User login
Ultrasound can be a useful tool in detecting subclinical juvenile psoriatic arthritis
Key clinical point: Musculoskeletal ultrasound along with physical examination can help identify juvenile psoriatic arthritis in pediatric patients with skin psoriasis showing musculoskeletal symptoms.
Major finding: Ultrasound evaluation showed higher number of joint and enthesitis abnormalities in pediatric patients with psoriasis who were symptomatic vs asymptomatic for musculoskeletal pain or swelling (all P ≤ .01). The concordance for detecting synovitis and enthesitis between physical and ultrasound examination was 82%.
Study details: Findings are from a cross-sectional study including 57 pediatric patients with psoriasis and no previous diagnosis of juvenile idiopathic arthritis or any systemic disease-causing articular manifestations who underwent ultrasound evaluation and clinical examination.
Disclosures: This study was supported by the PARTNER Fellowship program created with an unrestricted grant by Celgene-AMGEN, with L Coronel as a PARTNER fellow. Two authors declared ties with various sources, including Amgen. Two authors declared no conflicts of interest.
Source: Coronel L et al. Prevalence of ultrasound and clinical findings suggestive of inflammatory arthritis in children with skin psoriasis. Rheumatology (Oxford). 2023 (Aug 4). doi: 10.1093/rheumatology/kead398
Key clinical point: Musculoskeletal ultrasound along with physical examination can help identify juvenile psoriatic arthritis in pediatric patients with skin psoriasis showing musculoskeletal symptoms.
Major finding: Ultrasound evaluation showed higher number of joint and enthesitis abnormalities in pediatric patients with psoriasis who were symptomatic vs asymptomatic for musculoskeletal pain or swelling (all P ≤ .01). The concordance for detecting synovitis and enthesitis between physical and ultrasound examination was 82%.
Study details: Findings are from a cross-sectional study including 57 pediatric patients with psoriasis and no previous diagnosis of juvenile idiopathic arthritis or any systemic disease-causing articular manifestations who underwent ultrasound evaluation and clinical examination.
Disclosures: This study was supported by the PARTNER Fellowship program created with an unrestricted grant by Celgene-AMGEN, with L Coronel as a PARTNER fellow. Two authors declared ties with various sources, including Amgen. Two authors declared no conflicts of interest.
Source: Coronel L et al. Prevalence of ultrasound and clinical findings suggestive of inflammatory arthritis in children with skin psoriasis. Rheumatology (Oxford). 2023 (Aug 4). doi: 10.1093/rheumatology/kead398
Key clinical point: Musculoskeletal ultrasound along with physical examination can help identify juvenile psoriatic arthritis in pediatric patients with skin psoriasis showing musculoskeletal symptoms.
Major finding: Ultrasound evaluation showed higher number of joint and enthesitis abnormalities in pediatric patients with psoriasis who were symptomatic vs asymptomatic for musculoskeletal pain or swelling (all P ≤ .01). The concordance for detecting synovitis and enthesitis between physical and ultrasound examination was 82%.
Study details: Findings are from a cross-sectional study including 57 pediatric patients with psoriasis and no previous diagnosis of juvenile idiopathic arthritis or any systemic disease-causing articular manifestations who underwent ultrasound evaluation and clinical examination.
Disclosures: This study was supported by the PARTNER Fellowship program created with an unrestricted grant by Celgene-AMGEN, with L Coronel as a PARTNER fellow. Two authors declared ties with various sources, including Amgen. Two authors declared no conflicts of interest.
Source: Coronel L et al. Prevalence of ultrasound and clinical findings suggestive of inflammatory arthritis in children with skin psoriasis. Rheumatology (Oxford). 2023 (Aug 4). doi: 10.1093/rheumatology/kead398
Factors associated with depressive symptoms in PsA
Key clinical point: Depression is prevalent in patients with psoriatic arthritis (PsA), with patients not engaging in sports activities, experiencing fatigue, and having functional impairment being more likely to suffer from depression.
Major finding: Overall, 8.2% and 20.9% of patients with PsA had severe and moderate depressive symptoms, respectively. The odds of having depressive symptoms were higher in patients with functional impairment (odds ratio [OR] 1.08; P < .0001) and those experiencing fatigue (OR 1.56; P < .0001), whereas those engaging in sports for at least 1 hour/week were less likely to be depressed (OR 0.61; P = .0017).
Study details: This study included 1225 patients with PsA and 1245 patients with axial spondyloarthritis from the RABBIT-SpA cohort.
Disclosures: This study received open access funding from Projekt Deal, and RABBIT-SpA is supported by a joint, unconditional grant from AbbVie, Amgen, Biogen, and various other sources. The authors declared no conflicts of interest.
Source: Reich A et al. Depressive symptoms are associated with fatigue, poorer functional status and less engagement in sports in axSpA and PsA: An analysis from the RABBIT-SpA cohort. Arthritis Res Ther. 2023;25:136 (Aug 2). doi: 10.1186/s13075-023-03127-2.
Key clinical point: Depression is prevalent in patients with psoriatic arthritis (PsA), with patients not engaging in sports activities, experiencing fatigue, and having functional impairment being more likely to suffer from depression.
Major finding: Overall, 8.2% and 20.9% of patients with PsA had severe and moderate depressive symptoms, respectively. The odds of having depressive symptoms were higher in patients with functional impairment (odds ratio [OR] 1.08; P < .0001) and those experiencing fatigue (OR 1.56; P < .0001), whereas those engaging in sports for at least 1 hour/week were less likely to be depressed (OR 0.61; P = .0017).
