User login
Cervical Length Measurement Predicts Preterm Delivery
Major Finding: Measurements of vaginal cervical length using a disposable probe were similar to fetal fibronectin tests in predicting the likelihood of preterm delivery in women with threatened preterm labor.
Data Source: Study of 52 women with threatened preterm labor.
Disclosures: One of Dr. Burwick's associates in the study, Dr. Michael Ross, is medical director of CerviLenz Inc., the company that makes the device, and provided it free to the study. The investigators reported having no other conflicts of interest.
SAN FRANCISCO — A disposable probe that measures vaginal cervical length during speculum examination appeared to be similar in efficacy to fetal fibronectin testing for predicting the likelihood of preterm delivery, in a study of 52 at-risk women.
A cervical length less than 30 mm as measured by the CerviLenz probe correlated with fetal fibronectin positivity and with preterm birth within 7 days, Dr. Richard M. Burwick reported at the meeting.
The sensitivity for preterm delivery within 7 days was 67% with either a CerviLenz measurement of less than 30 mm or fetal fibronectin positivity. The specificity was 83% with the CerviLenz probe and 78% with fetal fibronectin, reported Dr. Burwick, who led the study at Harbor-UCLA Medical Center before moving to Brigham and Women's Hospital, Boston.
The positive predictive value for preterm delivery within 7 days was 22% with a CerviLenz measurement of less than 30 mm and 17% with fetal fibronectin positivity, and the negative predictive value was 97% in each group.
Both measures were less accurate in predicting delivery prior to 37 weeks' gestation. Sensitivity was 29% with a CerviLenz measurement of vaginal cervical length less than 30 mm and 40% with fetal fibronectin positivity. Specificity was 81% and 80%, respectively. The positive predictive value for delivery before 37 weeks was 22% with the CerviLenz measure and 33% with fetal fibronectin results, and the negative predictive value was 85% and 84%, respectively.
“Symptomatic women with a CerviLenz cervical length of less than 30 mm should undergo further observation and consideration of tocolytic and maternal glucocorticoid therapy,” Dr. Burwick said.
Immediate and quantifiable measures of cervical length using the CerviLenz probe may be less variable than the most common way of measuring—by digital exam—and speedier than waiting for fetal fibronectin results, he suggested. The CerviLenz probe also can be used after intercourse or bleeding, he added.
In a previous study by some of the same investigators in the current study, the CerviLenz probe had good predictive value compared with transvaginal ultrasound in identifying women with a cervix shorter than 30 mm (J. Reprod. Med. 2007;52:385-9).
The study enrolled women with singletons at 24-34 weeks' gestational age who were at risk for preterm delivery, with uterine contractions, less than 3 cm cervical dilation, intact membranes, and no vaginal bleeding or recent intercourse. During a speculum examination, fetal fibronectin was collected, cervical length was measured by the CerviLenz probe, and cervical cultures were taken in most patients.
Fetal fibronectin tests in 49 patients were positive in 12 (24%), and CerviLenz measurements were less than 30 mm in 9 of 43 patients (21%). The mean cervical length measurement was 34 mm. The cohort was predominantly Hispanic (38 patients), and 15 patients had a prior preterm birth.
Previous data have shown that only 21%-27% of women with symptoms of preterm labor will have preterm births. Cervical length and cervical effacement help assess preterm birth risk in symptomatic women.
In asymptomatic women, cervical length shorter than 25 mm has been linked to a sixfold increase in risk for preterm birth.
Sensitivity for preterm delivery within 7 days was 67% with either the CerviLenz probe or fetal fibronectin.
Source DR. BURWICK
Major Finding: Measurements of vaginal cervical length using a disposable probe were similar to fetal fibronectin tests in predicting the likelihood of preterm delivery in women with threatened preterm labor.
Data Source: Study of 52 women with threatened preterm labor.
Disclosures: One of Dr. Burwick's associates in the study, Dr. Michael Ross, is medical director of CerviLenz Inc., the company that makes the device, and provided it free to the study. The investigators reported having no other conflicts of interest.
SAN FRANCISCO — A disposable probe that measures vaginal cervical length during speculum examination appeared to be similar in efficacy to fetal fibronectin testing for predicting the likelihood of preterm delivery, in a study of 52 at-risk women.
A cervical length less than 30 mm as measured by the CerviLenz probe correlated with fetal fibronectin positivity and with preterm birth within 7 days, Dr. Richard M. Burwick reported at the meeting.
The sensitivity for preterm delivery within 7 days was 67% with either a CerviLenz measurement of less than 30 mm or fetal fibronectin positivity. The specificity was 83% with the CerviLenz probe and 78% with fetal fibronectin, reported Dr. Burwick, who led the study at Harbor-UCLA Medical Center before moving to Brigham and Women's Hospital, Boston.
The positive predictive value for preterm delivery within 7 days was 22% with a CerviLenz measurement of less than 30 mm and 17% with fetal fibronectin positivity, and the negative predictive value was 97% in each group.
Both measures were less accurate in predicting delivery prior to 37 weeks' gestation. Sensitivity was 29% with a CerviLenz measurement of vaginal cervical length less than 30 mm and 40% with fetal fibronectin positivity. Specificity was 81% and 80%, respectively. The positive predictive value for delivery before 37 weeks was 22% with the CerviLenz measure and 33% with fetal fibronectin results, and the negative predictive value was 85% and 84%, respectively.
“Symptomatic women with a CerviLenz cervical length of less than 30 mm should undergo further observation and consideration of tocolytic and maternal glucocorticoid therapy,” Dr. Burwick said.
Immediate and quantifiable measures of cervical length using the CerviLenz probe may be less variable than the most common way of measuring—by digital exam—and speedier than waiting for fetal fibronectin results, he suggested. The CerviLenz probe also can be used after intercourse or bleeding, he added.
In a previous study by some of the same investigators in the current study, the CerviLenz probe had good predictive value compared with transvaginal ultrasound in identifying women with a cervix shorter than 30 mm (J. Reprod. Med. 2007;52:385-9).
The study enrolled women with singletons at 24-34 weeks' gestational age who were at risk for preterm delivery, with uterine contractions, less than 3 cm cervical dilation, intact membranes, and no vaginal bleeding or recent intercourse. During a speculum examination, fetal fibronectin was collected, cervical length was measured by the CerviLenz probe, and cervical cultures were taken in most patients.
Fetal fibronectin tests in 49 patients were positive in 12 (24%), and CerviLenz measurements were less than 30 mm in 9 of 43 patients (21%). The mean cervical length measurement was 34 mm. The cohort was predominantly Hispanic (38 patients), and 15 patients had a prior preterm birth.
Previous data have shown that only 21%-27% of women with symptoms of preterm labor will have preterm births. Cervical length and cervical effacement help assess preterm birth risk in symptomatic women.
In asymptomatic women, cervical length shorter than 25 mm has been linked to a sixfold increase in risk for preterm birth.
Sensitivity for preterm delivery within 7 days was 67% with either the CerviLenz probe or fetal fibronectin.
Source DR. BURWICK
Major Finding: Measurements of vaginal cervical length using a disposable probe were similar to fetal fibronectin tests in predicting the likelihood of preterm delivery in women with threatened preterm labor.
Data Source: Study of 52 women with threatened preterm labor.
Disclosures: One of Dr. Burwick's associates in the study, Dr. Michael Ross, is medical director of CerviLenz Inc., the company that makes the device, and provided it free to the study. The investigators reported having no other conflicts of interest.
SAN FRANCISCO — A disposable probe that measures vaginal cervical length during speculum examination appeared to be similar in efficacy to fetal fibronectin testing for predicting the likelihood of preterm delivery, in a study of 52 at-risk women.
A cervical length less than 30 mm as measured by the CerviLenz probe correlated with fetal fibronectin positivity and with preterm birth within 7 days, Dr. Richard M. Burwick reported at the meeting.
The sensitivity for preterm delivery within 7 days was 67% with either a CerviLenz measurement of less than 30 mm or fetal fibronectin positivity. The specificity was 83% with the CerviLenz probe and 78% with fetal fibronectin, reported Dr. Burwick, who led the study at Harbor-UCLA Medical Center before moving to Brigham and Women's Hospital, Boston.
The positive predictive value for preterm delivery within 7 days was 22% with a CerviLenz measurement of less than 30 mm and 17% with fetal fibronectin positivity, and the negative predictive value was 97% in each group.
Both measures were less accurate in predicting delivery prior to 37 weeks' gestation. Sensitivity was 29% with a CerviLenz measurement of vaginal cervical length less than 30 mm and 40% with fetal fibronectin positivity. Specificity was 81% and 80%, respectively. The positive predictive value for delivery before 37 weeks was 22% with the CerviLenz measure and 33% with fetal fibronectin results, and the negative predictive value was 85% and 84%, respectively.
“Symptomatic women with a CerviLenz cervical length of less than 30 mm should undergo further observation and consideration of tocolytic and maternal glucocorticoid therapy,” Dr. Burwick said.
Immediate and quantifiable measures of cervical length using the CerviLenz probe may be less variable than the most common way of measuring—by digital exam—and speedier than waiting for fetal fibronectin results, he suggested. The CerviLenz probe also can be used after intercourse or bleeding, he added.
In a previous study by some of the same investigators in the current study, the CerviLenz probe had good predictive value compared with transvaginal ultrasound in identifying women with a cervix shorter than 30 mm (J. Reprod. Med. 2007;52:385-9).
The study enrolled women with singletons at 24-34 weeks' gestational age who were at risk for preterm delivery, with uterine contractions, less than 3 cm cervical dilation, intact membranes, and no vaginal bleeding or recent intercourse. During a speculum examination, fetal fibronectin was collected, cervical length was measured by the CerviLenz probe, and cervical cultures were taken in most patients.
Fetal fibronectin tests in 49 patients were positive in 12 (24%), and CerviLenz measurements were less than 30 mm in 9 of 43 patients (21%). The mean cervical length measurement was 34 mm. The cohort was predominantly Hispanic (38 patients), and 15 patients had a prior preterm birth.
Previous data have shown that only 21%-27% of women with symptoms of preterm labor will have preterm births. Cervical length and cervical effacement help assess preterm birth risk in symptomatic women.
In asymptomatic women, cervical length shorter than 25 mm has been linked to a sixfold increase in risk for preterm birth.
Sensitivity for preterm delivery within 7 days was 67% with either the CerviLenz probe or fetal fibronectin.
Source DR. BURWICK
From the Annual Meeting of the American College of Obstetricians and Gynecologists
Use of Form Improves Dystocia Documentation
Major Finding: Before use of the form, 39% of physicians documented estimated fetal weight, compared with 84% after adoption. The rate of documenting the head-to-shoulder delivery interval increased from 15% in the earlier time period to 77% after adoption of the form.
Data Source: Comparison of records for 100 deliveries with shoulder dystocia before use of the standardized form and 80 deliveries with shoulder dystocia afterward at one institution.
Disclosures: None was reported.
SAN FRANCISCO — Adoption of a standardized form for use during deliveries involving shoulder dystocia significantly improved documentation of some information at one institution, according to Dr. Vasiliki A. Moragianni.
A comparison of charts for 100 patients with deliveries involving shoulder dystocia before implementation of the standardized form in August 2003 and charts for 80 patients after adoption of the form found significant increases in the proportion of charts documenting the head-to-shoulder delivery interval and the estimated fetal weight.
Better documentation of shoulder dystocia cases could reduce legal risk, said Dr. Moragianni, whose presentation earned a second-place prize for clinical investigation at the meeting.
Before use of the form, 39 of 100 physicians (39%) documented estimated fetal weight, compared with 67 of 80 physicians (84%) after adoption of the form. The rate of documenting the head-to-shoulder delivery interval increased from 15 physicians (15%) in the earlier time period to 62 of 80 (77%) after adoption of the form.
The results show that adding a standardized form for shoulder dystocia cases improves documentation beyond what's recorded in standardized delivery forms already in use, she said. The standardized form for shoulder dystocia improved documentation of an item already included in the delivery form (estimated fetal weight) and an item not previously included (head-to-shoulder delivery interval), reported Dr. Moragianni of Beth Israel Deaconess Medical Center, Boston.
The investigators selected charts from the 2% of deliveries that involve shoulder dystocia at Abington (Pa.) Memorial Hospital, where Dr. Moragianni completed her residency training. The new form included documentation of estimated fetal weight, umbilical cord pH, the maneuvers used in managing shoulder dystocia, the head-to-shoulder delivery interval, the duration of the second stage of labor, and estimated blood loss.
Except for the head-to-shoulder delivery interval and estimated fetal weight, documentation did not change significantly between the two time periods.
Documentation of maneuvers used for shoulder dystocia included the type and the order of maneuvers tried, and adjuncts to maneuvers, but not the exact timing of maneuvers. The form did include records of delivery times for the head and shoulders.
The study's findings may be limited by the small number of cases, the retrospective design, and the concurrent “drills” for managing shoulder dystocia that were held at the hospital, she said.
The form might be improved by adding documentation of the team members involved in managing the case of shoulder dystocia and their training levels, the fetal head position (whether the presenting shoulder was anterior or posterior), and the condition of the infant's arms immediately following surgery, Dr. Moragianni added.
“Every obstetrician in the hospital is completing the form,” she noted.
Shoulder dystocia was the third-leading cause of obstetric litigation in one analysis, accounting for 14% of obstetric lawsuits. Poor documentation drove 54% of these cases, which resulted in an average payout of $429,000 (Obstet. Gynecol. 2008;112:1279-83), she noted.
Shoulder dystocia was the third-leading causeof obstetric litigation in one analysis.
Source DR. MORAGIANNI
Major Finding: Before use of the form, 39% of physicians documented estimated fetal weight, compared with 84% after adoption. The rate of documenting the head-to-shoulder delivery interval increased from 15% in the earlier time period to 77% after adoption of the form.
Data Source: Comparison of records for 100 deliveries with shoulder dystocia before use of the standardized form and 80 deliveries with shoulder dystocia afterward at one institution.
Disclosures: None was reported.
SAN FRANCISCO — Adoption of a standardized form for use during deliveries involving shoulder dystocia significantly improved documentation of some information at one institution, according to Dr. Vasiliki A. Moragianni.
A comparison of charts for 100 patients with deliveries involving shoulder dystocia before implementation of the standardized form in August 2003 and charts for 80 patients after adoption of the form found significant increases in the proportion of charts documenting the head-to-shoulder delivery interval and the estimated fetal weight.
Better documentation of shoulder dystocia cases could reduce legal risk, said Dr. Moragianni, whose presentation earned a second-place prize for clinical investigation at the meeting.
Before use of the form, 39 of 100 physicians (39%) documented estimated fetal weight, compared with 67 of 80 physicians (84%) after adoption of the form. The rate of documenting the head-to-shoulder delivery interval increased from 15 physicians (15%) in the earlier time period to 62 of 80 (77%) after adoption of the form.
The results show that adding a standardized form for shoulder dystocia cases improves documentation beyond what's recorded in standardized delivery forms already in use, she said. The standardized form for shoulder dystocia improved documentation of an item already included in the delivery form (estimated fetal weight) and an item not previously included (head-to-shoulder delivery interval), reported Dr. Moragianni of Beth Israel Deaconess Medical Center, Boston.
The investigators selected charts from the 2% of deliveries that involve shoulder dystocia at Abington (Pa.) Memorial Hospital, where Dr. Moragianni completed her residency training. The new form included documentation of estimated fetal weight, umbilical cord pH, the maneuvers used in managing shoulder dystocia, the head-to-shoulder delivery interval, the duration of the second stage of labor, and estimated blood loss.
Except for the head-to-shoulder delivery interval and estimated fetal weight, documentation did not change significantly between the two time periods.
Documentation of maneuvers used for shoulder dystocia included the type and the order of maneuvers tried, and adjuncts to maneuvers, but not the exact timing of maneuvers. The form did include records of delivery times for the head and shoulders.
The study's findings may be limited by the small number of cases, the retrospective design, and the concurrent “drills” for managing shoulder dystocia that were held at the hospital, she said.
The form might be improved by adding documentation of the team members involved in managing the case of shoulder dystocia and their training levels, the fetal head position (whether the presenting shoulder was anterior or posterior), and the condition of the infant's arms immediately following surgery, Dr. Moragianni added.
“Every obstetrician in the hospital is completing the form,” she noted.
Shoulder dystocia was the third-leading cause of obstetric litigation in one analysis, accounting for 14% of obstetric lawsuits. Poor documentation drove 54% of these cases, which resulted in an average payout of $429,000 (Obstet. Gynecol. 2008;112:1279-83), she noted.
Shoulder dystocia was the third-leading causeof obstetric litigation in one analysis.
Source DR. MORAGIANNI
Major Finding: Before use of the form, 39% of physicians documented estimated fetal weight, compared with 84% after adoption. The rate of documenting the head-to-shoulder delivery interval increased from 15% in the earlier time period to 77% after adoption of the form.
Data Source: Comparison of records for 100 deliveries with shoulder dystocia before use of the standardized form and 80 deliveries with shoulder dystocia afterward at one institution.
Disclosures: None was reported.
SAN FRANCISCO — Adoption of a standardized form for use during deliveries involving shoulder dystocia significantly improved documentation of some information at one institution, according to Dr. Vasiliki A. Moragianni.
A comparison of charts for 100 patients with deliveries involving shoulder dystocia before implementation of the standardized form in August 2003 and charts for 80 patients after adoption of the form found significant increases in the proportion of charts documenting the head-to-shoulder delivery interval and the estimated fetal weight.
Better documentation of shoulder dystocia cases could reduce legal risk, said Dr. Moragianni, whose presentation earned a second-place prize for clinical investigation at the meeting.
Before use of the form, 39 of 100 physicians (39%) documented estimated fetal weight, compared with 67 of 80 physicians (84%) after adoption of the form. The rate of documenting the head-to-shoulder delivery interval increased from 15 physicians (15%) in the earlier time period to 62 of 80 (77%) after adoption of the form.
The results show that adding a standardized form for shoulder dystocia cases improves documentation beyond what's recorded in standardized delivery forms already in use, she said. The standardized form for shoulder dystocia improved documentation of an item already included in the delivery form (estimated fetal weight) and an item not previously included (head-to-shoulder delivery interval), reported Dr. Moragianni of Beth Israel Deaconess Medical Center, Boston.
The investigators selected charts from the 2% of deliveries that involve shoulder dystocia at Abington (Pa.) Memorial Hospital, where Dr. Moragianni completed her residency training. The new form included documentation of estimated fetal weight, umbilical cord pH, the maneuvers used in managing shoulder dystocia, the head-to-shoulder delivery interval, the duration of the second stage of labor, and estimated blood loss.
Except for the head-to-shoulder delivery interval and estimated fetal weight, documentation did not change significantly between the two time periods.
Documentation of maneuvers used for shoulder dystocia included the type and the order of maneuvers tried, and adjuncts to maneuvers, but not the exact timing of maneuvers. The form did include records of delivery times for the head and shoulders.
The study's findings may be limited by the small number of cases, the retrospective design, and the concurrent “drills” for managing shoulder dystocia that were held at the hospital, she said.
The form might be improved by adding documentation of the team members involved in managing the case of shoulder dystocia and their training levels, the fetal head position (whether the presenting shoulder was anterior or posterior), and the condition of the infant's arms immediately following surgery, Dr. Moragianni added.
“Every obstetrician in the hospital is completing the form,” she noted.
Shoulder dystocia was the third-leading cause of obstetric litigation in one analysis, accounting for 14% of obstetric lawsuits. Poor documentation drove 54% of these cases, which resulted in an average payout of $429,000 (Obstet. Gynecol. 2008;112:1279-83), she noted.
Shoulder dystocia was the third-leading causeof obstetric litigation in one analysis.
Source DR. MORAGIANNI
From the Annual Meeting of the American College of Obstetricians and Gynecologists
Screening Tool Predicted Pain After C-Section
Major Finding: A 1-minute, 3-question screening tool predicted individuals in the top 20th percentile of pain scores, with a sensitivity of 67% and a specificity of 72%.
Data Source: Study of 192 women who underwent elective C-sections with spinal morphine.
Disclosures: None was reported.
SAN ANTONIO — A 1-minute, three-question screening tool accurately predicted post–cesarean section pain in a study of 192 women who underwent elective cesarean sections with spinal morphine.
The quick, simple screen was nearly as sensitive and specific in predicting pain after delivery as was a 120-minute battery of tests used in a previous study (Anesthesiology 2006;104:417-25).
“We were looking for a simple, clinically useful way to identify patients at high risk for severe acute pain and subsequent complications who might benefit from earlier intervention,” said Dr. Ashley M. Tonidandel of Wake Forest University, Winston-Salem, N.C.
The three screening questions that were used in the investigation addressed the three strongest independent predictors of postcesarean pain and narcotic usage identified in the study published in Anesthesiology and in another study (Clin. J. Pain 2009;25:455-60).
Those predictors were anticipated pain levelafollowing surgery (on a scale of 0-100), anxiety regarding the upcoming surgery (1-100), and estimated pain medication usage (much less to much more than average).
The study participants had a mean age of 30 years and a mean body mass index of 34 kg/m
At 24 hours following surgery, the mean evoked pain score was 44 (out of 100). The patients used an average of 19 morphine equivalents over 24 hours. More than half of the participants (54%) received a total of 200 mcg of spinal morphine, according to Dr. Tonidandel.
Anxiety, anticipated pain, and expected medication usage were all significantly related to evoked pain and 24-hour morphine equivalents, with high scores on any two of the three items significantly predicting high pain scores.
Overall, the model accounted for 22% of the variance in predicted pain scores, which is “pretty good considering the 22%-28% of variance from the 120-minute battery of questions,” Dr. Tonidandel commented at the meeting.
Using a regression equation, the screening tool predicted individuals in the top 20th percentile of pain scores with a sensitivity of 67% and a specificity of 72%. With the 120-minute screen, those values were 70% and 76%, respectively, she said.
Identifying women at high risk for severe postpartum pain is especially important because previous studies have shown that women who have it are in turn at greater risk for the development of chronic pain (Pain 2008;140:87-94).
In the future, adding genetic information to the model might further increase its accuracy, although “our aim was to simplify,” she said.
Major Finding: A 1-minute, 3-question screening tool predicted individuals in the top 20th percentile of pain scores, with a sensitivity of 67% and a specificity of 72%.
Data Source: Study of 192 women who underwent elective C-sections with spinal morphine.
Disclosures: None was reported.
SAN ANTONIO — A 1-minute, three-question screening tool accurately predicted post–cesarean section pain in a study of 192 women who underwent elective cesarean sections with spinal morphine.
The quick, simple screen was nearly as sensitive and specific in predicting pain after delivery as was a 120-minute battery of tests used in a previous study (Anesthesiology 2006;104:417-25).
“We were looking for a simple, clinically useful way to identify patients at high risk for severe acute pain and subsequent complications who might benefit from earlier intervention,” said Dr. Ashley M. Tonidandel of Wake Forest University, Winston-Salem, N.C.
The three screening questions that were used in the investigation addressed the three strongest independent predictors of postcesarean pain and narcotic usage identified in the study published in Anesthesiology and in another study (Clin. J. Pain 2009;25:455-60).
Those predictors were anticipated pain levelafollowing surgery (on a scale of 0-100), anxiety regarding the upcoming surgery (1-100), and estimated pain medication usage (much less to much more than average).
The study participants had a mean age of 30 years and a mean body mass index of 34 kg/m
At 24 hours following surgery, the mean evoked pain score was 44 (out of 100). The patients used an average of 19 morphine equivalents over 24 hours. More than half of the participants (54%) received a total of 200 mcg of spinal morphine, according to Dr. Tonidandel.
Anxiety, anticipated pain, and expected medication usage were all significantly related to evoked pain and 24-hour morphine equivalents, with high scores on any two of the three items significantly predicting high pain scores.
Overall, the model accounted for 22% of the variance in predicted pain scores, which is “pretty good considering the 22%-28% of variance from the 120-minute battery of questions,” Dr. Tonidandel commented at the meeting.
Using a regression equation, the screening tool predicted individuals in the top 20th percentile of pain scores with a sensitivity of 67% and a specificity of 72%. With the 120-minute screen, those values were 70% and 76%, respectively, she said.
Identifying women at high risk for severe postpartum pain is especially important because previous studies have shown that women who have it are in turn at greater risk for the development of chronic pain (Pain 2008;140:87-94).
In the future, adding genetic information to the model might further increase its accuracy, although “our aim was to simplify,” she said.
Major Finding: A 1-minute, 3-question screening tool predicted individuals in the top 20th percentile of pain scores, with a sensitivity of 67% and a specificity of 72%.
Data Source: Study of 192 women who underwent elective C-sections with spinal morphine.
Disclosures: None was reported.
SAN ANTONIO — A 1-minute, three-question screening tool accurately predicted post–cesarean section pain in a study of 192 women who underwent elective cesarean sections with spinal morphine.
The quick, simple screen was nearly as sensitive and specific in predicting pain after delivery as was a 120-minute battery of tests used in a previous study (Anesthesiology 2006;104:417-25).
“We were looking for a simple, clinically useful way to identify patients at high risk for severe acute pain and subsequent complications who might benefit from earlier intervention,” said Dr. Ashley M. Tonidandel of Wake Forest University, Winston-Salem, N.C.
The three screening questions that were used in the investigation addressed the three strongest independent predictors of postcesarean pain and narcotic usage identified in the study published in Anesthesiology and in another study (Clin. J. Pain 2009;25:455-60).
Those predictors were anticipated pain levelafollowing surgery (on a scale of 0-100), anxiety regarding the upcoming surgery (1-100), and estimated pain medication usage (much less to much more than average).
The study participants had a mean age of 30 years and a mean body mass index of 34 kg/m
At 24 hours following surgery, the mean evoked pain score was 44 (out of 100). The patients used an average of 19 morphine equivalents over 24 hours. More than half of the participants (54%) received a total of 200 mcg of spinal morphine, according to Dr. Tonidandel.
Anxiety, anticipated pain, and expected medication usage were all significantly related to evoked pain and 24-hour morphine equivalents, with high scores on any two of the three items significantly predicting high pain scores.
Overall, the model accounted for 22% of the variance in predicted pain scores, which is “pretty good considering the 22%-28% of variance from the 120-minute battery of questions,” Dr. Tonidandel commented at the meeting.
Using a regression equation, the screening tool predicted individuals in the top 20th percentile of pain scores with a sensitivity of 67% and a specificity of 72%. With the 120-minute screen, those values were 70% and 76%, respectively, she said.
Identifying women at high risk for severe postpartum pain is especially important because previous studies have shown that women who have it are in turn at greater risk for the development of chronic pain (Pain 2008;140:87-94).
In the future, adding genetic information to the model might further increase its accuracy, although “our aim was to simplify,” she said.
