User login
CHMP recommends conditional approval of drug for CLL

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of venetoclax (Venclyxto™).
The CHMP is recommending that venetoclax receive conditional marketing authorization to treat adults with chronic lymphocytic leukemia (CLL) who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with an inhibitor of the B-cell receptor pathway.
The CHMP is also recommending the conditional authorization of venetoclax as a treatment for adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemo immunotherapy and treatment with an inhibitor of the B-cell receptor pathway.
The European Commission (EC) will review the CHMP’s opinion and is expected to make a final decision about venetoclax in late 2016.
If the EC follows the CHMP’s recommendations, venetoclax will become the first BCL-2 inhibitor approved for use in Europe. The authorization will be valid in all member states of the European Union, as well as Iceland, Liechtenstein, and Norway.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently approved for use in Argentina, Canada, Puerto Rico, and the US. The drug is being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of venetoclax (Venclyxto™).
The CHMP is recommending that venetoclax receive conditional marketing authorization to treat adults with chronic lymphocytic leukemia (CLL) who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with an inhibitor of the B-cell receptor pathway.
The CHMP is also recommending the conditional authorization of venetoclax as a treatment for adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemo immunotherapy and treatment with an inhibitor of the B-cell receptor pathway.
The European Commission (EC) will review the CHMP’s opinion and is expected to make a final decision about venetoclax in late 2016.
If the EC follows the CHMP’s recommendations, venetoclax will become the first BCL-2 inhibitor approved for use in Europe. The authorization will be valid in all member states of the European Union, as well as Iceland, Liechtenstein, and Norway.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently approved for use in Argentina, Canada, Puerto Rico, and the US. The drug is being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of venetoclax (Venclyxto™).
The CHMP is recommending that venetoclax receive conditional marketing authorization to treat adults with chronic lymphocytic leukemia (CLL) who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with an inhibitor of the B-cell receptor pathway.
The CHMP is also recommending the conditional authorization of venetoclax as a treatment for adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemo immunotherapy and treatment with an inhibitor of the B-cell receptor pathway.
The European Commission (EC) will review the CHMP’s opinion and is expected to make a final decision about venetoclax in late 2016.
If the EC follows the CHMP’s recommendations, venetoclax will become the first BCL-2 inhibitor approved for use in Europe. The authorization will be valid in all member states of the European Union, as well as Iceland, Liechtenstein, and Norway.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently approved for use in Argentina, Canada, Puerto Rico, and the US. The drug is being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()
How a protein regulates blood cell fate
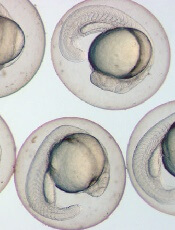
Image by Ian Johnston
Results of preclinical research explain the role endoglin plays in hematopoietic cell fate during embryogenesis.
Previous research showed that endoglin, a cell surface protein belonging to the TGF-beta receptor complex, is required for early hematopoietic lineage specification.
A new study shows that endoglin modulates the BMP and Wnt signaling pathways to encourage progenitor cells to develop into blood cells rather than cardiac cells.
This study was published in Nature Communications.
“During the early stages of development, cells have to make decisions very quickly,” said study author Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis.
“Fine-tuning of these early cell fate decisions can be easily disrupted by levels of key proteins within these cells. When one cell type is favored, this implies less of another. In this case, high levels of endoglin expression enhance the cell differentiation into blood cells, whereas cardiac cells are in deficit.”
Dr Perlingeiro and her colleagues made this discovery studying zebrafish and mouse models. The team wanted to pinpoint the mechanism underlying the dual function of endoglin in blood cell and cardiac cell fate.
The researchers found that endoglin is expressed in early mesoderm, and it marks both hematopoietic and cardiac progenitors.
Experiments showed that high levels of endoglin increase hematopoiesis while inhibiting cardiogenesis. And the levels of endoglin determine the activation of the BMP and Wnt signaling pathways.
With further investigation, the researchers identified JDP2, a member of the AP-1 transcription factor family, as an endoglin-dependent downstream target of Wnt signaling.
The team found that JDP2 expression is sufficient to establish blood cell fate when BMP and Wnt crosstalk is disturbed.
“The blood and heart systems are the first organs to develop in mammals, but the mechanisms regulating these earliest cell fate decisions are poorly understood,” Dr Perlingeiro said.
“By using multiple model systems, combined with specialized cell sorting technology and sequencing tools, our findings help uncover mechanisms previously unseen in the few cells engaged in these early development decisions.” ![]()

Image by Ian Johnston
Results of preclinical research explain the role endoglin plays in hematopoietic cell fate during embryogenesis.
Previous research showed that endoglin, a cell surface protein belonging to the TGF-beta receptor complex, is required for early hematopoietic lineage specification.
A new study shows that endoglin modulates the BMP and Wnt signaling pathways to encourage progenitor cells to develop into blood cells rather than cardiac cells.
This study was published in Nature Communications.
“During the early stages of development, cells have to make decisions very quickly,” said study author Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis.
“Fine-tuning of these early cell fate decisions can be easily disrupted by levels of key proteins within these cells. When one cell type is favored, this implies less of another. In this case, high levels of endoglin expression enhance the cell differentiation into blood cells, whereas cardiac cells are in deficit.”
Dr Perlingeiro and her colleagues made this discovery studying zebrafish and mouse models. The team wanted to pinpoint the mechanism underlying the dual function of endoglin in blood cell and cardiac cell fate.
The researchers found that endoglin is expressed in early mesoderm, and it marks both hematopoietic and cardiac progenitors.
Experiments showed that high levels of endoglin increase hematopoiesis while inhibiting cardiogenesis. And the levels of endoglin determine the activation of the BMP and Wnt signaling pathways.
With further investigation, the researchers identified JDP2, a member of the AP-1 transcription factor family, as an endoglin-dependent downstream target of Wnt signaling.
The team found that JDP2 expression is sufficient to establish blood cell fate when BMP and Wnt crosstalk is disturbed.
“The blood and heart systems are the first organs to develop in mammals, but the mechanisms regulating these earliest cell fate decisions are poorly understood,” Dr Perlingeiro said.
“By using multiple model systems, combined with specialized cell sorting technology and sequencing tools, our findings help uncover mechanisms previously unseen in the few cells engaged in these early development decisions.” ![]()

