User login
Focus groups seek transgender experience with HIV prevention
A pair of focus groups explored the experience of transgender patients with HIV prevention, finding many were discouraged by experiences of care that was not culturally competent and affirming.
The findings, including other important themes, were published in Pediatrics.
The pair of online asynchronous focus groups, conducted by Holly B. Fontenot, PhD, RN/NP, of the Fenway Institute in Boston, and colleagues, sought input from 30 transgender participants from across the United States. Eleven were aged 13-18 years, and 19 were aged 18-24 years, with an average age of 19. Most (70%) were white, and the remainder were African American (7%), Asian American (3%), multiracial (17%), and other (3%); 10% identified as Hispanic. Participants were given multiple options for reporting gender identity; 27% reported identifying as transgender males, 17% reported identifying as transgender females, and the rest identified with other terms, including 27% using one or more terms.
The quantitative analysis found four common themes, which the study explored in depth: “barriers to self-efficacy in sexual decision making; safety concerns, fear, and other challenges in forming romantic and/or sexual relationships; need for support and education; and desire for affirmative and culturally competent experiences and interactions.”
Based on their findings, the authors suggested ways of improving transgender youth experiences:
- Increasing provider knowledge and skills in providing affirming care through transgender health education programs.
- Addressing the barriers, such as stigma and lack of accessibility.
- Expanding sexual health education to be more inclusive regarding gender identities, sexual orientations, and definitions of sex.
Providers also need to include information on sexually transmitted infection and HIV prevention, including “discussion of safer sexual behaviors, negotiation and consent, sexual and physical assault, condoms, lubrication, STI and HIV testing, human papillomavirus vaccination, and PrEP [preexposure prophylaxis]” the authors emphasized.
Dr. Fontenot and associates determined that this study’s findings were consistent with what’s known about adult transgender patients, but this study provides more context regarding transgender youth experiences.
“It is important to elicit transgender youth experiences and perspectives related to HIV risk and preventive services,” they concluded. “This study provided a greater understanding of barriers to and facilitators of youth obtaining HIV preventive services and sexual health education.”
Limitations of the study included that non–English speaking participants were excluded, and that participants were predominantly white, non-Hispanic, and assigned female sex at birth.
This study was funded by the Centers for Disease Control and Prevention and NORC at The University of Chicago. The authors had no relevant financial disclosures.
SOURCE: Fontenot HB et al., Pediatrics. 2020. doi: 10.1542/peds.2019-2204.
A pair of focus groups explored the experience of transgender patients with HIV prevention, finding many were discouraged by experiences of care that was not culturally competent and affirming.
The findings, including other important themes, were published in Pediatrics.
The pair of online asynchronous focus groups, conducted by Holly B. Fontenot, PhD, RN/NP, of the Fenway Institute in Boston, and colleagues, sought input from 30 transgender participants from across the United States. Eleven were aged 13-18 years, and 19 were aged 18-24 years, with an average age of 19. Most (70%) were white, and the remainder were African American (7%), Asian American (3%), multiracial (17%), and other (3%); 10% identified as Hispanic. Participants were given multiple options for reporting gender identity; 27% reported identifying as transgender males, 17% reported identifying as transgender females, and the rest identified with other terms, including 27% using one or more terms.
The quantitative analysis found four common themes, which the study explored in depth: “barriers to self-efficacy in sexual decision making; safety concerns, fear, and other challenges in forming romantic and/or sexual relationships; need for support and education; and desire for affirmative and culturally competent experiences and interactions.”
Based on their findings, the authors suggested ways of improving transgender youth experiences:
- Increasing provider knowledge and skills in providing affirming care through transgender health education programs.
- Addressing the barriers, such as stigma and lack of accessibility.
- Expanding sexual health education to be more inclusive regarding gender identities, sexual orientations, and definitions of sex.
Providers also need to include information on sexually transmitted infection and HIV prevention, including “discussion of safer sexual behaviors, negotiation and consent, sexual and physical assault, condoms, lubrication, STI and HIV testing, human papillomavirus vaccination, and PrEP [preexposure prophylaxis]” the authors emphasized.
Dr. Fontenot and associates determined that this study’s findings were consistent with what’s known about adult transgender patients, but this study provides more context regarding transgender youth experiences.
“It is important to elicit transgender youth experiences and perspectives related to HIV risk and preventive services,” they concluded. “This study provided a greater understanding of barriers to and facilitators of youth obtaining HIV preventive services and sexual health education.”
Limitations of the study included that non–English speaking participants were excluded, and that participants were predominantly white, non-Hispanic, and assigned female sex at birth.
This study was funded by the Centers for Disease Control and Prevention and NORC at The University of Chicago. The authors had no relevant financial disclosures.
SOURCE: Fontenot HB et al., Pediatrics. 2020. doi: 10.1542/peds.2019-2204.
A pair of focus groups explored the experience of transgender patients with HIV prevention, finding many were discouraged by experiences of care that was not culturally competent and affirming.
The findings, including other important themes, were published in Pediatrics.
The pair of online asynchronous focus groups, conducted by Holly B. Fontenot, PhD, RN/NP, of the Fenway Institute in Boston, and colleagues, sought input from 30 transgender participants from across the United States. Eleven were aged 13-18 years, and 19 were aged 18-24 years, with an average age of 19. Most (70%) were white, and the remainder were African American (7%), Asian American (3%), multiracial (17%), and other (3%); 10% identified as Hispanic. Participants were given multiple options for reporting gender identity; 27% reported identifying as transgender males, 17% reported identifying as transgender females, and the rest identified with other terms, including 27% using one or more terms.
The quantitative analysis found four common themes, which the study explored in depth: “barriers to self-efficacy in sexual decision making; safety concerns, fear, and other challenges in forming romantic and/or sexual relationships; need for support and education; and desire for affirmative and culturally competent experiences and interactions.”
Based on their findings, the authors suggested ways of improving transgender youth experiences:
- Increasing provider knowledge and skills in providing affirming care through transgender health education programs.
- Addressing the barriers, such as stigma and lack of accessibility.
- Expanding sexual health education to be more inclusive regarding gender identities, sexual orientations, and definitions of sex.
Providers also need to include information on sexually transmitted infection and HIV prevention, including “discussion of safer sexual behaviors, negotiation and consent, sexual and physical assault, condoms, lubrication, STI and HIV testing, human papillomavirus vaccination, and PrEP [preexposure prophylaxis]” the authors emphasized.
Dr. Fontenot and associates determined that this study’s findings were consistent with what’s known about adult transgender patients, but this study provides more context regarding transgender youth experiences.
“It is important to elicit transgender youth experiences and perspectives related to HIV risk and preventive services,” they concluded. “This study provided a greater understanding of barriers to and facilitators of youth obtaining HIV preventive services and sexual health education.”
Limitations of the study included that non–English speaking participants were excluded, and that participants were predominantly white, non-Hispanic, and assigned female sex at birth.
This study was funded by the Centers for Disease Control and Prevention and NORC at The University of Chicago. The authors had no relevant financial disclosures.
SOURCE: Fontenot HB et al., Pediatrics. 2020. doi: 10.1542/peds.2019-2204.
FROM PEDIATRICS
Requests for crowd diagnoses of STDs common on social media
Requests for crowd diagnosis of sexually transmitted diseases were frequent on a social media website, new research found.
The social media website Reddit, which currently has 330 million monthly active users, is home to more than 230 health-related subreddits, including r/STD, a forum that allows users to publicly share “stories, concerns, and questions” about “anything and everything STD related,” Alicia L. Nobles, PhD, of the department of medicine at the University of California, San Diego, and associates wrote in a research letter published in JAMA.
Dr. Noble and associates conducted an analysis of all posts published to r/STD from the subreddit’s inception during November 2010–February 2019, a total of 16,979 posts. Three coauthors independently coded each post, recording whether or not a post requested a crowd diagnosis, and if so, whether that request was made to obtain a second opinion after a visit to a health care professional.
About 58% of posts requested a crowd diagnosis, 31% of which included an image of the physical signs. One-fifth of the requests for a crowd diagnosis were seeking a second opinion after a previous diagnosis by a health care professional. Nearly 90% of all crowd-diagnosis requests received at least one reply (mean responses, 1.7), with a median response time of 3.04 hours. About 80% of requests were answered in less than 1 day.
While crowd diagnoses do seem to be popular and have the benefits of anonymity, rapid response, and multiple opinions, the accuracy of crowd diagnoses is unknown given the limited information responders operate with and the potential lack of responder medical training, the study authors noted. Misdiagnosis could allow further disease transmission, and third parties viewing posts could incorrectly self-diagnose their own condition.
“Health care professionals could partner with social media outlets to promote the potential benefits of crowd diagnosis while suppressing potential harms, for example by having trained professionals respond to posts to better diagnose and make referrals to health care centers,” Dr. Nobles and associates concluded.
One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.
SOURCE: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
Requests for crowd diagnosis of sexually transmitted diseases were frequent on a social media website, new research found.
The social media website Reddit, which currently has 330 million monthly active users, is home to more than 230 health-related subreddits, including r/STD, a forum that allows users to publicly share “stories, concerns, and questions” about “anything and everything STD related,” Alicia L. Nobles, PhD, of the department of medicine at the University of California, San Diego, and associates wrote in a research letter published in JAMA.
Dr. Noble and associates conducted an analysis of all posts published to r/STD from the subreddit’s inception during November 2010–February 2019, a total of 16,979 posts. Three coauthors independently coded each post, recording whether or not a post requested a crowd diagnosis, and if so, whether that request was made to obtain a second opinion after a visit to a health care professional.
About 58% of posts requested a crowd diagnosis, 31% of which included an image of the physical signs. One-fifth of the requests for a crowd diagnosis were seeking a second opinion after a previous diagnosis by a health care professional. Nearly 90% of all crowd-diagnosis requests received at least one reply (mean responses, 1.7), with a median response time of 3.04 hours. About 80% of requests were answered in less than 1 day.
While crowd diagnoses do seem to be popular and have the benefits of anonymity, rapid response, and multiple opinions, the accuracy of crowd diagnoses is unknown given the limited information responders operate with and the potential lack of responder medical training, the study authors noted. Misdiagnosis could allow further disease transmission, and third parties viewing posts could incorrectly self-diagnose their own condition.
