User login
Finger prick yields ample iPSCs for banking

Credit: Salk Institute
Researchers say they’ve discovered an easy way to collect large quantities of viable, bankable stem cells.
Donors prick their own fingers to provide a single drop of blood, and the team generates induced pluripotent stem cells (iPSCs) from that sample.
“We show that a single drop of blood from a finger-prick sample is sufficient for performing cellular reprogramming, DNA sequencing, and blood typing in parallel,” said Jonathan Yuin-Han Loh, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore.
“Our strategy has the potential of facilitating the development of large-scale human iPSC banking worldwide.”
The researchers described this strategy in STEM CELLS Translational Medicine.
“We gradually reduced the starting volume of blood (collected using a needle) and confirmed that reprogramming can be achieved with as little as 0.25 milliliters,” said Hong Kee Tan, a research officer in the Loh lab.
And this made the team wonder whether a do-it-yourself approach to blood collection might work too.
“To test this idea, we asked donors to prick their own fingers in a normal room environment and collect a single drop of blood sample into a tube,” Tan said. “The tube was placed on ice and delivered to the lab for reprogramming.”
The cells were treated with a buffer at 12-, 24- or 48-hour increments and observed under the microscope for viability and signs of contamination. After 12 days of expansion in medium, the cells appeared healthy and were actively dividing.
The researchers then succeeded in forcing the cells to become mesodermal, endodermal, and neural cells. They were also able to produce cells that gave rise to rhythmically beating cardiomyocytes.
The team said there was no noticeable reduction in reprogramming efficiency between the freshly collected finger-prick samples and the do-it-yourself samples.
“[W]e derived healthy iPSCs from tiny volumes of venipuncture and a single drop from finger-prick blood samples,” Dr Loh said. “We also report a high reprogramming yield of 100 to 600 colonies per milliliter of blood.” ![]()

Credit: Salk Institute
Researchers say they’ve discovered an easy way to collect large quantities of viable, bankable stem cells.
Donors prick their own fingers to provide a single drop of blood, and the team generates induced pluripotent stem cells (iPSCs) from that sample.
“We show that a single drop of blood from a finger-prick sample is sufficient for performing cellular reprogramming, DNA sequencing, and blood typing in parallel,” said Jonathan Yuin-Han Loh, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore.
“Our strategy has the potential of facilitating the development of large-scale human iPSC banking worldwide.”
The researchers described this strategy in STEM CELLS Translational Medicine.
“We gradually reduced the starting volume of blood (collected using a needle) and confirmed that reprogramming can be achieved with as little as 0.25 milliliters,” said Hong Kee Tan, a research officer in the Loh lab.
And this made the team wonder whether a do-it-yourself approach to blood collection might work too.
“To test this idea, we asked donors to prick their own fingers in a normal room environment and collect a single drop of blood sample into a tube,” Tan said. “The tube was placed on ice and delivered to the lab for reprogramming.”
The cells were treated with a buffer at 12-, 24- or 48-hour increments and observed under the microscope for viability and signs of contamination. After 12 days of expansion in medium, the cells appeared healthy and were actively dividing.
The researchers then succeeded in forcing the cells to become mesodermal, endodermal, and neural cells. They were also able to produce cells that gave rise to rhythmically beating cardiomyocytes.
The team said there was no noticeable reduction in reprogramming efficiency between the freshly collected finger-prick samples and the do-it-yourself samples.
“[W]e derived healthy iPSCs from tiny volumes of venipuncture and a single drop from finger-prick blood samples,” Dr Loh said. “We also report a high reprogramming yield of 100 to 600 colonies per milliliter of blood.” ![]()

Credit: Salk Institute
Researchers say they’ve discovered an easy way to collect large quantities of viable, bankable stem cells.
Donors prick their own fingers to provide a single drop of blood, and the team generates induced pluripotent stem cells (iPSCs) from that sample.
“We show that a single drop of blood from a finger-prick sample is sufficient for performing cellular reprogramming, DNA sequencing, and blood typing in parallel,” said Jonathan Yuin-Han Loh, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore.
“Our strategy has the potential of facilitating the development of large-scale human iPSC banking worldwide.”
The researchers described this strategy in STEM CELLS Translational Medicine.
“We gradually reduced the starting volume of blood (collected using a needle) and confirmed that reprogramming can be achieved with as little as 0.25 milliliters,” said Hong Kee Tan, a research officer in the Loh lab.
And this made the team wonder whether a do-it-yourself approach to blood collection might work too.
“To test this idea, we asked donors to prick their own fingers in a normal room environment and collect a single drop of blood sample into a tube,” Tan said. “The tube was placed on ice and delivered to the lab for reprogramming.”
The cells were treated with a buffer at 12-, 24- or 48-hour increments and observed under the microscope for viability and signs of contamination. After 12 days of expansion in medium, the cells appeared healthy and were actively dividing.
The researchers then succeeded in forcing the cells to become mesodermal, endodermal, and neural cells. They were also able to produce cells that gave rise to rhythmically beating cardiomyocytes.
The team said there was no noticeable reduction in reprogramming efficiency between the freshly collected finger-prick samples and the do-it-yourself samples.
“[W]e derived healthy iPSCs from tiny volumes of venipuncture and a single drop from finger-prick blood samples,” Dr Loh said. “We also report a high reprogramming yield of 100 to 600 colonies per milliliter of blood.” ![]()
FDA approves IV formulation of antifungal agent

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()
Investigation reveals ‘inappropriate data handling’ but no misconduct
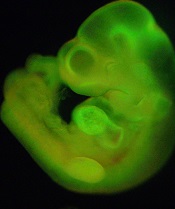
generated from STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has released early results of its investigation into allegations of misconduct leveled against the creators of STAP cells (stimulus-triggered acquisition of pluripotency cells).
RIKEN has confirmed 2 cases of “inappropriate data handling” but said the circumstances did not constitute misconduct.
The investigation is ongoing, with 4 issues—including charges of plagiarism and doctored figures—still to be resolved.
Research prompts questions, criticism
The investigation began shortly after a group of RIKEN scientists and colleagues from a few other institutions announced their creation of STAP cells.
The researchers said they could induce pluripotency in somatic cells by introducing the cells to a low-pH environment, and they reported this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
In light of these issues, one of the study authors recently called for the research to be retracted.
Teruhiko Wakayama, PhD, formerly of RIKEN but now a professor at the University of Yamanashi, said there are “too many uncertainties” surrounding the research at this point. After a retraction, the researchers could collect new data and images to ensure their accuracy and resubmit the research for publication.
On the other hand, fellow study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston, has said a retraction is unnecessary.
“I firmly believe that the questions and concerns raised about our STAP cell paper published in Nature do not affect our findings or conclusions,” Dr Vacanti said.
Investigation launched
In response to the questions and allegations, RIKEN formed a committee to investigate the possibility of misconduct.
The investigation is focusing on 4 of the researchers involved: Haruko Obokata, PhD; Yoshiki Sasai, MD, PhD; Hitoshi Niwa, MD, PhD; and Dr Wakayama.
The committee is also looking into 6 issues with the research, 2 of which have been resolved.
Resolved issues
(1) Critics have questioned the “unnatural appearance of colored cell parts shown by arrows in d2 and d3 images of Figure 1f” in the article.
RIKEN concluded that the process of preparing these images did not constitute fabrication within the context of research misconduct.
(2) Questions have been raised about a “strong resemblance between the rightmost panel in Figure 1b and the lower panel in Figure 2g, both showing a fluorescence image of mice placenta” in the letter.
There is no reference to the figures in the figure legends or the main body of text, and RIKEN does define this sort of discrepancy as fabrication. However, the researchers claimed they had intended to delete one of the figures prior to publication but forgot, and there is no evidence to contradict that explanation. So RIKEN concluded that no malice was intended, and this should not be considered misconduct.
Issues under investigation
(1) In Figure 1i of the article, lane 3 appears to have been inserted.
(2) A part of the article’s “Methods” section on karyotyping analysis appears to have been copied from another paper.
(3) Some of the description of karyotyping in the “Methods” section of the article is different from the procedure the researchers followed.
(4) In the article, the image of differentiated cells for Figures 2d and 2e and the image of chimera mouse immunostaining data are incorrect, and investigation revealed that these images closely resemble images Dr Obokata used in her doctoral dissertation.
Next steps
RIKEN said it will continue with the investigation and issue a full report upon its completion. The institute also aims to determine whether the STAP cell experiments can be reproduced.
“The reproducibility and credibility of the STAP phenomenon must be rigorously validated, not only by RIKEN scientists, but also by others,” said RIKEN President Ryoji Noyori, PhD.
“I have instructed our people to cooperate fully with researchers at outside institutions in their efforts to replicate the STAP cell results.”
Dr Noyori added that RIKEN is prepared to withdraw the Nature papers and take “strict disciplinary action” against the researchers involved if the investigation reveals deliberate misconduct. ![]()

generated from STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has released early results of its investigation into allegations of misconduct leveled against the creators of STAP cells (stimulus-triggered acquisition of pluripotency cells).
RIKEN has confirmed 2 cases of “inappropriate data handling” but said the circumstances did not constitute misconduct.
The investigation is ongoing, with 4 issues—including charges of plagiarism and doctored figures—still to be resolved.
Research prompts questions, criticism
The investigation began shortly after a group of RIKEN scientists and colleagues from a few other institutions announced their creation of STAP cells.
The researchers said they could induce pluripotency in somatic cells by introducing the cells to a low-pH environment, and they reported this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
In light of these issues, one of the study authors recently called for the research to be retracted.
Teruhiko Wakayama, PhD, formerly of RIKEN but now a professor at the University of Yamanashi, said there are “too many uncertainties” surrounding the research at this point. After a retraction, the researchers could collect new data and images to ensure their accuracy and resubmit the research for publication.
On the other hand, fellow study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston, has said a retraction is unnecessary.
“I firmly believe that the questions and concerns raised about our STAP cell paper published in Nature do not affect our findings or conclusions,” Dr Vacanti said.
Investigation launched
In response to the questions and allegations, RIKEN formed a committee to investigate the possibility of misconduct.
The investigation is focusing on 4 of the researchers involved: Haruko Obokata, PhD; Yoshiki Sasai, MD, PhD; Hitoshi Niwa, MD, PhD; and Dr Wakayama.
The committee is also looking into 6 issues with the research, 2 of which have been resolved.
Resolved issues
(1) Critics have questioned the “unnatural appearance of colored cell parts shown by arrows in d2 and d3 images of Figure 1f” in the article.
RIKEN concluded that the process of preparing these images did not constitute fabrication within the context of research misconduct.
(2) Questions have been raised about a “strong resemblance between the rightmost panel in Figure 1b and the lower panel in Figure 2g, both showing a fluorescence image of mice placenta” in the letter.
There is no reference to the figures in the figure legends or the main body of text, and RIKEN does define this sort of discrepancy as fabrication. However, the researchers claimed they had intended to delete one of the figures prior to publication but forgot, and there is no evidence to contradict that explanation. So RIKEN concluded that no malice was intended, and this should not be considered misconduct.
Issues under investigation
(1) In Figure 1i of the article, lane 3 appears to have been inserted.
(2) A part of the article’s “Methods” section on karyotyping analysis appears to have been copied from another paper.
(3) Some of the description of karyotyping in the “Methods” section of the article is different from the procedure the researchers followed.
(4) In the article, the image of differentiated cells for Figures 2d and 2e and the image of chimera mouse immunostaining data are incorrect, and investigation revealed that these images closely resemble images Dr Obokata used in her doctoral dissertation.
