User login
FDA approves IV formulation of drug for CINV
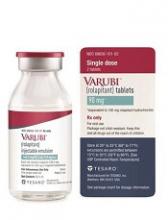
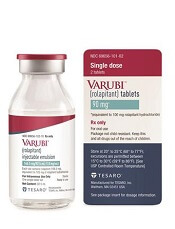
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
Hemophilia B therapy approved in Saudi Arabia
The Saudi Food and Drug Authority has approved eftrenonacog alfa (Alprolix®) for the treatment of hemophilia B in the Kingdom of Saudi Arabia.
Eftrenonacog alfa is a recombinant factor IX Fc fusion protein indicated for on-demand treatment and prophylaxis in hemophilia B patients of all ages.
Eftrenonacog alfa is engineered by fusing factor IX to the Fc portion of immunoglobulin G subclass 1. This enables eftrenonacog alfa to use a naturally occurring pathway to prolong the time the therapy remains in the body.
Eftrenonacog alfa is approved to treat hemophilia B in the European Union, Iceland, Liechtenstein, Kuwait, Norway, and Switzerland (where it is marketed by Sobi). The product is also approved in the US, Canada, Japan, Australia, New Zealand, and other countries (where it is marketed by Bioverativ).
The approvals of eftrenonacog alfa are based on results from a pair of phase 3 trials—the B-LONG study and the Kids B-LONG study.
B-LONG study
The B-LONG study included 123 males with severe hemophilia B who were 12 years of age or older. They had no current or previous factor IX inhibitors and a history of 100 or more documented prior exposure days to factor IX products.
Patients received eftrenonacog alfa in 1 of 4 treatment arms:
- Weekly prophylaxis starting at 50 IU/kg, with pharmacokinetic (PK)-driven dose adjustments (n=63)
- Individualized interval prophylaxis starting at 100 IU/kg every 10 days, with PK-driven interval adjustments (n=29)
- On-demand treatment at 20 IU/kg to 100 IU/kg (n=27)
- Perioperative management (n=12, including 8 from arms 1-3).
Researchers assessed control of bleeding in all patients who experienced a bleeding episode while on study. In total, 90.4% of bleeding episodes were controlled by a single injection of eftrenonacog alfa.
The overall median annualized bleeding rates (ABRs)—including spontaneous and traumatic bleeds—were 2.95 in the weekly prophylaxis arm, 1.38 in the individualized interval prophylaxis arm, and 17.69 in the episodic treatment arm.
The perioperative management arm consisted of 12 patients undergoing 14 major surgical procedures. The treating physicians rated the hemostatic efficacy of eftrenonacog alfa as “excellent” or “good” in all surgeries.
Eftrenonacog alfa was considered generally well-tolerated. None of the patients developed inhibitors, and none reported anaphylaxis.
The most common adverse events—with an incidence of 5% or greater—occurring outside of the perioperative management arm were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event may have been drug-related. The patient experienced obstructive uropathy in the setting of hematuria. However, he continued to receive eftrenonacog alfa, and the event resolved with medical management.
Kids B-LONG
In Kids B-LONG, researchers tested eftrenonacog alfa in 30 previously treated males younger than 12 who had severe hemophilia B. Patients had at least 50 prior exposure days to factor IX therapies.
Children who received eftrenonacog alfa prophylactically had an overall median ABR of 1.97. The median ABR for spontaneous joint bleeds was 0.
Approximately 33% of patients did not experience any bleeding episodes. About 92% of bleeding episodes were controlled by 1 or 2 injections of eftrenonacog alfa.
None of the patients developed inhibitors. There were no treatment-related serious adverse events and no cases of serious allergic reactions or vascular thrombotic events.
None of the patients discontinued the study due to an adverse event. One adverse event—decreased appetite occurring in 1 patient—was considered related to eftrenonacog alfa.
The pattern of treatment-emergent adverse events in this study was generally consistent with results seen in adolescents and adults in the B-LONG study. ![]()
The Saudi Food and Drug Authority has approved eftrenonacog alfa (Alprolix®) for the treatment of hemophilia B in the Kingdom of Saudi Arabia.
Eftrenonacog alfa is a recombinant factor IX Fc fusion protein indicated for on-demand treatment and prophylaxis in hemophilia B patients of all ages.
Eftrenonacog alfa is engineered by fusing factor IX to the Fc portion of immunoglobulin G subclass 1. This enables eftrenonacog alfa to use a naturally occurring pathway to prolong the time the therapy remains in the body.
Eftrenonacog alfa is approved to treat hemophilia B in the European Union, Iceland, Liechtenstein, Kuwait, Norway, and Switzerland (where it is marketed by Sobi). The product is also approved in the US, Canada, Japan, Australia, New Zealand, and other countries (where it is marketed by Bioverativ).
The approvals of eftrenonacog alfa are based on results from a pair of phase 3 trials—the B-LONG study and the Kids B-LONG study.
B-LONG study
The B-LONG study included 123 males with severe hemophilia B who were 12 years of age or older. They had no current or previous factor IX inhibitors and a history of 100 or more documented prior exposure days to factor IX products.
Patients received eftrenonacog alfa in 1 of 4 treatment arms:
- Weekly prophylaxis starting at 50 IU/kg, with pharmacokinetic (PK)-driven dose adjustments (n=63)
- Individualized interval prophylaxis starting at 100 IU/kg every 10 days, with PK-driven interval adjustments (n=29)
- On-demand treatment at 20 IU/kg to 100 IU/kg (n=27)
- Perioperative management (n=12, including 8 from arms 1-3).
Researchers assessed control of bleeding in all patients who experienced a bleeding episode while on study. In total, 90.4% of bleeding episodes were controlled by a single injection of eftrenonacog alfa.
The overall median annualized bleeding rates (ABRs)—including spontaneous and traumatic bleeds—were 2.95 in the weekly prophylaxis arm, 1.38 in the individualized interval prophylaxis arm, and 17.69 in the episodic treatment arm.
