User login
FDA updates warning about Treanda

Photo by Rhoda Baer
Last March, the US Food and Drug Administration (FDA) issued a statement warning healthcare professionals not to use the chemotherapy drug Treanda Injection (bendamustine hydrochloride) with closed system transfer devices (CSTDs), adapters, and syringes containing polycarbonate or acrylonitrile-butadiene-styrene (ABS).
Now, the FDA is providing a list of devices that were tested and deemed compatible with the drug (see the tables below).
The devices were tested by Treanda’s manufacturer, Teva Pharmaceuticals.
Treanda is used to treat patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Treanda is available in 2 formulations: a solution, Treanda Injection (45 mg/0.5 mL or 180 mg/2 mL solution), and a lyophilized powder, Treanda for Injection (25 mg/vial or 100 mg/vial lyophilized powder). The information discussed here is referring to compatibility with the solution, Treanda Injection.
Treanda Injection contains N, N-dimethylacetamide (DMA), which is incompatible with devices that contain polycarbonate or ABS. Devices including CSTDs, adapters, and syringes that contain polycarbonate or ABS have been shown to dissolve when they come in contact with DMA in the drug.
This incompatibility leads to device failure, such as leaking, breaking, or operational failure of CSTD components; possible product contamination; and potential serious adverse health consequences to practitioners, such as skin reactions, or to patients, including the risk of small blood vessel blockage if the product is contaminated with dissolved ABS or polycarbonate.
Users should contact device manufacturers prior to using the specific devices listed below to ensure there have been no changes made to the material composition of the devices and that the devices are compatible with Treanda use.
Table 1. The compatibility of Treanda Injection with specific CSTDs, syringes, vial adapters, and gloves (based on testing conducted by Teva from February 2015 through June 2015).
| Component tested | Component brand name (part number) |
| Closed system transfer devices (CSTDs) | BD Phaseal System consisting of:
BD Phaseal Protector P14 (REF 515100), BD Phaseal Injector Luer Lock N35 (REF 515003), BD Phaseal Infusion Adapter C100 (REF 515306), BD syringe 5 mL (REF 309646 and 309657) |
| Vial adapters | Baxter CHEMO-AIDE Dispensing Pin (REF 2N9106)
Medimop Swabable Vial Adapter (REF 8070101) Alaris Smartsite (REF 2202E and 2203E) |
| Polypropylene syringes | BD (Becton Dickinson), 5 mL (REF 309646) and 3 mL (REF 309657)
Covidien Monoject, 5 mL (REF 1180600777) and 3 mL (REF 1180300777) B. Braun, 5 mL (REF 4617053V-02) and 3 mL (REF 4610303-02) Air-Tite Norm Jet, 5 mL (REF 4050.X00V0) and 3 mL (REF 4020.X00V0) Medline, 5 mL (REF SYR105010) and 3 mL (REF SYR103010) Terumo, 5 mL (REF SS-05L) |
| Disposable gloves* | ChemoPlus (REF CT0194-1)
EP-Blue (REF 181350) Jackson Safety G29 (REF 49824) NeoPro (REF NPG-888) NitriDerm (REF 182350) Purple (REF 50604) Purple KC 500 (REF 55084) UltraSense EC (REF USE-880) |
*Part numbers reflect a specific size glove used in the compatibility tests.
Table 2. The IV administration set found to be compatible with Treanda Injection after dilution in a 500 mL 0.9% sodium chloride IV infusion bags (based on testing conducted by Teva from February 2015 through June 2015*).
| Component tested | Brand name (part number) |
| IV administration sets | B. Braun Safeline (REF NF3482) and AdditIV (REF V1921)
Baxter DuoVent Spike (REF 2C7575) and Clearlink System (2H8480) BD Phaseal Secondary set (REF 515301) ICU Medical Clave (REF CH3011) |
*Compatibility studies did not include testing with 2.5% dextrose/0.45% sodium chloride injection. However, the results of these studies are not expected to change. So either diluent, 0.9% sodium chloride or 2.5% dextrose/0.45% sodium chloride injection, can be used with Treanda injection.
The FDA required label changes for both the solution and the powder formulations of Treanda to include information for safe preparation and handling for IV administration. See the full prescribing information for details.
For more details on the compatibility of Treanda Injection with specific CSTDs, syringes, vial adapters, gloves, and IV administration sets, see Teva’s Dear Health Care Provider letter.
Adverse events or quality problems associated with the use of Treanda products can be reported to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

Photo by Rhoda Baer
Last March, the US Food and Drug Administration (FDA) issued a statement warning healthcare professionals not to use the chemotherapy drug Treanda Injection (bendamustine hydrochloride) with closed system transfer devices (CSTDs), adapters, and syringes containing polycarbonate or acrylonitrile-butadiene-styrene (ABS).
Now, the FDA is providing a list of devices that were tested and deemed compatible with the drug (see the tables below).
The devices were tested by Treanda’s manufacturer, Teva Pharmaceuticals.
Treanda is used to treat patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Treanda is available in 2 formulations: a solution, Treanda Injection (45 mg/0.5 mL or 180 mg/2 mL solution), and a lyophilized powder, Treanda for Injection (25 mg/vial or 100 mg/vial lyophilized powder). The information discussed here is referring to compatibility with the solution, Treanda Injection.
Treanda Injection contains N, N-dimethylacetamide (DMA), which is incompatible with devices that contain polycarbonate or ABS. Devices including CSTDs, adapters, and syringes that contain polycarbonate or ABS have been shown to dissolve when they come in contact with DMA in the drug.
This incompatibility leads to device failure, such as leaking, breaking, or operational failure of CSTD components; possible product contamination; and potential serious adverse health consequences to practitioners, such as skin reactions, or to patients, including the risk of small blood vessel blockage if the product is contaminated with dissolved ABS or polycarbonate.
Users should contact device manufacturers prior to using the specific devices listed below to ensure there have been no changes made to the material composition of the devices and that the devices are compatible with Treanda use.
Table 1. The compatibility of Treanda Injection with specific CSTDs, syringes, vial adapters, and gloves (based on testing conducted by Teva from February 2015 through June 2015).
| Component tested | Component brand name (part number) |
| Closed system transfer devices (CSTDs) | BD Phaseal System consisting of:
BD Phaseal Protector P14 (REF 515100), BD Phaseal Injector Luer Lock N35 (REF 515003), BD Phaseal Infusion Adapter C100 (REF 515306), BD syringe 5 mL (REF 309646 and 309657) |
| Vial adapters | Baxter CHEMO-AIDE Dispensing Pin (REF 2N9106)
Medimop Swabable Vial Adapter (REF 8070101) Alaris Smartsite (REF 2202E and 2203E) |
| Polypropylene syringes | BD (Becton Dickinson), 5 mL (REF 309646) and 3 mL (REF 309657)
Covidien Monoject, 5 mL (REF 1180600777) and 3 mL (REF 1180300777) B. Braun, 5 mL (REF 4617053V-02) and 3 mL (REF 4610303-02) Air-Tite Norm Jet, 5 mL (REF 4050.X00V0) and 3 mL (REF 4020.X00V0) Medline, 5 mL (REF SYR105010) and 3 mL (REF SYR103010) Terumo, 5 mL (REF SS-05L) |
| Disposable gloves* | ChemoPlus (REF CT0194-1)
EP-Blue (REF 181350) Jackson Safety G29 (REF 49824) NeoPro (REF NPG-888) NitriDerm (REF 182350) Purple (REF 50604) Purple KC 500 (REF 55084) UltraSense EC (REF USE-880) |
*Part numbers reflect a specific size glove used in the compatibility tests.
Table 2. The IV administration set found to be compatible with Treanda Injection after dilution in a 500 mL 0.9% sodium chloride IV infusion bags (based on testing conducted by Teva from February 2015 through June 2015*).
| Component tested | Brand name (part number) |
| IV administration sets | B. Braun Safeline (REF NF3482) and AdditIV (REF V1921)
Baxter DuoVent Spike (REF 2C7575) and Clearlink System (2H8480) BD Phaseal Secondary set (REF 515301) ICU Medical Clave (REF CH3011) |
*Compatibility studies did not include testing with 2.5% dextrose/0.45% sodium chloride injection. However, the results of these studies are not expected to change. So either diluent, 0.9% sodium chloride or 2.5% dextrose/0.45% sodium chloride injection, can be used with Treanda injection.
The FDA required label changes for both the solution and the powder formulations of Treanda to include information for safe preparation and handling for IV administration. See the full prescribing information for details.
For more details on the compatibility of Treanda Injection with specific CSTDs, syringes, vial adapters, gloves, and IV administration sets, see Teva’s Dear Health Care Provider letter.
Adverse events or quality problems associated with the use of Treanda products can be reported to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

