User login
How to Initiate a VTE Quality Improvement Project
While VTE sometimes occurs in spite of the best available prophylaxis, there are many lost opportunities to optimize prevention and reduce VTE risk factors in virtually every hospital. Reaching a meaningful improvement in VTE prevention requires an empowered, interdisciplinary team approach supported by the institution to standardize processes, monitor, and measure VTE process and outcomes, implement institutional policies, and educate providers and patients.
In particular, Greg Maynard, MD, MSc, SFHM, director of the University of California San Diego Center for Innovation and Improvement Science, and senior medical officer of the Society of Hospital Medicine’s Center for Hospital Innovation and Improvement, suggests reviewing guidelines and regulatory materials that focus on the implications for implementation. Then, summarize the evidence into a VTE prevention protocol.
A VTE prevention protocol includes a VTE risk assessment, bleeding risk assessment, and clinical decision support (CDS) on prophylactic choices based on this combination of VTE and bleeding risk factors. The VTE protocol CDS must be available at crucial junctures of care, such as admission to the hospital, transfer to different levels of care, and post-operatively.
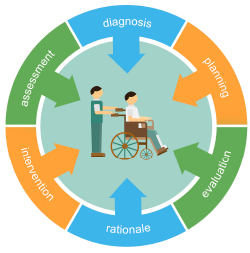
“This VTE protocol guidance is most often embedded in order sets that are commonly used [or mandated for use] in these settings, essentially ‘hard-wiring’ the VTE risk assessment into the process,” Dr. Maynard says.
Risk assessment is essential, as there are harms, costs, and discomfort associated with prophylactic methods. For some inpatients, the risk of anticoagulant prophylaxis may outweigh the risk
of hospital-acquired VTE. No perfect VTE risk assessment tool exists, and there is always inherent tension between the desire to provide comprehensive, detailed guidance and the need to keep the process simple to understand and measure.
Principles for the effective implementation of reliable interventions generally favor simple models, with more complicated models reserved for settings with advanced methods to make the models easier for the end user.
“Order sets with CDS are of no use if they are not used correctly and reliably, so monitoring this process is crucial,” Dr. Maynard says.
No matter which VTE risk assessment model is used, every effort should be made to enhance ease of use for the ordering provider. This may include carving out special populations such as obstetric patients and major orthopedic, trauma, cardiovascular surgery, and neurosurgery patients for modified VTE risk assessment and order sets, Dr. Maynard says, which allows for streamlining and simplification of VTE prevention order sets.
Successful integration of a VTE prevention protocol into heavily utilized admission and transfer order sets serves as a foundational beginning point for VTE prevention efforts, rather than the end point.
“Even if every patient has the best prophylaxis ordered on admission, other problems can lead to VTE during the hospital stay or after discharge,”
Dr. Maynard says.
For example:
- Bleeding and VTE risk factors can change several times during a hospital stay, but reassessment does not occur;
- Patients are not optimally mobilized;
- Adherence to ordered mechanical prophylaxis is notoriously low; and
- Overutilization of peripherally inserted central catheter lines or other central venous catheters contributes to upper extremity DVT.
VTE prevention programs should address these pitfalls, in addition to implementing order sets.
Publicly reported measures and the CMS core measures set a relatively low bar for performance and are inadequate to drive breakthrough levels of improvement, Dr. Maynard adds. The adequacy of VTE prophylaxis should be assessed not only on admission or transfer to the intensive care unit but also across the hospital stay. Month-to-month reporting is important to follow progress, but at least some measures should drive concurrent intervention to address deficits in prophylaxis in real time. This method of active surveillance (also known as measure-vention), along with multiple other measurement methods that go beyond the core measures, is often necessary to secure real improvement.
An extensive update and revision of the Agency for Healthcare Research and Quality/Society of Hospital Medicine VTE Prevention Implementation Guide will be released by early spring. It will provide comprehensive coverage of these concepts.
Karen Appold is a freelance medical writer in Pennsylvania.
While VTE sometimes occurs in spite of the best available prophylaxis, there are many lost opportunities to optimize prevention and reduce VTE risk factors in virtually every hospital. Reaching a meaningful improvement in VTE prevention requires an empowered, interdisciplinary team approach supported by the institution to standardize processes, monitor, and measure VTE process and outcomes, implement institutional policies, and educate providers and patients.
In particular, Greg Maynard, MD, MSc, SFHM, director of the University of California San Diego Center for Innovation and Improvement Science, and senior medical officer of the Society of Hospital Medicine’s Center for Hospital Innovation and Improvement, suggests reviewing guidelines and regulatory materials that focus on the implications for implementation. Then, summarize the evidence into a VTE prevention protocol.
A VTE prevention protocol includes a VTE risk assessment, bleeding risk assessment, and clinical decision support (CDS) on prophylactic choices based on this combination of VTE and bleeding risk factors. The VTE protocol CDS must be available at crucial junctures of care, such as admission to the hospital, transfer to different levels of care, and post-operatively.

“This VTE protocol guidance is most often embedded in order sets that are commonly used [or mandated for use] in these settings, essentially ‘hard-wiring’ the VTE risk assessment into the process,” Dr. Maynard says.
Risk assessment is essential, as there are harms, costs, and discomfort associated with prophylactic methods. For some inpatients, the risk of anticoagulant prophylaxis may outweigh the risk
of hospital-acquired VTE. No perfect VTE risk assessment tool exists, and there is always inherent tension between the desire to provide comprehensive, detailed guidance and the need to keep the process simple to understand and measure.
Principles for the effective implementation of reliable interventions generally favor simple models, with more complicated models reserved for settings with advanced methods to make the models easier for the end user.
“Order sets with CDS are of no use if they are not used correctly and reliably, so monitoring this process is crucial,” Dr. Maynard says.
No matter which VTE risk assessment model is used, every effort should be made to enhance ease of use for the ordering provider. This may include carving out special populations such as obstetric patients and major orthopedic, trauma, cardiovascular surgery, and neurosurgery patients for modified VTE risk assessment and order sets, Dr. Maynard says, which allows for streamlining and simplification of VTE prevention order sets.
Successful integration of a VTE prevention protocol into heavily utilized admission and transfer order sets serves as a foundational beginning point for VTE prevention efforts, rather than the end point.
“Even if every patient has the best prophylaxis ordered on admission, other problems can lead to VTE during the hospital stay or after discharge,”
Dr. Maynard says.
For example:
- Bleeding and VTE risk factors can change several times during a hospital stay, but reassessment does not occur;
- Patients are not optimally mobilized;
- Adherence to ordered mechanical prophylaxis is notoriously low; and
- Overutilization of peripherally inserted central catheter lines or other central venous catheters contributes to upper extremity DVT.
VTE prevention programs should address these pitfalls, in addition to implementing order sets.
Publicly reported measures and the CMS core measures set a relatively low bar for performance and are inadequate to drive breakthrough levels of improvement, Dr. Maynard adds. The adequacy of VTE prophylaxis should be assessed not only on admission or transfer to the intensive care unit but also across the hospital stay. Month-to-month reporting is important to follow progress, but at least some measures should drive concurrent intervention to address deficits in prophylaxis in real time. This method of active surveillance (also known as measure-vention), along with multiple other measurement methods that go beyond the core measures, is often necessary to secure real improvement.
An extensive update and revision of the Agency for Healthcare Research and Quality/Society of Hospital Medicine VTE Prevention Implementation Guide will be released by early spring. It will provide comprehensive coverage of these concepts.
Karen Appold is a freelance medical writer in Pennsylvania.