Study details: This study included 1225 patients with PsA and 1245 patients with axial spondyloarthritis from the RABBIT-SpA cohort.
Disclosures: This study received open access funding from Projekt Deal, and RABBIT-SpA is supported by a joint, unconditional grant from AbbVie, Amgen, Biogen, and various other sources. The authors declared no conflicts of interest.
Source: Reich A et al. Depressive symptoms are associated with fatigue, poorer functional status and less engagement in sports in axSpA and PsA: An analysis from the RABBIT-SpA cohort. Arthritis Res Ther. 2023;25:136 (Aug 2). doi: 10.1186/s13075-023-03127-2.
Key clinical point: Depression is prevalent in patients with psoriatic arthritis (PsA), with patients not engaging in sports activities, experiencing fatigue, and having functional impairment being more likely to suffer from depression.
Major finding: Overall, 8.2% and 20.9% of patients with PsA had severe and moderate depressive symptoms, respectively. The odds of having depressive symptoms were higher in patients with functional impairment (odds ratio [OR] 1.08; P < .0001) and those experiencing fatigue (OR 1.56; P < .0001), whereas those engaging in sports for at least 1 hour/week were less likely to be depressed (OR 0.61; P = .0017).
Study details: This study included 1225 patients with PsA and 1245 patients with axial spondyloarthritis from the RABBIT-SpA cohort.
Disclosures: This study received open access funding from Projekt Deal, and RABBIT-SpA is supported by a joint, unconditional grant from AbbVie, Amgen, Biogen, and various other sources. The authors declared no conflicts of interest.
Source: Reich A et al. Depressive symptoms are associated with fatigue, poorer functional status and less engagement in sports in axSpA and PsA: An analysis from the RABBIT-SpA cohort. Arthritis Res Ther. 2023;25:136 (Aug 2). doi: 10.1186/s13075-023-03127-2.
Guselkumab modulates immune cell composition that may drive clinical response in PsA
Key clinical point: Patients with psoriatic arthritis (PsA) have dysregulated immune cell profiles that were partially normalized to the levels in control individuals after guselkumab treatment, with this effect being more pronounced among American College of Rheumatology 20 (ACR20) responders.
Major finding: At baseline, 355 and 314 PsA-related genes were upregulated and downregulated, respectively, in patients with PsA vs control individuals, with guselkumab treatment modulating the expression of 82% of upregulated and 77% of downregulated genes at week 24. The ACR20 responders showed a significant decrease in gene set enrichment scores for upregulated PsA-associated genes after 24 weeks of guselkumab treatment (all P ≤ .05).
Study details: This study evaluated whole blood transcriptome profiles of 673 patients with PsA from the DISCOVER-1 and DISCOVER-2 studies. The patients received guselkumab or placebo and there were 21 matched healthy control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC. Nine authors declared being employees of Janssen R&D and owning stock or stock options in Johnson & Johnson. The other authors reported ties with Janssen or other sources.
Source: Siebert S et al. Guselkumab modulates differentially expressed genes in blood of patients with psoriatic arthritis: Results from two phase 3, randomized, placebo-controlled trials. ACR Open Rheumatol. 2023 (Aug 8). doi: 10.1002/acr2.11589
Key clinical point: Patients with psoriatic arthritis (PsA) have dysregulated immune cell profiles that were partially normalized to the levels in control individuals after guselkumab treatment, with this effect being more pronounced among American College of Rheumatology 20 (ACR20) responders.
Major finding: At baseline, 355 and 314 PsA-related genes were upregulated and downregulated, respectively, in patients with PsA vs control individuals, with guselkumab treatment modulating the expression of 82% of upregulated and 77% of downregulated genes at week 24. The ACR20 responders showed a significant decrease in gene set enrichment scores for upregulated PsA-associated genes after 24 weeks of guselkumab treatment (all P ≤ .05).
Study details: This study evaluated whole blood transcriptome profiles of 673 patients with PsA from the DISCOVER-1 and DISCOVER-2 studies. The patients received guselkumab or placebo and there were 21 matched healthy control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC. Nine authors declared being employees of Janssen R&D and owning stock or stock options in Johnson & Johnson. The other authors reported ties with Janssen or other sources.
Source: Siebert S et al. Guselkumab modulates differentially expressed genes in blood of patients with psoriatic arthritis: Results from two phase 3, randomized, placebo-controlled trials. ACR Open Rheumatol. 2023 (Aug 8). doi: 10.1002/acr2.11589
Key clinical point: Patients with psoriatic arthritis (PsA) have dysregulated immune cell profiles that were partially normalized to the levels in control individuals after guselkumab treatment, with this effect being more pronounced among American College of Rheumatology 20 (ACR20) responders.
Major finding: At baseline, 355 and 314 PsA-related genes were upregulated and downregulated, respectively, in patients with PsA vs control individuals, with guselkumab treatment modulating the expression of 82% of upregulated and 77% of downregulated genes at week 24. The ACR20 responders showed a significant decrease in gene set enrichment scores for upregulated PsA-associated genes after 24 weeks of guselkumab treatment (all P ≤ .05).
Study details: This study evaluated whole blood transcriptome profiles of 673 patients with PsA from the DISCOVER-1 and DISCOVER-2 studies. The patients received guselkumab or placebo and there were 21 matched healthy control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC. Nine authors declared being employees of Janssen R&D and owning stock or stock options in Johnson & Johnson. The other authors reported ties with Janssen or other sources.