Photo by Rhoda Baer
Last March, the US Food and Drug Administration (FDA) issued a statement warning healthcare professionals not to use the chemotherapy drug Treanda Injection (bendamustine hydrochloride) with closed system transfer devices (CSTDs), adapters, and syringes containing polycarbonate or acrylonitrile-butadiene-styrene (ABS).
Now, the FDA is providing a list of devices that were tested and deemed compatible with the drug (see the tables below).
The devices were tested by Treanda’s manufacturer, Teva Pharmaceuticals.
Treanda is used to treat patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Treanda is available in 2 formulations: a solution, Treanda Injection (45 mg/0.5 mL or 180 mg/2 mL solution), and a lyophilized powder, Treanda for Injection (25 mg/vial or 100 mg/vial lyophilized powder). The information discussed here is referring to compatibility with the solution, Treanda Injection.
Treanda Injection contains N, N-dimethylacetamide (DMA), which is incompatible with devices that contain polycarbonate or ABS. Devices including CSTDs, adapters, and syringes that contain polycarbonate or ABS have been shown to dissolve when they come in contact with DMA in the drug.
This incompatibility leads to device failure, such as leaking, breaking, or operational failure of CSTD components; possible product contamination; and potential serious adverse health consequences to practitioners, such as skin reactions, or to patients, including the risk of small blood vessel blockage if the product is contaminated with dissolved ABS or polycarbonate.
Users should contact device manufacturers prior to using the specific devices listed below to ensure there have been no changes made to the material composition of the devices and that the devices are compatible with Treanda use.
Table 1. The compatibility of Treanda Injection with specific CSTDs, syringes, vial adapters, and gloves (based on testing conducted by Teva from February 2015 through June 2015).
| Component tested | Component brand name (part number) |
| Closed system transfer devices (CSTDs) | BD Phaseal System consisting of:
BD Phaseal Protector P14 (REF 515100), BD Phaseal Injector Luer Lock N35 (REF 515003), BD Phaseal Infusion Adapter C100 (REF 515306), BD syringe 5 mL (REF 309646 and 309657) |
| Vial adapters | Baxter CHEMO-AIDE Dispensing Pin (REF 2N9106)
Medimop Swabable Vial Adapter (REF 8070101) Alaris Smartsite (REF 2202E and 2203E) |
| Polypropylene syringes | BD (Becton Dickinson), 5 mL (REF 309646) and 3 mL (REF 309657)
Covidien Monoject, 5 mL (REF 1180600777) and 3 mL (REF 1180300777) B. Braun, 5 mL (REF 4617053V-02) and 3 mL (REF 4610303-02) Air-Tite Norm Jet, 5 mL (REF 4050.X00V0) and 3 mL (REF 4020.X00V0) Medline, 5 mL (REF SYR105010) and 3 mL (REF SYR103010) Terumo, 5 mL (REF SS-05L) |
| Disposable gloves* | ChemoPlus (REF CT0194-1)
EP-Blue (REF 181350) Jackson Safety G29 (REF 49824) NeoPro (REF NPG-888) NitriDerm (REF 182350) Purple (REF 50604) Purple KC 500 (REF 55084) UltraSense EC (REF USE-880) |
*Part numbers reflect a specific size glove used in the compatibility tests.
Table 2. The IV administration set found to be compatible with Treanda Injection after dilution in a 500 mL 0.9% sodium chloride IV infusion bags (based on testing conducted by Teva from February 2015 through June 2015*).
| Component tested | Brand name (part number) |
| IV administration sets | B. Braun Safeline (REF NF3482) and AdditIV (REF V1921)
Baxter DuoVent Spike (REF 2C7575) and Clearlink System (2H8480) BD Phaseal Secondary set (REF 515301) ICU Medical Clave (REF CH3011) |
*Compatibility studies did not include testing with 2.5% dextrose/0.45% sodium chloride injection. However, the results of these studies are not expected to change. So either diluent, 0.9% sodium chloride or 2.5% dextrose/0.45% sodium chloride injection, can be used with Treanda injection.
The FDA required label changes for both the solution and the powder formulations of Treanda to include information for safe preparation and handling for IV administration. See the full prescribing information for details.
For more details on the compatibility of Treanda Injection with specific CSTDs, syringes, vial adapters, gloves, and IV administration sets, see Teva’s Dear Health Care Provider letter.
Adverse events or quality problems associated with the use of Treanda products can be reported to the FDA’s MedWatch Adverse Event Reporting Program. ![]()
Drug deemed ‘breakthrough’ for hemophilia A with inhibitors

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for ACE910 to prevent bleeding in hemophilia A patients age 12 and older who have factor VIII inhibitors.
ACE910 is the first factor VIIIa-mimetic bispecific antibody to be investigated for the prophylactic treatment of hemophilia A.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
The breakthrough therapy designation for ACE910 was granted based on results of a phase 1 study of ACE910 in patients with severe hemophilia A.
About ACE910
ACE910 is an investigational, humanized, bispecific monoclonal antibody engineered to simultaneously bind factors IXa and X. ACE910 thereby mimics the cofactor function of factor VIII and is designed to promote blood coagulation in hemophilia A patients, regardless of whether they have developed inhibitors to factor VIII.
ACE910 is administered subcutaneously once weekly. As it is distinct in structure from factor VIII, it is not expected to lead to the formation of factor VIII inhibitors.
ACE910 was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Genentech.
ACE910 research
Results of the phase 1 trial suggested that once-weekly, subcutaneous administration of ACE910 can reduce annualized bleeding rates (ABRs) in adults and adolescents with severe hemophilia A, with or without factor VIII inhibitors.
At ISTH 2015 (abstract AS017), researchers presented data on 18 Japanese patients with severe hemophilia A (factor VIII: C<1%, ages 12 to 58 years).
Patients received once-weekly subcutaneous ACE910 at one of the following dose levels: 0.3 mg/kg (cohort 1), 1 mg/kg (cohort 2), or 3 mg/kg (cohort 3). There were 6 patients in each cohort.
The patients were followed for 5.6 months to 18.5 months.
Efficacy
Whether or not they had inhibitors, patients experienced a decrease in ABR with ACE910. The changes in ABR per treatment cohort and according to inhibitor status are as follows:
| Treatment/patient type | N | ABR reduction | Median ABR change |
| Cohort 1 (0.3 mg/kg) without inhibitors | 2/6 | 22.8%-82.7% | 32.5→1.7 |
| Cohort 1 (0.3 mg/kg) with inhibitors | 4/6 | 49.3%-100% | |
| Cohort 2 (1 mg/kg) without inhibitors | 2/6 | 79.6%-100% | 18.3→0 |
| Cohort 2 (1 mg/kg) with inhibitors | 4/6 | 87.0%-100% | |
| Cohort 3 (3 mg/kg) without inhibitors | 3/6 | 0%*-100% | 15.2→0 |
| Cohort 3 (3 mg/kg) with inhibitors | 3/6 | 93.0%-100% |
*One patient did not report bleeding episodes at baseline or during the study.
Safety
There were 93 adverse events. The researchers said all events were of mild or moderate intensity. One patient discontinued ACE910 due to mild injection-site redness.
There were no thromboembolic events, even when ACE910 was given concomitantly with factor VIII products or bypassing agents as episodic treatment for breakthrough bleeds.
Three patients developed anti-ACE910 antibodies, but they did not affect ACE910 pharmacokinetics or pharmacodynamics.
Genentech is planning to initiate a phase 3 trial of ACE910 in patients with hemophilia A and factor VIII inhibitors by the end of 2015, a phase 3 trial in patients without inhibitors in 2016, and a trial in pediatric patients with hemophilia A in 2016. ![]()