Image by Ian Johnston
Results of preclinical research explain the role endoglin plays in hematopoietic cell fate during embryogenesis.
Previous research showed that endoglin, a cell surface protein belonging to the TGF-beta receptor complex, is required for early hematopoietic lineage specification.
A new study shows that endoglin modulates the BMP and Wnt signaling pathways to encourage progenitor cells to develop into blood cells rather than cardiac cells.
This study was published in Nature Communications.
“During the early stages of development, cells have to make decisions very quickly,” said study author Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis.
“Fine-tuning of these early cell fate decisions can be easily disrupted by levels of key proteins within these cells. When one cell type is favored, this implies less of another. In this case, high levels of endoglin expression enhance the cell differentiation into blood cells, whereas cardiac cells are in deficit.”
Dr Perlingeiro and her colleagues made this discovery studying zebrafish and mouse models. The team wanted to pinpoint the mechanism underlying the dual function of endoglin in blood cell and cardiac cell fate.
The researchers found that endoglin is expressed in early mesoderm, and it marks both hematopoietic and cardiac progenitors.
Experiments showed that high levels of endoglin increase hematopoiesis while inhibiting cardiogenesis. And the levels of endoglin determine the activation of the BMP and Wnt signaling pathways.
With further investigation, the researchers identified JDP2, a member of the AP-1 transcription factor family, as an endoglin-dependent downstream target of Wnt signaling.
The team found that JDP2 expression is sufficient to establish blood cell fate when BMP and Wnt crosstalk is disturbed.
“The blood and heart systems are the first organs to develop in mammals, but the mechanisms regulating these earliest cell fate decisions are poorly understood,” Dr Perlingeiro said.
“By using multiple model systems, combined with specialized cell sorting technology and sequencing tools, our findings help uncover mechanisms previously unseen in the few cells engaged in these early development decisions.” ![]()
FDA grants priority review for MM drug

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has granted priority review for part of a supplemental biologics license application (sBLA) for daratumumab (Darzalex®).
The priority review pertains to the use of daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat patients with multiple myeloma (MM) who have received at least 1 prior therapy.
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The FDA has assigned a Prescription Drug User Fee Act target date of February 17, 2017, to make a decision on daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone.
Standard review
The FDA has also granted a standard review period for part of the sBLA.
The standard review pertains to the use of daratumumab in combination with pomalidomide and dexamethasone to treat patients with relapsed or refractory MM who have received at least 2 prior therapies, including a proteasome inhibitor and an immunomodulatory agent.
The Prescription Drug User Fee Act date for the combination of daratumumab with pomalidomide and dexamethasone is June 17, 2017.
Trial data
The sBLA submission included data from a pair of phase 3 studies:
- The CASTOR study, in which researchers compared the combination of daratumumab, bortezomib, and dexamethasone to bortezomib and dexamethasone in patients with relapsed or refractory MM
- The POLLUX study, in which researchers compared daratumumab in combination with lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with relapsed or refractory MM.
The sBLA submission also included data from a phase 1 study of daratumumab in combination with pomalidomide and dexamethasone in patients with relapsed or refractory MM.
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab already has accelerated approval in the US as monotherapy for MM patients who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab to develop, manufacture, and commercialize daratumumab.
For more information on daratumumab, visit www.DARZALEX.com. ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has granted priority review for part of a supplemental biologics license application (sBLA) for daratumumab (Darzalex®).
The priority review pertains to the use of daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat patients with multiple myeloma (MM) who have received at least 1 prior therapy.
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The FDA has assigned a Prescription Drug User Fee Act target date of February 17, 2017, to make a decision on daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone.
Standard review
The FDA has also granted a standard review period for part of the sBLA.
The standard review pertains to the use of daratumumab in combination with pomalidomide and dexamethasone to treat patients with relapsed or refractory MM who have received at least 2 prior therapies, including a proteasome inhibitor and an immunomodulatory agent.
The Prescription Drug User Fee Act date for the combination of daratumumab with pomalidomide and dexamethasone is June 17, 2017.
Trial data
The sBLA submission included data from a pair of phase 3 studies:
- The CASTOR study, in which researchers compared the combination of daratumumab, bortezomib, and dexamethasone to bortezomib and dexamethasone in patients with relapsed or refractory MM
- The POLLUX study, in which researchers compared daratumumab in combination with lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with relapsed or refractory MM.
The sBLA submission also included data from a phase 1 study of daratumumab in combination with pomalidomide and dexamethasone in patients with relapsed or refractory MM.
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab already has accelerated approval in the US as monotherapy for MM patients who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab to develop, manufacture, and commercialize daratumumab.
For more information on daratumumab, visit www.DARZALEX.com. ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has granted priority review for part of a supplemental biologics license application (sBLA) for daratumumab (Darzalex®).
The priority review pertains to the use of daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat patients with multiple myeloma (MM) who have received at least 1 prior therapy.
To grant an application priority review, the FDA must believe the drug would provide a significant improvement in the treatment, diagnosis, or prevention of a serious condition.
The priority review designation means the FDA’s goal is to take action on an application within 6 months, rather than the 10 months typically taken for a standard review.
The FDA has assigned a Prescription Drug User Fee Act target date of February 17, 2017, to make a decision on daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone.
Standard review
The FDA has also granted a standard review period for part of the sBLA.
The standard review pertains to the use of daratumumab in combination with pomalidomide and dexamethasone to treat patients with relapsed or refractory MM who have received at least 2 prior therapies, including a proteasome inhibitor and an immunomodulatory agent.
The Prescription Drug User Fee Act date for the combination of daratumumab with pomalidomide and dexamethasone is June 17, 2017.
Trial data
The sBLA submission included data from a pair of phase 3 studies:
- The CASTOR study, in which researchers compared the combination of daratumumab, bortezomib, and dexamethasone to bortezomib and dexamethasone in patients with relapsed or refractory MM
- The POLLUX study, in which researchers compared daratumumab in combination with lenalidomide and dexamethasone to lenalidomide and dexamethasone in patients with relapsed or refractory MM.
The sBLA submission also included data from a phase 1 study of daratumumab in combination with pomalidomide and dexamethasone in patients with relapsed or refractory MM.
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab already has accelerated approval in the US as monotherapy for MM patients who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab to develop, manufacture, and commercialize daratumumab.
For more information on daratumumab, visit www.DARZALEX.com. ![]()
Drug granted conditional approval to treat CLL in Canada

