User login
Can we reduce the rate of scheduled births that occur earlier than 39 weeks of gestation?
This interesting study from the Ohio Perinatal Quality Collaborative (OPQC) tackles an important topic—reducing the number of scheduled deliveries that take place before 39 weeks’ gestation. Infants born before 39 weeks have a higher rate of NICU admission in addition to measurable morbidity. The March of Dimes and other organizations have recognized the reduction of these births as an important goal, and ACOG recently refined and clarified the medical indications for them.1
Details of the study
Twenty maternity hospitals in Ohio collected baseline data on the rate of scheduled delivery between 36.0 and 38.6 weeks’ gestation over a 60-day period. Members of the OPQC (which included perinatologists) then visited each participating hospital to introduce the project and explain how it would be sustained using techniques from the Institute for Healthcare Improvement Breakthrough Series.2 Each hospital developed a three-person team—a physician, a nurse, and a data manager—to lead the effort. OPQC faculty developed a list of interventions, including the following broad techniques:
- promote optimal determination of gestational age using ultrasonography (US)
- use ACOG criteria for the indication and timing of scheduled births
- educate physicians, nurses, and pregnant women of the risks and benefits of birth between 36.0 and 38.6 weeks’ gestation
- improve communication between obstetricians and pediatricians through chart documentation of clear patient hand-offs and monthly reporting of statistics to physicians, nurses, and administrators
- include scheduled births in an overall “culture of safety,” with regular discussion of the matter at department and quality meetings.
I offer five key points about any program to reduce the number of scheduled births between 36.0 and 38.6 weeks’ gestation:
- it requires a lot of work and commitment on the part of all members of the health care team to change a behavior. Educate team members about the goal and the reasons behind it, and encourage them to contribute ideas to achieve it
- consider cooperating with state agencies to obtain data that can be used as baseline information and to measure the effect of an intervention
- a “one size fits all” approach is unlikely to be effective. Each hospital and physician is unique, and interventions should be individualized
- although the result of this 14-month study is impressive, real change must be maintained over the long term. Keep this in mind when you are planning interventions
- efforts such as this one have a financial cost associated with them, and I would hope that the government would contribute funding.
—GEORGE A. MACONES, MD, MSCE
Staff at participating hospitals had much latitude in selecting and refining interventions for their institution. After implementation of specific initiatives, each hospital tracked and reported relevant outcomes, especially the rate of scheduled deliveries between 36.0 and 38.6 weeks’ gestation. Each hospital received a monthly report, and staff members were encouraged to share the results internally.
Dramatic result was validated
The result of this initiative is impressive: The rate of inappropriate scheduled births was reduced from 25% to less than 5% over 14 months. This finding was validated in two ways:
- by checking Ohio birth certificate data
- by determining whether there was a concomitant rise in the rate of deliveries between 39 and 41 weeks’ gestation in the same hospitals.
Both validations confirmed that the OPQC interventions were significantly effective.
Project was not truly “statewide”
No study is perfect. A potential limitation of this study is the fact that it did not include all hospitals in the state of Ohio—only a sampling of larger hospitals. This makes it unclear whether the approach would be as effective in smaller institutions. Moreover, it is possible that physicians simply got better at documenting a reason for scheduled delivery as the study progressed—although I doubt that this reason alone would explain the striking findings.
1. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin#107, August 2009: Induction of labor. Obstet Gynecol. 2009;114(2, part 1):386-397.
2. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement Website. 2003. http://www.ihi.org/IHI/Results/WhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchieving+BreakthroughImprovement.htm. Accessed May 4, 2010.
This interesting study from the Ohio Perinatal Quality Collaborative (OPQC) tackles an important topic—reducing the number of scheduled deliveries that take place before 39 weeks’ gestation. Infants born before 39 weeks have a higher rate of NICU admission in addition to measurable morbidity. The March of Dimes and other organizations have recognized the reduction of these births as an important goal, and ACOG recently refined and clarified the medical indications for them.1
Details of the study
Twenty maternity hospitals in Ohio collected baseline data on the rate of scheduled delivery between 36.0 and 38.6 weeks’ gestation over a 60-day period. Members of the OPQC (which included perinatologists) then visited each participating hospital to introduce the project and explain how it would be sustained using techniques from the Institute for Healthcare Improvement Breakthrough Series.2 Each hospital developed a three-person team—a physician, a nurse, and a data manager—to lead the effort. OPQC faculty developed a list of interventions, including the following broad techniques:
- promote optimal determination of gestational age using ultrasonography (US)
- use ACOG criteria for the indication and timing of scheduled births
- educate physicians, nurses, and pregnant women of the risks and benefits of birth between 36.0 and 38.6 weeks’ gestation
- improve communication between obstetricians and pediatricians through chart documentation of clear patient hand-offs and monthly reporting of statistics to physicians, nurses, and administrators
- include scheduled births in an overall “culture of safety,” with regular discussion of the matter at department and quality meetings.
I offer five key points about any program to reduce the number of scheduled births between 36.0 and 38.6 weeks’ gestation:
- it requires a lot of work and commitment on the part of all members of the health care team to change a behavior. Educate team members about the goal and the reasons behind it, and encourage them to contribute ideas to achieve it
- consider cooperating with state agencies to obtain data that can be used as baseline information and to measure the effect of an intervention
- a “one size fits all” approach is unlikely to be effective. Each hospital and physician is unique, and interventions should be individualized
- although the result of this 14-month study is impressive, real change must be maintained over the long term. Keep this in mind when you are planning interventions
- efforts such as this one have a financial cost associated with them, and I would hope that the government would contribute funding.
—GEORGE A. MACONES, MD, MSCE
Staff at participating hospitals had much latitude in selecting and refining interventions for their institution. After implementation of specific initiatives, each hospital tracked and reported relevant outcomes, especially the rate of scheduled deliveries between 36.0 and 38.6 weeks’ gestation. Each hospital received a monthly report, and staff members were encouraged to share the results internally.
Dramatic result was validated
The result of this initiative is impressive: The rate of inappropriate scheduled births was reduced from 25% to less than 5% over 14 months. This finding was validated in two ways:
- by checking Ohio birth certificate data
- by determining whether there was a concomitant rise in the rate of deliveries between 39 and 41 weeks’ gestation in the same hospitals.
Both validations confirmed that the OPQC interventions were significantly effective.
Project was not truly “statewide”
No study is perfect. A potential limitation of this study is the fact that it did not include all hospitals in the state of Ohio—only a sampling of larger hospitals. This makes it unclear whether the approach would be as effective in smaller institutions. Moreover, it is possible that physicians simply got better at documenting a reason for scheduled delivery as the study progressed—although I doubt that this reason alone would explain the striking findings.
This interesting study from the Ohio Perinatal Quality Collaborative (OPQC) tackles an important topic—reducing the number of scheduled deliveries that take place before 39 weeks’ gestation. Infants born before 39 weeks have a higher rate of NICU admission in addition to measurable morbidity. The March of Dimes and other organizations have recognized the reduction of these births as an important goal, and ACOG recently refined and clarified the medical indications for them.1
Details of the study
Twenty maternity hospitals in Ohio collected baseline data on the rate of scheduled delivery between 36.0 and 38.6 weeks’ gestation over a 60-day period. Members of the OPQC (which included perinatologists) then visited each participating hospital to introduce the project and explain how it would be sustained using techniques from the Institute for Healthcare Improvement Breakthrough Series.2 Each hospital developed a three-person team—a physician, a nurse, and a data manager—to lead the effort. OPQC faculty developed a list of interventions, including the following broad techniques:
- promote optimal determination of gestational age using ultrasonography (US)
- use ACOG criteria for the indication and timing of scheduled births
- educate physicians, nurses, and pregnant women of the risks and benefits of birth between 36.0 and 38.6 weeks’ gestation
- improve communication between obstetricians and pediatricians through chart documentation of clear patient hand-offs and monthly reporting of statistics to physicians, nurses, and administrators
- include scheduled births in an overall “culture of safety,” with regular discussion of the matter at department and quality meetings.
I offer five key points about any program to reduce the number of scheduled births between 36.0 and 38.6 weeks’ gestation:
- it requires a lot of work and commitment on the part of all members of the health care team to change a behavior. Educate team members about the goal and the reasons behind it, and encourage them to contribute ideas to achieve it
- consider cooperating with state agencies to obtain data that can be used as baseline information and to measure the effect of an intervention
- a “one size fits all” approach is unlikely to be effective. Each hospital and physician is unique, and interventions should be individualized
- although the result of this 14-month study is impressive, real change must be maintained over the long term. Keep this in mind when you are planning interventions
- efforts such as this one have a financial cost associated with them, and I would hope that the government would contribute funding.
—GEORGE A. MACONES, MD, MSCE
Staff at participating hospitals had much latitude in selecting and refining interventions for their institution. After implementation of specific initiatives, each hospital tracked and reported relevant outcomes, especially the rate of scheduled deliveries between 36.0 and 38.6 weeks’ gestation. Each hospital received a monthly report, and staff members were encouraged to share the results internally.
Dramatic result was validated
The result of this initiative is impressive: The rate of inappropriate scheduled births was reduced from 25% to less than 5% over 14 months. This finding was validated in two ways:
- by checking Ohio birth certificate data
- by determining whether there was a concomitant rise in the rate of deliveries between 39 and 41 weeks’ gestation in the same hospitals.
Both validations confirmed that the OPQC interventions were significantly effective.
Project was not truly “statewide”
No study is perfect. A potential limitation of this study is the fact that it did not include all hospitals in the state of Ohio—only a sampling of larger hospitals. This makes it unclear whether the approach would be as effective in smaller institutions. Moreover, it is possible that physicians simply got better at documenting a reason for scheduled delivery as the study progressed—although I doubt that this reason alone would explain the striking findings.
1. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin#107, August 2009: Induction of labor. Obstet Gynecol. 2009;114(2, part 1):386-397.
2. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement Website. 2003. http://www.ihi.org/IHI/Results/WhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchieving+BreakthroughImprovement.htm. Accessed May 4, 2010.
1. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin#107, August 2009: Induction of labor. Obstet Gynecol. 2009;114(2, part 1):386-397.
2. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement Website. 2003. http://www.ihi.org/IHI/Results/WhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchieving+BreakthroughImprovement.htm. Accessed May 4, 2010.
How did we arrive at a worldwide epidemic of vitamin D deficiency?
The news is troubling: Humans are, today, absolutely deficient in vitamin D, and evidence is accumulating that this deficiency is damaging the health of our patients and their children. How did we arrive at such a state?
Sources are numerous but lifestyle and miscalculation confound intake
We have several main sources of vitamin D:
- fatty fish (e.g., salmon, which contains 500 IU in 3 oz)
- eggs (25 IU in one yolk)
- vitamin D-enriched milk products (cow’s milk, 100 IU in every 8 oz)
- vitamin D supplements
- exposure to sunlight.
On the whole, we’ve markedly reduced our exposure to sunlight as we’ve changed from living outdoors in rural agrarian communities to an indoor urban lifestyle. Dermatologists have long crusaded against exposure to sunlight as a way to reduce our risk of skin cancer. And milk intake has dropped significantly over the past decade.
To those shifts, add the fact that the US government and its advisory councils have, historically, recommended an intake of vitamin D—200 IU/d for children and 400 IU/d for adults—that is too low to prevent vitamin D deficiency.
In short, our low exposure to sunlight and our low intake of vitamin D have caused an epidemic of vitamin D deficiency. Here is a look at key facets of the problem; the benefits of maintaining adequate stores of vitamin D; and recommendations for ending the epidemic.
Pregnant women are vitamin D deficient, most studies show
Measurement of circulating 25-hydroxyvitamin D (25OH vitamin D) is an accepted method of assessing vitamin D physiologic status. Many authorities believe that 1) a 25OH vitamin D concentration >30 ng/mL indicates adequate vitamin D stores and that 2) a level <20 ng/mL clearly represents vitamin D deficiency. In a recent study of pregnant women from Finland, more than 70% of subjects were vitamin D deficient.1
In turn, many of the newborns of subjects in the Finnish study were also vitamin D deficient.1
Preventing preeclampsia. Does vitamin D supplementation in pregnant women reduce their risk of preeclampsia? We don’t know—no randomized clinical trial has demonstrated such an effect. But investigators in several observational studies have reported that a low maternal serum concentration of 25OH vitamin D is associated with an increased risk of preeclampsia.2,3
In one such study, an imputed total vitamin D intake of 600 to 800 IU/d was associated with a 24% reduction in the risk of preeclampsia from what was seen when total vitamin D intake was 200 IU/d.4
Many infants are vitamin D deficient
Bone mass is reduced in children who are vitamin D deficient.1 Historically, the American Academy of Pediatrics (AAP) has asserted that vitamin D intake of 200 IU/d was adequate for infants,5 but the Academy recently changed its recommendation to daily supplementation with 400 IU/d for infants, beginning soon after birth.6
A recent survey showed that the majority of children do not receive adequate vitamin D supplementation.7
Lactation and vitamin D deficiency. The concentration of 25OH vitamin D in breast milk correlates with maternal vitamin D stores. Because most pregnant women are vitamin D deficient, their infants are, when breast-fed, also at higher risk of vitamin D deficiency.8,9
Authorities recommend that all infants who are being breast-fed receive vitamin D supplementation with 400 IU/d.
Vitamin D supplements and toxicity
The two commonly available forms of supplemental vitamin D are ergocalciferol (D2) and cholecalciferol (D3). Both are effective supplements,1 although some authorities contend that cholecalciferol may be slightly better absorbed.2
Commercial laboratories typically measure and report 1) total 25OH vitamin D as a single value, or 2) two values, one for 25OH vitamin D2 and one for 25OH vitamin D3. If two values are reported, you should add them together to assess the total concentration of 25OH vitamin D. Most authorities believe that a 25OH vitamin D level >30 ng/mL is normal and a value <20 ng/mL is clearly abnormally low.
For nonpregnant women who have a 25OH vitamin D level <20 ng/mL, some authorities recommend a weekly dosage of 50,000 IU of vitamin D for 8 weeks followed by a repeat measurement of 25OH vitamin D. If the post-treatment 25OH vitamin D level is >30 ng/mL, a daily dosage of 800 IU is initiated. If the vitamin D level is still very low, the 8-week course of high-dose vitamin D may be repeated.
For pregnant women, some authorities recommend a daily dose of 2,000 IU of vitamin D. This can be achieved by taking a prenatal vitamin (vitamin D, 400 IU) and two capsules of vitamin D, 800 IU per capsule, daily. Toxicity is poorly understood. The dose of vitamin D that is toxic is not well defined. In 1997, the Institute of Medicine of the National Academy of Sciences concluded that the “tolerable upper intake level” for vitamin D was 2,000 IU daily.3 Recent data suggest that dosages as high as 10,000 IU/d taken for as long as 5 months are not toxic.4,5
Excessive vitamin D intake, especially when combined with calcium supplementation, may be associated with hypercalcemia, hypercalciuria, and kidney stones.
References
1. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvtiamn D. J Clin Endocrinol Metab. 2008;93(3):677-681.
2. Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387-5391.
3. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference intakes for calcium phosphorus, magnesium, vitamin D and fluoride. National Academy Press, Washington DC 1997.
4. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204-210.
5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Which women are at high risk of vitamin D deficiency?
Women whose skin is darkly pigmented are at high risk of vitamin D deficiency; they require approximately three times the amount of exposure to sunlight as women with lightly pigmented skin to generate the same amount of vitamin D.10 In one study of pregnant adolescent African-American women, 46% had a 25OH vitamin D level <20 ng/mL.11
Women who wear concealing clothes, such as a burka, are also at increased risk of vitamin D deficiency.12,13
Women living in poverty may have dietary and lifestyle patterns that limit vitamin D intake and exposure to sun. In a study conducted in Camden, New Jersey, total vitamin D intake was reported to be low in African-American and Hispanic women.14
Musculoskeletal health in women. Many young women are deficient in vitamin D. In a recent study of 16- to 22-year-old women living in sun-drenched California, 59% of subjects had, surprisingly, a 25OH vitamin D level <30 ng/mL; 41% had a level <20 ng/mL.15
Of interest, women in this study who had a low vitamin D level tended to have increased fat infiltration in muscle at the mid-thigh (detected by computed tomographic scanning). Based on other studies, it is now thought that fat infiltration reduces muscle strength and undermines physical performance, including athletic performance. In a study of young adolescents, a positive relationship was detected between the vitamin D level and enhanced muscle function, including muscle power, velocity, and jump height.16
Osteoporosis. Many postmenopausal women are vitamin D deficient. A low level of vitamin D is associated with decreased intestinal calcium absorption, a negative calcium balance, and a rise in the parathyroid hormone level, which accelerates bone resorption.
A total calcium intake of approximately 1,500 mg/d in postmenopausal women is associated with positive calcium balance. A serum 25OH vitamin D level of about 20 to 40 ng/mL maximally suppresses PTH secretion.
A low 25OH vitamin D level is associated with an increased risk of hip fracture17; adequate calcium and vitamin D supplementation reduces the risk of osteoporotic fractures in the elderly. The authors of a meta-analysis of seven randomized trials reported that the risk of fracture was reduced about 35% when women were given vitamin D supplementation at 700 to 800 IU/d—but that risk was not reduced at a dosage of 400 IU/d.18 Similar findings have been reported in other meta-analyses.19
A note of caution: In one randomized trial, supplementation with vitamin D and calcium was associated with a 17% increase in the risk of kidney stones.20
Colon cancer. In prospective observational studies, a strong inverse relationship has been observed between levels of 25OH vitamin D and the risk of colon cancer.
For example, in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, the vitamin D level was measured in health study participants, and analysis of the relationship between this level and new, incident cases of colon cancer revealed that 25OH vitamin D levels >30 ng/mL were associated with a 12% decrease in the risk of colon cancer, compared to subjects with levels of 20 to 30 ng/mL.21 For subjects who had a 25OH vitamin D level >40 ng/mL, the risk of colon cancer was reduced by 23%.
A prospective randomized trial would be required, however, to prove that vitamin D has a protective effect on the risk of colon cancer.
A taste one doesn’t soon forget—forgotten
Throughout the 1950s, I remember the mandatory weekly dose of natural cod liver oil, a rich source of vitamin D. Somehow, with a movement away from that weekly regimen, and miscalculation of what constitutes optimal vitamin D supplementation, we’ve entered a period of worldwide vitamin D deficiency.
It is clear that for most women, vitamin D supplementation at 400 IU/d is inadequate to prevent deficiency. Most women should consider a vitamin D dosage of 800 to 1,000 IU/d. Measuring the 25OH vitamin D level, with the aim of providing supplemental vitamin D to achieve a value >30 ng/mL, will help end the epidemic.22
1. Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95(4):1749-1757.
2. Halhali A, Tovar AT, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab. 2000;85(5):1828-1833.
3. Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517-3522.
4. Haugen M, Brantsaeter AL, Trogstad L, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20(5):720-726.
5. Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496-506.
6. Wagner CL, Greer FR. For American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142-1152.
7. Perrine CG, Sharma AJ, Jefferds ME, Serdula MD, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627-632.
8. Seth A, Marwaha RK, Singla B, et al. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22(3):241-246.
9. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2009;202(5):429.e1-9.
10. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
11. Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O’Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23(1):45-52.
12. Al-Turki HA, Sadat-Ali M, AL-Elq AH, Al-Mulhim FA, Al-Ali AK. 25-Hydroxyvitamin D levels among healthy Saudi Arabian women. Saudi Med J. 2008;29(12):1765-1768.
13. Dijkstra SH, van Beek A, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL. High prevalence of vitamin D deficiency in newborn infants of high-risk mothers. Arch Dis Child. 2007;92(9):750-753.
14. Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev. 2009;85(4):231-234.
15. Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95(4):1595-1601.
16. Ward KA, Das G, Berry JL, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94(2):559-563.
17. Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242-250.
18. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257-2264.
19. Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415-1423.
20. Jackson RD, LaCroix AZ, Gass M, et al. For Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
21. Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and the risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500.-doi: 10.1136/bmj.b5500.
22. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis Int. 2005;16(7):713-716.
The news is troubling: Humans are, today, absolutely deficient in vitamin D, and evidence is accumulating that this deficiency is damaging the health of our patients and their children. How did we arrive at such a state?
Sources are numerous but lifestyle and miscalculation confound intake
We have several main sources of vitamin D:
- fatty fish (e.g., salmon, which contains 500 IU in 3 oz)
- eggs (25 IU in one yolk)
- vitamin D-enriched milk products (cow’s milk, 100 IU in every 8 oz)
- vitamin D supplements
- exposure to sunlight.
On the whole, we’ve markedly reduced our exposure to sunlight as we’ve changed from living outdoors in rural agrarian communities to an indoor urban lifestyle. Dermatologists have long crusaded against exposure to sunlight as a way to reduce our risk of skin cancer. And milk intake has dropped significantly over the past decade.
To those shifts, add the fact that the US government and its advisory councils have, historically, recommended an intake of vitamin D—200 IU/d for children and 400 IU/d for adults—that is too low to prevent vitamin D deficiency.
In short, our low exposure to sunlight and our low intake of vitamin D have caused an epidemic of vitamin D deficiency. Here is a look at key facets of the problem; the benefits of maintaining adequate stores of vitamin D; and recommendations for ending the epidemic.
Pregnant women are vitamin D deficient, most studies show
Measurement of circulating 25-hydroxyvitamin D (25OH vitamin D) is an accepted method of assessing vitamin D physiologic status. Many authorities believe that 1) a 25OH vitamin D concentration >30 ng/mL indicates adequate vitamin D stores and that 2) a level <20 ng/mL clearly represents vitamin D deficiency. In a recent study of pregnant women from Finland, more than 70% of subjects were vitamin D deficient.1
In turn, many of the newborns of subjects in the Finnish study were also vitamin D deficient.1
Preventing preeclampsia. Does vitamin D supplementation in pregnant women reduce their risk of preeclampsia? We don’t know—no randomized clinical trial has demonstrated such an effect. But investigators in several observational studies have reported that a low maternal serum concentration of 25OH vitamin D is associated with an increased risk of preeclampsia.2,3
In one such study, an imputed total vitamin D intake of 600 to 800 IU/d was associated with a 24% reduction in the risk of preeclampsia from what was seen when total vitamin D intake was 200 IU/d.4
Many infants are vitamin D deficient
Bone mass is reduced in children who are vitamin D deficient.1 Historically, the American Academy of Pediatrics (AAP) has asserted that vitamin D intake of 200 IU/d was adequate for infants,5 but the Academy recently changed its recommendation to daily supplementation with 400 IU/d for infants, beginning soon after birth.6
A recent survey showed that the majority of children do not receive adequate vitamin D supplementation.7
Lactation and vitamin D deficiency. The concentration of 25OH vitamin D in breast milk correlates with maternal vitamin D stores. Because most pregnant women are vitamin D deficient, their infants are, when breast-fed, also at higher risk of vitamin D deficiency.8,9
Authorities recommend that all infants who are being breast-fed receive vitamin D supplementation with 400 IU/d.
Vitamin D supplements and toxicity
The two commonly available forms of supplemental vitamin D are ergocalciferol (D2) and cholecalciferol (D3). Both are effective supplements,1 although some authorities contend that cholecalciferol may be slightly better absorbed.2
Commercial laboratories typically measure and report 1) total 25OH vitamin D as a single value, or 2) two values, one for 25OH vitamin D2 and one for 25OH vitamin D3. If two values are reported, you should add them together to assess the total concentration of 25OH vitamin D. Most authorities believe that a 25OH vitamin D level >30 ng/mL is normal and a value <20 ng/mL is clearly abnormally low.
For nonpregnant women who have a 25OH vitamin D level <20 ng/mL, some authorities recommend a weekly dosage of 50,000 IU of vitamin D for 8 weeks followed by a repeat measurement of 25OH vitamin D. If the post-treatment 25OH vitamin D level is >30 ng/mL, a daily dosage of 800 IU is initiated. If the vitamin D level is still very low, the 8-week course of high-dose vitamin D may be repeated.
For pregnant women, some authorities recommend a daily dose of 2,000 IU of vitamin D. This can be achieved by taking a prenatal vitamin (vitamin D, 400 IU) and two capsules of vitamin D, 800 IU per capsule, daily. Toxicity is poorly understood. The dose of vitamin D that is toxic is not well defined. In 1997, the Institute of Medicine of the National Academy of Sciences concluded that the “tolerable upper intake level” for vitamin D was 2,000 IU daily.3 Recent data suggest that dosages as high as 10,000 IU/d taken for as long as 5 months are not toxic.4,5
Excessive vitamin D intake, especially when combined with calcium supplementation, may be associated with hypercalcemia, hypercalciuria, and kidney stones.
References
1. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvtiamn D. J Clin Endocrinol Metab. 2008;93(3):677-681.
2. Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387-5391.
3. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference intakes for calcium phosphorus, magnesium, vitamin D and fluoride. National Academy Press, Washington DC 1997.
4. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204-210.
5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Which women are at high risk of vitamin D deficiency?
Women whose skin is darkly pigmented are at high risk of vitamin D deficiency; they require approximately three times the amount of exposure to sunlight as women with lightly pigmented skin to generate the same amount of vitamin D.10 In one study of pregnant adolescent African-American women, 46% had a 25OH vitamin D level <20 ng/mL.11
Women who wear concealing clothes, such as a burka, are also at increased risk of vitamin D deficiency.12,13
Women living in poverty may have dietary and lifestyle patterns that limit vitamin D intake and exposure to sun. In a study conducted in Camden, New Jersey, total vitamin D intake was reported to be low in African-American and Hispanic women.14
Musculoskeletal health in women. Many young women are deficient in vitamin D. In a recent study of 16- to 22-year-old women living in sun-drenched California, 59% of subjects had, surprisingly, a 25OH vitamin D level <30 ng/mL; 41% had a level <20 ng/mL.15
Of interest, women in this study who had a low vitamin D level tended to have increased fat infiltration in muscle at the mid-thigh (detected by computed tomographic scanning). Based on other studies, it is now thought that fat infiltration reduces muscle strength and undermines physical performance, including athletic performance. In a study of young adolescents, a positive relationship was detected between the vitamin D level and enhanced muscle function, including muscle power, velocity, and jump height.16
Osteoporosis. Many postmenopausal women are vitamin D deficient. A low level of vitamin D is associated with decreased intestinal calcium absorption, a negative calcium balance, and a rise in the parathyroid hormone level, which accelerates bone resorption.
A total calcium intake of approximately 1,500 mg/d in postmenopausal women is associated with positive calcium balance. A serum 25OH vitamin D level of about 20 to 40 ng/mL maximally suppresses PTH secretion.
A low 25OH vitamin D level is associated with an increased risk of hip fracture17; adequate calcium and vitamin D supplementation reduces the risk of osteoporotic fractures in the elderly. The authors of a meta-analysis of seven randomized trials reported that the risk of fracture was reduced about 35% when women were given vitamin D supplementation at 700 to 800 IU/d—but that risk was not reduced at a dosage of 400 IU/d.18 Similar findings have been reported in other meta-analyses.19
A note of caution: In one randomized trial, supplementation with vitamin D and calcium was associated with a 17% increase in the risk of kidney stones.20
Colon cancer. In prospective observational studies, a strong inverse relationship has been observed between levels of 25OH vitamin D and the risk of colon cancer.
For example, in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, the vitamin D level was measured in health study participants, and analysis of the relationship between this level and new, incident cases of colon cancer revealed that 25OH vitamin D levels >30 ng/mL were associated with a 12% decrease in the risk of colon cancer, compared to subjects with levels of 20 to 30 ng/mL.21 For subjects who had a 25OH vitamin D level >40 ng/mL, the risk of colon cancer was reduced by 23%.
A prospective randomized trial would be required, however, to prove that vitamin D has a protective effect on the risk of colon cancer.
A taste one doesn’t soon forget—forgotten
Throughout the 1950s, I remember the mandatory weekly dose of natural cod liver oil, a rich source of vitamin D. Somehow, with a movement away from that weekly regimen, and miscalculation of what constitutes optimal vitamin D supplementation, we’ve entered a period of worldwide vitamin D deficiency.
It is clear that for most women, vitamin D supplementation at 400 IU/d is inadequate to prevent deficiency. Most women should consider a vitamin D dosage of 800 to 1,000 IU/d. Measuring the 25OH vitamin D level, with the aim of providing supplemental vitamin D to achieve a value >30 ng/mL, will help end the epidemic.22
The news is troubling: Humans are, today, absolutely deficient in vitamin D, and evidence is accumulating that this deficiency is damaging the health of our patients and their children. How did we arrive at such a state?
Sources are numerous but lifestyle and miscalculation confound intake
We have several main sources of vitamin D:
- fatty fish (e.g., salmon, which contains 500 IU in 3 oz)
- eggs (25 IU in one yolk)
- vitamin D-enriched milk products (cow’s milk, 100 IU in every 8 oz)
- vitamin D supplements
- exposure to sunlight.
On the whole, we’ve markedly reduced our exposure to sunlight as we’ve changed from living outdoors in rural agrarian communities to an indoor urban lifestyle. Dermatologists have long crusaded against exposure to sunlight as a way to reduce our risk of skin cancer. And milk intake has dropped significantly over the past decade.
To those shifts, add the fact that the US government and its advisory councils have, historically, recommended an intake of vitamin D—200 IU/d for children and 400 IU/d for adults—that is too low to prevent vitamin D deficiency.
In short, our low exposure to sunlight and our low intake of vitamin D have caused an epidemic of vitamin D deficiency. Here is a look at key facets of the problem; the benefits of maintaining adequate stores of vitamin D; and recommendations for ending the epidemic.
Pregnant women are vitamin D deficient, most studies show
Measurement of circulating 25-hydroxyvitamin D (25OH vitamin D) is an accepted method of assessing vitamin D physiologic status. Many authorities believe that 1) a 25OH vitamin D concentration >30 ng/mL indicates adequate vitamin D stores and that 2) a level <20 ng/mL clearly represents vitamin D deficiency. In a recent study of pregnant women from Finland, more than 70% of subjects were vitamin D deficient.1
In turn, many of the newborns of subjects in the Finnish study were also vitamin D deficient.1
Preventing preeclampsia. Does vitamin D supplementation in pregnant women reduce their risk of preeclampsia? We don’t know—no randomized clinical trial has demonstrated such an effect. But investigators in several observational studies have reported that a low maternal serum concentration of 25OH vitamin D is associated with an increased risk of preeclampsia.2,3
In one such study, an imputed total vitamin D intake of 600 to 800 IU/d was associated with a 24% reduction in the risk of preeclampsia from what was seen when total vitamin D intake was 200 IU/d.4
Many infants are vitamin D deficient
Bone mass is reduced in children who are vitamin D deficient.1 Historically, the American Academy of Pediatrics (AAP) has asserted that vitamin D intake of 200 IU/d was adequate for infants,5 but the Academy recently changed its recommendation to daily supplementation with 400 IU/d for infants, beginning soon after birth.6
A recent survey showed that the majority of children do not receive adequate vitamin D supplementation.7
Lactation and vitamin D deficiency. The concentration of 25OH vitamin D in breast milk correlates with maternal vitamin D stores. Because most pregnant women are vitamin D deficient, their infants are, when breast-fed, also at higher risk of vitamin D deficiency.8,9
Authorities recommend that all infants who are being breast-fed receive vitamin D supplementation with 400 IU/d.
Vitamin D supplements and toxicity
The two commonly available forms of supplemental vitamin D are ergocalciferol (D2) and cholecalciferol (D3). Both are effective supplements,1 although some authorities contend that cholecalciferol may be slightly better absorbed.2
Commercial laboratories typically measure and report 1) total 25OH vitamin D as a single value, or 2) two values, one for 25OH vitamin D2 and one for 25OH vitamin D3. If two values are reported, you should add them together to assess the total concentration of 25OH vitamin D. Most authorities believe that a 25OH vitamin D level >30 ng/mL is normal and a value <20 ng/mL is clearly abnormally low.
For nonpregnant women who have a 25OH vitamin D level <20 ng/mL, some authorities recommend a weekly dosage of 50,000 IU of vitamin D for 8 weeks followed by a repeat measurement of 25OH vitamin D. If the post-treatment 25OH vitamin D level is >30 ng/mL, a daily dosage of 800 IU is initiated. If the vitamin D level is still very low, the 8-week course of high-dose vitamin D may be repeated.
For pregnant women, some authorities recommend a daily dose of 2,000 IU of vitamin D. This can be achieved by taking a prenatal vitamin (vitamin D, 400 IU) and two capsules of vitamin D, 800 IU per capsule, daily. Toxicity is poorly understood. The dose of vitamin D that is toxic is not well defined. In 1997, the Institute of Medicine of the National Academy of Sciences concluded that the “tolerable upper intake level” for vitamin D was 2,000 IU daily.3 Recent data suggest that dosages as high as 10,000 IU/d taken for as long as 5 months are not toxic.4,5
Excessive vitamin D intake, especially when combined with calcium supplementation, may be associated with hypercalcemia, hypercalciuria, and kidney stones.
References
1. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvtiamn D. J Clin Endocrinol Metab. 2008;93(3):677-681.
2. Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387-5391.
3. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference intakes for calcium phosphorus, magnesium, vitamin D and fluoride. National Academy Press, Washington DC 1997.
4. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204-210.
5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Which women are at high risk of vitamin D deficiency?
Women whose skin is darkly pigmented are at high risk of vitamin D deficiency; they require approximately three times the amount of exposure to sunlight as women with lightly pigmented skin to generate the same amount of vitamin D.10 In one study of pregnant adolescent African-American women, 46% had a 25OH vitamin D level <20 ng/mL.11
Women who wear concealing clothes, such as a burka, are also at increased risk of vitamin D deficiency.12,13
Women living in poverty may have dietary and lifestyle patterns that limit vitamin D intake and exposure to sun. In a study conducted in Camden, New Jersey, total vitamin D intake was reported to be low in African-American and Hispanic women.14
Musculoskeletal health in women. Many young women are deficient in vitamin D. In a recent study of 16- to 22-year-old women living in sun-drenched California, 59% of subjects had, surprisingly, a 25OH vitamin D level <30 ng/mL; 41% had a level <20 ng/mL.15
Of interest, women in this study who had a low vitamin D level tended to have increased fat infiltration in muscle at the mid-thigh (detected by computed tomographic scanning). Based on other studies, it is now thought that fat infiltration reduces muscle strength and undermines physical performance, including athletic performance. In a study of young adolescents, a positive relationship was detected between the vitamin D level and enhanced muscle function, including muscle power, velocity, and jump height.16
Osteoporosis. Many postmenopausal women are vitamin D deficient. A low level of vitamin D is associated with decreased intestinal calcium absorption, a negative calcium balance, and a rise in the parathyroid hormone level, which accelerates bone resorption.
A total calcium intake of approximately 1,500 mg/d in postmenopausal women is associated with positive calcium balance. A serum 25OH vitamin D level of about 20 to 40 ng/mL maximally suppresses PTH secretion.
A low 25OH vitamin D level is associated with an increased risk of hip fracture17; adequate calcium and vitamin D supplementation reduces the risk of osteoporotic fractures in the elderly. The authors of a meta-analysis of seven randomized trials reported that the risk of fracture was reduced about 35% when women were given vitamin D supplementation at 700 to 800 IU/d—but that risk was not reduced at a dosage of 400 IU/d.18 Similar findings have been reported in other meta-analyses.19
A note of caution: In one randomized trial, supplementation with vitamin D and calcium was associated with a 17% increase in the risk of kidney stones.20
Colon cancer. In prospective observational studies, a strong inverse relationship has been observed between levels of 25OH vitamin D and the risk of colon cancer.
For example, in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, the vitamin D level was measured in health study participants, and analysis of the relationship between this level and new, incident cases of colon cancer revealed that 25OH vitamin D levels >30 ng/mL were associated with a 12% decrease in the risk of colon cancer, compared to subjects with levels of 20 to 30 ng/mL.21 For subjects who had a 25OH vitamin D level >40 ng/mL, the risk of colon cancer was reduced by 23%.
A prospective randomized trial would be required, however, to prove that vitamin D has a protective effect on the risk of colon cancer.
A taste one doesn’t soon forget—forgotten
Throughout the 1950s, I remember the mandatory weekly dose of natural cod liver oil, a rich source of vitamin D. Somehow, with a movement away from that weekly regimen, and miscalculation of what constitutes optimal vitamin D supplementation, we’ve entered a period of worldwide vitamin D deficiency.
It is clear that for most women, vitamin D supplementation at 400 IU/d is inadequate to prevent deficiency. Most women should consider a vitamin D dosage of 800 to 1,000 IU/d. Measuring the 25OH vitamin D level, with the aim of providing supplemental vitamin D to achieve a value >30 ng/mL, will help end the epidemic.22
1. Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95(4):1749-1757.
2. Halhali A, Tovar AT, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab. 2000;85(5):1828-1833.
3. Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517-3522.
4. Haugen M, Brantsaeter AL, Trogstad L, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20(5):720-726.
5. Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496-506.
6. Wagner CL, Greer FR. For American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142-1152.
7. Perrine CG, Sharma AJ, Jefferds ME, Serdula MD, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627-632.
8. Seth A, Marwaha RK, Singla B, et al. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22(3):241-246.
9. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2009;202(5):429.e1-9.
10. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
11. Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O’Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23(1):45-52.
12. Al-Turki HA, Sadat-Ali M, AL-Elq AH, Al-Mulhim FA, Al-Ali AK. 25-Hydroxyvitamin D levels among healthy Saudi Arabian women. Saudi Med J. 2008;29(12):1765-1768.
13. Dijkstra SH, van Beek A, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL. High prevalence of vitamin D deficiency in newborn infants of high-risk mothers. Arch Dis Child. 2007;92(9):750-753.
14. Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev. 2009;85(4):231-234.
15. Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95(4):1595-1601.
16. Ward KA, Das G, Berry JL, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94(2):559-563.
17. Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242-250.
18. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257-2264.
19. Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415-1423.
20. Jackson RD, LaCroix AZ, Gass M, et al. For Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
21. Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and the risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500.-doi: 10.1136/bmj.b5500.
22. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis Int. 2005;16(7):713-716.
1. Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95(4):1749-1757.
2. Halhali A, Tovar AT, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab. 2000;85(5):1828-1833.
3. Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517-3522.
4. Haugen M, Brantsaeter AL, Trogstad L, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20(5):720-726.
5. Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496-506.
6. Wagner CL, Greer FR. For American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142-1152.
7. Perrine CG, Sharma AJ, Jefferds ME, Serdula MD, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627-632.
8. Seth A, Marwaha RK, Singla B, et al. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22(3):241-246.
9. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2009;202(5):429.e1-9.
10. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
11. Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O’Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23(1):45-52.
12. Al-Turki HA, Sadat-Ali M, AL-Elq AH, Al-Mulhim FA, Al-Ali AK. 25-Hydroxyvitamin D levels among healthy Saudi Arabian women. Saudi Med J. 2008;29(12):1765-1768.
13. Dijkstra SH, van Beek A, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL. High prevalence of vitamin D deficiency in newborn infants of high-risk mothers. Arch Dis Child. 2007;92(9):750-753.
14. Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev. 2009;85(4):231-234.
15. Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95(4):1595-1601.
16. Ward KA, Das G, Berry JL, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94(2):559-563.
17. Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242-250.
18. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257-2264.
19. Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415-1423.
20. Jackson RD, LaCroix AZ, Gass M, et al. For Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
21. Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and the risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500.-doi: 10.1136/bmj.b5500.
22. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis Int. 2005;16(7):713-716.
6 skin disorders of pregnancy: A management guide
The dermatoses of pregnancy are a poorly understood group of conditions. Their only common feature is a tendency to appear during pregnancy.
Only three of these conditions are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. To make management even more complex, two patients—mother and fetus—need to be considered in decisions about care.
Who manages these patients is another matter. These conditions fall into overlapping areas of health care, where family physicians, obstetricians, and dermatologists all might have some share in responsibility for diagnosis and treatment. You need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require referral to a specialist. This review and the handy TABLE, will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| Pemphigoid gestationis3,5 | Erythematous papules that progress to vesicles and bullae, in a periumbilical distribution that spares the face, palms, and soles |
|
| Frequent; skips a pregnancy 8% of the time |
| Pruritic urticarial papules and plaques of pregnancy8-10 | Urticarial papules and plaques on the abdomen, legs, arms, buttocks, chest, and back |
| Topical corticosteroids and antihistamines | Uncommon |
| Intrahepatic cholestasis of pregnancy14,17,19-22 | No primary lesions; secondary excoriations in any area that the patient can reach |
| Ursodeoxycholic acid, 450-1,200 mg/d | Frequent |
| Eczema of pregnancy/pruritus of pregnancy4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most often on extensor surfaces of the arms and legs or on the abdomen |
| Symptomatic treatment with topical corticosteroids or antihistamines | Frequent |
| Acute pustular psoriasis of pregnancy26-28 | Erythematous plaques and pustules that start on the inner thighs and groin and spread to the trunk and extremities |
|
| Unknown |
| Pruritic folliculitis of pregnancy24,28 | Papules and pustules concentrated around hair follicles, often beginning on the abdomen and spreading to the extremities |
| Topical corticosteroids | Unknown |
DERMATOSES UNIQUE TO PREGNANCY
1. Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 Time of onset is usually about the 21st week of gestation, although, in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). Lesions of PG tend to spare the face, palms, and soles. Mucosal surfaces are involved in fewer than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4
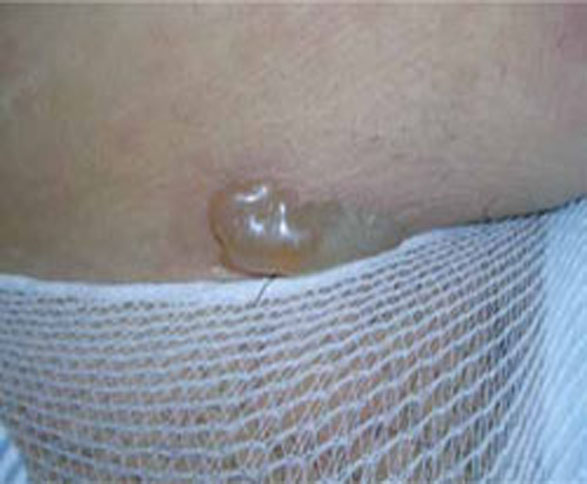
FIGURE 1 Pemphigoid gestationis
As the disease progresses, bullous lesions tend to develop.
Pathophysiology. The pathophysiology of PG is nearly identical to that of bullous pemphigoid, a blistering skin disorder seen more often in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target in several subepidermal blistering diseases.
Differential diagnosis. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3
Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg/d of prednisone. Oral corticosteroids are generally most effective at ameliorating symptoms. Prednisone at a dosage of 40 to 80 mg/d for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activities and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can be passively transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 The disease tends to recur in future pregnancies.
2. Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides its acronym PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name suggests, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). Lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps you to differentiate PUPPP from PG, in which lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in one study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.

