User login
What We Have Learned About Combining a Ketogenic Diet and Chemoimmunotherapy: A Case Report and Review of Literature
Originally developed for the treatment of refractory epilepsy, the ketogenic diet is distinguished by its high-fat, moderate-protein, and low-carbohydrate food program. Preclinical models provide emerging evidence that a ketogenic diet can have therapeutic potential for a broad range of cancers. The Warburg effect is a condition where cancer cells increase the uptake and fermentation of glucose to produce lactate for their metabolism, which is called aerobic glycolysis. Lactate is the key driver of cancer angiogenesis and proliferation.1,2
The ketogenic diet promotes a metabolic shift from glycolysis to mitochondrial metabolism in normal cells while cancer cells have dysfunction in their mitochondria due to damage in cellular respiration. The ketogenic diet creates a metabolic state whereby blood glucose levels are reduced, and blood ketone bodies (D-β-hydroxybutyrate and acetoacetate) are elevated. In normal cells, the ketogenic diet causes a decrease in glucose intake for glycolysis, which makes them unable to produce enough substrate to enter the tricarboxylic acid (TCA) cycle for adenosine triphosphate (ATP) production. Fatty acid oxidation plays a key role in ketone body synthesis as a “super fuel” that enter the TCA cycle as an alternative pathway to generate ATP. On the other hand, cancer cells are unable to use ketone bodies to produce ATP for energy and metabolism due to mitochondrial defects. Lack of energy subsequently leads to the inhibition of proliferation and survival of cancer cells.3,4
We previously published a safety and feasibility study of the Modified Atkins Diet in metastatic cancer patients after failure of chemotherapy at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System.1 None of the patients were on chemotherapy at the time of enrollment. The Modified Atkins Diet consists of 60% fat, 30% protein, and 10% carbohydrates and is more tolerable than the ketogenic diet due to higher amounts of protein. Six of 11 patients (54%) had stable disease and partial response on positron emission tomography/computed tomography (PET/CT). Our study showed that patients who lost at least 10% of their body weight had improvement in quality of life (QOL) and cancer response.1 Here we present a case of a veteran with extensive metastatic colon cancer on concurrent ketogenic diet and chemotherapy subsequently followed by concurrent ketogenic diet and immunotherapy at Veterans Affairs Central California Health Care Systems (VACCHCS) in Fresno.
CASE PRESENTATION
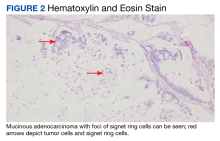
A 69-year-old veteran had iron deficiency anemia (hemoglobin, 6.5 g/dL) about 5 years previously. He underwent a colonoscopy that revealed a near circumferential ulcerated mass measuring 7 cm in the transverse colon. Biopsy results showed mucinous adenocarcinoma of the colon with a foci of signet ring cells (Figure 2).
The patient received adjuvant treatment with FOLFOX (fluorouracil, leucovorin calcium, and oxaliplatin), but within several months he developed pancreatic and worsening omental metastasis seen on PET/CT. He was then started on FOLFIRI (fluorouracil, leucovorin calcium, and irinotecan hydrochloride) plus bevacizumab 16 months after his initial diagnosis. He underwent a pancreatic mastectomy that confirmed adenocarcinoma 9 months later. Afterward, he briefly resumed FOLFIRI and bevacizumab. Next-generation sequencing testing with Foundation One CDx revealed a wild-type (WT) KRAS with a high degree of tumor mutation burden of 37 muts/Mb, BRAF V600E mutation, and high microsatellite instability (MSI-H).
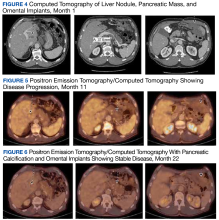
Due to disease progression, the patient’s treatment was changed to encorafenib and cetuximab for 4 months before progressing again with new liver mass and mediastinal lymphadenopathy. He then received pembrolizumab for 4 months until PET/CT showed progression and his carcinoembryonic antigen (CEA) increased from 95 to 1031 ng/mL by January 2021 (Figure 4).
The patient was started on trifluridine/tipiracil, and bevacizumab while concurrently initiating the ketogenic diet in January 2021. Laboratory tests drawn after 1 week of strict dietary ketogenic diet adherence showed low-level ketosis with a glucose ketone index (GKI) of 8.2 (Table 1).
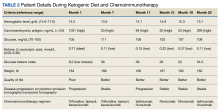
A follow-up PET/CT showed disease progression along with a CEA of 94 ng/mL after 10 months of chemotherapy plus the ketogenic diet (Table 2).
The patient continued to experience excellent QOL based on the QOL Eastern Cooperative Oncology Group (ECOG) core quality of life questionnaire (QLC-C30) forms, which he completed every 3 months. Twenty-two months after starting the ketogenic diet, the patient’s CEA increased to 293 ng/mL although PET/CT continues to show stable disease (Figures 4, 5, and 6).
DISCUSSION
The purpose of this case report is to describe whether a patient receiving active cancer treatment was able to tolerate the ketogenic diet in conjunction with chemotherapy or immunotherapy. Most literature published on the subject evaluated the tolerability and response of the ketogenic diet after the failure of standard therapy. Our patient was diagnosed with stage III mucinous colon adenocarcinoma. He received adjuvant chemotherapy but quickly developed metastatic disease to the pancreas and omentum. We started him on encorafenib and cetuximab based on the BEACON study that showed improvement in response rate and survival when compared with standard chemotherapy for patients with BRAF V600E mutation.5 Unfortunately, his cancer quickly progressed within 4 months and again did not respond to pembrolizumab despite MSI-H, which lasted for another 4 months.
