User login
Surgery may be avoided in early rectal cancer
NATIONAL HARBOR, MD. – It may sound like heresy, but select patients with locally advanced rectal cancer may be spared surgery and its associated complications, a cancer surgeon suggested at the annual Society of Surgical Oncology Cancer Symposium.
Approximately 10%-25% of patients with locally advanced rectal cancer will have clinical complete responses (cCR) to neoadjuvant chemotherapy and radiation, said Dr. Philip B. Paty, an attending surgeon in the colorectal surgery service at Memorial Sloan-Kettering Cancer Center in New York City.
"The vast majority of these patients will avoid rectal resection, at least within the first 5 years," Dr. Paty added.
Although local failure occurs in 10%-25% of patients, most of the failures occur within the first 18 months, and most of these cases can be salvaged with R0 resections. Patients treated with nonoperative management appear to have rates of distant recurrence and survival similar to those of patients with pathologic complete responses (pCR) treated with total mesorectal resection, he said.
If surgery is required, local excision may be sufficient for some patients with stage T1 lesions and a select few with T2 lesions, said Dr. Heidi Nelson, professor of surgery in the department of colon and rectal surgery at the Mayo Clinic in Rochester, Minn.
If a patient has a favorable T1 lesion and would otherwise face a life-altering procedure such as abdominal perineal resection (APR) and colostomy, the surgeon should at least show the patient the data and discuss local excision as a safe and effective alternative with results comparable to more extensive resections, she said.
T2 lesions are more problematic, but a select few patients with this tumor type might be spared the morbidity of standard rectal resection, she added.
Hold the surgery?
Dr. Paty noted that, with standard management of stage T3 or T4 rectal cancers, the combination of neoadjuvant chemoradiotherapy, surgery, and adjuvant chemotherapy resulted in a 76% overall survival rate with less than 0.3% local recurrence after 5 years (Ann. Surg. 2005;241:829-36).
"What we have not dwelt on much is the morbidity of surgery, which is very significant. Having a rectal resection is a life-changing event for every patient that has one. Surgeons know that, and patients know that even better than surgeons," he said.
Rectal resections are associated with significant perioperative morbidity, colostomy, altered bowel function, sexual dysfunction, and infertility, he noted.
Pathologic complete responses to neoadjuvant therapy occur in 10%-44% of patients, and patients who have a pCR have markedly better oncologic outcomes than patients with less robust responses.
Of course, pCR can only be determined after surgery, raising the question of whether a clinical CR is sufficient for determining whether a patient might be spared rectal resection.
There are currently more data on pCR in rectal cancer than cCR, "probably because clinical CR criteria right now are quite stringent; we don’t want to not operate on patients who have disease," Dr. Paty said.
His criteria for clinical complete response include a flat mucosa with no nodularity or mass on digital rectal examination. Smooth induration or minor scarring without nodularity is acceptable, "but it has to have a benign feel to it," he said.
In addition, on proctoscopy the mucosa must appear normal and flat, and if a scar is present it should be pale or white in appearance. Alternatively, there can be telangiectasias, he said.
"What’s not clear is whether ulceration is an exclusion criterion [for nonoperative management]. For me it is. Any time I see ulceration I feel there is something ongoing in that tumor that is not resolved and I don’t feel comfortable calling it a complete response," Dr. Paty commented.
Take local route
When surgery is required, local excision rather than total mesorectal resection may suffice, Dr. Nelson said. Suitable patients may be those who are frail or elderly or have limited life expectancy or serious medical conditions that might preclude more extensive surgery.
Tumors that may be good candidates for local excision include smaller lesions (less than 2-3 cm) below the peritoneal reflection that are not amenable to lower anterior resection. The tumors should be subject to full thickness excision, and the team should be able to confirm negative margins, she noted.
Favorable pathologic findings include well-differentiated tumors with the absence of lymphovascular invasion, mucinous features, or signet ring features, she said.
"Local excision really just takes care of the primary, of course. It doesn’t deal with the lymphatics, which is always the hidden game," Dr. Nelson said.
She noted that a 1989 study showed that the likelihood of untreated lymph node disease in patients who had undergone local excision was 0% for patients with T1 tumors, 28% for those with T2 tumors, 36% for T3, and 53% for T4 lesions, showing a significant increase in risk associated with tumor depth (Cancer 1989;63:1421-9).
"If you start tackling anything above a T2 lesion, you’re probably going to be missing lymphatic disease. It’s of relevance because it will form the site of recurrent disease," she said.
For patients with T1 tumors, overall survival is the same, but disease-free survival and local recurrence rates favor standard resection over local excision. "Selection criteria must be much more restrictive when it comes to a T2 lesion," Dr. Nelson said. "I’m pretty reticent to use it in my own practice. I have to really choose the tumor well and choose the patient well to want to do that with some assurance that it’s the right decision."
She pointed to a study published in 2000 comparing patients who underwent either local excision or standard resection for rectal cancer (Dis. Colon Rectum 2000;43:1064-71). Over about 4.5 years of follow-up, local recurrence for patients with T2 lesions was 47% if they had received local excision, compared with 6% for those who had standard resections. Respective overall survival rates were 65% and 81%.
Dr. Paty and Dr. Nelson reported having no financial disclosures.
NATIONAL HARBOR, MD. – It may sound like heresy, but select patients with locally advanced rectal cancer may be spared surgery and its associated complications, a cancer surgeon suggested at the annual Society of Surgical Oncology Cancer Symposium.
Approximately 10%-25% of patients with locally advanced rectal cancer will have clinical complete responses (cCR) to neoadjuvant chemotherapy and radiation, said Dr. Philip B. Paty, an attending surgeon in the colorectal surgery service at Memorial Sloan-Kettering Cancer Center in New York City.
"The vast majority of these patients will avoid rectal resection, at least within the first 5 years," Dr. Paty added.
Although local failure occurs in 10%-25% of patients, most of the failures occur within the first 18 months, and most of these cases can be salvaged with R0 resections. Patients treated with nonoperative management appear to have rates of distant recurrence and survival similar to those of patients with pathologic complete responses (pCR) treated with total mesorectal resection, he said.
If surgery is required, local excision may be sufficient for some patients with stage T1 lesions and a select few with T2 lesions, said Dr. Heidi Nelson, professor of surgery in the department of colon and rectal surgery at the Mayo Clinic in Rochester, Minn.
If a patient has a favorable T1 lesion and would otherwise face a life-altering procedure such as abdominal perineal resection (APR) and colostomy, the surgeon should at least show the patient the data and discuss local excision as a safe and effective alternative with results comparable to more extensive resections, she said.
T2 lesions are more problematic, but a select few patients with this tumor type might be spared the morbidity of standard rectal resection, she added.
Hold the surgery?
Dr. Paty noted that, with standard management of stage T3 or T4 rectal cancers, the combination of neoadjuvant chemoradiotherapy, surgery, and adjuvant chemotherapy resulted in a 76% overall survival rate with less than 0.3% local recurrence after 5 years (Ann. Surg. 2005;241:829-36).
"What we have not dwelt on much is the morbidity of surgery, which is very significant. Having a rectal resection is a life-changing event for every patient that has one. Surgeons know that, and patients know that even better than surgeons," he said.
Rectal resections are associated with significant perioperative morbidity, colostomy, altered bowel function, sexual dysfunction, and infertility, he noted.
Pathologic complete responses to neoadjuvant therapy occur in 10%-44% of patients, and patients who have a pCR have markedly better oncologic outcomes than patients with less robust responses.
Of course, pCR can only be determined after surgery, raising the question of whether a clinical CR is sufficient for determining whether a patient might be spared rectal resection.
There are currently more data on pCR in rectal cancer than cCR, "probably because clinical CR criteria right now are quite stringent; we don’t want to not operate on patients who have disease," Dr. Paty said.
His criteria for clinical complete response include a flat mucosa with no nodularity or mass on digital rectal examination. Smooth induration or minor scarring without nodularity is acceptable, "but it has to have a benign feel to it," he said.
In addition, on proctoscopy the mucosa must appear normal and flat, and if a scar is present it should be pale or white in appearance. Alternatively, there can be telangiectasias, he said.
"What’s not clear is whether ulceration is an exclusion criterion [for nonoperative management]. For me it is. Any time I see ulceration I feel there is something ongoing in that tumor that is not resolved and I don’t feel comfortable calling it a complete response," Dr. Paty commented.
Take local route
When surgery is required, local excision rather than total mesorectal resection may suffice, Dr. Nelson said. Suitable patients may be those who are frail or elderly or have limited life expectancy or serious medical conditions that might preclude more extensive surgery.
Tumors that may be good candidates for local excision include smaller lesions (less than 2-3 cm) below the peritoneal reflection that are not amenable to lower anterior resection. The tumors should be subject to full thickness excision, and the team should be able to confirm negative margins, she noted.
Favorable pathologic findings include well-differentiated tumors with the absence of lymphovascular invasion, mucinous features, or signet ring features, she said.
"Local excision really just takes care of the primary, of course. It doesn’t deal with the lymphatics, which is always the hidden game," Dr. Nelson said.
She noted that a 1989 study showed that the likelihood of untreated lymph node disease in patients who had undergone local excision was 0% for patients with T1 tumors, 28% for those with T2 tumors, 36% for T3, and 53% for T4 lesions, showing a significant increase in risk associated with tumor depth (Cancer 1989;63:1421-9).
"If you start tackling anything above a T2 lesion, you’re probably going to be missing lymphatic disease. It’s of relevance because it will form the site of recurrent disease," she said.
For patients with T1 tumors, overall survival is the same, but disease-free survival and local recurrence rates favor standard resection over local excision. "Selection criteria must be much more restrictive when it comes to a T2 lesion," Dr. Nelson said. "I’m pretty reticent to use it in my own practice. I have to really choose the tumor well and choose the patient well to want to do that with some assurance that it’s the right decision."
She pointed to a study published in 2000 comparing patients who underwent either local excision or standard resection for rectal cancer (Dis. Colon Rectum 2000;43:1064-71). Over about 4.5 years of follow-up, local recurrence for patients with T2 lesions was 47% if they had received local excision, compared with 6% for those who had standard resections. Respective overall survival rates were 65% and 81%.
Dr. Paty and Dr. Nelson reported having no financial disclosures.
NATIONAL HARBOR, MD. – It may sound like heresy, but select patients with locally advanced rectal cancer may be spared surgery and its associated complications, a cancer surgeon suggested at the annual Society of Surgical Oncology Cancer Symposium.
Approximately 10%-25% of patients with locally advanced rectal cancer will have clinical complete responses (cCR) to neoadjuvant chemotherapy and radiation, said Dr. Philip B. Paty, an attending surgeon in the colorectal surgery service at Memorial Sloan-Kettering Cancer Center in New York City.
"The vast majority of these patients will avoid rectal resection, at least within the first 5 years," Dr. Paty added.
Although local failure occurs in 10%-25% of patients, most of the failures occur within the first 18 months, and most of these cases can be salvaged with R0 resections. Patients treated with nonoperative management appear to have rates of distant recurrence and survival similar to those of patients with pathologic complete responses (pCR) treated with total mesorectal resection, he said.
If surgery is required, local excision may be sufficient for some patients with stage T1 lesions and a select few with T2 lesions, said Dr. Heidi Nelson, professor of surgery in the department of colon and rectal surgery at the Mayo Clinic in Rochester, Minn.
If a patient has a favorable T1 lesion and would otherwise face a life-altering procedure such as abdominal perineal resection (APR) and colostomy, the surgeon should at least show the patient the data and discuss local excision as a safe and effective alternative with results comparable to more extensive resections, she said.
T2 lesions are more problematic, but a select few patients with this tumor type might be spared the morbidity of standard rectal resection, she added.
Hold the surgery?
Dr. Paty noted that, with standard management of stage T3 or T4 rectal cancers, the combination of neoadjuvant chemoradiotherapy, surgery, and adjuvant chemotherapy resulted in a 76% overall survival rate with less than 0.3% local recurrence after 5 years (Ann. Surg. 2005;241:829-36).
"What we have not dwelt on much is the morbidity of surgery, which is very significant. Having a rectal resection is a life-changing event for every patient that has one. Surgeons know that, and patients know that even better than surgeons," he said.
Rectal resections are associated with significant perioperative morbidity, colostomy, altered bowel function, sexual dysfunction, and infertility, he noted.
Pathologic complete responses to neoadjuvant therapy occur in 10%-44% of patients, and patients who have a pCR have markedly better oncologic outcomes than patients with less robust responses.
Of course, pCR can only be determined after surgery, raising the question of whether a clinical CR is sufficient for determining whether a patient might be spared rectal resection.
There are currently more data on pCR in rectal cancer than cCR, "probably because clinical CR criteria right now are quite stringent; we don’t want to not operate on patients who have disease," Dr. Paty said.
His criteria for clinical complete response include a flat mucosa with no nodularity or mass on digital rectal examination. Smooth induration or minor scarring without nodularity is acceptable, "but it has to have a benign feel to it," he said.
In addition, on proctoscopy the mucosa must appear normal and flat, and if a scar is present it should be pale or white in appearance. Alternatively, there can be telangiectasias, he said.
"What’s not clear is whether ulceration is an exclusion criterion [for nonoperative management]. For me it is. Any time I see ulceration I feel there is something ongoing in that tumor that is not resolved and I don’t feel comfortable calling it a complete response," Dr. Paty commented.
Take local route
When surgery is required, local excision rather than total mesorectal resection may suffice, Dr. Nelson said. Suitable patients may be those who are frail or elderly or have limited life expectancy or serious medical conditions that might preclude more extensive surgery.
Tumors that may be good candidates for local excision include smaller lesions (less than 2-3 cm) below the peritoneal reflection that are not amenable to lower anterior resection. The tumors should be subject to full thickness excision, and the team should be able to confirm negative margins, she noted.
Favorable pathologic findings include well-differentiated tumors with the absence of lymphovascular invasion, mucinous features, or signet ring features, she said.
"Local excision really just takes care of the primary, of course. It doesn’t deal with the lymphatics, which is always the hidden game," Dr. Nelson said.
She noted that a 1989 study showed that the likelihood of untreated lymph node disease in patients who had undergone local excision was 0% for patients with T1 tumors, 28% for those with T2 tumors, 36% for T3, and 53% for T4 lesions, showing a significant increase in risk associated with tumor depth (Cancer 1989;63:1421-9).
"If you start tackling anything above a T2 lesion, you’re probably going to be missing lymphatic disease. It’s of relevance because it will form the site of recurrent disease," she said.
For patients with T1 tumors, overall survival is the same, but disease-free survival and local recurrence rates favor standard resection over local excision. "Selection criteria must be much more restrictive when it comes to a T2 lesion," Dr. Nelson said. "I’m pretty reticent to use it in my own practice. I have to really choose the tumor well and choose the patient well to want to do that with some assurance that it’s the right decision."