FIGURE 2 Pruritic urticarial papules and plaques of pregnancy
The itchy, red papules of PUPP often coalesce into plaques.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in six of 10 PUPPP sufferers, but found none in any of 26 controls—pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential diagnosis. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can be diagnosed only by clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild-to-potent topical corticosteroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and, occasionally, low-dose systemic corticosteroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmaceutical treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. If there is any question about the diagnosis, however, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
3. Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. Intrahepatic cholestasis of pregnancy (ICP) is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 of every 10,000 pregnancies in Europe and 70 of every 10,000 in the United States.12 In 80% of patients, time of onset is after the 30th week.14
Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14
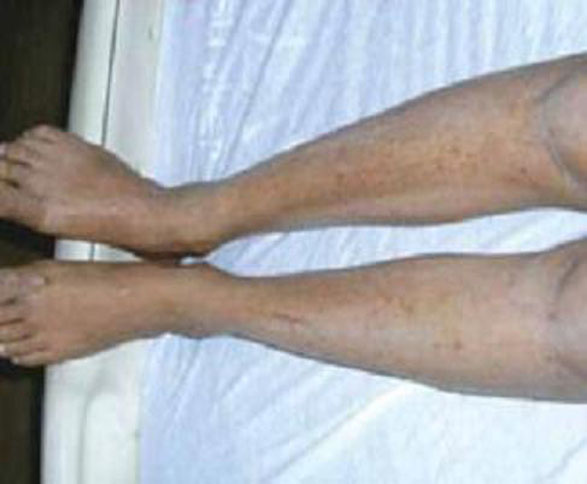
FIGURE 3 Intrahepatic cholestasis of pregnancy
ICP lacks primary lesions. Shown here are the secondary erythema and excoriations that results from scratching the intense pruritis.
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in one half of cases; cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential diagnosis. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased levels of serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 µmol/L, with an upper limit of 11 µmol/L. The average value in women who have ICP is 47 µmol/L.17
Although serum bile acids remain the gold standard, a recent study showed that elevated urine bile acids have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In four controlled trials, UDCA caused a sustained decrease in serum bile acids.19-22 The dosage used in these trials ranged from 450 to 1,200 mg/d.
Before UDCA treatment was available, ICP was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, the onset of action of cholestyramine is slow.3
Elective delivery is indicated for ICP, particularly in patients who have a significant clinical presentation.12 Delivery for ICP should be performed around weeks 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with ICP (see the next paragraph), the condition should be managed by a clinician experienced with the disease—likely, a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor.14 Fortunately, the rate of preterm labor correlates strongly with the level of bile acids, so that as bile acid levels are reduced with UDCA treatment, the rate of preterm labor also falls. Management of ICP has reduced the rate of perinatal death to 3.5%. No evidence of fetal growth retardation has been noted.14
DERMATOSES TRIGGERED BY PREGNANCY
4. Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23
In some patients, at least, EP/PP may be preexisting conditions that are exacerbated by pregnancy. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 The tendency of the condition to be made markedly worse by pregnancy, however, leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 Although many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, the two conditions have the highest prevalence of all pregnancy-induced dermatoses.
PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,4,23
Presentation. The typical presentation is grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. Lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.
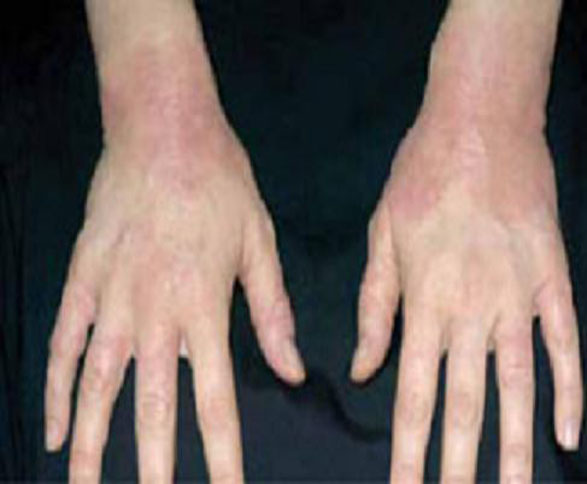
FIGURE 4 Eczema of pregnancy
EP/PP typically manifests as grouped, crusted, erythematous papules, patches, and plaques, often with excoriations.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who develop EP/PP have a history of atopy.10
Differential diagnosis. Conditions that need to be considered in making the diagnosis include Tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. The history and the physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are nonspecific. Correlation of EP/PP with increased IgE is marginal, at best.24,25
Treatment. These conditions are treated symptomatically with topical corticosteroids or systemic antihistamines.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with EP/PP.
5. Acute pustular psoriasis of pregnancy
Whether or not acute pustular psoriasis of pregnancy (APPP) is actually a pregnancy-induced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy but simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis and usually ceases when the pregnancy ends. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, leads us to include it here.26
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. Characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As plaques enlarge, their center becomes eroded and crusted.
The nails may become onycholytic. Hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful. Flu-like symptoms are often present.27
Pathophysiology. The pathophysiology of APPP is unknown.
Differential diagnosis. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. The clinical history and an association with systemic illness are the basis for a diagnosis of APPP. Cultures of pustules are negative for any infective pathology, although, as the disease progresses, pustules may become superinfected. Laboratory testing may show an increased erythrocyte sedimentation rate, hypocalcemia, and a low level of vitamin D.
Treatment. Prednisone, 15 to 60 mg/d, is often sufficient to control the disease.27 Cyclosporine, 100 mg twice daily, has also been shown to be useful.28 Cyclosporine in pregnancy is a Category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but risk appears minimal.6
Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, because delivery will almost always lead to swift resolution.
Sequelae. A number of case reports link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.27,29,30 The condition is too rare, however, for good data on specific sequelae. Although APPP does give significant cause for concern, it appears that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.31 This statistic has not been borne out in practice. It does appear that the mother will frequently suffer systemic symptoms, including fever and malaise.
6. Pruritic folliculitis of pregnancy
Accounts of the prevalence of pruritic folliculitis of pregnancy (PFP) vary widely. Some sources report fewer than 30 cases in all of the literature; others indicate that the prevalence is equivalent to that of PG—one in every 10,000 pregnancies.3,11 PFP most often presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often, lesions begin on the abdomen and spread to the extremities.24,28 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health than distressed by the symptoms.
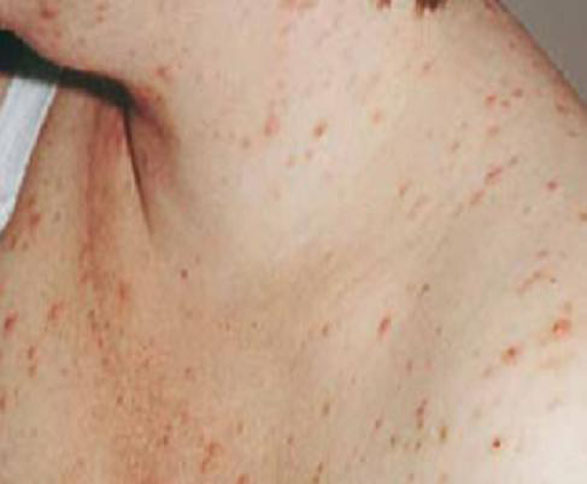
FIGURE 5 Pruritic folliculitis of pregnancy
The papules and pustules of PFP are concentrated around hair follicles.
Pathophysiology. Like many other dermatoses of pregnancy, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,28
Differential diagnosis. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third-trimester onset. No specific laboratory or histologic analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with a low- or midpotency topical corticosteroid, such as triamcinolone or desonide. A benzoyl peroxide wash can also be effective.
Sequelae. One study reports an increased incidence of low birth weight, but no associated morbidity or mortality has been reported in recent studies.24
- Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms
- The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines
- Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality
1. Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103(4):757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183(2):483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188(4):1083-1092.
4. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17(3):172-181.
5. Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8(2):214-224.
6. Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24(2):167-197, vi.
7. Hern S, Harman K, Bhogal BS, Black MM. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23(4):185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352(9144):1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154(1):54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130(6):734-739.
12. McDonald JA. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14(6):515-518.
13. Ohel I, Levy A, Silberstein T, Holcberg G, Sheiner E. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal Fetal Neonat Med. 2006;19(5):305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142(6 Pt 1):621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21(4):905-921.
17. Lammert F, Marschall H, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33(6):1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, Liu M, Javitt NB. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35(6):486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27(6):1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105(11):1205-1207.
22. Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42(6):1399-1405.
23. Ambros-Rudolph C, Müllegger M, Vaughan-Jones S, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395-404.
24. Vaughan Jones SA, Hern S, Nelson-Piercy C, Seed PT, Black MM. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141(1):71-81.
25. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8(3):405-412.
26. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3(4):449-451.
27. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24(2):101-104.
28. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43(1 Pt 1):132-134.
29. Sahin HG, Sahin HA, Metin A, Zeteroglu S, Ugras S. Recurrent impetigo herpetiformis in a pregnant adolescent: case report. Eur J Obstet Gynecol Reprod Biol. 2002;101:201-203.
30. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69(2):153-154.
31. Wade T, Wade S, Jones H. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52(2):233-242.
The dermatoses of pregnancy are a poorly understood group of conditions. Their only common feature is a tendency to appear during pregnancy.
Only three of these conditions are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. To make management even more complex, two patients—mother and fetus—need to be considered in decisions about care.
Who manages these patients is another matter. These conditions fall into overlapping areas of health care, where family physicians, obstetricians, and dermatologists all might have some share in responsibility for diagnosis and treatment. You need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require referral to a specialist. This review and the handy TABLE, will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| Pemphigoid gestationis3,5 | Erythematous papules that progress to vesicles and bullae, in a periumbilical distribution that spares the face, palms, and soles |
|
| Frequent; skips a pregnancy 8% of the time |
| Pruritic urticarial papules and plaques of pregnancy8-10 | Urticarial papules and plaques on the abdomen, legs, arms, buttocks, chest, and back |
| Topical corticosteroids and antihistamines | Uncommon |
| Intrahepatic cholestasis of pregnancy14,17,19-22 | No primary lesions; secondary excoriations in any area that the patient can reach |
| Ursodeoxycholic acid, 450-1,200 mg/d | Frequent |
| Eczema of pregnancy/pruritus of pregnancy4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most often on extensor surfaces of the arms and legs or on the abdomen |
| Symptomatic treatment with topical corticosteroids or antihistamines | Frequent |
| Acute pustular psoriasis of pregnancy26-28 | Erythematous plaques and pustules that start on the inner thighs and groin and spread to the trunk and extremities |
|
| Unknown |
| Pruritic folliculitis of pregnancy24,28 | Papules and pustules concentrated around hair follicles, often beginning on the abdomen and spreading to the extremities |
| Topical corticosteroids | Unknown |
DERMATOSES UNIQUE TO PREGNANCY
1. Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 Time of onset is usually about the 21st week of gestation, although, in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). Lesions of PG tend to spare the face, palms, and soles. Mucosal surfaces are involved in fewer than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4

