User login
When is it safe to resume anticoagulation in my patient with hemorrhagic stroke?
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Balancing risk is critical to decision making
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
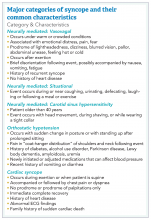
How to manage a patient presenting with syncope
Case
A 38-year-old construction worker without significant medical history presents following witnessed syncope at her job, after standing for at least 2 hours on a particularly warm day. She reported an episode of syncope under similar circumstances 2 months prior. With each episode, she experienced “tunneling” of peripheral vision, then loss of consciousness without palpitations or incontinence. Her physical exam, vital signs (including orthostatic blood pressures), labs, and ECG were unremarkable.
Brief overview
The American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines define syncope as “a symptom that presents with an abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery” with cerebral hypoperfusion as the presumed mechanism.1 Furthermore, “there should not be clinical features of other nonsyncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (that is, pseudosyncope).”1
A careful history revolving around the patient’s behavior prior to, during, and following the event, a thorough past medical history, and a review of current medications are essential. Potential obstacles in obtaining details of the event include lack of witnesses, patient’s inability to recall the experience, and inaccurate description of convulsive syncope as a “seizure” by bystanders.2
Overview of data
Obtaining a detailed history is crucial to understanding both the etiology of the syncopal event and determining which patients are at high risk for adverse outcomes. The etiology of syncope can be determined by history alone in 26% of patients younger than 65 years.3 Data on the prevalence of syncope by cause varies widely. As a general rule, in younger patients, especially those under 40 years of age, neurally mediated syncope is most common. As patients age, orthostatic hypotension and cardiac causes (including arrhythmias and structural diseases) occur more frequently, though neurally mediated syncope is still the most common.
Many of these predictors, however, would raise the clinical suspicion of most hospitalists for adverse outcomes in their hospitalized patients independent of the presence or absence of syncope. In fact, a meta-analysis has concluded that “None of the evaluated prediction tools (SFSR, EGSYS) performed better than clinical judgment in identifying serious outcomes during emergency department stay, and at 10 and 30 days after syncope.”6
Once the patient is hospitalized, further evaluation should be based on a careful history and physical examination. Standard evaluation also includes careful review of medications, an ECG to exclude findings suggestive of arrhythmias as well as structural or coronary artery disease, and orthostatic blood pressure measurements.1 Additional tests should be considered as deemed appropriate. For example, in patients over 40 years of age without history of carotid artery disease or stroke and in whom no carotid artery bruit is appreciated, a carotid sinus massage may be considered. The correct technique is to massage the sinus on the right then left, each for 5 seconds in both supine and standing positions with continuous heart rate and frequent blood pressure monitoring. Reproduction of syncope, especially concurrent with a cardiac pause of greater than 3 seconds and a systolic blood pressure drop of greater than 50 mmHg, is considered a positive test. Tilt-table testing should be considered in those for whom neurally mediated syncope is suspected but not confirmed, or in patients who might benefit from further elucidation of their prodromal symptoms.
Of note, a study recently published in the New England Journal of Medicine suggests that the prevalence of PE in patients (median age, 80 years) presenting with a first episode of syncope was 17%, a rate that is substantially higher than historically presumed.8 Although the prevalence of PE was highest among patients presenting with syncope of unclear origin (25%), nearly 13% of patients with other explanations for syncope also had PE.
Application of data
Bottom Line
Dr. Roberts, Dr. Krason, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Shen W-K et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;70(5):e39-e110.
2. Sheldon R. How to differentiate syncope from seizure. Cardiol Clin. 2015 Aug;33(3):377-85.
3. Del Rosso A et al. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005 Nov 15;96(10):1431-5.
4. Blanc JJ. Syncope: Definition, epidemiology, and classification. Cardiol Clin. 2015 Aug;33(3):341-5.
5. Matthews IG et al. Syncope in the older person. Cardiol Clin. 2015 Aug;33(3):411-21.
6. Costantino G et al. Syncope risk stratification tools vs clinical judgment: An individual patient data meta-analysis. Am J Med. 2014 Nov;127(11):1126.e13-25.
7. Chiu DT et al. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47:113-8.
8. Prandoni P et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016 Oct;375(20):1524-31.
9. Sheldon RS et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can J Cardiol. 2011 Mar-Apr;27(2):246-253.
10. Moya A et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J. 2009 Nov;30(21):2631-71.
Additional reading
1. Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol. 2012 May; 59(18):1583-91.
2. Rosanio S et al. Syncope in adults: systematic review and proposal of a diagnostic and therapeutic algorithm. Int J Cardiol. 2013 Jan;162(3):149-57.
