User login
Combined hormonal contraceptives and migraine: An update on the evidence
Combined hormonal contraceptives are contraindicated in women who have migraine with aura because they pose a risk of stroke. But how great is the risk, and how strong is the evidence, particularly with today’s low-dose contraceptives? Can we view migraine with aura as a relative contraindication rather than an absolute one?
This article reviews migraine diagnosis, the effects of estrogen and the menstrual cycle on migraine, the evidence of stroke risk with combined hormonal contraceptive use, and how the frequency of aura may affect risk. It offers practical advice on choosing contraceptive formulations and counseling patients on risks and benefits.
WHAT THE GUIDELINES SAY
Current guidelines restrict the use of combined hormonal contraceptives in the setting of migraine with aura, but not in migraine without aura.
A practice bulletin from the American College of Obstetrics and Gynecology in 2010 noted that extended-cycle or continuous hormonal contraceptives, including oral and parenteral products, might provide relief of migraines by eliminating the drops in estrogen levels that precipitate them.1 However, the bulletin also cautioned that though cerebrovascular accidents in women are rare, the impact of a stroke is so devastating that clinicians should consider intrauterine devices, progestin-only options, and other nonestrogen methods in women who have migraine with focal neurologic signs, women who smoke, and women age 35 or older.1
In 2016, the US Centers for Disease Control and Prevention published updates to its medical eligibility criteria for contraceptive use in various medical conditions. In the case of migraine without aura, the guidelines note no limitation to the use of combined hormonal contraceptives, regardless of the patient’s age. In the case of migraine with aura, the consensus was that the risk associated with combined hormonal contraception typically outweighs its benefits, noting “an unacceptable health risk if the contraceptive method is used.”2
We believe a fresh look at the data is warranted.
EARLY ORAL CONTRACEPTIVES WERE ALL HIGH-DOSE
This issue first surfaced in the decade and a half after the initial launch of oral contraceptives in 1960. The products then were all high-dose pills, containing up to 150 µg of mestranol. In subsequent decades, the dose of estrogen was successively reduced, so that now some pills contain only 10 µg of ethinyl estradiol. High-dose pills—which today contain 50 µg of ethinyl estradiol—account for less than 1% of pills currently sold in the United States and have been eliminated in many countries.
DIAGNOSTIC CRITERIA FOR MIGRAINE
According to the International Classification of Headache Disorders (ICHD),3 the diagnosis of migraine requires 2 of the 4 following criteria:
- Unilateral location
- Pulsating or throbbing pain
- Pain of at least moderate intensity
- Pain aggravated by activity, or causing a preference to avoid activity.
An additional criterion is either nausea or a combination of photophobia and phonophobia with the episode. This criterion can be met if the patient prefers to avoid bright lights and loud noises during an attack.
Headache experts have suggested that patients with a stable pattern of episodic, disabling headache and normal findings on physical examination should be considered to have migraine if there is no contradictory evidence.4,5
Migraine with aura requires at least 2 of the following 4 characteristics3:
- 1 aura symptom, spreading gradually over 5 minutes, or 2 or more aura symptoms occurring in succession, or both
- Each aura symptom lasting 5 to 60 minutes (not “a few seconds,” not “hours”)
- The aura followed by the onset of headache within 60 minutes
- At least 1 aura symptom is unilateral.
Visual blurring, floaters, or split-second flashes before or during a migraine headache do not meet the criteria for aura.
MIGRAINE IS COMMON AND UNDERRECOGNIZED
In a study of 1,203 patients seeking care from a primary care provider for headache,6 94% of the 377 who turned in a diary with enough data to make a diagnosis were diagnosed with a migraine or probable migraine by an expert panel. A quarter of patients who likely had migraine based on an expert review of symptoms did not receive a migraine diagnosis at the time of their office visit.
Similarly, in a large epidemiologic study,7 30,758 adults were asked if they had headaches and, if so, how they named them. Headaches were reported by 23,564 of the participants and were subsequently diagnosed by formal ICHD criteria. Of the 3,074 individuals who met the criteria for migraine, only 53.4% correctly recognized their headaches as migraine. The most common erroneous labels were “sinus headache” and “stress headache.”7
HOW ESTROGEN AFFECTS MIGRAINE
Of note, migraine can be exacerbated during times of cycle irregularity, such as adolescence and perimenopause, the 2 times during a woman’s life associated with the highest risk of unintended pregnancy.10,11
STROKE RISK: ESTROGEN DOSE MATTERS
Shortly after the first combined oral contraceptives were released, reports of adverse events began to appear, although serious events were relatively rare. In response, prescribing guidelines advised against giving oral contraceptives to women with a history of deep vein thrombosis, myocardial infarction, stroke, or hypertension. Also, over the years, the hormonal content of the formulations was successively reduced, and with each reduction in estrogen, a decrease was observed in venous thrombosis and pulmonary embolism.12,13 Current low-dose formulations are considerably safer than high-dose options but are not entirely without risk.14
Stroke risk with combined oral contraceptives was first highlighted in a landmark article in 1975.15 However, the authors were unable to correlate the risk with the estrogen concentration of the pill, since 23 of the 25 women who suffered thrombotic stroke while taking the mestranol-containing formulation took 100-μg pills, and all 20 women who had strokes while taking the ethinyl estradiol formulation took 50-μg pills. Thus, by today’s standards, they were all taking high-dose pills. The risk of thrombotic stroke was 4 to 5 times higher in users than in nonusers.
In 1996, a study from the World Health Organization16 reported an increased risk of stroke with high-dose combined oral contraceptives (odds ratio [OR] 5.30, 95% confidence interval [CI] 2.56–11.0). With preparations containing less than 50 μg of ethinyl estradiol, the risk was not statistically significant (OR 1.53, 95% CI 0.71–3.31). These numbers were for Europe only; in developing countries, the risk was elevated regardless of dose, presumably due to additional risk factors in combined oral contraceptive users. The majority of strokes were in smokers taking 50-μg pills, with an average age greater than 35.
In 2002, a 5-year case-control study in Denmark found that the risk of stroke with combined oral contraceptives correlated directly with the estrogen content, from no increased risk with the newest and lowest-dose formulation (containing ethinyl estradiol 20 µg) to an OR of 4.5 with the older high-dose (50 µg) formulations.17
Reassuringly, a 2012 retrospective review of the Danish national registry13 revealed a low absolute risk of arterial events in users of combined oral contraceptives: 21.4 per 100,000 person-years for thrombotic stroke, and 10.1 per 100,000 person-years for myocardial infarction. Further, these risks were substantially lower with 20-μg ethinyl estradiol products than with those containing 30 to 40 μg.13 An important limitation of this large database review is that it did not control for important stroke risk factors such as obesity and smoking.
Although international studies14,16 continue to show a small but increased risk, more than 30 years have passed since a US study found an increased risk of stroke with combined oral contraceptives.
The discrepancy between US and international studies is possibly explained by the strong relative contraindication in the United States to the use of combined oral contraceptives in smokers over the age of 35 and the more prevalent use of high-dose pills in international studies. High-dose pills had been used in most of the stroke cases in the 1996 World Health Organization study16 but were used by only 0.7% of the women in the case and control groups in 2 pooled US studies from the same time period.18 Similarly, in these US studies, only 17% of the women were smokers on combined oral contraceptives, whereas in the international study, 51% of the women who had strokes and 38% of those in the control groups were smokers.