FIGURE 1 Pemphigoid gestationis
As the disease progresses, bullous lesions tend to develop.
Pathophysiology. The pathophysiology of PG is nearly identical to that of bullous pemphigoid, a blistering skin disorder seen more often in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target in several subepidermal blistering diseases.
Differential diagnosis. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3
Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg/d of prednisone. Oral corticosteroids are generally most effective at ameliorating symptoms. Prednisone at a dosage of 40 to 80 mg/d for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activities and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can be passively transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 The disease tends to recur in future pregnancies.
2. Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides its acronym PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name suggests, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). Lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps you to differentiate PUPPP from PG, in which lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in one study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.

FIGURE 2 Pruritic urticarial papules and plaques of pregnancy
The itchy, red papules of PUPP often coalesce into plaques.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in six of 10 PUPPP sufferers, but found none in any of 26 controls—pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential diagnosis. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can be diagnosed only by clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild-to-potent topical corticosteroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and, occasionally, low-dose systemic corticosteroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmaceutical treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. If there is any question about the diagnosis, however, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
3. Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. Intrahepatic cholestasis of pregnancy (ICP) is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 of every 10,000 pregnancies in Europe and 70 of every 10,000 in the United States.12 In 80% of patients, time of onset is after the 30th week.14
Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14

FIGURE 3 Intrahepatic cholestasis of pregnancy
ICP lacks primary lesions. Shown here are the secondary erythema and excoriations that results from scratching the intense pruritis.
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in one half of cases; cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential diagnosis. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased levels of serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 µmol/L, with an upper limit of 11 µmol/L. The average value in women who have ICP is 47 µmol/L.17
Although serum bile acids remain the gold standard, a recent study showed that elevated urine bile acids have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In four controlled trials, UDCA caused a sustained decrease in serum bile acids.19-22 The dosage used in these trials ranged from 450 to 1,200 mg/d.
Before UDCA treatment was available, ICP was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, the onset of action of cholestyramine is slow.3
Elective delivery is indicated for ICP, particularly in patients who have a significant clinical presentation.12 Delivery for ICP should be performed around weeks 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with ICP (see the next paragraph), the condition should be managed by a clinician experienced with the disease—likely, a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor.14 Fortunately, the rate of preterm labor correlates strongly with the level of bile acids, so that as bile acid levels are reduced with UDCA treatment, the rate of preterm labor also falls. Management of ICP has reduced the rate of perinatal death to 3.5%. No evidence of fetal growth retardation has been noted.14
DERMATOSES TRIGGERED BY PREGNANCY
4. Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23
In some patients, at least, EP/PP may be preexisting conditions that are exacerbated by pregnancy. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 The tendency of the condition to be made markedly worse by pregnancy, however, leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 Although many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, the two conditions have the highest prevalence of all pregnancy-induced dermatoses.
PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,4,23
Presentation. The typical presentation is grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. Lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.

FIGURE 4 Eczema of pregnancy
EP/PP typically manifests as grouped, crusted, erythematous papules, patches, and plaques, often with excoriations.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who develop EP/PP have a history of atopy.10
Differential diagnosis. Conditions that need to be considered in making the diagnosis include Tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. The history and the physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are nonspecific. Correlation of EP/PP with increased IgE is marginal, at best.24,25
Treatment. These conditions are treated symptomatically with topical corticosteroids or systemic antihistamines.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with EP/PP.
5. Acute pustular psoriasis of pregnancy
Whether or not acute pustular psoriasis of pregnancy (APPP) is actually a pregnancy-induced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy but simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis and usually ceases when the pregnancy ends. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, leads us to include it here.26
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. Characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As plaques enlarge, their center becomes eroded and crusted.
The nails may become onycholytic. Hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful. Flu-like symptoms are often present.27
Pathophysiology. The pathophysiology of APPP is unknown.
Differential diagnosis. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. The clinical history and an association with systemic illness are the basis for a diagnosis of APPP. Cultures of pustules are negative for any infective pathology, although, as the disease progresses, pustules may become superinfected. Laboratory testing may show an increased erythrocyte sedimentation rate, hypocalcemia, and a low level of vitamin D.
Treatment. Prednisone, 15 to 60 mg/d, is often sufficient to control the disease.27 Cyclosporine, 100 mg twice daily, has also been shown to be useful.28 Cyclosporine in pregnancy is a Category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but risk appears minimal.6
Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, because delivery will almost always lead to swift resolution.
Sequelae. A number of case reports link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.27,29,30 The condition is too rare, however, for good data on specific sequelae. Although APPP does give significant cause for concern, it appears that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.31 This statistic has not been borne out in practice. It does appear that the mother will frequently suffer systemic symptoms, including fever and malaise.
6. Pruritic folliculitis of pregnancy
Accounts of the prevalence of pruritic folliculitis of pregnancy (PFP) vary widely. Some sources report fewer than 30 cases in all of the literature; others indicate that the prevalence is equivalent to that of PG—one in every 10,000 pregnancies.3,11 PFP most often presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often, lesions begin on the abdomen and spread to the extremities.24,28 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health than distressed by the symptoms.

FIGURE 5 Pruritic folliculitis of pregnancy
The papules and pustules of PFP are concentrated around hair follicles.
Pathophysiology. Like many other dermatoses of pregnancy, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,28
Differential diagnosis. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third-trimester onset. No specific laboratory or histologic analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with a low- or midpotency topical corticosteroid, such as triamcinolone or desonide. A benzoyl peroxide wash can also be effective.
Sequelae. One study reports an increased incidence of low birth weight, but no associated morbidity or mortality has been reported in recent studies.24
- Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms
- The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines
- Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality
The dermatoses of pregnancy are a poorly understood group of conditions. Their only common feature is a tendency to appear during pregnancy.
Only three of these conditions are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. To make management even more complex, two patients—mother and fetus—need to be considered in decisions about care.
Who manages these patients is another matter. These conditions fall into overlapping areas of health care, where family physicians, obstetricians, and dermatologists all might have some share in responsibility for diagnosis and treatment. You need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require referral to a specialist. This review and the handy TABLE, will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| Pemphigoid gestationis3,5 | Erythematous papules that progress to vesicles and bullae, in a periumbilical distribution that spares the face, palms, and soles |
|
| Frequent; skips a pregnancy 8% of the time |
| Pruritic urticarial papules and plaques of pregnancy8-10 | Urticarial papules and plaques on the abdomen, legs, arms, buttocks, chest, and back |
| Topical corticosteroids and antihistamines | Uncommon |
| Intrahepatic cholestasis of pregnancy14,17,19-22 | No primary lesions; secondary excoriations in any area that the patient can reach |
| Ursodeoxycholic acid, 450-1,200 mg/d | Frequent |
| Eczema of pregnancy/pruritus of pregnancy4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most often on extensor surfaces of the arms and legs or on the abdomen |
| Symptomatic treatment with topical corticosteroids or antihistamines | Frequent |
| Acute pustular psoriasis of pregnancy26-28 | Erythematous plaques and pustules that start on the inner thighs and groin and spread to the trunk and extremities |
|
| Unknown |
| Pruritic folliculitis of pregnancy24,28 | Papules and pustules concentrated around hair follicles, often beginning on the abdomen and spreading to the extremities |
| Topical corticosteroids | Unknown |
DERMATOSES UNIQUE TO PREGNANCY
1. Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 Time of onset is usually about the 21st week of gestation, although, in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). Lesions of PG tend to spare the face, palms, and soles. Mucosal surfaces are involved in fewer than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4

FIGURE 1 Pemphigoid gestationis
As the disease progresses, bullous lesions tend to develop.
Pathophysiology. The pathophysiology of PG is nearly identical to that of bullous pemphigoid, a blistering skin disorder seen more often in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target in several subepidermal blistering diseases.
Differential diagnosis. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3
Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg/d of prednisone. Oral corticosteroids are generally most effective at ameliorating symptoms. Prednisone at a dosage of 40 to 80 mg/d for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activities and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can be passively transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 The disease tends to recur in future pregnancies.
2. Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides its acronym PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name suggests, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). Lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps you to differentiate PUPPP from PG, in which lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in one study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.

FIGURE 2 Pruritic urticarial papules and plaques of pregnancy
The itchy, red papules of PUPP often coalesce into plaques.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in six of 10 PUPPP sufferers, but found none in any of 26 controls—pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential diagnosis. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can be diagnosed only by clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild-to-potent topical corticosteroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and, occasionally, low-dose systemic corticosteroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmaceutical treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. If there is any question about the diagnosis, however, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
3. Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. Intrahepatic cholestasis of pregnancy (ICP) is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 of every 10,000 pregnancies in Europe and 70 of every 10,000 in the United States.12 In 80% of patients, time of onset is after the 30th week.14
Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14

FIGURE 3 Intrahepatic cholestasis of pregnancy
ICP lacks primary lesions. Shown here are the secondary erythema and excoriations that results from scratching the intense pruritis.
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in one half of cases; cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential diagnosis. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased levels of serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 µmol/L, with an upper limit of 11 µmol/L. The average value in women who have ICP is 47 µmol/L.17
Although serum bile acids remain the gold standard, a recent study showed that elevated urine bile acids have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In four controlled trials, UDCA caused a sustained decrease in serum bile acids.19-22 The dosage used in these trials ranged from 450 to 1,200 mg/d.
Before UDCA treatment was available, ICP was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, the onset of action of cholestyramine is slow.3
Elective delivery is indicated for ICP, particularly in patients who have a significant clinical presentation.12 Delivery for ICP should be performed around weeks 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with ICP (see the next paragraph), the condition should be managed by a clinician experienced with the disease—likely, a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor.14 Fortunately, the rate of preterm labor correlates strongly with the level of bile acids, so that as bile acid levels are reduced with UDCA treatment, the rate of preterm labor also falls. Management of ICP has reduced the rate of perinatal death to 3.5%. No evidence of fetal growth retardation has been noted.14
DERMATOSES TRIGGERED BY PREGNANCY
4. Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23
In some patients, at least, EP/PP may be preexisting conditions that are exacerbated by pregnancy. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 The tendency of the condition to be made markedly worse by pregnancy, however, leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 Although many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, the two conditions have the highest prevalence of all pregnancy-induced dermatoses.
PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,4,23
Presentation. The typical presentation is grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. Lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.

FIGURE 4 Eczema of pregnancy
EP/PP typically manifests as grouped, crusted, erythematous papules, patches, and plaques, often with excoriations.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who develop EP/PP have a history of atopy.10
Differential diagnosis. Conditions that need to be considered in making the diagnosis include Tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. The history and the physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are nonspecific. Correlation of EP/PP with increased IgE is marginal, at best.24,25
Treatment. These conditions are treated symptomatically with topical corticosteroids or systemic antihistamines.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with EP/PP.
5. Acute pustular psoriasis of pregnancy
Whether or not acute pustular psoriasis of pregnancy (APPP) is actually a pregnancy-induced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy but simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis and usually ceases when the pregnancy ends. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, leads us to include it here.26
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. Characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As plaques enlarge, their center becomes eroded and crusted.
The nails may become onycholytic. Hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful. Flu-like symptoms are often present.27
Pathophysiology. The pathophysiology of APPP is unknown.
Differential diagnosis. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. The clinical history and an association with systemic illness are the basis for a diagnosis of APPP. Cultures of pustules are negative for any infective pathology, although, as the disease progresses, pustules may become superinfected. Laboratory testing may show an increased erythrocyte sedimentation rate, hypocalcemia, and a low level of vitamin D.
Treatment. Prednisone, 15 to 60 mg/d, is often sufficient to control the disease.27 Cyclosporine, 100 mg twice daily, has also been shown to be useful.28 Cyclosporine in pregnancy is a Category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but risk appears minimal.6
Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, because delivery will almost always lead to swift resolution.
Sequelae. A number of case reports link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.27,29,30 The condition is too rare, however, for good data on specific sequelae. Although APPP does give significant cause for concern, it appears that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.31 This statistic has not been borne out in practice. It does appear that the mother will frequently suffer systemic symptoms, including fever and malaise.
6. Pruritic folliculitis of pregnancy
Accounts of the prevalence of pruritic folliculitis of pregnancy (PFP) vary widely. Some sources report fewer than 30 cases in all of the literature; others indicate that the prevalence is equivalent to that of PG—one in every 10,000 pregnancies.3,11 PFP most often presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often, lesions begin on the abdomen and spread to the extremities.24,28 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health than distressed by the symptoms.

