User login
Converging Crises: Caring for Hospitalized Adults With Substance Use Disorder in the Time of COVID-19
The spread of SARS-CoV-2, the pathogen behind the COVID-19 pandemic, has converged with an unrelenting addiction epidemic. These combined crises will have profound effects on people with substance use disorders (SUD) and people in recovery. Hospitals—which were already hit hard by the addiction epidemic—are the last line of defense in the COVID-19 pandemic. Hospitalists have an important role in balancing the effects of these intersecting, synergistic crises.
People with SUD are disproportionately affected by major medical illnesses, including infections such as hepatitis C, HIV, and cardiovascular, pulmonary, and liver diseases.1 They also experience high rates of hospitalization due to drug-related infections, injury, and overdose.2 People with SUD commonly have intersecting vulnerabilities that may affect their healthcare experience and health outcomes, including housing and food insecurity, mental illness, and experiences of racism, incarceration, and other trauma. They may also harbor mistrust of healthcare providers because of previous negative encounters and discrimination with health systems.3 These vulnerabilities increase risks for COVID-19 morbidity and mortality.4,5 The COVID-19 pandemic may drive increases in use and harms from SUD among patients who already have an SUD, with widespread job loss, insurance loss,6 anxiety, and social isolation on the rise. We may also see increases in return to use among people in recovery or new substance use among those without a history of SUD.
The intersecting crises of SUD and COVID-19 are important for people with SUD and for public health. In this perspective, we describe how the COVID-19 pandemic has affected people with SUD and share practical resources for hospital providers to improve care for people with SUD during the pandemic and beyond.
CONTEXTUALIZING COVID-19 AND SUD RISK
Mistrust of Hospitals and Healthcare Providers
Fear of stigmatization is an ongoing problem for people with SUD, who often experience discrimination in hospitals and, as a result, may avoid hospital care.7 Much of this stigma is based on the false but persistent belief—widespread even among healthcare providers—that addiction is the result of bad choices and limited willpower; however, the science is clear that addiction is a disorder with neurobiological, genetic, and environmental underpinnings.3 These attitudes are likely to be amplified during COVID-19, as patients and providers experience higher levels of stress.
Increased Risks of Substance Use
Typically, people who use drugs are counseled to use with others nearby so that they might administer naloxone or call 911 in the event of an overdose.8 With physical distancing, people may be more likely to use alone. COVID-19 also introduces uncertainty into the drug supply chain through changes in drug production and trafficking.9 Further, access to alcohol may be limited as liquor stores close and public transportation becomes less available. As has been shown in other complex emergencies (such as social, political, economic, and environmental disasters), these barriers to obtaining substances may increase risks for withdrawal, for needing to exchange sex for money or drugs, for sharing syringes or drug preparation equipment,10 or for consuming other available sources of substances, like rubbing alcohol or hand sanitizer. COVID-19 may also increase risk for depression, anxiety, social isolation, and suicidality, all of which increase risk for return to use and overdose.
Changes to the Treatment Milieu
Many of the resources and services that people who use substances rely on to keep safe may be disrupted by COVID-19. Social distancing—the cornerstone of mitigating COVID-19 spread—may be challenging among people with SUD. Though federal regulations around methadone dispensing and buprenorphine prescribing have loosened in response to the pandemic,11 individuals in treatment may still be required to provide urine drug screens or be physically present to receive methadone doses, sometimes daily and in crowded waiting rooms.
Recovery support groups such as Alcoholics Anonymous (AA) and Self-Management and Recovery Training (SMART) provide social connection and are the foundation of many people’s recovery. While many in-person meetings have rapidly transformed to online and telephone support, they remain inaccessible to the most marginalized members of communities: people without smart phones, computers, or internet. This digital shift may also disproportionately affect older adults, people with limited English proficiency, and people with low technological literacy. Limits for other resources, such as syringe service programs, community centers, food pantries, housing shelters, and other places that people depend on for clean water, food, showers, soap, and safer spaces to use, may limit services or close altogether; those that remain open may see an unprecedented rise in need for services as millions of Americans file for unemployment. For many, anxiety about the pandemic, unemployment, financial strain, increased isolation, family stressors, illness, and community losses can lead to enormous personal distress and trigger return to use; loss of a recovery network may further exacerbate this.
Intersectionality of SUD and Other Structural Inequities
Many of the inequities that increase people’s risk for undertreated SUD also increase risk for COVID-19 infection, including racism,12 poverty, and homelessness.4 “Stay home and stay safe” is not an option for people who are unsheltered or whose homes are unsafe because of risks of physical, sexual, or emotional violence. Poverty commonly forces people to live in crowded communal apartments or shelters, rely on public transportation, wait in long lines at food pantries, and continue to work, even if unwell. Many shelters have had to reduce the number of people they serve to reduce crowding and support social distancing, which further compounds risks of unstable housing. Unfortunately, the same structural inequities that exacerbate SUD worsen the COVID-19 crisis.13
ROLE FOR HOSPITALISTS
The intersecting vulnerabilities of SUD and COVID-19 heighten an already urgent need to address SUD among hospitalized patients.14 While COVID-19 may increase harms of substance use, it may also increase people’s readiness to engage in treatment given changes to the drug supply and patient’s concerns about health risks. As such, it is even more critical to make treatment readily accessible and support harm reduction. Hospitalists can take important, actionable steps for patients with SUD—many of which are good general practices14 (Appendix Table).
Hospitalists should do the following:
1. Identify and treat acute withdrawal.15
2. Manage acute pain, including providing high-dose opioids if needed.16 Both practices (1 and 2) are evidence-based, can promote patient’s trust in providers,17 and can help avoid patients leaving against medical advice (AMA). Leaving AMA can lead to poor individual health and further threaten public health if patients leave with undiagnosed or unmanaged COVID-19 infection.
3. Encourage their hospitals to provide patients with tablets or other means to communicate with family, friends, and recovery supports via videolink, and refer patients to virtual peer support and recovery meetings during hospitalization.18 These practices may further support patients in tolerating hospitalization and prevent AMA discharge.
4. Initiate medication for addiction during admission and refer to addictions treatment after discharge. COVID-19–related regulatory changes such as expanded telehealth buprenorphine options and fewer daily dosing requirements for methadone may make this easier. Further, hospitalists should offer medication for alcohol and tobacco use disorders,15 especially given heightened possibility of unhealthy alcohol use and the respiratory complications associated with both tobacco and COVID-19.
5. Assess mental health and suicide risks19 given their association with social isolation, job loss, and financial insecurity.
6. Discuss relapse prevention among people in recovery.
7. Assess overdose risk and promote harm reduction.19 Specifically, this may include counseling patients to avoid sharing smoking supplies to avoid COVID-19 transmission, identifying places to access clean syringes, prescribing naloxone,20 and providing supports so that, if patients need to use alone, they can do so more safely.21
8. Consider high-risk transitions that may be exacerbated by COVID-19. COVID-19 may make safe discharge plans among people experiencing homelessness very challenging. Some communities are rapidly repurposing existing spaces or building new ones to care for people without a safe place to recover after acute hospitalization, yet many communities have no such resources. Hospital teams should consider the possibility that community services and SUD treatment resources may change rapidly during the pandemic. Hospitals can maintain updated resource lists and consider partnering with state and local health departments to improve safe care for people experiencing homelessness or lacking basic services.
COVID-19 is putting enormous strain on many US hospitals. Hospital-based addictions care is under resourced in the best of times,14 and while some hospitals have addiction consult services, many do not. To what degree hospitalists and hospital teams can address anything beyond COVID-19 emergencies will vary based on settings and resources. Furthermore, we recognize that who performs various activities will depend on individual hospital’s resources and practices. Addiction consult services, if available, can play a critical role, as can hospital social workers and care managers, nurses, residents, students, and other members of the healthcare team.
Finally, though COVID-19 adds tremendous stress to hospitals, permanent improvements in SUD treatment systems such as telephone visits for buprenorphine or eased methadone restrictions may emerge that could reduce barriers to hospital-based addictions care.11 Leveraging these changes now may help hospital providers to better support patients long-term.
CONCLUSION
Hospitalization can be a challenging time for patients with SUD and for the hospital teams who care for them. These tensions are exacerbated by the COVID-19 pandemic, yet hospitalists play a critical role in addressing the converging crises of SUD and COVID-19. Providing comprehensive, compassionate, evidence-based care for hospitalized patients with SUD is important for both individual and community health during COVID-19.
Acknowledgments
The authors would like to thank Alisa Patten for help preparing this manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
Dr King received grant support from the National Institutes of Health (UG1DA015815) and the National Institute on Drug Abuse (R01DA037441). Dr Snyder received a Public Health Institute grant payable to her institution.
1. Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and opioid use disorders, and disease burden in an integrated health care system. J Addict Med. 2017;11(1):3-9. https://doi.org/10.1097/adm.0000000000000260
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
3. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018
4. Ahmed F, Ahmed N, Pissarides C, Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5(5):e240. https://doi.org/10.1016/s2468-2667(20)30085-2
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648
6. Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173(1):63-64. https://doi.org/10.7326/m20-1491
7. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010
8. Harm Reduction Coalition. Accessed April 24, 2020. https://harmreduction.org/
9. COVID-19 and the drug supply chain: from production and trafficking to use. Global Research Network, United Nations Office on Drugs and Crime; 2020. Accessed June 4, 2020. http://www.unodc.org/documents/data-and-analysis/covid/Covid-19-and-drug-supply-chain-Mai2020.pdf
10. Pouget ER, Sandoval M, Nikolopoulos GK, Friedman SR. Immediate impact of hurricane Sandy on people who inject drugs in New York City. Subst Use Misuse. 2015;50(7):878-884. https://doi.org/10.3109/10826084.2015.978675
11. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Substance Abuse and Mental Health Services Administration. Updated April 21, 2020. Accessed March 27, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
12. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
13. Baggett TP, Lewis E, Gaeta JM. Epidemiology of COVID-19 among people experiencing homelessness: early evidence from Boston. Ann Fam Med. Preprint posted April 4, 2020. http://hdl.handle.net/2027.42/154734
14. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
15. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001
16. Quality & Science. American Society of Addiction Medicine. Accessed April 24, 2020. https://www.asam.org/Quality-Science/quality
17. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. Published online December 12, 2019. https://doi.org/10.1007/s11606-019-05311-0
18. Digital Recovery Support Services. Recovery Link. Accessed April 24, 2020. https://myrecoverylink.com/digital-recovery-support/
19. Publications and Digital Products: Suicide Assessment Five-Step Evaluation and Triage for Clinicians. Substance Abuse and Mental Health Administration. September 2009. Accessed April 4, 2020. https://store.samhsa.gov/product/SAFE-T-Pocket-Card-Suicide-Assessment-Five-Step-Evaluation-and-Triage-for-Clinicians/sma09-4432
20. Prescribe to Prevent: Prescribe Naloxone, Save a Life. Accessed April 24, 2020. https://prescribetoprevent.org/
21. Never Use Alone. Accessed April 24, 2020. https://neverusealone.com/
The spread of SARS-CoV-2, the pathogen behind the COVID-19 pandemic, has converged with an unrelenting addiction epidemic. These combined crises will have profound effects on people with substance use disorders (SUD) and people in recovery. Hospitals—which were already hit hard by the addiction epidemic—are the last line of defense in the COVID-19 pandemic. Hospitalists have an important role in balancing the effects of these intersecting, synergistic crises.
People with SUD are disproportionately affected by major medical illnesses, including infections such as hepatitis C, HIV, and cardiovascular, pulmonary, and liver diseases.1 They also experience high rates of hospitalization due to drug-related infections, injury, and overdose.2 People with SUD commonly have intersecting vulnerabilities that may affect their healthcare experience and health outcomes, including housing and food insecurity, mental illness, and experiences of racism, incarceration, and other trauma. They may also harbor mistrust of healthcare providers because of previous negative encounters and discrimination with health systems.3 These vulnerabilities increase risks for COVID-19 morbidity and mortality.4,5 The COVID-19 pandemic may drive increases in use and harms from SUD among patients who already have an SUD, with widespread job loss, insurance loss,6 anxiety, and social isolation on the rise. We may also see increases in return to use among people in recovery or new substance use among those without a history of SUD.
The intersecting crises of SUD and COVID-19 are important for people with SUD and for public health. In this perspective, we describe how the COVID-19 pandemic has affected people with SUD and share practical resources for hospital providers to improve care for people with SUD during the pandemic and beyond.
CONTEXTUALIZING COVID-19 AND SUD RISK
Mistrust of Hospitals and Healthcare Providers
Fear of stigmatization is an ongoing problem for people with SUD, who often experience discrimination in hospitals and, as a result, may avoid hospital care.7 Much of this stigma is based on the false but persistent belief—widespread even among healthcare providers—that addiction is the result of bad choices and limited willpower; however, the science is clear that addiction is a disorder with neurobiological, genetic, and environmental underpinnings.3 These attitudes are likely to be amplified during COVID-19, as patients and providers experience higher levels of stress.
Increased Risks of Substance Use
Typically, people who use drugs are counseled to use with others nearby so that they might administer naloxone or call 911 in the event of an overdose.8 With physical distancing, people may be more likely to use alone. COVID-19 also introduces uncertainty into the drug supply chain through changes in drug production and trafficking.9 Further, access to alcohol may be limited as liquor stores close and public transportation becomes less available. As has been shown in other complex emergencies (such as social, political, economic, and environmental disasters), these barriers to obtaining substances may increase risks for withdrawal, for needing to exchange sex for money or drugs, for sharing syringes or drug preparation equipment,10 or for consuming other available sources of substances, like rubbing alcohol or hand sanitizer. COVID-19 may also increase risk for depression, anxiety, social isolation, and suicidality, all of which increase risk for return to use and overdose.
Changes to the Treatment Milieu
Many of the resources and services that people who use substances rely on to keep safe may be disrupted by COVID-19. Social distancing—the cornerstone of mitigating COVID-19 spread—may be challenging among people with SUD. Though federal regulations around methadone dispensing and buprenorphine prescribing have loosened in response to the pandemic,11 individuals in treatment may still be required to provide urine drug screens or be physically present to receive methadone doses, sometimes daily and in crowded waiting rooms.
Recovery support groups such as Alcoholics Anonymous (AA) and Self-Management and Recovery Training (SMART) provide social connection and are the foundation of many people’s recovery. While many in-person meetings have rapidly transformed to online and telephone support, they remain inaccessible to the most marginalized members of communities: people without smart phones, computers, or internet. This digital shift may also disproportionately affect older adults, people with limited English proficiency, and people with low technological literacy. Limits for other resources, such as syringe service programs, community centers, food pantries, housing shelters, and other places that people depend on for clean water, food, showers, soap, and safer spaces to use, may limit services or close altogether; those that remain open may see an unprecedented rise in need for services as millions of Americans file for unemployment. For many, anxiety about the pandemic, unemployment, financial strain, increased isolation, family stressors, illness, and community losses can lead to enormous personal distress and trigger return to use; loss of a recovery network may further exacerbate this.
Intersectionality of SUD and Other Structural Inequities
Many of the inequities that increase people’s risk for undertreated SUD also increase risk for COVID-19 infection, including racism,12 poverty, and homelessness.4 “Stay home and stay safe” is not an option for people who are unsheltered or whose homes are unsafe because of risks of physical, sexual, or emotional violence. Poverty commonly forces people to live in crowded communal apartments or shelters, rely on public transportation, wait in long lines at food pantries, and continue to work, even if unwell. Many shelters have had to reduce the number of people they serve to reduce crowding and support social distancing, which further compounds risks of unstable housing. Unfortunately, the same structural inequities that exacerbate SUD worsen the COVID-19 crisis.13
ROLE FOR HOSPITALISTS
The intersecting vulnerabilities of SUD and COVID-19 heighten an already urgent need to address SUD among hospitalized patients.14 While COVID-19 may increase harms of substance use, it may also increase people’s readiness to engage in treatment given changes to the drug supply and patient’s concerns about health risks. As such, it is even more critical to make treatment readily accessible and support harm reduction. Hospitalists can take important, actionable steps for patients with SUD—many of which are good general practices14 (Appendix Table).
Hospitalists should do the following:
1. Identify and treat acute withdrawal.15
2. Manage acute pain, including providing high-dose opioids if needed.16 Both practices (1 and 2) are evidence-based, can promote patient’s trust in providers,17 and can help avoid patients leaving against medical advice (AMA). Leaving AMA can lead to poor individual health and further threaten public health if patients leave with undiagnosed or unmanaged COVID-19 infection.
3. Encourage their hospitals to provide patients with tablets or other means to communicate with family, friends, and recovery supports via videolink, and refer patients to virtual peer support and recovery meetings during hospitalization.18 These practices may further support patients in tolerating hospitalization and prevent AMA discharge.
