manner in which an intrauterine pressure catheter is placed),5 2) stabilizing the balloon in position by maintaining the hand in the vagina, and 3) ensuring that the balloon does not “pop” out of the lower uterus as the balloon is filled.Clinician #3 simultaneously begins to instill sterile fluid into the balloon. This team member can be a nurse, medical assistant, or surgical technician.
My recommendation. Consider using simulation training to practice the team approach to placing the Bakri balloon. This is an effective method of improving team coordination.
TIP: Instill at least 150 mL of fluid
In short, use more fluid, not less. Although one study showed that most clinicians fill the balloon to a median volume of 300 mL,6 I’ve observed that some timidly instill the balloon with 80 to 120 mL of fluid. I think this is too little to maximize the effectiveness of the balloon for a postpartum patient who has massive hemorrhage.
Studies have shown that filling the balloon to at least 150 mL noticeably increases tamponade pressure.7
TIP: Place a pack in the upper vagina to stabilize the balloon in position
It usually takes only a few minutes after the Bakri balloon is filled to determine whether it is going to control uterine bleeding—what is called a tamponade test. If the balloon does control bleeding, you can place a vaginal pack of ribbon gauze to help ensure that the balloon does not slip down through the dilated postpartum cervix.
To avoid obscuring continued bleeding, however, do not place vaginal packing until you have obtained a positive tamponade test.
TIP: Check the hemoglobin concentration, platelet count, and coagulation status
The trauma literature demonstrates that optimal patient outcomes are realized when the following targets are maintained during resuscitation of patients who have massive hemorrhage8:
- hematocrit, ≥21%
- platelet count, ≥50 × 103/μL
- fibrinogen ≥100 mg/dL
- International Normalized Ratio (INR) ≤1.5.
These targets are achieved by appropriate transfusion of red blood cells, platelets, fresh frozen plasma, cryoprecipitate, and purified recombinant fibrinogen concentrate (RiaSTAP). Many trauma specialists recommend transfusion of a 1:1 ratio of RBCs and fresh frozen plasma in cases of major hemorrhage until coagulation status is normalized.
My recommendation. Often, it takes as long as 1 hour (sometimes, longer) to get results of Stat coagulation tests from the lab. While you are waiting, obtain clinical evidence of clotting function by filling a red-top tube with the patient’s blood. Adequate function is signaled by formation of a stable clot within 10 minutes.
TIP: Practice your team’s response to PPH with simulation
Last, I urge clinicians in every birthing unit to use simulation exercises to improve all facets of their response to severe postpartum hemorrhage.9
Summing up
Death following PPH remains a major cause of maternal mortality in developed and developing countries. The appropriateness of your response within 10 minutes of the onset of PPH is critical to ensuring a successful outcome and minimizing adverse events.10 To help, use the Bakri Postpartum Balloon often, and use it early.
What are your tips and tricks for effective use of intra-uterine balloon tamponade in postpartum hemorrhage?
To enter your response, click here or send your pearl to [email protected], with your name and location of practice.
We’ll publish a sampling of bylined contributions in an upcoming issue of OBG Management, with appreciation.
1. Sibai BM. 10 practical evidence-based recommendations for managing severe postpartum hemorrhage. OBG Manage. 2011;23(6):44-48.
2. Barbieri RL. Give a uterotonic routinely during the third stage of labor. OBG Manage. 2007;19(05):6-13.
3. Jangsten E, Mattsson LA, Lyckestam I, Hellstrom AL, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG. 2011;118(3):362-369.
4. Barbieri RL. You should add the Bakri balloon to your treatment for OB bleeds. OBG Manage. 2009;21(2):6-10.
5. Dabelea V, Schultze PM, McDuffie RS, Jr. Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol. 2007;24(6):359-364.
6. Vitthala S, Tsoumpou I, Anjum ZK, Aziz NA. Use of Bakri balloon in post-partum hemorrhage: a series of 15 cases. Aust NZ J Obstet Gynecol. 2009;49(2):191-194.
7. Georgiou C. Intraluminal pressure readings during the establishment of a positive “tamponade test” in the management of postpartum hemorrhage. BJOG. 2010;117(3):295-303.
8. Barbieri RL. Control of massive hemorrhage: Lessons from Iraq reach the US labor and delivery suite. OBG Manage. 2007;19(7):8-16.
9. Skupski DW, Lowenwirt IP, Weinbaum FI, Brodsky D, Danek M, Eglinton GS. Improving hospital systems for the care of women with major obstetric hemorrhage. Obstet Gynecol. 2006;107(5):977-983.
10. Driessen M, Bouvier-Colle MH, Dupont C, et al. Pithagore6 Group Postpartum hemorrhage resulting from uterine atony after vaginal delivery: factors associated with severity. Obstet Gynecol. 2011;117(1):21-31.
- Read a related case in July’s Medical Verdicts
- “10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage”
Baha M. Sibai, MD (June 2011) - “Postpartum hemorrhage: 11 critical questions, answered by an expert”
Q&A with Haywood L. Brown, MD (January 2011) - “What you can do to optimize blood conservation in ObGyn practice”
Eric J. Bieber, MD; Linda Scott, RN; Corinna Muller, DO; Nancy Nuss, RN; and Edie L. Derian, MD (February 2010) - “Planning reduces the risk of maternal death. This tool helps.”
Robert L. Barbieri, MD (Editorial; August 2009) - “Consider retroperitoneal packing for postpartum hemorrhage”
Maj. William R. Fulton, DO (July 2008)
Obstetricians know that postpartum hemorrhage (PPH) must be treated decisively and swiftly.1 To review:
Active management of the third stage after vaginal delivery, with a uterotonic such as oxytocin, helps to reduce the frequency of PPH2; in a recent randomized trial involving vaginal delivery, the rate of PPH was 10% in women who received postpartum oxytocin and 17% in those who did not (P<.001).3
When hemorrhage occurs despite oxytocin, having been given postpartum, the standard treatment algorithm (see the TABLE) calls for:
- uterine massage
- additional uterotonics
- identification and repair of vaginal and cervical lacerations
- removal of any retained products of conception.
Sequential interventions for managing postpartum hemorrhage
| This sequence of steps applies 1) after vaginal delivery (including the left hand-side interventions) and 2) after cesarean delivery, with the abdominal incision still open (including the right hand-side interventions). | |
| Hemorrhage after vaginal birth | Hemorrhage after cesarean delivery |
| Administer oxytocin | |
| Perform uterine massage | |
| Administer additional uterotonics (methergine, misoprostol, carboprost [Hemabate]) | |
| Bring 2 units of packed RBCs and 2 units of fresh frozen plasma (FFP) to point of care Transfuse based on the clinical condition Consider transfusing RBCs and FFP at a 1:1 ratio until clotting parameters are evaluated Obtain Stat clotting studies Start an additional intravenous line | |
| Move the patient to the operating room |
|
| Repair any tears |
|
| Place uterine compression suture(s), such as a B-Lynch suture |
| Place an intrauterine balloon | Consider bilateral ligation of the internal iliac artery |
| If indicated, call for additional specialists: second anesthesiologist, gyn surgeon, interventional radiologist, blood bank director | |
| Selective embolization by interventional radiology | Hysterectomy |
| Exploratory laparotomy—follow steps (along the right-hand side of this table) for treating hemorrhage after cesarean delivery | Pelvic packing |
| Adapted from: California Maternal Quality Care Collaborative (www.CMQCC.org). | |
Placing an intrauterine balloon, such as the Bakri Postpartum Balloon (Cook Medical) or the BT-Cath (Utah Medical Products), is strongly recommended if these steps do not control bleeding.
In this Editorial, I review tips and tricks for using the Bakri balloon, building on my earlier OBG Management Editorial (February 2009), in which I outlined a basic approach to intrauterine balloon tamponade.4
TIP: Place the Bakri balloon early in the PPH treatment algorithm
For postpartum hemorrhage after vaginal delivery, typical initial steps include, as noted, fundal massage, administration of uterotonics, and curettage to remove retained products of conception. If these steps are ineffective, don’t wait: Immediately consider placing a Bakri balloon. Time is precious, and wasting time with less effective interventions results in excessive blood loss and consumption of clotting factors and increases the risk of a coagulopathy.
I’ve found that clinicians often spend too much time trying to determine whether postpartum bleeding originates in the uterus or from a cervical or vaginal laceration. But heavy bleeding can obscure anatomic structures, making it difficult to identify the site of bleeding with precision. Fruitlessly perseverating to differentiate uterine, cervical, and upper vaginal sources of bleeding can waste valuable time and lead to unnecessary blood loss.
By placing a Bakri balloon and inflating it early in the PPH treatment algorithm, you will significantly reduce uterine bleeding. You will also be able to assess the cervix and upper vagina more effectively for lacerations.
My recommendation. Place the Bakri balloon as soon as it is apparent that bleeding is so heavy that you are going to have difficulty assessing the cervix and upper vagina for lacerations. Note that this stands in contrast to the usual recommendation that you assess the cervix and vagina for lacerations before you place the Bakri balloon.
TIP: Have 3 clinicians place the balloon
Teamwork is a key to success here: The Bakri balloon is most quickly and elegantly inserted and inflated when three clinicians team up, as follows:
Clinician#1 scans the uterus, assessing for retained products of conception and providing real-time imaging as the balloon is placed and inflated. This team member provides feedback to the others about correct placement and filling of the balloon.
Clinician#2 inserts the balloon into the uterus by placing her hand into the vagina and guiding the balloon into the proper intrauterine position. (Most often, clinician#2 is the delivering clinician.) This technique is performed in a manner similar to how one places an intrauterine pressure catheter.5
Clinician #2 stabilizes the balloon in position by maintaining her hand in the vagina. She ensures that the balloon does not “pop” out of the lower uterus as the balloon is filled (FIGURE).

Manual insertion of a Bakri balloon
Insert the balloon into the uterus by 1) placing your hand into the vagina and guiding the balloon into the proper intrauterine position (similar to the manner in which an intrauterine pressure catheter is placed),5 2) stabilizing the balloon in position by maintaining the hand in the vagina, and 3) ensuring that the balloon does not “pop” out of the lower uterus as the balloon is filled.Clinician #3 simultaneously begins to instill sterile fluid into the balloon. This team member can be a nurse, medical assistant, or surgical technician.
My recommendation. Consider using simulation training to practice the team approach to placing the Bakri balloon. This is an effective method of improving team coordination.
TIP: Instill at least 150 mL of fluid
In short, use more fluid, not less. Although one study showed that most clinicians fill the balloon to a median volume of 300 mL,6 I’ve observed that some timidly instill the balloon with 80 to 120 mL of fluid. I think this is too little to maximize the effectiveness of the balloon for a postpartum patient who has massive hemorrhage.
Studies have shown that filling the balloon to at least 150 mL noticeably increases tamponade pressure.7
TIP: Place a pack in the upper vagina to stabilize the balloon in position
It usually takes only a few minutes after the Bakri balloon is filled to determine whether it is going to control uterine bleeding—what is called a tamponade test. If the balloon does control bleeding, you can place a vaginal pack of ribbon gauze to help ensure that the balloon does not slip down through the dilated postpartum cervix.
To avoid obscuring continued bleeding, however, do not place vaginal packing until you have obtained a positive tamponade test.
TIP: Check the hemoglobin concentration, platelet count, and coagulation status
The trauma literature demonstrates that optimal patient outcomes are realized when the following targets are maintained during resuscitation of patients who have massive hemorrhage8:
- hematocrit, ≥21%
- platelet count, ≥50 × 103/μL
- fibrinogen ≥100 mg/dL
- International Normalized Ratio (INR) ≤1.5.
These targets are achieved by appropriate transfusion of red blood cells, platelets, fresh frozen plasma, cryoprecipitate, and purified recombinant fibrinogen concentrate (RiaSTAP). Many trauma specialists recommend transfusion of a 1:1 ratio of RBCs and fresh frozen plasma in cases of major hemorrhage until coagulation status is normalized.
My recommendation. Often, it takes as long as 1 hour (sometimes, longer) to get results of Stat coagulation tests from the lab. While you are waiting, obtain clinical evidence of clotting function by filling a red-top tube with the patient’s blood. Adequate function is signaled by formation of a stable clot within 10 minutes.
TIP: Practice your team’s response to PPH with simulation
Last, I urge clinicians in every birthing unit to use simulation exercises to improve all facets of their response to severe postpartum hemorrhage.9
Summing up
Death following PPH remains a major cause of maternal mortality in developed and developing countries. The appropriateness of your response within 10 minutes of the onset of PPH is critical to ensuring a successful outcome and minimizing adverse events.10 To help, use the Bakri Postpartum Balloon often, and use it early.
What are your tips and tricks for effective use of intra-uterine balloon tamponade in postpartum hemorrhage?
To enter your response, click here or send your pearl to [email protected], with your name and location of practice.
We’ll publish a sampling of bylined contributions in an upcoming issue of OBG Management, with appreciation.
- Read a related case in July’s Medical Verdicts
- “10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage”
Baha M. Sibai, MD (June 2011) - “Postpartum hemorrhage: 11 critical questions, answered by an expert”
Q&A with Haywood L. Brown, MD (January 2011) - “What you can do to optimize blood conservation in ObGyn practice”
Eric J. Bieber, MD; Linda Scott, RN; Corinna Muller, DO; Nancy Nuss, RN; and Edie L. Derian, MD (February 2010) - “Planning reduces the risk of maternal death. This tool helps.”