FIGURE 5 Pruritic folliculitis of pregnancy
The papules and pustules of PFP are concentrated around hair follicles.
Pathophysiology. Like many other dermatoses of pregnancy, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,28
Differential diagnosis. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third-trimester onset. No specific laboratory or histologic analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with a low- or midpotency topical corticosteroid, such as triamcinolone or desonide. A benzoyl peroxide wash can also be effective.
Sequelae. One study reports an increased incidence of low birth weight, but no associated morbidity or mortality has been reported in recent studies.24
- Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms
- The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines
- Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality
1. Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103(4):757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183(2):483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188(4):1083-1092.
4. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17(3):172-181.
5. Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8(2):214-224.
6. Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24(2):167-197, vi.
7. Hern S, Harman K, Bhogal BS, Black MM. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23(4):185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352(9144):1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154(1):54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130(6):734-739.
12. McDonald JA. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14(6):515-518.
13. Ohel I, Levy A, Silberstein T, Holcberg G, Sheiner E. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal Fetal Neonat Med. 2006;19(5):305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142(6 Pt 1):621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21(4):905-921.
17. Lammert F, Marschall H, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33(6):1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, Liu M, Javitt NB. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35(6):486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27(6):1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105(11):1205-1207.
22. Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42(6):1399-1405.
23. Ambros-Rudolph C, Müllegger M, Vaughan-Jones S, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395-404.
24. Vaughan Jones SA, Hern S, Nelson-Piercy C, Seed PT, Black MM. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141(1):71-81.
25. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8(3):405-412.
26. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3(4):449-451.
27. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24(2):101-104.
28. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43(1 Pt 1):132-134.
29. Sahin HG, Sahin HA, Metin A, Zeteroglu S, Ugras S. Recurrent impetigo herpetiformis in a pregnant adolescent: case report. Eur J Obstet Gynecol Reprod Biol. 2002;101:201-203.
30. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69(2):153-154.
31. Wade T, Wade S, Jones H. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52(2):233-242.
1. Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103(4):757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183(2):483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188(4):1083-1092.
4. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17(3):172-181.
5. Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8(2):214-224.
6. Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24(2):167-197, vi.
7. Hern S, Harman K, Bhogal BS, Black MM. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23(4):185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352(9144):1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154(1):54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130(6):734-739.
12. McDonald JA. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14(6):515-518.
13. Ohel I, Levy A, Silberstein T, Holcberg G, Sheiner E. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal Fetal Neonat Med. 2006;19(5):305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142(6 Pt 1):621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21(4):905-921.
17. Lammert F, Marschall H, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33(6):1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, Liu M, Javitt NB. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35(6):486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27(6):1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105(11):1205-1207.
22. Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42(6):1399-1405.
23. Ambros-Rudolph C, Müllegger M, Vaughan-Jones S, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395-404.
24. Vaughan Jones SA, Hern S, Nelson-Piercy C, Seed PT, Black MM. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141(1):71-81.
25. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8(3):405-412.
26. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3(4):449-451.
27. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24(2):101-104.
28. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43(1 Pt 1):132-134.
29. Sahin HG, Sahin HA, Metin A, Zeteroglu S, Ugras S. Recurrent impetigo herpetiformis in a pregnant adolescent: case report. Eur J Obstet Gynecol Reprod Biol. 2002;101:201-203.
30. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69(2):153-154.
31. Wade T, Wade S, Jones H. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52(2):233-242.
Rare Fetal Sacral Appendage Studied
Major Finding: All seven fetuses with fetal sacral appendages had significant associated anomalies, and only one is surviving.
Data Source: A single-center study of seven fetuses with fetal sacral appendages evaluated over a 9-year period.
Disclosures: None was reported.
SAN DIEGO — A fetal sacral appendage carries a guarded prognosis, especially in the setting of associated anomalies, results from a single-center study demonstrated.
“Fetal sonography is still the primary mode for identifying the extension,” Lori J. Dobson said during the meeting. “It is important to measure fetal growth and look at the umbilical cord Doppler [scans], as well as to survey for additional anomalies. We did show that it is important to do a fetal MRI as it can help identify additional anomalies and help determine the position of the spinal cord conus.”
A fetal sacral appendage—also referred to as a fetal “tail”—is part of normal embryonic development, expected to resolve by the 8th week of gestation. While more than 30 published studies of prenatally diagnosed fetal sacral appendages have appeared in the medical literature, “the majority of cases are isolated, with no other structural anomalies reported,” said Ms. Dobson, a certified genetic counselor in the advanced fetal care center at Children's Hospital Boston. “And in most cases the appendage had resolved by the second trimester.”
She and her coinvestigators, some of whom performed fetal imaging, set out to describe the associated anomalies, etiology, and clinical outcome for a subset of the 3,641 patients evaluated at the hospital's advanced fetal care center over a 9-year period that ended in December 2009. Ms. Dobson reported that of the 3,641 patients, 7 fetuses (0.19%) had sacral appendages that ranged in size from 2.1 to 4.5 mm. The researchers assessed findings on fetal sonography and fetal MRI, results of genetic testing, and clinical outcomes.
“We noted that in all seven cases there was an extension of the coccyx causing a protrusion or a tenting of the skin,” she said. “Based on this, we propose the term sacrococcygeal extension to better describe the physical finding and its etiology. This is also a more patient-friendly term than fetal tail to use in counseling with parents.”
The average gestational age at diagnosis was 19 weeks. “Because we are a tertiary care referral center, it is not uncommon for us to only see patients in the second trimester or after,” she noted. “That may represent a bias in our sample. We did not have any cases that were diagnosed in the first or third trimester.”
All seven of the fetuses with sacral appendages had significant associated anomalies including severe growth restriction and neurologic, spinal, craniofacial, cardiac, renal, and musculoskeletal abnormalities. There were two cases of trisomy 13 and one case of Pfeiffer syndrome (craniosynostosis). “Most of our cases had multiple organ systems involved,” Ms. Dobson said.
She went on to report that three of the fetuses died in utero, two underwent elective termination, and one fetus with trisomy 13 was delivered at 36 weeks' gestation and died on the first day of life. The remaining case carried to term, and the infant is doing well at 5 months old, but does have a sacral appendage with a tethered spinal cord and an abnormal distal spine.
This sagittal view shows a fetal sacrococcygeal extension on ultrasound at 19 weeks', 4 days' gestation.
Source Courtesy Lori J. Dobson
Major Finding: All seven fetuses with fetal sacral appendages had significant associated anomalies, and only one is surviving.
Data Source: A single-center study of seven fetuses with fetal sacral appendages evaluated over a 9-year period.
Disclosures: None was reported.
SAN DIEGO — A fetal sacral appendage carries a guarded prognosis, especially in the setting of associated anomalies, results from a single-center study demonstrated.
“Fetal sonography is still the primary mode for identifying the extension,” Lori J. Dobson said during the meeting. “It is important to measure fetal growth and look at the umbilical cord Doppler [scans], as well as to survey for additional anomalies. We did show that it is important to do a fetal MRI as it can help identify additional anomalies and help determine the position of the spinal cord conus.”
A fetal sacral appendage—also referred to as a fetal “tail”—is part of normal embryonic development, expected to resolve by the 8th week of gestation. While more than 30 published studies of prenatally diagnosed fetal sacral appendages have appeared in the medical literature, “the majority of cases are isolated, with no other structural anomalies reported,” said Ms. Dobson, a certified genetic counselor in the advanced fetal care center at Children's Hospital Boston. “And in most cases the appendage had resolved by the second trimester.”
She and her coinvestigators, some of whom performed fetal imaging, set out to describe the associated anomalies, etiology, and clinical outcome for a subset of the 3,641 patients evaluated at the hospital's advanced fetal care center over a 9-year period that ended in December 2009. Ms. Dobson reported that of the 3,641 patients, 7 fetuses (0.19%) had sacral appendages that ranged in size from 2.1 to 4.5 mm. The researchers assessed findings on fetal sonography and fetal MRI, results of genetic testing, and clinical outcomes.
“We noted that in all seven cases there was an extension of the coccyx causing a protrusion or a tenting of the skin,” she said. “Based on this, we propose the term sacrococcygeal extension to better describe the physical finding and its etiology. This is also a more patient-friendly term than fetal tail to use in counseling with parents.”
The average gestational age at diagnosis was 19 weeks. “Because we are a tertiary care referral center, it is not uncommon for us to only see patients in the second trimester or after,” she noted. “That may represent a bias in our sample. We did not have any cases that were diagnosed in the first or third trimester.”
All seven of the fetuses with sacral appendages had significant associated anomalies including severe growth restriction and neurologic, spinal, craniofacial, cardiac, renal, and musculoskeletal abnormalities. There were two cases of trisomy 13 and one case of Pfeiffer syndrome (craniosynostosis). “Most of our cases had multiple organ systems involved,” Ms. Dobson said.
She went on to report that three of the fetuses died in utero, two underwent elective termination, and one fetus with trisomy 13 was delivered at 36 weeks' gestation and died on the first day of life. The remaining case carried to term, and the infant is doing well at 5 months old, but does have a sacral appendage with a tethered spinal cord and an abnormal distal spine.
This sagittal view shows a fetal sacrococcygeal extension on ultrasound at 19 weeks', 4 days' gestation.
Source Courtesy Lori J. Dobson
Major Finding: All seven fetuses with fetal sacral appendages had significant associated anomalies, and only one is surviving.
Data Source: A single-center study of seven fetuses with fetal sacral appendages evaluated over a 9-year period.
Disclosures: None was reported.
SAN DIEGO — A fetal sacral appendage carries a guarded prognosis, especially in the setting of associated anomalies, results from a single-center study demonstrated.
“Fetal sonography is still the primary mode for identifying the extension,” Lori J. Dobson said during the meeting. “It is important to measure fetal growth and look at the umbilical cord Doppler [scans], as well as to survey for additional anomalies. We did show that it is important to do a fetal MRI as it can help identify additional anomalies and help determine the position of the spinal cord conus.”
A fetal sacral appendage—also referred to as a fetal “tail”—is part of normal embryonic development, expected to resolve by the 8th week of gestation. While more than 30 published studies of prenatally diagnosed fetal sacral appendages have appeared in the medical literature, “the majority of cases are isolated, with no other structural anomalies reported,” said Ms. Dobson, a certified genetic counselor in the advanced fetal care center at Children's Hospital Boston. “And in most cases the appendage had resolved by the second trimester.”
She and her coinvestigators, some of whom performed fetal imaging, set out to describe the associated anomalies, etiology, and clinical outcome for a subset of the 3,641 patients evaluated at the hospital's advanced fetal care center over a 9-year period that ended in December 2009. Ms. Dobson reported that of the 3,641 patients, 7 fetuses (0.19%) had sacral appendages that ranged in size from 2.1 to 4.5 mm. The researchers assessed findings on fetal sonography and fetal MRI, results of genetic testing, and clinical outcomes.
“We noted that in all seven cases there was an extension of the coccyx causing a protrusion or a tenting of the skin,” she said. “Based on this, we propose the term sacrococcygeal extension to better describe the physical finding and its etiology. This is also a more patient-friendly term than fetal tail to use in counseling with parents.”
The average gestational age at diagnosis was 19 weeks. “Because we are a tertiary care referral center, it is not uncommon for us to only see patients in the second trimester or after,” she noted. “That may represent a bias in our sample. We did not have any cases that were diagnosed in the first or third trimester.”
All seven of the fetuses with sacral appendages had significant associated anomalies including severe growth restriction and neurologic, spinal, craniofacial, cardiac, renal, and musculoskeletal abnormalities. There were two cases of trisomy 13 and one case of Pfeiffer syndrome (craniosynostosis). “Most of our cases had multiple organ systems involved,” Ms. Dobson said.
She went on to report that three of the fetuses died in utero, two underwent elective termination, and one fetus with trisomy 13 was delivered at 36 weeks' gestation and died on the first day of life. The remaining case carried to term, and the infant is doing well at 5 months old, but does have a sacral appendage with a tethered spinal cord and an abnormal distal spine.
This sagittal view shows a fetal sacrococcygeal extension on ultrasound at 19 weeks', 4 days' gestation.
Source Courtesy Lori J. Dobson
From the annual meeting of the American Institute of Ultrasound in Medicine
Training Boosts Bedside Ultrasound Use in ED
SAN DIEGO — After a simple training intervention, emergency physicians at a large tertiary-care hospital performed more than twice as many focused bedside ultrasound exams on pregnant patients as before the training.
“Pregnant women who get the ultrasound by the emergency physician are usually in and out of the department in 20-30 minutes,” Dr. Michael Antonis said in an interview during a poster session.
“Basically, you're looking for a heartbeat and anything worrisome in the adnexa,” explained Dr. Antonis, ultrasound fellowship director at the Georgetown University/Washington Hospital Center's emergency medicine residency program, Washington. “With those questions answered, they're out the door in 20-30 minutes. You don't have to send them to radiology. It's something that's done right at the bedside.”
In a 9-month study led by Dr. Antonis's associate, Dr. Elizabeth Pontius, a third-year resident in the emergency medicine residency program, researchers developed a training program for attending physicians in the ED. The program consisted of two modules: an online training module on how to use the bedside ultrasound machine in general, and a second training module describing how to use the machine for transabdominal and endocavity scans during pregnancy. Next, the attending physicians were assigned to dedicated “sounding” shifts with Dr. Antonis and Dr. Carolyn Phillips, also of Washington Hospital Center, in which they learned how to perform focused ultrasound exams.
After that training, Dr. Antonis and his associates reviewed all ultrasound exams during weekly quality-assurance reviews, and the total number of scans performed by each physician during the intervention period was counted. Before the intervention period, 31 physicians had performed a total of 645 transabdominal or endocavity pregnancy ultrasound exams. After the intervention, 34 physicians had performed 2,350 exams. That translated into a 264% increase in the number of scans performed during the 9-month intervention.
Disclosures: None was reported.
SAN DIEGO — After a simple training intervention, emergency physicians at a large tertiary-care hospital performed more than twice as many focused bedside ultrasound exams on pregnant patients as before the training.
“Pregnant women who get the ultrasound by the emergency physician are usually in and out of the department in 20-30 minutes,” Dr. Michael Antonis said in an interview during a poster session.
“Basically, you're looking for a heartbeat and anything worrisome in the adnexa,” explained Dr. Antonis, ultrasound fellowship director at the Georgetown University/Washington Hospital Center's emergency medicine residency program, Washington. “With those questions answered, they're out the door in 20-30 minutes. You don't have to send them to radiology. It's something that's done right at the bedside.”
In a 9-month study led by Dr. Antonis's associate, Dr. Elizabeth Pontius, a third-year resident in the emergency medicine residency program, researchers developed a training program for attending physicians in the ED. The program consisted of two modules: an online training module on how to use the bedside ultrasound machine in general, and a second training module describing how to use the machine for transabdominal and endocavity scans during pregnancy. Next, the attending physicians were assigned to dedicated “sounding” shifts with Dr. Antonis and Dr. Carolyn Phillips, also of Washington Hospital Center, in which they learned how to perform focused ultrasound exams.
After that training, Dr. Antonis and his associates reviewed all ultrasound exams during weekly quality-assurance reviews, and the total number of scans performed by each physician during the intervention period was counted. Before the intervention period, 31 physicians had performed a total of 645 transabdominal or endocavity pregnancy ultrasound exams. After the intervention, 34 physicians had performed 2,350 exams. That translated into a 264% increase in the number of scans performed during the 9-month intervention.
Disclosures: None was reported.
SAN DIEGO — After a simple training intervention, emergency physicians at a large tertiary-care hospital performed more than twice as many focused bedside ultrasound exams on pregnant patients as before the training.
“Pregnant women who get the ultrasound by the emergency physician are usually in and out of the department in 20-30 minutes,” Dr. Michael Antonis said in an interview during a poster session.
“Basically, you're looking for a heartbeat and anything worrisome in the adnexa,” explained Dr. Antonis, ultrasound fellowship director at the Georgetown University/Washington Hospital Center's emergency medicine residency program, Washington. “With those questions answered, they're out the door in 20-30 minutes. You don't have to send them to radiology. It's something that's done right at the bedside.”
In a 9-month study led by Dr. Antonis's associate, Dr. Elizabeth Pontius, a third-year resident in the emergency medicine residency program, researchers developed a training program for attending physicians in the ED. The program consisted of two modules: an online training module on how to use the bedside ultrasound machine in general, and a second training module describing how to use the machine for transabdominal and endocavity scans during pregnancy. Next, the attending physicians were assigned to dedicated “sounding” shifts with Dr. Antonis and Dr. Carolyn Phillips, also of Washington Hospital Center, in which they learned how to perform focused ultrasound exams.
After that training, Dr. Antonis and his associates reviewed all ultrasound exams during weekly quality-assurance reviews, and the total number of scans performed by each physician during the intervention period was counted. Before the intervention period, 31 physicians had performed a total of 645 transabdominal or endocavity pregnancy ultrasound exams. After the intervention, 34 physicians had performed 2,350 exams. That translated into a 264% increase in the number of scans performed during the 9-month intervention.
Disclosures: None was reported.
From the annual meeting of the American Institute for Ultrasound in Medicine
Vitamins C, E: No Effect on Preeclampsia
Major Finding: The rates of preeclampsia were not significantly different between groups, occurring in 7.2% of the women receiving vitamin C and vitamin E and 6.7% of the placebo group.
Data Source: A large multicenter trial of 10,154 low-risk, nulliparous women from 16 clinical centers.
Disclosures: The study was supported by grants from the National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; and the National Center for Research Resources. Some of the investigators disclosed financial conflicts.
Daily supplementation with vitamins C and E starting between 9 and 16 weeks' gestation did not reduce the rate of pregnancy-associated hypertension, according to a large multicenter trial in low-risk, nulliparous women.
The findings “provide no support for the use of vitamin C and E supplementation in pregnancy to reduce the risk of preeclampsia or its complications,” wrote Dr. James M. Roberts of the University of Pittsburgh and his colleagues (N. Engl. J. Med. 2010;362:1282-91).
The study randomized 10,154 nulliparous women from 16 clinical centers. All women had singleton pregnancies, with gestational age at randomization ranging between 9 weeks, 0 days and 16 weeks, 6 days. The women were randomly assigned to take 1,000 mg of vitamin C and 400 IU of vitamin E daily, or matching placebo, until the end of their pregnancies. They returned any unused study drug each month and received a new batch, at which time they reported any side effects, and had their blood pressure and urine protein levels measured.
The primary outcome of the study was a composite of pregnancy-associated hypertension and serious adverse outcomes in the mother, fetus, or neonate, while the secondary outcomes included preeclampsia and other maternal and neonatal outcomes.
After some subjects were lost to follow-up or removed, a total of 4,993 women from the vitamin arm and 4,976 from the placebo arm were included in the final analysis.
Neither the primary or secondary outcomes of the study were significantly affected by vitamin treatment. A total of 6.1% of the vitamin group and 5.7% of the placebo group met criteria for the primary outcome. Similarly, the rates of the secondary outcome, preeclampsia, were not significantly different between groups—occurring in 7.2% of the vitamin group and 6.7% of the placebo group.
Several other studies have found a similar lack of benefit to antioxidant vitamins in terms of altering the risk of hypertension in pregnancy, and the authors suggested several possible explanations.
First, although there is evidence of oxidative stress in preeclampsia, it might not necessarily be important in the pathophysiology of the disease. Or, perhaps it is relevant, but only to a subset of preeclamptic women.
Yet another suggestion was that supplemental vitamin C and E may not be beneficial if women already have adequate concentrations at baseline. It has been suggested that the therapeutic antioxidant window might be between 8 and 10 weeks' gestation at the initiation of intervillous blood flow. However a post hoc subgroup analysis limited to women who were treated before 13 weeks' gestation showed no difference in outcome.
This and other studies have found no benefit of the antioxidant vitamins C and E in altering the risk of hypertension in pregnancy.
Source James E. Reinaker/Elsevier Global Medical News
Major Finding: The rates of preeclampsia were not significantly different between groups, occurring in 7.2% of the women receiving vitamin C and vitamin E and 6.7% of the placebo group.
Data Source: A large multicenter trial of 10,154 low-risk, nulliparous women from 16 clinical centers.
Disclosures: The study was supported by grants from the National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; and the National Center for Research Resources. Some of the investigators disclosed financial conflicts.
Daily supplementation with vitamins C and E starting between 9 and 16 weeks' gestation did not reduce the rate of pregnancy-associated hypertension, according to a large multicenter trial in low-risk, nulliparous women.
The findings “provide no support for the use of vitamin C and E supplementation in pregnancy to reduce the risk of preeclampsia or its complications,” wrote Dr. James M. Roberts of the University of Pittsburgh and his colleagues (N. Engl. J. Med. 2010;362:1282-91).
The study randomized 10,154 nulliparous women from 16 clinical centers. All women had singleton pregnancies, with gestational age at randomization ranging between 9 weeks, 0 days and 16 weeks, 6 days. The women were randomly assigned to take 1,000 mg of vitamin C and 400 IU of vitamin E daily, or matching placebo, until the end of their pregnancies. They returned any unused study drug each month and received a new batch, at which time they reported any side effects, and had their blood pressure and urine protein levels measured.
The primary outcome of the study was a composite of pregnancy-associated hypertension and serious adverse outcomes in the mother, fetus, or neonate, while the secondary outcomes included preeclampsia and other maternal and neonatal outcomes.
After some subjects were lost to follow-up or removed, a total of 4,993 women from the vitamin arm and 4,976 from the placebo arm were included in the final analysis.
Neither the primary or secondary outcomes of the study were significantly affected by vitamin treatment. A total of 6.1% of the vitamin group and 5.7% of the placebo group met criteria for the primary outcome. Similarly, the rates of the secondary outcome, preeclampsia, were not significantly different between groups—occurring in 7.2% of the vitamin group and 6.7% of the placebo group.
Several other studies have found a similar lack of benefit to antioxidant vitamins in terms of altering the risk of hypertension in pregnancy, and the authors suggested several possible explanations.
First, although there is evidence of oxidative stress in preeclampsia, it might not necessarily be important in the pathophysiology of the disease. Or, perhaps it is relevant, but only to a subset of preeclamptic women.
Yet another suggestion was that supplemental vitamin C and E may not be beneficial if women already have adequate concentrations at baseline. It has been suggested that the therapeutic antioxidant window might be between 8 and 10 weeks' gestation at the initiation of intervillous blood flow. However a post hoc subgroup analysis limited to women who were treated before 13 weeks' gestation showed no difference in outcome.
This and other studies have found no benefit of the antioxidant vitamins C and E in altering the risk of hypertension in pregnancy.
Source James E. Reinaker/Elsevier Global Medical News
Major Finding: The rates of preeclampsia were not significantly different between groups, occurring in 7.2% of the women receiving vitamin C and vitamin E and 6.7% of the placebo group.
Data Source: A large multicenter trial of 10,154 low-risk, nulliparous women from 16 clinical centers.
Disclosures: The study was supported by grants from the National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; and the National Center for Research Resources. Some of the investigators disclosed financial conflicts.
Daily supplementation with vitamins C and E starting between 9 and 16 weeks' gestation did not reduce the rate of pregnancy-associated hypertension, according to a large multicenter trial in low-risk, nulliparous women.
The findings “provide no support for the use of vitamin C and E supplementation in pregnancy to reduce the risk of preeclampsia or its complications,” wrote Dr. James M. Roberts of the University of Pittsburgh and his colleagues (N. Engl. J. Med. 2010;362:1282-91).
The study randomized 10,154 nulliparous women from 16 clinical centers. All women had singleton pregnancies, with gestational age at randomization ranging between 9 weeks, 0 days and 16 weeks, 6 days. The women were randomly assigned to take 1,000 mg of vitamin C and 400 IU of vitamin E daily, or matching placebo, until the end of their pregnancies. They returned any unused study drug each month and received a new batch, at which time they reported any side effects, and had their blood pressure and urine protein levels measured.
The primary outcome of the study was a composite of pregnancy-associated hypertension and serious adverse outcomes in the mother, fetus, or neonate, while the secondary outcomes included preeclampsia and other maternal and neonatal outcomes.
After some subjects were lost to follow-up or removed, a total of 4,993 women from the vitamin arm and 4,976 from the placebo arm were included in the final analysis.
Neither the primary or secondary outcomes of the study were significantly affected by vitamin treatment. A total of 6.1% of the vitamin group and 5.7% of the placebo group met criteria for the primary outcome. Similarly, the rates of the secondary outcome, preeclampsia, were not significantly different between groups—occurring in 7.2% of the vitamin group and 6.7% of the placebo group.
Several other studies have found a similar lack of benefit to antioxidant vitamins in terms of altering the risk of hypertension in pregnancy, and the authors suggested several possible explanations.
First, although there is evidence of oxidative stress in preeclampsia, it might not necessarily be important in the pathophysiology of the disease. Or, perhaps it is relevant, but only to a subset of preeclamptic women.
Yet another suggestion was that supplemental vitamin C and E may not be beneficial if women already have adequate concentrations at baseline. It has been suggested that the therapeutic antioxidant window might be between 8 and 10 weeks' gestation at the initiation of intervillous blood flow. However a post hoc subgroup analysis limited to women who were treated before 13 weeks' gestation showed no difference in outcome.
This and other studies have found no benefit of the antioxidant vitamins C and E in altering the risk of hypertension in pregnancy.
Source James E. Reinaker/Elsevier Global Medical News
From the New England Journal of Medicine
Pregnancy Possible After Fibroid Embolization : Uterine fibroid embolization is not the contraindication to conception it was thought to be.
Major Finding: Of the more than half of women who became pregnant after uterine fibroid embolization, 30 women had successful live births and seven pregnancies are ongoing; there were five abortions (one induced and four spontaneous) and one stillbirth.
Data Source: A study of 74 women who underwent UFE.
Disclosures: None was reported.
TAMPA — Pregnancy rates following treatment with uterine fibroid embolization are comparable to those with myomectomy, offering hope for women who choose embolization but still want to conceive, study results showed.
“Uterine fibroid embolization [UFE] is not a contraindication in patients who want to conceive,” Dr. Joao-Martins Pisco said at the meeting.
The fertility rate in a small population of women who underwent UFE was comparable to that reported for myomectomy—58% vs. 57%.
Dr. Pisco reported on 74 women who underwent UFE but still wished to become pregnant. More than half of the women (58%) had spontaneous pregnancies following the procedure. They ranged in age from 29 to 43 years (mean age, 36 years).
UFE is typically offered to women who no longer wish to become pregnant, and myomectomy is usually offered to women who still wish to become pregnant.
However, there are limited data on fertility rates and pregnancy outcomes following UFE to support this practice, said Dr. Pisco, an interventional radiologist at St. Louis Hospital in Lisbon.
None of the women in this series had been able to conceive prior to UFE. Before the procedure, the women were informed of the uncertain effect of UFE on fertility and pregnancy.
Polyvinyl alcohol particles or Embozene microspheres were used to embolize the uterine arteries.
The mean size of the dominant fibroid was 151 cc. The women were cautioned to wait at least 6 months before trying to conceive.
In all, 30 women (84%) had successful live births. Two of these babies (7%)were born prematurely. There were five abortions—one induced and four spontaneous.
One stillbirth occurred in a woman who had previously undergone five myomectomies and who had conceived through in vitro fertilization.
Seven of the remaining pregnancies are ongoing.
Dr. Pisco noted that larger, multicenter, randomized prospective studies are needed comparing UFE and myomectomy.
Major Finding: Of the more than half of women who became pregnant after uterine fibroid embolization, 30 women had successful live births and seven pregnancies are ongoing; there were five abortions (one induced and four spontaneous) and one stillbirth.
Data Source: A study of 74 women who underwent UFE.
Disclosures: None was reported.
TAMPA — Pregnancy rates following treatment with uterine fibroid embolization are comparable to those with myomectomy, offering hope for women who choose embolization but still want to conceive, study results showed.
“Uterine fibroid embolization [UFE] is not a contraindication in patients who want to conceive,” Dr. Joao-Martins Pisco said at the meeting.
The fertility rate in a small population of women who underwent UFE was comparable to that reported for myomectomy—58% vs. 57%.
Dr. Pisco reported on 74 women who underwent UFE but still wished to become pregnant. More than half of the women (58%) had spontaneous pregnancies following the procedure. They ranged in age from 29 to 43 years (mean age, 36 years).
UFE is typically offered to women who no longer wish to become pregnant, and myomectomy is usually offered to women who still wish to become pregnant.
However, there are limited data on fertility rates and pregnancy outcomes following UFE to support this practice, said Dr. Pisco, an interventional radiologist at St. Louis Hospital in Lisbon.
None of the women in this series had been able to conceive prior to UFE. Before the procedure, the women were informed of the uncertain effect of UFE on fertility and pregnancy.
Polyvinyl alcohol particles or Embozene microspheres were used to embolize the uterine arteries.
The mean size of the dominant fibroid was 151 cc. The women were cautioned to wait at least 6 months before trying to conceive.
In all, 30 women (84%) had successful live births. Two of these babies (7%)were born prematurely. There were five abortions—one induced and four spontaneous.
One stillbirth occurred in a woman who had previously undergone five myomectomies and who had conceived through in vitro fertilization.
Seven of the remaining pregnancies are ongoing.
Dr. Pisco noted that larger, multicenter, randomized prospective studies are needed comparing UFE and myomectomy.
Major Finding: Of the more than half of women who became pregnant after uterine fibroid embolization, 30 women had successful live births and seven pregnancies are ongoing; there were five abortions (one induced and four spontaneous) and one stillbirth.
Data Source: A study of 74 women who underwent UFE.
Disclosures: None was reported.
TAMPA — Pregnancy rates following treatment with uterine fibroid embolization are comparable to those with myomectomy, offering hope for women who choose embolization but still want to conceive, study results showed.
“Uterine fibroid embolization [UFE] is not a contraindication in patients who want to conceive,” Dr. Joao-Martins Pisco said at the meeting.
The fertility rate in a small population of women who underwent UFE was comparable to that reported for myomectomy—58% vs. 57%.
Dr. Pisco reported on 74 women who underwent UFE but still wished to become pregnant. More than half of the women (58%) had spontaneous pregnancies following the procedure. They ranged in age from 29 to 43 years (mean age, 36 years).
UFE is typically offered to women who no longer wish to become pregnant, and myomectomy is usually offered to women who still wish to become pregnant.
However, there are limited data on fertility rates and pregnancy outcomes following UFE to support this practice, said Dr. Pisco, an interventional radiologist at St. Louis Hospital in Lisbon.
None of the women in this series had been able to conceive prior to UFE. Before the procedure, the women were informed of the uncertain effect of UFE on fertility and pregnancy.
Polyvinyl alcohol particles or Embozene microspheres were used to embolize the uterine arteries.
The mean size of the dominant fibroid was 151 cc. The women were cautioned to wait at least 6 months before trying to conceive.
In all, 30 women (84%) had successful live births. Two of these babies (7%)were born prematurely. There were five abortions—one induced and four spontaneous.
One stillbirth occurred in a woman who had previously undergone five myomectomies and who had conceived through in vitro fertilization.
Seven of the remaining pregnancies are ongoing.
Dr. Pisco noted that larger, multicenter, randomized prospective studies are needed comparing UFE and myomectomy.
From the annual meeting of the Society of Interventional Radiology
Consensus panel proposes new diagnostic criteria for gestational diabetes
To and fro, obstetricians and endocrinologists have long debated the relative value of diagnosing and treating gestational diabetes mellitus (GDM). No doubt, significant health advantages can follow from identifying and treating women who have GDM, including:
- protecting the fetus from macrosomia and a lifetime of excess body fat and obesity
- avoiding birth injury, such as shoulder dystocia, and life-long paralytic disability
- early recognition of a group of women at risk of type 2 diabetes mellitus, which can result in cardiovascular disease and premature death when undertreated.1-6
Setting thresholds is a key sticking point
A fundamental issue with establishing diagnostic criteria for GDM, however, is that a continuum relationship exists between, on one hand, the maternal circulating glucose concentration below a level diagnostic of type 2 diabetes mellitus and, on the other hand, such outcomes as macrosomia, neonatal hyperglycemia, preeclampsia, preterm delivery, shoulder dystocia, birth injury, hyperbilirubinemia, and admission to a neonatal intensive care nursery. That is why there’s been a need for an expert consensus panel to establish glucose cutoffs that separate a “normal” state from GDM, based on an analysis of benefits and risks.
In June 2008, the International Association of Diabetes and Pregnancy Study Group convened 225 experts, from 40 countries, to review data and establish new criteria for diagnosing GDM.7 The panel decided that its target for detailed analysis should be a maternal glucose concentration that resulted in an increased risk of 1.75 for various adverse outcomes.
PART 1: New criteria for making a diagnosis of GDM
Consequently, the Study Group consensus panel concluded that GDM should be diagnosed when any one of three tests is abnormal:
- fasting venous plasma glucose ≥92 mg/dL but <126 mg/dL
- 1-hour glucose after a 75-g oral glucose load (the oral glucose tolerance test [OGTT]) ≥180 mg/dL
- 2-hour glucose after a 75-g OGTT ≥153 mg/dL.
Note the implications of these conclusions on diagnosis: Among the findings of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study,4 8.3% of subjects had a fasting venous plasma glucose ≥92 mg/dL, and would be diagnosed with GDM, and an additional 7.8% had a 1-hour or 2-hour glucose above threshold limits after an OGTT. In total, therefore, 11.1% of women in the HAPO study had one elevated result; 3.9% had two elevated results; and 1.1% had all three results elevated.
PART 2: New criteria for diagnosing overt diabetes in pregnancy
The Study Group consensus panel recommended using the following tests and thresholds to diagnose overt diabetes (not GDM) in pregnancy:
- fasting venous plasma glucose ≥126 mg/dL
- hemoglobin A1c ≥6.5%
- random plasma glucose ≥200 mg/dL.
If one of the tests listed above is abnormal, a confirmatory test is clinically appropriate.
Testing during first and second trimesters
The consensus panel recommends that, at the first prenatal visit, you measure the fasting venous plasma glucose, hemoglobin A1c, or random plasma glucose in either all women or high-risk women only. If the result indicates overt diabetes, provide treatment and follow-up as is standard for a pregnant woman who has pregestational diabetes mellitus. If the result is not diagnostic of overt diabetes and the fasting blood glucose level is ≥92 mg/dL but <126 mg/dL, then GDM should be diagnosed. If the fasting venous plasma glucose is <92 mg/dL, then you should perform a 75-g OGTT at 24 to 28 weeks’ gestation.
The percentage of pregnant women who have gestational diabetes mellitus (GDM) is increasing: In Massachusetts, from 1998 to 2006, the rate rose from 3.4% to 4.9%.
In part, we’ve seen this rise because minority women, older women, and overweight women—all of whom are at increased risk of GDM—account for a growing percentage of pregnant women.1 Given ongoing change in these birth demographics, the increase in the rate of GDM over the past decade will likely continue—even accelerate.
We can make a difference. Here is how.
Effective interventions for preventing GDM are to 1) optimize metabolic conditioning and body mass before pregnancy and 2) exercise and limit weight gain, consistent with fetal health, during pregnancy.2
In a large cohort study, a BMI >25 kg/m2 was associated with a relative risk of having a diagnosis of GDM of 2.25, compared with the risk in women whose BMI was <25 kg/m2.3
Abdominal obesity, as measured by waist-hip ratio, also appears to be independently associated with an increased risk of GDM. In a small clinical trial, exercise training during pregnancy—comprising 200 minutes of cycling a week at 65% of predicted aerobic capacity—reduced birth weight by 4%, improved maternal insulin sensitivity, and reduced concentrations of fetal cord insulin-like growth factors I and II.4
References
1. Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288-2293.
2. Morisset AS, St-Yves A, Veillette J, et al. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev. 2010;26(1):17-25.
3. Yeung EH, Hu FB, Solomon CG, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. 2010;53(4):668-678.
4. Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity [published online ahead of print March 24, 2010]. J Clin Endocrinol Metab. 2010;doi:10.1210/jc.2009-2255.
The panel recommends that, at 24 to 28 weeks’ gestation, a 2-hour, 75-g OGTT be performed following an overnight fast on all women not previously diagnosed with overt diabetes or GDM during the first-trimester testing. Based on the results of the 75-g OGTT, diabetes would be diagnosed if the fasting venous plasma glucose is ≥126 mg/dL. You would diagnose GDM if the 1-hour fasting venous plasma glucose is ≥180 mg/dL or the 2-hour result is ≥153 mg/dL.
Although the panel did not recommend applying the following piece of information in clinical practice, it noted that, if the fasting venous plasma glucose is ≤80 mg/dL in the first trimester, 1) it is unlikely that the patient will have an adverse pregnancy outcome attributable to hyperglycemia and 2) it might be possible to avoid the second-trimester OGTT in this select group
These proposals probably won’t end the back-and-forth
Proponents and skeptics are likely to continue their back-and-forth about the right approach to diagnosing and treating GDM. It’s likely that additional research is needed to more firmly establish a quantitative relationship between the newly proposed criteria for diagnosing GDM, and various fetal, childhood, and maternal outcomes. In addition, more research is needed to identify the most cost-effective approach to diagnosing and treating GDM.
When it comes to GDM, are you an OBskeptitrician or an OBconvert?
1. Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4 to 7 years of age. Diabetes Care. 1999;22(8):1284-1291.
2. Schaefer-Graf UM, Buhrer C, Pawliczak J, et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28(7):1745-1750.
3. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486.
4. Metzger BE, Lowe LP, Dyer AR, et al. for the HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002.
5. Landon MB, Spong CY, Thorn E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
6. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453-459.
7. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682.
To and fro, obstetricians and endocrinologists have long debated the relative value of diagnosing and treating gestational diabetes mellitus (GDM). No doubt, significant health advantages can follow from identifying and treating women who have GDM, including:
- protecting the fetus from macrosomia and a lifetime of excess body fat and obesity
- avoiding birth injury, such as shoulder dystocia, and life-long paralytic disability
- early recognition of a group of women at risk of type 2 diabetes mellitus, which can result in cardiovascular disease and premature death when undertreated.1-6
Setting thresholds is a key sticking point
A fundamental issue with establishing diagnostic criteria for GDM, however, is that a continuum relationship exists between, on one hand, the maternal circulating glucose concentration below a level diagnostic of type 2 diabetes mellitus and, on the other hand, such outcomes as macrosomia, neonatal hyperglycemia, preeclampsia, preterm delivery, shoulder dystocia, birth injury, hyperbilirubinemia, and admission to a neonatal intensive care nursery. That is why there’s been a need for an expert consensus panel to establish glucose cutoffs that separate a “normal” state from GDM, based on an analysis of benefits and risks.
In June 2008, the International Association of Diabetes and Pregnancy Study Group convened 225 experts, from 40 countries, to review data and establish new criteria for diagnosing GDM.7 The panel decided that its target for detailed analysis should be a maternal glucose concentration that resulted in an increased risk of 1.75 for various adverse outcomes.
PART 1: New criteria for making a diagnosis of GDM
Consequently, the Study Group consensus panel concluded that GDM should be diagnosed when any one of three tests is abnormal:
- fasting venous plasma glucose ≥92 mg/dL but <126 mg/dL
- 1-hour glucose after a 75-g oral glucose load (the oral glucose tolerance test [OGTT]) ≥180 mg/dL
- 2-hour glucose after a 75-g OGTT ≥153 mg/dL.
Note the implications of these conclusions on diagnosis: Among the findings of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study,4 8.3% of subjects had a fasting venous plasma glucose ≥92 mg/dL, and would be diagnosed with GDM, and an additional 7.8% had a 1-hour or 2-hour glucose above threshold limits after an OGTT. In total, therefore, 11.1% of women in the HAPO study had one elevated result; 3.9% had two elevated results; and 1.1% had all three results elevated.
PART 2: New criteria for diagnosing overt diabetes in pregnancy
The Study Group consensus panel recommended using the following tests and thresholds to diagnose overt diabetes (not GDM) in pregnancy:
- fasting venous plasma glucose ≥126 mg/dL
- hemoglobin A1c ≥6.5%
- random plasma glucose ≥200 mg/dL.
If one of the tests listed above is abnormal, a confirmatory test is clinically appropriate.
Testing during first and second trimesters
The consensus panel recommends that, at the first prenatal visit, you measure the fasting venous plasma glucose, hemoglobin A1c, or random plasma glucose in either all women or high-risk women only. If the result indicates overt diabetes, provide treatment and follow-up as is standard for a pregnant woman who has pregestational diabetes mellitus. If the result is not diagnostic of overt diabetes and the fasting blood glucose level is ≥92 mg/dL but <126 mg/dL, then GDM should be diagnosed. If the fasting venous plasma glucose is <92 mg/dL, then you should perform a 75-g OGTT at 24 to 28 weeks’ gestation.
The percentage of pregnant women who have gestational diabetes mellitus (GDM) is increasing: In Massachusetts, from 1998 to 2006, the rate rose from 3.4% to 4.9%.
In part, we’ve seen this rise because minority women, older women, and overweight women—all of whom are at increased risk of GDM—account for a growing percentage of pregnant women.1 Given ongoing change in these birth demographics, the increase in the rate of GDM over the past decade will likely continue—even accelerate.
We can make a difference. Here is how.
Effective interventions for preventing GDM are to 1) optimize metabolic conditioning and body mass before pregnancy and 2) exercise and limit weight gain, consistent with fetal health, during pregnancy.2
In a large cohort study, a BMI >25 kg/m2 was associated with a relative risk of having a diagnosis of GDM of 2.25, compared with the risk in women whose BMI was <25 kg/m2.3
Abdominal obesity, as measured by waist-hip ratio, also appears to be independently associated with an increased risk of GDM. In a small clinical trial, exercise training during pregnancy—comprising 200 minutes of cycling a week at 65% of predicted aerobic capacity—reduced birth weight by 4%, improved maternal insulin sensitivity, and reduced concentrations of fetal cord insulin-like growth factors I and II.4
References
1. Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288-2293.
2. Morisset AS, St-Yves A, Veillette J, et al. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev. 2010;26(1):17-25.
3. Yeung EH, Hu FB, Solomon CG, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. 2010;53(4):668-678.
4. Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity [published online ahead of print March 24, 2010]. J Clin Endocrinol Metab. 2010;doi:10.1210/jc.2009-2255.
The panel recommends that, at 24 to 28 weeks’ gestation, a 2-hour, 75-g OGTT be performed following an overnight fast on all women not previously diagnosed with overt diabetes or GDM during the first-trimester testing. Based on the results of the 75-g OGTT, diabetes would be diagnosed if the fasting venous plasma glucose is ≥126 mg/dL. You would diagnose GDM if the 1-hour fasting venous plasma glucose is ≥180 mg/dL or the 2-hour result is ≥153 mg/dL.
Although the panel did not recommend applying the following piece of information in clinical practice, it noted that, if the fasting venous plasma glucose is ≤80 mg/dL in the first trimester, 1) it is unlikely that the patient will have an adverse pregnancy outcome attributable to hyperglycemia and 2) it might be possible to avoid the second-trimester OGTT in this select group
These proposals probably won’t end the back-and-forth
Proponents and skeptics are likely to continue their back-and-forth about the right approach to diagnosing and treating GDM. It’s likely that additional research is needed to more firmly establish a quantitative relationship between the newly proposed criteria for diagnosing GDM, and various fetal, childhood, and maternal outcomes. In addition, more research is needed to identify the most cost-effective approach to diagnosing and treating GDM.
When it comes to GDM, are you an OBskeptitrician or an OBconvert?
To and fro, obstetricians and endocrinologists have long debated the relative value of diagnosing and treating gestational diabetes mellitus (GDM). No doubt, significant health advantages can follow from identifying and treating women who have GDM, including:
- protecting the fetus from macrosomia and a lifetime of excess body fat and obesity
- avoiding birth injury, such as shoulder dystocia, and life-long paralytic disability
- early recognition of a group of women at risk of type 2 diabetes mellitus, which can result in cardiovascular disease and premature death when undertreated.1-6
Setting thresholds is a key sticking point
A fundamental issue with establishing diagnostic criteria for GDM, however, is that a continuum relationship exists between, on one hand, the maternal circulating glucose concentration below a level diagnostic of type 2 diabetes mellitus and, on the other hand, such outcomes as macrosomia, neonatal hyperglycemia, preeclampsia, preterm delivery, shoulder dystocia, birth injury, hyperbilirubinemia, and admission to a neonatal intensive care nursery. That is why there’s been a need for an expert consensus panel to establish glucose cutoffs that separate a “normal” state from GDM, based on an analysis of benefits and risks.
In June 2008, the International Association of Diabetes and Pregnancy Study Group convened 225 experts, from 40 countries, to review data and establish new criteria for diagnosing GDM.7 The panel decided that its target for detailed analysis should be a maternal glucose concentration that resulted in an increased risk of 1.75 for various adverse outcomes.
PART 1: New criteria for making a diagnosis of GDM
Consequently, the Study Group consensus panel concluded that GDM should be diagnosed when any one of three tests is abnormal:
- fasting venous plasma glucose ≥92 mg/dL but <126 mg/dL
- 1-hour glucose after a 75-g oral glucose load (the oral glucose tolerance test [OGTT]) ≥180 mg/dL
- 2-hour glucose after a 75-g OGTT ≥153 mg/dL.
Note the implications of these conclusions on diagnosis: Among the findings of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study,4 8.3% of subjects had a fasting venous plasma glucose ≥92 mg/dL, and would be diagnosed with GDM, and an additional 7.8% had a 1-hour or 2-hour glucose above threshold limits after an OGTT. In total, therefore, 11.1% of women in the HAPO study had one elevated result; 3.9% had two elevated results; and 1.1% had all three results elevated.
PART 2: New criteria for diagnosing overt diabetes in pregnancy
The Study Group consensus panel recommended using the following tests and thresholds to diagnose overt diabetes (not GDM) in pregnancy:
- fasting venous plasma glucose ≥126 mg/dL
- hemoglobin A1c ≥6.5%
- random plasma glucose ≥200 mg/dL.
If one of the tests listed above is abnormal, a confirmatory test is clinically appropriate.
Testing during first and second trimesters
The consensus panel recommends that, at the first prenatal visit, you measure the fasting venous plasma glucose, hemoglobin A1c, or random plasma glucose in either all women or high-risk women only. If the result indicates overt diabetes, provide treatment and follow-up as is standard for a pregnant woman who has pregestational diabetes mellitus. If the result is not diagnostic of overt diabetes and the fasting blood glucose level is ≥92 mg/dL but <126 mg/dL, then GDM should be diagnosed. If the fasting venous plasma glucose is <92 mg/dL, then you should perform a 75-g OGTT at 24 to 28 weeks’ gestation.
The percentage of pregnant women who have gestational diabetes mellitus (GDM) is increasing: In Massachusetts, from 1998 to 2006, the rate rose from 3.4% to 4.9%.
In part, we’ve seen this rise because minority women, older women, and overweight women—all of whom are at increased risk of GDM—account for a growing percentage of pregnant women.1 Given ongoing change in these birth demographics, the increase in the rate of GDM over the past decade will likely continue—even accelerate.
We can make a difference. Here is how.
Effective interventions for preventing GDM are to 1) optimize metabolic conditioning and body mass before pregnancy and 2) exercise and limit weight gain, consistent with fetal health, during pregnancy.2
In a large cohort study, a BMI >25 kg/m2 was associated with a relative risk of having a diagnosis of GDM of 2.25, compared with the risk in women whose BMI was <25 kg/m2.3
Abdominal obesity, as measured by waist-hip ratio, also appears to be independently associated with an increased risk of GDM. In a small clinical trial, exercise training during pregnancy—comprising 200 minutes of cycling a week at 65% of predicted aerobic capacity—reduced birth weight by 4%, improved maternal insulin sensitivity, and reduced concentrations of fetal cord insulin-like growth factors I and II.4
References
1. Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288-2293.
2. Morisset AS, St-Yves A, Veillette J, et al. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev. 2010;26(1):17-25.
3. Yeung EH, Hu FB, Solomon CG, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. 2010;53(4):668-678.
4. Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity [published online ahead of print March 24, 2010]. J Clin Endocrinol Metab. 2010;doi:10.1210/jc.2009-2255.
The panel recommends that, at 24 to 28 weeks’ gestation, a 2-hour, 75-g OGTT be performed following an overnight fast on all women not previously diagnosed with overt diabetes or GDM during the first-trimester testing. Based on the results of the 75-g OGTT, diabetes would be diagnosed if the fasting venous plasma glucose is ≥126 mg/dL. You would diagnose GDM if the 1-hour fasting venous plasma glucose is ≥180 mg/dL or the 2-hour result is ≥153 mg/dL.
Although the panel did not recommend applying the following piece of information in clinical practice, it noted that, if the fasting venous plasma glucose is ≤80 mg/dL in the first trimester, 1) it is unlikely that the patient will have an adverse pregnancy outcome attributable to hyperglycemia and 2) it might be possible to avoid the second-trimester OGTT in this select group
These proposals probably won’t end the back-and-forth
Proponents and skeptics are likely to continue their back-and-forth about the right approach to diagnosing and treating GDM. It’s likely that additional research is needed to more firmly establish a quantitative relationship between the newly proposed criteria for diagnosing GDM, and various fetal, childhood, and maternal outcomes. In addition, more research is needed to identify the most cost-effective approach to diagnosing and treating GDM.
When it comes to GDM, are you an OBskeptitrician or an OBconvert?
1. Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4 to 7 years of age. Diabetes Care. 1999;22(8):1284-1291.
2. Schaefer-Graf UM, Buhrer C, Pawliczak J, et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28(7):1745-1750.
3. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486.
4. Metzger BE, Lowe LP, Dyer AR, et al. for the HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002.
5. Landon MB, Spong CY, Thorn E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
6. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453-459.
7. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682.
1. Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4 to 7 years of age. Diabetes Care. 1999;22(8):1284-1291.
2. Schaefer-Graf UM, Buhrer C, Pawliczak J, et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28(7):1745-1750.
3. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486.
4. Metzger BE, Lowe LP, Dyer AR, et al. for the HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002.
5. Landon MB, Spong CY, Thorn E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
6. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453-459.
7. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682.
What can be safer than having a baby in the USA?
A recent report1 by the National Highway Traffic Safety Administration revealed that deaths from automobile accidents have declined strikingly since 1954, reaching the lowest level ever recorded today: 1.16 fatalities for every 100 million vehicle-miles traveled. Why this improvement? Reasons include an increase in seat belt use and a lower rate of drunk driving, both secondary effects of better law enforcement, safer roads, and people just driving less.
There are lessons to be learned from this success that can be applied to obstetrical care and, potentially, to lower the unacceptably high rate of maternal mortality in this country.
Things aren’t working as we need them to
Truly, it is a reflection of a broken health care system that we, as health care providers, cannot say that we have seen a dramatic decrease in maternal mortality. In 2006, the latest year for which statistics are available, maternal mortality in the United States was at the highest level recorded since 1991: 13.3 deaths for every 100,000 live births (in California, the rate is 16.9 for every 100,000!).
Remember the Healthy People 2010 benchmark of 4.3 maternal deaths for every 100,000 live births? Clearly, we will not see that number any time in the near future; achieving it is as likely to happen as getting the national cesarean delivery rate to fall below 15% from the current level above 30%, or the return of vaginal breech deliveries!
Maternal mortality numbers are even more distressing when you appreciate that 1) African American women have three to four times the death rate from pregnancy complications that white women do and 2) minorities (African Americans, Latinos) are having the majority of pregnancies.