The perioperative management arm consisted of 12 patients undergoing 14 major surgical procedures. The treating physicians rated the hemostatic efficacy of eftrenonacog alfa as “excellent” or “good” in all surgeries.
Eftrenonacog alfa was considered generally well-tolerated. None of the patients developed inhibitors, and none reported anaphylaxis.
The most common adverse events—with an incidence of 5% or greater—occurring outside of the perioperative management arm were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event may have been drug-related. The patient experienced obstructive uropathy in the setting of hematuria. However, he continued to receive eftrenonacog alfa, and the event resolved with medical management.
Kids B-LONG
In Kids B-LONG, researchers tested eftrenonacog alfa in 30 previously treated males younger than 12 who had severe hemophilia B. Patients had at least 50 prior exposure days to factor IX therapies.
Children who received eftrenonacog alfa prophylactically had an overall median ABR of 1.97. The median ABR for spontaneous joint bleeds was 0.
Approximately 33% of patients did not experience any bleeding episodes. About 92% of bleeding episodes were controlled by 1 or 2 injections of eftrenonacog alfa.
None of the patients developed inhibitors. There were no treatment-related serious adverse events and no cases of serious allergic reactions or vascular thrombotic events.
None of the patients discontinued the study due to an adverse event. One adverse event—decreased appetite occurring in 1 patient—was considered related to eftrenonacog alfa.
The pattern of treatment-emergent adverse events in this study was generally consistent with results seen in adolescents and adults in the B-LONG study. ![]()
The Saudi Food and Drug Authority has approved eftrenonacog alfa (Alprolix®) for the treatment of hemophilia B in the Kingdom of Saudi Arabia.
Eftrenonacog alfa is a recombinant factor IX Fc fusion protein indicated for on-demand treatment and prophylaxis in hemophilia B patients of all ages.
Eftrenonacog alfa is engineered by fusing factor IX to the Fc portion of immunoglobulin G subclass 1. This enables eftrenonacog alfa to use a naturally occurring pathway to prolong the time the therapy remains in the body.
Eftrenonacog alfa is approved to treat hemophilia B in the European Union, Iceland, Liechtenstein, Kuwait, Norway, and Switzerland (where it is marketed by Sobi). The product is also approved in the US, Canada, Japan, Australia, New Zealand, and other countries (where it is marketed by Bioverativ).
The approvals of eftrenonacog alfa are based on results from a pair of phase 3 trials—the B-LONG study and the Kids B-LONG study.
B-LONG study
The B-LONG study included 123 males with severe hemophilia B who were 12 years of age or older. They had no current or previous factor IX inhibitors and a history of 100 or more documented prior exposure days to factor IX products.
Patients received eftrenonacog alfa in 1 of 4 treatment arms:
- Weekly prophylaxis starting at 50 IU/kg, with pharmacokinetic (PK)-driven dose adjustments (n=63)
- Individualized interval prophylaxis starting at 100 IU/kg every 10 days, with PK-driven interval adjustments (n=29)
- On-demand treatment at 20 IU/kg to 100 IU/kg (n=27)
- Perioperative management (n=12, including 8 from arms 1-3).
Researchers assessed control of bleeding in all patients who experienced a bleeding episode while on study. In total, 90.4% of bleeding episodes were controlled by a single injection of eftrenonacog alfa.
The overall median annualized bleeding rates (ABRs)—including spontaneous and traumatic bleeds—were 2.95 in the weekly prophylaxis arm, 1.38 in the individualized interval prophylaxis arm, and 17.69 in the episodic treatment arm.
The perioperative management arm consisted of 12 patients undergoing 14 major surgical procedures. The treating physicians rated the hemostatic efficacy of eftrenonacog alfa as “excellent” or “good” in all surgeries.
Eftrenonacog alfa was considered generally well-tolerated. None of the patients developed inhibitors, and none reported anaphylaxis.
The most common adverse events—with an incidence of 5% or greater—occurring outside of the perioperative management arm were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event may have been drug-related. The patient experienced obstructive uropathy in the setting of hematuria. However, he continued to receive eftrenonacog alfa, and the event resolved with medical management.
Kids B-LONG
In Kids B-LONG, researchers tested eftrenonacog alfa in 30 previously treated males younger than 12 who had severe hemophilia B. Patients had at least 50 prior exposure days to factor IX therapies.
Children who received eftrenonacog alfa prophylactically had an overall median ABR of 1.97. The median ABR for spontaneous joint bleeds was 0.
Approximately 33% of patients did not experience any bleeding episodes. About 92% of bleeding episodes were controlled by 1 or 2 injections of eftrenonacog alfa.
None of the patients developed inhibitors. There were no treatment-related serious adverse events and no cases of serious allergic reactions or vascular thrombotic events.
None of the patients discontinued the study due to an adverse event. One adverse event—decreased appetite occurring in 1 patient—was considered related to eftrenonacog alfa.
The pattern of treatment-emergent adverse events in this study was generally consistent with results seen in adolescents and adults in the B-LONG study. ![]()
CHMP recommends new formulation of pegaspargase
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for lyophilized pegaspargase (ONCASPAR).
If approved, the product would be used as a component of antineoplastic therapy in patients of all ages who have acute lymphoblastic leukemia (ALL).
The product is a freeze-dried formulation of liquid pegaspargase, which is already approved for the aforementioned indication.
The CHMP’s recommendation regarding lyophilized pegaspargase will be submitted to the European Commission (EC).
The EC typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The EC’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation regarding lyophilized pegaspargase is based on analytical and nonclinical studies, which indicate that lyophilized pegaspargase is comparable to the liquid formulation.
Once reconstituted, lyophilized pegaspargase demonstrates similar pharmacokinetics and pharmacodynamics as liquid pegaspargase.