Case
A 38-year-old construction worker without significant medical history presents following witnessed syncope at her job, after standing for at least 2 hours on a particularly warm day. She reported an episode of syncope under similar circumstances 2 months prior. With each episode, she experienced “tunneling” of peripheral vision, then loss of consciousness without palpitations or incontinence. Her physical exam, vital signs (including orthostatic blood pressures), labs, and ECG were unremarkable.
Brief overview
The American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines define syncope as “a symptom that presents with an abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery” with cerebral hypoperfusion as the presumed mechanism.1 Furthermore, “there should not be clinical features of other nonsyncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (that is, pseudosyncope).”1
A careful history revolving around the patient’s behavior prior to, during, and following the event, a thorough past medical history, and a review of current medications are essential. Potential obstacles in obtaining details of the event include lack of witnesses, patient’s inability to recall the experience, and inaccurate description of convulsive syncope as a “seizure” by bystanders.2
Overview of data
Obtaining a detailed history is crucial to understanding both the etiology of the syncopal event and determining which patients are at high risk for adverse outcomes. The etiology of syncope can be determined by history alone in 26% of patients younger than 65 years.3 Data on the prevalence of syncope by cause varies widely. As a general rule, in younger patients, especially those under 40 years of age, neurally mediated syncope is most common. As patients age, orthostatic hypotension and cardiac causes (including arrhythmias and structural diseases) occur more frequently, though neurally mediated syncope is still the most common.
Many of these predictors, however, would raise the clinical suspicion of most hospitalists for adverse outcomes in their hospitalized patients independent of the presence or absence of syncope. In fact, a meta-analysis has concluded that “None of the evaluated prediction tools (SFSR, EGSYS) performed better than clinical judgment in identifying serious outcomes during emergency department stay, and at 10 and 30 days after syncope.”6
Once the patient is hospitalized, further evaluation should be based on a careful history and physical examination. Standard evaluation also includes careful review of medications, an ECG to exclude findings suggestive of arrhythmias as well as structural or coronary artery disease, and orthostatic blood pressure measurements.1 Additional tests should be considered as deemed appropriate. For example, in patients over 40 years of age without history of carotid artery disease or stroke and in whom no carotid artery bruit is appreciated, a carotid sinus massage may be considered. The correct technique is to massage the sinus on the right then left, each for 5 seconds in both supine and standing positions with continuous heart rate and frequent blood pressure monitoring. Reproduction of syncope, especially concurrent with a cardiac pause of greater than 3 seconds and a systolic blood pressure drop of greater than 50 mmHg, is considered a positive test. Tilt-table testing should be considered in those for whom neurally mediated syncope is suspected but not confirmed, or in patients who might benefit from further elucidation of their prodromal symptoms.
Of note, a study recently published in the New England Journal of Medicine suggests that the prevalence of PE in patients (median age, 80 years) presenting with a first episode of syncope was 17%, a rate that is substantially higher than historically presumed.8 Although the prevalence of PE was highest among patients presenting with syncope of unclear origin (25%), nearly 13% of patients with other explanations for syncope also had PE.
Application of data
Bottom Line
Dr. Roberts, Dr. Krason, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Shen W-K et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;70(5):e39-e110.
2. Sheldon R. How to differentiate syncope from seizure. Cardiol Clin. 2015 Aug;33(3):377-85.
3. Del Rosso A et al. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005 Nov 15;96(10):1431-5.
4. Blanc JJ. Syncope: Definition, epidemiology, and classification. Cardiol Clin. 2015 Aug;33(3):341-5.
5. Matthews IG et al. Syncope in the older person. Cardiol Clin. 2015 Aug;33(3):411-21.
6. Costantino G et al. Syncope risk stratification tools vs clinical judgment: An individual patient data meta-analysis. Am J Med. 2014 Nov;127(11):1126.e13-25.
7. Chiu DT et al. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47:113-8.
8. Prandoni P et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016 Oct;375(20):1524-31.
9. Sheldon RS et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can J Cardiol. 2011 Mar-Apr;27(2):246-253.
10. Moya A et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J. 2009 Nov;30(21):2631-71.
Additional reading
1. Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol. 2012 May; 59(18):1583-91.
2. Rosanio S et al. Syncope in adults: systematic review and proposal of a diagnostic and therapeutic algorithm. Int J Cardiol. 2013 Jan;162(3):149-57.
Case
A 38-year-old construction worker without significant medical history presents following witnessed syncope at her job, after standing for at least 2 hours on a particularly warm day. She reported an episode of syncope under similar circumstances 2 months prior. With each episode, she experienced “tunneling” of peripheral vision, then loss of consciousness without palpitations or incontinence. Her physical exam, vital signs (including orthostatic blood pressures), labs, and ECG were unremarkable.