Robert L. Barbieri, MD (Editorial; August 2009) - “Consider retroperitoneal packing for postpartum hemorrhage”
Maj. William R. Fulton, DO (July 2008)
Obstetricians know that postpartum hemorrhage (PPH) must be treated decisively and swiftly.1 To review:
Active management of the third stage after vaginal delivery, with a uterotonic such as oxytocin, helps to reduce the frequency of PPH2; in a recent randomized trial involving vaginal delivery, the rate of PPH was 10% in women who received postpartum oxytocin and 17% in those who did not (P<.001).3
When hemorrhage occurs despite oxytocin, having been given postpartum, the standard treatment algorithm (see the TABLE) calls for:
- uterine massage
- additional uterotonics
- identification and repair of vaginal and cervical lacerations
- removal of any retained products of conception.
Sequential interventions for managing postpartum hemorrhage
| This sequence of steps applies 1) after vaginal delivery (including the left hand-side interventions) and 2) after cesarean delivery, with the abdominal incision still open (including the right hand-side interventions). | |
| Hemorrhage after vaginal birth | Hemorrhage after cesarean delivery |
| Administer oxytocin | |
| Perform uterine massage | |
| Administer additional uterotonics (methergine, misoprostol, carboprost [Hemabate]) | |
| Bring 2 units of packed RBCs and 2 units of fresh frozen plasma (FFP) to point of care Transfuse based on the clinical condition Consider transfusing RBCs and FFP at a 1:1 ratio until clotting parameters are evaluated Obtain Stat clotting studies Start an additional intravenous line | |
| Move the patient to the operating room |
|
| Repair any tears |
|
| Place uterine compression suture(s), such as a B-Lynch suture |
| Place an intrauterine balloon | Consider bilateral ligation of the internal iliac artery |
| If indicated, call for additional specialists: second anesthesiologist, gyn surgeon, interventional radiologist, blood bank director | |
| Selective embolization by interventional radiology | Hysterectomy |
| Exploratory laparotomy—follow steps (along the right-hand side of this table) for treating hemorrhage after cesarean delivery | Pelvic packing |
| Adapted from: California Maternal Quality Care Collaborative (www.CMQCC.org). | |
Placing an intrauterine balloon, such as the Bakri Postpartum Balloon (Cook Medical) or the BT-Cath (Utah Medical Products), is strongly recommended if these steps do not control bleeding.
In this Editorial, I review tips and tricks for using the Bakri balloon, building on my earlier OBG Management Editorial (February 2009), in which I outlined a basic approach to intrauterine balloon tamponade.4
TIP: Place the Bakri balloon early in the PPH treatment algorithm
For postpartum hemorrhage after vaginal delivery, typical initial steps include, as noted, fundal massage, administration of uterotonics, and curettage to remove retained products of conception. If these steps are ineffective, don’t wait: Immediately consider placing a Bakri balloon. Time is precious, and wasting time with less effective interventions results in excessive blood loss and consumption of clotting factors and increases the risk of a coagulopathy.
I’ve found that clinicians often spend too much time trying to determine whether postpartum bleeding originates in the uterus or from a cervical or vaginal laceration. But heavy bleeding can obscure anatomic structures, making it difficult to identify the site of bleeding with precision. Fruitlessly perseverating to differentiate uterine, cervical, and upper vaginal sources of bleeding can waste valuable time and lead to unnecessary blood loss.
By placing a Bakri balloon and inflating it early in the PPH treatment algorithm, you will significantly reduce uterine bleeding. You will also be able to assess the cervix and upper vagina more effectively for lacerations.
My recommendation. Place the Bakri balloon as soon as it is apparent that bleeding is so heavy that you are going to have difficulty assessing the cervix and upper vagina for lacerations. Note that this stands in contrast to the usual recommendation that you assess the cervix and vagina for lacerations before you place the Bakri balloon.
TIP: Have 3 clinicians place the balloon
Teamwork is a key to success here: The Bakri balloon is most quickly and elegantly inserted and inflated when three clinicians team up, as follows:
Clinician#1 scans the uterus, assessing for retained products of conception and providing real-time imaging as the balloon is placed and inflated. This team member provides feedback to the others about correct placement and filling of the balloon.
Clinician#2 inserts the balloon into the uterus by placing her hand into the vagina and guiding the balloon into the proper intrauterine position. (Most often, clinician#2 is the delivering clinician.) This technique is performed in a manner similar to how one places an intrauterine pressure catheter.5
Clinician #2 stabilizes the balloon in position by maintaining her hand in the vagina. She ensures that the balloon does not “pop” out of the lower uterus as the balloon is filled (FIGURE).

Manual insertion of a Bakri balloon
Insert the balloon into the uterus by 1) placing your hand into the vagina and guiding the balloon into the proper intrauterine position (similar to the manner in which an intrauterine pressure catheter is placed),5 2) stabilizing the balloon in position by maintaining the hand in the vagina, and 3) ensuring that the balloon does not “pop” out of the lower uterus as the balloon is filled.Clinician #3 simultaneously begins to instill sterile fluid into the balloon. This team member can be a nurse, medical assistant, or surgical technician.
My recommendation. Consider using simulation training to practice the team approach to placing the Bakri balloon. This is an effective method of improving team coordination.
TIP: Instill at least 150 mL of fluid
In short, use more fluid, not less. Although one study showed that most clinicians fill the balloon to a median volume of 300 mL,6 I’ve observed that some timidly instill the balloon with 80 to 120 mL of fluid. I think this is too little to maximize the effectiveness of the balloon for a postpartum patient who has massive hemorrhage.
Studies have shown that filling the balloon to at least 150 mL noticeably increases tamponade pressure.7
TIP: Place a pack in the upper vagina to stabilize the balloon in position
It usually takes only a few minutes after the Bakri balloon is filled to determine whether it is going to control uterine bleeding—what is called a tamponade test. If the balloon does control bleeding, you can place a vaginal pack of ribbon gauze to help ensure that the balloon does not slip down through the dilated postpartum cervix.
To avoid obscuring continued bleeding, however, do not place vaginal packing until you have obtained a positive tamponade test.
TIP: Check the hemoglobin concentration, platelet count, and coagulation status
The trauma literature demonstrates that optimal patient outcomes are realized when the following targets are maintained during resuscitation of patients who have massive hemorrhage8:
- hematocrit, ≥21%
- platelet count, ≥50 × 103/μL
- fibrinogen ≥100 mg/dL
- International Normalized Ratio (INR) ≤1.5.
These targets are achieved by appropriate transfusion of red blood cells, platelets, fresh frozen plasma, cryoprecipitate, and purified recombinant fibrinogen concentrate (RiaSTAP). Many trauma specialists recommend transfusion of a 1:1 ratio of RBCs and fresh frozen plasma in cases of major hemorrhage until coagulation status is normalized.
My recommendation. Often, it takes as long as 1 hour (sometimes, longer) to get results of Stat coagulation tests from the lab. While you are waiting, obtain clinical evidence of clotting function by filling a red-top tube with the patient’s blood. Adequate function is signaled by formation of a stable clot within 10 minutes.
TIP: Practice your team’s response to PPH with simulation
Last, I urge clinicians in every birthing unit to use simulation exercises to improve all facets of their response to severe postpartum hemorrhage.9
Summing up
Death following PPH remains a major cause of maternal mortality in developed and developing countries. The appropriateness of your response within 10 minutes of the onset of PPH is critical to ensuring a successful outcome and minimizing adverse events.10 To help, use the Bakri Postpartum Balloon often, and use it early.
What are your tips and tricks for effective use of intra-uterine balloon tamponade in postpartum hemorrhage?
To enter your response, click here or send your pearl to [email protected], with your name and location of practice.
We’ll publish a sampling of bylined contributions in an upcoming issue of OBG Management, with appreciation.
1. Sibai BM. 10 practical evidence-based recommendations for managing severe postpartum hemorrhage. OBG Manage. 2011;23(6):44-48.
2. Barbieri RL. Give a uterotonic routinely during the third stage of labor. OBG Manage. 2007;19(05):6-13.
3. Jangsten E, Mattsson LA, Lyckestam I, Hellstrom AL, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG. 2011;118(3):362-369.
4. Barbieri RL. You should add the Bakri balloon to your treatment for OB bleeds. OBG Manage. 2009;21(2):6-10.
5. Dabelea V, Schultze PM, McDuffie RS, Jr. Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol. 2007;24(6):359-364.
6. Vitthala S, Tsoumpou I, Anjum ZK, Aziz NA. Use of Bakri balloon in post-partum hemorrhage: a series of 15 cases. Aust NZ J Obstet Gynecol. 2009;49(2):191-194.
7. Georgiou C. Intraluminal pressure readings during the establishment of a positive “tamponade test” in the management of postpartum hemorrhage. BJOG. 2010;117(3):295-303.
8. Barbieri RL. Control of massive hemorrhage: Lessons from Iraq reach the US labor and delivery suite. OBG Manage. 2007;19(7):8-16.
9. Skupski DW, Lowenwirt IP, Weinbaum FI, Brodsky D, Danek M, Eglinton GS. Improving hospital systems for the care of women with major obstetric hemorrhage. Obstet Gynecol. 2006;107(5):977-983.
10. Driessen M, Bouvier-Colle MH, Dupont C, et al. Pithagore6 Group Postpartum hemorrhage resulting from uterine atony after vaginal delivery: factors associated with severity. Obstet Gynecol. 2011;117(1):21-31.
1. Sibai BM. 10 practical evidence-based recommendations for managing severe postpartum hemorrhage. OBG Manage. 2011;23(6):44-48.
2. Barbieri RL. Give a uterotonic routinely during the third stage of labor. OBG Manage. 2007;19(05):6-13.
3. Jangsten E, Mattsson LA, Lyckestam I, Hellstrom AL, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG. 2011;118(3):362-369.
4. Barbieri RL. You should add the Bakri balloon to your treatment for OB bleeds. OBG Manage. 2009;21(2):6-10.
5. Dabelea V, Schultze PM, McDuffie RS, Jr. Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol. 2007;24(6):359-364.
6. Vitthala S, Tsoumpou I, Anjum ZK, Aziz NA. Use of Bakri balloon in post-partum hemorrhage: a series of 15 cases. Aust NZ J Obstet Gynecol. 2009;49(2):191-194.
7. Georgiou C. Intraluminal pressure readings during the establishment of a positive “tamponade test” in the management of postpartum hemorrhage. BJOG. 2010;117(3):295-303.
8. Barbieri RL. Control of massive hemorrhage: Lessons from Iraq reach the US labor and delivery suite. OBG Manage. 2007;19(7):8-16.
9. Skupski DW, Lowenwirt IP, Weinbaum FI, Brodsky D, Danek M, Eglinton GS. Improving hospital systems for the care of women with major obstetric hemorrhage. Obstet Gynecol. 2006;107(5):977-983.
10. Driessen M, Bouvier-Colle MH, Dupont C, et al. Pithagore6 Group Postpartum hemorrhage resulting from uterine atony after vaginal delivery: factors associated with severity. Obstet Gynecol. 2011;117(1):21-31.
ACPA-Negative RA Up in First Postpartum Year
Major Finding: During the year following giving birth, women had a statistically significant, 2.4-fold increased risk of incident ACPA-negative rheumatoid arthritis, compared with nulliparous women.
Data Source: Case-control study of 1,205 Swedish women enrolled in the Epidemiological Investigation of Rheumatoid Arthritis study during 1996–2006.
Disclosures: Dr. Bengtsson said that she had no relevant financial disclosures.
LONDON – Women who give birth to a child face a twofold increased risk of incident anticitrullinated peptide antibody–negative rheumatoid arthritis, compared with nulliparous women, but they have no increased risk for developing ACPA-positive disease, based on results from a Swedish epidemiologic study.
The finding is consistent with a report last year from a Norwegian study that women face about a twofold increased risk for incident rheumatoid arthritis (RA) during the first 2 years after giving birth to a child, compared with their RA risk 2–4 years post partum (Ann. Rheum. Dis. 2010;69:332–6). The reason why the new analysis, which included more than 1,200 cases and controls, showed a different relationship between partum and the onset of ACPA-positive RA and ACPA-negative RA remains unclear, according to Camilla Bengtsson, Ph.D.
“Why there is only an association with ACPA-negative disease, and which biological mechanisms are involved remains to be elucidated,” said Dr. Bengtsson, a researcher at the Karolinska Institute in Stockholm. The way in which this finding might apply to practice also remains unclear, she added.
Dr. Bengtsson's analysis failed to show an increased incidence of any form of RA in women who were more than a year out from their delivery.
The study used data and blood specimens from Swedish women aged 18–50 years who were enrolled in the Epidemiological Investigation of RA (EIRA) study during 1996–2006. Among the women with incident RA enrolled in EIRA, 547 (95%) agreed to participate and provide blood specimens, and among the control women in the study, 658 (81%) provided blood. The analysis divided the cases and controls into subgroups based on their partum status. The 547 women with new-onset RA included 360 who had given birth and 187 who had not. The parous women included 226 with ACPA-positive RA and 134 with the ACPA-negative form. Among the nulliparous women with RA, 127 had the ACPA-positive form and 60 were ACPA negative.
Among the controls with no RA, 431 had given birth and 227 had never given birth.
The case-control analysis showed that among all women with incident RA, birth status during the year preceding a new RA diagnosis had no statistically significant relationship with RA onset. However, among women who developed ACPA-negative RA, their risk spiked by a statistically significant, 2.4-fold rate during the year following partum, compared with nulliparous women. In contrast, the incidence of ACPA-positive RA showed no significant relationship to partum status during the preceding year.
Further analysis examined the timing between delivery and onset of ACPA-negative RA more closely. Again, the analysis showed that, during the year following giving birth, women faced a statistically significant, 2.4-fold elevated risk for incident ACPA-negative RA, compared with nulliparous women. During the 2–10 years following giving birth, the rate of incident ACPA-negative RA dropped to a 50% higher risk, compared with nulliparous women, but this difference was not considered statistically significant. And women more than 10 years out from their most recent delivery had a risk for incident ACPA-negative RA identical to the nulliparous women, Dr. Bengtsson reported.
