User login
Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules (FULL)
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
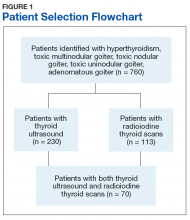
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
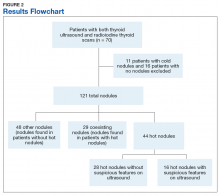
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.