User login
Unsuspected aspect of immune regulation revealed

Immunologists may have discovered an additional role for B cells. Their research suggests the cells participate in the development of regulatory T cells (Tregs).
Until now, the only non-thymic cells known to aid Treg production were dendritic cells, which travel to the thymus to deliver antigens.
The new research, published in the Journal of Immunology, suggests B cells can do the same thing.
B cells were previously thought to specialize only in antibody production. With their newly discovered role, the cells become much more interesting and complex characters, according to the researchers.
The findings mean B cells could have useful applications for treating transplant patients and those with autoimmune disorders.
“Regulatory T cells are critical in the outcome of an immune response, so anything that regulates them becomes very interesting to immunologists,” said study author Shane Grey, PhD, of the Garvan Institute of Medical Research in Darlinghurst, New South Wales, Australia.
“Right now, there are clinical trials around the world looking to expand populations of these cells in patients. Researchers are also working on ways to grow regulatory cells in the laboratory—to infuse into patients as therapy. Our finding suggests it should be possible to set up systems that harness B cells to expand regulatory cells.”
Dr Grey and his colleagues worked with mice genetically modified to express high levels of BAFF, which increases B-cell survival. The higher number of B cells overall allowed researchers to track the activity of B cells in the thymus.
“It has been known for years that some B cells travel to the thymus, but no one has understood why,” said study author Stacey Walters, also of the Garvan Institute of Medical Research.
“Our experiments showed clearly that B cells participated in the creation of regulatory T cells. The more B cells that were in the thymus, the higher the number of regulatory cells generated. That direct correlation raises interesting possibilities. One possibility is using BAFF, a non-toxic substance, to ramp up the B-cell count of patients before transplant procedures.”
Research has suggested that Tregs can reduce the risk of graft-vs-host disease, promote enhanced immune reconstitution, and decrease the incidence of infectious complications in stem cell transplant recipients. And several studies have shown that high levels of Tregs can prevent graft rejection after solid organ transplant. ![]()

Immunologists may have discovered an additional role for B cells. Their research suggests the cells participate in the development of regulatory T cells (Tregs).
Until now, the only non-thymic cells known to aid Treg production were dendritic cells, which travel to the thymus to deliver antigens.
The new research, published in the Journal of Immunology, suggests B cells can do the same thing.
B cells were previously thought to specialize only in antibody production. With their newly discovered role, the cells become much more interesting and complex characters, according to the researchers.
The findings mean B cells could have useful applications for treating transplant patients and those with autoimmune disorders.
“Regulatory T cells are critical in the outcome of an immune response, so anything that regulates them becomes very interesting to immunologists,” said study author Shane Grey, PhD, of the Garvan Institute of Medical Research in Darlinghurst, New South Wales, Australia.
“Right now, there are clinical trials around the world looking to expand populations of these cells in patients. Researchers are also working on ways to grow regulatory cells in the laboratory—to infuse into patients as therapy. Our finding suggests it should be possible to set up systems that harness B cells to expand regulatory cells.”
Dr Grey and his colleagues worked with mice genetically modified to express high levels of BAFF, which increases B-cell survival. The higher number of B cells overall allowed researchers to track the activity of B cells in the thymus.
“It has been known for years that some B cells travel to the thymus, but no one has understood why,” said study author Stacey Walters, also of the Garvan Institute of Medical Research.
“Our experiments showed clearly that B cells participated in the creation of regulatory T cells. The more B cells that were in the thymus, the higher the number of regulatory cells generated. That direct correlation raises interesting possibilities. One possibility is using BAFF, a non-toxic substance, to ramp up the B-cell count of patients before transplant procedures.”
Research has suggested that Tregs can reduce the risk of graft-vs-host disease, promote enhanced immune reconstitution, and decrease the incidence of infectious complications in stem cell transplant recipients. And several studies have shown that high levels of Tregs can prevent graft rejection after solid organ transplant. ![]()

Immunologists may have discovered an additional role for B cells. Their research suggests the cells participate in the development of regulatory T cells (Tregs).
Until now, the only non-thymic cells known to aid Treg production were dendritic cells, which travel to the thymus to deliver antigens.
The new research, published in the Journal of Immunology, suggests B cells can do the same thing.
B cells were previously thought to specialize only in antibody production. With their newly discovered role, the cells become much more interesting and complex characters, according to the researchers.
The findings mean B cells could have useful applications for treating transplant patients and those with autoimmune disorders.
“Regulatory T cells are critical in the outcome of an immune response, so anything that regulates them becomes very interesting to immunologists,” said study author Shane Grey, PhD, of the Garvan Institute of Medical Research in Darlinghurst, New South Wales, Australia.
“Right now, there are clinical trials around the world looking to expand populations of these cells in patients. Researchers are also working on ways to grow regulatory cells in the laboratory—to infuse into patients as therapy. Our finding suggests it should be possible to set up systems that harness B cells to expand regulatory cells.”
Dr Grey and his colleagues worked with mice genetically modified to express high levels of BAFF, which increases B-cell survival. The higher number of B cells overall allowed researchers to track the activity of B cells in the thymus.
“It has been known for years that some B cells travel to the thymus, but no one has understood why,” said study author Stacey Walters, also of the Garvan Institute of Medical Research.
“Our experiments showed clearly that B cells participated in the creation of regulatory T cells. The more B cells that were in the thymus, the higher the number of regulatory cells generated. That direct correlation raises interesting possibilities. One possibility is using BAFF, a non-toxic substance, to ramp up the B-cell count of patients before transplant procedures.”
Research has suggested that Tregs can reduce the risk of graft-vs-host disease, promote enhanced immune reconstitution, and decrease the incidence of infectious complications in stem cell transplant recipients. And several studies have shown that high levels of Tregs can prevent graft rejection after solid organ transplant. ![]()
HSCT regimen could be ‘transformative’ for SCD

pre-HSCT (top) and post-HSCT
Credit: NIH Molecular and
Clinical Hematology Branch
In a small study, a nonmyeloablative hematopoietic stem cell transplant (HSCT) regimen reversed sickle cell disease (SCD) phenotype in a majority of adult patients, some of whom also had thalassemia.
Half of the patients were able to stop taking immunosuppressants and did not develop graft-vs-host disease (GVHD).
There were adverse events associated with the regimen, but the researchers believe it shows promise and could be “transformative” for patients with severe SCD.
The team described the regimen and its effects in JAMA.
Matthew M. Hsieh, MD, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, and his colleagues first explored a nonmyeloablative HSCT approach in a pilot group of 10 adults with severe SCD.
The regimen had few toxic effects, but all patients continued taking immunosuppression medication. The researchers have since revised the protocol to include an option to stop immunosuppression after 1 year in patients with donor CD3 engraftment of greater than 50% and normalization of hemoglobin.
In JAMA, the team described the outcomes for 20 additional patients with severe SCD, with or without thalassemia, along with updated results from the first 10 patients.
All 30 patients (ages 16-65 years) were enrolled in the study from July 2004 to October 2013. Two patients had heterozygous hemoglobin S and C, 1 patient had HbSβ+-thalassemia, 1 patient had HbSβ0- thalassemia, and 1 had transfusion-dependent β-thalassemia intermedia. The remaining patients had homozygous hemoglobin S.
Patients received alemtuzumab (1mg/kg in divided doses), total-body irradiation (300 cGy), sirolimus, and an infusion of unmanipulated, filgrastim-mobilized peripheral blood stem cells (5.5-31.7 × 106 cells/kg) from HLA-matched siblings.
There were 38 serious adverse events. The most common were pain-related (n=15), transplant-related infections (n=6), abdominal events (n=6), and toxic effects associated with sirolimus (n=5).
As of October 25, 2013, 29 patients were still alive, with a median follow-up of 3.4 years. Twenty-six patients (87%) had long-term stable donor engraftment without acute or chronic GVHD.
Hemoglobin levels improved after HSCT. At 1 year, 25 patients (83%) had full donor-type hemoglobin. Fifteen engrafted patients discontinued immunosuppression medication and did not develop GVHD.
“Typically, stem cell recipients must take immunosuppressants all their lives,” Dr Hsieh noted. “That the patients who discontinued this medication were able to do so safely points to the stability of the partial transplant regimen.”
Hospitalization rates also decreased following HSCT. The average annual hospitalization rate was 3.2 the year before HSCT, 0.63 the first year after, 0.19 the second year after, and 0.11 the third year after transplant.
“One of the most debilitating effects of sickle cell disease is the often relentless pain,” Dr Hsieh pointed out. “Following the transplant, we saw a significant decrease in hospitalizations and narcotics to control that pain.”
Eleven patients were taking narcotics long-term at the time of transplant. During the week they were hospitalized and received their HSCT, the average narcotics use per week was 639 mg of intravenous morphine-equivalent dose. The dosage decreased to 140 mg at 6 months after the transplant.
“The devastating complications associated with sickle cell disease can deeply affect quality of life, ability to work, and long-term well-being,” said study author Griffin P. Rodgers, MD, director of the National Institute of Diabetes and Digestive and Kidney Diseases.
“This study represents an important advance in our efforts to make a potentially transformative treatment available to a wider range of people, especially those who could not tolerate a standard stem cell transplant or long-term use of immunosuppressants.” ![]()