While VTE sometimes occurs in spite of the best available prophylaxis, there are many lost opportunities to optimize prevention and reduce VTE risk factors in virtually every hospital. Reaching a meaningful improvement in VTE prevention requires an empowered, interdisciplinary team approach supported by the institution to standardize processes, monitor, and measure VTE process and outcomes, implement institutional policies, and educate providers and patients.
In particular, Greg Maynard, MD, MSc, SFHM, director of the University of California San Diego Center for Innovation and Improvement Science, and senior medical officer of the Society of Hospital Medicine’s Center for Hospital Innovation and Improvement, suggests reviewing guidelines and regulatory materials that focus on the implications for implementation. Then, summarize the evidence into a VTE prevention protocol.
A VTE prevention protocol includes a VTE risk assessment, bleeding risk assessment, and clinical decision support (CDS) on prophylactic choices based on this combination of VTE and bleeding risk factors. The VTE protocol CDS must be available at crucial junctures of care, such as admission to the hospital, transfer to different levels of care, and post-operatively.

“This VTE protocol guidance is most often embedded in order sets that are commonly used [or mandated for use] in these settings, essentially ‘hard-wiring’ the VTE risk assessment into the process,” Dr. Maynard says.
Risk assessment is essential, as there are harms, costs, and discomfort associated with prophylactic methods. For some inpatients, the risk of anticoagulant prophylaxis may outweigh the risk
of hospital-acquired VTE. No perfect VTE risk assessment tool exists, and there is always inherent tension between the desire to provide comprehensive, detailed guidance and the need to keep the process simple to understand and measure.
Principles for the effective implementation of reliable interventions generally favor simple models, with more complicated models reserved for settings with advanced methods to make the models easier for the end user.
“Order sets with CDS are of no use if they are not used correctly and reliably, so monitoring this process is crucial,” Dr. Maynard says.
No matter which VTE risk assessment model is used, every effort should be made to enhance ease of use for the ordering provider. This may include carving out special populations such as obstetric patients and major orthopedic, trauma, cardiovascular surgery, and neurosurgery patients for modified VTE risk assessment and order sets, Dr. Maynard says, which allows for streamlining and simplification of VTE prevention order sets.
Successful integration of a VTE prevention protocol into heavily utilized admission and transfer order sets serves as a foundational beginning point for VTE prevention efforts, rather than the end point.
“Even if every patient has the best prophylaxis ordered on admission, other problems can lead to VTE during the hospital stay or after discharge,”
Dr. Maynard says.
For example:
- Bleeding and VTE risk factors can change several times during a hospital stay, but reassessment does not occur;
- Patients are not optimally mobilized;
- Adherence to ordered mechanical prophylaxis is notoriously low; and
- Overutilization of peripherally inserted central catheter lines or other central venous catheters contributes to upper extremity DVT.
VTE prevention programs should address these pitfalls, in addition to implementing order sets.
Publicly reported measures and the CMS core measures set a relatively low bar for performance and are inadequate to drive breakthrough levels of improvement, Dr. Maynard adds. The adequacy of VTE prophylaxis should be assessed not only on admission or transfer to the intensive care unit but also across the hospital stay. Month-to-month reporting is important to follow progress, but at least some measures should drive concurrent intervention to address deficits in prophylaxis in real time. This method of active surveillance (also known as measure-vention), along with multiple other measurement methods that go beyond the core measures, is often necessary to secure real improvement.
An extensive update and revision of the Agency for Healthcare Research and Quality/Society of Hospital Medicine VTE Prevention Implementation Guide will be released by early spring. It will provide comprehensive coverage of these concepts.
Karen Appold is a freelance medical writer in Pennsylvania.
LISTEN NOW: Peter Pronovost, MD, PhD, Explains Hospitalists' Role in Improving the U.S. Healthcare System
Early Warning System Boosts Sepsis Detection, Care
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
LISTEN NOW: Kristen Kulasa, MD, Explains How Hospitalists Can Work with Nutritionists and Dieticians
Kristen Kulasa, MD, assistant clinical professor of medicine and director of Inpatient Glycemic Control, Division of Endocrinology, Diabetes, and Metabolism at the University of California in San Diego, provides tips on how hospitalists can work with nutritionists and dieticians for the betterment of diabetic patients. As a mentor for SHM's care coordination program on inpatient diabetes, Dr. Kulasa offers hospitalists advice in treating diabetic patients. She points to SHM’s website, which has a lot of resources to help hospitalists feel comfortable with insulin dosing.
Kristen Kulasa, MD, assistant clinical professor of medicine and director of Inpatient Glycemic Control, Division of Endocrinology, Diabetes, and Metabolism at the University of California in San Diego, provides tips on how hospitalists can work with nutritionists and dieticians for the betterment of diabetic patients. As a mentor for SHM's care coordination program on inpatient diabetes, Dr. Kulasa offers hospitalists advice in treating diabetic patients. She points to SHM’s website, which has a lot of resources to help hospitalists feel comfortable with insulin dosing.
Kristen Kulasa, MD, assistant clinical professor of medicine and director of Inpatient Glycemic Control, Division of Endocrinology, Diabetes, and Metabolism at the University of California in San Diego, provides tips on how hospitalists can work with nutritionists and dieticians for the betterment of diabetic patients. As a mentor for SHM's care coordination program on inpatient diabetes, Dr. Kulasa offers hospitalists advice in treating diabetic patients. She points to SHM’s website, which has a lot of resources to help hospitalists feel comfortable with insulin dosing.
Primary-Care Physicians Weigh in on Quality of Care Transitions
A new study on transitions of care gives hospitalists a view from the other side.
Published recently online in the Journal of Hospital Medicine, the authors surveyed 22 primary-care physician leaders in California-based post-discharge clinics and asked them about ways to improve care transitions.
Physicians' responses focused on several areas that need work, most notably aligned financial incentives, regulations to standardize interoperability among electronic health records (EHR) and data sharing, and more opportunities for professional networking, the authors note.
Although the qualitative study takes a broad view of the healthcare system, its lead author says hospitalists should view "systems change" as a long-term goal achievable via incremental improvements that can start now.
"National policy change is needed to move the needle for the whole health system," says hospitalist Oanh Kieu Nguyen, MD, MAS, of the University of Texas Southwestern Medical Center in Dallas. "But locally, you can innovate within these domains and start to make changes to improve practice settings more immediately. National policy to align financial incentives and improve EHR interoperability will be key to helping local changes take hold and spread across systems. Otherwise, there will continue to be a lot of variability and fragmentation around care transitions on a national level."
Dr. Nguyen, who has practiced as both a hospitalist and PCP, says that because policies and studies on post-discharge care transitions primarily have focused on the hospital perspective, it is important to gain an understanding of the primary-care point of view.
"As a hospitalist, it's really easy to get caught up in just wanting to get patients teed up and sent home. Once they're out, we think they're no longer really our problem," Dr. Nguyen adds. "It's easy to forget that primary care is an important part of the other side of the equation. The way our healthcare system is designed doesn't really give physicians an incentive to look at the whole picture of a patient across all the environments they're in."
Many hospitalists are sharing their challenges and successes in care transitions through HMX. Join the conversation now.
Visit our website for more information on transitions of care.
A new study on transitions of care gives hospitalists a view from the other side.
Published recently online in the Journal of Hospital Medicine, the authors surveyed 22 primary-care physician leaders in California-based post-discharge clinics and asked them about ways to improve care transitions.
Physicians' responses focused on several areas that need work, most notably aligned financial incentives, regulations to standardize interoperability among electronic health records (EHR) and data sharing, and more opportunities for professional networking, the authors note.
Although the qualitative study takes a broad view of the healthcare system, its lead author says hospitalists should view "systems change" as a long-term goal achievable via incremental improvements that can start now.