A large US study19 reviewing 3.6 million woman-years of use found no increased stroke risk (OR 0.96) in current users of low-dose combined oral contraceptives, results similar to those of a pooled analysis of US studies.18 Though this pooled analysis showed an adjusted increased risk of ischemic stroke in women reporting a history of migraine (OR 2.08, 95% CI 1.19–3.65), these conclusions were based on only 4 cases. The prevalence of migraine was identical in women who did or did not have strokes, 7.8% vs 7.7%, respectively, but the risk was judged to be increased after adjusting for other factors. But one important factor was not adjusted for: only 11 of the 1,017 women in the case and control groups were using 50-μg ethinyl estradiol pills, and 4 of the strokes were in this group of 11 women.
STROKE RISK INCREASES WITH FREQUENCY OF MIGRAINE AURA
Use of combined hormonal contraceptives in women who have migraine with aura remains controversial, based on good evidence that aura increases stroke risk20 and good evidence that high-dose oral contraceptives increase stroke risk.15
A cohort study encompassing more than 470,000 person-years with a median follow-up of 26 years found that while migraine without aura conferred no increase in risk of all-cause mortality, migraine with aura did.21
The longitudinal Women’s Health Study analyzed data from 27,798 women over age 45 and found that migraine with aura conferred an increased risk of cardiovascular disease (including stroke) that varied directly with aura frequency.22 Aura frequency less than once a month conferred a risk 2 times higher than in women without migraine, and the risk was more than 4 times higher when aura frequency exceeded once a week.
Similarly, an analysis of the World Health Organization study of stroke in young women found that the adjusted risk of ischemic stroke was significantly and directly associated with aura frequency.20
Potential explanations for this increased risk with greater aura frequency include changes induced during spreading cortical depression, shared genetic predispositions, and common underlying comorbidities such as patent foramen ovale.23–26
Though studies have shown that combined oral contraceptives in continuous regimens27 or in regimens that minimize drops in estrogen levels28 can help improve general headache and menstrual-related migraine, these studies have excluded patients who have migraine with aura.
In a pilot study,29 28 women referred to a tertiary headache clinic who had migraine with aura and intractable menstrual-related migraine were offered combined hormonal contraception in the form of a vaginal ring that releases only 15 μg ethinyl estradiol per 24 hours, thereby reducing peak estrogen exposure to a level lower than those encountered with the native menstrual cycle (with the suppression of ovulation). The women used this continuous ultra-low-dose hormonal contraception without placebo days. After a mean follow-up of 8 months, this regimen reduced aura frequency from a baseline average of 3.2 per month to only 0.2 per month. No woman had an increase in aura frequency, and menstrual-related migraine was eliminated in 21 (91.3%) of the 23 evaluable patients.
CHOOSING THE OPTIMAL CONTRACEPTIVE FORMULATION
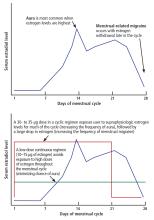
Today, ultra-low-dose combined oral contraceptives (containing 10–15 µg of ethinyl estradiol) inhibit ovulation with doses of estrogen that are in a midphysiologic range. Consequently, they expose women to lower peak concentrations of estrogen than they would experience in their natural menstrual cycle (Figure 1). If a combined oral contraceptive is used in women with migraine with aura, lower estrogen doses (≤ 20 µg ethinyl estradiol) are preferred to decrease aura frequency and minimize the risk of stroke associated with high-dose ethinyl estradiol formulations.
Does the progestin matter?
Though there has been debate about whether different types of progestins alter the risk of venous thromboembolism,30,31 the chosen progestin does not seem to affect arterial risks such as stroke and myocardial infarction.14
All current guidelines note that progestin-only pills can be safely offered to women with migraine with aura. However, progestin-only pills have a shorter half-life than combined hormonal contraceptives and must be taken consistently and on time to ensure contraceptive efficacy and minimize abnormal bleeding. Patients who cannot adhere to a strict daily pill regimen may increase their risk of unintended pregnancy. In addition, progestin-only pills do not help with reducing episodes of migraine because they prevent ovulation only about half of the time.2 In contrast, a progestin-only arm implant is not only considered safe to use in women with migraine with aura, it may also prevent ovulation more reliably. Though progestin arm implants have the potential to reduce menstrual migraine and aura, this requires further study to confirm.
For menstrual-related migraine
In clinical practice, providers may offer certain combined hormonal contraceptives to women with debilitating menstrual-related migraine to prevent attacks. Although menstrual-related migraine rarely if ever is accompanied by aura, these patients may still have migraine with aura at other times of the month.
In women with menstrual-related migraine, any decrease in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-withdrawal migraine. All currently available regimens of combined hormonal contraceptives that follow a 21-days-on, 7-days-off plan entail a drop in ethinyl estradiol of more than 10 µg (Figure 1).
Continuous regimens: Who needs a menstrual cycle anyway?
Of note: ultra-low-estrogen combined hormonal contraceptives that have placebo intervals may not inhibit ovulation consistently in all women.32 Contraceptive efficacy is still maintained, as contraception does not require inhibition of ovulation. Other mechanisms such as thickening of cervical mucus help with pregnancy prevention.
However, if ovulation is not inhibited, the consequent postovulatory decline in estrogen will continue to contribute to estrogen-withdrawal migraine.33,34 Reducing the number of placebo days may help inhibit ovulation. Adding back adequate estrogen during the placebo break (eg, either 0.9 mg conjugated equine estrogen with a 20-µg ethinyl estradiol combined oral contraceptive, or 0.075 mg transdermal 17B estradiol with a 15-µg combined hormonal contraceptive) can prevent these migraines.33,34
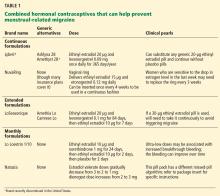
Some extended-cycle regimens, which give 4 withdrawal bleeds per year, will likewise prevent estrogen-withdrawal migraine if the decline in estrogen is limited to 10 µg (Table 1). Unfortunately, most extended regimens (Seasonale, Seasonique, and their generics) entail a 20- or 30-µg drop.
Continuous or extended-cycle regimens can be prescribed using any generic 20-µg combined hormonal contraceptive that the patient tolerates, along with specific instructions on the prescription to take the pills in a continuous fashion, eg, “Do not take the placebo pills; start the next pill pack immediately after 21 days.”
Postmenopausal hormone therapy
Neither smoking nor migraine is a contraindication to the use of postmenopausal hormone therapy, which is substantially lower in dosage than combined hormonal contraceptives.
