User login
When and how to image a suspected broken rib
A 70-year-old man falls in his bathroom and subsequently presents to an urgent care clinic. Among his complaints is right-sided chest pain. On physical examination he has point tenderness over the lateral right thorax with some superficial swelling and bruising. The chest is normal on auscultation.
Should this patient undergo imaging to determine if he has a rib fracture? And which imaging study would be appropriate?
This article outlines the use of various imaging tests in the evaluation of suspected rib fractures and recommends an approach to management. This article does not address fractures in children.
MANY CAUSES OF RIB FRACTURES
Trauma, the most common cause of rib fractures, includes penetrating injuries and blunt injury to the chest wall. Between 10% and 66% of traumatic injuries result in rib fractures. 1 Traumatic injury can result from motor vehicle accidents, assault, sports, cardiopulmonary resuscitation, physical abuse (“nonaccidental” trauma), and, rarely, severe paroxysms of coughing.2
Cancer can cause pathologic fractures of the rib.
Stress fractures of the ribs are more likely to occur in high-level athletes whose activity involves repetitive musculoskeletal loading, although they can also occur in people with repetitive coughing paroxysms.3 Sports and activities that result in stress fractures include rowing, pitching or throwing, basketball, weight-lifting, ballet, golf, gymnastics, and swimming.4
WHICH RIB IS BROKEN?
The fourth through 10th ribs are the most often fractured. Fractures of the first through the third ribs can be associated with underlying nerve and vascular injuries, and fractures of the 10th through 12th ribs are associated with damage to abdominal organs,5 most commonly the liver, spleen, kidneys, and diaphragm.3
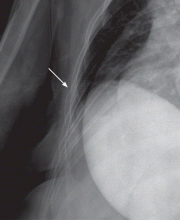
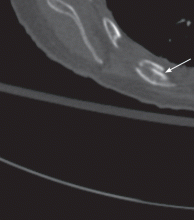
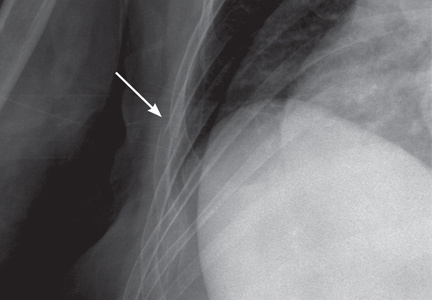
Fractures of the costal cartilage can occur by any of the mechanisms described above. The true incidence of costal cartilage fractures is not known because plain radiography, the traditional method of evaluation, does not reliably detect them.
WHY CONFIRM A RIB FRACTURE?
For many rib fractures without associated injury, a radiographic diagnosis has little impact on patient management, which consists mainly of pain control. But knowing whether a patient has a broken rib can often be important.
To detect associated injury. The rate of associated injury in patients with rib fractures is high.6 Potentially severe complications include:
- Pneumothorax
- Hemothorax
- Pulmonary contusion
- Flail chest
- Pneumonia
- Vascular and nerve damage (especially with trauma to the upper chest or the first through third ribs)
- Abdominal organ injury (particularly with trauma to the lower thorax or lower ribs).
The absence of a rib fracture does not preclude these conditions, however.
To prevent complications. Even in the absence of associated injuries, radiographic confirmation of a rib fracture can help prevent complications such as atelectasis and is particularly important in patients with comorbidities such as chronic obstructive pulmonary disease, cardiac disease, hepatic disease, renal disease, dementia, and coagulopathy.1
To document the injury. Radiographic documentation of a rib fracture may be required for medical-legal issues in cases of assault, motor vehicle accident, occupational injury, and abuse.
To help manage pain. Confirmation of rib fracture can facilitate pain management, particularly in patients with undiagnosed fractures with long-standing refractory pain. For example, conservative pain control with nonsteroidal anti-inflammatory drugs may be sufficient for a soft-tissue injury but may not be enough for a rib fracture. Intravenous narcotics or nerve blocks might be preferable.3,7 Controlling pain helps limit the incidence of associated complications.
To count how many ribs are broken. The more ribs broken, the greater the likelihood of illness and death in certain populations, such as the elderly. One study8 found that patients over age 45 with more than four broken ribs are at a significantly higher risk of prolonged stay in the intensive care unit, prolonged ventilator support, and prolonged overall hospital stay.
Knowing the number of ribs fractured may also influence other treatment decisions, such as whether to transfer the patient to a trauma center: a study showed that the more ribs broken, the greater the death rate, and that more than three rib fractures may indicate the need to transfer to a trauma center.6
HOW TO DIAGNOSE A BROKEN RIB
Signs and symptoms are unreliable but important
Clinical symptoms do not reliably tell us if a rib is broken.9,10 Nevertheless, the history and physical examination can uncover possible complications or associated injuries,10,11 such as flail chest, pneumothorax, or vascular injury.
Classic clinical signs and symptoms of rib fracture include point tenderness, focally referred pain with general chest compression, splinting, bony crepitus, and ecchymosis.9 A history of a motor vehicle accident (especially on a motorcycle) or other injury due to rapid deceleration, a fall from higher than 20 feet, a gunshot wound, assault, or a crushing injury would indicate a greater risk of complications.
Signs of complications may include decreased oxygen saturation, decreased or absent breath sounds, dullness or hyperresonance to percussion, tracheal deviation, hypotension, arrythmia, subcutaneous emphysema, neck vein distension, neck hematoma, a focal neurologic deficit below the clavicles or in the upper extremities, and flail chest.11 Flail chest results from multiple fractures in the same rib, so that a segment of chest wall does not contribute to breathing.
