User login
Bedside Ultrasound for Pulsatile Hand Mass
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
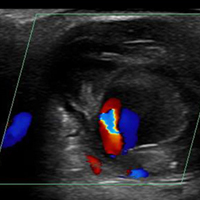
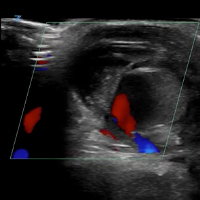
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
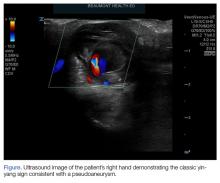
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
VIDEO: Ultrasound with Doppler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Initial Bedside Ultrasound of Pulsatile Hand Mass