ADVISING PATIENTS ON RISKS VS BENEFITS
It is important to remember that the risks of unintended pregnancy are always greater than the risks of any contraceptive, especially in women with chronic medical conditions, including those who have migraine with aura. Other benefits include the following:
Lower mortality risk. A 2010 analysis demonstrated that in nearly 46,000 women followed since 1968, those taking combined oral contraceptives had statistically significantly lower death rates from any cause and a lower risk of death from cancer and cardiovascular diseases than women who had never taken combined oral contraceptives.36
Stroke. Though the absolute risk of stroke to an individual woman taking a low-dose or ultra-low-dose combined hormonal contraceptive has been shown to be similar to that in women who are not taking combined hormonal contraception, its impact on an otherwise healthy woman could be devastating. Clinicians must remember that current guidelines still caution against prescribing combined hormonal contraceptives in women with migraine with aura and thus should counsel their patients accordingly and document the discussion in the medical record.
Noncontraceptive benefits. Women may be prescribed a combined hormonal contraceptive for benefits beyond contraception. The obvious reasons include beneficial effects on endometriosis, anemia, acne, hirsutism, dysmenorrhea, and prevention of ovarian cysts. But other important major benefits2 include substantial reductions in the risk of ovarian cancer (> 50% decrease after 10 years)37 and endometrial cancer (additional 24% reduction for each 5 years of use),38 and a modest decrease in the risk of colon cancer (37% less risk in ever-users).39 Further, combined oral contraceptive use has been associated with a decrease in mortality rates,40,41 with no increased risk of nonreproductive cancers.41
Ultra-low-dose, continuous formulations may benefit women by decreasing the frequency of migraine with aura and menstrual-related migraine. There is no evidence that reducing aura frequency also reduces stroke risk, but this represents an important area for future research.
WHAT WOULD WE DO?
For a patient who has a history of migraine with aura, if the goal is only to prevent pregnancy, we would recommend another contraceptive option that does not involve estrogen. However, we would consider prescribing a combined hormonal contraceptive in a low-dose regimen if the patient prefers this regimen for other health benefits (eg, acne control), if she has no other risk factors for stroke, and if she gives her informed consent after a discussion of the risks and benefits. Women who have menstrual-related migraine refractory to or who cannot tolerate other migraine therapies are often willing to try a low-dose estrogen-containing contraceptive for control of their migraine, especially if they have tried it in the past and believe that it helped prevent migraine. Patients should have follow-up within 3 months to discuss whether they have benefited from the regimen in terms of headache frequency or severity.
- ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol 2010; 115:206–218.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 2016; 65:1–104.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33:629–808.
- Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the Spectrum study. Neurology 2002; 58(suppl 6):S27–S31.
- Lipton RB, Stewart WF, Cady R, et al. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000; 40:783–791.
- Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004; 44:856–864.
- Lipton RB, Stewart WF, Liberman JN. Self-awareness of migraine: interpreting the labels that headache sufferers apply to their headaches. Neurology 2002; 58(suppl 6):S21–S26.
- Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Curr Opin Neurol 2014; 27:315–324.
- Calhoun AH. Menstrual migraine: update on pathophysiology and approach to therapy and management. Curr Treat Options Neurol 2012; 14:1–14.
- McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med 2015; 162:ITC1–ITC15.
- O’Brien HL, Cohen JM. Young adults with headaches: the transition from adolescents to adults. Headache 2015; 55:1404–1409.
- Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J (Clin Res Ed) 1986; 292:526.
- Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- MacGregor EA. Contraception and headache. Headache 2013; 53:247–276.
- Oral contraceptives and stroke in young women. Associated risk factors. JAMA 1975; 231:718–722.
- Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1996; 348:498–505.
- Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception 2002; 65:197–205.
- Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke 1998; 29:2277–2284.
- Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996; 335:8–15.
- Donaghy M, Chang CL, Poulter N; European Collaborators of the World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry 2002; 73:747–750.
- Gudmundsson LS, Scher AI, Aspelund T, et al. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ 2010; 341:c3966.
- Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005; 64:1020–1026.
- Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008; 70:1510–1517.
- Kunz GA, Liang G, Cuculi F, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006; 152:190–195.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA 2006; 296:283–291.
- Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A. The migraine-ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med 2009; 9:215–226.
- Sulak P, Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache 2007; 47:27–37.
- Nappi RE, Terreno E, Sances G, et al. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 2013; 88:369–375.
- Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache 2012; 52:1246–1253.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG 2013; 120:801–810.
- Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014; 89:253–263.
- Benson LS, Micks EA. Why stop now? Extended and continuous regimens of combined hormonal contraceptive methods. Obstet Gynecol Clin North Am 2015; 42:669–681.
- Mannix LK, Calhoun AH. Menstrual migraine. Curr Treat Options Neurol 2004; 6:489–498.
- Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004; 97:819–822.
- Calhoun AH. Current topics and controversies in menstrual migraine. Headache 2012; 52(suppl 1):8–11.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927.
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol 2013; 122:139 -147.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015; 16:1061–1070.
- Fernandez E, La Vecchia C, Franceschi S, et al. Oral contraceptive use and risk of colorectal cancer. Epidemiology 1998; 9:295–300.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association Contraceptive Study. Contraception 2013; 88:678–683.
Combined hormonal contraceptives are contraindicated in women who have migraine with aura because they pose a risk of stroke. But how great is the risk, and how strong is the evidence, particularly with today’s low-dose contraceptives? Can we view migraine with aura as a relative contraindication rather than an absolute one?
This article reviews migraine diagnosis, the effects of estrogen and the menstrual cycle on migraine, the evidence of stroke risk with combined hormonal contraceptive use, and how the frequency of aura may affect risk. It offers practical advice on choosing contraceptive formulations and counseling patients on risks and benefits.
WHAT THE GUIDELINES SAY
Current guidelines restrict the use of combined hormonal contraceptives in the setting of migraine with aura, but not in migraine without aura.
A practice bulletin from the American College of Obstetrics and Gynecology in 2010 noted that extended-cycle or continuous hormonal contraceptives, including oral and parenteral products, might provide relief of migraines by eliminating the drops in estrogen levels that precipitate them.1 However, the bulletin also cautioned that though cerebrovascular accidents in women are rare, the impact of a stroke is so devastating that clinicians should consider intrauterine devices, progestin-only options, and other nonestrogen methods in women who have migraine with focal neurologic signs, women who smoke, and women age 35 or older.1
In 2016, the US Centers for Disease Control and Prevention published updates to its medical eligibility criteria for contraceptive use in various medical conditions. In the case of migraine without aura, the guidelines note no limitation to the use of combined hormonal contraceptives, regardless of the patient’s age. In the case of migraine with aura, the consensus was that the risk associated with combined hormonal contraception typically outweighs its benefits, noting “an unacceptable health risk if the contraceptive method is used.”2
We believe a fresh look at the data is warranted.
EARLY ORAL CONTRACEPTIVES WERE ALL HIGH-DOSE
This issue first surfaced in the decade and a half after the initial launch of oral contraceptives in 1960. The products then were all high-dose pills, containing up to 150 µg of mestranol. In subsequent decades, the dose of estrogen was successively reduced, so that now some pills contain only 10 µg of ethinyl estradiol. High-dose pills—which today contain 50 µg of ethinyl estradiol—account for less than 1% of pills currently sold in the United States and have been eliminated in many countries.
DIAGNOSTIC CRITERIA FOR MIGRAINE
According to the International Classification of Headache Disorders (ICHD),3 the diagnosis of migraine requires 2 of the 4 following criteria:
- Unilateral location
- Pulsating or throbbing pain
- Pain of at least moderate intensity
- Pain aggravated by activity, or causing a preference to avoid activity.