We suggested the ketogenic diet and the patient agreed. He started the diet along with trifluridine/tipiracil, and bevacizumab in January 2021. The patient’s metastatic cancer stabilized for 9 months until his disease progressed again. He was started on doublet immune checkpoint inhibitors ipilimumab and nivolumab based on his MSI-H and high tumor mutation burden with the continuation of the ketogenic diet until now. The CheckMate 142 study revealed that the combination of ipilimumab and nivolumab in patients with MSI-H previously treated for metastatic colon cancer showed some benefit.6
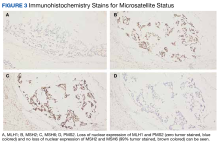
Our patient had the loss of nuclear expression of MLH1 and PMS2 (zero tumor stained) but no evidence of the loss expression of MSH2 and MSH6 genes (99% tumor stained). About 8% to 12% of patients with metastatic colon cancer have BRAF V600E mutations that are usually mucinous type, poorly differentiated, and located in the right side of the colon, which portends to a poor prognosis. Tumor DNA mismatch repair damage results in genetic hypermutability and leads to MSI that is sensitive to treatment with checkpoint inhibitors, as in our patient. Only about 3% of MSI-H tumors are due to germline mutations such as Lynch syndrome (hereditary nonpolyposis colorectal cancer). The presence of both MLH1 hypermethylation and BRAF mutation, as in our patient, is a strong indication of somatic rather than germline mutation.7
GKI, which represents the ratio of glucose to ketone, was developed to evaluate the efficacy of the ketogenic diet. This index measures the degree of metabolic stress on tumor cells through the decrease of glucose levels and increase of ketone bodies. A GKI of ≤ 1.0 has been suggested as the ideal therapeutic goal for cancer management.8 As levels of blood glucose decline, the blood levels of ketone bodies should rise. These 2 lines should eventually intersect at a certain point beyond which one enters the therapeutic zone or therapeutic ketosis zone. This is when tumor growth is expected to slow or cease.9 The patient’s ketone (β-hydroxybutyrate) level was initially high (0.71 mmol/L) with a GKI of 8.2. (low ketotic level), which meant he tolerated a rather strict diet for the first several months. This was also reflected in his 18 lb weight loss (almost 10% of body weight) and cancer stabilization, as in our previous publication.1 Unfortunately, the patient was unable to maintain high ketone and lower GKI levels due to fatigue from depleted carbohydrate intake. He added some carbohydrate snacks in between meals, which improved the fatigue. His ketone level has been < 0.5 mmol/L ever since, albeit his disease continues to be stable. The patient continues his daily work and reports a better QOL, based on the ECOG QLC-C30 form that he completed every 3 months.10 Currently, the patient is still receiving ipilimumab and nivolumab while maintaining the ketogenic diet with stable metastatic disease on PET/CT.
Ketogenic Diet and Cellular Mechanism of Action
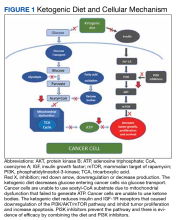
PI3K/Akt (phosphatidylinositol-3-kinase) signaling is one of the most important intracellular pathways for tumor cells. It leads to the inhibition of apoptosis and the promotion of cell proliferation, metabolism, and angiogenesis. Deregulation of the PI3K pathway either via amplification of PI3K by tyrosine kinase growth factor receptors or inactivation of the tumor suppressor phosphatase and tensin homolog (PTEN), which is the negative regulator of the PI3K pathway, contributes to the development of cancer cells.11
A study by Goncalves and colleagues revealed an interesting relationship between the PI3K pathway and the benefit of the ketogenic diet to slow tumor growth. PI3K inhibitors inhibit glucose uptake into skeletal muscle and adipose tissue that activate hepatic glycogenolysis. This event results in hyperglycemia due to the pancreas releasing very high levels of insulin into the blood (hyperinsulinemia) that subsequently reactivate PI3K signaling and cause resistance to PI3K inhibitors. The ketogenic diet reportedly minimized the hyperglycemia and hyperinsulinemia induced by the PI3K inhibitor and enhanced the efficacy of PI3K inhibitors in tumor models. Studies combining PI3K inhibitors and ketogenic diet are underway. Hence, combining the ketogenic diet with chemotherapy or other novel treatment should be the focus of ketogenic diet trials.12,13
Ketogenic Diet and Oncology Studies
The impact of the ketogenic diet on the growth of murine pancreatic tumors was evaluated by Yang and colleagues. The ketogenic diet decreased glucose concentration that enters the TCA cycle and increased fatty acid oxidation that produces β-hydroxybutyrate. This event promotes the generation of ATP, although with only modest elevations of NADH with less impact on tumor growth. The combination of ketogenic diet and standard chemotherapy substantially raised tumor NADH and suppressed the growth of murine tumor cells, they noted.14 Furukawa and colleagues compared 10 patients with metastatic colon cancer receiving chemotherapy plus the modified medium-chain triglyceride ketogenic diet for 1 year with 14 patients receiving chemotherapy only. The ketogenic diet group exhibited a response rate of 60% with 5 patients achieving a complete response and a disease control rate of 70%, while the chemotherapy-alone group showed a response rate of only 21% with no complete response and a disease control rate of 64%.15
The ketogenic diet also reportedly stimulates cytokine and CD4+ and CD8+ T-cell production that stimulates T-cell killing activity. The ketogenic diet may overcome several immune escape mechanisms by downregulating the expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on tumor-infiltrating lymphocytes.16 Our patient tolerated the combination of the ketogenic diet with ipilimumab (CTLA-4 inhibitor) and nivolumab (PD-1 inhibitor) without significant toxicities and stabilization of his disease.
Future Directions
We originally presented the abstract and poster of this case report at the Association of VA Hematology/Oncology annual meeting in San Diego, California, in September 2022.17 Based on our previous experience, we are now using a modified Atkins diet, which is a less strict diet consisting of 60% fat, 30% protein, and 10% carbohydrates combined with chemotherapy and/or immunotherapy. The composition of fat to carbohydrate plus protein in the traditional ketogenic diet is usually 4:1 or 3:1, while in modified Atkins diet the ratio is 1:1 or 2:1. The benefit of the modified Atkins diet is that patients can consume more protein than a strict ketogenic diet and they can be more liberal in carbohydrate allowances. We are about to open a study protocol of combining a modified Atkin diet and chemotherapy and/or immunotherapy as a first-line treatment for veterans with all types of advanced or metastatic solid tumors at VACCHCS. The study protocol was approved by the VA Office of Research and Development and has been submitted to the VACCHCS Institutional Review Board for review. Once approved, we will start patient recruitment.