“Lyophilized ONCASPAR builds on more than a decade of data and research with liquid ONCASPAR, and, with no change in dosing regimen, it offers a 3-times longer shelf life,” said Howard B. Mayer, MD, of Shire, the company that developed lyophilized pegaspargase.
“Prolonging shelf life to 24 months for this critically important therapy facilitates management of product inventory by enabling greater flexibility and longer-term planning. Once approved, with the extended shelf life of lyophilized ONCASPAR, we also hope to improve access to the medicine for ALL patients in countries currently not offering liquid ONCASPAR.”
Lyophilized pegaspargase works in the same way as the liquid formulation. It rapidly depletes serum L-asparagine levels and interferes with protein synthesis, thereby depriving lymphoblasts of asparaginase and resulting in cell death. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for lyophilized pegaspargase (ONCASPAR).
If approved, the product would be used as a component of antineoplastic therapy in patients of all ages who have acute lymphoblastic leukemia (ALL).
The product is a freeze-dried formulation of liquid pegaspargase, which is already approved for the aforementioned indication.
The CHMP’s recommendation regarding lyophilized pegaspargase will be submitted to the European Commission (EC).
The EC typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The EC’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation regarding lyophilized pegaspargase is based on analytical and nonclinical studies, which indicate that lyophilized pegaspargase is comparable to the liquid formulation.
Once reconstituted, lyophilized pegaspargase demonstrates similar pharmacokinetics and pharmacodynamics as liquid pegaspargase.
“Lyophilized ONCASPAR builds on more than a decade of data and research with liquid ONCASPAR, and, with no change in dosing regimen, it offers a 3-times longer shelf life,” said Howard B. Mayer, MD, of Shire, the company that developed lyophilized pegaspargase.
“Prolonging shelf life to 24 months for this critically important therapy facilitates management of product inventory by enabling greater flexibility and longer-term planning. Once approved, with the extended shelf life of lyophilized ONCASPAR, we also hope to improve access to the medicine for ALL patients in countries currently not offering liquid ONCASPAR.”
Lyophilized pegaspargase works in the same way as the liquid formulation. It rapidly depletes serum L-asparagine levels and interferes with protein synthesis, thereby depriving lymphoblasts of asparaginase and resulting in cell death. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for lyophilized pegaspargase (ONCASPAR).
If approved, the product would be used as a component of antineoplastic therapy in patients of all ages who have acute lymphoblastic leukemia (ALL).
The product is a freeze-dried formulation of liquid pegaspargase, which is already approved for the aforementioned indication.
The CHMP’s recommendation regarding lyophilized pegaspargase will be submitted to the European Commission (EC).
The EC typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The EC’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation regarding lyophilized pegaspargase is based on analytical and nonclinical studies, which indicate that lyophilized pegaspargase is comparable to the liquid formulation.
Once reconstituted, lyophilized pegaspargase demonstrates similar pharmacokinetics and pharmacodynamics as liquid pegaspargase.
“Lyophilized ONCASPAR builds on more than a decade of data and research with liquid ONCASPAR, and, with no change in dosing regimen, it offers a 3-times longer shelf life,” said Howard B. Mayer, MD, of Shire, the company that developed lyophilized pegaspargase.
“Prolonging shelf life to 24 months for this critically important therapy facilitates management of product inventory by enabling greater flexibility and longer-term planning. Once approved, with the extended shelf life of lyophilized ONCASPAR, we also hope to improve access to the medicine for ALL patients in countries currently not offering liquid ONCASPAR.”
Lyophilized pegaspargase works in the same way as the liquid formulation. It rapidly depletes serum L-asparagine levels and interferes with protein synthesis, thereby depriving lymphoblasts of asparaginase and resulting in cell death. ![]()
Japan approves product for hemophilia A
Japan’s Ministry of Health, Labor and Welfare has approved lonoctocog alfa (AFSTYLA®), a recombinant single-chain coagulation factor VIII product, for use in patients with hemophilia A.
The product is approved for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand treatment and control of bleeding, and for perioperative management.
Lonoctocog alfa is the first and only single-chain recombinant factor VIII product specifically designed to treat hemophilia A.
According to CSL Behring, the company developing lonoctocog alfa, the product was designed to provide greater molecular stability and longer duration of action. Lonoctocog alfa uses a covalent bond to form one structural entity, a single polypeptide chain, to improve the stability of factor VIII and provide factor VIII activity with the option of twice-weekly dosing.
Lonoctocog alfa is also approved in the European Union, US, Canada, Switzerland, and Australia.
AFFINITY trials
Japan’s approval of lonoctocog alfa is based on results from the AFFINITY clinical development program, which includes a trial of children (n=84) and a trial of adolescents and adults (n=175).
Among patients who received lonoctocog alfa prophylactically in these trials, the median annualized bleeding rate was 1.14 in the adults/adolescents and 3.69 in children younger than 12.
In all, there were 1195 bleeding events—848 in the adults/adolescents and 347 in the children.
Ninety-four percent of bleeds in adults/adolescents and 96% of bleeds in pediatric patients were effectively controlled with no more than 2 infusions of lonoctocog alfa weekly.
Eighty-one percent of bleeds in adults/adolescents and 86% of bleeds in pediatric patients were controlled by a single infusion.
Researchers assessed safety in 258 patients from both studies. Adverse reactions occurred in 14 patients and included hypersensitivity (n=4), dizziness (n=2), paresthesia (n=1), rash (n=1), erythema (n=1), pruritus (n=1), pyrexia (n=1), injection-site pain (n=1), chills (n=1), and feeling hot (n=1).
One patient withdrew from treatment due to hypersensitivity.
None of the patients developed neutralizing antibodies to factor VIII or antibodies to host cell proteins. There were no reports of anaphylaxis or thrombosis.
