User login
Pigmented Lesion on the Forearm
The Diagnosis: Monsel Solution Reaction
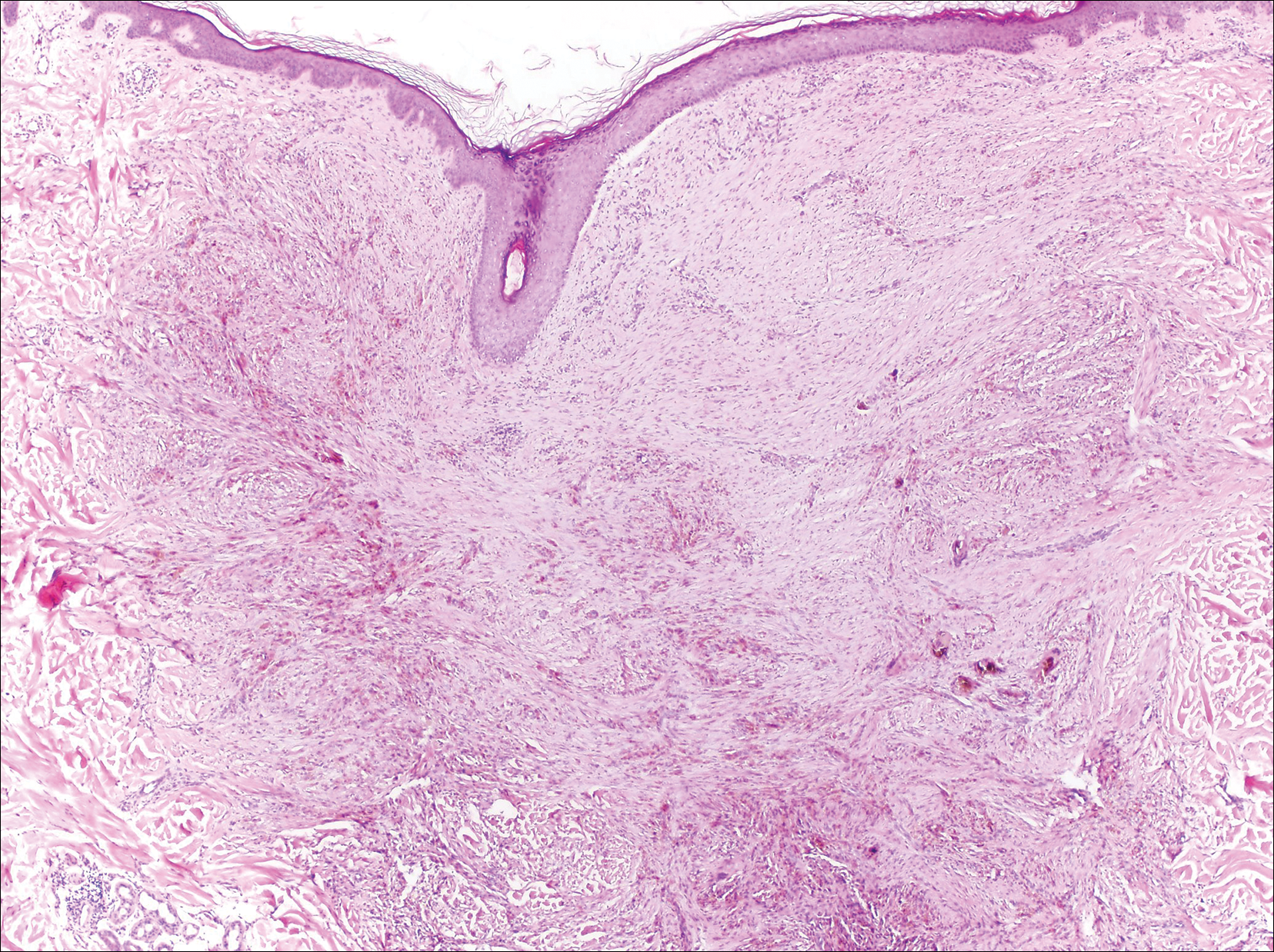
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
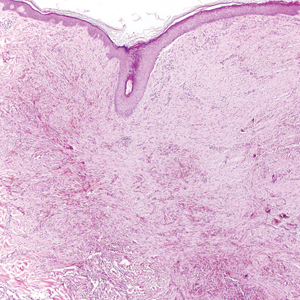
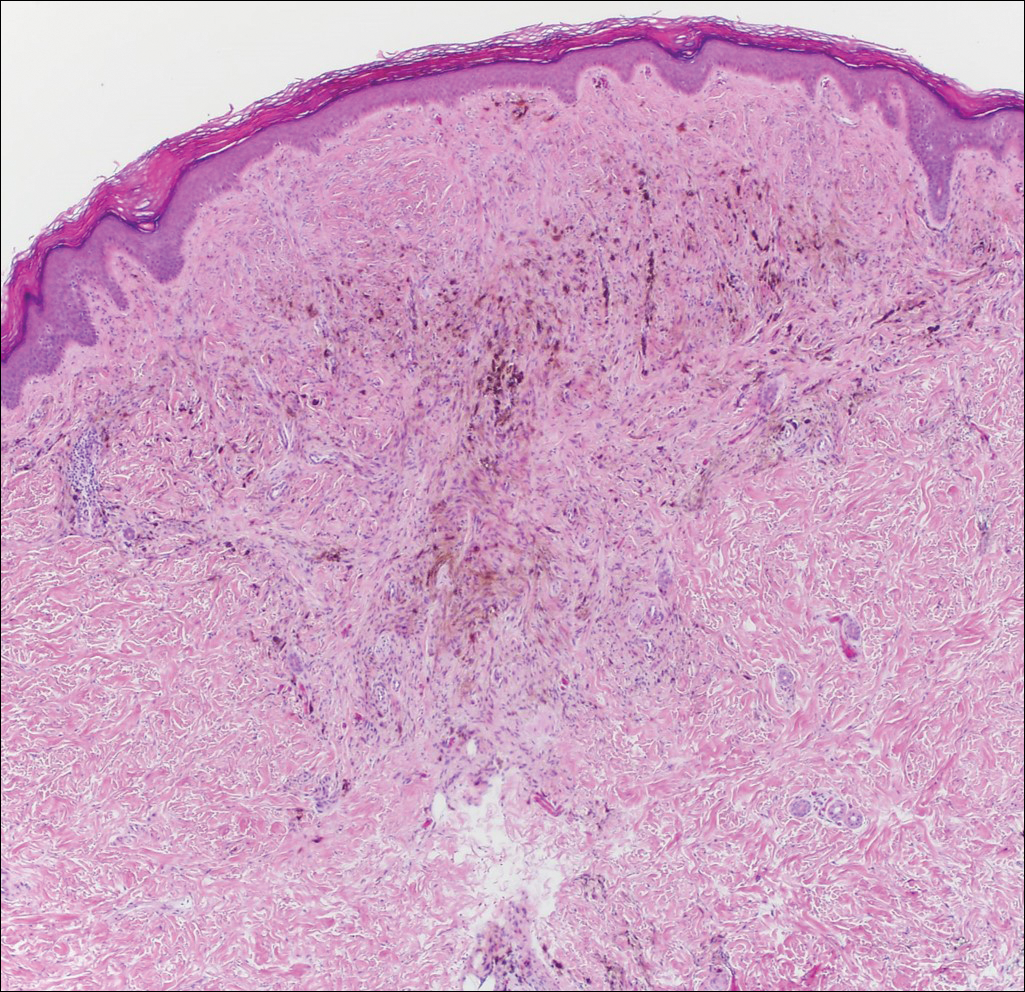
