User login
Identifying characteristics of difficult-to-treat PsA in real-world conditions
Key clinical point: Difficult-to-treat (D2T) psoriatic arthritis (PsA), a condition characterized by the failure of ≥2 targeted synthetic or biologic disease-modifying antirheumatic drugs (ts/bDMARD), is associated with a higher prevalence of axial involvement, structural damage, and treatment discontinuation.
Major finding: Peripheral structural damage (odds ratio [OR] 2.57; P = .020), axial involvement (OR 2.37; P = .035), and the discontinuation of bDMARD due to poor dermatological control (OR 2.99; P = .008) were more prevalent in patients with D2T PsA vs non-D2T PsA.
Study details: Findings are from a retrospective cohort study including 150 patients with PsA who initiated treatment with ts/bDMARD and were followed up for ≥2 years, of whom 49 patients had D2T PsA.
Disclosures: This study did not receive any funding. Three authors declared receiving honorary fees or research grants or serving as advisory board members for various sources.
Source: Philippoteaux C et al. Characteristics of difficult-to-treat psoriatic arthritis: A comparative analysis. Semin Arthritis Rheum. 2023;63:152275 (Oct 5). doi: 10.1016/j.semarthrit.2023.152275
Key clinical point: Difficult-to-treat (D2T) psoriatic arthritis (PsA), a condition characterized by the failure of ≥2 targeted synthetic or biologic disease-modifying antirheumatic drugs (ts/bDMARD), is associated with a higher prevalence of axial involvement, structural damage, and treatment discontinuation.
Major finding: Peripheral structural damage (odds ratio [OR] 2.57; P = .020), axial involvement (OR 2.37; P = .035), and the discontinuation of bDMARD due to poor dermatological control (OR 2.99; P = .008) were more prevalent in patients with D2T PsA vs non-D2T PsA.
Study details: Findings are from a retrospective cohort study including 150 patients with PsA who initiated treatment with ts/bDMARD and were followed up for ≥2 years, of whom 49 patients had D2T PsA.
Disclosures: This study did not receive any funding. Three authors declared receiving honorary fees or research grants or serving as advisory board members for various sources.
Source: Philippoteaux C et al. Characteristics of difficult-to-treat psoriatic arthritis: A comparative analysis. Semin Arthritis Rheum. 2023;63:152275 (Oct 5). doi: 10.1016/j.semarthrit.2023.152275
Key clinical point: Difficult-to-treat (D2T) psoriatic arthritis (PsA), a condition characterized by the failure of ≥2 targeted synthetic or biologic disease-modifying antirheumatic drugs (ts/bDMARD), is associated with a higher prevalence of axial involvement, structural damage, and treatment discontinuation.
Major finding: Peripheral structural damage (odds ratio [OR] 2.57; P = .020), axial involvement (OR 2.37; P = .035), and the discontinuation of bDMARD due to poor dermatological control (OR 2.99; P = .008) were more prevalent in patients with D2T PsA vs non-D2T PsA.
Study details: Findings are from a retrospective cohort study including 150 patients with PsA who initiated treatment with ts/bDMARD and were followed up for ≥2 years, of whom 49 patients had D2T PsA.
Disclosures: This study did not receive any funding. Three authors declared receiving honorary fees or research grants or serving as advisory board members for various sources.
Source: Philippoteaux C et al. Characteristics of difficult-to-treat psoriatic arthritis: A comparative analysis. Semin Arthritis Rheum. 2023;63:152275 (Oct 5). doi: 10.1016/j.semarthrit.2023.152275
What factors are responsible for a delayed diagnosis of PsA?
Key clinical point: Approximately one-third of patients with psoriatic arthritis (PsA) reported a diagnostic delay of >2 years, which can be attributed to a number of clinical and demographic factors.
Major finding: The mean diagnostic delay period was 35.1 months. A diagnostic delay of >2 years was seen in 32.98% of patients, with the occurrence of arthritis symptoms before skin manifestations (odds ratio [OR] 1.72; 95% CI 1.20-2.46) and low back pain at first visit (OR 1.60; 95% CI 1.21-2.11) being significant factors associated with this delay. However, generalized-type psoriasis was negatively associated with the diagnostic delay of >2 years (OR 0.25; 95% CI 0.07-0.98).
Study details: Findings are from a cross-sectional study including 1134 patients with PsA.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Kılıç G et al. Diagnostic delay in psoriatic arthritis: Insights from a nationwide multicenter study. Rheumatol Int. 2023 (Oct 8). doi: 10.1007/s00296-023-05479-z
Key clinical point: Approximately one-third of patients with psoriatic arthritis (PsA) reported a diagnostic delay of >2 years, which can be attributed to a number of clinical and demographic factors.
Major finding: The mean diagnostic delay period was 35.1 months. A diagnostic delay of >2 years was seen in 32.98% of patients, with the occurrence of arthritis symptoms before skin manifestations (odds ratio [OR] 1.72; 95% CI 1.20-2.46) and low back pain at first visit (OR 1.60; 95% CI 1.21-2.11) being significant factors associated with this delay. However, generalized-type psoriasis was negatively associated with the diagnostic delay of >2 years (OR 0.25; 95% CI 0.07-0.98).