Results from the trial of adolescents/adults were published in Blood in August 2016. Results from the trial of children were published in the Journal of Thrombosis and Haemostasis in March 2017. ![]()
Japan’s Ministry of Health, Labor and Welfare has approved lonoctocog alfa (AFSTYLA®), a recombinant single-chain coagulation factor VIII product, for use in patients with hemophilia A.
The product is approved for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand treatment and control of bleeding, and for perioperative management.
Lonoctocog alfa is the first and only single-chain recombinant factor VIII product specifically designed to treat hemophilia A.
According to CSL Behring, the company developing lonoctocog alfa, the product was designed to provide greater molecular stability and longer duration of action. Lonoctocog alfa uses a covalent bond to form one structural entity, a single polypeptide chain, to improve the stability of factor VIII and provide factor VIII activity with the option of twice-weekly dosing.
Lonoctocog alfa is also approved in the European Union, US, Canada, Switzerland, and Australia.
AFFINITY trials
Japan’s approval of lonoctocog alfa is based on results from the AFFINITY clinical development program, which includes a trial of children (n=84) and a trial of adolescents and adults (n=175).
Among patients who received lonoctocog alfa prophylactically in these trials, the median annualized bleeding rate was 1.14 in the adults/adolescents and 3.69 in children younger than 12.
In all, there were 1195 bleeding events—848 in the adults/adolescents and 347 in the children.
Ninety-four percent of bleeds in adults/adolescents and 96% of bleeds in pediatric patients were effectively controlled with no more than 2 infusions of lonoctocog alfa weekly.
Eighty-one percent of bleeds in adults/adolescents and 86% of bleeds in pediatric patients were controlled by a single infusion.
Researchers assessed safety in 258 patients from both studies. Adverse reactions occurred in 14 patients and included hypersensitivity (n=4), dizziness (n=2), paresthesia (n=1), rash (n=1), erythema (n=1), pruritus (n=1), pyrexia (n=1), injection-site pain (n=1), chills (n=1), and feeling hot (n=1).
One patient withdrew from treatment due to hypersensitivity.
None of the patients developed neutralizing antibodies to factor VIII or antibodies to host cell proteins. There were no reports of anaphylaxis or thrombosis.
Results from the trial of adolescents/adults were published in Blood in August 2016. Results from the trial of children were published in the Journal of Thrombosis and Haemostasis in March 2017. ![]()
Japan’s Ministry of Health, Labor and Welfare has approved lonoctocog alfa (AFSTYLA®), a recombinant single-chain coagulation factor VIII product, for use in patients with hemophilia A.
The product is approved for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand treatment and control of bleeding, and for perioperative management.
Lonoctocog alfa is the first and only single-chain recombinant factor VIII product specifically designed to treat hemophilia A.
According to CSL Behring, the company developing lonoctocog alfa, the product was designed to provide greater molecular stability and longer duration of action. Lonoctocog alfa uses a covalent bond to form one structural entity, a single polypeptide chain, to improve the stability of factor VIII and provide factor VIII activity with the option of twice-weekly dosing.
Lonoctocog alfa is also approved in the European Union, US, Canada, Switzerland, and Australia.
AFFINITY trials
Japan’s approval of lonoctocog alfa is based on results from the AFFINITY clinical development program, which includes a trial of children (n=84) and a trial of adolescents and adults (n=175).
Among patients who received lonoctocog alfa prophylactically in these trials, the median annualized bleeding rate was 1.14 in the adults/adolescents and 3.69 in children younger than 12.
In all, there were 1195 bleeding events—848 in the adults/adolescents and 347 in the children.
Ninety-four percent of bleeds in adults/adolescents and 96% of bleeds in pediatric patients were effectively controlled with no more than 2 infusions of lonoctocog alfa weekly.
Eighty-one percent of bleeds in adults/adolescents and 86% of bleeds in pediatric patients were controlled by a single infusion.
Researchers assessed safety in 258 patients from both studies. Adverse reactions occurred in 14 patients and included hypersensitivity (n=4), dizziness (n=2), paresthesia (n=1), rash (n=1), erythema (n=1), pruritus (n=1), pyrexia (n=1), injection-site pain (n=1), chills (n=1), and feeling hot (n=1).
One patient withdrew from treatment due to hypersensitivity.
None of the patients developed neutralizing antibodies to factor VIII or antibodies to host cell proteins. There were no reports of anaphylaxis or thrombosis.
Results from the trial of adolescents/adults were published in Blood in August 2016. Results from the trial of children were published in the Journal of Thrombosis and Haemostasis in March 2017. ![]()
FDA improves public access to AE data
The US Food and Drug Administration (FDA) has launched a new search tool designed to improve access to data in the FDA’s Adverse Event Reporting System (FAERS).
FAERS includes data on adverse events (AEs) associated with drug and biologic products.
The new FAERs public dashboard allows users to search for and organize data by criteria such as drug/biological product, age of the patient, type of AE, year the AE occurred, or within a specific time frame.
The FDA intends for this tool to increase transparency by making it easier for people to see reports the agency receives.
The FDA hopes this, in turn, will spur the submission of more detailed and complete reports from consumers, healthcare professionals, and others.
The FDA uses FAERS for surveillance, such as looking for new safety concerns that might be related to a marketed product, evaluating a manufacturer’s compliance with reporting regulations, and responding to outside requests for information.
The reports in FAERS are evaluated by clinical reviewers in the FDA’s Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research to monitor the safety of products after they are marketed. If a potential safety concern is identified in FAERS, further evaluation is performed.
“Our focus on safety extends beyond approval,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
“In fact, our staff spends a lot of time looking at FAERS reports received regarding approved drug and biologic products, and these reports can be very valuable components of our safety assessments. By giving people a better understanding of these data, and the associated limitations, we hope the new interface will encourage people to submit more complete reports.”
The FDA encourages healthcare professionals and consumers to report AEs or quality problems related to drugs and biologics to the FDA’s MedWatch Adverse Event Reporting Program.