Brief overview
The American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines define syncope as “a symptom that presents with an abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery” with cerebral hypoperfusion as the presumed mechanism.1 Furthermore, “there should not be clinical features of other nonsyncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (that is, pseudosyncope).”1
A careful history revolving around the patient’s behavior prior to, during, and following the event, a thorough past medical history, and a review of current medications are essential. Potential obstacles in obtaining details of the event include lack of witnesses, patient’s inability to recall the experience, and inaccurate description of convulsive syncope as a “seizure” by bystanders.2
Overview of data
Obtaining a detailed history is crucial to understanding both the etiology of the syncopal event and determining which patients are at high risk for adverse outcomes. The etiology of syncope can be determined by history alone in 26% of patients younger than 65 years.3 Data on the prevalence of syncope by cause varies widely. As a general rule, in younger patients, especially those under 40 years of age, neurally mediated syncope is most common. As patients age, orthostatic hypotension and cardiac causes (including arrhythmias and structural diseases) occur more frequently, though neurally mediated syncope is still the most common.
Many of these predictors, however, would raise the clinical suspicion of most hospitalists for adverse outcomes in their hospitalized patients independent of the presence or absence of syncope. In fact, a meta-analysis has concluded that “None of the evaluated prediction tools (SFSR, EGSYS) performed better than clinical judgment in identifying serious outcomes during emergency department stay, and at 10 and 30 days after syncope.”6
Once the patient is hospitalized, further evaluation should be based on a careful history and physical examination. Standard evaluation also includes careful review of medications, an ECG to exclude findings suggestive of arrhythmias as well as structural or coronary artery disease, and orthostatic blood pressure measurements.1 Additional tests should be considered as deemed appropriate. For example, in patients over 40 years of age without history of carotid artery disease or stroke and in whom no carotid artery bruit is appreciated, a carotid sinus massage may be considered. The correct technique is to massage the sinus on the right then left, each for 5 seconds in both supine and standing positions with continuous heart rate and frequent blood pressure monitoring. Reproduction of syncope, especially concurrent with a cardiac pause of greater than 3 seconds and a systolic blood pressure drop of greater than 50 mmHg, is considered a positive test. Tilt-table testing should be considered in those for whom neurally mediated syncope is suspected but not confirmed, or in patients who might benefit from further elucidation of their prodromal symptoms.
Of note, a study recently published in the New England Journal of Medicine suggests that the prevalence of PE in patients (median age, 80 years) presenting with a first episode of syncope was 17%, a rate that is substantially higher than historically presumed.8 Although the prevalence of PE was highest among patients presenting with syncope of unclear origin (25%), nearly 13% of patients with other explanations for syncope also had PE.
Application of data
Bottom Line
Dr. Roberts, Dr. Krason, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Shen W-K et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;70(5):e39-e110.
2. Sheldon R. How to differentiate syncope from seizure. Cardiol Clin. 2015 Aug;33(3):377-85.
3. Del Rosso A et al. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005 Nov 15;96(10):1431-5.
4. Blanc JJ. Syncope: Definition, epidemiology, and classification. Cardiol Clin. 2015 Aug;33(3):341-5.
5. Matthews IG et al. Syncope in the older person. Cardiol Clin. 2015 Aug;33(3):411-21.
6. Costantino G et al. Syncope risk stratification tools vs clinical judgment: An individual patient data meta-analysis. Am J Med. 2014 Nov;127(11):1126.e13-25.
7. Chiu DT et al. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47:113-8.
8. Prandoni P et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016 Oct;375(20):1524-31.
9. Sheldon RS et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can J Cardiol. 2011 Mar-Apr;27(2):246-253.
10. Moya A et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J. 2009 Nov;30(21):2631-71.
Additional reading
1. Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol. 2012 May; 59(18):1583-91.
2. Rosanio S et al. Syncope in adults: systematic review and proposal of a diagnostic and therapeutic algorithm. Int J Cardiol. 2013 Jan;162(3):149-57.
When should I transfuse a patient who has anemia?
Clinical case
A 48-year-old man with cirrhosis and esophageal varices presents to the emergency department with hematochezia and hematemesis. Upon arrival to the floor, he has another bout of hematochezia and hematemesis. He appears pale. His pulse is 90 beats per minute, his blood pressure is 100/60 mmHg, and his respiratory rate is 14 breaths per minute. A complete blood count reveals a hemoglobin level of 7.8 g/dL and hematocrit of 23.5%. Should he receive a blood transfusion?