"National policy change is needed to move the needle for the whole health system," says hospitalist Oanh Kieu Nguyen, MD, MAS, of the University of Texas Southwestern Medical Center in Dallas. "But locally, you can innovate within these domains and start to make changes to improve practice settings more immediately. National policy to align financial incentives and improve EHR interoperability will be key to helping local changes take hold and spread across systems. Otherwise, there will continue to be a lot of variability and fragmentation around care transitions on a national level."
Dr. Nguyen, who has practiced as both a hospitalist and PCP, says that because policies and studies on post-discharge care transitions primarily have focused on the hospital perspective, it is important to gain an understanding of the primary-care point of view.
"As a hospitalist, it's really easy to get caught up in just wanting to get patients teed up and sent home. Once they're out, we think they're no longer really our problem," Dr. Nguyen adds. "It's easy to forget that primary care is an important part of the other side of the equation. The way our healthcare system is designed doesn't really give physicians an incentive to look at the whole picture of a patient across all the environments they're in."
Many hospitalists are sharing their challenges and successes in care transitions through HMX. Join the conversation now.
Visit our website for more information on transitions of care.
A new study on transitions of care gives hospitalists a view from the other side.
Published recently online in the Journal of Hospital Medicine, the authors surveyed 22 primary-care physician leaders in California-based post-discharge clinics and asked them about ways to improve care transitions.
Physicians' responses focused on several areas that need work, most notably aligned financial incentives, regulations to standardize interoperability among electronic health records (EHR) and data sharing, and more opportunities for professional networking, the authors note.
Although the qualitative study takes a broad view of the healthcare system, its lead author says hospitalists should view "systems change" as a long-term goal achievable via incremental improvements that can start now.
"National policy change is needed to move the needle for the whole health system," says hospitalist Oanh Kieu Nguyen, MD, MAS, of the University of Texas Southwestern Medical Center in Dallas. "But locally, you can innovate within these domains and start to make changes to improve practice settings more immediately. National policy to align financial incentives and improve EHR interoperability will be key to helping local changes take hold and spread across systems. Otherwise, there will continue to be a lot of variability and fragmentation around care transitions on a national level."
Dr. Nguyen, who has practiced as both a hospitalist and PCP, says that because policies and studies on post-discharge care transitions primarily have focused on the hospital perspective, it is important to gain an understanding of the primary-care point of view.
"As a hospitalist, it's really easy to get caught up in just wanting to get patients teed up and sent home. Once they're out, we think they're no longer really our problem," Dr. Nguyen adds. "It's easy to forget that primary care is an important part of the other side of the equation. The way our healthcare system is designed doesn't really give physicians an incentive to look at the whole picture of a patient across all the environments they're in."
Many hospitalists are sharing their challenges and successes in care transitions through HMX. Join the conversation now.
Visit our website for more information on transitions of care.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Teaching Value Project, Choosing Wisely Competition Accepting Applications for 2015
Costs of Care (www.costsofcare.org), a nonprofit dedicated to empowering patients and their caregivers to deflate medical bills, and the American Board of Internal Medicine (ABIM) Foundation plan to launch their second annual Teaching Value and Choosing Wisely Competition this fall to highlight healthcare innovations promoting value. The submission deadline is Feb. 1, 2015.
The Teaching Value Project, the main educational arm of Costs of Care, has developed web-based video training modules based on actual cases, along with other educational materials to help residents and medical students learn to make clinical decisions optimizing quality and cost, says Christopher Moriates, MD, hospitalist and co-chair of the University of California-San Francisco’s High-Value Care Committee.
Details of the contest will be posted at www.teachingvalue.org. For more information, send an e-mail to [email protected].
Larry Beresford is a freelance writer in Alameda, Calif.
Costs of Care (www.costsofcare.org), a nonprofit dedicated to empowering patients and their caregivers to deflate medical bills, and the American Board of Internal Medicine (ABIM) Foundation plan to launch their second annual Teaching Value and Choosing Wisely Competition this fall to highlight healthcare innovations promoting value. The submission deadline is Feb. 1, 2015.
The Teaching Value Project, the main educational arm of Costs of Care, has developed web-based video training modules based on actual cases, along with other educational materials to help residents and medical students learn to make clinical decisions optimizing quality and cost, says Christopher Moriates, MD, hospitalist and co-chair of the University of California-San Francisco’s High-Value Care Committee.
Details of the contest will be posted at www.teachingvalue.org. For more information, send an e-mail to [email protected].
Larry Beresford is a freelance writer in Alameda, Calif.
Costs of Care (www.costsofcare.org), a nonprofit dedicated to empowering patients and their caregivers to deflate medical bills, and the American Board of Internal Medicine (ABIM) Foundation plan to launch their second annual Teaching Value and Choosing Wisely Competition this fall to highlight healthcare innovations promoting value. The submission deadline is Feb. 1, 2015.
The Teaching Value Project, the main educational arm of Costs of Care, has developed web-based video training modules based on actual cases, along with other educational materials to help residents and medical students learn to make clinical decisions optimizing quality and cost, says Christopher Moriates, MD, hospitalist and co-chair of the University of California-San Francisco’s High-Value Care Committee.
Details of the contest will be posted at www.teachingvalue.org. For more information, send an e-mail to [email protected].
Larry Beresford is a freelance writer in Alameda, Calif.
Better Prescription Practices Can Curb Antibiotic Resistance
Overuse of antibiotics is fueling antimicrobial resistance, posing a threat to people around the world and prompting increased attention to antibiotic stewardship practices. Good stewardship requires hospitals and clinicians to adopt coordinated interventions that focus on reducing inappropriate antibiotic prescribing while remaining focused on the health of patients.
Although it can seem overwhelming to physicians with busy workloads and sick patients to engage in these practices, not addressing the issue of responsible antibiotic prescribing is putting patients at risk.
“We know development of resistance is complicated,” says Arjun Srinivasan, MD, FSHEA, associate director for the CDC’s Healthcare Associated Infection Prevention Program and medical director of Get Smart for Healthcare in the CDC’s division of Healthcare Quality Promotion. Dr. Srinivasan is one of the authors of a recent CDC report on antibiotic prescribing practices across the U.S. “Nonetheless, we know that overuse of antibiotics leads to increases in resistance. We also know that if we can improve the way we prescribe them, we can reduce antibiotic resistance.”
The CDC recommends that hospitals adopt, at a minimum, the following antibiotic stewardship checklist:
- Commit leadership: Dedicate necessary human, financial, and information technology resources.
- Create accountability: Appoint a single leader responsible for program outcomes. Physicians have proven successful in this role.
- Provide drug expertise: Appoint a single pharmacist leader to support improved prescribing.
- Act: Take at least one prescribing improvement action, such as requiring reassessment within 48 hours to check drug choice, dose, and duration.
- Track: Monitor prescribing and antibiotic resistance patterns.
- Report: Regularly report to staff on prescribing and resistance patterns, as well as steps to improve.
- Educate: Offer education about antibiotic resistance and improving prescribing practices.
- Work with other healthcare facilities to prevent infections, transmission, and resistance.
These practices are not just the domain of infectious disease clinicians, either, says Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System and past president of the Society for Healthcare Epidemiology of America. In 1992, Dr. Fishman helped establish an antibiotic stewardship program at Penn, working with infectious disease staff to identify and adopt best practices tailored to their needs.
Their efforts have shown promise in improving the health of their patients, he says, and many institutions that adopt stewardship programs typically see cost savings, too.
“These programs do usually end up decreasing drug costs but also increasing the quality of care,”
Dr. Fishman says. “If you can cut out 30% of unnecessary drugs, you cut drug costs. To me, that meets the true definition of value in healthcare.”