4. Initiate medication for addiction during admission and refer to addictions treatment after discharge. COVID-19–related regulatory changes such as expanded telehealth buprenorphine options and fewer daily dosing requirements for methadone may make this easier. Further, hospitalists should offer medication for alcohol and tobacco use disorders,15 especially given heightened possibility of unhealthy alcohol use and the respiratory complications associated with both tobacco and COVID-19.
5. Assess mental health and suicide risks19 given their association with social isolation, job loss, and financial insecurity.
6. Discuss relapse prevention among people in recovery.
7. Assess overdose risk and promote harm reduction.19 Specifically, this may include counseling patients to avoid sharing smoking supplies to avoid COVID-19 transmission, identifying places to access clean syringes, prescribing naloxone,20 and providing supports so that, if patients need to use alone, they can do so more safely.21
8. Consider high-risk transitions that may be exacerbated by COVID-19. COVID-19 may make safe discharge plans among people experiencing homelessness very challenging. Some communities are rapidly repurposing existing spaces or building new ones to care for people without a safe place to recover after acute hospitalization, yet many communities have no such resources. Hospital teams should consider the possibility that community services and SUD treatment resources may change rapidly during the pandemic. Hospitals can maintain updated resource lists and consider partnering with state and local health departments to improve safe care for people experiencing homelessness or lacking basic services.
COVID-19 is putting enormous strain on many US hospitals. Hospital-based addictions care is under resourced in the best of times,14 and while some hospitals have addiction consult services, many do not. To what degree hospitalists and hospital teams can address anything beyond COVID-19 emergencies will vary based on settings and resources. Furthermore, we recognize that who performs various activities will depend on individual hospital’s resources and practices. Addiction consult services, if available, can play a critical role, as can hospital social workers and care managers, nurses, residents, students, and other members of the healthcare team.
Finally, though COVID-19 adds tremendous stress to hospitals, permanent improvements in SUD treatment systems such as telephone visits for buprenorphine or eased methadone restrictions may emerge that could reduce barriers to hospital-based addictions care.11 Leveraging these changes now may help hospital providers to better support patients long-term.
CONCLUSION
Hospitalization can be a challenging time for patients with SUD and for the hospital teams who care for them. These tensions are exacerbated by the COVID-19 pandemic, yet hospitalists play a critical role in addressing the converging crises of SUD and COVID-19. Providing comprehensive, compassionate, evidence-based care for hospitalized patients with SUD is important for both individual and community health during COVID-19.
Acknowledgments
The authors would like to thank Alisa Patten for help preparing this manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
Dr King received grant support from the National Institutes of Health (UG1DA015815) and the National Institute on Drug Abuse (R01DA037441). Dr Snyder received a Public Health Institute grant payable to her institution.
The spread of SARS-CoV-2, the pathogen behind the COVID-19 pandemic, has converged with an unrelenting addiction epidemic. These combined crises will have profound effects on people with substance use disorders (SUD) and people in recovery. Hospitals—which were already hit hard by the addiction epidemic—are the last line of defense in the COVID-19 pandemic. Hospitalists have an important role in balancing the effects of these intersecting, synergistic crises.
People with SUD are disproportionately affected by major medical illnesses, including infections such as hepatitis C, HIV, and cardiovascular, pulmonary, and liver diseases.1 They also experience high rates of hospitalization due to drug-related infections, injury, and overdose.2 People with SUD commonly have intersecting vulnerabilities that may affect their healthcare experience and health outcomes, including housing and food insecurity, mental illness, and experiences of racism, incarceration, and other trauma. They may also harbor mistrust of healthcare providers because of previous negative encounters and discrimination with health systems.3 These vulnerabilities increase risks for COVID-19 morbidity and mortality.4,5 The COVID-19 pandemic may drive increases in use and harms from SUD among patients who already have an SUD, with widespread job loss, insurance loss,6 anxiety, and social isolation on the rise. We may also see increases in return to use among people in recovery or new substance use among those without a history of SUD.
The intersecting crises of SUD and COVID-19 are important for people with SUD and for public health. In this perspective, we describe how the COVID-19 pandemic has affected people with SUD and share practical resources for hospital providers to improve care for people with SUD during the pandemic and beyond.
CONTEXTUALIZING COVID-19 AND SUD RISK
Mistrust of Hospitals and Healthcare Providers
Fear of stigmatization is an ongoing problem for people with SUD, who often experience discrimination in hospitals and, as a result, may avoid hospital care.7 Much of this stigma is based on the false but persistent belief—widespread even among healthcare providers—that addiction is the result of bad choices and limited willpower; however, the science is clear that addiction is a disorder with neurobiological, genetic, and environmental underpinnings.3 These attitudes are likely to be amplified during COVID-19, as patients and providers experience higher levels of stress.
Increased Risks of Substance Use
Typically, people who use drugs are counseled to use with others nearby so that they might administer naloxone or call 911 in the event of an overdose.8 With physical distancing, people may be more likely to use alone. COVID-19 also introduces uncertainty into the drug supply chain through changes in drug production and trafficking.9 Further, access to alcohol may be limited as liquor stores close and public transportation becomes less available. As has been shown in other complex emergencies (such as social, political, economic, and environmental disasters), these barriers to obtaining substances may increase risks for withdrawal, for needing to exchange sex for money or drugs, for sharing syringes or drug preparation equipment,10 or for consuming other available sources of substances, like rubbing alcohol or hand sanitizer. COVID-19 may also increase risk for depression, anxiety, social isolation, and suicidality, all of which increase risk for return to use and overdose.
Changes to the Treatment Milieu
Many of the resources and services that people who use substances rely on to keep safe may be disrupted by COVID-19. Social distancing—the cornerstone of mitigating COVID-19 spread—may be challenging among people with SUD. Though federal regulations around methadone dispensing and buprenorphine prescribing have loosened in response to the pandemic,11 individuals in treatment may still be required to provide urine drug screens or be physically present to receive methadone doses, sometimes daily and in crowded waiting rooms.
Recovery support groups such as Alcoholics Anonymous (AA) and Self-Management and Recovery Training (SMART) provide social connection and are the foundation of many people’s recovery. While many in-person meetings have rapidly transformed to online and telephone support, they remain inaccessible to the most marginalized members of communities: people without smart phones, computers, or internet. This digital shift may also disproportionately affect older adults, people with limited English proficiency, and people with low technological literacy. Limits for other resources, such as syringe service programs, community centers, food pantries, housing shelters, and other places that people depend on for clean water, food, showers, soap, and safer spaces to use, may limit services or close altogether; those that remain open may see an unprecedented rise in need for services as millions of Americans file for unemployment. For many, anxiety about the pandemic, unemployment, financial strain, increased isolation, family stressors, illness, and community losses can lead to enormous personal distress and trigger return to use; loss of a recovery network may further exacerbate this.
Intersectionality of SUD and Other Structural Inequities
Many of the inequities that increase people’s risk for undertreated SUD also increase risk for COVID-19 infection, including racism,12 poverty, and homelessness.4 “Stay home and stay safe” is not an option for people who are unsheltered or whose homes are unsafe because of risks of physical, sexual, or emotional violence. Poverty commonly forces people to live in crowded communal apartments or shelters, rely on public transportation, wait in long lines at food pantries, and continue to work, even if unwell. Many shelters have had to reduce the number of people they serve to reduce crowding and support social distancing, which further compounds risks of unstable housing. Unfortunately, the same structural inequities that exacerbate SUD worsen the COVID-19 crisis.13
ROLE FOR HOSPITALISTS
The intersecting vulnerabilities of SUD and COVID-19 heighten an already urgent need to address SUD among hospitalized patients.14 While COVID-19 may increase harms of substance use, it may also increase people’s readiness to engage in treatment given changes to the drug supply and patient’s concerns about health risks. As such, it is even more critical to make treatment readily accessible and support harm reduction. Hospitalists can take important, actionable steps for patients with SUD—many of which are good general practices14 (Appendix Table).
Hospitalists should do the following:
1. Identify and treat acute withdrawal.15
2. Manage acute pain, including providing high-dose opioids if needed.16 Both practices (1 and 2) are evidence-based, can promote patient’s trust in providers,17 and can help avoid patients leaving against medical advice (AMA). Leaving AMA can lead to poor individual health and further threaten public health if patients leave with undiagnosed or unmanaged COVID-19 infection.
3. Encourage their hospitals to provide patients with tablets or other means to communicate with family, friends, and recovery supports via videolink, and refer patients to virtual peer support and recovery meetings during hospitalization.18 These practices may further support patients in tolerating hospitalization and prevent AMA discharge.
4. Initiate medication for addiction during admission and refer to addictions treatment after discharge. COVID-19–related regulatory changes such as expanded telehealth buprenorphine options and fewer daily dosing requirements for methadone may make this easier. Further, hospitalists should offer medication for alcohol and tobacco use disorders,15 especially given heightened possibility of unhealthy alcohol use and the respiratory complications associated with both tobacco and COVID-19.
5. Assess mental health and suicide risks19 given their association with social isolation, job loss, and financial insecurity.
6. Discuss relapse prevention among people in recovery.
7. Assess overdose risk and promote harm reduction.19 Specifically, this may include counseling patients to avoid sharing smoking supplies to avoid COVID-19 transmission, identifying places to access clean syringes, prescribing naloxone,20 and providing supports so that, if patients need to use alone, they can do so more safely.21
8. Consider high-risk transitions that may be exacerbated by COVID-19. COVID-19 may make safe discharge plans among people experiencing homelessness very challenging. Some communities are rapidly repurposing existing spaces or building new ones to care for people without a safe place to recover after acute hospitalization, yet many communities have no such resources. Hospital teams should consider the possibility that community services and SUD treatment resources may change rapidly during the pandemic. Hospitals can maintain updated resource lists and consider partnering with state and local health departments to improve safe care for people experiencing homelessness or lacking basic services.
COVID-19 is putting enormous strain on many US hospitals. Hospital-based addictions care is under resourced in the best of times,14 and while some hospitals have addiction consult services, many do not. To what degree hospitalists and hospital teams can address anything beyond COVID-19 emergencies will vary based on settings and resources. Furthermore, we recognize that who performs various activities will depend on individual hospital’s resources and practices. Addiction consult services, if available, can play a critical role, as can hospital social workers and care managers, nurses, residents, students, and other members of the healthcare team.
Finally, though COVID-19 adds tremendous stress to hospitals, permanent improvements in SUD treatment systems such as telephone visits for buprenorphine or eased methadone restrictions may emerge that could reduce barriers to hospital-based addictions care.11 Leveraging these changes now may help hospital providers to better support patients long-term.
CONCLUSION
Hospitalization can be a challenging time for patients with SUD and for the hospital teams who care for them. These tensions are exacerbated by the COVID-19 pandemic, yet hospitalists play a critical role in addressing the converging crises of SUD and COVID-19. Providing comprehensive, compassionate, evidence-based care for hospitalized patients with SUD is important for both individual and community health during COVID-19.
Acknowledgments
The authors would like to thank Alisa Patten for help preparing this manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
Dr King received grant support from the National Institutes of Health (UG1DA015815) and the National Institute on Drug Abuse (R01DA037441). Dr Snyder received a Public Health Institute grant payable to her institution.
1. Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and opioid use disorders, and disease burden in an integrated health care system. J Addict Med. 2017;11(1):3-9. https://doi.org/10.1097/adm.0000000000000260
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
3. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018
4. Ahmed F, Ahmed N, Pissarides C, Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5(5):e240. https://doi.org/10.1016/s2468-2667(20)30085-2
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648
6. Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173(1):63-64. https://doi.org/10.7326/m20-1491
7. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010
8. Harm Reduction Coalition. Accessed April 24, 2020. https://harmreduction.org/
9. COVID-19 and the drug supply chain: from production and trafficking to use. Global Research Network, United Nations Office on Drugs and Crime; 2020. Accessed June 4, 2020. http://www.unodc.org/documents/data-and-analysis/covid/Covid-19-and-drug-supply-chain-Mai2020.pdf
10. Pouget ER, Sandoval M, Nikolopoulos GK, Friedman SR. Immediate impact of hurricane Sandy on people who inject drugs in New York City. Subst Use Misuse. 2015;50(7):878-884. https://doi.org/10.3109/10826084.2015.978675
11. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Substance Abuse and Mental Health Services Administration. Updated April 21, 2020. Accessed March 27, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
12. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
13. Baggett TP, Lewis E, Gaeta JM. Epidemiology of COVID-19 among people experiencing homelessness: early evidence from Boston. Ann Fam Med. Preprint posted April 4, 2020. http://hdl.handle.net/2027.42/154734
14. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
15. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001
16. Quality & Science. American Society of Addiction Medicine. Accessed April 24, 2020. https://www.asam.org/Quality-Science/quality
17. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. Published online December 12, 2019. https://doi.org/10.1007/s11606-019-05311-0
18. Digital Recovery Support Services. Recovery Link. Accessed April 24, 2020. https://myrecoverylink.com/digital-recovery-support/
19. Publications and Digital Products: Suicide Assessment Five-Step Evaluation and Triage for Clinicians. Substance Abuse and Mental Health Administration. September 2009. Accessed April 4, 2020. https://store.samhsa.gov/product/SAFE-T-Pocket-Card-Suicide-Assessment-Five-Step-Evaluation-and-Triage-for-Clinicians/sma09-4432
20. Prescribe to Prevent: Prescribe Naloxone, Save a Life. Accessed April 24, 2020. https://prescribetoprevent.org/
21. Never Use Alone. Accessed April 24, 2020. https://neverusealone.com/
1. Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and opioid use disorders, and disease burden in an integrated health care system. J Addict Med. 2017;11(1):3-9. https://doi.org/10.1097/adm.0000000000000260
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
3. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018
4. Ahmed F, Ahmed N, Pissarides C, Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5(5):e240. https://doi.org/10.1016/s2468-2667(20)30085-2
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648
6. Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173(1):63-64. https://doi.org/10.7326/m20-1491
7. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010
8. Harm Reduction Coalition. Accessed April 24, 2020. https://harmreduction.org/
9. COVID-19 and the drug supply chain: from production and trafficking to use. Global Research Network, United Nations Office on Drugs and Crime; 2020. Accessed June 4, 2020. http://www.unodc.org/documents/data-and-analysis/covid/Covid-19-and-drug-supply-chain-Mai2020.pdf
10. Pouget ER, Sandoval M, Nikolopoulos GK, Friedman SR. Immediate impact of hurricane Sandy on people who inject drugs in New York City. Subst Use Misuse. 2015;50(7):878-884. https://doi.org/10.3109/10826084.2015.978675
11. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Substance Abuse and Mental Health Services Administration. Updated April 21, 2020. Accessed March 27, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
12. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
13. Baggett TP, Lewis E, Gaeta JM. Epidemiology of COVID-19 among people experiencing homelessness: early evidence from Boston. Ann Fam Med. Preprint posted April 4, 2020. http://hdl.handle.net/2027.42/154734
14. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
15. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001
16. Quality & Science. American Society of Addiction Medicine. Accessed April 24, 2020. https://www.asam.org/Quality-Science/quality
17. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. Published online December 12, 2019. https://doi.org/10.1007/s11606-019-05311-0
18. Digital Recovery Support Services. Recovery Link. Accessed April 24, 2020. https://myrecoverylink.com/digital-recovery-support/
19. Publications and Digital Products: Suicide Assessment Five-Step Evaluation and Triage for Clinicians. Substance Abuse and Mental Health Administration. September 2009. Accessed April 4, 2020. https://store.samhsa.gov/product/SAFE-T-Pocket-Card-Suicide-Assessment-Five-Step-Evaluation-and-Triage-for-Clinicians/sma09-4432
20. Prescribe to Prevent: Prescribe Naloxone, Save a Life. Accessed April 24, 2020. https://prescribetoprevent.org/
21. Never Use Alone. Accessed April 24, 2020. https://neverusealone.com/
© 2020 Society of Hospital Medicine
Leadership & Professional Development: Engaging Patients as Stakeholders
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
© 2020 Society of Hospital Medicine
A Call to Action: Hospitalists’ Role in Addressing Substance Use Disorder
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
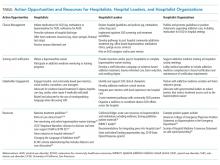
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
© 2020 Society of Hospital Medicine
“We’ve Learned It’s a Medical Illness, Not a Moral Choice”: Qualitative Study of the Effects of a Multicomponent Addiction Intervention on Hospital Providers’ Attitudes and Experiences
Substance use disorders (SUD) represent a national epidemic with death rates exceeding those of HIV at its peak.1 Hospitals are increasingly filled with people suffering from medical complications of addiction.2,3 While the US health system spends billions of dollars annually on hospital care for medical problems resulting from SUD,4 most hospitals lack expertise or care systems to directly address SUD or connect people to treatment after discharge. 5,6
Patients with SUD often feel stigmatized in healthcare settings and want providers who understand SUD and how to treat it.7 Providers feel underprepared8 and commonly have negative attitudes toward patients with SUD.9,10 Caring for patients can be a source of resentment, dissatisfaction, and burnout.9 Such negative attitudes can adversely affect patient care. Studies show that patients who perceive discrimination by providers are less likely to complete treatment11 and providers’ negative attitudes may disempower patients.9
Evaluations of hospital interventions for adults with SUD focus primarily on patient-level outcomes of SUD severity,12 healthcare utilization,13 and treatment engagement.14,15 Little is known about how such interventions can affect interprofessional providers’ attitudes and experiences, or how systems-level interventions influence hospital culture.16
We performed a qualitative study of multidisciplinary hospital providers to 1) understand the challenges that hospital providers face in managing care for patients with SUD, and 2) explore how integrating SUD treatment in a hospital setting affects providers’ attitudes, experiences, and perceptions of the care environment. This study was part of a formative evaluation of the Improving Addiction Care Team (IMPACT). IMPACT includes a hospital-based, interprofessional addiction medicine consultation service and rapid-access pathways to community addiction care after hospitalization.17. IMPACT is an intensive intervention that includes SUD assessments, withdrawal management, medications for addiction (eg, methadone, buprenorphine induction), counseling and behavioral SUD treatment, peer engagement and support, and linkages to community-based addiction care. We described the rationale and design of IMPACT in earlier publications.7,17
METHODS
Setting
We conducted in-person interviews and focus groups (FGs) with interprofessional hospital providers at a single urban academic medical center between February and July 2016, six months after starting IMPACT implementation. Oregon Health and Science University’s (OHSU) institutional review board approved the protocol.