Next steps
RIKEN said it will continue with the investigation and issue a full report upon its completion. The institute also aims to determine whether the STAP cell experiments can be reproduced.
“The reproducibility and credibility of the STAP phenomenon must be rigorously validated, not only by RIKEN scientists, but also by others,” said RIKEN President Ryoji Noyori, PhD.
“I have instructed our people to cooperate fully with researchers at outside institutions in their efforts to replicate the STAP cell results.”
Dr Noyori added that RIKEN is prepared to withdraw the Nature papers and take “strict disciplinary action” against the researchers involved if the investigation reveals deliberate misconduct. ![]()

generated from STAP cells
Credit: Haruko Obokata
The Japanese research institute RIKEN has released early results of its investigation into allegations of misconduct leveled against the creators of STAP cells (stimulus-triggered acquisition of pluripotency cells).
RIKEN has confirmed 2 cases of “inappropriate data handling” but said the circumstances did not constitute misconduct.
The investigation is ongoing, with 4 issues—including charges of plagiarism and doctored figures—still to be resolved.
Research prompts questions, criticism
The investigation began shortly after a group of RIKEN scientists and colleagues from a few other institutions announced their creation of STAP cells.
The researchers said they could induce pluripotency in somatic cells by introducing the cells to a low-pH environment, and they reported this discovery in an article and a letter to Nature.
Not long after the papers were published, members of the scientific community began questioning the validity of the research, citing issues with images, possible plagiarism, and an inability to replicate the experiments described.
In light of these issues, one of the study authors recently called for the research to be retracted.
Teruhiko Wakayama, PhD, formerly of RIKEN but now a professor at the University of Yamanashi, said there are “too many uncertainties” surrounding the research at this point. After a retraction, the researchers could collect new data and images to ensure their accuracy and resubmit the research for publication.
On the other hand, fellow study author Charles Vacanti, MD, of Brigham and Women’s Hospital in Boston, has said a retraction is unnecessary.
“I firmly believe that the questions and concerns raised about our STAP cell paper published in Nature do not affect our findings or conclusions,” Dr Vacanti said.
Investigation launched
In response to the questions and allegations, RIKEN formed a committee to investigate the possibility of misconduct.
The investigation is focusing on 4 of the researchers involved: Haruko Obokata, PhD; Yoshiki Sasai, MD, PhD; Hitoshi Niwa, MD, PhD; and Dr Wakayama.
The committee is also looking into 6 issues with the research, 2 of which have been resolved.
Resolved issues
(1) Critics have questioned the “unnatural appearance of colored cell parts shown by arrows in d2 and d3 images of Figure 1f” in the article.
RIKEN concluded that the process of preparing these images did not constitute fabrication within the context of research misconduct.
(2) Questions have been raised about a “strong resemblance between the rightmost panel in Figure 1b and the lower panel in Figure 2g, both showing a fluorescence image of mice placenta” in the letter.
There is no reference to the figures in the figure legends or the main body of text, and RIKEN does define this sort of discrepancy as fabrication. However, the researchers claimed they had intended to delete one of the figures prior to publication but forgot, and there is no evidence to contradict that explanation. So RIKEN concluded that no malice was intended, and this should not be considered misconduct.
Issues under investigation
(1) In Figure 1i of the article, lane 3 appears to have been inserted.
(2) A part of the article’s “Methods” section on karyotyping analysis appears to have been copied from another paper.
(3) Some of the description of karyotyping in the “Methods” section of the article is different from the procedure the researchers followed.
(4) In the article, the image of differentiated cells for Figures 2d and 2e and the image of chimera mouse immunostaining data are incorrect, and investigation revealed that these images closely resemble images Dr Obokata used in her doctoral dissertation.
Next steps
RIKEN said it will continue with the investigation and issue a full report upon its completion. The institute also aims to determine whether the STAP cell experiments can be reproduced.
“The reproducibility and credibility of the STAP phenomenon must be rigorously validated, not only by RIKEN scientists, but also by others,” said RIKEN President Ryoji Noyori, PhD.
“I have instructed our people to cooperate fully with researchers at outside institutions in their efforts to replicate the STAP cell results.”
Dr Noyori added that RIKEN is prepared to withdraw the Nature papers and take “strict disciplinary action” against the researchers involved if the investigation reveals deliberate misconduct. ![]()
Team finds hidden reservoir of HCMV

Credit: Chad McNeeley
Researchers have found evidence suggesting that perivascular mesenchymal stromal cells (MSCs) are a reservoir of human cytomegalovirus (HCMV).
This opens up the possibility of therapeutically targeting these cells, which surround blood vessels in the organs and can be found in the bone marrow.
If effective, such a treatment method could prove life-saving for individuals who experience HCMV reactivation, such as transplant recipients and patients receiving chemotherapy.
“There are antiviral medications designed to prevent HCMV from re-activating, but HCMV infection remains one of the major complications after both organ and bone marrow transplants,” said study author Graca Almeida-Porada, MD, PhD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“The question scientists have been asking for years is, ‘Where does the virus hide when it is latent?’ Maybe if we knew, we could target it.”
Previous research showed that hematopoietic stem cells can harbor HCMV. Dr Almeida-Porada and her colleagues hypothesized that other cell populations may also harbor the virus, and they suspected that perivascular MSCs were a likely culprit.
The team’s suspicions were confirmed when testing revealed that perivascular MSCs are susceptible to HCMV infection and that the virus can grow within these cells.
The researchers also compared the susceptibility of perivascular MSCs from the liver, brain, lung, and bone marrow. And they found the highest rate of HCMV infection in cells from the lung.
“This may explain why pneumonia is the primary manifestation of the HCMV infection in bone marrow transplant recipients,” Dr Almeida-Porada said.
To expand upon these findings, she and her colleagues analyzed bone marrow samples from 19 healthy individuals who had tested positive for HCMV. Quantitative PCR revealed HCMV DNA in perivascular MSCs from 7 of the subjects.
This suggests bone marrow-derived perivascular MSCs may be a natural HCMV reservoir, according to the researchers.
“We have found another source of cells that can harbor HCMV virus,” Dr Almeida-Porada concluded. “Knowing the identity of the cells opens the possibility of targeting treatments to stop its re-activation.”
Dr Almeida-Porada and her colleagues recounted their discoveries in the American Journal of Transplantation. ![]()

Credit: Chad McNeeley
Researchers have found evidence suggesting that perivascular mesenchymal stromal cells (MSCs) are a reservoir of human cytomegalovirus (HCMV).
This opens up the possibility of therapeutically targeting these cells, which surround blood vessels in the organs and can be found in the bone marrow.
If effective, such a treatment method could prove life-saving for individuals who experience HCMV reactivation, such as transplant recipients and patients receiving chemotherapy.
“There are antiviral medications designed to prevent HCMV from re-activating, but HCMV infection remains one of the major complications after both organ and bone marrow transplants,” said study author Graca Almeida-Porada, MD, PhD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“The question scientists have been asking for years is, ‘Where does the virus hide when it is latent?’ Maybe if we knew, we could target it.”
Previous research showed that hematopoietic stem cells can harbor HCMV. Dr Almeida-Porada and her colleagues hypothesized that other cell populations may also harbor the virus, and they suspected that perivascular MSCs were a likely culprit.
The team’s suspicions were confirmed when testing revealed that perivascular MSCs are susceptible to HCMV infection and that the virus can grow within these cells.
The researchers also compared the susceptibility of perivascular MSCs from the liver, brain, lung, and bone marrow. And they found the highest rate of HCMV infection in cells from the lung.
“This may explain why pneumonia is the primary manifestation of the HCMV infection in bone marrow transplant recipients,” Dr Almeida-Porada said.
To expand upon these findings, she and her colleagues analyzed bone marrow samples from 19 healthy individuals who had tested positive for HCMV. Quantitative PCR revealed HCMV DNA in perivascular MSCs from 7 of the subjects.
This suggests bone marrow-derived perivascular MSCs may be a natural HCMV reservoir, according to the researchers.
“We have found another source of cells that can harbor HCMV virus,” Dr Almeida-Porada concluded. “Knowing the identity of the cells opens the possibility of targeting treatments to stop its re-activation.”
Dr Almeida-Porada and her colleagues recounted their discoveries in the American Journal of Transplantation. ![]()

Credit: Chad McNeeley
Researchers have found evidence suggesting that perivascular mesenchymal stromal cells (MSCs) are a reservoir of human cytomegalovirus (HCMV).
This opens up the possibility of therapeutically targeting these cells, which surround blood vessels in the organs and can be found in the bone marrow.
If effective, such a treatment method could prove life-saving for individuals who experience HCMV reactivation, such as transplant recipients and patients receiving chemotherapy.
“There are antiviral medications designed to prevent HCMV from re-activating, but HCMV infection remains one of the major complications after both organ and bone marrow transplants,” said study author Graca Almeida-Porada, MD, PhD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“The question scientists have been asking for years is, ‘Where does the virus hide when it is latent?’ Maybe if we knew, we could target it.”
Previous research showed that hematopoietic stem cells can harbor HCMV. Dr Almeida-Porada and her colleagues hypothesized that other cell populations may also harbor the virus, and they suspected that perivascular MSCs were a likely culprit.
The team’s suspicions were confirmed when testing revealed that perivascular MSCs are susceptible to HCMV infection and that the virus can grow within these cells.
The researchers also compared the susceptibility of perivascular MSCs from the liver, brain, lung, and bone marrow. And they found the highest rate of HCMV infection in cells from the lung.
“This may explain why pneumonia is the primary manifestation of the HCMV infection in bone marrow transplant recipients,” Dr Almeida-Porada said.
To expand upon these findings, she and her colleagues analyzed bone marrow samples from 19 healthy individuals who had tested positive for HCMV. Quantitative PCR revealed HCMV DNA in perivascular MSCs from 7 of the subjects.
This suggests bone marrow-derived perivascular MSCs may be a natural HCMV reservoir, according to the researchers.
“We have found another source of cells that can harbor HCMV virus,” Dr Almeida-Porada concluded. “Knowing the identity of the cells opens the possibility of targeting treatments to stop its re-activation.”
Dr Almeida-Porada and her colleagues recounted their discoveries in the American Journal of Transplantation. ![]()
Analysis details effects of HLA mismatch
GRAPEVINE, TEXAS—An analysis of more than 8000 patients provides insights regarding HLA disparity that may help optimize outcomes in individuals undergoing unrelated-donor hematopoietic stem cell transplant.
The study showed that a single allele- or antigen-level HLA mismatch (7/8) increased the risk for acute and chronic graft-vs-host disease (GVHD) and worsened survival rates.
However, there were no locus-specific effects on survival, and there was no impact of allele- vs antigen-level mismatch on survival.
In addition, patients with an 8/8 matched graft had an increased risk of acute GVHD if they had a DQB1 mismatch or a DPB1 mismatch. DPB1 mismatch also decreased the risk of relapse, and nonpermissive DPB1 mismatch was associated with worse survival.
“Thus, we believe that consideration of DPB1 in donor selection may permit skewing toward donors with permissive DPB1 mismatch and may improve outcomes,” said study investigator Joseph Pidala, MD, of the H. Lee Moffitt Cancer Center in Tampa, Florida.
He presented these findings at the 2014 BMT Tandem Meetings as abstract 5, which was designated one of the meeting’s “Best Abstracts.”
Patient characteristics
Dr Pidala and his colleagues evaluated data from 8003 adult and pediatric patients who had undergone their first myeloablative, unrelated transplant between 1999 and 2011. The patients had been diagnosed with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome.
Patients and their donors had high-resolution typing for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Most cases were 8/8 matched (n=5449), followed by 7/8 (n=2071) and 6/8 (n=483).
