User login
Agent Orange Exposure, Transformation From MGUS to Multiple Myeloma, and Outcomes in Veterans
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
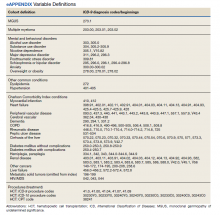
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
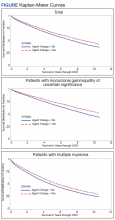
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
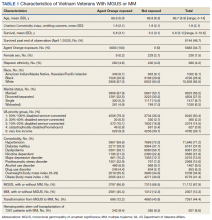
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
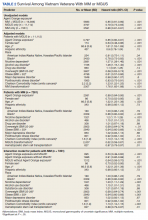
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760