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for ACE910 to prevent bleeding in hemophilia A patients age 12 and older who have factor VIII inhibitors.
ACE910 is the first factor VIIIa-mimetic bispecific antibody to be investigated for the prophylactic treatment of hemophilia A.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
The breakthrough therapy designation for ACE910 was granted based on results of a phase 1 study of ACE910 in patients with severe hemophilia A.
About ACE910
ACE910 is an investigational, humanized, bispecific monoclonal antibody engineered to simultaneously bind factors IXa and X. ACE910 thereby mimics the cofactor function of factor VIII and is designed to promote blood coagulation in hemophilia A patients, regardless of whether they have developed inhibitors to factor VIII.
ACE910 is administered subcutaneously once weekly. As it is distinct in structure from factor VIII, it is not expected to lead to the formation of factor VIII inhibitors.
ACE910 was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Genentech.
ACE910 research
Results of the phase 1 trial suggested that once-weekly, subcutaneous administration of ACE910 can reduce annualized bleeding rates (ABRs) in adults and adolescents with severe hemophilia A, with or without factor VIII inhibitors.
At ISTH 2015 (abstract AS017), researchers presented data on 18 Japanese patients with severe hemophilia A (factor VIII: C<1%, ages 12 to 58 years).
Patients received once-weekly subcutaneous ACE910 at one of the following dose levels: 0.3 mg/kg (cohort 1), 1 mg/kg (cohort 2), or 3 mg/kg (cohort 3). There were 6 patients in each cohort.
The patients were followed for 5.6 months to 18.5 months.
Efficacy
Whether or not they had inhibitors, patients experienced a decrease in ABR with ACE910. The changes in ABR per treatment cohort and according to inhibitor status are as follows:
| Treatment/patient type | N | ABR reduction | Median ABR change |
| Cohort 1 (0.3 mg/kg) without inhibitors | 2/6 | 22.8%-82.7% | 32.5→1.7 |
| Cohort 1 (0.3 mg/kg) with inhibitors | 4/6 | 49.3%-100% | |
| Cohort 2 (1 mg/kg) without inhibitors | 2/6 | 79.6%-100% | 18.3→0 |
| Cohort 2 (1 mg/kg) with inhibitors | 4/6 | 87.0%-100% | |
| Cohort 3 (3 mg/kg) without inhibitors | 3/6 | 0%*-100% | 15.2→0 |
| Cohort 3 (3 mg/kg) with inhibitors | 3/6 | 93.0%-100% |
*One patient did not report bleeding episodes at baseline or during the study.
Safety
There were 93 adverse events. The researchers said all events were of mild or moderate intensity. One patient discontinued ACE910 due to mild injection-site redness.
There were no thromboembolic events, even when ACE910 was given concomitantly with factor VIII products or bypassing agents as episodic treatment for breakthrough bleeds.
Three patients developed anti-ACE910 antibodies, but they did not affect ACE910 pharmacokinetics or pharmacodynamics.
Genentech is planning to initiate a phase 3 trial of ACE910 in patients with hemophilia A and factor VIII inhibitors by the end of 2015, a phase 3 trial in patients without inhibitors in 2016, and a trial in pediatric patients with hemophilia A in 2016. ![]()

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for ACE910 to prevent bleeding in hemophilia A patients age 12 and older who have factor VIII inhibitors.
ACE910 is the first factor VIIIa-mimetic bispecific antibody to be investigated for the prophylactic treatment of hemophilia A.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
The breakthrough therapy designation for ACE910 was granted based on results of a phase 1 study of ACE910 in patients with severe hemophilia A.
About ACE910
ACE910 is an investigational, humanized, bispecific monoclonal antibody engineered to simultaneously bind factors IXa and X. ACE910 thereby mimics the cofactor function of factor VIII and is designed to promote blood coagulation in hemophilia A patients, regardless of whether they have developed inhibitors to factor VIII.
ACE910 is administered subcutaneously once weekly. As it is distinct in structure from factor VIII, it is not expected to lead to the formation of factor VIII inhibitors.
ACE910 was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Genentech.
ACE910 research
Results of the phase 1 trial suggested that once-weekly, subcutaneous administration of ACE910 can reduce annualized bleeding rates (ABRs) in adults and adolescents with severe hemophilia A, with or without factor VIII inhibitors.
At ISTH 2015 (abstract AS017), researchers presented data on 18 Japanese patients with severe hemophilia A (factor VIII: C<1%, ages 12 to 58 years).
Patients received once-weekly subcutaneous ACE910 at one of the following dose levels: 0.3 mg/kg (cohort 1), 1 mg/kg (cohort 2), or 3 mg/kg (cohort 3). There were 6 patients in each cohort.
The patients were followed for 5.6 months to 18.5 months.
Efficacy
Whether or not they had inhibitors, patients experienced a decrease in ABR with ACE910. The changes in ABR per treatment cohort and according to inhibitor status are as follows:
| Treatment/patient type | N | ABR reduction | Median ABR change |
| Cohort 1 (0.3 mg/kg) without inhibitors | 2/6 | 22.8%-82.7% | 32.5→1.7 |
| Cohort 1 (0.3 mg/kg) with inhibitors | 4/6 | 49.3%-100% | |
| Cohort 2 (1 mg/kg) without inhibitors | 2/6 | 79.6%-100% | 18.3→0 |
| Cohort 2 (1 mg/kg) with inhibitors | 4/6 | 87.0%-100% | |
| Cohort 3 (3 mg/kg) without inhibitors | 3/6 | 0%*-100% | 15.2→0 |
| Cohort 3 (3 mg/kg) with inhibitors | 3/6 | 93.0%-100% |
*One patient did not report bleeding episodes at baseline or during the study.
Safety
There were 93 adverse events. The researchers said all events were of mild or moderate intensity. One patient discontinued ACE910 due to mild injection-site redness.
There were no thromboembolic events, even when ACE910 was given concomitantly with factor VIII products or bypassing agents as episodic treatment for breakthrough bleeds.
Three patients developed anti-ACE910 antibodies, but they did not affect ACE910 pharmacokinetics or pharmacodynamics.
Genentech is planning to initiate a phase 3 trial of ACE910 in patients with hemophilia A and factor VIII inhibitors by the end of 2015, a phase 3 trial in patients without inhibitors in 2016, and a trial in pediatric patients with hemophilia A in 2016. ![]()
FDA approves drug to prevent delayed CINV in adults