In a society supplied with well-trained obstetricians, marked improvements in the safety of anesthesia, and a plethora of maternal-fetal medicine specialists, we must do some serious introspection and ask ourselves: Why are so many young women dying? And what can we do to prevent their deaths?
Why the rise in maternal deaths?
Multiple reasons are cited for the rise in maternal mortality. They include:
- difficult access to care
- racial discrimination
- increase in substance abuse
- barriers to communication with non–English-speaking patients
- lack of health insurance
- inflexible appointment hours
- lack of transportation to visits
- serious comorbidities (e.g., diabetes).
Each of these has some validity. But my opinion (and many of you will disagree with me) is that our society has not addressed the real issues driving the problem—issues that are difficult to face:
First, some women have too many pregnancies.
Second, certain women who have serious medical conditions, including morbid obesity and substance abuse, are having pregnancies when they should not have any at all.
It is an interesting observation that a person needs a license to drive a car or hunt deer but only a simple act of intercourse with no contraception to become pregnant.
What I’ve learned from my work
I have spent more than 35 years caring for pregnant women of low socioeconomic status. I am proud to have played that role. The two constant themes in my work with these patients are that 1) they feel a lack of control over most aspects of their life and 2) they lack self-esteem.
Pregnancy, however, is the one thing that they can control. It is the one time when others become interested in their welfare, and their best opportunity to have a something (a baby) that is all theirs.
But their adherence to appointments and laboratory testing is often poor. And their use of contraception between pregnancies is sporadic.
The 18-year-old woman who is in her second pregnancy often has a mother who is in her early 30s and a grandmother around 50. For these women, and for their children and grandchildren, the cycle of poverty is repeated endlessly. No matter how much money or effort is put into providing resources to care for them, little has changed in the past 30 years, and there is, I believe, little hope for change in the future. The only solutions that I can discern that will decisively break the cycle of poverty, lower the rate of maternal mortality, and improve the well being of women and their newborns are education and contraception.
My three-pronged proposal
Several actions can improve the outcome of a pregnancy for mothers of low socioeconomic status and their babies. I understand that many people will find these actions coercive and prejudicial, which truly they are not.
- All forms of contraception should be free for women, and access to contraception should be easy. Such a policy would result in fewer unintended pregnancies and abortions and better pregnancy spacing. The expense of free and universal contraception offers a great return on investment compared to the cost of care for a premature baby who spends any length of time in the neonatal intensive care unit.
Young women should be compensated for using whatever contraceptive they choose by being given vouchers that can be redeemed for material goods at select venues or free minutes for their cellular phones.
- All women who qualify for Medicaid coverage of pregnancy should be aggressively encouraged to have a preconception visit at least 3 to 6 months before they plan to become pregnant to assess any risks to themselves or their fetus and have a program put in place to maximize outcomes. This encouragement can take place at any visit in which contraception is discussed.
At this visit, an attempt can be made to optimize their clinical condition by:
- counseling them about drug and alcohol use and smoking
- assessing their risk of genetic disorders
- initiating folic acid and dietary modifications
- performing appropriate screening tests (i.e., blood glucose).
Appropriate consideration should be given, and discussion held, during the visit about whether the patient should even consider becoming pregnant. She should be given a realistic assessment of the risk of pregnancy and childbirth to her and the baby—including the potential for death.
The preconception assessment should be conducted by a physician, a midwife, or a nurse practitioner who has not been involved in the care of the patient. Doing so will minimize the introduction of any bias into the conversation by a treating physician.
When it is in a woman’s medical best interest not to conceive, she should become eligible for expedited adoption and be compensated for each reproductive year in which she does not conceive.
Last, a strong financial incentive should be offered to women who complete this preconception evaluation.
Many will say that such a program is unfair and prejudicial to women of lower socioeconomic status. But a precedent exists: Medicaid prohibits the performance of a sterilization procedure unless a signed permit has been in place 30 days or longer.
- Pregnant women who adhere to a prenatal care plan should be compensated with vouchers that can be redeemed for baby items, maternity clothes, or food for the family at select venues. They should also be compensated for keeping prenatal appointments; obtaining timely laboratory tests; attending prenatal classes; avoiding drugs, alcohol and smoking; returning for postpartum assessment; and using reliable contraception.
Education + contraception = fewer deaths?
Would such a plan work? I am convinced that it is worth trying. What do you think? Send your comments to me at [email protected]!
Reference
1. Traffic safety facts. Early estimate of motor vehicle traffic fatalities in 2009. National Highway Traffic Safety Administration.http://www-nrd.nhtsa.dot.gov/Pubs/811291.PDF. March 2010.
A recent report1 by the National Highway Traffic Safety Administration revealed that deaths from automobile accidents have declined strikingly since 1954, reaching the lowest level ever recorded today: 1.16 fatalities for every 100 million vehicle-miles traveled. Why this improvement? Reasons include an increase in seat belt use and a lower rate of drunk driving, both secondary effects of better law enforcement, safer roads, and people just driving less.
There are lessons to be learned from this success that can be applied to obstetrical care and, potentially, to lower the unacceptably high rate of maternal mortality in this country.
Things aren’t working as we need them to
Truly, it is a reflection of a broken health care system that we, as health care providers, cannot say that we have seen a dramatic decrease in maternal mortality. In 2006, the latest year for which statistics are available, maternal mortality in the United States was at the highest level recorded since 1991: 13.3 deaths for every 100,000 live births (in California, the rate is 16.9 for every 100,000!).
Remember the Healthy People 2010 benchmark of 4.3 maternal deaths for every 100,000 live births? Clearly, we will not see that number any time in the near future; achieving it is as likely to happen as getting the national cesarean delivery rate to fall below 15% from the current level above 30%, or the return of vaginal breech deliveries!
Maternal mortality numbers are even more distressing when you appreciate that 1) African American women have three to four times the death rate from pregnancy complications that white women do and 2) minorities (African Americans, Latinos) are having the majority of pregnancies.

