User login
Reply to “In Reference to 'Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine'”
Hall et al. draw attention to the important question of whether some patients may benefit from a naloxone prescription when discharged from the hospital with a short-term opioid prescription for acute pain. Although all members of the working group agreed that naloxone is appropriate in some cases, we were hesitant to recommend this as a standard practice for several reasons.
First, the intent of our Consensus Statement1 was to synthesize and summarize the areas of consensus in existing guidelines; none of the existing guidelines included in our systematic review make a recommendation for naloxone prescription in the setting of short-term opioid use for acute pain.2 We believe that this may relate to the fact that the risk factors for overdose and the threshold of risk above which naloxone would be beneficial have yet to be defined for this population and are likely to differ from those defined in patients using opioids chronically.
Additionally, if practitioners follow the recommendations to limit prescribing for acute pain to the minimum dose and duration of an opioid that was presumably administered in the hospital with an observed response, then the risk of overdose and the potential benefit of naloxone will decrease. Furthermore, emerging data from randomized controlled trials demonstrating noninferiority of nonopioid analgesics in the management of acute pain suggest that we should not so readily presume opioids to be the necessary or the best option.3-5 Data questioning the benefits of opioids over other safer therapies have particularly important implications for patients in whom the risks are felt to be high enough to warrant consideration of naloxone.
Disclosures
Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role in the Journal of Hospital Medicine (unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding
Dr. Herzig is funded by a grant number K23AG042459 from the National Institute on Aging. Dr. Mosher is supported in part by the Department of Veterans Affairs Office of Academic Affiliations and the Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). The views expressed in this manuscript do not necessarily represent the views of the funding agencies.
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. doi: 10.12788/jhm.2980. PubMed
2. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines.. J Hosp Med. 2018;13(4):256-262. doi: 10.12788/jhm.2979. PubMed
3. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. doi: 10.1001/jama.2017.16190. PubMed
4. Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emerg Med Australas. 2016;28(6):666-672. doi: 10.1111/1742-6723.12672 PubMed
5. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. doi: 10.1002/14651858.CD004137.pub3 PubMed
Hall et al. draw attention to the important question of whether some patients may benefit from a naloxone prescription when discharged from the hospital with a short-term opioid prescription for acute pain. Although all members of the working group agreed that naloxone is appropriate in some cases, we were hesitant to recommend this as a standard practice for several reasons.
First, the intent of our Consensus Statement1 was to synthesize and summarize the areas of consensus in existing guidelines; none of the existing guidelines included in our systematic review make a recommendation for naloxone prescription in the setting of short-term opioid use for acute pain.2 We believe that this may relate to the fact that the risk factors for overdose and the threshold of risk above which naloxone would be beneficial have yet to be defined for this population and are likely to differ from those defined in patients using opioids chronically.
Additionally, if practitioners follow the recommendations to limit prescribing for acute pain to the minimum dose and duration of an opioid that was presumably administered in the hospital with an observed response, then the risk of overdose and the potential benefit of naloxone will decrease. Furthermore, emerging data from randomized controlled trials demonstrating noninferiority of nonopioid analgesics in the management of acute pain suggest that we should not so readily presume opioids to be the necessary or the best option.3-5 Data questioning the benefits of opioids over other safer therapies have particularly important implications for patients in whom the risks are felt to be high enough to warrant consideration of naloxone.
Disclosures
Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role in the Journal of Hospital Medicine (unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding
Dr. Herzig is funded by a grant number K23AG042459 from the National Institute on Aging. Dr. Mosher is supported in part by the Department of Veterans Affairs Office of Academic Affiliations and the Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). The views expressed in this manuscript do not necessarily represent the views of the funding agencies.
Hall et al. draw attention to the important question of whether some patients may benefit from a naloxone prescription when discharged from the hospital with a short-term opioid prescription for acute pain. Although all members of the working group agreed that naloxone is appropriate in some cases, we were hesitant to recommend this as a standard practice for several reasons.
First, the intent of our Consensus Statement1 was to synthesize and summarize the areas of consensus in existing guidelines; none of the existing guidelines included in our systematic review make a recommendation for naloxone prescription in the setting of short-term opioid use for acute pain.2 We believe that this may relate to the fact that the risk factors for overdose and the threshold of risk above which naloxone would be beneficial have yet to be defined for this population and are likely to differ from those defined in patients using opioids chronically.
Additionally, if practitioners follow the recommendations to limit prescribing for acute pain to the minimum dose and duration of an opioid that was presumably administered in the hospital with an observed response, then the risk of overdose and the potential benefit of naloxone will decrease. Furthermore, emerging data from randomized controlled trials demonstrating noninferiority of nonopioid analgesics in the management of acute pain suggest that we should not so readily presume opioids to be the necessary or the best option.3-5 Data questioning the benefits of opioids over other safer therapies have particularly important implications for patients in whom the risks are felt to be high enough to warrant consideration of naloxone.
Disclosures
Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role in the Journal of Hospital Medicine (unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding
Dr. Herzig is funded by a grant number K23AG042459 from the National Institute on Aging. Dr. Mosher is supported in part by the Department of Veterans Affairs Office of Academic Affiliations and the Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). The views expressed in this manuscript do not necessarily represent the views of the funding agencies.
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. doi: 10.12788/jhm.2980. PubMed
2. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines.. J Hosp Med. 2018;13(4):256-262. doi: 10.12788/jhm.2979. PubMed
3. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. doi: 10.1001/jama.2017.16190. PubMed
4. Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emerg Med Australas. 2016;28(6):666-672. doi: 10.1111/1742-6723.12672 PubMed
5. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. doi: 10.1002/14651858.CD004137.pub3 PubMed
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. doi: 10.12788/jhm.2980. PubMed
2. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines.. J Hosp Med. 2018;13(4):256-262. doi: 10.12788/jhm.2979. PubMed
3. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. doi: 10.1001/jama.2017.16190. PubMed
4. Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emerg Med Australas. 2016;28(6):666-672. doi: 10.1111/1742-6723.12672 PubMed
5. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. doi: 10.1002/14651858.CD004137.pub3 PubMed
Safe Opioid Prescribing for Acute Noncancer Pain in Hospitalized Adults: A Systematic Review of Existing Guidelines
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
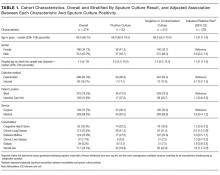
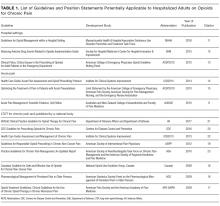
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
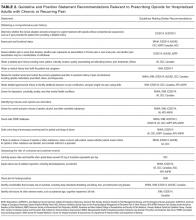
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
© 2018 Society of Hospital Medicine
Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement From the Society of Hospital Medicine
Since the initial reports of an emerging opioid epidemic in the early 2000s, intense focus on improving opioid prescribing in outpatient settings has culminated in new guidelines for chronic pain.1,2 Although opioid stewardship in the setting of chronic pain is of paramount importance in curbing the ongoing epidemic, long-term prescription opioid use often begins with treatment of acute pain.1 In addition to differences in recommended management strategies for acute and chronic pain, there are unique aspects and challenges to pain management in the acute-care setting.
Opioids are commonly used for the treatment of acute pain in hospitalized patients, often at high doses.3 Recent reports highlight that hospital use of opioids impacts downstream use.4-6 Additionally, opioid prescribing practices vary between hospital-based providers and hospitals,3,7 highlighting the need for prescribing standards and guidance. To our knowledge, there are no existing guidelines for improving the safety of opioid use in hospitalized patients outside of the intensive care or immediate perioperative settings.
The Society of Hospital Medicine (SHM) convened a working group to systematically review existing guidelines and develop a consensus statement to assist clinicians in safe opioid use for acute, noncancer pain in hospitalized adults.
Consensus Statement Purpose and Scope
The purpose of this Consensus Statement is to present clinical recommendations on the safe use of opioids for the treatment of acute, noncancer pain in hospitalized adults. The guidance is intended for clinicians practicing medicine in the inpatient setting (eg, hospitalists, primary care physicians, family physicians, nurse practitioners, and physician assistants) and is intended to apply to hospitalized adults with acute, noncancer pain (ie, pain that typically lasts <3 months or during the period of normal tissue healing) outside of the palliative, end-of-life, and intensive care settings.
Consensus Statement Development
Our working group included experts in opioid use in the hospital setting, defined by 1) engagement in the clinical practice of hospital medicine and 2) involvement in clinical research related to usage patterns and clinical outcomes of opioid use in hospitalized patients (see Appendix Table 1). The SHM provided administrative assistance with the project and funded the in-person working group meeting, but it had no role in formulating the recommendations. The SHM Board of Directors provided approval of the Consensus Statement without modification.
An overview of the sequential steps in the Consensus Statement development process is described below; details of the methods and results can be found in the Appendix (eMethods).
Performing the Systematic Review
Drafting the Consensus Statement
After performing the systematic review, the working group drafted and iteratively revised a set of recommendations using a variation of the Delphi Method8 to identify consensus among group members.
External Review
Following agreement on a draft set of recommendations, we obtained feedback from external groups, including 1) individuals involved in the SHM’s Reducing Adverse Drug Events Related to Opioids (RADEO) initiative, including those involved in the development of the implementation guide and site leads for the Mentored Implementation program, 2) SHM members, SHM Patient-Family Advisory Council (PFAC) members, and leaders of other relevant professional societies, and 3) peer-reviewers at the Journal of Hospital Medicine.
RESULTS
Deciding Whether to Use Opioids During Hospitalization
1. SHM recommends that clinicians limit the use of opioids to patients with 1) severe pain or 2) moderate pain that has not responded to nonopioid therapy, or where nonopioid therapy is contraindicated or anticipated to be ineffective.
Opioids are associated with several well-recognized risks ranging from mild to severe, including nausea, constipation, urinary retention, falls, delirium, sedation, physical dependence, addiction, respiratory depression, and death. Given these risks, the risk-to-benefit ratio is generally not favorable at lower levels of pain severity. Furthermore, for most painful conditions, including those causing severe pain, nonopioid analgesics, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), have been demonstrated to be equally or more effective with less risk of harm than opioids.9-13 Clinicians should consider drug–drug and drug–disease associations when deciding between these different therapies and make a determination in each patient regarding whether the benefits outweigh the risks. Often, drug–disease interactions do not represent absolute contraindications, and risks can be mitigated by adhering to dosage limits and, with respect to NSAIDs, 1) monitoring renal function, 2) monitoring volume status in patients with congestive heart failure, and 3) considering a selective cyclooxygenase-2 (COX-2) inhibitor rather than a nonselective NSAID or pairing the NSAID with an acid-suppressive medication in patients with a history of peptic ulcer disease or at elevated risk for gastroduodenal disease. For these reasons, a trial of nonopioid therapy (including pharmacologic and nonpharmacologic modalities) should always be considered before using opioids for pain of any severity. This does not imply that a trial of nonopioid therapy must be performed in all patients, but rather, that the likelihood of benefit and associated risks of opioid and nonopioid therapy should be considered for all patients in determining the best initial management strategy.
2. SHM recommends that clinicians use extra caution when administering opioids to patients with risk factors for opioid-related adverse events.
Several factors have been consistently demonstrated to increase the risk of opioid-related adverse events–most importantly, respiratory depression and overdose–in varied patient populations and settings, including age 65 years and older,1,14-17 renal insufficiency,1,14,18 hepatic insufficiency,1,14 chronic respiratory failure (including chronic obstructive pulmonary disease, sleep apnea, etc.), and receipt of other central nervous system (CNS) depressant medications (including, but not limited to, benzodiazepines).1,18-20 History of any substance use disorder and psychiatric disorders have been associated with an increased risk for the development of opioid use disorder.21-24 These factors should be weighed against the benefits when deciding on opioid appropriateness in a given patient. However, identification of these risks should not preclude opioids as part of pain management. When a decision is made to use opioids in patients with these risk factors, clinicians should 1) use a reduced starting dose (generally, at least a 50% reduction in the usual starting dose) and 2) consider closer monitoring for adverse effects (eg, more frequent nursing assessments, capnography, or more frequent outpatient visits).
3. SHM recommends that clinicians review the information contained in the prescription drug monitoring program (PDMP) database to inform decision-making around opioid therapy.
Although data on the impact of use of the state PDMP database on prescribing practices or patient outcomes are limited, PDMP use has been advocated by multiple guidelines on acute pain management.25-27 The PDMP provides information that can be useful in several ways, including 1) confirmation of prior opioid exposure and dosage, which should be used to guide appropriate dosage selection in the hospital, 2) identification of existing controlled substance prescriptions, which should be considered in prescribing decisions in the hospital and on discharge, and 3) identification of signs of aberrant behavior. For example, the identification of controlled substance prescriptions written by multiple different clinicians can facilitate early identification of potential diversion or evolving or existing opioid use disorder and the opportunity for intervention,28 which may include referral to support services, initiation of medication-assisted treatment, and/or pain specialist consultation when available. Concerns regarding evolving or existing opioid use disorder should prompt further discussion between the clinician and the patient, both to clarify their understanding of their recent prescription history and to discuss concerns for patient safety related to the increased risk of opioid-related adverse effects (including respiratory depression and overdose) among patients with controlled substance prescriptions written by multiple providers. Although such concerns should not automatically preclude the use of opioids for acute pain in the hospital setting, they should be considered in the assessment of whether the benefits of opioid therapy outweigh the risks for a given patient.
4. SHM recommends that clinicians educate patients and families or caregivers about the potential risks and side effects of opioid therapy as well as alternative pharmacologic and nonpharmacologic therapies for managing pain.
Patients are often unaware of the risks of opioid therapy (see Consensus Statement 1 for key risks),29 or that there are often equally effective alternative therapies. As with any therapy associated with substantial risk, clinicians should discuss these risks with patients and/or caregivers at the outset of therapy, as well as the potential benefits of nonopioid pharmacologic and nonpharmacologic therapies for managing pain. Patients should be informed that they may request nonopioid therapy in lieu of opioids, even for severe pain.
Once a Decision Has Been Made to Use Opioids During Hospitalization
5. SHM recommends that clinicians use the lowest effective opioid dose for the shortest duration possible.
Higher opioid doses are associated with an increased incidence of opioid-related adverse events, particularly overdose, in studies of both inpatient and outpatient populations.1,17,19,30,31 Studies in the inpatient and outpatient settings consistently demonstrate that risk increases with dosage.19,30,31 Clinicians should reduce the usual starting dose by at least 50% among patients with conditions that increase susceptibility to opioid-related adverse events (see Consensus Statement 2). The ongoing need for opioids should be re-assessed regularly-at least daily-during the hospitalization, with attempts at tapering as healing occurs and/or pain and function improve.
6. SHM recommends that clinicians use immediate-release opioid formulations and avoid initiation of long-acting or extended-release formulations (including transdermal fentanyl) for treatment of acute pain.
Studies in outpatient settings demonstrate that the use of long-acting opioids is associated with greater risk for overdose–especially in opioid-naïve patients–and long-term use.32,33 Further, hospitalized patients frequently have fluctuating renal function and rapidly changing pain levels. We therefore recommend that initiation of long-acting opioids be avoided for the treatment of acute, noncancer pain in hospitalized medical patients. It is important to note that although we recommend avoiding initiation of long-acting opioids for the treatment of acute, noncancer pain, there are circumstances outside of the scope of this Consensus Statement for which initiation of long-acting opioids may be indicated, including the treatment of opioid withdrawal. We also do not recommend discontinuation of long-acting or extended-release opioids in patients who are taking these medications for chronic pain at the time of hospital admission (unless there are concerns regarding adverse effects or drug–disease interactions).
7. SHM recommends that clinicians use the oral route of administration whenever possible. Intravenous opioids should be reserved for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal malabsorption, or when immediate pain control and/or rapid dose titration is necessary.
Intravenous opioid administration is associated with an increased risk of side effects, adverse events, and medication errors.34-36 Additionally, studies demonstrate that in general, the addiction potential of medications is greater the more rapid the onset of action (the onset of action is 5–10 min for intravenous and 15–30 minutes for oral administration).37,38 Furthermore, the duration of action is greater for oral compared to that of intravenous administration, potentially allowing for more consistent pain relief and less frequent administrations. As such, intravenous administration should be reserved for situations when oral administration is not possible or likely to be ineffective, or when immediate pain control and/or rapid titration is necessary.
8. SHM recommends that clinicians use an opioid equivalency table or calculator to understand the relative potency of different opioids 1) when initiating opioid therapy, 2) when changing from one route of administration to another, and 3) when changing from one opioid to another. When changing from one opioid to another, clinicians should generally reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids as well as possible incomplete cross-tolerance.
Most errors causing preventable adverse drug events in hospitals occur at the ordering stage.39,40 Clinicians are often unaware of the potency of different types of opioids relative to each other or to morphine (ie, morphine equivalent dose), which can lead to inadvertent overdose when initiating therapy with nonmorphine opioids and when converting from one opioid to another. To facilitate safe opioid use, we recommend that clinicians use one of several available opioid equivalency tables or calculators to better understand the relative potencies of opioids and to inform both starting dose calculations and conversions between opioids and routes of administration. When converting from one opioid to another, we caution clinicians to reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids and the potential for incomplete cross-tolerance, wherein tolerance to a currently administered opioid does not extend completely to other opioids. Clinicians should use extreme caution when performing conversions to and from methadone and consider consultation with a hospital pharmacist or a pain management specialist, when available, to assist with conversion decisions and calculations.
9. SHM recommends that clinicians pair opioids with scheduled nonopioid analgesic medications, unless contraindicated, and always consider pairing with nonpharmacologic pain management strategies (ie, multimodal analgesia).
Concurrent receipt of opioids and nonopioid analgesic medications (including acetaminophen, NSAIDs, and gabapentin or pregabalin, depending on the underlying pathophysiology of the pain) has been demonstrated to reduce total opioid requirements and improve pain management.41,42 Clinicians should be familiar with contraindications and maximum dosage recommendations for each of these adjunctive nonopioid medications. We recommend separate orders for each, rather than using drug formulations that combine opioids and nonopioid analgesics in the same pill, due to the risk of inadvertently exceeding the maximum recommended doses of the nonopioid analgesic (particularly acetaminophen) with combination products. We recommend that nonopioid analgesics be ordered at a scheduled frequency, rather than as needed, to facilitate consistent administration that is not dependent on opioid administration. Topical agents, including lidocaine and capsaicin, should also be considered. Nonpharmacologic pain management strategies can include procedure-based (eg, regional and local anesthesia) and nonprocedure-based therapies depending on the underlying condition and institutional availability. Although few studies have assessed the benefit of nonpharmacologic, nonprocedure-based therapies for the treatment of acute pain in hospitalized patients, the lack of harm associated with their use argues for their adoption. Simple nonpharmacologic therapies that can usually be provided to patients in any hospital setting include music therapy, cold or hot packs, chaplain or social work visits (possibly including mindfulness training),43 and physical therapy, among others.
10. SHM recommends that, unless contraindicated, clinicians order a bowel regimen to prevent opioid-induced constipation in patients receiving opioids.
Constipation is a common adverse effect of opioid therapy and results from the activation of mu opioid receptors in the colon, resulting in decreased peristalsis. Hospitalized patients are already prone to constipation due to their often-limited physical mobility. To mitigate this complication, we recommend the administration of a bowel regimen to all hospitalized medical patients receiving opioid therapy, provided the patient is not having diarrhea. Given the mechanism of opioid-induced constipation, stimulant laxatives (eg, senna, bisacodyl) have been recommended for this purpose.44 Osmotic laxatives (eg, polyethylene glycol, lactulose) have demonstrated efficacy for the treatment of constipation more generally (ie, not necessarily opioid-induced constipation). Stool softeners, although frequently used in the inpatient setting, are not recommended due to limited and conflicting evidence for efficacy in prevention or treatment of constipation.45 Bowel movements should be tracked during hospitalization, and the bowel regimen modified accordingly.
11. SHM recommends that clinicians limit co-administration of opioids with other central nervous system depressant medications to the extent possible.
This combination has been demonstrated to increase the risk of opioid-related adverse events in multiple settings of care, including during hospitalization.1,18,19 Although benzodiazepines have received the most attention in this respect, other medications with CNS depressant properties may also increase the risk, including, but not limited to, nonbenzodiazepine sedative-hypnotics (eg, zolpidem, zaleplon, zopiclone), muscle relaxants, sedating antidepressants, antipsychotics, and antihistamines.18,19,46 For some patients, the combination will be unavoidable, and we do not suggest routine discontinuation of longstanding medications that preexisted hospitalization, given the risks of withdrawal and/or worsening of the underlying condition for which these medications are prescribed. Rather, clinicians should carefully consider the necessity of each medication class with input from the patient’s outpatient providers, taper the frequency and/or the dose of CNS depressants when appropriate and feasible, and avoid new coprescriptions to the extent possible, both during hospitalization and on hospital discharge.
12. SHM recommends that clinicians work with patients and families or caregivers to establish realistic goals and expectations of opioid therapy and the expected course of recovery.
Discussing expectations at the start of therapy is important to facilitate a clear understanding of how meaningful improvement will be defined and measured during the hospitalization and how long the patient is anticipated to require opioid therapy. Meaningful improvement should be defined to include improvement in both pain and function. Clinicians should discuss with patients 1) that the goal of opioid therapy is tolerability of pain such that meaningful improvement in function can be achieved and 2) that a decrease in pain intensity in the absence of improved function is not considered meaningful improvement in most situations and should prompt reevaluation of the appropriateness of continued opioid therapy as well as close follow-up with a clinician following hospital discharge. Discussions regarding the expected course of recovery should include that acute pain is expected to resolve as the underlying medical condition improves and that although pain may persist beyond the hospitalization, pain that is severe enough to require opioids will often be resolved or almost resolved by the time of hospital discharge.
13. SHM recommends that clinicians monitor the response to opioid therapy, including assessment for functional improvement and development of adverse effects.
Pain severity and function should be assessed at least daily, and improvement in reported pain severity without improvement in function over several days should, in most circumstances, prompt reconsideration of ongoing opioid therapy and reconsideration of the underlying etiology of pain. Although hospital-specific functional measures in the setting of acute pain have not yet been validated, we suggest that such measures and goals should be individualized based on preexisting function and may include the ability to sit up or move in bed, move to a chair, work with physical therapy, or ambulate in the hallway. Protocols for the assessment for adverse effects are not well established. Because sedation typically precedes respiratory depression, it is generally recommended that patients are evaluated (eg, by nursing staff) for sedation after each opioid administration (10–20 minutes for intravenous and 30–60 minutes for oral administration based on the time-to-peak effect). Whether certain patients may benefit from more intensive respiratory monitoring, such as pulse oximetry or capnography, is an area of active investigation and not yet established.
Prescribing at the Time of Hospital Discharge
14. SHM recommends that clinicians ask patients about any existing opioid supply at home and account for any such supply when issuing an opioid prescription on discharge.
Even in the setting of acute pain, patients may have previously received an opioid prescription from an outpatient clinician prior to hospitalization. Unused prescription opioids create the possibility of both overdose (when patients take multiple opioids concurrently, intentionally or inadvertently) and diversion (many adults with prescription opioid misuse obtained their opioids from a friend or a relative who may or may not have known that this occurred47). The PDMP database can provide information related to the potential existence of any prior opioid supplies, which should be confirmed with the patient and considered when providing a new prescription on hospital discharge. Information on proper disposal should be provided if use of the preexisting opioid is no longer intended.
15. SHM recommends that clinicians prescribe the minimum quantity of opioids anticipated to be necessary based on the expected course and duration of pain that is severe enough to require opioid therapy after hospital discharge.
16. SHM recommends that clinicians ensure that patients and families or caregivers receive information regarding how to minimize the risks of opioid therapy for themselves, their families, and their communities. This includes but is not limited to 1) how to take their opioids correctly (the planned medications, doses, schedule); 2) that they should take the minimum quantity necessary to achieve tolerable levels of pain and meaningful functional improvement, reducing the dose and/or frequency as pain and function improve; 3) how to safeguard their supply and dispose of any unused supply; 4) that they should avoid agents that may potentiate the sedative effect of opioids, including sleeping medication and alcohol; 5) that they should avoid driving or operating heavy machinery while taking opioids; and 6) that they should seek help if they begin to experience any potential adverse effects, with inclusion of information on early warning signs.
Clear and concise patient instructions on home opioid dosing and administration will limit opioid-related adverse events and dosing errors upon hospital discharge. Each of these recommendations derive from one or more of the existing guidelines and reflect the transfer of responsibility for safe opioid use practices that occurs as patients transition from a closely monitored inpatient setting to the more self-regulated home environment.
DISCUSSION AND AREAS FOR FUTURE RESEARCH
This Consensus Statement reflects a synthesis of the key recommendations from a systematic review of existing guidelines on acute pain management, adapted for a hospital-specific scope of practice. Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.
Although these recommendations are not intended to apply to the immediate perioperative setting (ie, care in the postanesthesia care unit), many of the recommendations in the existing guidelines upon which this Consensus Statement was based were intended for the postoperative setting, and, as others have noted, recommendations in this setting are mostly comparable to those for treating acute pain more generally.27 Those interested in pain management in the postoperative setting specifically may wish to review the recent guidelines released by the American Pain Society,50 the content of which is in close alignment with our Consensus Statement.
Several important issues were raised during the extensive external feedback process undertaken as part of the development of this Consensus Statement. Although many issues were incorporated into the recommendations, there were several suggestions for which we felt the evidence base was not sufficient to allow a clear or valid recommendation to be made. For example, several reviewers requested endorsement of specific patient education tools and opioid equivalency calculators. In the absence of tools specifically validated for this purpose, we felt that the evidence was insufficient to make specific recommendations. Validating such tools for use in the inpatient setting should be an area of future investigation. In the meantime, we note that there are several existing and widely available resources for both patient education (ie, opioid information sheets, including opioid risks, safe containment and disposal, and safe use practices) and opioid equivalency calculations that clinicians and hospitals can adapt for their purposes.
Several individuals suggested recommendations on communication with outpatient continuity providers around opioid management decisions during hospitalization and on discharge. Although we believe that it is of paramount importance for outpatient providers to be aware of and have input into these decisions, the optimal timing and the method for such communication are unclear and likely to be institution-specific depending on the availability and integration of electronic records across care settings. We recommend that clinicians use their judgment as to the best format and timing for assuring that outpatient physicians are aware of and have input into these important management decisions with downstream consequences.
Concerns were also raised about the time required to complete the recommended practices and the importance of emphasizing the need for a team-based approach in this realm. We agree wholeheartedly with this sentiment and believe that many of the recommended practices can and should be automated and/or shared across the care team. For example, PDMPs allow prescribers to appoint delegates to check the PDMP on their behalf. Additionally, we suggest that hospitals work to develop systems to assist care teams with performance of these tasks in a standardized and streamlined manner (eg, integrating access to the PDMP and opioid equivalency tables within the electronic health record and developing standard patient educational handouts). Provision of written materials on opioid risks, side effects, and safety practices may be helpful in facilitating consistent messaging and efficient workflow for members of the care team.
Finally, the working group carefully considered whether to include a recommendation regarding naloxone prescribing at the time of hospital discharge. The provision of naloxone kits to laypersons through Overdose Education and Naloxone Distribution Programs has been shown to reduce opioid overdose deaths51,52 and hospitalizations53,54 and is both safe and cost-effective.55 The Centers for Disease Control and Preventionrecommend that clinicians “consider offering naloxone to patients with a history of overdose, a current or past substance use disorder, receipt of ≥50 mg of morphine equivalents per day or concurrent benzodiazepine use.”1 However, these recommendations are intended for patients on chronic opioid therapy; presently, no clear evidence exists to guide decisions about the benefits and costs associated with prescribing naloxone in the setting of short-term opioid therapy for acute pain. Further research in this area is warranted.
The greatest limitation of this Consensus Statement is the lack of high-quality studies informing most of the recommendations in the guidelines upon which our Consensus Statement was based. The majority of recommendations in the existing guidelines were based on expert opinion alone. Additional research is necessary before evidence-based recommendations can be formulated.
Accordingly, the working group identified several key areas for future research, in addition to those noted above. First, ongoing efforts to develop and evaluate the effectiveness of nonopioid and nonpharmacologic management strategies for acute pain in hospitalized patients are necessary. Second, studies identifying the risk factors for opioid-related adverse events in hospitalized patients would help inform management decisions and allow deployment of resources and specialized monitoring strategies to patients at heightened risk. The working group also noted the need for research investigating the impact of PDMP use on management decisions and downstream outcomes among hospitalized patients. Finally, conversations around pain management and concerns related to aberrant behaviors are often challenging in the hospital setting owing to the brief, high-intensity nature of the care and the lack of a longstanding therapeutic alliance. There is a great need to develop strategies and language to facilitate these conversations.
In conclusion, until more high-quality evidence becomes available, clinicians can use the recommendations contained in this Consensus Statement along with their clinical judgment and consultation with pharmacists, interventional pain specialists, and other staff (eg, social work, nursing) to help facilitate consistent, high-quality care across providers and hospitals. We believe that doing so will help increase the appropriateness of opioid therapy, minimize adverse events, facilitate shared decision-making, and foster stronger therapeutic alliances at the outset of the hospitalization for patients suffering from acute pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from the SHM for their facilitation of this project and dedication to this purpose.
The authors would also like to thank the many individuals who provided comments on the draft recommendations, including the participants in the SHM RADEO program; the SHM members; the representatives of specialty societies, including the American Academy of Family Physicians, the American College of Physicians, the American Hospital Association, the American Society of Addiction Medicine, the American Society of Anesthesiologists, the American Society of Health-System Pharmacists, the Society of Critical Care Medicine, and the Society of General Internal Medicine; and the representatives of patient advocacy groups, including SHM PFAC, Regions Hospital Patient and Family Advisory Committee, Patient and Family Centered Care Council of St. Louis Children’s Hospital, Missouri Family Partnership, and Parent and Family Care.
Disclosures: Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena reports receiving consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics, a consultancy to the life sciences industry (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance with the project and funded the in-person working group meeting but had no role in or influence on developing the content of the recommendations themselves. The SHM Board of Directors provided approval to submit the manuscript for publication without modification. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported in part by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, and reporting of the study
1. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States. JAMA. 2016;315(15):1624-1645. PubMed
55. Coffin PO, Sullivan SD. COst-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9. PubMed
54. Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR. 2015;64(23):631-635. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
52. Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Substance abuse 2015;36(2):240-253. PubMed
51. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. PubMed
50. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. PubMed
49. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine 2007;32(19):2127-2132. PubMed
48. Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine 2008;33(2):199-204. PubMed
47. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. PubMed
46. Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58-64. PubMed
45. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. PubMed
44. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159-180. PubMed
43. Garland EL, Baker AK, Larsen P, et al. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med. 2017;32(10):1106-1113. PubMed
42. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial [published online ahead of print December 13, 2017]. JAMA Surg. doi: 10.1001/jamasurg.2017.4915 PubMed
41. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-273. PubMed
40. Davies ED, Schneider F, Childs S, et al. A prevalence study of errors in opioid prescribing in a large teaching hospital. Int J Clin Pract. 2011;65(9):923-929. PubMed
39. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29-34. PubMed
38. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. PubMed
37. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. PubMed
36. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. PubMed
35. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. PubMed
34. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. PubMed
33. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32(1):21-27. PubMed
Since the initial reports of an emerging opioid epidemic in the early 2000s, intense focus on improving opioid prescribing in outpatient settings has culminated in new guidelines for chronic pain.1,2 Although opioid stewardship in the setting of chronic pain is of paramount importance in curbing the ongoing epidemic, long-term prescription opioid use often begins with treatment of acute pain.1 In addition to differences in recommended management strategies for acute and chronic pain, there are unique aspects and challenges to pain management in the acute-care setting.
Opioids are commonly used for the treatment of acute pain in hospitalized patients, often at high doses.3 Recent reports highlight that hospital use of opioids impacts downstream use.4-6 Additionally, opioid prescribing practices vary between hospital-based providers and hospitals,3,7 highlighting the need for prescribing standards and guidance. To our knowledge, there are no existing guidelines for improving the safety of opioid use in hospitalized patients outside of the intensive care or immediate perioperative settings.
The Society of Hospital Medicine (SHM) convened a working group to systematically review existing guidelines and develop a consensus statement to assist clinicians in safe opioid use for acute, noncancer pain in hospitalized adults.
Consensus Statement Purpose and Scope
The purpose of this Consensus Statement is to present clinical recommendations on the safe use of opioids for the treatment of acute, noncancer pain in hospitalized adults. The guidance is intended for clinicians practicing medicine in the inpatient setting (eg, hospitalists, primary care physicians, family physicians, nurse practitioners, and physician assistants) and is intended to apply to hospitalized adults with acute, noncancer pain (ie, pain that typically lasts <3 months or during the period of normal tissue healing) outside of the palliative, end-of-life, and intensive care settings.
Consensus Statement Development
Our working group included experts in opioid use in the hospital setting, defined by 1) engagement in the clinical practice of hospital medicine and 2) involvement in clinical research related to usage patterns and clinical outcomes of opioid use in hospitalized patients (see Appendix Table 1). The SHM provided administrative assistance with the project and funded the in-person working group meeting, but it had no role in formulating the recommendations. The SHM Board of Directors provided approval of the Consensus Statement without modification.