She pointed to a study published in 2000 comparing patients who underwent either local excision or standard resection for rectal cancer (Dis. Colon Rectum 2000;43:1064-71). Over about 4.5 years of follow-up, local recurrence for patients with T2 lesions was 47% if they had received local excision, compared with 6% for those who had standard resections. Respective overall survival rates were 65% and 81%.
Dr. Paty and Dr. Nelson reported having no financial disclosures.
EXPERT ANALYSIS FROM SSO 2013
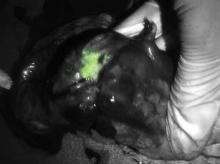
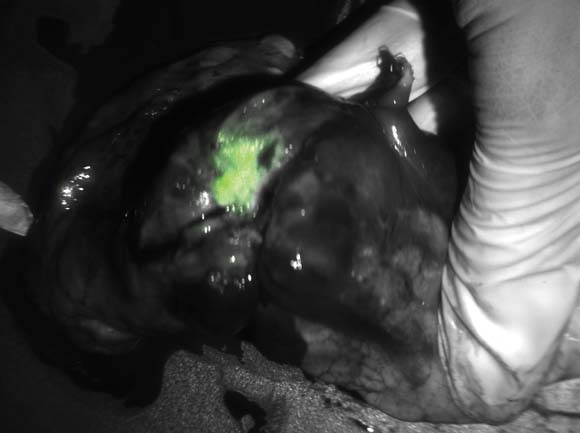
Green glow the tumors during surgery
NATIONAL HARBOR, MD – Seeing is believing, especially when enhanced visualization of tumors during surgery helps improve chances for complete resection, investigators said at the annual Society of Surgical Oncology Cancer Symposium.
With near-infrared (NIR) fluorescence imaging and a portable camera that can be used in an operating room, surgeons can eliminate some of the guesswork involved in identifying involved surgical margins or lymph nodes, researchers from the U.S. and the Netherlands reported in oral and poster sessions.
In early human trials, a small, portable infrared camera has been successful at identifying dye-impregnated tumors – including noncontiguous pockets of malignancy – during surgery to resect squamous cell carcinomas and adenocarcinomas of the lung, reported Dr. Sunil Singhal, of the department of surgery at the University of Pennsylvania, Philadelphia.
"Even in this day and age, surgeons leave behind disease in 40% of the cases, and in about a quarter of those cases the tumor was within two centimeters of where the surgeon was working," Dr. Singhal said.
To improve the odds, he and his colleagues have been investigating optical contrast agents that can be delivered safely to tumors and cause them to fluoresce under light in the near-infrared portion of the spectrum. In preclinical studies with dogs, they found that indocyanine green had the right combination of toxicity, photostability, pharmacokinetics, and cost. The dye, currently used in retinal angiography, has an emission profile that makes it easy for observers to discriminate between the fluorescing dye and blood or tissues, Dr. Singhal said.
They also developed an intraoperative device, dubbed the "FloCam," which consists of a light source and near-infrared (NIR) camera that sits above the patient and sends images to a computer monitor showing the operation in NIR.
In animal studies, the system found evidence of residual disease that was not visible to the naked eye or on x-ray microtomography. On pathologic examination, they saw that the dye was "remarkably precise in delineating margins from normal surrounding tissues," particularly in tumors with neovascular features.
Dr. Singhal said that the imaging technique has been effective at identifying tumor sites during surgery in 36 of 38 patients in early human trials, failing only for 1 patient with melanoma, and for 1 with a sarcoma.
One patient was a 64-year-old nonsmoking man who presented with a cough and was found to have a 2.5 cm right upper lobe lung tumor. Evaluation of the mediastinum with imaging and pathology samples was negative for malignancy, but during surgery, the dye highlighted previously undetected tumor hotspots in the right lower lobe.
"This is another patient who would have gone home [with a diagnosis of] stage 1A. I would have walked down to the recovery room, say ‘I cured you,’ and he would come back 1 year later with metastatic disease and die. This patient, who had minimal disease when we discovered it, got chemotherapy and is still alive at the 1-year mark," Dr. Singhal said.
Green hybrid
In a separate study, investigators in the Netherlands reported on improved intraoperative sentinel node identification and harvesting using a novel hybrid radiopharmaceutical tracer combining indocyanine green with technetium-99m in a nanocolloid suspension.
They found that in 96 patients with malignant melanomas of the head and neck, trunk, or extremities, the hybrid tracer, facilitated both preoperative SPECT/CT imaging and intraoperative radio- and fluorescence-guide sentinel node biopsy in all patients.
"The hybrid tracer was found to be particularly useful for the detection of sentinel nodes in the neck, and for sentinel nodes that failed to accumulate patent blue dye," wrote Dr. Oscar R. Brouwer from the division of nuclear medicine at the Netherlands Cancer Institute in Amsterdam, and colleagues in a scientific poster.
Dr. Singhal’s studies were supported by the Society of Surgical Oncology and the University of Pennsylvania. He reported having no financial disclosures. Dr. Brouwer’s study was supported by the Netherlands Cancer Institute. He reported having no financial disclosures.
NATIONAL HARBOR, MD – Seeing is believing, especially when enhanced visualization of tumors during surgery helps improve chances for complete resection, investigators said at the annual Society of Surgical Oncology Cancer Symposium.
With near-infrared (NIR) fluorescence imaging and a portable camera that can be used in an operating room, surgeons can eliminate some of the guesswork involved in identifying involved surgical margins or lymph nodes, researchers from the U.S. and the Netherlands reported in oral and poster sessions.
In early human trials, a small, portable infrared camera has been successful at identifying dye-impregnated tumors – including noncontiguous pockets of malignancy – during surgery to resect squamous cell carcinomas and adenocarcinomas of the lung, reported Dr. Sunil Singhal, of the department of surgery at the University of Pennsylvania, Philadelphia.
"Even in this day and age, surgeons leave behind disease in 40% of the cases, and in about a quarter of those cases the tumor was within two centimeters of where the surgeon was working," Dr. Singhal said.
To improve the odds, he and his colleagues have been investigating optical contrast agents that can be delivered safely to tumors and cause them to fluoresce under light in the near-infrared portion of the spectrum. In preclinical studies with dogs, they found that indocyanine green had the right combination of toxicity, photostability, pharmacokinetics, and cost. The dye, currently used in retinal angiography, has an emission profile that makes it easy for observers to discriminate between the fluorescing dye and blood or tissues, Dr. Singhal said.
They also developed an intraoperative device, dubbed the "FloCam," which consists of a light source and near-infrared (NIR) camera that sits above the patient and sends images to a computer monitor showing the operation in NIR.
In animal studies, the system found evidence of residual disease that was not visible to the naked eye or on x-ray microtomography. On pathologic examination, they saw that the dye was "remarkably precise in delineating margins from normal surrounding tissues," particularly in tumors with neovascular features.
Dr. Singhal said that the imaging technique has been effective at identifying tumor sites during surgery in 36 of 38 patients in early human trials, failing only for 1 patient with melanoma, and for 1 with a sarcoma.
One patient was a 64-year-old nonsmoking man who presented with a cough and was found to have a 2.5 cm right upper lobe lung tumor. Evaluation of the mediastinum with imaging and pathology samples was negative for malignancy, but during surgery, the dye highlighted previously undetected tumor hotspots in the right lower lobe.
"This is another patient who would have gone home [with a diagnosis of] stage 1A. I would have walked down to the recovery room, say ‘I cured you,’ and he would come back 1 year later with metastatic disease and die. This patient, who had minimal disease when we discovered it, got chemotherapy and is still alive at the 1-year mark," Dr. Singhal said.
Green hybrid
In a separate study, investigators in the Netherlands reported on improved intraoperative sentinel node identification and harvesting using a novel hybrid radiopharmaceutical tracer combining indocyanine green with technetium-99m in a nanocolloid suspension.
They found that in 96 patients with malignant melanomas of the head and neck, trunk, or extremities, the hybrid tracer, facilitated both preoperative SPECT/CT imaging and intraoperative radio- and fluorescence-guide sentinel node biopsy in all patients.
"The hybrid tracer was found to be particularly useful for the detection of sentinel nodes in the neck, and for sentinel nodes that failed to accumulate patent blue dye," wrote Dr. Oscar R. Brouwer from the division of nuclear medicine at the Netherlands Cancer Institute in Amsterdam, and colleagues in a scientific poster.
Dr. Singhal’s studies were supported by the Society of Surgical Oncology and the University of Pennsylvania. He reported having no financial disclosures. Dr. Brouwer’s study was supported by the Netherlands Cancer Institute. He reported having no financial disclosures.
NATIONAL HARBOR, MD – Seeing is believing, especially when enhanced visualization of tumors during surgery helps improve chances for complete resection, investigators said at the annual Society of Surgical Oncology Cancer Symposium.
With near-infrared (NIR) fluorescence imaging and a portable camera that can be used in an operating room, surgeons can eliminate some of the guesswork involved in identifying involved surgical margins or lymph nodes, researchers from the U.S. and the Netherlands reported in oral and poster sessions.
In early human trials, a small, portable infrared camera has been successful at identifying dye-impregnated tumors – including noncontiguous pockets of malignancy – during surgery to resect squamous cell carcinomas and adenocarcinomas of the lung, reported Dr. Sunil Singhal, of the department of surgery at the University of Pennsylvania, Philadelphia.
"Even in this day and age, surgeons leave behind disease in 40% of the cases, and in about a quarter of those cases the tumor was within two centimeters of where the surgeon was working," Dr. Singhal said.
To improve the odds, he and his colleagues have been investigating optical contrast agents that can be delivered safely to tumors and cause them to fluoresce under light in the near-infrared portion of the spectrum. In preclinical studies with dogs, they found that indocyanine green had the right combination of toxicity, photostability, pharmacokinetics, and cost. The dye, currently used in retinal angiography, has an emission profile that makes it easy for observers to discriminate between the fluorescing dye and blood or tissues, Dr. Singhal said.
They also developed an intraoperative device, dubbed the "FloCam," which consists of a light source and near-infrared (NIR) camera that sits above the patient and sends images to a computer monitor showing the operation in NIR.
In animal studies, the system found evidence of residual disease that was not visible to the naked eye or on x-ray microtomography. On pathologic examination, they saw that the dye was "remarkably precise in delineating margins from normal surrounding tissues," particularly in tumors with neovascular features.
Dr. Singhal said that the imaging technique has been effective at identifying tumor sites during surgery in 36 of 38 patients in early human trials, failing only for 1 patient with melanoma, and for 1 with a sarcoma.
One patient was a 64-year-old nonsmoking man who presented with a cough and was found to have a 2.5 cm right upper lobe lung tumor. Evaluation of the mediastinum with imaging and pathology samples was negative for malignancy, but during surgery, the dye highlighted previously undetected tumor hotspots in the right lower lobe.
"This is another patient who would have gone home [with a diagnosis of] stage 1A. I would have walked down to the recovery room, say ‘I cured you,’ and he would come back 1 year later with metastatic disease and die. This patient, who had minimal disease when we discovered it, got chemotherapy and is still alive at the 1-year mark," Dr. Singhal said.
Green hybrid
In a separate study, investigators in the Netherlands reported on improved intraoperative sentinel node identification and harvesting using a novel hybrid radiopharmaceutical tracer combining indocyanine green with technetium-99m in a nanocolloid suspension.
They found that in 96 patients with malignant melanomas of the head and neck, trunk, or extremities, the hybrid tracer, facilitated both preoperative SPECT/CT imaging and intraoperative radio- and fluorescence-guide sentinel node biopsy in all patients.
"The hybrid tracer was found to be particularly useful for the detection of sentinel nodes in the neck, and for sentinel nodes that failed to accumulate patent blue dye," wrote Dr. Oscar R. Brouwer from the division of nuclear medicine at the Netherlands Cancer Institute in Amsterdam, and colleagues in a scientific poster.
Dr. Singhal’s studies were supported by the Society of Surgical Oncology and the University of Pennsylvania. He reported having no financial disclosures. Dr. Brouwer’s study was supported by the Netherlands Cancer Institute. He reported having no financial disclosures.
AT SSO 2013
Major finding: Indocyanine green dye highlights tumors for intraoperative visualization and more complete resection with the aid of a portable near-infrared camera.
Data source: Review of research and early clinical studies in patients with lung tumors; case series investigating the use of a hybrid radio-labeled and fluorescent tracer for evaluating lymph nodes in patients with melanomas.
Disclosures: Dr. Singhal’s studies were supported by the Society of Surgical Oncology and the University of Pennsylvania. He reported having no financial disclosures. Dr. Brouwer's study was supported by the Netherlands Cancer Institute. He reported having no financial disclosures.
Contralateral prophylactic mastectomy adds complications
NATIONAL HARBOR, MD. – The rate of contralateral prophylactic mastectomies is rising, even though there is no evidence for a survival benefit.
From 1998 through 2007, contralateral prophylactic mastectomies (CPM) were performed within 1 year of unilateral mastectomies in 21% of those with ductal carcinoma in situ (DCIS) and in 17% of those with stage I-III breast cancer who were treated at one of 10 National Comprehensive Cancer Network (NCCN) centers.
But in an analysis of overall survival for patients with stages I-III invasive breast cancer, there was so significant difference in overall survival for patients who underwent a CPM, compared with those who underwent only unilateral mastectomy, regardless of whether they had received neoadjuvant chemotherapy, reported Dr. William E. Carson III, professor of surgery at the Ohio State University Comprehensive Cancer Center in Columbus.
In addition, CPMs are associated with a significantly greater risk of complications than unilateral mastectomies, including increased risk for major complications requiring reoperation and rehospitalization, said Dr. Megan Miller, a surgery resident at the University of Chicago, Illinois.
"CPM patients are 1.5 times more likely to have any complication, and 2.6 times more likely to have a major complication than unilateral mastectomy patients," she said at the annual Society of Surgical Oncology Cancer Symposium.
Among patients who underwent CPM, almost 40% of the complications occurred on the side of the body without cancer, she noted.
SSO position statement
A 2007 position statement from the Society of Surgical Oncology (SSO) states that in patients with a current or prior diagnosis, CPM may be indicated for risk reduction in cases where surveillance is difficult or for reconstructive issues such as symmetry and balance, Dr. Carson noted.
Two studies using Surveillance, Epidemiology, and End Results (SEER) data (Tuttle et al. [J. Clin. Oncology 2009;27:1362-7]); Bedrosian et al. [J. Natl. Cancer Inst. 2010;102:401-9]) and one from his own center (Jones et al. [Ann. Surg. Oncol. 2009;16:2691-6]) showed about a 10% increase in the rate of CPM over a decade. Younger women with higher levels of education were more likely to seek CPM.
To see whether this trend extended to NCCN centers, Dr. Carson and his colleagues reviewed data on 1,309 women with DCIS, and 7,044 with stage I-III breast cancer who underwent unilateral mastectomy from 1998 through 2007 at one of 10 designated centers.
