User login
Center of Excellence site
Key strategies for managing breast cancer brain metastases
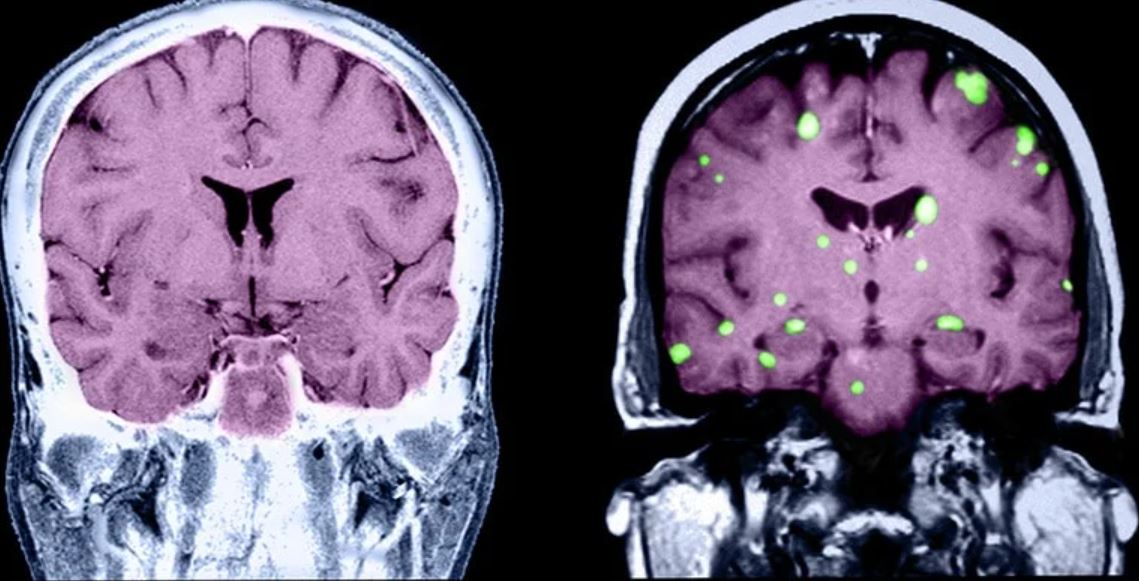
Brain metastases remain a frequent and often fatal consequence of metastatic breast cancer (MBC). MBC carries a median survival of about 3 years, but that rate drops significantly when cancer cells move to the brain. A recent analysis estimates median survival in patients with brain metastases ranges from 6 months in triple-negative breast cancer (TNBC) to 21 months in human epidermal growth factor receptor 2 (HER2)–positive disease.
This news organization spoke to Kevin M. Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University, about the risk for brain metastases in patients with MBC, strategies for screening and treatment, and the work being done to achieve a better understanding of the disease.
Question: Before we dig into strategies to manage MBC brain metastasis, let’s talk about the risks. When and how often do brain metastases present in patients with MBC? What factors increase the likelihood of developing brain metastasis?
Dr. Kalinsky: The biggest risk factor for MBC spreading to the central nervous system (CNS), which includes the brain and spine, is breast cancer subtype. For patients with metastatic TNBC, the risk for brain metastasis can be more than 50%. For patients with HER2-positive disease, the risk may be slightly lower, with estimates in the range of 25%-50%, whereas the likelihood of brain metastasis in patients with hormone receptor–positive MBC is significantly lower at close to 14%. In addition, patients with metastatic TNBC may have brain metastases a little earlier in their disease progression compared with patients with HER2-positive or estrogen receptor–positive breast cancers, where brain metastases generally develop a little later in the disease course.
At what point is it recommended to screen patients with MBC for brain metastasis?
Current guidelines suggest that we scan for brain metastasis in the presence of new neurologic symptoms, such as headache, dizziness, or weakness in the arms or legs. MRI, in particular, is useful for evaluating brain metastasis, especially for smaller lesions, but lesions are sometimes detected through CT imaging of the head, too.
That’s where the guidelines are now. But as our systemic agents improve, there’s always the possibility these recommendations will be revisited and potentially include imaging as screening tools in asymptomatic patients, as well.
How do you assess which patients with MBC should receive local therapy?
Increasingly, because our systemic therapies in breast cancer are getting better in terms of crossing the blood-brain barrier, we think about local therapy on a case-by-case basis. We think about it with the question of whether we delay surgery or radiation — whole brain radiation, in particular — given concerns surrounding the side effects of these modalities, namely cognitive dysfunction for radiation and increased risk of bleeding and infection for surgery.
Giving a patient-directed local therapy, such as Gamma Knife radiosurgery or whole-brain radiotherapy, ultimately depends on the burden of brain metastasis, the status of systemic disease outside of the brain, and the number and size of the lesions seen on imaging. If, for instance, a patient has a large lesion that will immediately impact their neurologic status, we may opt to resect the lesion. If there are innumerable lesions, some of which are large, we may do whole-brain radiotherapy. If, however, a patient has systemic disease that is largely under control but is experiencing local progression in the brain, we may use local radiotherapy while continuing systemic therapy.
What about systemic therapies that cross the blood-brain barrier? What’s available now and how do you choose among the options?The subtype of breast cancer informs treatment with systemic therapies. For instance, patients with HER2-positive disease may receive oral tyrosine kinase inhibitors, such as tucatinib, neratinib, and lapatinib, which have strong CNS penetration. For patients with estrogen receptor–positive, HER2-negative MBC, estrogen therapies including aromatase inhibitors, as well as targeted therapies such as the mTOR inhibitor everolimus, have good CNS penetration. For patients with metastatic TNBC, we have chemotherapies that cross the blood-brain barrier, such as capecitabine and platinum-based chemotherapy.
Evidence suggests that tumors in the brain may harbor different genetic abnormalities from tumors in the breast. How do you consider the potential genetic heterogeneity in CNS tumors vs. the primary breast tumor?When a patient’s disease has spread to the brain, we may preferentially use agents we know cross the blood-brain barrier, so we can obtain systemic control both intracranially and extracranially. If we have already resected or biopsied cancerous brain tissue, it’s good to check the tumor’s estrogen receptor, progesterone receptor, and HER2 status and do next-generation sequencing to see if the tumor has any other targetable mutations, such as PIK3CA mutations.
But when a patient has multiple lesions, we don’t go in and biopsy all of them to check for heterogeneity. We have to make decisions based on samples we have. In cases where we start systemic therapy and notice one lesion is not responding to these agents while others are, the nonresponsive lesion may be an outlier in terms of its biologic characteristics. It may be worth targeting that lesion for biopsy and further sequencing to determine the next best systemic approach.
How do quality of life considerations factor into the management of patients with MBC brain metastases?
We use a multidisciplinary approach when treating patients. This means patient care involves a team of experts, which can include medical oncologists, radiation oncologists, and neuro-oncologists who help determine a treatment plan that takes factors such as survival and quality of life into account.
This is why, for example, we try to delay whole brain radiotherapy when we can. The HER2CLIMB study, which led to the approval of tucatinib as a treatment option for patients with HER2-positive MBC, showed us that patients with treated or untreated brain metastases receiving systemic therapy before local therapy could benefit from the combination of tucatinib, trastuzumab, and capecitabine. These patients exhibited a median progression-free survival of 7.6 months compared with 5.4 months in the placebo group.
HER2CLIMB has been practice changing because it showed us that tucatinib has good CNS activity in patients with brain metastases. The HER2CLIMB findings raise an important question: As our systemic therapies improve, how aggressive do we need to be with local therapy? Can we push off modalities like whole-brain radiotherapy, which are associated with toxicity?
This study also highlights how important it is for patients with metastatic disease to seek clinical trials. Although some trials exclude patients with brain metastases and others may have criteria that require the stability of brain metastasis for a certain amount of time, the knowledge gained can be invaluable.
Where are some of the main gaps in our understanding of brain metastases in patients with MBC?
One issue is our understanding of tropism to the brain. In other words, why does MBC spread to the brain? Once we understand this key piece, we can work on developing more effective therapies and therapeutic combinations to block brain metastasis.
For hormone receptor–positive disease, in particular, a central question is whether the current antiestrogen therapies — such as selective estrogen receptor degraders like fulvestrant, as well as targeted AKT inhibitors — have the potential to affect brain tumor activity. The same holds true for TNBC, where antibody drug conjugates and immunotherapies are being evaluated for treatment of brain tumors. For patients with HER2-positive MBC that has spread to the brain, understanding the continued role for tyrosine kinase inhibitors, such as tucatinib and neratinib, as well as whether antibody drug conjugates, including trastuzumab deruxtecan and trastuzumab emtansine, have CNS activity are important areas to explore further.
The CompassHER2 trial, going on now, is randomizing patients with residual HER2-positive disease after neoadjuvant chemotherapy and HER2-targeted therapy to receive trastuzumab emtansine with or without tucatinib. One of the core questions of this study is whether trastuzumab emtansine/tucatinib lowers the rate of brain metastasis and the incidence of systemic metastasis.
Another area in MBC that requires greater scrutiny is patients who develop leptomeningeal disease, which is when cancer cells spread to the cerebrospinal fluid. These patients have a particularly poor prognosis, and it would be helpful to evaluate the efficacy of existing therapies, but these patients are often excluded from clinical trials.
Overall, the ultimate goal in these endeavors is to decrease the rate of metastasis to the brain and improve survival and quality of life in patients with MBC who do experience brain metastases.
A version of this article first appeared on Medscape.com.
Brain metastases remain a frequent and often fatal consequence of metastatic breast cancer (MBC). MBC carries a median survival of about 3 years, but that rate drops significantly when cancer cells move to the brain. A recent analysis estimates median survival in patients with brain metastases ranges from 6 months in triple-negative breast cancer (TNBC) to 21 months in human epidermal growth factor receptor 2 (HER2)–positive disease.
This news organization spoke to Kevin M. Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University, about the risk for brain metastases in patients with MBC, strategies for screening and treatment, and the work being done to achieve a better understanding of the disease.
Question: Before we dig into strategies to manage MBC brain metastasis, let’s talk about the risks. When and how often do brain metastases present in patients with MBC? What factors increase the likelihood of developing brain metastasis?
Dr. Kalinsky: The biggest risk factor for MBC spreading to the central nervous system (CNS), which includes the brain and spine, is breast cancer subtype. For patients with metastatic TNBC, the risk for brain metastasis can be more than 50%. For patients with HER2-positive disease, the risk may be slightly lower, with estimates in the range of 25%-50%, whereas the likelihood of brain metastasis in patients with hormone receptor–positive MBC is significantly lower at close to 14%. In addition, patients with metastatic TNBC may have brain metastases a little earlier in their disease progression compared with patients with HER2-positive or estrogen receptor–positive breast cancers, where brain metastases generally develop a little later in the disease course.
At what point is it recommended to screen patients with MBC for brain metastasis?
Current guidelines suggest that we scan for brain metastasis in the presence of new neurologic symptoms, such as headache, dizziness, or weakness in the arms or legs. MRI, in particular, is useful for evaluating brain metastasis, especially for smaller lesions, but lesions are sometimes detected through CT imaging of the head, too.
That’s where the guidelines are now. But as our systemic agents improve, there’s always the possibility these recommendations will be revisited and potentially include imaging as screening tools in asymptomatic patients, as well.
How do you assess which patients with MBC should receive local therapy?
Increasingly, because our systemic therapies in breast cancer are getting better in terms of crossing the blood-brain barrier, we think about local therapy on a case-by-case basis. We think about it with the question of whether we delay surgery or radiation — whole brain radiation, in particular — given concerns surrounding the side effects of these modalities, namely cognitive dysfunction for radiation and increased risk of bleeding and infection for surgery.
Giving a patient-directed local therapy, such as Gamma Knife radiosurgery or whole-brain radiotherapy, ultimately depends on the burden of brain metastasis, the status of systemic disease outside of the brain, and the number and size of the lesions seen on imaging. If, for instance, a patient has a large lesion that will immediately impact their neurologic status, we may opt to resect the lesion. If there are innumerable lesions, some of which are large, we may do whole-brain radiotherapy. If, however, a patient has systemic disease that is largely under control but is experiencing local progression in the brain, we may use local radiotherapy while continuing systemic therapy.
What about systemic therapies that cross the blood-brain barrier? What’s available now and how do you choose among the options?The subtype of breast cancer informs treatment with systemic therapies. For instance, patients with HER2-positive disease may receive oral tyrosine kinase inhibitors, such as tucatinib, neratinib, and lapatinib, which have strong CNS penetration. For patients with estrogen receptor–positive, HER2-negative MBC, estrogen therapies including aromatase inhibitors, as well as targeted therapies such as the mTOR inhibitor everolimus, have good CNS penetration. For patients with metastatic TNBC, we have chemotherapies that cross the blood-brain barrier, such as capecitabine and platinum-based chemotherapy.
Evidence suggests that tumors in the brain may harbor different genetic abnormalities from tumors in the breast. How do you consider the potential genetic heterogeneity in CNS tumors vs. the primary breast tumor?When a patient’s disease has spread to the brain, we may preferentially use agents we know cross the blood-brain barrier, so we can obtain systemic control both intracranially and extracranially. If we have already resected or biopsied cancerous brain tissue, it’s good to check the tumor’s estrogen receptor, progesterone receptor, and HER2 status and do next-generation sequencing to see if the tumor has any other targetable mutations, such as PIK3CA mutations.
But when a patient has multiple lesions, we don’t go in and biopsy all of them to check for heterogeneity. We have to make decisions based on samples we have. In cases where we start systemic therapy and notice one lesion is not responding to these agents while others are, the nonresponsive lesion may be an outlier in terms of its biologic characteristics. It may be worth targeting that lesion for biopsy and further sequencing to determine the next best systemic approach.
How do quality of life considerations factor into the management of patients with MBC brain metastases?
We use a multidisciplinary approach when treating patients. This means patient care involves a team of experts, which can include medical oncologists, radiation oncologists, and neuro-oncologists who help determine a treatment plan that takes factors such as survival and quality of life into account.
This is why, for example, we try to delay whole brain radiotherapy when we can. The HER2CLIMB study, which led to the approval of tucatinib as a treatment option for patients with HER2-positive MBC, showed us that patients with treated or untreated brain metastases receiving systemic therapy before local therapy could benefit from the combination of tucatinib, trastuzumab, and capecitabine. These patients exhibited a median progression-free survival of 7.6 months compared with 5.4 months in the placebo group.
HER2CLIMB has been practice changing because it showed us that tucatinib has good CNS activity in patients with brain metastases. The HER2CLIMB findings raise an important question: As our systemic therapies improve, how aggressive do we need to be with local therapy? Can we push off modalities like whole-brain radiotherapy, which are associated with toxicity?
This study also highlights how important it is for patients with metastatic disease to seek clinical trials. Although some trials exclude patients with brain metastases and others may have criteria that require the stability of brain metastasis for a certain amount of time, the knowledge gained can be invaluable.
Where are some of the main gaps in our understanding of brain metastases in patients with MBC?
One issue is our understanding of tropism to the brain. In other words, why does MBC spread to the brain? Once we understand this key piece, we can work on developing more effective therapies and therapeutic combinations to block brain metastasis.
For hormone receptor–positive disease, in particular, a central question is whether the current antiestrogen therapies — such as selective estrogen receptor degraders like fulvestrant, as well as targeted AKT inhibitors — have the potential to affect brain tumor activity. The same holds true for TNBC, where antibody drug conjugates and immunotherapies are being evaluated for treatment of brain tumors. For patients with HER2-positive MBC that has spread to the brain, understanding the continued role for tyrosine kinase inhibitors, such as tucatinib and neratinib, as well as whether antibody drug conjugates, including trastuzumab deruxtecan and trastuzumab emtansine, have CNS activity are important areas to explore further.
The CompassHER2 trial, going on now, is randomizing patients with residual HER2-positive disease after neoadjuvant chemotherapy and HER2-targeted therapy to receive trastuzumab emtansine with or without tucatinib. One of the core questions of this study is whether trastuzumab emtansine/tucatinib lowers the rate of brain metastasis and the incidence of systemic metastasis.
Another area in MBC that requires greater scrutiny is patients who develop leptomeningeal disease, which is when cancer cells spread to the cerebrospinal fluid. These patients have a particularly poor prognosis, and it would be helpful to evaluate the efficacy of existing therapies, but these patients are often excluded from clinical trials.
Overall, the ultimate goal in these endeavors is to decrease the rate of metastasis to the brain and improve survival and quality of life in patients with MBC who do experience brain metastases.
A version of this article first appeared on Medscape.com.
Brain metastases remain a frequent and often fatal consequence of metastatic breast cancer (MBC). MBC carries a median survival of about 3 years, but that rate drops significantly when cancer cells move to the brain. A recent analysis estimates median survival in patients with brain metastases ranges from 6 months in triple-negative breast cancer (TNBC) to 21 months in human epidermal growth factor receptor 2 (HER2)–positive disease.
This news organization spoke to Kevin M. Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University, about the risk for brain metastases in patients with MBC, strategies for screening and treatment, and the work being done to achieve a better understanding of the disease.
Question: Before we dig into strategies to manage MBC brain metastasis, let’s talk about the risks. When and how often do brain metastases present in patients with MBC? What factors increase the likelihood of developing brain metastasis?
Dr. Kalinsky: The biggest risk factor for MBC spreading to the central nervous system (CNS), which includes the brain and spine, is breast cancer subtype. For patients with metastatic TNBC, the risk for brain metastasis can be more than 50%. For patients with HER2-positive disease, the risk may be slightly lower, with estimates in the range of 25%-50%, whereas the likelihood of brain metastasis in patients with hormone receptor–positive MBC is significantly lower at close to 14%. In addition, patients with metastatic TNBC may have brain metastases a little earlier in their disease progression compared with patients with HER2-positive or estrogen receptor–positive breast cancers, where brain metastases generally develop a little later in the disease course.
At what point is it recommended to screen patients with MBC for brain metastasis?