Participants
We conducted 12 individual informant interviews (IIs) and 6 (FGs) (each comprising 3-6 participants) with a wide range of providers, including physicians, nurses, social workers, residents, patient advocates, case managers, and pharmacists. In total, 34 providers participated. We used purposive sampling to choose participants with experience both caring for patients with SUD and with exposure to IMPACT. Participant characteristics are summarized in Table 1.
Data Collection
We employed 2 different types of interviews. In situations where multiple providers occupied a similar role (eg, social workers), we chose to use a focus group format to elicit a range of perspectives and experiences through participant interaction.18 We conducted individual interviews to gain input from key informants who had unique roles in the program (eg, a cardiac surgeon) and to include providers who would otherwise be unable to participate due to scheduling barriers (eg, residents). We interviewed all participants using a semi-structured interview guide that was developed by an interdisciplinary team, including expert qualitative researchers, IMPACT clinical team members, and other OHSU clinicians (Appendix A). An interviewer who was not a part of the IMPACT clinical team asked all participants about their experience caring for patients with SUD, their experience with IMPACT, and how they might improve care. FGs lasted between 41-57 minutes, and individual key informant interviews lasted between 11-38 minutes. We ended recruitment after reaching theme saturation. Our goal was to achieve saturation across the sample as a whole and not within distinct participant groups. We noted if certain themes were more salient for 1 particular group. We audio-recorded all interviews and FGs. Recordings were transcribed, de-identified, and transferred to ATLAS.ti for data analysis.
Analysis
We conducted a thematic analysis using an inductive approach at the semantic level.19 Using an iterative process, we generated a preliminary coding schema after reviewing an initial selection of transcripts. Coders then independently coded transcripts and met in dyads to both discuss and reconcile codes, and resolve any discrepancies through discussion until reaching a consensus. One coder (DC) coded all transcripts; 3 coders (EP, SPP, MR) divided the transcripts evenly. All authors met periodically to discuss codebook revisions and emergent themes. We identified themes that represented patterns, had meaning to study participants, and captured important findings related to our research questions.19
As expected, the style of IIs differed from that of FGs and informants were able to provide information specific to their roles. Overall, the information provided by IIs was complementary to that of FGs and helped triangulate findings. Thus, we combined them in the results.18
RESULTS
We organized our findings into 3 main groupings, including (1) care before IMPACT, (2) care with IMPACT, and (3) perceived limitations of IMPACT. We included a table (Table 2) with additional quotations, beyond those in the body of the results, to support emergent themes described below.
Care before IMPACT
Providers felt hospitalization did not address addiction for many reasons, including ethical and legal concerns, medical knowledge gaps, and lack of treatment options.
Before IMPACT, many participants noted that hospitalization ignored or avoided addressing addiction, leading to a chaotic care environment that adversely affected patient care and provider experience. As one social worker stated, “prior to IMPACT we provided assessments, and we provided resources. But we didn’t address addiction.”
Providers cited multiple explanations for this, including the common misperception that using methadone to treat withdrawal violated federal regulations, and concerns about the ethicality of using opioids in patients with SUD. Across disciplines, providers described a “huge knowledge gap” and little confidence in addressing withdrawal, complex chronic pain, medications for addiction, and challenging patient behaviors. Providers also described limited expertise and scarce treatment options as a deterrent. As one attending reflected, “I would ask those questions [about SUD] before, but then … I had the information, but I couldn’t do anything with it.”
Providers felt the failure to address SUD adversely affected patient care, leading to untreated withdrawal, disruptive behaviors, and patients leaving against medical advice (AMA).
Participants across disciplines described wide variability in the medical management of SUD, particularly around the management of opioid withdrawal and pain, with some providers who “simply wouldn’t prescribe methadone or any opiates” and others who prescribed high doses without anticipating risks. As one attending recalled:
“You would see this pattern, especially in the intravenous drug-using population: left AMA, left AMA, left AMA … nine times out of ten, nobody was dealing with the fact that they were gonna go into withdrawal.”
Respondents recalled that disruptive behaviors from patients’ active use or withdrawal frequently threatened safety; imposed a tremendous burden on staff time and morale; and were a consistent source of providers’ distress. As one patient advocate explained:
“[Providers] get called to the unit because the person is yelling and throwing things or comes back after being gone for a long period and appears impaired … it often blows up, and they get discharged or they leave against medical advice or they go out and don’t come back. We don’t really know what happened to them, and they’re vulnerable. And the staff are vulnerable. And other patients are distressed by the disruption and commotion.”
Absent standards and systems to address SUD, providers felt they were “left to their own,” resulting in a reactive and chaotic care environment.
Providers noted inconsistent rules and policies regarding smoke breaks, room searches, and visitors. As a result, care felt “reckless and risky” and led to a “nonalliance” across disciplines. Providers frequently described inconsistent and loose expectations until an event -- often active use – triggered an ad hoc ratcheting up of the rules, damaging patient-provider relationships and limiting providers’ ability to provide medical care. Facing these conflicts, “staff gets escalated, and everybody gets kind of spun up.” As one attending reflected:
“I could not get any sort of engagement even in just her medical issues … I was trying to talk to her and educate her about heart failure and salt intake and food intake, but every time I walked in the room … I’d have to come in and be like, ‘your UDS [urine drug screen] was positive again, so here’s the changes to your behavioral plan, and OK, let’s talk about your heart failure …’ At that point, the relationship had completely disintegrated until it was very nonproductive.”
Providers described widespread “moral distress,” burnout, and feelings of futility before IMPACT.
Consequently, providers felt that caring for people with SUD was “very emotionally draining and very time consuming.” As one patient advocate described:
“We’ve been watching staff try to manage these patients for years without the experts and the resources and the skills that they need … As a result, there was a crescendo effect of moral distress, and [staff] bring in all of their past experiences which influence the interaction … Some staff are very skilled, but you also saw some really punitive responses.”
Many felt that providing intensive medical care without addressing people’s underlying SUD was a waste of time and resources. As one cardiac surgeon reflected:
“[Patients] ended up either dead or reinfected. Nobody wanted to do stuff because we felt it was futile. Well, of course, it’s futile …. you’re basically trying to fix the symptoms. It’s like having a leaky roof and just running around with a bunch of buckets, which is like surgery. You gotta fix the roof…otherwise they will continue to inject bacteria into their bodies.”
Care with IMPACT:
Providers felt integrating hospital-based systems to address SUD legitimized addiction as a treatable disease.
Participants described IMPACT as a “sea change” that “completely reframes” addiction as “a medical condition that actually has a treatment.” As one social worker observed, “when it’s somebody in a white coat with expertise who’s talking to another doctor it really can shift mindsets in an amazing way.” Others echoed this, stating that an addiction team “legitimized the fact that this is an actual disease that we need to treat - and a failure to treat it is a failure to be a good doctor.”
Providers felt that by addressing addiction directly, “IMPACT elevated the consciousness of providers and nurses … that substance use disorders are brain disorders and not bad behavior.” They described that this legitimization, combined with seeing firsthand the stabilizing effects of medications for addiction, allowed providers to understand SUD as a chronic disease, and not a moral failing.
Providers felt IMPACT improved patient engagement and humanized care by treating withdrawal, directly communicating about SUD, and modeling compassionate care.
Providers noted that treating withdrawal had a dramatic effect on patient engagement and care. One surgeon explained, “by managing their opioid dependence and other substance abuse issues … it’s easier for the staff to take care of them, it’s safer, and the patients feel better taken care of because the staff will engage with them.” Many noted that conflict-ridden “conversations were able to go to the side, and we were able to talk about other things to build rapport.” Others noted that this shift felt like “more productive time.”
In addition, providers repeatedly emphasized that having clear hospital standards and a process to engage patients “really helps … establish rapport with patients: ‘This is how we work this. These are your boundaries. And this is what will happen if you push those boundaries.’ There it is.” Providers attributed improved patient-provider communication to “frank conversation,” “the right amount of empathy,” and a less judgmental environment. As one attending described, “I don’t know if it gives them a voice or allows us to hear them better … but something’s happening with communication.”
Many participants highlighted that IMPACT modeled compassionate bedside interactions, exposed the role of trauma in many patients’ lives, and helped providers see SUD as a disease spectrum. One attending noted that to “actually appreciate the subtleties – just the severity of the disorder – has been powerful.” One resident said:
“There’s definitely a lot of stigma around patients with use disorders that probably shows itself in subtle ways throughout their hospitalization. I think IMPACT does a good job … keeping the patient in the center and keeping their use disorder contextualized in the greater person … [IMPACT] role models bedside interactions and how to treat people like humans.”
Providers valued post-hospital SUD treatment pathways.
Providers valued previously nonexistent post-hospital SUD treatment pathways, stating “this relationship with [community treatment] … it’s like an answer to prayers,” and “this isn’t just like we’re being nicer.” One attending described:
“Starting them on [methadone or buprenorphine-naloxone] and then making the next step in the outpatient world happen has been huge. That transition is so critical … that’s been probably the biggest impact.”
Providers felt relief after IMPACT implementation.
Providers felt that by addressing SUD treatment gaps and providing addiction expertise, IMPACT helped alleviate the previously widespread feelings of “moral distress.” One resident explained “having [IMPACT] as a lifeline, it just feels so good.” As an infectious disease consultant noted, “it makes people more open to treating people if they don’t feel isolated and out of their depth.” Others noted that IMPACT supported better multidisciplinary collaboration, which “reduced a lot of tension between the teams.” One nurse summarized:
“I think you feel more empowered when you’ve got the right medication, … the knowledge, and you feel like you have the resources. You actually feel like you’re making a difference.”
Respondents acknowledged that even with IMPACT, some patients leave AMA or relapse. However, by understanding addiction as a relapsing and remitting disease, providers reconceptualized “success,” further reducing feelings of emotional burnout and stress: “there will be ups and downs, it’s not gonna be a straight linear success.” One case manager reflected,
“Maybe that’s part of the nature of the illness, you progress, and then you kind of hold your breath and then it slips again … at least with IMPACT at the table I can say we’ve done the best we can for this person.”
Perceived limitations of IMPACT:
Providers noted several key limitations of IMPACT, including that hospital-based interventions do not address poverty and have limited ability to address socioeconomic determinants such as “social support, … housing, or nutrition.” Providers also felt that IMPACT had limited ability to alleviate patients’ feelings of boredom and isolation associated with prolonged hospitalization, and that IMPACT had limited effectiveness for highly ambivalent patients (Table 2).
Finally, while many described increased confidence managing SUD after working with IMPACT, others cautioned against deferring too much to specialists. As one resident doctor said:
“We shouldn’t forget that all providers should know how to handle some form of people with addiction … I just don’t want it to be like, ‘oh, well, no, I don’t need to think about this … because we have an addiction specialist.’”
Participants across disciplines repeatedly suggested formal, ongoing initiatives to educate and train providers to manage SUD independently.
DISCUSSION
This study explores provider perspectives on care for hospitalized adults with SUD. Before IMPACT, providers felt care was chaotic, unsafe, and frustrating. Providers perceived variable care quality, resulting in untreated withdrawal, inconsistent care plans, and poor patient outcomes, leading to widespread “moral distress” and feelings of futility among providers. Yet this experience was not inevitable. Providers described that a hospital-based intervention to treat SUD reframed addiction as a treatable chronic disease, transformed culture, and improved patient care and provider experience.
Our findings are consistent with and build on previous research in several ways. First, widespread anxiety and difficulty managing patients with SUD was not unique to our hospital. In a systematic review, van Boekel and colleagues describe that healthcare providers perceived violence, manipulation, and poor motivation as factors impeding care for patients with SUD.9 Our study demonstrates the resulting feelings of powerlessness and frustration may be alleviated through an intervention that provides SUD care.
Second, our study is consistent with a recent survey-based study by Wakeman and colleagues that found that a hospital-based SUD intervention improved providers’ feelings of preparedness and satisfaction.20 Our study provides a rich qualitative description and elucidates mechanisms by which such interventions may work.
The finding that a hospital-based SUD intervention can shift providers’ views of addiction is important. Earlier studies have shown that providers who perceive addiction as a choice are more likely to have negative attitudes toward people with SUD.11 While our intervention did not provide formal education aimed at changing attitudes, participants reported that seeing firsthand effects of treatment on patient behaviors was a powerful tool that radically shifted providers’ understanding and reduced stigma.
Stigma can occur at both individual and organizational levels. Structural stigma refers to practices, policies, and norms of institutions that exclude needs of a particular group.21 The absence of systems to address SUD sends a message to both patients and providers that addiction is a not a treatable or worthy disease. IMPACT was in and of itself a systems-level intervention; by creating a consultation service, hospital-wide policies, and pathways to care after hospitalization, IMPACT ‘legitimized’ SUD and reduced institutional stigma.
Several studies have shown the feasibility and effectiveness of starting medications for addiction (MAT) in the hospital.13-15 Our study builds on this work by highlighting systems-level elements valued by providers. These elements may be important to support and scale widespread adoption of MAT in hospitals. Specifically, providers felt that IMPACT’s attention to hospital policies, use of addiction medicine specialists, and direct linkages to outpatient SUD treatment proved instrumental in shifting care systems.
Our study has several limitations. As a single-site study, our goal was not generalizability, but transferability. As such, we aimed to obtain rich, in-depth information that can inform implementation of similar efforts. Because our study was conducted after the implementation of IMPACT, providers’ perspectives on care before IMPACT may have been influenced by the intervention. However, this also strengthens our findings by allowing participants the opportunity for insights under a different system. It likely leads to distinct findings compared to what we might have uncovered in a pre-post study. While respondents noted perceived limitations of IMPACT, there were few instances of negative remarks in the data we collected. It is possible that providers with more negative interpretations chose not to participate in interviews; however, we elicited wide viewpoints and encouraged participants to share both strengths and weaknesses. Finally, IMPACT implementation depends on regional as well as local factors such as Medicaid expansion, community treatment resources, and the existence of addiction medicine expertise that will differ across settings.
Despite these limitations, our study has several important implications. For clinical practice, our findings highlight the importance of treating withdrawal to address challenging patient behaviors and the value of integrating MAT into the hospital setting. Our findings also underscore the role of expert consultation for addiction. Importantly, our results emphasize that reframing SUD as a brain disease can have significant implications for clinical care and providers’ well-being. Provider distress is not inevitable and can change with the right support and systems.
At the hospital and health systems level, our findings suggest that hospitals can and should address SUD. This may include forming interprofessional teams with SUD expertise, providing standardized guidelines for addiction care such as patient safety plans and methadone policies, and creating rapid-access pathways to outpatient SUD care. By addressing SUD, hospitals may simultaneously improve care and reduce provider burnout. Providers’ important concerns about shifting SUD treatment to a specialty team and their discomfort managing SUD pre-IMPACT suggest the need to integrate SUD education across all levels of interprofessional education. Furthermore, provider concerns that IMPACT has limited ability to engage ambivalent patients underscores the need for hospital-based approaches that emphasize harm reduction strategies.
