User login
A Mission for Graduate Medical Education at VA
More than 65% of all physicians who train in the U.S. rotate through a VA hospital at some point during their training. In 2015 alone, more than 43,000 residents received some or all of their clinical training through VA.1 Of the approximately 120 VAMCs that hold academic affiliations
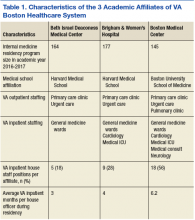
with medical schools and residency training programs, several hold affiliations with multiple institutions, including VA Boston Healthcare System (VABHS) in Massachusetts. The West Roxbury campus is the home of VA Boston’s acute care hospital, where residents and fellows from Boston Medical Center (BMC), Beth Israel Deaconess Medical Center (BIDMC), and Brigham and Women’s Hospital (BWH) train together. These are 3 of the largest medical training programs in Boston, though each provides a unique training experience for residents due to differences in patient population, faculty expertise, and hospital network affiliations (Table 1).
This diversity brings differences in cultural norms, institutional preferences, and educational expectations. Furthermore, residents from different programs who work together at VA Boston are often meeting one another for the first time, as opportunities for interinstitutional collaboration among these 3 training programs do not exist outside of VA. This training environment presents both an opportunity
and a challenge for medical educators: offering the best possible learning experience for physiciansin-training from multiple programs while providing the best possible care for U.S. veterans.
To guide educators charged with meeting this challenge, the VA Office of Academic Affiliations put forth a mission statement describing its overarching teaching mission (Table 2).2
To address this gap, chief medical residents from the 3 affiliate residency training programs came together to develop a shared mission statement for what we envision as the educational experience for all medical trainees rotating through VABHS (Table 2). In this article, we describe the development of a mission statement for graduate medical education in internal medicine at VABHS and provides examples of how our mission statement guided educational programming.
Methods
Whereas the affiliated institutions assign generic competency-based learning objectives to rotations at VABHS, no specific overarching educational objectives for residents have been defined previously. The directors of the internal medicine residency programs at each of the VABHS affiliate institutions grant their respective VA-based chief medical residents the autonomy to deliver graduate medical education at VA as they see fit, in collaboration with their colleagues from the other affiliated institutions and the VA director of medical resident education. This autonomy and flexibility allowed each of the chief medical residents to articulate an individual vision for VA graduate medical education based on their affiliate program’s goals, values, and mission.
At the beginning of the 2016/2017 academic year, in partnership with the director of medical resident education at VABHS, the chief medical residents met to reconcile these into a single shared mission statement. Special attention was paid to educational gaps at each affiliate institution that could be filled while residents were rotating at VABHS. Once all educational goals and priorities of the shared mission statement were identified, the chief medical residents and director of medical resident education adopted the mission statement as the blueprint for all educational programming for the academic year. Progress toward enacting the various components of the mission statement was reviewed monthly and changes in educational programming to ensure adequate emphasis of all components were made accordingly.
Results
Our first goal was to promote the personal and professional development of residents who rotate through VABHS, particularly interns, in a setting that fosters cross-institutional collaboration, respect, and friendship. The West Roxbury campus of VABHS is the only hospital in the city where internal medicine residents from 3 large training programs work together on teams that have been intentionally built to place residents from different institutions with one another. In educational conferences, we encouraged residents from different training programs to share their experiences with patient populations that others may not see at their home institutions, based on the specialized care that each institution provides. The conferences also give residents the opportunity to provide and receive near-peer teaching in a collegial environment.
Our second goal was to maintain an environment of educational excellence. We produced thought-provoking conferences that prioritized inspiring curiosity and teaching systems of thought over the dissemination of facts. We regularly focused on the broader context of medicine in case conferences and journal club, including topics such as public health, health policy, advocacy, health economics, quality improvement (QI), and high-value care. Our morning reports were interactive and participatory, emphasizing both technical skill practice and sophisticated clinical reasoning.
We embraced the principles of cognitive learning theory by priming learners with preconference “teasers” that previewed conference topics to be discussed. Every Friday, we played a medical version of Jeopardy!, which used spaced learning to consolidate the week’s teaching points in a fun, collaborative, and collegial atmosphere. Our dedicated patient safety conference gave residents the chance to use QI tools to dissect and tackle real problems in the hospital, and our monthly Morbidity and Mortality conference served as inspiration for many of the resident-driven QI projects.
Our third goal was to challenge physicians to provide the best possible care to veterans, including learning about issues unique to this often-marginalized population. We emphasized that training at a VA hospital is a privilege and that the best way to honor our veterans is to take advantage of the unique learning opportunities available at VA. To that end, we exposed residents to veteran-specific educational content, ranging from the structure and payment model of VHA to service-related medical conditions, such as posttraumatic stress disorder, other mental health issues, traumatic brain injury, Agent Orange exposure, and Gulf War Syndrome.
Discussion
Findings from the recently published Accreditation Council for Graduate Medical Education’s (ACGME) 2016 Clinical Learning Environment Review (CLER) Report support the need for mission statements like ours to guide the delivery of graduate medical education.3 A major finding of this report was that the development and implementation of graduate medical education largely occurs separately from other areas of organizational and strategic focus within clinical learning environments. Our mission statement has served as a road map for aligning the delivery of graduate medical education at VABHS with the specific strengths of the clinical learning environment that VA affords.
Additionally, the 2016 CLER report identified a lack of specificity in training on health care disparities and cultural competency for the specific populations served by the surveyed residency programs. The emphasis we placed on learning about issues specific to the care of the veteran population highlights the potential for other mission statements like ours to bridge the gap between articulation and execution of educational priorities. Finally, through the academic partnerships it holds with more than 90% of medical schools in the U.S., VA already has an integral role in both undergraduate and graduate medical education that positions its hospitals as ideal training environments in which to address shortcomings in medical training like those identified by the ACGME.4
Conclusion
We propose this mission statement as a model for the delivery of graduate medical education throughout all VA hospitals with academic affiliations and especially those where trainees from multiple institutions work together. As embodied in our mission statement, our goal was to provide a clinical training experience at VA that complements that of our residents’ home institutions and fosters a respect for and interest in the special care provided at VA. The development of a shared mission statement provides an invaluable tool in accomplishing that goal. We encourage chief medical residents and other leaders in medical education in all specialties at VAMCs to develop their own mission statements that reflect and embody the values of each affiliated training program. For our residents, rotating at VA is an opportunity to learn the practice of medicine for veterans, rather than practicing medicine on veterans. It is our sincere hope that shaping our residents’ educational experience in this fashion will foster a greater appreciation for the care of our nation’s veterans.
1. VA Office of Academic Affiliations. 2015 statistics: health professions trainees. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Published 2016. Accessed September 18, 2017.
2. VA Office of Academic Affiliations. Mission of the Office of Academic Affiliations. http://www.va.gov/oaa/oaa_mission.asp. Updated June 23, 2017. Accessed September 18, 2017.
3. Accreditation Council for Graduate Medical Education. Clinical learning environment review – national report of findings 2016 – executive summary. https://www.acgme.org/Portals/0/PDFs/CLER/ACGME-CLER-ExecutiveSummary.pdf. Published 2016. Accessed September 18, 2017.
4. Association of American Medical Colleges. The VA and academic medicine: partners in health care, training, and research. https://www.aamc.org/download/385612/data/07182014.pdf. Accessed September 14, 2017.
More than 65% of all physicians who train in the U.S. rotate through a VA hospital at some point during their training. In 2015 alone, more than 43,000 residents received some or all of their clinical training through VA.1 Of the approximately 120 VAMCs that hold academic affiliations
with medical schools and residency training programs, several hold affiliations with multiple institutions, including VA Boston Healthcare System (VABHS) in Massachusetts. The West Roxbury campus is the home of VA Boston’s acute care hospital, where residents and fellows from Boston Medical Center (BMC), Beth Israel Deaconess Medical Center (BIDMC), and Brigham and Women’s Hospital (BWH) train together. These are 3 of the largest medical training programs in Boston, though each provides a unique training experience for residents due to differences in patient population, faculty expertise, and hospital network affiliations (Table 1).
This diversity brings differences in cultural norms, institutional preferences, and educational expectations. Furthermore, residents from different programs who work together at VA Boston are often meeting one another for the first time, as opportunities for interinstitutional collaboration among these 3 training programs do not exist outside of VA. This training environment presents both an opportunity
and a challenge for medical educators: offering the best possible learning experience for physiciansin-training from multiple programs while providing the best possible care for U.S. veterans.
To guide educators charged with meeting this challenge, the VA Office of Academic Affiliations put forth a mission statement describing its overarching teaching mission (Table 2).2
To address this gap, chief medical residents from the 3 affiliate residency training programs came together to develop a shared mission statement for what we envision as the educational experience for all medical trainees rotating through VABHS (Table 2). In this article, we describe the development of a mission statement for graduate medical education in internal medicine at VABHS and provides examples of how our mission statement guided educational programming.
Methods
Whereas the affiliated institutions assign generic competency-based learning objectives to rotations at VABHS, no specific overarching educational objectives for residents have been defined previously. The directors of the internal medicine residency programs at each of the VABHS affiliate institutions grant their respective VA-based chief medical residents the autonomy to deliver graduate medical education at VA as they see fit, in collaboration with their colleagues from the other affiliated institutions and the VA director of medical resident education. This autonomy and flexibility allowed each of the chief medical residents to articulate an individual vision for VA graduate medical education based on their affiliate program’s goals, values, and mission.
At the beginning of the 2016/2017 academic year, in partnership with the director of medical resident education at VABHS, the chief medical residents met to reconcile these into a single shared mission statement. Special attention was paid to educational gaps at each affiliate institution that could be filled while residents were rotating at VABHS. Once all educational goals and priorities of the shared mission statement were identified, the chief medical residents and director of medical resident education adopted the mission statement as the blueprint for all educational programming for the academic year. Progress toward enacting the various components of the mission statement was reviewed monthly and changes in educational programming to ensure adequate emphasis of all components were made accordingly.
Results
Our first goal was to promote the personal and professional development of residents who rotate through VABHS, particularly interns, in a setting that fosters cross-institutional collaboration, respect, and friendship. The West Roxbury campus of VABHS is the only hospital in the city where internal medicine residents from 3 large training programs work together on teams that have been intentionally built to place residents from different institutions with one another. In educational conferences, we encouraged residents from different training programs to share their experiences with patient populations that others may not see at their home institutions, based on the specialized care that each institution provides. The conferences also give residents the opportunity to provide and receive near-peer teaching in a collegial environment.
Our second goal was to maintain an environment of educational excellence. We produced thought-provoking conferences that prioritized inspiring curiosity and teaching systems of thought over the dissemination of facts. We regularly focused on the broader context of medicine in case conferences and journal club, including topics such as public health, health policy, advocacy, health economics, quality improvement (QI), and high-value care. Our morning reports were interactive and participatory, emphasizing both technical skill practice and sophisticated clinical reasoning.
We embraced the principles of cognitive learning theory by priming learners with preconference “teasers” that previewed conference topics to be discussed. Every Friday, we played a medical version of Jeopardy!, which used spaced learning to consolidate the week’s teaching points in a fun, collaborative, and collegial atmosphere. Our dedicated patient safety conference gave residents the chance to use QI tools to dissect and tackle real problems in the hospital, and our monthly Morbidity and Mortality conference served as inspiration for many of the resident-driven QI projects.
Our third goal was to challenge physicians to provide the best possible care to veterans, including learning about issues unique to this often-marginalized population. We emphasized that training at a VA hospital is a privilege and that the best way to honor our veterans is to take advantage of the unique learning opportunities available at VA. To that end, we exposed residents to veteran-specific educational content, ranging from the structure and payment model of VHA to service-related medical conditions, such as posttraumatic stress disorder, other mental health issues, traumatic brain injury, Agent Orange exposure, and Gulf War Syndrome.
Discussion
Findings from the recently published Accreditation Council for Graduate Medical Education’s (ACGME) 2016 Clinical Learning Environment Review (CLER) Report support the need for mission statements like ours to guide the delivery of graduate medical education.3 A major finding of this report was that the development and implementation of graduate medical education largely occurs separately from other areas of organizational and strategic focus within clinical learning environments. Our mission statement has served as a road map for aligning the delivery of graduate medical education at VABHS with the specific strengths of the clinical learning environment that VA affords.
Additionally, the 2016 CLER report identified a lack of specificity in training on health care disparities and cultural competency for the specific populations served by the surveyed residency programs. The emphasis we placed on learning about issues specific to the care of the veteran population highlights the potential for other mission statements like ours to bridge the gap between articulation and execution of educational priorities. Finally, through the academic partnerships it holds with more than 90% of medical schools in the U.S., VA already has an integral role in both undergraduate and graduate medical education that positions its hospitals as ideal training environments in which to address shortcomings in medical training like those identified by the ACGME.4
Conclusion
We propose this mission statement as a model for the delivery of graduate medical education throughout all VA hospitals with academic affiliations and especially those where trainees from multiple institutions work together. As embodied in our mission statement, our goal was to provide a clinical training experience at VA that complements that of our residents’ home institutions and fosters a respect for and interest in the special care provided at VA. The development of a shared mission statement provides an invaluable tool in accomplishing that goal. We encourage chief medical residents and other leaders in medical education in all specialties at VAMCs to develop their own mission statements that reflect and embody the values of each affiliated training program. For our residents, rotating at VA is an opportunity to learn the practice of medicine for veterans, rather than practicing medicine on veterans. It is our sincere hope that shaping our residents’ educational experience in this fashion will foster a greater appreciation for the care of our nation’s veterans.
More than 65% of all physicians who train in the U.S. rotate through a VA hospital at some point during their training. In 2015 alone, more than 43,000 residents received some or all of their clinical training through VA.1 Of the approximately 120 VAMCs that hold academic affiliations
with medical schools and residency training programs, several hold affiliations with multiple institutions, including VA Boston Healthcare System (VABHS) in Massachusetts. The West Roxbury campus is the home of VA Boston’s acute care hospital, where residents and fellows from Boston Medical Center (BMC), Beth Israel Deaconess Medical Center (BIDMC), and Brigham and Women’s Hospital (BWH) train together. These are 3 of the largest medical training programs in Boston, though each provides a unique training experience for residents due to differences in patient population, faculty expertise, and hospital network affiliations (Table 1).
This diversity brings differences in cultural norms, institutional preferences, and educational expectations. Furthermore, residents from different programs who work together at VA Boston are often meeting one another for the first time, as opportunities for interinstitutional collaboration among these 3 training programs do not exist outside of VA. This training environment presents both an opportunity
and a challenge for medical educators: offering the best possible learning experience for physiciansin-training from multiple programs while providing the best possible care for U.S. veterans.
To guide educators charged with meeting this challenge, the VA Office of Academic Affiliations put forth a mission statement describing its overarching teaching mission (Table 2).2
To address this gap, chief medical residents from the 3 affiliate residency training programs came together to develop a shared mission statement for what we envision as the educational experience for all medical trainees rotating through VABHS (Table 2). In this article, we describe the development of a mission statement for graduate medical education in internal medicine at VABHS and provides examples of how our mission statement guided educational programming.
Methods
Whereas the affiliated institutions assign generic competency-based learning objectives to rotations at VABHS, no specific overarching educational objectives for residents have been defined previously. The directors of the internal medicine residency programs at each of the VABHS affiliate institutions grant their respective VA-based chief medical residents the autonomy to deliver graduate medical education at VA as they see fit, in collaboration with their colleagues from the other affiliated institutions and the VA director of medical resident education. This autonomy and flexibility allowed each of the chief medical residents to articulate an individual vision for VA graduate medical education based on their affiliate program’s goals, values, and mission.
At the beginning of the 2016/2017 academic year, in partnership with the director of medical resident education at VABHS, the chief medical residents met to reconcile these into a single shared mission statement. Special attention was paid to educational gaps at each affiliate institution that could be filled while residents were rotating at VABHS. Once all educational goals and priorities of the shared mission statement were identified, the chief medical residents and director of medical resident education adopted the mission statement as the blueprint for all educational programming for the academic year. Progress toward enacting the various components of the mission statement was reviewed monthly and changes in educational programming to ensure adequate emphasis of all components were made accordingly.
Results
Our first goal was to promote the personal and professional development of residents who rotate through VABHS, particularly interns, in a setting that fosters cross-institutional collaboration, respect, and friendship. The West Roxbury campus of VABHS is the only hospital in the city where internal medicine residents from 3 large training programs work together on teams that have been intentionally built to place residents from different institutions with one another. In educational conferences, we encouraged residents from different training programs to share their experiences with patient populations that others may not see at their home institutions, based on the specialized care that each institution provides. The conferences also give residents the opportunity to provide and receive near-peer teaching in a collegial environment.
Our second goal was to maintain an environment of educational excellence. We produced thought-provoking conferences that prioritized inspiring curiosity and teaching systems of thought over the dissemination of facts. We regularly focused on the broader context of medicine in case conferences and journal club, including topics such as public health, health policy, advocacy, health economics, quality improvement (QI), and high-value care. Our morning reports were interactive and participatory, emphasizing both technical skill practice and sophisticated clinical reasoning.
We embraced the principles of cognitive learning theory by priming learners with preconference “teasers” that previewed conference topics to be discussed. Every Friday, we played a medical version of Jeopardy!, which used spaced learning to consolidate the week’s teaching points in a fun, collaborative, and collegial atmosphere. Our dedicated patient safety conference gave residents the chance to use QI tools to dissect and tackle real problems in the hospital, and our monthly Morbidity and Mortality conference served as inspiration for many of the resident-driven QI projects.
Our third goal was to challenge physicians to provide the best possible care to veterans, including learning about issues unique to this often-marginalized population. We emphasized that training at a VA hospital is a privilege and that the best way to honor our veterans is to take advantage of the unique learning opportunities available at VA. To that end, we exposed residents to veteran-specific educational content, ranging from the structure and payment model of VHA to service-related medical conditions, such as posttraumatic stress disorder, other mental health issues, traumatic brain injury, Agent Orange exposure, and Gulf War Syndrome.
Discussion
Findings from the recently published Accreditation Council for Graduate Medical Education’s (ACGME) 2016 Clinical Learning Environment Review (CLER) Report support the need for mission statements like ours to guide the delivery of graduate medical education.3 A major finding of this report was that the development and implementation of graduate medical education largely occurs separately from other areas of organizational and strategic focus within clinical learning environments. Our mission statement has served as a road map for aligning the delivery of graduate medical education at VABHS with the specific strengths of the clinical learning environment that VA affords.
Additionally, the 2016 CLER report identified a lack of specificity in training on health care disparities and cultural competency for the specific populations served by the surveyed residency programs. The emphasis we placed on learning about issues specific to the care of the veteran population highlights the potential for other mission statements like ours to bridge the gap between articulation and execution of educational priorities. Finally, through the academic partnerships it holds with more than 90% of medical schools in the U.S., VA already has an integral role in both undergraduate and graduate medical education that positions its hospitals as ideal training environments in which to address shortcomings in medical training like those identified by the ACGME.4
Conclusion
We propose this mission statement as a model for the delivery of graduate medical education throughout all VA hospitals with academic affiliations and especially those where trainees from multiple institutions work together. As embodied in our mission statement, our goal was to provide a clinical training experience at VA that complements that of our residents’ home institutions and fosters a respect for and interest in the special care provided at VA. The development of a shared mission statement provides an invaluable tool in accomplishing that goal. We encourage chief medical residents and other leaders in medical education in all specialties at VAMCs to develop their own mission statements that reflect and embody the values of each affiliated training program. For our residents, rotating at VA is an opportunity to learn the practice of medicine for veterans, rather than practicing medicine on veterans. It is our sincere hope that shaping our residents’ educational experience in this fashion will foster a greater appreciation for the care of our nation’s veterans.
1. VA Office of Academic Affiliations. 2015 statistics: health professions trainees. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Published 2016. Accessed September 18, 2017.
2. VA Office of Academic Affiliations. Mission of the Office of Academic Affiliations. http://www.va.gov/oaa/oaa_mission.asp. Updated June 23, 2017. Accessed September 18, 2017.
3. Accreditation Council for Graduate Medical Education. Clinical learning environment review – national report of findings 2016 – executive summary. https://www.acgme.org/Portals/0/PDFs/CLER/ACGME-CLER-ExecutiveSummary.pdf. Published 2016. Accessed September 18, 2017.
4. Association of American Medical Colleges. The VA and academic medicine: partners in health care, training, and research. https://www.aamc.org/download/385612/data/07182014.pdf. Accessed September 14, 2017.
1. VA Office of Academic Affiliations. 2015 statistics: health professions trainees. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Published 2016. Accessed September 18, 2017.
2. VA Office of Academic Affiliations. Mission of the Office of Academic Affiliations. http://www.va.gov/oaa/oaa_mission.asp. Updated June 23, 2017. Accessed September 18, 2017.
3. Accreditation Council for Graduate Medical Education. Clinical learning environment review – national report of findings 2016 – executive summary. https://www.acgme.org/Portals/0/PDFs/CLER/ACGME-CLER-ExecutiveSummary.pdf. Published 2016. Accessed September 18, 2017.
4. Association of American Medical Colleges. The VA and academic medicine: partners in health care, training, and research. https://www.aamc.org/download/385612/data/07182014.pdf. Accessed September 14, 2017.
A Veteran With Alcohol Use Disorder and Acute Pancreatitis
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
Boston VA Medical Forum: HIV-Positive Veteran With Progressive Visual Changes
►Lakshmana Swamy, MD, chief medical resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. Dr. Serrao, when you hear about vision changes in a patient with HIV, what differential diagnosis is generated? What epidemiologic or historical factors can help distinguish these entities?
►Richard Serrao, MD, Infectious Disease Service, VABHS and assistant professor of medicine, Boston University School of Medicine. The differential diagnoses for vision changes in a patient with HIV is based on the overall immunosuppression of the patient: the lower the patient’s CD4 count, the higher the number of etiologies.1 The portions of the visual pathway as well as the pattern of vision loss are useful in narrowing the differential. For example, monocular visual disturbances with dermatomal vesicles within the ophthalmic division of the trigeminal nerve strongly implicates varicella zoster retinitis or keratitis; abducens nerve palsy could suggest granulomatous basilar meningitis from cryptococcosis. Likewise, ongoing fevers in an advanced AIDS patient with concomitant colitis, hepatitis, and pneumonitis is strongly suspicious for cytomegalovirus (CMV) retinitis with wide dissemination.
Geographic epidemiologic factors can suggest pathogens more prevalent to certain regions of the world, such as histoplasma chorioretinitis in a resident of the central and eastern U.S. or tuberculosis in a returning traveler. Likewise, a cat owner or one who consumes steak tartare increases the likelihood for toxoplasma retinochoroiditis, or syphilis in men who have sex with men (MSM) in the U.S. given that the majority of new cases occur in this patient population. Other clues one should consider include the presence of splinter hemorrhages in the extremities in an intravenous drug user, raising the possibility of embolic endophthalmitis from bacterial or fungal endocarditis. A variety of other diagnoses can certainly occur as a result of drug treatment (uveitis from rifampin, for example), immune reconstitution from HAART, infections with other HIV-associated pathogens, such as Pneumocystis jiroveci, and many non-HIV-related ocular diseases.
►Dr. Swamy. Dr. Butler, what concerns do you have when you hear about an HIV-infected patient with vision loss from the ophthalmology perspective?
►Nicholas Butler, MD, Ophthalmology Service, Uveitis and Ocular Immunology, VABHS and assistant professor of ophthalmology, Harvard Medical School. Of course, patients with HIV suffer from common causes of vision loss—cataract, glaucoma, diabetes, macular degeneration, for instance—just like those without HIV infection. If there is no significant immunodeficiency, then the patient’s HIV status would be less relevant, and these more common causes of vision loss should be pursued. My first task would be to determine the patient’s most recent CD4 T-cell count.
Assuming an HIV-positive individual is experiencing visual symptoms related to his/her underlying HIV infection (especially in the setting of CD4 counts < 200 cells/mm3), ocular opportunistic infections (OOI) come to mind first. Despite a reduction in incidence of 75% to 80% in the HAART-era, CMV retinitis remains the most common OOI in patients with AIDS and carries the greatest risk of ocular morbidity.2 In fact, based on enrollment data for the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), the prevalence of CMV retinitis among patients with AIDS is more than 20-fold higher than all other ocular complications of AIDS (OOIs and ocular neoplastic disease), including Kaposi sarcoma, lymphoma, herpes zoster ophthalmicus, ocular syphilis, ocular toxoplasma, necrotizing herpetic retinitis, cryptococcal choroiditis, and pneumocystis choroiditis.3 Beyond ocular opportunistic infections, the most common retinal finding in HIV-positive people is HIV retinopathy, nonspecific microvascular findings in the retina affecting nearly 70% of those with advanced HIV disease. Fortunately, HIV retinopathy is generally asymptomatic.4
►Dr. Swamy. Thank you for those explanations. Based on Dr. Serrao’s differential, it is worth noting that this patient is MSM. He was evaluated in urgent care with the initial examination showing a temperature of 98.0° F, pulse 83 beats per minute, and blood pressure 110/70 mm Hg. The eye exam showed no injection with normal extraocular movements. Initial laboratory data were notable for a CD4 count of 730 cells/mm3 with fewer than 20 HIV viral copies/mL. Cytomegalovirus immunoglobulin G (IgG) was positive, and immunoglobulin M (IgM) was negative. A Lyme antibody was positive with negative IgM and IgG by Western blot. Additional tests can be seen in Tables 1 and 2. The patient has good immunologic and virologic control. How does this change your thinking about the case?
►Dr. Serrao. His CD4 count is well above 350, increasing the likelihood of a relatively uncomplicated course and treatment. Cytomegalovirus antibodies reflect prior infection. As CMV generally does not manifest with disease of any variety (including CMV retinitis) at this high CD4 count, one can presume he does not have CMV retinitis as a cause for his visual changes. CMV retinitis occurs mainly when substantial CD4 depletion has occurred (typically less than 50 cells/mm3). A positive Lyme antibody screen, not specific to Lyme, can be falsely positive in other treponema diseases (eg, Treponema pallidum, the etiologic organism of syphilis) as evidenced by negative confirmatory Western blot IgG and IgM. Antineutrophil cystoplasmic antibodies, lysozyme, angiotensin-converting enzyme, rapid plasma reagin (RPR), herpes simplex virus, toxoplasma are generally included in the workup for the differential of uveitis, retinitis, choroiditis, etc.
►Dr. Swamy. Based on the visual changes, the patient was referred for urgent ophthalmologic evaluation. Dr. Butler, when should a generalist consider urgent ophthalmology referral?
►Dr. Butler. In general, all patients with acute (and significant) vision loss should be referred immediately to an ophthalmologist. The challenge for the general practitioner is determining the true extent of the reported vision loss. If possible, some assessment of visual acuity should be obtained, testing each eye independently and with the correct glasses correction (ie, the patient’s distance glasses if the test object is 12 feet or more from the patient or their reading glasses if the test object is held inside arm’s length). If the general practitioner does not have access to an eye chart or near card, any assessment of vision with an appropriate description will be useful (eg, the patient can quickly count fingers at 15 feet in the unaffected eye, but the eye with reported vision loss cannot reliably count fingers outside of 2 feet). Additional ocular symptoms associated with the vision loss, such as pain, redness, photophobia, new flashes or floaters, increase the urgency of the referral. The threshold for referral for any ocular complaint is lower compared with that of the general population for those with evidence of immunodeficiency, such as for this patient with HIV. Any CD4 count < 200 cells/mm3 should raise the practitioner’s concern for an ocular opportunistic infection, with the greatest concern with CD4 counts < 50 cells/mm3.
►Dr. Swamy. The patient underwent further testing in the ophthalmology clinic. Dr. Butler, can you please interpret the funduscopic exam?
►Dr. Butler. Both eyes demonstrate findings (microaneurysms and small dot-blot hemorrhages) consistent with moderate nonproliferative diabetic retinopathy (Figure 1A, white arrows). HIV-associated retinopathy could produce similar findings, but it is not generally seen with CD4 counts > 200 cells/mm3. Additionally, in the left eye, there is a diffuse patch of retinal whitening (retinitis) associated with the inferotemporal vascular arcades (Figure 1B, white arrows). The entire area involved is poorly circumscribed and the whitening is subtle in areas. Overlying some areas of deeper, ground-glass whitening there are scattered, punctate white spots (Figure 1B, green arrows). Wickremasinghe and colleagues described this pattern of retinitis and suggested that it had a high positive-predictive value in the diagnosis of ocular syphilis.5
►Dr. Swamy. The patient then underwent fluorescein angiography and optical coherence tomography (OCT). Dr. Butler, what did the fluorescein angiography show?
►Dr. Butler. The fluorescein angiogram in both eyes revealed leakage of dye consistent with diabetic retinopathy, with the right eye (OD) worse than the left (OS). Additionally, the areas of active retinitis in the left eye displayed gradual staining with leopard-spot changes, along with late leakage of fluorescein dye, indicating vasculopathy in the infected area (Figure 2, arrows). The patient also underwent OCT in the left eye (images not displayed) demonstrating vitreous cells (vitritis), patches of inner retinal thickening with hyperreflectivity, and hyperreflective nodules at the level of the retinal pigment epithelium with overlying photoreceptor disruption. These OCT findings are fairly stereotypic for syphilitic chorioretinitis.6
►Dr. Swamy. Based on the ophthalmic findings, a diagnosis of ocular syphilis was made. Dr. Serrao, what should internists consider as they evaluate and manage a patient with ocular syphilis?
►Dr. Serrao. Although isolated ocular involvement from syphilis is possible, the majority of patients (up to 85%) with HIV can present with concomitant central nervous system infection and about 30% present with symptomatic neurosyphilis (a typical late manifestation of this disease) that reflects the aggressiveness, accelerated course and propensity for wide dissemination of syphilis in this patient population.7
The presence of concomitant cutaneous rashes should prompt universal precautions, because transmission can occur via skin to skin contact. Clinicians should watch for the Jarisch-Herxheimer reaction during treatment, a syndrome of fever, myalgias, and headache, which results from circulating cytokines produced because of rapidly dying spirochetes that could mimic a penicillin drug reaction, yet is treated supportively.
As syphilis is sexually acquired, clinicians should test for coexistent sexually transmitted infections, vaccinate for those that are preventable (eg, hepatitis B), notify sexual partners via assistance from local departments of public health, and assess for coexistent drug use and offer counseling in order to optimize risk reduction. Special attention should be paid to virologic control of HIV since some studies have shown an increase in the propensity for breakthrough HIV viremia while on effective ART.9 This should warrant counseling for ongoing optimal ART adherence and close monitoring in the follow-up visits with a provider specialized in the treatment of syphilis and HIV.
►Dr. Swamy. A lumbar puncture is performed with the results listed in Table 2. Dr. Serrao, is the CSF consistent with neurosyphilis? What would you do next?
►Dr. Serrao. The lumbar puncture is inflammatory with a lymphocytic predominance, consistent with active ocular/neurosyphilis. The CSF Venereal Disease Research Laboratory test is specific but not sensitive so a negative value does not rule out the presence of central nervous system infection.10 The CSF fluorescent treponemal antibody (CSF FTA-ABS) is sensitive but not specific. In this case, the ocular findings, positive serum RPR, CSF lymphocytic predominance, and CSF FTA ABS strongly supports the diagnosis of ocular/early neurosyphilis in a patient with HIV infection in whom early aggressive treatment is warranted to prevent rapid progression/potential loss of vision.11
►Dr. Swamy. Dr. Butler, how does syphilis behave in the eye as compared to other infectious or inflammatory diseases? Do visual symptoms respond well to treatment?
►Dr. Butler. As opposed to the dramatic reduction in rates and severity of CMV retinitis, HAART has had a negligible effect on ocular syphilis in the setting of HIV coinfection; in fact, rates of syphilis, including ocular syphilis, are currently surging world-wide, and HIV coinfection portends a worse prognosis.12 This is especially true among gay men. More so, there appears to be no correlation between CD4 count and incidence of developing ocular syphilis, as opposed to CMV retinitis, which occurs far more frequently in those with CD4 counts < 50 cells/mm3. In keeping with its epithet as one of the “Great Imitators,” syphilis can affect virtually every tissue of the eye—conjunctiva, sclera, cornea, iris, lens, vitreous, retina, choroid, optic nerve—unlike other OOI, such as CMV or toxoplasma, which generally hone to the retina. Nonetheless, various findings and patterns on clinical exam and ancillary testing, such as the more recently described punctate inner retinitis (as seen in our patient) and the more classic acute syphilitic posterior placoid chorioretinitis, carry high specificity for ocular syphilis.13
Patients with ocular syphilis should be treated according to neurosyphilis treatment protocols. In general, these patients respond very well to treatment with resolution of the ocular findings and recovery of complete, or nearly so, visual function, as long as an excessive delay between diagnosis and proper treatment does not occur.14
►Dr. Swamy. Following this testing, the patient completed 14 days of IV penicillin with resolution of symptoms. He had no further vision complaints. He was started on Triumeq (abacavir, dolutegravir, and lamivudine) with good adherence to therapy. Dr. Serrao, in 2016 the CDC released a clinical advisory about ocular syphilis. Can you tell us about why this is an important diagnosis to be aware of today?
►Dr. Serrao. As with any disease, the epidemiologic characteristics of an infection like syphilis allow the clinician to more carefully entertain such a diagnosis in any one individual by improving the index of suspicion for a particular disease. Awareness of an increase in ocular syphilis in HIV positive MSM allows for a more timely assessment and subsequent treatment with the goal of preventing loss of vision.15
1. Cunningham ET Jr, Margolis TP. Ocular manifestations of HIV infection. N Engl J Med. 1998;339(4):236-244.
2. Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12(14):1931-1933.
3. Gangaputra S, Drye L, Vaidya V, Thorne JE, Jabs DA, Lyon AT. Non-cytomegalovirus ocular opportunistic infections in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2013;155(2):206-212.e205.
4. Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780-786.
5. Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, Zamir E. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009;116(6):1195-1200.
6. Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4(1):2.
7. Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113(11):872-881.
8. Lukehart SA, Hook EW 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109(11):855-862.
9. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
10. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012;39(6):453-457.
11. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis: a systematic review. Sex Transm Dis. 2012;39(4):291-297.
12. Butler NJ, Thorne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23(6):517-522.
13. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288-1297.
14. Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25(6):513-518.
15. Clinical Advisory: Ocular Syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Accessed September 11, 2017.
►Lakshmana Swamy, MD, chief medical resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. Dr. Serrao, when you hear about vision changes in a patient with HIV, what differential diagnosis is generated? What epidemiologic or historical factors can help distinguish these entities?
►Richard Serrao, MD, Infectious Disease Service, VABHS and assistant professor of medicine, Boston University School of Medicine. The differential diagnoses for vision changes in a patient with HIV is based on the overall immunosuppression of the patient: the lower the patient’s CD4 count, the higher the number of etiologies.1 The portions of the visual pathway as well as the pattern of vision loss are useful in narrowing the differential. For example, monocular visual disturbances with dermatomal vesicles within the ophthalmic division of the trigeminal nerve strongly implicates varicella zoster retinitis or keratitis; abducens nerve palsy could suggest granulomatous basilar meningitis from cryptococcosis. Likewise, ongoing fevers in an advanced AIDS patient with concomitant colitis, hepatitis, and pneumonitis is strongly suspicious for cytomegalovirus (CMV) retinitis with wide dissemination.
Geographic epidemiologic factors can suggest pathogens more prevalent to certain regions of the world, such as histoplasma chorioretinitis in a resident of the central and eastern U.S. or tuberculosis in a returning traveler. Likewise, a cat owner or one who consumes steak tartare increases the likelihood for toxoplasma retinochoroiditis, or syphilis in men who have sex with men (MSM) in the U.S. given that the majority of new cases occur in this patient population. Other clues one should consider include the presence of splinter hemorrhages in the extremities in an intravenous drug user, raising the possibility of embolic endophthalmitis from bacterial or fungal endocarditis. A variety of other diagnoses can certainly occur as a result of drug treatment (uveitis from rifampin, for example), immune reconstitution from HAART, infections with other HIV-associated pathogens, such as Pneumocystis jiroveci, and many non-HIV-related ocular diseases.
►Dr. Swamy. Dr. Butler, what concerns do you have when you hear about an HIV-infected patient with vision loss from the ophthalmology perspective?
►Nicholas Butler, MD, Ophthalmology Service, Uveitis and Ocular Immunology, VABHS and assistant professor of ophthalmology, Harvard Medical School. Of course, patients with HIV suffer from common causes of vision loss—cataract, glaucoma, diabetes, macular degeneration, for instance—just like those without HIV infection. If there is no significant immunodeficiency, then the patient’s HIV status would be less relevant, and these more common causes of vision loss should be pursued. My first task would be to determine the patient’s most recent CD4 T-cell count.
Assuming an HIV-positive individual is experiencing visual symptoms related to his/her underlying HIV infection (especially in the setting of CD4 counts < 200 cells/mm3), ocular opportunistic infections (OOI) come to mind first. Despite a reduction in incidence of 75% to 80% in the HAART-era, CMV retinitis remains the most common OOI in patients with AIDS and carries the greatest risk of ocular morbidity.2 In fact, based on enrollment data for the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), the prevalence of CMV retinitis among patients with AIDS is more than 20-fold higher than all other ocular complications of AIDS (OOIs and ocular neoplastic disease), including Kaposi sarcoma, lymphoma, herpes zoster ophthalmicus, ocular syphilis, ocular toxoplasma, necrotizing herpetic retinitis, cryptococcal choroiditis, and pneumocystis choroiditis.3 Beyond ocular opportunistic infections, the most common retinal finding in HIV-positive people is HIV retinopathy, nonspecific microvascular findings in the retina affecting nearly 70% of those with advanced HIV disease. Fortunately, HIV retinopathy is generally asymptomatic.4
►Dr. Swamy. Thank you for those explanations. Based on Dr. Serrao’s differential, it is worth noting that this patient is MSM. He was evaluated in urgent care with the initial examination showing a temperature of 98.0° F, pulse 83 beats per minute, and blood pressure 110/70 mm Hg. The eye exam showed no injection with normal extraocular movements. Initial laboratory data were notable for a CD4 count of 730 cells/mm3 with fewer than 20 HIV viral copies/mL. Cytomegalovirus immunoglobulin G (IgG) was positive, and immunoglobulin M (IgM) was negative. A Lyme antibody was positive with negative IgM and IgG by Western blot. Additional tests can be seen in Tables 1 and 2. The patient has good immunologic and virologic control. How does this change your thinking about the case?
►Dr. Serrao. His CD4 count is well above 350, increasing the likelihood of a relatively uncomplicated course and treatment. Cytomegalovirus antibodies reflect prior infection. As CMV generally does not manifest with disease of any variety (including CMV retinitis) at this high CD4 count, one can presume he does not have CMV retinitis as a cause for his visual changes. CMV retinitis occurs mainly when substantial CD4 depletion has occurred (typically less than 50 cells/mm3). A positive Lyme antibody screen, not specific to Lyme, can be falsely positive in other treponema diseases (eg, Treponema pallidum, the etiologic organism of syphilis) as evidenced by negative confirmatory Western blot IgG and IgM. Antineutrophil cystoplasmic antibodies, lysozyme, angiotensin-converting enzyme, rapid plasma reagin (RPR), herpes simplex virus, toxoplasma are generally included in the workup for the differential of uveitis, retinitis, choroiditis, etc.
►Dr. Swamy. Based on the visual changes, the patient was referred for urgent ophthalmologic evaluation. Dr. Butler, when should a generalist consider urgent ophthalmology referral?
►Dr. Butler. In general, all patients with acute (and significant) vision loss should be referred immediately to an ophthalmologist. The challenge for the general practitioner is determining the true extent of the reported vision loss. If possible, some assessment of visual acuity should be obtained, testing each eye independently and with the correct glasses correction (ie, the patient’s distance glasses if the test object is 12 feet or more from the patient or their reading glasses if the test object is held inside arm’s length). If the general practitioner does not have access to an eye chart or near card, any assessment of vision with an appropriate description will be useful (eg, the patient can quickly count fingers at 15 feet in the unaffected eye, but the eye with reported vision loss cannot reliably count fingers outside of 2 feet). Additional ocular symptoms associated with the vision loss, such as pain, redness, photophobia, new flashes or floaters, increase the urgency of the referral. The threshold for referral for any ocular complaint is lower compared with that of the general population for those with evidence of immunodeficiency, such as for this patient with HIV. Any CD4 count < 200 cells/mm3 should raise the practitioner’s concern for an ocular opportunistic infection, with the greatest concern with CD4 counts < 50 cells/mm3.
►Dr. Swamy. The patient underwent further testing in the ophthalmology clinic. Dr. Butler, can you please interpret the funduscopic exam?
►Dr. Butler. Both eyes demonstrate findings (microaneurysms and small dot-blot hemorrhages) consistent with moderate nonproliferative diabetic retinopathy (Figure 1A, white arrows). HIV-associated retinopathy could produce similar findings, but it is not generally seen with CD4 counts > 200 cells/mm3. Additionally, in the left eye, there is a diffuse patch of retinal whitening (retinitis) associated with the inferotemporal vascular arcades (Figure 1B, white arrows). The entire area involved is poorly circumscribed and the whitening is subtle in areas. Overlying some areas of deeper, ground-glass whitening there are scattered, punctate white spots (Figure 1B, green arrows). Wickremasinghe and colleagues described this pattern of retinitis and suggested that it had a high positive-predictive value in the diagnosis of ocular syphilis.5
►Dr. Swamy. The patient then underwent fluorescein angiography and optical coherence tomography (OCT). Dr. Butler, what did the fluorescein angiography show?