Major Finding: During the year following giving birth, women had a statistically significant, 2.4-fold increased risk of incident ACPA-negative rheumatoid arthritis, compared with nulliparous women.
Data Source: Case-control study of 1,205 Swedish women enrolled in the Epidemiological Investigation of Rheumatoid Arthritis study during 1996–2006.
Disclosures: Dr. Bengtsson said that she had no relevant financial disclosures.
LONDON – Women who give birth to a child face a twofold increased risk of incident anticitrullinated peptide antibody–negative rheumatoid arthritis, compared with nulliparous women, but they have no increased risk for developing ACPA-positive disease, based on results from a Swedish epidemiologic study.
The finding is consistent with a report last year from a Norwegian study that women face about a twofold increased risk for incident rheumatoid arthritis (RA) during the first 2 years after giving birth to a child, compared with their RA risk 2–4 years post partum (Ann. Rheum. Dis. 2010;69:332–6). The reason why the new analysis, which included more than 1,200 cases and controls, showed a different relationship between partum and the onset of ACPA-positive RA and ACPA-negative RA remains unclear, according to Camilla Bengtsson, Ph.D.
“Why there is only an association with ACPA-negative disease, and which biological mechanisms are involved remains to be elucidated,” said Dr. Bengtsson, a researcher at the Karolinska Institute in Stockholm. The way in which this finding might apply to practice also remains unclear, she added.
Dr. Bengtsson's analysis failed to show an increased incidence of any form of RA in women who were more than a year out from their delivery.
The study used data and blood specimens from Swedish women aged 18–50 years who were enrolled in the Epidemiological Investigation of RA (EIRA) study during 1996–2006. Among the women with incident RA enrolled in EIRA, 547 (95%) agreed to participate and provide blood specimens, and among the control women in the study, 658 (81%) provided blood. The analysis divided the cases and controls into subgroups based on their partum status. The 547 women with new-onset RA included 360 who had given birth and 187 who had not. The parous women included 226 with ACPA-positive RA and 134 with the ACPA-negative form. Among the nulliparous women with RA, 127 had the ACPA-positive form and 60 were ACPA negative.
Among the controls with no RA, 431 had given birth and 227 had never given birth.
The case-control analysis showed that among all women with incident RA, birth status during the year preceding a new RA diagnosis had no statistically significant relationship with RA onset. However, among women who developed ACPA-negative RA, their risk spiked by a statistically significant, 2.4-fold rate during the year following partum, compared with nulliparous women. In contrast, the incidence of ACPA-positive RA showed no significant relationship to partum status during the preceding year.
Further analysis examined the timing between delivery and onset of ACPA-negative RA more closely. Again, the analysis showed that, during the year following giving birth, women faced a statistically significant, 2.4-fold elevated risk for incident ACPA-negative RA, compared with nulliparous women. During the 2–10 years following giving birth, the rate of incident ACPA-negative RA dropped to a 50% higher risk, compared with nulliparous women, but this difference was not considered statistically significant. And women more than 10 years out from their most recent delivery had a risk for incident ACPA-negative RA identical to the nulliparous women, Dr. Bengtsson reported.
Major Finding: During the year following giving birth, women had a statistically significant, 2.4-fold increased risk of incident ACPA-negative rheumatoid arthritis, compared with nulliparous women.
Data Source: Case-control study of 1,205 Swedish women enrolled in the Epidemiological Investigation of Rheumatoid Arthritis study during 1996–2006.
Disclosures: Dr. Bengtsson said that she had no relevant financial disclosures.
LONDON – Women who give birth to a child face a twofold increased risk of incident anticitrullinated peptide antibody–negative rheumatoid arthritis, compared with nulliparous women, but they have no increased risk for developing ACPA-positive disease, based on results from a Swedish epidemiologic study.
The finding is consistent with a report last year from a Norwegian study that women face about a twofold increased risk for incident rheumatoid arthritis (RA) during the first 2 years after giving birth to a child, compared with their RA risk 2–4 years post partum (Ann. Rheum. Dis. 2010;69:332–6). The reason why the new analysis, which included more than 1,200 cases and controls, showed a different relationship between partum and the onset of ACPA-positive RA and ACPA-negative RA remains unclear, according to Camilla Bengtsson, Ph.D.
“Why there is only an association with ACPA-negative disease, and which biological mechanisms are involved remains to be elucidated,” said Dr. Bengtsson, a researcher at the Karolinska Institute in Stockholm. The way in which this finding might apply to practice also remains unclear, she added.
Dr. Bengtsson's analysis failed to show an increased incidence of any form of RA in women who were more than a year out from their delivery.
The study used data and blood specimens from Swedish women aged 18–50 years who were enrolled in the Epidemiological Investigation of RA (EIRA) study during 1996–2006. Among the women with incident RA enrolled in EIRA, 547 (95%) agreed to participate and provide blood specimens, and among the control women in the study, 658 (81%) provided blood. The analysis divided the cases and controls into subgroups based on their partum status. The 547 women with new-onset RA included 360 who had given birth and 187 who had not. The parous women included 226 with ACPA-positive RA and 134 with the ACPA-negative form. Among the nulliparous women with RA, 127 had the ACPA-positive form and 60 were ACPA negative.
Among the controls with no RA, 431 had given birth and 227 had never given birth.
The case-control analysis showed that among all women with incident RA, birth status during the year preceding a new RA diagnosis had no statistically significant relationship with RA onset. However, among women who developed ACPA-negative RA, their risk spiked by a statistically significant, 2.4-fold rate during the year following partum, compared with nulliparous women. In contrast, the incidence of ACPA-positive RA showed no significant relationship to partum status during the preceding year.
Further analysis examined the timing between delivery and onset of ACPA-negative RA more closely. Again, the analysis showed that, during the year following giving birth, women faced a statistically significant, 2.4-fold elevated risk for incident ACPA-negative RA, compared with nulliparous women. During the 2–10 years following giving birth, the rate of incident ACPA-negative RA dropped to a 50% higher risk, compared with nulliparous women, but this difference was not considered statistically significant. And women more than 10 years out from their most recent delivery had a risk for incident ACPA-negative RA identical to the nulliparous women, Dr. Bengtsson reported.
From the Annual European Congress of Rheumatology
Unintended Pregnancies Carry Big Price Tag
Taxpayers spend more than $11 billion each year as a result of unintended pregnancies, according to new data from two separate studies.
The estimates are based on public insurance costs for pregnancies and infant care in the first year. Researchers from the Guttmacher Institute used state-level data from 2006 to come up with a national estimate of $11.1 billion in public spending on unintended pregnancies. In a separate study, researchers at the Brookings Institution came up with their figures by using 2001 national data on publicly financed unintended pregnancies, resulting in average spending of $11.3 billion annually. Both studies were published in the June issue of Perspectives on Sexual and Reproductive Health.
Researchers from the Guttmacher Institute found that public programs such as Medicaid and the Children's Health Insurance Program bear the brunt of the nation's costs for unintended pregnancies (Perspect. Sex. Reprod. Health 2011;43:94–102 [doi:10.1363/4309411]). While 38% of U.S. births result from unintended pregnancies, births from unintended pregnancies make up about half of publicly funded births. But reducing unintended pregnancies also will require major new public investments, the Guttmacher researchers wrote, including increasing access to family planning services and comprehensive sex education. The Affordable Care Act may help, too, they said, by expanding insurance coverage and giving new authority to states to expand Medicaid eligibility for family planning services.
While preventing unintended pregnancies would require an up-front investment, the researchers at the Brookings Institution said it would be more than offset by potential savings. They estimated that if unintended pregnancies could be prevented altogether, with some being delayed until the women were ready to be pregnant, it could save taxpayers about $5.6 billion annually (Perspect. Sex. Reprod. Health 2011;43:88–93 [doi: 10.1363/4308811]).
Taxpayers spend more than $11 billion each year as a result of unintended pregnancies, according to new data from two separate studies.
The estimates are based on public insurance costs for pregnancies and infant care in the first year. Researchers from the Guttmacher Institute used state-level data from 2006 to come up with a national estimate of $11.1 billion in public spending on unintended pregnancies. In a separate study, researchers at the Brookings Institution came up with their figures by using 2001 national data on publicly financed unintended pregnancies, resulting in average spending of $11.3 billion annually. Both studies were published in the June issue of Perspectives on Sexual and Reproductive Health.
Researchers from the Guttmacher Institute found that public programs such as Medicaid and the Children's Health Insurance Program bear the brunt of the nation's costs for unintended pregnancies (Perspect. Sex. Reprod. Health 2011;43:94–102 [doi:10.1363/4309411]). While 38% of U.S. births result from unintended pregnancies, births from unintended pregnancies make up about half of publicly funded births. But reducing unintended pregnancies also will require major new public investments, the Guttmacher researchers wrote, including increasing access to family planning services and comprehensive sex education. The Affordable Care Act may help, too, they said, by expanding insurance coverage and giving new authority to states to expand Medicaid eligibility for family planning services.
While preventing unintended pregnancies would require an up-front investment, the researchers at the Brookings Institution said it would be more than offset by potential savings. They estimated that if unintended pregnancies could be prevented altogether, with some being delayed until the women were ready to be pregnant, it could save taxpayers about $5.6 billion annually (Perspect. Sex. Reprod. Health 2011;43:88–93 [doi: 10.1363/4308811]).
Taxpayers spend more than $11 billion each year as a result of unintended pregnancies, according to new data from two separate studies.
The estimates are based on public insurance costs for pregnancies and infant care in the first year. Researchers from the Guttmacher Institute used state-level data from 2006 to come up with a national estimate of $11.1 billion in public spending on unintended pregnancies. In a separate study, researchers at the Brookings Institution came up with their figures by using 2001 national data on publicly financed unintended pregnancies, resulting in average spending of $11.3 billion annually. Both studies were published in the June issue of Perspectives on Sexual and Reproductive Health.
Researchers from the Guttmacher Institute found that public programs such as Medicaid and the Children's Health Insurance Program bear the brunt of the nation's costs for unintended pregnancies (Perspect. Sex. Reprod. Health 2011;43:94–102 [doi:10.1363/4309411]). While 38% of U.S. births result from unintended pregnancies, births from unintended pregnancies make up about half of publicly funded births. But reducing unintended pregnancies also will require major new public investments, the Guttmacher researchers wrote, including increasing access to family planning services and comprehensive sex education. The Affordable Care Act may help, too, they said, by expanding insurance coverage and giving new authority to states to expand Medicaid eligibility for family planning services.
While preventing unintended pregnancies would require an up-front investment, the researchers at the Brookings Institution said it would be more than offset by potential savings. They estimated that if unintended pregnancies could be prevented altogether, with some being delayed until the women were ready to be pregnant, it could save taxpayers about $5.6 billion annually (Perspect. Sex. Reprod. Health 2011;43:88–93 [doi: 10.1363/4308811]).
Shoulder Dystocia Protocol Reduces Injuries : The rate of obstetric brachial plexus injury fell by nearly three-fourths in this study.
Major Finding: The rate of obstetric brachial plexus injury in cases of shoulder dystocia fell from 40% before implementation of the Code D protocol to 14% afterward (P less than .01).
Data Source: A retrospective cohort study of 11,862 vaginal deliveries of singleton, live born infants.
Disclosures: Dr. Inglis did not report any relevant financial disclosures.
SAN FRANCISCO – A simple, standardized protocol for managing shoulder dystocia, called Code D, reduced the incidence of obstetric brachial plexus injury, according to a study reported at the meeting.
Investigators retrospectively assessed the impact of the protocol – which entails mobilization of experienced staff, a hands-off pause for assessment, and varied maneuvers – in a cohort of nearly 12,000 vaginal deliveries.
Study results showed that with use of the protocol, the rate of obstetric brachial plexus injury (Erb's palsy) among cases of shoulder dystocia fell by nearly three-fourths, from 40% before the protocol's implementation to 14% afterward.
“A standardized and simple protocol to manage shoulder dystocia appears to reduce the risk of Erb's palsy,” said lead investigator Dr. Steven R. Inglis.
“We were unable to tell which part of the protocol really was helping us,” he added, so further research is needed to determine the responsible components and maneuvers.
Rates of both shoulder dystocia and brachial plexus injury appear to be on the rise, in part because of increasing maternal obesity and diabetes, as well as increasing fetal macrosomia, according to Dr. Inglis, chairman of the department of ob.gyn. at the Jamaica (N.Y.) Hospital Medical Center.
These complications not only can be associated with long-term morbidity, but also account for a substantial share of obstetricians' liability payouts, according to Dr. Inglis.
Many strategies for managing shoulder dystocia have been introduced, but few of them have been studied to assess their impact on important neonatal outcomes, he said.
Dr. Inglis and his colleagues determined the rate of brachial plexus injury at Jamaica Hospital Medical Center before and after implementation of the Code D shoulder dystocia protocol. The protocol emphasized a stepwise team approach to management, conducted in a calm and relaxed environment.
Code D training was provided to all labor and delivery staff including attending and resident physicians, midwives, and nurses. “I don't think anybody else has really included nurses,” he commented. “I think they were a key part of it.”
Training included didactic presentations followed by hands-on practice with a manikin. “Everybody had to go through shoulder dystocia once or twice and get it done right according to our protocol,” Dr. Inglis explained.
When the staff diagnosed dystocia (tight or difficult shoulders, or the so-called turtle sign requiring additional maneuvers to achieve delivery), they activated the Code D protocol, which summoned to the room the most experienced available obstetrician, and also an anesthesiologist, a neonatologist, and a nurse.