An additional criterion is either nausea or a combination of photophobia and phonophobia with the episode. This criterion can be met if the patient prefers to avoid bright lights and loud noises during an attack.
Headache experts have suggested that patients with a stable pattern of episodic, disabling headache and normal findings on physical examination should be considered to have migraine if there is no contradictory evidence.4,5
Migraine with aura requires at least 2 of the following 4 characteristics3:
- 1 aura symptom, spreading gradually over 5 minutes, or 2 or more aura symptoms occurring in succession, or both
- Each aura symptom lasting 5 to 60 minutes (not “a few seconds,” not “hours”)
- The aura followed by the onset of headache within 60 minutes
- At least 1 aura symptom is unilateral.
Visual blurring, floaters, or split-second flashes before or during a migraine headache do not meet the criteria for aura.
MIGRAINE IS COMMON AND UNDERRECOGNIZED
In a study of 1,203 patients seeking care from a primary care provider for headache,6 94% of the 377 who turned in a diary with enough data to make a diagnosis were diagnosed with a migraine or probable migraine by an expert panel. A quarter of patients who likely had migraine based on an expert review of symptoms did not receive a migraine diagnosis at the time of their office visit.
Similarly, in a large epidemiologic study,7 30,758 adults were asked if they had headaches and, if so, how they named them. Headaches were reported by 23,564 of the participants and were subsequently diagnosed by formal ICHD criteria. Of the 3,074 individuals who met the criteria for migraine, only 53.4% correctly recognized their headaches as migraine. The most common erroneous labels were “sinus headache” and “stress headache.”7
HOW ESTROGEN AFFECTS MIGRAINE
Of note, migraine can be exacerbated during times of cycle irregularity, such as adolescence and perimenopause, the 2 times during a woman’s life associated with the highest risk of unintended pregnancy.10,11
STROKE RISK: ESTROGEN DOSE MATTERS
Shortly after the first combined oral contraceptives were released, reports of adverse events began to appear, although serious events were relatively rare. In response, prescribing guidelines advised against giving oral contraceptives to women with a history of deep vein thrombosis, myocardial infarction, stroke, or hypertension. Also, over the years, the hormonal content of the formulations was successively reduced, and with each reduction in estrogen, a decrease was observed in venous thrombosis and pulmonary embolism.12,13 Current low-dose formulations are considerably safer than high-dose options but are not entirely without risk.14
Stroke risk with combined oral contraceptives was first highlighted in a landmark article in 1975.15 However, the authors were unable to correlate the risk with the estrogen concentration of the pill, since 23 of the 25 women who suffered thrombotic stroke while taking the mestranol-containing formulation took 100-μg pills, and all 20 women who had strokes while taking the ethinyl estradiol formulation took 50-μg pills. Thus, by today’s standards, they were all taking high-dose pills. The risk of thrombotic stroke was 4 to 5 times higher in users than in nonusers.
In 1996, a study from the World Health Organization16 reported an increased risk of stroke with high-dose combined oral contraceptives (odds ratio [OR] 5.30, 95% confidence interval [CI] 2.56–11.0). With preparations containing less than 50 μg of ethinyl estradiol, the risk was not statistically significant (OR 1.53, 95% CI 0.71–3.31). These numbers were for Europe only; in developing countries, the risk was elevated regardless of dose, presumably due to additional risk factors in combined oral contraceptive users. The majority of strokes were in smokers taking 50-μg pills, with an average age greater than 35.
In 2002, a 5-year case-control study in Denmark found that the risk of stroke with combined oral contraceptives correlated directly with the estrogen content, from no increased risk with the newest and lowest-dose formulation (containing ethinyl estradiol 20 µg) to an OR of 4.5 with the older high-dose (50 µg) formulations.17
Reassuringly, a 2012 retrospective review of the Danish national registry13 revealed a low absolute risk of arterial events in users of combined oral contraceptives: 21.4 per 100,000 person-years for thrombotic stroke, and 10.1 per 100,000 person-years for myocardial infarction. Further, these risks were substantially lower with 20-μg ethinyl estradiol products than with those containing 30 to 40 μg.13 An important limitation of this large database review is that it did not control for important stroke risk factors such as obesity and smoking.
Although international studies14,16 continue to show a small but increased risk, more than 30 years have passed since a US study found an increased risk of stroke with combined oral contraceptives.
The discrepancy between US and international studies is possibly explained by the strong relative contraindication in the United States to the use of combined oral contraceptives in smokers over the age of 35 and the more prevalent use of high-dose pills in international studies. High-dose pills had been used in most of the stroke cases in the 1996 World Health Organization study16 but were used by only 0.7% of the women in the case and control groups in 2 pooled US studies from the same time period.18 Similarly, in these US studies, only 17% of the women were smokers on combined oral contraceptives, whereas in the international study, 51% of the women who had strokes and 38% of those in the control groups were smokers.
A large US study19 reviewing 3.6 million woman-years of use found no increased stroke risk (OR 0.96) in current users of low-dose combined oral contraceptives, results similar to those of a pooled analysis of US studies.18 Though this pooled analysis showed an adjusted increased risk of ischemic stroke in women reporting a history of migraine (OR 2.08, 95% CI 1.19–3.65), these conclusions were based on only 4 cases. The prevalence of migraine was identical in women who did or did not have strokes, 7.8% vs 7.7%, respectively, but the risk was judged to be increased after adjusting for other factors. But one important factor was not adjusted for: only 11 of the 1,017 women in the case and control groups were using 50-μg ethinyl estradiol pills, and 4 of the strokes were in this group of 11 women.
STROKE RISK INCREASES WITH FREQUENCY OF MIGRAINE AURA
Use of combined hormonal contraceptives in women who have migraine with aura remains controversial, based on good evidence that aura increases stroke risk20 and good evidence that high-dose oral contraceptives increase stroke risk.15
A cohort study encompassing more than 470,000 person-years with a median follow-up of 26 years found that while migraine without aura conferred no increase in risk of all-cause mortality, migraine with aura did.21
The longitudinal Women’s Health Study analyzed data from 27,798 women over age 45 and found that migraine with aura conferred an increased risk of cardiovascular disease (including stroke) that varied directly with aura frequency.22 Aura frequency less than once a month conferred a risk 2 times higher than in women without migraine, and the risk was more than 4 times higher when aura frequency exceeded once a week.
Similarly, an analysis of the World Health Organization study of stroke in young women found that the adjusted risk of ischemic stroke was significantly and directly associated with aura frequency.20
Potential explanations for this increased risk with greater aura frequency include changes induced during spreading cortical depression, shared genetic predispositions, and common underlying comorbidities such as patent foramen ovale.23–26
Though studies have shown that combined oral contraceptives in continuous regimens27 or in regimens that minimize drops in estrogen levels28 can help improve general headache and menstrual-related migraine, these studies have excluded patients who have migraine with aura.