►Dr. Butler. The fluorescein angiogram in both eyes revealed leakage of dye consistent with diabetic retinopathy, with the right eye (OD) worse than the left (OS). Additionally, the areas of active retinitis in the left eye displayed gradual staining with leopard-spot changes, along with late leakage of fluorescein dye, indicating vasculopathy in the infected area (Figure 2, arrows). The patient also underwent OCT in the left eye (images not displayed) demonstrating vitreous cells (vitritis), patches of inner retinal thickening with hyperreflectivity, and hyperreflective nodules at the level of the retinal pigment epithelium with overlying photoreceptor disruption. These OCT findings are fairly stereotypic for syphilitic chorioretinitis.6
►Dr. Swamy. Based on the ophthalmic findings, a diagnosis of ocular syphilis was made. Dr. Serrao, what should internists consider as they evaluate and manage a patient with ocular syphilis?
►Dr. Serrao. Although isolated ocular involvement from syphilis is possible, the majority of patients (up to 85%) with HIV can present with concomitant central nervous system infection and about 30% present with symptomatic neurosyphilis (a typical late manifestation of this disease) that reflects the aggressiveness, accelerated course and propensity for wide dissemination of syphilis in this patient population.7
The presence of concomitant cutaneous rashes should prompt universal precautions, because transmission can occur via skin to skin contact. Clinicians should watch for the Jarisch-Herxheimer reaction during treatment, a syndrome of fever, myalgias, and headache, which results from circulating cytokines produced because of rapidly dying spirochetes that could mimic a penicillin drug reaction, yet is treated supportively.
As syphilis is sexually acquired, clinicians should test for coexistent sexually transmitted infections, vaccinate for those that are preventable (eg, hepatitis B), notify sexual partners via assistance from local departments of public health, and assess for coexistent drug use and offer counseling in order to optimize risk reduction. Special attention should be paid to virologic control of HIV since some studies have shown an increase in the propensity for breakthrough HIV viremia while on effective ART.9 This should warrant counseling for ongoing optimal ART adherence and close monitoring in the follow-up visits with a provider specialized in the treatment of syphilis and HIV.
►Dr. Swamy. A lumbar puncture is performed with the results listed in Table 2. Dr. Serrao, is the CSF consistent with neurosyphilis? What would you do next?
►Dr. Serrao. The lumbar puncture is inflammatory with a lymphocytic predominance, consistent with active ocular/neurosyphilis. The CSF Venereal Disease Research Laboratory test is specific but not sensitive so a negative value does not rule out the presence of central nervous system infection.10 The CSF fluorescent treponemal antibody (CSF FTA-ABS) is sensitive but not specific. In this case, the ocular findings, positive serum RPR, CSF lymphocytic predominance, and CSF FTA ABS strongly supports the diagnosis of ocular/early neurosyphilis in a patient with HIV infection in whom early aggressive treatment is warranted to prevent rapid progression/potential loss of vision.11
►Dr. Swamy. Dr. Butler, how does syphilis behave in the eye as compared to other infectious or inflammatory diseases? Do visual symptoms respond well to treatment?
►Dr. Butler. As opposed to the dramatic reduction in rates and severity of CMV retinitis, HAART has had a negligible effect on ocular syphilis in the setting of HIV coinfection; in fact, rates of syphilis, including ocular syphilis, are currently surging world-wide, and HIV coinfection portends a worse prognosis.12 This is especially true among gay men. More so, there appears to be no correlation between CD4 count and incidence of developing ocular syphilis, as opposed to CMV retinitis, which occurs far more frequently in those with CD4 counts < 50 cells/mm3. In keeping with its epithet as one of the “Great Imitators,” syphilis can affect virtually every tissue of the eye—conjunctiva, sclera, cornea, iris, lens, vitreous, retina, choroid, optic nerve—unlike other OOI, such as CMV or toxoplasma, which generally hone to the retina. Nonetheless, various findings and patterns on clinical exam and ancillary testing, such as the more recently described punctate inner retinitis (as seen in our patient) and the more classic acute syphilitic posterior placoid chorioretinitis, carry high specificity for ocular syphilis.13
Patients with ocular syphilis should be treated according to neurosyphilis treatment protocols. In general, these patients respond very well to treatment with resolution of the ocular findings and recovery of complete, or nearly so, visual function, as long as an excessive delay between diagnosis and proper treatment does not occur.14
►Dr. Swamy. Following this testing, the patient completed 14 days of IV penicillin with resolution of symptoms. He had no further vision complaints. He was started on Triumeq (abacavir, dolutegravir, and lamivudine) with good adherence to therapy. Dr. Serrao, in 2016 the CDC released a clinical advisory about ocular syphilis. Can you tell us about why this is an important diagnosis to be aware of today?
►Dr. Serrao. As with any disease, the epidemiologic characteristics of an infection like syphilis allow the clinician to more carefully entertain such a diagnosis in any one individual by improving the index of suspicion for a particular disease. Awareness of an increase in ocular syphilis in HIV positive MSM allows for a more timely assessment and subsequent treatment with the goal of preventing loss of vision.15
►Lakshmana Swamy, MD, chief medical resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. Dr. Serrao, when you hear about vision changes in a patient with HIV, what differential diagnosis is generated? What epidemiologic or historical factors can help distinguish these entities?
►Richard Serrao, MD, Infectious Disease Service, VABHS and assistant professor of medicine, Boston University School of Medicine. The differential diagnoses for vision changes in a patient with HIV is based on the overall immunosuppression of the patient: the lower the patient’s CD4 count, the higher the number of etiologies.1 The portions of the visual pathway as well as the pattern of vision loss are useful in narrowing the differential. For example, monocular visual disturbances with dermatomal vesicles within the ophthalmic division of the trigeminal nerve strongly implicates varicella zoster retinitis or keratitis; abducens nerve palsy could suggest granulomatous basilar meningitis from cryptococcosis. Likewise, ongoing fevers in an advanced AIDS patient with concomitant colitis, hepatitis, and pneumonitis is strongly suspicious for cytomegalovirus (CMV) retinitis with wide dissemination.
Geographic epidemiologic factors can suggest pathogens more prevalent to certain regions of the world, such as histoplasma chorioretinitis in a resident of the central and eastern U.S. or tuberculosis in a returning traveler. Likewise, a cat owner or one who consumes steak tartare increases the likelihood for toxoplasma retinochoroiditis, or syphilis in men who have sex with men (MSM) in the U.S. given that the majority of new cases occur in this patient population. Other clues one should consider include the presence of splinter hemorrhages in the extremities in an intravenous drug user, raising the possibility of embolic endophthalmitis from bacterial or fungal endocarditis. A variety of other diagnoses can certainly occur as a result of drug treatment (uveitis from rifampin, for example), immune reconstitution from HAART, infections with other HIV-associated pathogens, such as Pneumocystis jiroveci, and many non-HIV-related ocular diseases.
►Dr. Swamy. Dr. Butler, what concerns do you have when you hear about an HIV-infected patient with vision loss from the ophthalmology perspective?
►Nicholas Butler, MD, Ophthalmology Service, Uveitis and Ocular Immunology, VABHS and assistant professor of ophthalmology, Harvard Medical School. Of course, patients with HIV suffer from common causes of vision loss—cataract, glaucoma, diabetes, macular degeneration, for instance—just like those without HIV infection. If there is no significant immunodeficiency, then the patient’s HIV status would be less relevant, and these more common causes of vision loss should be pursued. My first task would be to determine the patient’s most recent CD4 T-cell count.
Assuming an HIV-positive individual is experiencing visual symptoms related to his/her underlying HIV infection (especially in the setting of CD4 counts < 200 cells/mm3), ocular opportunistic infections (OOI) come to mind first. Despite a reduction in incidence of 75% to 80% in the HAART-era, CMV retinitis remains the most common OOI in patients with AIDS and carries the greatest risk of ocular morbidity.2 In fact, based on enrollment data for the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), the prevalence of CMV retinitis among patients with AIDS is more than 20-fold higher than all other ocular complications of AIDS (OOIs and ocular neoplastic disease), including Kaposi sarcoma, lymphoma, herpes zoster ophthalmicus, ocular syphilis, ocular toxoplasma, necrotizing herpetic retinitis, cryptococcal choroiditis, and pneumocystis choroiditis.3 Beyond ocular opportunistic infections, the most common retinal finding in HIV-positive people is HIV retinopathy, nonspecific microvascular findings in the retina affecting nearly 70% of those with advanced HIV disease. Fortunately, HIV retinopathy is generally asymptomatic.4
►Dr. Swamy. Thank you for those explanations. Based on Dr. Serrao’s differential, it is worth noting that this patient is MSM. He was evaluated in urgent care with the initial examination showing a temperature of 98.0° F, pulse 83 beats per minute, and blood pressure 110/70 mm Hg. The eye exam showed no injection with normal extraocular movements. Initial laboratory data were notable for a CD4 count of 730 cells/mm3 with fewer than 20 HIV viral copies/mL. Cytomegalovirus immunoglobulin G (IgG) was positive, and immunoglobulin M (IgM) was negative. A Lyme antibody was positive with negative IgM and IgG by Western blot. Additional tests can be seen in Tables 1 and 2. The patient has good immunologic and virologic control. How does this change your thinking about the case?
►Dr. Serrao. His CD4 count is well above 350, increasing the likelihood of a relatively uncomplicated course and treatment. Cytomegalovirus antibodies reflect prior infection. As CMV generally does not manifest with disease of any variety (including CMV retinitis) at this high CD4 count, one can presume he does not have CMV retinitis as a cause for his visual changes. CMV retinitis occurs mainly when substantial CD4 depletion has occurred (typically less than 50 cells/mm3). A positive Lyme antibody screen, not specific to Lyme, can be falsely positive in other treponema diseases (eg, Treponema pallidum, the etiologic organism of syphilis) as evidenced by negative confirmatory Western blot IgG and IgM. Antineutrophil cystoplasmic antibodies, lysozyme, angiotensin-converting enzyme, rapid plasma reagin (RPR), herpes simplex virus, toxoplasma are generally included in the workup for the differential of uveitis, retinitis, choroiditis, etc.
►Dr. Swamy. Based on the visual changes, the patient was referred for urgent ophthalmologic evaluation. Dr. Butler, when should a generalist consider urgent ophthalmology referral?
►Dr. Butler. In general, all patients with acute (and significant) vision loss should be referred immediately to an ophthalmologist. The challenge for the general practitioner is determining the true extent of the reported vision loss. If possible, some assessment of visual acuity should be obtained, testing each eye independently and with the correct glasses correction (ie, the patient’s distance glasses if the test object is 12 feet or more from the patient or their reading glasses if the test object is held inside arm’s length). If the general practitioner does not have access to an eye chart or near card, any assessment of vision with an appropriate description will be useful (eg, the patient can quickly count fingers at 15 feet in the unaffected eye, but the eye with reported vision loss cannot reliably count fingers outside of 2 feet). Additional ocular symptoms associated with the vision loss, such as pain, redness, photophobia, new flashes or floaters, increase the urgency of the referral. The threshold for referral for any ocular complaint is lower compared with that of the general population for those with evidence of immunodeficiency, such as for this patient with HIV. Any CD4 count < 200 cells/mm3 should raise the practitioner’s concern for an ocular opportunistic infection, with the greatest concern with CD4 counts < 50 cells/mm3.
►Dr. Swamy. The patient underwent further testing in the ophthalmology clinic. Dr. Butler, can you please interpret the funduscopic exam?
►Dr. Butler. Both eyes demonstrate findings (microaneurysms and small dot-blot hemorrhages) consistent with moderate nonproliferative diabetic retinopathy (Figure 1A, white arrows). HIV-associated retinopathy could produce similar findings, but it is not generally seen with CD4 counts > 200 cells/mm3. Additionally, in the left eye, there is a diffuse patch of retinal whitening (retinitis) associated with the inferotemporal vascular arcades (Figure 1B, white arrows). The entire area involved is poorly circumscribed and the whitening is subtle in areas. Overlying some areas of deeper, ground-glass whitening there are scattered, punctate white spots (Figure 1B, green arrows). Wickremasinghe and colleagues described this pattern of retinitis and suggested that it had a high positive-predictive value in the diagnosis of ocular syphilis.5
►Dr. Swamy. The patient then underwent fluorescein angiography and optical coherence tomography (OCT). Dr. Butler, what did the fluorescein angiography show?
►Dr. Butler. The fluorescein angiogram in both eyes revealed leakage of dye consistent with diabetic retinopathy, with the right eye (OD) worse than the left (OS). Additionally, the areas of active retinitis in the left eye displayed gradual staining with leopard-spot changes, along with late leakage of fluorescein dye, indicating vasculopathy in the infected area (Figure 2, arrows). The patient also underwent OCT in the left eye (images not displayed) demonstrating vitreous cells (vitritis), patches of inner retinal thickening with hyperreflectivity, and hyperreflective nodules at the level of the retinal pigment epithelium with overlying photoreceptor disruption. These OCT findings are fairly stereotypic for syphilitic chorioretinitis.6
►Dr. Swamy. Based on the ophthalmic findings, a diagnosis of ocular syphilis was made. Dr. Serrao, what should internists consider as they evaluate and manage a patient with ocular syphilis?
►Dr. Serrao. Although isolated ocular involvement from syphilis is possible, the majority of patients (up to 85%) with HIV can present with concomitant central nervous system infection and about 30% present with symptomatic neurosyphilis (a typical late manifestation of this disease) that reflects the aggressiveness, accelerated course and propensity for wide dissemination of syphilis in this patient population.7
The presence of concomitant cutaneous rashes should prompt universal precautions, because transmission can occur via skin to skin contact. Clinicians should watch for the Jarisch-Herxheimer reaction during treatment, a syndrome of fever, myalgias, and headache, which results from circulating cytokines produced because of rapidly dying spirochetes that could mimic a penicillin drug reaction, yet is treated supportively.
As syphilis is sexually acquired, clinicians should test for coexistent sexually transmitted infections, vaccinate for those that are preventable (eg, hepatitis B), notify sexual partners via assistance from local departments of public health, and assess for coexistent drug use and offer counseling in order to optimize risk reduction. Special attention should be paid to virologic control of HIV since some studies have shown an increase in the propensity for breakthrough HIV viremia while on effective ART.9 This should warrant counseling for ongoing optimal ART adherence and close monitoring in the follow-up visits with a provider specialized in the treatment of syphilis and HIV.
►Dr. Swamy. A lumbar puncture is performed with the results listed in Table 2. Dr. Serrao, is the CSF consistent with neurosyphilis? What would you do next?
►Dr. Serrao. The lumbar puncture is inflammatory with a lymphocytic predominance, consistent with active ocular/neurosyphilis. The CSF Venereal Disease Research Laboratory test is specific but not sensitive so a negative value does not rule out the presence of central nervous system infection.10 The CSF fluorescent treponemal antibody (CSF FTA-ABS) is sensitive but not specific. In this case, the ocular findings, positive serum RPR, CSF lymphocytic predominance, and CSF FTA ABS strongly supports the diagnosis of ocular/early neurosyphilis in a patient with HIV infection in whom early aggressive treatment is warranted to prevent rapid progression/potential loss of vision.11
►Dr. Swamy. Dr. Butler, how does syphilis behave in the eye as compared to other infectious or inflammatory diseases? Do visual symptoms respond well to treatment?
►Dr. Butler. As opposed to the dramatic reduction in rates and severity of CMV retinitis, HAART has had a negligible effect on ocular syphilis in the setting of HIV coinfection; in fact, rates of syphilis, including ocular syphilis, are currently surging world-wide, and HIV coinfection portends a worse prognosis.12 This is especially true among gay men. More so, there appears to be no correlation between CD4 count and incidence of developing ocular syphilis, as opposed to CMV retinitis, which occurs far more frequently in those with CD4 counts < 50 cells/mm3. In keeping with its epithet as one of the “Great Imitators,” syphilis can affect virtually every tissue of the eye—conjunctiva, sclera, cornea, iris, lens, vitreous, retina, choroid, optic nerve—unlike other OOI, such as CMV or toxoplasma, which generally hone to the retina. Nonetheless, various findings and patterns on clinical exam and ancillary testing, such as the more recently described punctate inner retinitis (as seen in our patient) and the more classic acute syphilitic posterior placoid chorioretinitis, carry high specificity for ocular syphilis.13
Patients with ocular syphilis should be treated according to neurosyphilis treatment protocols. In general, these patients respond very well to treatment with resolution of the ocular findings and recovery of complete, or nearly so, visual function, as long as an excessive delay between diagnosis and proper treatment does not occur.14
►Dr. Swamy. Following this testing, the patient completed 14 days of IV penicillin with resolution of symptoms. He had no further vision complaints. He was started on Triumeq (abacavir, dolutegravir, and lamivudine) with good adherence to therapy. Dr. Serrao, in 2016 the CDC released a clinical advisory about ocular syphilis. Can you tell us about why this is an important diagnosis to be aware of today?
►Dr. Serrao. As with any disease, the epidemiologic characteristics of an infection like syphilis allow the clinician to more carefully entertain such a diagnosis in any one individual by improving the index of suspicion for a particular disease. Awareness of an increase in ocular syphilis in HIV positive MSM allows for a more timely assessment and subsequent treatment with the goal of preventing loss of vision.15
1. Cunningham ET Jr, Margolis TP. Ocular manifestations of HIV infection. N Engl J Med. 1998;339(4):236-244.
2. Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12(14):1931-1933.
3. Gangaputra S, Drye L, Vaidya V, Thorne JE, Jabs DA, Lyon AT. Non-cytomegalovirus ocular opportunistic infections in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2013;155(2):206-212.e205.
4. Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780-786.
5. Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, Zamir E. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009;116(6):1195-1200.
6. Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4(1):2.
7. Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113(11):872-881.
8. Lukehart SA, Hook EW 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109(11):855-862.
9. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
10. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012;39(6):453-457.
11. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis: a systematic review. Sex Transm Dis. 2012;39(4):291-297.
12. Butler NJ, Thorne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23(6):517-522.
13. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288-1297.
14. Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25(6):513-518.
15. Clinical Advisory: Ocular Syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Accessed September 11, 2017.
1. Cunningham ET Jr, Margolis TP. Ocular manifestations of HIV infection. N Engl J Med. 1998;339(4):236-244.
2. Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12(14):1931-1933.
3. Gangaputra S, Drye L, Vaidya V, Thorne JE, Jabs DA, Lyon AT. Non-cytomegalovirus ocular opportunistic infections in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2013;155(2):206-212.e205.
4. Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780-786.
5. Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, Zamir E. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009;116(6):1195-1200.
6. Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4(1):2.
7. Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113(11):872-881.
8. Lukehart SA, Hook EW 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109(11):855-862.
9. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
10. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012;39(6):453-457.
11. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis: a systematic review. Sex Transm Dis. 2012;39(4):291-297.
12. Butler NJ, Thorne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23(6):517-522.
13. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288-1297.
14. Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25(6):513-518.
15. Clinical Advisory: Ocular Syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Accessed September 11, 2017.
Yield of Blood Cultures
Blood cultures are the gold standard test for the diagnosis of bloodstream infections (BSI). Given the high mortality associated with BSI,[1, 2, 3] physicians have a low threshold to obtain blood cultures.[4, 5] Unfortunately, physicians are poor at predicting which hospitalized patients have BSI,[6, 7] and published guidelines do not provide clear indications for the use of blood cultures.[8] As a result, current practice follows a culture if spikes paradigm, whereby inpatient providers often obtain blood cultures in the setting of any fever. This is the most common anticipatory guidance communicated between providers, involving up to 75% of written sign‐out instructions.[9] The result is a low rate of true positive blood cultures (5%10%)[10, 11, 12] with only a slightly lower rate of false positive blood cultures (contaminants).[12, 13, 14] False positive blood cultures often lead to repeat blood cultures, unnecessary antibiotic use, and increased hospital cost and length of stay.[13]
Over the last several years, there has been an increased emphasis on practicing high‐value care by avoiding unnecessary and duplicate testing. In 2012, the American Board of Internal Medicine introduced the Choosing Wisely campaign, with specific initiatives to reduce medical waste and overuse. Given the low yield of blood cultures, guidance on patients in whom blood cultures are most appropriate would be welcome. Studies assessing risk factors for bacteremia have led to the development of multiple stratification systems without overall consensus.[10, 15, 16, 17, 18, 19, 20] Furthermore, much of the current literature on blood culture utilization includes cultures drawn in the emergency department (ED) or intensive care unit setting (ICU).[10, 18, 19, 20] Less is known regarding the rates of positivity and utility for blood cultures drawn on patients hospitalized on an acute care medical ward.
Our study had 3 main objectives: (1) determine the rates of true positive and false positive blood cultures among hospitalized medical patients, (2) determine the ability of physician‐selected indications and patient characteristics to predict BSI, and (3) identify populations in which blood cultures may not be necessary.
PATIENTS AND METHODS
Study Design
We conducted a prospective cohort study of all hospitalized medical patients for whom blood cultures were ordered and received by the microbiology laboratory. This investigation was approved by the Veterans Affairs (VA) Boston Healthcare System internal review board.
Patients and Setting
During a 7‐month period (October 1, 2014April 15, 2015), all blood culture orders were reviewed for indication and result each day (and on Monday for weekend blood cultures) at a large VA teaching hospital (approximately 6200 admissions each year). As part of the electronic medical order, providers selected from among a list of common indications. Options included various clinical signs and diagnoses, and providers could select more than 1 indication. Each blood culture order triggered a phlebotomist to draw 2 separate blood culture sets (each set consisted of 1 aerobic and 1 anaerobic blood culture bottle).
Inclusion criteria included admission to 1 of 5 general medical service teams or 1 of 2 cardiology teams. Given that the study hospital does not have dedicated subspecialty service teams (with the exception of cardiology), all patients with medical diagnoses are cared for on the general medical service.
Predictor and Outcome Variables
Patient characteristics were obtained via chart review. Fever was defined as a single temperature greater than 100.4F within 24 hours prior to a blood culture order. Leukocytosis was defined as a white blood cell count greater than 10,000 within 24 hours of a blood culture order. Patients were considered to have received antibiotics if an order for an antibacterial or antifungal agent was active within 72 hours prior to the blood culture order. Each blood culture order was assigned a working diagnosis that prompted the order. These working diagnoses were identified by chart review as documented under the provider's assessment and plan and were not necessarily the primary diagnosis prompting hospitalization.
Classification of positive blood cultures into true and false positive was determined by consensus among the microbiology and the infectious disease departments after review of clinical and laboratory data, consistent with a previously established practice at the hospital. A true negative culture consisted of any culture that was not a true positive or a false positive. A blood culture order was defined as an electronic entry and included all sets of blood cultures drawn as a result of that order. Consistent with previous literature, a blood culture episode was defined as all blood cultures ordered within a 48‐hour period starting at the time of the first culture.[10] For patients with multiple admissions during the study period, each admission was considered a unique patient.
Statistical Analysis
Rates of true and false positivity of blood cultures were calculated. In addition, positive likelihood ratios (LR+) for true positive blood cultures were calculated using JMP statistical software (SAS Institute, Inc., Cary, NC).
RESULTS
Overall
A total of 576 blood culture orders (467 blood culture episodes) were completed on 363 hospitalized medical patients during the study period. Five hundred forty orders were placed on patients on general medical services and 36 orders on patients on the cardiology services. Four hundred eighty‐seven (85%) orders resulted in 2 sets of cultures being drawn, 87 (15%) resulted in 1 set of cultures, and 2 (0.3%) resulted in 3 sets of cultures. The median time between admission and culture draw was 2 days (range, 072 days), with 57% of cultures drawn during hospital day 0 to 2, 24.5% drawn between hospital day 3 to 7, and 19.4% drawn after hospital day 7. The average age of the patients was 70.4 years, and 94% were men. Additional patient characteristics are shown in Table 1.
| Clinical Characteristic | Total, n = 363 (%) | True Positive Blood Cultures, n = 14 (%) | P Value |
|---|---|---|---|
| |||
| Mean age, y | 70.4 | 73.9 | 0.4 |
| Male sex | 350 (96%) | 14 (100%) | 1 |
| White race | 308 (85%) | 11 (79%) | 0.7 |
| Location prior to admission | |||
| Community | 276 (76%) | 11 (79%) | 1 |
| Hospital | 51 (14%) | 1 (7%) | 0.7 |
| Long‐term care facility | 36 (10%) | 2 (14%) | 0.6 |
| Comorbidities | |||
| Diabetes | 136 (37%) | 5 (36%) | 1 |
| Malignancy | 100 (28%) | 4 (31%) | 1 |
| Alcohol abuse | 89 (25%) | 2 (14%) | 0.5 |
| Cirrhosis | 31 (9%) | 1 (7%) | 1 |
| End‐stage renal disease | 21 (6%) | 1 (7%) | 1 |
| Active drug use* | 16 (4%) | 1 (7%) | 0.5 |
| Catheter | 93 (26%) | 3 (21%) | 0.8 |
| Recent hospitalization | 145 (40%) | 6 (43%) | 1 |
| History of MRSA colonization | 72 (20%) | 5 (36%) | 0.16 |
| Cultures drawn in emergency department | 69 (19%) | 6 (43%) | 0.03 |
The true positive and false positive rates per blood culture order were 3.6% (21/576) and 2.3% (13/576), respectively (Table 2). Similar values were seen per blood cultures episode (3.4% and 2.7%, respectively). The true positive blood culture rates per order and episode were significantly lower than those drawn on emergency room patients during the study period (41/570, 7.2%, P < 0.05).
| Total, n (%) | True Positive, n (%) | False Positive, n (%) | True Negative, n (%) | |
|---|---|---|---|---|
| ||||
| Per patient | 363 | 14 (3.8) | 13 (3.6) | 336 (92.6) |
| Per blood culture episode | 467 | 16 (3.4) | 13 (2.7) | 438 (93.8) |
| Per blood culture order | 576 | 21 (3.6) | 13 (2.3) | 542 (94.1) |
| Rates per blood culture order | ||||
| Physician‐selected indication, n = 530 | ||||
| Fever | 136 (25.6) | 3 (2.2) | 3 (2.2) | 130 (95.6) |
| Fever and additional indication(s) | 118 (22.2) | 5 (4.2) | 3 (2.5) | 110 (93.2) |
| Fever and leukocytosis | 50 (9.4) | 4 (8.0) | 3 (6.0) | 43 (86.0) |
| Leukocytosis | 50 (9.4) | 2 (4.0) | 0 (0) | 48 (96.0) |
| Follow‐up previous positive | 60 (11.3) | 7 (11.7) | 0 (0) | 53 (88.3) |
| Working diagnosis, n = 576 | ||||
| Pneumonia | 101 (17.5) | 0 (0) | 4 (3.9) | 97 (96.0) |
| Bacteremia/endocarditis | 97 (16.8) | 12 (12.3) | 1 (1.0) | 84 (86.6) |
| Urinary tract infection* | 95 (16.4) | 5 (5.3) | 2 (2.1) | 88 (92.6) |
| Other infection | 46 (8.0) | 0 (0) | 0 (0) | 46 (100) |
| Skin and soft‐tissue infection | 39 (6.8) | 1 (2.6) | 0 (0) | 38 (97.4) |
| Neutropenic fever | 28 (4.9) | 0 (0) | 0 (0) | 28 (100) |
| Sepsis | 27 (4.7) | 0 (0) | 0 (0) | 27 (100) |
| Fever | 18 (3.1) | 1 (5.5) | 1 (5.5) | 16 (88.9) |
| Bone and join infection | 15 (2.6) | 1 (6.7) | 0 (0) | 14 (93.3) |
| Postoperative fever | 9 (1.6) | 0 (0) | 0 (0) | 9 (100) |
| Noninfectious diagnosis | 101 (17.5) | 1 (1.0) | 5 (5.0) | 95 (94.1) |
| Antibiotic exposure | ||||
| Yes | 354 (61.5) | 5 (1.4) | 5 (1.4) | 344 (97.1) |
| No | 222 (38.6) | 16 (7.2) | 8 (3.6) | 198 (89.1) |
| Previous documented positive culture via chart review | ||||
| Yes | 155 (26.9) | 9 (5.8) | 2 (1.3) | 144 (92.9) |
| No | 421 (73.1) | 12 (2.9) | 11 (2.6) | 398 (94.5) |
| LR+ (95% CI), True Positive Blood Culture | LR+ (95% CI), False Positive Blood Culture | |
|---|---|---|
| ||
| Physician‐selected indication | ||
| Fever | 0.6 (0.21.7) | 0.9 (0.32.5) |
| Fever and additional indication(s) | 1.1 (0.52.4) | 1.0 (0.42.8) |
| Fever and leukocytosis | 2.2 (0.95.6) | 2.5 (0.97.1) |
| Leukocytosis | 1.1 (0.34.0) | 0.4 (0.05.6) |
| Follow‐up previous positive | 3.4 (1.86.5) | 0.3 (0.04.7) |
| Diagnosis | ||
| Pneumonia | 0.1 (0.01.9) | 1.8 (0.84.1) |
| Bacteremia/endocarditis | 3.7 (2.55.7) | 0.5 (0.13.0) |
| Urinary tract infection | 1.5 (0.73.2) | 0.9 (0.33.4) |
| Noninfectious diagnosis | 0.3 (0.01.8) | 2.3 (1.14.6) |
| Recent antibiotic exposure | ||
| Yes | 0.4 (0.20.8) | 0.6 (0.31.2) |
| No | 2.1 (1.62.7) | 1.6 (1.02.5) |
| No with fever | 2.4 (1.24.9) | 0.8 (0.23.6) |
| No with fever and leukocytosis | 5.6 (1.818.2) | 0.4 (0.12.6) |
| Prior positive cultures | ||
| Yes | 1.6 (1.02.7) | 0.6 (0.22.0) |
For the true positive cultures, gram‐positive organisms were isolated most frequently (14/21, 67%) with Staphylococcus aureus identified in 2/21 (10%) positive cultures and Enterococcus faecalis identified in 7/21 (33%) positive cultures. Gram‐negative organisms were isolated in 6/21 (29%) cultures, and 1/21 (5%) culture grew 2 organisms (Enterococcus faecalis and Nocardia). The majority of false positive cultures isolated 1 or more species of coagulase‐negative Staphylococcus (11/13, 85%).
Predictors of True Bacteremia
The 4 most common working diagnoses prompting a blood culture order were pneumonia, bacteremia/endocarditis, urinary tract infection, and a noninfectious diagnosis (eg, syncope), with each prompting approximately 17% of the total orders (Table 2). Of these, only a primary diagnosis of bacteremia/endocarditis was predictive of a true positive culture, yielding a rate of 12.3% (LR+ 3.7, 95% confidence interval [CI]: 2.5‐5.7). No other diagnosis was predictive of true positivity. A diagnosis of pneumonia yielded no true positive and 4 false positive blood cultures (3.9%), whereas a noninfectious diagnosis yielded only 1 true positive (1.0%) and 5 false positives (5.0%). The positive likelihood ratios for these 2 diagnoses were 0.1 (95% CI: 0.00‐1.9) and 0.3 (95% CI: 0.04‐1.8), respectively.
Indications were selected for 530 of 576 (92%) blood culture orders (Table 2). The most common indication was fever alone (25.6%), followed by fever with an additional indication (22.2%), follow‐up positive blood cultures (11.3%), fever and leukocytosis (9.4%), and leukocytosis alone (9.4%). Only follow‐up positive blood cultures was predictive of a true positive, with a LR+ of 3.4 (95% CI: 1.8‐6.5).
A total of 14 patients (3.9%) had true positive blood cultures. For these patients, 10/14 (71%) had 1 true positive blood culture, 3/14 (21%) had 2 true positive blood cultures, and 1/14 (7%) had 5 true positive blood cultures. The average number of cultures drawn was 4.9. The clinical characteristic most predictive of a true positive blood culture was the absence of recent antibiotic administration. If the blood culture was ordered on a patient not receiving antibiotics (true positivity rate 7.2%, 16/222), the LR+ was 2.1 (95% CI: 1.6‐2.7). In a patient not receiving antibiotics who was also noted to have fever and leukocytosis (true positivity rate 17.6%, 3/17), the LR+ was 5.6 (95% CI: 1.8‐18.2). Conversely, patients receiving antibiotics were rarely found to have true positive blood cultures (true positivity rate 1.4%, 5/354) with a LR+ of 0.4 (95% CI: 0.2‐0.8).
DISCUSSION
In this prospective study, we determined the diagnostic yield of blood cultures ordered on hospitalized medical patients to be low, with just 3.6% of orders identifying a true BSI. This was coupled with a similar false positive rate of 2.3%. Our study found rates of true positive blood cultures much lower in hospitalized medical patients than in rates previously described when ED and ICU patients were included.[11, 16]
Although ordering blood cultures is a routine clinical behavior when there is concern for an infection, a clinician's ability to subjectively predict who has a BSI only improves the likelihood 2‐fold.[6] Despite the availability of multiple scoring systems to aid the clinicians,[10, 21, 22] our study found that over 50% of cultures were ordered in the setting of fever or leukocytosis, potentially demonstrating a triggered response to an event, rather than a decision based on probabilities. This common clinician instinct to culture if spikes is an ineffective practice if not coupled with additional clinical information. In fact, in 1 retrospective study, there was no association between fever spike and blood culture positivity.[23]
Our study suggests that objective and easily obtainable clinical characteristics may be effective in helping determine the probability of blood cultures revealing a BSI. Although more robust prediction models have value, they often require multiple inputs, limiting their utility to the bedside clinician. Stratifying patients by either antibiotic exposure or working diagnosis may provide the most benefit for the hospitalized medical patient. For those on antibiotics, the yield of true positive blood cultures is so low that they are unlikely to provide clinically useful information. In fact, although nearly two‐thirds of cultures were obtained after antibiotic exposure, only 1 (0.2%) of these patients had a culture that provided additional information regarding a BSI. Bacteremia had already been established for the other 4 patients. These results are similar to a prior study, which concluded that physicians should wait 72 hours from time of preantibiotic cultures before considering additional blood cultures given the lack of additional information provided.[24]
The working diagnosis also drives the probability of a positive blood culture. As has been shown with other studies, blood cultures are unlikely to diagnose a BSI for patients being treated for either cellulitis or pneumonia.[25, 26, 27] In our study, the working diagnosis prompting the most blood cultures was pneumonia, with the false positive rate exceeding the true positive rate, a finding consistent with previous literature. This situation may lead to the addition of unnecessary antibiotics while waiting for a positive culture to be confirmed as a false positive (eg, vancomycin for a preliminary culture showing gram‐positive cocci in clusters).
There are a number of limitations to our study. Physician‐chosen indication may not correlate with the actual clinical picture and/or may not represent the full set of variables involved in the clinical decision to order a blood culture. However, the subjective clinical indication and the objective clinical criteria found in the chart provided similar LRs. Our study did not evaluate the potential harm of not ordering a blood culture. We also did not assess the value of a true negative culture particularly in patients with endovascular infections where additional cultures are often required to document clearance of bacteremia. Lastly, our study applies to patients on a hospitalized medical service and was performed at a VA hospital with a specific population of elderly male patients, which may limit the generalizability of our results.
Despite these limitations, this study benefits from its prospective design, along with the fact that >90% of blood culture orders placed included a corresponding indication. This provides insight into physician clinical reasoning at the time the blood culture was ordered. In addition, our ability to calculate likelihood ratios provides bedside physicians with an easy and powerful way of modifying the probability of BSI prior to ordering blood cultures, aiding them in providing high‐value clinical care while potentially reducing testing overuse.
The acceptability of not obtaining blood cultures may vary by clinical experience and by specialty. Physicians must weigh the low true positive rate against the consequences of missing a BSI. Although not a substitute for clinical judgement, the LRs in this study can provide a framework to aid in clinical decision making. For example, assuming a pretest probability of 3.6% (the rate of true positive for our entire cohort), blood cultures may not be equally as compelling in 2 similar patients with fever. The first is not on antibiotics and also has a leukocytosis. The second is being treated for pneumonia and is already on antibiotics. For the first patient, using a LR+ of 5.6 (for the fever and leukocytosis in the absence of antibiotics) modifies the patient's probability of a true positive blood culture to 17.3%. Blood cultures should be ordered. In contrast, for the second patient, using a LR+ of 0.4 (for the presence of antibiotics) decreases the patient's probability of a true positive blood culture to 1.5%. Armed with these data, the bedside clinician can now decide whether this rate of true positivity warrants blood cultures. For some, this rate will be comfortably low. For others, this rate will not assuage them; only the negative culture will. Our data are not meant to make this decision, but may aid in making it a probability‐based decision.
Disclosures
Presented in part at the Infectious Diseases Society of America Annual Meeting in San Diego, California in 2015. This material is the result of work supported in part with resources and the use of facilities at the VA Boston HCS, West Roxbury, MA. Katherine Linsenmeyer, MD, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
- , . Population‐based epidemiology and microbiology of community‐onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664.
- , , , et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602.
- , , , . The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5(1):54–70.
- , , , et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- , , , et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278(3):234–240.
- , , , . Predicting bacteremia in older patients. J Am Geriatr Soc. 1995;43(3):230–235.
- , , , , , . Febrile inpatients: house officers' use of blood cultures. J Gen Intern Med. 1987;2(5):293–297.
- , , , et al. Executive summary: a guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57(4):485–488.
- , , , , . What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248–255.
- , , , . Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113(7):495–500.
- , . Blood cultures. Ann Intern Med. 1987;106(2):246–253.
- , , , et al. Reducing blood culture contamination by a simple informational intervention. J Clin Microbiol. 2010;48(12):4552–4558.
- , , . Contaminant blood cultures and resource utilization. The true consequences of false‐positive results. JAMA. 1991;265(3):365–369.
- . Blood culture contaminants. J Hosp Infect. 2014;87(1):1–10.
- , , , , , . The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117–123.
- , , , et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997;176(6):1538–1551.
- , . The systemic inflammatory response syndrome as a predictor of bacteraemia and outcome from sepsis. QJM. 1996;89(7):515–522.
- , , , , . Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35(3):255–264.
- , , , , , . Factors associated with positive blood cultures in outpatients with suspected bacteremia. Eur J Clin Microbiol Infect Dis. 2011;30(12):1615–1619.
- , , , et al. Two rules for early prediction of bacteremia: testing in a university and a community hospital. J Gen Intern Med. 1996;11(2):98–103.
- , , , . Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308(5):502–511.
- , , , et al. Clinical prediction rules for bacteremia and in‐hospital death based on clinical data at the time of blood withdrawal for culture: an evaluation of their development and use. J Eval Clin Pract. 2006;12(6):692–703.
- , , , et al. Timing of specimen collection for blood cultures from febrile patients with bacteremia. J Clin Microbiol. 2008;46(4):1381–1385.
- , , , . Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis. 2001;32(11):1651–1655.
- , , , , . Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks. Chest. 1995;108(4):932–936.
- . Blood cultures in community‐acquired pneumonia: are we ready to quit? Chest. 2003;123(4):977–978.
- . Blood cultures for community‐acquired pneumonia: piecing together a mosaic for doing less. Am J Respir Crit Care Med. 2004;169(3):327–328.
Blood cultures are the gold standard test for the diagnosis of bloodstream infections (BSI). Given the high mortality associated with BSI,[1, 2, 3] physicians have a low threshold to obtain blood cultures.[4, 5] Unfortunately, physicians are poor at predicting which hospitalized patients have BSI,[6, 7] and published guidelines do not provide clear indications for the use of blood cultures.[8] As a result, current practice follows a culture if spikes paradigm, whereby inpatient providers often obtain blood cultures in the setting of any fever. This is the most common anticipatory guidance communicated between providers, involving up to 75% of written sign‐out instructions.[9] The result is a low rate of true positive blood cultures (5%10%)[10, 11, 12] with only a slightly lower rate of false positive blood cultures (contaminants).[12, 13, 14] False positive blood cultures often lead to repeat blood cultures, unnecessary antibiotic use, and increased hospital cost and length of stay.[13]
Over the last several years, there has been an increased emphasis on practicing high‐value care by avoiding unnecessary and duplicate testing. In 2012, the American Board of Internal Medicine introduced the Choosing Wisely campaign, with specific initiatives to reduce medical waste and overuse. Given the low yield of blood cultures, guidance on patients in whom blood cultures are most appropriate would be welcome. Studies assessing risk factors for bacteremia have led to the development of multiple stratification systems without overall consensus.[10, 15, 16, 17, 18, 19, 20] Furthermore, much of the current literature on blood culture utilization includes cultures drawn in the emergency department (ED) or intensive care unit setting (ICU).[10, 18, 19, 20] Less is known regarding the rates of positivity and utility for blood cultures drawn on patients hospitalized on an acute care medical ward.
Our study had 3 main objectives: (1) determine the rates of true positive and false positive blood cultures among hospitalized medical patients, (2) determine the ability of physician‐selected indications and patient characteristics to predict BSI, and (3) identify populations in which blood cultures may not be necessary.
PATIENTS AND METHODS
Study Design
We conducted a prospective cohort study of all hospitalized medical patients for whom blood cultures were ordered and received by the microbiology laboratory. This investigation was approved by the Veterans Affairs (VA) Boston Healthcare System internal review board.
Patients and Setting
During a 7‐month period (October 1, 2014April 15, 2015), all blood culture orders were reviewed for indication and result each day (and on Monday for weekend blood cultures) at a large VA teaching hospital (approximately 6200 admissions each year). As part of the electronic medical order, providers selected from among a list of common indications. Options included various clinical signs and diagnoses, and providers could select more than 1 indication. Each blood culture order triggered a phlebotomist to draw 2 separate blood culture sets (each set consisted of 1 aerobic and 1 anaerobic blood culture bottle).
Inclusion criteria included admission to 1 of 5 general medical service teams or 1 of 2 cardiology teams. Given that the study hospital does not have dedicated subspecialty service teams (with the exception of cardiology), all patients with medical diagnoses are cared for on the general medical service.
Predictor and Outcome Variables
Patient characteristics were obtained via chart review. Fever was defined as a single temperature greater than 100.4F within 24 hours prior to a blood culture order. Leukocytosis was defined as a white blood cell count greater than 10,000 within 24 hours of a blood culture order. Patients were considered to have received antibiotics if an order for an antibacterial or antifungal agent was active within 72 hours prior to the blood culture order. Each blood culture order was assigned a working diagnosis that prompted the order. These working diagnoses were identified by chart review as documented under the provider's assessment and plan and were not necessarily the primary diagnosis prompting hospitalization.