“Those with a greater extent of HLA mismatch were younger, less likely to be Caucasian, they differed according to disease indication for transplant, and had a greater proportion of advanced disease stage,” Dr Pidala noted.
“Additionally, those with a greater extent of HLA mismatch were more likely to receive bone marrow grafts in comparison to peripheral blood, total body irradiation, and also to receive in vivo T-cell depletion.”
Furthermore, there was a declining proportion of 6/8 match over time. It decreased from 49% (1999-2002) to 37% (2003-2006) to 14% (2007-2011).
Effects of HLA mismatch
In all analyses, the researchers considered findings significant if the P value was less than 0.01.
Compared to an 8/8 matched graft, receiving a 7/8 graft was significantly associated with an increase in acute grade 2-4, acute grade 3-4, and chronic GVHD; higher transplant-related mortality (TRM); lower disease-free survival (DFS) among patients with early stage or intermediate (but not advanced) disease; and lower overall survival (OS) regardless of disease stage.
Receiving a 6/8 graft was significantly associated with an increase in acute grade 2-4 and 3-4 GVHD, increased TRM, decreased DFS (early stage or intermediate disease), and decreased OS (early stage or intermediate disease).
“In no cases did we find [that mismatch had] an impact on the incidence of primary disease relapse,” Dr Pidala said. “Comparing 7/8 to 6/8 cases, we found that those with 7/8 had improved transplant-related mortality and disease-free and overall survival [only] in the context of early stage disease.”
The team also confirmed these findings in an analysis of 5846 unique cases. In this cohort, 2528 patients were 8/8 matched, 882 were 7/8, and 157 were 6/8.
Locus-specific effects
Mismatch at HLA-A (n=743) was significantly associated with an increase in acute grade 2-4/3-4 and chronic GVHD, increased TRM, decreased DFS, and decreased OS.
Mismatch at HLA-B (n=345) was significantly associated with an increase in grade 2-4/3-4 acute GHVD, chronic GVHD, and TRM. And mismatch at HLA-C (n=766) was significantly associated with an increase in acute grade 3-4 GVHD and TRM and a decrease in DFS and OS.
There were no significant differences in the case of DRB1 mismatch. However, Dr Pidala noted that this group had the smallest number of patients, at 217.
“I’d also like to point out that in direct, pair-wise comparisons across the mismatched loci, we did not observe any significant differences in overall survival,” Dr Pidala said. “Similarly, we found no impact of allele- vs antigen-level mismatch on overall survival.”
DP and DQ mismatch
Among 8/8 matched cases, DPB1 mismatch led to a significantly increased risk for acute grade 2-4 and 3-4 GHVD, as well as a significant reduction in the risk of relapse. This was the case whether patients had a single- or double-allele mismatch.
The addition of DQB1 mismatch significantly increased the risk of grade 2-4 acute GVHD among 8/8 matched cases only if it was a single-antigen mismatch.
Neither DPB1 nor DQB1 mismatch had a significant effect on OS, DFS, or TRM in 8/8 matched cases. And neither DPB1 nor DQB1 mismatch had a significant effect on outcomes of patients who received 7/8 matched grafts.
Among 8/8 matched cases, those with permissive DPB1 mismatch had a significantly lower risk of acute grade 2-4 and 3-4 GVHD and a significantly higher risk of relapse than patients with nonpermissive DPB1 mismatch. Patients with nonpermissive mismatch also had significantly higher TRM and significantly lower DFS and OS.
However, there were no significant differences between permissive and nonpermissive mismatches for patients with 7/8 matched grafts.
Treatment implications
Based on these results and those of previous studies, Dr Pidala said we can conclude that 8/8 matched grafts confer better survival than mismatched grafts. And a permissive DPB1 mismatch can further improve survival in 8/8 cases.
Among patients receiving a 7/8 matched graft, it is preferable to identify those with HLA-C 03:03/03:04 mismatches, as these patients appear to have mortality rates comparable to 8/8 matched cases.
Among 7/8 matched cases, we should avoid nonpermissive DPB1 mismatch, having 3 or more low-expression loci mismatches (DP, DQ, DRB3/4/5), and nonpermissive allele combinations and amino acid substitutions. ![]()
GRAPEVINE, TEXAS—An analysis of more than 8000 patients provides insights regarding HLA disparity that may help optimize outcomes in individuals undergoing unrelated-donor hematopoietic stem cell transplant.
The study showed that a single allele- or antigen-level HLA mismatch (7/8) increased the risk for acute and chronic graft-vs-host disease (GVHD) and worsened survival rates.
However, there were no locus-specific effects on survival, and there was no impact of allele- vs antigen-level mismatch on survival.
In addition, patients with an 8/8 matched graft had an increased risk of acute GVHD if they had a DQB1 mismatch or a DPB1 mismatch. DPB1 mismatch also decreased the risk of relapse, and nonpermissive DPB1 mismatch was associated with worse survival.
“Thus, we believe that consideration of DPB1 in donor selection may permit skewing toward donors with permissive DPB1 mismatch and may improve outcomes,” said study investigator Joseph Pidala, MD, of the H. Lee Moffitt Cancer Center in Tampa, Florida.
He presented these findings at the 2014 BMT Tandem Meetings as abstract 5, which was designated one of the meeting’s “Best Abstracts.”
Patient characteristics
Dr Pidala and his colleagues evaluated data from 8003 adult and pediatric patients who had undergone their first myeloablative, unrelated transplant between 1999 and 2011. The patients had been diagnosed with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome.
Patients and their donors had high-resolution typing for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Most cases were 8/8 matched (n=5449), followed by 7/8 (n=2071) and 6/8 (n=483).
“Those with a greater extent of HLA mismatch were younger, less likely to be Caucasian, they differed according to disease indication for transplant, and had a greater proportion of advanced disease stage,” Dr Pidala noted.
“Additionally, those with a greater extent of HLA mismatch were more likely to receive bone marrow grafts in comparison to peripheral blood, total body irradiation, and also to receive in vivo T-cell depletion.”
Furthermore, there was a declining proportion of 6/8 match over time. It decreased from 49% (1999-2002) to 37% (2003-2006) to 14% (2007-2011).
Effects of HLA mismatch
In all analyses, the researchers considered findings significant if the P value was less than 0.01.
Compared to an 8/8 matched graft, receiving a 7/8 graft was significantly associated with an increase in acute grade 2-4, acute grade 3-4, and chronic GVHD; higher transplant-related mortality (TRM); lower disease-free survival (DFS) among patients with early stage or intermediate (but not advanced) disease; and lower overall survival (OS) regardless of disease stage.
Receiving a 6/8 graft was significantly associated with an increase in acute grade 2-4 and 3-4 GVHD, increased TRM, decreased DFS (early stage or intermediate disease), and decreased OS (early stage or intermediate disease).
“In no cases did we find [that mismatch had] an impact on the incidence of primary disease relapse,” Dr Pidala said. “Comparing 7/8 to 6/8 cases, we found that those with 7/8 had improved transplant-related mortality and disease-free and overall survival [only] in the context of early stage disease.”
The team also confirmed these findings in an analysis of 5846 unique cases. In this cohort, 2528 patients were 8/8 matched, 882 were 7/8, and 157 were 6/8.
Locus-specific effects
Mismatch at HLA-A (n=743) was significantly associated with an increase in acute grade 2-4/3-4 and chronic GVHD, increased TRM, decreased DFS, and decreased OS.
Mismatch at HLA-B (n=345) was significantly associated with an increase in grade 2-4/3-4 acute GHVD, chronic GVHD, and TRM. And mismatch at HLA-C (n=766) was significantly associated with an increase in acute grade 3-4 GVHD and TRM and a decrease in DFS and OS.
There were no significant differences in the case of DRB1 mismatch. However, Dr Pidala noted that this group had the smallest number of patients, at 217.
“I’d also like to point out that in direct, pair-wise comparisons across the mismatched loci, we did not observe any significant differences in overall survival,” Dr Pidala said. “Similarly, we found no impact of allele- vs antigen-level mismatch on overall survival.”
DP and DQ mismatch
Among 8/8 matched cases, DPB1 mismatch led to a significantly increased risk for acute grade 2-4 and 3-4 GHVD, as well as a significant reduction in the risk of relapse. This was the case whether patients had a single- or double-allele mismatch.
The addition of DQB1 mismatch significantly increased the risk of grade 2-4 acute GVHD among 8/8 matched cases only if it was a single-antigen mismatch.
Neither DPB1 nor DQB1 mismatch had a significant effect on OS, DFS, or TRM in 8/8 matched cases. And neither DPB1 nor DQB1 mismatch had a significant effect on outcomes of patients who received 7/8 matched grafts.
Among 8/8 matched cases, those with permissive DPB1 mismatch had a significantly lower risk of acute grade 2-4 and 3-4 GVHD and a significantly higher risk of relapse than patients with nonpermissive DPB1 mismatch. Patients with nonpermissive mismatch also had significantly higher TRM and significantly lower DFS and OS.
However, there were no significant differences between permissive and nonpermissive mismatches for patients with 7/8 matched grafts.
Treatment implications
Based on these results and those of previous studies, Dr Pidala said we can conclude that 8/8 matched grafts confer better survival than mismatched grafts. And a permissive DPB1 mismatch can further improve survival in 8/8 cases.
Among patients receiving a 7/8 matched graft, it is preferable to identify those with HLA-C 03:03/03:04 mismatches, as these patients appear to have mortality rates comparable to 8/8 matched cases.
Among 7/8 matched cases, we should avoid nonpermissive DPB1 mismatch, having 3 or more low-expression loci mismatches (DP, DQ, DRB3/4/5), and nonpermissive allele combinations and amino acid substitutions. ![]()
GRAPEVINE, TEXAS—An analysis of more than 8000 patients provides insights regarding HLA disparity that may help optimize outcomes in individuals undergoing unrelated-donor hematopoietic stem cell transplant.
The study showed that a single allele- or antigen-level HLA mismatch (7/8) increased the risk for acute and chronic graft-vs-host disease (GVHD) and worsened survival rates.
However, there were no locus-specific effects on survival, and there was no impact of allele- vs antigen-level mismatch on survival.
In addition, patients with an 8/8 matched graft had an increased risk of acute GVHD if they had a DQB1 mismatch or a DPB1 mismatch. DPB1 mismatch also decreased the risk of relapse, and nonpermissive DPB1 mismatch was associated with worse survival.
“Thus, we believe that consideration of DPB1 in donor selection may permit skewing toward donors with permissive DPB1 mismatch and may improve outcomes,” said study investigator Joseph Pidala, MD, of the H. Lee Moffitt Cancer Center in Tampa, Florida.
He presented these findings at the 2014 BMT Tandem Meetings as abstract 5, which was designated one of the meeting’s “Best Abstracts.”
Patient characteristics
Dr Pidala and his colleagues evaluated data from 8003 adult and pediatric patients who had undergone their first myeloablative, unrelated transplant between 1999 and 2011. The patients had been diagnosed with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome.
Patients and their donors had high-resolution typing for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Most cases were 8/8 matched (n=5449), followed by 7/8 (n=2071) and 6/8 (n=483).
“Those with a greater extent of HLA mismatch were younger, less likely to be Caucasian, they differed according to disease indication for transplant, and had a greater proportion of advanced disease stage,” Dr Pidala noted.
“Additionally, those with a greater extent of HLA mismatch were more likely to receive bone marrow grafts in comparison to peripheral blood, total body irradiation, and also to receive in vivo T-cell depletion.”