Introduction
Anemia is one of the most frequent conditions in hospitalized patients. Anemia is variably associated with morbidity and mortality depending on chronicity, etiology, and associated comorbidities. Before the 1980s, standard practice was to transfuse all patients to a hemoglobin level greater than 10 g/dL and/or a hematocrit greater than 30%. However, with concerns about the potential adverse effects and cost of transfusions, the safety and effectiveness of liberal versus restrictive transfusion thresholds became the subject of many studies.
The 2016 the AABB (formerly American Association of Blood Banks) guidelines focused on the evidence for hemodynamically stable and asymptomatic hospitalized patients. The guidelines are based on randomized controlled trials that measured mortality as the primary endpoint. Most trials and guidelines reinforce that if a patient is symptomatic or hemodynamically unstable from anemia or hemorrhage, RBC transfusion is appropriate irrespective of hemoglobin level.
Overview of the data
Critically ill patients
The Transfusion Requirements in Critical Care (TRICC) trial, published in 1999, was the first large clinical trial examining the safety of restrictive transfusion thresholds in critically ill patients.3
The subsequent Transfusion Requirements in Septic Shock (TRISS) study involved patients with septic shock and similarly found that patients assigned to a restrictive strategy (transfusion for hemoglobin less than 7 g/dL) had similar outcomes to patients assigned to a liberal strategy (transfusion for hemoglobin less than 9 g/dL). The patients in the restrictive group received fewer transfusions, but had similar rates of 90-day mortality, use of life support, and number of days alive and out of the hospital.4
These large randomized controlled trials in critically ill patients served as the basis for subsequent studies in patient populations outside of the ICU.
Acute upper GI bleed
Acute upper gastrointestinal bleeding (UGIB) is one of the most common indications for RBC transfusion.
The TRIGGER trial, a cluster randomized multicenter study published in 2015, also found no difference in clinical outcomes, including mortality, between a restrictive strategy and liberal strategy for transfusion of patients with UGIB.6
Perioperative patients
Transfusion thresholds have been studied in large randomized trials for perioperative patients undergoing cardiac and orthopedic surgery.
The Transfusion Requirements after Cardiac Surgery (TRACS) trial, published in 2010, randomized patients undergoing cardiac surgery at a single center to a liberal strategy of blood transfusion (to maintain a hematocrit 30% or greater) or a restrictive strategy (hematocrit 24% or greater).7 Mortality and severe morbidity rates were noninferior in the restrictive strategy group. Mean hemoglobin concentrations were 10.5 g/dL in the liberal-strategy group and 9.1 g/dL in the restrictive-strategy group. Independent of transfusion strategy, the number of transfused red blood cell units was an independent risk factor for clinical complications and death at 30 days.
Based on these two trials, as well as other smaller randomized controlled trials and observational studies, the AABB guidelines recommend a restrictive RBC transfusion threshold of 8 g/dL for patients undergoing cardiac or orthopedic surgery.1
Acute coronary syndrome, stable coronary artery disease, and congestive heart failure
Anemia is an independent predictor of major adverse cardiovascular events in patients with acute coronary syndrome.9 However, it remains controversial if transfusion has benefit or causes harm in patients with acute coronary syndrome. No randomized controlled trials have yet been published on this topic, and observational studies and subgroups from randomized controlled trials have yielded mixed results.
Similarly, there are no randomized controlled trials examining liberal versus restrictive transfusion goals for asymptomatic hospitalized patients with stable coronary artery disease (CAD). However, patients with CAD were included in the TRICC and FOCUS trials.3,8 Of patients enrolled in the TRICC trial, 26% had a primary or secondary diagnosis of cardiac disease; subgroup analysis found no significant differences in 30-day mortality between treatment groups, similar to that of the entire study population.3 In a subgroup analysis of patients with “cardiovascular disease” the FOCUS trial found no difference in outcomes between a restrictive and liberal transfusion strategy, although there was a marginally higher incidence of myocardial infarction in the restrictive arm in the entire study population.8
Although some studies have included patients with congestive heart failure (CHF) as a subgroup, these subgroups are often not powered to show clinically meaningful differences. The risk of volume overload is one explanation for why transfusions may be harmful for patients with CHF.
Based on these limited available data, the AABB guidelines recommend a “restrictive RBC transfusion threshold (hemoglobin level of 8 g/dL)” for patients “with preexisting cardiovascular disease,” without distinguishing among patients with acute coronary syndrome, stable CAD, and CHF, but adds that the “threshold of 7 g/dL is likely comparable with 8 g/dL, but randomized controlled trial evidence is not available for all patient categories.”1
Of note, certain patient populations, such as those with end-stage renal disease, oncology patients (especially those undergoing active chemotherapy), and those with comorbid conditions such as thrombocytopenia and coagulopathy are specifically excluded from the AABB guidelines. The reader is referred to guidelines from respective specialty societies for further guidance.