From the Annual Meeting of the Society for Obstetric Anesthesia and Perinatology
Does vaginal birth after cesarean have a future?
Once again, vaginal birth after cesarean, or VBAC—sometimes referred to as a trial of labor after cesarean, or TOLAC—has arisen as a topic of interest in obstetrics, as demonstrated in this issue of OBG Management.1 I say “once again” because, frankly, I thought that the matter had become irrelevant—reminiscent of a debate over vaginal breech delivery in the 1970s and 1980s now largely resolved in the United States, thanks to evidence-based randomized clinical trials.
I thought the issue was closed when, in 2005, the chair of ACOG’s Committee on Obstetric Practice was quoted in USA Today: “… the VBAC rupture rate may seem quite low but it’s damn high if you’re the one.” And later in the same article: “I think VBAC is dead.”
And I considered VBAC finished when I compared the target VBAC rate established in the US Department of Health and Human Services’s Healthy People 2010 report against the astounding data that we see reported today:
- In 1998, the US primary cesarean delivery rate was 18%; the Healthy People 2010 target was 15%. Today, that rate exceeds 25%.
- In 1998, the repeat cesarean delivery rate was 72%; again, the Healthy People 2010 target was 63%. In 2003, however, the repeat cesarean rate had climbed to 88.7%—and today, that rate exceeds 90%.
Called “reasonable” for many women
Yet, in a recent report, a consensus panel convened by The National Institutes of Health declares that VBAC is a “reasonable option” for many pregnant women. The panel encourages physicians to incorporate evidence-based data into the counseling they provide to patients.2
But even our own College admits to a paucity of high-quality evidence about VBAC. A 2009 ACOG Practice Bulletin says that “despite thousands of citations in the world’s literature there are currently no randomized trials comparing maternal or neonatal outcomes for both repeat cesarean delivery and VBAC.”3
So the question remains: How can medical science help patients and physicians make the best decisions about VBAC? Let me try to provide an answer here. Some of the ideas I draw on are discussed by Dr. Aviva Lee-Parritz in her article beginning on page 17.
What are the risks?
The true risks of VBAC are unknown. However, we do know—all the data are in agreement—that elective repeat cesarean delivery, performed at the appropriate gestational age, is safer for fetus and newborn than a trial of labor.4
We also know that most mothers accept a greater burden of risk for themselves if there is potential benefit for their newborn. (An example is expectant management of severe preeclampsia remote from term, when a delay in delivery offers no maternal benefit but does offer potential benefit to the newborn.) With VBAC, mothers must be willing to accept the risks of the procedure; better ways to assess that risk have been proposed to help them make a decision.5
What are the chances of success?
It amazes me when the quoted VBAC success rate at a given hospital exceeds the likelihood there of successful vaginal delivery of a nullipara. I see such data reported often.
Be certain that your patients know the hospital-specific cesarean delivery rate and VBAC success rate—and if you don’t have those data, then tell the patient that you don’t. It doesn’t make sense to quote an 85% VBAC success rate if your institution’s primary cesarean delivery rate is 25%.
What does VBAC cost?
The data with which to answer this question are hard to obtain cleanly; ultimately, however, the choices we make should be based on proper medical decision-making, not cost. That said, I remain unconvinced that VBAC overall offers significant savings over repeat cesarean delivery when total cost (not just the cost of postpartum care or the cost of post-delivery length of stay) is examined.
Furthermore, the expense of settling malpractice claims of “VBACs gone awry” is never included in estimates of the cost of care.
How are VBACs reimbursed?
The current structure of reimbursement for health care doesn’t favor VBAC. In most regions of the country, 1) physicians’ reimbursement for performing a VBAC is either the same as, or lower than, it is for cesarean delivery and 2) most hospitals enjoy a greater margin on the hospital stay postcesarean than after a vaginal delivery.
Given the increased time involved in managing a VBAC, a change in reimbursement to recognize the greater effort and exposure to liability would be a reasonable step for payers—if there is true interest in reversing the trend away from VBAC that we’re seeing.
How great are concerns over liability?
In every data set that I have reviewed, perinatal morbidity and mortality are clearly higher in the VBAC group than in the repeat cesarean group. In essence, the central issue with VBAC is uterine rupture and all the complications that can flow from that event.6
A problem for small hospitals. ACOG has already issued guidelines for what care should be “readily available” in a hospital that offers VBAC. For the College to retreat from these recommendations in an effort to increase acceptance of VBAC among smaller community hospitals—many of which are without students, residents, fellows, or myriad other support personnel—would, I think, be disingenuous and ill-advised. Add to this recent data suggesting that peripartum hysterectomy (for which VBAC patients are at increased risk) is best done in a high-volume hospital setting7 and you further reduce the likelihood that smaller community hospitals will ever embrace VBAC.
How well do patients accept VBAC?
It’s tough to sell a product that people don’t want. My anecdotal experience (meaning that my conclusions are unencumbered by data) is that informed health care personnel who themselves have had a cesarean delivery almost uniformly select cesarean delivery subsequently. They know the data and they’re aware of the risks. Often, they aren’t planning on having more than two children, so the problem of placenta accreta in the future doesn’t apply.
These observations suggest, to me, that maybe 1) we need to do a better job counseling patients or 2) our society’s value system overwhelmingly favors predictability of delivery and safety of the newborn at the expense of even a slight increase in risk to the mother.
Alas, common sense is the most difficult thing to legislate
VBAC was, and is, a good idea. It’s based on sound principles and good intentions.
Recall that, in 1970, our dictum was “once a section always a section.” The cesarean delivery rate in the United States was 5%, and we didn’t need to worry about VBAC.
VBAC became popular only as the primary cesarean rate began to rise above 15%; at that time, strict rules accompanied the procedure: no oxytocin or epidural anesthesia, and, in many institutions, x-ray pelvimetry was required to document “adequacy” of the pelvis.
Now, we’ve moved to the other end of the spectrum: It seems we offer VBAC to anyone who wants it, regardless of comorbidities.
Can we compromise?
I support a middle-of-the-road position that strongly encourages VBAC for women who:
- have no comorbidities
- have had a prior VBAC or previous vaginal delivery of a term baby and
- who have had no more than one prior cesarean delivery.
On the other hand, VBAC should be discouraged for women who:
- have a body mass index >40
- are post-term
- present at term with premature rupture of the membranes, an unengaged vertex, or an unfavorable cervix or
- have any other condition that might make emergency cesarean delivery more difficult and, therefore, best avoided.
Such risk assessment approaches have already been proposed.5
Applying common sense to the matter, we might be able to agree on a solution that makes VBAC attractive and, more important, safe for our patients and for us. Furthermore, we must diligently keep track of our own data on maternal and neonatal outcomes so that we can most appropriately counsel our patients.
It’s up to us to determine whether VBAC should stay or go
I estimate that we have a window of opportunity of 5 to 10 years to resolve whether VBAC remains part of practice. If we don’t take that opportunity, we’ll be left with a generation of physicians who have little or no experience performing the procedure. VBAC will disappear, in a self-fulfilling prophecy—which, when you think about what happened with vaginal breech delivery, may not be a bad thing.
1. Lee-Parritz A. When is VBAC appropriate? OBG Manage. 2010;22(7):17-24.
2. National Institutes of Health Consensus Development conference statement: vaginal birth after cesarean: new insights March 8–10, 2010 Obstet Gynecol. 2010;115(6):1279-1295.
3. Vaginal birth after previous cesarean. ACOG Practice Bulletin #54. Obstet Gynecol. 2004;104(1):203-212.
4. Guise JM, Denman MA, Emeis C, et al. Vaginal birth after cesarean; new insights on maternal and neonatal outcomes. Obstet Gynecol. 2010;115(6):1267-1278.
5. Grobman WA, Lai Y, Landon MB, et al. Can a prediction model for vaginal birth after cesarean also predict the probability of morbidity related to a trial of labor? Am J Obstet Gynecol. 2009;200(1):56-e1-e6.
6. Scott JR. Solving the vaginal birth after cesarean dilemma. Obstet Gynecol. 2010;115(6):1112-1113.
7. Wright J, Herzog T, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol. 2010;115(6):1194-1200.
Once again, vaginal birth after cesarean, or VBAC—sometimes referred to as a trial of labor after cesarean, or TOLAC—has arisen as a topic of interest in obstetrics, as demonstrated in this issue of OBG Management.1 I say “once again” because, frankly, I thought that the matter had become irrelevant—reminiscent of a debate over vaginal breech delivery in the 1970s and 1980s now largely resolved in the United States, thanks to evidence-based randomized clinical trials.
I thought the issue was closed when, in 2005, the chair of ACOG’s Committee on Obstetric Practice was quoted in USA Today: “… the VBAC rupture rate may seem quite low but it’s damn high if you’re the one.” And later in the same article: “I think VBAC is dead.”
And I considered VBAC finished when I compared the target VBAC rate established in the US Department of Health and Human Services’s Healthy People 2010 report against the astounding data that we see reported today:
- In 1998, the US primary cesarean delivery rate was 18%; the Healthy People 2010 target was 15%. Today, that rate exceeds 25%.
- In 1998, the repeat cesarean delivery rate was 72%; again, the Healthy People 2010 target was 63%. In 2003, however, the repeat cesarean rate had climbed to 88.7%—and today, that rate exceeds 90%.
Called “reasonable” for many women
Yet, in a recent report, a consensus panel convened by The National Institutes of Health declares that VBAC is a “reasonable option” for many pregnant women. The panel encourages physicians to incorporate evidence-based data into the counseling they provide to patients.2
But even our own College admits to a paucity of high-quality evidence about VBAC. A 2009 ACOG Practice Bulletin says that “despite thousands of citations in the world’s literature there are currently no randomized trials comparing maternal or neonatal outcomes for both repeat cesarean delivery and VBAC.”3
So the question remains: How can medical science help patients and physicians make the best decisions about VBAC? Let me try to provide an answer here. Some of the ideas I draw on are discussed by Dr. Aviva Lee-Parritz in her article beginning on page 17.
What are the risks?
The true risks of VBAC are unknown. However, we do know—all the data are in agreement—that elective repeat cesarean delivery, performed at the appropriate gestational age, is safer for fetus and newborn than a trial of labor.4
We also know that most mothers accept a greater burden of risk for themselves if there is potential benefit for their newborn. (An example is expectant management of severe preeclampsia remote from term, when a delay in delivery offers no maternal benefit but does offer potential benefit to the newborn.) With VBAC, mothers must be willing to accept the risks of the procedure; better ways to assess that risk have been proposed to help them make a decision.5
What are the chances of success?
It amazes me when the quoted VBAC success rate at a given hospital exceeds the likelihood there of successful vaginal delivery of a nullipara. I see such data reported often.
Be certain that your patients know the hospital-specific cesarean delivery rate and VBAC success rate—and if you don’t have those data, then tell the patient that you don’t. It doesn’t make sense to quote an 85% VBAC success rate if your institution’s primary cesarean delivery rate is 25%.
What does VBAC cost?
The data with which to answer this question are hard to obtain cleanly; ultimately, however, the choices we make should be based on proper medical decision-making, not cost. That said, I remain unconvinced that VBAC overall offers significant savings over repeat cesarean delivery when total cost (not just the cost of postpartum care or the cost of post-delivery length of stay) is examined.
Furthermore, the expense of settling malpractice claims of “VBACs gone awry” is never included in estimates of the cost of care.
How are VBACs reimbursed?
The current structure of reimbursement for health care doesn’t favor VBAC. In most regions of the country, 1) physicians’ reimbursement for performing a VBAC is either the same as, or lower than, it is for cesarean delivery and 2) most hospitals enjoy a greater margin on the hospital stay postcesarean than after a vaginal delivery.
Given the increased time involved in managing a VBAC, a change in reimbursement to recognize the greater effort and exposure to liability would be a reasonable step for payers—if there is true interest in reversing the trend away from VBAC that we’re seeing.
How great are concerns over liability?
In every data set that I have reviewed, perinatal morbidity and mortality are clearly higher in the VBAC group than in the repeat cesarean group. In essence, the central issue with VBAC is uterine rupture and all the complications that can flow from that event.6
A problem for small hospitals. ACOG has already issued guidelines for what care should be “readily available” in a hospital that offers VBAC. For the College to retreat from these recommendations in an effort to increase acceptance of VBAC among smaller community hospitals—many of which are without students, residents, fellows, or myriad other support personnel—would, I think, be disingenuous and ill-advised. Add to this recent data suggesting that peripartum hysterectomy (for which VBAC patients are at increased risk) is best done in a high-volume hospital setting7 and you further reduce the likelihood that smaller community hospitals will ever embrace VBAC.
How well do patients accept VBAC?
It’s tough to sell a product that people don’t want. My anecdotal experience (meaning that my conclusions are unencumbered by data) is that informed health care personnel who themselves have had a cesarean delivery almost uniformly select cesarean delivery subsequently. They know the data and they’re aware of the risks. Often, they aren’t planning on having more than two children, so the problem of placenta accreta in the future doesn’t apply.
These observations suggest, to me, that maybe 1) we need to do a better job counseling patients or 2) our society’s value system overwhelmingly favors predictability of delivery and safety of the newborn at the expense of even a slight increase in risk to the mother.
Alas, common sense is the most difficult thing to legislate
VBAC was, and is, a good idea. It’s based on sound principles and good intentions.
Recall that, in 1970, our dictum was “once a section always a section.” The cesarean delivery rate in the United States was 5%, and we didn’t need to worry about VBAC.
VBAC became popular only as the primary cesarean rate began to rise above 15%; at that time, strict rules accompanied the procedure: no oxytocin or epidural anesthesia, and, in many institutions, x-ray pelvimetry was required to document “adequacy” of the pelvis.
Now, we’ve moved to the other end of the spectrum: It seems we offer VBAC to anyone who wants it, regardless of comorbidities.
Can we compromise?
I support a middle-of-the-road position that strongly encourages VBAC for women who:
- have no comorbidities
- have had a prior VBAC or previous vaginal delivery of a term baby and
- who have had no more than one prior cesarean delivery.
On the other hand, VBAC should be discouraged for women who:
- have a body mass index >40
- are post-term
- present at term with premature rupture of the membranes, an unengaged vertex, or an unfavorable cervix or
- have any other condition that might make emergency cesarean delivery more difficult and, therefore, best avoided.
Such risk assessment approaches have already been proposed.5
Applying common sense to the matter, we might be able to agree on a solution that makes VBAC attractive and, more important, safe for our patients and for us. Furthermore, we must diligently keep track of our own data on maternal and neonatal outcomes so that we can most appropriately counsel our patients.
It’s up to us to determine whether VBAC should stay or go
I estimate that we have a window of opportunity of 5 to 10 years to resolve whether VBAC remains part of practice. If we don’t take that opportunity, we’ll be left with a generation of physicians who have little or no experience performing the procedure. VBAC will disappear, in a self-fulfilling prophecy—which, when you think about what happened with vaginal breech delivery, may not be a bad thing.
Once again, vaginal birth after cesarean, or VBAC—sometimes referred to as a trial of labor after cesarean, or TOLAC—has arisen as a topic of interest in obstetrics, as demonstrated in this issue of OBG Management.1 I say “once again” because, frankly, I thought that the matter had become irrelevant—reminiscent of a debate over vaginal breech delivery in the 1970s and 1980s now largely resolved in the United States, thanks to evidence-based randomized clinical trials.
I thought the issue was closed when, in 2005, the chair of ACOG’s Committee on Obstetric Practice was quoted in USA Today: “… the VBAC rupture rate may seem quite low but it’s damn high if you’re the one.” And later in the same article: “I think VBAC is dead.”
And I considered VBAC finished when I compared the target VBAC rate established in the US Department of Health and Human Services’s Healthy People 2010 report against the astounding data that we see reported today:
- In 1998, the US primary cesarean delivery rate was 18%; the Healthy People 2010 target was 15%. Today, that rate exceeds 25%.
- In 1998, the repeat cesarean delivery rate was 72%; again, the Healthy People 2010 target was 63%. In 2003, however, the repeat cesarean rate had climbed to 88.7%—and today, that rate exceeds 90%.
Called “reasonable” for many women
Yet, in a recent report, a consensus panel convened by The National Institutes of Health declares that VBAC is a “reasonable option” for many pregnant women. The panel encourages physicians to incorporate evidence-based data into the counseling they provide to patients.2
But even our own College admits to a paucity of high-quality evidence about VBAC. A 2009 ACOG Practice Bulletin says that “despite thousands of citations in the world’s literature there are currently no randomized trials comparing maternal or neonatal outcomes for both repeat cesarean delivery and VBAC.”3
So the question remains: How can medical science help patients and physicians make the best decisions about VBAC? Let me try to provide an answer here. Some of the ideas I draw on are discussed by Dr. Aviva Lee-Parritz in her article beginning on page 17.
What are the risks?
The true risks of VBAC are unknown. However, we do know—all the data are in agreement—that elective repeat cesarean delivery, performed at the appropriate gestational age, is safer for fetus and newborn than a trial of labor.4
We also know that most mothers accept a greater burden of risk for themselves if there is potential benefit for their newborn. (An example is expectant management of severe preeclampsia remote from term, when a delay in delivery offers no maternal benefit but does offer potential benefit to the newborn.) With VBAC, mothers must be willing to accept the risks of the procedure; better ways to assess that risk have been proposed to help them make a decision.5
What are the chances of success?
It amazes me when the quoted VBAC success rate at a given hospital exceeds the likelihood there of successful vaginal delivery of a nullipara. I see such data reported often.
Be certain that your patients know the hospital-specific cesarean delivery rate and VBAC success rate—and if you don’t have those data, then tell the patient that you don’t. It doesn’t make sense to quote an 85% VBAC success rate if your institution’s primary cesarean delivery rate is 25%.
What does VBAC cost?
The data with which to answer this question are hard to obtain cleanly; ultimately, however, the choices we make should be based on proper medical decision-making, not cost. That said, I remain unconvinced that VBAC overall offers significant savings over repeat cesarean delivery when total cost (not just the cost of postpartum care or the cost of post-delivery length of stay) is examined.
Furthermore, the expense of settling malpractice claims of “VBACs gone awry” is never included in estimates of the cost of care.
How are VBACs reimbursed?
The current structure of reimbursement for health care doesn’t favor VBAC. In most regions of the country, 1) physicians’ reimbursement for performing a VBAC is either the same as, or lower than, it is for cesarean delivery and 2) most hospitals enjoy a greater margin on the hospital stay postcesarean than after a vaginal delivery.
Given the increased time involved in managing a VBAC, a change in reimbursement to recognize the greater effort and exposure to liability would be a reasonable step for payers—if there is true interest in reversing the trend away from VBAC that we’re seeing.
How great are concerns over liability?
In every data set that I have reviewed, perinatal morbidity and mortality are clearly higher in the VBAC group than in the repeat cesarean group. In essence, the central issue with VBAC is uterine rupture and all the complications that can flow from that event.6
A problem for small hospitals. ACOG has already issued guidelines for what care should be “readily available” in a hospital that offers VBAC. For the College to retreat from these recommendations in an effort to increase acceptance of VBAC among smaller community hospitals—many of which are without students, residents, fellows, or myriad other support personnel—would, I think, be disingenuous and ill-advised. Add to this recent data suggesting that peripartum hysterectomy (for which VBAC patients are at increased risk) is best done in a high-volume hospital setting7 and you further reduce the likelihood that smaller community hospitals will ever embrace VBAC.
How well do patients accept VBAC?
It’s tough to sell a product that people don’t want. My anecdotal experience (meaning that my conclusions are unencumbered by data) is that informed health care personnel who themselves have had a cesarean delivery almost uniformly select cesarean delivery subsequently. They know the data and they’re aware of the risks. Often, they aren’t planning on having more than two children, so the problem of placenta accreta in the future doesn’t apply.
These observations suggest, to me, that maybe 1) we need to do a better job counseling patients or 2) our society’s value system overwhelmingly favors predictability of delivery and safety of the newborn at the expense of even a slight increase in risk to the mother.
Alas, common sense is the most difficult thing to legislate
VBAC was, and is, a good idea. It’s based on sound principles and good intentions.
Recall that, in 1970, our dictum was “once a section always a section.” The cesarean delivery rate in the United States was 5%, and we didn’t need to worry about VBAC.
VBAC became popular only as the primary cesarean rate began to rise above 15%; at that time, strict rules accompanied the procedure: no oxytocin or epidural anesthesia, and, in many institutions, x-ray pelvimetry was required to document “adequacy” of the pelvis.
Now, we’ve moved to the other end of the spectrum: It seems we offer VBAC to anyone who wants it, regardless of comorbidities.
Can we compromise?
I support a middle-of-the-road position that strongly encourages VBAC for women who:
- have no comorbidities
- have had a prior VBAC or previous vaginal delivery of a term baby and
- who have had no more than one prior cesarean delivery.
On the other hand, VBAC should be discouraged for women who:
- have a body mass index >40
- are post-term
- present at term with premature rupture of the membranes, an unengaged vertex, or an unfavorable cervix or
- have any other condition that might make emergency cesarean delivery more difficult and, therefore, best avoided.
Such risk assessment approaches have already been proposed.5
Applying common sense to the matter, we might be able to agree on a solution that makes VBAC attractive and, more important, safe for our patients and for us. Furthermore, we must diligently keep track of our own data on maternal and neonatal outcomes so that we can most appropriately counsel our patients.
It’s up to us to determine whether VBAC should stay or go
I estimate that we have a window of opportunity of 5 to 10 years to resolve whether VBAC remains part of practice. If we don’t take that opportunity, we’ll be left with a generation of physicians who have little or no experience performing the procedure. VBAC will disappear, in a self-fulfilling prophecy—which, when you think about what happened with vaginal breech delivery, may not be a bad thing.
1. Lee-Parritz A. When is VBAC appropriate? OBG Manage. 2010;22(7):17-24.
2. National Institutes of Health Consensus Development conference statement: vaginal birth after cesarean: new insights March 8–10, 2010 Obstet Gynecol. 2010;115(6):1279-1295.
3. Vaginal birth after previous cesarean. ACOG Practice Bulletin #54. Obstet Gynecol. 2004;104(1):203-212.
4. Guise JM, Denman MA, Emeis C, et al. Vaginal birth after cesarean; new insights on maternal and neonatal outcomes. Obstet Gynecol. 2010;115(6):1267-1278.
5. Grobman WA, Lai Y, Landon MB, et al. Can a prediction model for vaginal birth after cesarean also predict the probability of morbidity related to a trial of labor? Am J Obstet Gynecol. 2009;200(1):56-e1-e6.
6. Scott JR. Solving the vaginal birth after cesarean dilemma. Obstet Gynecol. 2010;115(6):1112-1113.
7. Wright J, Herzog T, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol. 2010;115(6):1194-1200.
1. Lee-Parritz A. When is VBAC appropriate? OBG Manage. 2010;22(7):17-24.
2. National Institutes of Health Consensus Development conference statement: vaginal birth after cesarean: new insights March 8–10, 2010 Obstet Gynecol. 2010;115(6):1279-1295.
3. Vaginal birth after previous cesarean. ACOG Practice Bulletin #54. Obstet Gynecol. 2004;104(1):203-212.
4. Guise JM, Denman MA, Emeis C, et al. Vaginal birth after cesarean; new insights on maternal and neonatal outcomes. Obstet Gynecol. 2010;115(6):1267-1278.
5. Grobman WA, Lai Y, Landon MB, et al. Can a prediction model for vaginal birth after cesarean also predict the probability of morbidity related to a trial of labor? Am J Obstet Gynecol. 2009;200(1):56-e1-e6.
6. Scott JR. Solving the vaginal birth after cesarean dilemma. Obstet Gynecol. 2010;115(6):1112-1113.
7. Wright J, Herzog T, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol. 2010;115(6):1194-1200.
When is VBAC appropriate?
At first glance, the issue of vaginal birth after cesarean delivery (VBAC) appears to boil down to a simple question: Should I attempt it, or shouldn’t I?
On deeper inspection, the decision becomes extremely complex, and the evidence can be confusing.
Both planned elective repeat cesarean and planned VBAC are associated with harms as well as benefits. Most experts would agree than an uncomplicated vaginal delivery poses little risk to mother and baby, and that a planned repeat cesarean delivery at term carries some risk to the mother.
The greatest risks for both mother and baby arise when a trial of labor fails and cesarean delivery becomes necessary for maternal or fetal indications. Risks to the mother are largely operative in nature, and the primary risk to the fetus is uterine rupture. However, maternal and fetal risks cannot be truly separated. Uterine rupture not only compromises the fetus in utero but has a severe impact on maternal hemodynamic stability, just as a fetal hypoxic ischemic insult secondary to uterine rupture can have lifelong psychological and social consequences for the mother and family.
We are fortunate that serious adverse outcomes of VBAC are rare. Nevertheless, the only predictable delivery method is planned elective repeat cesarean. Uncertainty over the likelihood of success of VBAC arises when relative risk is confused with absolute risk.
In this article, I examine the literature on the route of delivery after cesarean to assess the overall safety of a trial of labor in various settings and populations.
Data on VBAC are limited
We lack randomized, controlled trials and valid animal studies that assess fetal and maternal outcomes of elective repeat cesarean versus planned vaginal delivery. The vast majority of studies of VBAC are retrospective or cohort studies, which have inherent potential for bias. Many studies lack a standardized definition of adverse outcomes or lack direct evidence that adverse outcomes are wholly attributable to the trial of labor. No studies compare women who are similar in all characteristics except their mode of delivery.
Nor do we fully understand how women choose a course of action after cesarean delivery—except that the decision is almost always multifactorial. Competing voices—health care provider, family members, friends, media, and a woman’s own memory of her previous delivery—and her emotional state—all contribute to the decision.
Clearly, a trial of labor after cesarean delivery can be safe for many women. Successful vaginal delivery is associated with a very low risk of adverse outcomes and may be associated with a lower risk of minor morbidity than is elective repeat cesarean. In fact, the overall success rate for a trial of labor after cesarean is not that different from the success rate for nulliparous women undergoing induction of labor.19 Even so, patients should understand that operative delivery may be necessary, and the physician and hospital must be prepared for this eventuality in accordance with ACOG guidelines.
As I interpret the data, if a woman has undergone one low transverse cesarean delivery for a nonrecurring condition and a nonmacrosomic fetus, a trial of labor after the spontaneous onset of labor should be strongly encouraged. If she has already delivered vaginally in the past, or had a successful VBAC, she is an even better candidate for a trial of labor. In such a case, labor induction with mechanical cervical ripening or appropriate use of oxytocin, or both, may still be appropriate, but the likelihood of success is lower.
If a woman has a history of more than one cesarean delivery without a vaginal birth, she may be better served by scheduled repeat cesarean delivery. The same holds true for women who have a history of preterm cesarean delivery, a short interpregnancy interval, suspected macrosomia, or an unengaged fetal vertex.