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved rolapitant (Varubi) for use in adult cancer patients receiving initial and repeat courses of emetogenic chemotherapy.
Rolapitant is to be used in combination with other antiemetic agents to prevent delayed chemotherapy-induced nausea and vomiting (CINV).
Tesaro, Inc., the company developing rolapitant, plans to launch the drug in the fourth quarter of this year.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 (NK-1) receptors, with a plasma half-life of approximately 7 days. Activation of NK-1 receptors plays a central role in CINV, particularly in the delayed phase (the 25- to 120-hour period after chemotherapy administration).
Rolapitant comes in tablet form. The recommended dose is 180 mg, given approximately 1 to 2 hours prior to chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone. No dosage adjustment is required for dexamethasone when administering rolapitant.
Rolapitant inhibits the CYP2D6 enzyme, so it is contraindicated with the use of thioridazine, a drug metabolized by the CYP2D6 enzyme. Use of these drugs together may increase the amount of thioridazine in the blood and cause an abnormal heart rhythm that can be serious.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were recently published in The Lancet Oncology.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (≥3%) were neutropenia (9% rolapitant and 8% control), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy regimens. Results from this trial were recently published in The Lancet Oncology.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (≥3%) were decreased appetite (9% rolapitant and 7% control), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%).
The full prescribing information for rolapitant is available at www.varubirx.com. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved rolapitant (Varubi) for use in adult cancer patients receiving initial and repeat courses of emetogenic chemotherapy.
Rolapitant is to be used in combination with other antiemetic agents to prevent delayed chemotherapy-induced nausea and vomiting (CINV).
Tesaro, Inc., the company developing rolapitant, plans to launch the drug in the fourth quarter of this year.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 (NK-1) receptors, with a plasma half-life of approximately 7 days. Activation of NK-1 receptors plays a central role in CINV, particularly in the delayed phase (the 25- to 120-hour period after chemotherapy administration).
Rolapitant comes in tablet form. The recommended dose is 180 mg, given approximately 1 to 2 hours prior to chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone. No dosage adjustment is required for dexamethasone when administering rolapitant.
Rolapitant inhibits the CYP2D6 enzyme, so it is contraindicated with the use of thioridazine, a drug metabolized by the CYP2D6 enzyme. Use of these drugs together may increase the amount of thioridazine in the blood and cause an abnormal heart rhythm that can be serious.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were recently published in The Lancet Oncology.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (≥3%) were neutropenia (9% rolapitant and 8% control), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy regimens. Results from this trial were recently published in The Lancet Oncology.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (≥3%) were decreased appetite (9% rolapitant and 7% control), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%).
The full prescribing information for rolapitant is available at www.varubirx.com. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved rolapitant (Varubi) for use in adult cancer patients receiving initial and repeat courses of emetogenic chemotherapy.
Rolapitant is to be used in combination with other antiemetic agents to prevent delayed chemotherapy-induced nausea and vomiting (CINV).
Tesaro, Inc., the company developing rolapitant, plans to launch the drug in the fourth quarter of this year.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 (NK-1) receptors, with a plasma half-life of approximately 7 days. Activation of NK-1 receptors plays a central role in CINV, particularly in the delayed phase (the 25- to 120-hour period after chemotherapy administration).
Rolapitant comes in tablet form. The recommended dose is 180 mg, given approximately 1 to 2 hours prior to chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone. No dosage adjustment is required for dexamethasone when administering rolapitant.
Rolapitant inhibits the CYP2D6 enzyme, so it is contraindicated with the use of thioridazine, a drug metabolized by the CYP2D6 enzyme. Use of these drugs together may increase the amount of thioridazine in the blood and cause an abnormal heart rhythm that can be serious.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were recently published in The Lancet Oncology.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (≥3%) were neutropenia (9% rolapitant and 8% control), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy regimens. Results from this trial were recently published in The Lancet Oncology.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (≥3%) were decreased appetite (9% rolapitant and 7% control), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%).
The full prescribing information for rolapitant is available at www.varubirx.com. ![]()
Eltrombopag approved to treat SAA in EU

The European Commission has approved eltrombopag (Revolade) for the treatment of adults with severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant.
Eltrombopag is the first therapy approved in the European Union (EU) for this patient population.
The approval applies to all 28 EU member states plus Iceland, Norway, and Liechtenstein.
Trials of eltrombopag in SAA
The European Commission’s approval is based primarily on results of a phase 2 pilot study (NCT00922883) conducted by the National Heart, Lung and Blood Institute at the National Institutes of Health.
Results from the ongoing study were published in NEJM in 2012 and Blood in 2013. The trial has enrolled 43 patients with SAA who had an insufficient response to at least 1 prior immunosuppressive therapy and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) had received at least 2 prior immunosuppressive therapies.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least 1 lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow-up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 1 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments.
The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()

The European Commission has approved eltrombopag (Revolade) for the treatment of adults with severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant.
Eltrombopag is the first therapy approved in the European Union (EU) for this patient population.
The approval applies to all 28 EU member states plus Iceland, Norway, and Liechtenstein.
Trials of eltrombopag in SAA
The European Commission’s approval is based primarily on results of a phase 2 pilot study (NCT00922883) conducted by the National Heart, Lung and Blood Institute at the National Institutes of Health.
Results from the ongoing study were published in NEJM in 2012 and Blood in 2013. The trial has enrolled 43 patients with SAA who had an insufficient response to at least 1 prior immunosuppressive therapy and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) had received at least 2 prior immunosuppressive therapies.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least 1 lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow-up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 1 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments.
The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()

The European Commission has approved eltrombopag (Revolade) for the treatment of adults with severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant.
Eltrombopag is the first therapy approved in the European Union (EU) for this patient population.
The approval applies to all 28 EU member states plus Iceland, Norway, and Liechtenstein.
Trials of eltrombopag in SAA
The European Commission’s approval is based primarily on results of a phase 2 pilot study (NCT00922883) conducted by the National Heart, Lung and Blood Institute at the National Institutes of Health.
Results from the ongoing study were published in NEJM in 2012 and Blood in 2013. The trial has enrolled 43 patients with SAA who had an insufficient response to at least 1 prior immunosuppressive therapy and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) had received at least 2 prior immunosuppressive therapies.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least 1 lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow-up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 1 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments.
The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()
FDA expands use of eltrombopag

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved an expanded use for eltrombopag (Promacta) to include children 1 year of age and older with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
The updated label also includes a new oral suspension formulation of eltrombopag designed for younger children who may not be able to swallow tablets.
Eltrombopag was previously approved by the FDA in a tablet formulation in June 2015 for ITP patients ages 6 and older and in 2008 for use in adults with ITP.
The label expansion of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT or AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient (1%).
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study.
Prescribing information
The recommended dose and schedule of eltrombopag for pediatric patients age 6 and older is 50 mg daily or 25 mg daily of the tablet formulation for patients with East Asian ancestry. The recommended dose for all patients age 1 to 5 years is 25 mg daily of the powder for oral suspension formulation.
Eltrombopag is marketed as Promacta in the US and Revolade in most other countries. For more information on the drug, see the full prescribing information. ![]()

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved an expanded use for eltrombopag (Promacta) to include children 1 year of age and older with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
The updated label also includes a new oral suspension formulation of eltrombopag designed for younger children who may not be able to swallow tablets.
Eltrombopag was previously approved by the FDA in a tablet formulation in June 2015 for ITP patients ages 6 and older and in 2008 for use in adults with ITP.
The label expansion of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT or AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient (1%).
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study.
Prescribing information
The recommended dose and schedule of eltrombopag for pediatric patients age 6 and older is 50 mg daily or 25 mg daily of the tablet formulation for patients with East Asian ancestry. The recommended dose for all patients age 1 to 5 years is 25 mg daily of the powder for oral suspension formulation.
Eltrombopag is marketed as Promacta in the US and Revolade in most other countries. For more information on the drug, see the full prescribing information. ![]()

