User login
Thoracic Oncology & Chest Imaging Network
Lung Cancer Section
What is comprehensive biomarker testing and who should order it? For non–small cell lung cancer, comprehensive biomarker testing is generally defined as testing eligible patients for all biomarkers that direct the use of FDA-approved therapies (Mileham KF, et al. Cancer Med. 2022;11[2]:530. What comprises comprehensive testing has changed over time and will likely continue to change as advances in biomarkers, therapies, and indications for their use continue to evolve. There are also some potential benefits to testing biomarkers without FDA-approved therapies, such as assessing eligibility for treatment as part of a clinical trial or for identifying treatment options that gain FDA-approval in the future. As for who should be responsible for biomarker test ordering, this remains unclear and variable between institutions and practices (Fox AH, et al. Chest. 2021;160[6]:2293). All subspecialties involved, including pulmonology, pathology, interventional radiology, surgery, and oncology, have the potential for knowledge gaps surrounding biomarker testing (Gregg JP, et al. Transl Lung Cancer Res. 2019;8[3]:286; Smeltzer MP, et al. J Thorac Oncol. 2020;15[9]:1434). Those obtaining diagnostic tissue, including pulmonologists, surgeons, and interventional radiologists may not appreciate the downstream use of each biomarker but are in the place to order testing as soon as the time of biopsy. Pathologists may be unaware of clinical aspects to the patient’s case, such as the suspected clinical stage of disease. Oncologists arguably have the best chance of having the expertise to order testing but, ideally, biomarker results would be available by the time a patient meets with an oncologist to discuss treatment options. There is no perfect solution to this question at present, but if you are involved with the diagnosis of lung cancer, you should collaborate with your multidisciplinary team to streamline testing and strategize how to best serve patients.
Adam Fox, MD
Section Fellow-in-Training
Lung Cancer Section
What is comprehensive biomarker testing and who should order it? For non–small cell lung cancer, comprehensive biomarker testing is generally defined as testing eligible patients for all biomarkers that direct the use of FDA-approved therapies (Mileham KF, et al. Cancer Med. 2022;11[2]:530. What comprises comprehensive testing has changed over time and will likely continue to change as advances in biomarkers, therapies, and indications for their use continue to evolve. There are also some potential benefits to testing biomarkers without FDA-approved therapies, such as assessing eligibility for treatment as part of a clinical trial or for identifying treatment options that gain FDA-approval in the future. As for who should be responsible for biomarker test ordering, this remains unclear and variable between institutions and practices (Fox AH, et al. Chest. 2021;160[6]:2293). All subspecialties involved, including pulmonology, pathology, interventional radiology, surgery, and oncology, have the potential for knowledge gaps surrounding biomarker testing (Gregg JP, et al. Transl Lung Cancer Res. 2019;8[3]:286; Smeltzer MP, et al. J Thorac Oncol. 2020;15[9]:1434). Those obtaining diagnostic tissue, including pulmonologists, surgeons, and interventional radiologists may not appreciate the downstream use of each biomarker but are in the place to order testing as soon as the time of biopsy. Pathologists may be unaware of clinical aspects to the patient’s case, such as the suspected clinical stage of disease. Oncologists arguably have the best chance of having the expertise to order testing but, ideally, biomarker results would be available by the time a patient meets with an oncologist to discuss treatment options. There is no perfect solution to this question at present, but if you are involved with the diagnosis of lung cancer, you should collaborate with your multidisciplinary team to streamline testing and strategize how to best serve patients.
Adam Fox, MD
Section Fellow-in-Training
Lung Cancer Section
What is comprehensive biomarker testing and who should order it? For non–small cell lung cancer, comprehensive biomarker testing is generally defined as testing eligible patients for all biomarkers that direct the use of FDA-approved therapies (Mileham KF, et al. Cancer Med. 2022;11[2]:530. What comprises comprehensive testing has changed over time and will likely continue to change as advances in biomarkers, therapies, and indications for their use continue to evolve. There are also some potential benefits to testing biomarkers without FDA-approved therapies, such as assessing eligibility for treatment as part of a clinical trial or for identifying treatment options that gain FDA-approval in the future. As for who should be responsible for biomarker test ordering, this remains unclear and variable between institutions and practices (Fox AH, et al. Chest. 2021;160[6]:2293). All subspecialties involved, including pulmonology, pathology, interventional radiology, surgery, and oncology, have the potential for knowledge gaps surrounding biomarker testing (Gregg JP, et al. Transl Lung Cancer Res. 2019;8[3]:286; Smeltzer MP, et al. J Thorac Oncol. 2020;15[9]:1434). Those obtaining diagnostic tissue, including pulmonologists, surgeons, and interventional radiologists may not appreciate the downstream use of each biomarker but are in the place to order testing as soon as the time of biopsy. Pathologists may be unaware of clinical aspects to the patient’s case, such as the suspected clinical stage of disease. Oncologists arguably have the best chance of having the expertise to order testing but, ideally, biomarker results would be available by the time a patient meets with an oncologist to discuss treatment options. There is no perfect solution to this question at present, but if you are involved with the diagnosis of lung cancer, you should collaborate with your multidisciplinary team to streamline testing and strategize how to best serve patients.