“Health care professionals could partner with social media outlets to promote the potential benefits of crowd diagnosis while suppressing potential harms, for example by having trained professionals respond to posts to better diagnose and make referrals to health care centers,” Dr. Nobles and associates concluded.
One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.
SOURCE: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
Requests for crowd diagnosis of sexually transmitted diseases were frequent on a social media website, new research found.
The social media website Reddit, which currently has 330 million monthly active users, is home to more than 230 health-related subreddits, including r/STD, a forum that allows users to publicly share “stories, concerns, and questions” about “anything and everything STD related,” Alicia L. Nobles, PhD, of the department of medicine at the University of California, San Diego, and associates wrote in a research letter published in JAMA.
Dr. Noble and associates conducted an analysis of all posts published to r/STD from the subreddit’s inception during November 2010–February 2019, a total of 16,979 posts. Three coauthors independently coded each post, recording whether or not a post requested a crowd diagnosis, and if so, whether that request was made to obtain a second opinion after a visit to a health care professional.
About 58% of posts requested a crowd diagnosis, 31% of which included an image of the physical signs. One-fifth of the requests for a crowd diagnosis were seeking a second opinion after a previous diagnosis by a health care professional. Nearly 90% of all crowd-diagnosis requests received at least one reply (mean responses, 1.7), with a median response time of 3.04 hours. About 80% of requests were answered in less than 1 day.
While crowd diagnoses do seem to be popular and have the benefits of anonymity, rapid response, and multiple opinions, the accuracy of crowd diagnoses is unknown given the limited information responders operate with and the potential lack of responder medical training, the study authors noted. Misdiagnosis could allow further disease transmission, and third parties viewing posts could incorrectly self-diagnose their own condition.
“Health care professionals could partner with social media outlets to promote the potential benefits of crowd diagnosis while suppressing potential harms, for example by having trained professionals respond to posts to better diagnose and make referrals to health care centers,” Dr. Nobles and associates concluded.
One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.
SOURCE: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
FROM JAMA
Key clinical point: Crowd-diagnosis requests of STDs are popular on a social media–based health forum.
Major finding: Nearly 60% of r/STD posts were a request for diagnosis, 87% of which received a reply (mean responses, 1.7; mean response time, 3.0 hours).
Study details: A review of 16,979 posts on the subreddit r/STD.
Disclosures: One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.Source: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
Survey: Most physicians who treat STIs in their offices lack key injectable drugs
The majority of physicians surveyed who treat sexually transmitted infections (STIs) in their offices reported that they did not have on-site availability of the two primary injectable drugs for syphilis and gonorrhea, according to researchers from the Centers for Disease Control and Prevention.
This lack of drug availability for immediate treatment is significant because STIs are on the rise in the United States. The numbers of reported cases of Neisseria gonorrhoeae and Treponema pallidum infections dramatically increased between 2013 and 2017, at 75% higher for gonorrhea and 153% higher for syphilis (primary and secondary), according to a research letter in the November issue of Emerging Infectious Diseases.
Optimal, same-day treatment of bacterial STIs with a highly effective regimen is critical for national STI control efforts and can help mitigate the development of drug resistance, the researchers stated. The recommended first-line treatment for uncomplicated gonorrhea is intramuscular ceftriaxone (250 mg), and for primary and secondary syphilis, it’s intramuscular penicillin G benzathine (2.5 million units), instead of using oral antimicrobial drug alternatives, which have been known to facilitate the development of drug resistance.
William S. Pearson, PhD, of the CDC and colleagues examined the on-site availability of the two injectable therapeutic agents among physicians who treated STIs in their office. They used the 2016 Physician Induction File of the National Ambulatory Medical Care Survey to assess the number of physicians who treat patients with STIs and had injectable antimicrobial drugs available on site. A total of 1,030 physicians (46.2% unweighted response rate), which represents an estimated 330,581 physicians in the United States, completed the Physician Induction File in 2016.
In this survey, physicians who reported evaluating or treating patients for STIs were asked which antimicrobial drugs they had available on site for same-day management of gonorrhea and syphilis, including intramuscular ceftriaxone and penicillin G benzathine at the recommended doses.
The researchers used this information to determine national estimates of reported on-site, same-day availability for these antimicrobial drugs and stratified results by patient-centered medical homes (PCMH) designation and U.S. region. They used multiple logistic regression models to determine if PCMH designation and region were predictive of on-site availability of the two medications.
An estimated 45.2% (149,483) of office-based physicians indicated that they evaluate patients for STIs in their offices. Of these, 77.9% reported not having penicillin G benzathine available on site, and 56.1% reported not having ceftriaxone.
Geographic differences in drug availability were not statistically significant. In addition, physicians in offices not designated PCMHs were more likely than those in offices designated as PCMHs to report lacking on-site availability of ceftriaxone (odds ratio, 2.03) and penicillin G benzathine (OR, 3.20).
“The costs of obtaining and carrying these medications, as well as issues of storage and shelf-life, should be explored to determine if these factors are barriers. In addition, the implications of prescribing alternative treatments or delaying care in situations when medications are not readily available on site should be further explored. Mitigating the lack of medication availability to treat these infections will help public health officials stop the rise in STI disease,” the researchers concluded.
The authors are all employees of the CDC and did not provide other disclosures.
SOURCE: Pearson WS et al. Emerg Infect Dis. 2019. doi: 10.3201/eid2511.190764.
The majority of physicians surveyed who treat sexually transmitted infections (STIs) in their offices reported that they did not have on-site availability of the two primary injectable drugs for syphilis and gonorrhea, according to researchers from the Centers for Disease Control and Prevention.
This lack of drug availability for immediate treatment is significant because STIs are on the rise in the United States. The numbers of reported cases of Neisseria gonorrhoeae and Treponema pallidum infections dramatically increased between 2013 and 2017, at 75% higher for gonorrhea and 153% higher for syphilis (primary and secondary), according to a research letter in the November issue of Emerging Infectious Diseases.
Optimal, same-day treatment of bacterial STIs with a highly effective regimen is critical for national STI control efforts and can help mitigate the development of drug resistance, the researchers stated. The recommended first-line treatment for uncomplicated gonorrhea is intramuscular ceftriaxone (250 mg), and for primary and secondary syphilis, it’s intramuscular penicillin G benzathine (2.5 million units), instead of using oral antimicrobial drug alternatives, which have been known to facilitate the development of drug resistance.
William S. Pearson, PhD, of the CDC and colleagues examined the on-site availability of the two injectable therapeutic agents among physicians who treated STIs in their office. They used the 2016 Physician Induction File of the National Ambulatory Medical Care Survey to assess the number of physicians who treat patients with STIs and had injectable antimicrobial drugs available on site. A total of 1,030 physicians (46.2% unweighted response rate), which represents an estimated 330,581 physicians in the United States, completed the Physician Induction File in 2016.
In this survey, physicians who reported evaluating or treating patients for STIs were asked which antimicrobial drugs they had available on site for same-day management of gonorrhea and syphilis, including intramuscular ceftriaxone and penicillin G benzathine at the recommended doses.
The researchers used this information to determine national estimates of reported on-site, same-day availability for these antimicrobial drugs and stratified results by patient-centered medical homes (PCMH) designation and U.S. region. They used multiple logistic regression models to determine if PCMH designation and region were predictive of on-site availability of the two medications.
An estimated 45.2% (149,483) of office-based physicians indicated that they evaluate patients for STIs in their offices. Of these, 77.9% reported not having penicillin G benzathine available on site, and 56.1% reported not having ceftriaxone.
Geographic differences in drug availability were not statistically significant. In addition, physicians in offices not designated PCMHs were more likely than those in offices designated as PCMHs to report lacking on-site availability of ceftriaxone (odds ratio, 2.03) and penicillin G benzathine (OR, 3.20).
“The costs of obtaining and carrying these medications, as well as issues of storage and shelf-life, should be explored to determine if these factors are barriers. In addition, the implications of prescribing alternative treatments or delaying care in situations when medications are not readily available on site should be further explored. Mitigating the lack of medication availability to treat these infections will help public health officials stop the rise in STI disease,” the researchers concluded.
The authors are all employees of the CDC and did not provide other disclosures.
SOURCE: Pearson WS et al. Emerg Infect Dis. 2019. doi: 10.3201/eid2511.190764.
The majority of physicians surveyed who treat sexually transmitted infections (STIs) in their offices reported that they did not have on-site availability of the two primary injectable drugs for syphilis and gonorrhea, according to researchers from the Centers for Disease Control and Prevention.
This lack of drug availability for immediate treatment is significant because STIs are on the rise in the United States. The numbers of reported cases of Neisseria gonorrhoeae and Treponema pallidum infections dramatically increased between 2013 and 2017, at 75% higher for gonorrhea and 153% higher for syphilis (primary and secondary), according to a research letter in the November issue of Emerging Infectious Diseases.
Optimal, same-day treatment of bacterial STIs with a highly effective regimen is critical for national STI control efforts and can help mitigate the development of drug resistance, the researchers stated. The recommended first-line treatment for uncomplicated gonorrhea is intramuscular ceftriaxone (250 mg), and for primary and secondary syphilis, it’s intramuscular penicillin G benzathine (2.5 million units), instead of using oral antimicrobial drug alternatives, which have been known to facilitate the development of drug resistance.
William S. Pearson, PhD, of the CDC and colleagues examined the on-site availability of the two injectable therapeutic agents among physicians who treated STIs in their office. They used the 2016 Physician Induction File of the National Ambulatory Medical Care Survey to assess the number of physicians who treat patients with STIs and had injectable antimicrobial drugs available on site. A total of 1,030 physicians (46.2% unweighted response rate), which represents an estimated 330,581 physicians in the United States, completed the Physician Induction File in 2016.
In this survey, physicians who reported evaluating or treating patients for STIs were asked which antimicrobial drugs they had available on site for same-day management of gonorrhea and syphilis, including intramuscular ceftriaxone and penicillin G benzathine at the recommended doses.
The researchers used this information to determine national estimates of reported on-site, same-day availability for these antimicrobial drugs and stratified results by patient-centered medical homes (PCMH) designation and U.S. region. They used multiple logistic regression models to determine if PCMH designation and region were predictive of on-site availability of the two medications.
