User login
Clinical staging of early esophageal cancer unreliable
LOS ANGELES – Clinical staging of T2N0 esophageal cancer remains unreliable, despite advances in staging techniques, a study has shown.
The addition of endoscopic ultrasound and PET/CT has improved the ability to clinically stage esophageal cancer overall, but clinical staging of T2N0 disease has generally been less reliable than staging of more advanced disease. The subset of patients with T2N0 disease, which accounts for only a small portion of esophageal resections, has been a difficult group to study because single-center investigations involve a limited number of patients, Dr. Traves D. Crabtree said at the annual meeting of the Society of Thoracic Surgeons (STS), in the prestigious Richard E. Clark Paper for General Thoracic Surgery.
Dr. Crabtree of Washington University, St. Louis, and his colleagues examined the adequacy of clinical staging of T2N0 disease using the STS General Thoracic Surgery Database.
The researchers identified 810 patients clinically staged as T2N0 from 2002 to 2011 and excluded 58 because of inadequate pathologic staging data. Clinical stage, pathologic stage, and preoperative characteristics were recorded for each patient and multivariable analysis was used to identify factors associated with upstaging at the time of surgery.
Among 752 patients with clinically staged T2N0, the investigators found that 482 went directly to surgery. Of these, 27.4% (132) were confirmed as pathologic T2N0. A total of 25.9% (125) were downstaged (T1N0), while 46.7% (225) were upstaged (T3-4N0 or TanyN1-3). Tumor depth (pT3-4) accounted for 18.2% of upstaging while nodal upstaging occurred in approximately 82%. When logistic regression was used, male sex was associated with upstaging (odds ratio = 1.85, P = .024). By analyzing the part of the database that included tumor grade (between the years 2009 and 2011), the investigators found that a higher histologic grade was significantly associated with upstaging (P = .004).
"Over one-third of surgeons have opted to treat T2N0 disease with induction therapy, despite the fact that one-quarter of these patients will be pT1N0," he said.
"This is the first large-scale multi-institutional study of clinical T2N0 patients using the STS General Thoracic Surgery Database. These data highlight the inaccuracy associated with clinical staging of T2N0 esophageal cancer and may influence the surgeon’s decision-making process in choosing a treatment regimen for these patients," Dr. Crabtree added in an interview.
"Given the current limitations of clinical staging of T2N0 patients, the incidence of occult nodal disease, and the similar perioperative outcomes among patients treated with and without induction therapy, these patients may more likely be treated with induction therapy in the future. Additional studies are needed to compare long-term outcomes between patients receiving induction therapy, vs. those clinical T2N0 patients going directly to surgery, before a definitive recommendation can be made," he concluded.
Dr. Crabtree reported that he had no relevant financial disclosures.
LOS ANGELES – Clinical staging of T2N0 esophageal cancer remains unreliable, despite advances in staging techniques, a study has shown.
The addition of endoscopic ultrasound and PET/CT has improved the ability to clinically stage esophageal cancer overall, but clinical staging of T2N0 disease has generally been less reliable than staging of more advanced disease. The subset of patients with T2N0 disease, which accounts for only a small portion of esophageal resections, has been a difficult group to study because single-center investigations involve a limited number of patients, Dr. Traves D. Crabtree said at the annual meeting of the Society of Thoracic Surgeons (STS), in the prestigious Richard E. Clark Paper for General Thoracic Surgery.
Dr. Crabtree of Washington University, St. Louis, and his colleagues examined the adequacy of clinical staging of T2N0 disease using the STS General Thoracic Surgery Database.
The researchers identified 810 patients clinically staged as T2N0 from 2002 to 2011 and excluded 58 because of inadequate pathologic staging data. Clinical stage, pathologic stage, and preoperative characteristics were recorded for each patient and multivariable analysis was used to identify factors associated with upstaging at the time of surgery.
Among 752 patients with clinically staged T2N0, the investigators found that 482 went directly to surgery. Of these, 27.4% (132) were confirmed as pathologic T2N0. A total of 25.9% (125) were downstaged (T1N0), while 46.7% (225) were upstaged (T3-4N0 or TanyN1-3). Tumor depth (pT3-4) accounted for 18.2% of upstaging while nodal upstaging occurred in approximately 82%. When logistic regression was used, male sex was associated with upstaging (odds ratio = 1.85, P = .024). By analyzing the part of the database that included tumor grade (between the years 2009 and 2011), the investigators found that a higher histologic grade was significantly associated with upstaging (P = .004).
"Over one-third of surgeons have opted to treat T2N0 disease with induction therapy, despite the fact that one-quarter of these patients will be pT1N0," he said.
"This is the first large-scale multi-institutional study of clinical T2N0 patients using the STS General Thoracic Surgery Database. These data highlight the inaccuracy associated with clinical staging of T2N0 esophageal cancer and may influence the surgeon’s decision-making process in choosing a treatment regimen for these patients," Dr. Crabtree added in an interview.
"Given the current limitations of clinical staging of T2N0 patients, the incidence of occult nodal disease, and the similar perioperative outcomes among patients treated with and without induction therapy, these patients may more likely be treated with induction therapy in the future. Additional studies are needed to compare long-term outcomes between patients receiving induction therapy, vs. those clinical T2N0 patients going directly to surgery, before a definitive recommendation can be made," he concluded.
Dr. Crabtree reported that he had no relevant financial disclosures.
LOS ANGELES – Clinical staging of T2N0 esophageal cancer remains unreliable, despite advances in staging techniques, a study has shown.
The addition of endoscopic ultrasound and PET/CT has improved the ability to clinically stage esophageal cancer overall, but clinical staging of T2N0 disease has generally been less reliable than staging of more advanced disease. The subset of patients with T2N0 disease, which accounts for only a small portion of esophageal resections, has been a difficult group to study because single-center investigations involve a limited number of patients, Dr. Traves D. Crabtree said at the annual meeting of the Society of Thoracic Surgeons (STS), in the prestigious Richard E. Clark Paper for General Thoracic Surgery.
Dr. Crabtree of Washington University, St. Louis, and his colleagues examined the adequacy of clinical staging of T2N0 disease using the STS General Thoracic Surgery Database.
The researchers identified 810 patients clinically staged as T2N0 from 2002 to 2011 and excluded 58 because of inadequate pathologic staging data. Clinical stage, pathologic stage, and preoperative characteristics were recorded for each patient and multivariable analysis was used to identify factors associated with upstaging at the time of surgery.
Among 752 patients with clinically staged T2N0, the investigators found that 482 went directly to surgery. Of these, 27.4% (132) were confirmed as pathologic T2N0. A total of 25.9% (125) were downstaged (T1N0), while 46.7% (225) were upstaged (T3-4N0 or TanyN1-3). Tumor depth (pT3-4) accounted for 18.2% of upstaging while nodal upstaging occurred in approximately 82%. When logistic regression was used, male sex was associated with upstaging (odds ratio = 1.85, P = .024). By analyzing the part of the database that included tumor grade (between the years 2009 and 2011), the investigators found that a higher histologic grade was significantly associated with upstaging (P = .004).
"Over one-third of surgeons have opted to treat T2N0 disease with induction therapy, despite the fact that one-quarter of these patients will be pT1N0," he said.
"This is the first large-scale multi-institutional study of clinical T2N0 patients using the STS General Thoracic Surgery Database. These data highlight the inaccuracy associated with clinical staging of T2N0 esophageal cancer and may influence the surgeon’s decision-making process in choosing a treatment regimen for these patients," Dr. Crabtree added in an interview.
"Given the current limitations of clinical staging of T2N0 patients, the incidence of occult nodal disease, and the similar perioperative outcomes among patients treated with and without induction therapy, these patients may more likely be treated with induction therapy in the future. Additional studies are needed to compare long-term outcomes between patients receiving induction therapy, vs. those clinical T2N0 patients going directly to surgery, before a definitive recommendation can be made," he concluded.
Dr. Crabtree reported that he had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY OF THORACIC SURGESONS
Major Finding: Of 482 patients who went directly to surgery, 26% were downstaged, while 47% were upstaged.
Data Source: A retrospective, database analysis of 810 patients clinically staged as T2N0 from 2002 to 2011.
Disclosures: Dr. Crabtree reported that he had no relevant financial disclosures.
Survival shorter in young gastric cancer patients
Patients with gastric adenocarcinoma who were diagnosed before age 40 years died significantly sooner than did older patients, based on the results of a single-center, retrospective study of 520 cases.
Patients diagnosed before age 40 survived a median of 5 months after diagnosis; 24% were alive at 1 year. Older patients survived a median of 8 months, and 39% were alive at 1 year, reported Dr. Bryan Goldner and his associates.
Surgical exploration was often futile in the younger patients, said Dr. Goldner of Harbor-UCLA Medical Center in Torrance, Calif. Surgery removed all tumors with histologically free margins (R0 resection) in 33% of younger patients, compared with 60% of older patients, he reported in a poster presentation at a meeting on gastrointestinal cancers sponsored by the American Society of Clinical Oncology.
Recent reports in the medical literature suggest that younger patients with gastric adenocarcinoma are more likely to be diagnosed with node-positive and metastatic disease. One retrospective study of 350 patients found that younger patients had more aggressive gastric adenocarcinomas and died sooner than older patients (Arch. Surg. 2009;144:506-10).
A separate retrospective study of 33,236 U.S. patients found that younger patients presented with more advanced gastric adenocarcinoma but had better survival outcomes than older patients when stratified by disease stage (Ann. Surg. Oncol. 2011;18:2800-7).
Gastric cancer might not be suspected in younger patients, which could result in young patients going undiagnosed until their cancers are more advanced, Dr. Goldner suggested.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Goldner reported having no financial disclosures.
On Twitter @sherryboschert
Patients with gastric adenocarcinoma who were diagnosed before age 40 years died significantly sooner than did older patients, based on the results of a single-center, retrospective study of 520 cases.
Patients diagnosed before age 40 survived a median of 5 months after diagnosis; 24% were alive at 1 year. Older patients survived a median of 8 months, and 39% were alive at 1 year, reported Dr. Bryan Goldner and his associates.
Surgical exploration was often futile in the younger patients, said Dr. Goldner of Harbor-UCLA Medical Center in Torrance, Calif. Surgery removed all tumors with histologically free margins (R0 resection) in 33% of younger patients, compared with 60% of older patients, he reported in a poster presentation at a meeting on gastrointestinal cancers sponsored by the American Society of Clinical Oncology.
Recent reports in the medical literature suggest that younger patients with gastric adenocarcinoma are more likely to be diagnosed with node-positive and metastatic disease. One retrospective study of 350 patients found that younger patients had more aggressive gastric adenocarcinomas and died sooner than older patients (Arch. Surg. 2009;144:506-10).
A separate retrospective study of 33,236 U.S. patients found that younger patients presented with more advanced gastric adenocarcinoma but had better survival outcomes than older patients when stratified by disease stage (Ann. Surg. Oncol. 2011;18:2800-7).
Gastric cancer might not be suspected in younger patients, which could result in young patients going undiagnosed until their cancers are more advanced, Dr. Goldner suggested.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Goldner reported having no financial disclosures.
On Twitter @sherryboschert
Patients with gastric adenocarcinoma who were diagnosed before age 40 years died significantly sooner than did older patients, based on the results of a single-center, retrospective study of 520 cases.
Patients diagnosed before age 40 survived a median of 5 months after diagnosis; 24% were alive at 1 year. Older patients survived a median of 8 months, and 39% were alive at 1 year, reported Dr. Bryan Goldner and his associates.
Surgical exploration was often futile in the younger patients, said Dr. Goldner of Harbor-UCLA Medical Center in Torrance, Calif. Surgery removed all tumors with histologically free margins (R0 resection) in 33% of younger patients, compared with 60% of older patients, he reported in a poster presentation at a meeting on gastrointestinal cancers sponsored by the American Society of Clinical Oncology.
Recent reports in the medical literature suggest that younger patients with gastric adenocarcinoma are more likely to be diagnosed with node-positive and metastatic disease. One retrospective study of 350 patients found that younger patients had more aggressive gastric adenocarcinomas and died sooner than older patients (Arch. Surg. 2009;144:506-10).
A separate retrospective study of 33,236 U.S. patients found that younger patients presented with more advanced gastric adenocarcinoma but had better survival outcomes than older patients when stratified by disease stage (Ann. Surg. Oncol. 2011;18:2800-7).
Gastric cancer might not be suspected in younger patients, which could result in young patients going undiagnosed until their cancers are more advanced, Dr. Goldner suggested.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Goldner reported having no financial disclosures.
On Twitter @sherryboschert
AT A MEETING ON GASTROINTESTINAL CANCERS SPONSORED BY THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Patients diagnosed with gastric adenocarcinoma before age 40 years lived a median of 5 months; older patients lived a median of 8 months.
Data Source: Retrospective study of 520 patients with gastric cancer at one public hospital.
Disclosures: Dr. Goldner reported having no financial disclosures.
Assay may target early lung cancers for adjuvant therapy
LOS ANGELES – A novel genetic assay helps identify patients with early, aggressive lung cancer who might benefit from adjuvant therapy.
The assay, marketed as Pervino Lung RS by Life Technologies, is the only lung cancer signature to undergo blinded validation in two large cohorts from different countries, one in the United States and one in China (Lancet 2012;379:823-32).
It assesses expression of 14 genes involved in lung cancer tumorigenesis, including ones on the EGFR and KRAS signaling pathways. The assay provides considerably more prognostic information than do conventional criteria proposed by the National Comprehensive Cancer Network (NCCN) as defining high-risk tumors warranting treatment, according to Dr. Johannes R. Kratz, who reported the data at the annual meeting of the Society of Thoracic Surgeons.
The assay results were used to stratify the 269 study patients who had undergone resection of T1a node-negative and nonmetastatic, nonsquamous, non–small cell lung cancer (NSCLC) into groups with distinctly different 5-year survival rates.