Source: Siebert S et al. Guselkumab modulates differentially expressed genes in blood of patients with psoriatic arthritis: Results from two phase 3, randomized, placebo-controlled trials. ACR Open Rheumatol. 2023 (Aug 8). doi: 10.1002/acr2.11589
Structured weight loss intervention reduces serum IL-23 levels in obese patients with PsA
Key clinical point: A structured Very Low Energy Diet intervention was associated with decrease in cytokines and leptins and increase in adipokines along with significant weight loss in patients with psoriatic arthritis (PsA) and obesity, highlighting the anti-inflammatory effect of weight loss in PsA.
Major finding: At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, whereas that of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals (P < .001 for all). The change in body mass index correlated positively with reduction in serum interleukin-23 (P < .001) and improvement in PsA disease activity (P = .003).
Study details: Findings are from a prospective interventional weight loss study that included patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on the Very Low Energy Diet (640 kcal/day) intervention.
Disclosures: This study was financed by grants from Swedish state and other sources, and open access funding was provided by the University of Gothenburg. The authors declared no conflicts of interest.
Source: Landgren AJ et al. Serum IL-23 significantly decreased in obese patients with psoriatic arthritis six months after a structured weight loss intervention. Arthritis Res Ther. 2023;25:131 (Jul 27). doi: 10.1186/s13075-023-03105-8
Key clinical point: A structured Very Low Energy Diet intervention was associated with decrease in cytokines and leptins and increase in adipokines along with significant weight loss in patients with psoriatic arthritis (PsA) and obesity, highlighting the anti-inflammatory effect of weight loss in PsA.
Major finding: At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, whereas that of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals (P < .001 for all). The change in body mass index correlated positively with reduction in serum interleukin-23 (P < .001) and improvement in PsA disease activity (P = .003).
Study details: Findings are from a prospective interventional weight loss study that included patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on the Very Low Energy Diet (640 kcal/day) intervention.
Disclosures: This study was financed by grants from Swedish state and other sources, and open access funding was provided by the University of Gothenburg. The authors declared no conflicts of interest.
Source: Landgren AJ et al. Serum IL-23 significantly decreased in obese patients with psoriatic arthritis six months after a structured weight loss intervention. Arthritis Res Ther. 2023;25:131 (Jul 27). doi: 10.1186/s13075-023-03105-8
Key clinical point: A structured Very Low Energy Diet intervention was associated with decrease in cytokines and leptins and increase in adipokines along with significant weight loss in patients with psoriatic arthritis (PsA) and obesity, highlighting the anti-inflammatory effect of weight loss in PsA.
Major finding: At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, whereas that of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals (P < .001 for all). The change in body mass index correlated positively with reduction in serum interleukin-23 (P < .001) and improvement in PsA disease activity (P = .003).
Study details: Findings are from a prospective interventional weight loss study that included patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on the Very Low Energy Diet (640 kcal/day) intervention.
Disclosures: This study was financed by grants from Swedish state and other sources, and open access funding was provided by the University of Gothenburg. The authors declared no conflicts of interest.
Source: Landgren AJ et al. Serum IL-23 significantly decreased in obese patients with psoriatic arthritis six months after a structured weight loss intervention. Arthritis Res Ther. 2023;25:131 (Jul 27). doi: 10.1186/s13075-023-03105-8
Psoriasis affects well-being and clinical outcomes in juvenile PsA
Key clinical point: Children and young people with juvenile psoriatic arthritis (JPsA) have poorer well-being than other subsets of juvenile idiopathic arthritis (JIA), with having psoriasis at JPsA diagnosis linked with more depressive symptoms.
Major finding: Children with JPsA vs other JIA categories had 2.35-times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores (P = .013), and children with psoriasis at JPsA diagnosis scored, on average, 10 points higher on the baseline Moods and Feelings Questionnaire (co-efficient 9.77; P = .039).
Study details: This study evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis.
Disclosures: This study was funded by the Cecil King Memorial Fund and other sources. The authors declared no conflicts of interest.
Source: Low JM et al for the CAPS Principal Investigators. The impact of psoriasis on wellbeing and clinical outcomes in juvenile psoriatic arthritis. Rheumatology (Oxford). 2023 (Jul 19). doi: 10.1093/rheumatology/kead370
Key clinical point: Children and young people with juvenile psoriatic arthritis (JPsA) have poorer well-being than other subsets of juvenile idiopathic arthritis (JIA), with having psoriasis at JPsA diagnosis linked with more depressive symptoms.
Major finding: Children with JPsA vs other JIA categories had 2.35-times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores (P = .013), and children with psoriasis at JPsA diagnosis scored, on average, 10 points higher on the baseline Moods and Feelings Questionnaire (co-efficient 9.77; P = .039).
Study details: This study evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis.
Disclosures: This study was funded by the Cecil King Memorial Fund and other sources. The authors declared no conflicts of interest.
Source: Low JM et al for the CAPS Principal Investigators. The impact of psoriasis on wellbeing and clinical outcomes in juvenile psoriatic arthritis. Rheumatology (Oxford). 2023 (Jul 19). doi: 10.1093/rheumatology/kead370
Key clinical point: Children and young people with juvenile psoriatic arthritis (JPsA) have poorer well-being than other subsets of juvenile idiopathic arthritis (JIA), with having psoriasis at JPsA diagnosis linked with more depressive symptoms.
Major finding: Children with JPsA vs other JIA categories had 2.35-times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores (P = .013), and children with psoriasis at JPsA diagnosis scored, on average, 10 points higher on the baseline Moods and Feelings Questionnaire (co-efficient 9.77; P = .039).
Study details: This study evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis.
Disclosures: This study was funded by the Cecil King Memorial Fund and other sources. The authors declared no conflicts of interest.