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved an expanded use for eltrombopag (Promacta) to include children 1 year of age and older with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
The updated label also includes a new oral suspension formulation of eltrombopag designed for younger children who may not be able to swallow tablets.
Eltrombopag was previously approved by the FDA in a tablet formulation in June 2015 for ITP patients ages 6 and older and in 2008 for use in adults with ITP.
The label expansion of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT or AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient (1%).
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study.
Prescribing information
The recommended dose and schedule of eltrombopag for pediatric patients age 6 and older is 50 mg daily or 25 mg daily of the tablet formulation for patients with East Asian ancestry. The recommended dose for all patients age 1 to 5 years is 25 mg daily of the powder for oral suspension formulation.
Eltrombopag is marketed as Promacta in the US and Revolade in most other countries. For more information on the drug, see the full prescribing information. ![]()
Drug gets orphan designation for CDI
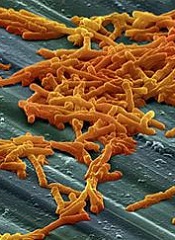
The US Food and Drug Administration (FDA) has granted orphan designation to SER-109 for the prevention of recurrent Clostridium difficile infection (CDI) in adults.
SER-109 is a microbiome therapeutic designed to treat recurrent CDI by correcting dysbiosis of the human microbiome.
In a single dose of 4 capsules, SER-109 re-introduces an ecology of purified bacterial spores that should restore the microbiome to a healthy state, allowing it to carry out key biological functions, including resisting Clostridium difficile.
“SER-109 is intended to re-introduce essential bacteria that restore the body’s natural resistance to CDI by re-establishing the ecology of the colonic microbiome,” explained Roger Pomerantz, MD, of Seres Therapeutics, Inc., the company developing SER-109.
“Because we’re focused on treating the underlying cause of the disease, we believe we have the potential to break the cycle of recurrent CDI and have a significant impact for patients.”
SER-109 is currently being investigated in a phase 2 trial. In addition to orphan designation, SER-109 has breakthrough designation from the FDA.
Trials of SER-109
Researchers reported phase 1/2 results with SER-109 at the 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy.
The study had 2 cohorts containing 15 patients each. Patients were between 18 and 90 years old, had 3 or more laboratory-confirmed CDI episodes over 1 year, had a life expectancy greater than 3 months, and were able to give informed consent.
Patients in cohort 1 received a mean SER-109 dose of 1.5 x 109 spores, and those in cohort 2 received a mean dose of 1 x 108 spores. SER-109 was deemed effective if patients did not have a CDI recurrence in the 8-week period after they received SER-109.
In cohort 1, 87% of patients (13/15) achieved the efficacy endpoint. Two patients had transient, self-limited diarrhea with a positive C difficile test, but both reached the week 8 endpoint without needing antibiotic therapy for CDI. Thus, in cohort 1, the clinical cure rate was 100%.
In cohort 2, 93% of patients (14/15) reached the 8-week endpoint CDI-free. One patient failed per protocol.
The researchers said there were no drug-related serious adverse events in this trial.
Seres Therapeutics is currently conducting a multicenter, randomized, placebo-controlled, phase 2 study (ECOSPOR) to assess the efficacy and safety of SER-109 in preventing recurrent CDI. The company expects results from this study to be available mid-2016.
About orphan and breakthrough designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
The benefits of breakthrough designation include the same benefits as fast track designation—priority review of a new drug application, rolling review, etc.—plus an organizational commitment involving the FDA’s senior managers with more intensive guidance from the FDA. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation to SER-109 for the prevention of recurrent Clostridium difficile infection (CDI) in adults.
SER-109 is a microbiome therapeutic designed to treat recurrent CDI by correcting dysbiosis of the human microbiome.
In a single dose of 4 capsules, SER-109 re-introduces an ecology of purified bacterial spores that should restore the microbiome to a healthy state, allowing it to carry out key biological functions, including resisting Clostridium difficile.
“SER-109 is intended to re-introduce essential bacteria that restore the body’s natural resistance to CDI by re-establishing the ecology of the colonic microbiome,” explained Roger Pomerantz, MD, of Seres Therapeutics, Inc., the company developing SER-109.
“Because we’re focused on treating the underlying cause of the disease, we believe we have the potential to break the cycle of recurrent CDI and have a significant impact for patients.”
SER-109 is currently being investigated in a phase 2 trial. In addition to orphan designation, SER-109 has breakthrough designation from the FDA.
Trials of SER-109
Researchers reported phase 1/2 results with SER-109 at the 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy.
The study had 2 cohorts containing 15 patients each. Patients were between 18 and 90 years old, had 3 or more laboratory-confirmed CDI episodes over 1 year, had a life expectancy greater than 3 months, and were able to give informed consent.
Patients in cohort 1 received a mean SER-109 dose of 1.5 x 109 spores, and those in cohort 2 received a mean dose of 1 x 108 spores. SER-109 was deemed effective if patients did not have a CDI recurrence in the 8-week period after they received SER-109.
In cohort 1, 87% of patients (13/15) achieved the efficacy endpoint. Two patients had transient, self-limited diarrhea with a positive C difficile test, but both reached the week 8 endpoint without needing antibiotic therapy for CDI. Thus, in cohort 1, the clinical cure rate was 100%.
In cohort 2, 93% of patients (14/15) reached the 8-week endpoint CDI-free. One patient failed per protocol.
The researchers said there were no drug-related serious adverse events in this trial.
Seres Therapeutics is currently conducting a multicenter, randomized, placebo-controlled, phase 2 study (ECOSPOR) to assess the efficacy and safety of SER-109 in preventing recurrent CDI. The company expects results from this study to be available mid-2016.
About orphan and breakthrough designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
The benefits of breakthrough designation include the same benefits as fast track designation—priority review of a new drug application, rolling review, etc.—plus an organizational commitment involving the FDA’s senior managers with more intensive guidance from the FDA. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation to SER-109 for the prevention of recurrent Clostridium difficile infection (CDI) in adults.
SER-109 is a microbiome therapeutic designed to treat recurrent CDI by correcting dysbiosis of the human microbiome.
In a single dose of 4 capsules, SER-109 re-introduces an ecology of purified bacterial spores that should restore the microbiome to a healthy state, allowing it to carry out key biological functions, including resisting Clostridium difficile.
“SER-109 is intended to re-introduce essential bacteria that restore the body’s natural resistance to CDI by re-establishing the ecology of the colonic microbiome,” explained Roger Pomerantz, MD, of Seres Therapeutics, Inc., the company developing SER-109.
“Because we’re focused on treating the underlying cause of the disease, we believe we have the potential to break the cycle of recurrent CDI and have a significant impact for patients.”
SER-109 is currently being investigated in a phase 2 trial. In addition to orphan designation, SER-109 has breakthrough designation from the FDA.
Trials of SER-109
Researchers reported phase 1/2 results with SER-109 at the 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy.
The study had 2 cohorts containing 15 patients each. Patients were between 18 and 90 years old, had 3 or more laboratory-confirmed CDI episodes over 1 year, had a life expectancy greater than 3 months, and were able to give informed consent.
Patients in cohort 1 received a mean SER-109 dose of 1.5 x 109 spores, and those in cohort 2 received a mean dose of 1 x 108 spores. SER-109 was deemed effective if patients did not have a CDI recurrence in the 8-week period after they received SER-109.
In cohort 1, 87% of patients (13/15) achieved the efficacy endpoint. Two patients had transient, self-limited diarrhea with a positive C difficile test, but both reached the week 8 endpoint without needing antibiotic therapy for CDI. Thus, in cohort 1, the clinical cure rate was 100%.
In cohort 2, 93% of patients (14/15) reached the 8-week endpoint CDI-free. One patient failed per protocol.
The researchers said there were no drug-related serious adverse events in this trial.
Seres Therapeutics is currently conducting a multicenter, randomized, placebo-controlled, phase 2 study (ECOSPOR) to assess the efficacy and safety of SER-109 in preventing recurrent CDI. The company expects results from this study to be available mid-2016.
About orphan and breakthrough designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
The benefits of breakthrough designation include the same benefits as fast track designation—priority review of a new drug application, rolling review, etc.—plus an organizational commitment involving the FDA’s senior managers with more intensive guidance from the FDA. ![]()
FDA approves brentuximab vedotin as consolidation

Photo from Business Wire
The US Food and Drug Administration (FDA) has approved brentuximab vedotin (Adcetris) for use as consolidation treatment after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with classical Hodgkin lymphoma (HL) at high risk of relapse or progression.
The drug is the first FDA-approved consolidation option available to these patients.
The approval was based on results of the phase 3 AETHERA trial.
Results from this trial also served to convert a prior accelerated approval of brentuximab vedotin to regular approval. The drug is now fully approved for the treatment of classical HL patients who have failed auto-HSCT and those who have failed at least 2 prior multi-agent chemotherapy regimens and are not candidates for auto-HSCT.
Brentuximab vedotin is also FDA-approved to treat patients with systemic anaplastic large-cell lymphoma who have failed at least 1 prior multi-agent chemotherapy regimen. The drug has accelerated approval for this indication based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
AETHERA trial
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following auto-HSCT. Results from the trial were published in The Lancet in March and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment in the AETHERA trial if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-auto-HSCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for patients who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()