Study details: Findings are from a cross-sectional study including 1134 patients with PsA.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Kılıç G et al. Diagnostic delay in psoriatic arthritis: Insights from a nationwide multicenter study. Rheumatol Int. 2023 (Oct 8). doi: 10.1007/s00296-023-05479-z
Key clinical point: Approximately one-third of patients with psoriatic arthritis (PsA) reported a diagnostic delay of >2 years, which can be attributed to a number of clinical and demographic factors.
Major finding: The mean diagnostic delay period was 35.1 months. A diagnostic delay of >2 years was seen in 32.98% of patients, with the occurrence of arthritis symptoms before skin manifestations (odds ratio [OR] 1.72; 95% CI 1.20-2.46) and low back pain at first visit (OR 1.60; 95% CI 1.21-2.11) being significant factors associated with this delay. However, generalized-type psoriasis was negatively associated with the diagnostic delay of >2 years (OR 0.25; 95% CI 0.07-0.98).
Study details: Findings are from a cross-sectional study including 1134 patients with PsA.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Kılıç G et al. Diagnostic delay in psoriatic arthritis: Insights from a nationwide multicenter study. Rheumatol Int. 2023 (Oct 8). doi: 10.1007/s00296-023-05479-z
Durable improvement in axial symptoms with guselkumab in PsA
Key clinical point: In patients with active psoriatic arthritis (PsA) and imaging-confirmed sacroiliitis, 100 mg guselkumab every 4 weeks (Q4W) or every 8 weeks (Q8W) yielded clinically meaningful and sustained improvements in axial symptoms through 2 years.
Major finding: At week 24, guselkumab Q4W and Q8W vs placebo showed significantly greater least-squares mean improvements (−2.5 and −2.4, respectively, vs −1.2; P < .001) in the total Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, with further improvements in the mean total BASDAI score for each group at week 100 (~3.0 points).
Study details: This post hoc analysis of the DISCOVER-2 study included 246 biologic-naive patients with active PsA and sacroiliitis who were randomized to guselkumab Q4W (n = 82), guselkumab Q8W (n = 68), or placebo with crossover to guselkumab Q4W at week 24 (n = 96).
Disclosures: This study was funded by Janssen Research & Development, LLC. Five authors declared employment with Janssen and stockownership in Johnson & Johnson, and others reported ties with various sources, including Janssen.
Source: Mease PJ et al. Efficacy of guselkumab on axial-related symptoms through up to 2 years in adults with active psoriatic arthritis in the phase 3, randomized, placebo-controlled DISCOVER-2 study. Rheumatol Ther. 2023 (Oct 11). doi: 10.1007/s40744-023-00592-8
Key clinical point: In patients with active psoriatic arthritis (PsA) and imaging-confirmed sacroiliitis, 100 mg guselkumab every 4 weeks (Q4W) or every 8 weeks (Q8W) yielded clinically meaningful and sustained improvements in axial symptoms through 2 years.
Major finding: At week 24, guselkumab Q4W and Q8W vs placebo showed significantly greater least-squares mean improvements (−2.5 and −2.4, respectively, vs −1.2; P < .001) in the total Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, with further improvements in the mean total BASDAI score for each group at week 100 (~3.0 points).
Study details: This post hoc analysis of the DISCOVER-2 study included 246 biologic-naive patients with active PsA and sacroiliitis who were randomized to guselkumab Q4W (n = 82), guselkumab Q8W (n = 68), or placebo with crossover to guselkumab Q4W at week 24 (n = 96).
Disclosures: This study was funded by Janssen Research & Development, LLC. Five authors declared employment with Janssen and stockownership in Johnson & Johnson, and others reported ties with various sources, including Janssen.
Source: Mease PJ et al. Efficacy of guselkumab on axial-related symptoms through up to 2 years in adults with active psoriatic arthritis in the phase 3, randomized, placebo-controlled DISCOVER-2 study. Rheumatol Ther. 2023 (Oct 11). doi: 10.1007/s40744-023-00592-8
Key clinical point: In patients with active psoriatic arthritis (PsA) and imaging-confirmed sacroiliitis, 100 mg guselkumab every 4 weeks (Q4W) or every 8 weeks (Q8W) yielded clinically meaningful and sustained improvements in axial symptoms through 2 years.
Major finding: At week 24, guselkumab Q4W and Q8W vs placebo showed significantly greater least-squares mean improvements (−2.5 and −2.4, respectively, vs −1.2; P < .001) in the total Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, with further improvements in the mean total BASDAI score for each group at week 100 (~3.0 points).
Study details: This post hoc analysis of the DISCOVER-2 study included 246 biologic-naive patients with active PsA and sacroiliitis who were randomized to guselkumab Q4W (n = 82), guselkumab Q8W (n = 68), or placebo with crossover to guselkumab Q4W at week 24 (n = 96).
Disclosures: This study was funded by Janssen Research & Development, LLC. Five authors declared employment with Janssen and stockownership in Johnson & Johnson, and others reported ties with various sources, including Janssen.