Adam Fox, MD
Section Fellow-in-Training
Chest Infections & Disaster Response Network
Chest Infections Section
An evolving diagnostic tool: Microbial cell-free DNA
The diagnosis of the microbial etiology of pneumonia remains a significant challenge with <50% yield of blood and sputum cultures in most studies. More reliable samples, like bronchoalveolar lavage, require invasive procedures. Undifferentiated pneumonia hampers antimicrobial stewardship and increases the risk of suboptimal treatment. New diagnostic tools that detect degraded microbial DNA in plasma, known as microbial cell-free DNA (cfDNA), may offer improved diagnostic yield. Through metagenomic next-generation approaches, these tools sequence DNA fragments to identify viral, bacterial, and fungal sequences.
Earlier studies of cfDNA in pneumonia have been mixed, correctly identifying the pathogen in 55% to 86% of cases – though notably cfDNA was superior to PCR and cultures and provided early detection of VAP in some cases (Farnaes L, et al. Diagn Microbiol Infect Dis. 2019;94:188; Langelier C, et al. Am J Respir Crit Care Med. 2020;201:491). However, a recent study of cfDNA in severe complicated pediatric pneumonia had promising results with significant clinical impact. cfDNA provided an accurate microbial diagnosis in 89% of cases, with it being the only positive study in 70% of cases. Further, cfDNA narrowed the antimicrobial regimen in 81% of cases (Dworsky ZD, et al. Hosp Pediatr. 2022;12:377).
The use of cfDNA is still in its infancy. Limitations, such as a lack of validated thresholds to differentiate colonization vs infection are noted given its detection sensitivity. Its utility, including ideal timing and patient population, needs further investigation. However, diagnostic cfDNA may soon provide earlier and less invasive microbial diagnostics in patients with chest infections and beyond.
Gregory Wigger, MD
Section Fellow-in-Training
Chest Infections Section
An evolving diagnostic tool: Microbial cell-free DNA
The diagnosis of the microbial etiology of pneumonia remains a significant challenge with <50% yield of blood and sputum cultures in most studies. More reliable samples, like bronchoalveolar lavage, require invasive procedures. Undifferentiated pneumonia hampers antimicrobial stewardship and increases the risk of suboptimal treatment. New diagnostic tools that detect degraded microbial DNA in plasma, known as microbial cell-free DNA (cfDNA), may offer improved diagnostic yield. Through metagenomic next-generation approaches, these tools sequence DNA fragments to identify viral, bacterial, and fungal sequences.
Earlier studies of cfDNA in pneumonia have been mixed, correctly identifying the pathogen in 55% to 86% of cases – though notably cfDNA was superior to PCR and cultures and provided early detection of VAP in some cases (Farnaes L, et al. Diagn Microbiol Infect Dis. 2019;94:188; Langelier C, et al. Am J Respir Crit Care Med. 2020;201:491). However, a recent study of cfDNA in severe complicated pediatric pneumonia had promising results with significant clinical impact. cfDNA provided an accurate microbial diagnosis in 89% of cases, with it being the only positive study in 70% of cases. Further, cfDNA narrowed the antimicrobial regimen in 81% of cases (Dworsky ZD, et al. Hosp Pediatr. 2022;12:377).
The use of cfDNA is still in its infancy. Limitations, such as a lack of validated thresholds to differentiate colonization vs infection are noted given its detection sensitivity. Its utility, including ideal timing and patient population, needs further investigation. However, diagnostic cfDNA may soon provide earlier and less invasive microbial diagnostics in patients with chest infections and beyond.
Gregory Wigger, MD
Section Fellow-in-Training
Chest Infections Section
An evolving diagnostic tool: Microbial cell-free DNA
The diagnosis of the microbial etiology of pneumonia remains a significant challenge with <50% yield of blood and sputum cultures in most studies. More reliable samples, like bronchoalveolar lavage, require invasive procedures. Undifferentiated pneumonia hampers antimicrobial stewardship and increases the risk of suboptimal treatment. New diagnostic tools that detect degraded microbial DNA in plasma, known as microbial cell-free DNA (cfDNA), may offer improved diagnostic yield. Through metagenomic next-generation approaches, these tools sequence DNA fragments to identify viral, bacterial, and fungal sequences.
Earlier studies of cfDNA in pneumonia have been mixed, correctly identifying the pathogen in 55% to 86% of cases – though notably cfDNA was superior to PCR and cultures and provided early detection of VAP in some cases (Farnaes L, et al. Diagn Microbiol Infect Dis. 2019;94:188; Langelier C, et al. Am J Respir Crit Care Med. 2020;201:491). However, a recent study of cfDNA in severe complicated pediatric pneumonia had promising results with significant clinical impact. cfDNA provided an accurate microbial diagnosis in 89% of cases, with it being the only positive study in 70% of cases. Further, cfDNA narrowed the antimicrobial regimen in 81% of cases (Dworsky ZD, et al. Hosp Pediatr. 2022;12:377).