Compared with their counterparts in the low-risk group, those in the intermediate- and high-risk groups had a respective doubling and more than tripling of the risk of death, said Dr. Kratz, who was the study’s lead investigator.
As a result of recommendations for CT screening in patients at high risk for lung cancer, resections of small node-negative tumors that are in fact deadly are likely to increase, he observed. Nearly 30% of all patients with stage IA tumors – the lowest level in the current classification system – will nonetheless die in the subsequent 5 years.
"These tumors with highly aggressive tumor biology can now be identified reliably with a prognostic gene signature. The identification of these small but deadly tumors may allow for personalized patient prognosis and could allow us to maximize the benefit of the early detection of these small but deadly tumors via low-dose CT screening," he added.
The current postoperative standard of care for stage IA disease is simply observation, according to Dr. Kratz, a former surgical resident at the Massachusetts General Hospital in Boston, and now a postdoctoral fellow at the University of California, San Francisco.
However, "we should strongly consider changing the way we think about patients with high-risk T1a tumors," he recommended. To that end, a randomized controlled trial of assay-guided adjuvant chemotherapy for early lung cancer is underway in China among roughly 1,000 patients.
Dr. Kratz said that studies to date have not examined a potential prognostic role of the assay in EGFR (epidermal growth factor receptor) mutations. "We haven’t performed an additional mutation analysis on these patients’ EGFR. The original assay was designed to work on patients with resected paraffin-embedded specimens and not fresh-frozen tissue specimens. As a result, it is difficult for us to do extensive EGFR mutation analysis. But that’s definitely something to consider, and it would be nice to explore that association."
It remains to be seen whether the assay, in fact, predicts chemotherapy benefit, he acknowledged in a related press conference. But research has suggested that such prognostic signatures in lung cancer are also predictive (J. Clin. Oncol. 2010;28:4417-24). "That is what we hope to show in the China trial as well," he said.
In the reported study, patients with T1a tumors were drawn from the initial validation cohorts. Fully 40% were under age 60. "This is important, because ... we’d like to be more aggressive in younger patients, both because they can tolerate it and we are more likely to treat them more aggressively," he noted.
The patients’ actual 5-year mortality rate was 32% overall, showing that "these tumors are as deadly as advertised."
The main study results, reported at the meeting and also published (JAMA 2012;308:1629-31), showed that the 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
In multivariate analyses, relative to their counterparts in the low-risk group, patients in the intermediate-risk group had a 2.0-fold higher risk of death (P = .04) and patients in the high-risk group had a 3.3-fold higher risk (P = .00).
The assay also showed good risk discrimination in analyses restricted to the smallest of tumors, those measuring 1.5 cm or less (P = .001 for difference across groups) and even those measuring 1.0 cm or less (P = .008).
And when compared with tumor size alone, the combination of the assay and tumor size significantly improved on the identification of patients who died (c-statistic, 0.68 vs. 0.57; P less than .0001).
Although these T1a tumors can be ablated nonoperatively, their genetic makeup offers a rich source of information about their subsequent behavior, Dr. Kratz said.
"Despite the popularity and endorsement of our radiology colleagues for techniques such as stereotactic radiation for small T1aN0M0 tumors, we should remember that these techniques don’t provide us with potentially important lung tissue that can provide prognostic and predictive information," he commented.
Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
LOS ANGELES – A novel genetic assay helps identify patients with early, aggressive lung cancer who might benefit from adjuvant therapy.
The assay, marketed as Pervino Lung RS by Life Technologies, is the only lung cancer signature to undergo blinded validation in two large cohorts from different countries, one in the United States and one in China (Lancet 2012;379:823-32).
It assesses expression of 14 genes involved in lung cancer tumorigenesis, including ones on the EGFR and KRAS signaling pathways. The assay provides considerably more prognostic information than do conventional criteria proposed by the National Comprehensive Cancer Network (NCCN) as defining high-risk tumors warranting treatment, according to Dr. Johannes R. Kratz, who reported the data at the annual meeting of the Society of Thoracic Surgeons.
The assay results were used to stratify the 269 study patients who had undergone resection of T1a node-negative and nonmetastatic, nonsquamous, non–small cell lung cancer (NSCLC) into groups with distinctly different 5-year survival rates.
Compared with their counterparts in the low-risk group, those in the intermediate- and high-risk groups had a respective doubling and more than tripling of the risk of death, said Dr. Kratz, who was the study’s lead investigator.
As a result of recommendations for CT screening in patients at high risk for lung cancer, resections of small node-negative tumors that are in fact deadly are likely to increase, he observed. Nearly 30% of all patients with stage IA tumors – the lowest level in the current classification system – will nonetheless die in the subsequent 5 years.
"These tumors with highly aggressive tumor biology can now be identified reliably with a prognostic gene signature. The identification of these small but deadly tumors may allow for personalized patient prognosis and could allow us to maximize the benefit of the early detection of these small but deadly tumors via low-dose CT screening," he added.
The current postoperative standard of care for stage IA disease is simply observation, according to Dr. Kratz, a former surgical resident at the Massachusetts General Hospital in Boston, and now a postdoctoral fellow at the University of California, San Francisco.
However, "we should strongly consider changing the way we think about patients with high-risk T1a tumors," he recommended. To that end, a randomized controlled trial of assay-guided adjuvant chemotherapy for early lung cancer is underway in China among roughly 1,000 patients.
Dr. Kratz said that studies to date have not examined a potential prognostic role of the assay in EGFR (epidermal growth factor receptor) mutations. "We haven’t performed an additional mutation analysis on these patients’ EGFR. The original assay was designed to work on patients with resected paraffin-embedded specimens and not fresh-frozen tissue specimens. As a result, it is difficult for us to do extensive EGFR mutation analysis. But that’s definitely something to consider, and it would be nice to explore that association."
It remains to be seen whether the assay, in fact, predicts chemotherapy benefit, he acknowledged in a related press conference. But research has suggested that such prognostic signatures in lung cancer are also predictive (J. Clin. Oncol. 2010;28:4417-24). "That is what we hope to show in the China trial as well," he said.
In the reported study, patients with T1a tumors were drawn from the initial validation cohorts. Fully 40% were under age 60. "This is important, because ... we’d like to be more aggressive in younger patients, both because they can tolerate it and we are more likely to treat them more aggressively," he noted.
The patients’ actual 5-year mortality rate was 32% overall, showing that "these tumors are as deadly as advertised."
The main study results, reported at the meeting and also published (JAMA 2012;308:1629-31), showed that the 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
In multivariate analyses, relative to their counterparts in the low-risk group, patients in the intermediate-risk group had a 2.0-fold higher risk of death (P = .04) and patients in the high-risk group had a 3.3-fold higher risk (P = .00).
The assay also showed good risk discrimination in analyses restricted to the smallest of tumors, those measuring 1.5 cm or less (P = .001 for difference across groups) and even those measuring 1.0 cm or less (P = .008).
And when compared with tumor size alone, the combination of the assay and tumor size significantly improved on the identification of patients who died (c-statistic, 0.68 vs. 0.57; P less than .0001).
Although these T1a tumors can be ablated nonoperatively, their genetic makeup offers a rich source of information about their subsequent behavior, Dr. Kratz said.
"Despite the popularity and endorsement of our radiology colleagues for techniques such as stereotactic radiation for small T1aN0M0 tumors, we should remember that these techniques don’t provide us with potentially important lung tissue that can provide prognostic and predictive information," he commented.
Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
LOS ANGELES – A novel genetic assay helps identify patients with early, aggressive lung cancer who might benefit from adjuvant therapy.
The assay, marketed as Pervino Lung RS by Life Technologies, is the only lung cancer signature to undergo blinded validation in two large cohorts from different countries, one in the United States and one in China (Lancet 2012;379:823-32).
It assesses expression of 14 genes involved in lung cancer tumorigenesis, including ones on the EGFR and KRAS signaling pathways. The assay provides considerably more prognostic information than do conventional criteria proposed by the National Comprehensive Cancer Network (NCCN) as defining high-risk tumors warranting treatment, according to Dr. Johannes R. Kratz, who reported the data at the annual meeting of the Society of Thoracic Surgeons.
The assay results were used to stratify the 269 study patients who had undergone resection of T1a node-negative and nonmetastatic, nonsquamous, non–small cell lung cancer (NSCLC) into groups with distinctly different 5-year survival rates.
Compared with their counterparts in the low-risk group, those in the intermediate- and high-risk groups had a respective doubling and more than tripling of the risk of death, said Dr. Kratz, who was the study’s lead investigator.
As a result of recommendations for CT screening in patients at high risk for lung cancer, resections of small node-negative tumors that are in fact deadly are likely to increase, he observed. Nearly 30% of all patients with stage IA tumors – the lowest level in the current classification system – will nonetheless die in the subsequent 5 years.
"These tumors with highly aggressive tumor biology can now be identified reliably with a prognostic gene signature. The identification of these small but deadly tumors may allow for personalized patient prognosis and could allow us to maximize the benefit of the early detection of these small but deadly tumors via low-dose CT screening," he added.
The current postoperative standard of care for stage IA disease is simply observation, according to Dr. Kratz, a former surgical resident at the Massachusetts General Hospital in Boston, and now a postdoctoral fellow at the University of California, San Francisco.
However, "we should strongly consider changing the way we think about patients with high-risk T1a tumors," he recommended. To that end, a randomized controlled trial of assay-guided adjuvant chemotherapy for early lung cancer is underway in China among roughly 1,000 patients.
Dr. Kratz said that studies to date have not examined a potential prognostic role of the assay in EGFR (epidermal growth factor receptor) mutations. "We haven’t performed an additional mutation analysis on these patients’ EGFR. The original assay was designed to work on patients with resected paraffin-embedded specimens and not fresh-frozen tissue specimens. As a result, it is difficult for us to do extensive EGFR mutation analysis. But that’s definitely something to consider, and it would be nice to explore that association."
It remains to be seen whether the assay, in fact, predicts chemotherapy benefit, he acknowledged in a related press conference. But research has suggested that such prognostic signatures in lung cancer are also predictive (J. Clin. Oncol. 2010;28:4417-24). "That is what we hope to show in the China trial as well," he said.
In the reported study, patients with T1a tumors were drawn from the initial validation cohorts. Fully 40% were under age 60. "This is important, because ... we’d like to be more aggressive in younger patients, both because they can tolerate it and we are more likely to treat them more aggressively," he noted.
The patients’ actual 5-year mortality rate was 32% overall, showing that "these tumors are as deadly as advertised."
The main study results, reported at the meeting and also published (JAMA 2012;308:1629-31), showed that the 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
In multivariate analyses, relative to their counterparts in the low-risk group, patients in the intermediate-risk group had a 2.0-fold higher risk of death (P = .04) and patients in the high-risk group had a 3.3-fold higher risk (P = .00).
The assay also showed good risk discrimination in analyses restricted to the smallest of tumors, those measuring 1.5 cm or less (P = .001 for difference across groups) and even those measuring 1.0 cm or less (P = .008).
And when compared with tumor size alone, the combination of the assay and tumor size significantly improved on the identification of patients who died (c-statistic, 0.68 vs. 0.57; P less than .0001).
Although these T1a tumors can be ablated nonoperatively, their genetic makeup offers a rich source of information about their subsequent behavior, Dr. Kratz said.
"Despite the popularity and endorsement of our radiology colleagues for techniques such as stereotactic radiation for small T1aN0M0 tumors, we should remember that these techniques don’t provide us with potentially important lung tissue that can provide prognostic and predictive information," he commented.
Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
AT THE ANNUAL MEETING OF THE SOCIETY OF THORACIC SURGEONS
Major Finding: The 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
Data Source: A cohort study of 269 patients who underwent resection of T1aN0M0 nonsquamous NSCLC.
Disclosures: Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
Breast-conserving therapy improved survival over mastectomy
Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer, compared with women who underwent mastectomy, Dr. E. Shelley Hwang and her colleagues reported Jan. 28 in the online issue of Cancer.
The 3-year disease-specific survival benefit for breast-conserving therapy (BCT) was most pronounced for chronic respiratory disease, for which lumpectomy was associated with a 54% decreased risk of death; heart disease, with a 49% decreased risk; and cerebrovascular disease, with a decreased risk of 36%.
The survival benefit varied with age, tumor size, and hormone receptor status but was significant in every subgroup, Dr. Hwang and her colleagues wrote (Cancer Jan. 28, 2013 [doi:10.1002/cncr.27795]).
"Our findings have important implications for understanding the overall benefit of BCT at the population level," wrote Dr. Hwang of the Duke University Comprehensive Cancer Center, Durham, N.C., and her coauthors. "These results provide confidence in the efficacy of BCT even among younger patients with HR-negative disease thought to be at relatively higher risk for local failure."
The team reviewed the records of 112,154 women who were treated for a new, unilateral T1/T2 stage I or II breast cancer diagnosed from 1990 to 2004. Most of these women (61,771) underwent a lumpectomy and radiation; the remainder underwent mastectomy without radiation. They were followed for a median of 10 years.
About a quarter of each group was younger than 50 years when diagnosed; another quarter was aged 70-80 years. A small portion (6%) was younger than 40 years.
Surgical approach evolved over the study period. Breast-conserving therapy increased from 37% in 1990-92 to 62% by 2002-04, while the rate of mastectomies declined.
The median tumor size was 1.5 cm; patients with larger tumors were more likely to have mastectomies. "Interestingly, the use of BCT varied by age even among tumors [smaller than and equal to] 2 cm where the youngest and oldest age groups had the lowest BCT rate. In [tumors larger than] 2 cm, BCT rate declined by age," Dr. Hwang and her associates said.
Over the follow-up period, there were 31,416 deaths; 39% of these were caused by breast cancer; 5-year overall survival was 89%.
