User login
Implantable Antibiotic Sponge Fails to Reduce Surgical Wound Infection Rate
The use of implantable gentamicin-impregnated collagen sponges did not reduce the sternal wound infection rate following cardiac surgery in a prospective, randomized, controlled trial of 1,502 patients who were at high risk of infection because of diabetes and/or obesity.
Dr. Elliott Bennett-Guerrero of Duke University in Durham, N.C., and his colleagues reported their findings Aug. 18 in JAMA.
The findings directly contradicted those of a 2005 Swedish study, which found that the sponges reduced surgical wound infections by 53%, and which served as strong preliminary data for the current trial (Ann. Thoracic. Surg. 2005;79:153-62).
The sponges are approved in 54 countries, and the current phase III study was designed to "confirm these promising data and support regulatory approval in the United States." However, the overall wound infection rates at 90 days were statistically similar at 8.4% and 8.7%, respectively, in patients who received standard care plus surgical site implantation of two of the topical antibiotic sponges, and in patients who received standard care with no additional intervention, researchers found. There were 753 patients in the sponge group and 749 in the standard care group,
Like the overall wound infection rates, the superficial and deep sternal wound infection rates were also similar in the treatment and control groups, at 6.5% and 6.1%, and 1.9% and 2.5%, respectively. Additionally, no differences were seen between the treatment and control groups in regard to the rehospitalization rate for sternal wound infection (3.1% and 3.2%, respectively), or in ASEPSIS score (mean, 1.9 and. 2.0, respectively) the investigators found (JAMA 2010;304:755-62).
The ASEPSIS score is a measure of additional treatment, presence of serous discharge, erythema, purulent exudates, separation of the deep tissues, isolation of bacteria, and duration of inpatient stay. Points are assigned for each variable and the scale has a minimum score of 0, with no theoretical maximum, the investigators explained.
Patients in the single-blind study were enrolled at 48 U.S. centers between Dec. 21, 2007, and March 11, 2009. Those randomized to the treatment group underwent insertion of two sponges with a total gentamicin dose of 260 mg, which was the same dose used in the Swedish study. Both groups received standardized care with prophylactic systemic antibiotics and rigid sternal fixation, with the treatment group also undergoing implantation with the sponges.
The groups were balanced regarding baseline characteristics, including age, weight, diabetes, and smoking history, and also in regard to perioperative variables.
As for why the antibiotic sponges failed to reduce infection rates in this study as they did in the Swedish study, the investigators suggested that important quality control measures used in their study – but not in the Swedish study – might be to blame. They cited measures including on-site monitoring and source data verification, central adjudication of outcomes by an independent blinded committee, and the inclusion of a large number of hospitals (48 in the U.S. study, compared with 2 in the Swedish study).
The researchers also wrote that ethnic and regional differences, such as varying distribution of bacterial pathogens between countries, could also explain the differing outcomes. For example, no cases of methicillin-resistant Staphylococcus aureus occurred in the Swedish trial, but in the U.S. study 6.3% of patients had MRSA growth from sternal wound infection, which may not be sensitive to gentamicin.
"Thus, variations in the distribution of bacterial pathogens among countries could affect efficacy in a trial of infection prevention such as the present one" they said, also noting that the findings underscore the importance of large validation trials, as "positive single-center trials are often not confirmed in larger multicenter trials."
Disclosures: The U.S. study was sponsored by Innocoll Technologies Ltd., the maker of the gentamicin-collagen sponges, and Dr. Bennett-Guerrero reported that he is in discussions with Excited States LLC regarding a consulting agreement to advise on a clinical trial involving surgical wound infection prevention. Another author on the study, Dr. T. Bruce Ferguson Jr., reported receiving honoraria from Innocoll Technologies Ltd. for serving on a steering committee.
The use of implantable gentamicin-impregnated collagen sponges did not reduce the sternal wound infection rate following cardiac surgery in a prospective, randomized, controlled trial of 1,502 patients who were at high risk of infection because of diabetes and/or obesity.
Dr. Elliott Bennett-Guerrero of Duke University in Durham, N.C., and his colleagues reported their findings Aug. 18 in JAMA.
The findings directly contradicted those of a 2005 Swedish study, which found that the sponges reduced surgical wound infections by 53%, and which served as strong preliminary data for the current trial (Ann. Thoracic. Surg. 2005;79:153-62).
The sponges are approved in 54 countries, and the current phase III study was designed to "confirm these promising data and support regulatory approval in the United States." However, the overall wound infection rates at 90 days were statistically similar at 8.4% and 8.7%, respectively, in patients who received standard care plus surgical site implantation of two of the topical antibiotic sponges, and in patients who received standard care with no additional intervention, researchers found. There were 753 patients in the sponge group and 749 in the standard care group,
Like the overall wound infection rates, the superficial and deep sternal wound infection rates were also similar in the treatment and control groups, at 6.5% and 6.1%, and 1.9% and 2.5%, respectively. Additionally, no differences were seen between the treatment and control groups in regard to the rehospitalization rate for sternal wound infection (3.1% and 3.2%, respectively), or in ASEPSIS score (mean, 1.9 and. 2.0, respectively) the investigators found (JAMA 2010;304:755-62).
The ASEPSIS score is a measure of additional treatment, presence of serous discharge, erythema, purulent exudates, separation of the deep tissues, isolation of bacteria, and duration of inpatient stay. Points are assigned for each variable and the scale has a minimum score of 0, with no theoretical maximum, the investigators explained.
Patients in the single-blind study were enrolled at 48 U.S. centers between Dec. 21, 2007, and March 11, 2009. Those randomized to the treatment group underwent insertion of two sponges with a total gentamicin dose of 260 mg, which was the same dose used in the Swedish study. Both groups received standardized care with prophylactic systemic antibiotics and rigid sternal fixation, with the treatment group also undergoing implantation with the sponges.
The groups were balanced regarding baseline characteristics, including age, weight, diabetes, and smoking history, and also in regard to perioperative variables.
As for why the antibiotic sponges failed to reduce infection rates in this study as they did in the Swedish study, the investigators suggested that important quality control measures used in their study – but not in the Swedish study – might be to blame. They cited measures including on-site monitoring and source data verification, central adjudication of outcomes by an independent blinded committee, and the inclusion of a large number of hospitals (48 in the U.S. study, compared with 2 in the Swedish study).
The researchers also wrote that ethnic and regional differences, such as varying distribution of bacterial pathogens between countries, could also explain the differing outcomes. For example, no cases of methicillin-resistant Staphylococcus aureus occurred in the Swedish trial, but in the U.S. study 6.3% of patients had MRSA growth from sternal wound infection, which may not be sensitive to gentamicin.
"Thus, variations in the distribution of bacterial pathogens among countries could affect efficacy in a trial of infection prevention such as the present one" they said, also noting that the findings underscore the importance of large validation trials, as "positive single-center trials are often not confirmed in larger multicenter trials."
Disclosures: The U.S. study was sponsored by Innocoll Technologies Ltd., the maker of the gentamicin-collagen sponges, and Dr. Bennett-Guerrero reported that he is in discussions with Excited States LLC regarding a consulting agreement to advise on a clinical trial involving surgical wound infection prevention. Another author on the study, Dr. T. Bruce Ferguson Jr., reported receiving honoraria from Innocoll Technologies Ltd. for serving on a steering committee.
The use of implantable gentamicin-impregnated collagen sponges did not reduce the sternal wound infection rate following cardiac surgery in a prospective, randomized, controlled trial of 1,502 patients who were at high risk of infection because of diabetes and/or obesity.
Dr. Elliott Bennett-Guerrero of Duke University in Durham, N.C., and his colleagues reported their findings Aug. 18 in JAMA.
The findings directly contradicted those of a 2005 Swedish study, which found that the sponges reduced surgical wound infections by 53%, and which served as strong preliminary data for the current trial (Ann. Thoracic. Surg. 2005;79:153-62).
The sponges are approved in 54 countries, and the current phase III study was designed to "confirm these promising data and support regulatory approval in the United States." However, the overall wound infection rates at 90 days were statistically similar at 8.4% and 8.7%, respectively, in patients who received standard care plus surgical site implantation of two of the topical antibiotic sponges, and in patients who received standard care with no additional intervention, researchers found. There were 753 patients in the sponge group and 749 in the standard care group,
Like the overall wound infection rates, the superficial and deep sternal wound infection rates were also similar in the treatment and control groups, at 6.5% and 6.1%, and 1.9% and 2.5%, respectively. Additionally, no differences were seen between the treatment and control groups in regard to the rehospitalization rate for sternal wound infection (3.1% and 3.2%, respectively), or in ASEPSIS score (mean, 1.9 and. 2.0, respectively) the investigators found (JAMA 2010;304:755-62).