The use of cfDNA is still in its infancy. Limitations, such as a lack of validated thresholds to differentiate colonization vs infection are noted given its detection sensitivity. Its utility, including ideal timing and patient population, needs further investigation. However, diagnostic cfDNA may soon provide earlier and less invasive microbial diagnostics in patients with chest infections and beyond.
Gregory Wigger, MD
Section Fellow-in-Training
Sleep Medicine Network
Nonrespiratory Sleep Section
Sleep in cancer patients
Sleep disturbance is among the most common symptoms in patients with cancer with an estimated prevalence of up to two out of three patients experiencing sleep disruption during their cancer journey.1,23,4
Common sleep disorders in cancer patients:
Insomnia: Cancer patients have at least a two-fold higher incidence of insomnia compared with the general population.5,6 Predisposing factors may include age, the presence of hyper-arousability,a prior history of insomnia, or a preexisting psychiatric disorder. Cancer-related factors include surgery, hospitalization, chemotherapy, hormonal therapy, radiation therapy, and use of steroids.7 If sedative-hypnotics are considered, they should be used in conjunction with cognitive and behavioral therapy for insomnia (CBT-I). Recent meta-analyses provide data to support a strong recommendation to utilize CBT-I to treat insomnia in cancer patients.6,8,9
Hypersomnolence: Hypersomnolence or excessive daytime sleepiness is a common symptom noted among cancer patients.10 Hypersomnia related to cancer can be often classified as either hypersomnia due to a medical condition or hypersomnia due to a drug or substance, especially for those patients taking opioid or other sedative medications.
Movement Disorders: Sleep movement disorders occur in patients with cancer and may be primary or attributable to chemotherapy-related neuropathy from therapy regimens, including platinum compounds, taxanes, vinca alkaloids, proteasome inhibitors, or thalidomide-based agents.11,12
Obstructive sleep apnea (OSA): OSA occurs in patients with cancer and may be increased in patients with specific cancers such as head and neck tumors.13 Patients with sleep apnea have a five-fold increased risk of cancer-related mortality, and several studies show an increased incidence of cancer in those with sleep apnea.14-16There is an increasing realization that not only sleep apnea, but sleep disturbance, in general, may be oncogenic based on increased autonomic tone, chronic stress, variation in the pituitary-hypothalamic axis, as well as circadian mechanisms.17
Early recognition/treatment of sleep issues is essential to improve quality of life in cancer patients.
Diwakar Balachandran, MD, FCCP
Member-at-Large
References
1. Balachandran DD, Miller MA, Faiz SA, Yennurajalingam S, Innominato PF. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr Treat Options Oncol. 2021;22(9):81.
2. Yennurajalingam S, Balachandran D, Pedraza Cardozo SL, et al. Patient-reported sleep disturbance in advanced cancer: frequency, predictors and screening performance of the Edmonton Symptom Assessment System sleep item. BMJ Support Palliat Care. 2017;7(3):274-80.
3. Harris B, Ross J, Sanchez-Reilly S. Sleeping in the arms of cancer: A review of sleeping disorders among patients with cancer. Cancer J. 2014;20(5):299-305.
4. Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27(7):2747-53.
5. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292-8.
6. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583-90.
7. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895-908.
8. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113-24.
9. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-8.
10. Jaumally BA, Das A, Cassell NC, et al. Excessive daytime sleepiness in cancer patients. Sleep Breath. 2021;25(2):1063-7.
11. Gewandter JS, Kleckner AS, Marshall JH, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: an NIH Collaboratory study of claims data. Support Care Cancer. 2020;28(6):2553-62.
12. St Germain DC, O’Mara AM, Robinson JL, Torres AD, Minasian LM. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer. 2020;126(20):4602-13.
13. Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist. 2014;19(11):1200-6.
14. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99-105.
15. Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742-8.
16. Martinez-Garcia MA, Campos-Rodriguez F, Barbe F. Cancer and OSA: Current evidence from human studies. Chest. 2016;150(2):451-63.
17. Gozal D, Farre R, Nieto FJ. Putative links between sleep apnea and cancer: From hypotheses to evolving evidence. Chest. 2015;148(5):1140-7.
Nonrespiratory Sleep Section
Sleep in cancer patients
Sleep disturbance is among the most common symptoms in patients with cancer with an estimated prevalence of up to two out of three patients experiencing sleep disruption during their cancer journey.1,23,4
Common sleep disorders in cancer patients:
Insomnia: Cancer patients have at least a two-fold higher incidence of insomnia compared with the general population.5,6 Predisposing factors may include age, the presence of hyper-arousability,a prior history of insomnia, or a preexisting psychiatric disorder. Cancer-related factors include surgery, hospitalization, chemotherapy, hormonal therapy, radiation therapy, and use of steroids.7 If sedative-hypnotics are considered, they should be used in conjunction with cognitive and behavioral therapy for insomnia (CBT-I). Recent meta-analyses provide data to support a strong recommendation to utilize CBT-I to treat insomnia in cancer patients.6,8,9
Hypersomnolence: Hypersomnolence or excessive daytime sleepiness is a common symptom noted among cancer patients.10 Hypersomnia related to cancer can be often classified as either hypersomnia due to a medical condition or hypersomnia due to a drug or substance, especially for those patients taking opioid or other sedative medications.