Decision-making about delivery should be shared between the provider and patient, after thorough counseling about the risks and benefits in language the patient can easily comprehend.
It would be best to avoid having to make a decision about VBAC by preventing the initial cesarean delivery.
How risky is repeat cesarean?
We are all acutely aware of the skyrocketing rate of cesarean delivery, which reaches 35% to 41% in some areas. Most studies indicate that approximately 50% of all cesarean deliveries are repeat cesarean deliveries. Besides the risks associated with the operation itself, planned repeat cesarean has significant downstream implications for the mother and baby—and for society. For example, multiple cesarean deliveries pose an ever greater risk of abnormal placentation and maternal hemorrhage. Cesarean delivery without labor can also heighten the risk of neonatal respiratory compromise, temperature instability, and slow feeding.1 Cesarean delivery and its longer attendant hospitalization markedly increase costs throughout an already strapped health care system.
On balance, any cesarean delivery imparts an increased risk of maternal morbidity and mortality, compared with vaginal delivery, as well as an increased risk of complications, such as placenta previa and placenta accreta, in subsequent pregnancies.
What are the risks of a trial of labor?
A prospective, 4-year observational study conducted at 19 academic medical centers under the auspices of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network compared the outcomes of 17,898 women undergoing a trial of labor after cesarean delivery with those of 15,801 women having elective repeat cesarean.2 Symptomatic uterine rupture occurred in 0.7% of the women attempting a trial of labor, with no occurrences in the elective cesarean group. Blood transfusion and endomyometritis were more common in the group undergoing a trial of labor, and this difference was statistically significant. These findings are in concordance with those of earlier studies.
The two groups in this study were not exactly the same; more women undergoing a trial of labor had had a previous vaginal delivery. Significant adverse maternal outcomes, such as endomyometritis, uterine rupture, hysterectomy, and the need for transfusion, were much more likely in a failed trial of labor than in a successful one.
The same study found a 0.46% risk of hypoxic-ischemic encephalopathy, which was most likely to occur after symptomatic uterine rupture (7 of 12 cases). No cases of hypoxic-ischemic encephalopathy occurred among women undergoing planned cesarean delivery. Multivariate logistic regression analysis determined that the risks of stillbirth, neonatal death, and hypoxic-ischemic encephalopathy in term infants were increased in the group undergoing a trial of labor, compared with elective repeat cesarean (odds ratio [OR], 2.72; 95% confidence interval [CI], 1.49–4.97).
Can we predict the success of a trial of labor?
Combined success rates from a large number of prospective cohort studies suggest an overall rate of 75.9%. Many clinical characteristics may increase the likelihood of success of a trial of labor after cesarean. In this section, I describe these characteristics and sift the data we have about them.
A history of vaginal delivery ups the odds of success
Women who have delivered vaginally have a much lower risk of rupture during a trial of labor after cesarean than women who have not. Women who have delivered vaginally are also four times more likely to have a successful VBAC. A multicenter, prospective study found a VBAC success rate of 86% among women who had already delivered vaginally, and a success rate of 90% among women who had a history of successful VBAC.3
Many aspects of the cesarean delivery have continuing impact
The type of labor that occurred in the cesarean delivery may help predict subsequent complications and the ultimate success of a trial of labor. For example, induced labor or no labor prior to cesarean delivery is associated with a 2.25-fold risk of uterine rupture in a subsequent trial of labor, compared with a history of spontaneous labor.4
In addition, several studies have demonstrated that the indication for the first cesarean delivery has a bearing on the success of a subsequent trial of labor. For example, an indication of shoulder dystocia reduces the success rate of a subsequent trial of labor by one third.2
Even a brief trial of labor before the cesarean may increase the success of a subsequent trial of labor. One study found that cervical dilation to 8 cm or greater was independently predictive of successful VBAC among women who had a nonrecurring indication for the initial cesarean delivery.5
When the cesarean delivery involves a preterm infant, the risk of uterine rupture during a subsequent trial of labor may increase if the infant is at term. Conversely, the risk of uterine rupture is lower when a term cesarean is followed by a preterm trial of labor.6
A vertical hysterotomy may preclude VBAC
A previous classical hysterotomy is generally an absolute contraindication for a trial of labor because rupture may occur in as many as 14% of women who have this type of scar.
Low transverse hysterotomy does not appear to confer excess risk during a subsequent trial of labor. Less clear is whether a low vertical hysterotomy poses a risk of rupture. In a 2004 prospective cohort study, the rate of uterine rupture among women who had a transverse hysterotomy scar was 0.7%, compared with 2.0% for a low vertical scar. Any difference in the rate of uterine rupture in retrospective studies may be attributable, at least in part, to the subjective nature of the definition of “low vertical” because there is no precise or objective way to ensure that the vertical hysterotomy did not breach the contractile portion of the uterus (FIGURE).
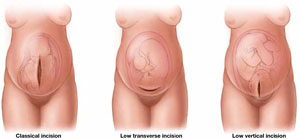
Type of prior hysterotomy influences the VBAC decision
A classical uterine incision is an absolute contraindication to vaginal birth after cesarean (VBAC). A trial of labor is thought to be safe in women who have had a low transverse hysterotomy. The jury is still out on the safety of VBAC in a woman who has had a low vertical incision, however, because of uncertainty over whether the contractile portion of the uterus is involved.
Are multiple cesareans a contraindication to VBAC?
Experts disagree as to whether more than one previous cesarean delivery before a trial of labor increases the risk of uterine rupture. One retrospective study showed no difference in the rate of rupture between women who had a single previous cesarean and those who had more than one.7 A larger prospective study showed a modest increase in the risk of rupture (OR, 1.16) among women who had undergone more than one cesarean—but no decrease in the chance of success.8
Most large retrospective and prospective studies include patients who have had more than one previous cesarean delivery, but their numbers remain low; therefore, statistical significance cannot be determined.
Induction or augmentation of labor may lower odds of success
The likelihood of successful VBAC may be reduced when labor is augmented or induced. The picture is unclear because most studies that have focused on cervical ripening and induction of labor in VBAC are small.
Bujold compared pregnancy outcomes of three groups of women:
- those who underwent cervical ripening via Foley catheter
- those who had amniotomy and oxytocin administration
- those who entered labor spontaneously.
No difference in the rate of uterine rupture was found among the groups. However, the group that underwent cervical ripening had a significantly lower rate of success.9
A large case-control study found no increase in the rate of rupture when oxytocin or prostaglandins were administered, but the rate tripled when both were used together.10
A small, nested, case-control study found an increased risk of uterine rupture only when oxytocin was administered at a rate exceeding 20 mU/mL.11
More than 90% of hysterotomies are transverse
When the obstetric history is incomplete, the clinician may not know what type of hysterotomy was used in the previous cesarean delivery. Most experts believe that VBAC is acceptable when the previous cesarean involved a low transverse hysterotomy. The risk may be much higher with other types of incisions. Today, however, with modern techniques in place, we can assume that more than 90% of hysterotomies are of the low transverse type.
At least one study suggests that the risk of uterine rupture during vaginal birth after cesarean is acceptably low when the type of hysterotomy is unknown. That study explored the effect of augmentation of labor with oxytocin among women who had an unknown scar and found an increased risk of rupture, compared with women who were managed expectantly. However, the overall rate of uterine rupture did not differ from the rate expected when the hysterotomy is known to be of the low transverse type.12
VBAC for twins is rare
Because few women carrying twins attempt VBAC, we have little data to guide counseling on success and complication rates. A multicenter, retrospective, cohort study explored delivery outcomes of 25,005 women who had undergone at least one previous cesarean. Of these women, 24,307 had a singleton pregnancy, and 535 were carrying twins. Women who had a twin gestation were 40% less likely to attempt a trial of labor, but those who did had a chance of success and risk of uterine rupture similar to those of women with a singleton gestation. Women carrying twins who underwent a trial of labor had an elevated risk of requiring transfusion, compared with those carrying singletons, but this risk was similar to that of women delivering twins by elective repeat cesarean. In fact, women who delivered twins by repeat cesarean tended to have more maternal morbidity overall than those who had a trial of labor.13
A short interpregnancy interval precludes VBAC
Data indicate that a trial of labor after cesarean should be avoided in women who have a brief interpregnancy interval. Several retrospective studies had found an increased risk of uterine rupture, as well as a host of other adverse outcomes, among these women. Using 12 months as a reference point, women who had an interpregnancy interval shorter than 6 months had triple the risk of uterine rupture.14 Although the mechanism is unknown, rupture is presumably the result of incomplete healing of the hysterotomy.
Macrosomia may not increase the risk of rupture
Women who are thought to have a macrosomic fetus may be encouraged to attempt VBAC, if they so desire. Macrosomia is a minor risk factor for failure of a trial of labor, but it does not necessarily increase the risk of uterine rupture.15
Elkousy examined VBAC success rates by birth weight, indication for the previous cesarean delivery, and pregnancy history. Not surprisingly, increased birth weight or a history of cephalopelvic disproportion reduced the rate of success, but a history of vaginal delivery negated that risk of failure. A history of successful VBAC improved the chance of success to more than 90%—even when the birth weight exceeded 4,000 g—and the success rate reached 82% when the birth weight exceeded 4,500 g.16
Physician and hospital attitudes toward vaginal birth after cesarean delivery (VBAC) may be a major determinant of its frequency and success. Many forces oppose women who desire a trial of labor after cesarean. Hospitals and insurers make it increasingly difficult to offer a trial of labor, and strict interpretation of ACOG’s guidelines requiring personnel to be “immediately available” during a trial of labor has caused many smaller and isolated hospitals to stop offering this option. The number of women who attempt VBAC has plummeted.20
Two recent surveys by ACOG indicate that an alarming number of providers have stopped offering VBAC because of a lack of insurance and fear of legal liability. As providers offer a trial of labor less and less, skills decline, and so does mentorship of younger physicians.
The NIH weighs in
In March 2010, the National Institutes of Health (NIH) convened a consensus development conference on the topic of VBAC. A panel of health professionals and public representatives reviewed the medical literature and produced a consensus statement. Their conclusion:
- Given the available evidence, [a trial of labor] is a reasonable option for many pregnant women with a prior low transverse uterine incision. The data reviewed in this report show that both [a trial of labor] and elective repeat cesarean for a pregnant woman with a prior transverse uterine incision have important risks and benefits and that these risks and benefits differ for the woman and her fetus.
The panel’s goal was to help women who have a history of cesarean delivery make an informed, evidence-based decision about the subsequent mode of delivery. The panel also acknowledged the general lack of high-quality evidence to confidently quantify the risks and benefits of a trial of labor versus planned repeat cesarean delivery.21
For another point of view on vaginal birth after cesarean, see the Editorial, “Does vaginal birth after cesarean have a future?” by John T. Repke, MD, of the OBG Management Board of Editors.
Repeat cesarean is probably best for obese gravidas
Obesity increases the likelihood of cesarean delivery in all circumstances, so it is not surprising that it is a risk factor for a failed trial of labor after cesarean. Obesity also increases the risks of anesthesia and surgery. Because of these risks, most clinicians opt to deliver obese patients by scheduled elective cesarean rather than risk having to perform emergent cesarean delivery in the case of acute fetal compromise or uterine rupture.
Race is not a risk factor for rupture
Race is probably not a significant independent risk factor for failure of VBAC. A secondary analysis of a multicenter, retrospective, cohort study found that black women were somewhat more likely to fail a trial of labor than white women (OR, 1.50; 95% CI, 1.29–1.74), after adjustment for confounding variables. However, black women undergoing a trial of labor were 40% less likely to suffer a uterine rupture than white women were.17
When comorbidities are well managed, VBAC remains an option
In general, a trial of labor in women who have well managed chronic medical disease does not pose undue risk to mother or baby.
In a population-based, retrospective cohort study using discharge data from California, Gregory and coworkers attempted to delineate clinical variables that might be associated with VBAC success and complications. They examined a wide range of maternal conditions, from diabetes to chorioamnionitis, as well as fetal conditions, such as oligohydramnios and unengaged vertex. Mothers were stratified into low- and high-risk groups, and multivariate logistic regression was performed. Low-risk patients had a 73.7% success rate, whereas high-risk patients had a 50% success rate. Not surprisingly, women who had a fetus with an unengaged vertex had a 9.8% chance of success and an eightfold increase in the risk of uterine rupture.18
1. Tita AT, Landon MB, Spong CY, et al. For Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111-1120.
2. Landon MB, Hauth JC, Leveno KJ, et al. For NICHD MFMU Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
3. Landon MB, Leindecker S, Spong CY, et al. For NICHD MFMU Network. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. Am J Obst Gynecol. 2005;193(3 Pt 2):1016-1023.
4. Algert CS, Morris JM, Simpson JM, Ford JB, Roberts CL. Labor before a primary cesarean delivery: reduced risk of uterine rupture in a subsequent trial of labor for vaginal birth after cesarean. Obstet Gynecol. 2008;112(5):1061-1066.
5. Kwon JY, Jo YS, Lee GS, Kim SJ, Shin JC, Lee Y. Cervical dilation at the time of cesarean section may affect the success of subsequent vaginal delivery. J Matern Fetal Neonatal Med. 2009;22(11):1057-1062.
6. Sciscione AC, Landon MB, Leveno KJ, et al. For NICHD MFMU Network. Previous preterm delivery and risk of subsequent uterine rupture. Obstet Gynecol. 2008;111(3):648-653.
7. Landon MB, Spong CY, Thom E, et al. For NICHD MFMU Network. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet Gynecol. 2006;108(1):12-20.
8. Macones GA, Cahill A, Pare E, et al. Obstetric outcomes in women with two prior cesarean deliveries: is vaginal birth after cesarean delivery a viable option? Am J Obstet Gynecol. 2005;192(4):1223-1229.
9. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103(1):18-23.
10. Macones G, Peipert J, Nelson D, et al. Maternal complications with vaginal birth after cesarean delivery: a multicenter study. Am J Obstet Gynecol. 2005;193(5):1656-1662.
11. Cahill A, Stamilio D, Odibo A, Peipert J, Stevens E, Macones G. Does a maximum dose of oxytocin affect risk for uterine rupture in candidates for vaginal birth after cesarean delivery? Am J Obstet Gynecol. 2007;197(5):495.e1-e5.
12. Grubb DK, Kjos SL, Paul RH. Latent labor with an unknown uterine scar. Obstet Gynecol. 1996;88(3):351-355.
13. Cahill A, Stamilio DM, Paré E, et al. Vaginal birth after cesarean (VBAC) attempt in twin pregnancies: is it safe? Am J Obstet Gynecol. 2005;193(3 Pt 2):1050-1055.
14. Stamilio DM, DeFranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075.-
15. Zelop CM, Shipp TD, Repke JT, Cohen A, Lieberman E. Outcomes of trial of labor following previous cesarean delivery among women with fetuses weighing >4000 g. Am J Obstet Gynecol. 2001;185(4):903-905.
16. Elkousy M, Sammel M, Stevens E, Peipert J, Macones G. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188(3):824-830.
17. Cahill AG, Stamilio DM, Odibo AO, Peipert J, Stevens E, Macones GA. Racial disparity in the success and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2008;111(3):654-658.
18. Gregory KD, Korst LM, Fridman M, et al. Vaginal birth after cesarean: clinical risk factors associated with adverse outcome. Am J Obstet Gynecol. 2008;198(4):452.e1-e12.
19. Smith GCS, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287(20):2684-2690.
20. Hamilton BE, Martin JA, Sutton PD. For US Department of Health and Human Services. Births: preliminary data for 2002. Natl Vital Stat Rep. 2003;51(11):1-20.
21. National Institutes of Health Consensus Development Conference Statement. Vaginal Birth after Cesarean: New Insights. Bethesda, Md: NIH; 2010. http://consensus.nih.gov/2010/images/vbac/vbac_statement.pdf. Accessed June 16, 2010.
At first glance, the issue of vaginal birth after cesarean delivery (VBAC) appears to boil down to a simple question: Should I attempt it, or shouldn’t I?
On deeper inspection, the decision becomes extremely complex, and the evidence can be confusing.
Both planned elective repeat cesarean and planned VBAC are associated with harms as well as benefits. Most experts would agree than an uncomplicated vaginal delivery poses little risk to mother and baby, and that a planned repeat cesarean delivery at term carries some risk to the mother.
The greatest risks for both mother and baby arise when a trial of labor fails and cesarean delivery becomes necessary for maternal or fetal indications. Risks to the mother are largely operative in nature, and the primary risk to the fetus is uterine rupture. However, maternal and fetal risks cannot be truly separated. Uterine rupture not only compromises the fetus in utero but has a severe impact on maternal hemodynamic stability, just as a fetal hypoxic ischemic insult secondary to uterine rupture can have lifelong psychological and social consequences for the mother and family.
We are fortunate that serious adverse outcomes of VBAC are rare. Nevertheless, the only predictable delivery method is planned elective repeat cesarean. Uncertainty over the likelihood of success of VBAC arises when relative risk is confused with absolute risk.
In this article, I examine the literature on the route of delivery after cesarean to assess the overall safety of a trial of labor in various settings and populations.
Data on VBAC are limited
We lack randomized, controlled trials and valid animal studies that assess fetal and maternal outcomes of elective repeat cesarean versus planned vaginal delivery. The vast majority of studies of VBAC are retrospective or cohort studies, which have inherent potential for bias. Many studies lack a standardized definition of adverse outcomes or lack direct evidence that adverse outcomes are wholly attributable to the trial of labor. No studies compare women who are similar in all characteristics except their mode of delivery.
Nor do we fully understand how women choose a course of action after cesarean delivery—except that the decision is almost always multifactorial. Competing voices—health care provider, family members, friends, media, and a woman’s own memory of her previous delivery—and her emotional state—all contribute to the decision.
Clearly, a trial of labor after cesarean delivery can be safe for many women. Successful vaginal delivery is associated with a very low risk of adverse outcomes and may be associated with a lower risk of minor morbidity than is elective repeat cesarean. In fact, the overall success rate for a trial of labor after cesarean is not that different from the success rate for nulliparous women undergoing induction of labor.19 Even so, patients should understand that operative delivery may be necessary, and the physician and hospital must be prepared for this eventuality in accordance with ACOG guidelines.
As I interpret the data, if a woman has undergone one low transverse cesarean delivery for a nonrecurring condition and a nonmacrosomic fetus, a trial of labor after the spontaneous onset of labor should be strongly encouraged. If she has already delivered vaginally in the past, or had a successful VBAC, she is an even better candidate for a trial of labor. In such a case, labor induction with mechanical cervical ripening or appropriate use of oxytocin, or both, may still be appropriate, but the likelihood of success is lower.
If a woman has a history of more than one cesarean delivery without a vaginal birth, she may be better served by scheduled repeat cesarean delivery. The same holds true for women who have a history of preterm cesarean delivery, a short interpregnancy interval, suspected macrosomia, or an unengaged fetal vertex.
Decision-making about delivery should be shared between the provider and patient, after thorough counseling about the risks and benefits in language the patient can easily comprehend.
It would be best to avoid having to make a decision about VBAC by preventing the initial cesarean delivery.
How risky is repeat cesarean?
We are all acutely aware of the skyrocketing rate of cesarean delivery, which reaches 35% to 41% in some areas. Most studies indicate that approximately 50% of all cesarean deliveries are repeat cesarean deliveries. Besides the risks associated with the operation itself, planned repeat cesarean has significant downstream implications for the mother and baby—and for society. For example, multiple cesarean deliveries pose an ever greater risk of abnormal placentation and maternal hemorrhage. Cesarean delivery without labor can also heighten the risk of neonatal respiratory compromise, temperature instability, and slow feeding.1 Cesarean delivery and its longer attendant hospitalization markedly increase costs throughout an already strapped health care system.
On balance, any cesarean delivery imparts an increased risk of maternal morbidity and mortality, compared with vaginal delivery, as well as an increased risk of complications, such as placenta previa and placenta accreta, in subsequent pregnancies.
What are the risks of a trial of labor?
A prospective, 4-year observational study conducted at 19 academic medical centers under the auspices of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network compared the outcomes of 17,898 women undergoing a trial of labor after cesarean delivery with those of 15,801 women having elective repeat cesarean.2 Symptomatic uterine rupture occurred in 0.7% of the women attempting a trial of labor, with no occurrences in the elective cesarean group. Blood transfusion and endomyometritis were more common in the group undergoing a trial of labor, and this difference was statistically significant. These findings are in concordance with those of earlier studies.
The two groups in this study were not exactly the same; more women undergoing a trial of labor had had a previous vaginal delivery. Significant adverse maternal outcomes, such as endomyometritis, uterine rupture, hysterectomy, and the need for transfusion, were much more likely in a failed trial of labor than in a successful one.
The same study found a 0.46% risk of hypoxic-ischemic encephalopathy, which was most likely to occur after symptomatic uterine rupture (7 of 12 cases). No cases of hypoxic-ischemic encephalopathy occurred among women undergoing planned cesarean delivery. Multivariate logistic regression analysis determined that the risks of stillbirth, neonatal death, and hypoxic-ischemic encephalopathy in term infants were increased in the group undergoing a trial of labor, compared with elective repeat cesarean (odds ratio [OR], 2.72; 95% confidence interval [CI], 1.49–4.97).
Can we predict the success of a trial of labor?
Combined success rates from a large number of prospective cohort studies suggest an overall rate of 75.9%. Many clinical characteristics may increase the likelihood of success of a trial of labor after cesarean. In this section, I describe these characteristics and sift the data we have about them.
A history of vaginal delivery ups the odds of success
Women who have delivered vaginally have a much lower risk of rupture during a trial of labor after cesarean than women who have not. Women who have delivered vaginally are also four times more likely to have a successful VBAC. A multicenter, prospective study found a VBAC success rate of 86% among women who had already delivered vaginally, and a success rate of 90% among women who had a history of successful VBAC.3
Many aspects of the cesarean delivery have continuing impact
The type of labor that occurred in the cesarean delivery may help predict subsequent complications and the ultimate success of a trial of labor. For example, induced labor or no labor prior to cesarean delivery is associated with a 2.25-fold risk of uterine rupture in a subsequent trial of labor, compared with a history of spontaneous labor.4
In addition, several studies have demonstrated that the indication for the first cesarean delivery has a bearing on the success of a subsequent trial of labor. For example, an indication of shoulder dystocia reduces the success rate of a subsequent trial of labor by one third.2
Even a brief trial of labor before the cesarean may increase the success of a subsequent trial of labor. One study found that cervical dilation to 8 cm or greater was independently predictive of successful VBAC among women who had a nonrecurring indication for the initial cesarean delivery.5
When the cesarean delivery involves a preterm infant, the risk of uterine rupture during a subsequent trial of labor may increase if the infant is at term. Conversely, the risk of uterine rupture is lower when a term cesarean is followed by a preterm trial of labor.6
A vertical hysterotomy may preclude VBAC
A previous classical hysterotomy is generally an absolute contraindication for a trial of labor because rupture may occur in as many as 14% of women who have this type of scar.
Low transverse hysterotomy does not appear to confer excess risk during a subsequent trial of labor. Less clear is whether a low vertical hysterotomy poses a risk of rupture. In a 2004 prospective cohort study, the rate of uterine rupture among women who had a transverse hysterotomy scar was 0.7%, compared with 2.0% for a low vertical scar. Any difference in the rate of uterine rupture in retrospective studies may be attributable, at least in part, to the subjective nature of the definition of “low vertical” because there is no precise or objective way to ensure that the vertical hysterotomy did not breach the contractile portion of the uterus (FIGURE).

Type of prior hysterotomy influences the VBAC decision
A classical uterine incision is an absolute contraindication to vaginal birth after cesarean (VBAC). A trial of labor is thought to be safe in women who have had a low transverse hysterotomy. The jury is still out on the safety of VBAC in a woman who has had a low vertical incision, however, because of uncertainty over whether the contractile portion of the uterus is involved.
Are multiple cesareans a contraindication to VBAC?
Experts disagree as to whether more than one previous cesarean delivery before a trial of labor increases the risk of uterine rupture. One retrospective study showed no difference in the rate of rupture between women who had a single previous cesarean and those who had more than one.7 A larger prospective study showed a modest increase in the risk of rupture (OR, 1.16) among women who had undergone more than one cesarean—but no decrease in the chance of success.8
Most large retrospective and prospective studies include patients who have had more than one previous cesarean delivery, but their numbers remain low; therefore, statistical significance cannot be determined.
Induction or augmentation of labor may lower odds of success
The likelihood of successful VBAC may be reduced when labor is augmented or induced. The picture is unclear because most studies that have focused on cervical ripening and induction of labor in VBAC are small.
Bujold compared pregnancy outcomes of three groups of women:
- those who underwent cervical ripening via Foley catheter
- those who had amniotomy and oxytocin administration
- those who entered labor spontaneously.
No difference in the rate of uterine rupture was found among the groups. However, the group that underwent cervical ripening had a significantly lower rate of success.9
A large case-control study found no increase in the rate of rupture when oxytocin or prostaglandins were administered, but the rate tripled when both were used together.10
A small, nested, case-control study found an increased risk of uterine rupture only when oxytocin was administered at a rate exceeding 20 mU/mL.11
More than 90% of hysterotomies are transverse
When the obstetric history is incomplete, the clinician may not know what type of hysterotomy was used in the previous cesarean delivery. Most experts believe that VBAC is acceptable when the previous cesarean involved a low transverse hysterotomy. The risk may be much higher with other types of incisions. Today, however, with modern techniques in place, we can assume that more than 90% of hysterotomies are of the low transverse type.
At least one study suggests that the risk of uterine rupture during vaginal birth after cesarean is acceptably low when the type of hysterotomy is unknown. That study explored the effect of augmentation of labor with oxytocin among women who had an unknown scar and found an increased risk of rupture, compared with women who were managed expectantly. However, the overall rate of uterine rupture did not differ from the rate expected when the hysterotomy is known to be of the low transverse type.12
VBAC for twins is rare
Because few women carrying twins attempt VBAC, we have little data to guide counseling on success and complication rates. A multicenter, retrospective, cohort study explored delivery outcomes of 25,005 women who had undergone at least one previous cesarean. Of these women, 24,307 had a singleton pregnancy, and 535 were carrying twins. Women who had a twin gestation were 40% less likely to attempt a trial of labor, but those who did had a chance of success and risk of uterine rupture similar to those of women with a singleton gestation. Women carrying twins who underwent a trial of labor had an elevated risk of requiring transfusion, compared with those carrying singletons, but this risk was similar to that of women delivering twins by elective repeat cesarean. In fact, women who delivered twins by repeat cesarean tended to have more maternal morbidity overall than those who had a trial of labor.13
A short interpregnancy interval precludes VBAC
Data indicate that a trial of labor after cesarean should be avoided in women who have a brief interpregnancy interval. Several retrospective studies had found an increased risk of uterine rupture, as well as a host of other adverse outcomes, among these women. Using 12 months as a reference point, women who had an interpregnancy interval shorter than 6 months had triple the risk of uterine rupture.14 Although the mechanism is unknown, rupture is presumably the result of incomplete healing of the hysterotomy.