The ASEPSIS score is a measure of additional treatment, presence of serous discharge, erythema, purulent exudates, separation of the deep tissues, isolation of bacteria, and duration of inpatient stay. Points are assigned for each variable and the scale has a minimum score of 0, with no theoretical maximum, the investigators explained.
Patients in the single-blind study were enrolled at 48 U.S. centers between Dec. 21, 2007, and March 11, 2009. Those randomized to the treatment group underwent insertion of two sponges with a total gentamicin dose of 260 mg, which was the same dose used in the Swedish study. Both groups received standardized care with prophylactic systemic antibiotics and rigid sternal fixation, with the treatment group also undergoing implantation with the sponges.
The groups were balanced regarding baseline characteristics, including age, weight, diabetes, and smoking history, and also in regard to perioperative variables.
As for why the antibiotic sponges failed to reduce infection rates in this study as they did in the Swedish study, the investigators suggested that important quality control measures used in their study – but not in the Swedish study – might be to blame. They cited measures including on-site monitoring and source data verification, central adjudication of outcomes by an independent blinded committee, and the inclusion of a large number of hospitals (48 in the U.S. study, compared with 2 in the Swedish study).
The researchers also wrote that ethnic and regional differences, such as varying distribution of bacterial pathogens between countries, could also explain the differing outcomes. For example, no cases of methicillin-resistant Staphylococcus aureus occurred in the Swedish trial, but in the U.S. study 6.3% of patients had MRSA growth from sternal wound infection, which may not be sensitive to gentamicin.
"Thus, variations in the distribution of bacterial pathogens among countries could affect efficacy in a trial of infection prevention such as the present one" they said, also noting that the findings underscore the importance of large validation trials, as "positive single-center trials are often not confirmed in larger multicenter trials."
Disclosures: The U.S. study was sponsored by Innocoll Technologies Ltd., the maker of the gentamicin-collagen sponges, and Dr. Bennett-Guerrero reported that he is in discussions with Excited States LLC regarding a consulting agreement to advise on a clinical trial involving surgical wound infection prevention. Another author on the study, Dr. T. Bruce Ferguson Jr., reported receiving honoraria from Innocoll Technologies Ltd. for serving on a steering committee.
Confrontational Coping, Depression May Delay Diabetic Ulcer Healing
Patients with diabetes who have a confrontational coping style or who are depressed may be more likely to have impaired healing of their foot ulcers, based on findings from a prospective observational study.
“Psychological interventions that reduce depression and promote effective coping could significantly improve healing rates in this patient group,” wrote Dr. Kavita Vedhara of the University of Nottingham (England) and colleagues.
On average, 4%-10% of diabetic patients with diabetes mellitus have foot ulceration. “The emotional, physical, and financial costs are considerable, with foot ulcer patients reporting greater depression and poorer quality of life,” the authors wrote. Meanwhile, the complications cost the health care system millions of dollars, the researchers added (Diabetologia 2010;53:1590-8).
For 24 weeks, the investigators assessed 93 patients – 68 men with a mean age of 60 years – with neuropathic or neuroischemic diabetic foot ulcers. The patients were recruited from podiatry clinics between 2002 and 2008.
Clinical and demographic determinants of healing, psychological distress, coping styles, salivary cortisol, and levels of two types of matrix metalloproteinases were evaluated at baseline. The ulcers were assessed at 6, 12, and 24 weeks post baseline.
Of the patients with complete data for the primary analysis, 56 had a healed ulcer by week 24, and 37 remained unhealed. Of the unhealed patients, 14 had an amputation and 3 died during the follow-up period.
The primary analysis showed that the patients who had a tendency toward confrontational coping were less likely to have a healed ulcer by week 24. The results also showed that patients who exhibited clinical depression had smaller changes in ulcer size over time. In addition, patients with unhealed ulcers had lower levels of evening cortisol; higher levels of pro-MMP2, a type of matrix metalloproteinase; and a greater cortisol awakening response at baseline.
“The present study suggests that depression and coping style are associated with a greater likelihood of diabetic foot ulcers not healing over a 6-month period, albeit through seemingly independent pathways, and that cortisol and pro-MMP2 may be among the mechanisms underlying these relationships,” the researchers wrote.
The study was limited by the sample size and by the fact that the assessment of wound size was restricted to the surface of the wounds and did not include measures below the skin.
Because the study is one of the few to investigate the relationship between stress and healing of diabetic foot ulcers, the authors concluded that more research is needed to “elucidate these mechanisms and to develop interventions, in particular those geared to modifying coping style and distress ... in order to improve clinical outcomes in this patient group.”
The investigators reported having no conflicts of interest.
Patients with diabetes who have a confrontational coping style or who are depressed may be more likely to have impaired healing of their foot ulcers, based on findings from a prospective observational study.
“Psychological interventions that reduce depression and promote effective coping could significantly improve healing rates in this patient group,” wrote Dr. Kavita Vedhara of the University of Nottingham (England) and colleagues.
On average, 4%-10% of diabetic patients with diabetes mellitus have foot ulceration. “The emotional, physical, and financial costs are considerable, with foot ulcer patients reporting greater depression and poorer quality of life,” the authors wrote. Meanwhile, the complications cost the health care system millions of dollars, the researchers added (Diabetologia 2010;53:1590-8).
For 24 weeks, the investigators assessed 93 patients – 68 men with a mean age of 60 years – with neuropathic or neuroischemic diabetic foot ulcers. The patients were recruited from podiatry clinics between 2002 and 2008.
Clinical and demographic determinants of healing, psychological distress, coping styles, salivary cortisol, and levels of two types of matrix metalloproteinases were evaluated at baseline. The ulcers were assessed at 6, 12, and 24 weeks post baseline.
Of the patients with complete data for the primary analysis, 56 had a healed ulcer by week 24, and 37 remained unhealed. Of the unhealed patients, 14 had an amputation and 3 died during the follow-up period.
The primary analysis showed that the patients who had a tendency toward confrontational coping were less likely to have a healed ulcer by week 24. The results also showed that patients who exhibited clinical depression had smaller changes in ulcer size over time. In addition, patients with unhealed ulcers had lower levels of evening cortisol; higher levels of pro-MMP2, a type of matrix metalloproteinase; and a greater cortisol awakening response at baseline.
“The present study suggests that depression and coping style are associated with a greater likelihood of diabetic foot ulcers not healing over a 6-month period, albeit through seemingly independent pathways, and that cortisol and pro-MMP2 may be among the mechanisms underlying these relationships,” the researchers wrote.
The study was limited by the sample size and by the fact that the assessment of wound size was restricted to the surface of the wounds and did not include measures below the skin.
Because the study is one of the few to investigate the relationship between stress and healing of diabetic foot ulcers, the authors concluded that more research is needed to “elucidate these mechanisms and to develop interventions, in particular those geared to modifying coping style and distress ... in order to improve clinical outcomes in this patient group.”
The investigators reported having no conflicts of interest.
Patients with diabetes who have a confrontational coping style or who are depressed may be more likely to have impaired healing of their foot ulcers, based on findings from a prospective observational study.
“Psychological interventions that reduce depression and promote effective coping could significantly improve healing rates in this patient group,” wrote Dr. Kavita Vedhara of the University of Nottingham (England) and colleagues.
On average, 4%-10% of diabetic patients with diabetes mellitus have foot ulceration. “The emotional, physical, and financial costs are considerable, with foot ulcer patients reporting greater depression and poorer quality of life,” the authors wrote. Meanwhile, the complications cost the health care system millions of dollars, the researchers added (Diabetologia 2010;53:1590-8).
For 24 weeks, the investigators assessed 93 patients – 68 men with a mean age of 60 years – with neuropathic or neuroischemic diabetic foot ulcers. The patients were recruited from podiatry clinics between 2002 and 2008.
Clinical and demographic determinants of healing, psychological distress, coping styles, salivary cortisol, and levels of two types of matrix metalloproteinases were evaluated at baseline. The ulcers were assessed at 6, 12, and 24 weeks post baseline.
Of the patients with complete data for the primary analysis, 56 had a healed ulcer by week 24, and 37 remained unhealed. Of the unhealed patients, 14 had an amputation and 3 died during the follow-up period.
The primary analysis showed that the patients who had a tendency toward confrontational coping were less likely to have a healed ulcer by week 24. The results also showed that patients who exhibited clinical depression had smaller changes in ulcer size over time. In addition, patients with unhealed ulcers had lower levels of evening cortisol; higher levels of pro-MMP2, a type of matrix metalloproteinase; and a greater cortisol awakening response at baseline.