Movement Disorders: Sleep movement disorders occur in patients with cancer and may be primary or attributable to chemotherapy-related neuropathy from therapy regimens, including platinum compounds, taxanes, vinca alkaloids, proteasome inhibitors, or thalidomide-based agents.11,12
Obstructive sleep apnea (OSA): OSA occurs in patients with cancer and may be increased in patients with specific cancers such as head and neck tumors.13 Patients with sleep apnea have a five-fold increased risk of cancer-related mortality, and several studies show an increased incidence of cancer in those with sleep apnea.14-16There is an increasing realization that not only sleep apnea, but sleep disturbance, in general, may be oncogenic based on increased autonomic tone, chronic stress, variation in the pituitary-hypothalamic axis, as well as circadian mechanisms.17
Early recognition/treatment of sleep issues is essential to improve quality of life in cancer patients.
Diwakar Balachandran, MD, FCCP
Member-at-Large
References
1. Balachandran DD, Miller MA, Faiz SA, Yennurajalingam S, Innominato PF. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr Treat Options Oncol. 2021;22(9):81.
2. Yennurajalingam S, Balachandran D, Pedraza Cardozo SL, et al. Patient-reported sleep disturbance in advanced cancer: frequency, predictors and screening performance of the Edmonton Symptom Assessment System sleep item. BMJ Support Palliat Care. 2017;7(3):274-80.
3. Harris B, Ross J, Sanchez-Reilly S. Sleeping in the arms of cancer: A review of sleeping disorders among patients with cancer. Cancer J. 2014;20(5):299-305.
4. Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27(7):2747-53.
5. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292-8.
6. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583-90.
7. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895-908.
8. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113-24.
9. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-8.
10. Jaumally BA, Das A, Cassell NC, et al. Excessive daytime sleepiness in cancer patients. Sleep Breath. 2021;25(2):1063-7.
11. Gewandter JS, Kleckner AS, Marshall JH, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: an NIH Collaboratory study of claims data. Support Care Cancer. 2020;28(6):2553-62.
12. St Germain DC, O’Mara AM, Robinson JL, Torres AD, Minasian LM. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer. 2020;126(20):4602-13.
13. Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist. 2014;19(11):1200-6.
14. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99-105.
15. Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742-8.
16. Martinez-Garcia MA, Campos-Rodriguez F, Barbe F. Cancer and OSA: Current evidence from human studies. Chest. 2016;150(2):451-63.
17. Gozal D, Farre R, Nieto FJ. Putative links between sleep apnea and cancer: From hypotheses to evolving evidence. Chest. 2015;148(5):1140-7.
Nonrespiratory Sleep Section
Sleep in cancer patients
Sleep disturbance is among the most common symptoms in patients with cancer with an estimated prevalence of up to two out of three patients experiencing sleep disruption during their cancer journey.1,23,4
Common sleep disorders in cancer patients:
Insomnia: Cancer patients have at least a two-fold higher incidence of insomnia compared with the general population.5,6 Predisposing factors may include age, the presence of hyper-arousability,a prior history of insomnia, or a preexisting psychiatric disorder. Cancer-related factors include surgery, hospitalization, chemotherapy, hormonal therapy, radiation therapy, and use of steroids.7 If sedative-hypnotics are considered, they should be used in conjunction with cognitive and behavioral therapy for insomnia (CBT-I). Recent meta-analyses provide data to support a strong recommendation to utilize CBT-I to treat insomnia in cancer patients.6,8,9
Hypersomnolence: Hypersomnolence or excessive daytime sleepiness is a common symptom noted among cancer patients.10 Hypersomnia related to cancer can be often classified as either hypersomnia due to a medical condition or hypersomnia due to a drug or substance, especially for those patients taking opioid or other sedative medications.
Movement Disorders: Sleep movement disorders occur in patients with cancer and may be primary or attributable to chemotherapy-related neuropathy from therapy regimens, including platinum compounds, taxanes, vinca alkaloids, proteasome inhibitors, or thalidomide-based agents.11,12
Obstructive sleep apnea (OSA): OSA occurs in patients with cancer and may be increased in patients with specific cancers such as head and neck tumors.13 Patients with sleep apnea have a five-fold increased risk of cancer-related mortality, and several studies show an increased incidence of cancer in those with sleep apnea.14-16There is an increasing realization that not only sleep apnea, but sleep disturbance, in general, may be oncogenic based on increased autonomic tone, chronic stress, variation in the pituitary-hypothalamic axis, as well as circadian mechanisms.17
Early recognition/treatment of sleep issues is essential to improve quality of life in cancer patients.