Macrosomia may not increase the risk of rupture
Women who are thought to have a macrosomic fetus may be encouraged to attempt VBAC, if they so desire. Macrosomia is a minor risk factor for failure of a trial of labor, but it does not necessarily increase the risk of uterine rupture.15
Elkousy examined VBAC success rates by birth weight, indication for the previous cesarean delivery, and pregnancy history. Not surprisingly, increased birth weight or a history of cephalopelvic disproportion reduced the rate of success, but a history of vaginal delivery negated that risk of failure. A history of successful VBAC improved the chance of success to more than 90%—even when the birth weight exceeded 4,000 g—and the success rate reached 82% when the birth weight exceeded 4,500 g.16
Physician and hospital attitudes toward vaginal birth after cesarean delivery (VBAC) may be a major determinant of its frequency and success. Many forces oppose women who desire a trial of labor after cesarean. Hospitals and insurers make it increasingly difficult to offer a trial of labor, and strict interpretation of ACOG’s guidelines requiring personnel to be “immediately available” during a trial of labor has caused many smaller and isolated hospitals to stop offering this option. The number of women who attempt VBAC has plummeted.20
Two recent surveys by ACOG indicate that an alarming number of providers have stopped offering VBAC because of a lack of insurance and fear of legal liability. As providers offer a trial of labor less and less, skills decline, and so does mentorship of younger physicians.
The NIH weighs in
In March 2010, the National Institutes of Health (NIH) convened a consensus development conference on the topic of VBAC. A panel of health professionals and public representatives reviewed the medical literature and produced a consensus statement. Their conclusion:
- Given the available evidence, [a trial of labor] is a reasonable option for many pregnant women with a prior low transverse uterine incision. The data reviewed in this report show that both [a trial of labor] and elective repeat cesarean for a pregnant woman with a prior transverse uterine incision have important risks and benefits and that these risks and benefits differ for the woman and her fetus.
The panel’s goal was to help women who have a history of cesarean delivery make an informed, evidence-based decision about the subsequent mode of delivery. The panel also acknowledged the general lack of high-quality evidence to confidently quantify the risks and benefits of a trial of labor versus planned repeat cesarean delivery.21
For another point of view on vaginal birth after cesarean, see the Editorial, “Does vaginal birth after cesarean have a future?” by John T. Repke, MD, of the OBG Management Board of Editors.
Repeat cesarean is probably best for obese gravidas
Obesity increases the likelihood of cesarean delivery in all circumstances, so it is not surprising that it is a risk factor for a failed trial of labor after cesarean. Obesity also increases the risks of anesthesia and surgery. Because of these risks, most clinicians opt to deliver obese patients by scheduled elective cesarean rather than risk having to perform emergent cesarean delivery in the case of acute fetal compromise or uterine rupture.
Race is not a risk factor for rupture
Race is probably not a significant independent risk factor for failure of VBAC. A secondary analysis of a multicenter, retrospective, cohort study found that black women were somewhat more likely to fail a trial of labor than white women (OR, 1.50; 95% CI, 1.29–1.74), after adjustment for confounding variables. However, black women undergoing a trial of labor were 40% less likely to suffer a uterine rupture than white women were.17
When comorbidities are well managed, VBAC remains an option
In general, a trial of labor in women who have well managed chronic medical disease does not pose undue risk to mother or baby.
In a population-based, retrospective cohort study using discharge data from California, Gregory and coworkers attempted to delineate clinical variables that might be associated with VBAC success and complications. They examined a wide range of maternal conditions, from diabetes to chorioamnionitis, as well as fetal conditions, such as oligohydramnios and unengaged vertex. Mothers were stratified into low- and high-risk groups, and multivariate logistic regression was performed. Low-risk patients had a 73.7% success rate, whereas high-risk patients had a 50% success rate. Not surprisingly, women who had a fetus with an unengaged vertex had a 9.8% chance of success and an eightfold increase in the risk of uterine rupture.18
At first glance, the issue of vaginal birth after cesarean delivery (VBAC) appears to boil down to a simple question: Should I attempt it, or shouldn’t I?
On deeper inspection, the decision becomes extremely complex, and the evidence can be confusing.
Both planned elective repeat cesarean and planned VBAC are associated with harms as well as benefits. Most experts would agree than an uncomplicated vaginal delivery poses little risk to mother and baby, and that a planned repeat cesarean delivery at term carries some risk to the mother.
The greatest risks for both mother and baby arise when a trial of labor fails and cesarean delivery becomes necessary for maternal or fetal indications. Risks to the mother are largely operative in nature, and the primary risk to the fetus is uterine rupture. However, maternal and fetal risks cannot be truly separated. Uterine rupture not only compromises the fetus in utero but has a severe impact on maternal hemodynamic stability, just as a fetal hypoxic ischemic insult secondary to uterine rupture can have lifelong psychological and social consequences for the mother and family.
We are fortunate that serious adverse outcomes of VBAC are rare. Nevertheless, the only predictable delivery method is planned elective repeat cesarean. Uncertainty over the likelihood of success of VBAC arises when relative risk is confused with absolute risk.
In this article, I examine the literature on the route of delivery after cesarean to assess the overall safety of a trial of labor in various settings and populations.
Data on VBAC are limited
We lack randomized, controlled trials and valid animal studies that assess fetal and maternal outcomes of elective repeat cesarean versus planned vaginal delivery. The vast majority of studies of VBAC are retrospective or cohort studies, which have inherent potential for bias. Many studies lack a standardized definition of adverse outcomes or lack direct evidence that adverse outcomes are wholly attributable to the trial of labor. No studies compare women who are similar in all characteristics except their mode of delivery.
Nor do we fully understand how women choose a course of action after cesarean delivery—except that the decision is almost always multifactorial. Competing voices—health care provider, family members, friends, media, and a woman’s own memory of her previous delivery—and her emotional state—all contribute to the decision.
Clearly, a trial of labor after cesarean delivery can be safe for many women. Successful vaginal delivery is associated with a very low risk of adverse outcomes and may be associated with a lower risk of minor morbidity than is elective repeat cesarean. In fact, the overall success rate for a trial of labor after cesarean is not that different from the success rate for nulliparous women undergoing induction of labor.19 Even so, patients should understand that operative delivery may be necessary, and the physician and hospital must be prepared for this eventuality in accordance with ACOG guidelines.
As I interpret the data, if a woman has undergone one low transverse cesarean delivery for a nonrecurring condition and a nonmacrosomic fetus, a trial of labor after the spontaneous onset of labor should be strongly encouraged. If she has already delivered vaginally in the past, or had a successful VBAC, she is an even better candidate for a trial of labor. In such a case, labor induction with mechanical cervical ripening or appropriate use of oxytocin, or both, may still be appropriate, but the likelihood of success is lower.
If a woman has a history of more than one cesarean delivery without a vaginal birth, she may be better served by scheduled repeat cesarean delivery. The same holds true for women who have a history of preterm cesarean delivery, a short interpregnancy interval, suspected macrosomia, or an unengaged fetal vertex.
Decision-making about delivery should be shared between the provider and patient, after thorough counseling about the risks and benefits in language the patient can easily comprehend.
It would be best to avoid having to make a decision about VBAC by preventing the initial cesarean delivery.
How risky is repeat cesarean?
We are all acutely aware of the skyrocketing rate of cesarean delivery, which reaches 35% to 41% in some areas. Most studies indicate that approximately 50% of all cesarean deliveries are repeat cesarean deliveries. Besides the risks associated with the operation itself, planned repeat cesarean has significant downstream implications for the mother and baby—and for society. For example, multiple cesarean deliveries pose an ever greater risk of abnormal placentation and maternal hemorrhage. Cesarean delivery without labor can also heighten the risk of neonatal respiratory compromise, temperature instability, and slow feeding.1 Cesarean delivery and its longer attendant hospitalization markedly increase costs throughout an already strapped health care system.
On balance, any cesarean delivery imparts an increased risk of maternal morbidity and mortality, compared with vaginal delivery, as well as an increased risk of complications, such as placenta previa and placenta accreta, in subsequent pregnancies.
What are the risks of a trial of labor?
A prospective, 4-year observational study conducted at 19 academic medical centers under the auspices of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network compared the outcomes of 17,898 women undergoing a trial of labor after cesarean delivery with those of 15,801 women having elective repeat cesarean.2 Symptomatic uterine rupture occurred in 0.7% of the women attempting a trial of labor, with no occurrences in the elective cesarean group. Blood transfusion and endomyometritis were more common in the group undergoing a trial of labor, and this difference was statistically significant. These findings are in concordance with those of earlier studies.
The two groups in this study were not exactly the same; more women undergoing a trial of labor had had a previous vaginal delivery. Significant adverse maternal outcomes, such as endomyometritis, uterine rupture, hysterectomy, and the need for transfusion, were much more likely in a failed trial of labor than in a successful one.
The same study found a 0.46% risk of hypoxic-ischemic encephalopathy, which was most likely to occur after symptomatic uterine rupture (7 of 12 cases). No cases of hypoxic-ischemic encephalopathy occurred among women undergoing planned cesarean delivery. Multivariate logistic regression analysis determined that the risks of stillbirth, neonatal death, and hypoxic-ischemic encephalopathy in term infants were increased in the group undergoing a trial of labor, compared with elective repeat cesarean (odds ratio [OR], 2.72; 95% confidence interval [CI], 1.49–4.97).
Can we predict the success of a trial of labor?
Combined success rates from a large number of prospective cohort studies suggest an overall rate of 75.9%. Many clinical characteristics may increase the likelihood of success of a trial of labor after cesarean. In this section, I describe these characteristics and sift the data we have about them.
A history of vaginal delivery ups the odds of success
Women who have delivered vaginally have a much lower risk of rupture during a trial of labor after cesarean than women who have not. Women who have delivered vaginally are also four times more likely to have a successful VBAC. A multicenter, prospective study found a VBAC success rate of 86% among women who had already delivered vaginally, and a success rate of 90% among women who had a history of successful VBAC.3
Many aspects of the cesarean delivery have continuing impact
The type of labor that occurred in the cesarean delivery may help predict subsequent complications and the ultimate success of a trial of labor. For example, induced labor or no labor prior to cesarean delivery is associated with a 2.25-fold risk of uterine rupture in a subsequent trial of labor, compared with a history of spontaneous labor.4
In addition, several studies have demonstrated that the indication for the first cesarean delivery has a bearing on the success of a subsequent trial of labor. For example, an indication of shoulder dystocia reduces the success rate of a subsequent trial of labor by one third.2
Even a brief trial of labor before the cesarean may increase the success of a subsequent trial of labor. One study found that cervical dilation to 8 cm or greater was independently predictive of successful VBAC among women who had a nonrecurring indication for the initial cesarean delivery.5
When the cesarean delivery involves a preterm infant, the risk of uterine rupture during a subsequent trial of labor may increase if the infant is at term. Conversely, the risk of uterine rupture is lower when a term cesarean is followed by a preterm trial of labor.6
A vertical hysterotomy may preclude VBAC
A previous classical hysterotomy is generally an absolute contraindication for a trial of labor because rupture may occur in as many as 14% of women who have this type of scar.
Low transverse hysterotomy does not appear to confer excess risk during a subsequent trial of labor. Less clear is whether a low vertical hysterotomy poses a risk of rupture. In a 2004 prospective cohort study, the rate of uterine rupture among women who had a transverse hysterotomy scar was 0.7%, compared with 2.0% for a low vertical scar. Any difference in the rate of uterine rupture in retrospective studies may be attributable, at least in part, to the subjective nature of the definition of “low vertical” because there is no precise or objective way to ensure that the vertical hysterotomy did not breach the contractile portion of the uterus (FIGURE).

Type of prior hysterotomy influences the VBAC decision
A classical uterine incision is an absolute contraindication to vaginal birth after cesarean (VBAC). A trial of labor is thought to be safe in women who have had a low transverse hysterotomy. The jury is still out on the safety of VBAC in a woman who has had a low vertical incision, however, because of uncertainty over whether the contractile portion of the uterus is involved.
Are multiple cesareans a contraindication to VBAC?
Experts disagree as to whether more than one previous cesarean delivery before a trial of labor increases the risk of uterine rupture. One retrospective study showed no difference in the rate of rupture between women who had a single previous cesarean and those who had more than one.7 A larger prospective study showed a modest increase in the risk of rupture (OR, 1.16) among women who had undergone more than one cesarean—but no decrease in the chance of success.8
Most large retrospective and prospective studies include patients who have had more than one previous cesarean delivery, but their numbers remain low; therefore, statistical significance cannot be determined.
Induction or augmentation of labor may lower odds of success
The likelihood of successful VBAC may be reduced when labor is augmented or induced. The picture is unclear because most studies that have focused on cervical ripening and induction of labor in VBAC are small.
Bujold compared pregnancy outcomes of three groups of women:
- those who underwent cervical ripening via Foley catheter
- those who had amniotomy and oxytocin administration
- those who entered labor spontaneously.
No difference in the rate of uterine rupture was found among the groups. However, the group that underwent cervical ripening had a significantly lower rate of success.9
A large case-control study found no increase in the rate of rupture when oxytocin or prostaglandins were administered, but the rate tripled when both were used together.10
A small, nested, case-control study found an increased risk of uterine rupture only when oxytocin was administered at a rate exceeding 20 mU/mL.11
More than 90% of hysterotomies are transverse
When the obstetric history is incomplete, the clinician may not know what type of hysterotomy was used in the previous cesarean delivery. Most experts believe that VBAC is acceptable when the previous cesarean involved a low transverse hysterotomy. The risk may be much higher with other types of incisions. Today, however, with modern techniques in place, we can assume that more than 90% of hysterotomies are of the low transverse type.
At least one study suggests that the risk of uterine rupture during vaginal birth after cesarean is acceptably low when the type of hysterotomy is unknown. That study explored the effect of augmentation of labor with oxytocin among women who had an unknown scar and found an increased risk of rupture, compared with women who were managed expectantly. However, the overall rate of uterine rupture did not differ from the rate expected when the hysterotomy is known to be of the low transverse type.12
VBAC for twins is rare
Because few women carrying twins attempt VBAC, we have little data to guide counseling on success and complication rates. A multicenter, retrospective, cohort study explored delivery outcomes of 25,005 women who had undergone at least one previous cesarean. Of these women, 24,307 had a singleton pregnancy, and 535 were carrying twins. Women who had a twin gestation were 40% less likely to attempt a trial of labor, but those who did had a chance of success and risk of uterine rupture similar to those of women with a singleton gestation. Women carrying twins who underwent a trial of labor had an elevated risk of requiring transfusion, compared with those carrying singletons, but this risk was similar to that of women delivering twins by elective repeat cesarean. In fact, women who delivered twins by repeat cesarean tended to have more maternal morbidity overall than those who had a trial of labor.13
A short interpregnancy interval precludes VBAC
Data indicate that a trial of labor after cesarean should be avoided in women who have a brief interpregnancy interval. Several retrospective studies had found an increased risk of uterine rupture, as well as a host of other adverse outcomes, among these women. Using 12 months as a reference point, women who had an interpregnancy interval shorter than 6 months had triple the risk of uterine rupture.14 Although the mechanism is unknown, rupture is presumably the result of incomplete healing of the hysterotomy.
Macrosomia may not increase the risk of rupture
Women who are thought to have a macrosomic fetus may be encouraged to attempt VBAC, if they so desire. Macrosomia is a minor risk factor for failure of a trial of labor, but it does not necessarily increase the risk of uterine rupture.15
Elkousy examined VBAC success rates by birth weight, indication for the previous cesarean delivery, and pregnancy history. Not surprisingly, increased birth weight or a history of cephalopelvic disproportion reduced the rate of success, but a history of vaginal delivery negated that risk of failure. A history of successful VBAC improved the chance of success to more than 90%—even when the birth weight exceeded 4,000 g—and the success rate reached 82% when the birth weight exceeded 4,500 g.16
Physician and hospital attitudes toward vaginal birth after cesarean delivery (VBAC) may be a major determinant of its frequency and success. Many forces oppose women who desire a trial of labor after cesarean. Hospitals and insurers make it increasingly difficult to offer a trial of labor, and strict interpretation of ACOG’s guidelines requiring personnel to be “immediately available” during a trial of labor has caused many smaller and isolated hospitals to stop offering this option. The number of women who attempt VBAC has plummeted.20
Two recent surveys by ACOG indicate that an alarming number of providers have stopped offering VBAC because of a lack of insurance and fear of legal liability. As providers offer a trial of labor less and less, skills decline, and so does mentorship of younger physicians.
The NIH weighs in
In March 2010, the National Institutes of Health (NIH) convened a consensus development conference on the topic of VBAC. A panel of health professionals and public representatives reviewed the medical literature and produced a consensus statement. Their conclusion:
- Given the available evidence, [a trial of labor] is a reasonable option for many pregnant women with a prior low transverse uterine incision. The data reviewed in this report show that both [a trial of labor] and elective repeat cesarean for a pregnant woman with a prior transverse uterine incision have important risks and benefits and that these risks and benefits differ for the woman and her fetus.
The panel’s goal was to help women who have a history of cesarean delivery make an informed, evidence-based decision about the subsequent mode of delivery. The panel also acknowledged the general lack of high-quality evidence to confidently quantify the risks and benefits of a trial of labor versus planned repeat cesarean delivery.21
For another point of view on vaginal birth after cesarean, see the Editorial, “Does vaginal birth after cesarean have a future?” by John T. Repke, MD, of the OBG Management Board of Editors.
Repeat cesarean is probably best for obese gravidas
Obesity increases the likelihood of cesarean delivery in all circumstances, so it is not surprising that it is a risk factor for a failed trial of labor after cesarean. Obesity also increases the risks of anesthesia and surgery. Because of these risks, most clinicians opt to deliver obese patients by scheduled elective cesarean rather than risk having to perform emergent cesarean delivery in the case of acute fetal compromise or uterine rupture.
Race is not a risk factor for rupture
Race is probably not a significant independent risk factor for failure of VBAC. A secondary analysis of a multicenter, retrospective, cohort study found that black women were somewhat more likely to fail a trial of labor than white women (OR, 1.50; 95% CI, 1.29–1.74), after adjustment for confounding variables. However, black women undergoing a trial of labor were 40% less likely to suffer a uterine rupture than white women were.17
When comorbidities are well managed, VBAC remains an option
In general, a trial of labor in women who have well managed chronic medical disease does not pose undue risk to mother or baby.
In a population-based, retrospective cohort study using discharge data from California, Gregory and coworkers attempted to delineate clinical variables that might be associated with VBAC success and complications. They examined a wide range of maternal conditions, from diabetes to chorioamnionitis, as well as fetal conditions, such as oligohydramnios and unengaged vertex. Mothers were stratified into low- and high-risk groups, and multivariate logistic regression was performed. Low-risk patients had a 73.7% success rate, whereas high-risk patients had a 50% success rate. Not surprisingly, women who had a fetus with an unengaged vertex had a 9.8% chance of success and an eightfold increase in the risk of uterine rupture.18
1. Tita AT, Landon MB, Spong CY, et al. For Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111-1120.
2. Landon MB, Hauth JC, Leveno KJ, et al. For NICHD MFMU Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
3. Landon MB, Leindecker S, Spong CY, et al. For NICHD MFMU Network. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. Am J Obst Gynecol. 2005;193(3 Pt 2):1016-1023.
4. Algert CS, Morris JM, Simpson JM, Ford JB, Roberts CL. Labor before a primary cesarean delivery: reduced risk of uterine rupture in a subsequent trial of labor for vaginal birth after cesarean. Obstet Gynecol. 2008;112(5):1061-1066.
5. Kwon JY, Jo YS, Lee GS, Kim SJ, Shin JC, Lee Y. Cervical dilation at the time of cesarean section may affect the success of subsequent vaginal delivery. J Matern Fetal Neonatal Med. 2009;22(11):1057-1062.
6. Sciscione AC, Landon MB, Leveno KJ, et al. For NICHD MFMU Network. Previous preterm delivery and risk of subsequent uterine rupture. Obstet Gynecol. 2008;111(3):648-653.
7. Landon MB, Spong CY, Thom E, et al. For NICHD MFMU Network. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet Gynecol. 2006;108(1):12-20.
8. Macones GA, Cahill A, Pare E, et al. Obstetric outcomes in women with two prior cesarean deliveries: is vaginal birth after cesarean delivery a viable option? Am J Obstet Gynecol. 2005;192(4):1223-1229.
9. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103(1):18-23.
10. Macones G, Peipert J, Nelson D, et al. Maternal complications with vaginal birth after cesarean delivery: a multicenter study. Am J Obstet Gynecol. 2005;193(5):1656-1662.
11. Cahill A, Stamilio D, Odibo A, Peipert J, Stevens E, Macones G. Does a maximum dose of oxytocin affect risk for uterine rupture in candidates for vaginal birth after cesarean delivery? Am J Obstet Gynecol. 2007;197(5):495.e1-e5.
12. Grubb DK, Kjos SL, Paul RH. Latent labor with an unknown uterine scar. Obstet Gynecol. 1996;88(3):351-355.
13. Cahill A, Stamilio DM, Paré E, et al. Vaginal birth after cesarean (VBAC) attempt in twin pregnancies: is it safe? Am J Obstet Gynecol. 2005;193(3 Pt 2):1050-1055.
14. Stamilio DM, DeFranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075.-
15. Zelop CM, Shipp TD, Repke JT, Cohen A, Lieberman E. Outcomes of trial of labor following previous cesarean delivery among women with fetuses weighing >4000 g. Am J Obstet Gynecol. 2001;185(4):903-905.
16. Elkousy M, Sammel M, Stevens E, Peipert J, Macones G. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188(3):824-830.
17. Cahill AG, Stamilio DM, Odibo AO, Peipert J, Stevens E, Macones GA. Racial disparity in the success and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2008;111(3):654-658.
18. Gregory KD, Korst LM, Fridman M, et al. Vaginal birth after cesarean: clinical risk factors associated with adverse outcome. Am J Obstet Gynecol. 2008;198(4):452.e1-e12.
19. Smith GCS, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287(20):2684-2690.
20. Hamilton BE, Martin JA, Sutton PD. For US Department of Health and Human Services. Births: preliminary data for 2002. Natl Vital Stat Rep. 2003;51(11):1-20.
21. National Institutes of Health Consensus Development Conference Statement. Vaginal Birth after Cesarean: New Insights. Bethesda, Md: NIH; 2010. http://consensus.nih.gov/2010/images/vbac/vbac_statement.pdf. Accessed June 16, 2010.
1. Tita AT, Landon MB, Spong CY, et al. For Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111-1120.
2. Landon MB, Hauth JC, Leveno KJ, et al. For NICHD MFMU Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
3. Landon MB, Leindecker S, Spong CY, et al. For NICHD MFMU Network. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. Am J Obst Gynecol. 2005;193(3 Pt 2):1016-1023.
4. Algert CS, Morris JM, Simpson JM, Ford JB, Roberts CL. Labor before a primary cesarean delivery: reduced risk of uterine rupture in a subsequent trial of labor for vaginal birth after cesarean. Obstet Gynecol. 2008;112(5):1061-1066.
5. Kwon JY, Jo YS, Lee GS, Kim SJ, Shin JC, Lee Y. Cervical dilation at the time of cesarean section may affect the success of subsequent vaginal delivery. J Matern Fetal Neonatal Med. 2009;22(11):1057-1062.
6. Sciscione AC, Landon MB, Leveno KJ, et al. For NICHD MFMU Network. Previous preterm delivery and risk of subsequent uterine rupture. Obstet Gynecol. 2008;111(3):648-653.
7. Landon MB, Spong CY, Thom E, et al. For NICHD MFMU Network. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet Gynecol. 2006;108(1):12-20.
8. Macones GA, Cahill A, Pare E, et al. Obstetric outcomes in women with two prior cesarean deliveries: is vaginal birth after cesarean delivery a viable option? Am J Obstet Gynecol. 2005;192(4):1223-1229.
9. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103(1):18-23.
10. Macones G, Peipert J, Nelson D, et al. Maternal complications with vaginal birth after cesarean delivery: a multicenter study. Am J Obstet Gynecol. 2005;193(5):1656-1662.
11. Cahill A, Stamilio D, Odibo A, Peipert J, Stevens E, Macones G. Does a maximum dose of oxytocin affect risk for uterine rupture in candidates for vaginal birth after cesarean delivery? Am J Obstet Gynecol. 2007;197(5):495.e1-e5.
12. Grubb DK, Kjos SL, Paul RH. Latent labor with an unknown uterine scar. Obstet Gynecol. 1996;88(3):351-355.
13. Cahill A, Stamilio DM, Paré E, et al. Vaginal birth after cesarean (VBAC) attempt in twin pregnancies: is it safe? Am J Obstet Gynecol. 2005;193(3 Pt 2):1050-1055.
14. Stamilio DM, DeFranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075.-
15. Zelop CM, Shipp TD, Repke JT, Cohen A, Lieberman E. Outcomes of trial of labor following previous cesarean delivery among women with fetuses weighing >4000 g. Am J Obstet Gynecol. 2001;185(4):903-905.
16. Elkousy M, Sammel M, Stevens E, Peipert J, Macones G. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188(3):824-830.
17. Cahill AG, Stamilio DM, Odibo AO, Peipert J, Stevens E, Macones GA. Racial disparity in the success and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2008;111(3):654-658.
18. Gregory KD, Korst LM, Fridman M, et al. Vaginal birth after cesarean: clinical risk factors associated with adverse outcome. Am J Obstet Gynecol. 2008;198(4):452.e1-e12.
19. Smith GCS, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287(20):2684-2690.
20. Hamilton BE, Martin JA, Sutton PD. For US Department of Health and Human Services. Births: preliminary data for 2002. Natl Vital Stat Rep. 2003;51(11):1-20.
21. National Institutes of Health Consensus Development Conference Statement. Vaginal Birth after Cesarean: New Insights. Bethesda, Md: NIH; 2010. http://consensus.nih.gov/2010/images/vbac/vbac_statement.pdf. Accessed June 16, 2010.
Study Looks at Oxygen Saturation in Preemies
Major Findings: Death before discharge occurred in 20% of the infants in the lower oxygen saturation, compared with 16% of those in the higher group. In contrast, severe retinopathy among the surviving infants occurred significantly less often in the lower group at 9%, compared with the higher group at 18%.
Data Source: The multicenter Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) comprising 1,316 preterm infants from 24 weeks to 27 weeks and 6 days gestation.
Disclosures: SUPPORT was funded by the National Institutes of Health and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute. One of the study co-authors, Dr. Krisa P. Van Meurs, disclosed receiving travel expenses from Ikaria Holdings. Dr. Colin Morley disclosed financial relationships with Dräger Medical and Fisher & Paykel.