In a society supplied with well-trained obstetricians, marked improvements in the safety of anesthesia, and a plethora of maternal-fetal medicine specialists, we must do some serious introspection and ask ourselves: Why are so many young women dying? And what can we do to prevent their deaths?
Why the rise in maternal deaths?
Multiple reasons are cited for the rise in maternal mortality. They include:
- difficult access to care
- racial discrimination
- increase in substance abuse
- barriers to communication with non–English-speaking patients
- lack of health insurance
- inflexible appointment hours
- lack of transportation to visits
- serious comorbidities (e.g., diabetes).
Each of these has some validity. But my opinion (and many of you will disagree with me) is that our society has not addressed the real issues driving the problem—issues that are difficult to face:
First, some women have too many pregnancies.
Second, certain women who have serious medical conditions, including morbid obesity and substance abuse, are having pregnancies when they should not have any at all.
It is an interesting observation that a person needs a license to drive a car or hunt deer but only a simple act of intercourse with no contraception to become pregnant.
What I’ve learned from my work
I have spent more than 35 years caring for pregnant women of low socioeconomic status. I am proud to have played that role. The two constant themes in my work with these patients are that 1) they feel a lack of control over most aspects of their life and 2) they lack self-esteem.
Pregnancy, however, is the one thing that they can control. It is the one time when others become interested in their welfare, and their best opportunity to have a something (a baby) that is all theirs.
But their adherence to appointments and laboratory testing is often poor. And their use of contraception between pregnancies is sporadic.
The 18-year-old woman who is in her second pregnancy often has a mother who is in her early 30s and a grandmother around 50. For these women, and for their children and grandchildren, the cycle of poverty is repeated endlessly. No matter how much money or effort is put into providing resources to care for them, little has changed in the past 30 years, and there is, I believe, little hope for change in the future. The only solutions that I can discern that will decisively break the cycle of poverty, lower the rate of maternal mortality, and improve the well being of women and their newborns are education and contraception.
My three-pronged proposal
Several actions can improve the outcome of a pregnancy for mothers of low socioeconomic status and their babies. I understand that many people will find these actions coercive and prejudicial, which truly they are not.
- All forms of contraception should be free for women, and access to contraception should be easy. Such a policy would result in fewer unintended pregnancies and abortions and better pregnancy spacing. The expense of free and universal contraception offers a great return on investment compared to the cost of care for a premature baby who spends any length of time in the neonatal intensive care unit.
Young women should be compensated for using whatever contraceptive they choose by being given vouchers that can be redeemed for material goods at select venues or free minutes for their cellular phones.
- All women who qualify for Medicaid coverage of pregnancy should be aggressively encouraged to have a preconception visit at least 3 to 6 months before they plan to become pregnant to assess any risks to themselves or their fetus and have a program put in place to maximize outcomes. This encouragement can take place at any visit in which contraception is discussed.
At this visit, an attempt can be made to optimize their clinical condition by:
- counseling them about drug and alcohol use and smoking
- assessing their risk of genetic disorders
- initiating folic acid and dietary modifications
- performing appropriate screening tests (i.e., blood glucose).
Appropriate consideration should be given, and discussion held, during the visit about whether the patient should even consider becoming pregnant. She should be given a realistic assessment of the risk of pregnancy and childbirth to her and the baby—including the potential for death.
The preconception assessment should be conducted by a physician, a midwife, or a nurse practitioner who has not been involved in the care of the patient. Doing so will minimize the introduction of any bias into the conversation by a treating physician.
When it is in a woman’s medical best interest not to conceive, she should become eligible for expedited adoption and be compensated for each reproductive year in which she does not conceive.
Last, a strong financial incentive should be offered to women who complete this preconception evaluation.
Many will say that such a program is unfair and prejudicial to women of lower socioeconomic status. But a precedent exists: Medicaid prohibits the performance of a sterilization procedure unless a signed permit has been in place 30 days or longer.
- Pregnant women who adhere to a prenatal care plan should be compensated with vouchers that can be redeemed for baby items, maternity clothes, or food for the family at select venues. They should also be compensated for keeping prenatal appointments; obtaining timely laboratory tests; attending prenatal classes; avoiding drugs, alcohol and smoking; returning for postpartum assessment; and using reliable contraception.
Education + contraception = fewer deaths?
Would such a plan work? I am convinced that it is worth trying. What do you think? Send your comments to me at [email protected]!
A recent report1 by the National Highway Traffic Safety Administration revealed that deaths from automobile accidents have declined strikingly since 1954, reaching the lowest level ever recorded today: 1.16 fatalities for every 100 million vehicle-miles traveled. Why this improvement? Reasons include an increase in seat belt use and a lower rate of drunk driving, both secondary effects of better law enforcement, safer roads, and people just driving less.
There are lessons to be learned from this success that can be applied to obstetrical care and, potentially, to lower the unacceptably high rate of maternal mortality in this country.
Things aren’t working as we need them to
Truly, it is a reflection of a broken health care system that we, as health care providers, cannot say that we have seen a dramatic decrease in maternal mortality. In 2006, the latest year for which statistics are available, maternal mortality in the United States was at the highest level recorded since 1991: 13.3 deaths for every 100,000 live births (in California, the rate is 16.9 for every 100,000!).
Remember the Healthy People 2010 benchmark of 4.3 maternal deaths for every 100,000 live births? Clearly, we will not see that number any time in the near future; achieving it is as likely to happen as getting the national cesarean delivery rate to fall below 15% from the current level above 30%, or the return of vaginal breech deliveries!
Maternal mortality numbers are even more distressing when you appreciate that 1) African American women have three to four times the death rate from pregnancy complications that white women do and 2) minorities (African Americans, Latinos) are having the majority of pregnancies.

In a society supplied with well-trained obstetricians, marked improvements in the safety of anesthesia, and a plethora of maternal-fetal medicine specialists, we must do some serious introspection and ask ourselves: Why are so many young women dying? And what can we do to prevent their deaths?
Why the rise in maternal deaths?
Multiple reasons are cited for the rise in maternal mortality. They include:
- difficult access to care
- racial discrimination
- increase in substance abuse
- barriers to communication with non–English-speaking patients
- lack of health insurance
- inflexible appointment hours
- lack of transportation to visits
- serious comorbidities (e.g., diabetes).
Each of these has some validity. But my opinion (and many of you will disagree with me) is that our society has not addressed the real issues driving the problem—issues that are difficult to face:
First, some women have too many pregnancies.
Second, certain women who have serious medical conditions, including morbid obesity and substance abuse, are having pregnancies when they should not have any at all.
It is an interesting observation that a person needs a license to drive a car or hunt deer but only a simple act of intercourse with no contraception to become pregnant.
What I’ve learned from my work
I have spent more than 35 years caring for pregnant women of low socioeconomic status. I am proud to have played that role. The two constant themes in my work with these patients are that 1) they feel a lack of control over most aspects of their life and 2) they lack self-esteem.
Pregnancy, however, is the one thing that they can control. It is the one time when others become interested in their welfare, and their best opportunity to have a something (a baby) that is all theirs.
But their adherence to appointments and laboratory testing is often poor. And their use of contraception between pregnancies is sporadic.
The 18-year-old woman who is in her second pregnancy often has a mother who is in her early 30s and a grandmother around 50. For these women, and for their children and grandchildren, the cycle of poverty is repeated endlessly. No matter how much money or effort is put into providing resources to care for them, little has changed in the past 30 years, and there is, I believe, little hope for change in the future. The only solutions that I can discern that will decisively break the cycle of poverty, lower the rate of maternal mortality, and improve the well being of women and their newborns are education and contraception.
My three-pronged proposal
Several actions can improve the outcome of a pregnancy for mothers of low socioeconomic status and their babies. I understand that many people will find these actions coercive and prejudicial, which truly they are not.
- All forms of contraception should be free for women, and access to contraception should be easy. Such a policy would result in fewer unintended pregnancies and abortions and better pregnancy spacing. The expense of free and universal contraception offers a great return on investment compared to the cost of care for a premature baby who spends any length of time in the neonatal intensive care unit.
Young women should be compensated for using whatever contraceptive they choose by being given vouchers that can be redeemed for material goods at select venues or free minutes for their cellular phones.
- All women who qualify for Medicaid coverage of pregnancy should be aggressively encouraged to have a preconception visit at least 3 to 6 months before they plan to become pregnant to assess any risks to themselves or their fetus and have a program put in place to maximize outcomes. This encouragement can take place at any visit in which contraception is discussed.
At this visit, an attempt can be made to optimize their clinical condition by:
- counseling them about drug and alcohol use and smoking
- assessing their risk of genetic disorders
- initiating folic acid and dietary modifications
- performing appropriate screening tests (i.e., blood glucose).
Appropriate consideration should be given, and discussion held, during the visit about whether the patient should even consider becoming pregnant. She should be given a realistic assessment of the risk of pregnancy and childbirth to her and the baby—including the potential for death.
The preconception assessment should be conducted by a physician, a midwife, or a nurse practitioner who has not been involved in the care of the patient. Doing so will minimize the introduction of any bias into the conversation by a treating physician.
When it is in a woman’s medical best interest not to conceive, she should become eligible for expedited adoption and be compensated for each reproductive year in which she does not conceive.
Last, a strong financial incentive should be offered to women who complete this preconception evaluation.
Many will say that such a program is unfair and prejudicial to women of lower socioeconomic status. But a precedent exists: Medicaid prohibits the performance of a sterilization procedure unless a signed permit has been in place 30 days or longer.
- Pregnant women who adhere to a prenatal care plan should be compensated with vouchers that can be redeemed for baby items, maternity clothes, or food for the family at select venues. They should also be compensated for keeping prenatal appointments; obtaining timely laboratory tests; attending prenatal classes; avoiding drugs, alcohol and smoking; returning for postpartum assessment; and using reliable contraception.
Education + contraception = fewer deaths?
Would such a plan work? I am convinced that it is worth trying. What do you think? Send your comments to me at [email protected]!
Reference
1. Traffic safety facts. Early estimate of motor vehicle traffic fatalities in 2009. National Highway Traffic Safety Administration.http://www-nrd.nhtsa.dot.gov/Pubs/811291.PDF. March 2010.
Reference
1. Traffic safety facts. Early estimate of motor vehicle traffic fatalities in 2009. National Highway Traffic Safety Administration.http://www-nrd.nhtsa.dot.gov/Pubs/811291.PDF. March 2010.
How to manage a short cervix to lower the risk of preterm delivery
CASE 1: Short cervix in the middle trimester
During routine second-trimester ultrasonography, a 34-year-old primigravida at 22 weeks’ gestation is found to have a cervix 15 mm in length. She has no other risk factors for spontaneous preterm birth.
What steps do you take to ensure that her pregnancy progresses uneventfully to term?
Cervical length is not routinely measured in low-risk women—i.e., those without a history of spontaneous preterm birth—but a short cervix is sometimes detected during ultrasonographic imaging for other indications, as it was in this case. When a short cervix is detected incidentally, I educate the patient to watch for early warning signs of preterm labor. I also recommend pelvic rest and a sedentary lifestyle.
The rate of preterm birth declined slightly in 2007—the first decrease in more than 20 years—but the phenomenon remains the leading cause of perinatal morbidity and mortality in developed nations.1 In the United States, more than 500,000 babies each year, or 12% to 13% of all births, are delivered before 37 weeks’ gestation. Most of these births are spontaneous and involve preterm labor or premature rupture of membranes. Medical costs for a preterm newborn exceed those of a term infant by a multiple of more than 10, and the average hospitalization exceeds that of a term infant by a multiple of more than six.
In this article, I discuss the rationale and technique for ultrasonographic cervical measurement to determine the likelihood of preterm birth. I also examine the data on the short cervix in various settings, and describe strategies for cervical assessment and preterm birth prevention, including cerclage and progesterone, framing the discussion in terms of gestational age.
Accurate and reproducible measurement of cervical length depends on correct technique. Use of transvaginal ultrasonography (TVUS) limits variations between measurements to 5% to 10%, a marked improvement over digital examination and transabdominal US.
Here are the five steps involved, in the order performed:
- Ensure that the patient’s bladder is empty. This precaution is necessary to prevent dynamic, or spontaneous, lengthening or shortening of the cervix.
- Counsel and position the patient. Explain the procedure to the patient and have her assume the dorsal lithotomy position.
- Introduce the probe into the anterior vaginal fornix using real-time visualization, and obtain a mid-sagittal view of the cervix. Withdraw the probe just enough to allow the image to blur, then advance the probe just enough for the image to regain clarity. This sequence prevents the practitioner from exerting excessive pressure on the cervix, which can falsely elongate it.
- Place an electronic caliper (on-screen) at the notch that represents the internal cervical os, and another at the external os (FIGURE 1).
- Measure the distance between the notches and report the shortest of three separately obtained measurements.
FIGURE 1 How to measure the cervix
Electronic calipers mark the internal and external os in this cervix measuring 42 mm via midsagittal transvaginal ultrasonography.
Watch for these pitfalls!
Before 20 weeks’ gestation, the lower uterine segment is not particularly well developed, making it difficult to reliably determine the location of the internal os (FIGURE 2). Moreover, focal myometrial contractions of the lower uterine segment, which are common, may give the false impression of increased cervical length or dilation of the internal os (FIGURE 2).24
FIGURE 2 Measurement may be difficult in early pregnancy
A. This transvaginal sonogram demonstrates the difficulty of determining the location of the internal os when the lower uterine segment is undeveloped (arrows). B. When lower uterine contractions occur with the anterior and posterior walls in opposition (arrowheads), transvaginal imaging may give the false appearance of dilation with funneling (arrow).
Why we assess cervical length
In the past, the cervix was viewed as either competent—i.e., capable of maintaining a pregnancy until term—or as “incompetent.” More recent evidence has broadened our understanding of cervical function, which is now viewed along a continuum.
In landmark research in the mid-1990s, investigators compared cervical lengths, measured via transvaginal ultrasonography (TVUS), between two groups of pregnant women—those who had a history of preterm birth and those who did not.
The result? Gestational age at delivery in the first pregnancy correlated significantly—and continuously—with cervical length between 20 and 30 weeks’ gestation in the subsequent pregnancy.2 Investigators also observed that the risk of spontaneous preterm birth increased with decreasing cervical length. A length of 25 mm (10th percentile) offered a clinically appropriate threshold for identification of the risk of preterm delivery.3
The value of cervical-length measurement lies in its high negative predictive value for recurrent spontaneous preterm birth.4 As a general rule of thumb, routine assessment of cervical length in asymptomatic women who do not have a history of preterm birth is not recommended because of the high rate of false-positive results and the low positive predictive value for preterm delivery. We also lack an evidence-based consensus on how to manage an abnormally short cervix in these women.
CASE RESOLVED
The patient is counseled to watch for signs of preterm labor and to remain sedentary. Her pregnancy progresses without incident until 40 weeks, when she undergoes induction of labor for oligohydramnios and delivers a healthy infant weighing 3,855 g.
When a short cervix is detected at less than 20 weeks
If a woman has a history of spontaneous preterm birth, a short cervix this early in gestation raises the question of cervical insufficiency. No objective criteria have been devised to identify this condition. Nor is there a widely accepted definition. A reasonable description does exist, however:
- …a clinical diagnosis characterized by recurrent painless dilation and spontaneous midtrimester birth, generally in the absence of predisposing conditions such as spontaneous membrane rupture, bleeding, and infection, characteristics that shift the presumed underlying cause away from cervical incompetence and support other components of the preterm birth syndrome.5
A patient who fits this description may be a candidate for cervical cerclage. Alternatively, it is reasonable to reassess the patient in 3 to 7 days, after restricting physical activity, keeping in mind the pitfalls of TVUS assessment of the lower uterine segment in early gestation (see the box on imaging). If cervical length remains short, consider cerclage.6,7
Watch for inflammation
Occasionally, echogenic material is observed in the amniotic fluid at the level of a short cervix. This debris is an inflammatory exudate of fibrin, white blood cells, and bacteria. The presence of this sludge (FIGURE 3) signifies a risk of preterm birth much greater than that associated with a short cervix alone.8 Because cerclage in the presence of inflammation may further heighten the risk of spontaneous preterm birth, I recommend caution.
FIGURE 3 Watch for signs of inflammation
When inflammatory exudate (asterisk) is identified at the level of a short cervix, the risk of spontaneous preterm birth is elevated beyond the risk associated with a short cervix alone.
When a short cervix is detected between 20 and 24 weeks
CASE 2: Is recurrent preterm birth likely?
A 30-year-old woman 21 weeks pregnant with her second child reports for TVUS. Because her first child was delivered preterm at 30 weeks, she has been undergoing periodic measurement of her cervix. Until today, it has been longer than 25 mm, but now it is 20 mm. What is the best strategy to avert another preterm birth?
If a short cervix (<25 mm) is noted in a high-risk patient at this gestational age, consider the possibility of preterm labor and ruptured membranes. If these conditions are present, they should be managed according to existing guidelines.4,9 If they are absent, consider cerclage.
A recent meta-analysis of randomized trials of cerclage for the prevention of preterm birth in a singleton, high-risk pregnancy with a short cervix suggests that cerclage is associated with a significantly lower risk of delivery before 35 weeks’ gestation (relative risk [RR], 0.61; 95% confidence interval [CI], 0.40–0.92). Among singleton pregnancies involving both a short cervix and a history of midtrimester loss, cerclage is again associated with a reduced likelihood of delivery before 35 weeks (39% vs 23.4%; number needed to treat [NNT], 8; RR, 0.57; 95% CI, 0.33–0.99).10 A recent randomized trial of cerclage versus no cerclage in women who had a history of spontaneous preterm birth produced similar findings.11 This trial is described in detail in the box.
Another randomized trial compared the relative merits of ultrasound-indicated cerclage for a cervix shorter than 20 mm to a history-indicated cerclage among women with a prior spontaneous preterm birth between 16 and 34 weeks. Thirty-nine of 123 women randomized to the former group received a cerclage, as did 25 of 125 subjects in the latter group. There were no significant differences in the primary outcome of delivery before 34 weeks or secondary measures of loss before 24 weeks, preterm premature membrane rupture, mean gestational age at delivery, or neonatal outcomes. This study was not designed or powered to evaluate the efficacy of cerclage in preventing recurrent, spontaneous preterm birth. It simply compares two ways of selecting high-risk women for cerclage.12
We lack data supporting placement of cerclage for an incidentally detected short cervix in women who lack a history of spontaneous preterm birth or midtrimester loss.7
Other groups known to be at increased risk of spontaneous preterm birth include women carrying twins and women who have undergone cervical cone biopsy or loop electrosurgical excision procedure (LEEP). Among twin gestations, cerclage for a short cervix is associated with an increased rate of preterm birth (<35 weeks).10 Cerclage for a short cervix has not been evaluated among women who have a history of LEEP.6
CASE RESOLVED
After preterm labor is ruled out, the patient is counseled about her options and chooses cervical cerclage. Her pregnancy proceeds uneventfully until 36 weeks’ gestation, when she delivers a healthy infant weighing 2,950 g.
When a short cervix is detected between 24 and 34 weeks
CASE 3: Is it preterm labor?
A primigravida at 31 weeks’ gestation presents to the labor floor reporting regular contractions. She denies bleeding or rupture of membranes. Contractions are noted at 3-minute intervals, and digital cervical examination reveals that she is dilated 1 cm, with 70% effacement. TVUS determines cervical length to be 17 mm.
How should she be managed?
From 24 to 34 weeks’ gestation, the prevention, diagnosis, and treatment of preterm labor become the main concerns. Because our ability to predict and prevent preterm birth is limited, clinical management focuses on a reliable diagnosis of preterm labor to allow for selective, timely interventions to optimize neonatal outcomes. These interventions include tocolysis to permit maternal transport; antibiotic prophylaxis for group B strep; and steroid administration to accelerate fetal lung maturity. Equally important is the ability to reliably rule out preterm labor among symptomatic (contracting) women to avoid the potential morbidity, cost, and inconvenience of these interventions.
Before 37 weeks, a diagnosis of preterm labor requires the following findings:
- six or more contractions per hour
- cervical dilation, as identified by digital examination, of at least 3 cm and 80% effacement.
This diagnosis is more reliable when ruptured membranes or vaginal bleeding are present.
The significance of contractions without these findings is less clear. Therefore, we follow an algorithm that incorporates TVUS measurement of cervical length and evaluation of fetal fibronectin (fFN) (FIGURE 4).13
fFN is a glycoprotein that is normally confined to the extracellular matrix of the fetal membranes between 24 and 34 weeks’ gestation. Detection of fFN in cervicovaginal secretions during this window is associated with an increased risk of spontaneous preterm birth, whereas its absence demonstrates a negative predictive value for delivery within 7 days of testing of more than 97%.
During initial assessment of a regularly contracting preterm patient, perform a vaginal speculum examination. If the membranes are intact, use a vaginal swab to assess the patient for the presence of fFN, and set the specimen aside. Also obtain a culture for group B strep.
If a digital cervical examination and the contraction pattern establish a diagnosis of preterm labor, administer a tocolytic, When fetal prophylactic antibiotics, and steroids. If the diagnosis remains unclear, evaluate the cervix via TVUS. A cervical length above 30 mm effectively rules out preterm labor and obviates the need to send the fFN swab for assessment. As a result, the patient can be managed expectantly.
In contrast, a cervical length below 20 mm effectively confirms the diagnosis of preterm labor, and treatment can proceed. Again, the fFN swab may be discarded. The swab is sent for processing only if cervical length is 20 mm to 30 mm (FIGURE 4). A positive fFN result leads to the presumptive diagnosis of preterm labor, whereas a negative result permits expectant management.
We also recommend that women who display symptoms of preterm labor be screened for asymptomatic bacteriuria. Identification and treatment of this condition significantly reduce the risk of preterm delivery (RR, 0.56; 95% CI, 0.43–0.73).14 In addition, diagnosis and treatment of bacterial vaginosis in symptomatic women who have a history of spontaneous preterm birth can also reduce the risk of recurrent preterm delivery (RR, 0.42; 95% CI, 0.27–0.67).15
Not all obstetric care providers have the resources necessary for TVUS assessment of cervical length. When that is the case, fFN offers high sensitivity and negative predictive value and can help guide initial clinical decision-making. Keep in mind, however, that not all facilities offer fFN testing. In addition, in some cases, cervical manipulation may have occurred before fFN testing was performed, precluding its validity. In such cases, the incorporation of TVUS assessment of cervical length into clinical evaluation may help guide decision-making.