Diwakar Balachandran, MD, FCCP
Member-at-Large
References
1. Balachandran DD, Miller MA, Faiz SA, Yennurajalingam S, Innominato PF. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr Treat Options Oncol. 2021;22(9):81.
2. Yennurajalingam S, Balachandran D, Pedraza Cardozo SL, et al. Patient-reported sleep disturbance in advanced cancer: frequency, predictors and screening performance of the Edmonton Symptom Assessment System sleep item. BMJ Support Palliat Care. 2017;7(3):274-80.
3. Harris B, Ross J, Sanchez-Reilly S. Sleeping in the arms of cancer: A review of sleeping disorders among patients with cancer. Cancer J. 2014;20(5):299-305.
4. Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27(7):2747-53.
5. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292-8.
6. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583-90.
7. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895-908.
8. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113-24.
9. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-8.
10. Jaumally BA, Das A, Cassell NC, et al. Excessive daytime sleepiness in cancer patients. Sleep Breath. 2021;25(2):1063-7.
11. Gewandter JS, Kleckner AS, Marshall JH, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: an NIH Collaboratory study of claims data. Support Care Cancer. 2020;28(6):2553-62.
12. St Germain DC, O’Mara AM, Robinson JL, Torres AD, Minasian LM. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer. 2020;126(20):4602-13.
13. Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist. 2014;19(11):1200-6.
14. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99-105.
15. Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742-8.
16. Martinez-Garcia MA, Campos-Rodriguez F, Barbe F. Cancer and OSA: Current evidence from human studies. Chest. 2016;150(2):451-63.
17. Gozal D, Farre R, Nieto FJ. Putative links between sleep apnea and cancer: From hypotheses to evolving evidence. Chest. 2015;148(5):1140-7.
Getting to know the incoming CHEST President
Q and A with Doreen J. Addrizzo-Harris, MD, FCCP
Starting January 1, 2023, current President-Elect Doreen J. Addrizzo-Harris, MD, FCCP, will become the new President of the American College of Chest Physicians. working as a Professor of Medicine at the NYU Grossman School of Medicine.
Before she steps into the role of President, we spoke with Dr. Addrizzo-Harris for a glimpse into what she looks to bring to the CHEST organization.
What would you like to accomplish as President of CHEST?
For my presidency, I want to continue the great trajectory CHEST is on by focusing on increasing membership, expanding our educational offerings and advancing our communication strategies, and continuing the initiatives that strive to make diversity seamless and a part of everything we do.
As many know, I have a very strong passion for the work of the CHEST Foundation, and, throughout my presidency, I will focus on how CHEST can support and integrate with the Foundation’s goal of improving patient care – whether it’s through supporting clinical research grants, expanding patient education and advocacy events, or through funding programs like the First 5 Minutes™, which touches on strengthening the rapport and trust between clinician and patient and enhances cultural competency by building an understanding of barriers to care. I can also see increasing patient involvement in CHEST to lend a unique perspective to upcoming initiatives.
Another key focus will be to strengthen and expand our membership through many venues.
We will focus on increasing physician membership of both new members and lapsed members but will also focus on increasing membership of those other providers who help us care for our patients, including advanced practice providers, respiratory therapists and more. CHEST is already an inclusive organization to a variety of health care providers, but we can do more.
My presidency will also focus on increasing collaborations with our sister societies to find new ways to reach fellows-in-training, as well as residents and medical students who are interested in pulmonary, critical care, or sleep medicine.
Along those lines, I’m also planning a dedicated focus on providing more opportunities to fellows and early career members. The goal is to enhance communications between trainees and key thought-leaders in a way that is simple, seamless, and welcoming. CHEST already does this better than anyone else, but an expanded offering, particularly in the area of career development, can help reach even more individuals – both on a national and on an international level. One such event was our successful Young Professionals Event at the Belmont event in New York City this past June.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your presidency?
CHEST has many strengths, but I think our greatest is the strength of our team – our members, our faculty, our volunteer leaders, and our staff.
To build on this, my presidency will include a strong communications strategy to reach, educate, and share the variety of opportunities with our members. I want to build on some of the excellent initiatives Dr. David Schulman started this year to continue engaging and showing our newer members, or soon-to-be members how to get involved with CHEST.
What are some challenges facing CHEST, and how will you address these challenges?
A challenge for all associations, CHEST included, will be redefining what associations look like in the wake of a global pandemic now that virtual and hybrid learning has become a part of what we do on a day-to-day basis. What will the CHEST Annual Meeting look like 3 years from now? What will keep learners coming to a physical meeting when so much is accessible on the internet? What will keep members engaged in settings where we no longer get together in-person – like the board review that is now virtual?
This all will take a lot of strategy, which is already being worked on. It will include ideas like enhancing the networking opportunities to extend beyond the annual meeting, strengthening our international strategy, and continuing to innovate in the area of medical education.