Classification of positive blood cultures into true and false positive was determined by consensus among the microbiology and the infectious disease departments after review of clinical and laboratory data, consistent with a previously established practice at the hospital. A true negative culture consisted of any culture that was not a true positive or a false positive. A blood culture order was defined as an electronic entry and included all sets of blood cultures drawn as a result of that order. Consistent with previous literature, a blood culture episode was defined as all blood cultures ordered within a 48‐hour period starting at the time of the first culture.[10] For patients with multiple admissions during the study period, each admission was considered a unique patient.
Statistical Analysis
Rates of true and false positivity of blood cultures were calculated. In addition, positive likelihood ratios (LR+) for true positive blood cultures were calculated using JMP statistical software (SAS Institute, Inc., Cary, NC).
RESULTS
Overall
A total of 576 blood culture orders (467 blood culture episodes) were completed on 363 hospitalized medical patients during the study period. Five hundred forty orders were placed on patients on general medical services and 36 orders on patients on the cardiology services. Four hundred eighty‐seven (85%) orders resulted in 2 sets of cultures being drawn, 87 (15%) resulted in 1 set of cultures, and 2 (0.3%) resulted in 3 sets of cultures. The median time between admission and culture draw was 2 days (range, 072 days), with 57% of cultures drawn during hospital day 0 to 2, 24.5% drawn between hospital day 3 to 7, and 19.4% drawn after hospital day 7. The average age of the patients was 70.4 years, and 94% were men. Additional patient characteristics are shown in Table 1.
| Clinical Characteristic | Total, n = 363 (%) | True Positive Blood Cultures, n = 14 (%) | P Value |
|---|---|---|---|
| |||
| Mean age, y | 70.4 | 73.9 | 0.4 |
| Male sex | 350 (96%) | 14 (100%) | 1 |
| White race | 308 (85%) | 11 (79%) | 0.7 |
| Location prior to admission | |||
| Community | 276 (76%) | 11 (79%) | 1 |
| Hospital | 51 (14%) | 1 (7%) | 0.7 |
| Long‐term care facility | 36 (10%) | 2 (14%) | 0.6 |
| Comorbidities | |||
| Diabetes | 136 (37%) | 5 (36%) | 1 |
| Malignancy | 100 (28%) | 4 (31%) | 1 |
| Alcohol abuse | 89 (25%) | 2 (14%) | 0.5 |
| Cirrhosis | 31 (9%) | 1 (7%) | 1 |
| End‐stage renal disease | 21 (6%) | 1 (7%) | 1 |
| Active drug use* | 16 (4%) | 1 (7%) | 0.5 |
| Catheter | 93 (26%) | 3 (21%) | 0.8 |
| Recent hospitalization | 145 (40%) | 6 (43%) | 1 |
| History of MRSA colonization | 72 (20%) | 5 (36%) | 0.16 |
| Cultures drawn in emergency department | 69 (19%) | 6 (43%) | 0.03 |
The true positive and false positive rates per blood culture order were 3.6% (21/576) and 2.3% (13/576), respectively (Table 2). Similar values were seen per blood cultures episode (3.4% and 2.7%, respectively). The true positive blood culture rates per order and episode were significantly lower than those drawn on emergency room patients during the study period (41/570, 7.2%, P < 0.05).
| Total, n (%) | True Positive, n (%) | False Positive, n (%) | True Negative, n (%) | |
|---|---|---|---|---|
| ||||
| Per patient | 363 | 14 (3.8) | 13 (3.6) | 336 (92.6) |
| Per blood culture episode | 467 | 16 (3.4) | 13 (2.7) | 438 (93.8) |
| Per blood culture order | 576 | 21 (3.6) | 13 (2.3) | 542 (94.1) |
| Rates per blood culture order | ||||
| Physician‐selected indication, n = 530 | ||||
| Fever | 136 (25.6) | 3 (2.2) | 3 (2.2) | 130 (95.6) |
| Fever and additional indication(s) | 118 (22.2) | 5 (4.2) | 3 (2.5) | 110 (93.2) |
| Fever and leukocytosis | 50 (9.4) | 4 (8.0) | 3 (6.0) | 43 (86.0) |
| Leukocytosis | 50 (9.4) | 2 (4.0) | 0 (0) | 48 (96.0) |
| Follow‐up previous positive | 60 (11.3) | 7 (11.7) | 0 (0) | 53 (88.3) |
| Working diagnosis, n = 576 | ||||
| Pneumonia | 101 (17.5) | 0 (0) | 4 (3.9) | 97 (96.0) |
| Bacteremia/endocarditis | 97 (16.8) | 12 (12.3) | 1 (1.0) | 84 (86.6) |
| Urinary tract infection* | 95 (16.4) | 5 (5.3) | 2 (2.1) | 88 (92.6) |
| Other infection | 46 (8.0) | 0 (0) | 0 (0) | 46 (100) |
| Skin and soft‐tissue infection | 39 (6.8) | 1 (2.6) | 0 (0) | 38 (97.4) |
| Neutropenic fever | 28 (4.9) | 0 (0) | 0 (0) | 28 (100) |
| Sepsis | 27 (4.7) | 0 (0) | 0 (0) | 27 (100) |
| Fever | 18 (3.1) | 1 (5.5) | 1 (5.5) | 16 (88.9) |
| Bone and join infection | 15 (2.6) | 1 (6.7) | 0 (0) | 14 (93.3) |
| Postoperative fever | 9 (1.6) | 0 (0) | 0 (0) | 9 (100) |
| Noninfectious diagnosis | 101 (17.5) | 1 (1.0) | 5 (5.0) | 95 (94.1) |
| Antibiotic exposure | ||||
| Yes | 354 (61.5) | 5 (1.4) | 5 (1.4) | 344 (97.1) |
| No | 222 (38.6) | 16 (7.2) | 8 (3.6) | 198 (89.1) |
| Previous documented positive culture via chart review | ||||
| Yes | 155 (26.9) | 9 (5.8) | 2 (1.3) | 144 (92.9) |
| No | 421 (73.1) | 12 (2.9) | 11 (2.6) | 398 (94.5) |
| LR+ (95% CI), True Positive Blood Culture | LR+ (95% CI), False Positive Blood Culture | |
|---|---|---|
| ||
| Physician‐selected indication | ||
| Fever | 0.6 (0.21.7) | 0.9 (0.32.5) |
| Fever and additional indication(s) | 1.1 (0.52.4) | 1.0 (0.42.8) |
| Fever and leukocytosis | 2.2 (0.95.6) | 2.5 (0.97.1) |
| Leukocytosis | 1.1 (0.34.0) | 0.4 (0.05.6) |
| Follow‐up previous positive | 3.4 (1.86.5) | 0.3 (0.04.7) |
| Diagnosis | ||
| Pneumonia | 0.1 (0.01.9) | 1.8 (0.84.1) |
| Bacteremia/endocarditis | 3.7 (2.55.7) | 0.5 (0.13.0) |
| Urinary tract infection | 1.5 (0.73.2) | 0.9 (0.33.4) |
| Noninfectious diagnosis | 0.3 (0.01.8) | 2.3 (1.14.6) |
| Recent antibiotic exposure | ||
| Yes | 0.4 (0.20.8) | 0.6 (0.31.2) |
| No | 2.1 (1.62.7) | 1.6 (1.02.5) |
| No with fever | 2.4 (1.24.9) | 0.8 (0.23.6) |
| No with fever and leukocytosis | 5.6 (1.818.2) | 0.4 (0.12.6) |
| Prior positive cultures | ||
| Yes | 1.6 (1.02.7) | 0.6 (0.22.0) |
For the true positive cultures, gram‐positive organisms were isolated most frequently (14/21, 67%) with Staphylococcus aureus identified in 2/21 (10%) positive cultures and Enterococcus faecalis identified in 7/21 (33%) positive cultures. Gram‐negative organisms were isolated in 6/21 (29%) cultures, and 1/21 (5%) culture grew 2 organisms (Enterococcus faecalis and Nocardia). The majority of false positive cultures isolated 1 or more species of coagulase‐negative Staphylococcus (11/13, 85%).
Predictors of True Bacteremia
The 4 most common working diagnoses prompting a blood culture order were pneumonia, bacteremia/endocarditis, urinary tract infection, and a noninfectious diagnosis (eg, syncope), with each prompting approximately 17% of the total orders (Table 2). Of these, only a primary diagnosis of bacteremia/endocarditis was predictive of a true positive culture, yielding a rate of 12.3% (LR+ 3.7, 95% confidence interval [CI]: 2.5‐5.7). No other diagnosis was predictive of true positivity. A diagnosis of pneumonia yielded no true positive and 4 false positive blood cultures (3.9%), whereas a noninfectious diagnosis yielded only 1 true positive (1.0%) and 5 false positives (5.0%). The positive likelihood ratios for these 2 diagnoses were 0.1 (95% CI: 0.00‐1.9) and 0.3 (95% CI: 0.04‐1.8), respectively.
Indications were selected for 530 of 576 (92%) blood culture orders (Table 2). The most common indication was fever alone (25.6%), followed by fever with an additional indication (22.2%), follow‐up positive blood cultures (11.3%), fever and leukocytosis (9.4%), and leukocytosis alone (9.4%). Only follow‐up positive blood cultures was predictive of a true positive, with a LR+ of 3.4 (95% CI: 1.8‐6.5).
A total of 14 patients (3.9%) had true positive blood cultures. For these patients, 10/14 (71%) had 1 true positive blood culture, 3/14 (21%) had 2 true positive blood cultures, and 1/14 (7%) had 5 true positive blood cultures. The average number of cultures drawn was 4.9. The clinical characteristic most predictive of a true positive blood culture was the absence of recent antibiotic administration. If the blood culture was ordered on a patient not receiving antibiotics (true positivity rate 7.2%, 16/222), the LR+ was 2.1 (95% CI: 1.6‐2.7). In a patient not receiving antibiotics who was also noted to have fever and leukocytosis (true positivity rate 17.6%, 3/17), the LR+ was 5.6 (95% CI: 1.8‐18.2). Conversely, patients receiving antibiotics were rarely found to have true positive blood cultures (true positivity rate 1.4%, 5/354) with a LR+ of 0.4 (95% CI: 0.2‐0.8).
DISCUSSION
In this prospective study, we determined the diagnostic yield of blood cultures ordered on hospitalized medical patients to be low, with just 3.6% of orders identifying a true BSI. This was coupled with a similar false positive rate of 2.3%. Our study found rates of true positive blood cultures much lower in hospitalized medical patients than in rates previously described when ED and ICU patients were included.[11, 16]
Although ordering blood cultures is a routine clinical behavior when there is concern for an infection, a clinician's ability to subjectively predict who has a BSI only improves the likelihood 2‐fold.[6] Despite the availability of multiple scoring systems to aid the clinicians,[10, 21, 22] our study found that over 50% of cultures were ordered in the setting of fever or leukocytosis, potentially demonstrating a triggered response to an event, rather than a decision based on probabilities. This common clinician instinct to culture if spikes is an ineffective practice if not coupled with additional clinical information. In fact, in 1 retrospective study, there was no association between fever spike and blood culture positivity.[23]
Our study suggests that objective and easily obtainable clinical characteristics may be effective in helping determine the probability of blood cultures revealing a BSI. Although more robust prediction models have value, they often require multiple inputs, limiting their utility to the bedside clinician. Stratifying patients by either antibiotic exposure or working diagnosis may provide the most benefit for the hospitalized medical patient. For those on antibiotics, the yield of true positive blood cultures is so low that they are unlikely to provide clinically useful information. In fact, although nearly two‐thirds of cultures were obtained after antibiotic exposure, only 1 (0.2%) of these patients had a culture that provided additional information regarding a BSI. Bacteremia had already been established for the other 4 patients. These results are similar to a prior study, which concluded that physicians should wait 72 hours from time of preantibiotic cultures before considering additional blood cultures given the lack of additional information provided.[24]
The working diagnosis also drives the probability of a positive blood culture. As has been shown with other studies, blood cultures are unlikely to diagnose a BSI for patients being treated for either cellulitis or pneumonia.[25, 26, 27] In our study, the working diagnosis prompting the most blood cultures was pneumonia, with the false positive rate exceeding the true positive rate, a finding consistent with previous literature. This situation may lead to the addition of unnecessary antibiotics while waiting for a positive culture to be confirmed as a false positive (eg, vancomycin for a preliminary culture showing gram‐positive cocci in clusters).
There are a number of limitations to our study. Physician‐chosen indication may not correlate with the actual clinical picture and/or may not represent the full set of variables involved in the clinical decision to order a blood culture. However, the subjective clinical indication and the objective clinical criteria found in the chart provided similar LRs. Our study did not evaluate the potential harm of not ordering a blood culture. We also did not assess the value of a true negative culture particularly in patients with endovascular infections where additional cultures are often required to document clearance of bacteremia. Lastly, our study applies to patients on a hospitalized medical service and was performed at a VA hospital with a specific population of elderly male patients, which may limit the generalizability of our results.
Despite these limitations, this study benefits from its prospective design, along with the fact that >90% of blood culture orders placed included a corresponding indication. This provides insight into physician clinical reasoning at the time the blood culture was ordered. In addition, our ability to calculate likelihood ratios provides bedside physicians with an easy and powerful way of modifying the probability of BSI prior to ordering blood cultures, aiding them in providing high‐value clinical care while potentially reducing testing overuse.
The acceptability of not obtaining blood cultures may vary by clinical experience and by specialty. Physicians must weigh the low true positive rate against the consequences of missing a BSI. Although not a substitute for clinical judgement, the LRs in this study can provide a framework to aid in clinical decision making. For example, assuming a pretest probability of 3.6% (the rate of true positive for our entire cohort), blood cultures may not be equally as compelling in 2 similar patients with fever. The first is not on antibiotics and also has a leukocytosis. The second is being treated for pneumonia and is already on antibiotics. For the first patient, using a LR+ of 5.6 (for the fever and leukocytosis in the absence of antibiotics) modifies the patient's probability of a true positive blood culture to 17.3%. Blood cultures should be ordered. In contrast, for the second patient, using a LR+ of 0.4 (for the presence of antibiotics) decreases the patient's probability of a true positive blood culture to 1.5%. Armed with these data, the bedside clinician can now decide whether this rate of true positivity warrants blood cultures. For some, this rate will be comfortably low. For others, this rate will not assuage them; only the negative culture will. Our data are not meant to make this decision, but may aid in making it a probability‐based decision.
Disclosures
Presented in part at the Infectious Diseases Society of America Annual Meeting in San Diego, California in 2015. This material is the result of work supported in part with resources and the use of facilities at the VA Boston HCS, West Roxbury, MA. Katherine Linsenmeyer, MD, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
Blood cultures are the gold standard test for the diagnosis of bloodstream infections (BSI). Given the high mortality associated with BSI,[1, 2, 3] physicians have a low threshold to obtain blood cultures.[4, 5] Unfortunately, physicians are poor at predicting which hospitalized patients have BSI,[6, 7] and published guidelines do not provide clear indications for the use of blood cultures.[8] As a result, current practice follows a culture if spikes paradigm, whereby inpatient providers often obtain blood cultures in the setting of any fever. This is the most common anticipatory guidance communicated between providers, involving up to 75% of written sign‐out instructions.[9] The result is a low rate of true positive blood cultures (5%10%)[10, 11, 12] with only a slightly lower rate of false positive blood cultures (contaminants).[12, 13, 14] False positive blood cultures often lead to repeat blood cultures, unnecessary antibiotic use, and increased hospital cost and length of stay.[13]
Over the last several years, there has been an increased emphasis on practicing high‐value care by avoiding unnecessary and duplicate testing. In 2012, the American Board of Internal Medicine introduced the Choosing Wisely campaign, with specific initiatives to reduce medical waste and overuse. Given the low yield of blood cultures, guidance on patients in whom blood cultures are most appropriate would be welcome. Studies assessing risk factors for bacteremia have led to the development of multiple stratification systems without overall consensus.[10, 15, 16, 17, 18, 19, 20] Furthermore, much of the current literature on blood culture utilization includes cultures drawn in the emergency department (ED) or intensive care unit setting (ICU).[10, 18, 19, 20] Less is known regarding the rates of positivity and utility for blood cultures drawn on patients hospitalized on an acute care medical ward.
Our study had 3 main objectives: (1) determine the rates of true positive and false positive blood cultures among hospitalized medical patients, (2) determine the ability of physician‐selected indications and patient characteristics to predict BSI, and (3) identify populations in which blood cultures may not be necessary.
PATIENTS AND METHODS
Study Design
We conducted a prospective cohort study of all hospitalized medical patients for whom blood cultures were ordered and received by the microbiology laboratory. This investigation was approved by the Veterans Affairs (VA) Boston Healthcare System internal review board.
Patients and Setting
During a 7‐month period (October 1, 2014April 15, 2015), all blood culture orders were reviewed for indication and result each day (and on Monday for weekend blood cultures) at a large VA teaching hospital (approximately 6200 admissions each year). As part of the electronic medical order, providers selected from among a list of common indications. Options included various clinical signs and diagnoses, and providers could select more than 1 indication. Each blood culture order triggered a phlebotomist to draw 2 separate blood culture sets (each set consisted of 1 aerobic and 1 anaerobic blood culture bottle).
Inclusion criteria included admission to 1 of 5 general medical service teams or 1 of 2 cardiology teams. Given that the study hospital does not have dedicated subspecialty service teams (with the exception of cardiology), all patients with medical diagnoses are cared for on the general medical service.
Predictor and Outcome Variables
Patient characteristics were obtained via chart review. Fever was defined as a single temperature greater than 100.4F within 24 hours prior to a blood culture order. Leukocytosis was defined as a white blood cell count greater than 10,000 within 24 hours of a blood culture order. Patients were considered to have received antibiotics if an order for an antibacterial or antifungal agent was active within 72 hours prior to the blood culture order. Each blood culture order was assigned a working diagnosis that prompted the order. These working diagnoses were identified by chart review as documented under the provider's assessment and plan and were not necessarily the primary diagnosis prompting hospitalization.
Classification of positive blood cultures into true and false positive was determined by consensus among the microbiology and the infectious disease departments after review of clinical and laboratory data, consistent with a previously established practice at the hospital. A true negative culture consisted of any culture that was not a true positive or a false positive. A blood culture order was defined as an electronic entry and included all sets of blood cultures drawn as a result of that order. Consistent with previous literature, a blood culture episode was defined as all blood cultures ordered within a 48‐hour period starting at the time of the first culture.[10] For patients with multiple admissions during the study period, each admission was considered a unique patient.
Statistical Analysis
Rates of true and false positivity of blood cultures were calculated. In addition, positive likelihood ratios (LR+) for true positive blood cultures were calculated using JMP statistical software (SAS Institute, Inc., Cary, NC).
RESULTS
Overall
A total of 576 blood culture orders (467 blood culture episodes) were completed on 363 hospitalized medical patients during the study period. Five hundred forty orders were placed on patients on general medical services and 36 orders on patients on the cardiology services. Four hundred eighty‐seven (85%) orders resulted in 2 sets of cultures being drawn, 87 (15%) resulted in 1 set of cultures, and 2 (0.3%) resulted in 3 sets of cultures. The median time between admission and culture draw was 2 days (range, 072 days), with 57% of cultures drawn during hospital day 0 to 2, 24.5% drawn between hospital day 3 to 7, and 19.4% drawn after hospital day 7. The average age of the patients was 70.4 years, and 94% were men. Additional patient characteristics are shown in Table 1.
| Clinical Characteristic | Total, n = 363 (%) | True Positive Blood Cultures, n = 14 (%) | P Value |
|---|---|---|---|
| |||
| Mean age, y | 70.4 | 73.9 | 0.4 |
| Male sex | 350 (96%) | 14 (100%) | 1 |
| White race | 308 (85%) | 11 (79%) | 0.7 |
| Location prior to admission | |||
| Community | 276 (76%) | 11 (79%) | 1 |
| Hospital | 51 (14%) | 1 (7%) | 0.7 |
| Long‐term care facility | 36 (10%) | 2 (14%) | 0.6 |
| Comorbidities | |||
| Diabetes | 136 (37%) | 5 (36%) | 1 |
| Malignancy | 100 (28%) | 4 (31%) | 1 |
| Alcohol abuse | 89 (25%) | 2 (14%) | 0.5 |
| Cirrhosis | 31 (9%) | 1 (7%) | 1 |
| End‐stage renal disease | 21 (6%) | 1 (7%) | 1 |
| Active drug use* | 16 (4%) | 1 (7%) | 0.5 |
| Catheter | 93 (26%) | 3 (21%) | 0.8 |
| Recent hospitalization | 145 (40%) | 6 (43%) | 1 |
| History of MRSA colonization | 72 (20%) | 5 (36%) | 0.16 |
| Cultures drawn in emergency department | 69 (19%) | 6 (43%) | 0.03 |
The true positive and false positive rates per blood culture order were 3.6% (21/576) and 2.3% (13/576), respectively (Table 2). Similar values were seen per blood cultures episode (3.4% and 2.7%, respectively). The true positive blood culture rates per order and episode were significantly lower than those drawn on emergency room patients during the study period (41/570, 7.2%, P < 0.05).
| Total, n (%) | True Positive, n (%) | False Positive, n (%) | True Negative, n (%) | |
|---|---|---|---|---|
| ||||
| Per patient | 363 | 14 (3.8) | 13 (3.6) | 336 (92.6) |
| Per blood culture episode | 467 | 16 (3.4) | 13 (2.7) | 438 (93.8) |
| Per blood culture order | 576 | 21 (3.6) | 13 (2.3) | 542 (94.1) |
| Rates per blood culture order | ||||
| Physician‐selected indication, n = 530 | ||||
| Fever | 136 (25.6) | 3 (2.2) | 3 (2.2) | 130 (95.6) |
| Fever and additional indication(s) | 118 (22.2) | 5 (4.2) | 3 (2.5) | 110 (93.2) |
| Fever and leukocytosis | 50 (9.4) | 4 (8.0) | 3 (6.0) | 43 (86.0) |
| Leukocytosis | 50 (9.4) | 2 (4.0) | 0 (0) | 48 (96.0) |
| Follow‐up previous positive | 60 (11.3) | 7 (11.7) | 0 (0) | 53 (88.3) |
| Working diagnosis, n = 576 | ||||
| Pneumonia | 101 (17.5) | 0 (0) | 4 (3.9) | 97 (96.0) |
| Bacteremia/endocarditis | 97 (16.8) | 12 (12.3) | 1 (1.0) | 84 (86.6) |
| Urinary tract infection* | 95 (16.4) | 5 (5.3) | 2 (2.1) | 88 (92.6) |
| Other infection | 46 (8.0) | 0 (0) | 0 (0) | 46 (100) |
| Skin and soft‐tissue infection | 39 (6.8) | 1 (2.6) | 0 (0) | 38 (97.4) |
| Neutropenic fever | 28 (4.9) | 0 (0) | 0 (0) | 28 (100) |
| Sepsis | 27 (4.7) | 0 (0) | 0 (0) | 27 (100) |
| Fever | 18 (3.1) | 1 (5.5) | 1 (5.5) | 16 (88.9) |
| Bone and join infection | 15 (2.6) | 1 (6.7) | 0 (0) | 14 (93.3) |
| Postoperative fever | 9 (1.6) | 0 (0) | 0 (0) | 9 (100) |
| Noninfectious diagnosis | 101 (17.5) | 1 (1.0) | 5 (5.0) | 95 (94.1) |
| Antibiotic exposure | ||||
| Yes | 354 (61.5) | 5 (1.4) | 5 (1.4) | 344 (97.1) |
| No | 222 (38.6) | 16 (7.2) | 8 (3.6) | 198 (89.1) |
| Previous documented positive culture via chart review | ||||
| Yes | 155 (26.9) | 9 (5.8) | 2 (1.3) | 144 (92.9) |
| No | 421 (73.1) | 12 (2.9) | 11 (2.6) | 398 (94.5) |
| LR+ (95% CI), True Positive Blood Culture | LR+ (95% CI), False Positive Blood Culture | |
|---|---|---|
| ||
| Physician‐selected indication | ||
| Fever | 0.6 (0.21.7) | 0.9 (0.32.5) |
| Fever and additional indication(s) | 1.1 (0.52.4) | 1.0 (0.42.8) |
| Fever and leukocytosis | 2.2 (0.95.6) | 2.5 (0.97.1) |
| Leukocytosis | 1.1 (0.34.0) | 0.4 (0.05.6) |
| Follow‐up previous positive | 3.4 (1.86.5) | 0.3 (0.04.7) |
| Diagnosis | ||
| Pneumonia | 0.1 (0.01.9) | 1.8 (0.84.1) |
| Bacteremia/endocarditis | 3.7 (2.55.7) | 0.5 (0.13.0) |
| Urinary tract infection | 1.5 (0.73.2) | 0.9 (0.33.4) |
| Noninfectious diagnosis | 0.3 (0.01.8) | 2.3 (1.14.6) |
| Recent antibiotic exposure | ||
| Yes | 0.4 (0.20.8) | 0.6 (0.31.2) |
| No | 2.1 (1.62.7) | 1.6 (1.02.5) |
| No with fever | 2.4 (1.24.9) | 0.8 (0.23.6) |
| No with fever and leukocytosis | 5.6 (1.818.2) | 0.4 (0.12.6) |
| Prior positive cultures | ||
| Yes | 1.6 (1.02.7) | 0.6 (0.22.0) |
For the true positive cultures, gram‐positive organisms were isolated most frequently (14/21, 67%) with Staphylococcus aureus identified in 2/21 (10%) positive cultures and Enterococcus faecalis identified in 7/21 (33%) positive cultures. Gram‐negative organisms were isolated in 6/21 (29%) cultures, and 1/21 (5%) culture grew 2 organisms (Enterococcus faecalis and Nocardia). The majority of false positive cultures isolated 1 or more species of coagulase‐negative Staphylococcus (11/13, 85%).
Predictors of True Bacteremia
The 4 most common working diagnoses prompting a blood culture order were pneumonia, bacteremia/endocarditis, urinary tract infection, and a noninfectious diagnosis (eg, syncope), with each prompting approximately 17% of the total orders (Table 2). Of these, only a primary diagnosis of bacteremia/endocarditis was predictive of a true positive culture, yielding a rate of 12.3% (LR+ 3.7, 95% confidence interval [CI]: 2.5‐5.7). No other diagnosis was predictive of true positivity. A diagnosis of pneumonia yielded no true positive and 4 false positive blood cultures (3.9%), whereas a noninfectious diagnosis yielded only 1 true positive (1.0%) and 5 false positives (5.0%). The positive likelihood ratios for these 2 diagnoses were 0.1 (95% CI: 0.00‐1.9) and 0.3 (95% CI: 0.04‐1.8), respectively.
Indications were selected for 530 of 576 (92%) blood culture orders (Table 2). The most common indication was fever alone (25.6%), followed by fever with an additional indication (22.2%), follow‐up positive blood cultures (11.3%), fever and leukocytosis (9.4%), and leukocytosis alone (9.4%). Only follow‐up positive blood cultures was predictive of a true positive, with a LR+ of 3.4 (95% CI: 1.8‐6.5).
A total of 14 patients (3.9%) had true positive blood cultures. For these patients, 10/14 (71%) had 1 true positive blood culture, 3/14 (21%) had 2 true positive blood cultures, and 1/14 (7%) had 5 true positive blood cultures. The average number of cultures drawn was 4.9. The clinical characteristic most predictive of a true positive blood culture was the absence of recent antibiotic administration. If the blood culture was ordered on a patient not receiving antibiotics (true positivity rate 7.2%, 16/222), the LR+ was 2.1 (95% CI: 1.6‐2.7). In a patient not receiving antibiotics who was also noted to have fever and leukocytosis (true positivity rate 17.6%, 3/17), the LR+ was 5.6 (95% CI: 1.8‐18.2). Conversely, patients receiving antibiotics were rarely found to have true positive blood cultures (true positivity rate 1.4%, 5/354) with a LR+ of 0.4 (95% CI: 0.2‐0.8).
DISCUSSION
In this prospective study, we determined the diagnostic yield of blood cultures ordered on hospitalized medical patients to be low, with just 3.6% of orders identifying a true BSI. This was coupled with a similar false positive rate of 2.3%. Our study found rates of true positive blood cultures much lower in hospitalized medical patients than in rates previously described when ED and ICU patients were included.[11, 16]
Although ordering blood cultures is a routine clinical behavior when there is concern for an infection, a clinician's ability to subjectively predict who has a BSI only improves the likelihood 2‐fold.[6] Despite the availability of multiple scoring systems to aid the clinicians,[10, 21, 22] our study found that over 50% of cultures were ordered in the setting of fever or leukocytosis, potentially demonstrating a triggered response to an event, rather than a decision based on probabilities. This common clinician instinct to culture if spikes is an ineffective practice if not coupled with additional clinical information. In fact, in 1 retrospective study, there was no association between fever spike and blood culture positivity.[23]
Our study suggests that objective and easily obtainable clinical characteristics may be effective in helping determine the probability of blood cultures revealing a BSI. Although more robust prediction models have value, they often require multiple inputs, limiting their utility to the bedside clinician. Stratifying patients by either antibiotic exposure or working diagnosis may provide the most benefit for the hospitalized medical patient. For those on antibiotics, the yield of true positive blood cultures is so low that they are unlikely to provide clinically useful information. In fact, although nearly two‐thirds of cultures were obtained after antibiotic exposure, only 1 (0.2%) of these patients had a culture that provided additional information regarding a BSI. Bacteremia had already been established for the other 4 patients. These results are similar to a prior study, which concluded that physicians should wait 72 hours from time of preantibiotic cultures before considering additional blood cultures given the lack of additional information provided.[24]
The working diagnosis also drives the probability of a positive blood culture. As has been shown with other studies, blood cultures are unlikely to diagnose a BSI for patients being treated for either cellulitis or pneumonia.[25, 26, 27] In our study, the working diagnosis prompting the most blood cultures was pneumonia, with the false positive rate exceeding the true positive rate, a finding consistent with previous literature. This situation may lead to the addition of unnecessary antibiotics while waiting for a positive culture to be confirmed as a false positive (eg, vancomycin for a preliminary culture showing gram‐positive cocci in clusters).
There are a number of limitations to our study. Physician‐chosen indication may not correlate with the actual clinical picture and/or may not represent the full set of variables involved in the clinical decision to order a blood culture. However, the subjective clinical indication and the objective clinical criteria found in the chart provided similar LRs. Our study did not evaluate the potential harm of not ordering a blood culture. We also did not assess the value of a true negative culture particularly in patients with endovascular infections where additional cultures are often required to document clearance of bacteremia. Lastly, our study applies to patients on a hospitalized medical service and was performed at a VA hospital with a specific population of elderly male patients, which may limit the generalizability of our results.
Despite these limitations, this study benefits from its prospective design, along with the fact that >90% of blood culture orders placed included a corresponding indication. This provides insight into physician clinical reasoning at the time the blood culture was ordered. In addition, our ability to calculate likelihood ratios provides bedside physicians with an easy and powerful way of modifying the probability of BSI prior to ordering blood cultures, aiding them in providing high‐value clinical care while potentially reducing testing overuse.
The acceptability of not obtaining blood cultures may vary by clinical experience and by specialty. Physicians must weigh the low true positive rate against the consequences of missing a BSI. Although not a substitute for clinical judgement, the LRs in this study can provide a framework to aid in clinical decision making. For example, assuming a pretest probability of 3.6% (the rate of true positive for our entire cohort), blood cultures may not be equally as compelling in 2 similar patients with fever. The first is not on antibiotics and also has a leukocytosis. The second is being treated for pneumonia and is already on antibiotics. For the first patient, using a LR+ of 5.6 (for the fever and leukocytosis in the absence of antibiotics) modifies the patient's probability of a true positive blood culture to 17.3%. Blood cultures should be ordered. In contrast, for the second patient, using a LR+ of 0.4 (for the presence of antibiotics) decreases the patient's probability of a true positive blood culture to 1.5%. Armed with these data, the bedside clinician can now decide whether this rate of true positivity warrants blood cultures. For some, this rate will be comfortably low. For others, this rate will not assuage them; only the negative culture will. Our data are not meant to make this decision, but may aid in making it a probability‐based decision.
Disclosures
Presented in part at the Infectious Diseases Society of America Annual Meeting in San Diego, California in 2015. This material is the result of work supported in part with resources and the use of facilities at the VA Boston HCS, West Roxbury, MA. Katherine Linsenmeyer, MD, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
- , . Population‐based epidemiology and microbiology of community‐onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664.
- , , , et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602.
- , , , . The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5(1):54–70.
- , , , et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- , , , et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278(3):234–240.
- , , , . Predicting bacteremia in older patients. J Am Geriatr Soc. 1995;43(3):230–235.
- , , , , , . Febrile inpatients: house officers' use of blood cultures. J Gen Intern Med. 1987;2(5):293–297.
- , , , et al. Executive summary: a guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57(4):485–488.
- , , , , . What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248–255.
- , , , . Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113(7):495–500.
- , . Blood cultures. Ann Intern Med. 1987;106(2):246–253.
- , , , et al. Reducing blood culture contamination by a simple informational intervention. J Clin Microbiol. 2010;48(12):4552–4558.
- , , . Contaminant blood cultures and resource utilization. The true consequences of false‐positive results. JAMA. 1991;265(3):365–369.
- . Blood culture contaminants. J Hosp Infect. 2014;87(1):1–10.
- , , , , , . The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117–123.
- , , , et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997;176(6):1538–1551.
- , . The systemic inflammatory response syndrome as a predictor of bacteraemia and outcome from sepsis. QJM. 1996;89(7):515–522.
- , , , , . Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35(3):255–264.
- , , , , , . Factors associated with positive blood cultures in outpatients with suspected bacteremia. Eur J Clin Microbiol Infect Dis. 2011;30(12):1615–1619.
- , , , et al. Two rules for early prediction of bacteremia: testing in a university and a community hospital. J Gen Intern Med. 1996;11(2):98–103.
- , , , . Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308(5):502–511.
- , , , et al. Clinical prediction rules for bacteremia and in‐hospital death based on clinical data at the time of blood withdrawal for culture: an evaluation of their development and use. J Eval Clin Pract. 2006;12(6):692–703.
- , , , et al. Timing of specimen collection for blood cultures from febrile patients with bacteremia. J Clin Microbiol. 2008;46(4):1381–1385.
- , , , . Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis. 2001;32(11):1651–1655.
- , , , , . Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks. Chest. 1995;108(4):932–936.
- . Blood cultures in community‐acquired pneumonia: are we ready to quit? Chest. 2003;123(4):977–978.
- . Blood cultures for community‐acquired pneumonia: piecing together a mosaic for doing less. Am J Respir Crit Care Med. 2004;169(3):327–328.
- , . Population‐based epidemiology and microbiology of community‐onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664.
- , , , et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602.
- , , , . The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5(1):54–70.
- , , , et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- , , , et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278(3):234–240.
- , , , . Predicting bacteremia in older patients. J Am Geriatr Soc. 1995;43(3):230–235.
- , , , , , . Febrile inpatients: house officers' use of blood cultures. J Gen Intern Med. 1987;2(5):293–297.
- , , , et al. Executive summary: a guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57(4):485–488.
- , , , , . What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248–255.
- , , , . Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113(7):495–500.
- , . Blood cultures. Ann Intern Med. 1987;106(2):246–253.
- , , , et al. Reducing blood culture contamination by a simple informational intervention. J Clin Microbiol. 2010;48(12):4552–4558.
- , , . Contaminant blood cultures and resource utilization. The true consequences of false‐positive results. JAMA. 1991;265(3):365–369.
- . Blood culture contaminants. J Hosp Infect. 2014;87(1):1–10.
- , , , , , . The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117–123.
- , , , et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997;176(6):1538–1551.
- , . The systemic inflammatory response syndrome as a predictor of bacteraemia and outcome from sepsis. QJM. 1996;89(7):515–522.
- , , , , . Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35(3):255–264.
- , , , , , . Factors associated with positive blood cultures in outpatients with suspected bacteremia. Eur J Clin Microbiol Infect Dis. 2011;30(12):1615–1619.
- , , , et al. Two rules for early prediction of bacteremia: testing in a university and a community hospital. J Gen Intern Med. 1996;11(2):98–103.
- , , , . Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308(5):502–511.
- , , , et al. Clinical prediction rules for bacteremia and in‐hospital death based on clinical data at the time of blood withdrawal for culture: an evaluation of their development and use. J Eval Clin Pract. 2006;12(6):692–703.
- , , , et al. Timing of specimen collection for blood cultures from febrile patients with bacteremia. J Clin Microbiol. 2008;46(4):1381–1385.
- , , , . Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis. 2001;32(11):1651–1655.
- , , , , . Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks. Chest. 1995;108(4):932–936.
- . Blood cultures in community‐acquired pneumonia: are we ready to quit? Chest. 2003;123(4):977–978.
- . Blood cultures for community‐acquired pneumonia: piecing together a mosaic for doing less. Am J Respir Crit Care Med. 2004;169(3):327–328.
© 2016 Society of Hospital Medicine
Serum and Red Blood Cell Folate Testing
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 65‐year‐old man is admitted with pneumonia. Review of the medical record reveals a persistent macrocytic anemia (hematocrit 29%, hemoglobin 9.3 g/dL, mean corpuscular volume [MCV] 105 fL) with a low reticulocyte count and normal peripheral blood smear. The provider contemplates ordering a serum folate or red blood cell (RBC) folate test to workup the persistent macrocytic anemia.
BACKGROUND
Folate is a water‐soluble B vitamin essential for the synthesis of DNA and for converting homocysteine to methionine. Folate deficiency is causally linked with both neural tube defects and megaloblastic anemia. Low levels of folate are associated with cardiovascular disease, colon cancer, neuropathy, depression, hypercoagulability, and cognitive decline, though there is a paucity of evidence showing causation or risk reduction with folate supplementation.[1] In patients with inadequate folate intake, the earliest sign is a decline in serum folate levels, followed by a fall in RBC folate levels. Only weeks later do macrocytosis, megaloblastic bone marrow, and finally anemia occur.[2] Given that humans are unable to synthesize folate and are therefore dependent on dietary sources, those with inadequate intake or absorption are at risk of folate deficiency.
WHY FOLATE TESTING IS ORDERED
In hospitalized patients, the most common indication for folate testing is anemia, either with or without macrocytosis.[3, 4] Given that at least 10% to 15% of hospitalized patients are anemic,[5, 6] it is unsurprising that folate testing is frequently performed. Despite the link between folate deficiency and megaloblastic anemia, >85% of patients evaluated for folate deficiency have normocytic or microcytic anemia.[3, 4] In addition, a study found that 30% of all folate testing was performed not as part of an anemia workup but in the evaluation of other comorbidities (eg, dementia and altered mental status) that are not causally linked to folate deficiency.[7]
WHY THERE IS NO REASON TO ORDER FOLATE TESTING
There are 2 reasons why testing hospitalized patients for folate deficiency does not contribute value: (1) the poor characteristics of the tests used and (2) the low prevalence of folate deficiency in the postfortification era.
There is no accepted gold standard for the diagnosis of folate deficiency, though biological assays are considered more accurate than the now more commonly used protein binding assays.[8] The lack of a gold standard limits the ability to fully quantify the sensitivity and specificity of either serum or RBC folate testing, though falsely low and high serum folate results can be seen. Falsely low serum levels (false positives) are found with heavy alcohol use and with certain anticonvulsant or antineoplastic drug use.[9] The low levels in these patients indicate low serum folate but do not necessarily reflect tissue stores. Serum folate levels may fall rapidly within a few days of the start of low dietary folate intake, resulting in low serum folate levels that also do not represent true folate deficiency.[10] On the other hand, intake of folatethrough a meal or ingestion of an oral supplementdirectly preceding evaluation of serum folate can lead to falsely elevated levels (false negatives).[10]
Although RBC folate reflects body stores and is largely unaffected by diet, the available tests also lack sensitivity and specificity.[11] Furthermore, serum folate levels and RBC folate levels correlate well.[12] Because RBC folate testing is more expensive than serum folate testing, has results that correlate well with serum folate testing, and is without significantly better test characteristics, there is no added value to using RBC folate testing as compared to serum folate testing.
In addition to the issues with available diagnostic tests, numerous studies now indicate that the rate of folate deficiency in the United States is exceptionally low. This is largely driven by the United States Food and Drug Administration's mandate that all grain products be fortified with 0.14 mg of folic acid per gram of grains.[13] Fortification has been overwhelmingly successful at increasing folic acid intake[14, 15] and reducing the incidence of neural tube defects.[16] Although the serum and RBC folate tests are prone to inaccuracies for an individual patient, population trends postfortification, coupled with the data on intake and rates of neural tube defects, make a strong argument that the prevalence of deficiency has decreased dramatically.
