User login
ACIP extends HPV vaccine coverage
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Syphilis rates high in HIV-positive women
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
REPORTING FROM CROI 2019
2019 ID update for dermatologists: Ticks are the “ride of choice” for arthropods
ORLANDO – New tricks from ticks, near-zero Zika, and the perils of personal grooming: Dermatologists have a lot to think about along the infectious disease spectrum in 2019, according to Justin Finch, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Anaphylaxis from alpha-gal syndrome is on the rise, caused in part by the geographic spread of the Lone Star tick. Beginning in 2006, isolated cases of an anaphylactic reaction to cetuximab, the epidermal growth factor receptor antagonist used to treat certain cancers, began to be seen in a curious geographic distribution. “The anaphylaxis cases were restricted to the southeastern United States, the home of the Lone Star tick,” said Dr. Finch, of the department of dermatology at the University of Connecticut, Farmington.
With some detective work, physicians and epidemiologists eventually determined that patients were reacting to an oligosaccharide called galactose-alpha–1,3-galactose (alpha-gal) found in cetuximab. This protein is also found in the meat of nonprimate mammals; individuals in the southeastern United States, where the Lone Star tick is endemic, had been sensitized via exposure to alpha-gal from Lone Star tick bites.
“Alpha-gal syndrome is on the rise,” said Dr. Finch, driven by the increased spread of this tick. Individuals who are sensitized develop delayed anaphylaxis 2-7 hours after ingesting red meat such as beef, pork, or lamb. “Ask about it,” said Dr. Finch, in patients who develop urticaria, dyspnea, angioedema, or hypotension without a clear offender. Because of the delay between allergen ingestion and anaphylaxis, it can be hard to connect the dots.
A number of drugs other than cetuximab contain alpha-gal, so patients must also be told to avoid these agents, said Dr. Finch, who noted that alpha-gal syndrome isn’t the only emerging culprit for tick-borne diseases. “The tick is the ride of choice for arthropod-borne diseases in the U.S.,” he added. “Year after year, tick-borne diseases top mosquito-borne diseases in the U.S.” Zika’s explosion in 2016 made that year the exception to the rule.
Now, Zika virus may be on the wane – the number of case reports have plummeted both in the United States and in Central and South America this past year – but it hasn’t completely gone away. “It looks like it fell off all the maps,” but the virus is still present at low levels, he said.
When Zika virus is symptomatic, there’s often a nonspecific maculopapular rash. Critically, Dr. Finch said, “women with a rash are four times as likely to have adverse congenital outcomes. This is the important point for us to take home as dermatologists. ... It’s really important to have a high index of suspicion and to screen these women as they are coming into our clinic.”
Turning back to ticks, Lyme disease continues to be a problem in endemic areas in the Northeast, the mid-Atlantic region, and the Midwest, said Dr. Finch, so it’s a perennial on the differential diagnosis for dermatologists.
An Asian tick new to North America was seen for the first time in New Jersey in the summer of 2017. The Asian longhorned tick carries a phlebovirus that causes severe fever with thrombocytopenia syndrome, a disease with a 15% fatality rate. The reservoir host of this virus in Asia isn’t known, said Dr. Finch, adding that no cases of the virus have yet been seen in the United States. As of November 2018, according to the Centers for Disease Control and Prevention, the tick had been found in nine states (Arkansas, Connecticut, Maryland, North Carolina, New Jersey, New York, Pennsylvania, Virginia, and West Virginia).
“What’s not on the rise? Pubic lice. We are destroying their natural habitat!” said Dr. Finch, citing surveys about personal grooming that show that more than 90% of women remove at least some of their pubic hair. Most college campuses are currently reporting essentially no cases of pubic lice, he noted.
However, the same personal grooming practices may be contributing to increases in molluscum contagiosum, herpes simplex virus, some strains of human papillomavirus, and cutaneous Streptococcus pyogenes infections, he said.
Another STI has had a resurgence in geographic pockets around the nation and among specific populations, said Dr. Finch. Syphilis is on the rise among gay and bisexual men and African Americans. Known as the “great imitator,” syphilis should be on the differential for dermatologists when the clinical picture isn’t quite adding up. “Think of this, and screen with an RPR [rapid plasma reagin],” he said.
Finally, an old enemy is back: A total of 11 measles outbreaks were reported in 2018. “We need to know about measles because of the complications,” said Dr. Finch. Even years later, such dire sequelae as subacute sclerosing panencephalitis can crop up, he added.