Furthermore, there was a declining proportion of 6/8 match over time. It decreased from 49% (1999-2002) to 37% (2003-2006) to 14% (2007-2011).
Effects of HLA mismatch
In all analyses, the researchers considered findings significant if the P value was less than 0.01.
Compared to an 8/8 matched graft, receiving a 7/8 graft was significantly associated with an increase in acute grade 2-4, acute grade 3-4, and chronic GVHD; higher transplant-related mortality (TRM); lower disease-free survival (DFS) among patients with early stage or intermediate (but not advanced) disease; and lower overall survival (OS) regardless of disease stage.
Receiving a 6/8 graft was significantly associated with an increase in acute grade 2-4 and 3-4 GVHD, increased TRM, decreased DFS (early stage or intermediate disease), and decreased OS (early stage or intermediate disease).
“In no cases did we find [that mismatch had] an impact on the incidence of primary disease relapse,” Dr Pidala said. “Comparing 7/8 to 6/8 cases, we found that those with 7/8 had improved transplant-related mortality and disease-free and overall survival [only] in the context of early stage disease.”
The team also confirmed these findings in an analysis of 5846 unique cases. In this cohort, 2528 patients were 8/8 matched, 882 were 7/8, and 157 were 6/8.
Locus-specific effects
Mismatch at HLA-A (n=743) was significantly associated with an increase in acute grade 2-4/3-4 and chronic GVHD, increased TRM, decreased DFS, and decreased OS.
Mismatch at HLA-B (n=345) was significantly associated with an increase in grade 2-4/3-4 acute GHVD, chronic GVHD, and TRM. And mismatch at HLA-C (n=766) was significantly associated with an increase in acute grade 3-4 GVHD and TRM and a decrease in DFS and OS.
There were no significant differences in the case of DRB1 mismatch. However, Dr Pidala noted that this group had the smallest number of patients, at 217.
“I’d also like to point out that in direct, pair-wise comparisons across the mismatched loci, we did not observe any significant differences in overall survival,” Dr Pidala said. “Similarly, we found no impact of allele- vs antigen-level mismatch on overall survival.”
DP and DQ mismatch
Among 8/8 matched cases, DPB1 mismatch led to a significantly increased risk for acute grade 2-4 and 3-4 GHVD, as well as a significant reduction in the risk of relapse. This was the case whether patients had a single- or double-allele mismatch.
The addition of DQB1 mismatch significantly increased the risk of grade 2-4 acute GVHD among 8/8 matched cases only if it was a single-antigen mismatch.
Neither DPB1 nor DQB1 mismatch had a significant effect on OS, DFS, or TRM in 8/8 matched cases. And neither DPB1 nor DQB1 mismatch had a significant effect on outcomes of patients who received 7/8 matched grafts.
Among 8/8 matched cases, those with permissive DPB1 mismatch had a significantly lower risk of acute grade 2-4 and 3-4 GVHD and a significantly higher risk of relapse than patients with nonpermissive DPB1 mismatch. Patients with nonpermissive mismatch also had significantly higher TRM and significantly lower DFS and OS.
However, there were no significant differences between permissive and nonpermissive mismatches for patients with 7/8 matched grafts.
Treatment implications
Based on these results and those of previous studies, Dr Pidala said we can conclude that 8/8 matched grafts confer better survival than mismatched grafts. And a permissive DPB1 mismatch can further improve survival in 8/8 cases.
Among patients receiving a 7/8 matched graft, it is preferable to identify those with HLA-C 03:03/03:04 mismatches, as these patients appear to have mortality rates comparable to 8/8 matched cases.
Among 7/8 matched cases, we should avoid nonpermissive DPB1 mismatch, having 3 or more low-expression loci mismatches (DP, DQ, DRB3/4/5), and nonpermissive allele combinations and amino acid substitutions. ![]()
T cells protect from GVHD, preserve GVT effect
GRAPEVINE, TEXAS—Adding specialized T cells to allogeneic bone marrow transplants can prevent graft-vs-host disease (GVHD) while preserving the graft-vs-tumor (GVT) effect, preclinical research suggests.
Donor-derived CD4+ invariant natural killer T cells (iNKT cells) helped protect mice from developing GVHD and lymphoma, thereby improving their survival.
As human CD4+ iNKT cells can be expanded in vitro, these findings may translate to the clinic, said study investigator Dominik Schneidawind, MD, of Stanford University in California.
Dr Schneidawind discussed this research at the 2014 BMT Tandem Meetings as abstract 2*, which was designated one of the meeting’s “Best Abstracts.”
Improved survival
Dr Schneidawind and his colleagues created a model of allogeneic bone marrow transplant in Balb/c (H-2Kd) mice. The mice were first irradiated with 8 Gy and then received T cell-depleted bone marrow, along with 1 x 106 CD4/CD8 T cells (Tcon) from C57Bl/6 (H-2Kb) donor mice and varying doses of CD4+ iNKT cells: 1 x 104 to 1 x 105.
Mice that received 100,000 CD4+ iNKT cells had significantly improved survival (P=0.002)—and better weight and GVHD scores—compared to mice that received bone marrow grafts alone. But a CD4+ iNKT dose as low as 10,000 cells also conferred a significant improvement in survival (P=0.03).
“In contrast, you need roughly 500,000 to 1 million donor-derived regulatory T cells to achieve a comparable survival benefit in this model of bone marrow transplantation,” Dr Schneidawind noted.
GVT effect
Dr Schneidawind and his colleagues also evaluated the impact of CD4+ iNKT cells on the GVT effect. They used luciferase-transfected BCL1 cells to induce lymphoma development, injecting the cells into mice 1 day before transplant.
Mice that received BCL1 cells, with or without CD4+ iNKT cells, showed increasing bioluminescence imaging (BLI) signals and all died from progressive lymphoma. The mice that received BLC1 and Tcon did not show an increase in BLI signals, but they all died from GVHD.
However, the mice that received BCL1, Tcon, and CD4+ iNKT cells didn’t show any sign of lymphoma or GVHD and survived through the whole experiment (P=0.002).
The investigators conducted the same experiment with the luciferase-positive A20 cell line. And they observed similar results. Mice that received A20 cells, Tcon, and CD4+ iNKT cells showed no sign of lymphoma and had significantly improved survival (P=0.002) over animals that received A20 and Tcon.
“Interestingly, animals that received A20 cells and 50,000 CD4+ iNKT cells only, without Tcon, showed a significant decrease in BLI signal and significantly improved survival,” Dr Schneidawind noted.
“So we conclude from these experiments that CD4+ iNKT cells do not abrogate the Tcon-mediated [GVT] effect but might exert some distinct antitumor activity by themselves.”
Mechanism of GVHD prevention
Lastly, Dr Schneidawind and his colleagues wanted to determine exactly how CD4+ iNKT cells prevent GVHD. The group’s experiments showed that the cells inhibit the proliferation of alloreactive T cells and promote the expansion of donor-derived CD4+ FoxP3+ Tregs.
The investigators decided to take a closer look at the Tregs, evaluating their role in GVHD-related death.
To do this, the team evaluated survival in 4 groups of mice: (1) irradiated controls; (2) mice that received only bone marrow; (3) mice that received Tcon, CD4+ iNKT cells, and a graft depleted of CD4+ FoxP3+ Tregs; and (4) mice that received Tcon, CD4+ iNKT cells, and a non-depleted graft.
“Animals that received a Treg-depleted graft and were treated with CD4+ iNKT [plus Tcon] did not show a significant improvement in survival [over controls],” Dr Schneidawind said.
“In contrast, animals that received a Treg-non-depleted graft showed a significant improvement in survival [P=0.006]. So we conclude that the expansion of these Tregs is necessary to protect from GVHD lethality and that they are required in this context.”
In closing, Dr Schneidawind noted that, although human CD4+ iNKT cells are rare, they can be isolated from peripheral blood and expanded in vitro. This suggests his group’s findings might ultimately prove useful for patients. ![]()
*Information in the abstract differs from that presented.
GRAPEVINE, TEXAS—Adding specialized T cells to allogeneic bone marrow transplants can prevent graft-vs-host disease (GVHD) while preserving the graft-vs-tumor (GVT) effect, preclinical research suggests.
Donor-derived CD4+ invariant natural killer T cells (iNKT cells) helped protect mice from developing GVHD and lymphoma, thereby improving their survival.
As human CD4+ iNKT cells can be expanded in vitro, these findings may translate to the clinic, said study investigator Dominik Schneidawind, MD, of Stanford University in California.
Dr Schneidawind discussed this research at the 2014 BMT Tandem Meetings as abstract 2*, which was designated one of the meeting’s “Best Abstracts.”
Improved survival
Dr Schneidawind and his colleagues created a model of allogeneic bone marrow transplant in Balb/c (H-2Kd) mice. The mice were first irradiated with 8 Gy and then received T cell-depleted bone marrow, along with 1 x 106 CD4/CD8 T cells (Tcon) from C57Bl/6 (H-2Kb) donor mice and varying doses of CD4+ iNKT cells: 1 x 104 to 1 x 105.
Mice that received 100,000 CD4+ iNKT cells had significantly improved survival (P=0.002)—and better weight and GVHD scores—compared to mice that received bone marrow grafts alone. But a CD4+ iNKT dose as low as 10,000 cells also conferred a significant improvement in survival (P=0.03).
“In contrast, you need roughly 500,000 to 1 million donor-derived regulatory T cells to achieve a comparable survival benefit in this model of bone marrow transplantation,” Dr Schneidawind noted.
GVT effect
Dr Schneidawind and his colleagues also evaluated the impact of CD4+ iNKT cells on the GVT effect. They used luciferase-transfected BCL1 cells to induce lymphoma development, injecting the cells into mice 1 day before transplant.
Mice that received BCL1 cells, with or without CD4+ iNKT cells, showed increasing bioluminescence imaging (BLI) signals and all died from progressive lymphoma. The mice that received BLC1 and Tcon did not show an increase in BLI signals, but they all died from GVHD.
However, the mice that received BCL1, Tcon, and CD4+ iNKT cells didn’t show any sign of lymphoma or GVHD and survived through the whole experiment (P=0.002).
The investigators conducted the same experiment with the luciferase-positive A20 cell line. And they observed similar results. Mice that received A20 cells, Tcon, and CD4+ iNKT cells showed no sign of lymphoma and had significantly improved survival (P=0.002) over animals that received A20 and Tcon.
“Interestingly, animals that received A20 cells and 50,000 CD4+ iNKT cells only, without Tcon, showed a significant decrease in BLI signal and significantly improved survival,” Dr Schneidawind noted.
“So we conclude from these experiments that CD4+ iNKT cells do not abrogate the Tcon-mediated [GVT] effect but might exert some distinct antitumor activity by themselves.”
Mechanism of GVHD prevention
Lastly, Dr Schneidawind and his colleagues wanted to determine exactly how CD4+ iNKT cells prevent GVHD. The group’s experiments showed that the cells inhibit the proliferation of alloreactive T cells and promote the expansion of donor-derived CD4+ FoxP3+ Tregs.
The investigators decided to take a closer look at the Tregs, evaluating their role in GVHD-related death.
To do this, the team evaluated survival in 4 groups of mice: (1) irradiated controls; (2) mice that received only bone marrow; (3) mice that received Tcon, CD4+ iNKT cells, and a graft depleted of CD4+ FoxP3+ Tregs; and (4) mice that received Tcon, CD4+ iNKT cells, and a non-depleted graft.
“Animals that received a Treg-depleted graft and were treated with CD4+ iNKT [plus Tcon] did not show a significant improvement in survival [over controls],” Dr Schneidawind said.