Source: Low JM et al for the CAPS Principal Investigators. The impact of psoriasis on wellbeing and clinical outcomes in juvenile psoriatic arthritis. Rheumatology (Oxford). 2023 (Jul 19). doi: 10.1093/rheumatology/kead370
DNA methylation markers may predict PsA development in patients with psoriasis
Key clinical point: Whole blood DNA methylation patterns measured before the onset of musculoskeletal symptoms can help distinguish patients with psoriasis who will develop psoriatic arthritis (PsA) from those who will not.
Major finding: The model based on 36 highly relevant methylation markers across 15 genes and intergenic regions (false discovery rate-adjusted P < .05; minimum change in methylation of 0.05) could identify patients with psoriasis who developed PsA from those who did not with an area under the receiver operating characteristic curve of 0.964.
Study details: Findings are from a nested case-control study including patients with psoriasis who later developed PsA (n = 58) matched with patients who did not develop PsA (n = 59).
Disclosures: This study was partly supported by an Early Career Research Grant awarded to RA Pollock from the National Psoriasis Foundation. The authors declared no conflicts of interest.
Source: Cruz-Correa OF et al. Prediction of psoriatic arthritis in patients with psoriasis using DNA methylation profiles. Arthritis Rheumatol. 2023 (Jul 18). doi: 10.1002/art.42654
Key clinical point: Whole blood DNA methylation patterns measured before the onset of musculoskeletal symptoms can help distinguish patients with psoriasis who will develop psoriatic arthritis (PsA) from those who will not.
Major finding: The model based on 36 highly relevant methylation markers across 15 genes and intergenic regions (false discovery rate-adjusted P < .05; minimum change in methylation of 0.05) could identify patients with psoriasis who developed PsA from those who did not with an area under the receiver operating characteristic curve of 0.964.
Study details: Findings are from a nested case-control study including patients with psoriasis who later developed PsA (n = 58) matched with patients who did not develop PsA (n = 59).
Disclosures: This study was partly supported by an Early Career Research Grant awarded to RA Pollock from the National Psoriasis Foundation. The authors declared no conflicts of interest.
Source: Cruz-Correa OF et al. Prediction of psoriatic arthritis in patients with psoriasis using DNA methylation profiles. Arthritis Rheumatol. 2023 (Jul 18). doi: 10.1002/art.42654
Key clinical point: Whole blood DNA methylation patterns measured before the onset of musculoskeletal symptoms can help distinguish patients with psoriasis who will develop psoriatic arthritis (PsA) from those who will not.
Major finding: The model based on 36 highly relevant methylation markers across 15 genes and intergenic regions (false discovery rate-adjusted P < .05; minimum change in methylation of 0.05) could identify patients with psoriasis who developed PsA from those who did not with an area under the receiver operating characteristic curve of 0.964.
Study details: Findings are from a nested case-control study including patients with psoriasis who later developed PsA (n = 58) matched with patients who did not develop PsA (n = 59).
Disclosures: This study was partly supported by an Early Career Research Grant awarded to RA Pollock from the National Psoriasis Foundation. The authors declared no conflicts of interest.
Source: Cruz-Correa OF et al. Prediction of psoriatic arthritis in patients with psoriasis using DNA methylation profiles. Arthritis Rheumatol. 2023 (Jul 18). doi: 10.1002/art.42654
PsA biomarkers move researchers closer to predictive test
In a new study, researchers report that they have found epigenetic methylation markers on 15 genes that appear to foreshadow psoriatic arthritis (PsA), a development that could bring scientists closer to developing a DNA test to predict which patients with psoriasis will develop the condition.
While no predictive test is in sight yet, the findings published in Arthritis & Rheumatology mark an important step, study lead author Omar F. Cruz-Correa, PhD, of the Psoriatic Arthritis Research Program in the University Health Network, Toronto, said in an interview. “In the future, markers like these could be measured by dermatologists and even general practitioners to help identify new psoriasis patients at a high risk of developing PsA,” he said. “Then both the health care team and the patients themselves could be more aware of their increased risk and the pressing need of closer monitoring for musculoskeletal symptoms. Once the first symptoms appear, treatment can be initiated early on, helping to prevent permanent joint damage.”
An estimated 30% of patients with psoriasis will develop PsA, too, putting them at higher risk of disability and death. According to Dr. Cruz-Correa, “one of the more pressing matters in PsA is the lack of means of predicting which psoriasis patients will develop PsA.”
DNA methylation, the topic of the new study, has already been linked to psoriasis and PsA. It’s “relatively easy to measure and helps regulate gene expression in response to environmental effects,” Dr. Cruz-Correa said. “DNA methylation is also appealing because it serves as an intermediary between environment and genetic factors as it’s transmitted between generations of cells and influenced by external factors.”
For the new study, researchers examined the DNA of 117 patients with psoriasis – 58 who went on to develop PsA (“converters”) and another 59 who were matched to converters but did not develop PsA (“nonconverters”). The patients were in a larger group of 700 patients with psoriasis who had the disease for a mean of about 17 years at the time of blood sampling.
Samples from converters were taken an average of 5.16 years (± 12.77 years) before PsA set in.
The researchers report that they found “36 highly relevant methylation markers … across 15 genes and several intergenic regions. A classification model relying on these markers identified converters and nonconverters with an area under the ROC curve of 0.9644.”
Statistically, this number is high and means that “the DNA methylation markers are really good at identifying psoriasis patients who will develop PsA and those that will not,” at least in this specific patient group, Dr. Cruz-Correa said.
At this point, the number of markers is a bit too high to develop a feasible DNA test to predict PsA, he said. “However, the results from our study have also pointed us toward some interesting metabolic pathways that may warrant further study.”