An overview of the sequential steps in the Consensus Statement development process is described below; details of the methods and results can be found in the Appendix (eMethods).
Performing the Systematic Review
Drafting the Consensus Statement
After performing the systematic review, the working group drafted and iteratively revised a set of recommendations using a variation of the Delphi Method8 to identify consensus among group members.
External Review
Following agreement on a draft set of recommendations, we obtained feedback from external groups, including 1) individuals involved in the SHM’s Reducing Adverse Drug Events Related to Opioids (RADEO) initiative, including those involved in the development of the implementation guide and site leads for the Mentored Implementation program, 2) SHM members, SHM Patient-Family Advisory Council (PFAC) members, and leaders of other relevant professional societies, and 3) peer-reviewers at the Journal of Hospital Medicine.
RESULTS
Deciding Whether to Use Opioids During Hospitalization
1. SHM recommends that clinicians limit the use of opioids to patients with 1) severe pain or 2) moderate pain that has not responded to nonopioid therapy, or where nonopioid therapy is contraindicated or anticipated to be ineffective.
Opioids are associated with several well-recognized risks ranging from mild to severe, including nausea, constipation, urinary retention, falls, delirium, sedation, physical dependence, addiction, respiratory depression, and death. Given these risks, the risk-to-benefit ratio is generally not favorable at lower levels of pain severity. Furthermore, for most painful conditions, including those causing severe pain, nonopioid analgesics, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), have been demonstrated to be equally or more effective with less risk of harm than opioids.9-13 Clinicians should consider drug–drug and drug–disease associations when deciding between these different therapies and make a determination in each patient regarding whether the benefits outweigh the risks. Often, drug–disease interactions do not represent absolute contraindications, and risks can be mitigated by adhering to dosage limits and, with respect to NSAIDs, 1) monitoring renal function, 2) monitoring volume status in patients with congestive heart failure, and 3) considering a selective cyclooxygenase-2 (COX-2) inhibitor rather than a nonselective NSAID or pairing the NSAID with an acid-suppressive medication in patients with a history of peptic ulcer disease or at elevated risk for gastroduodenal disease. For these reasons, a trial of nonopioid therapy (including pharmacologic and nonpharmacologic modalities) should always be considered before using opioids for pain of any severity. This does not imply that a trial of nonopioid therapy must be performed in all patients, but rather, that the likelihood of benefit and associated risks of opioid and nonopioid therapy should be considered for all patients in determining the best initial management strategy.
2. SHM recommends that clinicians use extra caution when administering opioids to patients with risk factors for opioid-related adverse events.
Several factors have been consistently demonstrated to increase the risk of opioid-related adverse events–most importantly, respiratory depression and overdose–in varied patient populations and settings, including age 65 years and older,1,14-17 renal insufficiency,1,14,18 hepatic insufficiency,1,14 chronic respiratory failure (including chronic obstructive pulmonary disease, sleep apnea, etc.), and receipt of other central nervous system (CNS) depressant medications (including, but not limited to, benzodiazepines).1,18-20 History of any substance use disorder and psychiatric disorders have been associated with an increased risk for the development of opioid use disorder.21-24 These factors should be weighed against the benefits when deciding on opioid appropriateness in a given patient. However, identification of these risks should not preclude opioids as part of pain management. When a decision is made to use opioids in patients with these risk factors, clinicians should 1) use a reduced starting dose (generally, at least a 50% reduction in the usual starting dose) and 2) consider closer monitoring for adverse effects (eg, more frequent nursing assessments, capnography, or more frequent outpatient visits).
3. SHM recommends that clinicians review the information contained in the prescription drug monitoring program (PDMP) database to inform decision-making around opioid therapy.
Although data on the impact of use of the state PDMP database on prescribing practices or patient outcomes are limited, PDMP use has been advocated by multiple guidelines on acute pain management.25-27 The PDMP provides information that can be useful in several ways, including 1) confirmation of prior opioid exposure and dosage, which should be used to guide appropriate dosage selection in the hospital, 2) identification of existing controlled substance prescriptions, which should be considered in prescribing decisions in the hospital and on discharge, and 3) identification of signs of aberrant behavior. For example, the identification of controlled substance prescriptions written by multiple different clinicians can facilitate early identification of potential diversion or evolving or existing opioid use disorder and the opportunity for intervention,28 which may include referral to support services, initiation of medication-assisted treatment, and/or pain specialist consultation when available. Concerns regarding evolving or existing opioid use disorder should prompt further discussion between the clinician and the patient, both to clarify their understanding of their recent prescription history and to discuss concerns for patient safety related to the increased risk of opioid-related adverse effects (including respiratory depression and overdose) among patients with controlled substance prescriptions written by multiple providers. Although such concerns should not automatically preclude the use of opioids for acute pain in the hospital setting, they should be considered in the assessment of whether the benefits of opioid therapy outweigh the risks for a given patient.
4. SHM recommends that clinicians educate patients and families or caregivers about the potential risks and side effects of opioid therapy as well as alternative pharmacologic and nonpharmacologic therapies for managing pain.
Patients are often unaware of the risks of opioid therapy (see Consensus Statement 1 for key risks),29 or that there are often equally effective alternative therapies. As with any therapy associated with substantial risk, clinicians should discuss these risks with patients and/or caregivers at the outset of therapy, as well as the potential benefits of nonopioid pharmacologic and nonpharmacologic therapies for managing pain. Patients should be informed that they may request nonopioid therapy in lieu of opioids, even for severe pain.
Once a Decision Has Been Made to Use Opioids During Hospitalization
5. SHM recommends that clinicians use the lowest effective opioid dose for the shortest duration possible.
Higher opioid doses are associated with an increased incidence of opioid-related adverse events, particularly overdose, in studies of both inpatient and outpatient populations.1,17,19,30,31 Studies in the inpatient and outpatient settings consistently demonstrate that risk increases with dosage.19,30,31 Clinicians should reduce the usual starting dose by at least 50% among patients with conditions that increase susceptibility to opioid-related adverse events (see Consensus Statement 2). The ongoing need for opioids should be re-assessed regularly-at least daily-during the hospitalization, with attempts at tapering as healing occurs and/or pain and function improve.
6. SHM recommends that clinicians use immediate-release opioid formulations and avoid initiation of long-acting or extended-release formulations (including transdermal fentanyl) for treatment of acute pain.
Studies in outpatient settings demonstrate that the use of long-acting opioids is associated with greater risk for overdose–especially in opioid-naïve patients–and long-term use.32,33 Further, hospitalized patients frequently have fluctuating renal function and rapidly changing pain levels. We therefore recommend that initiation of long-acting opioids be avoided for the treatment of acute, noncancer pain in hospitalized medical patients. It is important to note that although we recommend avoiding initiation of long-acting opioids for the treatment of acute, noncancer pain, there are circumstances outside of the scope of this Consensus Statement for which initiation of long-acting opioids may be indicated, including the treatment of opioid withdrawal. We also do not recommend discontinuation of long-acting or extended-release opioids in patients who are taking these medications for chronic pain at the time of hospital admission (unless there are concerns regarding adverse effects or drug–disease interactions).
7. SHM recommends that clinicians use the oral route of administration whenever possible. Intravenous opioids should be reserved for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal malabsorption, or when immediate pain control and/or rapid dose titration is necessary.
Intravenous opioid administration is associated with an increased risk of side effects, adverse events, and medication errors.34-36 Additionally, studies demonstrate that in general, the addiction potential of medications is greater the more rapid the onset of action (the onset of action is 5–10 min for intravenous and 15–30 minutes for oral administration).37,38 Furthermore, the duration of action is greater for oral compared to that of intravenous administration, potentially allowing for more consistent pain relief and less frequent administrations. As such, intravenous administration should be reserved for situations when oral administration is not possible or likely to be ineffective, or when immediate pain control and/or rapid titration is necessary.
8. SHM recommends that clinicians use an opioid equivalency table or calculator to understand the relative potency of different opioids 1) when initiating opioid therapy, 2) when changing from one route of administration to another, and 3) when changing from one opioid to another. When changing from one opioid to another, clinicians should generally reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids as well as possible incomplete cross-tolerance.
Most errors causing preventable adverse drug events in hospitals occur at the ordering stage.39,40 Clinicians are often unaware of the potency of different types of opioids relative to each other or to morphine (ie, morphine equivalent dose), which can lead to inadvertent overdose when initiating therapy with nonmorphine opioids and when converting from one opioid to another. To facilitate safe opioid use, we recommend that clinicians use one of several available opioid equivalency tables or calculators to better understand the relative potencies of opioids and to inform both starting dose calculations and conversions between opioids and routes of administration. When converting from one opioid to another, we caution clinicians to reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids and the potential for incomplete cross-tolerance, wherein tolerance to a currently administered opioid does not extend completely to other opioids. Clinicians should use extreme caution when performing conversions to and from methadone and consider consultation with a hospital pharmacist or a pain management specialist, when available, to assist with conversion decisions and calculations.
9. SHM recommends that clinicians pair opioids with scheduled nonopioid analgesic medications, unless contraindicated, and always consider pairing with nonpharmacologic pain management strategies (ie, multimodal analgesia).
Concurrent receipt of opioids and nonopioid analgesic medications (including acetaminophen, NSAIDs, and gabapentin or pregabalin, depending on the underlying pathophysiology of the pain) has been demonstrated to reduce total opioid requirements and improve pain management.41,42 Clinicians should be familiar with contraindications and maximum dosage recommendations for each of these adjunctive nonopioid medications. We recommend separate orders for each, rather than using drug formulations that combine opioids and nonopioid analgesics in the same pill, due to the risk of inadvertently exceeding the maximum recommended doses of the nonopioid analgesic (particularly acetaminophen) with combination products. We recommend that nonopioid analgesics be ordered at a scheduled frequency, rather than as needed, to facilitate consistent administration that is not dependent on opioid administration. Topical agents, including lidocaine and capsaicin, should also be considered. Nonpharmacologic pain management strategies can include procedure-based (eg, regional and local anesthesia) and nonprocedure-based therapies depending on the underlying condition and institutional availability. Although few studies have assessed the benefit of nonpharmacologic, nonprocedure-based therapies for the treatment of acute pain in hospitalized patients, the lack of harm associated with their use argues for their adoption. Simple nonpharmacologic therapies that can usually be provided to patients in any hospital setting include music therapy, cold or hot packs, chaplain or social work visits (possibly including mindfulness training),43 and physical therapy, among others.
10. SHM recommends that, unless contraindicated, clinicians order a bowel regimen to prevent opioid-induced constipation in patients receiving opioids.
Constipation is a common adverse effect of opioid therapy and results from the activation of mu opioid receptors in the colon, resulting in decreased peristalsis. Hospitalized patients are already prone to constipation due to their often-limited physical mobility. To mitigate this complication, we recommend the administration of a bowel regimen to all hospitalized medical patients receiving opioid therapy, provided the patient is not having diarrhea. Given the mechanism of opioid-induced constipation, stimulant laxatives (eg, senna, bisacodyl) have been recommended for this purpose.44 Osmotic laxatives (eg, polyethylene glycol, lactulose) have demonstrated efficacy for the treatment of constipation more generally (ie, not necessarily opioid-induced constipation). Stool softeners, although frequently used in the inpatient setting, are not recommended due to limited and conflicting evidence for efficacy in prevention or treatment of constipation.45 Bowel movements should be tracked during hospitalization, and the bowel regimen modified accordingly.
11. SHM recommends that clinicians limit co-administration of opioids with other central nervous system depressant medications to the extent possible.
This combination has been demonstrated to increase the risk of opioid-related adverse events in multiple settings of care, including during hospitalization.1,18,19 Although benzodiazepines have received the most attention in this respect, other medications with CNS depressant properties may also increase the risk, including, but not limited to, nonbenzodiazepine sedative-hypnotics (eg, zolpidem, zaleplon, zopiclone), muscle relaxants, sedating antidepressants, antipsychotics, and antihistamines.18,19,46 For some patients, the combination will be unavoidable, and we do not suggest routine discontinuation of longstanding medications that preexisted hospitalization, given the risks of withdrawal and/or worsening of the underlying condition for which these medications are prescribed. Rather, clinicians should carefully consider the necessity of each medication class with input from the patient’s outpatient providers, taper the frequency and/or the dose of CNS depressants when appropriate and feasible, and avoid new coprescriptions to the extent possible, both during hospitalization and on hospital discharge.
12. SHM recommends that clinicians work with patients and families or caregivers to establish realistic goals and expectations of opioid therapy and the expected course of recovery.
Discussing expectations at the start of therapy is important to facilitate a clear understanding of how meaningful improvement will be defined and measured during the hospitalization and how long the patient is anticipated to require opioid therapy. Meaningful improvement should be defined to include improvement in both pain and function. Clinicians should discuss with patients 1) that the goal of opioid therapy is tolerability of pain such that meaningful improvement in function can be achieved and 2) that a decrease in pain intensity in the absence of improved function is not considered meaningful improvement in most situations and should prompt reevaluation of the appropriateness of continued opioid therapy as well as close follow-up with a clinician following hospital discharge. Discussions regarding the expected course of recovery should include that acute pain is expected to resolve as the underlying medical condition improves and that although pain may persist beyond the hospitalization, pain that is severe enough to require opioids will often be resolved or almost resolved by the time of hospital discharge.
13. SHM recommends that clinicians monitor the response to opioid therapy, including assessment for functional improvement and development of adverse effects.
Pain severity and function should be assessed at least daily, and improvement in reported pain severity without improvement in function over several days should, in most circumstances, prompt reconsideration of ongoing opioid therapy and reconsideration of the underlying etiology of pain. Although hospital-specific functional measures in the setting of acute pain have not yet been validated, we suggest that such measures and goals should be individualized based on preexisting function and may include the ability to sit up or move in bed, move to a chair, work with physical therapy, or ambulate in the hallway. Protocols for the assessment for adverse effects are not well established. Because sedation typically precedes respiratory depression, it is generally recommended that patients are evaluated (eg, by nursing staff) for sedation after each opioid administration (10–20 minutes for intravenous and 30–60 minutes for oral administration based on the time-to-peak effect). Whether certain patients may benefit from more intensive respiratory monitoring, such as pulse oximetry or capnography, is an area of active investigation and not yet established.
Prescribing at the Time of Hospital Discharge
14. SHM recommends that clinicians ask patients about any existing opioid supply at home and account for any such supply when issuing an opioid prescription on discharge.
Even in the setting of acute pain, patients may have previously received an opioid prescription from an outpatient clinician prior to hospitalization. Unused prescription opioids create the possibility of both overdose (when patients take multiple opioids concurrently, intentionally or inadvertently) and diversion (many adults with prescription opioid misuse obtained their opioids from a friend or a relative who may or may not have known that this occurred47). The PDMP database can provide information related to the potential existence of any prior opioid supplies, which should be confirmed with the patient and considered when providing a new prescription on hospital discharge. Information on proper disposal should be provided if use of the preexisting opioid is no longer intended.
15. SHM recommends that clinicians prescribe the minimum quantity of opioids anticipated to be necessary based on the expected course and duration of pain that is severe enough to require opioid therapy after hospital discharge.
16. SHM recommends that clinicians ensure that patients and families or caregivers receive information regarding how to minimize the risks of opioid therapy for themselves, their families, and their communities. This includes but is not limited to 1) how to take their opioids correctly (the planned medications, doses, schedule); 2) that they should take the minimum quantity necessary to achieve tolerable levels of pain and meaningful functional improvement, reducing the dose and/or frequency as pain and function improve; 3) how to safeguard their supply and dispose of any unused supply; 4) that they should avoid agents that may potentiate the sedative effect of opioids, including sleeping medication and alcohol; 5) that they should avoid driving or operating heavy machinery while taking opioids; and 6) that they should seek help if they begin to experience any potential adverse effects, with inclusion of information on early warning signs.
Clear and concise patient instructions on home opioid dosing and administration will limit opioid-related adverse events and dosing errors upon hospital discharge. Each of these recommendations derive from one or more of the existing guidelines and reflect the transfer of responsibility for safe opioid use practices that occurs as patients transition from a closely monitored inpatient setting to the more self-regulated home environment.
DISCUSSION AND AREAS FOR FUTURE RESEARCH
This Consensus Statement reflects a synthesis of the key recommendations from a systematic review of existing guidelines on acute pain management, adapted for a hospital-specific scope of practice. Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.
Although these recommendations are not intended to apply to the immediate perioperative setting (ie, care in the postanesthesia care unit), many of the recommendations in the existing guidelines upon which this Consensus Statement was based were intended for the postoperative setting, and, as others have noted, recommendations in this setting are mostly comparable to those for treating acute pain more generally.27 Those interested in pain management in the postoperative setting specifically may wish to review the recent guidelines released by the American Pain Society,50 the content of which is in close alignment with our Consensus Statement.
Several important issues were raised during the extensive external feedback process undertaken as part of the development of this Consensus Statement. Although many issues were incorporated into the recommendations, there were several suggestions for which we felt the evidence base was not sufficient to allow a clear or valid recommendation to be made. For example, several reviewers requested endorsement of specific patient education tools and opioid equivalency calculators. In the absence of tools specifically validated for this purpose, we felt that the evidence was insufficient to make specific recommendations. Validating such tools for use in the inpatient setting should be an area of future investigation. In the meantime, we note that there are several existing and widely available resources for both patient education (ie, opioid information sheets, including opioid risks, safe containment and disposal, and safe use practices) and opioid equivalency calculations that clinicians and hospitals can adapt for their purposes.
Several individuals suggested recommendations on communication with outpatient continuity providers around opioid management decisions during hospitalization and on discharge. Although we believe that it is of paramount importance for outpatient providers to be aware of and have input into these decisions, the optimal timing and the method for such communication are unclear and likely to be institution-specific depending on the availability and integration of electronic records across care settings. We recommend that clinicians use their judgment as to the best format and timing for assuring that outpatient physicians are aware of and have input into these important management decisions with downstream consequences.
Concerns were also raised about the time required to complete the recommended practices and the importance of emphasizing the need for a team-based approach in this realm. We agree wholeheartedly with this sentiment and believe that many of the recommended practices can and should be automated and/or shared across the care team. For example, PDMPs allow prescribers to appoint delegates to check the PDMP on their behalf. Additionally, we suggest that hospitals work to develop systems to assist care teams with performance of these tasks in a standardized and streamlined manner (eg, integrating access to the PDMP and opioid equivalency tables within the electronic health record and developing standard patient educational handouts). Provision of written materials on opioid risks, side effects, and safety practices may be helpful in facilitating consistent messaging and efficient workflow for members of the care team.
Finally, the working group carefully considered whether to include a recommendation regarding naloxone prescribing at the time of hospital discharge. The provision of naloxone kits to laypersons through Overdose Education and Naloxone Distribution Programs has been shown to reduce opioid overdose deaths51,52 and hospitalizations53,54 and is both safe and cost-effective.55 The Centers for Disease Control and Preventionrecommend that clinicians “consider offering naloxone to patients with a history of overdose, a current or past substance use disorder, receipt of ≥50 mg of morphine equivalents per day or concurrent benzodiazepine use.”1 However, these recommendations are intended for patients on chronic opioid therapy; presently, no clear evidence exists to guide decisions about the benefits and costs associated with prescribing naloxone in the setting of short-term opioid therapy for acute pain. Further research in this area is warranted.
The greatest limitation of this Consensus Statement is the lack of high-quality studies informing most of the recommendations in the guidelines upon which our Consensus Statement was based. The majority of recommendations in the existing guidelines were based on expert opinion alone. Additional research is necessary before evidence-based recommendations can be formulated.
Accordingly, the working group identified several key areas for future research, in addition to those noted above. First, ongoing efforts to develop and evaluate the effectiveness of nonopioid and nonpharmacologic management strategies for acute pain in hospitalized patients are necessary. Second, studies identifying the risk factors for opioid-related adverse events in hospitalized patients would help inform management decisions and allow deployment of resources and specialized monitoring strategies to patients at heightened risk. The working group also noted the need for research investigating the impact of PDMP use on management decisions and downstream outcomes among hospitalized patients. Finally, conversations around pain management and concerns related to aberrant behaviors are often challenging in the hospital setting owing to the brief, high-intensity nature of the care and the lack of a longstanding therapeutic alliance. There is a great need to develop strategies and language to facilitate these conversations.
In conclusion, until more high-quality evidence becomes available, clinicians can use the recommendations contained in this Consensus Statement along with their clinical judgment and consultation with pharmacists, interventional pain specialists, and other staff (eg, social work, nursing) to help facilitate consistent, high-quality care across providers and hospitals. We believe that doing so will help increase the appropriateness of opioid therapy, minimize adverse events, facilitate shared decision-making, and foster stronger therapeutic alliances at the outset of the hospitalization for patients suffering from acute pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from the SHM for their facilitation of this project and dedication to this purpose.
The authors would also like to thank the many individuals who provided comments on the draft recommendations, including the participants in the SHM RADEO program; the SHM members; the representatives of specialty societies, including the American Academy of Family Physicians, the American College of Physicians, the American Hospital Association, the American Society of Addiction Medicine, the American Society of Anesthesiologists, the American Society of Health-System Pharmacists, the Society of Critical Care Medicine, and the Society of General Internal Medicine; and the representatives of patient advocacy groups, including SHM PFAC, Regions Hospital Patient and Family Advisory Committee, Patient and Family Centered Care Council of St. Louis Children’s Hospital, Missouri Family Partnership, and Parent and Family Care.
Disclosures: Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena reports receiving consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics, a consultancy to the life sciences industry (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance with the project and funded the in-person working group meeting but had no role in or influence on developing the content of the recommendations themselves. The SHM Board of Directors provided approval to submit the manuscript for publication without modification. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported in part by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, and reporting of the study
Since the initial reports of an emerging opioid epidemic in the early 2000s, intense focus on improving opioid prescribing in outpatient settings has culminated in new guidelines for chronic pain.1,2 Although opioid stewardship in the setting of chronic pain is of paramount importance in curbing the ongoing epidemic, long-term prescription opioid use often begins with treatment of acute pain.1 In addition to differences in recommended management strategies for acute and chronic pain, there are unique aspects and challenges to pain management in the acute-care setting.
Opioids are commonly used for the treatment of acute pain in hospitalized patients, often at high doses.3 Recent reports highlight that hospital use of opioids impacts downstream use.4-6 Additionally, opioid prescribing practices vary between hospital-based providers and hospitals,3,7 highlighting the need for prescribing standards and guidance. To our knowledge, there are no existing guidelines for improving the safety of opioid use in hospitalized patients outside of the intensive care or immediate perioperative settings.
The Society of Hospital Medicine (SHM) convened a working group to systematically review existing guidelines and develop a consensus statement to assist clinicians in safe opioid use for acute, noncancer pain in hospitalized adults.
Consensus Statement Purpose and Scope
The purpose of this Consensus Statement is to present clinical recommendations on the safe use of opioids for the treatment of acute, noncancer pain in hospitalized adults. The guidance is intended for clinicians practicing medicine in the inpatient setting (eg, hospitalists, primary care physicians, family physicians, nurse practitioners, and physician assistants) and is intended to apply to hospitalized adults with acute, noncancer pain (ie, pain that typically lasts <3 months or during the period of normal tissue healing) outside of the palliative, end-of-life, and intensive care settings.
Consensus Statement Development
Our working group included experts in opioid use in the hospital setting, defined by 1) engagement in the clinical practice of hospital medicine and 2) involvement in clinical research related to usage patterns and clinical outcomes of opioid use in hospitalized patients (see Appendix Table 1). The SHM provided administrative assistance with the project and funded the in-person working group meeting, but it had no role in formulating the recommendations. The SHM Board of Directors provided approval of the Consensus Statement without modification.
An overview of the sequential steps in the Consensus Statement development process is described below; details of the methods and results can be found in the Appendix (eMethods).
Performing the Systematic Review
Drafting the Consensus Statement
After performing the systematic review, the working group drafted and iteratively revised a set of recommendations using a variation of the Delphi Method8 to identify consensus among group members.
External Review
Following agreement on a draft set of recommendations, we obtained feedback from external groups, including 1) individuals involved in the SHM’s Reducing Adverse Drug Events Related to Opioids (RADEO) initiative, including those involved in the development of the implementation guide and site leads for the Mentored Implementation program, 2) SHM members, SHM Patient-Family Advisory Council (PFAC) members, and leaders of other relevant professional societies, and 3) peer-reviewers at the Journal of Hospital Medicine.
RESULTS
Deciding Whether to Use Opioids During Hospitalization
1. SHM recommends that clinicians limit the use of opioids to patients with 1) severe pain or 2) moderate pain that has not responded to nonopioid therapy, or where nonopioid therapy is contraindicated or anticipated to be ineffective.
Opioids are associated with several well-recognized risks ranging from mild to severe, including nausea, constipation, urinary retention, falls, delirium, sedation, physical dependence, addiction, respiratory depression, and death. Given these risks, the risk-to-benefit ratio is generally not favorable at lower levels of pain severity. Furthermore, for most painful conditions, including those causing severe pain, nonopioid analgesics, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), have been demonstrated to be equally or more effective with less risk of harm than opioids.9-13 Clinicians should consider drug–drug and drug–disease associations when deciding between these different therapies and make a determination in each patient regarding whether the benefits outweigh the risks. Often, drug–disease interactions do not represent absolute contraindications, and risks can be mitigated by adhering to dosage limits and, with respect to NSAIDs, 1) monitoring renal function, 2) monitoring volume status in patients with congestive heart failure, and 3) considering a selective cyclooxygenase-2 (COX-2) inhibitor rather than a nonselective NSAID or pairing the NSAID with an acid-suppressive medication in patients with a history of peptic ulcer disease or at elevated risk for gastroduodenal disease. For these reasons, a trial of nonopioid therapy (including pharmacologic and nonpharmacologic modalities) should always be considered before using opioids for pain of any severity. This does not imply that a trial of nonopioid therapy must be performed in all patients, but rather, that the likelihood of benefit and associated risks of opioid and nonopioid therapy should be considered for all patients in determining the best initial management strategy.
2. SHM recommends that clinicians use extra caution when administering opioids to patients with risk factors for opioid-related adverse events.
Several factors have been consistently demonstrated to increase the risk of opioid-related adverse events–most importantly, respiratory depression and overdose–in varied patient populations and settings, including age 65 years and older,1,14-17 renal insufficiency,1,14,18 hepatic insufficiency,1,14 chronic respiratory failure (including chronic obstructive pulmonary disease, sleep apnea, etc.), and receipt of other central nervous system (CNS) depressant medications (including, but not limited to, benzodiazepines).1,18-20 History of any substance use disorder and psychiatric disorders have been associated with an increased risk for the development of opioid use disorder.21-24 These factors should be weighed against the benefits when deciding on opioid appropriateness in a given patient. However, identification of these risks should not preclude opioids as part of pain management. When a decision is made to use opioids in patients with these risk factors, clinicians should 1) use a reduced starting dose (generally, at least a 50% reduction in the usual starting dose) and 2) consider closer monitoring for adverse effects (eg, more frequent nursing assessments, capnography, or more frequent outpatient visits).
3. SHM recommends that clinicians review the information contained in the prescription drug monitoring program (PDMP) database to inform decision-making around opioid therapy.
Although data on the impact of use of the state PDMP database on prescribing practices or patient outcomes are limited, PDMP use has been advocated by multiple guidelines on acute pain management.25-27 The PDMP provides information that can be useful in several ways, including 1) confirmation of prior opioid exposure and dosage, which should be used to guide appropriate dosage selection in the hospital, 2) identification of existing controlled substance prescriptions, which should be considered in prescribing decisions in the hospital and on discharge, and 3) identification of signs of aberrant behavior. For example, the identification of controlled substance prescriptions written by multiple different clinicians can facilitate early identification of potential diversion or evolving or existing opioid use disorder and the opportunity for intervention,28 which may include referral to support services, initiation of medication-assisted treatment, and/or pain specialist consultation when available. Concerns regarding evolving or existing opioid use disorder should prompt further discussion between the clinician and the patient, both to clarify their understanding of their recent prescription history and to discuss concerns for patient safety related to the increased risk of opioid-related adverse effects (including respiratory depression and overdose) among patients with controlled substance prescriptions written by multiple providers. Although such concerns should not automatically preclude the use of opioids for acute pain in the hospital setting, they should be considered in the assessment of whether the benefits of opioid therapy outweigh the risks for a given patient.
4. SHM recommends that clinicians educate patients and families or caregivers about the potential risks and side effects of opioid therapy as well as alternative pharmacologic and nonpharmacologic therapies for managing pain.
Patients are often unaware of the risks of opioid therapy (see Consensus Statement 1 for key risks),29 or that there are often equally effective alternative therapies. As with any therapy associated with substantial risk, clinicians should discuss these risks with patients and/or caregivers at the outset of therapy, as well as the potential benefits of nonopioid pharmacologic and nonpharmacologic therapies for managing pain. Patients should be informed that they may request nonopioid therapy in lieu of opioids, even for severe pain.
Once a Decision Has Been Made to Use Opioids During Hospitalization
5. SHM recommends that clinicians use the lowest effective opioid dose for the shortest duration possible.
Higher opioid doses are associated with an increased incidence of opioid-related adverse events, particularly overdose, in studies of both inpatient and outpatient populations.1,17,19,30,31 Studies in the inpatient and outpatient settings consistently demonstrate that risk increases with dosage.19,30,31 Clinicians should reduce the usual starting dose by at least 50% among patients with conditions that increase susceptibility to opioid-related adverse events (see Consensus Statement 2). The ongoing need for opioids should be re-assessed regularly-at least daily-during the hospitalization, with attempts at tapering as healing occurs and/or pain and function improve.
6. SHM recommends that clinicians use immediate-release opioid formulations and avoid initiation of long-acting or extended-release formulations (including transdermal fentanyl) for treatment of acute pain.
Studies in outpatient settings demonstrate that the use of long-acting opioids is associated with greater risk for overdose–especially in opioid-naïve patients–and long-term use.32,33 Further, hospitalized patients frequently have fluctuating renal function and rapidly changing pain levels. We therefore recommend that initiation of long-acting opioids be avoided for the treatment of acute, noncancer pain in hospitalized medical patients. It is important to note that although we recommend avoiding initiation of long-acting opioids for the treatment of acute, noncancer pain, there are circumstances outside of the scope of this Consensus Statement for which initiation of long-acting opioids may be indicated, including the treatment of opioid withdrawal. We also do not recommend discontinuation of long-acting or extended-release opioids in patients who are taking these medications for chronic pain at the time of hospital admission (unless there are concerns regarding adverse effects or drug–disease interactions).
7. SHM recommends that clinicians use the oral route of administration whenever possible. Intravenous opioids should be reserved for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal malabsorption, or when immediate pain control and/or rapid dose titration is necessary.
Intravenous opioid administration is associated with an increased risk of side effects, adverse events, and medication errors.34-36 Additionally, studies demonstrate that in general, the addiction potential of medications is greater the more rapid the onset of action (the onset of action is 5–10 min for intravenous and 15–30 minutes for oral administration).37,38 Furthermore, the duration of action is greater for oral compared to that of intravenous administration, potentially allowing for more consistent pain relief and less frequent administrations. As such, intravenous administration should be reserved for situations when oral administration is not possible or likely to be ineffective, or when immediate pain control and/or rapid titration is necessary.
8. SHM recommends that clinicians use an opioid equivalency table or calculator to understand the relative potency of different opioids 1) when initiating opioid therapy, 2) when changing from one route of administration to another, and 3) when changing from one opioid to another. When changing from one opioid to another, clinicians should generally reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids as well as possible incomplete cross-tolerance.
Most errors causing preventable adverse drug events in hospitals occur at the ordering stage.39,40 Clinicians are often unaware of the potency of different types of opioids relative to each other or to morphine (ie, morphine equivalent dose), which can lead to inadvertent overdose when initiating therapy with nonmorphine opioids and when converting from one opioid to another. To facilitate safe opioid use, we recommend that clinicians use one of several available opioid equivalency tables or calculators to better understand the relative potencies of opioids and to inform both starting dose calculations and conversions between opioids and routes of administration. When converting from one opioid to another, we caution clinicians to reduce the dose of the new opioid by at least 25%–50% of the calculated equianalgesic dose to account for interindividual variability in the response to opioids and the potential for incomplete cross-tolerance, wherein tolerance to a currently administered opioid does not extend completely to other opioids. Clinicians should use extreme caution when performing conversions to and from methadone and consider consultation with a hospital pharmacist or a pain management specialist, when available, to assist with conversion decisions and calculations.
9. SHM recommends that clinicians pair opioids with scheduled nonopioid analgesic medications, unless contraindicated, and always consider pairing with nonpharmacologic pain management strategies (ie, multimodal analgesia).