An estimated 45.2% (149,483) of office-based physicians indicated that they evaluate patients for STIs in their offices. Of these, 77.9% reported not having penicillin G benzathine available on site, and 56.1% reported not having ceftriaxone.
Geographic differences in drug availability were not statistically significant. In addition, physicians in offices not designated PCMHs were more likely than those in offices designated as PCMHs to report lacking on-site availability of ceftriaxone (odds ratio, 2.03) and penicillin G benzathine (OR, 3.20).
“The costs of obtaining and carrying these medications, as well as issues of storage and shelf-life, should be explored to determine if these factors are barriers. In addition, the implications of prescribing alternative treatments or delaying care in situations when medications are not readily available on site should be further explored. Mitigating the lack of medication availability to treat these infections will help public health officials stop the rise in STI disease,” the researchers concluded.
The authors are all employees of the CDC and did not provide other disclosures.
SOURCE: Pearson WS et al. Emerg Infect Dis. 2019. doi: 10.3201/eid2511.190764.
FROM EMERGING INFECTIOUS DISEASES
Short-term statin use linked to risk of skin and soft tissue infections
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
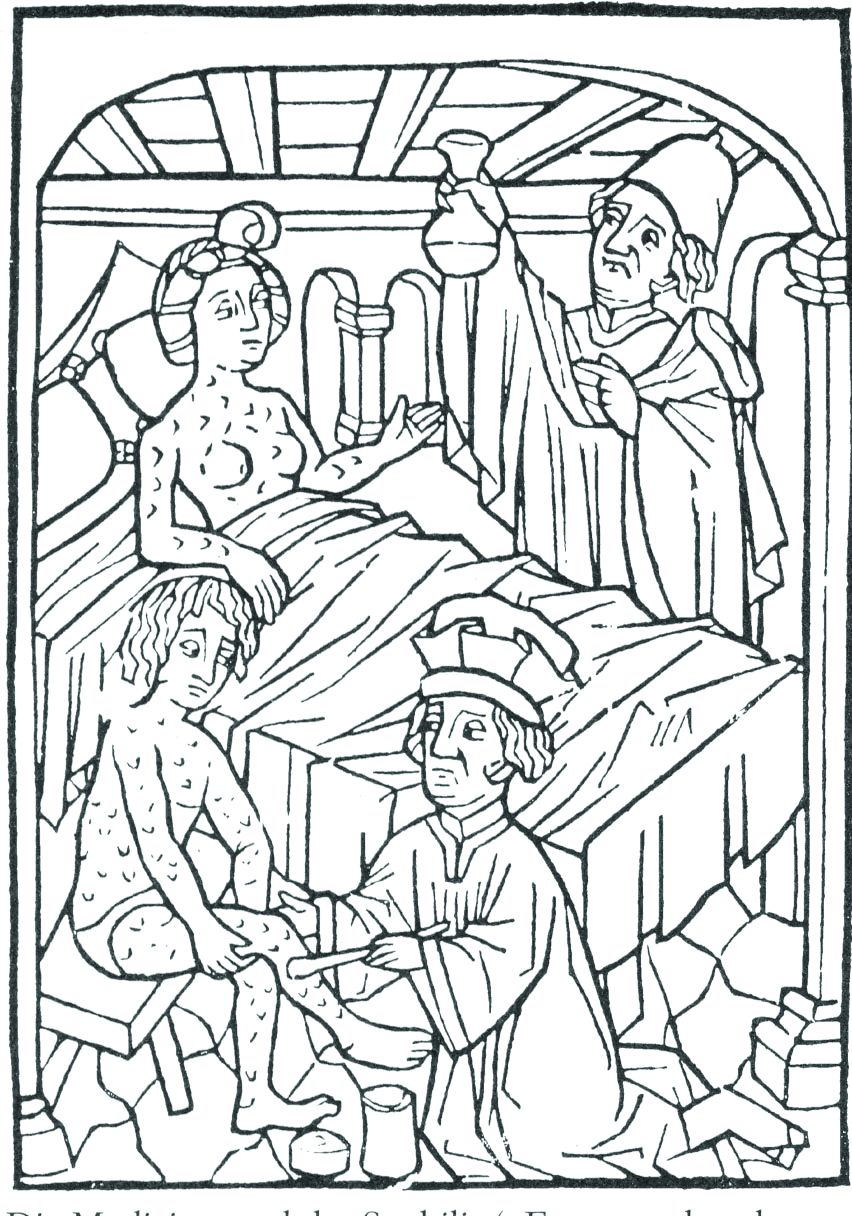
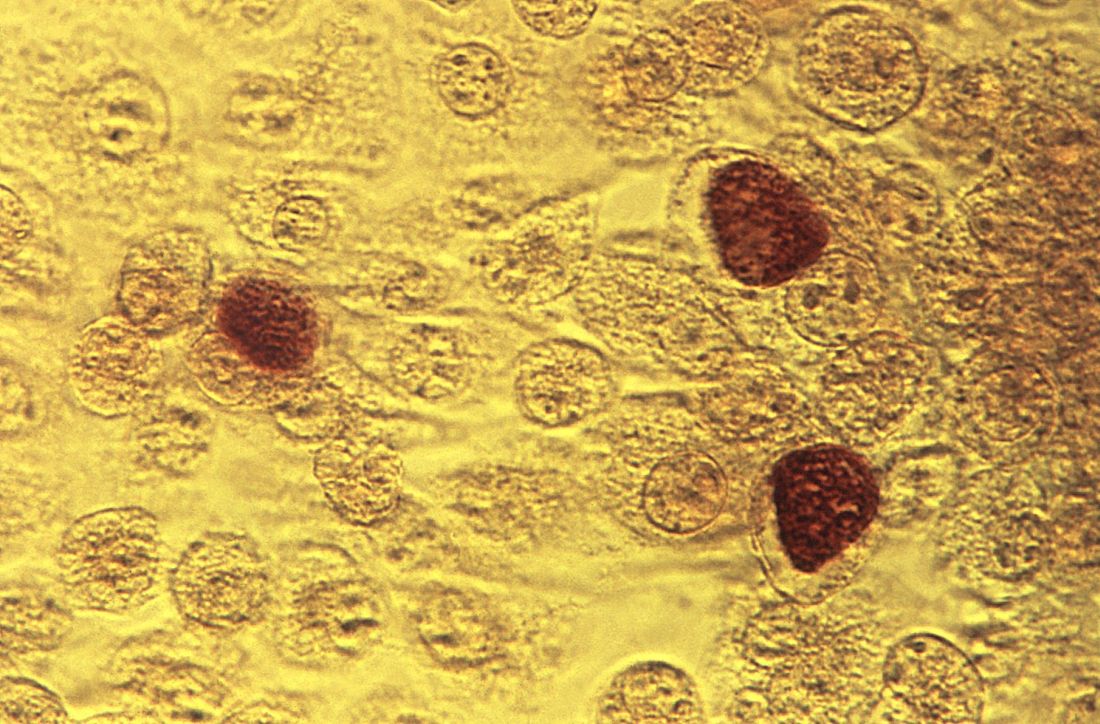
ID Blog: The story of syphilis, part III
The tortured road to successful treatment
It is rare in this modern era for medicine to confront an infectious disease for which there is no cure. Today, there are comparatively few infectious diseases (in the developed world and in places where money is no object) for which medicine cannot offer at least a glimmer of hope to infected patients. Even at its most futile, modern medicine has achieved vast improvements in the efficacy of palliative care. But it wasn’t that long ago that HIV infection was a nearly inevitable death sentence from the complications of AIDS, with no available treatments. And however monstrous that suffering and death, which still continues in many areas of the developing world, it was decades rather than centuries before modern medicine came up with effective treatments. Recently, there is even significant hope on the Ebola virus front that curative treatments may soon become available.
Medicine has always been in the business of hope, even when true cures were not available. Today that hope is less often misplaced. But in previous centuries, the need to offer hope to – and perhaps to make money from – desperate patients was a hallmark of the doctor’s trade.
It was this need to give patients hope and for doctors to feel that they were being effective that led to some highly dubious and desperate efforts to cure syphilis throughout history. These efforts meant centuries of fruitless torture for countless patients until the rise of modern antibiotics.
For the most part, what we now look upon as horrors and insanity in treatment were the result of misguided scientific theories, half-baked folk wisdom, and the generally well-intentioned efforts of medical practitioners at a cure. There were the charlatans as well, seeking a quick buck from the truly hopeless.
However, the social stigma of syphilis as a venereal disease played a role in the courses of treatment.
By the 15th century, syphilis was recognized as being spread by sexual intercourse, and in a situation analogous with the early AIDS epidemic, “16th- and 17th-century writers and physicians were divided on the moral aspects of syphilis. Some thought it was a divine punishment for sin – and as such only harsh treatments would cure it – or that people with syphilis shouldn’t be treated at all.”
Mercury rising
In its earliest manifestations, syphilis was considered untreatable. In 1496, Sebastian Brandt, wrote a poem entitled “De pestilentiali Scorra sive mala de Franzos” detailing the disease’s early spread across Europe and how doctors had no remedy for it.