Using lower target ranges of oxygen saturation in extremely preterm infants reduces the risk of severe retinopathy from oxygen toxicity, but increases the risk of death before discharge, according to one of two trials within the same neonatal study.
In the second trial, continuous positive airway pressure (CPAP) was determined to be an effective alternative to early surfactant administration followed by conventional intubation, with fewer complications.
Researchers in the multicenter Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) used a 2×2 factorial design to compare two target ranges of oxygen saturation in 1,316 infants who were born between 24 weeks and 27 weeks 6 days of gestation. In addition, they compared intubation and surfactant treatment initiated within 1 hour after birth and CPAP treatment initiated in the delivery room with subsequent use of a limited ventilation strategy.
For the oxygen range arm of the study, the infants were randomly assigned to the lower oxygen saturation target range of 85%-89% or the higher target range of 91%-95%. At the same time, they were randomly assigned to receive the oxygen through a ventilator or through a CPAP machine.
The primary outcome of the oxygen saturation investigation was a composite of severe retinopathy of prematurity (defined as the presence of threshold retinopathy, the need for surgical ophthalmologic intervention, or the use of bevacizumab), death before hospital discharge, or both, wrote Dr. Waldemar A. Carlo of the University of Alabama at Birmingham, and colleagues.
Although there was no overall difference between the two oxygen saturation groups using the composite outcome measure, marked differences were observed when the two components of the measure were considered independently, the authors wrote. Specifically, death before discharge occurred in 20% of the infants in the lower target group compared with 16% of those in the higher group. In contrast, severe retinopathy among the surviving infants occurred significantly less often in the lower group, at 9%, compared with the higher group, at 18%. These findings, the authors note, “add to the concern that oxygen restriction may increase the rate of death among preterm infants.” As such, they advise exercising caution when considering a strategy that targets oxygen levels in the low range (N. Engl. J. Med. 2010;362:1959-69).
For the CPAP vs. early intubation/surfactant study arm, the primary outcome was death or bronchopulmonary dysplasia, defined by the need for supplemental oxygen at 36 weeks, wrote Dr. Neil N. Finer of the University of California at San Diego, and colleagues. After adjusting for gestational age, medical center, and family clustering, the need for supplemental oxygen was similar in both groups, although the CPAP infants less frequently required intubation or postnatal corticosteroids for bronchopulmonary dysplasia than the surfactant group, and they also required fewer days of mechanical ventilation and were more likely to be alive by day 7, the authors wrote.
Considering the lower complication rate, the findings “support consideration of CPAP as an alternative to routine intubation and surfactant administration in preterm infants,” the authors wrote (N. Engl. J. Med. 2010;362:1970-9).
In an accompanying editorial, Dr. Colin J. Morley of the University of Melbourne stressed that caution is warranted in interpreting the “most important outcome” linking the lower target oxygen saturation range and death before discharge. “Additional research is needed to clarify this finding,” he said. “There were no significant differences between the groups in short-term outcomes that have been associated with relative ischemia.”
Major Findings: Death before discharge occurred in 20% of the infants in the lower oxygen saturation, compared with 16% of those in the higher group. In contrast, severe retinopathy among the surviving infants occurred significantly less often in the lower group at 9%, compared with the higher group at 18%.
Data Source: The multicenter Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) comprising 1,316 preterm infants from 24 weeks to 27 weeks and 6 days gestation.
Disclosures: SUPPORT was funded by the National Institutes of Health and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute. One of the study co-authors, Dr. Krisa P. Van Meurs, disclosed receiving travel expenses from Ikaria Holdings. Dr. Colin Morley disclosed financial relationships with Dräger Medical and Fisher & Paykel.
Using lower target ranges of oxygen saturation in extremely preterm infants reduces the risk of severe retinopathy from oxygen toxicity, but increases the risk of death before discharge, according to one of two trials within the same neonatal study.
In the second trial, continuous positive airway pressure (CPAP) was determined to be an effective alternative to early surfactant administration followed by conventional intubation, with fewer complications.
Researchers in the multicenter Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) used a 2×2 factorial design to compare two target ranges of oxygen saturation in 1,316 infants who were born between 24 weeks and 27 weeks 6 days of gestation. In addition, they compared intubation and surfactant treatment initiated within 1 hour after birth and CPAP treatment initiated in the delivery room with subsequent use of a limited ventilation strategy.
For the oxygen range arm of the study, the infants were randomly assigned to the lower oxygen saturation target range of 85%-89% or the higher target range of 91%-95%. At the same time, they were randomly assigned to receive the oxygen through a ventilator or through a CPAP machine.
The primary outcome of the oxygen saturation investigation was a composite of severe retinopathy of prematurity (defined as the presence of threshold retinopathy, the need for surgical ophthalmologic intervention, or the use of bevacizumab), death before hospital discharge, or both, wrote Dr. Waldemar A. Carlo of the University of Alabama at Birmingham, and colleagues.
Although there was no overall difference between the two oxygen saturation groups using the composite outcome measure, marked differences were observed when the two components of the measure were considered independently, the authors wrote. Specifically, death before discharge occurred in 20% of the infants in the lower target group compared with 16% of those in the higher group. In contrast, severe retinopathy among the surviving infants occurred significantly less often in the lower group, at 9%, compared with the higher group, at 18%. These findings, the authors note, “add to the concern that oxygen restriction may increase the rate of death among preterm infants.” As such, they advise exercising caution when considering a strategy that targets oxygen levels in the low range (N. Engl. J. Med. 2010;362:1959-69).
For the CPAP vs. early intubation/surfactant study arm, the primary outcome was death or bronchopulmonary dysplasia, defined by the need for supplemental oxygen at 36 weeks, wrote Dr. Neil N. Finer of the University of California at San Diego, and colleagues. After adjusting for gestational age, medical center, and family clustering, the need for supplemental oxygen was similar in both groups, although the CPAP infants less frequently required intubation or postnatal corticosteroids for bronchopulmonary dysplasia than the surfactant group, and they also required fewer days of mechanical ventilation and were more likely to be alive by day 7, the authors wrote.
Considering the lower complication rate, the findings “support consideration of CPAP as an alternative to routine intubation and surfactant administration in preterm infants,” the authors wrote (N. Engl. J. Med. 2010;362:1970-9).
In an accompanying editorial, Dr. Colin J. Morley of the University of Melbourne stressed that caution is warranted in interpreting the “most important outcome” linking the lower target oxygen saturation range and death before discharge. “Additional research is needed to clarify this finding,” he said. “There were no significant differences between the groups in short-term outcomes that have been associated with relative ischemia.”
Major Findings: Death before discharge occurred in 20% of the infants in the lower oxygen saturation, compared with 16% of those in the higher group. In contrast, severe retinopathy among the surviving infants occurred significantly less often in the lower group at 9%, compared with the higher group at 18%.
Data Source: The multicenter Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) comprising 1,316 preterm infants from 24 weeks to 27 weeks and 6 days gestation.
Disclosures: SUPPORT was funded by the National Institutes of Health and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute. One of the study co-authors, Dr. Krisa P. Van Meurs, disclosed receiving travel expenses from Ikaria Holdings. Dr. Colin Morley disclosed financial relationships with Dräger Medical and Fisher & Paykel.
Using lower target ranges of oxygen saturation in extremely preterm infants reduces the risk of severe retinopathy from oxygen toxicity, but increases the risk of death before discharge, according to one of two trials within the same neonatal study.
In the second trial, continuous positive airway pressure (CPAP) was determined to be an effective alternative to early surfactant administration followed by conventional intubation, with fewer complications.
Researchers in the multicenter Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) used a 2×2 factorial design to compare two target ranges of oxygen saturation in 1,316 infants who were born between 24 weeks and 27 weeks 6 days of gestation. In addition, they compared intubation and surfactant treatment initiated within 1 hour after birth and CPAP treatment initiated in the delivery room with subsequent use of a limited ventilation strategy.
For the oxygen range arm of the study, the infants were randomly assigned to the lower oxygen saturation target range of 85%-89% or the higher target range of 91%-95%. At the same time, they were randomly assigned to receive the oxygen through a ventilator or through a CPAP machine.
The primary outcome of the oxygen saturation investigation was a composite of severe retinopathy of prematurity (defined as the presence of threshold retinopathy, the need for surgical ophthalmologic intervention, or the use of bevacizumab), death before hospital discharge, or both, wrote Dr. Waldemar A. Carlo of the University of Alabama at Birmingham, and colleagues.
Although there was no overall difference between the two oxygen saturation groups using the composite outcome measure, marked differences were observed when the two components of the measure were considered independently, the authors wrote. Specifically, death before discharge occurred in 20% of the infants in the lower target group compared with 16% of those in the higher group. In contrast, severe retinopathy among the surviving infants occurred significantly less often in the lower group, at 9%, compared with the higher group, at 18%. These findings, the authors note, “add to the concern that oxygen restriction may increase the rate of death among preterm infants.” As such, they advise exercising caution when considering a strategy that targets oxygen levels in the low range (N. Engl. J. Med. 2010;362:1959-69).
For the CPAP vs. early intubation/surfactant study arm, the primary outcome was death or bronchopulmonary dysplasia, defined by the need for supplemental oxygen at 36 weeks, wrote Dr. Neil N. Finer of the University of California at San Diego, and colleagues. After adjusting for gestational age, medical center, and family clustering, the need for supplemental oxygen was similar in both groups, although the CPAP infants less frequently required intubation or postnatal corticosteroids for bronchopulmonary dysplasia than the surfactant group, and they also required fewer days of mechanical ventilation and were more likely to be alive by day 7, the authors wrote.
Considering the lower complication rate, the findings “support consideration of CPAP as an alternative to routine intubation and surfactant administration in preterm infants,” the authors wrote (N. Engl. J. Med. 2010;362:1970-9).
In an accompanying editorial, Dr. Colin J. Morley of the University of Melbourne stressed that caution is warranted in interpreting the “most important outcome” linking the lower target oxygen saturation range and death before discharge. “Additional research is needed to clarify this finding,” he said. “There were no significant differences between the groups in short-term outcomes that have been associated with relative ischemia.”
From the New England Journal of Medicine
Labetalol Doesn't Affect Nonstress Tests
Major Finding: The rate of reactive nonstress tests in pregnant women with chronic hypertension was not significantly different in 76 patients treated with labetalol (84%), compared with 36 treated with methyldopa (81%).
Data Source: Retrospective study of all pregnant women treated for chronic hypertension at one institution from January 2003 to September 2007.
Disclosures: None was reported.
SAN FRANCISCO — Results of nonstress tests in 112 pregnant women being treated for chronic hypertension from January 2003 to September 2007 did not differ significantly in patients on labetalol, compared with those on methyldopa, results of a retrospective study found.
“Attending physicians should feel comfortable using labetalol or methyldopa for pregnant patients with hypertension. Those medications have no effect on the baby,” Dr. Ramata Niang said in an interview at her prize-winning poster presentation at the meeting.
She and her associates had hypothesized that treatment with labetalol would increase the rate of nonreactive nonstress tests, but found no evidence of that.
Nonstress tests were reactive in 84% of 76 patients on labetalol and in 81% of 36 patients on methyldopa, a difference that was not statistically significant, reported Dr. Niang, an ob.gyn. at the University of Illinois at Chicago.
Among U.S. pregnant women, 10% have hypertension, which has been associated with an increased risk for perinatal morbidity and mortality. Traditionally, methyldopa has been used to treat hypertension during pregnancy, but in recent years more physicians have begun using beta-blockers or other medications. Labetalol is both a selective alpha-blocker and a nonselective beta-blocker that decreases systemic vascular resistance without changing maternal cardiac output.
Investigators used the average of nonstress test results for each patient to categorize results as reactive or nonreactive. The study started with charts on 188 women treated for hypertension during pregnancy and excluded women with multiple-gestation pregnancies, other antihypertensive treatment, or incomplete prenatal testing charts, to focus on the remaining 112 patients.
There were no significant differences between the two treatment groups in maternal age (29 years for women on labetalol and 31 years for those on methyldopa), gestational age at delivery (37 and 38 weeks), birth weight (2,823 g and 3,048 g), or the rate of preeclampsia (less than 1% in both groups).
Black patients made up 74% of the labetalol group and 53% of the methyldopa group, while white patients made up 12% and 23% of the two groups, respectively; Hispanics made up 6% of the labetalol group and 15% of the methyldopa group, with other races/ethnicities accounting for the remainder.
'Attending physicians should feel comfortable using labetalol or methyldopa.'
Source DR. NIANG
Major Finding: The rate of reactive nonstress tests in pregnant women with chronic hypertension was not significantly different in 76 patients treated with labetalol (84%), compared with 36 treated with methyldopa (81%).
Data Source: Retrospective study of all pregnant women treated for chronic hypertension at one institution from January 2003 to September 2007.
Disclosures: None was reported.
SAN FRANCISCO — Results of nonstress tests in 112 pregnant women being treated for chronic hypertension from January 2003 to September 2007 did not differ significantly in patients on labetalol, compared with those on methyldopa, results of a retrospective study found.
“Attending physicians should feel comfortable using labetalol or methyldopa for pregnant patients with hypertension. Those medications have no effect on the baby,” Dr. Ramata Niang said in an interview at her prize-winning poster presentation at the meeting.
She and her associates had hypothesized that treatment with labetalol would increase the rate of nonreactive nonstress tests, but found no evidence of that.
Nonstress tests were reactive in 84% of 76 patients on labetalol and in 81% of 36 patients on methyldopa, a difference that was not statistically significant, reported Dr. Niang, an ob.gyn. at the University of Illinois at Chicago.
Among U.S. pregnant women, 10% have hypertension, which has been associated with an increased risk for perinatal morbidity and mortality. Traditionally, methyldopa has been used to treat hypertension during pregnancy, but in recent years more physicians have begun using beta-blockers or other medications. Labetalol is both a selective alpha-blocker and a nonselective beta-blocker that decreases systemic vascular resistance without changing maternal cardiac output.
Investigators used the average of nonstress test results for each patient to categorize results as reactive or nonreactive. The study started with charts on 188 women treated for hypertension during pregnancy and excluded women with multiple-gestation pregnancies, other antihypertensive treatment, or incomplete prenatal testing charts, to focus on the remaining 112 patients.
There were no significant differences between the two treatment groups in maternal age (29 years for women on labetalol and 31 years for those on methyldopa), gestational age at delivery (37 and 38 weeks), birth weight (2,823 g and 3,048 g), or the rate of preeclampsia (less than 1% in both groups).
Black patients made up 74% of the labetalol group and 53% of the methyldopa group, while white patients made up 12% and 23% of the two groups, respectively; Hispanics made up 6% of the labetalol group and 15% of the methyldopa group, with other races/ethnicities accounting for the remainder.
'Attending physicians should feel comfortable using labetalol or methyldopa.'
Source DR. NIANG
Major Finding: The rate of reactive nonstress tests in pregnant women with chronic hypertension was not significantly different in 76 patients treated with labetalol (84%), compared with 36 treated with methyldopa (81%).
Data Source: Retrospective study of all pregnant women treated for chronic hypertension at one institution from January 2003 to September 2007.
Disclosures: None was reported.
SAN FRANCISCO — Results of nonstress tests in 112 pregnant women being treated for chronic hypertension from January 2003 to September 2007 did not differ significantly in patients on labetalol, compared with those on methyldopa, results of a retrospective study found.
“Attending physicians should feel comfortable using labetalol or methyldopa for pregnant patients with hypertension. Those medications have no effect on the baby,” Dr. Ramata Niang said in an interview at her prize-winning poster presentation at the meeting.
She and her associates had hypothesized that treatment with labetalol would increase the rate of nonreactive nonstress tests, but found no evidence of that.
Nonstress tests were reactive in 84% of 76 patients on labetalol and in 81% of 36 patients on methyldopa, a difference that was not statistically significant, reported Dr. Niang, an ob.gyn. at the University of Illinois at Chicago.
Among U.S. pregnant women, 10% have hypertension, which has been associated with an increased risk for perinatal morbidity and mortality. Traditionally, methyldopa has been used to treat hypertension during pregnancy, but in recent years more physicians have begun using beta-blockers or other medications. Labetalol is both a selective alpha-blocker and a nonselective beta-blocker that decreases systemic vascular resistance without changing maternal cardiac output.
Investigators used the average of nonstress test results for each patient to categorize results as reactive or nonreactive. The study started with charts on 188 women treated for hypertension during pregnancy and excluded women with multiple-gestation pregnancies, other antihypertensive treatment, or incomplete prenatal testing charts, to focus on the remaining 112 patients.
There were no significant differences between the two treatment groups in maternal age (29 years for women on labetalol and 31 years for those on methyldopa), gestational age at delivery (37 and 38 weeks), birth weight (2,823 g and 3,048 g), or the rate of preeclampsia (less than 1% in both groups).
Black patients made up 74% of the labetalol group and 53% of the methyldopa group, while white patients made up 12% and 23% of the two groups, respectively; Hispanics made up 6% of the labetalol group and 15% of the methyldopa group, with other races/ethnicities accounting for the remainder.
'Attending physicians should feel comfortable using labetalol or methyldopa.'
Source DR. NIANG
From the annual meeting of the American College of Obstetricians and Gynecologists
Obstetric Anesthesia Complications Database Is Established
Major Finding: High spinal block occurred with 1 of every 4,300 regional anesthetics. Other anesthesia-related serious complications were far less common, including respiratory distress during labor and delivery in 1 in 10,000, unrecognized spinal catheter in 1 in 15,000, and severe neurologic injury in 1 in 36,000.
Data Source: The Society for Obstetric Anesthesia and Perinatology Serious Complication Repository.
Disclosures: None was reported.
SAN ANTONIO — Serious obstetric complications occur in approximately 1 in 1,900 deliveries and serious anesthesia-related complications in about 1 in 3,000, according to data from a large multisite repository.
The Society for Obstetric Anesthesia and Perinatology Serious Complication Repository (SOAP SCORE) project was established in 2004 with the aim of gathering accurate data on the incidence of both overall obstetric- and obstetric anesthesia–related complications, as well as additional information about the complications that could inform future obstetric anesthesia practice.
Until now, complication rates reported in the literature have varied dramatically. “From this database, we can tell you that the rate of serious anesthesia-related obstetric complications is very low,” said Dr. Robert D'Angelo, professor of anesthesiology at Wake Forest University, Winston-Salem, N.C.
Data were collected on a quarterly basis from 25 institutions between Oct. 1, 2004, and June 30, 2009. Of a total 307,500 deliveries, approximately 257,000 (84%) involved anesthesia and 96,000 were cesarean sections. Regional anesthesia was used in about 76% of the vaginal deliveries and in 69% of the C-sections. There were a total of 158 serious complications, for a rate of 1 in 1,900 deliveries. Of those, 84 were deemed to be related to anesthesia, for a rate of 1 in 3,000. Failed regional anesthesia occurred in 1.7%.
Failed intubation occurred with 1 of every 533 general anesthetics, and high spinal block in 1 of every 4,300 regional anesthetics. Other anesthesia-related serious complications were far less common, including respiratory distress during labor and delivery in 1 in 10,000, unrecognized spinal catheter in 1 in 15,000, and severe neurologic injury in 1 in 36,000. The rate of epidural abscess/meningitis related to anesthesia was 1 in 63,000, while the rates of anesthesia-related myocardial infarction and cardiac arrest were both just 1 in 128,000. Rarest of all was epidural hematoma, in 1 in 250,000.
There were 30 maternal deaths and 5 cases of anaphylaxis, but none was deemed to be anesthesia related. There were no aspirations, Dr. D'Angelo said.
High spinal block was the only anesthesia-related complication reported in large enough numbers to allow for generalizations regarding associated risk factors. Of the 58 high spinal blocks reported, 14 were the result of an unrecognized spinal catheter. Of the remaining 44, known risk factors were identified in 32. The most common of these were obesity and spinal anesthesia following failed epidural.
Data were also collected on postdural puncture headaches (PDPH). Of the 1,647 reported PDPH, 917 (56%) were treated with epidural blood patch, and 98 (11% of EBP) required a repeat blood patch.
In October 2008, the American Society of Anesthesiologists formed the Anesthesia Quality Institute (www.aqihq.org
They plan to publish their first set of findings by January 2011, Dr. D'Angelo said in an interview.
'The rate of serious anesthesia-related obstetric complications is very low.'
Source DR. D'ANGELO
Major Finding: High spinal block occurred with 1 of every 4,300 regional anesthetics. Other anesthesia-related serious complications were far less common, including respiratory distress during labor and delivery in 1 in 10,000, unrecognized spinal catheter in 1 in 15,000, and severe neurologic injury in 1 in 36,000.
Data Source: The Society for Obstetric Anesthesia and Perinatology Serious Complication Repository.
Disclosures: None was reported.
SAN ANTONIO — Serious obstetric complications occur in approximately 1 in 1,900 deliveries and serious anesthesia-related complications in about 1 in 3,000, according to data from a large multisite repository.
The Society for Obstetric Anesthesia and Perinatology Serious Complication Repository (SOAP SCORE) project was established in 2004 with the aim of gathering accurate data on the incidence of both overall obstetric- and obstetric anesthesia–related complications, as well as additional information about the complications that could inform future obstetric anesthesia practice.
Until now, complication rates reported in the literature have varied dramatically. “From this database, we can tell you that the rate of serious anesthesia-related obstetric complications is very low,” said Dr. Robert D'Angelo, professor of anesthesiology at Wake Forest University, Winston-Salem, N.C.
Data were collected on a quarterly basis from 25 institutions between Oct. 1, 2004, and June 30, 2009. Of a total 307,500 deliveries, approximately 257,000 (84%) involved anesthesia and 96,000 were cesarean sections. Regional anesthesia was used in about 76% of the vaginal deliveries and in 69% of the C-sections. There were a total of 158 serious complications, for a rate of 1 in 1,900 deliveries. Of those, 84 were deemed to be related to anesthesia, for a rate of 1 in 3,000. Failed regional anesthesia occurred in 1.7%.
Failed intubation occurred with 1 of every 533 general anesthetics, and high spinal block in 1 of every 4,300 regional anesthetics. Other anesthesia-related serious complications were far less common, including respiratory distress during labor and delivery in 1 in 10,000, unrecognized spinal catheter in 1 in 15,000, and severe neurologic injury in 1 in 36,000. The rate of epidural abscess/meningitis related to anesthesia was 1 in 63,000, while the rates of anesthesia-related myocardial infarction and cardiac arrest were both just 1 in 128,000. Rarest of all was epidural hematoma, in 1 in 250,000.
There were 30 maternal deaths and 5 cases of anaphylaxis, but none was deemed to be anesthesia related. There were no aspirations, Dr. D'Angelo said.
High spinal block was the only anesthesia-related complication reported in large enough numbers to allow for generalizations regarding associated risk factors. Of the 58 high spinal blocks reported, 14 were the result of an unrecognized spinal catheter. Of the remaining 44, known risk factors were identified in 32. The most common of these were obesity and spinal anesthesia following failed epidural.
Data were also collected on postdural puncture headaches (PDPH). Of the 1,647 reported PDPH, 917 (56%) were treated with epidural blood patch, and 98 (11% of EBP) required a repeat blood patch.
In October 2008, the American Society of Anesthesiologists formed the Anesthesia Quality Institute (www.aqihq.org
They plan to publish their first set of findings by January 2011, Dr. D'Angelo said in an interview.
'The rate of serious anesthesia-related obstetric complications is very low.'
Source DR. D'ANGELO
Major Finding: High spinal block occurred with 1 of every 4,300 regional anesthetics. Other anesthesia-related serious complications were far less common, including respiratory distress during labor and delivery in 1 in 10,000, unrecognized spinal catheter in 1 in 15,000, and severe neurologic injury in 1 in 36,000.
Data Source: The Society for Obstetric Anesthesia and Perinatology Serious Complication Repository.
Disclosures: None was reported.
SAN ANTONIO — Serious obstetric complications occur in approximately 1 in 1,900 deliveries and serious anesthesia-related complications in about 1 in 3,000, according to data from a large multisite repository.
The Society for Obstetric Anesthesia and Perinatology Serious Complication Repository (SOAP SCORE) project was established in 2004 with the aim of gathering accurate data on the incidence of both overall obstetric- and obstetric anesthesia–related complications, as well as additional information about the complications that could inform future obstetric anesthesia practice.
Until now, complication rates reported in the literature have varied dramatically. “From this database, we can tell you that the rate of serious anesthesia-related obstetric complications is very low,” said Dr. Robert D'Angelo, professor of anesthesiology at Wake Forest University, Winston-Salem, N.C.
Data were collected on a quarterly basis from 25 institutions between Oct. 1, 2004, and June 30, 2009. Of a total 307,500 deliveries, approximately 257,000 (84%) involved anesthesia and 96,000 were cesarean sections. Regional anesthesia was used in about 76% of the vaginal deliveries and in 69% of the C-sections. There were a total of 158 serious complications, for a rate of 1 in 1,900 deliveries. Of those, 84 were deemed to be related to anesthesia, for a rate of 1 in 3,000. Failed regional anesthesia occurred in 1.7%.
Failed intubation occurred with 1 of every 533 general anesthetics, and high spinal block in 1 of every 4,300 regional anesthetics. Other anesthesia-related serious complications were far less common, including respiratory distress during labor and delivery in 1 in 10,000, unrecognized spinal catheter in 1 in 15,000, and severe neurologic injury in 1 in 36,000. The rate of epidural abscess/meningitis related to anesthesia was 1 in 63,000, while the rates of anesthesia-related myocardial infarction and cardiac arrest were both just 1 in 128,000. Rarest of all was epidural hematoma, in 1 in 250,000.
There were 30 maternal deaths and 5 cases of anaphylaxis, but none was deemed to be anesthesia related. There were no aspirations, Dr. D'Angelo said.
High spinal block was the only anesthesia-related complication reported in large enough numbers to allow for generalizations regarding associated risk factors. Of the 58 high spinal blocks reported, 14 were the result of an unrecognized spinal catheter. Of the remaining 44, known risk factors were identified in 32. The most common of these were obesity and spinal anesthesia following failed epidural.
Data were also collected on postdural puncture headaches (PDPH). Of the 1,647 reported PDPH, 917 (56%) were treated with epidural blood patch, and 98 (11% of EBP) required a repeat blood patch.
In October 2008, the American Society of Anesthesiologists formed the Anesthesia Quality Institute (www.aqihq.org
They plan to publish their first set of findings by January 2011, Dr. D'Angelo said in an interview.
'The rate of serious anesthesia-related obstetric complications is very low.'
Source DR. D'ANGELO
From the annual meeting of the Society for Obstetric Anesthesiology and Perinatology
Failed Spinal Anesthesia Tied to Cold Temperature
Major Finding: Fourteen cases of failed spinal anesthesia were attributed to chemical alteration from cold temperature exposure.