In a pilot study,29 28 women referred to a tertiary headache clinic who had migraine with aura and intractable menstrual-related migraine were offered combined hormonal contraception in the form of a vaginal ring that releases only 15 μg ethinyl estradiol per 24 hours, thereby reducing peak estrogen exposure to a level lower than those encountered with the native menstrual cycle (with the suppression of ovulation). The women used this continuous ultra-low-dose hormonal contraception without placebo days. After a mean follow-up of 8 months, this regimen reduced aura frequency from a baseline average of 3.2 per month to only 0.2 per month. No woman had an increase in aura frequency, and menstrual-related migraine was eliminated in 21 (91.3%) of the 23 evaluable patients.
CHOOSING THE OPTIMAL CONTRACEPTIVE FORMULATION
Today, ultra-low-dose combined oral contraceptives (containing 10–15 µg of ethinyl estradiol) inhibit ovulation with doses of estrogen that are in a midphysiologic range. Consequently, they expose women to lower peak concentrations of estrogen than they would experience in their natural menstrual cycle (Figure 1). If a combined oral contraceptive is used in women with migraine with aura, lower estrogen doses (≤ 20 µg ethinyl estradiol) are preferred to decrease aura frequency and minimize the risk of stroke associated with high-dose ethinyl estradiol formulations.
Does the progestin matter?
Though there has been debate about whether different types of progestins alter the risk of venous thromboembolism,30,31 the chosen progestin does not seem to affect arterial risks such as stroke and myocardial infarction.14
All current guidelines note that progestin-only pills can be safely offered to women with migraine with aura. However, progestin-only pills have a shorter half-life than combined hormonal contraceptives and must be taken consistently and on time to ensure contraceptive efficacy and minimize abnormal bleeding. Patients who cannot adhere to a strict daily pill regimen may increase their risk of unintended pregnancy. In addition, progestin-only pills do not help with reducing episodes of migraine because they prevent ovulation only about half of the time.2 In contrast, a progestin-only arm implant is not only considered safe to use in women with migraine with aura, it may also prevent ovulation more reliably. Though progestin arm implants have the potential to reduce menstrual migraine and aura, this requires further study to confirm.
For menstrual-related migraine
In clinical practice, providers may offer certain combined hormonal contraceptives to women with debilitating menstrual-related migraine to prevent attacks. Although menstrual-related migraine rarely if ever is accompanied by aura, these patients may still have migraine with aura at other times of the month.
In women with menstrual-related migraine, any decrease in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-withdrawal migraine. All currently available regimens of combined hormonal contraceptives that follow a 21-days-on, 7-days-off plan entail a drop in ethinyl estradiol of more than 10 µg (Figure 1).
Continuous regimens: Who needs a menstrual cycle anyway?
Of note: ultra-low-estrogen combined hormonal contraceptives that have placebo intervals may not inhibit ovulation consistently in all women.32 Contraceptive efficacy is still maintained, as contraception does not require inhibition of ovulation. Other mechanisms such as thickening of cervical mucus help with pregnancy prevention.
However, if ovulation is not inhibited, the consequent postovulatory decline in estrogen will continue to contribute to estrogen-withdrawal migraine.33,34 Reducing the number of placebo days may help inhibit ovulation. Adding back adequate estrogen during the placebo break (eg, either 0.9 mg conjugated equine estrogen with a 20-µg ethinyl estradiol combined oral contraceptive, or 0.075 mg transdermal 17B estradiol with a 15-µg combined hormonal contraceptive) can prevent these migraines.33,34
Some extended-cycle regimens, which give 4 withdrawal bleeds per year, will likewise prevent estrogen-withdrawal migraine if the decline in estrogen is limited to 10 µg (Table 1). Unfortunately, most extended regimens (Seasonale, Seasonique, and their generics) entail a 20- or 30-µg drop.
Continuous or extended-cycle regimens can be prescribed using any generic 20-µg combined hormonal contraceptive that the patient tolerates, along with specific instructions on the prescription to take the pills in a continuous fashion, eg, “Do not take the placebo pills; start the next pill pack immediately after 21 days.”
Postmenopausal hormone therapy
Neither smoking nor migraine is a contraindication to the use of postmenopausal hormone therapy, which is substantially lower in dosage than combined hormonal contraceptives.
ADVISING PATIENTS ON RISKS VS BENEFITS
It is important to remember that the risks of unintended pregnancy are always greater than the risks of any contraceptive, especially in women with chronic medical conditions, including those who have migraine with aura. Other benefits include the following:
Lower mortality risk. A 2010 analysis demonstrated that in nearly 46,000 women followed since 1968, those taking combined oral contraceptives had statistically significantly lower death rates from any cause and a lower risk of death from cancer and cardiovascular diseases than women who had never taken combined oral contraceptives.36
Stroke. Though the absolute risk of stroke to an individual woman taking a low-dose or ultra-low-dose combined hormonal contraceptive has been shown to be similar to that in women who are not taking combined hormonal contraception, its impact on an otherwise healthy woman could be devastating. Clinicians must remember that current guidelines still caution against prescribing combined hormonal contraceptives in women with migraine with aura and thus should counsel their patients accordingly and document the discussion in the medical record.
Noncontraceptive benefits. Women may be prescribed a combined hormonal contraceptive for benefits beyond contraception. The obvious reasons include beneficial effects on endometriosis, anemia, acne, hirsutism, dysmenorrhea, and prevention of ovarian cysts. But other important major benefits2 include substantial reductions in the risk of ovarian cancer (> 50% decrease after 10 years)37 and endometrial cancer (additional 24% reduction for each 5 years of use),38 and a modest decrease in the risk of colon cancer (37% less risk in ever-users).39 Further, combined oral contraceptive use has been associated with a decrease in mortality rates,40,41 with no increased risk of nonreproductive cancers.41
Ultra-low-dose, continuous formulations may benefit women by decreasing the frequency of migraine with aura and menstrual-related migraine. There is no evidence that reducing aura frequency also reduces stroke risk, but this represents an important area for future research.
WHAT WOULD WE DO?
For a patient who has a history of migraine with aura, if the goal is only to prevent pregnancy, we would recommend another contraceptive option that does not involve estrogen. However, we would consider prescribing a combined hormonal contraceptive in a low-dose regimen if the patient prefers this regimen for other health benefits (eg, acne control), if she has no other risk factors for stroke, and if she gives her informed consent after a discussion of the risks and benefits. Women who have menstrual-related migraine refractory to or who cannot tolerate other migraine therapies are often willing to try a low-dose estrogen-containing contraceptive for control of their migraine, especially if they have tried it in the past and believe that it helped prevent migraine. Patients should have follow-up within 3 months to discuss whether they have benefited from the regimen in terms of headache frequency or severity.
Combined hormonal contraceptives are contraindicated in women who have migraine with aura because they pose a risk of stroke. But how great is the risk, and how strong is the evidence, particularly with today’s low-dose contraceptives? Can we view migraine with aura as a relative contraindication rather than an absolute one?
This article reviews migraine diagnosis, the effects of estrogen and the menstrual cycle on migraine, the evidence of stroke risk with combined hormonal contraceptive use, and how the frequency of aura may affect risk. It offers practical advice on choosing contraceptive formulations and counseling patients on risks and benefits.