In addition to the FAERS database for drugs and biologics, the FDA has AE reporting programs and databases for foods, dietary supplements, and cosmetics (CFSAN Adverse Event Reporting System [CAERS]), medical devices (Manufacturer and User Facility Device Experience [MAUDE]), and vaccines (Vaccine Adverse Event Reporting System [VAERS], which the FDA co-manages with the Centers for Disease Control and Prevention). ![]()
The US Food and Drug Administration (FDA) has launched a new search tool designed to improve access to data in the FDA’s Adverse Event Reporting System (FAERS).
FAERS includes data on adverse events (AEs) associated with drug and biologic products.
The new FAERs public dashboard allows users to search for and organize data by criteria such as drug/biological product, age of the patient, type of AE, year the AE occurred, or within a specific time frame.
The FDA intends for this tool to increase transparency by making it easier for people to see reports the agency receives.
The FDA hopes this, in turn, will spur the submission of more detailed and complete reports from consumers, healthcare professionals, and others.
The FDA uses FAERS for surveillance, such as looking for new safety concerns that might be related to a marketed product, evaluating a manufacturer’s compliance with reporting regulations, and responding to outside requests for information.
The reports in FAERS are evaluated by clinical reviewers in the FDA’s Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research to monitor the safety of products after they are marketed. If a potential safety concern is identified in FAERS, further evaluation is performed.
“Our focus on safety extends beyond approval,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
“In fact, our staff spends a lot of time looking at FAERS reports received regarding approved drug and biologic products, and these reports can be very valuable components of our safety assessments. By giving people a better understanding of these data, and the associated limitations, we hope the new interface will encourage people to submit more complete reports.”
The FDA encourages healthcare professionals and consumers to report AEs or quality problems related to drugs and biologics to the FDA’s MedWatch Adverse Event Reporting Program.
In addition to the FAERS database for drugs and biologics, the FDA has AE reporting programs and databases for foods, dietary supplements, and cosmetics (CFSAN Adverse Event Reporting System [CAERS]), medical devices (Manufacturer and User Facility Device Experience [MAUDE]), and vaccines (Vaccine Adverse Event Reporting System [VAERS], which the FDA co-manages with the Centers for Disease Control and Prevention). ![]()
The US Food and Drug Administration (FDA) has launched a new search tool designed to improve access to data in the FDA’s Adverse Event Reporting System (FAERS).
FAERS includes data on adverse events (AEs) associated with drug and biologic products.
The new FAERs public dashboard allows users to search for and organize data by criteria such as drug/biological product, age of the patient, type of AE, year the AE occurred, or within a specific time frame.
The FDA intends for this tool to increase transparency by making it easier for people to see reports the agency receives.
The FDA hopes this, in turn, will spur the submission of more detailed and complete reports from consumers, healthcare professionals, and others.
The FDA uses FAERS for surveillance, such as looking for new safety concerns that might be related to a marketed product, evaluating a manufacturer’s compliance with reporting regulations, and responding to outside requests for information.
The reports in FAERS are evaluated by clinical reviewers in the FDA’s Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research to monitor the safety of products after they are marketed. If a potential safety concern is identified in FAERS, further evaluation is performed.
“Our focus on safety extends beyond approval,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
“In fact, our staff spends a lot of time looking at FAERS reports received regarding approved drug and biologic products, and these reports can be very valuable components of our safety assessments. By giving people a better understanding of these data, and the associated limitations, we hope the new interface will encourage people to submit more complete reports.”
The FDA encourages healthcare professionals and consumers to report AEs or quality problems related to drugs and biologics to the FDA’s MedWatch Adverse Event Reporting Program.
In addition to the FAERS database for drugs and biologics, the FDA has AE reporting programs and databases for foods, dietary supplements, and cosmetics (CFSAN Adverse Event Reporting System [CAERS]), medical devices (Manufacturer and User Facility Device Experience [MAUDE]), and vaccines (Vaccine Adverse Event Reporting System [VAERS], which the FDA co-manages with the Centers for Disease Control and Prevention). ![]()
Daratumumab combos approved to treat MM in Japan
The Ministry of Health, Labor and Welfare in Japan has approved the use of daratumumab (DARZALEX®) in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with relapsed or refractory multiple myeloma (MM).
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
The drug is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
The approval of daratumumab is based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The Ministry of Health, Labor and Welfare in Japan has approved the use of daratumumab (DARZALEX®) in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with relapsed or refractory multiple myeloma (MM).
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
The drug is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
The approval of daratumumab is based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The Ministry of Health, Labor and Welfare in Japan has approved the use of daratumumab (DARZALEX®) in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with relapsed or refractory multiple myeloma (MM).
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
The drug is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
The approval of daratumumab is based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
SCD drug receives rare pediatric disease designation
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to Altemia™ soft gelatin capsules for the treatment of children with sickle cell disease (SCD).
Altemia (formerly SC411) is being developed by Sancilio Pharmaceuticals Company, Inc. (SPCI) to treat SCD patients between the ages of 5 and 17 years.
Altemia consists of a mixture of fatty acids, primarily in the form of Ethyl Cervonate™ (a proprietary blend of docosahexaenoic acid and other omega-3 fatty acids), and surface active agents formulated using Advanced Lipid Technologies®.
According to SPCI, Advanced Lipid Technologies are proprietary formulation and manufacturing techniques used to create lipophilic drug products capable of increased bioavailability, avoidance of the first pass effect, and elimination of the food effects commonly associated with oral administration.
Altemia is designed to replenish the lipids destroyed by sickle hemoglobin. The product is intended to be taken once daily to reduce vaso-occlusive crises, anemia, organ damage, and other complications of SCD.
Altemia also has orphan drug designation from the FDA.