FIGURE 4 How to identify preterm labor at 24 to 34 weeks
CASE RESOLVED
The determination of short cervical length (17 mm) by TVUS confirms the diagnosis of preterm labor. The patient is admitted to the hospital and treated with a tocolytic, prophylactic antibiotics, and steroids. Three days later, preterm labor recurs, and she delivers an otherwise healthy infant. Future pregnancies will be managed according to the algorithm presented in FIGURE 5.
Cerclage may benefit women who have a history of spontaneous preterm birth
A recent multicenter randomized trial evaluated the efficacy of cerclage in preventing preterm birth among 302 women who had a history of spontaneous preterm birth before 34 weeks’ gestation.11 Any woman who had a cervix shorter than 25 mm between 16-0/7 and 22-6/7 weeks’ gestation was randomized to cerclage or no cerclage.
Cerclage did not significantly reduce preterm delivery before 35 weeks’ gestation, the primary outcome. Thirty-two percent of women who received cerclage and 42% of women who did not receive cerclage delivered before 35 weeks (P = .09). However, among women who had a cervix shorter than 15 mm at randomization, cerclage reduced the rate of delivery before 35 weeks by more than 75% (P = .006). Cerclage also reduced the rate of spontaneous birth before 37 weeks, compared with no cerclage (45% vs 60%; P = .01), as well as the rate of previable preterm birth before 24 weeks (6.1% vs 14%; P = .03) and the rate of perinatal death (8.8% vs 16%; P = .046).
As this study demonstrates, ultrasonographically indicated cerclage produces a number of highly clinically significant benefits in women who have a history of spontaneous preterm birth. Another important question is whether supplemental progesterone offers additional benefit beyond that conferred by cerclage in this population.
The role of progesterone in preventing preterm birth
CASE 4: History of preterm and term delivery
A woman in her third pregnancy is referred for placement of cervical cerclage, based on her obstetric history. Her first pregnancy was marked by preterm labor at 26 weeks, resulting in spontaneous preterm birth at 28 weeks. In her second pregnancy, she had cerclage placed electively at 13 weeks and delivered spontaneously at 40 weeks with no complications of pregnancy.
Is another cerclage indicated—or would progesterone be more effective?
Supplemental 17-hydroxyprogesterone caproate, given weekly in an intramuscular dosage of 250 mg, significantly reduced the rate of recurrent spontaneous preterm birth when it was administered from 16 to 36 weeks’ gestation in women carrying a singleton fetus.16 The protective effect of progesterone is most apparent in women who have a history of very preterm delivery (<32 weeks). No such benefit has been observed in twin and triplet gestations, however.17-19
Among women who do benefit from progesterone, the effect may vary. For this reason, researchers have explored the measurement of midtrimester cervical length as a means of stratifying response to progesterone.
For example, investigators randomized women who had a history of spontaneous preterm birth to daily treatment with 90 mg of vaginal progesterone gel or placebo, starting between 16 and 22-6/7 weeks and continuing until 37 weeks or delivery, whichever came first. No difference in the rate of delivery at or before 32 weeks was observed. However, a secondary analysis among progesterone-treated women who had a cervical length below 28 mm found significant declines in the rate of delivery at or before 32 weeks, admission to a NICU, and length of stay.20,21
In another trial, investigators assessed cervical length via TVUS between 20 and 25 weeks’ gestation in a general obstetric population that included women carrying twins. Women who had a cervical length below 15 mm were offered randomization to daily oral progesterone (200 mg) or placebo from 24 weeks to 33-6/7 weeks’ gestation. The rate of spontaneous preterm birth was significantly lower in the progesterone group (19% vs 34%).22
Because the ideal formulation of progesterone for the prevention of preterm birth is unknown, ACOG recommends restricting its use to women who have a documented history of spontaneous preterm birth at less than 37 weeks’ gestation.23 My practice follows the protocol of Meis and colleagues within the framework of an overall systematic algorithm (FIGURE 5).

FIGURE 5 Cervical length in the second trimester
*For a singleton gestation only.
CASE RESOLVED
The ObGyn reviews the patient’s obstetric history and determines that the first pregnancy was more suggestive of preterm labor than cervical insufficiency. Therefore, the ObGyn opts for progesterone rather than cerclage to prolong the gestation. The patient begins weekly injections of 17-hydroxyprogesterone caproate, starting at 16 weeks’ gestation, with TVUS measurement of cervical length every 2 weeks. The cervix remains longer than 25 mm through 24 weeks’ gestation, at which time TVUS assessment is stopped. Progesterone injections continue through 36 weeks, and the patient spontaneously delivers a healthy 3,700-g infant at term.
1. Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2007. National vital statistics reports; 2009;57(12). National Center for Health Statistics Web site. http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf. March 18, 2009. Accessed March 30, 2010.
2. Iams JD, Johnson FF, Sonek J, et al. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172(4 pt 1):1097-1106.
3. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
4. Clinical Management Guidelines for Obstetrician-Gynecologists. No. 43, May 2003: Management of Preterm Labor. Obstet Gynecol. 2003;101(5 pt 1):1039-1047.
5. Owen J, Iams JD, Hauth JC. Vaginal sonography and cervical incompetence. Am J Obstet Gynecol. 2003;188(2):586-596.
6. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100(6):1313-1327.
7. Clinical Management Guidelines for Obstetrician-Gynecologists. No. 48, Nov 2003: Cervical insufficiency. Obstet Gynecol. 2003;102(5 pt 1):1091-1099.
8. Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706-714.
9. ACOG Practice Bulletin No. 80. Premature rupture of membranes. Obstet Gynecol. 2007;109(4):1007-1020.DOI:10.1097/01.AOG.0000263888.69178.1f.
10. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106(1):181-189.
11. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened mid-trimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-e8.
12. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized control trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-e6.
13. Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol. 2003;101(2):402-412.
14. Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73(4):576-582.
15. Leitich H, Brunbauer M, Bodnar-Aderl B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol. 2003;188(3):752-758.
16. Meis PJ, Klebanoff M, Thom E, et al. For National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm birth by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
17. Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034-2040.
18. Rouse DJ, Caritis SN, Peaceman AM, et al. For National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454-461.
19. Caritis SN, Rouse DJ, Peaceman AM, et al. for Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Medicine Units Network (MFMU). Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113(2 pt 1):285-292.
20. O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):687-696.
21. DeFranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):697-705.
22. Fonseca EB, Celik E, Parra M, et al. For Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462-469.
23. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963.-
24. Yost NP, Bloom SL, Twickler DM, Leveno KJ. Pitfalls in ultrasonic cervical length measurement for predicting preterm birth. Obstet Gynecol. 1999;93(4):510-516.
CASE 1: Short cervix in the middle trimester
During routine second-trimester ultrasonography, a 34-year-old primigravida at 22 weeks’ gestation is found to have a cervix 15 mm in length. She has no other risk factors for spontaneous preterm birth.
What steps do you take to ensure that her pregnancy progresses uneventfully to term?
Cervical length is not routinely measured in low-risk women—i.e., those without a history of spontaneous preterm birth—but a short cervix is sometimes detected during ultrasonographic imaging for other indications, as it was in this case. When a short cervix is detected incidentally, I educate the patient to watch for early warning signs of preterm labor. I also recommend pelvic rest and a sedentary lifestyle.
The rate of preterm birth declined slightly in 2007—the first decrease in more than 20 years—but the phenomenon remains the leading cause of perinatal morbidity and mortality in developed nations.1 In the United States, more than 500,000 babies each year, or 12% to 13% of all births, are delivered before 37 weeks’ gestation. Most of these births are spontaneous and involve preterm labor or premature rupture of membranes. Medical costs for a preterm newborn exceed those of a term infant by a multiple of more than 10, and the average hospitalization exceeds that of a term infant by a multiple of more than six.
In this article, I discuss the rationale and technique for ultrasonographic cervical measurement to determine the likelihood of preterm birth. I also examine the data on the short cervix in various settings, and describe strategies for cervical assessment and preterm birth prevention, including cerclage and progesterone, framing the discussion in terms of gestational age.
Accurate and reproducible measurement of cervical length depends on correct technique. Use of transvaginal ultrasonography (TVUS) limits variations between measurements to 5% to 10%, a marked improvement over digital examination and transabdominal US.
Here are the five steps involved, in the order performed:
- Ensure that the patient’s bladder is empty. This precaution is necessary to prevent dynamic, or spontaneous, lengthening or shortening of the cervix.
- Counsel and position the patient. Explain the procedure to the patient and have her assume the dorsal lithotomy position.
- Introduce the probe into the anterior vaginal fornix using real-time visualization, and obtain a mid-sagittal view of the cervix. Withdraw the probe just enough to allow the image to blur, then advance the probe just enough for the image to regain clarity. This sequence prevents the practitioner from exerting excessive pressure on the cervix, which can falsely elongate it.
- Place an electronic caliper (on-screen) at the notch that represents the internal cervical os, and another at the external os (FIGURE 1).
- Measure the distance between the notches and report the shortest of three separately obtained measurements.

FIGURE 1 How to measure the cervix
Electronic calipers mark the internal and external os in this cervix measuring 42 mm via midsagittal transvaginal ultrasonography.
Watch for these pitfalls!
Before 20 weeks’ gestation, the lower uterine segment is not particularly well developed, making it difficult to reliably determine the location of the internal os (FIGURE 2). Moreover, focal myometrial contractions of the lower uterine segment, which are common, may give the false impression of increased cervical length or dilation of the internal os (FIGURE 2).24

FIGURE 2 Measurement may be difficult in early pregnancy
A. This transvaginal sonogram demonstrates the difficulty of determining the location of the internal os when the lower uterine segment is undeveloped (arrows). B. When lower uterine contractions occur with the anterior and posterior walls in opposition (arrowheads), transvaginal imaging may give the false appearance of dilation with funneling (arrow).
Why we assess cervical length
In the past, the cervix was viewed as either competent—i.e., capable of maintaining a pregnancy until term—or as “incompetent.” More recent evidence has broadened our understanding of cervical function, which is now viewed along a continuum.
In landmark research in the mid-1990s, investigators compared cervical lengths, measured via transvaginal ultrasonography (TVUS), between two groups of pregnant women—those who had a history of preterm birth and those who did not.
The result? Gestational age at delivery in the first pregnancy correlated significantly—and continuously—with cervical length between 20 and 30 weeks’ gestation in the subsequent pregnancy.2 Investigators also observed that the risk of spontaneous preterm birth increased with decreasing cervical length. A length of 25 mm (10th percentile) offered a clinically appropriate threshold for identification of the risk of preterm delivery.3
The value of cervical-length measurement lies in its high negative predictive value for recurrent spontaneous preterm birth.4 As a general rule of thumb, routine assessment of cervical length in asymptomatic women who do not have a history of preterm birth is not recommended because of the high rate of false-positive results and the low positive predictive value for preterm delivery. We also lack an evidence-based consensus on how to manage an abnormally short cervix in these women.
CASE RESOLVED
The patient is counseled to watch for signs of preterm labor and to remain sedentary. Her pregnancy progresses without incident until 40 weeks, when she undergoes induction of labor for oligohydramnios and delivers a healthy infant weighing 3,855 g.
When a short cervix is detected at less than 20 weeks
If a woman has a history of spontaneous preterm birth, a short cervix this early in gestation raises the question of cervical insufficiency. No objective criteria have been devised to identify this condition. Nor is there a widely accepted definition. A reasonable description does exist, however:
- …a clinical diagnosis characterized by recurrent painless dilation and spontaneous midtrimester birth, generally in the absence of predisposing conditions such as spontaneous membrane rupture, bleeding, and infection, characteristics that shift the presumed underlying cause away from cervical incompetence and support other components of the preterm birth syndrome.5
A patient who fits this description may be a candidate for cervical cerclage. Alternatively, it is reasonable to reassess the patient in 3 to 7 days, after restricting physical activity, keeping in mind the pitfalls of TVUS assessment of the lower uterine segment in early gestation (see the box on imaging). If cervical length remains short, consider cerclage.6,7
Watch for inflammation
Occasionally, echogenic material is observed in the amniotic fluid at the level of a short cervix. This debris is an inflammatory exudate of fibrin, white blood cells, and bacteria. The presence of this sludge (FIGURE 3) signifies a risk of preterm birth much greater than that associated with a short cervix alone.8 Because cerclage in the presence of inflammation may further heighten the risk of spontaneous preterm birth, I recommend caution.

FIGURE 3 Watch for signs of inflammation
When inflammatory exudate (asterisk) is identified at the level of a short cervix, the risk of spontaneous preterm birth is elevated beyond the risk associated with a short cervix alone.
When a short cervix is detected between 20 and 24 weeks
CASE 2: Is recurrent preterm birth likely?
A 30-year-old woman 21 weeks pregnant with her second child reports for TVUS. Because her first child was delivered preterm at 30 weeks, she has been undergoing periodic measurement of her cervix. Until today, it has been longer than 25 mm, but now it is 20 mm. What is the best strategy to avert another preterm birth?
If a short cervix (<25 mm) is noted in a high-risk patient at this gestational age, consider the possibility of preterm labor and ruptured membranes. If these conditions are present, they should be managed according to existing guidelines.4,9 If they are absent, consider cerclage.
A recent meta-analysis of randomized trials of cerclage for the prevention of preterm birth in a singleton, high-risk pregnancy with a short cervix suggests that cerclage is associated with a significantly lower risk of delivery before 35 weeks’ gestation (relative risk [RR], 0.61; 95% confidence interval [CI], 0.40–0.92). Among singleton pregnancies involving both a short cervix and a history of midtrimester loss, cerclage is again associated with a reduced likelihood of delivery before 35 weeks (39% vs 23.4%; number needed to treat [NNT], 8; RR, 0.57; 95% CI, 0.33–0.99).10 A recent randomized trial of cerclage versus no cerclage in women who had a history of spontaneous preterm birth produced similar findings.11 This trial is described in detail in the box.
Another randomized trial compared the relative merits of ultrasound-indicated cerclage for a cervix shorter than 20 mm to a history-indicated cerclage among women with a prior spontaneous preterm birth between 16 and 34 weeks. Thirty-nine of 123 women randomized to the former group received a cerclage, as did 25 of 125 subjects in the latter group. There were no significant differences in the primary outcome of delivery before 34 weeks or secondary measures of loss before 24 weeks, preterm premature membrane rupture, mean gestational age at delivery, or neonatal outcomes. This study was not designed or powered to evaluate the efficacy of cerclage in preventing recurrent, spontaneous preterm birth. It simply compares two ways of selecting high-risk women for cerclage.12
We lack data supporting placement of cerclage for an incidentally detected short cervix in women who lack a history of spontaneous preterm birth or midtrimester loss.7
Other groups known to be at increased risk of spontaneous preterm birth include women carrying twins and women who have undergone cervical cone biopsy or loop electrosurgical excision procedure (LEEP). Among twin gestations, cerclage for a short cervix is associated with an increased rate of preterm birth (<35 weeks).10 Cerclage for a short cervix has not been evaluated among women who have a history of LEEP.6
CASE RESOLVED
After preterm labor is ruled out, the patient is counseled about her options and chooses cervical cerclage. Her pregnancy proceeds uneventfully until 36 weeks’ gestation, when she delivers a healthy infant weighing 2,950 g.
When a short cervix is detected between 24 and 34 weeks
CASE 3: Is it preterm labor?
A primigravida at 31 weeks’ gestation presents to the labor floor reporting regular contractions. She denies bleeding or rupture of membranes. Contractions are noted at 3-minute intervals, and digital cervical examination reveals that she is dilated 1 cm, with 70% effacement. TVUS determines cervical length to be 17 mm.
How should she be managed?
From 24 to 34 weeks’ gestation, the prevention, diagnosis, and treatment of preterm labor become the main concerns. Because our ability to predict and prevent preterm birth is limited, clinical management focuses on a reliable diagnosis of preterm labor to allow for selective, timely interventions to optimize neonatal outcomes. These interventions include tocolysis to permit maternal transport; antibiotic prophylaxis for group B strep; and steroid administration to accelerate fetal lung maturity. Equally important is the ability to reliably rule out preterm labor among symptomatic (contracting) women to avoid the potential morbidity, cost, and inconvenience of these interventions.
Before 37 weeks, a diagnosis of preterm labor requires the following findings:
- six or more contractions per hour
- cervical dilation, as identified by digital examination, of at least 3 cm and 80% effacement.
This diagnosis is more reliable when ruptured membranes or vaginal bleeding are present.
The significance of contractions without these findings is less clear. Therefore, we follow an algorithm that incorporates TVUS measurement of cervical length and evaluation of fetal fibronectin (fFN) (FIGURE 4).13
fFN is a glycoprotein that is normally confined to the extracellular matrix of the fetal membranes between 24 and 34 weeks’ gestation. Detection of fFN in cervicovaginal secretions during this window is associated with an increased risk of spontaneous preterm birth, whereas its absence demonstrates a negative predictive value for delivery within 7 days of testing of more than 97%.
During initial assessment of a regularly contracting preterm patient, perform a vaginal speculum examination. If the membranes are intact, use a vaginal swab to assess the patient for the presence of fFN, and set the specimen aside. Also obtain a culture for group B strep.
If a digital cervical examination and the contraction pattern establish a diagnosis of preterm labor, administer a tocolytic, When fetal prophylactic antibiotics, and steroids. If the diagnosis remains unclear, evaluate the cervix via TVUS. A cervical length above 30 mm effectively rules out preterm labor and obviates the need to send the fFN swab for assessment. As a result, the patient can be managed expectantly.
In contrast, a cervical length below 20 mm effectively confirms the diagnosis of preterm labor, and treatment can proceed. Again, the fFN swab may be discarded. The swab is sent for processing only if cervical length is 20 mm to 30 mm (FIGURE 4). A positive fFN result leads to the presumptive diagnosis of preterm labor, whereas a negative result permits expectant management.
We also recommend that women who display symptoms of preterm labor be screened for asymptomatic bacteriuria. Identification and treatment of this condition significantly reduce the risk of preterm delivery (RR, 0.56; 95% CI, 0.43–0.73).14 In addition, diagnosis and treatment of bacterial vaginosis in symptomatic women who have a history of spontaneous preterm birth can also reduce the risk of recurrent preterm delivery (RR, 0.42; 95% CI, 0.27–0.67).15
Not all obstetric care providers have the resources necessary for TVUS assessment of cervical length. When that is the case, fFN offers high sensitivity and negative predictive value and can help guide initial clinical decision-making. Keep in mind, however, that not all facilities offer fFN testing. In addition, in some cases, cervical manipulation may have occurred before fFN testing was performed, precluding its validity. In such cases, the incorporation of TVUS assessment of cervical length into clinical evaluation may help guide decision-making.