Current guidelines suggest that we scan for brain metastasis in the presence of new neurologic symptoms, such as headache, dizziness, or weakness in the arms or legs. MRI, in particular, is useful for evaluating brain metastasis, especially for smaller lesions, but lesions are sometimes detected through CT imaging of the head, too.
That’s where the guidelines are now. But as our systemic agents improve, there’s always the possibility these recommendations will be revisited and potentially include imaging as screening tools in asymptomatic patients, as well.
How do you assess which patients with MBC should receive local therapy?
Increasingly, because our systemic therapies in breast cancer are getting better in terms of crossing the blood-brain barrier, we think about local therapy on a case-by-case basis. We think about it with the question of whether we delay surgery or radiation — whole brain radiation, in particular — given concerns surrounding the side effects of these modalities, namely cognitive dysfunction for radiation and increased risk of bleeding and infection for surgery.
Giving a patient-directed local therapy, such as Gamma Knife radiosurgery or whole-brain radiotherapy, ultimately depends on the burden of brain metastasis, the status of systemic disease outside of the brain, and the number and size of the lesions seen on imaging. If, for instance, a patient has a large lesion that will immediately impact their neurologic status, we may opt to resect the lesion. If there are innumerable lesions, some of which are large, we may do whole-brain radiotherapy. If, however, a patient has systemic disease that is largely under control but is experiencing local progression in the brain, we may use local radiotherapy while continuing systemic therapy.
What about systemic therapies that cross the blood-brain barrier? What’s available now and how do you choose among the options?The subtype of breast cancer informs treatment with systemic therapies. For instance, patients with HER2-positive disease may receive oral tyrosine kinase inhibitors, such as tucatinib, neratinib, and lapatinib, which have strong CNS penetration. For patients with estrogen receptor–positive, HER2-negative MBC, estrogen therapies including aromatase inhibitors, as well as targeted therapies such as the mTOR inhibitor everolimus, have good CNS penetration. For patients with metastatic TNBC, we have chemotherapies that cross the blood-brain barrier, such as capecitabine and platinum-based chemotherapy.
Evidence suggests that tumors in the brain may harbor different genetic abnormalities from tumors in the breast. How do you consider the potential genetic heterogeneity in CNS tumors vs. the primary breast tumor?When a patient’s disease has spread to the brain, we may preferentially use agents we know cross the blood-brain barrier, so we can obtain systemic control both intracranially and extracranially. If we have already resected or biopsied cancerous brain tissue, it’s good to check the tumor’s estrogen receptor, progesterone receptor, and HER2 status and do next-generation sequencing to see if the tumor has any other targetable mutations, such as PIK3CA mutations.
But when a patient has multiple lesions, we don’t go in and biopsy all of them to check for heterogeneity. We have to make decisions based on samples we have. In cases where we start systemic therapy and notice one lesion is not responding to these agents while others are, the nonresponsive lesion may be an outlier in terms of its biologic characteristics. It may be worth targeting that lesion for biopsy and further sequencing to determine the next best systemic approach.
How do quality of life considerations factor into the management of patients with MBC brain metastases?
We use a multidisciplinary approach when treating patients. This means patient care involves a team of experts, which can include medical oncologists, radiation oncologists, and neuro-oncologists who help determine a treatment plan that takes factors such as survival and quality of life into account.
This is why, for example, we try to delay whole brain radiotherapy when we can. The HER2CLIMB study, which led to the approval of tucatinib as a treatment option for patients with HER2-positive MBC, showed us that patients with treated or untreated brain metastases receiving systemic therapy before local therapy could benefit from the combination of tucatinib, trastuzumab, and capecitabine. These patients exhibited a median progression-free survival of 7.6 months compared with 5.4 months in the placebo group.
HER2CLIMB has been practice changing because it showed us that tucatinib has good CNS activity in patients with brain metastases. The HER2CLIMB findings raise an important question: As our systemic therapies improve, how aggressive do we need to be with local therapy? Can we push off modalities like whole-brain radiotherapy, which are associated with toxicity?
This study also highlights how important it is for patients with metastatic disease to seek clinical trials. Although some trials exclude patients with brain metastases and others may have criteria that require the stability of brain metastasis for a certain amount of time, the knowledge gained can be invaluable.
Where are some of the main gaps in our understanding of brain metastases in patients with MBC?
One issue is our understanding of tropism to the brain. In other words, why does MBC spread to the brain? Once we understand this key piece, we can work on developing more effective therapies and therapeutic combinations to block brain metastasis.
For hormone receptor–positive disease, in particular, a central question is whether the current antiestrogen therapies — such as selective estrogen receptor degraders like fulvestrant, as well as targeted AKT inhibitors — have the potential to affect brain tumor activity. The same holds true for TNBC, where antibody drug conjugates and immunotherapies are being evaluated for treatment of brain tumors. For patients with HER2-positive MBC that has spread to the brain, understanding the continued role for tyrosine kinase inhibitors, such as tucatinib and neratinib, as well as whether antibody drug conjugates, including trastuzumab deruxtecan and trastuzumab emtansine, have CNS activity are important areas to explore further.
The CompassHER2 trial, going on now, is randomizing patients with residual HER2-positive disease after neoadjuvant chemotherapy and HER2-targeted therapy to receive trastuzumab emtansine with or without tucatinib. One of the core questions of this study is whether trastuzumab emtansine/tucatinib lowers the rate of brain metastasis and the incidence of systemic metastasis.
Another area in MBC that requires greater scrutiny is patients who develop leptomeningeal disease, which is when cancer cells spread to the cerebrospinal fluid. These patients have a particularly poor prognosis, and it would be helpful to evaluate the efficacy of existing therapies, but these patients are often excluded from clinical trials.
Overall, the ultimate goal in these endeavors is to decrease the rate of metastasis to the brain and improve survival and quality of life in patients with MBC who do experience brain metastases.
A version of this article first appeared on Medscape.com.
CONCERT: Better QoL but not survival with cabazitaxel in metastatic HER2– breast cancer
For patients with HER2-negative metastatic breast cancer, first line chemotherapy with cabazitaxel (Jevtana) every 3 weeks offers efficacy comparable to that of once-weekly paclitaxel, but with lower risk for peripheral neuropathy and better patient-reported quality of life, investigators in the multicenter CONCERT trial found.
In an open-label clinical trial of 158 patients from 14 hospitals in the United Kingdom, there was no difference in the primary endpoint of progression-free survival (PFS) or a secondary overall survival endpoint between patients randomly assigned to initial chemotherapy with cabazitaxel every 3 weeks or weekly paclitaxel, reported Amit Bahl, MD, of University Hospital Bristol, England, and colleagues.
“Cabazitaxel is safe and well tolerated for metastatic breast cancer and requires fewer hospital visits than weekly paclitaxel, which is very important for patients and health care providers, but more so in the current situation,” he said in an oral abstract session at the American Society of Clinical Oncology annual meeting (Abstract 1008).
Cabazitaxel is currently approved in the United States and Europe in combination with prednisone for treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. It is not currently approved for the treatment of metastatic breast cancer, but has been explored for this indication in clinical trials.
“In the metastatic setting, where patients continue on treatment pretty much indefinitely until disease progression or unacceptable toxicity, the use of an every-3-week regimen could be attractive, because it means less visits for the patients, and it appears that this drug has lower toxicity in terms of peripheral neuropathy,” said breast cancer specialist Aditya Bardia, MD, MPH, who was not involved in the study.
Dr. Bardia, of Mass General Cancer Center in Boston, commented on the study in an interview.
Although paclitaxel is commonly used as first-line chemotherapy for HER2-negative metastatic breast cancer, it is associated with only modest response rates, ranging from 21.5% to 53.7% and carries significant risk of peripheral neuropathy, Dr. Bahl and colleagues noted.
“There is an unmet need for an alternative first-line cytotoxic chemotherapy agent, and cabazitaxel is a taxoid agent which has showed promising results in phase 2 trial of metastatic breast cancer patients in the second-line setting, even those with taxane resistance,” he said.
Open-label trial
To see whether cabazitaxel could meet those requirements, the investigators conducted a phase 2 randomized trial in which patients with HER2-negative metastatic breast cancer not previously treated with cytotoxic chemotherapy were assigned, 79 in each arm, to receive cabazitaxel 25 mg/m2 every 3 weeks, or paclitaxel 80 mg/m2 weekly.
The median patient age was 56 years in the cabazitaxel group and 61 years in the paclitaxel group. Roughly two-thirds of patients in each arm had Eastern Cooperative Oncology Group performance status 0, and the remainder had ECOG performance status 1.
In each arm, the median time on treatment was 15 weeks, but treatment delays and dose reductions were more common among patients on paclitaxel than cabazitaxel (61% vs. 39%, and 37% vs. 24%, respectively).
There were 149 PFS events at the time of the analysis. The median PFS with cabazitaxel was 6.7 months vs. 5.8 months with paclitaxel. This difference was not statistically significant. Median overall survival was 20.6 months in the cabazitaxel arm, vs. 18.2 months 20.0 months, respectively.
Similarly, there were no significant differences in either the overall response rates (42% vs. 37%), or time to response.
There were no complete responses with cabazitaxel vs. two (2.5%) with paclitaxel. The respective partial response rates were 41.8% vs. 34.2%.
In a subgroup analysis of PFS, there were no significant between-arm differences, except for an improved PFS in patients 65 and older with cabazitaxel (hazard ratio 0.45, 95% confidence interval, 0.25-0.80).
Quality of life favors cabazitaxel
Grade 3 or greater adverse events occurred in 42% of patients on cabazitaxel vs. 51% on paclitaxel. Diarrhea, febrile neutropenia, and nausea were the most common grade 3 or greater events in the cabazitaxel arm, whereas grade 3 or greater lung infection and peripheral neuropathy were more common with paclitaxel.
Sensory peripheral neuropathy of any grade occurred in 16% of patients assigned to cabazitaxel, compared with 54% assigned to paclitaxel. The respective rates of alopecia were 27% and 42%.
Over the course of treatment, the mean EuroQuol EQ-5D-5L single index utility score and visual analogue scale score were higher with cabazitaxel arm compared to paclitaxel, suggesting better patient quality of life with cabazitaxel.
In addition, throughout treatment patients in the cabazitaxel arm reported significantly better scores on The Functional Assessment of Cancer Therapy – Breast (FACT-B) breast cancer subscale, Dr. Bahl said.
Second-line may be better
ASCO invited discussant Marleen Kok, MD, PhD, from the Netherlands Cancer Institute in Amsterdam, pointed out that in the phase 2 GENEVIEVE trial comparing the efficacy and safety of cabazitaxel versus weekly paclitaxel as neoadjuvant treatment in patients with triple negative or luminal B/HER2 normal breast cancer the pathologic complete response rate with cabazitaxel was 1.2%, compared with 11% with paclitaxel.
“This GENEVIEVE trial, together with the CONCERT trial, suggests that there is not a big role for cabazitaxel to be used upfront before other taxanes,” she said.
However, in a phase 2 study of cabazitaxel as second-line therapy in patients with HER2-negative metastatic breast cancer who had previously been treated with taxanes, the overall response rate was 23%, “which is still of interest and importance for our patients,” she added.
Dr. Kok did not address quality of life differences between the regimens, however.
In a side note, Dr. Bardia said that “if there were an oral form of paclitaxel, that would certainly be very welcome, in that an oral drug is more convenient for patients, and would require fewer visits to the hospital.”
The CONCERT trial was funded by an investigator-sponsored study grant from Sanofi. Dr. Bahl disclosed honoraria and institutional research funding from Sanofi/Aventis and others, and travel expenses from Bayer and Roche. Dr. Kok disclosed a consulting or advisory role for Bristol Myers Squibb/Medarex, and institutional research funding from that company and others. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
For patients with HER2-negative metastatic breast cancer, first line chemotherapy with cabazitaxel (Jevtana) every 3 weeks offers efficacy comparable to that of once-weekly paclitaxel, but with lower risk for peripheral neuropathy and better patient-reported quality of life, investigators in the multicenter CONCERT trial found.
In an open-label clinical trial of 158 patients from 14 hospitals in the United Kingdom, there was no difference in the primary endpoint of progression-free survival (PFS) or a secondary overall survival endpoint between patients randomly assigned to initial chemotherapy with cabazitaxel every 3 weeks or weekly paclitaxel, reported Amit Bahl, MD, of University Hospital Bristol, England, and colleagues.
“Cabazitaxel is safe and well tolerated for metastatic breast cancer and requires fewer hospital visits than weekly paclitaxel, which is very important for patients and health care providers, but more so in the current situation,” he said in an oral abstract session at the American Society of Clinical Oncology annual meeting (Abstract 1008).
Cabazitaxel is currently approved in the United States and Europe in combination with prednisone for treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. It is not currently approved for the treatment of metastatic breast cancer, but has been explored for this indication in clinical trials.
“In the metastatic setting, where patients continue on treatment pretty much indefinitely until disease progression or unacceptable toxicity, the use of an every-3-week regimen could be attractive, because it means less visits for the patients, and it appears that this drug has lower toxicity in terms of peripheral neuropathy,” said breast cancer specialist Aditya Bardia, MD, MPH, who was not involved in the study.
Dr. Bardia, of Mass General Cancer Center in Boston, commented on the study in an interview.
Although paclitaxel is commonly used as first-line chemotherapy for HER2-negative metastatic breast cancer, it is associated with only modest response rates, ranging from 21.5% to 53.7% and carries significant risk of peripheral neuropathy, Dr. Bahl and colleagues noted.
“There is an unmet need for an alternative first-line cytotoxic chemotherapy agent, and cabazitaxel is a taxoid agent which has showed promising results in phase 2 trial of metastatic breast cancer patients in the second-line setting, even those with taxane resistance,” he said.
Open-label trial
To see whether cabazitaxel could meet those requirements, the investigators conducted a phase 2 randomized trial in which patients with HER2-negative metastatic breast cancer not previously treated with cytotoxic chemotherapy were assigned, 79 in each arm, to receive cabazitaxel 25 mg/m2 every 3 weeks, or paclitaxel 80 mg/m2 weekly.
The median patient age was 56 years in the cabazitaxel group and 61 years in the paclitaxel group. Roughly two-thirds of patients in each arm had Eastern Cooperative Oncology Group performance status 0, and the remainder had ECOG performance status 1.
In each arm, the median time on treatment was 15 weeks, but treatment delays and dose reductions were more common among patients on paclitaxel than cabazitaxel (61% vs. 39%, and 37% vs. 24%, respectively).
There were 149 PFS events at the time of the analysis. The median PFS with cabazitaxel was 6.7 months vs. 5.8 months with paclitaxel. This difference was not statistically significant. Median overall survival was 20.6 months in the cabazitaxel arm, vs. 18.2 months 20.0 months, respectively.
Similarly, there were no significant differences in either the overall response rates (42% vs. 37%), or time to response.
There were no complete responses with cabazitaxel vs. two (2.5%) with paclitaxel. The respective partial response rates were 41.8% vs. 34.2%.
In a subgroup analysis of PFS, there were no significant between-arm differences, except for an improved PFS in patients 65 and older with cabazitaxel (hazard ratio 0.45, 95% confidence interval, 0.25-0.80).
Quality of life favors cabazitaxel
Grade 3 or greater adverse events occurred in 42% of patients on cabazitaxel vs. 51% on paclitaxel. Diarrhea, febrile neutropenia, and nausea were the most common grade 3 or greater events in the cabazitaxel arm, whereas grade 3 or greater lung infection and peripheral neuropathy were more common with paclitaxel.
Sensory peripheral neuropathy of any grade occurred in 16% of patients assigned to cabazitaxel, compared with 54% assigned to paclitaxel. The respective rates of alopecia were 27% and 42%.
Over the course of treatment, the mean EuroQuol EQ-5D-5L single index utility score and visual analogue scale score were higher with cabazitaxel arm compared to paclitaxel, suggesting better patient quality of life with cabazitaxel.
In addition, throughout treatment patients in the cabazitaxel arm reported significantly better scores on The Functional Assessment of Cancer Therapy – Breast (FACT-B) breast cancer subscale, Dr. Bahl said.
Second-line may be better
ASCO invited discussant Marleen Kok, MD, PhD, from the Netherlands Cancer Institute in Amsterdam, pointed out that in the phase 2 GENEVIEVE trial comparing the efficacy and safety of cabazitaxel versus weekly paclitaxel as neoadjuvant treatment in patients with triple negative or luminal B/HER2 normal breast cancer the pathologic complete response rate with cabazitaxel was 1.2%, compared with 11% with paclitaxel.
“This GENEVIEVE trial, together with the CONCERT trial, suggests that there is not a big role for cabazitaxel to be used upfront before other taxanes,” she said.
However, in a phase 2 study of cabazitaxel as second-line therapy in patients with HER2-negative metastatic breast cancer who had previously been treated with taxanes, the overall response rate was 23%, “which is still of interest and importance for our patients,” she added.
Dr. Kok did not address quality of life differences between the regimens, however.
In a side note, Dr. Bardia said that “if there were an oral form of paclitaxel, that would certainly be very welcome, in that an oral drug is more convenient for patients, and would require fewer visits to the hospital.”
The CONCERT trial was funded by an investigator-sponsored study grant from Sanofi. Dr. Bahl disclosed honoraria and institutional research funding from Sanofi/Aventis and others, and travel expenses from Bayer and Roche. Dr. Kok disclosed a consulting or advisory role for Bristol Myers Squibb/Medarex, and institutional research funding from that company and others. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
For patients with HER2-negative metastatic breast cancer, first line chemotherapy with cabazitaxel (Jevtana) every 3 weeks offers efficacy comparable to that of once-weekly paclitaxel, but with lower risk for peripheral neuropathy and better patient-reported quality of life, investigators in the multicenter CONCERT trial found.
In an open-label clinical trial of 158 patients from 14 hospitals in the United Kingdom, there was no difference in the primary endpoint of progression-free survival (PFS) or a secondary overall survival endpoint between patients randomly assigned to initial chemotherapy with cabazitaxel every 3 weeks or weekly paclitaxel, reported Amit Bahl, MD, of University Hospital Bristol, England, and colleagues.