In all, 273 of the women diagnosed with DCIS (21%) had a contralateral prophylactic mastectomy, as did 1,199 (17%) of the women with a diagnosis of stage I-III invasive disease. Median follow-up was more than 4 years for both groups.
In a multivariate analysis, factors that significantly predicted the likelihood of CPM included age younger than 50 years, Caucasian race, MRI as the method of detection, and tumor size of 1 cm or smaller. In women with invasive disease, years of education, node-negative status, and no immediate reconstruction were also significant predictors of CPM (P less than .0001 for all variables).
Use of CPM varied widely by institution from 8.2%-34.7% of women with DCIS, and from 3.6%-30.8% of patients with stage I-III disease. As other studies have shown, the use of CPM increased over time, from 15% for DCIS in 1998 to 27% in 2007. For patients with invasive breast cancer, the respective increase was from 8% to 26%. The most pronounced increases were among patients younger than 50 years, Dr. Carson noted.
When they looked at overall survival in a multivariate Cox regression model adjusted for age, race, tumor size, nodal status, tumor grade, histology, and treatment, they found that there was no significant survival advantage for unilateral mastectomy plus CPM, compared with unilateral mastectomy alone.
Complications, complications
Dr. Miller and her colleagues retrospectively reviewed 600 patients who underwent either unilateral mastectomy (391) or CPM (209) at their center from January 2009 through March 2012. They looked at major complications such as seroma or hematoma requiring reoperations, infections requiring hospital admission, total nipple or flap necrosis, and bleeding requiring transfusion; and minor complications such as seromas and hematomas requiring aspiration, infections requiring oral antibiotics, partial nipple or flap necrosis, minor bleeding, and delayed wound healing.
The percentage of patients experiencing any complications was 29% for patients who had a unilateral mastectomy, compared with 42% of those who underwent CPM (P less than .001). Major complications occurred in 4.1% and 14%, respectively (P less than .001). Rates of minor complications were identical between the groups, at 15% each.
Multiple major complications were seen in 4.9% of unilateral patients, compared with 9.1% of CPM patients (P = .043).
Among the CPM patients, 40% of complications occurred on the CPM side.
In a multivariate analysis controlling for age, body mass index, diabetes, previous radiation, smoking history and reconstruction type, CPM was associated with an odds ratio for any complication of 1.5 (P = .029) and 2.6 for major complications (P = .007).
"We believe that patients considering CPM should be made aware of these risks, and certainly more research is needed on patient decision pathways and shared decision making," Dr. Miller said.
Both Dr. Carson’s and Dr. Miller’s studies were internally funded. Dr. Carson disclosed serving on the NCCN Board of Directors. Dr. Miller reported having no financial disclosures.
NATIONAL HARBOR, MD. – The rate of contralateral prophylactic mastectomies is rising, even though there is no evidence for a survival benefit.
From 1998 through 2007, contralateral prophylactic mastectomies (CPM) were performed within 1 year of unilateral mastectomies in 21% of those with ductal carcinoma in situ (DCIS) and in 17% of those with stage I-III breast cancer who were treated at one of 10 National Comprehensive Cancer Network (NCCN) centers.
But in an analysis of overall survival for patients with stages I-III invasive breast cancer, there was so significant difference in overall survival for patients who underwent a CPM, compared with those who underwent only unilateral mastectomy, regardless of whether they had received neoadjuvant chemotherapy, reported Dr. William E. Carson III, professor of surgery at the Ohio State University Comprehensive Cancer Center in Columbus.
In addition, CPMs are associated with a significantly greater risk of complications than unilateral mastectomies, including increased risk for major complications requiring reoperation and rehospitalization, said Dr. Megan Miller, a surgery resident at the University of Chicago, Illinois.
"CPM patients are 1.5 times more likely to have any complication, and 2.6 times more likely to have a major complication than unilateral mastectomy patients," she said at the annual Society of Surgical Oncology Cancer Symposium.
Among patients who underwent CPM, almost 40% of the complications occurred on the side of the body without cancer, she noted.
SSO position statement
A 2007 position statement from the Society of Surgical Oncology (SSO) states that in patients with a current or prior diagnosis, CPM may be indicated for risk reduction in cases where surveillance is difficult or for reconstructive issues such as symmetry and balance, Dr. Carson noted.
Two studies using Surveillance, Epidemiology, and End Results (SEER) data (Tuttle et al. [J. Clin. Oncology 2009;27:1362-7]); Bedrosian et al. [J. Natl. Cancer Inst. 2010;102:401-9]) and one from his own center (Jones et al. [Ann. Surg. Oncol. 2009;16:2691-6]) showed about a 10% increase in the rate of CPM over a decade. Younger women with higher levels of education were more likely to seek CPM.
To see whether this trend extended to NCCN centers, Dr. Carson and his colleagues reviewed data on 1,309 women with DCIS, and 7,044 with stage I-III breast cancer who underwent unilateral mastectomy from 1998 through 2007 at one of 10 designated centers.
In all, 273 of the women diagnosed with DCIS (21%) had a contralateral prophylactic mastectomy, as did 1,199 (17%) of the women with a diagnosis of stage I-III invasive disease. Median follow-up was more than 4 years for both groups.
In a multivariate analysis, factors that significantly predicted the likelihood of CPM included age younger than 50 years, Caucasian race, MRI as the method of detection, and tumor size of 1 cm or smaller. In women with invasive disease, years of education, node-negative status, and no immediate reconstruction were also significant predictors of CPM (P less than .0001 for all variables).
Use of CPM varied widely by institution from 8.2%-34.7% of women with DCIS, and from 3.6%-30.8% of patients with stage I-III disease. As other studies have shown, the use of CPM increased over time, from 15% for DCIS in 1998 to 27% in 2007. For patients with invasive breast cancer, the respective increase was from 8% to 26%. The most pronounced increases were among patients younger than 50 years, Dr. Carson noted.
When they looked at overall survival in a multivariate Cox regression model adjusted for age, race, tumor size, nodal status, tumor grade, histology, and treatment, they found that there was no significant survival advantage for unilateral mastectomy plus CPM, compared with unilateral mastectomy alone.
Complications, complications
Dr. Miller and her colleagues retrospectively reviewed 600 patients who underwent either unilateral mastectomy (391) or CPM (209) at their center from January 2009 through March 2012. They looked at major complications such as seroma or hematoma requiring reoperations, infections requiring hospital admission, total nipple or flap necrosis, and bleeding requiring transfusion; and minor complications such as seromas and hematomas requiring aspiration, infections requiring oral antibiotics, partial nipple or flap necrosis, minor bleeding, and delayed wound healing.
The percentage of patients experiencing any complications was 29% for patients who had a unilateral mastectomy, compared with 42% of those who underwent CPM (P less than .001). Major complications occurred in 4.1% and 14%, respectively (P less than .001). Rates of minor complications were identical between the groups, at 15% each.
Multiple major complications were seen in 4.9% of unilateral patients, compared with 9.1% of CPM patients (P = .043).
Among the CPM patients, 40% of complications occurred on the CPM side.
In a multivariate analysis controlling for age, body mass index, diabetes, previous radiation, smoking history and reconstruction type, CPM was associated with an odds ratio for any complication of 1.5 (P = .029) and 2.6 for major complications (P = .007).
"We believe that patients considering CPM should be made aware of these risks, and certainly more research is needed on patient decision pathways and shared decision making," Dr. Miller said.
Both Dr. Carson’s and Dr. Miller’s studies were internally funded. Dr. Carson disclosed serving on the NCCN Board of Directors. Dr. Miller reported having no financial disclosures.
NATIONAL HARBOR, MD. – The rate of contralateral prophylactic mastectomies is rising, even though there is no evidence for a survival benefit.
From 1998 through 2007, contralateral prophylactic mastectomies (CPM) were performed within 1 year of unilateral mastectomies in 21% of those with ductal carcinoma in situ (DCIS) and in 17% of those with stage I-III breast cancer who were treated at one of 10 National Comprehensive Cancer Network (NCCN) centers.
But in an analysis of overall survival for patients with stages I-III invasive breast cancer, there was so significant difference in overall survival for patients who underwent a CPM, compared with those who underwent only unilateral mastectomy, regardless of whether they had received neoadjuvant chemotherapy, reported Dr. William E. Carson III, professor of surgery at the Ohio State University Comprehensive Cancer Center in Columbus.
In addition, CPMs are associated with a significantly greater risk of complications than unilateral mastectomies, including increased risk for major complications requiring reoperation and rehospitalization, said Dr. Megan Miller, a surgery resident at the University of Chicago, Illinois.
"CPM patients are 1.5 times more likely to have any complication, and 2.6 times more likely to have a major complication than unilateral mastectomy patients," she said at the annual Society of Surgical Oncology Cancer Symposium.
Among patients who underwent CPM, almost 40% of the complications occurred on the side of the body without cancer, she noted.
SSO position statement
A 2007 position statement from the Society of Surgical Oncology (SSO) states that in patients with a current or prior diagnosis, CPM may be indicated for risk reduction in cases where surveillance is difficult or for reconstructive issues such as symmetry and balance, Dr. Carson noted.
Two studies using Surveillance, Epidemiology, and End Results (SEER) data (Tuttle et al. [J. Clin. Oncology 2009;27:1362-7]); Bedrosian et al. [J. Natl. Cancer Inst. 2010;102:401-9]) and one from his own center (Jones et al. [Ann. Surg. Oncol. 2009;16:2691-6]) showed about a 10% increase in the rate of CPM over a decade. Younger women with higher levels of education were more likely to seek CPM.
To see whether this trend extended to NCCN centers, Dr. Carson and his colleagues reviewed data on 1,309 women with DCIS, and 7,044 with stage I-III breast cancer who underwent unilateral mastectomy from 1998 through 2007 at one of 10 designated centers.
In all, 273 of the women diagnosed with DCIS (21%) had a contralateral prophylactic mastectomy, as did 1,199 (17%) of the women with a diagnosis of stage I-III invasive disease. Median follow-up was more than 4 years for both groups.
In a multivariate analysis, factors that significantly predicted the likelihood of CPM included age younger than 50 years, Caucasian race, MRI as the method of detection, and tumor size of 1 cm or smaller. In women with invasive disease, years of education, node-negative status, and no immediate reconstruction were also significant predictors of CPM (P less than .0001 for all variables).
Use of CPM varied widely by institution from 8.2%-34.7% of women with DCIS, and from 3.6%-30.8% of patients with stage I-III disease. As other studies have shown, the use of CPM increased over time, from 15% for DCIS in 1998 to 27% in 2007. For patients with invasive breast cancer, the respective increase was from 8% to 26%. The most pronounced increases were among patients younger than 50 years, Dr. Carson noted.
When they looked at overall survival in a multivariate Cox regression model adjusted for age, race, tumor size, nodal status, tumor grade, histology, and treatment, they found that there was no significant survival advantage for unilateral mastectomy plus CPM, compared with unilateral mastectomy alone.
Complications, complications
Dr. Miller and her colleagues retrospectively reviewed 600 patients who underwent either unilateral mastectomy (391) or CPM (209) at their center from January 2009 through March 2012. They looked at major complications such as seroma or hematoma requiring reoperations, infections requiring hospital admission, total nipple or flap necrosis, and bleeding requiring transfusion; and minor complications such as seromas and hematomas requiring aspiration, infections requiring oral antibiotics, partial nipple or flap necrosis, minor bleeding, and delayed wound healing.
The percentage of patients experiencing any complications was 29% for patients who had a unilateral mastectomy, compared with 42% of those who underwent CPM (P less than .001). Major complications occurred in 4.1% and 14%, respectively (P less than .001). Rates of minor complications were identical between the groups, at 15% each.
Multiple major complications were seen in 4.9% of unilateral patients, compared with 9.1% of CPM patients (P = .043).
Among the CPM patients, 40% of complications occurred on the CPM side.
In a multivariate analysis controlling for age, body mass index, diabetes, previous radiation, smoking history and reconstruction type, CPM was associated with an odds ratio for any complication of 1.5 (P = .029) and 2.6 for major complications (P = .007).
"We believe that patients considering CPM should be made aware of these risks, and certainly more research is needed on patient decision pathways and shared decision making," Dr. Miller said.
Both Dr. Carson’s and Dr. Miller’s studies were internally funded. Dr. Carson disclosed serving on the NCCN Board of Directors. Dr. Miller reported having no financial disclosures.
AT SSO 2013
Major finding: The percentage of patients experiencing any complications was 29% for patients who had a unilateral mastectomy, compared with 42% of those who also underwent a contralateral prophylactic mastectomy (P less than .001).
Data source: Retrospective studies of data on patients with breast cancer treated at 10 NCI-designated comprehensive cancer centers and at a single institution.
Disclosures: Both Dr. Carson’s and Dr. Milller’s studies were internally funded. Dr. Carson disclosed serving on the NCCN Board of Directors. Dr. Miller reported having no financial disclosures.
Guideline nonadherence linked to increased ovarian cancer deaths
LOS ANGELES – Guideline-adherent treatment can make the difference between life and death in patients with ovarian cancer, and it often hinges on where and from whom patients receive care, new data suggest.
In a retrospective, population-based study of more than 13,000 patients with epithelial ovarian cancer, only about 40% of patients received treatment adhering to that recommended by the National Comprehensive Cancer Network (NCCN).
Patients were more likely to receive guideline-adherent treatment if they went to high-volume hospitals (those treating at least 20 such patients each year) and high-volume physicians (those treating at least 10 such patients each year), according to results reported at the annual meeting of the Society of Gynecologic Oncology*. Still, in absolute terms, only about half of patients treated in high-volume hospitals or by high-volume surgeons received adherent treatment.
Compared with their counterparts who received guideline-adherent treatment, patients who received nonadherent treatment had a 33% higher risk of dying from their disease in the subsequent 5 years.
"NCCN guideline adherence predicts improved survival," lead investigator Dr. Robert E. Bristow commented in an interview. "A minority of patients is getting access to guideline care, and increased efforts to direct ovarian cancer patients to high-volume providers are warranted."
From a population-based perspective, much greater gains in survival can be achieved by centralizing ovarian cancer care to gynecologic oncologists and high-volume hospitals than through new chemotherapy drugs or experimental treatments, according to Dr. Bristow, who is director of the division of gynecologic oncology at the University of California, Irvine, medical center. The success of this model "has been demonstrated in Norway, where nonaccredited providers are not paid for any ovarian cancer care they deliver."
That said, the data cannot be used to discern the reasons for the overall poor rate of guideline adherence.