Source: Mease PJ et al. Efficacy of guselkumab on axial-related symptoms through up to 2 years in adults with active psoriatic arthritis in the phase 3, randomized, placebo-controlled DISCOVER-2 study. Rheumatol Ther. 2023 (Oct 11). doi: 10.1007/s40744-023-00592-8
Preliminary results of a real-world study confirm the efficacy and safety of upadacitinib in PsA
Key clinical point: The interim analysis of a real-world study confirmed the efficacy and safety of upadacitinib in patients with active psoriatic arthritis (PsA) who showed inadequate response or intolerance to conventional synthetic disease-modifying antirheumatic drugs (csDMARD) or biological DMARD (bDMARD).
Major finding: The proportion of patients treated with upadacitinib who achieved minimal disease activity was considerably higher at week 12 vs baseline (39.8% vs 2.7%), with the effect being maintained till week 24 (39.1%). No new adverse events were reported.
Study details: This 24-week interim analysis of the UPJOINT study included 296 patients with active oligoarticular or polyarticular PsA who were refractory to csDMARD or bDMARD and received upadacitinib.
Disclosures: This study was funded by AbbVie. Five authors declared being employees of or owing stocks or stock options in AbbVie. The other authors declared ties with various sources, including AbbVie.
Source: Werner SG et al. Treatment with upadacitinib in active psoriatic arthritis: Efficacy and safety data of the first 192 patients from the UPJOINT study, a multicentre, observational study in clinical practice. Rheumatol Ther. 2023 (Sep 11). doi: 10.1007/s40744-023-00589-3
Key clinical point: The interim analysis of a real-world study confirmed the efficacy and safety of upadacitinib in patients with active psoriatic arthritis (PsA) who showed inadequate response or intolerance to conventional synthetic disease-modifying antirheumatic drugs (csDMARD) or biological DMARD (bDMARD).
Major finding: The proportion of patients treated with upadacitinib who achieved minimal disease activity was considerably higher at week 12 vs baseline (39.8% vs 2.7%), with the effect being maintained till week 24 (39.1%). No new adverse events were reported.
Study details: This 24-week interim analysis of the UPJOINT study included 296 patients with active oligoarticular or polyarticular PsA who were refractory to csDMARD or bDMARD and received upadacitinib.
Disclosures: This study was funded by AbbVie. Five authors declared being employees of or owing stocks or stock options in AbbVie. The other authors declared ties with various sources, including AbbVie.
Source: Werner SG et al. Treatment with upadacitinib in active psoriatic arthritis: Efficacy and safety data of the first 192 patients from the UPJOINT study, a multicentre, observational study in clinical practice. Rheumatol Ther. 2023 (Sep 11). doi: 10.1007/s40744-023-00589-3
Key clinical point: The interim analysis of a real-world study confirmed the efficacy and safety of upadacitinib in patients with active psoriatic arthritis (PsA) who showed inadequate response or intolerance to conventional synthetic disease-modifying antirheumatic drugs (csDMARD) or biological DMARD (bDMARD).
Major finding: The proportion of patients treated with upadacitinib who achieved minimal disease activity was considerably higher at week 12 vs baseline (39.8% vs 2.7%), with the effect being maintained till week 24 (39.1%). No new adverse events were reported.
Study details: This 24-week interim analysis of the UPJOINT study included 296 patients with active oligoarticular or polyarticular PsA who were refractory to csDMARD or bDMARD and received upadacitinib.
Disclosures: This study was funded by AbbVie. Five authors declared being employees of or owing stocks or stock options in AbbVie. The other authors declared ties with various sources, including AbbVie.
Source: Werner SG et al. Treatment with upadacitinib in active psoriatic arthritis: Efficacy and safety data of the first 192 patients from the UPJOINT study, a multicentre, observational study in clinical practice. Rheumatol Ther. 2023 (Sep 11). doi: 10.1007/s40744-023-00589-3
IL-23 inhibitors effective and safe for PsA patients in real-world settings
Key clinical point: This real-world study confirmed the efficacy and safety of the interleukin (IL)-23 inhibitors guselkumab and risankizumab in patients with psoriatic arthritis (PsA).
Major finding: Mean Disease Activity Index for PsA (DAPSA) score was 43.6 at baseline and reduced significantly at 4 (36.8), 16 (23.6), and 24 (20.5) weeks of treatment (all P < .001). At 24 weeks, 29.2% and 16.7% of patients reported low disease activity and remission, respectively. None of the patients discontinued treatment due to safety concerns.
Study details: Findings are from a single-center retrospective study including 59 patients with moderate-to-severe plaque psoriasis who received guselkumab or risankizumab, of which 24 patients had concomitant PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Vaiopoulos AG et al. Real-world data show high efficacy of IL23 inhibitors guselkumab and risankizumab in psoriatic arthritis and difficult-to-treat areas. Int J Dermatol. 2023;62:1404-1413 (Sep 25). doi: 10.1111/ijd.16849
Key clinical point: This real-world study confirmed the efficacy and safety of the interleukin (IL)-23 inhibitors guselkumab and risankizumab in patients with psoriatic arthritis (PsA).
Major finding: Mean Disease Activity Index for PsA (DAPSA) score was 43.6 at baseline and reduced significantly at 4 (36.8), 16 (23.6), and 24 (20.5) weeks of treatment (all P < .001). At 24 weeks, 29.2% and 16.7% of patients reported low disease activity and remission, respectively. None of the patients discontinued treatment due to safety concerns.