pre-HSCT (top) and post-HSCT
Credit: NIH Molecular and
Clinical Hematology Branch
In a small study, a nonmyeloablative hematopoietic stem cell transplant (HSCT) regimen reversed sickle cell disease (SCD) phenotype in a majority of adult patients, some of whom also had thalassemia.
Half of the patients were able to stop taking immunosuppressants and did not develop graft-vs-host disease (GVHD).
There were adverse events associated with the regimen, but the researchers believe it shows promise and could be “transformative” for patients with severe SCD.
The team described the regimen and its effects in JAMA.
Matthew M. Hsieh, MD, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, and his colleagues first explored a nonmyeloablative HSCT approach in a pilot group of 10 adults with severe SCD.
The regimen had few toxic effects, but all patients continued taking immunosuppression medication. The researchers have since revised the protocol to include an option to stop immunosuppression after 1 year in patients with donor CD3 engraftment of greater than 50% and normalization of hemoglobin.
In JAMA, the team described the outcomes for 20 additional patients with severe SCD, with or without thalassemia, along with updated results from the first 10 patients.
All 30 patients (ages 16-65 years) were enrolled in the study from July 2004 to October 2013. Two patients had heterozygous hemoglobin S and C, 1 patient had HbSβ+-thalassemia, 1 patient had HbSβ0- thalassemia, and 1 had transfusion-dependent β-thalassemia intermedia. The remaining patients had homozygous hemoglobin S.
Patients received alemtuzumab (1mg/kg in divided doses), total-body irradiation (300 cGy), sirolimus, and an infusion of unmanipulated, filgrastim-mobilized peripheral blood stem cells (5.5-31.7 × 106 cells/kg) from HLA-matched siblings.
There were 38 serious adverse events. The most common were pain-related (n=15), transplant-related infections (n=6), abdominal events (n=6), and toxic effects associated with sirolimus (n=5).
As of October 25, 2013, 29 patients were still alive, with a median follow-up of 3.4 years. Twenty-six patients (87%) had long-term stable donor engraftment without acute or chronic GVHD.
Hemoglobin levels improved after HSCT. At 1 year, 25 patients (83%) had full donor-type hemoglobin. Fifteen engrafted patients discontinued immunosuppression medication and did not develop GVHD.
“Typically, stem cell recipients must take immunosuppressants all their lives,” Dr Hsieh noted. “That the patients who discontinued this medication were able to do so safely points to the stability of the partial transplant regimen.”
Hospitalization rates also decreased following HSCT. The average annual hospitalization rate was 3.2 the year before HSCT, 0.63 the first year after, 0.19 the second year after, and 0.11 the third year after transplant.
“One of the most debilitating effects of sickle cell disease is the often relentless pain,” Dr Hsieh pointed out. “Following the transplant, we saw a significant decrease in hospitalizations and narcotics to control that pain.”
Eleven patients were taking narcotics long-term at the time of transplant. During the week they were hospitalized and received their HSCT, the average narcotics use per week was 639 mg of intravenous morphine-equivalent dose. The dosage decreased to 140 mg at 6 months after the transplant.
“The devastating complications associated with sickle cell disease can deeply affect quality of life, ability to work, and long-term well-being,” said study author Griffin P. Rodgers, MD, director of the National Institute of Diabetes and Digestive and Kidney Diseases.
“This study represents an important advance in our efforts to make a potentially transformative treatment available to a wider range of people, especially those who could not tolerate a standard stem cell transplant or long-term use of immunosuppressants.” ![]()

pre-HSCT (top) and post-HSCT
Credit: NIH Molecular and
Clinical Hematology Branch
In a small study, a nonmyeloablative hematopoietic stem cell transplant (HSCT) regimen reversed sickle cell disease (SCD) phenotype in a majority of adult patients, some of whom also had thalassemia.
Half of the patients were able to stop taking immunosuppressants and did not develop graft-vs-host disease (GVHD).
There were adverse events associated with the regimen, but the researchers believe it shows promise and could be “transformative” for patients with severe SCD.
The team described the regimen and its effects in JAMA.
Matthew M. Hsieh, MD, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, and his colleagues first explored a nonmyeloablative HSCT approach in a pilot group of 10 adults with severe SCD.
The regimen had few toxic effects, but all patients continued taking immunosuppression medication. The researchers have since revised the protocol to include an option to stop immunosuppression after 1 year in patients with donor CD3 engraftment of greater than 50% and normalization of hemoglobin.
In JAMA, the team described the outcomes for 20 additional patients with severe SCD, with or without thalassemia, along with updated results from the first 10 patients.
All 30 patients (ages 16-65 years) were enrolled in the study from July 2004 to October 2013. Two patients had heterozygous hemoglobin S and C, 1 patient had HbSβ+-thalassemia, 1 patient had HbSβ0- thalassemia, and 1 had transfusion-dependent β-thalassemia intermedia. The remaining patients had homozygous hemoglobin S.
Patients received alemtuzumab (1mg/kg in divided doses), total-body irradiation (300 cGy), sirolimus, and an infusion of unmanipulated, filgrastim-mobilized peripheral blood stem cells (5.5-31.7 × 106 cells/kg) from HLA-matched siblings.
There were 38 serious adverse events. The most common were pain-related (n=15), transplant-related infections (n=6), abdominal events (n=6), and toxic effects associated with sirolimus (n=5).
As of October 25, 2013, 29 patients were still alive, with a median follow-up of 3.4 years. Twenty-six patients (87%) had long-term stable donor engraftment without acute or chronic GVHD.
Hemoglobin levels improved after HSCT. At 1 year, 25 patients (83%) had full donor-type hemoglobin. Fifteen engrafted patients discontinued immunosuppression medication and did not develop GVHD.
“Typically, stem cell recipients must take immunosuppressants all their lives,” Dr Hsieh noted. “That the patients who discontinued this medication were able to do so safely points to the stability of the partial transplant regimen.”
Hospitalization rates also decreased following HSCT. The average annual hospitalization rate was 3.2 the year before HSCT, 0.63 the first year after, 0.19 the second year after, and 0.11 the third year after transplant.
“One of the most debilitating effects of sickle cell disease is the often relentless pain,” Dr Hsieh pointed out. “Following the transplant, we saw a significant decrease in hospitalizations and narcotics to control that pain.”
Eleven patients were taking narcotics long-term at the time of transplant. During the week they were hospitalized and received their HSCT, the average narcotics use per week was 639 mg of intravenous morphine-equivalent dose. The dosage decreased to 140 mg at 6 months after the transplant.
“The devastating complications associated with sickle cell disease can deeply affect quality of life, ability to work, and long-term well-being,” said study author Griffin P. Rodgers, MD, director of the National Institute of Diabetes and Digestive and Kidney Diseases.
“This study represents an important advance in our efforts to make a potentially transformative treatment available to a wider range of people, especially those who could not tolerate a standard stem cell transplant or long-term use of immunosuppressants.” ![]()
Nature retracts STAP cell papers
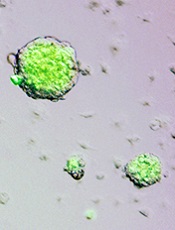
Credit: Haruko Obokata
The journal Nature has retracted the papers it published several months ago on stimulus-triggered acquisition of pluripotency (STAP) cells.
In January, Nature published an article and a letter in which researchers claimed they could create STAP cells—ie, induce pluripotency in somatic cells by exposing them to a low-pH environment.
Not long after the papers were published, however, members of the scientific community began to question the validity of the research.
They voiced concerns about published images, possible plagiarism, and an inability to replicate the experiments described.
So the Japanese institute RIKEN, where most of the study’s investigators are employed, launched an investigation.
In April, RIKEN’s investigative committee concluded that lead study author Haruko Obokata, PhD, and some of her colleagues were guilty of misconduct and/or negligence.
Dr Obokata appealed the findings, saying the acts of misconduct were simply mistakes and that STAP cells do exist.
But the committee decided another investigation is not warranted, and RIKEN called for a retraction of the Nature papers.
Today, Nature published the retractions, which can be viewed here and here. An editorial on the subject is available here.
As for the future of STAP cells, RIKEN is currently attempting to recreate Dr Obokata’s experiments and determine if the cells do exist. The organization plans to release an interim report on this attempt in late July or early August.
Other researchers said they have tried and failed to replicate Dr Obokata’s experiments. One group detailed their failed attempt in F1000Research. ![]()