And finally, what do you ask of the members and Fellows of CHEST to support you during your presidency?
I ask that everyone get involved. Please reach out if you have questions. I am (and all our leaders are) very accessible and we can connect you with the right people to get you engaged. Also, please spread the word. Tell your colleagues, trainees, etc., how great CHEST is and get them involved with CHEST too. We have so much to offer.#
Q and A with Doreen J. Addrizzo-Harris, MD, FCCP
Q and A with Doreen J. Addrizzo-Harris, MD, FCCP
Starting January 1, 2023, current President-Elect Doreen J. Addrizzo-Harris, MD, FCCP, will become the new President of the American College of Chest Physicians. working as a Professor of Medicine at the NYU Grossman School of Medicine.
Before she steps into the role of President, we spoke with Dr. Addrizzo-Harris for a glimpse into what she looks to bring to the CHEST organization.
What would you like to accomplish as President of CHEST?
For my presidency, I want to continue the great trajectory CHEST is on by focusing on increasing membership, expanding our educational offerings and advancing our communication strategies, and continuing the initiatives that strive to make diversity seamless and a part of everything we do.
As many know, I have a very strong passion for the work of the CHEST Foundation, and, throughout my presidency, I will focus on how CHEST can support and integrate with the Foundation’s goal of improving patient care – whether it’s through supporting clinical research grants, expanding patient education and advocacy events, or through funding programs like the First 5 Minutes™, which touches on strengthening the rapport and trust between clinician and patient and enhances cultural competency by building an understanding of barriers to care. I can also see increasing patient involvement in CHEST to lend a unique perspective to upcoming initiatives.
Another key focus will be to strengthen and expand our membership through many venues.
We will focus on increasing physician membership of both new members and lapsed members but will also focus on increasing membership of those other providers who help us care for our patients, including advanced practice providers, respiratory therapists and more. CHEST is already an inclusive organization to a variety of health care providers, but we can do more.
My presidency will also focus on increasing collaborations with our sister societies to find new ways to reach fellows-in-training, as well as residents and medical students who are interested in pulmonary, critical care, or sleep medicine.
Along those lines, I’m also planning a dedicated focus on providing more opportunities to fellows and early career members. The goal is to enhance communications between trainees and key thought-leaders in a way that is simple, seamless, and welcoming. CHEST already does this better than anyone else, but an expanded offering, particularly in the area of career development, can help reach even more individuals – both on a national and on an international level. One such event was our successful Young Professionals Event at the Belmont event in New York City this past June.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your presidency?
CHEST has many strengths, but I think our greatest is the strength of our team – our members, our faculty, our volunteer leaders, and our staff.
To build on this, my presidency will include a strong communications strategy to reach, educate, and share the variety of opportunities with our members. I want to build on some of the excellent initiatives Dr. David Schulman started this year to continue engaging and showing our newer members, or soon-to-be members how to get involved with CHEST.
What are some challenges facing CHEST, and how will you address these challenges?
A challenge for all associations, CHEST included, will be redefining what associations look like in the wake of a global pandemic now that virtual and hybrid learning has become a part of what we do on a day-to-day basis. What will the CHEST Annual Meeting look like 3 years from now? What will keep learners coming to a physical meeting when so much is accessible on the internet? What will keep members engaged in settings where we no longer get together in-person – like the board review that is now virtual?
This all will take a lot of strategy, which is already being worked on. It will include ideas like enhancing the networking opportunities to extend beyond the annual meeting, strengthening our international strategy, and continuing to innovate in the area of medical education.
And finally, what do you ask of the members and Fellows of CHEST to support you during your presidency?
I ask that everyone get involved. Please reach out if you have questions. I am (and all our leaders are) very accessible and we can connect you with the right people to get you engaged. Also, please spread the word. Tell your colleagues, trainees, etc., how great CHEST is and get them involved with CHEST too. We have so much to offer.#
Starting January 1, 2023, current President-Elect Doreen J. Addrizzo-Harris, MD, FCCP, will become the new President of the American College of Chest Physicians. working as a Professor of Medicine at the NYU Grossman School of Medicine.
Before she steps into the role of President, we spoke with Dr. Addrizzo-Harris for a glimpse into what she looks to bring to the CHEST organization.
What would you like to accomplish as President of CHEST?
For my presidency, I want to continue the great trajectory CHEST is on by focusing on increasing membership, expanding our educational offerings and advancing our communication strategies, and continuing the initiatives that strive to make diversity seamless and a part of everything we do.
As many know, I have a very strong passion for the work of the CHEST Foundation, and, throughout my presidency, I will focus on how CHEST can support and integrate with the Foundation’s goal of improving patient care – whether it’s through supporting clinical research grants, expanding patient education and advocacy events, or through funding programs like the First 5 Minutes™, which touches on strengthening the rapport and trust between clinician and patient and enhances cultural competency by building an understanding of barriers to care. I can also see increasing patient involvement in CHEST to lend a unique perspective to upcoming initiatives.