CONCLUSIONS
Cancer cells have defects in their mitochondria that prevent them from generating energy for metabolism in the absence of glucose. They also depend on the PI3K signaling pathway to survive. The ketogenic diet has the advantage of affecting cancer cell growth by exploiting these mitochondrial defects and blocking hyperglycemia. There is growing evidence that the ketogenic diet is feasible, tolerable, and reportedly inhibits cancer growth. Our case report and previous publications suggest that the ketogenic diet can be added to chemotherapy and/or immunotherapy as an adjunct to standard-of-care cancer treatment while maintaining good QOL. We are planning to open a clinical trial using the modified Atkins diet in conjunction with active cancer treatments as first-line therapy for metastatic solid tumors at the VACCHCS. We are also working closely with researchers from several veteran hospitals to do a diet collaborative study. We believe the ketogenic diet is an important part of cancer treatment and has a promising future. More research should be dedicated to this very interesting field.
Acknowledgments
We previously presented this case report in an abstract and poster at the September 2022 AVAHO meeting in San Diego, California.
1. Tan-Shalaby JL, Carrick J, Edinger K, et al. Modified Atkins diet in advanced malignancies-final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr Metab (Lond). 2016;13:52. Published 2016 Aug 12. doi:10.1186/s12986-016-0113-y
2. Talib WH, Mahmod AI, Kamal A, et al. Ketogenic diet in cancer prevention and therapy: molecular targets and therapeutic opportunities. Curr Issues Mol Biol. 2021;43(2):558-589. Published 2021 Jul 3. doi:10.3390/cimb43020042
3. Tan-Shalaby J. Ketogenic diets and cancer: emerging evidence. Fed Pract. 2017;34(suppl 1):37S-42S.
4. Cortez NE, Mackenzie GG. Ketogenic diets in pancreatic cancer and associated cachexia: cellular mechanisms and clinical perspectives. Nutrients. 2021;13(9):3202. Published 2021 Sep 15. doi:10.3390/nu13093202
5. Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273-284. doi:10.1200/JCO.20.02088
6. André T, Lonardi S, Wong KYM, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol. 2022;33(10):1052-1060. doi:10.1016/j.annonc.2022.06.008
7. Grassi E, Corbelli J, Papiani G, Barbera MA, Gazzaneo F, Tamberi S. Current therapeutic strategies in BRAF-mutant metastatic colorectal cancer. Front Oncol. 2021;11:601722. Published 2021 Jun 23. doi:10.3389/fonc.2021.601722
8. Seyfried TN, Mukherjee P, Iyikesici MS, et al. Consideration of ketogenic metabolic therapy as a complementary or alternative approach for managing breast cancer. Front Nutr. 2020;7:21. Published 2020 Mar 11. doi:10.3389/fnut.2020.00021
9. Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr Metab (Lond). 2015;12:12. Published 2015 Mar 11. doi:10.1186/s12986-015-0009-2
10. Fayers P, Bottomley A; EORTC Quality of Life Group; Quality of Life Unit. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38(suppl 4):S125-S133. doi:10.1016/s0959-8049(01)00448-8
11. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. Published 2019 Feb 19. doi:10.1186/s12943-019-0954-x
12. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379(21):2052-2062. doi:10.1056/NEJMra1704560
13. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer-where do we stand?. Mol Metab. 2020;33:102-121. doi:10.1016/j.molmet.2019.06.026
14. Yang L, TeSlaa T, Ng S, et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 2022;3(2):119-136. doi:10.1016/j.medj.2021.12.008
15. Furukawa K, Shigematus K, Iwase Y, et al. Clinical effects of one year of chemotherapy with a modified medium-chain triglyceride ketogenic diet on the recurrence of stage IV colon cancer. J Clin Oncol. 2018;36(suppl 15):e15709. doi:10.1200/JCO.2018.36.15_suppl.e15709
16. Zhang X, Li H, Lv X, et al. Impact of diets on response to immune checkpoint inhibitors (ICIs) therapy against tumors. Life (Basel). 2022;12(3):409. Published 2022 Mar 11. doi:10.3390/life12030409
17. Liman, A, Hwang A, Means J, Newson J. Ketogenic diet and cancer: a case report and feasibility study at VA Central California Healthcare System. Fed Pract. 2022;39(suppl 4):S18.
Originally developed for the treatment of refractory epilepsy, the ketogenic diet is distinguished by its high-fat, moderate-protein, and low-carbohydrate food program. Preclinical models provide emerging evidence that a ketogenic diet can have therapeutic potential for a broad range of cancers. The Warburg effect is a condition where cancer cells increase the uptake and fermentation of glucose to produce lactate for their metabolism, which is called aerobic glycolysis. Lactate is the key driver of cancer angiogenesis and proliferation.1,2
The ketogenic diet promotes a metabolic shift from glycolysis to mitochondrial metabolism in normal cells while cancer cells have dysfunction in their mitochondria due to damage in cellular respiration. The ketogenic diet creates a metabolic state whereby blood glucose levels are reduced, and blood ketone bodies (D-β-hydroxybutyrate and acetoacetate) are elevated. In normal cells, the ketogenic diet causes a decrease in glucose intake for glycolysis, which makes them unable to produce enough substrate to enter the tricarboxylic acid (TCA) cycle for adenosine triphosphate (ATP) production. Fatty acid oxidation plays a key role in ketone body synthesis as a “super fuel” that enter the TCA cycle as an alternative pathway to generate ATP. On the other hand, cancer cells are unable to use ketone bodies to produce ATP for energy and metabolism due to mitochondrial defects. Lack of energy subsequently leads to the inhibition of proliferation and survival of cancer cells.3,4
We previously published a safety and feasibility study of the Modified Atkins Diet in metastatic cancer patients after failure of chemotherapy at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System.1 None of the patients were on chemotherapy at the time of enrollment. The Modified Atkins Diet consists of 60% fat, 30% protein, and 10% carbohydrates and is more tolerable than the ketogenic diet due to higher amounts of protein. Six of 11 patients (54%) had stable disease and partial response on positron emission tomography/computed tomography (PET/CT). Our study showed that patients who lost at least 10% of their body weight had improvement in quality of life (QOL) and cancer response.1 Here we present a case of a veteran with extensive metastatic colon cancer on concurrent ketogenic diet and chemotherapy subsequently followed by concurrent ketogenic diet and immunotherapy at Veterans Affairs Central California Health Care Systems (VACCHCS) in Fresno.