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()
Factor IX therapy approved in Australia

The Australian Therapeutic Goods Administration has approved albutrepenonacog alfa (Idelvion) to treat hemophilia B patients of all ages.
Albutrepenonacog alfa is a fusion protein linking recombinant coagulation factor IX with recombinant albumin.
The product is now approved in Australia for use as routine prophylaxis to prevent and reduce the frequency of bleeding, for on-demand control of bleeding, and for perioperative management of
bleeding.
Albutrepenonacog alfa has also been approved in Canada, the European Union, Japan, Switzerland, and the US.
Albutrepenonacog alfa is being developed by CSL Behring.
The company says albutrepenonacog alfa is the first and only Australian-registered factor IX therapy that delivers high-level protection from bleeding with up to 14-day dosing for appropriate patients.
According to CSL Behring, albutrepenonacog alfa can deliver high-level protection by maintaining factor IX activity levels at an average of 20% in patients treated prophylactically every 7 days and an average of 12% in patients treated prophylactically every 14 days.
“The Australian Haemophilia Centre Directors’ Organisation (AHCDO) views the development of effective long-acting clotting factor concentrates as a major step forward in the management of our patients with hemophilia,” said Simon McRae, MMBS, consultant hematologist and chairman of AHCDO.
“The ability to maintain clotting factor levels above a level that prevent the vast majority of bleeding events with less frequent infusions has the potential to improve long-term outcomes in individuals with hemophilia.”
Phase 3 trial
The Therapeutic Goods Administration approved albutrepenonacog alfa based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were published in Blood. The study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache.
“I have seen first-hand the benefits Idelvion has had on children with hemophilia B,” said PROLONG-9FP investigator Julie Curtin, MBBS, PhD, of The Children’s Hospital at Westmead in New South Wales.
“Idelvion has enabled children on regular treatment with factor IX to reduce the frequency of infusions without increasing their risk of bleeding. For a child to only need an injection every 1-2 weeks is a good step forward in the management of hemophilia B, which I welcome, and I am sure my patients will welcome this improvement too.” ![]()

The Australian Therapeutic Goods Administration has approved albutrepenonacog alfa (Idelvion) to treat hemophilia B patients of all ages.
Albutrepenonacog alfa is a fusion protein linking recombinant coagulation factor IX with recombinant albumin.
The product is now approved in Australia for use as routine prophylaxis to prevent and reduce the frequency of bleeding, for on-demand control of bleeding, and for perioperative management of
bleeding.
Albutrepenonacog alfa has also been approved in Canada, the European Union, Japan, Switzerland, and the US.
Albutrepenonacog alfa is being developed by CSL Behring.
The company says albutrepenonacog alfa is the first and only Australian-registered factor IX therapy that delivers high-level protection from bleeding with up to 14-day dosing for appropriate patients.
According to CSL Behring, albutrepenonacog alfa can deliver high-level protection by maintaining factor IX activity levels at an average of 20% in patients treated prophylactically every 7 days and an average of 12% in patients treated prophylactically every 14 days.
“The Australian Haemophilia Centre Directors’ Organisation (AHCDO) views the development of effective long-acting clotting factor concentrates as a major step forward in the management of our patients with hemophilia,” said Simon McRae, MMBS, consultant hematologist and chairman of AHCDO.
“The ability to maintain clotting factor levels above a level that prevent the vast majority of bleeding events with less frequent infusions has the potential to improve long-term outcomes in individuals with hemophilia.”
Phase 3 trial
The Therapeutic Goods Administration approved albutrepenonacog alfa based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were published in Blood. The study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache.
“I have seen first-hand the benefits Idelvion has had on children with hemophilia B,” said PROLONG-9FP investigator Julie Curtin, MBBS, PhD, of The Children’s Hospital at Westmead in New South Wales.
“Idelvion has enabled children on regular treatment with factor IX to reduce the frequency of infusions without increasing their risk of bleeding. For a child to only need an injection every 1-2 weeks is a good step forward in the management of hemophilia B, which I welcome, and I am sure my patients will welcome this improvement too.” ![]()