Concurrent receipt of opioids and nonopioid analgesic medications (including acetaminophen, NSAIDs, and gabapentin or pregabalin, depending on the underlying pathophysiology of the pain) has been demonstrated to reduce total opioid requirements and improve pain management.41,42 Clinicians should be familiar with contraindications and maximum dosage recommendations for each of these adjunctive nonopioid medications. We recommend separate orders for each, rather than using drug formulations that combine opioids and nonopioid analgesics in the same pill, due to the risk of inadvertently exceeding the maximum recommended doses of the nonopioid analgesic (particularly acetaminophen) with combination products. We recommend that nonopioid analgesics be ordered at a scheduled frequency, rather than as needed, to facilitate consistent administration that is not dependent on opioid administration. Topical agents, including lidocaine and capsaicin, should also be considered. Nonpharmacologic pain management strategies can include procedure-based (eg, regional and local anesthesia) and nonprocedure-based therapies depending on the underlying condition and institutional availability. Although few studies have assessed the benefit of nonpharmacologic, nonprocedure-based therapies for the treatment of acute pain in hospitalized patients, the lack of harm associated with their use argues for their adoption. Simple nonpharmacologic therapies that can usually be provided to patients in any hospital setting include music therapy, cold or hot packs, chaplain or social work visits (possibly including mindfulness training),43 and physical therapy, among others.
10. SHM recommends that, unless contraindicated, clinicians order a bowel regimen to prevent opioid-induced constipation in patients receiving opioids.
Constipation is a common adverse effect of opioid therapy and results from the activation of mu opioid receptors in the colon, resulting in decreased peristalsis. Hospitalized patients are already prone to constipation due to their often-limited physical mobility. To mitigate this complication, we recommend the administration of a bowel regimen to all hospitalized medical patients receiving opioid therapy, provided the patient is not having diarrhea. Given the mechanism of opioid-induced constipation, stimulant laxatives (eg, senna, bisacodyl) have been recommended for this purpose.44 Osmotic laxatives (eg, polyethylene glycol, lactulose) have demonstrated efficacy for the treatment of constipation more generally (ie, not necessarily opioid-induced constipation). Stool softeners, although frequently used in the inpatient setting, are not recommended due to limited and conflicting evidence for efficacy in prevention or treatment of constipation.45 Bowel movements should be tracked during hospitalization, and the bowel regimen modified accordingly.
11. SHM recommends that clinicians limit co-administration of opioids with other central nervous system depressant medications to the extent possible.
This combination has been demonstrated to increase the risk of opioid-related adverse events in multiple settings of care, including during hospitalization.1,18,19 Although benzodiazepines have received the most attention in this respect, other medications with CNS depressant properties may also increase the risk, including, but not limited to, nonbenzodiazepine sedative-hypnotics (eg, zolpidem, zaleplon, zopiclone), muscle relaxants, sedating antidepressants, antipsychotics, and antihistamines.18,19,46 For some patients, the combination will be unavoidable, and we do not suggest routine discontinuation of longstanding medications that preexisted hospitalization, given the risks of withdrawal and/or worsening of the underlying condition for which these medications are prescribed. Rather, clinicians should carefully consider the necessity of each medication class with input from the patient’s outpatient providers, taper the frequency and/or the dose of CNS depressants when appropriate and feasible, and avoid new coprescriptions to the extent possible, both during hospitalization and on hospital discharge.
12. SHM recommends that clinicians work with patients and families or caregivers to establish realistic goals and expectations of opioid therapy and the expected course of recovery.
Discussing expectations at the start of therapy is important to facilitate a clear understanding of how meaningful improvement will be defined and measured during the hospitalization and how long the patient is anticipated to require opioid therapy. Meaningful improvement should be defined to include improvement in both pain and function. Clinicians should discuss with patients 1) that the goal of opioid therapy is tolerability of pain such that meaningful improvement in function can be achieved and 2) that a decrease in pain intensity in the absence of improved function is not considered meaningful improvement in most situations and should prompt reevaluation of the appropriateness of continued opioid therapy as well as close follow-up with a clinician following hospital discharge. Discussions regarding the expected course of recovery should include that acute pain is expected to resolve as the underlying medical condition improves and that although pain may persist beyond the hospitalization, pain that is severe enough to require opioids will often be resolved or almost resolved by the time of hospital discharge.
13. SHM recommends that clinicians monitor the response to opioid therapy, including assessment for functional improvement and development of adverse effects.
Pain severity and function should be assessed at least daily, and improvement in reported pain severity without improvement in function over several days should, in most circumstances, prompt reconsideration of ongoing opioid therapy and reconsideration of the underlying etiology of pain. Although hospital-specific functional measures in the setting of acute pain have not yet been validated, we suggest that such measures and goals should be individualized based on preexisting function and may include the ability to sit up or move in bed, move to a chair, work with physical therapy, or ambulate in the hallway. Protocols for the assessment for adverse effects are not well established. Because sedation typically precedes respiratory depression, it is generally recommended that patients are evaluated (eg, by nursing staff) for sedation after each opioid administration (10–20 minutes for intravenous and 30–60 minutes for oral administration based on the time-to-peak effect). Whether certain patients may benefit from more intensive respiratory monitoring, such as pulse oximetry or capnography, is an area of active investigation and not yet established.
Prescribing at the Time of Hospital Discharge
14. SHM recommends that clinicians ask patients about any existing opioid supply at home and account for any such supply when issuing an opioid prescription on discharge.
Even in the setting of acute pain, patients may have previously received an opioid prescription from an outpatient clinician prior to hospitalization. Unused prescription opioids create the possibility of both overdose (when patients take multiple opioids concurrently, intentionally or inadvertently) and diversion (many adults with prescription opioid misuse obtained their opioids from a friend or a relative who may or may not have known that this occurred47). The PDMP database can provide information related to the potential existence of any prior opioid supplies, which should be confirmed with the patient and considered when providing a new prescription on hospital discharge. Information on proper disposal should be provided if use of the preexisting opioid is no longer intended.
15. SHM recommends that clinicians prescribe the minimum quantity of opioids anticipated to be necessary based on the expected course and duration of pain that is severe enough to require opioid therapy after hospital discharge.
16. SHM recommends that clinicians ensure that patients and families or caregivers receive information regarding how to minimize the risks of opioid therapy for themselves, their families, and their communities. This includes but is not limited to 1) how to take their opioids correctly (the planned medications, doses, schedule); 2) that they should take the minimum quantity necessary to achieve tolerable levels of pain and meaningful functional improvement, reducing the dose and/or frequency as pain and function improve; 3) how to safeguard their supply and dispose of any unused supply; 4) that they should avoid agents that may potentiate the sedative effect of opioids, including sleeping medication and alcohol; 5) that they should avoid driving or operating heavy machinery while taking opioids; and 6) that they should seek help if they begin to experience any potential adverse effects, with inclusion of information on early warning signs.
Clear and concise patient instructions on home opioid dosing and administration will limit opioid-related adverse events and dosing errors upon hospital discharge. Each of these recommendations derive from one or more of the existing guidelines and reflect the transfer of responsibility for safe opioid use practices that occurs as patients transition from a closely monitored inpatient setting to the more self-regulated home environment.
DISCUSSION AND AREAS FOR FUTURE RESEARCH
This Consensus Statement reflects a synthesis of the key recommendations from a systematic review of existing guidelines on acute pain management, adapted for a hospital-specific scope of practice. Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.
Although these recommendations are not intended to apply to the immediate perioperative setting (ie, care in the postanesthesia care unit), many of the recommendations in the existing guidelines upon which this Consensus Statement was based were intended for the postoperative setting, and, as others have noted, recommendations in this setting are mostly comparable to those for treating acute pain more generally.27 Those interested in pain management in the postoperative setting specifically may wish to review the recent guidelines released by the American Pain Society,50 the content of which is in close alignment with our Consensus Statement.
Several important issues were raised during the extensive external feedback process undertaken as part of the development of this Consensus Statement. Although many issues were incorporated into the recommendations, there were several suggestions for which we felt the evidence base was not sufficient to allow a clear or valid recommendation to be made. For example, several reviewers requested endorsement of specific patient education tools and opioid equivalency calculators. In the absence of tools specifically validated for this purpose, we felt that the evidence was insufficient to make specific recommendations. Validating such tools for use in the inpatient setting should be an area of future investigation. In the meantime, we note that there are several existing and widely available resources for both patient education (ie, opioid information sheets, including opioid risks, safe containment and disposal, and safe use practices) and opioid equivalency calculations that clinicians and hospitals can adapt for their purposes.
Several individuals suggested recommendations on communication with outpatient continuity providers around opioid management decisions during hospitalization and on discharge. Although we believe that it is of paramount importance for outpatient providers to be aware of and have input into these decisions, the optimal timing and the method for such communication are unclear and likely to be institution-specific depending on the availability and integration of electronic records across care settings. We recommend that clinicians use their judgment as to the best format and timing for assuring that outpatient physicians are aware of and have input into these important management decisions with downstream consequences.
Concerns were also raised about the time required to complete the recommended practices and the importance of emphasizing the need for a team-based approach in this realm. We agree wholeheartedly with this sentiment and believe that many of the recommended practices can and should be automated and/or shared across the care team. For example, PDMPs allow prescribers to appoint delegates to check the PDMP on their behalf. Additionally, we suggest that hospitals work to develop systems to assist care teams with performance of these tasks in a standardized and streamlined manner (eg, integrating access to the PDMP and opioid equivalency tables within the electronic health record and developing standard patient educational handouts). Provision of written materials on opioid risks, side effects, and safety practices may be helpful in facilitating consistent messaging and efficient workflow for members of the care team.
Finally, the working group carefully considered whether to include a recommendation regarding naloxone prescribing at the time of hospital discharge. The provision of naloxone kits to laypersons through Overdose Education and Naloxone Distribution Programs has been shown to reduce opioid overdose deaths51,52 and hospitalizations53,54 and is both safe and cost-effective.55 The Centers for Disease Control and Preventionrecommend that clinicians “consider offering naloxone to patients with a history of overdose, a current or past substance use disorder, receipt of ≥50 mg of morphine equivalents per day or concurrent benzodiazepine use.”1 However, these recommendations are intended for patients on chronic opioid therapy; presently, no clear evidence exists to guide decisions about the benefits and costs associated with prescribing naloxone in the setting of short-term opioid therapy for acute pain. Further research in this area is warranted.
The greatest limitation of this Consensus Statement is the lack of high-quality studies informing most of the recommendations in the guidelines upon which our Consensus Statement was based. The majority of recommendations in the existing guidelines were based on expert opinion alone. Additional research is necessary before evidence-based recommendations can be formulated.
Accordingly, the working group identified several key areas for future research, in addition to those noted above. First, ongoing efforts to develop and evaluate the effectiveness of nonopioid and nonpharmacologic management strategies for acute pain in hospitalized patients are necessary. Second, studies identifying the risk factors for opioid-related adverse events in hospitalized patients would help inform management decisions and allow deployment of resources and specialized monitoring strategies to patients at heightened risk. The working group also noted the need for research investigating the impact of PDMP use on management decisions and downstream outcomes among hospitalized patients. Finally, conversations around pain management and concerns related to aberrant behaviors are often challenging in the hospital setting owing to the brief, high-intensity nature of the care and the lack of a longstanding therapeutic alliance. There is a great need to develop strategies and language to facilitate these conversations.
In conclusion, until more high-quality evidence becomes available, clinicians can use the recommendations contained in this Consensus Statement along with their clinical judgment and consultation with pharmacists, interventional pain specialists, and other staff (eg, social work, nursing) to help facilitate consistent, high-quality care across providers and hospitals. We believe that doing so will help increase the appropriateness of opioid therapy, minimize adverse events, facilitate shared decision-making, and foster stronger therapeutic alliances at the outset of the hospitalization for patients suffering from acute pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from the SHM for their facilitation of this project and dedication to this purpose.
The authors would also like to thank the many individuals who provided comments on the draft recommendations, including the participants in the SHM RADEO program; the SHM members; the representatives of specialty societies, including the American Academy of Family Physicians, the American College of Physicians, the American Hospital Association, the American Society of Addiction Medicine, the American Society of Anesthesiologists, the American Society of Health-System Pharmacists, the Society of Critical Care Medicine, and the Society of General Internal Medicine; and the representatives of patient advocacy groups, including SHM PFAC, Regions Hospital Patient and Family Advisory Committee, Patient and Family Centered Care Council of St. Louis Children’s Hospital, Missouri Family Partnership, and Parent and Family Care.
Disclosures: Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena reports receiving consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics, a consultancy to the life sciences industry (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance with the project and funded the in-person working group meeting but had no role in or influence on developing the content of the recommendations themselves. The SHM Board of Directors provided approval to submit the manuscript for publication without modification. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported in part by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, and reporting of the study
1. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States. JAMA. 2016;315(15):1624-1645. PubMed
55. Coffin PO, Sullivan SD. COst-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9. PubMed
54. Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR. 2015;64(23):631-635. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
52. Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Substance abuse 2015;36(2):240-253. PubMed
51. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. PubMed
50. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. PubMed
49. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine 2007;32(19):2127-2132. PubMed
48. Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine 2008;33(2):199-204. PubMed
47. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. PubMed
46. Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58-64. PubMed
45. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. PubMed
44. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159-180. PubMed
43. Garland EL, Baker AK, Larsen P, et al. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med. 2017;32(10):1106-1113. PubMed
42. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial [published online ahead of print December 13, 2017]. JAMA Surg. doi: 10.1001/jamasurg.2017.4915 PubMed
41. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-273. PubMed
40. Davies ED, Schneider F, Childs S, et al. A prevalence study of errors in opioid prescribing in a large teaching hospital. Int J Clin Pract. 2011;65(9):923-929. PubMed
39. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29-34. PubMed
38. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. PubMed
37. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. PubMed
36. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. PubMed
35. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. PubMed
34. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. PubMed
33. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32(1):21-27. PubMed
1. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States. JAMA. 2016;315(15):1624-1645. PubMed
55. Coffin PO, Sullivan SD. COst-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9. PubMed
54. Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR. 2015;64(23):631-635. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
52. Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Substance abuse 2015;36(2):240-253. PubMed
51. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. PubMed
50. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. PubMed
49. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine 2007;32(19):2127-2132. PubMed
48. Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine 2008;33(2):199-204. PubMed
47. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. PubMed
46. Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58-64. PubMed
45. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. PubMed
44. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159-180. PubMed
43. Garland EL, Baker AK, Larsen P, et al. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med. 2017;32(10):1106-1113. PubMed
42. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial [published online ahead of print December 13, 2017]. JAMA Surg. doi: 10.1001/jamasurg.2017.4915 PubMed
41. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-273. PubMed
40. Davies ED, Schneider F, Childs S, et al. A prevalence study of errors in opioid prescribing in a large teaching hospital. Int J Clin Pract. 2011;65(9):923-929. PubMed
39. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29-34. PubMed
38. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. PubMed
37. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. PubMed
36. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. PubMed
35. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. PubMed
34. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. PubMed
33. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32(1):21-27. PubMed
© 2018 Society of Hospital Medicine
The Diagnostic Yield of Noninvasive Microbiologic Sputum Sampling in a Cohort of Patients with Clinically Diagnosed Hospital-Acquired Pneumonia
Pneumonia is a major cause of hospitalization, mortality, and healthcare cost. 1,2 The diagnosis involves clinical features plus radiographic evidence of infection. Hospital-acquired pneumonia (HAP) is defined by the Infectious Disease Society of America (IDSA) as a pneumonia that occurs ≥48 hours after admission and is not associated with mechanical ventilation. 3
IDSA recommendations suggest that patients with suspected HAP be treated based on results of noninvasively obtained sputum cultures rather than being treated empirically. 3 This recommendation is graded as weak with low-quality evidence based on a lack of both evidence showing that respiratory cultures improve clinical outcomes and studies examining the yield of noninvasive collection methods. 4,5 However, resistant pathogens lead to a risk of inadequate empiric therapy, which is associated with increased mortality. 6 Culture data may provide an opportunity for escalation or de-escalation of antibiotic coverage. IDSA recommendations for microbiologic sampling are thus aimed at increasing appropriate coverage and minimizing unnecessary antibiotic exposure.
While the yield and clinical utility of sputum culture in community-acquired pneumonia has been studied extensively, data examining the yield of sputum culture in HAP (non–ventilator-associated pneumonia [non-VAP]) are sparse. In 1 small single-center study, researchers demonstrated positive sputum cultures in 17/35 (48.6%) patients with radiographically confirmed cases of HAP, 7 while in another study, researchers demonstrated positive sputum cultures in 57/63 (90.5%). 8 We aimed to identify the frequency with which sputum cultures positively identify an organism, identify predictors of positive sputum cultures, and characterize the microbiology of sputum cultures in a large cohort of HAP cases.
METHODS
We conducted a retrospective cohort study of patients admitted to a large academic medical center in Boston, Massachusetts, from January 2007 to July 2013. All patients ≥18 years of age were eligible for inclusion. We excluded outside hospital transfers, those with a length of hospitalization <48 hours, and psychiatric admissions.
The study was approved by the institutional review board at the Beth Israel Deaconess Medical Center and granted a waiver of informed consent. Data were collected from electronic databases and supplemented by chart review.
Hospital-Acquired Pneumonia
We defined HAP as pneumonia occurring at least 48 hours after admission, consistent with American Thoracic Society and IDSA criteria.3 To identify cases, we reviewed the charts of all admissions identified as having a discharge International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for bacterial pneumonia (481, 482, 483, 485, 486, 507), indicated as not “present-on-admission.” We validated that the treating clinician had clinically diagnosed pneumonia and initiated antibiotics for this purpose by performing chart review. We reviewed the radiologist interpretation of radiographs surrounding the date of the clinical diagnosis of pneumonia to confirm the presence of a new opacity. Uncertain cases (with respect to either the presence of pneumonia or the timing of the diagnosis) were reviewed by a second member of the study team and, in the case of disagreement, adjudicated by a third member of the study team. Only the first clinically validated HAP per hospitalization was included in the analysis. To focus on HAP rather than VAP, we excluded hospitalizations in which the date of a procedure code for mechanical ventilation preceded the date of pneumonia diagnosis.
Microbiology
In our analysis, we used sputum samples obtained from expectorated or induced samples to evaluate the yield of noninvasive sputum sampling, as recommended by the IDSA. We included sputum samples collected ≥48 hours after admission and within 7 days of the clinical diagnosis of HAP. Sputum samples with >10 epithelial cells per high-power field (hpf) were considered to be contaminated. Among noncontaminated samples, positive sputum cultures were defined as those with a microbiologic diagnosis other than “oral flora,” while those with no growth or growth of oral flora or only yeast were considered to be negative. The hospital’s microbiology laboratory does not routinely provide species identification for Gram-negative rods (GNRs) growing on culture in the presence of growth of ≥3 other colony types. We considered such GNRs (not further speciated) to represent a positive culture result in our analysis given that colonization versus pathogenicity is a clinical distinction and, as such, these results may impact antibiotic choice.
Statistical Analysis
Data were analyzed by using SAS software, version 9.3. We used a 2-sided P value of <0.05 to indicate statistical significance for all comparisons. We used the χ2 test and the nonparametric median test for unadjusted comparisons.
To identify predictors of a positive (versus negative or contaminated) sputum culture among patients with HAP, we used a generalized estimating equation model with a Poisson distribution error term, log link, and first-order autoregressive correlation structure to account for multiple sputum specimens per patient. We combined culture negative and contaminated samples to highlight the clinical utility of sputum culture in a real-world setting. Potential predictors chosen based on clinical grounds included all variables listed in Table 1. We defined comorbidities specified in Table 1 via ICD-9-CM secondary diagnosis codes and diagnosis related groups (DRGs) using Healthcare Cost and Utilization Project Comorbidity Software, version 3.7, based on the work of Elixhauser et al.9,10; dialysis use was defined by an ICD-9-CM procedure code of 39.95; inpatient steroid use was defined by a hospital pharmacy charge for a systemic steroid in the 7 days preceding the sputum sample.
RESULTS
There were 230,635 hospitalizations of patients ≥18 years of age from January 2007 to July 2013. After excluding outside hospital transfers (n = 14,422), hospitalizations <48 hours in duration (n = 59,774), and psychiatric hospitalizations (n = 9887), there were 146,552 hospitalizations in the cohort.
The top 3 bacterial organisms cultured from sputum samples were GNRs not further speciated (25.9%), Staphylococcus aureus (21.0%), and Pseudomonas aeruginosa (14.8%). The frequencies of isolated microorganisms are presented in Table 2.
In an adjusted analysis (Table 1), the significant predictors of a positive sputum culture were chronic lung disease (relative risk [RR] = 2.0; 95% confidence interval [CI], 1.2-3.4) and steroid use (RR = 1.8; 95% CI, 1.1-3.2).
DISCUSSION
To our knowledge, our study is the first to assess the predictors of positive sputum culture among patients with HAP (non-VAP) who had sputum samples obtained noninvasively, and this study is larger than prior studies in which researchers reported on sputum culture yield in HAP. Sputum samples were obtained in 29.4% cases of clinically diagnosed HAP. Although 87% of specimens obtained were culture-negative or contaminated, 13% yielded a bacterial organism. Although we do not report the antibiotic sensitivity patterns of the isolated organisms, the organisms identified frequently demonstrate antibiotic resistance, highlighting the potential for both antibiotic escalation and de-escalation based on sputum culture. In a multivariable model, presence of chronic lung disease and steroid use in the preceding week were both significantly associated with culture positivity.
The retrospective nature of the study raises the possibility of selection bias from systematic differences between the 29.4% of patients with HAP who had sputum collected and those who did not. Patients with sputum cultures were similar to patients without cultures in most measured characteristics, but we are unable to know what the yield of noninvasive sputum culture would have been had all patients with HAP been sampled. As such, our findings reflect the yield of sputum culture among patients with HAP for whom cultures were successfully obtained. It is not clear why only 29.4% of HAP patients received IDSA guideline-concordant care, but similar rates of culture use are reported elsewhere.7 While physician decision-making could have contributed to this finding, it is also possible that many sick, hospitalized patients are simply unable to produce sputum for analysis. In future studies, researchers should examine barriers to guideline-concordant care.
We considered a culture result of GNRs (not further speciated) as positive in our analysis because this result indicates growth of mixed bacterial types, the pathogenicity of which is a clinical determination. Physicians may request speciation and antibiotic sensitivities and, as such, these results have the potential to impact antibiotic choice. Had we considered such cultures to be negative or contaminated, the rate of culture positivity would have been only slightly reduced from 63/478 (13.2%) to 50/478 (10.5%).
The strengths of our study include the chart-based validation of administratively identified cases of pneumonia and a large cohort. There are also limitations. The single-center nature of the study has implications for pretest probability and generalizability. Additionally, in our study, we did not examine outcomes among patients treated empirically versus those treated based on sputum culture results. Finally, our reliance on administrative codes to identify cases of HAP for subsequent validation could have resulted in incomplete capture of HAP cases.
In conclusion, in our study, we provide an estimate of the diagnostic yield of sputum culture in a large cohort with chart-validated HAP, a description of HAP microbiology, and predictors of positive sputum culture. Thirteen percent of patients who had sputum culture testing received a microbiologic diagnosis. Because of the relative ease of obtaining a sputum sample and the microbiologic distribution in our study (representing a mix of commonly drug-resistant pathogens and more typical community-acquired pathogens), we suggest that sputum culture in HAP is a useful diagnostic tool with the potential to inform antibiotic escalation or de-escalation.
Acknowledgments
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant number K24AG035075 from the National Institute on Aging. The funding organizations had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure
No conflicts of interest apply for any of the authors.
1. Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: Final Data for 2009. Natl Vital Stat Rep. 2011;60(3):1-116. PubMed
2. Bonafede MM, Suaya JA, Wilson KL, Mannino DM, Polsky D. Incidence and cost of CAP in a large working-age population. Am J Manag Care. 2012;18(7):380-387. PubMed
3. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. PubMed
4. Wahl WL, Franklin GA, Brandt MM, et al. Does bronchoalveolar lavage enhance our ability to treat ventilator-associated pneumonia in a trauma-burn intensive care unit? J Trauma. 2003;54(4):633-638. PubMed
5. Herer B, Fuhrman C, Demontrond D, Gazevic Z, Housset B, Chouaïd C. Diagnosis of nosocomial pneumonia in medical ward: Repeatability of the protected specimen brush. Eur Respir J. 2001;18(1):157-163. PubMed
6. Chung DR, Song JH, Kim SH, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409-1417. PubMed
7. Russell CD, Koch O, Laurenson IF, O’Shea DT, Sutherland R, Mackintosh CL. Diagnosis and features of hospital-acquired pneumonia: a retrospective cohort study. J Hosp Infect. 2016;92(3):273-279. PubMed
8. Messika J, Stoclin A, Bouvard E, et al. The Challenging Diagnosis of Non-Community-Acquired Pneumonia in Non-Mechanically Ventilated Subjects: Value of Microbiological Investigation. Respir Care. 2016;61(2):225-234. PubMed
9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
10. HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP). January 2013. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed on March 15, 2016.
Pneumonia is a major cause of hospitalization, mortality, and healthcare cost. 1,2 The diagnosis involves clinical features plus radiographic evidence of infection. Hospital-acquired pneumonia (HAP) is defined by the Infectious Disease Society of America (IDSA) as a pneumonia that occurs ≥48 hours after admission and is not associated with mechanical ventilation. 3
IDSA recommendations suggest that patients with suspected HAP be treated based on results of noninvasively obtained sputum cultures rather than being treated empirically. 3 This recommendation is graded as weak with low-quality evidence based on a lack of both evidence showing that respiratory cultures improve clinical outcomes and studies examining the yield of noninvasive collection methods. 4,5 However, resistant pathogens lead to a risk of inadequate empiric therapy, which is associated with increased mortality. 6 Culture data may provide an opportunity for escalation or de-escalation of antibiotic coverage. IDSA recommendations for microbiologic sampling are thus aimed at increasing appropriate coverage and minimizing unnecessary antibiotic exposure.
While the yield and clinical utility of sputum culture in community-acquired pneumonia has been studied extensively, data examining the yield of sputum culture in HAP (non–ventilator-associated pneumonia [non-VAP]) are sparse. In 1 small single-center study, researchers demonstrated positive sputum cultures in 17/35 (48.6%) patients with radiographically confirmed cases of HAP, 7 while in another study, researchers demonstrated positive sputum cultures in 57/63 (90.5%). 8 We aimed to identify the frequency with which sputum cultures positively identify an organism, identify predictors of positive sputum cultures, and characterize the microbiology of sputum cultures in a large cohort of HAP cases.
METHODS
We conducted a retrospective cohort study of patients admitted to a large academic medical center in Boston, Massachusetts, from January 2007 to July 2013. All patients ≥18 years of age were eligible for inclusion. We excluded outside hospital transfers, those with a length of hospitalization <48 hours, and psychiatric admissions.
The study was approved by the institutional review board at the Beth Israel Deaconess Medical Center and granted a waiver of informed consent. Data were collected from electronic databases and supplemented by chart review.
Hospital-Acquired Pneumonia
We defined HAP as pneumonia occurring at least 48 hours after admission, consistent with American Thoracic Society and IDSA criteria.3 To identify cases, we reviewed the charts of all admissions identified as having a discharge International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for bacterial pneumonia (481, 482, 483, 485, 486, 507), indicated as not “present-on-admission.” We validated that the treating clinician had clinically diagnosed pneumonia and initiated antibiotics for this purpose by performing chart review. We reviewed the radiologist interpretation of radiographs surrounding the date of the clinical diagnosis of pneumonia to confirm the presence of a new opacity. Uncertain cases (with respect to either the presence of pneumonia or the timing of the diagnosis) were reviewed by a second member of the study team and, in the case of disagreement, adjudicated by a third member of the study team. Only the first clinically validated HAP per hospitalization was included in the analysis. To focus on HAP rather than VAP, we excluded hospitalizations in which the date of a procedure code for mechanical ventilation preceded the date of pneumonia diagnosis.
Microbiology
In our analysis, we used sputum samples obtained from expectorated or induced samples to evaluate the yield of noninvasive sputum sampling, as recommended by the IDSA. We included sputum samples collected ≥48 hours after admission and within 7 days of the clinical diagnosis of HAP. Sputum samples with >10 epithelial cells per high-power field (hpf) were considered to be contaminated. Among noncontaminated samples, positive sputum cultures were defined as those with a microbiologic diagnosis other than “oral flora,” while those with no growth or growth of oral flora or only yeast were considered to be negative. The hospital’s microbiology laboratory does not routinely provide species identification for Gram-negative rods (GNRs) growing on culture in the presence of growth of ≥3 other colony types. We considered such GNRs (not further speciated) to represent a positive culture result in our analysis given that colonization versus pathogenicity is a clinical distinction and, as such, these results may impact antibiotic choice.
Statistical Analysis
Data were analyzed by using SAS software, version 9.3. We used a 2-sided P value of <0.05 to indicate statistical significance for all comparisons. We used the χ2 test and the nonparametric median test for unadjusted comparisons.
To identify predictors of a positive (versus negative or contaminated) sputum culture among patients with HAP, we used a generalized estimating equation model with a Poisson distribution error term, log link, and first-order autoregressive correlation structure to account for multiple sputum specimens per patient. We combined culture negative and contaminated samples to highlight the clinical utility of sputum culture in a real-world setting. Potential predictors chosen based on clinical grounds included all variables listed in Table 1. We defined comorbidities specified in Table 1 via ICD-9-CM secondary diagnosis codes and diagnosis related groups (DRGs) using Healthcare Cost and Utilization Project Comorbidity Software, version 3.7, based on the work of Elixhauser et al.9,10; dialysis use was defined by an ICD-9-CM procedure code of 39.95; inpatient steroid use was defined by a hospital pharmacy charge for a systemic steroid in the 7 days preceding the sputum sample.
RESULTS
There were 230,635 hospitalizations of patients ≥18 years of age from January 2007 to July 2013. After excluding outside hospital transfers (n = 14,422), hospitalizations <48 hours in duration (n = 59,774), and psychiatric hospitalizations (n = 9887), there were 146,552 hospitalizations in the cohort.
The top 3 bacterial organisms cultured from sputum samples were GNRs not further speciated (25.9%), Staphylococcus aureus (21.0%), and Pseudomonas aeruginosa (14.8%). The frequencies of isolated microorganisms are presented in Table 2.
In an adjusted analysis (Table 1), the significant predictors of a positive sputum culture were chronic lung disease (relative risk [RR] = 2.0; 95% confidence interval [CI], 1.2-3.4) and steroid use (RR = 1.8; 95% CI, 1.1-3.2).
DISCUSSION
To our knowledge, our study is the first to assess the predictors of positive sputum culture among patients with HAP (non-VAP) who had sputum samples obtained noninvasively, and this study is larger than prior studies in which researchers reported on sputum culture yield in HAP. Sputum samples were obtained in 29.4% cases of clinically diagnosed HAP. Although 87% of specimens obtained were culture-negative or contaminated, 13% yielded a bacterial organism. Although we do not report the antibiotic sensitivity patterns of the isolated organisms, the organisms identified frequently demonstrate antibiotic resistance, highlighting the potential for both antibiotic escalation and de-escalation based on sputum culture. In a multivariable model, presence of chronic lung disease and steroid use in the preceding week were both significantly associated with culture positivity.
The retrospective nature of the study raises the possibility of selection bias from systematic differences between the 29.4% of patients with HAP who had sputum collected and those who did not. Patients with sputum cultures were similar to patients without cultures in most measured characteristics, but we are unable to know what the yield of noninvasive sputum culture would have been had all patients with HAP been sampled. As such, our findings reflect the yield of sputum culture among patients with HAP for whom cultures were successfully obtained. It is not clear why only 29.4% of HAP patients received IDSA guideline-concordant care, but similar rates of culture use are reported elsewhere.7 While physician decision-making could have contributed to this finding, it is also possible that many sick, hospitalized patients are simply unable to produce sputum for analysis. In future studies, researchers should examine barriers to guideline-concordant care.
We considered a culture result of GNRs (not further speciated) as positive in our analysis because this result indicates growth of mixed bacterial types, the pathogenicity of which is a clinical determination. Physicians may request speciation and antibiotic sensitivities and, as such, these results have the potential to impact antibiotic choice. Had we considered such cultures to be negative or contaminated, the rate of culture positivity would have been only slightly reduced from 63/478 (13.2%) to 50/478 (10.5%).
The strengths of our study include the chart-based validation of administratively identified cases of pneumonia and a large cohort. There are also limitations. The single-center nature of the study has implications for pretest probability and generalizability. Additionally, in our study, we did not examine outcomes among patients treated empirically versus those treated based on sputum culture results. Finally, our reliance on administrative codes to identify cases of HAP for subsequent validation could have resulted in incomplete capture of HAP cases.
In conclusion, in our study, we provide an estimate of the diagnostic yield of sputum culture in a large cohort with chart-validated HAP, a description of HAP microbiology, and predictors of positive sputum culture. Thirteen percent of patients who had sputum culture testing received a microbiologic diagnosis. Because of the relative ease of obtaining a sputum sample and the microbiologic distribution in our study (representing a mix of commonly drug-resistant pathogens and more typical community-acquired pathogens), we suggest that sputum culture in HAP is a useful diagnostic tool with the potential to inform antibiotic escalation or de-escalation.
Acknowledgments
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant number K24AG035075 from the National Institute on Aging. The funding organizations had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure
No conflicts of interest apply for any of the authors.