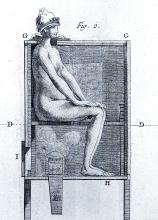
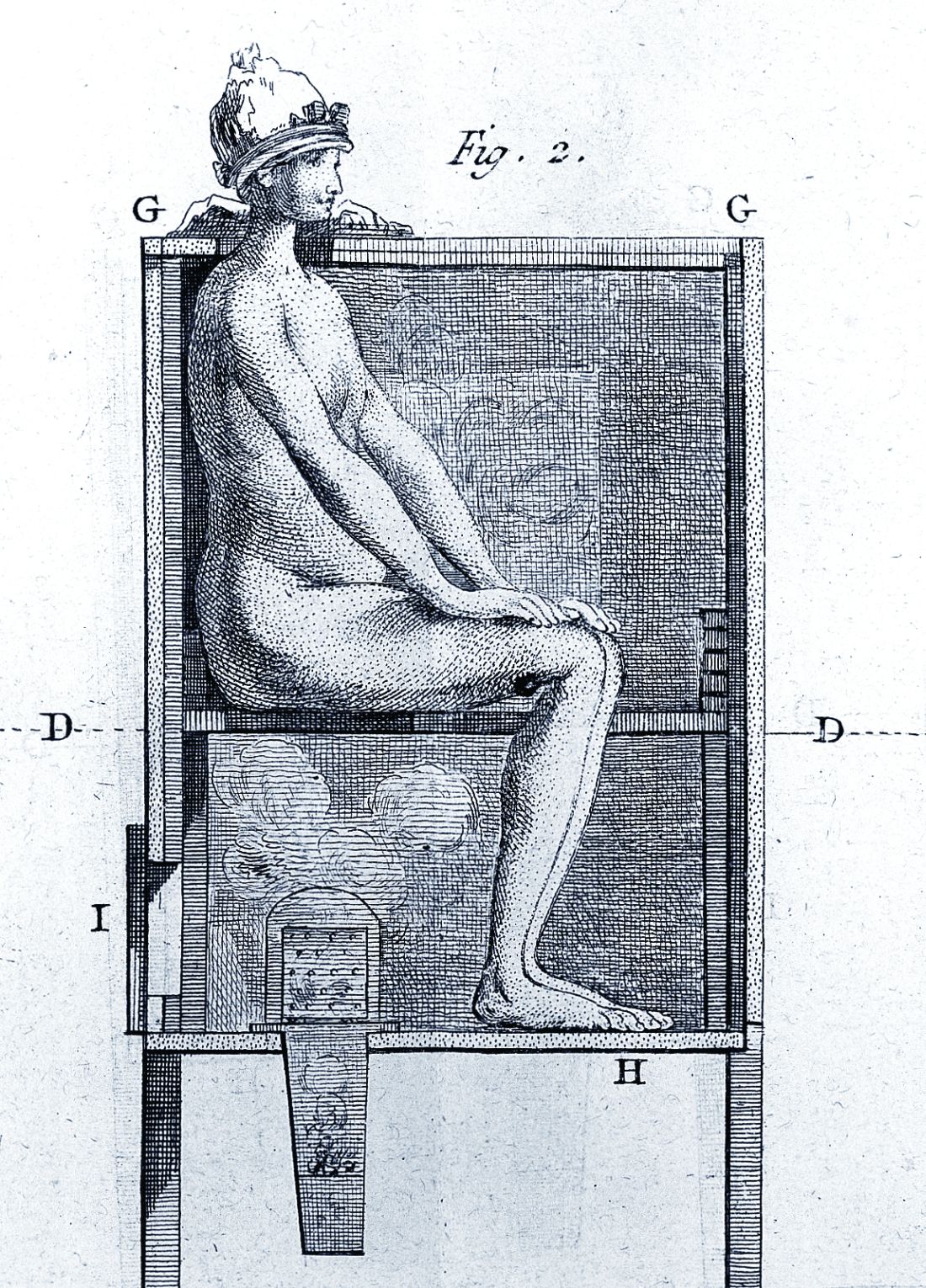
However, it wasn’t long before desperate physicians turned their quest for a cure to a reliable old standby treatment of the period – mercury, which had a history of being used for skin diseases. Mercury salves had been in use in the Arab world for leprosy and eczema, among other skin afflictions, and had been brought to Europe with the return of the medieval crusaders. Another way elemental mercury was administered was through the use of heated cinnabar (HgS), which gave off mercury vapors that could be absorbed by breathing and through the skin. In the 16th century, doctors would place a syphilis-infected individual inside an ovenlike chamber over pans of cinnabar, which were then heated at the person’s feet.
Oral mercury treatments were promoted by Paracelsus (1493?-1541), an alchemist and physician who prescribed calomel (HgCl), or mercury chloride, pills. Mercury treatment, administered at almost inevitably toxic doses, led to ulcerations of the lips, tongue, palate, and jaw; tooth loss; and fetid breath and excessive salivation. This last symptom was, in fact, considered the endpoint in mercury therapy for syphilis, which was “originally judged to be a copious secretion of saliva – ‘some few liters per diem.’ ” Even as recent as the late 19th century and early 20th century, syphilitic patients such as Oscar Wilde (whose teeth were blackened by the treatment), were prescribed calomel.
Looking to the “holy wood”
By 1519, an alternative treatment to mercury was available. In that year, Ulrich von Hutton, a German scholar who suffered from the “great pox,” described its treatment with guaiacum sanctum, or holy wood, in “De Morbo Gallico.” Four years later, despite such treatment, he was dead from the disease himself. But the lack of efficacy did not stop the faith that doctors placed in this botanical cure.
Holy wood was an herbal treatment derived from the bark of trees from the Guaiacum family. It was brought back on trading ships from the Caribbean and South America, the origin of syphilis’s foothold in Europe and the rest of the world. The use of holy wood matched a then-current theory that the cure to a disease could be found in the area from which it came. Other botanicals from around the world were also tried, but never came into routine use.
Guaiacum was the first treatment given to sufferers of syphilis in the Blatterhaus (pox hospital) in Augsburg after 1522, according to information from the archives at the Edward Worth Library in Dublin. The botanical therapy was given as a hot drink and followed by a sweating cure. Guaiacum extract acted as a sudorific, a compound which induces sweating when ingested. Even though the use of Guaiacum was initially popular, it was replaced almost exclusively by the use of mercury.
“Give me fever”
In the late 1800s, Julius Wagner von Jauregg (1857-1940), a Viennese neurologist, observed that Austrian army officers with neurosyphilis did not become partially paralyzed if they had also contracted malaria or relapsing fever. He initiated clinical trials in which he induced fever in syphilitics with tuberculin (1-10 mg) and observed in many the remissions their neuropsychiatric symptoms and signs. He also injected neurosyphilitic patients with a mild form of malaria to induce fever, which could then be suppressed with quinine treatment.
“Other physicians soon began using malariotherapy in uncontrolled studies of neurosyphilitics and reported clinical success rates of 33%-51% and only a 5% mortality. Persons with tabes dorsalis (the “wasting” paralysis of neurosyphilis) were hospitalized for 3 weeks of alternate-day fever therapy involving 5-hour long hot baths and extended periods wrapped in heavy blankets,” according to C.T. Ambrose, MD, of the University of Kentucky, Lexington.
A 1931 medical text summarizes in 35 studies involving 2,356 cases of general paresis treated with malaria and reported a 27.5% “full remission,” he added. A bacterial treatment developed in this period used a course of 18-23 injections of killed typhoid cells administered every 2-3 days in order to produce a fever of 103°–104°F. Animal studies of rabbits infected with syphilis showed that high temperatures could be curative.
Dr. Ambrose suggests that 16th-century syphilitics who had been subjected to mercury fumigation in ovenlike chambers endured severe sweating conditions and – for those who survived – the prolonged elevated body temperature (not the mercury) may have proved curative. Fever “was the common therapeutic denominator in the cinnabar-oven treatment, botanical sudorifics (guaiacum, China root), malarial infections (natural and iatrogenic), and bacterial (tuberculin) vaccine therapy.”
Prelude to modern antibiotics
German bacteriologist/immunologist Paul Ehrlich, MD, (1854-1915) investigated the use of atoxyl (sodium arsanilate) in syphilis, but the metallic drug had severe side effects, injuring the optic nerve and causing blindness. To overcome this problem, Ehrlich and his coworkers synthesized and tested related organic arsenicals. The antisyphilitic activity of arsphenamine (compound 606) was discovered by Sahachiro Hata, MD, (1879-1938) in 1909. This compound, known as Salvarsan, became “Dr. Ehrlich’s Magic Bullet,” for the treatment of syphilis in the 1910s, and it, and later, the less-toxic compound neoarsphenamine (compound 914) became mainstays of successful clinical treatment until the development and use of penicillin in the 1940s.
Selected sources
Ambrose, CT. Pre-antibiotic therapy of syphilis. NESSA J Infect Dis Immunology. 2016. 1(1);1-20.
Frith J. Syphilis: Its early history and treatment until penicillin and the debate on its origins. J Mil Veterans Health. 2012;20(4):49-58.
Tognotti B. The rise and fall of syphilis in Renaissance Italy. J Med Humanit. 2009 Jun;30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
The tortured road to successful treatment
The tortured road to successful treatment
It is rare in this modern era for medicine to confront an infectious disease for which there is no cure. Today, there are comparatively few infectious diseases (in the developed world and in places where money is no object) for which medicine cannot offer at least a glimmer of hope to infected patients. Even at its most futile, modern medicine has achieved vast improvements in the efficacy of palliative care. But it wasn’t that long ago that HIV infection was a nearly inevitable death sentence from the complications of AIDS, with no available treatments. And however monstrous that suffering and death, which still continues in many areas of the developing world, it was decades rather than centuries before modern medicine came up with effective treatments. Recently, there is even significant hope on the Ebola virus front that curative treatments may soon become available.
Medicine has always been in the business of hope, even when true cures were not available. Today that hope is less often misplaced. But in previous centuries, the need to offer hope to – and perhaps to make money from – desperate patients was a hallmark of the doctor’s trade.
It was this need to give patients hope and for doctors to feel that they were being effective that led to some highly dubious and desperate efforts to cure syphilis throughout history. These efforts meant centuries of fruitless torture for countless patients until the rise of modern antibiotics.
For the most part, what we now look upon as horrors and insanity in treatment were the result of misguided scientific theories, half-baked folk wisdom, and the generally well-intentioned efforts of medical practitioners at a cure. There were the charlatans as well, seeking a quick buck from the truly hopeless.
However, the social stigma of syphilis as a venereal disease played a role in the courses of treatment.
By the 15th century, syphilis was recognized as being spread by sexual intercourse, and in a situation analogous with the early AIDS epidemic, “16th- and 17th-century writers and physicians were divided on the moral aspects of syphilis. Some thought it was a divine punishment for sin – and as such only harsh treatments would cure it – or that people with syphilis shouldn’t be treated at all.”
Mercury rising
In its earliest manifestations, syphilis was considered untreatable. In 1496, Sebastian Brandt, wrote a poem entitled “De pestilentiali Scorra sive mala de Franzos” detailing the disease’s early spread across Europe and how doctors had no remedy for it.