After a 2-week incubation period, measles begins with a fever and cough, congestion, and conjunctivitis. The rash begins on the head and spreads inferiorly by day 3. As the rash blooms, the classic morbilliform eruption becomes apparent. A biopsy of affected skin will be nonspecific; measles is diagnosed with a nasopharyngeal culture and serologic assay. Dr. Finch pointed out that dermatologists are unlikely to see measles in its earliest stages because their expertise will be called on only after it becomes clear that the patient is not experiencing just a mild illness with a viral exanthem.
When there’s suspicion for measles, a full-body skin exam is needed. “Koplik’s spots – the gray white papules on the buccal mucosa – are not pathognomonic in themselves, but in the clinical scenario of a person with measles” they can help the dermatologist make a definitive call, he said.
Vitamin A can be given to a patient with active measles, but prevention via immunization at age 12 months and 5 years is the only way to stop the disease, Dr. Finch noted.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – New tricks from ticks, near-zero Zika, and the perils of personal grooming: Dermatologists have a lot to think about along the infectious disease spectrum in 2019, according to Justin Finch, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Anaphylaxis from alpha-gal syndrome is on the rise, caused in part by the geographic spread of the Lone Star tick. Beginning in 2006, isolated cases of an anaphylactic reaction to cetuximab, the epidermal growth factor receptor antagonist used to treat certain cancers, began to be seen in a curious geographic distribution. “The anaphylaxis cases were restricted to the southeastern United States, the home of the Lone Star tick,” said Dr. Finch, of the department of dermatology at the University of Connecticut, Farmington.
With some detective work, physicians and epidemiologists eventually determined that patients were reacting to an oligosaccharide called galactose-alpha–1,3-galactose (alpha-gal) found in cetuximab. This protein is also found in the meat of nonprimate mammals; individuals in the southeastern United States, where the Lone Star tick is endemic, had been sensitized via exposure to alpha-gal from Lone Star tick bites.
“Alpha-gal syndrome is on the rise,” said Dr. Finch, driven by the increased spread of this tick. Individuals who are sensitized develop delayed anaphylaxis 2-7 hours after ingesting red meat such as beef, pork, or lamb. “Ask about it,” said Dr. Finch, in patients who develop urticaria, dyspnea, angioedema, or hypotension without a clear offender. Because of the delay between allergen ingestion and anaphylaxis, it can be hard to connect the dots.
A number of drugs other than cetuximab contain alpha-gal, so patients must also be told to avoid these agents, said Dr. Finch, who noted that alpha-gal syndrome isn’t the only emerging culprit for tick-borne diseases. “The tick is the ride of choice for arthropod-borne diseases in the U.S.,” he added. “Year after year, tick-borne diseases top mosquito-borne diseases in the U.S.” Zika’s explosion in 2016 made that year the exception to the rule.
Now, Zika virus may be on the wane – the number of case reports have plummeted both in the United States and in Central and South America this past year – but it hasn’t completely gone away. “It looks like it fell off all the maps,” but the virus is still present at low levels, he said.
When Zika virus is symptomatic, there’s often a nonspecific maculopapular rash. Critically, Dr. Finch said, “women with a rash are four times as likely to have adverse congenital outcomes. This is the important point for us to take home as dermatologists. ... It’s really important to have a high index of suspicion and to screen these women as they are coming into our clinic.”
Turning back to ticks, Lyme disease continues to be a problem in endemic areas in the Northeast, the mid-Atlantic region, and the Midwest, said Dr. Finch, so it’s a perennial on the differential diagnosis for dermatologists.
An Asian tick new to North America was seen for the first time in New Jersey in the summer of 2017. The Asian longhorned tick carries a phlebovirus that causes severe fever with thrombocytopenia syndrome, a disease with a 15% fatality rate. The reservoir host of this virus in Asia isn’t known, said Dr. Finch, adding that no cases of the virus have yet been seen in the United States. As of November 2018, according to the Centers for Disease Control and Prevention, the tick had been found in nine states (Arkansas, Connecticut, Maryland, North Carolina, New Jersey, New York, Pennsylvania, Virginia, and West Virginia).
“What’s not on the rise? Pubic lice. We are destroying their natural habitat!” said Dr. Finch, citing surveys about personal grooming that show that more than 90% of women remove at least some of their pubic hair. Most college campuses are currently reporting essentially no cases of pubic lice, he noted.
However, the same personal grooming practices may be contributing to increases in molluscum contagiosum, herpes simplex virus, some strains of human papillomavirus, and cutaneous Streptococcus pyogenes infections, he said.