What’s next?
The first step forward “is the validation of these predictive DNA methylation markers in a wider population of patients with varied clinical and demographic characteristics. This would help assess the potential for generalization of such a test,” Dr. Cruz-Correa said. “A second step is to assess the potential impact of these methylation markers on disease activity and treatment response, which are clinical outcomes of great importance to patients.”
Meanwhile, he said, “there are ongoing efforts to shed light into how DNA methylation integrates with other epigenetic mechanisms like micro-RNAs to regulate gene expression in concert with one another. An integrative look into these mechanisms may be able to give insight into the pathogenesis of psoriatic disease in a way that has not been possible before.”
In an interview, Johann E. Gudjonsson, MD, PhD, professor of skin molecular immunology at the University of Michigan, Ann Arbor, said the study “is interesting and important as it indicates that there are changes in the blood that occur before the development of psoriatic arthritis. However, it does not provide much in terms of novel insights into the mechanisms involved and is still a long way away from being useful as a clinical predictor or biomarker.”
The National Psoriasis Foundation, Krembil Foundation, and Canadian Institutes of Health Research provided support for the study. Dr. Cruz-Correa reports support from the National Psoriasis Foundation and the Arthritis Society. Dr. Gudjonsson has no relevant financial relationships.
In a new study, researchers report that they have found epigenetic methylation markers on 15 genes that appear to foreshadow psoriatic arthritis (PsA), a development that could bring scientists closer to developing a DNA test to predict which patients with psoriasis will develop the condition.
While no predictive test is in sight yet, the findings published in Arthritis & Rheumatology mark an important step, study lead author Omar F. Cruz-Correa, PhD, of the Psoriatic Arthritis Research Program in the University Health Network, Toronto, said in an interview. “In the future, markers like these could be measured by dermatologists and even general practitioners to help identify new psoriasis patients at a high risk of developing PsA,” he said. “Then both the health care team and the patients themselves could be more aware of their increased risk and the pressing need of closer monitoring for musculoskeletal symptoms. Once the first symptoms appear, treatment can be initiated early on, helping to prevent permanent joint damage.”
An estimated 30% of patients with psoriasis will develop PsA, too, putting them at higher risk of disability and death. According to Dr. Cruz-Correa, “one of the more pressing matters in PsA is the lack of means of predicting which psoriasis patients will develop PsA.”
DNA methylation, the topic of the new study, has already been linked to psoriasis and PsA. It’s “relatively easy to measure and helps regulate gene expression in response to environmental effects,” Dr. Cruz-Correa said. “DNA methylation is also appealing because it serves as an intermediary between environment and genetic factors as it’s transmitted between generations of cells and influenced by external factors.”
For the new study, researchers examined the DNA of 117 patients with psoriasis – 58 who went on to develop PsA (“converters”) and another 59 who were matched to converters but did not develop PsA (“nonconverters”). The patients were in a larger group of 700 patients with psoriasis who had the disease for a mean of about 17 years at the time of blood sampling.
Samples from converters were taken an average of 5.16 years (± 12.77 years) before PsA set in.
The researchers report that they found “36 highly relevant methylation markers … across 15 genes and several intergenic regions. A classification model relying on these markers identified converters and nonconverters with an area under the ROC curve of 0.9644.”
Statistically, this number is high and means that “the DNA methylation markers are really good at identifying psoriasis patients who will develop PsA and those that will not,” at least in this specific patient group, Dr. Cruz-Correa said.
At this point, the number of markers is a bit too high to develop a feasible DNA test to predict PsA, he said. “However, the results from our study have also pointed us toward some interesting metabolic pathways that may warrant further study.”
What’s next?
The first step forward “is the validation of these predictive DNA methylation markers in a wider population of patients with varied clinical and demographic characteristics. This would help assess the potential for generalization of such a test,” Dr. Cruz-Correa said. “A second step is to assess the potential impact of these methylation markers on disease activity and treatment response, which are clinical outcomes of great importance to patients.”
Meanwhile, he said, “there are ongoing efforts to shed light into how DNA methylation integrates with other epigenetic mechanisms like micro-RNAs to regulate gene expression in concert with one another. An integrative look into these mechanisms may be able to give insight into the pathogenesis of psoriatic disease in a way that has not been possible before.”
In an interview, Johann E. Gudjonsson, MD, PhD, professor of skin molecular immunology at the University of Michigan, Ann Arbor, said the study “is interesting and important as it indicates that there are changes in the blood that occur before the development of psoriatic arthritis. However, it does not provide much in terms of novel insights into the mechanisms involved and is still a long way away from being useful as a clinical predictor or biomarker.”
The National Psoriasis Foundation, Krembil Foundation, and Canadian Institutes of Health Research provided support for the study. Dr. Cruz-Correa reports support from the National Psoriasis Foundation and the Arthritis Society. Dr. Gudjonsson has no relevant financial relationships.
In a new study, researchers report that they have found epigenetic methylation markers on 15 genes that appear to foreshadow psoriatic arthritis (PsA), a development that could bring scientists closer to developing a DNA test to predict which patients with psoriasis will develop the condition.
While no predictive test is in sight yet, the findings published in Arthritis & Rheumatology mark an important step, study lead author Omar F. Cruz-Correa, PhD, of the Psoriatic Arthritis Research Program in the University Health Network, Toronto, said in an interview. “In the future, markers like these could be measured by dermatologists and even general practitioners to help identify new psoriasis patients at a high risk of developing PsA,” he said. “Then both the health care team and the patients themselves could be more aware of their increased risk and the pressing need of closer monitoring for musculoskeletal symptoms. Once the first symptoms appear, treatment can be initiated early on, helping to prevent permanent joint damage.”