However, it wasn’t long before desperate physicians turned their quest for a cure to a reliable old standby treatment of the period – mercury, which had a history of being used for skin diseases. Mercury salves had been in use in the Arab world for leprosy and eczema, among other skin afflictions, and had been brought to Europe with the return of the medieval crusaders. Another way elemental mercury was administered was through the use of heated cinnabar (HgS), which gave off mercury vapors that could be absorbed by breathing and through the skin. In the 16th century, doctors would place a syphilis-infected individual inside an ovenlike chamber over pans of cinnabar, which were then heated at the person’s feet.
Oral mercury treatments were promoted by Paracelsus (1493?-1541), an alchemist and physician who prescribed calomel (HgCl), or mercury chloride, pills. Mercury treatment, administered at almost inevitably toxic doses, led to ulcerations of the lips, tongue, palate, and jaw; tooth loss; and fetid breath and excessive salivation. This last symptom was, in fact, considered the endpoint in mercury therapy for syphilis, which was “originally judged to be a copious secretion of saliva – ‘some few liters per diem.’ ” Even as recent as the late 19th century and early 20th century, syphilitic patients such as Oscar Wilde (whose teeth were blackened by the treatment), were prescribed calomel.
Looking to the “holy wood”
By 1519, an alternative treatment to mercury was available. In that year, Ulrich von Hutton, a German scholar who suffered from the “great pox,” described its treatment with guaiacum sanctum, or holy wood, in “De Morbo Gallico.” Four years later, despite such treatment, he was dead from the disease himself. But the lack of efficacy did not stop the faith that doctors placed in this botanical cure.
Holy wood was an herbal treatment derived from the bark of trees from the Guaiacum family. It was brought back on trading ships from the Caribbean and South America, the origin of syphilis’s foothold in Europe and the rest of the world. The use of holy wood matched a then-current theory that the cure to a disease could be found in the area from which it came. Other botanicals from around the world were also tried, but never came into routine use.
Guaiacum was the first treatment given to sufferers of syphilis in the Blatterhaus (pox hospital) in Augsburg after 1522, according to information from the archives at the Edward Worth Library in Dublin. The botanical therapy was given as a hot drink and followed by a sweating cure. Guaiacum extract acted as a sudorific, a compound which induces sweating when ingested. Even though the use of Guaiacum was initially popular, it was replaced almost exclusively by the use of mercury.
“Give me fever”
In the late 1800s, Julius Wagner von Jauregg (1857-1940), a Viennese neurologist, observed that Austrian army officers with neurosyphilis did not become partially paralyzed if they had also contracted malaria or relapsing fever. He initiated clinical trials in which he induced fever in syphilitics with tuberculin (1-10 mg) and observed in many the remissions their neuropsychiatric symptoms and signs. He also injected neurosyphilitic patients with a mild form of malaria to induce fever, which could then be suppressed with quinine treatment.
“Other physicians soon began using malariotherapy in uncontrolled studies of neurosyphilitics and reported clinical success rates of 33%-51% and only a 5% mortality. Persons with tabes dorsalis (the “wasting” paralysis of neurosyphilis) were hospitalized for 3 weeks of alternate-day fever therapy involving 5-hour long hot baths and extended periods wrapped in heavy blankets,” according to C.T. Ambrose, MD, of the University of Kentucky, Lexington.
A 1931 medical text summarizes in 35 studies involving 2,356 cases of general paresis treated with malaria and reported a 27.5% “full remission,” he added. A bacterial treatment developed in this period used a course of 18-23 injections of killed typhoid cells administered every 2-3 days in order to produce a fever of 103°–104°F. Animal studies of rabbits infected with syphilis showed that high temperatures could be curative.
Dr. Ambrose suggests that 16th-century syphilitics who had been subjected to mercury fumigation in ovenlike chambers endured severe sweating conditions and – for those who survived – the prolonged elevated body temperature (not the mercury) may have proved curative. Fever “was the common therapeutic denominator in the cinnabar-oven treatment, botanical sudorifics (guaiacum, China root), malarial infections (natural and iatrogenic), and bacterial (tuberculin) vaccine therapy.”
Prelude to modern antibiotics
German bacteriologist/immunologist Paul Ehrlich, MD, (1854-1915) investigated the use of atoxyl (sodium arsanilate) in syphilis, but the metallic drug had severe side effects, injuring the optic nerve and causing blindness. To overcome this problem, Ehrlich and his coworkers synthesized and tested related organic arsenicals. The antisyphilitic activity of arsphenamine (compound 606) was discovered by Sahachiro Hata, MD, (1879-1938) in 1909. This compound, known as Salvarsan, became “Dr. Ehrlich’s Magic Bullet,” for the treatment of syphilis in the 1910s, and it, and later, the less-toxic compound neoarsphenamine (compound 914) became mainstays of successful clinical treatment until the development and use of penicillin in the 1940s.
Selected sources
Ambrose, CT. Pre-antibiotic therapy of syphilis. NESSA J Infect Dis Immunology. 2016. 1(1);1-20.
Frith J. Syphilis: Its early history and treatment until penicillin and the debate on its origins. J Mil Veterans Health. 2012;20(4):49-58.
Tognotti B. The rise and fall of syphilis in Renaissance Italy. J Med Humanit. 2009 Jun;30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
It is rare in this modern era for medicine to confront an infectious disease for which there is no cure. Today, there are comparatively few infectious diseases (in the developed world and in places where money is no object) for which medicine cannot offer at least a glimmer of hope to infected patients. Even at its most futile, modern medicine has achieved vast improvements in the efficacy of palliative care. But it wasn’t that long ago that HIV infection was a nearly inevitable death sentence from the complications of AIDS, with no available treatments. And however monstrous that suffering and death, which still continues in many areas of the developing world, it was decades rather than centuries before modern medicine came up with effective treatments. Recently, there is even significant hope on the Ebola virus front that curative treatments may soon become available.
Medicine has always been in the business of hope, even when true cures were not available. Today that hope is less often misplaced. But in previous centuries, the need to offer hope to – and perhaps to make money from – desperate patients was a hallmark of the doctor’s trade.
It was this need to give patients hope and for doctors to feel that they were being effective that led to some highly dubious and desperate efforts to cure syphilis throughout history. These efforts meant centuries of fruitless torture for countless patients until the rise of modern antibiotics.
For the most part, what we now look upon as horrors and insanity in treatment were the result of misguided scientific theories, half-baked folk wisdom, and the generally well-intentioned efforts of medical practitioners at a cure. There were the charlatans as well, seeking a quick buck from the truly hopeless.
However, the social stigma of syphilis as a venereal disease played a role in the courses of treatment.
By the 15th century, syphilis was recognized as being spread by sexual intercourse, and in a situation analogous with the early AIDS epidemic, “16th- and 17th-century writers and physicians were divided on the moral aspects of syphilis. Some thought it was a divine punishment for sin – and as such only harsh treatments would cure it – or that people with syphilis shouldn’t be treated at all.”
Mercury rising
In its earliest manifestations, syphilis was considered untreatable. In 1496, Sebastian Brandt, wrote a poem entitled “De pestilentiali Scorra sive mala de Franzos” detailing the disease’s early spread across Europe and how doctors had no remedy for it.
However, it wasn’t long before desperate physicians turned their quest for a cure to a reliable old standby treatment of the period – mercury, which had a history of being used for skin diseases. Mercury salves had been in use in the Arab world for leprosy and eczema, among other skin afflictions, and had been brought to Europe with the return of the medieval crusaders. Another way elemental mercury was administered was through the use of heated cinnabar (HgS), which gave off mercury vapors that could be absorbed by breathing and through the skin. In the 16th century, doctors would place a syphilis-infected individual inside an ovenlike chamber over pans of cinnabar, which were then heated at the person’s feet.
Oral mercury treatments were promoted by Paracelsus (1493?-1541), an alchemist and physician who prescribed calomel (HgCl), or mercury chloride, pills. Mercury treatment, administered at almost inevitably toxic doses, led to ulcerations of the lips, tongue, palate, and jaw; tooth loss; and fetid breath and excessive salivation. This last symptom was, in fact, considered the endpoint in mercury therapy for syphilis, which was “originally judged to be a copious secretion of saliva – ‘some few liters per diem.’ ” Even as recent as the late 19th century and early 20th century, syphilitic patients such as Oscar Wilde (whose teeth were blackened by the treatment), were prescribed calomel.
Looking to the “holy wood”
By 1519, an alternative treatment to mercury was available. In that year, Ulrich von Hutton, a German scholar who suffered from the “great pox,” described its treatment with guaiacum sanctum, or holy wood, in “De Morbo Gallico.” Four years later, despite such treatment, he was dead from the disease himself. But the lack of efficacy did not stop the faith that doctors placed in this botanical cure.
Holy wood was an herbal treatment derived from the bark of trees from the Guaiacum family. It was brought back on trading ships from the Caribbean and South America, the origin of syphilis’s foothold in Europe and the rest of the world. The use of holy wood matched a then-current theory that the cure to a disease could be found in the area from which it came. Other botanicals from around the world were also tried, but never came into routine use.
Guaiacum was the first treatment given to sufferers of syphilis in the Blatterhaus (pox hospital) in Augsburg after 1522, according to information from the archives at the Edward Worth Library in Dublin. The botanical therapy was given as a hot drink and followed by a sweating cure. Guaiacum extract acted as a sudorific, a compound which induces sweating when ingested. Even though the use of Guaiacum was initially popular, it was replaced almost exclusively by the use of mercury.
“Give me fever”
In the late 1800s, Julius Wagner von Jauregg (1857-1940), a Viennese neurologist, observed that Austrian army officers with neurosyphilis did not become partially paralyzed if they had also contracted malaria or relapsing fever. He initiated clinical trials in which he induced fever in syphilitics with tuberculin (1-10 mg) and observed in many the remissions their neuropsychiatric symptoms and signs. He also injected neurosyphilitic patients with a mild form of malaria to induce fever, which could then be suppressed with quinine treatment.
“Other physicians soon began using malariotherapy in uncontrolled studies of neurosyphilitics and reported clinical success rates of 33%-51% and only a 5% mortality. Persons with tabes dorsalis (the “wasting” paralysis of neurosyphilis) were hospitalized for 3 weeks of alternate-day fever therapy involving 5-hour long hot baths and extended periods wrapped in heavy blankets,” according to C.T. Ambrose, MD, of the University of Kentucky, Lexington.