Further research is needed into the correlation of clinical symptoms with rib fractures. Much of the evidence that clinical symptoms correlate poorly with fractures comes from studies that used plain radiography to detect the fractures. However, ultrasonography and computed tomography (CT) can detect fractures that plain radiography cannot, and studies using these newer imaging tests may reveal a better correlation between clinical symptoms and rib fracture than previously thought.6
Chest radiography may miss 50% of rib fractures, but is still useful
Plain radiography of the chest with or without oblique views and optimized by the technologist for bony detail (“bone technique”) has historically been the imaging test of choice. However, it may miss up to 50% of fractures.10 Furthermore, it is not sensitive for costal cartilage3 or stress fractures.
Despite these limitations, plain radiography is vitally important in diagnosing complications and associated injuries such as a pneumothorax, hemothorax, pulmonary contusion, pneumomediastinum, or pneumoperitoneum. Also, a widened mediastinum could indicate aortic injury.
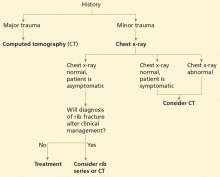
Currently, a standard chest x-ray is often the initial study of choice in the evaluation of chest pain and in cases of minor blunt trauma. If rib fractures are suspected clinically, a rib series can be of benefit. A rib series consists of a marker placed over the region of interest, oblique views, and optimization of the radiograph by the technologist to highlight bony detail. The decision to image a rib fracture in the absence of other underlying abnormalities or associated injuries depends on the clinical scenario.
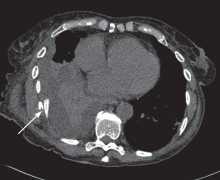
Computed tomography provides more detail
While CT appears to be the best imaging test for evaluating for rib fractures and associated injuries, it is relatively costly, is time-consuming, is not always available, and exposes the patient to a significant amount of radiation.
Also, while CT plays a vital role in major and penetrating trauma of the chest or abdomen, its use in other situations is more limited. Again, the issue of clinical impact of a diagnosis of rib fracture comes into play, and in this setting CT competes with plain radiography and ultrasonography, which are less costly and involve less or no radiation exposure.
Ultrasonography has advantages but is not widely used
Ultrasonography can be used to look for broken ribs and costal cartilage fractures. Associated injuries such as pneumothorax, hemothorax, and abdominal organ injury can also be evaluated. Studies have found it to be much more sensitive than plain radiography in detecting rib fractures,3,15 whereas other studies have suggested it is only equally sensitive or slightly better.7 It also has the advantage of not using radiation.
Because of a number of disadvantages, ultrasonography is rarely used in the evaluation of rib fracture. It is time-consuming and more costly than plain radiography. It is often not readily available. It can be painful, making it impractical for trauma patients. Its results depend greatly on the skill of the technician, and it is unable to adequately assess certain portions of the thorax (eg, the first rib under the clavicle, and the upper ribs under the scapula).7,15 Although able to detect some associated injuries, ultrasonography is not as sensitive and comprehensive as plain radiography and CT. Its role is therefore limited to situations in which the diagnosis of a rib fracture alone, in an accessible rib, is important.
Bone scan: Sensitive but not specific
Technetium Tc 99m methylene diphosphonate bone scanning can be used to look for bone pathology, including rib fractures. Bone scans are sensitive but not specific, and abnormal uptake generates an extensive differential diagnosis.16 Single-photon emission CT, or SPECT, can help localize the abnormality. 4 Because a hot spot on a bone scan can represent a number of conditions besides rib fractures, including cancer, focal sclerosis, and focal osteosclerosis, bone scanning is not routinely used for evaluating rib fractures, although it is very sensitive for stress fractures.
Occasionally, in a patient undergoing a bone scan as part of a workup for cancer, a scan shows a lesion that might be a rib fracture. In this case, one should correlate the results with those of plain radiography or CT.16
Magnetic resonance imaging: no role yet in rib fracture evaluation
MRI is not considered appropriate for evaluating rib fractures. It may be useful if there is concern about soft-tissue or vascular abnormalities. Beyond this, further research is needed to elucidate its role in rib fracture.
THE CHOICE OF TEST DEPENDS ON THE SITUATION
In patients with penetrating or major chest or abdominal trauma, CT is the study of choice. It provides the most information about associated injuries, and it accurately detects rib fractures. This helps target treatment of associated injuries, and helps identify patients at higher risk, such as those with significant vascular, pulmonary, or abdominal injuries and those with a greater number of fractures. An unstable, critically injured patient would not be a candidate for CT because of the risk of transport to the scanner; chest radiography would have to suffice in these cases.
In cases of minor blunt trauma when there is little suspicion of associated injuries or complications, plain radiography is likely sufficient. If there is suspicion of a rib fracture alone and confirmation is of clinical importance (eg, in the elderly or those with long-standing refractory pain, or when certain pain management treatments are being considered), then oblique radiographic views, bone technique, and marker placement over the concerning region are recommended. The role of ultrasonography in this setting is still up for debate.
In cases of suspected rib fracture with longstanding pain refractory to conservative pain management, plain radiography with oblique views, bone technique, and marker placement is useful. If the radiograph is negative or if there is a high suspicion of cartilage fracture, CT or ultrasonography may be of benefit only if the diagnosis will alter clinical management.
If stress fracture is suspected, a nuclear bone scan may be helpful to first detect an abnormality, and CT may then be used for correlation if needed.
CASE CONCLUDED: LIVING WITH UNCERTAINTY
As for the 70-year-old man presented at the beginning of this article, the first question is whether we suspect an associated injury on the basis of clinical features. If we had clinical findings suspicious for pneumothorax or hemothorax, plain radiography of the chest would be indicated. Since the patient was not involved in major trauma, a CT scan is not indicated as the first study.