Credit: Haruko Obokata
The journal Nature has retracted the papers it published several months ago on stimulus-triggered acquisition of pluripotency (STAP) cells.
In January, Nature published an article and a letter in which researchers claimed they could create STAP cells—ie, induce pluripotency in somatic cells by exposing them to a low-pH environment.
Not long after the papers were published, however, members of the scientific community began to question the validity of the research.
They voiced concerns about published images, possible plagiarism, and an inability to replicate the experiments described.
So the Japanese institute RIKEN, where most of the study’s investigators are employed, launched an investigation.
In April, RIKEN’s investigative committee concluded that lead study author Haruko Obokata, PhD, and some of her colleagues were guilty of misconduct and/or negligence.
Dr Obokata appealed the findings, saying the acts of misconduct were simply mistakes and that STAP cells do exist.
But the committee decided another investigation is not warranted, and RIKEN called for a retraction of the Nature papers.
Today, Nature published the retractions, which can be viewed here and here. An editorial on the subject is available here.
As for the future of STAP cells, RIKEN is currently attempting to recreate Dr Obokata’s experiments and determine if the cells do exist. The organization plans to release an interim report on this attempt in late July or early August.
Other researchers said they have tried and failed to replicate Dr Obokata’s experiments. One group detailed their failed attempt in F1000Research. ![]()

Credit: Haruko Obokata
The journal Nature has retracted the papers it published several months ago on stimulus-triggered acquisition of pluripotency (STAP) cells.
In January, Nature published an article and a letter in which researchers claimed they could create STAP cells—ie, induce pluripotency in somatic cells by exposing them to a low-pH environment.
Not long after the papers were published, however, members of the scientific community began to question the validity of the research.
They voiced concerns about published images, possible plagiarism, and an inability to replicate the experiments described.
So the Japanese institute RIKEN, where most of the study’s investigators are employed, launched an investigation.
In April, RIKEN’s investigative committee concluded that lead study author Haruko Obokata, PhD, and some of her colleagues were guilty of misconduct and/or negligence.
Dr Obokata appealed the findings, saying the acts of misconduct were simply mistakes and that STAP cells do exist.
But the committee decided another investigation is not warranted, and RIKEN called for a retraction of the Nature papers.
Today, Nature published the retractions, which can be viewed here and here. An editorial on the subject is available here.
As for the future of STAP cells, RIKEN is currently attempting to recreate Dr Obokata’s experiments and determine if the cells do exist. The organization plans to release an interim report on this attempt in late July or early August.
Other researchers said they have tried and failed to replicate Dr Obokata’s experiments. One group detailed their failed attempt in F1000Research. ![]()
Enhancing gene delivery to HSCs

Credit: Chad McNeeley
Scientists say they’ve overcome a major hurdle to developing gene therapies for blood disorders.
They found the drug rapamycin could help them bypass the natural defenses of hematopoietic stem cells (HSCs) and deliver therapeutic doses of disease-fighting genes, without compromising HSC function.
The team believes this discovery could lead to more effective and affordable long-term treatments for disorders such as leukemia and sickle cell anemia.
Bruce Torbett, PhD, of The Scripps Research Institute in La Jolla, California, and his colleagues reported their findings in Blood.
Past research showed that HIV vectors can deliver genes to HSCs. However, when scientists extract HSCs from the body for gene therapy, HIV vectors are usually able to deliver genes to about 30% to 40% of the cells.
For leukemia, leukodystrophy, or genetic diseases where treatment requires a reasonable number of healthy cells derived from stem cells, this number may be too low for therapeutic purposes.
This limitation prompted Dr Torbett and his colleagues to test whether rapamycin could improve delivery of a gene to HSCs. Rapamycin was selected based on its ability to control virus entry and slow cell growth.
The researchers began by isolating stem cells from cord blood samples. They exposed the HSCs to rapamycin and HIV vectors engineered to deliver a gene for a green florescent protein. This fluorescence provided a visual marker that helped the team track gene delivery.
They saw a big difference in both mouse and human stem cells treated with rapamycin, where therapeutic genes were inserted into up to 80% of cells. This property had never been connected to rapamycin before.
The researchers also found that rapamycin can keep HSCs from differentiating as quickly when taken out of the body for gene therapy.
“We wanted to make sure the conditions we will use preserve stem cells, so if we transplant them back into our animal models, they act just like the original stem cells,” Dr Torbett said. “We showed that, in 2 sets of animal models, stem cells remain and produce gene-modified cells.”
The scientists hope these methods could someday be useful in the clinic.
“Our methods could reduce costs and the amount of preparation that goes into modifying blood stem cells using viral vector gene therapy,” said Cathy Wang, also of The Scripps Research Institute. “It would make gene therapy accessible to a lot more patients.”
She said the team’s next steps are to carry out preclinical studies using rapamycin with stem cells in other animal models and then test the method in humans. The researchers are also working to delineate the dual pathways of rapamycin’s mechanism of action in HSCs. ![]()

Credit: Chad McNeeley
Scientists say they’ve overcome a major hurdle to developing gene therapies for blood disorders.
They found the drug rapamycin could help them bypass the natural defenses of hematopoietic stem cells (HSCs) and deliver therapeutic doses of disease-fighting genes, without compromising HSC function.
The team believes this discovery could lead to more effective and affordable long-term treatments for disorders such as leukemia and sickle cell anemia.
Bruce Torbett, PhD, of The Scripps Research Institute in La Jolla, California, and his colleagues reported their findings in Blood.
Past research showed that HIV vectors can deliver genes to HSCs. However, when scientists extract HSCs from the body for gene therapy, HIV vectors are usually able to deliver genes to about 30% to 40% of the cells.
For leukemia, leukodystrophy, or genetic diseases where treatment requires a reasonable number of healthy cells derived from stem cells, this number may be too low for therapeutic purposes.
This limitation prompted Dr Torbett and his colleagues to test whether rapamycin could improve delivery of a gene to HSCs. Rapamycin was selected based on its ability to control virus entry and slow cell growth.
The researchers began by isolating stem cells from cord blood samples. They exposed the HSCs to rapamycin and HIV vectors engineered to deliver a gene for a green florescent protein. This fluorescence provided a visual marker that helped the team track gene delivery.
They saw a big difference in both mouse and human stem cells treated with rapamycin, where therapeutic genes were inserted into up to 80% of cells. This property had never been connected to rapamycin before.
The researchers also found that rapamycin can keep HSCs from differentiating as quickly when taken out of the body for gene therapy.
“We wanted to make sure the conditions we will use preserve stem cells, so if we transplant them back into our animal models, they act just like the original stem cells,” Dr Torbett said. “We showed that, in 2 sets of animal models, stem cells remain and produce gene-modified cells.”
The scientists hope these methods could someday be useful in the clinic.
“Our methods could reduce costs and the amount of preparation that goes into modifying blood stem cells using viral vector gene therapy,” said Cathy Wang, also of The Scripps Research Institute. “It would make gene therapy accessible to a lot more patients.”
She said the team’s next steps are to carry out preclinical studies using rapamycin with stem cells in other animal models and then test the method in humans. The researchers are also working to delineate the dual pathways of rapamycin’s mechanism of action in HSCs. ![]()