Photo from Business Wire
The US Food and Drug Administration (FDA) has approved brentuximab vedotin (Adcetris) for use as consolidation treatment after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with classical Hodgkin lymphoma (HL) at high risk of relapse or progression.
The drug is the first FDA-approved consolidation option available to these patients.
The approval was based on results of the phase 3 AETHERA trial.
Results from this trial also served to convert a prior accelerated approval of brentuximab vedotin to regular approval. The drug is now fully approved for the treatment of classical HL patients who have failed auto-HSCT and those who have failed at least 2 prior multi-agent chemotherapy regimens and are not candidates for auto-HSCT.
Brentuximab vedotin is also FDA-approved to treat patients with systemic anaplastic large-cell lymphoma who have failed at least 1 prior multi-agent chemotherapy regimen. The drug has accelerated approval for this indication based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
AETHERA trial
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following auto-HSCT. Results from the trial were published in The Lancet in March and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment in the AETHERA trial if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-auto-HSCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for patients who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()

Photo from Business Wire
The US Food and Drug Administration (FDA) has approved brentuximab vedotin (Adcetris) for use as consolidation treatment after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with classical Hodgkin lymphoma (HL) at high risk of relapse or progression.
The drug is the first FDA-approved consolidation option available to these patients.
The approval was based on results of the phase 3 AETHERA trial.
Results from this trial also served to convert a prior accelerated approval of brentuximab vedotin to regular approval. The drug is now fully approved for the treatment of classical HL patients who have failed auto-HSCT and those who have failed at least 2 prior multi-agent chemotherapy regimens and are not candidates for auto-HSCT.
Brentuximab vedotin is also FDA-approved to treat patients with systemic anaplastic large-cell lymphoma who have failed at least 1 prior multi-agent chemotherapy regimen. The drug has accelerated approval for this indication based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
AETHERA trial
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following auto-HSCT. Results from the trial were published in The Lancet in March and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment in the AETHERA trial if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-auto-HSCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for patients who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138).
FDA approves new formulation of pain patch for cancer patients

receiving treatment
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a new formulation of fentanyl buccal soluble film CII (Onsolis), a patch used to manage breakthrough pain in adult cancer patients who are opioid-tolerant.
This decision allows BioDelivery Sciences International, Inc. (BDSI), the company developing Onsolis, to bring the product back to the US marketplace.
However, the company said this will not happen before 2016.
Onsolis is an opioid agonist indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and are tolerant to opioid therapy for their underlying persistent cancer pain.
Onsolis utilizes BioErodible MucoAdhesive drug delivery technology, which consists of a small, bioerodible polymer film that is applied to the inner lining of the cheek. Onsolis is the only differentiated fentanyl-containing product for this indication that provides buccal administration.
Onsolis off the US market
Onsolis was originally approved by the FDA in July 2009, but BDSI stopped manufacturing the product in March 2012, after the FDA uncovered 2 issues with Onsolis.
The FDA found that, during Onsolis’s 24-month shelf-life, microscopic crystals formed on the product, and the color faded slightly. BDSI said these changes did not affect the product’s underlying integrity or safety, but the FDA thought the fading color in particular might cause patients to question the product’s efficacy.
So the FDA required that Onsolis be modified before additional product could be manufactured and distributed. Supplies of Onsolis that were already on the market remained on the market.
An analysis by BDSI showed that the changes in Onsolis were related to an excipient used in the manufacturing process that could be removed to resolve the problem.
The excipient was specific to the manufacture of Onsolis in the US. Therefore, it did not impact the launch of Breakyl, which is the brand name for Onsolis in the European Union.
Return to market
After BDSI reformulated Onsolis to prevent the aforementioned changes in the product’s appearance, the FDA approved the product’s return to market.
“We are pleased to have obtained FDA approval of our [supplemental new drug application] and to now be in a position to move toward returning Onsolis to the US marketplace,” said Mark A. Sirgo, PharmD, President and Chief Executive Officer of BDSI.
“Although we have options for Onsolis, including commercializing it on our own, our current plan is to determine the value we can secure in a partnership with a company that has access to the target physician audience. We have been engaged with a number of potential partners, and, with this approval, we can now proceed forward with those discussions in earnest. We will provide more definitive timing in the near future about the reintroduction, but this would not be prior to 2016.”
Once Onsolis does return to the market, it will only be available via the Transmucosal Immediate Release Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS) program. This is an FDA-required program designed to mitigate the risk of misuse, abuse, addiction, overdose, and serious complications due to medication errors with the use of TIRF medicines.
Outpatients, healthcare professionals who prescribe to outpatients, pharmacies, and distributors must enroll in the program to receive Onsolis. Further information is available at www.TIRFREMSAccess.com.

receiving treatment
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a new formulation of fentanyl buccal soluble film CII (Onsolis), a patch used to manage breakthrough pain in adult cancer patients who are opioid-tolerant.
This decision allows BioDelivery Sciences International, Inc. (BDSI), the company developing Onsolis, to bring the product back to the US marketplace.
However, the company said this will not happen before 2016.
Onsolis is an opioid agonist indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and are tolerant to opioid therapy for their underlying persistent cancer pain.
Onsolis utilizes BioErodible MucoAdhesive drug delivery technology, which consists of a small, bioerodible polymer film that is applied to the inner lining of the cheek. Onsolis is the only differentiated fentanyl-containing product for this indication that provides buccal administration.
Onsolis off the US market
Onsolis was originally approved by the FDA in July 2009, but BDSI stopped manufacturing the product in March 2012, after the FDA uncovered 2 issues with Onsolis.
The FDA found that, during Onsolis’s 24-month shelf-life, microscopic crystals formed on the product, and the color faded slightly. BDSI said these changes did not affect the product’s underlying integrity or safety, but the FDA thought the fading color in particular might cause patients to question the product’s efficacy.
So the FDA required that Onsolis be modified before additional product could be manufactured and distributed. Supplies of Onsolis that were already on the market remained on the market.
An analysis by BDSI showed that the changes in Onsolis were related to an excipient used in the manufacturing process that could be removed to resolve the problem.
The excipient was specific to the manufacture of Onsolis in the US. Therefore, it did not impact the launch of Breakyl, which is the brand name for Onsolis in the European Union.
Return to market
After BDSI reformulated Onsolis to prevent the aforementioned changes in the product’s appearance, the FDA approved the product’s return to market.
“We are pleased to have obtained FDA approval of our [supplemental new drug application] and to now be in a position to move toward returning Onsolis to the US marketplace,” said Mark A. Sirgo, PharmD, President and Chief Executive Officer of BDSI.
“Although we have options for Onsolis, including commercializing it on our own, our current plan is to determine the value we can secure in a partnership with a company that has access to the target physician audience. We have been engaged with a number of potential partners, and, with this approval, we can now proceed forward with those discussions in earnest. We will provide more definitive timing in the near future about the reintroduction, but this would not be prior to 2016.”
Once Onsolis does return to the market, it will only be available via the Transmucosal Immediate Release Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS) program. This is an FDA-required program designed to mitigate the risk of misuse, abuse, addiction, overdose, and serious complications due to medication errors with the use of TIRF medicines.
Outpatients, healthcare professionals who prescribe to outpatients, pharmacies, and distributors must enroll in the program to receive Onsolis. Further information is available at www.TIRFREMSAccess.com.