Our patient has clinical findings suggesting a rib fracture without associated injury. In this setting, routine posteroanterior and lateral chest radiography would be useful to rule out major associated injuries and, perhaps, to find a rib fracture. If the chest film is normal and rib fracture is still suspected, we must decide whether the diagnosis would alter our clinical management. Our patient would likely be treated the same regardless of whether or not he has a fracture; therefore, we would prescribe pain management.
Chest radiography was performed to rule out associated injuries, especially since the patient was elderly, but the chest x-ray did not reveal anything. On follow-up approximately 1 month later, he appeared improved, with less pain and tenderness. This may be due to healing of a rib fracture or healing of his soft-tissue injury. We will never know whether he truly had a fracture, but it is irrelevant to his care.
- Bergeron E, Lavoie A, Clas D, et al. Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma 2003; 54:478–485.
- Lederer W, Mair D, Rabl W, Baubin M. Frequency of rib and sternum fractures associated with out-of-hospital cardiopulmonary resuscitation is underestimated by conventional chest x-ray. Resuscitation 2004; 60:157–162.
- Kara M, Dikmen E, Erdal HH, Simsir I, Kara SA. Disclosure of unnoticed rib fractures with the use of ultrasonography in minor blunt chest trauma. Eur J Cardiothorac Surg 2003; 24:608–613.
- Connolly LP, Connolly SA. Rib stress fractures. Clin Nucl Med 2004; 29:614–616.
- Bansidhar BJ, Lagares-Garcia JA, Miller SL. Clinical rib fractures: are follow-up chest x-rays a waste of resources? Am Surg 2002; 68:449–453.
- Stawicki SP, Grossman MD, Hoey BA, Miller DL, Reed JF. Rib fractures in the elderly: a marker of injury severity. J Am Geriatr Soc 2004; 52:805–808.
- Hurley ME, Keye GD, Hamilton S. Is ultrasound really helpful in the detection of rib fractures? Injury 2004; 35:562–566.
- Holcomb JB, McMullin NR, Kozar RA, Lygas MH, Moore FA. Morbidity from rib fractures increases after age 45. J Am Coll Surg 2003; 196:549–555.
- Deluca SA, Rhea JT, O’Malley TO. Radiographic evaluation of rib fractures. AJR Am J Roentgenol 1982; 138:91–92.
- Dubinsky I, Low A. Non-life threatening blunt chest trauma: appropriate investigation and treatment. Am J Emerg Med 1997; 15:240–243.
- Sears BW, Luchette FA, Esposito TJ, et al. Old fashion clinical judgment in the era of protocols: is mandatory chest x-ray necessary in injured patients? J Trauma 2005; 59:324–332.
- Traub M, Stevenson M, McEvoy S, et al. The use of chest computed tomography versus chest x-ray in patients with major blunt trauma. Injury 2007; 38:43–47.
- Trupka A, Waydhas C, Hallfeldt KK, Nast-Kolb D, Pfeifer KJ, Schweiberer L. Value of thoracic computed tomography in the first assessment of severely injured patients with blunt chest trauma: results of a prospective study. J Trauma 1997; 43:405–412.
- Malghem J, Vande Berg B, Lecouvet F, Maldague B. Costal cartilage fractures as revealed on CT and sonography. AJR Am J Roentgenol 2001; 176:429–432.
- Griffith JF, Rainer TH, Ching AS, Law KL, Cocks RA, Metreweli C. Sonography compared with radiography in revealing acute rib fracture. AJR Am J Roentgenol 1999; 173:1603–1609.
- Niitsu M, Takeda T. Solitary hot spots in the ribs on bone scan: value of thin-section reformatted computed tomography to exclude radiography negative fractures. J Comput Assist Tomogr 2003; 27:469–474.
A 70-year-old man falls in his bathroom and subsequently presents to an urgent care clinic. Among his complaints is right-sided chest pain. On physical examination he has point tenderness over the lateral right thorax with some superficial swelling and bruising. The chest is normal on auscultation.
Should this patient undergo imaging to determine if he has a rib fracture? And which imaging study would be appropriate?
This article outlines the use of various imaging tests in the evaluation of suspected rib fractures and recommends an approach to management. This article does not address fractures in children.
MANY CAUSES OF RIB FRACTURES
Trauma, the most common cause of rib fractures, includes penetrating injuries and blunt injury to the chest wall. Between 10% and 66% of traumatic injuries result in rib fractures. 1 Traumatic injury can result from motor vehicle accidents, assault, sports, cardiopulmonary resuscitation, physical abuse (“nonaccidental” trauma), and, rarely, severe paroxysms of coughing.2
Cancer can cause pathologic fractures of the rib.
Stress fractures of the ribs are more likely to occur in high-level athletes whose activity involves repetitive musculoskeletal loading, although they can also occur in people with repetitive coughing paroxysms.3 Sports and activities that result in stress fractures include rowing, pitching or throwing, basketball, weight-lifting, ballet, golf, gymnastics, and swimming.4
WHICH RIB IS BROKEN?
The fourth through 10th ribs are the most often fractured. Fractures of the first through the third ribs can be associated with underlying nerve and vascular injuries, and fractures of the 10th through 12th ribs are associated with damage to abdominal organs,5 most commonly the liver, spleen, kidneys, and diaphragm.3
Fractures of the costal cartilage can occur by any of the mechanisms described above. The true incidence of costal cartilage fractures is not known because plain radiography, the traditional method of evaluation, does not reliably detect them.