A 1931 medical text summarizes in 35 studies involving 2,356 cases of general paresis treated with malaria and reported a 27.5% “full remission,” he added. A bacterial treatment developed in this period used a course of 18-23 injections of killed typhoid cells administered every 2-3 days in order to produce a fever of 103°–104°F. Animal studies of rabbits infected with syphilis showed that high temperatures could be curative.
Dr. Ambrose suggests that 16th-century syphilitics who had been subjected to mercury fumigation in ovenlike chambers endured severe sweating conditions and – for those who survived – the prolonged elevated body temperature (not the mercury) may have proved curative. Fever “was the common therapeutic denominator in the cinnabar-oven treatment, botanical sudorifics (guaiacum, China root), malarial infections (natural and iatrogenic), and bacterial (tuberculin) vaccine therapy.”
Prelude to modern antibiotics
German bacteriologist/immunologist Paul Ehrlich, MD, (1854-1915) investigated the use of atoxyl (sodium arsanilate) in syphilis, but the metallic drug had severe side effects, injuring the optic nerve and causing blindness. To overcome this problem, Ehrlich and his coworkers synthesized and tested related organic arsenicals. The antisyphilitic activity of arsphenamine (compound 606) was discovered by Sahachiro Hata, MD, (1879-1938) in 1909. This compound, known as Salvarsan, became “Dr. Ehrlich’s Magic Bullet,” for the treatment of syphilis in the 1910s, and it, and later, the less-toxic compound neoarsphenamine (compound 914) became mainstays of successful clinical treatment until the development and use of penicillin in the 1940s.
Selected sources
Ambrose, CT. Pre-antibiotic therapy of syphilis. NESSA J Infect Dis Immunology. 2016. 1(1);1-20.
Frith J. Syphilis: Its early history and treatment until penicillin and the debate on its origins. J Mil Veterans Health. 2012;20(4):49-58.
Tognotti B. The rise and fall of syphilis in Renaissance Italy. J Med Humanit. 2009 Jun;30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Does this patient have bacterial conjunctivitis?
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
ID Blog: The story of syphilis, part II
From epidemic to endemic curse
Evolution is an amazing thing, and its more fascinating aspects are never more apparent than in the endless genetic dance between host and pathogen. And certainly, our fascination with the dance is not merely an intellectual exercise. The evolution of disease is perhaps one of the starkest examples of human misery writ large across the pages of recorded history.
In particular, the evolution of syphilis from dramatically visible, epidemic terror to silent, endemic, and long-term killer is one of the most striking examples of host-pathogen evolution. It is an example noteworthy not only for the profound transformation that occurred, but for the speed of the change, beginning so fast that it was noticed and detailed by physicians at the time as occurring over less than a human generation rather than centuries.
This very speed of the change makes it relatively certain that it was not the human species that evolved resistance, but rather that the syphilis-causing spirochetes transformed in virulence within almost the blink of an evolutionary eye – an epidemiologic mystery of profound importance to the countless lives involved.
Syphilis was a dramatic new phenomenon in the Old World of the late 15th and early 16th centuries – a hitherto unknown disease of terrible guise and rapid dissemination. It was noted and discussed throughout many of the writings of the time, so much so that one of the first detailed patient accounts in recorded history of the experience of a disease was written in response to a syphilis infection.
In 1498, Tommaso di Silvestro, an Italian notary, described his symptoms in depth: “I remember how I, Ser Tomaso, during the day 27th of April 1498, coming back from the fair in Foligno, started to feel pain in the virga [a contemporary euphemism for penis]. And then the pain grew in intensity. Then in June I started to feel the pains of the French disease. And all my body filled with pustules and crusts. I had pains in the right and left arms, in the entire arm, from the shoulder to the hand, I was filled with pain to the bones and I never found peace. And then I had pains in the right knee and all my body got full of boils, at the back at the front and behind.”
Alessandro Benedetti (1450-1512), a military surgeon and chronicler of the expedition of Charles VIII, wrote in 1497 that sufferers lost hands, feet, eyes, and noses to the disease, such that it made “the entire body so repulsive to look at and causes such great suffering.”Another common characteristic was a foul smell typical of the infected.
Careful analysis by historians has shown that, according to records from the time period, 10-15 years after the start of the epidemic in the late 15th century, there was a noticeable decline in disease virulence.
As one historian put it: “Many physicians and contemporary observers noticed the progressive decline in the severity of the disease. Many symptoms were less severe, and the rash, of a reddish color, did not cause itching.” Girolamo Fracastoro writes about some of these transformations, stating that “in the first epidemic periods the pustules were filthier,’ while they were ‘harder and drier’ afterwards.” Similarly, the historian and scholar Bernardino Cirillo dell’Aquila (1500-1575), writing in the 1530s, stated: “This horrible disease in different periods (1494) till the present had different alterations and different effects depending on the complications, and now many people just lose their hair and nothing else.”
As added documentation of the change, the chaplain of the infamous conquistador Hernàn Cortés reported that syphilis was less severe in his time than earlier. He wrote that: “at the beginning this disease was very violent, dirty and indecent; now it is no longer so severe and so indecent.”
The medical literature of the time confirmed that the fever, characteristic of the second stage of the disease, “was less violent, while even the rashes were just a ‘reddening.’ Moreover, the gummy tumors appeared only in a limited number of cases.”
According to another historian, “By the middle of the 16th century, the generation of physicians born between the end of the 15th century and the first decades of the 16th century considered the exceptional virulence manifested by syphilis when it first appeared to be ancient history.”
And Ambroise Paré (1510-1590), a renowned French surgeon, stated: “Today it is much less serious and easier to heal than it was in the past... It is obviously becoming much milder … so that it seems it should disappear in the future.”
Lacking detailed genetic analysis of the changing pathogen, if one were to speculate on why the virulence of syphilis decreased so rapidly, I suggest, in a Just-So story fashion, that one might merely speculate on the evolutionary wisdom of an STD that commonly turned its victims into foul-smelling, scabrous, agonized, and lethargic individuals who lost body parts, including their genitals, according to some reports. None of these outcomes, of course, would be conducive to the natural spread of the disease. In addition, this is a good case for sexual selection as well as early death of the host, which are two main engineers of evolutionary change.
But for whatever reason, the presentation of syphilis changed dramatically over a relatively short period of time, and as the disease was still spreading through a previously unexposed population, a change in pathogenicity rather than host immunity seems the most logical explanation.
As syphilis evolved from its initial onslaught, it showed new and hitherto unseen symptoms, including the aforementioned hair loss, and other manifestations such as tinnitus. Soon it was presenting so many systemic phenotypes similar to the effects of other diseases that Sir William Osler (1849-1919) ultimately proposed that syphilis should be described as the “Great Imitator.”
The evolution of syphilis from epidemic to endemic does not diminish the horrors of those afflicted with active tertiary syphilis, but as the disease transformed, these effects were greatly postponed and occurred less commonly, compared with their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
Although still lethal, especially in its congenital form, by the end of the 16th century, syphilis had completed its rapid evolution from a devastating, highly visible plague to the covert disease “so sinful that it could not be discussed by name.” It would remain so until the rise of modern antibiotics finally provided a reliable cure. Active tertiary syphilis remained a severe affliction, but the effects were postponed from their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
So, syphilis remains a unique example of host-pathogen evolution, an endemic part of the global human condition, battled by physicians in mostly futile efforts for nearly 500 years, and a disease tracking closely with the rise of modern medicine.
References
Frith J. 2012. Syphilis – Its Early History and Treatment Until Penicillin and the Debate on its Origins. J Military and Veteran’s Health. 20(4):49-58.
Tognoti B. 2009. The Rise and Fall of Syphilis in Renaissance Italy. J Med Humanit. 30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington, D.C.
From epidemic to endemic curse
From epidemic to endemic curse
Evolution is an amazing thing, and its more fascinating aspects are never more apparent than in the endless genetic dance between host and pathogen. And certainly, our fascination with the dance is not merely an intellectual exercise. The evolution of disease is perhaps one of the starkest examples of human misery writ large across the pages of recorded history.
In particular, the evolution of syphilis from dramatically visible, epidemic terror to silent, endemic, and long-term killer is one of the most striking examples of host-pathogen evolution. It is an example noteworthy not only for the profound transformation that occurred, but for the speed of the change, beginning so fast that it was noticed and detailed by physicians at the time as occurring over less than a human generation rather than centuries.
This very speed of the change makes it relatively certain that it was not the human species that evolved resistance, but rather that the syphilis-causing spirochetes transformed in virulence within almost the blink of an evolutionary eye – an epidemiologic mystery of profound importance to the countless lives involved.
Syphilis was a dramatic new phenomenon in the Old World of the late 15th and early 16th centuries – a hitherto unknown disease of terrible guise and rapid dissemination. It was noted and discussed throughout many of the writings of the time, so much so that one of the first detailed patient accounts in recorded history of the experience of a disease was written in response to a syphilis infection.
In 1498, Tommaso di Silvestro, an Italian notary, described his symptoms in depth: “I remember how I, Ser Tomaso, during the day 27th of April 1498, coming back from the fair in Foligno, started to feel pain in the virga [a contemporary euphemism for penis]. And then the pain grew in intensity. Then in June I started to feel the pains of the French disease. And all my body filled with pustules and crusts. I had pains in the right and left arms, in the entire arm, from the shoulder to the hand, I was filled with pain to the bones and I never found peace. And then I had pains in the right knee and all my body got full of boils, at the back at the front and behind.”
Alessandro Benedetti (1450-1512), a military surgeon and chronicler of the expedition of Charles VIII, wrote in 1497 that sufferers lost hands, feet, eyes, and noses to the disease, such that it made “the entire body so repulsive to look at and causes such great suffering.”Another common characteristic was a foul smell typical of the infected.
Careful analysis by historians has shown that, according to records from the time period, 10-15 years after the start of the epidemic in the late 15th century, there was a noticeable decline in disease virulence.