“Cabazitaxel is safe and well tolerated for metastatic breast cancer and requires fewer hospital visits than weekly paclitaxel, which is very important for patients and health care providers, but more so in the current situation,” he said in an oral abstract session at the American Society of Clinical Oncology annual meeting (Abstract 1008).
Cabazitaxel is currently approved in the United States and Europe in combination with prednisone for treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. It is not currently approved for the treatment of metastatic breast cancer, but has been explored for this indication in clinical trials.
“In the metastatic setting, where patients continue on treatment pretty much indefinitely until disease progression or unacceptable toxicity, the use of an every-3-week regimen could be attractive, because it means less visits for the patients, and it appears that this drug has lower toxicity in terms of peripheral neuropathy,” said breast cancer specialist Aditya Bardia, MD, MPH, who was not involved in the study.
Dr. Bardia, of Mass General Cancer Center in Boston, commented on the study in an interview.
Although paclitaxel is commonly used as first-line chemotherapy for HER2-negative metastatic breast cancer, it is associated with only modest response rates, ranging from 21.5% to 53.7% and carries significant risk of peripheral neuropathy, Dr. Bahl and colleagues noted.
“There is an unmet need for an alternative first-line cytotoxic chemotherapy agent, and cabazitaxel is a taxoid agent which has showed promising results in phase 2 trial of metastatic breast cancer patients in the second-line setting, even those with taxane resistance,” he said.
Open-label trial
To see whether cabazitaxel could meet those requirements, the investigators conducted a phase 2 randomized trial in which patients with HER2-negative metastatic breast cancer not previously treated with cytotoxic chemotherapy were assigned, 79 in each arm, to receive cabazitaxel 25 mg/m2 every 3 weeks, or paclitaxel 80 mg/m2 weekly.
The median patient age was 56 years in the cabazitaxel group and 61 years in the paclitaxel group. Roughly two-thirds of patients in each arm had Eastern Cooperative Oncology Group performance status 0, and the remainder had ECOG performance status 1.
In each arm, the median time on treatment was 15 weeks, but treatment delays and dose reductions were more common among patients on paclitaxel than cabazitaxel (61% vs. 39%, and 37% vs. 24%, respectively).
There were 149 PFS events at the time of the analysis. The median PFS with cabazitaxel was 6.7 months vs. 5.8 months with paclitaxel. This difference was not statistically significant. Median overall survival was 20.6 months in the cabazitaxel arm, vs. 18.2 months 20.0 months, respectively.
Similarly, there were no significant differences in either the overall response rates (42% vs. 37%), or time to response.
There were no complete responses with cabazitaxel vs. two (2.5%) with paclitaxel. The respective partial response rates were 41.8% vs. 34.2%.
In a subgroup analysis of PFS, there were no significant between-arm differences, except for an improved PFS in patients 65 and older with cabazitaxel (hazard ratio 0.45, 95% confidence interval, 0.25-0.80).
Quality of life favors cabazitaxel
Grade 3 or greater adverse events occurred in 42% of patients on cabazitaxel vs. 51% on paclitaxel. Diarrhea, febrile neutropenia, and nausea were the most common grade 3 or greater events in the cabazitaxel arm, whereas grade 3 or greater lung infection and peripheral neuropathy were more common with paclitaxel.
Sensory peripheral neuropathy of any grade occurred in 16% of patients assigned to cabazitaxel, compared with 54% assigned to paclitaxel. The respective rates of alopecia were 27% and 42%.
Over the course of treatment, the mean EuroQuol EQ-5D-5L single index utility score and visual analogue scale score were higher with cabazitaxel arm compared to paclitaxel, suggesting better patient quality of life with cabazitaxel.
In addition, throughout treatment patients in the cabazitaxel arm reported significantly better scores on The Functional Assessment of Cancer Therapy – Breast (FACT-B) breast cancer subscale, Dr. Bahl said.
Second-line may be better
ASCO invited discussant Marleen Kok, MD, PhD, from the Netherlands Cancer Institute in Amsterdam, pointed out that in the phase 2 GENEVIEVE trial comparing the efficacy and safety of cabazitaxel versus weekly paclitaxel as neoadjuvant treatment in patients with triple negative or luminal B/HER2 normal breast cancer the pathologic complete response rate with cabazitaxel was 1.2%, compared with 11% with paclitaxel.
“This GENEVIEVE trial, together with the CONCERT trial, suggests that there is not a big role for cabazitaxel to be used upfront before other taxanes,” she said.
However, in a phase 2 study of cabazitaxel as second-line therapy in patients with HER2-negative metastatic breast cancer who had previously been treated with taxanes, the overall response rate was 23%, “which is still of interest and importance for our patients,” she added.
Dr. Kok did not address quality of life differences between the regimens, however.
In a side note, Dr. Bardia said that “if there were an oral form of paclitaxel, that would certainly be very welcome, in that an oral drug is more convenient for patients, and would require fewer visits to the hospital.”
The CONCERT trial was funded by an investigator-sponsored study grant from Sanofi. Dr. Bahl disclosed honoraria and institutional research funding from Sanofi/Aventis and others, and travel expenses from Bayer and Roche. Dr. Kok disclosed a consulting or advisory role for Bristol Myers Squibb/Medarex, and institutional research funding from that company and others. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
FROM ASCO 2021
ASCO 2021: Breast cancer sessions not to miss
This transcript has been edited for clarity.

Hello. It’s Dr. Kathy Miller from Indiana University.
I have to admit that time has snuck up on me this year. It is already time for the American Society of Clinical Oncology Annual Meeting.
I found it hard to keep track of time this year with the pandemic. Many of the things that help mark the passage of time haven’t happened, have happened at different times of the year than is typical, or have happened in different ways that just haven’t had the same impact in my brain.
Just recently, I was taking a look through the breast cancer program at ASCO and there is a special clinical science symposium that I want to make sure you know about and tune into. It’s the sort of session that might not otherwise reach you.
This has been a year of incredible turmoil and critical thinking about issues of race, ethnicity, justice, and how we can make sure that the medical care we’re providing is inclusive and equitable. How we can make sure we are giving the best outcome to all of our patients.
This special clinical science symposium this year includes several presentations that will delve into how genetically determined ancestry and socially determined race might impact the outcome of our patients. This is a tangled web that is difficult to unpack and separate, but there are clear distinctions here: The genes we inherit do affect how we metabolize drugs, what side effects we might have from drugs, and what drugs might be the best choices for us.
Our socially determined race affects how the world interacts with us. Those biases, be they conscious or unconscious, can affect where we live, where we go to school, how people treat us, what opportunities we have, and how the medical system treats us. They’re related, but they’re not the same. Tune into that clinical science symposium to begin thinking about those differences and how we can make sure we give our patients the best care.
There are other high-profile presentations that you’re going to want to see as well, looking at how we can optimize therapy in patients with HER2-positive disease and beginning to think about who might not need chemotherapy to have an excellent outcome in early-stage disease.
Also, we will be thinking about those patients with triple-negative disease who have residual disease after neoadjuvant chemotherapy. We were all caught off guard with the results of the CREATE-X trial, quite frankly, several years ago.
This year we will hear the results of a postneoadjuvant trial coordinated by the Eastern Cooperative Oncology Group comparing platinum therapy with capecitabine. Tune in to think more about whether capecitabine really should be the standard of care in this population.
As always, I’m interested in your thoughts before or after ASCO. What stood out for you this year in breast cancer? Drop us a comment and let us know about these sessions and what else you found worthwhile.
Dr. Miller is associate director of clinical research and codirector of the breast cancer program at the Melvin and Bren Simon Cancer Center at Indiana University, Indianapolis. Her career has combined both laboratory and clinical research in breast cancer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.

Hello. It’s Dr. Kathy Miller from Indiana University.
I have to admit that time has snuck up on me this year. It is already time for the American Society of Clinical Oncology Annual Meeting.
I found it hard to keep track of time this year with the pandemic. Many of the things that help mark the passage of time haven’t happened, have happened at different times of the year than is typical, or have happened in different ways that just haven’t had the same impact in my brain.
Just recently, I was taking a look through the breast cancer program at ASCO and there is a special clinical science symposium that I want to make sure you know about and tune into. It’s the sort of session that might not otherwise reach you.
This has been a year of incredible turmoil and critical thinking about issues of race, ethnicity, justice, and how we can make sure that the medical care we’re providing is inclusive and equitable. How we can make sure we are giving the best outcome to all of our patients.
This special clinical science symposium this year includes several presentations that will delve into how genetically determined ancestry and socially determined race might impact the outcome of our patients. This is a tangled web that is difficult to unpack and separate, but there are clear distinctions here: The genes we inherit do affect how we metabolize drugs, what side effects we might have from drugs, and what drugs might be the best choices for us.
Our socially determined race affects how the world interacts with us. Those biases, be they conscious or unconscious, can affect where we live, where we go to school, how people treat us, what opportunities we have, and how the medical system treats us. They’re related, but they’re not the same. Tune into that clinical science symposium to begin thinking about those differences and how we can make sure we give our patients the best care.
There are other high-profile presentations that you’re going to want to see as well, looking at how we can optimize therapy in patients with HER2-positive disease and beginning to think about who might not need chemotherapy to have an excellent outcome in early-stage disease.
Also, we will be thinking about those patients with triple-negative disease who have residual disease after neoadjuvant chemotherapy. We were all caught off guard with the results of the CREATE-X trial, quite frankly, several years ago.
This year we will hear the results of a postneoadjuvant trial coordinated by the Eastern Cooperative Oncology Group comparing platinum therapy with capecitabine. Tune in to think more about whether capecitabine really should be the standard of care in this population.
As always, I’m interested in your thoughts before or after ASCO. What stood out for you this year in breast cancer? Drop us a comment and let us know about these sessions and what else you found worthwhile.
Dr. Miller is associate director of clinical research and codirector of the breast cancer program at the Melvin and Bren Simon Cancer Center at Indiana University, Indianapolis. Her career has combined both laboratory and clinical research in breast cancer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.

Hello. It’s Dr. Kathy Miller from Indiana University.
I have to admit that time has snuck up on me this year. It is already time for the American Society of Clinical Oncology Annual Meeting.
I found it hard to keep track of time this year with the pandemic. Many of the things that help mark the passage of time haven’t happened, have happened at different times of the year than is typical, or have happened in different ways that just haven’t had the same impact in my brain.
Just recently, I was taking a look through the breast cancer program at ASCO and there is a special clinical science symposium that I want to make sure you know about and tune into. It’s the sort of session that might not otherwise reach you.
This has been a year of incredible turmoil and critical thinking about issues of race, ethnicity, justice, and how we can make sure that the medical care we’re providing is inclusive and equitable. How we can make sure we are giving the best outcome to all of our patients.
This special clinical science symposium this year includes several presentations that will delve into how genetically determined ancestry and socially determined race might impact the outcome of our patients. This is a tangled web that is difficult to unpack and separate, but there are clear distinctions here: The genes we inherit do affect how we metabolize drugs, what side effects we might have from drugs, and what drugs might be the best choices for us.
Our socially determined race affects how the world interacts with us. Those biases, be they conscious or unconscious, can affect where we live, where we go to school, how people treat us, what opportunities we have, and how the medical system treats us. They’re related, but they’re not the same. Tune into that clinical science symposium to begin thinking about those differences and how we can make sure we give our patients the best care.
There are other high-profile presentations that you’re going to want to see as well, looking at how we can optimize therapy in patients with HER2-positive disease and beginning to think about who might not need chemotherapy to have an excellent outcome in early-stage disease.
Also, we will be thinking about those patients with triple-negative disease who have residual disease after neoadjuvant chemotherapy. We were all caught off guard with the results of the CREATE-X trial, quite frankly, several years ago.
This year we will hear the results of a postneoadjuvant trial coordinated by the Eastern Cooperative Oncology Group comparing platinum therapy with capecitabine. Tune in to think more about whether capecitabine really should be the standard of care in this population.
As always, I’m interested in your thoughts before or after ASCO. What stood out for you this year in breast cancer? Drop us a comment and let us know about these sessions and what else you found worthwhile.
Dr. Miller is associate director of clinical research and codirector of the breast cancer program at the Melvin and Bren Simon Cancer Center at Indiana University, Indianapolis. Her career has combined both laboratory and clinical research in breast cancer.
A version of this article first appeared on Medscape.com.
IL-6 levels predict distant breast cancer recurrence
The inflammatory cytokine interleukin 6 may be a biomarker for distant recurrence of breast cancer among patients treated for stage II-III HER2-negative disease, investigators have found.
In a case-control study of 498 women with breast cancer treated with surgery and adjuvant chemotherapy, as well as endocrine therapy for women with estrogen receptor (ER)–positive tumors, those with higher serum levels of IL-6 at diagnosis had a significantly greater risk for disease recurrence than women with lower levels of the cytokine, Joseph A. Sparano, MD, from the Albert Einstein College of Medicine and Montefiore Medical Center, New York, and colleagues reported.
“This analysis provides level 1B evidence indicating that higher levels of the cytokine IL-6 at diagnosis are associated with a significantly higher distant recurrence risk in high-risk stage II-III breast cancer despite optimal adjuvant systemic therapy,” they wrote in a study presented in a poster discussion session at the American Society of Clinical Oncology Annual Meeting.(Abstract 520)
In an interview, Dr. Sparano said that their findings first need to be validated in a larger study.
“When validated, I think the other key issue is to try to understand what the best cut point for identifying high risk is, “ he said.
If further studies confirm that higher IL-6 levels are prognostic for worse outcomes, it might be possible to use levels of the cytokine as a biomarker to predict for therapies targeting the IL-6/Janus kinase/STAT3 pathway.
“There are trials ongoing testing IL-6 antibodies in combination chemotherapy, and this could be a rational biomarker to identify which patients would be more likely to benefit from that approach,” he said.
Systemic inflammation
Systemic inflammation is suspected as a contributing factor to cancer progression and disease recurrence, Dr. Sparano and colleagues noted.
To test their hypothesis that inflammatory cytokines and/or chemokines could be associated with distant recurrence, they conducted a case-control study with 249 matched pairs of patients enrolled in a phase 3 trial of adjuvant chemotherapy for lymph-node positive and high-risk lymph-node negative breast cancer (NCT00433511).
The patients all had surgery and adjuvant chemotherapy with doxorubicin, cyclophosphamide, and paclitaxel with or without bevacizumab, and endocrine therapy for patients whose tumors were ER positive.
They used propensity score matching to pair each patient with distant recurrence to one without, with covariates including post versus premenopausal or perimenopausal status, estrogen and/or progesterone receptor positivity, tumor size (less than 2 cm, greater than 2-5 cm, or greater than 5 cm) nodal status, and grade.
The only biomarker that met the prespecified boundary for statistical significance (P < .0014) was IL-6, with a hazard ratio for distant recurrence of 1.37 (P = .0006).
The median and mean values for IL-6 were 0.95 and 7.5 pg/mL, respectively
Other substances associated with distant recurrence (with a two-sided P value < .05) were macrophage-derived chemokine/CCL22 (HR, 1.90; P = .0098), IL-17A, a T-helper cell inflammatory cytokine (HR, 1.36; P = .0052), and the cytokine vascular endothelial growth factor A (VEGF-A, HR, 1.13; P = 0.037).
There was no statistical interaction between VEGF-A levels and the benefit of bevacizumab.
Prognostic value, not clinical utility
“This is a nice abstract. It looks at inflammatory cytokines and provides evidence that inflammatory cytokines, particularly IL-6, could have a prognostic role in predicting risk of recurrence in HER2-negative disease, and the team did a very nice job in multivariate analysis looking at different factors,” said Aditya Bardia, MD, MPH, from the Mass General Cancer Center in Boston, the invited discussant for the study.*
In an interview, Dr. Bardia said that the finding “provides prognostic value, but does not provide clinical utility. It’s unclear if we used this assay and it identified that a patient was at high risk of recurrence whether we could change that. Is there any intervention that could be done to potentially alter the course of disease, alter the natural history? That’s unknown.”
He agreed with Dr. Sparano and colleagues that validation of the finding was still needed, ideally in a prospective or retrospective cohort study.
The study was supported by grants from the National Cancer Institute, Komen Foundation, and Breast Cancer Research Foundation. Dr. Sparano disclosed relationships with multiple companies. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
*Correction, 6/4/21: An earlier version of this article misstated Dr. Bardia's name.
The inflammatory cytokine interleukin 6 may be a biomarker for distant recurrence of breast cancer among patients treated for stage II-III HER2-negative disease, investigators have found.
In a case-control study of 498 women with breast cancer treated with surgery and adjuvant chemotherapy, as well as endocrine therapy for women with estrogen receptor (ER)–positive tumors, those with higher serum levels of IL-6 at diagnosis had a significantly greater risk for disease recurrence than women with lower levels of the cytokine, Joseph A. Sparano, MD, from the Albert Einstein College of Medicine and Montefiore Medical Center, New York, and colleagues reported.
“This analysis provides level 1B evidence indicating that higher levels of the cytokine IL-6 at diagnosis are associated with a significantly higher distant recurrence risk in high-risk stage II-III breast cancer despite optimal adjuvant systemic therapy,” they wrote in a study presented in a poster discussion session at the American Society of Clinical Oncology Annual Meeting.(Abstract 520)
In an interview, Dr. Sparano said that their findings first need to be validated in a larger study.
“When validated, I think the other key issue is to try to understand what the best cut point for identifying high risk is, “ he said.
If further studies confirm that higher IL-6 levels are prognostic for worse outcomes, it might be possible to use levels of the cytokine as a biomarker to predict for therapies targeting the IL-6/Janus kinase/STAT3 pathway.
“There are trials ongoing testing IL-6 antibodies in combination chemotherapy, and this could be a rational biomarker to identify which patients would be more likely to benefit from that approach,” he said.
Systemic inflammation
Systemic inflammation is suspected as a contributing factor to cancer progression and disease recurrence, Dr. Sparano and colleagues noted.