"In population-based data sets, you don’t have the granularity of data to tease out the nuances that might contribute to risk, like an infirm 85-year-old woman who can’t tolerate major surgery and aggressive surgery. We were not able to control for that," Dr. Bristow noted. Yet "only about 20% of patients had access to high-volume providers, and since high-volume providers are more likely to deliver appropriate care, the lack of access to these physicians and hospitals is probably the biggest reason (for nonadherence). By ensuring that we do everything possible to get ovarian cancer patients to the physicians and centers that are best equipped to take care of them, we will maximize each patient’s chance for the best possible outcome."
Analyses were based on 13,321 patients with epithelial ovarian cancer having data in the California Cancer Registry for the years 1999 through 2006. They had a median age of 61 years; 70% had stage III or IV disease, and 42% had serous tumor histology.
Among patients having data on these measures, 81% were treated at low-volume hospitals and 79% by low-volume surgeons. In multivariate analyses, patients were significantly more likely to receive nonadherent treatment if they were treated in low-volume hospitals (odds ratio, 1.83) or by low-volume physicians (OR, 1.19).
Overall, 37% of the patients received treatment recommended by NCCN guidelines. The 5-year disease-specific survival rate was 45% for the cohort overall. In multivariate analyses, patients had significantly higher odds of ovarian cancer death if they received nonadherent treatment (hazard ratio, 1.33), and if they were treated at a low-volume hospital (HR, 1.08) or by a low-volume physician (HR, 1.18).
"We are in the infancy of defining quality care for ovarian cancer," concluded Dr. Bristow. "We need to develop risk-adjusted models for comparison, to make sure we are comparing apples to apples, so to speak. We need to become more sophisticated in our measurement and reporting. Ideally, one day, everyone’s quality performance measures will be publicly available and patients and payers can choose for themselves where to go for care."
The investigators plan future research on such models and on universal reporting requirements. "There are also critical issues of racial and socioeconomic disparities in ovarian cancer care and outcomes that we are investigating," he said.
Dr. Bristow disclosed no relevant conflicts of interest.
Correction, 3/28/2013: An earlier version of this story misstated the name of the Society of Gynecologic Oncology.

|
|
Dr. Maurie Markman comments:
The report from Bristow, et al., is provocative and raises reasonable
questions regarding the quality of care provided to patients with
ovarian cancer. However, it is critical to acknowledge that while this
report suggests an association between "guideline adherence" and
clinical outcome, it does not in any way demonstrate the inferior
outcome actually resulted from the lack of guideline adherence. For
example, it is highly likely that patients with more advanced disease or
with clinically relevant co-morbidity were less likely to
undergo primary cytoreductive surgery, and these factors are known to be
independently associated with inferior survival. Large databases, as
employed in this analysis, will almost certainly be unable to capture
these clinical factors (for example, performance status, presence of
massive ascites, or large-volume pleural effusion) that will influence
both the decision to perform surgery and the survival outcome.
Therefore, while this study requires follow-up evaluation, it would be
premature to believe outcomes would improve simply because of physician
adherence to a declared "guideline." In fact, inappropriate adherence
that goes against a physician’s clinical judgment may result in a worse
outcome for an individual patient.
Dr. Markman is the senior
vice president of clinical affairs and national director of medical
oncology for the Cancer Treatment Centers of America.

|
|
Dr. Maurie Markman comments:
The report from Bristow, et al., is provocative and raises reasonable
questions regarding the quality of care provided to patients with
ovarian cancer. However, it is critical to acknowledge that while this
report suggests an association between "guideline adherence" and
clinical outcome, it does not in any way demonstrate the inferior
outcome actually resulted from the lack of guideline adherence. For
example, it is highly likely that patients with more advanced disease or
with clinically relevant co-morbidity were less likely to
undergo primary cytoreductive surgery, and these factors are known to be
independently associated with inferior survival. Large databases, as
employed in this analysis, will almost certainly be unable to capture
these clinical factors (for example, performance status, presence of
massive ascites, or large-volume pleural effusion) that will influence
both the decision to perform surgery and the survival outcome.
Therefore, while this study requires follow-up evaluation, it would be
premature to believe outcomes would improve simply because of physician
adherence to a declared "guideline." In fact, inappropriate adherence
that goes against a physician’s clinical judgment may result in a worse
outcome for an individual patient.
Dr. Markman is the senior
vice president of clinical affairs and national director of medical
oncology for the Cancer Treatment Centers of America.

|
|
Dr. Maurie Markman comments:
The report from Bristow, et al., is provocative and raises reasonable
questions regarding the quality of care provided to patients with
ovarian cancer. However, it is critical to acknowledge that while this
report suggests an association between "guideline adherence" and
clinical outcome, it does not in any way demonstrate the inferior
outcome actually resulted from the lack of guideline adherence. For
example, it is highly likely that patients with more advanced disease or
with clinically relevant co-morbidity were less likely to
undergo primary cytoreductive surgery, and these factors are known to be
independently associated with inferior survival. Large databases, as
employed in this analysis, will almost certainly be unable to capture
these clinical factors (for example, performance status, presence of
massive ascites, or large-volume pleural effusion) that will influence
both the decision to perform surgery and the survival outcome.
Therefore, while this study requires follow-up evaluation, it would be
premature to believe outcomes would improve simply because of physician
adherence to a declared "guideline." In fact, inappropriate adherence
that goes against a physician’s clinical judgment may result in a worse
outcome for an individual patient.
Dr. Markman is the senior
vice president of clinical affairs and national director of medical
oncology for the Cancer Treatment Centers of America.
LOS ANGELES – Guideline-adherent treatment can make the difference between life and death in patients with ovarian cancer, and it often hinges on where and from whom patients receive care, new data suggest.
In a retrospective, population-based study of more than 13,000 patients with epithelial ovarian cancer, only about 40% of patients received treatment adhering to that recommended by the National Comprehensive Cancer Network (NCCN).
Patients were more likely to receive guideline-adherent treatment if they went to high-volume hospitals (those treating at least 20 such patients each year) and high-volume physicians (those treating at least 10 such patients each year), according to results reported at the annual meeting of the Society of Gynecologic Oncology*. Still, in absolute terms, only about half of patients treated in high-volume hospitals or by high-volume surgeons received adherent treatment.
Compared with their counterparts who received guideline-adherent treatment, patients who received nonadherent treatment had a 33% higher risk of dying from their disease in the subsequent 5 years.
"NCCN guideline adherence predicts improved survival," lead investigator Dr. Robert E. Bristow commented in an interview. "A minority of patients is getting access to guideline care, and increased efforts to direct ovarian cancer patients to high-volume providers are warranted."
From a population-based perspective, much greater gains in survival can be achieved by centralizing ovarian cancer care to gynecologic oncologists and high-volume hospitals than through new chemotherapy drugs or experimental treatments, according to Dr. Bristow, who is director of the division of gynecologic oncology at the University of California, Irvine, medical center. The success of this model "has been demonstrated in Norway, where nonaccredited providers are not paid for any ovarian cancer care they deliver."
That said, the data cannot be used to discern the reasons for the overall poor rate of guideline adherence.
"In population-based data sets, you don’t have the granularity of data to tease out the nuances that might contribute to risk, like an infirm 85-year-old woman who can’t tolerate major surgery and aggressive surgery. We were not able to control for that," Dr. Bristow noted. Yet "only about 20% of patients had access to high-volume providers, and since high-volume providers are more likely to deliver appropriate care, the lack of access to these physicians and hospitals is probably the biggest reason (for nonadherence). By ensuring that we do everything possible to get ovarian cancer patients to the physicians and centers that are best equipped to take care of them, we will maximize each patient’s chance for the best possible outcome."
Analyses were based on 13,321 patients with epithelial ovarian cancer having data in the California Cancer Registry for the years 1999 through 2006. They had a median age of 61 years; 70% had stage III or IV disease, and 42% had serous tumor histology.
Among patients having data on these measures, 81% were treated at low-volume hospitals and 79% by low-volume surgeons. In multivariate analyses, patients were significantly more likely to receive nonadherent treatment if they were treated in low-volume hospitals (odds ratio, 1.83) or by low-volume physicians (OR, 1.19).
Overall, 37% of the patients received treatment recommended by NCCN guidelines. The 5-year disease-specific survival rate was 45% for the cohort overall. In multivariate analyses, patients had significantly higher odds of ovarian cancer death if they received nonadherent treatment (hazard ratio, 1.33), and if they were treated at a low-volume hospital (HR, 1.08) or by a low-volume physician (HR, 1.18).
"We are in the infancy of defining quality care for ovarian cancer," concluded Dr. Bristow. "We need to develop risk-adjusted models for comparison, to make sure we are comparing apples to apples, so to speak. We need to become more sophisticated in our measurement and reporting. Ideally, one day, everyone’s quality performance measures will be publicly available and patients and payers can choose for themselves where to go for care."
The investigators plan future research on such models and on universal reporting requirements. "There are also critical issues of racial and socioeconomic disparities in ovarian cancer care and outcomes that we are investigating," he said.
Dr. Bristow disclosed no relevant conflicts of interest.
Correction, 3/28/2013: An earlier version of this story misstated the name of the Society of Gynecologic Oncology.
LOS ANGELES – Guideline-adherent treatment can make the difference between life and death in patients with ovarian cancer, and it often hinges on where and from whom patients receive care, new data suggest.
In a retrospective, population-based study of more than 13,000 patients with epithelial ovarian cancer, only about 40% of patients received treatment adhering to that recommended by the National Comprehensive Cancer Network (NCCN).
Patients were more likely to receive guideline-adherent treatment if they went to high-volume hospitals (those treating at least 20 such patients each year) and high-volume physicians (those treating at least 10 such patients each year), according to results reported at the annual meeting of the Society of Gynecologic Oncology*. Still, in absolute terms, only about half of patients treated in high-volume hospitals or by high-volume surgeons received adherent treatment.
Compared with their counterparts who received guideline-adherent treatment, patients who received nonadherent treatment had a 33% higher risk of dying from their disease in the subsequent 5 years.
"NCCN guideline adherence predicts improved survival," lead investigator Dr. Robert E. Bristow commented in an interview. "A minority of patients is getting access to guideline care, and increased efforts to direct ovarian cancer patients to high-volume providers are warranted."
From a population-based perspective, much greater gains in survival can be achieved by centralizing ovarian cancer care to gynecologic oncologists and high-volume hospitals than through new chemotherapy drugs or experimental treatments, according to Dr. Bristow, who is director of the division of gynecologic oncology at the University of California, Irvine, medical center. The success of this model "has been demonstrated in Norway, where nonaccredited providers are not paid for any ovarian cancer care they deliver."
That said, the data cannot be used to discern the reasons for the overall poor rate of guideline adherence.
"In population-based data sets, you don’t have the granularity of data to tease out the nuances that might contribute to risk, like an infirm 85-year-old woman who can’t tolerate major surgery and aggressive surgery. We were not able to control for that," Dr. Bristow noted. Yet "only about 20% of patients had access to high-volume providers, and since high-volume providers are more likely to deliver appropriate care, the lack of access to these physicians and hospitals is probably the biggest reason (for nonadherence). By ensuring that we do everything possible to get ovarian cancer patients to the physicians and centers that are best equipped to take care of them, we will maximize each patient’s chance for the best possible outcome."
Analyses were based on 13,321 patients with epithelial ovarian cancer having data in the California Cancer Registry for the years 1999 through 2006. They had a median age of 61 years; 70% had stage III or IV disease, and 42% had serous tumor histology.
Among patients having data on these measures, 81% were treated at low-volume hospitals and 79% by low-volume surgeons. In multivariate analyses, patients were significantly more likely to receive nonadherent treatment if they were treated in low-volume hospitals (odds ratio, 1.83) or by low-volume physicians (OR, 1.19).
Overall, 37% of the patients received treatment recommended by NCCN guidelines. The 5-year disease-specific survival rate was 45% for the cohort overall. In multivariate analyses, patients had significantly higher odds of ovarian cancer death if they received nonadherent treatment (hazard ratio, 1.33), and if they were treated at a low-volume hospital (HR, 1.08) or by a low-volume physician (HR, 1.18).
"We are in the infancy of defining quality care for ovarian cancer," concluded Dr. Bristow. "We need to develop risk-adjusted models for comparison, to make sure we are comparing apples to apples, so to speak. We need to become more sophisticated in our measurement and reporting. Ideally, one day, everyone’s quality performance measures will be publicly available and patients and payers can choose for themselves where to go for care."
The investigators plan future research on such models and on universal reporting requirements. "There are also critical issues of racial and socioeconomic disparities in ovarian cancer care and outcomes that we are investigating," he said.
Dr. Bristow disclosed no relevant conflicts of interest.
Correction, 3/28/2013: An earlier version of this story misstated the name of the Society of Gynecologic Oncology.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Major finding: In multivariate analyses, patients had significantly higher odds of ovarian cancer death if they received nonadherent treatment (hazard ratio, 1.33), and if they were treated at a low-volume hospital (HR, 1.08) or by a low-volume physician (HR, 1.18).
Data source: A retrospective population-based cohort study of 13,321 patients with epithelial ovarian cancer from the California Cancer Registry
Disclosures: Dr. Bristow disclosed no relevant conflicts of interest.
Breast cancer: Cardiac risk increases with radiation dose to heart
The risk of major ischemic coronary events was significantly and proportionately associated with the estimated mean radiation dose to the heart in a study of women in Sweden and Denmark who received radiotherapy for breast cancer over a 43-year period.
"The risk of a major coronary event increased linearly with the mean dose to the heart," reported Sarah Darby, Ph.D., of the University of Oxford (England), and her associates. The risk began to increase within the first 5 years of treatment and continued to increase for at least 20 years.
The findings make it possible for a woman to estimate her absolute risk of radiation-related ischemic heart disease, the authors wrote. "This absolute risk can be weighed against the probable absolute reduction in her risk of recurrence or death from breast cancer that would be achieved without radiotherapy" (N. Engl. J. Med. 2013;368:987-98 [doi: 10.1056/NEJMoa1209825]).
The population-based study included 2,168 women who had been treated with external-beam radiation for invasive breast cancer between 1958 and 2001, and were enrolled in the Swedish National Cancer Register or the Danish Breast Cancer Cooperative Group. The 963 women who were subsequently diagnosed with a major coronary event (myocardial infarction, coronary revascularization, or death from ischemic heart disease, but not angina) were compared with 1,205 controls.
The major coronary events were diagnosed in the first decade after breast cancer diagnosis in 44% of patients; 33% of events were diagnosed 10-19 years after breast cancer diagnosis; and 23% occurred 20 or more years later. Of the cases, 54% died of ischemic heart disease.
The estimated mean radiation dose to the heart overall was 4.9 Gy (range, 0.03-27.72 Gy). For those with cancer in their left breast, the mean dose exposure to the heart was 6.6 Gy; for those with right-breast tumors, it was 2.9 Gy. Major coronary events were significantly higher among the women with radiation to the left breast.
The estimated dose to the heart of women who are currently treated with radiotherapy ranges from 1 to 5 Gy, the authors said.