Study details: Findings are from a single-center retrospective study including 59 patients with moderate-to-severe plaque psoriasis who received guselkumab or risankizumab, of which 24 patients had concomitant PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Vaiopoulos AG et al. Real-world data show high efficacy of IL23 inhibitors guselkumab and risankizumab in psoriatic arthritis and difficult-to-treat areas. Int J Dermatol. 2023;62:1404-1413 (Sep 25). doi: 10.1111/ijd.16849
Key clinical point: This real-world study confirmed the efficacy and safety of the interleukin (IL)-23 inhibitors guselkumab and risankizumab in patients with psoriatic arthritis (PsA).
Major finding: Mean Disease Activity Index for PsA (DAPSA) score was 43.6 at baseline and reduced significantly at 4 (36.8), 16 (23.6), and 24 (20.5) weeks of treatment (all P < .001). At 24 weeks, 29.2% and 16.7% of patients reported low disease activity and remission, respectively. None of the patients discontinued treatment due to safety concerns.
Study details: Findings are from a single-center retrospective study including 59 patients with moderate-to-severe plaque psoriasis who received guselkumab or risankizumab, of which 24 patients had concomitant PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Vaiopoulos AG et al. Real-world data show high efficacy of IL23 inhibitors guselkumab and risankizumab in psoriatic arthritis and difficult-to-treat areas. Int J Dermatol. 2023;62:1404-1413 (Sep 25). doi: 10.1111/ijd.16849
Factors affecting clinical response to fecal microbial transplantation in PsA
Key clinical point: Intestinal permeability as well as fecal and plasma metabolomic profiles significantly affected the clinical response to fecal microbial transplantation (FMT) or sham transplantation in patients having peripheral moderate-to-high psoriatic arthritis (PsA) despite receiving methotrexate for ≥3 months.
Major finding: Lactulose-to-mannitol ratio was higher at week 26 (0.027 vs 0.012; P = .013), indicating greater small intestinal permeability; moreover, fecal (P < .0001) and plasma (P = .005) metabolomic profiles differed in patients who needed vs did not need treatment intensification after undergoing an FMT or sham transplantation.
Study details: This exploratory study of the FLORA trial included 31 patients who had peripheral moderate-to-high PsA despite ≥3 months of methotrexate treatment and were randomly assigned to undergo either gastroscopic-guided single-donor FMT (n = 15) or sham transplantation (n = 16).
Disclosures: This study was supported by Sygeforsikringen “danmark” and other sources. Three authors declared receiving financial support, grants, or lectureship awards from various sources.
Source: Kragsnaes MS et al. Small intestinal permeability and metabolomic profiles in feces and plasma associate with clinical response in patients with active psoriatic arthritis participating in a fecal microbiota transplantation trial: Exploratory findings from the FLORA trial. ACR Open Rheumatol. 2023 (Sep 22). doi: 10.1002/acr2.11604
Key clinical point: Intestinal permeability as well as fecal and plasma metabolomic profiles significantly affected the clinical response to fecal microbial transplantation (FMT) or sham transplantation in patients having peripheral moderate-to-high psoriatic arthritis (PsA) despite receiving methotrexate for ≥3 months.
Major finding: Lactulose-to-mannitol ratio was higher at week 26 (0.027 vs 0.012; P = .013), indicating greater small intestinal permeability; moreover, fecal (P < .0001) and plasma (P = .005) metabolomic profiles differed in patients who needed vs did not need treatment intensification after undergoing an FMT or sham transplantation.
Study details: This exploratory study of the FLORA trial included 31 patients who had peripheral moderate-to-high PsA despite ≥3 months of methotrexate treatment and were randomly assigned to undergo either gastroscopic-guided single-donor FMT (n = 15) or sham transplantation (n = 16).
Disclosures: This study was supported by Sygeforsikringen “danmark” and other sources. Three authors declared receiving financial support, grants, or lectureship awards from various sources.
Source: Kragsnaes MS et al. Small intestinal permeability and metabolomic profiles in feces and plasma associate with clinical response in patients with active psoriatic arthritis participating in a fecal microbiota transplantation trial: Exploratory findings from the FLORA trial. ACR Open Rheumatol. 2023 (Sep 22). doi: 10.1002/acr2.11604
Key clinical point: Intestinal permeability as well as fecal and plasma metabolomic profiles significantly affected the clinical response to fecal microbial transplantation (FMT) or sham transplantation in patients having peripheral moderate-to-high psoriatic arthritis (PsA) despite receiving methotrexate for ≥3 months.
Major finding: Lactulose-to-mannitol ratio was higher at week 26 (0.027 vs 0.012; P = .013), indicating greater small intestinal permeability; moreover, fecal (P < .0001) and plasma (P = .005) metabolomic profiles differed in patients who needed vs did not need treatment intensification after undergoing an FMT or sham transplantation.
Study details: This exploratory study of the FLORA trial included 31 patients who had peripheral moderate-to-high PsA despite ≥3 months of methotrexate treatment and were randomly assigned to undergo either gastroscopic-guided single-donor FMT (n = 15) or sham transplantation (n = 16).
Disclosures: This study was supported by Sygeforsikringen “danmark” and other sources. Three authors declared receiving financial support, grants, or lectureship awards from various sources.