Back to our case
This 48-year-old man with cirrhosis and esophageal varices presenting with ongoing blood loss due to hematochezia and hematemesis with a hemoglobin of 7.8 g/dL should be transfused due to his active bleeding; his hemoglobin level should not be a determining factor under such circumstances. This distinction is one of the most fundamental in interpreting the guidelines, that is, patients with hemodynamic insult, symptoms, and/or ongoing bleeding should be evaluated clinically, independent of their hemoglobin level.
Bottom line
Although recent guidelines generally favor a more restrictive hemoglobin goal threshold, in the presence of active blood loss or hemodynamic instability, blood transfusion should be considered independent of the initial hemoglobin level.
Key Points
- The AABB guidelines recommend transfusing stable general medical inpatients to a hemoglobin level of 7 g/dL.
- For patients undergoing orthopedic surgery, cardiac surgery, and those with preexisting cardiovascular disease, a transfusion threshold of 8 g/dL is recommended.
- Patients with hemodynamic instability or active blood loss should be transfused based on clinical criteria, and not absolute hemoglobin levels.
- Patients with acute coronary syndrome, severe thrombocytopenia, and chronic transfusion–dependent anemia are excluded from these recommendations.
- Further studies are needed to further refine these recommendations to specific patient populations in maximizing the benefits and minimizing the risks of blood transfusions.
Quiz
In which of the following scenarios is RBC transfusion LEAST indicated?
- A. Active diverticular bleed, heart rate 125 beats/minute, blood pressure 85/65 mm HG, hemoglobin 10.5 g/dL
- B. Septic shock in the ICU, hemoglobin 6.7 g/dL
- C. Pulmonary embolism in the setting of stable coronary artery disease and hemoglobin 8.7 g/dL
- D. Lymphoma on chemotherapy, hemoglobin 7.5 g/d, patient becomes dizzy when standing
Answer: C – A hemoglobin transfusion goal of 8.0 g/dL is recommended for patients with cardiovascular disease. The patient in answer choice A has active blood loss, tachycardia, and hypotension, all pointing to the need for blood transfusion irrespective of an initial hemoglobin level greater than 8.0 g/dL. The patient in answer choice B has a hemoglobin level less than 7.0 g/dL, and the patient in choice D is symptomatic, possibly from anemia, as well as on chemotherapy, therefore making transfusion a reasonable option.
Dr. Sampat, Dr. Berger, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. Carson JL et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-35.
2. Dwyre DM et al. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92-8.
3. Hébert PC et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17.
4. Holst LB et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-91.
5. Villanueva C et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.
6. Jairath V et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-44.
7. Hajjar LA et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-67.
8. Carson JL et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-62.
9. Sabatine MS et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-9.
Clinical case
A 48-year-old man with cirrhosis and esophageal varices presents to the emergency department with hematochezia and hematemesis. Upon arrival to the floor, he has another bout of hematochezia and hematemesis. He appears pale. His pulse is 90 beats per minute, his blood pressure is 100/60 mmHg, and his respiratory rate is 14 breaths per minute. A complete blood count reveals a hemoglobin level of 7.8 g/dL and hematocrit of 23.5%. Should he receive a blood transfusion?
Introduction
Anemia is one of the most frequent conditions in hospitalized patients. Anemia is variably associated with morbidity and mortality depending on chronicity, etiology, and associated comorbidities. Before the 1980s, standard practice was to transfuse all patients to a hemoglobin level greater than 10 g/dL and/or a hematocrit greater than 30%. However, with concerns about the potential adverse effects and cost of transfusions, the safety and effectiveness of liberal versus restrictive transfusion thresholds became the subject of many studies.
The 2016 the AABB (formerly American Association of Blood Banks) guidelines focused on the evidence for hemodynamically stable and asymptomatic hospitalized patients. The guidelines are based on randomized controlled trials that measured mortality as the primary endpoint. Most trials and guidelines reinforce that if a patient is symptomatic or hemodynamically unstable from anemia or hemorrhage, RBC transfusion is appropriate irrespective of hemoglobin level.
Overview of the data
Critically ill patients
The Transfusion Requirements in Critical Care (TRICC) trial, published in 1999, was the first large clinical trial examining the safety of restrictive transfusion thresholds in critically ill patients.3
The subsequent Transfusion Requirements in Septic Shock (TRISS) study involved patients with septic shock and similarly found that patients assigned to a restrictive strategy (transfusion for hemoglobin less than 7 g/dL) had similar outcomes to patients assigned to a liberal strategy (transfusion for hemoglobin less than 9 g/dL). The patients in the restrictive group received fewer transfusions, but had similar rates of 90-day mortality, use of life support, and number of days alive and out of the hospital.4
These large randomized controlled trials in critically ill patients served as the basis for subsequent studies in patient populations outside of the ICU.