For each 1-Gy increase in the mean dose of radiation to the heart, the rate of major coronary events increased by 7.4%, which was a highly statistically significant finding. Compared with controls who had no cardiac dose, the rate of major coronary events increased by 10% among those exposed to a mean radiation dose of less than 2 Gy, by 30% among those exposed to 2-4 Gy, by 40% among those exposed to 5-9 Gy, and by 116% in those exposed to 10 Gy or more.
Among women with a history of ischemic heart disease, the risk of major coronary events was almost sevenfold higher than it was in women with no history of ischemic heart disease. This risk was increased by about 13-fold during the first 10 years after treatment and was about twofold higher in later years.
"Absolute increases in risk for a given dose to the heart were larger for women with preexisting risk factors," they wrote, so "clinicians may wish to consider cardiac dose and cardiac risk factors as well as tumor control when making decisions about the use of radiotherapy for breast cancer."
Among the strengths of the study was that the analysis included all women who were documented as having received radiotherapy for breast cancer in the two countries during the time period studied. The authors cautioned against applying the results to breast cancer patients who are treated before age 30 because few women in this age group were included in the study.
The study was supported by the Oxford University Clinical Trial Service Unit from Cancer Research UK, the British Heart Foundation, and the UK Medical Research Council.
While the results of the study are interesting, they likely overestimate the risk of coronary events associated with contemporary radiation therapy. Of greater concern, the findings could be misinterpreted and could deter women from having potentially lifesaving treatment.
Increases in the rate of major coronary events – about 20% higher per 1 Gy – were far greater among the women diagnosed in the 1980s, who drive much of the increase in risk. There was barely an increase in the rate of events – 0.85% per 1 Gy – among those diagnosed in the 1990s, for example.
Most of the data are taken from a time when radiation was administered by techniques that differ from those used today, which are associated with a lot less scatter to the heart. Three-dimensional, CT-based planning was not used for the women in the study, which the authors acknowledged was a limitation. Also, the dose was estimated using radiotherapy charts, which are notoriously inaccurate.
Further, there is now a better understanding of which patients are likely to have a survival benefit from radiation. Correctly targeted radiation therapy plays an incredibly important role in the excellent results we see today, with the majority of breast cancer patients surviving their disease.
Dr. Hope Rugo is professor of medicine at the University of California, San Francisco, and director of breast oncology and clinical trials education at the UCSF Helen Diller Family Comprehensive Cancer Center. She had no relevant financial disclosures.
While the results of the study are interesting, they likely overestimate the risk of coronary events associated with contemporary radiation therapy. Of greater concern, the findings could be misinterpreted and could deter women from having potentially lifesaving treatment.
Increases in the rate of major coronary events – about 20% higher per 1 Gy – were far greater among the women diagnosed in the 1980s, who drive much of the increase in risk. There was barely an increase in the rate of events – 0.85% per 1 Gy – among those diagnosed in the 1990s, for example.
Most of the data are taken from a time when radiation was administered by techniques that differ from those used today, which are associated with a lot less scatter to the heart. Three-dimensional, CT-based planning was not used for the women in the study, which the authors acknowledged was a limitation. Also, the dose was estimated using radiotherapy charts, which are notoriously inaccurate.
Further, there is now a better understanding of which patients are likely to have a survival benefit from radiation. Correctly targeted radiation therapy plays an incredibly important role in the excellent results we see today, with the majority of breast cancer patients surviving their disease.
Dr. Hope Rugo is professor of medicine at the University of California, San Francisco, and director of breast oncology and clinical trials education at the UCSF Helen Diller Family Comprehensive Cancer Center. She had no relevant financial disclosures.
While the results of the study are interesting, they likely overestimate the risk of coronary events associated with contemporary radiation therapy. Of greater concern, the findings could be misinterpreted and could deter women from having potentially lifesaving treatment.
Increases in the rate of major coronary events – about 20% higher per 1 Gy – were far greater among the women diagnosed in the 1980s, who drive much of the increase in risk. There was barely an increase in the rate of events – 0.85% per 1 Gy – among those diagnosed in the 1990s, for example.
Most of the data are taken from a time when radiation was administered by techniques that differ from those used today, which are associated with a lot less scatter to the heart. Three-dimensional, CT-based planning was not used for the women in the study, which the authors acknowledged was a limitation. Also, the dose was estimated using radiotherapy charts, which are notoriously inaccurate.
Further, there is now a better understanding of which patients are likely to have a survival benefit from radiation. Correctly targeted radiation therapy plays an incredibly important role in the excellent results we see today, with the majority of breast cancer patients surviving their disease.
Dr. Hope Rugo is professor of medicine at the University of California, San Francisco, and director of breast oncology and clinical trials education at the UCSF Helen Diller Family Comprehensive Cancer Center. She had no relevant financial disclosures.
The risk of major ischemic coronary events was significantly and proportionately associated with the estimated mean radiation dose to the heart in a study of women in Sweden and Denmark who received radiotherapy for breast cancer over a 43-year period.
"The risk of a major coronary event increased linearly with the mean dose to the heart," reported Sarah Darby, Ph.D., of the University of Oxford (England), and her associates. The risk began to increase within the first 5 years of treatment and continued to increase for at least 20 years.
The findings make it possible for a woman to estimate her absolute risk of radiation-related ischemic heart disease, the authors wrote. "This absolute risk can be weighed against the probable absolute reduction in her risk of recurrence or death from breast cancer that would be achieved without radiotherapy" (N. Engl. J. Med. 2013;368:987-98 [doi: 10.1056/NEJMoa1209825]).
The population-based study included 2,168 women who had been treated with external-beam radiation for invasive breast cancer between 1958 and 2001, and were enrolled in the Swedish National Cancer Register or the Danish Breast Cancer Cooperative Group. The 963 women who were subsequently diagnosed with a major coronary event (myocardial infarction, coronary revascularization, or death from ischemic heart disease, but not angina) were compared with 1,205 controls.
The major coronary events were diagnosed in the first decade after breast cancer diagnosis in 44% of patients; 33% of events were diagnosed 10-19 years after breast cancer diagnosis; and 23% occurred 20 or more years later. Of the cases, 54% died of ischemic heart disease.
The estimated mean radiation dose to the heart overall was 4.9 Gy (range, 0.03-27.72 Gy). For those with cancer in their left breast, the mean dose exposure to the heart was 6.6 Gy; for those with right-breast tumors, it was 2.9 Gy. Major coronary events were significantly higher among the women with radiation to the left breast.
The estimated dose to the heart of women who are currently treated with radiotherapy ranges from 1 to 5 Gy, the authors said.
For each 1-Gy increase in the mean dose of radiation to the heart, the rate of major coronary events increased by 7.4%, which was a highly statistically significant finding. Compared with controls who had no cardiac dose, the rate of major coronary events increased by 10% among those exposed to a mean radiation dose of less than 2 Gy, by 30% among those exposed to 2-4 Gy, by 40% among those exposed to 5-9 Gy, and by 116% in those exposed to 10 Gy or more.
Among women with a history of ischemic heart disease, the risk of major coronary events was almost sevenfold higher than it was in women with no history of ischemic heart disease. This risk was increased by about 13-fold during the first 10 years after treatment and was about twofold higher in later years.
"Absolute increases in risk for a given dose to the heart were larger for women with preexisting risk factors," they wrote, so "clinicians may wish to consider cardiac dose and cardiac risk factors as well as tumor control when making decisions about the use of radiotherapy for breast cancer."
Among the strengths of the study was that the analysis included all women who were documented as having received radiotherapy for breast cancer in the two countries during the time period studied. The authors cautioned against applying the results to breast cancer patients who are treated before age 30 because few women in this age group were included in the study.
The study was supported by the Oxford University Clinical Trial Service Unit from Cancer Research UK, the British Heart Foundation, and the UK Medical Research Council.
The risk of major ischemic coronary events was significantly and proportionately associated with the estimated mean radiation dose to the heart in a study of women in Sweden and Denmark who received radiotherapy for breast cancer over a 43-year period.
"The risk of a major coronary event increased linearly with the mean dose to the heart," reported Sarah Darby, Ph.D., of the University of Oxford (England), and her associates. The risk began to increase within the first 5 years of treatment and continued to increase for at least 20 years.
The findings make it possible for a woman to estimate her absolute risk of radiation-related ischemic heart disease, the authors wrote. "This absolute risk can be weighed against the probable absolute reduction in her risk of recurrence or death from breast cancer that would be achieved without radiotherapy" (N. Engl. J. Med. 2013;368:987-98 [doi: 10.1056/NEJMoa1209825]).
The population-based study included 2,168 women who had been treated with external-beam radiation for invasive breast cancer between 1958 and 2001, and were enrolled in the Swedish National Cancer Register or the Danish Breast Cancer Cooperative Group. The 963 women who were subsequently diagnosed with a major coronary event (myocardial infarction, coronary revascularization, or death from ischemic heart disease, but not angina) were compared with 1,205 controls.
The major coronary events were diagnosed in the first decade after breast cancer diagnosis in 44% of patients; 33% of events were diagnosed 10-19 years after breast cancer diagnosis; and 23% occurred 20 or more years later. Of the cases, 54% died of ischemic heart disease.
The estimated mean radiation dose to the heart overall was 4.9 Gy (range, 0.03-27.72 Gy). For those with cancer in their left breast, the mean dose exposure to the heart was 6.6 Gy; for those with right-breast tumors, it was 2.9 Gy. Major coronary events were significantly higher among the women with radiation to the left breast.
The estimated dose to the heart of women who are currently treated with radiotherapy ranges from 1 to 5 Gy, the authors said.
For each 1-Gy increase in the mean dose of radiation to the heart, the rate of major coronary events increased by 7.4%, which was a highly statistically significant finding. Compared with controls who had no cardiac dose, the rate of major coronary events increased by 10% among those exposed to a mean radiation dose of less than 2 Gy, by 30% among those exposed to 2-4 Gy, by 40% among those exposed to 5-9 Gy, and by 116% in those exposed to 10 Gy or more.
Among women with a history of ischemic heart disease, the risk of major coronary events was almost sevenfold higher than it was in women with no history of ischemic heart disease. This risk was increased by about 13-fold during the first 10 years after treatment and was about twofold higher in later years.
"Absolute increases in risk for a given dose to the heart were larger for women with preexisting risk factors," they wrote, so "clinicians may wish to consider cardiac dose and cardiac risk factors as well as tumor control when making decisions about the use of radiotherapy for breast cancer."
Among the strengths of the study was that the analysis included all women who were documented as having received radiotherapy for breast cancer in the two countries during the time period studied. The authors cautioned against applying the results to breast cancer patients who are treated before age 30 because few women in this age group were included in the study.
The study was supported by the Oxford University Clinical Trial Service Unit from Cancer Research UK, the British Heart Foundation, and the UK Medical Research Council.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: The risk of major coronary events increased by 7.4% per 1 Gy of radiation received as treatment for breast cancer.
Data source: A population-based, case-control study in 2,168 women treated with external-beam radiation therapy for invasive breast cancer in Sweden and Denmark between 1958 and 2001.
Disclosures: The study was supported by the Oxford University Clinical Trial Service Unit from Cancer Research UK, the British Heart Foundation, and the UK Medical Research Council.
Lipid metabolism genes linked to breast cancer subtype
Fine-needle aspirant samples taken from the healthy contralateral breast of patients undergoing surgery for breast cancer contained newly identified genetic markers that were expressed differently in estrogen receptor–negative and estrogen receptor–positive tumors.
The findings suggest that a metabolic derangement in lipid processing may precede the development of a breast tumor.
All of these genes are involved in lipid metabolism, an unexpected finding that speaks to the long-observed relationship between weight and breast cancer risk, Dr. Seema Khan and her colleagues wrote in the March issue of Cancer Prevention Research (2013 [doi:10.1158/1940-6207.CAPR-12-0304]).
"This was interesting because obesity is a breast cancer risk factor for postmenopausal women, but obese women are generally thought to be at increased risk for hormone-sensitive cancer," Khan said in a press statement. "We were surprised to see that some of these genes that are associated with lipid metabolism, or the metabolism of fats, are actually more highly expressed in the unaffected breasts of women with estrogen receptor–negative breast cancer."
The investigators, all from Northwestern University in Chicago, conducted their initial analysis on a set of 30 breast cancer patients – 15 with ER-negative tumors and 15 with ER-positive tumors. They then validated their results on 36-subjects, 12 with ER-negative cancers, 12 with ER-positive cancers, and 12 controls. All of the women in the study were matched for age, menopausal status, weight, and, in the patients, HER2 status. All subjects were followed for a minimum of 3 years.
Based on RNA extracted from fine-needle aspirations of the subjects’ contralateral breasts, eight unique genes were identified. There was significant differential expression of the genes between the groups. All of the genes were directly involved in lipid metabolism, and seven of them were significantly more common in ER-negative tumors.
Similar results were observed in the validation group, with all eight genes observed to be significantly more common in the ER-negative group than in the ER-positive group.
When the ER-negative cases were compared with the controls, four genes were significantly overexpressed and were observed to be up to six times more common in cases. The genetic markers were similarly expressed in ER-positive cases and in controls, however.
Two of the remaining four genes were significantly underexpressed in ER-positive cases, compared with controls (three and six times less likely to occur, respectively), indicating that both genes may protect against the development of ER-negative tumors. Of the remaining two genes, one was significantly under-expressed in both ER-negative and ER-positive groups, compared with controls (15 and 11 times less common, respectively). This, the authors said, indicates that the gene may protect against both types of cancer.
There were no significant associations between the final gene and either cancer subtype.
A clustering analysis of the eight genes separated the cases into low- mid- and high-expression groups, and also successfully separated the controls from the cases. In this analysis, 70% of the cases in the low-expression group were ER-positive; 67% of the cases in the mid-expression group were ER-positive; and 88% of the cases in the high-expression group were ER-negative.
The analysis also identified a high- and a low-expression group among the 12 controls. Four of these control cases had high-expression profiles, similar to those of the high-expression cases. The cytology of these four samples was atypical in two, borderline in one, and benign in one. The other eight samples had low gene expression and all had benign cytology.
"The potential involvement of lipid metabolism–related genes to ER-negative breast cancer is unexpected, though evidence pointing to a link of lipid/steroid metabolism with ER-negative breast cancer risk and outcomes exists," the authors said. "ER-negative/PR (progesterone receptor)-negative tumors are more common in obese premenopausal women, and large hip circumference has a particularly strong association with premenopausal ER-negative/PR-negative breast cancer. The functions of these genes in relation to lipid modification and elimination, and to transportation and detoxification of distinct lipid compounds, suggest that their expression results in a specific microenvironment of steroid hormone metabolites, which may determine whether initiated cells progress to ER-positive or ER-negative tumors."
None of the authors declared any financial relationships. The study was funded by the Lynn Sage Cancer Research Foundation, the Avon Foundation, and a private contribution.