Source: Kragsnaes MS et al. Small intestinal permeability and metabolomic profiles in feces and plasma associate with clinical response in patients with active psoriatic arthritis participating in a fecal microbiota transplantation trial: Exploratory findings from the FLORA trial. ACR Open Rheumatol. 2023 (Sep 22). doi: 10.1002/acr2.11604
IL-17 and IL-23(p19) inhibitors as effective as TNF inhibitors in PsA
Key clinical point: In patients with psoriatic arthritis (PsA), year-long treatment with interleukin-17 inhibitors (IL-17i) and IL-23(p19) inhibitors (IL23-[19]i), led to comparable drug survival and clinical response outcomes as tumor necrosis factor inhibitor (TNFi) treatment.
Major finding: At 12 months, the drug withdrawal rates for IL-17i (hazard ratio [HR] 1.36; 95% CI 0.59-3.14) and IL23-(19)i (HR 0.56; 95% CI 0.10-3.24) were comparable with those for TNFi. Moreover, the proportion of patients who achieved ≥50% improvement in the American College of Rheumatology scores was similar across the IL-17i, IL-23(19)i, and TNFi groups (19%, 29%, and 19%, respectively).
Study details: Findings are from a target trial emulation study including 109 patients with PsA who presented with peripheral manifestations and initiated either TNFi (n = 75), IL-7i (n = 26), or IL-23(19)i (n = 8).
Disclosures: This study was supported by the Danish Rheumatism Association and other sources. Some authors declared serving as speakers or consultants for or receiving research grants, fees, or other support from various sources.
Source: Stisen ZR et al. Tolerability and comparative effectiveness of TNF-, IL-17-, and IL-23(p19) inhibitors in psoriatic arthritis: A target trial emulation study. Rheumatology (Oxford). 2023 (Sep 15). doi: 10.1093/rheumatology/kead488
Key clinical point: In patients with psoriatic arthritis (PsA), year-long treatment with interleukin-17 inhibitors (IL-17i) and IL-23(p19) inhibitors (IL23-[19]i), led to comparable drug survival and clinical response outcomes as tumor necrosis factor inhibitor (TNFi) treatment.
Major finding: At 12 months, the drug withdrawal rates for IL-17i (hazard ratio [HR] 1.36; 95% CI 0.59-3.14) and IL23-(19)i (HR 0.56; 95% CI 0.10-3.24) were comparable with those for TNFi. Moreover, the proportion of patients who achieved ≥50% improvement in the American College of Rheumatology scores was similar across the IL-17i, IL-23(19)i, and TNFi groups (19%, 29%, and 19%, respectively).
Study details: Findings are from a target trial emulation study including 109 patients with PsA who presented with peripheral manifestations and initiated either TNFi (n = 75), IL-7i (n = 26), or IL-23(19)i (n = 8).
Disclosures: This study was supported by the Danish Rheumatism Association and other sources. Some authors declared serving as speakers or consultants for or receiving research grants, fees, or other support from various sources.
Source: Stisen ZR et al. Tolerability and comparative effectiveness of TNF-, IL-17-, and IL-23(p19) inhibitors in psoriatic arthritis: A target trial emulation study. Rheumatology (Oxford). 2023 (Sep 15). doi: 10.1093/rheumatology/kead488
Key clinical point: In patients with psoriatic arthritis (PsA), year-long treatment with interleukin-17 inhibitors (IL-17i) and IL-23(p19) inhibitors (IL23-[19]i), led to comparable drug survival and clinical response outcomes as tumor necrosis factor inhibitor (TNFi) treatment.
Major finding: At 12 months, the drug withdrawal rates for IL-17i (hazard ratio [HR] 1.36; 95% CI 0.59-3.14) and IL23-(19)i (HR 0.56; 95% CI 0.10-3.24) were comparable with those for TNFi. Moreover, the proportion of patients who achieved ≥50% improvement in the American College of Rheumatology scores was similar across the IL-17i, IL-23(19)i, and TNFi groups (19%, 29%, and 19%, respectively).
Study details: Findings are from a target trial emulation study including 109 patients with PsA who presented with peripheral manifestations and initiated either TNFi (n = 75), IL-7i (n = 26), or IL-23(19)i (n = 8).
Disclosures: This study was supported by the Danish Rheumatism Association and other sources. Some authors declared serving as speakers or consultants for or receiving research grants, fees, or other support from various sources.
Source: Stisen ZR et al. Tolerability and comparative effectiveness of TNF-, IL-17-, and IL-23(p19) inhibitors in psoriatic arthritis: A target trial emulation study. Rheumatology (Oxford). 2023 (Sep 15). doi: 10.1093/rheumatology/kead488
Bimekizumab offers sustained clinical response in bDMARD-naive PsA patients
Key clinical point: Bimekizumab led to sustained improvements in clinical response up to week 52 and had a consistent safety profile in patients with active psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD).
Major finding: At week 16, 43.9% of patients receiving bimekizumab achieved ≥50% improvement in the American College of Rheumatology scores (ACR50), with the response being maintained until week 52 (54.5%). Among patients who switched from placebo to bimekizumab at week 16, a similar proportion (53.0%) achieved ACR50 at week 52. No new safety signals were observed.
Study details: Findings are from the phase 3 BE OPTIMAL study including 852 bDMARD-naive patients with active PsA who were randomly assigned to receive bimekizumab, adalimumab, or placebo.