Staff were taught, first, to assess – using a hands-off pause during which there was no maternal pushing, application of fundal pressure, or head traction – the orientation of the infant's back and shoulders, and to announce it to the delivery team.
This hands-off period lasted just a few seconds, according to Dr. Inglis. “You basically want to stop, take a deep breath, collect yourself, make sure you are following the protocol, and then go on.”
Staff then began one of several maneuvers performed in an order of their choice, including rotating the shoulders to the oblique position, changing maternal position, implementing the corkscrew maneuver, and delivering the posterior arm.
“Each should last no longer than 30 seconds, and you could go back to a maneuver if it didn't work the first time,” Dr. Inglis said. Suprapubic pressure also could be used.
To assess the impact of the Code D protocol, the investigators retrospectively reviewed medical records for mothers and their singleton, live born, nonbreech infants delivered vaginally between August 2003 and December 2009. Analyses were based on 6,269 deliveries in the pretraining period before September 2006, and 5,593 deliveries in the posttraining period.
Study results showed that the rate of shoulder dystocia did not differ significantly between periods: This complication occurred in 83 or 1.32% of deliveries in the former period, and in 75 or 1.34% of deliveries in the latter period. However, the percentage of cases of shoulder dystocia that resulted in brachial plexus injury was 40% in the pretraining period, compared with just 14% in the posttraining period.
Among the cases of shoulder dystocia, those in the pretraining period had a higher maternal body mass index (33.4 vs. 30.3 kg/m
But in a logistic regression analysis, use of the shoulder dystocia protocol was still associated with a reduced risk of obstetric brachial plexus injury.
The interval between delivery of the infant's head and body in cases of shoulder dystocia was longer in the posttraining period than in the pretraining period (2.0 minutes vs. 1.5 minutes).
“We wanted everyone to go slowly, so we were actually happy to see that the head-body interval went up,” commented Dr. Inglis. “That certainly didn't seem to worsen the risk of Erb's palsy.”
Study results also showed that staff were more likely to use the Rubin maneuver and posterior arm delivery in the posttraining vs. pretraining period, and were less likely to use the McRoberts maneuver.
Major Finding: The rate of obstetric brachial plexus injury in cases of shoulder dystocia fell from 40% before implementation of the Code D protocol to 14% afterward (P less than .01).
Data Source: A retrospective cohort study of 11,862 vaginal deliveries of singleton, live born infants.
Disclosures: Dr. Inglis did not report any relevant financial disclosures.
SAN FRANCISCO – A simple, standardized protocol for managing shoulder dystocia, called Code D, reduced the incidence of obstetric brachial plexus injury, according to a study reported at the meeting.
Investigators retrospectively assessed the impact of the protocol – which entails mobilization of experienced staff, a hands-off pause for assessment, and varied maneuvers – in a cohort of nearly 12,000 vaginal deliveries.
Study results showed that with use of the protocol, the rate of obstetric brachial plexus injury (Erb's palsy) among cases of shoulder dystocia fell by nearly three-fourths, from 40% before the protocol's implementation to 14% afterward.
“A standardized and simple protocol to manage shoulder dystocia appears to reduce the risk of Erb's palsy,” said lead investigator Dr. Steven R. Inglis.
“We were unable to tell which part of the protocol really was helping us,” he added, so further research is needed to determine the responsible components and maneuvers.
Rates of both shoulder dystocia and brachial plexus injury appear to be on the rise, in part because of increasing maternal obesity and diabetes, as well as increasing fetal macrosomia, according to Dr. Inglis, chairman of the department of ob.gyn. at the Jamaica (N.Y.) Hospital Medical Center.
These complications not only can be associated with long-term morbidity, but also account for a substantial share of obstetricians' liability payouts, according to Dr. Inglis.
Many strategies for managing shoulder dystocia have been introduced, but few of them have been studied to assess their impact on important neonatal outcomes, he said.
Dr. Inglis and his colleagues determined the rate of brachial plexus injury at Jamaica Hospital Medical Center before and after implementation of the Code D shoulder dystocia protocol. The protocol emphasized a stepwise team approach to management, conducted in a calm and relaxed environment.
Code D training was provided to all labor and delivery staff including attending and resident physicians, midwives, and nurses. “I don't think anybody else has really included nurses,” he commented. “I think they were a key part of it.”
Training included didactic presentations followed by hands-on practice with a manikin. “Everybody had to go through shoulder dystocia once or twice and get it done right according to our protocol,” Dr. Inglis explained.
When the staff diagnosed dystocia (tight or difficult shoulders, or the so-called turtle sign requiring additional maneuvers to achieve delivery), they activated the Code D protocol, which summoned to the room the most experienced available obstetrician, and also an anesthesiologist, a neonatologist, and a nurse.
Staff were taught, first, to assess – using a hands-off pause during which there was no maternal pushing, application of fundal pressure, or head traction – the orientation of the infant's back and shoulders, and to announce it to the delivery team.
This hands-off period lasted just a few seconds, according to Dr. Inglis. “You basically want to stop, take a deep breath, collect yourself, make sure you are following the protocol, and then go on.”
Staff then began one of several maneuvers performed in an order of their choice, including rotating the shoulders to the oblique position, changing maternal position, implementing the corkscrew maneuver, and delivering the posterior arm.
“Each should last no longer than 30 seconds, and you could go back to a maneuver if it didn't work the first time,” Dr. Inglis said. Suprapubic pressure also could be used.
To assess the impact of the Code D protocol, the investigators retrospectively reviewed medical records for mothers and their singleton, live born, nonbreech infants delivered vaginally between August 2003 and December 2009. Analyses were based on 6,269 deliveries in the pretraining period before September 2006, and 5,593 deliveries in the posttraining period.
Study results showed that the rate of shoulder dystocia did not differ significantly between periods: This complication occurred in 83 or 1.32% of deliveries in the former period, and in 75 or 1.34% of deliveries in the latter period. However, the percentage of cases of shoulder dystocia that resulted in brachial plexus injury was 40% in the pretraining period, compared with just 14% in the posttraining period.
Among the cases of shoulder dystocia, those in the pretraining period had a higher maternal body mass index (33.4 vs. 30.3 kg/m
But in a logistic regression analysis, use of the shoulder dystocia protocol was still associated with a reduced risk of obstetric brachial plexus injury.
The interval between delivery of the infant's head and body in cases of shoulder dystocia was longer in the posttraining period than in the pretraining period (2.0 minutes vs. 1.5 minutes).
“We wanted everyone to go slowly, so we were actually happy to see that the head-body interval went up,” commented Dr. Inglis. “That certainly didn't seem to worsen the risk of Erb's palsy.”
Study results also showed that staff were more likely to use the Rubin maneuver and posterior arm delivery in the posttraining vs. pretraining period, and were less likely to use the McRoberts maneuver.
Major Finding: The rate of obstetric brachial plexus injury in cases of shoulder dystocia fell from 40% before implementation of the Code D protocol to 14% afterward (P less than .01).
Data Source: A retrospective cohort study of 11,862 vaginal deliveries of singleton, live born infants.
Disclosures: Dr. Inglis did not report any relevant financial disclosures.
SAN FRANCISCO – A simple, standardized protocol for managing shoulder dystocia, called Code D, reduced the incidence of obstetric brachial plexus injury, according to a study reported at the meeting.
Investigators retrospectively assessed the impact of the protocol – which entails mobilization of experienced staff, a hands-off pause for assessment, and varied maneuvers – in a cohort of nearly 12,000 vaginal deliveries.
Study results showed that with use of the protocol, the rate of obstetric brachial plexus injury (Erb's palsy) among cases of shoulder dystocia fell by nearly three-fourths, from 40% before the protocol's implementation to 14% afterward.
“A standardized and simple protocol to manage shoulder dystocia appears to reduce the risk of Erb's palsy,” said lead investigator Dr. Steven R. Inglis.
“We were unable to tell which part of the protocol really was helping us,” he added, so further research is needed to determine the responsible components and maneuvers.
Rates of both shoulder dystocia and brachial plexus injury appear to be on the rise, in part because of increasing maternal obesity and diabetes, as well as increasing fetal macrosomia, according to Dr. Inglis, chairman of the department of ob.gyn. at the Jamaica (N.Y.) Hospital Medical Center.
These complications not only can be associated with long-term morbidity, but also account for a substantial share of obstetricians' liability payouts, according to Dr. Inglis.
Many strategies for managing shoulder dystocia have been introduced, but few of them have been studied to assess their impact on important neonatal outcomes, he said.
Dr. Inglis and his colleagues determined the rate of brachial plexus injury at Jamaica Hospital Medical Center before and after implementation of the Code D shoulder dystocia protocol. The protocol emphasized a stepwise team approach to management, conducted in a calm and relaxed environment.
Code D training was provided to all labor and delivery staff including attending and resident physicians, midwives, and nurses. “I don't think anybody else has really included nurses,” he commented. “I think they were a key part of it.”
Training included didactic presentations followed by hands-on practice with a manikin. “Everybody had to go through shoulder dystocia once or twice and get it done right according to our protocol,” Dr. Inglis explained.
When the staff diagnosed dystocia (tight or difficult shoulders, or the so-called turtle sign requiring additional maneuvers to achieve delivery), they activated the Code D protocol, which summoned to the room the most experienced available obstetrician, and also an anesthesiologist, a neonatologist, and a nurse.
Staff were taught, first, to assess – using a hands-off pause during which there was no maternal pushing, application of fundal pressure, or head traction – the orientation of the infant's back and shoulders, and to announce it to the delivery team.
This hands-off period lasted just a few seconds, according to Dr. Inglis. “You basically want to stop, take a deep breath, collect yourself, make sure you are following the protocol, and then go on.”
Staff then began one of several maneuvers performed in an order of their choice, including rotating the shoulders to the oblique position, changing maternal position, implementing the corkscrew maneuver, and delivering the posterior arm.
“Each should last no longer than 30 seconds, and you could go back to a maneuver if it didn't work the first time,” Dr. Inglis said. Suprapubic pressure also could be used.
To assess the impact of the Code D protocol, the investigators retrospectively reviewed medical records for mothers and their singleton, live born, nonbreech infants delivered vaginally between August 2003 and December 2009. Analyses were based on 6,269 deliveries in the pretraining period before September 2006, and 5,593 deliveries in the posttraining period.
Study results showed that the rate of shoulder dystocia did not differ significantly between periods: This complication occurred in 83 or 1.32% of deliveries in the former period, and in 75 or 1.34% of deliveries in the latter period. However, the percentage of cases of shoulder dystocia that resulted in brachial plexus injury was 40% in the pretraining period, compared with just 14% in the posttraining period.
Among the cases of shoulder dystocia, those in the pretraining period had a higher maternal body mass index (33.4 vs. 30.3 kg/m
But in a logistic regression analysis, use of the shoulder dystocia protocol was still associated with a reduced risk of obstetric brachial plexus injury.
The interval between delivery of the infant's head and body in cases of shoulder dystocia was longer in the posttraining period than in the pretraining period (2.0 minutes vs. 1.5 minutes).
“We wanted everyone to go slowly, so we were actually happy to see that the head-body interval went up,” commented Dr. Inglis. “That certainly didn't seem to worsen the risk of Erb's palsy.”
Study results also showed that staff were more likely to use the Rubin maneuver and posterior arm delivery in the posttraining vs. pretraining period, and were less likely to use the McRoberts maneuver.
From the Annual Meeting of the Society for Maternal-Fetal Medicine
Try Vaginal-Perianal GBS Cultures
Major Finding: More than half (60%) of women reported no pain with the vaginal-perianal method, compared with 18% with the vaginal-rectal method. The sensitivity and specificity of the vaginal-perianal method were 91% and 99%.
Data Source: A study of 200 women at least 18 years of age with an average gestational age of 35.9 weeks.
Disclosures: Dr. Trappe reported that she had no relevant financial disclosures.
WASHINGTON – Vaginal-perianal cultures for group B streptococcus may be a more comfortable option for pregnant women and have comparable accuracy to vaginal-anal cultures, a study of 200 women has shown.
“Vaginal-perianal cultures may be a reasonable, patient-preferred alternative for the recommended vaginal-rectal cultures of GBS during pregnancy,” said Dr. Karen L. Trappe of Riverside Methodist Hospital in Columbus, Ohio.
It's estimated that 10%–30% of pregnant women are colonized with GBS, which is an established cause of neonatal morbidity and mortality. In 2002, the Centers for Disease Control and Prevention advised universal prenatal screening and the American College of Obstetricians and Gynecologists published a committee opinion outlining the collection of GBS cultures between 35 and 37 weeks' gestation (Obstet. Gynecol. 2002;100:1405–12). GBS cultures are to be obtained by a swab of the lower vagina (vaginal introitus) followed by the rectum (insert swab through anal sphincter). This collection method was based on a 1977 study suggesting that the GI tract was the primary site of GBS colonization. However, vaginal-anal cultures are uncomfortable for patients.
Dr. Trappe and her coinvestigators studied whether vaginal-perianal cultures were equally effective in identifying GBS and were more comfortable for patients. They included women in the study if they were at least 18 years old, were to undergo routine GBS culture, and spoke English, Spanish, or Somali. The researchers collected data from 200 patients. The average maternal age was 26 years, and the average gestational age was 35.9 weeks. In terms of ethnicity, half (49%) of patients where white, followed by black (23%), Hispanic (14%), Asian (1%), and other (13%). Although inclusion criteria were for 35–37 weeks' gestation, seven women outside of this range were enrolled – three at 34 weeks', three at 38 weeks', and one at 39 weeks' gestation.
The vaginal-perianal specimen was collected first, followed by the recommended vaginal-rectal specimen. Women were asked to rate pain on a 0–10 scale for each collection method. They also were asked if one method was more uncomfortable than the other. The overall agreement rate between the two collection methods was 96.5%. The sensitivity and specificity of the vaginal-perianal method were 91% and 99%.