Credit: Chad McNeeley
Scientists say they’ve overcome a major hurdle to developing gene therapies for blood disorders.
They found the drug rapamycin could help them bypass the natural defenses of hematopoietic stem cells (HSCs) and deliver therapeutic doses of disease-fighting genes, without compromising HSC function.
The team believes this discovery could lead to more effective and affordable long-term treatments for disorders such as leukemia and sickle cell anemia.
Bruce Torbett, PhD, of The Scripps Research Institute in La Jolla, California, and his colleagues reported their findings in Blood.
Past research showed that HIV vectors can deliver genes to HSCs. However, when scientists extract HSCs from the body for gene therapy, HIV vectors are usually able to deliver genes to about 30% to 40% of the cells.
For leukemia, leukodystrophy, or genetic diseases where treatment requires a reasonable number of healthy cells derived from stem cells, this number may be too low for therapeutic purposes.
This limitation prompted Dr Torbett and his colleagues to test whether rapamycin could improve delivery of a gene to HSCs. Rapamycin was selected based on its ability to control virus entry and slow cell growth.
The researchers began by isolating stem cells from cord blood samples. They exposed the HSCs to rapamycin and HIV vectors engineered to deliver a gene for a green florescent protein. This fluorescence provided a visual marker that helped the team track gene delivery.
They saw a big difference in both mouse and human stem cells treated with rapamycin, where therapeutic genes were inserted into up to 80% of cells. This property had never been connected to rapamycin before.
The researchers also found that rapamycin can keep HSCs from differentiating as quickly when taken out of the body for gene therapy.
“We wanted to make sure the conditions we will use preserve stem cells, so if we transplant them back into our animal models, they act just like the original stem cells,” Dr Torbett said. “We showed that, in 2 sets of animal models, stem cells remain and produce gene-modified cells.”
The scientists hope these methods could someday be useful in the clinic.
“Our methods could reduce costs and the amount of preparation that goes into modifying blood stem cells using viral vector gene therapy,” said Cathy Wang, also of The Scripps Research Institute. “It would make gene therapy accessible to a lot more patients.”
She said the team’s next steps are to carry out preclinical studies using rapamycin with stem cells in other animal models and then test the method in humans. The researchers are also working to delineate the dual pathways of rapamycin’s mechanism of action in HSCs. ![]()
VSTs can target up to 5 viruses

for Cell and Gene Therapy
at Baylor College of Medicine
New virus-specific T-cells (VSTs) can effectively target multiple viruses, thereby clearing and preventing infections in transplant recipients, researchers have reported in Science Translational Medicine.
VSTs have proven effective in previous studies, but they are typically costly, can take months to produce, and often target a single virus.
The new VSTs took about 10 days to produce, are more cost-effective than standard therapy, and can target up to 5 viruses, according to the researchers.
Ann Leen, PhD, of the Baylor College of Medicine in Houston, and her colleagues developed the technique to produce the VSTs, which can target Epstein-Barr virus (EBV), adenovirus (ADV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV6).
“Unlike conventional antiviral drugs, our therapy improves virus-specific T-cell immunity—the root cause of post-transplant viral infections—providing both an effective and safe strategy to treat viruses,” Dr Leen said.
“Additionally, we can readily produce both individualized products and T-cell banks for third-party use, facilitating the extension of T-cell therapy to a standard of care for transplant recipients.”
The researchers generated 48 VST cell lines by stimulating peripheral blood mononuclear cells from allogeneic donors with overlapping peptide libraries that incorporate EBV, CMV, ADV, BKV, and HHV6 antigens.
The team then tested these VSTs in 11 patients who had received allogeneic transplants to treat leukemia, lymphoma, sickle cell disease, and other hematologic and immunodeficient disorders.
Eight of the patients had up to 4 active infections with the targeted viruses. Three patients received the VSTs to prevent infection.
The VSTs produced an overall 94% virological and clinical response that was sustained long-term. Of the 3 patients who received VSTs prophylactically, all remained free of infection for more than 3 months after infusion.
There were no immediate infusion-related toxicities. One patient developed de novo graft-vs-host disease of the skin that improved with topical steroids.
Two patients who received VSTs as prophylaxis developed transplant-associated microangiopathy, but the researchers considered this to be unrelated to VST infusion.
They noted that this is the first time BKV and HHV6 reactivations have been controlled using VSTs. Dr Leen’s team had previously reported promising results with VSTs targeting EBV, CMV, and ADV.
“This study translated improved manufacturing techniques developed in Dr Leen’s laboratory to the clinic and showed that virus-specific T cells produced with the new method could target new viruses and be ready for clinical use after 10 days,” said Helen Heslop, MD, also of Baylor College of Medicine.
“These advances mean that this therapy could be available for more patients to treat viral infections and provide long-lasting protection.” ![]()

for Cell and Gene Therapy
at Baylor College of Medicine
New virus-specific T-cells (VSTs) can effectively target multiple viruses, thereby clearing and preventing infections in transplant recipients, researchers have reported in Science Translational Medicine.
VSTs have proven effective in previous studies, but they are typically costly, can take months to produce, and often target a single virus.
The new VSTs took about 10 days to produce, are more cost-effective than standard therapy, and can target up to 5 viruses, according to the researchers.
Ann Leen, PhD, of the Baylor College of Medicine in Houston, and her colleagues developed the technique to produce the VSTs, which can target Epstein-Barr virus (EBV), adenovirus (ADV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV6).
“Unlike conventional antiviral drugs, our therapy improves virus-specific T-cell immunity—the root cause of post-transplant viral infections—providing both an effective and safe strategy to treat viruses,” Dr Leen said.
“Additionally, we can readily produce both individualized products and T-cell banks for third-party use, facilitating the extension of T-cell therapy to a standard of care for transplant recipients.”
The researchers generated 48 VST cell lines by stimulating peripheral blood mononuclear cells from allogeneic donors with overlapping peptide libraries that incorporate EBV, CMV, ADV, BKV, and HHV6 antigens.
The team then tested these VSTs in 11 patients who had received allogeneic transplants to treat leukemia, lymphoma, sickle cell disease, and other hematologic and immunodeficient disorders.
Eight of the patients had up to 4 active infections with the targeted viruses. Three patients received the VSTs to prevent infection.
The VSTs produced an overall 94% virological and clinical response that was sustained long-term. Of the 3 patients who received VSTs prophylactically, all remained free of infection for more than 3 months after infusion.
There were no immediate infusion-related toxicities. One patient developed de novo graft-vs-host disease of the skin that improved with topical steroids.
Two patients who received VSTs as prophylaxis developed transplant-associated microangiopathy, but the researchers considered this to be unrelated to VST infusion.
They noted that this is the first time BKV and HHV6 reactivations have been controlled using VSTs. Dr Leen’s team had previously reported promising results with VSTs targeting EBV, CMV, and ADV.
“This study translated improved manufacturing techniques developed in Dr Leen’s laboratory to the clinic and showed that virus-specific T cells produced with the new method could target new viruses and be ready for clinical use after 10 days,” said Helen Heslop, MD, also of Baylor College of Medicine.
“These advances mean that this therapy could be available for more patients to treat viral infections and provide long-lasting protection.” ![]()