Similar to these population‐based trends, studies of hospital‐based laboratories have shown a marked decrease in the rate of low serum and RBC folate levels, making for a very low pretest probability for folate deficiency (Table 1). Even before fortification had been fully implemented, a study of outpatients and inpatients cared for at 3 hospitals in Denver, Colorado in 1996 found that just 1.9% of patients had low serum folate levels and 4.4% had low RBC folate levels.[17] A retrospective study of 26,662 patients in 1998 showed a rate of serum deficiency (<2.7 ng/mL) of 0.3%.[18] The authors also found that despite a decline in rate of serum deficiency from 1.3% to 0.3% between 1994 and 1998, the total number of serum folate tests performed increased by 84%. A similar study found just 0.4% of 1007 patients with low serum folate levels (<3.0 ng/mL).[7] Parallel results have been seen in other countries after implementation of folate fortification with a cohort of 2154 Canadian patients reporting low serum folate (<6.8 nmol/L) and RBC folate (<417 nmol/L) levels in just 0.5% and 0.7% of patients, respectively.[19]
| Author, Study Year | Year of Testing | Country | Population | Serum Folate | Red Blood Cell Folate | ||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Samples (n) | Low (%) | Patients (n) | Samples (n) | Low (%) | ||||
| Latif et al., [4] | 2001 | United States | Inpatient/outpatient | 4,315 | 4,689 | 1.6 | 1,215 | 1,335 | 1.2 |
| Shojania et al., 2010[19] | 2001 | Canada | Inpatient/outpatient | 2,154 | 0.5 | 560 | 0.7 | ||
| Ashraf et al., [7] | 2002 | United States | Inpatient/outpatient | 980 | 1,007 | 0.4 | |||
| Gudgeon et al., 2014[20] | 2010 | Canada | Inpatient | 2,563 | 0.2 | ||||
| Theisen‐Toupal et al., [3] | 2011 | United States | Inpatient/emergency department | 1,944 | 2,093 | 0.1 | |||
Few studies have looked exclusively at hospitalized and emergency room patients. In an evaluation of 2093 serum folate tests performed on hospitalized or emergency room patients (98.1% of whom were admitted) in 2011, only 2 (0.1%) deficient levels (<3 ng/mL) were identified, 1 of which was associated with a macrocytic anemia.[3] A similar study of RBC folate levels in 2562 patients at 3 Canadian hospitals found just 4 (0.16%) levels to be low (<254 nmol/L), only 1 of which was associated with macrocytic anemia.[20]
When examining only patients with macrocytic anemia, the rates of folate deficiency are only slightly higher than the general population. As noted above, each of the 2 studies of inpatients uncovered just 1 patient with macrocytic anemia and concomitant low serum or RBC folate levels.[3, 20] Other studies reveal rates of serum folate deficiency in patients with macrocytic anemia and macrocytosis of 2.8%[7] and 1%,[21] respectively, and RBC folate deficiency rates in patients with macrocytosis of 1.8%.[22] Patients with extreme macrocytosis (MCV >130) represent 1 subset of patients with a high pretest probability of low serum folate, with 1 study reporting low levels in 37% of patients.[23]
Despite the relatively inexpensive cost per serum and RBC folate test, expenses per test that result in an abnormally low level are significant. As the pretest probability for folate deficiency is extremely low, tests must be ordered on a large number of patients to find 1 patient with levels suggesting deficiency. For example, a study found that an institution charged $151 per serum folate test, which amounted to $158,000 per deficient result.[3] The institutional cost was <$2.00 per serum folate test and <$2093 per deficient result. Another study reported the institutional cost of RBC folate to be $12.54 per test and $8035 per deficient result.[20] The charges and costs are institution specific and will vary. However, in light of the low pretest probability of testing, any expense associated with these tests represents low value.
WHAT YOU SHOULD DO INSTEAD
The clinician in our case presentation is facing a common scenarioa patient with persistent anemia without a known etiology. The treatment of suspected or confirmed folate deficiency includes improving diet or adding a folic acid supplement, a low‐cost (as little $0.01 per tablet) intervention. Furthermore, other at‐risk patients (eg, those with sickle cell disease, alcoholism, or malabsorption) may be candidates for long‐term supplementation regardless of serum folate and/or RBC folate testing results.
Folate deficiency in patients living in the United States and Canada is exceedingly rare, making the pretest probability of testing low. Furthermore, even patients with typical hematologic characteristics for folate deficiency (anemia and macrocytosis) are unlikely to have folate deficiency. Importantly, there are no nonhematologic indications to test for folate deficiency, and testing those patients, just as in the general population, yields an extremely low rate of folate deficiency. The tests themselves are unreliable and inaccurate, and fortunately, treatment is cheap, easy to administer, and can be done empirically. In other words, testing for folate deficiency is a Thing We Do for No Reason.
RECOMMENDATIONS
In patients suspected of having folate deficiency or who are at high risk of folate deficiency (eg, diet poor in folate‐rich or folic acid fortified foods), treat with a diet containing folate or folic acid fortified foods and/or a supplement containing 400 to 1000 g of folic acid. Approximately 1 to 2 weeks following initiation of treatment, a complete blood count should be performed to evaluate for an appropriate increase in hematocrit/hemoglobin and decrease in MCV.[24] Once a full hematologic response is seen, treatment beyond this time is not required unless the cause (eg, malnutrition) persists.
Serum folate and RBC folate tests should not be routinely ordered. Even in those with macrocytic anemia, the pretest probability of folate deficiency remains low. Although testing may suggest a folate deficiency, it is still more likely there is another cause for the patient's anemia. This places providers at risk for premature closure. For patients such as the one presented in the case presentation, obtaining B12 levels is of greater importance, given the higher prevalence and the risks of untreated deficiency.
For patients in whom the pretest probability of folate deficiency is high (eg, those with an MCV >130), obtain fasting serum folate levels on samples taken before supplementation has begun or a diet administered.
Disclosures
Dr. Feldman is a consultant to Maven Medical, LLC. Maven Medical is a healthcare software startup.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71(1‐2):121–138.
- Experimental nutritional folate deficiency in man. Trans Assoc Am Physicians. 1962;75:307–320.
- , , Utility, charge, and cost of inpatient and emergency department serum folate testing. J Hosp Med. 2013;8(2):91–95.
- , , , Is there a role for folate determinations in current clinical practice in the USA? Clin Lab Haematol. 2004;26(6):379–383.
- , , , , Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- Healthcare Cost and Utilization Project (HCUP). HCUP facts and figures: statistics on hospital‐based care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , Clinical utility of folic acid testing for patients with anemia or dementia. J Gen Intern Med. 2008;23(6):824–826.
- Utility of measuring serum or red blood cell folate in the era of folate fortification of flour. Clin Biochem. 2014;47(7‐8):533–538.
- Kelley's Textbook of Internal Medicine. Philadelphia, PA: Lippincott Williams 2000.
- Problems in the diagnosis and investigation of megaloblastic anemia. Can Med Assoc J. 1980;122(9):999–1004.
- Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–1298.
- , Erythrocyte folate levels: a clinical study. Am J Hematol. 1991;36(2):116–21.
- US Food and Drug Administration. Food standards: amendments of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1996;61:8781–8797.
- , Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. 2003;77(1):221–225.
- , , , , , Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002;132(9):2792–2798.
- , , , , Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986.
- , Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110(2):88–90.
- , , , Trends in serum folate after food fortification. Lancet. 1999;354(9182):915–916.
- , Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010;3:22.
- , Folate testing in hospital inpatients. Am J Med. 2015;128(1):56–59.
- , , , , Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci. 2000;319(6):343–352.
- , , Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007;131(3):477–480.
- , , , et al. Etiologies and diagnostic work‐up of extreme macrocytosis defined by an erythrocyte mean corpuscular volume over 130°fL: s study of 109 patients. Am J Hematol. 2014;89(6):665–666.
- , , , et al. Best practice in primary care pathology: review 1. J Clin Pathol. 2005;58(10):1016–1024.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 65‐year‐old man is admitted with pneumonia. Review of the medical record reveals a persistent macrocytic anemia (hematocrit 29%, hemoglobin 9.3 g/dL, mean corpuscular volume [MCV] 105 fL) with a low reticulocyte count and normal peripheral blood smear. The provider contemplates ordering a serum folate or red blood cell (RBC) folate test to workup the persistent macrocytic anemia.
BACKGROUND
Folate is a water‐soluble B vitamin essential for the synthesis of DNA and for converting homocysteine to methionine. Folate deficiency is causally linked with both neural tube defects and megaloblastic anemia. Low levels of folate are associated with cardiovascular disease, colon cancer, neuropathy, depression, hypercoagulability, and cognitive decline, though there is a paucity of evidence showing causation or risk reduction with folate supplementation.[1] In patients with inadequate folate intake, the earliest sign is a decline in serum folate levels, followed by a fall in RBC folate levels. Only weeks later do macrocytosis, megaloblastic bone marrow, and finally anemia occur.[2] Given that humans are unable to synthesize folate and are therefore dependent on dietary sources, those with inadequate intake or absorption are at risk of folate deficiency.
WHY FOLATE TESTING IS ORDERED
In hospitalized patients, the most common indication for folate testing is anemia, either with or without macrocytosis.[3, 4] Given that at least 10% to 15% of hospitalized patients are anemic,[5, 6] it is unsurprising that folate testing is frequently performed. Despite the link between folate deficiency and megaloblastic anemia, >85% of patients evaluated for folate deficiency have normocytic or microcytic anemia.[3, 4] In addition, a study found that 30% of all folate testing was performed not as part of an anemia workup but in the evaluation of other comorbidities (eg, dementia and altered mental status) that are not causally linked to folate deficiency.[7]
WHY THERE IS NO REASON TO ORDER FOLATE TESTING
There are 2 reasons why testing hospitalized patients for folate deficiency does not contribute value: (1) the poor characteristics of the tests used and (2) the low prevalence of folate deficiency in the postfortification era.
There is no accepted gold standard for the diagnosis of folate deficiency, though biological assays are considered more accurate than the now more commonly used protein binding assays.[8] The lack of a gold standard limits the ability to fully quantify the sensitivity and specificity of either serum or RBC folate testing, though falsely low and high serum folate results can be seen. Falsely low serum levels (false positives) are found with heavy alcohol use and with certain anticonvulsant or antineoplastic drug use.[9] The low levels in these patients indicate low serum folate but do not necessarily reflect tissue stores. Serum folate levels may fall rapidly within a few days of the start of low dietary folate intake, resulting in low serum folate levels that also do not represent true folate deficiency.[10] On the other hand, intake of folatethrough a meal or ingestion of an oral supplementdirectly preceding evaluation of serum folate can lead to falsely elevated levels (false negatives).[10]
Although RBC folate reflects body stores and is largely unaffected by diet, the available tests also lack sensitivity and specificity.[11] Furthermore, serum folate levels and RBC folate levels correlate well.[12] Because RBC folate testing is more expensive than serum folate testing, has results that correlate well with serum folate testing, and is without significantly better test characteristics, there is no added value to using RBC folate testing as compared to serum folate testing.
In addition to the issues with available diagnostic tests, numerous studies now indicate that the rate of folate deficiency in the United States is exceptionally low. This is largely driven by the United States Food and Drug Administration's mandate that all grain products be fortified with 0.14 mg of folic acid per gram of grains.[13] Fortification has been overwhelmingly successful at increasing folic acid intake[14, 15] and reducing the incidence of neural tube defects.[16] Although the serum and RBC folate tests are prone to inaccuracies for an individual patient, population trends postfortification, coupled with the data on intake and rates of neural tube defects, make a strong argument that the prevalence of deficiency has decreased dramatically.
Similar to these population‐based trends, studies of hospital‐based laboratories have shown a marked decrease in the rate of low serum and RBC folate levels, making for a very low pretest probability for folate deficiency (Table 1). Even before fortification had been fully implemented, a study of outpatients and inpatients cared for at 3 hospitals in Denver, Colorado in 1996 found that just 1.9% of patients had low serum folate levels and 4.4% had low RBC folate levels.[17] A retrospective study of 26,662 patients in 1998 showed a rate of serum deficiency (<2.7 ng/mL) of 0.3%.[18] The authors also found that despite a decline in rate of serum deficiency from 1.3% to 0.3% between 1994 and 1998, the total number of serum folate tests performed increased by 84%. A similar study found just 0.4% of 1007 patients with low serum folate levels (<3.0 ng/mL).[7] Parallel results have been seen in other countries after implementation of folate fortification with a cohort of 2154 Canadian patients reporting low serum folate (<6.8 nmol/L) and RBC folate (<417 nmol/L) levels in just 0.5% and 0.7% of patients, respectively.[19]
| Author, Study Year | Year of Testing | Country | Population | Serum Folate | Red Blood Cell Folate | ||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Samples (n) | Low (%) | Patients (n) | Samples (n) | Low (%) | ||||
| Latif et al., [4] | 2001 | United States | Inpatient/outpatient | 4,315 | 4,689 | 1.6 | 1,215 | 1,335 | 1.2 |
| Shojania et al., 2010[19] | 2001 | Canada | Inpatient/outpatient | 2,154 | 0.5 | 560 | 0.7 | ||
| Ashraf et al., [7] | 2002 | United States | Inpatient/outpatient | 980 | 1,007 | 0.4 | |||
| Gudgeon et al., 2014[20] | 2010 | Canada | Inpatient | 2,563 | 0.2 | ||||
| Theisen‐Toupal et al., [3] | 2011 | United States | Inpatient/emergency department | 1,944 | 2,093 | 0.1 | |||
Few studies have looked exclusively at hospitalized and emergency room patients. In an evaluation of 2093 serum folate tests performed on hospitalized or emergency room patients (98.1% of whom were admitted) in 2011, only 2 (0.1%) deficient levels (<3 ng/mL) were identified, 1 of which was associated with a macrocytic anemia.[3] A similar study of RBC folate levels in 2562 patients at 3 Canadian hospitals found just 4 (0.16%) levels to be low (<254 nmol/L), only 1 of which was associated with macrocytic anemia.[20]
When examining only patients with macrocytic anemia, the rates of folate deficiency are only slightly higher than the general population. As noted above, each of the 2 studies of inpatients uncovered just 1 patient with macrocytic anemia and concomitant low serum or RBC folate levels.[3, 20] Other studies reveal rates of serum folate deficiency in patients with macrocytic anemia and macrocytosis of 2.8%[7] and 1%,[21] respectively, and RBC folate deficiency rates in patients with macrocytosis of 1.8%.[22] Patients with extreme macrocytosis (MCV >130) represent 1 subset of patients with a high pretest probability of low serum folate, with 1 study reporting low levels in 37% of patients.[23]
Despite the relatively inexpensive cost per serum and RBC folate test, expenses per test that result in an abnormally low level are significant. As the pretest probability for folate deficiency is extremely low, tests must be ordered on a large number of patients to find 1 patient with levels suggesting deficiency. For example, a study found that an institution charged $151 per serum folate test, which amounted to $158,000 per deficient result.[3] The institutional cost was <$2.00 per serum folate test and <$2093 per deficient result. Another study reported the institutional cost of RBC folate to be $12.54 per test and $8035 per deficient result.[20] The charges and costs are institution specific and will vary. However, in light of the low pretest probability of testing, any expense associated with these tests represents low value.
WHAT YOU SHOULD DO INSTEAD
The clinician in our case presentation is facing a common scenarioa patient with persistent anemia without a known etiology. The treatment of suspected or confirmed folate deficiency includes improving diet or adding a folic acid supplement, a low‐cost (as little $0.01 per tablet) intervention. Furthermore, other at‐risk patients (eg, those with sickle cell disease, alcoholism, or malabsorption) may be candidates for long‐term supplementation regardless of serum folate and/or RBC folate testing results.
Folate deficiency in patients living in the United States and Canada is exceedingly rare, making the pretest probability of testing low. Furthermore, even patients with typical hematologic characteristics for folate deficiency (anemia and macrocytosis) are unlikely to have folate deficiency. Importantly, there are no nonhematologic indications to test for folate deficiency, and testing those patients, just as in the general population, yields an extremely low rate of folate deficiency. The tests themselves are unreliable and inaccurate, and fortunately, treatment is cheap, easy to administer, and can be done empirically. In other words, testing for folate deficiency is a Thing We Do for No Reason.
RECOMMENDATIONS
In patients suspected of having folate deficiency or who are at high risk of folate deficiency (eg, diet poor in folate‐rich or folic acid fortified foods), treat with a diet containing folate or folic acid fortified foods and/or a supplement containing 400 to 1000 g of folic acid. Approximately 1 to 2 weeks following initiation of treatment, a complete blood count should be performed to evaluate for an appropriate increase in hematocrit/hemoglobin and decrease in MCV.[24] Once a full hematologic response is seen, treatment beyond this time is not required unless the cause (eg, malnutrition) persists.
Serum folate and RBC folate tests should not be routinely ordered. Even in those with macrocytic anemia, the pretest probability of folate deficiency remains low. Although testing may suggest a folate deficiency, it is still more likely there is another cause for the patient's anemia. This places providers at risk for premature closure. For patients such as the one presented in the case presentation, obtaining B12 levels is of greater importance, given the higher prevalence and the risks of untreated deficiency.
For patients in whom the pretest probability of folate deficiency is high (eg, those with an MCV >130), obtain fasting serum folate levels on samples taken before supplementation has begun or a diet administered.
Disclosures
Dr. Feldman is a consultant to Maven Medical, LLC. Maven Medical is a healthcare software startup.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 65‐year‐old man is admitted with pneumonia. Review of the medical record reveals a persistent macrocytic anemia (hematocrit 29%, hemoglobin 9.3 g/dL, mean corpuscular volume [MCV] 105 fL) with a low reticulocyte count and normal peripheral blood smear. The provider contemplates ordering a serum folate or red blood cell (RBC) folate test to workup the persistent macrocytic anemia.
BACKGROUND
Folate is a water‐soluble B vitamin essential for the synthesis of DNA and for converting homocysteine to methionine. Folate deficiency is causally linked with both neural tube defects and megaloblastic anemia. Low levels of folate are associated with cardiovascular disease, colon cancer, neuropathy, depression, hypercoagulability, and cognitive decline, though there is a paucity of evidence showing causation or risk reduction with folate supplementation.[1] In patients with inadequate folate intake, the earliest sign is a decline in serum folate levels, followed by a fall in RBC folate levels. Only weeks later do macrocytosis, megaloblastic bone marrow, and finally anemia occur.[2] Given that humans are unable to synthesize folate and are therefore dependent on dietary sources, those with inadequate intake or absorption are at risk of folate deficiency.
WHY FOLATE TESTING IS ORDERED
In hospitalized patients, the most common indication for folate testing is anemia, either with or without macrocytosis.[3, 4] Given that at least 10% to 15% of hospitalized patients are anemic,[5, 6] it is unsurprising that folate testing is frequently performed. Despite the link between folate deficiency and megaloblastic anemia, >85% of patients evaluated for folate deficiency have normocytic or microcytic anemia.[3, 4] In addition, a study found that 30% of all folate testing was performed not as part of an anemia workup but in the evaluation of other comorbidities (eg, dementia and altered mental status) that are not causally linked to folate deficiency.[7]
WHY THERE IS NO REASON TO ORDER FOLATE TESTING
There are 2 reasons why testing hospitalized patients for folate deficiency does not contribute value: (1) the poor characteristics of the tests used and (2) the low prevalence of folate deficiency in the postfortification era.
There is no accepted gold standard for the diagnosis of folate deficiency, though biological assays are considered more accurate than the now more commonly used protein binding assays.[8] The lack of a gold standard limits the ability to fully quantify the sensitivity and specificity of either serum or RBC folate testing, though falsely low and high serum folate results can be seen. Falsely low serum levels (false positives) are found with heavy alcohol use and with certain anticonvulsant or antineoplastic drug use.[9] The low levels in these patients indicate low serum folate but do not necessarily reflect tissue stores. Serum folate levels may fall rapidly within a few days of the start of low dietary folate intake, resulting in low serum folate levels that also do not represent true folate deficiency.[10] On the other hand, intake of folatethrough a meal or ingestion of an oral supplementdirectly preceding evaluation of serum folate can lead to falsely elevated levels (false negatives).[10]
Although RBC folate reflects body stores and is largely unaffected by diet, the available tests also lack sensitivity and specificity.[11] Furthermore, serum folate levels and RBC folate levels correlate well.[12] Because RBC folate testing is more expensive than serum folate testing, has results that correlate well with serum folate testing, and is without significantly better test characteristics, there is no added value to using RBC folate testing as compared to serum folate testing.
In addition to the issues with available diagnostic tests, numerous studies now indicate that the rate of folate deficiency in the United States is exceptionally low. This is largely driven by the United States Food and Drug Administration's mandate that all grain products be fortified with 0.14 mg of folic acid per gram of grains.[13] Fortification has been overwhelmingly successful at increasing folic acid intake[14, 15] and reducing the incidence of neural tube defects.[16] Although the serum and RBC folate tests are prone to inaccuracies for an individual patient, population trends postfortification, coupled with the data on intake and rates of neural tube defects, make a strong argument that the prevalence of deficiency has decreased dramatically.
Similar to these population‐based trends, studies of hospital‐based laboratories have shown a marked decrease in the rate of low serum and RBC folate levels, making for a very low pretest probability for folate deficiency (Table 1). Even before fortification had been fully implemented, a study of outpatients and inpatients cared for at 3 hospitals in Denver, Colorado in 1996 found that just 1.9% of patients had low serum folate levels and 4.4% had low RBC folate levels.[17] A retrospective study of 26,662 patients in 1998 showed a rate of serum deficiency (<2.7 ng/mL) of 0.3%.[18] The authors also found that despite a decline in rate of serum deficiency from 1.3% to 0.3% between 1994 and 1998, the total number of serum folate tests performed increased by 84%. A similar study found just 0.4% of 1007 patients with low serum folate levels (<3.0 ng/mL).[7] Parallel results have been seen in other countries after implementation of folate fortification with a cohort of 2154 Canadian patients reporting low serum folate (<6.8 nmol/L) and RBC folate (<417 nmol/L) levels in just 0.5% and 0.7% of patients, respectively.[19]
| Author, Study Year | Year of Testing | Country | Population | Serum Folate | Red Blood Cell Folate | ||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Samples (n) | Low (%) | Patients (n) | Samples (n) | Low (%) | ||||
| Latif et al., [4] | 2001 | United States | Inpatient/outpatient | 4,315 | 4,689 | 1.6 | 1,215 | 1,335 | 1.2 |
| Shojania et al., 2010[19] | 2001 | Canada | Inpatient/outpatient | 2,154 | 0.5 | 560 | 0.7 | ||
| Ashraf et al., [7] | 2002 | United States | Inpatient/outpatient | 980 | 1,007 | 0.4 | |||
| Gudgeon et al., 2014[20] | 2010 | Canada | Inpatient | 2,563 | 0.2 | ||||
| Theisen‐Toupal et al., [3] | 2011 | United States | Inpatient/emergency department | 1,944 | 2,093 | 0.1 | |||
Few studies have looked exclusively at hospitalized and emergency room patients. In an evaluation of 2093 serum folate tests performed on hospitalized or emergency room patients (98.1% of whom were admitted) in 2011, only 2 (0.1%) deficient levels (<3 ng/mL) were identified, 1 of which was associated with a macrocytic anemia.[3] A similar study of RBC folate levels in 2562 patients at 3 Canadian hospitals found just 4 (0.16%) levels to be low (<254 nmol/L), only 1 of which was associated with macrocytic anemia.[20]
When examining only patients with macrocytic anemia, the rates of folate deficiency are only slightly higher than the general population. As noted above, each of the 2 studies of inpatients uncovered just 1 patient with macrocytic anemia and concomitant low serum or RBC folate levels.[3, 20] Other studies reveal rates of serum folate deficiency in patients with macrocytic anemia and macrocytosis of 2.8%[7] and 1%,[21] respectively, and RBC folate deficiency rates in patients with macrocytosis of 1.8%.[22] Patients with extreme macrocytosis (MCV >130) represent 1 subset of patients with a high pretest probability of low serum folate, with 1 study reporting low levels in 37% of patients.[23]
Despite the relatively inexpensive cost per serum and RBC folate test, expenses per test that result in an abnormally low level are significant. As the pretest probability for folate deficiency is extremely low, tests must be ordered on a large number of patients to find 1 patient with levels suggesting deficiency. For example, a study found that an institution charged $151 per serum folate test, which amounted to $158,000 per deficient result.[3] The institutional cost was <$2.00 per serum folate test and <$2093 per deficient result. Another study reported the institutional cost of RBC folate to be $12.54 per test and $8035 per deficient result.[20] The charges and costs are institution specific and will vary. However, in light of the low pretest probability of testing, any expense associated with these tests represents low value.
WHAT YOU SHOULD DO INSTEAD
The clinician in our case presentation is facing a common scenarioa patient with persistent anemia without a known etiology. The treatment of suspected or confirmed folate deficiency includes improving diet or adding a folic acid supplement, a low‐cost (as little $0.01 per tablet) intervention. Furthermore, other at‐risk patients (eg, those with sickle cell disease, alcoholism, or malabsorption) may be candidates for long‐term supplementation regardless of serum folate and/or RBC folate testing results.
Folate deficiency in patients living in the United States and Canada is exceedingly rare, making the pretest probability of testing low. Furthermore, even patients with typical hematologic characteristics for folate deficiency (anemia and macrocytosis) are unlikely to have folate deficiency. Importantly, there are no nonhematologic indications to test for folate deficiency, and testing those patients, just as in the general population, yields an extremely low rate of folate deficiency. The tests themselves are unreliable and inaccurate, and fortunately, treatment is cheap, easy to administer, and can be done empirically. In other words, testing for folate deficiency is a Thing We Do for No Reason.
RECOMMENDATIONS
In patients suspected of having folate deficiency or who are at high risk of folate deficiency (eg, diet poor in folate‐rich or folic acid fortified foods), treat with a diet containing folate or folic acid fortified foods and/or a supplement containing 400 to 1000 g of folic acid. Approximately 1 to 2 weeks following initiation of treatment, a complete blood count should be performed to evaluate for an appropriate increase in hematocrit/hemoglobin and decrease in MCV.[24] Once a full hematologic response is seen, treatment beyond this time is not required unless the cause (eg, malnutrition) persists.
Serum folate and RBC folate tests should not be routinely ordered. Even in those with macrocytic anemia, the pretest probability of folate deficiency remains low. Although testing may suggest a folate deficiency, it is still more likely there is another cause for the patient's anemia. This places providers at risk for premature closure. For patients such as the one presented in the case presentation, obtaining B12 levels is of greater importance, given the higher prevalence and the risks of untreated deficiency.
For patients in whom the pretest probability of folate deficiency is high (eg, those with an MCV >130), obtain fasting serum folate levels on samples taken before supplementation has begun or a diet administered.
Disclosures
Dr. Feldman is a consultant to Maven Medical, LLC. Maven Medical is a healthcare software startup.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71(1‐2):121–138.
- Experimental nutritional folate deficiency in man. Trans Assoc Am Physicians. 1962;75:307–320.
- , , Utility, charge, and cost of inpatient and emergency department serum folate testing. J Hosp Med. 2013;8(2):91–95.
- , , , Is there a role for folate determinations in current clinical practice in the USA? Clin Lab Haematol. 2004;26(6):379–383.
- , , , , Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- Healthcare Cost and Utilization Project (HCUP). HCUP facts and figures: statistics on hospital‐based care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , Clinical utility of folic acid testing for patients with anemia or dementia. J Gen Intern Med. 2008;23(6):824–826.
- Utility of measuring serum or red blood cell folate in the era of folate fortification of flour. Clin Biochem. 2014;47(7‐8):533–538.
- Kelley's Textbook of Internal Medicine. Philadelphia, PA: Lippincott Williams 2000.
- Problems in the diagnosis and investigation of megaloblastic anemia. Can Med Assoc J. 1980;122(9):999–1004.
- Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–1298.
- , Erythrocyte folate levels: a clinical study. Am J Hematol. 1991;36(2):116–21.
- US Food and Drug Administration. Food standards: amendments of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1996;61:8781–8797.
- , Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. 2003;77(1):221–225.
- , , , , , Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002;132(9):2792–2798.
- , , , , Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986.
- , Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110(2):88–90.
- , , , Trends in serum folate after food fortification. Lancet. 1999;354(9182):915–916.
- , Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010;3:22.
- , Folate testing in hospital inpatients. Am J Med. 2015;128(1):56–59.
- , , , , Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci. 2000;319(6):343–352.
- , , Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007;131(3):477–480.
- , , , et al. Etiologies and diagnostic work‐up of extreme macrocytosis defined by an erythrocyte mean corpuscular volume over 130°fL: s study of 109 patients. Am J Hematol. 2014;89(6):665–666.
- , , , et al. Best practice in primary care pathology: review 1. J Clin Pathol. 2005;58(10):1016–1024.
- Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71(1‐2):121–138.
- Experimental nutritional folate deficiency in man. Trans Assoc Am Physicians. 1962;75:307–320.
- , , Utility, charge, and cost of inpatient and emergency department serum folate testing. J Hosp Med. 2013;8(2):91–95.
- , , , Is there a role for folate determinations in current clinical practice in the USA? Clin Lab Haematol. 2004;26(6):379–383.
- , , , , Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- Healthcare Cost and Utilization Project (HCUP). HCUP facts and figures: statistics on hospital‐based care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , Clinical utility of folic acid testing for patients with anemia or dementia. J Gen Intern Med. 2008;23(6):824–826.
- Utility of measuring serum or red blood cell folate in the era of folate fortification of flour. Clin Biochem. 2014;47(7‐8):533–538.
- Kelley's Textbook of Internal Medicine. Philadelphia, PA: Lippincott Williams 2000.
- Problems in the diagnosis and investigation of megaloblastic anemia. Can Med Assoc J. 1980;122(9):999–1004.
- Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–1298.
- , Erythrocyte folate levels: a clinical study. Am J Hematol. 1991;36(2):116–21.
- US Food and Drug Administration. Food standards: amendments of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1996;61:8781–8797.
- , Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. 2003;77(1):221–225.
- , , , , , Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002;132(9):2792–2798.
- , , , , Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986.
- , Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110(2):88–90.
- , , , Trends in serum folate after food fortification. Lancet. 1999;354(9182):915–916.
- , Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010;3:22.
- , Folate testing in hospital inpatients. Am J Med. 2015;128(1):56–59.
- , , , , Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci. 2000;319(6):343–352.
- , , Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007;131(3):477–480.
- , , , et al. Etiologies and diagnostic work‐up of extreme macrocytosis defined by an erythrocyte mean corpuscular volume over 130°fL: s study of 109 patients. Am J Hematol. 2014;89(6):665–666.
- , , , et al. Best practice in primary care pathology: review 1. J Clin Pathol. 2005;58(10):1016–1024.
© 2015 Society of Hospital Medicine
Code Status Documentation
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
Differentiating DNI From DNR
Since the introduction of defibrillation and closed chest cardiopulmonary resuscitation (CPR) in the 1950s, the ability to revive an arrested heart has been a realized possibility. Around the same time, endotracheal intubation with mechanical ventilation (MV) came into wide practice, allowing doctors to augment or even replace their patients' breathing. But just as the 1950s and 1960s saw the rise of these enhanced medical techniques, they also saw the increased importance of medical ethicsin particular, patient autonomy. A natural reaction to medicine's use of CPR and MV was the advent of advance directives and more specific do‐not‐resuscitate (DNR) and do‐not‐intubate (DNI) orders meant to protect a patient's ability to remain autonomous with their end of life decisions.[1]
Unfortunately, the code status discussions that lead to these orders often collapse cardiac arrest with prearrest respiratory failure and CPR with MV.[2, 3] This is a problem for a number of reasons. First, cardiac arrest and prearrest respiratory failure are unique end points, and though their respective treatments (CPR and MV) are often required simultaneously for an individual patient, they are distinct medical interventions with different goals, indications, and associated disease states. Although MV is typically a part of the cadre of interventions meant to ensure continued tissue oxygenation in the setting of a cardiac arrest, this accounts for <2% of indications for MV.[4] The vast majority of MV is used to treat prearrest causes of respiratory failure, such as pneumonia, congestive heart failure, acute exacerbations of chronic obstructive pulmonary disease, and following surgery.[4]
We do not believe these differences are adequately reflected in typical code status discussions.[2, 3] One study using audio‐recorded admission encounters included transcripts of hospitalist‐led code status discussions that all resembled the following: Physician: [I]f an emergency were to happenand your heart would (stop) or your breathing became so difficult that you needed to be attached to machines, would you want the nurses and doctors to attempt heroic measures to try to restart your heart and attach you to a breathing machine?[2] It would come as little surprise if a patient hearing this assumed that just 1 question were being asked and that decisions relating to any cause of respiratory failure (including prearrest causes) were being made. In practice, many physicians then extrapolate DNR orders to other treatment decision (including MV) and interpret them as precluding intubation, even for prearrest states.[5, 6, 7]
A second issue is that the mortality associated with cardiopulmonary arrest requiring CPR and prearrest respiratory failure requiring MV are not equal. Though the mortality after in‐hospital cardiac arrest has decreased over the last decade, it remains >75%.[8] The outcomes for MV for isolated respiratory failure, on the other hand, are not as grim; studies of the general population typically report mortality rates <40%. Despite this, descriptions of outcomes are often left out of goals of care discussions.[9, 10] For example, Sharma et al. recently reported that only one‐third of residents, including those who had undergone training on goals of care discussions, discussed outcomes.[9] And when outcomes are included, they are typically for CPR but not MV as an independent intervention for prearrest respiratory failure.[10] Given that many of the conditions that lead to respiratory failure are among the most common reasons for hospitalization,[11] distinguishing between decisions regarding CPR and prearrest MV with discussion of their associated outcomes is of particular importance to hospitalists. Failing to do so impedes patients from making informed autonomous decisions that incorporate an accurate understanding of the treatments being discussed.
Imagine you are caring for a 75‐year‐old man with a history of coronary artery disease and congestive heart failure now admitted with pneumonia. Given his age, admitting diagnosis, and comorbidities, you feel it would be appropriate to engage him in a discussion of goals of care. His chances of survival with near return to baseline after a cardiac arrest requiring CPR are not the same as his chances of surviving an episode of worsening pneumonia requiring MV. To discuss cardiac arrest and prearrest respiratory failure in the same breath, without acknowledging the differences, is misleading. Based on his goals and values, this patient may see a trial of MV as acceptable. One recent study supports this hypothesis, as 28% of hospitalized patients with a combined DNR/DNI order would accept a trial of MV for pneumonia.[12] If the genesis of these orders was our desire to ensure that patients' autonomous preferences are respected, we must actually know those preferences, and those preferences should be based on adequate information about the expected outcomes, highlighting the differences outlined above.
Some may consider separating CPR from MVtherefore allowing for more clearly separate DNR and DNI ordersproblematic, as it may result in a menu of choices for patients. However, although CPR and MV may be performed at the same time for the same patient, they do not overlap in 100% of their occurrences. This is conceptually different from discussing whether to use epinephrine versus vasopressin, for example, or offering options such as chest compressions alone. More clearly separating CPR from MV would not be dissimilar to what is done with renal dialysis; a patient may wish to be DNR while still electing to undergo dialysis for failing kidneys. Though the discussions surrounding renal dialysis are less urgent, this alone does not adequately explain why the topic is not routinely collapsed into the discussion of CPR. Instead, renal dialysis is an intervention with unique indications, goals, and outcomes; this is what prompts the separation. The same is true of MV.
No matter the situation, code status discussions should focus on determining an individual patient's values and goals of care and should guide physicians in provision (or omission) of certain interventions. For the patient with pneumonia described above, his goal may be to promote quality of life over extension of life. Although this may prompt a recommendation to forego CPR, (if it were felt that his quality of life, even after successful return of spontaneous circulation, would be low), it may not be inconsistent for him to accept a trial of MV were his pneumonia to get worse (if it were felt that he could quickly improve and return to a quality of life close to what he experienced before the episode of pneumonia). We recommend that when discussing options with patients, the indications for and outcomes of CPR and MV be more clearly separated. It may be as simple as saying, there are 2 different situations I would like to discuss with you, followed by a discussion of the associated scenarios and likely outcomes in the best judgment of the care team. For a hospitalist, framing the discussion of MV around anticipated causes of pre‐arrest respiratory failure (eg, pneumonia, acute pulmonary edema) is essential.
In conclusion, if DNR and DNI orders are going to meet their promise of ensuring patients make informed decisions congruent with their goals, then the discussions from which they follow will need to more clearly acknowledge the important differences in indications and outcomes. Although a patient's goals should still be the framework upon which decisions regarding interventions are made, an important distinction should be made between cardiopulmonary arrest and prearrest respiratory failure, with a more explicit accompanying discussion of how the corresponding interventions fit within the patient's overall goals of care.
Acknowledgements
The authors thank Rafael Campo, MD, and Sharon H. Chou, MD, for their suggestions and critical reading of this manuscript.
Disclosures:
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging.
- , , . Orders not to resuscitate. N Engl J Med. 1976;295(7):364–366.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA. 2002;287(3):345–355.
- , . The effect of do‐not‐resuscitate orders on physician decision‐making. J Am Geriatr Soc. 2002;50(12):2057–2061.
- , , . Hospital do‐not‐resuscitate orders: why they have failed and how to fix them. J Gen Intern Med. 2011;26(7):791–797.
- , , . Clinician perspectives regarding the do‐not‐resuscitate order. JAMA Pediatr. 2013;167(10):954–958.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , , , . Unpacking resident‐led code status discussions: results from a mixed methods study. J Gen Intern Med. 2014;29(5):750–7.
- , , , et al. If asked, hospitalized patients will choose whether to receive life‐sustaining therapies. J Hosp Med. 2006;1(3):161–167.
- Healthcare Cost and Utilization Project (HCUP). HCUP Facts and Figures: Statistics on Hospital‐Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Preferences for resuscitation and intubation among patients with do‐not‐resuscitate/do‐not‐intubate orders. Mayo Clin Proceed. 2013;88(7):658–665.
Since the introduction of defibrillation and closed chest cardiopulmonary resuscitation (CPR) in the 1950s, the ability to revive an arrested heart has been a realized possibility. Around the same time, endotracheal intubation with mechanical ventilation (MV) came into wide practice, allowing doctors to augment or even replace their patients' breathing. But just as the 1950s and 1960s saw the rise of these enhanced medical techniques, they also saw the increased importance of medical ethicsin particular, patient autonomy. A natural reaction to medicine's use of CPR and MV was the advent of advance directives and more specific do‐not‐resuscitate (DNR) and do‐not‐intubate (DNI) orders meant to protect a patient's ability to remain autonomous with their end of life decisions.[1]
Unfortunately, the code status discussions that lead to these orders often collapse cardiac arrest with prearrest respiratory failure and CPR with MV.[2, 3] This is a problem for a number of reasons. First, cardiac arrest and prearrest respiratory failure are unique end points, and though their respective treatments (CPR and MV) are often required simultaneously for an individual patient, they are distinct medical interventions with different goals, indications, and associated disease states. Although MV is typically a part of the cadre of interventions meant to ensure continued tissue oxygenation in the setting of a cardiac arrest, this accounts for <2% of indications for MV.[4] The vast majority of MV is used to treat prearrest causes of respiratory failure, such as pneumonia, congestive heart failure, acute exacerbations of chronic obstructive pulmonary disease, and following surgery.[4]
We do not believe these differences are adequately reflected in typical code status discussions.[2, 3] One study using audio‐recorded admission encounters included transcripts of hospitalist‐led code status discussions that all resembled the following: Physician: [I]f an emergency were to happenand your heart would (stop) or your breathing became so difficult that you needed to be attached to machines, would you want the nurses and doctors to attempt heroic measures to try to restart your heart and attach you to a breathing machine?[2] It would come as little surprise if a patient hearing this assumed that just 1 question were being asked and that decisions relating to any cause of respiratory failure (including prearrest causes) were being made. In practice, many physicians then extrapolate DNR orders to other treatment decision (including MV) and interpret them as precluding intubation, even for prearrest states.[5, 6, 7]
A second issue is that the mortality associated with cardiopulmonary arrest requiring CPR and prearrest respiratory failure requiring MV are not equal. Though the mortality after in‐hospital cardiac arrest has decreased over the last decade, it remains >75%.[8] The outcomes for MV for isolated respiratory failure, on the other hand, are not as grim; studies of the general population typically report mortality rates <40%. Despite this, descriptions of outcomes are often left out of goals of care discussions.[9, 10] For example, Sharma et al. recently reported that only one‐third of residents, including those who had undergone training on goals of care discussions, discussed outcomes.[9] And when outcomes are included, they are typically for CPR but not MV as an independent intervention for prearrest respiratory failure.[10] Given that many of the conditions that lead to respiratory failure are among the most common reasons for hospitalization,[11] distinguishing between decisions regarding CPR and prearrest MV with discussion of their associated outcomes is of particular importance to hospitalists. Failing to do so impedes patients from making informed autonomous decisions that incorporate an accurate understanding of the treatments being discussed.
Imagine you are caring for a 75‐year‐old man with a history of coronary artery disease and congestive heart failure now admitted with pneumonia. Given his age, admitting diagnosis, and comorbidities, you feel it would be appropriate to engage him in a discussion of goals of care. His chances of survival with near return to baseline after a cardiac arrest requiring CPR are not the same as his chances of surviving an episode of worsening pneumonia requiring MV. To discuss cardiac arrest and prearrest respiratory failure in the same breath, without acknowledging the differences, is misleading. Based on his goals and values, this patient may see a trial of MV as acceptable. One recent study supports this hypothesis, as 28% of hospitalized patients with a combined DNR/DNI order would accept a trial of MV for pneumonia.[12] If the genesis of these orders was our desire to ensure that patients' autonomous preferences are respected, we must actually know those preferences, and those preferences should be based on adequate information about the expected outcomes, highlighting the differences outlined above.
Some may consider separating CPR from MVtherefore allowing for more clearly separate DNR and DNI ordersproblematic, as it may result in a menu of choices for patients. However, although CPR and MV may be performed at the same time for the same patient, they do not overlap in 100% of their occurrences. This is conceptually different from discussing whether to use epinephrine versus vasopressin, for example, or offering options such as chest compressions alone. More clearly separating CPR from MV would not be dissimilar to what is done with renal dialysis; a patient may wish to be DNR while still electing to undergo dialysis for failing kidneys. Though the discussions surrounding renal dialysis are less urgent, this alone does not adequately explain why the topic is not routinely collapsed into the discussion of CPR. Instead, renal dialysis is an intervention with unique indications, goals, and outcomes; this is what prompts the separation. The same is true of MV.