Acute upper GI bleed
Acute upper gastrointestinal bleeding (UGIB) is one of the most common indications for RBC transfusion.
The TRIGGER trial, a cluster randomized multicenter study published in 2015, also found no difference in clinical outcomes, including mortality, between a restrictive strategy and liberal strategy for transfusion of patients with UGIB.6
Perioperative patients
Transfusion thresholds have been studied in large randomized trials for perioperative patients undergoing cardiac and orthopedic surgery.
The Transfusion Requirements after Cardiac Surgery (TRACS) trial, published in 2010, randomized patients undergoing cardiac surgery at a single center to a liberal strategy of blood transfusion (to maintain a hematocrit 30% or greater) or a restrictive strategy (hematocrit 24% or greater).7 Mortality and severe morbidity rates were noninferior in the restrictive strategy group. Mean hemoglobin concentrations were 10.5 g/dL in the liberal-strategy group and 9.1 g/dL in the restrictive-strategy group. Independent of transfusion strategy, the number of transfused red blood cell units was an independent risk factor for clinical complications and death at 30 days.
Based on these two trials, as well as other smaller randomized controlled trials and observational studies, the AABB guidelines recommend a restrictive RBC transfusion threshold of 8 g/dL for patients undergoing cardiac or orthopedic surgery.1
Acute coronary syndrome, stable coronary artery disease, and congestive heart failure
Anemia is an independent predictor of major adverse cardiovascular events in patients with acute coronary syndrome.9 However, it remains controversial if transfusion has benefit or causes harm in patients with acute coronary syndrome. No randomized controlled trials have yet been published on this topic, and observational studies and subgroups from randomized controlled trials have yielded mixed results.
Similarly, there are no randomized controlled trials examining liberal versus restrictive transfusion goals for asymptomatic hospitalized patients with stable coronary artery disease (CAD). However, patients with CAD were included in the TRICC and FOCUS trials.3,8 Of patients enrolled in the TRICC trial, 26% had a primary or secondary diagnosis of cardiac disease; subgroup analysis found no significant differences in 30-day mortality between treatment groups, similar to that of the entire study population.3 In a subgroup analysis of patients with “cardiovascular disease” the FOCUS trial found no difference in outcomes between a restrictive and liberal transfusion strategy, although there was a marginally higher incidence of myocardial infarction in the restrictive arm in the entire study population.8
Although some studies have included patients with congestive heart failure (CHF) as a subgroup, these subgroups are often not powered to show clinically meaningful differences. The risk of volume overload is one explanation for why transfusions may be harmful for patients with CHF.
Based on these limited available data, the AABB guidelines recommend a “restrictive RBC transfusion threshold (hemoglobin level of 8 g/dL)” for patients “with preexisting cardiovascular disease,” without distinguishing among patients with acute coronary syndrome, stable CAD, and CHF, but adds that the “threshold of 7 g/dL is likely comparable with 8 g/dL, but randomized controlled trial evidence is not available for all patient categories.”1
Of note, certain patient populations, such as those with end-stage renal disease, oncology patients (especially those undergoing active chemotherapy), and those with comorbid conditions such as thrombocytopenia and coagulopathy are specifically excluded from the AABB guidelines. The reader is referred to guidelines from respective specialty societies for further guidance.
Back to our case
This 48-year-old man with cirrhosis and esophageal varices presenting with ongoing blood loss due to hematochezia and hematemesis with a hemoglobin of 7.8 g/dL should be transfused due to his active bleeding; his hemoglobin level should not be a determining factor under such circumstances. This distinction is one of the most fundamental in interpreting the guidelines, that is, patients with hemodynamic insult, symptoms, and/or ongoing bleeding should be evaluated clinically, independent of their hemoglobin level.
Bottom line
Although recent guidelines generally favor a more restrictive hemoglobin goal threshold, in the presence of active blood loss or hemodynamic instability, blood transfusion should be considered independent of the initial hemoglobin level.
Key Points
- The AABB guidelines recommend transfusing stable general medical inpatients to a hemoglobin level of 7 g/dL.
- For patients undergoing orthopedic surgery, cardiac surgery, and those with preexisting cardiovascular disease, a transfusion threshold of 8 g/dL is recommended.
- Patients with hemodynamic instability or active blood loss should be transfused based on clinical criteria, and not absolute hemoglobin levels.
- Patients with acute coronary syndrome, severe thrombocytopenia, and chronic transfusion–dependent anemia are excluded from these recommendations.
- Further studies are needed to further refine these recommendations to specific patient populations in maximizing the benefits and minimizing the risks of blood transfusions.