FIGURE 4 How to identify preterm labor at 24 to 34 weeks
CASE RESOLVED
The determination of short cervical length (17 mm) by TVUS confirms the diagnosis of preterm labor. The patient is admitted to the hospital and treated with a tocolytic, prophylactic antibiotics, and steroids. Three days later, preterm labor recurs, and she delivers an otherwise healthy infant. Future pregnancies will be managed according to the algorithm presented in FIGURE 5.
Cerclage may benefit women who have a history of spontaneous preterm birth
A recent multicenter randomized trial evaluated the efficacy of cerclage in preventing preterm birth among 302 women who had a history of spontaneous preterm birth before 34 weeks’ gestation.11 Any woman who had a cervix shorter than 25 mm between 16-0/7 and 22-6/7 weeks’ gestation was randomized to cerclage or no cerclage.
Cerclage did not significantly reduce preterm delivery before 35 weeks’ gestation, the primary outcome. Thirty-two percent of women who received cerclage and 42% of women who did not receive cerclage delivered before 35 weeks (P = .09). However, among women who had a cervix shorter than 15 mm at randomization, cerclage reduced the rate of delivery before 35 weeks by more than 75% (P = .006). Cerclage also reduced the rate of spontaneous birth before 37 weeks, compared with no cerclage (45% vs 60%; P = .01), as well as the rate of previable preterm birth before 24 weeks (6.1% vs 14%; P = .03) and the rate of perinatal death (8.8% vs 16%; P = .046).
As this study demonstrates, ultrasonographically indicated cerclage produces a number of highly clinically significant benefits in women who have a history of spontaneous preterm birth. Another important question is whether supplemental progesterone offers additional benefit beyond that conferred by cerclage in this population.
The role of progesterone in preventing preterm birth
CASE 4: History of preterm and term delivery
A woman in her third pregnancy is referred for placement of cervical cerclage, based on her obstetric history. Her first pregnancy was marked by preterm labor at 26 weeks, resulting in spontaneous preterm birth at 28 weeks. In her second pregnancy, she had cerclage placed electively at 13 weeks and delivered spontaneously at 40 weeks with no complications of pregnancy.
Is another cerclage indicated—or would progesterone be more effective?
Supplemental 17-hydroxyprogesterone caproate, given weekly in an intramuscular dosage of 250 mg, significantly reduced the rate of recurrent spontaneous preterm birth when it was administered from 16 to 36 weeks’ gestation in women carrying a singleton fetus.16 The protective effect of progesterone is most apparent in women who have a history of very preterm delivery (<32 weeks). No such benefit has been observed in twin and triplet gestations, however.17-19
Among women who do benefit from progesterone, the effect may vary. For this reason, researchers have explored the measurement of midtrimester cervical length as a means of stratifying response to progesterone.
For example, investigators randomized women who had a history of spontaneous preterm birth to daily treatment with 90 mg of vaginal progesterone gel or placebo, starting between 16 and 22-6/7 weeks and continuing until 37 weeks or delivery, whichever came first. No difference in the rate of delivery at or before 32 weeks was observed. However, a secondary analysis among progesterone-treated women who had a cervical length below 28 mm found significant declines in the rate of delivery at or before 32 weeks, admission to a NICU, and length of stay.20,21
In another trial, investigators assessed cervical length via TVUS between 20 and 25 weeks’ gestation in a general obstetric population that included women carrying twins. Women who had a cervical length below 15 mm were offered randomization to daily oral progesterone (200 mg) or placebo from 24 weeks to 33-6/7 weeks’ gestation. The rate of spontaneous preterm birth was significantly lower in the progesterone group (19% vs 34%).22
Because the ideal formulation of progesterone for the prevention of preterm birth is unknown, ACOG recommends restricting its use to women who have a documented history of spontaneous preterm birth at less than 37 weeks’ gestation.23 My practice follows the protocol of Meis and colleagues within the framework of an overall systematic algorithm (FIGURE 5).

FIGURE 5 Cervical length in the second trimester
*For a singleton gestation only.
CASE RESOLVED
The ObGyn reviews the patient’s obstetric history and determines that the first pregnancy was more suggestive of preterm labor than cervical insufficiency. Therefore, the ObGyn opts for progesterone rather than cerclage to prolong the gestation. The patient begins weekly injections of 17-hydroxyprogesterone caproate, starting at 16 weeks’ gestation, with TVUS measurement of cervical length every 2 weeks. The cervix remains longer than 25 mm through 24 weeks’ gestation, at which time TVUS assessment is stopped. Progesterone injections continue through 36 weeks, and the patient spontaneously delivers a healthy 3,700-g infant at term.
CASE 1: Short cervix in the middle trimester
During routine second-trimester ultrasonography, a 34-year-old primigravida at 22 weeks’ gestation is found to have a cervix 15 mm in length. She has no other risk factors for spontaneous preterm birth.
What steps do you take to ensure that her pregnancy progresses uneventfully to term?
Cervical length is not routinely measured in low-risk women—i.e., those without a history of spontaneous preterm birth—but a short cervix is sometimes detected during ultrasonographic imaging for other indications, as it was in this case. When a short cervix is detected incidentally, I educate the patient to watch for early warning signs of preterm labor. I also recommend pelvic rest and a sedentary lifestyle.
The rate of preterm birth declined slightly in 2007—the first decrease in more than 20 years—but the phenomenon remains the leading cause of perinatal morbidity and mortality in developed nations.1 In the United States, more than 500,000 babies each year, or 12% to 13% of all births, are delivered before 37 weeks’ gestation. Most of these births are spontaneous and involve preterm labor or premature rupture of membranes. Medical costs for a preterm newborn exceed those of a term infant by a multiple of more than 10, and the average hospitalization exceeds that of a term infant by a multiple of more than six.
In this article, I discuss the rationale and technique for ultrasonographic cervical measurement to determine the likelihood of preterm birth. I also examine the data on the short cervix in various settings, and describe strategies for cervical assessment and preterm birth prevention, including cerclage and progesterone, framing the discussion in terms of gestational age.
Accurate and reproducible measurement of cervical length depends on correct technique. Use of transvaginal ultrasonography (TVUS) limits variations between measurements to 5% to 10%, a marked improvement over digital examination and transabdominal US.
Here are the five steps involved, in the order performed:
- Ensure that the patient’s bladder is empty. This precaution is necessary to prevent dynamic, or spontaneous, lengthening or shortening of the cervix.
- Counsel and position the patient. Explain the procedure to the patient and have her assume the dorsal lithotomy position.
- Introduce the probe into the anterior vaginal fornix using real-time visualization, and obtain a mid-sagittal view of the cervix. Withdraw the probe just enough to allow the image to blur, then advance the probe just enough for the image to regain clarity. This sequence prevents the practitioner from exerting excessive pressure on the cervix, which can falsely elongate it.
- Place an electronic caliper (on-screen) at the notch that represents the internal cervical os, and another at the external os (FIGURE 1).
- Measure the distance between the notches and report the shortest of three separately obtained measurements.

FIGURE 1 How to measure the cervix
Electronic calipers mark the internal and external os in this cervix measuring 42 mm via midsagittal transvaginal ultrasonography.
Watch for these pitfalls!
Before 20 weeks’ gestation, the lower uterine segment is not particularly well developed, making it difficult to reliably determine the location of the internal os (FIGURE 2). Moreover, focal myometrial contractions of the lower uterine segment, which are common, may give the false impression of increased cervical length or dilation of the internal os (FIGURE 2).24

FIGURE 2 Measurement may be difficult in early pregnancy
A. This transvaginal sonogram demonstrates the difficulty of determining the location of the internal os when the lower uterine segment is undeveloped (arrows). B. When lower uterine contractions occur with the anterior and posterior walls in opposition (arrowheads), transvaginal imaging may give the false appearance of dilation with funneling (arrow).
Why we assess cervical length
In the past, the cervix was viewed as either competent—i.e., capable of maintaining a pregnancy until term—or as “incompetent.” More recent evidence has broadened our understanding of cervical function, which is now viewed along a continuum.
In landmark research in the mid-1990s, investigators compared cervical lengths, measured via transvaginal ultrasonography (TVUS), between two groups of pregnant women—those who had a history of preterm birth and those who did not.
The result? Gestational age at delivery in the first pregnancy correlated significantly—and continuously—with cervical length between 20 and 30 weeks’ gestation in the subsequent pregnancy.2 Investigators also observed that the risk of spontaneous preterm birth increased with decreasing cervical length. A length of 25 mm (10th percentile) offered a clinically appropriate threshold for identification of the risk of preterm delivery.3
The value of cervical-length measurement lies in its high negative predictive value for recurrent spontaneous preterm birth.4 As a general rule of thumb, routine assessment of cervical length in asymptomatic women who do not have a history of preterm birth is not recommended because of the high rate of false-positive results and the low positive predictive value for preterm delivery. We also lack an evidence-based consensus on how to manage an abnormally short cervix in these women.
CASE RESOLVED
The patient is counseled to watch for signs of preterm labor and to remain sedentary. Her pregnancy progresses without incident until 40 weeks, when she undergoes induction of labor for oligohydramnios and delivers a healthy infant weighing 3,855 g.
When a short cervix is detected at less than 20 weeks
If a woman has a history of spontaneous preterm birth, a short cervix this early in gestation raises the question of cervical insufficiency. No objective criteria have been devised to identify this condition. Nor is there a widely accepted definition. A reasonable description does exist, however:
- …a clinical diagnosis characterized by recurrent painless dilation and spontaneous midtrimester birth, generally in the absence of predisposing conditions such as spontaneous membrane rupture, bleeding, and infection, characteristics that shift the presumed underlying cause away from cervical incompetence and support other components of the preterm birth syndrome.5
A patient who fits this description may be a candidate for cervical cerclage. Alternatively, it is reasonable to reassess the patient in 3 to 7 days, after restricting physical activity, keeping in mind the pitfalls of TVUS assessment of the lower uterine segment in early gestation (see the box on imaging). If cervical length remains short, consider cerclage.6,7
Watch for inflammation
Occasionally, echogenic material is observed in the amniotic fluid at the level of a short cervix. This debris is an inflammatory exudate of fibrin, white blood cells, and bacteria. The presence of this sludge (FIGURE 3) signifies a risk of preterm birth much greater than that associated with a short cervix alone.8 Because cerclage in the presence of inflammation may further heighten the risk of spontaneous preterm birth, I recommend caution.

FIGURE 3 Watch for signs of inflammation
When inflammatory exudate (asterisk) is identified at the level of a short cervix, the risk of spontaneous preterm birth is elevated beyond the risk associated with a short cervix alone.
When a short cervix is detected between 20 and 24 weeks
CASE 2: Is recurrent preterm birth likely?
A 30-year-old woman 21 weeks pregnant with her second child reports for TVUS. Because her first child was delivered preterm at 30 weeks, she has been undergoing periodic measurement of her cervix. Until today, it has been longer than 25 mm, but now it is 20 mm. What is the best strategy to avert another preterm birth?
If a short cervix (<25 mm) is noted in a high-risk patient at this gestational age, consider the possibility of preterm labor and ruptured membranes. If these conditions are present, they should be managed according to existing guidelines.4,9 If they are absent, consider cerclage.
A recent meta-analysis of randomized trials of cerclage for the prevention of preterm birth in a singleton, high-risk pregnancy with a short cervix suggests that cerclage is associated with a significantly lower risk of delivery before 35 weeks’ gestation (relative risk [RR], 0.61; 95% confidence interval [CI], 0.40–0.92). Among singleton pregnancies involving both a short cervix and a history of midtrimester loss, cerclage is again associated with a reduced likelihood of delivery before 35 weeks (39% vs 23.4%; number needed to treat [NNT], 8; RR, 0.57; 95% CI, 0.33–0.99).10 A recent randomized trial of cerclage versus no cerclage in women who had a history of spontaneous preterm birth produced similar findings.11 This trial is described in detail in the box.
Another randomized trial compared the relative merits of ultrasound-indicated cerclage for a cervix shorter than 20 mm to a history-indicated cerclage among women with a prior spontaneous preterm birth between 16 and 34 weeks. Thirty-nine of 123 women randomized to the former group received a cerclage, as did 25 of 125 subjects in the latter group. There were no significant differences in the primary outcome of delivery before 34 weeks or secondary measures of loss before 24 weeks, preterm premature membrane rupture, mean gestational age at delivery, or neonatal outcomes. This study was not designed or powered to evaluate the efficacy of cerclage in preventing recurrent, spontaneous preterm birth. It simply compares two ways of selecting high-risk women for cerclage.12
We lack data supporting placement of cerclage for an incidentally detected short cervix in women who lack a history of spontaneous preterm birth or midtrimester loss.7
Other groups known to be at increased risk of spontaneous preterm birth include women carrying twins and women who have undergone cervical cone biopsy or loop electrosurgical excision procedure (LEEP). Among twin gestations, cerclage for a short cervix is associated with an increased rate of preterm birth (<35 weeks).10 Cerclage for a short cervix has not been evaluated among women who have a history of LEEP.6
CASE RESOLVED
After preterm labor is ruled out, the patient is counseled about her options and chooses cervical cerclage. Her pregnancy proceeds uneventfully until 36 weeks’ gestation, when she delivers a healthy infant weighing 2,950 g.
When a short cervix is detected between 24 and 34 weeks
CASE 3: Is it preterm labor?
A primigravida at 31 weeks’ gestation presents to the labor floor reporting regular contractions. She denies bleeding or rupture of membranes. Contractions are noted at 3-minute intervals, and digital cervical examination reveals that she is dilated 1 cm, with 70% effacement. TVUS determines cervical length to be 17 mm.
How should she be managed?
From 24 to 34 weeks’ gestation, the prevention, diagnosis, and treatment of preterm labor become the main concerns. Because our ability to predict and prevent preterm birth is limited, clinical management focuses on a reliable diagnosis of preterm labor to allow for selective, timely interventions to optimize neonatal outcomes. These interventions include tocolysis to permit maternal transport; antibiotic prophylaxis for group B strep; and steroid administration to accelerate fetal lung maturity. Equally important is the ability to reliably rule out preterm labor among symptomatic (contracting) women to avoid the potential morbidity, cost, and inconvenience of these interventions.
Before 37 weeks, a diagnosis of preterm labor requires the following findings:
- six or more contractions per hour
- cervical dilation, as identified by digital examination, of at least 3 cm and 80% effacement.
This diagnosis is more reliable when ruptured membranes or vaginal bleeding are present.
The significance of contractions without these findings is less clear. Therefore, we follow an algorithm that incorporates TVUS measurement of cervical length and evaluation of fetal fibronectin (fFN) (FIGURE 4).13
fFN is a glycoprotein that is normally confined to the extracellular matrix of the fetal membranes between 24 and 34 weeks’ gestation. Detection of fFN in cervicovaginal secretions during this window is associated with an increased risk of spontaneous preterm birth, whereas its absence demonstrates a negative predictive value for delivery within 7 days of testing of more than 97%.
During initial assessment of a regularly contracting preterm patient, perform a vaginal speculum examination. If the membranes are intact, use a vaginal swab to assess the patient for the presence of fFN, and set the specimen aside. Also obtain a culture for group B strep.
If a digital cervical examination and the contraction pattern establish a diagnosis of preterm labor, administer a tocolytic, When fetal prophylactic antibiotics, and steroids. If the diagnosis remains unclear, evaluate the cervix via TVUS. A cervical length above 30 mm effectively rules out preterm labor and obviates the need to send the fFN swab for assessment. As a result, the patient can be managed expectantly.
In contrast, a cervical length below 20 mm effectively confirms the diagnosis of preterm labor, and treatment can proceed. Again, the fFN swab may be discarded. The swab is sent for processing only if cervical length is 20 mm to 30 mm (FIGURE 4). A positive fFN result leads to the presumptive diagnosis of preterm labor, whereas a negative result permits expectant management.
We also recommend that women who display symptoms of preterm labor be screened for asymptomatic bacteriuria. Identification and treatment of this condition significantly reduce the risk of preterm delivery (RR, 0.56; 95% CI, 0.43–0.73).14 In addition, diagnosis and treatment of bacterial vaginosis in symptomatic women who have a history of spontaneous preterm birth can also reduce the risk of recurrent preterm delivery (RR, 0.42; 95% CI, 0.27–0.67).15
Not all obstetric care providers have the resources necessary for TVUS assessment of cervical length. When that is the case, fFN offers high sensitivity and negative predictive value and can help guide initial clinical decision-making. Keep in mind, however, that not all facilities offer fFN testing. In addition, in some cases, cervical manipulation may have occurred before fFN testing was performed, precluding its validity. In such cases, the incorporation of TVUS assessment of cervical length into clinical evaluation may help guide decision-making.

FIGURE 4 How to identify preterm labor at 24 to 34 weeks
CASE RESOLVED
The determination of short cervical length (17 mm) by TVUS confirms the diagnosis of preterm labor. The patient is admitted to the hospital and treated with a tocolytic, prophylactic antibiotics, and steroids. Three days later, preterm labor recurs, and she delivers an otherwise healthy infant. Future pregnancies will be managed according to the algorithm presented in FIGURE 5.
Cerclage may benefit women who have a history of spontaneous preterm birth
A recent multicenter randomized trial evaluated the efficacy of cerclage in preventing preterm birth among 302 women who had a history of spontaneous preterm birth before 34 weeks’ gestation.11 Any woman who had a cervix shorter than 25 mm between 16-0/7 and 22-6/7 weeks’ gestation was randomized to cerclage or no cerclage.
Cerclage did not significantly reduce preterm delivery before 35 weeks’ gestation, the primary outcome. Thirty-two percent of women who received cerclage and 42% of women who did not receive cerclage delivered before 35 weeks (P = .09). However, among women who had a cervix shorter than 15 mm at randomization, cerclage reduced the rate of delivery before 35 weeks by more than 75% (P = .006). Cerclage also reduced the rate of spontaneous birth before 37 weeks, compared with no cerclage (45% vs 60%; P = .01), as well as the rate of previable preterm birth before 24 weeks (6.1% vs 14%; P = .03) and the rate of perinatal death (8.8% vs 16%; P = .046).
As this study demonstrates, ultrasonographically indicated cerclage produces a number of highly clinically significant benefits in women who have a history of spontaneous preterm birth. Another important question is whether supplemental progesterone offers additional benefit beyond that conferred by cerclage in this population.
The role of progesterone in preventing preterm birth
CASE 4: History of preterm and term delivery
A woman in her third pregnancy is referred for placement of cervical cerclage, based on her obstetric history. Her first pregnancy was marked by preterm labor at 26 weeks, resulting in spontaneous preterm birth at 28 weeks. In her second pregnancy, she had cerclage placed electively at 13 weeks and delivered spontaneously at 40 weeks with no complications of pregnancy.
Is another cerclage indicated—or would progesterone be more effective?
Supplemental 17-hydroxyprogesterone caproate, given weekly in an intramuscular dosage of 250 mg, significantly reduced the rate of recurrent spontaneous preterm birth when it was administered from 16 to 36 weeks’ gestation in women carrying a singleton fetus.16 The protective effect of progesterone is most apparent in women who have a history of very preterm delivery (<32 weeks). No such benefit has been observed in twin and triplet gestations, however.17-19
Among women who do benefit from progesterone, the effect may vary. For this reason, researchers have explored the measurement of midtrimester cervical length as a means of stratifying response to progesterone.
For example, investigators randomized women who had a history of spontaneous preterm birth to daily treatment with 90 mg of vaginal progesterone gel or placebo, starting between 16 and 22-6/7 weeks and continuing until 37 weeks or delivery, whichever came first. No difference in the rate of delivery at or before 32 weeks was observed. However, a secondary analysis among progesterone-treated women who had a cervical length below 28 mm found significant declines in the rate of delivery at or before 32 weeks, admission to a NICU, and length of stay.20,21
In another trial, investigators assessed cervical length via TVUS between 20 and 25 weeks’ gestation in a general obstetric population that included women carrying twins. Women who had a cervical length below 15 mm were offered randomization to daily oral progesterone (200 mg) or placebo from 24 weeks to 33-6/7 weeks’ gestation. The rate of spontaneous preterm birth was significantly lower in the progesterone group (19% vs 34%).22
Because the ideal formulation of progesterone for the prevention of preterm birth is unknown, ACOG recommends restricting its use to women who have a documented history of spontaneous preterm birth at less than 37 weeks’ gestation.23 My practice follows the protocol of Meis and colleagues within the framework of an overall systematic algorithm (FIGURE 5).

FIGURE 5 Cervical length in the second trimester
*For a singleton gestation only.
CASE RESOLVED
The ObGyn reviews the patient’s obstetric history and determines that the first pregnancy was more suggestive of preterm labor than cervical insufficiency. Therefore, the ObGyn opts for progesterone rather than cerclage to prolong the gestation. The patient begins weekly injections of 17-hydroxyprogesterone caproate, starting at 16 weeks’ gestation, with TVUS measurement of cervical length every 2 weeks. The cervix remains longer than 25 mm through 24 weeks’ gestation, at which time TVUS assessment is stopped. Progesterone injections continue through 36 weeks, and the patient spontaneously delivers a healthy 3,700-g infant at term.
1. Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2007. National vital statistics reports; 2009;57(12). National Center for Health Statistics Web site. http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf. March 18, 2009. Accessed March 30, 2010.
2. Iams JD, Johnson FF, Sonek J, et al. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172(4 pt 1):1097-1106.
3. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
4. Clinical Management Guidelines for Obstetrician-Gynecologists. No. 43, May 2003: Management of Preterm Labor. Obstet Gynecol. 2003;101(5 pt 1):1039-1047.
5. Owen J, Iams JD, Hauth JC. Vaginal sonography and cervical incompetence. Am J Obstet Gynecol. 2003;188(2):586-596.
6. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100(6):1313-1327.
7. Clinical Management Guidelines for Obstetrician-Gynecologists. No. 48, Nov 2003: Cervical insufficiency. Obstet Gynecol. 2003;102(5 pt 1):1091-1099.
8. Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706-714.
9. ACOG Practice Bulletin No. 80. Premature rupture of membranes. Obstet Gynecol. 2007;109(4):1007-1020.DOI:10.1097/01.AOG.0000263888.69178.1f.
10. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106(1):181-189.
11. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened mid-trimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-e8.
12. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized control trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-e6.
13. Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol. 2003;101(2):402-412.
14. Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73(4):576-582.
15. Leitich H, Brunbauer M, Bodnar-Aderl B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol. 2003;188(3):752-758.
16. Meis PJ, Klebanoff M, Thom E, et al. For National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm birth by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
17. Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034-2040.
18. Rouse DJ, Caritis SN, Peaceman AM, et al. For National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454-461.
19. Caritis SN, Rouse DJ, Peaceman AM, et al. for Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Medicine Units Network (MFMU). Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113(2 pt 1):285-292.
20. O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):687-696.
21. DeFranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):697-705.
22. Fonseca EB, Celik E, Parra M, et al. For Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462-469.
23. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963.-
24. Yost NP, Bloom SL, Twickler DM, Leveno KJ. Pitfalls in ultrasonic cervical length measurement for predicting preterm birth. Obstet Gynecol. 1999;93(4):510-516.
1. Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2007. National vital statistics reports; 2009;57(12). National Center for Health Statistics Web site. http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf. March 18, 2009. Accessed March 30, 2010.
2. Iams JD, Johnson FF, Sonek J, et al. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172(4 pt 1):1097-1106.
3. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
4. Clinical Management Guidelines for Obstetrician-Gynecologists. No. 43, May 2003: Management of Preterm Labor. Obstet Gynecol. 2003;101(5 pt 1):1039-1047.
5. Owen J, Iams JD, Hauth JC. Vaginal sonography and cervical incompetence. Am J Obstet Gynecol. 2003;188(2):586-596.
6. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100(6):1313-1327.
7. Clinical Management Guidelines for Obstetrician-Gynecologists. No. 48, Nov 2003: Cervical insufficiency. Obstet Gynecol. 2003;102(5 pt 1):1091-1099.
8. Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706-714.
9. ACOG Practice Bulletin No. 80. Premature rupture of membranes. Obstet Gynecol. 2007;109(4):1007-1020.DOI:10.1097/01.AOG.0000263888.69178.1f.
10. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106(1):181-189.
11. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened mid-trimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-e8.
12. Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized control trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol. 2009;200(6):623.e1-e6.
13. Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol. 2003;101(2):402-412.
14. Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73(4):576-582.
15. Leitich H, Brunbauer M, Bodnar-Aderl B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol. 2003;188(3):752-758.
16. Meis PJ, Klebanoff M, Thom E, et al. For National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm birth by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
17. Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034-2040.
18. Rouse DJ, Caritis SN, Peaceman AM, et al. For National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454-461.
19. Caritis SN, Rouse DJ, Peaceman AM, et al. for Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Medicine Units Network (MFMU). Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113(2 pt 1):285-292.
20. O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):687-696.
21. DeFranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):697-705.
22. Fonseca EB, Celik E, Parra M, et al. For Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462-469.
23. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963.-
24. Yost NP, Bloom SL, Twickler DM, Leveno KJ. Pitfalls in ultrasonic cervical length measurement for predicting preterm birth. Obstet Gynecol. 1999;93(4):510-516.