An estimated 30% of patients with psoriasis will develop PsA, too, putting them at higher risk of disability and death. According to Dr. Cruz-Correa, “one of the more pressing matters in PsA is the lack of means of predicting which psoriasis patients will develop PsA.”
DNA methylation, the topic of the new study, has already been linked to psoriasis and PsA. It’s “relatively easy to measure and helps regulate gene expression in response to environmental effects,” Dr. Cruz-Correa said. “DNA methylation is also appealing because it serves as an intermediary between environment and genetic factors as it’s transmitted between generations of cells and influenced by external factors.”
For the new study, researchers examined the DNA of 117 patients with psoriasis – 58 who went on to develop PsA (“converters”) and another 59 who were matched to converters but did not develop PsA (“nonconverters”). The patients were in a larger group of 700 patients with psoriasis who had the disease for a mean of about 17 years at the time of blood sampling.
Samples from converters were taken an average of 5.16 years (± 12.77 years) before PsA set in.
The researchers report that they found “36 highly relevant methylation markers … across 15 genes and several intergenic regions. A classification model relying on these markers identified converters and nonconverters with an area under the ROC curve of 0.9644.”
Statistically, this number is high and means that “the DNA methylation markers are really good at identifying psoriasis patients who will develop PsA and those that will not,” at least in this specific patient group, Dr. Cruz-Correa said.
At this point, the number of markers is a bit too high to develop a feasible DNA test to predict PsA, he said. “However, the results from our study have also pointed us toward some interesting metabolic pathways that may warrant further study.”
What’s next?
The first step forward “is the validation of these predictive DNA methylation markers in a wider population of patients with varied clinical and demographic characteristics. This would help assess the potential for generalization of such a test,” Dr. Cruz-Correa said. “A second step is to assess the potential impact of these methylation markers on disease activity and treatment response, which are clinical outcomes of great importance to patients.”
Meanwhile, he said, “there are ongoing efforts to shed light into how DNA methylation integrates with other epigenetic mechanisms like micro-RNAs to regulate gene expression in concert with one another. An integrative look into these mechanisms may be able to give insight into the pathogenesis of psoriatic disease in a way that has not been possible before.”
In an interview, Johann E. Gudjonsson, MD, PhD, professor of skin molecular immunology at the University of Michigan, Ann Arbor, said the study “is interesting and important as it indicates that there are changes in the blood that occur before the development of psoriatic arthritis. However, it does not provide much in terms of novel insights into the mechanisms involved and is still a long way away from being useful as a clinical predictor or biomarker.”
The National Psoriasis Foundation, Krembil Foundation, and Canadian Institutes of Health Research provided support for the study. Dr. Cruz-Correa reports support from the National Psoriasis Foundation and the Arthritis Society. Dr. Gudjonsson has no relevant financial relationships.
FROM ARTHRITIS & RHEUMATOLOGY
Study validates use of new psoriatic arthritis prediction tool
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
FROM ARTHRITIS AND RHEUMATOLOGY
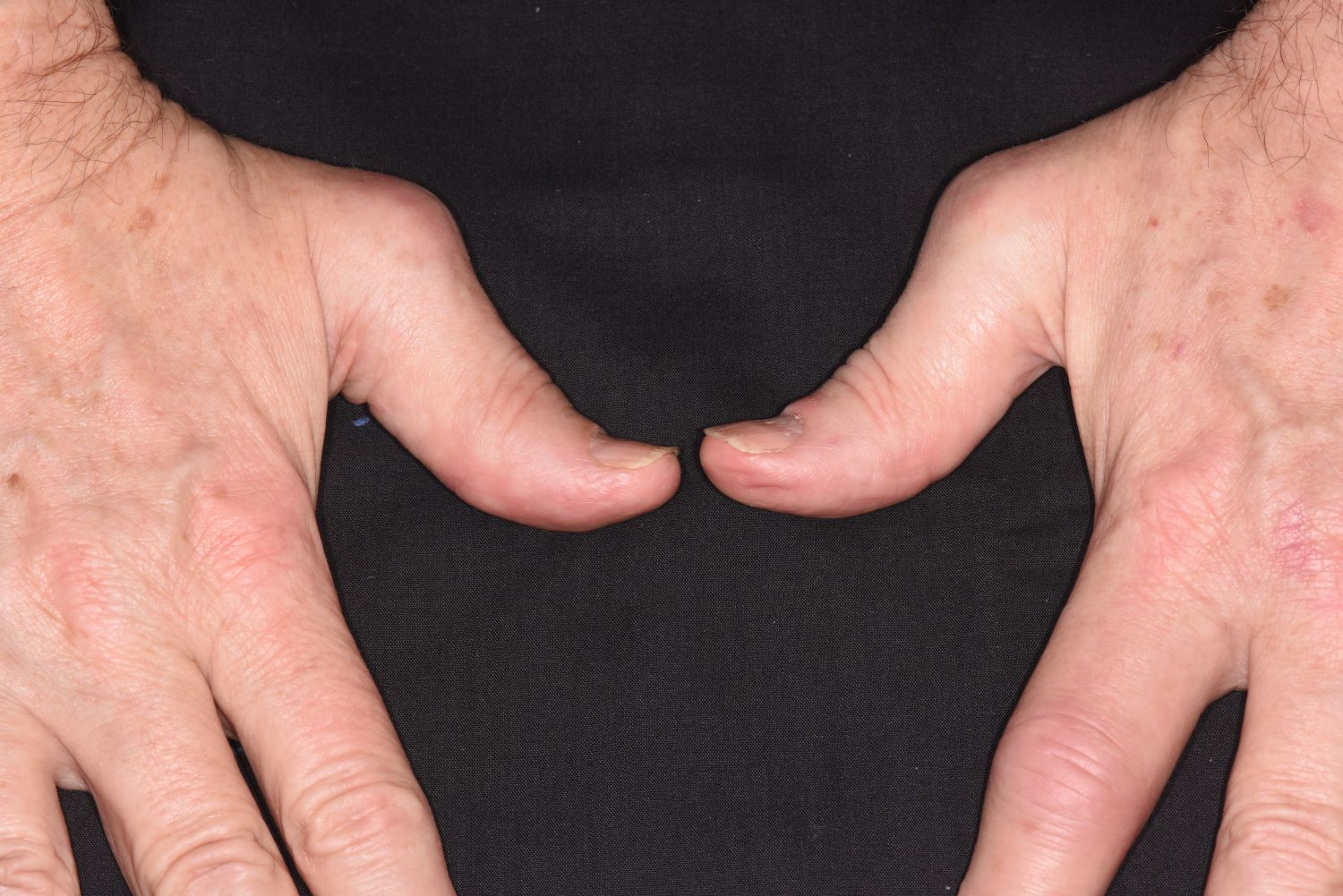
History of plaque psoriasis
The patient's history of psoriasis, along with his current skin and scalp plaque flares, symmetrical joint symptomatology, laboratory studies, and x-rays, suggest a diagnosis of symmetrical psoriatic arthritis (PsA). The rheumatologist considers ordering additional imaging to assess for subclinical enthesitis and dactylitis, and discusses treatment next steps with the patient, given inadequate control with a TNF inhibitor.