As one historian put it: “Many physicians and contemporary observers noticed the progressive decline in the severity of the disease. Many symptoms were less severe, and the rash, of a reddish color, did not cause itching.” Girolamo Fracastoro writes about some of these transformations, stating that “in the first epidemic periods the pustules were filthier,’ while they were ‘harder and drier’ afterwards.” Similarly, the historian and scholar Bernardino Cirillo dell’Aquila (1500-1575), writing in the 1530s, stated: “This horrible disease in different periods (1494) till the present had different alterations and different effects depending on the complications, and now many people just lose their hair and nothing else.”
As added documentation of the change, the chaplain of the infamous conquistador Hernàn Cortés reported that syphilis was less severe in his time than earlier. He wrote that: “at the beginning this disease was very violent, dirty and indecent; now it is no longer so severe and so indecent.”
The medical literature of the time confirmed that the fever, characteristic of the second stage of the disease, “was less violent, while even the rashes were just a ‘reddening.’ Moreover, the gummy tumors appeared only in a limited number of cases.”
According to another historian, “By the middle of the 16th century, the generation of physicians born between the end of the 15th century and the first decades of the 16th century considered the exceptional virulence manifested by syphilis when it first appeared to be ancient history.”
And Ambroise Paré (1510-1590), a renowned French surgeon, stated: “Today it is much less serious and easier to heal than it was in the past... It is obviously becoming much milder … so that it seems it should disappear in the future.”
Lacking detailed genetic analysis of the changing pathogen, if one were to speculate on why the virulence of syphilis decreased so rapidly, I suggest, in a Just-So story fashion, that one might merely speculate on the evolutionary wisdom of an STD that commonly turned its victims into foul-smelling, scabrous, agonized, and lethargic individuals who lost body parts, including their genitals, according to some reports. None of these outcomes, of course, would be conducive to the natural spread of the disease. In addition, this is a good case for sexual selection as well as early death of the host, which are two main engineers of evolutionary change.
But for whatever reason, the presentation of syphilis changed dramatically over a relatively short period of time, and as the disease was still spreading through a previously unexposed population, a change in pathogenicity rather than host immunity seems the most logical explanation.
As syphilis evolved from its initial onslaught, it showed new and hitherto unseen symptoms, including the aforementioned hair loss, and other manifestations such as tinnitus. Soon it was presenting so many systemic phenotypes similar to the effects of other diseases that Sir William Osler (1849-1919) ultimately proposed that syphilis should be described as the “Great Imitator.”
The evolution of syphilis from epidemic to endemic does not diminish the horrors of those afflicted with active tertiary syphilis, but as the disease transformed, these effects were greatly postponed and occurred less commonly, compared with their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
Although still lethal, especially in its congenital form, by the end of the 16th century, syphilis had completed its rapid evolution from a devastating, highly visible plague to the covert disease “so sinful that it could not be discussed by name.” It would remain so until the rise of modern antibiotics finally provided a reliable cure. Active tertiary syphilis remained a severe affliction, but the effects were postponed from their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
So, syphilis remains a unique example of host-pathogen evolution, an endemic part of the global human condition, battled by physicians in mostly futile efforts for nearly 500 years, and a disease tracking closely with the rise of modern medicine.
References
Frith J. 2012. Syphilis – Its Early History and Treatment Until Penicillin and the Debate on its Origins. J Military and Veteran’s Health. 20(4):49-58.
Tognoti B. 2009. The Rise and Fall of Syphilis in Renaissance Italy. J Med Humanit. 30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington, D.C.
Evolution is an amazing thing, and its more fascinating aspects are never more apparent than in the endless genetic dance between host and pathogen. And certainly, our fascination with the dance is not merely an intellectual exercise. The evolution of disease is perhaps one of the starkest examples of human misery writ large across the pages of recorded history.
In particular, the evolution of syphilis from dramatically visible, epidemic terror to silent, endemic, and long-term killer is one of the most striking examples of host-pathogen evolution. It is an example noteworthy not only for the profound transformation that occurred, but for the speed of the change, beginning so fast that it was noticed and detailed by physicians at the time as occurring over less than a human generation rather than centuries.
This very speed of the change makes it relatively certain that it was not the human species that evolved resistance, but rather that the syphilis-causing spirochetes transformed in virulence within almost the blink of an evolutionary eye – an epidemiologic mystery of profound importance to the countless lives involved.
Syphilis was a dramatic new phenomenon in the Old World of the late 15th and early 16th centuries – a hitherto unknown disease of terrible guise and rapid dissemination. It was noted and discussed throughout many of the writings of the time, so much so that one of the first detailed patient accounts in recorded history of the experience of a disease was written in response to a syphilis infection.
In 1498, Tommaso di Silvestro, an Italian notary, described his symptoms in depth: “I remember how I, Ser Tomaso, during the day 27th of April 1498, coming back from the fair in Foligno, started to feel pain in the virga [a contemporary euphemism for penis]. And then the pain grew in intensity. Then in June I started to feel the pains of the French disease. And all my body filled with pustules and crusts. I had pains in the right and left arms, in the entire arm, from the shoulder to the hand, I was filled with pain to the bones and I never found peace. And then I had pains in the right knee and all my body got full of boils, at the back at the front and behind.”
Alessandro Benedetti (1450-1512), a military surgeon and chronicler of the expedition of Charles VIII, wrote in 1497 that sufferers lost hands, feet, eyes, and noses to the disease, such that it made “the entire body so repulsive to look at and causes such great suffering.”Another common characteristic was a foul smell typical of the infected.
Careful analysis by historians has shown that, according to records from the time period, 10-15 years after the start of the epidemic in the late 15th century, there was a noticeable decline in disease virulence.
As one historian put it: “Many physicians and contemporary observers noticed the progressive decline in the severity of the disease. Many symptoms were less severe, and the rash, of a reddish color, did not cause itching.” Girolamo Fracastoro writes about some of these transformations, stating that “in the first epidemic periods the pustules were filthier,’ while they were ‘harder and drier’ afterwards.” Similarly, the historian and scholar Bernardino Cirillo dell’Aquila (1500-1575), writing in the 1530s, stated: “This horrible disease in different periods (1494) till the present had different alterations and different effects depending on the complications, and now many people just lose their hair and nothing else.”
As added documentation of the change, the chaplain of the infamous conquistador Hernàn Cortés reported that syphilis was less severe in his time than earlier. He wrote that: “at the beginning this disease was very violent, dirty and indecent; now it is no longer so severe and so indecent.”
The medical literature of the time confirmed that the fever, characteristic of the second stage of the disease, “was less violent, while even the rashes were just a ‘reddening.’ Moreover, the gummy tumors appeared only in a limited number of cases.”
According to another historian, “By the middle of the 16th century, the generation of physicians born between the end of the 15th century and the first decades of the 16th century considered the exceptional virulence manifested by syphilis when it first appeared to be ancient history.”
And Ambroise Paré (1510-1590), a renowned French surgeon, stated: “Today it is much less serious and easier to heal than it was in the past... It is obviously becoming much milder … so that it seems it should disappear in the future.”
Lacking detailed genetic analysis of the changing pathogen, if one were to speculate on why the virulence of syphilis decreased so rapidly, I suggest, in a Just-So story fashion, that one might merely speculate on the evolutionary wisdom of an STD that commonly turned its victims into foul-smelling, scabrous, agonized, and lethargic individuals who lost body parts, including their genitals, according to some reports. None of these outcomes, of course, would be conducive to the natural spread of the disease. In addition, this is a good case for sexual selection as well as early death of the host, which are two main engineers of evolutionary change.
But for whatever reason, the presentation of syphilis changed dramatically over a relatively short period of time, and as the disease was still spreading through a previously unexposed population, a change in pathogenicity rather than host immunity seems the most logical explanation.
As syphilis evolved from its initial onslaught, it showed new and hitherto unseen symptoms, including the aforementioned hair loss, and other manifestations such as tinnitus. Soon it was presenting so many systemic phenotypes similar to the effects of other diseases that Sir William Osler (1849-1919) ultimately proposed that syphilis should be described as the “Great Imitator.”
The evolution of syphilis from epidemic to endemic does not diminish the horrors of those afflicted with active tertiary syphilis, but as the disease transformed, these effects were greatly postponed and occurred less commonly, compared with their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
Although still lethal, especially in its congenital form, by the end of the 16th century, syphilis had completed its rapid evolution from a devastating, highly visible plague to the covert disease “so sinful that it could not be discussed by name.” It would remain so until the rise of modern antibiotics finally provided a reliable cure. Active tertiary syphilis remained a severe affliction, but the effects were postponed from their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
So, syphilis remains a unique example of host-pathogen evolution, an endemic part of the global human condition, battled by physicians in mostly futile efforts for nearly 500 years, and a disease tracking closely with the rise of modern medicine.
References
Frith J. 2012. Syphilis – Its Early History and Treatment Until Penicillin and the Debate on its Origins. J Military and Veteran’s Health. 20(4):49-58.
Tognoti B. 2009. The Rise and Fall of Syphilis in Renaissance Italy. J Med Humanit. 30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington, D.C.
Chlamydia trachomatis is associated with adverse reproductive health outcomes
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
FROM CLINICAL INFECTIOUS DISEASES
ID Blog: The story of syphilis, part I
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
Rise of a global scourge
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
In vasculitis, the skin tells the story
MILAN – , Robert Micheletti, MD, said at the World Congress of Dermatology.
In granulomatous vasculitis, histiocytes and giant cells can play a significant role, explained Dr. Micheletti, director of the cutaneous vasculitis clinic at the University of Pennsylvania, Philadelphia. The condition may be secondary to an autoimmune disease such as lupus erythematosus or RA; a granulomatous disease such as Crohn’s disease or sarcoidosis; infections such as tuberculosis, a fungal disease, or herpes or zoster viruses, or lymphoma, Dr. Micheletti said.
However, a primary systemic vasculitis such as granulomatosis with polyangiitis (GPA; formerly known as Wegener’s polyangiitis) or eosinophilic granulomatosis with polyangiitis (EGPA; also known as Churg-Strauss vasculitis), giant cell arteritis, or Takayasu arteritis may also be responsible, he said. Occasionally, the culprit can also be a drug-induced vasculitis.
The physical examination gives clues to the size of involved vessels, which in turn helps to classify the vasculitis, Dr. Micheletti said.
