User login
Antiviral agent may prevent CMV infection
An antiviral agent can reduce the incidence of cytomegalovirus (CMV) infection in patients receiving an allogeneic hematopoietic stem cell
transplant, according to a study published in The New England Journal of Medicine.
The agent, letermovir, proved more effective than placebo in preventing CMV, and the highest dose tested, 240 mg/day, was most effective.
The most common adverse events were gastrointestinal disorders and infections.
However, the incidence of events was similar among treated patients and those in the placebo arm.
Roy F. Chemaly, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted this randomized, double-blind, phase 2 trial. It was funded by AiCuris, the company that was developing letermovir before Merck purchased worldwide rights to develop and commercialize the drug in 2012.
The researchers evaluated the effect of letermovir on the incidence and time-to-onset of CMV prophylaxis failure in CMV-seropositive, matched transplant recipients.
The study included 131 patients. For 12 weeks after engraftment, they received placebo (n=33) or letermovir at 60 mg/day (n=33), 120 mg/day (n=31), or 240 mg/day (n=34).
Efficacy analysis
The primary endpoint was all-cause prophylaxis failure, which was defined as discontinuation of the study drug due to CMV antigen or CMV DNA detection, end-organ disease, or any other causes unrelated to CMV.
The primary efficacy analysis population was a modified intention-to-treat population, which included all patients who received at least 1 dose of the study drug and had at least 1 measurement of the CMV viral load during the study.
The incidence of all-cause prophylaxis failure was significantly lower in the groups that received letermovir at doses of 120 mg/day or 240 mg/day, when compared with the placebo group—32% and 29% vs 64%; P=0.01 and P=0.007, respectively.
The time to the onset of prophylaxis failure was significantly shorter in the 240-mg group (range, 1 to 8 days) than in the placebo group (range, 1 to 21 days; P=0.002).
However, comparisons with the placebo group were not significant for the 60-mg group (range, 1 to 42 days; P=0.15) or the 120-mg group (range, 1 to 15 days; P=0.13).
The incidence of virologic failure was lower in the 240-mg group (6%) than in the 120-mg group (19%), the 60-mg group (21%), or the placebo group (36%).
Virologic failure was defined as either detectable CMV antigen or DNA in the blood at 2 consecutive time points (with at least 1 instance confirmed by the central lab), leading to discontinuation of the study drug and the administration of rescue medication or the development of CMV end-organ disease.
Safety analysis
Nearly all of the patients had at least 1 adverse event during treatment—94% in the 60-mg and 120-mg groups and 100% in the 240-mg and placebo groups. Most events were mild or moderate.
However, 24% of letermovir-treated patients and 30% of those who received placebo experienced severe adverse events during treatment. Investigators, who were blinded to treatment, considered 17% of the severe events in the letermovir group to be drug-related and 33% of severe events in the placebo group to be drug-related.
Most adverse events were gastrointestinal disorders—diarrhea, nausea, and vomiting—which occurred in 66% of letermovir-treated patients and 61% of patients in the placebo group. Infections—mostly CMV—were also common, occurring in 59% of letermovir-treated patients and 76% of patients in the placebo group.
Five patients died during the trial. None of the deaths were thought to be related to treatment or to CMV. ![]()
An antiviral agent can reduce the incidence of cytomegalovirus (CMV) infection in patients receiving an allogeneic hematopoietic stem cell
transplant, according to a study published in The New England Journal of Medicine.
The agent, letermovir, proved more effective than placebo in preventing CMV, and the highest dose tested, 240 mg/day, was most effective.
The most common adverse events were gastrointestinal disorders and infections.
However, the incidence of events was similar among treated patients and those in the placebo arm.
Roy F. Chemaly, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted this randomized, double-blind, phase 2 trial. It was funded by AiCuris, the company that was developing letermovir before Merck purchased worldwide rights to develop and commercialize the drug in 2012.
The researchers evaluated the effect of letermovir on the incidence and time-to-onset of CMV prophylaxis failure in CMV-seropositive, matched transplant recipients.
The study included 131 patients. For 12 weeks after engraftment, they received placebo (n=33) or letermovir at 60 mg/day (n=33), 120 mg/day (n=31), or 240 mg/day (n=34).
Efficacy analysis
The primary endpoint was all-cause prophylaxis failure, which was defined as discontinuation of the study drug due to CMV antigen or CMV DNA detection, end-organ disease, or any other causes unrelated to CMV.
The primary efficacy analysis population was a modified intention-to-treat population, which included all patients who received at least 1 dose of the study drug and had at least 1 measurement of the CMV viral load during the study.
The incidence of all-cause prophylaxis failure was significantly lower in the groups that received letermovir at doses of 120 mg/day or 240 mg/day, when compared with the placebo group—32% and 29% vs 64%; P=0.01 and P=0.007, respectively.
The time to the onset of prophylaxis failure was significantly shorter in the 240-mg group (range, 1 to 8 days) than in the placebo group (range, 1 to 21 days; P=0.002).
However, comparisons with the placebo group were not significant for the 60-mg group (range, 1 to 42 days; P=0.15) or the 120-mg group (range, 1 to 15 days; P=0.13).
The incidence of virologic failure was lower in the 240-mg group (6%) than in the 120-mg group (19%), the 60-mg group (21%), or the placebo group (36%).
Virologic failure was defined as either detectable CMV antigen or DNA in the blood at 2 consecutive time points (with at least 1 instance confirmed by the central lab), leading to discontinuation of the study drug and the administration of rescue medication or the development of CMV end-organ disease.
Safety analysis
Nearly all of the patients had at least 1 adverse event during treatment—94% in the 60-mg and 120-mg groups and 100% in the 240-mg and placebo groups. Most events were mild or moderate.
However, 24% of letermovir-treated patients and 30% of those who received placebo experienced severe adverse events during treatment. Investigators, who were blinded to treatment, considered 17% of the severe events in the letermovir group to be drug-related and 33% of severe events in the placebo group to be drug-related.
Most adverse events were gastrointestinal disorders—diarrhea, nausea, and vomiting—which occurred in 66% of letermovir-treated patients and 61% of patients in the placebo group. Infections—mostly CMV—were also common, occurring in 59% of letermovir-treated patients and 76% of patients in the placebo group.
Five patients died during the trial. None of the deaths were thought to be related to treatment or to CMV. ![]()
An antiviral agent can reduce the incidence of cytomegalovirus (CMV) infection in patients receiving an allogeneic hematopoietic stem cell
transplant, according to a study published in The New England Journal of Medicine.
The agent, letermovir, proved more effective than placebo in preventing CMV, and the highest dose tested, 240 mg/day, was most effective.
The most common adverse events were gastrointestinal disorders and infections.
However, the incidence of events was similar among treated patients and those in the placebo arm.
Roy F. Chemaly, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted this randomized, double-blind, phase 2 trial. It was funded by AiCuris, the company that was developing letermovir before Merck purchased worldwide rights to develop and commercialize the drug in 2012.
The researchers evaluated the effect of letermovir on the incidence and time-to-onset of CMV prophylaxis failure in CMV-seropositive, matched transplant recipients.
The study included 131 patients. For 12 weeks after engraftment, they received placebo (n=33) or letermovir at 60 mg/day (n=33), 120 mg/day (n=31), or 240 mg/day (n=34).
Efficacy analysis
The primary endpoint was all-cause prophylaxis failure, which was defined as discontinuation of the study drug due to CMV antigen or CMV DNA detection, end-organ disease, or any other causes unrelated to CMV.
The primary efficacy analysis population was a modified intention-to-treat population, which included all patients who received at least 1 dose of the study drug and had at least 1 measurement of the CMV viral load during the study.
The incidence of all-cause prophylaxis failure was significantly lower in the groups that received letermovir at doses of 120 mg/day or 240 mg/day, when compared with the placebo group—32% and 29% vs 64%; P=0.01 and P=0.007, respectively.
The time to the onset of prophylaxis failure was significantly shorter in the 240-mg group (range, 1 to 8 days) than in the placebo group (range, 1 to 21 days; P=0.002).
However, comparisons with the placebo group were not significant for the 60-mg group (range, 1 to 42 days; P=0.15) or the 120-mg group (range, 1 to 15 days; P=0.13).
The incidence of virologic failure was lower in the 240-mg group (6%) than in the 120-mg group (19%), the 60-mg group (21%), or the placebo group (36%).
Virologic failure was defined as either detectable CMV antigen or DNA in the blood at 2 consecutive time points (with at least 1 instance confirmed by the central lab), leading to discontinuation of the study drug and the administration of rescue medication or the development of CMV end-organ disease.
Safety analysis
Nearly all of the patients had at least 1 adverse event during treatment—94% in the 60-mg and 120-mg groups and 100% in the 240-mg and placebo groups. Most events were mild or moderate.
However, 24% of letermovir-treated patients and 30% of those who received placebo experienced severe adverse events during treatment. Investigators, who were blinded to treatment, considered 17% of the severe events in the letermovir group to be drug-related and 33% of severe events in the placebo group to be drug-related.
Most adverse events were gastrointestinal disorders—diarrhea, nausea, and vomiting—which occurred in 66% of letermovir-treated patients and 61% of patients in the placebo group. Infections—mostly CMV—were also common, occurring in 59% of letermovir-treated patients and 76% of patients in the placebo group.
Five patients died during the trial. None of the deaths were thought to be related to treatment or to CMV. ![]()
Wnt pathway appears key to cell reprogramming