To test their hypothesis that inflammatory cytokines and/or chemokines could be associated with distant recurrence, they conducted a case-control study with 249 matched pairs of patients enrolled in a phase 3 trial of adjuvant chemotherapy for lymph-node positive and high-risk lymph-node negative breast cancer (NCT00433511).
The patients all had surgery and adjuvant chemotherapy with doxorubicin, cyclophosphamide, and paclitaxel with or without bevacizumab, and endocrine therapy for patients whose tumors were ER positive.
They used propensity score matching to pair each patient with distant recurrence to one without, with covariates including post versus premenopausal or perimenopausal status, estrogen and/or progesterone receptor positivity, tumor size (less than 2 cm, greater than 2-5 cm, or greater than 5 cm) nodal status, and grade.
The only biomarker that met the prespecified boundary for statistical significance (P < .0014) was IL-6, with a hazard ratio for distant recurrence of 1.37 (P = .0006).
The median and mean values for IL-6 were 0.95 and 7.5 pg/mL, respectively
Other substances associated with distant recurrence (with a two-sided P value < .05) were macrophage-derived chemokine/CCL22 (HR, 1.90; P = .0098), IL-17A, a T-helper cell inflammatory cytokine (HR, 1.36; P = .0052), and the cytokine vascular endothelial growth factor A (VEGF-A, HR, 1.13; P = 0.037).
There was no statistical interaction between VEGF-A levels and the benefit of bevacizumab.
Prognostic value, not clinical utility
“This is a nice abstract. It looks at inflammatory cytokines and provides evidence that inflammatory cytokines, particularly IL-6, could have a prognostic role in predicting risk of recurrence in HER2-negative disease, and the team did a very nice job in multivariate analysis looking at different factors,” said Aditya Bardia, MD, MPH, from the Mass General Cancer Center in Boston, the invited discussant for the study.*
In an interview, Dr. Bardia said that the finding “provides prognostic value, but does not provide clinical utility. It’s unclear if we used this assay and it identified that a patient was at high risk of recurrence whether we could change that. Is there any intervention that could be done to potentially alter the course of disease, alter the natural history? That’s unknown.”
He agreed with Dr. Sparano and colleagues that validation of the finding was still needed, ideally in a prospective or retrospective cohort study.
The study was supported by grants from the National Cancer Institute, Komen Foundation, and Breast Cancer Research Foundation. Dr. Sparano disclosed relationships with multiple companies. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
*Correction, 6/4/21: An earlier version of this article misstated Dr. Bardia's name.
The inflammatory cytokine interleukin 6 may be a biomarker for distant recurrence of breast cancer among patients treated for stage II-III HER2-negative disease, investigators have found.
In a case-control study of 498 women with breast cancer treated with surgery and adjuvant chemotherapy, as well as endocrine therapy for women with estrogen receptor (ER)–positive tumors, those with higher serum levels of IL-6 at diagnosis had a significantly greater risk for disease recurrence than women with lower levels of the cytokine, Joseph A. Sparano, MD, from the Albert Einstein College of Medicine and Montefiore Medical Center, New York, and colleagues reported.
“This analysis provides level 1B evidence indicating that higher levels of the cytokine IL-6 at diagnosis are associated with a significantly higher distant recurrence risk in high-risk stage II-III breast cancer despite optimal adjuvant systemic therapy,” they wrote in a study presented in a poster discussion session at the American Society of Clinical Oncology Annual Meeting.(Abstract 520)
In an interview, Dr. Sparano said that their findings first need to be validated in a larger study.
“When validated, I think the other key issue is to try to understand what the best cut point for identifying high risk is, “ he said.
If further studies confirm that higher IL-6 levels are prognostic for worse outcomes, it might be possible to use levels of the cytokine as a biomarker to predict for therapies targeting the IL-6/Janus kinase/STAT3 pathway.
“There are trials ongoing testing IL-6 antibodies in combination chemotherapy, and this could be a rational biomarker to identify which patients would be more likely to benefit from that approach,” he said.
Systemic inflammation
Systemic inflammation is suspected as a contributing factor to cancer progression and disease recurrence, Dr. Sparano and colleagues noted.
To test their hypothesis that inflammatory cytokines and/or chemokines could be associated with distant recurrence, they conducted a case-control study with 249 matched pairs of patients enrolled in a phase 3 trial of adjuvant chemotherapy for lymph-node positive and high-risk lymph-node negative breast cancer (NCT00433511).
The patients all had surgery and adjuvant chemotherapy with doxorubicin, cyclophosphamide, and paclitaxel with or without bevacizumab, and endocrine therapy for patients whose tumors were ER positive.
They used propensity score matching to pair each patient with distant recurrence to one without, with covariates including post versus premenopausal or perimenopausal status, estrogen and/or progesterone receptor positivity, tumor size (less than 2 cm, greater than 2-5 cm, or greater than 5 cm) nodal status, and grade.
The only biomarker that met the prespecified boundary for statistical significance (P < .0014) was IL-6, with a hazard ratio for distant recurrence of 1.37 (P = .0006).
The median and mean values for IL-6 were 0.95 and 7.5 pg/mL, respectively
Other substances associated with distant recurrence (with a two-sided P value < .05) were macrophage-derived chemokine/CCL22 (HR, 1.90; P = .0098), IL-17A, a T-helper cell inflammatory cytokine (HR, 1.36; P = .0052), and the cytokine vascular endothelial growth factor A (VEGF-A, HR, 1.13; P = 0.037).
There was no statistical interaction between VEGF-A levels and the benefit of bevacizumab.
Prognostic value, not clinical utility
“This is a nice abstract. It looks at inflammatory cytokines and provides evidence that inflammatory cytokines, particularly IL-6, could have a prognostic role in predicting risk of recurrence in HER2-negative disease, and the team did a very nice job in multivariate analysis looking at different factors,” said Aditya Bardia, MD, MPH, from the Mass General Cancer Center in Boston, the invited discussant for the study.*
In an interview, Dr. Bardia said that the finding “provides prognostic value, but does not provide clinical utility. It’s unclear if we used this assay and it identified that a patient was at high risk of recurrence whether we could change that. Is there any intervention that could be done to potentially alter the course of disease, alter the natural history? That’s unknown.”
He agreed with Dr. Sparano and colleagues that validation of the finding was still needed, ideally in a prospective or retrospective cohort study.
The study was supported by grants from the National Cancer Institute, Komen Foundation, and Breast Cancer Research Foundation. Dr. Sparano disclosed relationships with multiple companies. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
*Correction, 6/4/21: An earlier version of this article misstated Dr. Bardia's name.
FROM ASCO 2021
Trastuzumab deruxtecan-related lung disease in MBC patients can occur anytime in first year
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
FROM ESMO BREAST CANCER 2021
Endocrine therapy benefits in premenopausal breast cancer differ by molecular risk
The long-term benefits of endocrine therapy in premenopausal breast cancer appear to differ according to whether patients are categorized as high or low molecular risk using the 70-gene signature (MammaPrint).
Based upon data from patients who had participated in the Stockholm tamoxifen (STO-5) trial, high-risk patients significantly benefited from goserelin treatment, whereas low-risk patients benefited more from tamoxifen treatment when compared with no endocrine therapy.
“Goserelin, tamoxifen, and the combination of the two, reduced the 20-year risk of distant occurrences and fatal breast cancer, compared to no endocrine therapy,” Annelie Johansson, MSc, said at the European Society for Medical Oncology: Breast Cancer virtual meeting.
“Our findings indicate that the long-term endocrine therapy benefit in premenopausal patients is influenced by molecular risk classification and thus tumor characteristics,” she added.
Ms. Johansson, a postdoctoral researcher in genomic breast cancer at the Karolinska Institutet in Stockholm, reported the results of the analysis as a late-breaking abstract at the meeting.
“I think this is an innovative translational study trying to use the multigene assay results to look at differential endocrine therapy effects,” said Prudence Francis, MD, the invited discussant for study.
However, there are relatively few patients in the various subgroups being tested, she added. “We’ve also got short duration of tamoxifen, only 2 years, we’ve got prior chemotherapy in some patients and absence of HER2 therapy, all of which might influence outcomes.”
As a result, Dr. Francis, who is head of medical oncology at the Peter MacCallum Cancer Centre and a consultant Medical Oncologist at St. Vincent’s Hospital Melbourne, called the findings purely “hypothesis generating.”
Study details and results
The analysis was based on data from the STO-5 trial, which had recruited just over 900 patients between 1990 and 1997. Patients were stratified according to their lymph node status and some received chemotherapy with or without locoregional radiotherapy before being randomized to one of four study arms: goserelin alone, tamoxifen alone, the combination of the two, or no endocrine therapy.
Ms. Johansson noted that they were able to obtain the primary tumor blocks from 729 patients in the past year, of whom 610 were estrogen receptor positive. The analysis according to the 70-gene signature was then based on data from 465 patients: 131 had been treated with goserelin, 105 with tamoxifen, 120 with both, and 109 had received no endocrine treatment.
We have complete 20-year follow-up from high-quality Swedish National registries,” Ms. Johansson said, observing that the median age in the trial was 46 years.
Before stratifying patients into high and low risk using the 70-gene signature, the risk for having a distant recurrence, compared with no endocrine therapy was reduced by 52% with goserelin (hazard ratio, .48), 41% with tamoxifen (HR, 0.59), and 33% with both in combination (HR, 0.67).
After stratification, however, goserelin was associated with a 78% reduction of distant recurrence versus no endocrine treatment in high-risk patients (HR, 0.22) and a 20% reduction in low-risk patients (HR, 0.80).
Results in high- and low-risk patients with tamoxifen versus no endocrine treatment were a respective 31% reduction (HR, 0.69) and 62% reduction (HR, 0.38), and a respective 36% (HR, 0.64) and 28% (HR, 0.72) for the combination.
A further analysis was performed to compare between the active treatment arms, and this suggested a greater benefit of goserelin in patients at high risk when compared with both tamoxifen (HR, 0.30) and the combination (HR, 0.33).
Dr. Francis commented: “it is a bit surprising to find that goserelin appeared to be also better than the combination,” and it is something that the research team is looking into.
“One hypothesis might be if you look how the different treatments are working,” Ms. Johansson said. “Goserelin is very efficient in lowering the estrogen levels in premenopausal patients, suppressing the ovarian production of estrogen whereas tamoxifen can act both as an antagonist and agonist.
“So, we are thinking that maybe the addition of tamoxifen, with the agonistic properties of tamoxifen, might then make the goserelin not as efficient. But that’s of course, just a hypothesis right now and we need to look into this further,” she said.
The work was funded by The Swedish Research Council (Vetenskapsrådet), The Swedish Research Council for Health, Working life and Welfare, and the Swedish Cancer Society (Cancerfonden). Ms. Johansson had no personal disclosures; one of the coauthors was a coinventor of MammaPrint. Dr. Francis disclosed receiving travel support for overseas lectures from Ipsen and Novartis and acting as a medical oncology editor for Elsevier.
The long-term benefits of endocrine therapy in premenopausal breast cancer appear to differ according to whether patients are categorized as high or low molecular risk using the 70-gene signature (MammaPrint).
Based upon data from patients who had participated in the Stockholm tamoxifen (STO-5) trial, high-risk patients significantly benefited from goserelin treatment, whereas low-risk patients benefited more from tamoxifen treatment when compared with no endocrine therapy.
“Goserelin, tamoxifen, and the combination of the two, reduced the 20-year risk of distant occurrences and fatal breast cancer, compared to no endocrine therapy,” Annelie Johansson, MSc, said at the European Society for Medical Oncology: Breast Cancer virtual meeting.
“Our findings indicate that the long-term endocrine therapy benefit in premenopausal patients is influenced by molecular risk classification and thus tumor characteristics,” she added.
Ms. Johansson, a postdoctoral researcher in genomic breast cancer at the Karolinska Institutet in Stockholm, reported the results of the analysis as a late-breaking abstract at the meeting.
“I think this is an innovative translational study trying to use the multigene assay results to look at differential endocrine therapy effects,” said Prudence Francis, MD, the invited discussant for study.
However, there are relatively few patients in the various subgroups being tested, she added. “We’ve also got short duration of tamoxifen, only 2 years, we’ve got prior chemotherapy in some patients and absence of HER2 therapy, all of which might influence outcomes.”
As a result, Dr. Francis, who is head of medical oncology at the Peter MacCallum Cancer Centre and a consultant Medical Oncologist at St. Vincent’s Hospital Melbourne, called the findings purely “hypothesis generating.”
Study details and results
The analysis was based on data from the STO-5 trial, which had recruited just over 900 patients between 1990 and 1997. Patients were stratified according to their lymph node status and some received chemotherapy with or without locoregional radiotherapy before being randomized to one of four study arms: goserelin alone, tamoxifen alone, the combination of the two, or no endocrine therapy.
Ms. Johansson noted that they were able to obtain the primary tumor blocks from 729 patients in the past year, of whom 610 were estrogen receptor positive. The analysis according to the 70-gene signature was then based on data from 465 patients: 131 had been treated with goserelin, 105 with tamoxifen, 120 with both, and 109 had received no endocrine treatment.
We have complete 20-year follow-up from high-quality Swedish National registries,” Ms. Johansson said, observing that the median age in the trial was 46 years.
Before stratifying patients into high and low risk using the 70-gene signature, the risk for having a distant recurrence, compared with no endocrine therapy was reduced by 52% with goserelin (hazard ratio, .48), 41% with tamoxifen (HR, 0.59), and 33% with both in combination (HR, 0.67).
After stratification, however, goserelin was associated with a 78% reduction of distant recurrence versus no endocrine treatment in high-risk patients (HR, 0.22) and a 20% reduction in low-risk patients (HR, 0.80).
Results in high- and low-risk patients with tamoxifen versus no endocrine treatment were a respective 31% reduction (HR, 0.69) and 62% reduction (HR, 0.38), and a respective 36% (HR, 0.64) and 28% (HR, 0.72) for the combination.
A further analysis was performed to compare between the active treatment arms, and this suggested a greater benefit of goserelin in patients at high risk when compared with both tamoxifen (HR, 0.30) and the combination (HR, 0.33).
Dr. Francis commented: “it is a bit surprising to find that goserelin appeared to be also better than the combination,” and it is something that the research team is looking into.
“One hypothesis might be if you look how the different treatments are working,” Ms. Johansson said. “Goserelin is very efficient in lowering the estrogen levels in premenopausal patients, suppressing the ovarian production of estrogen whereas tamoxifen can act both as an antagonist and agonist.
“So, we are thinking that maybe the addition of tamoxifen, with the agonistic properties of tamoxifen, might then make the goserelin not as efficient. But that’s of course, just a hypothesis right now and we need to look into this further,” she said.
The work was funded by The Swedish Research Council (Vetenskapsrådet), The Swedish Research Council for Health, Working life and Welfare, and the Swedish Cancer Society (Cancerfonden). Ms. Johansson had no personal disclosures; one of the coauthors was a coinventor of MammaPrint. Dr. Francis disclosed receiving travel support for overseas lectures from Ipsen and Novartis and acting as a medical oncology editor for Elsevier.
The long-term benefits of endocrine therapy in premenopausal breast cancer appear to differ according to whether patients are categorized as high or low molecular risk using the 70-gene signature (MammaPrint).
Based upon data from patients who had participated in the Stockholm tamoxifen (STO-5) trial, high-risk patients significantly benefited from goserelin treatment, whereas low-risk patients benefited more from tamoxifen treatment when compared with no endocrine therapy.
“Goserelin, tamoxifen, and the combination of the two, reduced the 20-year risk of distant occurrences and fatal breast cancer, compared to no endocrine therapy,” Annelie Johansson, MSc, said at the European Society for Medical Oncology: Breast Cancer virtual meeting.
“Our findings indicate that the long-term endocrine therapy benefit in premenopausal patients is influenced by molecular risk classification and thus tumor characteristics,” she added.
Ms. Johansson, a postdoctoral researcher in genomic breast cancer at the Karolinska Institutet in Stockholm, reported the results of the analysis as a late-breaking abstract at the meeting.
“I think this is an innovative translational study trying to use the multigene assay results to look at differential endocrine therapy effects,” said Prudence Francis, MD, the invited discussant for study.
However, there are relatively few patients in the various subgroups being tested, she added. “We’ve also got short duration of tamoxifen, only 2 years, we’ve got prior chemotherapy in some patients and absence of HER2 therapy, all of which might influence outcomes.”
As a result, Dr. Francis, who is head of medical oncology at the Peter MacCallum Cancer Centre and a consultant Medical Oncologist at St. Vincent’s Hospital Melbourne, called the findings purely “hypothesis generating.”
Study details and results
The analysis was based on data from the STO-5 trial, which had recruited just over 900 patients between 1990 and 1997. Patients were stratified according to their lymph node status and some received chemotherapy with or without locoregional radiotherapy before being randomized to one of four study arms: goserelin alone, tamoxifen alone, the combination of the two, or no endocrine therapy.
Ms. Johansson noted that they were able to obtain the primary tumor blocks from 729 patients in the past year, of whom 610 were estrogen receptor positive. The analysis according to the 70-gene signature was then based on data from 465 patients: 131 had been treated with goserelin, 105 with tamoxifen, 120 with both, and 109 had received no endocrine treatment.
We have complete 20-year follow-up from high-quality Swedish National registries,” Ms. Johansson said, observing that the median age in the trial was 46 years.
Before stratifying patients into high and low risk using the 70-gene signature, the risk for having a distant recurrence, compared with no endocrine therapy was reduced by 52% with goserelin (hazard ratio, .48), 41% with tamoxifen (HR, 0.59), and 33% with both in combination (HR, 0.67).
After stratification, however, goserelin was associated with a 78% reduction of distant recurrence versus no endocrine treatment in high-risk patients (HR, 0.22) and a 20% reduction in low-risk patients (HR, 0.80).
Results in high- and low-risk patients with tamoxifen versus no endocrine treatment were a respective 31% reduction (HR, 0.69) and 62% reduction (HR, 0.38), and a respective 36% (HR, 0.64) and 28% (HR, 0.72) for the combination.