WHY CONFIRM A RIB FRACTURE?
For many rib fractures without associated injury, a radiographic diagnosis has little impact on patient management, which consists mainly of pain control. But knowing whether a patient has a broken rib can often be important.
To detect associated injury. The rate of associated injury in patients with rib fractures is high.6 Potentially severe complications include:
- Pneumothorax
- Hemothorax
- Pulmonary contusion
- Flail chest
- Pneumonia
- Vascular and nerve damage (especially with trauma to the upper chest or the first through third ribs)
- Abdominal organ injury (particularly with trauma to the lower thorax or lower ribs).
The absence of a rib fracture does not preclude these conditions, however.
To prevent complications. Even in the absence of associated injuries, radiographic confirmation of a rib fracture can help prevent complications such as atelectasis and is particularly important in patients with comorbidities such as chronic obstructive pulmonary disease, cardiac disease, hepatic disease, renal disease, dementia, and coagulopathy.1
To document the injury. Radiographic documentation of a rib fracture may be required for medical-legal issues in cases of assault, motor vehicle accident, occupational injury, and abuse.
To help manage pain. Confirmation of rib fracture can facilitate pain management, particularly in patients with undiagnosed fractures with long-standing refractory pain. For example, conservative pain control with nonsteroidal anti-inflammatory drugs may be sufficient for a soft-tissue injury but may not be enough for a rib fracture. Intravenous narcotics or nerve blocks might be preferable.3,7 Controlling pain helps limit the incidence of associated complications.
To count how many ribs are broken. The more ribs broken, the greater the likelihood of illness and death in certain populations, such as the elderly. One study8 found that patients over age 45 with more than four broken ribs are at a significantly higher risk of prolonged stay in the intensive care unit, prolonged ventilator support, and prolonged overall hospital stay.
Knowing the number of ribs fractured may also influence other treatment decisions, such as whether to transfer the patient to a trauma center: a study showed that the more ribs broken, the greater the death rate, and that more than three rib fractures may indicate the need to transfer to a trauma center.6
HOW TO DIAGNOSE A BROKEN RIB
Signs and symptoms are unreliable but important
Clinical symptoms do not reliably tell us if a rib is broken.9,10 Nevertheless, the history and physical examination can uncover possible complications or associated injuries,10,11 such as flail chest, pneumothorax, or vascular injury.
Classic clinical signs and symptoms of rib fracture include point tenderness, focally referred pain with general chest compression, splinting, bony crepitus, and ecchymosis.9 A history of a motor vehicle accident (especially on a motorcycle) or other injury due to rapid deceleration, a fall from higher than 20 feet, a gunshot wound, assault, or a crushing injury would indicate a greater risk of complications.
Signs of complications may include decreased oxygen saturation, decreased or absent breath sounds, dullness or hyperresonance to percussion, tracheal deviation, hypotension, arrythmia, subcutaneous emphysema, neck vein distension, neck hematoma, a focal neurologic deficit below the clavicles or in the upper extremities, and flail chest.11 Flail chest results from multiple fractures in the same rib, so that a segment of chest wall does not contribute to breathing.
Further research is needed into the correlation of clinical symptoms with rib fractures. Much of the evidence that clinical symptoms correlate poorly with fractures comes from studies that used plain radiography to detect the fractures. However, ultrasonography and computed tomography (CT) can detect fractures that plain radiography cannot, and studies using these newer imaging tests may reveal a better correlation between clinical symptoms and rib fracture than previously thought.6
Chest radiography may miss 50% of rib fractures, but is still useful
Plain radiography of the chest with or without oblique views and optimized by the technologist for bony detail (“bone technique”) has historically been the imaging test of choice. However, it may miss up to 50% of fractures.10 Furthermore, it is not sensitive for costal cartilage3 or stress fractures.
Despite these limitations, plain radiography is vitally important in diagnosing complications and associated injuries such as a pneumothorax, hemothorax, pulmonary contusion, pneumomediastinum, or pneumoperitoneum. Also, a widened mediastinum could indicate aortic injury.
Currently, a standard chest x-ray is often the initial study of choice in the evaluation of chest pain and in cases of minor blunt trauma. If rib fractures are suspected clinically, a rib series can be of benefit. A rib series consists of a marker placed over the region of interest, oblique views, and optimization of the radiograph by the technologist to highlight bony detail. The decision to image a rib fracture in the absence of other underlying abnormalities or associated injuries depends on the clinical scenario.
Computed tomography provides more detail
While CT appears to be the best imaging test for evaluating for rib fractures and associated injuries, it is relatively costly, is time-consuming, is not always available, and exposes the patient to a significant amount of radiation.
Also, while CT plays a vital role in major and penetrating trauma of the chest or abdomen, its use in other situations is more limited. Again, the issue of clinical impact of a diagnosis of rib fracture comes into play, and in this setting CT competes with plain radiography and ultrasonography, which are less costly and involve less or no radiation exposure.
Ultrasonography has advantages but is not widely used
Ultrasonography can be used to look for broken ribs and costal cartilage fractures. Associated injuries such as pneumothorax, hemothorax, and abdominal organ injury can also be evaluated. Studies have found it to be much more sensitive than plain radiography in detecting rib fractures,3,15 whereas other studies have suggested it is only equally sensitive or slightly better.7 It also has the advantage of not using radiation.
Because of a number of disadvantages, ultrasonography is rarely used in the evaluation of rib fracture. It is time-consuming and more costly than plain radiography. It is often not readily available. It can be painful, making it impractical for trauma patients. Its results depend greatly on the skill of the technician, and it is unable to adequately assess certain portions of the thorax (eg, the first rib under the clavicle, and the upper ribs under the scapula).7,15 Although able to detect some associated injuries, ultrasonography is not as sensitive and comprehensive as plain radiography and CT. Its role is therefore limited to situations in which the diagnosis of a rib fracture alone, in an accessible rib, is important.