Salk Institute
The Wnt signaling pathway plays a key role in the generation of induced pluripotent stem cells (iPSCs), according to a study published in Stem Cell Reports.
Researchers found they could increase the efficiency of the cell reprogramming process by inhibiting the Wnt pathway.
“[U]ntil now, this was a very inefficient process,” said study author Ilda Theka, a PhD student at the Centre for Genomic Regulation in Barcelona, Spain.
“There are many groups trying to understand the mechanism by which adult cells become pluripotent and what blocks that process and makes only a small percentage of cells end up being reprogrammed. We are providing information on why it happens.”
The researchers studied how the Wnt pathway behaves throughout the process of transforming mature cells into iPSCs, which usually lasts 2 weeks. It’s a dynamic process that produces oscillations from the pathway, which is not active all the time.
“We have seen that there are two phases and that, in each one of them, Wnt fulfils a different function,” Theka said. “And we have shown that, by inhibiting it at the beginning of the process and activating it at the end, we can increase the efficiency of reprogramming and obtain a larger number of pluripotent cells.”
The team also discovered that the exact moment when the Wnt pathway is activated is crucial. Activating the pathway too early makes the cells begin to differentiate, and they are not reprogrammed.
To artificially control the pathway, the researchers used a molecule called Iwp2, a Wnt-secretion inhibitor that does not permanently alter the cells. ![]()

Salk Institute
The Wnt signaling pathway plays a key role in the generation of induced pluripotent stem cells (iPSCs), according to a study published in Stem Cell Reports.
Researchers found they could increase the efficiency of the cell reprogramming process by inhibiting the Wnt pathway.
“[U]ntil now, this was a very inefficient process,” said study author Ilda Theka, a PhD student at the Centre for Genomic Regulation in Barcelona, Spain.
“There are many groups trying to understand the mechanism by which adult cells become pluripotent and what blocks that process and makes only a small percentage of cells end up being reprogrammed. We are providing information on why it happens.”
The researchers studied how the Wnt pathway behaves throughout the process of transforming mature cells into iPSCs, which usually lasts 2 weeks. It’s a dynamic process that produces oscillations from the pathway, which is not active all the time.
“We have seen that there are two phases and that, in each one of them, Wnt fulfils a different function,” Theka said. “And we have shown that, by inhibiting it at the beginning of the process and activating it at the end, we can increase the efficiency of reprogramming and obtain a larger number of pluripotent cells.”
The team also discovered that the exact moment when the Wnt pathway is activated is crucial. Activating the pathway too early makes the cells begin to differentiate, and they are not reprogrammed.
To artificially control the pathway, the researchers used a molecule called Iwp2, a Wnt-secretion inhibitor that does not permanently alter the cells. ![]()

Salk Institute
The Wnt signaling pathway plays a key role in the generation of induced pluripotent stem cells (iPSCs), according to a study published in Stem Cell Reports.
Researchers found they could increase the efficiency of the cell reprogramming process by inhibiting the Wnt pathway.
“[U]ntil now, this was a very inefficient process,” said study author Ilda Theka, a PhD student at the Centre for Genomic Regulation in Barcelona, Spain.
“There are many groups trying to understand the mechanism by which adult cells become pluripotent and what blocks that process and makes only a small percentage of cells end up being reprogrammed. We are providing information on why it happens.”
The researchers studied how the Wnt pathway behaves throughout the process of transforming mature cells into iPSCs, which usually lasts 2 weeks. It’s a dynamic process that produces oscillations from the pathway, which is not active all the time.
“We have seen that there are two phases and that, in each one of them, Wnt fulfils a different function,” Theka said. “And we have shown that, by inhibiting it at the beginning of the process and activating it at the end, we can increase the efficiency of reprogramming and obtain a larger number of pluripotent cells.”
The team also discovered that the exact moment when the Wnt pathway is activated is crucial. Activating the pathway too early makes the cells begin to differentiate, and they are not reprogrammed.
To artificially control the pathway, the researchers used a molecule called Iwp2, a Wnt-secretion inhibitor that does not permanently alter the cells. ![]()
Artificial bone marrow seems just like the real thing

Credit: James Weaver,
Harvard’s Wyss Institute
Researchers have created a device that reproduces the structure, function, and cellular make-up of bone marrow, according to a paper published
in Nature Methods.
The device, dubbed “bone marrow on a chip,” could serve as a new tool for testing the effects of radiation and other toxic agents on whole bone marrow.
The researchers believe this bone marrow on a chip could provide an alternative to animal testing, although the device itself was generated in mice.
The team also thinks that, in the future, the engineered bone marrow could be used to maintain a cancer patient’s own marrow temporarily during radiation or high-dose chemotherapy.
In an initial test, the engineered bone marrow withered in response to radiation, just as natural bone marrow does. And, as in natural marrow, granulocyte colony-stimulating factor conferred a protective effect on the engineered marrow.
“Bone marrow is an incredibly complex organ that is responsible for producing all of the blood cell types in our body, and our bone marrow chips are able to recapitulate this complexity in its entirety and maintain it in a functional form in vitro,” said study author Donald Ingber, MD, PhD, of the Wyss Institute for Biologically Inspired Engineering at Harvard University in Boston.
Dr Ingber leads an effort to develop human organs on chips—small microfluidic devices that mimic the physiology of living organs. To build these devices, researchers combine multiple types of cells from an organ on a plastic microfluidic device, while steadily supplying nutrients, removing waste, and applying mechanical forces the tissues would face in the body.
But bone marrow is so complex that Dr Ingber and his colleagues needed a new approach to mimic organ function. Rather than trying to reproduce such a complex structure cell by cell, the team used mice.
Specifically, the researchers packed dried bone powder into an open, ring-shaped mold the size of a coin battery and implanted the mold under the skin on the animal’s back.
After 8 weeks, the team surgically removed the disk-shaped bone that had formed in the mold and examined it with a specialized CAT scanner. The scan showed a honeycomb-like structure that looked identical to natural trabecular bone.
The marrow looked like the real thing as well. When the researchers stained the tissue and examined it under a microscope, the marrow was packed with blood cells, just like marrow from a living mouse.
And when the team sorted the bone marrow cells by type and tallied their numbers, the mix of different types of blood and immune cells in the engineered bone marrow was identical to that in a mouse thighbone.
To sustain the engineered bone marrow outside of a living animal, the researchers surgically removed the engineered bone from mice, then placed it in a microfluidic device that mimics the circulation the tissue would experience in the body.
Marrow in the device remained healthy for up to 1 week. This is typically long enough to test the toxicity and effectiveness of a new drug, the team said.
The device also passed an initial test of its drug-testing capabilities. Like marrow from live mice, this engineered marrow was susceptible to radiation, but granulocyte colony-stimulating factor conferred a protective effect.
The researchers believe that, in the future, they could potentially grow human bone marrow in immune-deficient mice. And their bone marrow on a chip could generate blood cells, which could circulate in an artificial circulatory system to supply a network of other organs on chips. ![]()

Credit: James Weaver,
Harvard’s Wyss Institute
Researchers have created a device that reproduces the structure, function, and cellular make-up of bone marrow, according to a paper published
in Nature Methods.
The device, dubbed “bone marrow on a chip,” could serve as a new tool for testing the effects of radiation and other toxic agents on whole bone marrow.
The researchers believe this bone marrow on a chip could provide an alternative to animal testing, although the device itself was generated in mice.
The team also thinks that, in the future, the engineered bone marrow could be used to maintain a cancer patient’s own marrow temporarily during radiation or high-dose chemotherapy.
In an initial test, the engineered bone marrow withered in response to radiation, just as natural bone marrow does. And, as in natural marrow, granulocyte colony-stimulating factor conferred a protective effect on the engineered marrow.
“Bone marrow is an incredibly complex organ that is responsible for producing all of the blood cell types in our body, and our bone marrow chips are able to recapitulate this complexity in its entirety and maintain it in a functional form in vitro,” said study author Donald Ingber, MD, PhD, of the Wyss Institute for Biologically Inspired Engineering at Harvard University in Boston.
Dr Ingber leads an effort to develop human organs on chips—small microfluidic devices that mimic the physiology of living organs. To build these devices, researchers combine multiple types of cells from an organ on a plastic microfluidic device, while steadily supplying nutrients, removing waste, and applying mechanical forces the tissues would face in the body.
But bone marrow is so complex that Dr Ingber and his colleagues needed a new approach to mimic organ function. Rather than trying to reproduce such a complex structure cell by cell, the team used mice.
Specifically, the researchers packed dried bone powder into an open, ring-shaped mold the size of a coin battery and implanted the mold under the skin on the animal’s back.
After 8 weeks, the team surgically removed the disk-shaped bone that had formed in the mold and examined it with a specialized CAT scanner. The scan showed a honeycomb-like structure that looked identical to natural trabecular bone.
The marrow looked like the real thing as well. When the researchers stained the tissue and examined it under a microscope, the marrow was packed with blood cells, just like marrow from a living mouse.
And when the team sorted the bone marrow cells by type and tallied their numbers, the mix of different types of blood and immune cells in the engineered bone marrow was identical to that in a mouse thighbone.
To sustain the engineered bone marrow outside of a living animal, the researchers surgically removed the engineered bone from mice, then placed it in a microfluidic device that mimics the circulation the tissue would experience in the body.
Marrow in the device remained healthy for up to 1 week. This is typically long enough to test the toxicity and effectiveness of a new drug, the team said.
The device also passed an initial test of its drug-testing capabilities. Like marrow from live mice, this engineered marrow was susceptible to radiation, but granulocyte colony-stimulating factor conferred a protective effect.
The researchers believe that, in the future, they could potentially grow human bone marrow in immune-deficient mice. And their bone marrow on a chip could generate blood cells, which could circulate in an artificial circulatory system to supply a network of other organs on chips. ![]()