for Cell and Gene Therapy
at Baylor College of Medicine
New virus-specific T-cells (VSTs) can effectively target multiple viruses, thereby clearing and preventing infections in transplant recipients, researchers have reported in Science Translational Medicine.
VSTs have proven effective in previous studies, but they are typically costly, can take months to produce, and often target a single virus.
The new VSTs took about 10 days to produce, are more cost-effective than standard therapy, and can target up to 5 viruses, according to the researchers.
Ann Leen, PhD, of the Baylor College of Medicine in Houston, and her colleagues developed the technique to produce the VSTs, which can target Epstein-Barr virus (EBV), adenovirus (ADV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV6).
“Unlike conventional antiviral drugs, our therapy improves virus-specific T-cell immunity—the root cause of post-transplant viral infections—providing both an effective and safe strategy to treat viruses,” Dr Leen said.
“Additionally, we can readily produce both individualized products and T-cell banks for third-party use, facilitating the extension of T-cell therapy to a standard of care for transplant recipients.”
The researchers generated 48 VST cell lines by stimulating peripheral blood mononuclear cells from allogeneic donors with overlapping peptide libraries that incorporate EBV, CMV, ADV, BKV, and HHV6 antigens.
The team then tested these VSTs in 11 patients who had received allogeneic transplants to treat leukemia, lymphoma, sickle cell disease, and other hematologic and immunodeficient disorders.
Eight of the patients had up to 4 active infections with the targeted viruses. Three patients received the VSTs to prevent infection.
The VSTs produced an overall 94% virological and clinical response that was sustained long-term. Of the 3 patients who received VSTs prophylactically, all remained free of infection for more than 3 months after infusion.
There were no immediate infusion-related toxicities. One patient developed de novo graft-vs-host disease of the skin that improved with topical steroids.
Two patients who received VSTs as prophylaxis developed transplant-associated microangiopathy, but the researchers considered this to be unrelated to VST infusion.
They noted that this is the first time BKV and HHV6 reactivations have been controlled using VSTs. Dr Leen’s team had previously reported promising results with VSTs targeting EBV, CMV, and ADV.
“This study translated improved manufacturing techniques developed in Dr Leen’s laboratory to the clinic and showed that virus-specific T cells produced with the new method could target new viruses and be ready for clinical use after 10 days,” said Helen Heslop, MD, also of Baylor College of Medicine.
“These advances mean that this therapy could be available for more patients to treat viral infections and provide long-lasting protection.” ![]()
Low-dose T-cell transfer can fight CMV

CMV infection
A new study indicates that transferring low doses of immune cells may be sufficient to protect against cytomegalovirus (CMV) infection in patients receiving allogeneic stem cell transplant.
The researchers noted that the adoptive transfer of donor-derived, virus-specific, memory T cells is a promising strategy for treating and preventing CMV infection.
But transferring too many of these cells can increase the risk of graft-vs-host disease.
So Christian Stemberger, PhD, of Technische Universitaet Muenchen in Germany, and his colleagues set out to establish a lower limit for successful adoptive T-cell therapy.
The team reported their results—observed in mice and 2 human patients—in Blood.
The researchers first conducted low-dose CD8+ T-cell transfers in the murine Listeria monocytogenes infection model.
And they found these MHC-Streptamer-enriched, antigen-specific, CD62Lhi, CD8+ memory T cells proliferated, differentiated, and protected against Listeria monocytogenes infections.
“The most astonishing result was that the offspring cells of just 1 transferred donor cell were enough to completely protect the animals,” Dr Stemberger said.
Next, the researchers used virus-specific T cells to treat 2 critically ill pediatric patients—1 with severe combined immunodeficiency (SCID) and the other with B-cell acute lymphoblastic leukemia.
The patients had received allogeneic transplants and subsequently developed CMV infections.
The patients received low-dose transfers of Streptamer-enriched, CMV-specific, CD8+ T cells—3750 cells per kilogram of body weight for the SCID patient and 5130 cells per kilogram of body weight for the leukemia patient.
In both patients, the researchers observed “strong, pathogen-specific T-cell expansion.” The CMV-specific, CD8+ T cells proliferated, and the CMV viral load decreased. Furthermore, neither patient developed graft-vs-host disease.
“It is a great advantage that even just a few cells can provide protection [from CMV],” said study author Michael Neuenhahn, Dr med, also of Technische Universitaet Muenchen.
“This means that the cells can be used for preventive treatment in low doses that are gentler on the organism.”
The researchers are now testing the potential of the CMV-specific, CD8+ T cells in a clinical study. ![]()

CMV infection
A new study indicates that transferring low doses of immune cells may be sufficient to protect against cytomegalovirus (CMV) infection in patients receiving allogeneic stem cell transplant.
The researchers noted that the adoptive transfer of donor-derived, virus-specific, memory T cells is a promising strategy for treating and preventing CMV infection.
But transferring too many of these cells can increase the risk of graft-vs-host disease.
So Christian Stemberger, PhD, of Technische Universitaet Muenchen in Germany, and his colleagues set out to establish a lower limit for successful adoptive T-cell therapy.
The team reported their results—observed in mice and 2 human patients—in Blood.
The researchers first conducted low-dose CD8+ T-cell transfers in the murine Listeria monocytogenes infection model.
And they found these MHC-Streptamer-enriched, antigen-specific, CD62Lhi, CD8+ memory T cells proliferated, differentiated, and protected against Listeria monocytogenes infections.
“The most astonishing result was that the offspring cells of just 1 transferred donor cell were enough to completely protect the animals,” Dr Stemberger said.
Next, the researchers used virus-specific T cells to treat 2 critically ill pediatric patients—1 with severe combined immunodeficiency (SCID) and the other with B-cell acute lymphoblastic leukemia.
The patients had received allogeneic transplants and subsequently developed CMV infections.
The patients received low-dose transfers of Streptamer-enriched, CMV-specific, CD8+ T cells—3750 cells per kilogram of body weight for the SCID patient and 5130 cells per kilogram of body weight for the leukemia patient.
In both patients, the researchers observed “strong, pathogen-specific T-cell expansion.” The CMV-specific, CD8+ T cells proliferated, and the CMV viral load decreased. Furthermore, neither patient developed graft-vs-host disease.
“It is a great advantage that even just a few cells can provide protection [from CMV],” said study author Michael Neuenhahn, Dr med, also of Technische Universitaet Muenchen.
“This means that the cells can be used for preventive treatment in low doses that are gentler on the organism.”
The researchers are now testing the potential of the CMV-specific, CD8+ T cells in a clinical study. ![]()

CMV infection
A new study indicates that transferring low doses of immune cells may be sufficient to protect against cytomegalovirus (CMV) infection in patients receiving allogeneic stem cell transplant.
The researchers noted that the adoptive transfer of donor-derived, virus-specific, memory T cells is a promising strategy for treating and preventing CMV infection.
But transferring too many of these cells can increase the risk of graft-vs-host disease.
So Christian Stemberger, PhD, of Technische Universitaet Muenchen in Germany, and his colleagues set out to establish a lower limit for successful adoptive T-cell therapy.
The team reported their results—observed in mice and 2 human patients—in Blood.
The researchers first conducted low-dose CD8+ T-cell transfers in the murine Listeria monocytogenes infection model.
And they found these MHC-Streptamer-enriched, antigen-specific, CD62Lhi, CD8+ memory T cells proliferated, differentiated, and protected against Listeria monocytogenes infections.
“The most astonishing result was that the offspring cells of just 1 transferred donor cell were enough to completely protect the animals,” Dr Stemberger said.
Next, the researchers used virus-specific T cells to treat 2 critically ill pediatric patients—1 with severe combined immunodeficiency (SCID) and the other with B-cell acute lymphoblastic leukemia.
The patients had received allogeneic transplants and subsequently developed CMV infections.
The patients received low-dose transfers of Streptamer-enriched, CMV-specific, CD8+ T cells—3750 cells per kilogram of body weight for the SCID patient and 5130 cells per kilogram of body weight for the leukemia patient.
In both patients, the researchers observed “strong, pathogen-specific T-cell expansion.” The CMV-specific, CD8+ T cells proliferated, and the CMV viral load decreased. Furthermore, neither patient developed graft-vs-host disease.
“It is a great advantage that even just a few cells can provide protection [from CMV],” said study author Michael Neuenhahn, Dr med, also of Technische Universitaet Muenchen.
“This means that the cells can be used for preventive treatment in low doses that are gentler on the organism.”
The researchers are now testing the potential of the CMV-specific, CD8+ T cells in a clinical study. ![]()
Gut bacteria may help predict survival after allo-SCT