Bone scan: Sensitive but not specific
Technetium Tc 99m methylene diphosphonate bone scanning can be used to look for bone pathology, including rib fractures. Bone scans are sensitive but not specific, and abnormal uptake generates an extensive differential diagnosis.16 Single-photon emission CT, or SPECT, can help localize the abnormality. 4 Because a hot spot on a bone scan can represent a number of conditions besides rib fractures, including cancer, focal sclerosis, and focal osteosclerosis, bone scanning is not routinely used for evaluating rib fractures, although it is very sensitive for stress fractures.
Occasionally, in a patient undergoing a bone scan as part of a workup for cancer, a scan shows a lesion that might be a rib fracture. In this case, one should correlate the results with those of plain radiography or CT.16
Magnetic resonance imaging: no role yet in rib fracture evaluation
MRI is not considered appropriate for evaluating rib fractures. It may be useful if there is concern about soft-tissue or vascular abnormalities. Beyond this, further research is needed to elucidate its role in rib fracture.
THE CHOICE OF TEST DEPENDS ON THE SITUATION
In patients with penetrating or major chest or abdominal trauma, CT is the study of choice. It provides the most information about associated injuries, and it accurately detects rib fractures. This helps target treatment of associated injuries, and helps identify patients at higher risk, such as those with significant vascular, pulmonary, or abdominal injuries and those with a greater number of fractures. An unstable, critically injured patient would not be a candidate for CT because of the risk of transport to the scanner; chest radiography would have to suffice in these cases.
In cases of minor blunt trauma when there is little suspicion of associated injuries or complications, plain radiography is likely sufficient. If there is suspicion of a rib fracture alone and confirmation is of clinical importance (eg, in the elderly or those with long-standing refractory pain, or when certain pain management treatments are being considered), then oblique radiographic views, bone technique, and marker placement over the concerning region are recommended. The role of ultrasonography in this setting is still up for debate.
In cases of suspected rib fracture with longstanding pain refractory to conservative pain management, plain radiography with oblique views, bone technique, and marker placement is useful. If the radiograph is negative or if there is a high suspicion of cartilage fracture, CT or ultrasonography may be of benefit only if the diagnosis will alter clinical management.
If stress fracture is suspected, a nuclear bone scan may be helpful to first detect an abnormality, and CT may then be used for correlation if needed.
CASE CONCLUDED: LIVING WITH UNCERTAINTY
As for the 70-year-old man presented at the beginning of this article, the first question is whether we suspect an associated injury on the basis of clinical features. If we had clinical findings suspicious for pneumothorax or hemothorax, plain radiography of the chest would be indicated. Since the patient was not involved in major trauma, a CT scan is not indicated as the first study.
Our patient has clinical findings suggesting a rib fracture without associated injury. In this setting, routine posteroanterior and lateral chest radiography would be useful to rule out major associated injuries and, perhaps, to find a rib fracture. If the chest film is normal and rib fracture is still suspected, we must decide whether the diagnosis would alter our clinical management. Our patient would likely be treated the same regardless of whether or not he has a fracture; therefore, we would prescribe pain management.
Chest radiography was performed to rule out associated injuries, especially since the patient was elderly, but the chest x-ray did not reveal anything. On follow-up approximately 1 month later, he appeared improved, with less pain and tenderness. This may be due to healing of a rib fracture or healing of his soft-tissue injury. We will never know whether he truly had a fracture, but it is irrelevant to his care.
A 70-year-old man falls in his bathroom and subsequently presents to an urgent care clinic. Among his complaints is right-sided chest pain. On physical examination he has point tenderness over the lateral right thorax with some superficial swelling and bruising. The chest is normal on auscultation.
Should this patient undergo imaging to determine if he has a rib fracture? And which imaging study would be appropriate?
This article outlines the use of various imaging tests in the evaluation of suspected rib fractures and recommends an approach to management. This article does not address fractures in children.
MANY CAUSES OF RIB FRACTURES
Trauma, the most common cause of rib fractures, includes penetrating injuries and blunt injury to the chest wall. Between 10% and 66% of traumatic injuries result in rib fractures. 1 Traumatic injury can result from motor vehicle accidents, assault, sports, cardiopulmonary resuscitation, physical abuse (“nonaccidental” trauma), and, rarely, severe paroxysms of coughing.2
Cancer can cause pathologic fractures of the rib.
Stress fractures of the ribs are more likely to occur in high-level athletes whose activity involves repetitive musculoskeletal loading, although they can also occur in people with repetitive coughing paroxysms.3 Sports and activities that result in stress fractures include rowing, pitching or throwing, basketball, weight-lifting, ballet, golf, gymnastics, and swimming.4
WHICH RIB IS BROKEN?
The fourth through 10th ribs are the most often fractured. Fractures of the first through the third ribs can be associated with underlying nerve and vascular injuries, and fractures of the 10th through 12th ribs are associated with damage to abdominal organs,5 most commonly the liver, spleen, kidneys, and diaphragm.3
Fractures of the costal cartilage can occur by any of the mechanisms described above. The true incidence of costal cartilage fractures is not known because plain radiography, the traditional method of evaluation, does not reliably detect them.
WHY CONFIRM A RIB FRACTURE?
For many rib fractures without associated injury, a radiographic diagnosis has little impact on patient management, which consists mainly of pain control. But knowing whether a patient has a broken rib can often be important.