Credit: James Weaver,
Harvard’s Wyss Institute
Researchers have created a device that reproduces the structure, function, and cellular make-up of bone marrow, according to a paper published
in Nature Methods.
The device, dubbed “bone marrow on a chip,” could serve as a new tool for testing the effects of radiation and other toxic agents on whole bone marrow.
The researchers believe this bone marrow on a chip could provide an alternative to animal testing, although the device itself was generated in mice.
The team also thinks that, in the future, the engineered bone marrow could be used to maintain a cancer patient’s own marrow temporarily during radiation or high-dose chemotherapy.
In an initial test, the engineered bone marrow withered in response to radiation, just as natural bone marrow does. And, as in natural marrow, granulocyte colony-stimulating factor conferred a protective effect on the engineered marrow.
“Bone marrow is an incredibly complex organ that is responsible for producing all of the blood cell types in our body, and our bone marrow chips are able to recapitulate this complexity in its entirety and maintain it in a functional form in vitro,” said study author Donald Ingber, MD, PhD, of the Wyss Institute for Biologically Inspired Engineering at Harvard University in Boston.
Dr Ingber leads an effort to develop human organs on chips—small microfluidic devices that mimic the physiology of living organs. To build these devices, researchers combine multiple types of cells from an organ on a plastic microfluidic device, while steadily supplying nutrients, removing waste, and applying mechanical forces the tissues would face in the body.
But bone marrow is so complex that Dr Ingber and his colleagues needed a new approach to mimic organ function. Rather than trying to reproduce such a complex structure cell by cell, the team used mice.
Specifically, the researchers packed dried bone powder into an open, ring-shaped mold the size of a coin battery and implanted the mold under the skin on the animal’s back.
After 8 weeks, the team surgically removed the disk-shaped bone that had formed in the mold and examined it with a specialized CAT scanner. The scan showed a honeycomb-like structure that looked identical to natural trabecular bone.
The marrow looked like the real thing as well. When the researchers stained the tissue and examined it under a microscope, the marrow was packed with blood cells, just like marrow from a living mouse.
And when the team sorted the bone marrow cells by type and tallied their numbers, the mix of different types of blood and immune cells in the engineered bone marrow was identical to that in a mouse thighbone.
To sustain the engineered bone marrow outside of a living animal, the researchers surgically removed the engineered bone from mice, then placed it in a microfluidic device that mimics the circulation the tissue would experience in the body.
Marrow in the device remained healthy for up to 1 week. This is typically long enough to test the toxicity and effectiveness of a new drug, the team said.
The device also passed an initial test of its drug-testing capabilities. Like marrow from live mice, this engineered marrow was susceptible to radiation, but granulocyte colony-stimulating factor conferred a protective effect.
The researchers believe that, in the future, they could potentially grow human bone marrow in immune-deficient mice. And their bone marrow on a chip could generate blood cells, which could circulate in an artificial circulatory system to supply a network of other organs on chips. ![]()
Protein is key to HSC recovery after chemo, radiation

in the bone marrow
The protein beta-catenin plays a critical role in promoting the recovery of hematopoietic stem cells (HSCs) after exposure to radiation or chemotherapy, according to preclinical research published in Genes & Development.
The study provides new insight into the impact of radiation and chemotherapy on cellular and molecular processes.
And it points to possibilities for improving HSC regeneration in cancer patients who have undergone these treatments.
Study investigators first used mouse models to show that exposure to radiation triggers activation of the Wnt signaling pathway in hematopoietic stem and progenitor cells.
“The Wnt pathway and its key mediator, beta catenin, are critical for embryonic development and establishment of the body plan,” explained Tannishtha Reya, PhD, of the University of California, San Diego.
“In addition, the Wnt pathway is activated in stem cells from many tissues and is needed for their continued maintenance.”
Dr Reya and her colleagues then found that mice deficient in beta-catenin lacked the ability to activate canonical Wnt signaling. They also suffered from impaired HSC regeneration and bone marrow recovery after radiation or chemotherapy.
Mouse HSCs without beta-catenin could not suppress the production of reactive oxygen species or resolve DNA double-strand breaks. As a result, they could not recover effectively after radiation exposure or treatment with the chemotherapeutic agent fluorouracil.
“Our work shows that Wnt signaling is important in the mammalian hematopoietic system and is critical for recovery from chemotherapy and radiation,” Dr Reya said. “There are 2 major reasons why accelerating regeneration is important clinically.”
“One is that, after cancer patients are irradiated and transplanted with stem cells, the rate and extent of recovery is often not sufficient to protect the patient from anemia or infections . . . . Identifying signals that can boost regeneration after the bone marrow is severely damaged may help improve outcomes after transplantation.”
“Second, doses of chemotherapy and radiation used to treat cancer are often limited by the collateral damage they cause to normal tissues. If we can improve and accelerate recovery, we might be able to use higher doses of radiation or chemotherapy and reduce the risk of cancer relapse.”
Dr Reya added that this research suggests HSC regeneration could be accelerated by modulating the Wnt pathway, either by delivering additional Wnt proteins directly to patients or through drugs that activate the pathway. ![]()

in the bone marrow
The protein beta-catenin plays a critical role in promoting the recovery of hematopoietic stem cells (HSCs) after exposure to radiation or chemotherapy, according to preclinical research published in Genes & Development.
The study provides new insight into the impact of radiation and chemotherapy on cellular and molecular processes.
And it points to possibilities for improving HSC regeneration in cancer patients who have undergone these treatments.
Study investigators first used mouse models to show that exposure to radiation triggers activation of the Wnt signaling pathway in hematopoietic stem and progenitor cells.
“The Wnt pathway and its key mediator, beta catenin, are critical for embryonic development and establishment of the body plan,” explained Tannishtha Reya, PhD, of the University of California, San Diego.
“In addition, the Wnt pathway is activated in stem cells from many tissues and is needed for their continued maintenance.”
Dr Reya and her colleagues then found that mice deficient in beta-catenin lacked the ability to activate canonical Wnt signaling. They also suffered from impaired HSC regeneration and bone marrow recovery after radiation or chemotherapy.
Mouse HSCs without beta-catenin could not suppress the production of reactive oxygen species or resolve DNA double-strand breaks. As a result, they could not recover effectively after radiation exposure or treatment with the chemotherapeutic agent fluorouracil.
“Our work shows that Wnt signaling is important in the mammalian hematopoietic system and is critical for recovery from chemotherapy and radiation,” Dr Reya said. “There are 2 major reasons why accelerating regeneration is important clinically.”
“One is that, after cancer patients are irradiated and transplanted with stem cells, the rate and extent of recovery is often not sufficient to protect the patient from anemia or infections . . . . Identifying signals that can boost regeneration after the bone marrow is severely damaged may help improve outcomes after transplantation.”
“Second, doses of chemotherapy and radiation used to treat cancer are often limited by the collateral damage they cause to normal tissues. If we can improve and accelerate recovery, we might be able to use higher doses of radiation or chemotherapy and reduce the risk of cancer relapse.”
Dr Reya added that this research suggests HSC regeneration could be accelerated by modulating the Wnt pathway, either by delivering additional Wnt proteins directly to patients or through drugs that activate the pathway. ![]()

in the bone marrow
The protein beta-catenin plays a critical role in promoting the recovery of hematopoietic stem cells (HSCs) after exposure to radiation or chemotherapy, according to preclinical research published in Genes & Development.
The study provides new insight into the impact of radiation and chemotherapy on cellular and molecular processes.
And it points to possibilities for improving HSC regeneration in cancer patients who have undergone these treatments.
Study investigators first used mouse models to show that exposure to radiation triggers activation of the Wnt signaling pathway in hematopoietic stem and progenitor cells.
“The Wnt pathway and its key mediator, beta catenin, are critical for embryonic development and establishment of the body plan,” explained Tannishtha Reya, PhD, of the University of California, San Diego.
“In addition, the Wnt pathway is activated in stem cells from many tissues and is needed for their continued maintenance.”
Dr Reya and her colleagues then found that mice deficient in beta-catenin lacked the ability to activate canonical Wnt signaling. They also suffered from impaired HSC regeneration and bone marrow recovery after radiation or chemotherapy.
Mouse HSCs without beta-catenin could not suppress the production of reactive oxygen species or resolve DNA double-strand breaks. As a result, they could not recover effectively after radiation exposure or treatment with the chemotherapeutic agent fluorouracil.
“Our work shows that Wnt signaling is important in the mammalian hematopoietic system and is critical for recovery from chemotherapy and radiation,” Dr Reya said. “There are 2 major reasons why accelerating regeneration is important clinically.”
“One is that, after cancer patients are irradiated and transplanted with stem cells, the rate and extent of recovery is often not sufficient to protect the patient from anemia or infections . . . . Identifying signals that can boost regeneration after the bone marrow is severely damaged may help improve outcomes after transplantation.”
“Second, doses of chemotherapy and radiation used to treat cancer are often limited by the collateral damage they cause to normal tissues. If we can improve and accelerate recovery, we might be able to use higher doses of radiation or chemotherapy and reduce the risk of cancer relapse.”
Dr Reya added that this research suggests HSC regeneration could be accelerated by modulating the Wnt pathway, either by delivering additional Wnt proteins directly to patients or through drugs that activate the pathway. ![]()
Maintaining stem cell pluripotency