No matter the situation, code status discussions should focus on determining an individual patient's values and goals of care and should guide physicians in provision (or omission) of certain interventions. For the patient with pneumonia described above, his goal may be to promote quality of life over extension of life. Although this may prompt a recommendation to forego CPR, (if it were felt that his quality of life, even after successful return of spontaneous circulation, would be low), it may not be inconsistent for him to accept a trial of MV were his pneumonia to get worse (if it were felt that he could quickly improve and return to a quality of life close to what he experienced before the episode of pneumonia). We recommend that when discussing options with patients, the indications for and outcomes of CPR and MV be more clearly separated. It may be as simple as saying, there are 2 different situations I would like to discuss with you, followed by a discussion of the associated scenarios and likely outcomes in the best judgment of the care team. For a hospitalist, framing the discussion of MV around anticipated causes of pre‐arrest respiratory failure (eg, pneumonia, acute pulmonary edema) is essential.
In conclusion, if DNR and DNI orders are going to meet their promise of ensuring patients make informed decisions congruent with their goals, then the discussions from which they follow will need to more clearly acknowledge the important differences in indications and outcomes. Although a patient's goals should still be the framework upon which decisions regarding interventions are made, an important distinction should be made between cardiopulmonary arrest and prearrest respiratory failure, with a more explicit accompanying discussion of how the corresponding interventions fit within the patient's overall goals of care.
Acknowledgements
The authors thank Rafael Campo, MD, and Sharon H. Chou, MD, for their suggestions and critical reading of this manuscript.
Disclosures:
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging.
Since the introduction of defibrillation and closed chest cardiopulmonary resuscitation (CPR) in the 1950s, the ability to revive an arrested heart has been a realized possibility. Around the same time, endotracheal intubation with mechanical ventilation (MV) came into wide practice, allowing doctors to augment or even replace their patients' breathing. But just as the 1950s and 1960s saw the rise of these enhanced medical techniques, they also saw the increased importance of medical ethicsin particular, patient autonomy. A natural reaction to medicine's use of CPR and MV was the advent of advance directives and more specific do‐not‐resuscitate (DNR) and do‐not‐intubate (DNI) orders meant to protect a patient's ability to remain autonomous with their end of life decisions.[1]
Unfortunately, the code status discussions that lead to these orders often collapse cardiac arrest with prearrest respiratory failure and CPR with MV.[2, 3] This is a problem for a number of reasons. First, cardiac arrest and prearrest respiratory failure are unique end points, and though their respective treatments (CPR and MV) are often required simultaneously for an individual patient, they are distinct medical interventions with different goals, indications, and associated disease states. Although MV is typically a part of the cadre of interventions meant to ensure continued tissue oxygenation in the setting of a cardiac arrest, this accounts for <2% of indications for MV.[4] The vast majority of MV is used to treat prearrest causes of respiratory failure, such as pneumonia, congestive heart failure, acute exacerbations of chronic obstructive pulmonary disease, and following surgery.[4]
We do not believe these differences are adequately reflected in typical code status discussions.[2, 3] One study using audio‐recorded admission encounters included transcripts of hospitalist‐led code status discussions that all resembled the following: Physician: [I]f an emergency were to happenand your heart would (stop) or your breathing became so difficult that you needed to be attached to machines, would you want the nurses and doctors to attempt heroic measures to try to restart your heart and attach you to a breathing machine?[2] It would come as little surprise if a patient hearing this assumed that just 1 question were being asked and that decisions relating to any cause of respiratory failure (including prearrest causes) were being made. In practice, many physicians then extrapolate DNR orders to other treatment decision (including MV) and interpret them as precluding intubation, even for prearrest states.[5, 6, 7]
A second issue is that the mortality associated with cardiopulmonary arrest requiring CPR and prearrest respiratory failure requiring MV are not equal. Though the mortality after in‐hospital cardiac arrest has decreased over the last decade, it remains >75%.[8] The outcomes for MV for isolated respiratory failure, on the other hand, are not as grim; studies of the general population typically report mortality rates <40%. Despite this, descriptions of outcomes are often left out of goals of care discussions.[9, 10] For example, Sharma et al. recently reported that only one‐third of residents, including those who had undergone training on goals of care discussions, discussed outcomes.[9] And when outcomes are included, they are typically for CPR but not MV as an independent intervention for prearrest respiratory failure.[10] Given that many of the conditions that lead to respiratory failure are among the most common reasons for hospitalization,[11] distinguishing between decisions regarding CPR and prearrest MV with discussion of their associated outcomes is of particular importance to hospitalists. Failing to do so impedes patients from making informed autonomous decisions that incorporate an accurate understanding of the treatments being discussed.
Imagine you are caring for a 75‐year‐old man with a history of coronary artery disease and congestive heart failure now admitted with pneumonia. Given his age, admitting diagnosis, and comorbidities, you feel it would be appropriate to engage him in a discussion of goals of care. His chances of survival with near return to baseline after a cardiac arrest requiring CPR are not the same as his chances of surviving an episode of worsening pneumonia requiring MV. To discuss cardiac arrest and prearrest respiratory failure in the same breath, without acknowledging the differences, is misleading. Based on his goals and values, this patient may see a trial of MV as acceptable. One recent study supports this hypothesis, as 28% of hospitalized patients with a combined DNR/DNI order would accept a trial of MV for pneumonia.[12] If the genesis of these orders was our desire to ensure that patients' autonomous preferences are respected, we must actually know those preferences, and those preferences should be based on adequate information about the expected outcomes, highlighting the differences outlined above.
Some may consider separating CPR from MVtherefore allowing for more clearly separate DNR and DNI ordersproblematic, as it may result in a menu of choices for patients. However, although CPR and MV may be performed at the same time for the same patient, they do not overlap in 100% of their occurrences. This is conceptually different from discussing whether to use epinephrine versus vasopressin, for example, or offering options such as chest compressions alone. More clearly separating CPR from MV would not be dissimilar to what is done with renal dialysis; a patient may wish to be DNR while still electing to undergo dialysis for failing kidneys. Though the discussions surrounding renal dialysis are less urgent, this alone does not adequately explain why the topic is not routinely collapsed into the discussion of CPR. Instead, renal dialysis is an intervention with unique indications, goals, and outcomes; this is what prompts the separation. The same is true of MV.
No matter the situation, code status discussions should focus on determining an individual patient's values and goals of care and should guide physicians in provision (or omission) of certain interventions. For the patient with pneumonia described above, his goal may be to promote quality of life over extension of life. Although this may prompt a recommendation to forego CPR, (if it were felt that his quality of life, even after successful return of spontaneous circulation, would be low), it may not be inconsistent for him to accept a trial of MV were his pneumonia to get worse (if it were felt that he could quickly improve and return to a quality of life close to what he experienced before the episode of pneumonia). We recommend that when discussing options with patients, the indications for and outcomes of CPR and MV be more clearly separated. It may be as simple as saying, there are 2 different situations I would like to discuss with you, followed by a discussion of the associated scenarios and likely outcomes in the best judgment of the care team. For a hospitalist, framing the discussion of MV around anticipated causes of pre‐arrest respiratory failure (eg, pneumonia, acute pulmonary edema) is essential.
In conclusion, if DNR and DNI orders are going to meet their promise of ensuring patients make informed decisions congruent with their goals, then the discussions from which they follow will need to more clearly acknowledge the important differences in indications and outcomes. Although a patient's goals should still be the framework upon which decisions regarding interventions are made, an important distinction should be made between cardiopulmonary arrest and prearrest respiratory failure, with a more explicit accompanying discussion of how the corresponding interventions fit within the patient's overall goals of care.
Acknowledgements
The authors thank Rafael Campo, MD, and Sharon H. Chou, MD, for their suggestions and critical reading of this manuscript.
Disclosures:
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging.
- , , . Orders not to resuscitate. N Engl J Med. 1976;295(7):364–366.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA. 2002;287(3):345–355.
- , . The effect of do‐not‐resuscitate orders on physician decision‐making. J Am Geriatr Soc. 2002;50(12):2057–2061.
- , , . Hospital do‐not‐resuscitate orders: why they have failed and how to fix them. J Gen Intern Med. 2011;26(7):791–797.
- , , . Clinician perspectives regarding the do‐not‐resuscitate order. JAMA Pediatr. 2013;167(10):954–958.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , , , . Unpacking resident‐led code status discussions: results from a mixed methods study. J Gen Intern Med. 2014;29(5):750–7.
- , , , et al. If asked, hospitalized patients will choose whether to receive life‐sustaining therapies. J Hosp Med. 2006;1(3):161–167.
- Healthcare Cost and Utilization Project (HCUP). HCUP Facts and Figures: Statistics on Hospital‐Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Preferences for resuscitation and intubation among patients with do‐not‐resuscitate/do‐not‐intubate orders. Mayo Clin Proceed. 2013;88(7):658–665.
- , , . Orders not to resuscitate. N Engl J Med. 1976;295(7):364–366.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA. 2002;287(3):345–355.
- , . The effect of do‐not‐resuscitate orders on physician decision‐making. J Am Geriatr Soc. 2002;50(12):2057–2061.
- , , . Hospital do‐not‐resuscitate orders: why they have failed and how to fix them. J Gen Intern Med. 2011;26(7):791–797.
- , , . Clinician perspectives regarding the do‐not‐resuscitate order. JAMA Pediatr. 2013;167(10):954–958.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , , , . Unpacking resident‐led code status discussions: results from a mixed methods study. J Gen Intern Med. 2014;29(5):750–7.
- , , , et al. If asked, hospitalized patients will choose whether to receive life‐sustaining therapies. J Hosp Med. 2006;1(3):161–167.
- Healthcare Cost and Utilization Project (HCUP). HCUP Facts and Figures: Statistics on Hospital‐Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Preferences for resuscitation and intubation among patients with do‐not‐resuscitate/do‐not‐intubate orders. Mayo Clin Proceed. 2013;88(7):658–665.
Head CT for the Inpatient With Delirium
Delirium is a common and costly problem in hospitalized medical patients. It is present on admission in 10% to 31% of cases and develops in up to 56% of patients during hospitalization.[1, 2] Prompt identification and treatment of the cause of delirium is important, because delirium is associated with increased morbidity and mortality, long‐term cognitive impairment, higher cost of care, increased length of stay, and more frequent discharge to an extended care facility.[3, 4, 5, 6]
Delirium can be caused or worsened by a variety of factors including adverse drug events, metabolic abnormalities, infections, immobilization, the use of tethers (eg, physical restraints, bladder catheters, telemetry), and disruption of sleepwake cycle.[7] An appropriate history, medication review, physical examination, and tailored laboratory evaluation is sufficient workup in the majority of cases.[8] However, neurologic processes, such as intracranial mass, intracranial hemorrhage, or stroke, can also present as delirium and require head imaging for diagnosis.
Because head imaging is a costly limited resource, a number of studies have aimed to determine which patients with delirium require this evaluation. The majority of research has focused on head computed tomography (CT) in patients presenting for evaluation to the emergency department (ED). In ED patients presenting with delirium, acute confusion, or altered mental status, head imaging identifies acute intracranial pathologic findings in 14% to 39% of cases.[9, 10, 11, 12, 13, 14] Only 2 studies have evaluated patients with delirium who have already been admitted to the hospital. One study involved patients admitted to a neurology unit with acute confusion and found that the yield of head imaging (head CT and magnetic resonance imaging) was 14% for acute intracranial pathology.[15] Another study reviewed patients admitted to an acute delirium unit and found a similar rate of positive findings on head CT (14.5%).[16] Neither study specified whether the head imaging occurred during initial presentation or later in the hospitalization.
Factors that increase the likelihood that delirium is caused by acute intracranial pathology include acute neurologic deficit, recent history of fall or head trauma, and significantly impaired consciousness.[9, 10, 11, 12, 13, 14, 15, 16, 17] Based on these findings, current guidelines and expert clinical statements recommend head imaging for patients with acute neurologic deficit, recent head trauma, or recent fall.[18, 19, 20]
Expert clinical statements also recommend imaging in cases where the cause is unidentified after appropriate medical testing or where delirium continues despite treatment.[8, 21] Yet the utility of head CT performed for nonresolving delirium or delirium that develops during hospitalization in the absence of recent fall, head trauma, or new neurologic deficits is not known. Our study aimed to determine the diagnostic yield of performing a head CT in this patient population. We hypothesized that the diagnostic yield of head CT in this population would be low.
METHODS
Study Design
We conducted a retrospective medical record review of hospitalized general medicine patients with head CT imaging performed for the evaluation of delirium. The study was reviewed by the internal review board and determined to be exempt.
Setting and Eligibility Criteria
The study was conducted at a large academic medical center in Boston, Massachusetts. All patients admitted to general medicine, nephrology, hepatology, cardiology, or oncology services with head CT studies performed from January 1, 2010 through November 30, 2012 were included in this retrospective, observational cohort study. Data were extracted using a defined instrument developed for this study with outcome measures predefined. Head CT imaging acquired for patients in the intensive care unit were not included in the review. The medical records were evaluated to determine indication. To be included in the study, the indication for the scan had to be delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness. In addition, the patient must have been admitted for at least 24 hours prior to the completion of the head CT scan. Scans were excluded if there was documentation in the medical record of a fall, head trauma, or new neurologic deficit within the preceding 2 weeks, or an admitting diagnosis of intracranial pathology (eg, stroke or subdural hematoma). If a patient had multiple head CT studies completed for the indication of delirium, each study was included. However, once a head CT study returned positive or equivocal for an acute intracranial process, subsequent head CT studies for the indication of delirium were not included in the analysis.
Outcome Measures
A positive head CT was defined as an intracranial process that could explain delirium (eg, intracranial hemorrhage or stroke). An equivocal head CT was defined as the presence of a finding of unclear significance in relation to delirium (eg, hypodensity of unknown etiology or clinical significance). Chronic head CT findings were noted to be intracranial pathologic findings of a chronic nature that did not meet criteria for either a positive or equivocal image (eg, chronic small vessel ischemic disease or atrophy). A normal study was without positive, equivocal, or chronic findings.
Data Collection and Statistical Analysis
Using the medical center's clinical informatics infrastructure, an experienced clinical informaticist (R.A.) compiled a list of all head CT imaging studies performed during the study period in hospitalized medical patients. An experienced hospital medicine physician (J.T.) conducted the medical record review and determined if each head CT performed met eligibility criteria. For each included study, the following information was collected: date of admission, date of head CT, date of onset of delirium, indication for obtaining head CT scan, head CT results, age, gender, race/ethnicity (patient reported), presence of dementia (if documented in the medical record), active cancer, use of anticoagulants (defined as factor Xa inhibitors, low molecular weight heparin, direct thrombin inhibitor, or vitamin K antagonist) with documentation of internationalized normalized ratio (INR), partial thromboplastin time (PTT) prothrombin time and platelet count, active infection, history of stroke, and change in clinical management. Descriptive statistics were used to analyze data. Median and interquartile range were used to describe results for age and time from admission to head CT performed due to skewed distribution of results.
RESULTS
Of 1714 head CT studies performed on hospitalized medical patients from January 1, 2010 to November 30, 2012, 398 studies were performed for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness in patients who were admitted for >24 hours. One hundred seventy‐eight studies were excluded (137 for admitting diagnosis of intracranial process, recent fall, or head trauma, and 41 for new neurologic deficit). There were 220 scans included in the study performed on 210 patients.
Table 1 displays characteristics of the 210 patients who underwent CT head imaging. Of the 42 patients on anticoagulation, 15 were potentially supratherapeutic; 10 were on warfarin (INR range, 3.37.7) and 5 were on intravenous heparin infusion (PTT range, 101>150 seconds). None of these individuals had positive or equivocal findings on head CT.
| Characteristic | N=210 |
|---|---|
| |
| Age, median (IQR) | 70 (5980) |
| Male, n (%) | 96 (45.7) |
| Race/ethnicity, n (%) | |
| White | 147 (70.0) |
| African American | 44 (21.0) |
| Hispanic | 4 (1.9) |
| Asian | 3 (1.4) |
| Unknown | 9 (4.3) |
| Other | 3 (1.4) |
| Comorbidities, n (%) | |
| Dementia | 30 (14.3) |
| Active cancer | 49 (23.3) |
| Anticoagulation | 42 (20.0) |
| Active infection | 105 (50.0) |
| History of stroke | 41 (19.5) |
| Days from admission to head CT, median (IQR) | 4 (38) |
| Days from delirium onset to head CT, median (IQR) | 2 (14) |
The main outcomes of the 220 included head CT scans and a separate analysis of the 60 head CT scans performed for indications of somnolence or unresponsiveness are shown in Table 2. The 6 (2.7%) positive and 4 (1.8%) equivocal head CT findings are listed in Table 3. Of the 3 positive results in patients on anticoagulation, 2 were on warfarin with an INR of 2.1 and 2.4, respectively, and another was on warfarin and therapeutic enoxaparin (dosed 1 mg/kg twice daily) with an INR of 1.6. The median time from admission to positive head CT was 8 days, with a range of 2 to 28 days. All of the positive head CT studies resulted in change of management. All equivocal head CT studies resulted in repeat imaging. None of these repeat head imaging studies diagnosed acute intracranial pathology. Chronic findings identified included 111 (50.5%) involution or atrophy, 95 (43.2%) small vessel ischemic disease, 31 (14.1%) prior stroke, and 18 (8.2%) other chronic abnormalities (eg, cyst or meningioma).
| Indication | Delirium, N=220 (100%)* | Somnolence or Unresponsiveness, N=60 (27.2%) |
|---|---|---|
| ||
| Outcome | ||
| Positive | 6 (2.7) | 0 |
| Equivocal | 4 (1.8) | 1 (1.6) |
| Chronic | 162 (73.6) | 41 (68.3) |
| Normal | 48 (21.8) | 18 (30.0) |
| CT Head Findings | Age (Sex) | Days From Onset | Change in Management | Outcome | |
|---|---|---|---|---|---|
| |||||
| Positive | |||||
| Case 1 | Subarachnoid hemorrhage in right frontal and temporal lobes | 64 (M) | 2 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 2 | Intraparenchymal hemorrhage with mild shift and vasogenic edema | 62 (M) | 1 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 3 | Subacute subdural hematoma | 62 (M) | 5 | Neurosurgery consult | Discharged with outpatient follow‐up |
| Case 4 | Acute infarct or mass | 73 (F) | 2 | Neurology consult, palliative care consult | Transitioned to comfort‐focused care, discharged |
| Case 5 | 4 mm focus concerning for hemorrhagic metastatic focus | 50 (M) | 3 | Neurosurgery consult, MRI | Discharged with outpatient follow‐up |
| Case 6 | Left occipital lobe parenchymal hemorrhage | 81 (F) | 1 | Neurosurgery consult, neurology consult | Transitioned to comfort‐focused care, died 6 days later |
| Equivocal | |||||
| Case 1 | Several white matter hypodensities of uncertain etiology | 70 (F) | 1 | MRI | MRI with chronic small vessel ischemia |
| Case 2 | Colloid cyst likely although cannot rule out intraventricular hemorrhage | 59 (F) | 1 | Repeat head CT | Repeat imaging with equivocal findings, no additional evaluation |
| Case 3 | Questionable hypodensity, either hemorrhagic contusion or artifact | 52 (M) | 3 | Repeat head CT | Repeat imaging normal |
| Case 4 | Ill‐defined hypodensity in left basal ganglia, no clear acute process | 74 (F) | 0 | MRI | MRI with chronic small vessel ischemia |
DISCUSSION
In this retrospective review, we determined that there is a low diagnostic yield of head CT imaging for identifying the cause of nonresolving or new‐onset delirium in hospitalized medical patients. Only 2.7% of head CT scans resulted in identifying an acute intracranial process. Because of the low number of positive results, no risk factor associations could be made from our study.
The low diagnostic yield of head imaging in hospitalized patients with delirium is particularly important for clinicians who care for hospitalized medical patients. Prior to this study, the yield of head CT scans in hospitalized medical patients with nonresolving or new‐onset delirium was unknown. In cases with known risk factors, such as recent fall, head trauma, or acute neurologic deficit, the guidelines recommend head CT imaging.[18, 19, 20] However, in the absence of these findings, the guidelines do not make any recommendation regarding when and in whom to perform head imaging. Expert statements recommend considering head CT imaging when the cause is not identified after appropriate testing or delirium continues despite treatment.[8, 21] Given these recommendations and lack of data, there is no clear standard of care for ordering head CT imaging when hospitalized patients experience delirium in the absence of known risk factors. The low diagnostic yield in this study suggests that head CT imaging is unlikely to diagnose the cause of delirium in hospitalized patients with nonresolving or new‐onset delirium.
The diagnostic yield of head CT for diagnosis of acute intracranial process in delirium was lower in our study than prior studies, which found between 14.0 and 39.1%.[9, 10, 11, 12, 13, 14, 15, 16] This was expected, as our study excluded patients with new neurologic deficits, recent fall or trauma, or an admitting diagnosis of an intracranial process. Even with these exclusions, we still allowed for a number of findings that prior studies considered to be high risk for intracranial pathology, such as age over 73 years, use of anticoagulation, and deterioration in consciousness level or Glasgow coma score under 14.[10, 11, 16] The inclusion and exclusion criteria were designed to create a generalizable study population without a clear standard of care based on current guidelines and expert statements.
Though the rate of positive findings found in our study is low, it likely overestimates the overall yield of head CT in hospitalized patients with delirium. This is because most hospitalized patients with delirium never receive head imaging. Presumably, ordering clinicians have deemed these patients to be at higher risk for intracranial processes than the average hospitalized patient with delirium who does not receive a head CT. Thus, the true rate of positive findings in head CT imaging in delirious hospitalized medical patients is likely lower than what we identified.
Although head CT had a low diagnostic yield, the positive and equivocal studies had a high impact on clinical care. All of the positive and equivocal head CT results produced a change in management. The equivocal findings led to repeat head imaging; however, none of the repeat images identified the cause of delirium. The positive results produced a more significant change in management, ranging from a higher platelet transfusion target, reversal of anticoagulation, repeat advanced head imaging, neurosurgery consultation, and a change in goals of care to a focus on comfort. No patients in our study underwent neurosurgical intervention.
The challenge for inpatient clinicians is to weigh the low diagnostic yield of head CT with the consequences of a missed or delayed diagnosis of an acute intracranial process. The low diagnostic yield leads to unnecessary cost, resource utilization, radiation exposure, and downstream evaluation of insignificant or indeterminate results when head CT is performed. Alternatively, a missed or delayed diagnosis can lead to potentially reversible morbidity and mortality. Given this, we feel that the routine use of head CT in the evaluation of delirium in hospitalized patients is unnecessary. However, there may be a subset of patients with delirium with an increased risk of acute intracranial processes that would benefit from head imaging. Further research is needed to identify this high‐risk population.
There are a number of limitations to our study. It is a retrospective chart review, which introduces a possibility of bias and relies on proper and thorough documentation. In addition, the diagnosis of delirium was made by individual clinicians without the use of a standardized delirium assessment tool. Furthermore, it is possible there may have been CT scans that were not identified due to mischaracterization of indication, or studies may have been included in individuals with new neurologic deficit or recent fall or trauma that were not documented or clinically appreciated. Finally, the study was conducted on medicine and medical subspecialty patients at a single academic tertiary care institution, potentially limiting the generalizability to patients in other settings.
In conclusion, our study suggests that the diagnostic yield of head CT to evaluate delirium in hospitalized patients in the absence of recent fall, head trauma, or new neurologic deficit is low. The routine use of head CT in evaluation of these individuals is unnecessary. However, there may be a subset of high‐risk individuals in which head CT imaging would be indicated. Further research is needed to identify these high‐risk individuals.
Disclosures
Jesse Theisen‐Toupal, MD, has no conflicts of interest to disclose. Anthony Breu is a contributor to Practical Reviews in Hospital Medicine but has no conflicts of interest. Melissa Mattison, MD, is a contributor to UpToDate and Practical Reviews in Hospital Medicine but has no conflicts of interest. Ramy Arnaout, MD, has no conflicts of interest to disclose.
Delirium is a common and costly problem in hospitalized medical patients. It is present on admission in 10% to 31% of cases and develops in up to 56% of patients during hospitalization.[1, 2] Prompt identification and treatment of the cause of delirium is important, because delirium is associated with increased morbidity and mortality, long‐term cognitive impairment, higher cost of care, increased length of stay, and more frequent discharge to an extended care facility.[3, 4, 5, 6]
Delirium can be caused or worsened by a variety of factors including adverse drug events, metabolic abnormalities, infections, immobilization, the use of tethers (eg, physical restraints, bladder catheters, telemetry), and disruption of sleepwake cycle.[7] An appropriate history, medication review, physical examination, and tailored laboratory evaluation is sufficient workup in the majority of cases.[8] However, neurologic processes, such as intracranial mass, intracranial hemorrhage, or stroke, can also present as delirium and require head imaging for diagnosis.
Because head imaging is a costly limited resource, a number of studies have aimed to determine which patients with delirium require this evaluation. The majority of research has focused on head computed tomography (CT) in patients presenting for evaluation to the emergency department (ED). In ED patients presenting with delirium, acute confusion, or altered mental status, head imaging identifies acute intracranial pathologic findings in 14% to 39% of cases.[9, 10, 11, 12, 13, 14] Only 2 studies have evaluated patients with delirium who have already been admitted to the hospital. One study involved patients admitted to a neurology unit with acute confusion and found that the yield of head imaging (head CT and magnetic resonance imaging) was 14% for acute intracranial pathology.[15] Another study reviewed patients admitted to an acute delirium unit and found a similar rate of positive findings on head CT (14.5%).[16] Neither study specified whether the head imaging occurred during initial presentation or later in the hospitalization.
Factors that increase the likelihood that delirium is caused by acute intracranial pathology include acute neurologic deficit, recent history of fall or head trauma, and significantly impaired consciousness.[9, 10, 11, 12, 13, 14, 15, 16, 17] Based on these findings, current guidelines and expert clinical statements recommend head imaging for patients with acute neurologic deficit, recent head trauma, or recent fall.[18, 19, 20]
Expert clinical statements also recommend imaging in cases where the cause is unidentified after appropriate medical testing or where delirium continues despite treatment.[8, 21] Yet the utility of head CT performed for nonresolving delirium or delirium that develops during hospitalization in the absence of recent fall, head trauma, or new neurologic deficits is not known. Our study aimed to determine the diagnostic yield of performing a head CT in this patient population. We hypothesized that the diagnostic yield of head CT in this population would be low.
METHODS
Study Design
We conducted a retrospective medical record review of hospitalized general medicine patients with head CT imaging performed for the evaluation of delirium. The study was reviewed by the internal review board and determined to be exempt.
Setting and Eligibility Criteria
The study was conducted at a large academic medical center in Boston, Massachusetts. All patients admitted to general medicine, nephrology, hepatology, cardiology, or oncology services with head CT studies performed from January 1, 2010 through November 30, 2012 were included in this retrospective, observational cohort study. Data were extracted using a defined instrument developed for this study with outcome measures predefined. Head CT imaging acquired for patients in the intensive care unit were not included in the review. The medical records were evaluated to determine indication. To be included in the study, the indication for the scan had to be delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness. In addition, the patient must have been admitted for at least 24 hours prior to the completion of the head CT scan. Scans were excluded if there was documentation in the medical record of a fall, head trauma, or new neurologic deficit within the preceding 2 weeks, or an admitting diagnosis of intracranial pathology (eg, stroke or subdural hematoma). If a patient had multiple head CT studies completed for the indication of delirium, each study was included. However, once a head CT study returned positive or equivocal for an acute intracranial process, subsequent head CT studies for the indication of delirium were not included in the analysis.
Outcome Measures
A positive head CT was defined as an intracranial process that could explain delirium (eg, intracranial hemorrhage or stroke). An equivocal head CT was defined as the presence of a finding of unclear significance in relation to delirium (eg, hypodensity of unknown etiology or clinical significance). Chronic head CT findings were noted to be intracranial pathologic findings of a chronic nature that did not meet criteria for either a positive or equivocal image (eg, chronic small vessel ischemic disease or atrophy). A normal study was without positive, equivocal, or chronic findings.
Data Collection and Statistical Analysis
Using the medical center's clinical informatics infrastructure, an experienced clinical informaticist (R.A.) compiled a list of all head CT imaging studies performed during the study period in hospitalized medical patients. An experienced hospital medicine physician (J.T.) conducted the medical record review and determined if each head CT performed met eligibility criteria. For each included study, the following information was collected: date of admission, date of head CT, date of onset of delirium, indication for obtaining head CT scan, head CT results, age, gender, race/ethnicity (patient reported), presence of dementia (if documented in the medical record), active cancer, use of anticoagulants (defined as factor Xa inhibitors, low molecular weight heparin, direct thrombin inhibitor, or vitamin K antagonist) with documentation of internationalized normalized ratio (INR), partial thromboplastin time (PTT) prothrombin time and platelet count, active infection, history of stroke, and change in clinical management. Descriptive statistics were used to analyze data. Median and interquartile range were used to describe results for age and time from admission to head CT performed due to skewed distribution of results.
RESULTS
Of 1714 head CT studies performed on hospitalized medical patients from January 1, 2010 to November 30, 2012, 398 studies were performed for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness in patients who were admitted for >24 hours. One hundred seventy‐eight studies were excluded (137 for admitting diagnosis of intracranial process, recent fall, or head trauma, and 41 for new neurologic deficit). There were 220 scans included in the study performed on 210 patients.
Table 1 displays characteristics of the 210 patients who underwent CT head imaging. Of the 42 patients on anticoagulation, 15 were potentially supratherapeutic; 10 were on warfarin (INR range, 3.37.7) and 5 were on intravenous heparin infusion (PTT range, 101>150 seconds). None of these individuals had positive or equivocal findings on head CT.
| Characteristic | N=210 |
|---|---|
| |
| Age, median (IQR) | 70 (5980) |
| Male, n (%) | 96 (45.7) |
| Race/ethnicity, n (%) | |
| White | 147 (70.0) |
| African American | 44 (21.0) |
| Hispanic | 4 (1.9) |
| Asian | 3 (1.4) |
| Unknown | 9 (4.3) |
| Other | 3 (1.4) |
| Comorbidities, n (%) | |
| Dementia | 30 (14.3) |
| Active cancer | 49 (23.3) |
| Anticoagulation | 42 (20.0) |
| Active infection | 105 (50.0) |
| History of stroke | 41 (19.5) |
| Days from admission to head CT, median (IQR) | 4 (38) |
| Days from delirium onset to head CT, median (IQR) | 2 (14) |
The main outcomes of the 220 included head CT scans and a separate analysis of the 60 head CT scans performed for indications of somnolence or unresponsiveness are shown in Table 2. The 6 (2.7%) positive and 4 (1.8%) equivocal head CT findings are listed in Table 3. Of the 3 positive results in patients on anticoagulation, 2 were on warfarin with an INR of 2.1 and 2.4, respectively, and another was on warfarin and therapeutic enoxaparin (dosed 1 mg/kg twice daily) with an INR of 1.6. The median time from admission to positive head CT was 8 days, with a range of 2 to 28 days. All of the positive head CT studies resulted in change of management. All equivocal head CT studies resulted in repeat imaging. None of these repeat head imaging studies diagnosed acute intracranial pathology. Chronic findings identified included 111 (50.5%) involution or atrophy, 95 (43.2%) small vessel ischemic disease, 31 (14.1%) prior stroke, and 18 (8.2%) other chronic abnormalities (eg, cyst or meningioma).
| Indication | Delirium, N=220 (100%)* | Somnolence or Unresponsiveness, N=60 (27.2%) |
|---|---|---|
| ||
| Outcome | ||
| Positive | 6 (2.7) | 0 |
| Equivocal | 4 (1.8) | 1 (1.6) |
| Chronic | 162 (73.6) | 41 (68.3) |
| Normal | 48 (21.8) | 18 (30.0) |
| CT Head Findings | Age (Sex) | Days From Onset | Change in Management | Outcome | |
|---|---|---|---|---|---|
| |||||
| Positive | |||||
| Case 1 | Subarachnoid hemorrhage in right frontal and temporal lobes | 64 (M) | 2 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 2 | Intraparenchymal hemorrhage with mild shift and vasogenic edema | 62 (M) | 1 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 3 | Subacute subdural hematoma | 62 (M) | 5 | Neurosurgery consult | Discharged with outpatient follow‐up |
| Case 4 | Acute infarct or mass | 73 (F) | 2 | Neurology consult, palliative care consult | Transitioned to comfort‐focused care, discharged |
| Case 5 | 4 mm focus concerning for hemorrhagic metastatic focus | 50 (M) | 3 | Neurosurgery consult, MRI | Discharged with outpatient follow‐up |
| Case 6 | Left occipital lobe parenchymal hemorrhage | 81 (F) | 1 | Neurosurgery consult, neurology consult | Transitioned to comfort‐focused care, died 6 days later |
| Equivocal | |||||
| Case 1 | Several white matter hypodensities of uncertain etiology | 70 (F) | 1 | MRI | MRI with chronic small vessel ischemia |
| Case 2 | Colloid cyst likely although cannot rule out intraventricular hemorrhage | 59 (F) | 1 | Repeat head CT | Repeat imaging with equivocal findings, no additional evaluation |
| Case 3 | Questionable hypodensity, either hemorrhagic contusion or artifact | 52 (M) | 3 | Repeat head CT | Repeat imaging normal |
| Case 4 | Ill‐defined hypodensity in left basal ganglia, no clear acute process | 74 (F) | 0 | MRI | MRI with chronic small vessel ischemia |
DISCUSSION
In this retrospective review, we determined that there is a low diagnostic yield of head CT imaging for identifying the cause of nonresolving or new‐onset delirium in hospitalized medical patients. Only 2.7% of head CT scans resulted in identifying an acute intracranial process. Because of the low number of positive results, no risk factor associations could be made from our study.
The low diagnostic yield of head imaging in hospitalized patients with delirium is particularly important for clinicians who care for hospitalized medical patients. Prior to this study, the yield of head CT scans in hospitalized medical patients with nonresolving or new‐onset delirium was unknown. In cases with known risk factors, such as recent fall, head trauma, or acute neurologic deficit, the guidelines recommend head CT imaging.[18, 19, 20] However, in the absence of these findings, the guidelines do not make any recommendation regarding when and in whom to perform head imaging. Expert statements recommend considering head CT imaging when the cause is not identified after appropriate testing or delirium continues despite treatment.[8, 21] Given these recommendations and lack of data, there is no clear standard of care for ordering head CT imaging when hospitalized patients experience delirium in the absence of known risk factors. The low diagnostic yield in this study suggests that head CT imaging is unlikely to diagnose the cause of delirium in hospitalized patients with nonresolving or new‐onset delirium.
The diagnostic yield of head CT for diagnosis of acute intracranial process in delirium was lower in our study than prior studies, which found between 14.0 and 39.1%.[9, 10, 11, 12, 13, 14, 15, 16] This was expected, as our study excluded patients with new neurologic deficits, recent fall or trauma, or an admitting diagnosis of an intracranial process. Even with these exclusions, we still allowed for a number of findings that prior studies considered to be high risk for intracranial pathology, such as age over 73 years, use of anticoagulation, and deterioration in consciousness level or Glasgow coma score under 14.[10, 11, 16] The inclusion and exclusion criteria were designed to create a generalizable study population without a clear standard of care based on current guidelines and expert statements.
Though the rate of positive findings found in our study is low, it likely overestimates the overall yield of head CT in hospitalized patients with delirium. This is because most hospitalized patients with delirium never receive head imaging. Presumably, ordering clinicians have deemed these patients to be at higher risk for intracranial processes than the average hospitalized patient with delirium who does not receive a head CT. Thus, the true rate of positive findings in head CT imaging in delirious hospitalized medical patients is likely lower than what we identified.
Although head CT had a low diagnostic yield, the positive and equivocal studies had a high impact on clinical care. All of the positive and equivocal head CT results produced a change in management. The equivocal findings led to repeat head imaging; however, none of the repeat images identified the cause of delirium. The positive results produced a more significant change in management, ranging from a higher platelet transfusion target, reversal of anticoagulation, repeat advanced head imaging, neurosurgery consultation, and a change in goals of care to a focus on comfort. No patients in our study underwent neurosurgical intervention.
The challenge for inpatient clinicians is to weigh the low diagnostic yield of head CT with the consequences of a missed or delayed diagnosis of an acute intracranial process. The low diagnostic yield leads to unnecessary cost, resource utilization, radiation exposure, and downstream evaluation of insignificant or indeterminate results when head CT is performed. Alternatively, a missed or delayed diagnosis can lead to potentially reversible morbidity and mortality. Given this, we feel that the routine use of head CT in the evaluation of delirium in hospitalized patients is unnecessary. However, there may be a subset of patients with delirium with an increased risk of acute intracranial processes that would benefit from head imaging. Further research is needed to identify this high‐risk population.
There are a number of limitations to our study. It is a retrospective chart review, which introduces a possibility of bias and relies on proper and thorough documentation. In addition, the diagnosis of delirium was made by individual clinicians without the use of a standardized delirium assessment tool. Furthermore, it is possible there may have been CT scans that were not identified due to mischaracterization of indication, or studies may have been included in individuals with new neurologic deficit or recent fall or trauma that were not documented or clinically appreciated. Finally, the study was conducted on medicine and medical subspecialty patients at a single academic tertiary care institution, potentially limiting the generalizability to patients in other settings.
In conclusion, our study suggests that the diagnostic yield of head CT to evaluate delirium in hospitalized patients in the absence of recent fall, head trauma, or new neurologic deficit is low. The routine use of head CT in evaluation of these individuals is unnecessary. However, there may be a subset of high‐risk individuals in which head CT imaging would be indicated. Further research is needed to identify these high‐risk individuals.
Disclosures
Jesse Theisen‐Toupal, MD, has no conflicts of interest to disclose. Anthony Breu is a contributor to Practical Reviews in Hospital Medicine but has no conflicts of interest. Melissa Mattison, MD, is a contributor to UpToDate and Practical Reviews in Hospital Medicine but has no conflicts of interest. Ramy Arnaout, MD, has no conflicts of interest to disclose.
Delirium is a common and costly problem in hospitalized medical patients. It is present on admission in 10% to 31% of cases and develops in up to 56% of patients during hospitalization.[1, 2] Prompt identification and treatment of the cause of delirium is important, because delirium is associated with increased morbidity and mortality, long‐term cognitive impairment, higher cost of care, increased length of stay, and more frequent discharge to an extended care facility.[3, 4, 5, 6]
Delirium can be caused or worsened by a variety of factors including adverse drug events, metabolic abnormalities, infections, immobilization, the use of tethers (eg, physical restraints, bladder catheters, telemetry), and disruption of sleepwake cycle.[7] An appropriate history, medication review, physical examination, and tailored laboratory evaluation is sufficient workup in the majority of cases.[8] However, neurologic processes, such as intracranial mass, intracranial hemorrhage, or stroke, can also present as delirium and require head imaging for diagnosis.
Because head imaging is a costly limited resource, a number of studies have aimed to determine which patients with delirium require this evaluation. The majority of research has focused on head computed tomography (CT) in patients presenting for evaluation to the emergency department (ED). In ED patients presenting with delirium, acute confusion, or altered mental status, head imaging identifies acute intracranial pathologic findings in 14% to 39% of cases.[9, 10, 11, 12, 13, 14] Only 2 studies have evaluated patients with delirium who have already been admitted to the hospital. One study involved patients admitted to a neurology unit with acute confusion and found that the yield of head imaging (head CT and magnetic resonance imaging) was 14% for acute intracranial pathology.[15] Another study reviewed patients admitted to an acute delirium unit and found a similar rate of positive findings on head CT (14.5%).[16] Neither study specified whether the head imaging occurred during initial presentation or later in the hospitalization.
Factors that increase the likelihood that delirium is caused by acute intracranial pathology include acute neurologic deficit, recent history of fall or head trauma, and significantly impaired consciousness.[9, 10, 11, 12, 13, 14, 15, 16, 17] Based on these findings, current guidelines and expert clinical statements recommend head imaging for patients with acute neurologic deficit, recent head trauma, or recent fall.[18, 19, 20]
Expert clinical statements also recommend imaging in cases where the cause is unidentified after appropriate medical testing or where delirium continues despite treatment.[8, 21] Yet the utility of head CT performed for nonresolving delirium or delirium that develops during hospitalization in the absence of recent fall, head trauma, or new neurologic deficits is not known. Our study aimed to determine the diagnostic yield of performing a head CT in this patient population. We hypothesized that the diagnostic yield of head CT in this population would be low.
METHODS
Study Design
We conducted a retrospective medical record review of hospitalized general medicine patients with head CT imaging performed for the evaluation of delirium. The study was reviewed by the internal review board and determined to be exempt.
Setting and Eligibility Criteria
The study was conducted at a large academic medical center in Boston, Massachusetts. All patients admitted to general medicine, nephrology, hepatology, cardiology, or oncology services with head CT studies performed from January 1, 2010 through November 30, 2012 were included in this retrospective, observational cohort study. Data were extracted using a defined instrument developed for this study with outcome measures predefined. Head CT imaging acquired for patients in the intensive care unit were not included in the review. The medical records were evaluated to determine indication. To be included in the study, the indication for the scan had to be delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness. In addition, the patient must have been admitted for at least 24 hours prior to the completion of the head CT scan. Scans were excluded if there was documentation in the medical record of a fall, head trauma, or new neurologic deficit within the preceding 2 weeks, or an admitting diagnosis of intracranial pathology (eg, stroke or subdural hematoma). If a patient had multiple head CT studies completed for the indication of delirium, each study was included. However, once a head CT study returned positive or equivocal for an acute intracranial process, subsequent head CT studies for the indication of delirium were not included in the analysis.
Outcome Measures
A positive head CT was defined as an intracranial process that could explain delirium (eg, intracranial hemorrhage or stroke). An equivocal head CT was defined as the presence of a finding of unclear significance in relation to delirium (eg, hypodensity of unknown etiology or clinical significance). Chronic head CT findings were noted to be intracranial pathologic findings of a chronic nature that did not meet criteria for either a positive or equivocal image (eg, chronic small vessel ischemic disease or atrophy). A normal study was without positive, equivocal, or chronic findings.