Pneumonia is a major cause of hospitalization, mortality, and healthcare cost. 1,2 The diagnosis involves clinical features plus radiographic evidence of infection. Hospital-acquired pneumonia (HAP) is defined by the Infectious Disease Society of America (IDSA) as a pneumonia that occurs ≥48 hours after admission and is not associated with mechanical ventilation. 3
IDSA recommendations suggest that patients with suspected HAP be treated based on results of noninvasively obtained sputum cultures rather than being treated empirically. 3 This recommendation is graded as weak with low-quality evidence based on a lack of both evidence showing that respiratory cultures improve clinical outcomes and studies examining the yield of noninvasive collection methods. 4,5 However, resistant pathogens lead to a risk of inadequate empiric therapy, which is associated with increased mortality. 6 Culture data may provide an opportunity for escalation or de-escalation of antibiotic coverage. IDSA recommendations for microbiologic sampling are thus aimed at increasing appropriate coverage and minimizing unnecessary antibiotic exposure.
While the yield and clinical utility of sputum culture in community-acquired pneumonia has been studied extensively, data examining the yield of sputum culture in HAP (non–ventilator-associated pneumonia [non-VAP]) are sparse. In 1 small single-center study, researchers demonstrated positive sputum cultures in 17/35 (48.6%) patients with radiographically confirmed cases of HAP, 7 while in another study, researchers demonstrated positive sputum cultures in 57/63 (90.5%). 8 We aimed to identify the frequency with which sputum cultures positively identify an organism, identify predictors of positive sputum cultures, and characterize the microbiology of sputum cultures in a large cohort of HAP cases.
METHODS
We conducted a retrospective cohort study of patients admitted to a large academic medical center in Boston, Massachusetts, from January 2007 to July 2013. All patients ≥18 years of age were eligible for inclusion. We excluded outside hospital transfers, those with a length of hospitalization <48 hours, and psychiatric admissions.
The study was approved by the institutional review board at the Beth Israel Deaconess Medical Center and granted a waiver of informed consent. Data were collected from electronic databases and supplemented by chart review.
Hospital-Acquired Pneumonia
We defined HAP as pneumonia occurring at least 48 hours after admission, consistent with American Thoracic Society and IDSA criteria.3 To identify cases, we reviewed the charts of all admissions identified as having a discharge International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for bacterial pneumonia (481, 482, 483, 485, 486, 507), indicated as not “present-on-admission.” We validated that the treating clinician had clinically diagnosed pneumonia and initiated antibiotics for this purpose by performing chart review. We reviewed the radiologist interpretation of radiographs surrounding the date of the clinical diagnosis of pneumonia to confirm the presence of a new opacity. Uncertain cases (with respect to either the presence of pneumonia or the timing of the diagnosis) were reviewed by a second member of the study team and, in the case of disagreement, adjudicated by a third member of the study team. Only the first clinically validated HAP per hospitalization was included in the analysis. To focus on HAP rather than VAP, we excluded hospitalizations in which the date of a procedure code for mechanical ventilation preceded the date of pneumonia diagnosis.
Microbiology
In our analysis, we used sputum samples obtained from expectorated or induced samples to evaluate the yield of noninvasive sputum sampling, as recommended by the IDSA. We included sputum samples collected ≥48 hours after admission and within 7 days of the clinical diagnosis of HAP. Sputum samples with >10 epithelial cells per high-power field (hpf) were considered to be contaminated. Among noncontaminated samples, positive sputum cultures were defined as those with a microbiologic diagnosis other than “oral flora,” while those with no growth or growth of oral flora or only yeast were considered to be negative. The hospital’s microbiology laboratory does not routinely provide species identification for Gram-negative rods (GNRs) growing on culture in the presence of growth of ≥3 other colony types. We considered such GNRs (not further speciated) to represent a positive culture result in our analysis given that colonization versus pathogenicity is a clinical distinction and, as such, these results may impact antibiotic choice.
Statistical Analysis
Data were analyzed by using SAS software, version 9.3. We used a 2-sided P value of <0.05 to indicate statistical significance for all comparisons. We used the χ2 test and the nonparametric median test for unadjusted comparisons.
To identify predictors of a positive (versus negative or contaminated) sputum culture among patients with HAP, we used a generalized estimating equation model with a Poisson distribution error term, log link, and first-order autoregressive correlation structure to account for multiple sputum specimens per patient. We combined culture negative and contaminated samples to highlight the clinical utility of sputum culture in a real-world setting. Potential predictors chosen based on clinical grounds included all variables listed in Table 1. We defined comorbidities specified in Table 1 via ICD-9-CM secondary diagnosis codes and diagnosis related groups (DRGs) using Healthcare Cost and Utilization Project Comorbidity Software, version 3.7, based on the work of Elixhauser et al.9,10; dialysis use was defined by an ICD-9-CM procedure code of 39.95; inpatient steroid use was defined by a hospital pharmacy charge for a systemic steroid in the 7 days preceding the sputum sample.
RESULTS
There were 230,635 hospitalizations of patients ≥18 years of age from January 2007 to July 2013. After excluding outside hospital transfers (n = 14,422), hospitalizations <48 hours in duration (n = 59,774), and psychiatric hospitalizations (n = 9887), there were 146,552 hospitalizations in the cohort.
The top 3 bacterial organisms cultured from sputum samples were GNRs not further speciated (25.9%), Staphylococcus aureus (21.0%), and Pseudomonas aeruginosa (14.8%). The frequencies of isolated microorganisms are presented in Table 2.
In an adjusted analysis (Table 1), the significant predictors of a positive sputum culture were chronic lung disease (relative risk [RR] = 2.0; 95% confidence interval [CI], 1.2-3.4) and steroid use (RR = 1.8; 95% CI, 1.1-3.2).
DISCUSSION
To our knowledge, our study is the first to assess the predictors of positive sputum culture among patients with HAP (non-VAP) who had sputum samples obtained noninvasively, and this study is larger than prior studies in which researchers reported on sputum culture yield in HAP. Sputum samples were obtained in 29.4% cases of clinically diagnosed HAP. Although 87% of specimens obtained were culture-negative or contaminated, 13% yielded a bacterial organism. Although we do not report the antibiotic sensitivity patterns of the isolated organisms, the organisms identified frequently demonstrate antibiotic resistance, highlighting the potential for both antibiotic escalation and de-escalation based on sputum culture. In a multivariable model, presence of chronic lung disease and steroid use in the preceding week were both significantly associated with culture positivity.
The retrospective nature of the study raises the possibility of selection bias from systematic differences between the 29.4% of patients with HAP who had sputum collected and those who did not. Patients with sputum cultures were similar to patients without cultures in most measured characteristics, but we are unable to know what the yield of noninvasive sputum culture would have been had all patients with HAP been sampled. As such, our findings reflect the yield of sputum culture among patients with HAP for whom cultures were successfully obtained. It is not clear why only 29.4% of HAP patients received IDSA guideline-concordant care, but similar rates of culture use are reported elsewhere.7 While physician decision-making could have contributed to this finding, it is also possible that many sick, hospitalized patients are simply unable to produce sputum for analysis. In future studies, researchers should examine barriers to guideline-concordant care.
We considered a culture result of GNRs (not further speciated) as positive in our analysis because this result indicates growth of mixed bacterial types, the pathogenicity of which is a clinical determination. Physicians may request speciation and antibiotic sensitivities and, as such, these results have the potential to impact antibiotic choice. Had we considered such cultures to be negative or contaminated, the rate of culture positivity would have been only slightly reduced from 63/478 (13.2%) to 50/478 (10.5%).
The strengths of our study include the chart-based validation of administratively identified cases of pneumonia and a large cohort. There are also limitations. The single-center nature of the study has implications for pretest probability and generalizability. Additionally, in our study, we did not examine outcomes among patients treated empirically versus those treated based on sputum culture results. Finally, our reliance on administrative codes to identify cases of HAP for subsequent validation could have resulted in incomplete capture of HAP cases.
In conclusion, in our study, we provide an estimate of the diagnostic yield of sputum culture in a large cohort with chart-validated HAP, a description of HAP microbiology, and predictors of positive sputum culture. Thirteen percent of patients who had sputum culture testing received a microbiologic diagnosis. Because of the relative ease of obtaining a sputum sample and the microbiologic distribution in our study (representing a mix of commonly drug-resistant pathogens and more typical community-acquired pathogens), we suggest that sputum culture in HAP is a useful diagnostic tool with the potential to inform antibiotic escalation or de-escalation.
Acknowledgments
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant number K24AG035075 from the National Institute on Aging. The funding organizations had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure
No conflicts of interest apply for any of the authors.
1. Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: Final Data for 2009. Natl Vital Stat Rep. 2011;60(3):1-116. PubMed
2. Bonafede MM, Suaya JA, Wilson KL, Mannino DM, Polsky D. Incidence and cost of CAP in a large working-age population. Am J Manag Care. 2012;18(7):380-387. PubMed
3. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. PubMed
4. Wahl WL, Franklin GA, Brandt MM, et al. Does bronchoalveolar lavage enhance our ability to treat ventilator-associated pneumonia in a trauma-burn intensive care unit? J Trauma. 2003;54(4):633-638. PubMed
5. Herer B, Fuhrman C, Demontrond D, Gazevic Z, Housset B, Chouaïd C. Diagnosis of nosocomial pneumonia in medical ward: Repeatability of the protected specimen brush. Eur Respir J. 2001;18(1):157-163. PubMed
6. Chung DR, Song JH, Kim SH, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409-1417. PubMed
7. Russell CD, Koch O, Laurenson IF, O’Shea DT, Sutherland R, Mackintosh CL. Diagnosis and features of hospital-acquired pneumonia: a retrospective cohort study. J Hosp Infect. 2016;92(3):273-279. PubMed
8. Messika J, Stoclin A, Bouvard E, et al. The Challenging Diagnosis of Non-Community-Acquired Pneumonia in Non-Mechanically Ventilated Subjects: Value of Microbiological Investigation. Respir Care. 2016;61(2):225-234. PubMed
9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
10. HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP). January 2013. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed on March 15, 2016.
1. Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: Final Data for 2009. Natl Vital Stat Rep. 2011;60(3):1-116. PubMed
2. Bonafede MM, Suaya JA, Wilson KL, Mannino DM, Polsky D. Incidence and cost of CAP in a large working-age population. Am J Manag Care. 2012;18(7):380-387. PubMed
3. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. PubMed
4. Wahl WL, Franklin GA, Brandt MM, et al. Does bronchoalveolar lavage enhance our ability to treat ventilator-associated pneumonia in a trauma-burn intensive care unit? J Trauma. 2003;54(4):633-638. PubMed
5. Herer B, Fuhrman C, Demontrond D, Gazevic Z, Housset B, Chouaïd C. Diagnosis of nosocomial pneumonia in medical ward: Repeatability of the protected specimen brush. Eur Respir J. 2001;18(1):157-163. PubMed
6. Chung DR, Song JH, Kim SH, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409-1417. PubMed
7. Russell CD, Koch O, Laurenson IF, O’Shea DT, Sutherland R, Mackintosh CL. Diagnosis and features of hospital-acquired pneumonia: a retrospective cohort study. J Hosp Infect. 2016;92(3):273-279. PubMed
8. Messika J, Stoclin A, Bouvard E, et al. The Challenging Diagnosis of Non-Community-Acquired Pneumonia in Non-Mechanically Ventilated Subjects: Value of Microbiological Investigation. Respir Care. 2016;61(2):225-234. PubMed
9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
10. HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP). January 2013. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed on March 15, 2016.
© 2018 Society of Hospital Medicine
The Evaluation of Medical Inpatients Who Are Admitted on Long-term Opioid Therapy for Chronic Pain
Hospitalists face complex questions about how to evaluate and treat the large number of individuals who are admitted on long-term opioid therapy (LTOT, defined as lasting 3 months or longer) for chronic noncancer pain. A recent study at one Veterans Affairs hospital, found 26% of medical inpatients were on LTOT.1 Over the last 2 decades, use of LTOT has risen substantially in the United States, including among middle-aged and older adults.2 Concurrently, inpatient hospitalizations related to the overuse of prescription opioids, including overdose, dependence, abuse, and adverse drug events, have increased by 153%.3 Individuals on LTOT can also be hospitalized for exacerbations of the opioid-treated chronic pain condition or unrelated conditions. In addition to affecting rates of hospitalization, use of LTOT is associated with higher rates of in-hospital adverse events, longer hospital stays, and higher readmission rates.1,4,5
Physicians find managing chronic pain to be stressful, are often concerned about misuse and addiction, and believe their training in opioid prescribing is inadequate.6 Hospitalists report confidence in assessing and prescribing opioids for acute pain but limited success and satisfaction with treating exacerbations of chronic pain.7 Although half of all hospitalized patients receive opioids,5 little information is available to guide the care of hospitalized medical patients on LTOT for chronic noncancer pain.8,9
Our multispecialty team sought to synthesize guideline recommendations and primary literature relevant to the assessment of medical inpatients on LTOT to assist practitioners balance effective pain treatment and opioid risk reduction. This article addresses obtaining a comprehensive pain history, identifying misuse and opioid use disorders, assessing the risk of overdose and adverse drug events, gauging the risk of withdrawal, and based on such findings, appraise indications for opioid therapy. Other authors have recently published narrative reviews on the management of acute pain in hospitalized patients with opioid dependence and the inpatient management of opioid use disorder.10,11
METHODS
To identify primary literature, we searched PubMed, EMBASE, The Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Health Economic Evaluations Database, key meeting abstracts, and hand searches. To identify guidelines, we searched PubMed, National Guidelines Clearinghouse, specialty societies’ websites, the Centers for Disease Control and Prevention (CDC), the United Kingdom National Institute for Health and Care Excellence, the Canadian Medical Association, and the Australian Government National Health and Medical Research Council. Search terms related to opioids and chronic pain, which was last updated in October 2016.12
We selected English-language documents on opioids and chronic pain among adults, excluding pain in the setting of procedures, labor and delivery, life-limiting illness, or specific conditions. For primary literature, we considered intervention studies of any design that addressed pain management among hospitalized medical patients. We included guidelines and specialty society position statements published after January 1, 2009, that addressed pain in the hospital setting, acute pain in any setting, or chronic pain in the outpatient setting if published by a national body. Due to the paucity of documents specific to inpatient care, we used a narrative review format to synthesize information. Dual reviewers extracted guideline recommendations potentially relevant to medical inpatients on LTOT. We also summarize relevant assessment instruments, emphasizing very brief screening instruments, which may be more likely to be used by busy hospitalists.
RESULTS
DISCUSSION
Obtaining a Comprehensive Pain History
Hospitalists newly evaluating patients on LTOT often face a dual challenge: deciding if the patient has an immediate indication for additional opioids and if the current long-term opioid regimen should be altered or discontinued. In general, opioids are an accepted short-term treatment for moderate to severe acute pain but their role in chronic noncancer pain is controversial. Newly released guidelines by the CDC recommend initiating LTOT as a last resort, and the Departments of Veterans Affairs and Defense guidelines recommend against initiation of LTOT.22,23
A key first step, therefore, is distinguishing between acute and chronic pain. Among patients on LTOT, pain can represent a new acute pain condition, an exacerbation of chronic pain, opioid-induced hyperalgesia, or opioid withdrawal. Acute pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in relation to such damage.26 In contrast, chronic pain is a complex response that may not be related to actual or ongoing tissue damage, and is influenced by physiological, contextual, and psychological factors. Two acute pain guidelines and 1 chronic pain guideline recommend distinguishing acute and chronic pain,9,16,21 3 chronic pain guidelines reinforce the importance of obtaining a pain history (including timing, intensity, frequency, onset, etc),20,22,23 and 6 guidelines recommend ascertaining a history of prior pain-related treatments.9,13,14,16,20,22 Inquiring how the current pain compares with symptoms “on a good day,” what activities the patient can usually perform, and what the patient does outside the hospital to cope with pain can serve as entry into this conversation.
In addition to function, 5 guidelines, including 2 specific guidelines for acute pain or the hospital setting, recommend obtaining a detailed psychosocial history to identify life stressors and gain insight into the patient’s coping skills.14,16,19,20,22 Psychiatric symptoms can intensify the experience of pain or hamper coping ability. Anxiety, depression, and insomnia frequently coexist in patients with chronic pain.31 As such, 3 hospital setting/acute pain guidelines and 3 chronic pain guidelines recommend screening for mental health issues including anxiety and depression.13,14,16,20,22,23 Several depression screening instruments have been validated among inpatients,32 and there are validated single-item, self-administered instruments for both depression and anxiety (Table 3).32,33
Although obtaining a comprehensive history before making treatment decisions is ideal, some patients present in extremis. In emergency departments, some guidelines endorse prompt administration of analgesics based on patient self-report, prior to establishing a diagnosis.17 Given concerns about the growing prevalence of opioid use disorders, several states now recommend emergency medicine prescribers screen for misuse before giving opioids and avoid parenteral opioids for acute exacerbations of chronic pain.34 Treatments received in emergency departments set patients’ expectations for the care they receive during hospitalization, and hospitalists may find it necessary to explain therapies appropriate for urgent management are not intended to be sustained.
Identifying Misuse and Opioid Use Disorders
Nonmedical use of prescription opioids and opioid use disorders have more than doubled over the last decade.35 Five guidelines, including 3 specific guidelines for acute pain or the hospital setting, recommend screening for opioid misuse.13,14,16,19,23 Many states mandate practitioners assess patients for substance use disorders before prescribing controlled substances.36 Instruments to identify aberrant and risky use include the Current Opioid Misuse Measure,37 Prescription Drug Use Questionnaire,38 Addiction Behaviors Checklist,39 Screening Tool for Abuse,40 and the Self-Administered Single-Item Screening Question (Table 3).41 However, the evidence for these and other tools is limited and absent for the inpatient setting.21,42
In addition to obtaining a history from the patient, 4 guidelines specific to hospital settings/acute pain and 4 chronic pain guidelines recommend practitioners access prescription drug monitoring programs (PDMPs).13-16,19,21-24 PDMPs exist in all states except Missouri, and about half of states mandate practitioners check the PDMP database in certain circumstances.36 Studies examining the effects of PDMPs on prescribing are limited, but checking these databases can uncover concerning patterns including overlapping prescriptions or multiple prescribers.43 PDMPs can also confirm reported medication doses, for which patient report may be less reliable.
Two hospital/acute pain guidelines and 5 chronic pain guidelines also recommend urine drug testing, although differing on when and whom to test, with some favoring universal screening.11,20,23 Screening hospitalized patients may reveal substances not reported by patients, but medications administered in emergency departments can confound results. Furthermore, the commonly used immunoassay does not distinguish heroin from prescription opioids, nor detect hydrocodone, oxycodone, methadone, buprenorphine, or certain benzodiazepines. Chromatography/mass spectrometry assays can but are often not available from hospital laboratories. The differential for unexpected results includes substance use, self treatment of uncontrolled pain, diversion, or laboratory error.20
If concerning opioid use is identified, 3 hospital setting/acute pain specific guidelines and the CDC guideline recommend sharing concerns with patients and assessing for a substance use disorder.9,13,16,22 Determining whether patients have an opioid use disorder that meets the criteria in the Diagnostic and Statistical Manual, 5th Edition44 can be challenging. Patients may minimize or deny symptoms or fear that the stigma of an opioid use disorder will lead to dismissive or subpar care. Additionally, substance use disorders are subject to federal confidentiality regulations, which can hamper acquisition of information from providers.45 Thus, hospitalists may find specialty consultation helpful to confirm the diagnosis.
Assessing the Risk of Overdose and Adverse Drug Events
Oversedation, respiratory depression, and death can result from iatrogenic or self-administered opioid overdose in the hospital.5 Patient factors that increase this risk among outpatients include a prior history of overdose, preexisting substance use disorders, cognitive impairment, mood and personality disorders, chronic kidney disease, sleep apnea, obstructive lung disease, and recent abstinence from opioids.12 Medication factors include concomitant use of benzodiazepines and other central nervous system depressants, including alcohol; recent initiation of long-acting opioids; use of fentanyl patches, immediate-release fentanyl, or methadone; rapid titration; switching opioids without adequate dose reduction; pharmacokinetic drug–drug interactions; and, importantly, higher doses.12,22 Two guidelines specific to acute pain and hospital settings and 5 chronic pain guidelines recommend screening for use of benzodiazepines among patients on LTOT.13,14,16,18-20,22,21
The CDC guideline recommends careful assessment when doses exceed 50 mg of morphine equivalents per day and avoiding doses above 90 mg per day due to the heightened risk of overdose.22 In the hospital, 23% of patients receive doses at or above 100 mg of morphine equivalents per day,5 and concurrent use of central nervous system depressants is common. Changes in kidney and liver function during acute illness may impact opioid metabolism and contribute to overdose.
In addition to overdose, opioids are leading causes of adverse drug events during hospitalization.46 Most studies have focused on surgical patients reporting common opioid-related events as nausea/vomiting, pruritus, rash, mental status changes, respiratory depression, ileus, and urinary retention.47 Hospitalized patients may also exhibit chronic adverse effects due to LTOT. At least one-third of patients on LTOT eventually stop because of adverse effects, such as endocrinopathies, sleep disordered breathing, constipation, fractures, falls, and mental status changes.48 Patients may lack awareness that their symptoms are attributable to opioids and are willing to reduce their opioid use once informed, especially when alternatives are offered to alleviate pain.
Gauging the Risk of Withdrawal
Sudden discontinuation of LTOT by patients, practitioners, or intercurrent events can have unanticipated and undesirable consequences. Withdrawal is not only distressing for patients; it can be dangerous because patients may resort to illicit use, diversion of opioids, or masking opioid withdrawal with other substances such as alcohol. The anxiety and distress associated with withdrawal, or anticipatory fear about withdrawal, can undermine therapeutic alliance and interfere with processes of care. Reviewed guidelines did not offer recommendations regarding withdrawal risk or specific strategies for avoidance. There is no specific prior dose threshold or degree of reduction in opioids that puts patients at risk for withdrawal, in part due to patients’ beliefs, expectations, and differences in response to opioid formulations. Symptoms of opioid withdrawal have been compared to a severe case of influenza, including stomach cramps, nausea and vomiting, diarrhea, tremor and muscle twitching, sweating, restlessness, yawning, tachycardia, anxiety and irritability, bone and joint aches, runny nose, tearing, and piloerection.49 The Clinical Opiate Withdrawal Scale (COWS)49 and the Clinical Institute Narcotic Assessment51 are clinician-administered tools to assess opioid withdrawal similar to the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised,52 to monitor for withdrawal in the inpatient setting.
Synthesizing and Appraising the Indications for Opioid Therapy
For medical inpatients who report adequate pain control and functional outcomes on current doses of LTOT, without evidence of misuse, the pragmatic approach is to continue the treatment plan established by the outpatient clinician rather than escalating or tapering the dose. If opioids are prescribed at discharge, 3 hospital setting/acute pain guidelines and the CDC guideline recommend prescribing the lowest effective dose of immediate release opioids for 3 to 7 days.13,15,16,22
When patients exhibit evidence of an opioid use disorder, have a history of serious overdose, or are experiencing intolerable opioid-related adverse events, the hospitalist may conclude the harms of LTOT outweigh the benefits. For these patients, opioid treatment in the hospital can be aimed at preventing withdrawal, avoiding the perpetuation of inappropriate opioid use, managing other acute medical conditions, and communicating with outpatient prescribers. For patients with misuse, discontinuing opioids is potentially harmful and may be perceived as punitive. Hospitalists should consider consulting addiction or mental health specialists to assist with formulating a plan of care. However, such specialists may not be available in smaller or rural hospitals and referral at discharge can be challenging.53
Beginning to taper opioids during the hospitalization can be appropriate when patients are motivated and can transition to an outpatient provider who will supervise the taper. In ambulatory settings, tapers of 10% to 30% every 2 to 5 days are generally well tolerated.54 If patients started tapering opioids under supervision of an outpatient provider prior to hospitalization; ideally, the taper can be continued during hospitalization with close coordination with the outpatient clinician.
Unfortunately, many patients on LTOT are admitted with new sources of acute pain and or exacerbations of chronic pain, and some have concomitant substance use disorders; we plan to address the management of these complex situations in future work.
Despite the frequency with which patients on LTOT are hospitalized for nonsurgical stays and the challenges inherent in evaluating pain and assessing the possibility of substance use disorders, no formal guidelines or empirical research studies pertain to this population. Guidelines in this review were developed for hospital settings and acute pain in the absence of LTOT, and for outpatient care of patients on LTOT. We also included a nonsystematic synthesis of literature that varied in relevance to medical inpatients on LTOT.
CONCLUSIONS
Although inpatient assessment and treatment of patients with LTOT remains an underresearched area, we were able to extract and synthesize recommendations from 14 guideline statements and apply these to the assessment of patients with LTOT in the inpatient setting. Hospitalists frequently encounter patients on LTOT for chronic nonmalignant pain and are faced with complex decisions about the effectiveness and safety of LTOT; appropriate patient assessment is fundamental to making these decisions. Key guideline recommendations relevant to inpatient assessment include assessing both pain and functional status, differentiating acute from chronic pain, ascertaining preadmission pain treatment history, obtaining a psychosocial history, screening for mental health issues such as depression and anxiety, screening for substance use disorders, checking state prescription drug monitoring databases, ordering urine drug immunoassays, detecting use of sedative-hypnotics, identifying medical conditions associated with increased risk of overdose and adverse events, and appraising the potential benefits and harms of opioid therapy. Although approaches to assessing medical inpatients on LTOT can be extrapolated from outpatient guidelines, observational studies, and small studies in surgical populations, more work is needed to address these critical topics for inpatients on LTOT.
Disclosure
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. All other authors have no relevant conflicts of interest with the work.
1. Mosher HJ, Jiang L, Sarrazin MSV, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and Characteristics of Hospitalized Adults on Chronic Opioid Therapy. J Hosp Med. 2014;9(2):82-87. PubMed
2. Campbell CI, Weisner C, Leresche L, et al. Age and Gender Trends in Long-Term Opioid Analgesic Use for Noncancer Pain. Am J Public Health. 2010;100(12):2541-2547. PubMed
3. Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R. Hospital Inpatient Utilization Related to Opioid Overuse among Adults, 1993–2012. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
33. Young QR, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-Item Measures for Depression and Anxiety: Validation of the Screening Tool for Psychological Distress in an Inpatient Cardiology Setting. Eur J Cardiovasc Nurs. 2015;14(6):544-551. PubMed
Hospitalists face complex questions about how to evaluate and treat the large number of individuals who are admitted on long-term opioid therapy (LTOT, defined as lasting 3 months or longer) for chronic noncancer pain. A recent study at one Veterans Affairs hospital, found 26% of medical inpatients were on LTOT.1 Over the last 2 decades, use of LTOT has risen substantially in the United States, including among middle-aged and older adults.2 Concurrently, inpatient hospitalizations related to the overuse of prescription opioids, including overdose, dependence, abuse, and adverse drug events, have increased by 153%.3 Individuals on LTOT can also be hospitalized for exacerbations of the opioid-treated chronic pain condition or unrelated conditions. In addition to affecting rates of hospitalization, use of LTOT is associated with higher rates of in-hospital adverse events, longer hospital stays, and higher readmission rates.1,4,5
Physicians find managing chronic pain to be stressful, are often concerned about misuse and addiction, and believe their training in opioid prescribing is inadequate.6 Hospitalists report confidence in assessing and prescribing opioids for acute pain but limited success and satisfaction with treating exacerbations of chronic pain.7 Although half of all hospitalized patients receive opioids,5 little information is available to guide the care of hospitalized medical patients on LTOT for chronic noncancer pain.8,9
Our multispecialty team sought to synthesize guideline recommendations and primary literature relevant to the assessment of medical inpatients on LTOT to assist practitioners balance effective pain treatment and opioid risk reduction. This article addresses obtaining a comprehensive pain history, identifying misuse and opioid use disorders, assessing the risk of overdose and adverse drug events, gauging the risk of withdrawal, and based on such findings, appraise indications for opioid therapy. Other authors have recently published narrative reviews on the management of acute pain in hospitalized patients with opioid dependence and the inpatient management of opioid use disorder.10,11
METHODS
To identify primary literature, we searched PubMed, EMBASE, The Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Health Economic Evaluations Database, key meeting abstracts, and hand searches. To identify guidelines, we searched PubMed, National Guidelines Clearinghouse, specialty societies’ websites, the Centers for Disease Control and Prevention (CDC), the United Kingdom National Institute for Health and Care Excellence, the Canadian Medical Association, and the Australian Government National Health and Medical Research Council. Search terms related to opioids and chronic pain, which was last updated in October 2016.12
We selected English-language documents on opioids and chronic pain among adults, excluding pain in the setting of procedures, labor and delivery, life-limiting illness, or specific conditions. For primary literature, we considered intervention studies of any design that addressed pain management among hospitalized medical patients. We included guidelines and specialty society position statements published after January 1, 2009, that addressed pain in the hospital setting, acute pain in any setting, or chronic pain in the outpatient setting if published by a national body. Due to the paucity of documents specific to inpatient care, we used a narrative review format to synthesize information. Dual reviewers extracted guideline recommendations potentially relevant to medical inpatients on LTOT. We also summarize relevant assessment instruments, emphasizing very brief screening instruments, which may be more likely to be used by busy hospitalists.
RESULTS
DISCUSSION
Obtaining a Comprehensive Pain History
Hospitalists newly evaluating patients on LTOT often face a dual challenge: deciding if the patient has an immediate indication for additional opioids and if the current long-term opioid regimen should be altered or discontinued. In general, opioids are an accepted short-term treatment for moderate to severe acute pain but their role in chronic noncancer pain is controversial. Newly released guidelines by the CDC recommend initiating LTOT as a last resort, and the Departments of Veterans Affairs and Defense guidelines recommend against initiation of LTOT.22,23
A key first step, therefore, is distinguishing between acute and chronic pain. Among patients on LTOT, pain can represent a new acute pain condition, an exacerbation of chronic pain, opioid-induced hyperalgesia, or opioid withdrawal. Acute pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in relation to such damage.26 In contrast, chronic pain is a complex response that may not be related to actual or ongoing tissue damage, and is influenced by physiological, contextual, and psychological factors. Two acute pain guidelines and 1 chronic pain guideline recommend distinguishing acute and chronic pain,9,16,21 3 chronic pain guidelines reinforce the importance of obtaining a pain history (including timing, intensity, frequency, onset, etc),20,22,23 and 6 guidelines recommend ascertaining a history of prior pain-related treatments.9,13,14,16,20,22 Inquiring how the current pain compares with symptoms “on a good day,” what activities the patient can usually perform, and what the patient does outside the hospital to cope with pain can serve as entry into this conversation.
In addition to function, 5 guidelines, including 2 specific guidelines for acute pain or the hospital setting, recommend obtaining a detailed psychosocial history to identify life stressors and gain insight into the patient’s coping skills.14,16,19,20,22 Psychiatric symptoms can intensify the experience of pain or hamper coping ability. Anxiety, depression, and insomnia frequently coexist in patients with chronic pain.31 As such, 3 hospital setting/acute pain guidelines and 3 chronic pain guidelines recommend screening for mental health issues including anxiety and depression.13,14,16,20,22,23 Several depression screening instruments have been validated among inpatients,32 and there are validated single-item, self-administered instruments for both depression and anxiety (Table 3).32,33
Although obtaining a comprehensive history before making treatment decisions is ideal, some patients present in extremis. In emergency departments, some guidelines endorse prompt administration of analgesics based on patient self-report, prior to establishing a diagnosis.17 Given concerns about the growing prevalence of opioid use disorders, several states now recommend emergency medicine prescribers screen for misuse before giving opioids and avoid parenteral opioids for acute exacerbations of chronic pain.34 Treatments received in emergency departments set patients’ expectations for the care they receive during hospitalization, and hospitalists may find it necessary to explain therapies appropriate for urgent management are not intended to be sustained.
Identifying Misuse and Opioid Use Disorders
Nonmedical use of prescription opioids and opioid use disorders have more than doubled over the last decade.35 Five guidelines, including 3 specific guidelines for acute pain or the hospital setting, recommend screening for opioid misuse.13,14,16,19,23 Many states mandate practitioners assess patients for substance use disorders before prescribing controlled substances.36 Instruments to identify aberrant and risky use include the Current Opioid Misuse Measure,37 Prescription Drug Use Questionnaire,38 Addiction Behaviors Checklist,39 Screening Tool for Abuse,40 and the Self-Administered Single-Item Screening Question (Table 3).41 However, the evidence for these and other tools is limited and absent for the inpatient setting.21,42
In addition to obtaining a history from the patient, 4 guidelines specific to hospital settings/acute pain and 4 chronic pain guidelines recommend practitioners access prescription drug monitoring programs (PDMPs).13-16,19,21-24 PDMPs exist in all states except Missouri, and about half of states mandate practitioners check the PDMP database in certain circumstances.36 Studies examining the effects of PDMPs on prescribing are limited, but checking these databases can uncover concerning patterns including overlapping prescriptions or multiple prescribers.43 PDMPs can also confirm reported medication doses, for which patient report may be less reliable.