Salk Institute
Retrotransposons—viral elements incorporated into the human genome—may play a key role in maintaining stem cell pluripotency, a new study suggests.
Previous research indicated that an important fraction of mammalian transcriptomes consists of transcripts derived from retrotransposon elements, but the biological function of these transcripts was unknown.
Now, experiments in induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) have provided some insight into the transcripts’ function.
Piero Carninci, PhD, of RIKEN Center for Life Science Technologies in Yokohama, Japan, and his colleagues described these findings in Nature Genetics.
The researchers found that thousands of transcripts in stem cells that have not yet been annotated are transcribed from retrotransposons, presumably to elicit nuclear functions. These transcripts were expressed in iPSCs and ESCs but not in differentiated cells.
Furthermore, several of the transcripts were shown to be involved in the maintenance of pluripotency. Degrading them using RNA interference caused iPSCs to lose their pluripotency and differentiate.
The researchers said these transcripts appear to have been recruited, both in the human and mouse genome, where they are used to maintain the pluripotency of stem cells. But more research is needed to determine exactly how and why this occurs.
“Our work has just begun to unravel the scale of unexpected functions carried out by retrotransposons and their derived transcripts in stem cell biology,” Dr Carninci said.
“We were extremely surprised to learn from our data that what was once considered genetic junk—namely, ancient retroviruses that were thought to just parasite the genome—are, in reality, symbiotic elements that work closely with other genes to maintain [iPSCs and ESCs] in their undifferentiated state.” ![]()

Salk Institute
Retrotransposons—viral elements incorporated into the human genome—may play a key role in maintaining stem cell pluripotency, a new study suggests.
Previous research indicated that an important fraction of mammalian transcriptomes consists of transcripts derived from retrotransposon elements, but the biological function of these transcripts was unknown.
Now, experiments in induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) have provided some insight into the transcripts’ function.
Piero Carninci, PhD, of RIKEN Center for Life Science Technologies in Yokohama, Japan, and his colleagues described these findings in Nature Genetics.
The researchers found that thousands of transcripts in stem cells that have not yet been annotated are transcribed from retrotransposons, presumably to elicit nuclear functions. These transcripts were expressed in iPSCs and ESCs but not in differentiated cells.
Furthermore, several of the transcripts were shown to be involved in the maintenance of pluripotency. Degrading them using RNA interference caused iPSCs to lose their pluripotency and differentiate.
The researchers said these transcripts appear to have been recruited, both in the human and mouse genome, where they are used to maintain the pluripotency of stem cells. But more research is needed to determine exactly how and why this occurs.
“Our work has just begun to unravel the scale of unexpected functions carried out by retrotransposons and their derived transcripts in stem cell biology,” Dr Carninci said.
“We were extremely surprised to learn from our data that what was once considered genetic junk—namely, ancient retroviruses that were thought to just parasite the genome—are, in reality, symbiotic elements that work closely with other genes to maintain [iPSCs and ESCs] in their undifferentiated state.” ![]()

Salk Institute
Retrotransposons—viral elements incorporated into the human genome—may play a key role in maintaining stem cell pluripotency, a new study suggests.
Previous research indicated that an important fraction of mammalian transcriptomes consists of transcripts derived from retrotransposon elements, but the biological function of these transcripts was unknown.
Now, experiments in induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) have provided some insight into the transcripts’ function.
Piero Carninci, PhD, of RIKEN Center for Life Science Technologies in Yokohama, Japan, and his colleagues described these findings in Nature Genetics.
The researchers found that thousands of transcripts in stem cells that have not yet been annotated are transcribed from retrotransposons, presumably to elicit nuclear functions. These transcripts were expressed in iPSCs and ESCs but not in differentiated cells.
Furthermore, several of the transcripts were shown to be involved in the maintenance of pluripotency. Degrading them using RNA interference caused iPSCs to lose their pluripotency and differentiate.
The researchers said these transcripts appear to have been recruited, both in the human and mouse genome, where they are used to maintain the pluripotency of stem cells. But more research is needed to determine exactly how and why this occurs.
“Our work has just begun to unravel the scale of unexpected functions carried out by retrotransposons and their derived transcripts in stem cell biology,” Dr Carninci said.
“We were extremely surprised to learn from our data that what was once considered genetic junk—namely, ancient retroviruses that were thought to just parasite the genome—are, in reality, symbiotic elements that work closely with other genes to maintain [iPSCs and ESCs] in their undifferentiated state.” ![]()
HDAC inhibitors aid expansion of HSCs from cord blood

Credit: NHS
New research suggests that histone deacetylase (HDAC) inhibitors can be used to expand hematopoietic stem cells (HSCs) isolated from cord blood.
Investigators found that valproic acid (VPA), in particular, could greatly expand HSCs from cord blood.
These HSCs expressed markers of pluripotency and were more efficient than conventionally expanded HSCs in repopulating the bone marrow and establishing hematopoiesis in immune-deficient mice.
Pratima Chaurasia, PhD, of the Mount Sinai School of Medicine in New York, and colleagues reported these results in The Journal of Clinical Investigation.
For several decades, investigators have used a variety of strategies to expand the numbers of HSCs isolated from cord blood, with limited success. Evidence has suggested the accumulation of epigenetic modifications influences preservation of stem-cell characteristics in HSC daughter cells.
So Dr Chaurasia’s group tested the effects of HDAC inhibitors on CD34+ cells derived from cord blood. The team primed the cells for 16 hours with cytokines, then treated the cells for 7 days, with or without additional cytokines and in the presence or absence of HDAC inhibitors.
The inhibitors included VPA, scriptaid (SCR), trichostatin A, suberoylanilide hydroxamic acid, CAY10433 (C433), CAY10398, and CAY10603 .
VPA, SCR, and C433 were the most active inhibitors. Treatment with these agents led to a similar percentage of CD34+CD90+ cells—75.2% ± 10.7%, 73.4% ± 13.9%, and 70.1% ± 18.4%, respectively—which was significantly higher than control conditions—16.2% ± 9.2% (P<0.0001).
VPA, SCR, and C433 also generated a greater absolute number of CD34+ and CD34+CD90+ cells per cord blood collection, when compared to control conditions (P≤0.0007). And the inhibitors generated a greater absolute number of CD34+CD90+CD184+ cells (P<0.0001)
The investigators conducted subsequent experiments with VPA only. And they found that VPA was more effective under serum-free conditions and in the presence of cytokines.
Additional experiments revealed that VPA influences the expression of pluripotency genes—SOX2, OCT4, and NANOG. And these genes proved essential for the expansion of CD34+ CD90+ cells.
Lastly, the investigators tested VPA-expanded cells by transplanting them into immune-deficient mice. The cells were more efficient than conventionally expanded cord blood HSCs in repopulating the bone marrow and establishing hematopoietic populations.
Specifically, at 13 to 14 weeks after transplant, VPA-treated cord blood CD34+ cells resulted in a greater degree of human CD45+ cell chimerism—32.2% ± 11.3%—when compared to primary cord blood CD34+ cells—19.4% ± 4.9%—and to cells from control cultures—13.2% ± 6.4% (P=0.006 and P=0.0008, respectively).
The investigators evaluated the self-renewal potential of the expanded grafts by transplanting donor-derived cells from primary recipients into secondary recipients.
After 15 to 16 weeks, the secondary recipients transplanted with VPA-treated cord blood CD34+ cells had achieved the greatest degree of human CD45+ cell chimerism, compared to primary cord blood CD34+ cells and cells from control cultures (P<0.0001).
The team also noted that the VPA-treated cells belonged to multiple hematopoietic lineages. And this pattern was distinct from that observed in mice that had received primary cord blood CD34+ cells or cells from control cultures (P<0.0001).
In addition, the VPA-treated HSCs did not cause hematologic malignancies or teratomas in the mice.
The investigators said these results suggest that cord blood cells can be epigenetically reprogrammed by VPA to generate greater numbers of functional HSCs for transplantation.
In a related commentary, Hal Broxmeyer, PhD, of the Indiana University School of Medicine in Indianapolis, discussed how these findings enhance our understanding of HSC function and could potentially provide clinical benefit. ![]()