“The present study suggests that depression and coping style are associated with a greater likelihood of diabetic foot ulcers not healing over a 6-month period, albeit through seemingly independent pathways, and that cortisol and pro-MMP2 may be among the mechanisms underlying these relationships,” the researchers wrote.
The study was limited by the sample size and by the fact that the assessment of wound size was restricted to the surface of the wounds and did not include measures below the skin.
Because the study is one of the few to investigate the relationship between stress and healing of diabetic foot ulcers, the authors concluded that more research is needed to “elucidate these mechanisms and to develop interventions, in particular those geared to modifying coping style and distress ... in order to improve clinical outcomes in this patient group.”
The investigators reported having no conflicts of interest.
Beta-HPVs Increase Likelihood of Squamous Cell Skin Cancers
A new population-based study has demonstrated an association between a group of human papillomaviruses viruses and an increased incidence of squamous cell carcinomas, adding weight to the emerging body of evidence that one genus of HPV may play a role in nonmelanocytic skin cancers.
The findings, published online July 9 in BMJ (BMJ2010 July 9 [doi:10.1136/bmj.c2986]), showed that seropositivity to more than one of a host of 16 beta HPV types increased the likelihood of being diagnosed with a squamous cell carcinoma. Moreover, the risk increased with the number of beta-HPV types to which a person tested positive. No particular type among the 16 examined was found to increase the risk more than another; the risk merely increased with the number of infections.
People with antibodies to two or three types of beta-HPVs were 1.4 times more likely to have been diagnosed with a squamous cell carcinoma than were healthy controls, after adjustment for factors such as age, sex, and smoking. People positive for more than eight types of beta HPV were 1.7 times more likely to have been diagnosed with a skin SCC. However, no link was found between seropositivity to beta HPVs and basal cell cancers.
For their research, the largest study to date to associate beta HPV and squamous cell skin cancer in a general population, epidemiologist Margaret Karagas, Ph.D., of Dartmouth Medical School, Lebanon, New Hampshire, and colleagues identified and analyzed, from an existing cohort in and around the state, 663 previously diagnosed cases of squamous cell carcinoma and 898 cases of basal cell carcinoma, along with 805 randomly chosen healthy controls from the same region.
Study participants were aged between 25 and 75 years and nearly all were white. None of their carcinomas occurred in genital regions. All were interviewed and had venous blood drawn and analyzed for the presence of beta HPV antibodies. A handful of cases—seven squamous cell and two basal cell, along with seven healthy controls—could not be included in the analysis because of problems with their samples.
Dr. Karagas and colleagues performed subanalyses to identify confounding factors that would influence the risk of developing the cancers. “All risk estimates ultimately were adjusted for or stratified by age, sex, and sun sensitivity, and additionally for cigarette smoking for cases of squamous cell carcinoma,” they wrote. “No other factors appreciably influenced the results.”
Of particular interest to the investigators, though, was one subgroup of people taking glucocorticoids. Previous research had demonstrated links between immunosuppressed organ transplant recipients, beta HPV positivity, and another type of nonmelanocytic skin cancer (Transplantation 1996;61:715-21), so Dr. Karagas’ team identified cases and controls who had never had an organ transplant but had taken systemic glucocorticoids to treat other conditions to determine whether glucocorticoid use, and not transplantation, might be the factor affecting the association.
They found that people who reported having taken glucocorticoids for at least a month did in fact have a higher incidence of squamous cell carcinoma with beta HPV positivity. Notably, the investigators wrote, the odds ratio for squamous cell carcinoma with beta HPV positivity was 3.21 among participants with a history of prolonged glucocorticoid use, and 1.23 among those without such a history.
The finding, they wrote, “suggest that the known association between the human papillomavirus and occurrence of skin cancer in the presence of immunosuppression extends to drugs more commonly used by the general population.”
Another potential risk factor Dr. Karagas and colleagues identified was ultraviolet exposure. Though cases with a history of severe sunburn did not evidence higher cancer risk than that of controls with the same history, the findings that sun-sensitive people were at higher risk “provide some evidence of an interaction between exposure to ultraviolet light and human papillomavirus related risk of squamous cell carcinoma,” they wrote.
However, the investigators cautioned, the findings related to the sun-sensitive and glucocorticoid subgroups had limited statistical power. They also noted that because the vast majority of subjects were white, the findings could not necessarily be generalized further.
Neither Dr. Karagas nor her colleagues declared any conflicts of interest.
A new population-based study has demonstrated an association between a group of human papillomaviruses viruses and an increased incidence of squamous cell carcinomas, adding weight to the emerging body of evidence that one genus of HPV may play a role in nonmelanocytic skin cancers.
The findings, published online July 9 in BMJ (BMJ2010 July 9 [doi:10.1136/bmj.c2986]), showed that seropositivity to more than one of a host of 16 beta HPV types increased the likelihood of being diagnosed with a squamous cell carcinoma. Moreover, the risk increased with the number of beta-HPV types to which a person tested positive. No particular type among the 16 examined was found to increase the risk more than another; the risk merely increased with the number of infections.
People with antibodies to two or three types of beta-HPVs were 1.4 times more likely to have been diagnosed with a squamous cell carcinoma than were healthy controls, after adjustment for factors such as age, sex, and smoking. People positive for more than eight types of beta HPV were 1.7 times more likely to have been diagnosed with a skin SCC. However, no link was found between seropositivity to beta HPVs and basal cell cancers.
For their research, the largest study to date to associate beta HPV and squamous cell skin cancer in a general population, epidemiologist Margaret Karagas, Ph.D., of Dartmouth Medical School, Lebanon, New Hampshire, and colleagues identified and analyzed, from an existing cohort in and around the state, 663 previously diagnosed cases of squamous cell carcinoma and 898 cases of basal cell carcinoma, along with 805 randomly chosen healthy controls from the same region.
Study participants were aged between 25 and 75 years and nearly all were white. None of their carcinomas occurred in genital regions. All were interviewed and had venous blood drawn and analyzed for the presence of beta HPV antibodies. A handful of cases—seven squamous cell and two basal cell, along with seven healthy controls—could not be included in the analysis because of problems with their samples.
Dr. Karagas and colleagues performed subanalyses to identify confounding factors that would influence the risk of developing the cancers. “All risk estimates ultimately were adjusted for or stratified by age, sex, and sun sensitivity, and additionally for cigarette smoking for cases of squamous cell carcinoma,” they wrote. “No other factors appreciably influenced the results.”
Of particular interest to the investigators, though, was one subgroup of people taking glucocorticoids. Previous research had demonstrated links between immunosuppressed organ transplant recipients, beta HPV positivity, and another type of nonmelanocytic skin cancer (Transplantation 1996;61:715-21), so Dr. Karagas’ team identified cases and controls who had never had an organ transplant but had taken systemic glucocorticoids to treat other conditions to determine whether glucocorticoid use, and not transplantation, might be the factor affecting the association.
They found that people who reported having taken glucocorticoids for at least a month did in fact have a higher incidence of squamous cell carcinoma with beta HPV positivity. Notably, the investigators wrote, the odds ratio for squamous cell carcinoma with beta HPV positivity was 3.21 among participants with a history of prolonged glucocorticoid use, and 1.23 among those without such a history.
The finding, they wrote, “suggest that the known association between the human papillomavirus and occurrence of skin cancer in the presence of immunosuppression extends to drugs more commonly used by the general population.”
Another potential risk factor Dr. Karagas and colleagues identified was ultraviolet exposure. Though cases with a history of severe sunburn did not evidence higher cancer risk than that of controls with the same history, the findings that sun-sensitive people were at higher risk “provide some evidence of an interaction between exposure to ultraviolet light and human papillomavirus related risk of squamous cell carcinoma,” they wrote.
However, the investigators cautioned, the findings related to the sun-sensitive and glucocorticoid subgroups had limited statistical power. They also noted that because the vast majority of subjects were white, the findings could not necessarily be generalized further.
Neither Dr. Karagas nor her colleagues declared any conflicts of interest.
A new population-based study has demonstrated an association between a group of human papillomaviruses viruses and an increased incidence of squamous cell carcinomas, adding weight to the emerging body of evidence that one genus of HPV may play a role in nonmelanocytic skin cancers.
The findings, published online July 9 in BMJ (BMJ2010 July 9 [doi:10.1136/bmj.c2986]), showed that seropositivity to more than one of a host of 16 beta HPV types increased the likelihood of being diagnosed with a squamous cell carcinoma. Moreover, the risk increased with the number of beta-HPV types to which a person tested positive. No particular type among the 16 examined was found to increase the risk more than another; the risk merely increased with the number of infections.
People with antibodies to two or three types of beta-HPVs were 1.4 times more likely to have been diagnosed with a squamous cell carcinoma than were healthy controls, after adjustment for factors such as age, sex, and smoking. People positive for more than eight types of beta HPV were 1.7 times more likely to have been diagnosed with a skin SCC. However, no link was found between seropositivity to beta HPVs and basal cell cancers.