As the SUD epidemic worsens, SUD-related hospitalizations are skyrocketing, and people are dying at unprecedented rates.2,3 Many efforts to address SUD have been in primary care or community settings. While important, many people with SUD are unable or unlikely to seek primary care. 22 Hospitals need a workforce and systems that can address both the physical and behavioral health needs of this population. By implementing SUD improvements, hospitals can support staff and reduce burnout, better engage patients, improve care, and reduce stigma from this devastating disease
Disclosures
The authors have no conflicts of interest to disclose.
1. Rossen L, Bastian B, Warner M, Khan D, Chong Y. Drug poisoning mortality: United States, 1999-2015. 2017; https://www.cdc.gov/nchs/data-visualization/drug-poisoning-mortality/. Accessed 7-11, 2017.
2. Tedesco D, Asch SM, Curtin C, et al. Opioid abuse and poisoning: trends in inpatient and emergency department discharges. Health Aff (Millwood). 2017;36(10):1748-1753. http:// doi.org/10.1377/hlthaff.2017.0260. PubMed
3. Weiss AJ, Elixhauser A, Barrett ML, Steiner CA, Bailey MK, O’Malley L. Statistical Brief #219: Opioid-Related Inpatient Stays and Emergency Department Visits by State, 2009-2014. 2017; https://hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp?utm_source=AHRQ&utm_medium=EN-2&utm_term=&utm_content=2&utm_campaign=AHRQ_EN12_20_2016. Accessed July 11, 2017. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. http:// doi.org/10.1377/hlthaff.2015.1424. PubMed
5. Infectious Diseases Society of America Emerging Infections Network. Report for Query: ‘Injection Drug Use (IDU) and Infectious Disease Practice’. 2017; https://www.int-med.uiowa.edu/Research/EIN/FinalReport_IDUandID.pdf. Accessed July 11, 2017.
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. http:// doi.org/10.1016/j.amjmed.2015.09.024. PubMed
7. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an Experience, a Life Learning Experience”: A qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. http:// doi.org/10.1007/s11606-016-3919-4. PubMed
8. Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: Results from a survey of general internists. Subst Abus. 2016;37(4):635-641. http:// doi.org/10.1080/08897077.2016.1187240. PubMed
9. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. http:// doi.org/10.1016/j.drugalcdep.2013.02.018 PubMed
10. Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327-333. http:// doi.org/10.1046/j.1525-1497.2002.10625.x. PubMed
11. Brener L, Von Hippel W, Kippax S, Preacher KJ. The role of physician and nurse attitudes in the health care of injecting drug users. Subst Use Misuse. 2010;45(7-8):1007-1018. http:// doi.org/10.3109/10826081003659543. PubMed
12. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. http:// doi.org/10.1007/s11606-017-4077-z. PubMed
13. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. http:// doi.org/10.1007/s11606-014-2968-9. PubMed
14. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. http:// doi.org/10.1001/jamainternmed.2014.2556. PubMed
15. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. http:// doi.org/10.1007/s11606-010-1311-3. PubMed
16. Parmelli E, Flodgren G, Beyer F, Baillie N, Schaafsma ME, Eccles MP. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(1):33. http:// doi.org/10.1186/1748-5908-6-33. PubMed
17. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the improving addiction care team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. http:// doi.org/10.12788/jhm.2736. PubMed
18. Lambert SD, Loiselle CG. Combining individual interviews and focus groups to enhance data richness. J Adv Nurs. 2008;62(2):228-237. http:// doi.org/10.1111/j.1365-2648.2007.04559.x. PubMed
19. Braun VC, Victoria. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:25. http://dx.doi.org/10.1191/1478088706qp063oa.
20. Wakeman SE, Kanter GP, Donelan K. Institutional substance use disorder intervention improves general internist preparedness, attitudes, and clinical practice. J Addict Med. 2017;11(4):308-314. http:// doi.org/10.1097/ADM.0000000000000314. PubMed
21. Paterson B, Hirsch G, Andres K. Structural factors that promote stigmatization of drug users with hepatitis C in hospital emergency departments. Int J Drug Policy. 2013;24(5):471-478. http:// doi.org/10.1016/j.drugpo.2013.01.008 PubMed
22. Ross LE, Vigod S, Wishart J, et al. Barriers and facilitators to primary care for people with mental health and/or substance use issues: a qualitative study. BMC Fam Pract. 2015;16:135. http:// doi.org/10.1186/s12875-015-0353-3. PubMed
Substance use disorders (SUD) represent a national epidemic with death rates exceeding those of HIV at its peak.1 Hospitals are increasingly filled with people suffering from medical complications of addiction.2,3 While the US health system spends billions of dollars annually on hospital care for medical problems resulting from SUD,4 most hospitals lack expertise or care systems to directly address SUD or connect people to treatment after discharge. 5,6
Patients with SUD often feel stigmatized in healthcare settings and want providers who understand SUD and how to treat it.7 Providers feel underprepared8 and commonly have negative attitudes toward patients with SUD.9,10 Caring for patients can be a source of resentment, dissatisfaction, and burnout.9 Such negative attitudes can adversely affect patient care. Studies show that patients who perceive discrimination by providers are less likely to complete treatment11 and providers’ negative attitudes may disempower patients.9
Evaluations of hospital interventions for adults with SUD focus primarily on patient-level outcomes of SUD severity,12 healthcare utilization,13 and treatment engagement.14,15 Little is known about how such interventions can affect interprofessional providers’ attitudes and experiences, or how systems-level interventions influence hospital culture.16
We performed a qualitative study of multidisciplinary hospital providers to 1) understand the challenges that hospital providers face in managing care for patients with SUD, and 2) explore how integrating SUD treatment in a hospital setting affects providers’ attitudes, experiences, and perceptions of the care environment. This study was part of a formative evaluation of the Improving Addiction Care Team (IMPACT). IMPACT includes a hospital-based, interprofessional addiction medicine consultation service and rapid-access pathways to community addiction care after hospitalization.17. IMPACT is an intensive intervention that includes SUD assessments, withdrawal management, medications for addiction (eg, methadone, buprenorphine induction), counseling and behavioral SUD treatment, peer engagement and support, and linkages to community-based addiction care. We described the rationale and design of IMPACT in earlier publications.7,17
METHODS
Setting
We conducted in-person interviews and focus groups (FGs) with interprofessional hospital providers at a single urban academic medical center between February and July 2016, six months after starting IMPACT implementation. Oregon Health and Science University’s (OHSU) institutional review board approved the protocol.
Participants
We conducted 12 individual informant interviews (IIs) and 6 (FGs) (each comprising 3-6 participants) with a wide range of providers, including physicians, nurses, social workers, residents, patient advocates, case managers, and pharmacists. In total, 34 providers participated. We used purposive sampling to choose participants with experience both caring for patients with SUD and with exposure to IMPACT. Participant characteristics are summarized in Table 1.
Data Collection
We employed 2 different types of interviews. In situations where multiple providers occupied a similar role (eg, social workers), we chose to use a focus group format to elicit a range of perspectives and experiences through participant interaction.18 We conducted individual interviews to gain input from key informants who had unique roles in the program (eg, a cardiac surgeon) and to include providers who would otherwise be unable to participate due to scheduling barriers (eg, residents). We interviewed all participants using a semi-structured interview guide that was developed by an interdisciplinary team, including expert qualitative researchers, IMPACT clinical team members, and other OHSU clinicians (Appendix A). An interviewer who was not a part of the IMPACT clinical team asked all participants about their experience caring for patients with SUD, their experience with IMPACT, and how they might improve care. FGs lasted between 41-57 minutes, and individual key informant interviews lasted between 11-38 minutes. We ended recruitment after reaching theme saturation. Our goal was to achieve saturation across the sample as a whole and not within distinct participant groups. We noted if certain themes were more salient for 1 particular group. We audio-recorded all interviews and FGs. Recordings were transcribed, de-identified, and transferred to ATLAS.ti for data analysis.
Analysis
We conducted a thematic analysis using an inductive approach at the semantic level.19 Using an iterative process, we generated a preliminary coding schema after reviewing an initial selection of transcripts. Coders then independently coded transcripts and met in dyads to both discuss and reconcile codes, and resolve any discrepancies through discussion until reaching a consensus. One coder (DC) coded all transcripts; 3 coders (EP, SPP, MR) divided the transcripts evenly. All authors met periodically to discuss codebook revisions and emergent themes. We identified themes that represented patterns, had meaning to study participants, and captured important findings related to our research questions.19
As expected, the style of IIs differed from that of FGs and informants were able to provide information specific to their roles. Overall, the information provided by IIs was complementary to that of FGs and helped triangulate findings. Thus, we combined them in the results.18
RESULTS
We organized our findings into 3 main groupings, including (1) care before IMPACT, (2) care with IMPACT, and (3) perceived limitations of IMPACT. We included a table (Table 2) with additional quotations, beyond those in the body of the results, to support emergent themes described below.
Care before IMPACT
Providers felt hospitalization did not address addiction for many reasons, including ethical and legal concerns, medical knowledge gaps, and lack of treatment options.
Before IMPACT, many participants noted that hospitalization ignored or avoided addressing addiction, leading to a chaotic care environment that adversely affected patient care and provider experience. As one social worker stated, “prior to IMPACT we provided assessments, and we provided resources. But we didn’t address addiction.”
Providers cited multiple explanations for this, including the common misperception that using methadone to treat withdrawal violated federal regulations, and concerns about the ethicality of using opioids in patients with SUD. Across disciplines, providers described a “huge knowledge gap” and little confidence in addressing withdrawal, complex chronic pain, medications for addiction, and challenging patient behaviors. Providers also described limited expertise and scarce treatment options as a deterrent. As one attending reflected, “I would ask those questions [about SUD] before, but then … I had the information, but I couldn’t do anything with it.”
Providers felt the failure to address SUD adversely affected patient care, leading to untreated withdrawal, disruptive behaviors, and patients leaving against medical advice (AMA).
Participants across disciplines described wide variability in the medical management of SUD, particularly around the management of opioid withdrawal and pain, with some providers who “simply wouldn’t prescribe methadone or any opiates” and others who prescribed high doses without anticipating risks. As one attending recalled:
“You would see this pattern, especially in the intravenous drug-using population: left AMA, left AMA, left AMA … nine times out of ten, nobody was dealing with the fact that they were gonna go into withdrawal.”
Respondents recalled that disruptive behaviors from patients’ active use or withdrawal frequently threatened safety; imposed a tremendous burden on staff time and morale; and were a consistent source of providers’ distress. As one patient advocate explained:
“[Providers] get called to the unit because the person is yelling and throwing things or comes back after being gone for a long period and appears impaired … it often blows up, and they get discharged or they leave against medical advice or they go out and don’t come back. We don’t really know what happened to them, and they’re vulnerable. And the staff are vulnerable. And other patients are distressed by the disruption and commotion.”
Absent standards and systems to address SUD, providers felt they were “left to their own,” resulting in a reactive and chaotic care environment.
Providers noted inconsistent rules and policies regarding smoke breaks, room searches, and visitors. As a result, care felt “reckless and risky” and led to a “nonalliance” across disciplines. Providers frequently described inconsistent and loose expectations until an event -- often active use – triggered an ad hoc ratcheting up of the rules, damaging patient-provider relationships and limiting providers’ ability to provide medical care. Facing these conflicts, “staff gets escalated, and everybody gets kind of spun up.” As one attending reflected:
“I could not get any sort of engagement even in just her medical issues … I was trying to talk to her and educate her about heart failure and salt intake and food intake, but every time I walked in the room … I’d have to come in and be like, ‘your UDS [urine drug screen] was positive again, so here’s the changes to your behavioral plan, and OK, let’s talk about your heart failure …’ At that point, the relationship had completely disintegrated until it was very nonproductive.”
Providers described widespread “moral distress,” burnout, and feelings of futility before IMPACT.
Consequently, providers felt that caring for people with SUD was “very emotionally draining and very time consuming.” As one patient advocate described:
“We’ve been watching staff try to manage these patients for years without the experts and the resources and the skills that they need … As a result, there was a crescendo effect of moral distress, and [staff] bring in all of their past experiences which influence the interaction … Some staff are very skilled, but you also saw some really punitive responses.”
Many felt that providing intensive medical care without addressing people’s underlying SUD was a waste of time and resources. As one cardiac surgeon reflected:
“[Patients] ended up either dead or reinfected. Nobody wanted to do stuff because we felt it was futile. Well, of course, it’s futile …. you’re basically trying to fix the symptoms. It’s like having a leaky roof and just running around with a bunch of buckets, which is like surgery. You gotta fix the roof…otherwise they will continue to inject bacteria into their bodies.”
Care with IMPACT:
Providers felt integrating hospital-based systems to address SUD legitimized addiction as a treatable disease.
Participants described IMPACT as a “sea change” that “completely reframes” addiction as “a medical condition that actually has a treatment.” As one social worker observed, “when it’s somebody in a white coat with expertise who’s talking to another doctor it really can shift mindsets in an amazing way.” Others echoed this, stating that an addiction team “legitimized the fact that this is an actual disease that we need to treat - and a failure to treat it is a failure to be a good doctor.”
Providers felt that by addressing addiction directly, “IMPACT elevated the consciousness of providers and nurses … that substance use disorders are brain disorders and not bad behavior.” They described that this legitimization, combined with seeing firsthand the stabilizing effects of medications for addiction, allowed providers to understand SUD as a chronic disease, and not a moral failing.
Providers felt IMPACT improved patient engagement and humanized care by treating withdrawal, directly communicating about SUD, and modeling compassionate care.
Providers noted that treating withdrawal had a dramatic effect on patient engagement and care. One surgeon explained, “by managing their opioid dependence and other substance abuse issues … it’s easier for the staff to take care of them, it’s safer, and the patients feel better taken care of because the staff will engage with them.” Many noted that conflict-ridden “conversations were able to go to the side, and we were able to talk about other things to build rapport.” Others noted that this shift felt like “more productive time.”
In addition, providers repeatedly emphasized that having clear hospital standards and a process to engage patients “really helps … establish rapport with patients: ‘This is how we work this. These are your boundaries. And this is what will happen if you push those boundaries.’ There it is.” Providers attributed improved patient-provider communication to “frank conversation,” “the right amount of empathy,” and a less judgmental environment. As one attending described, “I don’t know if it gives them a voice or allows us to hear them better … but something’s happening with communication.”
Many participants highlighted that IMPACT modeled compassionate bedside interactions, exposed the role of trauma in many patients’ lives, and helped providers see SUD as a disease spectrum. One attending noted that to “actually appreciate the subtleties – just the severity of the disorder – has been powerful.” One resident said:
“There’s definitely a lot of stigma around patients with use disorders that probably shows itself in subtle ways throughout their hospitalization. I think IMPACT does a good job … keeping the patient in the center and keeping their use disorder contextualized in the greater person … [IMPACT] role models bedside interactions and how to treat people like humans.”
Providers valued post-hospital SUD treatment pathways.
Providers valued previously nonexistent post-hospital SUD treatment pathways, stating “this relationship with [community treatment] … it’s like an answer to prayers,” and “this isn’t just like we’re being nicer.” One attending described:
“Starting them on [methadone or buprenorphine-naloxone] and then making the next step in the outpatient world happen has been huge. That transition is so critical … that’s been probably the biggest impact.”
Providers felt relief after IMPACT implementation.
Providers felt that by addressing SUD treatment gaps and providing addiction expertise, IMPACT helped alleviate the previously widespread feelings of “moral distress.” One resident explained “having [IMPACT] as a lifeline, it just feels so good.” As an infectious disease consultant noted, “it makes people more open to treating people if they don’t feel isolated and out of their depth.” Others noted that IMPACT supported better multidisciplinary collaboration, which “reduced a lot of tension between the teams.” One nurse summarized:
“I think you feel more empowered when you’ve got the right medication, … the knowledge, and you feel like you have the resources. You actually feel like you’re making a difference.”
Respondents acknowledged that even with IMPACT, some patients leave AMA or relapse. However, by understanding addiction as a relapsing and remitting disease, providers reconceptualized “success,” further reducing feelings of emotional burnout and stress: “there will be ups and downs, it’s not gonna be a straight linear success.” One case manager reflected,
“Maybe that’s part of the nature of the illness, you progress, and then you kind of hold your breath and then it slips again … at least with IMPACT at the table I can say we’ve done the best we can for this person.”
Perceived limitations of IMPACT:
Providers noted several key limitations of IMPACT, including that hospital-based interventions do not address poverty and have limited ability to address socioeconomic determinants such as “social support, … housing, or nutrition.” Providers also felt that IMPACT had limited ability to alleviate patients’ feelings of boredom and isolation associated with prolonged hospitalization, and that IMPACT had limited effectiveness for highly ambivalent patients (Table 2).
Finally, while many described increased confidence managing SUD after working with IMPACT, others cautioned against deferring too much to specialists. As one resident doctor said:
“We shouldn’t forget that all providers should know how to handle some form of people with addiction … I just don’t want it to be like, ‘oh, well, no, I don’t need to think about this … because we have an addiction specialist.’”
Participants across disciplines repeatedly suggested formal, ongoing initiatives to educate and train providers to manage SUD independently.
DISCUSSION
This study explores provider perspectives on care for hospitalized adults with SUD. Before IMPACT, providers felt care was chaotic, unsafe, and frustrating. Providers perceived variable care quality, resulting in untreated withdrawal, inconsistent care plans, and poor patient outcomes, leading to widespread “moral distress” and feelings of futility among providers. Yet this experience was not inevitable. Providers described that a hospital-based intervention to treat SUD reframed addiction as a treatable chronic disease, transformed culture, and improved patient care and provider experience.
Our findings are consistent with and build on previous research in several ways. First, widespread anxiety and difficulty managing patients with SUD was not unique to our hospital. In a systematic review, van Boekel and colleagues describe that healthcare providers perceived violence, manipulation, and poor motivation as factors impeding care for patients with SUD.9 Our study demonstrates the resulting feelings of powerlessness and frustration may be alleviated through an intervention that provides SUD care.
Second, our study is consistent with a recent survey-based study by Wakeman and colleagues that found that a hospital-based SUD intervention improved providers’ feelings of preparedness and satisfaction.20 Our study provides a rich qualitative description and elucidates mechanisms by which such interventions may work.
The finding that a hospital-based SUD intervention can shift providers’ views of addiction is important. Earlier studies have shown that providers who perceive addiction as a choice are more likely to have negative attitudes toward people with SUD.11 While our intervention did not provide formal education aimed at changing attitudes, participants reported that seeing firsthand effects of treatment on patient behaviors was a powerful tool that radically shifted providers’ understanding and reduced stigma.
Stigma can occur at both individual and organizational levels. Structural stigma refers to practices, policies, and norms of institutions that exclude needs of a particular group.21 The absence of systems to address SUD sends a message to both patients and providers that addiction is a not a treatable or worthy disease. IMPACT was in and of itself a systems-level intervention; by creating a consultation service, hospital-wide policies, and pathways to care after hospitalization, IMPACT ‘legitimized’ SUD and reduced institutional stigma.
Several studies have shown the feasibility and effectiveness of starting medications for addiction (MAT) in the hospital.13-15 Our study builds on this work by highlighting systems-level elements valued by providers. These elements may be important to support and scale widespread adoption of MAT in hospitals. Specifically, providers felt that IMPACT’s attention to hospital policies, use of addiction medicine specialists, and direct linkages to outpatient SUD treatment proved instrumental in shifting care systems.
Our study has several limitations. As a single-site study, our goal was not generalizability, but transferability. As such, we aimed to obtain rich, in-depth information that can inform implementation of similar efforts. Because our study was conducted after the implementation of IMPACT, providers’ perspectives on care before IMPACT may have been influenced by the intervention. However, this also strengthens our findings by allowing participants the opportunity for insights under a different system. It likely leads to distinct findings compared to what we might have uncovered in a pre-post study. While respondents noted perceived limitations of IMPACT, there were few instances of negative remarks in the data we collected. It is possible that providers with more negative interpretations chose not to participate in interviews; however, we elicited wide viewpoints and encouraged participants to share both strengths and weaknesses. Finally, IMPACT implementation depends on regional as well as local factors such as Medicaid expansion, community treatment resources, and the existence of addiction medicine expertise that will differ across settings.
Despite these limitations, our study has several important implications. For clinical practice, our findings highlight the importance of treating withdrawal to address challenging patient behaviors and the value of integrating MAT into the hospital setting. Our findings also underscore the role of expert consultation for addiction. Importantly, our results emphasize that reframing SUD as a brain disease can have significant implications for clinical care and providers’ well-being. Provider distress is not inevitable and can change with the right support and systems.
At the hospital and health systems level, our findings suggest that hospitals can and should address SUD. This may include forming interprofessional teams with SUD expertise, providing standardized guidelines for addiction care such as patient safety plans and methadone policies, and creating rapid-access pathways to outpatient SUD care. By addressing SUD, hospitals may simultaneously improve care and reduce provider burnout. Providers’ important concerns about shifting SUD treatment to a specialty team and their discomfort managing SUD pre-IMPACT suggest the need to integrate SUD education across all levels of interprofessional education. Furthermore, provider concerns that IMPACT has limited ability to engage ambivalent patients underscores the need for hospital-based approaches that emphasize harm reduction strategies.
As the SUD epidemic worsens, SUD-related hospitalizations are skyrocketing, and people are dying at unprecedented rates.2,3 Many efforts to address SUD have been in primary care or community settings. While important, many people with SUD are unable or unlikely to seek primary care. 22 Hospitals need a workforce and systems that can address both the physical and behavioral health needs of this population. By implementing SUD improvements, hospitals can support staff and reduce burnout, better engage patients, improve care, and reduce stigma from this devastating disease
Disclosures
The authors have no conflicts of interest to disclose.
Substance use disorders (SUD) represent a national epidemic with death rates exceeding those of HIV at its peak.1 Hospitals are increasingly filled with people suffering from medical complications of addiction.2,3 While the US health system spends billions of dollars annually on hospital care for medical problems resulting from SUD,4 most hospitals lack expertise or care systems to directly address SUD or connect people to treatment after discharge. 5,6
Patients with SUD often feel stigmatized in healthcare settings and want providers who understand SUD and how to treat it.7 Providers feel underprepared8 and commonly have negative attitudes toward patients with SUD.9,10 Caring for patients can be a source of resentment, dissatisfaction, and burnout.9 Such negative attitudes can adversely affect patient care. Studies show that patients who perceive discrimination by providers are less likely to complete treatment11 and providers’ negative attitudes may disempower patients.9
Evaluations of hospital interventions for adults with SUD focus primarily on patient-level outcomes of SUD severity,12 healthcare utilization,13 and treatment engagement.14,15 Little is known about how such interventions can affect interprofessional providers’ attitudes and experiences, or how systems-level interventions influence hospital culture.16
We performed a qualitative study of multidisciplinary hospital providers to 1) understand the challenges that hospital providers face in managing care for patients with SUD, and 2) explore how integrating SUD treatment in a hospital setting affects providers’ attitudes, experiences, and perceptions of the care environment. This study was part of a formative evaluation of the Improving Addiction Care Team (IMPACT). IMPACT includes a hospital-based, interprofessional addiction medicine consultation service and rapid-access pathways to community addiction care after hospitalization.17. IMPACT is an intensive intervention that includes SUD assessments, withdrawal management, medications for addiction (eg, methadone, buprenorphine induction), counseling and behavioral SUD treatment, peer engagement and support, and linkages to community-based addiction care. We described the rationale and design of IMPACT in earlier publications.7,17
METHODS
Setting
We conducted in-person interviews and focus groups (FGs) with interprofessional hospital providers at a single urban academic medical center between February and July 2016, six months after starting IMPACT implementation. Oregon Health and Science University’s (OHSU) institutional review board approved the protocol.
Participants
We conducted 12 individual informant interviews (IIs) and 6 (FGs) (each comprising 3-6 participants) with a wide range of providers, including physicians, nurses, social workers, residents, patient advocates, case managers, and pharmacists. In total, 34 providers participated. We used purposive sampling to choose participants with experience both caring for patients with SUD and with exposure to IMPACT. Participant characteristics are summarized in Table 1.
Data Collection
We employed 2 different types of interviews. In situations where multiple providers occupied a similar role (eg, social workers), we chose to use a focus group format to elicit a range of perspectives and experiences through participant interaction.18 We conducted individual interviews to gain input from key informants who had unique roles in the program (eg, a cardiac surgeon) and to include providers who would otherwise be unable to participate due to scheduling barriers (eg, residents). We interviewed all participants using a semi-structured interview guide that was developed by an interdisciplinary team, including expert qualitative researchers, IMPACT clinical team members, and other OHSU clinicians (Appendix A). An interviewer who was not a part of the IMPACT clinical team asked all participants about their experience caring for patients with SUD, their experience with IMPACT, and how they might improve care. FGs lasted between 41-57 minutes, and individual key informant interviews lasted between 11-38 minutes. We ended recruitment after reaching theme saturation. Our goal was to achieve saturation across the sample as a whole and not within distinct participant groups. We noted if certain themes were more salient for 1 particular group. We audio-recorded all interviews and FGs. Recordings were transcribed, de-identified, and transferred to ATLAS.ti for data analysis.
Analysis
We conducted a thematic analysis using an inductive approach at the semantic level.19 Using an iterative process, we generated a preliminary coding schema after reviewing an initial selection of transcripts. Coders then independently coded transcripts and met in dyads to both discuss and reconcile codes, and resolve any discrepancies through discussion until reaching a consensus. One coder (DC) coded all transcripts; 3 coders (EP, SPP, MR) divided the transcripts evenly. All authors met periodically to discuss codebook revisions and emergent themes. We identified themes that represented patterns, had meaning to study participants, and captured important findings related to our research questions.19
As expected, the style of IIs differed from that of FGs and informants were able to provide information specific to their roles. Overall, the information provided by IIs was complementary to that of FGs and helped triangulate findings. Thus, we combined them in the results.18
RESULTS
We organized our findings into 3 main groupings, including (1) care before IMPACT, (2) care with IMPACT, and (3) perceived limitations of IMPACT. We included a table (Table 2) with additional quotations, beyond those in the body of the results, to support emergent themes described below.
Care before IMPACT
Providers felt hospitalization did not address addiction for many reasons, including ethical and legal concerns, medical knowledge gaps, and lack of treatment options.
Before IMPACT, many participants noted that hospitalization ignored or avoided addressing addiction, leading to a chaotic care environment that adversely affected patient care and provider experience. As one social worker stated, “prior to IMPACT we provided assessments, and we provided resources. But we didn’t address addiction.”
Providers cited multiple explanations for this, including the common misperception that using methadone to treat withdrawal violated federal regulations, and concerns about the ethicality of using opioids in patients with SUD. Across disciplines, providers described a “huge knowledge gap” and little confidence in addressing withdrawal, complex chronic pain, medications for addiction, and challenging patient behaviors. Providers also described limited expertise and scarce treatment options as a deterrent. As one attending reflected, “I would ask those questions [about SUD] before, but then … I had the information, but I couldn’t do anything with it.”
Providers felt the failure to address SUD adversely affected patient care, leading to untreated withdrawal, disruptive behaviors, and patients leaving against medical advice (AMA).
Participants across disciplines described wide variability in the medical management of SUD, particularly around the management of opioid withdrawal and pain, with some providers who “simply wouldn’t prescribe methadone or any opiates” and others who prescribed high doses without anticipating risks. As one attending recalled:
“You would see this pattern, especially in the intravenous drug-using population: left AMA, left AMA, left AMA … nine times out of ten, nobody was dealing with the fact that they were gonna go into withdrawal.”
Respondents recalled that disruptive behaviors from patients’ active use or withdrawal frequently threatened safety; imposed a tremendous burden on staff time and morale; and were a consistent source of providers’ distress. As one patient advocate explained:
“[Providers] get called to the unit because the person is yelling and throwing things or comes back after being gone for a long period and appears impaired … it often blows up, and they get discharged or they leave against medical advice or they go out and don’t come back. We don’t really know what happened to them, and they’re vulnerable. And the staff are vulnerable. And other patients are distressed by the disruption and commotion.”
Absent standards and systems to address SUD, providers felt they were “left to their own,” resulting in a reactive and chaotic care environment.
Providers noted inconsistent rules and policies regarding smoke breaks, room searches, and visitors. As a result, care felt “reckless and risky” and led to a “nonalliance” across disciplines. Providers frequently described inconsistent and loose expectations until an event -- often active use – triggered an ad hoc ratcheting up of the rules, damaging patient-provider relationships and limiting providers’ ability to provide medical care. Facing these conflicts, “staff gets escalated, and everybody gets kind of spun up.” As one attending reflected:
“I could not get any sort of engagement even in just her medical issues … I was trying to talk to her and educate her about heart failure and salt intake and food intake, but every time I walked in the room … I’d have to come in and be like, ‘your UDS [urine drug screen] was positive again, so here’s the changes to your behavioral plan, and OK, let’s talk about your heart failure …’ At that point, the relationship had completely disintegrated until it was very nonproductive.”
Providers described widespread “moral distress,” burnout, and feelings of futility before IMPACT.
Consequently, providers felt that caring for people with SUD was “very emotionally draining and very time consuming.” As one patient advocate described:
“We’ve been watching staff try to manage these patients for years without the experts and the resources and the skills that they need … As a result, there was a crescendo effect of moral distress, and [staff] bring in all of their past experiences which influence the interaction … Some staff are very skilled, but you also saw some really punitive responses.”
Many felt that providing intensive medical care without addressing people’s underlying SUD was a waste of time and resources. As one cardiac surgeon reflected:
“[Patients] ended up either dead or reinfected. Nobody wanted to do stuff because we felt it was futile. Well, of course, it’s futile …. you’re basically trying to fix the symptoms. It’s like having a leaky roof and just running around with a bunch of buckets, which is like surgery. You gotta fix the roof…otherwise they will continue to inject bacteria into their bodies.”
Care with IMPACT:
Providers felt integrating hospital-based systems to address SUD legitimized addiction as a treatable disease.
Participants described IMPACT as a “sea change” that “completely reframes” addiction as “a medical condition that actually has a treatment.” As one social worker observed, “when it’s somebody in a white coat with expertise who’s talking to another doctor it really can shift mindsets in an amazing way.” Others echoed this, stating that an addiction team “legitimized the fact that this is an actual disease that we need to treat - and a failure to treat it is a failure to be a good doctor.”
Providers felt that by addressing addiction directly, “IMPACT elevated the consciousness of providers and nurses … that substance use disorders are brain disorders and not bad behavior.” They described that this legitimization, combined with seeing firsthand the stabilizing effects of medications for addiction, allowed providers to understand SUD as a chronic disease, and not a moral failing.
Providers felt IMPACT improved patient engagement and humanized care by treating withdrawal, directly communicating about SUD, and modeling compassionate care.
Providers noted that treating withdrawal had a dramatic effect on patient engagement and care. One surgeon explained, “by managing their opioid dependence and other substance abuse issues … it’s easier for the staff to take care of them, it’s safer, and the patients feel better taken care of because the staff will engage with them.” Many noted that conflict-ridden “conversations were able to go to the side, and we were able to talk about other things to build rapport.” Others noted that this shift felt like “more productive time.”
In addition, providers repeatedly emphasized that having clear hospital standards and a process to engage patients “really helps … establish rapport with patients: ‘This is how we work this. These are your boundaries. And this is what will happen if you push those boundaries.’ There it is.” Providers attributed improved patient-provider communication to “frank conversation,” “the right amount of empathy,” and a less judgmental environment. As one attending described, “I don’t know if it gives them a voice or allows us to hear them better … but something’s happening with communication.”
Many participants highlighted that IMPACT modeled compassionate bedside interactions, exposed the role of trauma in many patients’ lives, and helped providers see SUD as a disease spectrum. One attending noted that to “actually appreciate the subtleties – just the severity of the disorder – has been powerful.” One resident said:
“There’s definitely a lot of stigma around patients with use disorders that probably shows itself in subtle ways throughout their hospitalization. I think IMPACT does a good job … keeping the patient in the center and keeping their use disorder contextualized in the greater person … [IMPACT] role models bedside interactions and how to treat people like humans.”
Providers valued post-hospital SUD treatment pathways.
Providers valued previously nonexistent post-hospital SUD treatment pathways, stating “this relationship with [community treatment] … it’s like an answer to prayers,” and “this isn’t just like we’re being nicer.” One attending described:
“Starting them on [methadone or buprenorphine-naloxone] and then making the next step in the outpatient world happen has been huge. That transition is so critical … that’s been probably the biggest impact.”
Providers felt relief after IMPACT implementation.
Providers felt that by addressing SUD treatment gaps and providing addiction expertise, IMPACT helped alleviate the previously widespread feelings of “moral distress.” One resident explained “having [IMPACT] as a lifeline, it just feels so good.” As an infectious disease consultant noted, “it makes people more open to treating people if they don’t feel isolated and out of their depth.” Others noted that IMPACT supported better multidisciplinary collaboration, which “reduced a lot of tension between the teams.” One nurse summarized:
“I think you feel more empowered when you’ve got the right medication, … the knowledge, and you feel like you have the resources. You actually feel like you’re making a difference.”
Respondents acknowledged that even with IMPACT, some patients leave AMA or relapse. However, by understanding addiction as a relapsing and remitting disease, providers reconceptualized “success,” further reducing feelings of emotional burnout and stress: “there will be ups and downs, it’s not gonna be a straight linear success.” One case manager reflected,
“Maybe that’s part of the nature of the illness, you progress, and then you kind of hold your breath and then it slips again … at least with IMPACT at the table I can say we’ve done the best we can for this person.”
Perceived limitations of IMPACT:
Providers noted several key limitations of IMPACT, including that hospital-based interventions do not address poverty and have limited ability to address socioeconomic determinants such as “social support, … housing, or nutrition.” Providers also felt that IMPACT had limited ability to alleviate patients’ feelings of boredom and isolation associated with prolonged hospitalization, and that IMPACT had limited effectiveness for highly ambivalent patients (Table 2).
Finally, while many described increased confidence managing SUD after working with IMPACT, others cautioned against deferring too much to specialists. As one resident doctor said:
“We shouldn’t forget that all providers should know how to handle some form of people with addiction … I just don’t want it to be like, ‘oh, well, no, I don’t need to think about this … because we have an addiction specialist.’”
Participants across disciplines repeatedly suggested formal, ongoing initiatives to educate and train providers to manage SUD independently.
DISCUSSION
This study explores provider perspectives on care for hospitalized adults with SUD. Before IMPACT, providers felt care was chaotic, unsafe, and frustrating. Providers perceived variable care quality, resulting in untreated withdrawal, inconsistent care plans, and poor patient outcomes, leading to widespread “moral distress” and feelings of futility among providers. Yet this experience was not inevitable. Providers described that a hospital-based intervention to treat SUD reframed addiction as a treatable chronic disease, transformed culture, and improved patient care and provider experience.
Our findings are consistent with and build on previous research in several ways. First, widespread anxiety and difficulty managing patients with SUD was not unique to our hospital. In a systematic review, van Boekel and colleagues describe that healthcare providers perceived violence, manipulation, and poor motivation as factors impeding care for patients with SUD.9 Our study demonstrates the resulting feelings of powerlessness and frustration may be alleviated through an intervention that provides SUD care.
Second, our study is consistent with a recent survey-based study by Wakeman and colleagues that found that a hospital-based SUD intervention improved providers’ feelings of preparedness and satisfaction.20 Our study provides a rich qualitative description and elucidates mechanisms by which such interventions may work.
The finding that a hospital-based SUD intervention can shift providers’ views of addiction is important. Earlier studies have shown that providers who perceive addiction as a choice are more likely to have negative attitudes toward people with SUD.11 While our intervention did not provide formal education aimed at changing attitudes, participants reported that seeing firsthand effects of treatment on patient behaviors was a powerful tool that radically shifted providers’ understanding and reduced stigma.
Stigma can occur at both individual and organizational levels. Structural stigma refers to practices, policies, and norms of institutions that exclude needs of a particular group.21 The absence of systems to address SUD sends a message to both patients and providers that addiction is a not a treatable or worthy disease. IMPACT was in and of itself a systems-level intervention; by creating a consultation service, hospital-wide policies, and pathways to care after hospitalization, IMPACT ‘legitimized’ SUD and reduced institutional stigma.
Several studies have shown the feasibility and effectiveness of starting medications for addiction (MAT) in the hospital.13-15 Our study builds on this work by highlighting systems-level elements valued by providers. These elements may be important to support and scale widespread adoption of MAT in hospitals. Specifically, providers felt that IMPACT’s attention to hospital policies, use of addiction medicine specialists, and direct linkages to outpatient SUD treatment proved instrumental in shifting care systems.
Our study has several limitations. As a single-site study, our goal was not generalizability, but transferability. As such, we aimed to obtain rich, in-depth information that can inform implementation of similar efforts. Because our study was conducted after the implementation of IMPACT, providers’ perspectives on care before IMPACT may have been influenced by the intervention. However, this also strengthens our findings by allowing participants the opportunity for insights under a different system. It likely leads to distinct findings compared to what we might have uncovered in a pre-post study. While respondents noted perceived limitations of IMPACT, there were few instances of negative remarks in the data we collected. It is possible that providers with more negative interpretations chose not to participate in interviews; however, we elicited wide viewpoints and encouraged participants to share both strengths and weaknesses. Finally, IMPACT implementation depends on regional as well as local factors such as Medicaid expansion, community treatment resources, and the existence of addiction medicine expertise that will differ across settings.
Despite these limitations, our study has several important implications. For clinical practice, our findings highlight the importance of treating withdrawal to address challenging patient behaviors and the value of integrating MAT into the hospital setting. Our findings also underscore the role of expert consultation for addiction. Importantly, our results emphasize that reframing SUD as a brain disease can have significant implications for clinical care and providers’ well-being. Provider distress is not inevitable and can change with the right support and systems.
At the hospital and health systems level, our findings suggest that hospitals can and should address SUD. This may include forming interprofessional teams with SUD expertise, providing standardized guidelines for addiction care such as patient safety plans and methadone policies, and creating rapid-access pathways to outpatient SUD care. By addressing SUD, hospitals may simultaneously improve care and reduce provider burnout. Providers’ important concerns about shifting SUD treatment to a specialty team and their discomfort managing SUD pre-IMPACT suggest the need to integrate SUD education across all levels of interprofessional education. Furthermore, provider concerns that IMPACT has limited ability to engage ambivalent patients underscores the need for hospital-based approaches that emphasize harm reduction strategies.
As the SUD epidemic worsens, SUD-related hospitalizations are skyrocketing, and people are dying at unprecedented rates.2,3 Many efforts to address SUD have been in primary care or community settings. While important, many people with SUD are unable or unlikely to seek primary care. 22 Hospitals need a workforce and systems that can address both the physical and behavioral health needs of this population. By implementing SUD improvements, hospitals can support staff and reduce burnout, better engage patients, improve care, and reduce stigma from this devastating disease
Disclosures
The authors have no conflicts of interest to disclose.
1. Rossen L, Bastian B, Warner M, Khan D, Chong Y. Drug poisoning mortality: United States, 1999-2015. 2017; https://www.cdc.gov/nchs/data-visualization/drug-poisoning-mortality/. Accessed 7-11, 2017.
2. Tedesco D, Asch SM, Curtin C, et al. Opioid abuse and poisoning: trends in inpatient and emergency department discharges. Health Aff (Millwood). 2017;36(10):1748-1753. http:// doi.org/10.1377/hlthaff.2017.0260. PubMed
3. Weiss AJ, Elixhauser A, Barrett ML, Steiner CA, Bailey MK, O’Malley L. Statistical Brief #219: Opioid-Related Inpatient Stays and Emergency Department Visits by State, 2009-2014. 2017; https://hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp?utm_source=AHRQ&utm_medium=EN-2&utm_term=&utm_content=2&utm_campaign=AHRQ_EN12_20_2016. Accessed July 11, 2017. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. http:// doi.org/10.1377/hlthaff.2015.1424. PubMed
5. Infectious Diseases Society of America Emerging Infections Network. Report for Query: ‘Injection Drug Use (IDU) and Infectious Disease Practice’. 2017; https://www.int-med.uiowa.edu/Research/EIN/FinalReport_IDUandID.pdf. Accessed July 11, 2017.
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. http:// doi.org/10.1016/j.amjmed.2015.09.024. PubMed
7. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an Experience, a Life Learning Experience”: A qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. http:// doi.org/10.1007/s11606-016-3919-4. PubMed
8. Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: Results from a survey of general internists. Subst Abus. 2016;37(4):635-641. http:// doi.org/10.1080/08897077.2016.1187240. PubMed
9. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. http:// doi.org/10.1016/j.drugalcdep.2013.02.018 PubMed
10. Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327-333. http:// doi.org/10.1046/j.1525-1497.2002.10625.x. PubMed
11. Brener L, Von Hippel W, Kippax S, Preacher KJ. The role of physician and nurse attitudes in the health care of injecting drug users. Subst Use Misuse. 2010;45(7-8):1007-1018. http:// doi.org/10.3109/10826081003659543. PubMed
12. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. http:// doi.org/10.1007/s11606-017-4077-z. PubMed
13. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. http:// doi.org/10.1007/s11606-014-2968-9. PubMed
14. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. http:// doi.org/10.1001/jamainternmed.2014.2556. PubMed
15. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. http:// doi.org/10.1007/s11606-010-1311-3. PubMed
16. Parmelli E, Flodgren G, Beyer F, Baillie N, Schaafsma ME, Eccles MP. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(1):33. http:// doi.org/10.1186/1748-5908-6-33. PubMed
17. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the improving addiction care team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. http:// doi.org/10.12788/jhm.2736. PubMed
18. Lambert SD, Loiselle CG. Combining individual interviews and focus groups to enhance data richness. J Adv Nurs. 2008;62(2):228-237. http:// doi.org/10.1111/j.1365-2648.2007.04559.x. PubMed
19. Braun VC, Victoria. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:25. http://dx.doi.org/10.1191/1478088706qp063oa.
20. Wakeman SE, Kanter GP, Donelan K. Institutional substance use disorder intervention improves general internist preparedness, attitudes, and clinical practice. J Addict Med. 2017;11(4):308-314. http:// doi.org/10.1097/ADM.0000000000000314. PubMed
21. Paterson B, Hirsch G, Andres K. Structural factors that promote stigmatization of drug users with hepatitis C in hospital emergency departments. Int J Drug Policy. 2013;24(5):471-478. http:// doi.org/10.1016/j.drugpo.2013.01.008 PubMed
22. Ross LE, Vigod S, Wishart J, et al. Barriers and facilitators to primary care for people with mental health and/or substance use issues: a qualitative study. BMC Fam Pract. 2015;16:135. http:// doi.org/10.1186/s12875-015-0353-3. PubMed
1. Rossen L, Bastian B, Warner M, Khan D, Chong Y. Drug poisoning mortality: United States, 1999-2015. 2017; https://www.cdc.gov/nchs/data-visualization/drug-poisoning-mortality/. Accessed 7-11, 2017.
2. Tedesco D, Asch SM, Curtin C, et al. Opioid abuse and poisoning: trends in inpatient and emergency department discharges. Health Aff (Millwood). 2017;36(10):1748-1753. http:// doi.org/10.1377/hlthaff.2017.0260. PubMed
3. Weiss AJ, Elixhauser A, Barrett ML, Steiner CA, Bailey MK, O’Malley L. Statistical Brief #219: Opioid-Related Inpatient Stays and Emergency Department Visits by State, 2009-2014. 2017; https://hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp?utm_source=AHRQ&utm_medium=EN-2&utm_term=&utm_content=2&utm_campaign=AHRQ_EN12_20_2016. Accessed July 11, 2017. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. http:// doi.org/10.1377/hlthaff.2015.1424. PubMed
5. Infectious Diseases Society of America Emerging Infections Network. Report for Query: ‘Injection Drug Use (IDU) and Infectious Disease Practice’. 2017; https://www.int-med.uiowa.edu/Research/EIN/FinalReport_IDUandID.pdf. Accessed July 11, 2017.
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. http:// doi.org/10.1016/j.amjmed.2015.09.024. PubMed
7. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an Experience, a Life Learning Experience”: A qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. http:// doi.org/10.1007/s11606-016-3919-4. PubMed
8. Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: Results from a survey of general internists. Subst Abus. 2016;37(4):635-641. http:// doi.org/10.1080/08897077.2016.1187240. PubMed
9. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. http:// doi.org/10.1016/j.drugalcdep.2013.02.018 PubMed
10. Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327-333. http:// doi.org/10.1046/j.1525-1497.2002.10625.x. PubMed
11. Brener L, Von Hippel W, Kippax S, Preacher KJ. The role of physician and nurse attitudes in the health care of injecting drug users. Subst Use Misuse. 2010;45(7-8):1007-1018. http:// doi.org/10.3109/10826081003659543. PubMed
12. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. http:// doi.org/10.1007/s11606-017-4077-z. PubMed
13. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. http:// doi.org/10.1007/s11606-014-2968-9. PubMed
14. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. http:// doi.org/10.1001/jamainternmed.2014.2556. PubMed
15. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. http:// doi.org/10.1007/s11606-010-1311-3. PubMed
16. Parmelli E, Flodgren G, Beyer F, Baillie N, Schaafsma ME, Eccles MP. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(1):33. http:// doi.org/10.1186/1748-5908-6-33. PubMed
17. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the improving addiction care team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. http:// doi.org/10.12788/jhm.2736. PubMed
18. Lambert SD, Loiselle CG. Combining individual interviews and focus groups to enhance data richness. J Adv Nurs. 2008;62(2):228-237. http:// doi.org/10.1111/j.1365-2648.2007.04559.x. PubMed
19. Braun VC, Victoria. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:25. http://dx.doi.org/10.1191/1478088706qp063oa.
20. Wakeman SE, Kanter GP, Donelan K. Institutional substance use disorder intervention improves general internist preparedness, attitudes, and clinical practice. J Addict Med. 2017;11(4):308-314. http:// doi.org/10.1097/ADM.0000000000000314. PubMed
21. Paterson B, Hirsch G, Andres K. Structural factors that promote stigmatization of drug users with hepatitis C in hospital emergency departments. Int J Drug Policy. 2013;24(5):471-478. http:// doi.org/10.1016/j.drugpo.2013.01.008 PubMed
22. Ross LE, Vigod S, Wishart J, et al. Barriers and facilitators to primary care for people with mental health and/or substance use issues: a qualitative study. BMC Fam Pract. 2015;16:135. http:// doi.org/10.1186/s12875-015-0353-3. PubMed
© 2018 Society of Hospital Medicine
Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder
Addiction is a national epidemic that represents both a pressing need and a significant burden to the healthcare system.1 Hospitals are increasingly filled with people admitted for medical complications of substance use disorders (SUD).2People with SUD have longer lengths of stay (LOS) and high readmission rates.3 Hospitalization often does not address the root cause—the SUD. For example, many hospitals replace heart valves and deliver prolonged courses of intravenous (IV) antibiotics for endocarditis from injection drug use but do not offer addiction medicine consultation, medication for addiction treatment (MAT), or linkage to posthospital SUD treatment.4,5
Hospitalization can provide reachable moments for initiating addiction care.6 Medications for opioid7 and alcohol use disorders8 can be started during hospitalization, promoting engagement in outpatient SUD care7 and increased uptake of MAT,7-9 and reducing readmissions.8,10 Yet, medications for SUD are underprescribed,11,12 and most hospitals lack inpatient addiction medicine services and pathways to timely SUD care after discharge. Furthermore, traditional SUD treatment programs are often not equipped to manage medically complex patients or they have long waitlists.13 Most behavioral-physical health integration occurs in ambulatory settings. This fails to engage patients who do not access primary care. There is an urgent need for models that can improve care for hospitalized patients with SUD.
Here, we describe our experience using patient needs assessment to engage stakeholders and drive local systems change. We also describe the resulting care model, the Improving Addiction Care Team (IMPACT). Our experience provides a potentially useful example to other hospitals and communities seeking to address the national SUD epidemic.
METHODS
Setting
In 2012, Oregon transformed its Medicaid system by establishing 16 regional “coordinated care organizations” (CCOs) to improve outcomes and slow healthcare spending.14 In a CCO environment, hospitals assume increased financial risk, yet reforms have focused on the outpatient setting. Therefore, executive leadership at Oregon Health & Science University (OHSU), an urban academic medical center, asked clinician-leaders to design point-of-care improvements for Medicaid-funded adults and build on existing models to improve care for socioeconomically vulnerable adults.15,16 One priority that emerged was to make improvements for hospitalized adults with SUD. Of the adult inpatients at OHSU, 30% have Medicaid and 15% have SUD by administrative data alone. Before we started our work, OHSU lacked inpatient addiction medicine services.
Local Needs Assessment
To understand local needs and opportunities, we surveyed hospitalized adults with SUD. We used the electronic health record to generate a list of inpatients flagged by nurses for risky alcohol or drug use. A research assistant screened consecutive adults (≥18 years old) and invited those who screened positive for alcohol use (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C])17 or drug use (single-item screener)18 to participate. We excluded non-English speakers, incarcerated adults, people using only marijuana or tobacco, psychiatry inpatients, and people unable to consent. Surveys assessed social and demographic factors, healthcare utilization, substance use severity, and treatment experience. Participants who reported high-risk illicit drug or alcohol use19 were asked to indicate their readiness to change on a 3-point scale developed for this study. Response range included: no interest, interest in cutting back, or interest in quitting. A subset of participants completed in-depth qualitative interviews exploring patient perceptions of substance use treatment needs.20 We obtained hospital administrative data from hospital financial services.
Partner Engagement
We identified community partners with which we had an individual or organizational relationship and a common interest and potential for collaboration. All invited partners agreed to attend initial meetings. We convened leadership and frontline staff across partners. OHSU staff included hospital nursing and social work leaders; infectious disease, hospitalist, and addiction physicians; and health services researchers. Community organizations included Central City Concern (CCC), a community organization serving people facing homelessness and addiction; CODA, Inc., a nonprofit SUD treatment agency; and Coram/CVS infusion pharmacy.
Collectively, we reviewed needs assessment findings and examples from the literature7-9 to develop strategies to address patient and system needs. We used patient narratives to foster alignment and prioritized areas in which integration could improve quality and costs. We assumed we would petition OHSU and/or Medicaid CCOs to finance efforts and saved potentially challenging budget discussions for later, when partnerships would be more developed. Our task force attended more than 3 large-group meetings and numerous small-group meetings to develop IMPACT.
RESULTS
Needs Assessment
Between September 2014 and April 2015, a research assistant approached 326 patients. Of these, 235 (72%) met study inclusion criteria, and 185 (78%) agreed to participate (Table 1). Of people who reported any substance use within the preceding 3 months, 58% of alcohol users and 67% of drug users said they were interested in cutting back or quitting. Fifty-four percent of participants with moderate- to high-risk opioid use and 16% with moderate- to high-risk alcohol use reported strong interest in MAT. In qualitative interviews, participants described inadequately treated withdrawal, the importance of trust and choice, and long wait times as a barriers to entering treatment after hospital discharge.20
Administrative data revealed high rates of hospital readmissions and longer than expected LOS (Figure). Mean LOS was 10.26 days—4 days more than medicine patients’. Mean LOS was high among participants who required long-term IV antibiotics, particularly those with endocarditis or osteomyelitis (21.75 days; range, 1.00-51.00 days). We excluded one outlier with a 116-day hospitalization.
Intervention Design
Mapping needs to intervention components. We mapped needs assessment findings to 3 main IMPACT components: inpatient addiction medicine consultation service, pathways to posthospital SUD treatment, and medically enhanced residential treatment (MERT) (Table 2).
Inpatient addiction medicine consultation service. We developed this service to address patients’ report of high readiness to change and interest in starting MAT in the hospital. Community partners highlighted the need for peers to increase engagement and trust. Therefore, we included a physician, a social worker, and two peers on our team. The inpatient service engages patients, advises on withdrawal and pain, performs SUD assessments, initiates MAT, and provides counseling and treatment.
Pathways to posthospital SUD treatment. As pathways from hospital to community SUD treatment were lacking, and long administrative wait times limited access to community treatment, we employed “in-reach” liaisons—community SUD treatment staff who perform in-hospital assessments to triage and coordinate care across systems. Given that patients value having treatment choices, we linked pathways to an array of MAT and abstinence-based treatments, including office-based, intensive outpatient and residential levels of care. For patients who live outside the Portland area, we developed relationships with rural stakeholders and engaged the help of the Oregon State Opioid Authority in introducing our program to SUD treatment providers around the state.
Medically Enhanced Residential Treatment (MERT). In many cases where patients required prolonged courses of IV antibiotics, hospital stays were longer for two reasons: At-home central-line self-administration of antibiotics was deemed unsafe, and patients were denied admission to a skilled nursing facility due to history of substance use. These long LOS create an opportunity to initiate and engage patients in treatment, and to render savings by shifting care to a residential addiction treatment setting that can accommodate IV antibiotic administration and MAT. We increased residential staffing and collaborated with a home infusion pharmacy to administer daily infusions on site.
Funding the Intervention
We used administrative data to estimate potential savings and tailored a business case to CCO and hospital payers. The CCO business case centered on hospitalization as an opportunity to engage out-of-treatment adults and potentially reduce high-cost readmissions by managing physical and behavioral health needs. Working within budgeting time lines, we used data from the first 165 participants. These participants had 137 readmissions over a mean observation period of 4.5 months. Mean charge per readmission was $31,157 (range, $699-$206,596) and was highest for people with endocarditis (mean, $55,493; range, $23,204-$145,066) and osteomyelitis (mean, $68,774; range, $29,359-$124,481). We estimated that a 10% reduction in 6-month readmissions could avoid $674,863 in charges.
For the hospital, the primary financial incentive was reduced LOS. Given the possibility of shortening hospitalization through MERT, we estimated a 20% mean LOS reduction; for budgeting, we estimated a conservative 10% reduction. A 10% mean LOS reduction would free 205 bed-days (10% × 10.26 days mean LOS × 200 patients) and create space for another 32 inpatient admissions in year 1, assuming no change from medical patients’ 6.26 days mean LOS. The future of bundled payments further bolstered our business case, as did the potential to improve care quality, reduce nonproductive staff time, and increase institutional learning about SUD. Overall program costs approximated projected savings, and the hospital and a local CCO agreed to equally share the costs of the intervention (Table 2).
DISCUSSION
We have described an innovative approach to developing an SUD intervention for hospitalized adults. Using a process of broad stakeholder engagement, data-driven understanding of population needs, and analysis of financial incentives, we built consensus and secured funding for a multicomponent intervention across hospital and post–acute care settings. Other studies have demonstrated the feasibility and efficacy of starting a single medication for a specific indication7-9 (eg, methadone for opioid use disorder), yet strategies for expanding SUD services in hospitals and facilitating posthospital treatment linkages remain scarce.21 Our model addresses a widespread need and could be adapted to other hospitals, SUD treatment organizations, and Medicaid payers.
Our experience has several limitations. First, it took place at a single academic medical center in Oregon, a Medicaid expansion state. Second, our needs assessment involved a convenience sample of limited racial/ethnic diversity. Third, almost all patients had insurance, which could limit generalizability. Fourth, to secure funding, it was essential we had a clinical champion who was persuasive with hospital and CCO leadership; though increasing disease burden and skyrocketing costs2 may drive administrators’ increased demand for ways to address SUD in hospitalized adults.
Our experience has several key implications. First, diverse partners were vital at all stages of program design, suggesting hospitals should look beyond traditional healthcare partners to address the SUD epidemic. Second, an interprofessional team that includes physicians, social workers, and peers may better engage patients and address complex system needs. Finally, a planned IMPACT evaluation will assess effects on substance use, healthcare use, and costs.
The United States faces a burgeoning SUD epidemic. Our experience describes an innovative care model and supports the idea that hospitals may play a leading role in convening partners, providing treatment, and driving population health improvements for adults with SUD.
Acknowledgment
The authors would like to acknowledge Peter Rapp and Thomas Yackel for leadership support; Tara Williams for administrative data support; Sarann Bielavitz and Naomi Wright for project management support, and Lynn Smith-Stott and Maria Michalczyk for help with model design. This work was presented at the American Society of Addiction Medicine national conference in Baltimore, MD in April 2016.
Disclosure
This work was funded by Oregon Health & Science University and CareOregon. The authors have no conflicts of interest to disclose.
1. Volkow N, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370:2063-2066. PubMed
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. PubMed
3. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. PubMed
4. Rosenthal ES, Karchmer AW, Thiesen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. PubMed
5. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;379(9):811-813. PubMed
6. Pollini RA, O’Toole TP, Ford D, Bigelow G. Does this patient really want treatment? Factors associated with baseline and evolving readiness for change among hospitalized substance using adults interested in treatment. Addict Behav. 2006;31(10):1904-1918. PubMed
7. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
8. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol and dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
9. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. PubMed
10. Pecoraro A, Horton T, Ewen E, et al. Early data from Project Engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract. 2012;7:20. PubMed
11. National Center on Addiction and Substance Abuse; Addiction Medicine: Closing the Gap between Science and Practice. June 2012. http://www.centeronaddiction.org/addiction-research/reports/addiction-medicine-closing-gap-between-science-and-practice. Accessed May 2, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. Results From the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Dept of Health and Human Services; 2011. NSDUH series H-41, HHS publication SMA 11-4658. https://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. Published September 2011. Accessed March 31, 2017.
13. Vestal C. Few doctors are willing, able to prescribe powerful anti-addiction drugs. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/01/15/few-doctors-are-willing-able-to-prescribe-powerful-anti-addiction-drugs. Published January 15, 2016. Accessed May 2, 2016.
14. McConnell KJ. Oregon’s Medicaid coordinated care organizations. JAMA. 2016;315(9):869-870. PubMed
15. Englander H, Kansagara D. Planning and designing the Care Transitions Innovation (C-TraIn) for uninsured and Medicaid patients. J Hosp Med. 2012;7(7):524-529. PubMed
16. Englander H, Michaels L, Chan B, Kansagara D. The Care Transitions Innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. PubMed
17. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. PubMed
18. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155-1160. PubMed
19. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039-1047. PubMed
20. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. PubMed
21. Gryczynski J, Schwartz RP, O’Grady KE, Restivo L, Mitchell SG, Jaffe JH. Understanding patterns of high-cost health care use across different substance user groups. Health Aff (Millwood). 2016.;35(1):12-19. PubMed
Addiction is a national epidemic that represents both a pressing need and a significant burden to the healthcare system.1 Hospitals are increasingly filled with people admitted for medical complications of substance use disorders (SUD).2People with SUD have longer lengths of stay (LOS) and high readmission rates.3 Hospitalization often does not address the root cause—the SUD. For example, many hospitals replace heart valves and deliver prolonged courses of intravenous (IV) antibiotics for endocarditis from injection drug use but do not offer addiction medicine consultation, medication for addiction treatment (MAT), or linkage to posthospital SUD treatment.4,5
Hospitalization can provide reachable moments for initiating addiction care.6 Medications for opioid7 and alcohol use disorders8 can be started during hospitalization, promoting engagement in outpatient SUD care7 and increased uptake of MAT,7-9 and reducing readmissions.8,10 Yet, medications for SUD are underprescribed,11,12 and most hospitals lack inpatient addiction medicine services and pathways to timely SUD care after discharge. Furthermore, traditional SUD treatment programs are often not equipped to manage medically complex patients or they have long waitlists.13 Most behavioral-physical health integration occurs in ambulatory settings. This fails to engage patients who do not access primary care. There is an urgent need for models that can improve care for hospitalized patients with SUD.
Here, we describe our experience using patient needs assessment to engage stakeholders and drive local systems change. We also describe the resulting care model, the Improving Addiction Care Team (IMPACT). Our experience provides a potentially useful example to other hospitals and communities seeking to address the national SUD epidemic.
METHODS
Setting
In 2012, Oregon transformed its Medicaid system by establishing 16 regional “coordinated care organizations” (CCOs) to improve outcomes and slow healthcare spending.14 In a CCO environment, hospitals assume increased financial risk, yet reforms have focused on the outpatient setting. Therefore, executive leadership at Oregon Health & Science University (OHSU), an urban academic medical center, asked clinician-leaders to design point-of-care improvements for Medicaid-funded adults and build on existing models to improve care for socioeconomically vulnerable adults.15,16 One priority that emerged was to make improvements for hospitalized adults with SUD. Of the adult inpatients at OHSU, 30% have Medicaid and 15% have SUD by administrative data alone. Before we started our work, OHSU lacked inpatient addiction medicine services.
Local Needs Assessment
To understand local needs and opportunities, we surveyed hospitalized adults with SUD. We used the electronic health record to generate a list of inpatients flagged by nurses for risky alcohol or drug use. A research assistant screened consecutive adults (≥18 years old) and invited those who screened positive for alcohol use (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C])17 or drug use (single-item screener)18 to participate. We excluded non-English speakers, incarcerated adults, people using only marijuana or tobacco, psychiatry inpatients, and people unable to consent. Surveys assessed social and demographic factors, healthcare utilization, substance use severity, and treatment experience. Participants who reported high-risk illicit drug or alcohol use19 were asked to indicate their readiness to change on a 3-point scale developed for this study. Response range included: no interest, interest in cutting back, or interest in quitting. A subset of participants completed in-depth qualitative interviews exploring patient perceptions of substance use treatment needs.20 We obtained hospital administrative data from hospital financial services.
Partner Engagement
We identified community partners with which we had an individual or organizational relationship and a common interest and potential for collaboration. All invited partners agreed to attend initial meetings. We convened leadership and frontline staff across partners. OHSU staff included hospital nursing and social work leaders; infectious disease, hospitalist, and addiction physicians; and health services researchers. Community organizations included Central City Concern (CCC), a community organization serving people facing homelessness and addiction; CODA, Inc., a nonprofit SUD treatment agency; and Coram/CVS infusion pharmacy.
Collectively, we reviewed needs assessment findings and examples from the literature7-9 to develop strategies to address patient and system needs. We used patient narratives to foster alignment and prioritized areas in which integration could improve quality and costs. We assumed we would petition OHSU and/or Medicaid CCOs to finance efforts and saved potentially challenging budget discussions for later, when partnerships would be more developed. Our task force attended more than 3 large-group meetings and numerous small-group meetings to develop IMPACT.
RESULTS
Needs Assessment
Between September 2014 and April 2015, a research assistant approached 326 patients. Of these, 235 (72%) met study inclusion criteria, and 185 (78%) agreed to participate (Table 1). Of people who reported any substance use within the preceding 3 months, 58% of alcohol users and 67% of drug users said they were interested in cutting back or quitting. Fifty-four percent of participants with moderate- to high-risk opioid use and 16% with moderate- to high-risk alcohol use reported strong interest in MAT. In qualitative interviews, participants described inadequately treated withdrawal, the importance of trust and choice, and long wait times as a barriers to entering treatment after hospital discharge.20
Administrative data revealed high rates of hospital readmissions and longer than expected LOS (Figure). Mean LOS was 10.26 days—4 days more than medicine patients’. Mean LOS was high among participants who required long-term IV antibiotics, particularly those with endocarditis or osteomyelitis (21.75 days; range, 1.00-51.00 days). We excluded one outlier with a 116-day hospitalization.
Intervention Design
Mapping needs to intervention components. We mapped needs assessment findings to 3 main IMPACT components: inpatient addiction medicine consultation service, pathways to posthospital SUD treatment, and medically enhanced residential treatment (MERT) (Table 2).
Inpatient addiction medicine consultation service. We developed this service to address patients’ report of high readiness to change and interest in starting MAT in the hospital. Community partners highlighted the need for peers to increase engagement and trust. Therefore, we included a physician, a social worker, and two peers on our team. The inpatient service engages patients, advises on withdrawal and pain, performs SUD assessments, initiates MAT, and provides counseling and treatment.
Pathways to posthospital SUD treatment. As pathways from hospital to community SUD treatment were lacking, and long administrative wait times limited access to community treatment, we employed “in-reach” liaisons—community SUD treatment staff who perform in-hospital assessments to triage and coordinate care across systems. Given that patients value having treatment choices, we linked pathways to an array of MAT and abstinence-based treatments, including office-based, intensive outpatient and residential levels of care. For patients who live outside the Portland area, we developed relationships with rural stakeholders and engaged the help of the Oregon State Opioid Authority in introducing our program to SUD treatment providers around the state.
Medically Enhanced Residential Treatment (MERT). In many cases where patients required prolonged courses of IV antibiotics, hospital stays were longer for two reasons: At-home central-line self-administration of antibiotics was deemed unsafe, and patients were denied admission to a skilled nursing facility due to history of substance use. These long LOS create an opportunity to initiate and engage patients in treatment, and to render savings by shifting care to a residential addiction treatment setting that can accommodate IV antibiotic administration and MAT. We increased residential staffing and collaborated with a home infusion pharmacy to administer daily infusions on site.
Funding the Intervention
We used administrative data to estimate potential savings and tailored a business case to CCO and hospital payers. The CCO business case centered on hospitalization as an opportunity to engage out-of-treatment adults and potentially reduce high-cost readmissions by managing physical and behavioral health needs. Working within budgeting time lines, we used data from the first 165 participants. These participants had 137 readmissions over a mean observation period of 4.5 months. Mean charge per readmission was $31,157 (range, $699-$206,596) and was highest for people with endocarditis (mean, $55,493; range, $23,204-$145,066) and osteomyelitis (mean, $68,774; range, $29,359-$124,481). We estimated that a 10% reduction in 6-month readmissions could avoid $674,863 in charges.