CASE PRESENTATION
A 69-year-old veteran had iron deficiency anemia (hemoglobin, 6.5 g/dL) about 5 years previously. He underwent a colonoscopy that revealed a near circumferential ulcerated mass measuring 7 cm in the transverse colon. Biopsy results showed mucinous adenocarcinoma of the colon with a foci of signet ring cells (Figure 2).
The patient received adjuvant treatment with FOLFOX (fluorouracil, leucovorin calcium, and oxaliplatin), but within several months he developed pancreatic and worsening omental metastasis seen on PET/CT. He was then started on FOLFIRI (fluorouracil, leucovorin calcium, and irinotecan hydrochloride) plus bevacizumab 16 months after his initial diagnosis. He underwent a pancreatic mastectomy that confirmed adenocarcinoma 9 months later. Afterward, he briefly resumed FOLFIRI and bevacizumab. Next-generation sequencing testing with Foundation One CDx revealed a wild-type (WT) KRAS with a high degree of tumor mutation burden of 37 muts/Mb, BRAF V600E mutation, and high microsatellite instability (MSI-H).
Due to disease progression, the patient’s treatment was changed to encorafenib and cetuximab for 4 months before progressing again with new liver mass and mediastinal lymphadenopathy. He then received pembrolizumab for 4 months until PET/CT showed progression and his carcinoembryonic antigen (CEA) increased from 95 to 1031 ng/mL by January 2021 (Figure 4).
The patient was started on trifluridine/tipiracil, and bevacizumab while concurrently initiating the ketogenic diet in January 2021. Laboratory tests drawn after 1 week of strict dietary ketogenic diet adherence showed low-level ketosis with a glucose ketone index (GKI) of 8.2 (Table 1).
A follow-up PET/CT showed disease progression along with a CEA of 94 ng/mL after 10 months of chemotherapy plus the ketogenic diet (Table 2).
The patient continued to experience excellent QOL based on the QOL Eastern Cooperative Oncology Group (ECOG) core quality of life questionnaire (QLC-C30) forms, which he completed every 3 months. Twenty-two months after starting the ketogenic diet, the patient’s CEA increased to 293 ng/mL although PET/CT continues to show stable disease (Figures 4, 5, and 6).
DISCUSSION
The purpose of this case report is to describe whether a patient receiving active cancer treatment was able to tolerate the ketogenic diet in conjunction with chemotherapy or immunotherapy. Most literature published on the subject evaluated the tolerability and response of the ketogenic diet after the failure of standard therapy. Our patient was diagnosed with stage III mucinous colon adenocarcinoma. He received adjuvant chemotherapy but quickly developed metastatic disease to the pancreas and omentum. We started him on encorafenib and cetuximab based on the BEACON study that showed improvement in response rate and survival when compared with standard chemotherapy for patients with BRAF V600E mutation.5 Unfortunately, his cancer quickly progressed within 4 months and again did not respond to pembrolizumab despite MSI-H, which lasted for another 4 months.
We suggested the ketogenic diet and the patient agreed. He started the diet along with trifluridine/tipiracil, and bevacizumab in January 2021. The patient’s metastatic cancer stabilized for 9 months until his disease progressed again. He was started on doublet immune checkpoint inhibitors ipilimumab and nivolumab based on his MSI-H and high tumor mutation burden with the continuation of the ketogenic diet until now. The CheckMate 142 study revealed that the combination of ipilimumab and nivolumab in patients with MSI-H previously treated for metastatic colon cancer showed some benefit.6
Our patient had the loss of nuclear expression of MLH1 and PMS2 (zero tumor stained) but no evidence of the loss expression of MSH2 and MSH6 genes (99% tumor stained). About 8% to 12% of patients with metastatic colon cancer have BRAF V600E mutations that are usually mucinous type, poorly differentiated, and located in the right side of the colon, which portends to a poor prognosis. Tumor DNA mismatch repair damage results in genetic hypermutability and leads to MSI that is sensitive to treatment with checkpoint inhibitors, as in our patient. Only about 3% of MSI-H tumors are due to germline mutations such as Lynch syndrome (hereditary nonpolyposis colorectal cancer). The presence of both MLH1 hypermethylation and BRAF mutation, as in our patient, is a strong indication of somatic rather than germline mutation.7
GKI, which represents the ratio of glucose to ketone, was developed to evaluate the efficacy of the ketogenic diet. This index measures the degree of metabolic stress on tumor cells through the decrease of glucose levels and increase of ketone bodies. A GKI of ≤ 1.0 has been suggested as the ideal therapeutic goal for cancer management.8 As levels of blood glucose decline, the blood levels of ketone bodies should rise. These 2 lines should eventually intersect at a certain point beyond which one enters the therapeutic zone or therapeutic ketosis zone. This is when tumor growth is expected to slow or cease.9 The patient’s ketone (β-hydroxybutyrate) level was initially high (0.71 mmol/L) with a GKI of 8.2. (low ketotic level), which meant he tolerated a rather strict diet for the first several months. This was also reflected in his 18 lb weight loss (almost 10% of body weight) and cancer stabilization, as in our previous publication.1 Unfortunately, the patient was unable to maintain high ketone and lower GKI levels due to fatigue from depleted carbohydrate intake. He added some carbohydrate snacks in between meals, which improved the fatigue. His ketone level has been < 0.5 mmol/L ever since, albeit his disease continues to be stable. The patient continues his daily work and reports a better QOL, based on the ECOG QLC-C30 form that he completed every 3 months.10 Currently, the patient is still receiving ipilimumab and nivolumab while maintaining the ketogenic diet with stable metastatic disease on PET/CT.