receiving treatment
Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a new formulation of fentanyl buccal soluble film CII (Onsolis), a patch used to manage breakthrough pain in adult cancer patients who are opioid-tolerant.
This decision allows BioDelivery Sciences International, Inc. (BDSI), the company developing Onsolis, to bring the product back to the US marketplace.
However, the company said this will not happen before 2016.
Onsolis is an opioid agonist indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and are tolerant to opioid therapy for their underlying persistent cancer pain.
Onsolis utilizes BioErodible MucoAdhesive drug delivery technology, which consists of a small, bioerodible polymer film that is applied to the inner lining of the cheek. Onsolis is the only differentiated fentanyl-containing product for this indication that provides buccal administration.
Onsolis off the US market
Onsolis was originally approved by the FDA in July 2009, but BDSI stopped manufacturing the product in March 2012, after the FDA uncovered 2 issues with Onsolis.
The FDA found that, during Onsolis’s 24-month shelf-life, microscopic crystals formed on the product, and the color faded slightly. BDSI said these changes did not affect the product’s underlying integrity or safety, but the FDA thought the fading color in particular might cause patients to question the product’s efficacy.
So the FDA required that Onsolis be modified before additional product could be manufactured and distributed. Supplies of Onsolis that were already on the market remained on the market.
An analysis by BDSI showed that the changes in Onsolis were related to an excipient used in the manufacturing process that could be removed to resolve the problem.
The excipient was specific to the manufacture of Onsolis in the US. Therefore, it did not impact the launch of Breakyl, which is the brand name for Onsolis in the European Union.
Return to market
After BDSI reformulated Onsolis to prevent the aforementioned changes in the product’s appearance, the FDA approved the product’s return to market.
“We are pleased to have obtained FDA approval of our [supplemental new drug application] and to now be in a position to move toward returning Onsolis to the US marketplace,” said Mark A. Sirgo, PharmD, President and Chief Executive Officer of BDSI.
“Although we have options for Onsolis, including commercializing it on our own, our current plan is to determine the value we can secure in a partnership with a company that has access to the target physician audience. We have been engaged with a number of potential partners, and, with this approval, we can now proceed forward with those discussions in earnest. We will provide more definitive timing in the near future about the reintroduction, but this would not be prior to 2016.”
Once Onsolis does return to the market, it will only be available via the Transmucosal Immediate Release Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS) program. This is an FDA-required program designed to mitigate the risk of misuse, abuse, addiction, overdose, and serious complications due to medication errors with the use of TIRF medicines.
Outpatients, healthcare professionals who prescribe to outpatients, pharmacies, and distributors must enroll in the program to receive Onsolis. Further information is available at www.TIRFREMSAccess.com.
FDA expands indication for VWD drug

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has expanded the approved indication for a human von Willebrand factor/coagulation factor VIII complex (Wilate) to include prevention of excessive bleeding during and after minor and major surgery in adult and pediatric patients with von Willebrand disease (VWD).
The product was previously approved for the treatment of spontaneous and trauma-induced bleeding episodes in patients with severe VWD, as well as patients with mild or moderate VWD in whom the use of desmopressin is known or suspected to be ineffective or contraindicated.
About Wilate
Wilate is a plasma-derived, highly purified concentrate of freeze-dried human von Willebrand factor and coagulation factor VIII. Two virus-inactivation steps are incorporated into the manufacturing process of the product: a solvent/detergent and terminal dry-heat treatment.
Investigators evaluated Wilate’s safety and efficacy in surgical procedures in a single-arm, phase 3 study known as WONDERS. The investigators enrolled 41 patients, 30 of whom completed the trial.
The patients had type 1, type 2 or type 3 VWD. They had a median age of 39.7, and most were female. All patients underwent surgery—21 major and 9 minor surgeries.
The hemostatic efficacy of Wilate was assessed intra-operatively by the surgeon and post-operatively by investigators. The overall hemostatic efficacy—success or failure—of Wilate was based on both assessments, using a 4-point ordinal efficacy scale.
In the 29 evaluable surgeries, the success rate was 96.7%. Wilate was successful in 100% of minor surgeries (n=9) and 95.2% of major surgeries (n=20).
There were 2 serious adverse events—erosive gastritis and vaginal hemorrhage. Nonserious events included nausea (7 cases in 6 patients), vomiting (n=6), pain (n=4), pyrexia (n=4), procedural pain (10 cases in 8 patients), decrease in hemoglobin (6 cases in 4 patients), and hypertension (n=4).
Wilate is under development by Octapharma. For more information on the product, visit www.WILATEusa.com.

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has expanded the approved indication for a human von Willebrand factor/coagulation factor VIII complex (Wilate) to include prevention of excessive bleeding during and after minor and major surgery in adult and pediatric patients with von Willebrand disease (VWD).
The product was previously approved for the treatment of spontaneous and trauma-induced bleeding episodes in patients with severe VWD, as well as patients with mild or moderate VWD in whom the use of desmopressin is known or suspected to be ineffective or contraindicated.
About Wilate
Wilate is a plasma-derived, highly purified concentrate of freeze-dried human von Willebrand factor and coagulation factor VIII. Two virus-inactivation steps are incorporated into the manufacturing process of the product: a solvent/detergent and terminal dry-heat treatment.
Investigators evaluated Wilate’s safety and efficacy in surgical procedures in a single-arm, phase 3 study known as WONDERS. The investigators enrolled 41 patients, 30 of whom completed the trial.
The patients had type 1, type 2 or type 3 VWD. They had a median age of 39.7, and most were female. All patients underwent surgery—21 major and 9 minor surgeries.
The hemostatic efficacy of Wilate was assessed intra-operatively by the surgeon and post-operatively by investigators. The overall hemostatic efficacy—success or failure—of Wilate was based on both assessments, using a 4-point ordinal efficacy scale.
In the 29 evaluable surgeries, the success rate was 96.7%. Wilate was successful in 100% of minor surgeries (n=9) and 95.2% of major surgeries (n=20).
There were 2 serious adverse events—erosive gastritis and vaginal hemorrhage. Nonserious events included nausea (7 cases in 6 patients), vomiting (n=6), pain (n=4), pyrexia (n=4), procedural pain (10 cases in 8 patients), decrease in hemoglobin (6 cases in 4 patients), and hypertension (n=4).
Wilate is under development by Octapharma. For more information on the product, visit www.WILATEusa.com.

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has expanded the approved indication for a human von Willebrand factor/coagulation factor VIII complex (Wilate) to include prevention of excessive bleeding during and after minor and major surgery in adult and pediatric patients with von Willebrand disease (VWD).
The product was previously approved for the treatment of spontaneous and trauma-induced bleeding episodes in patients with severe VWD, as well as patients with mild or moderate VWD in whom the use of desmopressin is known or suspected to be ineffective or contraindicated.
About Wilate
Wilate is a plasma-derived, highly purified concentrate of freeze-dried human von Willebrand factor and coagulation factor VIII. Two virus-inactivation steps are incorporated into the manufacturing process of the product: a solvent/detergent and terminal dry-heat treatment.
Investigators evaluated Wilate’s safety and efficacy in surgical procedures in a single-arm, phase 3 study known as WONDERS. The investigators enrolled 41 patients, 30 of whom completed the trial.
The patients had type 1, type 2 or type 3 VWD. They had a median age of 39.7, and most were female. All patients underwent surgery—21 major and 9 minor surgeries.
The hemostatic efficacy of Wilate was assessed intra-operatively by the surgeon and post-operatively by investigators. The overall hemostatic efficacy—success or failure—of Wilate was based on both assessments, using a 4-point ordinal efficacy scale.
In the 29 evaluable surgeries, the success rate was 96.7%. Wilate was successful in 100% of minor surgeries (n=9) and 95.2% of major surgeries (n=20).
There were 2 serious adverse events—erosive gastritis and vaginal hemorrhage. Nonserious events included nausea (7 cases in 6 patients), vomiting (n=6), pain (n=4), pyrexia (n=4), procedural pain (10 cases in 8 patients), decrease in hemoglobin (6 cases in 4 patients), and hypertension (n=4).
Wilate is under development by Octapharma. For more information on the product, visit www.WILATEusa.com.
Rivaroxaban monitoring kit launched in Europe