Data Collection and Statistical Analysis
Using the medical center's clinical informatics infrastructure, an experienced clinical informaticist (R.A.) compiled a list of all head CT imaging studies performed during the study period in hospitalized medical patients. An experienced hospital medicine physician (J.T.) conducted the medical record review and determined if each head CT performed met eligibility criteria. For each included study, the following information was collected: date of admission, date of head CT, date of onset of delirium, indication for obtaining head CT scan, head CT results, age, gender, race/ethnicity (patient reported), presence of dementia (if documented in the medical record), active cancer, use of anticoagulants (defined as factor Xa inhibitors, low molecular weight heparin, direct thrombin inhibitor, or vitamin K antagonist) with documentation of internationalized normalized ratio (INR), partial thromboplastin time (PTT) prothrombin time and platelet count, active infection, history of stroke, and change in clinical management. Descriptive statistics were used to analyze data. Median and interquartile range were used to describe results for age and time from admission to head CT performed due to skewed distribution of results.
RESULTS
Of 1714 head CT studies performed on hospitalized medical patients from January 1, 2010 to November 30, 2012, 398 studies were performed for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness in patients who were admitted for >24 hours. One hundred seventy‐eight studies were excluded (137 for admitting diagnosis of intracranial process, recent fall, or head trauma, and 41 for new neurologic deficit). There were 220 scans included in the study performed on 210 patients.
Table 1 displays characteristics of the 210 patients who underwent CT head imaging. Of the 42 patients on anticoagulation, 15 were potentially supratherapeutic; 10 were on warfarin (INR range, 3.37.7) and 5 were on intravenous heparin infusion (PTT range, 101>150 seconds). None of these individuals had positive or equivocal findings on head CT.
| Characteristic | N=210 |
|---|---|
| |
| Age, median (IQR) | 70 (5980) |
| Male, n (%) | 96 (45.7) |
| Race/ethnicity, n (%) | |
| White | 147 (70.0) |
| African American | 44 (21.0) |
| Hispanic | 4 (1.9) |
| Asian | 3 (1.4) |
| Unknown | 9 (4.3) |
| Other | 3 (1.4) |
| Comorbidities, n (%) | |
| Dementia | 30 (14.3) |
| Active cancer | 49 (23.3) |
| Anticoagulation | 42 (20.0) |
| Active infection | 105 (50.0) |
| History of stroke | 41 (19.5) |
| Days from admission to head CT, median (IQR) | 4 (38) |
| Days from delirium onset to head CT, median (IQR) | 2 (14) |
The main outcomes of the 220 included head CT scans and a separate analysis of the 60 head CT scans performed for indications of somnolence or unresponsiveness are shown in Table 2. The 6 (2.7%) positive and 4 (1.8%) equivocal head CT findings are listed in Table 3. Of the 3 positive results in patients on anticoagulation, 2 were on warfarin with an INR of 2.1 and 2.4, respectively, and another was on warfarin and therapeutic enoxaparin (dosed 1 mg/kg twice daily) with an INR of 1.6. The median time from admission to positive head CT was 8 days, with a range of 2 to 28 days. All of the positive head CT studies resulted in change of management. All equivocal head CT studies resulted in repeat imaging. None of these repeat head imaging studies diagnosed acute intracranial pathology. Chronic findings identified included 111 (50.5%) involution or atrophy, 95 (43.2%) small vessel ischemic disease, 31 (14.1%) prior stroke, and 18 (8.2%) other chronic abnormalities (eg, cyst or meningioma).
| Indication | Delirium, N=220 (100%)* | Somnolence or Unresponsiveness, N=60 (27.2%) |
|---|---|---|
| ||
| Outcome | ||
| Positive | 6 (2.7) | 0 |
| Equivocal | 4 (1.8) | 1 (1.6) |
| Chronic | 162 (73.6) | 41 (68.3) |
| Normal | 48 (21.8) | 18 (30.0) |
| CT Head Findings | Age (Sex) | Days From Onset | Change in Management | Outcome | |
|---|---|---|---|---|---|
| |||||
| Positive | |||||
| Case 1 | Subarachnoid hemorrhage in right frontal and temporal lobes | 64 (M) | 2 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 2 | Intraparenchymal hemorrhage with mild shift and vasogenic edema | 62 (M) | 1 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 3 | Subacute subdural hematoma | 62 (M) | 5 | Neurosurgery consult | Discharged with outpatient follow‐up |
| Case 4 | Acute infarct or mass | 73 (F) | 2 | Neurology consult, palliative care consult | Transitioned to comfort‐focused care, discharged |
| Case 5 | 4 mm focus concerning for hemorrhagic metastatic focus | 50 (M) | 3 | Neurosurgery consult, MRI | Discharged with outpatient follow‐up |
| Case 6 | Left occipital lobe parenchymal hemorrhage | 81 (F) | 1 | Neurosurgery consult, neurology consult | Transitioned to comfort‐focused care, died 6 days later |
| Equivocal | |||||
| Case 1 | Several white matter hypodensities of uncertain etiology | 70 (F) | 1 | MRI | MRI with chronic small vessel ischemia |
| Case 2 | Colloid cyst likely although cannot rule out intraventricular hemorrhage | 59 (F) | 1 | Repeat head CT | Repeat imaging with equivocal findings, no additional evaluation |
| Case 3 | Questionable hypodensity, either hemorrhagic contusion or artifact | 52 (M) | 3 | Repeat head CT | Repeat imaging normal |
| Case 4 | Ill‐defined hypodensity in left basal ganglia, no clear acute process | 74 (F) | 0 | MRI | MRI with chronic small vessel ischemia |
DISCUSSION
In this retrospective review, we determined that there is a low diagnostic yield of head CT imaging for identifying the cause of nonresolving or new‐onset delirium in hospitalized medical patients. Only 2.7% of head CT scans resulted in identifying an acute intracranial process. Because of the low number of positive results, no risk factor associations could be made from our study.
The low diagnostic yield of head imaging in hospitalized patients with delirium is particularly important for clinicians who care for hospitalized medical patients. Prior to this study, the yield of head CT scans in hospitalized medical patients with nonresolving or new‐onset delirium was unknown. In cases with known risk factors, such as recent fall, head trauma, or acute neurologic deficit, the guidelines recommend head CT imaging.[18, 19, 20] However, in the absence of these findings, the guidelines do not make any recommendation regarding when and in whom to perform head imaging. Expert statements recommend considering head CT imaging when the cause is not identified after appropriate testing or delirium continues despite treatment.[8, 21] Given these recommendations and lack of data, there is no clear standard of care for ordering head CT imaging when hospitalized patients experience delirium in the absence of known risk factors. The low diagnostic yield in this study suggests that head CT imaging is unlikely to diagnose the cause of delirium in hospitalized patients with nonresolving or new‐onset delirium.
The diagnostic yield of head CT for diagnosis of acute intracranial process in delirium was lower in our study than prior studies, which found between 14.0 and 39.1%.[9, 10, 11, 12, 13, 14, 15, 16] This was expected, as our study excluded patients with new neurologic deficits, recent fall or trauma, or an admitting diagnosis of an intracranial process. Even with these exclusions, we still allowed for a number of findings that prior studies considered to be high risk for intracranial pathology, such as age over 73 years, use of anticoagulation, and deterioration in consciousness level or Glasgow coma score under 14.[10, 11, 16] The inclusion and exclusion criteria were designed to create a generalizable study population without a clear standard of care based on current guidelines and expert statements.
Though the rate of positive findings found in our study is low, it likely overestimates the overall yield of head CT in hospitalized patients with delirium. This is because most hospitalized patients with delirium never receive head imaging. Presumably, ordering clinicians have deemed these patients to be at higher risk for intracranial processes than the average hospitalized patient with delirium who does not receive a head CT. Thus, the true rate of positive findings in head CT imaging in delirious hospitalized medical patients is likely lower than what we identified.
Although head CT had a low diagnostic yield, the positive and equivocal studies had a high impact on clinical care. All of the positive and equivocal head CT results produced a change in management. The equivocal findings led to repeat head imaging; however, none of the repeat images identified the cause of delirium. The positive results produced a more significant change in management, ranging from a higher platelet transfusion target, reversal of anticoagulation, repeat advanced head imaging, neurosurgery consultation, and a change in goals of care to a focus on comfort. No patients in our study underwent neurosurgical intervention.
The challenge for inpatient clinicians is to weigh the low diagnostic yield of head CT with the consequences of a missed or delayed diagnosis of an acute intracranial process. The low diagnostic yield leads to unnecessary cost, resource utilization, radiation exposure, and downstream evaluation of insignificant or indeterminate results when head CT is performed. Alternatively, a missed or delayed diagnosis can lead to potentially reversible morbidity and mortality. Given this, we feel that the routine use of head CT in the evaluation of delirium in hospitalized patients is unnecessary. However, there may be a subset of patients with delirium with an increased risk of acute intracranial processes that would benefit from head imaging. Further research is needed to identify this high‐risk population.
There are a number of limitations to our study. It is a retrospective chart review, which introduces a possibility of bias and relies on proper and thorough documentation. In addition, the diagnosis of delirium was made by individual clinicians without the use of a standardized delirium assessment tool. Furthermore, it is possible there may have been CT scans that were not identified due to mischaracterization of indication, or studies may have been included in individuals with new neurologic deficit or recent fall or trauma that were not documented or clinically appreciated. Finally, the study was conducted on medicine and medical subspecialty patients at a single academic tertiary care institution, potentially limiting the generalizability to patients in other settings.
In conclusion, our study suggests that the diagnostic yield of head CT to evaluate delirium in hospitalized patients in the absence of recent fall, head trauma, or new neurologic deficit is low. The routine use of head CT in evaluation of these individuals is unnecessary. However, there may be a subset of high‐risk individuals in which head CT imaging would be indicated. Further research is needed to identify these high‐risk individuals.
Disclosures
Jesse Theisen‐Toupal, MD, has no conflicts of interest to disclose. Anthony Breu is a contributor to Practical Reviews in Hospital Medicine but has no conflicts of interest. Melissa Mattison, MD, is a contributor to UpToDate and Practical Reviews in Hospital Medicine but has no conflicts of interest. Ramy Arnaout, MD, has no conflicts of interest to disclose.
© 2014 Society of Hospital Medicine
Management of Chronic Conditions
Over the past 2 decades, the care of the hospitalized patient has changed dramatically. Hospitalists now account for the care of more than one‐third of general medicine inpatients, and this number is likely to grow.[1] The emergence of hospital medicine has resulted in a partnership between primary care physicians (PCPs) and hospitalists wherein hospitalists focus on acute medical issues requiring hospitalization, whereas more chronic issues unrelated to the reason for hospitalization remain largely the domain of the PCP.[2, 3]
However, several evolving financial and quality incentives have already begun to blur the distinction between inpatient and outpatient care. First, as private and public payers increasingly scrutinize readmission rates, it has become clear that the responsibility for patient outcomes extends beyond the day of discharge.[4] The birth of Accountable Care Organizations and patient‐centered medical homes may further blur distinctions between what has traditionally constituted inpatient and outpatient care.[5] Bundled payments may force providers to ensure that each visit, whether hospital‐ or clinic‐based, is taken as an opportunity to enact meaningful change.[6] The Centers for Medicare and Medicaid Services (CMS) are already tracking hospital performance on institution of medical therapy for certain conditions regardless of their relatedness to the reason for hospitalization.[7]
No published literature has yet examined the attitudes of inpatient and outpatient providers regarding this issue. Through a case‐based survey conducted at 3 large urban academic medical centers, we aimed to assess opinions among hospitalists and PCPs regarding the role of hospitalists in the management of conditions unrelated to the reason for admission. Our study had 2 main objectives: (1) to determine whether surveyed physicians were more likely to rate an inpatient intervention as appropriate when it related to the reason for admission as compared to interventions unrelated to the reason for admission; and (2) to determine whether these attitudes differed between PCPs and hospitalists.
METHODS
Setting and Subjects
We surveyed hospitalists and hospital‐based PCPs at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women's Hospital, and Massachusetts General Hospital, 3 large academic medical centers in Boston, Massachusetts. Each hospitalist group includes both teaching and nonteaching services and admits patients from both the surveyed hospital‐based PCP groups and other nonhospital‐based PCP groups. All 3 study sites use electronic medical records with patient information for each hospital‐based PCP available to treating hospitalists.
Survey Design
Using a commercially available online product (SurveyMonkey, Palo Alto, CA), we created a 3‐part case‐based survey instrument. The first section included demographic questions regarding age, sex, primary clinical role (hospitalist or PCP), prior experience as a PCP (for hospitalists only) or a hospitalist (for PCPs only; defined as a position with >30% of clinical time as the attending of record in the inpatient setting), years of clinical experience, and hospital affiliation.
The second section aimed to indirectly assess physician opinions on the appropriateness of inpatient management of conditions unrelated to the reason for admission. It consisted of 6 paired case scenarios, each with an inpatient management decision for a hypothetical hospitalist (Table 1). For each pair, 1 case dealt with management of the condition prompting admission (eg, starting aspirin in a patient admitted with acute nonST‐elevation myocardial infarction). The partner case involved the same intervention (eg, starting aspirin) but for a patient with a chronic condition (eg, history of prior myocardial infarction) and an alternate admitting diagnosis (eg, cellulitis). In an attempt to mitigate concerns regarding the flow of information and communication between providers, the survey asked respondents to assume that the hospitalist has access to the patient's outpatient electronic medical record, and that the hospitalist communicates the details of any hospitalizations at the time of discharge. For each case, the physician was asked to rate the appropriateness of enacting the intervention without discussing it with the PCP on a 5‐point scale from very inappropriate to very appropriate. When a physician answered that an intervention was inappropriate or very inappropriate, an additional question soliciting reasons for inappropriateness was included, with multiple predefined answer choices, as well as the option of a free‐text reply under the other designation.
| |
| Starting aspirin (related to the reason for admission) | A 60‐year‐old patient is admitted with a nonST‐elevation MI, medically managed without cardiac catheterization or percutaneous coronary intervention. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting aspirin (unrelated to the reason for admission) | A 60‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed is admitted to the hospital for treatment of cellulitis. The hospitalist notes the patient is not on aspirin at home. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting spironolactone (related to the reason for admission) | A 70‐year‐old patient with a past medical history significant for NYHA class II congestive heart failure (LVEF of 20%) is admitted for acute on chronic, left‐sided systolic congestive heart failure. The patient has been maintained on furosemide, metoprolol, and lisinopril. Admission serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting spironolactone (unrelated to the reason for admission) | A 70‐year‐old patient with a past history of NYHA class II congestive heart failure (LVEF of 20%) on furosemide, metoprolol, and lisinopril is admitted with pneumonia. Serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting warfarin (related to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension and diabetes is admitted with new atrial fibrillation. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Starting warfarin (unrelated to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension, diabetes, and atrial fibrillation is admitted with pneumonia. The patient is not anticoagulation therapy. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Stopping proton pump inhibitor (related to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of Clostridium difficile colitis. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping proton pump inhibitor (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of a urinary tract infection. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping statin or fibrate (related to the reason for admission) | A 60‐year‐old patient with a history of hyperlipidemia is admitted with an elevated creatine kinase to 5000. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent serum LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Stopping statin or fibrate (unrelated to the reason for admission) | A 60‐year‐old patient is admitted with an acute diarrheal illness. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Changing statin (related to the reason for admission) | A 65‐year‐old patient with a past medical history of hyperlipidemia on maximum‐dose simvastatin is admitted with a nonST‐elevation MI. The patient's cholesterol is noted to be above goal. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
| Changing statin (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed and hyperlipidemia on maximum‐dose simvastatin is admitted with pneumonia. Incidentally, the hospitalist notes that the patient's cholesterol has been above goal for the last 2 years. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
The third section aimed to directly assess physicians' opinions. It consisted of questions regarding the appropriateness of inpatient management of conditions related to and unrelated to a patient's reason for admission.
Prior to administration, we conducted focus groups of hospitalists and PCPs to help hypothesize current physician perceptions on inpatient management, assess physician understanding of survey cases and questions, and to evaluate survey length.
Survey Administration
Between October 23, 2012 and November 10, 2012, 3 emails containing a link to the online survey were sent to all hospitalist and hospital‐based PCPs at the 3 study institutions. The BIDMC Committee on Clinical Investigations, to whom authority was ceded by the remaining 2 study institutions, certified this research protocol as exempt.
Statistical Analysis
We hypothesized that respondents as a whole would be more likely to rate an intervention as appropriate or very appropriate if it was related to the reason for admission, compared to unrelated, and that there would be no difference between PCPs and hospitalists.
We used 2 and Fisher exact tests (where applicable) to compare categorical variables, and a nonparametric median test for continuous variables. We used the Fisher exact test to compare the percent of respondents rating each intervention as appropriate or very appropriate by relatedness or unrelatedness to the reason for admission, and by PCP vs hospitalist. To derive the relative risk (RR) of rating each intervention as appropriate or very appropriate by PCPs compared to hospitalists, adjusting for potential confounders including years out of residency and sex, we used multivariable generalized estimating equation models, each with a Poisson distribution error term, a log link, and an exchangeable working correlation structure to account for dependency of observations arising from clustering at either the hospital or participant level, depending on the comparison: for comparisons within a given case, we controlled for clustering at the hospital level; for comparisons of cases in aggregate, owing to multiple responses from each participant, we controlled for clustering at the individual level.
Assuming a 50% response rate from both PCPs and hospitalists, and that 50% of PCPs would rate a given intervention as appropriate, we calculated that we would have 90% power to detect a 50% increase in the proportion of hospitalists rating an intervention as appropriate as compared to PCPs, using an of .05.
RESULTS
Demographics
One hundred sixty‐two out of 295 providers (55%) responded to the survey (Table 2). The response rate did not differ between hospitalists (70 out of 128; 55%) and PCPs (92 out of 167; 55%). Female respondents made up 58.7% of the PCP and 50.0% of the hospitalist groups (P=0.34). On average, PCPs were older (P<0.001) with a greater median number of years since graduation from residency (P<0.001). A greater percentage of hospitalists spent more than three‐quarters of their time clinically (42.9% vs 19.6%, P=0.009).
| Total, n=162 (100.0%) | PCP, n=92 (6.8%) | Hospitalist, n=70 (43.2%) | P Valuea | |
|---|---|---|---|---|
| ||||
| Hospital, n (%) | ||||
| BIDMC | 79 (48.8) | 48 (60.8) | 31 (39.2) | 0.115 |
| BWH | 36 (22.2) | 15 (41.7) | 21 (58.3) | |
| MGH | 47 (29.0) | 29 (61.7) | 18 (38.3) | |
| Sex, n (%) | ||||
| Male | 73 (45.1) | 38 (41.3) | 35 (50.0) | 0.339 |
| Female | 89 (54.9) | 54 (58.7) | 35 (50.0) | |
| Age interval, y, n (%) | ||||
| 2534 | 36 (22.2) | 9 (9.8) | 27 (38.6) | <0.001 |
| 3544 | 67 (41.4) | 34 (37.0) | 33 (47.1) | |
| 4554 | 35 (21.6) | 29 (31.5) | 6 (8.6) | |
| 5564 | 19 (11.7) | 16 (17.4) | 3 (4.3) | |
| 6574 | 5 (3.1) | 4 (4.4) | 1 (1.4) | |
| Years out of residency, median (IQR) | 10 (417) | 15 (74) | 5 (211) | <0.001 |
| Clinical FTE, n (%) | ||||
| 0.25 | 30 (18.6) | 22 (23.9) | 8 (11.4) | 0.009 |
| 0.260.50 | 41 (25.3) | 25 (27.2) | 16 (22.9) | |
| 0.510.75 | 43 (26.5) | 27 (29.4) | 16 (22.9) | |
| >0.75 | 48 (29.6) | 18 (19.6) | 30 (42.9) | |
| Worked as PCP?b | ||||
| Yes | 6 (8.6) | |||
| No | 64 (91.4) | |||
| Worked as hospitalist? | ||||
| Yes | 11 (12.0) | |||
| No | 81 (88.0) | |||
| AOR for admitted patients | ||||
| Always | 16 (17.4) | |||
| Mostly | 8 (8.7) | |||
| Rarely | 7 (7.6) | |||
| Never | 60 (65.2) | |||
Appropriateness of Inpatient Management Based on Admitting Diagnosis
For each of the 6 case pairings individually and in aggregate, respondents were significantly more likely to deem the intervention appropriate or very appropriate if it was related to the reason for admission, compared to those interventions unrelated to the reason for admission (in aggregate, 78.9% vs 38.8% respectively, P<0.001). For example, whereas 96.9% felt that the addition of aspirin in a patient admitted with acute myocardial infarction (MI) was appropriate, only 54.3% felt it appropriate to start aspirin in a patient with a prior history of MI admitted with cellulitis (P<0.001). Significant differences (all P values <0.001) were seen for all case pairs: starting spironolactone (68.1% when related to the reason for reason for admission vs 43.1% when unrelated to reason for admission); starting warfarin (62.3% vs 23.3%), stopping proton pump inhibitor (72.3% vs 42.8%), stopping statin or fibrate (90.6% vs 28.3%), and changing statin (83.0% vs 40.5%).
Appropriateness of Inpatient Management based on Primary Role
Table 3 compares the percent of PCPs and hospitalists rating each intervention as appropriate or very appropriate, by relatedness of the intervention to the reason for admission. In both unadjusted and adjusted comparisons for all cases in aggregate, PCPs were significantly more likely than hospitalists to rate the inpatient interventions as appropriate or very appropriate when the intervention was related to the reason for admission (83.4% of PCP responses vs 73.0% of hospitalist responses, P<0.001; RR: 1.2, 95% confidence interval [CI]: 1.11.3), unrelated to the reason for admission (44.7% vs 31.1%, P<0.001; RR: 1.5, 95% CI: 1.11.9), and overall (64.1% vs 52.1%, P<0.001; RR: 1.3, 95% CI: 1.11.4).
| Relationship to Admission Diagnosis | PCP, n (%) | Hospitalist, n (%) | P Value | Adjusted RR | 95% CI |
|---|---|---|---|---|---|
| |||||
| Related | 453 (83.4) | 303 (73.0) | <0.001 | 1.2a | 1.11.3 |
| Unrelated | 242 (44.7) | 129 (31.1) | <0.001 | 1.5a | 1.11.9 |
| Overall | 695 (64.1) | 432 (52.1) | <0.001 | 1.3b | 1.11.4 |
Reasons for Inappropriate Designation
Among those respondents rating an intervention as inappropriate or very inappropriate, the 3 most common reasons selected as explanation for perceived inappropriateness from our predefined answer choices were: This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible (chosen by physicians in 49.4% of instances); I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (35.7%); and Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications (34.6%). The least common explanation chosen was I do not believe this is an appropriate pharmacologic intervention for this particular medical problem (6.5%). See Table 4 for a complete list of explanations, overall and stratified by PCP/hospitalist.
| Predefined Reason for Inappropriateness | Total, n=583 (%) | PCP, n=318 (%) | Hospitalist, n=265 (%) | P Value |
|---|---|---|---|---|
| ||||
| This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible. | 288 (49.4) | 151 (47.5) | 137 (51.7) | 0.32 |
| I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision. | 208 (35.7) | 98 (30.8) | 110 (41.5) | 0.009 |
| Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications. | 201 (34.5) | 125 (39.3) | 76 (28.7) | 0.009 |
| I am not confident that the hospitalist will adequately review the medical history necessary to make this decision. | 184 (31.6) | 130 (40.9) | 54 (20.4) | <0.001 |
| Even if the hospitalist has all of the medical history, I do not believe hospitalization is the right time to start this new medication | 106 (21.4) | 69 (21.7) | 56 (21.1) | 0.92 |
| I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient. | 106 (18.2) | 85 (26.7) | 21 (7.9) | <0.001 |
| The benefit of this medication will be too remote to justify starting it in the acute setting. | 66 (11.3) | 40 (12.6) | 26 (9.8) | 0.36 |
| I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. | 38 (6.5) | 27 (8.5) | 11 (4.2) | 0.04 |
There were significant differences in the proportion of PCPs and hospitalists choosing several of the prespecified reasons for inappropriateness. Although hospitalists were more likely than PCPs to select I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (chosen by 41.5% of hospitalists vs 30.8% of PCPs, P=0.009), PCPs were more likely than hospitalists to select, I am not confident that the hospitalist will adequately review the medical history necessary to make this decision (chosen by 40.9% of PCPs vs 20.4% of hospitalists, P<0.001) and I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient (26.7% of PCPs vs 9.8% of hospitalists, P<0.001).
Opinions on Current Management of Conditions Related and Unrelated to Admission
A minority of PCPs and hospitalists agreed or strongly agreed that hospitalists should play a larger role in the management of medical conditions unrelated to the reason for admission (28.1% of PCPs vs 34.8% of hospitalists; P=0.39).
DISCUSSION
In this survey‐based study of PCPs and hospitalists across 3 Boston‐area academic medical centers, we found that: (1) physicians were more likely to see inpatient interventions as appropriate when those interventions dealt with the reason for admission as compared to interventions unrelated to the reason for admission; and (2) PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when they targeted chronic conditions unrelated to the reason for admission. To our knowledge, this study represents the first investigation into the attitudes of PCPs and hospitalists regarding the inpatient management of conditions unrelated to the reason for admission.
That surveyed physicians, regardless of role, were less likely to report an intervention unrelated to the reason for hospitalization as appropriateeven those with likely mortality benefitsuggests that opportunities to affect meaningful change may be missed in a healthcare system that adheres to strict inpatient and outpatient roles. For several of the cases, a change in therapy could lead to benefit soon after implementation. For example, aldosterone antagonists reduce mortality as early as 1 month after initiation in select patients.[8] If a major goal of inpatient care is to reduce 30‐day mortality, it could be argued that hospitalists should more actively adjust congestive heart failure therapy in appropriate inpatients, even when this is not their admitting diagnosis.
For some conditions, CMS is already tracking hospital performance. Since 2003, hospitals have been required to document whether a patient with congestive heart failure (either acute or chronic and regardless of the relationship to admission) was prescribed an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the time of discharge.[7] CMS has determined that the proven benefits of ACE inhibitors and ARBs confer hospital accountability for their inclusion in appropriate patients, independent of the acuity of heart failure. There are many potential therapeutic maneuvers on which health systems (and their physicians) may be graded, and accepting the view that a hospitalization provides a window of opportunity for medical optimization may allow for more fruitful interventions and more patient‐centered care.
Despite the potential benefits of addressing chronic medical issues during hospitalization, there are important limitations on what can and/or should be done in the hospital setting. Hospitalizations are a time of fluctuating clinical status, which continues beyond discharge and is often accompanied by several medication changes.[9] In our study, more than 20% of those who believed that a medication intervention was inappropriate selected I do not believe hospitalization is the right time to start this new medication as one of their explanations. Although some medication interventions have been shown in randomized controlled trials to reduce short‐term mortality, the ability to generalize these findings to the average hospitalized patient with multiple comorbidities, concurrent medication changes, and rapidly fluctuating clinical status is limited. Furthermore, there are interventions most would agree should not be dealt with in the hospital (eg, screening colonoscopy) and encounters that may be too short to allow for change (eg, 24‐hour observation). These issues notwithstanding, the average 4‐day hospitalization likely provides an opportunity for monitored change that may currently be underutilized.
Our study suggests several additional explanations for physicians' current practice and opinions. Only 6.5% of respondents who answered that an intervention was inappropriate indicated as a justification that I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. This suggests that the hesitancy has little to do with a lack of benefit but instead relates to systems issues (eg, access to all pertinent records and concerns regarding follow‐up testing) and perceived limitations to what a hospitalist should and should not do without actively involving the PCP. There are likely additional concerns that the medical record and/or patient histories do not fully outline the rationale for exclusion or inclusion of particular medications. Advances in information technology that enhance information exchange and enable streamlined communication may help to address these perceived barriers. However, an additional barrier may be trust, as PCPs appear more concerned that hospitalists will not review all the pertinent records or discuss risks and benefits before enacting important medication changes. Increased attempts at communication between hospitalists and outpatient providers may help to build trust and alleviate concerns regarding the loss of information that often occurs both on admission and at discharge.
We also noted that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when targeting chronic conditions unrelated to the reason for admission. It may be that PCPs, with an increasing number of problems to address per outpatient visit,[10, 11] are more open to hospitalists managing any medical problems during their patients' admissions. At the same time, with increased acuity[12, 13, 14] and shortened length of stays,[15, 16] hospitalists have only a finite amount of time to ensure acute issues are managed, leaving potentially modifiable chronic conditions to the outpatient setting. These differences aside, a minority of both PCPs and hospitalists in our study were ready to embrace the idea of hospitalists playing a larger role in the management of conditions unrelated to the reason for hospitalization.
Even though our study benefits from its multisite design, there are a number of limitations. First, although we crafted our survey with input from general medicine focus groups, our survey instrument has not been validated. In addition, the cases are necessarily contrived and do not take into account the complexities of inpatient medicine. Furthermore, though our goal was to create paired cases that isolate a management decision as being simply based on whether it was related or unrelated to the reason for admission, it is possible that other factors, not captured by our survey, influenced the responses. For example, the benefits of aspirin as part of secondary prevention are not equal to the benefits in an acute MI.[17]
In an attempt to isolate the hospitalists' role in these management decisions, respondents were instructed to assume that the decisions were being made without discussing it with the primary care physician, but that the hospitalist would communicate the details of any hospitalization at the time of discharge. They were also instructed to assume that the hospitalist has access to the patient's outpatient electronic medical record. These assumptions were made to address concerns regarding the flow of information and communication, and to simulate the ideal system from a communication and information accessibility standpoint. Had these assumptions not been placed, the responses may have differed. It is likely that PCPs and hospitalists practicing in systems without shared, accessible inpatient/outpatient medical records would be even more reluctant to enact medication changes unrelated to the reason for admission.
Along the same lines, our physician cohort consisted of several metropolitan academic physician groups, in which hospitalists have had a presence for almost 20 years. As a result, our findings may not be generalizable to other academic hospitals, community‐based hospitalist programs, or nonhospital‐based PCP practices. Finally, we do not know whether survey nonresponders differed from responders in ways that could have meaningfully affected our results.
In conclusion, our findings suggest that both PCPs and hospitalists see the management of conditions unrelated to the reason for admission as less appropriate than the management of conditions related to the reason for admission. Our findings also suggest that PCPs may be more open to this practice when compared to hospitalists. Failure to capitalize on opportunities for meaningful medical interventions, independent of patient location, suggests a possible lack of patient centeredness in the current partnership between PCPs and hospitalists. Further studies should examine existing barriers and investigate interventions designed to address those barriers, in an effort to improve both quality of care and the degree of patient‐centeredness in our current healthcare system.
Disclosures: Dr. Herzig is supported by a grant from the National Institute on Aging (K23 AG042459). Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions: study concept and design, Breu, Allen‐Dicker, Mueller, Herzig; acquisition of data, Breu, Allen‐Dicker, Mueller, Palamara, Herzig; analysis and interpretation of data, Breu, Allen‐Dicker, Hinami, Herzig; drafting of the manuscript, Breu; critical revision of the manuscript for important intellectual content, Breu, Allen‐Dicker, Mueller, Palamara, Hinami, Herzig; statistical analysis, Allen‐Dicker, Hinami, Herzig; study supervision, Breu, Herzig. This study was presented as a poster at the Society of Hospital Medicine National Meeting, Washington, DC, May 17, 2013.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , . A national strategy to put accountable care into practice. Health Aff (Millwood). 2010;29(5):982–990.
- . Keeping score under a global payment system. N Engl J Med. 2012;366(5):393–395.
- Reporting Hospital Quality Data for Annual Payment Update. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/Downloads/HospitalRHQDAPU200808. Accessed December 18, 2013.
- , , , et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- , , , , , . How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461.
- , , . Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169(20):1866–1872.
- , , , The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23(12):2058–2065.
- , , . Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief. 2012;(100):1–8.
- , , . Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7(1):82.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391–395.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
Over the past 2 decades, the care of the hospitalized patient has changed dramatically. Hospitalists now account for the care of more than one‐third of general medicine inpatients, and this number is likely to grow.[1] The emergence of hospital medicine has resulted in a partnership between primary care physicians (PCPs) and hospitalists wherein hospitalists focus on acute medical issues requiring hospitalization, whereas more chronic issues unrelated to the reason for hospitalization remain largely the domain of the PCP.[2, 3]
However, several evolving financial and quality incentives have already begun to blur the distinction between inpatient and outpatient care. First, as private and public payers increasingly scrutinize readmission rates, it has become clear that the responsibility for patient outcomes extends beyond the day of discharge.[4] The birth of Accountable Care Organizations and patient‐centered medical homes may further blur distinctions between what has traditionally constituted inpatient and outpatient care.[5] Bundled payments may force providers to ensure that each visit, whether hospital‐ or clinic‐based, is taken as an opportunity to enact meaningful change.[6] The Centers for Medicare and Medicaid Services (CMS) are already tracking hospital performance on institution of medical therapy for certain conditions regardless of their relatedness to the reason for hospitalization.[7]
No published literature has yet examined the attitudes of inpatient and outpatient providers regarding this issue. Through a case‐based survey conducted at 3 large urban academic medical centers, we aimed to assess opinions among hospitalists and PCPs regarding the role of hospitalists in the management of conditions unrelated to the reason for admission. Our study had 2 main objectives: (1) to determine whether surveyed physicians were more likely to rate an inpatient intervention as appropriate when it related to the reason for admission as compared to interventions unrelated to the reason for admission; and (2) to determine whether these attitudes differed between PCPs and hospitalists.
METHODS
Setting and Subjects
We surveyed hospitalists and hospital‐based PCPs at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women's Hospital, and Massachusetts General Hospital, 3 large academic medical centers in Boston, Massachusetts. Each hospitalist group includes both teaching and nonteaching services and admits patients from both the surveyed hospital‐based PCP groups and other nonhospital‐based PCP groups. All 3 study sites use electronic medical records with patient information for each hospital‐based PCP available to treating hospitalists.
Survey Design
Using a commercially available online product (SurveyMonkey, Palo Alto, CA), we created a 3‐part case‐based survey instrument. The first section included demographic questions regarding age, sex, primary clinical role (hospitalist or PCP), prior experience as a PCP (for hospitalists only) or a hospitalist (for PCPs only; defined as a position with >30% of clinical time as the attending of record in the inpatient setting), years of clinical experience, and hospital affiliation.
The second section aimed to indirectly assess physician opinions on the appropriateness of inpatient management of conditions unrelated to the reason for admission. It consisted of 6 paired case scenarios, each with an inpatient management decision for a hypothetical hospitalist (Table 1). For each pair, 1 case dealt with management of the condition prompting admission (eg, starting aspirin in a patient admitted with acute nonST‐elevation myocardial infarction). The partner case involved the same intervention (eg, starting aspirin) but for a patient with a chronic condition (eg, history of prior myocardial infarction) and an alternate admitting diagnosis (eg, cellulitis). In an attempt to mitigate concerns regarding the flow of information and communication between providers, the survey asked respondents to assume that the hospitalist has access to the patient's outpatient electronic medical record, and that the hospitalist communicates the details of any hospitalizations at the time of discharge. For each case, the physician was asked to rate the appropriateness of enacting the intervention without discussing it with the PCP on a 5‐point scale from very inappropriate to very appropriate. When a physician answered that an intervention was inappropriate or very inappropriate, an additional question soliciting reasons for inappropriateness was included, with multiple predefined answer choices, as well as the option of a free‐text reply under the other designation.
| |
| Starting aspirin (related to the reason for admission) | A 60‐year‐old patient is admitted with a nonST‐elevation MI, medically managed without cardiac catheterization or percutaneous coronary intervention. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting aspirin (unrelated to the reason for admission) | A 60‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed is admitted to the hospital for treatment of cellulitis. The hospitalist notes the patient is not on aspirin at home. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting spironolactone (related to the reason for admission) | A 70‐year‐old patient with a past medical history significant for NYHA class II congestive heart failure (LVEF of 20%) is admitted for acute on chronic, left‐sided systolic congestive heart failure. The patient has been maintained on furosemide, metoprolol, and lisinopril. Admission serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting spironolactone (unrelated to the reason for admission) | A 70‐year‐old patient with a past history of NYHA class II congestive heart failure (LVEF of 20%) on furosemide, metoprolol, and lisinopril is admitted with pneumonia. Serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting warfarin (related to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension and diabetes is admitted with new atrial fibrillation. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Starting warfarin (unrelated to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension, diabetes, and atrial fibrillation is admitted with pneumonia. The patient is not anticoagulation therapy. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Stopping proton pump inhibitor (related to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of Clostridium difficile colitis. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping proton pump inhibitor (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of a urinary tract infection. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping statin or fibrate (related to the reason for admission) | A 60‐year‐old patient with a history of hyperlipidemia is admitted with an elevated creatine kinase to 5000. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent serum LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Stopping statin or fibrate (unrelated to the reason for admission) | A 60‐year‐old patient is admitted with an acute diarrheal illness. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Changing statin (related to the reason for admission) | A 65‐year‐old patient with a past medical history of hyperlipidemia on maximum‐dose simvastatin is admitted with a nonST‐elevation MI. The patient's cholesterol is noted to be above goal. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
| Changing statin (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed and hyperlipidemia on maximum‐dose simvastatin is admitted with pneumonia. Incidentally, the hospitalist notes that the patient's cholesterol has been above goal for the last 2 years. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
The third section aimed to directly assess physicians' opinions. It consisted of questions regarding the appropriateness of inpatient management of conditions related to and unrelated to a patient's reason for admission.
Prior to administration, we conducted focus groups of hospitalists and PCPs to help hypothesize current physician perceptions on inpatient management, assess physician understanding of survey cases and questions, and to evaluate survey length.
Survey Administration
Between October 23, 2012 and November 10, 2012, 3 emails containing a link to the online survey were sent to all hospitalist and hospital‐based PCPs at the 3 study institutions. The BIDMC Committee on Clinical Investigations, to whom authority was ceded by the remaining 2 study institutions, certified this research protocol as exempt.
Statistical Analysis
We hypothesized that respondents as a whole would be more likely to rate an intervention as appropriate or very appropriate if it was related to the reason for admission, compared to unrelated, and that there would be no difference between PCPs and hospitalists.
We used 2 and Fisher exact tests (where applicable) to compare categorical variables, and a nonparametric median test for continuous variables. We used the Fisher exact test to compare the percent of respondents rating each intervention as appropriate or very appropriate by relatedness or unrelatedness to the reason for admission, and by PCP vs hospitalist. To derive the relative risk (RR) of rating each intervention as appropriate or very appropriate by PCPs compared to hospitalists, adjusting for potential confounders including years out of residency and sex, we used multivariable generalized estimating equation models, each with a Poisson distribution error term, a log link, and an exchangeable working correlation structure to account for dependency of observations arising from clustering at either the hospital or participant level, depending on the comparison: for comparisons within a given case, we controlled for clustering at the hospital level; for comparisons of cases in aggregate, owing to multiple responses from each participant, we controlled for clustering at the individual level.
Assuming a 50% response rate from both PCPs and hospitalists, and that 50% of PCPs would rate a given intervention as appropriate, we calculated that we would have 90% power to detect a 50% increase in the proportion of hospitalists rating an intervention as appropriate as compared to PCPs, using an of .05.
RESULTS
Demographics
One hundred sixty‐two out of 295 providers (55%) responded to the survey (Table 2). The response rate did not differ between hospitalists (70 out of 128; 55%) and PCPs (92 out of 167; 55%). Female respondents made up 58.7% of the PCP and 50.0% of the hospitalist groups (P=0.34). On average, PCPs were older (P<0.001) with a greater median number of years since graduation from residency (P<0.001). A greater percentage of hospitalists spent more than three‐quarters of their time clinically (42.9% vs 19.6%, P=0.009).
| Total, n=162 (100.0%) | PCP, n=92 (6.8%) | Hospitalist, n=70 (43.2%) | P Valuea | |
|---|---|---|---|---|
| ||||
| Hospital, n (%) | ||||
| BIDMC | 79 (48.8) | 48 (60.8) | 31 (39.2) | 0.115 |
| BWH | 36 (22.2) | 15 (41.7) | 21 (58.3) | |
| MGH | 47 (29.0) | 29 (61.7) | 18 (38.3) | |
| Sex, n (%) | ||||
| Male | 73 (45.1) | 38 (41.3) | 35 (50.0) | 0.339 |
| Female | 89 (54.9) | 54 (58.7) | 35 (50.0) | |
| Age interval, y, n (%) | ||||
| 2534 | 36 (22.2) | 9 (9.8) | 27 (38.6) | <0.001 |
| 3544 | 67 (41.4) | 34 (37.0) | 33 (47.1) | |
| 4554 | 35 (21.6) | 29 (31.5) | 6 (8.6) | |
| 5564 | 19 (11.7) | 16 (17.4) | 3 (4.3) | |
| 6574 | 5 (3.1) | 4 (4.4) | 1 (1.4) | |
| Years out of residency, median (IQR) | 10 (417) | 15 (74) | 5 (211) | <0.001 |
| Clinical FTE, n (%) | ||||
| 0.25 | 30 (18.6) | 22 (23.9) | 8 (11.4) | 0.009 |
| 0.260.50 | 41 (25.3) | 25 (27.2) | 16 (22.9) | |
| 0.510.75 | 43 (26.5) | 27 (29.4) | 16 (22.9) | |
| >0.75 | 48 (29.6) | 18 (19.6) | 30 (42.9) | |
| Worked as PCP?b | ||||
| Yes | 6 (8.6) | |||
| No | 64 (91.4) | |||
| Worked as hospitalist? | ||||
| Yes | 11 (12.0) | |||
| No | 81 (88.0) | |||
| AOR for admitted patients | ||||
| Always | 16 (17.4) | |||
| Mostly | 8 (8.7) | |||
| Rarely | 7 (7.6) | |||
| Never | 60 (65.2) | |||
Appropriateness of Inpatient Management Based on Admitting Diagnosis
For each of the 6 case pairings individually and in aggregate, respondents were significantly more likely to deem the intervention appropriate or very appropriate if it was related to the reason for admission, compared to those interventions unrelated to the reason for admission (in aggregate, 78.9% vs 38.8% respectively, P<0.001). For example, whereas 96.9% felt that the addition of aspirin in a patient admitted with acute myocardial infarction (MI) was appropriate, only 54.3% felt it appropriate to start aspirin in a patient with a prior history of MI admitted with cellulitis (P<0.001). Significant differences (all P values <0.001) were seen for all case pairs: starting spironolactone (68.1% when related to the reason for reason for admission vs 43.1% when unrelated to reason for admission); starting warfarin (62.3% vs 23.3%), stopping proton pump inhibitor (72.3% vs 42.8%), stopping statin or fibrate (90.6% vs 28.3%), and changing statin (83.0% vs 40.5%).
