User login
Positivity Rates in Oropharyngeal and Nonoropharyngeal Head and Neck Cancer in the VA
Head and neck cancer (HNC) continues to be a major health issue with an estimated 51,540 cases in the US in 2018, making it the eighth most common cancer among men with an estimated 4% of all new cancer diagnoses.1 Over the past decade, human papillomavirus (HPV) has emerged as a major prognostic factor for survival in squamous cell carcinomas of the oropharynx. Patients who are HPV-positive (HPV+) have a much higher survival rate than patients who have HPV-negative (HPV-) cancers of the oropharynx. The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual has 2 distinct stagings for HPV+ and HPV- oropharyngeal tumors using p16-positivity (p16+) as a surrogate marker.2
Squamous cell carcinomas of the oropharynx that are HPV+ have about half the risk of death of HPV- tumors, are highly responsive to treatment, and are more often seen in younger and healthier patients with little to no tobacco use.2,3 As such, there also is a movement to de-escalate HPV+ oropharyngeal cancers with multiple trials by either replacing cytotoxic chemotherapy with a targeted agent (cisplatin vs cetuximab in RTOG 1016) or reducing the radiation dose (ECOG 1308, NRG HN002, Quarterback, and OPTIMA trials).3
The focus of many epidemiologic studies has been in the HNC general population. A recent epidemiologic analysis of the HNC general population found a p16 positivity rate of 60% in oropharyngeal squamous cell carcinomas (OPSCC) and 10% in nonoropharyngeal squamous cell carcinomas (NOPSCC).4 There has been a lack of studies focusing on the US Department of Veterans Administration (VA) population. The VA HNC population consists mostly of older white male smokers; whereas the rise of OPSCC in the general population consists primarily of males aged < 60 years often with little or no tobacco use.5 Furthermore, the importance of p16 positivity in NOPSCC also may be prognostic.6 Population data on this subset in the VA are lacking as well.This study’s purpose is to analyze the p16 positivity rate in both the OPSCC and NOPSCC in the VA population. Elucidation of epidemiologic factors that are associated with these groups may bring to light important differences between the VA and general HNC populations.
Methods
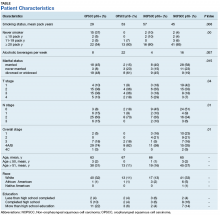
A review of the Kansas City VA Medical Center database for patients with HNC was performed from 2011 to 2017. The review consisted of 183 patient records (second primaries were scored separately), and 123 were deemed eligible for the study. Epidemiologic data were collected, including site, OPSCC vs NOPSCC, age, race, education level, tobacco use, alcohol use, TNM stage, and marital status (Table).
Results
The NOPSCC p16+ group had the greatest mean pack-year use (57). The lowest was in the OPSCC p16+ group (29). The OPSCC p16+ group had 37% never smokers compared with ≤ 10% for the other groups. Both the OPSCC and NOPSCC p16- groups had much more alcohol use per week than that of the p16+ groups. The differences in marital status included a lower rate of never married individuals in the p16+ group and a higher rate of marriage in the NOPSCC p16- group. The T stage distribution within the OPSCC groups was similar, but NOPSCC groups saw more T1 lesions in the NOPSCC p16- group (42% p16- vs 18% p16+). Conversely, more T4 lesions were found in the NOPSCC p16+ patients (7% p16- vs 29% p16+).