Fine-needle aspirant samples taken from the healthy contralateral breast of patients undergoing surgery for breast cancer contained newly identified genetic markers that were expressed differently in estrogen receptor–negative and estrogen receptor–positive tumors.
The findings suggest that a metabolic derangement in lipid processing may precede the development of a breast tumor.
All of these genes are involved in lipid metabolism, an unexpected finding that speaks to the long-observed relationship between weight and breast cancer risk, Dr. Seema Khan and her colleagues wrote in the March issue of Cancer Prevention Research (2013 [doi:10.1158/1940-6207.CAPR-12-0304]).
"This was interesting because obesity is a breast cancer risk factor for postmenopausal women, but obese women are generally thought to be at increased risk for hormone-sensitive cancer," Khan said in a press statement. "We were surprised to see that some of these genes that are associated with lipid metabolism, or the metabolism of fats, are actually more highly expressed in the unaffected breasts of women with estrogen receptor–negative breast cancer."
The investigators, all from Northwestern University in Chicago, conducted their initial analysis on a set of 30 breast cancer patients – 15 with ER-negative tumors and 15 with ER-positive tumors. They then validated their results on 36-subjects, 12 with ER-negative cancers, 12 with ER-positive cancers, and 12 controls. All of the women in the study were matched for age, menopausal status, weight, and, in the patients, HER2 status. All subjects were followed for a minimum of 3 years.
Based on RNA extracted from fine-needle aspirations of the subjects’ contralateral breasts, eight unique genes were identified. There was significant differential expression of the genes between the groups. All of the genes were directly involved in lipid metabolism, and seven of them were significantly more common in ER-negative tumors.
Similar results were observed in the validation group, with all eight genes observed to be significantly more common in the ER-negative group than in the ER-positive group.
When the ER-negative cases were compared with the controls, four genes were significantly overexpressed and were observed to be up to six times more common in cases. The genetic markers were similarly expressed in ER-positive cases and in controls, however.
Two of the remaining four genes were significantly underexpressed in ER-positive cases, compared with controls (three and six times less likely to occur, respectively), indicating that both genes may protect against the development of ER-negative tumors. Of the remaining two genes, one was significantly under-expressed in both ER-negative and ER-positive groups, compared with controls (15 and 11 times less common, respectively). This, the authors said, indicates that the gene may protect against both types of cancer.
There were no significant associations between the final gene and either cancer subtype.
A clustering analysis of the eight genes separated the cases into low- mid- and high-expression groups, and also successfully separated the controls from the cases. In this analysis, 70% of the cases in the low-expression group were ER-positive; 67% of the cases in the mid-expression group were ER-positive; and 88% of the cases in the high-expression group were ER-negative.
The analysis also identified a high- and a low-expression group among the 12 controls. Four of these control cases had high-expression profiles, similar to those of the high-expression cases. The cytology of these four samples was atypical in two, borderline in one, and benign in one. The other eight samples had low gene expression and all had benign cytology.
"The potential involvement of lipid metabolism–related genes to ER-negative breast cancer is unexpected, though evidence pointing to a link of lipid/steroid metabolism with ER-negative breast cancer risk and outcomes exists," the authors said. "ER-negative/PR (progesterone receptor)-negative tumors are more common in obese premenopausal women, and large hip circumference has a particularly strong association with premenopausal ER-negative/PR-negative breast cancer. The functions of these genes in relation to lipid modification and elimination, and to transportation and detoxification of distinct lipid compounds, suggest that their expression results in a specific microenvironment of steroid hormone metabolites, which may determine whether initiated cells progress to ER-positive or ER-negative tumors."
None of the authors declared any financial relationships. The study was funded by the Lynn Sage Cancer Research Foundation, the Avon Foundation, and a private contribution.
Fine-needle aspirant samples taken from the healthy contralateral breast of patients undergoing surgery for breast cancer contained newly identified genetic markers that were expressed differently in estrogen receptor–negative and estrogen receptor–positive tumors.
The findings suggest that a metabolic derangement in lipid processing may precede the development of a breast tumor.
All of these genes are involved in lipid metabolism, an unexpected finding that speaks to the long-observed relationship between weight and breast cancer risk, Dr. Seema Khan and her colleagues wrote in the March issue of Cancer Prevention Research (2013 [doi:10.1158/1940-6207.CAPR-12-0304]).
"This was interesting because obesity is a breast cancer risk factor for postmenopausal women, but obese women are generally thought to be at increased risk for hormone-sensitive cancer," Khan said in a press statement. "We were surprised to see that some of these genes that are associated with lipid metabolism, or the metabolism of fats, are actually more highly expressed in the unaffected breasts of women with estrogen receptor–negative breast cancer."
The investigators, all from Northwestern University in Chicago, conducted their initial analysis on a set of 30 breast cancer patients – 15 with ER-negative tumors and 15 with ER-positive tumors. They then validated their results on 36-subjects, 12 with ER-negative cancers, 12 with ER-positive cancers, and 12 controls. All of the women in the study were matched for age, menopausal status, weight, and, in the patients, HER2 status. All subjects were followed for a minimum of 3 years.
Based on RNA extracted from fine-needle aspirations of the subjects’ contralateral breasts, eight unique genes were identified. There was significant differential expression of the genes between the groups. All of the genes were directly involved in lipid metabolism, and seven of them were significantly more common in ER-negative tumors.
Similar results were observed in the validation group, with all eight genes observed to be significantly more common in the ER-negative group than in the ER-positive group.
When the ER-negative cases were compared with the controls, four genes were significantly overexpressed and were observed to be up to six times more common in cases. The genetic markers were similarly expressed in ER-positive cases and in controls, however.
Two of the remaining four genes were significantly underexpressed in ER-positive cases, compared with controls (three and six times less likely to occur, respectively), indicating that both genes may protect against the development of ER-negative tumors. Of the remaining two genes, one was significantly under-expressed in both ER-negative and ER-positive groups, compared with controls (15 and 11 times less common, respectively). This, the authors said, indicates that the gene may protect against both types of cancer.
There were no significant associations between the final gene and either cancer subtype.
A clustering analysis of the eight genes separated the cases into low- mid- and high-expression groups, and also successfully separated the controls from the cases. In this analysis, 70% of the cases in the low-expression group were ER-positive; 67% of the cases in the mid-expression group were ER-positive; and 88% of the cases in the high-expression group were ER-negative.
The analysis also identified a high- and a low-expression group among the 12 controls. Four of these control cases had high-expression profiles, similar to those of the high-expression cases. The cytology of these four samples was atypical in two, borderline in one, and benign in one. The other eight samples had low gene expression and all had benign cytology.
"The potential involvement of lipid metabolism–related genes to ER-negative breast cancer is unexpected, though evidence pointing to a link of lipid/steroid metabolism with ER-negative breast cancer risk and outcomes exists," the authors said. "ER-negative/PR (progesterone receptor)-negative tumors are more common in obese premenopausal women, and large hip circumference has a particularly strong association with premenopausal ER-negative/PR-negative breast cancer. The functions of these genes in relation to lipid modification and elimination, and to transportation and detoxification of distinct lipid compounds, suggest that their expression results in a specific microenvironment of steroid hormone metabolites, which may determine whether initiated cells progress to ER-positive or ER-negative tumors."
None of the authors declared any financial relationships. The study was funded by the Lynn Sage Cancer Research Foundation, the Avon Foundation, and a private contribution.
FROM CANCER PREVENTION RESEARCH
Major finding: A clustering analysis of eight genes separated cases into expression groups; 70% of the low-expression cases had ER-positive tumors, 67% of the mid-expression cases had ER-positive tumors; and 88% of the high-expression group had ER-negative tumors.
Data source: The findings are from investigation and validation groups that comprised a total of 24 cases and 12 controls.
Disclosures: None of the authors declared any financial relationships. The study was funded by the Lynn Sage Cancer Research Foundation, the Avon Foundation, and a private contribution.
MVAC plus cystectomy boosts bladder cancer survival
ORLANDO – Cisplatin-based neoadjuvant chemotherapy plus cystectomy improved the overall survival of patients with muscle-invasive bladder cancer beyond that achieved with cystectomy alone; however, the results did not reach statistical significance in the Japan Clinical Oncology Group Study, JCOG0209.
In a randomized trial of 130 patients accrued over a 6-year period, overall survival at 5 years was 72.3% for patients who had two cycles of chemotherapy followed by radical cystectomy and 62.4% for patients who had radical cystectomy alone. The overall survival results were not significant because of insufficient sample size, according to Dr. Hiroshi Kitamura of Sapporo (Japan) Medical University.
Median progression-free survival time, however, was significantly longer at 99 months in the group who received neoadjuvant chemotherapy plus cystectomy compared with 78 months in those who had radical cystectomy alone (HR = 0.61, P = .04). Progression-free survival at 5 years was 69.1% and 56.4%, respectively.
The Japan Clinical Oncology Group Study, JCOG0209, examined the MVAC regimen of methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin followed by radical cystectomy. MVAC comprised methotrexate (30 mg/m2) on days 1, 15, and 22; vinblastine (3 mg/m2) on days 2, 15, and 22; Adriamycin (30 mg/m2) on day 2; and cisplatin (70 mg/m2) on day 2.
Because neoadjuvant therapy with gemcitabine and cisplatin is now widely used to treat invasive bladder cancer, and considered to provide a 5%-8% overall survival advantage, the Data and Safety Monitoring Committee recommended early publication of the results, Dr. Kitamura said at the annual Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology. The findings indicate that the MVAC regimen can still be considered promising.
"Although (the chemotherapy regimen) was two cycles, and the study was underpowered," chemotherapy plus radical cystectomy were confirmed as the standard of care for muscle-invasive bladder cancer, commented Dr. Dean Bajorin of Memorial Sloan-Kettering Cancer Center, New York, who was not involved in the study. "I do not think we need to include nontreatment arms in these trials anymore."
Between March 2003 and March 2009, researchers randomized 130 patients to receive two cycles of MVAC neoadjuvant chemotherapy followed by radical cystectomy (64 patients) or to radical cystectomy alone (66 patients). Patient registration was terminated early because of slow accrual.
The primary endpoint was overall survival. Secondary endpoints were progression-free survival, surgery-related complication rate, adverse events during neoadjuvant chemotherapy, the rate of no residual tumor in radical cystectomy specimens (pT0), and quality of life.
Results at the second interim analysis showed that overall survival was better with MVAC plus surgery (median, 102 months) than with radical cystectomy alone (median, 81 months), but the difference did not achieve statistical significance because the sample size was small (HR = 0.65, P = .07).
At the time of radical cystectomy, clinical stage was pT0 in 37% of the MVAC arm and 9% in the radical cystectomy arm (P less than .01).
Dr. Kitamura said he had no relationships to disclose. Dr. Bajorin disclosed that he has been a consultant or adviser for Bristol-Myers Squibb, Dendreon, Lilly, and Novartis. He has received honoraria from Lilly, and research funding from Amgen, Dendreon, Genentech, Genta, GlaxoSmithKline, and Novartis.
On Twitter @naseemsmiller
ORLANDO – Cisplatin-based neoadjuvant chemotherapy plus cystectomy improved the overall survival of patients with muscle-invasive bladder cancer beyond that achieved with cystectomy alone; however, the results did not reach statistical significance in the Japan Clinical Oncology Group Study, JCOG0209.
In a randomized trial of 130 patients accrued over a 6-year period, overall survival at 5 years was 72.3% for patients who had two cycles of chemotherapy followed by radical cystectomy and 62.4% for patients who had radical cystectomy alone. The overall survival results were not significant because of insufficient sample size, according to Dr. Hiroshi Kitamura of Sapporo (Japan) Medical University.
Median progression-free survival time, however, was significantly longer at 99 months in the group who received neoadjuvant chemotherapy plus cystectomy compared with 78 months in those who had radical cystectomy alone (HR = 0.61, P = .04). Progression-free survival at 5 years was 69.1% and 56.4%, respectively.
The Japan Clinical Oncology Group Study, JCOG0209, examined the MVAC regimen of methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin followed by radical cystectomy. MVAC comprised methotrexate (30 mg/m2) on days 1, 15, and 22; vinblastine (3 mg/m2) on days 2, 15, and 22; Adriamycin (30 mg/m2) on day 2; and cisplatin (70 mg/m2) on day 2.
Because neoadjuvant therapy with gemcitabine and cisplatin is now widely used to treat invasive bladder cancer, and considered to provide a 5%-8% overall survival advantage, the Data and Safety Monitoring Committee recommended early publication of the results, Dr. Kitamura said at the annual Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology. The findings indicate that the MVAC regimen can still be considered promising.
"Although (the chemotherapy regimen) was two cycles, and the study was underpowered," chemotherapy plus radical cystectomy were confirmed as the standard of care for muscle-invasive bladder cancer, commented Dr. Dean Bajorin of Memorial Sloan-Kettering Cancer Center, New York, who was not involved in the study. "I do not think we need to include nontreatment arms in these trials anymore."
Between March 2003 and March 2009, researchers randomized 130 patients to receive two cycles of MVAC neoadjuvant chemotherapy followed by radical cystectomy (64 patients) or to radical cystectomy alone (66 patients). Patient registration was terminated early because of slow accrual.
The primary endpoint was overall survival. Secondary endpoints were progression-free survival, surgery-related complication rate, adverse events during neoadjuvant chemotherapy, the rate of no residual tumor in radical cystectomy specimens (pT0), and quality of life.
Results at the second interim analysis showed that overall survival was better with MVAC plus surgery (median, 102 months) than with radical cystectomy alone (median, 81 months), but the difference did not achieve statistical significance because the sample size was small (HR = 0.65, P = .07).
At the time of radical cystectomy, clinical stage was pT0 in 37% of the MVAC arm and 9% in the radical cystectomy arm (P less than .01).
Dr. Kitamura said he had no relationships to disclose. Dr. Bajorin disclosed that he has been a consultant or adviser for Bristol-Myers Squibb, Dendreon, Lilly, and Novartis. He has received honoraria from Lilly, and research funding from Amgen, Dendreon, Genentech, Genta, GlaxoSmithKline, and Novartis.
On Twitter @naseemsmiller
ORLANDO – Cisplatin-based neoadjuvant chemotherapy plus cystectomy improved the overall survival of patients with muscle-invasive bladder cancer beyond that achieved with cystectomy alone; however, the results did not reach statistical significance in the Japan Clinical Oncology Group Study, JCOG0209.
In a randomized trial of 130 patients accrued over a 6-year period, overall survival at 5 years was 72.3% for patients who had two cycles of chemotherapy followed by radical cystectomy and 62.4% for patients who had radical cystectomy alone. The overall survival results were not significant because of insufficient sample size, according to Dr. Hiroshi Kitamura of Sapporo (Japan) Medical University.