To further explore the treatment-mortality interaction, the investigators divided the cohort into four groups according to age and tumor characteristics:
• 50 years or older, hormone receptor negative.
• 50 years or older, hormone receptor negative.
• Younger than 50 years, hormone receptor positive.
• Younger than 50 years, hormone receptor positive.
Women 50 years and older with HR-positive tumors who had BCT experienced the greatest survival benefit, compared with mastectomy patients (hazard ratio, 0.81). Women younger than 50 years with HR- positive tumors experienced the smallest benefit (HR, 0.93), but one which was still statistically significant.
The investigators also looked at 3-year overall and disease-specific survival. "Notably, BCT was associated with significantly lower 3-year mortality rates from all causes," including heart disease (HR, 0.51), chronic respiratory disease (HR, 0.46), and cerebrovascular disease (HR, 0.64).
The findings align with those of randomized trials showing the benefits of BCT, the authors noted.
"Despite this, recent studies have shown an increased rate of mastectomy for patient subgroups including younger women with early-stage tumors, many of which would have presumably been amenable to BCT," they wrote. This could be the result of a perception that women with unfavorable characteristics, like younger age and high-risk tumors, don’t do as well with BCT.
The investigators noted that some differences in disease burden at baseline could have contributed to the findings.
"Interestingly, for every cause of mortality that we evaluated, women who had mastectomy were more likely to die within 3 years of their breast cancer diagnosis than women who chose BCT. Based on these findings, it is reasonable to infer that the mastectomy group was likely to have a greater burden of nonfatal comorbidities at presentation, and that this factor may well have influenced surgical decision-making. Nevertheless, this factor alone cannot account for why women with mastectomy had lower [disease specific survival] after adjusting for age and tumor characteristics," Dr. Hwang and her associates said.
Based on the strong associations with survival, "these findings support the notion that BCT, when combined with radiation, confers at least equivalent and perhaps even superior survival to mastectomy as definitive breast cancer treatment," they said.
The National Cancer Institute funded the study. Dr. Hwang had no financial disclosures.
This article is interesting and important, but I don’t see any mention of systemic therapy, which has a huge impact on breast cancer–specific survival. Confounding factors could have created bias in this registry analysis that makes interpretation of improved survival impossible.

|
|
Having said that, I believe this is an important publication. The benefits of mastectomy are clearly overemphasized, and mastectomy is used in many situations where breast-conserving surgery will result in at least an identical outcome. Patients often understand that their outcome will be improved if more surgery is done, or if they remove the offending breast.
Education for patients in this regard is critical. Surgeons and medical oncologists are critical components of a change in practice – a change that has the potential to significantly improve quality of life and cosmetic outcome for women with breast cancer, representing over 200,000 patients per year in the United States. Hopefully, data such as these will disabuse practitioners of the all-too-common approach that mastectomy is an easier, simpler solution than breast-conserving surgery when managing early-stage breast cancer.
Hope S. Rugo, M.D., associate editor of The Oncology Report, is professor of medicine and director of breast oncology and clinical trials education at the comprehensive cancer center of the University of California, San Francisco.
This article is interesting and important, but I don’t see any mention of systemic therapy, which has a huge impact on breast cancer–specific survival. Confounding factors could have created bias in this registry analysis that makes interpretation of improved survival impossible.

|
|
Having said that, I believe this is an important publication. The benefits of mastectomy are clearly overemphasized, and mastectomy is used in many situations where breast-conserving surgery will result in at least an identical outcome. Patients often understand that their outcome will be improved if more surgery is done, or if they remove the offending breast.
Education for patients in this regard is critical. Surgeons and medical oncologists are critical components of a change in practice – a change that has the potential to significantly improve quality of life and cosmetic outcome for women with breast cancer, representing over 200,000 patients per year in the United States. Hopefully, data such as these will disabuse practitioners of the all-too-common approach that mastectomy is an easier, simpler solution than breast-conserving surgery when managing early-stage breast cancer.
Hope S. Rugo, M.D., associate editor of The Oncology Report, is professor of medicine and director of breast oncology and clinical trials education at the comprehensive cancer center of the University of California, San Francisco.
This article is interesting and important, but I don’t see any mention of systemic therapy, which has a huge impact on breast cancer–specific survival. Confounding factors could have created bias in this registry analysis that makes interpretation of improved survival impossible.

|
|
Having said that, I believe this is an important publication. The benefits of mastectomy are clearly overemphasized, and mastectomy is used in many situations where breast-conserving surgery will result in at least an identical outcome. Patients often understand that their outcome will be improved if more surgery is done, or if they remove the offending breast.
Education for patients in this regard is critical. Surgeons and medical oncologists are critical components of a change in practice – a change that has the potential to significantly improve quality of life and cosmetic outcome for women with breast cancer, representing over 200,000 patients per year in the United States. Hopefully, data such as these will disabuse practitioners of the all-too-common approach that mastectomy is an easier, simpler solution than breast-conserving surgery when managing early-stage breast cancer.
Hope S. Rugo, M.D., associate editor of The Oncology Report, is professor of medicine and director of breast oncology and clinical trials education at the comprehensive cancer center of the University of California, San Francisco.
Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer, compared with women who underwent mastectomy, Dr. E. Shelley Hwang and her colleagues reported Jan. 28 in the online issue of Cancer.
The 3-year disease-specific survival benefit for breast-conserving therapy (BCT) was most pronounced for chronic respiratory disease, for which lumpectomy was associated with a 54% decreased risk of death; heart disease, with a 49% decreased risk; and cerebrovascular disease, with a decreased risk of 36%.
The survival benefit varied with age, tumor size, and hormone receptor status but was significant in every subgroup, Dr. Hwang and her colleagues wrote (Cancer Jan. 28, 2013 [doi:10.1002/cncr.27795]).
"Our findings have important implications for understanding the overall benefit of BCT at the population level," wrote Dr. Hwang of the Duke University Comprehensive Cancer Center, Durham, N.C., and her coauthors. "These results provide confidence in the efficacy of BCT even among younger patients with HR-negative disease thought to be at relatively higher risk for local failure."
The team reviewed the records of 112,154 women who were treated for a new, unilateral T1/T2 stage I or II breast cancer diagnosed from 1990 to 2004. Most of these women (61,771) underwent a lumpectomy and radiation; the remainder underwent mastectomy without radiation. They were followed for a median of 10 years.
About a quarter of each group was younger than 50 years when diagnosed; another quarter was aged 70-80 years. A small portion (6%) was younger than 40 years.
Surgical approach evolved over the study period. Breast-conserving therapy increased from 37% in 1990-92 to 62% by 2002-04, while the rate of mastectomies declined.
The median tumor size was 1.5 cm; patients with larger tumors were more likely to have mastectomies. "Interestingly, the use of BCT varied by age even among tumors [smaller than and equal to] 2 cm where the youngest and oldest age groups had the lowest BCT rate. In [tumors larger than] 2 cm, BCT rate declined by age," Dr. Hwang and her associates said.
Over the follow-up period, there were 31,416 deaths; 39% of these were caused by breast cancer; 5-year overall survival was 89%.
To further explore the treatment-mortality interaction, the investigators divided the cohort into four groups according to age and tumor characteristics:
• 50 years or older, hormone receptor negative.
• 50 years or older, hormone receptor negative.
• Younger than 50 years, hormone receptor positive.
• Younger than 50 years, hormone receptor positive.
Women 50 years and older with HR-positive tumors who had BCT experienced the greatest survival benefit, compared with mastectomy patients (hazard ratio, 0.81). Women younger than 50 years with HR- positive tumors experienced the smallest benefit (HR, 0.93), but one which was still statistically significant.
The investigators also looked at 3-year overall and disease-specific survival. "Notably, BCT was associated with significantly lower 3-year mortality rates from all causes," including heart disease (HR, 0.51), chronic respiratory disease (HR, 0.46), and cerebrovascular disease (HR, 0.64).
The findings align with those of randomized trials showing the benefits of BCT, the authors noted.
"Despite this, recent studies have shown an increased rate of mastectomy for patient subgroups including younger women with early-stage tumors, many of which would have presumably been amenable to BCT," they wrote. This could be the result of a perception that women with unfavorable characteristics, like younger age and high-risk tumors, don’t do as well with BCT.
The investigators noted that some differences in disease burden at baseline could have contributed to the findings.
"Interestingly, for every cause of mortality that we evaluated, women who had mastectomy were more likely to die within 3 years of their breast cancer diagnosis than women who chose BCT. Based on these findings, it is reasonable to infer that the mastectomy group was likely to have a greater burden of nonfatal comorbidities at presentation, and that this factor may well have influenced surgical decision-making. Nevertheless, this factor alone cannot account for why women with mastectomy had lower [disease specific survival] after adjusting for age and tumor characteristics," Dr. Hwang and her associates said.
Based on the strong associations with survival, "these findings support the notion that BCT, when combined with radiation, confers at least equivalent and perhaps even superior survival to mastectomy as definitive breast cancer treatment," they said.
The National Cancer Institute funded the study. Dr. Hwang had no financial disclosures.
Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer, compared with women who underwent mastectomy, Dr. E. Shelley Hwang and her colleagues reported Jan. 28 in the online issue of Cancer.
The 3-year disease-specific survival benefit for breast-conserving therapy (BCT) was most pronounced for chronic respiratory disease, for which lumpectomy was associated with a 54% decreased risk of death; heart disease, with a 49% decreased risk; and cerebrovascular disease, with a decreased risk of 36%.
The survival benefit varied with age, tumor size, and hormone receptor status but was significant in every subgroup, Dr. Hwang and her colleagues wrote (Cancer Jan. 28, 2013 [doi:10.1002/cncr.27795]).
"Our findings have important implications for understanding the overall benefit of BCT at the population level," wrote Dr. Hwang of the Duke University Comprehensive Cancer Center, Durham, N.C., and her coauthors. "These results provide confidence in the efficacy of BCT even among younger patients with HR-negative disease thought to be at relatively higher risk for local failure."
The team reviewed the records of 112,154 women who were treated for a new, unilateral T1/T2 stage I or II breast cancer diagnosed from 1990 to 2004. Most of these women (61,771) underwent a lumpectomy and radiation; the remainder underwent mastectomy without radiation. They were followed for a median of 10 years.
About a quarter of each group was younger than 50 years when diagnosed; another quarter was aged 70-80 years. A small portion (6%) was younger than 40 years.
Surgical approach evolved over the study period. Breast-conserving therapy increased from 37% in 1990-92 to 62% by 2002-04, while the rate of mastectomies declined.
The median tumor size was 1.5 cm; patients with larger tumors were more likely to have mastectomies. "Interestingly, the use of BCT varied by age even among tumors [smaller than and equal to] 2 cm where the youngest and oldest age groups had the lowest BCT rate. In [tumors larger than] 2 cm, BCT rate declined by age," Dr. Hwang and her associates said.
Over the follow-up period, there were 31,416 deaths; 39% of these were caused by breast cancer; 5-year overall survival was 89%.
To further explore the treatment-mortality interaction, the investigators divided the cohort into four groups according to age and tumor characteristics:
• 50 years or older, hormone receptor negative.
• 50 years or older, hormone receptor negative.
• Younger than 50 years, hormone receptor positive.
• Younger than 50 years, hormone receptor positive.
Women 50 years and older with HR-positive tumors who had BCT experienced the greatest survival benefit, compared with mastectomy patients (hazard ratio, 0.81). Women younger than 50 years with HR- positive tumors experienced the smallest benefit (HR, 0.93), but one which was still statistically significant.
The investigators also looked at 3-year overall and disease-specific survival. "Notably, BCT was associated with significantly lower 3-year mortality rates from all causes," including heart disease (HR, 0.51), chronic respiratory disease (HR, 0.46), and cerebrovascular disease (HR, 0.64).
The findings align with those of randomized trials showing the benefits of BCT, the authors noted.
"Despite this, recent studies have shown an increased rate of mastectomy for patient subgroups including younger women with early-stage tumors, many of which would have presumably been amenable to BCT," they wrote. This could be the result of a perception that women with unfavorable characteristics, like younger age and high-risk tumors, don’t do as well with BCT.
The investigators noted that some differences in disease burden at baseline could have contributed to the findings.
"Interestingly, for every cause of mortality that we evaluated, women who had mastectomy were more likely to die within 3 years of their breast cancer diagnosis than women who chose BCT. Based on these findings, it is reasonable to infer that the mastectomy group was likely to have a greater burden of nonfatal comorbidities at presentation, and that this factor may well have influenced surgical decision-making. Nevertheless, this factor alone cannot account for why women with mastectomy had lower [disease specific survival] after adjusting for age and tumor characteristics," Dr. Hwang and her associates said.
Based on the strong associations with survival, "these findings support the notion that BCT, when combined with radiation, confers at least equivalent and perhaps even superior survival to mastectomy as definitive breast cancer treatment," they said.
The National Cancer Institute funded the study. Dr. Hwang had no financial disclosures.
FROM CANCER
Major Finding: Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer than women who underwent mastectomy.
Data Source: Multivariate survival analysis including more than 112,000 women.
Disclosures: The National Cancer Institute funded the work. None of the authors had any financial disclosures.
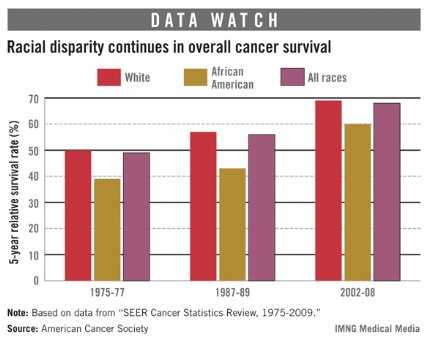
Cancer survival still lower for African Americans
The 5-year cancer survival rate for African Americans has risen 54% since the 1970s, but continues to be lower than that of whites, according to a report from the American Cancer Society.