Discussion
The overall HPV positivity rate in the general population of patients with HNC has been reported as between 57% and 72% for OPSCC and between 1.3% and 7% for NOPSCC.6 One study, however, examined the p16 positivity rate in NOPSCC patients enrolled in major trials (RTOG 0129, 0234, and 0522 studies) and found that up to 19.3% of NOPSCC patients had p16 positivity.6 Even with the near 20% rate in those aforementioned trials that are above the reported norm, the current study found that nearly 30% of its VA population had p16+ NOPSCC. It has been shown that regardless of site, HPV-driven head and neck tumors share a similar gene expression and DNA methylation profiles (nonkeratinizing, basaloid histopathologic features, and lack of TP53 or CDKN2A alterations).5 p16+ NOPSCC has a different immune microenvironment with less lymphocyte infiltration, and there is some debate in the literature about the effects on tumor outcomes for NOPSCC cancer.5
In the aforementioned RTOG trials, p16- NOPSCC had worse outcomes compared with those of p16+ NOPSCC.6 This result is in contrast to the Danish Head and Neck Cancer Group (DAHANCA) and the combined Johns Hopkins University (JHU) and University of California, San Francisco (UCSF) data that found no difference between p16+ NOPSCC or p16- NOPSCC.7,8 In regards to race, this study did not find any differences. Another UCSF and JHU study showed lower p16+ rates in African American patients with OPSCC, but no distinction between race in the NOPSCC group. This result is consistent with the data in the current study as the distribution of race was no different among the 4 groups; however, this study's cohort was 90% white, 10% African American, and only < 1% Native American.4 This study's cohort population also was consistent with HPV-positive tumors presenting with earlier T, but higher N staging.9
Smoking is known to decrease survival in HPV-positive HNC, with the RTOG 0129 study separating head and neck tumors into low, medium, and high risk, based on HPV status, smoking, and stage.10 Although the average smoking pack-years in the current study’s OPC p16+ group was high at 29 pack-years, there was still a significant number of nonsmokers in that same group (37%). The University of Michigan conducted a study that had a similar profile of patients with an average age of 56.5 and 32.4% never smokers in their p16+ OPSCC cohort; thus, the VA p16+ OPSCC group in this study may be similar to the general population's p16+ OPSCC group.11 Nonmonogamous relationships also have been shown to be a risk factor for HPV positivity, and there was a difference in marital status (assuming it was a surrogate for monogamy) between the 4 groups; however, in contrast, the p16+ group in the current study had a high number of married patients, 45% in OPC p16+ group, and may not have been a good surrogate for monogamy in this VA population.
Limitations
Limitations of this study include all the caveats that come with a retrospective study, such as confounding variables, unbalanced groups, and selection bias. A detailed sexual history was not included, although it is well known that sexual activity is linked with oral HPV positivity.12 Human papillomavirus positivity based on p16 immunohistochemical analysis also was used as a surrogate marker for HPV instead of DNA in situ hybridization. The data also may be skewed due to the study patient’s being predominantly white and male: Both groups have a higher predilection for HPV-driven HNCs.13
Conclusion
The proportion of p16+ VA OPSCC cases was similar to that of the general population at 75% with 37% never smokers, but the percentage in NOPSCC was higher at 29% with only 10% never smokers. The p16+ NOPSCC also presented with more T4 lesions and a higher overall stage compared with p16- NOPSCC. Further studies are needed to compare these subgroups in the VA and in the general HNC populations.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122-137.
3. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11.
4. D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177.
5. Chakravarthy A, Henderson S, Thirdborough SM, et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34(34):4132-4141.
6. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930-3938.
7. Lassen P, Primdahl H, Johansen J, et al; Danish Head and Neck Cancer Group (DAHANCA). Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310-316.
8. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566-1575.
9. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299-309.
10. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35.
11. Maxwell, JH, Kumar B, Feng FY, et al. Tobacco use in HPV-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226-1235.
12. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703.
13. Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50(6):565-574.
Head and neck cancer (HNC) continues to be a major health issue with an estimated 51,540 cases in the US in 2018, making it the eighth most common cancer among men with an estimated 4% of all new cancer diagnoses.1 Over the past decade, human papillomavirus (HPV) has emerged as a major prognostic factor for survival in squamous cell carcinomas of the oropharynx. Patients who are HPV-positive (HPV+) have a much higher survival rate than patients who have HPV-negative (HPV-) cancers of the oropharynx. The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual has 2 distinct stagings for HPV+ and HPV- oropharyngeal tumors using p16-positivity (p16+) as a surrogate marker.2
Squamous cell carcinomas of the oropharynx that are HPV+ have about half the risk of death of HPV- tumors, are highly responsive to treatment, and are more often seen in younger and healthier patients with little to no tobacco use.2,3 As such, there also is a movement to de-escalate HPV+ oropharyngeal cancers with multiple trials by either replacing cytotoxic chemotherapy with a targeted agent (cisplatin vs cetuximab in RTOG 1016) or reducing the radiation dose (ECOG 1308, NRG HN002, Quarterback, and OPTIMA trials).3
The focus of many epidemiologic studies has been in the HNC general population. A recent epidemiologic analysis of the HNC general population found a p16 positivity rate of 60% in oropharyngeal squamous cell carcinomas (OPSCC) and 10% in nonoropharyngeal squamous cell carcinomas (NOPSCC).4 There has been a lack of studies focusing on the US Department of Veterans Administration (VA) population. The VA HNC population consists mostly of older white male smokers; whereas the rise of OPSCC in the general population consists primarily of males aged < 60 years often with little or no tobacco use.5 Furthermore, the importance of p16 positivity in NOPSCC also may be prognostic.6 Population data on this subset in the VA are lacking as well.This study’s purpose is to analyze the p16 positivity rate in both the OPSCC and NOPSCC in the VA population. Elucidation of epidemiologic factors that are associated with these groups may bring to light important differences between the VA and general HNC populations.