Quiz
In which of the following scenarios is RBC transfusion LEAST indicated?
- A. Active diverticular bleed, heart rate 125 beats/minute, blood pressure 85/65 mm HG, hemoglobin 10.5 g/dL
- B. Septic shock in the ICU, hemoglobin 6.7 g/dL
- C. Pulmonary embolism in the setting of stable coronary artery disease and hemoglobin 8.7 g/dL
- D. Lymphoma on chemotherapy, hemoglobin 7.5 g/d, patient becomes dizzy when standing
Answer: C – A hemoglobin transfusion goal of 8.0 g/dL is recommended for patients with cardiovascular disease. The patient in answer choice A has active blood loss, tachycardia, and hypotension, all pointing to the need for blood transfusion irrespective of an initial hemoglobin level greater than 8.0 g/dL. The patient in answer choice B has a hemoglobin level less than 7.0 g/dL, and the patient in choice D is symptomatic, possibly from anemia, as well as on chemotherapy, therefore making transfusion a reasonable option.
Dr. Sampat, Dr. Berger, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. Carson JL et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-35.
2. Dwyre DM et al. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92-8.
3. Hébert PC et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17.
4. Holst LB et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-91.
5. Villanueva C et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.
6. Jairath V et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-44.
7. Hajjar LA et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-67.
8. Carson JL et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-62.
9. Sabatine MS et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-9.
Clinical case
A 48-year-old man with cirrhosis and esophageal varices presents to the emergency department with hematochezia and hematemesis. Upon arrival to the floor, he has another bout of hematochezia and hematemesis. He appears pale. His pulse is 90 beats per minute, his blood pressure is 100/60 mmHg, and his respiratory rate is 14 breaths per minute. A complete blood count reveals a hemoglobin level of 7.8 g/dL and hematocrit of 23.5%. Should he receive a blood transfusion?
Introduction
Anemia is one of the most frequent conditions in hospitalized patients. Anemia is variably associated with morbidity and mortality depending on chronicity, etiology, and associated comorbidities. Before the 1980s, standard practice was to transfuse all patients to a hemoglobin level greater than 10 g/dL and/or a hematocrit greater than 30%. However, with concerns about the potential adverse effects and cost of transfusions, the safety and effectiveness of liberal versus restrictive transfusion thresholds became the subject of many studies.
The 2016 the AABB (formerly American Association of Blood Banks) guidelines focused on the evidence for hemodynamically stable and asymptomatic hospitalized patients. The guidelines are based on randomized controlled trials that measured mortality as the primary endpoint. Most trials and guidelines reinforce that if a patient is symptomatic or hemodynamically unstable from anemia or hemorrhage, RBC transfusion is appropriate irrespective of hemoglobin level.
Overview of the data
Critically ill patients
The Transfusion Requirements in Critical Care (TRICC) trial, published in 1999, was the first large clinical trial examining the safety of restrictive transfusion thresholds in critically ill patients.3
The subsequent Transfusion Requirements in Septic Shock (TRISS) study involved patients with septic shock and similarly found that patients assigned to a restrictive strategy (transfusion for hemoglobin less than 7 g/dL) had similar outcomes to patients assigned to a liberal strategy (transfusion for hemoglobin less than 9 g/dL). The patients in the restrictive group received fewer transfusions, but had similar rates of 90-day mortality, use of life support, and number of days alive and out of the hospital.4
These large randomized controlled trials in critically ill patients served as the basis for subsequent studies in patient populations outside of the ICU.
Acute upper GI bleed
Acute upper gastrointestinal bleeding (UGIB) is one of the most common indications for RBC transfusion.
The TRIGGER trial, a cluster randomized multicenter study published in 2015, also found no difference in clinical outcomes, including mortality, between a restrictive strategy and liberal strategy for transfusion of patients with UGIB.6
Perioperative patients
Transfusion thresholds have been studied in large randomized trials for perioperative patients undergoing cardiac and orthopedic surgery.
The Transfusion Requirements after Cardiac Surgery (TRACS) trial, published in 2010, randomized patients undergoing cardiac surgery at a single center to a liberal strategy of blood transfusion (to maintain a hematocrit 30% or greater) or a restrictive strategy (hematocrit 24% or greater).7 Mortality and severe morbidity rates were noninferior in the restrictive strategy group. Mean hemoglobin concentrations were 10.5 g/dL in the liberal-strategy group and 9.1 g/dL in the restrictive-strategy group. Independent of transfusion strategy, the number of transfused red blood cell units was an independent risk factor for clinical complications and death at 30 days.