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees and shareholders of UCB Pharma. The other authors declared ties with several sources, including UCB Pharma.
Source: Ritchlin CT et al. Bimekizumab treatment in biologic DMARD-naïve patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis. 2023;82:1404-1414 (Sep 11). doi: 10.1136/ard-2023-224431
Key clinical point: Bimekizumab led to sustained improvements in clinical response up to week 52 and had a consistent safety profile in patients with active psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD).
Major finding: At week 16, 43.9% of patients receiving bimekizumab achieved ≥50% improvement in the American College of Rheumatology scores (ACR50), with the response being maintained until week 52 (54.5%). Among patients who switched from placebo to bimekizumab at week 16, a similar proportion (53.0%) achieved ACR50 at week 52. No new safety signals were observed.
Study details: Findings are from the phase 3 BE OPTIMAL study including 852 bDMARD-naive patients with active PsA who were randomly assigned to receive bimekizumab, adalimumab, or placebo.
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees and shareholders of UCB Pharma. The other authors declared ties with several sources, including UCB Pharma.
Source: Ritchlin CT et al. Bimekizumab treatment in biologic DMARD-naïve patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis. 2023;82:1404-1414 (Sep 11). doi: 10.1136/ard-2023-224431
Key clinical point: Bimekizumab led to sustained improvements in clinical response up to week 52 and had a consistent safety profile in patients with active psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD).
Major finding: At week 16, 43.9% of patients receiving bimekizumab achieved ≥50% improvement in the American College of Rheumatology scores (ACR50), with the response being maintained until week 52 (54.5%). Among patients who switched from placebo to bimekizumab at week 16, a similar proportion (53.0%) achieved ACR50 at week 52. No new safety signals were observed.
Study details: Findings are from the phase 3 BE OPTIMAL study including 852 bDMARD-naive patients with active PsA who were randomly assigned to receive bimekizumab, adalimumab, or placebo.
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees and shareholders of UCB Pharma. The other authors declared ties with several sources, including UCB Pharma.
Source: Ritchlin CT et al. Bimekizumab treatment in biologic DMARD-naïve patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis. 2023;82:1404-1414 (Sep 11). doi: 10.1136/ard-2023-224431
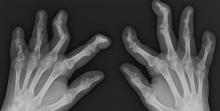
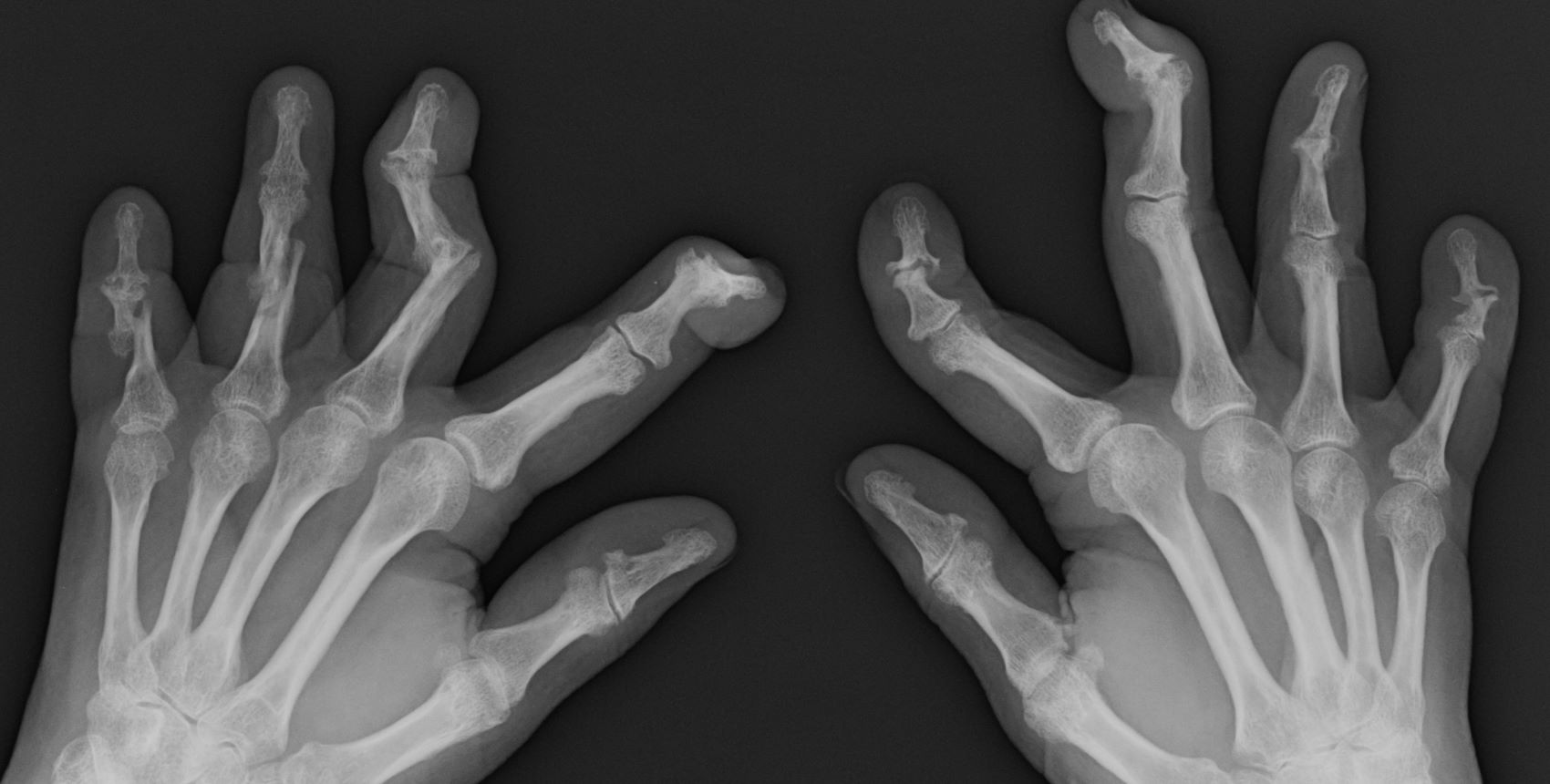
Pain in fingers for several months
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man presents with pain in fingers of several months' duration, which is moderately relieved with over-the-counter naproxen. He is concerned about "crooked" fingers and is worried that his work will be affected. He is overweight (BMI, 28.8), hypertensive, hypercholesterolemic, and a nonsmoker. The patient also reports a 6-month history of itchy "scalp" behind the ears, not relieved with dandruff shampoos. Physical exam reveals advanced deformity in index and middle fingers of both hands and no evident deformity in the wrists or metacarpophalangeal joints. Nails are pitted and discolored. Scalp behind ears shows well-demarcated, scaly patches. Lab work, radiography, and biopsy of the retroauricular area are ordered.
FDA approves bimekizumab for moderate to severe plaque psoriasis in adults
, the manufacturer announced in a press release.
The indication is for adults who are candidates for systemic therapy or phototherapy.
With this approval, bimekizumab becomes the only interleukin (IL)-17A and IL-17F inhibitor approved for the treatment of these patients. Psoriasis affects more than 7.5 million U.S. adults, according to the National Psoriasis Foundation.