Another key focus will be to strengthen and expand our membership through many venues.
We will focus on increasing physician membership of both new members and lapsed members but will also focus on increasing membership of those other providers who help us care for our patients, including advanced practice providers, respiratory therapists and more. CHEST is already an inclusive organization to a variety of health care providers, but we can do more.
My presidency will also focus on increasing collaborations with our sister societies to find new ways to reach fellows-in-training, as well as residents and medical students who are interested in pulmonary, critical care, or sleep medicine.
Along those lines, I’m also planning a dedicated focus on providing more opportunities to fellows and early career members. The goal is to enhance communications between trainees and key thought-leaders in a way that is simple, seamless, and welcoming. CHEST already does this better than anyone else, but an expanded offering, particularly in the area of career development, can help reach even more individuals – both on a national and on an international level. One such event was our successful Young Professionals Event at the Belmont event in New York City this past June.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your presidency?
CHEST has many strengths, but I think our greatest is the strength of our team – our members, our faculty, our volunteer leaders, and our staff.
To build on this, my presidency will include a strong communications strategy to reach, educate, and share the variety of opportunities with our members. I want to build on some of the excellent initiatives Dr. David Schulman started this year to continue engaging and showing our newer members, or soon-to-be members how to get involved with CHEST.
What are some challenges facing CHEST, and how will you address these challenges?
A challenge for all associations, CHEST included, will be redefining what associations look like in the wake of a global pandemic now that virtual and hybrid learning has become a part of what we do on a day-to-day basis. What will the CHEST Annual Meeting look like 3 years from now? What will keep learners coming to a physical meeting when so much is accessible on the internet? What will keep members engaged in settings where we no longer get together in-person – like the board review that is now virtual?
This all will take a lot of strategy, which is already being worked on. It will include ideas like enhancing the networking opportunities to extend beyond the annual meeting, strengthening our international strategy, and continuing to innovate in the area of medical education.
And finally, what do you ask of the members and Fellows of CHEST to support you during your presidency?
I ask that everyone get involved. Please reach out if you have questions. I am (and all our leaders are) very accessible and we can connect you with the right people to get you engaged. Also, please spread the word. Tell your colleagues, trainees, etc., how great CHEST is and get them involved with CHEST too. We have so much to offer.#
The LIVE CHEST Challenge Championship is back!
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
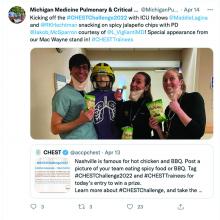
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
What are we missing when it comes to obstructive sleep apnea and atrial fibrillation?
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Now accepting applications for summer undergraduate research award
Eight undergraduate students from groups traditionally underrepresented in biomedical research will have the opportunity to perform 10 weeks of research related to digestive diseases alongside an established investigator. Recipients will also receive a $5,400 stipend and funding to offset travel and meal expenses.
Students may independently secure support from an AGA member mentor or choose from our list of participating mentors. Past recipients are eligible to apply!
Additional information about the award, including application requirements and a downloadable preview, are available in the request for applications. Please see important dates below.
- Dec. 14, 2022 - Online applications close at 11:59 p.m. ET.
- March 2023 - Applicants are notified of their status.
- May-August 2023 - Recipients perform summer research with mentors.
AGA gratefully acknowledges the Aman Armaan Ahmed Family for supporting this program.
Eight undergraduate students from groups traditionally underrepresented in biomedical research will have the opportunity to perform 10 weeks of research related to digestive diseases alongside an established investigator. Recipients will also receive a $5,400 stipend and funding to offset travel and meal expenses.
Students may independently secure support from an AGA member mentor or choose from our list of participating mentors. Past recipients are eligible to apply!
Additional information about the award, including application requirements and a downloadable preview, are available in the request for applications. Please see important dates below.
- Dec. 14, 2022 - Online applications close at 11:59 p.m. ET.
- March 2023 - Applicants are notified of their status.
- May-August 2023 - Recipients perform summer research with mentors.
AGA gratefully acknowledges the Aman Armaan Ahmed Family for supporting this program.
Eight undergraduate students from groups traditionally underrepresented in biomedical research will have the opportunity to perform 10 weeks of research related to digestive diseases alongside an established investigator. Recipients will also receive a $5,400 stipend and funding to offset travel and meal expenses.
Students may independently secure support from an AGA member mentor or choose from our list of participating mentors. Past recipients are eligible to apply!
Additional information about the award, including application requirements and a downloadable preview, are available in the request for applications. Please see important dates below.
- Dec. 14, 2022 - Online applications close at 11:59 p.m. ET.
- March 2023 - Applicants are notified of their status.
- May-August 2023 - Recipients perform summer research with mentors.
AGA gratefully acknowledges the Aman Armaan Ahmed Family for supporting this program.
AGA to host women’s regional workshops across the U.S. this fall
The AGA Women in GI Regional Workshops –
Registration is now open for the Midwest and Northeast workshops.