Based on these two trials, as well as other smaller randomized controlled trials and observational studies, the AABB guidelines recommend a restrictive RBC transfusion threshold of 8 g/dL for patients undergoing cardiac or orthopedic surgery.1
Acute coronary syndrome, stable coronary artery disease, and congestive heart failure
Anemia is an independent predictor of major adverse cardiovascular events in patients with acute coronary syndrome.9 However, it remains controversial if transfusion has benefit or causes harm in patients with acute coronary syndrome. No randomized controlled trials have yet been published on this topic, and observational studies and subgroups from randomized controlled trials have yielded mixed results.
Similarly, there are no randomized controlled trials examining liberal versus restrictive transfusion goals for asymptomatic hospitalized patients with stable coronary artery disease (CAD). However, patients with CAD were included in the TRICC and FOCUS trials.3,8 Of patients enrolled in the TRICC trial, 26% had a primary or secondary diagnosis of cardiac disease; subgroup analysis found no significant differences in 30-day mortality between treatment groups, similar to that of the entire study population.3 In a subgroup analysis of patients with “cardiovascular disease” the FOCUS trial found no difference in outcomes between a restrictive and liberal transfusion strategy, although there was a marginally higher incidence of myocardial infarction in the restrictive arm in the entire study population.8
Although some studies have included patients with congestive heart failure (CHF) as a subgroup, these subgroups are often not powered to show clinically meaningful differences. The risk of volume overload is one explanation for why transfusions may be harmful for patients with CHF.
Based on these limited available data, the AABB guidelines recommend a “restrictive RBC transfusion threshold (hemoglobin level of 8 g/dL)” for patients “with preexisting cardiovascular disease,” without distinguishing among patients with acute coronary syndrome, stable CAD, and CHF, but adds that the “threshold of 7 g/dL is likely comparable with 8 g/dL, but randomized controlled trial evidence is not available for all patient categories.”1
Of note, certain patient populations, such as those with end-stage renal disease, oncology patients (especially those undergoing active chemotherapy), and those with comorbid conditions such as thrombocytopenia and coagulopathy are specifically excluded from the AABB guidelines. The reader is referred to guidelines from respective specialty societies for further guidance.
Back to our case
This 48-year-old man with cirrhosis and esophageal varices presenting with ongoing blood loss due to hematochezia and hematemesis with a hemoglobin of 7.8 g/dL should be transfused due to his active bleeding; his hemoglobin level should not be a determining factor under such circumstances. This distinction is one of the most fundamental in interpreting the guidelines, that is, patients with hemodynamic insult, symptoms, and/or ongoing bleeding should be evaluated clinically, independent of their hemoglobin level.
Bottom line
Although recent guidelines generally favor a more restrictive hemoglobin goal threshold, in the presence of active blood loss or hemodynamic instability, blood transfusion should be considered independent of the initial hemoglobin level.
Key Points
- The AABB guidelines recommend transfusing stable general medical inpatients to a hemoglobin level of 7 g/dL.
- For patients undergoing orthopedic surgery, cardiac surgery, and those with preexisting cardiovascular disease, a transfusion threshold of 8 g/dL is recommended.
- Patients with hemodynamic instability or active blood loss should be transfused based on clinical criteria, and not absolute hemoglobin levels.
- Patients with acute coronary syndrome, severe thrombocytopenia, and chronic transfusion–dependent anemia are excluded from these recommendations.
- Further studies are needed to further refine these recommendations to specific patient populations in maximizing the benefits and minimizing the risks of blood transfusions.
Quiz
In which of the following scenarios is RBC transfusion LEAST indicated?
- A. Active diverticular bleed, heart rate 125 beats/minute, blood pressure 85/65 mm HG, hemoglobin 10.5 g/dL
- B. Septic shock in the ICU, hemoglobin 6.7 g/dL
- C. Pulmonary embolism in the setting of stable coronary artery disease and hemoglobin 8.7 g/dL
- D. Lymphoma on chemotherapy, hemoglobin 7.5 g/d, patient becomes dizzy when standing
Answer: C – A hemoglobin transfusion goal of 8.0 g/dL is recommended for patients with cardiovascular disease. The patient in answer choice A has active blood loss, tachycardia, and hypotension, all pointing to the need for blood transfusion irrespective of an initial hemoglobin level greater than 8.0 g/dL. The patient in answer choice B has a hemoglobin level less than 7.0 g/dL, and the patient in choice D is symptomatic, possibly from anemia, as well as on chemotherapy, therefore making transfusion a reasonable option.
Dr. Sampat, Dr. Berger, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. Carson JL et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-35.
2. Dwyre DM et al. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92-8.
3. Hébert PC et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17.
4. Holst LB et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-91.
5. Villanueva C et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.
6. Jairath V et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-44.
7. Hajjar LA et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-67.
8. Carson JL et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-62.
9. Sabatine MS et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-9.