Two hospital/acute pain guidelines and 5 chronic pain guidelines also recommend urine drug testing, although differing on when and whom to test, with some favoring universal screening.11,20,23 Screening hospitalized patients may reveal substances not reported by patients, but medications administered in emergency departments can confound results. Furthermore, the commonly used immunoassay does not distinguish heroin from prescription opioids, nor detect hydrocodone, oxycodone, methadone, buprenorphine, or certain benzodiazepines. Chromatography/mass spectrometry assays can but are often not available from hospital laboratories. The differential for unexpected results includes substance use, self treatment of uncontrolled pain, diversion, or laboratory error.20
If concerning opioid use is identified, 3 hospital setting/acute pain specific guidelines and the CDC guideline recommend sharing concerns with patients and assessing for a substance use disorder.9,13,16,22 Determining whether patients have an opioid use disorder that meets the criteria in the Diagnostic and Statistical Manual, 5th Edition44 can be challenging. Patients may minimize or deny symptoms or fear that the stigma of an opioid use disorder will lead to dismissive or subpar care. Additionally, substance use disorders are subject to federal confidentiality regulations, which can hamper acquisition of information from providers.45 Thus, hospitalists may find specialty consultation helpful to confirm the diagnosis.
Assessing the Risk of Overdose and Adverse Drug Events
Oversedation, respiratory depression, and death can result from iatrogenic or self-administered opioid overdose in the hospital.5 Patient factors that increase this risk among outpatients include a prior history of overdose, preexisting substance use disorders, cognitive impairment, mood and personality disorders, chronic kidney disease, sleep apnea, obstructive lung disease, and recent abstinence from opioids.12 Medication factors include concomitant use of benzodiazepines and other central nervous system depressants, including alcohol; recent initiation of long-acting opioids; use of fentanyl patches, immediate-release fentanyl, or methadone; rapid titration; switching opioids without adequate dose reduction; pharmacokinetic drug–drug interactions; and, importantly, higher doses.12,22 Two guidelines specific to acute pain and hospital settings and 5 chronic pain guidelines recommend screening for use of benzodiazepines among patients on LTOT.13,14,16,18-20,22,21
The CDC guideline recommends careful assessment when doses exceed 50 mg of morphine equivalents per day and avoiding doses above 90 mg per day due to the heightened risk of overdose.22 In the hospital, 23% of patients receive doses at or above 100 mg of morphine equivalents per day,5 and concurrent use of central nervous system depressants is common. Changes in kidney and liver function during acute illness may impact opioid metabolism and contribute to overdose.
In addition to overdose, opioids are leading causes of adverse drug events during hospitalization.46 Most studies have focused on surgical patients reporting common opioid-related events as nausea/vomiting, pruritus, rash, mental status changes, respiratory depression, ileus, and urinary retention.47 Hospitalized patients may also exhibit chronic adverse effects due to LTOT. At least one-third of patients on LTOT eventually stop because of adverse effects, such as endocrinopathies, sleep disordered breathing, constipation, fractures, falls, and mental status changes.48 Patients may lack awareness that their symptoms are attributable to opioids and are willing to reduce their opioid use once informed, especially when alternatives are offered to alleviate pain.
Gauging the Risk of Withdrawal
Sudden discontinuation of LTOT by patients, practitioners, or intercurrent events can have unanticipated and undesirable consequences. Withdrawal is not only distressing for patients; it can be dangerous because patients may resort to illicit use, diversion of opioids, or masking opioid withdrawal with other substances such as alcohol. The anxiety and distress associated with withdrawal, or anticipatory fear about withdrawal, can undermine therapeutic alliance and interfere with processes of care. Reviewed guidelines did not offer recommendations regarding withdrawal risk or specific strategies for avoidance. There is no specific prior dose threshold or degree of reduction in opioids that puts patients at risk for withdrawal, in part due to patients’ beliefs, expectations, and differences in response to opioid formulations. Symptoms of opioid withdrawal have been compared to a severe case of influenza, including stomach cramps, nausea and vomiting, diarrhea, tremor and muscle twitching, sweating, restlessness, yawning, tachycardia, anxiety and irritability, bone and joint aches, runny nose, tearing, and piloerection.49 The Clinical Opiate Withdrawal Scale (COWS)49 and the Clinical Institute Narcotic Assessment51 are clinician-administered tools to assess opioid withdrawal similar to the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised,52 to monitor for withdrawal in the inpatient setting.
Synthesizing and Appraising the Indications for Opioid Therapy
For medical inpatients who report adequate pain control and functional outcomes on current doses of LTOT, without evidence of misuse, the pragmatic approach is to continue the treatment plan established by the outpatient clinician rather than escalating or tapering the dose. If opioids are prescribed at discharge, 3 hospital setting/acute pain guidelines and the CDC guideline recommend prescribing the lowest effective dose of immediate release opioids for 3 to 7 days.13,15,16,22
When patients exhibit evidence of an opioid use disorder, have a history of serious overdose, or are experiencing intolerable opioid-related adverse events, the hospitalist may conclude the harms of LTOT outweigh the benefits. For these patients, opioid treatment in the hospital can be aimed at preventing withdrawal, avoiding the perpetuation of inappropriate opioid use, managing other acute medical conditions, and communicating with outpatient prescribers. For patients with misuse, discontinuing opioids is potentially harmful and may be perceived as punitive. Hospitalists should consider consulting addiction or mental health specialists to assist with formulating a plan of care. However, such specialists may not be available in smaller or rural hospitals and referral at discharge can be challenging.53
Beginning to taper opioids during the hospitalization can be appropriate when patients are motivated and can transition to an outpatient provider who will supervise the taper. In ambulatory settings, tapers of 10% to 30% every 2 to 5 days are generally well tolerated.54 If patients started tapering opioids under supervision of an outpatient provider prior to hospitalization; ideally, the taper can be continued during hospitalization with close coordination with the outpatient clinician.
Unfortunately, many patients on LTOT are admitted with new sources of acute pain and or exacerbations of chronic pain, and some have concomitant substance use disorders; we plan to address the management of these complex situations in future work.
Despite the frequency with which patients on LTOT are hospitalized for nonsurgical stays and the challenges inherent in evaluating pain and assessing the possibility of substance use disorders, no formal guidelines or empirical research studies pertain to this population. Guidelines in this review were developed for hospital settings and acute pain in the absence of LTOT, and for outpatient care of patients on LTOT. We also included a nonsystematic synthesis of literature that varied in relevance to medical inpatients on LTOT.
CONCLUSIONS
Although inpatient assessment and treatment of patients with LTOT remains an underresearched area, we were able to extract and synthesize recommendations from 14 guideline statements and apply these to the assessment of patients with LTOT in the inpatient setting. Hospitalists frequently encounter patients on LTOT for chronic nonmalignant pain and are faced with complex decisions about the effectiveness and safety of LTOT; appropriate patient assessment is fundamental to making these decisions. Key guideline recommendations relevant to inpatient assessment include assessing both pain and functional status, differentiating acute from chronic pain, ascertaining preadmission pain treatment history, obtaining a psychosocial history, screening for mental health issues such as depression and anxiety, screening for substance use disorders, checking state prescription drug monitoring databases, ordering urine drug immunoassays, detecting use of sedative-hypnotics, identifying medical conditions associated with increased risk of overdose and adverse events, and appraising the potential benefits and harms of opioid therapy. Although approaches to assessing medical inpatients on LTOT can be extrapolated from outpatient guidelines, observational studies, and small studies in surgical populations, more work is needed to address these critical topics for inpatients on LTOT.
Disclosure
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. All other authors have no relevant conflicts of interest with the work.
Hospitalists face complex questions about how to evaluate and treat the large number of individuals who are admitted on long-term opioid therapy (LTOT, defined as lasting 3 months or longer) for chronic noncancer pain. A recent study at one Veterans Affairs hospital, found 26% of medical inpatients were on LTOT.1 Over the last 2 decades, use of LTOT has risen substantially in the United States, including among middle-aged and older adults.2 Concurrently, inpatient hospitalizations related to the overuse of prescription opioids, including overdose, dependence, abuse, and adverse drug events, have increased by 153%.3 Individuals on LTOT can also be hospitalized for exacerbations of the opioid-treated chronic pain condition or unrelated conditions. In addition to affecting rates of hospitalization, use of LTOT is associated with higher rates of in-hospital adverse events, longer hospital stays, and higher readmission rates.1,4,5
Physicians find managing chronic pain to be stressful, are often concerned about misuse and addiction, and believe their training in opioid prescribing is inadequate.6 Hospitalists report confidence in assessing and prescribing opioids for acute pain but limited success and satisfaction with treating exacerbations of chronic pain.7 Although half of all hospitalized patients receive opioids,5 little information is available to guide the care of hospitalized medical patients on LTOT for chronic noncancer pain.8,9
Our multispecialty team sought to synthesize guideline recommendations and primary literature relevant to the assessment of medical inpatients on LTOT to assist practitioners balance effective pain treatment and opioid risk reduction. This article addresses obtaining a comprehensive pain history, identifying misuse and opioid use disorders, assessing the risk of overdose and adverse drug events, gauging the risk of withdrawal, and based on such findings, appraise indications for opioid therapy. Other authors have recently published narrative reviews on the management of acute pain in hospitalized patients with opioid dependence and the inpatient management of opioid use disorder.10,11
METHODS
To identify primary literature, we searched PubMed, EMBASE, The Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Health Economic Evaluations Database, key meeting abstracts, and hand searches. To identify guidelines, we searched PubMed, National Guidelines Clearinghouse, specialty societies’ websites, the Centers for Disease Control and Prevention (CDC), the United Kingdom National Institute for Health and Care Excellence, the Canadian Medical Association, and the Australian Government National Health and Medical Research Council. Search terms related to opioids and chronic pain, which was last updated in October 2016.12
We selected English-language documents on opioids and chronic pain among adults, excluding pain in the setting of procedures, labor and delivery, life-limiting illness, or specific conditions. For primary literature, we considered intervention studies of any design that addressed pain management among hospitalized medical patients. We included guidelines and specialty society position statements published after January 1, 2009, that addressed pain in the hospital setting, acute pain in any setting, or chronic pain in the outpatient setting if published by a national body. Due to the paucity of documents specific to inpatient care, we used a narrative review format to synthesize information. Dual reviewers extracted guideline recommendations potentially relevant to medical inpatients on LTOT. We also summarize relevant assessment instruments, emphasizing very brief screening instruments, which may be more likely to be used by busy hospitalists.
RESULTS
DISCUSSION
Obtaining a Comprehensive Pain History
Hospitalists newly evaluating patients on LTOT often face a dual challenge: deciding if the patient has an immediate indication for additional opioids and if the current long-term opioid regimen should be altered or discontinued. In general, opioids are an accepted short-term treatment for moderate to severe acute pain but their role in chronic noncancer pain is controversial. Newly released guidelines by the CDC recommend initiating LTOT as a last resort, and the Departments of Veterans Affairs and Defense guidelines recommend against initiation of LTOT.22,23
A key first step, therefore, is distinguishing between acute and chronic pain. Among patients on LTOT, pain can represent a new acute pain condition, an exacerbation of chronic pain, opioid-induced hyperalgesia, or opioid withdrawal. Acute pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in relation to such damage.26 In contrast, chronic pain is a complex response that may not be related to actual or ongoing tissue damage, and is influenced by physiological, contextual, and psychological factors. Two acute pain guidelines and 1 chronic pain guideline recommend distinguishing acute and chronic pain,9,16,21 3 chronic pain guidelines reinforce the importance of obtaining a pain history (including timing, intensity, frequency, onset, etc),20,22,23 and 6 guidelines recommend ascertaining a history of prior pain-related treatments.9,13,14,16,20,22 Inquiring how the current pain compares with symptoms “on a good day,” what activities the patient can usually perform, and what the patient does outside the hospital to cope with pain can serve as entry into this conversation.
In addition to function, 5 guidelines, including 2 specific guidelines for acute pain or the hospital setting, recommend obtaining a detailed psychosocial history to identify life stressors and gain insight into the patient’s coping skills.14,16,19,20,22 Psychiatric symptoms can intensify the experience of pain or hamper coping ability. Anxiety, depression, and insomnia frequently coexist in patients with chronic pain.31 As such, 3 hospital setting/acute pain guidelines and 3 chronic pain guidelines recommend screening for mental health issues including anxiety and depression.13,14,16,20,22,23 Several depression screening instruments have been validated among inpatients,32 and there are validated single-item, self-administered instruments for both depression and anxiety (Table 3).32,33
Although obtaining a comprehensive history before making treatment decisions is ideal, some patients present in extremis. In emergency departments, some guidelines endorse prompt administration of analgesics based on patient self-report, prior to establishing a diagnosis.17 Given concerns about the growing prevalence of opioid use disorders, several states now recommend emergency medicine prescribers screen for misuse before giving opioids and avoid parenteral opioids for acute exacerbations of chronic pain.34 Treatments received in emergency departments set patients’ expectations for the care they receive during hospitalization, and hospitalists may find it necessary to explain therapies appropriate for urgent management are not intended to be sustained.
Identifying Misuse and Opioid Use Disorders
Nonmedical use of prescription opioids and opioid use disorders have more than doubled over the last decade.35 Five guidelines, including 3 specific guidelines for acute pain or the hospital setting, recommend screening for opioid misuse.13,14,16,19,23 Many states mandate practitioners assess patients for substance use disorders before prescribing controlled substances.36 Instruments to identify aberrant and risky use include the Current Opioid Misuse Measure,37 Prescription Drug Use Questionnaire,38 Addiction Behaviors Checklist,39 Screening Tool for Abuse,40 and the Self-Administered Single-Item Screening Question (Table 3).41 However, the evidence for these and other tools is limited and absent for the inpatient setting.21,42
In addition to obtaining a history from the patient, 4 guidelines specific to hospital settings/acute pain and 4 chronic pain guidelines recommend practitioners access prescription drug monitoring programs (PDMPs).13-16,19,21-24 PDMPs exist in all states except Missouri, and about half of states mandate practitioners check the PDMP database in certain circumstances.36 Studies examining the effects of PDMPs on prescribing are limited, but checking these databases can uncover concerning patterns including overlapping prescriptions or multiple prescribers.43 PDMPs can also confirm reported medication doses, for which patient report may be less reliable.
Two hospital/acute pain guidelines and 5 chronic pain guidelines also recommend urine drug testing, although differing on when and whom to test, with some favoring universal screening.11,20,23 Screening hospitalized patients may reveal substances not reported by patients, but medications administered in emergency departments can confound results. Furthermore, the commonly used immunoassay does not distinguish heroin from prescription opioids, nor detect hydrocodone, oxycodone, methadone, buprenorphine, or certain benzodiazepines. Chromatography/mass spectrometry assays can but are often not available from hospital laboratories. The differential for unexpected results includes substance use, self treatment of uncontrolled pain, diversion, or laboratory error.20
If concerning opioid use is identified, 3 hospital setting/acute pain specific guidelines and the CDC guideline recommend sharing concerns with patients and assessing for a substance use disorder.9,13,16,22 Determining whether patients have an opioid use disorder that meets the criteria in the Diagnostic and Statistical Manual, 5th Edition44 can be challenging. Patients may minimize or deny symptoms or fear that the stigma of an opioid use disorder will lead to dismissive or subpar care. Additionally, substance use disorders are subject to federal confidentiality regulations, which can hamper acquisition of information from providers.45 Thus, hospitalists may find specialty consultation helpful to confirm the diagnosis.
Assessing the Risk of Overdose and Adverse Drug Events
Oversedation, respiratory depression, and death can result from iatrogenic or self-administered opioid overdose in the hospital.5 Patient factors that increase this risk among outpatients include a prior history of overdose, preexisting substance use disorders, cognitive impairment, mood and personality disorders, chronic kidney disease, sleep apnea, obstructive lung disease, and recent abstinence from opioids.12 Medication factors include concomitant use of benzodiazepines and other central nervous system depressants, including alcohol; recent initiation of long-acting opioids; use of fentanyl patches, immediate-release fentanyl, or methadone; rapid titration; switching opioids without adequate dose reduction; pharmacokinetic drug–drug interactions; and, importantly, higher doses.12,22 Two guidelines specific to acute pain and hospital settings and 5 chronic pain guidelines recommend screening for use of benzodiazepines among patients on LTOT.13,14,16,18-20,22,21
The CDC guideline recommends careful assessment when doses exceed 50 mg of morphine equivalents per day and avoiding doses above 90 mg per day due to the heightened risk of overdose.22 In the hospital, 23% of patients receive doses at or above 100 mg of morphine equivalents per day,5 and concurrent use of central nervous system depressants is common. Changes in kidney and liver function during acute illness may impact opioid metabolism and contribute to overdose.
In addition to overdose, opioids are leading causes of adverse drug events during hospitalization.46 Most studies have focused on surgical patients reporting common opioid-related events as nausea/vomiting, pruritus, rash, mental status changes, respiratory depression, ileus, and urinary retention.47 Hospitalized patients may also exhibit chronic adverse effects due to LTOT. At least one-third of patients on LTOT eventually stop because of adverse effects, such as endocrinopathies, sleep disordered breathing, constipation, fractures, falls, and mental status changes.48 Patients may lack awareness that their symptoms are attributable to opioids and are willing to reduce their opioid use once informed, especially when alternatives are offered to alleviate pain.
Gauging the Risk of Withdrawal
Sudden discontinuation of LTOT by patients, practitioners, or intercurrent events can have unanticipated and undesirable consequences. Withdrawal is not only distressing for patients; it can be dangerous because patients may resort to illicit use, diversion of opioids, or masking opioid withdrawal with other substances such as alcohol. The anxiety and distress associated with withdrawal, or anticipatory fear about withdrawal, can undermine therapeutic alliance and interfere with processes of care. Reviewed guidelines did not offer recommendations regarding withdrawal risk or specific strategies for avoidance. There is no specific prior dose threshold or degree of reduction in opioids that puts patients at risk for withdrawal, in part due to patients’ beliefs, expectations, and differences in response to opioid formulations. Symptoms of opioid withdrawal have been compared to a severe case of influenza, including stomach cramps, nausea and vomiting, diarrhea, tremor and muscle twitching, sweating, restlessness, yawning, tachycardia, anxiety and irritability, bone and joint aches, runny nose, tearing, and piloerection.49 The Clinical Opiate Withdrawal Scale (COWS)49 and the Clinical Institute Narcotic Assessment51 are clinician-administered tools to assess opioid withdrawal similar to the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised,52 to monitor for withdrawal in the inpatient setting.
Synthesizing and Appraising the Indications for Opioid Therapy
For medical inpatients who report adequate pain control and functional outcomes on current doses of LTOT, without evidence of misuse, the pragmatic approach is to continue the treatment plan established by the outpatient clinician rather than escalating or tapering the dose. If opioids are prescribed at discharge, 3 hospital setting/acute pain guidelines and the CDC guideline recommend prescribing the lowest effective dose of immediate release opioids for 3 to 7 days.13,15,16,22
When patients exhibit evidence of an opioid use disorder, have a history of serious overdose, or are experiencing intolerable opioid-related adverse events, the hospitalist may conclude the harms of LTOT outweigh the benefits. For these patients, opioid treatment in the hospital can be aimed at preventing withdrawal, avoiding the perpetuation of inappropriate opioid use, managing other acute medical conditions, and communicating with outpatient prescribers. For patients with misuse, discontinuing opioids is potentially harmful and may be perceived as punitive. Hospitalists should consider consulting addiction or mental health specialists to assist with formulating a plan of care. However, such specialists may not be available in smaller or rural hospitals and referral at discharge can be challenging.53
Beginning to taper opioids during the hospitalization can be appropriate when patients are motivated and can transition to an outpatient provider who will supervise the taper. In ambulatory settings, tapers of 10% to 30% every 2 to 5 days are generally well tolerated.54 If patients started tapering opioids under supervision of an outpatient provider prior to hospitalization; ideally, the taper can be continued during hospitalization with close coordination with the outpatient clinician.
Unfortunately, many patients on LTOT are admitted with new sources of acute pain and or exacerbations of chronic pain, and some have concomitant substance use disorders; we plan to address the management of these complex situations in future work.
Despite the frequency with which patients on LTOT are hospitalized for nonsurgical stays and the challenges inherent in evaluating pain and assessing the possibility of substance use disorders, no formal guidelines or empirical research studies pertain to this population. Guidelines in this review were developed for hospital settings and acute pain in the absence of LTOT, and for outpatient care of patients on LTOT. We also included a nonsystematic synthesis of literature that varied in relevance to medical inpatients on LTOT.
CONCLUSIONS
Although inpatient assessment and treatment of patients with LTOT remains an underresearched area, we were able to extract and synthesize recommendations from 14 guideline statements and apply these to the assessment of patients with LTOT in the inpatient setting. Hospitalists frequently encounter patients on LTOT for chronic nonmalignant pain and are faced with complex decisions about the effectiveness and safety of LTOT; appropriate patient assessment is fundamental to making these decisions. Key guideline recommendations relevant to inpatient assessment include assessing both pain and functional status, differentiating acute from chronic pain, ascertaining preadmission pain treatment history, obtaining a psychosocial history, screening for mental health issues such as depression and anxiety, screening for substance use disorders, checking state prescription drug monitoring databases, ordering urine drug immunoassays, detecting use of sedative-hypnotics, identifying medical conditions associated with increased risk of overdose and adverse events, and appraising the potential benefits and harms of opioid therapy. Although approaches to assessing medical inpatients on LTOT can be extrapolated from outpatient guidelines, observational studies, and small studies in surgical populations, more work is needed to address these critical topics for inpatients on LTOT.
Disclosure
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. All other authors have no relevant conflicts of interest with the work.
1. Mosher HJ, Jiang L, Sarrazin MSV, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and Characteristics of Hospitalized Adults on Chronic Opioid Therapy. J Hosp Med. 2014;9(2):82-87. PubMed
2. Campbell CI, Weisner C, Leresche L, et al. Age and Gender Trends in Long-Term Opioid Analgesic Use for Noncancer Pain. Am J Public Health. 2010;100(12):2541-2547. PubMed
3. Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R. Hospital Inpatient Utilization Related to Opioid Overuse among Adults, 1993–2012. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
33. Young QR, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-Item Measures for Depression and Anxiety: Validation of the Screening Tool for Psychological Distress in an Inpatient Cardiology Setting. Eur J Cardiovasc Nurs. 2015;14(6):544-551. PubMed
1. Mosher HJ, Jiang L, Sarrazin MSV, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and Characteristics of Hospitalized Adults on Chronic Opioid Therapy. J Hosp Med. 2014;9(2):82-87. PubMed
2. Campbell CI, Weisner C, Leresche L, et al. Age and Gender Trends in Long-Term Opioid Analgesic Use for Noncancer Pain. Am J Public Health. 2010;100(12):2541-2547. PubMed
3. Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R. Hospital Inpatient Utilization Related to Opioid Overuse among Adults, 1993–2012. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
33. Young QR, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-Item Measures for Depression and Anxiety: Validation of the Screening Tool for Psychological Distress in an Inpatient Cardiology Setting. Eur J Cardiovasc Nurs. 2015;14(6):544-551. PubMed
© 2018 Society of Hospital Medicine
Perceived safety and value of inpatient “very important person” services
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
© 2017 Society of Hospital Medicine
Nondirected testing for inpatients with severe liver injury
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
© 2017 Society of Hospital Medicine
Inpatient Opioid Prescribing
Since initial reports describing an emerging opioid epidemic in the early 2000s,[1] we have seen a flurry of studies characterizing the scope and impact of the problem and calling for actions to stem the rising tide.[2] However, most of these studies, even the recently issued Centers for Disease Control and Prevention (CDC) guidelines, have focused on the outpatient setting,[3] rendering the inpatient setting somewhat of an opioid prescribing black box.
Recently, however, several studies have highlighted both the scope and downstream impact of opioid prescribing in the inpatient setting. We now know that more than half of hospitalized patients in the United States are exposed to opioid medications during their hospitalization,[4] the majority of which are new initiations in patients without opioid receipt in the year preceding their hospitalization.[5] Among opioid nave patients admitted to the hospital, one‐quarter go on to receive a script for an opioid in the 72 hours after hospital discharge, and 4% have ongoing use 1 year after discharge.[5] Although this may seem like a relatively small percentage, when you consider that there are about 40 million discharges from US medical centers each year, the majority of which are opioid nave prior to hospitalization, this becomes a large absolute number. Taken together, these studies suggest that inpatient prescribing contributes substantially to more chronic opioid use. Accordingly, reigning in inpatient prescribing may be a crucial step in curbing the opioid epidemic as a whole.
In this issue of the Journal of Hospital Medicine, Calcaterra et al.,[6] in a qualitative analysis of hospitalist perceptions of opioid prescribing, draw attention to the bidirectional pull exerted on physicians by the need to adequately treat pain as mandated by the Joint Commission,[7] while minimizing exposure to medications fraught with a wide array of adverse effects, ranging from constipation to addiction to death. What often ensues is a haphazardly choreographed negotiation between 2 parties, 1 of which, in the setting of addiction, may not know what is best for him/herself, and the other of which is caught between the desire to relieve suffering and the desire to do no harm.
At the center of all this is the fact that pain itself is a nebulous concept, defined and experienced in a multitude of different ways by different people and cultures. For some, there is no distinction between psychological and physical pain. Without sufficient objective measures of pain, we must rely on the patient to convey their degree of suffering, and then use our clinical judgment to decide whether pain is severe enough and risks are low enough to use medications with physiological effects that are identical to heroin.
This study adds important information to the opioid prescribing equation, in that understanding the drivers of physician decision making in this realm is an important prelude to developing strategies that effectively promote more standardized and appropriate opioid prescribing. This is the first study to specifically investigate perceptions of hospitalists. Although their study involved only 25 hospitalists, raising questions of validity and generalizability, as a practicing hospitalist, I anticipate that their findings will resonate widely with other hospitalists across the country. First, although the hospitalists in their study were generally comfortable using opioids for acute pain, they found managing acute pain exacerbations in patients with chronic pain more challenging. Second, negative prior experiences related to opioid prescribing strongly inform future prescribing. Third, opioids are often used as a tool to facilitate discharges and prevent readmissions.
There are several important implications arising from each of these 3 identified emergent themes.
First, although hospitalists felt generally comfortable in prescribing opioids for acute pain in patients not on chronic opioids, in reality, prescribing opioids for acute pain, even in opioid nave patients, is neither straightforward nor done safely. It is important we recognize that our prescribing practices as hospitalists, even for acute pain in opioid nave patients, contribute to adverse events, and promote and propagate addiction. We can do better. Akin to the recent CDC guidelines,[3] prescribing guidelines specifically directed at the hospital setting are necessary. An effective set of guidelines would both promote more standardized and safer prescribing practices, as well as provide support for physician decision making in this realm. Such guidelines would help provide ground rules and a framework from which physicians could draw during those challenging discussions with patients suffering from chronic pain.
Second, many of the negative prior experiences described by the hospitalists in this study as shaping future behavior could have been avoided with enhanced, system‐wide safety measures directed at each of the steps in the medication use continuum, from prescribing to administration. For example, mandatory use of electronic prescribing of controlled substances can prevent patients from tampering with prescriptions.[8] Monitored ingestion can prevent misuse and diversion. Additional safety measures that should be widely adopted in the inpatient setting include integration and mandatory review of the State Prescription Drug Monitoring Program when prescribing opioids on admission and discharge, and clinical decision support to promote safe prescribing decisions related to dose, route, and monitoring practices. Incorporation of these and other safety measures in a systematic way will ultimately improve the experience and outcomes for both patients and physicians.
Finally, opioids are used as a tool to facilitate discharge, in part because it is much harder to discuss a decision not to prescribe opioids with a patient expressing suffering than it is to just provide a limited supply and get them back to their longitudinal provider. Physicians often lack the vocabulary necessary to effectively navigate such discussions. We need to make these discussions easier, through physician education and training regarding how to speak to patients about pain management. A shared, standard vocabulary specific to the inpatient setting should be developed and disseminated for discussing with patients (1) expectations related to pain management, (2) potential benefits and risks of opioids, (3) concerns over addiction, and (4) discontinuing/tapering opioids.
In conclusion, if we are to effectively curb the opioid epidemic, the inpatient setting cannot remain a black box. Standardizing opioid prescribing in the hospital will require a concerted effort by hospitalists and other physicians, nurses, pharmacists, and regulatory bodies, with important input from patients as well as longitudinal providers in the outpatient setting, to assure appropriate navigation during transitions of care. Together, we can turn haphazard negotiation into coordinated comanagement, ultimately promoting individual and public health.
Disclosures: Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The article contents are solely the responsibility of the author and do not necessarily represent the views of the funding organization. Dr. Herzig has no conflicts to disclose.
- , . Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31(6):506–511.
- . A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985.
- , , . CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645.
- , , , , . Opioid utilization and opioid‐related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73–81.
- , , , , , . Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478–485.
- , , , et al. The hospitalist perspective on opioid prescribing: a qualitative analysis. J Hosp Med. 2016;11(8):536–542.
- The Joint Commission. Facts about pain management. Available at: http://www.jointcommission.org/pain_management. Accessed April 3, 2016.
- Drug Enforcement Administration, Department of Justice. Electronic prescriptions for controlled substances. Available at: http://www.deadiversion.usdoj.gov/fed_regs/rules/2010/fr0331.htm. Published March 31, 2010. Accessed April 3, 2016.
Since initial reports describing an emerging opioid epidemic in the early 2000s,[1] we have seen a flurry of studies characterizing the scope and impact of the problem and calling for actions to stem the rising tide.[2] However, most of these studies, even the recently issued Centers for Disease Control and Prevention (CDC) guidelines, have focused on the outpatient setting,[3] rendering the inpatient setting somewhat of an opioid prescribing black box.
Recently, however, several studies have highlighted both the scope and downstream impact of opioid prescribing in the inpatient setting. We now know that more than half of hospitalized patients in the United States are exposed to opioid medications during their hospitalization,[4] the majority of which are new initiations in patients without opioid receipt in the year preceding their hospitalization.[5] Among opioid nave patients admitted to the hospital, one‐quarter go on to receive a script for an opioid in the 72 hours after hospital discharge, and 4% have ongoing use 1 year after discharge.[5] Although this may seem like a relatively small percentage, when you consider that there are about 40 million discharges from US medical centers each year, the majority of which are opioid nave prior to hospitalization, this becomes a large absolute number. Taken together, these studies suggest that inpatient prescribing contributes substantially to more chronic opioid use. Accordingly, reigning in inpatient prescribing may be a crucial step in curbing the opioid epidemic as a whole.
In this issue of the Journal of Hospital Medicine, Calcaterra et al.,[6] in a qualitative analysis of hospitalist perceptions of opioid prescribing, draw attention to the bidirectional pull exerted on physicians by the need to adequately treat pain as mandated by the Joint Commission,[7] while minimizing exposure to medications fraught with a wide array of adverse effects, ranging from constipation to addiction to death. What often ensues is a haphazardly choreographed negotiation between 2 parties, 1 of which, in the setting of addiction, may not know what is best for him/herself, and the other of which is caught between the desire to relieve suffering and the desire to do no harm.
At the center of all this is the fact that pain itself is a nebulous concept, defined and experienced in a multitude of different ways by different people and cultures. For some, there is no distinction between psychological and physical pain. Without sufficient objective measures of pain, we must rely on the patient to convey their degree of suffering, and then use our clinical judgment to decide whether pain is severe enough and risks are low enough to use medications with physiological effects that are identical to heroin.
This study adds important information to the opioid prescribing equation, in that understanding the drivers of physician decision making in this realm is an important prelude to developing strategies that effectively promote more standardized and appropriate opioid prescribing. This is the first study to specifically investigate perceptions of hospitalists. Although their study involved only 25 hospitalists, raising questions of validity and generalizability, as a practicing hospitalist, I anticipate that their findings will resonate widely with other hospitalists across the country. First, although the hospitalists in their study were generally comfortable using opioids for acute pain, they found managing acute pain exacerbations in patients with chronic pain more challenging. Second, negative prior experiences related to opioid prescribing strongly inform future prescribing. Third, opioids are often used as a tool to facilitate discharges and prevent readmissions.
There are several important implications arising from each of these 3 identified emergent themes.
First, although hospitalists felt generally comfortable in prescribing opioids for acute pain in patients not on chronic opioids, in reality, prescribing opioids for acute pain, even in opioid nave patients, is neither straightforward nor done safely. It is important we recognize that our prescribing practices as hospitalists, even for acute pain in opioid nave patients, contribute to adverse events, and promote and propagate addiction. We can do better. Akin to the recent CDC guidelines,[3] prescribing guidelines specifically directed at the hospital setting are necessary. An effective set of guidelines would both promote more standardized and safer prescribing practices, as well as provide support for physician decision making in this realm. Such guidelines would help provide ground rules and a framework from which physicians could draw during those challenging discussions with patients suffering from chronic pain.
Second, many of the negative prior experiences described by the hospitalists in this study as shaping future behavior could have been avoided with enhanced, system‐wide safety measures directed at each of the steps in the medication use continuum, from prescribing to administration. For example, mandatory use of electronic prescribing of controlled substances can prevent patients from tampering with prescriptions.[8] Monitored ingestion can prevent misuse and diversion. Additional safety measures that should be widely adopted in the inpatient setting include integration and mandatory review of the State Prescription Drug Monitoring Program when prescribing opioids on admission and discharge, and clinical decision support to promote safe prescribing decisions related to dose, route, and monitoring practices. Incorporation of these and other safety measures in a systematic way will ultimately improve the experience and outcomes for both patients and physicians.
Finally, opioids are used as a tool to facilitate discharge, in part because it is much harder to discuss a decision not to prescribe opioids with a patient expressing suffering than it is to just provide a limited supply and get them back to their longitudinal provider. Physicians often lack the vocabulary necessary to effectively navigate such discussions. We need to make these discussions easier, through physician education and training regarding how to speak to patients about pain management. A shared, standard vocabulary specific to the inpatient setting should be developed and disseminated for discussing with patients (1) expectations related to pain management, (2) potential benefits and risks of opioids, (3) concerns over addiction, and (4) discontinuing/tapering opioids.
In conclusion, if we are to effectively curb the opioid epidemic, the inpatient setting cannot remain a black box. Standardizing opioid prescribing in the hospital will require a concerted effort by hospitalists and other physicians, nurses, pharmacists, and regulatory bodies, with important input from patients as well as longitudinal providers in the outpatient setting, to assure appropriate navigation during transitions of care. Together, we can turn haphazard negotiation into coordinated comanagement, ultimately promoting individual and public health.
Disclosures: Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The article contents are solely the responsibility of the author and do not necessarily represent the views of the funding organization. Dr. Herzig has no conflicts to disclose.
Since initial reports describing an emerging opioid epidemic in the early 2000s,[1] we have seen a flurry of studies characterizing the scope and impact of the problem and calling for actions to stem the rising tide.[2] However, most of these studies, even the recently issued Centers for Disease Control and Prevention (CDC) guidelines, have focused on the outpatient setting,[3] rendering the inpatient setting somewhat of an opioid prescribing black box.
Recently, however, several studies have highlighted both the scope and downstream impact of opioid prescribing in the inpatient setting. We now know that more than half of hospitalized patients in the United States are exposed to opioid medications during their hospitalization,[4] the majority of which are new initiations in patients without opioid receipt in the year preceding their hospitalization.[5] Among opioid nave patients admitted to the hospital, one‐quarter go on to receive a script for an opioid in the 72 hours after hospital discharge, and 4% have ongoing use 1 year after discharge.[5] Although this may seem like a relatively small percentage, when you consider that there are about 40 million discharges from US medical centers each year, the majority of which are opioid nave prior to hospitalization, this becomes a large absolute number. Taken together, these studies suggest that inpatient prescribing contributes substantially to more chronic opioid use. Accordingly, reigning in inpatient prescribing may be a crucial step in curbing the opioid epidemic as a whole.
In this issue of the Journal of Hospital Medicine, Calcaterra et al.,[6] in a qualitative analysis of hospitalist perceptions of opioid prescribing, draw attention to the bidirectional pull exerted on physicians by the need to adequately treat pain as mandated by the Joint Commission,[7] while minimizing exposure to medications fraught with a wide array of adverse effects, ranging from constipation to addiction to death. What often ensues is a haphazardly choreographed negotiation between 2 parties, 1 of which, in the setting of addiction, may not know what is best for him/herself, and the other of which is caught between the desire to relieve suffering and the desire to do no harm.
At the center of all this is the fact that pain itself is a nebulous concept, defined and experienced in a multitude of different ways by different people and cultures. For some, there is no distinction between psychological and physical pain. Without sufficient objective measures of pain, we must rely on the patient to convey their degree of suffering, and then use our clinical judgment to decide whether pain is severe enough and risks are low enough to use medications with physiological effects that are identical to heroin.
This study adds important information to the opioid prescribing equation, in that understanding the drivers of physician decision making in this realm is an important prelude to developing strategies that effectively promote more standardized and appropriate opioid prescribing. This is the first study to specifically investigate perceptions of hospitalists. Although their study involved only 25 hospitalists, raising questions of validity and generalizability, as a practicing hospitalist, I anticipate that their findings will resonate widely with other hospitalists across the country. First, although the hospitalists in their study were generally comfortable using opioids for acute pain, they found managing acute pain exacerbations in patients with chronic pain more challenging. Second, negative prior experiences related to opioid prescribing strongly inform future prescribing. Third, opioids are often used as a tool to facilitate discharges and prevent readmissions.
There are several important implications arising from each of these 3 identified emergent themes.
First, although hospitalists felt generally comfortable in prescribing opioids for acute pain in patients not on chronic opioids, in reality, prescribing opioids for acute pain, even in opioid nave patients, is neither straightforward nor done safely. It is important we recognize that our prescribing practices as hospitalists, even for acute pain in opioid nave patients, contribute to adverse events, and promote and propagate addiction. We can do better. Akin to the recent CDC guidelines,[3] prescribing guidelines specifically directed at the hospital setting are necessary. An effective set of guidelines would both promote more standardized and safer prescribing practices, as well as provide support for physician decision making in this realm. Such guidelines would help provide ground rules and a framework from which physicians could draw during those challenging discussions with patients suffering from chronic pain.
Second, many of the negative prior experiences described by the hospitalists in this study as shaping future behavior could have been avoided with enhanced, system‐wide safety measures directed at each of the steps in the medication use continuum, from prescribing to administration. For example, mandatory use of electronic prescribing of controlled substances can prevent patients from tampering with prescriptions.[8] Monitored ingestion can prevent misuse and diversion. Additional safety measures that should be widely adopted in the inpatient setting include integration and mandatory review of the State Prescription Drug Monitoring Program when prescribing opioids on admission and discharge, and clinical decision support to promote safe prescribing decisions related to dose, route, and monitoring practices. Incorporation of these and other safety measures in a systematic way will ultimately improve the experience and outcomes for both patients and physicians.
Finally, opioids are used as a tool to facilitate discharge, in part because it is much harder to discuss a decision not to prescribe opioids with a patient expressing suffering than it is to just provide a limited supply and get them back to their longitudinal provider. Physicians often lack the vocabulary necessary to effectively navigate such discussions. We need to make these discussions easier, through physician education and training regarding how to speak to patients about pain management. A shared, standard vocabulary specific to the inpatient setting should be developed and disseminated for discussing with patients (1) expectations related to pain management, (2) potential benefits and risks of opioids, (3) concerns over addiction, and (4) discontinuing/tapering opioids.
In conclusion, if we are to effectively curb the opioid epidemic, the inpatient setting cannot remain a black box. Standardizing opioid prescribing in the hospital will require a concerted effort by hospitalists and other physicians, nurses, pharmacists, and regulatory bodies, with important input from patients as well as longitudinal providers in the outpatient setting, to assure appropriate navigation during transitions of care. Together, we can turn haphazard negotiation into coordinated comanagement, ultimately promoting individual and public health.
Disclosures: Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The article contents are solely the responsibility of the author and do not necessarily represent the views of the funding organization. Dr. Herzig has no conflicts to disclose.
- , . Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31(6):506–511.
- . A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985.
- , , . CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645.
- , , , , . Opioid utilization and opioid‐related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73–81.
- , , , , , . Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478–485.
- , , , et al. The hospitalist perspective on opioid prescribing: a qualitative analysis. J Hosp Med. 2016;11(8):536–542.
- The Joint Commission. Facts about pain management. Available at: http://www.jointcommission.org/pain_management. Accessed April 3, 2016.
- Drug Enforcement Administration, Department of Justice. Electronic prescriptions for controlled substances. Available at: http://www.deadiversion.usdoj.gov/fed_regs/rules/2010/fr0331.htm. Published March 31, 2010. Accessed April 3, 2016.
- , . Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31(6):506–511.
- . A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985.
- , , . CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645.
- , , , , . Opioid utilization and opioid‐related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73–81.
- , , , , , . Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478–485.
- , , , et al. The hospitalist perspective on opioid prescribing: a qualitative analysis. J Hosp Med. 2016;11(8):536–542.
- The Joint Commission. Facts about pain management. Available at: http://www.jointcommission.org/pain_management. Accessed April 3, 2016.
- Drug Enforcement Administration, Department of Justice. Electronic prescriptions for controlled substances. Available at: http://www.deadiversion.usdoj.gov/fed_regs/rules/2010/fr0331.htm. Published March 31, 2010. Accessed April 3, 2016.
Hospital Antipsychotic Use
Antipsychotic medications are frequently used off label for management of behavioral symptoms associated with delirium and/or dementia. Despite regulations designed to curb inappropriate prescribing of these medications in nursing homes, substantial levels of use and variation in use have been observed in this setting.[1] Although antipsychotic medications are also frequently used in the hospital, the scope and variation in use have not been adequately investigated. Given the lack of oversight for medication prescribing in the hospital setting and the frequency of delirium, occurring in 15% to 26% of hospitalized older adults,[2, 3, 4] off‐label use of antipsychotic medications and variation in use could be substantial.
Because variation in practice is known to increase in the setting of controversy or lack of clarity regarding appropriate management,[5] large degrees of variation can draw attention to priority areas for clinical effectiveness studies, and the need for guidelines, clinical decision support, or regulatory oversight. In the absence of clear guidelines for the use of antipsychotic medication in nonpsychiatric hospitalized patients, we hypothesized that significant variation in use would persist after controlling for patient characteristics. Using a large, nationally representative cohort of admissions to 300 hospitals from July 2009 to June 2010, we sought to investigate prescribing patterns and hospital variation in use of antipsychotic medications in nonpsychiatric admissions to US hospitals.
METHODS
Setting and Data Collection
We conducted a retrospective cohort study using data from 300 US, nonfederal, acute care facilities contributing to the database maintained by Premier (Premier Healthcare Solutions, Inc., Charlotte, NC). This nationally representative database, created to measure healthcare utilization and quality of care, is drawn from voluntarily participating hospitals and contains data on approximately 1 in every 4 discharges nationwide.[6] Participating hospitals are similar in geographic distribution and urban/rural status to hospitals nationwide, although large, nonteaching hospitals are slightly over‐represented. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center.
Inclusion and Exclusion Criteria
We studied a cohort of all adult (18 years) nonpsychiatric admissions to participating hospitals from July 1, 2009 through June 30, 2010. We excluded patients admitted to a psychiatry service or with any discharge diagnosis of a psychotic disorder (defined by the Elixhauser comorbidity Psychoses: 295.00‐298.9, 299.10‐299.11), because we were interested in use of antipsychotics for conditions other than primary psychiatric disorders. We also excluded patients with a charge for labor and delivery owing to the nonrepresentativeness of this patient population for the general hospitalized patient. We excluded admissions with unknown gender, and admissions with a length of stay greater than 365 days, as these admissions are not representative of the typical admission to an acute care hospital. We also excluded hospitals contributing less than 100 admissions owing to lack of precision in corresponding hospital prescribing rates.
Antipsychotic Medication Utilization
In‐hospital antipsychotic use was ascertained from pharmacy charges, reflecting each medication dispensed during the hospitalization. We categorized antipsychotic medications as typical (haloperidol, loxapine, thioridazine, molindone, thiothixine, pimozide, fluphenazine, trifluoperazine, chlorpromazine, and perphenazine) and atypical (aripiprazole, asenapine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone) based on classification by the Food and Drug Administration.[7, 8] We excluded prochlorperazine (Compazine) from our typical antipsychotic definition, as this medication is almost exclusively used as an antiemetic.
In the absence of guidelines for use of antipsychotic agents in hospitalized patients, we used the Centers for Medicare and Medicaid Services (CMS) guidelines for long‐term care facilities to define measures of potentially excessive dosing in the hospital setting.[9] These guidelines define the daily dosage levels of antipsychotics above which the medical necessity of the higher dose should be explained in the medical record. We defined any daily dosage above these specified levels as a potentially excessive daily dose.
Characteristics Associated With Use
We investigated the association between antipsychotic use and patient and hospital characteristics, selected based on clinical grounds. Patient characteristics included: (1) demographic variables such as age (<65, 6574, 75+ years), gender, self‐reported race, marital status, and primary insurance; (2) admission characteristic variables, including admitting department (surgical vs nonsurgical, defined by a surgical attending of record and presence of operating room charges), whether the patient spent any time in the intensive care unit (ICU), and whether they received mechanical ventilation; and (3) potential indications for use, including delirium (included delirium superimposed upon dementia), dementia (without delirium), and insomnia (see Supporting Information, Appendix, in the online version of this article for International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] codes). Hospital characteristics included number of beds, urban versus rural status, teaching status, and US Census region.
Statistical Analysis
We report the proportion of admissions with in‐hospital use of any antipsychotic, and the number of days of exposure, overall and stratified by typical and atypical.
We determined potentially excessive dosing by taking the sum of the doses for a specific antipsychotic charged on a given day and comparing it to the CMS guidelines for long‐term care settings described above. We report the percentage of exposed admissions with at least 1 day of potentially excessive dosing.
All multivariable models below were operationalized as generalized estimating equations with a Poisson error term, log link, robust variance estimator,[10] and an exchangeable correlation structure to account for repeated admissions of the same patient.
We investigated patient and hospital characteristics associated with use of any antipsychotic medication using a multivariable model that simultaneously included all patient and hospital characteristics in Table 1 as independent variables.
| % of Cohort | Any Exposure, % | Typical Exposure, % | Atypical Exposure, % | |
|---|---|---|---|---|
| ||||
| Patient characteristics | ||||
| Age group, y | ||||
| <65 | 52.1 | 4.6 | 2.0 | 3.1 |
| 6574 | 18.5 | 5.2 | 2.7 | 3.1 |
| 75+ | 29.4 | 8.8 | 4.6 | 5.4 |
| Gender | ||||
| Male | 43.8 | 6.6 | 3.6 | 3.8 |
| Female | 56.2 | 5.5 | 2.3 | 3.8 |
| Race | ||||
| White | 64.6 | 6.1 | 2.9 | 4.0 |
| Black | 13.5 | 5.5 | 2.8 | 3.3 |
| Hispanic | 5.0 | 4.9 | 2.2 | 3.2 |
| Other | 19.9 | 6.1 | 3.1 | 3.7 |
| Marital Status | ||||
| Married | 42.5 | 4.6 | 2.4 | 2.7 |
| Single | 46.7 | 7.2 | 3.2 | 4.7 |
| Unknown/other | 10.8 | 6.4 | 3.1 | 4.1 |
| Primary insurance | ||||
| Private (commercial) | 28.8 | 3.0 | 1.5 | 1.8 |
| Medicaid | 10.3 | 6.4 | 2.4 | 4.6 |
| Medicare managed | 10.6 | 7.1 | 4.1 | 4.0 |
| Medicare traditional | 40.9 | 8.0 | 3.7 | 5.3 |
| Self‐pay or other | 9.4 | 4.3 | 2.5 | 2.2 |
| Admitting department | ||||
| Surgical | 60.6 | 5.8 | 3.1 | 3.4 |
| Nonsurgical | 39.4 | 6.2 | 2.4 | 4.4 |
| Any ICU stay | 16.6 | 10.4 | 7.2 | 4.9 |
| Mechanical ventilation | 4.7 | 17.4 | 12.9 | 7.9 |
| Diagnoses | ||||
| Delirium | 3.2 | 28.6 | 19.4 | 15.7 |
| Dementia | 3.1 | 27.4 | 12.0 | 20.2 |
| Insomnia | 1.3 | 10.2 | 3.9 | 7.5 |
| Discharge disposition | ||||
| Home | 77.9 | 3.8 | 1.6 | 2.5 |
| SNF/Rehab | 15.5 | 13.7 | 6.8 | 9.0 |
| Hospice | 1.7 | 16.0 | 10.3 | 8.1 |
| Other | 4.9 | 11.6 | 7.6 | 5.7 |
| Hospital characteristics, % | ||||
| No. of beds | ||||
| 200 | 14.1 | 6.1 | 2.8 | 3.8 |
| 201300 | 18.6 | 6.1 | 2.9 | 3.9 |
| 301500 | 37.7 | 5.9 | 2.9 | 3.7 |
| 500+ | 29.7 | 5.9 | 2.8 | 3.8 |
| Population served | ||||
| Urban | 89.4 | 6.0 | 2.9 | 3.8 |
| Rural | 10.6 | 5.8 | 2.4 | 3.9 |
| Teaching status | ||||
| Teaching | 39.2 | 5.8 | 2.9 | 3.7 |
| Nonteaching | 60.8 | 6.0 | 2.8 | 3.9 |
| US Census region | ||||
| West | 16.9 | 5.9 | 3.2 | 3.5 |
| Northeast | 20.1 | 6.1 | 2.9 | 3.9 |
| Midwest | 21.9 | 5.7 | 2.5 | 3.8 |
| South | 41.0 | 6.1 | 2.9 | 3.9 |
To determine hospital variation in antipsychotic use, we first determined the proportion of admissions at each hospital with at least 1 charge for antipsychotic medication. We then divided hospitals into quintiles based on their facility‐level antipsychotic prescribing rates and assigned all admissions to their corresponding hospital quintile. We then used a multivariable model to measure the adjusted association between prescribing quintile and patient‐level receipt of antipsychotic medication, controlling for all patient characteristics listed in Table 1 (except discharge disposition), and comorbidities using the Healthcare Cost and Utilization Project Comorbidity Software version 3.7 (Agency for Healthcare Research and Quality, Rockville, MD).[11] We used the lowest prescribing quintile as the reference group. We also report in the Supporting Information, Appendix, in the online version of this article, the distribution of prescribing rates for the hospitals in our cohort before and after adjustment for patient characteristics. For both approaches, we conducted stratified analyses in admissions with delirium and dementia.
All analyses were carried out using SAS software (SAS Institute Inc., Cary, NC).
RESULTS
Admission Characteristics
There were 3,190,934 admissions aged 18 years and over to 300 hospitals from July 1, 2009 to June 30, 2010. After excluding admissions with unknown gender (n = 17), length of stay greater than 365 days (n = 25), charges for labor and delivery (n = 323,111) or a psychiatric attending of record or psychiatric comorbidity (n = 172,669), and admissions to hospitals with fewer than 100 admissions (n = 31), our cohort included 2,695,081 admissions. The median age was 63 years (25th, 75th percentile 48, 77 years), and 1,514,986 (56%) were women. Table 1 shows the overall admission characteristics of the cohort and the percent exposed to antipsychotics among each patient and hospital characteristic.
Antipsychotic Use
There were 160,773 (6%) admissions with antipsychotic exposure. Among exposed admissions, 102,148 (64%) received atypical and 76,979 (48%) received typical antipsychotics, with 18,354 (11%) exposed to both. The median (25th, 75th percentile) length of stay among exposed was 5 days (3, 9 days), and the median (25th, 75th percentile) number of days of exposure was 3 (1, 5 days) overall, 3 days (2, 6 days) for atypical and 2 days (1, 3 days) for typical exposure.
Among admissions aged 65 to 74 years, 25,855 (5%) were exposed. Among admissions aged 75 years or older, 69,792 (9%) were exposed. Among admissions with delirium, exposure occurred in 24,787 (29%), with 13,640 (55%) receiving atypical, 16,828 (68%) receiving typical, and 5681 (23%) exposed to both. Among admissions with dementia, exposure occurred in 23,179 (27%), with 17,068 (74%) receiving atypical, 10,108 (44%) receiving typical, and 3997 (17%) exposed to both.
Use of Specific Drugs and Potentially Excessive Dosing
Table 2 demonstrates the most commonly used antipsychotic medications and the rates of potentially excessive dosing. Quetiapine and olanzapine were the most commonly used atypical antipsychotics, and haloperidol represented the majority of typical antipsychotic use. Among admissions with antipsychotic exposure, 47% received at least 1 potentially excessive daily dose, 18% of those with atypical exposure and 79% of those with typical exposure. Among admissions aged 65 years and up (n = 1,291,375), the prevalence of potentially excessive dosing was almost identical; 46% received at least 1 daily dose in excess of the recommended daily dose, 11% of those with atypical exposure and 79% of those with typical exposure.
| Agent |
Overall Prevalence,N = 2,695,081 |
% of Exposed With Potentially Excessive Dosing* | ||
|---|---|---|---|---|
| Within 100% of Recommended DD* | 101% to 150% of Recommended DD* | >150% of Recommended DD* | ||
| ||||
| Any antipsychotic | 6.0 | 52.9 | 20.2 | 26.9 |
| Atypical | 3.8 | 82.0 | 5.4 | 12.6 |
| Quetiapine (200) | 1.8 | 81.7 | 5.7 | 12.6 |
| Olanzapine (10) | 0.6 | 73.7 | 7.3 | 19.0 |
| Risperidone (2) | 0.9 | 79.2 | 6.8 | 14.0 |
| Other | 0.7 | 98.3 | 0.1 | 1.6 |
| Typical | 2.9 | 21.1 | 37.0 | 41.9 |
| Haloperidol (4) | 2.5 | 13.2 | 41.3 | 45.5 |
| Chlorpromazine (75) | 0.3 | 76.0 | 9.8 | 14.2 |
| Other | 0.4 | 89.1 | 2.9 | 8.0 |
Characteristics Associated With Antipsychotic Use
Among the patient and hospital characteristics included in our analysis, the 5 characteristics most strongly associated with antipsychotic exposure after adjustment were (Table 3): delirium (relative risk [RR]: 2.93, 95% confidence interval [CI]: 2.88‐2.98); dementia (RR: 2.78, 95% CI: 2.72‐2.83); insurance status, with higher risk among patients with traditional Medicare (RR: 2.09, 95% CI: 2.04‐2.13), Medicare managed (RR: 1.98, 95% CI: 1.93‐2.03), Medicaid (RR: 1.84, 95% CI: 1.80‐1.88), and self‐pay/other (RR: 1.26, 95% CI: 1.23‐1.29) compared to private (commercial) insurance; use of mechanical ventilation (RR: 1.84, 95% CI: 1.81‐1.87); and any ICU stay (RR: 1.53, 95% CI: 1.51‐1.55).
| Unadjusted RR of Receiving Any Antipsychotic [95% CI] | Adjusted RR of Receiving Any Antipsychotic [95% CI]* | |
|---|---|---|
| ||
| Age group, y, % | ||
| <65 | Reference | Reference |
| 6574 | 1.12 [1.10,1.14] | 0.74 [0.72, 0.75] |
| 75+ | 1.90 [1.88,1.92] | 1.03 [1.01, 1.05] |
| Gender | ||
| Female | Reference | Reference |
| Male | 1.19 [1.18,1.20] | 1.27 [1.26, 1.28] |
| Race | ||
| White | Reference | Reference |
| Black | 0.91 [0.90,0.92] | 0.85 [0.83, 0.86] |
| Hispanic | 0.80 [0.78,0.82] | 0.79 [0.76, 0.81] |
| Other | 0.99 [0.98,1.00] | 0.96 [0.95, 0.98] |
| Marital status | ||
| Married | Reference | Reference |
| Single | 1.57 [1.55,1.59] | 1.43 [1.42, 1.45] |
| Unknown/other | 1.41 [1.39,1.43] | 1.27 [1.24, 1.29] |
| Primary insurance | ||
| Private (commercial) | Reference | Reference |
| Medicaid | 2.13 [2.09,2.17] | 1.84 [1.80, 1.88] |
| Medicare managed | 2.35 [2.31,2.39] | 1.98 [1.93, 2.03] |
| Medicare traditional | 2.65 [2.61,2.69] | 2.09 [2.04, 2.13] |
| Self‐pay or other | 1.41 [1.38,1.44] | 1.26 [1.23, 1.29] |
| Admitting department | ||
| Surgical | Reference | Reference |
| Nonsurgical | 1.06 [1.05,1.07] | 1.05 [1.03, 1.06] |
| Any ICU stay | 2.05 [2.03,2.07] | 1.53 [1.51, 1.55] |
| Mechanical ventilation | 3.22 [3.18,3.26] | 1.84 [1.81, 1.87] |
| Diagnoses | ||
| Delirium | 5.48 [5.42, 5.45] | 2.93 [2.88, 2.98] |
| Dementia | 5.21 [5.15,5.27] | 2.78 [2.72, 2.83] |
| Insomnia | 1.72 [1.67,1.78] | 1.51 [1.45, 1.57] |
| No. of beds | ||
| 200 | Reference | Reference |
| 201300 | 1.01 [0.99,1.03] | 0.96 [0.94, 0.98] |
| 301500 | 0.98 [0.97,1.00] | 0.93 [0.91, 0.95] |
| 500+ | 0.97 [0.96,0.98] | 0.91 [0.90, 0.93] |
| Population served | ||
| Urban | Reference | Reference |
| Rural | 0.96 [0.95,0.98] | 0.91 [0.89, 0.93] |
| Teaching status | ||
| Teaching | Reference | Reference |
| Nonteaching | 1.03 [1.02,1.04] | 0.98 [0.97, 1.00] |
| US Census region | ||
| West | Reference | Reference |
| Northeast | 1.03 [1.01,1.05] | 1.04 [1.02, 1.06] |
| Midwest | 0.95 [0.94,0.97] | 0.93 [0.91, 0.94] |
| South | 1.02 [1.01,1.03] | 1.07 [1.05, 1.09] |
Hospital Variation in Antipsychotic Use
Figure 1 demonstrates the antipsychotic prescribing rate at each hospital in our cohort, and the corresponding quintiles. Patients admitted to hospitals in the highest prescribing quintile were more than twice as likely to be exposed to antipsychotics compared to patients admitted to hospitals in the lowest prescribing quintile, even after adjustment for patient characteristics and comorbidities (Table 4). This relationship was similar across subgroups of admissions with delirium and dementia (see Supporting Information, Appendix, in the online version of this article for the distribution of hospital antipsychotic prescribing rates before and after adjustment for patient characteristics).
| Admissions, No. (% of Total) | Unadjusted RR of Exposure [95% CI] | Adjusted RR of exposure [95% CI]* | |
|---|---|---|---|
| |||
| Overall | |||
| Q1 | 431,017 (16%) | Reference | Reference |
| Q2 | 630,486 (23%) | 1.67 [1.63, 1.71] | 1.59 [1.55, 1.62] |
| Q3 | 548,337 (20%) | 1.93 [1.88, 1.97] | 1.84 [1.80, 1.88] |
| Q4 | 639,027 (24%) | 2.16 [2.12, 2.21] | 2.07 [2.03, 2.12] |
| Q5 | 446,214 (17%) | 2.83 [2.77, 2.89] | 2.56 [2.50, 2.61] |
| Delirium | |||
| Q1 | 12,878 (15%) | Reference | Reference |
| Q2 | 20,588 (24%) | 1.58 [1.51, 1.65] | 1.58 [1.51, 1.65] |
| Q3 | 17,402 (20%) | 1.71 [1.64, 1.80] | 1.73 [1.65, 1.82] |
| Q4 | 20,943 (24%) | 2.01 [1.92, 2.10] | 1.99 [1.91, 2.08] |
| Q5 | 14,883 (17%) | 2.15 [2.05, 2.25] | 2.16 [2.07, 2.26] |
| Dementia | |||
| Q1 | 28,290 (15%) | Reference | Reference |
| Q2 | 42,018 (22%) | 1.43 [1.36, 1.50] | 1.40 [1.34, 1.47] |
| Q3 | 38,593 (21%) | 1.61 [1.53, 1.69] | 1.59 [1.51, 1.66] |
| Q4 | 44,638 (24%) | 1.69 [1.62, 1.77] | 1.69 [1.61, 1.77] |
| Q5 | 34,442 (18%) | 1.92 [1.83, 2.01] | 1.90 [1.81, 1.99] |
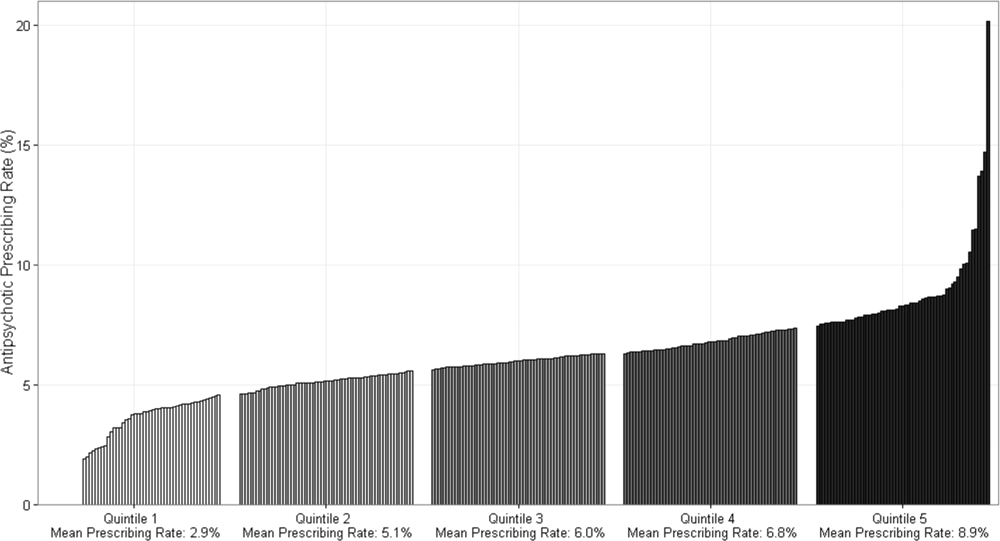
DISCUSSION
In this cohort of nonpsychiatric admissions to 300 US hospitals, antipsychotic medications were used in 6% of admissions, with atypical antipsychotics representing the majority of use. Potentially excessive daily doses based on CMS recommendations for long‐term care facilities occurred in almost half of admissions with any antipsychotic exposure, and in 87% of admissions with haloperidol exposure specifically. We found variation in hospital use of antipsychotics that was not fully accounted for by measured patient characteristics, and which persisted among subgroups of admissions with delirium and/or dementia. Although unmeasured patient characteristics or different billing practices between hospitals are potential explanations, our findings also raise the possibility of different hospital antipsychotic prescribing cultures. These findings provide new information regarding the scope of prescribing in US hospitals, and draw attention to the need for additional studies to better define what constitutes appropriate use of antipsychotics in the hospital setting.
A recent single‐center study at a large academic medical center found an overall antipsychotic exposure rate of 9% of nonpsychiatric admissions.[12] Our finding that 6% of admissions in this multicenter cohort were exposed to antipsychotics is slightly lower, but similar to the previous estimate. Assuming 37 million discharges from US hospitals each year,[13] our study suggests that more than 2 million hospitalized patients receive antipsychotics annually. With around 1.4 million residents in nursing homes on any given day,[14] and an exposure rate of 25% to 30% in that setting,[15, 16, 17] our study suggests that the number of patients exposed in the hospital setting is greater than the number exposed in the nursing home setting, the site of care for which prescribing regulations have been focused thus far.
Because our dataset does not contain preadmission medications, we were unable to specifically investigate new initiation. In the prior single‐center study, approximately 55% of overall use in the hospital setting was new initiation,[12] which would suggest that antipsychotics are newly initiated in around 1 million admissions each year in the hospital. Although we are unable to determine reason for use in our analysis, delirium was a strong predictor of antipsychotic use in our multivariable model, and prior studies have demonstrated delirium to be the most common reason for antipsychotic initiation in hospitalized patients,[12, 18] an indication for which efficacy/effectiveness data are lacking. A recent systematic review of antipsychotics for the treatment of delirium in older adults concluded that because of severe methodological limitations, the small number of existing studies on this topic do not support the use of antipsychotics in the treatment of delirium in older hospitalized adults.[19] Our results further highlight the need for randomized placebo‐controlled trials of antipsychotics in treatment of delirium.
We found variation in antipsychotic use between hospitals that was not fully explained by patient characteristics. Insufficient data to inform clinical decisions surrounding management of agitated delirium/dementia and lack of clear criteria by which to judge appropriateness of antipsychotic use may contribute to this variation. Some variation may relate to resource allocation at different hospitals, and the feasibility of implementing nonpharmacologic management options across settings. Our results collectively highlight the need for studies evaluating the efficacy/effectiveness of antipsychotics in the treatment of delirium and drivers of physician decision‐making in this realm, as well as the need for greater hospital investment in nonpharmacologic delirium‐prevention programs, which have been shown to be effective in prevention of delirium in hospitalized patients.[20]
We observed high levels of potentially excessive daily dosing using cutoffs applied in the long‐term care setting. The majority of the potentially excessive doses were in the setting of typical antipsychotic use, and haloperidol specifically, where doses exceeded 4 mg on at least 1 day in 87% of exposed admissions. Of note, the threshold for haloperidol dosage above which justification is required was decreased from 4 to 2 mg per day in the 2015 update to the CMS guidelines.[21] For the present analysis, we used the guidelines that were contemporaneous to our cohort; we are unable to determine current rates of potentially inappropriate dosages in the present analysis, but given the high prevalence in 2009 to 2010, and the lowering of the dosage threshold since then, it is unlikely that any decrease in use would be enough to substantially reduce the estimate. Whether these high dosages are actually inappropriate in the hospital setting is not established, and we were not able to review medical records to determine whether justification for use of such doses was documented.[22, 23] It is possible that hospitalized patients with altered pharmacodynamics and greater severity of illness could require larger doses of these medications; however, this is an area in need of further investigation, and current critical care guidelines note the lack of sufficient data upon which to justify use of haloperidol in the prevention or treatment of delirium in ICU patients.[24, 25]
The dosages in use are concerning given that the risk of extrapyramidal side effects increases with increasing dose, and prior studies have demonstrated an association between increased dose of antipsychotics and increased risk of other adverse events, including hip fracture and sudden cardiac death.[22, 23] Further, despite these known risks, studies have demonstrated failure to follow recommendations to mitigate risk,[26] such as electrocardiogram monitoring in individuals receiving intravenous haloperidol.[27] Our results suggest that physicians are similarly not following recommendations to use lower doses of haloperidol when treating older patients, given the almost identical incidence of potentially excessive dosing among admissions of patients aged 65 years and older in our cohort.[25] Clinical decision support prompts have been effective at increasing appropriate use of antipsychotic medications in several single‐center analyses,[28, 29, 30] and widespread implementation of such support with a focus on haloperidol dosing should be considered on the basis of our results.