Appropriateness of Inpatient Management based on Primary Role
Table 3 compares the percent of PCPs and hospitalists rating each intervention as appropriate or very appropriate, by relatedness of the intervention to the reason for admission. In both unadjusted and adjusted comparisons for all cases in aggregate, PCPs were significantly more likely than hospitalists to rate the inpatient interventions as appropriate or very appropriate when the intervention was related to the reason for admission (83.4% of PCP responses vs 73.0% of hospitalist responses, P<0.001; RR: 1.2, 95% confidence interval [CI]: 1.11.3), unrelated to the reason for admission (44.7% vs 31.1%, P<0.001; RR: 1.5, 95% CI: 1.11.9), and overall (64.1% vs 52.1%, P<0.001; RR: 1.3, 95% CI: 1.11.4).
| Relationship to Admission Diagnosis | PCP, n (%) | Hospitalist, n (%) | P Value | Adjusted RR | 95% CI |
|---|---|---|---|---|---|
| |||||
| Related | 453 (83.4) | 303 (73.0) | <0.001 | 1.2a | 1.11.3 |
| Unrelated | 242 (44.7) | 129 (31.1) | <0.001 | 1.5a | 1.11.9 |
| Overall | 695 (64.1) | 432 (52.1) | <0.001 | 1.3b | 1.11.4 |
Reasons for Inappropriate Designation
Among those respondents rating an intervention as inappropriate or very inappropriate, the 3 most common reasons selected as explanation for perceived inappropriateness from our predefined answer choices were: This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible (chosen by physicians in 49.4% of instances); I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (35.7%); and Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications (34.6%). The least common explanation chosen was I do not believe this is an appropriate pharmacologic intervention for this particular medical problem (6.5%). See Table 4 for a complete list of explanations, overall and stratified by PCP/hospitalist.
| Predefined Reason for Inappropriateness | Total, n=583 (%) | PCP, n=318 (%) | Hospitalist, n=265 (%) | P Value |
|---|---|---|---|---|
| ||||
| This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible. | 288 (49.4) | 151 (47.5) | 137 (51.7) | 0.32 |
| I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision. | 208 (35.7) | 98 (30.8) | 110 (41.5) | 0.009 |
| Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications. | 201 (34.5) | 125 (39.3) | 76 (28.7) | 0.009 |
| I am not confident that the hospitalist will adequately review the medical history necessary to make this decision. | 184 (31.6) | 130 (40.9) | 54 (20.4) | <0.001 |
| Even if the hospitalist has all of the medical history, I do not believe hospitalization is the right time to start this new medication | 106 (21.4) | 69 (21.7) | 56 (21.1) | 0.92 |
| I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient. | 106 (18.2) | 85 (26.7) | 21 (7.9) | <0.001 |
| The benefit of this medication will be too remote to justify starting it in the acute setting. | 66 (11.3) | 40 (12.6) | 26 (9.8) | 0.36 |
| I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. | 38 (6.5) | 27 (8.5) | 11 (4.2) | 0.04 |
There were significant differences in the proportion of PCPs and hospitalists choosing several of the prespecified reasons for inappropriateness. Although hospitalists were more likely than PCPs to select I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (chosen by 41.5% of hospitalists vs 30.8% of PCPs, P=0.009), PCPs were more likely than hospitalists to select, I am not confident that the hospitalist will adequately review the medical history necessary to make this decision (chosen by 40.9% of PCPs vs 20.4% of hospitalists, P<0.001) and I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient (26.7% of PCPs vs 9.8% of hospitalists, P<0.001).
Opinions on Current Management of Conditions Related and Unrelated to Admission
A minority of PCPs and hospitalists agreed or strongly agreed that hospitalists should play a larger role in the management of medical conditions unrelated to the reason for admission (28.1% of PCPs vs 34.8% of hospitalists; P=0.39).
DISCUSSION
In this survey‐based study of PCPs and hospitalists across 3 Boston‐area academic medical centers, we found that: (1) physicians were more likely to see inpatient interventions as appropriate when those interventions dealt with the reason for admission as compared to interventions unrelated to the reason for admission; and (2) PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when they targeted chronic conditions unrelated to the reason for admission. To our knowledge, this study represents the first investigation into the attitudes of PCPs and hospitalists regarding the inpatient management of conditions unrelated to the reason for admission.
That surveyed physicians, regardless of role, were less likely to report an intervention unrelated to the reason for hospitalization as appropriateeven those with likely mortality benefitsuggests that opportunities to affect meaningful change may be missed in a healthcare system that adheres to strict inpatient and outpatient roles. For several of the cases, a change in therapy could lead to benefit soon after implementation. For example, aldosterone antagonists reduce mortality as early as 1 month after initiation in select patients.[8] If a major goal of inpatient care is to reduce 30‐day mortality, it could be argued that hospitalists should more actively adjust congestive heart failure therapy in appropriate inpatients, even when this is not their admitting diagnosis.
For some conditions, CMS is already tracking hospital performance. Since 2003, hospitals have been required to document whether a patient with congestive heart failure (either acute or chronic and regardless of the relationship to admission) was prescribed an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the time of discharge.[7] CMS has determined that the proven benefits of ACE inhibitors and ARBs confer hospital accountability for their inclusion in appropriate patients, independent of the acuity of heart failure. There are many potential therapeutic maneuvers on which health systems (and their physicians) may be graded, and accepting the view that a hospitalization provides a window of opportunity for medical optimization may allow for more fruitful interventions and more patient‐centered care.
Despite the potential benefits of addressing chronic medical issues during hospitalization, there are important limitations on what can and/or should be done in the hospital setting. Hospitalizations are a time of fluctuating clinical status, which continues beyond discharge and is often accompanied by several medication changes.[9] In our study, more than 20% of those who believed that a medication intervention was inappropriate selected I do not believe hospitalization is the right time to start this new medication as one of their explanations. Although some medication interventions have been shown in randomized controlled trials to reduce short‐term mortality, the ability to generalize these findings to the average hospitalized patient with multiple comorbidities, concurrent medication changes, and rapidly fluctuating clinical status is limited. Furthermore, there are interventions most would agree should not be dealt with in the hospital (eg, screening colonoscopy) and encounters that may be too short to allow for change (eg, 24‐hour observation). These issues notwithstanding, the average 4‐day hospitalization likely provides an opportunity for monitored change that may currently be underutilized.
Our study suggests several additional explanations for physicians' current practice and opinions. Only 6.5% of respondents who answered that an intervention was inappropriate indicated as a justification that I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. This suggests that the hesitancy has little to do with a lack of benefit but instead relates to systems issues (eg, access to all pertinent records and concerns regarding follow‐up testing) and perceived limitations to what a hospitalist should and should not do without actively involving the PCP. There are likely additional concerns that the medical record and/or patient histories do not fully outline the rationale for exclusion or inclusion of particular medications. Advances in information technology that enhance information exchange and enable streamlined communication may help to address these perceived barriers. However, an additional barrier may be trust, as PCPs appear more concerned that hospitalists will not review all the pertinent records or discuss risks and benefits before enacting important medication changes. Increased attempts at communication between hospitalists and outpatient providers may help to build trust and alleviate concerns regarding the loss of information that often occurs both on admission and at discharge.
We also noted that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when targeting chronic conditions unrelated to the reason for admission. It may be that PCPs, with an increasing number of problems to address per outpatient visit,[10, 11] are more open to hospitalists managing any medical problems during their patients' admissions. At the same time, with increased acuity[12, 13, 14] and shortened length of stays,[15, 16] hospitalists have only a finite amount of time to ensure acute issues are managed, leaving potentially modifiable chronic conditions to the outpatient setting. These differences aside, a minority of both PCPs and hospitalists in our study were ready to embrace the idea of hospitalists playing a larger role in the management of conditions unrelated to the reason for hospitalization.
Even though our study benefits from its multisite design, there are a number of limitations. First, although we crafted our survey with input from general medicine focus groups, our survey instrument has not been validated. In addition, the cases are necessarily contrived and do not take into account the complexities of inpatient medicine. Furthermore, though our goal was to create paired cases that isolate a management decision as being simply based on whether it was related or unrelated to the reason for admission, it is possible that other factors, not captured by our survey, influenced the responses. For example, the benefits of aspirin as part of secondary prevention are not equal to the benefits in an acute MI.[17]
In an attempt to isolate the hospitalists' role in these management decisions, respondents were instructed to assume that the decisions were being made without discussing it with the primary care physician, but that the hospitalist would communicate the details of any hospitalization at the time of discharge. They were also instructed to assume that the hospitalist has access to the patient's outpatient electronic medical record. These assumptions were made to address concerns regarding the flow of information and communication, and to simulate the ideal system from a communication and information accessibility standpoint. Had these assumptions not been placed, the responses may have differed. It is likely that PCPs and hospitalists practicing in systems without shared, accessible inpatient/outpatient medical records would be even more reluctant to enact medication changes unrelated to the reason for admission.
Along the same lines, our physician cohort consisted of several metropolitan academic physician groups, in which hospitalists have had a presence for almost 20 years. As a result, our findings may not be generalizable to other academic hospitals, community‐based hospitalist programs, or nonhospital‐based PCP practices. Finally, we do not know whether survey nonresponders differed from responders in ways that could have meaningfully affected our results.
In conclusion, our findings suggest that both PCPs and hospitalists see the management of conditions unrelated to the reason for admission as less appropriate than the management of conditions related to the reason for admission. Our findings also suggest that PCPs may be more open to this practice when compared to hospitalists. Failure to capitalize on opportunities for meaningful medical interventions, independent of patient location, suggests a possible lack of patient centeredness in the current partnership between PCPs and hospitalists. Further studies should examine existing barriers and investigate interventions designed to address those barriers, in an effort to improve both quality of care and the degree of patient‐centeredness in our current healthcare system.
Disclosures: Dr. Herzig is supported by a grant from the National Institute on Aging (K23 AG042459). Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions: study concept and design, Breu, Allen‐Dicker, Mueller, Herzig; acquisition of data, Breu, Allen‐Dicker, Mueller, Palamara, Herzig; analysis and interpretation of data, Breu, Allen‐Dicker, Hinami, Herzig; drafting of the manuscript, Breu; critical revision of the manuscript for important intellectual content, Breu, Allen‐Dicker, Mueller, Palamara, Hinami, Herzig; statistical analysis, Allen‐Dicker, Hinami, Herzig; study supervision, Breu, Herzig. This study was presented as a poster at the Society of Hospital Medicine National Meeting, Washington, DC, May 17, 2013.
Over the past 2 decades, the care of the hospitalized patient has changed dramatically. Hospitalists now account for the care of more than one‐third of general medicine inpatients, and this number is likely to grow.[1] The emergence of hospital medicine has resulted in a partnership between primary care physicians (PCPs) and hospitalists wherein hospitalists focus on acute medical issues requiring hospitalization, whereas more chronic issues unrelated to the reason for hospitalization remain largely the domain of the PCP.[2, 3]
However, several evolving financial and quality incentives have already begun to blur the distinction between inpatient and outpatient care. First, as private and public payers increasingly scrutinize readmission rates, it has become clear that the responsibility for patient outcomes extends beyond the day of discharge.[4] The birth of Accountable Care Organizations and patient‐centered medical homes may further blur distinctions between what has traditionally constituted inpatient and outpatient care.[5] Bundled payments may force providers to ensure that each visit, whether hospital‐ or clinic‐based, is taken as an opportunity to enact meaningful change.[6] The Centers for Medicare and Medicaid Services (CMS) are already tracking hospital performance on institution of medical therapy for certain conditions regardless of their relatedness to the reason for hospitalization.[7]
No published literature has yet examined the attitudes of inpatient and outpatient providers regarding this issue. Through a case‐based survey conducted at 3 large urban academic medical centers, we aimed to assess opinions among hospitalists and PCPs regarding the role of hospitalists in the management of conditions unrelated to the reason for admission. Our study had 2 main objectives: (1) to determine whether surveyed physicians were more likely to rate an inpatient intervention as appropriate when it related to the reason for admission as compared to interventions unrelated to the reason for admission; and (2) to determine whether these attitudes differed between PCPs and hospitalists.
METHODS
Setting and Subjects
We surveyed hospitalists and hospital‐based PCPs at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women's Hospital, and Massachusetts General Hospital, 3 large academic medical centers in Boston, Massachusetts. Each hospitalist group includes both teaching and nonteaching services and admits patients from both the surveyed hospital‐based PCP groups and other nonhospital‐based PCP groups. All 3 study sites use electronic medical records with patient information for each hospital‐based PCP available to treating hospitalists.
Survey Design
Using a commercially available online product (SurveyMonkey, Palo Alto, CA), we created a 3‐part case‐based survey instrument. The first section included demographic questions regarding age, sex, primary clinical role (hospitalist or PCP), prior experience as a PCP (for hospitalists only) or a hospitalist (for PCPs only; defined as a position with >30% of clinical time as the attending of record in the inpatient setting), years of clinical experience, and hospital affiliation.
The second section aimed to indirectly assess physician opinions on the appropriateness of inpatient management of conditions unrelated to the reason for admission. It consisted of 6 paired case scenarios, each with an inpatient management decision for a hypothetical hospitalist (Table 1). For each pair, 1 case dealt with management of the condition prompting admission (eg, starting aspirin in a patient admitted with acute nonST‐elevation myocardial infarction). The partner case involved the same intervention (eg, starting aspirin) but for a patient with a chronic condition (eg, history of prior myocardial infarction) and an alternate admitting diagnosis (eg, cellulitis). In an attempt to mitigate concerns regarding the flow of information and communication between providers, the survey asked respondents to assume that the hospitalist has access to the patient's outpatient electronic medical record, and that the hospitalist communicates the details of any hospitalizations at the time of discharge. For each case, the physician was asked to rate the appropriateness of enacting the intervention without discussing it with the PCP on a 5‐point scale from very inappropriate to very appropriate. When a physician answered that an intervention was inappropriate or very inappropriate, an additional question soliciting reasons for inappropriateness was included, with multiple predefined answer choices, as well as the option of a free‐text reply under the other designation.
| |
| Starting aspirin (related to the reason for admission) | A 60‐year‐old patient is admitted with a nonST‐elevation MI, medically managed without cardiac catheterization or percutaneous coronary intervention. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting aspirin (unrelated to the reason for admission) | A 60‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed is admitted to the hospital for treatment of cellulitis. The hospitalist notes the patient is not on aspirin at home. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting spironolactone (related to the reason for admission) | A 70‐year‐old patient with a past medical history significant for NYHA class II congestive heart failure (LVEF of 20%) is admitted for acute on chronic, left‐sided systolic congestive heart failure. The patient has been maintained on furosemide, metoprolol, and lisinopril. Admission serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting spironolactone (unrelated to the reason for admission) | A 70‐year‐old patient with a past history of NYHA class II congestive heart failure (LVEF of 20%) on furosemide, metoprolol, and lisinopril is admitted with pneumonia. Serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting warfarin (related to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension and diabetes is admitted with new atrial fibrillation. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Starting warfarin (unrelated to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension, diabetes, and atrial fibrillation is admitted with pneumonia. The patient is not anticoagulation therapy. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Stopping proton pump inhibitor (related to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of Clostridium difficile colitis. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping proton pump inhibitor (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of a urinary tract infection. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping statin or fibrate (related to the reason for admission) | A 60‐year‐old patient with a history of hyperlipidemia is admitted with an elevated creatine kinase to 5000. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent serum LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Stopping statin or fibrate (unrelated to the reason for admission) | A 60‐year‐old patient is admitted with an acute diarrheal illness. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Changing statin (related to the reason for admission) | A 65‐year‐old patient with a past medical history of hyperlipidemia on maximum‐dose simvastatin is admitted with a nonST‐elevation MI. The patient's cholesterol is noted to be above goal. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
| Changing statin (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed and hyperlipidemia on maximum‐dose simvastatin is admitted with pneumonia. Incidentally, the hospitalist notes that the patient's cholesterol has been above goal for the last 2 years. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
The third section aimed to directly assess physicians' opinions. It consisted of questions regarding the appropriateness of inpatient management of conditions related to and unrelated to a patient's reason for admission.
Prior to administration, we conducted focus groups of hospitalists and PCPs to help hypothesize current physician perceptions on inpatient management, assess physician understanding of survey cases and questions, and to evaluate survey length.
Survey Administration
Between October 23, 2012 and November 10, 2012, 3 emails containing a link to the online survey were sent to all hospitalist and hospital‐based PCPs at the 3 study institutions. The BIDMC Committee on Clinical Investigations, to whom authority was ceded by the remaining 2 study institutions, certified this research protocol as exempt.
Statistical Analysis
We hypothesized that respondents as a whole would be more likely to rate an intervention as appropriate or very appropriate if it was related to the reason for admission, compared to unrelated, and that there would be no difference between PCPs and hospitalists.
We used 2 and Fisher exact tests (where applicable) to compare categorical variables, and a nonparametric median test for continuous variables. We used the Fisher exact test to compare the percent of respondents rating each intervention as appropriate or very appropriate by relatedness or unrelatedness to the reason for admission, and by PCP vs hospitalist. To derive the relative risk (RR) of rating each intervention as appropriate or very appropriate by PCPs compared to hospitalists, adjusting for potential confounders including years out of residency and sex, we used multivariable generalized estimating equation models, each with a Poisson distribution error term, a log link, and an exchangeable working correlation structure to account for dependency of observations arising from clustering at either the hospital or participant level, depending on the comparison: for comparisons within a given case, we controlled for clustering at the hospital level; for comparisons of cases in aggregate, owing to multiple responses from each participant, we controlled for clustering at the individual level.
Assuming a 50% response rate from both PCPs and hospitalists, and that 50% of PCPs would rate a given intervention as appropriate, we calculated that we would have 90% power to detect a 50% increase in the proportion of hospitalists rating an intervention as appropriate as compared to PCPs, using an of .05.
RESULTS
Demographics
One hundred sixty‐two out of 295 providers (55%) responded to the survey (Table 2). The response rate did not differ between hospitalists (70 out of 128; 55%) and PCPs (92 out of 167; 55%). Female respondents made up 58.7% of the PCP and 50.0% of the hospitalist groups (P=0.34). On average, PCPs were older (P<0.001) with a greater median number of years since graduation from residency (P<0.001). A greater percentage of hospitalists spent more than three‐quarters of their time clinically (42.9% vs 19.6%, P=0.009).
| Total, n=162 (100.0%) | PCP, n=92 (6.8%) | Hospitalist, n=70 (43.2%) | P Valuea | |
|---|---|---|---|---|
| ||||
| Hospital, n (%) | ||||
| BIDMC | 79 (48.8) | 48 (60.8) | 31 (39.2) | 0.115 |
| BWH | 36 (22.2) | 15 (41.7) | 21 (58.3) | |
| MGH | 47 (29.0) | 29 (61.7) | 18 (38.3) | |
| Sex, n (%) | ||||
| Male | 73 (45.1) | 38 (41.3) | 35 (50.0) | 0.339 |
| Female | 89 (54.9) | 54 (58.7) | 35 (50.0) | |
| Age interval, y, n (%) | ||||
| 2534 | 36 (22.2) | 9 (9.8) | 27 (38.6) | <0.001 |
| 3544 | 67 (41.4) | 34 (37.0) | 33 (47.1) | |
| 4554 | 35 (21.6) | 29 (31.5) | 6 (8.6) | |
| 5564 | 19 (11.7) | 16 (17.4) | 3 (4.3) | |
| 6574 | 5 (3.1) | 4 (4.4) | 1 (1.4) | |
| Years out of residency, median (IQR) | 10 (417) | 15 (74) | 5 (211) | <0.001 |
| Clinical FTE, n (%) | ||||
| 0.25 | 30 (18.6) | 22 (23.9) | 8 (11.4) | 0.009 |
| 0.260.50 | 41 (25.3) | 25 (27.2) | 16 (22.9) | |
| 0.510.75 | 43 (26.5) | 27 (29.4) | 16 (22.9) | |
| >0.75 | 48 (29.6) | 18 (19.6) | 30 (42.9) | |
| Worked as PCP?b | ||||
| Yes | 6 (8.6) | |||
| No | 64 (91.4) | |||
| Worked as hospitalist? | ||||
| Yes | 11 (12.0) | |||
| No | 81 (88.0) | |||
| AOR for admitted patients | ||||
| Always | 16 (17.4) | |||
| Mostly | 8 (8.7) | |||
| Rarely | 7 (7.6) | |||
| Never | 60 (65.2) | |||
Appropriateness of Inpatient Management Based on Admitting Diagnosis
For each of the 6 case pairings individually and in aggregate, respondents were significantly more likely to deem the intervention appropriate or very appropriate if it was related to the reason for admission, compared to those interventions unrelated to the reason for admission (in aggregate, 78.9% vs 38.8% respectively, P<0.001). For example, whereas 96.9% felt that the addition of aspirin in a patient admitted with acute myocardial infarction (MI) was appropriate, only 54.3% felt it appropriate to start aspirin in a patient with a prior history of MI admitted with cellulitis (P<0.001). Significant differences (all P values <0.001) were seen for all case pairs: starting spironolactone (68.1% when related to the reason for reason for admission vs 43.1% when unrelated to reason for admission); starting warfarin (62.3% vs 23.3%), stopping proton pump inhibitor (72.3% vs 42.8%), stopping statin or fibrate (90.6% vs 28.3%), and changing statin (83.0% vs 40.5%).
Appropriateness of Inpatient Management based on Primary Role
Table 3 compares the percent of PCPs and hospitalists rating each intervention as appropriate or very appropriate, by relatedness of the intervention to the reason for admission. In both unadjusted and adjusted comparisons for all cases in aggregate, PCPs were significantly more likely than hospitalists to rate the inpatient interventions as appropriate or very appropriate when the intervention was related to the reason for admission (83.4% of PCP responses vs 73.0% of hospitalist responses, P<0.001; RR: 1.2, 95% confidence interval [CI]: 1.11.3), unrelated to the reason for admission (44.7% vs 31.1%, P<0.001; RR: 1.5, 95% CI: 1.11.9), and overall (64.1% vs 52.1%, P<0.001; RR: 1.3, 95% CI: 1.11.4).
| Relationship to Admission Diagnosis | PCP, n (%) | Hospitalist, n (%) | P Value | Adjusted RR | 95% CI |
|---|---|---|---|---|---|
| |||||
| Related | 453 (83.4) | 303 (73.0) | <0.001 | 1.2a | 1.11.3 |
| Unrelated | 242 (44.7) | 129 (31.1) | <0.001 | 1.5a | 1.11.9 |
| Overall | 695 (64.1) | 432 (52.1) | <0.001 | 1.3b | 1.11.4 |
Reasons for Inappropriate Designation
Among those respondents rating an intervention as inappropriate or very inappropriate, the 3 most common reasons selected as explanation for perceived inappropriateness from our predefined answer choices were: This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible (chosen by physicians in 49.4% of instances); I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (35.7%); and Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications (34.6%). The least common explanation chosen was I do not believe this is an appropriate pharmacologic intervention for this particular medical problem (6.5%). See Table 4 for a complete list of explanations, overall and stratified by PCP/hospitalist.
| Predefined Reason for Inappropriateness | Total, n=583 (%) | PCP, n=318 (%) | Hospitalist, n=265 (%) | P Value |
|---|---|---|---|---|
| ||||
| This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible. | 288 (49.4) | 151 (47.5) | 137 (51.7) | 0.32 |
| I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision. | 208 (35.7) | 98 (30.8) | 110 (41.5) | 0.009 |
| Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications. | 201 (34.5) | 125 (39.3) | 76 (28.7) | 0.009 |
| I am not confident that the hospitalist will adequately review the medical history necessary to make this decision. | 184 (31.6) | 130 (40.9) | 54 (20.4) | <0.001 |
| Even if the hospitalist has all of the medical history, I do not believe hospitalization is the right time to start this new medication | 106 (21.4) | 69 (21.7) | 56 (21.1) | 0.92 |
| I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient. | 106 (18.2) | 85 (26.7) | 21 (7.9) | <0.001 |
| The benefit of this medication will be too remote to justify starting it in the acute setting. | 66 (11.3) | 40 (12.6) | 26 (9.8) | 0.36 |
| I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. | 38 (6.5) | 27 (8.5) | 11 (4.2) | 0.04 |
There were significant differences in the proportion of PCPs and hospitalists choosing several of the prespecified reasons for inappropriateness. Although hospitalists were more likely than PCPs to select I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (chosen by 41.5% of hospitalists vs 30.8% of PCPs, P=0.009), PCPs were more likely than hospitalists to select, I am not confident that the hospitalist will adequately review the medical history necessary to make this decision (chosen by 40.9% of PCPs vs 20.4% of hospitalists, P<0.001) and I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient (26.7% of PCPs vs 9.8% of hospitalists, P<0.001).
Opinions on Current Management of Conditions Related and Unrelated to Admission
A minority of PCPs and hospitalists agreed or strongly agreed that hospitalists should play a larger role in the management of medical conditions unrelated to the reason for admission (28.1% of PCPs vs 34.8% of hospitalists; P=0.39).
DISCUSSION
In this survey‐based study of PCPs and hospitalists across 3 Boston‐area academic medical centers, we found that: (1) physicians were more likely to see inpatient interventions as appropriate when those interventions dealt with the reason for admission as compared to interventions unrelated to the reason for admission; and (2) PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when they targeted chronic conditions unrelated to the reason for admission. To our knowledge, this study represents the first investigation into the attitudes of PCPs and hospitalists regarding the inpatient management of conditions unrelated to the reason for admission.
That surveyed physicians, regardless of role, were less likely to report an intervention unrelated to the reason for hospitalization as appropriateeven those with likely mortality benefitsuggests that opportunities to affect meaningful change may be missed in a healthcare system that adheres to strict inpatient and outpatient roles. For several of the cases, a change in therapy could lead to benefit soon after implementation. For example, aldosterone antagonists reduce mortality as early as 1 month after initiation in select patients.[8] If a major goal of inpatient care is to reduce 30‐day mortality, it could be argued that hospitalists should more actively adjust congestive heart failure therapy in appropriate inpatients, even when this is not their admitting diagnosis.
For some conditions, CMS is already tracking hospital performance. Since 2003, hospitals have been required to document whether a patient with congestive heart failure (either acute or chronic and regardless of the relationship to admission) was prescribed an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the time of discharge.[7] CMS has determined that the proven benefits of ACE inhibitors and ARBs confer hospital accountability for their inclusion in appropriate patients, independent of the acuity of heart failure. There are many potential therapeutic maneuvers on which health systems (and their physicians) may be graded, and accepting the view that a hospitalization provides a window of opportunity for medical optimization may allow for more fruitful interventions and more patient‐centered care.
Despite the potential benefits of addressing chronic medical issues during hospitalization, there are important limitations on what can and/or should be done in the hospital setting. Hospitalizations are a time of fluctuating clinical status, which continues beyond discharge and is often accompanied by several medication changes.[9] In our study, more than 20% of those who believed that a medication intervention was inappropriate selected I do not believe hospitalization is the right time to start this new medication as one of their explanations. Although some medication interventions have been shown in randomized controlled trials to reduce short‐term mortality, the ability to generalize these findings to the average hospitalized patient with multiple comorbidities, concurrent medication changes, and rapidly fluctuating clinical status is limited. Furthermore, there are interventions most would agree should not be dealt with in the hospital (eg, screening colonoscopy) and encounters that may be too short to allow for change (eg, 24‐hour observation). These issues notwithstanding, the average 4‐day hospitalization likely provides an opportunity for monitored change that may currently be underutilized.
Our study suggests several additional explanations for physicians' current practice and opinions. Only 6.5% of respondents who answered that an intervention was inappropriate indicated as a justification that I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. This suggests that the hesitancy has little to do with a lack of benefit but instead relates to systems issues (eg, access to all pertinent records and concerns regarding follow‐up testing) and perceived limitations to what a hospitalist should and should not do without actively involving the PCP. There are likely additional concerns that the medical record and/or patient histories do not fully outline the rationale for exclusion or inclusion of particular medications. Advances in information technology that enhance information exchange and enable streamlined communication may help to address these perceived barriers. However, an additional barrier may be trust, as PCPs appear more concerned that hospitalists will not review all the pertinent records or discuss risks and benefits before enacting important medication changes. Increased attempts at communication between hospitalists and outpatient providers may help to build trust and alleviate concerns regarding the loss of information that often occurs both on admission and at discharge.
We also noted that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when targeting chronic conditions unrelated to the reason for admission. It may be that PCPs, with an increasing number of problems to address per outpatient visit,[10, 11] are more open to hospitalists managing any medical problems during their patients' admissions. At the same time, with increased acuity[12, 13, 14] and shortened length of stays,[15, 16] hospitalists have only a finite amount of time to ensure acute issues are managed, leaving potentially modifiable chronic conditions to the outpatient setting. These differences aside, a minority of both PCPs and hospitalists in our study were ready to embrace the idea of hospitalists playing a larger role in the management of conditions unrelated to the reason for hospitalization.
Even though our study benefits from its multisite design, there are a number of limitations. First, although we crafted our survey with input from general medicine focus groups, our survey instrument has not been validated. In addition, the cases are necessarily contrived and do not take into account the complexities of inpatient medicine. Furthermore, though our goal was to create paired cases that isolate a management decision as being simply based on whether it was related or unrelated to the reason for admission, it is possible that other factors, not captured by our survey, influenced the responses. For example, the benefits of aspirin as part of secondary prevention are not equal to the benefits in an acute MI.[17]
In an attempt to isolate the hospitalists' role in these management decisions, respondents were instructed to assume that the decisions were being made without discussing it with the primary care physician, but that the hospitalist would communicate the details of any hospitalization at the time of discharge. They were also instructed to assume that the hospitalist has access to the patient's outpatient electronic medical record. These assumptions were made to address concerns regarding the flow of information and communication, and to simulate the ideal system from a communication and information accessibility standpoint. Had these assumptions not been placed, the responses may have differed. It is likely that PCPs and hospitalists practicing in systems without shared, accessible inpatient/outpatient medical records would be even more reluctant to enact medication changes unrelated to the reason for admission.
Along the same lines, our physician cohort consisted of several metropolitan academic physician groups, in which hospitalists have had a presence for almost 20 years. As a result, our findings may not be generalizable to other academic hospitals, community‐based hospitalist programs, or nonhospital‐based PCP practices. Finally, we do not know whether survey nonresponders differed from responders in ways that could have meaningfully affected our results.
In conclusion, our findings suggest that both PCPs and hospitalists see the management of conditions unrelated to the reason for admission as less appropriate than the management of conditions related to the reason for admission. Our findings also suggest that PCPs may be more open to this practice when compared to hospitalists. Failure to capitalize on opportunities for meaningful medical interventions, independent of patient location, suggests a possible lack of patient centeredness in the current partnership between PCPs and hospitalists. Further studies should examine existing barriers and investigate interventions designed to address those barriers, in an effort to improve both quality of care and the degree of patient‐centeredness in our current healthcare system.
Disclosures: Dr. Herzig is supported by a grant from the National Institute on Aging (K23 AG042459). Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions: study concept and design, Breu, Allen‐Dicker, Mueller, Herzig; acquisition of data, Breu, Allen‐Dicker, Mueller, Palamara, Herzig; analysis and interpretation of data, Breu, Allen‐Dicker, Hinami, Herzig; drafting of the manuscript, Breu; critical revision of the manuscript for important intellectual content, Breu, Allen‐Dicker, Mueller, Palamara, Hinami, Herzig; statistical analysis, Allen‐Dicker, Hinami, Herzig; study supervision, Breu, Herzig. This study was presented as a poster at the Society of Hospital Medicine National Meeting, Washington, DC, May 17, 2013.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , . A national strategy to put accountable care into practice. Health Aff (Millwood). 2010;29(5):982–990.
- . Keeping score under a global payment system. N Engl J Med. 2012;366(5):393–395.
- Reporting Hospital Quality Data for Annual Payment Update. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/Downloads/HospitalRHQDAPU200808. Accessed December 18, 2013.
- , , , et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- , , , , , . How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461.
- , , . Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169(20):1866–1872.
- , , , The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23(12):2058–2065.
- , , . Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief. 2012;(100):1–8.
- , , . Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7(1):82.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391–395.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , . A national strategy to put accountable care into practice. Health Aff (Millwood). 2010;29(5):982–990.
- . Keeping score under a global payment system. N Engl J Med. 2012;366(5):393–395.
- Reporting Hospital Quality Data for Annual Payment Update. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/Downloads/HospitalRHQDAPU200808. Accessed December 18, 2013.
- , , , et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- , , , , , . How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461.
- , , . Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169(20):1866–1872.
- , , , The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23(12):2058–2065.
- , , . Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief. 2012;(100):1–8.
- , , . Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7(1):82.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391–395.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
© 2014 Society of Hospital Medicine
Analysis of Serum Folate Testing
Folate deficiency has been associated with a number of medical conditions. It is well established that folate deficiency leads to macrocytic anemia,[1, 2] and that supplementation of folic acid during pregnancy leads to decreased rates of neural tube defects.[3] Folate deficiency has also been hypothesized to affect other conditions including dementia, delirium, peripheral neuropathy, depression, cancer, and cardiovascular disease.[4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18] Most of these latter assertions are based on case reports or observational studies, with randomized controlled trials failing to demonstrate benefit of folic acid supplementation.[19, 20, 21]
Prior to mandatory folic acid fortification in the United States, the prevalence of folate deficiency was estimated to be between 3% and 16%.[16, 22, 23] In a study conducted prior to fortification, serum folate levels were evaluated in patients presenting with macrocytosis and anemia.[24] The study found that 2.3% of patients were serum folate deficient, with a change in management occurring in 24% of the deficient patients. The study also found that patients were charged $9979 per result that changed physician management.
In 1998, mandatory folic acid fortification began in the United States, and the prevalence of folate deficiency in the general population decreased to an estimated 0.5%.[23, 25] In a postfortification study, serum folate levels were evaluated in patients with anemia, dementia, or altered mental status.[26] The overall rate of serum folate deficiency was 0.4%, with the authors concluding that there was a lack of utility in serum folate testing. Despite this, algorithms addressing the evaluation of anemia continue to include serum folate levels.[2, 27, 28]
To our knowledge, the use of serum folate testing in the inpatient and emergency department population has never been independently evaluated. In our study, we aimed to characterize the indications, rate of deficiency, charge and cost per deficient result, and change in management per deficient result in inpatient and emergency department serum folate testing. We hypothesized that serum folate testing in these populations would have poor utility and would not be cost‐effective for any indication.
METHODS
We conducted a retrospective review of all serum folate tests ordered in inpatient units and the emergency department at a large academic medical center in Boston, Massachusetts from January 1, 2011 through December 31, 2011. The test was considered to be an inpatient or emergency department test based on the location of the blood draw on which the test was performed. Serum folate values were determined using a chemiluminescent competitive binding protein assay on an E170 analyzer as prescribed by the manufacturer (Roche Diagnostics, Indianapolis, IN). We defined serum folate levels as deficient (3.0 ng/mL), low‐normal (3.0 ng/mL3.9 ng/mL),[26] normal (4.0 ng/mL20.0 ng/mL), and high (>20.0 ng/mL). Erythrocyte folate levels are not routinely ordered at our institution and were not measured in our study.[29] Macrocytosis was defined as mean corpuscular volume of >99 fL. Vitamin B12 deficiency was defined as vitamin B12 level of under 200 pg/mL or vitamin B12 level of 200 to 300 pg/mL, with a methylmalonic acid >270 nmol/L and a normal homocysteine level (514 mol/L).[30, 31]
We evaluated 250 randomly selected serum folate levels and all deficient or low‐normal serum folate levels and recorded indication, comorbidities, age, sex, race or ethnicity, hemoglobin, hematocrit, mean corpuscular volume, vitamin B12 level, folic acid supplement on presentation, and folic acid supplement on discharge. Indications were determined by chart review. If serum folate was checked at the same time as iron studies, it was assumed that the indication was anemia without macrocytosis or anemia with macrocytosis unless otherwise documented. Comorbidities were selected based on historical risk factors and included depression, peripheral neuropathy, intestinal surgery, gastric bypass, cirrhosis, inflammatory bowel disease, celiac disease, delirium, dementia, alcohol abuse, malnutrition, anemia, end‐stage renal disease, vitamin B12 deficiency, or current use of phenytoin, valproic acid, or methotrexate.[32]
A charge analysis was performed using the same methodology as Robinson and Mladenovic.[24] We defined the charge of serum folate testing as our institution's charge to the patient or payer, which was $151.00 per test. Because hospital charges are variable, we also made a second calculation based on the charge per patient or payer from the Robinson and Mladenovic study,[24] which was $71.00. The analytical cost to our hospital of performing each serum folate test was <$2.00. We determined the total charge and cost for all serum folate tests and the charge and cost per deficient result.
The study was reviewed by the institutional review board and determined to be exempt.
RESULTS
In 2011, a total of 2093 serum folate levels were obtained on 1944 inpatients and emergency department patients. Of the total patients, 79.9% were inpatients and 20.1% were emergency department patients. Of the patients with tests performed in the emergency department, 98.1% were admitted to an inpatient unit.
Of the 250 random chart reviews, all had normal or high serum folate levels. The demographics, indications, and comorbidities are listed in Table 1. The most common indications were anemia without macrocytosis (43.2%), anemia with macrocytosis (13.2%; mean corpuscular volume [MCV], 106.8 fL), delirium (12.0%), malnutrition (6.4%), and peripheral neuropathy (6.0%). The other indications included thrombocytopenia, macrocytosis (without anemia), methotrexate use, alcohol abuse, frequent falls, syncope, headache, lethargy, optic nerve neuropathy, paranoia, psychosis, leukopenia, anxiety, and suicidal ideation. All of these individual indications were 2% of total reviewed indications. There were 16 cases (6.4%) without a documented indication.
| |
| Age, median, y | 66.0 |
| Male sex, % | 50.8 |
| Race or ethnicity, % | |
| White | 76.0 |
| Black or African American | 12.0 |
| Asian | 4.4 |
| Hispanic | 4.0 |
| Unknown or declined | 2.0 |
| Other | 1.6 |
| Indications, %* | |
| Anemia without macrocytosis | 43.2 |
| Anemia with macrocytosis | 13.2 |
| Delirium | 12.0 |
| Malnutrition | 6.4 |
| Peripheral neuropathy | 6.0 |
| Depression | 3.6 |
| Dementia | 3.2 |
| Pancytopenia | 2.4 |
| Other | 10.4 |
| Unknown | 6.4 |
| Comorbidities, % | |
| Depression | 23.2 |
| Alcohol abuse | 18.4 |
| Chronic anemia | 11.2 |
| Malnutrition | 9.6 |
| Prior intestinal surgery | 8.8 |
| Peripheral neuropathy | 6.0 |
| Dementia | 5.6 |
| Gastric bypass surgery | 4.4 |
| End‐stage renal disease | 4.0 |
| End‐stage liver disease | 3.6 |
| Use of phenytoin | 3.2 |
| Inflammatory bowel disease | 2.4 |
| Use of valproic acid | 2.0 |
| Celiac disease | 1.2 |
Of the 2093 serum folate levels, there were 2 deficient (0.1%), 7 low‐normal (0.3%), 1487 normal (71.1%), and 597 high (28.5%) levels (Table 2). There were 128 patients (6.6%) who had more than 1 serum folate level checked within the prior 12 months, with 1 patient having 5 levels obtained during that time period. All of the deficient and low‐normal serum folate results are listed in Table 3. Of the 9 deficient or low‐normal serum folate levels, 8 had comorbid risk factors for folate deficiency. One of the deficient cases was on folic acid and multivitamin supplementation on presentation, although nonadherence with these supplements was documented in the medical record. The other deficient case was not on folic acid supplementation and did not receive folic acid supplementation for the deficient result. Vitamin B12 levels were checked simultaneously to serum folate levels in 85.2% of cases and within 6 months in 99.2% of cases. Of these patients, 2.0% were found to have vitamin B12 deficiency.
| |
| Total tests | 2093 |
| Total patients | 1944 |
| Low (%) | 2 (0.1) |
| Low‐normal (%) | 7 (0.3) |
| Normal (%) | 1487 (71.0) |
| High (%) | 597 (28.5) |
| MCV (StDev) | 92.1 (9.2) |
| Age, y | Sex | Folate (ng/mL) | Indication | Comorbidities | Hgb (g/dL) | MCV (fL) | |
|---|---|---|---|---|---|---|---|
| |||||||
| Deficient results | |||||||
| Case 1 | 35 | Male | 2.6 | Stroke workup | Phenytoin, depression | 16.0 | 91 |
| Case 2 | 63 | Male | 2.9 | Macrocytic anemia | Alcohol abuse, acute GI bleed | 7.7 | 119 |
| Low‐normal results | |||||||
| Case 3 | 64 | Male | 3.3 | Macrocytic anemia | Cirrhosis, alcohol abuse | 12.3 | 109 |
| Case 4 | 42 | Male | 3.4 | Pancytopenia | HIV, B12 deficiency | 7.5 | 93 |
| Case 5 | 58 | Male | 3.4 | Depression | Depression, alcohol abuse | 13.8 | 98 |
| Case 6 | 56 | Female | 3.5 | Depression | Alcohol abuse | ||
| Case 7 | 85 | Male | 3.6 | Delirium | Depression | 10.5 | 91 |
| Case 8 | 81 | Female | 3.6 | Anemia | Chronic anemia | 9.1 | 95 |
| Case 9 | 63 | Male | 3.9 | Anemia | Chronic anemia, malnutrition | 7.6 | 88 |
Based on our institution's charge for serum folate, a total of $316,043 was charged for the 2093 serum folate tests. The amount charged per deficient result was $158,022. Substituting the charge from the Robinson and Mladenovic study,[24] we calculated the corresponding total charge and charge per deficient result as $149,545 and $74,772, respectively. The actual total cost to our hospital was <$4186, with a cost per deficient test of <$2093.