Data Source: Experience at Magee-Women's Hospital, Pittsburgh.
Disclosures: None was reported.
SAN ANTONIO — A cluster of 14 cases of failed spinal anesthesia among women undergoing C-sections in Pittsburgh during the winter of 2008-2009 was linked to chemical alteration that is believed to be the result of exposure of the anesthetic to subfreezing temperatures during the shipping process.
Failed spinal anesthesia can have significant clinical consequences, possibly necessitating redosing, conversion to general anesthesia, or pain during surgery.
“Efforts to identify and reduce the incidence of failed neuraxial anesthesia are of utmost importance, considering the increased use of these techniques in obstetric cases,” Dr. Manuel C. Vallejo Jr. said at the meeting.
The 14 cases, spanning an 8-week period, were all associated with three particular lots of unexpired BD spinal anesthesia trays. The cases were all under the care of experienced staff anesthesiologists and were associated with standardized neuraxial technique using hyperbaric bupivacaine 12 mg (0.75% with dextrose 8.25%) plus 20 mcg fentanyl plus 0.2 mg Duramorph (morphine). A 25-gauge Whitacre needle was used, and the free flow of cerebrospinal fluid was confirmed before and after the intrathecal drug placement, Dr. Vallejo reported.
His team at Magee-Women's Hospital contacted the bupivacaine manufacturer, Hospira Inc.; the spinal kit manufacturer, BD (Becton, Dickinson, and Co.); and the Food and Drug Administration, noting that the clustering of failures suggested problems with concentration/formulation or drug stability. Hospira tested samples from the drug lot and found no formulation or concentration issues.
For its part, BD replied that Hospira's testing showed that “all testing was within specification,” and that “we were unable to find the cause of the insufficient anesthesia identified in your report.”
The company also said that the drug supplier would “continue to monitor for the complaint condition through normal inspection and sampling procedures.”
The negative batch analysis, normal product potency, and unremarkable testing of retained samples by Hospira led Dr. Vallejo and his team to suspect that the problem was related to drug stability issues in transit and/or storage of the spinal kit.
There had been a cold spell starting during the first week of December, during which the temperature was never above freezing, ranging from 15.4° to 30.9° F.
The bupivacaine is made in Indianapolis by Hospira and is shipped to BD in New Jersey, where the spinal kits are assembled. The suspect kits had been shipped to a warehouse in Pittsburgh, loaded onto a nonenvironmentally controlled truck in the evening, and unloaded at Magee the following morning, thus having been stored for more than 9 hours at temperatures below freezing before delivery, Dr. Vallejo said.
A mass spectrometric analysis done at the University of Pittsburgh demonstrated that there had been chemical alteration of the bupivacaine involved. The suspect sample had a molecular mass to charge ratio of 276, compared with 289 for normal bupivacaine.
As a temporary solution, a separate bupivacaine ampule was affixed to the suspect spinal kits, and temperature indicator strips were used.
All 14 of the women and newborns were fine with no long-term problems, he noted.
BD now directly drop ships the packaged spinal kits to Magee-Women's. These measures have dramatically decreased but not eliminated the rate of failed spinal anesthetics, Dr. Vallejo commented, adding that other factors may also contribute, including technical failure, inadequate patient position, inadvertent subdural or epidural injection, inadequate intrathecal dose, and “rachi-resistance,” a phenomenon described in 1934 in which patients are “hyperresistant” to spinal anesthesia, possibly because of insensitive nerve roots.
“I think it's multifactorial, but we definitely showed a chemical alteration,” he noted.
During the question and answer period, an audience member from Toronto said that approximately 400 cases of failed spinal anesthesia had been reported in the province of Ontario in October 2009 and were also suspected to be a result of cold temperature exposure.
“There's definitely a problem,” Dr. Vallejo replied.
Asked to comment specifically for this story, BD replied in part: “Patient safety and quality of our products are BD's foremost priorities. The causes of failed regional anesthesia are multifactorial and an industrywide phenomenon. They are typically not associated with any single manufacturer's product.”
“Evaluations of our complaint records do not show a correlation between the shipment of product during cold or hot months and complaint rates. The rate remains stable across the year.
“We are conducting a more extensive, controlled study to evaluate this hypothesis. When this investigation is completed, BD will take all actions deemed necessary and appropriate by the study, if any.”
Major Finding: Fourteen cases of failed spinal anesthesia were attributed to chemical alteration from cold temperature exposure.
Data Source: Experience at Magee-Women's Hospital, Pittsburgh.
Disclosures: None was reported.
SAN ANTONIO — A cluster of 14 cases of failed spinal anesthesia among women undergoing C-sections in Pittsburgh during the winter of 2008-2009 was linked to chemical alteration that is believed to be the result of exposure of the anesthetic to subfreezing temperatures during the shipping process.
Failed spinal anesthesia can have significant clinical consequences, possibly necessitating redosing, conversion to general anesthesia, or pain during surgery.
“Efforts to identify and reduce the incidence of failed neuraxial anesthesia are of utmost importance, considering the increased use of these techniques in obstetric cases,” Dr. Manuel C. Vallejo Jr. said at the meeting.
The 14 cases, spanning an 8-week period, were all associated with three particular lots of unexpired BD spinal anesthesia trays. The cases were all under the care of experienced staff anesthesiologists and were associated with standardized neuraxial technique using hyperbaric bupivacaine 12 mg (0.75% with dextrose 8.25%) plus 20 mcg fentanyl plus 0.2 mg Duramorph (morphine). A 25-gauge Whitacre needle was used, and the free flow of cerebrospinal fluid was confirmed before and after the intrathecal drug placement, Dr. Vallejo reported.
His team at Magee-Women's Hospital contacted the bupivacaine manufacturer, Hospira Inc.; the spinal kit manufacturer, BD (Becton, Dickinson, and Co.); and the Food and Drug Administration, noting that the clustering of failures suggested problems with concentration/formulation or drug stability. Hospira tested samples from the drug lot and found no formulation or concentration issues.
For its part, BD replied that Hospira's testing showed that “all testing was within specification,” and that “we were unable to find the cause of the insufficient anesthesia identified in your report.”
The company also said that the drug supplier would “continue to monitor for the complaint condition through normal inspection and sampling procedures.”
The negative batch analysis, normal product potency, and unremarkable testing of retained samples by Hospira led Dr. Vallejo and his team to suspect that the problem was related to drug stability issues in transit and/or storage of the spinal kit.
There had been a cold spell starting during the first week of December, during which the temperature was never above freezing, ranging from 15.4° to 30.9° F.
The bupivacaine is made in Indianapolis by Hospira and is shipped to BD in New Jersey, where the spinal kits are assembled. The suspect kits had been shipped to a warehouse in Pittsburgh, loaded onto a nonenvironmentally controlled truck in the evening, and unloaded at Magee the following morning, thus having been stored for more than 9 hours at temperatures below freezing before delivery, Dr. Vallejo said.
A mass spectrometric analysis done at the University of Pittsburgh demonstrated that there had been chemical alteration of the bupivacaine involved. The suspect sample had a molecular mass to charge ratio of 276, compared with 289 for normal bupivacaine.
As a temporary solution, a separate bupivacaine ampule was affixed to the suspect spinal kits, and temperature indicator strips were used.
All 14 of the women and newborns were fine with no long-term problems, he noted.
BD now directly drop ships the packaged spinal kits to Magee-Women's. These measures have dramatically decreased but not eliminated the rate of failed spinal anesthetics, Dr. Vallejo commented, adding that other factors may also contribute, including technical failure, inadequate patient position, inadvertent subdural or epidural injection, inadequate intrathecal dose, and “rachi-resistance,” a phenomenon described in 1934 in which patients are “hyperresistant” to spinal anesthesia, possibly because of insensitive nerve roots.
“I think it's multifactorial, but we definitely showed a chemical alteration,” he noted.
During the question and answer period, an audience member from Toronto said that approximately 400 cases of failed spinal anesthesia had been reported in the province of Ontario in October 2009 and were also suspected to be a result of cold temperature exposure.
“There's definitely a problem,” Dr. Vallejo replied.
Asked to comment specifically for this story, BD replied in part: “Patient safety and quality of our products are BD's foremost priorities. The causes of failed regional anesthesia are multifactorial and an industrywide phenomenon. They are typically not associated with any single manufacturer's product.”
“Evaluations of our complaint records do not show a correlation between the shipment of product during cold or hot months and complaint rates. The rate remains stable across the year.
“We are conducting a more extensive, controlled study to evaluate this hypothesis. When this investigation is completed, BD will take all actions deemed necessary and appropriate by the study, if any.”
Major Finding: Fourteen cases of failed spinal anesthesia were attributed to chemical alteration from cold temperature exposure.
Data Source: Experience at Magee-Women's Hospital, Pittsburgh.
Disclosures: None was reported.
SAN ANTONIO — A cluster of 14 cases of failed spinal anesthesia among women undergoing C-sections in Pittsburgh during the winter of 2008-2009 was linked to chemical alteration that is believed to be the result of exposure of the anesthetic to subfreezing temperatures during the shipping process.
Failed spinal anesthesia can have significant clinical consequences, possibly necessitating redosing, conversion to general anesthesia, or pain during surgery.
“Efforts to identify and reduce the incidence of failed neuraxial anesthesia are of utmost importance, considering the increased use of these techniques in obstetric cases,” Dr. Manuel C. Vallejo Jr. said at the meeting.
The 14 cases, spanning an 8-week period, were all associated with three particular lots of unexpired BD spinal anesthesia trays. The cases were all under the care of experienced staff anesthesiologists and were associated with standardized neuraxial technique using hyperbaric bupivacaine 12 mg (0.75% with dextrose 8.25%) plus 20 mcg fentanyl plus 0.2 mg Duramorph (morphine). A 25-gauge Whitacre needle was used, and the free flow of cerebrospinal fluid was confirmed before and after the intrathecal drug placement, Dr. Vallejo reported.
His team at Magee-Women's Hospital contacted the bupivacaine manufacturer, Hospira Inc.; the spinal kit manufacturer, BD (Becton, Dickinson, and Co.); and the Food and Drug Administration, noting that the clustering of failures suggested problems with concentration/formulation or drug stability. Hospira tested samples from the drug lot and found no formulation or concentration issues.
For its part, BD replied that Hospira's testing showed that “all testing was within specification,” and that “we were unable to find the cause of the insufficient anesthesia identified in your report.”
The company also said that the drug supplier would “continue to monitor for the complaint condition through normal inspection and sampling procedures.”
The negative batch analysis, normal product potency, and unremarkable testing of retained samples by Hospira led Dr. Vallejo and his team to suspect that the problem was related to drug stability issues in transit and/or storage of the spinal kit.
There had been a cold spell starting during the first week of December, during which the temperature was never above freezing, ranging from 15.4° to 30.9° F.
The bupivacaine is made in Indianapolis by Hospira and is shipped to BD in New Jersey, where the spinal kits are assembled. The suspect kits had been shipped to a warehouse in Pittsburgh, loaded onto a nonenvironmentally controlled truck in the evening, and unloaded at Magee the following morning, thus having been stored for more than 9 hours at temperatures below freezing before delivery, Dr. Vallejo said.
A mass spectrometric analysis done at the University of Pittsburgh demonstrated that there had been chemical alteration of the bupivacaine involved. The suspect sample had a molecular mass to charge ratio of 276, compared with 289 for normal bupivacaine.
As a temporary solution, a separate bupivacaine ampule was affixed to the suspect spinal kits, and temperature indicator strips were used.
All 14 of the women and newborns were fine with no long-term problems, he noted.
BD now directly drop ships the packaged spinal kits to Magee-Women's. These measures have dramatically decreased but not eliminated the rate of failed spinal anesthetics, Dr. Vallejo commented, adding that other factors may also contribute, including technical failure, inadequate patient position, inadvertent subdural or epidural injection, inadequate intrathecal dose, and “rachi-resistance,” a phenomenon described in 1934 in which patients are “hyperresistant” to spinal anesthesia, possibly because of insensitive nerve roots.
“I think it's multifactorial, but we definitely showed a chemical alteration,” he noted.
During the question and answer period, an audience member from Toronto said that approximately 400 cases of failed spinal anesthesia had been reported in the province of Ontario in October 2009 and were also suspected to be a result of cold temperature exposure.
“There's definitely a problem,” Dr. Vallejo replied.
Asked to comment specifically for this story, BD replied in part: “Patient safety and quality of our products are BD's foremost priorities. The causes of failed regional anesthesia are multifactorial and an industrywide phenomenon. They are typically not associated with any single manufacturer's product.”
“Evaluations of our complaint records do not show a correlation between the shipment of product during cold or hot months and complaint rates. The rate remains stable across the year.
“We are conducting a more extensive, controlled study to evaluate this hypothesis. When this investigation is completed, BD will take all actions deemed necessary and appropriate by the study, if any.”
From the annual meeting of the Society for Obstetric Anesthesia and Perinatology
UPDATE: INFECTIOUS DISEASE
Six recent articles stand out in the field of infectious disease:
- an assessment of outcomes of seriously ill patients who were hospitalized early in the course of the H1N1 influenza epidemic. The authors highlight major differences in the epidemiology of this infection, compared with regular seasonal flu
- an examination of outcomes of pregnant women who developed H1N1 influenza
- an exploration of the use of blunt needles during cesarean delivery to prevent glove perforation
- an evaluation of the utility of prophylactic antibiotics in ostensibly low-risk women undergoing scheduled cesarean delivery
- a look at the timing of antibiotic prophylaxis for cesarean delivery
- a comparison of skin preparation techniques in the prevention of surgical-site infection.
The focus on cesarean delivery in most of these studies seems particularly appropriate, now that this operation has become the most frequently performed major surgical procedure in US hospitals.
H1N1 virus hits hardest during pregnancy and chronic illness
Jain S, Kamimoto L, Bramley AM, et al, for 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944.
This retrospective survey of patients hospitalized for at least 24 hours for treatment of influenza-like illness included 272 patients who were given a diagnosis of H1N1 influenza, based on real-time, reverse-transcriptase, polymerase chain reaction assay. Sixty-seven (25%) of these patients were admitted to an ICU, and 19 (7%) died. All of the patients who died had been treated in an ICU, and two thirds had an underlying medical condition. Three of the deaths involved pregnant women. None of the patients who died received antiviral therapy within 48 hours of the onset of symptoms. Those who died were also less likely to have been vaccinated against seasonal influenza in 2008–2009.
Details of the trial
The 272 patients included in this study sample represented 25% of the total number of patients hospitalized in the United States for treatment of influenza between April and mid-June 2009. They exhibited the following characteristics:
- median age: 21 years
- race and ethnicity: 30% were Hispanic, and 27% were non-Hispanic white
- most common symptoms: fever and cough, although diarrhea or vomiting was reported in 39% of patients
- underlying medical illness: present in 73% (198 patients), including 60% of children and 83% of adults. At least two underlying medical conditions were present in 32% of patients. Asthma was the most common comorbid condition
- pregnancy: 18 patients were pregnant. Four of the pregnant patients also had asthma, and two had diabetes
- obesity: 29% of adults were obese. Morbid obesity was present in 26%. More than 75% of obese and morbidly obese patients had at least one underlying medical illness
- bloodwork at admission: 20% of patients were leukopenic; 37% were anemic; and 14% were thrombocytopenic
- chest film: 40% of patients who underwent chest radiography had findings consistent with pneumonia. Findings included bilateral infiltrates in 66 patients, a unilobar infiltrate in 26, and multilobar infiltrates in two
- antiviral therapy: 75% ultimately received antiviral drugs, with a median time from onset of illness to initiation of therapy of 3 days (range, 0–29 days). Only 39% received antiviral therapy within 48 hours of the onset of symptoms
- antibiotic therapy: 79% of patients received antibiotics for presumed superimposed bacterial infection. The most commonly used antibiotics were ceftriaxone, azithromycin, vancomycin, and levofloxacin.
Study offers 4 useful lessons
The study by Jain and colleagues offers clinically applicable lessons:
- it reinforces the point that children and young adults, including pregnant women, are at increased risk of serious morbidity and mortality
- it demonstrates that most seriously affected patients have at least one underlying medical condition, such as asthma
- it highlights the importance of pregnancy and morbid obesity as major conditions that contribute to serious complications from influenza. The 7% prevalence of pregnant patients is significantly higher than the 1% prevalence that would typically be expected with seasonal influenza. Similarly, the 26% prevalence of morbid obesity greatly exceeds the estimated 5% prevalence in the adult US population
- it confirms the importance of treating patients early in the course of their illness with antiviral drugs such as oseltamivir. Notably, none of the patients who died received treatment within 48 hours of the onset of illness, when the drugs are most likely to be effective.
How to treat H1N1 influenza
The vast majority of strains of the 2009 H1N1 virus are susceptible to oseltamivir and zanamivir, but essentially all strains are resistant to amantadine and rimantadine.1 Therefore, all individuals who are hospitalized should be treated with one of two regimens:
- oseltamivir, 75 mg orally, twice daily for at least 5 days
- zanamivir, 10 mg by inhalation, twice daily for at least 5 days.
These same regimens should be used for outpatients who are at high risk of complications.
Ideally, antiviral treatment should be administered within 48 hours of the onset of symptoms, but do not withhold treatment even if more than 48 hours have elapsed since the onset of illness.2,3
Both oseltamivir and zanamivir are also effective for prevention of infection in susceptible patients who have been exposed to H1N1 influenza. The appropriate dosage of oseltamivir for prophylaxis is 75 mg orally once daily for 10 days. The corresponding dosage of zanamivir is 10 mg by inhalation once daily for 10 days.1
The most effective method of prophylaxis, of course, is vaccination with the new H1N1 vaccine.4 There are two forms of the vaccine—a live virus nasal vaccine and an inactivated vaccine for intramuscular administration. Pregnant women should receive only the inactivated vaccine.
The key reservoirs of all influenza A viruses are migrating waterfowl, pigs, and humans. The current H1N1 strain of virus contains eight unique RNA segments that are a mixture of components from avian, pig, and human influenza viruses.2 The pandemic resulting from this virus is unusual because the continent of origin was North America (Mexico) rather than Asia, the season of origin was spring rather than fall, and the patients at greatest risk of dying have been children and young adults rather than infants and the elderly.3
Women who are pregnant or planning to become pregnant should be vaccinated against H1N1 influenza. Use the inactivated virus if a woman is already pregnant.
After exposure to H1N1 influenza, unvaccinated pregnant women and other patients at high risk of developing the virus should be given oseltamivir or zanamivir prophylactically, using the dosage and route of administration described above for prophylaxis.
Pregnant women and other high-risk patients who exhibit symptoms of H1N1 influenza should be given oseltamivir or zanamivir, using the dosage and route of administration described above for treatment, ideally within 48 hours of the onset of symptoms.
For pregnant and postpartum patients, base treatment of H1N1 flu on symptoms, not rapid tests
Louie JK, Acosta M, Jamieson DJ, et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35.
Louie and coworkers describe the outcome of a statewide surveillance program by the California Department of Public Health. They reviewed the medical records of 94 pregnant women, eight women who were within the first 2 weeks postpartum, and 137 nonpregnant women of reproductive age who were hospitalized with confirmed 2009 H1N1 influenza between April 23 and August 11, 2009.
Eighteen pregnant women and four postpartum patients (22%) required intensive care, and 16 (73%) of these women had to be ventilated mechanically. Of the 18 pregnant women who required treatment in the ICU, 12 delivered in the hospital, and four underwent emergent cesarean delivery in the ICU.
Eight (8%) of the 102 pregnant and postpartum patients died. None of these eight women received antiviral therapy within 48 hours of the onset of symptoms. In fact, for pregnant and postpartum patients, a delay in administration of antiviral therapy beyond 48 hours after the onset of symptoms produced a 4.3 relative risk of death (95% confidence interval [CI], 1.4–13.7), compared with patients who were treated early in the course of their infection.
Details of the trial
The women in this trial had the following characteristics:
- gestational age: five (5%) of the 94 pregnant women were in the first trimester, 35 (37%) were in the second trimester, and 54 (57%) were in the third trimester
- underlying conditions were present in 34% of the pregnant and postpartum women and 60% of nonpregnant women. These conditions placed them at increased risk of complications from influenza. The most common underlying condition was asthma
- antiviral therapy was administered to approximately 80% of both pregnant and nonpregnant women. However, only 50% of pregnant women and 34% of nonpregnant women received treatment within 48 hours of the onset of symptoms
- antibiotic therapy was given to 45% of pregnant women and 58% of nonpregnant women for presumed secondary bacterial infection
- false-negative test results: 153 women underwent rapid tests for influenza, 38% of which were falsely negative.
Treat pregnant patients expediently
This article is an excellent complement to the study by Jain and colleagues described on page 37. It strikingly illustrates the heightened risk of morbidity and mortality that pregnant women face when they develop H1N1 influenza. Louie and coworkers documented an influenza-specific mortality ratio (maternal deaths for every 100,000 live births) of 4.3. They also provide clear evidence of the perils of relying on rapid diagnostic tests and withholding antiviral treatment if the rapid test is negative. In their series, 38% of rapid tests were falsely negative. In pregnant women, when antiviral therapy was delayed more than 48 hours, the relative risk of death was 4.3, compared with patients who were treated within 48 hours of the onset of symptoms.
If there is a clinical suspicion of influenza in a pregnant or postpartum patient, treat her immediately with one of the antiviral regimens outlined on page 38—regardless of the outcome of the rapid test for influenza.
Blunt needles reduce needle sticks during cesarean delivery
Sullivan S, Williamson B, Wilson LK, Korde JE, Soper D. Blunt needles for reduction of needlestick injuries during cesarean delivery. Obstet Gynecol. 2009;114 (2 Pt 1):211–216.
Using glove perforation as a proxy for needlestick injuries, Sullivan and colleagues compared blunt needles with sharp needles during cesarean delivery. Ninety-seven women had all anatomic layers reapproximated using blunt needles, and 97 had them reapproximated using sharp needles. The overall glove perforation rate was 12.3%. For sharp needles, the perforation rate was 17.5%, and for blunt needles it was 7.2% (relative risk [RR], 0.66; 95% CI, 0.49–0.89). The key protective effect of the blunt needles was confined to the assistant surgeon (RR, 0.54; 95% CI, 0.41–0.71). The RR for glove perforation involving the primary surgeon was 0.8 (95% CI, 0.53–1.2).
Details of the trial
Glove type, number of gloves, needle size, and type and gauge of suture material were left to the discretion of the surgeon. Glove perforations were identified by filling the gloves with 1,000 mL of water and applying pressure to the palm and each finger. The secondary endpoint of the study was physician satisfaction with the needle. Primary and assistant surgeons reported comparable levels of dissatisfaction with blunt needles, compared with sharp needles (P < .001). However, 92% of primary surgeons and 93% of assistant surgeons rated the blunt needles as at least “acceptable” for use.
Needle stick has led to hepatitis B transmission
Earlier studies reported a rate of glove perforation of 20% to 26% during open abdominal procedures. In an investigation at our center, we noted glove perforation in 13% of cesarean deliveries.5 In this and another investigation, the frequency of perforation did not vary with the level of training of the surgeon or time of day of the procedure.5,6 The most common sites of perforation were the thumb, index finger, and middle finger of the non-dominant hand. The most common mechanism of injury was handling the needle with the operator’s gloved hand rather than with an instrument.
Double-gloving significantly reduces the risk of injury to the inner glove and, subsequently, to the surgeon’s skin. (Note: Double-gloving does not decrease tactile sensation or increase the risk of mishap.6)
The study by Sullivan and colleagues demonstrates that use of blunt needles offers an additional measure of protection against a penetrating injury to the surgeon’s bare skin. Although no surgeon has yet contracted HIV infection from a surgical needle, the transmission of hepatitis B via contaminated surgical needle has been well documented.
Prudence dictates that we use all proven measures to prevent intraoperative blood exposure. Use of blunt needles should be added to interventions such as double-gloving and use of a neutral zone in which to pass sharp objects.
Prophylactic antibiotics reduce postcesarean infection, even in low-risk women
Dinsmoor MJ, Gilbert S, Landon MB, et al, for Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
Infection is the most common postoperative complication of cesarean delivery, now the most frequently performed major operation in America. The principal infection is endometritis, followed by wound infection and urinary tract infection. The frequency of wound infection is on the rise because of the steadily increasing prevalence of obesity in the obstetric population.
Dinsmoor and coworkers conducted this secondary analysis using data from an earlier observational study of 9,432 women who underwent cesarean delivery before the onset of labor. Of these women, 6,006 (64%) received antibiotic prophylaxis.
Women treated prophylactically had a significantly lower rate of endometritis (adjusted odds ratio [OR], 0.40; 95% CI, 0.28–0.59) and of wound infection (adjusted OR, 0.49; 95% CI, 0.28–0.86). The frequency of other infection-related complications was not significantly reduced (adjusted OR, 0.39; 95% CI, 0.13–1.12).
Overall, the size of the effect for endometritis was small; endometritis developed in 2.0% of women in the group that received prophylaxis and 2.6% of women in the group that did not. The size of the effect was even smaller for wound infection.
In this uncontrolled series, 113 patients had to be treated to prevent one case of endometritis or wound infection.
Details of the trial
The original observational study from which this analysis derives was performed by the Maternal-Fetal Medicine Units Network at 13 centers in 1999–2000. The choice of antibiotics and the timing of administration were left to the discretion of the attending physician.
Principal endpoints were the occurrence of postoperative endometritis and wound infection. Secondary endpoints were less common infection-related complications such as maternal sepsis, fascial dehiscence or evisceration, necrotizing fasciitis, pelvic abscess, and septic pelvic vein thrombophlebitis.
Of the women who were given prophylactic antibiotics, 88% received only a cephalosporin, 7% received only a broad-spectrum penicillin, and 6% received other regimens. Approximately 1% of patients received more than one antibiotic for prophylaxis.
Averting infection pays dividends
More than 90% of patients who have endometritis respond promptly to broad-spectrum antibiotic therapy. However, some women with postcesarean endometritis develop serious complications such as septic shock, septic pelvic vein thrombophlebitis, and pelvic abscess.
Treatment of wound infection is not so straightforward as treatment of endometritis. Wound infections may well require surgical intervention to drain an incisional abscess. They also necessitate a change in antibiotic therapy, and they are one of the two most important risk factors for fascial dehiscence and intestinal evisceration.