Data from the Surveillance, Epidemiology, and End Results (SEER) database show that the 5-year relative survival rate in 2002-2008 was 60% for blacks, 69% for whites, and 68% for all races. Those numbers are all up significantly since 1975-77, when the rates for all cancers were 39% for African Americans, 50% for whites, and 49% for all races, the ACS reported.
The differences between blacks and whites varied among specific cancer sites. In 2002-2008, the 5-year survival for melanoma was 70% for blacks and 93% for whites. For colon cancer, the rate was 55% for blacks and 66% for whites. For kidney and renal pelvis cancer, however, the rates were much closer: 70% for blacks and 72% for whites. The only sites for which African Americans had higher survival rates were the brain (41%, compared with 34%) and the stomach (28%, compared with 27%), the ACS said.
The 5-year cancer survival rate for African Americans has risen 54% since the 1970s, but continues to be lower than that of whites, according to a report from the American Cancer Society.
Data from the Surveillance, Epidemiology, and End Results (SEER) database show that the 5-year relative survival rate in 2002-2008 was 60% for blacks, 69% for whites, and 68% for all races. Those numbers are all up significantly since 1975-77, when the rates for all cancers were 39% for African Americans, 50% for whites, and 49% for all races, the ACS reported.
The differences between blacks and whites varied among specific cancer sites. In 2002-2008, the 5-year survival for melanoma was 70% for blacks and 93% for whites. For colon cancer, the rate was 55% for blacks and 66% for whites. For kidney and renal pelvis cancer, however, the rates were much closer: 70% for blacks and 72% for whites. The only sites for which African Americans had higher survival rates were the brain (41%, compared with 34%) and the stomach (28%, compared with 27%), the ACS said.
The 5-year cancer survival rate for African Americans has risen 54% since the 1970s, but continues to be lower than that of whites, according to a report from the American Cancer Society.
Data from the Surveillance, Epidemiology, and End Results (SEER) database show that the 5-year relative survival rate in 2002-2008 was 60% for blacks, 69% for whites, and 68% for all races. Those numbers are all up significantly since 1975-77, when the rates for all cancers were 39% for African Americans, 50% for whites, and 49% for all races, the ACS reported.
The differences between blacks and whites varied among specific cancer sites. In 2002-2008, the 5-year survival for melanoma was 70% for blacks and 93% for whites. For colon cancer, the rate was 55% for blacks and 66% for whites. For kidney and renal pelvis cancer, however, the rates were much closer: 70% for blacks and 72% for whites. The only sites for which African Americans had higher survival rates were the brain (41%, compared with 34%) and the stomach (28%, compared with 27%), the ACS said.
New tool predicts late recurrence in breast cancer
SAN ANTONIO – A novel multigene signature known as the breast cancer index markedly outperformed the widely used Oncotype DX Recurrence Score and IHC4 tools in predicting late recurrences of estrogen receptor-positive/lymph node-negative breast cancer, Dr. Dennis C. Sgroi reported at the annual San Antonio Breast Cancer Symposium.
In a large clinical study, all three tools demonstrated significant prognostic value for early distant recurrences – that is, breast cancers recurring within 5 years of diagnosis. But only the breast cancer index (BCI) had sustained power for predicting distant recurrences arising during years 5-10. Both Oncotype DX and the IHC4 – a test based upon a tumor’s estrogen receptor, progesterone receptor, HER2, and Ki-67 status – lost their prognostic ability at about the 5-year mark, observed Dr. Sgroi of Massachusetts General Hospital, Boston.
More than half of all recurrences of estrogen receptor–positive early-stage breast cancer take place after 5 years of adjuvant tamoxifen or aromatase inhibitor therapy. Thus, the BCI fills a major need: a biomarker capable of identifying those estrogen receptor–positive breast cancer patients at increased risk for recurrence 5-10 years after their diagnosis.
The BCI includes the ratio of HOXB13-to-IL17BR gene expression and a five-gene molecular grade index shown to be predictive beyond tumor grade of distance metastasis. Dr. Sgroi and coinvestigators have previously established that these two biomarkers are complementary in their power to predict recurrences (Clin. Cancer Res. 2008;14:2601-8).
At the San Antonio symposium, Dr. Sgroi presented an analysis of the comparative prognostic performance of the BCI, the Oncotype DX recurrence score, and the IHC4 as applied to baseline tumor samples from 665 hormone receptor-positive participants in the randomized, multicenter TransATAC (Arimedex, Tamoxifen, Alone or Together) trial. The primary endpoint was distant recurrence.
The baseline BCI score differentiated three risk groups over the course of 10 years of follow-up in TransATAC: 58% of women fell into the low-risk group with a 4.2% risk of distant recurrence within 10 years, 25% had an intermediate score with an 18% risk, and 17% had a high score with a 30% risk.
In the first 5 years of follow-up, patients with a low- or intermediate-risk BCI had a distant recurrence risk of 1.3% and 5.6%, respectively. Thus, the BCI identified 83% of study participants as having, on average, a 5-year distant recurrence risk of less than 5%. In contrast, women with a high BCI score had a 5-year recurrence risk of 18%.
Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
In a multivariate analysis adjusted for the clinical treatment score – an algorithm based upon nodal status, tumor size and grade, age, and treatment (J. Clin. Oncol. 2011;29:4273-8) – the BCI, IHC4, and Oncotype DX displayed similar prognostic performance for distant recurrences in years 0-5. But the latter two tests lost their predictive capability in years 5-10.
Estrogen receptor–positive/node-negative patients have traditionally been considered at low risk. But, as shown in the TransATAC analysis, applying the BCI to primary tumor specimens from such patients enables physicians to identify two groups at the time of diagnosis: those who are at low risk for recurrence within the next 5 years and who are adequately treated with endocrine therapy alone; and a high-risk subgroup who should be considered for chemotherapy or some other additional therapy along with endocrine therapy, Dr. Sgroi concluded.
Moreover, the BCI score at diagnosis also permits identification of two distinct groups among patients remaining disease free at 5 years of follow up: those at low risk of late recurrence and who don’t need subsequent therapy; and those at roughly a threefold greater risk of late recurrence and are therefore candidates for further systemic adjuvant therapy.
"What that additional treatment should be is something we don’t know at this point. It might be extended adjuvant hormonal therapy alone or in combination with another therapeutic agent," he added.
In response to an audience question, Dr. Sgroi admitted that the exact biologic role of the genes incorporated in the BCI is "still a bit of a mystery," although it’s clear that they’re not solely involved in proliferation.
The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.
SAN ANTONIO – A novel multigene signature known as the breast cancer index markedly outperformed the widely used Oncotype DX Recurrence Score and IHC4 tools in predicting late recurrences of estrogen receptor-positive/lymph node-negative breast cancer, Dr. Dennis C. Sgroi reported at the annual San Antonio Breast Cancer Symposium.
In a large clinical study, all three tools demonstrated significant prognostic value for early distant recurrences – that is, breast cancers recurring within 5 years of diagnosis. But only the breast cancer index (BCI) had sustained power for predicting distant recurrences arising during years 5-10. Both Oncotype DX and the IHC4 – a test based upon a tumor’s estrogen receptor, progesterone receptor, HER2, and Ki-67 status – lost their prognostic ability at about the 5-year mark, observed Dr. Sgroi of Massachusetts General Hospital, Boston.
More than half of all recurrences of estrogen receptor–positive early-stage breast cancer take place after 5 years of adjuvant tamoxifen or aromatase inhibitor therapy. Thus, the BCI fills a major need: a biomarker capable of identifying those estrogen receptor–positive breast cancer patients at increased risk for recurrence 5-10 years after their diagnosis.
The BCI includes the ratio of HOXB13-to-IL17BR gene expression and a five-gene molecular grade index shown to be predictive beyond tumor grade of distance metastasis. Dr. Sgroi and coinvestigators have previously established that these two biomarkers are complementary in their power to predict recurrences (Clin. Cancer Res. 2008;14:2601-8).
At the San Antonio symposium, Dr. Sgroi presented an analysis of the comparative prognostic performance of the BCI, the Oncotype DX recurrence score, and the IHC4 as applied to baseline tumor samples from 665 hormone receptor-positive participants in the randomized, multicenter TransATAC (Arimedex, Tamoxifen, Alone or Together) trial. The primary endpoint was distant recurrence.
The baseline BCI score differentiated three risk groups over the course of 10 years of follow-up in TransATAC: 58% of women fell into the low-risk group with a 4.2% risk of distant recurrence within 10 years, 25% had an intermediate score with an 18% risk, and 17% had a high score with a 30% risk.
In the first 5 years of follow-up, patients with a low- or intermediate-risk BCI had a distant recurrence risk of 1.3% and 5.6%, respectively. Thus, the BCI identified 83% of study participants as having, on average, a 5-year distant recurrence risk of less than 5%. In contrast, women with a high BCI score had a 5-year recurrence risk of 18%.
Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
In a multivariate analysis adjusted for the clinical treatment score – an algorithm based upon nodal status, tumor size and grade, age, and treatment (J. Clin. Oncol. 2011;29:4273-8) – the BCI, IHC4, and Oncotype DX displayed similar prognostic performance for distant recurrences in years 0-5. But the latter two tests lost their predictive capability in years 5-10.
Estrogen receptor–positive/node-negative patients have traditionally been considered at low risk. But, as shown in the TransATAC analysis, applying the BCI to primary tumor specimens from such patients enables physicians to identify two groups at the time of diagnosis: those who are at low risk for recurrence within the next 5 years and who are adequately treated with endocrine therapy alone; and a high-risk subgroup who should be considered for chemotherapy or some other additional therapy along with endocrine therapy, Dr. Sgroi concluded.
Moreover, the BCI score at diagnosis also permits identification of two distinct groups among patients remaining disease free at 5 years of follow up: those at low risk of late recurrence and who don’t need subsequent therapy; and those at roughly a threefold greater risk of late recurrence and are therefore candidates for further systemic adjuvant therapy.
"What that additional treatment should be is something we don’t know at this point. It might be extended adjuvant hormonal therapy alone or in combination with another therapeutic agent," he added.
In response to an audience question, Dr. Sgroi admitted that the exact biologic role of the genes incorporated in the BCI is "still a bit of a mystery," although it’s clear that they’re not solely involved in proliferation.
The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.
SAN ANTONIO – A novel multigene signature known as the breast cancer index markedly outperformed the widely used Oncotype DX Recurrence Score and IHC4 tools in predicting late recurrences of estrogen receptor-positive/lymph node-negative breast cancer, Dr. Dennis C. Sgroi reported at the annual San Antonio Breast Cancer Symposium.
In a large clinical study, all three tools demonstrated significant prognostic value for early distant recurrences – that is, breast cancers recurring within 5 years of diagnosis. But only the breast cancer index (BCI) had sustained power for predicting distant recurrences arising during years 5-10. Both Oncotype DX and the IHC4 – a test based upon a tumor’s estrogen receptor, progesterone receptor, HER2, and Ki-67 status – lost their prognostic ability at about the 5-year mark, observed Dr. Sgroi of Massachusetts General Hospital, Boston.
More than half of all recurrences of estrogen receptor–positive early-stage breast cancer take place after 5 years of adjuvant tamoxifen or aromatase inhibitor therapy. Thus, the BCI fills a major need: a biomarker capable of identifying those estrogen receptor–positive breast cancer patients at increased risk for recurrence 5-10 years after their diagnosis.
The BCI includes the ratio of HOXB13-to-IL17BR gene expression and a five-gene molecular grade index shown to be predictive beyond tumor grade of distance metastasis. Dr. Sgroi and coinvestigators have previously established that these two biomarkers are complementary in their power to predict recurrences (Clin. Cancer Res. 2008;14:2601-8).
At the San Antonio symposium, Dr. Sgroi presented an analysis of the comparative prognostic performance of the BCI, the Oncotype DX recurrence score, and the IHC4 as applied to baseline tumor samples from 665 hormone receptor-positive participants in the randomized, multicenter TransATAC (Arimedex, Tamoxifen, Alone or Together) trial. The primary endpoint was distant recurrence.
The baseline BCI score differentiated three risk groups over the course of 10 years of follow-up in TransATAC: 58% of women fell into the low-risk group with a 4.2% risk of distant recurrence within 10 years, 25% had an intermediate score with an 18% risk, and 17% had a high score with a 30% risk.
In the first 5 years of follow-up, patients with a low- or intermediate-risk BCI had a distant recurrence risk of 1.3% and 5.6%, respectively. Thus, the BCI identified 83% of study participants as having, on average, a 5-year distant recurrence risk of less than 5%. In contrast, women with a high BCI score had a 5-year recurrence risk of 18%.
Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
In a multivariate analysis adjusted for the clinical treatment score – an algorithm based upon nodal status, tumor size and grade, age, and treatment (J. Clin. Oncol. 2011;29:4273-8) – the BCI, IHC4, and Oncotype DX displayed similar prognostic performance for distant recurrences in years 0-5. But the latter two tests lost their predictive capability in years 5-10.
Estrogen receptor–positive/node-negative patients have traditionally been considered at low risk. But, as shown in the TransATAC analysis, applying the BCI to primary tumor specimens from such patients enables physicians to identify two groups at the time of diagnosis: those who are at low risk for recurrence within the next 5 years and who are adequately treated with endocrine therapy alone; and a high-risk subgroup who should be considered for chemotherapy or some other additional therapy along with endocrine therapy, Dr. Sgroi concluded.
Moreover, the BCI score at diagnosis also permits identification of two distinct groups among patients remaining disease free at 5 years of follow up: those at low risk of late recurrence and who don’t need subsequent therapy; and those at roughly a threefold greater risk of late recurrence and are therefore candidates for further systemic adjuvant therapy.