For their research, the largest study to date to associate beta HPV and squamous cell skin cancer in a general population, epidemiologist Margaret Karagas, Ph.D., of Dartmouth Medical School, Lebanon, New Hampshire, and colleagues identified and analyzed, from an existing cohort in and around the state, 663 previously diagnosed cases of squamous cell carcinoma and 898 cases of basal cell carcinoma, along with 805 randomly chosen healthy controls from the same region.
Study participants were aged between 25 and 75 years and nearly all were white. None of their carcinomas occurred in genital regions. All were interviewed and had venous blood drawn and analyzed for the presence of beta HPV antibodies. A handful of cases—seven squamous cell and two basal cell, along with seven healthy controls—could not be included in the analysis because of problems with their samples.
Dr. Karagas and colleagues performed subanalyses to identify confounding factors that would influence the risk of developing the cancers. “All risk estimates ultimately were adjusted for or stratified by age, sex, and sun sensitivity, and additionally for cigarette smoking for cases of squamous cell carcinoma,” they wrote. “No other factors appreciably influenced the results.”
Of particular interest to the investigators, though, was one subgroup of people taking glucocorticoids. Previous research had demonstrated links between immunosuppressed organ transplant recipients, beta HPV positivity, and another type of nonmelanocytic skin cancer (Transplantation 1996;61:715-21), so Dr. Karagas’ team identified cases and controls who had never had an organ transplant but had taken systemic glucocorticoids to treat other conditions to determine whether glucocorticoid use, and not transplantation, might be the factor affecting the association.
They found that people who reported having taken glucocorticoids for at least a month did in fact have a higher incidence of squamous cell carcinoma with beta HPV positivity. Notably, the investigators wrote, the odds ratio for squamous cell carcinoma with beta HPV positivity was 3.21 among participants with a history of prolonged glucocorticoid use, and 1.23 among those without such a history.
The finding, they wrote, “suggest that the known association between the human papillomavirus and occurrence of skin cancer in the presence of immunosuppression extends to drugs more commonly used by the general population.”
Another potential risk factor Dr. Karagas and colleagues identified was ultraviolet exposure. Though cases with a history of severe sunburn did not evidence higher cancer risk than that of controls with the same history, the findings that sun-sensitive people were at higher risk “provide some evidence of an interaction between exposure to ultraviolet light and human papillomavirus related risk of squamous cell carcinoma,” they wrote.
However, the investigators cautioned, the findings related to the sun-sensitive and glucocorticoid subgroups had limited statistical power. They also noted that because the vast majority of subjects were white, the findings could not necessarily be generalized further.
Neither Dr. Karagas nor her colleagues declared any conflicts of interest.
Diffuse Nature of MRSA Abscesses Contribute to High Treatment Failures
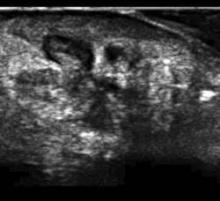
PHOENIX — Methicillin-resistant Staphylococcus aureus abscesses, when compared by ultrasound with those caused by other pathogens, are smaller and more likely to lack a defined edge.
They are also more likely to have edema in surrounding tissue planes as well as pus divided into multiple pockets within the abscess, according to an abscess ultrasound study presented at the Society for Academic Emergency Medicine’s annual meeting.
The characteristics could make it more likely that an abscess is caused by methicillin-resistant Staphylococcus aureus (MRSA), helping guide antibiotic selection pending culture and sensitivity reports, said the study’s presenter, Dr. Dana Resop, an emergency department physician at the University of Massachusetts, Worcester.
The findings also indicate that additional factors—in addition to thick pus—make MRSA abscesses harder to drain than those caused by other pathogens, according to Dr. Romolo J. Gaspari, also an emergency department physician at the school.
The doctors and their colleagues assessed ultrasound images of 254 abscess, blinded to culture results until after the assessments were complete; 124 were MRSA positive.
They found MRSA abscesses are more than twice as likely as those caused by other pathogens to have no defined edge, to have edema in surrounding tissue planes, and have extensions to the skin surface.
They were also smaller and less likely to have ultrasound edge artifacts, thin black lines extending from the abscess edge away from the ultrasound probe.
Though odds ratios were not significant for shape, 26.7% of MRSA abscesses were lobated or ill defined, compared with 11.3% of non-MRSA abscesses.
The less well-organized nature of MRSA abscesses makes it harder to evacuate purulent matter and could contribute to the higher treatment failure rates found in a second study by the team, presented by Dr. Gaspari.
The second study compared incision and drainage (I&D) to ultrasound-guided, 18-gauge needle aspiration for draining abscesses.
The study included 101 abscesses, 54 treated by I&D, the rest by needle aspiration. All patients were treated with antibiotics. Failure was defined as the need for a second drainage.
The team found that 39% of MRSA abscess failed I&D, compared with 11% of non-MRSA abscesses; 92% of MRSA abscesses failed needle aspiration, compared with 55% of non-MRSA abscesses.
Overall, “aspiration’s just not as good as I&D,” Dr. Gaspari said.
When asked by an audience member if he thought the high MRSA failure rates in the second study were related to the physical characteristics of MRSA abscesses found in the first, Dr. Gaspari said, “I would say yes.”
Dr. Gaspari and Dr. Resop reported that they had no disclosures. The studies had no outside funding.
PHOENIX — Methicillin-resistant Staphylococcus aureus abscesses, when compared by ultrasound with those caused by other pathogens, are smaller and more likely to lack a defined edge.
They are also more likely to have edema in surrounding tissue planes as well as pus divided into multiple pockets within the abscess, according to an abscess ultrasound study presented at the Society for Academic Emergency Medicine’s annual meeting.
The characteristics could make it more likely that an abscess is caused by methicillin-resistant Staphylococcus aureus (MRSA), helping guide antibiotic selection pending culture and sensitivity reports, said the study’s presenter, Dr. Dana Resop, an emergency department physician at the University of Massachusetts, Worcester.
The findings also indicate that additional factors—in addition to thick pus—make MRSA abscesses harder to drain than those caused by other pathogens, according to Dr. Romolo J. Gaspari, also an emergency department physician at the school.
The doctors and their colleagues assessed ultrasound images of 254 abscess, blinded to culture results until after the assessments were complete; 124 were MRSA positive.
They found MRSA abscesses are more than twice as likely as those caused by other pathogens to have no defined edge, to have edema in surrounding tissue planes, and have extensions to the skin surface.
They were also smaller and less likely to have ultrasound edge artifacts, thin black lines extending from the abscess edge away from the ultrasound probe.
Though odds ratios were not significant for shape, 26.7% of MRSA abscesses were lobated or ill defined, compared with 11.3% of non-MRSA abscesses.
The less well-organized nature of MRSA abscesses makes it harder to evacuate purulent matter and could contribute to the higher treatment failure rates found in a second study by the team, presented by Dr. Gaspari.
The second study compared incision and drainage (I&D) to ultrasound-guided, 18-gauge needle aspiration for draining abscesses.
The study included 101 abscesses, 54 treated by I&D, the rest by needle aspiration. All patients were treated with antibiotics. Failure was defined as the need for a second drainage.
The team found that 39% of MRSA abscess failed I&D, compared with 11% of non-MRSA abscesses; 92% of MRSA abscesses failed needle aspiration, compared with 55% of non-MRSA abscesses.
Overall, “aspiration’s just not as good as I&D,” Dr. Gaspari said.
When asked by an audience member if he thought the high MRSA failure rates in the second study were related to the physical characteristics of MRSA abscesses found in the first, Dr. Gaspari said, “I would say yes.”
Dr. Gaspari and Dr. Resop reported that they had no disclosures. The studies had no outside funding.
PHOENIX — Methicillin-resistant Staphylococcus aureus abscesses, when compared by ultrasound with those caused by other pathogens, are smaller and more likely to lack a defined edge.
They are also more likely to have edema in surrounding tissue planes as well as pus divided into multiple pockets within the abscess, according to an abscess ultrasound study presented at the Society for Academic Emergency Medicine’s annual meeting.
The characteristics could make it more likely that an abscess is caused by methicillin-resistant Staphylococcus aureus (MRSA), helping guide antibiotic selection pending culture and sensitivity reports, said the study’s presenter, Dr. Dana Resop, an emergency department physician at the University of Massachusetts, Worcester.
The findings also indicate that additional factors—in addition to thick pus—make MRSA abscesses harder to drain than those caused by other pathogens, according to Dr. Romolo J. Gaspari, also an emergency department physician at the school.