Multiple studies have confirmed that antibiotic prophylaxis significantly reduces the risk of endometritis and wound infection in women who undergo cesarean after the start of labor, with or without ruptured membranes.7,8 Recent publications have also demonstrated that prophylaxis before the start of surgery offers a greater protective effect than administration after the infant’s umbilical cord is clamped.9,10 Other investigations have demonstrated that broader-spectrum prophylaxis further improves outcomes in women undergoing cesarean delivery.11,12
Antibiotic prophylaxis reduces the frequency of postcesarean endometritis and wound infection, even in very low-risk patients. I strongly support the use of prophylactic antibiotics in all women undergoing cesarean delivery. I believe that the best available evidence supports the use of cefazolin (1 g) plus azithromycin (500 mg), administered intravenously before the start of surgery.9-12
Administer antibiotics before making the incision for greatest effectiveness
Owens SM, Brozanski BS, Meyn LA, Wisenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
In this retrospective investigation, Owens and colleagues compared antibiotic prophylaxis in two groups of women undergoing cesarean delivery:
- 4,229 women who received antibiotics after the infant’s umbilical cord was clamped, from July 2002 to November 2004 (Group 1)
- 4,781 women who received antibiotics before the skin was incised, from June 2005 to August 2007 (Group 2).
Both scheduled and unscheduled cesarean deliveries were included, as were women who received antibiotics intrapartum for group B streptococcus prophylaxis and treatment of chorioamnionitis. The most commonly used antibiotic was intravenous cefazolin (1 g).
After excluding women who received group B streptococcus prophylaxis or intrapartum treatment of chorioamnionitis, the authors demonstrated a nearly 50% reduction in the rate of endometritis among women who received antibiotics before surgery (OR, 0.54; 95% CI, 0.38–0.75). They also documented a 30% reduction in the rate of wound infection in these patients (OR, 0.72; 95% CI, 0.55–0.46).
Details of the trial
Principal outcome measures were the rates of maternal endometritis and wound infection and rates of proven and presumed neonatal infection. The mean age and racial distribution were similar in the two groups, but the percentage of patients treated on a resident teaching service was lower in Group 2 (14.9% vs. 18.9%; P < .001). The two groups did not differ in mean body mass index or in the percentage of patients who were in labor before surgery. Colonization with group B streptococcus was more common in Group 2 (24.4% vs. 22.2%; P = .5). However, chorioamnionitis was less prevalent in Group 2 (5.6% vs. 10.3%; P < .001).
The rates of culture-proven neonatal infection within the first 3 days of life (early-onset infection) were similar between groups (1.3% in Group 1 vs. 0.7% in Group 2). Culture-proven late-onset neonatal infection was less common in Group 2 (1.8% vs. 5.7%; P < .001). The groups did not differ in the proportion of newborns treated for presumed infection (24.1% in Group 1 vs. 22.2% in Group 2).
Plentiful data confirm the superiority of preoperative administration
Endometritis is the most common postoperative complication associated with cesarean delivery. Wound infection is less common but more likely to lead to prolonged postoperative morbidity and extended hospitalization. Reducing both of these complications is a critical clinical objective.
Virtually without exception, every investigation has confirmed that prophylactic antibiotics reduce the frequency of postcesarean endometritis and, usually, wound infection as well. One dose of a given antibiotic is clearly as effective as multiple doses.
Classic animal investigations by Burke demonstrated that prophylaxis was most effective when antibiotics were present in tissue prior to the surgical incision.13 Nevertheless, early investigators in obstetrics argued that preoperative exposure to antibiotics increased the likelihood that the neonate would require an evaluation for sepsis and that delaying antibiotics until after cord clamping did not compromise the effectiveness of prophylaxis.14,15
Sullivan and colleagues were the first authors to successfully challenge this dictum.9 In a well-designed investigation, they demonstrated that preoperative administration of antibiotics significantly reduces the frequency of endometritis (RR, 0.22) but not wound infection, and does not increase the need for neonatal sepsis evaluation. Kaimel and coworkers later confirmed these findings,16 and this study by Owen and associates offers additional proof of the effectiveness and safety of preoperative antibiotic administration.
I offer only one addendum to the conclusions presented by Owen and colleagues. Two recent investigations from the University of Alabama conclusively demonstrate that, by extending the spectrum of antibiotic coverage by combining azithromycin and cefazolin, we can further reduce postcesarean endometritis and wound infection.11,17 Accordingly, at our center, we now administer both intravenous (IV) azithromycin (500 mg over 1 hour) and IV cefazolin (1 g) approximately 30 to 60 minutes before the start of surgery.
Antibiotic prophylaxis reduces the rates of postcesarean endometritis and wound infection, and preoperative administration is superior to administration after cord clamping. Preoperative administration is also safe for the neonate.
Administer IV azithromycin (500 mg over 1 hour) and IV cefazolin (1 g) approximately 30 to 60 minutes before the start of surgery.
Chlorhexidine solutions are superior to povidone-iodine for surgical-site antisepsis
Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
This report is an excellent complement to the two studies discussed above, which focused on systemic antibiotic prophylaxis for the prevention of postcesarean infection. Here, Darouiche and colleagues conducted a randomized, prospective, unblinded, multi-center comparison of two skin preparations to prevent surgical-site infection:
- 2% chlorhexidine gluconate and 70% isopropyl alcohol (409 patients)
- 10% povidone-iodine solution (440 patients).
Participants underwent a variety of abdominal and nonabdominal (thoracic, gynecologic, and urologic) procedures. All patients received systemic antibiotic prophylaxis within 1 hour before the start of surgery.
The primary outcome measure was the occurrence of any surgical-site infection up to 30 days after surgery. This rate was lower among patients who received chlorhexidine-alcohol skin preparations than among those who received povidone-iodine (9.5% vs. 16.1%; P = .004).
Secondary endpoints were specific types of infection:
- superficial incisional infection (skin and subcutaneous tissue): lower among patients receiving chlorhexidine-alcohol (4.2% vs. 8.6%; P = .008)
- deep incisional infection (involving fascia and muscle): lower among patients receiving chlorhexidine-alcohol (1% vs. 3%; P = .05)
- organ-space infection (any organ or space other than the body wall): no significant difference between women treated with chlorhexidine-alcohol and those treated with povidone-iodine.
Seventeen patients would need to be treated with chlorhexidine-alcohol to prevent one surgical-site infection.
Chlorhexidine has a solid track record
The 41% reduction in the rate of surgical-site infection with chlorhexidine-alcohol (RR, 0.59; 95% CI, 0.41–0.85) is consistent with a 49% reduction in the risk of vascular catheter-related bacteremia using the same formulation.18 The findings are also consistent with a recent report showing that chlorhexidine was more effective than iodine-containing solutions in reducing bacterial concentration in the operative field in women undergoing vaginal hysterectomy.19
Darouiche and coworkers suggest that chlorhexidine is more effective because it has a more rapid onset of action and greater and more persistent antibacterial activity despite exposure to body fluids. Quite appropriately, they indicate that the solution used in their study is flammable, but they observed no adverse effects in a large sample of patients undergoing a variety of procedures.
I strongly recommend that chlorhexidine be used for all surgical skin preparation in obstetric and gynecologic patients. this intervention, along with consistent use of systemic antibiotic prophylaxis, should be highly effective in reducing the risk of superficial and deep abdominal wound infection.
1. Antiviral drugs for influenza. The Medical Letter. 2009;51(1325):89-92.
2. Wenzel RP, Edmond MB. Preparing for 2009 H1N1 influenza. N Engl J Med. 2009;361(20):1991-1993.
3. Perez-Padilla R, Rosa-Zambori D, deLeon SP, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680-689.
4. H1N1 vaccine for prevention of pandemic influenza. The Medical Letter. 2009;51(1322):77-78.
5. Chapman S, Duff P. Frequency of glove perforations and subsequent blood contact in association with selected obstetric surgical procedures. Am J Obstet Gynecol. 1993;168(5):1354-1357.
6. Lancaster C, Duff P. Single versus double-gloving for obstetric and gynecologic procedures. Am J Obstet Gynecol. 2007;196(5):e36-e37.
7. Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database of Systematic Reviews. 2002;(3):CD000933.-doi:10/1002/14651858.
8. Prophylactic antibiotics in labor and delivery. ACOG Practice Bulletin No. 47. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2003;102(4):875-882.
9. Sullivan SA, Smith T, Chang F, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1-e5.
10. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1-e6.
11. Tita ATN, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51-56.
12. Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1-e3.
13. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168.
14. Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before and after cord clamping. Obstet Gynecol. 1979;53(2):151-156.
15. Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151-154.
16. Kaimal AJ, Zlatnik MG, Chang YW, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical-site infections. Am J Obstet Gynecol. 2008;199(3):310.e1-e5.
17. Tita AT, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systemic review. Obstet Gynecol. 2009;113(3):675-682.
18. Chaiyakunapruk N, Veerstra DI, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136(11):792-801.
19. Culligan PJ, Kubik K, Murphy M, Blackwell L, Snyder J. A randomized trial that compared povidone iodine and chlorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol. 2005;192(2):422-425.
Six recent articles stand out in the field of infectious disease:
- an assessment of outcomes of seriously ill patients who were hospitalized early in the course of the H1N1 influenza epidemic. The authors highlight major differences in the epidemiology of this infection, compared with regular seasonal flu
- an examination of outcomes of pregnant women who developed H1N1 influenza
- an exploration of the use of blunt needles during cesarean delivery to prevent glove perforation
- an evaluation of the utility of prophylactic antibiotics in ostensibly low-risk women undergoing scheduled cesarean delivery
- a look at the timing of antibiotic prophylaxis for cesarean delivery
- a comparison of skin preparation techniques in the prevention of surgical-site infection.
The focus on cesarean delivery in most of these studies seems particularly appropriate, now that this operation has become the most frequently performed major surgical procedure in US hospitals.
H1N1 virus hits hardest during pregnancy and chronic illness
Jain S, Kamimoto L, Bramley AM, et al, for 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944.
This retrospective survey of patients hospitalized for at least 24 hours for treatment of influenza-like illness included 272 patients who were given a diagnosis of H1N1 influenza, based on real-time, reverse-transcriptase, polymerase chain reaction assay. Sixty-seven (25%) of these patients were admitted to an ICU, and 19 (7%) died. All of the patients who died had been treated in an ICU, and two thirds had an underlying medical condition. Three of the deaths involved pregnant women. None of the patients who died received antiviral therapy within 48 hours of the onset of symptoms. Those who died were also less likely to have been vaccinated against seasonal influenza in 2008–2009.
Details of the trial
The 272 patients included in this study sample represented 25% of the total number of patients hospitalized in the United States for treatment of influenza between April and mid-June 2009. They exhibited the following characteristics:
- median age: 21 years
- race and ethnicity: 30% were Hispanic, and 27% were non-Hispanic white
- most common symptoms: fever and cough, although diarrhea or vomiting was reported in 39% of patients
- underlying medical illness: present in 73% (198 patients), including 60% of children and 83% of adults. At least two underlying medical conditions were present in 32% of patients. Asthma was the most common comorbid condition
- pregnancy: 18 patients were pregnant. Four of the pregnant patients also had asthma, and two had diabetes
- obesity: 29% of adults were obese. Morbid obesity was present in 26%. More than 75% of obese and morbidly obese patients had at least one underlying medical illness
- bloodwork at admission: 20% of patients were leukopenic; 37% were anemic; and 14% were thrombocytopenic
- chest film: 40% of patients who underwent chest radiography had findings consistent with pneumonia. Findings included bilateral infiltrates in 66 patients, a unilobar infiltrate in 26, and multilobar infiltrates in two
- antiviral therapy: 75% ultimately received antiviral drugs, with a median time from onset of illness to initiation of therapy of 3 days (range, 0–29 days). Only 39% received antiviral therapy within 48 hours of the onset of symptoms
- antibiotic therapy: 79% of patients received antibiotics for presumed superimposed bacterial infection. The most commonly used antibiotics were ceftriaxone, azithromycin, vancomycin, and levofloxacin.
Study offers 4 useful lessons
The study by Jain and colleagues offers clinically applicable lessons:
- it reinforces the point that children and young adults, including pregnant women, are at increased risk of serious morbidity and mortality
- it demonstrates that most seriously affected patients have at least one underlying medical condition, such as asthma
- it highlights the importance of pregnancy and morbid obesity as major conditions that contribute to serious complications from influenza. The 7% prevalence of pregnant patients is significantly higher than the 1% prevalence that would typically be expected with seasonal influenza. Similarly, the 26% prevalence of morbid obesity greatly exceeds the estimated 5% prevalence in the adult US population
- it confirms the importance of treating patients early in the course of their illness with antiviral drugs such as oseltamivir. Notably, none of the patients who died received treatment within 48 hours of the onset of illness, when the drugs are most likely to be effective.
How to treat H1N1 influenza
The vast majority of strains of the 2009 H1N1 virus are susceptible to oseltamivir and zanamivir, but essentially all strains are resistant to amantadine and rimantadine.1 Therefore, all individuals who are hospitalized should be treated with one of two regimens:
- oseltamivir, 75 mg orally, twice daily for at least 5 days
- zanamivir, 10 mg by inhalation, twice daily for at least 5 days.
These same regimens should be used for outpatients who are at high risk of complications.
Ideally, antiviral treatment should be administered within 48 hours of the onset of symptoms, but do not withhold treatment even if more than 48 hours have elapsed since the onset of illness.2,3
Both oseltamivir and zanamivir are also effective for prevention of infection in susceptible patients who have been exposed to H1N1 influenza. The appropriate dosage of oseltamivir for prophylaxis is 75 mg orally once daily for 10 days. The corresponding dosage of zanamivir is 10 mg by inhalation once daily for 10 days.1
The most effective method of prophylaxis, of course, is vaccination with the new H1N1 vaccine.4 There are two forms of the vaccine—a live virus nasal vaccine and an inactivated vaccine for intramuscular administration. Pregnant women should receive only the inactivated vaccine.
The key reservoirs of all influenza A viruses are migrating waterfowl, pigs, and humans. The current H1N1 strain of virus contains eight unique RNA segments that are a mixture of components from avian, pig, and human influenza viruses.2 The pandemic resulting from this virus is unusual because the continent of origin was North America (Mexico) rather than Asia, the season of origin was spring rather than fall, and the patients at greatest risk of dying have been children and young adults rather than infants and the elderly.3
Women who are pregnant or planning to become pregnant should be vaccinated against H1N1 influenza. Use the inactivated virus if a woman is already pregnant.
After exposure to H1N1 influenza, unvaccinated pregnant women and other patients at high risk of developing the virus should be given oseltamivir or zanamivir prophylactically, using the dosage and route of administration described above for prophylaxis.
Pregnant women and other high-risk patients who exhibit symptoms of H1N1 influenza should be given oseltamivir or zanamivir, using the dosage and route of administration described above for treatment, ideally within 48 hours of the onset of symptoms.
For pregnant and postpartum patients, base treatment of H1N1 flu on symptoms, not rapid tests
Louie JK, Acosta M, Jamieson DJ, et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35.
Louie and coworkers describe the outcome of a statewide surveillance program by the California Department of Public Health. They reviewed the medical records of 94 pregnant women, eight women who were within the first 2 weeks postpartum, and 137 nonpregnant women of reproductive age who were hospitalized with confirmed 2009 H1N1 influenza between April 23 and August 11, 2009.
Eighteen pregnant women and four postpartum patients (22%) required intensive care, and 16 (73%) of these women had to be ventilated mechanically. Of the 18 pregnant women who required treatment in the ICU, 12 delivered in the hospital, and four underwent emergent cesarean delivery in the ICU.
Eight (8%) of the 102 pregnant and postpartum patients died. None of these eight women received antiviral therapy within 48 hours of the onset of symptoms. In fact, for pregnant and postpartum patients, a delay in administration of antiviral therapy beyond 48 hours after the onset of symptoms produced a 4.3 relative risk of death (95% confidence interval [CI], 1.4–13.7), compared with patients who were treated early in the course of their infection.
Details of the trial
The women in this trial had the following characteristics:
- gestational age: five (5%) of the 94 pregnant women were in the first trimester, 35 (37%) were in the second trimester, and 54 (57%) were in the third trimester
- underlying conditions were present in 34% of the pregnant and postpartum women and 60% of nonpregnant women. These conditions placed them at increased risk of complications from influenza. The most common underlying condition was asthma
- antiviral therapy was administered to approximately 80% of both pregnant and nonpregnant women. However, only 50% of pregnant women and 34% of nonpregnant women received treatment within 48 hours of the onset of symptoms
- antibiotic therapy was given to 45% of pregnant women and 58% of nonpregnant women for presumed secondary bacterial infection
- false-negative test results: 153 women underwent rapid tests for influenza, 38% of which were falsely negative.
Treat pregnant patients expediently
This article is an excellent complement to the study by Jain and colleagues described on page 37. It strikingly illustrates the heightened risk of morbidity and mortality that pregnant women face when they develop H1N1 influenza. Louie and coworkers documented an influenza-specific mortality ratio (maternal deaths for every 100,000 live births) of 4.3. They also provide clear evidence of the perils of relying on rapid diagnostic tests and withholding antiviral treatment if the rapid test is negative. In their series, 38% of rapid tests were falsely negative. In pregnant women, when antiviral therapy was delayed more than 48 hours, the relative risk of death was 4.3, compared with patients who were treated within 48 hours of the onset of symptoms.
If there is a clinical suspicion of influenza in a pregnant or postpartum patient, treat her immediately with one of the antiviral regimens outlined on page 38—regardless of the outcome of the rapid test for influenza.
Blunt needles reduce needle sticks during cesarean delivery
Sullivan S, Williamson B, Wilson LK, Korde JE, Soper D. Blunt needles for reduction of needlestick injuries during cesarean delivery. Obstet Gynecol. 2009;114 (2 Pt 1):211–216.
Using glove perforation as a proxy for needlestick injuries, Sullivan and colleagues compared blunt needles with sharp needles during cesarean delivery. Ninety-seven women had all anatomic layers reapproximated using blunt needles, and 97 had them reapproximated using sharp needles. The overall glove perforation rate was 12.3%. For sharp needles, the perforation rate was 17.5%, and for blunt needles it was 7.2% (relative risk [RR], 0.66; 95% CI, 0.49–0.89). The key protective effect of the blunt needles was confined to the assistant surgeon (RR, 0.54; 95% CI, 0.41–0.71). The RR for glove perforation involving the primary surgeon was 0.8 (95% CI, 0.53–1.2).
Details of the trial
Glove type, number of gloves, needle size, and type and gauge of suture material were left to the discretion of the surgeon. Glove perforations were identified by filling the gloves with 1,000 mL of water and applying pressure to the palm and each finger. The secondary endpoint of the study was physician satisfaction with the needle. Primary and assistant surgeons reported comparable levels of dissatisfaction with blunt needles, compared with sharp needles (P < .001). However, 92% of primary surgeons and 93% of assistant surgeons rated the blunt needles as at least “acceptable” for use.
Needle stick has led to hepatitis B transmission
Earlier studies reported a rate of glove perforation of 20% to 26% during open abdominal procedures. In an investigation at our center, we noted glove perforation in 13% of cesarean deliveries.5 In this and another investigation, the frequency of perforation did not vary with the level of training of the surgeon or time of day of the procedure.5,6 The most common sites of perforation were the thumb, index finger, and middle finger of the non-dominant hand. The most common mechanism of injury was handling the needle with the operator’s gloved hand rather than with an instrument.
Double-gloving significantly reduces the risk of injury to the inner glove and, subsequently, to the surgeon’s skin. (Note: Double-gloving does not decrease tactile sensation or increase the risk of mishap.6)
The study by Sullivan and colleagues demonstrates that use of blunt needles offers an additional measure of protection against a penetrating injury to the surgeon’s bare skin. Although no surgeon has yet contracted HIV infection from a surgical needle, the transmission of hepatitis B via contaminated surgical needle has been well documented.
Prudence dictates that we use all proven measures to prevent intraoperative blood exposure. Use of blunt needles should be added to interventions such as double-gloving and use of a neutral zone in which to pass sharp objects.
Prophylactic antibiotics reduce postcesarean infection, even in low-risk women
Dinsmoor MJ, Gilbert S, Landon MB, et al, for Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
Infection is the most common postoperative complication of cesarean delivery, now the most frequently performed major operation in America. The principal infection is endometritis, followed by wound infection and urinary tract infection. The frequency of wound infection is on the rise because of the steadily increasing prevalence of obesity in the obstetric population.
Dinsmoor and coworkers conducted this secondary analysis using data from an earlier observational study of 9,432 women who underwent cesarean delivery before the onset of labor. Of these women, 6,006 (64%) received antibiotic prophylaxis.
Women treated prophylactically had a significantly lower rate of endometritis (adjusted odds ratio [OR], 0.40; 95% CI, 0.28–0.59) and of wound infection (adjusted OR, 0.49; 95% CI, 0.28–0.86). The frequency of other infection-related complications was not significantly reduced (adjusted OR, 0.39; 95% CI, 0.13–1.12).
Overall, the size of the effect for endometritis was small; endometritis developed in 2.0% of women in the group that received prophylaxis and 2.6% of women in the group that did not. The size of the effect was even smaller for wound infection.
In this uncontrolled series, 113 patients had to be treated to prevent one case of endometritis or wound infection.
Details of the trial
The original observational study from which this analysis derives was performed by the Maternal-Fetal Medicine Units Network at 13 centers in 1999–2000. The choice of antibiotics and the timing of administration were left to the discretion of the attending physician.
Principal endpoints were the occurrence of postoperative endometritis and wound infection. Secondary endpoints were less common infection-related complications such as maternal sepsis, fascial dehiscence or evisceration, necrotizing fasciitis, pelvic abscess, and septic pelvic vein thrombophlebitis.
Of the women who were given prophylactic antibiotics, 88% received only a cephalosporin, 7% received only a broad-spectrum penicillin, and 6% received other regimens. Approximately 1% of patients received more than one antibiotic for prophylaxis.
Averting infection pays dividends
More than 90% of patients who have endometritis respond promptly to broad-spectrum antibiotic therapy. However, some women with postcesarean endometritis develop serious complications such as septic shock, septic pelvic vein thrombophlebitis, and pelvic abscess.
Treatment of wound infection is not so straightforward as treatment of endometritis. Wound infections may well require surgical intervention to drain an incisional abscess. They also necessitate a change in antibiotic therapy, and they are one of the two most important risk factors for fascial dehiscence and intestinal evisceration.
Multiple studies have confirmed that antibiotic prophylaxis significantly reduces the risk of endometritis and wound infection in women who undergo cesarean after the start of labor, with or without ruptured membranes.7,8 Recent publications have also demonstrated that prophylaxis before the start of surgery offers a greater protective effect than administration after the infant’s umbilical cord is clamped.9,10 Other investigations have demonstrated that broader-spectrum prophylaxis further improves outcomes in women undergoing cesarean delivery.11,12
Antibiotic prophylaxis reduces the frequency of postcesarean endometritis and wound infection, even in very low-risk patients. I strongly support the use of prophylactic antibiotics in all women undergoing cesarean delivery. I believe that the best available evidence supports the use of cefazolin (1 g) plus azithromycin (500 mg), administered intravenously before the start of surgery.9-12
Administer antibiotics before making the incision for greatest effectiveness
Owens SM, Brozanski BS, Meyn LA, Wisenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
In this retrospective investigation, Owens and colleagues compared antibiotic prophylaxis in two groups of women undergoing cesarean delivery:
- 4,229 women who received antibiotics after the infant’s umbilical cord was clamped, from July 2002 to November 2004 (Group 1)
- 4,781 women who received antibiotics before the skin was incised, from June 2005 to August 2007 (Group 2).
Both scheduled and unscheduled cesarean deliveries were included, as were women who received antibiotics intrapartum for group B streptococcus prophylaxis and treatment of chorioamnionitis. The most commonly used antibiotic was intravenous cefazolin (1 g).
After excluding women who received group B streptococcus prophylaxis or intrapartum treatment of chorioamnionitis, the authors demonstrated a nearly 50% reduction in the rate of endometritis among women who received antibiotics before surgery (OR, 0.54; 95% CI, 0.38–0.75). They also documented a 30% reduction in the rate of wound infection in these patients (OR, 0.72; 95% CI, 0.55–0.46).
Details of the trial
Principal outcome measures were the rates of maternal endometritis and wound infection and rates of proven and presumed neonatal infection. The mean age and racial distribution were similar in the two groups, but the percentage of patients treated on a resident teaching service was lower in Group 2 (14.9% vs. 18.9%; P < .001). The two groups did not differ in mean body mass index or in the percentage of patients who were in labor before surgery. Colonization with group B streptococcus was more common in Group 2 (24.4% vs. 22.2%; P = .5). However, chorioamnionitis was less prevalent in Group 2 (5.6% vs. 10.3%; P < .001).
The rates of culture-proven neonatal infection within the first 3 days of life (early-onset infection) were similar between groups (1.3% in Group 1 vs. 0.7% in Group 2). Culture-proven late-onset neonatal infection was less common in Group 2 (1.8% vs. 5.7%; P < .001). The groups did not differ in the proportion of newborns treated for presumed infection (24.1% in Group 1 vs. 22.2% in Group 2).
Plentiful data confirm the superiority of preoperative administration
Endometritis is the most common postoperative complication associated with cesarean delivery. Wound infection is less common but more likely to lead to prolonged postoperative morbidity and extended hospitalization. Reducing both of these complications is a critical clinical objective.
Virtually without exception, every investigation has confirmed that prophylactic antibiotics reduce the frequency of postcesarean endometritis and, usually, wound infection as well. One dose of a given antibiotic is clearly as effective as multiple doses.
Classic animal investigations by Burke demonstrated that prophylaxis was most effective when antibiotics were present in tissue prior to the surgical incision.13 Nevertheless, early investigators in obstetrics argued that preoperative exposure to antibiotics increased the likelihood that the neonate would require an evaluation for sepsis and that delaying antibiotics until after cord clamping did not compromise the effectiveness of prophylaxis.14,15
Sullivan and colleagues were the first authors to successfully challenge this dictum.9 In a well-designed investigation, they demonstrated that preoperative administration of antibiotics significantly reduces the frequency of endometritis (RR, 0.22) but not wound infection, and does not increase the need for neonatal sepsis evaluation. Kaimel and coworkers later confirmed these findings,16 and this study by Owen and associates offers additional proof of the effectiveness and safety of preoperative antibiotic administration.
I offer only one addendum to the conclusions presented by Owen and colleagues. Two recent investigations from the University of Alabama conclusively demonstrate that, by extending the spectrum of antibiotic coverage by combining azithromycin and cefazolin, we can further reduce postcesarean endometritis and wound infection.11,17 Accordingly, at our center, we now administer both intravenous (IV) azithromycin (500 mg over 1 hour) and IV cefazolin (1 g) approximately 30 to 60 minutes before the start of surgery.
Antibiotic prophylaxis reduces the rates of postcesarean endometritis and wound infection, and preoperative administration is superior to administration after cord clamping. Preoperative administration is also safe for the neonate.
Administer IV azithromycin (500 mg over 1 hour) and IV cefazolin (1 g) approximately 30 to 60 minutes before the start of surgery.