The Australian Therapeutic Goods Administration has approved albutrepenonacog alfa (Idelvion) to treat hemophilia B patients of all ages.
Albutrepenonacog alfa is a fusion protein linking recombinant coagulation factor IX with recombinant albumin.
The product is now approved in Australia for use as routine prophylaxis to prevent and reduce the frequency of bleeding, for on-demand control of bleeding, and for perioperative management of
bleeding.
Albutrepenonacog alfa has also been approved in Canada, the European Union, Japan, Switzerland, and the US.
Albutrepenonacog alfa is being developed by CSL Behring.
The company says albutrepenonacog alfa is the first and only Australian-registered factor IX therapy that delivers high-level protection from bleeding with up to 14-day dosing for appropriate patients.
According to CSL Behring, albutrepenonacog alfa can deliver high-level protection by maintaining factor IX activity levels at an average of 20% in patients treated prophylactically every 7 days and an average of 12% in patients treated prophylactically every 14 days.
“The Australian Haemophilia Centre Directors’ Organisation (AHCDO) views the development of effective long-acting clotting factor concentrates as a major step forward in the management of our patients with hemophilia,” said Simon McRae, MMBS, consultant hematologist and chairman of AHCDO.
“The ability to maintain clotting factor levels above a level that prevent the vast majority of bleeding events with less frequent infusions has the potential to improve long-term outcomes in individuals with hemophilia.”
Phase 3 trial
The Therapeutic Goods Administration approved albutrepenonacog alfa based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were published in Blood. The study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache.
“I have seen first-hand the benefits Idelvion has had on children with hemophilia B,” said PROLONG-9FP investigator Julie Curtin, MBBS, PhD, of The Children’s Hospital at Westmead in New South Wales.
“Idelvion has enabled children on regular treatment with factor IX to reduce the frequency of infusions without increasing their risk of bleeding. For a child to only need an injection every 1-2 weeks is a good step forward in the management of hemophilia B, which I welcome, and I am sure my patients will welcome this improvement too.” ![]()
Medicare doesn’t lower TKI costs enough, study suggests

cut in half with a pill splitter
Photo by Patrick Pelletier
Significant out-of-pocket costs may delay treatment for Medicare beneficiaries with chronic myeloid leukemia (CML), according to a study published in the Journal of Clinical Oncology.
Researchers studied 393 patients with CML who had federally funded health insurance—specifically, a Medicare Part D plan.
Nearly a third of these patients did not start tyrosine kinase inhibitor (TKI) treatment within 6 months of their diagnosis.
However, patients who had access to subsidies that help cover treatment costs had a shorter median time to the start of therapy.
“There are 2 troubling findings here,” said study author Aaron Winn, a doctoral student at the University of North Carolina at Chapel Hill.
“First, we are seeing that more than 30% of people aren’t starting therapy within 6 months. Second, we are seeing long delays in starting drugs for people without subsidies. This is very concerning as these delays may be an indicator that the patient is trying to find funds to pay for their first treatment.”
Medicare Part D and TKIs
Previous studies have shown that patients insured through Medicare Part D have out of-pocket costs of nearly $3000 for the first month’s supply of a TKI.
According to researchers, the high upfront costs are due to the Medicare Part D benefit design, which requires patients to pay a larger share of medication costs until they have paid at least $4850 out-of-pocket in a year (cost in 2016). After that, patients pay 5% of the monthly drug costs.
In order to qualify for Medicare Part D’s low-income subsidy, an individual must have an annual income of less than $17,820 and assets of less than $13,640 (figures for 2016).
“Once you’re on Medicare Part D, there really aren’t ways to minimize these out-of-pocket costs, other than subsidies,” said Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“One of the challenges is that when the Medicare benefit was designed, I don’t think they were really considering these very expensive therapies. The benefit design makes a lot more sense when you’re looking at drugs that cost several hundred dollars versus several thousand dollars or more. We really need to think carefully about how much these high out-of-pocket costs are impacting patients’ access to life-saving drugs.”
Study results
For this study, Dr Dusetzina and her colleagues evaluated data on 393 patients who were diagnosed with CML between 2007 and 2011. The patients’ median age was 77, 47% were married, 48% were male, and 85% were white.
All of the patients were enrolled in Medicare Part D, and 40% qualified for subsidies to lower drug costs.
Of all the patients, there were 32% who had not started treatment with a first-line TKI (imatinib, nilotinib, or dasatinib) within 6 months of diagnosis.
However, having access to subsidies was associated with a shorter time to the start of treatment. The median time to the start of treatment was 58 days for patients with subsidies and 108 days for patients without them.
While the gap between the 2 groups widened after diagnosis, eventually, patients without subsidies did catch up, Dr Dusetzina said.
Ninety days from diagnosis, 48% of patients without subsidies had started treatment, compared to 63% of patients with subsidies. At 6 months from diagnosis, 64% of patients without subsidies had started treatment, compared to 65% of patients with subsidies.
Dr Dusetzina said patients without subsidies could be catching up as they find the financial resources to help cover those initial costs. But overall, patients with subsidies were 35% more likely to start TKI treatment faster.
“We recognize that people have a high cost to even start therapy, and this study really demonstrates the difference between people with and without a subsidy in initiating therapy,” Dr Dusetzina said. “The out-of-pocket costs may be delaying people starting these life-saving drugs.” ![]()