The diversity of gut microbiota in patients receiving allogeneic stem cell transplants (allo-SCTs) may be an important predictor of their survival, according to a study published in Blood.
Previous studies have suggested the intensive treatment given to allo-SCT recipients can destroy a significant portion of their gut microbiota and reduce its overall diversity.
And disturbances of the gut microbiota have been associated with complications such as bloodstream infections and graft-vs-host disease.
“While the link between gut microbiota and complications in allogeneic SCT has been previously established, until this point, it has remained unclear whether the gut bacteria of transplant recipients could predict their survival,” said study author Ying Taur, MD, of Memorial Sloan-Kettering Cancer Center in New York.
“This study sought to further explore the potential connection between transplantation, gut bacteria, and overall survival.”
To that end, the researchers collected fecal specimens from 80 patients undergoing allo-SCT and sequenced each sample’s bacterial DNA. Specimens were collected within 7 days of engraftment, the point at which the researchers speculated that microbiota diversity would be greatest following pre-transplant conditioning.
The team compared patient outcomes based on diversity of microbiota in their specimens, grouping subjects into high-, intermediate-, and low-microbiota-diversity categories.
At engraftment, 34 patients (42.5%) had low diversity, 20 (25%) had intermediate diversity, and 26 (32.5%) had high diversity. The analysis continued for up to 3 years or until death or last follow-up.
Following their analyses, the researchers found a strong connection between post-transplant gut microbiota diversity and outcomes, observing overall survival rates of 36%, 60%, and 67% among the low-, intermediate-, and high-diversity groups, respectively.
Furthermore, diversity was particularly associated with transplant-related outcomes. Patients with low microbiota diversity were approximately 5 times more likely to die of transplant-related causes within the follow-up period than those with more diverse gut bacteria.
“These results further underscore the significance of the gut microbiota in allogeneic stem cell transplant,” Dr Taur said. “A major question is whether we can improve outcomes by preserving diversity within the gut microbiota.”
“One possible strategy is to find ways to perform transplants in a manner that minimizes damage to the gut microbiota. Another approach would be to replenish the gut with beneficial microbes that are lost after this procedure is performed. We hope that this study will inspire additional research that will further examine the role and importance of the gut microbiota to stem cell transplant outcome.” ![]()

The diversity of gut microbiota in patients receiving allogeneic stem cell transplants (allo-SCTs) may be an important predictor of their survival, according to a study published in Blood.
Previous studies have suggested the intensive treatment given to allo-SCT recipients can destroy a significant portion of their gut microbiota and reduce its overall diversity.
And disturbances of the gut microbiota have been associated with complications such as bloodstream infections and graft-vs-host disease.
“While the link between gut microbiota and complications in allogeneic SCT has been previously established, until this point, it has remained unclear whether the gut bacteria of transplant recipients could predict their survival,” said study author Ying Taur, MD, of Memorial Sloan-Kettering Cancer Center in New York.
“This study sought to further explore the potential connection between transplantation, gut bacteria, and overall survival.”
To that end, the researchers collected fecal specimens from 80 patients undergoing allo-SCT and sequenced each sample’s bacterial DNA. Specimens were collected within 7 days of engraftment, the point at which the researchers speculated that microbiota diversity would be greatest following pre-transplant conditioning.
The team compared patient outcomes based on diversity of microbiota in their specimens, grouping subjects into high-, intermediate-, and low-microbiota-diversity categories.
At engraftment, 34 patients (42.5%) had low diversity, 20 (25%) had intermediate diversity, and 26 (32.5%) had high diversity. The analysis continued for up to 3 years or until death or last follow-up.
Following their analyses, the researchers found a strong connection between post-transplant gut microbiota diversity and outcomes, observing overall survival rates of 36%, 60%, and 67% among the low-, intermediate-, and high-diversity groups, respectively.
Furthermore, diversity was particularly associated with transplant-related outcomes. Patients with low microbiota diversity were approximately 5 times more likely to die of transplant-related causes within the follow-up period than those with more diverse gut bacteria.
“These results further underscore the significance of the gut microbiota in allogeneic stem cell transplant,” Dr Taur said. “A major question is whether we can improve outcomes by preserving diversity within the gut microbiota.”
“One possible strategy is to find ways to perform transplants in a manner that minimizes damage to the gut microbiota. Another approach would be to replenish the gut with beneficial microbes that are lost after this procedure is performed. We hope that this study will inspire additional research that will further examine the role and importance of the gut microbiota to stem cell transplant outcome.” ![]()

The diversity of gut microbiota in patients receiving allogeneic stem cell transplants (allo-SCTs) may be an important predictor of their survival, according to a study published in Blood.
Previous studies have suggested the intensive treatment given to allo-SCT recipients can destroy a significant portion of their gut microbiota and reduce its overall diversity.
And disturbances of the gut microbiota have been associated with complications such as bloodstream infections and graft-vs-host disease.
“While the link between gut microbiota and complications in allogeneic SCT has been previously established, until this point, it has remained unclear whether the gut bacteria of transplant recipients could predict their survival,” said study author Ying Taur, MD, of Memorial Sloan-Kettering Cancer Center in New York.
“This study sought to further explore the potential connection between transplantation, gut bacteria, and overall survival.”
To that end, the researchers collected fecal specimens from 80 patients undergoing allo-SCT and sequenced each sample’s bacterial DNA. Specimens were collected within 7 days of engraftment, the point at which the researchers speculated that microbiota diversity would be greatest following pre-transplant conditioning.
The team compared patient outcomes based on diversity of microbiota in their specimens, grouping subjects into high-, intermediate-, and low-microbiota-diversity categories.
At engraftment, 34 patients (42.5%) had low diversity, 20 (25%) had intermediate diversity, and 26 (32.5%) had high diversity. The analysis continued for up to 3 years or until death or last follow-up.
Following their analyses, the researchers found a strong connection between post-transplant gut microbiota diversity and outcomes, observing overall survival rates of 36%, 60%, and 67% among the low-, intermediate-, and high-diversity groups, respectively.
Furthermore, diversity was particularly associated with transplant-related outcomes. Patients with low microbiota diversity were approximately 5 times more likely to die of transplant-related causes within the follow-up period than those with more diverse gut bacteria.
“These results further underscore the significance of the gut microbiota in allogeneic stem cell transplant,” Dr Taur said. “A major question is whether we can improve outcomes by preserving diversity within the gut microbiota.”
“One possible strategy is to find ways to perform transplants in a manner that minimizes damage to the gut microbiota. Another approach would be to replenish the gut with beneficial microbes that are lost after this procedure is performed. We hope that this study will inspire additional research that will further examine the role and importance of the gut microbiota to stem cell transplant outcome.”
MRD test can predict HSCT outcomes in ALL patients