In one study that looked at stewardship-related cost reduction, primarily among larger healthcare settings, the average annual savings from reduced inappropriate antibiotic prescribing ranged from $200,000 to $900,000.
The recent CDC report, to which Dr. Srinivasan contributed, was published March 4 in Vital Signs. The study found that as many as a third of antibiotics prescribed are done so inappropriately. According to experts, hospitals and other healthcare institutions need to develop processes and standards to assist physicians in efforts to be responsible antibiotic prescribers.
“Sometimes, when you’re focusing on other issues, antibiotics are a bit of an afterthought,” says Scott Flanders, MD, FACP, MHM, professor of internal medicine and director of hospital medicine at University of Michigan Medical School in Ann Arbor.
“If there is not a checklist of processes [and] things are not accounted for in a systematic way, it doesn’t happen.”
Dr. Flanders and colleague Sanjay Saint, MD, MPH, the University of Michigan George Dock Collegiate professor of internal medicine and associate chief of medicine at the VA Ann Arbor Healthcare System, recently published an article in the Journal of the American Medical Association Internal Medicine in which they recommend the following:
- Antimicrobial stewardship programs, which aim to develop guidelines and implement programs that help optimize antibiotic use among hospitalized patients, should partner with front-line clinicians to tackle the problem.
- Clinicians should better document aspects of antibiotic use that can be shared with other providers caring for the same patient throughout his or her hospital stay and after discharge.
- Clinicians should take an “antibiotic time-out” after 48-72 hours of a patient’s use of antibiotics to reassess the use of these drugs.
- Treatment and its duration should be in line with evidence-based guidelines, and institutions should work to clearly identify appropriate treatment duration.
- Improved diagnostic tests should be available to physicians.
- Target diagnostic error by working to improve how physicians think when considering whether to provide antibiotics.
- Develop performance measures that highlight common conditions in which antibiotics are overprescribed, to shine a brighter light on the problem.
“I think we can make a lot of progress,” Dr. Flanders says. “The problem is complex; it developed over decades, and any solutions are unlikely to solve the problem immediately. But there are several examples of institutions and hospitals making significant inroads in a short period of time.” —KAT
Overuse of antibiotics is fueling antimicrobial resistance, posing a threat to people around the world and prompting increased attention to antibiotic stewardship practices. Good stewardship requires hospitals and clinicians to adopt coordinated interventions that focus on reducing inappropriate antibiotic prescribing while remaining focused on the health of patients.
Although it can seem overwhelming to physicians with busy workloads and sick patients to engage in these practices, not addressing the issue of responsible antibiotic prescribing is putting patients at risk.
“We know development of resistance is complicated,” says Arjun Srinivasan, MD, FSHEA, associate director for the CDC’s Healthcare Associated Infection Prevention Program and medical director of Get Smart for Healthcare in the CDC’s division of Healthcare Quality Promotion. Dr. Srinivasan is one of the authors of a recent CDC report on antibiotic prescribing practices across the U.S. “Nonetheless, we know that overuse of antibiotics leads to increases in resistance. We also know that if we can improve the way we prescribe them, we can reduce antibiotic resistance.”
The CDC recommends that hospitals adopt, at a minimum, the following antibiotic stewardship checklist:
- Commit leadership: Dedicate necessary human, financial, and information technology resources.
- Create accountability: Appoint a single leader responsible for program outcomes. Physicians have proven successful in this role.
- Provide drug expertise: Appoint a single pharmacist leader to support improved prescribing.
- Act: Take at least one prescribing improvement action, such as requiring reassessment within 48 hours to check drug choice, dose, and duration.
- Track: Monitor prescribing and antibiotic resistance patterns.
- Report: Regularly report to staff on prescribing and resistance patterns, as well as steps to improve.
- Educate: Offer education about antibiotic resistance and improving prescribing practices.
- Work with other healthcare facilities to prevent infections, transmission, and resistance.
These practices are not just the domain of infectious disease clinicians, either, says Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System and past president of the Society for Healthcare Epidemiology of America. In 1992, Dr. Fishman helped establish an antibiotic stewardship program at Penn, working with infectious disease staff to identify and adopt best practices tailored to their needs.
Their efforts have shown promise in improving the health of their patients, he says, and many institutions that adopt stewardship programs typically see cost savings, too.
“These programs do usually end up decreasing drug costs but also increasing the quality of care,”
Dr. Fishman says. “If you can cut out 30% of unnecessary drugs, you cut drug costs. To me, that meets the true definition of value in healthcare.”
In one study that looked at stewardship-related cost reduction, primarily among larger healthcare settings, the average annual savings from reduced inappropriate antibiotic prescribing ranged from $200,000 to $900,000.
The recent CDC report, to which Dr. Srinivasan contributed, was published March 4 in Vital Signs. The study found that as many as a third of antibiotics prescribed are done so inappropriately. According to experts, hospitals and other healthcare institutions need to develop processes and standards to assist physicians in efforts to be responsible antibiotic prescribers.
“Sometimes, when you’re focusing on other issues, antibiotics are a bit of an afterthought,” says Scott Flanders, MD, FACP, MHM, professor of internal medicine and director of hospital medicine at University of Michigan Medical School in Ann Arbor.
“If there is not a checklist of processes [and] things are not accounted for in a systematic way, it doesn’t happen.”
Dr. Flanders and colleague Sanjay Saint, MD, MPH, the University of Michigan George Dock Collegiate professor of internal medicine and associate chief of medicine at the VA Ann Arbor Healthcare System, recently published an article in the Journal of the American Medical Association Internal Medicine in which they recommend the following:
- Antimicrobial stewardship programs, which aim to develop guidelines and implement programs that help optimize antibiotic use among hospitalized patients, should partner with front-line clinicians to tackle the problem.
- Clinicians should better document aspects of antibiotic use that can be shared with other providers caring for the same patient throughout his or her hospital stay and after discharge.
- Clinicians should take an “antibiotic time-out” after 48-72 hours of a patient’s use of antibiotics to reassess the use of these drugs.
- Treatment and its duration should be in line with evidence-based guidelines, and institutions should work to clearly identify appropriate treatment duration.
- Improved diagnostic tests should be available to physicians.
- Target diagnostic error by working to improve how physicians think when considering whether to provide antibiotics.
- Develop performance measures that highlight common conditions in which antibiotics are overprescribed, to shine a brighter light on the problem.
“I think we can make a lot of progress,” Dr. Flanders says. “The problem is complex; it developed over decades, and any solutions are unlikely to solve the problem immediately. But there are several examples of institutions and hospitals making significant inroads in a short period of time.” —KAT
Overuse of antibiotics is fueling antimicrobial resistance, posing a threat to people around the world and prompting increased attention to antibiotic stewardship practices. Good stewardship requires hospitals and clinicians to adopt coordinated interventions that focus on reducing inappropriate antibiotic prescribing while remaining focused on the health of patients.
Although it can seem overwhelming to physicians with busy workloads and sick patients to engage in these practices, not addressing the issue of responsible antibiotic prescribing is putting patients at risk.
“We know development of resistance is complicated,” says Arjun Srinivasan, MD, FSHEA, associate director for the CDC’s Healthcare Associated Infection Prevention Program and medical director of Get Smart for Healthcare in the CDC’s division of Healthcare Quality Promotion. Dr. Srinivasan is one of the authors of a recent CDC report on antibiotic prescribing practices across the U.S. “Nonetheless, we know that overuse of antibiotics leads to increases in resistance. We also know that if we can improve the way we prescribe them, we can reduce antibiotic resistance.”