DISCUSSION
Serum folate levels are often obtained when evaluating anemia without macrocytosis and anemia with macrocytosis.[2] They are also frequently obtained in the evaluation of delirium and dementia. A prior study evaluated both inpatient and outpatient serum folate levels in anemia, dementia, and altered mental status and found only 0.4% of serum folate results to be deficient.[26] In their study, the indications for serum folate tests were anemia or macrocytic anemia (60%) and dementia or altered mental status (30%).
We found the indications for serum folate testing in inpatients and emergency department patients to be different than prior studies. The majority of tests were done to evaluate anemia without macrocytosis (43.2%) or anemia with macrocytosis (13.2%). Lower percentages were done for the evaluation of delirium (12.0%) or dementia (3.2%). In addition, there were multiple indications that have not been noted in previous studies, including depression, peripheral neuropathy, malnutrition, pancytopenia, and others. These accounted for 28.0% of all indications. The reason for the difference in indications compared to prior studies is unknown but may be related to our cohort of exclusively inpatients and emergency department patients. Also, we observed a high concurrence of serum folate and vitamin B12 testing, with 85.2% of serum folate levels ordered at the same time as vitamin B12 levels. It appears that the tests are often ordered together even when the indication suggests that vitamin B12 alone may be more appropriate, such as peripheral neuropathy.
We found that serum folate deficiency was rare, occurring in only 2 of 2093 results. Furthermore, the deficient serum folate results may have been checked for inappropriate indications. The first deficient result was noted as part of a stroke workup in a patient not taking folic acid supplementation. Current guidelines do not recommend serum folate testing in patients with new stroke.[33] In the second deficient case, serum folate testing was performed for evaluation of macrocytic anemia with an MCV of 119 fL. Although reasonable, this was an alcoholic patient presenting with acute gastrointestinal bleeding already on folic acid and multivitamin supplementation and known nonadherence with these supplements. In neither case was there a change in management based on the deficient result.
Given the low rate of serum folate deficiency and the lack of change in management based on deficient results, we conclude that there is a low utility of serum folate testing for any indication in inpatients and emergency department patients in folic acid‐fortified countries. Based on prior studies, and supported by our results, there is no evidence for checking serum folate levels in delirium, dementia, peripheral neuropathy, malnutrition, or any of the other indications. In addition, our results demonstrate a low utility even in patients with anemia or macrocytic anemia.
The rate of serum folate deficiency in our study was significantly lower than prior studies.[24, 26] There may have been geographical factors that led to a lower prevalence of folate deficiency in our study population. Our cohort included inpatients and emergency department patients, whereas previous studies had a majority of outpatients. It is known that serum folate levels can rapidly fluctuate with proper nutrition.[34] It may be that our patients received nutrition in the hospital that corrected serum folate levels prior to laboratory testing.
In addition to the low utility of serum folate testing, the charge per deficient result in our study ($158,022) was more than 100‐fold higher than that in the Robinson and Mladenovic study ($1321).[24] Even when correcting for variability in hospital charges by using the charge from the latter study, the charge per deficient serum folate test remained 50‐fold higher ($74,772). This implies that the increase in charge per deficient result was driven in part by a decreased rate of deficient tests. Folic acid fortification is likely responsible for some of the decrease. However, we believe another source is the excessive ordering of serum folate tests in patients without previously accepted indications. Because no change in management was made for the deficient patients in our study, the charge per serum folate deficient result that changed management approached infinity. This compares to $9979 in the Robinson and Mladenovic analysis.[24]
The cost to the hospital of a serum folate test was much lower than the charge, and estimated to be <$2093 per deficient result. Because serum folate tests are performed on a highly automated, random access analyzer that performs thousands of other measurements daily, the capital and labor costs for each test was well below $0.50 combined. With the addition of reagent costs, our total cost for each serum folate measurement was <$2.00. It is somewhat difficult to extrapolate these values to other hospitals, as exact costs and charges are variable. Nonetheless, the exceptionally low utility of serum folate testing makes the costs associated with these tests excessive.
Our study has several limitations. We conducted our study at a single institution in a country with mandatory folic acid fortification. Our results may not be generalizable to other institutions or patient populations, such as those in countries without mandatory folic acid fortification. Only 259 (12.4%) charts were reviewed, and indications were determined in 93.6% of charts, which may have caused our frequency to vary from the true frequency. Additionally, the low rate of deficient serum folate results limited our ability to identify associations with deficiency. Further evaluation for geographic variations of serum folate deficiency may reveal variable rates.
We conclude that in folic acid fortified countries, the rate of serum folate deficiency is increasingly rare, and the charge to patients and payers per deficient result is exceptionally high. In addition, testing in our study did not change clinical management, which makes the costs associated with these test wasteful. Further evaluation of serum folate testing of inpatients and emergency department patients in folic acid fortified countries is warranted; however, based on our results the utility appears poor for all indications.
Disclosure
Nothing to report.
- , . The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181–186.
- , , , et al. Harrison's Principles of Internal Medicine. New York, NY:McGraw‐Hill Professional;2004.
- , . Folic acid, pernicious anaemia, and prevention of neural tube defects. Lancet. 1994;343(8893):307.
- , , , et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high‐functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118(2):161–167.
- , . Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7(6):659–664.
- , , , , , . Plasma homocysteine levels and cognitive status in long‐term stay geriatric patients: a cross‐sectional study. Arch Gerontol Geriatr. 2005;40(2):129–138.
- . Folate responsive neuropathy. Presse Med. 1994;23(3):131–137.
- , . Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol (Oxford). 2005;19(1):59–65.
- , , , et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94(7):3290–3295.
- , , , et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997;65(1):46–52.
- , , , , , . Folate intake and carcinogenesis of the colon and rectum. Int J Epidemiol. 1991;20(2):368–374.
- , . Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr Cancer. 2006;56(1):11–21.
- , , , et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129(7):517–524.
- , , , , . Serum homocysteine and folate but not vitamin B12 are predictors of CHD mortality in older adults [published online ahead of print September 29, 2011]. Eur J Cardiovasc Prev Rehabil. doi: 10.1177/1741826711424568.
- , , , , , . Plasma homocyst(e)ine levels in men with premature coronary artery disease. J Am Coll Cardiol. 1990;16(5):1114–1119.
- , , , et al. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition. 2000;16(6):434–438.
- , , , , , . Low serum folate concentrations are associated with an excess incidence of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Clin Nutr. 2000;54(5):424–428.
- , , , , . Dietary folate and the risk of nonfatal myocardial infarction. Epidemiology. 2002;13(6):700–706.
- , , , et al.;Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and b vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577.
- , , , , , . A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354(26):2764–2772.
- , . Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514.
- , , , . Does the mean corpuscular volume help physicians evaluate hospitalized patients with anemia?J Gen Intern Med. 1990;5(3):187–191.
- , , , , . Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82(2):442–450.
- , . Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110(2):88–90.
- , , , et al. Blood folate levels: the latest NHANES results. NCHS Data Brief. 2008;(6):1–8.
- , , . Clinical utility of folic acid testing for patients with anemia or dementia. J Gen Intern Med. 2008;23(6):824–826.
- . Anemia in adults: a contemporary approach to diagnosis. Mayo Clin Proc 2003;78(10):1274–1280.
- . Anemia in the elderly. Am Fam Physician. 2000;62(7):1565–1572.
- , . Red cell or serum folate? Results from the National Pathology Alliance benchmarking review. J Clin Pathol. 2003;56(12):924–926.
- , , , , , . Hematology. Philadelphia, PA:Churchill Livingstone;2012.
- , , , . Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96(3):239–246.
- . Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–1298.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–1711.
- , , , . Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. J Nutr. 2006;136(12):3074–3078.
Folate deficiency has been associated with a number of medical conditions. It is well established that folate deficiency leads to macrocytic anemia,[1, 2] and that supplementation of folic acid during pregnancy leads to decreased rates of neural tube defects.[3] Folate deficiency has also been hypothesized to affect other conditions including dementia, delirium, peripheral neuropathy, depression, cancer, and cardiovascular disease.[4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18] Most of these latter assertions are based on case reports or observational studies, with randomized controlled trials failing to demonstrate benefit of folic acid supplementation.[19, 20, 21]
Prior to mandatory folic acid fortification in the United States, the prevalence of folate deficiency was estimated to be between 3% and 16%.[16, 22, 23] In a study conducted prior to fortification, serum folate levels were evaluated in patients presenting with macrocytosis and anemia.[24] The study found that 2.3% of patients were serum folate deficient, with a change in management occurring in 24% of the deficient patients. The study also found that patients were charged $9979 per result that changed physician management.
In 1998, mandatory folic acid fortification began in the United States, and the prevalence of folate deficiency in the general population decreased to an estimated 0.5%.[23, 25] In a postfortification study, serum folate levels were evaluated in patients with anemia, dementia, or altered mental status.[26] The overall rate of serum folate deficiency was 0.4%, with the authors concluding that there was a lack of utility in serum folate testing. Despite this, algorithms addressing the evaluation of anemia continue to include serum folate levels.[2, 27, 28]
To our knowledge, the use of serum folate testing in the inpatient and emergency department population has never been independently evaluated. In our study, we aimed to characterize the indications, rate of deficiency, charge and cost per deficient result, and change in management per deficient result in inpatient and emergency department serum folate testing. We hypothesized that serum folate testing in these populations would have poor utility and would not be cost‐effective for any indication.
METHODS
We conducted a retrospective review of all serum folate tests ordered in inpatient units and the emergency department at a large academic medical center in Boston, Massachusetts from January 1, 2011 through December 31, 2011. The test was considered to be an inpatient or emergency department test based on the location of the blood draw on which the test was performed. Serum folate values were determined using a chemiluminescent competitive binding protein assay on an E170 analyzer as prescribed by the manufacturer (Roche Diagnostics, Indianapolis, IN). We defined serum folate levels as deficient (3.0 ng/mL), low‐normal (3.0 ng/mL3.9 ng/mL),[26] normal (4.0 ng/mL20.0 ng/mL), and high (>20.0 ng/mL). Erythrocyte folate levels are not routinely ordered at our institution and were not measured in our study.[29] Macrocytosis was defined as mean corpuscular volume of >99 fL. Vitamin B12 deficiency was defined as vitamin B12 level of under 200 pg/mL or vitamin B12 level of 200 to 300 pg/mL, with a methylmalonic acid >270 nmol/L and a normal homocysteine level (514 mol/L).[30, 31]
We evaluated 250 randomly selected serum folate levels and all deficient or low‐normal serum folate levels and recorded indication, comorbidities, age, sex, race or ethnicity, hemoglobin, hematocrit, mean corpuscular volume, vitamin B12 level, folic acid supplement on presentation, and folic acid supplement on discharge. Indications were determined by chart review. If serum folate was checked at the same time as iron studies, it was assumed that the indication was anemia without macrocytosis or anemia with macrocytosis unless otherwise documented. Comorbidities were selected based on historical risk factors and included depression, peripheral neuropathy, intestinal surgery, gastric bypass, cirrhosis, inflammatory bowel disease, celiac disease, delirium, dementia, alcohol abuse, malnutrition, anemia, end‐stage renal disease, vitamin B12 deficiency, or current use of phenytoin, valproic acid, or methotrexate.[32]
A charge analysis was performed using the same methodology as Robinson and Mladenovic.[24] We defined the charge of serum folate testing as our institution's charge to the patient or payer, which was $151.00 per test. Because hospital charges are variable, we also made a second calculation based on the charge per patient or payer from the Robinson and Mladenovic study,[24] which was $71.00. The analytical cost to our hospital of performing each serum folate test was <$2.00. We determined the total charge and cost for all serum folate tests and the charge and cost per deficient result.
The study was reviewed by the institutional review board and determined to be exempt.
RESULTS
In 2011, a total of 2093 serum folate levels were obtained on 1944 inpatients and emergency department patients. Of the total patients, 79.9% were inpatients and 20.1% were emergency department patients. Of the patients with tests performed in the emergency department, 98.1% were admitted to an inpatient unit.
Of the 250 random chart reviews, all had normal or high serum folate levels. The demographics, indications, and comorbidities are listed in Table 1. The most common indications were anemia without macrocytosis (43.2%), anemia with macrocytosis (13.2%; mean corpuscular volume [MCV], 106.8 fL), delirium (12.0%), malnutrition (6.4%), and peripheral neuropathy (6.0%). The other indications included thrombocytopenia, macrocytosis (without anemia), methotrexate use, alcohol abuse, frequent falls, syncope, headache, lethargy, optic nerve neuropathy, paranoia, psychosis, leukopenia, anxiety, and suicidal ideation. All of these individual indications were 2% of total reviewed indications. There were 16 cases (6.4%) without a documented indication.
| |
| Age, median, y | 66.0 |
| Male sex, % | 50.8 |
| Race or ethnicity, % | |
| White | 76.0 |
| Black or African American | 12.0 |
| Asian | 4.4 |
| Hispanic | 4.0 |
| Unknown or declined | 2.0 |
| Other | 1.6 |
| Indications, %* | |
| Anemia without macrocytosis | 43.2 |
| Anemia with macrocytosis | 13.2 |
| Delirium | 12.0 |
| Malnutrition | 6.4 |
| Peripheral neuropathy | 6.0 |
| Depression | 3.6 |
| Dementia | 3.2 |
| Pancytopenia | 2.4 |
| Other | 10.4 |
| Unknown | 6.4 |
| Comorbidities, % | |
| Depression | 23.2 |
| Alcohol abuse | 18.4 |
| Chronic anemia | 11.2 |
| Malnutrition | 9.6 |
| Prior intestinal surgery | 8.8 |
| Peripheral neuropathy | 6.0 |
| Dementia | 5.6 |
| Gastric bypass surgery | 4.4 |
| End‐stage renal disease | 4.0 |
| End‐stage liver disease | 3.6 |
| Use of phenytoin | 3.2 |
| Inflammatory bowel disease | 2.4 |
| Use of valproic acid | 2.0 |
| Celiac disease | 1.2 |
Of the 2093 serum folate levels, there were 2 deficient (0.1%), 7 low‐normal (0.3%), 1487 normal (71.1%), and 597 high (28.5%) levels (Table 2). There were 128 patients (6.6%) who had more than 1 serum folate level checked within the prior 12 months, with 1 patient having 5 levels obtained during that time period. All of the deficient and low‐normal serum folate results are listed in Table 3. Of the 9 deficient or low‐normal serum folate levels, 8 had comorbid risk factors for folate deficiency. One of the deficient cases was on folic acid and multivitamin supplementation on presentation, although nonadherence with these supplements was documented in the medical record. The other deficient case was not on folic acid supplementation and did not receive folic acid supplementation for the deficient result. Vitamin B12 levels were checked simultaneously to serum folate levels in 85.2% of cases and within 6 months in 99.2% of cases. Of these patients, 2.0% were found to have vitamin B12 deficiency.
| |
| Total tests | 2093 |
| Total patients | 1944 |
| Low (%) | 2 (0.1) |
| Low‐normal (%) | 7 (0.3) |
| Normal (%) | 1487 (71.0) |
| High (%) | 597 (28.5) |
| MCV (StDev) | 92.1 (9.2) |
| Age, y | Sex | Folate (ng/mL) | Indication | Comorbidities | Hgb (g/dL) | MCV (fL) | |
|---|---|---|---|---|---|---|---|
| |||||||
| Deficient results | |||||||
| Case 1 | 35 | Male | 2.6 | Stroke workup | Phenytoin, depression | 16.0 | 91 |
| Case 2 | 63 | Male | 2.9 | Macrocytic anemia | Alcohol abuse, acute GI bleed | 7.7 | 119 |
| Low‐normal results | |||||||
| Case 3 | 64 | Male | 3.3 | Macrocytic anemia | Cirrhosis, alcohol abuse | 12.3 | 109 |
| Case 4 | 42 | Male | 3.4 | Pancytopenia | HIV, B12 deficiency | 7.5 | 93 |
| Case 5 | 58 | Male | 3.4 | Depression | Depression, alcohol abuse | 13.8 | 98 |
| Case 6 | 56 | Female | 3.5 | Depression | Alcohol abuse | ||
| Case 7 | 85 | Male | 3.6 | Delirium | Depression | 10.5 | 91 |
| Case 8 | 81 | Female | 3.6 | Anemia | Chronic anemia | 9.1 | 95 |
| Case 9 | 63 | Male | 3.9 | Anemia | Chronic anemia, malnutrition | 7.6 | 88 |
Based on our institution's charge for serum folate, a total of $316,043 was charged for the 2093 serum folate tests. The amount charged per deficient result was $158,022. Substituting the charge from the Robinson and Mladenovic study,[24] we calculated the corresponding total charge and charge per deficient result as $149,545 and $74,772, respectively. The actual total cost to our hospital was <$4186, with a cost per deficient test of <$2093.
DISCUSSION
Serum folate levels are often obtained when evaluating anemia without macrocytosis and anemia with macrocytosis.[2] They are also frequently obtained in the evaluation of delirium and dementia. A prior study evaluated both inpatient and outpatient serum folate levels in anemia, dementia, and altered mental status and found only 0.4% of serum folate results to be deficient.[26] In their study, the indications for serum folate tests were anemia or macrocytic anemia (60%) and dementia or altered mental status (30%).
We found the indications for serum folate testing in inpatients and emergency department patients to be different than prior studies. The majority of tests were done to evaluate anemia without macrocytosis (43.2%) or anemia with macrocytosis (13.2%). Lower percentages were done for the evaluation of delirium (12.0%) or dementia (3.2%). In addition, there were multiple indications that have not been noted in previous studies, including depression, peripheral neuropathy, malnutrition, pancytopenia, and others. These accounted for 28.0% of all indications. The reason for the difference in indications compared to prior studies is unknown but may be related to our cohort of exclusively inpatients and emergency department patients. Also, we observed a high concurrence of serum folate and vitamin B12 testing, with 85.2% of serum folate levels ordered at the same time as vitamin B12 levels. It appears that the tests are often ordered together even when the indication suggests that vitamin B12 alone may be more appropriate, such as peripheral neuropathy.
We found that serum folate deficiency was rare, occurring in only 2 of 2093 results. Furthermore, the deficient serum folate results may have been checked for inappropriate indications. The first deficient result was noted as part of a stroke workup in a patient not taking folic acid supplementation. Current guidelines do not recommend serum folate testing in patients with new stroke.[33] In the second deficient case, serum folate testing was performed for evaluation of macrocytic anemia with an MCV of 119 fL. Although reasonable, this was an alcoholic patient presenting with acute gastrointestinal bleeding already on folic acid and multivitamin supplementation and known nonadherence with these supplements. In neither case was there a change in management based on the deficient result.
Given the low rate of serum folate deficiency and the lack of change in management based on deficient results, we conclude that there is a low utility of serum folate testing for any indication in inpatients and emergency department patients in folic acid‐fortified countries. Based on prior studies, and supported by our results, there is no evidence for checking serum folate levels in delirium, dementia, peripheral neuropathy, malnutrition, or any of the other indications. In addition, our results demonstrate a low utility even in patients with anemia or macrocytic anemia.
The rate of serum folate deficiency in our study was significantly lower than prior studies.[24, 26] There may have been geographical factors that led to a lower prevalence of folate deficiency in our study population. Our cohort included inpatients and emergency department patients, whereas previous studies had a majority of outpatients. It is known that serum folate levels can rapidly fluctuate with proper nutrition.[34] It may be that our patients received nutrition in the hospital that corrected serum folate levels prior to laboratory testing.
In addition to the low utility of serum folate testing, the charge per deficient result in our study ($158,022) was more than 100‐fold higher than that in the Robinson and Mladenovic study ($1321).[24] Even when correcting for variability in hospital charges by using the charge from the latter study, the charge per deficient serum folate test remained 50‐fold higher ($74,772). This implies that the increase in charge per deficient result was driven in part by a decreased rate of deficient tests. Folic acid fortification is likely responsible for some of the decrease. However, we believe another source is the excessive ordering of serum folate tests in patients without previously accepted indications. Because no change in management was made for the deficient patients in our study, the charge per serum folate deficient result that changed management approached infinity. This compares to $9979 in the Robinson and Mladenovic analysis.[24]
The cost to the hospital of a serum folate test was much lower than the charge, and estimated to be <$2093 per deficient result. Because serum folate tests are performed on a highly automated, random access analyzer that performs thousands of other measurements daily, the capital and labor costs for each test was well below $0.50 combined. With the addition of reagent costs, our total cost for each serum folate measurement was <$2.00. It is somewhat difficult to extrapolate these values to other hospitals, as exact costs and charges are variable. Nonetheless, the exceptionally low utility of serum folate testing makes the costs associated with these tests excessive.
Our study has several limitations. We conducted our study at a single institution in a country with mandatory folic acid fortification. Our results may not be generalizable to other institutions or patient populations, such as those in countries without mandatory folic acid fortification. Only 259 (12.4%) charts were reviewed, and indications were determined in 93.6% of charts, which may have caused our frequency to vary from the true frequency. Additionally, the low rate of deficient serum folate results limited our ability to identify associations with deficiency. Further evaluation for geographic variations of serum folate deficiency may reveal variable rates.
We conclude that in folic acid fortified countries, the rate of serum folate deficiency is increasingly rare, and the charge to patients and payers per deficient result is exceptionally high. In addition, testing in our study did not change clinical management, which makes the costs associated with these test wasteful. Further evaluation of serum folate testing of inpatients and emergency department patients in folic acid fortified countries is warranted; however, based on our results the utility appears poor for all indications.
Disclosure
Nothing to report.
Folate deficiency has been associated with a number of medical conditions. It is well established that folate deficiency leads to macrocytic anemia,[1, 2] and that supplementation of folic acid during pregnancy leads to decreased rates of neural tube defects.[3] Folate deficiency has also been hypothesized to affect other conditions including dementia, delirium, peripheral neuropathy, depression, cancer, and cardiovascular disease.[4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18] Most of these latter assertions are based on case reports or observational studies, with randomized controlled trials failing to demonstrate benefit of folic acid supplementation.[19, 20, 21]
Prior to mandatory folic acid fortification in the United States, the prevalence of folate deficiency was estimated to be between 3% and 16%.[16, 22, 23] In a study conducted prior to fortification, serum folate levels were evaluated in patients presenting with macrocytosis and anemia.[24] The study found that 2.3% of patients were serum folate deficient, with a change in management occurring in 24% of the deficient patients. The study also found that patients were charged $9979 per result that changed physician management.
In 1998, mandatory folic acid fortification began in the United States, and the prevalence of folate deficiency in the general population decreased to an estimated 0.5%.[23, 25] In a postfortification study, serum folate levels were evaluated in patients with anemia, dementia, or altered mental status.[26] The overall rate of serum folate deficiency was 0.4%, with the authors concluding that there was a lack of utility in serum folate testing. Despite this, algorithms addressing the evaluation of anemia continue to include serum folate levels.[2, 27, 28]
To our knowledge, the use of serum folate testing in the inpatient and emergency department population has never been independently evaluated. In our study, we aimed to characterize the indications, rate of deficiency, charge and cost per deficient result, and change in management per deficient result in inpatient and emergency department serum folate testing. We hypothesized that serum folate testing in these populations would have poor utility and would not be cost‐effective for any indication.
METHODS
We conducted a retrospective review of all serum folate tests ordered in inpatient units and the emergency department at a large academic medical center in Boston, Massachusetts from January 1, 2011 through December 31, 2011. The test was considered to be an inpatient or emergency department test based on the location of the blood draw on which the test was performed. Serum folate values were determined using a chemiluminescent competitive binding protein assay on an E170 analyzer as prescribed by the manufacturer (Roche Diagnostics, Indianapolis, IN). We defined serum folate levels as deficient (3.0 ng/mL), low‐normal (3.0 ng/mL3.9 ng/mL),[26] normal (4.0 ng/mL20.0 ng/mL), and high (>20.0 ng/mL). Erythrocyte folate levels are not routinely ordered at our institution and were not measured in our study.[29] Macrocytosis was defined as mean corpuscular volume of >99 fL. Vitamin B12 deficiency was defined as vitamin B12 level of under 200 pg/mL or vitamin B12 level of 200 to 300 pg/mL, with a methylmalonic acid >270 nmol/L and a normal homocysteine level (514 mol/L).[30, 31]
We evaluated 250 randomly selected serum folate levels and all deficient or low‐normal serum folate levels and recorded indication, comorbidities, age, sex, race or ethnicity, hemoglobin, hematocrit, mean corpuscular volume, vitamin B12 level, folic acid supplement on presentation, and folic acid supplement on discharge. Indications were determined by chart review. If serum folate was checked at the same time as iron studies, it was assumed that the indication was anemia without macrocytosis or anemia with macrocytosis unless otherwise documented. Comorbidities were selected based on historical risk factors and included depression, peripheral neuropathy, intestinal surgery, gastric bypass, cirrhosis, inflammatory bowel disease, celiac disease, delirium, dementia, alcohol abuse, malnutrition, anemia, end‐stage renal disease, vitamin B12 deficiency, or current use of phenytoin, valproic acid, or methotrexate.[32]
A charge analysis was performed using the same methodology as Robinson and Mladenovic.[24] We defined the charge of serum folate testing as our institution's charge to the patient or payer, which was $151.00 per test. Because hospital charges are variable, we also made a second calculation based on the charge per patient or payer from the Robinson and Mladenovic study,[24] which was $71.00. The analytical cost to our hospital of performing each serum folate test was <$2.00. We determined the total charge and cost for all serum folate tests and the charge and cost per deficient result.
The study was reviewed by the institutional review board and determined to be exempt.
RESULTS
In 2011, a total of 2093 serum folate levels were obtained on 1944 inpatients and emergency department patients. Of the total patients, 79.9% were inpatients and 20.1% were emergency department patients. Of the patients with tests performed in the emergency department, 98.1% were admitted to an inpatient unit.
Of the 250 random chart reviews, all had normal or high serum folate levels. The demographics, indications, and comorbidities are listed in Table 1. The most common indications were anemia without macrocytosis (43.2%), anemia with macrocytosis (13.2%; mean corpuscular volume [MCV], 106.8 fL), delirium (12.0%), malnutrition (6.4%), and peripheral neuropathy (6.0%). The other indications included thrombocytopenia, macrocytosis (without anemia), methotrexate use, alcohol abuse, frequent falls, syncope, headache, lethargy, optic nerve neuropathy, paranoia, psychosis, leukopenia, anxiety, and suicidal ideation. All of these individual indications were 2% of total reviewed indications. There were 16 cases (6.4%) without a documented indication.
| |
| Age, median, y | 66.0 |
| Male sex, % | 50.8 |
| Race or ethnicity, % | |
| White | 76.0 |
| Black or African American | 12.0 |
| Asian | 4.4 |
| Hispanic | 4.0 |
| Unknown or declined | 2.0 |
| Other | 1.6 |
| Indications, %* | |
| Anemia without macrocytosis | 43.2 |
| Anemia with macrocytosis | 13.2 |
| Delirium | 12.0 |
| Malnutrition | 6.4 |
| Peripheral neuropathy | 6.0 |
| Depression | 3.6 |
| Dementia | 3.2 |
| Pancytopenia | 2.4 |
| Other | 10.4 |
| Unknown | 6.4 |
| Comorbidities, % | |
| Depression | 23.2 |
| Alcohol abuse | 18.4 |
| Chronic anemia | 11.2 |
| Malnutrition | 9.6 |
| Prior intestinal surgery | 8.8 |
| Peripheral neuropathy | 6.0 |
| Dementia | 5.6 |
| Gastric bypass surgery | 4.4 |
| End‐stage renal disease | 4.0 |
| End‐stage liver disease | 3.6 |
| Use of phenytoin | 3.2 |
| Inflammatory bowel disease | 2.4 |
| Use of valproic acid | 2.0 |
| Celiac disease | 1.2 |
Of the 2093 serum folate levels, there were 2 deficient (0.1%), 7 low‐normal (0.3%), 1487 normal (71.1%), and 597 high (28.5%) levels (Table 2). There were 128 patients (6.6%) who had more than 1 serum folate level checked within the prior 12 months, with 1 patient having 5 levels obtained during that time period. All of the deficient and low‐normal serum folate results are listed in Table 3. Of the 9 deficient or low‐normal serum folate levels, 8 had comorbid risk factors for folate deficiency. One of the deficient cases was on folic acid and multivitamin supplementation on presentation, although nonadherence with these supplements was documented in the medical record. The other deficient case was not on folic acid supplementation and did not receive folic acid supplementation for the deficient result. Vitamin B12 levels were checked simultaneously to serum folate levels in 85.2% of cases and within 6 months in 99.2% of cases. Of these patients, 2.0% were found to have vitamin B12 deficiency.
| |
| Total tests | 2093 |
| Total patients | 1944 |
| Low (%) | 2 (0.1) |
| Low‐normal (%) | 7 (0.3) |
| Normal (%) | 1487 (71.0) |
| High (%) | 597 (28.5) |
| MCV (StDev) | 92.1 (9.2) |
| Age, y | Sex | Folate (ng/mL) | Indication | Comorbidities | Hgb (g/dL) | MCV (fL) | |
|---|---|---|---|---|---|---|---|
| |||||||
| Deficient results | |||||||
| Case 1 | 35 | Male | 2.6 | Stroke workup | Phenytoin, depression | 16.0 | 91 |
| Case 2 | 63 | Male | 2.9 | Macrocytic anemia | Alcohol abuse, acute GI bleed | 7.7 | 119 |
| Low‐normal results | |||||||
| Case 3 | 64 | Male | 3.3 | Macrocytic anemia | Cirrhosis, alcohol abuse | 12.3 | 109 |
| Case 4 | 42 | Male | 3.4 | Pancytopenia | HIV, B12 deficiency | 7.5 | 93 |
| Case 5 | 58 | Male | 3.4 | Depression | Depression, alcohol abuse | 13.8 | 98 |
| Case 6 | 56 | Female | 3.5 | Depression | Alcohol abuse | ||
| Case 7 | 85 | Male | 3.6 | Delirium | Depression | 10.5 | 91 |
| Case 8 | 81 | Female | 3.6 | Anemia | Chronic anemia | 9.1 | 95 |
| Case 9 | 63 | Male | 3.9 | Anemia | Chronic anemia, malnutrition | 7.6 | 88 |
Based on our institution's charge for serum folate, a total of $316,043 was charged for the 2093 serum folate tests. The amount charged per deficient result was $158,022. Substituting the charge from the Robinson and Mladenovic study,[24] we calculated the corresponding total charge and charge per deficient result as $149,545 and $74,772, respectively. The actual total cost to our hospital was <$4186, with a cost per deficient test of <$2093.
DISCUSSION
Serum folate levels are often obtained when evaluating anemia without macrocytosis and anemia with macrocytosis.[2] They are also frequently obtained in the evaluation of delirium and dementia. A prior study evaluated both inpatient and outpatient serum folate levels in anemia, dementia, and altered mental status and found only 0.4% of serum folate results to be deficient.[26] In their study, the indications for serum folate tests were anemia or macrocytic anemia (60%) and dementia or altered mental status (30%).
We found the indications for serum folate testing in inpatients and emergency department patients to be different than prior studies. The majority of tests were done to evaluate anemia without macrocytosis (43.2%) or anemia with macrocytosis (13.2%). Lower percentages were done for the evaluation of delirium (12.0%) or dementia (3.2%). In addition, there were multiple indications that have not been noted in previous studies, including depression, peripheral neuropathy, malnutrition, pancytopenia, and others. These accounted for 28.0% of all indications. The reason for the difference in indications compared to prior studies is unknown but may be related to our cohort of exclusively inpatients and emergency department patients. Also, we observed a high concurrence of serum folate and vitamin B12 testing, with 85.2% of serum folate levels ordered at the same time as vitamin B12 levels. It appears that the tests are often ordered together even when the indication suggests that vitamin B12 alone may be more appropriate, such as peripheral neuropathy.
We found that serum folate deficiency was rare, occurring in only 2 of 2093 results. Furthermore, the deficient serum folate results may have been checked for inappropriate indications. The first deficient result was noted as part of a stroke workup in a patient not taking folic acid supplementation. Current guidelines do not recommend serum folate testing in patients with new stroke.[33] In the second deficient case, serum folate testing was performed for evaluation of macrocytic anemia with an MCV of 119 fL. Although reasonable, this was an alcoholic patient presenting with acute gastrointestinal bleeding already on folic acid and multivitamin supplementation and known nonadherence with these supplements. In neither case was there a change in management based on the deficient result.
Given the low rate of serum folate deficiency and the lack of change in management based on deficient results, we conclude that there is a low utility of serum folate testing for any indication in inpatients and emergency department patients in folic acid‐fortified countries. Based on prior studies, and supported by our results, there is no evidence for checking serum folate levels in delirium, dementia, peripheral neuropathy, malnutrition, or any of the other indications. In addition, our results demonstrate a low utility even in patients with anemia or macrocytic anemia.
The rate of serum folate deficiency in our study was significantly lower than prior studies.[24, 26] There may have been geographical factors that led to a lower prevalence of folate deficiency in our study population. Our cohort included inpatients and emergency department patients, whereas previous studies had a majority of outpatients. It is known that serum folate levels can rapidly fluctuate with proper nutrition.[34] It may be that our patients received nutrition in the hospital that corrected serum folate levels prior to laboratory testing.
In addition to the low utility of serum folate testing, the charge per deficient result in our study ($158,022) was more than 100‐fold higher than that in the Robinson and Mladenovic study ($1321).[24] Even when correcting for variability in hospital charges by using the charge from the latter study, the charge per deficient serum folate test remained 50‐fold higher ($74,772). This implies that the increase in charge per deficient result was driven in part by a decreased rate of deficient tests. Folic acid fortification is likely responsible for some of the decrease. However, we believe another source is the excessive ordering of serum folate tests in patients without previously accepted indications. Because no change in management was made for the deficient patients in our study, the charge per serum folate deficient result that changed management approached infinity. This compares to $9979 in the Robinson and Mladenovic analysis.[24]
The cost to the hospital of a serum folate test was much lower than the charge, and estimated to be <$2093 per deficient result. Because serum folate tests are performed on a highly automated, random access analyzer that performs thousands of other measurements daily, the capital and labor costs for each test was well below $0.50 combined. With the addition of reagent costs, our total cost for each serum folate measurement was <$2.00. It is somewhat difficult to extrapolate these values to other hospitals, as exact costs and charges are variable. Nonetheless, the exceptionally low utility of serum folate testing makes the costs associated with these tests excessive.
Our study has several limitations. We conducted our study at a single institution in a country with mandatory folic acid fortification. Our results may not be generalizable to other institutions or patient populations, such as those in countries without mandatory folic acid fortification. Only 259 (12.4%) charts were reviewed, and indications were determined in 93.6% of charts, which may have caused our frequency to vary from the true frequency. Additionally, the low rate of deficient serum folate results limited our ability to identify associations with deficiency. Further evaluation for geographic variations of serum folate deficiency may reveal variable rates.
We conclude that in folic acid fortified countries, the rate of serum folate deficiency is increasingly rare, and the charge to patients and payers per deficient result is exceptionally high. In addition, testing in our study did not change clinical management, which makes the costs associated with these test wasteful. Further evaluation of serum folate testing of inpatients and emergency department patients in folic acid fortified countries is warranted; however, based on our results the utility appears poor for all indications.
Disclosure
Nothing to report.
- , . The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181–186.
- , , , et al. Harrison's Principles of Internal Medicine. New York, NY:McGraw‐Hill Professional;2004.
- , . Folic acid, pernicious anaemia, and prevention of neural tube defects. Lancet. 1994;343(8893):307.
- , , , et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high‐functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118(2):161–167.
- , . Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7(6):659–664.
- , , , , , . Plasma homocysteine levels and cognitive status in long‐term stay geriatric patients: a cross‐sectional study. Arch Gerontol Geriatr. 2005;40(2):129–138.
- . Folate responsive neuropathy. Presse Med. 1994;23(3):131–137.
- , . Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol (Oxford). 2005;19(1):59–65.
- , , , et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94(7):3290–3295.
- , , , et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997;65(1):46–52.
- , , , , , . Folate intake and carcinogenesis of the colon and rectum. Int J Epidemiol. 1991;20(2):368–374.
- , . Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr Cancer. 2006;56(1):11–21.
- , , , et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129(7):517–524.
- , , , , . Serum homocysteine and folate but not vitamin B12 are predictors of CHD mortality in older adults [published online ahead of print September 29, 2011]. Eur J Cardiovasc Prev Rehabil. doi: 10.1177/1741826711424568.
- , , , , , . Plasma homocyst(e)ine levels in men with premature coronary artery disease. J Am Coll Cardiol. 1990;16(5):1114–1119.
- , , , et al. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition. 2000;16(6):434–438.
- , , , , , . Low serum folate concentrations are associated with an excess incidence of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Clin Nutr. 2000;54(5):424–428.
- , , , , . Dietary folate and the risk of nonfatal myocardial infarction. Epidemiology. 2002;13(6):700–706.
- , , , et al.;Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and b vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577.
- , , , , , . A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354(26):2764–2772.
- , . Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514.
- , , , . Does the mean corpuscular volume help physicians evaluate hospitalized patients with anemia?J Gen Intern Med. 1990;5(3):187–191.
- , , , , . Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82(2):442–450.
- , . Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110(2):88–90.
- , , , et al. Blood folate levels: the latest NHANES results. NCHS Data Brief. 2008;(6):1–8.
- , , . Clinical utility of folic acid testing for patients with anemia or dementia. J Gen Intern Med. 2008;23(6):824–826.
- . Anemia in adults: a contemporary approach to diagnosis. Mayo Clin Proc 2003;78(10):1274–1280.
- . Anemia in the elderly. Am Fam Physician. 2000;62(7):1565–1572.
- , . Red cell or serum folate? Results from the National Pathology Alliance benchmarking review. J Clin Pathol. 2003;56(12):924–926.
- , , , , , . Hematology. Philadelphia, PA:Churchill Livingstone;2012.
- , , , . Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96(3):239–246.
- . Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–1298.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–1711.
- , , , . Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. J Nutr. 2006;136(12):3074–3078.
- , . The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181–186.
- , , , et al. Harrison's Principles of Internal Medicine. New York, NY:McGraw‐Hill Professional;2004.
- , . Folic acid, pernicious anaemia, and prevention of neural tube defects. Lancet. 1994;343(8893):307.
- , , , et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high‐functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118(2):161–167.
- , . Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7(6):659–664.
- , , , , , . Plasma homocysteine levels and cognitive status in long‐term stay geriatric patients: a cross‐sectional study. Arch Gerontol Geriatr. 2005;40(2):129–138.
- . Folate responsive neuropathy. Presse Med. 1994;23(3):131–137.
- , . Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol (Oxford). 2005;19(1):59–65.
- , , , et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94(7):3290–3295.
- , , , et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997;65(1):46–52.
- , , , , , . Folate intake and carcinogenesis of the colon and rectum. Int J Epidemiol. 1991;20(2):368–374.
- , . Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr Cancer. 2006;56(1):11–21.
- , , , et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129(7):517–524.
- , , , , . Serum homocysteine and folate but not vitamin B12 are predictors of CHD mortality in older adults [published online ahead of print September 29, 2011]. Eur J Cardiovasc Prev Rehabil. doi: 10.1177/1741826711424568.
- , , , , , . Plasma homocyst(e)ine levels in men with premature coronary artery disease. J Am Coll Cardiol. 1990;16(5):1114–1119.
- , , , et al. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition. 2000;16(6):434–438.
- , , , , , . Low serum folate concentrations are associated with an excess incidence of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Clin Nutr. 2000;54(5):424–428.
- , , , , . Dietary folate and the risk of nonfatal myocardial infarction. Epidemiology. 2002;13(6):700–706.
- , , , et al.;Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and b vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577.
- , , , , , . A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354(26):2764–2772.
- , . Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514.
- , , , . Does the mean corpuscular volume help physicians evaluate hospitalized patients with anemia?J Gen Intern Med. 1990;5(3):187–191.
- , , , , . Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82(2):442–450.
- , . Lack of clinical utility of folate levels in the evaluation of macrocytosis or anemia. Am J Med. 2001;110(2):88–90.
- , , , et al. Blood folate levels: the latest NHANES results. NCHS Data Brief. 2008;(6):1–8.
- , , . Clinical utility of folic acid testing for patients with anemia or dementia. J Gen Intern Med. 2008;23(6):824–826.
- . Anemia in adults: a contemporary approach to diagnosis. Mayo Clin Proc 2003;78(10):1274–1280.
- . Anemia in the elderly. Am Fam Physician. 2000;62(7):1565–1572.
- , . Red cell or serum folate? Results from the National Pathology Alliance benchmarking review. J Clin Pathol. 2003;56(12):924–926.
- , , , , , . Hematology. Philadelphia, PA:Churchill Livingstone;2012.
- , , , . Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96(3):239–246.
- . Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–1298.
- , , , et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–1711.
- , , , . Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. J Nutr. 2006;136(12):3074–3078.
Copyright © 2012 Society of Hospital Medicine