Methods
A review of the Kansas City VA Medical Center database for patients with HNC was performed from 2011 to 2017. The review consisted of 183 patient records (second primaries were scored separately), and 123 were deemed eligible for the study. Epidemiologic data were collected, including site, OPSCC vs NOPSCC, age, race, education level, tobacco use, alcohol use, TNM stage, and marital status (Table).
Results
The NOPSCC p16+ group had the greatest mean pack-year use (57). The lowest was in the OPSCC p16+ group (29). The OPSCC p16+ group had 37% never smokers compared with ≤ 10% for the other groups. Both the OPSCC and NOPSCC p16- groups had much more alcohol use per week than that of the p16+ groups. The differences in marital status included a lower rate of never married individuals in the p16+ group and a higher rate of marriage in the NOPSCC p16- group. The T stage distribution within the OPSCC groups was similar, but NOPSCC groups saw more T1 lesions in the NOPSCC p16- group (42% p16- vs 18% p16+). Conversely, more T4 lesions were found in the NOPSCC p16+ patients (7% p16- vs 29% p16+).
Discussion
The overall HPV positivity rate in the general population of patients with HNC has been reported as between 57% and 72% for OPSCC and between 1.3% and 7% for NOPSCC.6 One study, however, examined the p16 positivity rate in NOPSCC patients enrolled in major trials (RTOG 0129, 0234, and 0522 studies) and found that up to 19.3% of NOPSCC patients had p16 positivity.6 Even with the near 20% rate in those aforementioned trials that are above the reported norm, the current study found that nearly 30% of its VA population had p16+ NOPSCC. It has been shown that regardless of site, HPV-driven head and neck tumors share a similar gene expression and DNA methylation profiles (nonkeratinizing, basaloid histopathologic features, and lack of TP53 or CDKN2A alterations).5 p16+ NOPSCC has a different immune microenvironment with less lymphocyte infiltration, and there is some debate in the literature about the effects on tumor outcomes for NOPSCC cancer.5
In the aforementioned RTOG trials, p16- NOPSCC had worse outcomes compared with those of p16+ NOPSCC.6 This result is in contrast to the Danish Head and Neck Cancer Group (DAHANCA) and the combined Johns Hopkins University (JHU) and University of California, San Francisco (UCSF) data that found no difference between p16+ NOPSCC or p16- NOPSCC.7,8 In regards to race, this study did not find any differences. Another UCSF and JHU study showed lower p16+ rates in African American patients with OPSCC, but no distinction between race in the NOPSCC group. This result is consistent with the data in the current study as the distribution of race was no different among the 4 groups; however, this study's cohort was 90% white, 10% African American, and only < 1% Native American.4 This study's cohort population also was consistent with HPV-positive tumors presenting with earlier T, but higher N staging.9
Smoking is known to decrease survival in HPV-positive HNC, with the RTOG 0129 study separating head and neck tumors into low, medium, and high risk, based on HPV status, smoking, and stage.10 Although the average smoking pack-years in the current study’s OPC p16+ group was high at 29 pack-years, there was still a significant number of nonsmokers in that same group (37%). The University of Michigan conducted a study that had a similar profile of patients with an average age of 56.5 and 32.4% never smokers in their p16+ OPSCC cohort; thus, the VA p16+ OPSCC group in this study may be similar to the general population's p16+ OPSCC group.11 Nonmonogamous relationships also have been shown to be a risk factor for HPV positivity, and there was a difference in marital status (assuming it was a surrogate for monogamy) between the 4 groups; however, in contrast, the p16+ group in the current study had a high number of married patients, 45% in OPC p16+ group, and may not have been a good surrogate for monogamy in this VA population.