The CDC recommends that hospitals adopt, at a minimum, the following antibiotic stewardship checklist:
- Commit leadership: Dedicate necessary human, financial, and information technology resources.
- Create accountability: Appoint a single leader responsible for program outcomes. Physicians have proven successful in this role.
- Provide drug expertise: Appoint a single pharmacist leader to support improved prescribing.
- Act: Take at least one prescribing improvement action, such as requiring reassessment within 48 hours to check drug choice, dose, and duration.
- Track: Monitor prescribing and antibiotic resistance patterns.
- Report: Regularly report to staff on prescribing and resistance patterns, as well as steps to improve.
- Educate: Offer education about antibiotic resistance and improving prescribing practices.
- Work with other healthcare facilities to prevent infections, transmission, and resistance.
These practices are not just the domain of infectious disease clinicians, either, says Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System and past president of the Society for Healthcare Epidemiology of America. In 1992, Dr. Fishman helped establish an antibiotic stewardship program at Penn, working with infectious disease staff to identify and adopt best practices tailored to their needs.
Their efforts have shown promise in improving the health of their patients, he says, and many institutions that adopt stewardship programs typically see cost savings, too.
“These programs do usually end up decreasing drug costs but also increasing the quality of care,”
Dr. Fishman says. “If you can cut out 30% of unnecessary drugs, you cut drug costs. To me, that meets the true definition of value in healthcare.”
In one study that looked at stewardship-related cost reduction, primarily among larger healthcare settings, the average annual savings from reduced inappropriate antibiotic prescribing ranged from $200,000 to $900,000.
The recent CDC report, to which Dr. Srinivasan contributed, was published March 4 in Vital Signs. The study found that as many as a third of antibiotics prescribed are done so inappropriately. According to experts, hospitals and other healthcare institutions need to develop processes and standards to assist physicians in efforts to be responsible antibiotic prescribers.
“Sometimes, when you’re focusing on other issues, antibiotics are a bit of an afterthought,” says Scott Flanders, MD, FACP, MHM, professor of internal medicine and director of hospital medicine at University of Michigan Medical School in Ann Arbor.
“If there is not a checklist of processes [and] things are not accounted for in a systematic way, it doesn’t happen.”
Dr. Flanders and colleague Sanjay Saint, MD, MPH, the University of Michigan George Dock Collegiate professor of internal medicine and associate chief of medicine at the VA Ann Arbor Healthcare System, recently published an article in the Journal of the American Medical Association Internal Medicine in which they recommend the following:
- Antimicrobial stewardship programs, which aim to develop guidelines and implement programs that help optimize antibiotic use among hospitalized patients, should partner with front-line clinicians to tackle the problem.
- Clinicians should better document aspects of antibiotic use that can be shared with other providers caring for the same patient throughout his or her hospital stay and after discharge.
- Clinicians should take an “antibiotic time-out” after 48-72 hours of a patient’s use of antibiotics to reassess the use of these drugs.
- Treatment and its duration should be in line with evidence-based guidelines, and institutions should work to clearly identify appropriate treatment duration.
- Improved diagnostic tests should be available to physicians.
- Target diagnostic error by working to improve how physicians think when considering whether to provide antibiotics.
- Develop performance measures that highlight common conditions in which antibiotics are overprescribed, to shine a brighter light on the problem.
“I think we can make a lot of progress,” Dr. Flanders says. “The problem is complex; it developed over decades, and any solutions are unlikely to solve the problem immediately. But there are several examples of institutions and hospitals making significant inroads in a short period of time.” —KAT
Hospitalists Adopt Strategies to Become More Responsible Prescribers of Antibiotics
A recent CDC study found that nearly a third of antibiotics might be inappropriately prescribed.1 The report also found wide variation in antibiotic prescribing practices for patients in similar treatment areas in hospitals across the country.
Across the globe, antibiotic resistance has become a daunting threat. Some public health officials have labeled it a crisis, and improper prescribing and use of antibiotics is at least partly to blame, experts say.
“We’re dangerously close to a pre-antibiotic era where we don’t have antibiotics to treat common infections,” says Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System and past president of the Society for Healthcare Epidemiology of America. “We are seeing more and more infections, usually hospital-based, caused by bacterial resistance to most, if not all, of the antibiotics that we have.”
It’s an issue hospitalists around the country are championing.
“I think for a long time there’s been a misperception that antibiotic stewardship is at odds with hospitalists, who are managing very busy patient loads and managing inpatient prescribing,” says Arjun Srinivasan, MD, FSHEA, associate director for the CDC’s Healthcare Associated Infection Prevention Program and medical director of Get Smart for Healthcare in the division of Healthcare Quality Promotion at the CDC. Dr. Srinivasan is one of the authors of the new CDC study.
But “they have taken that ball and run with it,” says Dr. Srinivasan, who has worked with the Society of Hospital Medicine to address antibiotic resistance issues.
The goals of the study, published in the CDC’s Vital Signs on March 4, 2014, were to evaluate the extent and rationale for the prescribing of antibiotics in U.S. hospitals, while demonstrating opportunities for improvement in prescribing practices.
—Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System
Study authors analyzed data from the Truven Health MarketScan Hospital Drug Database and the CDC’s Emerging Infection Program and, using a model based on the data, demonstrated that a 30% reduction in broad-spectrum antibiotics use would decrease Clostridium difficile infection (CDI) by 26%. Overall antibiotic use would drop by 5%.
According to the CDC, antibiotics are among the most frequent causes of adverse drug events among hospitalized patients in the U.S., and complications like CDI can be deadly. In fact, 250,000 hospitalized patients are infected with CDI each year, resulting in 14,000 deaths.
“We’re really at a critical juncture in healthcare now,” Dr. Fishman says. “The field of stewardship has evolved mainly in academic tertiary care settings. The CDC report is timely because it highlights the necessity of making sure antibiotics are used appropriately in all healthcare settings.”
Take a Break
One of the ways in which hospitalists have addressed the need for more appropriate antibiotic prescribing in their institutions is the practice of an “antibiotic time-out.”
“After some point, when the dust settles at about 48-72 hours, you can evaluate the patient’s progress, evaluate their studies, [and] you may have culture results,” says Scott Flanders, MD, FACP, MHM, professor of internal medicine and director of hospital medicine at the University of Michigan Medical School in Ann Arbor. At that point, physicians can decide whether to maintain a patient on the original antibiotic, alter the duration of treatment, or take them off the treatment altogether.
Dr. Flanders and a colleague published an editorial in the Journal of the American Medical Association Internal Medicine that coincided with the CDC report.2 A 2007 study published in Clinical Infectious Diseases found that the choice of antibiotic agent or duration of treatment can be incorrect in as many as half of all cases in which antibiotics are prescribed.3
Dr. Flanders, a past president of SHM who has worked extensively with the CDC and the Institute for Healthcare Improvement, was behind the development of the time-out strategy. Dr. Srinivasan says the clinical utility of the method was “eye-opening.”
The strategy, which has taken hold among hospital groups the CDC has worked with, has demonstrated that stewardship and patient management are not at odds, Dr. Srinivasan says. Despite patient sign-outs and hand-offs, the time-out strategy allows any clinician to track a patient’s antibiotic status and reevaluate the treatment plan.
Having a process is critical to more responsible prescribing practices, Dr. Flanders says. He attributes much of the variability in antibiotics prescribing among similar departments at hospitals across the country to a lack of standards, though he noted that variability in patient populations undoubtedly plays a role.
Lack of Stats
The CDC report showed up to a threefold difference in the number of antibiotics prescribed to patients in similar hospital settings at hospitals across the country. The reasons for this are not known, Dr. Fishman says.