To detect associated injury. The rate of associated injury in patients with rib fractures is high.6 Potentially severe complications include:
- Pneumothorax
- Hemothorax
- Pulmonary contusion
- Flail chest
- Pneumonia
- Vascular and nerve damage (especially with trauma to the upper chest or the first through third ribs)
- Abdominal organ injury (particularly with trauma to the lower thorax or lower ribs).
The absence of a rib fracture does not preclude these conditions, however.
To prevent complications. Even in the absence of associated injuries, radiographic confirmation of a rib fracture can help prevent complications such as atelectasis and is particularly important in patients with comorbidities such as chronic obstructive pulmonary disease, cardiac disease, hepatic disease, renal disease, dementia, and coagulopathy.1
To document the injury. Radiographic documentation of a rib fracture may be required for medical-legal issues in cases of assault, motor vehicle accident, occupational injury, and abuse.
To help manage pain. Confirmation of rib fracture can facilitate pain management, particularly in patients with undiagnosed fractures with long-standing refractory pain. For example, conservative pain control with nonsteroidal anti-inflammatory drugs may be sufficient for a soft-tissue injury but may not be enough for a rib fracture. Intravenous narcotics or nerve blocks might be preferable.3,7 Controlling pain helps limit the incidence of associated complications.
To count how many ribs are broken. The more ribs broken, the greater the likelihood of illness and death in certain populations, such as the elderly. One study8 found that patients over age 45 with more than four broken ribs are at a significantly higher risk of prolonged stay in the intensive care unit, prolonged ventilator support, and prolonged overall hospital stay.
Knowing the number of ribs fractured may also influence other treatment decisions, such as whether to transfer the patient to a trauma center: a study showed that the more ribs broken, the greater the death rate, and that more than three rib fractures may indicate the need to transfer to a trauma center.6
HOW TO DIAGNOSE A BROKEN RIB
Signs and symptoms are unreliable but important
Clinical symptoms do not reliably tell us if a rib is broken.9,10 Nevertheless, the history and physical examination can uncover possible complications or associated injuries,10,11 such as flail chest, pneumothorax, or vascular injury.
Classic clinical signs and symptoms of rib fracture include point tenderness, focally referred pain with general chest compression, splinting, bony crepitus, and ecchymosis.9 A history of a motor vehicle accident (especially on a motorcycle) or other injury due to rapid deceleration, a fall from higher than 20 feet, a gunshot wound, assault, or a crushing injury would indicate a greater risk of complications.
Signs of complications may include decreased oxygen saturation, decreased or absent breath sounds, dullness or hyperresonance to percussion, tracheal deviation, hypotension, arrythmia, subcutaneous emphysema, neck vein distension, neck hematoma, a focal neurologic deficit below the clavicles or in the upper extremities, and flail chest.11 Flail chest results from multiple fractures in the same rib, so that a segment of chest wall does not contribute to breathing.
Further research is needed into the correlation of clinical symptoms with rib fractures. Much of the evidence that clinical symptoms correlate poorly with fractures comes from studies that used plain radiography to detect the fractures. However, ultrasonography and computed tomography (CT) can detect fractures that plain radiography cannot, and studies using these newer imaging tests may reveal a better correlation between clinical symptoms and rib fracture than previously thought.6
Chest radiography may miss 50% of rib fractures, but is still useful
Plain radiography of the chest with or without oblique views and optimized by the technologist for bony detail (“bone technique”) has historically been the imaging test of choice. However, it may miss up to 50% of fractures.10 Furthermore, it is not sensitive for costal cartilage3 or stress fractures.
Despite these limitations, plain radiography is vitally important in diagnosing complications and associated injuries such as a pneumothorax, hemothorax, pulmonary contusion, pneumomediastinum, or pneumoperitoneum. Also, a widened mediastinum could indicate aortic injury.
Currently, a standard chest x-ray is often the initial study of choice in the evaluation of chest pain and in cases of minor blunt trauma. If rib fractures are suspected clinically, a rib series can be of benefit. A rib series consists of a marker placed over the region of interest, oblique views, and optimization of the radiograph by the technologist to highlight bony detail. The decision to image a rib fracture in the absence of other underlying abnormalities or associated injuries depends on the clinical scenario.
Computed tomography provides more detail
While CT appears to be the best imaging test for evaluating for rib fractures and associated injuries, it is relatively costly, is time-consuming, is not always available, and exposes the patient to a significant amount of radiation.
Also, while CT plays a vital role in major and penetrating trauma of the chest or abdomen, its use in other situations is more limited. Again, the issue of clinical impact of a diagnosis of rib fracture comes into play, and in this setting CT competes with plain radiography and ultrasonography, which are less costly and involve less or no radiation exposure.
Ultrasonography has advantages but is not widely used
Ultrasonography can be used to look for broken ribs and costal cartilage fractures. Associated injuries such as pneumothorax, hemothorax, and abdominal organ injury can also be evaluated. Studies have found it to be much more sensitive than plain radiography in detecting rib fractures,3,15 whereas other studies have suggested it is only equally sensitive or slightly better.7 It also has the advantage of not using radiation.
Because of a number of disadvantages, ultrasonography is rarely used in the evaluation of rib fracture. It is time-consuming and more costly than plain radiography. It is often not readily available. It can be painful, making it impractical for trauma patients. Its results depend greatly on the skill of the technician, and it is unable to adequately assess certain portions of the thorax (eg, the first rib under the clavicle, and the upper ribs under the scapula).7,15 Although able to detect some associated injuries, ultrasonography is not as sensitive and comprehensive as plain radiography and CT. Its role is therefore limited to situations in which the diagnosis of a rib fracture alone, in an accessible rib, is important.