A further analysis was performed to compare between the active treatment arms, and this suggested a greater benefit of goserelin in patients at high risk when compared with both tamoxifen (HR, 0.30) and the combination (HR, 0.33).
Dr. Francis commented: “it is a bit surprising to find that goserelin appeared to be also better than the combination,” and it is something that the research team is looking into.
“One hypothesis might be if you look how the different treatments are working,” Ms. Johansson said. “Goserelin is very efficient in lowering the estrogen levels in premenopausal patients, suppressing the ovarian production of estrogen whereas tamoxifen can act both as an antagonist and agonist.
“So, we are thinking that maybe the addition of tamoxifen, with the agonistic properties of tamoxifen, might then make the goserelin not as efficient. But that’s of course, just a hypothesis right now and we need to look into this further,” she said.
The work was funded by The Swedish Research Council (Vetenskapsrådet), The Swedish Research Council for Health, Working life and Welfare, and the Swedish Cancer Society (Cancerfonden). Ms. Johansson had no personal disclosures; one of the coauthors was a coinventor of MammaPrint. Dr. Francis disclosed receiving travel support for overseas lectures from Ipsen and Novartis and acting as a medical oncology editor for Elsevier.
FROM ESMO BREAST CANCER 2021
Evolving strategies in sequencing for HER2+ MBC therapy
The landscape for therapies targeting HER2-positive metastatic breast cancer (MBC) has evolved rapidly in the past few years. In a 12-month window, the U.S. Food and Drug Administration approved four agents targeting human epidermal growth factor 2 (HER2)–positive MBC, starting with trastuzumab deruxtecan in December 2019, followed by neratinib and tucatinib a few months later, and margetuximab last December.
Although first-line therapy for the majority of patients continues to be the CLEOPATRA regimen — the monoclonal antibodies trastuzumab and pertuzumab plus a taxane, such as docetaxel or paclitaxel — .
“We have been really fortunate to see a number of highly effective new therapies approved for HER2-positive MBC in the past year, and this has given us even more options to offer our patients,” remarked Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
What considerations do experts weigh when sequencing HER2-positive MBC?
For Kelly McCann, MD, PhD, the order largely depends on balancing two factors: regimens that will provide the best efficacy in terms of patient survival and quality of life. “In the metastatic setting, I know I’m going to end up using all of the available medications one after the other, so the order that allows patients to continue living their best life for as long as possible is essential,” commented Dr. McCann, a hematologist/oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles.
A new second-line option?
Before the wave of drug approvals for metastatic HER2-positive disease last year, oncologists routinely looked to trastuzumab emtansine (T-DM1) as second-line therapy.
But tucatinib may also now be considered in the second-line setting, after results from the HER2CLIMB trial. The decision between tucatinib and T-DM1 largely comes down to the presence or absence of brain metastases.
“T-DM1 is well-tolerated, so it’s still my go-to in the second-line setting unless my patient has a brain metastasis, in which case I opt for tucatinib,” Dr. McCann noted, adding that the HER2-specific oral tyrosine kinase inhibitor (TKI) not only crosses the blood-brain barrier but is also effective in patients with untreated brain metastases.
In HER2CLIMB, tucatinib exhibited strong efficacy in patients with advanced HER2-positive disease, including those with previously treated or untreated brain metastases. The randomized controlled trial, which paired tucatinib with trastuzumab and capecitabine, showed median progression-free survival of 7.8 months in 410 patients with HER2-positive MBC compared with 5.6 months in the 202 patients receiving the placebo regimen. The tucatinib cohort showed an overall survival advantage compared with the placebo group (21.9 vs 17.4 months).
Perhaps the most notable finding occurred in patients with brain or central nervous system (CNS) involvement, which develops in as many as half of patients with HER-positive MBC and is associated with shorter survival. In the HER2CLIMB trial, median progression-free survival was 7.6 months in patients with brain metastases compared with 5.4 months in the placebo group.
A follow-up exploratory analysis, which focused on 291 patients with brain metastases, found that adding tucatinib reduced the risk for intracranial progression by two thirds and death by almost half. In patients with active brain metastases, median progression-free survival reached 9.5 months vs 4.1 months in the placebo group. Those with stable metastases also benefited from tucatinib, with median progression-free survival of 13.9 vs 5.6 months in the placebo group.
On the basis of the results, the authors concluded that this randomized trial was the first to demonstrate improvements in both CNS progression–free survival and overall survival in patients with HER2-positive MBC and brain metastases.
Evolving options in the third-line setting and after
For third-line therapy and beyond, oncologists have an array of newer agents to choose from alongside longer-standing options — which include trastuzumab plus lapatinib, trastuzumab or lapatinib plus capecitabine, as well as T-DM1, if not given as second-line therapy.
According to Dr. McCann, the antibody-drug conjugate trastuzumab deruxtecan has been a particularly exciting addition to third-line treatment. In the phase 2 DESTINY-01 trial, more than 60% of a heavily pretreated population showed an objective response to trastuzumab deruxtecan, with a median response duration of almost 15 months and a median progression-free survival of 16.4 months. Longer-term follow-up results, presented in December at the 2020 San Antonio Breast Cancer Symposium, revealed progression-free survival of 19.4 months and preliminary median overall survival of 24.6 months.
Neratinib, the second TKI to bridge the blood-brain barrier in HER2-positive disease, was also approved for third-line use; however, Sayeh Lavasani, MD, MS, said she is more likely to consider this agent later in the sequence, potentially in the fourth-line setting and beyond, given the more robust outcomes observed in the HER2CLIMB tucatinib trial.
“Neratinib improved progression-free survival and time to intervention for CNS metastasis but, unlike tucatinib, did not demonstrate an overall survival benefit,” remarked Dr. Lavasani, a medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
More specifically, the phase 3 NALA trial, which randomly assigned patients to receive neratinib plus capecitabine or lapatinib plus capecitabine, reported progression-free survival of 8.8 months in the neratinib group compared with 6.6 months in the control arm but no significant gains in overall survival (hazard ratio, 0.88; P = .2098).
The fourth recently approved drug, margetuximab, has not yet made a significant mark on sequencing decisions for Dr. McCann.
“Margetuximab could have been a potential game changer, but clinical trial results were underwhelming,” she said.
In the phase 3 randomized clinical SOPHIA trial, margetuximab plus chemotherapy prolonged median progression-free survival by just over 1 month compared with trastuzumab plus chemotherapy. Preliminary overall survival data showed a slight, but not significant, benefit in the margetuximab group (21.6 vs 19.8 months).
For Dr. Lavasani, the presence of brain metastases is the most important consideration when weighing sequencing options. “For some of my patients with HER2-positive MBC, it’s ultimately disease progression in the brain that takes their life,” she said.
Aside from CNS metastases, specific sequencing choices may vary on the basis of drug-related tolerance as well as patient preferences. “It is critical to get a patient’s input in treatment selection,” Dr. Nanda remarked. “Given the number of effective treatments for HER2-positive MBC and the lack of data to guide how to sequence these regimens, it is important to ask patients what their preferences are.”
Dr. McCann agreed, noting that “a patient with HER2-positive MBC typically has a life expectancy measured in years, which is also why sequencing should be influenced by quality of life considerations.”
Convenience, side-effect profile, and financial toxicity should factor into clinical decision-making, according to Dr. Nanda. Some patients may, for instance, prefer a combination of tucatinib, capecitabine, and trastuzumab over trastuzumab deruxtecan to avoid hair loss and the risk for interstitial lung disease, which has been reported in more than 13% of patients, whereas others may prefer trastuzumab deruxtecan to avoid the possibility of diarrhea.
Taxanes come with a high risk for infusion reactions — which occur in about 30% of patients — and can cause neuropathy as well as hair loss and severe gastrointestinal side effects. In first-line care, Dr. McCann typically stops the taxane at some point for toxicity reasons and continues with trastuzumab plus pertuzumab until disease progression.
Even with an array of new options for treating metastatic HER2-positive disease, ultimately drug resistance does occur, Dr. Lavasani cautioned. Several ongoing trials are exploring new combinations of existing drugs to see whether those variations move the needle on survival outcomes. The HER2CLIMB-04 trial, for instance, is pairing tucatinib with trastuzumab deruxtecan, whereas HER2CLIMB-02 is pairing tucatinib with T-DM1.
But given progress in drug development in just the past few years, Lisa A. Carey, MD, deputy director of Clinical Sciences at the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, sees a promising future for treating metastatic HER2-positive disease. “There is so much going on in the HER2-positive MBC therapeutics space that almost every 6 months, oncologists have to regroup and reevaluate treatment and sequencing, which is a great position to be in,” Dr. Carey noted.
A version of this article first appeared on Medscape.com .
The landscape for therapies targeting HER2-positive metastatic breast cancer (MBC) has evolved rapidly in the past few years. In a 12-month window, the U.S. Food and Drug Administration approved four agents targeting human epidermal growth factor 2 (HER2)–positive MBC, starting with trastuzumab deruxtecan in December 2019, followed by neratinib and tucatinib a few months later, and margetuximab last December.
Although first-line therapy for the majority of patients continues to be the CLEOPATRA regimen — the monoclonal antibodies trastuzumab and pertuzumab plus a taxane, such as docetaxel or paclitaxel — .
“We have been really fortunate to see a number of highly effective new therapies approved for HER2-positive MBC in the past year, and this has given us even more options to offer our patients,” remarked Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
What considerations do experts weigh when sequencing HER2-positive MBC?
For Kelly McCann, MD, PhD, the order largely depends on balancing two factors: regimens that will provide the best efficacy in terms of patient survival and quality of life. “In the metastatic setting, I know I’m going to end up using all of the available medications one after the other, so the order that allows patients to continue living their best life for as long as possible is essential,” commented Dr. McCann, a hematologist/oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles.
A new second-line option?
Before the wave of drug approvals for metastatic HER2-positive disease last year, oncologists routinely looked to trastuzumab emtansine (T-DM1) as second-line therapy.
But tucatinib may also now be considered in the second-line setting, after results from the HER2CLIMB trial. The decision between tucatinib and T-DM1 largely comes down to the presence or absence of brain metastases.
“T-DM1 is well-tolerated, so it’s still my go-to in the second-line setting unless my patient has a brain metastasis, in which case I opt for tucatinib,” Dr. McCann noted, adding that the HER2-specific oral tyrosine kinase inhibitor (TKI) not only crosses the blood-brain barrier but is also effective in patients with untreated brain metastases.
In HER2CLIMB, tucatinib exhibited strong efficacy in patients with advanced HER2-positive disease, including those with previously treated or untreated brain metastases. The randomized controlled trial, which paired tucatinib with trastuzumab and capecitabine, showed median progression-free survival of 7.8 months in 410 patients with HER2-positive MBC compared with 5.6 months in the 202 patients receiving the placebo regimen. The tucatinib cohort showed an overall survival advantage compared with the placebo group (21.9 vs 17.4 months).
Perhaps the most notable finding occurred in patients with brain or central nervous system (CNS) involvement, which develops in as many as half of patients with HER-positive MBC and is associated with shorter survival. In the HER2CLIMB trial, median progression-free survival was 7.6 months in patients with brain metastases compared with 5.4 months in the placebo group.
A follow-up exploratory analysis, which focused on 291 patients with brain metastases, found that adding tucatinib reduced the risk for intracranial progression by two thirds and death by almost half. In patients with active brain metastases, median progression-free survival reached 9.5 months vs 4.1 months in the placebo group. Those with stable metastases also benefited from tucatinib, with median progression-free survival of 13.9 vs 5.6 months in the placebo group.
On the basis of the results, the authors concluded that this randomized trial was the first to demonstrate improvements in both CNS progression–free survival and overall survival in patients with HER2-positive MBC and brain metastases.
Evolving options in the third-line setting and after
For third-line therapy and beyond, oncologists have an array of newer agents to choose from alongside longer-standing options — which include trastuzumab plus lapatinib, trastuzumab or lapatinib plus capecitabine, as well as T-DM1, if not given as second-line therapy.
According to Dr. McCann, the antibody-drug conjugate trastuzumab deruxtecan has been a particularly exciting addition to third-line treatment. In the phase 2 DESTINY-01 trial, more than 60% of a heavily pretreated population showed an objective response to trastuzumab deruxtecan, with a median response duration of almost 15 months and a median progression-free survival of 16.4 months. Longer-term follow-up results, presented in December at the 2020 San Antonio Breast Cancer Symposium, revealed progression-free survival of 19.4 months and preliminary median overall survival of 24.6 months.
Neratinib, the second TKI to bridge the blood-brain barrier in HER2-positive disease, was also approved for third-line use; however, Sayeh Lavasani, MD, MS, said she is more likely to consider this agent later in the sequence, potentially in the fourth-line setting and beyond, given the more robust outcomes observed in the HER2CLIMB tucatinib trial.
“Neratinib improved progression-free survival and time to intervention for CNS metastasis but, unlike tucatinib, did not demonstrate an overall survival benefit,” remarked Dr. Lavasani, a medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
More specifically, the phase 3 NALA trial, which randomly assigned patients to receive neratinib plus capecitabine or lapatinib plus capecitabine, reported progression-free survival of 8.8 months in the neratinib group compared with 6.6 months in the control arm but no significant gains in overall survival (hazard ratio, 0.88; P = .2098).
The fourth recently approved drug, margetuximab, has not yet made a significant mark on sequencing decisions for Dr. McCann.
“Margetuximab could have been a potential game changer, but clinical trial results were underwhelming,” she said.
In the phase 3 randomized clinical SOPHIA trial, margetuximab plus chemotherapy prolonged median progression-free survival by just over 1 month compared with trastuzumab plus chemotherapy. Preliminary overall survival data showed a slight, but not significant, benefit in the margetuximab group (21.6 vs 19.8 months).
For Dr. Lavasani, the presence of brain metastases is the most important consideration when weighing sequencing options. “For some of my patients with HER2-positive MBC, it’s ultimately disease progression in the brain that takes their life,” she said.
Aside from CNS metastases, specific sequencing choices may vary on the basis of drug-related tolerance as well as patient preferences. “It is critical to get a patient’s input in treatment selection,” Dr. Nanda remarked. “Given the number of effective treatments for HER2-positive MBC and the lack of data to guide how to sequence these regimens, it is important to ask patients what their preferences are.”
Dr. McCann agreed, noting that “a patient with HER2-positive MBC typically has a life expectancy measured in years, which is also why sequencing should be influenced by quality of life considerations.”
Convenience, side-effect profile, and financial toxicity should factor into clinical decision-making, according to Dr. Nanda. Some patients may, for instance, prefer a combination of tucatinib, capecitabine, and trastuzumab over trastuzumab deruxtecan to avoid hair loss and the risk for interstitial lung disease, which has been reported in more than 13% of patients, whereas others may prefer trastuzumab deruxtecan to avoid the possibility of diarrhea.
Taxanes come with a high risk for infusion reactions — which occur in about 30% of patients — and can cause neuropathy as well as hair loss and severe gastrointestinal side effects. In first-line care, Dr. McCann typically stops the taxane at some point for toxicity reasons and continues with trastuzumab plus pertuzumab until disease progression.
Even with an array of new options for treating metastatic HER2-positive disease, ultimately drug resistance does occur, Dr. Lavasani cautioned. Several ongoing trials are exploring new combinations of existing drugs to see whether those variations move the needle on survival outcomes. The HER2CLIMB-04 trial, for instance, is pairing tucatinib with trastuzumab deruxtecan, whereas HER2CLIMB-02 is pairing tucatinib with T-DM1.
But given progress in drug development in just the past few years, Lisa A. Carey, MD, deputy director of Clinical Sciences at the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, sees a promising future for treating metastatic HER2-positive disease. “There is so much going on in the HER2-positive MBC therapeutics space that almost every 6 months, oncologists have to regroup and reevaluate treatment and sequencing, which is a great position to be in,” Dr. Carey noted.
A version of this article first appeared on Medscape.com .
The landscape for therapies targeting HER2-positive metastatic breast cancer (MBC) has evolved rapidly in the past few years. In a 12-month window, the U.S. Food and Drug Administration approved four agents targeting human epidermal growth factor 2 (HER2)–positive MBC, starting with trastuzumab deruxtecan in December 2019, followed by neratinib and tucatinib a few months later, and margetuximab last December.
Although first-line therapy for the majority of patients continues to be the CLEOPATRA regimen — the monoclonal antibodies trastuzumab and pertuzumab plus a taxane, such as docetaxel or paclitaxel — .
“We have been really fortunate to see a number of highly effective new therapies approved for HER2-positive MBC in the past year, and this has given us even more options to offer our patients,” remarked Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
What considerations do experts weigh when sequencing HER2-positive MBC?
For Kelly McCann, MD, PhD, the order largely depends on balancing two factors: regimens that will provide the best efficacy in terms of patient survival and quality of life. “In the metastatic setting, I know I’m going to end up using all of the available medications one after the other, so the order that allows patients to continue living their best life for as long as possible is essential,” commented Dr. McCann, a hematologist/oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles.
A new second-line option?
Before the wave of drug approvals for metastatic HER2-positive disease last year, oncologists routinely looked to trastuzumab emtansine (T-DM1) as second-line therapy.
But tucatinib may also now be considered in the second-line setting, after results from the HER2CLIMB trial. The decision between tucatinib and T-DM1 largely comes down to the presence or absence of brain metastases.
“T-DM1 is well-tolerated, so it’s still my go-to in the second-line setting unless my patient has a brain metastasis, in which case I opt for tucatinib,” Dr. McCann noted, adding that the HER2-specific oral tyrosine kinase inhibitor (TKI) not only crosses the blood-brain barrier but is also effective in patients with untreated brain metastases.
In HER2CLIMB, tucatinib exhibited strong efficacy in patients with advanced HER2-positive disease, including those with previously treated or untreated brain metastases. The randomized controlled trial, which paired tucatinib with trastuzumab and capecitabine, showed median progression-free survival of 7.8 months in 410 patients with HER2-positive MBC compared with 5.6 months in the 202 patients receiving the placebo regimen. The tucatinib cohort showed an overall survival advantage compared with the placebo group (21.9 vs 17.4 months).