“Patients also reported statistically greater pain and discomfort with the vaginal-rectal culture collection, with over two-thirds of patients reporting less discomfort with the vaginal-perianal method,” Dr. Trappe said at the meeting. More than half (60%) of women reported no pain with the vaginal-perianal method, compared with 18% with the vaginal-rectal method. “Patients indicated a preference for the vaginal-perianal collection method based on pain and discomfort surveys,” she noted.
Major Finding: More than half (60%) of women reported no pain with the vaginal-perianal method, compared with 18% with the vaginal-rectal method. The sensitivity and specificity of the vaginal-perianal method were 91% and 99%.
Data Source: A study of 200 women at least 18 years of age with an average gestational age of 35.9 weeks.
Disclosures: Dr. Trappe reported that she had no relevant financial disclosures.
WASHINGTON – Vaginal-perianal cultures for group B streptococcus may be a more comfortable option for pregnant women and have comparable accuracy to vaginal-anal cultures, a study of 200 women has shown.
“Vaginal-perianal cultures may be a reasonable, patient-preferred alternative for the recommended vaginal-rectal cultures of GBS during pregnancy,” said Dr. Karen L. Trappe of Riverside Methodist Hospital in Columbus, Ohio.
It's estimated that 10%–30% of pregnant women are colonized with GBS, which is an established cause of neonatal morbidity and mortality. In 2002, the Centers for Disease Control and Prevention advised universal prenatal screening and the American College of Obstetricians and Gynecologists published a committee opinion outlining the collection of GBS cultures between 35 and 37 weeks' gestation (Obstet. Gynecol. 2002;100:1405–12). GBS cultures are to be obtained by a swab of the lower vagina (vaginal introitus) followed by the rectum (insert swab through anal sphincter). This collection method was based on a 1977 study suggesting that the GI tract was the primary site of GBS colonization. However, vaginal-anal cultures are uncomfortable for patients.
Dr. Trappe and her coinvestigators studied whether vaginal-perianal cultures were equally effective in identifying GBS and were more comfortable for patients. They included women in the study if they were at least 18 years old, were to undergo routine GBS culture, and spoke English, Spanish, or Somali. The researchers collected data from 200 patients. The average maternal age was 26 years, and the average gestational age was 35.9 weeks. In terms of ethnicity, half (49%) of patients where white, followed by black (23%), Hispanic (14%), Asian (1%), and other (13%). Although inclusion criteria were for 35–37 weeks' gestation, seven women outside of this range were enrolled – three at 34 weeks', three at 38 weeks', and one at 39 weeks' gestation.
The vaginal-perianal specimen was collected first, followed by the recommended vaginal-rectal specimen. Women were asked to rate pain on a 0–10 scale for each collection method. They also were asked if one method was more uncomfortable than the other. The overall agreement rate between the two collection methods was 96.5%. The sensitivity and specificity of the vaginal-perianal method were 91% and 99%.
“Patients also reported statistically greater pain and discomfort with the vaginal-rectal culture collection, with over two-thirds of patients reporting less discomfort with the vaginal-perianal method,” Dr. Trappe said at the meeting. More than half (60%) of women reported no pain with the vaginal-perianal method, compared with 18% with the vaginal-rectal method. “Patients indicated a preference for the vaginal-perianal collection method based on pain and discomfort surveys,” she noted.
Major Finding: More than half (60%) of women reported no pain with the vaginal-perianal method, compared with 18% with the vaginal-rectal method. The sensitivity and specificity of the vaginal-perianal method were 91% and 99%.
Data Source: A study of 200 women at least 18 years of age with an average gestational age of 35.9 weeks.
Disclosures: Dr. Trappe reported that she had no relevant financial disclosures.
WASHINGTON – Vaginal-perianal cultures for group B streptococcus may be a more comfortable option for pregnant women and have comparable accuracy to vaginal-anal cultures, a study of 200 women has shown.
“Vaginal-perianal cultures may be a reasonable, patient-preferred alternative for the recommended vaginal-rectal cultures of GBS during pregnancy,” said Dr. Karen L. Trappe of Riverside Methodist Hospital in Columbus, Ohio.
It's estimated that 10%–30% of pregnant women are colonized with GBS, which is an established cause of neonatal morbidity and mortality. In 2002, the Centers for Disease Control and Prevention advised universal prenatal screening and the American College of Obstetricians and Gynecologists published a committee opinion outlining the collection of GBS cultures between 35 and 37 weeks' gestation (Obstet. Gynecol. 2002;100:1405–12). GBS cultures are to be obtained by a swab of the lower vagina (vaginal introitus) followed by the rectum (insert swab through anal sphincter). This collection method was based on a 1977 study suggesting that the GI tract was the primary site of GBS colonization. However, vaginal-anal cultures are uncomfortable for patients.
Dr. Trappe and her coinvestigators studied whether vaginal-perianal cultures were equally effective in identifying GBS and were more comfortable for patients. They included women in the study if they were at least 18 years old, were to undergo routine GBS culture, and spoke English, Spanish, or Somali. The researchers collected data from 200 patients. The average maternal age was 26 years, and the average gestational age was 35.9 weeks. In terms of ethnicity, half (49%) of patients where white, followed by black (23%), Hispanic (14%), Asian (1%), and other (13%). Although inclusion criteria were for 35–37 weeks' gestation, seven women outside of this range were enrolled – three at 34 weeks', three at 38 weeks', and one at 39 weeks' gestation.
The vaginal-perianal specimen was collected first, followed by the recommended vaginal-rectal specimen. Women were asked to rate pain on a 0–10 scale for each collection method. They also were asked if one method was more uncomfortable than the other. The overall agreement rate between the two collection methods was 96.5%. The sensitivity and specificity of the vaginal-perianal method were 91% and 99%.
“Patients also reported statistically greater pain and discomfort with the vaginal-rectal culture collection, with over two-thirds of patients reporting less discomfort with the vaginal-perianal method,” Dr. Trappe said at the meeting. More than half (60%) of women reported no pain with the vaginal-perianal method, compared with 18% with the vaginal-rectal method. “Patients indicated a preference for the vaginal-perianal collection method based on pain and discomfort surveys,” she noted.
From the Annual Meeting of the American College of Obstetricians and Gynecologists
Type 2, Gestational Diabetes Are Genetically Linked
WASHINGTON – Most of the gene variations identified thus far as risk factors for type 2 diabetes also appear to increase risk for gestational diabetes – a trend that reaffirms the importance of taking family histories in obstetrical practice, Dr. Alan R. Shuldiner said.
Hundreds of candidate genes for type 2 diabetes have been analyzed in association studies over the past several years, and more recently, whole genome approaches have identified close to 40 genes with variations that increase the risk of type 2 diabetes, he explained at the meeting. Moreover, “most of these genetic variants that have also been looked at in [studies of] gestational diabetes all seem to increase risk there as well.”
While the utility of genetic screening in obstetrics needs to be investigated, it's clear that “people who have a family history of type 2 diabetes are probably at increased risk for gestational diabetes,” he said in an interview.
“From a genetic point of view, recent research reaffirms the importance of clinicians asking about family history,” said Dr. Shuldiner, who directs the program in personalized medicine and chairs the division of endocrinology, diabetes, and nutrition at the University of Maryland, Baltimore.
“Until recently, we really didn't know [about this interface],” he said. “It was possible that the genetic factors contributing to gestational diabetes would be very different and distinct from those contributing to type 2 diabetes. So far, that appears not to be the case.”
Most recently, an analysis of more than 5,500 pregnant women participating in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study demonstrated that a common maternal variant of the TCF7L2 gene is associated with a higher risk of gestational diabetes, as defined by the new International Association of Diabetes and Pregnancy Study Groupshand thus a higher risk of adverse pregnancy outcomes, he told meeting participants.
The risk-conferring variants of the TCF7L2 gene appear to be associated with impaired beta-cell function rather than insulin resistance, he noted.
An earlier report on TCF7L2 polymorphisms and progression to diabetes from the Diabetes Prevention Program Research Group showed that patients with the TCF7L2 variant are at increased risk of developing diabetes but “may be superresponders to lifestyle interventions,” Dr. Shuldiner said.
It is findings like these that may, with further research, lead to future recommendations for genetic screening.
Growing evidence on the effects of mutations in the glucokinase (GCK) gene, which appear to account for approximately 5% of gestational diabetes cases in white mothers, may similarly drive screening efforts in the future, he said. (Glucokinase is an enzyme present in pancreatic beta cells required for proper glucose sensing and insulin secretion.)
In a small study conducted in the United Kingdom, maternal hyperglycemia due to a GCK mutation – with no GCK mutation in the fetus – has been shown to result in higher birth weights, while inheritance by the fetus of a paternal GCK mutation appears to result in significant reductions in birth weight. “Screening for GCK mutations could potentially be useful in guiding therapy so that the baby has a normal birth weight,” said Dr. Shuldiner, also John L. Whitehurst Professor of Medicine and professor of physiology. “The data so far suggest that if both mom and the fetus have a GCK mutation, you may want to forego treatment [with oral hypoglycemic agents or insulin], and even put mom on a high-carb diet, because the baby needs a high glucose level.”
Glucokinase mutations are also associated with maturity-onset diabetes of the young (MODY), which begins before the age of 25 and which we “now know is a heterogeneous group of disorders” resulting in mutations in any of at least eight different genes, he said.
In fact, many experts refer to MODY as being either “glucokinase diabetes” (resulting from mutations in the gene that encodes the glycolytic enzyme glucokinase) or “transcription factor diabetes” (resulting from mutations in genes that encode various transcription factors). Unlike GCK MODY, transcription factor MODY is characterized by hyperglycemia that progressively worsens and often requires treatment with oral hypoglycemic agents or insulin.
Dr. Shuldiner reported that he had no relevant financial disclosures.
WASHINGTON – Most of the gene variations identified thus far as risk factors for type 2 diabetes also appear to increase risk for gestational diabetes – a trend that reaffirms the importance of taking family histories in obstetrical practice, Dr. Alan R. Shuldiner said.
Hundreds of candidate genes for type 2 diabetes have been analyzed in association studies over the past several years, and more recently, whole genome approaches have identified close to 40 genes with variations that increase the risk of type 2 diabetes, he explained at the meeting. Moreover, “most of these genetic variants that have also been looked at in [studies of] gestational diabetes all seem to increase risk there as well.”
While the utility of genetic screening in obstetrics needs to be investigated, it's clear that “people who have a family history of type 2 diabetes are probably at increased risk for gestational diabetes,” he said in an interview.
“From a genetic point of view, recent research reaffirms the importance of clinicians asking about family history,” said Dr. Shuldiner, who directs the program in personalized medicine and chairs the division of endocrinology, diabetes, and nutrition at the University of Maryland, Baltimore.
“Until recently, we really didn't know [about this interface],” he said. “It was possible that the genetic factors contributing to gestational diabetes would be very different and distinct from those contributing to type 2 diabetes. So far, that appears not to be the case.”
Most recently, an analysis of more than 5,500 pregnant women participating in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study demonstrated that a common maternal variant of the TCF7L2 gene is associated with a higher risk of gestational diabetes, as defined by the new International Association of Diabetes and Pregnancy Study Groupshand thus a higher risk of adverse pregnancy outcomes, he told meeting participants.
The risk-conferring variants of the TCF7L2 gene appear to be associated with impaired beta-cell function rather than insulin resistance, he noted.
An earlier report on TCF7L2 polymorphisms and progression to diabetes from the Diabetes Prevention Program Research Group showed that patients with the TCF7L2 variant are at increased risk of developing diabetes but “may be superresponders to lifestyle interventions,” Dr. Shuldiner said.
It is findings like these that may, with further research, lead to future recommendations for genetic screening.
Growing evidence on the effects of mutations in the glucokinase (GCK) gene, which appear to account for approximately 5% of gestational diabetes cases in white mothers, may similarly drive screening efforts in the future, he said. (Glucokinase is an enzyme present in pancreatic beta cells required for proper glucose sensing and insulin secretion.)
In a small study conducted in the United Kingdom, maternal hyperglycemia due to a GCK mutation – with no GCK mutation in the fetus – has been shown to result in higher birth weights, while inheritance by the fetus of a paternal GCK mutation appears to result in significant reductions in birth weight. “Screening for GCK mutations could potentially be useful in guiding therapy so that the baby has a normal birth weight,” said Dr. Shuldiner, also John L. Whitehurst Professor of Medicine and professor of physiology. “The data so far suggest that if both mom and the fetus have a GCK mutation, you may want to forego treatment [with oral hypoglycemic agents or insulin], and even put mom on a high-carb diet, because the baby needs a high glucose level.”
Glucokinase mutations are also associated with maturity-onset diabetes of the young (MODY), which begins before the age of 25 and which we “now know is a heterogeneous group of disorders” resulting in mutations in any of at least eight different genes, he said.
In fact, many experts refer to MODY as being either “glucokinase diabetes” (resulting from mutations in the gene that encodes the glycolytic enzyme glucokinase) or “transcription factor diabetes” (resulting from mutations in genes that encode various transcription factors). Unlike GCK MODY, transcription factor MODY is characterized by hyperglycemia that progressively worsens and often requires treatment with oral hypoglycemic agents or insulin.
Dr. Shuldiner reported that he had no relevant financial disclosures.
WASHINGTON – Most of the gene variations identified thus far as risk factors for type 2 diabetes also appear to increase risk for gestational diabetes – a trend that reaffirms the importance of taking family histories in obstetrical practice, Dr. Alan R. Shuldiner said.