“The main reason we don’t know is we don’t have a good mechanism in the U.S. right now to monitor antibiotics use,” he explains. “We don’t have a way for healthcare facilities to benchmark their use.”
Without good strategies to monitor and develop more responsible antibiotics prescription practices, Dr. Flanders believes many physicians find themselves trapped by the “chagrin” of not prescribing.
“Patients often enter the hospital without a clear diagnosis,” he says. “They are quite ill. They may have a serious bacterial infection, and, in diagnosing them, we can’t guess wrong and make the decision to withhold antibiotics, only to find out later the patient is infected.
“We know delays increase mortality, and that’s not an acceptable option.”

—Scott Flanders, MD, FACP, MHM, professor of internal medicine, director of hospital medicine, University of Michigan Medical School, Ann Arbor, past president, SHM
Beyond the Bedside
Many physicians fail to consider the bigger societal implications when prescribing antibiotics for sick patients in their charge, because their responsibility is, first and foremost, to that individual. But, Dr. Srinivasan says, “good antibiotic stewardship is beneficial to the patient lying in the bed in front of you, because every day we are confronted with C. diff. infections, adverse drug events, all of these issues.”
Strategies and processes help hospitalists make the best decision for their patients at the time they require care, while providing room for adaptation and the improvements that serve all patients.
Some institutions use interventions like prospective audit and feedback monitoring to help physicians become more responsible antibiotic prescribers, says Dr. Fishman, who worked with infectious disease specialists at the University of Pennsylvania in the early 1990s to develop a stewardship program there.
“In our institution, we see better outcomes—lower complications—usually associated with a decreased length of stay, at least in the ICU for critically ill patients—and increased cure rates,” he says.
Stewardship efforts take investment on the part of the hospital. Dr. Fishman cited a recent study at the Children’s Hospital of Pennsylvania that looked at whether a particular education strategy the hospital implemented actually led to improvements.4
“It was successful in intervening in this problem [of inappropriate prescribing] in pediatricians, but it did take ongoing education of both healthcare providers and patients,” he says, noting that large financial and time investments are necessary for the ongoing training and follow-up that is necessary.
And patients need to be educated, too.
“It takes a minute to write that prescription and probably 15 or 20 minutes not to write it,” Dr. Fishman says. “We need to educate patients about potential complications of antibiotics use, as well as the signs and symptoms of infection.”
The CDC report is a call to action for all healthcare providers to consider how they can become better antibiotic stewards. There are very few new antibiotics on the market and little in the pipeline. All providers must do what they can to preserve the antibiotics we currently have, Dr. Fishman says.
“There is opportunity, and I think hospitalists are up to the challenge,” Dr. Flanders says. “They are doing lots of work to improve quality across lots of domains in their hospitals. I think this is an area where attention is deserved.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report. Vital signs: improving antibiotic use among hospitalized patients. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm6309a4.htm?s_cid=mm6309a4_w. Accessed August 31, 2014.
- Flanders SA, Saint S. Why does antrimicrobial overuse in hospitalized patients persist? JAMA Internal Medicine online. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1838720. Accessed August 31, 2014.
- Dellit TH, Owens RC, McGowan JE, et al. Clinical Infectious Diseases online. Available at: http://cid.oxfordjournals.org/content/44/2/159.full. Accessed August 31, 2014.
- Gerber JS, Prasad PA, Fiks A, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians. JAMA. 2013;309(22):2345-2352.
A recent CDC study found that nearly a third of antibiotics might be inappropriately prescribed.1 The report also found wide variation in antibiotic prescribing practices for patients in similar treatment areas in hospitals across the country.
Across the globe, antibiotic resistance has become a daunting threat. Some public health officials have labeled it a crisis, and improper prescribing and use of antibiotics is at least partly to blame, experts say.
“We’re dangerously close to a pre-antibiotic era where we don’t have antibiotics to treat common infections,” says Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System and past president of the Society for Healthcare Epidemiology of America. “We are seeing more and more infections, usually hospital-based, caused by bacterial resistance to most, if not all, of the antibiotics that we have.”
It’s an issue hospitalists around the country are championing.
“I think for a long time there’s been a misperception that antibiotic stewardship is at odds with hospitalists, who are managing very busy patient loads and managing inpatient prescribing,” says Arjun Srinivasan, MD, FSHEA, associate director for the CDC’s Healthcare Associated Infection Prevention Program and medical director of Get Smart for Healthcare in the division of Healthcare Quality Promotion at the CDC. Dr. Srinivasan is one of the authors of the new CDC study.
But “they have taken that ball and run with it,” says Dr. Srinivasan, who has worked with the Society of Hospital Medicine to address antibiotic resistance issues.
The goals of the study, published in the CDC’s Vital Signs on March 4, 2014, were to evaluate the extent and rationale for the prescribing of antibiotics in U.S. hospitals, while demonstrating opportunities for improvement in prescribing practices.
—Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System
Study authors analyzed data from the Truven Health MarketScan Hospital Drug Database and the CDC’s Emerging Infection Program and, using a model based on the data, demonstrated that a 30% reduction in broad-spectrum antibiotics use would decrease Clostridium difficile infection (CDI) by 26%. Overall antibiotic use would drop by 5%.
According to the CDC, antibiotics are among the most frequent causes of adverse drug events among hospitalized patients in the U.S., and complications like CDI can be deadly. In fact, 250,000 hospitalized patients are infected with CDI each year, resulting in 14,000 deaths.
“We’re really at a critical juncture in healthcare now,” Dr. Fishman says. “The field of stewardship has evolved mainly in academic tertiary care settings. The CDC report is timely because it highlights the necessity of making sure antibiotics are used appropriately in all healthcare settings.”
Take a Break
One of the ways in which hospitalists have addressed the need for more appropriate antibiotic prescribing in their institutions is the practice of an “antibiotic time-out.”
“After some point, when the dust settles at about 48-72 hours, you can evaluate the patient’s progress, evaluate their studies, [and] you may have culture results,” says Scott Flanders, MD, FACP, MHM, professor of internal medicine and director of hospital medicine at the University of Michigan Medical School in Ann Arbor. At that point, physicians can decide whether to maintain a patient on the original antibiotic, alter the duration of treatment, or take them off the treatment altogether.
Dr. Flanders and a colleague published an editorial in the Journal of the American Medical Association Internal Medicine that coincided with the CDC report.2 A 2007 study published in Clinical Infectious Diseases found that the choice of antibiotic agent or duration of treatment can be incorrect in as many as half of all cases in which antibiotics are prescribed.3
Dr. Flanders, a past president of SHM who has worked extensively with the CDC and the Institute for Healthcare Improvement, was behind the development of the time-out strategy. Dr. Srinivasan says the clinical utility of the method was “eye-opening.”
The strategy, which has taken hold among hospital groups the CDC has worked with, has demonstrated that stewardship and patient management are not at odds, Dr. Srinivasan says. Despite patient sign-outs and hand-offs, the time-out strategy allows any clinician to track a patient’s antibiotic status and reevaluate the treatment plan.
Having a process is critical to more responsible prescribing practices, Dr. Flanders says. He attributes much of the variability in antibiotics prescribing among similar departments at hospitals across the country to a lack of standards, though he noted that variability in patient populations undoubtedly plays a role.
Lack of Stats
The CDC report showed up to a threefold difference in the number of antibiotics prescribed to patients in similar hospital settings at hospitals across the country. The reasons for this are not known, Dr. Fishman says.
“The main reason we don’t know is we don’t have a good mechanism in the U.S. right now to monitor antibiotics use,” he explains. “We don’t have a way for healthcare facilities to benchmark their use.”