cut in half with a pill splitter
Photo by Patrick Pelletier
Significant out-of-pocket costs may delay treatment for Medicare beneficiaries with chronic myeloid leukemia (CML), according to a study published in the Journal of Clinical Oncology.
Researchers studied 393 patients with CML who had federally funded health insurance—specifically, a Medicare Part D plan.
Nearly a third of these patients did not start tyrosine kinase inhibitor (TKI) treatment within 6 months of their diagnosis.
However, patients who had access to subsidies that help cover treatment costs had a shorter median time to the start of therapy.
“There are 2 troubling findings here,” said study author Aaron Winn, a doctoral student at the University of North Carolina at Chapel Hill.
“First, we are seeing that more than 30% of people aren’t starting therapy within 6 months. Second, we are seeing long delays in starting drugs for people without subsidies. This is very concerning as these delays may be an indicator that the patient is trying to find funds to pay for their first treatment.”
Medicare Part D and TKIs
Previous studies have shown that patients insured through Medicare Part D have out of-pocket costs of nearly $3000 for the first month’s supply of a TKI.
According to researchers, the high upfront costs are due to the Medicare Part D benefit design, which requires patients to pay a larger share of medication costs until they have paid at least $4850 out-of-pocket in a year (cost in 2016). After that, patients pay 5% of the monthly drug costs.
In order to qualify for Medicare Part D’s low-income subsidy, an individual must have an annual income of less than $17,820 and assets of less than $13,640 (figures for 2016).
“Once you’re on Medicare Part D, there really aren’t ways to minimize these out-of-pocket costs, other than subsidies,” said Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“One of the challenges is that when the Medicare benefit was designed, I don’t think they were really considering these very expensive therapies. The benefit design makes a lot more sense when you’re looking at drugs that cost several hundred dollars versus several thousand dollars or more. We really need to think carefully about how much these high out-of-pocket costs are impacting patients’ access to life-saving drugs.”
Study results
For this study, Dr Dusetzina and her colleagues evaluated data on 393 patients who were diagnosed with CML between 2007 and 2011. The patients’ median age was 77, 47% were married, 48% were male, and 85% were white.
All of the patients were enrolled in Medicare Part D, and 40% qualified for subsidies to lower drug costs.
Of all the patients, there were 32% who had not started treatment with a first-line TKI (imatinib, nilotinib, or dasatinib) within 6 months of diagnosis.
However, having access to subsidies was associated with a shorter time to the start of treatment. The median time to the start of treatment was 58 days for patients with subsidies and 108 days for patients without them.
While the gap between the 2 groups widened after diagnosis, eventually, patients without subsidies did catch up, Dr Dusetzina said.
Ninety days from diagnosis, 48% of patients without subsidies had started treatment, compared to 63% of patients with subsidies. At 6 months from diagnosis, 64% of patients without subsidies had started treatment, compared to 65% of patients with subsidies.
Dr Dusetzina said patients without subsidies could be catching up as they find the financial resources to help cover those initial costs. But overall, patients with subsidies were 35% more likely to start TKI treatment faster.
“We recognize that people have a high cost to even start therapy, and this study really demonstrates the difference between people with and without a subsidy in initiating therapy,” Dr Dusetzina said. “The out-of-pocket costs may be delaying people starting these life-saving drugs.” ![]()

cut in half with a pill splitter
Photo by Patrick Pelletier
Significant out-of-pocket costs may delay treatment for Medicare beneficiaries with chronic myeloid leukemia (CML), according to a study published in the Journal of Clinical Oncology.
Researchers studied 393 patients with CML who had federally funded health insurance—specifically, a Medicare Part D plan.
Nearly a third of these patients did not start tyrosine kinase inhibitor (TKI) treatment within 6 months of their diagnosis.
However, patients who had access to subsidies that help cover treatment costs had a shorter median time to the start of therapy.
“There are 2 troubling findings here,” said study author Aaron Winn, a doctoral student at the University of North Carolina at Chapel Hill.
“First, we are seeing that more than 30% of people aren’t starting therapy within 6 months. Second, we are seeing long delays in starting drugs for people without subsidies. This is very concerning as these delays may be an indicator that the patient is trying to find funds to pay for their first treatment.”
Medicare Part D and TKIs
Previous studies have shown that patients insured through Medicare Part D have out of-pocket costs of nearly $3000 for the first month’s supply of a TKI.
According to researchers, the high upfront costs are due to the Medicare Part D benefit design, which requires patients to pay a larger share of medication costs until they have paid at least $4850 out-of-pocket in a year (cost in 2016). After that, patients pay 5% of the monthly drug costs.
In order to qualify for Medicare Part D’s low-income subsidy, an individual must have an annual income of less than $17,820 and assets of less than $13,640 (figures for 2016).
“Once you’re on Medicare Part D, there really aren’t ways to minimize these out-of-pocket costs, other than subsidies,” said Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
“One of the challenges is that when the Medicare benefit was designed, I don’t think they were really considering these very expensive therapies. The benefit design makes a lot more sense when you’re looking at drugs that cost several hundred dollars versus several thousand dollars or more. We really need to think carefully about how much these high out-of-pocket costs are impacting patients’ access to life-saving drugs.”
Study results
For this study, Dr Dusetzina and her colleagues evaluated data on 393 patients who were diagnosed with CML between 2007 and 2011. The patients’ median age was 77, 47% were married, 48% were male, and 85% were white.
All of the patients were enrolled in Medicare Part D, and 40% qualified for subsidies to lower drug costs.
Of all the patients, there were 32% who had not started treatment with a first-line TKI (imatinib, nilotinib, or dasatinib) within 6 months of diagnosis.
However, having access to subsidies was associated with a shorter time to the start of treatment. The median time to the start of treatment was 58 days for patients with subsidies and 108 days for patients without them.
While the gap between the 2 groups widened after diagnosis, eventually, patients without subsidies did catch up, Dr Dusetzina said.
Ninety days from diagnosis, 48% of patients without subsidies had started treatment, compared to 63% of patients with subsidies. At 6 months from diagnosis, 64% of patients without subsidies had started treatment, compared to 65% of patients with subsidies.
Dr Dusetzina said patients without subsidies could be catching up as they find the financial resources to help cover those initial costs. But overall, patients with subsidies were 35% more likely to start TKI treatment faster.
“We recognize that people have a high cost to even start therapy, and this study really demonstrates the difference between people with and without a subsidy in initiating therapy,” Dr Dusetzina said. “The out-of-pocket costs may be delaying people starting these life-saving drugs.” ![]()
Factor IX therapy approved in Japan

Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved albutrepenonacog alfa (Idelvion) to treat and prevent bleeding in children and adults with hemophilia B.
Albutrepenonacog alfa is a fusion protein linking recombinant coagulation factor IX with recombinant albumin.
The product is now approved for use as routine prophylaxis, for on-demand control of bleeding, and for perioperative management of bleeding.
Albutrepenonacog alfa is being developed by CSL Behring.
The company says the product is the first and only hemophilia B therapy with up to 14-day dosing intervals.
According to CSL Behring, albutrepenonacog alfa can deliver high-level protection from bleeds by maintaining factor IX activity levels at an average of 20% in patients treated prophylactically every 7 days and an average of 12% in patients treated prophylactically every 14 days.
Albutrepenonacog alfa has also been approved in Canada, the European Union, Switzerland, and the US.
Phase 3 trial
The MHLW approved albutrepenonacog alfa based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were published in Blood. The study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()

Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved albutrepenonacog alfa (Idelvion) to treat and prevent bleeding in children and adults with hemophilia B.
Albutrepenonacog alfa is a fusion protein linking recombinant coagulation factor IX with recombinant albumin.
The product is now approved for use as routine prophylaxis, for on-demand control of bleeding, and for perioperative management of bleeding.
Albutrepenonacog alfa is being developed by CSL Behring.
The company says the product is the first and only hemophilia B therapy with up to 14-day dosing intervals.
According to CSL Behring, albutrepenonacog alfa can deliver high-level protection from bleeds by maintaining factor IX activity levels at an average of 20% in patients treated prophylactically every 7 days and an average of 12% in patients treated prophylactically every 14 days.
Albutrepenonacog alfa has also been approved in Canada, the European Union, Switzerland, and the US.
Phase 3 trial
The MHLW approved albutrepenonacog alfa based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were published in Blood. The study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()

Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved albutrepenonacog alfa (Idelvion) to treat and prevent bleeding in children and adults with hemophilia B.
Albutrepenonacog alfa is a fusion protein linking recombinant coagulation factor IX with recombinant albumin.
The product is now approved for use as routine prophylaxis, for on-demand control of bleeding, and for perioperative management of bleeding.
Albutrepenonacog alfa is being developed by CSL Behring.
The company says the product is the first and only hemophilia B therapy with up to 14-day dosing intervals.
According to CSL Behring, albutrepenonacog alfa can deliver high-level protection from bleeds by maintaining factor IX activity levels at an average of 20% in patients treated prophylactically every 7 days and an average of 12% in patients treated prophylactically every 14 days.
Albutrepenonacog alfa has also been approved in Canada, the European Union, Switzerland, and the US.
Phase 3 trial
The MHLW approved albutrepenonacog alfa based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were published in Blood. The study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache.
Ponatinib approved to treat CML, ALL in Japan

Image from UCSD
The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has approved 2 uses of the tyrosine kinase inhibitor (TKI) ponatinib (Iclusig®).
The drug is now approved to treat recurrent or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myeloid leukemia (CML) that was resistant to or intolerant of prior treatment.
Ponatinib will be manufactured and sold by Otsuka Pharmaceutical Co., Ltd.
Due to the limited existing treatment options for patients in Japan, Otsuka said it will provide access to ponatinib free of charge as soon as procedures are in place from an ethical standpoint.
This program will be offered at medical institutions where clinical trials of ponatinib were performed and which are amenable to accepting the drug access program until the product is listed on the Japan National Health Insurance price list.
About ponatinib
Ponatinib is a TKI discovered by ARIAD Pharmaceuticals, Inc. The drug has demonstrated activity against native and mutated BCR-ABL and other kinases.
The PMDA’s approval of ponatinib for CML and Ph+ ALL is based on data from a phase 1/2 trial of Japanese patients, a phase 1 trial, and the phase 2 PACE trial.
Extended follow-up data from the PACE trial, collected in 2013, suggested ponatinib can increase the risk of thrombotic events. When these data came to light, officials in the European Union and the US, where ponatinib had already been approved, began to investigate the drug.
Ponatinib was pulled from the US market for a little over 2 months, and trials of the TKI were placed on partial hold while the US Food and Drug Administration evaluated the drug’s safety. Ponatinib went back on the market in January 2014, with new safety measures in place.
Ponatinib was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of the TKI. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided its benefits outweigh its risks.
In addition to the European Union and the US, ponatinib has been approved in Australia, Canada, Israel, and Switzerland.

Image from UCSD
The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has approved 2 uses of the tyrosine kinase inhibitor (TKI) ponatinib (Iclusig®).
The drug is now approved to treat recurrent or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myeloid leukemia (CML) that was resistant to or intolerant of prior treatment.
Ponatinib will be manufactured and sold by Otsuka Pharmaceutical Co., Ltd.
Due to the limited existing treatment options for patients in Japan, Otsuka said it will provide access to ponatinib free of charge as soon as procedures are in place from an ethical standpoint.
This program will be offered at medical institutions where clinical trials of ponatinib were performed and which are amenable to accepting the drug access program until the product is listed on the Japan National Health Insurance price list.
About ponatinib
Ponatinib is a TKI discovered by ARIAD Pharmaceuticals, Inc. The drug has demonstrated activity against native and mutated BCR-ABL and other kinases.
The PMDA’s approval of ponatinib for CML and Ph+ ALL is based on data from a phase 1/2 trial of Japanese patients, a phase 1 trial, and the phase 2 PACE trial.
Extended follow-up data from the PACE trial, collected in 2013, suggested ponatinib can increase the risk of thrombotic events. When these data came to light, officials in the European Union and the US, where ponatinib had already been approved, began to investigate the drug.
Ponatinib was pulled from the US market for a little over 2 months, and trials of the TKI were placed on partial hold while the US Food and Drug Administration evaluated the drug’s safety. Ponatinib went back on the market in January 2014, with new safety measures in place.
Ponatinib was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of the TKI. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided its benefits outweigh its risks.
In addition to the European Union and the US, ponatinib has been approved in Australia, Canada, Israel, and Switzerland.