Limitations
Limitations of this study include all the caveats that come with a retrospective study, such as confounding variables, unbalanced groups, and selection bias. A detailed sexual history was not included, although it is well known that sexual activity is linked with oral HPV positivity.12 Human papillomavirus positivity based on p16 immunohistochemical analysis also was used as a surrogate marker for HPV instead of DNA in situ hybridization. The data also may be skewed due to the study patient’s being predominantly white and male: Both groups have a higher predilection for HPV-driven HNCs.13
Conclusion
The proportion of p16+ VA OPSCC cases was similar to that of the general population at 75% with 37% never smokers, but the percentage in NOPSCC was higher at 29% with only 10% never smokers. The p16+ NOPSCC also presented with more T4 lesions and a higher overall stage compared with p16- NOPSCC. Further studies are needed to compare these subgroups in the VA and in the general HNC populations.
Head and neck cancer (HNC) continues to be a major health issue with an estimated 51,540 cases in the US in 2018, making it the eighth most common cancer among men with an estimated 4% of all new cancer diagnoses.1 Over the past decade, human papillomavirus (HPV) has emerged as a major prognostic factor for survival in squamous cell carcinomas of the oropharynx. Patients who are HPV-positive (HPV+) have a much higher survival rate than patients who have HPV-negative (HPV-) cancers of the oropharynx. The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual has 2 distinct stagings for HPV+ and HPV- oropharyngeal tumors using p16-positivity (p16+) as a surrogate marker.2
Squamous cell carcinomas of the oropharynx that are HPV+ have about half the risk of death of HPV- tumors, are highly responsive to treatment, and are more often seen in younger and healthier patients with little to no tobacco use.2,3 As such, there also is a movement to de-escalate HPV+ oropharyngeal cancers with multiple trials by either replacing cytotoxic chemotherapy with a targeted agent (cisplatin vs cetuximab in RTOG 1016) or reducing the radiation dose (ECOG 1308, NRG HN002, Quarterback, and OPTIMA trials).3
The focus of many epidemiologic studies has been in the HNC general population. A recent epidemiologic analysis of the HNC general population found a p16 positivity rate of 60% in oropharyngeal squamous cell carcinomas (OPSCC) and 10% in nonoropharyngeal squamous cell carcinomas (NOPSCC).4 There has been a lack of studies focusing on the US Department of Veterans Administration (VA) population. The VA HNC population consists mostly of older white male smokers; whereas the rise of OPSCC in the general population consists primarily of males aged < 60 years often with little or no tobacco use.5 Furthermore, the importance of p16 positivity in NOPSCC also may be prognostic.6 Population data on this subset in the VA are lacking as well.This study’s purpose is to analyze the p16 positivity rate in both the OPSCC and NOPSCC in the VA population. Elucidation of epidemiologic factors that are associated with these groups may bring to light important differences between the VA and general HNC populations.
Methods
A review of the Kansas City VA Medical Center database for patients with HNC was performed from 2011 to 2017. The review consisted of 183 patient records (second primaries were scored separately), and 123 were deemed eligible for the study. Epidemiologic data were collected, including site, OPSCC vs NOPSCC, age, race, education level, tobacco use, alcohol use, TNM stage, and marital status (Table).
Results
The NOPSCC p16+ group had the greatest mean pack-year use (57). The lowest was in the OPSCC p16+ group (29). The OPSCC p16+ group had 37% never smokers compared with ≤ 10% for the other groups. Both the OPSCC and NOPSCC p16- groups had much more alcohol use per week than that of the p16+ groups. The differences in marital status included a lower rate of never married individuals in the p16+ group and a higher rate of marriage in the NOPSCC p16- group. The T stage distribution within the OPSCC groups was similar, but NOPSCC groups saw more T1 lesions in the NOPSCC p16- group (42% p16- vs 18% p16+). Conversely, more T4 lesions were found in the NOPSCC p16+ patients (7% p16- vs 29% p16+).