“In contrast, animals that received a Treg-non-depleted graft showed a significant improvement in survival [P=0.006]. So we conclude that the expansion of these Tregs is necessary to protect from GVHD lethality and that they are required in this context.”
In closing, Dr Schneidawind noted that, although human CD4+ iNKT cells are rare, they can be isolated from peripheral blood and expanded in vitro. This suggests his group’s findings might ultimately prove useful for patients. ![]()
*Information in the abstract differs from that presented.
GRAPEVINE, TEXAS—Adding specialized T cells to allogeneic bone marrow transplants can prevent graft-vs-host disease (GVHD) while preserving the graft-vs-tumor (GVT) effect, preclinical research suggests.
Donor-derived CD4+ invariant natural killer T cells (iNKT cells) helped protect mice from developing GVHD and lymphoma, thereby improving their survival.
As human CD4+ iNKT cells can be expanded in vitro, these findings may translate to the clinic, said study investigator Dominik Schneidawind, MD, of Stanford University in California.
Dr Schneidawind discussed this research at the 2014 BMT Tandem Meetings as abstract 2*, which was designated one of the meeting’s “Best Abstracts.”
Improved survival
Dr Schneidawind and his colleagues created a model of allogeneic bone marrow transplant in Balb/c (H-2Kd) mice. The mice were first irradiated with 8 Gy and then received T cell-depleted bone marrow, along with 1 x 106 CD4/CD8 T cells (Tcon) from C57Bl/6 (H-2Kb) donor mice and varying doses of CD4+ iNKT cells: 1 x 104 to 1 x 105.
Mice that received 100,000 CD4+ iNKT cells had significantly improved survival (P=0.002)—and better weight and GVHD scores—compared to mice that received bone marrow grafts alone. But a CD4+ iNKT dose as low as 10,000 cells also conferred a significant improvement in survival (P=0.03).
“In contrast, you need roughly 500,000 to 1 million donor-derived regulatory T cells to achieve a comparable survival benefit in this model of bone marrow transplantation,” Dr Schneidawind noted.
GVT effect
Dr Schneidawind and his colleagues also evaluated the impact of CD4+ iNKT cells on the GVT effect. They used luciferase-transfected BCL1 cells to induce lymphoma development, injecting the cells into mice 1 day before transplant.
Mice that received BCL1 cells, with or without CD4+ iNKT cells, showed increasing bioluminescence imaging (BLI) signals and all died from progressive lymphoma. The mice that received BLC1 and Tcon did not show an increase in BLI signals, but they all died from GVHD.
However, the mice that received BCL1, Tcon, and CD4+ iNKT cells didn’t show any sign of lymphoma or GVHD and survived through the whole experiment (P=0.002).
The investigators conducted the same experiment with the luciferase-positive A20 cell line. And they observed similar results. Mice that received A20 cells, Tcon, and CD4+ iNKT cells showed no sign of lymphoma and had significantly improved survival (P=0.002) over animals that received A20 and Tcon.
“Interestingly, animals that received A20 cells and 50,000 CD4+ iNKT cells only, without Tcon, showed a significant decrease in BLI signal and significantly improved survival,” Dr Schneidawind noted.
“So we conclude from these experiments that CD4+ iNKT cells do not abrogate the Tcon-mediated [GVT] effect but might exert some distinct antitumor activity by themselves.”
Mechanism of GVHD prevention
Lastly, Dr Schneidawind and his colleagues wanted to determine exactly how CD4+ iNKT cells prevent GVHD. The group’s experiments showed that the cells inhibit the proliferation of alloreactive T cells and promote the expansion of donor-derived CD4+ FoxP3+ Tregs.
The investigators decided to take a closer look at the Tregs, evaluating their role in GVHD-related death.
To do this, the team evaluated survival in 4 groups of mice: (1) irradiated controls; (2) mice that received only bone marrow; (3) mice that received Tcon, CD4+ iNKT cells, and a graft depleted of CD4+ FoxP3+ Tregs; and (4) mice that received Tcon, CD4+ iNKT cells, and a non-depleted graft.
“Animals that received a Treg-depleted graft and were treated with CD4+ iNKT [plus Tcon] did not show a significant improvement in survival [over controls],” Dr Schneidawind said.
“In contrast, animals that received a Treg-non-depleted graft showed a significant improvement in survival [P=0.006]. So we conclude that the expansion of these Tregs is necessary to protect from GVHD lethality and that they are required in this context.”
In closing, Dr Schneidawind noted that, although human CD4+ iNKT cells are rare, they can be isolated from peripheral blood and expanded in vitro. This suggests his group’s findings might ultimately prove useful for patients. ![]()
*Information in the abstract differs from that presented.
Order of Cy, TBI doesn’t impact HSCT outcome
GRAPEVINE, TEXAS—The order in which patients receive cyclophosphamide (Cy) and total body irradiation (TBI) does not affect the outcome of hematopoietic stem cell transplant (HSCT), researchers have reported.
In a large, retrospective study, the rates of relapse, survival, chronic graft-vs-host disease (GVHD), and other complications were similar whether patients received Cy-TBI or TBI-Cy.
However, receiving Cy-TBI was associated with a slight decrease in the risk of grade 2-4 acute GVHD.
Jennifer L. Holter-Chakrabarty, MD, of the University of Oklahoma in Oklahoma City, presented these findings at the 2014 BMT Tandem Meetings as abstract 13.
She noted that other researchers have investigated the impact of TBI/Cy order, but the results have not provided definitive answers.
“Mouse models show that TBI-Cy is superior, with reduced lung toxicity and increased incidence of bone marrow damage,” Dr Holter-Chakrabarty said.
“Cy-TBI, however, in a retrospective study, showed improved anti-leukemic effect, as well as increased incidence of sinusoidal obstructive syndrome. So for this reason, we wanted to look at the CIBMTR database and compare the order of cyclophosphamide and TBI.”
She and her colleagues analyzed data from 1769 HSCT recipients who were younger than 60 years of age and had been reported to the CIBMTR from 2003 to 2010. Patients had been diagnosed with acute myeloid leukemia (AML, n=945) or acute lymphoblastic leukemia (ALL, n=824) and were in their first or second remission.
They had received TBI doses of at least 1200 cGy, followed by related or unrelated bone marrow or peripheral blood stem cell grafts. Patients who had received cord blood, haploidentical, or T-cell-depleted grafts were excluded.
In all, 948 patients received Cy-TBI, and 821 received TBI-Cy. The 2 cohorts had comparable patient-, disease- and transplant-related characteristics.
The sequence of TBI and Cy did not significantly affect the rates of relapse, leukemia-free survival, or overall survival. And it had no significant impact on transplant-related complications, with the exception of acute grade 2-4 GVHD.
At 100 days, the rate of grade 2-4 acute GVHD was 39% in the Cy-TBI group and 45% in the TBI-Cy group (P=0.01). But the rates of grade 3-4 acute GVHD were 16% and 15%, respectively (P=0.62).
At 100 days, the incidence of veno-occlusive disease and sinusoidal obstructive syndrome was 4% in the Cy-TBI group and 6% in the TBI-Cy group (P=0.082). At 1 year, the incidence of interstitial pneumonia syndrome was 6% and 5%, respectively (P=0.370).
At 3 years, the relapse rate was 27% in the Cy-TBI group and 29% in the TBI-Cy group (P=0.34). Leukemia-free survival was 48% and 49%, respectively (P=0.27). And overall survival was 53% and 52%, respectively (P=0.62).
Transplant-related mortality at 3 years was 24% and 23%, respectively (P=0.67). And the rate of chronic GVHD was 45% and 47%, respectively (P=0.39).
“So, in this population, we see no difference,” Dr Holter-Chakrabarty said. “Cy-TBI or TBI-Cy—it seems to not make a difference either way in relapse, treatment-related mortality, [chronic] graft-vs-host disease, acute 3 and 4 [GVHD], and survival. However, it does give us an idea that 2-4 acute GVHD may be lower in Cy-TBI.” ![]()
GRAPEVINE, TEXAS—The order in which patients receive cyclophosphamide (Cy) and total body irradiation (TBI) does not affect the outcome of hematopoietic stem cell transplant (HSCT), researchers have reported.
In a large, retrospective study, the rates of relapse, survival, chronic graft-vs-host disease (GVHD), and other complications were similar whether patients received Cy-TBI or TBI-Cy.
However, receiving Cy-TBI was associated with a slight decrease in the risk of grade 2-4 acute GVHD.
Jennifer L. Holter-Chakrabarty, MD, of the University of Oklahoma in Oklahoma City, presented these findings at the 2014 BMT Tandem Meetings as abstract 13.
She noted that other researchers have investigated the impact of TBI/Cy order, but the results have not provided definitive answers.
“Mouse models show that TBI-Cy is superior, with reduced lung toxicity and increased incidence of bone marrow damage,” Dr Holter-Chakrabarty said.
“Cy-TBI, however, in a retrospective study, showed improved anti-leukemic effect, as well as increased incidence of sinusoidal obstructive syndrome. So for this reason, we wanted to look at the CIBMTR database and compare the order of cyclophosphamide and TBI.”
She and her colleagues analyzed data from 1769 HSCT recipients who were younger than 60 years of age and had been reported to the CIBMTR from 2003 to 2010. Patients had been diagnosed with acute myeloid leukemia (AML, n=945) or acute lymphoblastic leukemia (ALL, n=824) and were in their first or second remission.
They had received TBI doses of at least 1200 cGy, followed by related or unrelated bone marrow or peripheral blood stem cell grafts. Patients who had received cord blood, haploidentical, or T-cell-depleted grafts were excluded.
In all, 948 patients received Cy-TBI, and 821 received TBI-Cy. The 2 cohorts had comparable patient-, disease- and transplant-related characteristics.
The sequence of TBI and Cy did not significantly affect the rates of relapse, leukemia-free survival, or overall survival. And it had no significant impact on transplant-related complications, with the exception of acute grade 2-4 GVHD.
At 100 days, the rate of grade 2-4 acute GVHD was 39% in the Cy-TBI group and 45% in the TBI-Cy group (P=0.01). But the rates of grade 3-4 acute GVHD were 16% and 15%, respectively (P=0.62).
At 100 days, the incidence of veno-occlusive disease and sinusoidal obstructive syndrome was 4% in the Cy-TBI group and 6% in the TBI-Cy group (P=0.082). At 1 year, the incidence of interstitial pneumonia syndrome was 6% and 5%, respectively (P=0.370).
At 3 years, the relapse rate was 27% in the Cy-TBI group and 29% in the TBI-Cy group (P=0.34). Leukemia-free survival was 48% and 49%, respectively (P=0.27). And overall survival was 53% and 52%, respectively (P=0.62).
Transplant-related mortality at 3 years was 24% and 23%, respectively (P=0.67). And the rate of chronic GVHD was 45% and 47%, respectively (P=0.39).
“So, in this population, we see no difference,” Dr Holter-Chakrabarty said. “Cy-TBI or TBI-Cy—it seems to not make a difference either way in relapse, treatment-related mortality, [chronic] graft-vs-host disease, acute 3 and 4 [GVHD], and survival. However, it does give us an idea that 2-4 acute GVHD may be lower in Cy-TBI.” ![]()
GRAPEVINE, TEXAS—The order in which patients receive cyclophosphamide (Cy) and total body irradiation (TBI) does not affect the outcome of hematopoietic stem cell transplant (HSCT), researchers have reported.
In a large, retrospective study, the rates of relapse, survival, chronic graft-vs-host disease (GVHD), and other complications were similar whether patients received Cy-TBI or TBI-Cy.
However, receiving Cy-TBI was associated with a slight decrease in the risk of grade 2-4 acute GVHD.