SPCI is currently conducting a phase 2 trial of Altemia. In this randomized, double-blind, placebo-controlled trial, researchers are evaluating the efficacy and safety of Altemia in pediatric patients with SCD.
The company plans to report top-line results from the study, known as the SCOT trial, early in the fourth quarter of this year.
About rare pediatric disease designation
Rare pediatric disease designation is granted to drugs that show promise to treat diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 or younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, if a drug with rare pediatric disease designation is approved, the drug’s developer may qualify for a voucher that can be redeemed to obtain priority review for any subsequent marketing application. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to Altemia™ soft gelatin capsules for the treatment of children with sickle cell disease (SCD).
Altemia (formerly SC411) is being developed by Sancilio Pharmaceuticals Company, Inc. (SPCI) to treat SCD patients between the ages of 5 and 17 years.
Altemia consists of a mixture of fatty acids, primarily in the form of Ethyl Cervonate™ (a proprietary blend of docosahexaenoic acid and other omega-3 fatty acids), and surface active agents formulated using Advanced Lipid Technologies®.
According to SPCI, Advanced Lipid Technologies are proprietary formulation and manufacturing techniques used to create lipophilic drug products capable of increased bioavailability, avoidance of the first pass effect, and elimination of the food effects commonly associated with oral administration.
Altemia is designed to replenish the lipids destroyed by sickle hemoglobin. The product is intended to be taken once daily to reduce vaso-occlusive crises, anemia, organ damage, and other complications of SCD.
Altemia also has orphan drug designation from the FDA.
SPCI is currently conducting a phase 2 trial of Altemia. In this randomized, double-blind, placebo-controlled trial, researchers are evaluating the efficacy and safety of Altemia in pediatric patients with SCD.
The company plans to report top-line results from the study, known as the SCOT trial, early in the fourth quarter of this year.
About rare pediatric disease designation
Rare pediatric disease designation is granted to drugs that show promise to treat diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 or younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, if a drug with rare pediatric disease designation is approved, the drug’s developer may qualify for a voucher that can be redeemed to obtain priority review for any subsequent marketing application. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to Altemia™ soft gelatin capsules for the treatment of children with sickle cell disease (SCD).
Altemia (formerly SC411) is being developed by Sancilio Pharmaceuticals Company, Inc. (SPCI) to treat SCD patients between the ages of 5 and 17 years.
Altemia consists of a mixture of fatty acids, primarily in the form of Ethyl Cervonate™ (a proprietary blend of docosahexaenoic acid and other omega-3 fatty acids), and surface active agents formulated using Advanced Lipid Technologies®.
According to SPCI, Advanced Lipid Technologies are proprietary formulation and manufacturing techniques used to create lipophilic drug products capable of increased bioavailability, avoidance of the first pass effect, and elimination of the food effects commonly associated with oral administration.
Altemia is designed to replenish the lipids destroyed by sickle hemoglobin. The product is intended to be taken once daily to reduce vaso-occlusive crises, anemia, organ damage, and other complications of SCD.
Altemia also has orphan drug designation from the FDA.
SPCI is currently conducting a phase 2 trial of Altemia. In this randomized, double-blind, placebo-controlled trial, researchers are evaluating the efficacy and safety of Altemia in pediatric patients with SCD.
The company plans to report top-line results from the study, known as the SCOT trial, early in the fourth quarter of this year.
About rare pediatric disease designation
Rare pediatric disease designation is granted to drugs that show promise to treat diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 or younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, if a drug with rare pediatric disease designation is approved, the drug’s developer may qualify for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
Midostaurin approved to treat AML, SM in Europe
The European Commission has approved the multi-targeted kinase inhibitor midostaurin (Rydapt®) to treat acute myeloid leukemia (AML) and 3 types of advanced systemic mastocytosis (SM).
Midostaurin is approved to treat adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive. In these patients, midostaurin can be used in combination with standard daunorubicin and cytarabine induction, followed by high-dose cytarabine consolidation. Patients who achieve a complete response can then receive midostaurin as maintenance therapy.
Midostaurin is also approved as monotherapy for adults with aggressive SM (ASM), SM with associated hematological neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin in AML
The approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were published in NEJM last month.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
The median overall survival was significantly longer in the midostaurin arm than the placebo arm—74.7 months and 25.6 months, respectively (hazard ratio=0.77, P=0.016).
And the median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infection.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The approval of midostaurin in advanced SM is based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3. Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 responses (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders.
Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage.
The European Commission has approved the multi-targeted kinase inhibitor midostaurin (Rydapt®) to treat acute myeloid leukemia (AML) and 3 types of advanced systemic mastocytosis (SM).
Midostaurin is approved to treat adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive. In these patients, midostaurin can be used in combination with standard daunorubicin and cytarabine induction, followed by high-dose cytarabine consolidation. Patients who achieve a complete response can then receive midostaurin as maintenance therapy.
Midostaurin is also approved as monotherapy for adults with aggressive SM (ASM), SM with associated hematological neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin in AML
The approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were published in NEJM last month.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
The median overall survival was significantly longer in the midostaurin arm than the placebo arm—74.7 months and 25.6 months, respectively (hazard ratio=0.77, P=0.016).
And the median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infection.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The approval of midostaurin in advanced SM is based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3. Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 responses (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders.
Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage.
The European Commission has approved the multi-targeted kinase inhibitor midostaurin (Rydapt®) to treat acute myeloid leukemia (AML) and 3 types of advanced systemic mastocytosis (SM).
Midostaurin is approved to treat adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive. In these patients, midostaurin can be used in combination with standard daunorubicin and cytarabine induction, followed by high-dose cytarabine consolidation. Patients who achieve a complete response can then receive midostaurin as maintenance therapy.
Midostaurin is also approved as monotherapy for adults with aggressive SM (ASM), SM with associated hematological neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin in AML
The approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were published in NEJM last month.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
The median overall survival was significantly longer in the midostaurin arm than the placebo arm—74.7 months and 25.6 months, respectively (hazard ratio=0.77, P=0.016).