Credit: Graham Colm
A method of measuring minimal residual disease (MRD) can predict transplant outcomes in adults with acute lymphoblastic leukemia (ALL), according to a study published in Biology of Blood and Marrow Transplantation.
Researchers used the test, called LymphoSIGHT, to evaluate MRD in patient blood samples.
The test successfully predicted both relapse and survival and detected evidence of disease a median of 3 months prior to relapse.
This research was conducted by Aaron C. Logan, MD, PhD, of the University of California, San Francisco, and his colleagues. It was sponsored by Sequenta, Inc., makers of the LymphoSIGHT test.
The researchers used LymphoSIGHT to analyze 237 blood samples from 29 adults who underwent allogeneic hematopoietic stem cell transplant (HSCT) to treat ALL.
The LymphoSIGHT test consists of a 2-step process. First, cancer cell DNA sequences are identified in a diagnostic sample. Follow-up samples are then screened for these sequences to detect MRD.
The results, which are generated in 7 days using Sequenta’s CLIA-certified laboratory, are provided in a report that shows a patient’s MRD status and level, as well as MRD trends over time.
The researchers found the test could quantify MRD in 93% of patients. MRD positivity was defined as more than 1 leukemia cell per 1 million white blood cells.
Patients who were MRD-positive before HSCT conditioning were significantly more likely than MRD-negative patients to relapse after transplant (hazard ratio=7.7, P=0.003).
And patients who were MRD-positive in the first 90 days after transplant had a significantly higher risk of relapse than patients who were MRD-negative (hazard ratio=14; P<0.0001).
Patients who were MRD-positive at any point after HSCT (17 of 25 evaluable patients) all relapsed and died from their disease. Their median survival was 359 days (range, 85-1991 days). The median lead-time from MRD detection to clinical relapse was 89 days (range, 0-207 days).
Of the 8 patients who remained MRD-negative, 6 were still alive at a median of 1853 days post-HSCT (range, 1641-2732). Two patients died from complications of graft-vs-host disease, without evidence of leukemia recurrence.
The researchers said these results suggest the LymphoSIGHT test can help physicians understand treatment responses and patient prognoses, as well as guide treatment decisions.

Credit: Graham Colm
A method of measuring minimal residual disease (MRD) can predict transplant outcomes in adults with acute lymphoblastic leukemia (ALL), according to a study published in Biology of Blood and Marrow Transplantation.
Researchers used the test, called LymphoSIGHT, to evaluate MRD in patient blood samples.
The test successfully predicted both relapse and survival and detected evidence of disease a median of 3 months prior to relapse.
This research was conducted by Aaron C. Logan, MD, PhD, of the University of California, San Francisco, and his colleagues. It was sponsored by Sequenta, Inc., makers of the LymphoSIGHT test.
The researchers used LymphoSIGHT to analyze 237 blood samples from 29 adults who underwent allogeneic hematopoietic stem cell transplant (HSCT) to treat ALL.
The LymphoSIGHT test consists of a 2-step process. First, cancer cell DNA sequences are identified in a diagnostic sample. Follow-up samples are then screened for these sequences to detect MRD.
The results, which are generated in 7 days using Sequenta’s CLIA-certified laboratory, are provided in a report that shows a patient’s MRD status and level, as well as MRD trends over time.
The researchers found the test could quantify MRD in 93% of patients. MRD positivity was defined as more than 1 leukemia cell per 1 million white blood cells.
Patients who were MRD-positive before HSCT conditioning were significantly more likely than MRD-negative patients to relapse after transplant (hazard ratio=7.7, P=0.003).
And patients who were MRD-positive in the first 90 days after transplant had a significantly higher risk of relapse than patients who were MRD-negative (hazard ratio=14; P<0.0001).
Patients who were MRD-positive at any point after HSCT (17 of 25 evaluable patients) all relapsed and died from their disease. Their median survival was 359 days (range, 85-1991 days). The median lead-time from MRD detection to clinical relapse was 89 days (range, 0-207 days).
Of the 8 patients who remained MRD-negative, 6 were still alive at a median of 1853 days post-HSCT (range, 1641-2732). Two patients died from complications of graft-vs-host disease, without evidence of leukemia recurrence.
The researchers said these results suggest the LymphoSIGHT test can help physicians understand treatment responses and patient prognoses, as well as guide treatment decisions.

Credit: Graham Colm
A method of measuring minimal residual disease (MRD) can predict transplant outcomes in adults with acute lymphoblastic leukemia (ALL), according to a study published in Biology of Blood and Marrow Transplantation.
Researchers used the test, called LymphoSIGHT, to evaluate MRD in patient blood samples.
The test successfully predicted both relapse and survival and detected evidence of disease a median of 3 months prior to relapse.
This research was conducted by Aaron C. Logan, MD, PhD, of the University of California, San Francisco, and his colleagues. It was sponsored by Sequenta, Inc., makers of the LymphoSIGHT test.
The researchers used LymphoSIGHT to analyze 237 blood samples from 29 adults who underwent allogeneic hematopoietic stem cell transplant (HSCT) to treat ALL.
The LymphoSIGHT test consists of a 2-step process. First, cancer cell DNA sequences are identified in a diagnostic sample. Follow-up samples are then screened for these sequences to detect MRD.
The results, which are generated in 7 days using Sequenta’s CLIA-certified laboratory, are provided in a report that shows a patient’s MRD status and level, as well as MRD trends over time.
The researchers found the test could quantify MRD in 93% of patients. MRD positivity was defined as more than 1 leukemia cell per 1 million white blood cells.
Patients who were MRD-positive before HSCT conditioning were significantly more likely than MRD-negative patients to relapse after transplant (hazard ratio=7.7, P=0.003).
And patients who were MRD-positive in the first 90 days after transplant had a significantly higher risk of relapse than patients who were MRD-negative (hazard ratio=14; P<0.0001).
Patients who were MRD-positive at any point after HSCT (17 of 25 evaluable patients) all relapsed and died from their disease. Their median survival was 359 days (range, 85-1991 days). The median lead-time from MRD detection to clinical relapse was 89 days (range, 0-207 days).
Of the 8 patients who remained MRD-negative, 6 were still alive at a median of 1853 days post-HSCT (range, 1641-2732). Two patients died from complications of graft-vs-host disease, without evidence of leukemia recurrence.
The researchers said these results suggest the LymphoSIGHT test can help physicians understand treatment responses and patient prognoses, as well as guide treatment decisions.
Paper recounts failed attempt to create STAP cells

Credit: NIH
A group of researchers who tried, and failed, to replicate the STAP cell phenomenon have detailed their work in F1000Research.
Kenneth Ka Ho Lee, PhD, of the Chinese University of Hong Kong, and his colleagues attempted to create STAP (stimulus-triggered acquisition of pluripotency) cells using the method described in a recent Nature paper.
The paper’s authors had reported they could induce pluripotency in somatic cells by bathing them in acid.
However, not long after the paper and a related letter were published, members of the scientific community voiced concerns about the publications—such as suspicions of plagiarism and the possibility of doctored images—and began to question the findings.
Then, an investigation by the Japanese research institute RIKEN (where many of the researchers are employed) suggested that at least some of the paper’s authors were guilty of misconduct and/or negligence.
However, the study’s lead author, Haruko Obokata, PhD, has maintained that, despite errors in the paper, STAP cells can be created.
Dr Lee’s experiments suggest otherwise—or at least that the cells cannot be created using the methods outlined in the Nature paper.
Dr Lee and his colleagues reported that carefully replicating the original acid-treatment method does not induce pluripotency in 2 types of mouse somatic cells.
Using both white blood cells isolated from the spleen of neonatal mice—the same cells used in the original study—and lung fibroblasts, the team was unable to replicate the original findings.
They’ve published a full account of this work in F1000Research. The journal also employs open peer review by invited experts, which occurs after publication and is published in full online alongside the paper.
Dr Lee’s study will now undergo this process, and readers interested to see referees’ views as they come in can follow the paper by clicking “Track” on the published article.

