User login
A 44-year-old man with hemoptysis: A review of pertinent imaging studies and radiographic interventions
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
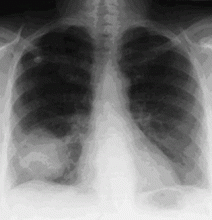
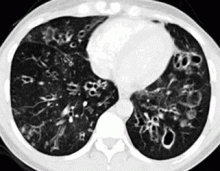
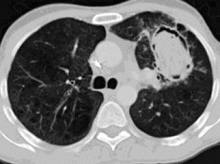
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
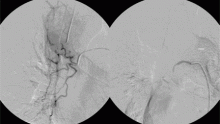
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
KEY POINTS
- We recommend chest radiography in the initial stages of evaluation of hemoptysis, whether the hemoptysis is massive or nonmassive.
- In cases of hemoptysis that is intermittent (whether massive or nonmassive) in patients whose condition is stable, CT, multidetector CT angiography, and bronchoscopy are all useful.
- In cases of hemoptysis that is active, persistent, and massive, multidetector CT angiography, bronchoscopy, and conventional bronchial angiography are all useful, depending on the hemodynamic stability of the patient.
- Bronchial artery embolization is the preferred noninvasive first-line treatment for hemoptysis and offers an excellent alternative to surgery for patients who are poor candidates for surgery.