Hundreds of candidate genes for type 2 diabetes have been analyzed in association studies over the past several years, and more recently, whole genome approaches have identified close to 40 genes with variations that increase the risk of type 2 diabetes, he explained at the meeting. Moreover, “most of these genetic variants that have also been looked at in [studies of] gestational diabetes all seem to increase risk there as well.”
While the utility of genetic screening in obstetrics needs to be investigated, it's clear that “people who have a family history of type 2 diabetes are probably at increased risk for gestational diabetes,” he said in an interview.
“From a genetic point of view, recent research reaffirms the importance of clinicians asking about family history,” said Dr. Shuldiner, who directs the program in personalized medicine and chairs the division of endocrinology, diabetes, and nutrition at the University of Maryland, Baltimore.
“Until recently, we really didn't know [about this interface],” he said. “It was possible that the genetic factors contributing to gestational diabetes would be very different and distinct from those contributing to type 2 diabetes. So far, that appears not to be the case.”
Most recently, an analysis of more than 5,500 pregnant women participating in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study demonstrated that a common maternal variant of the TCF7L2 gene is associated with a higher risk of gestational diabetes, as defined by the new International Association of Diabetes and Pregnancy Study Groupshand thus a higher risk of adverse pregnancy outcomes, he told meeting participants.
The risk-conferring variants of the TCF7L2 gene appear to be associated with impaired beta-cell function rather than insulin resistance, he noted.
An earlier report on TCF7L2 polymorphisms and progression to diabetes from the Diabetes Prevention Program Research Group showed that patients with the TCF7L2 variant are at increased risk of developing diabetes but “may be superresponders to lifestyle interventions,” Dr. Shuldiner said.
It is findings like these that may, with further research, lead to future recommendations for genetic screening.
Growing evidence on the effects of mutations in the glucokinase (GCK) gene, which appear to account for approximately 5% of gestational diabetes cases in white mothers, may similarly drive screening efforts in the future, he said. (Glucokinase is an enzyme present in pancreatic beta cells required for proper glucose sensing and insulin secretion.)
In a small study conducted in the United Kingdom, maternal hyperglycemia due to a GCK mutation – with no GCK mutation in the fetus – has been shown to result in higher birth weights, while inheritance by the fetus of a paternal GCK mutation appears to result in significant reductions in birth weight. “Screening for GCK mutations could potentially be useful in guiding therapy so that the baby has a normal birth weight,” said Dr. Shuldiner, also John L. Whitehurst Professor of Medicine and professor of physiology. “The data so far suggest that if both mom and the fetus have a GCK mutation, you may want to forego treatment [with oral hypoglycemic agents or insulin], and even put mom on a high-carb diet, because the baby needs a high glucose level.”
Glucokinase mutations are also associated with maturity-onset diabetes of the young (MODY), which begins before the age of 25 and which we “now know is a heterogeneous group of disorders” resulting in mutations in any of at least eight different genes, he said.
In fact, many experts refer to MODY as being either “glucokinase diabetes” (resulting from mutations in the gene that encodes the glycolytic enzyme glucokinase) or “transcription factor diabetes” (resulting from mutations in genes that encode various transcription factors). Unlike GCK MODY, transcription factor MODY is characterized by hyperglycemia that progressively worsens and often requires treatment with oral hypoglycemic agents or insulin.
Dr. Shuldiner reported that he had no relevant financial disclosures.
Expert Analysis from the Annual Meeting of the Diabetes in Pregnancy Study Group of North America
Experts Debate Insulin Analogue Use in Pregnancy : While the analogues appear safe, there is reason to be cautious until more data are available.
WASHINGTON – Short-acting insulin analogues appear to be safe in insulin-requiring pregnancies and have clinical advantages, including increased freedom of meal timing, better matching of insulin dose with meal content, and improved glycemic control with a reduction in the frequency of hypoglyemic events, said Dr. Marshall W. Carpenter.
The analogues lispro (Humalog) and aspart (Novolog) may be advantageous, for instance, for the pregnant woman “with a toddler around who may not know when she is going to sit down to eat,” Dr. Carpenter said at the meeting.
“The benefit is reflected in the [higher, faster] peak insulin values seen with both lispro and aspart compared with human insulin,” said Dr. Carpenter of the department of obstetrics and gynecology at Tufts University, Boston.
Dr. Virginia R. Lupo, who chairs the department of obstetrics and gynecology at the Hennepin County Medical Center in Minneapolis, offered a different take on the utility of short-acting insulin analogues.
Diabetes disproportionately affects women who have annual household incomes below $25,000 and who are more likely to be black, Hispanic, American Indian, or Asian Pacific Islanders than white, she said. For many of these women, a regimen including short-acting insulin analogues is too complex for their lifestyle, eating habits, functional health literacy, and other life circumstances.
“A lot of my patients eat by grazing – there are no distinct meal times,” she said. And because of evening-long food availability and ingestion, these patients “require elevated basal insulin through the evenings.”
“I'm not convinced that insulin analogues are the right way to go,” she said. “I like the idea of NPH twice a day, before breakfast and before supper. It's better to take [insulin] twice a day than not take it five times a day.”
The utility of long-acting insulin analogues in pregnancy, Dr. Carpenter said, is still uncertain considering the lack of substantive real-life clinical experience with these analogues and the safety implications raised in the literature thus far. “And really,” he added in an interview after the meeting, “there's no evidence that glargine (Lantus) offers any benefit over NPH insulin – and NPH insulin is cheaper.”
Questions about the safety of analogues overall stem from the molecular modifications involved in their creation, and specifically the implications of modifying the C-terminal end of the insulin beta chain. Such modifications appear to increase affinity for the insulinlike growth factor–1 (IGF-1) receptor – a receptor that has “a broad array of effects,” from induction of mitogenesis and inhibition of apoptosis, to stimulation of angiogenesis, he said.
Analogues' increased “stimulation of [this receptor] has thus appropriately raised concerns about safety,” he said.
While the current safety profile of the short-acting analogues “suggests no independent effect on retinopathic change or carcinogenesis,” there is reason to be cautious about long-acting analogues until more data are available, he said.
A study published in 2000 comparing the toxicopharmacologic properties of insulin analogues showed that glargine had a six- to eightfold increase in IGF-1 receptor affinity and associated mitogenic potency compared with human insulin, he noted. The two rapidly acting insulin analogues resembled human insulin on all parameters, except for a slightly elevated IGF-1 receptor affinity for lispro (Diabetes 2000;49:999–1005).
A possible association of lispro with proliferative retinopathy was “put on the map” more than a decade ago when Dr. John L. Kitzmiller and his colleagues reported that 3 out of 10 lispro-treated patients with no detected background retinopathy progressed to proliferative retinopathy during pregnancy (Diabetes Care 1999;22:874–5), Dr. Carpenter said.
Studies and commentary since then have shown no adverse impact of insulin analogues on the progression of diabetic retinopathy in pregnant patients, he said. A Finnish study of 69 pregnant women treated with either lispro or conventional human insulin, for instance, showed no significant differences in retinopathy progression (Diabetes Care 2003;26:1193–8).
Experts have also noted that the hemaglobin A1c levels in women in Dr. Kitzmiller's series were initially high, indicative of poor prepregnancy metabolic control, which raises the question of whether the rapid change to euglycemic control may have been the primary contributor to the advancing retinopathy among these patients rather than a specific lispro effect.
Regardless of insulin choice, rapid tightening of glycemic control is among the predictors of proliferative diabetic retinopathy during pregnancy, along with the duration of diabetes, HbA1c or plasma glucose at the onset of care, and other factors, he said. “We really ought to have informed consent for the rapid achievement of normal blood sugars from a nonpregnant state to a pregnant state … for patients who are in denial before becoming pregnant, with very poor metabolic control, and who are then enlisted in very careful management to dramatically improve their glycemic control,” Dr. Carpenter said in an interview. “These are the patients we know are at risk of worsening retinopathy.”
Dr. Lupo and Dr. Carpenter said they had no relevant financial disclosures.
WASHINGTON – Short-acting insulin analogues appear to be safe in insulin-requiring pregnancies and have clinical advantages, including increased freedom of meal timing, better matching of insulin dose with meal content, and improved glycemic control with a reduction in the frequency of hypoglyemic events, said Dr. Marshall W. Carpenter.
The analogues lispro (Humalog) and aspart (Novolog) may be advantageous, for instance, for the pregnant woman “with a toddler around who may not know when she is going to sit down to eat,” Dr. Carpenter said at the meeting.
“The benefit is reflected in the [higher, faster] peak insulin values seen with both lispro and aspart compared with human insulin,” said Dr. Carpenter of the department of obstetrics and gynecology at Tufts University, Boston.
Dr. Virginia R. Lupo, who chairs the department of obstetrics and gynecology at the Hennepin County Medical Center in Minneapolis, offered a different take on the utility of short-acting insulin analogues.
Diabetes disproportionately affects women who have annual household incomes below $25,000 and who are more likely to be black, Hispanic, American Indian, or Asian Pacific Islanders than white, she said. For many of these women, a regimen including short-acting insulin analogues is too complex for their lifestyle, eating habits, functional health literacy, and other life circumstances.
“A lot of my patients eat by grazing – there are no distinct meal times,” she said. And because of evening-long food availability and ingestion, these patients “require elevated basal insulin through the evenings.”
“I'm not convinced that insulin analogues are the right way to go,” she said. “I like the idea of NPH twice a day, before breakfast and before supper. It's better to take [insulin] twice a day than not take it five times a day.”
The utility of long-acting insulin analogues in pregnancy, Dr. Carpenter said, is still uncertain considering the lack of substantive real-life clinical experience with these analogues and the safety implications raised in the literature thus far. “And really,” he added in an interview after the meeting, “there's no evidence that glargine (Lantus) offers any benefit over NPH insulin – and NPH insulin is cheaper.”
Questions about the safety of analogues overall stem from the molecular modifications involved in their creation, and specifically the implications of modifying the C-terminal end of the insulin beta chain. Such modifications appear to increase affinity for the insulinlike growth factor–1 (IGF-1) receptor – a receptor that has “a broad array of effects,” from induction of mitogenesis and inhibition of apoptosis, to stimulation of angiogenesis, he said.
Analogues' increased “stimulation of [this receptor] has thus appropriately raised concerns about safety,” he said.
While the current safety profile of the short-acting analogues “suggests no independent effect on retinopathic change or carcinogenesis,” there is reason to be cautious about long-acting analogues until more data are available, he said.
A study published in 2000 comparing the toxicopharmacologic properties of insulin analogues showed that glargine had a six- to eightfold increase in IGF-1 receptor affinity and associated mitogenic potency compared with human insulin, he noted. The two rapidly acting insulin analogues resembled human insulin on all parameters, except for a slightly elevated IGF-1 receptor affinity for lispro (Diabetes 2000;49:999–1005).
A possible association of lispro with proliferative retinopathy was “put on the map” more than a decade ago when Dr. John L. Kitzmiller and his colleagues reported that 3 out of 10 lispro-treated patients with no detected background retinopathy progressed to proliferative retinopathy during pregnancy (Diabetes Care 1999;22:874–5), Dr. Carpenter said.
Studies and commentary since then have shown no adverse impact of insulin analogues on the progression of diabetic retinopathy in pregnant patients, he said. A Finnish study of 69 pregnant women treated with either lispro or conventional human insulin, for instance, showed no significant differences in retinopathy progression (Diabetes Care 2003;26:1193–8).
Experts have also noted that the hemaglobin A1c levels in women in Dr. Kitzmiller's series were initially high, indicative of poor prepregnancy metabolic control, which raises the question of whether the rapid change to euglycemic control may have been the primary contributor to the advancing retinopathy among these patients rather than a specific lispro effect.
Regardless of insulin choice, rapid tightening of glycemic control is among the predictors of proliferative diabetic retinopathy during pregnancy, along with the duration of diabetes, HbA1c or plasma glucose at the onset of care, and other factors, he said. “We really ought to have informed consent for the rapid achievement of normal blood sugars from a nonpregnant state to a pregnant state … for patients who are in denial before becoming pregnant, with very poor metabolic control, and who are then enlisted in very careful management to dramatically improve their glycemic control,” Dr. Carpenter said in an interview. “These are the patients we know are at risk of worsening retinopathy.”
Dr. Lupo and Dr. Carpenter said they had no relevant financial disclosures.
WASHINGTON – Short-acting insulin analogues appear to be safe in insulin-requiring pregnancies and have clinical advantages, including increased freedom of meal timing, better matching of insulin dose with meal content, and improved glycemic control with a reduction in the frequency of hypoglyemic events, said Dr. Marshall W. Carpenter.
The analogues lispro (Humalog) and aspart (Novolog) may be advantageous, for instance, for the pregnant woman “with a toddler around who may not know when she is going to sit down to eat,” Dr. Carpenter said at the meeting.
“The benefit is reflected in the [higher, faster] peak insulin values seen with both lispro and aspart compared with human insulin,” said Dr. Carpenter of the department of obstetrics and gynecology at Tufts University, Boston.
Dr. Virginia R. Lupo, who chairs the department of obstetrics and gynecology at the Hennepin County Medical Center in Minneapolis, offered a different take on the utility of short-acting insulin analogues.
Diabetes disproportionately affects women who have annual household incomes below $25,000 and who are more likely to be black, Hispanic, American Indian, or Asian Pacific Islanders than white, she said. For many of these women, a regimen including short-acting insulin analogues is too complex for their lifestyle, eating habits, functional health literacy, and other life circumstances.
“A lot of my patients eat by grazing – there are no distinct meal times,” she said. And because of evening-long food availability and ingestion, these patients “require elevated basal insulin through the evenings.”
“I'm not convinced that insulin analogues are the right way to go,” she said. “I like the idea of NPH twice a day, before breakfast and before supper. It's better to take [insulin] twice a day than not take it five times a day.”