WHAT THE GUIDELINES SAY
Current guidelines restrict the use of combined hormonal contraceptives in the setting of migraine with aura, but not in migraine without aura.
A practice bulletin from the American College of Obstetrics and Gynecology in 2010 noted that extended-cycle or continuous hormonal contraceptives, including oral and parenteral products, might provide relief of migraines by eliminating the drops in estrogen levels that precipitate them.1 However, the bulletin also cautioned that though cerebrovascular accidents in women are rare, the impact of a stroke is so devastating that clinicians should consider intrauterine devices, progestin-only options, and other nonestrogen methods in women who have migraine with focal neurologic signs, women who smoke, and women age 35 or older.1
In 2016, the US Centers for Disease Control and Prevention published updates to its medical eligibility criteria for contraceptive use in various medical conditions. In the case of migraine without aura, the guidelines note no limitation to the use of combined hormonal contraceptives, regardless of the patient’s age. In the case of migraine with aura, the consensus was that the risk associated with combined hormonal contraception typically outweighs its benefits, noting “an unacceptable health risk if the contraceptive method is used.”2
We believe a fresh look at the data is warranted.
EARLY ORAL CONTRACEPTIVES WERE ALL HIGH-DOSE
This issue first surfaced in the decade and a half after the initial launch of oral contraceptives in 1960. The products then were all high-dose pills, containing up to 150 µg of mestranol. In subsequent decades, the dose of estrogen was successively reduced, so that now some pills contain only 10 µg of ethinyl estradiol. High-dose pills—which today contain 50 µg of ethinyl estradiol—account for less than 1% of pills currently sold in the United States and have been eliminated in many countries.
DIAGNOSTIC CRITERIA FOR MIGRAINE
According to the International Classification of Headache Disorders (ICHD),3 the diagnosis of migraine requires 2 of the 4 following criteria:
- Unilateral location
- Pulsating or throbbing pain
- Pain of at least moderate intensity
- Pain aggravated by activity, or causing a preference to avoid activity.
An additional criterion is either nausea or a combination of photophobia and phonophobia with the episode. This criterion can be met if the patient prefers to avoid bright lights and loud noises during an attack.
Headache experts have suggested that patients with a stable pattern of episodic, disabling headache and normal findings on physical examination should be considered to have migraine if there is no contradictory evidence.4,5
Migraine with aura requires at least 2 of the following 4 characteristics3:
- 1 aura symptom, spreading gradually over 5 minutes, or 2 or more aura symptoms occurring in succession, or both
- Each aura symptom lasting 5 to 60 minutes (not “a few seconds,” not “hours”)
- The aura followed by the onset of headache within 60 minutes
- At least 1 aura symptom is unilateral.
Visual blurring, floaters, or split-second flashes before or during a migraine headache do not meet the criteria for aura.
MIGRAINE IS COMMON AND UNDERRECOGNIZED
In a study of 1,203 patients seeking care from a primary care provider for headache,6 94% of the 377 who turned in a diary with enough data to make a diagnosis were diagnosed with a migraine or probable migraine by an expert panel. A quarter of patients who likely had migraine based on an expert review of symptoms did not receive a migraine diagnosis at the time of their office visit.
Similarly, in a large epidemiologic study,7 30,758 adults were asked if they had headaches and, if so, how they named them. Headaches were reported by 23,564 of the participants and were subsequently diagnosed by formal ICHD criteria. Of the 3,074 individuals who met the criteria for migraine, only 53.4% correctly recognized their headaches as migraine. The most common erroneous labels were “sinus headache” and “stress headache.”7
HOW ESTROGEN AFFECTS MIGRAINE
Of note, migraine can be exacerbated during times of cycle irregularity, such as adolescence and perimenopause, the 2 times during a woman’s life associated with the highest risk of unintended pregnancy.10,11
STROKE RISK: ESTROGEN DOSE MATTERS
Shortly after the first combined oral contraceptives were released, reports of adverse events began to appear, although serious events were relatively rare. In response, prescribing guidelines advised against giving oral contraceptives to women with a history of deep vein thrombosis, myocardial infarction, stroke, or hypertension. Also, over the years, the hormonal content of the formulations was successively reduced, and with each reduction in estrogen, a decrease was observed in venous thrombosis and pulmonary embolism.12,13 Current low-dose formulations are considerably safer than high-dose options but are not entirely without risk.14
Stroke risk with combined oral contraceptives was first highlighted in a landmark article in 1975.15 However, the authors were unable to correlate the risk with the estrogen concentration of the pill, since 23 of the 25 women who suffered thrombotic stroke while taking the mestranol-containing formulation took 100-μg pills, and all 20 women who had strokes while taking the ethinyl estradiol formulation took 50-μg pills. Thus, by today’s standards, they were all taking high-dose pills. The risk of thrombotic stroke was 4 to 5 times higher in users than in nonusers.
In 1996, a study from the World Health Organization16 reported an increased risk of stroke with high-dose combined oral contraceptives (odds ratio [OR] 5.30, 95% confidence interval [CI] 2.56–11.0). With preparations containing less than 50 μg of ethinyl estradiol, the risk was not statistically significant (OR 1.53, 95% CI 0.71–3.31). These numbers were for Europe only; in developing countries, the risk was elevated regardless of dose, presumably due to additional risk factors in combined oral contraceptive users. The majority of strokes were in smokers taking 50-μg pills, with an average age greater than 35.
In 2002, a 5-year case-control study in Denmark found that the risk of stroke with combined oral contraceptives correlated directly with the estrogen content, from no increased risk with the newest and lowest-dose formulation (containing ethinyl estradiol 20 µg) to an OR of 4.5 with the older high-dose (50 µg) formulations.17
Reassuringly, a 2012 retrospective review of the Danish national registry13 revealed a low absolute risk of arterial events in users of combined oral contraceptives: 21.4 per 100,000 person-years for thrombotic stroke, and 10.1 per 100,000 person-years for myocardial infarction. Further, these risks were substantially lower with 20-μg ethinyl estradiol products than with those containing 30 to 40 μg.13 An important limitation of this large database review is that it did not control for important stroke risk factors such as obesity and smoking.
Although international studies14,16 continue to show a small but increased risk, more than 30 years have passed since a US study found an increased risk of stroke with combined oral contraceptives.
The discrepancy between US and international studies is possibly explained by the strong relative contraindication in the United States to the use of combined oral contraceptives in smokers over the age of 35 and the more prevalent use of high-dose pills in international studies. High-dose pills had been used in most of the stroke cases in the 1996 World Health Organization study16 but were used by only 0.7% of the women in the case and control groups in 2 pooled US studies from the same time period.18 Similarly, in these US studies, only 17% of the women were smokers on combined oral contraceptives, whereas in the international study, 51% of the women who had strokes and 38% of those in the control groups were smokers.
A large US study19 reviewing 3.6 million woman-years of use found no increased stroke risk (OR 0.96) in current users of low-dose combined oral contraceptives, results similar to those of a pooled analysis of US studies.18 Though this pooled analysis showed an adjusted increased risk of ischemic stroke in women reporting a history of migraine (OR 2.08, 95% CI 1.19–3.65), these conclusions were based on only 4 cases. The prevalence of migraine was identical in women who did or did not have strokes, 7.8% vs 7.7%, respectively, but the risk was judged to be increased after adjusting for other factors. But one important factor was not adjusted for: only 11 of the 1,017 women in the case and control groups were using 50-μg ethinyl estradiol pills, and 4 of the strokes were in this group of 11 women.