The doctors and their colleagues assessed ultrasound images of 254 abscess, blinded to culture results until after the assessments were complete; 124 were MRSA positive.
They found MRSA abscesses are more than twice as likely as those caused by other pathogens to have no defined edge, to have edema in surrounding tissue planes, and have extensions to the skin surface.
They were also smaller and less likely to have ultrasound edge artifacts, thin black lines extending from the abscess edge away from the ultrasound probe.
Though odds ratios were not significant for shape, 26.7% of MRSA abscesses were lobated or ill defined, compared with 11.3% of non-MRSA abscesses.
The less well-organized nature of MRSA abscesses makes it harder to evacuate purulent matter and could contribute to the higher treatment failure rates found in a second study by the team, presented by Dr. Gaspari.
The second study compared incision and drainage (I&D) to ultrasound-guided, 18-gauge needle aspiration for draining abscesses.
The study included 101 abscesses, 54 treated by I&D, the rest by needle aspiration. All patients were treated with antibiotics. Failure was defined as the need for a second drainage.
The team found that 39% of MRSA abscess failed I&D, compared with 11% of non-MRSA abscesses; 92% of MRSA abscesses failed needle aspiration, compared with 55% of non-MRSA abscesses.
Overall, “aspiration’s just not as good as I&D,” Dr. Gaspari said.
When asked by an audience member if he thought the high MRSA failure rates in the second study were related to the physical characteristics of MRSA abscesses found in the first, Dr. Gaspari said, “I would say yes.”
Dr. Gaspari and Dr. Resop reported that they had no disclosures. The studies had no outside funding.
Burn Scar Treatment Called ‘Work in Progress’
PHOENIX — Burn scars rank as one of the most difficult dermatologic conditions to treat, Dr. Jill S. Waibel said at the annual meeting of the American Society for Laser Medicine and Surgery.
"Burn scars are the worst we see in clinical medicine," said Dr. Waibel, a dermatologist with a laser practice in Miami. "I believe that if we can treat a burn scar, we can treat any scar."
Under normal circumstances, wounded skin re-epithelizes from hair follicles and dermal glands, but because burn scars are often partially or completely deprived of their epidermal appendages, "healing is severely affected," she noted.
Thanks to surgical advances in the past decade, survival of burn patients has risen from about 30% to 95%. The types of scars they present with include hypertrophic, keloid, contracture, and atrophic.
Current efforts to treat burn scars fall into one of two camps: prevention of scar formation and late reconstruction of mature scars.
"We do have a model for a scarless wound," Dr. Waibel said. "A fetus in utero does not scar. We don't understand that process. There are also a number of topical applications to prevent scars at the time of the wound. Over 200 cytokines are involved in wound healing."
Research efforts are also under way on laser-assisted skin healing with a diode laser, she said, which alters the wound-healing process by thermal stress.
Current treatment for late reconstruction of mature scars includes surgery, followed by laser combination therapy. "I think fractional therapy is the … standard, but I really don't think we understand the mechanism of action in laser and scar reduction," Dr. Waibel commented. "I think we break it down into two areas: either fractional versus thermal, or probably it's fractional and thermal. The thermal effects are the most interesting. How much heat is required for the most constructive healing versus too much thermal injury? We need to look more at what that [ideal] temperature is."
She tells her burn scar patients to consider their treatment as a "work in progress" and asks them to give her a year before they start to assess efficacy. In 2005, her first burn patient underwent five treatments with a 1550-nm, nonablative, erbium-fiber fractional laser; intralesional Kenalog (triamcinolone); and a shave biopsy.
"We see functional improvement as well as cosmetic, especially with contracture scars, and we're working on some range-of-motion studies right now," Dr. Waibel said.
In a study presented at the society's 2009 meeting, Dr. Waibel and her associates presented results from a proof-of-concept study of 10 patients who had burn scars that were treated with a 1550-nm, nonablative, erbium-fiber fractional laser.
Objective scoring by blinded investigators of photos taken pretreatment and at 3 months posttreatment indicated that 78% of patients had excellent to moderate results.
Dr. Waibel acknowledged certain limitations in current efforts to improve treatment for patients with burn scars, including the need for surgery for anatomical fixes and the lack of understanding of the processes of scar formation and the laser effects on scars. "We need better technology, and we need to maximize treatment modalities," she said. "We really need to develop a scar laser. All of the lasers that we use for scars right now were invented for wrinkles."
Dr. Waibel has conducted research for Solta Medical and Sciton, and she has received honoraria from Lumenis for lectures.
PHOENIX — Burn scars rank as one of the most difficult dermatologic conditions to treat, Dr. Jill S. Waibel said at the annual meeting of the American Society for Laser Medicine and Surgery.
"Burn scars are the worst we see in clinical medicine," said Dr. Waibel, a dermatologist with a laser practice in Miami. "I believe that if we can treat a burn scar, we can treat any scar."
Under normal circumstances, wounded skin re-epithelizes from hair follicles and dermal glands, but because burn scars are often partially or completely deprived of their epidermal appendages, "healing is severely affected," she noted.
Thanks to surgical advances in the past decade, survival of burn patients has risen from about 30% to 95%. The types of scars they present with include hypertrophic, keloid, contracture, and atrophic.
Current efforts to treat burn scars fall into one of two camps: prevention of scar formation and late reconstruction of mature scars.
"We do have a model for a scarless wound," Dr. Waibel said. "A fetus in utero does not scar. We don't understand that process. There are also a number of topical applications to prevent scars at the time of the wound. Over 200 cytokines are involved in wound healing."
Research efforts are also under way on laser-assisted skin healing with a diode laser, she said, which alters the wound-healing process by thermal stress.
Current treatment for late reconstruction of mature scars includes surgery, followed by laser combination therapy. "I think fractional therapy is the … standard, but I really don't think we understand the mechanism of action in laser and scar reduction," Dr. Waibel commented. "I think we break it down into two areas: either fractional versus thermal, or probably it's fractional and thermal. The thermal effects are the most interesting. How much heat is required for the most constructive healing versus too much thermal injury? We need to look more at what that [ideal] temperature is."
She tells her burn scar patients to consider their treatment as a "work in progress" and asks them to give her a year before they start to assess efficacy. In 2005, her first burn patient underwent five treatments with a 1550-nm, nonablative, erbium-fiber fractional laser; intralesional Kenalog (triamcinolone); and a shave biopsy.
"We see functional improvement as well as cosmetic, especially with contracture scars, and we're working on some range-of-motion studies right now," Dr. Waibel said.
In a study presented at the society's 2009 meeting, Dr. Waibel and her associates presented results from a proof-of-concept study of 10 patients who had burn scars that were treated with a 1550-nm, nonablative, erbium-fiber fractional laser.
Objective scoring by blinded investigators of photos taken pretreatment and at 3 months posttreatment indicated that 78% of patients had excellent to moderate results.
Dr. Waibel acknowledged certain limitations in current efforts to improve treatment for patients with burn scars, including the need for surgery for anatomical fixes and the lack of understanding of the processes of scar formation and the laser effects on scars. "We need better technology, and we need to maximize treatment modalities," she said. "We really need to develop a scar laser. All of the lasers that we use for scars right now were invented for wrinkles."
Dr. Waibel has conducted research for Solta Medical and Sciton, and she has received honoraria from Lumenis for lectures.
PHOENIX — Burn scars rank as one of the most difficult dermatologic conditions to treat, Dr. Jill S. Waibel said at the annual meeting of the American Society for Laser Medicine and Surgery.
"Burn scars are the worst we see in clinical medicine," said Dr. Waibel, a dermatologist with a laser practice in Miami. "I believe that if we can treat a burn scar, we can treat any scar."
Under normal circumstances, wounded skin re-epithelizes from hair follicles and dermal glands, but because burn scars are often partially or completely deprived of their epidermal appendages, "healing is severely affected," she noted.
Thanks to surgical advances in the past decade, survival of burn patients has risen from about 30% to 95%. The types of scars they present with include hypertrophic, keloid, contracture, and atrophic.
Current efforts to treat burn scars fall into one of two camps: prevention of scar formation and late reconstruction of mature scars.
"We do have a model for a scarless wound," Dr. Waibel said. "A fetus in utero does not scar. We don't understand that process. There are also a number of topical applications to prevent scars at the time of the wound. Over 200 cytokines are involved in wound healing."
Research efforts are also under way on laser-assisted skin healing with a diode laser, she said, which alters the wound-healing process by thermal stress.
Current treatment for late reconstruction of mature scars includes surgery, followed by laser combination therapy. "I think fractional therapy is the … standard, but I really don't think we understand the mechanism of action in laser and scar reduction," Dr. Waibel commented. "I think we break it down into two areas: either fractional versus thermal, or probably it's fractional and thermal. The thermal effects are the most interesting. How much heat is required for the most constructive healing versus too much thermal injury? We need to look more at what that [ideal] temperature is."