Without good strategies to monitor and develop more responsible antibiotics prescription practices, Dr. Flanders believes many physicians find themselves trapped by the “chagrin” of not prescribing.
“Patients often enter the hospital without a clear diagnosis,” he says. “They are quite ill. They may have a serious bacterial infection, and, in diagnosing them, we can’t guess wrong and make the decision to withhold antibiotics, only to find out later the patient is infected.
“We know delays increase mortality, and that’s not an acceptable option.”

—Scott Flanders, MD, FACP, MHM, professor of internal medicine, director of hospital medicine, University of Michigan Medical School, Ann Arbor, past president, SHM
Beyond the Bedside
Many physicians fail to consider the bigger societal implications when prescribing antibiotics for sick patients in their charge, because their responsibility is, first and foremost, to that individual. But, Dr. Srinivasan says, “good antibiotic stewardship is beneficial to the patient lying in the bed in front of you, because every day we are confronted with C. diff. infections, adverse drug events, all of these issues.”
Strategies and processes help hospitalists make the best decision for their patients at the time they require care, while providing room for adaptation and the improvements that serve all patients.
Some institutions use interventions like prospective audit and feedback monitoring to help physicians become more responsible antibiotic prescribers, says Dr. Fishman, who worked with infectious disease specialists at the University of Pennsylvania in the early 1990s to develop a stewardship program there.
“In our institution, we see better outcomes—lower complications—usually associated with a decreased length of stay, at least in the ICU for critically ill patients—and increased cure rates,” he says.
Stewardship efforts take investment on the part of the hospital. Dr. Fishman cited a recent study at the Children’s Hospital of Pennsylvania that looked at whether a particular education strategy the hospital implemented actually led to improvements.4
“It was successful in intervening in this problem [of inappropriate prescribing] in pediatricians, but it did take ongoing education of both healthcare providers and patients,” he says, noting that large financial and time investments are necessary for the ongoing training and follow-up that is necessary.
And patients need to be educated, too.
“It takes a minute to write that prescription and probably 15 or 20 minutes not to write it,” Dr. Fishman says. “We need to educate patients about potential complications of antibiotics use, as well as the signs and symptoms of infection.”
The CDC report is a call to action for all healthcare providers to consider how they can become better antibiotic stewards. There are very few new antibiotics on the market and little in the pipeline. All providers must do what they can to preserve the antibiotics we currently have, Dr. Fishman says.
“There is opportunity, and I think hospitalists are up to the challenge,” Dr. Flanders says. “They are doing lots of work to improve quality across lots of domains in their hospitals. I think this is an area where attention is deserved.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report. Vital signs: improving antibiotic use among hospitalized patients. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm6309a4.htm?s_cid=mm6309a4_w. Accessed August 31, 2014.
- Flanders SA, Saint S. Why does antrimicrobial overuse in hospitalized patients persist? JAMA Internal Medicine online. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1838720. Accessed August 31, 2014.
- Dellit TH, Owens RC, McGowan JE, et al. Clinical Infectious Diseases online. Available at: http://cid.oxfordjournals.org/content/44/2/159.full. Accessed August 31, 2014.
- Gerber JS, Prasad PA, Fiks A, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians. JAMA. 2013;309(22):2345-2352.
A recent CDC study found that nearly a third of antibiotics might be inappropriately prescribed.1 The report also found wide variation in antibiotic prescribing practices for patients in similar treatment areas in hospitals across the country.
Across the globe, antibiotic resistance has become a daunting threat. Some public health officials have labeled it a crisis, and improper prescribing and use of antibiotics is at least partly to blame, experts say.
“We’re dangerously close to a pre-antibiotic era where we don’t have antibiotics to treat common infections,” says Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System and past president of the Society for Healthcare Epidemiology of America. “We are seeing more and more infections, usually hospital-based, caused by bacterial resistance to most, if not all, of the antibiotics that we have.”
It’s an issue hospitalists around the country are championing.
“I think for a long time there’s been a misperception that antibiotic stewardship is at odds with hospitalists, who are managing very busy patient loads and managing inpatient prescribing,” says Arjun Srinivasan, MD, FSHEA, associate director for the CDC’s Healthcare Associated Infection Prevention Program and medical director of Get Smart for Healthcare in the division of Healthcare Quality Promotion at the CDC. Dr. Srinivasan is one of the authors of the new CDC study.
But “they have taken that ball and run with it,” says Dr. Srinivasan, who has worked with the Society of Hospital Medicine to address antibiotic resistance issues.
The goals of the study, published in the CDC’s Vital Signs on March 4, 2014, were to evaluate the extent and rationale for the prescribing of antibiotics in U.S. hospitals, while demonstrating opportunities for improvement in prescribing practices.
—Neil Fishman, MD, chief patient safety officer and associate chief medical officer at the University of Pennsylvania Health System
Study authors analyzed data from the Truven Health MarketScan Hospital Drug Database and the CDC’s Emerging Infection Program and, using a model based on the data, demonstrated that a 30% reduction in broad-spectrum antibiotics use would decrease Clostridium difficile infection (CDI) by 26%. Overall antibiotic use would drop by 5%.
According to the CDC, antibiotics are among the most frequent causes of adverse drug events among hospitalized patients in the U.S., and complications like CDI can be deadly. In fact, 250,000 hospitalized patients are infected with CDI each year, resulting in 14,000 deaths.
“We’re really at a critical juncture in healthcare now,” Dr. Fishman says. “The field of stewardship has evolved mainly in academic tertiary care settings. The CDC report is timely because it highlights the necessity of making sure antibiotics are used appropriately in all healthcare settings.”
Take a Break
One of the ways in which hospitalists have addressed the need for more appropriate antibiotic prescribing in their institutions is the practice of an “antibiotic time-out.”
“After some point, when the dust settles at about 48-72 hours, you can evaluate the patient’s progress, evaluate their studies, [and] you may have culture results,” says Scott Flanders, MD, FACP, MHM, professor of internal medicine and director of hospital medicine at the University of Michigan Medical School in Ann Arbor. At that point, physicians can decide whether to maintain a patient on the original antibiotic, alter the duration of treatment, or take them off the treatment altogether.
Dr. Flanders and a colleague published an editorial in the Journal of the American Medical Association Internal Medicine that coincided with the CDC report.2 A 2007 study published in Clinical Infectious Diseases found that the choice of antibiotic agent or duration of treatment can be incorrect in as many as half of all cases in which antibiotics are prescribed.3
Dr. Flanders, a past president of SHM who has worked extensively with the CDC and the Institute for Healthcare Improvement, was behind the development of the time-out strategy. Dr. Srinivasan says the clinical utility of the method was “eye-opening.”
The strategy, which has taken hold among hospital groups the CDC has worked with, has demonstrated that stewardship and patient management are not at odds, Dr. Srinivasan says. Despite patient sign-outs and hand-offs, the time-out strategy allows any clinician to track a patient’s antibiotic status and reevaluate the treatment plan.
Having a process is critical to more responsible prescribing practices, Dr. Flanders says. He attributes much of the variability in antibiotics prescribing among similar departments at hospitals across the country to a lack of standards, though he noted that variability in patient populations undoubtedly plays a role.
Lack of Stats
The CDC report showed up to a threefold difference in the number of antibiotics prescribed to patients in similar hospital settings at hospitals across the country. The reasons for this are not known, Dr. Fishman says.
“The main reason we don’t know is we don’t have a good mechanism in the U.S. right now to monitor antibiotics use,” he explains. “We don’t have a way for healthcare facilities to benchmark their use.”
Without good strategies to monitor and develop more responsible antibiotics prescription practices, Dr. Flanders believes many physicians find themselves trapped by the “chagrin” of not prescribing.