“We have been eagerly awaiting bimekizumab,” Mark Lebwohl, MD, bimekizumab investigator and dean for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York City, said in the press release.
Dr. Lebwohl states that bimekizumab “achieved superior levels of skin clearance at week 16 compared to placebo and three existing biologics for psoriasis, with responses being rapid and lasting up to a year. Long-term data have also shown that the majority of patients maintained high levels of clinical response through three years.”
The most common adverse reactions (occurring in at least 1% of patients) are upper respiratory infections, oral candidiasis, headache, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other Candida infections, fatigue, and injection site reactions, according to the company, UCB.
Available in about 1 month in U.S.
Bimekizumab can be administered by a health care provider or it can be self-injected by a patient after training. It is available as a single-dose prefilled autoinjector and a single-dose prefilled syringe and will be available in the United States in about 1 month.
The recommended dosage of bimekizumab for patients with psoriasis is 320 mg (two subcutaneous injections of 160 mg each) at baseline, then on weeks 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing at least 120 kg (about 265 lb), a dosage of 320 mg every 4 weeks after week 16 may be considered, the company states.
Three phase 3 trials
Approval was based on three phase 3 multicenter, randomized, placebo and/or active comparator-controlled trials: bimekizumab versus placebo and ustekinumab (BE VIVID); versus placebo (BE READY); and versus adalimumab (BE SURE).
“All studies met their co-primary endpoints and all ranked secondary endpoints,” the company reports. Secondary endpoints included the Psoriasis Area and Severity Index (PASI) 75 at week 4 and PASI 100 (complete skin clearance) at week 16.
Highlights from the trials include the following results, according to UCB:
- Clear or almost clear skin: More than 8 out of 10 patients achieved a 90% or greater reduction from baseline in the PASI 90 and an Investigator’s Global Assessment score of 0/1 at week 16.
- Complete skin clearance: About 60% of patients achieved PASI 100 at week 16.
- Time to response: More than 70% of patients achieved PASI 75 at week 4 following one 320-mg dose.
Safety information
The safety information includes the statement that bimekizumab may increase the risk for suicidal ideation and behavior, though a causal association has not been established. Prescribers should advise patients, caregivers, and families “to monitor for emergence or worsening of depression, suicidal ideation, or other mood changes,” according to the prescribing information.
Bimekizumab is being studied for other conditions, including hidradenitis suppurativa. In the European Union, it was approved for the treatment of psoriasis in 2021 and for the treatment of psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Lebwohl is an investigator for UCB. He has not accepted any consulting payments from UCB.
A version of this article first appeared on Medscape.com.
, the manufacturer announced in a press release.
The indication is for adults who are candidates for systemic therapy or phototherapy.
With this approval, bimekizumab becomes the only interleukin (IL)-17A and IL-17F inhibitor approved for the treatment of these patients. Psoriasis affects more than 7.5 million U.S. adults, according to the National Psoriasis Foundation.
“We have been eagerly awaiting bimekizumab,” Mark Lebwohl, MD, bimekizumab investigator and dean for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York City, said in the press release.