Symmetrical polyarthritis is one of the most common types of PsA and involves five or more joints in the hands, wrists, ankles, and/or feet. Among patients with PsA, 60% to 80% experience plaque psoriasis before joint-symptom onset; time to joint-symptom onset in these patients typically occurs within 10 years of a plaque psoriasis diagnosis. Involvement of DIP joints differentiates PsA from rheumatoid arthritis, as does the absence of subcutaneous nodules and a negative result for rheumatoid factor. About 30% of all people with plaque psoriasis will develop PsA, which affects an estimated 1 million people in the United States annually. Symptoms typically appear between the ages of 35 and 55 years; women are more likely than men to develop symmetrical PsA.
There are no specific diagnostic tests for PsA. Rheumatologists generally use the assessment known as the Classification Criteria for Psoriatic Arthritis, (CASPAR), which can help reveal established inflammatory articular disease through a point system based on the presence/absence of various factors. On laboratory studies, the most common characteristic abnormalities of PsA are elevated ESR and CRP levels and negative rheumatoid factor in most patients. Other abnormalities that may be present in patients with PsA include elevated serum uric acid concentration and serum immunoglobulin A, and reduced levels of circulating immune complexes. Physicians also use imaging studies, such as radiography, ultrasonography, and MRI, to help differentiate PsA from other articular diseases.
While the pathogenesis of PsA remains unclear, research has shown that disease development is associated with a complex interplay of immune-mediated inflammatory responses; genetic and environmental factors may also be involved. In addition, patients with PsA are more likely to have a high risk for comorbidities, including obesity, type 2 diabetes, hypertension, hyperlipidemia, and cardiovascular events, compared with the general population.
When patients with PsA experience both skin and joint symptoms, a multidisciplinary approach to care is advised. Multidisciplinary teams play a key role in educating patients about their treatment plans and managing their PsA symptoms. The teams also help patients determine the best approaches to exercise to help maintain current joint function, as well as helpful adjustments in daily activities that will make it easier to accommodate their disease.
Nonsteroidal anti-inflammatory drugs, whether self-prescribed or prescribed by a physician, are a common initial treatment to manage joint symptoms of PsA. Current American College of Rheumatology treatment guidelines, however, encourage early treatment with disease-modifying antirheumatic drugs (DMARDs) because approximately 40% of patients with PsA develop erosive and deforming arthritis. Several DMARDs are available, including older drugs like methotrexate, as well as newer biologic agents, such as TNF inhibitors, interleukin (IL)-17 inhibitors, IL-12/23 inhibitors, and Janus kinase inhibitors. In addition, guidelines recommend early and customized physical therapy and rehabilitation approaches for patients with PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient's history of psoriasis, along with his current skin and scalp plaque flares, symmetrical joint symptomatology, laboratory studies, and x-rays, suggest a diagnosis of symmetrical psoriatic arthritis (PsA). The rheumatologist considers ordering additional imaging to assess for subclinical enthesitis and dactylitis, and discusses treatment next steps with the patient, given inadequate control with a TNF inhibitor.
Symmetrical polyarthritis is one of the most common types of PsA and involves five or more joints in the hands, wrists, ankles, and/or feet. Among patients with PsA, 60% to 80% experience plaque psoriasis before joint-symptom onset; time to joint-symptom onset in these patients typically occurs within 10 years of a plaque psoriasis diagnosis. Involvement of DIP joints differentiates PsA from rheumatoid arthritis, as does the absence of subcutaneous nodules and a negative result for rheumatoid factor. About 30% of all people with plaque psoriasis will develop PsA, which affects an estimated 1 million people in the United States annually. Symptoms typically appear between the ages of 35 and 55 years; women are more likely than men to develop symmetrical PsA.