The patient characteristics associated with antipsychotic use in this large, nationally representative analysis are consistent with those identified in prior single‐center analyses.[12, 18] Both prior analyses identified delirium as the most common reason for antipsychotic use, and dementia, intensive care unit stay, and mechanical ventilation were also previously identified as strong predictors of use that we believe hold face validity for the practicing hospitalist. On the other hand, some of the factors associated with antipsychotic use in our model cannot be readily explained, such as insurance status and race, and may be serving as proxies for other variables not included in our analysis. That nonwhite patients are less likely than white patients to receive antipsychotic medications in the hospital has been previously demonstrated,[12] and further investigation to understand this disparity is warranted.
Our study has several additional limitations. First, because our study is observational, the possibility of residual confounding exists, and we cannot rule out that there are other patient factors driving the hospital variation in antipsychotic use that we observed. Second, because guidelines do not exist for antipsychotic dosing in hospitalized patients, we could only comment on potentially excessive dosing, extrapolating from guidelines in the long‐term care setting. Whether such doses are actually excessive in hospitalized patients is not defined. Third, although Premier performs quality checks on charge and ICD‐9‐CM coding data submitted by participating hospitals, the validity of administrative data is uncertain. For example, the use of administrative data to identify delirium diagnoses is likely to have resulted in underestimation of delirium incidence among our different exposure groups. Delirium is likely to be coded more often in the setting of more severe or hyperactive cases, when antipsychotics are more likely to be utilized. This could result in an overestimation of the association between delirium and antipsychotic use. Additionally, differences in coding practices between hospitals for any of the variables in our models could explain some of the variation in antipsychotic prescribing that we observed. Finally, because we were unable to differentiate between new initiation and continuation of a preadmission antipsychotic, some of the variation that we observed is likely to reflect differences in outpatient antipsychotic prescribing practices.
In conclusion, in this large cohort of nonpsychiatric admissions to 300 US hospitals, we found that antipsychotic medication exposure was common, often at high daily doses. Delirium and dementia were the strongest predictors of use among the patient and hospital characteristics examined. The variation in antipsychotic prescribing that we observed was not fully accounted for by measured patient characteristics, and raises the possibility of differing hospital prescribing cultures. Our results draw attention to the need for additional research to better define what constitutes appropriate use of these potentially harmful medications in the hospital setting.
Disclosures: Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Herzig, Rothberg, Gurwitz, and Marcantonio. Acquisition of data: Dr. Herzig. Analysis of data: Mr. Guess. Interpretation of data: Drs. Herzig, Rothberg, Gurwitz, Marcantonio, and Mr. Guess. Drafting of the manuscript: Dr. Herzig. Critical revision of the manuscript for important intellectual content: Drs. Rothberg, Gurwitz, Marcantonio, and Mr. Guess. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant number K24AG035075 from the National Institute on Aging. The funding organizations had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- , , , et al. Variation in nursing home antipsychotic prescribing rates. Arch Intern Med. 2007;167(7):676–683.
- , . Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857.
- , , , , . A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474–481.
- , , , , , . Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42(8):809–815.
- , , , . Small‐area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982;307(21):1310–1314.
- Premier Research Services. Available at: https://www.premierinc.com/transforming‐healthcare/healthcare‐performance‐improvement/premier‐research‐services. Accessed March 15, 2016.
- U.S. Food and Drug Administration. Atypical antipsychotic drugs information. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm094303.htm. Accessed November 1, 2015.
- U.S. Food and Drug Administration. Information on conventional antipsychotics. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm107211. htm. Accessed November 1, 2015.
- Centers for Medicare and Medicaid Services. State Operations Manual. Appendix PP: guidance to surveyors for long‐term care facilities. Available at: https://www.cms.gov/Medicare/Provider‐Enrollment‐and‐Certification/GuidanceforLawsAndRegulations/Downloads/som107 ap_pp_guidelines_ltcf.pdf. Revised October 14, 2005. Accessed March 15, 2016.
- . A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706.
- Healthcare Cost and Utilization Project. Comorbidity software, version 3.7. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed March 15, 2016.
- , , , et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc. 2016;64(2):299–305.
- , . Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb180‐Hospitalizations‐United‐States‐2012.pdf. Published October 2014. Accessed June 29, 2015.
- , , , . Long‐term care services in the United States: 2013 overview. Vital Health Stat 3. 2013;(37):1–107. Available at: http://www.cdc.gov/nchs/data/nsltcp/long_term_care_services_2013.pdf. Accessed March 16, 2016.
- , , , et al. The quality of antipsychotic drug prescribing in nursing homes. Arch Intern Med. 2005;165(11):1280–1285.
- , , , , , . Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89–95.
- , , , , . Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770–w781.
- , , , , , . From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802–804.
- , , . Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269–S276.
- , , , et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta‐analysis. JAMA Intern Med. 2015;175(4):512–520.
- Centers for Medicare and Medicaid Services. State operations manual, appendix PP: guidance to surveyors for long‐term care facilities. Available at: https://www.cms.gov/Regulations‐and‐Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf. Revised October 9, 2015. Accessed February 22, 2016.
- , , , , . Psychotropic drug use and the risk of hip fracture. N Engl J Med. 1987;316(7):363–369.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , , , et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306.
- , , , , . Haloperidol overdosing in the treatment of agitated hospitalized older people with delirium: a retrospective chart review from a community teaching hospital. Drugs Aging. 2013;30(8):639–644.
- , , , . Unsafe use of intravenous haloperidol: evaluation of recommendation‐concordant care in hospitalized elderly adults. J Am Geriatr Soc. 2013;61(1):160–161.
- U.S. Food and Drug Administration. HALDOL brand of haloperidol injection. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/015923s082,018701s057lbl.pdf. Accessed February 23, 2016.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- , , , et al. A standardized, bundled approach to providing geriatric‐focused acute care. J Am Geriatr Soc. 2014;62(5):936–942.
- , , , . Don't fuel the fire: decreasing intravenous haloperidol use in high risk patients via a customized electronic alert. J Am Med Inform Assoc. 2014;21(6):1109–1112.
Antipsychotic medications are frequently used off label for management of behavioral symptoms associated with delirium and/or dementia. Despite regulations designed to curb inappropriate prescribing of these medications in nursing homes, substantial levels of use and variation in use have been observed in this setting.[1] Although antipsychotic medications are also frequently used in the hospital, the scope and variation in use have not been adequately investigated. Given the lack of oversight for medication prescribing in the hospital setting and the frequency of delirium, occurring in 15% to 26% of hospitalized older adults,[2, 3, 4] off‐label use of antipsychotic medications and variation in use could be substantial.
Because variation in practice is known to increase in the setting of controversy or lack of clarity regarding appropriate management,[5] large degrees of variation can draw attention to priority areas for clinical effectiveness studies, and the need for guidelines, clinical decision support, or regulatory oversight. In the absence of clear guidelines for the use of antipsychotic medication in nonpsychiatric hospitalized patients, we hypothesized that significant variation in use would persist after controlling for patient characteristics. Using a large, nationally representative cohort of admissions to 300 hospitals from July 2009 to June 2010, we sought to investigate prescribing patterns and hospital variation in use of antipsychotic medications in nonpsychiatric admissions to US hospitals.
METHODS
Setting and Data Collection
We conducted a retrospective cohort study using data from 300 US, nonfederal, acute care facilities contributing to the database maintained by Premier (Premier Healthcare Solutions, Inc., Charlotte, NC). This nationally representative database, created to measure healthcare utilization and quality of care, is drawn from voluntarily participating hospitals and contains data on approximately 1 in every 4 discharges nationwide.[6] Participating hospitals are similar in geographic distribution and urban/rural status to hospitals nationwide, although large, nonteaching hospitals are slightly over‐represented. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center.
Inclusion and Exclusion Criteria
We studied a cohort of all adult (18 years) nonpsychiatric admissions to participating hospitals from July 1, 2009 through June 30, 2010. We excluded patients admitted to a psychiatry service or with any discharge diagnosis of a psychotic disorder (defined by the Elixhauser comorbidity Psychoses: 295.00‐298.9, 299.10‐299.11), because we were interested in use of antipsychotics for conditions other than primary psychiatric disorders. We also excluded patients with a charge for labor and delivery owing to the nonrepresentativeness of this patient population for the general hospitalized patient. We excluded admissions with unknown gender, and admissions with a length of stay greater than 365 days, as these admissions are not representative of the typical admission to an acute care hospital. We also excluded hospitals contributing less than 100 admissions owing to lack of precision in corresponding hospital prescribing rates.
Antipsychotic Medication Utilization
In‐hospital antipsychotic use was ascertained from pharmacy charges, reflecting each medication dispensed during the hospitalization. We categorized antipsychotic medications as typical (haloperidol, loxapine, thioridazine, molindone, thiothixine, pimozide, fluphenazine, trifluoperazine, chlorpromazine, and perphenazine) and atypical (aripiprazole, asenapine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone) based on classification by the Food and Drug Administration.[7, 8] We excluded prochlorperazine (Compazine) from our typical antipsychotic definition, as this medication is almost exclusively used as an antiemetic.
In the absence of guidelines for use of antipsychotic agents in hospitalized patients, we used the Centers for Medicare and Medicaid Services (CMS) guidelines for long‐term care facilities to define measures of potentially excessive dosing in the hospital setting.[9] These guidelines define the daily dosage levels of antipsychotics above which the medical necessity of the higher dose should be explained in the medical record. We defined any daily dosage above these specified levels as a potentially excessive daily dose.
Characteristics Associated With Use
We investigated the association between antipsychotic use and patient and hospital characteristics, selected based on clinical grounds. Patient characteristics included: (1) demographic variables such as age (<65, 6574, 75+ years), gender, self‐reported race, marital status, and primary insurance; (2) admission characteristic variables, including admitting department (surgical vs nonsurgical, defined by a surgical attending of record and presence of operating room charges), whether the patient spent any time in the intensive care unit (ICU), and whether they received mechanical ventilation; and (3) potential indications for use, including delirium (included delirium superimposed upon dementia), dementia (without delirium), and insomnia (see Supporting Information, Appendix, in the online version of this article for International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] codes). Hospital characteristics included number of beds, urban versus rural status, teaching status, and US Census region.
Statistical Analysis
We report the proportion of admissions with in‐hospital use of any antipsychotic, and the number of days of exposure, overall and stratified by typical and atypical.
We determined potentially excessive dosing by taking the sum of the doses for a specific antipsychotic charged on a given day and comparing it to the CMS guidelines for long‐term care settings described above. We report the percentage of exposed admissions with at least 1 day of potentially excessive dosing.
All multivariable models below were operationalized as generalized estimating equations with a Poisson error term, log link, robust variance estimator,[10] and an exchangeable correlation structure to account for repeated admissions of the same patient.
We investigated patient and hospital characteristics associated with use of any antipsychotic medication using a multivariable model that simultaneously included all patient and hospital characteristics in Table 1 as independent variables.
| % of Cohort | Any Exposure, % | Typical Exposure, % | Atypical Exposure, % | |
|---|---|---|---|---|
| ||||
| Patient characteristics | ||||
| Age group, y | ||||
| <65 | 52.1 | 4.6 | 2.0 | 3.1 |
| 6574 | 18.5 | 5.2 | 2.7 | 3.1 |
| 75+ | 29.4 | 8.8 | 4.6 | 5.4 |
| Gender | ||||
| Male | 43.8 | 6.6 | 3.6 | 3.8 |
| Female | 56.2 | 5.5 | 2.3 | 3.8 |
| Race | ||||
| White | 64.6 | 6.1 | 2.9 | 4.0 |
| Black | 13.5 | 5.5 | 2.8 | 3.3 |
| Hispanic | 5.0 | 4.9 | 2.2 | 3.2 |
| Other | 19.9 | 6.1 | 3.1 | 3.7 |
| Marital Status | ||||
| Married | 42.5 | 4.6 | 2.4 | 2.7 |
| Single | 46.7 | 7.2 | 3.2 | 4.7 |
| Unknown/other | 10.8 | 6.4 | 3.1 | 4.1 |
| Primary insurance | ||||
| Private (commercial) | 28.8 | 3.0 | 1.5 | 1.8 |
| Medicaid | 10.3 | 6.4 | 2.4 | 4.6 |
| Medicare managed | 10.6 | 7.1 | 4.1 | 4.0 |
| Medicare traditional | 40.9 | 8.0 | 3.7 | 5.3 |
| Self‐pay or other | 9.4 | 4.3 | 2.5 | 2.2 |
| Admitting department | ||||
| Surgical | 60.6 | 5.8 | 3.1 | 3.4 |
| Nonsurgical | 39.4 | 6.2 | 2.4 | 4.4 |
| Any ICU stay | 16.6 | 10.4 | 7.2 | 4.9 |
| Mechanical ventilation | 4.7 | 17.4 | 12.9 | 7.9 |
| Diagnoses | ||||
| Delirium | 3.2 | 28.6 | 19.4 | 15.7 |
| Dementia | 3.1 | 27.4 | 12.0 | 20.2 |
| Insomnia | 1.3 | 10.2 | 3.9 | 7.5 |
| Discharge disposition | ||||
| Home | 77.9 | 3.8 | 1.6 | 2.5 |
| SNF/Rehab | 15.5 | 13.7 | 6.8 | 9.0 |
| Hospice | 1.7 | 16.0 | 10.3 | 8.1 |
| Other | 4.9 | 11.6 | 7.6 | 5.7 |
| Hospital characteristics, % | ||||
| No. of beds | ||||
| 200 | 14.1 | 6.1 | 2.8 | 3.8 |
| 201300 | 18.6 | 6.1 | 2.9 | 3.9 |
| 301500 | 37.7 | 5.9 | 2.9 | 3.7 |
| 500+ | 29.7 | 5.9 | 2.8 | 3.8 |
| Population served | ||||
| Urban | 89.4 | 6.0 | 2.9 | 3.8 |
| Rural | 10.6 | 5.8 | 2.4 | 3.9 |
| Teaching status | ||||
| Teaching | 39.2 | 5.8 | 2.9 | 3.7 |
| Nonteaching | 60.8 | 6.0 | 2.8 | 3.9 |
| US Census region | ||||
| West | 16.9 | 5.9 | 3.2 | 3.5 |
| Northeast | 20.1 | 6.1 | 2.9 | 3.9 |
| Midwest | 21.9 | 5.7 | 2.5 | 3.8 |
| South | 41.0 | 6.1 | 2.9 | 3.9 |
To determine hospital variation in antipsychotic use, we first determined the proportion of admissions at each hospital with at least 1 charge for antipsychotic medication. We then divided hospitals into quintiles based on their facility‐level antipsychotic prescribing rates and assigned all admissions to their corresponding hospital quintile. We then used a multivariable model to measure the adjusted association between prescribing quintile and patient‐level receipt of antipsychotic medication, controlling for all patient characteristics listed in Table 1 (except discharge disposition), and comorbidities using the Healthcare Cost and Utilization Project Comorbidity Software version 3.7 (Agency for Healthcare Research and Quality, Rockville, MD).[11] We used the lowest prescribing quintile as the reference group. We also report in the Supporting Information, Appendix, in the online version of this article, the distribution of prescribing rates for the hospitals in our cohort before and after adjustment for patient characteristics. For both approaches, we conducted stratified analyses in admissions with delirium and dementia.
All analyses were carried out using SAS software (SAS Institute Inc., Cary, NC).
RESULTS
Admission Characteristics
There were 3,190,934 admissions aged 18 years and over to 300 hospitals from July 1, 2009 to June 30, 2010. After excluding admissions with unknown gender (n = 17), length of stay greater than 365 days (n = 25), charges for labor and delivery (n = 323,111) or a psychiatric attending of record or psychiatric comorbidity (n = 172,669), and admissions to hospitals with fewer than 100 admissions (n = 31), our cohort included 2,695,081 admissions. The median age was 63 years (25th, 75th percentile 48, 77 years), and 1,514,986 (56%) were women. Table 1 shows the overall admission characteristics of the cohort and the percent exposed to antipsychotics among each patient and hospital characteristic.
Antipsychotic Use
There were 160,773 (6%) admissions with antipsychotic exposure. Among exposed admissions, 102,148 (64%) received atypical and 76,979 (48%) received typical antipsychotics, with 18,354 (11%) exposed to both. The median (25th, 75th percentile) length of stay among exposed was 5 days (3, 9 days), and the median (25th, 75th percentile) number of days of exposure was 3 (1, 5 days) overall, 3 days (2, 6 days) for atypical and 2 days (1, 3 days) for typical exposure.
Among admissions aged 65 to 74 years, 25,855 (5%) were exposed. Among admissions aged 75 years or older, 69,792 (9%) were exposed. Among admissions with delirium, exposure occurred in 24,787 (29%), with 13,640 (55%) receiving atypical, 16,828 (68%) receiving typical, and 5681 (23%) exposed to both. Among admissions with dementia, exposure occurred in 23,179 (27%), with 17,068 (74%) receiving atypical, 10,108 (44%) receiving typical, and 3997 (17%) exposed to both.
Use of Specific Drugs and Potentially Excessive Dosing
Table 2 demonstrates the most commonly used antipsychotic medications and the rates of potentially excessive dosing. Quetiapine and olanzapine were the most commonly used atypical antipsychotics, and haloperidol represented the majority of typical antipsychotic use. Among admissions with antipsychotic exposure, 47% received at least 1 potentially excessive daily dose, 18% of those with atypical exposure and 79% of those with typical exposure. Among admissions aged 65 years and up (n = 1,291,375), the prevalence of potentially excessive dosing was almost identical; 46% received at least 1 daily dose in excess of the recommended daily dose, 11% of those with atypical exposure and 79% of those with typical exposure.
| Agent |
Overall Prevalence,N = 2,695,081 |
% of Exposed With Potentially Excessive Dosing* | ||
|---|---|---|---|---|
| Within 100% of Recommended DD* | 101% to 150% of Recommended DD* | >150% of Recommended DD* | ||
| ||||
| Any antipsychotic | 6.0 | 52.9 | 20.2 | 26.9 |
| Atypical | 3.8 | 82.0 | 5.4 | 12.6 |
| Quetiapine (200) | 1.8 | 81.7 | 5.7 | 12.6 |
| Olanzapine (10) | 0.6 | 73.7 | 7.3 | 19.0 |
| Risperidone (2) | 0.9 | 79.2 | 6.8 | 14.0 |
| Other | 0.7 | 98.3 | 0.1 | 1.6 |
| Typical | 2.9 | 21.1 | 37.0 | 41.9 |
| Haloperidol (4) | 2.5 | 13.2 | 41.3 | 45.5 |
| Chlorpromazine (75) | 0.3 | 76.0 | 9.8 | 14.2 |
| Other | 0.4 | 89.1 | 2.9 | 8.0 |
Characteristics Associated With Antipsychotic Use
Among the patient and hospital characteristics included in our analysis, the 5 characteristics most strongly associated with antipsychotic exposure after adjustment were (Table 3): delirium (relative risk [RR]: 2.93, 95% confidence interval [CI]: 2.88‐2.98); dementia (RR: 2.78, 95% CI: 2.72‐2.83); insurance status, with higher risk among patients with traditional Medicare (RR: 2.09, 95% CI: 2.04‐2.13), Medicare managed (RR: 1.98, 95% CI: 1.93‐2.03), Medicaid (RR: 1.84, 95% CI: 1.80‐1.88), and self‐pay/other (RR: 1.26, 95% CI: 1.23‐1.29) compared to private (commercial) insurance; use of mechanical ventilation (RR: 1.84, 95% CI: 1.81‐1.87); and any ICU stay (RR: 1.53, 95% CI: 1.51‐1.55).
| Unadjusted RR of Receiving Any Antipsychotic [95% CI] | Adjusted RR of Receiving Any Antipsychotic [95% CI]* | |
|---|---|---|
| ||
| Age group, y, % | ||
| <65 | Reference | Reference |
| 6574 | 1.12 [1.10,1.14] | 0.74 [0.72, 0.75] |
| 75+ | 1.90 [1.88,1.92] | 1.03 [1.01, 1.05] |
| Gender | ||
| Female | Reference | Reference |
| Male | 1.19 [1.18,1.20] | 1.27 [1.26, 1.28] |
| Race | ||
| White | Reference | Reference |
| Black | 0.91 [0.90,0.92] | 0.85 [0.83, 0.86] |
| Hispanic | 0.80 [0.78,0.82] | 0.79 [0.76, 0.81] |
| Other | 0.99 [0.98,1.00] | 0.96 [0.95, 0.98] |
| Marital status | ||
| Married | Reference | Reference |
| Single | 1.57 [1.55,1.59] | 1.43 [1.42, 1.45] |
| Unknown/other | 1.41 [1.39,1.43] | 1.27 [1.24, 1.29] |
| Primary insurance | ||
| Private (commercial) | Reference | Reference |
| Medicaid | 2.13 [2.09,2.17] | 1.84 [1.80, 1.88] |
| Medicare managed | 2.35 [2.31,2.39] | 1.98 [1.93, 2.03] |
| Medicare traditional | 2.65 [2.61,2.69] | 2.09 [2.04, 2.13] |
| Self‐pay or other | 1.41 [1.38,1.44] | 1.26 [1.23, 1.29] |
| Admitting department | ||
| Surgical | Reference | Reference |
| Nonsurgical | 1.06 [1.05,1.07] | 1.05 [1.03, 1.06] |
| Any ICU stay | 2.05 [2.03,2.07] | 1.53 [1.51, 1.55] |
| Mechanical ventilation | 3.22 [3.18,3.26] | 1.84 [1.81, 1.87] |
| Diagnoses | ||
| Delirium | 5.48 [5.42, 5.45] | 2.93 [2.88, 2.98] |
| Dementia | 5.21 [5.15,5.27] | 2.78 [2.72, 2.83] |
| Insomnia | 1.72 [1.67,1.78] | 1.51 [1.45, 1.57] |
| No. of beds | ||
| 200 | Reference | Reference |
| 201300 | 1.01 [0.99,1.03] | 0.96 [0.94, 0.98] |
| 301500 | 0.98 [0.97,1.00] | 0.93 [0.91, 0.95] |
| 500+ | 0.97 [0.96,0.98] | 0.91 [0.90, 0.93] |
| Population served | ||
| Urban | Reference | Reference |
| Rural | 0.96 [0.95,0.98] | 0.91 [0.89, 0.93] |
| Teaching status | ||
| Teaching | Reference | Reference |
| Nonteaching | 1.03 [1.02,1.04] | 0.98 [0.97, 1.00] |
| US Census region | ||
| West | Reference | Reference |
| Northeast | 1.03 [1.01,1.05] | 1.04 [1.02, 1.06] |
| Midwest | 0.95 [0.94,0.97] | 0.93 [0.91, 0.94] |
| South | 1.02 [1.01,1.03] | 1.07 [1.05, 1.09] |
Hospital Variation in Antipsychotic Use
Figure 1 demonstrates the antipsychotic prescribing rate at each hospital in our cohort, and the corresponding quintiles. Patients admitted to hospitals in the highest prescribing quintile were more than twice as likely to be exposed to antipsychotics compared to patients admitted to hospitals in the lowest prescribing quintile, even after adjustment for patient characteristics and comorbidities (Table 4). This relationship was similar across subgroups of admissions with delirium and dementia (see Supporting Information, Appendix, in the online version of this article for the distribution of hospital antipsychotic prescribing rates before and after adjustment for patient characteristics).
| Admissions, No. (% of Total) | Unadjusted RR of Exposure [95% CI] | Adjusted RR of exposure [95% CI]* | |
|---|---|---|---|
| |||
| Overall | |||
| Q1 | 431,017 (16%) | Reference | Reference |
| Q2 | 630,486 (23%) | 1.67 [1.63, 1.71] | 1.59 [1.55, 1.62] |
| Q3 | 548,337 (20%) | 1.93 [1.88, 1.97] | 1.84 [1.80, 1.88] |
| Q4 | 639,027 (24%) | 2.16 [2.12, 2.21] | 2.07 [2.03, 2.12] |
| Q5 | 446,214 (17%) | 2.83 [2.77, 2.89] | 2.56 [2.50, 2.61] |
| Delirium | |||
| Q1 | 12,878 (15%) | Reference | Reference |
| Q2 | 20,588 (24%) | 1.58 [1.51, 1.65] | 1.58 [1.51, 1.65] |
| Q3 | 17,402 (20%) | 1.71 [1.64, 1.80] | 1.73 [1.65, 1.82] |
| Q4 | 20,943 (24%) | 2.01 [1.92, 2.10] | 1.99 [1.91, 2.08] |
| Q5 | 14,883 (17%) | 2.15 [2.05, 2.25] | 2.16 [2.07, 2.26] |
| Dementia | |||
| Q1 | 28,290 (15%) | Reference | Reference |
| Q2 | 42,018 (22%) | 1.43 [1.36, 1.50] | 1.40 [1.34, 1.47] |
| Q3 | 38,593 (21%) | 1.61 [1.53, 1.69] | 1.59 [1.51, 1.66] |
| Q4 | 44,638 (24%) | 1.69 [1.62, 1.77] | 1.69 [1.61, 1.77] |
| Q5 | 34,442 (18%) | 1.92 [1.83, 2.01] | 1.90 [1.81, 1.99] |

DISCUSSION
In this cohort of nonpsychiatric admissions to 300 US hospitals, antipsychotic medications were used in 6% of admissions, with atypical antipsychotics representing the majority of use. Potentially excessive daily doses based on CMS recommendations for long‐term care facilities occurred in almost half of admissions with any antipsychotic exposure, and in 87% of admissions with haloperidol exposure specifically. We found variation in hospital use of antipsychotics that was not fully accounted for by measured patient characteristics, and which persisted among subgroups of admissions with delirium and/or dementia. Although unmeasured patient characteristics or different billing practices between hospitals are potential explanations, our findings also raise the possibility of different hospital antipsychotic prescribing cultures. These findings provide new information regarding the scope of prescribing in US hospitals, and draw attention to the need for additional studies to better define what constitutes appropriate use of antipsychotics in the hospital setting.
A recent single‐center study at a large academic medical center found an overall antipsychotic exposure rate of 9% of nonpsychiatric admissions.[12] Our finding that 6% of admissions in this multicenter cohort were exposed to antipsychotics is slightly lower, but similar to the previous estimate. Assuming 37 million discharges from US hospitals each year,[13] our study suggests that more than 2 million hospitalized patients receive antipsychotics annually. With around 1.4 million residents in nursing homes on any given day,[14] and an exposure rate of 25% to 30% in that setting,[15, 16, 17] our study suggests that the number of patients exposed in the hospital setting is greater than the number exposed in the nursing home setting, the site of care for which prescribing regulations have been focused thus far.
Because our dataset does not contain preadmission medications, we were unable to specifically investigate new initiation. In the prior single‐center study, approximately 55% of overall use in the hospital setting was new initiation,[12] which would suggest that antipsychotics are newly initiated in around 1 million admissions each year in the hospital. Although we are unable to determine reason for use in our analysis, delirium was a strong predictor of antipsychotic use in our multivariable model, and prior studies have demonstrated delirium to be the most common reason for antipsychotic initiation in hospitalized patients,[12, 18] an indication for which efficacy/effectiveness data are lacking. A recent systematic review of antipsychotics for the treatment of delirium in older adults concluded that because of severe methodological limitations, the small number of existing studies on this topic do not support the use of antipsychotics in the treatment of delirium in older hospitalized adults.[19] Our results further highlight the need for randomized placebo‐controlled trials of antipsychotics in treatment of delirium.
We found variation in antipsychotic use between hospitals that was not fully explained by patient characteristics. Insufficient data to inform clinical decisions surrounding management of agitated delirium/dementia and lack of clear criteria by which to judge appropriateness of antipsychotic use may contribute to this variation. Some variation may relate to resource allocation at different hospitals, and the feasibility of implementing nonpharmacologic management options across settings. Our results collectively highlight the need for studies evaluating the efficacy/effectiveness of antipsychotics in the treatment of delirium and drivers of physician decision‐making in this realm, as well as the need for greater hospital investment in nonpharmacologic delirium‐prevention programs, which have been shown to be effective in prevention of delirium in hospitalized patients.[20]
We observed high levels of potentially excessive daily dosing using cutoffs applied in the long‐term care setting. The majority of the potentially excessive doses were in the setting of typical antipsychotic use, and haloperidol specifically, where doses exceeded 4 mg on at least 1 day in 87% of exposed admissions. Of note, the threshold for haloperidol dosage above which justification is required was decreased from 4 to 2 mg per day in the 2015 update to the CMS guidelines.[21] For the present analysis, we used the guidelines that were contemporaneous to our cohort; we are unable to determine current rates of potentially inappropriate dosages in the present analysis, but given the high prevalence in 2009 to 2010, and the lowering of the dosage threshold since then, it is unlikely that any decrease in use would be enough to substantially reduce the estimate. Whether these high dosages are actually inappropriate in the hospital setting is not established, and we were not able to review medical records to determine whether justification for use of such doses was documented.[22, 23] It is possible that hospitalized patients with altered pharmacodynamics and greater severity of illness could require larger doses of these medications; however, this is an area in need of further investigation, and current critical care guidelines note the lack of sufficient data upon which to justify use of haloperidol in the prevention or treatment of delirium in ICU patients.[24, 25]
The dosages in use are concerning given that the risk of extrapyramidal side effects increases with increasing dose, and prior studies have demonstrated an association between increased dose of antipsychotics and increased risk of other adverse events, including hip fracture and sudden cardiac death.[22, 23] Further, despite these known risks, studies have demonstrated failure to follow recommendations to mitigate risk,[26] such as electrocardiogram monitoring in individuals receiving intravenous haloperidol.[27] Our results suggest that physicians are similarly not following recommendations to use lower doses of haloperidol when treating older patients, given the almost identical incidence of potentially excessive dosing among admissions of patients aged 65 years and older in our cohort.[25] Clinical decision support prompts have been effective at increasing appropriate use of antipsychotic medications in several single‐center analyses,[28, 29, 30] and widespread implementation of such support with a focus on haloperidol dosing should be considered on the basis of our results.
The patient characteristics associated with antipsychotic use in this large, nationally representative analysis are consistent with those identified in prior single‐center analyses.[12, 18] Both prior analyses identified delirium as the most common reason for antipsychotic use, and dementia, intensive care unit stay, and mechanical ventilation were also previously identified as strong predictors of use that we believe hold face validity for the practicing hospitalist. On the other hand, some of the factors associated with antipsychotic use in our model cannot be readily explained, such as insurance status and race, and may be serving as proxies for other variables not included in our analysis. That nonwhite patients are less likely than white patients to receive antipsychotic medications in the hospital has been previously demonstrated,[12] and further investigation to understand this disparity is warranted.
Our study has several additional limitations. First, because our study is observational, the possibility of residual confounding exists, and we cannot rule out that there are other patient factors driving the hospital variation in antipsychotic use that we observed. Second, because guidelines do not exist for antipsychotic dosing in hospitalized patients, we could only comment on potentially excessive dosing, extrapolating from guidelines in the long‐term care setting. Whether such doses are actually excessive in hospitalized patients is not defined. Third, although Premier performs quality checks on charge and ICD‐9‐CM coding data submitted by participating hospitals, the validity of administrative data is uncertain. For example, the use of administrative data to identify delirium diagnoses is likely to have resulted in underestimation of delirium incidence among our different exposure groups. Delirium is likely to be coded more often in the setting of more severe or hyperactive cases, when antipsychotics are more likely to be utilized. This could result in an overestimation of the association between delirium and antipsychotic use. Additionally, differences in coding practices between hospitals for any of the variables in our models could explain some of the variation in antipsychotic prescribing that we observed. Finally, because we were unable to differentiate between new initiation and continuation of a preadmission antipsychotic, some of the variation that we observed is likely to reflect differences in outpatient antipsychotic prescribing practices.
In conclusion, in this large cohort of nonpsychiatric admissions to 300 US hospitals, we found that antipsychotic medication exposure was common, often at high daily doses. Delirium and dementia were the strongest predictors of use among the patient and hospital characteristics examined. The variation in antipsychotic prescribing that we observed was not fully accounted for by measured patient characteristics, and raises the possibility of differing hospital prescribing cultures. Our results draw attention to the need for additional research to better define what constitutes appropriate use of these potentially harmful medications in the hospital setting.