She tells her burn scar patients to consider their treatment as a "work in progress" and asks them to give her a year before they start to assess efficacy. In 2005, her first burn patient underwent five treatments with a 1550-nm, nonablative, erbium-fiber fractional laser; intralesional Kenalog (triamcinolone); and a shave biopsy.
"We see functional improvement as well as cosmetic, especially with contracture scars, and we're working on some range-of-motion studies right now," Dr. Waibel said.
In a study presented at the society's 2009 meeting, Dr. Waibel and her associates presented results from a proof-of-concept study of 10 patients who had burn scars that were treated with a 1550-nm, nonablative, erbium-fiber fractional laser.
Objective scoring by blinded investigators of photos taken pretreatment and at 3 months posttreatment indicated that 78% of patients had excellent to moderate results.
Dr. Waibel acknowledged certain limitations in current efforts to improve treatment for patients with burn scars, including the need for surgery for anatomical fixes and the lack of understanding of the processes of scar formation and the laser effects on scars. "We need better technology, and we need to maximize treatment modalities," she said. "We really need to develop a scar laser. All of the lasers that we use for scars right now were invented for wrinkles."
Dr. Waibel has conducted research for Solta Medical and Sciton, and she has received honoraria from Lumenis for lectures.
Veterans With Embedded Shrapnel Found to Have Strong Skin Reactions to Metal
MIAMI -- Veterans from the first Gulf War with embedded shrapnel have stronger skin reactions to metal allergy than those without shrapnel, according to the first study of cutaneous reactivity to traumatically implanted metals.
Dr. Marianna Shvartsbeyn and her colleagues patch tested 40 Gulf War I veterans to an extensive number of metal allergens and found that, overall, 25% were reactive to zinc and 12.5% to manganese.
The average number of patch test reactions for each group suggested that
reactions were stronger among the 18 veterans with shrapnel in their combat wounds (0.72), compared with the 22 who did not have shrapnel fragments (0.63).
Individuals with fragments also tended to react to more substances on the patch testing: The range of positive patch-test reactions was 0-4 for the group with fragments and 0-2 for the group without fragments, she noted.
A total of 56 shrapnel samples were analyzed for the study.
"The pattern of sensitivity fits with the metal components of the shrapnel extracted from the wounds of the soldiers," said Dr. Shvartsbeyn.
All participants were assessed in the spring of 2009 as part of the Depleted Uranium Follow-Up Program at the Baltimore VA Medical Center.
"Depleted uranium did not emerge as a sensitizer," said Dr. Shvartsbeyn, who was an internal medicine resident at the University of Maryland Medical Center, Baltimore, at the time of the study.
A meeting attendee asked about the source of the uranium. "It is in the bullets intended to penetrate armor and it is incorporated in some vehicle shields. Depleted uranium is a very dense metal," said session moderator Bryan Anderson of the Penn State Milton S. Hershey Medical Center, Hershey.
"Some participants had elevated urine uranium if they had retained depleted uranium shrapnel," Dr. Shvartsbeyn said, but none of these participants had significant patch- test reactions.
Embedded shrapnel can release metals over time and induce an immunologic reactivity in some people, the study suggests, said Dr. Shvartsbeyn, currently a resident in the department of pathology at New York University Medical Center, New York.
The veterans were patch tested to 42 reagents using an extended metal series (Dormer Laboratories Inc.), 50 allergens in the North American tray (Dormer), and soluble uranyl nitrate (SPI Supplies and Structure Probe Inc.). Only strong skin reactions of 1+ or above, or crescendo reactions, were considered positive.
Another meeting attendee commented that zinc often is a skin irritant. "We had a high rate of irritant reactions, but we only focused on 1+ reactions in this analysis," Dr. Shvartsbeyn replied.
Because the rates of reactivity to zinc and manganese in the general population are unknown, Dr. Shvartsbeyn and her coworkers also recruited a control group of 46 patients assessed for allergic contact dermatitis at the University of Maryland dermatology clinic from July to December 2009. This group demonstrated lower reactivity rates to zinc (8.6%) and to manganese (6.5%).
"A threefold reactivity in zinc and twofold reactivity to manganese [among the veterans] makes us think these could be the true sensitizers," she said.
MIAMI -- Veterans from the first Gulf War with embedded shrapnel have stronger skin reactions to metal allergy than those without shrapnel, according to the first study of cutaneous reactivity to traumatically implanted metals.
Dr. Marianna Shvartsbeyn and her colleagues patch tested 40 Gulf War I veterans to an extensive number of metal allergens and found that, overall, 25% were reactive to zinc and 12.5% to manganese.
The average number of patch test reactions for each group suggested that
reactions were stronger among the 18 veterans with shrapnel in their combat wounds (0.72), compared with the 22 who did not have shrapnel fragments (0.63).
Individuals with fragments also tended to react to more substances on the patch testing: The range of positive patch-test reactions was 0-4 for the group with fragments and 0-2 for the group without fragments, she noted.
A total of 56 shrapnel samples were analyzed for the study.
"The pattern of sensitivity fits with the metal components of the shrapnel extracted from the wounds of the soldiers," said Dr. Shvartsbeyn.
All participants were assessed in the spring of 2009 as part of the Depleted Uranium Follow-Up Program at the Baltimore VA Medical Center.
"Depleted uranium did not emerge as a sensitizer," said Dr. Shvartsbeyn, who was an internal medicine resident at the University of Maryland Medical Center, Baltimore, at the time of the study.
A meeting attendee asked about the source of the uranium. "It is in the bullets intended to penetrate armor and it is incorporated in some vehicle shields. Depleted uranium is a very dense metal," said session moderator Bryan Anderson of the Penn State Milton S. Hershey Medical Center, Hershey.
"Some participants had elevated urine uranium if they had retained depleted uranium shrapnel," Dr. Shvartsbeyn said, but none of these participants had significant patch- test reactions.
Embedded shrapnel can release metals over time and induce an immunologic reactivity in some people, the study suggests, said Dr. Shvartsbeyn, currently a resident in the department of pathology at New York University Medical Center, New York.
The veterans were patch tested to 42 reagents using an extended metal series (Dormer Laboratories Inc.), 50 allergens in the North American tray (Dormer), and soluble uranyl nitrate (SPI Supplies and Structure Probe Inc.). Only strong skin reactions of 1+ or above, or crescendo reactions, were considered positive.
Another meeting attendee commented that zinc often is a skin irritant. "We had a high rate of irritant reactions, but we only focused on 1+ reactions in this analysis," Dr. Shvartsbeyn replied.
Because the rates of reactivity to zinc and manganese in the general population are unknown, Dr. Shvartsbeyn and her coworkers also recruited a control group of 46 patients assessed for allergic contact dermatitis at the University of Maryland dermatology clinic from July to December 2009. This group demonstrated lower reactivity rates to zinc (8.6%) and to manganese (6.5%).
"A threefold reactivity in zinc and twofold reactivity to manganese [among the veterans] makes us think these could be the true sensitizers," she said.
MIAMI -- Veterans from the first Gulf War with embedded shrapnel have stronger skin reactions to metal allergy than those without shrapnel, according to the first study of cutaneous reactivity to traumatically implanted metals.
Dr. Marianna Shvartsbeyn and her colleagues patch tested 40 Gulf War I veterans to an extensive number of metal allergens and found that, overall, 25% were reactive to zinc and 12.5% to manganese.
The average number of patch test reactions for each group suggested that
reactions were stronger among the 18 veterans with shrapnel in their combat wounds (0.72), compared with the 22 who did not have shrapnel fragments (0.63).
Individuals with fragments also tended to react to more substances on the patch testing: The range of positive patch-test reactions was 0-4 for the group with fragments and 0-2 for the group without fragments, she noted.
A total of 56 shrapnel samples were analyzed for the study.
"The pattern of sensitivity fits with the metal components of the shrapnel extracted from the wounds of the soldiers," said Dr. Shvartsbeyn.
All participants were assessed in the spring of 2009 as part of the Depleted Uranium Follow-Up Program at the Baltimore VA Medical Center.
"Depleted uranium did not emerge as a sensitizer," said Dr. Shvartsbeyn, who was an internal medicine resident at the University of Maryland Medical Center, Baltimore, at the time of the study.
A meeting attendee asked about the source of the uranium. "It is in the bullets intended to penetrate armor and it is incorporated in some vehicle shields. Depleted uranium is a very dense metal," said session moderator Bryan Anderson of the Penn State Milton S. Hershey Medical Center, Hershey.
"Some participants had elevated urine uranium if they had retained depleted uranium shrapnel," Dr. Shvartsbeyn said, but none of these participants had significant patch- test reactions.