Ketogenic Diet and Cellular Mechanism of Action
PI3K/Akt (phosphatidylinositol-3-kinase) signaling is one of the most important intracellular pathways for tumor cells. It leads to the inhibition of apoptosis and the promotion of cell proliferation, metabolism, and angiogenesis. Deregulation of the PI3K pathway either via amplification of PI3K by tyrosine kinase growth factor receptors or inactivation of the tumor suppressor phosphatase and tensin homolog (PTEN), which is the negative regulator of the PI3K pathway, contributes to the development of cancer cells.11
A study by Goncalves and colleagues revealed an interesting relationship between the PI3K pathway and the benefit of the ketogenic diet to slow tumor growth. PI3K inhibitors inhibit glucose uptake into skeletal muscle and adipose tissue that activate hepatic glycogenolysis. This event results in hyperglycemia due to the pancreas releasing very high levels of insulin into the blood (hyperinsulinemia) that subsequently reactivate PI3K signaling and cause resistance to PI3K inhibitors. The ketogenic diet reportedly minimized the hyperglycemia and hyperinsulinemia induced by the PI3K inhibitor and enhanced the efficacy of PI3K inhibitors in tumor models. Studies combining PI3K inhibitors and ketogenic diet are underway. Hence, combining the ketogenic diet with chemotherapy or other novel treatment should be the focus of ketogenic diet trials.12,13
Ketogenic Diet and Oncology Studies
The impact of the ketogenic diet on the growth of murine pancreatic tumors was evaluated by Yang and colleagues. The ketogenic diet decreased glucose concentration that enters the TCA cycle and increased fatty acid oxidation that produces β-hydroxybutyrate. This event promotes the generation of ATP, although with only modest elevations of NADH with less impact on tumor growth. The combination of ketogenic diet and standard chemotherapy substantially raised tumor NADH and suppressed the growth of murine tumor cells, they noted.14 Furukawa and colleagues compared 10 patients with metastatic colon cancer receiving chemotherapy plus the modified medium-chain triglyceride ketogenic diet for 1 year with 14 patients receiving chemotherapy only. The ketogenic diet group exhibited a response rate of 60% with 5 patients achieving a complete response and a disease control rate of 70%, while the chemotherapy-alone group showed a response rate of only 21% with no complete response and a disease control rate of 64%.15
The ketogenic diet also reportedly stimulates cytokine and CD4+ and CD8+ T-cell production that stimulates T-cell killing activity. The ketogenic diet may overcome several immune escape mechanisms by downregulating the expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on tumor-infiltrating lymphocytes.16 Our patient tolerated the combination of the ketogenic diet with ipilimumab (CTLA-4 inhibitor) and nivolumab (PD-1 inhibitor) without significant toxicities and stabilization of his disease.
Future Directions
We originally presented the abstract and poster of this case report at the Association of VA Hematology/Oncology annual meeting in San Diego, California, in September 2022.17 Based on our previous experience, we are now using a modified Atkins diet, which is a less strict diet consisting of 60% fat, 30% protein, and 10% carbohydrates combined with chemotherapy and/or immunotherapy. The composition of fat to carbohydrate plus protein in the traditional ketogenic diet is usually 4:1 or 3:1, while in modified Atkins diet the ratio is 1:1 or 2:1. The benefit of the modified Atkins diet is that patients can consume more protein than a strict ketogenic diet and they can be more liberal in carbohydrate allowances. We are about to open a study protocol of combining a modified Atkin diet and chemotherapy and/or immunotherapy as a first-line treatment for veterans with all types of advanced or metastatic solid tumors at VACCHCS. The study protocol was approved by the VA Office of Research and Development and has been submitted to the VACCHCS Institutional Review Board for review. Once approved, we will start patient recruitment.
CONCLUSIONS
Cancer cells have defects in their mitochondria that prevent them from generating energy for metabolism in the absence of glucose. They also depend on the PI3K signaling pathway to survive. The ketogenic diet has the advantage of affecting cancer cell growth by exploiting these mitochondrial defects and blocking hyperglycemia. There is growing evidence that the ketogenic diet is feasible, tolerable, and reportedly inhibits cancer growth. Our case report and previous publications suggest that the ketogenic diet can be added to chemotherapy and/or immunotherapy as an adjunct to standard-of-care cancer treatment while maintaining good QOL. We are planning to open a clinical trial using the modified Atkins diet in conjunction with active cancer treatments as first-line therapy for metastatic solid tumors at the VACCHCS. We are also working closely with researchers from several veteran hospitals to do a diet collaborative study. We believe the ketogenic diet is an important part of cancer treatment and has a promising future. More research should be dedicated to this very interesting field.
Acknowledgments
We previously presented this case report in an abstract and poster at the September 2022 AVAHO meeting in San Diego, California.