Discussion
The overall HPV positivity rate in the general population of patients with HNC has been reported as between 57% and 72% for OPSCC and between 1.3% and 7% for NOPSCC.6 One study, however, examined the p16 positivity rate in NOPSCC patients enrolled in major trials (RTOG 0129, 0234, and 0522 studies) and found that up to 19.3% of NOPSCC patients had p16 positivity.6 Even with the near 20% rate in those aforementioned trials that are above the reported norm, the current study found that nearly 30% of its VA population had p16+ NOPSCC. It has been shown that regardless of site, HPV-driven head and neck tumors share a similar gene expression and DNA methylation profiles (nonkeratinizing, basaloid histopathologic features, and lack of TP53 or CDKN2A alterations).5 p16+ NOPSCC has a different immune microenvironment with less lymphocyte infiltration, and there is some debate in the literature about the effects on tumor outcomes for NOPSCC cancer.5
In the aforementioned RTOG trials, p16- NOPSCC had worse outcomes compared with those of p16+ NOPSCC.6 This result is in contrast to the Danish Head and Neck Cancer Group (DAHANCA) and the combined Johns Hopkins University (JHU) and University of California, San Francisco (UCSF) data that found no difference between p16+ NOPSCC or p16- NOPSCC.7,8 In regards to race, this study did not find any differences. Another UCSF and JHU study showed lower p16+ rates in African American patients with OPSCC, but no distinction between race in the NOPSCC group. This result is consistent with the data in the current study as the distribution of race was no different among the 4 groups; however, this study's cohort was 90% white, 10% African American, and only < 1% Native American.4 This study's cohort population also was consistent with HPV-positive tumors presenting with earlier T, but higher N staging.9
Smoking is known to decrease survival in HPV-positive HNC, with the RTOG 0129 study separating head and neck tumors into low, medium, and high risk, based on HPV status, smoking, and stage.10 Although the average smoking pack-years in the current study’s OPC p16+ group was high at 29 pack-years, there was still a significant number of nonsmokers in that same group (37%). The University of Michigan conducted a study that had a similar profile of patients with an average age of 56.5 and 32.4% never smokers in their p16+ OPSCC cohort; thus, the VA p16+ OPSCC group in this study may be similar to the general population's p16+ OPSCC group.11 Nonmonogamous relationships also have been shown to be a risk factor for HPV positivity, and there was a difference in marital status (assuming it was a surrogate for monogamy) between the 4 groups; however, in contrast, the p16+ group in the current study had a high number of married patients, 45% in OPC p16+ group, and may not have been a good surrogate for monogamy in this VA population.
Limitations
Limitations of this study include all the caveats that come with a retrospective study, such as confounding variables, unbalanced groups, and selection bias. A detailed sexual history was not included, although it is well known that sexual activity is linked with oral HPV positivity.12 Human papillomavirus positivity based on p16 immunohistochemical analysis also was used as a surrogate marker for HPV instead of DNA in situ hybridization. The data also may be skewed due to the study patient’s being predominantly white and male: Both groups have a higher predilection for HPV-driven HNCs.13
Conclusion
The proportion of p16+ VA OPSCC cases was similar to that of the general population at 75% with 37% never smokers, but the percentage in NOPSCC was higher at 29% with only 10% never smokers. The p16+ NOPSCC also presented with more T4 lesions and a higher overall stage compared with p16- NOPSCC. Further studies are needed to compare these subgroups in the VA and in the general HNC populations.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122-137.
3. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11.
4. D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177.
5. Chakravarthy A, Henderson S, Thirdborough SM, et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34(34):4132-4141.
6. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930-3938.
7. Lassen P, Primdahl H, Johansen J, et al; Danish Head and Neck Cancer Group (DAHANCA). Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310-316.
8. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566-1575.
9. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299-309.
10. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35.
11. Maxwell, JH, Kumar B, Feng FY, et al. Tobacco use in HPV-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226-1235.
12. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703.
13. Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50(6):565-574.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122-137.
3. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11.
4. D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177.
5. Chakravarthy A, Henderson S, Thirdborough SM, et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34(34):4132-4141.
6. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930-3938.
7. Lassen P, Primdahl H, Johansen J, et al; Danish Head and Neck Cancer Group (DAHANCA). Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310-316.
8. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566-1575.
9. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299-309.
10. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35.
11. Maxwell, JH, Kumar B, Feng FY, et al. Tobacco use in HPV-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226-1235.
12. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703.
13. Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50(6):565-574.