Perhaps the most notable finding occurred in patients with brain or central nervous system (CNS) involvement, which develops in as many as half of patients with HER-positive MBC and is associated with shorter survival. In the HER2CLIMB trial, median progression-free survival was 7.6 months in patients with brain metastases compared with 5.4 months in the placebo group.
A follow-up exploratory analysis, which focused on 291 patients with brain metastases, found that adding tucatinib reduced the risk for intracranial progression by two thirds and death by almost half. In patients with active brain metastases, median progression-free survival reached 9.5 months vs 4.1 months in the placebo group. Those with stable metastases also benefited from tucatinib, with median progression-free survival of 13.9 vs 5.6 months in the placebo group.
On the basis of the results, the authors concluded that this randomized trial was the first to demonstrate improvements in both CNS progression–free survival and overall survival in patients with HER2-positive MBC and brain metastases.
Evolving options in the third-line setting and after
For third-line therapy and beyond, oncologists have an array of newer agents to choose from alongside longer-standing options — which include trastuzumab plus lapatinib, trastuzumab or lapatinib plus capecitabine, as well as T-DM1, if not given as second-line therapy.
According to Dr. McCann, the antibody-drug conjugate trastuzumab deruxtecan has been a particularly exciting addition to third-line treatment. In the phase 2 DESTINY-01 trial, more than 60% of a heavily pretreated population showed an objective response to trastuzumab deruxtecan, with a median response duration of almost 15 months and a median progression-free survival of 16.4 months. Longer-term follow-up results, presented in December at the 2020 San Antonio Breast Cancer Symposium, revealed progression-free survival of 19.4 months and preliminary median overall survival of 24.6 months.
Neratinib, the second TKI to bridge the blood-brain barrier in HER2-positive disease, was also approved for third-line use; however, Sayeh Lavasani, MD, MS, said she is more likely to consider this agent later in the sequence, potentially in the fourth-line setting and beyond, given the more robust outcomes observed in the HER2CLIMB tucatinib trial.
“Neratinib improved progression-free survival and time to intervention for CNS metastasis but, unlike tucatinib, did not demonstrate an overall survival benefit,” remarked Dr. Lavasani, a medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
More specifically, the phase 3 NALA trial, which randomly assigned patients to receive neratinib plus capecitabine or lapatinib plus capecitabine, reported progression-free survival of 8.8 months in the neratinib group compared with 6.6 months in the control arm but no significant gains in overall survival (hazard ratio, 0.88; P = .2098).
The fourth recently approved drug, margetuximab, has not yet made a significant mark on sequencing decisions for Dr. McCann.
“Margetuximab could have been a potential game changer, but clinical trial results were underwhelming,” she said.
In the phase 3 randomized clinical SOPHIA trial, margetuximab plus chemotherapy prolonged median progression-free survival by just over 1 month compared with trastuzumab plus chemotherapy. Preliminary overall survival data showed a slight, but not significant, benefit in the margetuximab group (21.6 vs 19.8 months).
For Dr. Lavasani, the presence of brain metastases is the most important consideration when weighing sequencing options. “For some of my patients with HER2-positive MBC, it’s ultimately disease progression in the brain that takes their life,” she said.
Aside from CNS metastases, specific sequencing choices may vary on the basis of drug-related tolerance as well as patient preferences. “It is critical to get a patient’s input in treatment selection,” Dr. Nanda remarked. “Given the number of effective treatments for HER2-positive MBC and the lack of data to guide how to sequence these regimens, it is important to ask patients what their preferences are.”
Dr. McCann agreed, noting that “a patient with HER2-positive MBC typically has a life expectancy measured in years, which is also why sequencing should be influenced by quality of life considerations.”
Convenience, side-effect profile, and financial toxicity should factor into clinical decision-making, according to Dr. Nanda. Some patients may, for instance, prefer a combination of tucatinib, capecitabine, and trastuzumab over trastuzumab deruxtecan to avoid hair loss and the risk for interstitial lung disease, which has been reported in more than 13% of patients, whereas others may prefer trastuzumab deruxtecan to avoid the possibility of diarrhea.
Taxanes come with a high risk for infusion reactions — which occur in about 30% of patients — and can cause neuropathy as well as hair loss and severe gastrointestinal side effects. In first-line care, Dr. McCann typically stops the taxane at some point for toxicity reasons and continues with trastuzumab plus pertuzumab until disease progression.
Even with an array of new options for treating metastatic HER2-positive disease, ultimately drug resistance does occur, Dr. Lavasani cautioned. Several ongoing trials are exploring new combinations of existing drugs to see whether those variations move the needle on survival outcomes. The HER2CLIMB-04 trial, for instance, is pairing tucatinib with trastuzumab deruxtecan, whereas HER2CLIMB-02 is pairing tucatinib with T-DM1.
But given progress in drug development in just the past few years, Lisa A. Carey, MD, deputy director of Clinical Sciences at the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, sees a promising future for treating metastatic HER2-positive disease. “There is so much going on in the HER2-positive MBC therapeutics space that almost every 6 months, oncologists have to regroup and reevaluate treatment and sequencing, which is a great position to be in,” Dr. Carey noted.
A version of this article first appeared on Medscape.com .
New targeted treatments are major advances for HER2-positive breast cancer
Before 2001, HER2/neu-positive breast cancer (HER2+) was one of the most dreaded diagnoses a woman could face, as treatment was largely ineffective. The discovery of trastuzumab changed that dramatically.
Over the next 20 years, two additional HER2-targeted therapies – lapatinib and trastuzumab emtansine (TDM-1) – earned approval from the Food and Drug Administration for selected patients with early and late HER2+ breast cancer.
Since 2019, four additional HER2-targeted therapies have been approved by the FDA for HER2+ metastatic breast cancer (MBC), changing the treatment paradigm for those patients substantially.
The new agents are especially useful in certain patient populations. The agents offer the promise of improved survival for patients with recurrent metastatic disease and the potential for further reductions in relapse rates in earlier settings.
Trastuzumab deruxtecan
Trastuzumab deruxtecan is an antibody-drug conjugate that links three components: an anti-HER2 monoclonal antibody, a highly potent topoisomerase I inhibitor payload, and a tetrapeptide-based cleavable linker.
Trastuzumab deruxtecan has a high drug-to-antibody ratio. A membrane-permeable payload offers the potential for activity against adjacent HER2-negative cells in heterogeneous tumors. It has a long half-life (6 days).
Trastuzumab deruxtecan received accelerated approval from the FDA in December 2019 to treat patients with HER2+ MBC who have received two or more prior HER2-targeted regimens, based on the results of the DESTINY-Breast 01 trial.
DESTINY-Breast 01 trial
In the phase 2 DESTINY-Breast 01 trial, 184 patients with a median of six previous treatments received trastuzumab deruxtecan (5.4 mg/kg) intravenously every 21 days. There were 24 patients with treated, asymptomatic brain metastases who participated. Patients with untreated or symptomatic brain metastases were excluded.
Overall, a response to therapy was reported in 112 patients (60.9%), with 6.0% complete and 54.9% partial responses. Most of the patients for whom both baseline and postbaseline data were available had a reduction in tumor size.
The median time until response was 1.6 months, an interval that corresponded to the time until the first scheduled imaging. Three patients (1.6%) had progressive disease, and two patients (1.1%) could not be evaluated.
The median duration of follow-up was 11.1 months, and the median response duration was 14.8 months.
The median progression-free survival (PFS) was 16.4 months, and the median overall survival (OS) was not reached. The median PFS in the patients with brain involvement was 18.1 months.
The most common adverse events of grade 3 or higher were a decreased neutrophil count (20.7%), anemia (8.7%), and nausea (7.6%). Most concerning was that trastuzumab deruxtecan was associated with interstitial lung disease in 13.6% of patients.
Tucatinib
Tucatinib is an oral, highly selective HER2 tyrosine kinase inhibitor (TKI). In April 2020, it was approved by the FDA, in combination with trastuzumab and capecitabine, for adult patients with advanced unresectable or metastatic HER2+ breast cancer who have received one or more prior anti-HER2–based regimens for MBC. The approval included patients with brain metastases.
The recommended tucatinib dose is 300 mg orally twice a day in combination with trastuzumab (at the standard dose) and capecitabine (1,000 mg/m2 given orally twice daily on days 1-14) on a 21-day cycle, until disease progression or unacceptable toxicity.
HER2CLIMB trial
The study that led to the approval of tucatinib was the HER2CLIMB trial. The trial enrolled 612 HER2+ MBC patients who had prior treatment with trastuzumab, pertuzumab, and T-DM1. Patients had received a median of 4 (range, 2-17) prior lines of HER2-targeted therapy.
The patients were randomized 2:1 to receive trastuzumab plus capecitabine and either tucatinib or an identical placebo twice daily.
The primary endpoint was PFS, evaluated in the initial 480 randomized patients. The median PFS was 7.8 months in the tucatinib arm and 5.6 months in the control arm (hazard ratio, 0.54; 95% confidence interval, 0.42-0.71; P < .001).
The confirmed overall response rate for patients with measurable disease was 40.6% in the tucatinib arm and 22.8% in the control arm (P = .001). The proportion of patients still in response at 12 months was 33.1% and 12.3%, respectively.
The median OS was 21.9 months in the tucatinib arm and 17.4 months in the placebo arm (HR, 0.66; 95% CI, 0.50-0.88; P = .005). At 24 months, 44.9% and 26.6% of patients, respectively, were still alive.
The most common grade 3 or higher adverse events (in the tucatinib and placebo arms, respectively) were palmar-plantar erythrodysesthesia syndrome (13.1% vs. 9.1%), diarrhea (12.9% vs. 8.6%), elevations in ALT and AST (approximately 5% vs. 0.5% for each), and fatigue (4.7% vs. 4.1%).
Tucatinib in patients with brain involvement
A unique feature of the HER2CLIMB study was that patients with MBC and untreated, symptomatic brain metastases were eligible. Patients with active, untreated central nervous system disease are excluded from virtually all other trials, especially drug-approval trials.
There were 291 patients with brain metastases in HER2CLIMB, 198 (48%) in the tucatinib arm and 93 (46%) in the control arm.
The risk of intracranial progression or death was reduced by 68% in the tucatinib arm (HR, 0.32; 95% CI, 0.22 to 0.48; P < .0001).
The 1-year CNS-PFS rate was 40.2% in the tucatinib arm and 0% in the placebo arm. The median duration of CNS-PFS was 9.9 months and 4.2 months, respectively.
The risk of death was reduced by 42% in the tucatinib arm (HR, 0.58; 95% CI, 0.40-0.85; P = .005). The median OS was 18.1 months and 12.0 months, respectively.
There were more objective responses in the brain with tucatinib (47.3%) than with placebo (20.0%; P = .03). The median duration of response was 6.8 months and 3.0 months, respectively.
Particularly because of its CNS activity and lack of serious, long-term toxicity, tucatinib combination therapy represents an attractive new option for patients with HER2+ MBC.
Neratinib
Neratinib is an irreversible pan-HER TKI that was approved by the FDA in July 2017 for extended adjuvant therapy in patients with early-stage HER2+ breast cancer, following the use of trastuzumab-based therapy.
Long-term results of the ExteNet study led to the approval for use as extended adjuvant therapy.
In February 2020, neratinib was FDA approved in combination with capecitabine for patients with HER2+ MBC after two or more prior anti-HER2–based regimens. The more recent FDA approval was based on results of the NALA trial.
NALA trial
The phase 3 NALA trial included 621 patients with HER2+ MBC who had received at least two prior anti-HER2 based regimens.
Patients were randomized 1:1 to receive neratinib at 240 mg orally once daily on days 1-21 with capecitabine at 750 mg/m2 orally twice daily on days 1-14 or lapatinib at 1,250 mg orally once daily on days 1-21 with capecitabine at 1,000 mg/m2 orally twice daily on days 1-14 for each 21-day cycle. Patients were treated until disease progression or unacceptable toxicity.
The primary endpoints were PFS and OS by blinded, independent, central review.
The median PFS was 5.6 months in the neratinib arm and 5.5 months in the lapatinib arm (HR, 0.76; 95% CI, 0.63-0.93; P = .0059). The PFS rate at 12 months was 28.8% and 14.8%, respectively.
The median OS was 21.0 months in the neratinib arm and 18.7 months in the lapatinib arm (HR, 0.88; 95% CI, 0.72-1.07; P = .2086). The ORR was 32.8% and 26.7%, respectively. The median response duration was 8.5 months and 5.6 months, respectively.
Fewer interventions for CNS disease were required in the neratinib arm than in the lapatinib arm (cumulative incidence, 22.8% vs. 29.2%; P = .043).
The most frequently reported grade 3-4 adverse reactions for the neratinib combination were diarrhea, nausea, vomiting, fatigue, and decreased appetite.
Grade 3 diarrhea occurred in 24.4% of those in the neratinib arm and 12.5% of those in the lapatinib arm. Antidiarrheal medication was used by 98.3% of patients receiving neratinib and 62.1% of patients receiving lapatinib.
Margetuximab-cmkb
Margetuximab is a chimeric Fc-engineered anti-HER2 monoclonal antibody that targets the same epitope as trastuzumab and exerts similar antiproliferative effects.
Compared with trastuzumab, margetuximab has higher affinity for both 158V (high-binding) and 158F (low-binding) alleles of the activating Fc receptor, CD16A. As a result, margetuximab enhances innate immunity, including CD16A-mediated antibody-dependent cellular cytotoxicity, more effectively than trastuzumab. Margetuximab also potentiates adaptive immunity, including enhanced clonality of the T-cell repertoire and induction of HER2-specific T- and B-cell responses.
In December 2020, margetuximab, in combination with chemotherapy, was approved by the FDA for patients with HER2+ MBC after two or more prior anti-HER2 regimens, at least one of which was for metastatic disease. The approved dose is 15 mg/kg IV every 3 weeks.
The study that led to margetuximab’s approval was the phase 3 SOPHIA trial.
SOPHIA trial
SOPHIA was a randomized trial of 536 patients with HER2+ MBC who had received prior treatment with other anti-HER2 therapies, including one to three lines of therapy for MBC.
Patients were randomly assigned 1:1 to receive margetuximab plus chemotherapy or trastuzumab plus chemotherapy. Assignment was stratified by chemotherapy choice (capecitabine, eribulin, gemcitabine, or vinorelbine), the number of previous lines of therapy for MBC, and disease extent.
Co–primary outcome measures were PFS by blinded, independent, central review and OS.
At the second interim analysis, the median PFS was 5.8 months in the margetuximab arm and 4.9 months in the trastuzumab arm (HR, 0.76; 95% CI, 0.59-0.98; P = .033). Results were more impressive in patients with CD16A genotypes containing a 158F allele. In this group, the median PFS was 6.9 months with margetuximab and 5.1 months with trastuzumab (HR, 0.68, 95% CI, 0.52-0.90; P = .005).
At the second interim analysis, the median OS was 21.6 months in the margetuximab arm and 19.8 months in the trastuzumab arm (HR, 0.89; 95% CI, 0.69-1.13; P = .33).
Subgroup data showed no differences in OS between the two arms for any subgroup except HER2+ MBC patients with an IHC score of 2 or higher. This is consistent with the postulated mechanism of action of margetuximab.
The confirmed ORR was 25% in the margetuximab arm and 14% in the trastuzumab arm, with similar durations of response between the study arms.
The most common adverse events in both arms (≥20%), regardless of causality, were fatigue, nausea, diarrhea, and neutropenia. Vomiting was common in the margetuximab arm, and anemia was common in the trastuzumab arm.
Grade 3 or higher adverse events occurred in 53.8% of patients receiving margetuximab and 52.6% of those receiving trastuzumab.
In view of margetuximab’s modest benefits in the SOPHIA trial, the ultimate role for margetuximab in HER2+ MBC may be restricted to patients with the CD16A-158F allele. A neoadjuvant trial is planned in that population.
Take-home messages
There are legitimate arguments regarding whether curing MBC is within reach for certain patient subsets, but there is no argument about whether the outlook for patients with HER2+ MBC has improved dramatically in recent years; it has.
The approval of four unique, new agents for the treatment of women with HER2+ MBC in relapse provides further improvements in outcome for these patients and distinctly different opportunities for tailoring treatment to the special circumstances of each patient (e.g., whether brain metastases are present, desire for oral therapy, comorbidities, experience with prior chemotherapy, etc).
When considered along with the potential for incorporating these drugs in earlier settings in well-designed clinical trials, these new drugs offer great promise to a group of patients who faced a dismal outcome just 2 decades ago.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Before 2001, HER2/neu-positive breast cancer (HER2+) was one of the most dreaded diagnoses a woman could face, as treatment was largely ineffective. The discovery of trastuzumab changed that dramatically.
Over the next 20 years, two additional HER2-targeted therapies – lapatinib and trastuzumab emtansine (TDM-1) – earned approval from the Food and Drug Administration for selected patients with early and late HER2+ breast cancer.
Since 2019, four additional HER2-targeted therapies have been approved by the FDA for HER2+ metastatic breast cancer (MBC), changing the treatment paradigm for those patients substantially.
The new agents are especially useful in certain patient populations. The agents offer the promise of improved survival for patients with recurrent metastatic disease and the potential for further reductions in relapse rates in earlier settings.
Trastuzumab deruxtecan
Trastuzumab deruxtecan is an antibody-drug conjugate that links three components: an anti-HER2 monoclonal antibody, a highly potent topoisomerase I inhibitor payload, and a tetrapeptide-based cleavable linker.
Trastuzumab deruxtecan has a high drug-to-antibody ratio. A membrane-permeable payload offers the potential for activity against adjacent HER2-negative cells in heterogeneous tumors. It has a long half-life (6 days).
Trastuzumab deruxtecan received accelerated approval from the FDA in December 2019 to treat patients with HER2+ MBC who have received two or more prior HER2-targeted regimens, based on the results of the DESTINY-Breast 01 trial.