And the median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infection.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The approval of midostaurin in advanced SM is based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3. Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 responses (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders.
Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage.
CHMP recommends approval of generic imatinib
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Imatinib Teva B.V., a generic of Glivec.
The recommendation is that Imatinib Teva B.V. be approved to treat chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), gastrointestinal stromal tumors (GIST), and dermatofibrosarcoma protuberans (DFSP).
The European Commission typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The European Commission’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
If approved, Imatinib Teva B.V. will be available as capsules and film-coated tablets (100 mg and 400 mg). And it will be authorized:
- As monotherapy for pediatric patients with newly diagnosed, Philadelphia-chromosome-positive (Ph+) CML for whom bone marrow transplant is not considered the first line of treatment.
- As monotherapy for pediatric patients with Ph+ CML in chronic phase after failure of interferon-alpha therapy or in accelerated phase or blast crisis.
- As monotherapy for adults with Ph+ CML in blast crisis.
- Integrated with chemotherapy to treat adult and pediatric patients with newly diagnosed, Ph+ ALL.
- As monotherapy for adults with relapsed or refractory Ph+ ALL.
- As monotherapy for adults with MDS/MPNs associated with platelet-derived growth factor receptor gene re-arrangements.
- As monotherapy for adults with advanced HES and/or CEL with FIP1L1-PDGFRα rearrangement.
- As monotherapy for adults with Kit- (CD117-) positive, unresectable and/or metastatic malignant GISTs.
- For the adjuvant treatment of adults who are at significant risk of relapse following resection of Kit-positive GIST. Patients who have a low or very low risk of recurrence should not receive adjuvant treatment.
- As monotherapy for adults with unresectable DFSP and adults with recurrent and/or metastatic DFSP who are not eligible for surgery.
The CHMP said studies have demonstrated the satisfactory quality of Imatinib Teva B.V. and its bioequivalence to the reference product, Glivec.
In adult and pediatric patients, the effectiveness of imatinib is based on:
- Overall hematologic and cytogenetic response rates and progression-free survival in CML
- Hematologic and cytogenetic response rates in Ph+ ALL and MDS/MPNs
- Hematologic response rates in HES/CEL
- Objective response rates in adults with unresectable and/or metastatic GIST and DFSP
- Recurrence-free survival in adjuvant GIST.
The experience with imatinib in patients with MDS/MPNs associated with PDGFR gene re-arrangements is very limited. There are no controlled trials demonstrating a clinical benefit or increased survival for these diseases.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Imatinib Teva B.V., a generic of Glivec.
The recommendation is that Imatinib Teva B.V. be approved to treat chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), gastrointestinal stromal tumors (GIST), and dermatofibrosarcoma protuberans (DFSP).
The European Commission typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The European Commission’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
If approved, Imatinib Teva B.V. will be available as capsules and film-coated tablets (100 mg and 400 mg). And it will be authorized:
- As monotherapy for pediatric patients with newly diagnosed, Philadelphia-chromosome-positive (Ph+) CML for whom bone marrow transplant is not considered the first line of treatment.
- As monotherapy for pediatric patients with Ph+ CML in chronic phase after failure of interferon-alpha therapy or in accelerated phase or blast crisis.
- As monotherapy for adults with Ph+ CML in blast crisis.
- Integrated with chemotherapy to treat adult and pediatric patients with newly diagnosed, Ph+ ALL.
- As monotherapy for adults with relapsed or refractory Ph+ ALL.
- As monotherapy for adults with MDS/MPNs associated with platelet-derived growth factor receptor gene re-arrangements.
- As monotherapy for adults with advanced HES and/or CEL with FIP1L1-PDGFRα rearrangement.
- As monotherapy for adults with Kit- (CD117-) positive, unresectable and/or metastatic malignant GISTs.
- For the adjuvant treatment of adults who are at significant risk of relapse following resection of Kit-positive GIST. Patients who have a low or very low risk of recurrence should not receive adjuvant treatment.
- As monotherapy for adults with unresectable DFSP and adults with recurrent and/or metastatic DFSP who are not eligible for surgery.
The CHMP said studies have demonstrated the satisfactory quality of Imatinib Teva B.V. and its bioequivalence to the reference product, Glivec.
In adult and pediatric patients, the effectiveness of imatinib is based on:
- Overall hematologic and cytogenetic response rates and progression-free survival in CML
- Hematologic and cytogenetic response rates in Ph+ ALL and MDS/MPNs
- Hematologic response rates in HES/CEL
- Objective response rates in adults with unresectable and/or metastatic GIST and DFSP
- Recurrence-free survival in adjuvant GIST.
The experience with imatinib in patients with MDS/MPNs associated with PDGFR gene re-arrangements is very limited. There are no controlled trials demonstrating a clinical benefit or increased survival for these diseases.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Imatinib Teva B.V., a generic of Glivec.
The recommendation is that Imatinib Teva B.V. be approved to treat chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), gastrointestinal stromal tumors (GIST), and dermatofibrosarcoma protuberans (DFSP).
The European Commission typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The European Commission’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
If approved, Imatinib Teva B.V. will be available as capsules and film-coated tablets (100 mg and 400 mg). And it will be authorized:
- As monotherapy for pediatric patients with newly diagnosed, Philadelphia-chromosome-positive (Ph+) CML for whom bone marrow transplant is not considered the first line of treatment.
- As monotherapy for pediatric patients with Ph+ CML in chronic phase after failure of interferon-alpha therapy or in accelerated phase or blast crisis.
- As monotherapy for adults with Ph+ CML in blast crisis.
- Integrated with chemotherapy to treat adult and pediatric patients with newly diagnosed, Ph+ ALL.
- As monotherapy for adults with relapsed or refractory Ph+ ALL.