Credit: NHS
New research suggests that histone deacetylase (HDAC) inhibitors can be used to expand hematopoietic stem cells (HSCs) isolated from cord blood.
Investigators found that valproic acid (VPA), in particular, could greatly expand HSCs from cord blood.
These HSCs expressed markers of pluripotency and were more efficient than conventionally expanded HSCs in repopulating the bone marrow and establishing hematopoiesis in immune-deficient mice.
Pratima Chaurasia, PhD, of the Mount Sinai School of Medicine in New York, and colleagues reported these results in The Journal of Clinical Investigation.
For several decades, investigators have used a variety of strategies to expand the numbers of HSCs isolated from cord blood, with limited success. Evidence has suggested the accumulation of epigenetic modifications influences preservation of stem-cell characteristics in HSC daughter cells.
So Dr Chaurasia’s group tested the effects of HDAC inhibitors on CD34+ cells derived from cord blood. The team primed the cells for 16 hours with cytokines, then treated the cells for 7 days, with or without additional cytokines and in the presence or absence of HDAC inhibitors.
The inhibitors included VPA, scriptaid (SCR), trichostatin A, suberoylanilide hydroxamic acid, CAY10433 (C433), CAY10398, and CAY10603 .
VPA, SCR, and C433 were the most active inhibitors. Treatment with these agents led to a similar percentage of CD34+CD90+ cells—75.2% ± 10.7%, 73.4% ± 13.9%, and 70.1% ± 18.4%, respectively—which was significantly higher than control conditions—16.2% ± 9.2% (P<0.0001).
VPA, SCR, and C433 also generated a greater absolute number of CD34+ and CD34+CD90+ cells per cord blood collection, when compared to control conditions (P≤0.0007). And the inhibitors generated a greater absolute number of CD34+CD90+CD184+ cells (P<0.0001)
The investigators conducted subsequent experiments with VPA only. And they found that VPA was more effective under serum-free conditions and in the presence of cytokines.
Additional experiments revealed that VPA influences the expression of pluripotency genes—SOX2, OCT4, and NANOG. And these genes proved essential for the expansion of CD34+ CD90+ cells.
Lastly, the investigators tested VPA-expanded cells by transplanting them into immune-deficient mice. The cells were more efficient than conventionally expanded cord blood HSCs in repopulating the bone marrow and establishing hematopoietic populations.
Specifically, at 13 to 14 weeks after transplant, VPA-treated cord blood CD34+ cells resulted in a greater degree of human CD45+ cell chimerism—32.2% ± 11.3%—when compared to primary cord blood CD34+ cells—19.4% ± 4.9%—and to cells from control cultures—13.2% ± 6.4% (P=0.006 and P=0.0008, respectively).
The investigators evaluated the self-renewal potential of the expanded grafts by transplanting donor-derived cells from primary recipients into secondary recipients.
After 15 to 16 weeks, the secondary recipients transplanted with VPA-treated cord blood CD34+ cells had achieved the greatest degree of human CD45+ cell chimerism, compared to primary cord blood CD34+ cells and cells from control cultures (P<0.0001).
The team also noted that the VPA-treated cells belonged to multiple hematopoietic lineages. And this pattern was distinct from that observed in mice that had received primary cord blood CD34+ cells or cells from control cultures (P<0.0001).
In addition, the VPA-treated HSCs did not cause hematologic malignancies or teratomas in the mice.
The investigators said these results suggest that cord blood cells can be epigenetically reprogrammed by VPA to generate greater numbers of functional HSCs for transplantation.
In a related commentary, Hal Broxmeyer, PhD, of the Indiana University School of Medicine in Indianapolis, discussed how these findings enhance our understanding of HSC function and could potentially provide clinical benefit. ![]()

Credit: NHS
New research suggests that histone deacetylase (HDAC) inhibitors can be used to expand hematopoietic stem cells (HSCs) isolated from cord blood.
Investigators found that valproic acid (VPA), in particular, could greatly expand HSCs from cord blood.
These HSCs expressed markers of pluripotency and were more efficient than conventionally expanded HSCs in repopulating the bone marrow and establishing hematopoiesis in immune-deficient mice.
Pratima Chaurasia, PhD, of the Mount Sinai School of Medicine in New York, and colleagues reported these results in The Journal of Clinical Investigation.
For several decades, investigators have used a variety of strategies to expand the numbers of HSCs isolated from cord blood, with limited success. Evidence has suggested the accumulation of epigenetic modifications influences preservation of stem-cell characteristics in HSC daughter cells.
So Dr Chaurasia’s group tested the effects of HDAC inhibitors on CD34+ cells derived from cord blood. The team primed the cells for 16 hours with cytokines, then treated the cells for 7 days, with or without additional cytokines and in the presence or absence of HDAC inhibitors.
The inhibitors included VPA, scriptaid (SCR), trichostatin A, suberoylanilide hydroxamic acid, CAY10433 (C433), CAY10398, and CAY10603 .
VPA, SCR, and C433 were the most active inhibitors. Treatment with these agents led to a similar percentage of CD34+CD90+ cells—75.2% ± 10.7%, 73.4% ± 13.9%, and 70.1% ± 18.4%, respectively—which was significantly higher than control conditions—16.2% ± 9.2% (P<0.0001).
VPA, SCR, and C433 also generated a greater absolute number of CD34+ and CD34+CD90+ cells per cord blood collection, when compared to control conditions (P≤0.0007). And the inhibitors generated a greater absolute number of CD34+CD90+CD184+ cells (P<0.0001)
The investigators conducted subsequent experiments with VPA only. And they found that VPA was more effective under serum-free conditions and in the presence of cytokines.
Additional experiments revealed that VPA influences the expression of pluripotency genes—SOX2, OCT4, and NANOG. And these genes proved essential for the expansion of CD34+ CD90+ cells.
Lastly, the investigators tested VPA-expanded cells by transplanting them into immune-deficient mice. The cells were more efficient than conventionally expanded cord blood HSCs in repopulating the bone marrow and establishing hematopoietic populations.
Specifically, at 13 to 14 weeks after transplant, VPA-treated cord blood CD34+ cells resulted in a greater degree of human CD45+ cell chimerism—32.2% ± 11.3%—when compared to primary cord blood CD34+ cells—19.4% ± 4.9%—and to cells from control cultures—13.2% ± 6.4% (P=0.006 and P=0.0008, respectively).
The investigators evaluated the self-renewal potential of the expanded grafts by transplanting donor-derived cells from primary recipients into secondary recipients.
After 15 to 16 weeks, the secondary recipients transplanted with VPA-treated cord blood CD34+ cells had achieved the greatest degree of human CD45+ cell chimerism, compared to primary cord blood CD34+ cells and cells from control cultures (P<0.0001).
The team also noted that the VPA-treated cells belonged to multiple hematopoietic lineages. And this pattern was distinct from that observed in mice that had received primary cord blood CD34+ cells or cells from control cultures (P<0.0001).
In addition, the VPA-treated HSCs did not cause hematologic malignancies or teratomas in the mice.
The investigators said these results suggest that cord blood cells can be epigenetically reprogrammed by VPA to generate greater numbers of functional HSCs for transplantation.
In a related commentary, Hal Broxmeyer, PhD, of the Indiana University School of Medicine in Indianapolis, discussed how these findings enhance our understanding of HSC function and could potentially provide clinical benefit. ![]()
Team reprograms blood cells into HSCs in mice

Researchers have found a way to reprogram mature blood cells from mice into hematopoietic stem cells (HSCs), according to a paper published in Cell.
The team used 8 transcription factors to reprogram blood progenitor cells and mature mouse myeloid cells into HSCs.
These cells, called induced HSCs (iHSCs), have the functional hallmarks of natural HSCs, are able to self-renew like natural HSCs, and can give rise to all of the cellular components of the blood.
“Blood cell production invariably goes in one direction—from stem cells, to progenitors, to mature effector cells,” said study author Derrick J. Rossi, PhD, of Boston Children’s Hospital in Massachusetts.
“We wanted to reverse the process and derive HSCs from differentiated blood cells using transcription factors that we found were specific to HSCs.”
To that end, Dr Rossi and his colleagues screened gene expression in 40 different types of blood and blood progenitor cells from mice. From this screen, the team identified 36 transcription factors that are expressed in HSCs but not in the cells that arise from them.
In a series of mouse transplantation experiments, the researchers found that 6 of the 36 transcription factors—Hlf, Runx1t1, Pbx1, Lmo2, Zfp37, and Prdm5—plus 2 additional factors not originally identified in their screen—Mycn and Meis1—were sufficient to reprogram 2 kinds of blood progenitor cells—pro/pre-B cells and common myeloid progenitor cells—into iHSCs.
The team reprogrammed their source cells by exposing them to viruses containing the genes for all 8 transcription factors and a molecular switch that turned the factor genes on in the presence of doxycycline. They then transplanted the exposed cells into recipient mice and activated the genes by giving the mice doxycycline.
The resulting iHSCs were capable of generating the entire blood cell repertoire in the transplanted mice, showing they had gained the ability to differentiate into all blood lineages. Stem cells collected from those recipients were capable of reconstituting the blood of secondary transplant recipients, proving that the 8-factor cocktail could instill the capacity for self-renewal.
Taking the work a step further, the researchers treated mature mouse myeloid cells with the same 8-factor cocktail. The resulting iHSCs produced all of the blood lineages and could regenerate the blood of secondary transplant recipients.
Study author Stuart Orkin, MD, of the Dana-Farber Cancer Institute in Boston, noted that the use of mice as a kind of reactor for reprogramming marks a novel direction in HSC research.
“In the blood research field, no one has the conditions to expand HSCs in the tissue culture dish,” he said. “Instead, by letting the reprogramming occur in mice, Rossi takes advantage of the signaling and environmental cues HSCs would normally experience.”
Dr Orkin added that iHSCs are nearly indistinguishable from normal HSCs at the transcriptional level. Unfortunately, though, these findings are far from translation to the clinic.
Researchers must still ascertain the precise contribution each of the 8 transcription factors makes in the reprogramming process and determine whether approaches that do not rely on viruses and transcription factors can have similar success.
In addition, studies are needed to test whether these results can be achieved using human cells and if other, non-blood cells can be reprogrammed to iHSCs. ![]()