Another STI has had a resurgence in geographic pockets around the nation and among specific populations, said Dr. Finch. Syphilis is on the rise among gay and bisexual men and African Americans. Known as the “great imitator,” syphilis should be on the differential for dermatologists when the clinical picture isn’t quite adding up. “Think of this, and screen with an RPR [rapid plasma reagin],” he said.
Finally, an old enemy is back: A total of 11 measles outbreaks were reported in 2018. “We need to know about measles because of the complications,” said Dr. Finch. Even years later, such dire sequelae as subacute sclerosing panencephalitis can crop up, he added.
After a 2-week incubation period, measles begins with a fever and cough, congestion, and conjunctivitis. The rash begins on the head and spreads inferiorly by day 3. As the rash blooms, the classic morbilliform eruption becomes apparent. A biopsy of affected skin will be nonspecific; measles is diagnosed with a nasopharyngeal culture and serologic assay. Dr. Finch pointed out that dermatologists are unlikely to see measles in its earliest stages because their expertise will be called on only after it becomes clear that the patient is not experiencing just a mild illness with a viral exanthem.
When there’s suspicion for measles, a full-body skin exam is needed. “Koplik’s spots – the gray white papules on the buccal mucosa – are not pathognomonic in themselves, but in the clinical scenario of a person with measles” they can help the dermatologist make a definitive call, he said.
Vitamin A can be given to a patient with active measles, but prevention via immunization at age 12 months and 5 years is the only way to stop the disease, Dr. Finch noted.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – New tricks from ticks, near-zero Zika, and the perils of personal grooming: Dermatologists have a lot to think about along the infectious disease spectrum in 2019, according to Justin Finch, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Anaphylaxis from alpha-gal syndrome is on the rise, caused in part by the geographic spread of the Lone Star tick. Beginning in 2006, isolated cases of an anaphylactic reaction to cetuximab, the epidermal growth factor receptor antagonist used to treat certain cancers, began to be seen in a curious geographic distribution. “The anaphylaxis cases were restricted to the southeastern United States, the home of the Lone Star tick,” said Dr. Finch, of the department of dermatology at the University of Connecticut, Farmington.
With some detective work, physicians and epidemiologists eventually determined that patients were reacting to an oligosaccharide called galactose-alpha–1,3-galactose (alpha-gal) found in cetuximab. This protein is also found in the meat of nonprimate mammals; individuals in the southeastern United States, where the Lone Star tick is endemic, had been sensitized via exposure to alpha-gal from Lone Star tick bites.
“Alpha-gal syndrome is on the rise,” said Dr. Finch, driven by the increased spread of this tick. Individuals who are sensitized develop delayed anaphylaxis 2-7 hours after ingesting red meat such as beef, pork, or lamb. “Ask about it,” said Dr. Finch, in patients who develop urticaria, dyspnea, angioedema, or hypotension without a clear offender. Because of the delay between allergen ingestion and anaphylaxis, it can be hard to connect the dots.
A number of drugs other than cetuximab contain alpha-gal, so patients must also be told to avoid these agents, said Dr. Finch, who noted that alpha-gal syndrome isn’t the only emerging culprit for tick-borne diseases. “The tick is the ride of choice for arthropod-borne diseases in the U.S.,” he added. “Year after year, tick-borne diseases top mosquito-borne diseases in the U.S.” Zika’s explosion in 2016 made that year the exception to the rule.
Now, Zika virus may be on the wane – the number of case reports have plummeted both in the United States and in Central and South America this past year – but it hasn’t completely gone away. “It looks like it fell off all the maps,” but the virus is still present at low levels, he said.
When Zika virus is symptomatic, there’s often a nonspecific maculopapular rash. Critically, Dr. Finch said, “women with a rash are four times as likely to have adverse congenital outcomes. This is the important point for us to take home as dermatologists. ... It’s really important to have a high index of suspicion and to screen these women as they are coming into our clinic.”
Turning back to ticks, Lyme disease continues to be a problem in endemic areas in the Northeast, the mid-Atlantic region, and the Midwest, said Dr. Finch, so it’s a perennial on the differential diagnosis for dermatologists.
An Asian tick new to North America was seen for the first time in New Jersey in the summer of 2017. The Asian longhorned tick carries a phlebovirus that causes severe fever with thrombocytopenia syndrome, a disease with a 15% fatality rate. The reservoir host of this virus in Asia isn’t known, said Dr. Finch, adding that no cases of the virus have yet been seen in the United States. As of November 2018, according to the Centers for Disease Control and Prevention, the tick had been found in nine states (Arkansas, Connecticut, Maryland, North Carolina, New Jersey, New York, Pennsylvania, Virginia, and West Virginia).