- As monotherapy for adults with MDS/MPNs associated with platelet-derived growth factor receptor gene re-arrangements.
- As monotherapy for adults with advanced HES and/or CEL with FIP1L1-PDGFRα rearrangement.
- As monotherapy for adults with Kit- (CD117-) positive, unresectable and/or metastatic malignant GISTs.
- For the adjuvant treatment of adults who are at significant risk of relapse following resection of Kit-positive GIST. Patients who have a low or very low risk of recurrence should not receive adjuvant treatment.
- As monotherapy for adults with unresectable DFSP and adults with recurrent and/or metastatic DFSP who are not eligible for surgery.
The CHMP said studies have demonstrated the satisfactory quality of Imatinib Teva B.V. and its bioequivalence to the reference product, Glivec.
In adult and pediatric patients, the effectiveness of imatinib is based on:
- Overall hematologic and cytogenetic response rates and progression-free survival in CML
- Hematologic and cytogenetic response rates in Ph+ ALL and MDS/MPNs
- Hematologic response rates in HES/CEL
- Objective response rates in adults with unresectable and/or metastatic GIST and DFSP
- Recurrence-free survival in adjuvant GIST.
The experience with imatinib in patients with MDS/MPNs associated with PDGFR gene re-arrangements is very limited. There are no controlled trials demonstrating a clinical benefit or increased survival for these diseases.
CHMP advocates refusal of application for SM drug
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of the marketing authorization for masitinib (Masipro).
Masitinib is a tyrosine kinase inhibitor being developed by AB Science to treat adults with smoldering or indolent severe systemic mastocytosis (SM).
To support the application for masitinib, AB Science presented data from a study involving 135 SM patients who had severe symptoms, including at least one of the following: itching, hot flashes, depression, and tiredness.
Researchers compared masitinib to placebo in these patients, looking for improvements in any of the symptoms during the first 24 weeks of treatment.
The CHMP said it was concerned about the reliability of the study results because a routine good clinical practice inspection at the study sites revealed serious failings in the way the study had been conducted.
In addition, major changes were made to the study design while the study was underway, which made the results difficult to interpret.
Finally, data on the safety of masitinib were limited. And the CHMP was concerned about side effects, including neutropenia and harmful effects on the skin and liver, which were particularly relevant because masitinib was intended to be used long-term.
Therefore, the CHMP concluded the benefits of masitinib do not appear to outweigh the risks, and the committee recommended the drug be refused marketing authorization.
The CHMP informed AB Science of this negative opinion in May, and the company asked the committee to re-examine its opinion. However, the CHMP ultimately concluded that masitinib should be refused marketing authorization.
AB Science said this decision does not have any consequences for patients in clinical trials or compassionate use programs of masitinib.
The company also said it intends to initiate a confirmatory study in patients with smoldering or indolent severe SM that is unresponsive to optimal symptomatic treatment in order to confirm the results from the first pivotal study.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of the marketing authorization for masitinib (Masipro).
Masitinib is a tyrosine kinase inhibitor being developed by AB Science to treat adults with smoldering or indolent severe systemic mastocytosis (SM).
To support the application for masitinib, AB Science presented data from a study involving 135 SM patients who had severe symptoms, including at least one of the following: itching, hot flashes, depression, and tiredness.
Researchers compared masitinib to placebo in these patients, looking for improvements in any of the symptoms during the first 24 weeks of treatment.
The CHMP said it was concerned about the reliability of the study results because a routine good clinical practice inspection at the study sites revealed serious failings in the way the study had been conducted.
In addition, major changes were made to the study design while the study was underway, which made the results difficult to interpret.
Finally, data on the safety of masitinib were limited. And the CHMP was concerned about side effects, including neutropenia and harmful effects on the skin and liver, which were particularly relevant because masitinib was intended to be used long-term.
Therefore, the CHMP concluded the benefits of masitinib do not appear to outweigh the risks, and the committee recommended the drug be refused marketing authorization.
The CHMP informed AB Science of this negative opinion in May, and the company asked the committee to re-examine its opinion. However, the CHMP ultimately concluded that masitinib should be refused marketing authorization.
AB Science said this decision does not have any consequences for patients in clinical trials or compassionate use programs of masitinib.
The company also said it intends to initiate a confirmatory study in patients with smoldering or indolent severe SM that is unresponsive to optimal symptomatic treatment in order to confirm the results from the first pivotal study.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended refusal of the marketing authorization for masitinib (Masipro).
Masitinib is a tyrosine kinase inhibitor being developed by AB Science to treat adults with smoldering or indolent severe systemic mastocytosis (SM).
To support the application for masitinib, AB Science presented data from a study involving 135 SM patients who had severe symptoms, including at least one of the following: itching, hot flashes, depression, and tiredness.
Researchers compared masitinib to placebo in these patients, looking for improvements in any of the symptoms during the first 24 weeks of treatment.
The CHMP said it was concerned about the reliability of the study results because a routine good clinical practice inspection at the study sites revealed serious failings in the way the study had been conducted.
In addition, major changes were made to the study design while the study was underway, which made the results difficult to interpret.
Finally, data on the safety of masitinib were limited. And the CHMP was concerned about side effects, including neutropenia and harmful effects on the skin and liver, which were particularly relevant because masitinib was intended to be used long-term.
Therefore, the CHMP concluded the benefits of masitinib do not appear to outweigh the risks, and the committee recommended the drug be refused marketing authorization.
The CHMP informed AB Science of this negative opinion in May, and the company asked the committee to re-examine its opinion. However, the CHMP ultimately concluded that masitinib should be refused marketing authorization.
AB Science said this decision does not have any consequences for patients in clinical trials or compassionate use programs of masitinib.
The company also said it intends to initiate a confirmatory study in patients with smoldering or indolent severe SM that is unresponsive to optimal symptomatic treatment in order to confirm the results from the first pivotal study.