Originally developed for the treatment of refractory epilepsy, the ketogenic diet is distinguished by its high-fat, moderate-protein, and low-carbohydrate food program. Preclinical models provide emerging evidence that a ketogenic diet can have therapeutic potential for a broad range of cancers. The Warburg effect is a condition where cancer cells increase the uptake and fermentation of glucose to produce lactate for their metabolism, which is called aerobic glycolysis. Lactate is the key driver of cancer angiogenesis and proliferation.1,2
The ketogenic diet promotes a metabolic shift from glycolysis to mitochondrial metabolism in normal cells while cancer cells have dysfunction in their mitochondria due to damage in cellular respiration. The ketogenic diet creates a metabolic state whereby blood glucose levels are reduced, and blood ketone bodies (D-β-hydroxybutyrate and acetoacetate) are elevated. In normal cells, the ketogenic diet causes a decrease in glucose intake for glycolysis, which makes them unable to produce enough substrate to enter the tricarboxylic acid (TCA) cycle for adenosine triphosphate (ATP) production. Fatty acid oxidation plays a key role in ketone body synthesis as a “super fuel” that enter the TCA cycle as an alternative pathway to generate ATP. On the other hand, cancer cells are unable to use ketone bodies to produce ATP for energy and metabolism due to mitochondrial defects. Lack of energy subsequently leads to the inhibition of proliferation and survival of cancer cells.3,4
We previously published a safety and feasibility study of the Modified Atkins Diet in metastatic cancer patients after failure of chemotherapy at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System.1 None of the patients were on chemotherapy at the time of enrollment. The Modified Atkins Diet consists of 60% fat, 30% protein, and 10% carbohydrates and is more tolerable than the ketogenic diet due to higher amounts of protein. Six of 11 patients (54%) had stable disease and partial response on positron emission tomography/computed tomography (PET/CT). Our study showed that patients who lost at least 10% of their body weight had improvement in quality of life (QOL) and cancer response.1 Here we present a case of a veteran with extensive metastatic colon cancer on concurrent ketogenic diet and chemotherapy subsequently followed by concurrent ketogenic diet and immunotherapy at Veterans Affairs Central California Health Care Systems (VACCHCS) in Fresno.
CASE PRESENTATION
A 69-year-old veteran had iron deficiency anemia (hemoglobin, 6.5 g/dL) about 5 years previously. He underwent a colonoscopy that revealed a near circumferential ulcerated mass measuring 7 cm in the transverse colon. Biopsy results showed mucinous adenocarcinoma of the colon with a foci of signet ring cells (Figure 2).
The patient received adjuvant treatment with FOLFOX (fluorouracil, leucovorin calcium, and oxaliplatin), but within several months he developed pancreatic and worsening omental metastasis seen on PET/CT. He was then started on FOLFIRI (fluorouracil, leucovorin calcium, and irinotecan hydrochloride) plus bevacizumab 16 months after his initial diagnosis. He underwent a pancreatic mastectomy that confirmed adenocarcinoma 9 months later. Afterward, he briefly resumed FOLFIRI and bevacizumab. Next-generation sequencing testing with Foundation One CDx revealed a wild-type (WT) KRAS with a high degree of tumor mutation burden of 37 muts/Mb, BRAF V600E mutation, and high microsatellite instability (MSI-H).
Due to disease progression, the patient’s treatment was changed to encorafenib and cetuximab for 4 months before progressing again with new liver mass and mediastinal lymphadenopathy. He then received pembrolizumab for 4 months until PET/CT showed progression and his carcinoembryonic antigen (CEA) increased from 95 to 1031 ng/mL by January 2021 (Figure 4).
The patient was started on trifluridine/tipiracil, and bevacizumab while concurrently initiating the ketogenic diet in January 2021. Laboratory tests drawn after 1 week of strict dietary ketogenic diet adherence showed low-level ketosis with a glucose ketone index (GKI) of 8.2 (Table 1).
A follow-up PET/CT showed disease progression along with a CEA of 94 ng/mL after 10 months of chemotherapy plus the ketogenic diet (Table 2).
The patient continued to experience excellent QOL based on the QOL Eastern Cooperative Oncology Group (ECOG) core quality of life questionnaire (QLC-C30) forms, which he completed every 3 months. Twenty-two months after starting the ketogenic diet, the patient’s CEA increased to 293 ng/mL although PET/CT continues to show stable disease (Figures 4, 5, and 6).
DISCUSSION
The purpose of this case report is to describe whether a patient receiving active cancer treatment was able to tolerate the ketogenic diet in conjunction with chemotherapy or immunotherapy. Most literature published on the subject evaluated the tolerability and response of the ketogenic diet after the failure of standard therapy. Our patient was diagnosed with stage III mucinous colon adenocarcinoma. He received adjuvant chemotherapy but quickly developed metastatic disease to the pancreas and omentum. We started him on encorafenib and cetuximab based on the BEACON study that showed improvement in response rate and survival when compared with standard chemotherapy for patients with BRAF V600E mutation.5 Unfortunately, his cancer quickly progressed within 4 months and again did not respond to pembrolizumab despite MSI-H, which lasted for another 4 months.
We suggested the ketogenic diet and the patient agreed. He started the diet along with trifluridine/tipiracil, and bevacizumab in January 2021. The patient’s metastatic cancer stabilized for 9 months until his disease progressed again. He was started on doublet immune checkpoint inhibitors ipilimumab and nivolumab based on his MSI-H and high tumor mutation burden with the continuation of the ketogenic diet until now. The CheckMate 142 study revealed that the combination of ipilimumab and nivolumab in patients with MSI-H previously treated for metastatic colon cancer showed some benefit.6
Our patient had the loss of nuclear expression of MLH1 and PMS2 (zero tumor stained) but no evidence of the loss expression of MSH2 and MSH6 genes (99% tumor stained). About 8% to 12% of patients with metastatic colon cancer have BRAF V600E mutations that are usually mucinous type, poorly differentiated, and located in the right side of the colon, which portends to a poor prognosis. Tumor DNA mismatch repair damage results in genetic hypermutability and leads to MSI that is sensitive to treatment with checkpoint inhibitors, as in our patient. Only about 3% of MSI-H tumors are due to germline mutations such as Lynch syndrome (hereditary nonpolyposis colorectal cancer). The presence of both MLH1 hypermethylation and BRAF mutation, as in our patient, is a strong indication of somatic rather than germline mutation.7
GKI, which represents the ratio of glucose to ketone, was developed to evaluate the efficacy of the ketogenic diet. This index measures the degree of metabolic stress on tumor cells through the decrease of glucose levels and increase of ketone bodies. A GKI of ≤ 1.0 has been suggested as the ideal therapeutic goal for cancer management.8 As levels of blood glucose decline, the blood levels of ketone bodies should rise. These 2 lines should eventually intersect at a certain point beyond which one enters the therapeutic zone or therapeutic ketosis zone. This is when tumor growth is expected to slow or cease.9 The patient’s ketone (β-hydroxybutyrate) level was initially high (0.71 mmol/L) with a GKI of 8.2. (low ketotic level), which meant he tolerated a rather strict diet for the first several months. This was also reflected in his 18 lb weight loss (almost 10% of body weight) and cancer stabilization, as in our previous publication.1 Unfortunately, the patient was unable to maintain high ketone and lower GKI levels due to fatigue from depleted carbohydrate intake. He added some carbohydrate snacks in between meals, which improved the fatigue. His ketone level has been < 0.5 mmol/L ever since, albeit his disease continues to be stable. The patient continues his daily work and reports a better QOL, based on the ECOG QLC-C30 form that he completed every 3 months.10 Currently, the patient is still receiving ipilimumab and nivolumab while maintaining the ketogenic diet with stable metastatic disease on PET/CT.