Instrumentation Laboratory, a company that develops in vitro diagnostic instruments, has announced the commercialization of the HemosIL Rivaroxaban Testing Solution in Europe.
This testing kit consists of the HemosIL Liquid Anti-Xa Assay, Rivaroxaban Calibrators, and Rivaroxaban Controls, which can be used with ACL TOP Hemostasis Testing Systems to monitor patients taking the oral anticoagulant rivaroxaban (Xarelto).
The assay, calibrators, and controls are now CE IVD Marked under the European IVD Directive 98/79/EC.
This allows Instrumentation Laboratory to distribute the HemosIL Rivaroxaban Testing Solution in the European Union and other international territories.
Although monitoring is generally not required for patients on rivaroxaban, there are cases in which measuring rivaroxaban may be necessary.
This includes patients who present with bleeding, require reversal of anticoagulation, experience deteriorating renal function, or must undergo surgery or an invasive procedure and have taken rivaroxaban within 24 hours or longer if creatinine clearance is < 50 mL min-1.
Liquid Anti-Xa Assay
The HemosIL Liquid Anti-Xa kit is a one-stage chromogenic assay based on a synthetic chromogenic substrate and factor Xa inactivation. Rivaroxaban levels in patient plasma are measured automatically on an ACL TOP Hemostasis Testing System when this assay is calibrated with the HemosIL Rivaroxaban Calibrators.
The Anti-Xa Assay kit consists of:
- Factor Xa reagent: 5 x 2.5 mL vial of a liquid preparation containing purified bovine factor Xa (approximately 5.5 nkat/mL), Tris-Buffer, EDTA, dextran sulfate, sodium chloride, and bovine serum albumin.
- Chromogenic substrate: 5 x 3 mL vial of liquid chromogenic substrate S-2732 (approximately 1.2 mg/mL) and bulking agent.
Rivaroxaban Calibrators
The HemosIL Rivaroxaban Calibrators are intended for the calibration of the Liquid Anti-Xa Assay when testing for rivaroxaban on an ACL TOP Hemostasis Testing System.
Two levels of lyophilized calibrators prepared from human citrated plasma by means of a dedicated process at 2 different concentrations of rivaroxaban are used by the instrument to automatically prepare a calibration curve.
The Rivaroxaban Calibrator kit consists of:
- Rivaroxaban Calibrator 1: 5 x 1 mL vials of a lyophilized human plasma containing buffers and stabilizers.
- Rivaroxaban Calibrator 2: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, buffers, and stabilizers.
Rivaroxaban Controls
HemosIL Rivaroxaban Controls are intended for the quality control of the Liquid Anti-Xa Assay when testing for rivaroxaban on an ACL TOP Hemostasis Testing System.
Two levels of lyophilized controls are prepared from human citrated plasma by means of a dedicated process at 2 different concentrations of rivaroxaban. Use of both controls is recommended for a complete quality control program.
The Rivaroxaban Controls kit consists of:
- Rivaroxaban Low Control: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, stabilizers, and buffer solution.
- Rivaroxaban High Control: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, stabilizers, and buffer solution.

Instrumentation Laboratory, a company that develops in vitro diagnostic instruments, has announced the commercialization of the HemosIL Rivaroxaban Testing Solution in Europe.
This testing kit consists of the HemosIL Liquid Anti-Xa Assay, Rivaroxaban Calibrators, and Rivaroxaban Controls, which can be used with ACL TOP Hemostasis Testing Systems to monitor patients taking the oral anticoagulant rivaroxaban (Xarelto).
The assay, calibrators, and controls are now CE IVD Marked under the European IVD Directive 98/79/EC.
This allows Instrumentation Laboratory to distribute the HemosIL Rivaroxaban Testing Solution in the European Union and other international territories.
Although monitoring is generally not required for patients on rivaroxaban, there are cases in which measuring rivaroxaban may be necessary.
This includes patients who present with bleeding, require reversal of anticoagulation, experience deteriorating renal function, or must undergo surgery or an invasive procedure and have taken rivaroxaban within 24 hours or longer if creatinine clearance is < 50 mL min-1.
Liquid Anti-Xa Assay
The HemosIL Liquid Anti-Xa kit is a one-stage chromogenic assay based on a synthetic chromogenic substrate and factor Xa inactivation. Rivaroxaban levels in patient plasma are measured automatically on an ACL TOP Hemostasis Testing System when this assay is calibrated with the HemosIL Rivaroxaban Calibrators.
The Anti-Xa Assay kit consists of:
- Factor Xa reagent: 5 x 2.5 mL vial of a liquid preparation containing purified bovine factor Xa (approximately 5.5 nkat/mL), Tris-Buffer, EDTA, dextran sulfate, sodium chloride, and bovine serum albumin.
- Chromogenic substrate: 5 x 3 mL vial of liquid chromogenic substrate S-2732 (approximately 1.2 mg/mL) and bulking agent.
Rivaroxaban Calibrators
The HemosIL Rivaroxaban Calibrators are intended for the calibration of the Liquid Anti-Xa Assay when testing for rivaroxaban on an ACL TOP Hemostasis Testing System.
Two levels of lyophilized calibrators prepared from human citrated plasma by means of a dedicated process at 2 different concentrations of rivaroxaban are used by the instrument to automatically prepare a calibration curve.
The Rivaroxaban Calibrator kit consists of:
- Rivaroxaban Calibrator 1: 5 x 1 mL vials of a lyophilized human plasma containing buffers and stabilizers.
- Rivaroxaban Calibrator 2: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, buffers, and stabilizers.
Rivaroxaban Controls
HemosIL Rivaroxaban Controls are intended for the quality control of the Liquid Anti-Xa Assay when testing for rivaroxaban on an ACL TOP Hemostasis Testing System.
Two levels of lyophilized controls are prepared from human citrated plasma by means of a dedicated process at 2 different concentrations of rivaroxaban. Use of both controls is recommended for a complete quality control program.
The Rivaroxaban Controls kit consists of:
- Rivaroxaban Low Control: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, stabilizers, and buffer solution.
- Rivaroxaban High Control: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, stabilizers, and buffer solution.

Instrumentation Laboratory, a company that develops in vitro diagnostic instruments, has announced the commercialization of the HemosIL Rivaroxaban Testing Solution in Europe.
This testing kit consists of the HemosIL Liquid Anti-Xa Assay, Rivaroxaban Calibrators, and Rivaroxaban Controls, which can be used with ACL TOP Hemostasis Testing Systems to monitor patients taking the oral anticoagulant rivaroxaban (Xarelto).
The assay, calibrators, and controls are now CE IVD Marked under the European IVD Directive 98/79/EC.
This allows Instrumentation Laboratory to distribute the HemosIL Rivaroxaban Testing Solution in the European Union and other international territories.
Although monitoring is generally not required for patients on rivaroxaban, there are cases in which measuring rivaroxaban may be necessary.
This includes patients who present with bleeding, require reversal of anticoagulation, experience deteriorating renal function, or must undergo surgery or an invasive procedure and have taken rivaroxaban within 24 hours or longer if creatinine clearance is < 50 mL min-1.
Liquid Anti-Xa Assay
The HemosIL Liquid Anti-Xa kit is a one-stage chromogenic assay based on a synthetic chromogenic substrate and factor Xa inactivation. Rivaroxaban levels in patient plasma are measured automatically on an ACL TOP Hemostasis Testing System when this assay is calibrated with the HemosIL Rivaroxaban Calibrators.
The Anti-Xa Assay kit consists of:
- Factor Xa reagent: 5 x 2.5 mL vial of a liquid preparation containing purified bovine factor Xa (approximately 5.5 nkat/mL), Tris-Buffer, EDTA, dextran sulfate, sodium chloride, and bovine serum albumin.
- Chromogenic substrate: 5 x 3 mL vial of liquid chromogenic substrate S-2732 (approximately 1.2 mg/mL) and bulking agent.
Rivaroxaban Calibrators
The HemosIL Rivaroxaban Calibrators are intended for the calibration of the Liquid Anti-Xa Assay when testing for rivaroxaban on an ACL TOP Hemostasis Testing System.
Two levels of lyophilized calibrators prepared from human citrated plasma by means of a dedicated process at 2 different concentrations of rivaroxaban are used by the instrument to automatically prepare a calibration curve.
The Rivaroxaban Calibrator kit consists of:
- Rivaroxaban Calibrator 1: 5 x 1 mL vials of a lyophilized human plasma containing buffers and stabilizers.
- Rivaroxaban Calibrator 2: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, buffers, and stabilizers.
Rivaroxaban Controls
HemosIL Rivaroxaban Controls are intended for the quality control of the Liquid Anti-Xa Assay when testing for rivaroxaban on an ACL TOP Hemostasis Testing System.
Two levels of lyophilized controls are prepared from human citrated plasma by means of a dedicated process at 2 different concentrations of rivaroxaban. Use of both controls is recommended for a complete quality control program.
The Rivaroxaban Controls kit consists of:
- Rivaroxaban Low Control: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, stabilizers, and buffer solution.
- Rivaroxaban High Control: 5 x 1 mL vials of a lyophilized human plasma containing rivaroxaban, stabilizers, and buffer solution.