Disclosures: Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Herzig, Rothberg, Gurwitz, and Marcantonio. Acquisition of data: Dr. Herzig. Analysis of data: Mr. Guess. Interpretation of data: Drs. Herzig, Rothberg, Gurwitz, Marcantonio, and Mr. Guess. Drafting of the manuscript: Dr. Herzig. Critical revision of the manuscript for important intellectual content: Drs. Rothberg, Gurwitz, Marcantonio, and Mr. Guess. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant number K24AG035075 from the National Institute on Aging. The funding organizations had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
Antipsychotic medications are frequently used off label for management of behavioral symptoms associated with delirium and/or dementia. Despite regulations designed to curb inappropriate prescribing of these medications in nursing homes, substantial levels of use and variation in use have been observed in this setting.[1] Although antipsychotic medications are also frequently used in the hospital, the scope and variation in use have not been adequately investigated. Given the lack of oversight for medication prescribing in the hospital setting and the frequency of delirium, occurring in 15% to 26% of hospitalized older adults,[2, 3, 4] off‐label use of antipsychotic medications and variation in use could be substantial.
Because variation in practice is known to increase in the setting of controversy or lack of clarity regarding appropriate management,[5] large degrees of variation can draw attention to priority areas for clinical effectiveness studies, and the need for guidelines, clinical decision support, or regulatory oversight. In the absence of clear guidelines for the use of antipsychotic medication in nonpsychiatric hospitalized patients, we hypothesized that significant variation in use would persist after controlling for patient characteristics. Using a large, nationally representative cohort of admissions to 300 hospitals from July 2009 to June 2010, we sought to investigate prescribing patterns and hospital variation in use of antipsychotic medications in nonpsychiatric admissions to US hospitals.
METHODS
Setting and Data Collection
We conducted a retrospective cohort study using data from 300 US, nonfederal, acute care facilities contributing to the database maintained by Premier (Premier Healthcare Solutions, Inc., Charlotte, NC). This nationally representative database, created to measure healthcare utilization and quality of care, is drawn from voluntarily participating hospitals and contains data on approximately 1 in every 4 discharges nationwide.[6] Participating hospitals are similar in geographic distribution and urban/rural status to hospitals nationwide, although large, nonteaching hospitals are slightly over‐represented. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center.
Inclusion and Exclusion Criteria
We studied a cohort of all adult (18 years) nonpsychiatric admissions to participating hospitals from July 1, 2009 through June 30, 2010. We excluded patients admitted to a psychiatry service or with any discharge diagnosis of a psychotic disorder (defined by the Elixhauser comorbidity Psychoses: 295.00‐298.9, 299.10‐299.11), because we were interested in use of antipsychotics for conditions other than primary psychiatric disorders. We also excluded patients with a charge for labor and delivery owing to the nonrepresentativeness of this patient population for the general hospitalized patient. We excluded admissions with unknown gender, and admissions with a length of stay greater than 365 days, as these admissions are not representative of the typical admission to an acute care hospital. We also excluded hospitals contributing less than 100 admissions owing to lack of precision in corresponding hospital prescribing rates.
Antipsychotic Medication Utilization
In‐hospital antipsychotic use was ascertained from pharmacy charges, reflecting each medication dispensed during the hospitalization. We categorized antipsychotic medications as typical (haloperidol, loxapine, thioridazine, molindone, thiothixine, pimozide, fluphenazine, trifluoperazine, chlorpromazine, and perphenazine) and atypical (aripiprazole, asenapine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone) based on classification by the Food and Drug Administration.[7, 8] We excluded prochlorperazine (Compazine) from our typical antipsychotic definition, as this medication is almost exclusively used as an antiemetic.
In the absence of guidelines for use of antipsychotic agents in hospitalized patients, we used the Centers for Medicare and Medicaid Services (CMS) guidelines for long‐term care facilities to define measures of potentially excessive dosing in the hospital setting.[9] These guidelines define the daily dosage levels of antipsychotics above which the medical necessity of the higher dose should be explained in the medical record. We defined any daily dosage above these specified levels as a potentially excessive daily dose.
Characteristics Associated With Use
We investigated the association between antipsychotic use and patient and hospital characteristics, selected based on clinical grounds. Patient characteristics included: (1) demographic variables such as age (<65, 6574, 75+ years), gender, self‐reported race, marital status, and primary insurance; (2) admission characteristic variables, including admitting department (surgical vs nonsurgical, defined by a surgical attending of record and presence of operating room charges), whether the patient spent any time in the intensive care unit (ICU), and whether they received mechanical ventilation; and (3) potential indications for use, including delirium (included delirium superimposed upon dementia), dementia (without delirium), and insomnia (see Supporting Information, Appendix, in the online version of this article for International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] codes). Hospital characteristics included number of beds, urban versus rural status, teaching status, and US Census region.
Statistical Analysis
We report the proportion of admissions with in‐hospital use of any antipsychotic, and the number of days of exposure, overall and stratified by typical and atypical.
We determined potentially excessive dosing by taking the sum of the doses for a specific antipsychotic charged on a given day and comparing it to the CMS guidelines for long‐term care settings described above. We report the percentage of exposed admissions with at least 1 day of potentially excessive dosing.
All multivariable models below were operationalized as generalized estimating equations with a Poisson error term, log link, robust variance estimator,[10] and an exchangeable correlation structure to account for repeated admissions of the same patient.
We investigated patient and hospital characteristics associated with use of any antipsychotic medication using a multivariable model that simultaneously included all patient and hospital characteristics in Table 1 as independent variables.
| % of Cohort | Any Exposure, % | Typical Exposure, % | Atypical Exposure, % | |
|---|---|---|---|---|
| ||||
| Patient characteristics | ||||
| Age group, y | ||||
| <65 | 52.1 | 4.6 | 2.0 | 3.1 |
| 6574 | 18.5 | 5.2 | 2.7 | 3.1 |
| 75+ | 29.4 | 8.8 | 4.6 | 5.4 |
| Gender | ||||
| Male | 43.8 | 6.6 | 3.6 | 3.8 |
| Female | 56.2 | 5.5 | 2.3 | 3.8 |
| Race | ||||
| White | 64.6 | 6.1 | 2.9 | 4.0 |
| Black | 13.5 | 5.5 | 2.8 | 3.3 |
| Hispanic | 5.0 | 4.9 | 2.2 | 3.2 |
| Other | 19.9 | 6.1 | 3.1 | 3.7 |
| Marital Status | ||||
| Married | 42.5 | 4.6 | 2.4 | 2.7 |
| Single | 46.7 | 7.2 | 3.2 | 4.7 |
| Unknown/other | 10.8 | 6.4 | 3.1 | 4.1 |
| Primary insurance | ||||
| Private (commercial) | 28.8 | 3.0 | 1.5 | 1.8 |
| Medicaid | 10.3 | 6.4 | 2.4 | 4.6 |
| Medicare managed | 10.6 | 7.1 | 4.1 | 4.0 |
| Medicare traditional | 40.9 | 8.0 | 3.7 | 5.3 |
| Self‐pay or other | 9.4 | 4.3 | 2.5 | 2.2 |
| Admitting department | ||||
| Surgical | 60.6 | 5.8 | 3.1 | 3.4 |
| Nonsurgical | 39.4 | 6.2 | 2.4 | 4.4 |
| Any ICU stay | 16.6 | 10.4 | 7.2 | 4.9 |
| Mechanical ventilation | 4.7 | 17.4 | 12.9 | 7.9 |
| Diagnoses | ||||
| Delirium | 3.2 | 28.6 | 19.4 | 15.7 |
| Dementia | 3.1 | 27.4 | 12.0 | 20.2 |
| Insomnia | 1.3 | 10.2 | 3.9 | 7.5 |
| Discharge disposition | ||||
| Home | 77.9 | 3.8 | 1.6 | 2.5 |
| SNF/Rehab | 15.5 | 13.7 | 6.8 | 9.0 |
| Hospice | 1.7 | 16.0 | 10.3 | 8.1 |
| Other | 4.9 | 11.6 | 7.6 | 5.7 |
| Hospital characteristics, % | ||||
| No. of beds | ||||
| 200 | 14.1 | 6.1 | 2.8 | 3.8 |
| 201300 | 18.6 | 6.1 | 2.9 | 3.9 |
| 301500 | 37.7 | 5.9 | 2.9 | 3.7 |
| 500+ | 29.7 | 5.9 | 2.8 | 3.8 |
| Population served | ||||
| Urban | 89.4 | 6.0 | 2.9 | 3.8 |
| Rural | 10.6 | 5.8 | 2.4 | 3.9 |
| Teaching status | ||||
| Teaching | 39.2 | 5.8 | 2.9 | 3.7 |
| Nonteaching | 60.8 | 6.0 | 2.8 | 3.9 |
| US Census region | ||||
| West | 16.9 | 5.9 | 3.2 | 3.5 |
| Northeast | 20.1 | 6.1 | 2.9 | 3.9 |
| Midwest | 21.9 | 5.7 | 2.5 | 3.8 |
| South | 41.0 | 6.1 | 2.9 | 3.9 |
To determine hospital variation in antipsychotic use, we first determined the proportion of admissions at each hospital with at least 1 charge for antipsychotic medication. We then divided hospitals into quintiles based on their facility‐level antipsychotic prescribing rates and assigned all admissions to their corresponding hospital quintile. We then used a multivariable model to measure the adjusted association between prescribing quintile and patient‐level receipt of antipsychotic medication, controlling for all patient characteristics listed in Table 1 (except discharge disposition), and comorbidities using the Healthcare Cost and Utilization Project Comorbidity Software version 3.7 (Agency for Healthcare Research and Quality, Rockville, MD).[11] We used the lowest prescribing quintile as the reference group. We also report in the Supporting Information, Appendix, in the online version of this article, the distribution of prescribing rates for the hospitals in our cohort before and after adjustment for patient characteristics. For both approaches, we conducted stratified analyses in admissions with delirium and dementia.
All analyses were carried out using SAS software (SAS Institute Inc., Cary, NC).
RESULTS
Admission Characteristics
There were 3,190,934 admissions aged 18 years and over to 300 hospitals from July 1, 2009 to June 30, 2010. After excluding admissions with unknown gender (n = 17), length of stay greater than 365 days (n = 25), charges for labor and delivery (n = 323,111) or a psychiatric attending of record or psychiatric comorbidity (n = 172,669), and admissions to hospitals with fewer than 100 admissions (n = 31), our cohort included 2,695,081 admissions. The median age was 63 years (25th, 75th percentile 48, 77 years), and 1,514,986 (56%) were women. Table 1 shows the overall admission characteristics of the cohort and the percent exposed to antipsychotics among each patient and hospital characteristic.
Antipsychotic Use
There were 160,773 (6%) admissions with antipsychotic exposure. Among exposed admissions, 102,148 (64%) received atypical and 76,979 (48%) received typical antipsychotics, with 18,354 (11%) exposed to both. The median (25th, 75th percentile) length of stay among exposed was 5 days (3, 9 days), and the median (25th, 75th percentile) number of days of exposure was 3 (1, 5 days) overall, 3 days (2, 6 days) for atypical and 2 days (1, 3 days) for typical exposure.
Among admissions aged 65 to 74 years, 25,855 (5%) were exposed. Among admissions aged 75 years or older, 69,792 (9%) were exposed. Among admissions with delirium, exposure occurred in 24,787 (29%), with 13,640 (55%) receiving atypical, 16,828 (68%) receiving typical, and 5681 (23%) exposed to both. Among admissions with dementia, exposure occurred in 23,179 (27%), with 17,068 (74%) receiving atypical, 10,108 (44%) receiving typical, and 3997 (17%) exposed to both.
Use of Specific Drugs and Potentially Excessive Dosing
Table 2 demonstrates the most commonly used antipsychotic medications and the rates of potentially excessive dosing. Quetiapine and olanzapine were the most commonly used atypical antipsychotics, and haloperidol represented the majority of typical antipsychotic use. Among admissions with antipsychotic exposure, 47% received at least 1 potentially excessive daily dose, 18% of those with atypical exposure and 79% of those with typical exposure. Among admissions aged 65 years and up (n = 1,291,375), the prevalence of potentially excessive dosing was almost identical; 46% received at least 1 daily dose in excess of the recommended daily dose, 11% of those with atypical exposure and 79% of those with typical exposure.
| Agent |
Overall Prevalence,N = 2,695,081 |
% of Exposed With Potentially Excessive Dosing* | ||
|---|---|---|---|---|
| Within 100% of Recommended DD* | 101% to 150% of Recommended DD* | >150% of Recommended DD* | ||
| ||||
| Any antipsychotic | 6.0 | 52.9 | 20.2 | 26.9 |
| Atypical | 3.8 | 82.0 | 5.4 | 12.6 |
| Quetiapine (200) | 1.8 | 81.7 | 5.7 | 12.6 |
| Olanzapine (10) | 0.6 | 73.7 | 7.3 | 19.0 |
| Risperidone (2) | 0.9 | 79.2 | 6.8 | 14.0 |
| Other | 0.7 | 98.3 | 0.1 | 1.6 |
| Typical | 2.9 | 21.1 | 37.0 | 41.9 |
| Haloperidol (4) | 2.5 | 13.2 | 41.3 | 45.5 |
| Chlorpromazine (75) | 0.3 | 76.0 | 9.8 | 14.2 |
| Other | 0.4 | 89.1 | 2.9 | 8.0 |
Characteristics Associated With Antipsychotic Use
Among the patient and hospital characteristics included in our analysis, the 5 characteristics most strongly associated with antipsychotic exposure after adjustment were (Table 3): delirium (relative risk [RR]: 2.93, 95% confidence interval [CI]: 2.88‐2.98); dementia (RR: 2.78, 95% CI: 2.72‐2.83); insurance status, with higher risk among patients with traditional Medicare (RR: 2.09, 95% CI: 2.04‐2.13), Medicare managed (RR: 1.98, 95% CI: 1.93‐2.03), Medicaid (RR: 1.84, 95% CI: 1.80‐1.88), and self‐pay/other (RR: 1.26, 95% CI: 1.23‐1.29) compared to private (commercial) insurance; use of mechanical ventilation (RR: 1.84, 95% CI: 1.81‐1.87); and any ICU stay (RR: 1.53, 95% CI: 1.51‐1.55).
| Unadjusted RR of Receiving Any Antipsychotic [95% CI] | Adjusted RR of Receiving Any Antipsychotic [95% CI]* | |
|---|---|---|
| ||
| Age group, y, % | ||
| <65 | Reference | Reference |
| 6574 | 1.12 [1.10,1.14] | 0.74 [0.72, 0.75] |
| 75+ | 1.90 [1.88,1.92] | 1.03 [1.01, 1.05] |
| Gender | ||
| Female | Reference | Reference |
| Male | 1.19 [1.18,1.20] | 1.27 [1.26, 1.28] |
| Race | ||
| White | Reference | Reference |
| Black | 0.91 [0.90,0.92] | 0.85 [0.83, 0.86] |
| Hispanic | 0.80 [0.78,0.82] | 0.79 [0.76, 0.81] |
| Other | 0.99 [0.98,1.00] | 0.96 [0.95, 0.98] |
| Marital status | ||
| Married | Reference | Reference |
| Single | 1.57 [1.55,1.59] | 1.43 [1.42, 1.45] |
| Unknown/other | 1.41 [1.39,1.43] | 1.27 [1.24, 1.29] |
| Primary insurance | ||
| Private (commercial) | Reference | Reference |
| Medicaid | 2.13 [2.09,2.17] | 1.84 [1.80, 1.88] |
| Medicare managed | 2.35 [2.31,2.39] | 1.98 [1.93, 2.03] |
| Medicare traditional | 2.65 [2.61,2.69] | 2.09 [2.04, 2.13] |
| Self‐pay or other | 1.41 [1.38,1.44] | 1.26 [1.23, 1.29] |
| Admitting department | ||
| Surgical | Reference | Reference |
| Nonsurgical | 1.06 [1.05,1.07] | 1.05 [1.03, 1.06] |
| Any ICU stay | 2.05 [2.03,2.07] | 1.53 [1.51, 1.55] |
| Mechanical ventilation | 3.22 [3.18,3.26] | 1.84 [1.81, 1.87] |
| Diagnoses | ||
| Delirium | 5.48 [5.42, 5.45] | 2.93 [2.88, 2.98] |
| Dementia | 5.21 [5.15,5.27] | 2.78 [2.72, 2.83] |
| Insomnia | 1.72 [1.67,1.78] | 1.51 [1.45, 1.57] |
| No. of beds | ||
| 200 | Reference | Reference |
| 201300 | 1.01 [0.99,1.03] | 0.96 [0.94, 0.98] |
| 301500 | 0.98 [0.97,1.00] | 0.93 [0.91, 0.95] |
| 500+ | 0.97 [0.96,0.98] | 0.91 [0.90, 0.93] |
| Population served | ||
| Urban | Reference | Reference |
| Rural | 0.96 [0.95,0.98] | 0.91 [0.89, 0.93] |
| Teaching status | ||
| Teaching | Reference | Reference |
| Nonteaching | 1.03 [1.02,1.04] | 0.98 [0.97, 1.00] |
| US Census region | ||
| West | Reference | Reference |
| Northeast | 1.03 [1.01,1.05] | 1.04 [1.02, 1.06] |
| Midwest | 0.95 [0.94,0.97] | 0.93 [0.91, 0.94] |
| South | 1.02 [1.01,1.03] | 1.07 [1.05, 1.09] |
Hospital Variation in Antipsychotic Use
Figure 1 demonstrates the antipsychotic prescribing rate at each hospital in our cohort, and the corresponding quintiles. Patients admitted to hospitals in the highest prescribing quintile were more than twice as likely to be exposed to antipsychotics compared to patients admitted to hospitals in the lowest prescribing quintile, even after adjustment for patient characteristics and comorbidities (Table 4). This relationship was similar across subgroups of admissions with delirium and dementia (see Supporting Information, Appendix, in the online version of this article for the distribution of hospital antipsychotic prescribing rates before and after adjustment for patient characteristics).
| Admissions, No. (% of Total) | Unadjusted RR of Exposure [95% CI] | Adjusted RR of exposure [95% CI]* | |
|---|---|---|---|
| |||
| Overall | |||
| Q1 | 431,017 (16%) | Reference | Reference |
| Q2 | 630,486 (23%) | 1.67 [1.63, 1.71] | 1.59 [1.55, 1.62] |
| Q3 | 548,337 (20%) | 1.93 [1.88, 1.97] | 1.84 [1.80, 1.88] |
| Q4 | 639,027 (24%) | 2.16 [2.12, 2.21] | 2.07 [2.03, 2.12] |
| Q5 | 446,214 (17%) | 2.83 [2.77, 2.89] | 2.56 [2.50, 2.61] |
| Delirium | |||
| Q1 | 12,878 (15%) | Reference | Reference |
| Q2 | 20,588 (24%) | 1.58 [1.51, 1.65] | 1.58 [1.51, 1.65] |
| Q3 | 17,402 (20%) | 1.71 [1.64, 1.80] | 1.73 [1.65, 1.82] |
| Q4 | 20,943 (24%) | 2.01 [1.92, 2.10] | 1.99 [1.91, 2.08] |
| Q5 | 14,883 (17%) | 2.15 [2.05, 2.25] | 2.16 [2.07, 2.26] |
| Dementia | |||
| Q1 | 28,290 (15%) | Reference | Reference |
| Q2 | 42,018 (22%) | 1.43 [1.36, 1.50] | 1.40 [1.34, 1.47] |
| Q3 | 38,593 (21%) | 1.61 [1.53, 1.69] | 1.59 [1.51, 1.66] |
| Q4 | 44,638 (24%) | 1.69 [1.62, 1.77] | 1.69 [1.61, 1.77] |
| Q5 | 34,442 (18%) | 1.92 [1.83, 2.01] | 1.90 [1.81, 1.99] |

DISCUSSION
In this cohort of nonpsychiatric admissions to 300 US hospitals, antipsychotic medications were used in 6% of admissions, with atypical antipsychotics representing the majority of use. Potentially excessive daily doses based on CMS recommendations for long‐term care facilities occurred in almost half of admissions with any antipsychotic exposure, and in 87% of admissions with haloperidol exposure specifically. We found variation in hospital use of antipsychotics that was not fully accounted for by measured patient characteristics, and which persisted among subgroups of admissions with delirium and/or dementia. Although unmeasured patient characteristics or different billing practices between hospitals are potential explanations, our findings also raise the possibility of different hospital antipsychotic prescribing cultures. These findings provide new information regarding the scope of prescribing in US hospitals, and draw attention to the need for additional studies to better define what constitutes appropriate use of antipsychotics in the hospital setting.
A recent single‐center study at a large academic medical center found an overall antipsychotic exposure rate of 9% of nonpsychiatric admissions.[12] Our finding that 6% of admissions in this multicenter cohort were exposed to antipsychotics is slightly lower, but similar to the previous estimate. Assuming 37 million discharges from US hospitals each year,[13] our study suggests that more than 2 million hospitalized patients receive antipsychotics annually. With around 1.4 million residents in nursing homes on any given day,[14] and an exposure rate of 25% to 30% in that setting,[15, 16, 17] our study suggests that the number of patients exposed in the hospital setting is greater than the number exposed in the nursing home setting, the site of care for which prescribing regulations have been focused thus far.
Because our dataset does not contain preadmission medications, we were unable to specifically investigate new initiation. In the prior single‐center study, approximately 55% of overall use in the hospital setting was new initiation,[12] which would suggest that antipsychotics are newly initiated in around 1 million admissions each year in the hospital. Although we are unable to determine reason for use in our analysis, delirium was a strong predictor of antipsychotic use in our multivariable model, and prior studies have demonstrated delirium to be the most common reason for antipsychotic initiation in hospitalized patients,[12, 18] an indication for which efficacy/effectiveness data are lacking. A recent systematic review of antipsychotics for the treatment of delirium in older adults concluded that because of severe methodological limitations, the small number of existing studies on this topic do not support the use of antipsychotics in the treatment of delirium in older hospitalized adults.[19] Our results further highlight the need for randomized placebo‐controlled trials of antipsychotics in treatment of delirium.
We found variation in antipsychotic use between hospitals that was not fully explained by patient characteristics. Insufficient data to inform clinical decisions surrounding management of agitated delirium/dementia and lack of clear criteria by which to judge appropriateness of antipsychotic use may contribute to this variation. Some variation may relate to resource allocation at different hospitals, and the feasibility of implementing nonpharmacologic management options across settings. Our results collectively highlight the need for studies evaluating the efficacy/effectiveness of antipsychotics in the treatment of delirium and drivers of physician decision‐making in this realm, as well as the need for greater hospital investment in nonpharmacologic delirium‐prevention programs, which have been shown to be effective in prevention of delirium in hospitalized patients.[20]
We observed high levels of potentially excessive daily dosing using cutoffs applied in the long‐term care setting. The majority of the potentially excessive doses were in the setting of typical antipsychotic use, and haloperidol specifically, where doses exceeded 4 mg on at least 1 day in 87% of exposed admissions. Of note, the threshold for haloperidol dosage above which justification is required was decreased from 4 to 2 mg per day in the 2015 update to the CMS guidelines.[21] For the present analysis, we used the guidelines that were contemporaneous to our cohort; we are unable to determine current rates of potentially inappropriate dosages in the present analysis, but given the high prevalence in 2009 to 2010, and the lowering of the dosage threshold since then, it is unlikely that any decrease in use would be enough to substantially reduce the estimate. Whether these high dosages are actually inappropriate in the hospital setting is not established, and we were not able to review medical records to determine whether justification for use of such doses was documented.[22, 23] It is possible that hospitalized patients with altered pharmacodynamics and greater severity of illness could require larger doses of these medications; however, this is an area in need of further investigation, and current critical care guidelines note the lack of sufficient data upon which to justify use of haloperidol in the prevention or treatment of delirium in ICU patients.[24, 25]
The dosages in use are concerning given that the risk of extrapyramidal side effects increases with increasing dose, and prior studies have demonstrated an association between increased dose of antipsychotics and increased risk of other adverse events, including hip fracture and sudden cardiac death.[22, 23] Further, despite these known risks, studies have demonstrated failure to follow recommendations to mitigate risk,[26] such as electrocardiogram monitoring in individuals receiving intravenous haloperidol.[27] Our results suggest that physicians are similarly not following recommendations to use lower doses of haloperidol when treating older patients, given the almost identical incidence of potentially excessive dosing among admissions of patients aged 65 years and older in our cohort.[25] Clinical decision support prompts have been effective at increasing appropriate use of antipsychotic medications in several single‐center analyses,[28, 29, 30] and widespread implementation of such support with a focus on haloperidol dosing should be considered on the basis of our results.
The patient characteristics associated with antipsychotic use in this large, nationally representative analysis are consistent with those identified in prior single‐center analyses.[12, 18] Both prior analyses identified delirium as the most common reason for antipsychotic use, and dementia, intensive care unit stay, and mechanical ventilation were also previously identified as strong predictors of use that we believe hold face validity for the practicing hospitalist. On the other hand, some of the factors associated with antipsychotic use in our model cannot be readily explained, such as insurance status and race, and may be serving as proxies for other variables not included in our analysis. That nonwhite patients are less likely than white patients to receive antipsychotic medications in the hospital has been previously demonstrated,[12] and further investigation to understand this disparity is warranted.
Our study has several additional limitations. First, because our study is observational, the possibility of residual confounding exists, and we cannot rule out that there are other patient factors driving the hospital variation in antipsychotic use that we observed. Second, because guidelines do not exist for antipsychotic dosing in hospitalized patients, we could only comment on potentially excessive dosing, extrapolating from guidelines in the long‐term care setting. Whether such doses are actually excessive in hospitalized patients is not defined. Third, although Premier performs quality checks on charge and ICD‐9‐CM coding data submitted by participating hospitals, the validity of administrative data is uncertain. For example, the use of administrative data to identify delirium diagnoses is likely to have resulted in underestimation of delirium incidence among our different exposure groups. Delirium is likely to be coded more often in the setting of more severe or hyperactive cases, when antipsychotics are more likely to be utilized. This could result in an overestimation of the association between delirium and antipsychotic use. Additionally, differences in coding practices between hospitals for any of the variables in our models could explain some of the variation in antipsychotic prescribing that we observed. Finally, because we were unable to differentiate between new initiation and continuation of a preadmission antipsychotic, some of the variation that we observed is likely to reflect differences in outpatient antipsychotic prescribing practices.
In conclusion, in this large cohort of nonpsychiatric admissions to 300 US hospitals, we found that antipsychotic medication exposure was common, often at high daily doses. Delirium and dementia were the strongest predictors of use among the patient and hospital characteristics examined. The variation in antipsychotic prescribing that we observed was not fully accounted for by measured patient characteristics, and raises the possibility of differing hospital prescribing cultures. Our results draw attention to the need for additional research to better define what constitutes appropriate use of these potentially harmful medications in the hospital setting.
Disclosures: Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Herzig, Rothberg, Gurwitz, and Marcantonio. Acquisition of data: Dr. Herzig. Analysis of data: Mr. Guess. Interpretation of data: Drs. Herzig, Rothberg, Gurwitz, Marcantonio, and Mr. Guess. Drafting of the manuscript: Dr. Herzig. Critical revision of the manuscript for important intellectual content: Drs. Rothberg, Gurwitz, Marcantonio, and Mr. Guess. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant number K24AG035075 from the National Institute on Aging. The funding organizations had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- , , , et al. Variation in nursing home antipsychotic prescribing rates. Arch Intern Med. 2007;167(7):676–683.
- , . Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857.
- , , , , . A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474–481.
- , , , , , . Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42(8):809–815.
- , , , . Small‐area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982;307(21):1310–1314.
- Premier Research Services. Available at: https://www.premierinc.com/transforming‐healthcare/healthcare‐performance‐improvement/premier‐research‐services. Accessed March 15, 2016.
- U.S. Food and Drug Administration. Atypical antipsychotic drugs information. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm094303.htm. Accessed November 1, 2015.
- U.S. Food and Drug Administration. Information on conventional antipsychotics. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm107211. htm. Accessed November 1, 2015.
- Centers for Medicare and Medicaid Services. State Operations Manual. Appendix PP: guidance to surveyors for long‐term care facilities. Available at: https://www.cms.gov/Medicare/Provider‐Enrollment‐and‐Certification/GuidanceforLawsAndRegulations/Downloads/som107 ap_pp_guidelines_ltcf.pdf. Revised October 14, 2005. Accessed March 15, 2016.
- . A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706.
- Healthcare Cost and Utilization Project. Comorbidity software, version 3.7. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed March 15, 2016.
- , , , et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc. 2016;64(2):299–305.
- , . Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb180‐Hospitalizations‐United‐States‐2012.pdf. Published October 2014. Accessed June 29, 2015.
- , , , . Long‐term care services in the United States: 2013 overview. Vital Health Stat 3. 2013;(37):1–107. Available at: http://www.cdc.gov/nchs/data/nsltcp/long_term_care_services_2013.pdf. Accessed March 16, 2016.
- , , , et al. The quality of antipsychotic drug prescribing in nursing homes. Arch Intern Med. 2005;165(11):1280–1285.
- , , , , , . Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89–95.
- , , , , . Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770–w781.
- , , , , , . From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802–804.
- , , . Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269–S276.
- , , , et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta‐analysis. JAMA Intern Med. 2015;175(4):512–520.
- Centers for Medicare and Medicaid Services. State operations manual, appendix PP: guidance to surveyors for long‐term care facilities. Available at: https://www.cms.gov/Regulations‐and‐Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf. Revised October 9, 2015. Accessed February 22, 2016.
- , , , , . Psychotropic drug use and the risk of hip fracture. N Engl J Med. 1987;316(7):363–369.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , , , et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306.
- , , , , . Haloperidol overdosing in the treatment of agitated hospitalized older people with delirium: a retrospective chart review from a community teaching hospital. Drugs Aging. 2013;30(8):639–644.
- , , , . Unsafe use of intravenous haloperidol: evaluation of recommendation‐concordant care in hospitalized elderly adults. J Am Geriatr Soc. 2013;61(1):160–161.
- U.S. Food and Drug Administration. HALDOL brand of haloperidol injection. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/015923s082,018701s057lbl.pdf. Accessed February 23, 2016.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- , , , et al. A standardized, bundled approach to providing geriatric‐focused acute care. J Am Geriatr Soc. 2014;62(5):936–942.
- , , , . Don't fuel the fire: decreasing intravenous haloperidol use in high risk patients via a customized electronic alert. J Am Med Inform Assoc. 2014;21(6):1109–1112.
- , , , et al. Variation in nursing home antipsychotic prescribing rates. Arch Intern Med. 2007;167(7):676–683.
- , . Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857.
- , , , , . A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474–481.
- , , , , , . Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42(8):809–815.
- , , , . Small‐area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982;307(21):1310–1314.
- Premier Research Services. Available at: https://www.premierinc.com/transforming‐healthcare/healthcare‐performance‐improvement/premier‐research‐services. Accessed March 15, 2016.
- U.S. Food and Drug Administration. Atypical antipsychotic drugs information. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm094303.htm. Accessed November 1, 2015.
- U.S. Food and Drug Administration. Information on conventional antipsychotics. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm107211. htm. Accessed November 1, 2015.
- Centers for Medicare and Medicaid Services. State Operations Manual. Appendix PP: guidance to surveyors for long‐term care facilities. Available at: https://www.cms.gov/Medicare/Provider‐Enrollment‐and‐Certification/GuidanceforLawsAndRegulations/Downloads/som107 ap_pp_guidelines_ltcf.pdf. Revised October 14, 2005. Accessed March 15, 2016.
- . A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706.
- Healthcare Cost and Utilization Project. Comorbidity software, version 3.7. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed March 15, 2016.
- , , , et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc. 2016;64(2):299–305.
- , . Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb180‐Hospitalizations‐United‐States‐2012.pdf. Published October 2014. Accessed June 29, 2015.
- , , , . Long‐term care services in the United States: 2013 overview. Vital Health Stat 3. 2013;(37):1–107. Available at: http://www.cdc.gov/nchs/data/nsltcp/long_term_care_services_2013.pdf. Accessed March 16, 2016.
- , , , et al. The quality of antipsychotic drug prescribing in nursing homes. Arch Intern Med. 2005;165(11):1280–1285.
- , , , , , . Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89–95.
- , , , , . Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770–w781.
- , , , , , . From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802–804.
- , , . Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269–S276.
- , , , et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta‐analysis. JAMA Intern Med. 2015;175(4):512–520.
- Centers for Medicare and Medicaid Services. State operations manual, appendix PP: guidance to surveyors for long‐term care facilities. Available at: https://www.cms.gov/Regulations‐and‐Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf. Revised October 9, 2015. Accessed February 22, 2016.
- , , , , . Psychotropic drug use and the risk of hip fracture. N Engl J Med. 1987;316(7):363–369.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , , , et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306.
- , , , , . Haloperidol overdosing in the treatment of agitated hospitalized older people with delirium: a retrospective chart review from a community teaching hospital. Drugs Aging. 2013;30(8):639–644.
- , , , . Unsafe use of intravenous haloperidol: evaluation of recommendation‐concordant care in hospitalized elderly adults. J Am Geriatr Soc. 2013;61(1):160–161.
- U.S. Food and Drug Administration. HALDOL brand of haloperidol injection. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/015923s082,018701s057lbl.pdf. Accessed February 23, 2016.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- , , , et al. A standardized, bundled approach to providing geriatric‐focused acute care. J Am Geriatr Soc. 2014;62(5):936–942.
- , , , . Don't fuel the fire: decreasing intravenous haloperidol use in high risk patients via a customized electronic alert. J Am Med Inform Assoc. 2014;21(6):1109–1112.
Interhospital Transfer Patients
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
© 2015 Society of Hospital Medicine