STROKE RISK INCREASES WITH FREQUENCY OF MIGRAINE AURA
Use of combined hormonal contraceptives in women who have migraine with aura remains controversial, based on good evidence that aura increases stroke risk20 and good evidence that high-dose oral contraceptives increase stroke risk.15
A cohort study encompassing more than 470,000 person-years with a median follow-up of 26 years found that while migraine without aura conferred no increase in risk of all-cause mortality, migraine with aura did.21
The longitudinal Women’s Health Study analyzed data from 27,798 women over age 45 and found that migraine with aura conferred an increased risk of cardiovascular disease (including stroke) that varied directly with aura frequency.22 Aura frequency less than once a month conferred a risk 2 times higher than in women without migraine, and the risk was more than 4 times higher when aura frequency exceeded once a week.
Similarly, an analysis of the World Health Organization study of stroke in young women found that the adjusted risk of ischemic stroke was significantly and directly associated with aura frequency.20
Potential explanations for this increased risk with greater aura frequency include changes induced during spreading cortical depression, shared genetic predispositions, and common underlying comorbidities such as patent foramen ovale.23–26
Though studies have shown that combined oral contraceptives in continuous regimens27 or in regimens that minimize drops in estrogen levels28 can help improve general headache and menstrual-related migraine, these studies have excluded patients who have migraine with aura.
In a pilot study,29 28 women referred to a tertiary headache clinic who had migraine with aura and intractable menstrual-related migraine were offered combined hormonal contraception in the form of a vaginal ring that releases only 15 μg ethinyl estradiol per 24 hours, thereby reducing peak estrogen exposure to a level lower than those encountered with the native menstrual cycle (with the suppression of ovulation). The women used this continuous ultra-low-dose hormonal contraception without placebo days. After a mean follow-up of 8 months, this regimen reduced aura frequency from a baseline average of 3.2 per month to only 0.2 per month. No woman had an increase in aura frequency, and menstrual-related migraine was eliminated in 21 (91.3%) of the 23 evaluable patients.
CHOOSING THE OPTIMAL CONTRACEPTIVE FORMULATION
Today, ultra-low-dose combined oral contraceptives (containing 10–15 µg of ethinyl estradiol) inhibit ovulation with doses of estrogen that are in a midphysiologic range. Consequently, they expose women to lower peak concentrations of estrogen than they would experience in their natural menstrual cycle (Figure 1). If a combined oral contraceptive is used in women with migraine with aura, lower estrogen doses (≤ 20 µg ethinyl estradiol) are preferred to decrease aura frequency and minimize the risk of stroke associated with high-dose ethinyl estradiol formulations.
Does the progestin matter?
Though there has been debate about whether different types of progestins alter the risk of venous thromboembolism,30,31 the chosen progestin does not seem to affect arterial risks such as stroke and myocardial infarction.14
All current guidelines note that progestin-only pills can be safely offered to women with migraine with aura. However, progestin-only pills have a shorter half-life than combined hormonal contraceptives and must be taken consistently and on time to ensure contraceptive efficacy and minimize abnormal bleeding. Patients who cannot adhere to a strict daily pill regimen may increase their risk of unintended pregnancy. In addition, progestin-only pills do not help with reducing episodes of migraine because they prevent ovulation only about half of the time.2 In contrast, a progestin-only arm implant is not only considered safe to use in women with migraine with aura, it may also prevent ovulation more reliably. Though progestin arm implants have the potential to reduce menstrual migraine and aura, this requires further study to confirm.
For menstrual-related migraine
In clinical practice, providers may offer certain combined hormonal contraceptives to women with debilitating menstrual-related migraine to prevent attacks. Although menstrual-related migraine rarely if ever is accompanied by aura, these patients may still have migraine with aura at other times of the month.
In women with menstrual-related migraine, any decrease in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-withdrawal migraine. All currently available regimens of combined hormonal contraceptives that follow a 21-days-on, 7-days-off plan entail a drop in ethinyl estradiol of more than 10 µg (Figure 1).
Continuous regimens: Who needs a menstrual cycle anyway?
Of note: ultra-low-estrogen combined hormonal contraceptives that have placebo intervals may not inhibit ovulation consistently in all women.32 Contraceptive efficacy is still maintained, as contraception does not require inhibition of ovulation. Other mechanisms such as thickening of cervical mucus help with pregnancy prevention.
However, if ovulation is not inhibited, the consequent postovulatory decline in estrogen will continue to contribute to estrogen-withdrawal migraine.33,34 Reducing the number of placebo days may help inhibit ovulation. Adding back adequate estrogen during the placebo break (eg, either 0.9 mg conjugated equine estrogen with a 20-µg ethinyl estradiol combined oral contraceptive, or 0.075 mg transdermal 17B estradiol with a 15-µg combined hormonal contraceptive) can prevent these migraines.33,34
Some extended-cycle regimens, which give 4 withdrawal bleeds per year, will likewise prevent estrogen-withdrawal migraine if the decline in estrogen is limited to 10 µg (Table 1). Unfortunately, most extended regimens (Seasonale, Seasonique, and their generics) entail a 20- or 30-µg drop.
Continuous or extended-cycle regimens can be prescribed using any generic 20-µg combined hormonal contraceptive that the patient tolerates, along with specific instructions on the prescription to take the pills in a continuous fashion, eg, “Do not take the placebo pills; start the next pill pack immediately after 21 days.”
Postmenopausal hormone therapy
Neither smoking nor migraine is a contraindication to the use of postmenopausal hormone therapy, which is substantially lower in dosage than combined hormonal contraceptives.
ADVISING PATIENTS ON RISKS VS BENEFITS
It is important to remember that the risks of unintended pregnancy are always greater than the risks of any contraceptive, especially in women with chronic medical conditions, including those who have migraine with aura. Other benefits include the following:
Lower mortality risk. A 2010 analysis demonstrated that in nearly 46,000 women followed since 1968, those taking combined oral contraceptives had statistically significantly lower death rates from any cause and a lower risk of death from cancer and cardiovascular diseases than women who had never taken combined oral contraceptives.36
Stroke. Though the absolute risk of stroke to an individual woman taking a low-dose or ultra-low-dose combined hormonal contraceptive has been shown to be similar to that in women who are not taking combined hormonal contraception, its impact on an otherwise healthy woman could be devastating. Clinicians must remember that current guidelines still caution against prescribing combined hormonal contraceptives in women with migraine with aura and thus should counsel their patients accordingly and document the discussion in the medical record.