Researchers have found a way to reprogram mature blood cells from mice into hematopoietic stem cells (HSCs), according to a paper published in Cell.
The team used 8 transcription factors to reprogram blood progenitor cells and mature mouse myeloid cells into HSCs.
These cells, called induced HSCs (iHSCs), have the functional hallmarks of natural HSCs, are able to self-renew like natural HSCs, and can give rise to all of the cellular components of the blood.
“Blood cell production invariably goes in one direction—from stem cells, to progenitors, to mature effector cells,” said study author Derrick J. Rossi, PhD, of Boston Children’s Hospital in Massachusetts.
“We wanted to reverse the process and derive HSCs from differentiated blood cells using transcription factors that we found were specific to HSCs.”
To that end, Dr Rossi and his colleagues screened gene expression in 40 different types of blood and blood progenitor cells from mice. From this screen, the team identified 36 transcription factors that are expressed in HSCs but not in the cells that arise from them.
In a series of mouse transplantation experiments, the researchers found that 6 of the 36 transcription factors—Hlf, Runx1t1, Pbx1, Lmo2, Zfp37, and Prdm5—plus 2 additional factors not originally identified in their screen—Mycn and Meis1—were sufficient to reprogram 2 kinds of blood progenitor cells—pro/pre-B cells and common myeloid progenitor cells—into iHSCs.
The team reprogrammed their source cells by exposing them to viruses containing the genes for all 8 transcription factors and a molecular switch that turned the factor genes on in the presence of doxycycline. They then transplanted the exposed cells into recipient mice and activated the genes by giving the mice doxycycline.
The resulting iHSCs were capable of generating the entire blood cell repertoire in the transplanted mice, showing they had gained the ability to differentiate into all blood lineages. Stem cells collected from those recipients were capable of reconstituting the blood of secondary transplant recipients, proving that the 8-factor cocktail could instill the capacity for self-renewal.
Taking the work a step further, the researchers treated mature mouse myeloid cells with the same 8-factor cocktail. The resulting iHSCs produced all of the blood lineages and could regenerate the blood of secondary transplant recipients.
Study author Stuart Orkin, MD, of the Dana-Farber Cancer Institute in Boston, noted that the use of mice as a kind of reactor for reprogramming marks a novel direction in HSC research.
“In the blood research field, no one has the conditions to expand HSCs in the tissue culture dish,” he said. “Instead, by letting the reprogramming occur in mice, Rossi takes advantage of the signaling and environmental cues HSCs would normally experience.”
Dr Orkin added that iHSCs are nearly indistinguishable from normal HSCs at the transcriptional level. Unfortunately, though, these findings are far from translation to the clinic.
Researchers must still ascertain the precise contribution each of the 8 transcription factors makes in the reprogramming process and determine whether approaches that do not rely on viruses and transcription factors can have similar success.
In addition, studies are needed to test whether these results can be achieved using human cells and if other, non-blood cells can be reprogrammed to iHSCs. ![]()

Researchers have found a way to reprogram mature blood cells from mice into hematopoietic stem cells (HSCs), according to a paper published in Cell.
The team used 8 transcription factors to reprogram blood progenitor cells and mature mouse myeloid cells into HSCs.
These cells, called induced HSCs (iHSCs), have the functional hallmarks of natural HSCs, are able to self-renew like natural HSCs, and can give rise to all of the cellular components of the blood.
“Blood cell production invariably goes in one direction—from stem cells, to progenitors, to mature effector cells,” said study author Derrick J. Rossi, PhD, of Boston Children’s Hospital in Massachusetts.
“We wanted to reverse the process and derive HSCs from differentiated blood cells using transcription factors that we found were specific to HSCs.”
To that end, Dr Rossi and his colleagues screened gene expression in 40 different types of blood and blood progenitor cells from mice. From this screen, the team identified 36 transcription factors that are expressed in HSCs but not in the cells that arise from them.
In a series of mouse transplantation experiments, the researchers found that 6 of the 36 transcription factors—Hlf, Runx1t1, Pbx1, Lmo2, Zfp37, and Prdm5—plus 2 additional factors not originally identified in their screen—Mycn and Meis1—were sufficient to reprogram 2 kinds of blood progenitor cells—pro/pre-B cells and common myeloid progenitor cells—into iHSCs.
The team reprogrammed their source cells by exposing them to viruses containing the genes for all 8 transcription factors and a molecular switch that turned the factor genes on in the presence of doxycycline. They then transplanted the exposed cells into recipient mice and activated the genes by giving the mice doxycycline.
The resulting iHSCs were capable of generating the entire blood cell repertoire in the transplanted mice, showing they had gained the ability to differentiate into all blood lineages. Stem cells collected from those recipients were capable of reconstituting the blood of secondary transplant recipients, proving that the 8-factor cocktail could instill the capacity for self-renewal.
Taking the work a step further, the researchers treated mature mouse myeloid cells with the same 8-factor cocktail. The resulting iHSCs produced all of the blood lineages and could regenerate the blood of secondary transplant recipients.
Study author Stuart Orkin, MD, of the Dana-Farber Cancer Institute in Boston, noted that the use of mice as a kind of reactor for reprogramming marks a novel direction in HSC research.
“In the blood research field, no one has the conditions to expand HSCs in the tissue culture dish,” he said. “Instead, by letting the reprogramming occur in mice, Rossi takes advantage of the signaling and environmental cues HSCs would normally experience.”
Dr Orkin added that iHSCs are nearly indistinguishable from normal HSCs at the transcriptional level. Unfortunately, though, these findings are far from translation to the clinic.
Researchers must still ascertain the precise contribution each of the 8 transcription factors makes in the reprogramming process and determine whether approaches that do not rely on viruses and transcription factors can have similar success.
In addition, studies are needed to test whether these results can be achieved using human cells and if other, non-blood cells can be reprogrammed to iHSCs.
Cases of ‘misconduct’ were really mistakes, STAP cell researcher says

Associated Press
The scientist who led the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) has apologized for the errors in her published work but maintains that she is not guilty of misconduct.
She offered additional explanations for the errors and argued that they do not change the conclusion of the research—that the STAP phenomenon is real.
Earlier this month, Haruko Obokata, PhD, was accused of research misconduct by her institution, the RIKEN Center for Developmental Biology in Kobe, Japan.
RIKEN had launched an investigation into the STAP cell research (published in an article and a letter to Nature) after members of the scientific community began questioning its validity.
The researchers had claimed they could induce pluripotency in somatic cells by introducing them to a low-pH environment.
RIKEN investigated 6 issues with the research and ruled that there were 2 cases of data mishandling, 2 unintentional errors, and 2 cases of misconduct in the form of data manipulation.
In the first case of data manipulation, Dr Obokata switched 1 lane of a diagram for another. In the second, she used an image of a teratoma from her doctoral thesis.
Dr Obokata said the first manipulation was for the purpose of image clarity and not made with an intent to deceive readers. And the second “manipulation” was the result of a mix up in Power Point slides.
Dr Obokata also maintained that STAP cells do exist, and she has produced them more than 200 times. She added that she is willing to help scientists trying to replicate her experiments.
Furthermore, the notes that RIKEN reviewed in their investigation—2 notebooks that detail the STAP cell experiments—are not the complete set of notes Dr Obokata made. She said she has at least 4 or 5 other notebooks in different labs.
Dr Obokata has filed an appeal with RIKEN, asking the institute to reconsider its judgment. If RIKEN upholds the allegations of misconduct, Dr Obokata and 2 of her co-authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—will face disciplinary action.
Meanwhile, a group of RIKEN investigators is conducting research to determine if STAP cells can be created, but they expect the process to take up to a year.

Associated Press
The scientist who led the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) has apologized for the errors in her published work but maintains that she is not guilty of misconduct.
She offered additional explanations for the errors and argued that they do not change the conclusion of the research—that the STAP phenomenon is real.
Earlier this month, Haruko Obokata, PhD, was accused of research misconduct by her institution, the RIKEN Center for Developmental Biology in Kobe, Japan.
RIKEN had launched an investigation into the STAP cell research (published in an article and a letter to Nature) after members of the scientific community began questioning its validity.
The researchers had claimed they could induce pluripotency in somatic cells by introducing them to a low-pH environment.
RIKEN investigated 6 issues with the research and ruled that there were 2 cases of data mishandling, 2 unintentional errors, and 2 cases of misconduct in the form of data manipulation.
In the first case of data manipulation, Dr Obokata switched 1 lane of a diagram for another. In the second, she used an image of a teratoma from her doctoral thesis.
Dr Obokata said the first manipulation was for the purpose of image clarity and not made with an intent to deceive readers. And the second “manipulation” was the result of a mix up in Power Point slides.
Dr Obokata also maintained that STAP cells do exist, and she has produced them more than 200 times. She added that she is willing to help scientists trying to replicate her experiments.
Furthermore, the notes that RIKEN reviewed in their investigation—2 notebooks that detail the STAP cell experiments—are not the complete set of notes Dr Obokata made. She said she has at least 4 or 5 other notebooks in different labs.
Dr Obokata has filed an appeal with RIKEN, asking the institute to reconsider its judgment. If RIKEN upholds the allegations of misconduct, Dr Obokata and 2 of her co-authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—will face disciplinary action.
Meanwhile, a group of RIKEN investigators is conducting research to determine if STAP cells can be created, but they expect the process to take up to a year.