Embedded shrapnel can release metals over time and induce an immunologic reactivity in some people, the study suggests, said Dr. Shvartsbeyn, currently a resident in the department of pathology at New York University Medical Center, New York.
The veterans were patch tested to 42 reagents using an extended metal series (Dormer Laboratories Inc.), 50 allergens in the North American tray (Dormer), and soluble uranyl nitrate (SPI Supplies and Structure Probe Inc.). Only strong skin reactions of 1+ or above, or crescendo reactions, were considered positive.
Another meeting attendee commented that zinc often is a skin irritant. "We had a high rate of irritant reactions, but we only focused on 1+ reactions in this analysis," Dr. Shvartsbeyn replied.
Because the rates of reactivity to zinc and manganese in the general population are unknown, Dr. Shvartsbeyn and her coworkers also recruited a control group of 46 patients assessed for allergic contact dermatitis at the University of Maryland dermatology clinic from July to December 2009. This group demonstrated lower reactivity rates to zinc (8.6%) and to manganese (6.5%).
"A threefold reactivity in zinc and twofold reactivity to manganese [among the veterans] makes us think these could be the true sensitizers," she said.
A Review of Bioactive Materials and Chronic Wounds
Burned Children Benefit From Growth Hormone
INDIAN WELLS, CALIF. — Severe burns in children typically stunt growth in the first 2 years after injury, but growth curves can be normalized with some dosages of recombinant human growth hormone, a prospective, double-blind study of 206 patients found.
Children with burns covering more than 40% of their total body surface area were randomized to receive placebo or one of three doses (0.05, 0.1, or 0.2 mg/kg per day) of subcutaneous recombinant human growth hormone (rhGH) or placebo starting at discharge from the hospital through 1 year after being burned.
They were examined for treatment effects at 6, 9, 12, 18, and 24 months post burn.
The 105 patients on placebo were at the 33rd percentile for growth on standard growth curves at baseline and at all follow-up time points.
Growth returned to normal in 37 patients on 0.05 mg/kg per day rhGH at the 12- and 24-month follow-ups and at all follow-up time points in 41 patients in the 0.1-mg/kg per day rhGH group, with significantly better growth, compared with the placebo group, Dr. David N. Herndon and his associates reported at the annual meeting of the American Surgical Association.
Paradoxically, 23 patients given the highest dose of rhGH (0.2 mg/kg per day) showed less growth than did the other rhGH groups—even though the highest dose produced the greatest improvements in lean body mass and in some other parameters measured, said Dr. Herndon, chief of staff at the Shriners Burn Institute and professor and chair of surgery at the University of Texas Medical Branch, both in Galveston. Dr. Ludwik K. Branski, also of the university, was the lead investigator in the study.
The limited growth on high-dose rhGH might be related to a significant decrease in bone mineral content and a significant increase in levels of osteocalcin (a marker of bone resorption) in that group, he suggested. “This may be because we did not give them enough calcium or enough vitamin D to allow the growth-potentiating effects on bone to express themselves by increased bone mineral content,” he speculated.
The few adverse events all occurred in the high-dose group: hypercalcemia in two patients and glucose intolerance in one patient that necessitated discontinuation of rhGH.
In addition to measuring growth, rhGH levels, lean body mass, bone mineral content, and osteocalcin levels, the investigators assessed levels of insulin-like growth factor body protein 3 (a mediator of growth hormone's effects), resting energy expenditure, cardiac output, percent body fat, and parathyroid levels. “The 0.1-mg/kg per day dose had salutary effects in all body systems and did not reach a dose that was dangerous in any particular group,” and thus can be recommended for extensively burned children, Dr. Herndon said.
Commenting on the study, Dr. Basil A. Pruitt Jr. cautioned that more information is needed to evaluate the findings. “Since food intake and exercise can influence lean body mass and body weight,” it would have been helpful to have daily exercise and dietary logs in the study, said Dr. Pruitt of the University of Texas Health Sciences Center, San Antonio.
Although approximately two-thirds of each group were males, it also would be helpful to compare males with males and females with females to eliminate any potential sex bias, he suggested. Dr. Herndon said that analysis will be done.
Dr. Herndon and his associates reported having no conflicts of interest related to this study.
INDIAN WELLS, CALIF. — Severe burns in children typically stunt growth in the first 2 years after injury, but growth curves can be normalized with some dosages of recombinant human growth hormone, a prospective, double-blind study of 206 patients found.
Children with burns covering more than 40% of their total body surface area were randomized to receive placebo or one of three doses (0.05, 0.1, or 0.2 mg/kg per day) of subcutaneous recombinant human growth hormone (rhGH) or placebo starting at discharge from the hospital through 1 year after being burned.
They were examined for treatment effects at 6, 9, 12, 18, and 24 months post burn.
The 105 patients on placebo were at the 33rd percentile for growth on standard growth curves at baseline and at all follow-up time points.
Growth returned to normal in 37 patients on 0.05 mg/kg per day rhGH at the 12- and 24-month follow-ups and at all follow-up time points in 41 patients in the 0.1-mg/kg per day rhGH group, with significantly better growth, compared with the placebo group, Dr. David N. Herndon and his associates reported at the annual meeting of the American Surgical Association.
Paradoxically, 23 patients given the highest dose of rhGH (0.2 mg/kg per day) showed less growth than did the other rhGH groups—even though the highest dose produced the greatest improvements in lean body mass and in some other parameters measured, said Dr. Herndon, chief of staff at the Shriners Burn Institute and professor and chair of surgery at the University of Texas Medical Branch, both in Galveston. Dr. Ludwik K. Branski, also of the university, was the lead investigator in the study.
The limited growth on high-dose rhGH might be related to a significant decrease in bone mineral content and a significant increase in levels of osteocalcin (a marker of bone resorption) in that group, he suggested. “This may be because we did not give them enough calcium or enough vitamin D to allow the growth-potentiating effects on bone to express themselves by increased bone mineral content,” he speculated.
The few adverse events all occurred in the high-dose group: hypercalcemia in two patients and glucose intolerance in one patient that necessitated discontinuation of rhGH.
In addition to measuring growth, rhGH levels, lean body mass, bone mineral content, and osteocalcin levels, the investigators assessed levels of insulin-like growth factor body protein 3 (a mediator of growth hormone's effects), resting energy expenditure, cardiac output, percent body fat, and parathyroid levels. “The 0.1-mg/kg per day dose had salutary effects in all body systems and did not reach a dose that was dangerous in any particular group,” and thus can be recommended for extensively burned children, Dr. Herndon said.
Commenting on the study, Dr. Basil A. Pruitt Jr. cautioned that more information is needed to evaluate the findings. “Since food intake and exercise can influence lean body mass and body weight,” it would have been helpful to have daily exercise and dietary logs in the study, said Dr. Pruitt of the University of Texas Health Sciences Center, San Antonio.
Although approximately two-thirds of each group were males, it also would be helpful to compare males with males and females with females to eliminate any potential sex bias, he suggested. Dr. Herndon said that analysis will be done.
Dr. Herndon and his associates reported having no conflicts of interest related to this study.
INDIAN WELLS, CALIF. — Severe burns in children typically stunt growth in the first 2 years after injury, but growth curves can be normalized with some dosages of recombinant human growth hormone, a prospective, double-blind study of 206 patients found.
Children with burns covering more than 40% of their total body surface area were randomized to receive placebo or one of three doses (0.05, 0.1, or 0.2 mg/kg per day) of subcutaneous recombinant human growth hormone (rhGH) or placebo starting at discharge from the hospital through 1 year after being burned.
They were examined for treatment effects at 6, 9, 12, 18, and 24 months post burn.
The 105 patients on placebo were at the 33rd percentile for growth on standard growth curves at baseline and at all follow-up time points.
Growth returned to normal in 37 patients on 0.05 mg/kg per day rhGH at the 12- and 24-month follow-ups and at all follow-up time points in 41 patients in the 0.1-mg/kg per day rhGH group, with significantly better growth, compared with the placebo group, Dr. David N. Herndon and his associates reported at the annual meeting of the American Surgical Association.
Paradoxically, 23 patients given the highest dose of rhGH (0.2 mg/kg per day) showed less growth than did the other rhGH groups—even though the highest dose produced the greatest improvements in lean body mass and in some other parameters measured, said Dr. Herndon, chief of staff at the Shriners Burn Institute and professor and chair of surgery at the University of Texas Medical Branch, both in Galveston. Dr. Ludwik K. Branski, also of the university, was the lead investigator in the study.