Each workshop is an opportunity to gain new knowledge from a unique lineup of experts and various topics. Select attendees also have the opportunity to participate in the Women’s Leadership Collaboration Conference at AGA Headquarters (Dec. 2-3, 2022) to advance the work from the regional events nationally. To register and for more information on the regional workshops, please visit www.gastro.org/AGAWomensRegional.
The AGA Women in GI Regional Workshops –
Registration is now open for the Midwest and Northeast workshops.
Each workshop is an opportunity to gain new knowledge from a unique lineup of experts and various topics. Select attendees also have the opportunity to participate in the Women’s Leadership Collaboration Conference at AGA Headquarters (Dec. 2-3, 2022) to advance the work from the regional events nationally. To register and for more information on the regional workshops, please visit www.gastro.org/AGAWomensRegional.
The AGA Women in GI Regional Workshops –
Registration is now open for the Midwest and Northeast workshops.
Each workshop is an opportunity to gain new knowledge from a unique lineup of experts and various topics. Select attendees also have the opportunity to participate in the Women’s Leadership Collaboration Conference at AGA Headquarters (Dec. 2-3, 2022) to advance the work from the regional events nationally. To register and for more information on the regional workshops, please visit www.gastro.org/AGAWomensRegional.
Memorial and honorary gifts: A special tribute
Honor a family member, friend, or colleague while supporting the work of our mission through a gift to the AGA Research Foundation. Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit. The AGA Research Awards program recruits, retains, and supports the most promising investigators in gastroenterology and hepatology.
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help support researchers working toward developing new treatments and diagnostics for patients with GI conditions. Your gift will assist in fostering a new pipeline of scientists – the next generation of leaders in GI. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly, a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
Conclusion
Your gift directly supports talented young researchers working to advance our understanding of digestive diseases. Make a tax-deductible donation to help spur innovation. Donate today at www.gastro.org/donateonline.
Honor a family member, friend, or colleague while supporting the work of our mission through a gift to the AGA Research Foundation. Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit. The AGA Research Awards program recruits, retains, and supports the most promising investigators in gastroenterology and hepatology.
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help support researchers working toward developing new treatments and diagnostics for patients with GI conditions. Your gift will assist in fostering a new pipeline of scientists – the next generation of leaders in GI. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly, a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
Conclusion
Your gift directly supports talented young researchers working to advance our understanding of digestive diseases. Make a tax-deductible donation to help spur innovation. Donate today at www.gastro.org/donateonline.
Honor a family member, friend, or colleague while supporting the work of our mission through a gift to the AGA Research Foundation. Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit. The AGA Research Awards program recruits, retains, and supports the most promising investigators in gastroenterology and hepatology.
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help support researchers working toward developing new treatments and diagnostics for patients with GI conditions. Your gift will assist in fostering a new pipeline of scientists – the next generation of leaders in GI. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly, a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
Conclusion
Your gift directly supports talented young researchers working to advance our understanding of digestive diseases. Make a tax-deductible donation to help spur innovation. Donate today at www.gastro.org/donateonline.
CMS releases proposed payment rule
On July 15, for calendar year 2023.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, have identified the following top three takeaways:
Slight increase in ASC payments – The proposed ASC conversion factor increases 2.7% to $51.315 for ASCs that meet quality reporting requirements.
Slight increase in facility fees payments – Hospitals that meet quality reporting requirements also receive a 2.7% proposed increase, which translates to $86.785 – a stark difference from the ASC payment.
18% cuts to some motility and G-tube codes – Hospital outpatient facility payments for motility codes 91117 and 91122 and G-tube codes 43761-43763 could decrease by 18% because of proposed changes to their Ambulatory Payment Classification (APC) family.
On July 15, for calendar year 2023.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, have identified the following top three takeaways:
Slight increase in ASC payments – The proposed ASC conversion factor increases 2.7% to $51.315 for ASCs that meet quality reporting requirements.
Slight increase in facility fees payments – Hospitals that meet quality reporting requirements also receive a 2.7% proposed increase, which translates to $86.785 – a stark difference from the ASC payment.
18% cuts to some motility and G-tube codes – Hospital outpatient facility payments for motility codes 91117 and 91122 and G-tube codes 43761-43763 could decrease by 18% because of proposed changes to their Ambulatory Payment Classification (APC) family.
On July 15, for calendar year 2023.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, have identified the following top three takeaways:
Slight increase in ASC payments – The proposed ASC conversion factor increases 2.7% to $51.315 for ASCs that meet quality reporting requirements.
Slight increase in facility fees payments – Hospitals that meet quality reporting requirements also receive a 2.7% proposed increase, which translates to $86.785 – a stark difference from the ASC payment.
18% cuts to some motility and G-tube codes – Hospital outpatient facility payments for motility codes 91117 and 91122 and G-tube codes 43761-43763 could decrease by 18% because of proposed changes to their Ambulatory Payment Classification (APC) family.