Jennifer L. Holter-Chakrabarty, MD, of the University of Oklahoma in Oklahoma City, presented these findings at the 2014 BMT Tandem Meetings as abstract 13.
She noted that other researchers have investigated the impact of TBI/Cy order, but the results have not provided definitive answers.
“Mouse models show that TBI-Cy is superior, with reduced lung toxicity and increased incidence of bone marrow damage,” Dr Holter-Chakrabarty said.
“Cy-TBI, however, in a retrospective study, showed improved anti-leukemic effect, as well as increased incidence of sinusoidal obstructive syndrome. So for this reason, we wanted to look at the CIBMTR database and compare the order of cyclophosphamide and TBI.”
She and her colleagues analyzed data from 1769 HSCT recipients who were younger than 60 years of age and had been reported to the CIBMTR from 2003 to 2010. Patients had been diagnosed with acute myeloid leukemia (AML, n=945) or acute lymphoblastic leukemia (ALL, n=824) and were in their first or second remission.
They had received TBI doses of at least 1200 cGy, followed by related or unrelated bone marrow or peripheral blood stem cell grafts. Patients who had received cord blood, haploidentical, or T-cell-depleted grafts were excluded.
In all, 948 patients received Cy-TBI, and 821 received TBI-Cy. The 2 cohorts had comparable patient-, disease- and transplant-related characteristics.
The sequence of TBI and Cy did not significantly affect the rates of relapse, leukemia-free survival, or overall survival. And it had no significant impact on transplant-related complications, with the exception of acute grade 2-4 GVHD.
At 100 days, the rate of grade 2-4 acute GVHD was 39% in the Cy-TBI group and 45% in the TBI-Cy group (P=0.01). But the rates of grade 3-4 acute GVHD were 16% and 15%, respectively (P=0.62).
At 100 days, the incidence of veno-occlusive disease and sinusoidal obstructive syndrome was 4% in the Cy-TBI group and 6% in the TBI-Cy group (P=0.082). At 1 year, the incidence of interstitial pneumonia syndrome was 6% and 5%, respectively (P=0.370).
At 3 years, the relapse rate was 27% in the Cy-TBI group and 29% in the TBI-Cy group (P=0.34). Leukemia-free survival was 48% and 49%, respectively (P=0.27). And overall survival was 53% and 52%, respectively (P=0.62).
Transplant-related mortality at 3 years was 24% and 23%, respectively (P=0.67). And the rate of chronic GVHD was 45% and 47%, respectively (P=0.39).
“So, in this population, we see no difference,” Dr Holter-Chakrabarty said. “Cy-TBI or TBI-Cy—it seems to not make a difference either way in relapse, treatment-related mortality, [chronic] graft-vs-host disease, acute 3 and 4 [GVHD], and survival. However, it does give us an idea that 2-4 acute GVHD may be lower in Cy-TBI.”
Score can predict survival in GVHD patients

Credit: CDC
A scoring system that rates symptoms of pulmonary dysfunction can help predict survival in patients with chronic graft-vs-host disease (GVHD), new research suggests.
The National Institutes of Health (NIH) devised a scoring system whereby patients can rate their breathing difficulties on a scale of 0 to 3.
In a study of nearly 500 patients with chronic GVHD, this system proved more effective in predicting survival than other measures of pulmonary dysfunction.
A patient’s score was significantly associated with the risk of overall survival (OS) and non-relapse mortality (NRM).
Stephanie Lee, MD, MPH, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The researchers evaluated the utility of pulmonary function tests (PFTs) and symptom assessment in predicting the outcomes of 496 patients with chronic GVHD. The team looked at results of PFTs and the NIH lung scoring system, which has 2 parts.
One part is the NIH symptom-based lung score, which assigns the following numbers to breathing difficulties: 0 for no symptoms, 1 for shortness of breath climbing stairs, 2 for shortness of breath on flat ground, and 3 for shortness of breath at rest or requiring oxygen.
The second part of the system is the NIH PFT-based lung score, a lung function score calculated according to a patient’s forced expiratory volume in 1 second (FEV1) and diffusing capacity of carbon monoxide (DLCO), corrected for hemoglobin but not alveolar volume.
The researchers focused on a set of hypothesized associations between pulmonary measures and NRM, OS, patient-reported outcomes, and functional status.
The 7 measures of interest were:
- Obstructive lung disease based on PFTs
- Restrictive lung disease based on PFTs
- NIH PFT-based lung score
- NIH symptom-based lung score
- Clinical diagnosis of bronchiolitis obliterans syndrome
- Decrease in FEV1 or forced vital capacity (FVC) compared to enrollment
- Worsening of NIH symptom-based lung score by 1 point or greater compared with the first recorded score.
The researchers found that only the NIH symptom-based lung score was significantly associated with NRM (P=0.02), OS (P=0.02), patient-reported symptoms (P<0.001), and functional status (P<0.001).
In addition, worsening of the NIH symptom-based lung score over time was associated with higher NRM and lower OS.
None of the other measures studied were significantly associated with OS or NRM, although some were associated with patient-reported symptoms.
“The [NIH symptom-based lung score] turned out to be the most predictive,” Dr Lee said. “It’s just a question [and], therefore, easy to do and cost-effective. No special equipment is involved.”
This suggests there’s a simple way for physicians to detect pulmonary dysfunction earlier, she added. A patient’s doctor could follow up on a poor score with tests to determine the cause of the problem and identify the appropriate treatment.

Credit: CDC
A scoring system that rates symptoms of pulmonary dysfunction can help predict survival in patients with chronic graft-vs-host disease (GVHD), new research suggests.
The National Institutes of Health (NIH) devised a scoring system whereby patients can rate their breathing difficulties on a scale of 0 to 3.
In a study of nearly 500 patients with chronic GVHD, this system proved more effective in predicting survival than other measures of pulmonary dysfunction.
A patient’s score was significantly associated with the risk of overall survival (OS) and non-relapse mortality (NRM).
Stephanie Lee, MD, MPH, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The researchers evaluated the utility of pulmonary function tests (PFTs) and symptom assessment in predicting the outcomes of 496 patients with chronic GVHD. The team looked at results of PFTs and the NIH lung scoring system, which has 2 parts.
One part is the NIH symptom-based lung score, which assigns the following numbers to breathing difficulties: 0 for no symptoms, 1 for shortness of breath climbing stairs, 2 for shortness of breath on flat ground, and 3 for shortness of breath at rest or requiring oxygen.
The second part of the system is the NIH PFT-based lung score, a lung function score calculated according to a patient’s forced expiratory volume in 1 second (FEV1) and diffusing capacity of carbon monoxide (DLCO), corrected for hemoglobin but not alveolar volume.
The researchers focused on a set of hypothesized associations between pulmonary measures and NRM, OS, patient-reported outcomes, and functional status.
The 7 measures of interest were:
- Obstructive lung disease based on PFTs
- Restrictive lung disease based on PFTs
- NIH PFT-based lung score
- NIH symptom-based lung score
- Clinical diagnosis of bronchiolitis obliterans syndrome
- Decrease in FEV1 or forced vital capacity (FVC) compared to enrollment
- Worsening of NIH symptom-based lung score by 1 point or greater compared with the first recorded score.
The researchers found that only the NIH symptom-based lung score was significantly associated with NRM (P=0.02), OS (P=0.02), patient-reported symptoms (P<0.001), and functional status (P<0.001).
In addition, worsening of the NIH symptom-based lung score over time was associated with higher NRM and lower OS.
None of the other measures studied were significantly associated with OS or NRM, although some were associated with patient-reported symptoms.
“The [NIH symptom-based lung score] turned out to be the most predictive,” Dr Lee said. “It’s just a question [and], therefore, easy to do and cost-effective. No special equipment is involved.”
This suggests there’s a simple way for physicians to detect pulmonary dysfunction earlier, she added. A patient’s doctor could follow up on a poor score with tests to determine the cause of the problem and identify the appropriate treatment.

Credit: CDC
A scoring system that rates symptoms of pulmonary dysfunction can help predict survival in patients with chronic graft-vs-host disease (GVHD), new research suggests.
The National Institutes of Health (NIH) devised a scoring system whereby patients can rate their breathing difficulties on a scale of 0 to 3.
In a study of nearly 500 patients with chronic GVHD, this system proved more effective in predicting survival than other measures of pulmonary dysfunction.
A patient’s score was significantly associated with the risk of overall survival (OS) and non-relapse mortality (NRM).
Stephanie Lee, MD, MPH, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The researchers evaluated the utility of pulmonary function tests (PFTs) and symptom assessment in predicting the outcomes of 496 patients with chronic GVHD. The team looked at results of PFTs and the NIH lung scoring system, which has 2 parts.
One part is the NIH symptom-based lung score, which assigns the following numbers to breathing difficulties: 0 for no symptoms, 1 for shortness of breath climbing stairs, 2 for shortness of breath on flat ground, and 3 for shortness of breath at rest or requiring oxygen.
The second part of the system is the NIH PFT-based lung score, a lung function score calculated according to a patient’s forced expiratory volume in 1 second (FEV1) and diffusing capacity of carbon monoxide (DLCO), corrected for hemoglobin but not alveolar volume.
The researchers focused on a set of hypothesized associations between pulmonary measures and NRM, OS, patient-reported outcomes, and functional status.
The 7 measures of interest were:
- Obstructive lung disease based on PFTs
- Restrictive lung disease based on PFTs
- NIH PFT-based lung score
- NIH symptom-based lung score
- Clinical diagnosis of bronchiolitis obliterans syndrome
- Decrease in FEV1 or forced vital capacity (FVC) compared to enrollment
- Worsening of NIH symptom-based lung score by 1 point or greater compared with the first recorded score.
The researchers found that only the NIH symptom-based lung score was significantly associated with NRM (P=0.02), OS (P=0.02), patient-reported symptoms (P<0.001), and functional status (P<0.001).
In addition, worsening of the NIH symptom-based lung score over time was associated with higher NRM and lower OS.
None of the other measures studied were significantly associated with OS or NRM, although some were associated with patient-reported symptoms.
“The [NIH symptom-based lung score] turned out to be the most predictive,” Dr Lee said. “It’s just a question [and], therefore, easy to do and cost-effective. No special equipment is involved.”
This suggests there’s a simple way for physicians to detect pulmonary dysfunction earlier, she added. A patient’s doctor could follow up on a poor score with tests to determine the cause of the problem and identify the appropriate treatment.
RIT can improve transplant outcomes in NHL, CLL
GRAPEVINE, TEXAS—Administering radioimmunotherapy (RIT) prior to non-myeloablative allogeneic transplant (NMAT) can improve survival in patients with persistent disease, according to a speaker at the 2014 BMT Tandem Meetings.
Ryan Cassaday, MD, of the University of Washington in Seattle, noted that RIT-augmented NMAT can produce long-term remissions in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (B-NHL) or chronic lymphocytic leukemia (CLL).
But outcomes for patients with persistent disease are “underdescribed.”
So he and his colleagues set out to describe outcomes of NMAT for patients with persistent indolent B-NHL or CLL and estimate the impact of RIT in these patients.
Treatment details
The researchers retrospectively analyzed data from 89 patients who underwent NMAT from December 1998 to April 2009 and were followed until September 2013. Eighteen of the patients had received RIT as part of a prospective study (AK Gopal et al, Blood 2011).
The remaining 71 patients did not receive RIT but met eligibility criteria for that study. Specifically, they had a CD20+ B-cell malignancy, an HLA-matched peripheral blood stem cell donor, and persistent disease at NMAT. These control subjects received fludarabine (30 mg/m2 on days -7, -6, and -5) and 2 Gy of total body irradiation prior to NMAT.