Bone scan: Sensitive but not specific
Technetium Tc 99m methylene diphosphonate bone scanning can be used to look for bone pathology, including rib fractures. Bone scans are sensitive but not specific, and abnormal uptake generates an extensive differential diagnosis.16 Single-photon emission CT, or SPECT, can help localize the abnormality. 4 Because a hot spot on a bone scan can represent a number of conditions besides rib fractures, including cancer, focal sclerosis, and focal osteosclerosis, bone scanning is not routinely used for evaluating rib fractures, although it is very sensitive for stress fractures.
Occasionally, in a patient undergoing a bone scan as part of a workup for cancer, a scan shows a lesion that might be a rib fracture. In this case, one should correlate the results with those of plain radiography or CT.16
Magnetic resonance imaging: no role yet in rib fracture evaluation
MRI is not considered appropriate for evaluating rib fractures. It may be useful if there is concern about soft-tissue or vascular abnormalities. Beyond this, further research is needed to elucidate its role in rib fracture.
THE CHOICE OF TEST DEPENDS ON THE SITUATION
In patients with penetrating or major chest or abdominal trauma, CT is the study of choice. It provides the most information about associated injuries, and it accurately detects rib fractures. This helps target treatment of associated injuries, and helps identify patients at higher risk, such as those with significant vascular, pulmonary, or abdominal injuries and those with a greater number of fractures. An unstable, critically injured patient would not be a candidate for CT because of the risk of transport to the scanner; chest radiography would have to suffice in these cases.
In cases of minor blunt trauma when there is little suspicion of associated injuries or complications, plain radiography is likely sufficient. If there is suspicion of a rib fracture alone and confirmation is of clinical importance (eg, in the elderly or those with long-standing refractory pain, or when certain pain management treatments are being considered), then oblique radiographic views, bone technique, and marker placement over the concerning region are recommended. The role of ultrasonography in this setting is still up for debate.
In cases of suspected rib fracture with longstanding pain refractory to conservative pain management, plain radiography with oblique views, bone technique, and marker placement is useful. If the radiograph is negative or if there is a high suspicion of cartilage fracture, CT or ultrasonography may be of benefit only if the diagnosis will alter clinical management.
If stress fracture is suspected, a nuclear bone scan may be helpful to first detect an abnormality, and CT may then be used for correlation if needed.
CASE CONCLUDED: LIVING WITH UNCERTAINTY
As for the 70-year-old man presented at the beginning of this article, the first question is whether we suspect an associated injury on the basis of clinical features. If we had clinical findings suspicious for pneumothorax or hemothorax, plain radiography of the chest would be indicated. Since the patient was not involved in major trauma, a CT scan is not indicated as the first study.
Our patient has clinical findings suggesting a rib fracture without associated injury. In this setting, routine posteroanterior and lateral chest radiography would be useful to rule out major associated injuries and, perhaps, to find a rib fracture. If the chest film is normal and rib fracture is still suspected, we must decide whether the diagnosis would alter our clinical management. Our patient would likely be treated the same regardless of whether or not he has a fracture; therefore, we would prescribe pain management.
Chest radiography was performed to rule out associated injuries, especially since the patient was elderly, but the chest x-ray did not reveal anything. On follow-up approximately 1 month later, he appeared improved, with less pain and tenderness. This may be due to healing of a rib fracture or healing of his soft-tissue injury. We will never know whether he truly had a fracture, but it is irrelevant to his care.
- Bergeron E, Lavoie A, Clas D, et al. Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma 2003; 54:478–485.
- Lederer W, Mair D, Rabl W, Baubin M. Frequency of rib and sternum fractures associated with out-of-hospital cardiopulmonary resuscitation is underestimated by conventional chest x-ray. Resuscitation 2004; 60:157–162.
- Kara M, Dikmen E, Erdal HH, Simsir I, Kara SA. Disclosure of unnoticed rib fractures with the use of ultrasonography in minor blunt chest trauma. Eur J Cardiothorac Surg 2003; 24:608–613.
- Connolly LP, Connolly SA. Rib stress fractures. Clin Nucl Med 2004; 29:614–616.
- Bansidhar BJ, Lagares-Garcia JA, Miller SL. Clinical rib fractures: are follow-up chest x-rays a waste of resources? Am Surg 2002; 68:449–453.
- Stawicki SP, Grossman MD, Hoey BA, Miller DL, Reed JF. Rib fractures in the elderly: a marker of injury severity. J Am Geriatr Soc 2004; 52:805–808.
- Hurley ME, Keye GD, Hamilton S. Is ultrasound really helpful in the detection of rib fractures? Injury 2004; 35:562–566.
- Holcomb JB, McMullin NR, Kozar RA, Lygas MH, Moore FA. Morbidity from rib fractures increases after age 45. J Am Coll Surg 2003; 196:549–555.
- Deluca SA, Rhea JT, O’Malley TO. Radiographic evaluation of rib fractures. AJR Am J Roentgenol 1982; 138:91–92.
- Dubinsky I, Low A. Non-life threatening blunt chest trauma: appropriate investigation and treatment. Am J Emerg Med 1997; 15:240–243.
- Sears BW, Luchette FA, Esposito TJ, et al. Old fashion clinical judgment in the era of protocols: is mandatory chest x-ray necessary in injured patients? J Trauma 2005; 59:324–332.
- Traub M, Stevenson M, McEvoy S, et al. The use of chest computed tomography versus chest x-ray in patients with major blunt trauma. Injury 2007; 38:43–47.