Image from UCSD
The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has approved 2 uses of the tyrosine kinase inhibitor (TKI) ponatinib (Iclusig®).
The drug is now approved to treat recurrent or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myeloid leukemia (CML) that was resistant to or intolerant of prior treatment.
Ponatinib will be manufactured and sold by Otsuka Pharmaceutical Co., Ltd.
Due to the limited existing treatment options for patients in Japan, Otsuka said it will provide access to ponatinib free of charge as soon as procedures are in place from an ethical standpoint.
This program will be offered at medical institutions where clinical trials of ponatinib were performed and which are amenable to accepting the drug access program until the product is listed on the Japan National Health Insurance price list.
About ponatinib
Ponatinib is a TKI discovered by ARIAD Pharmaceuticals, Inc. The drug has demonstrated activity against native and mutated BCR-ABL and other kinases.
The PMDA’s approval of ponatinib for CML and Ph+ ALL is based on data from a phase 1/2 trial of Japanese patients, a phase 1 trial, and the phase 2 PACE trial.
Extended follow-up data from the PACE trial, collected in 2013, suggested ponatinib can increase the risk of thrombotic events. When these data came to light, officials in the European Union and the US, where ponatinib had already been approved, began to investigate the drug.
Ponatinib was pulled from the US market for a little over 2 months, and trials of the TKI were placed on partial hold while the US Food and Drug Administration evaluated the drug’s safety. Ponatinib went back on the market in January 2014, with new safety measures in place.
Ponatinib was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of the TKI. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided its benefits outweigh its risks.
In addition to the European Union and the US, ponatinib has been approved in Australia, Canada, Israel, and Switzerland.
Combo disappoints in newly diagnosed MM

Top-line results from the phase 3 CLARION trial suggest that treatment with carfilzomib, melphalan, and prednisone (KMP) is not superior to treatment with bortezomib, melphalan, and prednisone (VMP).
The trial was designed to compare KMP and VMP in patients with newly diagnosed multiple myeloma (MM) who were ineligible for hematopoietic stem cell transplant.
The results showed that progression-free survival (PFS) rates were similar with the 2 regimens.
And although overall survival data are not yet mature, there seems to be a trend favoring the VMP regimen.
Amgen, the company developing carfilzomib, released these results yesterday.
“The CLARION results, generated in the context of a melphalan-containing regimen, are disappointing, especially given the robust data we’ve seen in the second-line setting,” said Sean E. Harper, MD, executive vice president of Research and Development at Amgen.
“However, the myeloma landscape has changed dramatically since the design of the CLARION study, with very few newly diagnosed patients treated with
melphalan-based regimens, particularly in the US. We remain committed to exploring Kyprolis in combination with other agents to advance the treatment of multiple myeloma.”
Dr Harper said he could not comment on whether the CLARION trial will continue, as Amgen hopes to present data from the trial at the 2016 ASH Annual Meeting.
The CLARION trial is a head-to-head, randomized study in transplant-ineligible patients with newly diagnosed MM. A total of 955 patients were randomized 1:1 to receive KMP or VMP for 54 weeks. The median patient age was 72.
The trial did not meet the primary endpoint of superiority in PFS. The median PFS was 22.3 months in the KMP arm and 22.1 months in the VMP arm. The hazard ratio was 0.91 (95% CI, 0.75-1.10), and the difference between the arms was not statistically significant.
The data for overall survival, a secondary endpoint, are not yet mature. But the observed hazard ratio was 1.21 (95% CI, 0.90-1.64), and there was no significant difference between the treatment arms.
The incidence of grade 3 or higher adverse events was 74.7% in the KMP arm and 76.2% in the VMP arm.
The incidence of grade 2 or higher peripheral neuropathy, a secondary endpoint, was 2.5% in the KMP arm and 35.1% in the VMP arm.
Fatal treatment-emergent adverse events occurred in 6.5% of patients in the KMP arm and 4.3% of those in the VMP arm.

Top-line results from the phase 3 CLARION trial suggest that treatment with carfilzomib, melphalan, and prednisone (KMP) is not superior to treatment with bortezomib, melphalan, and prednisone (VMP).
The trial was designed to compare KMP and VMP in patients with newly diagnosed multiple myeloma (MM) who were ineligible for hematopoietic stem cell transplant.
The results showed that progression-free survival (PFS) rates were similar with the 2 regimens.
And although overall survival data are not yet mature, there seems to be a trend favoring the VMP regimen.
Amgen, the company developing carfilzomib, released these results yesterday.
“The CLARION results, generated in the context of a melphalan-containing regimen, are disappointing, especially given the robust data we’ve seen in the second-line setting,” said Sean E. Harper, MD, executive vice president of Research and Development at Amgen.
“However, the myeloma landscape has changed dramatically since the design of the CLARION study, with very few newly diagnosed patients treated with
melphalan-based regimens, particularly in the US. We remain committed to exploring Kyprolis in combination with other agents to advance the treatment of multiple myeloma.”
Dr Harper said he could not comment on whether the CLARION trial will continue, as Amgen hopes to present data from the trial at the 2016 ASH Annual Meeting.
The CLARION trial is a head-to-head, randomized study in transplant-ineligible patients with newly diagnosed MM. A total of 955 patients were randomized 1:1 to receive KMP or VMP for 54 weeks. The median patient age was 72.
The trial did not meet the primary endpoint of superiority in PFS. The median PFS was 22.3 months in the KMP arm and 22.1 months in the VMP arm. The hazard ratio was 0.91 (95% CI, 0.75-1.10), and the difference between the arms was not statistically significant.
The data for overall survival, a secondary endpoint, are not yet mature. But the observed hazard ratio was 1.21 (95% CI, 0.90-1.64), and there was no significant difference between the treatment arms.
The incidence of grade 3 or higher adverse events was 74.7% in the KMP arm and 76.2% in the VMP arm.
The incidence of grade 2 or higher peripheral neuropathy, a secondary endpoint, was 2.5% in the KMP arm and 35.1% in the VMP arm.
Fatal treatment-emergent adverse events occurred in 6.5% of patients in the KMP arm and 4.3% of those in the VMP arm.