Patients in the RIT group received the same treatment following RIT. On day -21, they received 250 mg/m2 of rituximab before an imaging dose of 111In-ibritumomab tiuxetan. And on day -14, they received 250 mg/m2 of rituximab and 0.4 mCi/kg of 90Y-ibritumomab tiuxetan.
Patient characteristics
In the RIT group, 10 patients had CLL/small lymphocytic lymphoma (SLL), 6 had follicular lymphoma (FL), 1 had marginal zone lymphoma (MZL), and 1 had hairy cell leukemia. As for controls, 52 had CLL/SLL, 18 had FL, and 1 had MZL.
“The majority of patients were male [74%] and a relatively young age, given the diseases being treated [median of 56 years],” Dr Cassaday said. “The majority of patients had previously received rituximab [88%], and patients were heavily pretreated [median of 4 prior therapies, range 1-12].”
There were no significant differences between the 2 treatment groups with regard to the aforementioned characteristics. However, there were some “striking differences” between the 2 groups, Dr Cassaday said, including characteristics that portend worse prognosis.
Specifically, RIT-treated patients had more bulky disease (> 5 cm) than controls (61% vs 15%, P<0.001) and more chemoresistant disease (81% vs 39%, P=0.003). And RIT patients were more likely to have HCT comorbidity index scores of 3 or higher (72% vs 37%, P=0.006), as well as pre-NMAT platelet counts less than 25k/μL (33% vs 7%, P=0.002).
RIT improves PFS, OS
The researchers conducted a multivariate analysis including the factors that differed significantly between the 2 treatment groups. And they found that only RIT was significantly associated with both progression-free survival (PFS) and overall survival (OS).
When calculating survival curves, the researchers adjusted for the imbalance in covariates between the treatment groups.
“[The adjusted survival rate] is essentially what one might expect had the RIT group had similar baseline characteristics as the control group,” Dr Cassaday explained.
Control subjects had a 3-year OS of 55%. For the RIT-treated patients, the unadjusted 3-year OS was 78% (P=0.20), and the adjusted OS was 87% (P=0.008).
The 3-year PFS was 44% for controls. For the RIT group, the unadjusted 3-year PFS was 56% (P=0.36), and the adjusted PFS was 71% (P=0.02).
The researchers also found that RIT did not increase the rate of non-relapse mortality. The unadjusted hazard ratio was 0.5 (P=0.32), and the adjusted hazard ratio was 0.4 (P=0.18).
“This analysis does have some limitations,” Dr Cassaday conceded. “Clearly, it does not replace the strength of evidence that would come from a randomized, controlled trial. And the relatively small sample size does limit our ability to look at a lot of different subgroups.”
In addition, the findings may not apply to other non-myeloablative regimens. And, due to the time frame of the study, the researchers could not account for the potential impact of newer agents.
Nevertheless, Dr Cassaday said the data suggest that RIT can improve the outcome of NMAT in patients with persistent indolent B-NHL or CLL. And a prospective, randomized study evaluating this approach is warranted.
Dr Cassaday presented this research at the 2014 BMT Tandem Meetings as abstract 75. Information in the abstract differs from that presented.
GRAPEVINE, TEXAS—Administering radioimmunotherapy (RIT) prior to non-myeloablative allogeneic transplant (NMAT) can improve survival in patients with persistent disease, according to a speaker at the 2014 BMT Tandem Meetings.
Ryan Cassaday, MD, of the University of Washington in Seattle, noted that RIT-augmented NMAT can produce long-term remissions in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (B-NHL) or chronic lymphocytic leukemia (CLL).
But outcomes for patients with persistent disease are “underdescribed.”
So he and his colleagues set out to describe outcomes of NMAT for patients with persistent indolent B-NHL or CLL and estimate the impact of RIT in these patients.
Treatment details
The researchers retrospectively analyzed data from 89 patients who underwent NMAT from December 1998 to April 2009 and were followed until September 2013. Eighteen of the patients had received RIT as part of a prospective study (AK Gopal et al, Blood 2011).
The remaining 71 patients did not receive RIT but met eligibility criteria for that study. Specifically, they had a CD20+ B-cell malignancy, an HLA-matched peripheral blood stem cell donor, and persistent disease at NMAT. These control subjects received fludarabine (30 mg/m2 on days -7, -6, and -5) and 2 Gy of total body irradiation prior to NMAT.
Patients in the RIT group received the same treatment following RIT. On day -21, they received 250 mg/m2 of rituximab before an imaging dose of 111In-ibritumomab tiuxetan. And on day -14, they received 250 mg/m2 of rituximab and 0.4 mCi/kg of 90Y-ibritumomab tiuxetan.
Patient characteristics
In the RIT group, 10 patients had CLL/small lymphocytic lymphoma (SLL), 6 had follicular lymphoma (FL), 1 had marginal zone lymphoma (MZL), and 1 had hairy cell leukemia. As for controls, 52 had CLL/SLL, 18 had FL, and 1 had MZL.
“The majority of patients were male [74%] and a relatively young age, given the diseases being treated [median of 56 years],” Dr Cassaday said. “The majority of patients had previously received rituximab [88%], and patients were heavily pretreated [median of 4 prior therapies, range 1-12].”
There were no significant differences between the 2 treatment groups with regard to the aforementioned characteristics. However, there were some “striking differences” between the 2 groups, Dr Cassaday said, including characteristics that portend worse prognosis.
Specifically, RIT-treated patients had more bulky disease (> 5 cm) than controls (61% vs 15%, P<0.001) and more chemoresistant disease (81% vs 39%, P=0.003). And RIT patients were more likely to have HCT comorbidity index scores of 3 or higher (72% vs 37%, P=0.006), as well as pre-NMAT platelet counts less than 25k/μL (33% vs 7%, P=0.002).
RIT improves PFS, OS
The researchers conducted a multivariate analysis including the factors that differed significantly between the 2 treatment groups. And they found that only RIT was significantly associated with both progression-free survival (PFS) and overall survival (OS).
When calculating survival curves, the researchers adjusted for the imbalance in covariates between the treatment groups.
“[The adjusted survival rate] is essentially what one might expect had the RIT group had similar baseline characteristics as the control group,” Dr Cassaday explained.
Control subjects had a 3-year OS of 55%. For the RIT-treated patients, the unadjusted 3-year OS was 78% (P=0.20), and the adjusted OS was 87% (P=0.008).
The 3-year PFS was 44% for controls. For the RIT group, the unadjusted 3-year PFS was 56% (P=0.36), and the adjusted PFS was 71% (P=0.02).
The researchers also found that RIT did not increase the rate of non-relapse mortality. The unadjusted hazard ratio was 0.5 (P=0.32), and the adjusted hazard ratio was 0.4 (P=0.18).
“This analysis does have some limitations,” Dr Cassaday conceded. “Clearly, it does not replace the strength of evidence that would come from a randomized, controlled trial. And the relatively small sample size does limit our ability to look at a lot of different subgroups.”
In addition, the findings may not apply to other non-myeloablative regimens. And, due to the time frame of the study, the researchers could not account for the potential impact of newer agents.
Nevertheless, Dr Cassaday said the data suggest that RIT can improve the outcome of NMAT in patients with persistent indolent B-NHL or CLL. And a prospective, randomized study evaluating this approach is warranted.
Dr Cassaday presented this research at the 2014 BMT Tandem Meetings as abstract 75. Information in the abstract differs from that presented.
GRAPEVINE, TEXAS—Administering radioimmunotherapy (RIT) prior to non-myeloablative allogeneic transplant (NMAT) can improve survival in patients with persistent disease, according to a speaker at the 2014 BMT Tandem Meetings.
Ryan Cassaday, MD, of the University of Washington in Seattle, noted that RIT-augmented NMAT can produce long-term remissions in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (B-NHL) or chronic lymphocytic leukemia (CLL).
But outcomes for patients with persistent disease are “underdescribed.”
So he and his colleagues set out to describe outcomes of NMAT for patients with persistent indolent B-NHL or CLL and estimate the impact of RIT in these patients.
Treatment details
The researchers retrospectively analyzed data from 89 patients who underwent NMAT from December 1998 to April 2009 and were followed until September 2013. Eighteen of the patients had received RIT as part of a prospective study (AK Gopal et al, Blood 2011).
The remaining 71 patients did not receive RIT but met eligibility criteria for that study. Specifically, they had a CD20+ B-cell malignancy, an HLA-matched peripheral blood stem cell donor, and persistent disease at NMAT. These control subjects received fludarabine (30 mg/m2 on days -7, -6, and -5) and 2 Gy of total body irradiation prior to NMAT.
Patients in the RIT group received the same treatment following RIT. On day -21, they received 250 mg/m2 of rituximab before an imaging dose of 111In-ibritumomab tiuxetan. And on day -14, they received 250 mg/m2 of rituximab and 0.4 mCi/kg of 90Y-ibritumomab tiuxetan.
Patient characteristics
In the RIT group, 10 patients had CLL/small lymphocytic lymphoma (SLL), 6 had follicular lymphoma (FL), 1 had marginal zone lymphoma (MZL), and 1 had hairy cell leukemia. As for controls, 52 had CLL/SLL, 18 had FL, and 1 had MZL.
“The majority of patients were male [74%] and a relatively young age, given the diseases being treated [median of 56 years],” Dr Cassaday said. “The majority of patients had previously received rituximab [88%], and patients were heavily pretreated [median of 4 prior therapies, range 1-12].”
There were no significant differences between the 2 treatment groups with regard to the aforementioned characteristics. However, there were some “striking differences” between the 2 groups, Dr Cassaday said, including characteristics that portend worse prognosis.
Specifically, RIT-treated patients had more bulky disease (> 5 cm) than controls (61% vs 15%, P<0.001) and more chemoresistant disease (81% vs 39%, P=0.003). And RIT patients were more likely to have HCT comorbidity index scores of 3 or higher (72% vs 37%, P=0.006), as well as pre-NMAT platelet counts less than 25k/μL (33% vs 7%, P=0.002).
RIT improves PFS, OS
The researchers conducted a multivariate analysis including the factors that differed significantly between the 2 treatment groups. And they found that only RIT was significantly associated with both progression-free survival (PFS) and overall survival (OS).
When calculating survival curves, the researchers adjusted for the imbalance in covariates between the treatment groups.
“[The adjusted survival rate] is essentially what one might expect had the RIT group had similar baseline characteristics as the control group,” Dr Cassaday explained.
Control subjects had a 3-year OS of 55%. For the RIT-treated patients, the unadjusted 3-year OS was 78% (P=0.20), and the adjusted OS was 87% (P=0.008).
The 3-year PFS was 44% for controls. For the RIT group, the unadjusted 3-year PFS was 56% (P=0.36), and the adjusted PFS was 71% (P=0.02).
The researchers also found that RIT did not increase the rate of non-relapse mortality. The unadjusted hazard ratio was 0.5 (P=0.32), and the adjusted hazard ratio was 0.4 (P=0.18).
“This analysis does have some limitations,” Dr Cassaday conceded. “Clearly, it does not replace the strength of evidence that would come from a randomized, controlled trial. And the relatively small sample size does limit our ability to look at a lot of different subgroups.”
In addition, the findings may not apply to other non-myeloablative regimens. And, due to the time frame of the study, the researchers could not account for the potential impact of newer agents.
Nevertheless, Dr Cassaday said the data suggest that RIT can improve the outcome of NMAT in patients with persistent indolent B-NHL or CLL. And a prospective, randomized study evaluating this approach is warranted.