“Patients often enter the hospital without a clear diagnosis,” he says. “They are quite ill. They may have a serious bacterial infection, and, in diagnosing them, we can’t guess wrong and make the decision to withhold antibiotics, only to find out later the patient is infected.
“We know delays increase mortality, and that’s not an acceptable option.”

—Scott Flanders, MD, FACP, MHM, professor of internal medicine, director of hospital medicine, University of Michigan Medical School, Ann Arbor, past president, SHM
Beyond the Bedside
Many physicians fail to consider the bigger societal implications when prescribing antibiotics for sick patients in their charge, because their responsibility is, first and foremost, to that individual. But, Dr. Srinivasan says, “good antibiotic stewardship is beneficial to the patient lying in the bed in front of you, because every day we are confronted with C. diff. infections, adverse drug events, all of these issues.”
Strategies and processes help hospitalists make the best decision for their patients at the time they require care, while providing room for adaptation and the improvements that serve all patients.
Some institutions use interventions like prospective audit and feedback monitoring to help physicians become more responsible antibiotic prescribers, says Dr. Fishman, who worked with infectious disease specialists at the University of Pennsylvania in the early 1990s to develop a stewardship program there.
“In our institution, we see better outcomes—lower complications—usually associated with a decreased length of stay, at least in the ICU for critically ill patients—and increased cure rates,” he says.
Stewardship efforts take investment on the part of the hospital. Dr. Fishman cited a recent study at the Children’s Hospital of Pennsylvania that looked at whether a particular education strategy the hospital implemented actually led to improvements.4
“It was successful in intervening in this problem [of inappropriate prescribing] in pediatricians, but it did take ongoing education of both healthcare providers and patients,” he says, noting that large financial and time investments are necessary for the ongoing training and follow-up that is necessary.
And patients need to be educated, too.
“It takes a minute to write that prescription and probably 15 or 20 minutes not to write it,” Dr. Fishman says. “We need to educate patients about potential complications of antibiotics use, as well as the signs and symptoms of infection.”
The CDC report is a call to action for all healthcare providers to consider how they can become better antibiotic stewards. There are very few new antibiotics on the market and little in the pipeline. All providers must do what they can to preserve the antibiotics we currently have, Dr. Fishman says.
“There is opportunity, and I think hospitalists are up to the challenge,” Dr. Flanders says. “They are doing lots of work to improve quality across lots of domains in their hospitals. I think this is an area where attention is deserved.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report. Vital signs: improving antibiotic use among hospitalized patients. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm6309a4.htm?s_cid=mm6309a4_w. Accessed August 31, 2014.
- Flanders SA, Saint S. Why does antrimicrobial overuse in hospitalized patients persist? JAMA Internal Medicine online. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1838720. Accessed August 31, 2014.
- Dellit TH, Owens RC, McGowan JE, et al. Clinical Infectious Diseases online. Available at: http://cid.oxfordjournals.org/content/44/2/159.full. Accessed August 31, 2014.
- Gerber JS, Prasad PA, Fiks A, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians. JAMA. 2013;309(22):2345-2352.
Derail Behavioral Emergencies in Hospitals
Summary
Behavioral emergencies occur when a patient is physically aggressive or potentially harmful to himself/herself or others. Although they may be rare, behavioral emergencies are high-risk situations, and untrained staff might be uncomfortable dealing with these events.
Patients with underlying psychiatric or developmental disorders, those who have ingested substances, or those who have a medication side effect are at the highest risk for becoming violent. Triggers for these events could be pain, hunger, isolation, change in routine, or even the hospital’s physical environment. Early warning signs for a behavioral emergency can include verbal threats, yelling, or silence. Physical signs may include pacing, crossed arms, furrowed brow, or throwing objects.
The first response to a potential behavioral emergency is to try to de-escalate the situation. Speak in a quiet, calm voice; back off and give personal space. Try to reduce a source of discomfort, and use distractions or rewards. If de-escalation is not successful and a patient becomes violent, the provider’s first role is to be safe: Get away and get help. Hospitals should have—or should develop—a violent patient response team, which may then physically restrain the patient. Medications can be used to treat medical issues but should not be used solely for chemical restraint.
Once a patient is safely restrained, a number of Joint Commission on Accreditation of Healthcare Organizations-mandated actions must occur. The legal guardian and attending of record must be notified. A debrief must occur regarding the events; this must be documented in the medical record. Finally, a strategy must be formulated to enable the patient to be safely removed from restraints as soon as it is safe.
The presenters demonstrated various personal safety techniques to escape from a violent patient, as well as the use of physical restraints. Participants engaged in a mock behavioral emergency to experience the chaos of these events.
Summary
Behavioral emergencies occur when a patient is physically aggressive or potentially harmful to himself/herself or others. Although they may be rare, behavioral emergencies are high-risk situations, and untrained staff might be uncomfortable dealing with these events.
Patients with underlying psychiatric or developmental disorders, those who have ingested substances, or those who have a medication side effect are at the highest risk for becoming violent. Triggers for these events could be pain, hunger, isolation, change in routine, or even the hospital’s physical environment. Early warning signs for a behavioral emergency can include verbal threats, yelling, or silence. Physical signs may include pacing, crossed arms, furrowed brow, or throwing objects.
The first response to a potential behavioral emergency is to try to de-escalate the situation. Speak in a quiet, calm voice; back off and give personal space. Try to reduce a source of discomfort, and use distractions or rewards. If de-escalation is not successful and a patient becomes violent, the provider’s first role is to be safe: Get away and get help. Hospitals should have—or should develop—a violent patient response team, which may then physically restrain the patient. Medications can be used to treat medical issues but should not be used solely for chemical restraint.
Once a patient is safely restrained, a number of Joint Commission on Accreditation of Healthcare Organizations-mandated actions must occur. The legal guardian and attending of record must be notified. A debrief must occur regarding the events; this must be documented in the medical record. Finally, a strategy must be formulated to enable the patient to be safely removed from restraints as soon as it is safe.
The presenters demonstrated various personal safety techniques to escape from a violent patient, as well as the use of physical restraints. Participants engaged in a mock behavioral emergency to experience the chaos of these events.
Summary
Behavioral emergencies occur when a patient is physically aggressive or potentially harmful to himself/herself or others. Although they may be rare, behavioral emergencies are high-risk situations, and untrained staff might be uncomfortable dealing with these events.
Patients with underlying psychiatric or developmental disorders, those who have ingested substances, or those who have a medication side effect are at the highest risk for becoming violent. Triggers for these events could be pain, hunger, isolation, change in routine, or even the hospital’s physical environment. Early warning signs for a behavioral emergency can include verbal threats, yelling, or silence. Physical signs may include pacing, crossed arms, furrowed brow, or throwing objects.
The first response to a potential behavioral emergency is to try to de-escalate the situation. Speak in a quiet, calm voice; back off and give personal space. Try to reduce a source of discomfort, and use distractions or rewards. If de-escalation is not successful and a patient becomes violent, the provider’s first role is to be safe: Get away and get help. Hospitals should have—or should develop—a violent patient response team, which may then physically restrain the patient. Medications can be used to treat medical issues but should not be used solely for chemical restraint.
Once a patient is safely restrained, a number of Joint Commission on Accreditation of Healthcare Organizations-mandated actions must occur. The legal guardian and attending of record must be notified. A debrief must occur regarding the events; this must be documented in the medical record. Finally, a strategy must be formulated to enable the patient to be safely removed from restraints as soon as it is safe.
The presenters demonstrated various personal safety techniques to escape from a violent patient, as well as the use of physical restraints. Participants engaged in a mock behavioral emergency to experience the chaos of these events.