When vasculitis affects small vessels, the skin findings will be palpable purpura, urticarial papules, vesicles, and petechiae, he said, adding that “The small vessel involvement accounts for the small size of the lesions, and complement cascade and inflammation account for the palpability of the lesions and the symptomatology.” As red blood cells extravasate from the affected vessels, nonblanching purpura develop, and gravity’s effect on the deposition of immune complex material dictates how lesions are distributed.
“Manifestations more typical of medium vessel vasculitis include subcutaneous nodules, livedo reticularis, retiform purpura, larger hemorrhagic bullae, and more significant ulceration and necrosis,” he said. “If such lesions are seen, suspect medium-vessel vasculitis or vasculitis overlapping small and medium vessels.” Cutaneous or systemic polyarteritis nodosa, antineutrophilic cytoplasmic autoantibody (ANCA)–associated vasculitis, and cryoglobulinemic vasculitis are examples, he added.
The particularities of renal manifestations of vasculitis also offer clues to the vessels involved. When a vasculitis patient has glomerulonephritis, suspect small-vessel involvement, Dr. Micheletti said. However, vasculitis affecting medium-sized vessels will cause renovascular hypertension and, potentially renal arterial aneurysms.
Nerves are typically spared in small-vessel vasculitis, while wrist or foot drop can be seen in mononeuritis multiplex.
Recently, the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) looked at more than 6,800 patients at over 130 sites around the world, proposing new classification criteria for ANCA-associated vasculitis (AAV) and large-vessel vasculitis. The study found that skin findings are common in AAV, with 30%-50% of cases presenting initially with skin lesions. Petechiae and/or purpura are the most common of the skin manifestations, he said. By contrast, for EGPA, allergic and nonspecific findings were the most common findings.
Although skin biopsy can confirm the diagnosis in up to 94% of AAV cases, it’s underutilized and performed in less than half (24%-44%) of cases, Dr. Micheletti said. The study’s findings “demonstrate the importance of a good skin exam, as well as its utility for diagnosis” of vasculitis, he said.
An additional finding form the DCVAS study was that skin lesions can give clues to severity of vasculitis: “Among 1,184 patients with ANCA-associated vasculitis, those with cutaneous involvement were more likely to have systemic manifestations of disease, more likely to have such severe manifestations as glomerulonephritis, alveolar hemorrhage, and mononeuritis,” said Dr. Micheletti, with a hazard ratio of 2.0 among those individuals who had EGPA or GPA.
“Skin findings have diagnostic and, potentially, prognostic importance,” he said. “Use the physician exam and your clinical acumen to your advantage,” but always confirm vasculitis with a biopsy. “Clinicopathologic correlation is key.” A simple urinalysis will screen for renal involvement, and is of “paramount importance,” he added.
Dr. Micheletti reported that he had no relevant disclosures.
MILAN – , Robert Micheletti, MD, said at the World Congress of Dermatology.
In granulomatous vasculitis, histiocytes and giant cells can play a significant role, explained Dr. Micheletti, director of the cutaneous vasculitis clinic at the University of Pennsylvania, Philadelphia. The condition may be secondary to an autoimmune disease such as lupus erythematosus or RA; a granulomatous disease such as Crohn’s disease or sarcoidosis; infections such as tuberculosis, a fungal disease, or herpes or zoster viruses, or lymphoma, Dr. Micheletti said.
However, a primary systemic vasculitis such as granulomatosis with polyangiitis (GPA; formerly known as Wegener’s polyangiitis) or eosinophilic granulomatosis with polyangiitis (EGPA; also known as Churg-Strauss vasculitis), giant cell arteritis, or Takayasu arteritis may also be responsible, he said. Occasionally, the culprit can also be a drug-induced vasculitis.
The physical examination gives clues to the size of involved vessels, which in turn helps to classify the vasculitis, Dr. Micheletti said.
When vasculitis affects small vessels, the skin findings will be palpable purpura, urticarial papules, vesicles, and petechiae, he said, adding that “The small vessel involvement accounts for the small size of the lesions, and complement cascade and inflammation account for the palpability of the lesions and the symptomatology.” As red blood cells extravasate from the affected vessels, nonblanching purpura develop, and gravity’s effect on the deposition of immune complex material dictates how lesions are distributed.
“Manifestations more typical of medium vessel vasculitis include subcutaneous nodules, livedo reticularis, retiform purpura, larger hemorrhagic bullae, and more significant ulceration and necrosis,” he said. “If such lesions are seen, suspect medium-vessel vasculitis or vasculitis overlapping small and medium vessels.” Cutaneous or systemic polyarteritis nodosa, antineutrophilic cytoplasmic autoantibody (ANCA)–associated vasculitis, and cryoglobulinemic vasculitis are examples, he added.
The particularities of renal manifestations of vasculitis also offer clues to the vessels involved. When a vasculitis patient has glomerulonephritis, suspect small-vessel involvement, Dr. Micheletti said. However, vasculitis affecting medium-sized vessels will cause renovascular hypertension and, potentially renal arterial aneurysms.
Nerves are typically spared in small-vessel vasculitis, while wrist or foot drop can be seen in mononeuritis multiplex.
Recently, the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) looked at more than 6,800 patients at over 130 sites around the world, proposing new classification criteria for ANCA-associated vasculitis (AAV) and large-vessel vasculitis. The study found that skin findings are common in AAV, with 30%-50% of cases presenting initially with skin lesions. Petechiae and/or purpura are the most common of the skin manifestations, he said. By contrast, for EGPA, allergic and nonspecific findings were the most common findings.
Although skin biopsy can confirm the diagnosis in up to 94% of AAV cases, it’s underutilized and performed in less than half (24%-44%) of cases, Dr. Micheletti said. The study’s findings “demonstrate the importance of a good skin exam, as well as its utility for diagnosis” of vasculitis, he said.
An additional finding form the DCVAS study was that skin lesions can give clues to severity of vasculitis: “Among 1,184 patients with ANCA-associated vasculitis, those with cutaneous involvement were more likely to have systemic manifestations of disease, more likely to have such severe manifestations as glomerulonephritis, alveolar hemorrhage, and mononeuritis,” said Dr. Micheletti, with a hazard ratio of 2.0 among those individuals who had EGPA or GPA.
“Skin findings have diagnostic and, potentially, prognostic importance,” he said. “Use the physician exam and your clinical acumen to your advantage,” but always confirm vasculitis with a biopsy. “Clinicopathologic correlation is key.” A simple urinalysis will screen for renal involvement, and is of “paramount importance,” he added.
Dr. Micheletti reported that he had no relevant disclosures.
MILAN – , Robert Micheletti, MD, said at the World Congress of Dermatology.
In granulomatous vasculitis, histiocytes and giant cells can play a significant role, explained Dr. Micheletti, director of the cutaneous vasculitis clinic at the University of Pennsylvania, Philadelphia. The condition may be secondary to an autoimmune disease such as lupus erythematosus or RA; a granulomatous disease such as Crohn’s disease or sarcoidosis; infections such as tuberculosis, a fungal disease, or herpes or zoster viruses, or lymphoma, Dr. Micheletti said.
However, a primary systemic vasculitis such as granulomatosis with polyangiitis (GPA; formerly known as Wegener’s polyangiitis) or eosinophilic granulomatosis with polyangiitis (EGPA; also known as Churg-Strauss vasculitis), giant cell arteritis, or Takayasu arteritis may also be responsible, he said. Occasionally, the culprit can also be a drug-induced vasculitis.
The physical examination gives clues to the size of involved vessels, which in turn helps to classify the vasculitis, Dr. Micheletti said.
When vasculitis affects small vessels, the skin findings will be palpable purpura, urticarial papules, vesicles, and petechiae, he said, adding that “The small vessel involvement accounts for the small size of the lesions, and complement cascade and inflammation account for the palpability of the lesions and the symptomatology.” As red blood cells extravasate from the affected vessels, nonblanching purpura develop, and gravity’s effect on the deposition of immune complex material dictates how lesions are distributed.
“Manifestations more typical of medium vessel vasculitis include subcutaneous nodules, livedo reticularis, retiform purpura, larger hemorrhagic bullae, and more significant ulceration and necrosis,” he said. “If such lesions are seen, suspect medium-vessel vasculitis or vasculitis overlapping small and medium vessels.” Cutaneous or systemic polyarteritis nodosa, antineutrophilic cytoplasmic autoantibody (ANCA)–associated vasculitis, and cryoglobulinemic vasculitis are examples, he added.
The particularities of renal manifestations of vasculitis also offer clues to the vessels involved. When a vasculitis patient has glomerulonephritis, suspect small-vessel involvement, Dr. Micheletti said. However, vasculitis affecting medium-sized vessels will cause renovascular hypertension and, potentially renal arterial aneurysms.
Nerves are typically spared in small-vessel vasculitis, while wrist or foot drop can be seen in mononeuritis multiplex.
Recently, the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) looked at more than 6,800 patients at over 130 sites around the world, proposing new classification criteria for ANCA-associated vasculitis (AAV) and large-vessel vasculitis. The study found that skin findings are common in AAV, with 30%-50% of cases presenting initially with skin lesions. Petechiae and/or purpura are the most common of the skin manifestations, he said. By contrast, for EGPA, allergic and nonspecific findings were the most common findings.
Although skin biopsy can confirm the diagnosis in up to 94% of AAV cases, it’s underutilized and performed in less than half (24%-44%) of cases, Dr. Micheletti said. The study’s findings “demonstrate the importance of a good skin exam, as well as its utility for diagnosis” of vasculitis, he said.
An additional finding form the DCVAS study was that skin lesions can give clues to severity of vasculitis: “Among 1,184 patients with ANCA-associated vasculitis, those with cutaneous involvement were more likely to have systemic manifestations of disease, more likely to have such severe manifestations as glomerulonephritis, alveolar hemorrhage, and mononeuritis,” said Dr. Micheletti, with a hazard ratio of 2.0 among those individuals who had EGPA or GPA.
“Skin findings have diagnostic and, potentially, prognostic importance,” he said. “Use the physician exam and your clinical acumen to your advantage,” but always confirm vasculitis with a biopsy. “Clinicopathologic correlation is key.” A simple urinalysis will screen for renal involvement, and is of “paramount importance,” he added.
Dr. Micheletti reported that he had no relevant disclosures.
AT WCD2019