DESTINY-Breast 01 trial
In the phase 2 DESTINY-Breast 01 trial, 184 patients with a median of six previous treatments received trastuzumab deruxtecan (5.4 mg/kg) intravenously every 21 days. There were 24 patients with treated, asymptomatic brain metastases who participated. Patients with untreated or symptomatic brain metastases were excluded.
Overall, a response to therapy was reported in 112 patients (60.9%), with 6.0% complete and 54.9% partial responses. Most of the patients for whom both baseline and postbaseline data were available had a reduction in tumor size.
The median time until response was 1.6 months, an interval that corresponded to the time until the first scheduled imaging. Three patients (1.6%) had progressive disease, and two patients (1.1%) could not be evaluated.
The median duration of follow-up was 11.1 months, and the median response duration was 14.8 months.
The median progression-free survival (PFS) was 16.4 months, and the median overall survival (OS) was not reached. The median PFS in the patients with brain involvement was 18.1 months.
The most common adverse events of grade 3 or higher were a decreased neutrophil count (20.7%), anemia (8.7%), and nausea (7.6%). Most concerning was that trastuzumab deruxtecan was associated with interstitial lung disease in 13.6% of patients.
Tucatinib
Tucatinib is an oral, highly selective HER2 tyrosine kinase inhibitor (TKI). In April 2020, it was approved by the FDA, in combination with trastuzumab and capecitabine, for adult patients with advanced unresectable or metastatic HER2+ breast cancer who have received one or more prior anti-HER2–based regimens for MBC. The approval included patients with brain metastases.
The recommended tucatinib dose is 300 mg orally twice a day in combination with trastuzumab (at the standard dose) and capecitabine (1,000 mg/m2 given orally twice daily on days 1-14) on a 21-day cycle, until disease progression or unacceptable toxicity.
HER2CLIMB trial
The study that led to the approval of tucatinib was the HER2CLIMB trial. The trial enrolled 612 HER2+ MBC patients who had prior treatment with trastuzumab, pertuzumab, and T-DM1. Patients had received a median of 4 (range, 2-17) prior lines of HER2-targeted therapy.
The patients were randomized 2:1 to receive trastuzumab plus capecitabine and either tucatinib or an identical placebo twice daily.
The primary endpoint was PFS, evaluated in the initial 480 randomized patients. The median PFS was 7.8 months in the tucatinib arm and 5.6 months in the control arm (hazard ratio, 0.54; 95% confidence interval, 0.42-0.71; P < .001).
The confirmed overall response rate for patients with measurable disease was 40.6% in the tucatinib arm and 22.8% in the control arm (P = .001). The proportion of patients still in response at 12 months was 33.1% and 12.3%, respectively.
The median OS was 21.9 months in the tucatinib arm and 17.4 months in the placebo arm (HR, 0.66; 95% CI, 0.50-0.88; P = .005). At 24 months, 44.9% and 26.6% of patients, respectively, were still alive.
The most common grade 3 or higher adverse events (in the tucatinib and placebo arms, respectively) were palmar-plantar erythrodysesthesia syndrome (13.1% vs. 9.1%), diarrhea (12.9% vs. 8.6%), elevations in ALT and AST (approximately 5% vs. 0.5% for each), and fatigue (4.7% vs. 4.1%).
Tucatinib in patients with brain involvement
A unique feature of the HER2CLIMB study was that patients with MBC and untreated, symptomatic brain metastases were eligible. Patients with active, untreated central nervous system disease are excluded from virtually all other trials, especially drug-approval trials.
There were 291 patients with brain metastases in HER2CLIMB, 198 (48%) in the tucatinib arm and 93 (46%) in the control arm.
The risk of intracranial progression or death was reduced by 68% in the tucatinib arm (HR, 0.32; 95% CI, 0.22 to 0.48; P < .0001).
The 1-year CNS-PFS rate was 40.2% in the tucatinib arm and 0% in the placebo arm. The median duration of CNS-PFS was 9.9 months and 4.2 months, respectively.
The risk of death was reduced by 42% in the tucatinib arm (HR, 0.58; 95% CI, 0.40-0.85; P = .005). The median OS was 18.1 months and 12.0 months, respectively.
There were more objective responses in the brain with tucatinib (47.3%) than with placebo (20.0%; P = .03). The median duration of response was 6.8 months and 3.0 months, respectively.
Particularly because of its CNS activity and lack of serious, long-term toxicity, tucatinib combination therapy represents an attractive new option for patients with HER2+ MBC.
Neratinib
Neratinib is an irreversible pan-HER TKI that was approved by the FDA in July 2017 for extended adjuvant therapy in patients with early-stage HER2+ breast cancer, following the use of trastuzumab-based therapy.
Long-term results of the ExteNet study led to the approval for use as extended adjuvant therapy.
In February 2020, neratinib was FDA approved in combination with capecitabine for patients with HER2+ MBC after two or more prior anti-HER2–based regimens. The more recent FDA approval was based on results of the NALA trial.
NALA trial
The phase 3 NALA trial included 621 patients with HER2+ MBC who had received at least two prior anti-HER2 based regimens.
Patients were randomized 1:1 to receive neratinib at 240 mg orally once daily on days 1-21 with capecitabine at 750 mg/m2 orally twice daily on days 1-14 or lapatinib at 1,250 mg orally once daily on days 1-21 with capecitabine at 1,000 mg/m2 orally twice daily on days 1-14 for each 21-day cycle. Patients were treated until disease progression or unacceptable toxicity.
The primary endpoints were PFS and OS by blinded, independent, central review.
The median PFS was 5.6 months in the neratinib arm and 5.5 months in the lapatinib arm (HR, 0.76; 95% CI, 0.63-0.93; P = .0059). The PFS rate at 12 months was 28.8% and 14.8%, respectively.
The median OS was 21.0 months in the neratinib arm and 18.7 months in the lapatinib arm (HR, 0.88; 95% CI, 0.72-1.07; P = .2086). The ORR was 32.8% and 26.7%, respectively. The median response duration was 8.5 months and 5.6 months, respectively.
Fewer interventions for CNS disease were required in the neratinib arm than in the lapatinib arm (cumulative incidence, 22.8% vs. 29.2%; P = .043).
The most frequently reported grade 3-4 adverse reactions for the neratinib combination were diarrhea, nausea, vomiting, fatigue, and decreased appetite.
Grade 3 diarrhea occurred in 24.4% of those in the neratinib arm and 12.5% of those in the lapatinib arm. Antidiarrheal medication was used by 98.3% of patients receiving neratinib and 62.1% of patients receiving lapatinib.
Margetuximab-cmkb
Margetuximab is a chimeric Fc-engineered anti-HER2 monoclonal antibody that targets the same epitope as trastuzumab and exerts similar antiproliferative effects.
Compared with trastuzumab, margetuximab has higher affinity for both 158V (high-binding) and 158F (low-binding) alleles of the activating Fc receptor, CD16A. As a result, margetuximab enhances innate immunity, including CD16A-mediated antibody-dependent cellular cytotoxicity, more effectively than trastuzumab. Margetuximab also potentiates adaptive immunity, including enhanced clonality of the T-cell repertoire and induction of HER2-specific T- and B-cell responses.
In December 2020, margetuximab, in combination with chemotherapy, was approved by the FDA for patients with HER2+ MBC after two or more prior anti-HER2 regimens, at least one of which was for metastatic disease. The approved dose is 15 mg/kg IV every 3 weeks.
The study that led to margetuximab’s approval was the phase 3 SOPHIA trial.
SOPHIA trial
SOPHIA was a randomized trial of 536 patients with HER2+ MBC who had received prior treatment with other anti-HER2 therapies, including one to three lines of therapy for MBC.
Patients were randomly assigned 1:1 to receive margetuximab plus chemotherapy or trastuzumab plus chemotherapy. Assignment was stratified by chemotherapy choice (capecitabine, eribulin, gemcitabine, or vinorelbine), the number of previous lines of therapy for MBC, and disease extent.
Co–primary outcome measures were PFS by blinded, independent, central review and OS.
At the second interim analysis, the median PFS was 5.8 months in the margetuximab arm and 4.9 months in the trastuzumab arm (HR, 0.76; 95% CI, 0.59-0.98; P = .033). Results were more impressive in patients with CD16A genotypes containing a 158F allele. In this group, the median PFS was 6.9 months with margetuximab and 5.1 months with trastuzumab (HR, 0.68, 95% CI, 0.52-0.90; P = .005).
At the second interim analysis, the median OS was 21.6 months in the margetuximab arm and 19.8 months in the trastuzumab arm (HR, 0.89; 95% CI, 0.69-1.13; P = .33).
Subgroup data showed no differences in OS between the two arms for any subgroup except HER2+ MBC patients with an IHC score of 2 or higher. This is consistent with the postulated mechanism of action of margetuximab.
The confirmed ORR was 25% in the margetuximab arm and 14% in the trastuzumab arm, with similar durations of response between the study arms.
The most common adverse events in both arms (≥20%), regardless of causality, were fatigue, nausea, diarrhea, and neutropenia. Vomiting was common in the margetuximab arm, and anemia was common in the trastuzumab arm.
Grade 3 or higher adverse events occurred in 53.8% of patients receiving margetuximab and 52.6% of those receiving trastuzumab.
In view of margetuximab’s modest benefits in the SOPHIA trial, the ultimate role for margetuximab in HER2+ MBC may be restricted to patients with the CD16A-158F allele. A neoadjuvant trial is planned in that population.
Take-home messages
There are legitimate arguments regarding whether curing MBC is within reach for certain patient subsets, but there is no argument about whether the outlook for patients with HER2+ MBC has improved dramatically in recent years; it has.
The approval of four unique, new agents for the treatment of women with HER2+ MBC in relapse provides further improvements in outcome for these patients and distinctly different opportunities for tailoring treatment to the special circumstances of each patient (e.g., whether brain metastases are present, desire for oral therapy, comorbidities, experience with prior chemotherapy, etc).
When considered along with the potential for incorporating these drugs in earlier settings in well-designed clinical trials, these new drugs offer great promise to a group of patients who faced a dismal outcome just 2 decades ago.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Before 2001, HER2/neu-positive breast cancer (HER2+) was one of the most dreaded diagnoses a woman could face, as treatment was largely ineffective. The discovery of trastuzumab changed that dramatically.
Over the next 20 years, two additional HER2-targeted therapies – lapatinib and trastuzumab emtansine (TDM-1) – earned approval from the Food and Drug Administration for selected patients with early and late HER2+ breast cancer.
Since 2019, four additional HER2-targeted therapies have been approved by the FDA for HER2+ metastatic breast cancer (MBC), changing the treatment paradigm for those patients substantially.
The new agents are especially useful in certain patient populations. The agents offer the promise of improved survival for patients with recurrent metastatic disease and the potential for further reductions in relapse rates in earlier settings.
Trastuzumab deruxtecan
Trastuzumab deruxtecan is an antibody-drug conjugate that links three components: an anti-HER2 monoclonal antibody, a highly potent topoisomerase I inhibitor payload, and a tetrapeptide-based cleavable linker.
Trastuzumab deruxtecan has a high drug-to-antibody ratio. A membrane-permeable payload offers the potential for activity against adjacent HER2-negative cells in heterogeneous tumors. It has a long half-life (6 days).
Trastuzumab deruxtecan received accelerated approval from the FDA in December 2019 to treat patients with HER2+ MBC who have received two or more prior HER2-targeted regimens, based on the results of the DESTINY-Breast 01 trial.
DESTINY-Breast 01 trial
In the phase 2 DESTINY-Breast 01 trial, 184 patients with a median of six previous treatments received trastuzumab deruxtecan (5.4 mg/kg) intravenously every 21 days. There were 24 patients with treated, asymptomatic brain metastases who participated. Patients with untreated or symptomatic brain metastases were excluded.
Overall, a response to therapy was reported in 112 patients (60.9%), with 6.0% complete and 54.9% partial responses. Most of the patients for whom both baseline and postbaseline data were available had a reduction in tumor size.
The median time until response was 1.6 months, an interval that corresponded to the time until the first scheduled imaging. Three patients (1.6%) had progressive disease, and two patients (1.1%) could not be evaluated.
The median duration of follow-up was 11.1 months, and the median response duration was 14.8 months.
The median progression-free survival (PFS) was 16.4 months, and the median overall survival (OS) was not reached. The median PFS in the patients with brain involvement was 18.1 months.
The most common adverse events of grade 3 or higher were a decreased neutrophil count (20.7%), anemia (8.7%), and nausea (7.6%). Most concerning was that trastuzumab deruxtecan was associated with interstitial lung disease in 13.6% of patients.
Tucatinib
Tucatinib is an oral, highly selective HER2 tyrosine kinase inhibitor (TKI). In April 2020, it was approved by the FDA, in combination with trastuzumab and capecitabine, for adult patients with advanced unresectable or metastatic HER2+ breast cancer who have received one or more prior anti-HER2–based regimens for MBC. The approval included patients with brain metastases.
The recommended tucatinib dose is 300 mg orally twice a day in combination with trastuzumab (at the standard dose) and capecitabine (1,000 mg/m2 given orally twice daily on days 1-14) on a 21-day cycle, until disease progression or unacceptable toxicity.
HER2CLIMB trial
The study that led to the approval of tucatinib was the HER2CLIMB trial. The trial enrolled 612 HER2+ MBC patients who had prior treatment with trastuzumab, pertuzumab, and T-DM1. Patients had received a median of 4 (range, 2-17) prior lines of HER2-targeted therapy.
The patients were randomized 2:1 to receive trastuzumab plus capecitabine and either tucatinib or an identical placebo twice daily.
The primary endpoint was PFS, evaluated in the initial 480 randomized patients. The median PFS was 7.8 months in the tucatinib arm and 5.6 months in the control arm (hazard ratio, 0.54; 95% confidence interval, 0.42-0.71; P < .001).
The confirmed overall response rate for patients with measurable disease was 40.6% in the tucatinib arm and 22.8% in the control arm (P = .001). The proportion of patients still in response at 12 months was 33.1% and 12.3%, respectively.
The median OS was 21.9 months in the tucatinib arm and 17.4 months in the placebo arm (HR, 0.66; 95% CI, 0.50-0.88; P = .005). At 24 months, 44.9% and 26.6% of patients, respectively, were still alive.
The most common grade 3 or higher adverse events (in the tucatinib and placebo arms, respectively) were palmar-plantar erythrodysesthesia syndrome (13.1% vs. 9.1%), diarrhea (12.9% vs. 8.6%), elevations in ALT and AST (approximately 5% vs. 0.5% for each), and fatigue (4.7% vs. 4.1%).
Tucatinib in patients with brain involvement
A unique feature of the HER2CLIMB study was that patients with MBC and untreated, symptomatic brain metastases were eligible. Patients with active, untreated central nervous system disease are excluded from virtually all other trials, especially drug-approval trials.
There were 291 patients with brain metastases in HER2CLIMB, 198 (48%) in the tucatinib arm and 93 (46%) in the control arm.
The risk of intracranial progression or death was reduced by 68% in the tucatinib arm (HR, 0.32; 95% CI, 0.22 to 0.48; P < .0001).
The 1-year CNS-PFS rate was 40.2% in the tucatinib arm and 0% in the placebo arm. The median duration of CNS-PFS was 9.9 months and 4.2 months, respectively.
The risk of death was reduced by 42% in the tucatinib arm (HR, 0.58; 95% CI, 0.40-0.85; P = .005). The median OS was 18.1 months and 12.0 months, respectively.
There were more objective responses in the brain with tucatinib (47.3%) than with placebo (20.0%; P = .03). The median duration of response was 6.8 months and 3.0 months, respectively.
Particularly because of its CNS activity and lack of serious, long-term toxicity, tucatinib combination therapy represents an attractive new option for patients with HER2+ MBC.
Neratinib
Neratinib is an irreversible pan-HER TKI that was approved by the FDA in July 2017 for extended adjuvant therapy in patients with early-stage HER2+ breast cancer, following the use of trastuzumab-based therapy.
Long-term results of the ExteNet study led to the approval for use as extended adjuvant therapy.
In February 2020, neratinib was FDA approved in combination with capecitabine for patients with HER2+ MBC after two or more prior anti-HER2–based regimens. The more recent FDA approval was based on results of the NALA trial.
NALA trial
The phase 3 NALA trial included 621 patients with HER2+ MBC who had received at least two prior anti-HER2 based regimens.
Patients were randomized 1:1 to receive neratinib at 240 mg orally once daily on days 1-21 with capecitabine at 750 mg/m2 orally twice daily on days 1-14 or lapatinib at 1,250 mg orally once daily on days 1-21 with capecitabine at 1,000 mg/m2 orally twice daily on days 1-14 for each 21-day cycle. Patients were treated until disease progression or unacceptable toxicity.
The primary endpoints were PFS and OS by blinded, independent, central review.
The median PFS was 5.6 months in the neratinib arm and 5.5 months in the lapatinib arm (HR, 0.76; 95% CI, 0.63-0.93; P = .0059). The PFS rate at 12 months was 28.8% and 14.8%, respectively.
The median OS was 21.0 months in the neratinib arm and 18.7 months in the lapatinib arm (HR, 0.88; 95% CI, 0.72-1.07; P = .2086). The ORR was 32.8% and 26.7%, respectively. The median response duration was 8.5 months and 5.6 months, respectively.
Fewer interventions for CNS disease were required in the neratinib arm than in the lapatinib arm (cumulative incidence, 22.8% vs. 29.2%; P = .043).
The most frequently reported grade 3-4 adverse reactions for the neratinib combination were diarrhea, nausea, vomiting, fatigue, and decreased appetite.
Grade 3 diarrhea occurred in 24.4% of those in the neratinib arm and 12.5% of those in the lapatinib arm. Antidiarrheal medication was used by 98.3% of patients receiving neratinib and 62.1% of patients receiving lapatinib.
Margetuximab-cmkb
Margetuximab is a chimeric Fc-engineered anti-HER2 monoclonal antibody that targets the same epitope as trastuzumab and exerts similar antiproliferative effects.