There are no specific diagnostic tests for PsA. Rheumatologists generally use the assessment known as the Classification Criteria for Psoriatic Arthritis, (CASPAR), which can help reveal established inflammatory articular disease through a point system based on the presence/absence of various factors. On laboratory studies, the most common characteristic abnormalities of PsA are elevated ESR and CRP levels and negative rheumatoid factor in most patients. Other abnormalities that may be present in patients with PsA include elevated serum uric acid concentration and serum immunoglobulin A, and reduced levels of circulating immune complexes. Physicians also use imaging studies, such as radiography, ultrasonography, and MRI, to help differentiate PsA from other articular diseases.
While the pathogenesis of PsA remains unclear, research has shown that disease development is associated with a complex interplay of immune-mediated inflammatory responses; genetic and environmental factors may also be involved. In addition, patients with PsA are more likely to have a high risk for comorbidities, including obesity, type 2 diabetes, hypertension, hyperlipidemia, and cardiovascular events, compared with the general population.
When patients with PsA experience both skin and joint symptoms, a multidisciplinary approach to care is advised. Multidisciplinary teams play a key role in educating patients about their treatment plans and managing their PsA symptoms. The teams also help patients determine the best approaches to exercise to help maintain current joint function, as well as helpful adjustments in daily activities that will make it easier to accommodate their disease.
Nonsteroidal anti-inflammatory drugs, whether self-prescribed or prescribed by a physician, are a common initial treatment to manage joint symptoms of PsA. Current American College of Rheumatology treatment guidelines, however, encourage early treatment with disease-modifying antirheumatic drugs (DMARDs) because approximately 40% of patients with PsA develop erosive and deforming arthritis. Several DMARDs are available, including older drugs like methotrexate, as well as newer biologic agents, such as TNF inhibitors, interleukin (IL)-17 inhibitors, IL-12/23 inhibitors, and Janus kinase inhibitors. In addition, guidelines recommend early and customized physical therapy and rehabilitation approaches for patients with PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient's history of psoriasis, along with his current skin and scalp plaque flares, symmetrical joint symptomatology, laboratory studies, and x-rays, suggest a diagnosis of symmetrical psoriatic arthritis (PsA). The rheumatologist considers ordering additional imaging to assess for subclinical enthesitis and dactylitis, and discusses treatment next steps with the patient, given inadequate control with a TNF inhibitor.
Symmetrical polyarthritis is one of the most common types of PsA and involves five or more joints in the hands, wrists, ankles, and/or feet. Among patients with PsA, 60% to 80% experience plaque psoriasis before joint-symptom onset; time to joint-symptom onset in these patients typically occurs within 10 years of a plaque psoriasis diagnosis. Involvement of DIP joints differentiates PsA from rheumatoid arthritis, as does the absence of subcutaneous nodules and a negative result for rheumatoid factor. About 30% of all people with plaque psoriasis will develop PsA, which affects an estimated 1 million people in the United States annually. Symptoms typically appear between the ages of 35 and 55 years; women are more likely than men to develop symmetrical PsA.
There are no specific diagnostic tests for PsA. Rheumatologists generally use the assessment known as the Classification Criteria for Psoriatic Arthritis, (CASPAR), which can help reveal established inflammatory articular disease through a point system based on the presence/absence of various factors. On laboratory studies, the most common characteristic abnormalities of PsA are elevated ESR and CRP levels and negative rheumatoid factor in most patients. Other abnormalities that may be present in patients with PsA include elevated serum uric acid concentration and serum immunoglobulin A, and reduced levels of circulating immune complexes. Physicians also use imaging studies, such as radiography, ultrasonography, and MRI, to help differentiate PsA from other articular diseases.
While the pathogenesis of PsA remains unclear, research has shown that disease development is associated with a complex interplay of immune-mediated inflammatory responses; genetic and environmental factors may also be involved. In addition, patients with PsA are more likely to have a high risk for comorbidities, including obesity, type 2 diabetes, hypertension, hyperlipidemia, and cardiovascular events, compared with the general population.
When patients with PsA experience both skin and joint symptoms, a multidisciplinary approach to care is advised. Multidisciplinary teams play a key role in educating patients about their treatment plans and managing their PsA symptoms. The teams also help patients determine the best approaches to exercise to help maintain current joint function, as well as helpful adjustments in daily activities that will make it easier to accommodate their disease.
Nonsteroidal anti-inflammatory drugs, whether self-prescribed or prescribed by a physician, are a common initial treatment to manage joint symptoms of PsA. Current American College of Rheumatology treatment guidelines, however, encourage early treatment with disease-modifying antirheumatic drugs (DMARDs) because approximately 40% of patients with PsA develop erosive and deforming arthritis. Several DMARDs are available, including older drugs like methotrexate, as well as newer biologic agents, such as TNF inhibitors, interleukin (IL)-17 inhibitors, IL-12/23 inhibitors, and Janus kinase inhibitors. In addition, guidelines recommend early and customized physical therapy and rehabilitation approaches for patients with PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 43-year-old White man with a 5-year history of plaque psoriasis presents to a rheumatologist on referral from his dermatologist. He had been taking a tumor necrosis factor (TNF) inhibitor, which had controlled his skin and scalp plaques since diagnosis. Lately, however, some of the plaques have begun to flare up, and the patient reports new tenderness and swelling in three of the same joints on his left and right hands and extensive fatigue. Additional medical history includes type 2 diabetes, which was diagnosed 3 years ago; soon thereafter, he started taking metformin with consistent disease control. The rheumatologist conducts a physical exam and orders laboratory studies and x-rays. Results of the laboratory studies reveal elevated levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Radiographs reveal joint-space narrowing in several distal interphalangeal (DIP) joints in both hands, with mild erosive disease.
What factors cause multiple biologic failure in psoriasis?
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY