User login
What Markers Are Helpful to Diagnose Infection in Tocilizumab Users?
TOPLINE:
Eosinopenia and low ratio between eosinophil count (EC) and neutrophil count (NC) are potential indicators of infection for patients with inflammatory disease who are treated with tocilizumab.
METHODOLOGY:
- The researchers reviewed data from 163 patients treated for an inflammatory disease (mostly rheumatoid arthritis) with tocilizumab at a single center between 2009 and 2020.
- The study population included 41 patients with unscheduled hospitalizations for suspected infections. Patients’ median age was 59 years, and 83% were female.
- The researchers assessed the association in tocilizumab-treated patients between infections and eosinopenia (defined as EC < 0.05 g/L) and a low ratio between EC and NC, defined as EC/NC × 1000 < 11.8.
TAKEAWAY:
- Infectious diseases were diagnosed in 20 of the hospitalized patients (49%); the most common diseases were pneumonia (30%), joint or bone infections (25%), and gastrointestinal tract infections (15%).
- The median absolute EC at hospital admission was significantly lower for patients with infections than for those without infections (0.06 g/L vs 0.20 g/L).
- The median EC/NC × 1000 ratios were significantly lower in infected patients vs noninfected patients (6.54 vs 48.50).
- No differences appeared between patients with and without infections in age, sex, type of inflammatory disease, and steroid treatment.
IN PRACTICE:
“This original study suggests that all those easily available parameters should be used to maximize [sensitivity] in the screening of infection in patients undergoing treatment with IL-6 pathway antagonists,” the researchers wrote.
SOURCE:
The lead author on the study was Audrey Glatre, MD, of University Hospital Centre Reims, France. The study was published online in RMD Open on February 9.
LIMITATIONS:
The retrospective, observational design; relatively small study population; and use of data from a single center were potential limitations of the findings.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Eosinopenia and low ratio between eosinophil count (EC) and neutrophil count (NC) are potential indicators of infection for patients with inflammatory disease who are treated with tocilizumab.
METHODOLOGY:
- The researchers reviewed data from 163 patients treated for an inflammatory disease (mostly rheumatoid arthritis) with tocilizumab at a single center between 2009 and 2020.
- The study population included 41 patients with unscheduled hospitalizations for suspected infections. Patients’ median age was 59 years, and 83% were female.
- The researchers assessed the association in tocilizumab-treated patients between infections and eosinopenia (defined as EC < 0.05 g/L) and a low ratio between EC and NC, defined as EC/NC × 1000 < 11.8.
TAKEAWAY:
- Infectious diseases were diagnosed in 20 of the hospitalized patients (49%); the most common diseases were pneumonia (30%), joint or bone infections (25%), and gastrointestinal tract infections (15%).
- The median absolute EC at hospital admission was significantly lower for patients with infections than for those without infections (0.06 g/L vs 0.20 g/L).
- The median EC/NC × 1000 ratios were significantly lower in infected patients vs noninfected patients (6.54 vs 48.50).
- No differences appeared between patients with and without infections in age, sex, type of inflammatory disease, and steroid treatment.
IN PRACTICE:
“This original study suggests that all those easily available parameters should be used to maximize [sensitivity] in the screening of infection in patients undergoing treatment with IL-6 pathway antagonists,” the researchers wrote.
SOURCE:
The lead author on the study was Audrey Glatre, MD, of University Hospital Centre Reims, France. The study was published online in RMD Open on February 9.
LIMITATIONS:
The retrospective, observational design; relatively small study population; and use of data from a single center were potential limitations of the findings.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Eosinopenia and low ratio between eosinophil count (EC) and neutrophil count (NC) are potential indicators of infection for patients with inflammatory disease who are treated with tocilizumab.
METHODOLOGY:
- The researchers reviewed data from 163 patients treated for an inflammatory disease (mostly rheumatoid arthritis) with tocilizumab at a single center between 2009 and 2020.
- The study population included 41 patients with unscheduled hospitalizations for suspected infections. Patients’ median age was 59 years, and 83% were female.
- The researchers assessed the association in tocilizumab-treated patients between infections and eosinopenia (defined as EC < 0.05 g/L) and a low ratio between EC and NC, defined as EC/NC × 1000 < 11.8.
TAKEAWAY:
- Infectious diseases were diagnosed in 20 of the hospitalized patients (49%); the most common diseases were pneumonia (30%), joint or bone infections (25%), and gastrointestinal tract infections (15%).
- The median absolute EC at hospital admission was significantly lower for patients with infections than for those without infections (0.06 g/L vs 0.20 g/L).
- The median EC/NC × 1000 ratios were significantly lower in infected patients vs noninfected patients (6.54 vs 48.50).
- No differences appeared between patients with and without infections in age, sex, type of inflammatory disease, and steroid treatment.
IN PRACTICE:
“This original study suggests that all those easily available parameters should be used to maximize [sensitivity] in the screening of infection in patients undergoing treatment with IL-6 pathway antagonists,” the researchers wrote.
SOURCE:
The lead author on the study was Audrey Glatre, MD, of University Hospital Centre Reims, France. The study was published online in RMD Open on February 9.
LIMITATIONS:
The retrospective, observational design; relatively small study population; and use of data from a single center were potential limitations of the findings.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Autoimmune Disease Risk May Rise Following Cushing Disease Remission After Surgery
Patients with Cushing disease have an increased risk for new-onset autoimmune disease in the 3 years after surgical remission, according to a new retrospective study published on February 20 in Annals of Internal Medicine.
Outcomes for patients with Cushing disease were compared against those with nonfunctioning pituitary adenomas (NFPAs). New-onset autoimmune disease occurred in 10.4% with Cushing disease and 1.6% among patients with NFPA (hazard ratio, 7.80; 95% CI, 2.88-21.10).
“Understanding and recognizing new and recurrent autoimmune disease in this setting is important to avoid misclassifying such patients with glucocorticoid withdrawal syndrome, which could result in failure to treat underlying autoimmune disease, as well as erroneous diagnosis of steroid withdrawal cases,” wrote Dennis Delasi Nyanyo of Massachusetts General Hospital and Harvard Medical School, Boston, and colleagues.
Given the general population’s annual incidence of major autoimmune diseases, estimated at about 100 cases per 100,000 people, and the 3-year incidence of 10.4% found in this study’s cohort, “our findings suggest that Cushing disease remission may trigger development of autoimmune disease,” the authors wrote.
Monitor Patients With Family History of Autoimmune Disease?
The study results were not necessarily surprising to Anthony P. Heaney, MD, PhD, an endocrinologist and professor of medicine at the University of California, Los Angeles, because past research has raised similar questions. The authors’ suggestion that the rapid postsurgical drop in cortisol that occurs as a result of treating Cushing disease becomes some sort of autoimmune trigger is interesting but remains speculative, Dr. Heaney pointed out.
If future evidence supports that possibility, “it would suggest, in terms of managing those patients in the postoperative setting, that there may be some merit to giving them higher concentrations of glucocorticoids for a short period of time,” Dr. Heaney said, thereby bringing their levels down more gradually rather than taking them off a cliff, in a sense. Or, if more evidence bears out the authors’ hypothesis, another approach might be treating patients with medicine to bring down the cortisol before surgery, though there are challenges to that approach, Dr. Heaney said.
At the same time, those who developed new autoimmune disease remain a small subset of patients with Cushing disease, so such approaches may become only potentially appropriate to consider in patients with risk factors, such as a family history of autoimmune disease.
The researchers conducted a retrospective chart review of adult patients who underwent transsphenoidal surgery for either Cushing disease or NFPA at Massachusetts General Hospital between 2005 and 2019.
The study involved 194 patients with Cushing disease who had postsurgical remission and at least one follow-up visit with a pituitary expert and 92 patients with NFPA who were matched to patients with Cushing disease based on age and sex. The authors regarded autoimmune disease diagnosed within 36 months of the surgery to be temporally associated with Cushing disease remission. Among the autoimmune diseases considered were “rheumatoid arthritis, Sjögren syndrome, systemic lupus erythematosus, autoimmune thyroiditis, celiac disease, psoriasis, vitiligo, autoimmune neuropathy, multiple sclerosis, myasthenia gravis, and ulcerative colitis.”
Patients differed in average body mass index and tumor size, but family history of autoimmune disease was similar in both groups. Average BMI was 34.5 in the Cushing group and 29.5 in the NFPA group. Average tumor size was 5.7 mm in the Cushing group and 21.3 mm in the NFPA group.
Before surgery, 2.9% of patients with Cushing disease and 15.4% of patients with NFPA had central hypothyroidism, and 8% in the Cushing group and 56.8% in the NFPA group had hyperprolactinemia. Central adrenal insufficiency occurred in 11% with NFPA and in all with Cushing disease, by definition.
After surgery, 93.8% in the Cushing group and 16.5% in the NFPA group had adrenal insufficiency. In addition, patients with Cushing disease had lower postsurgical nadir serum cortisol levels (63.8 nmol/L) than those with NFPA (282.3 nmol/L).
Of the 17 patients with Cushing disease — all women — who developed autoimmune disease within 3 years, 6 had a personal history of autoimmune disease and 7 had a family history of it. In addition, 41.2% of them had adrenal insufficiency when they developed the new autoimmune disease. Among the diseases were six autoimmune thyroiditis cases, three Sjögren syndrome cases, and two autoimmune seronegative spondyloarthropathy.
Dr. Heaney said he found it interesting that more than half of the new autoimmune diseases in patients with Cushing disease were related to the thyroid. “In this kind of setting, where you have a patient who has been producing too much steroid over a period of time and then you take that away, it’s almost like you release a brake on the TSH [thyroid-stimulating hormone],” Dr. Heaney said. “So, there’s probably some rebound in TSH that occurs, and that could be driving the thyroiditis, to some extent, that we see in these patients.”
Only one patient with NFPA developed new-onset autoimmune disease, a woman who developed Graves disease 22 months after surgery. When the researchers excluded patients in both groups with central hypothyroidism, new-onset autoimmune disease was still significantly higher (11.4%) in the Cushing group than in the NFPA group (1.9%; HR, 7.02; 95% CI, 2.54-19.39).
Could Postoperative Adrenal Insufficiency Contribute to Risk?
Within the Cushing cohort, those who developed autoimmune disease had a lower BMI (31.8 vs 34.8) and larger tumor size (7.2 vs 5.6 mm) than those who didn’t develop new autoimmune disease. Patients who developed autoimmune disease also had a lower baseline urine free cortisol ratio (2.7 vs 6.3) before surgery and more family history of autoimmune disease (41.2% vs 20.9%) than those who didn’t develop one.
“The higher prevalence of adrenal insufficiency and the lower nadir serum cortisol levels in the Cushing disease group suggest that the postoperative adrenal insufficiency in the Cushing disease group might have contributed to autoimmune disease pathogenesis,” the authors wrote. “This finding is clinically significant because cortisol plays a pivotal role in modulating the immune system.”
Most postoperative management among patients with Cushing disease was similar, with all but one patient receiving 0.5 or 1 mg daily dexamethasone within the first week after surgery. (The one outlier received 5 mg daily prednisone.) However, fewer patients who developed autoimmune disease (17.6%) received supraphysiologic doses of glucocorticoid — equivalent to at least 25 mg hydrocortisone — compared with patients who didn’t develop autoimmune disease (41.8%).
“Although the daily average hydrocortisone equivalent replacement doses within the first month and during long-term follow-up were within the physiologic range in both subgroups, patients with Cushing disease who had autoimmune disease received slightly lower doses of glucocorticoid replacement within the first month after surgery,” the authors reported. “The immediate postoperative period might be a critical window where supraphysiologic glucocorticoids seem to be protective with regard to development of autoimmune disease,” they wrote, though they acknowledged the study’s retrospective design as a limitation in drawing that conclusion.
At the least, they suggested that new symptoms in patients with Cushing disease, particularly those with a family history of autoimmune disease, should prompt investigation of potential autoimmune disease.
Recordati Rare Diseases funded the study. The research was also conducted with support from Harvard Catalyst (the Harvard Clinical and Translational Science Center) as well as financial contributions from Harvard University and its affiliated academic healthcare centers. One author reported holding stocks in Pfizer and Amgen, and another reported receiving consulting fees from Corcept. Dr. Heaney reported receiving institutional grants for trials from Corcept, Ascendis, Crinetics, and Sparrow Pharm; serving on the advisory board for Xeris, Recordati, Corcept, Novo Nordisk, Lundbeck, and Crinetics; and serving as a speaker for Chiesi, Novo Nordisk, and Corcept.
A version of this article appeared on Medscape.com.
Patients with Cushing disease have an increased risk for new-onset autoimmune disease in the 3 years after surgical remission, according to a new retrospective study published on February 20 in Annals of Internal Medicine.
Outcomes for patients with Cushing disease were compared against those with nonfunctioning pituitary adenomas (NFPAs). New-onset autoimmune disease occurred in 10.4% with Cushing disease and 1.6% among patients with NFPA (hazard ratio, 7.80; 95% CI, 2.88-21.10).
“Understanding and recognizing new and recurrent autoimmune disease in this setting is important to avoid misclassifying such patients with glucocorticoid withdrawal syndrome, which could result in failure to treat underlying autoimmune disease, as well as erroneous diagnosis of steroid withdrawal cases,” wrote Dennis Delasi Nyanyo of Massachusetts General Hospital and Harvard Medical School, Boston, and colleagues.
Given the general population’s annual incidence of major autoimmune diseases, estimated at about 100 cases per 100,000 people, and the 3-year incidence of 10.4% found in this study’s cohort, “our findings suggest that Cushing disease remission may trigger development of autoimmune disease,” the authors wrote.
Monitor Patients With Family History of Autoimmune Disease?
The study results were not necessarily surprising to Anthony P. Heaney, MD, PhD, an endocrinologist and professor of medicine at the University of California, Los Angeles, because past research has raised similar questions. The authors’ suggestion that the rapid postsurgical drop in cortisol that occurs as a result of treating Cushing disease becomes some sort of autoimmune trigger is interesting but remains speculative, Dr. Heaney pointed out.
If future evidence supports that possibility, “it would suggest, in terms of managing those patients in the postoperative setting, that there may be some merit to giving them higher concentrations of glucocorticoids for a short period of time,” Dr. Heaney said, thereby bringing their levels down more gradually rather than taking them off a cliff, in a sense. Or, if more evidence bears out the authors’ hypothesis, another approach might be treating patients with medicine to bring down the cortisol before surgery, though there are challenges to that approach, Dr. Heaney said.
At the same time, those who developed new autoimmune disease remain a small subset of patients with Cushing disease, so such approaches may become only potentially appropriate to consider in patients with risk factors, such as a family history of autoimmune disease.
The researchers conducted a retrospective chart review of adult patients who underwent transsphenoidal surgery for either Cushing disease or NFPA at Massachusetts General Hospital between 2005 and 2019.
The study involved 194 patients with Cushing disease who had postsurgical remission and at least one follow-up visit with a pituitary expert and 92 patients with NFPA who were matched to patients with Cushing disease based on age and sex. The authors regarded autoimmune disease diagnosed within 36 months of the surgery to be temporally associated with Cushing disease remission. Among the autoimmune diseases considered were “rheumatoid arthritis, Sjögren syndrome, systemic lupus erythematosus, autoimmune thyroiditis, celiac disease, psoriasis, vitiligo, autoimmune neuropathy, multiple sclerosis, myasthenia gravis, and ulcerative colitis.”
Patients differed in average body mass index and tumor size, but family history of autoimmune disease was similar in both groups. Average BMI was 34.5 in the Cushing group and 29.5 in the NFPA group. Average tumor size was 5.7 mm in the Cushing group and 21.3 mm in the NFPA group.
Before surgery, 2.9% of patients with Cushing disease and 15.4% of patients with NFPA had central hypothyroidism, and 8% in the Cushing group and 56.8% in the NFPA group had hyperprolactinemia. Central adrenal insufficiency occurred in 11% with NFPA and in all with Cushing disease, by definition.
After surgery, 93.8% in the Cushing group and 16.5% in the NFPA group had adrenal insufficiency. In addition, patients with Cushing disease had lower postsurgical nadir serum cortisol levels (63.8 nmol/L) than those with NFPA (282.3 nmol/L).
Of the 17 patients with Cushing disease — all women — who developed autoimmune disease within 3 years, 6 had a personal history of autoimmune disease and 7 had a family history of it. In addition, 41.2% of them had adrenal insufficiency when they developed the new autoimmune disease. Among the diseases were six autoimmune thyroiditis cases, three Sjögren syndrome cases, and two autoimmune seronegative spondyloarthropathy.
Dr. Heaney said he found it interesting that more than half of the new autoimmune diseases in patients with Cushing disease were related to the thyroid. “In this kind of setting, where you have a patient who has been producing too much steroid over a period of time and then you take that away, it’s almost like you release a brake on the TSH [thyroid-stimulating hormone],” Dr. Heaney said. “So, there’s probably some rebound in TSH that occurs, and that could be driving the thyroiditis, to some extent, that we see in these patients.”
Only one patient with NFPA developed new-onset autoimmune disease, a woman who developed Graves disease 22 months after surgery. When the researchers excluded patients in both groups with central hypothyroidism, new-onset autoimmune disease was still significantly higher (11.4%) in the Cushing group than in the NFPA group (1.9%; HR, 7.02; 95% CI, 2.54-19.39).
Could Postoperative Adrenal Insufficiency Contribute to Risk?
Within the Cushing cohort, those who developed autoimmune disease had a lower BMI (31.8 vs 34.8) and larger tumor size (7.2 vs 5.6 mm) than those who didn’t develop new autoimmune disease. Patients who developed autoimmune disease also had a lower baseline urine free cortisol ratio (2.7 vs 6.3) before surgery and more family history of autoimmune disease (41.2% vs 20.9%) than those who didn’t develop one.
“The higher prevalence of adrenal insufficiency and the lower nadir serum cortisol levels in the Cushing disease group suggest that the postoperative adrenal insufficiency in the Cushing disease group might have contributed to autoimmune disease pathogenesis,” the authors wrote. “This finding is clinically significant because cortisol plays a pivotal role in modulating the immune system.”
Most postoperative management among patients with Cushing disease was similar, with all but one patient receiving 0.5 or 1 mg daily dexamethasone within the first week after surgery. (The one outlier received 5 mg daily prednisone.) However, fewer patients who developed autoimmune disease (17.6%) received supraphysiologic doses of glucocorticoid — equivalent to at least 25 mg hydrocortisone — compared with patients who didn’t develop autoimmune disease (41.8%).
“Although the daily average hydrocortisone equivalent replacement doses within the first month and during long-term follow-up were within the physiologic range in both subgroups, patients with Cushing disease who had autoimmune disease received slightly lower doses of glucocorticoid replacement within the first month after surgery,” the authors reported. “The immediate postoperative period might be a critical window where supraphysiologic glucocorticoids seem to be protective with regard to development of autoimmune disease,” they wrote, though they acknowledged the study’s retrospective design as a limitation in drawing that conclusion.
At the least, they suggested that new symptoms in patients with Cushing disease, particularly those with a family history of autoimmune disease, should prompt investigation of potential autoimmune disease.
Recordati Rare Diseases funded the study. The research was also conducted with support from Harvard Catalyst (the Harvard Clinical and Translational Science Center) as well as financial contributions from Harvard University and its affiliated academic healthcare centers. One author reported holding stocks in Pfizer and Amgen, and another reported receiving consulting fees from Corcept. Dr. Heaney reported receiving institutional grants for trials from Corcept, Ascendis, Crinetics, and Sparrow Pharm; serving on the advisory board for Xeris, Recordati, Corcept, Novo Nordisk, Lundbeck, and Crinetics; and serving as a speaker for Chiesi, Novo Nordisk, and Corcept.
A version of this article appeared on Medscape.com.
Patients with Cushing disease have an increased risk for new-onset autoimmune disease in the 3 years after surgical remission, according to a new retrospective study published on February 20 in Annals of Internal Medicine.
Outcomes for patients with Cushing disease were compared against those with nonfunctioning pituitary adenomas (NFPAs). New-onset autoimmune disease occurred in 10.4% with Cushing disease and 1.6% among patients with NFPA (hazard ratio, 7.80; 95% CI, 2.88-21.10).
“Understanding and recognizing new and recurrent autoimmune disease in this setting is important to avoid misclassifying such patients with glucocorticoid withdrawal syndrome, which could result in failure to treat underlying autoimmune disease, as well as erroneous diagnosis of steroid withdrawal cases,” wrote Dennis Delasi Nyanyo of Massachusetts General Hospital and Harvard Medical School, Boston, and colleagues.
Given the general population’s annual incidence of major autoimmune diseases, estimated at about 100 cases per 100,000 people, and the 3-year incidence of 10.4% found in this study’s cohort, “our findings suggest that Cushing disease remission may trigger development of autoimmune disease,” the authors wrote.
Monitor Patients With Family History of Autoimmune Disease?
The study results were not necessarily surprising to Anthony P. Heaney, MD, PhD, an endocrinologist and professor of medicine at the University of California, Los Angeles, because past research has raised similar questions. The authors’ suggestion that the rapid postsurgical drop in cortisol that occurs as a result of treating Cushing disease becomes some sort of autoimmune trigger is interesting but remains speculative, Dr. Heaney pointed out.
If future evidence supports that possibility, “it would suggest, in terms of managing those patients in the postoperative setting, that there may be some merit to giving them higher concentrations of glucocorticoids for a short period of time,” Dr. Heaney said, thereby bringing their levels down more gradually rather than taking them off a cliff, in a sense. Or, if more evidence bears out the authors’ hypothesis, another approach might be treating patients with medicine to bring down the cortisol before surgery, though there are challenges to that approach, Dr. Heaney said.
At the same time, those who developed new autoimmune disease remain a small subset of patients with Cushing disease, so such approaches may become only potentially appropriate to consider in patients with risk factors, such as a family history of autoimmune disease.
The researchers conducted a retrospective chart review of adult patients who underwent transsphenoidal surgery for either Cushing disease or NFPA at Massachusetts General Hospital between 2005 and 2019.
The study involved 194 patients with Cushing disease who had postsurgical remission and at least one follow-up visit with a pituitary expert and 92 patients with NFPA who were matched to patients with Cushing disease based on age and sex. The authors regarded autoimmune disease diagnosed within 36 months of the surgery to be temporally associated with Cushing disease remission. Among the autoimmune diseases considered were “rheumatoid arthritis, Sjögren syndrome, systemic lupus erythematosus, autoimmune thyroiditis, celiac disease, psoriasis, vitiligo, autoimmune neuropathy, multiple sclerosis, myasthenia gravis, and ulcerative colitis.”
Patients differed in average body mass index and tumor size, but family history of autoimmune disease was similar in both groups. Average BMI was 34.5 in the Cushing group and 29.5 in the NFPA group. Average tumor size was 5.7 mm in the Cushing group and 21.3 mm in the NFPA group.
Before surgery, 2.9% of patients with Cushing disease and 15.4% of patients with NFPA had central hypothyroidism, and 8% in the Cushing group and 56.8% in the NFPA group had hyperprolactinemia. Central adrenal insufficiency occurred in 11% with NFPA and in all with Cushing disease, by definition.
After surgery, 93.8% in the Cushing group and 16.5% in the NFPA group had adrenal insufficiency. In addition, patients with Cushing disease had lower postsurgical nadir serum cortisol levels (63.8 nmol/L) than those with NFPA (282.3 nmol/L).
Of the 17 patients with Cushing disease — all women — who developed autoimmune disease within 3 years, 6 had a personal history of autoimmune disease and 7 had a family history of it. In addition, 41.2% of them had adrenal insufficiency when they developed the new autoimmune disease. Among the diseases were six autoimmune thyroiditis cases, three Sjögren syndrome cases, and two autoimmune seronegative spondyloarthropathy.
Dr. Heaney said he found it interesting that more than half of the new autoimmune diseases in patients with Cushing disease were related to the thyroid. “In this kind of setting, where you have a patient who has been producing too much steroid over a period of time and then you take that away, it’s almost like you release a brake on the TSH [thyroid-stimulating hormone],” Dr. Heaney said. “So, there’s probably some rebound in TSH that occurs, and that could be driving the thyroiditis, to some extent, that we see in these patients.”
Only one patient with NFPA developed new-onset autoimmune disease, a woman who developed Graves disease 22 months after surgery. When the researchers excluded patients in both groups with central hypothyroidism, new-onset autoimmune disease was still significantly higher (11.4%) in the Cushing group than in the NFPA group (1.9%; HR, 7.02; 95% CI, 2.54-19.39).
Could Postoperative Adrenal Insufficiency Contribute to Risk?
Within the Cushing cohort, those who developed autoimmune disease had a lower BMI (31.8 vs 34.8) and larger tumor size (7.2 vs 5.6 mm) than those who didn’t develop new autoimmune disease. Patients who developed autoimmune disease also had a lower baseline urine free cortisol ratio (2.7 vs 6.3) before surgery and more family history of autoimmune disease (41.2% vs 20.9%) than those who didn’t develop one.
“The higher prevalence of adrenal insufficiency and the lower nadir serum cortisol levels in the Cushing disease group suggest that the postoperative adrenal insufficiency in the Cushing disease group might have contributed to autoimmune disease pathogenesis,” the authors wrote. “This finding is clinically significant because cortisol plays a pivotal role in modulating the immune system.”
Most postoperative management among patients with Cushing disease was similar, with all but one patient receiving 0.5 or 1 mg daily dexamethasone within the first week after surgery. (The one outlier received 5 mg daily prednisone.) However, fewer patients who developed autoimmune disease (17.6%) received supraphysiologic doses of glucocorticoid — equivalent to at least 25 mg hydrocortisone — compared with patients who didn’t develop autoimmune disease (41.8%).
“Although the daily average hydrocortisone equivalent replacement doses within the first month and during long-term follow-up were within the physiologic range in both subgroups, patients with Cushing disease who had autoimmune disease received slightly lower doses of glucocorticoid replacement within the first month after surgery,” the authors reported. “The immediate postoperative period might be a critical window where supraphysiologic glucocorticoids seem to be protective with regard to development of autoimmune disease,” they wrote, though they acknowledged the study’s retrospective design as a limitation in drawing that conclusion.
At the least, they suggested that new symptoms in patients with Cushing disease, particularly those with a family history of autoimmune disease, should prompt investigation of potential autoimmune disease.
Recordati Rare Diseases funded the study. The research was also conducted with support from Harvard Catalyst (the Harvard Clinical and Translational Science Center) as well as financial contributions from Harvard University and its affiliated academic healthcare centers. One author reported holding stocks in Pfizer and Amgen, and another reported receiving consulting fees from Corcept. Dr. Heaney reported receiving institutional grants for trials from Corcept, Ascendis, Crinetics, and Sparrow Pharm; serving on the advisory board for Xeris, Recordati, Corcept, Novo Nordisk, Lundbeck, and Crinetics; and serving as a speaker for Chiesi, Novo Nordisk, and Corcept.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Comorbidities and Disease Type Weigh Heavily in Pregnancy Outcomes of Immune-Mediated Inflammatory Diseases
Comorbidities may play a large role in driving poor pregnancy outcomes in pregnant people with certain immune-mediated inflammatory diseases (IMIDs).
In a new study of 12 individual IMIDs, people with rheumatoid arthritis (RA) or inflammatory bowel disease (IBD) did not have signficantly increased risk for preterm birth (PTB) or low birth weight (LBW), compared with people who did not have an IMID, after adjusting for additional chronic conditions and other confounding factors.
The study was published online on February 1 in eClinicalMedicine.
While many studies have explored the relationships between pregnancy outcomes and IMIDs, “the impact of comorbidities on the relation between IMIDs and pregnancy course is insufficiently examined,” the authors wrote. These previous studies also tended to have a small sample size.
Pregnancy Outcome Risks Varied Between IMIDs
To remedy this, researchers used electronic health record data from Providence St Joseph Health — a multistate integrated healthcare system — to identify more than 365,000 pregnant people with live births between January 1, 2013, and December 31, 2022. The cohort included more than 5700 people with at least one of 12 IMIDs: Psoriasis, IBD, RA, spondyloarthritis (SpA), multiple sclerosis, systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), antiphospholipid syndrome (APS), Sjögren syndrome (SjS), vasculitis, sarcoidosis, and systemic sclerosis. The study included only live births with a gestational age of 20 weeks or greater.
Researchers compared maternal-fetal health outcomes between the two groups, controlling for comorbidities including diabetes, cardiovascular disease, chronic kidney disease, obesity, and depression. They also accounted for confounding variables including race, age, smoking status, and socioeconomic status.
In total, 83% of people in the IMID group had no immunomodulatory medication prescriptions during their pregnancy. Of the 17% taking medication, 48%-70% continued taking their medication until delivery. Most patients were White, comprising 62.9% of the non-IMID group and 73.1% of the IMID group.
After adjusting for comorbidities, patients with any of the 12 IMIDs had a 10%-20% higher risk for PTB, LBW, small for gestation age (SGA), and cesarean section than did comparators.
But these risks varied between IMIDs. Patients with RA and IBD did not have an increased risk for PTB or LBW. However, when researchers did not control for comorbidities, pregnancy risks were higher and showed statistical significance in these two groups.
“This suggests that for RA and IBD, comorbidities may be a more important factor for adverse outcomes than the underlying autoimmune disease,” senior author Jennifer Hadlock, MD, an associate professor and director of medical data science at the Institute for Systems Biology in Seattle, Washington, said in a video accompanying a press release.
Overall, the analysis found that women with IMIDs were approximately two to three times more likely to have chronic comorbidities than the control group.
Like previous studies, there was a strong association between SLE and APS and poor pregnancy outcomes, even after controlling for confounding factors. Patients with SpA had a 50% increased risk for PTB, while those with SLE and APS had more than a twofold higher risk. Patients with SLE were 90% more likely than comparators to deliver babies with an SGA condition, while RA patients had a 30% higher risk. SLE was the only condition with an increased risk for LBW (relative risk, 3.5). IBD, RA, PsA, SpA, SLE, APS, and SjS were all associated with a higher likelihood of delivery via cesarean section.
“The findings of this study reveal that the associations between IMIDs and adverse pregnancy outcomes are influenced by the specific type of IMIDs and the presence of comorbidities,” the authors wrote.
A Large Study, But How Representative Is It?
Asked to comment on the study, Catherine Sims, MD, a rheumatologist at Duke University Medical Center in Durham, North Carolina, noted that the analysis was much larger than many reproductive rheumatology studies, and “their statistics were phenomenal.”
She agreed that “not all autoimmune diseases are created equal when it comes to pregnancy-associated risks.” However, she added that this study’s patient population may not be totally representative of pregnant people with IMIDs or autoimmune diseases.
“We’re making generalizations about autoimmune diseases based on this demographic of White women who are not taking immunosuppression,” she said.
“We know that race and ethnicity play a huge role in pregnancy outcomes, and Black women have higher maternal and fetal morbidity and mortality, which is likely related to systemic racism and biases in the medical system,” she added. “While the study did control for sociodemographic factors, the population studied is not diverse.”
Only 17% of people with IMID in the cohort were on immunosuppressive medication, which could suggest low disease activity in the study population, Dr. Sims said. If the population generally had well-controlled disease, that could have positioned them for better pregnancy outcomes.
The authors noted that their analysis did not have information on IMID disease activity or severity — one of the limitations of the study.
However, the authors argued that the observed low prescription rate during the study may have increased poor pregnancy outcomes.
“Although this reflects real-world care in the population studied, results from this study may show higher risk than might be achieved with recommended care guidelines,” they wrote.
Ultimately, the authors argued that these findings show how co-occurring health conditions can affect pregnancy outcomes in autoimmune diseases, particularly for RA and IBD.
“There is a need to take comorbidities into consideration for guidelines for patients with inflammatory bowel disease and rheumatoid arthritis and when designing future research to investigate maternal health in patients with IMIDs,” they wrote.
The study was funded by the National Institutes of Health. Dr. Sims declared no relevant financial relationships. Dr. Hadlock has received research funding (paid to the institute) from Pfizer, Novartis, Janssen, Bristol-Myers Squibb, and Gilead.
A version of this article first appeared on Medscape.com.
Comorbidities may play a large role in driving poor pregnancy outcomes in pregnant people with certain immune-mediated inflammatory diseases (IMIDs).
In a new study of 12 individual IMIDs, people with rheumatoid arthritis (RA) or inflammatory bowel disease (IBD) did not have signficantly increased risk for preterm birth (PTB) or low birth weight (LBW), compared with people who did not have an IMID, after adjusting for additional chronic conditions and other confounding factors.
The study was published online on February 1 in eClinicalMedicine.
While many studies have explored the relationships between pregnancy outcomes and IMIDs, “the impact of comorbidities on the relation between IMIDs and pregnancy course is insufficiently examined,” the authors wrote. These previous studies also tended to have a small sample size.
Pregnancy Outcome Risks Varied Between IMIDs
To remedy this, researchers used electronic health record data from Providence St Joseph Health — a multistate integrated healthcare system — to identify more than 365,000 pregnant people with live births between January 1, 2013, and December 31, 2022. The cohort included more than 5700 people with at least one of 12 IMIDs: Psoriasis, IBD, RA, spondyloarthritis (SpA), multiple sclerosis, systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), antiphospholipid syndrome (APS), Sjögren syndrome (SjS), vasculitis, sarcoidosis, and systemic sclerosis. The study included only live births with a gestational age of 20 weeks or greater.
Researchers compared maternal-fetal health outcomes between the two groups, controlling for comorbidities including diabetes, cardiovascular disease, chronic kidney disease, obesity, and depression. They also accounted for confounding variables including race, age, smoking status, and socioeconomic status.
In total, 83% of people in the IMID group had no immunomodulatory medication prescriptions during their pregnancy. Of the 17% taking medication, 48%-70% continued taking their medication until delivery. Most patients were White, comprising 62.9% of the non-IMID group and 73.1% of the IMID group.
After adjusting for comorbidities, patients with any of the 12 IMIDs had a 10%-20% higher risk for PTB, LBW, small for gestation age (SGA), and cesarean section than did comparators.
But these risks varied between IMIDs. Patients with RA and IBD did not have an increased risk for PTB or LBW. However, when researchers did not control for comorbidities, pregnancy risks were higher and showed statistical significance in these two groups.
“This suggests that for RA and IBD, comorbidities may be a more important factor for adverse outcomes than the underlying autoimmune disease,” senior author Jennifer Hadlock, MD, an associate professor and director of medical data science at the Institute for Systems Biology in Seattle, Washington, said in a video accompanying a press release.
Overall, the analysis found that women with IMIDs were approximately two to three times more likely to have chronic comorbidities than the control group.
Like previous studies, there was a strong association between SLE and APS and poor pregnancy outcomes, even after controlling for confounding factors. Patients with SpA had a 50% increased risk for PTB, while those with SLE and APS had more than a twofold higher risk. Patients with SLE were 90% more likely than comparators to deliver babies with an SGA condition, while RA patients had a 30% higher risk. SLE was the only condition with an increased risk for LBW (relative risk, 3.5). IBD, RA, PsA, SpA, SLE, APS, and SjS were all associated with a higher likelihood of delivery via cesarean section.
“The findings of this study reveal that the associations between IMIDs and adverse pregnancy outcomes are influenced by the specific type of IMIDs and the presence of comorbidities,” the authors wrote.
A Large Study, But How Representative Is It?
Asked to comment on the study, Catherine Sims, MD, a rheumatologist at Duke University Medical Center in Durham, North Carolina, noted that the analysis was much larger than many reproductive rheumatology studies, and “their statistics were phenomenal.”
She agreed that “not all autoimmune diseases are created equal when it comes to pregnancy-associated risks.” However, she added that this study’s patient population may not be totally representative of pregnant people with IMIDs or autoimmune diseases.
“We’re making generalizations about autoimmune diseases based on this demographic of White women who are not taking immunosuppression,” she said.
“We know that race and ethnicity play a huge role in pregnancy outcomes, and Black women have higher maternal and fetal morbidity and mortality, which is likely related to systemic racism and biases in the medical system,” she added. “While the study did control for sociodemographic factors, the population studied is not diverse.”
Only 17% of people with IMID in the cohort were on immunosuppressive medication, which could suggest low disease activity in the study population, Dr. Sims said. If the population generally had well-controlled disease, that could have positioned them for better pregnancy outcomes.
The authors noted that their analysis did not have information on IMID disease activity or severity — one of the limitations of the study.
However, the authors argued that the observed low prescription rate during the study may have increased poor pregnancy outcomes.
“Although this reflects real-world care in the population studied, results from this study may show higher risk than might be achieved with recommended care guidelines,” they wrote.
Ultimately, the authors argued that these findings show how co-occurring health conditions can affect pregnancy outcomes in autoimmune diseases, particularly for RA and IBD.
“There is a need to take comorbidities into consideration for guidelines for patients with inflammatory bowel disease and rheumatoid arthritis and when designing future research to investigate maternal health in patients with IMIDs,” they wrote.
The study was funded by the National Institutes of Health. Dr. Sims declared no relevant financial relationships. Dr. Hadlock has received research funding (paid to the institute) from Pfizer, Novartis, Janssen, Bristol-Myers Squibb, and Gilead.
A version of this article first appeared on Medscape.com.
Comorbidities may play a large role in driving poor pregnancy outcomes in pregnant people with certain immune-mediated inflammatory diseases (IMIDs).
In a new study of 12 individual IMIDs, people with rheumatoid arthritis (RA) or inflammatory bowel disease (IBD) did not have signficantly increased risk for preterm birth (PTB) or low birth weight (LBW), compared with people who did not have an IMID, after adjusting for additional chronic conditions and other confounding factors.
The study was published online on February 1 in eClinicalMedicine.
While many studies have explored the relationships between pregnancy outcomes and IMIDs, “the impact of comorbidities on the relation between IMIDs and pregnancy course is insufficiently examined,” the authors wrote. These previous studies also tended to have a small sample size.
Pregnancy Outcome Risks Varied Between IMIDs
To remedy this, researchers used electronic health record data from Providence St Joseph Health — a multistate integrated healthcare system — to identify more than 365,000 pregnant people with live births between January 1, 2013, and December 31, 2022. The cohort included more than 5700 people with at least one of 12 IMIDs: Psoriasis, IBD, RA, spondyloarthritis (SpA), multiple sclerosis, systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), antiphospholipid syndrome (APS), Sjögren syndrome (SjS), vasculitis, sarcoidosis, and systemic sclerosis. The study included only live births with a gestational age of 20 weeks or greater.
Researchers compared maternal-fetal health outcomes between the two groups, controlling for comorbidities including diabetes, cardiovascular disease, chronic kidney disease, obesity, and depression. They also accounted for confounding variables including race, age, smoking status, and socioeconomic status.
In total, 83% of people in the IMID group had no immunomodulatory medication prescriptions during their pregnancy. Of the 17% taking medication, 48%-70% continued taking their medication until delivery. Most patients were White, comprising 62.9% of the non-IMID group and 73.1% of the IMID group.
After adjusting for comorbidities, patients with any of the 12 IMIDs had a 10%-20% higher risk for PTB, LBW, small for gestation age (SGA), and cesarean section than did comparators.
But these risks varied between IMIDs. Patients with RA and IBD did not have an increased risk for PTB or LBW. However, when researchers did not control for comorbidities, pregnancy risks were higher and showed statistical significance in these two groups.
“This suggests that for RA and IBD, comorbidities may be a more important factor for adverse outcomes than the underlying autoimmune disease,” senior author Jennifer Hadlock, MD, an associate professor and director of medical data science at the Institute for Systems Biology in Seattle, Washington, said in a video accompanying a press release.
Overall, the analysis found that women with IMIDs were approximately two to three times more likely to have chronic comorbidities than the control group.
Like previous studies, there was a strong association between SLE and APS and poor pregnancy outcomes, even after controlling for confounding factors. Patients with SpA had a 50% increased risk for PTB, while those with SLE and APS had more than a twofold higher risk. Patients with SLE were 90% more likely than comparators to deliver babies with an SGA condition, while RA patients had a 30% higher risk. SLE was the only condition with an increased risk for LBW (relative risk, 3.5). IBD, RA, PsA, SpA, SLE, APS, and SjS were all associated with a higher likelihood of delivery via cesarean section.
“The findings of this study reveal that the associations between IMIDs and adverse pregnancy outcomes are influenced by the specific type of IMIDs and the presence of comorbidities,” the authors wrote.
A Large Study, But How Representative Is It?
Asked to comment on the study, Catherine Sims, MD, a rheumatologist at Duke University Medical Center in Durham, North Carolina, noted that the analysis was much larger than many reproductive rheumatology studies, and “their statistics were phenomenal.”
She agreed that “not all autoimmune diseases are created equal when it comes to pregnancy-associated risks.” However, she added that this study’s patient population may not be totally representative of pregnant people with IMIDs or autoimmune diseases.
“We’re making generalizations about autoimmune diseases based on this demographic of White women who are not taking immunosuppression,” she said.
“We know that race and ethnicity play a huge role in pregnancy outcomes, and Black women have higher maternal and fetal morbidity and mortality, which is likely related to systemic racism and biases in the medical system,” she added. “While the study did control for sociodemographic factors, the population studied is not diverse.”
Only 17% of people with IMID in the cohort were on immunosuppressive medication, which could suggest low disease activity in the study population, Dr. Sims said. If the population generally had well-controlled disease, that could have positioned them for better pregnancy outcomes.
The authors noted that their analysis did not have information on IMID disease activity or severity — one of the limitations of the study.
However, the authors argued that the observed low prescription rate during the study may have increased poor pregnancy outcomes.
“Although this reflects real-world care in the population studied, results from this study may show higher risk than might be achieved with recommended care guidelines,” they wrote.
Ultimately, the authors argued that these findings show how co-occurring health conditions can affect pregnancy outcomes in autoimmune diseases, particularly for RA and IBD.
“There is a need to take comorbidities into consideration for guidelines for patients with inflammatory bowel disease and rheumatoid arthritis and when designing future research to investigate maternal health in patients with IMIDs,” they wrote.
The study was funded by the National Institutes of Health. Dr. Sims declared no relevant financial relationships. Dr. Hadlock has received research funding (paid to the institute) from Pfizer, Novartis, Janssen, Bristol-Myers Squibb, and Gilead.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
Breaking the Diagnostic Bottleneck in RA
As head of the clinical laboratory at the San Juan University Hospital in Alicante, Spain, Maria Salinas, PhD, is passionate about the role she and her colleagues can play in clinical decision-making.
Her mission is the identification of “hidden diseases,” as she calls them, chronic conditions for which early identification and intervention can change the course of the illness. Her lab has been a leader over the past decade in using technology to partner with clinicians to promote the appropriate use of testing and clinical decision-making.
An example of a disease ripe for this type of intervention is rheumatoid arthritis (RA), the most common form of autoimmune arthritis, affecting around 1.3 million people in the United States. The prognosis for patients is better the earlier treatment begins.
But the
Amy S. Kehl, MD, an attending rheumatologist at Cedars-Sinai Medical Center in Los Angeles, who also sees patients at Saint John’s Physician Partners in Santa Monica, California, recommends a workup for inflammatory arthritis for patients presenting with the new onset of joint pain and swelling, primarily of small joints, although larger joints can be involved. The workup includes markers of inflammation such as an erythrocyte sedimentation rate and C-reactive protein, which are typically elevated and can be used to monitor the progression of the disease. Similarly, the presence of anemia is consistent with RA and helpful in tracking response to treatment.
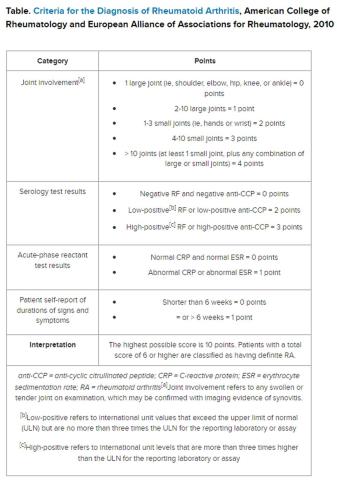
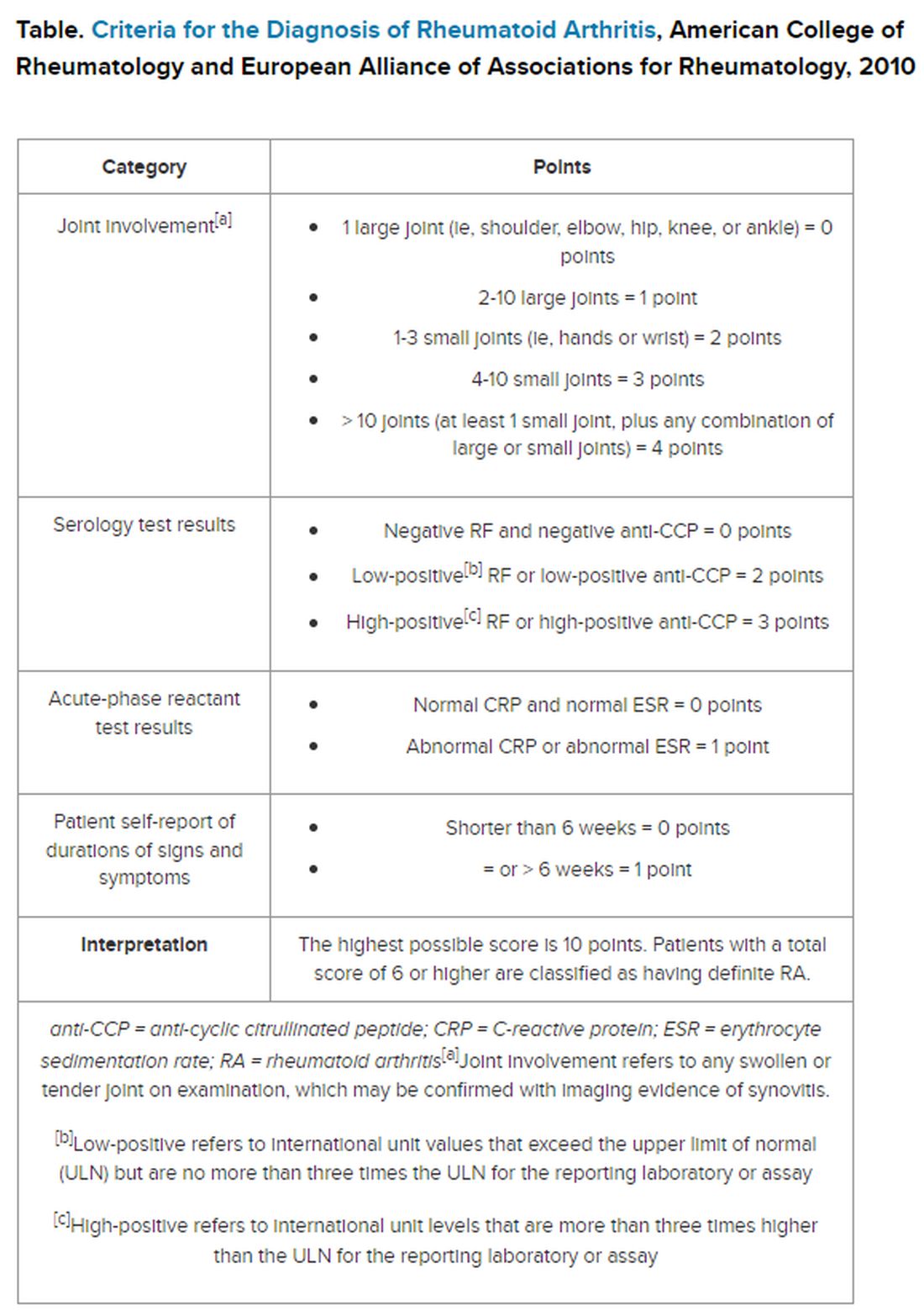
But pinning down the diagnosis requires the presence of autoimmune antibodies. Guidelines from the American College of Rheumatology require a positive result for either rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibody to definitively determine whether a patient has RA (Table).
“Classically, I find that the primary care physicians include a rheumatoid factor, not always a CCP, and may include other antibodies, including an ANA [antinuclear antibody] test, as part of that workup,” Dr. Kehl said. The problem with that strategy is that although the RF test does detect 60%-80% of patients with RA, it is positive in many other autoimmune conditions. Although the ANA might be positive in patients with RA, it is nonspecific and does not confirm the diagnosis of RA.
Up to 50% of autoimmune antibody tests are inappropriately ordered. And for rheumatologists, that leads to unnecessary referrals of patients with musculoskeletal complaints who do not meet objective clinical criteria for joint disease.
“These tests get ordered almost reflexively, and sometimes they’re ordered as part of a panel that includes a rheumatoid factor and an ANA, and it’s not necessarily going to be a high-yield test,” Dr. Kehl said. Superfluous tests and referrals often cause unnecessary anxiety in patients, as well as drive up costs, she added.
Dr. Salinas made the same observation in her hospital lab, which also serves nine primary care centers. She documented an upward trend in orders for RF testing in her hospital lab between 2011 and 2019. Dr. Salinas also noted that the anti-CCP antibody test was not commonly requested, although it has more utility in the diagnosis. Like the RF, it detects 50%-70% of patients with RA but has 95% specificity, resulting in far fewer false-positive results.
The appearance of both RF and anti-CCP antibodies often predicts a rapid progression to clinical disease. Dr. Kehl wants symptomatic patients with positive results for both markers to be seen right away by a rheumatologist. “We do know from studies that bony erosions can develop as early as a month or months after the onset of an inflammatory arthritis,” she said.
To address this need, Dr. Salinas worked with rheumatologists and primary care clinicians to develop an algorithm that called for reflex testing of samples from patients with positive RF results (> 30 IU/mL) for anti-CCP antibodies. If the anti-CCP antibody result was > 40 IU/mL, a comment in the lab report suggested rheumatology referral. The lab turned down requests to test sample for RF if the patient had a negative result in the previous 12 months — but it would perform the test if the clinician repeated the request.
The results were encouraging, Dr. Salinas said. “The main result in this study was that we really identified more hidden cases of patients with rheumatoid arthritis,” she told this news organization.
Compared with baseline trends, during the study period from April 2019 to January 2021 her lab demonstrated:
- Reduced RF tests conducted by canceling 16% of tests ordered for patients with negative RF result in the previous 12 months
- Fewer unnecessary referrals, from 22% in the baseline period to 8% during the intervention period
- A smaller percentage of missed patients, from 21% to 16%
To be sure, pre- and post-implementation comparisons are difficult when the implementation period happens to coincide with the emergence of SARS-CoV-2.
Although fewer patients were seen and fewer lab tests were ordered overall in Alicante during the COVID-19 pandemic, the proportion of tests ordered for RF testing dropped, and all the patients identified with double positives for RF and anti-CCP antibodies were referred to rheumatology, suggesting evidence of benefit.
Dr. Kehl said the practice of using clinical decision support systems could be used in the United States. “I thought this was an important study,” she said. Electronic health records systems “have all these capabilities where we can include best practice alerts when you order a test to make sure that it’s clinically warranted and cost-effective.”
Dr. Salinas and Dr. Kehl reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
As head of the clinical laboratory at the San Juan University Hospital in Alicante, Spain, Maria Salinas, PhD, is passionate about the role she and her colleagues can play in clinical decision-making.
Her mission is the identification of “hidden diseases,” as she calls them, chronic conditions for which early identification and intervention can change the course of the illness. Her lab has been a leader over the past decade in using technology to partner with clinicians to promote the appropriate use of testing and clinical decision-making.
An example of a disease ripe for this type of intervention is rheumatoid arthritis (RA), the most common form of autoimmune arthritis, affecting around 1.3 million people in the United States. The prognosis for patients is better the earlier treatment begins.
But the
Amy S. Kehl, MD, an attending rheumatologist at Cedars-Sinai Medical Center in Los Angeles, who also sees patients at Saint John’s Physician Partners in Santa Monica, California, recommends a workup for inflammatory arthritis for patients presenting with the new onset of joint pain and swelling, primarily of small joints, although larger joints can be involved. The workup includes markers of inflammation such as an erythrocyte sedimentation rate and C-reactive protein, which are typically elevated and can be used to monitor the progression of the disease. Similarly, the presence of anemia is consistent with RA and helpful in tracking response to treatment.
But pinning down the diagnosis requires the presence of autoimmune antibodies. Guidelines from the American College of Rheumatology require a positive result for either rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibody to definitively determine whether a patient has RA (Table).
“Classically, I find that the primary care physicians include a rheumatoid factor, not always a CCP, and may include other antibodies, including an ANA [antinuclear antibody] test, as part of that workup,” Dr. Kehl said. The problem with that strategy is that although the RF test does detect 60%-80% of patients with RA, it is positive in many other autoimmune conditions. Although the ANA might be positive in patients with RA, it is nonspecific and does not confirm the diagnosis of RA.
Up to 50% of autoimmune antibody tests are inappropriately ordered. And for rheumatologists, that leads to unnecessary referrals of patients with musculoskeletal complaints who do not meet objective clinical criteria for joint disease.
“These tests get ordered almost reflexively, and sometimes they’re ordered as part of a panel that includes a rheumatoid factor and an ANA, and it’s not necessarily going to be a high-yield test,” Dr. Kehl said. Superfluous tests and referrals often cause unnecessary anxiety in patients, as well as drive up costs, she added.
Dr. Salinas made the same observation in her hospital lab, which also serves nine primary care centers. She documented an upward trend in orders for RF testing in her hospital lab between 2011 and 2019. Dr. Salinas also noted that the anti-CCP antibody test was not commonly requested, although it has more utility in the diagnosis. Like the RF, it detects 50%-70% of patients with RA but has 95% specificity, resulting in far fewer false-positive results.
The appearance of both RF and anti-CCP antibodies often predicts a rapid progression to clinical disease. Dr. Kehl wants symptomatic patients with positive results for both markers to be seen right away by a rheumatologist. “We do know from studies that bony erosions can develop as early as a month or months after the onset of an inflammatory arthritis,” she said.
To address this need, Dr. Salinas worked with rheumatologists and primary care clinicians to develop an algorithm that called for reflex testing of samples from patients with positive RF results (> 30 IU/mL) for anti-CCP antibodies. If the anti-CCP antibody result was > 40 IU/mL, a comment in the lab report suggested rheumatology referral. The lab turned down requests to test sample for RF if the patient had a negative result in the previous 12 months — but it would perform the test if the clinician repeated the request.
The results were encouraging, Dr. Salinas said. “The main result in this study was that we really identified more hidden cases of patients with rheumatoid arthritis,” she told this news organization.
Compared with baseline trends, during the study period from April 2019 to January 2021 her lab demonstrated:
- Reduced RF tests conducted by canceling 16% of tests ordered for patients with negative RF result in the previous 12 months
- Fewer unnecessary referrals, from 22% in the baseline period to 8% during the intervention period
- A smaller percentage of missed patients, from 21% to 16%
To be sure, pre- and post-implementation comparisons are difficult when the implementation period happens to coincide with the emergence of SARS-CoV-2.
Although fewer patients were seen and fewer lab tests were ordered overall in Alicante during the COVID-19 pandemic, the proportion of tests ordered for RF testing dropped, and all the patients identified with double positives for RF and anti-CCP antibodies were referred to rheumatology, suggesting evidence of benefit.
Dr. Kehl said the practice of using clinical decision support systems could be used in the United States. “I thought this was an important study,” she said. Electronic health records systems “have all these capabilities where we can include best practice alerts when you order a test to make sure that it’s clinically warranted and cost-effective.”
Dr. Salinas and Dr. Kehl reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
As head of the clinical laboratory at the San Juan University Hospital in Alicante, Spain, Maria Salinas, PhD, is passionate about the role she and her colleagues can play in clinical decision-making.
Her mission is the identification of “hidden diseases,” as she calls them, chronic conditions for which early identification and intervention can change the course of the illness. Her lab has been a leader over the past decade in using technology to partner with clinicians to promote the appropriate use of testing and clinical decision-making.
An example of a disease ripe for this type of intervention is rheumatoid arthritis (RA), the most common form of autoimmune arthritis, affecting around 1.3 million people in the United States. The prognosis for patients is better the earlier treatment begins.
But the
Amy S. Kehl, MD, an attending rheumatologist at Cedars-Sinai Medical Center in Los Angeles, who also sees patients at Saint John’s Physician Partners in Santa Monica, California, recommends a workup for inflammatory arthritis for patients presenting with the new onset of joint pain and swelling, primarily of small joints, although larger joints can be involved. The workup includes markers of inflammation such as an erythrocyte sedimentation rate and C-reactive protein, which are typically elevated and can be used to monitor the progression of the disease. Similarly, the presence of anemia is consistent with RA and helpful in tracking response to treatment.
But pinning down the diagnosis requires the presence of autoimmune antibodies. Guidelines from the American College of Rheumatology require a positive result for either rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibody to definitively determine whether a patient has RA (Table).
“Classically, I find that the primary care physicians include a rheumatoid factor, not always a CCP, and may include other antibodies, including an ANA [antinuclear antibody] test, as part of that workup,” Dr. Kehl said. The problem with that strategy is that although the RF test does detect 60%-80% of patients with RA, it is positive in many other autoimmune conditions. Although the ANA might be positive in patients with RA, it is nonspecific and does not confirm the diagnosis of RA.
Up to 50% of autoimmune antibody tests are inappropriately ordered. And for rheumatologists, that leads to unnecessary referrals of patients with musculoskeletal complaints who do not meet objective clinical criteria for joint disease.
“These tests get ordered almost reflexively, and sometimes they’re ordered as part of a panel that includes a rheumatoid factor and an ANA, and it’s not necessarily going to be a high-yield test,” Dr. Kehl said. Superfluous tests and referrals often cause unnecessary anxiety in patients, as well as drive up costs, she added.
Dr. Salinas made the same observation in her hospital lab, which also serves nine primary care centers. She documented an upward trend in orders for RF testing in her hospital lab between 2011 and 2019. Dr. Salinas also noted that the anti-CCP antibody test was not commonly requested, although it has more utility in the diagnosis. Like the RF, it detects 50%-70% of patients with RA but has 95% specificity, resulting in far fewer false-positive results.
The appearance of both RF and anti-CCP antibodies often predicts a rapid progression to clinical disease. Dr. Kehl wants symptomatic patients with positive results for both markers to be seen right away by a rheumatologist. “We do know from studies that bony erosions can develop as early as a month or months after the onset of an inflammatory arthritis,” she said.
To address this need, Dr. Salinas worked with rheumatologists and primary care clinicians to develop an algorithm that called for reflex testing of samples from patients with positive RF results (> 30 IU/mL) for anti-CCP antibodies. If the anti-CCP antibody result was > 40 IU/mL, a comment in the lab report suggested rheumatology referral. The lab turned down requests to test sample for RF if the patient had a negative result in the previous 12 months — but it would perform the test if the clinician repeated the request.
The results were encouraging, Dr. Salinas said. “The main result in this study was that we really identified more hidden cases of patients with rheumatoid arthritis,” she told this news organization.
Compared with baseline trends, during the study period from April 2019 to January 2021 her lab demonstrated:
- Reduced RF tests conducted by canceling 16% of tests ordered for patients with negative RF result in the previous 12 months
- Fewer unnecessary referrals, from 22% in the baseline period to 8% during the intervention period
- A smaller percentage of missed patients, from 21% to 16%
To be sure, pre- and post-implementation comparisons are difficult when the implementation period happens to coincide with the emergence of SARS-CoV-2.
Although fewer patients were seen and fewer lab tests were ordered overall in Alicante during the COVID-19 pandemic, the proportion of tests ordered for RF testing dropped, and all the patients identified with double positives for RF and anti-CCP antibodies were referred to rheumatology, suggesting evidence of benefit.
Dr. Kehl said the practice of using clinical decision support systems could be used in the United States. “I thought this was an important study,” she said. Electronic health records systems “have all these capabilities where we can include best practice alerts when you order a test to make sure that it’s clinically warranted and cost-effective.”
Dr. Salinas and Dr. Kehl reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
A Paradox? Higher Male Fertility Seen With Inflammatory Arthritis
TOPLINE:
Men with an inflammatory arthritis (IA) diagnosis are less likely to be childless than healthy comparators, according to an epidemiological study.
METHODS:
- 10,865 men in the Norwegian Arthritis Registry were compared with 54,325 men without IA, matched by age and location.
- In the arthritis group, 37% had rheumatoid arthritis, 33% had psoriatic arthritis, and 30% had spondyloarthritis.
- Researchers used childlessness and number of children as proxies for male fertility.
TAKEAWAY:
- 21% of men with IA were childless compared with 27% in the healthy cohort (P < .001).
- On an average, a man with IA had 1.80 children whereas a man in the control group had 1.69 children (P < .001).
- These findings were consistent over time, but the most pronounced difference between groups was seen in men diagnosed after the year 2000.
IN PRACTICE:
The finding “is novel and generates new hypotheses regarding associations between fertility, inflammatory rheumatic diseases, and immune-modulating drugs,” the authors wrote.
SOURCE:
First author Gudrun David Sigmo, of the department of rheumatology at Stavanger (Norway) University Hospital, and colleagues had their work published online on January 23, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The analysis relied on administrative data, and researchers did not have data on confounding factors.
DISCLOSURES:
The study was funded by the nonprofit organizations Aslaug Anders fond, Astri og Edvard Riisøens legat, Det alminnelige medisinske forskningsfond, Pahles legat, and Fagsenter for medisins-ke kvalitetsregistre i Helse Vest. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Men with an inflammatory arthritis (IA) diagnosis are less likely to be childless than healthy comparators, according to an epidemiological study.
METHODS:
- 10,865 men in the Norwegian Arthritis Registry were compared with 54,325 men without IA, matched by age and location.
- In the arthritis group, 37% had rheumatoid arthritis, 33% had psoriatic arthritis, and 30% had spondyloarthritis.
- Researchers used childlessness and number of children as proxies for male fertility.
TAKEAWAY:
- 21% of men with IA were childless compared with 27% in the healthy cohort (P < .001).
- On an average, a man with IA had 1.80 children whereas a man in the control group had 1.69 children (P < .001).
- These findings were consistent over time, but the most pronounced difference between groups was seen in men diagnosed after the year 2000.
IN PRACTICE:
The finding “is novel and generates new hypotheses regarding associations between fertility, inflammatory rheumatic diseases, and immune-modulating drugs,” the authors wrote.
SOURCE:
First author Gudrun David Sigmo, of the department of rheumatology at Stavanger (Norway) University Hospital, and colleagues had their work published online on January 23, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The analysis relied on administrative data, and researchers did not have data on confounding factors.
DISCLOSURES:
The study was funded by the nonprofit organizations Aslaug Anders fond, Astri og Edvard Riisøens legat, Det alminnelige medisinske forskningsfond, Pahles legat, and Fagsenter for medisins-ke kvalitetsregistre i Helse Vest. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Men with an inflammatory arthritis (IA) diagnosis are less likely to be childless than healthy comparators, according to an epidemiological study.
METHODS:
- 10,865 men in the Norwegian Arthritis Registry were compared with 54,325 men without IA, matched by age and location.
- In the arthritis group, 37% had rheumatoid arthritis, 33% had psoriatic arthritis, and 30% had spondyloarthritis.
- Researchers used childlessness and number of children as proxies for male fertility.
TAKEAWAY:
- 21% of men with IA were childless compared with 27% in the healthy cohort (P < .001).
- On an average, a man with IA had 1.80 children whereas a man in the control group had 1.69 children (P < .001).
- These findings were consistent over time, but the most pronounced difference between groups was seen in men diagnosed after the year 2000.
IN PRACTICE:
The finding “is novel and generates new hypotheses regarding associations between fertility, inflammatory rheumatic diseases, and immune-modulating drugs,” the authors wrote.
SOURCE:
First author Gudrun David Sigmo, of the department of rheumatology at Stavanger (Norway) University Hospital, and colleagues had their work published online on January 23, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The analysis relied on administrative data, and researchers did not have data on confounding factors.
DISCLOSURES:
The study was funded by the nonprofit organizations Aslaug Anders fond, Astri og Edvard Riisøens legat, Det alminnelige medisinske forskningsfond, Pahles legat, and Fagsenter for medisins-ke kvalitetsregistre i Helse Vest. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Is RA Disease Activity Assessed Too Little After Starting TNFi?
TOPLINE:
Less than half of patients with rheumatoid arthritis (RA) initiating a first-line tumor necrosis factor inhibitor (TNFi) in clinical practice had a recorded composite disease activity assessment at the start of the treatment, and many remained on that treatment for years without evidence recorded in their electronic medical record of achieving low-disease activity or remission.
METHODOLOGY:
- Researchers reviewed data from 1651 adults aged 18 years and older with moderate to severe RA at baseline or follow-up in the electronic medical record database of the American Rheumatology Network, a large community network of independent practices with > 200 rheumatologists across the United States.
- Patients received a TNFi as their first advanced therapy between January 2014 and August 2021 and were assessed for measurement of disease activity with the Clinical Disease Activity Index (CDAI) or Routine Assessment of Patient Index Data 3 (RAPID3) at baseline and follow-up visits.
TAKEAWAY:
- Among the patients with moderate to severe RA, 47.2% of patients remained on first-line TNFi therapy 1 year after initiation despite no evidence of achieving treatment targets of low disease activity or remission (defined as CDAI ≤ 10 and/or RAPID3 ≤ 2).
- Approximately one third of patients remained on TNFi therapy for 2 (38.1%) or 3 (35.4%) years after initiation despite not achieving these targets. The median times to TNFi discontinuation was 30.4 months and to subsequent therapy initiation 68.3 months.
- A total of 52% discontinued their initial TNFi during the study period; among those who started a second therapy, 15% restarted the same TNFi, 45.6% started another TNFi, 27.6% started a non-TNFi biologic, and 11.5% started a Janus kinase inhibitor.
- The most common reported reasons for discontinuation were a combination of efficacy and intolerance, efficacy only, and intolerance only (26.9%, 25.3%, and 20.3%, respectively).
- Persistent pain was the most common reason for efficacy-related discontinuation (39.0%), followed by persistent inflammation/swelling and overall general discomfort (31.8% for both).
IN PRACTICE:
“Consistent monitoring of treatment response and timely switch to effective therapy as appropriate is needed in patients with RA initiating their first advanced therapies,” the researchers wrote.
SOURCE:
First author Colin Edgerton, MD, of Articularis Healthcare Group and American Rheumatology Network, Charleston, South Carolina, reported their work on January 14, 2024, in ACR Open Rheumatology.
LIMITATIONS:
The findings were limited by several factors including the retrospective design, incomplete data from electronic medical records, and reliance on physician documentation for drivers of discontinuation.
DISCLOSURES:
The study was supported by AbbVie. Lead author Edgerton also disclosed relationships with Novartis and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Less than half of patients with rheumatoid arthritis (RA) initiating a first-line tumor necrosis factor inhibitor (TNFi) in clinical practice had a recorded composite disease activity assessment at the start of the treatment, and many remained on that treatment for years without evidence recorded in their electronic medical record of achieving low-disease activity or remission.
METHODOLOGY:
- Researchers reviewed data from 1651 adults aged 18 years and older with moderate to severe RA at baseline or follow-up in the electronic medical record database of the American Rheumatology Network, a large community network of independent practices with > 200 rheumatologists across the United States.
- Patients received a TNFi as their first advanced therapy between January 2014 and August 2021 and were assessed for measurement of disease activity with the Clinical Disease Activity Index (CDAI) or Routine Assessment of Patient Index Data 3 (RAPID3) at baseline and follow-up visits.
TAKEAWAY:
- Among the patients with moderate to severe RA, 47.2% of patients remained on first-line TNFi therapy 1 year after initiation despite no evidence of achieving treatment targets of low disease activity or remission (defined as CDAI ≤ 10 and/or RAPID3 ≤ 2).
- Approximately one third of patients remained on TNFi therapy for 2 (38.1%) or 3 (35.4%) years after initiation despite not achieving these targets. The median times to TNFi discontinuation was 30.4 months and to subsequent therapy initiation 68.3 months.
- A total of 52% discontinued their initial TNFi during the study period; among those who started a second therapy, 15% restarted the same TNFi, 45.6% started another TNFi, 27.6% started a non-TNFi biologic, and 11.5% started a Janus kinase inhibitor.
- The most common reported reasons for discontinuation were a combination of efficacy and intolerance, efficacy only, and intolerance only (26.9%, 25.3%, and 20.3%, respectively).
- Persistent pain was the most common reason for efficacy-related discontinuation (39.0%), followed by persistent inflammation/swelling and overall general discomfort (31.8% for both).
IN PRACTICE:
“Consistent monitoring of treatment response and timely switch to effective therapy as appropriate is needed in patients with RA initiating their first advanced therapies,” the researchers wrote.
SOURCE:
First author Colin Edgerton, MD, of Articularis Healthcare Group and American Rheumatology Network, Charleston, South Carolina, reported their work on January 14, 2024, in ACR Open Rheumatology.
LIMITATIONS:
The findings were limited by several factors including the retrospective design, incomplete data from electronic medical records, and reliance on physician documentation for drivers of discontinuation.
DISCLOSURES:
The study was supported by AbbVie. Lead author Edgerton also disclosed relationships with Novartis and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Less than half of patients with rheumatoid arthritis (RA) initiating a first-line tumor necrosis factor inhibitor (TNFi) in clinical practice had a recorded composite disease activity assessment at the start of the treatment, and many remained on that treatment for years without evidence recorded in their electronic medical record of achieving low-disease activity or remission.
METHODOLOGY:
- Researchers reviewed data from 1651 adults aged 18 years and older with moderate to severe RA at baseline or follow-up in the electronic medical record database of the American Rheumatology Network, a large community network of independent practices with > 200 rheumatologists across the United States.
- Patients received a TNFi as their first advanced therapy between January 2014 and August 2021 and were assessed for measurement of disease activity with the Clinical Disease Activity Index (CDAI) or Routine Assessment of Patient Index Data 3 (RAPID3) at baseline and follow-up visits.
TAKEAWAY:
- Among the patients with moderate to severe RA, 47.2% of patients remained on first-line TNFi therapy 1 year after initiation despite no evidence of achieving treatment targets of low disease activity or remission (defined as CDAI ≤ 10 and/or RAPID3 ≤ 2).
- Approximately one third of patients remained on TNFi therapy for 2 (38.1%) or 3 (35.4%) years after initiation despite not achieving these targets. The median times to TNFi discontinuation was 30.4 months and to subsequent therapy initiation 68.3 months.
- A total of 52% discontinued their initial TNFi during the study period; among those who started a second therapy, 15% restarted the same TNFi, 45.6% started another TNFi, 27.6% started a non-TNFi biologic, and 11.5% started a Janus kinase inhibitor.
- The most common reported reasons for discontinuation were a combination of efficacy and intolerance, efficacy only, and intolerance only (26.9%, 25.3%, and 20.3%, respectively).
- Persistent pain was the most common reason for efficacy-related discontinuation (39.0%), followed by persistent inflammation/swelling and overall general discomfort (31.8% for both).
IN PRACTICE:
“Consistent monitoring of treatment response and timely switch to effective therapy as appropriate is needed in patients with RA initiating their first advanced therapies,” the researchers wrote.
SOURCE:
First author Colin Edgerton, MD, of Articularis Healthcare Group and American Rheumatology Network, Charleston, South Carolina, reported their work on January 14, 2024, in ACR Open Rheumatology.
LIMITATIONS:
The findings were limited by several factors including the retrospective design, incomplete data from electronic medical records, and reliance on physician documentation for drivers of discontinuation.
DISCLOSURES:
The study was supported by AbbVie. Lead author Edgerton also disclosed relationships with Novartis and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Corticosteroid Injections Don’t Move Blood Sugar for Most
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Patients With Severe RA Improve Functional Limitations With Exercise Therapy
TOPLINE:
Adults whose rheumatoid arthritis caused them severe functional limitations showed significant improvement in measures of function and quality of life following at least 1 year of a personalized, supervised exercise program than those who received usual care.
METHODOLOGY:
- Researchers randomized 217 adults with rheumatoid arthritis and severe functional limitations to an active exercise intervention delivered by a physical therapist (PT) or usual care; the mean age of the participants was approximately 59 years, and approximately 90% were female.
- The intervention consisted of individualized goal setting, active exercises adapted to functional limitations, and education about self-management of physical activity in two sessions per week for the first 12 weeks, followed by once weekly sessions with the option for additional sessions if needed. The primary care PTs in the Netherlands who treated the patients were primarily recruited through a national network of PTs with specific expertise regarding rheumatic diseases.
- In considering each participant’s three most limited activities, the study’s primary outcome at 52 weeks measured the change from the one ranked highest at baseline on the Patient-Specific Complaints Numeric Rating Scale (PSC1 NRS); secondary outcomes included changes in the NRS for participants’ second and third most difficult activities, as well as the Patient Reported Outcome Measurement Information System Physical Function-10, the Health Assessment Questionnaire-Disability Index, the Rheumatoid Arthritis Quality of Life Questionnaire, the 36-Item Short-Form Health Survey (SF-36) Physical and Mental Component Summary Scales (PCS and MCS), and the 6-minute walk test.
TAKEAWAY:
- At 52 weeks, the change in PSC1 NRS was significantly greater in the intervention group than in the usual care group, with a mean difference of −1.7 and a between-group effect size from baseline of 0.7.
- Improvements in secondary outcome measures at 52 weeks also were significantly greater in the intervention group than in the usual care group, with the exception of the SF-36 MCS, which showed no difference between the groups.
- A total of 89 participants in the intervention group and 45 participants in the usual care group responded to questions about muscle soreness and fatigue; 70% and 60%, and 71% and 64%, of each group reported these conditions, respectively.
IN PRACTICE:
“The completion of the trial substantiates the feasibility of recruiting and training primary care [physical therapists] to deliver a complex intervention,” although more research is needed to explore long-term outcomes and cost-effectiveness, the researchers wrote.
SOURCE:
The lead author on the study was Max M.H. Teuwen, MSc, a PhD candidate at Leiden University Medical Center, Leiden, the Netherlands. The study was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The participants were not blinded to their group, and blinded assessors became aware of the allocations, which might have impacted measurements; other limitations included lack of data on medication changes and the exclusion of physical activity amount as an outcome measure.
DISCLOSURES:
The study was supported by the Netherlands Organization for Health Research and Development; the Ministry of Health, Welfare and Sport; the Royal Dutch Society for Physical Therapy; and the Dutch Arthritis Society. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Adults whose rheumatoid arthritis caused them severe functional limitations showed significant improvement in measures of function and quality of life following at least 1 year of a personalized, supervised exercise program than those who received usual care.
METHODOLOGY:
- Researchers randomized 217 adults with rheumatoid arthritis and severe functional limitations to an active exercise intervention delivered by a physical therapist (PT) or usual care; the mean age of the participants was approximately 59 years, and approximately 90% were female.
- The intervention consisted of individualized goal setting, active exercises adapted to functional limitations, and education about self-management of physical activity in two sessions per week for the first 12 weeks, followed by once weekly sessions with the option for additional sessions if needed. The primary care PTs in the Netherlands who treated the patients were primarily recruited through a national network of PTs with specific expertise regarding rheumatic diseases.
- In considering each participant’s three most limited activities, the study’s primary outcome at 52 weeks measured the change from the one ranked highest at baseline on the Patient-Specific Complaints Numeric Rating Scale (PSC1 NRS); secondary outcomes included changes in the NRS for participants’ second and third most difficult activities, as well as the Patient Reported Outcome Measurement Information System Physical Function-10, the Health Assessment Questionnaire-Disability Index, the Rheumatoid Arthritis Quality of Life Questionnaire, the 36-Item Short-Form Health Survey (SF-36) Physical and Mental Component Summary Scales (PCS and MCS), and the 6-minute walk test.
TAKEAWAY:
- At 52 weeks, the change in PSC1 NRS was significantly greater in the intervention group than in the usual care group, with a mean difference of −1.7 and a between-group effect size from baseline of 0.7.
- Improvements in secondary outcome measures at 52 weeks also were significantly greater in the intervention group than in the usual care group, with the exception of the SF-36 MCS, which showed no difference between the groups.
- A total of 89 participants in the intervention group and 45 participants in the usual care group responded to questions about muscle soreness and fatigue; 70% and 60%, and 71% and 64%, of each group reported these conditions, respectively.
IN PRACTICE:
“The completion of the trial substantiates the feasibility of recruiting and training primary care [physical therapists] to deliver a complex intervention,” although more research is needed to explore long-term outcomes and cost-effectiveness, the researchers wrote.
SOURCE:
The lead author on the study was Max M.H. Teuwen, MSc, a PhD candidate at Leiden University Medical Center, Leiden, the Netherlands. The study was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The participants were not blinded to their group, and blinded assessors became aware of the allocations, which might have impacted measurements; other limitations included lack of data on medication changes and the exclusion of physical activity amount as an outcome measure.
DISCLOSURES:
The study was supported by the Netherlands Organization for Health Research and Development; the Ministry of Health, Welfare and Sport; the Royal Dutch Society for Physical Therapy; and the Dutch Arthritis Society. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Adults whose rheumatoid arthritis caused them severe functional limitations showed significant improvement in measures of function and quality of life following at least 1 year of a personalized, supervised exercise program than those who received usual care.
METHODOLOGY:
- Researchers randomized 217 adults with rheumatoid arthritis and severe functional limitations to an active exercise intervention delivered by a physical therapist (PT) or usual care; the mean age of the participants was approximately 59 years, and approximately 90% were female.
- The intervention consisted of individualized goal setting, active exercises adapted to functional limitations, and education about self-management of physical activity in two sessions per week for the first 12 weeks, followed by once weekly sessions with the option for additional sessions if needed. The primary care PTs in the Netherlands who treated the patients were primarily recruited through a national network of PTs with specific expertise regarding rheumatic diseases.
- In considering each participant’s three most limited activities, the study’s primary outcome at 52 weeks measured the change from the one ranked highest at baseline on the Patient-Specific Complaints Numeric Rating Scale (PSC1 NRS); secondary outcomes included changes in the NRS for participants’ second and third most difficult activities, as well as the Patient Reported Outcome Measurement Information System Physical Function-10, the Health Assessment Questionnaire-Disability Index, the Rheumatoid Arthritis Quality of Life Questionnaire, the 36-Item Short-Form Health Survey (SF-36) Physical and Mental Component Summary Scales (PCS and MCS), and the 6-minute walk test.
TAKEAWAY:
- At 52 weeks, the change in PSC1 NRS was significantly greater in the intervention group than in the usual care group, with a mean difference of −1.7 and a between-group effect size from baseline of 0.7.
- Improvements in secondary outcome measures at 52 weeks also were significantly greater in the intervention group than in the usual care group, with the exception of the SF-36 MCS, which showed no difference between the groups.
- A total of 89 participants in the intervention group and 45 participants in the usual care group responded to questions about muscle soreness and fatigue; 70% and 60%, and 71% and 64%, of each group reported these conditions, respectively.
IN PRACTICE:
“The completion of the trial substantiates the feasibility of recruiting and training primary care [physical therapists] to deliver a complex intervention,” although more research is needed to explore long-term outcomes and cost-effectiveness, the researchers wrote.
SOURCE:
The lead author on the study was Max M.H. Teuwen, MSc, a PhD candidate at Leiden University Medical Center, Leiden, the Netherlands. The study was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The participants were not blinded to their group, and blinded assessors became aware of the allocations, which might have impacted measurements; other limitations included lack of data on medication changes and the exclusion of physical activity amount as an outcome measure.