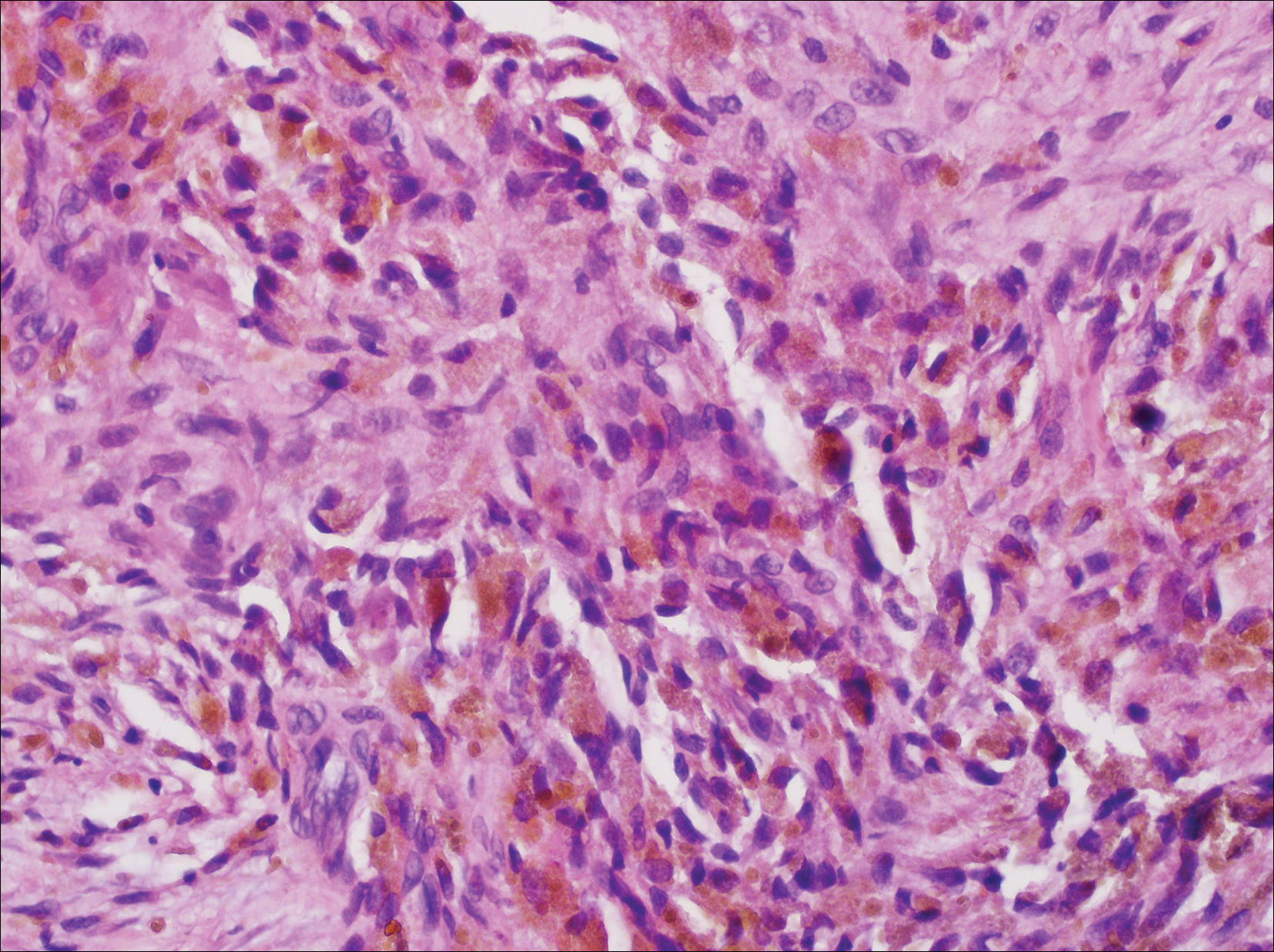
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3
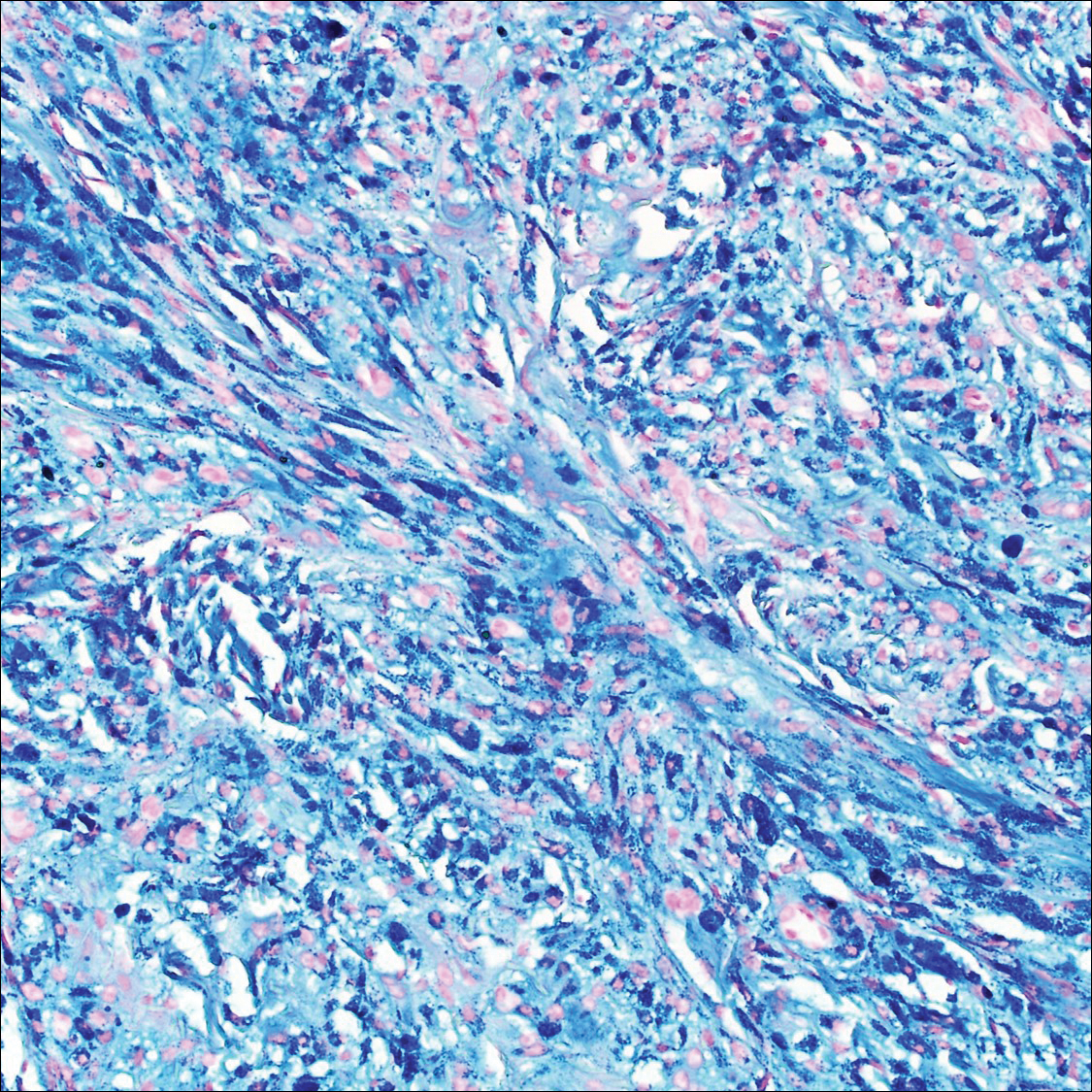
Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4
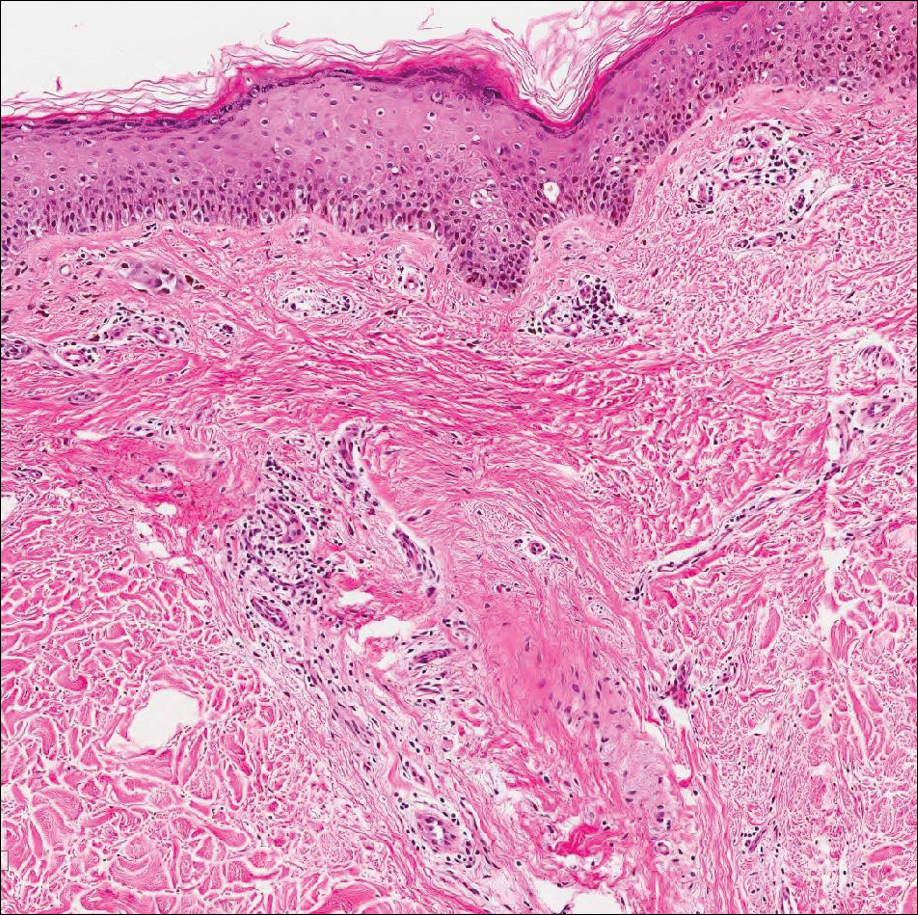
Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8
Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3

Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4

Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8

Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3

Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4

Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8

Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
A 67-year-old man presented to the dermatology clinic with a 2-cm pigmented lesion on the forearm. An excisional biopsy was obtained.