Dr. Lebwohl states that bimekizumab “achieved superior levels of skin clearance at week 16 compared to placebo and three existing biologics for psoriasis, with responses being rapid and lasting up to a year. Long-term data have also shown that the majority of patients maintained high levels of clinical response through three years.”
The most common adverse reactions (occurring in at least 1% of patients) are upper respiratory infections, oral candidiasis, headache, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other Candida infections, fatigue, and injection site reactions, according to the company, UCB.
Available in about 1 month in U.S.
Bimekizumab can be administered by a health care provider or it can be self-injected by a patient after training. It is available as a single-dose prefilled autoinjector and a single-dose prefilled syringe and will be available in the United States in about 1 month.
The recommended dosage of bimekizumab for patients with psoriasis is 320 mg (two subcutaneous injections of 160 mg each) at baseline, then on weeks 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing at least 120 kg (about 265 lb), a dosage of 320 mg every 4 weeks after week 16 may be considered, the company states.
Three phase 3 trials
Approval was based on three phase 3 multicenter, randomized, placebo and/or active comparator-controlled trials: bimekizumab versus placebo and ustekinumab (BE VIVID); versus placebo (BE READY); and versus adalimumab (BE SURE).
“All studies met their co-primary endpoints and all ranked secondary endpoints,” the company reports. Secondary endpoints included the Psoriasis Area and Severity Index (PASI) 75 at week 4 and PASI 100 (complete skin clearance) at week 16.
Highlights from the trials include the following results, according to UCB:
- Clear or almost clear skin: More than 8 out of 10 patients achieved a 90% or greater reduction from baseline in the PASI 90 and an Investigator’s Global Assessment score of 0/1 at week 16.
- Complete skin clearance: About 60% of patients achieved PASI 100 at week 16.
- Time to response: More than 70% of patients achieved PASI 75 at week 4 following one 320-mg dose.
Safety information
The safety information includes the statement that bimekizumab may increase the risk for suicidal ideation and behavior, though a causal association has not been established. Prescribers should advise patients, caregivers, and families “to monitor for emergence or worsening of depression, suicidal ideation, or other mood changes,” according to the prescribing information.
Bimekizumab is being studied for other conditions, including hidradenitis suppurativa. In the European Union, it was approved for the treatment of psoriasis in 2021 and for the treatment of psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Lebwohl is an investigator for UCB. He has not accepted any consulting payments from UCB.
A version of this article first appeared on Medscape.com.
, the manufacturer announced in a press release.
The indication is for adults who are candidates for systemic therapy or phototherapy.
With this approval, bimekizumab becomes the only interleukin (IL)-17A and IL-17F inhibitor approved for the treatment of these patients. Psoriasis affects more than 7.5 million U.S. adults, according to the National Psoriasis Foundation.
“We have been eagerly awaiting bimekizumab,” Mark Lebwohl, MD, bimekizumab investigator and dean for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York City, said in the press release.
Dr. Lebwohl states that bimekizumab “achieved superior levels of skin clearance at week 16 compared to placebo and three existing biologics for psoriasis, with responses being rapid and lasting up to a year. Long-term data have also shown that the majority of patients maintained high levels of clinical response through three years.”
The most common adverse reactions (occurring in at least 1% of patients) are upper respiratory infections, oral candidiasis, headache, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other Candida infections, fatigue, and injection site reactions, according to the company, UCB.
Available in about 1 month in U.S.
Bimekizumab can be administered by a health care provider or it can be self-injected by a patient after training. It is available as a single-dose prefilled autoinjector and a single-dose prefilled syringe and will be available in the United States in about 1 month.
The recommended dosage of bimekizumab for patients with psoriasis is 320 mg (two subcutaneous injections of 160 mg each) at baseline, then on weeks 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing at least 120 kg (about 265 lb), a dosage of 320 mg every 4 weeks after week 16 may be considered, the company states.
Three phase 3 trials
Approval was based on three phase 3 multicenter, randomized, placebo and/or active comparator-controlled trials: bimekizumab versus placebo and ustekinumab (BE VIVID); versus placebo (BE READY); and versus adalimumab (BE SURE).
“All studies met their co-primary endpoints and all ranked secondary endpoints,” the company reports. Secondary endpoints included the Psoriasis Area and Severity Index (PASI) 75 at week 4 and PASI 100 (complete skin clearance) at week 16.
Highlights from the trials include the following results, according to UCB:
- Clear or almost clear skin: More than 8 out of 10 patients achieved a 90% or greater reduction from baseline in the PASI 90 and an Investigator’s Global Assessment score of 0/1 at week 16.
- Complete skin clearance: About 60% of patients achieved PASI 100 at week 16.
- Time to response: More than 70% of patients achieved PASI 75 at week 4 following one 320-mg dose.
Safety information
The safety information includes the statement that bimekizumab may increase the risk for suicidal ideation and behavior, though a causal association has not been established. Prescribers should advise patients, caregivers, and families “to monitor for emergence or worsening of depression, suicidal ideation, or other mood changes,” according to the prescribing information.
Bimekizumab is being studied for other conditions, including hidradenitis suppurativa. In the European Union, it was approved for the treatment of psoriasis in 2021 and for the treatment of psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Lebwohl is an investigator for UCB. He has not accepted any consulting payments from UCB.
A version of this article first appeared on Medscape.com.