Noncontraceptive benefits. Women may be prescribed a combined hormonal contraceptive for benefits beyond contraception. The obvious reasons include beneficial effects on endometriosis, anemia, acne, hirsutism, dysmenorrhea, and prevention of ovarian cysts. But other important major benefits2 include substantial reductions in the risk of ovarian cancer (> 50% decrease after 10 years)37 and endometrial cancer (additional 24% reduction for each 5 years of use),38 and a modest decrease in the risk of colon cancer (37% less risk in ever-users).39 Further, combined oral contraceptive use has been associated with a decrease in mortality rates,40,41 with no increased risk of nonreproductive cancers.41
Ultra-low-dose, continuous formulations may benefit women by decreasing the frequency of migraine with aura and menstrual-related migraine. There is no evidence that reducing aura frequency also reduces stroke risk, but this represents an important area for future research.
WHAT WOULD WE DO?
For a patient who has a history of migraine with aura, if the goal is only to prevent pregnancy, we would recommend another contraceptive option that does not involve estrogen. However, we would consider prescribing a combined hormonal contraceptive in a low-dose regimen if the patient prefers this regimen for other health benefits (eg, acne control), if she has no other risk factors for stroke, and if she gives her informed consent after a discussion of the risks and benefits. Women who have menstrual-related migraine refractory to or who cannot tolerate other migraine therapies are often willing to try a low-dose estrogen-containing contraceptive for control of their migraine, especially if they have tried it in the past and believe that it helped prevent migraine. Patients should have follow-up within 3 months to discuss whether they have benefited from the regimen in terms of headache frequency or severity.
- ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol 2010; 115:206–218.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 2016; 65:1–104.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33:629–808.
- Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the Spectrum study. Neurology 2002; 58(suppl 6):S27–S31.
- Lipton RB, Stewart WF, Cady R, et al. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000; 40:783–791.
- Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004; 44:856–864.
- Lipton RB, Stewart WF, Liberman JN. Self-awareness of migraine: interpreting the labels that headache sufferers apply to their headaches. Neurology 2002; 58(suppl 6):S21–S26.
- Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Curr Opin Neurol 2014; 27:315–324.
- Calhoun AH. Menstrual migraine: update on pathophysiology and approach to therapy and management. Curr Treat Options Neurol 2012; 14:1–14.
- McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med 2015; 162:ITC1–ITC15.
- O’Brien HL, Cohen JM. Young adults with headaches: the transition from adolescents to adults. Headache 2015; 55:1404–1409.
- Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J (Clin Res Ed) 1986; 292:526.
- Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- MacGregor EA. Contraception and headache. Headache 2013; 53:247–276.
- Oral contraceptives and stroke in young women. Associated risk factors. JAMA 1975; 231:718–722.
- Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1996; 348:498–505.
- Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception 2002; 65:197–205.
- Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke 1998; 29:2277–2284.
- Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996; 335:8–15.
- Donaghy M, Chang CL, Poulter N; European Collaborators of the World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry 2002; 73:747–750.
- Gudmundsson LS, Scher AI, Aspelund T, et al. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ 2010; 341:c3966.
- Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005; 64:1020–1026.
- Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008; 70:1510–1517.
- Kunz GA, Liang G, Cuculi F, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006; 152:190–195.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA 2006; 296:283–291.
- Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A. The migraine-ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med 2009; 9:215–226.
- Sulak P, Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache 2007; 47:27–37.
- Nappi RE, Terreno E, Sances G, et al. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 2013; 88:369–375.
- Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache 2012; 52:1246–1253.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG 2013; 120:801–810.
- Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014; 89:253–263.
- Benson LS, Micks EA. Why stop now? Extended and continuous regimens of combined hormonal contraceptive methods. Obstet Gynecol Clin North Am 2015; 42:669–681.
- Mannix LK, Calhoun AH. Menstrual migraine. Curr Treat Options Neurol 2004; 6:489–498.
- Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004; 97:819–822.
- Calhoun AH. Current topics and controversies in menstrual migraine. Headache 2012; 52(suppl 1):8–11.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927.
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol 2013; 122:139 -147.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015; 16:1061–1070.
- Fernandez E, La Vecchia C, Franceschi S, et al. Oral contraceptive use and risk of colorectal cancer. Epidemiology 1998; 9:295–300.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association Contraceptive Study. Contraception 2013; 88:678–683.
- ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol 2010; 115:206–218.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 2016; 65:1–104.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33:629–808.
- Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the Spectrum study. Neurology 2002; 58(suppl 6):S27–S31.
- Lipton RB, Stewart WF, Cady R, et al. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000; 40:783–791.
- Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004; 44:856–864.
- Lipton RB, Stewart WF, Liberman JN. Self-awareness of migraine: interpreting the labels that headache sufferers apply to their headaches. Neurology 2002; 58(suppl 6):S21–S26.
- Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Curr Opin Neurol 2014; 27:315–324.
- Calhoun AH. Menstrual migraine: update on pathophysiology and approach to therapy and management. Curr Treat Options Neurol 2012; 14:1–14.
- McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med 2015; 162:ITC1–ITC15.
- O’Brien HL, Cohen JM. Young adults with headaches: the transition from adolescents to adults. Headache 2015; 55:1404–1409.
- Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J (Clin Res Ed) 1986; 292:526.
- Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- MacGregor EA. Contraception and headache. Headache 2013; 53:247–276.
- Oral contraceptives and stroke in young women. Associated risk factors. JAMA 1975; 231:718–722.
- Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1996; 348:498–505.
- Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception 2002; 65:197–205.
- Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke 1998; 29:2277–2284.
- Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996; 335:8–15.
- Donaghy M, Chang CL, Poulter N; European Collaborators of the World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry 2002; 73:747–750.
- Gudmundsson LS, Scher AI, Aspelund T, et al. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ 2010; 341:c3966.
- Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005; 64:1020–1026.
- Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008; 70:1510–1517.
- Kunz GA, Liang G, Cuculi F, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006; 152:190–195.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA 2006; 296:283–291.
- Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A. The migraine-ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med 2009; 9:215–226.
- Sulak P, Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache 2007; 47:27–37.
- Nappi RE, Terreno E, Sances G, et al. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 2013; 88:369–375.
- Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache 2012; 52:1246–1253.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG 2013; 120:801–810.
- Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014; 89:253–263.
- Benson LS, Micks EA. Why stop now? Extended and continuous regimens of combined hormonal contraceptive methods. Obstet Gynecol Clin North Am 2015; 42:669–681.
- Mannix LK, Calhoun AH. Menstrual migraine. Curr Treat Options Neurol 2004; 6:489–498.
- Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004; 97:819–822.
- Calhoun AH. Current topics and controversies in menstrual migraine. Headache 2012; 52(suppl 1):8–11.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927.
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol 2013; 122:139 -147.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015; 16:1061–1070.
- Fernandez E, La Vecchia C, Franceschi S, et al. Oral contraceptive use and risk of colorectal cancer. Epidemiology 1998; 9:295–300.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association Contraceptive Study. Contraception 2013; 88:678–683.
KEY POINTS
- There is no restriction on the use of combined hormonal contraceptives by women with migraine without aura, and the risk vs benefit for women with aura is debatable.
- Migraine with aura—but not migraine without aura—is associated with a twofold increased risk of ischemic stroke, although the absolute risk is small in healthy women who do not smoke.
- Combined hormonal contraceptives are associated with ischemic stroke, but the risk is dose-dependent. Ultra-low-dose formulations (containing ≤ 20 μg of ethinyl estradiol) do not pose an increased risk of stroke in healthy nonsmokers.