Top-line results from the phase 3 CLARION trial suggest that treatment with carfilzomib, melphalan, and prednisone (KMP) is not superior to treatment with bortezomib, melphalan, and prednisone (VMP).
The trial was designed to compare KMP and VMP in patients with newly diagnosed multiple myeloma (MM) who were ineligible for hematopoietic stem cell transplant.
The results showed that progression-free survival (PFS) rates were similar with the 2 regimens.
And although overall survival data are not yet mature, there seems to be a trend favoring the VMP regimen.
Amgen, the company developing carfilzomib, released these results yesterday.
“The CLARION results, generated in the context of a melphalan-containing regimen, are disappointing, especially given the robust data we’ve seen in the second-line setting,” said Sean E. Harper, MD, executive vice president of Research and Development at Amgen.
“However, the myeloma landscape has changed dramatically since the design of the CLARION study, with very few newly diagnosed patients treated with
melphalan-based regimens, particularly in the US. We remain committed to exploring Kyprolis in combination with other agents to advance the treatment of multiple myeloma.”
Dr Harper said he could not comment on whether the CLARION trial will continue, as Amgen hopes to present data from the trial at the 2016 ASH Annual Meeting.
The CLARION trial is a head-to-head, randomized study in transplant-ineligible patients with newly diagnosed MM. A total of 955 patients were randomized 1:1 to receive KMP or VMP for 54 weeks. The median patient age was 72.
The trial did not meet the primary endpoint of superiority in PFS. The median PFS was 22.3 months in the KMP arm and 22.1 months in the VMP arm. The hazard ratio was 0.91 (95% CI, 0.75-1.10), and the difference between the arms was not statistically significant.
The data for overall survival, a secondary endpoint, are not yet mature. But the observed hazard ratio was 1.21 (95% CI, 0.90-1.64), and there was no significant difference between the treatment arms.
The incidence of grade 3 or higher adverse events was 74.7% in the KMP arm and 76.2% in the VMP arm.
The incidence of grade 2 or higher peripheral neuropathy, a secondary endpoint, was 2.5% in the KMP arm and 35.1% in the VMP arm.
Fatal treatment-emergent adverse events occurred in 6.5% of patients in the KMP arm and 4.3% of those in the VMP arm.
Malaria vaccine receives fast track designation

Image by Ute Frevert
and Margaret Shear
The US Food and Drug Administration (FDA) has granted fast track designation to an investigational malaria vaccine.
Sanaria® PfSPZ Vaccine is composed of live but weakened Plasmodium falciparum sporozoites and is being developed by Sanaria, Inc.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
Trials of Sanaria® PfSPZ Vaccine
In a phase 1 study published in Science in 2013, 80% of patients who received higher doses of Sanaria® PfSPZ Vaccine were protected from malaria at 3 weeks after vaccination.
In another phase 1 study published in Nature Medicine in 2016, 4 doses of the vaccine protected 73% of subjects from malaria at 3 weeks and 55% of subjects at 21 weeks.
Five of the subjects without parasitemia at 21 weeks were exposed to malaria-carrying mosquitoes again at 59 weeks, and none developed parasitemia.
To date, 1165 volunteers have received Sanaria’s PfSPZ-based products in more than 2 dozen clinical trials in the US, Europe, and Africa.
Clinical trials are in progress in Tanzania, Kenya, Mali, Burkina Faso, Germany, and the US, and are intended to begin soon in Equatorial Guinea.

Image by Ute Frevert
and Margaret Shear
The US Food and Drug Administration (FDA) has granted fast track designation to an investigational malaria vaccine.
Sanaria® PfSPZ Vaccine is composed of live but weakened Plasmodium falciparum sporozoites and is being developed by Sanaria, Inc.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
Trials of Sanaria® PfSPZ Vaccine
In a phase 1 study published in Science in 2013, 80% of patients who received higher doses of Sanaria® PfSPZ Vaccine were protected from malaria at 3 weeks after vaccination.
In another phase 1 study published in Nature Medicine in 2016, 4 doses of the vaccine protected 73% of subjects from malaria at 3 weeks and 55% of subjects at 21 weeks.
Five of the subjects without parasitemia at 21 weeks were exposed to malaria-carrying mosquitoes again at 59 weeks, and none developed parasitemia.
To date, 1165 volunteers have received Sanaria’s PfSPZ-based products in more than 2 dozen clinical trials in the US, Europe, and Africa.
Clinical trials are in progress in Tanzania, Kenya, Mali, Burkina Faso, Germany, and the US, and are intended to begin soon in Equatorial Guinea.

Image by Ute Frevert
and Margaret Shear
The US Food and Drug Administration (FDA) has granted fast track designation to an investigational malaria vaccine.
Sanaria® PfSPZ Vaccine is composed of live but weakened Plasmodium falciparum sporozoites and is being developed by Sanaria, Inc.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
Trials of Sanaria® PfSPZ Vaccine
In a phase 1 study published in Science in 2013, 80% of patients who received higher doses of Sanaria® PfSPZ Vaccine were protected from malaria at 3 weeks after vaccination.
In another phase 1 study published in Nature Medicine in 2016, 4 doses of the vaccine protected 73% of subjects from malaria at 3 weeks and 55% of subjects at 21 weeks.
Five of the subjects without parasitemia at 21 weeks were exposed to malaria-carrying mosquitoes again at 59 weeks, and none developed parasitemia.
To date, 1165 volunteers have received Sanaria’s PfSPZ-based products in more than 2 dozen clinical trials in the US, Europe, and Africa.
Clinical trials are in progress in Tanzania, Kenya, Mali, Burkina Faso, Germany, and the US, and are intended to begin soon in Equatorial Guinea.