The utility of long-acting insulin analogues in pregnancy, Dr. Carpenter said, is still uncertain considering the lack of substantive real-life clinical experience with these analogues and the safety implications raised in the literature thus far. “And really,” he added in an interview after the meeting, “there's no evidence that glargine (Lantus) offers any benefit over NPH insulin – and NPH insulin is cheaper.”
Questions about the safety of analogues overall stem from the molecular modifications involved in their creation, and specifically the implications of modifying the C-terminal end of the insulin beta chain. Such modifications appear to increase affinity for the insulinlike growth factor–1 (IGF-1) receptor – a receptor that has “a broad array of effects,” from induction of mitogenesis and inhibition of apoptosis, to stimulation of angiogenesis, he said.
Analogues' increased “stimulation of [this receptor] has thus appropriately raised concerns about safety,” he said.
While the current safety profile of the short-acting analogues “suggests no independent effect on retinopathic change or carcinogenesis,” there is reason to be cautious about long-acting analogues until more data are available, he said.
A study published in 2000 comparing the toxicopharmacologic properties of insulin analogues showed that glargine had a six- to eightfold increase in IGF-1 receptor affinity and associated mitogenic potency compared with human insulin, he noted. The two rapidly acting insulin analogues resembled human insulin on all parameters, except for a slightly elevated IGF-1 receptor affinity for lispro (Diabetes 2000;49:999–1005).
A possible association of lispro with proliferative retinopathy was “put on the map” more than a decade ago when Dr. John L. Kitzmiller and his colleagues reported that 3 out of 10 lispro-treated patients with no detected background retinopathy progressed to proliferative retinopathy during pregnancy (Diabetes Care 1999;22:874–5), Dr. Carpenter said.
Studies and commentary since then have shown no adverse impact of insulin analogues on the progression of diabetic retinopathy in pregnant patients, he said. A Finnish study of 69 pregnant women treated with either lispro or conventional human insulin, for instance, showed no significant differences in retinopathy progression (Diabetes Care 2003;26:1193–8).
Experts have also noted that the hemaglobin A1c levels in women in Dr. Kitzmiller's series were initially high, indicative of poor prepregnancy metabolic control, which raises the question of whether the rapid change to euglycemic control may have been the primary contributor to the advancing retinopathy among these patients rather than a specific lispro effect.
Regardless of insulin choice, rapid tightening of glycemic control is among the predictors of proliferative diabetic retinopathy during pregnancy, along with the duration of diabetes, HbA1c or plasma glucose at the onset of care, and other factors, he said. “We really ought to have informed consent for the rapid achievement of normal blood sugars from a nonpregnant state to a pregnant state … for patients who are in denial before becoming pregnant, with very poor metabolic control, and who are then enlisted in very careful management to dramatically improve their glycemic control,” Dr. Carpenter said in an interview. “These are the patients we know are at risk of worsening retinopathy.”
Dr. Lupo and Dr. Carpenter said they had no relevant financial disclosures.
Expert Analysis from the Annual Meeting of the Diabetes in Pregnancy Study Group of North America
Pregnancy After Liver Transplant Raises Risk of Graft Loss
PHILADELPHIA – Women who become pregnant after receiving a transplanted liver face an elevated risk of graft rejection, especially during or immediately following the pregnancy, based on a review of 161 U.S. cases.
“The data suggest poorer outcomes for both mothers and their newborns in female liver recipients with risk factors for graft loss within 5 years post pregnancy,” Dr. Carlo B. Ramirez said at the meeting.
“The findings highlight the high-risk nature of this group, warranting closer follow-up of both mother and child,” said Dr. Ramirez, a transplant surgeon at Thomas Jefferson University, Philadelphia.
Of the 161 women who became pregnant following a liver transplant and were enrolled in the National Transplantation Pregnancy Registry (in place since 1991), 16 (10%) lost their graft within 5 years following their first posttransplant pregnancy. The pregnancy and the 3 months following pregnancy posed a particular risk, with half of the women who eventually lost their graft experiencing rejection during that time. In a multivariate model that took into account baseline risk factors, women with a liver transplant faced a 14-fold increased risk for graft loss during the pregnancy, Dr. Ramirez said.
“A lot of patients who have a stable equilibrium with their graft may destabilize under stress. It is possible that there is low-grade, clinically insignificant rejection in some of these patients prior to pregnancy” that then becomes exacerbated by the stress of pregnancy, commented Dr. Jean C. Emond, professor of surgery and director of transplantation at Columbia University in New York. Dr. Emond suggested that a liver biopsy prior to pregnancy might be warranted to assess the stability of the transplant.
Other risk factors for graft loss included younger age of the mother and low gestational age at the time of delivery. In the multivariate analysis, the risk for graft loss fell by a statistically significant 26% for each additional year of age for the mother. Graft loss fell by a statistically significant 5% for each additional week of gestational age when delivery occurred.
Among the 16 women who lost their graft during pregnancy or the following 5 years, their average age when they conceived was 22 years old, compared with an average age of 28 years among the 145 women who did not lose their graft. Average gestational age at delivery was 33 weeks among the women who lost their graft, and 37 weeks among the women who did not lose their graft.
The average age of the women at the time they received their liver transplant was 18 years among those who later lost their grafts, and 23 years among those who retained their grafts. However, the average time between transplantation and conception was an identical 4.3 years in both groups.
The only other risk factor for graft loss that approached statistical significance in the multivariate model was viral hepatitis as the etiologic agent for the liver failure that led to the transplants. Viral hepatitis was the cause of liver failure for six (38%) of the women who lost their grafts following pregnancy, and for 23 (16%) of the women who did not lose their grafts. In the multivariate model, viral hepatitis as the cause of liver failure was linked with a nearly fourfold increased risk for women losing their graft during or after pregnancy, but this relationship failed to meet the standard criterion for statistical significance, Dr. Ramirez said.
The congress was sponsored by the American Society of Transplant Surgeons. Dr. Ramirez said he had no disclosures. The National Transplantation Pregnancy Registry has been supported by grants from Novartis, Astellas, Genentech, Pfizer, Teva, and Sandoz.
PHILADELPHIA – Women who become pregnant after receiving a transplanted liver face an elevated risk of graft rejection, especially during or immediately following the pregnancy, based on a review of 161 U.S. cases.
“The data suggest poorer outcomes for both mothers and their newborns in female liver recipients with risk factors for graft loss within 5 years post pregnancy,” Dr. Carlo B. Ramirez said at the meeting.
“The findings highlight the high-risk nature of this group, warranting closer follow-up of both mother and child,” said Dr. Ramirez, a transplant surgeon at Thomas Jefferson University, Philadelphia.
Of the 161 women who became pregnant following a liver transplant and were enrolled in the National Transplantation Pregnancy Registry (in place since 1991), 16 (10%) lost their graft within 5 years following their first posttransplant pregnancy. The pregnancy and the 3 months following pregnancy posed a particular risk, with half of the women who eventually lost their graft experiencing rejection during that time. In a multivariate model that took into account baseline risk factors, women with a liver transplant faced a 14-fold increased risk for graft loss during the pregnancy, Dr. Ramirez said.
“A lot of patients who have a stable equilibrium with their graft may destabilize under stress. It is possible that there is low-grade, clinically insignificant rejection in some of these patients prior to pregnancy” that then becomes exacerbated by the stress of pregnancy, commented Dr. Jean C. Emond, professor of surgery and director of transplantation at Columbia University in New York. Dr. Emond suggested that a liver biopsy prior to pregnancy might be warranted to assess the stability of the transplant.
Other risk factors for graft loss included younger age of the mother and low gestational age at the time of delivery. In the multivariate analysis, the risk for graft loss fell by a statistically significant 26% for each additional year of age for the mother. Graft loss fell by a statistically significant 5% for each additional week of gestational age when delivery occurred.
Among the 16 women who lost their graft during pregnancy or the following 5 years, their average age when they conceived was 22 years old, compared with an average age of 28 years among the 145 women who did not lose their graft. Average gestational age at delivery was 33 weeks among the women who lost their graft, and 37 weeks among the women who did not lose their graft.
The average age of the women at the time they received their liver transplant was 18 years among those who later lost their grafts, and 23 years among those who retained their grafts. However, the average time between transplantation and conception was an identical 4.3 years in both groups.
The only other risk factor for graft loss that approached statistical significance in the multivariate model was viral hepatitis as the etiologic agent for the liver failure that led to the transplants. Viral hepatitis was the cause of liver failure for six (38%) of the women who lost their grafts following pregnancy, and for 23 (16%) of the women who did not lose their grafts. In the multivariate model, viral hepatitis as the cause of liver failure was linked with a nearly fourfold increased risk for women losing their graft during or after pregnancy, but this relationship failed to meet the standard criterion for statistical significance, Dr. Ramirez said.
The congress was sponsored by the American Society of Transplant Surgeons. Dr. Ramirez said he had no disclosures. The National Transplantation Pregnancy Registry has been supported by grants from Novartis, Astellas, Genentech, Pfizer, Teva, and Sandoz.
PHILADELPHIA – Women who become pregnant after receiving a transplanted liver face an elevated risk of graft rejection, especially during or immediately following the pregnancy, based on a review of 161 U.S. cases.
“The data suggest poorer outcomes for both mothers and their newborns in female liver recipients with risk factors for graft loss within 5 years post pregnancy,” Dr. Carlo B. Ramirez said at the meeting.
“The findings highlight the high-risk nature of this group, warranting closer follow-up of both mother and child,” said Dr. Ramirez, a transplant surgeon at Thomas Jefferson University, Philadelphia.
Of the 161 women who became pregnant following a liver transplant and were enrolled in the National Transplantation Pregnancy Registry (in place since 1991), 16 (10%) lost their graft within 5 years following their first posttransplant pregnancy. The pregnancy and the 3 months following pregnancy posed a particular risk, with half of the women who eventually lost their graft experiencing rejection during that time. In a multivariate model that took into account baseline risk factors, women with a liver transplant faced a 14-fold increased risk for graft loss during the pregnancy, Dr. Ramirez said.
“A lot of patients who have a stable equilibrium with their graft may destabilize under stress. It is possible that there is low-grade, clinically insignificant rejection in some of these patients prior to pregnancy” that then becomes exacerbated by the stress of pregnancy, commented Dr. Jean C. Emond, professor of surgery and director of transplantation at Columbia University in New York. Dr. Emond suggested that a liver biopsy prior to pregnancy might be warranted to assess the stability of the transplant.
Other risk factors for graft loss included younger age of the mother and low gestational age at the time of delivery. In the multivariate analysis, the risk for graft loss fell by a statistically significant 26% for each additional year of age for the mother. Graft loss fell by a statistically significant 5% for each additional week of gestational age when delivery occurred.
Among the 16 women who lost their graft during pregnancy or the following 5 years, their average age when they conceived was 22 years old, compared with an average age of 28 years among the 145 women who did not lose their graft. Average gestational age at delivery was 33 weeks among the women who lost their graft, and 37 weeks among the women who did not lose their graft.
The average age of the women at the time they received their liver transplant was 18 years among those who later lost their grafts, and 23 years among those who retained their grafts. However, the average time between transplantation and conception was an identical 4.3 years in both groups.
The only other risk factor for graft loss that approached statistical significance in the multivariate model was viral hepatitis as the etiologic agent for the liver failure that led to the transplants. Viral hepatitis was the cause of liver failure for six (38%) of the women who lost their grafts following pregnancy, and for 23 (16%) of the women who did not lose their grafts. In the multivariate model, viral hepatitis as the cause of liver failure was linked with a nearly fourfold increased risk for women losing their graft during or after pregnancy, but this relationship failed to meet the standard criterion for statistical significance, Dr. Ramirez said.
The congress was sponsored by the American Society of Transplant Surgeons. Dr. Ramirez said he had no disclosures. The National Transplantation Pregnancy Registry has been supported by grants from Novartis, Astellas, Genentech, Pfizer, Teva, and Sandoz.
From the American Transplant Congress
ACIP Recommends Prenatal Tdap Vaccine
ATLANTA – The Tdap vaccine should be given to pregnant women after 20 weeks' gestation to help prevent pertussis in the mothers and their newborns, the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices voted at its meeting.
The ACIP also voted that in special situations in which a pregnant women who has not previously received Tdap is in need of a tetanus booster (for wound management or if it's been more than 10 years since the previous Td), health care providers should administer Tdap during the third or late second trimester (after 20 weeks' gestation). However, the language may be revised to allow for earlier vaccination for urgent wound management in women prior to 20 weeks' gestation.
In addition, the ACIP recommended that pregnant women whose tetanus vaccination status is unknown or uncertain should received three vaccinations containing tetanus and reduced diphtheria toxoids during pregnancy. The recommended dosing schedule is 0, 4 weeks, and 6–12 months. Tdap should replace one dose of Td, preferably during the late second or third trimester.
As part of the same vote, the ACIP also voted to recommend “cocooning” (Tdap vaccination of adolescent and adult contacts of infants younger than 12 months) despite the lack of evidence for its effectiveness.
“The working group would never not recommend cocooning, but it is an insufficient national strategy to prevent pertussis morbidity and mortality for newborn infants,” noted Dr. Jennifer Liang of the CDC, who presented data on behalf of the ACIP pertussis vaccine working group. Cocooning has been recommended since 2005, but available data show poor uptake and no evidence that cocooning programs are sustainable.
The ACIP voted in favor of prenatal Tdap vaccination as preferable to postpartum vaccination when possible. “Postpartum vaccination is a suboptimal national strategy to prevent infant pertussis morbidity and mortality,” said Dr. Liang. “Vaccinating pregnant women during the late second or early third trimester is acceptably safe for both mother and fetus.”