Credit: NIH
A group of researchers who tried, and failed, to replicate the STAP cell phenomenon have detailed their work in F1000Research.
Kenneth Ka Ho Lee, PhD, of the Chinese University of Hong Kong, and his colleagues attempted to create STAP (stimulus-triggered acquisition of pluripotency) cells using the method described in a recent Nature paper.
The paper’s authors had reported they could induce pluripotency in somatic cells by bathing them in acid.
However, not long after the paper and a related letter were published, members of the scientific community voiced concerns about the publications—such as suspicions of plagiarism and the possibility of doctored images—and began to question the findings.
Then, an investigation by the Japanese research institute RIKEN (where many of the researchers are employed) suggested that at least some of the paper’s authors were guilty of misconduct and/or negligence.
However, the study’s lead author, Haruko Obokata, PhD, has maintained that, despite errors in the paper, STAP cells can be created.
Dr Lee’s experiments suggest otherwise—or at least that the cells cannot be created using the methods outlined in the Nature paper.
Dr Lee and his colleagues reported that carefully replicating the original acid-treatment method does not induce pluripotency in 2 types of mouse somatic cells.
Using both white blood cells isolated from the spleen of neonatal mice—the same cells used in the original study—and lung fibroblasts, the team was unable to replicate the original findings.
They’ve published a full account of this work in F1000Research. The journal also employs open peer review by invited experts, which occurs after publication and is published in full online alongside the paper.
Dr Lee’s study will now undergo this process, and readers interested to see referees’ views as they come in can follow the paper by clicking “Track” on the published article.

Credit: NIH
A group of researchers who tried, and failed, to replicate the STAP cell phenomenon have detailed their work in F1000Research.
Kenneth Ka Ho Lee, PhD, of the Chinese University of Hong Kong, and his colleagues attempted to create STAP (stimulus-triggered acquisition of pluripotency) cells using the method described in a recent Nature paper.
The paper’s authors had reported they could induce pluripotency in somatic cells by bathing them in acid.
However, not long after the paper and a related letter were published, members of the scientific community voiced concerns about the publications—such as suspicions of plagiarism and the possibility of doctored images—and began to question the findings.
Then, an investigation by the Japanese research institute RIKEN (where many of the researchers are employed) suggested that at least some of the paper’s authors were guilty of misconduct and/or negligence.
However, the study’s lead author, Haruko Obokata, PhD, has maintained that, despite errors in the paper, STAP cells can be created.
Dr Lee’s experiments suggest otherwise—or at least that the cells cannot be created using the methods outlined in the Nature paper.
Dr Lee and his colleagues reported that carefully replicating the original acid-treatment method does not induce pluripotency in 2 types of mouse somatic cells.
Using both white blood cells isolated from the spleen of neonatal mice—the same cells used in the original study—and lung fibroblasts, the team was unable to replicate the original findings.
They’ve published a full account of this work in F1000Research. The journal also employs open peer review by invited experts, which occurs after publication and is published in full online alongside the paper.
Dr Lee’s study will now undergo this process, and readers interested to see referees’ views as they come in can follow the paper by clicking “Track” on the published article.
RIKEN upholds misconduct allegations, calls for paper’s retraction

Credit: RIKEN
The Japanese research institute RIKEN has announced that it will not re-investigate the allegations of misconduct levelled against the researcher who claimed to have discovered a new method for inducing pluripotency in somatic cells.
In January, Haruko Obokata, PhD, and her colleagues reported the creation of stimulus-triggered acquisition of pluripotency (STAP) cells.
The team said they could induce pluripotency by exposing somatic cells to a low-pH environment.
But members of the scientific community voiced concerns about the research, so RIKEN launched an investigation.
In April, the investigative committee concluded that Dr Obokata and some of her colleagues were guilty of misconduct and/or negligence.
Dr Obokata appealed the findings, but the committee has decided another investigation is not warranted. RIKEN has also called for a retraction of the Nature paper in which the committee found evidence of misconduct.
Another RIKEN committee has already met to discuss possible disciplinary action for Dr Obokata and some of her colleagues. Potential punishments range from pay cuts to temporary suspension to disciplinary discharge.
In the meantime, a group headed by Shinichi Aizawa, PhD, special advisor to RIKEN, is attempting to verify the results of the STAP experiments and determine if the STAP phenomenon is, in fact, real.
As the events of this case unfolded, another possible case of misconduct surfaced at RIKEN.
The institute recently announced that Shunsuke Ishii, PhD, chairperson of the committee investigating misconduct in the STAP papers, had resigned from the committee amid concerns about one of his own papers.
RIKEN replaced Dr Ishii with Jun Watanabe, a lawyer already on the committee. And the institute has launched an investigation into Dr Ishii’s work.
Furthermore, RIKEN’s office for internal reform, headed by President Ryoji Noyori, PhD, has commissioned a committee of outside experts to deliberate and recommend measures to prevent research misconduct in the future.

Credit: RIKEN
The Japanese research institute RIKEN has announced that it will not re-investigate the allegations of misconduct levelled against the researcher who claimed to have discovered a new method for inducing pluripotency in somatic cells.
In January, Haruko Obokata, PhD, and her colleagues reported the creation of stimulus-triggered acquisition of pluripotency (STAP) cells.
The team said they could induce pluripotency by exposing somatic cells to a low-pH environment.
But members of the scientific community voiced concerns about the research, so RIKEN launched an investigation.
In April, the investigative committee concluded that Dr Obokata and some of her colleagues were guilty of misconduct and/or negligence.
Dr Obokata appealed the findings, but the committee has decided another investigation is not warranted. RIKEN has also called for a retraction of the Nature paper in which the committee found evidence of misconduct.
Another RIKEN committee has already met to discuss possible disciplinary action for Dr Obokata and some of her colleagues. Potential punishments range from pay cuts to temporary suspension to disciplinary discharge.
In the meantime, a group headed by Shinichi Aizawa, PhD, special advisor to RIKEN, is attempting to verify the results of the STAP experiments and determine if the STAP phenomenon is, in fact, real.
As the events of this case unfolded, another possible case of misconduct surfaced at RIKEN.
The institute recently announced that Shunsuke Ishii, PhD, chairperson of the committee investigating misconduct in the STAP papers, had resigned from the committee amid concerns about one of his own papers.
RIKEN replaced Dr Ishii with Jun Watanabe, a lawyer already on the committee. And the institute has launched an investigation into Dr Ishii’s work.
Furthermore, RIKEN’s office for internal reform, headed by President Ryoji Noyori, PhD, has commissioned a committee of outside experts to deliberate and recommend measures to prevent research misconduct in the future.

Credit: RIKEN
The Japanese research institute RIKEN has announced that it will not re-investigate the allegations of misconduct levelled against the researcher who claimed to have discovered a new method for inducing pluripotency in somatic cells.
In January, Haruko Obokata, PhD, and her colleagues reported the creation of stimulus-triggered acquisition of pluripotency (STAP) cells.
The team said they could induce pluripotency by exposing somatic cells to a low-pH environment.
But members of the scientific community voiced concerns about the research, so RIKEN launched an investigation.
In April, the investigative committee concluded that Dr Obokata and some of her colleagues were guilty of misconduct and/or negligence.
Dr Obokata appealed the findings, but the committee has decided another investigation is not warranted. RIKEN has also called for a retraction of the Nature paper in which the committee found evidence of misconduct.
Another RIKEN committee has already met to discuss possible disciplinary action for Dr Obokata and some of her colleagues. Potential punishments range from pay cuts to temporary suspension to disciplinary discharge.
In the meantime, a group headed by Shinichi Aizawa, PhD, special advisor to RIKEN, is attempting to verify the results of the STAP experiments and determine if the STAP phenomenon is, in fact, real.
As the events of this case unfolded, another possible case of misconduct surfaced at RIKEN.
The institute recently announced that Shunsuke Ishii, PhD, chairperson of the committee investigating misconduct in the STAP papers, had resigned from the committee amid concerns about one of his own papers.
RIKEN replaced Dr Ishii with Jun Watanabe, a lawyer already on the committee. And the institute has launched an investigation into Dr Ishii’s work.
Furthermore, RIKEN’s office for internal reform, headed by President Ryoji Noyori, PhD, has commissioned a committee of outside experts to deliberate and recommend measures to prevent research misconduct in the future.