DISCLOSURES:
The study was supported by the Netherlands Organization for Health Research and Development; the Ministry of Health, Welfare and Sport; the Royal Dutch Society for Physical Therapy; and the Dutch Arthritis Society. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Tool Uses Genetics to Assist With Diagnosis of Early Inflammatory Arthritis
A new diagnostic tool can effectively discriminate different rheumatologic conditions and could potentially aid in the diagnosis of early inflammatory arthritis.
The algorithm — called Genetic Probability tool (G-PROB) — uses genetic information to calculate the probability of certain diseases.
“At such an early stage of disease, it’s not always easy to determine what the final outcome will be with respect to final diagnosis,” said John Bowes, PhD, a senior lecturer in the division of musculoskeletal & dermatological sciences at the University of Manchester in the United Kingdom. He was a senior author of the newest study of G-PROB. “What we are hoping for here is that genetics can help [clinicians] with the decision-making process and hopefully accelerate the correct diagnosis and get individuals onto the correct treatment as early as possible.”
Creating the Algorithm
G-PROB was first developed by an international group of scientists with the goal of using genetic risk scores to predict the probabilities of common diagnoses for patients with early signs of arthritis, such as synovitis and joint swelling. According to the study authors, about 80% of these types of patients are eventually diagnosed with the following conditions: Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and gout.
The algorithm combines existing knowledge about single-nucleotide polymorphisms from prior genomic studies to create genetic risk scores — also called polygenic risk score (PRS) — for multiple diseases. Using these scores, the program then calculates the probabilities of certain diagnoses for a patient, based on the assumption that at least one disease was present.
In this first study, researchers trained the tool on simulated data and then tested it in three patient cohorts totaling about 1700 individuals from the Electronic Medical Records and Genomics database and Mass General Brigham Biobank. In the initial study, G-PROB identified a likely diagnosis in 45% of patients, with a positive predictive value (PPV) of 64%. Adding these genetic scores to clinical data improved diagnostic accuracy from 39% to 51%.
Validating G-PROB
But data from these biobanks may not necessarily be representative of early arthritis in patients appearing in outpatient clinics, noted Dr. Bowes. In this new study, researchers sought to independently validate the original study’s findings using data from the Norfolk Arthritis Register, a community-based, long-term observational study on inflammatory polyarthritis. The team applied G-PROB in this cohort and then compared the tool’s probabilities for common rheumatic conditions to the final clinician diagnosis.
The study ultimately included 1047 individuals with early inflammatory arthritis with genotype data. In the cohort, more than 70% (756 individuals) were diagnosed with RA. Of the remaining patients, 104 had PsA, 18 had SLE, 16 had AS, and 12 had gout. The research team also added an “other diseases” category to the algorithm. A total of 141 patients fell into this category and were diagnosed with diseases including chronic pain syndrome (52 individuals), polymyalgia rheumatica (29 individuals), and Sjögren’s syndrome (9 individuals).
G-PROB was best at excluding diagnoses: Probabilities under 5% for a single disease corresponded to a negative predictive value (NPV) of 96%. If probabilities for two diseases were both < 5%, the NPV was 94%.
For patients with a single probability above 50%, the tool had a PPV of 70.3%. In 55.7% of all patients, the disease with the highest probability ended up being the final diagnosis.
Generally, PRSs, as well as tests using biomarkers, were better at excluding diagnoses than affirming them, noted Matthew Brown, MBBS, MD, a professor of medicine at King’s College London, who was not involved with the research. If disease prevalence is low, then a test aimed at diagnosis of that disease would be better at excluding a diagnosis than affirming it, he explained.
However, he noted that G-PROB’s PPV may have performed better if researchers had started by using established PRS scores to form the algorithm, rather than developing these genetic scores independently using internal datasets.
Can G-PROB Improve Diagnosis?
The new study’s key contribution was that it independently validated findings from a previous study, noted Katherine Liao, MD, a rheumatologist at Brigham and Women’s Hospital in Boston, Massachusetts. She coauthored an accompanying editorial to the newest study and coauthored the original G-PROB paper.
This new study also brought up an important question about G-PROB that has yet to be tested: Will this tool help clinicians make more efficient and accurate diagnoses in practice?
A prospective trial would be necessary to begin answering this question, both Dr. Bowes and Dr. Liao agreed. For example, one clinician group would have access to G-PROB data, while another would not, and “see if that helps [the first group] make the diagnosis faster or more accurately,” Dr. Liao said.
Dr. Bowes was also interested in exploring if combining G-PROB with other clinical data would improve diagnostic performance.
“Genetics isn’t the full story,” he said. Dr. Bowes saw genetics as one additional, complementary tool in a clinician’s toolbox.
Future studies were needed to understand the clinical utility of genetic information in conjunction with current diagnostic practices, such as imaging, physical exams, and lab results, Dr. Liao and her editorial coauthors argued.
“For example, in cardiovascular disease, the clinical utility of polygenic risk scores has been defined by their ability to improve risk stratification beyond what is already achieved with more common risk factors and measures such as cholesterol levels, smoking status, and coronary calcium scores,” Dr. Liao and her coauthors wrote. “Similarly, a polygenic risk score for breast cancer would not be clinically implemented alone for risk prediction but rather as one risk factor among others, such as hormonal and reproductive factors and prior mammographic data.”
Future of Genetics in Rheumatology
An additional hurdle for using tools like G-PROB was that a patient must have undergone DNA sequencing, and these data must be available to clinicians. Even a decade ago, this type of testing may have seemed unrealistic to incorporate in daily practice, Dr. Liao noted, but technological advancements continue to make genetic sequencing more accessible to the public.
There are already efforts in the United Kingdom to incorporate genetics into healthcare, including trials for PRSs and heart disease, noted Dr. Bowes, as well as large-scale studies such as Our Future Health.
“As these population-based studies expand more, a high proportion of individuals should hopefully have access to this kind of data,” he said.
Brown added that genetic testing is already used to make rheumatology diagnoses.
“[HLA] B-27 testing, for example, is an extremely commonly used test to assist in the diagnosis of ankylosing spondylitis. Is it that different to change to a PRS as opposed to a straight HLA testing? I don’t think it is,” he said.
While there would need to be systematic training for clinicians to understand how to calculate and use PRSs in daily practice, Dr. Brown did not think this adjustment would be too difficult.
“There is a lot of exceptionalism about genetics, which is actually inappropriate,” he said. “This is actually just a quantitative score that should be easy for people to interpret.”
Dr. Bowes and Dr. Brown reported no relevant financial relationships. Dr. Liao worked as a consultant for UCB.
A version of this article appeared on Medscape.com.
A new diagnostic tool can effectively discriminate different rheumatologic conditions and could potentially aid in the diagnosis of early inflammatory arthritis.
The algorithm — called Genetic Probability tool (G-PROB) — uses genetic information to calculate the probability of certain diseases.
“At such an early stage of disease, it’s not always easy to determine what the final outcome will be with respect to final diagnosis,” said John Bowes, PhD, a senior lecturer in the division of musculoskeletal & dermatological sciences at the University of Manchester in the United Kingdom. He was a senior author of the newest study of G-PROB. “What we are hoping for here is that genetics can help [clinicians] with the decision-making process and hopefully accelerate the correct diagnosis and get individuals onto the correct treatment as early as possible.”
Creating the Algorithm
G-PROB was first developed by an international group of scientists with the goal of using genetic risk scores to predict the probabilities of common diagnoses for patients with early signs of arthritis, such as synovitis and joint swelling. According to the study authors, about 80% of these types of patients are eventually diagnosed with the following conditions: Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and gout.
The algorithm combines existing knowledge about single-nucleotide polymorphisms from prior genomic studies to create genetic risk scores — also called polygenic risk score (PRS) — for multiple diseases. Using these scores, the program then calculates the probabilities of certain diagnoses for a patient, based on the assumption that at least one disease was present.
In this first study, researchers trained the tool on simulated data and then tested it in three patient cohorts totaling about 1700 individuals from the Electronic Medical Records and Genomics database and Mass General Brigham Biobank. In the initial study, G-PROB identified a likely diagnosis in 45% of patients, with a positive predictive value (PPV) of 64%. Adding these genetic scores to clinical data improved diagnostic accuracy from 39% to 51%.
Validating G-PROB
But data from these biobanks may not necessarily be representative of early arthritis in patients appearing in outpatient clinics, noted Dr. Bowes. In this new study, researchers sought to independently validate the original study’s findings using data from the Norfolk Arthritis Register, a community-based, long-term observational study on inflammatory polyarthritis. The team applied G-PROB in this cohort and then compared the tool’s probabilities for common rheumatic conditions to the final clinician diagnosis.
The study ultimately included 1047 individuals with early inflammatory arthritis with genotype data. In the cohort, more than 70% (756 individuals) were diagnosed with RA. Of the remaining patients, 104 had PsA, 18 had SLE, 16 had AS, and 12 had gout. The research team also added an “other diseases” category to the algorithm. A total of 141 patients fell into this category and were diagnosed with diseases including chronic pain syndrome (52 individuals), polymyalgia rheumatica (29 individuals), and Sjögren’s syndrome (9 individuals).
G-PROB was best at excluding diagnoses: Probabilities under 5% for a single disease corresponded to a negative predictive value (NPV) of 96%. If probabilities for two diseases were both < 5%, the NPV was 94%.
For patients with a single probability above 50%, the tool had a PPV of 70.3%. In 55.7% of all patients, the disease with the highest probability ended up being the final diagnosis.
Generally, PRSs, as well as tests using biomarkers, were better at excluding diagnoses than affirming them, noted Matthew Brown, MBBS, MD, a professor of medicine at King’s College London, who was not involved with the research. If disease prevalence is low, then a test aimed at diagnosis of that disease would be better at excluding a diagnosis than affirming it, he explained.
However, he noted that G-PROB’s PPV may have performed better if researchers had started by using established PRS scores to form the algorithm, rather than developing these genetic scores independently using internal datasets.
Can G-PROB Improve Diagnosis?
The new study’s key contribution was that it independently validated findings from a previous study, noted Katherine Liao, MD, a rheumatologist at Brigham and Women’s Hospital in Boston, Massachusetts. She coauthored an accompanying editorial to the newest study and coauthored the original G-PROB paper.
This new study also brought up an important question about G-PROB that has yet to be tested: Will this tool help clinicians make more efficient and accurate diagnoses in practice?
A prospective trial would be necessary to begin answering this question, both Dr. Bowes and Dr. Liao agreed. For example, one clinician group would have access to G-PROB data, while another would not, and “see if that helps [the first group] make the diagnosis faster or more accurately,” Dr. Liao said.
Dr. Bowes was also interested in exploring if combining G-PROB with other clinical data would improve diagnostic performance.
“Genetics isn’t the full story,” he said. Dr. Bowes saw genetics as one additional, complementary tool in a clinician’s toolbox.
Future studies were needed to understand the clinical utility of genetic information in conjunction with current diagnostic practices, such as imaging, physical exams, and lab results, Dr. Liao and her editorial coauthors argued.
“For example, in cardiovascular disease, the clinical utility of polygenic risk scores has been defined by their ability to improve risk stratification beyond what is already achieved with more common risk factors and measures such as cholesterol levels, smoking status, and coronary calcium scores,” Dr. Liao and her coauthors wrote. “Similarly, a polygenic risk score for breast cancer would not be clinically implemented alone for risk prediction but rather as one risk factor among others, such as hormonal and reproductive factors and prior mammographic data.”
Future of Genetics in Rheumatology
An additional hurdle for using tools like G-PROB was that a patient must have undergone DNA sequencing, and these data must be available to clinicians. Even a decade ago, this type of testing may have seemed unrealistic to incorporate in daily practice, Dr. Liao noted, but technological advancements continue to make genetic sequencing more accessible to the public.
There are already efforts in the United Kingdom to incorporate genetics into healthcare, including trials for PRSs and heart disease, noted Dr. Bowes, as well as large-scale studies such as Our Future Health.
“As these population-based studies expand more, a high proportion of individuals should hopefully have access to this kind of data,” he said.
Brown added that genetic testing is already used to make rheumatology diagnoses.
“[HLA] B-27 testing, for example, is an extremely commonly used test to assist in the diagnosis of ankylosing spondylitis. Is it that different to change to a PRS as opposed to a straight HLA testing? I don’t think it is,” he said.
While there would need to be systematic training for clinicians to understand how to calculate and use PRSs in daily practice, Dr. Brown did not think this adjustment would be too difficult.
“There is a lot of exceptionalism about genetics, which is actually inappropriate,” he said. “This is actually just a quantitative score that should be easy for people to interpret.”
Dr. Bowes and Dr. Brown reported no relevant financial relationships. Dr. Liao worked as a consultant for UCB.
A version of this article appeared on Medscape.com.
A new diagnostic tool can effectively discriminate different rheumatologic conditions and could potentially aid in the diagnosis of early inflammatory arthritis.
The algorithm — called Genetic Probability tool (G-PROB) — uses genetic information to calculate the probability of certain diseases.
“At such an early stage of disease, it’s not always easy to determine what the final outcome will be with respect to final diagnosis,” said John Bowes, PhD, a senior lecturer in the division of musculoskeletal & dermatological sciences at the University of Manchester in the United Kingdom. He was a senior author of the newest study of G-PROB. “What we are hoping for here is that genetics can help [clinicians] with the decision-making process and hopefully accelerate the correct diagnosis and get individuals onto the correct treatment as early as possible.”