Ketogenic Diet and Cellular Mechanism of Action
PI3K/Akt (phosphatidylinositol-3-kinase) signaling is one of the most important intracellular pathways for tumor cells. It leads to the inhibition of apoptosis and the promotion of cell proliferation, metabolism, and angiogenesis. Deregulation of the PI3K pathway either via amplification of PI3K by tyrosine kinase growth factor receptors or inactivation of the tumor suppressor phosphatase and tensin homolog (PTEN), which is the negative regulator of the PI3K pathway, contributes to the development of cancer cells.11
A study by Goncalves and colleagues revealed an interesting relationship between the PI3K pathway and the benefit of the ketogenic diet to slow tumor growth. PI3K inhibitors inhibit glucose uptake into skeletal muscle and adipose tissue that activate hepatic glycogenolysis. This event results in hyperglycemia due to the pancreas releasing very high levels of insulin into the blood (hyperinsulinemia) that subsequently reactivate PI3K signaling and cause resistance to PI3K inhibitors. The ketogenic diet reportedly minimized the hyperglycemia and hyperinsulinemia induced by the PI3K inhibitor and enhanced the efficacy of PI3K inhibitors in tumor models. Studies combining PI3K inhibitors and ketogenic diet are underway. Hence, combining the ketogenic diet with chemotherapy or other novel treatment should be the focus of ketogenic diet trials.12,13
Ketogenic Diet and Oncology Studies
The impact of the ketogenic diet on the growth of murine pancreatic tumors was evaluated by Yang and colleagues. The ketogenic diet decreased glucose concentration that enters the TCA cycle and increased fatty acid oxidation that produces β-hydroxybutyrate. This event promotes the generation of ATP, although with only modest elevations of NADH with less impact on tumor growth. The combination of ketogenic diet and standard chemotherapy substantially raised tumor NADH and suppressed the growth of murine tumor cells, they noted.14 Furukawa and colleagues compared 10 patients with metastatic colon cancer receiving chemotherapy plus the modified medium-chain triglyceride ketogenic diet for 1 year with 14 patients receiving chemotherapy only. The ketogenic diet group exhibited a response rate of 60% with 5 patients achieving a complete response and a disease control rate of 70%, while the chemotherapy-alone group showed a response rate of only 21% with no complete response and a disease control rate of 64%.15
The ketogenic diet also reportedly stimulates cytokine and CD4+ and CD8+ T-cell production that stimulates T-cell killing activity. The ketogenic diet may overcome several immune escape mechanisms by downregulating the expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on tumor-infiltrating lymphocytes.16 Our patient tolerated the combination of the ketogenic diet with ipilimumab (CTLA-4 inhibitor) and nivolumab (PD-1 inhibitor) without significant toxicities and stabilization of his disease.
Future Directions
We originally presented the abstract and poster of this case report at the Association of VA Hematology/Oncology annual meeting in San Diego, California, in September 2022.17 Based on our previous experience, we are now using a modified Atkins diet, which is a less strict diet consisting of 60% fat, 30% protein, and 10% carbohydrates combined with chemotherapy and/or immunotherapy. The composition of fat to carbohydrate plus protein in the traditional ketogenic diet is usually 4:1 or 3:1, while in modified Atkins diet the ratio is 1:1 or 2:1. The benefit of the modified Atkins diet is that patients can consume more protein than a strict ketogenic diet and they can be more liberal in carbohydrate allowances. We are about to open a study protocol of combining a modified Atkin diet and chemotherapy and/or immunotherapy as a first-line treatment for veterans with all types of advanced or metastatic solid tumors at VACCHCS. The study protocol was approved by the VA Office of Research and Development and has been submitted to the VACCHCS Institutional Review Board for review. Once approved, we will start patient recruitment.
CONCLUSIONS
Cancer cells have defects in their mitochondria that prevent them from generating energy for metabolism in the absence of glucose. They also depend on the PI3K signaling pathway to survive. The ketogenic diet has the advantage of affecting cancer cell growth by exploiting these mitochondrial defects and blocking hyperglycemia. There is growing evidence that the ketogenic diet is feasible, tolerable, and reportedly inhibits cancer growth. Our case report and previous publications suggest that the ketogenic diet can be added to chemotherapy and/or immunotherapy as an adjunct to standard-of-care cancer treatment while maintaining good QOL. We are planning to open a clinical trial using the modified Atkins diet in conjunction with active cancer treatments as first-line therapy for metastatic solid tumors at the VACCHCS. We are also working closely with researchers from several veteran hospitals to do a diet collaborative study. We believe the ketogenic diet is an important part of cancer treatment and has a promising future. More research should be dedicated to this very interesting field.
Acknowledgments
We previously presented this case report in an abstract and poster at the September 2022 AVAHO meeting in San Diego, California.