Chlorhexidine solutions are superior to povidone-iodine for surgical-site antisepsis
Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
This report is an excellent complement to the two studies discussed above, which focused on systemic antibiotic prophylaxis for the prevention of postcesarean infection. Here, Darouiche and colleagues conducted a randomized, prospective, unblinded, multi-center comparison of two skin preparations to prevent surgical-site infection:
- 2% chlorhexidine gluconate and 70% isopropyl alcohol (409 patients)
- 10% povidone-iodine solution (440 patients).
Participants underwent a variety of abdominal and nonabdominal (thoracic, gynecologic, and urologic) procedures. All patients received systemic antibiotic prophylaxis within 1 hour before the start of surgery.
The primary outcome measure was the occurrence of any surgical-site infection up to 30 days after surgery. This rate was lower among patients who received chlorhexidine-alcohol skin preparations than among those who received povidone-iodine (9.5% vs. 16.1%; P = .004).
Secondary endpoints were specific types of infection:
- superficial incisional infection (skin and subcutaneous tissue): lower among patients receiving chlorhexidine-alcohol (4.2% vs. 8.6%; P = .008)
- deep incisional infection (involving fascia and muscle): lower among patients receiving chlorhexidine-alcohol (1% vs. 3%; P = .05)
- organ-space infection (any organ or space other than the body wall): no significant difference between women treated with chlorhexidine-alcohol and those treated with povidone-iodine.
Seventeen patients would need to be treated with chlorhexidine-alcohol to prevent one surgical-site infection.
Chlorhexidine has a solid track record
The 41% reduction in the rate of surgical-site infection with chlorhexidine-alcohol (RR, 0.59; 95% CI, 0.41–0.85) is consistent with a 49% reduction in the risk of vascular catheter-related bacteremia using the same formulation.18 The findings are also consistent with a recent report showing that chlorhexidine was more effective than iodine-containing solutions in reducing bacterial concentration in the operative field in women undergoing vaginal hysterectomy.19
Darouiche and coworkers suggest that chlorhexidine is more effective because it has a more rapid onset of action and greater and more persistent antibacterial activity despite exposure to body fluids. Quite appropriately, they indicate that the solution used in their study is flammable, but they observed no adverse effects in a large sample of patients undergoing a variety of procedures.
I strongly recommend that chlorhexidine be used for all surgical skin preparation in obstetric and gynecologic patients. this intervention, along with consistent use of systemic antibiotic prophylaxis, should be highly effective in reducing the risk of superficial and deep abdominal wound infection.
Six recent articles stand out in the field of infectious disease:
- an assessment of outcomes of seriously ill patients who were hospitalized early in the course of the H1N1 influenza epidemic. The authors highlight major differences in the epidemiology of this infection, compared with regular seasonal flu
- an examination of outcomes of pregnant women who developed H1N1 influenza
- an exploration of the use of blunt needles during cesarean delivery to prevent glove perforation
- an evaluation of the utility of prophylactic antibiotics in ostensibly low-risk women undergoing scheduled cesarean delivery
- a look at the timing of antibiotic prophylaxis for cesarean delivery
- a comparison of skin preparation techniques in the prevention of surgical-site infection.
The focus on cesarean delivery in most of these studies seems particularly appropriate, now that this operation has become the most frequently performed major surgical procedure in US hospitals.
H1N1 virus hits hardest during pregnancy and chronic illness
Jain S, Kamimoto L, Bramley AM, et al, for 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944.
This retrospective survey of patients hospitalized for at least 24 hours for treatment of influenza-like illness included 272 patients who were given a diagnosis of H1N1 influenza, based on real-time, reverse-transcriptase, polymerase chain reaction assay. Sixty-seven (25%) of these patients were admitted to an ICU, and 19 (7%) died. All of the patients who died had been treated in an ICU, and two thirds had an underlying medical condition. Three of the deaths involved pregnant women. None of the patients who died received antiviral therapy within 48 hours of the onset of symptoms. Those who died were also less likely to have been vaccinated against seasonal influenza in 2008–2009.
Details of the trial
The 272 patients included in this study sample represented 25% of the total number of patients hospitalized in the United States for treatment of influenza between April and mid-June 2009. They exhibited the following characteristics:
- median age: 21 years
- race and ethnicity: 30% were Hispanic, and 27% were non-Hispanic white
- most common symptoms: fever and cough, although diarrhea or vomiting was reported in 39% of patients
- underlying medical illness: present in 73% (198 patients), including 60% of children and 83% of adults. At least two underlying medical conditions were present in 32% of patients. Asthma was the most common comorbid condition
- pregnancy: 18 patients were pregnant. Four of the pregnant patients also had asthma, and two had diabetes
- obesity: 29% of adults were obese. Morbid obesity was present in 26%. More than 75% of obese and morbidly obese patients had at least one underlying medical illness
- bloodwork at admission: 20% of patients were leukopenic; 37% were anemic; and 14% were thrombocytopenic
- chest film: 40% of patients who underwent chest radiography had findings consistent with pneumonia. Findings included bilateral infiltrates in 66 patients, a unilobar infiltrate in 26, and multilobar infiltrates in two
- antiviral therapy: 75% ultimately received antiviral drugs, with a median time from onset of illness to initiation of therapy of 3 days (range, 0–29 days). Only 39% received antiviral therapy within 48 hours of the onset of symptoms
- antibiotic therapy: 79% of patients received antibiotics for presumed superimposed bacterial infection. The most commonly used antibiotics were ceftriaxone, azithromycin, vancomycin, and levofloxacin.
Study offers 4 useful lessons
The study by Jain and colleagues offers clinically applicable lessons:
- it reinforces the point that children and young adults, including pregnant women, are at increased risk of serious morbidity and mortality
- it demonstrates that most seriously affected patients have at least one underlying medical condition, such as asthma
- it highlights the importance of pregnancy and morbid obesity as major conditions that contribute to serious complications from influenza. The 7% prevalence of pregnant patients is significantly higher than the 1% prevalence that would typically be expected with seasonal influenza. Similarly, the 26% prevalence of morbid obesity greatly exceeds the estimated 5% prevalence in the adult US population
- it confirms the importance of treating patients early in the course of their illness with antiviral drugs such as oseltamivir. Notably, none of the patients who died received treatment within 48 hours of the onset of illness, when the drugs are most likely to be effective.
How to treat H1N1 influenza
The vast majority of strains of the 2009 H1N1 virus are susceptible to oseltamivir and zanamivir, but essentially all strains are resistant to amantadine and rimantadine.1 Therefore, all individuals who are hospitalized should be treated with one of two regimens:
- oseltamivir, 75 mg orally, twice daily for at least 5 days
- zanamivir, 10 mg by inhalation, twice daily for at least 5 days.
These same regimens should be used for outpatients who are at high risk of complications.
Ideally, antiviral treatment should be administered within 48 hours of the onset of symptoms, but do not withhold treatment even if more than 48 hours have elapsed since the onset of illness.2,3
Both oseltamivir and zanamivir are also effective for prevention of infection in susceptible patients who have been exposed to H1N1 influenza. The appropriate dosage of oseltamivir for prophylaxis is 75 mg orally once daily for 10 days. The corresponding dosage of zanamivir is 10 mg by inhalation once daily for 10 days.1
The most effective method of prophylaxis, of course, is vaccination with the new H1N1 vaccine.4 There are two forms of the vaccine—a live virus nasal vaccine and an inactivated vaccine for intramuscular administration. Pregnant women should receive only the inactivated vaccine.
The key reservoirs of all influenza A viruses are migrating waterfowl, pigs, and humans. The current H1N1 strain of virus contains eight unique RNA segments that are a mixture of components from avian, pig, and human influenza viruses.2 The pandemic resulting from this virus is unusual because the continent of origin was North America (Mexico) rather than Asia, the season of origin was spring rather than fall, and the patients at greatest risk of dying have been children and young adults rather than infants and the elderly.3
Women who are pregnant or planning to become pregnant should be vaccinated against H1N1 influenza. Use the inactivated virus if a woman is already pregnant.
After exposure to H1N1 influenza, unvaccinated pregnant women and other patients at high risk of developing the virus should be given oseltamivir or zanamivir prophylactically, using the dosage and route of administration described above for prophylaxis.
Pregnant women and other high-risk patients who exhibit symptoms of H1N1 influenza should be given oseltamivir or zanamivir, using the dosage and route of administration described above for treatment, ideally within 48 hours of the onset of symptoms.
For pregnant and postpartum patients, base treatment of H1N1 flu on symptoms, not rapid tests
Louie JK, Acosta M, Jamieson DJ, et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35.
Louie and coworkers describe the outcome of a statewide surveillance program by the California Department of Public Health. They reviewed the medical records of 94 pregnant women, eight women who were within the first 2 weeks postpartum, and 137 nonpregnant women of reproductive age who were hospitalized with confirmed 2009 H1N1 influenza between April 23 and August 11, 2009.
Eighteen pregnant women and four postpartum patients (22%) required intensive care, and 16 (73%) of these women had to be ventilated mechanically. Of the 18 pregnant women who required treatment in the ICU, 12 delivered in the hospital, and four underwent emergent cesarean delivery in the ICU.
Eight (8%) of the 102 pregnant and postpartum patients died. None of these eight women received antiviral therapy within 48 hours of the onset of symptoms. In fact, for pregnant and postpartum patients, a delay in administration of antiviral therapy beyond 48 hours after the onset of symptoms produced a 4.3 relative risk of death (95% confidence interval [CI], 1.4–13.7), compared with patients who were treated early in the course of their infection.
Details of the trial
The women in this trial had the following characteristics:
- gestational age: five (5%) of the 94 pregnant women were in the first trimester, 35 (37%) were in the second trimester, and 54 (57%) were in the third trimester
- underlying conditions were present in 34% of the pregnant and postpartum women and 60% of nonpregnant women. These conditions placed them at increased risk of complications from influenza. The most common underlying condition was asthma
- antiviral therapy was administered to approximately 80% of both pregnant and nonpregnant women. However, only 50% of pregnant women and 34% of nonpregnant women received treatment within 48 hours of the onset of symptoms
- antibiotic therapy was given to 45% of pregnant women and 58% of nonpregnant women for presumed secondary bacterial infection
- false-negative test results: 153 women underwent rapid tests for influenza, 38% of which were falsely negative.
Treat pregnant patients expediently
This article is an excellent complement to the study by Jain and colleagues described on page 37. It strikingly illustrates the heightened risk of morbidity and mortality that pregnant women face when they develop H1N1 influenza. Louie and coworkers documented an influenza-specific mortality ratio (maternal deaths for every 100,000 live births) of 4.3. They also provide clear evidence of the perils of relying on rapid diagnostic tests and withholding antiviral treatment if the rapid test is negative. In their series, 38% of rapid tests were falsely negative. In pregnant women, when antiviral therapy was delayed more than 48 hours, the relative risk of death was 4.3, compared with patients who were treated within 48 hours of the onset of symptoms.
If there is a clinical suspicion of influenza in a pregnant or postpartum patient, treat her immediately with one of the antiviral regimens outlined on page 38—regardless of the outcome of the rapid test for influenza.
Blunt needles reduce needle sticks during cesarean delivery
Sullivan S, Williamson B, Wilson LK, Korde JE, Soper D. Blunt needles for reduction of needlestick injuries during cesarean delivery. Obstet Gynecol. 2009;114 (2 Pt 1):211–216.
Using glove perforation as a proxy for needlestick injuries, Sullivan and colleagues compared blunt needles with sharp needles during cesarean delivery. Ninety-seven women had all anatomic layers reapproximated using blunt needles, and 97 had them reapproximated using sharp needles. The overall glove perforation rate was 12.3%. For sharp needles, the perforation rate was 17.5%, and for blunt needles it was 7.2% (relative risk [RR], 0.66; 95% CI, 0.49–0.89). The key protective effect of the blunt needles was confined to the assistant surgeon (RR, 0.54; 95% CI, 0.41–0.71). The RR for glove perforation involving the primary surgeon was 0.8 (95% CI, 0.53–1.2).
Details of the trial
Glove type, number of gloves, needle size, and type and gauge of suture material were left to the discretion of the surgeon. Glove perforations were identified by filling the gloves with 1,000 mL of water and applying pressure to the palm and each finger. The secondary endpoint of the study was physician satisfaction with the needle. Primary and assistant surgeons reported comparable levels of dissatisfaction with blunt needles, compared with sharp needles (P < .001). However, 92% of primary surgeons and 93% of assistant surgeons rated the blunt needles as at least “acceptable” for use.
Needle stick has led to hepatitis B transmission
Earlier studies reported a rate of glove perforation of 20% to 26% during open abdominal procedures. In an investigation at our center, we noted glove perforation in 13% of cesarean deliveries.5 In this and another investigation, the frequency of perforation did not vary with the level of training of the surgeon or time of day of the procedure.5,6 The most common sites of perforation were the thumb, index finger, and middle finger of the non-dominant hand. The most common mechanism of injury was handling the needle with the operator’s gloved hand rather than with an instrument.
Double-gloving significantly reduces the risk of injury to the inner glove and, subsequently, to the surgeon’s skin. (Note: Double-gloving does not decrease tactile sensation or increase the risk of mishap.6)
The study by Sullivan and colleagues demonstrates that use of blunt needles offers an additional measure of protection against a penetrating injury to the surgeon’s bare skin. Although no surgeon has yet contracted HIV infection from a surgical needle, the transmission of hepatitis B via contaminated surgical needle has been well documented.
Prudence dictates that we use all proven measures to prevent intraoperative blood exposure. Use of blunt needles should be added to interventions such as double-gloving and use of a neutral zone in which to pass sharp objects.
Prophylactic antibiotics reduce postcesarean infection, even in low-risk women
Dinsmoor MJ, Gilbert S, Landon MB, et al, for Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Perioperative antibiotic prophylaxis for nonlaboring cesarean delivery. Obstet Gynecol. 2009;114(4):752–756.
Infection is the most common postoperative complication of cesarean delivery, now the most frequently performed major operation in America. The principal infection is endometritis, followed by wound infection and urinary tract infection. The frequency of wound infection is on the rise because of the steadily increasing prevalence of obesity in the obstetric population.
Dinsmoor and coworkers conducted this secondary analysis using data from an earlier observational study of 9,432 women who underwent cesarean delivery before the onset of labor. Of these women, 6,006 (64%) received antibiotic prophylaxis.
Women treated prophylactically had a significantly lower rate of endometritis (adjusted odds ratio [OR], 0.40; 95% CI, 0.28–0.59) and of wound infection (adjusted OR, 0.49; 95% CI, 0.28–0.86). The frequency of other infection-related complications was not significantly reduced (adjusted OR, 0.39; 95% CI, 0.13–1.12).
Overall, the size of the effect for endometritis was small; endometritis developed in 2.0% of women in the group that received prophylaxis and 2.6% of women in the group that did not. The size of the effect was even smaller for wound infection.
In this uncontrolled series, 113 patients had to be treated to prevent one case of endometritis or wound infection.
Details of the trial
The original observational study from which this analysis derives was performed by the Maternal-Fetal Medicine Units Network at 13 centers in 1999–2000. The choice of antibiotics and the timing of administration were left to the discretion of the attending physician.
Principal endpoints were the occurrence of postoperative endometritis and wound infection. Secondary endpoints were less common infection-related complications such as maternal sepsis, fascial dehiscence or evisceration, necrotizing fasciitis, pelvic abscess, and septic pelvic vein thrombophlebitis.
Of the women who were given prophylactic antibiotics, 88% received only a cephalosporin, 7% received only a broad-spectrum penicillin, and 6% received other regimens. Approximately 1% of patients received more than one antibiotic for prophylaxis.
Averting infection pays dividends
More than 90% of patients who have endometritis respond promptly to broad-spectrum antibiotic therapy. However, some women with postcesarean endometritis develop serious complications such as septic shock, septic pelvic vein thrombophlebitis, and pelvic abscess.
Treatment of wound infection is not so straightforward as treatment of endometritis. Wound infections may well require surgical intervention to drain an incisional abscess. They also necessitate a change in antibiotic therapy, and they are one of the two most important risk factors for fascial dehiscence and intestinal evisceration.
Multiple studies have confirmed that antibiotic prophylaxis significantly reduces the risk of endometritis and wound infection in women who undergo cesarean after the start of labor, with or without ruptured membranes.7,8 Recent publications have also demonstrated that prophylaxis before the start of surgery offers a greater protective effect than administration after the infant’s umbilical cord is clamped.9,10 Other investigations have demonstrated that broader-spectrum prophylaxis further improves outcomes in women undergoing cesarean delivery.11,12
Antibiotic prophylaxis reduces the frequency of postcesarean endometritis and wound infection, even in very low-risk patients. I strongly support the use of prophylactic antibiotics in all women undergoing cesarean delivery. I believe that the best available evidence supports the use of cefazolin (1 g) plus azithromycin (500 mg), administered intravenously before the start of surgery.9-12
Administer antibiotics before making the incision for greatest effectiveness
Owens SM, Brozanski BS, Meyn LA, Wisenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstet Gynecol. 2009;114(3):573–579.
In this retrospective investigation, Owens and colleagues compared antibiotic prophylaxis in two groups of women undergoing cesarean delivery:
- 4,229 women who received antibiotics after the infant’s umbilical cord was clamped, from July 2002 to November 2004 (Group 1)
- 4,781 women who received antibiotics before the skin was incised, from June 2005 to August 2007 (Group 2).
Both scheduled and unscheduled cesarean deliveries were included, as were women who received antibiotics intrapartum for group B streptococcus prophylaxis and treatment of chorioamnionitis. The most commonly used antibiotic was intravenous cefazolin (1 g).
After excluding women who received group B streptococcus prophylaxis or intrapartum treatment of chorioamnionitis, the authors demonstrated a nearly 50% reduction in the rate of endometritis among women who received antibiotics before surgery (OR, 0.54; 95% CI, 0.38–0.75). They also documented a 30% reduction in the rate of wound infection in these patients (OR, 0.72; 95% CI, 0.55–0.46).
Details of the trial
Principal outcome measures were the rates of maternal endometritis and wound infection and rates of proven and presumed neonatal infection. The mean age and racial distribution were similar in the two groups, but the percentage of patients treated on a resident teaching service was lower in Group 2 (14.9% vs. 18.9%; P < .001). The two groups did not differ in mean body mass index or in the percentage of patients who were in labor before surgery. Colonization with group B streptococcus was more common in Group 2 (24.4% vs. 22.2%; P = .5). However, chorioamnionitis was less prevalent in Group 2 (5.6% vs. 10.3%; P < .001).
The rates of culture-proven neonatal infection within the first 3 days of life (early-onset infection) were similar between groups (1.3% in Group 1 vs. 0.7% in Group 2). Culture-proven late-onset neonatal infection was less common in Group 2 (1.8% vs. 5.7%; P < .001). The groups did not differ in the proportion of newborns treated for presumed infection (24.1% in Group 1 vs. 22.2% in Group 2).
Plentiful data confirm the superiority of preoperative administration
Endometritis is the most common postoperative complication associated with cesarean delivery. Wound infection is less common but more likely to lead to prolonged postoperative morbidity and extended hospitalization. Reducing both of these complications is a critical clinical objective.
Virtually without exception, every investigation has confirmed that prophylactic antibiotics reduce the frequency of postcesarean endometritis and, usually, wound infection as well. One dose of a given antibiotic is clearly as effective as multiple doses.
Classic animal investigations by Burke demonstrated that prophylaxis was most effective when antibiotics were present in tissue prior to the surgical incision.13 Nevertheless, early investigators in obstetrics argued that preoperative exposure to antibiotics increased the likelihood that the neonate would require an evaluation for sepsis and that delaying antibiotics until after cord clamping did not compromise the effectiveness of prophylaxis.14,15
Sullivan and colleagues were the first authors to successfully challenge this dictum.9 In a well-designed investigation, they demonstrated that preoperative administration of antibiotics significantly reduces the frequency of endometritis (RR, 0.22) but not wound infection, and does not increase the need for neonatal sepsis evaluation. Kaimel and coworkers later confirmed these findings,16 and this study by Owen and associates offers additional proof of the effectiveness and safety of preoperative antibiotic administration.
I offer only one addendum to the conclusions presented by Owen and colleagues. Two recent investigations from the University of Alabama conclusively demonstrate that, by extending the spectrum of antibiotic coverage by combining azithromycin and cefazolin, we can further reduce postcesarean endometritis and wound infection.11,17 Accordingly, at our center, we now administer both intravenous (IV) azithromycin (500 mg over 1 hour) and IV cefazolin (1 g) approximately 30 to 60 minutes before the start of surgery.
Antibiotic prophylaxis reduces the rates of postcesarean endometritis and wound infection, and preoperative administration is superior to administration after cord clamping. Preoperative administration is also safe for the neonate.
Administer IV azithromycin (500 mg over 1 hour) and IV cefazolin (1 g) approximately 30 to 60 minutes before the start of surgery.
Chlorhexidine solutions are superior to povidone-iodine for surgical-site antisepsis
Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
This report is an excellent complement to the two studies discussed above, which focused on systemic antibiotic prophylaxis for the prevention of postcesarean infection. Here, Darouiche and colleagues conducted a randomized, prospective, unblinded, multi-center comparison of two skin preparations to prevent surgical-site infection:
- 2% chlorhexidine gluconate and 70% isopropyl alcohol (409 patients)
- 10% povidone-iodine solution (440 patients).
Participants underwent a variety of abdominal and nonabdominal (thoracic, gynecologic, and urologic) procedures. All patients received systemic antibiotic prophylaxis within 1 hour before the start of surgery.
The primary outcome measure was the occurrence of any surgical-site infection up to 30 days after surgery. This rate was lower among patients who received chlorhexidine-alcohol skin preparations than among those who received povidone-iodine (9.5% vs. 16.1%; P = .004).
Secondary endpoints were specific types of infection:
- superficial incisional infection (skin and subcutaneous tissue): lower among patients receiving chlorhexidine-alcohol (4.2% vs. 8.6%; P = .008)
- deep incisional infection (involving fascia and muscle): lower among patients receiving chlorhexidine-alcohol (1% vs. 3%; P = .05)
- organ-space infection (any organ or space other than the body wall): no significant difference between women treated with chlorhexidine-alcohol and those treated with povidone-iodine.
Seventeen patients would need to be treated with chlorhexidine-alcohol to prevent one surgical-site infection.
Chlorhexidine has a solid track record
The 41% reduction in the rate of surgical-site infection with chlorhexidine-alcohol (RR, 0.59; 95% CI, 0.41–0.85) is consistent with a 49% reduction in the risk of vascular catheter-related bacteremia using the same formulation.18 The findings are also consistent with a recent report showing that chlorhexidine was more effective than iodine-containing solutions in reducing bacterial concentration in the operative field in women undergoing vaginal hysterectomy.19
Darouiche and coworkers suggest that chlorhexidine is more effective because it has a more rapid onset of action and greater and more persistent antibacterial activity despite exposure to body fluids. Quite appropriately, they indicate that the solution used in their study is flammable, but they observed no adverse effects in a large sample of patients undergoing a variety of procedures.
I strongly recommend that chlorhexidine be used for all surgical skin preparation in obstetric and gynecologic patients. this intervention, along with consistent use of systemic antibiotic prophylaxis, should be highly effective in reducing the risk of superficial and deep abdominal wound infection.
1. Antiviral drugs for influenza. The Medical Letter. 2009;51(1325):89-92.
2. Wenzel RP, Edmond MB. Preparing for 2009 H1N1 influenza. N Engl J Med. 2009;361(20):1991-1993.
3. Perez-Padilla R, Rosa-Zambori D, deLeon SP, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680-689.
4. H1N1 vaccine for prevention of pandemic influenza. The Medical Letter. 2009;51(1322):77-78.
5. Chapman S, Duff P. Frequency of glove perforations and subsequent blood contact in association with selected obstetric surgical procedures. Am J Obstet Gynecol. 1993;168(5):1354-1357.
6. Lancaster C, Duff P. Single versus double-gloving for obstetric and gynecologic procedures. Am J Obstet Gynecol. 2007;196(5):e36-e37.
7. Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database of Systematic Reviews. 2002;(3):CD000933.-doi:10/1002/14651858.
8. Prophylactic antibiotics in labor and delivery. ACOG Practice Bulletin No. 47. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2003;102(4):875-882.
9. Sullivan SA, Smith T, Chang F, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1-e5.
10. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1-e6.
11. Tita ATN, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51-56.
12. Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1-e3.
13. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168.
14. Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before and after cord clamping. Obstet Gynecol. 1979;53(2):151-156.
15. Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151-154.
16. Kaimal AJ, Zlatnik MG, Chang YW, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical-site infections. Am J Obstet Gynecol. 2008;199(3):310.e1-e5.
17. Tita AT, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systemic review. Obstet Gynecol. 2009;113(3):675-682.
18. Chaiyakunapruk N, Veerstra DI, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136(11):792-801.
19. Culligan PJ, Kubik K, Murphy M, Blackwell L, Snyder J. A randomized trial that compared povidone iodine and chlorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol. 2005;192(2):422-425.
1. Antiviral drugs for influenza. The Medical Letter. 2009;51(1325):89-92.
2. Wenzel RP, Edmond MB. Preparing for 2009 H1N1 influenza. N Engl J Med. 2009;361(20):1991-1993.
3. Perez-Padilla R, Rosa-Zambori D, deLeon SP, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680-689.
4. H1N1 vaccine for prevention of pandemic influenza. The Medical Letter. 2009;51(1322):77-78.
5. Chapman S, Duff P. Frequency of glove perforations and subsequent blood contact in association with selected obstetric surgical procedures. Am J Obstet Gynecol. 1993;168(5):1354-1357.
6. Lancaster C, Duff P. Single versus double-gloving for obstetric and gynecologic procedures. Am J Obstet Gynecol. 2007;196(5):e36-e37.
7. Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database of Systematic Reviews. 2002;(3):CD000933.-doi:10/1002/14651858.
8. Prophylactic antibiotics in labor and delivery. ACOG Practice Bulletin No. 47. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2003;102(4):875-882.
9. Sullivan SA, Smith T, Chang F, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2007;196(5):455.e1-e5.
10. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1-e6.
11. Tita ATN, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51-56.
12. Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199(3):303.e1-e3.
13. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168.
14. Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before and after cord clamping. Obstet Gynecol. 1979;53(2):151-156.
15. Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151-154.
16. Kaimal AJ, Zlatnik MG, Chang YW, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical-site infections. Am J Obstet Gynecol. 2008;199(3):310.e1-e5.
17. Tita AT, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systemic review. Obstet Gynecol. 2009;113(3):675-682.
18. Chaiyakunapruk N, Veerstra DI, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136(11):792-801.
19. Culligan PJ, Kubik K, Murphy M, Blackwell L, Snyder J. A randomized trial that compared povidone iodine and chlorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol. 2005;192(2):422-425.