"What that additional treatment should be is something we don’t know at this point. It might be extended adjuvant hormonal therapy alone or in combination with another therapeutic agent," he added.
In response to an audience question, Dr. Sgroi admitted that the exact biologic role of the genes incorporated in the BCI is "still a bit of a mystery," although it’s clear that they’re not solely involved in proliferation.
The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.
AT THE ANNUAL SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
Data Source: This study compared the prognostic performance of three biomarker-based tools in predicting the risk at 5-10 years of distant recurrence of breast cancer in TransATAC study participants.
Disclosures: The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.
Minimally invasive breast biopsy lags in Texas
PALM BEACH, FLA. – More than a fifth of women in Texas with image-detected breast abnormalities failed to undergo minimally invasive breast biopsy as recently as 2008, according to a review of statewide Medicare data, even though in 2005 a U.S. consensus panel declared the minimally invasive approach the procedure of choice and that few patients should have excisional biopsy as their initial procedure.
The analysis also revealed substantial disparities in use of minimally-invasive breast biopsy (MIBB) relative to open-surgical biopsy. In several rural health service areas (HSA) of Texas during 2005-2008, fewer than 40% of women undergoing biopsy of an image-detected breast abnormality had MIBB, Dr. Taylor S. Riall said at the annual meeting of the Southern Surgical Association. During 2005-2008, 5% of Texas HSAs had MIBB rates greater than 90%, the target set by U.S. cancer organizations. The researchers also identified low levels of MIBB use for Hispanic women, and women of low socioeconomic status.
"Our studies identify targets for interventions to improve MIBB rates, such as the Hispanic disparity and geographic variations in practice pattern," she said. "Our findings highlight that the strategies for intervention need to vary by geographic region and the underlying etiology of the failure to adopt this cost-effective practice," said Dr. Riall, a cancer surgeon at the University of Texas Medical Branch in Galveston.
"This is by far the most detailed study of MIBB [practice patterns] performed to date," commented Dr. Stephen Grobmyer, a surgical oncologist and director of breast services at the Cleveland Clinic.
The data documented that surgeons were an important contributor to MIBB underuse. Throughout the 9 years of data studied by Dr. Riall and her associates during 2001-2008, 70% of MIBB were performed by radiologists, while 26% were performed by surgeons. In contrast, surgeons performed 94% of open, excisional biopsies. When a woman’s breast mass was first identified by a surgeon, 44% of the women had MIBB; when first identified by a primary care physician, 58% had MIBB; when first identified by an oncologist, 59% had MIBB; and when first identified by a gynecologist, 67% had MIBB.
The low levels of MIBB use occurred despite increasingly strong recommendations during the period studied to move MIBB to the forefront of breast-abnormality assessment. In 2001, the first international consensus conference on image-detected breast cancer, organized by the University of Southern California, said that "percutaneous biopsy is the preferred initial diagnostic procedure in most patients with mammographically detected abnormalities"(J. Amer. Coll. Surg. 2001;193:297-302).
In 2005, the second international consensus conference on image-detected breast cancer racheted up the recommendation, saying "minimally invasive breast biopsy is the optimal tissue-acquisition method and the procedure of choice for image-detected breast abnormalities. It should be readily available to all patients with image-detected lesions" (J. Amer. Coll. Surg. 2005;201:586-597).
Although the third international consensus conference did not take place until 2009, the year after the end of the period studied by Dr. Riall, the statement at that time showed how MIBB had become the clear standard of care for biopsy of suspicious breast masses. The 2009 panel said that "percutaneous needle biopsy represents ‘best practice’ and should be the new ‘gold standard’ for initial diagnosis. It should essentially replace open biopsy in this role. The Panel called on the medical community to change their current practice if they are using open surgical breast biopsy as a standard diagnostic procedure. Surgeons should audit their practice and make adjustments to decrease their rate of open biopsy for initial diagnosis to less than 5% to 10%" (J. Amer. Coll. Surg. 2009;209:504-20).
"We need to get a message out to surgeons because they are the ones doing many of the open biopsies," Dr. Riall said in an interview. "Surgeons are a group to target, but we also need to target primary care physicians and other referring physicians so that they understand that MIBB is appropriate. The decision to do MIBB versus open biopsy should be made with the surgeon and with the oncologist who will ultimately treat the breast cancer; the decision should not be made just by a radiologist," who is usually the first person to see a mass when it is first detected by mammography.
Dr. Riall also stressed that the causes of MIBB underuse are multifactorial, and require multiple solutions.
"In very rural areas, the primary problem is access to mammography. In the cases where women cluster in primary care practices that don’t do MIBB, we need to provide better physician education. In regions where there is a high density of private practice surgeons, open biopsy is driven by reimbursement. I think there is an interaction of patient preference, surgeon preference, education and training, geographic region, and availability of radiologists and mammography facilities. Trying to dissect it is very hard."
Her study identified in Texas Medicare records 67,582 unique women aged 66 years or older who underwent 75,518 unique breast mass episodes during 2001-2008, including 49,653 (66%) of masses that underwent MIBB and 25,865 (34%) that underwent open surgical biopsy. Use of MIBB rose steadily during the period, starting at 44% of masses in 2001 and increasing to 79% by 2008.
Analysis of MIBB use by Medicare health service area showed stark geographic disparities, with MIBB use as low as 21% in one HSA. During 2005-2008, MIBB use remained at 40% or less in several HSA along the Rio Grande border and in East Texas, including the HSAs in the south Texas towns of McAllen and Harlingen. In contrast, the HSA immediately adjacent to these that includes Brownsville had a MIBB rate greater than 70%. The analysis also showed than many of the HSAs with the lowest rates of MIBB use were located in Texas regions with high Hispanic populations, Dr. Riall said.
Dr. Riall and Dr. Grobmyer had no disclosures.
Current guidelines strongly endorse minimally-invasive breast biopsy as the standard for establishing the histologic diagnosis of a breast mass before interventional treatment. Minimally-invasive breast biopsy reduces the interval between diagnosis and starting therapy, and reduces cost compared with an open technique.
The report by Dr. Riall and her associates also touches on a legal aspect that demands our attention. Currently, about 20% of U.S. medical litigation centers on cases involving breast cancer and delayed diagnosis of these cancers. The demographic disparities in care that they identified in their study mean that it is essential for us to identify and resolve the specific barriers to performing minimally-invasive breast biopsy in certain regions and among certain groups of patients. In doing this, we could better achieve the goal of using the minimally-invasive approach in greater than 90% of patients, both in Texas and throughout the United States.
The principle reason why these barriers exist is possibly related to improper insurance coverage and inadequate access to the necessary technology. It is not surprising to me that 70% of the minimally-invasive biopsies were performed by radiologists, while only 26% were done by surgeons. Our goal should be to make access to this contemporary technology available to the entire U.S. population.
Dr. Kirby I. Bland is a surgical oncologist and professor and chairman of surgery at the University of Alabama, Birmingham. He made these comments as a designated discussant of the report. He had no disclosures.
Current guidelines strongly endorse minimally-invasive breast biopsy as the standard for establishing the histologic diagnosis of a breast mass before interventional treatment. Minimally-invasive breast biopsy reduces the interval between diagnosis and starting therapy, and reduces cost compared with an open technique.
The report by Dr. Riall and her associates also touches on a legal aspect that demands our attention. Currently, about 20% of U.S. medical litigation centers on cases involving breast cancer and delayed diagnosis of these cancers. The demographic disparities in care that they identified in their study mean that it is essential for us to identify and resolve the specific barriers to performing minimally-invasive breast biopsy in certain regions and among certain groups of patients. In doing this, we could better achieve the goal of using the minimally-invasive approach in greater than 90% of patients, both in Texas and throughout the United States.
The principle reason why these barriers exist is possibly related to improper insurance coverage and inadequate access to the necessary technology. It is not surprising to me that 70% of the minimally-invasive biopsies were performed by radiologists, while only 26% were done by surgeons. Our goal should be to make access to this contemporary technology available to the entire U.S. population.
Dr. Kirby I. Bland is a surgical oncologist and professor and chairman of surgery at the University of Alabama, Birmingham. He made these comments as a designated discussant of the report. He had no disclosures.
Current guidelines strongly endorse minimally-invasive breast biopsy as the standard for establishing the histologic diagnosis of a breast mass before interventional treatment. Minimally-invasive breast biopsy reduces the interval between diagnosis and starting therapy, and reduces cost compared with an open technique.
The report by Dr. Riall and her associates also touches on a legal aspect that demands our attention. Currently, about 20% of U.S. medical litigation centers on cases involving breast cancer and delayed diagnosis of these cancers. The demographic disparities in care that they identified in their study mean that it is essential for us to identify and resolve the specific barriers to performing minimally-invasive breast biopsy in certain regions and among certain groups of patients. In doing this, we could better achieve the goal of using the minimally-invasive approach in greater than 90% of patients, both in Texas and throughout the United States.
The principle reason why these barriers exist is possibly related to improper insurance coverage and inadequate access to the necessary technology. It is not surprising to me that 70% of the minimally-invasive biopsies were performed by radiologists, while only 26% were done by surgeons. Our goal should be to make access to this contemporary technology available to the entire U.S. population.
Dr. Kirby I. Bland is a surgical oncologist and professor and chairman of surgery at the University of Alabama, Birmingham. He made these comments as a designated discussant of the report. He had no disclosures.
PALM BEACH, FLA. – More than a fifth of women in Texas with image-detected breast abnormalities failed to undergo minimally invasive breast biopsy as recently as 2008, according to a review of statewide Medicare data, even though in 2005 a U.S. consensus panel declared the minimally invasive approach the procedure of choice and that few patients should have excisional biopsy as their initial procedure.
The analysis also revealed substantial disparities in use of minimally-invasive breast biopsy (MIBB) relative to open-surgical biopsy. In several rural health service areas (HSA) of Texas during 2005-2008, fewer than 40% of women undergoing biopsy of an image-detected breast abnormality had MIBB, Dr. Taylor S. Riall said at the annual meeting of the Southern Surgical Association. During 2005-2008, 5% of Texas HSAs had MIBB rates greater than 90%, the target set by U.S. cancer organizations. The researchers also identified low levels of MIBB use for Hispanic women, and women of low socioeconomic status.
"Our studies identify targets for interventions to improve MIBB rates, such as the Hispanic disparity and geographic variations in practice pattern," she said. "Our findings highlight that the strategies for intervention need to vary by geographic region and the underlying etiology of the failure to adopt this cost-effective practice," said Dr. Riall, a cancer surgeon at the University of Texas Medical Branch in Galveston.
"This is by far the most detailed study of MIBB [practice patterns] performed to date," commented Dr. Stephen Grobmyer, a surgical oncologist and director of breast services at the Cleveland Clinic.
The data documented that surgeons were an important contributor to MIBB underuse. Throughout the 9 years of data studied by Dr. Riall and her associates during 2001-2008, 70% of MIBB were performed by radiologists, while 26% were performed by surgeons. In contrast, surgeons performed 94% of open, excisional biopsies. When a woman’s breast mass was first identified by a surgeon, 44% of the women had MIBB; when first identified by a primary care physician, 58% had MIBB; when first identified by an oncologist, 59% had MIBB; and when first identified by a gynecologist, 67% had MIBB.
The low levels of MIBB use occurred despite increasingly strong recommendations during the period studied to move MIBB to the forefront of breast-abnormality assessment. In 2001, the first international consensus conference on image-detected breast cancer, organized by the University of Southern California, said that "percutaneous biopsy is the preferred initial diagnostic procedure in most patients with mammographically detected abnormalities"(J. Amer. Coll. Surg. 2001;193:297-302).
In 2005, the second international consensus conference on image-detected breast cancer racheted up the recommendation, saying "minimally invasive breast biopsy is the optimal tissue-acquisition method and the procedure of choice for image-detected breast abnormalities. It should be readily available to all patients with image-detected lesions" (J. Amer. Coll. Surg. 2005;201:586-597).
Although the third international consensus conference did not take place until 2009, the year after the end of the period studied by Dr. Riall, the statement at that time showed how MIBB had become the clear standard of care for biopsy of suspicious breast masses. The 2009 panel said that "percutaneous needle biopsy represents ‘best practice’ and should be the new ‘gold standard’ for initial diagnosis. It should essentially replace open biopsy in this role. The Panel called on the medical community to change their current practice if they are using open surgical breast biopsy as a standard diagnostic procedure. Surgeons should audit their practice and make adjustments to decrease their rate of open biopsy for initial diagnosis to less than 5% to 10%" (J. Amer. Coll. Surg. 2009;209:504-20).
"We need to get a message out to surgeons because they are the ones doing many of the open biopsies," Dr. Riall said in an interview. "Surgeons are a group to target, but we also need to target primary care physicians and other referring physicians so that they understand that MIBB is appropriate. The decision to do MIBB versus open biopsy should be made with the surgeon and with the oncologist who will ultimately treat the breast cancer; the decision should not be made just by a radiologist," who is usually the first person to see a mass when it is first detected by mammography.
Dr. Riall also stressed that the causes of MIBB underuse are multifactorial, and require multiple solutions.
"In very rural areas, the primary problem is access to mammography. In the cases where women cluster in primary care practices that don’t do MIBB, we need to provide better physician education. In regions where there is a high density of private practice surgeons, open biopsy is driven by reimbursement. I think there is an interaction of patient preference, surgeon preference, education and training, geographic region, and availability of radiologists and mammography facilities. Trying to dissect it is very hard."
Her study identified in Texas Medicare records 67,582 unique women aged 66 years or older who underwent 75,518 unique breast mass episodes during 2001-2008, including 49,653 (66%) of masses that underwent MIBB and 25,865 (34%) that underwent open surgical biopsy. Use of MIBB rose steadily during the period, starting at 44% of masses in 2001 and increasing to 79% by 2008.