Dr Cassaday presented this research at the 2014 BMT Tandem Meetings as abstract 75. Information in the abstract differs from that presented.
Study links graft source to length of hospital stay
GRAPEVINE, TEXAS—Acute leukemia patients who undergo cord blood (CB) transplant have longer hospital stays than patients who receive other types of transplant, new research indicates.
The study also suggests the length of stay (LOS) is similar whether patients receive double or single CB grafts.
So it seems strategies are needed to decrease hospital stay after CB transplant, particularly as LOS drives the cost of care, said Karen K. Ballen, MD, of Massachusetts General Hospital in Boston.
Dr Ballen presented this research at the 2014 BMT Tandem Meetings as abstract 104.*
She and her colleagues studied patients diagnosed with acute leukemias who were transplanted at US centers and reported to the CIBMTR between 2008 and 2011.
Patients were eligible if they received an unrelated single or double CB transplant, an 8/8 matched unrelated donor (MUD) transplant with peripheral blood (PB) or bone marrow (BM), or a 7/8 MUD PB or BM graft.
In all, 1796 patients met these criteria. The researchers evaluated patients’ total hospital LOS in the first 100 days after transplant, compared LOS among graft sources, and looked for predictors of LOS in the first 100 days.
The team stratified patients according to age and conditioning regimen. Pediatric patients were classified as those aged 18 and younger, and they only received myeloablative conditioning (MAC). Adults received either MAC or reduced-intensity conditioning (RIC).
Pediatric patients
In a univariate analysis of the 368 pediatric patients, there was no significant difference in 100-day survival according to graft source (P=0.13).
However, patients who received single or double CB grafts had a significantly higher median total LOS by day 100 than patients who received 8/8 MUD BM, which was the only other graft source in this patient group (P=0.03).
Patients who received CB grafts also had significantly fewer days in which they were alive and not in the hospital (P=0.005).
“We wanted to account for patients whose length of stay was short because they actually died early after transplant,” Dr Ballen explained. “Therefore, we did an analysis of days alive and not in the hospital.”
In a multivariate analysis, pediatric patients who received CB grafts had significantly fewer days alive and out of the hospital than those who received 8/8 MUD BM (P=0.03).
Other factors associated with fewer days alive and out of the hospital were CMV positivity (P=0.01), black race (P=0.01), and a Karnofsky performance score of less than 80 (P=0.03).
Adults on MAC
In a univariate analysis of the 768 adults who received MAC, recipients of CB grafts had significantly worse 100-day survival than their peers (P<0.001), as well as a longer median LOS by day 100 (P<0.001) and fewer days alive and not in the hospital (P<0.001).
In a multivariate analysis, adults who received MAC had significantly fewer days alive and out of the hospital if they received CB grafts than if they received 8/8 MUD BM (P<0.001), 8/8 MUD PB (P<0.001), or 7/8 MUD PB (P=0.01), but not 7/8 MUD BM (P=0.49).
Other factors associated with fewer days alive and out of the hospital were black race (P=0.04), having acute lymphocytic leukemia rather than acute myeloid leukemia (P=0.01), and age 18-25 (P=0.01).
“We were a little surprised at these results—that the older patients actually spent more time alive and out of the hospital,” Dr Ballen said.
Adults on RIC
In a univariate analysis of the 660 adults who received RIC, recipients of CB grafts had significantly worse 100-day survival than their peers (P=0.017), as well as a longer median LOS by day 100 (P<0.001) and fewer days alive and not in the hospital (P<0.001).
In a multivariate analysis, adults who received RIC had significantly fewer days alive and out of the hospital if they received CB grafts than if they received 8/8 MUD PB (P<0.001) or 7/8 MUD PB (P<0.001).
No other factors were associated with the number of days these patients were alive and out of the hospital.
These results, when taken together, suggest that CB grafts are associated with longer hospital stays, independent of other factors.
“The majority of cost appears to be driven by the number of days in the hospital,” Dr Ballen noted. “So these data may be important for resource allocation, especially given the recent changes in the US healthcare system.”
*Information in the abstract differs from that presented at the meeting.
GRAPEVINE, TEXAS—Acute leukemia patients who undergo cord blood (CB) transplant have longer hospital stays than patients who receive other types of transplant, new research indicates.
The study also suggests the length of stay (LOS) is similar whether patients receive double or single CB grafts.
So it seems strategies are needed to decrease hospital stay after CB transplant, particularly as LOS drives the cost of care, said Karen K. Ballen, MD, of Massachusetts General Hospital in Boston.
Dr Ballen presented this research at the 2014 BMT Tandem Meetings as abstract 104.*
She and her colleagues studied patients diagnosed with acute leukemias who were transplanted at US centers and reported to the CIBMTR between 2008 and 2011.
Patients were eligible if they received an unrelated single or double CB transplant, an 8/8 matched unrelated donor (MUD) transplant with peripheral blood (PB) or bone marrow (BM), or a 7/8 MUD PB or BM graft.
In all, 1796 patients met these criteria. The researchers evaluated patients’ total hospital LOS in the first 100 days after transplant, compared LOS among graft sources, and looked for predictors of LOS in the first 100 days.
The team stratified patients according to age and conditioning regimen. Pediatric patients were classified as those aged 18 and younger, and they only received myeloablative conditioning (MAC). Adults received either MAC or reduced-intensity conditioning (RIC).
Pediatric patients
In a univariate analysis of the 368 pediatric patients, there was no significant difference in 100-day survival according to graft source (P=0.13).
However, patients who received single or double CB grafts had a significantly higher median total LOS by day 100 than patients who received 8/8 MUD BM, which was the only other graft source in this patient group (P=0.03).
Patients who received CB grafts also had significantly fewer days in which they were alive and not in the hospital (P=0.005).
“We wanted to account for patients whose length of stay was short because they actually died early after transplant,” Dr Ballen explained. “Therefore, we did an analysis of days alive and not in the hospital.”
In a multivariate analysis, pediatric patients who received CB grafts had significantly fewer days alive and out of the hospital than those who received 8/8 MUD BM (P=0.03).
Other factors associated with fewer days alive and out of the hospital were CMV positivity (P=0.01), black race (P=0.01), and a Karnofsky performance score of less than 80 (P=0.03).
Adults on MAC
In a univariate analysis of the 768 adults who received MAC, recipients of CB grafts had significantly worse 100-day survival than their peers (P<0.001), as well as a longer median LOS by day 100 (P<0.001) and fewer days alive and not in the hospital (P<0.001).
In a multivariate analysis, adults who received MAC had significantly fewer days alive and out of the hospital if they received CB grafts than if they received 8/8 MUD BM (P<0.001), 8/8 MUD PB (P<0.001), or 7/8 MUD PB (P=0.01), but not 7/8 MUD BM (P=0.49).
Other factors associated with fewer days alive and out of the hospital were black race (P=0.04), having acute lymphocytic leukemia rather than acute myeloid leukemia (P=0.01), and age 18-25 (P=0.01).
“We were a little surprised at these results—that the older patients actually spent more time alive and out of the hospital,” Dr Ballen said.
Adults on RIC
In a univariate analysis of the 660 adults who received RIC, recipients of CB grafts had significantly worse 100-day survival than their peers (P=0.017), as well as a longer median LOS by day 100 (P<0.001) and fewer days alive and not in the hospital (P<0.001).
In a multivariate analysis, adults who received RIC had significantly fewer days alive and out of the hospital if they received CB grafts than if they received 8/8 MUD PB (P<0.001) or 7/8 MUD PB (P<0.001).
No other factors were associated with the number of days these patients were alive and out of the hospital.
These results, when taken together, suggest that CB grafts are associated with longer hospital stays, independent of other factors.
“The majority of cost appears to be driven by the number of days in the hospital,” Dr Ballen noted. “So these data may be important for resource allocation, especially given the recent changes in the US healthcare system.”
*Information in the abstract differs from that presented at the meeting.
GRAPEVINE, TEXAS—Acute leukemia patients who undergo cord blood (CB) transplant have longer hospital stays than patients who receive other types of transplant, new research indicates.
The study also suggests the length of stay (LOS) is similar whether patients receive double or single CB grafts.
So it seems strategies are needed to decrease hospital stay after CB transplant, particularly as LOS drives the cost of care, said Karen K. Ballen, MD, of Massachusetts General Hospital in Boston.
Dr Ballen presented this research at the 2014 BMT Tandem Meetings as abstract 104.*
She and her colleagues studied patients diagnosed with acute leukemias who were transplanted at US centers and reported to the CIBMTR between 2008 and 2011.
Patients were eligible if they received an unrelated single or double CB transplant, an 8/8 matched unrelated donor (MUD) transplant with peripheral blood (PB) or bone marrow (BM), or a 7/8 MUD PB or BM graft.
In all, 1796 patients met these criteria. The researchers evaluated patients’ total hospital LOS in the first 100 days after transplant, compared LOS among graft sources, and looked for predictors of LOS in the first 100 days.
The team stratified patients according to age and conditioning regimen. Pediatric patients were classified as those aged 18 and younger, and they only received myeloablative conditioning (MAC). Adults received either MAC or reduced-intensity conditioning (RIC).
Pediatric patients
In a univariate analysis of the 368 pediatric patients, there was no significant difference in 100-day survival according to graft source (P=0.13).
However, patients who received single or double CB grafts had a significantly higher median total LOS by day 100 than patients who received 8/8 MUD BM, which was the only other graft source in this patient group (P=0.03).
Patients who received CB grafts also had significantly fewer days in which they were alive and not in the hospital (P=0.005).
“We wanted to account for patients whose length of stay was short because they actually died early after transplant,” Dr Ballen explained. “Therefore, we did an analysis of days alive and not in the hospital.”
In a multivariate analysis, pediatric patients who received CB grafts had significantly fewer days alive and out of the hospital than those who received 8/8 MUD BM (P=0.03).
Other factors associated with fewer days alive and out of the hospital were CMV positivity (P=0.01), black race (P=0.01), and a Karnofsky performance score of less than 80 (P=0.03).
Adults on MAC
In a univariate analysis of the 768 adults who received MAC, recipients of CB grafts had significantly worse 100-day survival than their peers (P<0.001), as well as a longer median LOS by day 100 (P<0.001) and fewer days alive and not in the hospital (P<0.001).
In a multivariate analysis, adults who received MAC had significantly fewer days alive and out of the hospital if they received CB grafts than if they received 8/8 MUD BM (P<0.001), 8/8 MUD PB (P<0.001), or 7/8 MUD PB (P=0.01), but not 7/8 MUD BM (P=0.49).
Other factors associated with fewer days alive and out of the hospital were black race (P=0.04), having acute lymphocytic leukemia rather than acute myeloid leukemia (P=0.01), and age 18-25 (P=0.01).
“We were a little surprised at these results—that the older patients actually spent more time alive and out of the hospital,” Dr Ballen said.
Adults on RIC
In a univariate analysis of the 660 adults who received RIC, recipients of CB grafts had significantly worse 100-day survival than their peers (P=0.017), as well as a longer median LOS by day 100 (P<0.001) and fewer days alive and not in the hospital (P<0.001).
In a multivariate analysis, adults who received RIC had significantly fewer days alive and out of the hospital if they received CB grafts than if they received 8/8 MUD PB (P<0.001) or 7/8 MUD PB (P<0.001).
No other factors were associated with the number of days these patients were alive and out of the hospital.
These results, when taken together, suggest that CB grafts are associated with longer hospital stays, independent of other factors.
“The majority of cost appears to be driven by the number of days in the hospital,” Dr Ballen noted. “So these data may be important for resource allocation, especially given the recent changes in the US healthcare system.”
*Information in the abstract differs from that presented at the meeting.