Median progression-free survival time, however, was significantly longer at 99 months in the group who received neoadjuvant chemotherapy plus cystectomy compared with 78 months in those who had radical cystectomy alone (HR = 0.61, P = .04). Progression-free survival at 5 years was 69.1% and 56.4%, respectively.
The Japan Clinical Oncology Group Study, JCOG0209, examined the MVAC regimen of methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin followed by radical cystectomy. MVAC comprised methotrexate (30 mg/m2) on days 1, 15, and 22; vinblastine (3 mg/m2) on days 2, 15, and 22; Adriamycin (30 mg/m2) on day 2; and cisplatin (70 mg/m2) on day 2.
Because neoadjuvant therapy with gemcitabine and cisplatin is now widely used to treat invasive bladder cancer, and considered to provide a 5%-8% overall survival advantage, the Data and Safety Monitoring Committee recommended early publication of the results, Dr. Kitamura said at the annual Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology. The findings indicate that the MVAC regimen can still be considered promising.
"Although (the chemotherapy regimen) was two cycles, and the study was underpowered," chemotherapy plus radical cystectomy were confirmed as the standard of care for muscle-invasive bladder cancer, commented Dr. Dean Bajorin of Memorial Sloan-Kettering Cancer Center, New York, who was not involved in the study. "I do not think we need to include nontreatment arms in these trials anymore."
Between March 2003 and March 2009, researchers randomized 130 patients to receive two cycles of MVAC neoadjuvant chemotherapy followed by radical cystectomy (64 patients) or to radical cystectomy alone (66 patients). Patient registration was terminated early because of slow accrual.
The primary endpoint was overall survival. Secondary endpoints were progression-free survival, surgery-related complication rate, adverse events during neoadjuvant chemotherapy, the rate of no residual tumor in radical cystectomy specimens (pT0), and quality of life.
Results at the second interim analysis showed that overall survival was better with MVAC plus surgery (median, 102 months) than with radical cystectomy alone (median, 81 months), but the difference did not achieve statistical significance because the sample size was small (HR = 0.65, P = .07).
At the time of radical cystectomy, clinical stage was pT0 in 37% of the MVAC arm and 9% in the radical cystectomy arm (P less than .01).
Dr. Kitamura said he had no relationships to disclose. Dr. Bajorin disclosed that he has been a consultant or adviser for Bristol-Myers Squibb, Dendreon, Lilly, and Novartis. He has received honoraria from Lilly, and research funding from Amgen, Dendreon, Genentech, Genta, GlaxoSmithKline, and Novartis.
On Twitter @naseemsmiller
AT ASCO GENITOURINARY CANCERS SYMPOSIUM
Major finding: Overall survival was better with MVAC plus radical cystectomy (median, 102 months) than with radical cystectomy alone, (median, 81 months), but the difference did not achieve statistical significance because the sample size was small (HR = 0.65, P = .07).
Data source: A study of 130 patients randomized to receive two cycles of MVAC neoadjuvant chemotherapy followed by radical cystectomy (64 patients), or radical cystectomy alone (66).
Disclosures: Dr. Kitamura said he had no relationships to disclose. Dr. Bajorin disclosed that he has been a consultant or adviser for Bristol-Myers Squibb, Dendreon, Lilly, and Novartis. He has received honoraria from Lilly, and research funding from Amgen, Dendreon, Genentech, Genta, GlaxoSmithKline, and Novartis.
Imaging agent approved for locating lymph nodes
A radioactive diagnostic imaging agent called Lymphoseek (technetium Tc 99m tilmanocept) Injection has been approved for locating lymph nodes in patients who have breast cancer or melanoma and are undergoing surgery to remove tumor-draining lymph nodes, the Food and Drug Administration announced.
Lymphoseek is the first new drug for lymph-node mapping to be approved in more than 30 years. Other FDA-approved drugs used for lymph-node mapping include sulfur colloid and isosulfan blue.
"To use Lymphoseek, doctors inject the drug into the tumor area and later, using a handheld radiation detector, find lymph nodes that have taken up Lymphoseek’s radioactivity," said Dr. Shaw Chen, deputy director of the Office of Drug Evaluation IV in the FDA’s Center for Drug Evaluation and Research.
Lymphoseek is marketed by Navidea Biopharmaceuticals. The manufacturer’s website notes that the ability to rapidly locate and biopsy sentinel nodes enables surgical management to be tailored specifically to each patient’s burden of disease.
In two clinical trials, 332 patients with melanoma or breast cancer were injected with Lymphoseek and blue dye. Surgeons subsequently removed suspected lymph nodes for pathologic examination. Confirmed lymph nodes were examined for their content of blue dye and Lymphoseek. The combination of Lymphoseek and blue dye localized most lymph nodes, although a notable number of nodes were localized only by Lymphoseek.
The most common side effects identified in clinical trials were pain and irritation at the injection site.
According to the manufacturer, a clinical trial involving patients with head and neck cancer is completing enrollment and is expected to be the subject of a future New Drug Application amendment. An initial Marketing Authorization Application filing in the European Union is anticipated by the end of 2012.
A radioactive diagnostic imaging agent called Lymphoseek (technetium Tc 99m tilmanocept) Injection has been approved for locating lymph nodes in patients who have breast cancer or melanoma and are undergoing surgery to remove tumor-draining lymph nodes, the Food and Drug Administration announced.
Lymphoseek is the first new drug for lymph-node mapping to be approved in more than 30 years. Other FDA-approved drugs used for lymph-node mapping include sulfur colloid and isosulfan blue.
"To use Lymphoseek, doctors inject the drug into the tumor area and later, using a handheld radiation detector, find lymph nodes that have taken up Lymphoseek’s radioactivity," said Dr. Shaw Chen, deputy director of the Office of Drug Evaluation IV in the FDA’s Center for Drug Evaluation and Research.
Lymphoseek is marketed by Navidea Biopharmaceuticals. The manufacturer’s website notes that the ability to rapidly locate and biopsy sentinel nodes enables surgical management to be tailored specifically to each patient’s burden of disease.
In two clinical trials, 332 patients with melanoma or breast cancer were injected with Lymphoseek and blue dye. Surgeons subsequently removed suspected lymph nodes for pathologic examination. Confirmed lymph nodes were examined for their content of blue dye and Lymphoseek. The combination of Lymphoseek and blue dye localized most lymph nodes, although a notable number of nodes were localized only by Lymphoseek.
The most common side effects identified in clinical trials were pain and irritation at the injection site.
According to the manufacturer, a clinical trial involving patients with head and neck cancer is completing enrollment and is expected to be the subject of a future New Drug Application amendment. An initial Marketing Authorization Application filing in the European Union is anticipated by the end of 2012.
A radioactive diagnostic imaging agent called Lymphoseek (technetium Tc 99m tilmanocept) Injection has been approved for locating lymph nodes in patients who have breast cancer or melanoma and are undergoing surgery to remove tumor-draining lymph nodes, the Food and Drug Administration announced.
Lymphoseek is the first new drug for lymph-node mapping to be approved in more than 30 years. Other FDA-approved drugs used for lymph-node mapping include sulfur colloid and isosulfan blue.
"To use Lymphoseek, doctors inject the drug into the tumor area and later, using a handheld radiation detector, find lymph nodes that have taken up Lymphoseek’s radioactivity," said Dr. Shaw Chen, deputy director of the Office of Drug Evaluation IV in the FDA’s Center for Drug Evaluation and Research.
Lymphoseek is marketed by Navidea Biopharmaceuticals. The manufacturer’s website notes that the ability to rapidly locate and biopsy sentinel nodes enables surgical management to be tailored specifically to each patient’s burden of disease.
In two clinical trials, 332 patients with melanoma or breast cancer were injected with Lymphoseek and blue dye. Surgeons subsequently removed suspected lymph nodes for pathologic examination. Confirmed lymph nodes were examined for their content of blue dye and Lymphoseek. The combination of Lymphoseek and blue dye localized most lymph nodes, although a notable number of nodes were localized only by Lymphoseek.
The most common side effects identified in clinical trials were pain and irritation at the injection site.
According to the manufacturer, a clinical trial involving patients with head and neck cancer is completing enrollment and is expected to be the subject of a future New Drug Application amendment. An initial Marketing Authorization Application filing in the European Union is anticipated by the end of 2012.
Third drug approved for metastatic, treatment-resistant GIST
Regorafenib, a multikinase inhibitor, has been approved as a treatment for locally advanced, unresectable, or metastatic gastrointestinal stromal tumor in people who have been treated with imatinib and sunitinib, the other two treatments approved for GIST, the Food and Drug Administration announced on Feb. 26.
Regorafenib was first approved in September as a treatment for metastatic colorectal cancer, and "provides an important new treatment option for patients with GIST in which other approved drugs are no longer effective," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. The recommended dose is 160 mg orally once a day for the first 21 days of each 28-day cycle, according to the prescribing information for regorafenib, which is marketed as Stivarga by Bayer HealthCare Pharmaceuticals.
Approval was based on the interim results of the phase III GRID (GIST – Regorafenib In Progressive Disease) study, comparing placebo plus best supportive care (BSC) to regorafenib plus BSC in 199 patients with locally advanced, unresectable, or metastatic GIST, previously treated with imatinib and sunitinib, according to the FDA statement, as well as the statement issued by the manufacturer. The median progression-free survival (the primary endpoint) was 4.8 months among those on regorafenib, compared with 0.8 months among those on placebo, a statistically significant difference (Lancet 381;9863:295-302). At the time of the planned interim analysis, there was no statistically significant difference in overall survival.
The most common adverse events associated with treatment, reported by at least 30% of those treated, included hand-foot syndrome, diarrhea, mucositis, dysphonia, asthenia/fatigue, hypertension, reduced appetite and food intake, and rash. Serious adverse events, affecting less than 1% of patients, included hepatotoxicity, severe bleeding, blistering and peeling of skin, very high blood pressures requiring emergency treatment, heart attacks, and intestinal perforations. The regorafenib label includes a boxed warning about the risk of hepatotoxicity associated with treatment, noting that severe and sometimes fatal hepatotoxicity has been reported in clinical trials, and that hepatic function should be monitored before and during treatment.
Regorafenib inhibits multiple kinases that are involved in normal cellular functions, as well as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment, according to the manufacturer.
Regorafenib was the focus of the FDA’s priority review program, which evaluates the drug in 6 months instead of the usual 12 months, and is designated for products "that may provide safe and effective therapy when no satisfactory alternative therapy exists, or offer significant improvement compared to marketed products," according to the FDA statement.
The FDA cites a National Cancer Institute estimate that 3,300-6,000 new cases of GIST are diagnosed every year in the United States, affecting mostly older adults. The previously approved colorectal cancer indication is for people who have metastatic colorectal cancer, who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.
Imatinib (Gleevec) and sunitinib (Sutent) are both orally administered kinase inhibitors.
Regorafenib, a multikinase inhibitor, has been approved as a treatment for locally advanced, unresectable, or metastatic gastrointestinal stromal tumor in people who have been treated with imatinib and sunitinib, the other two treatments approved for GIST, the Food and Drug Administration announced on Feb. 26.
Regorafenib was first approved in September as a treatment for metastatic colorectal cancer, and "provides an important new treatment option for patients with GIST in which other approved drugs are no longer effective," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. The recommended dose is 160 mg orally once a day for the first 21 days of each 28-day cycle, according to the prescribing information for regorafenib, which is marketed as Stivarga by Bayer HealthCare Pharmaceuticals.
Approval was based on the interim results of the phase III GRID (GIST – Regorafenib In Progressive Disease) study, comparing placebo plus best supportive care (BSC) to regorafenib plus BSC in 199 patients with locally advanced, unresectable, or metastatic GIST, previously treated with imatinib and sunitinib, according to the FDA statement, as well as the statement issued by the manufacturer. The median progression-free survival (the primary endpoint) was 4.8 months among those on regorafenib, compared with 0.8 months among those on placebo, a statistically significant difference (Lancet 381;9863:295-302). At the time of the planned interim analysis, there was no statistically significant difference in overall survival.
The most common adverse events associated with treatment, reported by at least 30% of those treated, included hand-foot syndrome, diarrhea, mucositis, dysphonia, asthenia/fatigue, hypertension, reduced appetite and food intake, and rash. Serious adverse events, affecting less than 1% of patients, included hepatotoxicity, severe bleeding, blistering and peeling of skin, very high blood pressures requiring emergency treatment, heart attacks, and intestinal perforations. The regorafenib label includes a boxed warning about the risk of hepatotoxicity associated with treatment, noting that severe and sometimes fatal hepatotoxicity has been reported in clinical trials, and that hepatic function should be monitored before and during treatment.
Regorafenib inhibits multiple kinases that are involved in normal cellular functions, as well as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment, according to the manufacturer.
Regorafenib was the focus of the FDA’s priority review program, which evaluates the drug in 6 months instead of the usual 12 months, and is designated for products "that may provide safe and effective therapy when no satisfactory alternative therapy exists, or offer significant improvement compared to marketed products," according to the FDA statement.
The FDA cites a National Cancer Institute estimate that 3,300-6,000 new cases of GIST are diagnosed every year in the United States, affecting mostly older adults. The previously approved colorectal cancer indication is for people who have metastatic colorectal cancer, who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.
Imatinib (Gleevec) and sunitinib (Sutent) are both orally administered kinase inhibitors.
Regorafenib, a multikinase inhibitor, has been approved as a treatment for locally advanced, unresectable, or metastatic gastrointestinal stromal tumor in people who have been treated with imatinib and sunitinib, the other two treatments approved for GIST, the Food and Drug Administration announced on Feb. 26.
Regorafenib was first approved in September as a treatment for metastatic colorectal cancer, and "provides an important new treatment option for patients with GIST in which other approved drugs are no longer effective," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. The recommended dose is 160 mg orally once a day for the first 21 days of each 28-day cycle, according to the prescribing information for regorafenib, which is marketed as Stivarga by Bayer HealthCare Pharmaceuticals.
Approval was based on the interim results of the phase III GRID (GIST – Regorafenib In Progressive Disease) study, comparing placebo plus best supportive care (BSC) to regorafenib plus BSC in 199 patients with locally advanced, unresectable, or metastatic GIST, previously treated with imatinib and sunitinib, according to the FDA statement, as well as the statement issued by the manufacturer. The median progression-free survival (the primary endpoint) was 4.8 months among those on regorafenib, compared with 0.8 months among those on placebo, a statistically significant difference (Lancet 381;9863:295-302). At the time of the planned interim analysis, there was no statistically significant difference in overall survival.
The most common adverse events associated with treatment, reported by at least 30% of those treated, included hand-foot syndrome, diarrhea, mucositis, dysphonia, asthenia/fatigue, hypertension, reduced appetite and food intake, and rash. Serious adverse events, affecting less than 1% of patients, included hepatotoxicity, severe bleeding, blistering and peeling of skin, very high blood pressures requiring emergency treatment, heart attacks, and intestinal perforations. The regorafenib label includes a boxed warning about the risk of hepatotoxicity associated with treatment, noting that severe and sometimes fatal hepatotoxicity has been reported in clinical trials, and that hepatic function should be monitored before and during treatment.