Compared with trastuzumab, margetuximab has higher affinity for both 158V (high-binding) and 158F (low-binding) alleles of the activating Fc receptor, CD16A. As a result, margetuximab enhances innate immunity, including CD16A-mediated antibody-dependent cellular cytotoxicity, more effectively than trastuzumab. Margetuximab also potentiates adaptive immunity, including enhanced clonality of the T-cell repertoire and induction of HER2-specific T- and B-cell responses.
In December 2020, margetuximab, in combination with chemotherapy, was approved by the FDA for patients with HER2+ MBC after two or more prior anti-HER2 regimens, at least one of which was for metastatic disease. The approved dose is 15 mg/kg IV every 3 weeks.
The study that led to margetuximab’s approval was the phase 3 SOPHIA trial.
SOPHIA trial
SOPHIA was a randomized trial of 536 patients with HER2+ MBC who had received prior treatment with other anti-HER2 therapies, including one to three lines of therapy for MBC.
Patients were randomly assigned 1:1 to receive margetuximab plus chemotherapy or trastuzumab plus chemotherapy. Assignment was stratified by chemotherapy choice (capecitabine, eribulin, gemcitabine, or vinorelbine), the number of previous lines of therapy for MBC, and disease extent.
Co–primary outcome measures were PFS by blinded, independent, central review and OS.
At the second interim analysis, the median PFS was 5.8 months in the margetuximab arm and 4.9 months in the trastuzumab arm (HR, 0.76; 95% CI, 0.59-0.98; P = .033). Results were more impressive in patients with CD16A genotypes containing a 158F allele. In this group, the median PFS was 6.9 months with margetuximab and 5.1 months with trastuzumab (HR, 0.68, 95% CI, 0.52-0.90; P = .005).
At the second interim analysis, the median OS was 21.6 months in the margetuximab arm and 19.8 months in the trastuzumab arm (HR, 0.89; 95% CI, 0.69-1.13; P = .33).
Subgroup data showed no differences in OS between the two arms for any subgroup except HER2+ MBC patients with an IHC score of 2 or higher. This is consistent with the postulated mechanism of action of margetuximab.
The confirmed ORR was 25% in the margetuximab arm and 14% in the trastuzumab arm, with similar durations of response between the study arms.
The most common adverse events in both arms (≥20%), regardless of causality, were fatigue, nausea, diarrhea, and neutropenia. Vomiting was common in the margetuximab arm, and anemia was common in the trastuzumab arm.
Grade 3 or higher adverse events occurred in 53.8% of patients receiving margetuximab and 52.6% of those receiving trastuzumab.
In view of margetuximab’s modest benefits in the SOPHIA trial, the ultimate role for margetuximab in HER2+ MBC may be restricted to patients with the CD16A-158F allele. A neoadjuvant trial is planned in that population.
Take-home messages
There are legitimate arguments regarding whether curing MBC is within reach for certain patient subsets, but there is no argument about whether the outlook for patients with HER2+ MBC has improved dramatically in recent years; it has.
The approval of four unique, new agents for the treatment of women with HER2+ MBC in relapse provides further improvements in outcome for these patients and distinctly different opportunities for tailoring treatment to the special circumstances of each patient (e.g., whether brain metastases are present, desire for oral therapy, comorbidities, experience with prior chemotherapy, etc).
When considered along with the potential for incorporating these drugs in earlier settings in well-designed clinical trials, these new drugs offer great promise to a group of patients who faced a dismal outcome just 2 decades ago.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
GELATO trial: Chemoimmunotherapy may help in metastatic invasive lobular breast cancer
The PD-L1 inhibitor atezolizumab (Tecentriq) combined with carboplatin has shown signs of clinical activity in women with metastatic invasive lobular breast cancer (ILC) according to the first results to come from the ongoing GELATO trial.
The 6-month objective response rate was 19%, based on 4 of 21 patients who could be evaluated exhibiting a partial response to the chemoimmunotherapy. A further two (10%) patients had stable disease, meaning that clinical benefit rate was 29%.
GELATO (AssessinG Efficacy of Carboplatin and ATezOlizumab in Metastatic Lobular Breast Cancer) is a phase 2 trial being conducted at four Dutch centers. The primary premise of the study is that “there’s an immune-related subtype of ILC,” researcher Leonie Voorwerk, BSc, reported at the European Society for Medical Oncology: Breast Cancer virtual meeting (Abstract LBA3).
This ILC subtype is “characterized by high expression of immune-related genes and high levels of TILs [tumor-infiltrating lymphocytes] and PDL-1,” said Ms. Voorwerk, a PhD student working with medical oncologist Marleen Kok, MD, PhD, at the Netherlands Cancer Institute in Amsterdam.
Furthermore, she added, in vitro data suggest sensitivity of immune-related-ILCs to platinum and there is preclinical work showing that there is synergy between platinum-based chemotherapy and checkpoint blockade.
First chemoimmunotherapy trial in lobular cancer setting
GELATO is a significant trial as it is “the first chemoimmunotherapy trial in metastatic lobular breast cancer,” said Sylvia Adams, MD, professor of medicine and director of the Breast Cancer Center at NYU Langone Health in New York City.
“Of note, the further research should include the immune-related genes and TMB [tumor mutational burden],” proposed Dr. Adams, who was not involved in the trial.
“We should look to tumor mutational burden because while it is not typically high in early disease, metastatic lesions can have higher TMB,” she explained. “Also, metastatic ILC is known to have higher tumor mutational burden compared to IDC [invasive ductal carcinoma], so this is an important thing along with the clinical factors as described in looking at outcomes.”
Trial design and patient characteristics
GELATO is a single-arm, nonrandomized trial in which 37 patients with metastatic ILC were screened for inclusion between November 2017 and January 2021. A total of 26 of these patients were registered for the trial, and 23 have so far received at least one cycle of atezolizumab.
Prerequisites for entry into the trial were that patients had to have negative or aberrant E-cadherin, a characteristic feature of ILC. Patients with estrogen receptor (ER)-positive (ER+) disease could be included, but they had to be proven to be resistant to endocrine therapies. No more than two prior lines of palliative chemotherapy were allowed, and all participants had to have lactose dehydrogenase levels of less than 2 times the upper limit of normal.
Patients were then treated with up to 12 cycles of weekly carboplatin (AUC = 1.5 mg/mL/min), with atezolizumab (1,200 mg) added in from cycle 3 onward. Treatment was continued until disease progression or unacceptable toxicity occurred.
“Baseline characteristics were mainly as expected for this patient population,” Ms. Voorwerk stated. Patients were aged 45-89 years, with a median of 60 years. Around half each had a WHO performance status of 0 or 1, and around half each had one to two or three or more metastatic sites; 78% had liver metastases.
“But I want to highlight that we included five patients with the triple-negative ILC,” said Ms. Voorwerk, also highlighting that approximately 50% of patients had received prior palliative chemotherapy. Later in her presentation she noted that four out of the six patients that showed any clinical benefit had triple negative disease.
Key findings and next steps
The primary endpoint was progression-free survival (PFS) at 6 months, with secondary endpoints of the best overall response rate, PFS at 1 year, overall survival, and safety.
While details of the latter three endpoints are yet to be reported, Ms. Voorwerk noted that there was a median duration of response of 12 weeks and the median PFS was 15 weeks. The primary endpoint of PFS was met as four patients were free of progression at 6 months and the statistical method used called for patients to be progression free at this time point.
“We observed that stromal TILs and CD8+ cells were not associated with clinical benefits,” said Ms. Voorwerk. There was, however, “a slight trend” toward higher PD-L1 expression in responding patients.
“Further translational research is needed to provide the rationale for new strategies to improve checkpoint blockade in patients with lobular breast cancer,” she concluded.
Dr. Adams concurred, adding that a future research question was whether either atezolizumab or carboplatin was contributing to the response. This is “difficult to tell as the study was a single arm trial.”
Another question, said Dr. Adams, is are “anti-CDK 4/6 inhibitors helpful in improving response rates and durability?” In the trial, 70% of patients had prior exposure to CDK 4/6 inhibitors.
The GELATO trial was sponsored by the Netherlands Cancer Institute with funding from Roche Pharma AG. Ms. Voorwerk had nothing to disclose. Dr. Adams disclosed uncompensated consulting or advisory roles with Bristol-Myers Squibb, Genentech, and Merck from whom she has received research funding. Dr. Adams also disclosed research funding from Amgen, Celgene, and Novartis.
The PD-L1 inhibitor atezolizumab (Tecentriq) combined with carboplatin has shown signs of clinical activity in women with metastatic invasive lobular breast cancer (ILC) according to the first results to come from the ongoing GELATO trial.
The 6-month objective response rate was 19%, based on 4 of 21 patients who could be evaluated exhibiting a partial response to the chemoimmunotherapy. A further two (10%) patients had stable disease, meaning that clinical benefit rate was 29%.
GELATO (AssessinG Efficacy of Carboplatin and ATezOlizumab in Metastatic Lobular Breast Cancer) is a phase 2 trial being conducted at four Dutch centers. The primary premise of the study is that “there’s an immune-related subtype of ILC,” researcher Leonie Voorwerk, BSc, reported at the European Society for Medical Oncology: Breast Cancer virtual meeting (Abstract LBA3).
This ILC subtype is “characterized by high expression of immune-related genes and high levels of TILs [tumor-infiltrating lymphocytes] and PDL-1,” said Ms. Voorwerk, a PhD student working with medical oncologist Marleen Kok, MD, PhD, at the Netherlands Cancer Institute in Amsterdam.
Furthermore, she added, in vitro data suggest sensitivity of immune-related-ILCs to platinum and there is preclinical work showing that there is synergy between platinum-based chemotherapy and checkpoint blockade.
First chemoimmunotherapy trial in lobular cancer setting
GELATO is a significant trial as it is “the first chemoimmunotherapy trial in metastatic lobular breast cancer,” said Sylvia Adams, MD, professor of medicine and director of the Breast Cancer Center at NYU Langone Health in New York City.
“Of note, the further research should include the immune-related genes and TMB [tumor mutational burden],” proposed Dr. Adams, who was not involved in the trial.
“We should look to tumor mutational burden because while it is not typically high in early disease, metastatic lesions can have higher TMB,” she explained. “Also, metastatic ILC is known to have higher tumor mutational burden compared to IDC [invasive ductal carcinoma], so this is an important thing along with the clinical factors as described in looking at outcomes.”
Trial design and patient characteristics
GELATO is a single-arm, nonrandomized trial in which 37 patients with metastatic ILC were screened for inclusion between November 2017 and January 2021. A total of 26 of these patients were registered for the trial, and 23 have so far received at least one cycle of atezolizumab.
Prerequisites for entry into the trial were that patients had to have negative or aberrant E-cadherin, a characteristic feature of ILC. Patients with estrogen receptor (ER)-positive (ER+) disease could be included, but they had to be proven to be resistant to endocrine therapies. No more than two prior lines of palliative chemotherapy were allowed, and all participants had to have lactose dehydrogenase levels of less than 2 times the upper limit of normal.
Patients were then treated with up to 12 cycles of weekly carboplatin (AUC = 1.5 mg/mL/min), with atezolizumab (1,200 mg) added in from cycle 3 onward. Treatment was continued until disease progression or unacceptable toxicity occurred.
“Baseline characteristics were mainly as expected for this patient population,” Ms. Voorwerk stated. Patients were aged 45-89 years, with a median of 60 years. Around half each had a WHO performance status of 0 or 1, and around half each had one to two or three or more metastatic sites; 78% had liver metastases.
“But I want to highlight that we included five patients with the triple-negative ILC,” said Ms. Voorwerk, also highlighting that approximately 50% of patients had received prior palliative chemotherapy. Later in her presentation she noted that four out of the six patients that showed any clinical benefit had triple negative disease.
Key findings and next steps
The primary endpoint was progression-free survival (PFS) at 6 months, with secondary endpoints of the best overall response rate, PFS at 1 year, overall survival, and safety.
While details of the latter three endpoints are yet to be reported, Ms. Voorwerk noted that there was a median duration of response of 12 weeks and the median PFS was 15 weeks. The primary endpoint of PFS was met as four patients were free of progression at 6 months and the statistical method used called for patients to be progression free at this time point.
“We observed that stromal TILs and CD8+ cells were not associated with clinical benefits,” said Ms. Voorwerk. There was, however, “a slight trend” toward higher PD-L1 expression in responding patients.
“Further translational research is needed to provide the rationale for new strategies to improve checkpoint blockade in patients with lobular breast cancer,” she concluded.
Dr. Adams concurred, adding that a future research question was whether either atezolizumab or carboplatin was contributing to the response. This is “difficult to tell as the study was a single arm trial.”
Another question, said Dr. Adams, is are “anti-CDK 4/6 inhibitors helpful in improving response rates and durability?” In the trial, 70% of patients had prior exposure to CDK 4/6 inhibitors.
The GELATO trial was sponsored by the Netherlands Cancer Institute with funding from Roche Pharma AG. Ms. Voorwerk had nothing to disclose. Dr. Adams disclosed uncompensated consulting or advisory roles with Bristol-Myers Squibb, Genentech, and Merck from whom she has received research funding. Dr. Adams also disclosed research funding from Amgen, Celgene, and Novartis.
The PD-L1 inhibitor atezolizumab (Tecentriq) combined with carboplatin has shown signs of clinical activity in women with metastatic invasive lobular breast cancer (ILC) according to the first results to come from the ongoing GELATO trial.
The 6-month objective response rate was 19%, based on 4 of 21 patients who could be evaluated exhibiting a partial response to the chemoimmunotherapy. A further two (10%) patients had stable disease, meaning that clinical benefit rate was 29%.
GELATO (AssessinG Efficacy of Carboplatin and ATezOlizumab in Metastatic Lobular Breast Cancer) is a phase 2 trial being conducted at four Dutch centers. The primary premise of the study is that “there’s an immune-related subtype of ILC,” researcher Leonie Voorwerk, BSc, reported at the European Society for Medical Oncology: Breast Cancer virtual meeting (Abstract LBA3).
This ILC subtype is “characterized by high expression of immune-related genes and high levels of TILs [tumor-infiltrating lymphocytes] and PDL-1,” said Ms. Voorwerk, a PhD student working with medical oncologist Marleen Kok, MD, PhD, at the Netherlands Cancer Institute in Amsterdam.
Furthermore, she added, in vitro data suggest sensitivity of immune-related-ILCs to platinum and there is preclinical work showing that there is synergy between platinum-based chemotherapy and checkpoint blockade.
First chemoimmunotherapy trial in lobular cancer setting
GELATO is a significant trial as it is “the first chemoimmunotherapy trial in metastatic lobular breast cancer,” said Sylvia Adams, MD, professor of medicine and director of the Breast Cancer Center at NYU Langone Health in New York City.
“Of note, the further research should include the immune-related genes and TMB [tumor mutational burden],” proposed Dr. Adams, who was not involved in the trial.
“We should look to tumor mutational burden because while it is not typically high in early disease, metastatic lesions can have higher TMB,” she explained. “Also, metastatic ILC is known to have higher tumor mutational burden compared to IDC [invasive ductal carcinoma], so this is an important thing along with the clinical factors as described in looking at outcomes.”
Trial design and patient characteristics
GELATO is a single-arm, nonrandomized trial in which 37 patients with metastatic ILC were screened for inclusion between November 2017 and January 2021. A total of 26 of these patients were registered for the trial, and 23 have so far received at least one cycle of atezolizumab.
Prerequisites for entry into the trial were that patients had to have negative or aberrant E-cadherin, a characteristic feature of ILC. Patients with estrogen receptor (ER)-positive (ER+) disease could be included, but they had to be proven to be resistant to endocrine therapies. No more than two prior lines of palliative chemotherapy were allowed, and all participants had to have lactose dehydrogenase levels of less than 2 times the upper limit of normal.
Patients were then treated with up to 12 cycles of weekly carboplatin (AUC = 1.5 mg/mL/min), with atezolizumab (1,200 mg) added in from cycle 3 onward. Treatment was continued until disease progression or unacceptable toxicity occurred.
“Baseline characteristics were mainly as expected for this patient population,” Ms. Voorwerk stated. Patients were aged 45-89 years, with a median of 60 years. Around half each had a WHO performance status of 0 or 1, and around half each had one to two or three or more metastatic sites; 78% had liver metastases.
“But I want to highlight that we included five patients with the triple-negative ILC,” said Ms. Voorwerk, also highlighting that approximately 50% of patients had received prior palliative chemotherapy. Later in her presentation she noted that four out of the six patients that showed any clinical benefit had triple negative disease.
Key findings and next steps
The primary endpoint was progression-free survival (PFS) at 6 months, with secondary endpoints of the best overall response rate, PFS at 1 year, overall survival, and safety.
While details of the latter three endpoints are yet to be reported, Ms. Voorwerk noted that there was a median duration of response of 12 weeks and the median PFS was 15 weeks. The primary endpoint of PFS was met as four patients were free of progression at 6 months and the statistical method used called for patients to be progression free at this time point.
“We observed that stromal TILs and CD8+ cells were not associated with clinical benefits,” said Ms. Voorwerk. There was, however, “a slight trend” toward higher PD-L1 expression in responding patients.
“Further translational research is needed to provide the rationale for new strategies to improve checkpoint blockade in patients with lobular breast cancer,” she concluded.
Dr. Adams concurred, adding that a future research question was whether either atezolizumab or carboplatin was contributing to the response. This is “difficult to tell as the study was a single arm trial.”
Another question, said Dr. Adams, is are “anti-CDK 4/6 inhibitors helpful in improving response rates and durability?” In the trial, 70% of patients had prior exposure to CDK 4/6 inhibitors.
The GELATO trial was sponsored by the Netherlands Cancer Institute with funding from Roche Pharma AG. Ms. Voorwerk had nothing to disclose. Dr. Adams disclosed uncompensated consulting or advisory roles with Bristol-Myers Squibb, Genentech, and Merck from whom she has received research funding. Dr. Adams also disclosed research funding from Amgen, Celgene, and Novartis.
FROM ESMO BREAST CANCER 2021
FDA panel votes against 2 cancer indications but backs 4 of 6
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.