Analysis of MIBB use by Medicare health service area showed stark geographic disparities, with MIBB use as low as 21% in one HSA. During 2005-2008, MIBB use remained at 40% or less in several HSA along the Rio Grande border and in East Texas, including the HSAs in the south Texas towns of McAllen and Harlingen. In contrast, the HSA immediately adjacent to these that includes Brownsville had a MIBB rate greater than 70%. The analysis also showed than many of the HSAs with the lowest rates of MIBB use were located in Texas regions with high Hispanic populations, Dr. Riall said.
Dr. Riall and Dr. Grobmyer had no disclosures.
PALM BEACH, FLA. – More than a fifth of women in Texas with image-detected breast abnormalities failed to undergo minimally invasive breast biopsy as recently as 2008, according to a review of statewide Medicare data, even though in 2005 a U.S. consensus panel declared the minimally invasive approach the procedure of choice and that few patients should have excisional biopsy as their initial procedure.
The analysis also revealed substantial disparities in use of minimally-invasive breast biopsy (MIBB) relative to open-surgical biopsy. In several rural health service areas (HSA) of Texas during 2005-2008, fewer than 40% of women undergoing biopsy of an image-detected breast abnormality had MIBB, Dr. Taylor S. Riall said at the annual meeting of the Southern Surgical Association. During 2005-2008, 5% of Texas HSAs had MIBB rates greater than 90%, the target set by U.S. cancer organizations. The researchers also identified low levels of MIBB use for Hispanic women, and women of low socioeconomic status.
"Our studies identify targets for interventions to improve MIBB rates, such as the Hispanic disparity and geographic variations in practice pattern," she said. "Our findings highlight that the strategies for intervention need to vary by geographic region and the underlying etiology of the failure to adopt this cost-effective practice," said Dr. Riall, a cancer surgeon at the University of Texas Medical Branch in Galveston.
"This is by far the most detailed study of MIBB [practice patterns] performed to date," commented Dr. Stephen Grobmyer, a surgical oncologist and director of breast services at the Cleveland Clinic.
The data documented that surgeons were an important contributor to MIBB underuse. Throughout the 9 years of data studied by Dr. Riall and her associates during 2001-2008, 70% of MIBB were performed by radiologists, while 26% were performed by surgeons. In contrast, surgeons performed 94% of open, excisional biopsies. When a woman’s breast mass was first identified by a surgeon, 44% of the women had MIBB; when first identified by a primary care physician, 58% had MIBB; when first identified by an oncologist, 59% had MIBB; and when first identified by a gynecologist, 67% had MIBB.
The low levels of MIBB use occurred despite increasingly strong recommendations during the period studied to move MIBB to the forefront of breast-abnormality assessment. In 2001, the first international consensus conference on image-detected breast cancer, organized by the University of Southern California, said that "percutaneous biopsy is the preferred initial diagnostic procedure in most patients with mammographically detected abnormalities"(J. Amer. Coll. Surg. 2001;193:297-302).
In 2005, the second international consensus conference on image-detected breast cancer racheted up the recommendation, saying "minimally invasive breast biopsy is the optimal tissue-acquisition method and the procedure of choice for image-detected breast abnormalities. It should be readily available to all patients with image-detected lesions" (J. Amer. Coll. Surg. 2005;201:586-597).
Although the third international consensus conference did not take place until 2009, the year after the end of the period studied by Dr. Riall, the statement at that time showed how MIBB had become the clear standard of care for biopsy of suspicious breast masses. The 2009 panel said that "percutaneous needle biopsy represents ‘best practice’ and should be the new ‘gold standard’ for initial diagnosis. It should essentially replace open biopsy in this role. The Panel called on the medical community to change their current practice if they are using open surgical breast biopsy as a standard diagnostic procedure. Surgeons should audit their practice and make adjustments to decrease their rate of open biopsy for initial diagnosis to less than 5% to 10%" (J. Amer. Coll. Surg. 2009;209:504-20).
"We need to get a message out to surgeons because they are the ones doing many of the open biopsies," Dr. Riall said in an interview. "Surgeons are a group to target, but we also need to target primary care physicians and other referring physicians so that they understand that MIBB is appropriate. The decision to do MIBB versus open biopsy should be made with the surgeon and with the oncologist who will ultimately treat the breast cancer; the decision should not be made just by a radiologist," who is usually the first person to see a mass when it is first detected by mammography.
Dr. Riall also stressed that the causes of MIBB underuse are multifactorial, and require multiple solutions.
"In very rural areas, the primary problem is access to mammography. In the cases where women cluster in primary care practices that don’t do MIBB, we need to provide better physician education. In regions where there is a high density of private practice surgeons, open biopsy is driven by reimbursement. I think there is an interaction of patient preference, surgeon preference, education and training, geographic region, and availability of radiologists and mammography facilities. Trying to dissect it is very hard."
Her study identified in Texas Medicare records 67,582 unique women aged 66 years or older who underwent 75,518 unique breast mass episodes during 2001-2008, including 49,653 (66%) of masses that underwent MIBB and 25,865 (34%) that underwent open surgical biopsy. Use of MIBB rose steadily during the period, starting at 44% of masses in 2001 and increasing to 79% by 2008.
Analysis of MIBB use by Medicare health service area showed stark geographic disparities, with MIBB use as low as 21% in one HSA. During 2005-2008, MIBB use remained at 40% or less in several HSA along the Rio Grande border and in East Texas, including the HSAs in the south Texas towns of McAllen and Harlingen. In contrast, the HSA immediately adjacent to these that includes Brownsville had a MIBB rate greater than 70%. The analysis also showed than many of the HSAs with the lowest rates of MIBB use were located in Texas regions with high Hispanic populations, Dr. Riall said.
Dr. Riall and Dr. Grobmyer had no disclosures.
AT THE ANNUAL MEETING OF THE SOUTHERN SURGICAL ASSOCIATION
Major Finding: During 2008, 79% of Texas women aged 66 years or older undergoing breast biopsy had a minimally-invasive procedure.
Data Source: A review of Texas Medicare claims data for 67,582 women who underwent a breast mass biopsy during 2001-2008.
Disclosures: Dr. Riall and Dr. Grobmyer had no disclosures.
Resecting residual gastrointestinal stromal tumors improved survival
SAN FRANCISCO – Surgically removing residual gastrointestinal stromal tumors in patients who respond to imatinib therapy significantly increased time to tumor progression to 88 months, compared with 43 months using imatinib alone, based on findings from a retrospective study of 134 patients.
After controlling for the effects of other risk factors, the surgery decreased threefold the likelihood of disease progression and decreased fivefold the risk of death, Dr. Seong Joon Park reported in a press briefing sponsored by the American Society of Clinical Oncology (ASCO). The press conference was held in advance of at a meeting on gastrointestinal cancers sponsored by ASCO and three other cancer organizations.
The findings support the widely adopted practice of removing residual tumors in these patients, despite the retrospective and observational design of the study, Dr. Park said. A prospective European study of similar design to this one terminated early due to poor patient enrollment. "It’s really hard to conduct a prospective study of this design," said Dr. Park of Asan Medical Center, Seoul, South Korea.
He and his associates reviewed the records of patients who showed at least 6 months of disease stabilization or response to imatinib (Gleevec) treatment, 92 of whom got the drug treatment alone and 42 of whom underwent surgery to remove residual tumors after a median of 19 months of imatinib therapy. The imatinib therapy was restarted after surgery. Median follow-up for the cohort as a whole was 59 months.
"This treatment strategy is worth trying as a clinical practice if the medical center is large enough to have an experienced multidisciplinary team and to have low morbidity and mortality associated with surgery," he said.
Each year, approximately 5,000 new cases of gastrointestinal stromal tumors are diagnosed in the United States, most often in the stomach and small intestine, though they can occur anywhere in or near the GI tract. Imatinib typically is first-line therapy, and 80%-85% of patients will respond to the treatment, he said. A majority of patients who respond to imatinib will have residual tumors, however, which are believed to contribute to the development of drug resistance, leading to the hypothesis that removing the residual tumors would improve survival.
In general, one-third of patients are candidates for surgical removal of residual lesions, depending on the tumor size and other tumor and patient characteristics, Dr. Park said.
The two patient groups in the study were similar except that the surgery group was significantly younger (51 vs. 58 years) and was less likely to have metastases in the peritoneum (41% in the surgery group vs. 61% in the control group).
As it is an aggressive and difficult treatment, surgery is more likely to be considered and recommended to younger patients who have a good performance status and, thus, less likely to be recommended in patients with multiple peritoneal metastases.
Factors associated with longer progression-free and overall survival included surgery and having an initial tumor size less than 150 mm, multivariate analyses showed. Female sex and having the KIT exon 11 mutation also were associated with longer progression-free survival. The researchers used propensity scores and inverse-probability-weighting adjustments to account for the effects of factors other than surgery.
The gastrointestinal cancers meeting, where Dr. Park will present the results, is cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Park reported having no financial disclosures.
On Twitter @sherryboschert
GI stromal tumors are an uncommon type of gastrointestinal tumor that can arise at many different places within the GI tract. This disease is notable because it’s really been a triumph of molecularly targeted therapy with imatinib (Gleevec), a drug that targets a particular molecular abnormality present in GI stromal tumors. Because of this, there is an extremely high response rate in patients with GI stromal tumors and drug therapy can control the disease for years.
Unfortunately, resistance ultimately develops to imatinib. This study provides provocative evidence that taking an aggressive approach surgically in addition to medical treatment with imatinib may result in longer survival of patients with GI stromal tumors.
Dr. Neal J. Meropol is chief of hematology and oncology at Case Western Reserve University, Cleveland. He gave these comments as moderator of the press briefing. He has been a consultant or advisor to Precision Therapeutics.
GI stromal tumors are an uncommon type of gastrointestinal tumor that can arise at many different places within the GI tract. This disease is notable because it’s really been a triumph of molecularly targeted therapy with imatinib (Gleevec), a drug that targets a particular molecular abnormality present in GI stromal tumors. Because of this, there is an extremely high response rate in patients with GI stromal tumors and drug therapy can control the disease for years.
Unfortunately, resistance ultimately develops to imatinib. This study provides provocative evidence that taking an aggressive approach surgically in addition to medical treatment with imatinib may result in longer survival of patients with GI stromal tumors.
Dr. Neal J. Meropol is chief of hematology and oncology at Case Western Reserve University, Cleveland. He gave these comments as moderator of the press briefing. He has been a consultant or advisor to Precision Therapeutics.
GI stromal tumors are an uncommon type of gastrointestinal tumor that can arise at many different places within the GI tract. This disease is notable because it’s really been a triumph of molecularly targeted therapy with imatinib (Gleevec), a drug that targets a particular molecular abnormality present in GI stromal tumors. Because of this, there is an extremely high response rate in patients with GI stromal tumors and drug therapy can control the disease for years.
Unfortunately, resistance ultimately develops to imatinib. This study provides provocative evidence that taking an aggressive approach surgically in addition to medical treatment with imatinib may result in longer survival of patients with GI stromal tumors.
Dr. Neal J. Meropol is chief of hematology and oncology at Case Western Reserve University, Cleveland. He gave these comments as moderator of the press briefing. He has been a consultant or advisor to Precision Therapeutics.
SAN FRANCISCO – Surgically removing residual gastrointestinal stromal tumors in patients who respond to imatinib therapy significantly increased time to tumor progression to 88 months, compared with 43 months using imatinib alone, based on findings from a retrospective study of 134 patients.
After controlling for the effects of other risk factors, the surgery decreased threefold the likelihood of disease progression and decreased fivefold the risk of death, Dr. Seong Joon Park reported in a press briefing sponsored by the American Society of Clinical Oncology (ASCO). The press conference was held in advance of at a meeting on gastrointestinal cancers sponsored by ASCO and three other cancer organizations.
The findings support the widely adopted practice of removing residual tumors in these patients, despite the retrospective and observational design of the study, Dr. Park said. A prospective European study of similar design to this one terminated early due to poor patient enrollment. "It’s really hard to conduct a prospective study of this design," said Dr. Park of Asan Medical Center, Seoul, South Korea.
He and his associates reviewed the records of patients who showed at least 6 months of disease stabilization or response to imatinib (Gleevec) treatment, 92 of whom got the drug treatment alone and 42 of whom underwent surgery to remove residual tumors after a median of 19 months of imatinib therapy. The imatinib therapy was restarted after surgery. Median follow-up for the cohort as a whole was 59 months.
"This treatment strategy is worth trying as a clinical practice if the medical center is large enough to have an experienced multidisciplinary team and to have low morbidity and mortality associated with surgery," he said.
Each year, approximately 5,000 new cases of gastrointestinal stromal tumors are diagnosed in the United States, most often in the stomach and small intestine, though they can occur anywhere in or near the GI tract. Imatinib typically is first-line therapy, and 80%-85% of patients will respond to the treatment, he said. A majority of patients who respond to imatinib will have residual tumors, however, which are believed to contribute to the development of drug resistance, leading to the hypothesis that removing the residual tumors would improve survival.
In general, one-third of patients are candidates for surgical removal of residual lesions, depending on the tumor size and other tumor and patient characteristics, Dr. Park said.
The two patient groups in the study were similar except that the surgery group was significantly younger (51 vs. 58 years) and was less likely to have metastases in the peritoneum (41% in the surgery group vs. 61% in the control group).
As it is an aggressive and difficult treatment, surgery is more likely to be considered and recommended to younger patients who have a good performance status and, thus, less likely to be recommended in patients with multiple peritoneal metastases.
Factors associated with longer progression-free and overall survival included surgery and having an initial tumor size less than 150 mm, multivariate analyses showed. Female sex and having the KIT exon 11 mutation also were associated with longer progression-free survival. The researchers used propensity scores and inverse-probability-weighting adjustments to account for the effects of factors other than surgery.