1. Tan-Shalaby JL, Carrick J, Edinger K, et al. Modified Atkins diet in advanced malignancies-final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr Metab (Lond). 2016;13:52. Published 2016 Aug 12. doi:10.1186/s12986-016-0113-y
2. Talib WH, Mahmod AI, Kamal A, et al. Ketogenic diet in cancer prevention and therapy: molecular targets and therapeutic opportunities. Curr Issues Mol Biol. 2021;43(2):558-589. Published 2021 Jul 3. doi:10.3390/cimb43020042
3. Tan-Shalaby J. Ketogenic diets and cancer: emerging evidence. Fed Pract. 2017;34(suppl 1):37S-42S.
4. Cortez NE, Mackenzie GG. Ketogenic diets in pancreatic cancer and associated cachexia: cellular mechanisms and clinical perspectives. Nutrients. 2021;13(9):3202. Published 2021 Sep 15. doi:10.3390/nu13093202
5. Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273-284. doi:10.1200/JCO.20.02088
6. André T, Lonardi S, Wong KYM, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol. 2022;33(10):1052-1060. doi:10.1016/j.annonc.2022.06.008
7. Grassi E, Corbelli J, Papiani G, Barbera MA, Gazzaneo F, Tamberi S. Current therapeutic strategies in BRAF-mutant metastatic colorectal cancer. Front Oncol. 2021;11:601722. Published 2021 Jun 23. doi:10.3389/fonc.2021.601722
8. Seyfried TN, Mukherjee P, Iyikesici MS, et al. Consideration of ketogenic metabolic therapy as a complementary or alternative approach for managing breast cancer. Front Nutr. 2020;7:21. Published 2020 Mar 11. doi:10.3389/fnut.2020.00021
9. Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr Metab (Lond). 2015;12:12. Published 2015 Mar 11. doi:10.1186/s12986-015-0009-2
10. Fayers P, Bottomley A; EORTC Quality of Life Group; Quality of Life Unit. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38(suppl 4):S125-S133. doi:10.1016/s0959-8049(01)00448-8
11. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. Published 2019 Feb 19. doi:10.1186/s12943-019-0954-x
12. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379(21):2052-2062. doi:10.1056/NEJMra1704560
13. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer-where do we stand?. Mol Metab. 2020;33:102-121. doi:10.1016/j.molmet.2019.06.026
14. Yang L, TeSlaa T, Ng S, et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 2022;3(2):119-136. doi:10.1016/j.medj.2021.12.008
15. Furukawa K, Shigematus K, Iwase Y, et al. Clinical effects of one year of chemotherapy with a modified medium-chain triglyceride ketogenic diet on the recurrence of stage IV colon cancer. J Clin Oncol. 2018;36(suppl 15):e15709. doi:10.1200/JCO.2018.36.15_suppl.e15709
16. Zhang X, Li H, Lv X, et al. Impact of diets on response to immune checkpoint inhibitors (ICIs) therapy against tumors. Life (Basel). 2022;12(3):409. Published 2022 Mar 11. doi:10.3390/life12030409
17. Liman, A, Hwang A, Means J, Newson J. Ketogenic diet and cancer: a case report and feasibility study at VA Central California Healthcare System. Fed Pract. 2022;39(suppl 4):S18.
1. Tan-Shalaby JL, Carrick J, Edinger K, et al. Modified Atkins diet in advanced malignancies-final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr Metab (Lond). 2016;13:52. Published 2016 Aug 12. doi:10.1186/s12986-016-0113-y
2. Talib WH, Mahmod AI, Kamal A, et al. Ketogenic diet in cancer prevention and therapy: molecular targets and therapeutic opportunities. Curr Issues Mol Biol. 2021;43(2):558-589. Published 2021 Jul 3. doi:10.3390/cimb43020042
3. Tan-Shalaby J. Ketogenic diets and cancer: emerging evidence. Fed Pract. 2017;34(suppl 1):37S-42S.
4. Cortez NE, Mackenzie GG. Ketogenic diets in pancreatic cancer and associated cachexia: cellular mechanisms and clinical perspectives. Nutrients. 2021;13(9):3202. Published 2021 Sep 15. doi:10.3390/nu13093202
5. Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273-284. doi:10.1200/JCO.20.02088
6. André T, Lonardi S, Wong KYM, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol. 2022;33(10):1052-1060. doi:10.1016/j.annonc.2022.06.008
7. Grassi E, Corbelli J, Papiani G, Barbera MA, Gazzaneo F, Tamberi S. Current therapeutic strategies in BRAF-mutant metastatic colorectal cancer. Front Oncol. 2021;11:601722. Published 2021 Jun 23. doi:10.3389/fonc.2021.601722
8. Seyfried TN, Mukherjee P, Iyikesici MS, et al. Consideration of ketogenic metabolic therapy as a complementary or alternative approach for managing breast cancer. Front Nutr. 2020;7:21. Published 2020 Mar 11. doi:10.3389/fnut.2020.00021
9. Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr Metab (Lond). 2015;12:12. Published 2015 Mar 11. doi:10.1186/s12986-015-0009-2
10. Fayers P, Bottomley A; EORTC Quality of Life Group; Quality of Life Unit. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38(suppl 4):S125-S133. doi:10.1016/s0959-8049(01)00448-8
11. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. Published 2019 Feb 19. doi:10.1186/s12943-019-0954-x
12. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379(21):2052-2062. doi:10.1056/NEJMra1704560
13. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer-where do we stand?. Mol Metab. 2020;33:102-121. doi:10.1016/j.molmet.2019.06.026
14. Yang L, TeSlaa T, Ng S, et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 2022;3(2):119-136. doi:10.1016/j.medj.2021.12.008
15. Furukawa K, Shigematus K, Iwase Y, et al. Clinical effects of one year of chemotherapy with a modified medium-chain triglyceride ketogenic diet on the recurrence of stage IV colon cancer. J Clin Oncol. 2018;36(suppl 15):e15709. doi:10.1200/JCO.2018.36.15_suppl.e15709
16. Zhang X, Li H, Lv X, et al. Impact of diets on response to immune checkpoint inhibitors (ICIs) therapy against tumors. Life (Basel). 2022;12(3):409. Published 2022 Mar 11. doi:10.3390/life12030409
17. Liman, A, Hwang A, Means J, Newson J. Ketogenic diet and cancer: a case report and feasibility study at VA Central California Healthcare System. Fed Pract. 2022;39(suppl 4):S18.