For the hospital, the primary financial incentive was reduced LOS. Given the possibility of shortening hospitalization through MERT, we estimated a 20% mean LOS reduction; for budgeting, we estimated a conservative 10% reduction. A 10% mean LOS reduction would free 205 bed-days (10% × 10.26 days mean LOS × 200 patients) and create space for another 32 inpatient admissions in year 1, assuming no change from medical patients’ 6.26 days mean LOS. The future of bundled payments further bolstered our business case, as did the potential to improve care quality, reduce nonproductive staff time, and increase institutional learning about SUD. Overall program costs approximated projected savings, and the hospital and a local CCO agreed to equally share the costs of the intervention (Table 2).
DISCUSSION
We have described an innovative approach to developing an SUD intervention for hospitalized adults. Using a process of broad stakeholder engagement, data-driven understanding of population needs, and analysis of financial incentives, we built consensus and secured funding for a multicomponent intervention across hospital and post–acute care settings. Other studies have demonstrated the feasibility and efficacy of starting a single medication for a specific indication7-9 (eg, methadone for opioid use disorder), yet strategies for expanding SUD services in hospitals and facilitating posthospital treatment linkages remain scarce.21 Our model addresses a widespread need and could be adapted to other hospitals, SUD treatment organizations, and Medicaid payers.
Our experience has several limitations. First, it took place at a single academic medical center in Oregon, a Medicaid expansion state. Second, our needs assessment involved a convenience sample of limited racial/ethnic diversity. Third, almost all patients had insurance, which could limit generalizability. Fourth, to secure funding, it was essential we had a clinical champion who was persuasive with hospital and CCO leadership; though increasing disease burden and skyrocketing costs2 may drive administrators’ increased demand for ways to address SUD in hospitalized adults.
Our experience has several key implications. First, diverse partners were vital at all stages of program design, suggesting hospitals should look beyond traditional healthcare partners to address the SUD epidemic. Second, an interprofessional team that includes physicians, social workers, and peers may better engage patients and address complex system needs. Finally, a planned IMPACT evaluation will assess effects on substance use, healthcare use, and costs.
The United States faces a burgeoning SUD epidemic. Our experience describes an innovative care model and supports the idea that hospitals may play a leading role in convening partners, providing treatment, and driving population health improvements for adults with SUD.
Acknowledgment
The authors would like to acknowledge Peter Rapp and Thomas Yackel for leadership support; Tara Williams for administrative data support; Sarann Bielavitz and Naomi Wright for project management support, and Lynn Smith-Stott and Maria Michalczyk for help with model design. This work was presented at the American Society of Addiction Medicine national conference in Baltimore, MD in April 2016.
Disclosure
This work was funded by Oregon Health & Science University and CareOregon. The authors have no conflicts of interest to disclose.
Addiction is a national epidemic that represents both a pressing need and a significant burden to the healthcare system.1 Hospitals are increasingly filled with people admitted for medical complications of substance use disorders (SUD).2People with SUD have longer lengths of stay (LOS) and high readmission rates.3 Hospitalization often does not address the root cause—the SUD. For example, many hospitals replace heart valves and deliver prolonged courses of intravenous (IV) antibiotics for endocarditis from injection drug use but do not offer addiction medicine consultation, medication for addiction treatment (MAT), or linkage to posthospital SUD treatment.4,5
Hospitalization can provide reachable moments for initiating addiction care.6 Medications for opioid7 and alcohol use disorders8 can be started during hospitalization, promoting engagement in outpatient SUD care7 and increased uptake of MAT,7-9 and reducing readmissions.8,10 Yet, medications for SUD are underprescribed,11,12 and most hospitals lack inpatient addiction medicine services and pathways to timely SUD care after discharge. Furthermore, traditional SUD treatment programs are often not equipped to manage medically complex patients or they have long waitlists.13 Most behavioral-physical health integration occurs in ambulatory settings. This fails to engage patients who do not access primary care. There is an urgent need for models that can improve care for hospitalized patients with SUD.
Here, we describe our experience using patient needs assessment to engage stakeholders and drive local systems change. We also describe the resulting care model, the Improving Addiction Care Team (IMPACT). Our experience provides a potentially useful example to other hospitals and communities seeking to address the national SUD epidemic.
METHODS
Setting
In 2012, Oregon transformed its Medicaid system by establishing 16 regional “coordinated care organizations” (CCOs) to improve outcomes and slow healthcare spending.14 In a CCO environment, hospitals assume increased financial risk, yet reforms have focused on the outpatient setting. Therefore, executive leadership at Oregon Health & Science University (OHSU), an urban academic medical center, asked clinician-leaders to design point-of-care improvements for Medicaid-funded adults and build on existing models to improve care for socioeconomically vulnerable adults.15,16 One priority that emerged was to make improvements for hospitalized adults with SUD. Of the adult inpatients at OHSU, 30% have Medicaid and 15% have SUD by administrative data alone. Before we started our work, OHSU lacked inpatient addiction medicine services.
Local Needs Assessment
To understand local needs and opportunities, we surveyed hospitalized adults with SUD. We used the electronic health record to generate a list of inpatients flagged by nurses for risky alcohol or drug use. A research assistant screened consecutive adults (≥18 years old) and invited those who screened positive for alcohol use (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C])17 or drug use (single-item screener)18 to participate. We excluded non-English speakers, incarcerated adults, people using only marijuana or tobacco, psychiatry inpatients, and people unable to consent. Surveys assessed social and demographic factors, healthcare utilization, substance use severity, and treatment experience. Participants who reported high-risk illicit drug or alcohol use19 were asked to indicate their readiness to change on a 3-point scale developed for this study. Response range included: no interest, interest in cutting back, or interest in quitting. A subset of participants completed in-depth qualitative interviews exploring patient perceptions of substance use treatment needs.20 We obtained hospital administrative data from hospital financial services.
Partner Engagement
We identified community partners with which we had an individual or organizational relationship and a common interest and potential for collaboration. All invited partners agreed to attend initial meetings. We convened leadership and frontline staff across partners. OHSU staff included hospital nursing and social work leaders; infectious disease, hospitalist, and addiction physicians; and health services researchers. Community organizations included Central City Concern (CCC), a community organization serving people facing homelessness and addiction; CODA, Inc., a nonprofit SUD treatment agency; and Coram/CVS infusion pharmacy.
Collectively, we reviewed needs assessment findings and examples from the literature7-9 to develop strategies to address patient and system needs. We used patient narratives to foster alignment and prioritized areas in which integration could improve quality and costs. We assumed we would petition OHSU and/or Medicaid CCOs to finance efforts and saved potentially challenging budget discussions for later, when partnerships would be more developed. Our task force attended more than 3 large-group meetings and numerous small-group meetings to develop IMPACT.
RESULTS
Needs Assessment
Between September 2014 and April 2015, a research assistant approached 326 patients. Of these, 235 (72%) met study inclusion criteria, and 185 (78%) agreed to participate (Table 1). Of people who reported any substance use within the preceding 3 months, 58% of alcohol users and 67% of drug users said they were interested in cutting back or quitting. Fifty-four percent of participants with moderate- to high-risk opioid use and 16% with moderate- to high-risk alcohol use reported strong interest in MAT. In qualitative interviews, participants described inadequately treated withdrawal, the importance of trust and choice, and long wait times as a barriers to entering treatment after hospital discharge.20
Administrative data revealed high rates of hospital readmissions and longer than expected LOS (Figure). Mean LOS was 10.26 days—4 days more than medicine patients’. Mean LOS was high among participants who required long-term IV antibiotics, particularly those with endocarditis or osteomyelitis (21.75 days; range, 1.00-51.00 days). We excluded one outlier with a 116-day hospitalization.
Intervention Design
Mapping needs to intervention components. We mapped needs assessment findings to 3 main IMPACT components: inpatient addiction medicine consultation service, pathways to posthospital SUD treatment, and medically enhanced residential treatment (MERT) (Table 2).
Inpatient addiction medicine consultation service. We developed this service to address patients’ report of high readiness to change and interest in starting MAT in the hospital. Community partners highlighted the need for peers to increase engagement and trust. Therefore, we included a physician, a social worker, and two peers on our team. The inpatient service engages patients, advises on withdrawal and pain, performs SUD assessments, initiates MAT, and provides counseling and treatment.
Pathways to posthospital SUD treatment. As pathways from hospital to community SUD treatment were lacking, and long administrative wait times limited access to community treatment, we employed “in-reach” liaisons—community SUD treatment staff who perform in-hospital assessments to triage and coordinate care across systems. Given that patients value having treatment choices, we linked pathways to an array of MAT and abstinence-based treatments, including office-based, intensive outpatient and residential levels of care. For patients who live outside the Portland area, we developed relationships with rural stakeholders and engaged the help of the Oregon State Opioid Authority in introducing our program to SUD treatment providers around the state.
Medically Enhanced Residential Treatment (MERT). In many cases where patients required prolonged courses of IV antibiotics, hospital stays were longer for two reasons: At-home central-line self-administration of antibiotics was deemed unsafe, and patients were denied admission to a skilled nursing facility due to history of substance use. These long LOS create an opportunity to initiate and engage patients in treatment, and to render savings by shifting care to a residential addiction treatment setting that can accommodate IV antibiotic administration and MAT. We increased residential staffing and collaborated with a home infusion pharmacy to administer daily infusions on site.
Funding the Intervention
We used administrative data to estimate potential savings and tailored a business case to CCO and hospital payers. The CCO business case centered on hospitalization as an opportunity to engage out-of-treatment adults and potentially reduce high-cost readmissions by managing physical and behavioral health needs. Working within budgeting time lines, we used data from the first 165 participants. These participants had 137 readmissions over a mean observation period of 4.5 months. Mean charge per readmission was $31,157 (range, $699-$206,596) and was highest for people with endocarditis (mean, $55,493; range, $23,204-$145,066) and osteomyelitis (mean, $68,774; range, $29,359-$124,481). We estimated that a 10% reduction in 6-month readmissions could avoid $674,863 in charges.
For the hospital, the primary financial incentive was reduced LOS. Given the possibility of shortening hospitalization through MERT, we estimated a 20% mean LOS reduction; for budgeting, we estimated a conservative 10% reduction. A 10% mean LOS reduction would free 205 bed-days (10% × 10.26 days mean LOS × 200 patients) and create space for another 32 inpatient admissions in year 1, assuming no change from medical patients’ 6.26 days mean LOS. The future of bundled payments further bolstered our business case, as did the potential to improve care quality, reduce nonproductive staff time, and increase institutional learning about SUD. Overall program costs approximated projected savings, and the hospital and a local CCO agreed to equally share the costs of the intervention (Table 2).
DISCUSSION
We have described an innovative approach to developing an SUD intervention for hospitalized adults. Using a process of broad stakeholder engagement, data-driven understanding of population needs, and analysis of financial incentives, we built consensus and secured funding for a multicomponent intervention across hospital and post–acute care settings. Other studies have demonstrated the feasibility and efficacy of starting a single medication for a specific indication7-9 (eg, methadone for opioid use disorder), yet strategies for expanding SUD services in hospitals and facilitating posthospital treatment linkages remain scarce.21 Our model addresses a widespread need and could be adapted to other hospitals, SUD treatment organizations, and Medicaid payers.
Our experience has several limitations. First, it took place at a single academic medical center in Oregon, a Medicaid expansion state. Second, our needs assessment involved a convenience sample of limited racial/ethnic diversity. Third, almost all patients had insurance, which could limit generalizability. Fourth, to secure funding, it was essential we had a clinical champion who was persuasive with hospital and CCO leadership; though increasing disease burden and skyrocketing costs2 may drive administrators’ increased demand for ways to address SUD in hospitalized adults.
Our experience has several key implications. First, diverse partners were vital at all stages of program design, suggesting hospitals should look beyond traditional healthcare partners to address the SUD epidemic. Second, an interprofessional team that includes physicians, social workers, and peers may better engage patients and address complex system needs. Finally, a planned IMPACT evaluation will assess effects on substance use, healthcare use, and costs.
The United States faces a burgeoning SUD epidemic. Our experience describes an innovative care model and supports the idea that hospitals may play a leading role in convening partners, providing treatment, and driving population health improvements for adults with SUD.
Acknowledgment
The authors would like to acknowledge Peter Rapp and Thomas Yackel for leadership support; Tara Williams for administrative data support; Sarann Bielavitz and Naomi Wright for project management support, and Lynn Smith-Stott and Maria Michalczyk for help with model design. This work was presented at the American Society of Addiction Medicine national conference in Baltimore, MD in April 2016.
Disclosure
This work was funded by Oregon Health & Science University and CareOregon. The authors have no conflicts of interest to disclose.
1. Volkow N, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370:2063-2066. PubMed
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. PubMed
3. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. PubMed
4. Rosenthal ES, Karchmer AW, Thiesen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. PubMed
5. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;379(9):811-813. PubMed
6. Pollini RA, O’Toole TP, Ford D, Bigelow G. Does this patient really want treatment? Factors associated with baseline and evolving readiness for change among hospitalized substance using adults interested in treatment. Addict Behav. 2006;31(10):1904-1918. PubMed
7. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
8. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol and dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
9. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. PubMed
10. Pecoraro A, Horton T, Ewen E, et al. Early data from Project Engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract. 2012;7:20. PubMed
11. National Center on Addiction and Substance Abuse; Addiction Medicine: Closing the Gap between Science and Practice. June 2012. http://www.centeronaddiction.org/addiction-research/reports/addiction-medicine-closing-gap-between-science-and-practice. Accessed May 2, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. Results From the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Dept of Health and Human Services; 2011. NSDUH series H-41, HHS publication SMA 11-4658. https://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. Published September 2011. Accessed March 31, 2017.
13. Vestal C. Few doctors are willing, able to prescribe powerful anti-addiction drugs. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/01/15/few-doctors-are-willing-able-to-prescribe-powerful-anti-addiction-drugs. Published January 15, 2016. Accessed May 2, 2016.
14. McConnell KJ. Oregon’s Medicaid coordinated care organizations. JAMA. 2016;315(9):869-870. PubMed
15. Englander H, Kansagara D. Planning and designing the Care Transitions Innovation (C-TraIn) for uninsured and Medicaid patients. J Hosp Med. 2012;7(7):524-529. PubMed
16. Englander H, Michaels L, Chan B, Kansagara D. The Care Transitions Innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. PubMed
17. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. PubMed
18. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155-1160. PubMed
19. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039-1047. PubMed
20. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. PubMed
21. Gryczynski J, Schwartz RP, O’Grady KE, Restivo L, Mitchell SG, Jaffe JH. Understanding patterns of high-cost health care use across different substance user groups. Health Aff (Millwood). 2016.;35(1):12-19. PubMed
1. Volkow N, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370:2063-2066. PubMed
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. PubMed
3. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. PubMed
4. Rosenthal ES, Karchmer AW, Thiesen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. PubMed
5. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;379(9):811-813. PubMed
6. Pollini RA, O’Toole TP, Ford D, Bigelow G. Does this patient really want treatment? Factors associated with baseline and evolving readiness for change among hospitalized substance using adults interested in treatment. Addict Behav. 2006;31(10):1904-1918. PubMed
7. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
8. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol and dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
9. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. PubMed
10. Pecoraro A, Horton T, Ewen E, et al. Early data from Project Engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract. 2012;7:20. PubMed
11. National Center on Addiction and Substance Abuse; Addiction Medicine: Closing the Gap between Science and Practice. June 2012. http://www.centeronaddiction.org/addiction-research/reports/addiction-medicine-closing-gap-between-science-and-practice. Accessed May 2, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. Results From the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Dept of Health and Human Services; 2011. NSDUH series H-41, HHS publication SMA 11-4658. https://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. Published September 2011. Accessed March 31, 2017.
13. Vestal C. Few doctors are willing, able to prescribe powerful anti-addiction drugs. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/01/15/few-doctors-are-willing-able-to-prescribe-powerful-anti-addiction-drugs. Published January 15, 2016. Accessed May 2, 2016.
14. McConnell KJ. Oregon’s Medicaid coordinated care organizations. JAMA. 2016;315(9):869-870. PubMed
15. Englander H, Kansagara D. Planning and designing the Care Transitions Innovation (C-TraIn) for uninsured and Medicaid patients. J Hosp Med. 2012;7(7):524-529. PubMed
16. Englander H, Michaels L, Chan B, Kansagara D. The Care Transitions Innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. PubMed
17. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. PubMed
18. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155-1160. PubMed
19. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039-1047. PubMed
20. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. PubMed
21. Gryczynski J, Schwartz RP, O’Grady KE, Restivo L, Mitchell SG, Jaffe JH. Understanding patterns of high-cost health care use across different substance user groups. Health Aff (Millwood). 2016.;35(1):12-19. PubMed
© 2017 Society of Hospital Medicine
Care Transitions from Hospital to Home
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.