Moving Tdap vaccination to the third trimester of pregnancy is the most cost effective of several options to protect pregnant women and newborns against pertussis, said Garrett R. Beeler Asay, Ph.D., also of the CDC. Using a simulated birth cohort model of approximately 4 million infants, the cost per quality-of-life-year saved was $414,442 for vaccination during pregnancy, compared to $1,174,143 for postpartum vaccination.
The ACIP also voted to include the recommendations in the Vaccines for Children program, to state that adolescents who are pregnant would receive Tdap in the same manner as adult pregnant women.
Neither Dr. Liang nor Dr. Beeler Asay had any relevant financial disclosures.
ATLANTA – The Tdap vaccine should be given to pregnant women after 20 weeks' gestation to help prevent pertussis in the mothers and their newborns, the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices voted at its meeting.
The ACIP also voted that in special situations in which a pregnant women who has not previously received Tdap is in need of a tetanus booster (for wound management or if it's been more than 10 years since the previous Td), health care providers should administer Tdap during the third or late second trimester (after 20 weeks' gestation). However, the language may be revised to allow for earlier vaccination for urgent wound management in women prior to 20 weeks' gestation.
In addition, the ACIP recommended that pregnant women whose tetanus vaccination status is unknown or uncertain should received three vaccinations containing tetanus and reduced diphtheria toxoids during pregnancy. The recommended dosing schedule is 0, 4 weeks, and 6–12 months. Tdap should replace one dose of Td, preferably during the late second or third trimester.
As part of the same vote, the ACIP also voted to recommend “cocooning” (Tdap vaccination of adolescent and adult contacts of infants younger than 12 months) despite the lack of evidence for its effectiveness.
“The working group would never not recommend cocooning, but it is an insufficient national strategy to prevent pertussis morbidity and mortality for newborn infants,” noted Dr. Jennifer Liang of the CDC, who presented data on behalf of the ACIP pertussis vaccine working group. Cocooning has been recommended since 2005, but available data show poor uptake and no evidence that cocooning programs are sustainable.
The ACIP voted in favor of prenatal Tdap vaccination as preferable to postpartum vaccination when possible. “Postpartum vaccination is a suboptimal national strategy to prevent infant pertussis morbidity and mortality,” said Dr. Liang. “Vaccinating pregnant women during the late second or early third trimester is acceptably safe for both mother and fetus.”
Moving Tdap vaccination to the third trimester of pregnancy is the most cost effective of several options to protect pregnant women and newborns against pertussis, said Garrett R. Beeler Asay, Ph.D., also of the CDC. Using a simulated birth cohort model of approximately 4 million infants, the cost per quality-of-life-year saved was $414,442 for vaccination during pregnancy, compared to $1,174,143 for postpartum vaccination.
The ACIP also voted to include the recommendations in the Vaccines for Children program, to state that adolescents who are pregnant would receive Tdap in the same manner as adult pregnant women.
Neither Dr. Liang nor Dr. Beeler Asay had any relevant financial disclosures.
ATLANTA – The Tdap vaccine should be given to pregnant women after 20 weeks' gestation to help prevent pertussis in the mothers and their newborns, the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices voted at its meeting.
The ACIP also voted that in special situations in which a pregnant women who has not previously received Tdap is in need of a tetanus booster (for wound management or if it's been more than 10 years since the previous Td), health care providers should administer Tdap during the third or late second trimester (after 20 weeks' gestation). However, the language may be revised to allow for earlier vaccination for urgent wound management in women prior to 20 weeks' gestation.
In addition, the ACIP recommended that pregnant women whose tetanus vaccination status is unknown or uncertain should received three vaccinations containing tetanus and reduced diphtheria toxoids during pregnancy. The recommended dosing schedule is 0, 4 weeks, and 6–12 months. Tdap should replace one dose of Td, preferably during the late second or third trimester.
As part of the same vote, the ACIP also voted to recommend “cocooning” (Tdap vaccination of adolescent and adult contacts of infants younger than 12 months) despite the lack of evidence for its effectiveness.
“The working group would never not recommend cocooning, but it is an insufficient national strategy to prevent pertussis morbidity and mortality for newborn infants,” noted Dr. Jennifer Liang of the CDC, who presented data on behalf of the ACIP pertussis vaccine working group. Cocooning has been recommended since 2005, but available data show poor uptake and no evidence that cocooning programs are sustainable.
The ACIP voted in favor of prenatal Tdap vaccination as preferable to postpartum vaccination when possible. “Postpartum vaccination is a suboptimal national strategy to prevent infant pertussis morbidity and mortality,” said Dr. Liang. “Vaccinating pregnant women during the late second or early third trimester is acceptably safe for both mother and fetus.”
Moving Tdap vaccination to the third trimester of pregnancy is the most cost effective of several options to protect pregnant women and newborns against pertussis, said Garrett R. Beeler Asay, Ph.D., also of the CDC. Using a simulated birth cohort model of approximately 4 million infants, the cost per quality-of-life-year saved was $414,442 for vaccination during pregnancy, compared to $1,174,143 for postpartum vaccination.
The ACIP also voted to include the recommendations in the Vaccines for Children program, to state that adolescents who are pregnant would receive Tdap in the same manner as adult pregnant women.
Neither Dr. Liang nor Dr. Beeler Asay had any relevant financial disclosures.
From the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices
Can cerclage prevent preterm birth in women who have a short cervix?
This meta-analysis by Berghella and colleagues adds to the debate about the role of cervical-length measurement in determining candidacy for cerclage in an effort to reduce the rate of preterm birth. The authors are clearly passionate about the prevention of preterm birth—as we all are—but the conclusions they reach must be questioned.
First, it is misleading to report these results as Level-1 evidence. A meta-analysis can never, strictly speaking, be Level-1 evidence, although it may be based on an analysis of Level-1 evidence.
Sound confusing? Let’s take, as an example, the study of the role of calcium supplementation in the prevention of preeclampsia. In JAMA, in 1996, a meta-analysis fairly conclusively demonstrated that calcium supplementation was effective in preventing preeclampsia (odds ratio, 0.38; 95% CI, 0.22–0.65).1 Yet, a subsequent large randomized trial failed to confirm the findings of this meta-analysis.2
The lesson here? Level-1 evidence consists of appropriately powered, large-scale, randomized clinical trials. To date, we lack such trials with respect to cervical-length measurement and indications for cerclage. In fact, two of the authors of this paper are “on the record” as saying this very thing.
A 2009 paper by Owen and coworkers demonstrated only that cerclage for a cervical length below 25 mm reduced previable birth and perinatal death, but did not prevent births before 35 weeks unless the cervical length was less than 15 mm—and that bit of information came from a secondary analysis of the data.3 In a follow-up study, Owen and coworkers concluded that cervical length did not predict preterm birth before 37, 35, or 28 weeks, whether or not cervical length was viewed as a continuum or was stratified.4 And in an earlier meta-analysis reported by Berghella and associates in 2005, the authors conclude that “…cerclage may reduce preterm birth, and a well-powered trial should be carried out on this group of patients.”5
We should continue to rely on clinical assessment and history to make cerclage decisions, a conclusion reached in a recent randomized, controlled trial.6
In the meantime, those of us who practice maternal-fetal medicine would be wise to stop spending time massaging the data (i.e., meta-analysis and secondary analyses) from trials that have already been performed and start spending time, effort, and money to conduct the well-powered trials that I (and Dr. Berghella and colleagues) believe that we need. This is not to say that cervical-length measurement is without value. We simply don’t yet have the strength of association to accurately determine what that value is—most certainly not in the form of a screening tool for low-risk populations.
—John T. Repke, MD
We want to hear from you! Tell us what you think.
1. Bucher HC, Guyatt GH, Cook RJ, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275(14):1113-1117.
2. Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69-76.
3. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
4. Owen J, Szychowski JM, Hankins G, et al. Does midtrimester cervical length ≥25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol. 2010;203(4):393.e1-5.
5. Berghella V, Obido AO, To MS, Rust OA, Althiusius SM. Cerclage for short cervix on ultrasound: meta-analysis of trials using individual patient level data. Obstet Gynecol. 2005;106(1):181-189.
6. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-6.
This meta-analysis by Berghella and colleagues adds to the debate about the role of cervical-length measurement in determining candidacy for cerclage in an effort to reduce the rate of preterm birth. The authors are clearly passionate about the prevention of preterm birth—as we all are—but the conclusions they reach must be questioned.
First, it is misleading to report these results as Level-1 evidence. A meta-analysis can never, strictly speaking, be Level-1 evidence, although it may be based on an analysis of Level-1 evidence.
Sound confusing? Let’s take, as an example, the study of the role of calcium supplementation in the prevention of preeclampsia. In JAMA, in 1996, a meta-analysis fairly conclusively demonstrated that calcium supplementation was effective in preventing preeclampsia (odds ratio, 0.38; 95% CI, 0.22–0.65).1 Yet, a subsequent large randomized trial failed to confirm the findings of this meta-analysis.2
The lesson here? Level-1 evidence consists of appropriately powered, large-scale, randomized clinical trials. To date, we lack such trials with respect to cervical-length measurement and indications for cerclage. In fact, two of the authors of this paper are “on the record” as saying this very thing.
A 2009 paper by Owen and coworkers demonstrated only that cerclage for a cervical length below 25 mm reduced previable birth and perinatal death, but did not prevent births before 35 weeks unless the cervical length was less than 15 mm—and that bit of information came from a secondary analysis of the data.3 In a follow-up study, Owen and coworkers concluded that cervical length did not predict preterm birth before 37, 35, or 28 weeks, whether or not cervical length was viewed as a continuum or was stratified.4 And in an earlier meta-analysis reported by Berghella and associates in 2005, the authors conclude that “…cerclage may reduce preterm birth, and a well-powered trial should be carried out on this group of patients.”5
We should continue to rely on clinical assessment and history to make cerclage decisions, a conclusion reached in a recent randomized, controlled trial.6
In the meantime, those of us who practice maternal-fetal medicine would be wise to stop spending time massaging the data (i.e., meta-analysis and secondary analyses) from trials that have already been performed and start spending time, effort, and money to conduct the well-powered trials that I (and Dr. Berghella and colleagues) believe that we need. This is not to say that cervical-length measurement is without value. We simply don’t yet have the strength of association to accurately determine what that value is—most certainly not in the form of a screening tool for low-risk populations.
—John T. Repke, MD
We want to hear from you! Tell us what you think.
This meta-analysis by Berghella and colleagues adds to the debate about the role of cervical-length measurement in determining candidacy for cerclage in an effort to reduce the rate of preterm birth. The authors are clearly passionate about the prevention of preterm birth—as we all are—but the conclusions they reach must be questioned.
First, it is misleading to report these results as Level-1 evidence. A meta-analysis can never, strictly speaking, be Level-1 evidence, although it may be based on an analysis of Level-1 evidence.
Sound confusing? Let’s take, as an example, the study of the role of calcium supplementation in the prevention of preeclampsia. In JAMA, in 1996, a meta-analysis fairly conclusively demonstrated that calcium supplementation was effective in preventing preeclampsia (odds ratio, 0.38; 95% CI, 0.22–0.65).1 Yet, a subsequent large randomized trial failed to confirm the findings of this meta-analysis.2
The lesson here? Level-1 evidence consists of appropriately powered, large-scale, randomized clinical trials. To date, we lack such trials with respect to cervical-length measurement and indications for cerclage. In fact, two of the authors of this paper are “on the record” as saying this very thing.
A 2009 paper by Owen and coworkers demonstrated only that cerclage for a cervical length below 25 mm reduced previable birth and perinatal death, but did not prevent births before 35 weeks unless the cervical length was less than 15 mm—and that bit of information came from a secondary analysis of the data.3 In a follow-up study, Owen and coworkers concluded that cervical length did not predict preterm birth before 37, 35, or 28 weeks, whether or not cervical length was viewed as a continuum or was stratified.4 And in an earlier meta-analysis reported by Berghella and associates in 2005, the authors conclude that “…cerclage may reduce preterm birth, and a well-powered trial should be carried out on this group of patients.”5
We should continue to rely on clinical assessment and history to make cerclage decisions, a conclusion reached in a recent randomized, controlled trial.6
In the meantime, those of us who practice maternal-fetal medicine would be wise to stop spending time massaging the data (i.e., meta-analysis and secondary analyses) from trials that have already been performed and start spending time, effort, and money to conduct the well-powered trials that I (and Dr. Berghella and colleagues) believe that we need. This is not to say that cervical-length measurement is without value. We simply don’t yet have the strength of association to accurately determine what that value is—most certainly not in the form of a screening tool for low-risk populations.
—John T. Repke, MD
We want to hear from you! Tell us what you think.
1. Bucher HC, Guyatt GH, Cook RJ, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275(14):1113-1117.
2. Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69-76.
3. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
4. Owen J, Szychowski JM, Hankins G, et al. Does midtrimester cervical length ≥25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol. 2010;203(4):393.e1-5.
5. Berghella V, Obido AO, To MS, Rust OA, Althiusius SM. Cerclage for short cervix on ultrasound: meta-analysis of trials using individual patient level data. Obstet Gynecol. 2005;106(1):181-189.
6. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-6.
1. Bucher HC, Guyatt GH, Cook RJ, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275(14):1113-1117.
2. Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69-76.
3. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
4. Owen J, Szychowski JM, Hankins G, et al. Does midtrimester cervical length ≥25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol. 2010;203(4):393.e1-5.
5. Berghella V, Obido AO, To MS, Rust OA, Althiusius SM. Cerclage for short cervix on ultrasound: meta-analysis of trials using individual patient level data. Obstet Gynecol. 2005;106(1):181-189.
6. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-6.