Creating the Algorithm
G-PROB was first developed by an international group of scientists with the goal of using genetic risk scores to predict the probabilities of common diagnoses for patients with early signs of arthritis, such as synovitis and joint swelling. According to the study authors, about 80% of these types of patients are eventually diagnosed with the following conditions: Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and gout.
The algorithm combines existing knowledge about single-nucleotide polymorphisms from prior genomic studies to create genetic risk scores — also called polygenic risk score (PRS) — for multiple diseases. Using these scores, the program then calculates the probabilities of certain diagnoses for a patient, based on the assumption that at least one disease was present.
In this first study, researchers trained the tool on simulated data and then tested it in three patient cohorts totaling about 1700 individuals from the Electronic Medical Records and Genomics database and Mass General Brigham Biobank. In the initial study, G-PROB identified a likely diagnosis in 45% of patients, with a positive predictive value (PPV) of 64%. Adding these genetic scores to clinical data improved diagnostic accuracy from 39% to 51%.
Validating G-PROB
But data from these biobanks may not necessarily be representative of early arthritis in patients appearing in outpatient clinics, noted Dr. Bowes. In this new study, researchers sought to independently validate the original study’s findings using data from the Norfolk Arthritis Register, a community-based, long-term observational study on inflammatory polyarthritis. The team applied G-PROB in this cohort and then compared the tool’s probabilities for common rheumatic conditions to the final clinician diagnosis.
The study ultimately included 1047 individuals with early inflammatory arthritis with genotype data. In the cohort, more than 70% (756 individuals) were diagnosed with RA. Of the remaining patients, 104 had PsA, 18 had SLE, 16 had AS, and 12 had gout. The research team also added an “other diseases” category to the algorithm. A total of 141 patients fell into this category and were diagnosed with diseases including chronic pain syndrome (52 individuals), polymyalgia rheumatica (29 individuals), and Sjögren’s syndrome (9 individuals).
G-PROB was best at excluding diagnoses: Probabilities under 5% for a single disease corresponded to a negative predictive value (NPV) of 96%. If probabilities for two diseases were both < 5%, the NPV was 94%.
For patients with a single probability above 50%, the tool had a PPV of 70.3%. In 55.7% of all patients, the disease with the highest probability ended up being the final diagnosis.
Generally, PRSs, as well as tests using biomarkers, were better at excluding diagnoses than affirming them, noted Matthew Brown, MBBS, MD, a professor of medicine at King’s College London, who was not involved with the research. If disease prevalence is low, then a test aimed at diagnosis of that disease would be better at excluding a diagnosis than affirming it, he explained.
However, he noted that G-PROB’s PPV may have performed better if researchers had started by using established PRS scores to form the algorithm, rather than developing these genetic scores independently using internal datasets.
Can G-PROB Improve Diagnosis?
The new study’s key contribution was that it independently validated findings from a previous study, noted Katherine Liao, MD, a rheumatologist at Brigham and Women’s Hospital in Boston, Massachusetts. She coauthored an accompanying editorial to the newest study and coauthored the original G-PROB paper.
This new study also brought up an important question about G-PROB that has yet to be tested: Will this tool help clinicians make more efficient and accurate diagnoses in practice?
A prospective trial would be necessary to begin answering this question, both Dr. Bowes and Dr. Liao agreed. For example, one clinician group would have access to G-PROB data, while another would not, and “see if that helps [the first group] make the diagnosis faster or more accurately,” Dr. Liao said.
Dr. Bowes was also interested in exploring if combining G-PROB with other clinical data would improve diagnostic performance.
“Genetics isn’t the full story,” he said. Dr. Bowes saw genetics as one additional, complementary tool in a clinician’s toolbox.
Future studies were needed to understand the clinical utility of genetic information in conjunction with current diagnostic practices, such as imaging, physical exams, and lab results, Dr. Liao and her editorial coauthors argued.
“For example, in cardiovascular disease, the clinical utility of polygenic risk scores has been defined by their ability to improve risk stratification beyond what is already achieved with more common risk factors and measures such as cholesterol levels, smoking status, and coronary calcium scores,” Dr. Liao and her coauthors wrote. “Similarly, a polygenic risk score for breast cancer would not be clinically implemented alone for risk prediction but rather as one risk factor among others, such as hormonal and reproductive factors and prior mammographic data.”
Future of Genetics in Rheumatology
An additional hurdle for using tools like G-PROB was that a patient must have undergone DNA sequencing, and these data must be available to clinicians. Even a decade ago, this type of testing may have seemed unrealistic to incorporate in daily practice, Dr. Liao noted, but technological advancements continue to make genetic sequencing more accessible to the public.
There are already efforts in the United Kingdom to incorporate genetics into healthcare, including trials for PRSs and heart disease, noted Dr. Bowes, as well as large-scale studies such as Our Future Health.
“As these population-based studies expand more, a high proportion of individuals should hopefully have access to this kind of data,” he said.
Brown added that genetic testing is already used to make rheumatology diagnoses.
“[HLA] B-27 testing, for example, is an extremely commonly used test to assist in the diagnosis of ankylosing spondylitis. Is it that different to change to a PRS as opposed to a straight HLA testing? I don’t think it is,” he said.
While there would need to be systematic training for clinicians to understand how to calculate and use PRSs in daily practice, Dr. Brown did not think this adjustment would be too difficult.
“There is a lot of exceptionalism about genetics, which is actually inappropriate,” he said. “This is actually just a quantitative score that should be easy for people to interpret.”
Dr. Bowes and Dr. Brown reported no relevant financial relationships. Dr. Liao worked as a consultant for UCB.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Obesity in Patients With RA Successfully Managed With Remote Diet, Exercise Intervention
TOPLINE:
A combination of remote, supervised aerobic training, resistance training, and a hypocaloric diet significantly improved cardiovascular risk factors in adults with rheumatoid arthritis (RA) and overweight or obesity.
METHODOLOGY:
- The researchers recruited 24 adults aged 60-80 years with RA who met criteria for overweight or obesity; participants were randomized to a Supervised Weight Loss and Exercise Training (SWET) or Counseling Health as Treatment (CHAT) program for 16 weeks.
- The SWET intervention included remote supervision of aerobic training of 150 minutes/week moderate-to-vigorous intensity, 2 days per week of resistance training, and a hypocaloric diet based on a weight loss goal of 7% of body weight. The CHAT patients served as controls and completed two lifestyle counseling sessions followed by monthly check-ins.
- The primary outcome was change in a composite measure of cardiovascular risk based on metabolic syndrome z-score (MSSc), a continuous weighted score of five metabolic syndrome components: Waist circumference, mean arterial blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol.
TAKEAWAY:
- Both groups showed improvement in the primary outcome of MSSc, with absolute changes from baseline of −1.67 for the SWET group and −1.34 for the CHAT group (P < .01 for both).
- Participants in the SWET group showed significantly more improvement in secondary outcome measures of body weight, fat mass, and disease activity score in 28 joints based on C-reactive protein (DAS28-CRP), as well as greater improvement in patient-reported physical and mental health, physical function, and fatigue, than those in the CHAT group, but the CHAT group improved significantly compared with their baseline.
- The strongest specific effects for the different components of the intervention were those of aerobic training on physical function and fatigue, resistance training on DAS28-CRP, and weight loss on MSSc.
- Neither group experienced significant changes in lean mass, absolute peak V02, unilateral isometric knee extension, or bilateral grip strength.
IN PRACTICE:
“Findings from our study indicate, at a minimum, integrating even 2 hours of healthy lifestyle counseling may improve RA management, let alone demonstrate the substantial impact that can be provided by a comprehensive, remotely supervised lifestyle intervention,” the researchers wrote.
SOURCE:
The lead author on the study was Brian J. Andonian, MD, of Duke University, Durham, North Carolina. The study was published online in ACR Open Rheumatology.
LIMITATIONS:
The small sample size was a limitation of the study findings, as was the lack of blinding and high level of motivation in the CHAT group, who had greater improvements than expected in weight loss and increased physical activity; the study also was conducted during the COVID-19 pandemic, with potential physical and mental effects on participants who tested positive during the study period.
DISCLOSURES:
The study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Claude D. Pepper Older Americans Independence Center of the US National Institute on Aging.
A version of this article appeared on Medscape.com.
TOPLINE:
A combination of remote, supervised aerobic training, resistance training, and a hypocaloric diet significantly improved cardiovascular risk factors in adults with rheumatoid arthritis (RA) and overweight or obesity.
METHODOLOGY:
- The researchers recruited 24 adults aged 60-80 years with RA who met criteria for overweight or obesity; participants were randomized to a Supervised Weight Loss and Exercise Training (SWET) or Counseling Health as Treatment (CHAT) program for 16 weeks.
- The SWET intervention included remote supervision of aerobic training of 150 minutes/week moderate-to-vigorous intensity, 2 days per week of resistance training, and a hypocaloric diet based on a weight loss goal of 7% of body weight. The CHAT patients served as controls and completed two lifestyle counseling sessions followed by monthly check-ins.
- The primary outcome was change in a composite measure of cardiovascular risk based on metabolic syndrome z-score (MSSc), a continuous weighted score of five metabolic syndrome components: Waist circumference, mean arterial blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol.
TAKEAWAY:
- Both groups showed improvement in the primary outcome of MSSc, with absolute changes from baseline of −1.67 for the SWET group and −1.34 for the CHAT group (P < .01 for both).
- Participants in the SWET group showed significantly more improvement in secondary outcome measures of body weight, fat mass, and disease activity score in 28 joints based on C-reactive protein (DAS28-CRP), as well as greater improvement in patient-reported physical and mental health, physical function, and fatigue, than those in the CHAT group, but the CHAT group improved significantly compared with their baseline.
- The strongest specific effects for the different components of the intervention were those of aerobic training on physical function and fatigue, resistance training on DAS28-CRP, and weight loss on MSSc.
- Neither group experienced significant changes in lean mass, absolute peak V02, unilateral isometric knee extension, or bilateral grip strength.
IN PRACTICE:
“Findings from our study indicate, at a minimum, integrating even 2 hours of healthy lifestyle counseling may improve RA management, let alone demonstrate the substantial impact that can be provided by a comprehensive, remotely supervised lifestyle intervention,” the researchers wrote.
SOURCE:
The lead author on the study was Brian J. Andonian, MD, of Duke University, Durham, North Carolina. The study was published online in ACR Open Rheumatology.
LIMITATIONS:
The small sample size was a limitation of the study findings, as was the lack of blinding and high level of motivation in the CHAT group, who had greater improvements than expected in weight loss and increased physical activity; the study also was conducted during the COVID-19 pandemic, with potential physical and mental effects on participants who tested positive during the study period.
DISCLOSURES:
The study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Claude D. Pepper Older Americans Independence Center of the US National Institute on Aging.
A version of this article appeared on Medscape.com.
TOPLINE:
A combination of remote, supervised aerobic training, resistance training, and a hypocaloric diet significantly improved cardiovascular risk factors in adults with rheumatoid arthritis (RA) and overweight or obesity.
METHODOLOGY:
- The researchers recruited 24 adults aged 60-80 years with RA who met criteria for overweight or obesity; participants were randomized to a Supervised Weight Loss and Exercise Training (SWET) or Counseling Health as Treatment (CHAT) program for 16 weeks.
- The SWET intervention included remote supervision of aerobic training of 150 minutes/week moderate-to-vigorous intensity, 2 days per week of resistance training, and a hypocaloric diet based on a weight loss goal of 7% of body weight. The CHAT patients served as controls and completed two lifestyle counseling sessions followed by monthly check-ins.
- The primary outcome was change in a composite measure of cardiovascular risk based on metabolic syndrome z-score (MSSc), a continuous weighted score of five metabolic syndrome components: Waist circumference, mean arterial blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol.
TAKEAWAY:
- Both groups showed improvement in the primary outcome of MSSc, with absolute changes from baseline of −1.67 for the SWET group and −1.34 for the CHAT group (P < .01 for both).
- Participants in the SWET group showed significantly more improvement in secondary outcome measures of body weight, fat mass, and disease activity score in 28 joints based on C-reactive protein (DAS28-CRP), as well as greater improvement in patient-reported physical and mental health, physical function, and fatigue, than those in the CHAT group, but the CHAT group improved significantly compared with their baseline.
- The strongest specific effects for the different components of the intervention were those of aerobic training on physical function and fatigue, resistance training on DAS28-CRP, and weight loss on MSSc.
- Neither group experienced significant changes in lean mass, absolute peak V02, unilateral isometric knee extension, or bilateral grip strength.
IN PRACTICE:
“Findings from our study indicate, at a minimum, integrating even 2 hours of healthy lifestyle counseling may improve RA management, let alone demonstrate the substantial impact that can be provided by a comprehensive, remotely supervised lifestyle intervention,” the researchers wrote.
SOURCE:
The lead author on the study was Brian J. Andonian, MD, of Duke University, Durham, North Carolina. The study was published online in ACR Open Rheumatology.
LIMITATIONS:
The small sample size was a limitation of the study findings, as was the lack of blinding and high level of motivation in the CHAT group, who had greater improvements than expected in weight loss and increased physical activity; the study also was conducted during the COVID-19 pandemic, with potential physical and mental effects on participants who tested positive during the study period.
DISCLOSURES:
The study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Claude D. Pepper Older Americans Independence Center of the US National Institute on Aging.
A version of this article appeared on Medscape.com.