- Trupka A, Waydhas C, Hallfeldt KK, Nast-Kolb D, Pfeifer KJ, Schweiberer L. Value of thoracic computed tomography in the first assessment of severely injured patients with blunt chest trauma: results of a prospective study. J Trauma 1997; 43:405–412.
- Malghem J, Vande Berg B, Lecouvet F, Maldague B. Costal cartilage fractures as revealed on CT and sonography. AJR Am J Roentgenol 2001; 176:429–432.
- Griffith JF, Rainer TH, Ching AS, Law KL, Cocks RA, Metreweli C. Sonography compared with radiography in revealing acute rib fracture. AJR Am J Roentgenol 1999; 173:1603–1609.
- Niitsu M, Takeda T. Solitary hot spots in the ribs on bone scan: value of thin-section reformatted computed tomography to exclude radiography negative fractures. J Comput Assist Tomogr 2003; 27:469–474.
- Bergeron E, Lavoie A, Clas D, et al. Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma 2003; 54:478–485.
- Lederer W, Mair D, Rabl W, Baubin M. Frequency of rib and sternum fractures associated with out-of-hospital cardiopulmonary resuscitation is underestimated by conventional chest x-ray. Resuscitation 2004; 60:157–162.
- Kara M, Dikmen E, Erdal HH, Simsir I, Kara SA. Disclosure of unnoticed rib fractures with the use of ultrasonography in minor blunt chest trauma. Eur J Cardiothorac Surg 2003; 24:608–613.
- Connolly LP, Connolly SA. Rib stress fractures. Clin Nucl Med 2004; 29:614–616.
- Bansidhar BJ, Lagares-Garcia JA, Miller SL. Clinical rib fractures: are follow-up chest x-rays a waste of resources? Am Surg 2002; 68:449–453.
- Stawicki SP, Grossman MD, Hoey BA, Miller DL, Reed JF. Rib fractures in the elderly: a marker of injury severity. J Am Geriatr Soc 2004; 52:805–808.
- Hurley ME, Keye GD, Hamilton S. Is ultrasound really helpful in the detection of rib fractures? Injury 2004; 35:562–566.
- Holcomb JB, McMullin NR, Kozar RA, Lygas MH, Moore FA. Morbidity from rib fractures increases after age 45. J Am Coll Surg 2003; 196:549–555.
- Deluca SA, Rhea JT, O’Malley TO. Radiographic evaluation of rib fractures. AJR Am J Roentgenol 1982; 138:91–92.
- Dubinsky I, Low A. Non-life threatening blunt chest trauma: appropriate investigation and treatment. Am J Emerg Med 1997; 15:240–243.
- Sears BW, Luchette FA, Esposito TJ, et al. Old fashion clinical judgment in the era of protocols: is mandatory chest x-ray necessary in injured patients? J Trauma 2005; 59:324–332.
- Traub M, Stevenson M, McEvoy S, et al. The use of chest computed tomography versus chest x-ray in patients with major blunt trauma. Injury 2007; 38:43–47.
- Trupka A, Waydhas C, Hallfeldt KK, Nast-Kolb D, Pfeifer KJ, Schweiberer L. Value of thoracic computed tomography in the first assessment of severely injured patients with blunt chest trauma: results of a prospective study. J Trauma 1997; 43:405–412.
- Malghem J, Vande Berg B, Lecouvet F, Maldague B. Costal cartilage fractures as revealed on CT and sonography. AJR Am J Roentgenol 2001; 176:429–432.
- Griffith JF, Rainer TH, Ching AS, Law KL, Cocks RA, Metreweli C. Sonography compared with radiography in revealing acute rib fracture. AJR Am J Roentgenol 1999; 173:1603–1609.
- Niitsu M, Takeda T. Solitary hot spots in the ribs on bone scan: value of thin-section reformatted computed tomography to exclude radiography negative fractures. J Comput Assist Tomogr 2003; 27:469–474.
KEY POINTS
- Knowing the number of ribs fractured may influence treatment decisions, such as whether to transfer a patient to a trauma center.
- Classic clinical signs and symptoms of rib fracture include point tenderness, focally referred pain with general chest compression, splinting, bony crepitus, and ecchymosis.
- In a patient with minor blunt trauma, when there is little suspicion of associated injury or complication, plain radiography is likely sufficient.
- Computed tomography is the imaging study of choice in patients with penetrating or major chest or abdominal trauma.
A 44-year-old man with hemoptysis: A review of pertinent imaging studies and radiographic interventions
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
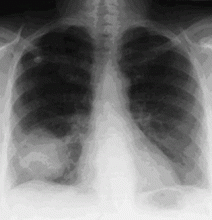
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
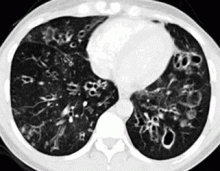
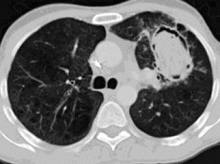
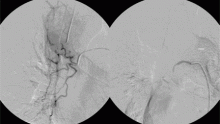
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
KEY POINTS
- We recommend chest radiography in the initial stages of evaluation of hemoptysis, whether the hemoptysis is massive or nonmassive.
- In cases of hemoptysis that is intermittent (whether massive or nonmassive) in patients whose condition is stable, CT, multidetector CT angiography, and bronchoscopy are all useful.
- In cases of hemoptysis that is active, persistent, and massive, multidetector CT angiography, bronchoscopy, and conventional bronchial angiography are all useful, depending on the hemodynamic stability of the patient.
- Bronchial artery embolization is the preferred noninvasive first-line treatment for hemoptysis and offers an excellent alternative to surgery for patients who are poor candidates for surgery.