“What’s not on the rise? Pubic lice. We are destroying their natural habitat!” said Dr. Finch, citing surveys about personal grooming that show that more than 90% of women remove at least some of their pubic hair. Most college campuses are currently reporting essentially no cases of pubic lice, he noted.
However, the same personal grooming practices may be contributing to increases in molluscum contagiosum, herpes simplex virus, some strains of human papillomavirus, and cutaneous Streptococcus pyogenes infections, he said.
Another STI has had a resurgence in geographic pockets around the nation and among specific populations, said Dr. Finch. Syphilis is on the rise among gay and bisexual men and African Americans. Known as the “great imitator,” syphilis should be on the differential for dermatologists when the clinical picture isn’t quite adding up. “Think of this, and screen with an RPR [rapid plasma reagin],” he said.
Finally, an old enemy is back: A total of 11 measles outbreaks were reported in 2018. “We need to know about measles because of the complications,” said Dr. Finch. Even years later, such dire sequelae as subacute sclerosing panencephalitis can crop up, he added.
After a 2-week incubation period, measles begins with a fever and cough, congestion, and conjunctivitis. The rash begins on the head and spreads inferiorly by day 3. As the rash blooms, the classic morbilliform eruption becomes apparent. A biopsy of affected skin will be nonspecific; measles is diagnosed with a nasopharyngeal culture and serologic assay. Dr. Finch pointed out that dermatologists are unlikely to see measles in its earliest stages because their expertise will be called on only after it becomes clear that the patient is not experiencing just a mild illness with a viral exanthem.
When there’s suspicion for measles, a full-body skin exam is needed. “Koplik’s spots – the gray white papules on the buccal mucosa – are not pathognomonic in themselves, but in the clinical scenario of a person with measles” they can help the dermatologist make a definitive call, he said.
Vitamin A can be given to a patient with active measles, but prevention via immunization at age 12 months and 5 years is the only way to stop the disease, Dr. Finch noted.
Dr. Finch reported that he has no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2019
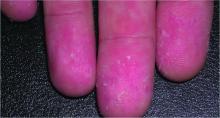
Digoxin-furosemide reduces viral load, diameter of cutaneous warts
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
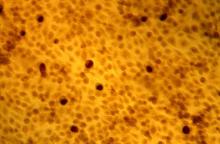
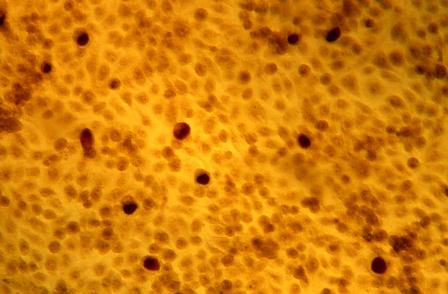
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The combination of digoxin and furosemide in a topical gel reduced the diameter of cutaneous warts caused by HPV.
Major finding: Wart diameter was reduced by a mean of 3 mm among those treated with the combination.
Study details: The randomized, placebo-controlled phase 2a study compared the furosemide-digoxin combination with the two components separately, and placebo separately, in 80 adults.
Disclosures: One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
Source: Rijsbergen M et al. J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
HIV testing low in U.S. women engaged in risky behavior
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point: Health care providers don’t ask sexually active women about risky behavior that would raise their risk of HIV infection.
Major finding: Of women who reported having anal sex, 19% reported that their providers asked about their types of intercourse.
Study details: Data from the 2011-2015 National Survey of Family Growth.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
FDA expands approval of 9-valent HPV vaccine
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
Age, risk factors should guide chlamydia, gonorrhea screening of HIV-infected women
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
REPORTING FROM THE 2018 STD PREVENTION CONFERENCE
Key clinical point: Targeted screening for chlamydia and gonorrhea in women with HIV based on age, risk is warranted.
Major finding: Chlamydia infections were seen in 16% and gonorrhea in 3.9% of HIV-infected women aged 18-24 years and in 1.1% and 0.7%, respectively, in women over age 50.
Study details: Data analysis of 5,084 women in 8 U.S. cities during 2007-2016.
Disclosures: Dr. Dionne-Odom reported that she had no relevant disclosures.
Syphilis surge drives USPSTF reaffirmation of early screening for all pregnant women
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
FROM JAMA