The limited growth on high-dose rhGH might be related to a significant decrease in bone mineral content and a significant increase in levels of osteocalcin (a marker of bone resorption) in that group, he suggested. “This may be because we did not give them enough calcium or enough vitamin D to allow the growth-potentiating effects on bone to express themselves by increased bone mineral content,” he speculated.
The few adverse events all occurred in the high-dose group: hypercalcemia in two patients and glucose intolerance in one patient that necessitated discontinuation of rhGH.
In addition to measuring growth, rhGH levels, lean body mass, bone mineral content, and osteocalcin levels, the investigators assessed levels of insulin-like growth factor body protein 3 (a mediator of growth hormone's effects), resting energy expenditure, cardiac output, percent body fat, and parathyroid levels. “The 0.1-mg/kg per day dose had salutary effects in all body systems and did not reach a dose that was dangerous in any particular group,” and thus can be recommended for extensively burned children, Dr. Herndon said.
Commenting on the study, Dr. Basil A. Pruitt Jr. cautioned that more information is needed to evaluate the findings. “Since food intake and exercise can influence lean body mass and body weight,” it would have been helpful to have daily exercise and dietary logs in the study, said Dr. Pruitt of the University of Texas Health Sciences Center, San Antonio.
Although approximately two-thirds of each group were males, it also would be helpful to compare males with males and females with females to eliminate any potential sex bias, he suggested. Dr. Herndon said that analysis will be done.
Dr. Herndon and his associates reported having no conflicts of interest related to this study.
Update on Management of Keloids
A. Paul Kelly, MD
Keloids are scars, unique to humans, that grow beyond the boundaries of a cutaneous injury, inflammation, burn, or surgical incision. Although benign, keloids are often aesthetically malignant. The etiology of keloids is uncertain. However, we do know that they occur more often in African-American and Asian than Caucasian patients. There is no one therapeutic modality that either prevents the formation of keloids or treats active or inactive lesions. Consequently, there are many therapeutic options. In this review, an approach to medical and surgical management of keloids is provided, as well as a review of experimental therapeutic modalities.
*For a PDF of the full article, click on the link to the left of this introduction.
A. Paul Kelly, MD
Keloids are scars, unique to humans, that grow beyond the boundaries of a cutaneous injury, inflammation, burn, or surgical incision. Although benign, keloids are often aesthetically malignant. The etiology of keloids is uncertain. However, we do know that they occur more often in African-American and Asian than Caucasian patients. There is no one therapeutic modality that either prevents the formation of keloids or treats active or inactive lesions. Consequently, there are many therapeutic options. In this review, an approach to medical and surgical management of keloids is provided, as well as a review of experimental therapeutic modalities.
*For a PDF of the full article, click on the link to the left of this introduction.
A. Paul Kelly, MD
Keloids are scars, unique to humans, that grow beyond the boundaries of a cutaneous injury, inflammation, burn, or surgical incision. Although benign, keloids are often aesthetically malignant. The etiology of keloids is uncertain. However, we do know that they occur more often in African-American and Asian than Caucasian patients. There is no one therapeutic modality that either prevents the formation of keloids or treats active or inactive lesions. Consequently, there are many therapeutic options. In this review, an approach to medical and surgical management of keloids is provided, as well as a review of experimental therapeutic modalities.
*For a PDF of the full article, click on the link to the left of this introduction.
Stem Cells, Skin Substitute Heal Scleroderma Wounds
SAN FRANCISCO — Chronic wounds due to scleroderma responded well to a combination of mesenchymal stem cells and bilayer bioengineered skin, in a study of 12 patients followed for up to 6 months.
Four wounds completely healed, and the others decreased in size enough that patients' quality of life was improved, Dr. Vincent Falanga said in a press briefing at the annual meeting of the American Academy of Dermatology.
He and his associates harvested the patients' own stem cells from a small amount of bone marrow taken from the hip. And they applied the stem cells to the wound with an innovative fibrin spray system that had not been used before in humans.
Using a pressurized double-barrel syringe, they placed the cells in fibrinogen in one barrel and in a thrombin solution in the other. This produced a fine spray, spreading the cells evenly over the wound and simultaneously combining the fibrinogen and thrombin to form fibrin, which glued the cells to the exposed wound tissue, said Dr. Falanga of Boston University and Roger Williams Medical Center, Providence, R.I.
In prior studies, Dr. Falanga determined that the spray alone would not be enough to encourage the stem cells to differentiate into skin cells. To teach the stem cells to become skin cells, he covered the spray with living bioengineered skin substitute, specifically a product from Organogenesis Inc. called Apligraf that contains two layers of living cells, one of fibroblasts and one of keratinocytes.
Dr. Falanga gave each patient three treatments, the maximum permitted by the Food and Drug Administration. He said that he thinks the patients would do even better with additional treatments and hopes to receive approval for this as his studies continue.
Dr. Falanga said he found it necessary to use quite a large number of stem cells, 1.5 million to 2 million/cm
He continued, “I think that this is a lesson that's going to be learned when we go into other areas of medicine. Whether it's skin, brain, spinal cord, we have to have some methods whereby we impart upon the stem cells a didactic component, a direction we want them to go to.”
Dr. Falanga disclosed that he is a former consultant to the company Organogenesis.
SAN FRANCISCO — Chronic wounds due to scleroderma responded well to a combination of mesenchymal stem cells and bilayer bioengineered skin, in a study of 12 patients followed for up to 6 months.
Four wounds completely healed, and the others decreased in size enough that patients' quality of life was improved, Dr. Vincent Falanga said in a press briefing at the annual meeting of the American Academy of Dermatology.
He and his associates harvested the patients' own stem cells from a small amount of bone marrow taken from the hip. And they applied the stem cells to the wound with an innovative fibrin spray system that had not been used before in humans.
Using a pressurized double-barrel syringe, they placed the cells in fibrinogen in one barrel and in a thrombin solution in the other. This produced a fine spray, spreading the cells evenly over the wound and simultaneously combining the fibrinogen and thrombin to form fibrin, which glued the cells to the exposed wound tissue, said Dr. Falanga of Boston University and Roger Williams Medical Center, Providence, R.I.
In prior studies, Dr. Falanga determined that the spray alone would not be enough to encourage the stem cells to differentiate into skin cells. To teach the stem cells to become skin cells, he covered the spray with living bioengineered skin substitute, specifically a product from Organogenesis Inc. called Apligraf that contains two layers of living cells, one of fibroblasts and one of keratinocytes.
Dr. Falanga gave each patient three treatments, the maximum permitted by the Food and Drug Administration. He said that he thinks the patients would do even better with additional treatments and hopes to receive approval for this as his studies continue.
Dr. Falanga said he found it necessary to use quite a large number of stem cells, 1.5 million to 2 million/cm
He continued, “I think that this is a lesson that's going to be learned when we go into other areas of medicine. Whether it's skin, brain, spinal cord, we have to have some methods whereby we impart upon the stem cells a didactic component, a direction we want them to go to.”
Dr. Falanga disclosed that he is a former consultant to the company Organogenesis.
SAN FRANCISCO — Chronic wounds due to scleroderma responded well to a combination of mesenchymal stem cells and bilayer bioengineered skin, in a study of 12 patients followed for up to 6 months.
Four wounds completely healed, and the others decreased in size enough that patients' quality of life was improved, Dr. Vincent Falanga said in a press briefing at the annual meeting of the American Academy of Dermatology.
He and his associates harvested the patients' own stem cells from a small amount of bone marrow taken from the hip. And they applied the stem cells to the wound with an innovative fibrin spray system that had not been used before in humans.
Using a pressurized double-barrel syringe, they placed the cells in fibrinogen in one barrel and in a thrombin solution in the other. This produced a fine spray, spreading the cells evenly over the wound and simultaneously combining the fibrinogen and thrombin to form fibrin, which glued the cells to the exposed wound tissue, said Dr. Falanga of Boston University and Roger Williams Medical Center, Providence, R.I.
In prior studies, Dr. Falanga determined that the spray alone would not be enough to encourage the stem cells to differentiate into skin cells. To teach the stem cells to become skin cells, he covered the spray with living bioengineered skin substitute, specifically a product from Organogenesis Inc. called Apligraf that contains two layers of living cells, one of fibroblasts and one of keratinocytes.
Dr. Falanga gave each patient three treatments, the maximum permitted by the Food and Drug Administration. He said that he thinks the patients would do even better with additional treatments and hopes to receive approval for this as his studies continue.
Dr. Falanga said he found it necessary to use quite a large number of stem cells, 1.5 million to 2 million/cm
He continued, “I think that this is a lesson that's going to be learned when we go into other areas of medicine. Whether it's skin, brain, spinal cord, we have to have some methods whereby we impart upon the stem cells a didactic component, a direction we want them to go to.”
Dr. Falanga disclosed that he is a former consultant to the company Organogenesis.