Associated Press
The scientist who led the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) has apologized for the errors in her published work but maintains that she is not guilty of misconduct.
She offered additional explanations for the errors and argued that they do not change the conclusion of the research—that the STAP phenomenon is real.
Earlier this month, Haruko Obokata, PhD, was accused of research misconduct by her institution, the RIKEN Center for Developmental Biology in Kobe, Japan.
RIKEN had launched an investigation into the STAP cell research (published in an article and a letter to Nature) after members of the scientific community began questioning its validity.
The researchers had claimed they could induce pluripotency in somatic cells by introducing them to a low-pH environment.
RIKEN investigated 6 issues with the research and ruled that there were 2 cases of data mishandling, 2 unintentional errors, and 2 cases of misconduct in the form of data manipulation.
In the first case of data manipulation, Dr Obokata switched 1 lane of a diagram for another. In the second, she used an image of a teratoma from her doctoral thesis.
Dr Obokata said the first manipulation was for the purpose of image clarity and not made with an intent to deceive readers. And the second “manipulation” was the result of a mix up in Power Point slides.
Dr Obokata also maintained that STAP cells do exist, and she has produced them more than 200 times. She added that she is willing to help scientists trying to replicate her experiments.
Furthermore, the notes that RIKEN reviewed in their investigation—2 notebooks that detail the STAP cell experiments—are not the complete set of notes Dr Obokata made. She said she has at least 4 or 5 other notebooks in different labs.
Dr Obokata has filed an appeal with RIKEN, asking the institute to reconsider its judgment. If RIKEN upholds the allegations of misconduct, Dr Obokata and 2 of her co-authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—will face disciplinary action.
Meanwhile, a group of RIKEN investigators is conducting research to determine if STAP cells can be created, but they expect the process to take up to a year.
Investigation suggests misconduct in STAP study
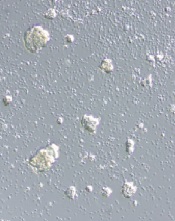
alleged STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has completed its investigation of the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) and claims that study authors are guilty of misconduct and negligence.
According to RIKEN, lead study author Haruko Obokata, PhD, is guilty of misconduct because she manipulated study data.
And 2 other authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—should have recognized the data manipulation.
Dr Obokata denied the allegations and said she plans to appeal this judgment. Drs Wakayama and Sasai wrote letters of apology, but Dr Sasai said he still believes the research is valid.
A group of RIKEN researchers has joined other groups in the attempt to replicate Dr Obokata’s experiments and determine if STAP cells can be generated.
About the investigation
RIKEN’s investigation began shortly after Dr Obokata and her colleagues reported the creation of STAP cells. The researchers said they could induce pluripotency by introducing somatic cells to a low-pH environment, and they described this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
So RIKEN launched an investigation into 6 issues with the papers. Early results of the investigation revealed 2 cases of “data mishandling” but no misconduct. And the final results of the investigation suggest 2 of the other issues were simply errors.
However, the 2 remaining issues constituted acts of misconduct, according to RIKEN. In the first case, Dr Obokata switched 1 lane of a diagram for another. She said it was for the purpose of image clarity, but the committee said this change was “intentionally misleading.”
Dr Obokata also used an image of a teratoma from her doctoral thesis (which involved different research). She said she mistakenly used the wrong image, but RIKEN said it was a fraudulent act because the caption on the image had been changed.
Next steps
It is still unclear whether the issues identified affect the results of this research. Dr Obokata and other study authors said the errors do not alter the study’s findings, and creating STAP cells is possible.
Other research groups have attempted to create STAP cells, and most results have suggested it is not possible when using the methods described in the Nature paper (or variations of those methods).
RIKEN has established an internal group that is attempting to verify the results of the STAP experiments. The group expects this process to take about a year.
As for dealing with the alleged misconduct, RIKEN said it will allow for an appeal. If the appeal is unsuccessful, the institution will call for the Nature papers to be retracted and take disciplinary action against Dr Obokata.
Drs Sasai and Wakayama may be subject to disciplinary measures as well. RIKEN said that, although the authors are not guilty of research misconduct, they “still bear heavy responsibility for their administrative negligence.”

alleged STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has completed its investigation of the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) and claims that study authors are guilty of misconduct and negligence.
According to RIKEN, lead study author Haruko Obokata, PhD, is guilty of misconduct because she manipulated study data.
And 2 other authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—should have recognized the data manipulation.
Dr Obokata denied the allegations and said she plans to appeal this judgment. Drs Wakayama and Sasai wrote letters of apology, but Dr Sasai said he still believes the research is valid.
A group of RIKEN researchers has joined other groups in the attempt to replicate Dr Obokata’s experiments and determine if STAP cells can be generated.
About the investigation
RIKEN’s investigation began shortly after Dr Obokata and her colleagues reported the creation of STAP cells. The researchers said they could induce pluripotency by introducing somatic cells to a low-pH environment, and they described this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
So RIKEN launched an investigation into 6 issues with the papers. Early results of the investigation revealed 2 cases of “data mishandling” but no misconduct. And the final results of the investigation suggest 2 of the other issues were simply errors.
However, the 2 remaining issues constituted acts of misconduct, according to RIKEN. In the first case, Dr Obokata switched 1 lane of a diagram for another. She said it was for the purpose of image clarity, but the committee said this change was “intentionally misleading.”
Dr Obokata also used an image of a teratoma from her doctoral thesis (which involved different research). She said she mistakenly used the wrong image, but RIKEN said it was a fraudulent act because the caption on the image had been changed.
Next steps
It is still unclear whether the issues identified affect the results of this research. Dr Obokata and other study authors said the errors do not alter the study’s findings, and creating STAP cells is possible.
Other research groups have attempted to create STAP cells, and most results have suggested it is not possible when using the methods described in the Nature paper (or variations of those methods).
RIKEN has established an internal group that is attempting to verify the results of the STAP experiments. The group expects this process to take about a year.
As for dealing with the alleged misconduct, RIKEN said it will allow for an appeal. If the appeal is unsuccessful, the institution will call for the Nature papers to be retracted and take disciplinary action against Dr Obokata.
Drs Sasai and Wakayama may be subject to disciplinary measures as well. RIKEN said that, although the authors are not guilty of research misconduct, they “still bear heavy responsibility for their administrative negligence.”

alleged STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has completed its investigation of the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) and claims that study authors are guilty of misconduct and negligence.
According to RIKEN, lead study author Haruko Obokata, PhD, is guilty of misconduct because she manipulated study data.
And 2 other authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—should have recognized the data manipulation.
Dr Obokata denied the allegations and said she plans to appeal this judgment. Drs Wakayama and Sasai wrote letters of apology, but Dr Sasai said he still believes the research is valid.
A group of RIKEN researchers has joined other groups in the attempt to replicate Dr Obokata’s experiments and determine if STAP cells can be generated.
About the investigation
RIKEN’s investigation began shortly after Dr Obokata and her colleagues reported the creation of STAP cells. The researchers said they could induce pluripotency by introducing somatic cells to a low-pH environment, and they described this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
So RIKEN launched an investigation into 6 issues with the papers. Early results of the investigation revealed 2 cases of “data mishandling” but no misconduct. And the final results of the investigation suggest 2 of the other issues were simply errors.
However, the 2 remaining issues constituted acts of misconduct, according to RIKEN. In the first case, Dr Obokata switched 1 lane of a diagram for another. She said it was for the purpose of image clarity, but the committee said this change was “intentionally misleading.”
Dr Obokata also used an image of a teratoma from her doctoral thesis (which involved different research). She said she mistakenly used the wrong image, but RIKEN said it was a fraudulent act because the caption on the image had been changed.
Next steps
It is still unclear whether the issues identified affect the results of this research. Dr Obokata and other study authors said the errors do not alter the study’s findings, and creating STAP cells is possible.
Other research groups have attempted to create STAP cells, and most results have suggested it is not possible when using the methods described in the Nature paper (or variations of those methods).
RIKEN has established an internal group that is attempting to verify the results of the STAP experiments. The group expects this process to take about a year.
As for dealing with the alleged misconduct, RIKEN said it will allow for an appeal. If the appeal is unsuccessful, the institution will call for the Nature papers to be retracted and take disciplinary action against Dr Obokata.
Drs Sasai and Wakayama may be subject to disciplinary measures as well. RIKEN said that, although the authors are not guilty of research misconduct, they “still bear heavy responsibility for their administrative negligence.”
Proteins appear necessary for stem cell formation