The gastrointestinal cancers meeting, where Dr. Park will present the results, is cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Park reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Surgically removing residual gastrointestinal stromal tumors in patients who respond to imatinib therapy significantly increased time to tumor progression to 88 months, compared with 43 months using imatinib alone, based on findings from a retrospective study of 134 patients.
After controlling for the effects of other risk factors, the surgery decreased threefold the likelihood of disease progression and decreased fivefold the risk of death, Dr. Seong Joon Park reported in a press briefing sponsored by the American Society of Clinical Oncology (ASCO). The press conference was held in advance of at a meeting on gastrointestinal cancers sponsored by ASCO and three other cancer organizations.
The findings support the widely adopted practice of removing residual tumors in these patients, despite the retrospective and observational design of the study, Dr. Park said. A prospective European study of similar design to this one terminated early due to poor patient enrollment. "It’s really hard to conduct a prospective study of this design," said Dr. Park of Asan Medical Center, Seoul, South Korea.
He and his associates reviewed the records of patients who showed at least 6 months of disease stabilization or response to imatinib (Gleevec) treatment, 92 of whom got the drug treatment alone and 42 of whom underwent surgery to remove residual tumors after a median of 19 months of imatinib therapy. The imatinib therapy was restarted after surgery. Median follow-up for the cohort as a whole was 59 months.
"This treatment strategy is worth trying as a clinical practice if the medical center is large enough to have an experienced multidisciplinary team and to have low morbidity and mortality associated with surgery," he said.
Each year, approximately 5,000 new cases of gastrointestinal stromal tumors are diagnosed in the United States, most often in the stomach and small intestine, though they can occur anywhere in or near the GI tract. Imatinib typically is first-line therapy, and 80%-85% of patients will respond to the treatment, he said. A majority of patients who respond to imatinib will have residual tumors, however, which are believed to contribute to the development of drug resistance, leading to the hypothesis that removing the residual tumors would improve survival.
In general, one-third of patients are candidates for surgical removal of residual lesions, depending on the tumor size and other tumor and patient characteristics, Dr. Park said.
The two patient groups in the study were similar except that the surgery group was significantly younger (51 vs. 58 years) and was less likely to have metastases in the peritoneum (41% in the surgery group vs. 61% in the control group).
As it is an aggressive and difficult treatment, surgery is more likely to be considered and recommended to younger patients who have a good performance status and, thus, less likely to be recommended in patients with multiple peritoneal metastases.
Factors associated with longer progression-free and overall survival included surgery and having an initial tumor size less than 150 mm, multivariate analyses showed. Female sex and having the KIT exon 11 mutation also were associated with longer progression-free survival. The researchers used propensity scores and inverse-probability-weighting adjustments to account for the effects of factors other than surgery.
The gastrointestinal cancers meeting, where Dr. Park will present the results, is cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Park reported having no financial disclosures.
On Twitter @sherryboschert
FROM A PRESS BRIEFING SPONSORED BY THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Time to tumor progression was 88 months in 42 patients who had surgery for residual disease and 42 months in 92 patients given imatinib alone. Metastases in the peritoneum were present in 41% of the imatinib-and-surgery group and 61% of the imatinib-only group.
Data Source: Retrospective study of 134 patients with metastatic or recurrent gastrointestinal tumors who showed at least 6 months of disease stabilization or response to imatinib, 42 of whom had residual tumors resected.
Disclosures: Dr. Park reported having no financial disclosures.
Neoadjuvant chemo renders SLN biopsy less reliable
SAN ANTONIO – The results of a sentinel node biopsy performed after neoadjuvant chemotherapy for breast cancer are often unreliable, new data suggest.
Researchers with the German SENTINA trial prospectively analyzed the impact of the timing of sentinel lymph node (SLN) biopsy on the accuracy and feasibility of the procedure in 1,737 patients with early breast cancer who received neoadjuvant chemotherapy.
Patients with initial clinically node-negative disease (cN0) underwent SLN biopsy before chemotherapy. If biopsy results were positive, they underwent biopsy along with an axillary dissection after chemotherapy. Patients with clinically node-positive disease (cN1) underwent chemotherapy followed by SLN biopsy plus axillary dissection if they had a downstaging to clinically node-negative disease.
The SLN biopsy procedure was standardized according to interdisciplinary consensus (Cancer 2005;103:451-61); radiocolloid tracer with lymphoscintigraphy was mandatory for identifying sentinel nodes, whereas blue dye was optional.
Study results showed that the false-negative rate was 51.6% in patients with cN0 disease who had an initial SLN biopsy showing nodal involvement and therefore went on to have another SLN biopsy after the chemotherapy.
SLN biopsy had a false-negative rate of 14.2% when performed after the neoadjuvant chemotherapy in patients with initial cN1 disease who had downstaging to cN0 disease, lead author Dr. Thorsten Kuehn reported at the San Antonio Breast Cancer Symposium.
This rate was up to twice as high as that seen historically in patients instead having the SLN biopsy at the time of primary surgery, which has been on the order of 7%-10%.
"The SLN biopsy as a diagnostic procedure is not a reliable tool in patients who convert on neoadjuvant chemotherapy from cN1 to cN0 compared to SLN biopsy in primary surgery," he said.
Within this downstaged group, data showed that the odds of a false-negative result were reduced by half when patients had more than one sentinel node examined.
"It strikes me that your conclusion should have been, if you take two or more sentinel nodes, this is a safe procedure," one attendee noted during a discussion session. "But if you take less than that, it’s not safe. Is that true?"
The study biopsies were adequate in that all patients had lymphoscintigraphy, contended Dr. Kuehn, who is head of the breast center and chief of the gynecology and obstetrics clinic at Klinikum Esslingen in Germany. "I don’t think you necessarily always have two sentinel nodes – you may have one sentinel node. And every institution or every surgeon had to prove that the sentinel nodes that were shown on lymphoscintigraphy had to be removed."
Another attendee wondered about the extent of residual axillary disease in the patients having false-positive SLN results.
"As a radiation oncologist, I am seeing a lot of patients who have had sentinel node biopsies done after neoadjuvant chemotherapy without an axillary dissection, and I’m being asked to irradiate the patient, which makes me uncomfortable because I don’t know what the volume of disease left is," he explained. "Do you have a sense of what the volume of disease is – micrometastases, macrometastases, number of nodes – in the patients with false-negative sentinel nodes, and do you also have a sense of what that volume is in the axillary dissection specimen outside the true-positive sentinel nodes?"
"We don’t have information on the size of the metastases. We have information on the number of involved non–sentinel nodes, and these numbers are from 1 to 11," Dr. Kuehn replied.
"What we want to say is SLN biopsy is a diagnostic tool, and this tool is worse after previous treatment. I can say I work with this tool, but it is not a good diagnostic tool," he added.
In the study, SLN detection rates, a measure of feasibility, also differed significantly across groups. Surgeons were able to detect these nodes in 99% of all SLN biopsies performed before any treatment, but in only 80% of those performed after a downstaging of disease with the neoadjuvant chemotherapy and in only 61% of the repeated SLN biopsies. The pattern of nodal uptake of the radiocolloid tracer mirrored these findings.
"Previous local and systemic treatment significantly impairs the tracer uptake and the detection rate," concluded Dr. Kuehn, who disclosed no conflicts of interest related to the research.
In a multivariate analysis in the downstaged group, none of a variety of tumor characteristics significantly predicted the ability to detect an SLN.
SAN ANTONIO – The results of a sentinel node biopsy performed after neoadjuvant chemotherapy for breast cancer are often unreliable, new data suggest.
Researchers with the German SENTINA trial prospectively analyzed the impact of the timing of sentinel lymph node (SLN) biopsy on the accuracy and feasibility of the procedure in 1,737 patients with early breast cancer who received neoadjuvant chemotherapy.
Patients with initial clinically node-negative disease (cN0) underwent SLN biopsy before chemotherapy. If biopsy results were positive, they underwent biopsy along with an axillary dissection after chemotherapy. Patients with clinically node-positive disease (cN1) underwent chemotherapy followed by SLN biopsy plus axillary dissection if they had a downstaging to clinically node-negative disease.
The SLN biopsy procedure was standardized according to interdisciplinary consensus (Cancer 2005;103:451-61); radiocolloid tracer with lymphoscintigraphy was mandatory for identifying sentinel nodes, whereas blue dye was optional.
Study results showed that the false-negative rate was 51.6% in patients with cN0 disease who had an initial SLN biopsy showing nodal involvement and therefore went on to have another SLN biopsy after the chemotherapy.
SLN biopsy had a false-negative rate of 14.2% when performed after the neoadjuvant chemotherapy in patients with initial cN1 disease who had downstaging to cN0 disease, lead author Dr. Thorsten Kuehn reported at the San Antonio Breast Cancer Symposium.
This rate was up to twice as high as that seen historically in patients instead having the SLN biopsy at the time of primary surgery, which has been on the order of 7%-10%.
"The SLN biopsy as a diagnostic procedure is not a reliable tool in patients who convert on neoadjuvant chemotherapy from cN1 to cN0 compared to SLN biopsy in primary surgery," he said.
Within this downstaged group, data showed that the odds of a false-negative result were reduced by half when patients had more than one sentinel node examined.
"It strikes me that your conclusion should have been, if you take two or more sentinel nodes, this is a safe procedure," one attendee noted during a discussion session. "But if you take less than that, it’s not safe. Is that true?"
The study biopsies were adequate in that all patients had lymphoscintigraphy, contended Dr. Kuehn, who is head of the breast center and chief of the gynecology and obstetrics clinic at Klinikum Esslingen in Germany. "I don’t think you necessarily always have two sentinel nodes – you may have one sentinel node. And every institution or every surgeon had to prove that the sentinel nodes that were shown on lymphoscintigraphy had to be removed."
Another attendee wondered about the extent of residual axillary disease in the patients having false-positive SLN results.
"As a radiation oncologist, I am seeing a lot of patients who have had sentinel node biopsies done after neoadjuvant chemotherapy without an axillary dissection, and I’m being asked to irradiate the patient, which makes me uncomfortable because I don’t know what the volume of disease left is," he explained. "Do you have a sense of what the volume of disease is – micrometastases, macrometastases, number of nodes – in the patients with false-negative sentinel nodes, and do you also have a sense of what that volume is in the axillary dissection specimen outside the true-positive sentinel nodes?"
"We don’t have information on the size of the metastases. We have information on the number of involved non–sentinel nodes, and these numbers are from 1 to 11," Dr. Kuehn replied.
"What we want to say is SLN biopsy is a diagnostic tool, and this tool is worse after previous treatment. I can say I work with this tool, but it is not a good diagnostic tool," he added.
In the study, SLN detection rates, a measure of feasibility, also differed significantly across groups. Surgeons were able to detect these nodes in 99% of all SLN biopsies performed before any treatment, but in only 80% of those performed after a downstaging of disease with the neoadjuvant chemotherapy and in only 61% of the repeated SLN biopsies. The pattern of nodal uptake of the radiocolloid tracer mirrored these findings.
"Previous local and systemic treatment significantly impairs the tracer uptake and the detection rate," concluded Dr. Kuehn, who disclosed no conflicts of interest related to the research.
In a multivariate analysis in the downstaged group, none of a variety of tumor characteristics significantly predicted the ability to detect an SLN.
SAN ANTONIO – The results of a sentinel node biopsy performed after neoadjuvant chemotherapy for breast cancer are often unreliable, new data suggest.
Researchers with the German SENTINA trial prospectively analyzed the impact of the timing of sentinel lymph node (SLN) biopsy on the accuracy and feasibility of the procedure in 1,737 patients with early breast cancer who received neoadjuvant chemotherapy.
Patients with initial clinically node-negative disease (cN0) underwent SLN biopsy before chemotherapy. If biopsy results were positive, they underwent biopsy along with an axillary dissection after chemotherapy. Patients with clinically node-positive disease (cN1) underwent chemotherapy followed by SLN biopsy plus axillary dissection if they had a downstaging to clinically node-negative disease.
The SLN biopsy procedure was standardized according to interdisciplinary consensus (Cancer 2005;103:451-61); radiocolloid tracer with lymphoscintigraphy was mandatory for identifying sentinel nodes, whereas blue dye was optional.
Study results showed that the false-negative rate was 51.6% in patients with cN0 disease who had an initial SLN biopsy showing nodal involvement and therefore went on to have another SLN biopsy after the chemotherapy.
SLN biopsy had a false-negative rate of 14.2% when performed after the neoadjuvant chemotherapy in patients with initial cN1 disease who had downstaging to cN0 disease, lead author Dr. Thorsten Kuehn reported at the San Antonio Breast Cancer Symposium.
This rate was up to twice as high as that seen historically in patients instead having the SLN biopsy at the time of primary surgery, which has been on the order of 7%-10%.
"The SLN biopsy as a diagnostic procedure is not a reliable tool in patients who convert on neoadjuvant chemotherapy from cN1 to cN0 compared to SLN biopsy in primary surgery," he said.
Within this downstaged group, data showed that the odds of a false-negative result were reduced by half when patients had more than one sentinel node examined.
"It strikes me that your conclusion should have been, if you take two or more sentinel nodes, this is a safe procedure," one attendee noted during a discussion session. "But if you take less than that, it’s not safe. Is that true?"
The study biopsies were adequate in that all patients had lymphoscintigraphy, contended Dr. Kuehn, who is head of the breast center and chief of the gynecology and obstetrics clinic at Klinikum Esslingen in Germany. "I don’t think you necessarily always have two sentinel nodes – you may have one sentinel node. And every institution or every surgeon had to prove that the sentinel nodes that were shown on lymphoscintigraphy had to be removed."
Another attendee wondered about the extent of residual axillary disease in the patients having false-positive SLN results.