Regorafenib inhibits multiple kinases that are involved in normal cellular functions, as well as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment, according to the manufacturer.
Regorafenib was the focus of the FDA’s priority review program, which evaluates the drug in 6 months instead of the usual 12 months, and is designated for products "that may provide safe and effective therapy when no satisfactory alternative therapy exists, or offer significant improvement compared to marketed products," according to the FDA statement.
The FDA cites a National Cancer Institute estimate that 3,300-6,000 new cases of GIST are diagnosed every year in the United States, affecting mostly older adults. The previously approved colorectal cancer indication is for people who have metastatic colorectal cancer, who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.
Imatinib (Gleevec) and sunitinib (Sutent) are both orally administered kinase inhibitors.
Selective resection feasible for esophageal cancer
SAN FRANCISCO – Five-year survival rates for 41 patients with locoregionally advanced esophageal cancer who underwent induction chemotherapy and definitive chemoradiotherapy were 53% for patients with a clinical complete response who avoided surgery, 33% for patients with suspected residual disease, and 41% for patients with suspected residual disease who were able to undergo surgery.
The multicenter phase II Radiation Therapy Oncology Group (RTOG) 0246 trial suggests that the organ-preserving strategy of selective esophageal resection after definitive chemoradiation can be an effective approach, Dr. Stephen Swisher said at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology (ASCO).
Forty-one of the 43 patients enrolled with nonmetastatic resectable esophageal cancer had enough data to be included in the final analysis. Thirty-seven patients completed both induction chemotherapy and definitive chemoradiotherapy. Four patients died from problems associated with treatment (10%), two related to induction chemotherapy, one related to chemoradiation, and one related to surgery.
The overall 5-year survival rate after a median follow-up of 6.7 years was 37% in an intention-to-treat analysis, which is similar to results of trials of other treatment modalities for locoregionally advanced esophageal cancer, he said. Patients with a clinical complete response to chemoradiotherapy had a median survival of 66 months, compared with 15 months for all patients with suspected residual disease and 36 months for patients with suspected residual disease who were able to undergo surgery, reported Dr. Swisher of the University of Texas M.D. Anderson Cancer Center, Houston, and his associates.
During enrollment in 2003-2006, 15 patients came from the M.D. Anderson Cancer Center; 18 other sites contributed 1-3 patients each. Twenty-nine patients had adenocarcinomas (73%). Pretreatment clinical stages were T3 or greater in 31 patients (76%) and N1 in 29 patients (71%).
All underwent two cycles of induction chemotherapy with 5-fluorouracil (5-FU), cisplatin, and paclitaxel, followed by concurrent chemoradiation and daily 5-FU with cisplatin over the first 5 days.
After definitive chemoradiation, patients underwent chest and abdominal imaging to look for residual locoregional disease using CT scans, esophageal ultrasound, and optional PET scans. Those with no suspected residual disease were observed and monitored for recurrence every 3 months for the first 6 months, then every 6 months for the next 18 months, and then yearly. Patients with suspected residual disease were considered for immediate surgery.
Ultimately, 21 patients (51% of the total) underwent surgery: 17 had selective esophagectomies due to suspected residual cancer; 1 patient with a clinical complete response after chemoradiotherapy requested surgery; and 3 patients who were thought to have clinical complete responses underwent salvage esophagectomies due to recurrent cancer 5-15 months after chemoradiotherapy.
Besides the 17 patients with suspected residual cancer who underwent surgery, 4 others with a noncomplete response to chemoradiotherapy did not have esophagectomies because of metastatic disease in 3 patients and inoperable local disease in 1 patient.
The investigators previously reported preliminary results showing an overall 1-year survival rate of 71% in the study.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Swisher reported having no financial disclosures.
On Twitter @sherryboschert
This study provides a start toward "what we should be doing in cancer service delivery," which is creating a customized or tailored approach to delivery of services, Dr. Donald Low said. Eliminating esophagectomy when feasible is "probably appropriate, considering the historical perspective of bad outcomes in esophageal resection."

|
Courtesy of the American Society of Clinical Oncology
|
The RTOG 0246 trial addresses an important clinical issue and looks at a new approach of neoadjuvant therapy and selective surgery. One of the study’s strengths is that surveillance was very scheduled and orchestrated – there was a branch point at which the decision was made to have or not have surgery. How that surveillance would fit into real-world practice is unclear and important to consider, he said. The long-term follow-up is another strength of the study.
On the other hand, it has several weaknesses. A large number of participating centers yielded a relatively limited number of study participants: 79% of the centers contributed fewer than two patients, and only the M.D. Anderson center contributed more than three patients. The cancers were mostly adenocarcinomas, but the study included some squamous cell cancers and esophageal or esophagogastric junction tumors – a mixed presentation that makes analysis of results more complex. There were no stage-by-stage comparisons of results, and the study included everything from T2 N0 to T3 N1 stage disease.
In addition, there was no reliable current methodology for accurately identifying complete pathological response without surgical resection. "Remember, that’s the important issue, because at some point you have to sit down with a physiologically intact person and give an impression as to whether they’ve had a complete response," he said.
Dr. Donald Low is head of the section on general thoracic surgery and thoracic oncology at Virginia Mason Medical Center, Seattle. This is a portion of his discussion of Dr. Swisher’s study at the meeting. Dr. Low has received honoraria from Boston Scientific.
This study provides a start toward "what we should be doing in cancer service delivery," which is creating a customized or tailored approach to delivery of services, Dr. Donald Low said. Eliminating esophagectomy when feasible is "probably appropriate, considering the historical perspective of bad outcomes in esophageal resection."

|
Courtesy of the American Society of Clinical Oncology
|
The RTOG 0246 trial addresses an important clinical issue and looks at a new approach of neoadjuvant therapy and selective surgery. One of the study’s strengths is that surveillance was very scheduled and orchestrated – there was a branch point at which the decision was made to have or not have surgery. How that surveillance would fit into real-world practice is unclear and important to consider, he said. The long-term follow-up is another strength of the study.
On the other hand, it has several weaknesses. A large number of participating centers yielded a relatively limited number of study participants: 79% of the centers contributed fewer than two patients, and only the M.D. Anderson center contributed more than three patients. The cancers were mostly adenocarcinomas, but the study included some squamous cell cancers and esophageal or esophagogastric junction tumors – a mixed presentation that makes analysis of results more complex. There were no stage-by-stage comparisons of results, and the study included everything from T2 N0 to T3 N1 stage disease.
In addition, there was no reliable current methodology for accurately identifying complete pathological response without surgical resection. "Remember, that’s the important issue, because at some point you have to sit down with a physiologically intact person and give an impression as to whether they’ve had a complete response," he said.
Dr. Donald Low is head of the section on general thoracic surgery and thoracic oncology at Virginia Mason Medical Center, Seattle. This is a portion of his discussion of Dr. Swisher’s study at the meeting. Dr. Low has received honoraria from Boston Scientific.
This study provides a start toward "what we should be doing in cancer service delivery," which is creating a customized or tailored approach to delivery of services, Dr. Donald Low said. Eliminating esophagectomy when feasible is "probably appropriate, considering the historical perspective of bad outcomes in esophageal resection."

|
Courtesy of the American Society of Clinical Oncology
|
The RTOG 0246 trial addresses an important clinical issue and looks at a new approach of neoadjuvant therapy and selective surgery. One of the study’s strengths is that surveillance was very scheduled and orchestrated – there was a branch point at which the decision was made to have or not have surgery. How that surveillance would fit into real-world practice is unclear and important to consider, he said. The long-term follow-up is another strength of the study.
On the other hand, it has several weaknesses. A large number of participating centers yielded a relatively limited number of study participants: 79% of the centers contributed fewer than two patients, and only the M.D. Anderson center contributed more than three patients. The cancers were mostly adenocarcinomas, but the study included some squamous cell cancers and esophageal or esophagogastric junction tumors – a mixed presentation that makes analysis of results more complex. There were no stage-by-stage comparisons of results, and the study included everything from T2 N0 to T3 N1 stage disease.
In addition, there was no reliable current methodology for accurately identifying complete pathological response without surgical resection. "Remember, that’s the important issue, because at some point you have to sit down with a physiologically intact person and give an impression as to whether they’ve had a complete response," he said.
Dr. Donald Low is head of the section on general thoracic surgery and thoracic oncology at Virginia Mason Medical Center, Seattle. This is a portion of his discussion of Dr. Swisher’s study at the meeting. Dr. Low has received honoraria from Boston Scientific.
SAN FRANCISCO – Five-year survival rates for 41 patients with locoregionally advanced esophageal cancer who underwent induction chemotherapy and definitive chemoradiotherapy were 53% for patients with a clinical complete response who avoided surgery, 33% for patients with suspected residual disease, and 41% for patients with suspected residual disease who were able to undergo surgery.
The multicenter phase II Radiation Therapy Oncology Group (RTOG) 0246 trial suggests that the organ-preserving strategy of selective esophageal resection after definitive chemoradiation can be an effective approach, Dr. Stephen Swisher said at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology (ASCO).
Forty-one of the 43 patients enrolled with nonmetastatic resectable esophageal cancer had enough data to be included in the final analysis. Thirty-seven patients completed both induction chemotherapy and definitive chemoradiotherapy. Four patients died from problems associated with treatment (10%), two related to induction chemotherapy, one related to chemoradiation, and one related to surgery.
The overall 5-year survival rate after a median follow-up of 6.7 years was 37% in an intention-to-treat analysis, which is similar to results of trials of other treatment modalities for locoregionally advanced esophageal cancer, he said. Patients with a clinical complete response to chemoradiotherapy had a median survival of 66 months, compared with 15 months for all patients with suspected residual disease and 36 months for patients with suspected residual disease who were able to undergo surgery, reported Dr. Swisher of the University of Texas M.D. Anderson Cancer Center, Houston, and his associates.
During enrollment in 2003-2006, 15 patients came from the M.D. Anderson Cancer Center; 18 other sites contributed 1-3 patients each. Twenty-nine patients had adenocarcinomas (73%). Pretreatment clinical stages were T3 or greater in 31 patients (76%) and N1 in 29 patients (71%).
All underwent two cycles of induction chemotherapy with 5-fluorouracil (5-FU), cisplatin, and paclitaxel, followed by concurrent chemoradiation and daily 5-FU with cisplatin over the first 5 days.
After definitive chemoradiation, patients underwent chest and abdominal imaging to look for residual locoregional disease using CT scans, esophageal ultrasound, and optional PET scans. Those with no suspected residual disease were observed and monitored for recurrence every 3 months for the first 6 months, then every 6 months for the next 18 months, and then yearly. Patients with suspected residual disease were considered for immediate surgery.
Ultimately, 21 patients (51% of the total) underwent surgery: 17 had selective esophagectomies due to suspected residual cancer; 1 patient with a clinical complete response after chemoradiotherapy requested surgery; and 3 patients who were thought to have clinical complete responses underwent salvage esophagectomies due to recurrent cancer 5-15 months after chemoradiotherapy.
Besides the 17 patients with suspected residual cancer who underwent surgery, 4 others with a noncomplete response to chemoradiotherapy did not have esophagectomies because of metastatic disease in 3 patients and inoperable local disease in 1 patient.
The investigators previously reported preliminary results showing an overall 1-year survival rate of 71% in the study.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Swisher reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Five-year survival rates for 41 patients with locoregionally advanced esophageal cancer who underwent induction chemotherapy and definitive chemoradiotherapy were 53% for patients with a clinical complete response who avoided surgery, 33% for patients with suspected residual disease, and 41% for patients with suspected residual disease who were able to undergo surgery.
The multicenter phase II Radiation Therapy Oncology Group (RTOG) 0246 trial suggests that the organ-preserving strategy of selective esophageal resection after definitive chemoradiation can be an effective approach, Dr. Stephen Swisher said at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology (ASCO).
Forty-one of the 43 patients enrolled with nonmetastatic resectable esophageal cancer had enough data to be included in the final analysis. Thirty-seven patients completed both induction chemotherapy and definitive chemoradiotherapy. Four patients died from problems associated with treatment (10%), two related to induction chemotherapy, one related to chemoradiation, and one related to surgery.
The overall 5-year survival rate after a median follow-up of 6.7 years was 37% in an intention-to-treat analysis, which is similar to results of trials of other treatment modalities for locoregionally advanced esophageal cancer, he said. Patients with a clinical complete response to chemoradiotherapy had a median survival of 66 months, compared with 15 months for all patients with suspected residual disease and 36 months for patients with suspected residual disease who were able to undergo surgery, reported Dr. Swisher of the University of Texas M.D. Anderson Cancer Center, Houston, and his associates.
During enrollment in 2003-2006, 15 patients came from the M.D. Anderson Cancer Center; 18 other sites contributed 1-3 patients each. Twenty-nine patients had adenocarcinomas (73%). Pretreatment clinical stages were T3 or greater in 31 patients (76%) and N1 in 29 patients (71%).
All underwent two cycles of induction chemotherapy with 5-fluorouracil (5-FU), cisplatin, and paclitaxel, followed by concurrent chemoradiation and daily 5-FU with cisplatin over the first 5 days.
After definitive chemoradiation, patients underwent chest and abdominal imaging to look for residual locoregional disease using CT scans, esophageal ultrasound, and optional PET scans. Those with no suspected residual disease were observed and monitored for recurrence every 3 months for the first 6 months, then every 6 months for the next 18 months, and then yearly. Patients with suspected residual disease were considered for immediate surgery.
Ultimately, 21 patients (51% of the total) underwent surgery: 17 had selective esophagectomies due to suspected residual cancer; 1 patient with a clinical complete response after chemoradiotherapy requested surgery; and 3 patients who were thought to have clinical complete responses underwent salvage esophagectomies due to recurrent cancer 5-15 months after chemoradiotherapy.
Besides the 17 patients with suspected residual cancer who underwent surgery, 4 others with a noncomplete response to chemoradiotherapy did not have esophagectomies because of metastatic disease in 3 patients and inoperable local disease in 1 patient.
The investigators previously reported preliminary results showing an overall 1-year survival rate of 71% in the study.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Swisher reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO GASTROINTESTINAL CANCERS SYMPOSIUM
Major finding: Five-year survival rates after definitive chemoradiotherapy were 53% with complete clinical responses to definitive chemoradiotherapy, 33% for those with noncomplete responses, and 41% for patients in the latter group who were able to undergo esophagectomy.
Data source: Prospective multicenter trial of selective surgery following definitive chemoradiotherapy in 41 patients with advanced locoregional esophageal cancer.
Disclosures: Dr. Swisher reported having no financial disclosures.