Credit: James Thomson
Proteins that regulate energy metabolism are essential for stem cell formation, according to a study published in Cell Stem Cell.
The researchers showed that hypoxia-induced factor 1α and 2α (HIF1α and HIF2α)—2 proteins that control how cells metabolize glucose—play a key role in the formation of stem cells.
The findings may advance our understanding of stem cell development, but they also suggest the proteins might be targets for new cancer therapies.
Julie Mathieu, PhD, of the University of Washington in Seattle, and her colleagues conducted this research, creating induced pluripotent stem cells (iPSCs) by reprogramming mature human tissue fibroblasts.
During reprogramming, the cells must go through a stage in which they shut down the metabolic pathway they use to generate energy from glucose that requires the presence of oxygen in mitochondria. The cells shift over to the glycolytic pathway, which generates less energy but does not require the presence of oxygen.
This shift may take place because in nature, embryonic and tissue stem cells often must survive in hypoxic conditions. This transition to a glycolytic state is of particular interest to cancer researchers because, as normal cells are transformed into cancer cells, they too go through a glycolytic phase.
For their study, Dr Mathieu and her colleagues focused on the function of HIF1α and HIF2α in this process. The researchers showed that each protein is required for iPSC generation.
To tease out the impact of HIF1α and 2α on cellular processes in more detail, the team stabilized the proteins in an active form and tested what each protein could do alone.
They found that when HIF1α was stabilized, the cells went into the glycolytic state and produced more iPSCs than normal. However, when the researchers activated HIF2α, the cells failed to develop into stem cells.
“This was a big surprise,” Dr Mathieu said. “These proteins are very similar, but HIF1α gives you lots of stem cells [and] HIF2α, none.”
If stabilized together, HIF2α won the battle, repressing all stem cell formation.
Further investigation revealed that HIF2α does indeed promote the shift to glycolysis in an early stage of the cells’ reprogramming. But if it persists too long, it has the opposite effect, blocking the progression to the stem cell state.
“HIF2α is like Darth Vader, originally a Jedi who falls to the dark side,” said study author Hannele Ruohola-Baker, PhD, also of the University of Washington.
“While HIF1α, the good guy, is beneficial for reprogramming throughout the process, HIF2α, if not eliminated, turns bad in the middle and represses pluripotency.”
HIF2α does this, in part, by upregulating production of the protein TRAIL, which is known to, among other things, induce apoptosis.
These findings suggest there may be proteins of other families that are playing alternating “good guy/bad guy” roles during stem cell development, according to study author Wenyu Zhou, PhD, of Stanford University in California.
“It is very intriguing that HIF2α has the capacity to both promote and repress pluripotency, doing so at different stages in a cellular reprogramming process,” she said.
The findings have implications for stem cell research, Dr Mathieu said. First, they indicate that it may be possible to use HIF1α to greatly increase the number of stem cells in a culture.
And second, they suggest it may be possible to induce stem cell formation with HIF proteins alone or in combination with other stimulating factors without inserting genes at the start of the reprogramming process.
But the findings may also have implications for cancer research. Both HIF1α and 2α are known to play a role in normal cells’ transformation to cancer stem cells. And the presence of activated HIF1α is known to be a marker for aggressive disease.
So the researchers believe it might be possible to interfere with cancer development by either blocking the effect of HIF1α in malignant cells early in the process or stimulating the effect of HIF2 at a later stage.

Credit: James Thomson
Proteins that regulate energy metabolism are essential for stem cell formation, according to a study published in Cell Stem Cell.
The researchers showed that hypoxia-induced factor 1α and 2α (HIF1α and HIF2α)—2 proteins that control how cells metabolize glucose—play a key role in the formation of stem cells.
The findings may advance our understanding of stem cell development, but they also suggest the proteins might be targets for new cancer therapies.
Julie Mathieu, PhD, of the University of Washington in Seattle, and her colleagues conducted this research, creating induced pluripotent stem cells (iPSCs) by reprogramming mature human tissue fibroblasts.
During reprogramming, the cells must go through a stage in which they shut down the metabolic pathway they use to generate energy from glucose that requires the presence of oxygen in mitochondria. The cells shift over to the glycolytic pathway, which generates less energy but does not require the presence of oxygen.
This shift may take place because in nature, embryonic and tissue stem cells often must survive in hypoxic conditions. This transition to a glycolytic state is of particular interest to cancer researchers because, as normal cells are transformed into cancer cells, they too go through a glycolytic phase.
For their study, Dr Mathieu and her colleagues focused on the function of HIF1α and HIF2α in this process. The researchers showed that each protein is required for iPSC generation.
To tease out the impact of HIF1α and 2α on cellular processes in more detail, the team stabilized the proteins in an active form and tested what each protein could do alone.
They found that when HIF1α was stabilized, the cells went into the glycolytic state and produced more iPSCs than normal. However, when the researchers activated HIF2α, the cells failed to develop into stem cells.
“This was a big surprise,” Dr Mathieu said. “These proteins are very similar, but HIF1α gives you lots of stem cells [and] HIF2α, none.”
If stabilized together, HIF2α won the battle, repressing all stem cell formation.
Further investigation revealed that HIF2α does indeed promote the shift to glycolysis in an early stage of the cells’ reprogramming. But if it persists too long, it has the opposite effect, blocking the progression to the stem cell state.
“HIF2α is like Darth Vader, originally a Jedi who falls to the dark side,” said study author Hannele Ruohola-Baker, PhD, also of the University of Washington.
“While HIF1α, the good guy, is beneficial for reprogramming throughout the process, HIF2α, if not eliminated, turns bad in the middle and represses pluripotency.”
HIF2α does this, in part, by upregulating production of the protein TRAIL, which is known to, among other things, induce apoptosis.
These findings suggest there may be proteins of other families that are playing alternating “good guy/bad guy” roles during stem cell development, according to study author Wenyu Zhou, PhD, of Stanford University in California.
“It is very intriguing that HIF2α has the capacity to both promote and repress pluripotency, doing so at different stages in a cellular reprogramming process,” she said.
The findings have implications for stem cell research, Dr Mathieu said. First, they indicate that it may be possible to use HIF1α to greatly increase the number of stem cells in a culture.
And second, they suggest it may be possible to induce stem cell formation with HIF proteins alone or in combination with other stimulating factors without inserting genes at the start of the reprogramming process.
But the findings may also have implications for cancer research. Both HIF1α and 2α are known to play a role in normal cells’ transformation to cancer stem cells. And the presence of activated HIF1α is known to be a marker for aggressive disease.
So the researchers believe it might be possible to interfere with cancer development by either blocking the effect of HIF1α in malignant cells early in the process or stimulating the effect of HIF2 at a later stage.

Credit: James Thomson
Proteins that regulate energy metabolism are essential for stem cell formation, according to a study published in Cell Stem Cell.
The researchers showed that hypoxia-induced factor 1α and 2α (HIF1α and HIF2α)—2 proteins that control how cells metabolize glucose—play a key role in the formation of stem cells.
The findings may advance our understanding of stem cell development, but they also suggest the proteins might be targets for new cancer therapies.
Julie Mathieu, PhD, of the University of Washington in Seattle, and her colleagues conducted this research, creating induced pluripotent stem cells (iPSCs) by reprogramming mature human tissue fibroblasts.
During reprogramming, the cells must go through a stage in which they shut down the metabolic pathway they use to generate energy from glucose that requires the presence of oxygen in mitochondria. The cells shift over to the glycolytic pathway, which generates less energy but does not require the presence of oxygen.
This shift may take place because in nature, embryonic and tissue stem cells often must survive in hypoxic conditions. This transition to a glycolytic state is of particular interest to cancer researchers because, as normal cells are transformed into cancer cells, they too go through a glycolytic phase.
For their study, Dr Mathieu and her colleagues focused on the function of HIF1α and HIF2α in this process. The researchers showed that each protein is required for iPSC generation.
To tease out the impact of HIF1α and 2α on cellular processes in more detail, the team stabilized the proteins in an active form and tested what each protein could do alone.
They found that when HIF1α was stabilized, the cells went into the glycolytic state and produced more iPSCs than normal. However, when the researchers activated HIF2α, the cells failed to develop into stem cells.
“This was a big surprise,” Dr Mathieu said. “These proteins are very similar, but HIF1α gives you lots of stem cells [and] HIF2α, none.”
If stabilized together, HIF2α won the battle, repressing all stem cell formation.
Further investigation revealed that HIF2α does indeed promote the shift to glycolysis in an early stage of the cells’ reprogramming. But if it persists too long, it has the opposite effect, blocking the progression to the stem cell state.
“HIF2α is like Darth Vader, originally a Jedi who falls to the dark side,” said study author Hannele Ruohola-Baker, PhD, also of the University of Washington.
“While HIF1α, the good guy, is beneficial for reprogramming throughout the process, HIF2α, if not eliminated, turns bad in the middle and represses pluripotency.”
HIF2α does this, in part, by upregulating production of the protein TRAIL, which is known to, among other things, induce apoptosis.
These findings suggest there may be proteins of other families that are playing alternating “good guy/bad guy” roles during stem cell development, according to study author Wenyu Zhou, PhD, of Stanford University in California.
“It is very intriguing that HIF2α has the capacity to both promote and repress pluripotency, doing so at different stages in a cellular reprogramming process,” she said.
The findings have implications for stem cell research, Dr Mathieu said. First, they indicate that it may be possible to use HIF1α to greatly increase the number of stem cells in a culture.
And second, they suggest it may be possible to induce stem cell formation with HIF proteins alone or in combination with other stimulating factors without inserting genes at the start of the reprogramming process.
But the findings may also have implications for cancer research. Both HIF1α and 2α are known to play a role in normal cells’ transformation to cancer stem cells. And the presence of activated HIF1α is known to be a marker for aggressive disease.
So the researchers believe it might be possible to interfere with cancer development by either blocking the effect of HIF1α in malignant cells early in the process or stimulating the effect of HIF2 at a later stage.