"As a radiation oncologist, I am seeing a lot of patients who have had sentinel node biopsies done after neoadjuvant chemotherapy without an axillary dissection, and I’m being asked to irradiate the patient, which makes me uncomfortable because I don’t know what the volume of disease left is," he explained. "Do you have a sense of what the volume of disease is – micrometastases, macrometastases, number of nodes – in the patients with false-negative sentinel nodes, and do you also have a sense of what that volume is in the axillary dissection specimen outside the true-positive sentinel nodes?"
"We don’t have information on the size of the metastases. We have information on the number of involved non–sentinel nodes, and these numbers are from 1 to 11," Dr. Kuehn replied.
"What we want to say is SLN biopsy is a diagnostic tool, and this tool is worse after previous treatment. I can say I work with this tool, but it is not a good diagnostic tool," he added.
In the study, SLN detection rates, a measure of feasibility, also differed significantly across groups. Surgeons were able to detect these nodes in 99% of all SLN biopsies performed before any treatment, but in only 80% of those performed after a downstaging of disease with the neoadjuvant chemotherapy and in only 61% of the repeated SLN biopsies. The pattern of nodal uptake of the radiocolloid tracer mirrored these findings.
"Previous local and systemic treatment significantly impairs the tracer uptake and the detection rate," concluded Dr. Kuehn, who disclosed no conflicts of interest related to the research.
In a multivariate analysis in the downstaged group, none of a variety of tumor characteristics significantly predicted the ability to detect an SLN.
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: The false-negative rates of SLN biopsy were 14% for patients who had a chemotherapy-induced downstaging from cN1 to cN0 disease and 52% for patients with cN0 disease who had an initial SLN biopsy showing nodal involvement and therefore went on to have another SLN biopsy after the chemotherapy.
Data Source: A prospective cohort study of 1,737 patients who received neoadjuvant chemotherapy for early breast cancer (the SENTINA trial).
Disclosures: Dr. Kuehn disclosed no relevant conflicts of interest.
Ki67 in pretreatment breast biopsy predicts prognosis
SAN ANTONIO – Immunohistochemical expression of the proliferation marker Ki67 in a pretreatment biopsy of breast cancer is valid as both a positive predictive marker and a negative prognostic marker of response to neoadjuvant therapy, said Dr. Carsten Denkert, senior pathologist and head of the translational cancer research group at the Institute of Pathology, Charité University Hospital, Berlin.
The findings are based on an analysis of data from the GeparTrio trial of the German Breast Group.
Among the eight molecular subtypes of breast cancer (hormone receptor positive/negative, HER2 positive/negative, and four subtypes created by combining these features), Ki67 was a positive predictive marker in six subtypes and a negative prognostic marker in the three hormone receptor–positive subtypes, Dr. Denkert said at the San Antonio Breast Cancer Symposium.
Across cutpoints for Ki67 positivity ranging from 10% to 45%, women with Ki67-positive tumors were significantly more likely to have a pathologic complete response (pCR) to neoadjuvant chemotherapy, but they also had significantly poorer disease-free and overall survival.
The analysis was unable to identify an optimal cutpoint for positivity, but the "results provide an explanation for the very different cutpoint results in various previous clinical studies," he said. As Ki67 is a continuous marker that measures tumor proliferation, cutpoints can be defined by the scientific community.
In the GeparTrio trial, the 1,166 patients all had tumors measuring at least 2 cm, underwent a core biopsy, received two cycles of neoadjuvant chemotherapy, underwent ultrasound assessment of response, and then received additional cycles of chemotherapy tailored to that response.
The research team led by Dr. Denkert performed immunohistochemical staining for Ki67 in the pretreatment core biopsy using the MIB-1 antibody and systematically assessed 94 cutpoints for Ki67 positivity between 0% and 100% for their association with outcomes.
Results showed that 93 of the cutpoints were significantly associated with pCR, 48 were significantly associated with disease-free survival, and 58 were significantly associated with overall survival. "So it is very difficult to decide which one is the optimal cutpoint," Dr. Denkert commented.
For further analyses, patients were split into three equal groups according to Ki67 cutpoint: low (less than or equal to 15%), intermediate (15.1%-35%), and high (greater than 35%).
In a multivariate analysis, these three groups differed significantly with respect to pCR rate (P less than .0005), median disease-free survival (P = .012), and median overall survival (P = .013).
In a discussion after the presentation, Dr. Steven Vogl of the Montefiore Medical Center, New York, referenced earlier reports from the same trial. "Getting a partial response for some of the hormone receptor–positive tumors was good in terms of prognosis, even though a pCR wasn’t achieved. Did you look at Ki67 in the non-pCR patients and see if that gave you any information as to their prognosis? That is, Ki67 at the time of the final surgical procedure."
"In fact, this was one of the aims of the large Ki67 program that we did on the core biopsies and also on the surgical tissues," Dr. Denkert replied. "So far, we have not yet been able to define Ki67 as a parameter for the effect observed with the hormone receptor–positive tumors in the GeparTrio trial. Very likely, we have to combine this parameter with other parameters, and we are currently working on this."
Although the 12th St. Gallen International Breast Cancer Conference (2011) Expert Panel has proposed that Ki67 may be able to differentiate between the luminal A and luminal B molecular subtypes of breast cancer (Ann. Oncol. 2011;22:1736-47), the optimal cutpoint of the percentage of positive cells for defining a tumor to be Ki67 positive is still debated, he noted.
Dr. Denkert disclosed that he receives research/grant support from Siemens Medical Solutions and Sividon Diagnostics; that he is a consultant for Celgene, Amgen, and Sividon Diagnostics; and that he is a shareholder in Sividon Diagnostics.
hormone receptor positive/negative, HER2 positive/negative, and four subtypes created by combining these features, San Antonio Breast Cancer Symposium, pathologic complete response, pCR, neoadjuvant chemotherapy,
SAN ANTONIO – Immunohistochemical expression of the proliferation marker Ki67 in a pretreatment biopsy of breast cancer is valid as both a positive predictive marker and a negative prognostic marker of response to neoadjuvant therapy, said Dr. Carsten Denkert, senior pathologist and head of the translational cancer research group at the Institute of Pathology, Charité University Hospital, Berlin.
The findings are based on an analysis of data from the GeparTrio trial of the German Breast Group.
Among the eight molecular subtypes of breast cancer (hormone receptor positive/negative, HER2 positive/negative, and four subtypes created by combining these features), Ki67 was a positive predictive marker in six subtypes and a negative prognostic marker in the three hormone receptor–positive subtypes, Dr. Denkert said at the San Antonio Breast Cancer Symposium.
Across cutpoints for Ki67 positivity ranging from 10% to 45%, women with Ki67-positive tumors were significantly more likely to have a pathologic complete response (pCR) to neoadjuvant chemotherapy, but they also had significantly poorer disease-free and overall survival.
The analysis was unable to identify an optimal cutpoint for positivity, but the "results provide an explanation for the very different cutpoint results in various previous clinical studies," he said. As Ki67 is a continuous marker that measures tumor proliferation, cutpoints can be defined by the scientific community.
In the GeparTrio trial, the 1,166 patients all had tumors measuring at least 2 cm, underwent a core biopsy, received two cycles of neoadjuvant chemotherapy, underwent ultrasound assessment of response, and then received additional cycles of chemotherapy tailored to that response.
The research team led by Dr. Denkert performed immunohistochemical staining for Ki67 in the pretreatment core biopsy using the MIB-1 antibody and systematically assessed 94 cutpoints for Ki67 positivity between 0% and 100% for their association with outcomes.
Results showed that 93 of the cutpoints were significantly associated with pCR, 48 were significantly associated with disease-free survival, and 58 were significantly associated with overall survival. "So it is very difficult to decide which one is the optimal cutpoint," Dr. Denkert commented.
For further analyses, patients were split into three equal groups according to Ki67 cutpoint: low (less than or equal to 15%), intermediate (15.1%-35%), and high (greater than 35%).
In a multivariate analysis, these three groups differed significantly with respect to pCR rate (P less than .0005), median disease-free survival (P = .012), and median overall survival (P = .013).
In a discussion after the presentation, Dr. Steven Vogl of the Montefiore Medical Center, New York, referenced earlier reports from the same trial. "Getting a partial response for some of the hormone receptor–positive tumors was good in terms of prognosis, even though a pCR wasn’t achieved. Did you look at Ki67 in the non-pCR patients and see if that gave you any information as to their prognosis? That is, Ki67 at the time of the final surgical procedure."
"In fact, this was one of the aims of the large Ki67 program that we did on the core biopsies and also on the surgical tissues," Dr. Denkert replied. "So far, we have not yet been able to define Ki67 as a parameter for the effect observed with the hormone receptor–positive tumors in the GeparTrio trial. Very likely, we have to combine this parameter with other parameters, and we are currently working on this."
Although the 12th St. Gallen International Breast Cancer Conference (2011) Expert Panel has proposed that Ki67 may be able to differentiate between the luminal A and luminal B molecular subtypes of breast cancer (Ann. Oncol. 2011;22:1736-47), the optimal cutpoint of the percentage of positive cells for defining a tumor to be Ki67 positive is still debated, he noted.
Dr. Denkert disclosed that he receives research/grant support from Siemens Medical Solutions and Sividon Diagnostics; that he is a consultant for Celgene, Amgen, and Sividon Diagnostics; and that he is a shareholder in Sividon Diagnostics.
SAN ANTONIO – Immunohistochemical expression of the proliferation marker Ki67 in a pretreatment biopsy of breast cancer is valid as both a positive predictive marker and a negative prognostic marker of response to neoadjuvant therapy, said Dr. Carsten Denkert, senior pathologist and head of the translational cancer research group at the Institute of Pathology, Charité University Hospital, Berlin.
The findings are based on an analysis of data from the GeparTrio trial of the German Breast Group.
Among the eight molecular subtypes of breast cancer (hormone receptor positive/negative, HER2 positive/negative, and four subtypes created by combining these features), Ki67 was a positive predictive marker in six subtypes and a negative prognostic marker in the three hormone receptor–positive subtypes, Dr. Denkert said at the San Antonio Breast Cancer Symposium.
Across cutpoints for Ki67 positivity ranging from 10% to 45%, women with Ki67-positive tumors were significantly more likely to have a pathologic complete response (pCR) to neoadjuvant chemotherapy, but they also had significantly poorer disease-free and overall survival.
The analysis was unable to identify an optimal cutpoint for positivity, but the "results provide an explanation for the very different cutpoint results in various previous clinical studies," he said. As Ki67 is a continuous marker that measures tumor proliferation, cutpoints can be defined by the scientific community.
In the GeparTrio trial, the 1,166 patients all had tumors measuring at least 2 cm, underwent a core biopsy, received two cycles of neoadjuvant chemotherapy, underwent ultrasound assessment of response, and then received additional cycles of chemotherapy tailored to that response.
The research team led by Dr. Denkert performed immunohistochemical staining for Ki67 in the pretreatment core biopsy using the MIB-1 antibody and systematically assessed 94 cutpoints for Ki67 positivity between 0% and 100% for their association with outcomes.
Results showed that 93 of the cutpoints were significantly associated with pCR, 48 were significantly associated with disease-free survival, and 58 were significantly associated with overall survival. "So it is very difficult to decide which one is the optimal cutpoint," Dr. Denkert commented.
For further analyses, patients were split into three equal groups according to Ki67 cutpoint: low (less than or equal to 15%), intermediate (15.1%-35%), and high (greater than 35%).
In a multivariate analysis, these three groups differed significantly with respect to pCR rate (P less than .0005), median disease-free survival (P = .012), and median overall survival (P = .013).
In a discussion after the presentation, Dr. Steven Vogl of the Montefiore Medical Center, New York, referenced earlier reports from the same trial. "Getting a partial response for some of the hormone receptor–positive tumors was good in terms of prognosis, even though a pCR wasn’t achieved. Did you look at Ki67 in the non-pCR patients and see if that gave you any information as to their prognosis? That is, Ki67 at the time of the final surgical procedure."
"In fact, this was one of the aims of the large Ki67 program that we did on the core biopsies and also on the surgical tissues," Dr. Denkert replied. "So far, we have not yet been able to define Ki67 as a parameter for the effect observed with the hormone receptor–positive tumors in the GeparTrio trial. Very likely, we have to combine this parameter with other parameters, and we are currently working on this."
Although the 12th St. Gallen International Breast Cancer Conference (2011) Expert Panel has proposed that Ki67 may be able to differentiate between the luminal A and luminal B molecular subtypes of breast cancer (Ann. Oncol. 2011;22:1736-47), the optimal cutpoint of the percentage of positive cells for defining a tumor to be Ki67 positive is still debated, he noted.
Dr. Denkert disclosed that he receives research/grant support from Siemens Medical Solutions and Sividon Diagnostics; that he is a consultant for Celgene, Amgen, and Sividon Diagnostics; and that he is a shareholder in Sividon Diagnostics.
hormone receptor positive/negative, HER2 positive/negative, and four subtypes created by combining these features, San Antonio Breast Cancer Symposium, pathologic complete response, pCR, neoadjuvant chemotherapy,
hormone receptor positive/negative, HER2 positive/negative, and four subtypes created by combining these features, San Antonio Breast Cancer Symposium, pathologic complete response, pCR, neoadjuvant chemotherapy,
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: When patients were split into three equal groups according to Ki67 cutpoints of low (less than or equal to 15%), intermediate (15.1%-35%), and high (greater than 35%), the three groups differed significantly with respect to pathologic complete response (P less than .0005), median disease-free survival (P = .012), and median overall survival (P = .013).
Data Source: A retrospective analysis among 1,166 women with early breast cancer who received neoadjuvant chemotherapy (the GeparTrio trial).
Disclosures: Dr. Denkert disclosed that he receives research/grant support from Siemens Medical Solutions and Sividon Diagnostics; that he is a consultant for Celgene, Amgen, and Sividon; and that he is a shareholder in Sividon.